User login
Moving forward with ICD-10: Capitalize on this extra time
Yes, we have been here before. Another day, another delay in implementing International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). But, do not expect another postponement. If you are already conducting training sessions to move to the new system come next October, continue to do so. If you have not yet started, now is the time to start. ICD-10-CM is coming to your practice, and it will change everything.
“Why the switch?” you ask?
This change in our diagnostic coding system is required to allow coding for increased specificity in the reporting of diseases and recently recognized conditions as well as to maintain our status with respect to the rest of the world (which has been using ICD-10 for years). It also will be essential to use this coding system with the electronic medical record (EMR), so that meaningful use can be demonstrated more easily. Keep in mind that failure to show meaningful use will lead to penalties in the future. This new system offers improvements over ICD-9-CM in coding primary care encounters, external causes of injury, mental disorders, neoplasms, obstetric complications, and preventive health. It also allows physicians to demonstrate severity of illness in a way that is not possible with ICD-9-CM.
There will be 65,000 more codes than currently exist in ICD-9-CM. No physician will be able to keep all of these code numbers handy, but by making changes to clinician documentation and applying diagnostic coding guidelines correctly within the framework of the new system, the transition will not be onerous. And consider that, while the number of new codes is great, the number of codes used in the typical ObGyn practice will be a fraction of that number.
Related article: As ICD-10 conversion nears, keep these factors in mind to ensure proper reimbursements in 2014. Barbara S. Levy, MD (Audiocast, January 2014)
For ICD-10, documentation is paramount
The most important issue when considering overall coding and practice changes will be recognizing that clinician documentation will be the key to coding the highest level of specificity—and this high level of specificity may be required by most payers when deciding to reimburse for treatments rendered. Complete documentation sets the stage for the severity of illness and should in fact result in fewer denials for medical necessity.
For the new process to work efficiently, however, without a lot of delays due to coders and billers having to get more information from clinician offices before sending out claims, your understanding of and “buy-in” to the more clinically specific documentation will be essential.
To explain, under ICD-9-CM coding, simply documenting amenorrhea was acceptable. But when we switch to ICD-10-CM, documentation will need to specify whether the amenorrhea was primary or secondary. This more specific diagnostic coding will make a difference in the health statistics we collect. These data are used for research and to make decisions about allocation of resources—all essential components to excellent quality patient care.
The codes themselves will look different, which may be why some are resisting the change. Instead of the up to five digits required in ICD-9-CM, ICD-10-CM will require up to seven characters. All of the ICD-10-CM codes begin with a letter, may require a placeholder code of “x” as part of the code number, and the seventh character can be either a number or a letter. For instance, with some ICD-10-CM diagnoses reported by ObGyns, a seventh character might require documentation of the encounter as being initial, subsequent, or a sequel; in other cases, that seventh character will be used to identify which fetus has the problem identified by the diagnostic code.
Related article: The economics of surgical gynecology: How we can not only survive, but thrive, in the 21st Century. Q&A with Barbara S. Levy, MD (Practice Management; February 2013)
Your understanding, although not a necessity, is best for all involved
In truth, most clinicians are not familiar with code formats and code numbers within our current ICD-9-CM code set. The expectation that you will suddenly become fluent in ICD-10-CM “code speak” is not realistic. But an understanding of the new codes in relation to documentation expectations will go a long way to making this transition as smooth as possible. For instance, when a patient currently presents reporting vaginal pain that is found to be due to erosion of a previously placed mesh, the code 628.31 (Erosion of implanted vaginal mesh and other prosthetic materials to surrounding organ or tissue) is reported. But in ICD-10-CM, the documentation would need to include whether this was an initial encounter and the code would become T83.711A (Erosion of implanted vaginal mesh and other prosthetic materials to surrounding organ or tissue, initial encounter).
Smart search. The good news is that most EMR products will have a “smart search” program available for clinicians to pick the correct code based on the search criteria. The bad news is that you will have to be a bit more exact in the search terms you use to make the process easy. For instance, the patient has pelvic pain but you search only on the term “pain.” That term by itself will result in about 100 codes to select from, and the order of the codes may mean that the correct code for pelvic pain is 25 codes down the list. However, if you instead search on the term “pelvic pain,” the one and only code for this condition will be listed and you can simply select it and move on.
