User login
NEED HAIR: Pinpointing the cause of a patient’s hair loss
Telogen effluvium (TE)—temporary hair loss due to the shedding of telogen (resting phase of hair cycle) hair after exposure to some form of stress—is one of the most common causes of diffuse non-scarring hair loss from the scalp.1 TE is more common than anagen (growing phase of hair cycle) hair loss.2 Hair loss can be triggered by numerous factors, including certain psychotropic medications.3,4 Mood stabilizers, such as valproic acid and lithium, are most commonly implicated. Hair loss also has been associated with the use of the first-generation antipsychotics haloperidol and chlorpromazine and the second-generation antipsychotics olanzapine, quetiapine, and risperidone.5-7 We recently cared for a woman with bipolar I disorder and generalized anxiety disorder who was prescribed lurasidone and later developed TE. Her hair loss completely resolved 4 months after lurasidone was discontinued.
TE can be triggered by events that interrupt the normal hair growth cycle. Typically, it is observed approximately 3 months after a triggering event and is usually self-limiting, lasting approximately 6 months.7 Diagnostic features include a strongly positive hair-pull test, a trichogram showing >25% telogen hair, and a biopsy showing an increase in telogen follicles.8,9 To conduct the hair-pull test, grasp approximately 40 to 60 hairs between the thumb and forefinger while slightly stretching the scalp to allow your fingers to slide along the length of the hair. Usually only 2 to 3 hairs in the telogen phase can be plucked via this method; if >10% of the hairs grasped can be plucked, this indicates a pathologic process.10
Significant hair loss, particularly among women, is a distressing adverse effect that can be an important moderator of compliance, treatment adherence, and relapse. To help clinicians narrow down the wide range of potential causes of a patient’s hair loss, we created the mnemonic NEED HAIR.
Nutritional: Iron deficiency anemia, a “crash” diet, zinc deficiency, vitamin B6 or B12 deficiency, chronic starvation, diarrhea, hypoproteinemia (metabolic or dietary origin), malabsorption, or heavy metal ingestion.1
Endocrine: Thyroid disorders and the early stage of androgenic alopecia.8,11
Environmental: Stress from a severe febrile illness, emotional stress, serious injuries, major surgery, large hemorrhage, and difficult labor.12
Drugs: Oral contraceptives, antithyroid drugs, retinoids (etretinate and acitretin), anticonvulsants, antidepressants, antipsychotics, hypolipidemic drugs, anticoagulants, antihypertensives (beta blockers, angiotensin-converting enzyme inhibitors), and cytotoxic drugs.1,4-7
Continue to: Hormonal fluctuations
Hormonal fluctuations: Polycystic ovarian syndrome and postpartum hormonal changes.11
Autoimmune: Lupus erythematosus, dermatomyositis, scleroderma, Hashimoto’s thyroiditis, Sjögren’s syndrome, inflammatory bowel disease, and autoimmune atrophic gastritis.12,13
Infections and chronic illnesses: Fungal infections (eg, tinea capitis), human immunodeficiency virus, syphilis, typhoid, malaria, tuberculosis, malignancy, renal failure, hepatic failure, and other chronic illnesses.1,9
Radiation: Radiation treatment and excessive UV exposure.12,14,15
Treatment for hair loss depends on the specific caus
1. Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venerol Leprol. 2013;79(5):591-603.
2. Harrison S, Bergfeld W. Diffuse hair loss: its triggers and management. Cleve Clin J Med. 2009;76(6):361-367.
3. Gautam M. Alopecia due to psychotropic medications. Ann Pharmacother. 1999;33(5):631-637.
4. Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12(1):35-42.
5. Kubota T, Ishikura T, Jibiki I. Alopecia areata associated with haloperidol. Jpn J Psychiatry Neurol. 1994;48(3):579-581.
6. McLean RM, Harrison-Woolrych M. Alopecia associated with quetiapine. Int Clin Psychopharmacol 2007;22(2):117-9.
7. Kulolu M, Korkmaz S, Kilic N, et al. Olanzapine induced hair loss: a case report. Bulletin of Clinical Psychopharmacology. 2012;22(4):362-365.
8. Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9(9):WE01-WE03.
9. Shrivastava SB. Diffuse hair loss in an adult female: approach to diagnosis and management. Indian J Dermatol Venerol Leprol. 2009;75(1):20-28; quiz 27-28.
10. Piérard GE, Piérard-Franchimont C, Marks R, et al; EEMCO group (European Expert Group on Efficacy Measurement of Cosmetics and Other Topical Products). EEMCO guidance for the assessment of hair shedding and alopecia. Skin Pharmacol Physiol. 2004;17(2):98-110.
11. Mirallas O, Grimalt R. The postpartum telogen effluvium fallacy. Skin Appendage Disord. 2016;1(4):198-201.
12. Rebora A. Proposing a simpler classification of telogen effluvium. Skin Appendage Disord. 2016;2(1-2):35-38.
13. Cassano N, Amerio P, D’Ovidio R, et al. Hair disorders associated with autoimmune connective tissue diseases. G Ital Dermatol Venereol. 2014;149(5):555-565.
14. Trüeb RM. Telogen effluvium: is there a need for a new classification? Skin Appendage Disord. 2016;2(1-2):39-44.
15. Ali SY, Singh G. Radiation-induced alopecia. Int J Trichology. 2010;2(2):118-119.
Telogen effluvium (TE)—temporary hair loss due to the shedding of telogen (resting phase of hair cycle) hair after exposure to some form of stress—is one of the most common causes of diffuse non-scarring hair loss from the scalp.1 TE is more common than anagen (growing phase of hair cycle) hair loss.2 Hair loss can be triggered by numerous factors, including certain psychotropic medications.3,4 Mood stabilizers, such as valproic acid and lithium, are most commonly implicated. Hair loss also has been associated with the use of the first-generation antipsychotics haloperidol and chlorpromazine and the second-generation antipsychotics olanzapine, quetiapine, and risperidone.5-7 We recently cared for a woman with bipolar I disorder and generalized anxiety disorder who was prescribed lurasidone and later developed TE. Her hair loss completely resolved 4 months after lurasidone was discontinued.
TE can be triggered by events that interrupt the normal hair growth cycle. Typically, it is observed approximately 3 months after a triggering event and is usually self-limiting, lasting approximately 6 months.7 Diagnostic features include a strongly positive hair-pull test, a trichogram showing >25% telogen hair, and a biopsy showing an increase in telogen follicles.8,9 To conduct the hair-pull test, grasp approximately 40 to 60 hairs between the thumb and forefinger while slightly stretching the scalp to allow your fingers to slide along the length of the hair. Usually only 2 to 3 hairs in the telogen phase can be plucked via this method; if >10% of the hairs grasped can be plucked, this indicates a pathologic process.10
Significant hair loss, particularly among women, is a distressing adverse effect that can be an important moderator of compliance, treatment adherence, and relapse. To help clinicians narrow down the wide range of potential causes of a patient’s hair loss, we created the mnemonic NEED HAIR.
Nutritional: Iron deficiency anemia, a “crash” diet, zinc deficiency, vitamin B6 or B12 deficiency, chronic starvation, diarrhea, hypoproteinemia (metabolic or dietary origin), malabsorption, or heavy metal ingestion.1
Endocrine: Thyroid disorders and the early stage of androgenic alopecia.8,11
Environmental: Stress from a severe febrile illness, emotional stress, serious injuries, major surgery, large hemorrhage, and difficult labor.12
Drugs: Oral contraceptives, antithyroid drugs, retinoids (etretinate and acitretin), anticonvulsants, antidepressants, antipsychotics, hypolipidemic drugs, anticoagulants, antihypertensives (beta blockers, angiotensin-converting enzyme inhibitors), and cytotoxic drugs.1,4-7
Continue to: Hormonal fluctuations
Hormonal fluctuations: Polycystic ovarian syndrome and postpartum hormonal changes.11
Autoimmune: Lupus erythematosus, dermatomyositis, scleroderma, Hashimoto’s thyroiditis, Sjögren’s syndrome, inflammatory bowel disease, and autoimmune atrophic gastritis.12,13
Infections and chronic illnesses: Fungal infections (eg, tinea capitis), human immunodeficiency virus, syphilis, typhoid, malaria, tuberculosis, malignancy, renal failure, hepatic failure, and other chronic illnesses.1,9
Radiation: Radiation treatment and excessive UV exposure.12,14,15
Treatment for hair loss depends on the specific caus
Telogen effluvium (TE)—temporary hair loss due to the shedding of telogen (resting phase of hair cycle) hair after exposure to some form of stress—is one of the most common causes of diffuse non-scarring hair loss from the scalp.1 TE is more common than anagen (growing phase of hair cycle) hair loss.2 Hair loss can be triggered by numerous factors, including certain psychotropic medications.3,4 Mood stabilizers, such as valproic acid and lithium, are most commonly implicated. Hair loss also has been associated with the use of the first-generation antipsychotics haloperidol and chlorpromazine and the second-generation antipsychotics olanzapine, quetiapine, and risperidone.5-7 We recently cared for a woman with bipolar I disorder and generalized anxiety disorder who was prescribed lurasidone and later developed TE. Her hair loss completely resolved 4 months after lurasidone was discontinued.
TE can be triggered by events that interrupt the normal hair growth cycle. Typically, it is observed approximately 3 months after a triggering event and is usually self-limiting, lasting approximately 6 months.7 Diagnostic features include a strongly positive hair-pull test, a trichogram showing >25% telogen hair, and a biopsy showing an increase in telogen follicles.8,9 To conduct the hair-pull test, grasp approximately 40 to 60 hairs between the thumb and forefinger while slightly stretching the scalp to allow your fingers to slide along the length of the hair. Usually only 2 to 3 hairs in the telogen phase can be plucked via this method; if >10% of the hairs grasped can be plucked, this indicates a pathologic process.10
Significant hair loss, particularly among women, is a distressing adverse effect that can be an important moderator of compliance, treatment adherence, and relapse. To help clinicians narrow down the wide range of potential causes of a patient’s hair loss, we created the mnemonic NEED HAIR.
Nutritional: Iron deficiency anemia, a “crash” diet, zinc deficiency, vitamin B6 or B12 deficiency, chronic starvation, diarrhea, hypoproteinemia (metabolic or dietary origin), malabsorption, or heavy metal ingestion.1
Endocrine: Thyroid disorders and the early stage of androgenic alopecia.8,11
Environmental: Stress from a severe febrile illness, emotional stress, serious injuries, major surgery, large hemorrhage, and difficult labor.12
Drugs: Oral contraceptives, antithyroid drugs, retinoids (etretinate and acitretin), anticonvulsants, antidepressants, antipsychotics, hypolipidemic drugs, anticoagulants, antihypertensives (beta blockers, angiotensin-converting enzyme inhibitors), and cytotoxic drugs.1,4-7
Continue to: Hormonal fluctuations
Hormonal fluctuations: Polycystic ovarian syndrome and postpartum hormonal changes.11
Autoimmune: Lupus erythematosus, dermatomyositis, scleroderma, Hashimoto’s thyroiditis, Sjögren’s syndrome, inflammatory bowel disease, and autoimmune atrophic gastritis.12,13
Infections and chronic illnesses: Fungal infections (eg, tinea capitis), human immunodeficiency virus, syphilis, typhoid, malaria, tuberculosis, malignancy, renal failure, hepatic failure, and other chronic illnesses.1,9
Radiation: Radiation treatment and excessive UV exposure.12,14,15
Treatment for hair loss depends on the specific caus
1. Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venerol Leprol. 2013;79(5):591-603.
2. Harrison S, Bergfeld W. Diffuse hair loss: its triggers and management. Cleve Clin J Med. 2009;76(6):361-367.
3. Gautam M. Alopecia due to psychotropic medications. Ann Pharmacother. 1999;33(5):631-637.
4. Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12(1):35-42.
5. Kubota T, Ishikura T, Jibiki I. Alopecia areata associated with haloperidol. Jpn J Psychiatry Neurol. 1994;48(3):579-581.
6. McLean RM, Harrison-Woolrych M. Alopecia associated with quetiapine. Int Clin Psychopharmacol 2007;22(2):117-9.
7. Kulolu M, Korkmaz S, Kilic N, et al. Olanzapine induced hair loss: a case report. Bulletin of Clinical Psychopharmacology. 2012;22(4):362-365.
8. Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9(9):WE01-WE03.
9. Shrivastava SB. Diffuse hair loss in an adult female: approach to diagnosis and management. Indian J Dermatol Venerol Leprol. 2009;75(1):20-28; quiz 27-28.
10. Piérard GE, Piérard-Franchimont C, Marks R, et al; EEMCO group (European Expert Group on Efficacy Measurement of Cosmetics and Other Topical Products). EEMCO guidance for the assessment of hair shedding and alopecia. Skin Pharmacol Physiol. 2004;17(2):98-110.
11. Mirallas O, Grimalt R. The postpartum telogen effluvium fallacy. Skin Appendage Disord. 2016;1(4):198-201.
12. Rebora A. Proposing a simpler classification of telogen effluvium. Skin Appendage Disord. 2016;2(1-2):35-38.
13. Cassano N, Amerio P, D’Ovidio R, et al. Hair disorders associated with autoimmune connective tissue diseases. G Ital Dermatol Venereol. 2014;149(5):555-565.
14. Trüeb RM. Telogen effluvium: is there a need for a new classification? Skin Appendage Disord. 2016;2(1-2):39-44.
15. Ali SY, Singh G. Radiation-induced alopecia. Int J Trichology. 2010;2(2):118-119.
1. Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venerol Leprol. 2013;79(5):591-603.
2. Harrison S, Bergfeld W. Diffuse hair loss: its triggers and management. Cleve Clin J Med. 2009;76(6):361-367.
3. Gautam M. Alopecia due to psychotropic medications. Ann Pharmacother. 1999;33(5):631-637.
4. Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12(1):35-42.
5. Kubota T, Ishikura T, Jibiki I. Alopecia areata associated with haloperidol. Jpn J Psychiatry Neurol. 1994;48(3):579-581.
6. McLean RM, Harrison-Woolrych M. Alopecia associated with quetiapine. Int Clin Psychopharmacol 2007;22(2):117-9.
7. Kulolu M, Korkmaz S, Kilic N, et al. Olanzapine induced hair loss: a case report. Bulletin of Clinical Psychopharmacology. 2012;22(4):362-365.
8. Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9(9):WE01-WE03.
9. Shrivastava SB. Diffuse hair loss in an adult female: approach to diagnosis and management. Indian J Dermatol Venerol Leprol. 2009;75(1):20-28; quiz 27-28.
10. Piérard GE, Piérard-Franchimont C, Marks R, et al; EEMCO group (European Expert Group on Efficacy Measurement of Cosmetics and Other Topical Products). EEMCO guidance for the assessment of hair shedding and alopecia. Skin Pharmacol Physiol. 2004;17(2):98-110.
11. Mirallas O, Grimalt R. The postpartum telogen effluvium fallacy. Skin Appendage Disord. 2016;1(4):198-201.
12. Rebora A. Proposing a simpler classification of telogen effluvium. Skin Appendage Disord. 2016;2(1-2):35-38.
13. Cassano N, Amerio P, D’Ovidio R, et al. Hair disorders associated with autoimmune connective tissue diseases. G Ital Dermatol Venereol. 2014;149(5):555-565.
14. Trüeb RM. Telogen effluvium: is there a need for a new classification? Skin Appendage Disord. 2016;2(1-2):39-44.
15. Ali SY, Singh G. Radiation-induced alopecia. Int J Trichology. 2010;2(2):118-119.
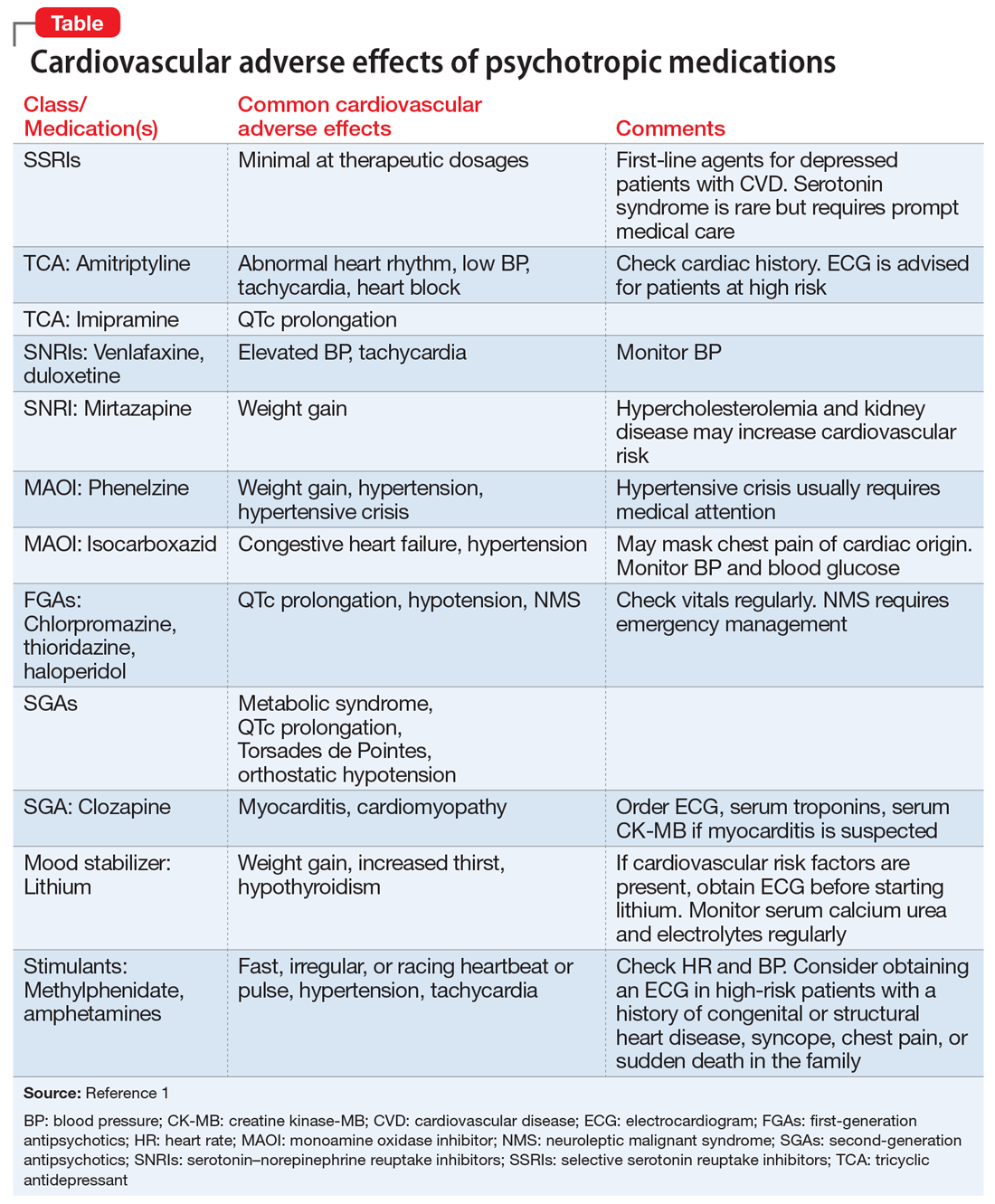
Cardiovascular adverse effects of psychotropics: What to look for
Most patients who take psychotropic medications are at low risk for cardiovascular adverse effects from these medications, and require only routine monitoring. However, patients with severe mental illness, those with a personal or family history of cardiovascular disease, or those receiving high doses or multiple medications are considered at high risk for morbidity and mortality from cardiovascular adverse effects. Such patients may need more careful cardiovascular monitoring.
To help identify important cardiovascular-related adverse effects of various psychotropics, we summarize these risks, and offer guidance about what you can do when your patient experiences them (Table).1
Antipsychotics and metabolic syndrome
Patients who take antipsychotics should be monitored for metabolic syndrome. The presence of 3 of the following 5 parameters is considered positive for metabolic syndrome2:
- fasting glucose ≥100 mg/dL or hemoglobin A1c ≥5.6%
- blood pressure ≥130/85 mm Hg
- triglycerides ≥150 mg/dL
- high-density lipoprotein cholesterol
- waist circumference ≥102 cm in men or ≥88 cm in women.
Stimulants and sudden cardiac death
Sudden cardiac death (SCD) in children and adolescents who take stimulants to treat attention-deficit/hyperactivity disorder is rare. For these patients, the risk of SCD is no higher than that of the general population.3 For patients who do not have any known cardiac risk factors, the American Academy of Pediatrics does not recommend performing any cardiac tests before starting stimulants.3
1. Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol. 2008;23(suppl 1):S3-S14.
2. Grundy SM, Brewer HB Jr, Cleeman JI, et al; National Heart, Lung, and Blood Institute; American Heart Association. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24(2):e13-e18.
3. American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines. Pediatrics. 2004;114(3):874-877.
Most patients who take psychotropic medications are at low risk for cardiovascular adverse effects from these medications, and require only routine monitoring. However, patients with severe mental illness, those with a personal or family history of cardiovascular disease, or those receiving high doses or multiple medications are considered at high risk for morbidity and mortality from cardiovascular adverse effects. Such patients may need more careful cardiovascular monitoring.
To help identify important cardiovascular-related adverse effects of various psychotropics, we summarize these risks, and offer guidance about what you can do when your patient experiences them (Table).1

Antipsychotics and metabolic syndrome
Patients who take antipsychotics should be monitored for metabolic syndrome. The presence of 3 of the following 5 parameters is considered positive for metabolic syndrome2:
- fasting glucose ≥100 mg/dL or hemoglobin A1c ≥5.6%
- blood pressure ≥130/85 mm Hg
- triglycerides ≥150 mg/dL
- high-density lipoprotein cholesterol
- waist circumference ≥102 cm in men or ≥88 cm in women.
Stimulants and sudden cardiac death
Sudden cardiac death (SCD) in children and adolescents who take stimulants to treat attention-deficit/hyperactivity disorder is rare. For these patients, the risk of SCD is no higher than that of the general population.3 For patients who do not have any known cardiac risk factors, the American Academy of Pediatrics does not recommend performing any cardiac tests before starting stimulants.3
Most patients who take psychotropic medications are at low risk for cardiovascular adverse effects from these medications, and require only routine monitoring. However, patients with severe mental illness, those with a personal or family history of cardiovascular disease, or those receiving high doses or multiple medications are considered at high risk for morbidity and mortality from cardiovascular adverse effects. Such patients may need more careful cardiovascular monitoring.
To help identify important cardiovascular-related adverse effects of various psychotropics, we summarize these risks, and offer guidance about what you can do when your patient experiences them (Table).1

