User login
Options for managing severe aortic stenosis: A case-based review
Surgical aortic valve replacement remains the gold standard treatment for symptomatic aortic valve stenosis in patients at low or moderate risk of surgical complications. But this is a disease of the elderly, many of whom are too frail or too sick to undergo surgery.
Now, patients who cannot undergo this surgery can be offered the less invasive option of transcatheter aortic valve replacement. Balloon valvuloplasty, sodium nitroprusside, and intra-aortic balloon counterpulsation can buy time for ill patients while more permanent mechanical interventions are being considered.
In this review, we will present several cases that highlight management choices for patients with severe aortic stenosis.
A PROGRESSIVE DISEASE OF THE ELDERLY
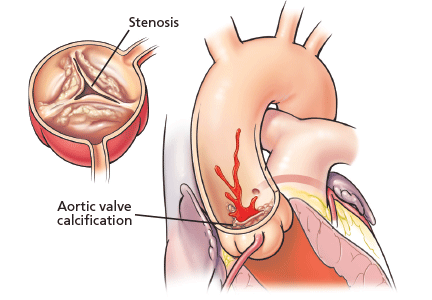
Aortic stenosis is the most common acquired valvular disease in the United States, and its incidence and prevalence are rising as the population ages. Epidemiologic studies suggest that 2% to 7% of all patients over age 65 have it.1,2
The natural history of the untreated disease is well established, with several case series showing an average decrease of 0.1 cm2 per year in aortic valve area and an increase of 7 mm Hg per year in the pressure gradient across the valve once the diagnosis is made.3,4 Development of angina, syncope, or heart failure is associated with adverse clinical outcomes, including death, and warrants prompt intervention with aortic valve replacement.5–7 Without intervention, the mortality rates reach as high as 75% in 3 years once symptoms develop.
Statins, bisphosphonates, and angiotensin-converting enzyme inhibitors have been used in attempts to slow or reverse the progression of aortic stenosis. However, studies of these drugs have had mixed results, and no definitive benefit has been shown.8–13 Surgical aortic valve replacement, on the other hand, normalizes the life expectancy of patients with aortic stenosis to that of age- and sex-matched controls and remains the gold standard therapy for patients who have symptoms.14
Traditionally, valve replacement has involved open heart surgery, since it requires direct visualization of the valve while the patient is on cardiopulmonary bypass. Unfortunately, many patients have multiple comorbid conditions and therefore are not candidates for open heart surgery. Options for these patients include aortic valvuloplasty and transcatheter aortic valve replacement. While there is considerable experience with aortic valvuloplasty, transcatheter aortic valve replacement is relatively new. In large randomized trials and registries, the transcatheter procedure has been shown to significantly improve long-term survival compared with medical management alone in inoperable patients and to have benefit similar to that of surgery in the high-risk population.15–17
CASE 1: SEVERE, SYMPTOMATIC STENOSIS IN A GOOD SURGICAL CANDIDATE
Mr. A, age 83, presents with shortness of breath and peripheral edema that have been worsening over the past several months. His pulse rate is 64 beats per minute and his blood pressure is 110/90 mm Hg. Auscultation reveals an absent aortic second heart sound with a late peaking systolic murmur that increases with expiration.
On echocardiography, his left ventricular ejection fraction is 55%, peak transaortic valve gradient 88 mm Hg, mean gradient 60 mm Hg, and effective valve area 0.6 cm2. He undergoes catheterization of the left side of his heart, which shows normal coronary arteries.
Mr. A also has hypertension and hyperlipidemia; his renal and pulmonary functions are normal.
How would you manage Mr. A’s aortic stenosis?
Symptomatic aortic stenosis leads to adverse clinical outcomes if managed medically without mechanical intervention,5–7 but patients who undergo aortic valve replacement have age-corrected postoperative survival rates that are nearly normal.14 Furthermore, thanks to improvements in surgical techniques and perioperative management, surgical mortality rates have decreased significantly in recent years and now range from 1% to 8%.18–20 The accumulated evidence showing clear superiority of a surgical approach over medical therapy has greatly simplified the therapeutic algorithm.21
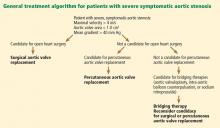
Consequently, the current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) give surgery a class I indication (evidence or general agreement that the procedure is beneficial, useful, and effective) for symptomatic severe aortic stenosis (Figure 1). This level of recommendation also applies to patients who have severe but asymptomatic aortic stenosis who are undergoing other types of cardiac surgery and also to patients with severe aortic stenosis and left ventricular dysfunction (defined as an ejection fraction < 50%).21
Mr. A was referred for surgical aortic valve replacement, given its clear survival benefit.
CASE 2: SYMPTOMS AND LEFT VENTRICULAR DYSFUNCTION
Ms. B, age 79, has hypertension and hyperlipidemia and now presents to the outpatient department with worsening shortness of breath and chest discomfort. Electrocardiography shows significant left ventricular hypertrophy and abnormal repolarization. Left heart catheterization reveals mild nonobstructive coronary artery disease.
Echocardiography reveals an ejection fraction of 25%, severe left ventricular hypertrophy, and global hypokinesis. The aortic valve leaflets appear heavily calcified, with restricted motion. The peak and mean gradients across the aortic valve are 40 and 28 mm Hg, and the valve area is 0.8 cm2. Right heart catheterization shows a cardiac output of 3.1 L/min.
Does this patient’s aortic stenosis account for her clinical presentation?
Managing patients who have suspected severe aortic stenosis, left ventricular dysfunction, and low aortic valve gradients can be challenging. Although data for surgical intervention are not as robust for these patient subsets as for patients like Mr. A, several case series have suggested that survival in these patients is significantly better with surgery than with medical therapy alone.22–27
Specific factors predict whether patients with ventricular dysfunction and low gradients will benefit from aortic valve replacement. Dobutamine stress echocardiography is helpful in distinguishing true severe aortic stenosis from “pseudostenosis,” in which leaflet motion is restricted due to primary cardiomyopathy and low flow. Distinguishing between true aortic stenosis and pseudostenosis is of paramount value, as surgery is associated with improved long-term outcomes in patients with true aortic stenosis (even though they are at higher surgical risk), whereas those with pseudostenosis will not benefit from surgery.28–31
Infusion of dobutamine increases the flow across the aortic valve (if the left ventricle has contractile reserve; more on this below), and an increasing valve area with increasing doses of dobutamine is consistent with pseudostenosis. In this situation, treatment of the underlying cardiomyopathy is indicated as opposed to replacement of the aortic valve (Figure 2).
Contractile reserve is defined as an increase in stroke volume (> 20%), valvular gradient (> 10 mm Hg), or peak velocity (> 0.6 m/s) with peak dobutamine infusion. The presence of contractile reserve in patients with aortic stenosis identifies a high-risk group that benefits from aortic valve replacement (Figure 2).
Treatment of patients who have inadequate reserve is controversial. In the absence of contractile reserve, an adjunct imaging study such as computed tomography may be of value in detecting calcified valve leaflets, as the presence of calcium is associated with true aortic stenosis. Comorbid conditions should be taken into account as well, given the higher surgical risk in this patient subset, as aortic valve replacement in this already high-risk group of patients might be futile in some cases.
The ACC/AHA guidelines now give dobutamine stress echocardiography a class IIa indication (meaning the weight of the evidence or opinion is in favor of usefulness or efficacy) for determination of contractile reserve and valvular stenosis for patients with an ejection fraction of 30% or less or a mean gradient of 40 mm Hg or less.21
Ms. B underwent dobutamine stress echocardiography. It showed increases in ejection fraction, stroke volume, and transvalvular gradients, indicating that she did have contractile reserve and true severe aortic stenosis. Consequently, she was referred for surgical aortic valve replacement.
CASE 3: MODERATE STENOSIS AND THREE-VESSEL CORONARY ARTERY DISEASE
Mr. C, age 81, has hypertension and hyperlipidemia. He now presents to the emergency department with chest discomfort that began suddenly, awakening him from sleep. His presenting electrocardiogram shows nonspecific changes, and he is diagnosed with non-ST-elevation myocardial infarction. He undergoes left heart catheterization, which reveals severe three-vessel coronary artery disease.
Echocardiography reveals an ejection fraction of 55% and aortic stenosis, with an aortic valve area of 1.2 cm2, a peak gradient of 44 mm Hg, and a mean gradient of 28 mm Hg.
How would you manage his aortic stenosis?
Moderate aortic stenosis in a patient who needs surgery for severe triple-vessel coronary artery disease, other valve diseases, or aortic disease raises the question of whether aortic valve replacement should be performed in conjunction with these surgeries. Although these patients would not otherwise qualify for aortic valve replacement, the fact that they will undergo a procedure that will expose them to the risks associated with open heart surgery makes them reasonable candidates. Even if the patient does not need aortic valve replacement right now, aortic stenosis progresses at a predictable rate—the valve area decreases by a mean of 0.1 cm2/year and the gradients increase by 7 mm Hg/year. Therefore, clinical judgment should be exercised so that the patient will not need to undergo open heart surgery again in the near future.
The ACC/AHA guidelines recommend aortic valve replacement for patients with moderate aortic stenosis undergoing coronary artery bypass grafting or surgery on the aorta or other heart valves, giving it a class IIa indication.21 This recommendation is based on several retrospective case series that evaluated survival, the need for reoperation for aortic valve replacement, or both in patients undergoing coronary artery bypass grafting.32–35
No data exist, however, on adding aortic valve replacement to coronary artery bypass grafting in cases of mild aortic stenosis. As a result, it is controversial and carries a class IIb recommendation (meaning that its usefulness or efficacy is less well established). The ACC/AHA guidelines state that aortic valve replacement “may be considered” in patients undergoing coronary artery bypass grafting who have mild aortic stenosis (mean gradient < 30 mm Hg or jet velocity < 3 m/s) when there is evidence, such as moderate or severe valve calcification, that progression may be rapid (level of evidence C: based only on consensus opinion of experts, case studies or standard of care).21
Mr. C, who has moderate aortic stenosis, underwent aortic valve replacement in conjunction with three-vessel bypass grafting.
CASE 4: ASYMPTOMATIC BUT SEVERE STENOSIS
Mr. D, age 74, has hypertension, hyperlipidemia, and aortic stenosis. He now presents to the outpatient department for his annual echocardiogram to follow his aortic stenosis. He has a sedentary lifestyle but feels well performing activities of daily living. He denies dyspnea on exertion, chest pain, or syncope.
His echocardiogram reveals an effective aortic valve area of 0.7 cm2, peak gradient 90 mm Hg, and mean gradient 70 mm Hg. There is evidence of severe left ventricular hypertrophy, and the valve leaflets show bulky calcification and severe restriction. An echocardiogram performed at the same institution a year earlier revealed gradients of 60 and 40 mm Hg.
Blood is drawn for laboratory tests, including N-terminal pro-brain natriuretic peptide, which is 350 pg/mL (reference range for his age < 125 pg/mL). He is referred for a treadmill stress test, which elicits symptoms at a moderate activity level.
How would you manage his aortic stenosis?
Aortic valve replacement can be considered in patients who have asymptomatic but severe aortic stenosis with preserved left ventricular function (class IIb indication).21
Clinical assessment of asymptomatic aortic stenosis can be challenging, however, as patients may underreport their symptoms or decrease their activity levels to avoid symptoms. Exercise testing in such patients can elicit symptoms, unmask diminished exercise capacity, and help determine if they should be referred for surgery.36,37 Natriuretic peptide levels have been shown to correlate with the severity of aortic stenosis,38,39 and more importantly, to help predict symptom onset, cardiac death, and need for aortic valve replacement.40–42
Some patients with asymptomatic but severe aortic stenosis are at higher risk of morbidity and death. High-risk subsets include patients with rapid progression of aortic stenosis and those with critical aortic stenosis characterized by an aortic valve area less than 0.60 cm2, mean gradient greater than 60 mm Hg, and jet velocity greater than 5.0 m/s. It is reasonable to offer these patients surgery if their expected operative mortality risk is less than 1.0%.21
Mr. D has evidence of rapid progression as defined by an increase in aortic jet velocity of more than 0.3 m/s/year. He is at low surgical risk and was referred for elective aortic valve replacement.
CASE 5: TOO FRAIL FOR SURGERY
Mr. E, age 84, has severe aortic stenosis (valve area 0.6 cm2, peak and mean gradients of 88 and 56 mm Hg), coronary artery disease status post coronary artery bypass grafting, moderate chronic obstructive pulmonary disease (forced expiratory volume in 1 second 0.8 L), chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, hyperlipidemia, and diabetes mellitus. He has preserved left ventricular function. He presents to the outpatient department with worsening shortness of breath and peripheral edema over the past several months. Your impression is that he is very frail. How would you manage Mr. E’s aortic stenosis?
Advances in surgical techniques and perioperative management over the years have enabled higher-risk patients to undergo surgical aortic valve replacement with excellent out-comes.18–20,43 Yet many patients still cannot undergo surgery because their risk is too high. Patients ineligible for surgery have traditionally been treated medically—with poor out-comes—or with balloon aortic valvuloplasty to palliate symptoms.
Transcatheter aortic valve replacement, approved by the US Food and Drug Administration (FDA) in 2011, now provides another option for these patients. In this procedure, a bioprosthetic valve mounted on a metal frame is implanted over the native stenotic valve.
Currently, the only FDA-approved and commercially available valve in the United States is the Edwards SAPIEN valve, which has bovine pericardial tissue leaflets fixed to a balloon-expandable stainless steel frame (Figure 3). In the Placement of Aortic Transcatheter Valves (PARTNER) trial,15 patients who could not undergo surgery who underwent transcatheter replacement with this valve had a significantly better survival rate than patients treated medically.15,17 Use of this valve has also been compared against conventional surgical aortic valve replacement in high-risk patients and was found to have similar long-term outcomes (Figure 4).16 It was on the basis of this trial that this valve was granted approval for patients who cannot undergo surgery.
The standard of care for high-risk patients remains surgical aortic valve replacement, although it remains to be seen whether transcatheter replacement will be made available as well to patients eligible for surgery in the near future. There are currently no randomized data for transcatheter aortic valve replacement in patients at moderate to low surgical risk, and these patients should not be considered for this procedure.
Although the initial studies are encouraging for patients who cannot undergo surgery and who are at high risk without it, several issues and concerns remain. Importantly, the long-term durability of the transcatheter valve and longer-term outcomes remain unknown. Furthermore, the risk of vascular complications remains high (10% to 15%), dictating the need for careful patient selection. There are also concerns about the risks of stroke and of paravalvular aortic insufficiency. These issues are being investigated and addressed, however, and we hope that with increasing operator experience and improvements in the technique, outcomes will be improved.
Which approach for transcatheter aortic valve replacement?
There are several considerations in determining a patient’s eligibility for transcatheter aortic valve replacement.
Initially, these valves were placed by a transvenous, transseptal approach, but now retrograde placement through the femoral artery has become standard. In this procedure, the device is advanced retrograde from the femoral artery through the aorta and placed across the native aortic valve under fluoroscopic and echocardiographic guidance.
Patients who are not eligible for transfemoral placement because of severe atherosclerosis, tortuosity, or ectasia of the iliofemoral artery or aorta can still undergo percutaneous treatment with a transapical approach. This is a hybrid surgical-transcatheter approach in which the valve is delivered through a sheath placed by left ventricular apical puncture.17,44
A newer approach gaining popularity is the transaortic technique, in which the ascending aorta is accessed directly through a ministernotomy and the delivery sheath is placed with a direct puncture. Other approaches are through the axillary and subclavian arteries.
Other valves are under development
Several other valves are under development and will likely change the landscape of transcatheter aortic valve replacement with improving outcomes. Valves that are available in the United States are shown in Figure 3. The CoreValve, consisting of porcine pericardial leaflets mounted on a self-expanding nitinol stent, is currently being studied in a trial in the United States, and the manufacturer (Medtronic) will seek approval when results are complete in the near future.
Mr. E was initially referred for surgery, but when deemed to be unable to undergo surgery was found to be a good candidate for transcatheter aortic valve replacement.
CASE 6: LIFE-LIMITING COMORBID ILLNESS
Mr. F, age 77, has multiple problems: severe aortic stenosis (aortic valve area 0.6 cm2; peak and mean gradients of 92 and 59 mm Hg), stage IV pancreatic cancer, coronary artery disease status post coronary artery bypass grafting, chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, and hyperlipidemia. He presents to the outpatient department with shortness of breath at rest, orthopnea, effort intolerance, and peripheral edema over the past several months.
On physical examination rales in both lung bases can be heard. Left heart catheterization shows patent bypass grafts.
How would you manage Mr. F’s aortic stenosis?
Aortic valve replacement is not considered an option in patients with noncardiac illnesses and comorbidities that are life-limiting in the near term. Under these circumstances, aortic valvuloplasty can be offered as a means of palliating symptoms or, if the comorbid conditions can be modified, as a bridge to more definitive treatment with aortic valve replacement.
Since first described in 1986,45 percutaneous aortic valvuloplasty has been studied in several case series and registries, with consistent findings. Acutely, it increases the valve area and lessens the gradients across the valve, relieving symptoms. The risk of death during the procedure ranged from 3% to 13.5% in several case series, with a 30-day survival rate greater than 85%.46 However, the hemodynamic and symptomatic improvement is only short-term, as valve area and gradients gradually worsen within several months.47,48 Consequently, balloon valvuloplasty is considered a palliative approach.
Mr. F has a potentially life-limiting illness, ie, cancer, which would make him a candidate for aortic valvuloplasty rather than replacement. He can be referred for evaluation for this procedure in hopes of palliating his symptoms by relieving his dyspnea and improving his quality of life.
CASE 7: HEMODYNAMIC INSTABILITY
Mr. G, age 87, is scheduled for surgical aortic valve replacement because of severe aortic stenosis (valve area 0.5 cm2, peak and mean gradients 89 and 45 mm Hg) with an ejection fraction of 30%.
Two weeks before his scheduled surgery he presents to the emergency department with worsening fluid overload and increasing shortness of breath. His initial laboratory work shows new-onset renal failure, and he has signs of hypoperfusion on physical examination. He is transferred to the cardiac intensive care unit for further care.
How would you manage his aortic stenosis?
Patients with decompensated aortic stenosis and hemodynamic instability are at extreme risk during surgery. Medical stabilization beforehand may mitigate the risks associated with surgical or transcatheter aortic valve replacement. Aortic valvuloplasty, treatment with sodium nitroprusside, and support with intra-aortic balloon counterpulsation may help stabilize patients in this “low-output” setting.
Sodium nitroprusside has long been used in low-output states. By relaxing vascular smooth muscle, it leads to increased venous capacitance, decreasing preload and congestion. It also decreases systemic vascular resistance with a subsequent decrease in afterload, which in turn improves systolic emptying. Together, these effects reduce systolic and diastolic wall stress, lower myocardial oxygen consumption, and ultimately increase cardiac output.49,50
These theoretical benefits translate to clinical improvement and increased cardiac output, as shown in a case series of 25 patients with severe aortic stenosis and left ventricular systolic dysfunction (ejection fraction 35%) presenting in a low-output state in the absence of hypotension.51 These findings have led to a ACC/AHA recommendation for the use of sodium nitroprusside in patients who have severe aortic stenosis presenting in low-output state with decompensated heart failure.21
Intra-aortic balloon counterpulsation, introduced in 1968, has been used in several clinical settings, including acute coronary syndromes, intractable ventricular arrhythmias, and refractory heart failure, and for support of hemodynamics in the perioperative setting. Its role in managing ventricular septal rupture and acute mitral regurgitation is well established. It reliably reduces afterload and improves coronary perfusion, augmenting the cardiac output. This in turn leads to improved systemic perfusion, which can buy time for a critically ill patient during which the primary disease process is addressed.
Recently, a case series in which intraaortic balloon counterpulsation devices were placed in patients with severe aortic stenosis and cardiogenic shock showed findings similar to those with sodium nitroprusside infusion. Specifically, their use was associated with improved cardiac indices and filling pressures with a decrease in systemic vascular resistance. These changes have led to increased cardiac performance, resulting in better systemic perfusion.52 Thus, intra-aortic balloon counterpulsation can be an option for stabilizing patients with severe aortic stenosis and cardiogenic shock.
Mr. G was treated with sodium nitroprusside and intravenous diuretics. He achieved symptomatic relief and his renal function returned to baseline. He subsequently underwent aortic valve replacement during the hospitalization.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009; 373:956–966.
- Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21:1220–1225.
- Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989; 13:545–550.
- Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95:2262–2270.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987; 8:471–483.
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J 1988; 9(suppl E):57–64.
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation 2001; 104:2205–2209.
- Cowell SJ, Newby DE, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352:2389–2397.
- Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359:1343–1356.
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007; 49:554–561.
- Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004; 110:1291–1295.
- O’Brien KD, Probstfield JL, Caulked MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 2005; 165:858–862.
- Lindblom D, Lindblom U, Qvist J, Lundström H. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990; 15:566–573.
- Makkar RR, Fontana G P, Jilaihawi H, et al; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012; 366:1696–1704.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Di Eusanio M, Fortuna D, Cristell D, et al; RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Contemporary outcomes of conventional aortic valve replacement in 638 octogenarians: insights from an Italian Regional Cardiac Surgery Registry (RERIC). Eur J Cardiothorac Surg 2012; 41:1247–1252.
- Di Eusanio M, Fortuna D, De Palma R, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011; 141:940–947.
- Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg 1999; 67:943–951.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52:e1–e142.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39:1356–1363.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Monin JL, Quéré J P, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J 2003; 4:264–270.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Ahmed AA, Graham AN, Lovell D, O’Kane HO. Management of mild to moderate aortic valve disease during coronary artery bypass grafting. Eur J Cardiothorac Surg 2003; 24:535–539.
- Verhoye J P, Merlicco F, Sami IM, et al. Aortic valve replacement for aortic stenosis after previous coronary artery bypass grafting: could early reoperation be prevented? J Heart Valve Dis 2006; 15:474–478.
- Hochrein J, Lucke JC, Harrison JK, et al. Mortality and need for reoperation in patients with mild-to-moderate asymptomatic aortic valve disease undergoing coronary artery bypass graft alone. Am Heart J 1999; 138:791–797.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Weber M, Hausen M, Arnold R, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide for conservatively and surgically treated patients with aortic valve stenosis. Heart 2006; 92:1639–1644.
- Gerber IL, Stewart RA, Legget ME, et al. Increased plasma natriuretic peptide levels refect symptom onset in aortic stenosis. Circulation 2003; 107:1884–1890.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol 2010; 105:383–388.
- Langanay T, Flécher E, Fouquet O, et al. Aortic valve replacement in the elderly: the real life. Ann Thorac Surg 2012; 93:70–77.
- Christofferson RD, Kapadia SR, Rajagopal V, Tuzcu EM. Emerging transcatheter therapies for aortic and mitral disease. Heart 2009; 95:148–155.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Bernard Y, Etievent J, Mourand JL, et al. Long-term results of percutaneous aortic valvuloplasty compared with aortic valve replacement in patients more than 75 years old. J Am Coll Cardiol 1992; 20:796–801.
- Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med 2008; 36(suppl 1):S95–S105.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003; 348:1756–1763.
- Aksoy O, Yousefzai R, Singh D, et al. Cardiogenic shock in the setting of severe aortic stenosis: role of intra-aortic balloon pump support. Heart 2011; 97:838–843.
Surgical aortic valve replacement remains the gold standard treatment for symptomatic aortic valve stenosis in patients at low or moderate risk of surgical complications. But this is a disease of the elderly, many of whom are too frail or too sick to undergo surgery.
Now, patients who cannot undergo this surgery can be offered the less invasive option of transcatheter aortic valve replacement. Balloon valvuloplasty, sodium nitroprusside, and intra-aortic balloon counterpulsation can buy time for ill patients while more permanent mechanical interventions are being considered.
In this review, we will present several cases that highlight management choices for patients with severe aortic stenosis.
A PROGRESSIVE DISEASE OF THE ELDERLY
Aortic stenosis is the most common acquired valvular disease in the United States, and its incidence and prevalence are rising as the population ages. Epidemiologic studies suggest that 2% to 7% of all patients over age 65 have it.1,2
The natural history of the untreated disease is well established, with several case series showing an average decrease of 0.1 cm2 per year in aortic valve area and an increase of 7 mm Hg per year in the pressure gradient across the valve once the diagnosis is made.3,4 Development of angina, syncope, or heart failure is associated with adverse clinical outcomes, including death, and warrants prompt intervention with aortic valve replacement.5–7 Without intervention, the mortality rates reach as high as 75% in 3 years once symptoms develop.
Statins, bisphosphonates, and angiotensin-converting enzyme inhibitors have been used in attempts to slow or reverse the progression of aortic stenosis. However, studies of these drugs have had mixed results, and no definitive benefit has been shown.8–13 Surgical aortic valve replacement, on the other hand, normalizes the life expectancy of patients with aortic stenosis to that of age- and sex-matched controls and remains the gold standard therapy for patients who have symptoms.14
Traditionally, valve replacement has involved open heart surgery, since it requires direct visualization of the valve while the patient is on cardiopulmonary bypass. Unfortunately, many patients have multiple comorbid conditions and therefore are not candidates for open heart surgery. Options for these patients include aortic valvuloplasty and transcatheter aortic valve replacement. While there is considerable experience with aortic valvuloplasty, transcatheter aortic valve replacement is relatively new. In large randomized trials and registries, the transcatheter procedure has been shown to significantly improve long-term survival compared with medical management alone in inoperable patients and to have benefit similar to that of surgery in the high-risk population.15–17
CASE 1: SEVERE, SYMPTOMATIC STENOSIS IN A GOOD SURGICAL CANDIDATE
Mr. A, age 83, presents with shortness of breath and peripheral edema that have been worsening over the past several months. His pulse rate is 64 beats per minute and his blood pressure is 110/90 mm Hg. Auscultation reveals an absent aortic second heart sound with a late peaking systolic murmur that increases with expiration.
On echocardiography, his left ventricular ejection fraction is 55%, peak transaortic valve gradient 88 mm Hg, mean gradient 60 mm Hg, and effective valve area 0.6 cm2. He undergoes catheterization of the left side of his heart, which shows normal coronary arteries.
Mr. A also has hypertension and hyperlipidemia; his renal and pulmonary functions are normal.
How would you manage Mr. A’s aortic stenosis?
Symptomatic aortic stenosis leads to adverse clinical outcomes if managed medically without mechanical intervention,5–7 but patients who undergo aortic valve replacement have age-corrected postoperative survival rates that are nearly normal.14 Furthermore, thanks to improvements in surgical techniques and perioperative management, surgical mortality rates have decreased significantly in recent years and now range from 1% to 8%.18–20 The accumulated evidence showing clear superiority of a surgical approach over medical therapy has greatly simplified the therapeutic algorithm.21
Consequently, the current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) give surgery a class I indication (evidence or general agreement that the procedure is beneficial, useful, and effective) for symptomatic severe aortic stenosis (Figure 1). This level of recommendation also applies to patients who have severe but asymptomatic aortic stenosis who are undergoing other types of cardiac surgery and also to patients with severe aortic stenosis and left ventricular dysfunction (defined as an ejection fraction < 50%).21
Mr. A was referred for surgical aortic valve replacement, given its clear survival benefit.
CASE 2: SYMPTOMS AND LEFT VENTRICULAR DYSFUNCTION
Ms. B, age 79, has hypertension and hyperlipidemia and now presents to the outpatient department with worsening shortness of breath and chest discomfort. Electrocardiography shows significant left ventricular hypertrophy and abnormal repolarization. Left heart catheterization reveals mild nonobstructive coronary artery disease.
Echocardiography reveals an ejection fraction of 25%, severe left ventricular hypertrophy, and global hypokinesis. The aortic valve leaflets appear heavily calcified, with restricted motion. The peak and mean gradients across the aortic valve are 40 and 28 mm Hg, and the valve area is 0.8 cm2. Right heart catheterization shows a cardiac output of 3.1 L/min.
Does this patient’s aortic stenosis account for her clinical presentation?
Managing patients who have suspected severe aortic stenosis, left ventricular dysfunction, and low aortic valve gradients can be challenging. Although data for surgical intervention are not as robust for these patient subsets as for patients like Mr. A, several case series have suggested that survival in these patients is significantly better with surgery than with medical therapy alone.22–27
Specific factors predict whether patients with ventricular dysfunction and low gradients will benefit from aortic valve replacement. Dobutamine stress echocardiography is helpful in distinguishing true severe aortic stenosis from “pseudostenosis,” in which leaflet motion is restricted due to primary cardiomyopathy and low flow. Distinguishing between true aortic stenosis and pseudostenosis is of paramount value, as surgery is associated with improved long-term outcomes in patients with true aortic stenosis (even though they are at higher surgical risk), whereas those with pseudostenosis will not benefit from surgery.28–31
Infusion of dobutamine increases the flow across the aortic valve (if the left ventricle has contractile reserve; more on this below), and an increasing valve area with increasing doses of dobutamine is consistent with pseudostenosis. In this situation, treatment of the underlying cardiomyopathy is indicated as opposed to replacement of the aortic valve (Figure 2).
Contractile reserve is defined as an increase in stroke volume (> 20%), valvular gradient (> 10 mm Hg), or peak velocity (> 0.6 m/s) with peak dobutamine infusion. The presence of contractile reserve in patients with aortic stenosis identifies a high-risk group that benefits from aortic valve replacement (Figure 2).
Treatment of patients who have inadequate reserve is controversial. In the absence of contractile reserve, an adjunct imaging study such as computed tomography may be of value in detecting calcified valve leaflets, as the presence of calcium is associated with true aortic stenosis. Comorbid conditions should be taken into account as well, given the higher surgical risk in this patient subset, as aortic valve replacement in this already high-risk group of patients might be futile in some cases.
The ACC/AHA guidelines now give dobutamine stress echocardiography a class IIa indication (meaning the weight of the evidence or opinion is in favor of usefulness or efficacy) for determination of contractile reserve and valvular stenosis for patients with an ejection fraction of 30% or less or a mean gradient of 40 mm Hg or less.21
Ms. B underwent dobutamine stress echocardiography. It showed increases in ejection fraction, stroke volume, and transvalvular gradients, indicating that she did have contractile reserve and true severe aortic stenosis. Consequently, she was referred for surgical aortic valve replacement.
CASE 3: MODERATE STENOSIS AND THREE-VESSEL CORONARY ARTERY DISEASE
Mr. C, age 81, has hypertension and hyperlipidemia. He now presents to the emergency department with chest discomfort that began suddenly, awakening him from sleep. His presenting electrocardiogram shows nonspecific changes, and he is diagnosed with non-ST-elevation myocardial infarction. He undergoes left heart catheterization, which reveals severe three-vessel coronary artery disease.
Echocardiography reveals an ejection fraction of 55% and aortic stenosis, with an aortic valve area of 1.2 cm2, a peak gradient of 44 mm Hg, and a mean gradient of 28 mm Hg.
How would you manage his aortic stenosis?
Moderate aortic stenosis in a patient who needs surgery for severe triple-vessel coronary artery disease, other valve diseases, or aortic disease raises the question of whether aortic valve replacement should be performed in conjunction with these surgeries. Although these patients would not otherwise qualify for aortic valve replacement, the fact that they will undergo a procedure that will expose them to the risks associated with open heart surgery makes them reasonable candidates. Even if the patient does not need aortic valve replacement right now, aortic stenosis progresses at a predictable rate—the valve area decreases by a mean of 0.1 cm2/year and the gradients increase by 7 mm Hg/year. Therefore, clinical judgment should be exercised so that the patient will not need to undergo open heart surgery again in the near future.
The ACC/AHA guidelines recommend aortic valve replacement for patients with moderate aortic stenosis undergoing coronary artery bypass grafting or surgery on the aorta or other heart valves, giving it a class IIa indication.21 This recommendation is based on several retrospective case series that evaluated survival, the need for reoperation for aortic valve replacement, or both in patients undergoing coronary artery bypass grafting.32–35
No data exist, however, on adding aortic valve replacement to coronary artery bypass grafting in cases of mild aortic stenosis. As a result, it is controversial and carries a class IIb recommendation (meaning that its usefulness or efficacy is less well established). The ACC/AHA guidelines state that aortic valve replacement “may be considered” in patients undergoing coronary artery bypass grafting who have mild aortic stenosis (mean gradient < 30 mm Hg or jet velocity < 3 m/s) when there is evidence, such as moderate or severe valve calcification, that progression may be rapid (level of evidence C: based only on consensus opinion of experts, case studies or standard of care).21
Mr. C, who has moderate aortic stenosis, underwent aortic valve replacement in conjunction with three-vessel bypass grafting.
CASE 4: ASYMPTOMATIC BUT SEVERE STENOSIS
Mr. D, age 74, has hypertension, hyperlipidemia, and aortic stenosis. He now presents to the outpatient department for his annual echocardiogram to follow his aortic stenosis. He has a sedentary lifestyle but feels well performing activities of daily living. He denies dyspnea on exertion, chest pain, or syncope.
His echocardiogram reveals an effective aortic valve area of 0.7 cm2, peak gradient 90 mm Hg, and mean gradient 70 mm Hg. There is evidence of severe left ventricular hypertrophy, and the valve leaflets show bulky calcification and severe restriction. An echocardiogram performed at the same institution a year earlier revealed gradients of 60 and 40 mm Hg.
Blood is drawn for laboratory tests, including N-terminal pro-brain natriuretic peptide, which is 350 pg/mL (reference range for his age < 125 pg/mL). He is referred for a treadmill stress test, which elicits symptoms at a moderate activity level.
How would you manage his aortic stenosis?
Aortic valve replacement can be considered in patients who have asymptomatic but severe aortic stenosis with preserved left ventricular function (class IIb indication).21
Clinical assessment of asymptomatic aortic stenosis can be challenging, however, as patients may underreport their symptoms or decrease their activity levels to avoid symptoms. Exercise testing in such patients can elicit symptoms, unmask diminished exercise capacity, and help determine if they should be referred for surgery.36,37 Natriuretic peptide levels have been shown to correlate with the severity of aortic stenosis,38,39 and more importantly, to help predict symptom onset, cardiac death, and need for aortic valve replacement.40–42
Some patients with asymptomatic but severe aortic stenosis are at higher risk of morbidity and death. High-risk subsets include patients with rapid progression of aortic stenosis and those with critical aortic stenosis characterized by an aortic valve area less than 0.60 cm2, mean gradient greater than 60 mm Hg, and jet velocity greater than 5.0 m/s. It is reasonable to offer these patients surgery if their expected operative mortality risk is less than 1.0%.21
Mr. D has evidence of rapid progression as defined by an increase in aortic jet velocity of more than 0.3 m/s/year. He is at low surgical risk and was referred for elective aortic valve replacement.
CASE 5: TOO FRAIL FOR SURGERY
Mr. E, age 84, has severe aortic stenosis (valve area 0.6 cm2, peak and mean gradients of 88 and 56 mm Hg), coronary artery disease status post coronary artery bypass grafting, moderate chronic obstructive pulmonary disease (forced expiratory volume in 1 second 0.8 L), chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, hyperlipidemia, and diabetes mellitus. He has preserved left ventricular function. He presents to the outpatient department with worsening shortness of breath and peripheral edema over the past several months. Your impression is that he is very frail. How would you manage Mr. E’s aortic stenosis?
Advances in surgical techniques and perioperative management over the years have enabled higher-risk patients to undergo surgical aortic valve replacement with excellent out-comes.18–20,43 Yet many patients still cannot undergo surgery because their risk is too high. Patients ineligible for surgery have traditionally been treated medically—with poor out-comes—or with balloon aortic valvuloplasty to palliate symptoms.
Transcatheter aortic valve replacement, approved by the US Food and Drug Administration (FDA) in 2011, now provides another option for these patients. In this procedure, a bioprosthetic valve mounted on a metal frame is implanted over the native stenotic valve.
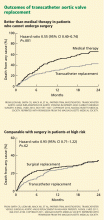
Currently, the only FDA-approved and commercially available valve in the United States is the Edwards SAPIEN valve, which has bovine pericardial tissue leaflets fixed to a balloon-expandable stainless steel frame (Figure 3). In the Placement of Aortic Transcatheter Valves (PARTNER) trial,15 patients who could not undergo surgery who underwent transcatheter replacement with this valve had a significantly better survival rate than patients treated medically.15,17 Use of this valve has also been compared against conventional surgical aortic valve replacement in high-risk patients and was found to have similar long-term outcomes (Figure 4).16 It was on the basis of this trial that this valve was granted approval for patients who cannot undergo surgery.
The standard of care for high-risk patients remains surgical aortic valve replacement, although it remains to be seen whether transcatheter replacement will be made available as well to patients eligible for surgery in the near future. There are currently no randomized data for transcatheter aortic valve replacement in patients at moderate to low surgical risk, and these patients should not be considered for this procedure.
Although the initial studies are encouraging for patients who cannot undergo surgery and who are at high risk without it, several issues and concerns remain. Importantly, the long-term durability of the transcatheter valve and longer-term outcomes remain unknown. Furthermore, the risk of vascular complications remains high (10% to 15%), dictating the need for careful patient selection. There are also concerns about the risks of stroke and of paravalvular aortic insufficiency. These issues are being investigated and addressed, however, and we hope that with increasing operator experience and improvements in the technique, outcomes will be improved.
Which approach for transcatheter aortic valve replacement?
There are several considerations in determining a patient’s eligibility for transcatheter aortic valve replacement.
Initially, these valves were placed by a transvenous, transseptal approach, but now retrograde placement through the femoral artery has become standard. In this procedure, the device is advanced retrograde from the femoral artery through the aorta and placed across the native aortic valve under fluoroscopic and echocardiographic guidance.
Patients who are not eligible for transfemoral placement because of severe atherosclerosis, tortuosity, or ectasia of the iliofemoral artery or aorta can still undergo percutaneous treatment with a transapical approach. This is a hybrid surgical-transcatheter approach in which the valve is delivered through a sheath placed by left ventricular apical puncture.17,44
A newer approach gaining popularity is the transaortic technique, in which the ascending aorta is accessed directly through a ministernotomy and the delivery sheath is placed with a direct puncture. Other approaches are through the axillary and subclavian arteries.