Develop cheat sheets. Health-care professionals who are not using an EMR or some sort of computerized code search program will have a harder time, but the use of multiple paper “cheat sheets” for general gynecology, family planning, surgical cases, urology, infertility, obstetrics, etc., will ease that burden. Practice management staff can develop these forms, built on the codes that are currently being reported by the clinician. Place all of the options to replace the older code on the sheet so the correct selection can be made.
For instance, if the provider previously had reported vaginitis with one code, when we move to ICD-10-CM the code would expand to four code selections based on documentation of acute vaginitis, subacute and chronic vaginitis, acute vulvitis, or subacute and chronic vulvitis. If you only had documented vaginitis in the medical record, this gives you the opportunity to refine the documentation to something more specific that supports selection of the correct code and supports the medical need for management options.
Related article: Dos, don’ts, and dollars: Making the switch to an HER. Neil H. Baum, MD; Paul Kepper, MS. (Practice Management; November 2013)
Take advantage of the extra time
Now that we have a delay in the rollout, take this time to critically examine your documentation styles, and practice selecting ICD-10-CM codes before it counts toward payment or nonpayment of a claim. When the time comes, your practice will be fluent in the new system and there will be no delays in getting claims out the door or payment due to incorrect diagnostic coding. In other words, practice makes perfect.
In fact, some ObGyn practices that were ready for the new system have decided to switch to ICD-10-CM coding as of October 1, 2014. They will code each encounter by reporting both the ICD-9-CM code and the ICD-10-CM code on the revised CMS claim form or electronic billing format that permits dual diagnostic coding. This type of experience will ensure that all physicians and other health-care professionals in the practice have ample opportunity to improve their documentation and make any adjustments before the 2015 deadline.
Related article: The 2014 CPT and Medicare code changes affecting ObGyn practice. Melanie Witt, RN, CPC, COBGC, MA (Reimbursement Adviser; January 2014)
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
Yes, we have been here before. Another day, another delay in implementing International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). But, do not expect another postponement. If you are already conducting training sessions to move to the new system come next October, continue to do so. If you have not yet started, now is the time to start. ICD-10-CM is coming to your practice, and it will change everything.
“Why the switch?” you ask?
This change in our diagnostic coding system is required to allow coding for increased specificity in the reporting of diseases and recently recognized conditions as well as to maintain our status with respect to the rest of the world (which has been using ICD-10 for years). It also will be essential to use this coding system with the electronic medical record (EMR), so that meaningful use can be demonstrated more easily. Keep in mind that failure to show meaningful use will lead to penalties in the future. This new system offers improvements over ICD-9-CM in coding primary care encounters, external causes of injury, mental disorders, neoplasms, obstetric complications, and preventive health. It also allows physicians to demonstrate severity of illness in a way that is not possible with ICD-9-CM.
There will be 65,000 more codes than currently exist in ICD-9-CM. No physician will be able to keep all of these code numbers handy, but by making changes to clinician documentation and applying diagnostic coding guidelines correctly within the framework of the new system, the transition will not be onerous. And consider that, while the number of new codes is great, the number of codes used in the typical ObGyn practice will be a fraction of that number.
Related article: As ICD-10 conversion nears, keep these factors in mind to ensure proper reimbursements in 2014. Barbara S. Levy, MD (Audiocast, January 2014)
For ICD-10, documentation is paramount
The most important issue when considering overall coding and practice changes will be recognizing that clinician documentation will be the key to coding the highest level of specificity—and this high level of specificity may be required by most payers when deciding to reimburse for treatments rendered. Complete documentation sets the stage for the severity of illness and should in fact result in fewer denials for medical necessity.
For the new process to work efficiently, however, without a lot of delays due to coders and billers having to get more information from clinician offices before sending out claims, your understanding of and “buy-in” to the more clinically specific documentation will be essential.