Antipsychotics and metabolic syndrome
Patients who take antipsychotics should be monitored for metabolic syndrome. The presence of 3 of the following 5 parameters is considered positive for metabolic syndrome2:
- fasting glucose ≥100 mg/dL or hemoglobin A1c ≥5.6%
- blood pressure ≥130/85 mm Hg
- triglycerides ≥150 mg/dL
- high-density lipoprotein cholesterol
- waist circumference ≥102 cm in men or ≥88 cm in women.
Stimulants and sudden cardiac death
Sudden cardiac death (SCD) in children and adolescents who take stimulants to treat attention-deficit/hyperactivity disorder is rare. For these patients, the risk of SCD is no higher than that of the general population.3 For patients who do not have any known cardiac risk factors, the American Academy of Pediatrics does not recommend performing any cardiac tests before starting stimulants.3
1. Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol. 2008;23(suppl 1):S3-S14.
2. Grundy SM, Brewer HB Jr, Cleeman JI, et al; National Heart, Lung, and Blood Institute; American Heart Association. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24(2):e13-e18.
3. American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines. Pediatrics. 2004;114(3):874-877.
1. Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol. 2008;23(suppl 1):S3-S14.
2. Grundy SM, Brewer HB Jr, Cleeman JI, et al; National Heart, Lung, and Blood Institute; American Heart Association. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24(2):e13-e18.
3. American Academy of Pediatrics Steering Committee on Quality Improvement and Management. Classifying recommendations for clinical practice guidelines. Pediatrics. 2004;114(3):874-877.
Dietary recommendations for patients with diabetes
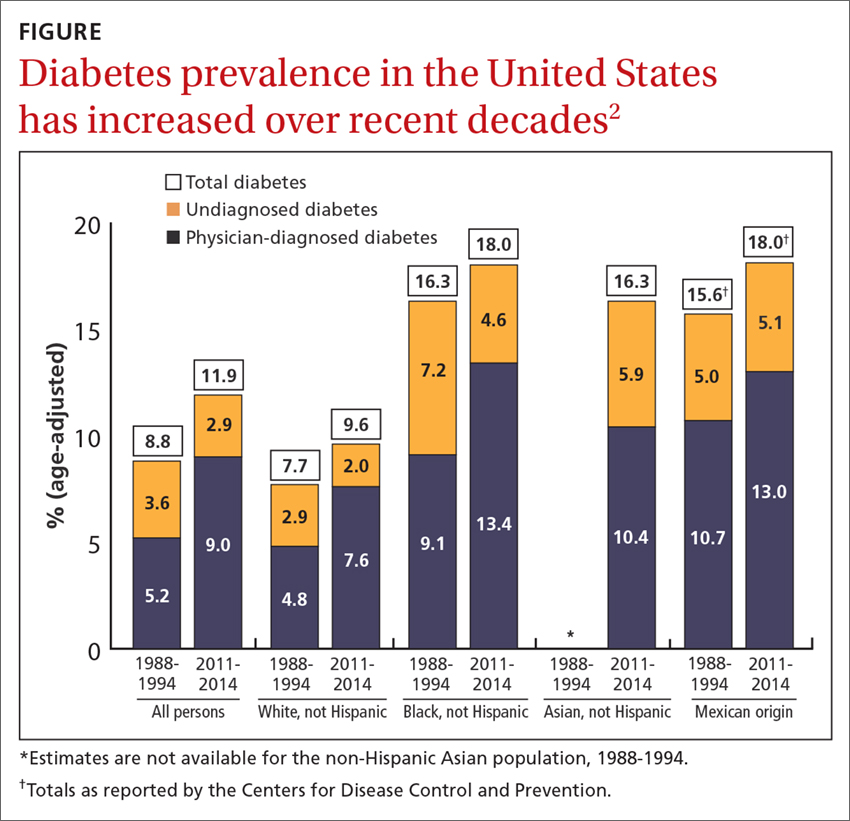
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2
Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
Thoracic aortic aneurysm: How to counsel, when to refer
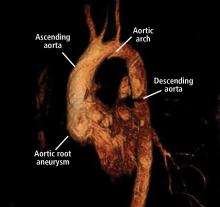
Thoracic aortic aneurysm (TAA) needs to be detected, monitored, and managed in a timely manner to prevent a serious consequence such as acute dissection or rupture. But only about 5% of patients experience symptoms before an acute event occurs, and for the other 95% the first “symptom” is often death.1 Most cases are detected either incidentally with echocardiography, computed tomography (CT), or magnetic resonance imaging (MRI) during workup for another condition. Patients may also be diagnosed during workup of a murmur or after a family member is found to have an aneurysm. Therefore, its true incidence is difficult to determine.2
With these facts in mind, how would you manage the following 2 cases?
Case 1: Bicuspid aortic valve, ascending aortic aneurysm
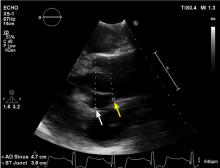
A 45-year-old man with stage 1 hypertension presents for evaluation of a bicuspid aortic valve and ascending aortic aneurysm. He has several first-degree relatives with similar conditions, and his brother recently underwent elective aortic repair. At the urging of his primary care physician, he underwent screening echocardiography, which demonstrated a “dilated root and ascending aorta” 4.6 cm in diameter. He presents today to discuss management options and how the aneurysm could affect his everyday life.
Case 2: Marfan syndrome in a young woman
A 24-year-old woman with Marfan syndrome diagnosed in adolescence presents for annual follow-up. She has many family members with the same condition, and several have undergone prophylactic aortic root repair. Her aortic root has been monitored annually for progression of dilation, and today it is 4.6 cm in diameter, a 3-mm increase from the last measurement. She has grade 2+ aortic insufficiency (on a scale of 1+ to 4+) based on echocardiography, but she has no symptoms. She is curious about what size her aortic root will need to reach for surgery to be considered.
LIKELY UNDERDETECTED
TAA is being detected more often than in the past thanks to better detection methods and heightened awareness among physicians and patients. While an incidence rate of 10.4 per 100,000 patient-years is often cited,3 this figure likely underestimates the true incidence of this clinically silent condition. The most robust data come from studies based on in-hospital diagnostic codes coupled with data from autopsies for out-of-hospital deaths.
Olsson et al,4 in a 2016 study in Sweden, found the incidence of TAA and aortic dissection to be 16.3 per 100,000 per year for men and 9.1 per 100,000 per year for women.
Clouse et al5 reported the incidence of thoracic aortic dissection as 3.5 per 100,000 patient-years, and the same figure for thoracic aortic rupture.
Aneurysmal disease accounts for 52,000 deaths per year in the United States, making it the 19th most common cause of death.6 These figures are likely lower than the true mortality rate for this condition, given that aortic dissection is often mistaken for acute myocardial infarction or other acute event if an autopsy is not done to confirm the cause of death.7
RISK FACTORS FOR THORACIC AORTIC ANEURYSM
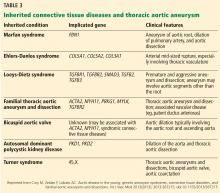
Risk factors for TAA include genetic conditions that lead to aortic medial weakness or destruction such as Loeys-Dietz syndrome and Marfan syndrome.2 In addition, family history is important even in the absence of known genetic mutations. Other risk factors include conditions that increase aortic wall stress, such as hypertension, cocaine abuse, extreme weightlifting, trauma, and aortic coarctation.2
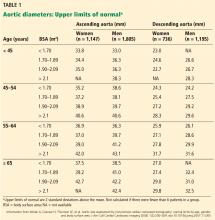
DIAMETER INCREASES WITH AGE, BODY SURFACE AREA
Normal dimensions for the aortic segments differ depending on age, sex, and body surface area.8,44,45 The size of the aortic root may also vary depending on how it is measured, due to the root’s trefoil shape. Measured sinus to sinus, the root is larger than when measured sinus to commissure on CT angiography or cardiac MRI. It is also larger when measured leading edge to leading edge than inner edge to inner edge on echocardiography.10
TAA is defined as an aortic diameter at least 50% greater than the upper limit of normal.8
Geometric changes in the curvature of the ascending aorta, aortic arch, and descending thoracic aorta can occur as the result of hypertension, atherosclerosis, or connective tissue disease.
HOW IS TAA DIAGNOSED?
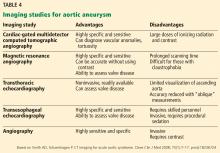
Imaging tests
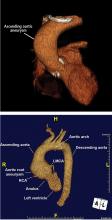
It is particularly important to obtain a gated CTA image in patients with aortic root aneurysm to avoid motion artifact and possible erroneous measurements. Gated CTA is done with electrocardiographic synchronization and allows for image processing to correct for cardiac motion.
HOW IS TAA CLASSIFIED?
TAA can be caused by a variety of inherited and sporadic conditions. These differences in pathogenesis lend themselves to classification of aneurysms into groups. Table 3 highlights the most common conditions associated with TAA.13
Bicuspid aortic valve aortopathy
From 1% to 2% of people have a bicuspid aortic valve, with a 3-to-1 male predominance.14,15 Aortic dilation occurs in 35% to 80% of people who have a bicuspid aortic valve, conferring a risk of dissection 8 times higher than in the general population.16–18
The pathogenic mechanisms that lead to this condition are widely debated, although a combination of genetic defects leading to intrinsic weakening of the aortic wall and hemodynamic effects likely contribute.19 Evidence of hemodynamic contributions to aortic dilation comes from findings that particular patterns of cusp fusion of the bicuspid aortic valve result in changes in transvalvular flow, placing more stress on specific regions of the ascending aorta.20,21 These hemodynamic alterations result in patterns of aortic dilation that depend on cusp fusion and the presence of valvular disease.
Multiple small studies found that replacing bicuspid aortic valves reduced the rate of aortic dilation, suggesting that hemodynamic factors may play a larger role than intrinsic wall properties in genetically susceptible individuals.22,23 However, larger studies are needed before any definitive conclusions can be made.
HOW IS ANEURYSM MANAGED ON AN OUTPATIENT BASIS?
Patients with a new diagnosis of TAA should be referred to a cardiologist with expertise in managing aortic disease or to a cardiac surgeon specializing in aortic surgery, depending on the initial size of the aneurysm.
Control blood pressure with beta-blockers
Medical management for patients with TAA has historically been limited to strict blood pressure control aimed at reducing aortic wall stress, mainly with beta-blockers.
Are angiotensin II receptor blockers (ARBs) beneficial? Studies in a mouse model of Marfan syndrome revealed that the ARB losartan attenuated aortic root growth.24 The results of early, small studies in humans were promising,25–27 but larger randomized trials have shown no advantage of losartan over beta-blockers in slowing aortic root growth.28 These negative results led many to question the effectiveness of losartan, although some point out that no studies have shown even beta-blockers to be beneficial in reducing the clinical end points of death or dissection.29 On the other hand, patients with certain FBN1 mutations respond more readily than others to losartan.30 Additional clinical trials of ARBs in Marfan syndrome are ongoing.
Current guidelines recommend stringent blood pressure control and smoking cessation for patients with a small aneurysm not requiring surgery and for those who are considered unsuitable for surgical or percutaneous intervention (level of evidence C, the lowest).2 For patients with TAA, it is considered reasonable to give beta-blockers. Angiotensin-converting enzyme inhibitors or ARBs may be used in combination with beta-blockers, titrated to the lowest tolerable blood pressure without adverse effects (level of evidence B).2
The recommended target blood pressure is less than 140/90 mm Hg, or 130/80 mm Hg in those with diabetes or chronic kidney disease (level of evidence B).2 However, we recommend more stringent blood pressure control: ie, less than 130/80 mm Hg for all patients with aortic aneurysm and a heart rate goal of 70 beats per minute or less, as tolerated.
Activity restriction
Activity restrictions for patients with TAA are largely based on theory, and certain activities may require more modification than others. For example, heavy lifting should be discouraged, as it may increase blood pressure significantly for short periods of time.2,31 The increased wall stress, in theory, could initiate dissection or rupture. However, moderate-intensity aerobic activity is rarely associated with significant elevations in blood pressure and should be encouraged. Stressful emotional states have been anecdotally associated with aortic dissection; thus, measures to reduce stress may offer some benefit.31
Our recommendations. While there are no published guidelines regarding activity restrictions in patients with TAA, we use a graded approach based on aortic diameter:
- 4.0 to 4.4 cm—lift no more than 75 pounds
- 4.5 to 5 cm—lift no more than 50 pounds
- 5 cm—lift no more than 25 pounds.
We also recommend not lifting anything heavier than half of one’s body weight and to avoid breath-holding or performing the Valsalva maneuver while lifting. Although these recommendations are somewhat arbitrary, based on theory and a large clinical experience at our aortic center, they seem reasonable and practical.
Activity restrictions should be stringent and individualized in patients with Marfan, Loeys-Dietz, or Ehlers-Danlos syndrome due to increased risk of dissection or rupture even if the aorta is normal in size.
We sometimes recommend exercise stress testing to assess the heart rate and blood pressure response to exercise, and we are developing research protocols to help tailor activity recommendations.
WHEN SHOULD A PATIENT BE REFERRED?
To a cardiologist at the time of diagnosis
As soon as TAA is diagnosed, the patient should be referred to a cardiologist who has special interest in aortic disease. This will allow for appropriate and timely decisions about medical management, imaging, follow-up, and referral to surgery. Additional recommendations for screening of family members and referral to clinical geneticists can be discussed at this juncture. Activity restrictions should be reviewed at the initial evaluation.
To a surgeon relatively early
Size thresholds for surgical intervention are discussed below, but one should not wait until these thresholds are reached to send the patient for surgical consultation. It is beneficial to the state of mind of a potential surgical candidate to have early discussions pertaining to the types of operations available, their outcomes, and associated risks and benefits. If a patient’s aortic size remains stable over time, he or she may be followed by the cardiologist until significant size or growth has been documented, at which time the patient and surgeon can reconvene to discuss options for definitive treatment.
To a clinical geneticist
If 1 or more first-degree relatives of a patient with TAA or dissection are found to have aneurysmal disease, referral to a clinical geneticist is very important for genetic testing of multiple genes that have been implicated in thoracic aortic aneurysm and dissection.
WHEN SHOULD TAA BE REPAIRED?
Surgery to prevent rupture or dissection remains the definitive treatment of TAA when size thresholds are reached, and symptomatic aneurysm should be operated on regardless of the size. However, rarely are thoracic aneurysms symptomatic unless they rupture or dissect. The size criteria are based on underlying genetic etiology if known and on the behavior and natural course of TAA.
Size and other factors
Treatment should be tailored to the patient’s clinical scenario, family history, and estimated risk of rupture or dissection, balanced against the individual center’s outcomes of elective aortic replacement.32 For example, young and otherwise healthy patients with TAA and a family history of aortic dissection (who may be more likely to have connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehler-Danlos syndrome) may elect to undergo repair when the aneurysm reaches or nearly reaches the diameter of that of the family member’s aorta when dissection occurred.2 On the other hand, TAA of degenerative etiology (eg, related to smoking or hypertension) measuring less than 5.5 cm in an older patient with comorbidities poses a lower risk of a catastrophic event such as dissection or rupture than the risk of surgery.11
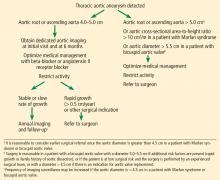
Thresholds for surgery. Once the diameter of the ascending aorta reaches 6 cm, the likelihood of an acute dissection is 31%.11 A similar threshold is reached for the descending aorta at a size of 7 cm.11 Therefore, to avoid high-risk emergency surgery on an acutely dissected aorta, surgery on an ascending aortic aneurysm of degenerative etiology is usually suggested when the aneurysm reaches 5.5 cm or a documented growth rate greater than 0.5 cm/year.2,33
Additionally, in patients already undergoing surgery for valvular or coronary disease, prophylactic aortic replacement is recommended if the ascending aorta is larger than 4.5 cm. The threshold for intervention is lower in patients with connective tissue disease (> 5.0 cm for Marfan syndrome, 4.4–4.6 cm for Loeys-Dietz syndrome).2,33
Observational studies suggest that the risk of aortic complications in patients with bicuspid aortic valve aortopathy is low overall, though significantly greater than in the general population.18,34,35 These findings led to changes in the 2014 American College of Cardiology/American Heart Association guidelines on valvular heart disease,36 suggesting a surgical threshold of 5.5 cm in the absence of significant valve disease or family history of dissection of an aorta of smaller diameter.
A 2015 study of dissection risk in patients with bicuspid aortic valve aortopathy by our group found a dramatic increase in risk of aortic dissection for ascending aortic diameters greater than 5.3 cm, and a gradual increase in risk for aortic root diameters greater than 5.0 cm.37 In addition, a near-constant 3% to 4% risk of dissection was present for aortic diameters ranging from 4.7 cm to 5.0 cm, revealing that watchful waiting carries its own inherent risks.37 In our surgical experience with this population, the hospital mortality rate and risk of stroke from aortic surgery were 0.25% and 0.75%, respectively.37 Thus, the decision to operate for aortic aneurysm in the setting of a bicuspid aortic valve should take into account patient-specific factors and institutional outcomes.
A statement of clarification in the American College of Cardiology/American Heart Association guidelines was published in 2015, recommending surgery for patients with an aortic diameter of 5.0 cm or greater if the patient is at low risk and the surgery is performed by an experienced surgical team at a center with established surgical expertise in this condition.38 However, current recommendations are for surgery at 5.5 cm if the above conditions are not met.
Ratio of aortic cross-sectional area to height
Although size alone has long been used to guide surgical intervention, a recent review from the International Registry of Aortic Dissection revealed that 59% of patients suffered aortic dissection at diameters less than 5.5 cm, and that patients with certain connective tissue diseases such as Loeys-Dietz syndrome or familial thoracic aneurysm and dissection had a documented propensity for dissection at smaller diameters.39–41
Size indices such as the aortic cross-sectional area indexed to height have been implemented in guidelines for certain patient populations (eg, 10 cm2/m in Marfan syndrome) and provide better risk stratification than size cutoffs alone.2,42
The ratio of aortic cross-sectional area to the patient’s height has also been applied to patients with bicuspid aortic valve-associated aortopathy and to those with a dilated aorta and a tricuspid aortic valve.43,44 Notably, a ratio greater than 10 cm2/m has been associated with aortic dissection in these groups, and this cutoff provides better stratification for prediction of death than traditional size metrics.27,28
HOW SHOULD PATIENTS BE SCREENED? WHAT FOLLOW-UP IS NECESSARY?
Initial screening and follow-up
Follow-up of TAA depends on the initial aortic size or rate of growth, or both. For patients presenting for the first time with TAA, it is reasonable to obtain definitive aortic imaging with CT or magnetic resonance angiography (MRA), then to repeat imaging at 6 months to document stability. If the aortic dimensions remain stable, then annual follow-up with CT or MRA is reasonable.2
Our flow chart of initial screening and follow-up is shown in Figure 5.
Screening of family members
In our center, we routinely recommend screening of all first-degree relatives of patients with TAA. Aortic imaging with echocardiography plus CT or MRI should be considered to detect asymptomatic disease.2 In patients with a strong family history (ie, multiple relatives affected with aortic aneurysm, dissection, or sudden cardiac death), genetic screening and testing for known mutations are recommended for the patient as well as for the family members.
If a mutation is identified in a family, then first-degree relatives should undergo genetic screening for the mutation and aortic imaging.2 Imaging in second-degree relatives may also be considered if one or more first-degree relatives are found to have aortic dilation.2
We recommend similar screening of first-degree family members of patients with bicuspid aortic valve aortopathy. In patients with young children, we recommend obtaining an echocardiogram of the child to look for a bicuspid aortic valve or aortic dilation. If an abnormality is detected or suspected, dedicated imaging with MRA to assess aortic dimensions is warranted.
BACK TO OUR PATIENT WITH A BICUSPID AORTIC VALVE
Our patient with a bicuspid aortic valve had a 4.6-cm root, an ascending aortic aneurysm, and several affected family members.
We would obtain dedicated aortic imaging at this patient’s initial visit with either gated CT with contrast or MRA, and we would obtain a cardioaortic surgery consult. We would repeat these studies at a follow-up visit 6 months later to detect any aortic growth compared with initial studies, and follow up annually thereafter. Echocardiography can also be done at the initial visit to determine if valvular disease is present that may influence clinical decisions.
Surgery would likely be recommended once the root reached a maximum area-to-height ratio greater than 10 cm2/m, or if the valve became severely dysfunctional during follow-up.
BACK TO OUR PATIENT WITH MARFAN SYNDROME
The young woman with Marfan syndrome has a 4.6-cm aortic root aneurysm and 2+ aortic insufficiency. Her question pertains to the threshold at which an operation would be considered. This question is complicated and is influenced by several concurrent clinical features in her presentation.
Starting with size criteria, patients with Marfan syndrome should be considered for elective aortic root repair at a diameter greater than 5 cm. However, an aortic cross-sectional area-to-height ratio greater than 10 cm2/m may provide a more robust metric for clinical decision-making than aortic diameter alone. Additional factors such as degree of aortic insufficiency and deleterious left ventricular remodeling may urge one to consider aortic root repair at a diameter of 4.5 cm.
These factors, including rate of growth and the surgeon’s assessment about his or her ability to preserve the aortic valve during repair, should be considered collectively in this scenario.
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010; 55(9):841–857. doi:10.1016/j.jacc.2009.08.084
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. Anesth Analg 2010; 111(2):279–315. doi:10.1213/ANE.0b013e3181dd869b
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280(22):1926–1929. pmid:9851478
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114(24):2611–2618. doi:10.1161/CIRCULATIONAHA.106.630400
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004; 79(2):176–180. pmid:14959911
- US Centers for Disease Control and Prevention (CDC). National Center for Injury Prevention and Control. WISQARS leading causes of death reports, 1999 – 2007. https://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. Accessed May 21, 2018.
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99(6):852–856. doi:10.1016/j.amjcard.2006.10.055
- Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014; 64(16):1725–1739. doi:10.1016/j.jacc.2014.08.025
- Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008; 1(2):200–209. doi:10.1016/j.jcmg.2007.11.005
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002; 74(5):S1877–S1880; discussion S1892–S1898. pmid:12440685
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75(1):7–17. pmid:18236724
- Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013(2013); 2013:267215. doi:10.1155/2013/267215
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39(12):1890–1900. doi:10.1016/S0735-1097(02)01886-7
- Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002; 106(8):900–904. pmid:12186790
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007; 31(3):397–405. doi:10.1016/j.ejcts.2006.12.006
- Jackson V, Petrini J, Caidahl K, et al. Bicuspid aortic valve leaflet morphology in relation to aortic root morphology: a study of 300 patients undergoing open-heart surgery. Eur J Cardiothorac Surg 2011; 40(3):e118–e124. doi:10.1016/j.ejcts.2011.04.014
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112. doi:10.1001/jama.2011.1286
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014; 370(20):1920–1929. doi:10.1056/NEJMra1207059
- Barker AJ, Markl M, Bürk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012; 5(4):457–466. doi:10.1161/CIRCIMAGING.112.973370
- Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010; 255(1):53–61. doi:10.1148/radiol.09091437
- Abdulkareem N, Soppa G, Jones S, Valencia O, Smelt J, Jahangiri M. Dilatation of the remaining aorta after aortic valve or aortic root replacement in patients with bicuspid aortic valve: a 5-year follow-up. Ann Thorac Surg 2013; 96(1):43–49. doi:10.1016/j.athoracsur.2013.03.086
- Regeer MV, Versteegh MI, Klautz RJ, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg 2016; 102(6):1981–1987. doi:10.1016/j.athoracsur.2016.05.038
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312(5770):117–121. doi:10.1126/science.1124287
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358(26):2787–2795. doi:10.1056/NEJMoa0706585
- Chiu HH, Wu MH, Wang JK, et al. Losartan added to ß-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013; 88(3):271–276. doi:10.1016/j.mayocp.2012.11.005
- Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013; 34(45):3491–3500. doi:10.1093/eurheartj/eht334
- Lacro RV, Dietz HC, Sleeper LA, et al; Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014; 371(22):2061–2071. doi:10.1056/NEJMoa1404731
- Ziganshin BA, Mukherjee SK, Elefteriades JA, et al. Atenolol versus losartan in Marfan’s syndrome (letters). N Engl J Med 2015; 372(10):977–981. doi:10.1056/NEJMc1500128
- Franken R, den Hartog AW, Radonic T, et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet 2015; 8(2):383–388. doi:10.1161/CIRCGENETICS.114.000950
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol 2008; 33(5):203–277. doi:10.1016/j.cpcardiol.2008.01.004
- Idrees JJ, Roselli EE, Lowry AM, et al. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101(6):2185–2192. doi:10.1016/j.athoracsur.2015.12.026
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016; 151(4):959–966. doi:10.1016/j.jtcvs.2015.12.001
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300(11):1317–1325. doi:10.1001/jama.300.11.1317
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002; 73(1):17–28. pmid:11834007
- Nishimura RA, Otto CM, Bono RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve–associated aneurysms. Ann Thorac Surg 2015; 100(5):1666–1674. doi:10.1016/j.athoracsur.2015.04.126
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 133(7):680–686. doi:10.1161/CIR.0000000000000331
- Pape LA, Tsai TT, Isselbacher EM, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116(10):1120–1127. doi:10.1161/CIRCULATIONAHA.107.702720
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355(8):788–798. doi:10.1056/NEJMoa055695
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39(12):1488–1493. doi:10.1038/ng.2007.6
- Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg 2002; 123(2):360–361. pmid:11828302
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003; 126(3):892–893. pmid:14502185
- Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134(22):1724–1737. doi:10.1161/CIRCULATIONAHA.116.022995
Thoracic aortic aneurysm (TAA) needs to be detected, monitored, and managed in a timely manner to prevent a serious consequence such as acute dissection or rupture. But only about 5% of patients experience symptoms before an acute event occurs, and for the other 95% the first “symptom” is often death.1 Most cases are detected either incidentally with echocardiography, computed tomography (CT), or magnetic resonance imaging (MRI) during workup for another condition. Patients may also be diagnosed during workup of a murmur or after a family member is found to have an aneurysm. Therefore, its true incidence is difficult to determine.2
With these facts in mind, how would you manage the following 2 cases?
Case 1: Bicuspid aortic valve, ascending aortic aneurysm
A 45-year-old man with stage 1 hypertension presents for evaluation of a bicuspid aortic valve and ascending aortic aneurysm. He has several first-degree relatives with similar conditions, and his brother recently underwent elective aortic repair. At the urging of his primary care physician, he underwent screening echocardiography, which demonstrated a “dilated root and ascending aorta” 4.6 cm in diameter. He presents today to discuss management options and how the aneurysm could affect his everyday life.
Case 2: Marfan syndrome in a young woman
A 24-year-old woman with Marfan syndrome diagnosed in adolescence presents for annual follow-up. She has many family members with the same condition, and several have undergone prophylactic aortic root repair. Her aortic root has been monitored annually for progression of dilation, and today it is 4.6 cm in diameter, a 3-mm increase from the last measurement. She has grade 2+ aortic insufficiency (on a scale of 1+ to 4+) based on echocardiography, but she has no symptoms. She is curious about what size her aortic root will need to reach for surgery to be considered.
LIKELY UNDERDETECTED
TAA is being detected more often than in the past thanks to better detection methods and heightened awareness among physicians and patients. While an incidence rate of 10.4 per 100,000 patient-years is often cited,3 this figure likely underestimates the true incidence of this clinically silent condition. The most robust data come from studies based on in-hospital diagnostic codes coupled with data from autopsies for out-of-hospital deaths.
Olsson et al,4 in a 2016 study in Sweden, found the incidence of TAA and aortic dissection to be 16.3 per 100,000 per year for men and 9.1 per 100,000 per year for women.
Clouse et al5 reported the incidence of thoracic aortic dissection as 3.5 per 100,000 patient-years, and the same figure for thoracic aortic rupture.
Aneurysmal disease accounts for 52,000 deaths per year in the United States, making it the 19th most common cause of death.6 These figures are likely lower than the true mortality rate for this condition, given that aortic dissection is often mistaken for acute myocardial infarction or other acute event if an autopsy is not done to confirm the cause of death.7
RISK FACTORS FOR THORACIC AORTIC ANEURYSM
Risk factors for TAA include genetic conditions that lead to aortic medial weakness or destruction such as Loeys-Dietz syndrome and Marfan syndrome.2 In addition, family history is important even in the absence of known genetic mutations. Other risk factors include conditions that increase aortic wall stress, such as hypertension, cocaine abuse, extreme weightlifting, trauma, and aortic coarctation.2
DIAMETER INCREASES WITH AGE, BODY SURFACE AREA
Normal dimensions for the aortic segments differ depending on age, sex, and body surface area.8,44,45 The size of the aortic root may also vary depending on how it is measured, due to the root’s trefoil shape. Measured sinus to sinus, the root is larger than when measured sinus to commissure on CT angiography or cardiac MRI. It is also larger when measured leading edge to leading edge than inner edge to inner edge on echocardiography.10
TAA is defined as an aortic diameter at least 50% greater than the upper limit of normal.8
Geometric changes in the curvature of the ascending aorta, aortic arch, and descending thoracic aorta can occur as the result of hypertension, atherosclerosis, or connective tissue disease.
HOW IS TAA DIAGNOSED?
Imaging tests
It is particularly important to obtain a gated CTA image in patients with aortic root aneurysm to avoid motion artifact and possible erroneous measurements. Gated CTA is done with electrocardiographic synchronization and allows for image processing to correct for cardiac motion.
HOW IS TAA CLASSIFIED?
TAA can be caused by a variety of inherited and sporadic conditions. These differences in pathogenesis lend themselves to classification of aneurysms into groups. Table 3 highlights the most common conditions associated with TAA.13
Bicuspid aortic valve aortopathy
From 1% to 2% of people have a bicuspid aortic valve, with a 3-to-1 male predominance.14,15 Aortic dilation occurs in 35% to 80% of people who have a bicuspid aortic valve, conferring a risk of dissection 8 times higher than in the general population.16–18
The pathogenic mechanisms that lead to this condition are widely debated, although a combination of genetic defects leading to intrinsic weakening of the aortic wall and hemodynamic effects likely contribute.19 Evidence of hemodynamic contributions to aortic dilation comes from findings that particular patterns of cusp fusion of the bicuspid aortic valve result in changes in transvalvular flow, placing more stress on specific regions of the ascending aorta.20,21 These hemodynamic alterations result in patterns of aortic dilation that depend on cusp fusion and the presence of valvular disease.
Multiple small studies found that replacing bicuspid aortic valves reduced the rate of aortic dilation, suggesting that hemodynamic factors may play a larger role than intrinsic wall properties in genetically susceptible individuals.22,23 However, larger studies are needed before any definitive conclusions can be made.
HOW IS ANEURYSM MANAGED ON AN OUTPATIENT BASIS?
Patients with a new diagnosis of TAA should be referred to a cardiologist with expertise in managing aortic disease or to a cardiac surgeon specializing in aortic surgery, depending on the initial size of the aneurysm.
Control blood pressure with beta-blockers
Medical management for patients with TAA has historically been limited to strict blood pressure control aimed at reducing aortic wall stress, mainly with beta-blockers.
Are angiotensin II receptor blockers (ARBs) beneficial? Studies in a mouse model of Marfan syndrome revealed that the ARB losartan attenuated aortic root growth.24 The results of early, small studies in humans were promising,25–27 but larger randomized trials have shown no advantage of losartan over beta-blockers in slowing aortic root growth.28 These negative results led many to question the effectiveness of losartan, although some point out that no studies have shown even beta-blockers to be beneficial in reducing the clinical end points of death or dissection.29 On the other hand, patients with certain FBN1 mutations respond more readily than others to losartan.30 Additional clinical trials of ARBs in Marfan syndrome are ongoing.
Current guidelines recommend stringent blood pressure control and smoking cessation for patients with a small aneurysm not requiring surgery and for those who are considered unsuitable for surgical or percutaneous intervention (level of evidence C, the lowest).2 For patients with TAA, it is considered reasonable to give beta-blockers. Angiotensin-converting enzyme inhibitors or ARBs may be used in combination with beta-blockers, titrated to the lowest tolerable blood pressure without adverse effects (level of evidence B).2
The recommended target blood pressure is less than 140/90 mm Hg, or 130/80 mm Hg in those with diabetes or chronic kidney disease (level of evidence B).2 However, we recommend more stringent blood pressure control: ie, less than 130/80 mm Hg for all patients with aortic aneurysm and a heart rate goal of 70 beats per minute or less, as tolerated.
Activity restriction
Activity restrictions for patients with TAA are largely based on theory, and certain activities may require more modification than others. For example, heavy lifting should be discouraged, as it may increase blood pressure significantly for short periods of time.2,31 The increased wall stress, in theory, could initiate dissection or rupture. However, moderate-intensity aerobic activity is rarely associated with significant elevations in blood pressure and should be encouraged. Stressful emotional states have been anecdotally associated with aortic dissection; thus, measures to reduce stress may offer some benefit.31
Our recommendations. While there are no published guidelines regarding activity restrictions in patients with TAA, we use a graded approach based on aortic diameter:
- 4.0 to 4.4 cm—lift no more than 75 pounds
- 4.5 to 5 cm—lift no more than 50 pounds
- 5 cm—lift no more than 25 pounds.
We also recommend not lifting anything heavier than half of one’s body weight and to avoid breath-holding or performing the Valsalva maneuver while lifting. Although these recommendations are somewhat arbitrary, based on theory and a large clinical experience at our aortic center, they seem reasonable and practical.
Activity restrictions should be stringent and individualized in patients with Marfan, Loeys-Dietz, or Ehlers-Danlos syndrome due to increased risk of dissection or rupture even if the aorta is normal in size.
We sometimes recommend exercise stress testing to assess the heart rate and blood pressure response to exercise, and we are developing research protocols to help tailor activity recommendations.
WHEN SHOULD A PATIENT BE REFERRED?
To a cardiologist at the time of diagnosis
As soon as TAA is diagnosed, the patient should be referred to a cardiologist who has special interest in aortic disease. This will allow for appropriate and timely decisions about medical management, imaging, follow-up, and referral to surgery. Additional recommendations for screening of family members and referral to clinical geneticists can be discussed at this juncture. Activity restrictions should be reviewed at the initial evaluation.
To a surgeon relatively early
Size thresholds for surgical intervention are discussed below, but one should not wait until these thresholds are reached to send the patient for surgical consultation. It is beneficial to the state of mind of a potential surgical candidate to have early discussions pertaining to the types of operations available, their outcomes, and associated risks and benefits. If a patient’s aortic size remains stable over time, he or she may be followed by the cardiologist until significant size or growth has been documented, at which time the patient and surgeon can reconvene to discuss options for definitive treatment.
To a clinical geneticist
If 1 or more first-degree relatives of a patient with TAA or dissection are found to have aneurysmal disease, referral to a clinical geneticist is very important for genetic testing of multiple genes that have been implicated in thoracic aortic aneurysm and dissection.
WHEN SHOULD TAA BE REPAIRED?
Surgery to prevent rupture or dissection remains the definitive treatment of TAA when size thresholds are reached, and symptomatic aneurysm should be operated on regardless of the size. However, rarely are thoracic aneurysms symptomatic unless they rupture or dissect. The size criteria are based on underlying genetic etiology if known and on the behavior and natural course of TAA.
Size and other factors
Treatment should be tailored to the patient’s clinical scenario, family history, and estimated risk of rupture or dissection, balanced against the individual center’s outcomes of elective aortic replacement.32 For example, young and otherwise healthy patients with TAA and a family history of aortic dissection (who may be more likely to have connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehler-Danlos syndrome) may elect to undergo repair when the aneurysm reaches or nearly reaches the diameter of that of the family member’s aorta when dissection occurred.2 On the other hand, TAA of degenerative etiology (eg, related to smoking or hypertension) measuring less than 5.5 cm in an older patient with comorbidities poses a lower risk of a catastrophic event such as dissection or rupture than the risk of surgery.11
Thresholds for surgery. Once the diameter of the ascending aorta reaches 6 cm, the likelihood of an acute dissection is 31%.11 A similar threshold is reached for the descending aorta at a size of 7 cm.11 Therefore, to avoid high-risk emergency surgery on an acutely dissected aorta, surgery on an ascending aortic aneurysm of degenerative etiology is usually suggested when the aneurysm reaches 5.5 cm or a documented growth rate greater than 0.5 cm/year.2,33
Additionally, in patients already undergoing surgery for valvular or coronary disease, prophylactic aortic replacement is recommended if the ascending aorta is larger than 4.5 cm. The threshold for intervention is lower in patients with connective tissue disease (> 5.0 cm for Marfan syndrome, 4.4–4.6 cm for Loeys-Dietz syndrome).2,33
Observational studies suggest that the risk of aortic complications in patients with bicuspid aortic valve aortopathy is low overall, though significantly greater than in the general population.18,34,35 These findings led to changes in the 2014 American College of Cardiology/American Heart Association guidelines on valvular heart disease,36 suggesting a surgical threshold of 5.5 cm in the absence of significant valve disease or family history of dissection of an aorta of smaller diameter.
A 2015 study of dissection risk in patients with bicuspid aortic valve aortopathy by our group found a dramatic increase in risk of aortic dissection for ascending aortic diameters greater than 5.3 cm, and a gradual increase in risk for aortic root diameters greater than 5.0 cm.37 In addition, a near-constant 3% to 4% risk of dissection was present for aortic diameters ranging from 4.7 cm to 5.0 cm, revealing that watchful waiting carries its own inherent risks.37 In our surgical experience with this population, the hospital mortality rate and risk of stroke from aortic surgery were 0.25% and 0.75%, respectively.37 Thus, the decision to operate for aortic aneurysm in the setting of a bicuspid aortic valve should take into account patient-specific factors and institutional outcomes.
A statement of clarification in the American College of Cardiology/American Heart Association guidelines was published in 2015, recommending surgery for patients with an aortic diameter of 5.0 cm or greater if the patient is at low risk and the surgery is performed by an experienced surgical team at a center with established surgical expertise in this condition.38 However, current recommendations are for surgery at 5.5 cm if the above conditions are not met.
Ratio of aortic cross-sectional area to height
Although size alone has long been used to guide surgical intervention, a recent review from the International Registry of Aortic Dissection revealed that 59% of patients suffered aortic dissection at diameters less than 5.5 cm, and that patients with certain connective tissue diseases such as Loeys-Dietz syndrome or familial thoracic aneurysm and dissection had a documented propensity for dissection at smaller diameters.39–41
Size indices such as the aortic cross-sectional area indexed to height have been implemented in guidelines for certain patient populations (eg, 10 cm2/m in Marfan syndrome) and provide better risk stratification than size cutoffs alone.2,42
The ratio of aortic cross-sectional area to the patient’s height has also been applied to patients with bicuspid aortic valve-associated aortopathy and to those with a dilated aorta and a tricuspid aortic valve.43,44 Notably, a ratio greater than 10 cm2/m has been associated with aortic dissection in these groups, and this cutoff provides better stratification for prediction of death than traditional size metrics.27,28
HOW SHOULD PATIENTS BE SCREENED? WHAT FOLLOW-UP IS NECESSARY?
Initial screening and follow-up
Follow-up of TAA depends on the initial aortic size or rate of growth, or both. For patients presenting for the first time with TAA, it is reasonable to obtain definitive aortic imaging with CT or magnetic resonance angiography (MRA), then to repeat imaging at 6 months to document stability. If the aortic dimensions remain stable, then annual follow-up with CT or MRA is reasonable.2
Our flow chart of initial screening and follow-up is shown in Figure 5.
Screening of family members
In our center, we routinely recommend screening of all first-degree relatives of patients with TAA. Aortic imaging with echocardiography plus CT or MRI should be considered to detect asymptomatic disease.2 In patients with a strong family history (ie, multiple relatives affected with aortic aneurysm, dissection, or sudden cardiac death), genetic screening and testing for known mutations are recommended for the patient as well as for the family members.
If a mutation is identified in a family, then first-degree relatives should undergo genetic screening for the mutation and aortic imaging.2 Imaging in second-degree relatives may also be considered if one or more first-degree relatives are found to have aortic dilation.2
We recommend similar screening of first-degree family members of patients with bicuspid aortic valve aortopathy. In patients with young children, we recommend obtaining an echocardiogram of the child to look for a bicuspid aortic valve or aortic dilation. If an abnormality is detected or suspected, dedicated imaging with MRA to assess aortic dimensions is warranted.
BACK TO OUR PATIENT WITH A BICUSPID AORTIC VALVE
Our patient with a bicuspid aortic valve had a 4.6-cm root, an ascending aortic aneurysm, and several affected family members.
We would obtain dedicated aortic imaging at this patient’s initial visit with either gated CT with contrast or MRA, and we would obtain a cardioaortic surgery consult. We would repeat these studies at a follow-up visit 6 months later to detect any aortic growth compared with initial studies, and follow up annually thereafter. Echocardiography can also be done at the initial visit to determine if valvular disease is present that may influence clinical decisions.
Surgery would likely be recommended once the root reached a maximum area-to-height ratio greater than 10 cm2/m, or if the valve became severely dysfunctional during follow-up.
BACK TO OUR PATIENT WITH MARFAN SYNDROME
The young woman with Marfan syndrome has a 4.6-cm aortic root aneurysm and 2+ aortic insufficiency. Her question pertains to the threshold at which an operation would be considered. This question is complicated and is influenced by several concurrent clinical features in her presentation.
Starting with size criteria, patients with Marfan syndrome should be considered for elective aortic root repair at a diameter greater than 5 cm. However, an aortic cross-sectional area-to-height ratio greater than 10 cm2/m may provide a more robust metric for clinical decision-making than aortic diameter alone. Additional factors such as degree of aortic insufficiency and deleterious left ventricular remodeling may urge one to consider aortic root repair at a diameter of 4.5 cm.
These factors, including rate of growth and the surgeon’s assessment about his or her ability to preserve the aortic valve during repair, should be considered collectively in this scenario.
Thoracic aortic aneurysm (TAA) needs to be detected, monitored, and managed in a timely manner to prevent a serious consequence such as acute dissection or rupture. But only about 5% of patients experience symptoms before an acute event occurs, and for the other 95% the first “symptom” is often death.1 Most cases are detected either incidentally with echocardiography, computed tomography (CT), or magnetic resonance imaging (MRI) during workup for another condition. Patients may also be diagnosed during workup of a murmur or after a family member is found to have an aneurysm. Therefore, its true incidence is difficult to determine.2
With these facts in mind, how would you manage the following 2 cases?
Case 1: Bicuspid aortic valve, ascending aortic aneurysm
A 45-year-old man with stage 1 hypertension presents for evaluation of a bicuspid aortic valve and ascending aortic aneurysm. He has several first-degree relatives with similar conditions, and his brother recently underwent elective aortic repair. At the urging of his primary care physician, he underwent screening echocardiography, which demonstrated a “dilated root and ascending aorta” 4.6 cm in diameter. He presents today to discuss management options and how the aneurysm could affect his everyday life.
Case 2: Marfan syndrome in a young woman
A 24-year-old woman with Marfan syndrome diagnosed in adolescence presents for annual follow-up. She has many family members with the same condition, and several have undergone prophylactic aortic root repair. Her aortic root has been monitored annually for progression of dilation, and today it is 4.6 cm in diameter, a 3-mm increase from the last measurement. She has grade 2+ aortic insufficiency (on a scale of 1+ to 4+) based on echocardiography, but she has no symptoms. She is curious about what size her aortic root will need to reach for surgery to be considered.
LIKELY UNDERDETECTED
TAA is being detected more often than in the past thanks to better detection methods and heightened awareness among physicians and patients. While an incidence rate of 10.4 per 100,000 patient-years is often cited,3 this figure likely underestimates the true incidence of this clinically silent condition. The most robust data come from studies based on in-hospital diagnostic codes coupled with data from autopsies for out-of-hospital deaths.
Olsson et al,4 in a 2016 study in Sweden, found the incidence of TAA and aortic dissection to be 16.3 per 100,000 per year for men and 9.1 per 100,000 per year for women.
Clouse et al5 reported the incidence of thoracic aortic dissection as 3.5 per 100,000 patient-years, and the same figure for thoracic aortic rupture.
Aneurysmal disease accounts for 52,000 deaths per year in the United States, making it the 19th most common cause of death.6 These figures are likely lower than the true mortality rate for this condition, given that aortic dissection is often mistaken for acute myocardial infarction or other acute event if an autopsy is not done to confirm the cause of death.7
RISK FACTORS FOR THORACIC AORTIC ANEURYSM
Risk factors for TAA include genetic conditions that lead to aortic medial weakness or destruction such as Loeys-Dietz syndrome and Marfan syndrome.2 In addition, family history is important even in the absence of known genetic mutations. Other risk factors include conditions that increase aortic wall stress, such as hypertension, cocaine abuse, extreme weightlifting, trauma, and aortic coarctation.2
DIAMETER INCREASES WITH AGE, BODY SURFACE AREA
Normal dimensions for the aortic segments differ depending on age, sex, and body surface area.8,44,45 The size of the aortic root may also vary depending on how it is measured, due to the root’s trefoil shape. Measured sinus to sinus, the root is larger than when measured sinus to commissure on CT angiography or cardiac MRI. It is also larger when measured leading edge to leading edge than inner edge to inner edge on echocardiography.10
TAA is defined as an aortic diameter at least 50% greater than the upper limit of normal.8
Geometric changes in the curvature of the ascending aorta, aortic arch, and descending thoracic aorta can occur as the result of hypertension, atherosclerosis, or connective tissue disease.
HOW IS TAA DIAGNOSED?
Imaging tests
It is particularly important to obtain a gated CTA image in patients with aortic root aneurysm to avoid motion artifact and possible erroneous measurements. Gated CTA is done with electrocardiographic synchronization and allows for image processing to correct for cardiac motion.
HOW IS TAA CLASSIFIED?
TAA can be caused by a variety of inherited and sporadic conditions. These differences in pathogenesis lend themselves to classification of aneurysms into groups. Table 3 highlights the most common conditions associated with TAA.13
Bicuspid aortic valve aortopathy
From 1% to 2% of people have a bicuspid aortic valve, with a 3-to-1 male predominance.14,15 Aortic dilation occurs in 35% to 80% of people who have a bicuspid aortic valve, conferring a risk of dissection 8 times higher than in the general population.16–18
The pathogenic mechanisms that lead to this condition are widely debated, although a combination of genetic defects leading to intrinsic weakening of the aortic wall and hemodynamic effects likely contribute.19 Evidence of hemodynamic contributions to aortic dilation comes from findings that particular patterns of cusp fusion of the bicuspid aortic valve result in changes in transvalvular flow, placing more stress on specific regions of the ascending aorta.20,21 These hemodynamic alterations result in patterns of aortic dilation that depend on cusp fusion and the presence of valvular disease.
Multiple small studies found that replacing bicuspid aortic valves reduced the rate of aortic dilation, suggesting that hemodynamic factors may play a larger role than intrinsic wall properties in genetically susceptible individuals.22,23 However, larger studies are needed before any definitive conclusions can be made.
HOW IS ANEURYSM MANAGED ON AN OUTPATIENT BASIS?
Patients with a new diagnosis of TAA should be referred to a cardiologist with expertise in managing aortic disease or to a cardiac surgeon specializing in aortic surgery, depending on the initial size of the aneurysm.
Control blood pressure with beta-blockers
Medical management for patients with TAA has historically been limited to strict blood pressure control aimed at reducing aortic wall stress, mainly with beta-blockers.
Are angiotensin II receptor blockers (ARBs) beneficial? Studies in a mouse model of Marfan syndrome revealed that the ARB losartan attenuated aortic root growth.24 The results of early, small studies in humans were promising,25–27 but larger randomized trials have shown no advantage of losartan over beta-blockers in slowing aortic root growth.28 These negative results led many to question the effectiveness of losartan, although some point out that no studies have shown even beta-blockers to be beneficial in reducing the clinical end points of death or dissection.29 On the other hand, patients with certain FBN1 mutations respond more readily than others to losartan.30 Additional clinical trials of ARBs in Marfan syndrome are ongoing.
Current guidelines recommend stringent blood pressure control and smoking cessation for patients with a small aneurysm not requiring surgery and for those who are considered unsuitable for surgical or percutaneous intervention (level of evidence C, the lowest).2 For patients with TAA, it is considered reasonable to give beta-blockers. Angiotensin-converting enzyme inhibitors or ARBs may be used in combination with beta-blockers, titrated to the lowest tolerable blood pressure without adverse effects (level of evidence B).2
The recommended target blood pressure is less than 140/90 mm Hg, or 130/80 mm Hg in those with diabetes or chronic kidney disease (level of evidence B).2 However, we recommend more stringent blood pressure control: ie, less than 130/80 mm Hg for all patients with aortic aneurysm and a heart rate goal of 70 beats per minute or less, as tolerated.
Activity restriction
Activity restrictions for patients with TAA are largely based on theory, and certain activities may require more modification than others. For example, heavy lifting should be discouraged, as it may increase blood pressure significantly for short periods of time.2,31 The increased wall stress, in theory, could initiate dissection or rupture. However, moderate-intensity aerobic activity is rarely associated with significant elevations in blood pressure and should be encouraged. Stressful emotional states have been anecdotally associated with aortic dissection; thus, measures to reduce stress may offer some benefit.31
Our recommendations. While there are no published guidelines regarding activity restrictions in patients with TAA, we use a graded approach based on aortic diameter:
- 4.0 to 4.4 cm—lift no more than 75 pounds
- 4.5 to 5 cm—lift no more than 50 pounds
- 5 cm—lift no more than 25 pounds.
We also recommend not lifting anything heavier than half of one’s body weight and to avoid breath-holding or performing the Valsalva maneuver while lifting. Although these recommendations are somewhat arbitrary, based on theory and a large clinical experience at our aortic center, they seem reasonable and practical.
Activity restrictions should be stringent and individualized in patients with Marfan, Loeys-Dietz, or Ehlers-Danlos syndrome due to increased risk of dissection or rupture even if the aorta is normal in size.
We sometimes recommend exercise stress testing to assess the heart rate and blood pressure response to exercise, and we are developing research protocols to help tailor activity recommendations.
WHEN SHOULD A PATIENT BE REFERRED?
To a cardiologist at the time of diagnosis
As soon as TAA is diagnosed, the patient should be referred to a cardiologist who has special interest in aortic disease. This will allow for appropriate and timely decisions about medical management, imaging, follow-up, and referral to surgery. Additional recommendations for screening of family members and referral to clinical geneticists can be discussed at this juncture. Activity restrictions should be reviewed at the initial evaluation.
To a surgeon relatively early
Size thresholds for surgical intervention are discussed below, but one should not wait until these thresholds are reached to send the patient for surgical consultation. It is beneficial to the state of mind of a potential surgical candidate to have early discussions pertaining to the types of operations available, their outcomes, and associated risks and benefits. If a patient’s aortic size remains stable over time, he or she may be followed by the cardiologist until significant size or growth has been documented, at which time the patient and surgeon can reconvene to discuss options for definitive treatment.
To a clinical geneticist
If 1 or more first-degree relatives of a patient with TAA or dissection are found to have aneurysmal disease, referral to a clinical geneticist is very important for genetic testing of multiple genes that have been implicated in thoracic aortic aneurysm and dissection.
WHEN SHOULD TAA BE REPAIRED?
Surgery to prevent rupture or dissection remains the definitive treatment of TAA when size thresholds are reached, and symptomatic aneurysm should be operated on regardless of the size. However, rarely are thoracic aneurysms symptomatic unless they rupture or dissect. The size criteria are based on underlying genetic etiology if known and on the behavior and natural course of TAA.
Size and other factors
Treatment should be tailored to the patient’s clinical scenario, family history, and estimated risk of rupture or dissection, balanced against the individual center’s outcomes of elective aortic replacement.32 For example, young and otherwise healthy patients with TAA and a family history of aortic dissection (who may be more likely to have connective tissue disorders such as Marfan syndrome, Loeys-Dietz syndrome, or vascular Ehler-Danlos syndrome) may elect to undergo repair when the aneurysm reaches or nearly reaches the diameter of that of the family member’s aorta when dissection occurred.2 On the other hand, TAA of degenerative etiology (eg, related to smoking or hypertension) measuring less than 5.5 cm in an older patient with comorbidities poses a lower risk of a catastrophic event such as dissection or rupture than the risk of surgery.11
Thresholds for surgery. Once the diameter of the ascending aorta reaches 6 cm, the likelihood of an acute dissection is 31%.11 A similar threshold is reached for the descending aorta at a size of 7 cm.11 Therefore, to avoid high-risk emergency surgery on an acutely dissected aorta, surgery on an ascending aortic aneurysm of degenerative etiology is usually suggested when the aneurysm reaches 5.5 cm or a documented growth rate greater than 0.5 cm/year.2,33
Additionally, in patients already undergoing surgery for valvular or coronary disease, prophylactic aortic replacement is recommended if the ascending aorta is larger than 4.5 cm. The threshold for intervention is lower in patients with connective tissue disease (> 5.0 cm for Marfan syndrome, 4.4–4.6 cm for Loeys-Dietz syndrome).2,33
Observational studies suggest that the risk of aortic complications in patients with bicuspid aortic valve aortopathy is low overall, though significantly greater than in the general population.18,34,35 These findings led to changes in the 2014 American College of Cardiology/American Heart Association guidelines on valvular heart disease,36 suggesting a surgical threshold of 5.5 cm in the absence of significant valve disease or family history of dissection of an aorta of smaller diameter.
A 2015 study of dissection risk in patients with bicuspid aortic valve aortopathy by our group found a dramatic increase in risk of aortic dissection for ascending aortic diameters greater than 5.3 cm, and a gradual increase in risk for aortic root diameters greater than 5.0 cm.37 In addition, a near-constant 3% to 4% risk of dissection was present for aortic diameters ranging from 4.7 cm to 5.0 cm, revealing that watchful waiting carries its own inherent risks.37 In our surgical experience with this population, the hospital mortality rate and risk of stroke from aortic surgery were 0.25% and 0.75%, respectively.37 Thus, the decision to operate for aortic aneurysm in the setting of a bicuspid aortic valve should take into account patient-specific factors and institutional outcomes.
A statement of clarification in the American College of Cardiology/American Heart Association guidelines was published in 2015, recommending surgery for patients with an aortic diameter of 5.0 cm or greater if the patient is at low risk and the surgery is performed by an experienced surgical team at a center with established surgical expertise in this condition.38 However, current recommendations are for surgery at 5.5 cm if the above conditions are not met.
Ratio of aortic cross-sectional area to height
Although size alone has long been used to guide surgical intervention, a recent review from the International Registry of Aortic Dissection revealed that 59% of patients suffered aortic dissection at diameters less than 5.5 cm, and that patients with certain connective tissue diseases such as Loeys-Dietz syndrome or familial thoracic aneurysm and dissection had a documented propensity for dissection at smaller diameters.39–41
Size indices such as the aortic cross-sectional area indexed to height have been implemented in guidelines for certain patient populations (eg, 10 cm2/m in Marfan syndrome) and provide better risk stratification than size cutoffs alone.2,42
The ratio of aortic cross-sectional area to the patient’s height has also been applied to patients with bicuspid aortic valve-associated aortopathy and to those with a dilated aorta and a tricuspid aortic valve.43,44 Notably, a ratio greater than 10 cm2/m has been associated with aortic dissection in these groups, and this cutoff provides better stratification for prediction of death than traditional size metrics.27,28
HOW SHOULD PATIENTS BE SCREENED? WHAT FOLLOW-UP IS NECESSARY?
Initial screening and follow-up
Follow-up of TAA depends on the initial aortic size or rate of growth, or both. For patients presenting for the first time with TAA, it is reasonable to obtain definitive aortic imaging with CT or magnetic resonance angiography (MRA), then to repeat imaging at 6 months to document stability. If the aortic dimensions remain stable, then annual follow-up with CT or MRA is reasonable.2
Our flow chart of initial screening and follow-up is shown in Figure 5.
Screening of family members
In our center, we routinely recommend screening of all first-degree relatives of patients with TAA. Aortic imaging with echocardiography plus CT or MRI should be considered to detect asymptomatic disease.2 In patients with a strong family history (ie, multiple relatives affected with aortic aneurysm, dissection, or sudden cardiac death), genetic screening and testing for known mutations are recommended for the patient as well as for the family members.
If a mutation is identified in a family, then first-degree relatives should undergo genetic screening for the mutation and aortic imaging.2 Imaging in second-degree relatives may also be considered if one or more first-degree relatives are found to have aortic dilation.2
We recommend similar screening of first-degree family members of patients with bicuspid aortic valve aortopathy. In patients with young children, we recommend obtaining an echocardiogram of the child to look for a bicuspid aortic valve or aortic dilation. If an abnormality is detected or suspected, dedicated imaging with MRA to assess aortic dimensions is warranted.
BACK TO OUR PATIENT WITH A BICUSPID AORTIC VALVE
Our patient with a bicuspid aortic valve had a 4.6-cm root, an ascending aortic aneurysm, and several affected family members.
We would obtain dedicated aortic imaging at this patient’s initial visit with either gated CT with contrast or MRA, and we would obtain a cardioaortic surgery consult. We would repeat these studies at a follow-up visit 6 months later to detect any aortic growth compared with initial studies, and follow up annually thereafter. Echocardiography can also be done at the initial visit to determine if valvular disease is present that may influence clinical decisions.
Surgery would likely be recommended once the root reached a maximum area-to-height ratio greater than 10 cm2/m, or if the valve became severely dysfunctional during follow-up.
BACK TO OUR PATIENT WITH MARFAN SYNDROME
The young woman with Marfan syndrome has a 4.6-cm aortic root aneurysm and 2+ aortic insufficiency. Her question pertains to the threshold at which an operation would be considered. This question is complicated and is influenced by several concurrent clinical features in her presentation.
Starting with size criteria, patients with Marfan syndrome should be considered for elective aortic root repair at a diameter greater than 5 cm. However, an aortic cross-sectional area-to-height ratio greater than 10 cm2/m may provide a more robust metric for clinical decision-making than aortic diameter alone. Additional factors such as degree of aortic insufficiency and deleterious left ventricular remodeling may urge one to consider aortic root repair at a diameter of 4.5 cm.
These factors, including rate of growth and the surgeon’s assessment about his or her ability to preserve the aortic valve during repair, should be considered collectively in this scenario.
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010; 55(9):841–857. doi:10.1016/j.jacc.2009.08.084
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. Anesth Analg 2010; 111(2):279–315. doi:10.1213/ANE.0b013e3181dd869b
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280(22):1926–1929. pmid:9851478
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114(24):2611–2618. doi:10.1161/CIRCULATIONAHA.106.630400
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004; 79(2):176–180. pmid:14959911
- US Centers for Disease Control and Prevention (CDC). National Center for Injury Prevention and Control. WISQARS leading causes of death reports, 1999 – 2007. https://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. Accessed May 21, 2018.
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99(6):852–856. doi:10.1016/j.amjcard.2006.10.055
- Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014; 64(16):1725–1739. doi:10.1016/j.jacc.2014.08.025
- Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008; 1(2):200–209. doi:10.1016/j.jcmg.2007.11.005
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002; 74(5):S1877–S1880; discussion S1892–S1898. pmid:12440685
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75(1):7–17. pmid:18236724
- Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013(2013); 2013:267215. doi:10.1155/2013/267215
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39(12):1890–1900. doi:10.1016/S0735-1097(02)01886-7
- Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002; 106(8):900–904. pmid:12186790
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007; 31(3):397–405. doi:10.1016/j.ejcts.2006.12.006
- Jackson V, Petrini J, Caidahl K, et al. Bicuspid aortic valve leaflet morphology in relation to aortic root morphology: a study of 300 patients undergoing open-heart surgery. Eur J Cardiothorac Surg 2011; 40(3):e118–e124. doi:10.1016/j.ejcts.2011.04.014
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112. doi:10.1001/jama.2011.1286
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014; 370(20):1920–1929. doi:10.1056/NEJMra1207059
- Barker AJ, Markl M, Bürk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012; 5(4):457–466. doi:10.1161/CIRCIMAGING.112.973370
- Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010; 255(1):53–61. doi:10.1148/radiol.09091437
- Abdulkareem N, Soppa G, Jones S, Valencia O, Smelt J, Jahangiri M. Dilatation of the remaining aorta after aortic valve or aortic root replacement in patients with bicuspid aortic valve: a 5-year follow-up. Ann Thorac Surg 2013; 96(1):43–49. doi:10.1016/j.athoracsur.2013.03.086
- Regeer MV, Versteegh MI, Klautz RJ, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg 2016; 102(6):1981–1987. doi:10.1016/j.athoracsur.2016.05.038
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312(5770):117–121. doi:10.1126/science.1124287
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358(26):2787–2795. doi:10.1056/NEJMoa0706585
- Chiu HH, Wu MH, Wang JK, et al. Losartan added to ß-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013; 88(3):271–276. doi:10.1016/j.mayocp.2012.11.005
- Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013; 34(45):3491–3500. doi:10.1093/eurheartj/eht334
- Lacro RV, Dietz HC, Sleeper LA, et al; Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014; 371(22):2061–2071. doi:10.1056/NEJMoa1404731
- Ziganshin BA, Mukherjee SK, Elefteriades JA, et al. Atenolol versus losartan in Marfan’s syndrome (letters). N Engl J Med 2015; 372(10):977–981. doi:10.1056/NEJMc1500128
- Franken R, den Hartog AW, Radonic T, et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet 2015; 8(2):383–388. doi:10.1161/CIRCGENETICS.114.000950
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol 2008; 33(5):203–277. doi:10.1016/j.cpcardiol.2008.01.004
- Idrees JJ, Roselli EE, Lowry AM, et al. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101(6):2185–2192. doi:10.1016/j.athoracsur.2015.12.026
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016; 151(4):959–966. doi:10.1016/j.jtcvs.2015.12.001
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300(11):1317–1325. doi:10.1001/jama.300.11.1317
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002; 73(1):17–28. pmid:11834007
- Nishimura RA, Otto CM, Bono RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve–associated aneurysms. Ann Thorac Surg 2015; 100(5):1666–1674. doi:10.1016/j.athoracsur.2015.04.126
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 133(7):680–686. doi:10.1161/CIR.0000000000000331
- Pape LA, Tsai TT, Isselbacher EM, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116(10):1120–1127. doi:10.1161/CIRCULATIONAHA.107.702720
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355(8):788–798. doi:10.1056/NEJMoa055695
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39(12):1488–1493. doi:10.1038/ng.2007.6
- Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg 2002; 123(2):360–361. pmid:11828302
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003; 126(3):892–893. pmid:14502185
- Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134(22):1724–1737. doi:10.1161/CIRCULATIONAHA.116.022995
- Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010; 55(9):841–857. doi:10.1016/j.jacc.2009.08.084
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. Anesth Analg 2010; 111(2):279–315. doi:10.1213/ANE.0b013e3181dd869b
- Clouse WD, Hallett JW Jr, Schaff HV, Gayari MM, Ilstrup DM, Melton LJ 3rd. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998; 280(22):1926–1929. pmid:9851478
- Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006; 114(24):2611–2618. doi:10.1161/CIRCULATIONAHA.106.630400
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004; 79(2):176–180. pmid:14959911
- US Centers for Disease Control and Prevention (CDC). National Center for Injury Prevention and Control. WISQARS leading causes of death reports, 1999 – 2007. https://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. Accessed May 21, 2018.
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol 2007; 99(6):852–856. doi:10.1016/j.amjcard.2006.10.055
- Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 2014; 64(16):1725–1739. doi:10.1016/j.jacc.2014.08.025
- Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging 2008; 1(2):200–209. doi:10.1016/j.jcmg.2007.11.005
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002; 74(5):S1877–S1880; discussion S1892–S1898. pmid:12440685
- Smith AD, Schoenhagen P. CT imaging for acute aortic syndrome. Cleve Clin J Med 2008; 75(1):7–17. pmid:18236724
- Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med 2013(2013); 2013:267215. doi:10.1155/2013/267215
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39(12):1890–1900. doi:10.1016/S0735-1097(02)01886-7
- Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002; 106(8):900–904. pmid:12186790
- Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 2007; 31(3):397–405. doi:10.1016/j.ejcts.2006.12.006
- Jackson V, Petrini J, Caidahl K, et al. Bicuspid aortic valve leaflet morphology in relation to aortic root morphology: a study of 300 patients undergoing open-heart surgery. Eur J Cardiothorac Surg 2011; 40(3):e118–e124. doi:10.1016/j.ejcts.2011.04.014
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306(10):1104–1112. doi:10.1001/jama.2011.1286
- Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med 2014; 370(20):1920–1929. doi:10.1056/NEJMra1207059
- Barker AJ, Markl M, Bürk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging 2012; 5(4):457–466. doi:10.1161/CIRCIMAGING.112.973370
- Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010; 255(1):53–61. doi:10.1148/radiol.09091437
- Abdulkareem N, Soppa G, Jones S, Valencia O, Smelt J, Jahangiri M. Dilatation of the remaining aorta after aortic valve or aortic root replacement in patients with bicuspid aortic valve: a 5-year follow-up. Ann Thorac Surg 2013; 96(1):43–49. doi:10.1016/j.athoracsur.2013.03.086
- Regeer MV, Versteegh MI, Klautz RJ, et al. Effect of aortic valve replacement on aortic root dilatation rate in patients with bicuspid and tricuspid aortic valves. Ann Thorac Surg 2016; 102(6):1981–1987. doi:10.1016/j.athoracsur.2016.05.038
- Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006; 312(5770):117–121. doi:10.1126/science.1124287
- Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC 3rd. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med 2008; 358(26):2787–2795. doi:10.1056/NEJMoa0706585
- Chiu HH, Wu MH, Wang JK, et al. Losartan added to ß-blockade therapy for aortic root dilation in Marfan syndrome: a randomized, open-label pilot study. Mayo Clin Proc 2013; 88(3):271–276. doi:10.1016/j.mayocp.2012.11.005
- Groenink M, den Hartog AW, Franken R, et al. Losartan reduces aortic dilatation rate in adults with Marfan syndrome: a randomized controlled trial. Eur Heart J 2013; 34(45):3491–3500. doi:10.1093/eurheartj/eht334
- Lacro RV, Dietz HC, Sleeper LA, et al; Pediatric Heart Network Investigators. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med 2014; 371(22):2061–2071. doi:10.1056/NEJMoa1404731
- Ziganshin BA, Mukherjee SK, Elefteriades JA, et al. Atenolol versus losartan in Marfan’s syndrome (letters). N Engl J Med 2015; 372(10):977–981. doi:10.1056/NEJMc1500128
- Franken R, den Hartog AW, Radonic T, et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circ Cardiovasc Genet 2015; 8(2):383–388. doi:10.1161/CIRCGENETICS.114.000950
- Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol 2008; 33(5):203–277. doi:10.1016/j.cpcardiol.2008.01.004
- Idrees JJ, Roselli EE, Lowry AM, et al. Outcomes after elective proximal aortic replacement: a matched comparison of isolated versus multicomponent operations. Ann Thorac Surg 2016; 101(6):2185–2192. doi:10.1016/j.athoracsur.2015.12.026
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016; 151(4):959–966. doi:10.1016/j.jtcvs.2015.12.001
- Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008; 300(11):1317–1325. doi:10.1001/jama.300.11.1317
- Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002; 73(1):17–28. pmid:11834007
- Nishimura RA, Otto CM, Bono RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American heart Association Task Force on Practice Guidelines. Circulation 2014; 129(23):2440–2492. doi:10.1161/CIR.0000000000000029
- Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve–associated aneurysms. Ann Thorac Surg 2015; 100(5):1666–1674. doi:10.1016/j.athoracsur.2015.04.126
- Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 133(7):680–686. doi:10.1161/CIR.0000000000000331
- Pape LA, Tsai TT, Isselbacher EM, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter > or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007; 116(10):1120–1127. doi:10.1161/CIRCULATIONAHA.107.702720
- Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355(8):788–798. doi:10.1056/NEJMoa055695
- Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007; 39(12):1488–1493. doi:10.1038/ng.2007.6
- Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg 2002; 123(2):360–361. pmid:11828302
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003; 126(3):892–893. pmid:14502185
- Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation 2016; 134(22):1724–1737. doi:10.1161/CIRCULATIONAHA.116.022995
KEY POINTS
- Screening and referral depend on clinical context. A size-based model to determine screening, referral, follow-up, and management serves most cases but should be modified in the context of connective tissue disease or family history of aneurysm and dissection.
- Medical management involves strict blood pressure and heart rate control with beta-blockers and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Activity modifications should be tailored to the individual, although extreme isometric exercises and heavy lifting should be discouraged.
- Patients with TAA should be followed up annually, unless the patient is presenting for initial evaluation or significant changes are seen with dedicated imaging.
Evaluating suspected pulmonary hypertension: A structured approach
Pulmonary arterial hypertension (PAH) is a hemodynamic disorder that affects small and medium-size pulmonary arteries through cellular proliferation and luminal narrowing.1 Increased pulmonary vascular resistance causes restricted blood flow in these arteries, leading to elevated pulmonary arterial pressure and afterload on the right ventricle. Despite advances in therapy, death usually occurs as a result of right ventricular failure.
- Group 1—PAH, due to narrowed pulmonary arteries
- Group 2—due to left heart disease
- Group 3—due to lung disease or hypoxia, or both
- Group 4—due to chronic thromboembolism or other pulmonary artery obstruction
- Group 5—due to uncertain or multifactorial causes.
Experts recognize the morbidity and mortality associated with pulmonary hypertension now more than in the past, and they emphasize recognizing it early. Guidelines for its diagnosis and treatment were updated in 2015.1
Below, we use a case to discuss recommendations for initial evaluation and classification of pulmonary hypertension, particularly PAH.
A PATIENT SUSPECTED OF HAVING PULMONARY HYPERTENSION
A 63-year-old woman with a 25-pack-year history of tobacco use, as well as pulmonary embolism and coronary artery disease, presents to her primary care physician with exertional dyspnea. She had been a clerk at a hardware store and physically active until she took early retirement 8 months ago because of increasing fatigue. She initially felt the fatigue was simply “a sign of getting old.”
Since retiring, she has noticed the slow onset of progressive dyspnea on exertion. She can no longer climb more than 1 flight of stairs or walk more than 1 block. She also complains of mild, fluctuating edema in her lower extremities over the past month. She quit smoking 8 years ago after undergoing placement of a drug-eluting stent in the mid-left circumflex artery. After this, she received clopidogrel and was followed by a cardiologist for 2 years but stopped taking the medication because of bruising. She has not seen her cardiologist in more than 5 years.
She underwent elective right total knee arthroplasty 3 years ago, complicated by acute deep vein thrombosis in the right common femoral vein. Computed tomography (CT) at that time did not reveal pulmonary emboli. She received warfarin therapy for 3 months.
She reports no current cough, chest pain, lightheadedness, or syncope. She has no orthopnea, and she feels normal at rest.
Her family history is unremarkable, and she has had no exposure to illicit substances, environmental toxins, or dietary supplements. She takes aspirin 81 mg daily, metoprolol 25 mg twice daily, lisinopril 10 mg daily, and simvastatin 40 mg at bedtime.
Her primary care physician detects a murmur in the left lower sternal border and sends her for transthoracic echocardiography, which demonstrates mild right ventricular dilation, right atrial dilation, and mildly reduced right ventricular function. The calculated right ventricular systolic pressure is 69 mm Hg. The left ventricle shows mild concentric hypertrophy; the left atrium is normal in size.
DIAGNOSTIC EVALUATION OF SUSPECTED PULMONARY HYPERTENSION
CLINICAL MANIFESTATIONS
Symptoms and signs. As in the patient described above, the first symptoms such as exertional dyspnea, fatigue, and lightheadedness usually arise in situations that call for increased cardiac output.4 As right ventricular function worsens, symptoms start to occur at rest, and signs of increased right ventricular preload appear, such as abdominal and lower-extremity edema and pericardial effusion. Syncope is a sign of severe right ventricular dysfunction.5
Physical examination. Look for signs of increased right ventricular loading and failure, eg:
- An accentuated intensity and persistent splitting of the second heart sound
- A prominent parasternal heave
- A prominent jugular “a” wave
- A systolic murmur along the left sternal border at the fourth intercostal space, which may worsen with breath-holding
- Pitting lower-extremity edema
- Hepatomegaly
- Hepatojugular reflux
- Hepatic pulsatility.6
ECHOCARDIOGRAPHY IN SUSPECTED PULMONARY HYPERTENSION
Many practitioners rely heavily on the estimated right ventricular systolic pressure in diagnosing pulmonary hypertension. In theory, this number should be nearly the same as the pulmonary arterial systolic pressure. However, technical and patient-related aspects of transthoracic echocardiography often limit accurate measurement of the right ventricular systolic pressure, and readings often differ from those measured with right heart catheterization.8
Our patient had a markedly elevated right ventricular systolic pressure and signs of right ventricular dysfunction, suggesting a high probability of pulmonary hypertension.
EVALUATING LEFT HEART DISEASE (WHO GROUP 2)
More than 75% of cases of pulmonary hypertension are directly related to left ventricular dysfunction or mitral or aortic valve disease (WHO group 2).1 Since group 2 differs markedly from group 1 (PAH) in its pathophysiology and treatment, it is important to distinguish between them.
Compared with WHO group 1 patients, those in group 2 tend to be older, more of them are male, and more of them have comorbidities such as metabolic syndrome, hypertension, and coronary artery disease.1,9 A combination of risk factors and clinical findings should be considered in identifying these patients.10
Transthoracic echocardiography is used to detect features of systolic and diastolic dysfunction. Left atrial enlargement is a clue that left heart disease may be present. In addition, signs of left ventricular or valvular dysfunction on electrocardiography or chest radiography are often helpful.
When estimated right ventricular systolic pressures are only minimally abnormal and no significant right ventricular dysfunction exists, further diagnostic evaluation is not warranted. However, because no single identifying feature or variable can readily distinguish group 2 from the other WHO groups, further evaluation should be considered if the right ventricular systolic pressure is significantly elevated or right ventricular dysfunction exists.
Our patient had several risk factors for left heart disease, including a history of smoking and coronary artery disease. Nonetheless, findings consistent with severe right ventricular dysfunction necessitated further evaluation for other possible causes of her suspected pulmonary hypertension.
Postcapillary pulmonary hypertension
In patients for whom further evaluation is pursued, the diagnosis of WHO group 2 pulmonary hypertension is ultimately based on findings consistent with postcapillary or “passive” pulmonary hypertension on right heart catheterization. Although mean pulmonary arterial pressures must be at least 25 mm Hg to certify the diagnosis of pulmonary hypertension, a pulmonary artery occlusion pressure greater than 15 mm Hg (normal 6–12) and pulmonary vascular resistance of 3 Wood units or less (normal 0.3–1.6) suggests the pulmonary hypertension is due to elevated left atrial pressure (ie, postcapillary) rather than precapillary pulmonary arterial remodeling.
Mixed pre- and postcapillary pulmonary hypertension
Distinguishing pulmonary venous hypertension from PAH is important, since their management differs. In particular, PAH-specific therapies (ie, prostacyclin analogues, prostaglandin I2 receptor agonists, endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and cyclic guanosine monophosphate stimulators) can have a detrimental effect in WHO group 2 patients by causing increased pulmonary capillary leakage with pulmonary edema.11,12
In some patients, chronic passive congestion in the pulmonary venous circulation causes additional disruption of the homeostatic milieu regulating precapillary smooth muscle and endothelial function. These changes result in structural remodeling of precapillary arterioles and increased precapillary vascular resistance, creating a “mixed” pulmonary hypertension with both pre- and postcapillary abnormalities.
There is controversy over the ideal way to identify these patients but little disagreement that they face a worse prognosis than those without precapillary remodeling.13 In light of this, efforts have been made to characterize this cohort.
Historically, mixed pre- and postcapillary pulmonary hypertension was defined as the combination of all of the following:
- Mean pulmonary arterial pressure ≥ 25 mm Hg
- Pulmonary artery occlusion pressure > 15 mm Hg
- Transpulmonary gradient (the mean pulmonary arterial pressure minus the pulmonary artery occlusion pressure) > 12 mm Hg.14
However, the utility of the transpulmonary gradient for distinguishing mixed pulmonary hypertension has been questioned because of concerns over its susceptibility to variations in stroke volume and loading conditions.15
The diastolic pulmonary gradient (the pulmonary arterial diastolic pressure minus the pulmonary artery occlusion pressure) has been proposed as an alternative to the transpulmonary gradient under the theory that it is less sensitive to fluctuation from variations in flow or loading.15
Current guidelines1 suggest that a patient who has all of the following should be considered to have mixed pulmonary hypertension:
- A mean pulmonary arterial pressure > 25 mm Hg
- A pulmonary artery occlusion pressure > 15 mm Hg
- A diastolic pulmonary gradient > 7 mm Hg or a pulmonary vascular resistance > 3 Wood units, or both.
Occult group 2 pulmonary hypertension
Currently, the diagnosis of WHO group 2 pulmonary hypertension is based on elevated resting pulmonary artery occlusion pressure. However, some patients with WHO group 2 pulmonary hypertension and transiently low preload from aggressive diuresis or fasting may have a low pulmonary artery occlusion pressure during right heart catheterization and be misdiagnosed as having WHO group 1 PAH.12,16
This concern was acknowledged in the 2015 Ambrisentan and Tadalafil in Patients With Pulmonary Arterial Hypertension (AMBITION) study after investigators changed the protocol to exclude patients who technically met the criteria for WHO group 1 PAH, but had borderline-elevated pulmonary artery occlusion pressure and additional risk factors worrisome for left heart disease and occult WHO group 2 pulmonary hypertension.17,18
Several strategies, including passive leg-raising, fluid challenge, and exercise during diagnostic right heart catheterization, have been proposed to better classify these patients.19 Unfortunately, due to a lack of standardization of normal values and methodology for executing these maneuvers, consensus is lacking over their routine use, and recommendations for their use have not been provided.1
EVALUATION OF LUNG DISEASE (WHO GROUP 3)
All patients with suspected pulmonary hypertension should also be assessed for underlying pulmonary parenchymal or physiologic disease.
WHO group 3 consists of pulmonary disorders that, over an extended time, can lead to pulmonary hypertension. The most common of these disorders include chronic obstructive pulmonary disease, interstitial lung disease, and combined pulmonary fibrosis and emphysema.1
Pulmonary hypertension in these patients is precapillary, and changes in pulmonary vascular resistance are influenced by multiple factors, the most significant of which is alveolar hypoxia. Hypoxia induces pulmonary artery vasoconstrictionn (in contrast to the reflexive hemodynamics seen in peripheral tissues, where systemic vascular tone is generally lower in states of hypoxia) as a mechanism to divert pulmonary blood flow to well-ventilated portions of the lung and maintain ventilation-perfusion matching.
Repeated chronic hypoxia also alters cellular structure and function of pulmonary vessels and leads to medial hypertrophy and increased vascular tone, thus contributing to the development of pulmonary hypertension in many of these patients.20
Obstructive sleep apnea. Up to 70% of patients with obstructive sleep apnea have pulmonary hypertension.21 Chronic repetitive hypoxia throughout the night increases the levels of reactive oxygen species and alters cellular and molecular signaling, thus inducing vascular remodeling. In addition, apneic events during sleep promote catecholamine-driven elevations in systemic blood pressure. Over time, patients are at higher risk of developing left ventricular dysfunction and concomitant postcapillary group 2 pulmonary hypertension.22 Because typical methods of obstructive sleep apnea screening (eg, the Epworth Sleep Scale) have been historically poor at discriminating PAH patients with obstructive sleep apnea from those without, patients diagnosed with PAH should be considered for formal sleep testing.23,24
Pulmonary function tests, chest imaging
Pulmonary function tests and high-resolution computed tomography are essential to any PAH evaluation and help to exclude WHO group 3 pulmonary hypertension.1
An abnormal result on CT or spirometry can help point toward parenchymal lung disease. Normal spirometry and lung volumes with an isolated reduction in the diffusing capacity of the lung for carbon monoxide (Dlco) is typical of patients with WHO group 1 PAH.
In our patient, CT of the chest did not show any evidence of parenchymal lung disease, and pulmonary function tests showed no evidence of obstruction or restriction. There was a moderate decrease in Dlco, which did not reach normal limits when adjusted for lung volumes. In this setting, further evaluation of her PAH was warranted.
EVALUATION OF THROMBOEMBOLIC DISEASE (WHO GROUP 4)
Once pulmonary hypertension due to underlying left heart disease or parenchymal lung disease has been excluded, testing for chronic thromboembolic pulmonary hypertension is necessary, even in the absence of prior known pulmonary embolism. Identifying these patients is paramount, as chronic thromboembolic pulmonary hypertension (WHO group 4) is the only type of pulmonary hypertension for which a definitive cure is available.26
Up to 9% of patients who survive acute pulmonary embolism exhibit features of chronic proximal thrombosis and remodeling of distal pulmonary arteries.27
It remains unknown exactly why some patients develop chronic thromboembolic pulmonary hypertension and others do not, but the pathophysiology involves inappropriate thrombus resolution after venous thromboembolic events. Monocyte recruitment (which plays an important role in thrombus resolution) is reduced, angiogenesis is impaired (preventing effective vascular collateralization), and abnormal fibroblast proliferation leads to distal pulmonary vascular wall thickening.28 There is some evidence of increased thrombophilic risk in this population, and approximately 10% to 20% of patients are positive for antiphospholipid antibodies or lupus anticoagulant.29,30
Patients with chronic thromboembolic pulmonary hypertension usually present with symptoms similar to those of WHO group 1 PAH. Up to one-quarter of patients have no recollection of prior pulmonary embolism.31 As the disease progresses, signs and symptoms related to elevated pulmonary vascular resistance and right ventricular dysfunction are common.32,33
Although thrombi usually resolve quickly, the diagnosis of chronic thromboembolic pulmonary hypertension should be made only after at least 3 months of appropriate anticoagulation to avoid treatment of transient hemodynamic changes often seen after an acute pulmonary embolism.1
Radiographic changes associated with chronic thromboembolic pulmonary hypertension are distinct from the intraluminal filling defects seen with acute thromboembolism, since chronic thrombi tend to become organized and eccentric. On imaging, one may see features of rapid luminal narrowing or eccentric filling defects rather than the conventional central filling defects of acute pulmonary embolism. These changes are often overlooked by radiologists who are not specifically looking for chronic thromboembolic pulmonary hypertension.34 For this reason, the sensitivity and specificity of identifying chronic thromboembolic disease using radionuclide ventilation-perfusion lung scanning is superior to that of CT angiography.
All patients with suspected PAH should undergo a ventilation-perfusion scan.1,35 In patients with ventilation-perfusion mismatch on radionuclide scanning, pulmonary angiography can fulfill multiple goals of measuring pulmonary arterial pressures, identifying the extent and location of chronic thromboemboli, and can determine whether surgical thromboendarterectomy is feasible.
If chronic thromboembolic pulmonary hypertension is identified, it is imperative that patients be referred to a center of excellence specializing in its management regardless of symptom severity, as surgery can be curative and may prevent development of progressive right ventricular dysfunction.36
Our patient’s ventilation-perfusion scan was normal, effectively ruling out the possibility of chronic thromboembolism as a cause of her pulmonary hypertension.
RIGHT HEART CATHETERIZATION
Once the above-mentioned conditions have been evaluated, patients with suspected PAH should be referred to a pulmonary hypertension center of excellence to undergo right heart catheterization. If this test reveals PAH, further vasoreactivity testing should be performed if the etiology of the PAH is considered to be idiopathic, heritable, or drug-induced.1
Vasoreactivity is most commonly tested using 20 ppm of inhaled nitric oxide, but alternative formulations including intravenous epoprostenol, intravenous adenosine, or inhaled iloprost are acceptable. Patients who have a positive vasoreactive test usually respond well to high-dose calcium channel blocker therapy and have a significantly better prognosis than other patients with PAH.37
Patients with WHO group 1 PAH who do not have idiopathic, heritable, or drug-induced PAH have not been shown to have favorable outcomes using calcium channel blockers even if they have a positive vasoreactive response. A positive vasoreactive response is defined as a drop in mean pulmonary arterial pressure of at least 10 mm Hg to an absolute level of 40 mm Hg or less. Cardiac output should be preserved or elevated compared with baseline values during the challenge.1
In reality, only 10% to 15% of patients with idiopathic PAH have a positive vasoreactive response, and half of these patients stop responding within 1 year.38 Therefore, clinicians should not assume that calcium channel blockers will be successful in the long term in a vasoreactive patient, and these patients should have follow-up right heart catheterization after 3 to 6 months and annually thereafter to ensure continued vasoreactivity.1
In patients who are no longer vasoreactive or whose functional status is worse than New York Heart Association functional class I or II, conventional PAH-specific therapy should be started.
LOOKING FOR CAUSES OF ‘IDIOPATHIC’ PAH
Pulmonary hypertension is considered the final common pathway of many varied diseases and syndromes, and therefore one cannot say it is idiopathic without making a robust effort to identify features of alternative causes and rule out other contributing factors.
Although the exact etiology of idiopathic PAH is unclear, well-characterized imbalances in vascular homeostasis have been identified. These include processes that promote vasoconstriction, cell proliferation, and thrombosis (thromboxane A2, endothelin-1, and serotonin) and those that suppress prostacyclin, nitric oxide, and vasoactive intestinal peptide-mediated vasodilation.1 Furthermore, an abnormal angiogenic response to hypoxia and vascular endothelial growth factor has been observed.39
Before considering a diagnosis of idiopathic PAH, a careful history is essential. Other causative agents include appetite-suppressing medications, such as fenfluramine derivatives or stimulants such as amphetamines. Human immunodeficiency virus (HIV) or hepatitis, a history of splenectomy, and prior thyroid or liver disease are also common causes of PAH. Joint pain, myalgias, Raynaud features, or a rash characteristic of connective tissue disease can be identified on history and physical examination. Worldwide, chronic exposure to high-altitude climates and exposure to schistosomiasis are significant causes of PAH, but are rarely seen in developed nations. Confirmatory serum tests for HIV, antinuclear antibody, scleroderma antibody, and thyroid function are essential.1
Genetic factors
If patients report having relatives with possible or probable PAH, genetic counseling is recommended, particularly for rare but causative gene mutations.
BMPR2, the gene that codes for the bone morphogenetic protein receptor type 2, can carry mutations with variable penetrance over the patient’s lifetime depending on other genetic polymorphisms, concurrent inflammation, and the patient’s sex.40
The population carrier estimates of BMPR2 mutations are only 0.001% to 0.01%, but mutations in this gene are identified in approximately 25% of nonfamilial PAH patients and in over 75% of those with a familial inheritance pattern. The BMPR2 protein is a part of the transforming growth factor beta family and is partially responsible for control of vascular cell proliferation. Mutations in this gene lead to PAH at a younger age than in those with mutation-negative idiopathic PAH and to a more severe clinical phenotype in terms of pulmonary vascular resistance and cardiac function.40
Other mutations. Although BMPR2 is the most commonly identified gene mutation in patients with PAH, other gene mutations within this family have also been recognized. These include mutations in the genes for activin receptor-like kinase 1 and endoglin, which, although better known for their association with hereditary hemorrhagic telangiectasia, can lead directly to PAH.40
More recently, a novel autosomal recessive gene mutation in eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) has been identified in patients with pulmonary veno-occlusive disease41 and pulmonary capillary hemangiomatosis,42 which are specific subclasses of WHO group 1 PAH. The mechanistic parallels between EIF2AK4 and these diseases are not clear, but the prevalence of disease in those with a familial inheritance pattern and an EIF2AK4 mutation is nearly 100%.41 Thus, identification of this mutation has been accepted as a way to confirm pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis in patients suspected of PAH with features of these diseases.43,44
GROUP 5: MISCELLANEOUS FORMS OF PULMONARY HYPERTENSION
WHO group 5 pulmonary hypertension encompasses disorders whose pathophysiology does not fit neatly within the context of the other pulmonary hypertension subtypes. Nonetheless, appreciation of these disorders is important in determining the etiology and appropriate therapy for patients with pulmonary hypertension. The mechanism driving abnormal pulmonary arterial pressures in patients with group 5 pulmonary hypertension is not always clear and may involve intrinsic or extrinsic factors.1
Diseases within group 5 include those that cause extrinsic compression of the pulmonary arteries (ie, fibrosing mediastinitis) or intrinsic elevations in pulmonary vascular resistance (sarcoidosis, pulmonary Langerhans cell histiocytosis, sickle cell anemia, polycythemia vera, and malignancy).
The most common cause of pulmonary hypertension in this category is sarcoidosis. Current theories suggest that, for most patients, invasion of granulomatous inflammation within the arterial walls induces PAH via fibrotic or inflammatory vascular occlusion. Extrinsic compression due to lymphadenopathy, right or left ventricular dysfunction due to cardiac myocite infiltration, and endothelin-induced pulmonary vasoconstriction are other possible links between the PAH and sarcoidosis.45
PROGNOSTIC RISK STRATIFICATION IN THE PATIENTS WITH PAH
Cardiac magnetic resonance imaging (MRI) has gained popularity as a noninvasive and reproducible alternative to echocardiography. Image fidelity and characterization of right ventricular function and right ventricular ejection fraction are all more accurate than with echocardiography, and serial MRI has proven valuable in its ability to guide patient prognosis.46
However, MRI is more expensive than echocardiography, and some patients cannot tolerate the procedure. In addition, for those who can tolerate it, MRI is not a suitable alternative to right heart catheterization, since it cannot accurately estimate pulmonary artery occlusion pressure or pulmonary arterial pressures.1 For these reasons, cardiac MRI use varies across pulmonary hypertension centers.
A goal of treatment is to reduce a patient’s risk. While no consensus has been achieved over which PAH-specific therapy to start with, evidence is robust that using more than 1 class of agent is beneficial, capitalizing on multiple therapeutic targets.17,47
In our patient, right heart catheterization revealed PAH with a mean pulmonary arterial pressure of 44 mm Hg, pulmonary artery occlusion pressure 6 mm Hg, and a cardiac index of 2.1 L/min/m2. Ancillary testing for alternative causes of PAH was unrevealing, as was vasoreactivity testing. Our patient could walk only 314 meters on her 6-minute walk test and had an initial NT-proBNP level of 750 ng/L.
Based on these and the findings during her evaluation, she would be classified as having intermediate-risk PAH with an estimated 1-year mortality risk of 5% to 10%.1 Appropriate therapy and follow-up would be guided by this determination. Specific therapy is beyond the scope of this article but we would start her on dual oral therapy with close follow-up to reassess her 1-year mortality risk. If there were no improvement over a short period of time, we would add further therapy.
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46(4):903–975. doi:10.1183/13993003.01032-2015
- Galiè N, Rubin LJ, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomized controlled trial. Lancet 2008; 371(9630):2093–2100. doi:10.1016/S0140-6736(08)60919-8
- Howard LS. Prognostic factors in pulmonary arterial hypertension: assessing the course of the disease. Eur Respir Rev 2011; 20:236–242. doi:10.1183/09059180.00006711
- Brown LM, Chen H, Halpern S, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL registry. Chest 2011; 140:19–26. doi:10.1378/chest.10-1166
- Elliot CG, Farber H, Frost A, Liou TG, Turner M. REVEAL Registry: medical history and time to diagnosis of enrolled patients. Chest 2007; 132(4):631a. doi:10.1378/chest.132.4_MeetingAbstracts.631a
- Minai OA, Budev MM. Diagnostic strategies for suspected pulmonary arterial hypertension: a primer for the internist. Cleve Clin J Med 2007; 74(10):737–747. pmid:17941295
- Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010; 137(2):376–387. doi:10.1378/chest.09-1140
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179(7):615–621. doi:10.1164/rccm.200811-1691OC
- Robbins IM, Newman JH, Johnson RF, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest 2009; 136(1):31–36. doi:10.1378/chest.08-2008
- Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016; 37(12):942–954. doi:10.1093/eurheartj/ehv512
- Opitz CF, Hoeper MM, Gibbs JSR, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016; 68:368–378. doi: 10.1016/j.jacc.2016.05.047
- Robbins IM, Hemnes AR, Pugh ME, et al. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail 2014; 7(1):116–122. doi:10.1161/CIRCHEARTFAILURE.113.000468
- Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013; 143(3):758–766. doi:10.1378/chest.12-1653
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT); Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009; 34(6):1219–1263. doi:10.1183/09031936.00139009
- Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013; 41(1):217–223. doi:10.1183/09031936.00074312
- Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013; 144(5):1521–1529. doi:10.1378/chest.12-3023
- Galiè N, Barberà JA, Frost AE, et al; AMBITION Investigators. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373(9):834–844. doi:10.1056/NEJMoa1413687
- Farr G, Shah K, Markley R, Abbate A, Salloum FN, Grinnan D. Development of pulmonary hypertension in heart failure with preserved ejection fraction. Prog Cardiovasc Dis 2016; 59(1):52–58. doi:10.1016/j.pcad.2016.06.002
- Hoeper MM, Barberà JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol 2009; 54(suppl 1):S85–S96. doi:10.1016/j.jacc.2009.04.008
- Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008; 32(5):1371–1385. doi:10.1183/09031936.00015608
- Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol 2009; 104(9):1300–1306. doi:10.1016/j.amjcard.2009.06.048
- Kholdani C, Fares WH, Mohsenin V. Pulmonary hypertension in obstructive sleep apnea: is it clinically significant? A critical analysis of the association and pathophysiology. Pulm Circ 2015; 5(2):220–227. doi:10.1086/679995
- Prisco DL, Sica AL, Talwar A, et al. Correlation of pulmonary hypertension severity with metrics of comorbid sleep-disordered breathing. Sleep Breath 2011; 15(4):633–639. doi:10.1007/s11325-010-0411-y
- Dumitrascu R, Tiede H, Eckermann J, et al. Sleep apnea in precapillary pulmonary hypertension. Sleep Med 2013; 14(3):247–251. doi:10.1016/j.sleep.2012.11.013
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167(5):735–740. doi:10.1164/rccm.200210-1130OC
- Pepke-Zaba J, Jansa P, Kim NH, Naeije R, Simonneau G. Chronic thromboembolic pulmonary hypertension: role of medical therapy. Eur Respir J 2013; 41(4):985–990. doi:10.1183/09031936.00201612
- Guérin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost 2014; 112(3):598–605. doi:10.1160/TH13-07-0538
- Lang IM, Pesavento R, Bonderman D, Yuan JX. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J 2013; 41(2):462–468. doi:10.1183/09031936.00049312
- Pepke-Zaba J. Diagnostic testing to guide the management of chronic thromboembolic pulmonary hypertension: state of the art. Eur Respir Rev 2010; 19(115):55–58. doi:10.1183/09059180.00007209
- Bonderman D, Turecek PL, Jakowitsch J, et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost 2003; 90(3):372–376. doi:10.1160/TH03-02-0067
- Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124(18):1973–1981. doi:10.1161/CIRCULATIONAHA.110.015008
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62:(suppl 25):D92–D99. doi:10.1016/j.jacc.2013.10.024
- Moser KM, Auger WR, Fedullo PF. Chronic major-vessel thromboembolic pulmonary hypertension. Circulation 1990; 81(6):1735–1743. pmid:2188751
- McNeil K, Dunning J. Chronic thromboembolic pulmonary hypertension (CTEPH). Heart 2007; 93(9):1152–1158. doi:10.1136/hrt.2004.053603
- Tunariu N, Gibbs SJ, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med 2007; 48(5):680–684. doi:10.2967/jnumed.106.039438
- Fedullo P, Kerr KM, Kim NH, Auger WR. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2011; 183(12):1605–1613. doi:10.1164/rccm.201011-1854CI
- Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 1992; 327(2):76–81. doi:10.1056/NEJM199207093270203
- Sitbon O, Humbert M, Jaıs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005; 111(23):3105–3111. doi:10.1161/CIRCULATIONAHA.104.488486
- Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol 2008; 51(16):1527–1538. doi:10.1016/j.jacc.2008.01.024
- Soubrier F, Chung WK, Machado R, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2013; 62(suppl 25):D13–D21. doi:10.1016/j.jacc.2013.10.035
- Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 2014; 46(1):65–69. doi: 10.1038/ng.2844
- Best DH, Sumner KL, Austin ED, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest 2014; 145(2):231–236. doi:10.1378/chest.13-2366
- Best DH, Sumner KL, Smith BP, et al. EIF2AK4 mutations in patients diagnosed with pulmonary arterial hypertension. Chest 2017; 151(4):821–828. doi:10.1016/j.chest.2016.11.014
- Hadinnapola C, Bleda M, Haimel M, et al; NIHR BioResource–Rare Diseases Consortium; UK National Cohort Study of Idiopathic and Heritable PAH. Phenotypic characterization of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation 2017; 136(21):2022–2033. doi:10.1161/CIRCULATIONAHA.117.028351
- Diaz-Guzman E, Farver C, Parambil J, Culver DA. Pulmonary hypertension caused by sarcoidosis. Clin Chest Med 2008; 29(3):549–563. doi:10.1016/j.ccm.2008.03.010
- Mauritz GJ, Kind T, Marcus JT, et al. Progressive changes in right ventricular geometric shortening and long-term survival in pulmonary arterial hypertension. Chest 2012; 141(4):935–943. doi:10.1378/chest.10-3277
- Galiè N, Palazzini M, Manes A. Pulmonary arterial hypertension: from the kingdom of the near-dead to multiple clinical trial meta-analyses. Eur Heart J 2010; 31(17):2080–2086. doi:10.1093/eurheartj/ehq152
Pulmonary arterial hypertension (PAH) is a hemodynamic disorder that affects small and medium-size pulmonary arteries through cellular proliferation and luminal narrowing.1 Increased pulmonary vascular resistance causes restricted blood flow in these arteries, leading to elevated pulmonary arterial pressure and afterload on the right ventricle. Despite advances in therapy, death usually occurs as a result of right ventricular failure.
- Group 1—PAH, due to narrowed pulmonary arteries
- Group 2—due to left heart disease
- Group 3—due to lung disease or hypoxia, or both
- Group 4—due to chronic thromboembolism or other pulmonary artery obstruction
- Group 5—due to uncertain or multifactorial causes.
Experts recognize the morbidity and mortality associated with pulmonary hypertension now more than in the past, and they emphasize recognizing it early. Guidelines for its diagnosis and treatment were updated in 2015.1
Below, we use a case to discuss recommendations for initial evaluation and classification of pulmonary hypertension, particularly PAH.
A PATIENT SUSPECTED OF HAVING PULMONARY HYPERTENSION
A 63-year-old woman with a 25-pack-year history of tobacco use, as well as pulmonary embolism and coronary artery disease, presents to her primary care physician with exertional dyspnea. She had been a clerk at a hardware store and physically active until she took early retirement 8 months ago because of increasing fatigue. She initially felt the fatigue was simply “a sign of getting old.”
Since retiring, she has noticed the slow onset of progressive dyspnea on exertion. She can no longer climb more than 1 flight of stairs or walk more than 1 block. She also complains of mild, fluctuating edema in her lower extremities over the past month. She quit smoking 8 years ago after undergoing placement of a drug-eluting stent in the mid-left circumflex artery. After this, she received clopidogrel and was followed by a cardiologist for 2 years but stopped taking the medication because of bruising. She has not seen her cardiologist in more than 5 years.
She underwent elective right total knee arthroplasty 3 years ago, complicated by acute deep vein thrombosis in the right common femoral vein. Computed tomography (CT) at that time did not reveal pulmonary emboli. She received warfarin therapy for 3 months.
She reports no current cough, chest pain, lightheadedness, or syncope. She has no orthopnea, and she feels normal at rest.
Her family history is unremarkable, and she has had no exposure to illicit substances, environmental toxins, or dietary supplements. She takes aspirin 81 mg daily, metoprolol 25 mg twice daily, lisinopril 10 mg daily, and simvastatin 40 mg at bedtime.
Her primary care physician detects a murmur in the left lower sternal border and sends her for transthoracic echocardiography, which demonstrates mild right ventricular dilation, right atrial dilation, and mildly reduced right ventricular function. The calculated right ventricular systolic pressure is 69 mm Hg. The left ventricle shows mild concentric hypertrophy; the left atrium is normal in size.
DIAGNOSTIC EVALUATION OF SUSPECTED PULMONARY HYPERTENSION
CLINICAL MANIFESTATIONS
Symptoms and signs. As in the patient described above, the first symptoms such as exertional dyspnea, fatigue, and lightheadedness usually arise in situations that call for increased cardiac output.4 As right ventricular function worsens, symptoms start to occur at rest, and signs of increased right ventricular preload appear, such as abdominal and lower-extremity edema and pericardial effusion. Syncope is a sign of severe right ventricular dysfunction.5
Physical examination. Look for signs of increased right ventricular loading and failure, eg:
- An accentuated intensity and persistent splitting of the second heart sound
- A prominent parasternal heave
- A prominent jugular “a” wave
- A systolic murmur along the left sternal border at the fourth intercostal space, which may worsen with breath-holding
- Pitting lower-extremity edema
- Hepatomegaly
- Hepatojugular reflux
- Hepatic pulsatility.6
ECHOCARDIOGRAPHY IN SUSPECTED PULMONARY HYPERTENSION
Many practitioners rely heavily on the estimated right ventricular systolic pressure in diagnosing pulmonary hypertension. In theory, this number should be nearly the same as the pulmonary arterial systolic pressure. However, technical and patient-related aspects of transthoracic echocardiography often limit accurate measurement of the right ventricular systolic pressure, and readings often differ from those measured with right heart catheterization.8
Our patient had a markedly elevated right ventricular systolic pressure and signs of right ventricular dysfunction, suggesting a high probability of pulmonary hypertension.
EVALUATING LEFT HEART DISEASE (WHO GROUP 2)
More than 75% of cases of pulmonary hypertension are directly related to left ventricular dysfunction or mitral or aortic valve disease (WHO group 2).1 Since group 2 differs markedly from group 1 (PAH) in its pathophysiology and treatment, it is important to distinguish between them.
Compared with WHO group 1 patients, those in group 2 tend to be older, more of them are male, and more of them have comorbidities such as metabolic syndrome, hypertension, and coronary artery disease.1,9 A combination of risk factors and clinical findings should be considered in identifying these patients.10
Transthoracic echocardiography is used to detect features of systolic and diastolic dysfunction. Left atrial enlargement is a clue that left heart disease may be present. In addition, signs of left ventricular or valvular dysfunction on electrocardiography or chest radiography are often helpful.
When estimated right ventricular systolic pressures are only minimally abnormal and no significant right ventricular dysfunction exists, further diagnostic evaluation is not warranted. However, because no single identifying feature or variable can readily distinguish group 2 from the other WHO groups, further evaluation should be considered if the right ventricular systolic pressure is significantly elevated or right ventricular dysfunction exists.
Our patient had several risk factors for left heart disease, including a history of smoking and coronary artery disease. Nonetheless, findings consistent with severe right ventricular dysfunction necessitated further evaluation for other possible causes of her suspected pulmonary hypertension.
Postcapillary pulmonary hypertension
In patients for whom further evaluation is pursued, the diagnosis of WHO group 2 pulmonary hypertension is ultimately based on findings consistent with postcapillary or “passive” pulmonary hypertension on right heart catheterization. Although mean pulmonary arterial pressures must be at least 25 mm Hg to certify the diagnosis of pulmonary hypertension, a pulmonary artery occlusion pressure greater than 15 mm Hg (normal 6–12) and pulmonary vascular resistance of 3 Wood units or less (normal 0.3–1.6) suggests the pulmonary hypertension is due to elevated left atrial pressure (ie, postcapillary) rather than precapillary pulmonary arterial remodeling.
Mixed pre- and postcapillary pulmonary hypertension
Distinguishing pulmonary venous hypertension from PAH is important, since their management differs. In particular, PAH-specific therapies (ie, prostacyclin analogues, prostaglandin I2 receptor agonists, endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and cyclic guanosine monophosphate stimulators) can have a detrimental effect in WHO group 2 patients by causing increased pulmonary capillary leakage with pulmonary edema.11,12
In some patients, chronic passive congestion in the pulmonary venous circulation causes additional disruption of the homeostatic milieu regulating precapillary smooth muscle and endothelial function. These changes result in structural remodeling of precapillary arterioles and increased precapillary vascular resistance, creating a “mixed” pulmonary hypertension with both pre- and postcapillary abnormalities.
There is controversy over the ideal way to identify these patients but little disagreement that they face a worse prognosis than those without precapillary remodeling.13 In light of this, efforts have been made to characterize this cohort.
Historically, mixed pre- and postcapillary pulmonary hypertension was defined as the combination of all of the following:
- Mean pulmonary arterial pressure ≥ 25 mm Hg
- Pulmonary artery occlusion pressure > 15 mm Hg
- Transpulmonary gradient (the mean pulmonary arterial pressure minus the pulmonary artery occlusion pressure) > 12 mm Hg.14
However, the utility of the transpulmonary gradient for distinguishing mixed pulmonary hypertension has been questioned because of concerns over its susceptibility to variations in stroke volume and loading conditions.15
The diastolic pulmonary gradient (the pulmonary arterial diastolic pressure minus the pulmonary artery occlusion pressure) has been proposed as an alternative to the transpulmonary gradient under the theory that it is less sensitive to fluctuation from variations in flow or loading.15
Current guidelines1 suggest that a patient who has all of the following should be considered to have mixed pulmonary hypertension:
- A mean pulmonary arterial pressure > 25 mm Hg
- A pulmonary artery occlusion pressure > 15 mm Hg
- A diastolic pulmonary gradient > 7 mm Hg or a pulmonary vascular resistance > 3 Wood units, or both.
Occult group 2 pulmonary hypertension
Currently, the diagnosis of WHO group 2 pulmonary hypertension is based on elevated resting pulmonary artery occlusion pressure. However, some patients with WHO group 2 pulmonary hypertension and transiently low preload from aggressive diuresis or fasting may have a low pulmonary artery occlusion pressure during right heart catheterization and be misdiagnosed as having WHO group 1 PAH.12,16
This concern was acknowledged in the 2015 Ambrisentan and Tadalafil in Patients With Pulmonary Arterial Hypertension (AMBITION) study after investigators changed the protocol to exclude patients who technically met the criteria for WHO group 1 PAH, but had borderline-elevated pulmonary artery occlusion pressure and additional risk factors worrisome for left heart disease and occult WHO group 2 pulmonary hypertension.17,18
Several strategies, including passive leg-raising, fluid challenge, and exercise during diagnostic right heart catheterization, have been proposed to better classify these patients.19 Unfortunately, due to a lack of standardization of normal values and methodology for executing these maneuvers, consensus is lacking over their routine use, and recommendations for their use have not been provided.1
EVALUATION OF LUNG DISEASE (WHO GROUP 3)
All patients with suspected pulmonary hypertension should also be assessed for underlying pulmonary parenchymal or physiologic disease.
WHO group 3 consists of pulmonary disorders that, over an extended time, can lead to pulmonary hypertension. The most common of these disorders include chronic obstructive pulmonary disease, interstitial lung disease, and combined pulmonary fibrosis and emphysema.1
Pulmonary hypertension in these patients is precapillary, and changes in pulmonary vascular resistance are influenced by multiple factors, the most significant of which is alveolar hypoxia. Hypoxia induces pulmonary artery vasoconstrictionn (in contrast to the reflexive hemodynamics seen in peripheral tissues, where systemic vascular tone is generally lower in states of hypoxia) as a mechanism to divert pulmonary blood flow to well-ventilated portions of the lung and maintain ventilation-perfusion matching.
Repeated chronic hypoxia also alters cellular structure and function of pulmonary vessels and leads to medial hypertrophy and increased vascular tone, thus contributing to the development of pulmonary hypertension in many of these patients.20
Obstructive sleep apnea. Up to 70% of patients with obstructive sleep apnea have pulmonary hypertension.21 Chronic repetitive hypoxia throughout the night increases the levels of reactive oxygen species and alters cellular and molecular signaling, thus inducing vascular remodeling. In addition, apneic events during sleep promote catecholamine-driven elevations in systemic blood pressure. Over time, patients are at higher risk of developing left ventricular dysfunction and concomitant postcapillary group 2 pulmonary hypertension.22 Because typical methods of obstructive sleep apnea screening (eg, the Epworth Sleep Scale) have been historically poor at discriminating PAH patients with obstructive sleep apnea from those without, patients diagnosed with PAH should be considered for formal sleep testing.23,24
Pulmonary function tests, chest imaging
Pulmonary function tests and high-resolution computed tomography are essential to any PAH evaluation and help to exclude WHO group 3 pulmonary hypertension.1
An abnormal result on CT or spirometry can help point toward parenchymal lung disease. Normal spirometry and lung volumes with an isolated reduction in the diffusing capacity of the lung for carbon monoxide (Dlco) is typical of patients with WHO group 1 PAH.
In our patient, CT of the chest did not show any evidence of parenchymal lung disease, and pulmonary function tests showed no evidence of obstruction or restriction. There was a moderate decrease in Dlco, which did not reach normal limits when adjusted for lung volumes. In this setting, further evaluation of her PAH was warranted.
EVALUATION OF THROMBOEMBOLIC DISEASE (WHO GROUP 4)
Once pulmonary hypertension due to underlying left heart disease or parenchymal lung disease has been excluded, testing for chronic thromboembolic pulmonary hypertension is necessary, even in the absence of prior known pulmonary embolism. Identifying these patients is paramount, as chronic thromboembolic pulmonary hypertension (WHO group 4) is the only type of pulmonary hypertension for which a definitive cure is available.26
Up to 9% of patients who survive acute pulmonary embolism exhibit features of chronic proximal thrombosis and remodeling of distal pulmonary arteries.27
It remains unknown exactly why some patients develop chronic thromboembolic pulmonary hypertension and others do not, but the pathophysiology involves inappropriate thrombus resolution after venous thromboembolic events. Monocyte recruitment (which plays an important role in thrombus resolution) is reduced, angiogenesis is impaired (preventing effective vascular collateralization), and abnormal fibroblast proliferation leads to distal pulmonary vascular wall thickening.28 There is some evidence of increased thrombophilic risk in this population, and approximately 10% to 20% of patients are positive for antiphospholipid antibodies or lupus anticoagulant.29,30
Patients with chronic thromboembolic pulmonary hypertension usually present with symptoms similar to those of WHO group 1 PAH. Up to one-quarter of patients have no recollection of prior pulmonary embolism.31 As the disease progresses, signs and symptoms related to elevated pulmonary vascular resistance and right ventricular dysfunction are common.32,33
Although thrombi usually resolve quickly, the diagnosis of chronic thromboembolic pulmonary hypertension should be made only after at least 3 months of appropriate anticoagulation to avoid treatment of transient hemodynamic changes often seen after an acute pulmonary embolism.1
Radiographic changes associated with chronic thromboembolic pulmonary hypertension are distinct from the intraluminal filling defects seen with acute thromboembolism, since chronic thrombi tend to become organized and eccentric. On imaging, one may see features of rapid luminal narrowing or eccentric filling defects rather than the conventional central filling defects of acute pulmonary embolism. These changes are often overlooked by radiologists who are not specifically looking for chronic thromboembolic pulmonary hypertension.34 For this reason, the sensitivity and specificity of identifying chronic thromboembolic disease using radionuclide ventilation-perfusion lung scanning is superior to that of CT angiography.
All patients with suspected PAH should undergo a ventilation-perfusion scan.1,35 In patients with ventilation-perfusion mismatch on radionuclide scanning, pulmonary angiography can fulfill multiple goals of measuring pulmonary arterial pressures, identifying the extent and location of chronic thromboemboli, and can determine whether surgical thromboendarterectomy is feasible.
If chronic thromboembolic pulmonary hypertension is identified, it is imperative that patients be referred to a center of excellence specializing in its management regardless of symptom severity, as surgery can be curative and may prevent development of progressive right ventricular dysfunction.36
Our patient’s ventilation-perfusion scan was normal, effectively ruling out the possibility of chronic thromboembolism as a cause of her pulmonary hypertension.
RIGHT HEART CATHETERIZATION
Once the above-mentioned conditions have been evaluated, patients with suspected PAH should be referred to a pulmonary hypertension center of excellence to undergo right heart catheterization. If this test reveals PAH, further vasoreactivity testing should be performed if the etiology of the PAH is considered to be idiopathic, heritable, or drug-induced.1
Vasoreactivity is most commonly tested using 20 ppm of inhaled nitric oxide, but alternative formulations including intravenous epoprostenol, intravenous adenosine, or inhaled iloprost are acceptable. Patients who have a positive vasoreactive test usually respond well to high-dose calcium channel blocker therapy and have a significantly better prognosis than other patients with PAH.37
Patients with WHO group 1 PAH who do not have idiopathic, heritable, or drug-induced PAH have not been shown to have favorable outcomes using calcium channel blockers even if they have a positive vasoreactive response. A positive vasoreactive response is defined as a drop in mean pulmonary arterial pressure of at least 10 mm Hg to an absolute level of 40 mm Hg or less. Cardiac output should be preserved or elevated compared with baseline values during the challenge.1
In reality, only 10% to 15% of patients with idiopathic PAH have a positive vasoreactive response, and half of these patients stop responding within 1 year.38 Therefore, clinicians should not assume that calcium channel blockers will be successful in the long term in a vasoreactive patient, and these patients should have follow-up right heart catheterization after 3 to 6 months and annually thereafter to ensure continued vasoreactivity.1
In patients who are no longer vasoreactive or whose functional status is worse than New York Heart Association functional class I or II, conventional PAH-specific therapy should be started.
LOOKING FOR CAUSES OF ‘IDIOPATHIC’ PAH
Pulmonary hypertension is considered the final common pathway of many varied diseases and syndromes, and therefore one cannot say it is idiopathic without making a robust effort to identify features of alternative causes and rule out other contributing factors.
Although the exact etiology of idiopathic PAH is unclear, well-characterized imbalances in vascular homeostasis have been identified. These include processes that promote vasoconstriction, cell proliferation, and thrombosis (thromboxane A2, endothelin-1, and serotonin) and those that suppress prostacyclin, nitric oxide, and vasoactive intestinal peptide-mediated vasodilation.1 Furthermore, an abnormal angiogenic response to hypoxia and vascular endothelial growth factor has been observed.39
Before considering a diagnosis of idiopathic PAH, a careful history is essential. Other causative agents include appetite-suppressing medications, such as fenfluramine derivatives or stimulants such as amphetamines. Human immunodeficiency virus (HIV) or hepatitis, a history of splenectomy, and prior thyroid or liver disease are also common causes of PAH. Joint pain, myalgias, Raynaud features, or a rash characteristic of connective tissue disease can be identified on history and physical examination. Worldwide, chronic exposure to high-altitude climates and exposure to schistosomiasis are significant causes of PAH, but are rarely seen in developed nations. Confirmatory serum tests for HIV, antinuclear antibody, scleroderma antibody, and thyroid function are essential.1
Genetic factors
If patients report having relatives with possible or probable PAH, genetic counseling is recommended, particularly for rare but causative gene mutations.
BMPR2, the gene that codes for the bone morphogenetic protein receptor type 2, can carry mutations with variable penetrance over the patient’s lifetime depending on other genetic polymorphisms, concurrent inflammation, and the patient’s sex.40
The population carrier estimates of BMPR2 mutations are only 0.001% to 0.01%, but mutations in this gene are identified in approximately 25% of nonfamilial PAH patients and in over 75% of those with a familial inheritance pattern. The BMPR2 protein is a part of the transforming growth factor beta family and is partially responsible for control of vascular cell proliferation. Mutations in this gene lead to PAH at a younger age than in those with mutation-negative idiopathic PAH and to a more severe clinical phenotype in terms of pulmonary vascular resistance and cardiac function.40
Other mutations. Although BMPR2 is the most commonly identified gene mutation in patients with PAH, other gene mutations within this family have also been recognized. These include mutations in the genes for activin receptor-like kinase 1 and endoglin, which, although better known for their association with hereditary hemorrhagic telangiectasia, can lead directly to PAH.40
More recently, a novel autosomal recessive gene mutation in eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) has been identified in patients with pulmonary veno-occlusive disease41 and pulmonary capillary hemangiomatosis,42 which are specific subclasses of WHO group 1 PAH. The mechanistic parallels between EIF2AK4 and these diseases are not clear, but the prevalence of disease in those with a familial inheritance pattern and an EIF2AK4 mutation is nearly 100%.41 Thus, identification of this mutation has been accepted as a way to confirm pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis in patients suspected of PAH with features of these diseases.43,44
GROUP 5: MISCELLANEOUS FORMS OF PULMONARY HYPERTENSION
WHO group 5 pulmonary hypertension encompasses disorders whose pathophysiology does not fit neatly within the context of the other pulmonary hypertension subtypes. Nonetheless, appreciation of these disorders is important in determining the etiology and appropriate therapy for patients with pulmonary hypertension. The mechanism driving abnormal pulmonary arterial pressures in patients with group 5 pulmonary hypertension is not always clear and may involve intrinsic or extrinsic factors.1
Diseases within group 5 include those that cause extrinsic compression of the pulmonary arteries (ie, fibrosing mediastinitis) or intrinsic elevations in pulmonary vascular resistance (sarcoidosis, pulmonary Langerhans cell histiocytosis, sickle cell anemia, polycythemia vera, and malignancy).
The most common cause of pulmonary hypertension in this category is sarcoidosis. Current theories suggest that, for most patients, invasion of granulomatous inflammation within the arterial walls induces PAH via fibrotic or inflammatory vascular occlusion. Extrinsic compression due to lymphadenopathy, right or left ventricular dysfunction due to cardiac myocite infiltration, and endothelin-induced pulmonary vasoconstriction are other possible links between the PAH and sarcoidosis.45
PROGNOSTIC RISK STRATIFICATION IN THE PATIENTS WITH PAH
Cardiac magnetic resonance imaging (MRI) has gained popularity as a noninvasive and reproducible alternative to echocardiography. Image fidelity and characterization of right ventricular function and right ventricular ejection fraction are all more accurate than with echocardiography, and serial MRI has proven valuable in its ability to guide patient prognosis.46
However, MRI is more expensive than echocardiography, and some patients cannot tolerate the procedure. In addition, for those who can tolerate it, MRI is not a suitable alternative to right heart catheterization, since it cannot accurately estimate pulmonary artery occlusion pressure or pulmonary arterial pressures.1 For these reasons, cardiac MRI use varies across pulmonary hypertension centers.
A goal of treatment is to reduce a patient’s risk. While no consensus has been achieved over which PAH-specific therapy to start with, evidence is robust that using more than 1 class of agent is beneficial, capitalizing on multiple therapeutic targets.17,47
In our patient, right heart catheterization revealed PAH with a mean pulmonary arterial pressure of 44 mm Hg, pulmonary artery occlusion pressure 6 mm Hg, and a cardiac index of 2.1 L/min/m2. Ancillary testing for alternative causes of PAH was unrevealing, as was vasoreactivity testing. Our patient could walk only 314 meters on her 6-minute walk test and had an initial NT-proBNP level of 750 ng/L.
Based on these and the findings during her evaluation, she would be classified as having intermediate-risk PAH with an estimated 1-year mortality risk of 5% to 10%.1 Appropriate therapy and follow-up would be guided by this determination. Specific therapy is beyond the scope of this article but we would start her on dual oral therapy with close follow-up to reassess her 1-year mortality risk. If there were no improvement over a short period of time, we would add further therapy.
Pulmonary arterial hypertension (PAH) is a hemodynamic disorder that affects small and medium-size pulmonary arteries through cellular proliferation and luminal narrowing.1 Increased pulmonary vascular resistance causes restricted blood flow in these arteries, leading to elevated pulmonary arterial pressure and afterload on the right ventricle. Despite advances in therapy, death usually occurs as a result of right ventricular failure.
- Group 1—PAH, due to narrowed pulmonary arteries
- Group 2—due to left heart disease
- Group 3—due to lung disease or hypoxia, or both
- Group 4—due to chronic thromboembolism or other pulmonary artery obstruction
- Group 5—due to uncertain or multifactorial causes.
Experts recognize the morbidity and mortality associated with pulmonary hypertension now more than in the past, and they emphasize recognizing it early. Guidelines for its diagnosis and treatment were updated in 2015.1
Below, we use a case to discuss recommendations for initial evaluation and classification of pulmonary hypertension, particularly PAH.
A PATIENT SUSPECTED OF HAVING PULMONARY HYPERTENSION
A 63-year-old woman with a 25-pack-year history of tobacco use, as well as pulmonary embolism and coronary artery disease, presents to her primary care physician with exertional dyspnea. She had been a clerk at a hardware store and physically active until she took early retirement 8 months ago because of increasing fatigue. She initially felt the fatigue was simply “a sign of getting old.”
Since retiring, she has noticed the slow onset of progressive dyspnea on exertion. She can no longer climb more than 1 flight of stairs or walk more than 1 block. She also complains of mild, fluctuating edema in her lower extremities over the past month. She quit smoking 8 years ago after undergoing placement of a drug-eluting stent in the mid-left circumflex artery. After this, she received clopidogrel and was followed by a cardiologist for 2 years but stopped taking the medication because of bruising. She has not seen her cardiologist in more than 5 years.
She underwent elective right total knee arthroplasty 3 years ago, complicated by acute deep vein thrombosis in the right common femoral vein. Computed tomography (CT) at that time did not reveal pulmonary emboli. She received warfarin therapy for 3 months.
She reports no current cough, chest pain, lightheadedness, or syncope. She has no orthopnea, and she feels normal at rest.
Her family history is unremarkable, and she has had no exposure to illicit substances, environmental toxins, or dietary supplements. She takes aspirin 81 mg daily, metoprolol 25 mg twice daily, lisinopril 10 mg daily, and simvastatin 40 mg at bedtime.
Her primary care physician detects a murmur in the left lower sternal border and sends her for transthoracic echocardiography, which demonstrates mild right ventricular dilation, right atrial dilation, and mildly reduced right ventricular function. The calculated right ventricular systolic pressure is 69 mm Hg. The left ventricle shows mild concentric hypertrophy; the left atrium is normal in size.
DIAGNOSTIC EVALUATION OF SUSPECTED PULMONARY HYPERTENSION
CLINICAL MANIFESTATIONS
Symptoms and signs. As in the patient described above, the first symptoms such as exertional dyspnea, fatigue, and lightheadedness usually arise in situations that call for increased cardiac output.4 As right ventricular function worsens, symptoms start to occur at rest, and signs of increased right ventricular preload appear, such as abdominal and lower-extremity edema and pericardial effusion. Syncope is a sign of severe right ventricular dysfunction.5
Physical examination. Look for signs of increased right ventricular loading and failure, eg:
- An accentuated intensity and persistent splitting of the second heart sound
- A prominent parasternal heave
- A prominent jugular “a” wave
- A systolic murmur along the left sternal border at the fourth intercostal space, which may worsen with breath-holding
- Pitting lower-extremity edema
- Hepatomegaly
- Hepatojugular reflux
- Hepatic pulsatility.6
ECHOCARDIOGRAPHY IN SUSPECTED PULMONARY HYPERTENSION
Many practitioners rely heavily on the estimated right ventricular systolic pressure in diagnosing pulmonary hypertension. In theory, this number should be nearly the same as the pulmonary arterial systolic pressure. However, technical and patient-related aspects of transthoracic echocardiography often limit accurate measurement of the right ventricular systolic pressure, and readings often differ from those measured with right heart catheterization.8
Our patient had a markedly elevated right ventricular systolic pressure and signs of right ventricular dysfunction, suggesting a high probability of pulmonary hypertension.
EVALUATING LEFT HEART DISEASE (WHO GROUP 2)
More than 75% of cases of pulmonary hypertension are directly related to left ventricular dysfunction or mitral or aortic valve disease (WHO group 2).1 Since group 2 differs markedly from group 1 (PAH) in its pathophysiology and treatment, it is important to distinguish between them.
Compared with WHO group 1 patients, those in group 2 tend to be older, more of them are male, and more of them have comorbidities such as metabolic syndrome, hypertension, and coronary artery disease.1,9 A combination of risk factors and clinical findings should be considered in identifying these patients.10
Transthoracic echocardiography is used to detect features of systolic and diastolic dysfunction. Left atrial enlargement is a clue that left heart disease may be present. In addition, signs of left ventricular or valvular dysfunction on electrocardiography or chest radiography are often helpful.
When estimated right ventricular systolic pressures are only minimally abnormal and no significant right ventricular dysfunction exists, further diagnostic evaluation is not warranted. However, because no single identifying feature or variable can readily distinguish group 2 from the other WHO groups, further evaluation should be considered if the right ventricular systolic pressure is significantly elevated or right ventricular dysfunction exists.
Our patient had several risk factors for left heart disease, including a history of smoking and coronary artery disease. Nonetheless, findings consistent with severe right ventricular dysfunction necessitated further evaluation for other possible causes of her suspected pulmonary hypertension.
Postcapillary pulmonary hypertension
In patients for whom further evaluation is pursued, the diagnosis of WHO group 2 pulmonary hypertension is ultimately based on findings consistent with postcapillary or “passive” pulmonary hypertension on right heart catheterization. Although mean pulmonary arterial pressures must be at least 25 mm Hg to certify the diagnosis of pulmonary hypertension, a pulmonary artery occlusion pressure greater than 15 mm Hg (normal 6–12) and pulmonary vascular resistance of 3 Wood units or less (normal 0.3–1.6) suggests the pulmonary hypertension is due to elevated left atrial pressure (ie, postcapillary) rather than precapillary pulmonary arterial remodeling.
Mixed pre- and postcapillary pulmonary hypertension
Distinguishing pulmonary venous hypertension from PAH is important, since their management differs. In particular, PAH-specific therapies (ie, prostacyclin analogues, prostaglandin I2 receptor agonists, endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and cyclic guanosine monophosphate stimulators) can have a detrimental effect in WHO group 2 patients by causing increased pulmonary capillary leakage with pulmonary edema.11,12
In some patients, chronic passive congestion in the pulmonary venous circulation causes additional disruption of the homeostatic milieu regulating precapillary smooth muscle and endothelial function. These changes result in structural remodeling of precapillary arterioles and increased precapillary vascular resistance, creating a “mixed” pulmonary hypertension with both pre- and postcapillary abnormalities.
There is controversy over the ideal way to identify these patients but little disagreement that they face a worse prognosis than those without precapillary remodeling.13 In light of this, efforts have been made to characterize this cohort.
Historically, mixed pre- and postcapillary pulmonary hypertension was defined as the combination of all of the following:
- Mean pulmonary arterial pressure ≥ 25 mm Hg
- Pulmonary artery occlusion pressure > 15 mm Hg
- Transpulmonary gradient (the mean pulmonary arterial pressure minus the pulmonary artery occlusion pressure) > 12 mm Hg.14
However, the utility of the transpulmonary gradient for distinguishing mixed pulmonary hypertension has been questioned because of concerns over its susceptibility to variations in stroke volume and loading conditions.15
The diastolic pulmonary gradient (the pulmonary arterial diastolic pressure minus the pulmonary artery occlusion pressure) has been proposed as an alternative to the transpulmonary gradient under the theory that it is less sensitive to fluctuation from variations in flow or loading.15
Current guidelines1 suggest that a patient who has all of the following should be considered to have mixed pulmonary hypertension:
- A mean pulmonary arterial pressure > 25 mm Hg
- A pulmonary artery occlusion pressure > 15 mm Hg
- A diastolic pulmonary gradient > 7 mm Hg or a pulmonary vascular resistance > 3 Wood units, or both.
Occult group 2 pulmonary hypertension
Currently, the diagnosis of WHO group 2 pulmonary hypertension is based on elevated resting pulmonary artery occlusion pressure. However, some patients with WHO group 2 pulmonary hypertension and transiently low preload from aggressive diuresis or fasting may have a low pulmonary artery occlusion pressure during right heart catheterization and be misdiagnosed as having WHO group 1 PAH.12,16
This concern was acknowledged in the 2015 Ambrisentan and Tadalafil in Patients With Pulmonary Arterial Hypertension (AMBITION) study after investigators changed the protocol to exclude patients who technically met the criteria for WHO group 1 PAH, but had borderline-elevated pulmonary artery occlusion pressure and additional risk factors worrisome for left heart disease and occult WHO group 2 pulmonary hypertension.17,18
Several strategies, including passive leg-raising, fluid challenge, and exercise during diagnostic right heart catheterization, have been proposed to better classify these patients.19 Unfortunately, due to a lack of standardization of normal values and methodology for executing these maneuvers, consensus is lacking over their routine use, and recommendations for their use have not been provided.1
EVALUATION OF LUNG DISEASE (WHO GROUP 3)
All patients with suspected pulmonary hypertension should also be assessed for underlying pulmonary parenchymal or physiologic disease.
WHO group 3 consists of pulmonary disorders that, over an extended time, can lead to pulmonary hypertension. The most common of these disorders include chronic obstructive pulmonary disease, interstitial lung disease, and combined pulmonary fibrosis and emphysema.1
Pulmonary hypertension in these patients is precapillary, and changes in pulmonary vascular resistance are influenced by multiple factors, the most significant of which is alveolar hypoxia. Hypoxia induces pulmonary artery vasoconstrictionn (in contrast to the reflexive hemodynamics seen in peripheral tissues, where systemic vascular tone is generally lower in states of hypoxia) as a mechanism to divert pulmonary blood flow to well-ventilated portions of the lung and maintain ventilation-perfusion matching.
Repeated chronic hypoxia also alters cellular structure and function of pulmonary vessels and leads to medial hypertrophy and increased vascular tone, thus contributing to the development of pulmonary hypertension in many of these patients.20
Obstructive sleep apnea. Up to 70% of patients with obstructive sleep apnea have pulmonary hypertension.21 Chronic repetitive hypoxia throughout the night increases the levels of reactive oxygen species and alters cellular and molecular signaling, thus inducing vascular remodeling. In addition, apneic events during sleep promote catecholamine-driven elevations in systemic blood pressure. Over time, patients are at higher risk of developing left ventricular dysfunction and concomitant postcapillary group 2 pulmonary hypertension.22 Because typical methods of obstructive sleep apnea screening (eg, the Epworth Sleep Scale) have been historically poor at discriminating PAH patients with obstructive sleep apnea from those without, patients diagnosed with PAH should be considered for formal sleep testing.23,24
Pulmonary function tests, chest imaging
Pulmonary function tests and high-resolution computed tomography are essential to any PAH evaluation and help to exclude WHO group 3 pulmonary hypertension.1
An abnormal result on CT or spirometry can help point toward parenchymal lung disease. Normal spirometry and lung volumes with an isolated reduction in the diffusing capacity of the lung for carbon monoxide (Dlco) is typical of patients with WHO group 1 PAH.
In our patient, CT of the chest did not show any evidence of parenchymal lung disease, and pulmonary function tests showed no evidence of obstruction or restriction. There was a moderate decrease in Dlco, which did not reach normal limits when adjusted for lung volumes. In this setting, further evaluation of her PAH was warranted.
EVALUATION OF THROMBOEMBOLIC DISEASE (WHO GROUP 4)
Once pulmonary hypertension due to underlying left heart disease or parenchymal lung disease has been excluded, testing for chronic thromboembolic pulmonary hypertension is necessary, even in the absence of prior known pulmonary embolism. Identifying these patients is paramount, as chronic thromboembolic pulmonary hypertension (WHO group 4) is the only type of pulmonary hypertension for which a definitive cure is available.26
Up to 9% of patients who survive acute pulmonary embolism exhibit features of chronic proximal thrombosis and remodeling of distal pulmonary arteries.27
It remains unknown exactly why some patients develop chronic thromboembolic pulmonary hypertension and others do not, but the pathophysiology involves inappropriate thrombus resolution after venous thromboembolic events. Monocyte recruitment (which plays an important role in thrombus resolution) is reduced, angiogenesis is impaired (preventing effective vascular collateralization), and abnormal fibroblast proliferation leads to distal pulmonary vascular wall thickening.28 There is some evidence of increased thrombophilic risk in this population, and approximately 10% to 20% of patients are positive for antiphospholipid antibodies or lupus anticoagulant.29,30
Patients with chronic thromboembolic pulmonary hypertension usually present with symptoms similar to those of WHO group 1 PAH. Up to one-quarter of patients have no recollection of prior pulmonary embolism.31 As the disease progresses, signs and symptoms related to elevated pulmonary vascular resistance and right ventricular dysfunction are common.32,33
Although thrombi usually resolve quickly, the diagnosis of chronic thromboembolic pulmonary hypertension should be made only after at least 3 months of appropriate anticoagulation to avoid treatment of transient hemodynamic changes often seen after an acute pulmonary embolism.1
Radiographic changes associated with chronic thromboembolic pulmonary hypertension are distinct from the intraluminal filling defects seen with acute thromboembolism, since chronic thrombi tend to become organized and eccentric. On imaging, one may see features of rapid luminal narrowing or eccentric filling defects rather than the conventional central filling defects of acute pulmonary embolism. These changes are often overlooked by radiologists who are not specifically looking for chronic thromboembolic pulmonary hypertension.34 For this reason, the sensitivity and specificity of identifying chronic thromboembolic disease using radionuclide ventilation-perfusion lung scanning is superior to that of CT angiography.
All patients with suspected PAH should undergo a ventilation-perfusion scan.1,35 In patients with ventilation-perfusion mismatch on radionuclide scanning, pulmonary angiography can fulfill multiple goals of measuring pulmonary arterial pressures, identifying the extent and location of chronic thromboemboli, and can determine whether surgical thromboendarterectomy is feasible.
If chronic thromboembolic pulmonary hypertension is identified, it is imperative that patients be referred to a center of excellence specializing in its management regardless of symptom severity, as surgery can be curative and may prevent development of progressive right ventricular dysfunction.36
Our patient’s ventilation-perfusion scan was normal, effectively ruling out the possibility of chronic thromboembolism as a cause of her pulmonary hypertension.
RIGHT HEART CATHETERIZATION
Once the above-mentioned conditions have been evaluated, patients with suspected PAH should be referred to a pulmonary hypertension center of excellence to undergo right heart catheterization. If this test reveals PAH, further vasoreactivity testing should be performed if the etiology of the PAH is considered to be idiopathic, heritable, or drug-induced.1
Vasoreactivity is most commonly tested using 20 ppm of inhaled nitric oxide, but alternative formulations including intravenous epoprostenol, intravenous adenosine, or inhaled iloprost are acceptable. Patients who have a positive vasoreactive test usually respond well to high-dose calcium channel blocker therapy and have a significantly better prognosis than other patients with PAH.37
Patients with WHO group 1 PAH who do not have idiopathic, heritable, or drug-induced PAH have not been shown to have favorable outcomes using calcium channel blockers even if they have a positive vasoreactive response. A positive vasoreactive response is defined as a drop in mean pulmonary arterial pressure of at least 10 mm Hg to an absolute level of 40 mm Hg or less. Cardiac output should be preserved or elevated compared with baseline values during the challenge.1
In reality, only 10% to 15% of patients with idiopathic PAH have a positive vasoreactive response, and half of these patients stop responding within 1 year.38 Therefore, clinicians should not assume that calcium channel blockers will be successful in the long term in a vasoreactive patient, and these patients should have follow-up right heart catheterization after 3 to 6 months and annually thereafter to ensure continued vasoreactivity.1
In patients who are no longer vasoreactive or whose functional status is worse than New York Heart Association functional class I or II, conventional PAH-specific therapy should be started.
LOOKING FOR CAUSES OF ‘IDIOPATHIC’ PAH
Pulmonary hypertension is considered the final common pathway of many varied diseases and syndromes, and therefore one cannot say it is idiopathic without making a robust effort to identify features of alternative causes and rule out other contributing factors.
Although the exact etiology of idiopathic PAH is unclear, well-characterized imbalances in vascular homeostasis have been identified. These include processes that promote vasoconstriction, cell proliferation, and thrombosis (thromboxane A2, endothelin-1, and serotonin) and those that suppress prostacyclin, nitric oxide, and vasoactive intestinal peptide-mediated vasodilation.1 Furthermore, an abnormal angiogenic response to hypoxia and vascular endothelial growth factor has been observed.39
Before considering a diagnosis of idiopathic PAH, a careful history is essential. Other causative agents include appetite-suppressing medications, such as fenfluramine derivatives or stimulants such as amphetamines. Human immunodeficiency virus (HIV) or hepatitis, a history of splenectomy, and prior thyroid or liver disease are also common causes of PAH. Joint pain, myalgias, Raynaud features, or a rash characteristic of connective tissue disease can be identified on history and physical examination. Worldwide, chronic exposure to high-altitude climates and exposure to schistosomiasis are significant causes of PAH, but are rarely seen in developed nations. Confirmatory serum tests for HIV, antinuclear antibody, scleroderma antibody, and thyroid function are essential.1
Genetic factors
If patients report having relatives with possible or probable PAH, genetic counseling is recommended, particularly for rare but causative gene mutations.
BMPR2, the gene that codes for the bone morphogenetic protein receptor type 2, can carry mutations with variable penetrance over the patient’s lifetime depending on other genetic polymorphisms, concurrent inflammation, and the patient’s sex.40
The population carrier estimates of BMPR2 mutations are only 0.001% to 0.01%, but mutations in this gene are identified in approximately 25% of nonfamilial PAH patients and in over 75% of those with a familial inheritance pattern. The BMPR2 protein is a part of the transforming growth factor beta family and is partially responsible for control of vascular cell proliferation. Mutations in this gene lead to PAH at a younger age than in those with mutation-negative idiopathic PAH and to a more severe clinical phenotype in terms of pulmonary vascular resistance and cardiac function.40
Other mutations. Although BMPR2 is the most commonly identified gene mutation in patients with PAH, other gene mutations within this family have also been recognized. These include mutations in the genes for activin receptor-like kinase 1 and endoglin, which, although better known for their association with hereditary hemorrhagic telangiectasia, can lead directly to PAH.40
More recently, a novel autosomal recessive gene mutation in eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) has been identified in patients with pulmonary veno-occlusive disease41 and pulmonary capillary hemangiomatosis,42 which are specific subclasses of WHO group 1 PAH. The mechanistic parallels between EIF2AK4 and these diseases are not clear, but the prevalence of disease in those with a familial inheritance pattern and an EIF2AK4 mutation is nearly 100%.41 Thus, identification of this mutation has been accepted as a way to confirm pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis in patients suspected of PAH with features of these diseases.43,44
GROUP 5: MISCELLANEOUS FORMS OF PULMONARY HYPERTENSION
WHO group 5 pulmonary hypertension encompasses disorders whose pathophysiology does not fit neatly within the context of the other pulmonary hypertension subtypes. Nonetheless, appreciation of these disorders is important in determining the etiology and appropriate therapy for patients with pulmonary hypertension. The mechanism driving abnormal pulmonary arterial pressures in patients with group 5 pulmonary hypertension is not always clear and may involve intrinsic or extrinsic factors.1
Diseases within group 5 include those that cause extrinsic compression of the pulmonary arteries (ie, fibrosing mediastinitis) or intrinsic elevations in pulmonary vascular resistance (sarcoidosis, pulmonary Langerhans cell histiocytosis, sickle cell anemia, polycythemia vera, and malignancy).
The most common cause of pulmonary hypertension in this category is sarcoidosis. Current theories suggest that, for most patients, invasion of granulomatous inflammation within the arterial walls induces PAH via fibrotic or inflammatory vascular occlusion. Extrinsic compression due to lymphadenopathy, right or left ventricular dysfunction due to cardiac myocite infiltration, and endothelin-induced pulmonary vasoconstriction are other possible links between the PAH and sarcoidosis.45
PROGNOSTIC RISK STRATIFICATION IN THE PATIENTS WITH PAH
Cardiac magnetic resonance imaging (MRI) has gained popularity as a noninvasive and reproducible alternative to echocardiography. Image fidelity and characterization of right ventricular function and right ventricular ejection fraction are all more accurate than with echocardiography, and serial MRI has proven valuable in its ability to guide patient prognosis.46
However, MRI is more expensive than echocardiography, and some patients cannot tolerate the procedure. In addition, for those who can tolerate it, MRI is not a suitable alternative to right heart catheterization, since it cannot accurately estimate pulmonary artery occlusion pressure or pulmonary arterial pressures.1 For these reasons, cardiac MRI use varies across pulmonary hypertension centers.
A goal of treatment is to reduce a patient’s risk. While no consensus has been achieved over which PAH-specific therapy to start with, evidence is robust that using more than 1 class of agent is beneficial, capitalizing on multiple therapeutic targets.17,47
In our patient, right heart catheterization revealed PAH with a mean pulmonary arterial pressure of 44 mm Hg, pulmonary artery occlusion pressure 6 mm Hg, and a cardiac index of 2.1 L/min/m2. Ancillary testing for alternative causes of PAH was unrevealing, as was vasoreactivity testing. Our patient could walk only 314 meters on her 6-minute walk test and had an initial NT-proBNP level of 750 ng/L.
Based on these and the findings during her evaluation, she would be classified as having intermediate-risk PAH with an estimated 1-year mortality risk of 5% to 10%.1 Appropriate therapy and follow-up would be guided by this determination. Specific therapy is beyond the scope of this article but we would start her on dual oral therapy with close follow-up to reassess her 1-year mortality risk. If there were no improvement over a short period of time, we would add further therapy.
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46(4):903–975. doi:10.1183/13993003.01032-2015
- Galiè N, Rubin LJ, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomized controlled trial. Lancet 2008; 371(9630):2093–2100. doi:10.1016/S0140-6736(08)60919-8
- Howard LS. Prognostic factors in pulmonary arterial hypertension: assessing the course of the disease. Eur Respir Rev 2011; 20:236–242. doi:10.1183/09059180.00006711
- Brown LM, Chen H, Halpern S, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL registry. Chest 2011; 140:19–26. doi:10.1378/chest.10-1166
- Elliot CG, Farber H, Frost A, Liou TG, Turner M. REVEAL Registry: medical history and time to diagnosis of enrolled patients. Chest 2007; 132(4):631a. doi:10.1378/chest.132.4_MeetingAbstracts.631a
- Minai OA, Budev MM. Diagnostic strategies for suspected pulmonary arterial hypertension: a primer for the internist. Cleve Clin J Med 2007; 74(10):737–747. pmid:17941295
- Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010; 137(2):376–387. doi:10.1378/chest.09-1140
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179(7):615–621. doi:10.1164/rccm.200811-1691OC
- Robbins IM, Newman JH, Johnson RF, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest 2009; 136(1):31–36. doi:10.1378/chest.08-2008
- Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016; 37(12):942–954. doi:10.1093/eurheartj/ehv512
- Opitz CF, Hoeper MM, Gibbs JSR, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016; 68:368–378. doi: 10.1016/j.jacc.2016.05.047
- Robbins IM, Hemnes AR, Pugh ME, et al. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail 2014; 7(1):116–122. doi:10.1161/CIRCHEARTFAILURE.113.000468
- Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013; 143(3):758–766. doi:10.1378/chest.12-1653
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT); Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009; 34(6):1219–1263. doi:10.1183/09031936.00139009
- Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013; 41(1):217–223. doi:10.1183/09031936.00074312
- Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013; 144(5):1521–1529. doi:10.1378/chest.12-3023
- Galiè N, Barberà JA, Frost AE, et al; AMBITION Investigators. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373(9):834–844. doi:10.1056/NEJMoa1413687
- Farr G, Shah K, Markley R, Abbate A, Salloum FN, Grinnan D. Development of pulmonary hypertension in heart failure with preserved ejection fraction. Prog Cardiovasc Dis 2016; 59(1):52–58. doi:10.1016/j.pcad.2016.06.002
- Hoeper MM, Barberà JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol 2009; 54(suppl 1):S85–S96. doi:10.1016/j.jacc.2009.04.008
- Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008; 32(5):1371–1385. doi:10.1183/09031936.00015608
- Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol 2009; 104(9):1300–1306. doi:10.1016/j.amjcard.2009.06.048
- Kholdani C, Fares WH, Mohsenin V. Pulmonary hypertension in obstructive sleep apnea: is it clinically significant? A critical analysis of the association and pathophysiology. Pulm Circ 2015; 5(2):220–227. doi:10.1086/679995
- Prisco DL, Sica AL, Talwar A, et al. Correlation of pulmonary hypertension severity with metrics of comorbid sleep-disordered breathing. Sleep Breath 2011; 15(4):633–639. doi:10.1007/s11325-010-0411-y
- Dumitrascu R, Tiede H, Eckermann J, et al. Sleep apnea in precapillary pulmonary hypertension. Sleep Med 2013; 14(3):247–251. doi:10.1016/j.sleep.2012.11.013
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167(5):735–740. doi:10.1164/rccm.200210-1130OC
- Pepke-Zaba J, Jansa P, Kim NH, Naeije R, Simonneau G. Chronic thromboembolic pulmonary hypertension: role of medical therapy. Eur Respir J 2013; 41(4):985–990. doi:10.1183/09031936.00201612
- Guérin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost 2014; 112(3):598–605. doi:10.1160/TH13-07-0538
- Lang IM, Pesavento R, Bonderman D, Yuan JX. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J 2013; 41(2):462–468. doi:10.1183/09031936.00049312
- Pepke-Zaba J. Diagnostic testing to guide the management of chronic thromboembolic pulmonary hypertension: state of the art. Eur Respir Rev 2010; 19(115):55–58. doi:10.1183/09059180.00007209
- Bonderman D, Turecek PL, Jakowitsch J, et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost 2003; 90(3):372–376. doi:10.1160/TH03-02-0067
- Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124(18):1973–1981. doi:10.1161/CIRCULATIONAHA.110.015008
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62:(suppl 25):D92–D99. doi:10.1016/j.jacc.2013.10.024
- Moser KM, Auger WR, Fedullo PF. Chronic major-vessel thromboembolic pulmonary hypertension. Circulation 1990; 81(6):1735–1743. pmid:2188751
- McNeil K, Dunning J. Chronic thromboembolic pulmonary hypertension (CTEPH). Heart 2007; 93(9):1152–1158. doi:10.1136/hrt.2004.053603
- Tunariu N, Gibbs SJ, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med 2007; 48(5):680–684. doi:10.2967/jnumed.106.039438
- Fedullo P, Kerr KM, Kim NH, Auger WR. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2011; 183(12):1605–1613. doi:10.1164/rccm.201011-1854CI
- Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 1992; 327(2):76–81. doi:10.1056/NEJM199207093270203
- Sitbon O, Humbert M, Jaıs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005; 111(23):3105–3111. doi:10.1161/CIRCULATIONAHA.104.488486
- Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol 2008; 51(16):1527–1538. doi:10.1016/j.jacc.2008.01.024
- Soubrier F, Chung WK, Machado R, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2013; 62(suppl 25):D13–D21. doi:10.1016/j.jacc.2013.10.035
- Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 2014; 46(1):65–69. doi: 10.1038/ng.2844
- Best DH, Sumner KL, Austin ED, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest 2014; 145(2):231–236. doi:10.1378/chest.13-2366
- Best DH, Sumner KL, Smith BP, et al. EIF2AK4 mutations in patients diagnosed with pulmonary arterial hypertension. Chest 2017; 151(4):821–828. doi:10.1016/j.chest.2016.11.014
- Hadinnapola C, Bleda M, Haimel M, et al; NIHR BioResource–Rare Diseases Consortium; UK National Cohort Study of Idiopathic and Heritable PAH. Phenotypic characterization of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation 2017; 136(21):2022–2033. doi:10.1161/CIRCULATIONAHA.117.028351
- Diaz-Guzman E, Farver C, Parambil J, Culver DA. Pulmonary hypertension caused by sarcoidosis. Clin Chest Med 2008; 29(3):549–563. doi:10.1016/j.ccm.2008.03.010
- Mauritz GJ, Kind T, Marcus JT, et al. Progressive changes in right ventricular geometric shortening and long-term survival in pulmonary arterial hypertension. Chest 2012; 141(4):935–943. doi:10.1378/chest.10-3277
- Galiè N, Palazzini M, Manes A. Pulmonary arterial hypertension: from the kingdom of the near-dead to multiple clinical trial meta-analyses. Eur Heart J 2010; 31(17):2080–2086. doi:10.1093/eurheartj/ehq152
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46(4):903–975. doi:10.1183/13993003.01032-2015
- Galiè N, Rubin LJ, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomized controlled trial. Lancet 2008; 371(9630):2093–2100. doi:10.1016/S0140-6736(08)60919-8
- Howard LS. Prognostic factors in pulmonary arterial hypertension: assessing the course of the disease. Eur Respir Rev 2011; 20:236–242. doi:10.1183/09059180.00006711
- Brown LM, Chen H, Halpern S, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL registry. Chest 2011; 140:19–26. doi:10.1378/chest.10-1166
- Elliot CG, Farber H, Frost A, Liou TG, Turner M. REVEAL Registry: medical history and time to diagnosis of enrolled patients. Chest 2007; 132(4):631a. doi:10.1378/chest.132.4_MeetingAbstracts.631a
- Minai OA, Budev MM. Diagnostic strategies for suspected pulmonary arterial hypertension: a primer for the internist. Cleve Clin J Med 2007; 74(10):737–747. pmid:17941295
- Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010; 137(2):376–387. doi:10.1378/chest.09-1140
- Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179(7):615–621. doi:10.1164/rccm.200811-1691OC
- Robbins IM, Newman JH, Johnson RF, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest 2009; 136(1):31–36. doi:10.1378/chest.08-2008
- Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016; 37(12):942–954. doi:10.1093/eurheartj/ehv512
- Opitz CF, Hoeper MM, Gibbs JSR, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016; 68:368–378. doi: 10.1016/j.jacc.2016.05.047
- Robbins IM, Hemnes AR, Pugh ME, et al. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail 2014; 7(1):116–122. doi:10.1161/CIRCHEARTFAILURE.113.000468
- Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013; 143(3):758–766. doi:10.1378/chest.12-1653
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT); Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009; 34(6):1219–1263. doi:10.1183/09031936.00139009
- Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013; 41(1):217–223. doi:10.1183/09031936.00074312
- Frost AE, Badesch DB, Miller DP, Benza RL, Meltzer LA, McGoon MD. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013; 144(5):1521–1529. doi:10.1378/chest.12-3023
- Galiè N, Barberà JA, Frost AE, et al; AMBITION Investigators. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373(9):834–844. doi:10.1056/NEJMoa1413687
- Farr G, Shah K, Markley R, Abbate A, Salloum FN, Grinnan D. Development of pulmonary hypertension in heart failure with preserved ejection fraction. Prog Cardiovasc Dis 2016; 59(1):52–58. doi:10.1016/j.pcad.2016.06.002
- Hoeper MM, Barberà JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol 2009; 54(suppl 1):S85–S96. doi:10.1016/j.jacc.2009.04.008
- Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008; 32(5):1371–1385. doi:10.1183/09031936.00015608
- Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol 2009; 104(9):1300–1306. doi:10.1016/j.amjcard.2009.06.048
- Kholdani C, Fares WH, Mohsenin V. Pulmonary hypertension in obstructive sleep apnea: is it clinically significant? A critical analysis of the association and pathophysiology. Pulm Circ 2015; 5(2):220–227. doi:10.1086/679995
- Prisco DL, Sica AL, Talwar A, et al. Correlation of pulmonary hypertension severity with metrics of comorbid sleep-disordered breathing. Sleep Breath 2011; 15(4):633–639. doi:10.1007/s11325-010-0411-y
- Dumitrascu R, Tiede H, Eckermann J, et al. Sleep apnea in precapillary pulmonary hypertension. Sleep Med 2013; 14(3):247–251. doi:10.1016/j.sleep.2012.11.013
- Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167(5):735–740. doi:10.1164/rccm.200210-1130OC
- Pepke-Zaba J, Jansa P, Kim NH, Naeije R, Simonneau G. Chronic thromboembolic pulmonary hypertension: role of medical therapy. Eur Respir J 2013; 41(4):985–990. doi:10.1183/09031936.00201612
- Guérin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost 2014; 112(3):598–605. doi:10.1160/TH13-07-0538
- Lang IM, Pesavento R, Bonderman D, Yuan JX. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J 2013; 41(2):462–468. doi:10.1183/09031936.00049312
- Pepke-Zaba J. Diagnostic testing to guide the management of chronic thromboembolic pulmonary hypertension: state of the art. Eur Respir Rev 2010; 19(115):55–58. doi:10.1183/09059180.00007209
- Bonderman D, Turecek PL, Jakowitsch J, et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost 2003; 90(3):372–376. doi:10.1160/TH03-02-0067
- Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124(18):1973–1981. doi:10.1161/CIRCULATIONAHA.110.015008
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62:(suppl 25):D92–D99. doi:10.1016/j.jacc.2013.10.024
- Moser KM, Auger WR, Fedullo PF. Chronic major-vessel thromboembolic pulmonary hypertension. Circulation 1990; 81(6):1735–1743. pmid:2188751
- McNeil K, Dunning J. Chronic thromboembolic pulmonary hypertension (CTEPH). Heart 2007; 93(9):1152–1158. doi:10.1136/hrt.2004.053603
- Tunariu N, Gibbs SJ, Win Z, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med 2007; 48(5):680–684. doi:10.2967/jnumed.106.039438
- Fedullo P, Kerr KM, Kim NH, Auger WR. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2011; 183(12):1605–1613. doi:10.1164/rccm.201011-1854CI
- Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 1992; 327(2):76–81. doi:10.1056/NEJM199207093270203
- Sitbon O, Humbert M, Jaıs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005; 111(23):3105–3111. doi:10.1161/CIRCULATIONAHA.104.488486
- Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol 2008; 51(16):1527–1538. doi:10.1016/j.jacc.2008.01.024
- Soubrier F, Chung WK, Machado R, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2013; 62(suppl 25):D13–D21. doi:10.1016/j.jacc.2013.10.035
- Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 2014; 46(1):65–69. doi: 10.1038/ng.2844
- Best DH, Sumner KL, Austin ED, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest 2014; 145(2):231–236. doi:10.1378/chest.13-2366
- Best DH, Sumner KL, Smith BP, et al. EIF2AK4 mutations in patients diagnosed with pulmonary arterial hypertension. Chest 2017; 151(4):821–828. doi:10.1016/j.chest.2016.11.014
- Hadinnapola C, Bleda M, Haimel M, et al; NIHR BioResource–Rare Diseases Consortium; UK National Cohort Study of Idiopathic and Heritable PAH. Phenotypic characterization of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation 2017; 136(21):2022–2033. doi:10.1161/CIRCULATIONAHA.117.028351
- Diaz-Guzman E, Farver C, Parambil J, Culver DA. Pulmonary hypertension caused by sarcoidosis. Clin Chest Med 2008; 29(3):549–563. doi:10.1016/j.ccm.2008.03.010
- Mauritz GJ, Kind T, Marcus JT, et al. Progressive changes in right ventricular geometric shortening and long-term survival in pulmonary arterial hypertension. Chest 2012; 141(4):935–943. doi:10.1378/chest.10-3277
- Galiè N, Palazzini M, Manes A. Pulmonary arterial hypertension: from the kingdom of the near-dead to multiple clinical trial meta-analyses. Eur Heart J 2010; 31(17):2080–2086. doi:10.1093/eurheartj/ehq152
KEY POINTS
- PAH has nonspecific symptoms, largely attributable to right ventricular dysfunction but seen in a host of other common cardiopulmonary ailments.
- In a patient suspected of having pulmonary hypertension, it is important to take a methodic diagnostic approach to identify underlying contributors and minimize unnecessary testing.
- Patients suspected of having PAH should be referred to a pulmonary hypertension center of excellence for evaluation and right heart catheterization.
- Once testing is complete, therapy and management should be guided both by data obtained during the initial evaluation and by factors with prognostic significance. This approach has changed PAH from a disease with a grim outlook to one in which appropriate evaluation and guidance can improve patient outcomes.
Working at a long-term psychiatric hospital? Consider your patient’s point of view
Working at a long-term psychiatric hospital can present challenges similar to those found in other institutions, such as correctional facilities1; however, in this setting, additional obstacles that could affect treatment may not readily come to mind. Following the 2 simple approaches described here can help you to understand your patient’s point of view and improve the treatment relationship.
Allow patients some control. Many patients in long-term psychiatric hospitals are prescribed medications that can result in metabolic complications such as weight gain or hyperlipidemia. To avoid these complications, we may need to institute dietary restrictions. Despite our explanations of why these restrictions are necessary, some patients may continue to insist on eating food that we believe will worsen their physical health; they may feel that they have little control in their lives and have nothing to look forward to except for what they can eat.2
For patients in long-term psychiatric hospitals, everyday life usually is structured from morning to evening. This includes when meals and snacks are served, as well as what they are allowed to eat. Food is a basic human necessity, and we often forget its psychological significance. Because most patients can control what they put in their mouths, food allows them to exert control in an environment where they may believe they have no influence. This may explain why patients insist on certain meals, purchase unhealthy food, or engage in a surreptitious snack distribution system with other patients. We usually can decide what and when we eat, but many of our hospitalized patients do not have that opportunity. Within reason, negotiating meals and snacks could provide patients with a sense of control, and might increase treatment compliance.2
Mind what you say. At the hospital, patients are acutely aware that we are there for a short period each day. For these patients, the hospital serves as their home. Many will live there for months to years; some will spend the remainder of their lives there. The way these patients view us can become adversely affected when they see that we occasionally bring a negative attitude toward having to spend the day in their living space, telling them how to behave and what to do. This daily temporary relationship between hospital staff and patients can greatly affect treatment.
Although the hospital can serve as a home, patients do not have input into how we should behave in their home. Be mindful of your actions and the comments you make while in the hospital. We would not appreciate someone making a negative comment about our homes, so it is likely that our patients do not want to hear us complain about the hospital. Furthermore, they likely do not enjoy hearing hospital staff discussing plans they have made in their personal lives. Many patients do not enjoy being in the hospital, and they could view such expressions as “rubbing it in,” which could adversely affect treatment.
1. Khajuria K. CORRECT: insights into working at correctional facilities. Current Psychiatry. 2017;16(2):54-55.
2. Joshi KG. Can I have cheese on my ham sandwich? BMJ. 2016;355:i6024. doi: 10.1136/bmj.i6024.
Working at a long-term psychiatric hospital can present challenges similar to those found in other institutions, such as correctional facilities1; however, in this setting, additional obstacles that could affect treatment may not readily come to mind. Following the 2 simple approaches described here can help you to understand your patient’s point of view and improve the treatment relationship.
Allow patients some control. Many patients in long-term psychiatric hospitals are prescribed medications that can result in metabolic complications such as weight gain or hyperlipidemia. To avoid these complications, we may need to institute dietary restrictions. Despite our explanations of why these restrictions are necessary, some patients may continue to insist on eating food that we believe will worsen their physical health; they may feel that they have little control in their lives and have nothing to look forward to except for what they can eat.2
For patients in long-term psychiatric hospitals, everyday life usually is structured from morning to evening. This includes when meals and snacks are served, as well as what they are allowed to eat. Food is a basic human necessity, and we often forget its psychological significance. Because most patients can control what they put in their mouths, food allows them to exert control in an environment where they may believe they have no influence. This may explain why patients insist on certain meals, purchase unhealthy food, or engage in a surreptitious snack distribution system with other patients. We usually can decide what and when we eat, but many of our hospitalized patients do not have that opportunity. Within reason, negotiating meals and snacks could provide patients with a sense of control, and might increase treatment compliance.2
Mind what you say. At the hospital, patients are acutely aware that we are there for a short period each day. For these patients, the hospital serves as their home. Many will live there for months to years; some will spend the remainder of their lives there. The way these patients view us can become adversely affected when they see that we occasionally bring a negative attitude toward having to spend the day in their living space, telling them how to behave and what to do. This daily temporary relationship between hospital staff and patients can greatly affect treatment.
Although the hospital can serve as a home, patients do not have input into how we should behave in their home. Be mindful of your actions and the comments you make while in the hospital. We would not appreciate someone making a negative comment about our homes, so it is likely that our patients do not want to hear us complain about the hospital. Furthermore, they likely do not enjoy hearing hospital staff discussing plans they have made in their personal lives. Many patients do not enjoy being in the hospital, and they could view such expressions as “rubbing it in,” which could adversely affect treatment.
Working at a long-term psychiatric hospital can present challenges similar to those found in other institutions, such as correctional facilities1; however, in this setting, additional obstacles that could affect treatment may not readily come to mind. Following the 2 simple approaches described here can help you to understand your patient’s point of view and improve the treatment relationship.
Allow patients some control. Many patients in long-term psychiatric hospitals are prescribed medications that can result in metabolic complications such as weight gain or hyperlipidemia. To avoid these complications, we may need to institute dietary restrictions. Despite our explanations of why these restrictions are necessary, some patients may continue to insist on eating food that we believe will worsen their physical health; they may feel that they have little control in their lives and have nothing to look forward to except for what they can eat.2
For patients in long-term psychiatric hospitals, everyday life usually is structured from morning to evening. This includes when meals and snacks are served, as well as what they are allowed to eat. Food is a basic human necessity, and we often forget its psychological significance. Because most patients can control what they put in their mouths, food allows them to exert control in an environment where they may believe they have no influence. This may explain why patients insist on certain meals, purchase unhealthy food, or engage in a surreptitious snack distribution system with other patients. We usually can decide what and when we eat, but many of our hospitalized patients do not have that opportunity. Within reason, negotiating meals and snacks could provide patients with a sense of control, and might increase treatment compliance.2
Mind what you say. At the hospital, patients are acutely aware that we are there for a short period each day. For these patients, the hospital serves as their home. Many will live there for months to years; some will spend the remainder of their lives there. The way these patients view us can become adversely affected when they see that we occasionally bring a negative attitude toward having to spend the day in their living space, telling them how to behave and what to do. This daily temporary relationship between hospital staff and patients can greatly affect treatment.
Although the hospital can serve as a home, patients do not have input into how we should behave in their home. Be mindful of your actions and the comments you make while in the hospital. We would not appreciate someone making a negative comment about our homes, so it is likely that our patients do not want to hear us complain about the hospital. Furthermore, they likely do not enjoy hearing hospital staff discussing plans they have made in their personal lives. Many patients do not enjoy being in the hospital, and they could view such expressions as “rubbing it in,” which could adversely affect treatment.
1. Khajuria K. CORRECT: insights into working at correctional facilities. Current Psychiatry. 2017;16(2):54-55.
2. Joshi KG. Can I have cheese on my ham sandwich? BMJ. 2016;355:i6024. doi: 10.1136/bmj.i6024.
1. Khajuria K. CORRECT: insights into working at correctional facilities. Current Psychiatry. 2017;16(2):54-55.
2. Joshi KG. Can I have cheese on my ham sandwich? BMJ. 2016;355:i6024. doi: 10.1136/bmj.i6024.
Bipolar disorder: How to avoid overdiagnosis
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
N-acetylcysteine: A potential treatment for substance use disorders
Pharmacologic treatment options for many substance use disorders (SUDs) are limited. This is especially true for cocaine use disorder and cannabis use disorder, for which there are no FDA-approved medications. FDA-approved medications for other SUDs often take the form of replacement or agonist therapies (eg, nicotine replacement therapy) that substitute the effects of the substance to aid in cessation. Other pharmacotherapies treat symptoms of withdrawal, reduce craving, or provide aversive counter-conditioning if the patient consumes the substance while on the medication (eg, disulfiram).
The over-the-counter (OTC) antioxidant N-acetylcysteine (NAC) may be a potential treatment for SUDs. Although NAC is not approved by the FDA for treating SUDs, its proposed mechanism of action differs from that of current FDA-approved medications for SUDs. NAC’s potential for broad applicability, favorable adverse-effect profile, accessibility, and low cost make it an intriguing option for patients with multiple comorbidities, and potentially for individuals with polysubstance use. This article reviews the current evidence supporting NAC for treating SUDs, to provide insight about which patients may benefit from NAC and under which circumstances they are most likely to benefit.
NAC may correct glutamate dysregulation
Approximately 85% of individuals with an SUD do not seek treatment for it, and those who do are older, have a longer history of use, have more severe dependence, and have sought treatment numerous times before.1 By the time most people seek treatment, years of chronic substance use have likely led to significant brain-related adaptations. Individuals with SUDs often indicate that their substance use began as a pleasurable activity—the effects of the drug were enjoyable and they were motivated to use it again. With repeated substance use, they may begin to develop a stronger urge to use the drug, driven not necessarily by a desire for pleasure, but by compulsion.2
Numerous neural adaptations underlie the transition from “liking” a substance to engaging in the compulsive use that is characteristic of an SUD.2 For example, repeated use of an addictive substance may result in excess glutamate in the nucleus accumbens,3,4 an area of the brain that plays a critical role in motivation and learning. As a result, it has been proposed that pharmacotherapies that help correct glutamate dysregulation may be effective in promoting abstinence or preventing relapse to a substance.5,6
NAC may reverse the neural dysfunction seen in SUDs. As an OTC antioxidant that impacts glutamatergic functioning in the brain, NAC has long been used to treat acetaminophen overdose; however, in recent years, researchers have begun to tap its potential for treating substance use and psychiatric disorders. NAC is thought to upregulate the glutamate transporter (GLT-1) that removes excess glutamate from the nucleus accumbens.6 Several published reviews provide more in-depth information about the neurobiology of NAC.6-10
The adverse-effect profile of NAC is relatively benign. Nausea, vomiting, diarrhea, and sleepiness are relatively infrequent and mild.11,12 The bioavailability of NAC is about 4% to 9%, with an approximate half-life of 6.25 hours when orally administered.13 Because NAC is classified as an OTC supplement, the potency and preparation may vary by supplier. To maximize consistency, NAC should be obtained from a supplier that meets United States Pharmacopeia (USP) standards.
NAC for SUDs: Emerging evidence
Several recent reviews have described the efficacy of NAC for SUDs and other psychiatric disorders. Here we summarize the current research examining the efficacy of NAC for stimulant (ie, cocaine and methamphetamine), cannabis, tobacco, and alcohol use disorders.
Continue to: Stimulant use disorders
Stimulant use disorders. The United Nations Office for Drugs and Crime estimates that worldwide, more than 18 million people use cocaine and more than 35 million use amphetamines.14 There are currently no FDA-approved treatments for stimulant use disorders, and clinicians treating patients with cocaine or amphetamine dependence often are at a loss for how best to promote abstinence. Recent studies suggest that NAC may decrease drug-seeking behavior and cravings in adults who seek treatment. The results of studies examining NAC for treating cocaine use and methamphetamine use are summarized in Table 115-17 and Table 2,18,19 respectively.
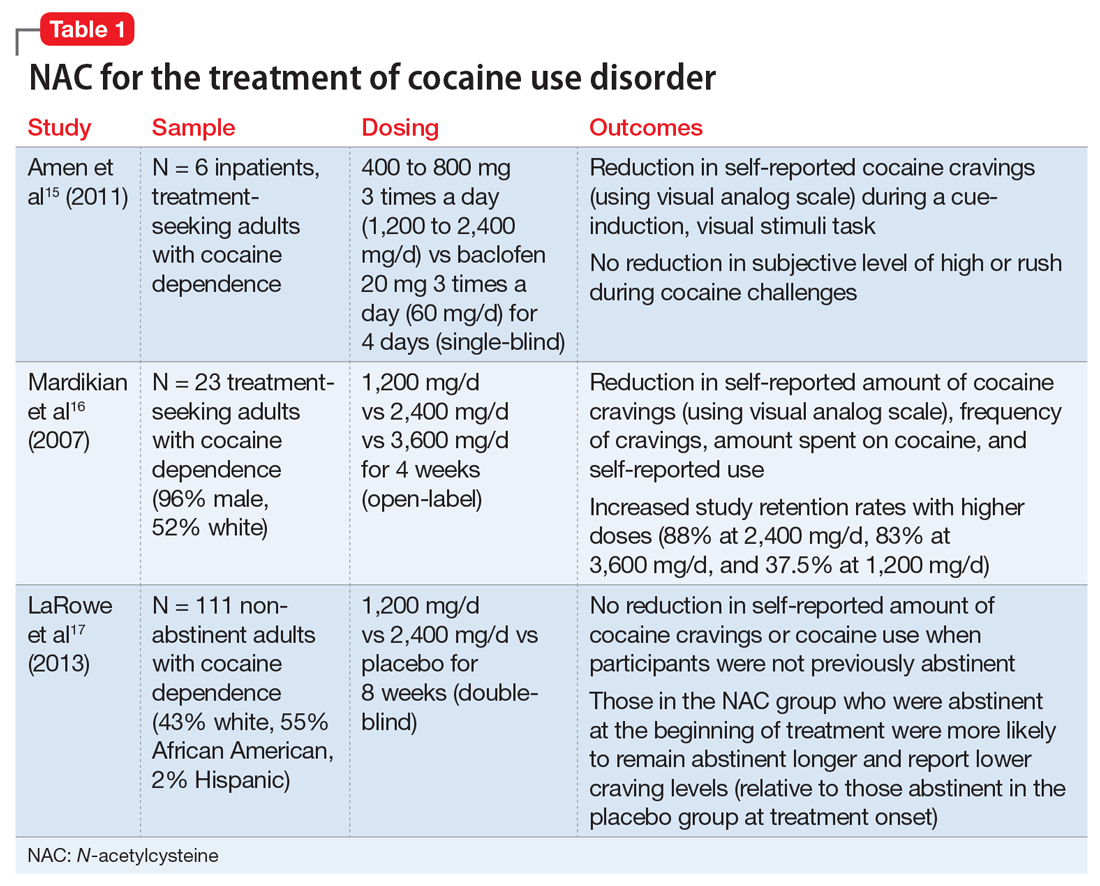
Cocaine cessation and relapse prevention. Several small pilot projects15,16 found that compared with placebo, various doses of NAC reduced craving (as measured with a visual analog scale). However, in a double-blind, placebo-controlled study, NAC did not decrease cravings or use after 8 weeks of treatment in individuals with cocaine use disorder who were still using cocaine (ie, they had not yet become abstinent). Interestingly, those who were abstinent when treatment began reported lower craving and remained abstinent longer if they received NAC (vs placebo), which suggests that NAC may be useful for preventing relapse.17
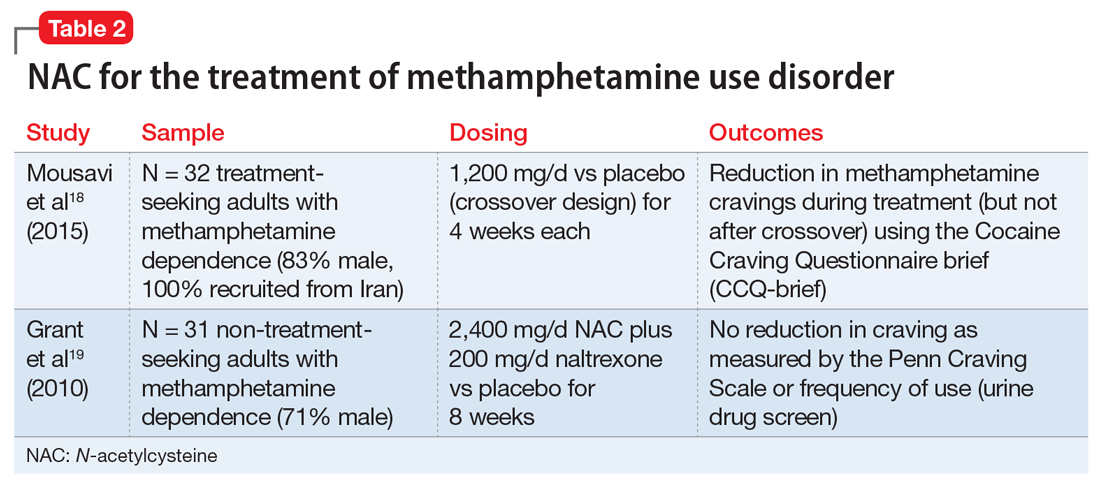
Methamphetamine cessation and relapse prevention. One study (N = 32) that evaluated the use of NAC, 1,200 mg/d for 4 weeks, vs placebo found reduced cravings among methamphetamine users who were seeking treatment.18 In contrast, a study of 31 methamphetamine users who were not seeking treatment evaluated the use of NAC, 2,400 mg/d, plus naltrexone, 200 mg/d, vs placebo for 8 weeks.19 It found no significant differences in craving or use patterns. Further research is needed to optimize the use of NAC for stimulant use disorders, and to better understand the role that abstinence plays.
Appropriate populations. The most support for use of NAC has been as an anti-relapse agent in treatment-seeking adults.
Continue to: Safety and dosing
Safety and dosing. Suggested dosages for the treatment of cocaine use disorder range from 1,200 to 3,600 mg/d (typically 600 to 1,800 mg twice daily, due to NAC’s short half-life), with higher retention rates noted in individuals who received 2,400 mg/d and 3,600 mg/d.16
Clinical implications. NAC is thought to act as an anti-relapse agent, rather than an agent that can help someone who is actively using stimulants to stop. Consequently, NAC will likely be most helpful for patients who are motivated to quit and are abstinent when they start taking NAC; however, this hypothesis needs further testing.
Cannabis use disorder
There are no FDA-approved treatments for cannabis use disorder. Individuals who use marijuana or other forms of cannabis may be less likely to report negative consequences or seek treatment compared with those who use other substances. Approximately 9% of individuals who use marijuana develop cannabis use disorder20; those who begin using marijuana earlier in adolescence are at increased risk.21 Commonly reported reasons for wanting to stop using marijuana include being concerned about health consequences, regaining or demonstrating self-control, saving money, avoiding legal consequences, obtaining or keeping employment, and reducing interpersonal conflict.22,23 Table 324-27 summarizes initial evidence that suggests NAC may be particularly useful in reducing marijuana use among adolescents (age 15 to 21).24,25
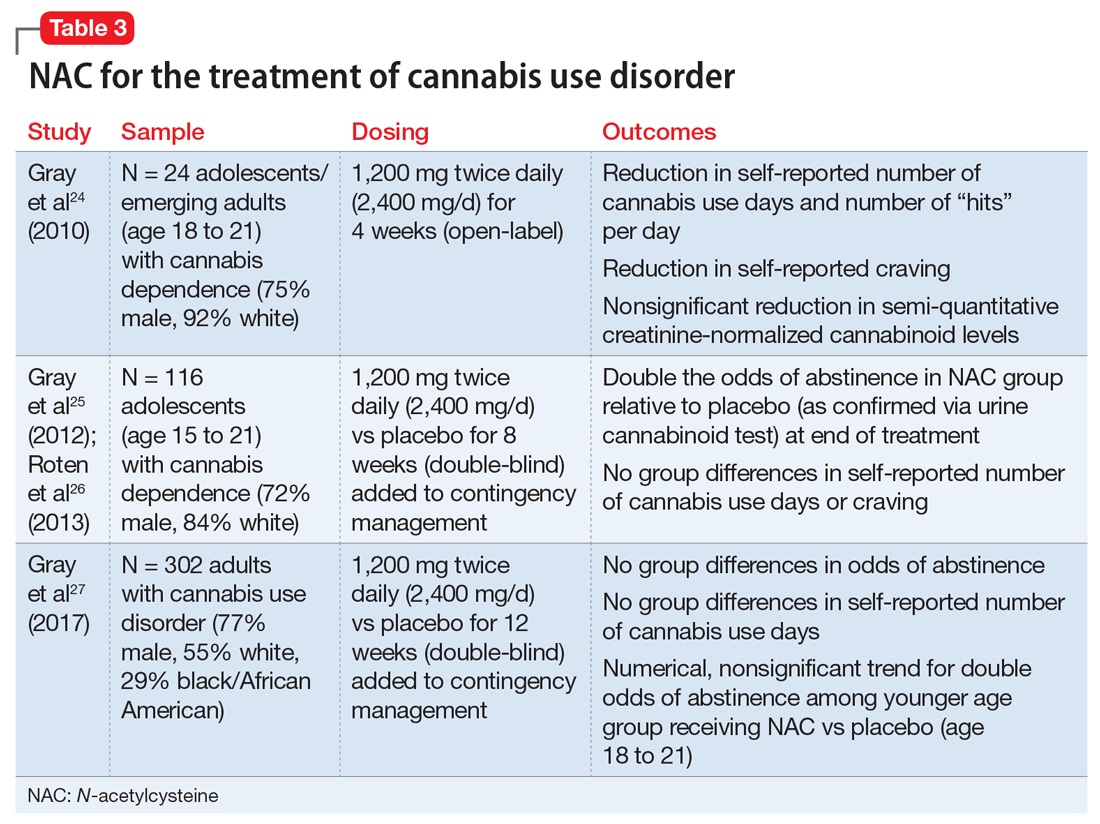
Cessation. An open-label, pilot clinical trial found significant reductions in self-reported marijuana use and craving—but not in biomarkers of use—among 24 adolescents after 4 weeks of NAC, 1,200 mg twice daily.24 In an 8-week, double-blind, randomized controlled trial of 116 adolescents, NAC, 1,200 mg twice daily, plus contingency management doubled the odds of abstinence, but had no effect on self-reported craving or use.25,26 In a sample of 302 adults, a 12-week trial of NAC, 1,200 mg twice daily, plus contingency management was no more effective than contingency management alone in promoting abstinence.27
Continue to: Appropriate populations
Appropriate populations. Evidence is stronger for use of NAC among adolescents (age 15 to 21) than for individuals older than age 21.25,27 Further research is needed to explore potential reasons for age-specific effects.
Safety and dosing. A safe and potentially efficacious dosage for the treatment of cannabis use disorder is 2,400 mg/d (1,200 mg twice daily).24,25,27
Clinical implications. Combined with contingency management, NAC might be efficacious for adolescents with cannabis use disorder, with treatment gains evident by the fourth week of treatment.24,25 To date, no clinical trials have examined the efficacy of NAC for treating cannabis use disorder without adjunctive contingency management, and research is needed to isolate the clinical effect of NAC among adolescents.
Tobacco use disorder
Cigarette smoking remains a leading cause of preventable death in the United States,28 and nearly 70% of people who start using tobacco become dependent.20 Existing FDA-approved treatments include nicotine replacement products, varenicline, and bupropion. Even though efficacious treatments exist, successful and sustained quit attempts are infrequent.29 NAC may exert a complementary effect to existing tobacco cessation interventions, such as varenicline.30 While these medications promote abstinence, NAC may be particularly beneficial in preventing relapse after abstinence has been achieved (Table 430-36).
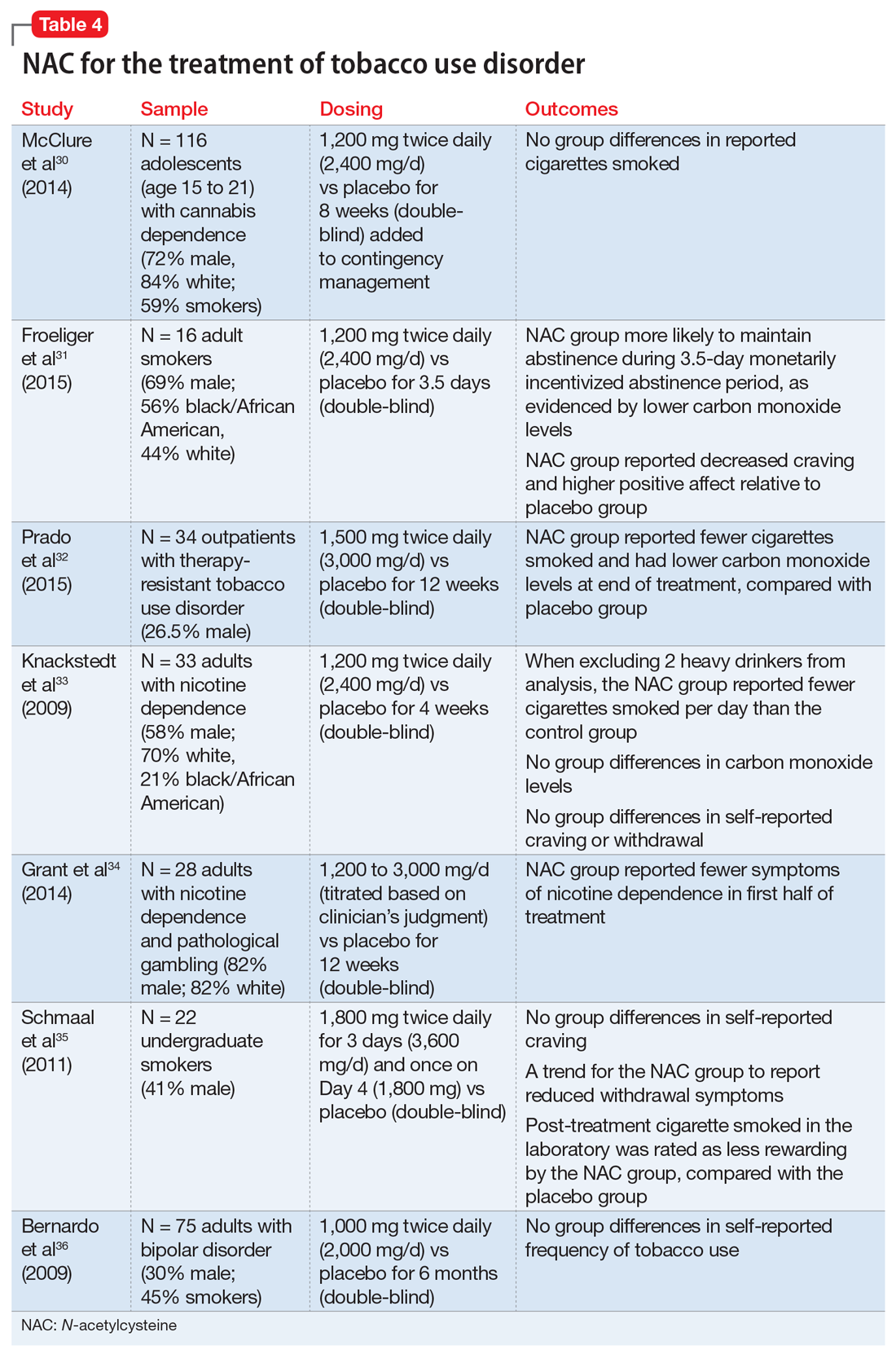
Continue to: Cessation and relapse prevention
Cessation and relapse prevention. Several pilot studies found that adult smokers who received NAC (alone or in combination with another treatment) had lower carbon monoxide levels,31,32 smoked fewer cigarettes,32,33 and had fewer self-reported symptoms of nicotine dependence34 and/or less craving for cigarettes.31 However, one study of 33 smokers did not find a reduction in craving or carbon monoxide for NAC compared with placebo.33 Another pilot study of 22 young adult smokers found that those who received NAC rated their first cigarette after treatment (smoked in the laboratory) as less rewarding, relative to smokers who received a placebo.35
Secondary analyses of adults with bipolar disorder36 and adolescents with cannabis use disorder37 found no decreases in tobacco use among those who received NAC compared with placebo. However, the studies in these analyses did not specifically recruit tobacco users, and participants who were tobacco users were not necessarily interested in quitting. This may partially explain discrepant findings.
Appropriate populations. NAC has been studied mostly in adult cigarette smokers.
Safety and dosing. Suggested dosages for treating tobacco use disorder range from 1,200 to 3,600 mg/d (600 to 1,800 mg twice daily).
Continue to: Clinical implications
Clinical implications. Data on NAC’s efficacy for tobacco use disorder come from small, pilot trials. Although initial evidence is promising, it is premature to suggest NAC for smoking cessation until a fully powered, randomized clinical trial provides evidence of efficacy.
Alcohol use disorder
Alcohol use disorders are widely prevalent; 13.9% of U.S. adults met criteria in the past year, and 29.1% of U.S. adults meet criteria in their lifetime.38 Alcohol use disorders can result in significant negative consequences, including relationship problems, violent behavior, medical problems, and death. Existing FDA-approved medications for alcohol use disorder include naltrexone, acamprosate, and disulfiram.
Due to the severe potential health consequences of alcohol, NAC has been examined as a possible aid in preventing relapse. However, most studies have been conducted using animals. Three studies have examined alcohol use in humans (Table 536,39,40). One was a pilot study,39 and the other 2 were secondary data analyses.36,40 None of them specifically focused on alcohol use disorders. A pilot study of 35 veterans with co-occurring posttraumautic stress disorder (PTSD) and SUDs (82% of whom had an alcohol use disorder) found that compared with placebo, NAC significantly decreased PTSD symptoms, craving, and depression.39 In a study of 75 adults with bipolar disorder, secondary alcohol use was not significantly reduced.36 However, one study suggested that NAC may decrease adolescent alcohol and marijuana co-use.40 Future work is needed to examine the potential clinical utility of NAC in individuals with alcohol use disorders.
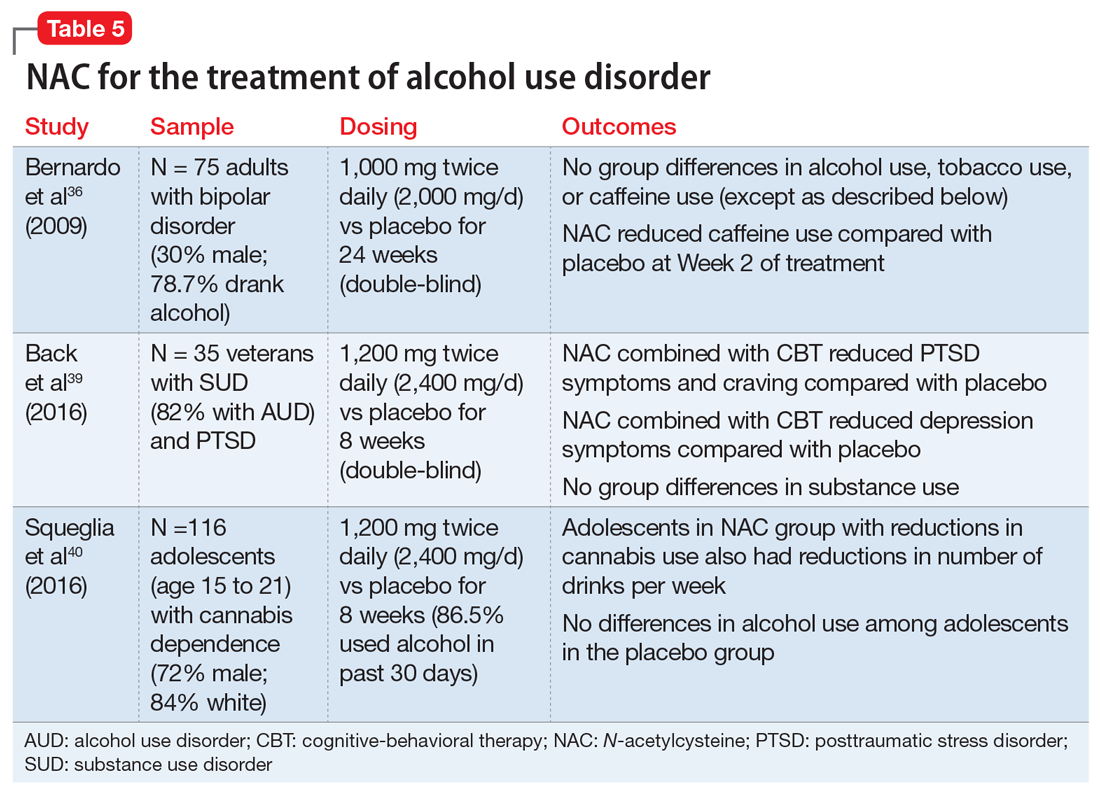
Findings from animal studies indicate that NAC may:
- reduce alcohol-seeking41
- reduce withdrawal symptoms42
- reduce the teratogenic effects of alcohol43
- prevent alcohol toxicity44
- reduce health-related consequences of alcohol (eg, myocardial oxidative stress45 and alcohol-related steatohepatitis46).
Continue to: Appropriate populations
Appropriate populations. Pilot studies have suggested that appropriate populations may include veterans with SUD and PTSD39 and adolescents with marijuana dependence who use alcohol.40
Safety and dosing. Suggested dosages for the treatment of alcohol use disorder based on these studies range from 1,000 to 2,400 mg/d (500 to 1,200 mg twice daily).
Clinical implications. Future work is needed to determine if NAC is effective for treating alcohol use disorders. Ongoing randomized clinical trials are examining the efficacy of NAC in reducing alcohol use among individuals with alcohol use disorder. It is premature to recommend NAC for treatment of alcohol use disorders.
Other psychiatric uses
Although we have highlighted NAC’s effect on glutamatergic transmission, evidence suggests that NAC may have multiple mechanisms of action that could impact psychiatric functioning. For example, NAC may also reverse oxidative stress, which is frequently observed in psychiatric disorders such as schizophrenia and bipolar disorder.10,12 NAC also has anti-inflammatory properties. When inflammatory pathways of the CNS are dysregulated, production of neurotransmitters may be impaired, resulting in depression-like symptoms.10,12,47 Preliminary evidence suggests that NAC may be effective in treating mood-related symptoms (eg, irritability, depression) in individuals with psychiatric disorders (eg, bipolar and depressive disorders, PTSD, and SUDs) and general symptoms of schizophrenia, obsessive-compulsive disorder, and trichotillomania, although mixed findings in controlled studies suggest a need for further research.12,39
Continue to: NAC: A promising candidate
NAC: A promising candidate
Initial evidence suggests NAC may be helpful for treating patients with SUDs. A patient seeking SUD treatment who is treated with NAC may experience a decreased drive, craving, or compulsion to use. Notably, NAC may be particularly useful in preventing relapse after an individual has achieved abstinence. Evidence suggests that NAC may be useful in the treatment of adults with cocaine use disorders who have achieved abstinence, and adolescents with cannabis use disorders. Preliminary results for adult tobacco use disorder are also promising. Human data examining the efficacy of NAC for alcohol use disorder is limited. Researchers’ ongoing challenge is to identify which patients with which SUDs are most likely to benefit from NAC, and to create clear clinical guidelines for the provider.
Bottom Line
N-acetylcysteine is likely to have modest effects for some patients who have a substance use disorder, particularly adults who use cocaine and adolescents who use marijuana. It may be useful in preventing relapse to substance use after an individual has achieved abstinence.
Related Resources
- Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
- Roberts-Wolfe D, Kalivas P. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
- National Institute on Drug Abuse. Treatment approaches for drug addiction. https://www.drugabuse.gov/publications/drugfacts/treatment-approaches-drug-addiction.
Drug Brand Names
Acamprosate • Campral
Acetaminophen • Tylenol
Baclofen • Lioresal
Bupropion • Zyban
Disulfiram • Antabuse
Naltrexone • Revia,Vivitrol
Varenicline • Chantix
1. Grella CE, Karno MP, Warda US, et al. Perceptions of need and help received for substance dependence in a national probability survey. Psychiatr Serv. 2009;60(8):1068-1074.
2. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23-50.
3. McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531-3537.
4. LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170-3177.
5. Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974-986.
6. Roberts-Wolfe D, Kalivas PW. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
7. Berk M, Malhi GS, Gray LJ, et al. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167-177.
8. McClure EA, Gipson CD, Malcolm RJ, et al. Potential role of N-acetylcysteine in the management of substance use disorders. CNS drugs. 2014;28(2):95-106.
9. Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
10. Minarini A, Ferrari S, Galletti M, et al. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol. 2017;13(3):279-292.
11. Grandjean EM, Berthet P, Ruffman R, et al. Efficacy of oral long-term N‑acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22(2):209‑221.
12. Rhodes K, Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Med. 2017;47(8):1619-1636.
13. Olsson B, Johansson M, Gabrielsson J, et al. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77-82.
14. United Nations Office on Drugs and Crime. World Drug Report 2016 (United Nations publication, Sales No. E.16.XI.7). https://www.unodc.org/doc/wdr2016/WORLD_DRUG_REPORT_2016_web.pdf. Published May 2016. Accessed April 26, 2018.
15. Amen SL, Piacentine LB, Ahmad ME, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36(4):871-878.
16. Mardikian PN, LaRowe SD, Hedden S, et al. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389-394.
17. LaRowe SD, Kalivas PW, Nicholas JS, et al. A double‐blind placebo‐controlled trial of N‐acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22(5):443-452.
18. Mousavi SG, Sharbafchi MR, Salehi M, et al. The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study. Arch Iran Med. 2015;18(1):28-33.
19. Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol. 2010;20(11):823-828.
20. Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011;115(1-2):120-130.
21. Chen CY, O’Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000-2001. Drug Alcohol Depend. 2005;79(1):11-22.
22. Copersino ML, Boyd SJ, Tashkin DP, et al. Quitting among non-treatment-seeking marijuana users: reasons and changes in other substance use. Am J Addict. 2006;15(4):297-302.
23. Weiner MD, Sussman S, McCuller WJ, et al. Factors in marijuana cessation among high-risk youth. J Drug Educ. 1999;29(4):337-357.
24. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
25. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
26. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
27. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;177:249-257.
28. Rostron B. Mortality risks associated with environmental tobacco smoke exposure in the United States. Nicotine Tob Res. 2013;15(10):1722-1728.
29. Centers for Disease Control and Prevention. Quitting smoking among adults – United States, 2001–2010. MMWR. 2011;60(44):1513-1519.
30. McClure EA, Baker NL, Gipson CD, et al. An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers. Am J Drug Alcohol Abuse. 2015;41(1):52-56.
31. Froeliger B, McConnell P, Stankeviciute N, et al. The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: a double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 2015;156:234-242.
32. Prado E, Maes M, Piccoli LG, et al. N-acetylcysteine for therapy-resistant tobacco use disorder: a pilot study. Redox Rep. 2015;20(5):215-222.
33. Knackstedt LA, LaRowe S, Mardikian P, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841-845.
34. Grant JE, Odlaug BL, Chamberlain SR, et al. A randomized, placebo-controlled trial of N-acetylcysteine plus imaginal desensitization for nicotine-dependent pathological gamblers. J Clin Psychiatry. 2014;75(1):39-45.
35. Schmaal L, Berk L, Hulstijn KP, et al. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addiction Res. 2011;17(4):211-216.
36. Bernardo M, Dodd S, Gama CS, et al. Effects of N‐acetylcysteine on substance use in bipolar disorder: a randomised placebo‐controlled clinical trial. Acta Neuropsychiatr. 2009;21(5):239-245.
37. McClure EA, Baker NL, Gray KM. Cigarette smoking during an N-acetylcysteine-assisted cannabis cessation trial in adolescents. Am J Drug Alcohol Abuse. 2014;40(4):285-291.
38. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
39. Back SE, McCauley JL, Korte KJ, et al. A double-blind randomized controlled pilot trial of N-acetylcysteine in veterans with PTSD and substance use disorders. J Clin Psychiatry. 2016;77(11):e1439-e1446.
40. Squeglia LM, Baker NL, McClure EA, et al. Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation. Addict Behav. 2016;63:172-177.
41. Lebourgeois S, González-Marín MC, Jeanblanc J, et al. Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration. Addict Biol. 2018;23(2):643-652.
42. Schneider R Jr, Santos CF, Clarimundo V, et al. N-acetylcysteine prevents behavioral and biochemical changes induced by alcohol cessation in rats. Alcohol. 2015;49(3):259-263.
43. Parnell SE, Sulik KK, Dehart DB, et al. Reduction of ethanol-induced ocular abnormalities in mice via dietary administration of N-acetylcysteine. Alcohol. 2010;44(7-8):699-705.
44. Ozkol H, Bulut G, Balahoroglu R, et al. Protective effects of Selenium, N-acetylcysteine and Vitamin E against acute ethanol intoxication in rats. Biol Trace Elem Res. 2017;175(1):177-185.
45. Seiva FR, Amauchi JF, Rocha KK, et al. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43(8):649-656.
46. Setshedi M, Longato L, Petersen DR, et al. Limited therapeutic effect of N‐acetylcysteine on hepatic insulin resistance in an experimental model of alcohol‐induced steatohepatitis. Alcohol Clin Exp Res. 2011;35(12):2139-2151.
47. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-741.
Pharmacologic treatment options for many substance use disorders (SUDs) are limited. This is especially true for cocaine use disorder and cannabis use disorder, for which there are no FDA-approved medications. FDA-approved medications for other SUDs often take the form of replacement or agonist therapies (eg, nicotine replacement therapy) that substitute the effects of the substance to aid in cessation. Other pharmacotherapies treat symptoms of withdrawal, reduce craving, or provide aversive counter-conditioning if the patient consumes the substance while on the medication (eg, disulfiram).
The over-the-counter (OTC) antioxidant N-acetylcysteine (NAC) may be a potential treatment for SUDs. Although NAC is not approved by the FDA for treating SUDs, its proposed mechanism of action differs from that of current FDA-approved medications for SUDs. NAC’s potential for broad applicability, favorable adverse-effect profile, accessibility, and low cost make it an intriguing option for patients with multiple comorbidities, and potentially for individuals with polysubstance use. This article reviews the current evidence supporting NAC for treating SUDs, to provide insight about which patients may benefit from NAC and under which circumstances they are most likely to benefit.
NAC may correct glutamate dysregulation
Approximately 85% of individuals with an SUD do not seek treatment for it, and those who do are older, have a longer history of use, have more severe dependence, and have sought treatment numerous times before.1 By the time most people seek treatment, years of chronic substance use have likely led to significant brain-related adaptations. Individuals with SUDs often indicate that their substance use began as a pleasurable activity—the effects of the drug were enjoyable and they were motivated to use it again. With repeated substance use, they may begin to develop a stronger urge to use the drug, driven not necessarily by a desire for pleasure, but by compulsion.2
Numerous neural adaptations underlie the transition from “liking” a substance to engaging in the compulsive use that is characteristic of an SUD.2 For example, repeated use of an addictive substance may result in excess glutamate in the nucleus accumbens,3,4 an area of the brain that plays a critical role in motivation and learning. As a result, it has been proposed that pharmacotherapies that help correct glutamate dysregulation may be effective in promoting abstinence or preventing relapse to a substance.5,6
NAC may reverse the neural dysfunction seen in SUDs. As an OTC antioxidant that impacts glutamatergic functioning in the brain, NAC has long been used to treat acetaminophen overdose; however, in recent years, researchers have begun to tap its potential for treating substance use and psychiatric disorders. NAC is thought to upregulate the glutamate transporter (GLT-1) that removes excess glutamate from the nucleus accumbens.6 Several published reviews provide more in-depth information about the neurobiology of NAC.6-10
The adverse-effect profile of NAC is relatively benign. Nausea, vomiting, diarrhea, and sleepiness are relatively infrequent and mild.11,12 The bioavailability of NAC is about 4% to 9%, with an approximate half-life of 6.25 hours when orally administered.13 Because NAC is classified as an OTC supplement, the potency and preparation may vary by supplier. To maximize consistency, NAC should be obtained from a supplier that meets United States Pharmacopeia (USP) standards.
NAC for SUDs: Emerging evidence
Several recent reviews have described the efficacy of NAC for SUDs and other psychiatric disorders. Here we summarize the current research examining the efficacy of NAC for stimulant (ie, cocaine and methamphetamine), cannabis, tobacco, and alcohol use disorders.
Continue to: Stimulant use disorders
Stimulant use disorders. The United Nations Office for Drugs and Crime estimates that worldwide, more than 18 million people use cocaine and more than 35 million use amphetamines.14 There are currently no FDA-approved treatments for stimulant use disorders, and clinicians treating patients with cocaine or amphetamine dependence often are at a loss for how best to promote abstinence. Recent studies suggest that NAC may decrease drug-seeking behavior and cravings in adults who seek treatment. The results of studies examining NAC for treating cocaine use and methamphetamine use are summarized in Table 115-17 and Table 2,18,19 respectively.