Other valves are under development
Several other valves are under development and will likely change the landscape of transcatheter aortic valve replacement with improving outcomes. Valves that are available in the United States are shown in Figure 3. The CoreValve, consisting of porcine pericardial leaflets mounted on a self-expanding nitinol stent, is currently being studied in a trial in the United States, and the manufacturer (Medtronic) will seek approval when results are complete in the near future.
Mr. E was initially referred for surgery, but when deemed to be unable to undergo surgery was found to be a good candidate for transcatheter aortic valve replacement.
CASE 6: LIFE-LIMITING COMORBID ILLNESS
Mr. F, age 77, has multiple problems: severe aortic stenosis (aortic valve area 0.6 cm2; peak and mean gradients of 92 and 59 mm Hg), stage IV pancreatic cancer, coronary artery disease status post coronary artery bypass grafting, chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, and hyperlipidemia. He presents to the outpatient department with shortness of breath at rest, orthopnea, effort intolerance, and peripheral edema over the past several months.
On physical examination rales in both lung bases can be heard. Left heart catheterization shows patent bypass grafts.
How would you manage Mr. F’s aortic stenosis?
Aortic valve replacement is not considered an option in patients with noncardiac illnesses and comorbidities that are life-limiting in the near term. Under these circumstances, aortic valvuloplasty can be offered as a means of palliating symptoms or, if the comorbid conditions can be modified, as a bridge to more definitive treatment with aortic valve replacement.
Since first described in 1986,45 percutaneous aortic valvuloplasty has been studied in several case series and registries, with consistent findings. Acutely, it increases the valve area and lessens the gradients across the valve, relieving symptoms. The risk of death during the procedure ranged from 3% to 13.5% in several case series, with a 30-day survival rate greater than 85%.46 However, the hemodynamic and symptomatic improvement is only short-term, as valve area and gradients gradually worsen within several months.47,48 Consequently, balloon valvuloplasty is considered a palliative approach.
Mr. F has a potentially life-limiting illness, ie, cancer, which would make him a candidate for aortic valvuloplasty rather than replacement. He can be referred for evaluation for this procedure in hopes of palliating his symptoms by relieving his dyspnea and improving his quality of life.
CASE 7: HEMODYNAMIC INSTABILITY
Mr. G, age 87, is scheduled for surgical aortic valve replacement because of severe aortic stenosis (valve area 0.5 cm2, peak and mean gradients 89 and 45 mm Hg) with an ejection fraction of 30%.
Two weeks before his scheduled surgery he presents to the emergency department with worsening fluid overload and increasing shortness of breath. His initial laboratory work shows new-onset renal failure, and he has signs of hypoperfusion on physical examination. He is transferred to the cardiac intensive care unit for further care.
How would you manage his aortic stenosis?
Patients with decompensated aortic stenosis and hemodynamic instability are at extreme risk during surgery. Medical stabilization beforehand may mitigate the risks associated with surgical or transcatheter aortic valve replacement. Aortic valvuloplasty, treatment with sodium nitroprusside, and support with intra-aortic balloon counterpulsation may help stabilize patients in this “low-output” setting.
Sodium nitroprusside has long been used in low-output states. By relaxing vascular smooth muscle, it leads to increased venous capacitance, decreasing preload and congestion. It also decreases systemic vascular resistance with a subsequent decrease in afterload, which in turn improves systolic emptying. Together, these effects reduce systolic and diastolic wall stress, lower myocardial oxygen consumption, and ultimately increase cardiac output.49,50
These theoretical benefits translate to clinical improvement and increased cardiac output, as shown in a case series of 25 patients with severe aortic stenosis and left ventricular systolic dysfunction (ejection fraction 35%) presenting in a low-output state in the absence of hypotension.51 These findings have led to a ACC/AHA recommendation for the use of sodium nitroprusside in patients who have severe aortic stenosis presenting in low-output state with decompensated heart failure.21
Intra-aortic balloon counterpulsation, introduced in 1968, has been used in several clinical settings, including acute coronary syndromes, intractable ventricular arrhythmias, and refractory heart failure, and for support of hemodynamics in the perioperative setting. Its role in managing ventricular septal rupture and acute mitral regurgitation is well established. It reliably reduces afterload and improves coronary perfusion, augmenting the cardiac output. This in turn leads to improved systemic perfusion, which can buy time for a critically ill patient during which the primary disease process is addressed.
Recently, a case series in which intraaortic balloon counterpulsation devices were placed in patients with severe aortic stenosis and cardiogenic shock showed findings similar to those with sodium nitroprusside infusion. Specifically, their use was associated with improved cardiac indices and filling pressures with a decrease in systemic vascular resistance. These changes have led to increased cardiac performance, resulting in better systemic perfusion.52 Thus, intra-aortic balloon counterpulsation can be an option for stabilizing patients with severe aortic stenosis and cardiogenic shock.
Mr. G was treated with sodium nitroprusside and intravenous diuretics. He achieved symptomatic relief and his renal function returned to baseline. He subsequently underwent aortic valve replacement during the hospitalization.
Surgical aortic valve replacement remains the gold standard treatment for symptomatic aortic valve stenosis in patients at low or moderate risk of surgical complications. But this is a disease of the elderly, many of whom are too frail or too sick to undergo surgery.
Now, patients who cannot undergo this surgery can be offered the less invasive option of transcatheter aortic valve replacement. Balloon valvuloplasty, sodium nitroprusside, and intra-aortic balloon counterpulsation can buy time for ill patients while more permanent mechanical interventions are being considered.
In this review, we will present several cases that highlight management choices for patients with severe aortic stenosis.
A PROGRESSIVE DISEASE OF THE ELDERLY
Aortic stenosis is the most common acquired valvular disease in the United States, and its incidence and prevalence are rising as the population ages. Epidemiologic studies suggest that 2% to 7% of all patients over age 65 have it.1,2
The natural history of the untreated disease is well established, with several case series showing an average decrease of 0.1 cm2 per year in aortic valve area and an increase of 7 mm Hg per year in the pressure gradient across the valve once the diagnosis is made.3,4 Development of angina, syncope, or heart failure is associated with adverse clinical outcomes, including death, and warrants prompt intervention with aortic valve replacement.5–7 Without intervention, the mortality rates reach as high as 75% in 3 years once symptoms develop.
Statins, bisphosphonates, and angiotensin-converting enzyme inhibitors have been used in attempts to slow or reverse the progression of aortic stenosis. However, studies of these drugs have had mixed results, and no definitive benefit has been shown.8–13 Surgical aortic valve replacement, on the other hand, normalizes the life expectancy of patients with aortic stenosis to that of age- and sex-matched controls and remains the gold standard therapy for patients who have symptoms.14
Traditionally, valve replacement has involved open heart surgery, since it requires direct visualization of the valve while the patient is on cardiopulmonary bypass. Unfortunately, many patients have multiple comorbid conditions and therefore are not candidates for open heart surgery. Options for these patients include aortic valvuloplasty and transcatheter aortic valve replacement. While there is considerable experience with aortic valvuloplasty, transcatheter aortic valve replacement is relatively new. In large randomized trials and registries, the transcatheter procedure has been shown to significantly improve long-term survival compared with medical management alone in inoperable patients and to have benefit similar to that of surgery in the high-risk population.15–17
CASE 1: SEVERE, SYMPTOMATIC STENOSIS IN A GOOD SURGICAL CANDIDATE
Mr. A, age 83, presents with shortness of breath and peripheral edema that have been worsening over the past several months. His pulse rate is 64 beats per minute and his blood pressure is 110/90 mm Hg. Auscultation reveals an absent aortic second heart sound with a late peaking systolic murmur that increases with expiration.
On echocardiography, his left ventricular ejection fraction is 55%, peak transaortic valve gradient 88 mm Hg, mean gradient 60 mm Hg, and effective valve area 0.6 cm2. He undergoes catheterization of the left side of his heart, which shows normal coronary arteries.
Mr. A also has hypertension and hyperlipidemia; his renal and pulmonary functions are normal.
How would you manage Mr. A’s aortic stenosis?
Symptomatic aortic stenosis leads to adverse clinical outcomes if managed medically without mechanical intervention,5–7 but patients who undergo aortic valve replacement have age-corrected postoperative survival rates that are nearly normal.14 Furthermore, thanks to improvements in surgical techniques and perioperative management, surgical mortality rates have decreased significantly in recent years and now range from 1% to 8%.18–20 The accumulated evidence showing clear superiority of a surgical approach over medical therapy has greatly simplified the therapeutic algorithm.21
Consequently, the current guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) give surgery a class I indication (evidence or general agreement that the procedure is beneficial, useful, and effective) for symptomatic severe aortic stenosis (Figure 1). This level of recommendation also applies to patients who have severe but asymptomatic aortic stenosis who are undergoing other types of cardiac surgery and also to patients with severe aortic stenosis and left ventricular dysfunction (defined as an ejection fraction < 50%).21
Mr. A was referred for surgical aortic valve replacement, given its clear survival benefit.
CASE 2: SYMPTOMS AND LEFT VENTRICULAR DYSFUNCTION
Ms. B, age 79, has hypertension and hyperlipidemia and now presents to the outpatient department with worsening shortness of breath and chest discomfort. Electrocardiography shows significant left ventricular hypertrophy and abnormal repolarization. Left heart catheterization reveals mild nonobstructive coronary artery disease.
Echocardiography reveals an ejection fraction of 25%, severe left ventricular hypertrophy, and global hypokinesis. The aortic valve leaflets appear heavily calcified, with restricted motion. The peak and mean gradients across the aortic valve are 40 and 28 mm Hg, and the valve area is 0.8 cm2. Right heart catheterization shows a cardiac output of 3.1 L/min.
Does this patient’s aortic stenosis account for her clinical presentation?
Managing patients who have suspected severe aortic stenosis, left ventricular dysfunction, and low aortic valve gradients can be challenging. Although data for surgical intervention are not as robust for these patient subsets as for patients like Mr. A, several case series have suggested that survival in these patients is significantly better with surgery than with medical therapy alone.22–27
Specific factors predict whether patients with ventricular dysfunction and low gradients will benefit from aortic valve replacement. Dobutamine stress echocardiography is helpful in distinguishing true severe aortic stenosis from “pseudostenosis,” in which leaflet motion is restricted due to primary cardiomyopathy and low flow. Distinguishing between true aortic stenosis and pseudostenosis is of paramount value, as surgery is associated with improved long-term outcomes in patients with true aortic stenosis (even though they are at higher surgical risk), whereas those with pseudostenosis will not benefit from surgery.28–31
Infusion of dobutamine increases the flow across the aortic valve (if the left ventricle has contractile reserve; more on this below), and an increasing valve area with increasing doses of dobutamine is consistent with pseudostenosis. In this situation, treatment of the underlying cardiomyopathy is indicated as opposed to replacement of the aortic valve (Figure 2).
Contractile reserve is defined as an increase in stroke volume (> 20%), valvular gradient (> 10 mm Hg), or peak velocity (> 0.6 m/s) with peak dobutamine infusion. The presence of contractile reserve in patients with aortic stenosis identifies a high-risk group that benefits from aortic valve replacement (Figure 2).
Treatment of patients who have inadequate reserve is controversial. In the absence of contractile reserve, an adjunct imaging study such as computed tomography may be of value in detecting calcified valve leaflets, as the presence of calcium is associated with true aortic stenosis. Comorbid conditions should be taken into account as well, given the higher surgical risk in this patient subset, as aortic valve replacement in this already high-risk group of patients might be futile in some cases.
The ACC/AHA guidelines now give dobutamine stress echocardiography a class IIa indication (meaning the weight of the evidence or opinion is in favor of usefulness or efficacy) for determination of contractile reserve and valvular stenosis for patients with an ejection fraction of 30% or less or a mean gradient of 40 mm Hg or less.21
Ms. B underwent dobutamine stress echocardiography. It showed increases in ejection fraction, stroke volume, and transvalvular gradients, indicating that she did have contractile reserve and true severe aortic stenosis. Consequently, she was referred for surgical aortic valve replacement.
CASE 3: MODERATE STENOSIS AND THREE-VESSEL CORONARY ARTERY DISEASE
Mr. C, age 81, has hypertension and hyperlipidemia. He now presents to the emergency department with chest discomfort that began suddenly, awakening him from sleep. His presenting electrocardiogram shows nonspecific changes, and he is diagnosed with non-ST-elevation myocardial infarction. He undergoes left heart catheterization, which reveals severe three-vessel coronary artery disease.
Echocardiography reveals an ejection fraction of 55% and aortic stenosis, with an aortic valve area of 1.2 cm2, a peak gradient of 44 mm Hg, and a mean gradient of 28 mm Hg.
How would you manage his aortic stenosis?
Moderate aortic stenosis in a patient who needs surgery for severe triple-vessel coronary artery disease, other valve diseases, or aortic disease raises the question of whether aortic valve replacement should be performed in conjunction with these surgeries. Although these patients would not otherwise qualify for aortic valve replacement, the fact that they will undergo a procedure that will expose them to the risks associated with open heart surgery makes them reasonable candidates. Even if the patient does not need aortic valve replacement right now, aortic stenosis progresses at a predictable rate—the valve area decreases by a mean of 0.1 cm2/year and the gradients increase by 7 mm Hg/year. Therefore, clinical judgment should be exercised so that the patient will not need to undergo open heart surgery again in the near future.
The ACC/AHA guidelines recommend aortic valve replacement for patients with moderate aortic stenosis undergoing coronary artery bypass grafting or surgery on the aorta or other heart valves, giving it a class IIa indication.21 This recommendation is based on several retrospective case series that evaluated survival, the need for reoperation for aortic valve replacement, or both in patients undergoing coronary artery bypass grafting.32–35
No data exist, however, on adding aortic valve replacement to coronary artery bypass grafting in cases of mild aortic stenosis. As a result, it is controversial and carries a class IIb recommendation (meaning that its usefulness or efficacy is less well established). The ACC/AHA guidelines state that aortic valve replacement “may be considered” in patients undergoing coronary artery bypass grafting who have mild aortic stenosis (mean gradient < 30 mm Hg or jet velocity < 3 m/s) when there is evidence, such as moderate or severe valve calcification, that progression may be rapid (level of evidence C: based only on consensus opinion of experts, case studies or standard of care).21
Mr. C, who has moderate aortic stenosis, underwent aortic valve replacement in conjunction with three-vessel bypass grafting.
CASE 4: ASYMPTOMATIC BUT SEVERE STENOSIS
Mr. D, age 74, has hypertension, hyperlipidemia, and aortic stenosis. He now presents to the outpatient department for his annual echocardiogram to follow his aortic stenosis. He has a sedentary lifestyle but feels well performing activities of daily living. He denies dyspnea on exertion, chest pain, or syncope.
His echocardiogram reveals an effective aortic valve area of 0.7 cm2, peak gradient 90 mm Hg, and mean gradient 70 mm Hg. There is evidence of severe left ventricular hypertrophy, and the valve leaflets show bulky calcification and severe restriction. An echocardiogram performed at the same institution a year earlier revealed gradients of 60 and 40 mm Hg.
Blood is drawn for laboratory tests, including N-terminal pro-brain natriuretic peptide, which is 350 pg/mL (reference range for his age < 125 pg/mL). He is referred for a treadmill stress test, which elicits symptoms at a moderate activity level.
How would you manage his aortic stenosis?
Aortic valve replacement can be considered in patients who have asymptomatic but severe aortic stenosis with preserved left ventricular function (class IIb indication).21
Clinical assessment of asymptomatic aortic stenosis can be challenging, however, as patients may underreport their symptoms or decrease their activity levels to avoid symptoms. Exercise testing in such patients can elicit symptoms, unmask diminished exercise capacity, and help determine if they should be referred for surgery.36,37 Natriuretic peptide levels have been shown to correlate with the severity of aortic stenosis,38,39 and more importantly, to help predict symptom onset, cardiac death, and need for aortic valve replacement.40–42
Some patients with asymptomatic but severe aortic stenosis are at higher risk of morbidity and death. High-risk subsets include patients with rapid progression of aortic stenosis and those with critical aortic stenosis characterized by an aortic valve area less than 0.60 cm2, mean gradient greater than 60 mm Hg, and jet velocity greater than 5.0 m/s. It is reasonable to offer these patients surgery if their expected operative mortality risk is less than 1.0%.21
Mr. D has evidence of rapid progression as defined by an increase in aortic jet velocity of more than 0.3 m/s/year. He is at low surgical risk and was referred for elective aortic valve replacement.
CASE 5: TOO FRAIL FOR SURGERY
Mr. E, age 84, has severe aortic stenosis (valve area 0.6 cm2, peak and mean gradients of 88 and 56 mm Hg), coronary artery disease status post coronary artery bypass grafting, moderate chronic obstructive pulmonary disease (forced expiratory volume in 1 second 0.8 L), chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, hyperlipidemia, and diabetes mellitus. He has preserved left ventricular function. He presents to the outpatient department with worsening shortness of breath and peripheral edema over the past several months. Your impression is that he is very frail. How would you manage Mr. E’s aortic stenosis?
Advances in surgical techniques and perioperative management over the years have enabled higher-risk patients to undergo surgical aortic valve replacement with excellent out-comes.18–20,43 Yet many patients still cannot undergo surgery because their risk is too high. Patients ineligible for surgery have traditionally been treated medically—with poor out-comes—or with balloon aortic valvuloplasty to palliate symptoms.
Transcatheter aortic valve replacement, approved by the US Food and Drug Administration (FDA) in 2011, now provides another option for these patients. In this procedure, a bioprosthetic valve mounted on a metal frame is implanted over the native stenotic valve.
Currently, the only FDA-approved and commercially available valve in the United States is the Edwards SAPIEN valve, which has bovine pericardial tissue leaflets fixed to a balloon-expandable stainless steel frame (Figure 3). In the Placement of Aortic Transcatheter Valves (PARTNER) trial,15 patients who could not undergo surgery who underwent transcatheter replacement with this valve had a significantly better survival rate than patients treated medically.15,17 Use of this valve has also been compared against conventional surgical aortic valve replacement in high-risk patients and was found to have similar long-term outcomes (Figure 4).16 It was on the basis of this trial that this valve was granted approval for patients who cannot undergo surgery.
The standard of care for high-risk patients remains surgical aortic valve replacement, although it remains to be seen whether transcatheter replacement will be made available as well to patients eligible for surgery in the near future. There are currently no randomized data for transcatheter aortic valve replacement in patients at moderate to low surgical risk, and these patients should not be considered for this procedure.
Although the initial studies are encouraging for patients who cannot undergo surgery and who are at high risk without it, several issues and concerns remain. Importantly, the long-term durability of the transcatheter valve and longer-term outcomes remain unknown. Furthermore, the risk of vascular complications remains high (10% to 15%), dictating the need for careful patient selection. There are also concerns about the risks of stroke and of paravalvular aortic insufficiency. These issues are being investigated and addressed, however, and we hope that with increasing operator experience and improvements in the technique, outcomes will be improved.
Which approach for transcatheter aortic valve replacement?
There are several considerations in determining a patient’s eligibility for transcatheter aortic valve replacement.
Initially, these valves were placed by a transvenous, transseptal approach, but now retrograde placement through the femoral artery has become standard. In this procedure, the device is advanced retrograde from the femoral artery through the aorta and placed across the native aortic valve under fluoroscopic and echocardiographic guidance.
Patients who are not eligible for transfemoral placement because of severe atherosclerosis, tortuosity, or ectasia of the iliofemoral artery or aorta can still undergo percutaneous treatment with a transapical approach. This is a hybrid surgical-transcatheter approach in which the valve is delivered through a sheath placed by left ventricular apical puncture.17,44
A newer approach gaining popularity is the transaortic technique, in which the ascending aorta is accessed directly through a ministernotomy and the delivery sheath is placed with a direct puncture. Other approaches are through the axillary and subclavian arteries.
Other valves are under development
Several other valves are under development and will likely change the landscape of transcatheter aortic valve replacement with improving outcomes. Valves that are available in the United States are shown in Figure 3. The CoreValve, consisting of porcine pericardial leaflets mounted on a self-expanding nitinol stent, is currently being studied in a trial in the United States, and the manufacturer (Medtronic) will seek approval when results are complete in the near future.
Mr. E was initially referred for surgery, but when deemed to be unable to undergo surgery was found to be a good candidate for transcatheter aortic valve replacement.
CASE 6: LIFE-LIMITING COMORBID ILLNESS
Mr. F, age 77, has multiple problems: severe aortic stenosis (aortic valve area 0.6 cm2; peak and mean gradients of 92 and 59 mm Hg), stage IV pancreatic cancer, coronary artery disease status post coronary artery bypass grafting, chronic kidney disease (serum creatinine 1.9 mg/dL), hypertension, and hyperlipidemia. He presents to the outpatient department with shortness of breath at rest, orthopnea, effort intolerance, and peripheral edema over the past several months.
On physical examination rales in both lung bases can be heard. Left heart catheterization shows patent bypass grafts.
How would you manage Mr. F’s aortic stenosis?
Aortic valve replacement is not considered an option in patients with noncardiac illnesses and comorbidities that are life-limiting in the near term. Under these circumstances, aortic valvuloplasty can be offered as a means of palliating symptoms or, if the comorbid conditions can be modified, as a bridge to more definitive treatment with aortic valve replacement.
Since first described in 1986,45 percutaneous aortic valvuloplasty has been studied in several case series and registries, with consistent findings. Acutely, it increases the valve area and lessens the gradients across the valve, relieving symptoms. The risk of death during the procedure ranged from 3% to 13.5% in several case series, with a 30-day survival rate greater than 85%.46 However, the hemodynamic and symptomatic improvement is only short-term, as valve area and gradients gradually worsen within several months.47,48 Consequently, balloon valvuloplasty is considered a palliative approach.
Mr. F has a potentially life-limiting illness, ie, cancer, which would make him a candidate for aortic valvuloplasty rather than replacement. He can be referred for evaluation for this procedure in hopes of palliating his symptoms by relieving his dyspnea and improving his quality of life.
CASE 7: HEMODYNAMIC INSTABILITY
Mr. G, age 87, is scheduled for surgical aortic valve replacement because of severe aortic stenosis (valve area 0.5 cm2, peak and mean gradients 89 and 45 mm Hg) with an ejection fraction of 30%.
Two weeks before his scheduled surgery he presents to the emergency department with worsening fluid overload and increasing shortness of breath. His initial laboratory work shows new-onset renal failure, and he has signs of hypoperfusion on physical examination. He is transferred to the cardiac intensive care unit for further care.
How would you manage his aortic stenosis?
Patients with decompensated aortic stenosis and hemodynamic instability are at extreme risk during surgery. Medical stabilization beforehand may mitigate the risks associated with surgical or transcatheter aortic valve replacement. Aortic valvuloplasty, treatment with sodium nitroprusside, and support with intra-aortic balloon counterpulsation may help stabilize patients in this “low-output” setting.
Sodium nitroprusside has long been used in low-output states. By relaxing vascular smooth muscle, it leads to increased venous capacitance, decreasing preload and congestion. It also decreases systemic vascular resistance with a subsequent decrease in afterload, which in turn improves systolic emptying. Together, these effects reduce systolic and diastolic wall stress, lower myocardial oxygen consumption, and ultimately increase cardiac output.49,50
These theoretical benefits translate to clinical improvement and increased cardiac output, as shown in a case series of 25 patients with severe aortic stenosis and left ventricular systolic dysfunction (ejection fraction 35%) presenting in a low-output state in the absence of hypotension.51 These findings have led to a ACC/AHA recommendation for the use of sodium nitroprusside in patients who have severe aortic stenosis presenting in low-output state with decompensated heart failure.21
Intra-aortic balloon counterpulsation, introduced in 1968, has been used in several clinical settings, including acute coronary syndromes, intractable ventricular arrhythmias, and refractory heart failure, and for support of hemodynamics in the perioperative setting. Its role in managing ventricular septal rupture and acute mitral regurgitation is well established. It reliably reduces afterload and improves coronary perfusion, augmenting the cardiac output. This in turn leads to improved systemic perfusion, which can buy time for a critically ill patient during which the primary disease process is addressed.
Recently, a case series in which intraaortic balloon counterpulsation devices were placed in patients with severe aortic stenosis and cardiogenic shock showed findings similar to those with sodium nitroprusside infusion. Specifically, their use was associated with improved cardiac indices and filling pressures with a decrease in systemic vascular resistance. These changes have led to increased cardiac performance, resulting in better systemic perfusion.52 Thus, intra-aortic balloon counterpulsation can be an option for stabilizing patients with severe aortic stenosis and cardiogenic shock.
Mr. G was treated with sodium nitroprusside and intravenous diuretics. He achieved symptomatic relief and his renal function returned to baseline. He subsequently underwent aortic valve replacement during the hospitalization.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009; 373:956–966.
- Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21:1220–1225.
- Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989; 13:545–550.
- Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95:2262–2270.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987; 8:471–483.
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J 1988; 9(suppl E):57–64.
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation 2001; 104:2205–2209.
- Cowell SJ, Newby DE, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352:2389–2397.
- Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359:1343–1356.
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007; 49:554–561.
- Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004; 110:1291–1295.
- O’Brien KD, Probstfield JL, Caulked MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 2005; 165:858–862.
- Lindblom D, Lindblom U, Qvist J, Lundström H. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990; 15:566–573.
- Makkar RR, Fontana G P, Jilaihawi H, et al; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012; 366:1696–1704.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Di Eusanio M, Fortuna D, Cristell D, et al; RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Contemporary outcomes of conventional aortic valve replacement in 638 octogenarians: insights from an Italian Regional Cardiac Surgery Registry (RERIC). Eur J Cardiothorac Surg 2012; 41:1247–1252.
- Di Eusanio M, Fortuna D, De Palma R, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011; 141:940–947.
- Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg 1999; 67:943–951.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52:e1–e142.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39:1356–1363.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Monin JL, Quéré J P, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J 2003; 4:264–270.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Ahmed AA, Graham AN, Lovell D, O’Kane HO. Management of mild to moderate aortic valve disease during coronary artery bypass grafting. Eur J Cardiothorac Surg 2003; 24:535–539.
- Verhoye J P, Merlicco F, Sami IM, et al. Aortic valve replacement for aortic stenosis after previous coronary artery bypass grafting: could early reoperation be prevented? J Heart Valve Dis 2006; 15:474–478.
- Hochrein J, Lucke JC, Harrison JK, et al. Mortality and need for reoperation in patients with mild-to-moderate asymptomatic aortic valve disease undergoing coronary artery bypass graft alone. Am Heart J 1999; 138:791–797.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Weber M, Hausen M, Arnold R, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide for conservatively and surgically treated patients with aortic valve stenosis. Heart 2006; 92:1639–1644.
- Gerber IL, Stewart RA, Legget ME, et al. Increased plasma natriuretic peptide levels refect symptom onset in aortic stenosis. Circulation 2003; 107:1884–1890.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol 2010; 105:383–388.
- Langanay T, Flécher E, Fouquet O, et al. Aortic valve replacement in the elderly: the real life. Ann Thorac Surg 2012; 93:70–77.
- Christofferson RD, Kapadia SR, Rajagopal V, Tuzcu EM. Emerging transcatheter therapies for aortic and mitral disease. Heart 2009; 95:148–155.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Bernard Y, Etievent J, Mourand JL, et al. Long-term results of percutaneous aortic valvuloplasty compared with aortic valve replacement in patients more than 75 years old. J Am Coll Cardiol 1992; 20:796–801.
- Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med 2008; 36(suppl 1):S95–S105.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003; 348:1756–1763.
- Aksoy O, Yousefzai R, Singh D, et al. Cardiogenic shock in the setting of severe aortic stenosis: role of intra-aortic balloon pump support. Heart 2011; 97:838–843.
- Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009; 373:956–966.
- Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993; 21:1220–1225.
- Otto CM, Pearlman AS, Gardner CL. Hemodynamic progression of aortic stenosis in adults assessed by Doppler echocardiography. J Am Coll Cardiol 1989; 13:545–550.
- Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997; 95:2262–2270.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987; 8:471–483.
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J 1988; 9(suppl E):57–64.
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation 2001; 104:2205–2209.
- Cowell SJ, Newby DE, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352:2389–2397.
- Rossebø AB, Pedersen TR, Boman K, et al; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359:1343–1356.
- Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007; 49:554–561.
- Rosenhek R, Rader F, Loho N, et al. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation 2004; 110:1291–1295.
- O’Brien KD, Probstfield JL, Caulked MT, et al. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 2005; 165:858–862.
- Lindblom D, Lindblom U, Qvist J, Lundström H. Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 1990; 15:566–573.
- Makkar RR, Fontana G P, Jilaihawi H, et al; PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012; 366:1696–1704.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Di Eusanio M, Fortuna D, Cristell D, et al; RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Contemporary outcomes of conventional aortic valve replacement in 638 octogenarians: insights from an Italian Regional Cardiac Surgery Registry (RERIC). Eur J Cardiothorac Surg 2012; 41:1247–1252.
- Di Eusanio M, Fortuna D, De Palma R, et al. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg 2011; 141:940–947.
- Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg 1999; 67:943–951.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52:e1–e142.
- Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007; 115:2856–2864.
- Vaquette B, Corbineau H, Laurent M, et al. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart 2005; 91:1324–1329.
- Connolly HM, Oh JK, Orszulak TA, et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction. Prognostic indicators. Circulation 1997; 95:2395–2400.
- Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation 2000; 101:1940–1946.
- Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002; 39:1356–1363.
- Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg 2008; 86:1781–1789.
- Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Rande JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients: risk stratification by low-dose dobutamine echocardiography. J Am Coll Cardiol 2001; 37:2101–2107.
- Monin JL, Quéré J P, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003; 108:319–324.
- Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J 2003; 4:264–270.
- Tribouilloy C, Lévy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865–1873.
- Ahmed AA, Graham AN, Lovell D, O’Kane HO. Management of mild to moderate aortic valve disease during coronary artery bypass grafting. Eur J Cardiothorac Surg 2003; 24:535–539.
- Verhoye J P, Merlicco F, Sami IM, et al. Aortic valve replacement for aortic stenosis after previous coronary artery bypass grafting: could early reoperation be prevented? J Heart Valve Dis 2006; 15:474–478.
- Hochrein J, Lucke JC, Harrison JK, et al. Mortality and need for reoperation in patients with mild-to-moderate asymptomatic aortic valve disease undergoing coronary artery bypass graft alone. Am Heart J 1999; 138:791–797.
- Pereira JJ, Balaban K, Lauer MS, Lytle B, Thomas JD, Garcia MJ. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735–742.
- Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart 2001; 86:381–386.
- Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005; 26:1309–1313.
- Weber M, Arnold R, Rau M, et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol 2004; 94:740–745.
- Weber M, Hausen M, Arnold R, et al. Prognostic value of N-terminal pro-B-type natriuretic peptide for conservatively and surgically treated patients with aortic valve stenosis. Heart 2006; 92:1639–1644.
- Gerber IL, Stewart RA, Legget ME, et al. Increased plasma natriuretic peptide levels refect symptom onset in aortic stenosis. Circulation 2003; 107:1884–1890.
- Bergler-Klein J, Klaar U, Heger M, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation 2004; 109:2302–2308.
- Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol 2010; 105:383–388.
- Langanay T, Flécher E, Fouquet O, et al. Aortic valve replacement in the elderly: the real life. Ann Thorac Surg 2012; 93:70–77.
- Christofferson RD, Kapadia SR, Rajagopal V, Tuzcu EM. Emerging transcatheter therapies for aortic and mitral disease. Heart 2009; 95:148–155.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Bernard Y, Etievent J, Mourand JL, et al. Long-term results of percutaneous aortic valvuloplasty compared with aortic valve replacement in patients more than 75 years old. J Am Coll Cardiol 1992; 20:796–801.
- Elkayam U, Janmohamed M, Habib M, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med 2008; 36(suppl 1):S95–S105.
- Popovic ZB, Khot UN, Novaro GM, et al. Effects of sodium nitroprusside in aortic stenosis associated with severe heart failure: pressure-volume loop analysis using a numerical model. Am J Physiol Heart Circ Physiol 2005; 288:H416–H423.
- Khot UN, Novaro GM, Popovic ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003; 348:1756–1763.
- Aksoy O, Yousefzai R, Singh D, et al. Cardiogenic shock in the setting of severe aortic stenosis: role of intra-aortic balloon pump support. Heart 2011; 97:838–843.
KEY POINTS
- Calcific aortic stenosis is the most common acquired valvular disease, and its prevalence is increasing as the population ages.
- Patients who have symptoms should be referred for aortic valve replacement. Patients who are not candidates for open heart surgery may be eligible for transcatheter aortic valve replacement.
- For high-risk patients with multiple comorbidities, “bridging” therapies such as aortic valvuloplasty are an option.
- In patients with aortic stenosis who present with hemodynamic instability and circulatory collapse, time can be gained with the use of intravenous sodium nitroprusside (in the absence of hypotension) or intra-aortic balloon counterpulsation while more definitive treatment decisions are being made.
Carbapenem-resistant Enterobacteriaceae: A menace to our most vulnerable patients
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
KEY POINTS
- The utility of carbapenems is being undermined by the emergence of resistance in Enterobacteriaceae and other bacteria.
- The clinical impact of CRE falls on elderly patients exposed to these organisms in hospitals and long-term care facilities. In this vulnerable group, invasive infections with CRE exact a high death rate.
- Long-term care facilities play an important role in the transmission dynamics of CRE.
- Tigecycline and colistin are treatments of last resort against infections caused by CRE. Their use in combination with other agents, especially carbapenems, may improve outcomes and needs to be explored further.
- Early detection of CRE in the microbiology laboratory is key to guiding infection control and treatment decisions and supporting surveillance efforts.
Vitamin D deficiency and psychiatric illness
In the United States, >50% of psychiatric inpatients have vitamin D deficiency—<30 nmol/L (<12 ng/mL).1 A growing body of literature has found associations between vitamin D deficiency and psychiatric illnesses, particularly depression. Several randomized controlled trials (RCTs) have demonstrated that vitamin D supplementation can benefit depression symptoms. In this article, we discuss the current literature on vitamin D and psychiatric illness, and provide practical information for clinicians on the use of vitamin D supplementation.
Biosynthesis of vitamin D
Biosynthesis of vitamin D begins with the sterol provitamin D3 molecule 7-dehydrocholesterol (Figure).2 When skin is exposed to sunlight, 7-dehydrocholesterol absorbs UV radiation and forms provitamin D3, which undergoes rapid transformation to vitamin D3.2 Vitamin D3 is released from the plasma membrane and enters systemic circulation in a protein-bound form that has a serum half-life of 36 to 78 hours.3 Vitamin D3 can be taken up by adipocytes and stored in fat deposits, where it has a half-life of approximately 2 months.4
Figure: Biosynthesis of vitamin D
Provitamin D3 (7-dehydrocholesterol) in the skin absorbs UV radiation and undergoes isomerization to form vitamin D3. Endogenously produced vitamin D3 along with dietary vitamin D2 and vitamin D3 absorbed in the gastrointestinal tract are metabolized in the liver to 25-hydroxyvitamin D (25[OH]D), which re-enters the circulation and is metabolized in the kidney and other tissues to the active metabolite 1,25-dihydroxyvitamin D (1,25[OH]2D). Catabolism of 25(OH)D and 1,25(OH)2D into biologically-inactive molecules is primarily mediated by the cytochrome P450 (CYP) enzymes CYP24 and CYP3A4.
Source: Reference 2Circulating vitamin D3 is metabolized in the liver by the enzyme vitamin D-25-hydroxylase to 25-hydroxyvitamin D (25[OH]D3), which has a serum half-life of approximately 15 days.4 Circulating 25(OH)D3 is not biologically active at the physiological level, and requires activation by conversion to 1,25-dihydroxyvitamin D (1,25[OH]2D3) in the kidneys by the enzyme 25(OH)D-1α-hydroxylase. Production of 1,25(OH)2D3 is regulated by serum phosphorus and parathyroid hormone levels and other factors.5 Catabolism of 1,25(OH)2D3 is rapid, with a serum half-life of 3.5 to 21 hours.6 Vitamin D2 is structurally similar to vitamin D3, but occurs primarily in fungi, yeasts, and some invertebrates.
Risk factors for deficiency
A patient’s vitamin D status is determined by measuring 25(OH)D (Box 1). Risk factors for vitamin D deficiency include conditions that affect cutaneous production (insufficient sunlight exposure), obesity, gastrointestinal disorders, aging, renal disorders, and medications (Table 1). 2,5,7,8 The link between sunscreen use, either alone or in cosmetics, and vitamin D deficiency continues to be debated. While controlled studies have found that application of sunscreen with high sun protection factor can significantly reduce vitamin D production, 9 studies in clinical populations have failed to confirm these findings. 10,11 See Box 2 for a discussion of these risk factors and Box 3 for a discussion of acute and long-term medical manifestations of deficiency.
Although 1,25-dihydroxyvitamin D (1,25[OH]2D3) is the biologically active form of vitamin D, its circulating half-life is only 4 to 6 hours.a,b Therefore, 25-hydroxyvitamin D (25[OH]D) is the principal vitamin D metabolite measured to determine vitamin D status. Vitamin D levels commonly are expressed as ng/mL or nmol/L; the conversion factor from ng/mL to nmol/L is 2.496. The Institute of Medicine has defined vitamin D deficiency as a serum 25(OH)D level of <30 nmol/L (<12 ng/mL).c However, many experts define vitamin D insufficiency as a 25(OH)D level of 21 to 29 ng/ml, and deficiency as <20 ng/mL.a,d The upper limit is more difficult to define, but symptoms of vitamin D intoxication appear with blood levels >150 to 200 ng/mL.a
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Aloia JF. Clinical review: the 2011 report on dietary reference intake for vitamin D: where do we go from here? J Clin Endocrinol Metab. 2011;96(10):2987-2996.