To explain, under ICD-9-CM coding, simply documenting amenorrhea was acceptable. But when we switch to ICD-10-CM, documentation will need to specify whether the amenorrhea was primary or secondary. This more specific diagnostic coding will make a difference in the health statistics we collect. These data are used for research and to make decisions about allocation of resources—all essential components to excellent quality patient care.
The codes themselves will look different, which may be why some are resisting the change. Instead of the up to five digits required in ICD-9-CM, ICD-10-CM will require up to seven characters. All of the ICD-10-CM codes begin with a letter, may require a placeholder code of “x” as part of the code number, and the seventh character can be either a number or a letter. For instance, with some ICD-10-CM diagnoses reported by ObGyns, a seventh character might require documentation of the encounter as being initial, subsequent, or a sequel; in other cases, that seventh character will be used to identify which fetus has the problem identified by the diagnostic code.
Related article: The economics of surgical gynecology: How we can not only survive, but thrive, in the 21st Century. Q&A with Barbara S. Levy, MD (Practice Management; February 2013)
Your understanding, although not a necessity, is best for all involved
In truth, most clinicians are not familiar with code formats and code numbers within our current ICD-9-CM code set. The expectation that you will suddenly become fluent in ICD-10-CM “code speak” is not realistic. But an understanding of the new codes in relation to documentation expectations will go a long way to making this transition as smooth as possible. For instance, when a patient currently presents reporting vaginal pain that is found to be due to erosion of a previously placed mesh, the code 628.31 (Erosion of implanted vaginal mesh and other prosthetic materials to surrounding organ or tissue) is reported. But in ICD-10-CM, the documentation would need to include whether this was an initial encounter and the code would become T83.711A (Erosion of implanted vaginal mesh and other prosthetic materials to surrounding organ or tissue, initial encounter).
Smart search. The good news is that most EMR products will have a “smart search” program available for clinicians to pick the correct code based on the search criteria. The bad news is that you will have to be a bit more exact in the search terms you use to make the process easy. For instance, the patient has pelvic pain but you search only on the term “pain.” That term by itself will result in about 100 codes to select from, and the order of the codes may mean that the correct code for pelvic pain is 25 codes down the list. However, if you instead search on the term “pelvic pain,” the one and only code for this condition will be listed and you can simply select it and move on.
Develop cheat sheets. Health-care professionals who are not using an EMR or some sort of computerized code search program will have a harder time, but the use of multiple paper “cheat sheets” for general gynecology, family planning, surgical cases, urology, infertility, obstetrics, etc., will ease that burden. Practice management staff can develop these forms, built on the codes that are currently being reported by the clinician. Place all of the options to replace the older code on the sheet so the correct selection can be made.
For instance, if the provider previously had reported vaginitis with one code, when we move to ICD-10-CM the code would expand to four code selections based on documentation of acute vaginitis, subacute and chronic vaginitis, acute vulvitis, or subacute and chronic vulvitis. If you only had documented vaginitis in the medical record, this gives you the opportunity to refine the documentation to something more specific that supports selection of the correct code and supports the medical need for management options.
Related article: Dos, don’ts, and dollars: Making the switch to an HER. Neil H. Baum, MD; Paul Kepper, MS. (Practice Management; November 2013)
Take advantage of the extra time
Now that we have a delay in the rollout, take this time to critically examine your documentation styles, and practice selecting ICD-10-CM codes before it counts toward payment or nonpayment of a claim. When the time comes, your practice will be fluent in the new system and there will be no delays in getting claims out the door or payment due to incorrect diagnostic coding. In other words, practice makes perfect.
In fact, some ObGyn practices that were ready for the new system have decided to switch to ICD-10-CM coding as of October 1, 2014. They will code each encounter by reporting both the ICD-9-CM code and the ICD-10-CM code on the revised CMS claim form or electronic billing format that permits dual diagnostic coding. This type of experience will ensure that all physicians and other health-care professionals in the practice have ample opportunity to improve their documentation and make any adjustments before the 2015 deadline.