Cocaine cessation and relapse prevention. Several small pilot projects15,16 found that compared with placebo, various doses of NAC reduced craving (as measured with a visual analog scale). However, in a double-blind, placebo-controlled study, NAC did not decrease cravings or use after 8 weeks of treatment in individuals with cocaine use disorder who were still using cocaine (ie, they had not yet become abstinent). Interestingly, those who were abstinent when treatment began reported lower craving and remained abstinent longer if they received NAC (vs placebo), which suggests that NAC may be useful for preventing relapse.17

Methamphetamine cessation and relapse prevention. One study (N = 32) that evaluated the use of NAC, 1,200 mg/d for 4 weeks, vs placebo found reduced cravings among methamphetamine users who were seeking treatment.18 In contrast, a study of 31 methamphetamine users who were not seeking treatment evaluated the use of NAC, 2,400 mg/d, plus naltrexone, 200 mg/d, vs placebo for 8 weeks.19 It found no significant differences in craving or use patterns. Further research is needed to optimize the use of NAC for stimulant use disorders, and to better understand the role that abstinence plays.
Appropriate populations. The most support for use of NAC has been as an anti-relapse agent in treatment-seeking adults.
Continue to: Safety and dosing
Safety and dosing. Suggested dosages for the treatment of cocaine use disorder range from 1,200 to 3,600 mg/d (typically 600 to 1,800 mg twice daily, due to NAC’s short half-life), with higher retention rates noted in individuals who received 2,400 mg/d and 3,600 mg/d.16
Clinical implications. NAC is thought to act as an anti-relapse agent, rather than an agent that can help someone who is actively using stimulants to stop. Consequently, NAC will likely be most helpful for patients who are motivated to quit and are abstinent when they start taking NAC; however, this hypothesis needs further testing.
Cannabis use disorder
There are no FDA-approved treatments for cannabis use disorder. Individuals who use marijuana or other forms of cannabis may be less likely to report negative consequences or seek treatment compared with those who use other substances. Approximately 9% of individuals who use marijuana develop cannabis use disorder20; those who begin using marijuana earlier in adolescence are at increased risk.21 Commonly reported reasons for wanting to stop using marijuana include being concerned about health consequences, regaining or demonstrating self-control, saving money, avoiding legal consequences, obtaining or keeping employment, and reducing interpersonal conflict.22,23 Table 324-27 summarizes initial evidence that suggests NAC may be particularly useful in reducing marijuana use among adolescents (age 15 to 21).24,25