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
Table 1
Risk factors associated with vitamin D deficiency
| Age (>65) |
| Insufficient sunlight |
| Breastfeeding |
| Dark skin |
| Malabsorption diseases |
| Obesity (BMI >30 kg/m2) |
| Use of medications that alter vitamin D metabolism (eg, anticonvulsants, glucocorticoids) |
| Hepatobiliary disease |
| Renal disease |
| BMI: body mass index Source: References 2,5,7,8 |
Any factor that diminishes UV radiation penetration into the skin will affect cutaneous synthesis of vitamin D.a,b For example, sunscreen with a sun protection factor of 15 can decrease vitamin D synthesis by 98%.c Geography and its impact on yearly sunlight exposure is a well-known factor in vitamin D deficiency. Individuals who live below a latitude of approximately 35° North—approximately the southern border of Tennessee and through Albuquerque, NM—receive sufficient UV radiation exposure to ensure adequate vitamin D production throughout the year, but at higher latitudes, adequate vitamin D is not produced during winter months.d Melanin affects UV radiation absorption in a manner that prevents vitamin D production, and increased skin pigmentation markedly reduces vitamin D synthesis.e African Americans with very dark skin have significantly diminished cutaneous production of vitamin D.e,f
Renal 1α-hydroxylase activity decreases with aging in parallel with age-related decreases in glomerular filtration.g In addition, aging is associated with increased clearance of 1,25-dihydroxyvitamin D (1,25[OH]2D3).h However, vitamin D absorption generally is adequate even at older ages.i Studies have shown that obese individuals tend to have lower serum concentrations of vitamin D and 25-hydroxyvitamin D (25[OH]D) than those at a normal weight.j,k Obese patients have been shown to have lower cutaneous production of vitamin D3 and display lower bioavailability of orally administered vitamin D2.j
For patients with chronic renal insufficiency, creatinine clearance is positively correlated with serum 1,25(OH)2D levels.l Any process that results in malabsorption of intestinal fat may impair vitamin D absorption. In patients with celiac disease, biliary obstruction, or chronic pancreatitis, absorption consistently is reduced.m Individuals taking bile acid-binding medications, such as cholestyramine for hypercholesterolemia, also may have impaired vitamin D absorption.n In addition, hepatobiliary disease is associated with low levels of 25(OH)D.o Some drugs that alter hepatic metabolism are associated with vitamin D deficiency, including anticonvulsants or glucocorticoids, which can increase catabolism or vitamin D.p
References
- Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29(6):361-368.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
- Clemens TL, Adams JS, Henderson SL, et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76.
- Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213-217.
- Slovik DM, Adams JS, Neer RM, et al. Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981;305(7):372-374.
- Armbrecht HJ, Zenser TV, Davis BB. Effect of age on the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by kidney of rat. J Clin Invest. 1980;66(5):1118-1123.
- Lips P, Wiersinga A, van Ginkel FC, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67(4):644-650.
- Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693.
- Bell NH, Epstein S, Greene A, et al. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370-373.
- Pitts TO, Piraino BH, Mitro R, et al. Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab. 1988;67(5):876-881.
- Thompson GR, Lewis B, Booth CC. Absorption of vitamin D3-3H in control subjects and patients with intestinal malabsorption. J Clin Invest. 1966;45(1):94-102.
- Lo CW, Paris PW, Clemens TL, et al. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42(4):644-649.
- Pappa HM, Bern E, Kamin D, et al. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008;24(2):176-183.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
Acute effects. Vitamin D deficiency produces a range of clinical effects.a-c One well-documented consequence of vitamin D deficiency is osteomalacia—bone demineralization—which produces characteristic bone deformity and growth retardation in children.d,e In adults, osteomalacia may manifest as diffuse pain bone discomfort and muscle aches that may resemble fibromyalgia or arthritis.f Because vitamin D receptors are present in skeletal muscle, deficiency also may lead to proximal muscle weakness; an increased risk of falls; global bone discomfort, often elicited with pressure over the sternum or tibia; and low back pain in older women.c,f
Long-term effects. A large epidemiologic study found that adults with 25-hydroxyvitamin D (25[OH]D) levels <21 ng/mL had an increased risk of hypertension, diabetes, obesity, and dyslipidemia.g Cardiovascular mortality was higher in individuals with 25(OH)D levels <10 ng/mL compared with those with >40 ng/mL.h Adolescents in the National Health and Nutrition Examination Survey-III with serum 25(OH)D levels <15 ng/mL were more likely to have elevated blood glucose levels than those with >26 ng/mL.i Other epidemiologic data have demonstrated associations of vitamin D deficiency with multiple sclerosis, seasonal allergies, asthma, and various infectious diseases.j,k
Because vitamin D is known to promote cellular differentiation and inhibit cellular proliferation, its role in cancer has been studied extensively. A recent meta-analysis of case-control studies found that the odds of colon cancer were reduced by >40% for each 20 ng/mL increase in serum 25(OH)D levels.l Another meta-analysis reported a lower risk of breast cancer among women in the highest quartile of 25(OH)D values compared with the lowest quartile.m
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Bordelon P, Ghetu MV, Langan RC. Recognition and management of vitamin D deficiency. Am Fam Physician. 2009;80(8):841-846.
- Hicks GE, Shardell M, Miller RR, et al. Associations between vitamin D status and pain in older adults: the Invecchiare in Chianti study. J Am Geriatr Soc. 2008;56(5):785-791.
- Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167(11):1159-1165.
- Ginde AA, Scragg R, Schwartz RS, et al. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57(9):1595-1603.
- Reis JP, von Mühlen D, Miller ER 3rd, et al. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124(3):e371-379.
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
- Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832-2838.
- Yin L, Grandi N, Raum E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30(2):113-125.
- Chen P, Hu P, Xie D, et al. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469-477.
Vitamin D’s role in the brain
Vitamin D’s role in psychiatric illnesses is suggested by region-specific expression of vitamin D receptors (VDR) in the cingulate cortex, thalamus, cerebellum, amygdala, and hippocampus.12 Most of these regions also express 1α-hydroxylase enzymes capable of metabolizing 25(OH)D to 1,25(OH)2D3, which suggests that vitamin D may have an autocrine or paracrine function in brain.13
Vitamin D regulates expression of tyrosine hydroxylase, the rate-limiting enzyme in the biosynthesis of dopamine, norepinephrine, and epinephrine.14 Vitamin D also promotes survival of monoaminergic neurons through upregulation of glial cell line-derived neurotrophic factor, which supports survival of midbrain dopaminergic neurons and confers resistance to neurotoxins that deplete dopaminergic neurons in Parkinson’s disease.15 Vitamin D also promotes neuronal survival by inhibiting oxidative pathways in the brain through inhibition of inducible nitric oxide synthase (reducing free radical formation)16 and upregulation of γ-glutamyl transpeptidase (increasing antioxidant production).17 Vitamin D may play a neuroprotective role through regulation of calcium channels. In vitro studies have shown that vitamin D downregulates expression of L-type calcium channels, conferring protection against excitatory neurotoxins in cultured neurons.18 Proteomic analysis of brain tissue in a rat model of developmental vitamin D revealed dysregulation of 36 brain proteins involved in many biologic pathways involved in calcium homeostasis, synaptic plasticity, and neurotransmission.19 Taken together, these findings suggest vitamin D has a neurosteroid-like role in the CNS.
Psychotic disorders
Several epidemiologic studies have linked low vitamin D levels to schizophrenia and other psychotic disorders. Researchers in Norway who used a structured clinical interview to identify psychosis consistently found low levels of 25(OH)D among immigrants and native Norwegians with psychotic symptoms.20 A study of 8,411 Swedish women found low vitamin D levels were associated with psychotic symptoms.21 The Finnish birth cohort study found that use of vitamin D supplementation during the first year of life reduced the incidence of schizophrenia.22 In another pilot study, researchers measured third-trimester serum 25(OH)D levels and found that low levels of maternal vitamin D may be associated with an increased risk of schizophrenia.23 These studies suggest that low prenatal vitamin D levels may adversely impact the developing brain, increasing the risk for adult-onset schizophrenia.
Cognitive dysfunction
Low vitamin D concentrations have been associated with impairments in cognitive functions such as memory and orientation,24 executive function impairments,25 and Alzheimer’s disease (AD).26 A large study conducted from 1998 to 2006 in Italy concluded that persons with severe vitamin D deficiency (<25 nmol/L) had a higher risk of substantial decline on Mini-Mental State Examination than those with sufficient levels (≥75 nmol/L).27 Other studies have linked low vitamin D levels to poor cognitive performance in depressed older adults.28 Low vitamin D levels in older women have been associated with risk of AD, but not with other dementias.29 Polymorphisms of VDR have been associated with depression and poor cognitive performance.30
Depression
Epidemiologic studies evaluating vitamin D deficiency have had conflicting results. The Third National Health and Nutrition Examination Survey, which used a sample of 7,970 non-institutionalized U.S. residents age 15 to 39, demonstrated that individuals with serum vitamin D ≤50 nmol/L are at a significantly higher risk of developing depression than those with vitamin D ≥75 nmol/L.31 A study of 1,282 adults age 65 to 95 in the Netherlands found that 25(OH)D levels were 14% lower in depressed patients compared with controls.32 However, a large epidemiologic study in China did not detect a relationship between vitamin D and depression in 3,262 men and women age 50 to 70.33 After researchers adjusted for geography, body mass index, physical activity, and smoking, 25(OH)D levels did not correlate significantly with the presence or severity of depression. In a case series,34 after 48 vitamin D-deficient depressed adolescents were given vitamin D3 over 3 months, there was a significant improvement in well-being, depressive symptoms, irritability, and fatigue.34 Other small, cross-sectional studies have examined associations between vitamin D status and depression with divergent results, which may reflect differences in population and methodology.
Prospective interventional studies. Although direct causal relationships are difficult to establish, several prospective studies have tested the hypothesis that treating vitamin D deficiency can improve depressive symptoms.
In a double-blind, controlled trial, Jorde et al35 randomized 441 individuals age 21 to 70 to vitamin D, 20,000 IU per week; vitamin D, 40,000 IU per week; or placebo for 1 year. Individuals with serum 25(OH)D levels <40 nmol/L scored significantly higher on depression rating scales than those with serum 25(OH)D levels ≥40 nmol/L at the end of the study. There was no significant improvement in depression ratings in the placebo group (Table 2).35 These results must be interpreted with care because depressive symptoms were secondary endpoints in this study.
Table 2
Effect of vitamin D supplementation on depressive symptoms in a controlled trial
| Vitamin D Supplementation | Serum 25(OH)D levels at baseline | BDI total score, Median and range at end of study | After 1 year of vitamin D supplementation |
|---|---|---|---|
| 20,000 IU/week | <40 nmol/L | Significantly higher (more depressive traits), 6.0 (0 to 23) | Significantly improved BDI score |
| 40,000 IU/week | ≥40 nmol/L | 4.5 (0 to 28) | Significantly improved BDI score |
| Placebo | - | - | No improvement in BDI score |
| 25(OH)D: 25-hydroxyvitamin D; BDI: Beck Depression Inventory Source: Reference 35 | |||
Kjærgaard et al36 systematically examined vitamin D levels in a case-control study followed by a randomized controlled trial (RCT) of vitamin D supplementation. In the case-control phase, participants with low 25(OH)D levels at baseline were significantly more depressed than participants with high 25(OH)D levels. Participants with low 25(OH)D levels were randomized to placebo or 40,000 IU vitamin D3 per week for 6 months. Low levels of vitamin D were strongly associated with depressive symptoms, but vitamin D supplementation did not have a significant effect on depressive symptom scores.
Seasonal affective disorder (SAD). Seasonal variation in vitamin D levels suggests that supplementation may help patients who have seasonal mood disturbances. In a randomized, double-blind study, 44 healthy individuals received vitamin D3, 400 IU/d, 800 IU/d, or no vitamin D3 for 5 days during late winter. Based on self-reports, vitamin D3 significantly enhanced positive affect and there was some evidence it reduced negative affect.37 In a pilot study of 9 women with serum vitamin D levels <40 ng/ml, vitamin D supplementation during winter was associated with an average 10-point decline in Beck Depression Inventory-II scores.38 In a prospective RCT of 15 individuals with SAD, all patients who received vitamin D improved in all outcome measures.39 Vieth40 randomized 82 adults with vitamin D deficiency to 600 IU/d or 4,000 IU/d of vitamin D3 for 3 months over 2 consecutive winters. Patients taking the higher dose showed some evidence of improved well-being compared with those taking the lower dose, although results were not significant for all comparisons. Two other trials did not observe any improvement in SAD symptoms with vitamin D treatment.41,42
Treating vitamin D deficiency
The Endocrine Society recently developed consensus guidelines for diagnosing and managing vitamin D deficiency.43 In addition, the Institute of Medicine of the National Academies recommends daily vitamin D supplementation to prevent deficiency:
- age <70: 400 IU/d
- age >70: 800 IU/d
- pregnant or lactating women: 600 IU/d
- upper limit: 4,000 IU/d.7
Higher doses may be used for patients deprived of sun exposure.8 A typical replacement regimen consists of oral ergocalciferol, 50,000 IU per week for 8 weeks.44 The optimal time for rechecking serum levels after repletion has not been clearly defined, but serum 25(OH)D levels should be measured again after therapy is completed. If values have not reached or exceeded 20 ng/mL, consider a second 8-week course of ergocalciferol (see the Box 1 for a discussion of measuring vitamin D levels). If serum 25(OH)D levels have not increased, the most likely cause is nonadherence or malabsorption.
Contraindications and toxicity. Contraindications to vitamin D supplementation include granulomatous diseases, sarcoidosis, metastatic bone disease, and Williams syndrome.45Table 345 lists signs of vitamin D toxicity. There is little risk of toxicity at dosages of up to 2,000 IU/d.46
Table 3
Signs of vitamin D toxicity
| Headache |
| Metallic taste |
| Nephrocalcinosis or vascular calcinosis |
| Pancreatitis |
| Nausea |
| Vomiting |
| Source: Reference 45 |
Related Resources
- LaFerney MC. Vitamin D deficiency in older adults. Current Psychiatry. 2012;11(11):63.
- National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional.
Drug Brand Names
- Cholestyramine • Questran
- Ergocalciferol • Calciferol, Drisdol
Disclosures
Dr. Harris is an employee of Rho, Chapel Hill, NC.
Dr. Jaiswal reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Holmes receives research support from Bristol-Myers Squibb, Elan, Merck, Otsuka, Shire, Takeda, and Theravance, and is on the speaker’s bureau for Forest Pharmaceuticals and PamLab.
Dr. Patkar is a consultant for Dey Pharmaceuticals, Forest, Gilead, and TTK Pharma and is on the speaker’s bureau and received honoraria from Alkermes, Bristol-Myers Squibb, Dey Pharmaceuticals, Pfizer, and Sunovion; and has received grant support from the Duke Endowment, Dey Pharmaceuticals, Envivo, Forest, Janssen, Lundbeck, The National Institutes of Health, the National Institute on Drug Abuse, National Institute on Alcohol Abuse and Alcoholism, Pfizer Inc., Shire, Sunovion, and Titan.
Dr. Weisler has been a consultant to, on the speaker’s bureaus of, and/or received research support from Abbott, Agency for Toxic Substances and Disease Registry, AstraZeneca, Biovail, Bristol-Myers Squibb, Burroughs Wellcome, Cenerx, Centers for Disease Control and Prevention, Cephalon, Ciba Geigy, CoMentis, Corcept, Cortex, Dainippon Sumitomo Pharma America, Eisai, Elan, Eli Lilly and Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen, Johnson & Johnson, Lundbeck, McNeil Pharmaceuticals, Medicinova, Medscape Advisory Board, Merck, National Institute of Mental Health, Neurochem, New River Pharmaceuticals, Novartis, Organon, Otsuka America Pharma, Pfizer Inc., Pharmacia, Repligen, Saegis, Sandoz, Sanofi, Sanofi-Synthelabo, Schwabe/Ingenix, Sepracor, Shire, Solvay, Sunovion, Synaptic, Takeda, TAP, Theravance, Transcept Pharma, TransTech, UCB Pharma, Validus, Vela, and Wyeth.
1. McCue RE, Charles RA, Orendain GC, et al. Vitamin D deficiency among psychiatric inpatients [published online April 19, 2012]. Prim Care Companion CNS Disord. doi: 10.4088/PCC.11m01230.
2. Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115-124.
3. Adams JS, Clemens TL, Parrish JA, et al. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med. 1982;306(12):722-725.
4. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S-586S.
5. Holick MF. Vitamin D: importance in the prevention of cancers type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
6. Fakih MG, Trump DL, Muindi JR, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(4):1216-1223.
7. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
8. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
9. Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
10. Norval M, Wulf HC. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol. 2009;161(4):732-736.
11. Linos E, Keiser E, Kanzler M, et al. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003-2006. Cancer Causes Control. 2012;23(1):133-140.
12. Prüfer K, Veenstra TD, Jirikowski GF, et al. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135-145.
13. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
14. Garcion E, Wion-Barbot N, Montero-Menei CN, et al. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100-105.
15. Smith MP, Fletcher-Turner A, Yurek DM, et al. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31(4):533-539.
16. Garcion E, Nataf S, Berod A, et al. 1,25-dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45(2):255-267.
17. Baas D, Prüfer K, Ittel ME, et al. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia. 2000;31(1):59-68.
18. Brewer LD, Thibault V, Chen KC, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21(1):98-108.
19. Almeras L, Eyles D, Benech P, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7(5):769-780.
20. Berg AO, Melle I, Torjesen PA, et al. A cross-sectional study of vitamin D deficiency among immigrants and Norwegians with psychosis compared to the general population. J Clin Psychiatry. 2010;71(12):1598-1604.
21. Hedelin M, Löf M, Olsson M, et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33,000 women from the general population. BMC Psychiatry. 2010;10:38.-
22. McGrath J, Saari K, Hakko H, et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res. 2004;67(2-3):237-245.
23. McGrath J, Eyles D, Mowry B, et al. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63(1-2):73-78.
24. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
25. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
26. Buell JS, Dawson-Hughes B, Scott TM, et al. 25-hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18-26.
27. Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135-1141.
28. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
29. Annweiler C, Rolland Y, Schott AM, et al. Higher vitamin D dietary intake is associated with lower risk of Alzheimer’s disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205-1211.
30. Kuningas M, Mooijaart SP, Jolles J, et al. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30(3):466-473.
31. Ganji V, Milone C, Cody MM, et al. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29.-
32. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
33. Pan A, Lu L, Franco OH, et al. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord. 2009;118(1-3):240-243.
34. Högberg G, Gustafsson SA, Hällström T, et al. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta Paediatr. 2012;101(7):779-783.
35. Jorde R, Sneve M, Figenschau Y, et al. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599-609.
36. Kjærgaard M, Waterloo K, Wang CE, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201(5):360-368.
37. Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology (Berl). 1998;135(4):319-223.
38. Shipowick CD, Moore CB, Corbett C, et al. Vitamin D and depressive symptoms in women during the winter: a pilot study. Appl Nurs Res. 2009;22(3):221-225.
39. Gloth FM 3rd, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3(1):5-7.
40. Vieth R, Kimball S, Hu A, et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8.-
41. Harris S, Dawson-Hughes B. Seasonal mood changes in 250 normal women. Psychiatry Res. 1993;49(1):77-87.
42. Dumville JC, Miles JN, Porthouse J, et al. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J Nutr Health Aging. 2006;10(2):151-153.
43. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
44. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
45. Schwalfenberg G. Not enough vitamin D: health consequences for Canadians. Can Fam Physician. 2007;53(5):841-854.
46. Norman AW, Bouillon R, Whiting SJ, et al. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103(3-5):204-205.
In the United States, >50% of psychiatric inpatients have vitamin D deficiency—<30 nmol/L (<12 ng/mL).1 A growing body of literature has found associations between vitamin D deficiency and psychiatric illnesses, particularly depression. Several randomized controlled trials (RCTs) have demonstrated that vitamin D supplementation can benefit depression symptoms. In this article, we discuss the current literature on vitamin D and psychiatric illness, and provide practical information for clinicians on the use of vitamin D supplementation.
Biosynthesis of vitamin D
Biosynthesis of vitamin D begins with the sterol provitamin D3 molecule 7-dehydrocholesterol (Figure).2 When skin is exposed to sunlight, 7-dehydrocholesterol absorbs UV radiation and forms provitamin D3, which undergoes rapid transformation to vitamin D3.2 Vitamin D3 is released from the plasma membrane and enters systemic circulation in a protein-bound form that has a serum half-life of 36 to 78 hours.3 Vitamin D3 can be taken up by adipocytes and stored in fat deposits, where it has a half-life of approximately 2 months.4

Figure: Biosynthesis of vitamin D
Provitamin D3 (7-dehydrocholesterol) in the skin absorbs UV radiation and undergoes isomerization to form vitamin D3. Endogenously produced vitamin D3 along with dietary vitamin D2 and vitamin D3 absorbed in the gastrointestinal tract are metabolized in the liver to 25-hydroxyvitamin D (25[OH]D), which re-enters the circulation and is metabolized in the kidney and other tissues to the active metabolite 1,25-dihydroxyvitamin D (1,25[OH]2D). Catabolism of 25(OH)D and 1,25(OH)2D into biologically-inactive molecules is primarily mediated by the cytochrome P450 (CYP) enzymes CYP24 and CYP3A4.
Source: Reference 2Circulating vitamin D3 is metabolized in the liver by the enzyme vitamin D-25-hydroxylase to 25-hydroxyvitamin D (25[OH]D3), which has a serum half-life of approximately 15 days.4 Circulating 25(OH)D3 is not biologically active at the physiological level, and requires activation by conversion to 1,25-dihydroxyvitamin D (1,25[OH]2D3) in the kidneys by the enzyme 25(OH)D-1α-hydroxylase. Production of 1,25(OH)2D3 is regulated by serum phosphorus and parathyroid hormone levels and other factors.5 Catabolism of 1,25(OH)2D3 is rapid, with a serum half-life of 3.5 to 21 hours.6 Vitamin D2 is structurally similar to vitamin D3, but occurs primarily in fungi, yeasts, and some invertebrates.
Risk factors for deficiency
A patient’s vitamin D status is determined by measuring 25(OH)D (Box 1). Risk factors for vitamin D deficiency include conditions that affect cutaneous production (insufficient sunlight exposure), obesity, gastrointestinal disorders, aging, renal disorders, and medications (Table 1). 2,5,7,8 The link between sunscreen use, either alone or in cosmetics, and vitamin D deficiency continues to be debated. While controlled studies have found that application of sunscreen with high sun protection factor can significantly reduce vitamin D production, 9 studies in clinical populations have failed to confirm these findings. 10,11 See Box 2 for a discussion of these risk factors and Box 3 for a discussion of acute and long-term medical manifestations of deficiency.
Although 1,25-dihydroxyvitamin D (1,25[OH]2D3) is the biologically active form of vitamin D, its circulating half-life is only 4 to 6 hours.a,b Therefore, 25-hydroxyvitamin D (25[OH]D) is the principal vitamin D metabolite measured to determine vitamin D status. Vitamin D levels commonly are expressed as ng/mL or nmol/L; the conversion factor from ng/mL to nmol/L is 2.496. The Institute of Medicine has defined vitamin D deficiency as a serum 25(OH)D level of <30 nmol/L (<12 ng/mL).c However, many experts define vitamin D insufficiency as a 25(OH)D level of 21 to 29 ng/ml, and deficiency as <20 ng/mL.a,d The upper limit is more difficult to define, but symptoms of vitamin D intoxication appear with blood levels >150 to 200 ng/mL.a
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Aloia JF. Clinical review: the 2011 report on dietary reference intake for vitamin D: where do we go from here? J Clin Endocrinol Metab. 2011;96(10):2987-2996.
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
Table 1
Risk factors associated with vitamin D deficiency
| Age (>65) |
| Insufficient sunlight |
| Breastfeeding |
| Dark skin |
| Malabsorption diseases |
| Obesity (BMI >30 kg/m2) |
| Use of medications that alter vitamin D metabolism (eg, anticonvulsants, glucocorticoids) |
| Hepatobiliary disease |
| Renal disease |
| BMI: body mass index Source: References 2,5,7,8 |
Any factor that diminishes UV radiation penetration into the skin will affect cutaneous synthesis of vitamin D.a,b For example, sunscreen with a sun protection factor of 15 can decrease vitamin D synthesis by 98%.c Geography and its impact on yearly sunlight exposure is a well-known factor in vitamin D deficiency. Individuals who live below a latitude of approximately 35° North—approximately the southern border of Tennessee and through Albuquerque, NM—receive sufficient UV radiation exposure to ensure adequate vitamin D production throughout the year, but at higher latitudes, adequate vitamin D is not produced during winter months.d Melanin affects UV radiation absorption in a manner that prevents vitamin D production, and increased skin pigmentation markedly reduces vitamin D synthesis.e African Americans with very dark skin have significantly diminished cutaneous production of vitamin D.e,f
Renal 1α-hydroxylase activity decreases with aging in parallel with age-related decreases in glomerular filtration.g In addition, aging is associated with increased clearance of 1,25-dihydroxyvitamin D (1,25[OH]2D3).h However, vitamin D absorption generally is adequate even at older ages.i Studies have shown that obese individuals tend to have lower serum concentrations of vitamin D and 25-hydroxyvitamin D (25[OH]D) than those at a normal weight.j,k Obese patients have been shown to have lower cutaneous production of vitamin D3 and display lower bioavailability of orally administered vitamin D2.j
For patients with chronic renal insufficiency, creatinine clearance is positively correlated with serum 1,25(OH)2D levels.l Any process that results in malabsorption of intestinal fat may impair vitamin D absorption. In patients with celiac disease, biliary obstruction, or chronic pancreatitis, absorption consistently is reduced.m Individuals taking bile acid-binding medications, such as cholestyramine for hypercholesterolemia, also may have impaired vitamin D absorption.n In addition, hepatobiliary disease is associated with low levels of 25(OH)D.o Some drugs that alter hepatic metabolism are associated with vitamin D deficiency, including anticonvulsants or glucocorticoids, which can increase catabolism or vitamin D.p
References
- Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29(6):361-368.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
- Clemens TL, Adams JS, Henderson SL, et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76.
- Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213-217.
- Slovik DM, Adams JS, Neer RM, et al. Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981;305(7):372-374.
- Armbrecht HJ, Zenser TV, Davis BB. Effect of age on the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by kidney of rat. J Clin Invest. 1980;66(5):1118-1123.
- Lips P, Wiersinga A, van Ginkel FC, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67(4):644-650.
- Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693.
- Bell NH, Epstein S, Greene A, et al. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370-373.
- Pitts TO, Piraino BH, Mitro R, et al. Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab. 1988;67(5):876-881.
- Thompson GR, Lewis B, Booth CC. Absorption of vitamin D3-3H in control subjects and patients with intestinal malabsorption. J Clin Invest. 1966;45(1):94-102.
- Lo CW, Paris PW, Clemens TL, et al. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42(4):644-649.
- Pappa HM, Bern E, Kamin D, et al. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008;24(2):176-183.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
Acute effects. Vitamin D deficiency produces a range of clinical effects.a-c One well-documented consequence of vitamin D deficiency is osteomalacia—bone demineralization—which produces characteristic bone deformity and growth retardation in children.d,e In adults, osteomalacia may manifest as diffuse pain bone discomfort and muscle aches that may resemble fibromyalgia or arthritis.f Because vitamin D receptors are present in skeletal muscle, deficiency also may lead to proximal muscle weakness; an increased risk of falls; global bone discomfort, often elicited with pressure over the sternum or tibia; and low back pain in older women.c,f
Long-term effects. A large epidemiologic study found that adults with 25-hydroxyvitamin D (25[OH]D) levels <21 ng/mL had an increased risk of hypertension, diabetes, obesity, and dyslipidemia.g Cardiovascular mortality was higher in individuals with 25(OH)D levels <10 ng/mL compared with those with >40 ng/mL.h Adolescents in the National Health and Nutrition Examination Survey-III with serum 25(OH)D levels <15 ng/mL were more likely to have elevated blood glucose levels than those with >26 ng/mL.i Other epidemiologic data have demonstrated associations of vitamin D deficiency with multiple sclerosis, seasonal allergies, asthma, and various infectious diseases.j,k
Because vitamin D is known to promote cellular differentiation and inhibit cellular proliferation, its role in cancer has been studied extensively. A recent meta-analysis of case-control studies found that the odds of colon cancer were reduced by >40% for each 20 ng/mL increase in serum 25(OH)D levels.l Another meta-analysis reported a lower risk of breast cancer among women in the highest quartile of 25(OH)D values compared with the lowest quartile.m
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Bordelon P, Ghetu MV, Langan RC. Recognition and management of vitamin D deficiency. Am Fam Physician. 2009;80(8):841-846.
- Hicks GE, Shardell M, Miller RR, et al. Associations between vitamin D status and pain in older adults: the Invecchiare in Chianti study. J Am Geriatr Soc. 2008;56(5):785-791.
- Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167(11):1159-1165.
- Ginde AA, Scragg R, Schwartz RS, et al. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57(9):1595-1603.
- Reis JP, von Mühlen D, Miller ER 3rd, et al. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124(3):e371-379.
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
- Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832-2838.
- Yin L, Grandi N, Raum E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30(2):113-125.
- Chen P, Hu P, Xie D, et al. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469-477.
Vitamin D’s role in the brain
Vitamin D’s role in psychiatric illnesses is suggested by region-specific expression of vitamin D receptors (VDR) in the cingulate cortex, thalamus, cerebellum, amygdala, and hippocampus.12 Most of these regions also express 1α-hydroxylase enzymes capable of metabolizing 25(OH)D to 1,25(OH)2D3, which suggests that vitamin D may have an autocrine or paracrine function in brain.13
Vitamin D regulates expression of tyrosine hydroxylase, the rate-limiting enzyme in the biosynthesis of dopamine, norepinephrine, and epinephrine.14 Vitamin D also promotes survival of monoaminergic neurons through upregulation of glial cell line-derived neurotrophic factor, which supports survival of midbrain dopaminergic neurons and confers resistance to neurotoxins that deplete dopaminergic neurons in Parkinson’s disease.15 Vitamin D also promotes neuronal survival by inhibiting oxidative pathways in the brain through inhibition of inducible nitric oxide synthase (reducing free radical formation)16 and upregulation of γ-glutamyl transpeptidase (increasing antioxidant production).17 Vitamin D may play a neuroprotective role through regulation of calcium channels. In vitro studies have shown that vitamin D downregulates expression of L-type calcium channels, conferring protection against excitatory neurotoxins in cultured neurons.18 Proteomic analysis of brain tissue in a rat model of developmental vitamin D revealed dysregulation of 36 brain proteins involved in many biologic pathways involved in calcium homeostasis, synaptic plasticity, and neurotransmission.19 Taken together, these findings suggest vitamin D has a neurosteroid-like role in the CNS.
Psychotic disorders
Several epidemiologic studies have linked low vitamin D levels to schizophrenia and other psychotic disorders. Researchers in Norway who used a structured clinical interview to identify psychosis consistently found low levels of 25(OH)D among immigrants and native Norwegians with psychotic symptoms.20 A study of 8,411 Swedish women found low vitamin D levels were associated with psychotic symptoms.21 The Finnish birth cohort study found that use of vitamin D supplementation during the first year of life reduced the incidence of schizophrenia.22 In another pilot study, researchers measured third-trimester serum 25(OH)D levels and found that low levels of maternal vitamin D may be associated with an increased risk of schizophrenia.23 These studies suggest that low prenatal vitamin D levels may adversely impact the developing brain, increasing the risk for adult-onset schizophrenia.
Cognitive dysfunction
Low vitamin D concentrations have been associated with impairments in cognitive functions such as memory and orientation,24 executive function impairments,25 and Alzheimer’s disease (AD).26 A large study conducted from 1998 to 2006 in Italy concluded that persons with severe vitamin D deficiency (<25 nmol/L) had a higher risk of substantial decline on Mini-Mental State Examination than those with sufficient levels (≥75 nmol/L).27 Other studies have linked low vitamin D levels to poor cognitive performance in depressed older adults.28 Low vitamin D levels in older women have been associated with risk of AD, but not with other dementias.29 Polymorphisms of VDR have been associated with depression and poor cognitive performance.30
Depression
Epidemiologic studies evaluating vitamin D deficiency have had conflicting results. The Third National Health and Nutrition Examination Survey, which used a sample of 7,970 non-institutionalized U.S. residents age 15 to 39, demonstrated that individuals with serum vitamin D ≤50 nmol/L are at a significantly higher risk of developing depression than those with vitamin D ≥75 nmol/L.31 A study of 1,282 adults age 65 to 95 in the Netherlands found that 25(OH)D levels were 14% lower in depressed patients compared with controls.32 However, a large epidemiologic study in China did not detect a relationship between vitamin D and depression in 3,262 men and women age 50 to 70.33 After researchers adjusted for geography, body mass index, physical activity, and smoking, 25(OH)D levels did not correlate significantly with the presence or severity of depression. In a case series,34 after 48 vitamin D-deficient depressed adolescents were given vitamin D3 over 3 months, there was a significant improvement in well-being, depressive symptoms, irritability, and fatigue.34 Other small, cross-sectional studies have examined associations between vitamin D status and depression with divergent results, which may reflect differences in population and methodology.
Prospective interventional studies. Although direct causal relationships are difficult to establish, several prospective studies have tested the hypothesis that treating vitamin D deficiency can improve depressive symptoms.
In a double-blind, controlled trial, Jorde et al35 randomized 441 individuals age 21 to 70 to vitamin D, 20,000 IU per week; vitamin D, 40,000 IU per week; or placebo for 1 year. Individuals with serum 25(OH)D levels <40 nmol/L scored significantly higher on depression rating scales than those with serum 25(OH)D levels ≥40 nmol/L at the end of the study. There was no significant improvement in depression ratings in the placebo group (Table 2).35 These results must be interpreted with care because depressive symptoms were secondary endpoints in this study.
Table 2
Effect of vitamin D supplementation on depressive symptoms in a controlled trial
| Vitamin D Supplementation | Serum 25(OH)D levels at baseline | BDI total score, Median and range at end of study | After 1 year of vitamin D supplementation |
|---|---|---|---|
| 20,000 IU/week | <40 nmol/L | Significantly higher (more depressive traits), 6.0 (0 to 23) | Significantly improved BDI score |
| 40,000 IU/week | ≥40 nmol/L | 4.5 (0 to 28) | Significantly improved BDI score |
| Placebo | - | - | No improvement in BDI score |
| 25(OH)D: 25-hydroxyvitamin D; BDI: Beck Depression Inventory Source: Reference 35 | |||
Kjærgaard et al36 systematically examined vitamin D levels in a case-control study followed by a randomized controlled trial (RCT) of vitamin D supplementation. In the case-control phase, participants with low 25(OH)D levels at baseline were significantly more depressed than participants with high 25(OH)D levels. Participants with low 25(OH)D levels were randomized to placebo or 40,000 IU vitamin D3 per week for 6 months. Low levels of vitamin D were strongly associated with depressive symptoms, but vitamin D supplementation did not have a significant effect on depressive symptom scores.
Seasonal affective disorder (SAD). Seasonal variation in vitamin D levels suggests that supplementation may help patients who have seasonal mood disturbances. In a randomized, double-blind study, 44 healthy individuals received vitamin D3, 400 IU/d, 800 IU/d, or no vitamin D3 for 5 days during late winter. Based on self-reports, vitamin D3 significantly enhanced positive affect and there was some evidence it reduced negative affect.37 In a pilot study of 9 women with serum vitamin D levels <40 ng/ml, vitamin D supplementation during winter was associated with an average 10-point decline in Beck Depression Inventory-II scores.38 In a prospective RCT of 15 individuals with SAD, all patients who received vitamin D improved in all outcome measures.39 Vieth40 randomized 82 adults with vitamin D deficiency to 600 IU/d or 4,000 IU/d of vitamin D3 for 3 months over 2 consecutive winters. Patients taking the higher dose showed some evidence of improved well-being compared with those taking the lower dose, although results were not significant for all comparisons. Two other trials did not observe any improvement in SAD symptoms with vitamin D treatment.41,42
Treating vitamin D deficiency
The Endocrine Society recently developed consensus guidelines for diagnosing and managing vitamin D deficiency.43 In addition, the Institute of Medicine of the National Academies recommends daily vitamin D supplementation to prevent deficiency:
- age <70: 400 IU/d
- age >70: 800 IU/d
- pregnant or lactating women: 600 IU/d
- upper limit: 4,000 IU/d.7
Higher doses may be used for patients deprived of sun exposure.8 A typical replacement regimen consists of oral ergocalciferol, 50,000 IU per week for 8 weeks.44 The optimal time for rechecking serum levels after repletion has not been clearly defined, but serum 25(OH)D levels should be measured again after therapy is completed. If values have not reached or exceeded 20 ng/mL, consider a second 8-week course of ergocalciferol (see the Box 1 for a discussion of measuring vitamin D levels). If serum 25(OH)D levels have not increased, the most likely cause is nonadherence or malabsorption.
Contraindications and toxicity. Contraindications to vitamin D supplementation include granulomatous diseases, sarcoidosis, metastatic bone disease, and Williams syndrome.45Table 345 lists signs of vitamin D toxicity. There is little risk of toxicity at dosages of up to 2,000 IU/d.46
Table 3
Signs of vitamin D toxicity
| Headache |
| Metallic taste |
| Nephrocalcinosis or vascular calcinosis |
| Pancreatitis |
| Nausea |
| Vomiting |
| Source: Reference 45 |
Related Resources
- LaFerney MC. Vitamin D deficiency in older adults. Current Psychiatry. 2012;11(11):63.
- National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional.
Drug Brand Names
- Cholestyramine • Questran
- Ergocalciferol • Calciferol, Drisdol
Disclosures
Dr. Harris is an employee of Rho, Chapel Hill, NC.