Related article: The 2014 CPT and Medicare code changes affecting ObGyn practice. Melanie Witt, RN, CPC, COBGC, MA (Reimbursement Adviser; January 2014)
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
Yes, we have been here before. Another day, another delay in implementing International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). But, do not expect another postponement. If you are already conducting training sessions to move to the new system come next October, continue to do so. If you have not yet started, now is the time to start. ICD-10-CM is coming to your practice, and it will change everything.
“Why the switch?” you ask?
This change in our diagnostic coding system is required to allow coding for increased specificity in the reporting of diseases and recently recognized conditions as well as to maintain our status with respect to the rest of the world (which has been using ICD-10 for years). It also will be essential to use this coding system with the electronic medical record (EMR), so that meaningful use can be demonstrated more easily. Keep in mind that failure to show meaningful use will lead to penalties in the future. This new system offers improvements over ICD-9-CM in coding primary care encounters, external causes of injury, mental disorders, neoplasms, obstetric complications, and preventive health. It also allows physicians to demonstrate severity of illness in a way that is not possible with ICD-9-CM.
There will be 65,000 more codes than currently exist in ICD-9-CM. No physician will be able to keep all of these code numbers handy, but by making changes to clinician documentation and applying diagnostic coding guidelines correctly within the framework of the new system, the transition will not be onerous. And consider that, while the number of new codes is great, the number of codes used in the typical ObGyn practice will be a fraction of that number.
Related article: As ICD-10 conversion nears, keep these factors in mind to ensure proper reimbursements in 2014. Barbara S. Levy, MD (Audiocast, January 2014)
For ICD-10, documentation is paramount
The most important issue when considering overall coding and practice changes will be recognizing that clinician documentation will be the key to coding the highest level of specificity—and this high level of specificity may be required by most payers when deciding to reimburse for treatments rendered. Complete documentation sets the stage for the severity of illness and should in fact result in fewer denials for medical necessity.
For the new process to work efficiently, however, without a lot of delays due to coders and billers having to get more information from clinician offices before sending out claims, your understanding of and “buy-in” to the more clinically specific documentation will be essential.
To explain, under ICD-9-CM coding, simply documenting amenorrhea was acceptable. But when we switch to ICD-10-CM, documentation will need to specify whether the amenorrhea was primary or secondary. This more specific diagnostic coding will make a difference in the health statistics we collect. These data are used for research and to make decisions about allocation of resources—all essential components to excellent quality patient care.
The codes themselves will look different, which may be why some are resisting the change. Instead of the up to five digits required in ICD-9-CM, ICD-10-CM will require up to seven characters. All of the ICD-10-CM codes begin with a letter, may require a placeholder code of “x” as part of the code number, and the seventh character can be either a number or a letter. For instance, with some ICD-10-CM diagnoses reported by ObGyns, a seventh character might require documentation of the encounter as being initial, subsequent, or a sequel; in other cases, that seventh character will be used to identify which fetus has the problem identified by the diagnostic code.
Related article: The economics of surgical gynecology: How we can not only survive, but thrive, in the 21st Century. Q&A with Barbara S. Levy, MD (Practice Management; February 2013)
Your understanding, although not a necessity, is best for all involved
In truth, most clinicians are not familiar with code formats and code numbers within our current ICD-9-CM code set. The expectation that you will suddenly become fluent in ICD-10-CM “code speak” is not realistic. But an understanding of the new codes in relation to documentation expectations will go a long way to making this transition as smooth as possible. For instance, when a patient currently presents reporting vaginal pain that is found to be due to erosion of a previously placed mesh, the code 628.31 (Erosion of implanted vaginal mesh and other prosthetic materials to surrounding organ or tissue) is reported. But in ICD-10-CM, the documentation would need to include whether this was an initial encounter and the code would become T83.711A (Erosion of implanted vaginal mesh and other prosthetic materials to surrounding organ or tissue, initial encounter).
Smart search. The good news is that most EMR products will have a “smart search” program available for clinicians to pick the correct code based on the search criteria. The bad news is that you will have to be a bit more exact in the search terms you use to make the process easy. For instance, the patient has pelvic pain but you search only on the term “pain.” That term by itself will result in about 100 codes to select from, and the order of the codes may mean that the correct code for pelvic pain is 25 codes down the list. However, if you instead search on the term “pelvic pain,” the one and only code for this condition will be listed and you can simply select it and move on.