Cessation. An open-label, pilot clinical trial found significant reductions in self-reported marijuana use and craving—but not in biomarkers of use—among 24 adolescents after 4 weeks of NAC, 1,200 mg twice daily.24 In an 8-week, double-blind, randomized controlled trial of 116 adolescents, NAC, 1,200 mg twice daily, plus contingency management doubled the odds of abstinence, but had no effect on self-reported craving or use.25,26 In a sample of 302 adults, a 12-week trial of NAC, 1,200 mg twice daily, plus contingency management was no more effective than contingency management alone in promoting abstinence.27
Continue to: Appropriate populations
Appropriate populations. Evidence is stronger for use of NAC among adolescents (age 15 to 21) than for individuals older than age 21.25,27 Further research is needed to explore potential reasons for age-specific effects.
Safety and dosing. A safe and potentially efficacious dosage for the treatment of cannabis use disorder is 2,400 mg/d (1,200 mg twice daily).24,25,27
Clinical implications. Combined with contingency management, NAC might be efficacious for adolescents with cannabis use disorder, with treatment gains evident by the fourth week of treatment.24,25 To date, no clinical trials have examined the efficacy of NAC for treating cannabis use disorder without adjunctive contingency management, and research is needed to isolate the clinical effect of NAC among adolescents.
Tobacco use disorder
Cigarette smoking remains a leading cause of preventable death in the United States,28 and nearly 70% of people who start using tobacco become dependent.20 Existing FDA-approved treatments include nicotine replacement products, varenicline, and bupropion. Even though efficacious treatments exist, successful and sustained quit attempts are infrequent.29 NAC may exert a complementary effect to existing tobacco cessation interventions, such as varenicline.30 While these medications promote abstinence, NAC may be particularly beneficial in preventing relapse after abstinence has been achieved (Table 430-36).