Dr. Jaiswal reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Holmes receives research support from Bristol-Myers Squibb, Elan, Merck, Otsuka, Shire, Takeda, and Theravance, and is on the speaker’s bureau for Forest Pharmaceuticals and PamLab.
Dr. Patkar is a consultant for Dey Pharmaceuticals, Forest, Gilead, and TTK Pharma and is on the speaker’s bureau and received honoraria from Alkermes, Bristol-Myers Squibb, Dey Pharmaceuticals, Pfizer, and Sunovion; and has received grant support from the Duke Endowment, Dey Pharmaceuticals, Envivo, Forest, Janssen, Lundbeck, The National Institutes of Health, the National Institute on Drug Abuse, National Institute on Alcohol Abuse and Alcoholism, Pfizer Inc., Shire, Sunovion, and Titan.
Dr. Weisler has been a consultant to, on the speaker’s bureaus of, and/or received research support from Abbott, Agency for Toxic Substances and Disease Registry, AstraZeneca, Biovail, Bristol-Myers Squibb, Burroughs Wellcome, Cenerx, Centers for Disease Control and Prevention, Cephalon, Ciba Geigy, CoMentis, Corcept, Cortex, Dainippon Sumitomo Pharma America, Eisai, Elan, Eli Lilly and Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen, Johnson & Johnson, Lundbeck, McNeil Pharmaceuticals, Medicinova, Medscape Advisory Board, Merck, National Institute of Mental Health, Neurochem, New River Pharmaceuticals, Novartis, Organon, Otsuka America Pharma, Pfizer Inc., Pharmacia, Repligen, Saegis, Sandoz, Sanofi, Sanofi-Synthelabo, Schwabe/Ingenix, Sepracor, Shire, Solvay, Sunovion, Synaptic, Takeda, TAP, Theravance, Transcept Pharma, TransTech, UCB Pharma, Validus, Vela, and Wyeth.
In the United States, >50% of psychiatric inpatients have vitamin D deficiency—<30 nmol/L (<12 ng/mL).1 A growing body of literature has found associations between vitamin D deficiency and psychiatric illnesses, particularly depression. Several randomized controlled trials (RCTs) have demonstrated that vitamin D supplementation can benefit depression symptoms. In this article, we discuss the current literature on vitamin D and psychiatric illness, and provide practical information for clinicians on the use of vitamin D supplementation.
Biosynthesis of vitamin D
Biosynthesis of vitamin D begins with the sterol provitamin D3 molecule 7-dehydrocholesterol (Figure).2 When skin is exposed to sunlight, 7-dehydrocholesterol absorbs UV radiation and forms provitamin D3, which undergoes rapid transformation to vitamin D3.2 Vitamin D3 is released from the plasma membrane and enters systemic circulation in a protein-bound form that has a serum half-life of 36 to 78 hours.3 Vitamin D3 can be taken up by adipocytes and stored in fat deposits, where it has a half-life of approximately 2 months.4

Figure: Biosynthesis of vitamin D
Provitamin D3 (7-dehydrocholesterol) in the skin absorbs UV radiation and undergoes isomerization to form vitamin D3. Endogenously produced vitamin D3 along with dietary vitamin D2 and vitamin D3 absorbed in the gastrointestinal tract are metabolized in the liver to 25-hydroxyvitamin D (25[OH]D), which re-enters the circulation and is metabolized in the kidney and other tissues to the active metabolite 1,25-dihydroxyvitamin D (1,25[OH]2D). Catabolism of 25(OH)D and 1,25(OH)2D into biologically-inactive molecules is primarily mediated by the cytochrome P450 (CYP) enzymes CYP24 and CYP3A4.
Source: Reference 2Circulating vitamin D3 is metabolized in the liver by the enzyme vitamin D-25-hydroxylase to 25-hydroxyvitamin D (25[OH]D3), which has a serum half-life of approximately 15 days.4 Circulating 25(OH)D3 is not biologically active at the physiological level, and requires activation by conversion to 1,25-dihydroxyvitamin D (1,25[OH]2D3) in the kidneys by the enzyme 25(OH)D-1α-hydroxylase. Production of 1,25(OH)2D3 is regulated by serum phosphorus and parathyroid hormone levels and other factors.5 Catabolism of 1,25(OH)2D3 is rapid, with a serum half-life of 3.5 to 21 hours.6 Vitamin D2 is structurally similar to vitamin D3, but occurs primarily in fungi, yeasts, and some invertebrates.
Risk factors for deficiency
A patient’s vitamin D status is determined by measuring 25(OH)D (Box 1). Risk factors for vitamin D deficiency include conditions that affect cutaneous production (insufficient sunlight exposure), obesity, gastrointestinal disorders, aging, renal disorders, and medications (Table 1). 2,5,7,8 The link between sunscreen use, either alone or in cosmetics, and vitamin D deficiency continues to be debated. While controlled studies have found that application of sunscreen with high sun protection factor can significantly reduce vitamin D production, 9 studies in clinical populations have failed to confirm these findings. 10,11 See Box 2 for a discussion of these risk factors and Box 3 for a discussion of acute and long-term medical manifestations of deficiency.
Although 1,25-dihydroxyvitamin D (1,25[OH]2D3) is the biologically active form of vitamin D, its circulating half-life is only 4 to 6 hours.a,b Therefore, 25-hydroxyvitamin D (25[OH]D) is the principal vitamin D metabolite measured to determine vitamin D status. Vitamin D levels commonly are expressed as ng/mL or nmol/L; the conversion factor from ng/mL to nmol/L is 2.496. The Institute of Medicine has defined vitamin D deficiency as a serum 25(OH)D level of <30 nmol/L (<12 ng/mL).c However, many experts define vitamin D insufficiency as a 25(OH)D level of 21 to 29 ng/ml, and deficiency as <20 ng/mL.a,d The upper limit is more difficult to define, but symptoms of vitamin D intoxication appear with blood levels >150 to 200 ng/mL.a
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Aloia JF. Clinical review: the 2011 report on dietary reference intake for vitamin D: where do we go from here? J Clin Endocrinol Metab. 2011;96(10):2987-2996.
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
Table 1
Risk factors associated with vitamin D deficiency
| Age (>65) |
| Insufficient sunlight |
| Breastfeeding |
| Dark skin |
| Malabsorption diseases |
| Obesity (BMI >30 kg/m2) |
| Use of medications that alter vitamin D metabolism (eg, anticonvulsants, glucocorticoids) |
| Hepatobiliary disease |
| Renal disease |
| BMI: body mass index Source: References 2,5,7,8 |
Any factor that diminishes UV radiation penetration into the skin will affect cutaneous synthesis of vitamin D.a,b For example, sunscreen with a sun protection factor of 15 can decrease vitamin D synthesis by 98%.c Geography and its impact on yearly sunlight exposure is a well-known factor in vitamin D deficiency. Individuals who live below a latitude of approximately 35° North—approximately the southern border of Tennessee and through Albuquerque, NM—receive sufficient UV radiation exposure to ensure adequate vitamin D production throughout the year, but at higher latitudes, adequate vitamin D is not produced during winter months.d Melanin affects UV radiation absorption in a manner that prevents vitamin D production, and increased skin pigmentation markedly reduces vitamin D synthesis.e African Americans with very dark skin have significantly diminished cutaneous production of vitamin D.e,f
Renal 1α-hydroxylase activity decreases with aging in parallel with age-related decreases in glomerular filtration.g In addition, aging is associated with increased clearance of 1,25-dihydroxyvitamin D (1,25[OH]2D3).h However, vitamin D absorption generally is adequate even at older ages.i Studies have shown that obese individuals tend to have lower serum concentrations of vitamin D and 25-hydroxyvitamin D (25[OH]D) than those at a normal weight.j,k Obese patients have been shown to have lower cutaneous production of vitamin D3 and display lower bioavailability of orally administered vitamin D2.j
For patients with chronic renal insufficiency, creatinine clearance is positively correlated with serum 1,25(OH)2D levels.l Any process that results in malabsorption of intestinal fat may impair vitamin D absorption. In patients with celiac disease, biliary obstruction, or chronic pancreatitis, absorption consistently is reduced.m Individuals taking bile acid-binding medications, such as cholestyramine for hypercholesterolemia, also may have impaired vitamin D absorption.n In addition, hepatobiliary disease is associated with low levels of 25(OH)D.o Some drugs that alter hepatic metabolism are associated with vitamin D deficiency, including anticonvulsants or glucocorticoids, which can increase catabolism or vitamin D.p
References
- Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29(6):361-368.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
- Clemens TL, Adams JS, Henderson SL, et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76.
- Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213-217.
- Slovik DM, Adams JS, Neer RM, et al. Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981;305(7):372-374.
- Armbrecht HJ, Zenser TV, Davis BB. Effect of age on the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by kidney of rat. J Clin Invest. 1980;66(5):1118-1123.
- Lips P, Wiersinga A, van Ginkel FC, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67(4):644-650.
- Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693.
- Bell NH, Epstein S, Greene A, et al. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370-373.
- Pitts TO, Piraino BH, Mitro R, et al. Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab. 1988;67(5):876-881.
- Thompson GR, Lewis B, Booth CC. Absorption of vitamin D3-3H in control subjects and patients with intestinal malabsorption. J Clin Invest. 1966;45(1):94-102.
- Lo CW, Paris PW, Clemens TL, et al. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42(4):644-649.
- Pappa HM, Bern E, Kamin D, et al. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008;24(2):176-183.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
Acute effects. Vitamin D deficiency produces a range of clinical effects.a-c One well-documented consequence of vitamin D deficiency is osteomalacia—bone demineralization—which produces characteristic bone deformity and growth retardation in children.d,e In adults, osteomalacia may manifest as diffuse pain bone discomfort and muscle aches that may resemble fibromyalgia or arthritis.f Because vitamin D receptors are present in skeletal muscle, deficiency also may lead to proximal muscle weakness; an increased risk of falls; global bone discomfort, often elicited with pressure over the sternum or tibia; and low back pain in older women.c,f
Long-term effects. A large epidemiologic study found that adults with 25-hydroxyvitamin D (25[OH]D) levels <21 ng/mL had an increased risk of hypertension, diabetes, obesity, and dyslipidemia.g Cardiovascular mortality was higher in individuals with 25(OH)D levels <10 ng/mL compared with those with >40 ng/mL.h Adolescents in the National Health and Nutrition Examination Survey-III with serum 25(OH)D levels <15 ng/mL were more likely to have elevated blood glucose levels than those with >26 ng/mL.i Other epidemiologic data have demonstrated associations of vitamin D deficiency with multiple sclerosis, seasonal allergies, asthma, and various infectious diseases.j,k
Because vitamin D is known to promote cellular differentiation and inhibit cellular proliferation, its role in cancer has been studied extensively. A recent meta-analysis of case-control studies found that the odds of colon cancer were reduced by >40% for each 20 ng/mL increase in serum 25(OH)D levels.l Another meta-analysis reported a lower risk of breast cancer among women in the highest quartile of 25(OH)D values compared with the lowest quartile.m
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Bordelon P, Ghetu MV, Langan RC. Recognition and management of vitamin D deficiency. Am Fam Physician. 2009;80(8):841-846.
- Hicks GE, Shardell M, Miller RR, et al. Associations between vitamin D status and pain in older adults: the Invecchiare in Chianti study. J Am Geriatr Soc. 2008;56(5):785-791.
- Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167(11):1159-1165.
- Ginde AA, Scragg R, Schwartz RS, et al. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57(9):1595-1603.
- Reis JP, von Mühlen D, Miller ER 3rd, et al. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124(3):e371-379.
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
- Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832-2838.
- Yin L, Grandi N, Raum E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30(2):113-125.
- Chen P, Hu P, Xie D, et al. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469-477.
Vitamin D’s role in the brain
Vitamin D’s role in psychiatric illnesses is suggested by region-specific expression of vitamin D receptors (VDR) in the cingulate cortex, thalamus, cerebellum, amygdala, and hippocampus.12 Most of these regions also express 1α-hydroxylase enzymes capable of metabolizing 25(OH)D to 1,25(OH)2D3, which suggests that vitamin D may have an autocrine or paracrine function in brain.13
Vitamin D regulates expression of tyrosine hydroxylase, the rate-limiting enzyme in the biosynthesis of dopamine, norepinephrine, and epinephrine.14 Vitamin D also promotes survival of monoaminergic neurons through upregulation of glial cell line-derived neurotrophic factor, which supports survival of midbrain dopaminergic neurons and confers resistance to neurotoxins that deplete dopaminergic neurons in Parkinson’s disease.15 Vitamin D also promotes neuronal survival by inhibiting oxidative pathways in the brain through inhibition of inducible nitric oxide synthase (reducing free radical formation)16 and upregulation of γ-glutamyl transpeptidase (increasing antioxidant production).17 Vitamin D may play a neuroprotective role through regulation of calcium channels. In vitro studies have shown that vitamin D downregulates expression of L-type calcium channels, conferring protection against excitatory neurotoxins in cultured neurons.18 Proteomic analysis of brain tissue in a rat model of developmental vitamin D revealed dysregulation of 36 brain proteins involved in many biologic pathways involved in calcium homeostasis, synaptic plasticity, and neurotransmission.19 Taken together, these findings suggest vitamin D has a neurosteroid-like role in the CNS.
Psychotic disorders
Several epidemiologic studies have linked low vitamin D levels to schizophrenia and other psychotic disorders. Researchers in Norway who used a structured clinical interview to identify psychosis consistently found low levels of 25(OH)D among immigrants and native Norwegians with psychotic symptoms.20 A study of 8,411 Swedish women found low vitamin D levels were associated with psychotic symptoms.21 The Finnish birth cohort study found that use of vitamin D supplementation during the first year of life reduced the incidence of schizophrenia.22 In another pilot study, researchers measured third-trimester serum 25(OH)D levels and found that low levels of maternal vitamin D may be associated with an increased risk of schizophrenia.23 These studies suggest that low prenatal vitamin D levels may adversely impact the developing brain, increasing the risk for adult-onset schizophrenia.
Cognitive dysfunction
Low vitamin D concentrations have been associated with impairments in cognitive functions such as memory and orientation,24 executive function impairments,25 and Alzheimer’s disease (AD).26 A large study conducted from 1998 to 2006 in Italy concluded that persons with severe vitamin D deficiency (<25 nmol/L) had a higher risk of substantial decline on Mini-Mental State Examination than those with sufficient levels (≥75 nmol/L).27 Other studies have linked low vitamin D levels to poor cognitive performance in depressed older adults.28 Low vitamin D levels in older women have been associated with risk of AD, but not with other dementias.29 Polymorphisms of VDR have been associated with depression and poor cognitive performance.30
Depression
Epidemiologic studies evaluating vitamin D deficiency have had conflicting results. The Third National Health and Nutrition Examination Survey, which used a sample of 7,970 non-institutionalized U.S. residents age 15 to 39, demonstrated that individuals with serum vitamin D ≤50 nmol/L are at a significantly higher risk of developing depression than those with vitamin D ≥75 nmol/L.31 A study of 1,282 adults age 65 to 95 in the Netherlands found that 25(OH)D levels were 14% lower in depressed patients compared with controls.32 However, a large epidemiologic study in China did not detect a relationship between vitamin D and depression in 3,262 men and women age 50 to 70.33 After researchers adjusted for geography, body mass index, physical activity, and smoking, 25(OH)D levels did not correlate significantly with the presence or severity of depression. In a case series,34 after 48 vitamin D-deficient depressed adolescents were given vitamin D3 over 3 months, there was a significant improvement in well-being, depressive symptoms, irritability, and fatigue.34 Other small, cross-sectional studies have examined associations between vitamin D status and depression with divergent results, which may reflect differences in population and methodology.
Prospective interventional studies. Although direct causal relationships are difficult to establish, several prospective studies have tested the hypothesis that treating vitamin D deficiency can improve depressive symptoms.
In a double-blind, controlled trial, Jorde et al35 randomized 441 individuals age 21 to 70 to vitamin D, 20,000 IU per week; vitamin D, 40,000 IU per week; or placebo for 1 year. Individuals with serum 25(OH)D levels <40 nmol/L scored significantly higher on depression rating scales than those with serum 25(OH)D levels ≥40 nmol/L at the end of the study. There was no significant improvement in depression ratings in the placebo group (Table 2).35 These results must be interpreted with care because depressive symptoms were secondary endpoints in this study.
Table 2
Effect of vitamin D supplementation on depressive symptoms in a controlled trial
| Vitamin D Supplementation | Serum 25(OH)D levels at baseline | BDI total score, Median and range at end of study | After 1 year of vitamin D supplementation |
|---|---|---|---|
| 20,000 IU/week | <40 nmol/L | Significantly higher (more depressive traits), 6.0 (0 to 23) | Significantly improved BDI score |
| 40,000 IU/week | ≥40 nmol/L | 4.5 (0 to 28) | Significantly improved BDI score |
| Placebo | - | - | No improvement in BDI score |
| 25(OH)D: 25-hydroxyvitamin D; BDI: Beck Depression Inventory Source: Reference 35 | |||
Kjærgaard et al36 systematically examined vitamin D levels in a case-control study followed by a randomized controlled trial (RCT) of vitamin D supplementation. In the case-control phase, participants with low 25(OH)D levels at baseline were significantly more depressed than participants with high 25(OH)D levels. Participants with low 25(OH)D levels were randomized to placebo or 40,000 IU vitamin D3 per week for 6 months. Low levels of vitamin D were strongly associated with depressive symptoms, but vitamin D supplementation did not have a significant effect on depressive symptom scores.
Seasonal affective disorder (SAD). Seasonal variation in vitamin D levels suggests that supplementation may help patients who have seasonal mood disturbances. In a randomized, double-blind study, 44 healthy individuals received vitamin D3, 400 IU/d, 800 IU/d, or no vitamin D3 for 5 days during late winter. Based on self-reports, vitamin D3 significantly enhanced positive affect and there was some evidence it reduced negative affect.37 In a pilot study of 9 women with serum vitamin D levels <40 ng/ml, vitamin D supplementation during winter was associated with an average 10-point decline in Beck Depression Inventory-II scores.38 In a prospective RCT of 15 individuals with SAD, all patients who received vitamin D improved in all outcome measures.39 Vieth40 randomized 82 adults with vitamin D deficiency to 600 IU/d or 4,000 IU/d of vitamin D3 for 3 months over 2 consecutive winters. Patients taking the higher dose showed some evidence of improved well-being compared with those taking the lower dose, although results were not significant for all comparisons. Two other trials did not observe any improvement in SAD symptoms with vitamin D treatment.41,42
Treating vitamin D deficiency
The Endocrine Society recently developed consensus guidelines for diagnosing and managing vitamin D deficiency.43 In addition, the Institute of Medicine of the National Academies recommends daily vitamin D supplementation to prevent deficiency:
- age <70: 400 IU/d
- age >70: 800 IU/d
- pregnant or lactating women: 600 IU/d
- upper limit: 4,000 IU/d.7
Higher doses may be used for patients deprived of sun exposure.8 A typical replacement regimen consists of oral ergocalciferol, 50,000 IU per week for 8 weeks.44 The optimal time for rechecking serum levels after repletion has not been clearly defined, but serum 25(OH)D levels should be measured again after therapy is completed. If values have not reached or exceeded 20 ng/mL, consider a second 8-week course of ergocalciferol (see the Box 1 for a discussion of measuring vitamin D levels). If serum 25(OH)D levels have not increased, the most likely cause is nonadherence or malabsorption.
Contraindications and toxicity. Contraindications to vitamin D supplementation include granulomatous diseases, sarcoidosis, metastatic bone disease, and Williams syndrome.45Table 345 lists signs of vitamin D toxicity. There is little risk of toxicity at dosages of up to 2,000 IU/d.46
Table 3
Signs of vitamin D toxicity
| Headache |
| Metallic taste |
| Nephrocalcinosis or vascular calcinosis |
| Pancreatitis |
| Nausea |
| Vomiting |
| Source: Reference 45 |
Related Resources
- LaFerney MC. Vitamin D deficiency in older adults. Current Psychiatry. 2012;11(11):63.
- National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional.
Drug Brand Names
- Cholestyramine • Questran
- Ergocalciferol • Calciferol, Drisdol
Disclosures
Dr. Harris is an employee of Rho, Chapel Hill, NC.
Dr. Jaiswal reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Holmes receives research support from Bristol-Myers Squibb, Elan, Merck, Otsuka, Shire, Takeda, and Theravance, and is on the speaker’s bureau for Forest Pharmaceuticals and PamLab.
Dr. Patkar is a consultant for Dey Pharmaceuticals, Forest, Gilead, and TTK Pharma and is on the speaker’s bureau and received honoraria from Alkermes, Bristol-Myers Squibb, Dey Pharmaceuticals, Pfizer, and Sunovion; and has received grant support from the Duke Endowment, Dey Pharmaceuticals, Envivo, Forest, Janssen, Lundbeck, The National Institutes of Health, the National Institute on Drug Abuse, National Institute on Alcohol Abuse and Alcoholism, Pfizer Inc., Shire, Sunovion, and Titan.
Dr. Weisler has been a consultant to, on the speaker’s bureaus of, and/or received research support from Abbott, Agency for Toxic Substances and Disease Registry, AstraZeneca, Biovail, Bristol-Myers Squibb, Burroughs Wellcome, Cenerx, Centers for Disease Control and Prevention, Cephalon, Ciba Geigy, CoMentis, Corcept, Cortex, Dainippon Sumitomo Pharma America, Eisai, Elan, Eli Lilly and Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen, Johnson & Johnson, Lundbeck, McNeil Pharmaceuticals, Medicinova, Medscape Advisory Board, Merck, National Institute of Mental Health, Neurochem, New River Pharmaceuticals, Novartis, Organon, Otsuka America Pharma, Pfizer Inc., Pharmacia, Repligen, Saegis, Sandoz, Sanofi, Sanofi-Synthelabo, Schwabe/Ingenix, Sepracor, Shire, Solvay, Sunovion, Synaptic, Takeda, TAP, Theravance, Transcept Pharma, TransTech, UCB Pharma, Validus, Vela, and Wyeth.
1. McCue RE, Charles RA, Orendain GC, et al. Vitamin D deficiency among psychiatric inpatients [published online April 19, 2012]. Prim Care Companion CNS Disord. doi: 10.4088/PCC.11m01230.
2. Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115-124.
3. Adams JS, Clemens TL, Parrish JA, et al. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med. 1982;306(12):722-725.
4. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S-586S.
5. Holick MF. Vitamin D: importance in the prevention of cancers type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
6. Fakih MG, Trump DL, Muindi JR, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(4):1216-1223.
7. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
8. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
9. Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
10. Norval M, Wulf HC. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol. 2009;161(4):732-736.
11. Linos E, Keiser E, Kanzler M, et al. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003-2006. Cancer Causes Control. 2012;23(1):133-140.
12. Prüfer K, Veenstra TD, Jirikowski GF, et al. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135-145.
13. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
14. Garcion E, Wion-Barbot N, Montero-Menei CN, et al. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100-105.
15. Smith MP, Fletcher-Turner A, Yurek DM, et al. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31(4):533-539.
16. Garcion E, Nataf S, Berod A, et al. 1,25-dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45(2):255-267.
17. Baas D, Prüfer K, Ittel ME, et al. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia. 2000;31(1):59-68.
18. Brewer LD, Thibault V, Chen KC, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21(1):98-108.
19. Almeras L, Eyles D, Benech P, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7(5):769-780.
20. Berg AO, Melle I, Torjesen PA, et al. A cross-sectional study of vitamin D deficiency among immigrants and Norwegians with psychosis compared to the general population. J Clin Psychiatry. 2010;71(12):1598-1604.
21. Hedelin M, Löf M, Olsson M, et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33,000 women from the general population. BMC Psychiatry. 2010;10:38.-
22. McGrath J, Saari K, Hakko H, et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res. 2004;67(2-3):237-245.
23. McGrath J, Eyles D, Mowry B, et al. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63(1-2):73-78.
24. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
25. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
26. Buell JS, Dawson-Hughes B, Scott TM, et al. 25-hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18-26.
27. Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135-1141.
28. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
29. Annweiler C, Rolland Y, Schott AM, et al. Higher vitamin D dietary intake is associated with lower risk of Alzheimer’s disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205-1211.
30. Kuningas M, Mooijaart SP, Jolles J, et al. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30(3):466-473.
31. Ganji V, Milone C, Cody MM, et al. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29.-
32. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
33. Pan A, Lu L, Franco OH, et al. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord. 2009;118(1-3):240-243.
34. Högberg G, Gustafsson SA, Hällström T, et al. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta Paediatr. 2012;101(7):779-783.
35. Jorde R, Sneve M, Figenschau Y, et al. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599-609.
36. Kjærgaard M, Waterloo K, Wang CE, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201(5):360-368.
37. Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology (Berl). 1998;135(4):319-223.
38. Shipowick CD, Moore CB, Corbett C, et al. Vitamin D and depressive symptoms in women during the winter: a pilot study. Appl Nurs Res. 2009;22(3):221-225.
39. Gloth FM 3rd, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3(1):5-7.
40. Vieth R, Kimball S, Hu A, et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8.-
41. Harris S, Dawson-Hughes B. Seasonal mood changes in 250 normal women. Psychiatry Res. 1993;49(1):77-87.
42. Dumville JC, Miles JN, Porthouse J, et al. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J Nutr Health Aging. 2006;10(2):151-153.
43. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
44. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
45. Schwalfenberg G. Not enough vitamin D: health consequences for Canadians. Can Fam Physician. 2007;53(5):841-854.
46. Norman AW, Bouillon R, Whiting SJ, et al. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103(3-5):204-205.
1. McCue RE, Charles RA, Orendain GC, et al. Vitamin D deficiency among psychiatric inpatients [published online April 19, 2012]. Prim Care Companion CNS Disord. doi: 10.4088/PCC.11m01230.
2. Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115-124.
3. Adams JS, Clemens TL, Parrish JA, et al. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med. 1982;306(12):722-725.
4. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S-586S.
5. Holick MF. Vitamin D: importance in the prevention of cancers type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
6. Fakih MG, Trump DL, Muindi JR, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(4):1216-1223.
7. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
8. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
9. Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
10. Norval M, Wulf HC. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol. 2009;161(4):732-736.
11. Linos E, Keiser E, Kanzler M, et al. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003-2006. Cancer Causes Control. 2012;23(1):133-140.
12. Prüfer K, Veenstra TD, Jirikowski GF, et al. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135-145.
13. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
14. Garcion E, Wion-Barbot N, Montero-Menei CN, et al. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100-105.
15. Smith MP, Fletcher-Turner A, Yurek DM, et al. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31(4):533-539.
16. Garcion E, Nataf S, Berod A, et al. 1,25-dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45(2):255-267.
17. Baas D, Prüfer K, Ittel ME, et al. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia. 2000;31(1):59-68.
18. Brewer LD, Thibault V, Chen KC, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21(1):98-108.
19. Almeras L, Eyles D, Benech P, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7(5):769-780.
20. Berg AO, Melle I, Torjesen PA, et al. A cross-sectional study of vitamin D deficiency among immigrants and Norwegians with psychosis compared to the general population. J Clin Psychiatry. 2010;71(12):1598-1604.
21. Hedelin M, Löf M, Olsson M, et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33,000 women from the general population. BMC Psychiatry. 2010;10:38.-
22. McGrath J, Saari K, Hakko H, et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res. 2004;67(2-3):237-245.
23. McGrath J, Eyles D, Mowry B, et al. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63(1-2):73-78.
24. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
25. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
26. Buell JS, Dawson-Hughes B, Scott TM, et al. 25-hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18-26.
27. Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135-1141.
28. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
29. Annweiler C, Rolland Y, Schott AM, et al. Higher vitamin D dietary intake is associated with lower risk of Alzheimer’s disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205-1211.
30. Kuningas M, Mooijaart SP, Jolles J, et al. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30(3):466-473.
31. Ganji V, Milone C, Cody MM, et al. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29.-
32. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
33. Pan A, Lu L, Franco OH, et al. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord. 2009;118(1-3):240-243.
34. Högberg G, Gustafsson SA, Hällström T, et al. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta Paediatr. 2012;101(7):779-783.
35. Jorde R, Sneve M, Figenschau Y, et al. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599-609.
36. Kjærgaard M, Waterloo K, Wang CE, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201(5):360-368.
37. Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology (Berl). 1998;135(4):319-223.
38. Shipowick CD, Moore CB, Corbett C, et al. Vitamin D and depressive symptoms in women during the winter: a pilot study. Appl Nurs Res. 2009;22(3):221-225.
39. Gloth FM 3rd, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3(1):5-7.
40. Vieth R, Kimball S, Hu A, et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8.-
41. Harris S, Dawson-Hughes B. Seasonal mood changes in 250 normal women. Psychiatry Res. 1993;49(1):77-87.
42. Dumville JC, Miles JN, Porthouse J, et al. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J Nutr Health Aging. 2006;10(2):151-153.
43. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
44. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
45. Schwalfenberg G. Not enough vitamin D: health consequences for Canadians. Can Fam Physician. 2007;53(5):841-854.
46. Norman AW, Bouillon R, Whiting SJ, et al. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103(3-5):204-205.
Strategies for treating depression in patients with hepatitis C
Mr. P, age 31, has been using heroin intravenously for 9 years. He smokes 1 pack of cigarettes daily, but denies using other substances, including alcohol. After an unintentional heroin overdose, Mr. P enrolls in a methadone maintenance treatment program (MMTP) that includes primary medical care and addiction medicine and psychiatric specialists, where he undergoes medical evaluation and screening for hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Laboratory data reveal that although Mr. P is HIV negative, he has been exposed to HCV and treatment is indicated.
Among the approximately 3 million people in the United States with chronic HCV—an enveloped, single-stranded RNA virus—there’s a high prevalence of premorbid psychopathology and substance abuse, as well as neuropsychiatric effects caused by HCV treatment.1-3 Because underdiagnosing and undertreating psychiatric disorders contributes to morbidity and mortality in HCV patients, early identification and prompt treatment is critical.
IV drug use is the most common route for HCV infection, accounting for 65% to 70% of infections.1 The prevalence of HCV among IV drug users is 28% to 90%.1 Once exposed to HCV, 75% to 85% of patients do not clear the initial infection and become chronically infected.
This article reviews the pathophysiology, identification, and management of psychiatric manifestations found among HCV patients and provides an understanding of how psychiatric symptoms manifest in HCV patients. This article also discusses HCV treatment and its neuropsychiatric side effects.
Testing for HCV
Chronically infected HCV patients may have few, if any, specific physical complaints, and often are diagnosed during screenings or other routine laboratory evaluations. The presence of risk factors, such as a history of injection drug use or receiving a blood transfusion before 1992,1 guides the decision to screen for HCV. Normal liver function test results should not preclude testing because many HCV-positive patients have transaminases within the normal range.4 Initial screening is via an antibody-mediated immunoassay that is highly specific and sensitive for past exposure to HCV (Table 1).4 However, a positive screen does not indicate the presence of active infection. Evidence of the virus via a viral assay will identify active HCV, but does not indicate need for treatment. Liver biopsy confirms the presence of liver injury and quantifies its extent. The severity of liver damage will determine whether treatment is needed. HCV genotyping determines the appropriate duration and dosage of pharmacotherapy.
Table 1
Tests to diagnose and evaluate HCV
| Test | Results |
|---|---|
| HCV antibody | Determines prior exposure to HCV |
| HCV viral assay | Evaluates for current HCV infection |
| Liver biopsy | Assesses level of liver damage |
| HCV genotyping | Provides data to determine duration and intensity of treatment and likelihood of treatment success |
| HCV: hepatitis C virus Source: Reference 4 | |
CASE CONTINUED: Mood improves, but fatigue persists
As part of pre-HCV treatment evaluation, Mr. P undergoes a psychiatric evaluation. He describes periods of low mood while actively engaged in drug use but has never received psychiatric treatment, experienced suicidal ideation, or attempted suicide. Since starting opioid agonist therapy, he reports improved mood but endorses continued mild fatigue and difficulty falling sleep. The psychiatrist determines Mr. P does not meet criteria for an axis I diagnosis other than a substance use disorder.
Although most HCV patients have few, if any, nonspecific physical symptoms, many have psychiatric symptoms or disorders before the HCV diagnosis is made or treatment is initiated; substance use disorders are most common. Batki et al1 found that 56% of HCV patients in an MMTP met criteria for a nonsubstance axis I disorder and 82% met criteria for such a disorder during their lifetime. Additionally, 66% of patients were taking psychiatric medications. Table 21,5,6 lists the rates of other psychiatric disorders found in patients with untreated HCV.
Table 2
Rates of psychiatric disorders in patients with untreated hepatitis C virus
| Disorder(s) | Current rate | Lifetime rate |
|---|---|---|
| Mood disorders | 34% to 35% | 67% |
| Major depressive disorder | 22% to 28% | 42% |
| Anxiety disorders | 26% to 44% | 63% |
| Antisocial personality disorder | No rates; lifetime diagnosis | 16% to 40% |
| Psychotic disorders | 9% to 17% | 11% |
| Substance use disorder | 56% | 56% to 86% |
| Source: References 1,5,6 | ||
Many patients with chronic HCV complain of chronic fatigue and deficiencies in attention, concentration, higher executive functions, learning ability, and memory that result in significant reduction in quality of life (Box 1).7-9 These findings have been found to be independent of the degree of liver disease and are seen in HCV patients with normal liver function.7,8
The pathophysiology of fatigue and neurocognitive dysfunction in hepatitis C virus (HCV) infection is unclear. However, the improvement of chronic fatigue in patients with HCV who receive ondansetron, a 5-hydroxytryptophan-3 receptor antagonist, has implicated abnormal monoaminergic function. Single-photon emission CT studies have found decreased midbrain serotonergic and striatal dopaminergic transmission in some HCV patients with cognitive deficits.7
Recently, data have been mounting on a direct neuropathic effect of HCV, with viral elements found in autopsy brain tissue and cerebrospinal fluid.8 Researchers have suggested that HCV may enter the CNS via a Trojan horse-like mechanism inside infected mononuclear cells.8 More recently, human brain microvascular endothelium, the major component of the blood-brain barrier, has been found to express all major viral receptors that would allow HCV infection of the CNS.9
CASE CONTINUED: Motivated and compliant
Since joining the MMTP 6 months ago, Mr. P has been motivated and compliant with all appointments and treatments. Routine urine toxicology screening supports his claim of abstinence. Mr. P begins HCV treatment while continuing follow-up with addiction medicine and psychiatric clinicians and maintains open communication with all treatment providers.
For many years the standard HCV treatment was pegylated interferon-α (IFN-α) and ribavirin. IFN-α is a proinflammatory cytokine with antiproliferative, antiviral, and immunoregulatory properties. The half-life of IFN-α significantly increases with pegylation, which allows for weekly injections.10,11 IFN-α usually is combined with ribavirin, which increases its efficacy as measured by the sustained virological response (SVR) compared with IFN-α alone. Depending on the virus genotype, treatment lasts 24 to 48 weeks; SVR rates range from 40% to 82%.11-13 In 2011, the FDA approved 2 agents—telaprevir and boceprevir—for adjunctive treatment of HCV genotype 1 infection. These 2 agents are protease inhibitors that when added to IFN-α and ribavirin increase the SVR rate in genotype 1 infection from 40% to 50% to approximately 75%.14,15
Although the neuropsychiatric side effects of telaprevir and boceprevir have not been determined, treating chronic HCV with IFN-α and ribavirin has been associated with multiple psychiatric symptoms, including depression, mania, suicidality, anxiety, and psychosis.11-14 Psychiatric symptoms are a common reason for discontinuing or reducing HCV treatment. Because of the high frequency of neuropsychiatric complications, some clinicians believe HCV patients with preexisting affective, psychotic, or substance use disorders should be excluded from HCV treatment. This has led to many HCV patients being untreated despite a lack of prospective, controlled data to support this opinion.12 To improve outcomes and decrease morbidity, providing appropriate psychiatric services appears to be more important than attempting to select lower-risk patients for antiviral therapy.1,12,16 The goals of psychiatric treatment should be to alleviate symptoms and allow patients to complete IFN-α therapy without interruption.16,17
Studies of high-risk patients who attend multidisciplinary treatment programs that can monitor adherence and efficacy and control side effects before and during HCV treatment have found psychiatric patients have similar adherence, compliance, and SVR rates and were not at increased risk of worsening depressive or psychotic symptoms compared with patients without a psychiatric history.12,18 Additionally, HCV patients with a psychiatric history are not at an increased risk of suicide.13,16 Similar findings have been observed in patients with active IV drug use or those receiving opioid agonist therapy. When HCV and substance use are treated simultaneously, patients can successfully complete HCV treatment with SVR rates comparable to those of patients not receiving opioid agonist therapy.19-21
CASE CONTINUED: Worsening symptoms
During a psychiatric follow-up 12 weeks after starting HCV treatment, Mr. P reports worsening depressive symptoms with low mood, decreased enjoyment of activities, poor sleep, low appetite, and fatigue. He shows no evidence of psychosis and denies suicidal ideation. We continue his HCV treatment, schedule more frequent psychiatric visits, and initiate citalopram, titrated to 40 mg/d.
Depressive symptoms, the most common neuropsychiatric manifestation of HCV, typically begin early in treatment, usually within the first 12 weeks. Two distinct symptom clusters are noted. A neurovegetative cluster characterized by reduced energy, anorexia, and psychomotor retardation typically begins within the first few months of treatment. Months later, a depression-specific syndrome appears that includes depressed mood, anxiety, and cognitive impairment.22
Depressive symptoms may occur in up to 60% of patients treated with IFN-α.11 When more rigorous depression measures are used, rates decrease to approximately 20% to 30%.11,13 Accurate diagnosis and treatment of emerging depressive symptoms is essential because untreated depression can lead to postponing or excluding patients from antiviral treatment.2 Screening instruments such as the Beck Depression Inventory-Second Edition (BDI-II) can be used to measure depressive symptoms in HCV patients with high sensitivity. However, because specificity has been low and somatic symptoms of chronic illness and depression often overlap, the BDI-II and other inventories may overestimate depression. Some researchers have suggested that focusing on questions targeting cognitive and affective symptoms rather than somatic ones may be a more valid measure of depression in patients undergoing immunotherapy for HCV.2
The immune system is implicated in IFN-α-induced depression because depressive symptoms share many features with a constellation of somatic and behavioral symptoms termed “sickness behavior.”11 These behaviors can occur when patients are exposed to cytokines that lead to a depressed level of functioning, which may allow the body to devote more energy to fighting illness. IFN-α, a cytokine, stimulates the immune system, which can lead to increases of interleukin (IL)-2, IL-6, and IL-10. Increased circulating levels of these ILs have been correlated with higher depression scores. Additionally, studies have found that patients who develop depression during IFN-α treatment have higher SVR rates, suggesting a more robust immune response.11,22 For a discussion of how serotonin metabolism and genetic polymorphisms also may help explain the prevalence of depression in patients with HCV, see Box 2.