Develop cheat sheets. Health-care professionals who are not using an EMR or some sort of computerized code search program will have a harder time, but the use of multiple paper “cheat sheets” for general gynecology, family planning, surgical cases, urology, infertility, obstetrics, etc., will ease that burden. Practice management staff can develop these forms, built on the codes that are currently being reported by the clinician. Place all of the options to replace the older code on the sheet so the correct selection can be made.
For instance, if the provider previously had reported vaginitis with one code, when we move to ICD-10-CM the code would expand to four code selections based on documentation of acute vaginitis, subacute and chronic vaginitis, acute vulvitis, or subacute and chronic vulvitis. If you only had documented vaginitis in the medical record, this gives you the opportunity to refine the documentation to something more specific that supports selection of the correct code and supports the medical need for management options.
Related article: Dos, don’ts, and dollars: Making the switch to an HER. Neil H. Baum, MD; Paul Kepper, MS. (Practice Management; November 2013)
Take advantage of the extra time
Now that we have a delay in the rollout, take this time to critically examine your documentation styles, and practice selecting ICD-10-CM codes before it counts toward payment or nonpayment of a claim. When the time comes, your practice will be fluent in the new system and there will be no delays in getting claims out the door or payment due to incorrect diagnostic coding. In other words, practice makes perfect.
In fact, some ObGyn practices that were ready for the new system have decided to switch to ICD-10-CM coding as of October 1, 2014. They will code each encounter by reporting both the ICD-9-CM code and the ICD-10-CM code on the revised CMS claim form or electronic billing format that permits dual diagnostic coding. This type of experience will ensure that all physicians and other health-care professionals in the practice have ample opportunity to improve their documentation and make any adjustments before the 2015 deadline.
Related article: The 2014 CPT and Medicare code changes affecting ObGyn practice. Melanie Witt, RN, CPC, COBGC, MA (Reimbursement Adviser; January 2014)
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
Cutaneous Melanoma
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Melanoma is the sixth most common cancer in the United States and the leading cause of deaths among all cutaneous malignancies. In 2012, it was estimated that approximately 75,000 individuals were diagnosed with melanoma and more than 9000 died. The incidence of melanoma is rising the fastest among all major malignancies, and the lifetime risk of melanoma among men and women now exceeds 1 in 68, as compared with 1:1500 in 1930.4 The incidence of melanoma is predicted to continue increasing, and there has been no corresponding decrease in mortality. This case-based review summarizes the etiology, risk factors, clinical presentation, and management of cutaneous melanomas, which comprise the majority of melanoma cases. The biology and management for other noncutaneous melanomas (such as mucosal or ocular melanomas) are beyond the scope of this review.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Melanoma is the sixth most common cancer in the United States and the leading cause of deaths among all cutaneous malignancies. In 2012, it was estimated that approximately 75,000 individuals were diagnosed with melanoma and more than 9000 died. The incidence of melanoma is rising the fastest among all major malignancies, and the lifetime risk of melanoma among men and women now exceeds 1 in 68, as compared with 1:1500 in 1930.4 The incidence of melanoma is predicted to continue increasing, and there has been no corresponding decrease in mortality. This case-based review summarizes the etiology, risk factors, clinical presentation, and management of cutaneous melanomas, which comprise the majority of melanoma cases. The biology and management for other noncutaneous melanomas (such as mucosal or ocular melanomas) are beyond the scope of this review.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Melanoma is the sixth most common cancer in the United States and the leading cause of deaths among all cutaneous malignancies. In 2012, it was estimated that approximately 75,000 individuals were diagnosed with melanoma and more than 9000 died. The incidence of melanoma is rising the fastest among all major malignancies, and the lifetime risk of melanoma among men and women now exceeds 1 in 68, as compared with 1:1500 in 1930.4 The incidence of melanoma is predicted to continue increasing, and there has been no corresponding decrease in mortality. This case-based review summarizes the etiology, risk factors, clinical presentation, and management of cutaneous melanomas, which comprise the majority of melanoma cases. The biology and management for other noncutaneous melanomas (such as mucosal or ocular melanomas) are beyond the scope of this review.