Continue to: Cessation and relapse prevention
Cessation and relapse prevention. Several pilot studies found that adult smokers who received NAC (alone or in combination with another treatment) had lower carbon monoxide levels,31,32 smoked fewer cigarettes,32,33 and had fewer self-reported symptoms of nicotine dependence34 and/or less craving for cigarettes.31 However, one study of 33 smokers did not find a reduction in craving or carbon monoxide for NAC compared with placebo.33 Another pilot study of 22 young adult smokers found that those who received NAC rated their first cigarette after treatment (smoked in the laboratory) as less rewarding, relative to smokers who received a placebo.35
Secondary analyses of adults with bipolar disorder36 and adolescents with cannabis use disorder37 found no decreases in tobacco use among those who received NAC compared with placebo. However, the studies in these analyses did not specifically recruit tobacco users, and participants who were tobacco users were not necessarily interested in quitting. This may partially explain discrepant findings.
Appropriate populations. NAC has been studied mostly in adult cigarette smokers.
Safety and dosing. Suggested dosages for treating tobacco use disorder range from 1,200 to 3,600 mg/d (600 to 1,800 mg twice daily).
Continue to: Clinical implications
Clinical implications. Data on NAC’s efficacy for tobacco use disorder come from small, pilot trials. Although initial evidence is promising, it is premature to suggest NAC for smoking cessation until a fully powered, randomized clinical trial provides evidence of efficacy.
Alcohol use disorder
Alcohol use disorders are widely prevalent; 13.9% of U.S. adults met criteria in the past year, and 29.1% of U.S. adults meet criteria in their lifetime.38 Alcohol use disorders can result in significant negative consequences, including relationship problems, violent behavior, medical problems, and death. Existing FDA-approved medications for alcohol use disorder include naltrexone, acamprosate, and disulfiram.
Due to the severe potential health consequences of alcohol, NAC has been examined as a possible aid in preventing relapse. However, most studies have been conducted using animals. Three studies have examined alcohol use in humans (Table 536,39,40). One was a pilot study,39 and the other 2 were secondary data analyses.36,40 None of them specifically focused on alcohol use disorders. A pilot study of 35 veterans with co-occurring posttraumautic stress disorder (PTSD) and SUDs (82% of whom had an alcohol use disorder) found that compared with placebo, NAC significantly decreased PTSD symptoms, craving, and depression.39 In a study of 75 adults with bipolar disorder, secondary alcohol use was not significantly reduced.36 However, one study suggested that NAC may decrease adolescent alcohol and marijuana co-use.40 Future work is needed to examine the potential clinical utility of NAC in individuals with alcohol use disorders.