Altered serotonin metabolism has been linked to depression in hepatitis C virus (HCV) patients treated with interferon-α (IFN-α). Tryptophan can be metabolized towards serotonin via tryptophan hydroxylase and niacin via indoleamine-2,3-dioxygenase (IDO) with kynurenine (KYN) and quinolinic acid (QUIN) as intermediaries. Introduction of IFN-α activates IDO, causing preferential conversion of tryptophan towards the niacin arm away from serotonin and leads to elevated levels of KYN and QUIN. KYN and QUIN are available centrally, are neurotoxic, and have been correlated with increased depressive symptoms in IFN-α-treated patients.a,b A tryptophan-deficient state is created, with less tryptophan being converted to serotonin and subsequently to its metabolite, 5-hydroxyindoleacetic acid (5-HIAA). Decreased levels of 5-HIAA in cerebrospinal fluid have been associated with higher depressive symptoms and higher rates of suicide.a,b
Several genetic polymorphisms may help identify patients at risk for developing IFN-α-induced depression. Genes for the 5’ promoter of the serotonin transporter (5-HTTLPR) have been investigated for roles in depression development in patients undergoing immunotherapy. Studies have found that persons with the short allele in the 5-HTTLPR gene are more likely to develop depression than those with the long allele. However, this has not been consistent across racial or ethnic groups.a,b Research also has associated the serotonin (5-HT) transporter, interferon receptor-A1, apolipoprotein ε4 allele, cyclooxygenase 2, and phospholipase A2 with development of a specific subgroup of symptoms.a
References
a. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
b. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
Treating depressed HCV patients
Antidepressants are the treatment of choice for IFN-α-induced depression. Most currently used antidepressants are effective22 and selective serotonin reuptake inhibitors are considered first choice.16 Antidepressant choice should be guided by principles similar to those used for patients without HCV: using side effects profiles to target specific symptoms and being mindful of pharmacokinetic properties.
Two treatment approaches have been investigated: prophylactic and symptomatic. A 2012 study23 of 181 HCV patients with no history of mental illness determined escitalopram, 10 mg/d, effectively reduced the incidence and severity of interferon-associated depression. Other studies examining prophylactic treatment of all patients who were to undergo interferon treatment found this approach did not prevent depressive episodes.24,25 However, antidepressants have been beneficial for patients with subsyndromal depressive symptoms at baseline26 and after clinically significant depressive symptoms emerge.27 Electroconvulsive therapy also has been reported to effectively treat depression in HCV patients undergoing antiviral therapy.28
CASE CONTINUED: Lingering symptoms
Mr. P responds to citalopram with an improvement in mood, anhedonia, and appetite, but he continues to complain of low energy and poor concentration. In an effort to target these symptoms, methylphenidate, titrated to 30 mg/d in divided doses, is added to his regimen, which rapidly improves his symptoms. Insomnia is treated successfully with trazodone, 50 mg/d. Mr. P frequently visits his psychiatrist, who monitors his depressive symptoms using the BDI-II. Mr. P completes HCV treatment without recurrence of depressive symptoms or relapse to heroin use.
Although antidepressants are effective for treating affective and cognitive symptoms, they are not as effective for fatigue and other neurovegetative symptoms.16,29 The psychostimulants methylphenidate and dextroamphetamine and the nonstimulant modafinil have been studied for treating depressive symptoms in medically ill patients and can be used to treat IFN-α-induced fatigue.16,22,29
IFN-α’s effect on serotonin metabolism leads to a tryptophan-deficient state because of increased catabolism as a result of activation of indoleamine-2,3-dioxygenase (IDO). This has led to use of tryptophan supplementation, either as augmentation or monotherapy, for managing depressive symptoms in patients treated with IFN-α. Schaefer et al30 reported 3 cases where tryptophan supplementation significantly decreased depressive symptoms. Other researchers have argued that supplementing tryptophan in the context of IDO activation can lead to greater production of kynurenine and quinolinic acid, which have been linked to increased depressive symptoms in patients receiving IFN-α.31 They argue that supplementation of 5-HTP, which is available as a dietary supplement without a prescription, can lead to increased serotonin levels and improvement in depressive symptoms.31
IFN-α treatment also is associated with mania and psychosis. The incidence, pathophysiology, and management of these treatment-emergent symptoms are not as well studied as IFN-α-induced depression. Mania and hypomania have been reported with interferon treatment, discontinuation of interferon, and use of antidepressants for interferon-induced depression.29,32 Psychosis, in association with mood symptoms or alone, has been reported to occur in <1% of treated patients.33 Treatment for mania and psychosis consists of decreasing or discontinuing immunotherapy and adding mood stabilizers and antipsychotics. Once immunotherapy is discontinued, mania and psychosis usually resolve, but prolonged duration of symptoms has been reported.29,32,33
Related Resources
- Centers for Disease Control and Prevention. Hepatitis C information for health professionals. www.cdc.gov/hepatitis/hcv/index.htm.
- Hep C Connection. www.hepc-connection.org.
- United States Department of Veterans Affairs. Hepatitis C. www.hepatitis.va.gov.
Drug Brand Names
- Boceprevir • Victrelis
- Citalopram • Celexa
- Dextroamphetamine • Dexedrine
- Escitalopram • Lexapro
- Interferon-α • Intron
- Methadone • Dolophine, Methadose
- Methylphenidate • Ritalin, Methylin, others
- Modafinil • Provigil
- Ondansetron • Zofran
- Ribavirin • Copegus, Rebetol, others
- Telaprevir • Incivek
- Trazodone • Desyrel, Oleptro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Batki SL, Canfield KM, Ploutz-Snyder R. Psychiatric and substance use disorders among methadone maintenance patients with chronic hepatitis C infection: effects on eligibility for hepatitis C treatment. Am J Addict. 2011;20(4):312-318.
2. Patterson AL, Morasco BJ, Fuller BE, et al. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33(4):354-362.
3. Maddur H, Kwo PY. Boceprevir. Hepatology. 2011;54(6):2254-2257.
4. Sylvestre D. Hepatitis C for addiction professionals. Addict Sci Clin Pract. 2007;4(1):34-41.
5. Dwight MM, Kowdley KV, Russo JE, et al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49(5):311-317.
6. Yovtcheva SP, Rifai MA, Moles JK, et al. Psychiatric comorbidity among hepatitis C-positive patients. Psychosomatics. 2001;42(5):411-415.
7. Weissenborn K, Ennen JC, Bokemeyer M, et al. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55(11):1624-1630.
8. Weissenborn K, Tryc AB, Heeren M, et al. Hepatitis C virus infection and the brain. Metab Brain Dis. 2009;24(1):197-210.
9. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142(3):634-643.e6.
10. Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006;43(2 suppl 1):S207-S220.
11. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
12. Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46(4):991-998.
13. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
14. Telaprevir (Incivek) and boceprevir (Victrelis) for chronic hepatitis C. Med Lett Drugs Ther. 2011;53(1369):57-59.
15. Nelson DR. The role of triple therapy with protease inhibitors in hepatitis C virus genotype 1 naïve patients. Liver Int. 2011;31(suppl 1):53-57.
16. Spennati A, Pariante CM. Withdrawing interferon-α from psychiatric patients: clinical care or unjustifiable stigma? [published online September 14 2012] Psychol Med. doi: 10. 1017/S0033291712001808.
17. Baraldi S, Hepgul N, Mondelli V, et al. Symptomatic treatment of interferon-α-induced depression in hepatitis C: a systematic review. J Clin Psychopharmacol. 2012;32(4):531-543.
18. Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37(2):443-451.
19. Harris KA, Jr, Arnsten JH, Litwin AH. Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. J Addict Med. 2010;4(1):20-26.
20. Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37(1):32-40.
21. Sasadeusz JJ, Dore G, Kronborg I, et al. Clinical experience with the treatment of hepatitis C infection in patients on opioid pharmacotherapy. Addiction. 2011;106(5):977-984.
22. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
23. Schaefer M, Sarkar R, Knop V, et al. Escitalopram for the prevention of peginterferon-α2a-associated depression in hepatitis C virus-infected patients without previous psychiatric disease: a randomized trial. Ann Intern Med. 2012;157(2):94-103.
24. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
25. Morasco BJ, Loftis JM, Indest DW, et al. Prophylactic antidepressant treatment in patients with hepatitis C on antiviral therapy: a double-blind, placebo-controlled trial. Psychosomatics. 2010;51(5):401-408.
26. Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25(10):1163-1174.
27. Kraus MR, Schäfer A, Schöttker K, et al. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomised, double-blind, placebo-controlled study. Gut. 2008;57(4):531-536.
28. Zincke MT, Kurani A, Istafanous R, et al. The successful use of electroconvulsive therapy in a patient with interferon-induced psychotic depression. J ECT. 2007;23(4):291-292.
29. Crone CC, Gabriel GM, Wise TN. Managing the neuropsychiatric side effects of interferon-based therapy for hepatitis C. Cleve Clin J Med. 2004;71(suppl 3):S27-S32.
30. Schaefer M, Winterer J, Sarkar R, et al. Three cases of successful tryptophan add-on or monotherapy of hepatitis C and IFNa-associated mood disorders. Psychosomatics. 2008;49(5):442-446.
31. Turner EH, Blackwell AD. 5-Hydroxytryptophan plus SSRIs for interferon-induced depression: synergistic mechanisms for normalizing synaptic serotonin. Med Hypotheses. 2005;65(1):138-144.
32. Onyike CU, Bonner JO, Lyketsos CG, et al. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;161(3):429-435.
33. Cheng YC, Chen CC, Ho AS, et al. Prolonged psychosis associated with interferon therapy in a patient with hepatitis C: case study and literature review. Psychosomatics. 2009;50(5):538-542.
Mr. P, age 31, has been using heroin intravenously for 9 years. He smokes 1 pack of cigarettes daily, but denies using other substances, including alcohol. After an unintentional heroin overdose, Mr. P enrolls in a methadone maintenance treatment program (MMTP) that includes primary medical care and addiction medicine and psychiatric specialists, where he undergoes medical evaluation and screening for hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Laboratory data reveal that although Mr. P is HIV negative, he has been exposed to HCV and treatment is indicated.
Among the approximately 3 million people in the United States with chronic HCV—an enveloped, single-stranded RNA virus—there’s a high prevalence of premorbid psychopathology and substance abuse, as well as neuropsychiatric effects caused by HCV treatment.1-3 Because underdiagnosing and undertreating psychiatric disorders contributes to morbidity and mortality in HCV patients, early identification and prompt treatment is critical.
IV drug use is the most common route for HCV infection, accounting for 65% to 70% of infections.1 The prevalence of HCV among IV drug users is 28% to 90%.1 Once exposed to HCV, 75% to 85% of patients do not clear the initial infection and become chronically infected.
This article reviews the pathophysiology, identification, and management of psychiatric manifestations found among HCV patients and provides an understanding of how psychiatric symptoms manifest in HCV patients. This article also discusses HCV treatment and its neuropsychiatric side effects.
Testing for HCV
Chronically infected HCV patients may have few, if any, specific physical complaints, and often are diagnosed during screenings or other routine laboratory evaluations. The presence of risk factors, such as a history of injection drug use or receiving a blood transfusion before 1992,1 guides the decision to screen for HCV. Normal liver function test results should not preclude testing because many HCV-positive patients have transaminases within the normal range.4 Initial screening is via an antibody-mediated immunoassay that is highly specific and sensitive for past exposure to HCV (Table 1).4 However, a positive screen does not indicate the presence of active infection. Evidence of the virus via a viral assay will identify active HCV, but does not indicate need for treatment. Liver biopsy confirms the presence of liver injury and quantifies its extent. The severity of liver damage will determine whether treatment is needed. HCV genotyping determines the appropriate duration and dosage of pharmacotherapy.
Table 1
Tests to diagnose and evaluate HCV
| Test | Results |
|---|---|
| HCV antibody | Determines prior exposure to HCV |
| HCV viral assay | Evaluates for current HCV infection |
| Liver biopsy | Assesses level of liver damage |
| HCV genotyping | Provides data to determine duration and intensity of treatment and likelihood of treatment success |
| HCV: hepatitis C virus Source: Reference 4 | |
CASE CONTINUED: Mood improves, but fatigue persists
As part of pre-HCV treatment evaluation, Mr. P undergoes a psychiatric evaluation. He describes periods of low mood while actively engaged in drug use but has never received psychiatric treatment, experienced suicidal ideation, or attempted suicide. Since starting opioid agonist therapy, he reports improved mood but endorses continued mild fatigue and difficulty falling sleep. The psychiatrist determines Mr. P does not meet criteria for an axis I diagnosis other than a substance use disorder.
Although most HCV patients have few, if any, nonspecific physical symptoms, many have psychiatric symptoms or disorders before the HCV diagnosis is made or treatment is initiated; substance use disorders are most common. Batki et al1 found that 56% of HCV patients in an MMTP met criteria for a nonsubstance axis I disorder and 82% met criteria for such a disorder during their lifetime. Additionally, 66% of patients were taking psychiatric medications. Table 21,5,6 lists the rates of other psychiatric disorders found in patients with untreated HCV.
Table 2
Rates of psychiatric disorders in patients with untreated hepatitis C virus
| Disorder(s) | Current rate | Lifetime rate |
|---|---|---|
| Mood disorders | 34% to 35% | 67% |
| Major depressive disorder | 22% to 28% | 42% |
| Anxiety disorders | 26% to 44% | 63% |
| Antisocial personality disorder | No rates; lifetime diagnosis | 16% to 40% |
| Psychotic disorders | 9% to 17% | 11% |
| Substance use disorder | 56% | 56% to 86% |
| Source: References 1,5,6 | ||
Many patients with chronic HCV complain of chronic fatigue and deficiencies in attention, concentration, higher executive functions, learning ability, and memory that result in significant reduction in quality of life (Box 1).7-9 These findings have been found to be independent of the degree of liver disease and are seen in HCV patients with normal liver function.7,8
The pathophysiology of fatigue and neurocognitive dysfunction in hepatitis C virus (HCV) infection is unclear. However, the improvement of chronic fatigue in patients with HCV who receive ondansetron, a 5-hydroxytryptophan-3 receptor antagonist, has implicated abnormal monoaminergic function. Single-photon emission CT studies have found decreased midbrain serotonergic and striatal dopaminergic transmission in some HCV patients with cognitive deficits.7
Recently, data have been mounting on a direct neuropathic effect of HCV, with viral elements found in autopsy brain tissue and cerebrospinal fluid.8 Researchers have suggested that HCV may enter the CNS via a Trojan horse-like mechanism inside infected mononuclear cells.8 More recently, human brain microvascular endothelium, the major component of the blood-brain barrier, has been found to express all major viral receptors that would allow HCV infection of the CNS.9
CASE CONTINUED: Motivated and compliant
Since joining the MMTP 6 months ago, Mr. P has been motivated and compliant with all appointments and treatments. Routine urine toxicology screening supports his claim of abstinence. Mr. P begins HCV treatment while continuing follow-up with addiction medicine and psychiatric clinicians and maintains open communication with all treatment providers.
For many years the standard HCV treatment was pegylated interferon-α (IFN-α) and ribavirin. IFN-α is a proinflammatory cytokine with antiproliferative, antiviral, and immunoregulatory properties. The half-life of IFN-α significantly increases with pegylation, which allows for weekly injections.10,11 IFN-α usually is combined with ribavirin, which increases its efficacy as measured by the sustained virological response (SVR) compared with IFN-α alone. Depending on the virus genotype, treatment lasts 24 to 48 weeks; SVR rates range from 40% to 82%.11-13 In 2011, the FDA approved 2 agents—telaprevir and boceprevir—for adjunctive treatment of HCV genotype 1 infection. These 2 agents are protease inhibitors that when added to IFN-α and ribavirin increase the SVR rate in genotype 1 infection from 40% to 50% to approximately 75%.14,15
Although the neuropsychiatric side effects of telaprevir and boceprevir have not been determined, treating chronic HCV with IFN-α and ribavirin has been associated with multiple psychiatric symptoms, including depression, mania, suicidality, anxiety, and psychosis.11-14 Psychiatric symptoms are a common reason for discontinuing or reducing HCV treatment. Because of the high frequency of neuropsychiatric complications, some clinicians believe HCV patients with preexisting affective, psychotic, or substance use disorders should be excluded from HCV treatment. This has led to many HCV patients being untreated despite a lack of prospective, controlled data to support this opinion.12 To improve outcomes and decrease morbidity, providing appropriate psychiatric services appears to be more important than attempting to select lower-risk patients for antiviral therapy.1,12,16 The goals of psychiatric treatment should be to alleviate symptoms and allow patients to complete IFN-α therapy without interruption.16,17
Studies of high-risk patients who attend multidisciplinary treatment programs that can monitor adherence and efficacy and control side effects before and during HCV treatment have found psychiatric patients have similar adherence, compliance, and SVR rates and were not at increased risk of worsening depressive or psychotic symptoms compared with patients without a psychiatric history.12,18 Additionally, HCV patients with a psychiatric history are not at an increased risk of suicide.13,16 Similar findings have been observed in patients with active IV drug use or those receiving opioid agonist therapy. When HCV and substance use are treated simultaneously, patients can successfully complete HCV treatment with SVR rates comparable to those of patients not receiving opioid agonist therapy.19-21
CASE CONTINUED: Worsening symptoms
During a psychiatric follow-up 12 weeks after starting HCV treatment, Mr. P reports worsening depressive symptoms with low mood, decreased enjoyment of activities, poor sleep, low appetite, and fatigue. He shows no evidence of psychosis and denies suicidal ideation. We continue his HCV treatment, schedule more frequent psychiatric visits, and initiate citalopram, titrated to 40 mg/d.
Depressive symptoms, the most common neuropsychiatric manifestation of HCV, typically begin early in treatment, usually within the first 12 weeks. Two distinct symptom clusters are noted. A neurovegetative cluster characterized by reduced energy, anorexia, and psychomotor retardation typically begins within the first few months of treatment. Months later, a depression-specific syndrome appears that includes depressed mood, anxiety, and cognitive impairment.22
Depressive symptoms may occur in up to 60% of patients treated with IFN-α.11 When more rigorous depression measures are used, rates decrease to approximately 20% to 30%.11,13 Accurate diagnosis and treatment of emerging depressive symptoms is essential because untreated depression can lead to postponing or excluding patients from antiviral treatment.2 Screening instruments such as the Beck Depression Inventory-Second Edition (BDI-II) can be used to measure depressive symptoms in HCV patients with high sensitivity. However, because specificity has been low and somatic symptoms of chronic illness and depression often overlap, the BDI-II and other inventories may overestimate depression. Some researchers have suggested that focusing on questions targeting cognitive and affective symptoms rather than somatic ones may be a more valid measure of depression in patients undergoing immunotherapy for HCV.2
The immune system is implicated in IFN-α-induced depression because depressive symptoms share many features with a constellation of somatic and behavioral symptoms termed “sickness behavior.”11 These behaviors can occur when patients are exposed to cytokines that lead to a depressed level of functioning, which may allow the body to devote more energy to fighting illness. IFN-α, a cytokine, stimulates the immune system, which can lead to increases of interleukin (IL)-2, IL-6, and IL-10. Increased circulating levels of these ILs have been correlated with higher depression scores. Additionally, studies have found that patients who develop depression during IFN-α treatment have higher SVR rates, suggesting a more robust immune response.11,22 For a discussion of how serotonin metabolism and genetic polymorphisms also may help explain the prevalence of depression in patients with HCV, see Box 2.
Altered serotonin metabolism has been linked to depression in hepatitis C virus (HCV) patients treated with interferon-α (IFN-α). Tryptophan can be metabolized towards serotonin via tryptophan hydroxylase and niacin via indoleamine-2,3-dioxygenase (IDO) with kynurenine (KYN) and quinolinic acid (QUIN) as intermediaries. Introduction of IFN-α activates IDO, causing preferential conversion of tryptophan towards the niacin arm away from serotonin and leads to elevated levels of KYN and QUIN. KYN and QUIN are available centrally, are neurotoxic, and have been correlated with increased depressive symptoms in IFN-α-treated patients.a,b A tryptophan-deficient state is created, with less tryptophan being converted to serotonin and subsequently to its metabolite, 5-hydroxyindoleacetic acid (5-HIAA). Decreased levels of 5-HIAA in cerebrospinal fluid have been associated with higher depressive symptoms and higher rates of suicide.a,b
Several genetic polymorphisms may help identify patients at risk for developing IFN-α-induced depression. Genes for the 5’ promoter of the serotonin transporter (5-HTTLPR) have been investigated for roles in depression development in patients undergoing immunotherapy. Studies have found that persons with the short allele in the 5-HTTLPR gene are more likely to develop depression than those with the long allele. However, this has not been consistent across racial or ethnic groups.a,b Research also has associated the serotonin (5-HT) transporter, interferon receptor-A1, apolipoprotein ε4 allele, cyclooxygenase 2, and phospholipase A2 with development of a specific subgroup of symptoms.a
References
a. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
b. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
Treating depressed HCV patients
Antidepressants are the treatment of choice for IFN-α-induced depression. Most currently used antidepressants are effective22 and selective serotonin reuptake inhibitors are considered first choice.16 Antidepressant choice should be guided by principles similar to those used for patients without HCV: using side effects profiles to target specific symptoms and being mindful of pharmacokinetic properties.
Two treatment approaches have been investigated: prophylactic and symptomatic. A 2012 study23 of 181 HCV patients with no history of mental illness determined escitalopram, 10 mg/d, effectively reduced the incidence and severity of interferon-associated depression. Other studies examining prophylactic treatment of all patients who were to undergo interferon treatment found this approach did not prevent depressive episodes.24,25 However, antidepressants have been beneficial for patients with subsyndromal depressive symptoms at baseline26 and after clinically significant depressive symptoms emerge.27 Electroconvulsive therapy also has been reported to effectively treat depression in HCV patients undergoing antiviral therapy.28
CASE CONTINUED: Lingering symptoms
Mr. P responds to citalopram with an improvement in mood, anhedonia, and appetite, but he continues to complain of low energy and poor concentration. In an effort to target these symptoms, methylphenidate, titrated to 30 mg/d in divided doses, is added to his regimen, which rapidly improves his symptoms. Insomnia is treated successfully with trazodone, 50 mg/d. Mr. P frequently visits his psychiatrist, who monitors his depressive symptoms using the BDI-II. Mr. P completes HCV treatment without recurrence of depressive symptoms or relapse to heroin use.
Although antidepressants are effective for treating affective and cognitive symptoms, they are not as effective for fatigue and other neurovegetative symptoms.16,29 The psychostimulants methylphenidate and dextroamphetamine and the nonstimulant modafinil have been studied for treating depressive symptoms in medically ill patients and can be used to treat IFN-α-induced fatigue.16,22,29
IFN-α’s effect on serotonin metabolism leads to a tryptophan-deficient state because of increased catabolism as a result of activation of indoleamine-2,3-dioxygenase (IDO). This has led to use of tryptophan supplementation, either as augmentation or monotherapy, for managing depressive symptoms in patients treated with IFN-α. Schaefer et al30 reported 3 cases where tryptophan supplementation significantly decreased depressive symptoms. Other researchers have argued that supplementing tryptophan in the context of IDO activation can lead to greater production of kynurenine and quinolinic acid, which have been linked to increased depressive symptoms in patients receiving IFN-α.31 They argue that supplementation of 5-HTP, which is available as a dietary supplement without a prescription, can lead to increased serotonin levels and improvement in depressive symptoms.31
IFN-α treatment also is associated with mania and psychosis. The incidence, pathophysiology, and management of these treatment-emergent symptoms are not as well studied as IFN-α-induced depression. Mania and hypomania have been reported with interferon treatment, discontinuation of interferon, and use of antidepressants for interferon-induced depression.29,32 Psychosis, in association with mood symptoms or alone, has been reported to occur in <1% of treated patients.33 Treatment for mania and psychosis consists of decreasing or discontinuing immunotherapy and adding mood stabilizers and antipsychotics. Once immunotherapy is discontinued, mania and psychosis usually resolve, but prolonged duration of symptoms has been reported.29,32,33
Related Resources
- Centers for Disease Control and Prevention. Hepatitis C information for health professionals. www.cdc.gov/hepatitis/hcv/index.htm.
- Hep C Connection. www.hepc-connection.org.
- United States Department of Veterans Affairs. Hepatitis C. www.hepatitis.va.gov.
Drug Brand Names
- Boceprevir • Victrelis
- Citalopram • Celexa
- Dextroamphetamine • Dexedrine
- Escitalopram • Lexapro
- Interferon-α • Intron
- Methadone • Dolophine, Methadose
- Methylphenidate • Ritalin, Methylin, others
- Modafinil • Provigil
- Ondansetron • Zofran
- Ribavirin • Copegus, Rebetol, others
- Telaprevir • Incivek
- Trazodone • Desyrel, Oleptro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. P, age 31, has been using heroin intravenously for 9 years. He smokes 1 pack of cigarettes daily, but denies using other substances, including alcohol. After an unintentional heroin overdose, Mr. P enrolls in a methadone maintenance treatment program (MMTP) that includes primary medical care and addiction medicine and psychiatric specialists, where he undergoes medical evaluation and screening for hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Laboratory data reveal that although Mr. P is HIV negative, he has been exposed to HCV and treatment is indicated.
Among the approximately 3 million people in the United States with chronic HCV—an enveloped, single-stranded RNA virus—there’s a high prevalence of premorbid psychopathology and substance abuse, as well as neuropsychiatric effects caused by HCV treatment.1-3 Because underdiagnosing and undertreating psychiatric disorders contributes to morbidity and mortality in HCV patients, early identification and prompt treatment is critical.
IV drug use is the most common route for HCV infection, accounting for 65% to 70% of infections.1 The prevalence of HCV among IV drug users is 28% to 90%.1 Once exposed to HCV, 75% to 85% of patients do not clear the initial infection and become chronically infected.
This article reviews the pathophysiology, identification, and management of psychiatric manifestations found among HCV patients and provides an understanding of how psychiatric symptoms manifest in HCV patients. This article also discusses HCV treatment and its neuropsychiatric side effects.
Testing for HCV
Chronically infected HCV patients may have few, if any, specific physical complaints, and often are diagnosed during screenings or other routine laboratory evaluations. The presence of risk factors, such as a history of injection drug use or receiving a blood transfusion before 1992,1 guides the decision to screen for HCV. Normal liver function test results should not preclude testing because many HCV-positive patients have transaminases within the normal range.4 Initial screening is via an antibody-mediated immunoassay that is highly specific and sensitive for past exposure to HCV (Table 1).4 However, a positive screen does not indicate the presence of active infection. Evidence of the virus via a viral assay will identify active HCV, but does not indicate need for treatment. Liver biopsy confirms the presence of liver injury and quantifies its extent. The severity of liver damage will determine whether treatment is needed. HCV genotyping determines the appropriate duration and dosage of pharmacotherapy.
Table 1
Tests to diagnose and evaluate HCV
| Test | Results |
|---|---|
| HCV antibody | Determines prior exposure to HCV |
| HCV viral assay | Evaluates for current HCV infection |
| Liver biopsy | Assesses level of liver damage |
| HCV genotyping | Provides data to determine duration and intensity of treatment and likelihood of treatment success |
| HCV: hepatitis C virus Source: Reference 4 | |
CASE CONTINUED: Mood improves, but fatigue persists
As part of pre-HCV treatment evaluation, Mr. P undergoes a psychiatric evaluation. He describes periods of low mood while actively engaged in drug use but has never received psychiatric treatment, experienced suicidal ideation, or attempted suicide. Since starting opioid agonist therapy, he reports improved mood but endorses continued mild fatigue and difficulty falling sleep. The psychiatrist determines Mr. P does not meet criteria for an axis I diagnosis other than a substance use disorder.
Although most HCV patients have few, if any, nonspecific physical symptoms, many have psychiatric symptoms or disorders before the HCV diagnosis is made or treatment is initiated; substance use disorders are most common. Batki et al1 found that 56% of HCV patients in an MMTP met criteria for a nonsubstance axis I disorder and 82% met criteria for such a disorder during their lifetime. Additionally, 66% of patients were taking psychiatric medications. Table 21,5,6 lists the rates of other psychiatric disorders found in patients with untreated HCV.
Table 2
Rates of psychiatric disorders in patients with untreated hepatitis C virus
| Disorder(s) | Current rate | Lifetime rate |
|---|---|---|
| Mood disorders | 34% to 35% | 67% |
| Major depressive disorder | 22% to 28% | 42% |
| Anxiety disorders | 26% to 44% | 63% |
| Antisocial personality disorder | No rates; lifetime diagnosis | 16% to 40% |
| Psychotic disorders | 9% to 17% | 11% |
| Substance use disorder | 56% | 56% to 86% |
| Source: References 1,5,6 | ||
Many patients with chronic HCV complain of chronic fatigue and deficiencies in attention, concentration, higher executive functions, learning ability, and memory that result in significant reduction in quality of life (Box 1).7-9 These findings have been found to be independent of the degree of liver disease and are seen in HCV patients with normal liver function.7,8
The pathophysiology of fatigue and neurocognitive dysfunction in hepatitis C virus (HCV) infection is unclear. However, the improvement of chronic fatigue in patients with HCV who receive ondansetron, a 5-hydroxytryptophan-3 receptor antagonist, has implicated abnormal monoaminergic function. Single-photon emission CT studies have found decreased midbrain serotonergic and striatal dopaminergic transmission in some HCV patients with cognitive deficits.7
Recently, data have been mounting on a direct neuropathic effect of HCV, with viral elements found in autopsy brain tissue and cerebrospinal fluid.8 Researchers have suggested that HCV may enter the CNS via a Trojan horse-like mechanism inside infected mononuclear cells.8 More recently, human brain microvascular endothelium, the major component of the blood-brain barrier, has been found to express all major viral receptors that would allow HCV infection of the CNS.9
CASE CONTINUED: Motivated and compliant
Since joining the MMTP 6 months ago, Mr. P has been motivated and compliant with all appointments and treatments. Routine urine toxicology screening supports his claim of abstinence. Mr. P begins HCV treatment while continuing follow-up with addiction medicine and psychiatric clinicians and maintains open communication with all treatment providers.
For many years the standard HCV treatment was pegylated interferon-α (IFN-α) and ribavirin. IFN-α is a proinflammatory cytokine with antiproliferative, antiviral, and immunoregulatory properties. The half-life of IFN-α significantly increases with pegylation, which allows for weekly injections.10,11 IFN-α usually is combined with ribavirin, which increases its efficacy as measured by the sustained virological response (SVR) compared with IFN-α alone. Depending on the virus genotype, treatment lasts 24 to 48 weeks; SVR rates range from 40% to 82%.11-13 In 2011, the FDA approved 2 agents—telaprevir and boceprevir—for adjunctive treatment of HCV genotype 1 infection. These 2 agents are protease inhibitors that when added to IFN-α and ribavirin increase the SVR rate in genotype 1 infection from 40% to 50% to approximately 75%.14,15
Although the neuropsychiatric side effects of telaprevir and boceprevir have not been determined, treating chronic HCV with IFN-α and ribavirin has been associated with multiple psychiatric symptoms, including depression, mania, suicidality, anxiety, and psychosis.11-14 Psychiatric symptoms are a common reason for discontinuing or reducing HCV treatment. Because of the high frequency of neuropsychiatric complications, some clinicians believe HCV patients with preexisting affective, psychotic, or substance use disorders should be excluded from HCV treatment. This has led to many HCV patients being untreated despite a lack of prospective, controlled data to support this opinion.12 To improve outcomes and decrease morbidity, providing appropriate psychiatric services appears to be more important than attempting to select lower-risk patients for antiviral therapy.1,12,16 The goals of psychiatric treatment should be to alleviate symptoms and allow patients to complete IFN-α therapy without interruption.16,17
Studies of high-risk patients who attend multidisciplinary treatment programs that can monitor adherence and efficacy and control side effects before and during HCV treatment have found psychiatric patients have similar adherence, compliance, and SVR rates and were not at increased risk of worsening depressive or psychotic symptoms compared with patients without a psychiatric history.12,18 Additionally, HCV patients with a psychiatric history are not at an increased risk of suicide.13,16 Similar findings have been observed in patients with active IV drug use or those receiving opioid agonist therapy. When HCV and substance use are treated simultaneously, patients can successfully complete HCV treatment with SVR rates comparable to those of patients not receiving opioid agonist therapy.19-21
CASE CONTINUED: Worsening symptoms
During a psychiatric follow-up 12 weeks after starting HCV treatment, Mr. P reports worsening depressive symptoms with low mood, decreased enjoyment of activities, poor sleep, low appetite, and fatigue. He shows no evidence of psychosis and denies suicidal ideation. We continue his HCV treatment, schedule more frequent psychiatric visits, and initiate citalopram, titrated to 40 mg/d.
Depressive symptoms, the most common neuropsychiatric manifestation of HCV, typically begin early in treatment, usually within the first 12 weeks. Two distinct symptom clusters are noted. A neurovegetative cluster characterized by reduced energy, anorexia, and psychomotor retardation typically begins within the first few months of treatment. Months later, a depression-specific syndrome appears that includes depressed mood, anxiety, and cognitive impairment.22
Depressive symptoms may occur in up to 60% of patients treated with IFN-α.11 When more rigorous depression measures are used, rates decrease to approximately 20% to 30%.11,13 Accurate diagnosis and treatment of emerging depressive symptoms is essential because untreated depression can lead to postponing or excluding patients from antiviral treatment.2 Screening instruments such as the Beck Depression Inventory-Second Edition (BDI-II) can be used to measure depressive symptoms in HCV patients with high sensitivity. However, because specificity has been low and somatic symptoms of chronic illness and depression often overlap, the BDI-II and other inventories may overestimate depression. Some researchers have suggested that focusing on questions targeting cognitive and affective symptoms rather than somatic ones may be a more valid measure of depression in patients undergoing immunotherapy for HCV.2
The immune system is implicated in IFN-α-induced depression because depressive symptoms share many features with a constellation of somatic and behavioral symptoms termed “sickness behavior.”11 These behaviors can occur when patients are exposed to cytokines that lead to a depressed level of functioning, which may allow the body to devote more energy to fighting illness. IFN-α, a cytokine, stimulates the immune system, which can lead to increases of interleukin (IL)-2, IL-6, and IL-10. Increased circulating levels of these ILs have been correlated with higher depression scores. Additionally, studies have found that patients who develop depression during IFN-α treatment have higher SVR rates, suggesting a more robust immune response.11,22 For a discussion of how serotonin metabolism and genetic polymorphisms also may help explain the prevalence of depression in patients with HCV, see Box 2.
Altered serotonin metabolism has been linked to depression in hepatitis C virus (HCV) patients treated with interferon-α (IFN-α). Tryptophan can be metabolized towards serotonin via tryptophan hydroxylase and niacin via indoleamine-2,3-dioxygenase (IDO) with kynurenine (KYN) and quinolinic acid (QUIN) as intermediaries. Introduction of IFN-α activates IDO, causing preferential conversion of tryptophan towards the niacin arm away from serotonin and leads to elevated levels of KYN and QUIN. KYN and QUIN are available centrally, are neurotoxic, and have been correlated with increased depressive symptoms in IFN-α-treated patients.a,b A tryptophan-deficient state is created, with less tryptophan being converted to serotonin and subsequently to its metabolite, 5-hydroxyindoleacetic acid (5-HIAA). Decreased levels of 5-HIAA in cerebrospinal fluid have been associated with higher depressive symptoms and higher rates of suicide.a,b
Several genetic polymorphisms may help identify patients at risk for developing IFN-α-induced depression. Genes for the 5’ promoter of the serotonin transporter (5-HTTLPR) have been investigated for roles in depression development in patients undergoing immunotherapy. Studies have found that persons with the short allele in the 5-HTTLPR gene are more likely to develop depression than those with the long allele. However, this has not been consistent across racial or ethnic groups.a,b Research also has associated the serotonin (5-HT) transporter, interferon receptor-A1, apolipoprotein ε4 allele, cyclooxygenase 2, and phospholipase A2 with development of a specific subgroup of symptoms.a
References
a. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
b. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
Treating depressed HCV patients
Antidepressants are the treatment of choice for IFN-α-induced depression. Most currently used antidepressants are effective22 and selective serotonin reuptake inhibitors are considered first choice.16 Antidepressant choice should be guided by principles similar to those used for patients without HCV: using side effects profiles to target specific symptoms and being mindful of pharmacokinetic properties.
Two treatment approaches have been investigated: prophylactic and symptomatic. A 2012 study23 of 181 HCV patients with no history of mental illness determined escitalopram, 10 mg/d, effectively reduced the incidence and severity of interferon-associated depression. Other studies examining prophylactic treatment of all patients who were to undergo interferon treatment found this approach did not prevent depressive episodes.24,25 However, antidepressants have been beneficial for patients with subsyndromal depressive symptoms at baseline26 and after clinically significant depressive symptoms emerge.27 Electroconvulsive therapy also has been reported to effectively treat depression in HCV patients undergoing antiviral therapy.28
CASE CONTINUED: Lingering symptoms
Mr. P responds to citalopram with an improvement in mood, anhedonia, and appetite, but he continues to complain of low energy and poor concentration. In an effort to target these symptoms, methylphenidate, titrated to 30 mg/d in divided doses, is added to his regimen, which rapidly improves his symptoms. Insomnia is treated successfully with trazodone, 50 mg/d. Mr. P frequently visits his psychiatrist, who monitors his depressive symptoms using the BDI-II. Mr. P completes HCV treatment without recurrence of depressive symptoms or relapse to heroin use.