To read the full article in PDF:
Intimate partner violence: How you can help female survivors
Also known as “domestic violence” and “spouse abuse,” intimate partner violence (IPV) is now the term defined by the US Centers for Disease Control and Prevention to include physical violence, sexual violence, threats of physical or sexual violence, and psychological or emotional abuse by a current or former spouse, common-law spouse, nonmarital dating partner, or boyfriend or girlfriend of the same or opposite sex.1 Although IPV is often hidden or kept secret by those affected, it is a highly prevalent issue, especially in women. Knowing how to broach the subject and provide appropriate support in a caring and nonjudgmental manner are the keys to helping a woman move forward in her readiness and ability to improve her situation.
See related patient information
ONE IN THREE WOMEN EXPERIENCES IPV IN HER LIFE
As clinicians, we have all seen patients who have been affected by IPV—even if we did not realize it at the time. Indeed, 36% of women in the United States (approximately 42.4 million) have experienced rape, physical violence, or stalking by an intimate partner in their lifetime, and 6% (approximately 7 million) have experienced these forms of IPV within the past 12 months.2
ASSOCIATION WITH MURDER
From 30% to 70% of women who are murdered are killed by a current or former intimate partner.3,4 Of those killed by their partner, two-thirds had previously reported physical assault, and 83% had been threatened by the man who eventually killed them.4 In another study, 44% of IPV murder victims had presented to an emergency department within 2 years of their murder.5
PHYSICAL EFFECTS NOT ALWAYS APPARENT
Although 41% of women who experience IPV suffer physical injury from their attacks, only 28% of those who are injured seek medical care.6 Because injuries are often absent or no longer apparent when an IPV victim decides to get help, it is important to be aware of the clinical signs associated with IPV:
- Gastrointestinal disorders7
- Depression8
- Anxiety
- Chronic pain syndromes9
- Substance abuse
- Suicidal ideation.10
In women of childbearing age, IPV is associated with unintended pregnancy, sexually transmitted infections, condom non-use,11,12 inconsistent condom use,13 and fear of talking about condom use.11,12 Coerced sexual experiences (eg, sexual intercourse that was not wanted or consented to) are common, with 28% to 42% of college women reporting at least one such experience. In more than three quarters of women who have been sexually assaulted, the first experience occurred before age 25.14,15
One-quarter of women ages 16 to 29 have experienced reproductive coercion, which includes birth control sabotage or pregnancy coercion by the active male partner.16 Among women reporting birth control sabotage, 79% had also been victims of physical or sexual IPV.16
The cost of providing health care to women experiencing IPV is 1.4 to 2.5 times higher than that of the nonabused population. Studies have shown that female victims of both physical and nonphysical (eg, emotional or verbal) IPV are more likely to use emergency, mental health, and outpatient health care services. The economic toll of IPV, including health care and costs from lost productivity and premature death, ranges from $2.3 to $8.3 billion per year.17,18
ASK FEMALE PATIENTS ABOUT IPV
In the early 1990s, various medical organizations began issuing policy statements that endorsed screening for IPV.19–22 Since 1992, the Joint Commission on Accreditation of Healthcare Organizations has required hospitals and clinics to provide assistance to those experiencing IPV.23 Although the United States Preventive Services Task Force initially found insufficient evidence to support regular IPV screening in health care settings,24–27 the group reversed its position in 2012 after a review of more recent studies. The group now recommends that clinicians address IPV with all women of childbearing age.28
A Cochrane review found that IPV screening increased identification of IPV survivors.29 Female participants in many studies wanted clinicians to ask routinely about violence and to provide information on community and legal resources.30,31
How should we ask about IPV?