Findings from animal studies indicate that NAC may:
- reduce alcohol-seeking41
- reduce withdrawal symptoms42
- reduce the teratogenic effects of alcohol43
- prevent alcohol toxicity44
- reduce health-related consequences of alcohol (eg, myocardial oxidative stress45 and alcohol-related steatohepatitis46).
Continue to: Appropriate populations
Appropriate populations. Pilot studies have suggested that appropriate populations may include veterans with SUD and PTSD39 and adolescents with marijuana dependence who use alcohol.40
Safety and dosing. Suggested dosages for the treatment of alcohol use disorder based on these studies range from 1,000 to 2,400 mg/d (500 to 1,200 mg twice daily).
Clinical implications. Future work is needed to determine if NAC is effective for treating alcohol use disorders. Ongoing randomized clinical trials are examining the efficacy of NAC in reducing alcohol use among individuals with alcohol use disorder. It is premature to recommend NAC for treatment of alcohol use disorders.
Other psychiatric uses
Although we have highlighted NAC’s effect on glutamatergic transmission, evidence suggests that NAC may have multiple mechanisms of action that could impact psychiatric functioning. For example, NAC may also reverse oxidative stress, which is frequently observed in psychiatric disorders such as schizophrenia and bipolar disorder.10,12 NAC also has anti-inflammatory properties. When inflammatory pathways of the CNS are dysregulated, production of neurotransmitters may be impaired, resulting in depression-like symptoms.10,12,47 Preliminary evidence suggests that NAC may be effective in treating mood-related symptoms (eg, irritability, depression) in individuals with psychiatric disorders (eg, bipolar and depressive disorders, PTSD, and SUDs) and general symptoms of schizophrenia, obsessive-compulsive disorder, and trichotillomania, although mixed findings in controlled studies suggest a need for further research.12,39
Continue to: NAC: A promising candidate
NAC: A promising candidate
Initial evidence suggests NAC may be helpful for treating patients with SUDs. A patient seeking SUD treatment who is treated with NAC may experience a decreased drive, craving, or compulsion to use. Notably, NAC may be particularly useful in preventing relapse after an individual has achieved abstinence. Evidence suggests that NAC may be useful in the treatment of adults with cocaine use disorders who have achieved abstinence, and adolescents with cannabis use disorders. Preliminary results for adult tobacco use disorder are also promising. Human data examining the efficacy of NAC for alcohol use disorder is limited. Researchers’ ongoing challenge is to identify which patients with which SUDs are most likely to benefit from NAC, and to create clear clinical guidelines for the provider.
Bottom Line
N-acetylcysteine is likely to have modest effects for some patients who have a substance use disorder, particularly adults who use cocaine and adolescents who use marijuana. It may be useful in preventing relapse to substance use after an individual has achieved abstinence.
Related Resources
- Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
- Roberts-Wolfe D, Kalivas P. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
- National Institute on Drug Abuse. Treatment approaches for drug addiction. https://www.drugabuse.gov/publications/drugfacts/treatment-approaches-drug-addiction.
Drug Brand Names
Acamprosate • Campral
Acetaminophen • Tylenol
Baclofen • Lioresal
Bupropion • Zyban
Disulfiram • Antabuse
Naltrexone • Revia,Vivitrol
Varenicline • Chantix
Pharmacologic treatment options for many substance use disorders (SUDs) are limited. This is especially true for cocaine use disorder and cannabis use disorder, for which there are no FDA-approved medications. FDA-approved medications for other SUDs often take the form of replacement or agonist therapies (eg, nicotine replacement therapy) that substitute the effects of the substance to aid in cessation. Other pharmacotherapies treat symptoms of withdrawal, reduce craving, or provide aversive counter-conditioning if the patient consumes the substance while on the medication (eg, disulfiram).
The over-the-counter (OTC) antioxidant N-acetylcysteine (NAC) may be a potential treatment for SUDs. Although NAC is not approved by the FDA for treating SUDs, its proposed mechanism of action differs from that of current FDA-approved medications for SUDs. NAC’s potential for broad applicability, favorable adverse-effect profile, accessibility, and low cost make it an intriguing option for patients with multiple comorbidities, and potentially for individuals with polysubstance use. This article reviews the current evidence supporting NAC for treating SUDs, to provide insight about which patients may benefit from NAC and under which circumstances they are most likely to benefit.
NAC may correct glutamate dysregulation
Approximately 85% of individuals with an SUD do not seek treatment for it, and those who do are older, have a longer history of use, have more severe dependence, and have sought treatment numerous times before.1 By the time most people seek treatment, years of chronic substance use have likely led to significant brain-related adaptations. Individuals with SUDs often indicate that their substance use began as a pleasurable activity—the effects of the drug were enjoyable and they were motivated to use it again. With repeated substance use, they may begin to develop a stronger urge to use the drug, driven not necessarily by a desire for pleasure, but by compulsion.2
Numerous neural adaptations underlie the transition from “liking” a substance to engaging in the compulsive use that is characteristic of an SUD.2 For example, repeated use of an addictive substance may result in excess glutamate in the nucleus accumbens,3,4 an area of the brain that plays a critical role in motivation and learning. As a result, it has been proposed that pharmacotherapies that help correct glutamate dysregulation may be effective in promoting abstinence or preventing relapse to a substance.5,6
NAC may reverse the neural dysfunction seen in SUDs. As an OTC antioxidant that impacts glutamatergic functioning in the brain, NAC has long been used to treat acetaminophen overdose; however, in recent years, researchers have begun to tap its potential for treating substance use and psychiatric disorders. NAC is thought to upregulate the glutamate transporter (GLT-1) that removes excess glutamate from the nucleus accumbens.6 Several published reviews provide more in-depth information about the neurobiology of NAC.6-10
The adverse-effect profile of NAC is relatively benign. Nausea, vomiting, diarrhea, and sleepiness are relatively infrequent and mild.11,12 The bioavailability of NAC is about 4% to 9%, with an approximate half-life of 6.25 hours when orally administered.13 Because NAC is classified as an OTC supplement, the potency and preparation may vary by supplier. To maximize consistency, NAC should be obtained from a supplier that meets United States Pharmacopeia (USP) standards.
NAC for SUDs: Emerging evidence
Several recent reviews have described the efficacy of NAC for SUDs and other psychiatric disorders. Here we summarize the current research examining the efficacy of NAC for stimulant (ie, cocaine and methamphetamine), cannabis, tobacco, and alcohol use disorders.
Continue to: Stimulant use disorders
Stimulant use disorders. The United Nations Office for Drugs and Crime estimates that worldwide, more than 18 million people use cocaine and more than 35 million use amphetamines.14 There are currently no FDA-approved treatments for stimulant use disorders, and clinicians treating patients with cocaine or amphetamine dependence often are at a loss for how best to promote abstinence. Recent studies suggest that NAC may decrease drug-seeking behavior and cravings in adults who seek treatment. The results of studies examining NAC for treating cocaine use and methamphetamine use are summarized in Table 115-17 and Table 2,18,19 respectively.

Cocaine cessation and relapse prevention. Several small pilot projects15,16 found that compared with placebo, various doses of NAC reduced craving (as measured with a visual analog scale). However, in a double-blind, placebo-controlled study, NAC did not decrease cravings or use after 8 weeks of treatment in individuals with cocaine use disorder who were still using cocaine (ie, they had not yet become abstinent). Interestingly, those who were abstinent when treatment began reported lower craving and remained abstinent longer if they received NAC (vs placebo), which suggests that NAC may be useful for preventing relapse.17

Methamphetamine cessation and relapse prevention. One study (N = 32) that evaluated the use of NAC, 1,200 mg/d for 4 weeks, vs placebo found reduced cravings among methamphetamine users who were seeking treatment.18 In contrast, a study of 31 methamphetamine users who were not seeking treatment evaluated the use of NAC, 2,400 mg/d, plus naltrexone, 200 mg/d, vs placebo for 8 weeks.19 It found no significant differences in craving or use patterns. Further research is needed to optimize the use of NAC for stimulant use disorders, and to better understand the role that abstinence plays.
Appropriate populations. The most support for use of NAC has been as an anti-relapse agent in treatment-seeking adults.
Continue to: Safety and dosing
Safety and dosing. Suggested dosages for the treatment of cocaine use disorder range from 1,200 to 3,600 mg/d (typically 600 to 1,800 mg twice daily, due to NAC’s short half-life), with higher retention rates noted in individuals who received 2,400 mg/d and 3,600 mg/d.16
Clinical implications. NAC is thought to act as an anti-relapse agent, rather than an agent that can help someone who is actively using stimulants to stop. Consequently, NAC will likely be most helpful for patients who are motivated to quit and are abstinent when they start taking NAC; however, this hypothesis needs further testing.
Cannabis use disorder
There are no FDA-approved treatments for cannabis use disorder. Individuals who use marijuana or other forms of cannabis may be less likely to report negative consequences or seek treatment compared with those who use other substances. Approximately 9% of individuals who use marijuana develop cannabis use disorder20; those who begin using marijuana earlier in adolescence are at increased risk.21 Commonly reported reasons for wanting to stop using marijuana include being concerned about health consequences, regaining or demonstrating self-control, saving money, avoiding legal consequences, obtaining or keeping employment, and reducing interpersonal conflict.22,23 Table 324-27 summarizes initial evidence that suggests NAC may be particularly useful in reducing marijuana use among adolescents (age 15 to 21).24,25

Cessation. An open-label, pilot clinical trial found significant reductions in self-reported marijuana use and craving—but not in biomarkers of use—among 24 adolescents after 4 weeks of NAC, 1,200 mg twice daily.24 In an 8-week, double-blind, randomized controlled trial of 116 adolescents, NAC, 1,200 mg twice daily, plus contingency management doubled the odds of abstinence, but had no effect on self-reported craving or use.25,26 In a sample of 302 adults, a 12-week trial of NAC, 1,200 mg twice daily, plus contingency management was no more effective than contingency management alone in promoting abstinence.27
Continue to: Appropriate populations
Appropriate populations. Evidence is stronger for use of NAC among adolescents (age 15 to 21) than for individuals older than age 21.25,27 Further research is needed to explore potential reasons for age-specific effects.
Safety and dosing. A safe and potentially efficacious dosage for the treatment of cannabis use disorder is 2,400 mg/d (1,200 mg twice daily).24,25,27
Clinical implications. Combined with contingency management, NAC might be efficacious for adolescents with cannabis use disorder, with treatment gains evident by the fourth week of treatment.24,25 To date, no clinical trials have examined the efficacy of NAC for treating cannabis use disorder without adjunctive contingency management, and research is needed to isolate the clinical effect of NAC among adolescents.
Tobacco use disorder
Cigarette smoking remains a leading cause of preventable death in the United States,28 and nearly 70% of people who start using tobacco become dependent.20 Existing FDA-approved treatments include nicotine replacement products, varenicline, and bupropion. Even though efficacious treatments exist, successful and sustained quit attempts are infrequent.29 NAC may exert a complementary effect to existing tobacco cessation interventions, such as varenicline.30 While these medications promote abstinence, NAC may be particularly beneficial in preventing relapse after abstinence has been achieved (Table 430-36).

Continue to: Cessation and relapse prevention
Cessation and relapse prevention. Several pilot studies found that adult smokers who received NAC (alone or in combination with another treatment) had lower carbon monoxide levels,31,32 smoked fewer cigarettes,32,33 and had fewer self-reported symptoms of nicotine dependence34 and/or less craving for cigarettes.31 However, one study of 33 smokers did not find a reduction in craving or carbon monoxide for NAC compared with placebo.33 Another pilot study of 22 young adult smokers found that those who received NAC rated their first cigarette after treatment (smoked in the laboratory) as less rewarding, relative to smokers who received a placebo.35
Secondary analyses of adults with bipolar disorder36 and adolescents with cannabis use disorder37 found no decreases in tobacco use among those who received NAC compared with placebo. However, the studies in these analyses did not specifically recruit tobacco users, and participants who were tobacco users were not necessarily interested in quitting. This may partially explain discrepant findings.
Appropriate populations. NAC has been studied mostly in adult cigarette smokers.
Safety and dosing. Suggested dosages for treating tobacco use disorder range from 1,200 to 3,600 mg/d (600 to 1,800 mg twice daily).
Continue to: Clinical implications
Clinical implications. Data on NAC’s efficacy for tobacco use disorder come from small, pilot trials. Although initial evidence is promising, it is premature to suggest NAC for smoking cessation until a fully powered, randomized clinical trial provides evidence of efficacy.
Alcohol use disorder
Alcohol use disorders are widely prevalent; 13.9% of U.S. adults met criteria in the past year, and 29.1% of U.S. adults meet criteria in their lifetime.38 Alcohol use disorders can result in significant negative consequences, including relationship problems, violent behavior, medical problems, and death. Existing FDA-approved medications for alcohol use disorder include naltrexone, acamprosate, and disulfiram.
Due to the severe potential health consequences of alcohol, NAC has been examined as a possible aid in preventing relapse. However, most studies have been conducted using animals. Three studies have examined alcohol use in humans (Table 536,39,40). One was a pilot study,39 and the other 2 were secondary data analyses.36,40 None of them specifically focused on alcohol use disorders. A pilot study of 35 veterans with co-occurring posttraumautic stress disorder (PTSD) and SUDs (82% of whom had an alcohol use disorder) found that compared with placebo, NAC significantly decreased PTSD symptoms, craving, and depression.39 In a study of 75 adults with bipolar disorder, secondary alcohol use was not significantly reduced.36 However, one study suggested that NAC may decrease adolescent alcohol and marijuana co-use.40 Future work is needed to examine the potential clinical utility of NAC in individuals with alcohol use disorders.