Although antidepressants are effective for treating affective and cognitive symptoms, they are not as effective for fatigue and other neurovegetative symptoms.16,29 The psychostimulants methylphenidate and dextroamphetamine and the nonstimulant modafinil have been studied for treating depressive symptoms in medically ill patients and can be used to treat IFN-α-induced fatigue.16,22,29
IFN-α’s effect on serotonin metabolism leads to a tryptophan-deficient state because of increased catabolism as a result of activation of indoleamine-2,3-dioxygenase (IDO). This has led to use of tryptophan supplementation, either as augmentation or monotherapy, for managing depressive symptoms in patients treated with IFN-α. Schaefer et al30 reported 3 cases where tryptophan supplementation significantly decreased depressive symptoms. Other researchers have argued that supplementing tryptophan in the context of IDO activation can lead to greater production of kynurenine and quinolinic acid, which have been linked to increased depressive symptoms in patients receiving IFN-α.31 They argue that supplementation of 5-HTP, which is available as a dietary supplement without a prescription, can lead to increased serotonin levels and improvement in depressive symptoms.31
IFN-α treatment also is associated with mania and psychosis. The incidence, pathophysiology, and management of these treatment-emergent symptoms are not as well studied as IFN-α-induced depression. Mania and hypomania have been reported with interferon treatment, discontinuation of interferon, and use of antidepressants for interferon-induced depression.29,32 Psychosis, in association with mood symptoms or alone, has been reported to occur in <1% of treated patients.33 Treatment for mania and psychosis consists of decreasing or discontinuing immunotherapy and adding mood stabilizers and antipsychotics. Once immunotherapy is discontinued, mania and psychosis usually resolve, but prolonged duration of symptoms has been reported.29,32,33
Related Resources
- Centers for Disease Control and Prevention. Hepatitis C information for health professionals. www.cdc.gov/hepatitis/hcv/index.htm.
- Hep C Connection. www.hepc-connection.org.
- United States Department of Veterans Affairs. Hepatitis C. www.hepatitis.va.gov.
Drug Brand Names
- Boceprevir • Victrelis
- Citalopram • Celexa
- Dextroamphetamine • Dexedrine
- Escitalopram • Lexapro
- Interferon-α • Intron
- Methadone • Dolophine, Methadose
- Methylphenidate • Ritalin, Methylin, others
- Modafinil • Provigil
- Ondansetron • Zofran
- Ribavirin • Copegus, Rebetol, others
- Telaprevir • Incivek
- Trazodone • Desyrel, Oleptro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Batki SL, Canfield KM, Ploutz-Snyder R. Psychiatric and substance use disorders among methadone maintenance patients with chronic hepatitis C infection: effects on eligibility for hepatitis C treatment. Am J Addict. 2011;20(4):312-318.
2. Patterson AL, Morasco BJ, Fuller BE, et al. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33(4):354-362.
3. Maddur H, Kwo PY. Boceprevir. Hepatology. 2011;54(6):2254-2257.
4. Sylvestre D. Hepatitis C for addiction professionals. Addict Sci Clin Pract. 2007;4(1):34-41.
5. Dwight MM, Kowdley KV, Russo JE, et al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49(5):311-317.
6. Yovtcheva SP, Rifai MA, Moles JK, et al. Psychiatric comorbidity among hepatitis C-positive patients. Psychosomatics. 2001;42(5):411-415.
7. Weissenborn K, Ennen JC, Bokemeyer M, et al. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55(11):1624-1630.
8. Weissenborn K, Tryc AB, Heeren M, et al. Hepatitis C virus infection and the brain. Metab Brain Dis. 2009;24(1):197-210.
9. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142(3):634-643.e6.
10. Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006;43(2 suppl 1):S207-S220.
11. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
12. Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46(4):991-998.
13. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
14. Telaprevir (Incivek) and boceprevir (Victrelis) for chronic hepatitis C. Med Lett Drugs Ther. 2011;53(1369):57-59.
15. Nelson DR. The role of triple therapy with protease inhibitors in hepatitis C virus genotype 1 naïve patients. Liver Int. 2011;31(suppl 1):53-57.
16. Spennati A, Pariante CM. Withdrawing interferon-α from psychiatric patients: clinical care or unjustifiable stigma? [published online September 14 2012] Psychol Med. doi: 10. 1017/S0033291712001808.
17. Baraldi S, Hepgul N, Mondelli V, et al. Symptomatic treatment of interferon-α-induced depression in hepatitis C: a systematic review. J Clin Psychopharmacol. 2012;32(4):531-543.
18. Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37(2):443-451.
19. Harris KA, Jr, Arnsten JH, Litwin AH. Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. J Addict Med. 2010;4(1):20-26.
20. Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37(1):32-40.
21. Sasadeusz JJ, Dore G, Kronborg I, et al. Clinical experience with the treatment of hepatitis C infection in patients on opioid pharmacotherapy. Addiction. 2011;106(5):977-984.
22. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
23. Schaefer M, Sarkar R, Knop V, et al. Escitalopram for the prevention of peginterferon-α2a-associated depression in hepatitis C virus-infected patients without previous psychiatric disease: a randomized trial. Ann Intern Med. 2012;157(2):94-103.
24. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
25. Morasco BJ, Loftis JM, Indest DW, et al. Prophylactic antidepressant treatment in patients with hepatitis C on antiviral therapy: a double-blind, placebo-controlled trial. Psychosomatics. 2010;51(5):401-408.
26. Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25(10):1163-1174.
27. Kraus MR, Schäfer A, Schöttker K, et al. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomised, double-blind, placebo-controlled study. Gut. 2008;57(4):531-536.
28. Zincke MT, Kurani A, Istafanous R, et al. The successful use of electroconvulsive therapy in a patient with interferon-induced psychotic depression. J ECT. 2007;23(4):291-292.
29. Crone CC, Gabriel GM, Wise TN. Managing the neuropsychiatric side effects of interferon-based therapy for hepatitis C. Cleve Clin J Med. 2004;71(suppl 3):S27-S32.
30. Schaefer M, Winterer J, Sarkar R, et al. Three cases of successful tryptophan add-on or monotherapy of hepatitis C and IFNa-associated mood disorders. Psychosomatics. 2008;49(5):442-446.
31. Turner EH, Blackwell AD. 5-Hydroxytryptophan plus SSRIs for interferon-induced depression: synergistic mechanisms for normalizing synaptic serotonin. Med Hypotheses. 2005;65(1):138-144.
32. Onyike CU, Bonner JO, Lyketsos CG, et al. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;161(3):429-435.
33. Cheng YC, Chen CC, Ho AS, et al. Prolonged psychosis associated with interferon therapy in a patient with hepatitis C: case study and literature review. Psychosomatics. 2009;50(5):538-542.
1. Batki SL, Canfield KM, Ploutz-Snyder R. Psychiatric and substance use disorders among methadone maintenance patients with chronic hepatitis C infection: effects on eligibility for hepatitis C treatment. Am J Addict. 2011;20(4):312-318.
2. Patterson AL, Morasco BJ, Fuller BE, et al. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33(4):354-362.
3. Maddur H, Kwo PY. Boceprevir. Hepatology. 2011;54(6):2254-2257.
4. Sylvestre D. Hepatitis C for addiction professionals. Addict Sci Clin Pract. 2007;4(1):34-41.
5. Dwight MM, Kowdley KV, Russo JE, et al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49(5):311-317.
6. Yovtcheva SP, Rifai MA, Moles JK, et al. Psychiatric comorbidity among hepatitis C-positive patients. Psychosomatics. 2001;42(5):411-415.
7. Weissenborn K, Ennen JC, Bokemeyer M, et al. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55(11):1624-1630.
8. Weissenborn K, Tryc AB, Heeren M, et al. Hepatitis C virus infection and the brain. Metab Brain Dis. 2009;24(1):197-210.
9. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142(3):634-643.e6.
10. Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006;43(2 suppl 1):S207-S220.
11. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
12. Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46(4):991-998.
13. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
14. Telaprevir (Incivek) and boceprevir (Victrelis) for chronic hepatitis C. Med Lett Drugs Ther. 2011;53(1369):57-59.
15. Nelson DR. The role of triple therapy with protease inhibitors in hepatitis C virus genotype 1 naïve patients. Liver Int. 2011;31(suppl 1):53-57.
16. Spennati A, Pariante CM. Withdrawing interferon-α from psychiatric patients: clinical care or unjustifiable stigma? [published online September 14 2012] Psychol Med. doi: 10. 1017/S0033291712001808.
17. Baraldi S, Hepgul N, Mondelli V, et al. Symptomatic treatment of interferon-α-induced depression in hepatitis C: a systematic review. J Clin Psychopharmacol. 2012;32(4):531-543.
18. Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37(2):443-451.
19. Harris KA, Jr, Arnsten JH, Litwin AH. Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. J Addict Med. 2010;4(1):20-26.
20. Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37(1):32-40.
21. Sasadeusz JJ, Dore G, Kronborg I, et al. Clinical experience with the treatment of hepatitis C infection in patients on opioid pharmacotherapy. Addiction. 2011;106(5):977-984.
22. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
23. Schaefer M, Sarkar R, Knop V, et al. Escitalopram for the prevention of peginterferon-α2a-associated depression in hepatitis C virus-infected patients without previous psychiatric disease: a randomized trial. Ann Intern Med. 2012;157(2):94-103.
24. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
25. Morasco BJ, Loftis JM, Indest DW, et al. Prophylactic antidepressant treatment in patients with hepatitis C on antiviral therapy: a double-blind, placebo-controlled trial. Psychosomatics. 2010;51(5):401-408.
26. Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25(10):1163-1174.
27. Kraus MR, Schäfer A, Schöttker K, et al. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomised, double-blind, placebo-controlled study. Gut. 2008;57(4):531-536.
28. Zincke MT, Kurani A, Istafanous R, et al. The successful use of electroconvulsive therapy in a patient with interferon-induced psychotic depression. J ECT. 2007;23(4):291-292.
29. Crone CC, Gabriel GM, Wise TN. Managing the neuropsychiatric side effects of interferon-based therapy for hepatitis C. Cleve Clin J Med. 2004;71(suppl 3):S27-S32.
30. Schaefer M, Winterer J, Sarkar R, et al. Three cases of successful tryptophan add-on or monotherapy of hepatitis C and IFNa-associated mood disorders. Psychosomatics. 2008;49(5):442-446.
31. Turner EH, Blackwell AD. 5-Hydroxytryptophan plus SSRIs for interferon-induced depression: synergistic mechanisms for normalizing synaptic serotonin. Med Hypotheses. 2005;65(1):138-144.
32. Onyike CU, Bonner JO, Lyketsos CG, et al. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;161(3):429-435.
33. Cheng YC, Chen CC, Ho AS, et al. Prolonged psychosis associated with interferon therapy in a patient with hepatitis C: case study and literature review. Psychosomatics. 2009;50(5):538-542.
Postpartum high blood pressure missed, mother suffers brain damage … and more
HOSPITALIZED TWICE FOR HYPERTENSION in the month before her child was born, a 41-year-old woman gave birth to a healthy baby by cesarean delivery. The mother was discharged 2 days later with a blood pressure (BP) of 130/90 mm Hg.
Three days later, she went to her ObGyn’s office because she was not feeling well and had extreme swelling. Her BP, taken twice by a nurse, read 170/88 mm Hg, and 168/90 mm Hg, but she was not examined by the ObGyn.
That evening, the patient had difficulty breathing and was taken to the emergency department (ED), where she was intubated. She went into cardiac arrest and suffered permanent brain damage after being without a pulse for 15 minutes. She was in a coma for 45 days. She is unable to walk without assistance, is legally blind, and her hands are so contorted that she cannot feed herself. She suffers from short-term memory loss and has difficulty speaking.
PATIENT’S CLAIM The ObGyn should have examined her when she was at the office. Her hypertension would have been properly treated and injuries avoided. She had classic signs of postpartum cardiomyopathy.
PHYSICIAN’S DEFENSE The patient had not come to the office because she was feeling ill, but to show off her baby and have her BP checked. If he had been advised of the BP readings, he would have examined her.
VERDICT A $5 million Georgia verdict was returned.
Cervical biopsy results improperly reported
A 44-YEAR-OLD WOMAN UNDERWENT a cervical biopsy in July 2007 performed by a pathologist. A few days later, the pathologist contacted the patient and reported that the biopsy revealed invasive cervical cancer that required immediate surgery. Several procedures were performed without any cancer ever being found.
A second opinion was sought, and it was determined that the cancer diagnosis was incorrect; another patient’s pathology had been reported as the patient’s.
PATIENT’S CLAIM The pathologist and hospital were negligent in reporting incorrect results of the cervical biopsy, which resulted in the patient’s physical and emotional injuries, including unnecessary surgical procedures and depression and anxiety.
DEFENDANTS’ DEFENSE The defendants did not oppose the patient’s motion for summary judgment on liability; the issue of damages was contested.
VERDICT The patient received summary judgment on liability. She then discontinued claims against the pathologist, and the matter proceeded on damages against the hospital. A $46,000 New York verdict was returned. Stipulated medical expenses were added to the verdict for a total recovery of $60,979.
Brachial plexus injury: child has significant functional disability
AT 38 6/7 WEEKS’ GESTATION, a 23-year-old woman went to the ED with contractions. She had pregestational diabetes mellitus. Her admitting glucose level was 143 mg/dL, and she had gained 25 lb during pregnancy. Her fundal height was 40 cm, and estimated fetal weight was 4000 g (8 lb 13 oz). A pelvic examination determined that she was 3 to 4 cm dilated, 100% effaced, and at minus-1 station. She was given oxytocin to aid labor. The ObGyn noted that overall fetal heart-rate tracings were reassuring, and that a pediatrician would be present for delivery due to suspected macrosomia. Shoulder dystocia occurred during delivery, but it was resolved in 40 seconds. The mother sustained a second-degree perineal laceration.
At birth, the baby’s left arm was limp. Apgar scores were 5 and 9 at 1 and 5 minutes, respectively. Her birth weight was 10 lb 2 oz. A brachial plexus injury was diagnosed, and she underwent surgery in October 2008. Despite successful nerve grafts at C5 and C6, the child has significant functional disability in the left arm.
PARENTS’ CLAIM A cesarean delivery should have been scheduled when a macrosomic fetus was suspected.
PHYSICIAN’S DEFENSE The case was settled during trial.
VERDICT A $1,475,000 Maryland settlement was reached.
DURING A MOTHER’S 38-WEEK PRENATAL VISIT, ultrasonography showed the baby was in breech position. The midwife offered two options: to schedule an external cephalic version procedure at 38 weeks or a cesarean delivery at 39 weeks. The parents agreed to schedule a cesarean delivery for 8 days later. The day before the scheduled birth, the mother awoke to find the umbilical cord between her legs. An emergency cesarean delivery was performed. The newborn required resuscitation and mechanical ventilation and suffered permanent brain damage attributed to hypoxia from umbilical cord prolapse.
PARENTS’ CLAIM The midwife’s negligence caused the baby’s injuries. Breech presentation put the pregnancy at high-risk. She did not have a physician examine the patient before scheduling a cesarean delivery and did not attempt to rotate the child back to a head-first position. She did not warn the parents about the risks of breech presentation and umbilical cord prolapse.
DEFENDANTS’ DEFENSE The choices given the parents were reasonable. Scheduling a cesarean delivery at 39 weeks was proper. A prolapsed cord is not predictable or preventable.
VERDICT A $12.6 million Pennsylvania verdict was returned against the midwife and the hospital; a confidential high/low agreement was reached.
Extensive adhesions result in bowel injury
A 58-YEAR-OLD WOMAN UNDERWENT exploratory laparotomy in May 2009. There were extensive adhesions, and the gynecologist used blind, blunt dissection to resect a large pelvic mass adhered to the sidewall. He had difficulty removing the specimen because it was too large to fit through the incision. A left salpingo-oophorectomy was also performed.
On the second postoperative day, the patient reported shortness of breath, intermittent chest pain, and had a fever of 103° F. The next day, she was unable to ambulate due to shortness of breath. CT results ruled out deep vein thrombosis or pulmonary embolism but revealed significantly decreased lung volume. She continued to experience shortness of breath and temperature spikes for 3 more days. She was discharged on the seventh postoperative day despite shortness of breath.
Two days later, she experienced severe abdominal pain and shortness of breath at home and returned to the ED by ambulance. A CT scan revealed free pelvic air, ascites, and extensive inflammatory changes, likely due to bowel perforation. She was intubated and airlifted to a regional trauma center. During exploratory surgery, the surgeon aspirated a foul-smelling fluid and identified a perforation at the rectosigmoid junction; a colostomy was created. The patient stayed in intensive care for 5 days, developed renal failure, and was transfused due to acute blood loss. She was hospitalized for 19 days. The colostomy was reversed in October 2009.
PATIENT’S CLAIM The ObGyn was negligent in injuring the bowel during surgery and in not recognizing the bowel injury and treating it in a timely manner.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $600,000 Virginia settlement was reached.
Pregnant woman stabbed: mother and baby die
A 20-YEAR-OLD WOMAN AT 30 WEEKS’ gestation was treated in the ED for a stab wound to the shoulder. The emergency medicine (EM) physician noted internal bleeding and a possible collapsed lung on radiographs, and began efforts to have the woman transferred. One facility declined because of her pregnancy. The patient was in pain and her ability to breathe declined. An airlift was finally arranged, but she suffered cardiac arrest as the helicopter arrived. A cesarean delivery was performed, but both the mother and baby died.
ESTATE’S CLAIM The EM physician was negligent in failing to perform a thoracotomy and arrange for a more timely transfer. The physician didn’t contact a hospital that was only 8 miles away.
DEFENDANTS’ DEFENSE The federal government, which operated the facility, admitted fault.
VERDICT A $7,267,390 Mississippi verdict included $5.45 million in noneconomic damages.
What caused child’s brain damage?
DURING LABOR AND DELIVERY, electronic fetal heart-rate monitoring indicated fetal distress. Meconium-stained fluid was present. The child was born with brain damage. It is unlikely that he will walk independently, talk in full sentences, or be able to perform daily activities independently.
PARENTS’ CLAIM The fetal heart-rate monitor indicated a need for emergency cesarean delivery. The quality and quantity of meconium should have alerted the caregivers to fetal distress and caused them to perform a cesarean delivery.
DEFENDANTS’ DEFENSE Fetal heart-rate strips did not indicate a need for emergency delivery until shortly before the delivery occurred. The underlying cause of the child’s injuries was an infection that spread to the brain and was irreversible.
VERDICT A $1.71 million Massachusetts verdict was returned.
When did bladder injury occur?
AN 84-YEAR-OLD WOMAN suffered recurrent bladder cancer. She underwent a cystoscopy, and then chemotherapy. Several weeks later, she was diagnosed with a bladder perforation became septic, and died.
ESTATE’S CLAIM The bladder was lacerated during cystoscopy; she would have survived if the laceration had been treated in a timely manner.
PHYSICIAN’S DEFENSE Bladder perforation during cystoscopy is a known risk of the procedure. However, the bladder was not perforated during cystoscopy; chemotherapy may have caused the perforation.
VERDICT A Michigan defense verdict was returned.
Fetal tracings poor: Why wasn’t an internal lead used?
AT 32 WEEKS’ GESTATION, a woman’s membranes ruptured, and she was admitted. Her ObGyn planned to induce labor at 34 weeks’ gestation. She experienced contractions on the morning of the scheduled induction. Although fetal heart-rate monitoring was reassuring, the fetus was in a compound position, with the chin leading. Labor progressed rapidly to 6-cm dilation. The fetal heart rate began to show recurrent mild variable decelerations that became increasingly deeper. Although the technical quality of the external monitoring was poor, no internal lead was applied.
After 3 hours, the tracing showed severe variable decelerations. The mother was fully dilated and began to push. The tracings were of poor quality, but interpretable portions showed minimal variability and significant decelerations during contractions. The fetal baseline heart rate became tachycardic. The obstetric nurse and resident continued to note abnormalities, but there is no evidence that they called the attending ObGyn. The fetal baseline heart rate reached 190 bpm with ongoing decelerations associated with contractions. Variability remained minimal to absent. After 2 hours of pushing, meconium-stained fluid was noted. The infant was born 1 hour later. The attending ObGyn was present for the last 30 minutes of labor.
The newborn’s Apgar scores were 1, 5, and 7, at 1, 5, and 10 minutes, respectively. His arterial cord pH was significantly low. MRI of the head showed subdural and intraventricular hemorrhage and evolving, profound hypoxic ischemic injury. At 1 year of age, the child suffers from a seizure disorder, cortical blindness, and severe developmental delays.
PARENTS’ CLAIM The nurse and resident failed to respond to fetal heart-rate abnormalities and failed to insert an internal lead to obtain better quality heart-rate tracings. They did not expedite delivery when fetal distress was evident.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $4.2 million Massachusetts settlement was reached.
Hypoxic ischemic encephalopathy
DUE TO PREECLAMPSIA, a woman was admitted to the hospital 5 weeks before her due date. Her condition was monitored for 2 weeks when it was decided to induce labor with oxytocin. After 3 hours in labor, the fetal heart-rate tracing began to show significant decelerations. The baby was born at 37 weeks’ gestation with severe hypoxic ischemic encephalopathy. The child died 2 years later from severe brain damage.
PARENTS’ CLAIM The ObGyns failed to respond to signs of fetal distress by performing an emergency cesarean. The brain images would have been different if a stroke-like event had occurred.
DEFENDANTS’ DEFENSE The fetus experienced an embolic process due to a compressed umbilical cord, resulting in a stroke-like vascular event, which led to the hypoxic ischemic encephalopathy.
VERDICT A $450,000 Wisconsin settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
HOSPITALIZED TWICE FOR HYPERTENSION in the month before her child was born, a 41-year-old woman gave birth to a healthy baby by cesarean delivery. The mother was discharged 2 days later with a blood pressure (BP) of 130/90 mm Hg.
Three days later, she went to her ObGyn’s office because she was not feeling well and had extreme swelling. Her BP, taken twice by a nurse, read 170/88 mm Hg, and 168/90 mm Hg, but she was not examined by the ObGyn.
That evening, the patient had difficulty breathing and was taken to the emergency department (ED), where she was intubated. She went into cardiac arrest and suffered permanent brain damage after being without a pulse for 15 minutes. She was in a coma for 45 days. She is unable to walk without assistance, is legally blind, and her hands are so contorted that she cannot feed herself. She suffers from short-term memory loss and has difficulty speaking.
PATIENT’S CLAIM The ObGyn should have examined her when she was at the office. Her hypertension would have been properly treated and injuries avoided. She had classic signs of postpartum cardiomyopathy.
PHYSICIAN’S DEFENSE The patient had not come to the office because she was feeling ill, but to show off her baby and have her BP checked. If he had been advised of the BP readings, he would have examined her.
VERDICT A $5 million Georgia verdict was returned.
Cervical biopsy results improperly reported
A 44-YEAR-OLD WOMAN UNDERWENT a cervical biopsy in July 2007 performed by a pathologist. A few days later, the pathologist contacted the patient and reported that the biopsy revealed invasive cervical cancer that required immediate surgery. Several procedures were performed without any cancer ever being found.
A second opinion was sought, and it was determined that the cancer diagnosis was incorrect; another patient’s pathology had been reported as the patient’s.
PATIENT’S CLAIM The pathologist and hospital were negligent in reporting incorrect results of the cervical biopsy, which resulted in the patient’s physical and emotional injuries, including unnecessary surgical procedures and depression and anxiety.
DEFENDANTS’ DEFENSE The defendants did not oppose the patient’s motion for summary judgment on liability; the issue of damages was contested.
VERDICT The patient received summary judgment on liability. She then discontinued claims against the pathologist, and the matter proceeded on damages against the hospital. A $46,000 New York verdict was returned. Stipulated medical expenses were added to the verdict for a total recovery of $60,979.
Brachial plexus injury: child has significant functional disability
AT 38 6/7 WEEKS’ GESTATION, a 23-year-old woman went to the ED with contractions. She had pregestational diabetes mellitus. Her admitting glucose level was 143 mg/dL, and she had gained 25 lb during pregnancy. Her fundal height was 40 cm, and estimated fetal weight was 4000 g (8 lb 13 oz). A pelvic examination determined that she was 3 to 4 cm dilated, 100% effaced, and at minus-1 station. She was given oxytocin to aid labor. The ObGyn noted that overall fetal heart-rate tracings were reassuring, and that a pediatrician would be present for delivery due to suspected macrosomia. Shoulder dystocia occurred during delivery, but it was resolved in 40 seconds. The mother sustained a second-degree perineal laceration.
At birth, the baby’s left arm was limp. Apgar scores were 5 and 9 at 1 and 5 minutes, respectively. Her birth weight was 10 lb 2 oz. A brachial plexus injury was diagnosed, and she underwent surgery in October 2008. Despite successful nerve grafts at C5 and C6, the child has significant functional disability in the left arm.
PARENTS’ CLAIM A cesarean delivery should have been scheduled when a macrosomic fetus was suspected.
PHYSICIAN’S DEFENSE The case was settled during trial.
VERDICT A $1,475,000 Maryland settlement was reached.
DURING A MOTHER’S 38-WEEK PRENATAL VISIT, ultrasonography showed the baby was in breech position. The midwife offered two options: to schedule an external cephalic version procedure at 38 weeks or a cesarean delivery at 39 weeks. The parents agreed to schedule a cesarean delivery for 8 days later. The day before the scheduled birth, the mother awoke to find the umbilical cord between her legs. An emergency cesarean delivery was performed. The newborn required resuscitation and mechanical ventilation and suffered permanent brain damage attributed to hypoxia from umbilical cord prolapse.
PARENTS’ CLAIM The midwife’s negligence caused the baby’s injuries. Breech presentation put the pregnancy at high-risk. She did not have a physician examine the patient before scheduling a cesarean delivery and did not attempt to rotate the child back to a head-first position. She did not warn the parents about the risks of breech presentation and umbilical cord prolapse.
DEFENDANTS’ DEFENSE The choices given the parents were reasonable. Scheduling a cesarean delivery at 39 weeks was proper. A prolapsed cord is not predictable or preventable.
VERDICT A $12.6 million Pennsylvania verdict was returned against the midwife and the hospital; a confidential high/low agreement was reached.
Extensive adhesions result in bowel injury
A 58-YEAR-OLD WOMAN UNDERWENT exploratory laparotomy in May 2009. There were extensive adhesions, and the gynecologist used blind, blunt dissection to resect a large pelvic mass adhered to the sidewall. He had difficulty removing the specimen because it was too large to fit through the incision. A left salpingo-oophorectomy was also performed.
On the second postoperative day, the patient reported shortness of breath, intermittent chest pain, and had a fever of 103° F. The next day, she was unable to ambulate due to shortness of breath. CT results ruled out deep vein thrombosis or pulmonary embolism but revealed significantly decreased lung volume. She continued to experience shortness of breath and temperature spikes for 3 more days. She was discharged on the seventh postoperative day despite shortness of breath.
Two days later, she experienced severe abdominal pain and shortness of breath at home and returned to the ED by ambulance. A CT scan revealed free pelvic air, ascites, and extensive inflammatory changes, likely due to bowel perforation. She was intubated and airlifted to a regional trauma center. During exploratory surgery, the surgeon aspirated a foul-smelling fluid and identified a perforation at the rectosigmoid junction; a colostomy was created. The patient stayed in intensive care for 5 days, developed renal failure, and was transfused due to acute blood loss. She was hospitalized for 19 days. The colostomy was reversed in October 2009.
PATIENT’S CLAIM The ObGyn was negligent in injuring the bowel during surgery and in not recognizing the bowel injury and treating it in a timely manner.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $600,000 Virginia settlement was reached.
Pregnant woman stabbed: mother and baby die
A 20-YEAR-OLD WOMAN AT 30 WEEKS’ gestation was treated in the ED for a stab wound to the shoulder. The emergency medicine (EM) physician noted internal bleeding and a possible collapsed lung on radiographs, and began efforts to have the woman transferred. One facility declined because of her pregnancy. The patient was in pain and her ability to breathe declined. An airlift was finally arranged, but she suffered cardiac arrest as the helicopter arrived. A cesarean delivery was performed, but both the mother and baby died.
ESTATE’S CLAIM The EM physician was negligent in failing to perform a thoracotomy and arrange for a more timely transfer. The physician didn’t contact a hospital that was only 8 miles away.
DEFENDANTS’ DEFENSE The federal government, which operated the facility, admitted fault.
VERDICT A $7,267,390 Mississippi verdict included $5.45 million in noneconomic damages.
What caused child’s brain damage?
DURING LABOR AND DELIVERY, electronic fetal heart-rate monitoring indicated fetal distress. Meconium-stained fluid was present. The child was born with brain damage. It is unlikely that he will walk independently, talk in full sentences, or be able to perform daily activities independently.
PARENTS’ CLAIM The fetal heart-rate monitor indicated a need for emergency cesarean delivery. The quality and quantity of meconium should have alerted the caregivers to fetal distress and caused them to perform a cesarean delivery.
DEFENDANTS’ DEFENSE Fetal heart-rate strips did not indicate a need for emergency delivery until shortly before the delivery occurred. The underlying cause of the child’s injuries was an infection that spread to the brain and was irreversible.
VERDICT A $1.71 million Massachusetts verdict was returned.
When did bladder injury occur?
AN 84-YEAR-OLD WOMAN suffered recurrent bladder cancer. She underwent a cystoscopy, and then chemotherapy. Several weeks later, she was diagnosed with a bladder perforation became septic, and died.
ESTATE’S CLAIM The bladder was lacerated during cystoscopy; she would have survived if the laceration had been treated in a timely manner.
PHYSICIAN’S DEFENSE Bladder perforation during cystoscopy is a known risk of the procedure. However, the bladder was not perforated during cystoscopy; chemotherapy may have caused the perforation.
VERDICT A Michigan defense verdict was returned.
Fetal tracings poor: Why wasn’t an internal lead used?
AT 32 WEEKS’ GESTATION, a woman’s membranes ruptured, and she was admitted. Her ObGyn planned to induce labor at 34 weeks’ gestation. She experienced contractions on the morning of the scheduled induction. Although fetal heart-rate monitoring was reassuring, the fetus was in a compound position, with the chin leading. Labor progressed rapidly to 6-cm dilation. The fetal heart rate began to show recurrent mild variable decelerations that became increasingly deeper. Although the technical quality of the external monitoring was poor, no internal lead was applied.
After 3 hours, the tracing showed severe variable decelerations. The mother was fully dilated and began to push. The tracings were of poor quality, but interpretable portions showed minimal variability and significant decelerations during contractions. The fetal baseline heart rate became tachycardic. The obstetric nurse and resident continued to note abnormalities, but there is no evidence that they called the attending ObGyn. The fetal baseline heart rate reached 190 bpm with ongoing decelerations associated with contractions. Variability remained minimal to absent. After 2 hours of pushing, meconium-stained fluid was noted. The infant was born 1 hour later. The attending ObGyn was present for the last 30 minutes of labor.
The newborn’s Apgar scores were 1, 5, and 7, at 1, 5, and 10 minutes, respectively. His arterial cord pH was significantly low. MRI of the head showed subdural and intraventricular hemorrhage and evolving, profound hypoxic ischemic injury. At 1 year of age, the child suffers from a seizure disorder, cortical blindness, and severe developmental delays.
PARENTS’ CLAIM The nurse and resident failed to respond to fetal heart-rate abnormalities and failed to insert an internal lead to obtain better quality heart-rate tracings. They did not expedite delivery when fetal distress was evident.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $4.2 million Massachusetts settlement was reached.
Hypoxic ischemic encephalopathy
DUE TO PREECLAMPSIA, a woman was admitted to the hospital 5 weeks before her due date. Her condition was monitored for 2 weeks when it was decided to induce labor with oxytocin. After 3 hours in labor, the fetal heart-rate tracing began to show significant decelerations. The baby was born at 37 weeks’ gestation with severe hypoxic ischemic encephalopathy. The child died 2 years later from severe brain damage.
PARENTS’ CLAIM The ObGyns failed to respond to signs of fetal distress by performing an emergency cesarean. The brain images would have been different if a stroke-like event had occurred.
DEFENDANTS’ DEFENSE The fetus experienced an embolic process due to a compressed umbilical cord, resulting in a stroke-like vascular event, which led to the hypoxic ischemic encephalopathy.
VERDICT A $450,000 Wisconsin settlement was reached.
HOSPITALIZED TWICE FOR HYPERTENSION in the month before her child was born, a 41-year-old woman gave birth to a healthy baby by cesarean delivery. The mother was discharged 2 days later with a blood pressure (BP) of 130/90 mm Hg.
Three days later, she went to her ObGyn’s office because she was not feeling well and had extreme swelling. Her BP, taken twice by a nurse, read 170/88 mm Hg, and 168/90 mm Hg, but she was not examined by the ObGyn.
That evening, the patient had difficulty breathing and was taken to the emergency department (ED), where she was intubated. She went into cardiac arrest and suffered permanent brain damage after being without a pulse for 15 minutes. She was in a coma for 45 days. She is unable to walk without assistance, is legally blind, and her hands are so contorted that she cannot feed herself. She suffers from short-term memory loss and has difficulty speaking.
PATIENT’S CLAIM The ObGyn should have examined her when she was at the office. Her hypertension would have been properly treated and injuries avoided. She had classic signs of postpartum cardiomyopathy.
PHYSICIAN’S DEFENSE The patient had not come to the office because she was feeling ill, but to show off her baby and have her BP checked. If he had been advised of the BP readings, he would have examined her.
VERDICT A $5 million Georgia verdict was returned.
Cervical biopsy results improperly reported
A 44-YEAR-OLD WOMAN UNDERWENT a cervical biopsy in July 2007 performed by a pathologist. A few days later, the pathologist contacted the patient and reported that the biopsy revealed invasive cervical cancer that required immediate surgery. Several procedures were performed without any cancer ever being found.
A second opinion was sought, and it was determined that the cancer diagnosis was incorrect; another patient’s pathology had been reported as the patient’s.
PATIENT’S CLAIM The pathologist and hospital were negligent in reporting incorrect results of the cervical biopsy, which resulted in the patient’s physical and emotional injuries, including unnecessary surgical procedures and depression and anxiety.
DEFENDANTS’ DEFENSE The defendants did not oppose the patient’s motion for summary judgment on liability; the issue of damages was contested.
VERDICT The patient received summary judgment on liability. She then discontinued claims against the pathologist, and the matter proceeded on damages against the hospital. A $46,000 New York verdict was returned. Stipulated medical expenses were added to the verdict for a total recovery of $60,979.
Brachial plexus injury: child has significant functional disability
AT 38 6/7 WEEKS’ GESTATION, a 23-year-old woman went to the ED with contractions. She had pregestational diabetes mellitus. Her admitting glucose level was 143 mg/dL, and she had gained 25 lb during pregnancy. Her fundal height was 40 cm, and estimated fetal weight was 4000 g (8 lb 13 oz). A pelvic examination determined that she was 3 to 4 cm dilated, 100% effaced, and at minus-1 station. She was given oxytocin to aid labor. The ObGyn noted that overall fetal heart-rate tracings were reassuring, and that a pediatrician would be present for delivery due to suspected macrosomia. Shoulder dystocia occurred during delivery, but it was resolved in 40 seconds. The mother sustained a second-degree perineal laceration.
At birth, the baby’s left arm was limp. Apgar scores were 5 and 9 at 1 and 5 minutes, respectively. Her birth weight was 10 lb 2 oz. A brachial plexus injury was diagnosed, and she underwent surgery in October 2008. Despite successful nerve grafts at C5 and C6, the child has significant functional disability in the left arm.
PARENTS’ CLAIM A cesarean delivery should have been scheduled when a macrosomic fetus was suspected.
PHYSICIAN’S DEFENSE The case was settled during trial.
VERDICT A $1,475,000 Maryland settlement was reached.
DURING A MOTHER’S 38-WEEK PRENATAL VISIT, ultrasonography showed the baby was in breech position. The midwife offered two options: to schedule an external cephalic version procedure at 38 weeks or a cesarean delivery at 39 weeks. The parents agreed to schedule a cesarean delivery for 8 days later. The day before the scheduled birth, the mother awoke to find the umbilical cord between her legs. An emergency cesarean delivery was performed. The newborn required resuscitation and mechanical ventilation and suffered permanent brain damage attributed to hypoxia from umbilical cord prolapse.
PARENTS’ CLAIM The midwife’s negligence caused the baby’s injuries. Breech presentation put the pregnancy at high-risk. She did not have a physician examine the patient before scheduling a cesarean delivery and did not attempt to rotate the child back to a head-first position. She did not warn the parents about the risks of breech presentation and umbilical cord prolapse.
DEFENDANTS’ DEFENSE The choices given the parents were reasonable. Scheduling a cesarean delivery at 39 weeks was proper. A prolapsed cord is not predictable or preventable.
VERDICT A $12.6 million Pennsylvania verdict was returned against the midwife and the hospital; a confidential high/low agreement was reached.
Extensive adhesions result in bowel injury
A 58-YEAR-OLD WOMAN UNDERWENT exploratory laparotomy in May 2009. There were extensive adhesions, and the gynecologist used blind, blunt dissection to resect a large pelvic mass adhered to the sidewall. He had difficulty removing the specimen because it was too large to fit through the incision. A left salpingo-oophorectomy was also performed.
On the second postoperative day, the patient reported shortness of breath, intermittent chest pain, and had a fever of 103° F. The next day, she was unable to ambulate due to shortness of breath. CT results ruled out deep vein thrombosis or pulmonary embolism but revealed significantly decreased lung volume. She continued to experience shortness of breath and temperature spikes for 3 more days. She was discharged on the seventh postoperative day despite shortness of breath.
Two days later, she experienced severe abdominal pain and shortness of breath at home and returned to the ED by ambulance. A CT scan revealed free pelvic air, ascites, and extensive inflammatory changes, likely due to bowel perforation. She was intubated and airlifted to a regional trauma center. During exploratory surgery, the surgeon aspirated a foul-smelling fluid and identified a perforation at the rectosigmoid junction; a colostomy was created. The patient stayed in intensive care for 5 days, developed renal failure, and was transfused due to acute blood loss. She was hospitalized for 19 days. The colostomy was reversed in October 2009.
PATIENT’S CLAIM The ObGyn was negligent in injuring the bowel during surgery and in not recognizing the bowel injury and treating it in a timely manner.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $600,000 Virginia settlement was reached.
Pregnant woman stabbed: mother and baby die
A 20-YEAR-OLD WOMAN AT 30 WEEKS’ gestation was treated in the ED for a stab wound to the shoulder. The emergency medicine (EM) physician noted internal bleeding and a possible collapsed lung on radiographs, and began efforts to have the woman transferred. One facility declined because of her pregnancy. The patient was in pain and her ability to breathe declined. An airlift was finally arranged, but she suffered cardiac arrest as the helicopter arrived. A cesarean delivery was performed, but both the mother and baby died.
ESTATE’S CLAIM The EM physician was negligent in failing to perform a thoracotomy and arrange for a more timely transfer. The physician didn’t contact a hospital that was only 8 miles away.
DEFENDANTS’ DEFENSE The federal government, which operated the facility, admitted fault.
VERDICT A $7,267,390 Mississippi verdict included $5.45 million in noneconomic damages.
What caused child’s brain damage?
DURING LABOR AND DELIVERY, electronic fetal heart-rate monitoring indicated fetal distress. Meconium-stained fluid was present. The child was born with brain damage. It is unlikely that he will walk independently, talk in full sentences, or be able to perform daily activities independently.
PARENTS’ CLAIM The fetal heart-rate monitor indicated a need for emergency cesarean delivery. The quality and quantity of meconium should have alerted the caregivers to fetal distress and caused them to perform a cesarean delivery.
DEFENDANTS’ DEFENSE Fetal heart-rate strips did not indicate a need for emergency delivery until shortly before the delivery occurred. The underlying cause of the child’s injuries was an infection that spread to the brain and was irreversible.
VERDICT A $1.71 million Massachusetts verdict was returned.
When did bladder injury occur?
AN 84-YEAR-OLD WOMAN suffered recurrent bladder cancer. She underwent a cystoscopy, and then chemotherapy. Several weeks later, she was diagnosed with a bladder perforation became septic, and died.
ESTATE’S CLAIM The bladder was lacerated during cystoscopy; she would have survived if the laceration had been treated in a timely manner.
PHYSICIAN’S DEFENSE Bladder perforation during cystoscopy is a known risk of the procedure. However, the bladder was not perforated during cystoscopy; chemotherapy may have caused the perforation.
VERDICT A Michigan defense verdict was returned.
Fetal tracings poor: Why wasn’t an internal lead used?
AT 32 WEEKS’ GESTATION, a woman’s membranes ruptured, and she was admitted. Her ObGyn planned to induce labor at 34 weeks’ gestation. She experienced contractions on the morning of the scheduled induction. Although fetal heart-rate monitoring was reassuring, the fetus was in a compound position, with the chin leading. Labor progressed rapidly to 6-cm dilation. The fetal heart rate began to show recurrent mild variable decelerations that became increasingly deeper. Although the technical quality of the external monitoring was poor, no internal lead was applied.
After 3 hours, the tracing showed severe variable decelerations. The mother was fully dilated and began to push. The tracings were of poor quality, but interpretable portions showed minimal variability and significant decelerations during contractions. The fetal baseline heart rate became tachycardic. The obstetric nurse and resident continued to note abnormalities, but there is no evidence that they called the attending ObGyn. The fetal baseline heart rate reached 190 bpm with ongoing decelerations associated with contractions. Variability remained minimal to absent. After 2 hours of pushing, meconium-stained fluid was noted. The infant was born 1 hour later. The attending ObGyn was present for the last 30 minutes of labor.
The newborn’s Apgar scores were 1, 5, and 7, at 1, 5, and 10 minutes, respectively. His arterial cord pH was significantly low. MRI of the head showed subdural and intraventricular hemorrhage and evolving, profound hypoxic ischemic injury. At 1 year of age, the child suffers from a seizure disorder, cortical blindness, and severe developmental delays.
PARENTS’ CLAIM The nurse and resident failed to respond to fetal heart-rate abnormalities and failed to insert an internal lead to obtain better quality heart-rate tracings. They did not expedite delivery when fetal distress was evident.
DEFENDANTS’ DEFENSE The case was settled during trial.
VERDICT A $4.2 million Massachusetts settlement was reached.
Hypoxic ischemic encephalopathy
DUE TO PREECLAMPSIA, a woman was admitted to the hospital 5 weeks before her due date. Her condition was monitored for 2 weeks when it was decided to induce labor with oxytocin. After 3 hours in labor, the fetal heart-rate tracing began to show significant decelerations. The baby was born at 37 weeks’ gestation with severe hypoxic ischemic encephalopathy. The child died 2 years later from severe brain damage.
PARENTS’ CLAIM The ObGyns failed to respond to signs of fetal distress by performing an emergency cesarean. The brain images would have been different if a stroke-like event had occurred.
DEFENDANTS’ DEFENSE The fetus experienced an embolic process due to a compressed umbilical cord, resulting in a stroke-like vascular event, which led to the hypoxic ischemic encephalopathy.
VERDICT A $450,000 Wisconsin settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
ACOG to legislators: Partnership, not interference
Who’s in charge here?
It’s a legitimate question being asked by more physicians in all areas of the country as they struggle to provide good quality care. Yes, physicians face longstanding payment and coverage issues, regulations, and the insurance bureaucracy. But more and more often, physicians are struggling to care for their patients in the face of legislative interference that reaches right into their exam rooms. Who’s in charge here, indeed?
In this article, I detail several examples of legislative interference and describe the response of the American Congress of Obstetricians and Gynecologists (ACOG). I also detail a very healthy partnership ACOG has undertaken with the US Department of Health and Human Services (HHS) and the March of Dimes to end early elective deliveries before 39 weeks of gestation.

Physician gag law passes in Florida
State lawmakers in Florida have decided that physicians should no longer ask about guns in the home when performing a child wellness exam. The use of bike helmets and exposure to secondhand smoke are childhood health concerns worth mentioning, but the importance of keeping guns unloaded and locked away is not.
Under the Firearm Owners’ Privacy Act, enacted in 2011, physicians in Florida could be fined or imprisoned for initiating this conversation, and could be charged with a third-degree felony punishable by a fine of up to $5 million. Thanks to public pushback, the law was amended to remove the criminal penalty. Instead, patients who feel “harassed” by their physicians’ questions about gun safety can complain to the Florida Board of Medicine, which can take disciplinary action against an offending physician.
In November 2012, ACOG joined an amicus brief in the case of Wollschlaeger v the State of Florida, asking the court to overturn the Florida bill, now known as the “physician gag law,” challenging, in part, the government’s right to interfere with a physician’s freedom of speech.
In another example of legislative interference, energy production politics gets in the way of doctors sharing relevant medical information with their patients. Four states—Colorado, Ohio, Pennsylvania, and Texas—prohibit physicians from disclosing information about exposure to chemicals used in hydraulic fracturing, or fracking. Scientific evidence shows that exposure to the chemicals used in fracking can result in a spectrum of health-care problems, from headaches to cancer. Can doctors talk about this with their patients? Not in these states.
While some states are trying to gag physicians by limiting what they can talk about with their patients, legislators in other states are considering requiring physicians to read, or offer to read, scripts to all patients who might have a terminal illness about end-of-life care options. Laws were enacted in California (2008) and New York (2011) to do just that. ObGyns are too familiar with legislatively mandated scripts; we know how inappropriate they are.
According to the Guttmacher Institute, in 2013, a number of states require abortion providers to read a script or provide written materials to patients seeking abortions; often these scripts contain medically inaccurate information. Twelve states require the physician to “inform” the patient about the ability of the fetus to feel pain, five states require the physician to claim that personhood begins at conception, and five states require doctors to say that abortion increases the risk of breast cancer. Six states require inaccurate information on the effects of abortion on future fertility.1
Serious penalties usually accompany these laws—financial fines, loss of licensure, and jail time. These and other legislative efforts infringe on physicians’ freedom of speech and force physicians to make terrible choices: Do you risk criminal prosecution or do you give your patient scientifically accurate and complete information? Do you adhere to your professional obligation to your patients, and risk putting your professional career on the line?
CLICK HERE to read other insightful articles by Ms. DiVenere.
Women’s reproductive health in the firing line
Nowhere is legislative interference more rampant than in the world of women’s health care. Over the past 2 years, an unprecedented number of bills have been introduced in the US Congress and statehouses restricting access to care for women and placing inappropriate requirements on physicians. The year 2011 was record-breaking in terms of abortion restrictions in the states, with 92 restrictions enacted.
In 2012, 42 states and the District of Columbia enacted 122 reproductive health provisions, one-third of them related to abortion restrictions. Forty-three new laws in 19 states were passed that restrict access to abortion. More than half of these new laws came from six states: Arizona was first with seven anti-abortion restrictions. Kansas, Louisiana, Oklahoma, South Dakota, and Wisconsin all had at least three.
In 2013, there have already been bills introduced in the US Congress and in the states that would:
- prohibit Title X family planning funds from going to clinics that provide abortions or prohibit funds from going to other entities that perform abortions (US Congress)
- repeal the Affordable Care Act, including the insurance protections and preventive services provisions that ACOG supports (US Congress)
- ban medical abortion (Mississippi)
- require women to undergo transvaginal ultrasound before having an abortion (Michigan)
- prohibit abortion after detection of a fetal heartbeat (at least three states: Arkansas, North Dakota, and Wyoming).
Few, if any, of these proposals are based on medical science. In fact, many of them run contrary to science and good patient-care principles. And although most of these efforts focus on reducing access to reproductive health care, including abortions, legislative interference is an issue of concern to physicians of all specialties, regardless of individual positions on life and choice.
What the medical community is doing
The American Medical Association (AMA) has made clear, consistent with the direction of its House of Delegates, that it fully opposes political interference in the patient-physician relationship. In 2012, the AMA unveiled its “Protect the sanctity of the patient-physician relationship” campaign with a panel that included Dr. Erin Tracy, chair of the Massachusetts Section of ACOG; Dr. Tim Bartholow, chief medical officer of the Wisconsin Medical Society; and Dr. H. Garry Gardner, chair of the American Academy of Pediatrics’ (AAP) Council on Injury, Violence, and Poison Prevention Executive Committee.
The AMA’s campaign is designed to educate physicians and to work with state medical societies and specialties to “articulate a compelling and comprehensive legal foundation to oppose legislation that encroaches on the sanctity of the patient-physician relationship.”
ACOG’s leadership has directly and forcefully pushed back on legislative encroachment. In 2012, ACOG Executive Vice President Hal C. Lawrence III, MD, and the executive leadership of the American Academy of Family Physicians (AAFP), AAP, the American College of Physicians, and the American College of Surgeons issued a joint statement that was published in the New England Journal of Medicine against legislative interference in the exam room.2
In addition, ACOG President James T. Breeden, MD, has written eight oped pieces, letters to the editor, and other public statements in venues with far and important reach, including the New York Times, USA Today, and Capitol Hill dailies (see the box). ACOG has also issued “Rapid Responses” to counter inaccurate statements about women’s health made in the media or on the campaign trail by state or national politicians.
In a paid ad message to the National Conference on State Legislatures (NCSL), which the NCSL refused to run, Dr. Breeden said, in part:
- Because we stand firmly for access to needed care, we also stand firmly against legislative interference with the patient-physician relationship. There’s only room for two people in our exam rooms: the patient and the caregiver. Lawmakers get in the way of good patient care when they try to force women to undergo transvaginal ultrasounds or other unnecessary medical procedures; when they try to close health clinics for specious reasons; or when they try to tell women that legislators know best.
Lawmakers can and do play a vitally productive and important role in ensuring public health. Lawmakers should not, however, attempt to define, mandate, or prohibit medical practices or require doctors to read a government script to their patients.
As ObGyns visit with state and federal legislators this year, our message is simple: Partnership with lawmakers, yes. Legislative interference, no.
Our campaign makes the point that there are a number of legitimate roles that state and federal governments play in public health. We welcome opportunities to partner with legislators on important women’s health-care needs. We draw the line at legislative interference of all stripes.
Here are just a few examples of ACOG’s many statements on behalf of women’s reproductive health
USA Today – Letter to the editor – May 21, 2012
“Politicians should not be legislating the practice of medicine or the doctor-patient relationship. We all need to speak up and take action when legislators pretend they know what’s best for women and their physicians.” —ACOG President James T. Breeden, MD
New York Times – Letter to the editor – June 4, 2012
“Politicians were not elected to, nor should they, legislate the practice of medicine or dictate the parameters of the doctor-patient relationship. Our message to politicians is unequivocal: Get out of our exam rooms.” —ACOG President James T. Breeden, MD
“Universal access to contraception could be a lifesaver” – Las Vegas Review Journal – July 22, 2012
“Contraception is a basic and essential element of women’s preventive health care and a basic public health necessity.” —ACOG President James T. Breeden, MD
ACOG Rapid Response to Rep. Todd Akin’s August 19, 2012 statement on “legitimate rape” – Issued August 20, 2012
“Absolutely no veracity to the claim … A woman who is raped has no control over ovulation, fertilization, or implantation of a fertilized egg. To suggest otherwise contradicts basic biological truths.”
ACOG Rapid Response to Rep. Joe Walsh’s October 18, 2012, statement that “Technology has advanced to the point that abortions are never needed to save the health or life of a mother” – Issued October 19, 2012
“Abortions are necessary in a number of circumstances to save the life of a woman or to preserve her health.”
A meaningful partnership: Strong Start
ACOG was invited to partner with the HHS and the March of Dimes on an initiative designed to bring about a meaningful and lasting improvement in maternity care: ending early elective deliveries before 39 weeks’ gestation. At the press conference announcing this partnership on the Strong Start initiative, Dr. Lawrence stood with HHS Secretary Kathleen Sebelius and said, in part:
- An ObGyn’s job is one of the most rewarding jobs on the planet, bringing little babies into the world. This job carries enormous responsibilities, too, ensuring the highest levels of health and safety for every mom and baby.
- The American College of Obstetricians and Gynecologists is proud to partner with the Department of Health and Human Services and the March of Dimes on one of the most certain ways of helping babies get a good start in life: Babies should not be delivered earlier than 39 weeks, unless pregnancy complications require otherwise to keep mother and child safe.
- Such a simple change, but one that can tremendously benefit children, families, our health system, and our society.
- Our joint initiative will help bring this important information to women and physicians across the nation, and has enormous potential to make a real and lasting change in how we care for expectant moms, and more importantly, how expectant moms expect us to care for them.
The Strong Start initiative is an all-too-rare example of a wonderful partnership between government, medicine, and the public to lead and create important change. This initiative goes far beyond the original press conference. Dr. Lawrence and other leaders have participated in webinars and interviews to spread the word. ACOG has developed patient education materials tailored to the message of no early elective deliveries before 39 weeks unless there is a maternal or fetal medical indication. Strong Start has provided funding to innovative maternity care models, including centering and pregnancy medical homes.
Earlier this year, Dr. Lawrence convened a Strong Start summit of the heads of the American Hospital Association, AAFP, AAP, the American Women’s Health and Neonatal Nurses Association, the American College of Nurse Midwives, the March of Dimes, and our federal partners, the Centers for Medicare and Medicaid Services and the Center for Medicare and Medicaid Innovation.
This one-day summit had a clear goal: gain unanimous agreement and commitment from the maternal care community to move our clinical knowledge into practice, ending nonmedically indicated early elective deliveries before 39 weeks.
Since 1979, ACOG has emphasized that labor should be induced “when the benefits of delivery to the fetus or the mother exceed the benefits of continuing the pregnancy,” and that pregnancies should be maintained until at least 39 weeks unless medical indications make early delivery necessary. This guidance was based on sound clinical knowledge in 1979, and today’s data are only more compelling. Somehow, however, early elective deliveries are still common and, for a variety of reasons, usually not related to infant or maternal health. This Strong Start summit is our specialty’s way of leading through partnership to encourage every maternity hospital in America to have in place a practice policy supporting no nonmedically indicated early elective deliveries before 39 weeks.
In many important ways, Strong Start is an example of the best that partnership with government and our colleagues has to offer.
The difference between partnership and interference is easy to see. That’s why we say: Partnership, yes. Interference, no.
We want to hear from you! Tell us what you think.
1. Guttmacher Institute. State Policies in Brief: Counseling and Waiting Period for Abortion. New York, NY, and Washington, DC: Guttmacher Institute; 2013.
2. Weinberger SE, Lawrence HC, III, Henley DE, et al. Legislative interference with the patient-physician relationship. N Engl J Med. 2012;367(16):1557-1559.
Who’s in charge here?
It’s a legitimate question being asked by more physicians in all areas of the country as they struggle to provide good quality care. Yes, physicians face longstanding payment and coverage issues, regulations, and the insurance bureaucracy. But more and more often, physicians are struggling to care for their patients in the face of legislative interference that reaches right into their exam rooms. Who’s in charge here, indeed?
In this article, I detail several examples of legislative interference and describe the response of the American Congress of Obstetricians and Gynecologists (ACOG). I also detail a very healthy partnership ACOG has undertaken with the US Department of Health and Human Services (HHS) and the March of Dimes to end early elective deliveries before 39 weeks of gestation.

Physician gag law passes in Florida
State lawmakers in Florida have decided that physicians should no longer ask about guns in the home when performing a child wellness exam. The use of bike helmets and exposure to secondhand smoke are childhood health concerns worth mentioning, but the importance of keeping guns unloaded and locked away is not.
Under the Firearm Owners’ Privacy Act, enacted in 2011, physicians in Florida could be fined or imprisoned for initiating this conversation, and could be charged with a third-degree felony punishable by a fine of up to $5 million. Thanks to public pushback, the law was amended to remove the criminal penalty. Instead, patients who feel “harassed” by their physicians’ questions about gun safety can complain to the Florida Board of Medicine, which can take disciplinary action against an offending physician.
In November 2012, ACOG joined an amicus brief in the case of Wollschlaeger v the State of Florida, asking the court to overturn the Florida bill, now known as the “physician gag law,” challenging, in part, the government’s right to interfere with a physician’s freedom of speech.
In another example of legislative interference, energy production politics gets in the way of doctors sharing relevant medical information with their patients. Four states—Colorado, Ohio, Pennsylvania, and Texas—prohibit physicians from disclosing information about exposure to chemicals used in hydraulic fracturing, or fracking. Scientific evidence shows that exposure to the chemicals used in fracking can result in a spectrum of health-care problems, from headaches to cancer. Can doctors talk about this with their patients? Not in these states.
While some states are trying to gag physicians by limiting what they can talk about with their patients, legislators in other states are considering requiring physicians to read, or offer to read, scripts to all patients who might have a terminal illness about end-of-life care options. Laws were enacted in California (2008) and New York (2011) to do just that. ObGyns are too familiar with legislatively mandated scripts; we know how inappropriate they are.
According to the Guttmacher Institute, in 2013, a number of states require abortion providers to read a script or provide written materials to patients seeking abortions; often these scripts contain medically inaccurate information. Twelve states require the physician to “inform” the patient about the ability of the fetus to feel pain, five states require the physician to claim that personhood begins at conception, and five states require doctors to say that abortion increases the risk of breast cancer. Six states require inaccurate information on the effects of abortion on future fertility.1
Serious penalties usually accompany these laws—financial fines, loss of licensure, and jail time. These and other legislative efforts infringe on physicians’ freedom of speech and force physicians to make terrible choices: Do you risk criminal prosecution or do you give your patient scientifically accurate and complete information? Do you adhere to your professional obligation to your patients, and risk putting your professional career on the line?
CLICK HERE to read other insightful articles by Ms. DiVenere.
Women’s reproductive health in the firing line
Nowhere is legislative interference more rampant than in the world of women’s health care. Over the past 2 years, an unprecedented number of bills have been introduced in the US Congress and statehouses restricting access to care for women and placing inappropriate requirements on physicians. The year 2011 was record-breaking in terms of abortion restrictions in the states, with 92 restrictions enacted.
In 2012, 42 states and the District of Columbia enacted 122 reproductive health provisions, one-third of them related to abortion restrictions. Forty-three new laws in 19 states were passed that restrict access to abortion. More than half of these new laws came from six states: Arizona was first with seven anti-abortion restrictions. Kansas, Louisiana, Oklahoma, South Dakota, and Wisconsin all had at least three.
In 2013, there have already been bills introduced in the US Congress and in the states that would:
- prohibit Title X family planning funds from going to clinics that provide abortions or prohibit funds from going to other entities that perform abortions (US Congress)
- repeal the Affordable Care Act, including the insurance protections and preventive services provisions that ACOG supports (US Congress)
- ban medical abortion (Mississippi)
- require women to undergo transvaginal ultrasound before having an abortion (Michigan)
- prohibit abortion after detection of a fetal heartbeat (at least three states: Arkansas, North Dakota, and Wyoming).
Few, if any, of these proposals are based on medical science. In fact, many of them run contrary to science and good patient-care principles. And although most of these efforts focus on reducing access to reproductive health care, including abortions, legislative interference is an issue of concern to physicians of all specialties, regardless of individual positions on life and choice.
What the medical community is doing
The American Medical Association (AMA) has made clear, consistent with the direction of its House of Delegates, that it fully opposes political interference in the patient-physician relationship. In 2012, the AMA unveiled its “Protect the sanctity of the patient-physician relationship” campaign with a panel that included Dr. Erin Tracy, chair of the Massachusetts Section of ACOG; Dr. Tim Bartholow, chief medical officer of the Wisconsin Medical Society; and Dr. H. Garry Gardner, chair of the American Academy of Pediatrics’ (AAP) Council on Injury, Violence, and Poison Prevention Executive Committee.
The AMA’s campaign is designed to educate physicians and to work with state medical societies and specialties to “articulate a compelling and comprehensive legal foundation to oppose legislation that encroaches on the sanctity of the patient-physician relationship.”
ACOG’s leadership has directly and forcefully pushed back on legislative encroachment. In 2012, ACOG Executive Vice President Hal C. Lawrence III, MD, and the executive leadership of the American Academy of Family Physicians (AAFP), AAP, the American College of Physicians, and the American College of Surgeons issued a joint statement that was published in the New England Journal of Medicine against legislative interference in the exam room.2
In addition, ACOG President James T. Breeden, MD, has written eight oped pieces, letters to the editor, and other public statements in venues with far and important reach, including the New York Times, USA Today, and Capitol Hill dailies (see the box). ACOG has also issued “Rapid Responses” to counter inaccurate statements about women’s health made in the media or on the campaign trail by state or national politicians.
In a paid ad message to the National Conference on State Legislatures (NCSL), which the NCSL refused to run, Dr. Breeden said, in part:
- Because we stand firmly for access to needed care, we also stand firmly against legislative interference with the patient-physician relationship. There’s only room for two people in our exam rooms: the patient and the caregiver. Lawmakers get in the way of good patient care when they try to force women to undergo transvaginal ultrasounds or other unnecessary medical procedures; when they try to close health clinics for specious reasons; or when they try to tell women that legislators know best.
Lawmakers can and do play a vitally productive and important role in ensuring public health. Lawmakers should not, however, attempt to define, mandate, or prohibit medical practices or require doctors to read a government script to their patients.
As ObGyns visit with state and federal legislators this year, our message is simple: Partnership with lawmakers, yes. Legislative interference, no.
Our campaign makes the point that there are a number of legitimate roles that state and federal governments play in public health. We welcome opportunities to partner with legislators on important women’s health-care needs. We draw the line at legislative interference of all stripes.
Here are just a few examples of ACOG’s many statements on behalf of women’s reproductive health
USA Today – Letter to the editor – May 21, 2012
“Politicians should not be legislating the practice of medicine or the doctor-patient relationship. We all need to speak up and take action when legislators pretend they know what’s best for women and their physicians.” —ACOG President James T. Breeden, MD
New York Times – Letter to the editor – June 4, 2012
“Politicians were not elected to, nor should they, legislate the practice of medicine or dictate the parameters of the doctor-patient relationship. Our message to politicians is unequivocal: Get out of our exam rooms.” —ACOG President James T. Breeden, MD
“Universal access to contraception could be a lifesaver” – Las Vegas Review Journal – July 22, 2012
“Contraception is a basic and essential element of women’s preventive health care and a basic public health necessity.” —ACOG President James T. Breeden, MD
ACOG Rapid Response to Rep. Todd Akin’s August 19, 2012 statement on “legitimate rape” – Issued August 20, 2012
“Absolutely no veracity to the claim … A woman who is raped has no control over ovulation, fertilization, or implantation of a fertilized egg. To suggest otherwise contradicts basic biological truths.”
ACOG Rapid Response to Rep. Joe Walsh’s October 18, 2012, statement that “Technology has advanced to the point that abortions are never needed to save the health or life of a mother” – Issued October 19, 2012
“Abortions are necessary in a number of circumstances to save the life of a woman or to preserve her health.”
A meaningful partnership: Strong Start
ACOG was invited to partner with the HHS and the March of Dimes on an initiative designed to bring about a meaningful and lasting improvement in maternity care: ending early elective deliveries before 39 weeks’ gestation. At the press conference announcing this partnership on the Strong Start initiative, Dr. Lawrence stood with HHS Secretary Kathleen Sebelius and said, in part:
- An ObGyn’s job is one of the most rewarding jobs on the planet, bringing little babies into the world. This job carries enormous responsibilities, too, ensuring the highest levels of health and safety for every mom and baby.
- The American College of Obstetricians and Gynecologists is proud to partner with the Department of Health and Human Services and the March of Dimes on one of the most certain ways of helping babies get a good start in life: Babies should not be delivered earlier than 39 weeks, unless pregnancy complications require otherwise to keep mother and child safe.
- Such a simple change, but one that can tremendously benefit children, families, our health system, and our society.
- Our joint initiative will help bring this important information to women and physicians across the nation, and has enormous potential to make a real and lasting change in how we care for expectant moms, and more importantly, how expectant moms expect us to care for them.
The Strong Start initiative is an all-too-rare example of a wonderful partnership between government, medicine, and the public to lead and create important change. This initiative goes far beyond the original press conference. Dr. Lawrence and other leaders have participated in webinars and interviews to spread the word. ACOG has developed patient education materials tailored to the message of no early elective deliveries before 39 weeks unless there is a maternal or fetal medical indication. Strong Start has provided funding to innovative maternity care models, including centering and pregnancy medical homes.
Earlier this year, Dr. Lawrence convened a Strong Start summit of the heads of the American Hospital Association, AAFP, AAP, the American Women’s Health and Neonatal Nurses Association, the American College of Nurse Midwives, the March of Dimes, and our federal partners, the Centers for Medicare and Medicaid Services and the Center for Medicare and Medicaid Innovation.
This one-day summit had a clear goal: gain unanimous agreement and commitment from the maternal care community to move our clinical knowledge into practice, ending nonmedically indicated early elective deliveries before 39 weeks.
Since 1979, ACOG has emphasized that labor should be induced “when the benefits of delivery to the fetus or the mother exceed the benefits of continuing the pregnancy,” and that pregnancies should be maintained until at least 39 weeks unless medical indications make early delivery necessary. This guidance was based on sound clinical knowledge in 1979, and today’s data are only more compelling. Somehow, however, early elective deliveries are still common and, for a variety of reasons, usually not related to infant or maternal health. This Strong Start summit is our specialty’s way of leading through partnership to encourage every maternity hospital in America to have in place a practice policy supporting no nonmedically indicated early elective deliveries before 39 weeks.
In many important ways, Strong Start is an example of the best that partnership with government and our colleagues has to offer.
The difference between partnership and interference is easy to see. That’s why we say: Partnership, yes. Interference, no.
We want to hear from you! Tell us what you think.
Who’s in charge here?
It’s a legitimate question being asked by more physicians in all areas of the country as they struggle to provide good quality care. Yes, physicians face longstanding payment and coverage issues, regulations, and the insurance bureaucracy. But more and more often, physicians are struggling to care for their patients in the face of legislative interference that reaches right into their exam rooms. Who’s in charge here, indeed?
In this article, I detail several examples of legislative interference and describe the response of the American Congress of Obstetricians and Gynecologists (ACOG). I also detail a very healthy partnership ACOG has undertaken with the US Department of Health and Human Services (HHS) and the March of Dimes to end early elective deliveries before 39 weeks of gestation.

Physician gag law passes in Florida
State lawmakers in Florida have decided that physicians should no longer ask about guns in the home when performing a child wellness exam. The use of bike helmets and exposure to secondhand smoke are childhood health concerns worth mentioning, but the importance of keeping guns unloaded and locked away is not.
Under the Firearm Owners’ Privacy Act, enacted in 2011, physicians in Florida could be fined or imprisoned for initiating this conversation, and could be charged with a third-degree felony punishable by a fine of up to $5 million. Thanks to public pushback, the law was amended to remove the criminal penalty. Instead, patients who feel “harassed” by their physicians’ questions about gun safety can complain to the Florida Board of Medicine, which can take disciplinary action against an offending physician.
In November 2012, ACOG joined an amicus brief in the case of Wollschlaeger v the State of Florida, asking the court to overturn the Florida bill, now known as the “physician gag law,” challenging, in part, the government’s right to interfere with a physician’s freedom of speech.
In another example of legislative interference, energy production politics gets in the way of doctors sharing relevant medical information with their patients. Four states—Colorado, Ohio, Pennsylvania, and Texas—prohibit physicians from disclosing information about exposure to chemicals used in hydraulic fracturing, or fracking. Scientific evidence shows that exposure to the chemicals used in fracking can result in a spectrum of health-care problems, from headaches to cancer. Can doctors talk about this with their patients? Not in these states.
While some states are trying to gag physicians by limiting what they can talk about with their patients, legislators in other states are considering requiring physicians to read, or offer to read, scripts to all patients who might have a terminal illness about end-of-life care options. Laws were enacted in California (2008) and New York (2011) to do just that. ObGyns are too familiar with legislatively mandated scripts; we know how inappropriate they are.
According to the Guttmacher Institute, in 2013, a number of states require abortion providers to read a script or provide written materials to patients seeking abortions; often these scripts contain medically inaccurate information. Twelve states require the physician to “inform” the patient about the ability of the fetus to feel pain, five states require the physician to claim that personhood begins at conception, and five states require doctors to say that abortion increases the risk of breast cancer. Six states require inaccurate information on the effects of abortion on future fertility.1
Serious penalties usually accompany these laws—financial fines, loss of licensure, and jail time. These and other legislative efforts infringe on physicians’ freedom of speech and force physicians to make terrible choices: Do you risk criminal prosecution or do you give your patient scientifically accurate and complete information? Do you adhere to your professional obligation to your patients, and risk putting your professional career on the line?
CLICK HERE to read other insightful articles by Ms. DiVenere.
Women’s reproductive health in the firing line
Nowhere is legislative interference more rampant than in the world of women’s health care. Over the past 2 years, an unprecedented number of bills have been introduced in the US Congress and statehouses restricting access to care for women and placing inappropriate requirements on physicians. The year 2011 was record-breaking in terms of abortion restrictions in the states, with 92 restrictions enacted.
In 2012, 42 states and the District of Columbia enacted 122 reproductive health provisions, one-third of them related to abortion restrictions. Forty-three new laws in 19 states were passed that restrict access to abortion. More than half of these new laws came from six states: Arizona was first with seven anti-abortion restrictions. Kansas, Louisiana, Oklahoma, South Dakota, and Wisconsin all had at least three.
In 2013, there have already been bills introduced in the US Congress and in the states that would:
- prohibit Title X family planning funds from going to clinics that provide abortions or prohibit funds from going to other entities that perform abortions (US Congress)
- repeal the Affordable Care Act, including the insurance protections and preventive services provisions that ACOG supports (US Congress)
- ban medical abortion (Mississippi)
- require women to undergo transvaginal ultrasound before having an abortion (Michigan)
- prohibit abortion after detection of a fetal heartbeat (at least three states: Arkansas, North Dakota, and Wyoming).
Few, if any, of these proposals are based on medical science. In fact, many of them run contrary to science and good patient-care principles. And although most of these efforts focus on reducing access to reproductive health care, including abortions, legislative interference is an issue of concern to physicians of all specialties, regardless of individual positions on life and choice.
What the medical community is doing
The American Medical Association (AMA) has made clear, consistent with the direction of its House of Delegates, that it fully opposes political interference in the patient-physician relationship. In 2012, the AMA unveiled its “Protect the sanctity of the patient-physician relationship” campaign with a panel that included Dr. Erin Tracy, chair of the Massachusetts Section of ACOG; Dr. Tim Bartholow, chief medical officer of the Wisconsin Medical Society; and Dr. H. Garry Gardner, chair of the American Academy of Pediatrics’ (AAP) Council on Injury, Violence, and Poison Prevention Executive Committee.
The AMA’s campaign is designed to educate physicians and to work with state medical societies and specialties to “articulate a compelling and comprehensive legal foundation to oppose legislation that encroaches on the sanctity of the patient-physician relationship.”
ACOG’s leadership has directly and forcefully pushed back on legislative encroachment. In 2012, ACOG Executive Vice President Hal C. Lawrence III, MD, and the executive leadership of the American Academy of Family Physicians (AAFP), AAP, the American College of Physicians, and the American College of Surgeons issued a joint statement that was published in the New England Journal of Medicine against legislative interference in the exam room.2
In addition, ACOG President James T. Breeden, MD, has written eight oped pieces, letters to the editor, and other public statements in venues with far and important reach, including the New York Times, USA Today, and Capitol Hill dailies (see the box). ACOG has also issued “Rapid Responses” to counter inaccurate statements about women’s health made in the media or on the campaign trail by state or national politicians.
In a paid ad message to the National Conference on State Legislatures (NCSL), which the NCSL refused to run, Dr. Breeden said, in part:
- Because we stand firmly for access to needed care, we also stand firmly against legislative interference with the patient-physician relationship. There’s only room for two people in our exam rooms: the patient and the caregiver. Lawmakers get in the way of good patient care when they try to force women to undergo transvaginal ultrasounds or other unnecessary medical procedures; when they try to close health clinics for specious reasons; or when they try to tell women that legislators know best.
Lawmakers can and do play a vitally productive and important role in ensuring public health. Lawmakers should not, however, attempt to define, mandate, or prohibit medical practices or require doctors to read a government script to their patients.
As ObGyns visit with state and federal legislators this year, our message is simple: Partnership with lawmakers, yes. Legislative interference, no.
Our campaign makes the point that there are a number of legitimate roles that state and federal governments play in public health. We welcome opportunities to partner with legislators on important women’s health-care needs. We draw the line at legislative interference of all stripes.
Here are just a few examples of ACOG’s many statements on behalf of women’s reproductive health
USA Today – Letter to the editor – May 21, 2012
“Politicians should not be legislating the practice of medicine or the doctor-patient relationship. We all need to speak up and take action when legislators pretend they know what’s best for women and their physicians.” —ACOG President James T. Breeden, MD
New York Times – Letter to the editor – June 4, 2012
“Politicians were not elected to, nor should they, legislate the practice of medicine or dictate the parameters of the doctor-patient relationship. Our message to politicians is unequivocal: Get out of our exam rooms.” —ACOG President James T. Breeden, MD
“Universal access to contraception could be a lifesaver” – Las Vegas Review Journal – July 22, 2012
“Contraception is a basic and essential element of women’s preventive health care and a basic public health necessity.” —ACOG President James T. Breeden, MD
ACOG Rapid Response to Rep. Todd Akin’s August 19, 2012 statement on “legitimate rape” – Issued August 20, 2012
“Absolutely no veracity to the claim … A woman who is raped has no control over ovulation, fertilization, or implantation of a fertilized egg. To suggest otherwise contradicts basic biological truths.”
ACOG Rapid Response to Rep. Joe Walsh’s October 18, 2012, statement that “Technology has advanced to the point that abortions are never needed to save the health or life of a mother” – Issued October 19, 2012
“Abortions are necessary in a number of circumstances to save the life of a woman or to preserve her health.”
A meaningful partnership: Strong Start
ACOG was invited to partner with the HHS and the March of Dimes on an initiative designed to bring about a meaningful and lasting improvement in maternity care: ending early elective deliveries before 39 weeks’ gestation. At the press conference announcing this partnership on the Strong Start initiative, Dr. Lawrence stood with HHS Secretary Kathleen Sebelius and said, in part:
- An ObGyn’s job is one of the most rewarding jobs on the planet, bringing little babies into the world. This job carries enormous responsibilities, too, ensuring the highest levels of health and safety for every mom and baby.
- The American College of Obstetricians and Gynecologists is proud to partner with the Department of Health and Human Services and the March of Dimes on one of the most certain ways of helping babies get a good start in life: Babies should not be delivered earlier than 39 weeks, unless pregnancy complications require otherwise to keep mother and child safe.
- Such a simple change, but one that can tremendously benefit children, families, our health system, and our society.
- Our joint initiative will help bring this important information to women and physicians across the nation, and has enormous potential to make a real and lasting change in how we care for expectant moms, and more importantly, how expectant moms expect us to care for them.
The Strong Start initiative is an all-too-rare example of a wonderful partnership between government, medicine, and the public to lead and create important change. This initiative goes far beyond the original press conference. Dr. Lawrence and other leaders have participated in webinars and interviews to spread the word. ACOG has developed patient education materials tailored to the message of no early elective deliveries before 39 weeks unless there is a maternal or fetal medical indication. Strong Start has provided funding to innovative maternity care models, including centering and pregnancy medical homes.