Findings from animal studies indicate that NAC may:
- reduce alcohol-seeking41
- reduce withdrawal symptoms42
- reduce the teratogenic effects of alcohol43
- prevent alcohol toxicity44
- reduce health-related consequences of alcohol (eg, myocardial oxidative stress45 and alcohol-related steatohepatitis46).
Continue to: Appropriate populations
Appropriate populations. Pilot studies have suggested that appropriate populations may include veterans with SUD and PTSD39 and adolescents with marijuana dependence who use alcohol.40
Safety and dosing. Suggested dosages for the treatment of alcohol use disorder based on these studies range from 1,000 to 2,400 mg/d (500 to 1,200 mg twice daily).
Clinical implications. Future work is needed to determine if NAC is effective for treating alcohol use disorders. Ongoing randomized clinical trials are examining the efficacy of NAC in reducing alcohol use among individuals with alcohol use disorder. It is premature to recommend NAC for treatment of alcohol use disorders.
Other psychiatric uses
Although we have highlighted NAC’s effect on glutamatergic transmission, evidence suggests that NAC may have multiple mechanisms of action that could impact psychiatric functioning. For example, NAC may also reverse oxidative stress, which is frequently observed in psychiatric disorders such as schizophrenia and bipolar disorder.10,12 NAC also has anti-inflammatory properties. When inflammatory pathways of the CNS are dysregulated, production of neurotransmitters may be impaired, resulting in depression-like symptoms.10,12,47 Preliminary evidence suggests that NAC may be effective in treating mood-related symptoms (eg, irritability, depression) in individuals with psychiatric disorders (eg, bipolar and depressive disorders, PTSD, and SUDs) and general symptoms of schizophrenia, obsessive-compulsive disorder, and trichotillomania, although mixed findings in controlled studies suggest a need for further research.12,39
Continue to: NAC: A promising candidate
NAC: A promising candidate
Initial evidence suggests NAC may be helpful for treating patients with SUDs. A patient seeking SUD treatment who is treated with NAC may experience a decreased drive, craving, or compulsion to use. Notably, NAC may be particularly useful in preventing relapse after an individual has achieved abstinence. Evidence suggests that NAC may be useful in the treatment of adults with cocaine use disorders who have achieved abstinence, and adolescents with cannabis use disorders. Preliminary results for adult tobacco use disorder are also promising. Human data examining the efficacy of NAC for alcohol use disorder is limited. Researchers’ ongoing challenge is to identify which patients with which SUDs are most likely to benefit from NAC, and to create clear clinical guidelines for the provider.
Bottom Line
N-acetylcysteine is likely to have modest effects for some patients who have a substance use disorder, particularly adults who use cocaine and adolescents who use marijuana. It may be useful in preventing relapse to substance use after an individual has achieved abstinence.
Related Resources
- Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
- Roberts-Wolfe D, Kalivas P. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
- National Institute on Drug Abuse. Treatment approaches for drug addiction. https://www.drugabuse.gov/publications/drugfacts/treatment-approaches-drug-addiction.
Drug Brand Names
Acamprosate • Campral
Acetaminophen • Tylenol
Baclofen • Lioresal
Bupropion • Zyban
Disulfiram • Antabuse
Naltrexone • Revia,Vivitrol
Varenicline • Chantix
1. Grella CE, Karno MP, Warda US, et al. Perceptions of need and help received for substance dependence in a national probability survey. Psychiatr Serv. 2009;60(8):1068-1074.
2. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23-50.
3. McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531-3537.
4. LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170-3177.
5. Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974-986.
6. Roberts-Wolfe D, Kalivas PW. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
7. Berk M, Malhi GS, Gray LJ, et al. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167-177.
8. McClure EA, Gipson CD, Malcolm RJ, et al. Potential role of N-acetylcysteine in the management of substance use disorders. CNS drugs. 2014;28(2):95-106.
9. Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
10. Minarini A, Ferrari S, Galletti M, et al. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol. 2017;13(3):279-292.
11. Grandjean EM, Berthet P, Ruffman R, et al. Efficacy of oral long-term N‑acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22(2):209‑221.
12. Rhodes K, Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Med. 2017;47(8):1619-1636.
13. Olsson B, Johansson M, Gabrielsson J, et al. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77-82.
14. United Nations Office on Drugs and Crime. World Drug Report 2016 (United Nations publication, Sales No. E.16.XI.7). https://www.unodc.org/doc/wdr2016/WORLD_DRUG_REPORT_2016_web.pdf. Published May 2016. Accessed April 26, 2018.
15. Amen SL, Piacentine LB, Ahmad ME, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36(4):871-878.
16. Mardikian PN, LaRowe SD, Hedden S, et al. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389-394.
17. LaRowe SD, Kalivas PW, Nicholas JS, et al. A double‐blind placebo‐controlled trial of N‐acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22(5):443-452.
18. Mousavi SG, Sharbafchi MR, Salehi M, et al. The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study. Arch Iran Med. 2015;18(1):28-33.
19. Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol. 2010;20(11):823-828.
20. Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011;115(1-2):120-130.
21. Chen CY, O’Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000-2001. Drug Alcohol Depend. 2005;79(1):11-22.
22. Copersino ML, Boyd SJ, Tashkin DP, et al. Quitting among non-treatment-seeking marijuana users: reasons and changes in other substance use. Am J Addict. 2006;15(4):297-302.
23. Weiner MD, Sussman S, McCuller WJ, et al. Factors in marijuana cessation among high-risk youth. J Drug Educ. 1999;29(4):337-357.
24. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
25. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
26. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
27. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;177:249-257.
28. Rostron B. Mortality risks associated with environmental tobacco smoke exposure in the United States. Nicotine Tob Res. 2013;15(10):1722-1728.
29. Centers for Disease Control and Prevention. Quitting smoking among adults – United States, 2001–2010. MMWR. 2011;60(44):1513-1519.
30. McClure EA, Baker NL, Gipson CD, et al. An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers. Am J Drug Alcohol Abuse. 2015;41(1):52-56.
31. Froeliger B, McConnell P, Stankeviciute N, et al. The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: a double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 2015;156:234-242.
32. Prado E, Maes M, Piccoli LG, et al. N-acetylcysteine for therapy-resistant tobacco use disorder: a pilot study. Redox Rep. 2015;20(5):215-222.
33. Knackstedt LA, LaRowe S, Mardikian P, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841-845.
34. Grant JE, Odlaug BL, Chamberlain SR, et al. A randomized, placebo-controlled trial of N-acetylcysteine plus imaginal desensitization for nicotine-dependent pathological gamblers. J Clin Psychiatry. 2014;75(1):39-45.
35. Schmaal L, Berk L, Hulstijn KP, et al. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addiction Res. 2011;17(4):211-216.
36. Bernardo M, Dodd S, Gama CS, et al. Effects of N‐acetylcysteine on substance use in bipolar disorder: a randomised placebo‐controlled clinical trial. Acta Neuropsychiatr. 2009;21(5):239-245.
37. McClure EA, Baker NL, Gray KM. Cigarette smoking during an N-acetylcysteine-assisted cannabis cessation trial in adolescents. Am J Drug Alcohol Abuse. 2014;40(4):285-291.
38. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
39. Back SE, McCauley JL, Korte KJ, et al. A double-blind randomized controlled pilot trial of N-acetylcysteine in veterans with PTSD and substance use disorders. J Clin Psychiatry. 2016;77(11):e1439-e1446.
40. Squeglia LM, Baker NL, McClure EA, et al. Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation. Addict Behav. 2016;63:172-177.
41. Lebourgeois S, González-Marín MC, Jeanblanc J, et al. Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration. Addict Biol. 2018;23(2):643-652.
42. Schneider R Jr, Santos CF, Clarimundo V, et al. N-acetylcysteine prevents behavioral and biochemical changes induced by alcohol cessation in rats. Alcohol. 2015;49(3):259-263.
43. Parnell SE, Sulik KK, Dehart DB, et al. Reduction of ethanol-induced ocular abnormalities in mice via dietary administration of N-acetylcysteine. Alcohol. 2010;44(7-8):699-705.
44. Ozkol H, Bulut G, Balahoroglu R, et al. Protective effects of Selenium, N-acetylcysteine and Vitamin E against acute ethanol intoxication in rats. Biol Trace Elem Res. 2017;175(1):177-185.
45. Seiva FR, Amauchi JF, Rocha KK, et al. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43(8):649-656.
46. Setshedi M, Longato L, Petersen DR, et al. Limited therapeutic effect of N‐acetylcysteine on hepatic insulin resistance in an experimental model of alcohol‐induced steatohepatitis. Alcohol Clin Exp Res. 2011;35(12):2139-2151.
47. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-741.
1. Grella CE, Karno MP, Warda US, et al. Perceptions of need and help received for substance dependence in a national probability survey. Psychiatr Serv. 2009;60(8):1068-1074.
2. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23-50.
3. McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531-3537.
4. LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170-3177.
5. Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974-986.
6. Roberts-Wolfe D, Kalivas PW. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
7. Berk M, Malhi GS, Gray LJ, et al. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167-177.
8. McClure EA, Gipson CD, Malcolm RJ, et al. Potential role of N-acetylcysteine in the management of substance use disorders. CNS drugs. 2014;28(2):95-106.
9. Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
10. Minarini A, Ferrari S, Galletti M, et al. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol. 2017;13(3):279-292.
11. Grandjean EM, Berthet P, Ruffman R, et al. Efficacy of oral long-term N‑acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22(2):209‑221.
12. Rhodes K, Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Med. 2017;47(8):1619-1636.
13. Olsson B, Johansson M, Gabrielsson J, et al. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77-82.
14. United Nations Office on Drugs and Crime. World Drug Report 2016 (United Nations publication, Sales No. E.16.XI.7). https://www.unodc.org/doc/wdr2016/WORLD_DRUG_REPORT_2016_web.pdf. Published May 2016. Accessed April 26, 2018.
15. Amen SL, Piacentine LB, Ahmad ME, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36(4):871-878.
16. Mardikian PN, LaRowe SD, Hedden S, et al. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389-394.
17. LaRowe SD, Kalivas PW, Nicholas JS, et al. A double‐blind placebo‐controlled trial of N‐acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22(5):443-452.
18. Mousavi SG, Sharbafchi MR, Salehi M, et al. The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study. Arch Iran Med. 2015;18(1):28-33.
19. Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol. 2010;20(11):823-828.
20. Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011;115(1-2):120-130.
21. Chen CY, O’Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000-2001. Drug Alcohol Depend. 2005;79(1):11-22.
22. Copersino ML, Boyd SJ, Tashkin DP, et al. Quitting among non-treatment-seeking marijuana users: reasons and changes in other substance use. Am J Addict. 2006;15(4):297-302.
23. Weiner MD, Sussman S, McCuller WJ, et al. Factors in marijuana cessation among high-risk youth. J Drug Educ. 1999;29(4):337-357.
24. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
25. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
26. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
27. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;177:249-257.
28. Rostron B. Mortality risks associated with environmental tobacco smoke exposure in the United States. Nicotine Tob Res. 2013;15(10):1722-1728.
29. Centers for Disease Control and Prevention. Quitting smoking among adults – United States, 2001–2010. MMWR. 2011;60(44):1513-1519.
30. McClure EA, Baker NL, Gipson CD, et al. An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers. Am J Drug Alcohol Abuse. 2015;41(1):52-56.
31. Froeliger B, McConnell P, Stankeviciute N, et al. The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: a double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 2015;156:234-242.
32. Prado E, Maes M, Piccoli LG, et al. N-acetylcysteine for therapy-resistant tobacco use disorder: a pilot study. Redox Rep. 2015;20(5):215-222.
33. Knackstedt LA, LaRowe S, Mardikian P, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841-845.
34. Grant JE, Odlaug BL, Chamberlain SR, et al. A randomized, placebo-controlled trial of N-acetylcysteine plus imaginal desensitization for nicotine-dependent pathological gamblers. J Clin Psychiatry. 2014;75(1):39-45.
35. Schmaal L, Berk L, Hulstijn KP, et al. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addiction Res. 2011;17(4):211-216.
36. Bernardo M, Dodd S, Gama CS, et al. Effects of N‐acetylcysteine on substance use in bipolar disorder: a randomised placebo‐controlled clinical trial. Acta Neuropsychiatr. 2009;21(5):239-245.
37. McClure EA, Baker NL, Gray KM. Cigarette smoking during an N-acetylcysteine-assisted cannabis cessation trial in adolescents. Am J Drug Alcohol Abuse. 2014;40(4):285-291.
38. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
39. Back SE, McCauley JL, Korte KJ, et al. A double-blind randomized controlled pilot trial of N-acetylcysteine in veterans with PTSD and substance use disorders. J Clin Psychiatry. 2016;77(11):e1439-e1446.
40. Squeglia LM, Baker NL, McClure EA, et al. Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation. Addict Behav. 2016;63:172-177.
41. Lebourgeois S, González-Marín MC, Jeanblanc J, et al. Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration. Addict Biol. 2018;23(2):643-652.
42. Schneider R Jr, Santos CF, Clarimundo V, et al. N-acetylcysteine prevents behavioral and biochemical changes induced by alcohol cessation in rats. Alcohol. 2015;49(3):259-263.
43. Parnell SE, Sulik KK, Dehart DB, et al. Reduction of ethanol-induced ocular abnormalities in mice via dietary administration of N-acetylcysteine. Alcohol. 2010;44(7-8):699-705.
44. Ozkol H, Bulut G, Balahoroglu R, et al. Protective effects of Selenium, N-acetylcysteine and Vitamin E against acute ethanol intoxication in rats. Biol Trace Elem Res. 2017;175(1):177-185.
45. Seiva FR, Amauchi JF, Rocha KK, et al. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43(8):649-656.
46. Setshedi M, Longato L, Petersen DR, et al. Limited therapeutic effect of N‐acetylcysteine on hepatic insulin resistance in an experimental model of alcohol‐induced steatohepatitis. Alcohol Clin Exp Res. 2011;35(12):2139-2151.
47. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-741.
Repetitive transcranial magnetic stimulation for tic disorders
Tourette syndrome (TS) is a chronic neuropsychiatric disorder occurring in early childhood or adolescence that’s characterized by multiple motor and vocal tics that are usually preceded by premonitory urges.1,2 Usually, the tics are repetitive, sudden, stereotypical, non-rhythmic movements and/or vocalizations.3,4 Individuals with TS and other tic disorders often experience impulsivity, aggression, obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder, and various mood and anxiety disorders.3 Psychosocial issues may include having low self-esteem, increased family conflict, and poor social skills. Males are affected 3 to 5 times more often than females.3
There is no definitive treatment for TS. Commonly used interventions are pharmacotherapy and/or behavioral therapy, which includes supportive psychotherapy, habit reversal training, exposure with response prevention, relaxation therapy, cognitive-behavioral therapy, and self-monitoring. Pharmacotherapy for TS and other tic disorders consists mainly of antipsychotics such as haloperidol, pimozide, and aripiprazole, and alpha-2 agonists (guanfacine and clonidine).4,8-10 Unfortunately, not all children respond to these medications, and these agents are associated with multiple adverse effects.11 Therefore, there is a need for additional treatment options for patients with TS and other tic disorders, especially those who are not helped by conventional treatments.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive therapeutic technique in which high-intensity magnetic impulses are delivered through an electromagnetic coil placed on the patient’s scalp to stimulate cortical neurons. The effect is determined by various parameters, including the intensity, frequency, pulse number, duration, coil location, and type of coil.3,8
rTMS is FDA-approved for treating depression, and has been used to treat anxiety disorders, Parkinson’s disease, chronic pain syndromes, and dystonia.12,13 Researchers have begun to evaluate the usefulness of rTMS for patients with TS or other tic disorders. In this article, we review the findings of 11 studies—9 clinical trials and 2 case studies—that evaluated rTMS as a treatment option for patients with tic disorders.
A proposed mechanism of action
TS is believed to be caused by multiple factors, including neurotransmitter imbalances and genetic, environmental, and psychosocial factors.14 Evidence strongly suggests the involvement of the motor cortex, basal ganglia, and reticular activating system in the expression of TS.2,15-17
Researchers have consistently identified networks of regions in the brain, including the supplementary motor area (SMA), that are active in the seconds before tics occur in patients with these disorders.6,18-22 The SMA modulates the way information is channeled between motor circuits, the limbic system, and the cognitive processes.3,23-26 The SMA can be used as a target for focal brain stimulation to modulate activity in those circuits and improve symptoms in resistant patients. Recent rTMS studies that targeted the SMA have found that stimulation to this area may be an effective way to treat TS.19,20,23,27
Continue to: rTMS for tics: Mixed evidence
rTMS for tics: Mixed evidence
We reviewed the results of 11 studies that described the use of rTMS for TS and other tic disorders (Table 11,24-26,28,29 and Table 23,8,23,27,30,31). They included:
- 2 double-blind, randomized controlled trials28,29
- 2 single-blind trials24-26
- 1 double-blind trial with an open-label extension1
- 4 open-label studies3,8,23,30
- 1 case series27 and 1 case report.31
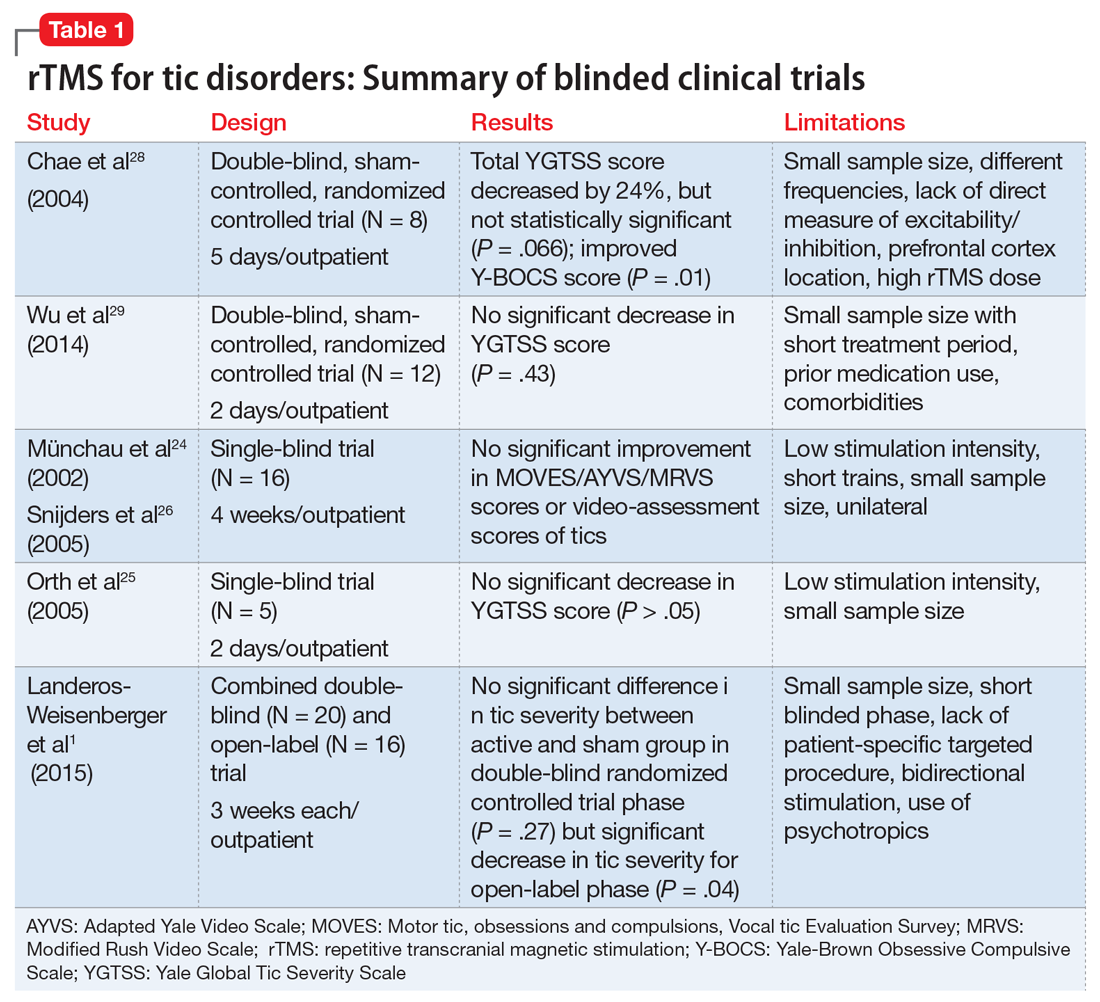
Study characteristics. In the 11 studies we reviewed, the duration of rTMS treatment varied from 2 days to 4 weeks. The pulses used were 900, 1,200, 1,800, and 2,400 per day, and the frequencies were 1 Hz, 4 Hz, 15 Hz and 30 Hz. Seven studies did not use placebo- or sham-controlled arms.1,3,8,23,27,30,31
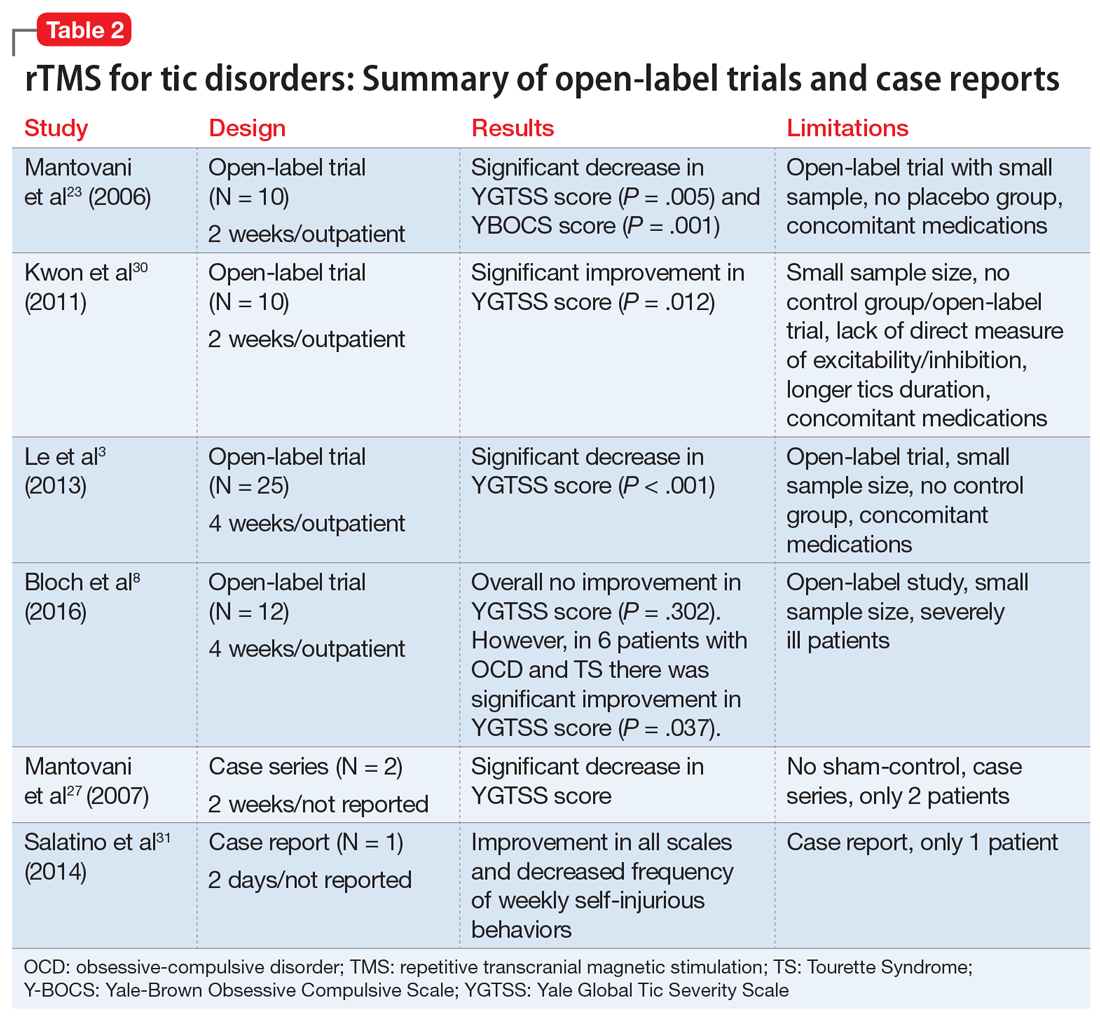
Efficacy. Two double-blind trials28,29 found no significant improvement in tic severity in patients treated with rTMS (P = .066 and P = .43, respectively). In addition, the 2 single-blind studies showed no beneficial effects of rTMS for patients with tics (P > .05).24-26 However, 3 of the 4 open-label studies found a significant improvement in tics.3,23,30 In one of the double-blind trials, researchers added an open-label extension phase.1 They found no significant results in the double-blind phase of the study (P = .27), but in the open-label phase, patients experienced a significant improvement in tic severity (P = .04).1 Lastly, the case series and case report found an improvement in tic severity and improvement in TS symptoms, respectively, with rTMS treatment.
rTMS might also improve symptoms of OCD that may co-occur with TS.8,23,28 Two studies found significant improvement in tic severity in a subgroup of patients suffering from comorbid OCD.8,28
Continue to: Safety profile and adverse effects
Safety profile and adverse effects. In the studies we reviewed, the adverse effects associated with rTMS included headache (45%),1,8,24,26,28,29 scalp pain (18%),8,30 self-injurious crisis (9%),31 abdominal pain (9%),29 red eyes (9%),29 neck pain (9%),1 muscle sprain (9%),1 tiredness (9%),24,26 and increase in motor excitability (9%).28 There were no severe adverse effects reported in any of the studies. The self-injurious crisis reported by a patient early in one study as a seizure was later ruled out after careful clinical and electroencephalographic evaluation. This patient demonstrated self-injurious behaviors prior to the treatment, and overall there was a reduction in frequency and intensity of self-injurious behavior as well as an improvement in tics.31
Dissimilar studies
There was great heterogeneity among the 11 studies we reviewed. One case series27 and one case report31 found significant improvement in tics, but these studies did not have control groups. Both studies employed rTMS with a frequency of 1 Hz and between 900 to 1,200 pulses per day. Three open-label studies that found significant improvement in tic severity used the same frequency of stimulation (1 Hz with 1,200 pulses per day).3,23,30 All studies we analyzed differed in the total number of rTMS sessions and number of trains per stimulation.
The studies also differed in terms of the age of the participants. Some studies focused primarily on pediatric patients,3,30 but many of them also included adults. The main limitations of the 11 studies included a small sample size,1,3,8,23-25,28-30 no placebo or controlled arm,1,3,8,23,27,30,31 concomitant psychiatric comorbidities8,28,29 or medications,1,3,23,29,30 low stimulation intensity,24-26 and use of short trains24,26 or unilateral cerebral stimulation.24,26 Among the blinded studies, limitations included a small sample size, prior medications used, comorbidities, low stimulation intensity, and high rTMS dose.1,24-26,28,29
A possible option for treatment-resistant tics
We cannot offer a definitive conclusion on the safety and effectiveness of rTMS for the treatment of TS and other tic disorders because of the inconsistent results, heterogeneity, and small sample sizes of the studies we analyzed. Higher-quality studies failed to find evidence supporting the use of rTMS for treating TS and other tics disorders, but open-label studies and case reports found significant improvements. In light of this evidence and the treatment’s relatively favorable adverse-effects profile, rTMS might be an option for certain patients with treatment-resistant tics, particularly those with comorbid OCD symptoms.
Continue to: Bottom Line
Bottom Line
The evidence for using repetitive transcranial stimulation (rTMS) to treat patients with Tourette syndrome and other tic disorders is mixed. Higher-quality studies have found no significant improvements, whereas open-label studies and case studies have. Although not recommended for the routine treatment of tic disorders, rTMS may be an option for patients with treatment-resistant tics, particularly those with comorbid obsessive-compulsive symptoms.
Related Resources
- Tourette Association of America. https://www.tourette.org/.
- Harris E. Children with tic disorders: How to match treatment with symptoms. Current Psychiatry. 2010;9(3):29-36.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres, Duraclon
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Pimozide • Orap
1. Landeros-Weisenberger A, Mantovani A, Motlagh MG, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimulat. 2015;8(3):574-581.
2. Kamble N, Netravathi M, Pal PK. Therapeutic applications of repetitive transcranial magnetic stimulation (rTMS) in movement disorders: a review. Parkinsonism Relat Disord. 2014;20(7):695-707.
3. Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257-262.
4. Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi:10.1136/bmj.f4964.
5. Leckman JF, Bloch MH, Scahill L, et al. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642-649.
6. Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65-69.
7. Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119-125.
8. Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. 2016;17(7):557-561.
9. Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97(5):166-175.
10. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
11. Du JC, Chiu TF, Lee KM, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51(5):255-264.
12. Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13(4):372-378.
13. Di Lazzaro V, Oliviero A, Berardelli A, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144(4):549-553.
14. Olson LL, Singer HS, Goodman WK, et al. Tourette syndrome: diagnosis, strategies, therapies, pathogenesis, and future research directions. J Child Neurol. 2006;21(8):630-641.
15. Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res. 2003;55(1):13-22.
16. Kurlan R. Hypothesis II: Tourette’s syndrome is part of a clinical spectrum that includes normal brain development. Arch Neurol. 1994;51(11):1145-1150.
17. Peterson BS. Neuroimaging in child and adolescent neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 1995;34(12):1560-1576.
18. Sheppard DM, Bradshaw JL, Purcell R, et al. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19(5):531-552.
19. Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(pt 8):2029-2037.
20. Hampson M, Tokoglu F, King RA, et al. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65(7):594-599.
21. Eichele H, Plessen KJ. Neural plasticity in functional and anatomical MRI studies of children with Tourette syndrome. Behav Neurol. 2013;27(1):33-45.
22. Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol. 2013;112:35-71.
23. Mantovani A, Lisanby SH, Pieraccini F, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. 2006;9(1):95-100.
24. Münchau A, Bloem BR, Thilo KV, et al. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59(11):1789-1791.
25. Orth M, Kirby R, Richardson MP, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. 2005;116(4):764-768.
26. Snijders AH, Bloem BR, Orth M, et al. Video assessment of rTMS for Tourette syndrome. J Neurol Neurosurg Psychiatry. 2005;76(12):1743-1744.
27. Mantovani A, Leckman JF, Grantz H, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118(10):2314-2315.
28. Chae JH, Nahas Z, Wassermann E, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol. 2004;17(2):109-117.
29. Wu SW, Maloney T, Gilbert DL, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7(2):212-218.
30. Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1-4.
31. Salatino A, Momo E, Nobili M, et al. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimulat. 2014;7(2):341-343.
Tourette syndrome (TS) is a chronic neuropsychiatric disorder occurring in early childhood or adolescence that’s characterized by multiple motor and vocal tics that are usually preceded by premonitory urges.1,2 Usually, the tics are repetitive, sudden, stereotypical, non-rhythmic movements and/or vocalizations.3,4 Individuals with TS and other tic disorders often experience impulsivity, aggression, obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder, and various mood and anxiety disorders.3 Psychosocial issues may include having low self-esteem, increased family conflict, and poor social skills. Males are affected 3 to 5 times more often than females.3
There is no definitive treatment for TS. Commonly used interventions are pharmacotherapy and/or behavioral therapy, which includes supportive psychotherapy, habit reversal training, exposure with response prevention, relaxation therapy, cognitive-behavioral therapy, and self-monitoring. Pharmacotherapy for TS and other tic disorders consists mainly of antipsychotics such as haloperidol, pimozide, and aripiprazole, and alpha-2 agonists (guanfacine and clonidine).4,8-10 Unfortunately, not all children respond to these medications, and these agents are associated with multiple adverse effects.11 Therefore, there is a need for additional treatment options for patients with TS and other tic disorders, especially those who are not helped by conventional treatments.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive therapeutic technique in which high-intensity magnetic impulses are delivered through an electromagnetic coil placed on the patient’s scalp to stimulate cortical neurons. The effect is determined by various parameters, including the intensity, frequency, pulse number, duration, coil location, and type of coil.3,8
rTMS is FDA-approved for treating depression, and has been used to treat anxiety disorders, Parkinson’s disease, chronic pain syndromes, and dystonia.12,13 Researchers have begun to evaluate the usefulness of rTMS for patients with TS or other tic disorders. In this article, we review the findings of 11 studies—9 clinical trials and 2 case studies—that evaluated rTMS as a treatment option for patients with tic disorders.
A proposed mechanism of action
TS is believed to be caused by multiple factors, including neurotransmitter imbalances and genetic, environmental, and psychosocial factors.14 Evidence strongly suggests the involvement of the motor cortex, basal ganglia, and reticular activating system in the expression of TS.2,15-17
Researchers have consistently identified networks of regions in the brain, including the supplementary motor area (SMA), that are active in the seconds before tics occur in patients with these disorders.6,18-22 The SMA modulates the way information is channeled between motor circuits, the limbic system, and the cognitive processes.3,23-26 The SMA can be used as a target for focal brain stimulation to modulate activity in those circuits and improve symptoms in resistant patients. Recent rTMS studies that targeted the SMA have found that stimulation to this area may be an effective way to treat TS.19,20,23,27
Continue to: rTMS for tics: Mixed evidence
rTMS for tics: Mixed evidence
We reviewed the results of 11 studies that described the use of rTMS for TS and other tic disorders (Table 11,24-26,28,29 and Table 23,8,23,27,30,31). They included:
- 2 double-blind, randomized controlled trials28,29
- 2 single-blind trials24-26
- 1 double-blind trial with an open-label extension1
- 4 open-label studies3,8,23,30
- 1 case series27 and 1 case report.31

Study characteristics. In the 11 studies we reviewed, the duration of rTMS treatment varied from 2 days to 4 weeks. The pulses used were 900, 1,200, 1,800, and 2,400 per day, and the frequencies were 1 Hz, 4 Hz, 15 Hz and 30 Hz. Seven studies did not use placebo- or sham-controlled arms.1,3,8,23,27,30,31

Efficacy. Two double-blind trials28,29 found no significant improvement in tic severity in patients treated with rTMS (P = .066 and P = .43, respectively). In addition, the 2 single-blind studies showed no beneficial effects of rTMS for patients with tics (P > .05).24-26 However, 3 of the 4 open-label studies found a significant improvement in tics.3,23,30 In one of the double-blind trials, researchers added an open-label extension phase.1 They found no significant results in the double-blind phase of the study (P = .27), but in the open-label phase, patients experienced a significant improvement in tic severity (P = .04).1 Lastly, the case series and case report found an improvement in tic severity and improvement in TS symptoms, respectively, with rTMS treatment.
rTMS might also improve symptoms of OCD that may co-occur with TS.8,23,28 Two studies found significant improvement in tic severity in a subgroup of patients suffering from comorbid OCD.8,28
Continue to: Safety profile and adverse effects
Safety profile and adverse effects. In the studies we reviewed, the adverse effects associated with rTMS included headache (45%),1,8,24,26,28,29 scalp pain (18%),8,30 self-injurious crisis (9%),31 abdominal pain (9%),29 red eyes (9%),29 neck pain (9%),1 muscle sprain (9%),1 tiredness (9%),24,26 and increase in motor excitability (9%).28 There were no severe adverse effects reported in any of the studies. The self-injurious crisis reported by a patient early in one study as a seizure was later ruled out after careful clinical and electroencephalographic evaluation. This patient demonstrated self-injurious behaviors prior to the treatment, and overall there was a reduction in frequency and intensity of self-injurious behavior as well as an improvement in tics.31
Dissimilar studies
There was great heterogeneity among the 11 studies we reviewed. One case series27 and one case report31 found significant improvement in tics, but these studies did not have control groups. Both studies employed rTMS with a frequency of 1 Hz and between 900 to 1,200 pulses per day. Three open-label studies that found significant improvement in tic severity used the same frequency of stimulation (1 Hz with 1,200 pulses per day).3,23,30 All studies we analyzed differed in the total number of rTMS sessions and number of trains per stimulation.
The studies also differed in terms of the age of the participants. Some studies focused primarily on pediatric patients,3,30 but many of them also included adults. The main limitations of the 11 studies included a small sample size,1,3,8,23-25,28-30 no placebo or controlled arm,1,3,8,23,27,30,31 concomitant psychiatric comorbidities8,28,29 or medications,1,3,23,29,30 low stimulation intensity,24-26 and use of short trains24,26 or unilateral cerebral stimulation.24,26 Among the blinded studies, limitations included a small sample size, prior medications used, comorbidities, low stimulation intensity, and high rTMS dose.1,24-26,28,29
A possible option for treatment-resistant tics
We cannot offer a definitive conclusion on the safety and effectiveness of rTMS for the treatment of TS and other tic disorders because of the inconsistent results, heterogeneity, and small sample sizes of the studies we analyzed. Higher-quality studies failed to find evidence supporting the use of rTMS for treating TS and other tics disorders, but open-label studies and case reports found significant improvements. In light of this evidence and the treatment’s relatively favorable adverse-effects profile, rTMS might be an option for certain patients with treatment-resistant tics, particularly those with comorbid OCD symptoms.
Continue to: Bottom Line
Bottom Line
The evidence for using repetitive transcranial stimulation (rTMS) to treat patients with Tourette syndrome and other tic disorders is mixed. Higher-quality studies have found no significant improvements, whereas open-label studies and case studies have. Although not recommended for the routine treatment of tic disorders, rTMS may be an option for patients with treatment-resistant tics, particularly those with comorbid obsessive-compulsive symptoms.
Related Resources
- Tourette Association of America. https://www.tourette.org/.
- Harris E. Children with tic disorders: How to match treatment with symptoms. Current Psychiatry. 2010;9(3):29-36.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres, Duraclon
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Pimozide • Orap
Tourette syndrome (TS) is a chronic neuropsychiatric disorder occurring in early childhood or adolescence that’s characterized by multiple motor and vocal tics that are usually preceded by premonitory urges.1,2 Usually, the tics are repetitive, sudden, stereotypical, non-rhythmic movements and/or vocalizations.3,4 Individuals with TS and other tic disorders often experience impulsivity, aggression, obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder, and various mood and anxiety disorders.3 Psychosocial issues may include having low self-esteem, increased family conflict, and poor social skills. Males are affected 3 to 5 times more often than females.3
There is no definitive treatment for TS. Commonly used interventions are pharmacotherapy and/or behavioral therapy, which includes supportive psychotherapy, habit reversal training, exposure with response prevention, relaxation therapy, cognitive-behavioral therapy, and self-monitoring. Pharmacotherapy for TS and other tic disorders consists mainly of antipsychotics such as haloperidol, pimozide, and aripiprazole, and alpha-2 agonists (guanfacine and clonidine).4,8-10 Unfortunately, not all children respond to these medications, and these agents are associated with multiple adverse effects.11 Therefore, there is a need for additional treatment options for patients with TS and other tic disorders, especially those who are not helped by conventional treatments.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive therapeutic technique in which high-intensity magnetic impulses are delivered through an electromagnetic coil placed on the patient’s scalp to stimulate cortical neurons. The effect is determined by various parameters, including the intensity, frequency, pulse number, duration, coil location, and type of coil.3,8
rTMS is FDA-approved for treating depression, and has been used to treat anxiety disorders, Parkinson’s disease, chronic pain syndromes, and dystonia.12,13 Researchers have begun to evaluate the usefulness of rTMS for patients with TS or other tic disorders. In this article, we review the findings of 11 studies—9 clinical trials and 2 case studies—that evaluated rTMS as a treatment option for patients with tic disorders.
A proposed mechanism of action
TS is believed to be caused by multiple factors, including neurotransmitter imbalances and genetic, environmental, and psychosocial factors.14 Evidence strongly suggests the involvement of the motor cortex, basal ganglia, and reticular activating system in the expression of TS.2,15-17
Researchers have consistently identified networks of regions in the brain, including the supplementary motor area (SMA), that are active in the seconds before tics occur in patients with these disorders.6,18-22 The SMA modulates the way information is channeled between motor circuits, the limbic system, and the cognitive processes.3,23-26 The SMA can be used as a target for focal brain stimulation to modulate activity in those circuits and improve symptoms in resistant patients. Recent rTMS studies that targeted the SMA have found that stimulation to this area may be an effective way to treat TS.19,20,23,27
Continue to: rTMS for tics: Mixed evidence
rTMS for tics: Mixed evidence
We reviewed the results of 11 studies that described the use of rTMS for TS and other tic disorders (Table 11,24-26,28,29 and Table 23,8,23,27,30,31). They included:
- 2 double-blind, randomized controlled trials28,29
- 2 single-blind trials24-26
- 1 double-blind trial with an open-label extension1
- 4 open-label studies3,8,23,30
- 1 case series27 and 1 case report.31

Study characteristics. In the 11 studies we reviewed, the duration of rTMS treatment varied from 2 days to 4 weeks. The pulses used were 900, 1,200, 1,800, and 2,400 per day, and the frequencies were 1 Hz, 4 Hz, 15 Hz and 30 Hz. Seven studies did not use placebo- or sham-controlled arms.1,3,8,23,27,30,31

Efficacy. Two double-blind trials28,29 found no significant improvement in tic severity in patients treated with rTMS (P = .066 and P = .43, respectively). In addition, the 2 single-blind studies showed no beneficial effects of rTMS for patients with tics (P > .05).24-26 However, 3 of the 4 open-label studies found a significant improvement in tics.3,23,30 In one of the double-blind trials, researchers added an open-label extension phase.1 They found no significant results in the double-blind phase of the study (P = .27), but in the open-label phase, patients experienced a significant improvement in tic severity (P = .04).1 Lastly, the case series and case report found an improvement in tic severity and improvement in TS symptoms, respectively, with rTMS treatment.
rTMS might also improve symptoms of OCD that may co-occur with TS.8,23,28 Two studies found significant improvement in tic severity in a subgroup of patients suffering from comorbid OCD.8,28
Continue to: Safety profile and adverse effects
Safety profile and adverse effects. In the studies we reviewed, the adverse effects associated with rTMS included headache (45%),1,8,24,26,28,29 scalp pain (18%),8,30 self-injurious crisis (9%),31 abdominal pain (9%),29 red eyes (9%),29 neck pain (9%),1 muscle sprain (9%),1 tiredness (9%),24,26 and increase in motor excitability (9%).28 There were no severe adverse effects reported in any of the studies. The self-injurious crisis reported by a patient early in one study as a seizure was later ruled out after careful clinical and electroencephalographic evaluation. This patient demonstrated self-injurious behaviors prior to the treatment, and overall there was a reduction in frequency and intensity of self-injurious behavior as well as an improvement in tics.31
Dissimilar studies
There was great heterogeneity among the 11 studies we reviewed. One case series27 and one case report31 found significant improvement in tics, but these studies did not have control groups. Both studies employed rTMS with a frequency of 1 Hz and between 900 to 1,200 pulses per day. Three open-label studies that found significant improvement in tic severity used the same frequency of stimulation (1 Hz with 1,200 pulses per day).3,23,30 All studies we analyzed differed in the total number of rTMS sessions and number of trains per stimulation.
The studies also differed in terms of the age of the participants. Some studies focused primarily on pediatric patients,3,30 but many of them also included adults. The main limitations of the 11 studies included a small sample size,1,3,8,23-25,28-30 no placebo or controlled arm,1,3,8,23,27,30,31 concomitant psychiatric comorbidities8,28,29 or medications,1,3,23,29,30 low stimulation intensity,24-26 and use of short trains24,26 or unilateral cerebral stimulation.24,26 Among the blinded studies, limitations included a small sample size, prior medications used, comorbidities, low stimulation intensity, and high rTMS dose.1,24-26,28,29
A possible option for treatment-resistant tics
We cannot offer a definitive conclusion on the safety and effectiveness of rTMS for the treatment of TS and other tic disorders because of the inconsistent results, heterogeneity, and small sample sizes of the studies we analyzed. Higher-quality studies failed to find evidence supporting the use of rTMS for treating TS and other tics disorders, but open-label studies and case reports found significant improvements. In light of this evidence and the treatment’s relatively favorable adverse-effects profile, rTMS might be an option for certain patients with treatment-resistant tics, particularly those with comorbid OCD symptoms.
Continue to: Bottom Line
Bottom Line
The evidence for using repetitive transcranial stimulation (rTMS) to treat patients with Tourette syndrome and other tic disorders is mixed. Higher-quality studies have found no significant improvements, whereas open-label studies and case studies have. Although not recommended for the routine treatment of tic disorders, rTMS may be an option for patients with treatment-resistant tics, particularly those with comorbid obsessive-compulsive symptoms.
Related Resources
- Tourette Association of America. https://www.tourette.org/.
- Harris E. Children with tic disorders: How to match treatment with symptoms. Current Psychiatry. 2010;9(3):29-36.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres, Duraclon
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Pimozide • Orap
1. Landeros-Weisenberger A, Mantovani A, Motlagh MG, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimulat. 2015;8(3):574-581.
2. Kamble N, Netravathi M, Pal PK. Therapeutic applications of repetitive transcranial magnetic stimulation (rTMS) in movement disorders: a review. Parkinsonism Relat Disord. 2014;20(7):695-707.
3. Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257-262.
4. Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi:10.1136/bmj.f4964.
5. Leckman JF, Bloch MH, Scahill L, et al. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642-649.
6. Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65-69.
7. Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119-125.
8. Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. 2016;17(7):557-561.
9. Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97(5):166-175.
10. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
11. Du JC, Chiu TF, Lee KM, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51(5):255-264.
12. Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13(4):372-378.
13. Di Lazzaro V, Oliviero A, Berardelli A, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144(4):549-553.
14. Olson LL, Singer HS, Goodman WK, et al. Tourette syndrome: diagnosis, strategies, therapies, pathogenesis, and future research directions. J Child Neurol. 2006;21(8):630-641.
15. Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res. 2003;55(1):13-22.
16. Kurlan R. Hypothesis II: Tourette’s syndrome is part of a clinical spectrum that includes normal brain development. Arch Neurol. 1994;51(11):1145-1150.
17. Peterson BS. Neuroimaging in child and adolescent neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 1995;34(12):1560-1576.
18. Sheppard DM, Bradshaw JL, Purcell R, et al. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19(5):531-552.
19. Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(pt 8):2029-2037.
20. Hampson M, Tokoglu F, King RA, et al. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65(7):594-599.
21. Eichele H, Plessen KJ. Neural plasticity in functional and anatomical MRI studies of children with Tourette syndrome. Behav Neurol. 2013;27(1):33-45.
22. Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol. 2013;112:35-71.
23. Mantovani A, Lisanby SH, Pieraccini F, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. 2006;9(1):95-100.
24. Münchau A, Bloem BR, Thilo KV, et al. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59(11):1789-1791.
25. Orth M, Kirby R, Richardson MP, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. 2005;116(4):764-768.
26. Snijders AH, Bloem BR, Orth M, et al. Video assessment of rTMS for Tourette syndrome. J Neurol Neurosurg Psychiatry. 2005;76(12):1743-1744.
27. Mantovani A, Leckman JF, Grantz H, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118(10):2314-2315.
28. Chae JH, Nahas Z, Wassermann E, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol. 2004;17(2):109-117.
29. Wu SW, Maloney T, Gilbert DL, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7(2):212-218.
30. Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1-4.
31. Salatino A, Momo E, Nobili M, et al. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimulat. 2014;7(2):341-343.
1. Landeros-Weisenberger A, Mantovani A, Motlagh MG, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimulat. 2015;8(3):574-581.
2. Kamble N, Netravathi M, Pal PK. Therapeutic applications of repetitive transcranial magnetic stimulation (rTMS) in movement disorders: a review. Parkinsonism Relat Disord. 2014;20(7):695-707.
3. Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257-262.
4. Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi:10.1136/bmj.f4964.
5. Leckman JF, Bloch MH, Scahill L, et al. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642-649.
6. Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65-69.
7. Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119-125.
8. Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. 2016;17(7):557-561.
9. Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97(5):166-175.
10. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
11. Du JC, Chiu TF, Lee KM, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51(5):255-264.
12. Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13(4):372-378.
13. Di Lazzaro V, Oliviero A, Berardelli A, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144(4):549-553.
14. Olson LL, Singer HS, Goodman WK, et al. Tourette syndrome: diagnosis, strategies, therapies, pathogenesis, and future research directions. J Child Neurol. 2006;21(8):630-641.
15. Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res. 2003;55(1):13-22.
16. Kurlan R. Hypothesis II: Tourette’s syndrome is part of a clinical spectrum that includes normal brain development. Arch Neurol. 1994;51(11):1145-1150.
17. Peterson BS. Neuroimaging in child and adolescent neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 1995;34(12):1560-1576.
18. Sheppard DM, Bradshaw JL, Purcell R, et al. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19(5):531-552.
19. Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(pt 8):2029-2037.
20. Hampson M, Tokoglu F, King RA, et al. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65(7):594-599.
21. Eichele H, Plessen KJ. Neural plasticity in functional and anatomical MRI studies of children with Tourette syndrome. Behav Neurol. 2013;27(1):33-45.
22. Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol. 2013;112:35-71.
23. Mantovani A, Lisanby SH, Pieraccini F, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. 2006;9(1):95-100.
24. Münchau A, Bloem BR, Thilo KV, et al. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59(11):1789-1791.
25. Orth M, Kirby R, Richardson MP, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. 2005;116(4):764-768.
26. Snijders AH, Bloem BR, Orth M, et al. Video assessment of rTMS for Tourette syndrome. J Neurol Neurosurg Psychiatry. 2005;76(12):1743-1744.
27. Mantovani A, Leckman JF, Grantz H, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118(10):2314-2315.
28. Chae JH, Nahas Z, Wassermann E, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol. 2004;17(2):109-117.
29. Wu SW, Maloney T, Gilbert DL, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7(2):212-218.
30. Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1-4.
31. Salatino A, Momo E, Nobili M, et al. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimulat. 2014;7(2):341-343.