Earlier this year, Dr. Lawrence convened a Strong Start summit of the heads of the American Hospital Association, AAFP, AAP, the American Women’s Health and Neonatal Nurses Association, the American College of Nurse Midwives, the March of Dimes, and our federal partners, the Centers for Medicare and Medicaid Services and the Center for Medicare and Medicaid Innovation.
This one-day summit had a clear goal: gain unanimous agreement and commitment from the maternal care community to move our clinical knowledge into practice, ending nonmedically indicated early elective deliveries before 39 weeks.
Since 1979, ACOG has emphasized that labor should be induced “when the benefits of delivery to the fetus or the mother exceed the benefits of continuing the pregnancy,” and that pregnancies should be maintained until at least 39 weeks unless medical indications make early delivery necessary. This guidance was based on sound clinical knowledge in 1979, and today’s data are only more compelling. Somehow, however, early elective deliveries are still common and, for a variety of reasons, usually not related to infant or maternal health. This Strong Start summit is our specialty’s way of leading through partnership to encourage every maternity hospital in America to have in place a practice policy supporting no nonmedically indicated early elective deliveries before 39 weeks.
In many important ways, Strong Start is an example of the best that partnership with government and our colleagues has to offer.
The difference between partnership and interference is easy to see. That’s why we say: Partnership, yes. Interference, no.
We want to hear from you! Tell us what you think.
1. Guttmacher Institute. State Policies in Brief: Counseling and Waiting Period for Abortion. New York, NY, and Washington, DC: Guttmacher Institute; 2013.
2. Weinberger SE, Lawrence HC, III, Henley DE, et al. Legislative interference with the patient-physician relationship. N Engl J Med. 2012;367(16):1557-1559.
1. Guttmacher Institute. State Policies in Brief: Counseling and Waiting Period for Abortion. New York, NY, and Washington, DC: Guttmacher Institute; 2013.
2. Weinberger SE, Lawrence HC, III, Henley DE, et al. Legislative interference with the patient-physician relationship. N Engl J Med. 2012;367(16):1557-1559.
New ‘legal’ highs: Kratom and methoxetamine
The demand for “legal highs”— intoxicating natural or synthetic substances that are not prohibited by law—continues to increase. Young adults may use these substances, which are widely available on the internet, at “head shops,” and at gas stations. Such substances frequently cause adverse medical and psychiatric effects, exemplified by recent reports concerning the dangers of using synthetic cannabinoids (eg, “Spice,” “K2”) and synthetic cathinones (“bath salts”). Although these 2 substances now are illegal in many jurisdictions, other novel substances of misuse remain legal and widely available, including Kratom and methoxetamine.
Because these substances usually are not detectable on standard urine toxicology screens, clinicians need to be aware of them to be able to take an accurate substance use history, consider possible dangerous interactions with prescribed psychotropics, and address medical and psychiatric complications.
Kratom is an herbal product derived from Mitragyna speciosa, a plant native to Southeast Asia. Traditionally used as a medicinal herb, it increasingly is being used for recreational purposes and remains legal and widely available in the United States. Kratom’s leaves contain multiple alkaloids, including mitragynine and 7-hydroxymitragynine, which are believed to act as agonists at the μ-opioid receptor. Mitragynine also may have agonist activity at post-synaptic α2-adrenergic receptors, as well as antagonist activity at 5-HT2A receptors.1 Mitragynine is 13 times more potent than morphine, and 7-hydroxymitragynine is 4 times more potent than mitragynine.2
Kratom is available as leaves, powdered leaves, or gum. It can be smoked, brewed into tea, or mixed with liquid and ingested. Effects are dose-dependent; lower doses tend to produce a stimulant effect and higher doses produce an opioid effect. A typical dose is 1 to 8 g.3 Users may take Kratom to experience euphoria or analgesia, or to self-treat opioid withdrawal symptoms.3 Kratom withdrawal syndrome shares many features of classic opioid withdrawal—diarrhea, rhinorrhea, cravings, anxiety, tremor, myalgia, sweating, and irritability—but has been reported to be less severe and shorter-lasting.1 Kratom withdrawal, like opioid withdrawal, may respond to supportive care in combination with opioid-replacement therapy. Airway management and naloxone treatment may be needed on an emergent basis if a user develops respiratory depression.2 There have been case reports of seizures occurring following Kratom use.2
Methoxetamine is a ketamine analog originally developed as an alternative to ketamine. It isn’t classified as a controlled substance in the United States and is available on the internet.2 Methoxetamine is a white powder typically snorted or taken sublingually, although it can be injected intramuscularly. Because methoxetamine’s structure is similar to ketamine, its mechanism of action is assumed to involve glutamate N-methyl-D-aspartate receptor antagonism and dopamine reuptake inhibition. Doses range from 20 to 100 mg orally and 10 to 50 mg when injected. Effects may not be apparent for 30 to 90 minutes after the drug is snorted, which may cause users to take another dose or ingest a different substance, possibly leading to synergistic adverse effects. Effects may emerge within 5 minutes when injected. The duration of effect generally is 5 to 7 hours—notably longer than ketamine—but as little as 1 hour when injected.
No clinical human or animal studies have been conducted on methoxetamine, which makes it difficult to ascertain the drug’s true clinical and toxic effects; instead, these effects must be surmised from user reports and case studies. Desired effects described by users are similar to those of ketamine: dissociation, short-term mood elevation, visual hallucinations, and alteration of sensory experiences. Reported adverse effects include catatonia, confusion, agitation, and depression.4 In addition, methoxetamine may induce sympathomimetic toxicity as evidenced by tachycardia and hypertension. Researchers have suggested that patients who experience methoxetamine toxicity and require emergency treatment be managed with supportive care and benzodiazepines.5
Staying current is key
A paucity of clinical research on these substances means their effects are poorly understood, which creates a dangerous situation for users and physicians. In addition, many users assume these substances are safer than illegal substances. New and potentially dangerous substances are being produced so quickly distributors are able to stay ahead of regulatory efforts. When one substance is declared illegal, another related substance quickly is available to take its place. To provide the best care for our patients, it is essential for psychiatrists to stay up-to-date about these novel substances.
Disclosure
Dr. Troy reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
2. Rosenbaum CD, Carreiro SP, Babu KM. Here today gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), Kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
3. Boyer EW, Babu KM, Macalino GE. Self-treatment of opioid withdrawal with a dietary supplement Kratom. Am J Addict. 2007;16(5):352-356.
4. Corazza O, Schifano F, Simonato P, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27(2):145-149.
5. Wood DM, Davies S, Puchnarewicz M, et al. Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol. 2012;68(5):853-856.
The demand for “legal highs”— intoxicating natural or synthetic substances that are not prohibited by law—continues to increase. Young adults may use these substances, which are widely available on the internet, at “head shops,” and at gas stations. Such substances frequently cause adverse medical and psychiatric effects, exemplified by recent reports concerning the dangers of using synthetic cannabinoids (eg, “Spice,” “K2”) and synthetic cathinones (“bath salts”). Although these 2 substances now are illegal in many jurisdictions, other novel substances of misuse remain legal and widely available, including Kratom and methoxetamine.
Because these substances usually are not detectable on standard urine toxicology screens, clinicians need to be aware of them to be able to take an accurate substance use history, consider possible dangerous interactions with prescribed psychotropics, and address medical and psychiatric complications.
Kratom is an herbal product derived from Mitragyna speciosa, a plant native to Southeast Asia. Traditionally used as a medicinal herb, it increasingly is being used for recreational purposes and remains legal and widely available in the United States. Kratom’s leaves contain multiple alkaloids, including mitragynine and 7-hydroxymitragynine, which are believed to act as agonists at the μ-opioid receptor. Mitragynine also may have agonist activity at post-synaptic α2-adrenergic receptors, as well as antagonist activity at 5-HT2A receptors.1 Mitragynine is 13 times more potent than morphine, and 7-hydroxymitragynine is 4 times more potent than mitragynine.2
Kratom is available as leaves, powdered leaves, or gum. It can be smoked, brewed into tea, or mixed with liquid and ingested. Effects are dose-dependent; lower doses tend to produce a stimulant effect and higher doses produce an opioid effect. A typical dose is 1 to 8 g.3 Users may take Kratom to experience euphoria or analgesia, or to self-treat opioid withdrawal symptoms.3 Kratom withdrawal syndrome shares many features of classic opioid withdrawal—diarrhea, rhinorrhea, cravings, anxiety, tremor, myalgia, sweating, and irritability—but has been reported to be less severe and shorter-lasting.1 Kratom withdrawal, like opioid withdrawal, may respond to supportive care in combination with opioid-replacement therapy. Airway management and naloxone treatment may be needed on an emergent basis if a user develops respiratory depression.2 There have been case reports of seizures occurring following Kratom use.2
Methoxetamine is a ketamine analog originally developed as an alternative to ketamine. It isn’t classified as a controlled substance in the United States and is available on the internet.2 Methoxetamine is a white powder typically snorted or taken sublingually, although it can be injected intramuscularly. Because methoxetamine’s structure is similar to ketamine, its mechanism of action is assumed to involve glutamate N-methyl-D-aspartate receptor antagonism and dopamine reuptake inhibition. Doses range from 20 to 100 mg orally and 10 to 50 mg when injected. Effects may not be apparent for 30 to 90 minutes after the drug is snorted, which may cause users to take another dose or ingest a different substance, possibly leading to synergistic adverse effects. Effects may emerge within 5 minutes when injected. The duration of effect generally is 5 to 7 hours—notably longer than ketamine—but as little as 1 hour when injected.
No clinical human or animal studies have been conducted on methoxetamine, which makes it difficult to ascertain the drug’s true clinical and toxic effects; instead, these effects must be surmised from user reports and case studies. Desired effects described by users are similar to those of ketamine: dissociation, short-term mood elevation, visual hallucinations, and alteration of sensory experiences. Reported adverse effects include catatonia, confusion, agitation, and depression.4 In addition, methoxetamine may induce sympathomimetic toxicity as evidenced by tachycardia and hypertension. Researchers have suggested that patients who experience methoxetamine toxicity and require emergency treatment be managed with supportive care and benzodiazepines.5
Staying current is key
A paucity of clinical research on these substances means their effects are poorly understood, which creates a dangerous situation for users and physicians. In addition, many users assume these substances are safer than illegal substances. New and potentially dangerous substances are being produced so quickly distributors are able to stay ahead of regulatory efforts. When one substance is declared illegal, another related substance quickly is available to take its place. To provide the best care for our patients, it is essential for psychiatrists to stay up-to-date about these novel substances.
Disclosure
Dr. Troy reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
The demand for “legal highs”— intoxicating natural or synthetic substances that are not prohibited by law—continues to increase. Young adults may use these substances, which are widely available on the internet, at “head shops,” and at gas stations. Such substances frequently cause adverse medical and psychiatric effects, exemplified by recent reports concerning the dangers of using synthetic cannabinoids (eg, “Spice,” “K2”) and synthetic cathinones (“bath salts”). Although these 2 substances now are illegal in many jurisdictions, other novel substances of misuse remain legal and widely available, including Kratom and methoxetamine.
Because these substances usually are not detectable on standard urine toxicology screens, clinicians need to be aware of them to be able to take an accurate substance use history, consider possible dangerous interactions with prescribed psychotropics, and address medical and psychiatric complications.
Kratom is an herbal product derived from Mitragyna speciosa, a plant native to Southeast Asia. Traditionally used as a medicinal herb, it increasingly is being used for recreational purposes and remains legal and widely available in the United States. Kratom’s leaves contain multiple alkaloids, including mitragynine and 7-hydroxymitragynine, which are believed to act as agonists at the μ-opioid receptor. Mitragynine also may have agonist activity at post-synaptic α2-adrenergic receptors, as well as antagonist activity at 5-HT2A receptors.1 Mitragynine is 13 times more potent than morphine, and 7-hydroxymitragynine is 4 times more potent than mitragynine.2
Kratom is available as leaves, powdered leaves, or gum. It can be smoked, brewed into tea, or mixed with liquid and ingested. Effects are dose-dependent; lower doses tend to produce a stimulant effect and higher doses produce an opioid effect. A typical dose is 1 to 8 g.3 Users may take Kratom to experience euphoria or analgesia, or to self-treat opioid withdrawal symptoms.3 Kratom withdrawal syndrome shares many features of classic opioid withdrawal—diarrhea, rhinorrhea, cravings, anxiety, tremor, myalgia, sweating, and irritability—but has been reported to be less severe and shorter-lasting.1 Kratom withdrawal, like opioid withdrawal, may respond to supportive care in combination with opioid-replacement therapy. Airway management and naloxone treatment may be needed on an emergent basis if a user develops respiratory depression.2 There have been case reports of seizures occurring following Kratom use.2
Methoxetamine is a ketamine analog originally developed as an alternative to ketamine. It isn’t classified as a controlled substance in the United States and is available on the internet.2 Methoxetamine is a white powder typically snorted or taken sublingually, although it can be injected intramuscularly. Because methoxetamine’s structure is similar to ketamine, its mechanism of action is assumed to involve glutamate N-methyl-D-aspartate receptor antagonism and dopamine reuptake inhibition. Doses range from 20 to 100 mg orally and 10 to 50 mg when injected. Effects may not be apparent for 30 to 90 minutes after the drug is snorted, which may cause users to take another dose or ingest a different substance, possibly leading to synergistic adverse effects. Effects may emerge within 5 minutes when injected. The duration of effect generally is 5 to 7 hours—notably longer than ketamine—but as little as 1 hour when injected.
No clinical human or animal studies have been conducted on methoxetamine, which makes it difficult to ascertain the drug’s true clinical and toxic effects; instead, these effects must be surmised from user reports and case studies. Desired effects described by users are similar to those of ketamine: dissociation, short-term mood elevation, visual hallucinations, and alteration of sensory experiences. Reported adverse effects include catatonia, confusion, agitation, and depression.4 In addition, methoxetamine may induce sympathomimetic toxicity as evidenced by tachycardia and hypertension. Researchers have suggested that patients who experience methoxetamine toxicity and require emergency treatment be managed with supportive care and benzodiazepines.5
Staying current is key
A paucity of clinical research on these substances means their effects are poorly understood, which creates a dangerous situation for users and physicians. In addition, many users assume these substances are safer than illegal substances. New and potentially dangerous substances are being produced so quickly distributors are able to stay ahead of regulatory efforts. When one substance is declared illegal, another related substance quickly is available to take its place. To provide the best care for our patients, it is essential for psychiatrists to stay up-to-date about these novel substances.
Disclosure
Dr. Troy reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
2. Rosenbaum CD, Carreiro SP, Babu KM. Here today gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), Kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
3. Boyer EW, Babu KM, Macalino GE. Self-treatment of opioid withdrawal with a dietary supplement Kratom. Am J Addict. 2007;16(5):352-356.
4. Corazza O, Schifano F, Simonato P, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27(2):145-149.
5. Wood DM, Davies S, Puchnarewicz M, et al. Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol. 2012;68(5):853-856.
1. McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. Eur Addict Res. 2010;16(4):229-231.
2. Rosenbaum CD, Carreiro SP, Babu KM. Here today gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), Kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
3. Boyer EW, Babu KM, Macalino GE. Self-treatment of opioid withdrawal with a dietary supplement Kratom. Am J Addict. 2007;16(5):352-356.
4. Corazza O, Schifano F, Simonato P, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27(2):145-149.
5. Wood DM, Davies S, Puchnarewicz M, et al. Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol. 2012;68(5):853-856.
MEAN: How to manage a child who bullies
A survey from the National Institute of Child Health and Human Development estimated that 20% of 6th through 10th graders admitted to bullying their classmates.1 In addition to an increased risk for personal injury, bullied children are more likely to report low self-esteem and emotional problems2 and often experience loneliness.1 In contrast, children who bully suffer in their school performance1 and are more likely to engage in drug use3 and violence4 later in life. Child psychiatrists often see both bullies and their victims.
Evidence-based recommendations are available to help educators improve the school climate5 and identify children who are at an increased risk for bullying,6 but research supporting specific clinical strategies for managing a child who bullies is limited. Establishing rapport and engaging a bully often is challenging; these difficulties further complicate assessment and successful management of such children.
We present the mnemonic MEAN to help clinicians assess and understand children who bully.
Model. Discuss, demonstrate, and practice models of alternative social skills and behaviors, including active listening, being open to others’ views, accepting failure, controlling impulses, developing problem-solving techniques, and treating others with respect.
Empathize. Encourage children who bully to explore their feelings about themselves—which may uncover poor self-esteem, anger, or guilt—and acknowledge the hurt they cause others by bullying. Focusing on the pain they inflict on others in the context of personal experiences of pain that likely is driving their aggression may enable bullies to empathize with their victims.
Assess. Help the bully assess the costs and benefits of his or her behavior. Point out what the bully stands to gain from ending his or her aggressive behavior, which likely already has resulted in lost recesses, after school detentions, missed sports practices, and the loss of privileges at home. Most importantly, assess and treat any underlying psychopathology, including mood and anxiety disorders.
Nurture. Aid the bully in identifying his or her prosocial strengths to build self-esteem and thereby reduce the need to commit aggressive acts as a means of gaining a sense of control or personal security. Disarm the child with your genuine concern for his or her well-being.
Using these psychotherapeutic techniques may enhance establishing rapport with a child who bullies and may improve outcomes.
Disclosures
Dr. Kepple reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Madaan receives grant or research support from Eli Lilly and Company, Forest Pharmaceuticals, Merck, Otsuka, Pfizer Inc., and Shire.
1. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285(16):2094-2100.
2. Guerra NG, Williams KR, Sadek S. Understanding bullying and victimization during childhood and adolescence: a mixed methods study. Child Dev. 2011;82(1):295-310.
3. Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009;34(6-7):561-567.
4. Duke NN, Pettingell SL, McMorris BJ, et al. Adolescent violence perpetration: associations with multiple types of adverse childhood experiences. Pediatrics. 2010;125(4):e778-e786.
5. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80(1):124-134.
6. Jansen DE, Veenstra R, Ormel J, et al. Early risk factors for being a bully, victim, or bully/victim in late elementary and early secondary education. The longitudinal TRAILS study. BMC Public Health. 2011;11:440.-
A survey from the National Institute of Child Health and Human Development estimated that 20% of 6th through 10th graders admitted to bullying their classmates.1 In addition to an increased risk for personal injury, bullied children are more likely to report low self-esteem and emotional problems2 and often experience loneliness.1 In contrast, children who bully suffer in their school performance1 and are more likely to engage in drug use3 and violence4 later in life. Child psychiatrists often see both bullies and their victims.
Evidence-based recommendations are available to help educators improve the school climate5 and identify children who are at an increased risk for bullying,6 but research supporting specific clinical strategies for managing a child who bullies is limited. Establishing rapport and engaging a bully often is challenging; these difficulties further complicate assessment and successful management of such children.
We present the mnemonic MEAN to help clinicians assess and understand children who bully.
Model. Discuss, demonstrate, and practice models of alternative social skills and behaviors, including active listening, being open to others’ views, accepting failure, controlling impulses, developing problem-solving techniques, and treating others with respect.
Empathize. Encourage children who bully to explore their feelings about themselves—which may uncover poor self-esteem, anger, or guilt—and acknowledge the hurt they cause others by bullying. Focusing on the pain they inflict on others in the context of personal experiences of pain that likely is driving their aggression may enable bullies to empathize with their victims.
Assess. Help the bully assess the costs and benefits of his or her behavior. Point out what the bully stands to gain from ending his or her aggressive behavior, which likely already has resulted in lost recesses, after school detentions, missed sports practices, and the loss of privileges at home. Most importantly, assess and treat any underlying psychopathology, including mood and anxiety disorders.
Nurture. Aid the bully in identifying his or her prosocial strengths to build self-esteem and thereby reduce the need to commit aggressive acts as a means of gaining a sense of control or personal security. Disarm the child with your genuine concern for his or her well-being.
Using these psychotherapeutic techniques may enhance establishing rapport with a child who bullies and may improve outcomes.
Disclosures
Dr. Kepple reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Madaan receives grant or research support from Eli Lilly and Company, Forest Pharmaceuticals, Merck, Otsuka, Pfizer Inc., and Shire.
A survey from the National Institute of Child Health and Human Development estimated that 20% of 6th through 10th graders admitted to bullying their classmates.1 In addition to an increased risk for personal injury, bullied children are more likely to report low self-esteem and emotional problems2 and often experience loneliness.1 In contrast, children who bully suffer in their school performance1 and are more likely to engage in drug use3 and violence4 later in life. Child psychiatrists often see both bullies and their victims.
Evidence-based recommendations are available to help educators improve the school climate5 and identify children who are at an increased risk for bullying,6 but research supporting specific clinical strategies for managing a child who bullies is limited. Establishing rapport and engaging a bully often is challenging; these difficulties further complicate assessment and successful management of such children.
We present the mnemonic MEAN to help clinicians assess and understand children who bully.
Model. Discuss, demonstrate, and practice models of alternative social skills and behaviors, including active listening, being open to others’ views, accepting failure, controlling impulses, developing problem-solving techniques, and treating others with respect.
Empathize. Encourage children who bully to explore their feelings about themselves—which may uncover poor self-esteem, anger, or guilt—and acknowledge the hurt they cause others by bullying. Focusing on the pain they inflict on others in the context of personal experiences of pain that likely is driving their aggression may enable bullies to empathize with their victims.
Assess. Help the bully assess the costs and benefits of his or her behavior. Point out what the bully stands to gain from ending his or her aggressive behavior, which likely already has resulted in lost recesses, after school detentions, missed sports practices, and the loss of privileges at home. Most importantly, assess and treat any underlying psychopathology, including mood and anxiety disorders.
Nurture. Aid the bully in identifying his or her prosocial strengths to build self-esteem and thereby reduce the need to commit aggressive acts as a means of gaining a sense of control or personal security. Disarm the child with your genuine concern for his or her well-being.
Using these psychotherapeutic techniques may enhance establishing rapport with a child who bullies and may improve outcomes.
Disclosures
Dr. Kepple reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Madaan receives grant or research support from Eli Lilly and Company, Forest Pharmaceuticals, Merck, Otsuka, Pfizer Inc., and Shire.
1. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285(16):2094-2100.
2. Guerra NG, Williams KR, Sadek S. Understanding bullying and victimization during childhood and adolescence: a mixed methods study. Child Dev. 2011;82(1):295-310.
3. Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009;34(6-7):561-567.
4. Duke NN, Pettingell SL, McMorris BJ, et al. Adolescent violence perpetration: associations with multiple types of adverse childhood experiences. Pediatrics. 2010;125(4):e778-e786.
5. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80(1):124-134.
6. Jansen DE, Veenstra R, Ormel J, et al. Early risk factors for being a bully, victim, or bully/victim in late elementary and early secondary education. The longitudinal TRAILS study. BMC Public Health. 2011;11:440.-
1. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285(16):2094-2100.
2. Guerra NG, Williams KR, Sadek S. Understanding bullying and victimization during childhood and adolescence: a mixed methods study. Child Dev. 2011;82(1):295-310.
3. Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009;34(6-7):561-567.
4. Duke NN, Pettingell SL, McMorris BJ, et al. Adolescent violence perpetration: associations with multiple types of adverse childhood experiences. Pediatrics. 2010;125(4):e778-e786.
5. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80(1):124-134.
6. Jansen DE, Veenstra R, Ormel J, et al. Early risk factors for being a bully, victim, or bully/victim in late elementary and early secondary education. The longitudinal TRAILS study. BMC Public Health. 2011;11:440.-
8 tips for talking to parents and children about school shootings
In the aftermath of a school shooting, parents and teachers may seek a psychiatrist’s advice on how to best discuss these incidents with children. We offer guidelines on what to tell concerned parents, educators, and other adults who may interact with children affected by a school shooting.
6 tips for interacting with children
1. Talk about the event. Instruct adults to ask children to share their feelings about the incident and to show genuine interest in listening to the child’s thoughts and point of view. Adults shouldn’t pretend the event hasn’t occurred or isn’t serious. Children may be more worried if they think adults are too afraid to tell them what is happening. It is important to gently correct any misinformation older students may have received via social media.1
2. Reinforce that home is a safe haven. Overwhelming emotions and uncertainty can bring about a sense of insecurity in children. Children may come home seeking a safe environment. Advise parents to plan a night where family members participate in a favorite family activity.1 Tell parents to remind their children that trust-worthy adults—parents, emergency workers, police, firefighters, doctors, and the military—are helping provide safety, comfort, and support.2
3. Limit television time. If children are exposed to the news, parents should watch it with them briefly, but avoid letting children rewatch the same event repetitively. Constant exposure to the event may heighten a child’s anxiety and fears.
4. Maintain a normal routine. Tell parents they should maintain, as best they can, their normal routine for dinner, homework, chores, and bedtime, but to remain flexible.2 Children may have a hard time concentrating on schoolwork or falling asleep. Advise parents to spend extra time reading or playing quiet games with their children, particularly at bedtime. These activities are calming, foster a sense of closeness and security, and reinforce a feeling of normalcy.
5. Encourage emotions. Instruct parents to explain to their children that all feelings are okay and normal, and to let children talk about their feelings and help put them into perspective.1 Children may need help in expressing these feelings, so be patient. If an incident happened at the child’s school, teachers and administrators may conduct group sessions to help children express their concerns about being back in school.
6. Seek creativity or spirituality. Encourage parents and other adults to provide a creative outlet for children, such as making get well cards or sending letters to the survivors and their families. Writing thank you letters to doctors, nurses, fire-fighters, and police officers also may be comforting.1,2 Suggest that parents encourage their children to pray or think hopeful thoughts for the victims and their families.
2 tips for interacting with adults
7. Recommend they take care of themselves. Explain to adult caregivers that because children learn by observing, they shouldn’t ignore their own feelings of anxiety, grief, and anger. By expressing their emotions in a productive manner, adults will be better able to support their children. Encourage adults to talk to friends, family, religious leaders, or mental health counselors.
8. Advise adults to be alert for children who may need professional help. Tell them to be vigilant when monitoring a child’s emotional state. Children who may benefit from mental health counseling after a tragedy may exhibit warning signs, such as changes in behavior, appetite, and sleep patterns, which may indicate the child is experiencing grief, anxiety, or discomfort.
Remind adults to be aware of children who are at greater risk for mental health issues, including those who are already struggling with other recent traumatic experiences—past traumatic experiences, personal loss, depression, or other mental illness.1 Be particularly observant for children who may be at risk of suicide.1,2 Professional counseling may be needed for a child who is experiencing an emotional reaction that lasts >1 month and is impacting his or her daily functioning.1
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychological Association. Helping your children manage distress in the aftermath of a shooting. http://www.apa.org/helpcenter/aftermath.aspx. Updated April 2011. Accessed February 15, 2013.
2. National Association of School Psychologists resources. A national tragedy: helping children cope. http://www.nasponline.org/resources/crisis_safety/terror_general.aspx. Published September 2001. Accessed February 15, 2013.
In the aftermath of a school shooting, parents and teachers may seek a psychiatrist’s advice on how to best discuss these incidents with children. We offer guidelines on what to tell concerned parents, educators, and other adults who may interact with children affected by a school shooting.
6 tips for interacting with children
1. Talk about the event. Instruct adults to ask children to share their feelings about the incident and to show genuine interest in listening to the child’s thoughts and point of view. Adults shouldn’t pretend the event hasn’t occurred or isn’t serious. Children may be more worried if they think adults are too afraid to tell them what is happening. It is important to gently correct any misinformation older students may have received via social media.1
2. Reinforce that home is a safe haven. Overwhelming emotions and uncertainty can bring about a sense of insecurity in children. Children may come home seeking a safe environment. Advise parents to plan a night where family members participate in a favorite family activity.1 Tell parents to remind their children that trust-worthy adults—parents, emergency workers, police, firefighters, doctors, and the military—are helping provide safety, comfort, and support.2
3. Limit television time. If children are exposed to the news, parents should watch it with them briefly, but avoid letting children rewatch the same event repetitively. Constant exposure to the event may heighten a child’s anxiety and fears.
4. Maintain a normal routine. Tell parents they should maintain, as best they can, their normal routine for dinner, homework, chores, and bedtime, but to remain flexible.2 Children may have a hard time concentrating on schoolwork or falling asleep. Advise parents to spend extra time reading or playing quiet games with their children, particularly at bedtime. These activities are calming, foster a sense of closeness and security, and reinforce a feeling of normalcy.
5. Encourage emotions. Instruct parents to explain to their children that all feelings are okay and normal, and to let children talk about their feelings and help put them into perspective.1 Children may need help in expressing these feelings, so be patient. If an incident happened at the child’s school, teachers and administrators may conduct group sessions to help children express their concerns about being back in school.
6. Seek creativity or spirituality. Encourage parents and other adults to provide a creative outlet for children, such as making get well cards or sending letters to the survivors and their families. Writing thank you letters to doctors, nurses, fire-fighters, and police officers also may be comforting.1,2 Suggest that parents encourage their children to pray or think hopeful thoughts for the victims and their families.
2 tips for interacting with adults
7. Recommend they take care of themselves. Explain to adult caregivers that because children learn by observing, they shouldn’t ignore their own feelings of anxiety, grief, and anger. By expressing their emotions in a productive manner, adults will be better able to support their children. Encourage adults to talk to friends, family, religious leaders, or mental health counselors.
8. Advise adults to be alert for children who may need professional help. Tell them to be vigilant when monitoring a child’s emotional state. Children who may benefit from mental health counseling after a tragedy may exhibit warning signs, such as changes in behavior, appetite, and sleep patterns, which may indicate the child is experiencing grief, anxiety, or discomfort.
Remind adults to be aware of children who are at greater risk for mental health issues, including those who are already struggling with other recent traumatic experiences—past traumatic experiences, personal loss, depression, or other mental illness.1 Be particularly observant for children who may be at risk of suicide.1,2 Professional counseling may be needed for a child who is experiencing an emotional reaction that lasts >1 month and is impacting his or her daily functioning.1
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In the aftermath of a school shooting, parents and teachers may seek a psychiatrist’s advice on how to best discuss these incidents with children. We offer guidelines on what to tell concerned parents, educators, and other adults who may interact with children affected by a school shooting.
6 tips for interacting with children
1. Talk about the event. Instruct adults to ask children to share their feelings about the incident and to show genuine interest in listening to the child’s thoughts and point of view. Adults shouldn’t pretend the event hasn’t occurred or isn’t serious. Children may be more worried if they think adults are too afraid to tell them what is happening. It is important to gently correct any misinformation older students may have received via social media.1
2. Reinforce that home is a safe haven. Overwhelming emotions and uncertainty can bring about a sense of insecurity in children. Children may come home seeking a safe environment. Advise parents to plan a night where family members participate in a favorite family activity.1 Tell parents to remind their children that trust-worthy adults—parents, emergency workers, police, firefighters, doctors, and the military—are helping provide safety, comfort, and support.2
3. Limit television time. If children are exposed to the news, parents should watch it with them briefly, but avoid letting children rewatch the same event repetitively. Constant exposure to the event may heighten a child’s anxiety and fears.
4. Maintain a normal routine. Tell parents they should maintain, as best they can, their normal routine for dinner, homework, chores, and bedtime, but to remain flexible.2 Children may have a hard time concentrating on schoolwork or falling asleep. Advise parents to spend extra time reading or playing quiet games with their children, particularly at bedtime. These activities are calming, foster a sense of closeness and security, and reinforce a feeling of normalcy.
5. Encourage emotions. Instruct parents to explain to their children that all feelings are okay and normal, and to let children talk about their feelings and help put them into perspective.1 Children may need help in expressing these feelings, so be patient. If an incident happened at the child’s school, teachers and administrators may conduct group sessions to help children express their concerns about being back in school.
6. Seek creativity or spirituality. Encourage parents and other adults to provide a creative outlet for children, such as making get well cards or sending letters to the survivors and their families. Writing thank you letters to doctors, nurses, fire-fighters, and police officers also may be comforting.1,2 Suggest that parents encourage their children to pray or think hopeful thoughts for the victims and their families.
2 tips for interacting with adults
7. Recommend they take care of themselves. Explain to adult caregivers that because children learn by observing, they shouldn’t ignore their own feelings of anxiety, grief, and anger. By expressing their emotions in a productive manner, adults will be better able to support their children. Encourage adults to talk to friends, family, religious leaders, or mental health counselors.
8. Advise adults to be alert for children who may need professional help. Tell them to be vigilant when monitoring a child’s emotional state. Children who may benefit from mental health counseling after a tragedy may exhibit warning signs, such as changes in behavior, appetite, and sleep patterns, which may indicate the child is experiencing grief, anxiety, or discomfort.
Remind adults to be aware of children who are at greater risk for mental health issues, including those who are already struggling with other recent traumatic experiences—past traumatic experiences, personal loss, depression, or other mental illness.1 Be particularly observant for children who may be at risk of suicide.1,2 Professional counseling may be needed for a child who is experiencing an emotional reaction that lasts >1 month and is impacting his or her daily functioning.1
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychological Association. Helping your children manage distress in the aftermath of a shooting. http://www.apa.org/helpcenter/aftermath.aspx. Updated April 2011. Accessed February 15, 2013.
2. National Association of School Psychologists resources. A national tragedy: helping children cope. http://www.nasponline.org/resources/crisis_safety/terror_general.aspx. Published September 2001. Accessed February 15, 2013.
1. American Psychological Association. Helping your children manage distress in the aftermath of a shooting. http://www.apa.org/helpcenter/aftermath.aspx. Updated April 2011. Accessed February 15, 2013.
2. National Association of School Psychologists resources. A national tragedy: helping children cope. http://www.nasponline.org/resources/crisis_safety/terror_general.aspx. Published September 2001. Accessed February 15, 2013.
Recent advances in the management of advanced non–small-cell lung cancer
Lung cancer is the leading cause of cancer-related mortality among men and women in the United States. Non–small-cell lung cancer (NSCLC) accounts for about 85% of all lung cancers. Most patients with NSCLC present with advanced disease and median overall survival in this incurable setting remain dismal. Accumulating evidence suggests that both histology and molecular signature have prognostic and predictive value for NSCLC. Recent advances in the molecular characterization of NSCLC tumors have made individualized treatment approaches feasible. Personalized chemotherapy and targeted biological therapy based on a tumor’s individual biologic and molecular profile can optimize efficacy while minimizing toxicity. Molecular testing for activating mutations in the epidermal growth factor receptor (EGFR) domain and EML4-ALK translocation are routinely used to guide therapeutic decisions. Several new treatments that irreversibly target EGFR family members are in development for patients with NSCLC. Novel EML4-ALK inhibitors such as LDK378 are promising agents with encouraging early efficacy data. KRAS mutations are the most common mutation in adenocarcinomas. Although no agents for this subset of NSCLC have been approved, there are several agents in clinical development, including selumetinib, an MEK inhibitor, that seem promising. A growing body of evidence suggests that NSCLC is subject to immune surveillance. Immunotherapeutic interventions, including vaccine therapy and antigen-independent immunomodulatory strategies, may improve outcomes in NSCLC. In this review, we summarize recent advances in non–small-cell lung cancer, with an emphasis on investigational strategies for individualized treatment.
*Click on the link to the left for a PDF of the full article.
Lung cancer is the leading cause of cancer-related mortality among men and women in the United States. Non–small-cell lung cancer (NSCLC) accounts for about 85% of all lung cancers. Most patients with NSCLC present with advanced disease and median overall survival in this incurable setting remain dismal. Accumulating evidence suggests that both histology and molecular signature have prognostic and predictive value for NSCLC. Recent advances in the molecular characterization of NSCLC tumors have made individualized treatment approaches feasible. Personalized chemotherapy and targeted biological therapy based on a tumor’s individual biologic and molecular profile can optimize efficacy while minimizing toxicity. Molecular testing for activating mutations in the epidermal growth factor receptor (EGFR) domain and EML4-ALK translocation are routinely used to guide therapeutic decisions. Several new treatments that irreversibly target EGFR family members are in development for patients with NSCLC. Novel EML4-ALK inhibitors such as LDK378 are promising agents with encouraging early efficacy data. KRAS mutations are the most common mutation in adenocarcinomas. Although no agents for this subset of NSCLC have been approved, there are several agents in clinical development, including selumetinib, an MEK inhibitor, that seem promising. A growing body of evidence suggests that NSCLC is subject to immune surveillance. Immunotherapeutic interventions, including vaccine therapy and antigen-independent immunomodulatory strategies, may improve outcomes in NSCLC. In this review, we summarize recent advances in non–small-cell lung cancer, with an emphasis on investigational strategies for individualized treatment.
*Click on the link to the left for a PDF of the full article.
Lung cancer is the leading cause of cancer-related mortality among men and women in the United States. Non–small-cell lung cancer (NSCLC) accounts for about 85% of all lung cancers. Most patients with NSCLC present with advanced disease and median overall survival in this incurable setting remain dismal. Accumulating evidence suggests that both histology and molecular signature have prognostic and predictive value for NSCLC. Recent advances in the molecular characterization of NSCLC tumors have made individualized treatment approaches feasible. Personalized chemotherapy and targeted biological therapy based on a tumor’s individual biologic and molecular profile can optimize efficacy while minimizing toxicity. Molecular testing for activating mutations in the epidermal growth factor receptor (EGFR) domain and EML4-ALK translocation are routinely used to guide therapeutic decisions. Several new treatments that irreversibly target EGFR family members are in development for patients with NSCLC. Novel EML4-ALK inhibitors such as LDK378 are promising agents with encouraging early efficacy data. KRAS mutations are the most common mutation in adenocarcinomas. Although no agents for this subset of NSCLC have been approved, there are several agents in clinical development, including selumetinib, an MEK inhibitor, that seem promising. A growing body of evidence suggests that NSCLC is subject to immune surveillance. Immunotherapeutic interventions, including vaccine therapy and antigen-independent immunomodulatory strategies, may improve outcomes in NSCLC. In this review, we summarize recent advances in non–small-cell lung cancer, with an emphasis on investigational strategies for individualized treatment.
*Click on the link to the left for a PDF of the full article.