Although various sets of screening questions and tools are available, no one instrument is considered better than the others. However, women experiencing IPV have specific preferences regarding how they want clinicians to ask and talk about the topic. In one survey, women who had experienced IPV preferred that clinicians ask about it as part of the complete medical history, as long as it did not create “an atmosphere of interrogation.”32
The style in which a clinician asks about IPV may make a difference as well. In focus groups, immigrant Latina and Asian women who had experienced IPV stated that clinicians could facilitate open communication by initiating the discussion and exhibiting compassionate and supportive behavior during the visit.33 Being able to see the same clinician at each visit also enhanced clinician-patient communication.33
In a study of IPV screening in emergency room settings, most clinicians asked about IPV in a perfunctory, direct manner—generally some variant of, “Are you a victim of domestic violence?” In this study, patient IPV disclosure occurred more often when clinicians used an open-ended approach such as, “Tell me what happened,” or when clinicians probed for possible IPV (eg, “What do you think may be causing some of this stress?”).34
In a focus group, female IPV survivors described feeling stigmatized or invalidated when clinicians were condescending, judgmental, or dismissive.35 Nonjudgmental and supportive communication decreased the women’s sense of isolation and led to positive outcomes such as increased awareness of IPV as a problem, decreased isolation, and feeling that the clinician cared.35
When addressing IPV, clinicians should explain why they are asking about it because it allows the woman to understand the context of the inquiry and to feel more comfortable about disclosing IPV. If the query is a regular part of a general screening or history-taking, for example, they should frame the question to make that point apparent. For example, “Because we know that many women in the United States experience physical, sexual or emotional violence from their romantic partners, I like to ask all of my patients whether they have been hurt or have felt threatened or afraid in a current or past relationship.”
In situations in which clinicians are concerned about IPV with a particular patient, they should explicitly share their concerns and desire to help the patient. One IPV survivor offered this advice: “Just look at the patient like she is your friend. Call her by her name. For instance, say ‘Sally, is he hurting you? Are you having problems? If you need help, I have some [phone] numbers.’ Personalize the encounter.”
It is also important to address IPV in a manner that ensures the patient’s safety, confidentiality, and dignity. When having this type of sensitive conversation, the patient should ideally be clothed and alone—without others present, particularly her partner. Professional interpreters should be available to women who do not speak English. The clinician should maintain eye contact, smile to communicate friendliness, and use a supportive tone.36
Just asking may be an intervention
Qualitative studies have suggested that just the act of asking about IPV in a nonjudgmental and compassionate manner is helpful to women experiencing IPV.35,37 Doing so not only helps women recognize the abuse, but also begins to decrease their sense of isolation and increase their awareness of helpful resources. It also gives the patient a sense that the clinician cares about her situation.35 As a result, experts have begun to recommend that health clinicians view asking about IPV not merely as a screening tool, but as a potentially therapeutic intervention in and of itself.35,37,38
HOW TO HELP
What to do when a woman discloses IPV
Female survivors, advocates, and health care clinicians who care for abused women suggest responding to a positive disclosure of IPV by providing the following:
Validation. The IPV perpetrator will often attempt to justify the violence and abuse by shifting some of the blame or responsibility for the violence onto the victim. This “brainwashing” leads to self-blame and a diminished sense of self-worth. However, clinicians can help reverse this mindset by acknowledging the woman’s disclosure and emphasizing that she did not deserve the abuse or violence. An example of such a statement is, “I am so very sorry that you went through that with your partner. You definitely did not deserve that. No one should ever be hurt by or afraid of the people who are supposed to love them.” Providing validation helps women recognize that the violence was a problem they did not deserve.38,39
Support. Women who have experienced IPV appreciate feeling supported and cared for by their health care clinician. Even if the patient is not ready to take any definitive action regarding her relationship or situation, knowing that her clinician and the health care setting are resources and sources of support is both comforting and empowering.35,40 Clinicians can communicate this support by stating, “I want you to know that whatever happens and whatever you decide, we are here for you.”
Respect for autonomy. Women experiencing IPV best understand their own situation and its various complexities and so they know best what they can do, cannot do, or need to do. As such, clinicians must respect a woman’s autonomy and preserve her ability to express her own needs and desires and make her own decisions. Prescribing a plan of action or giving commands to IPV victims could further perpetuate their sense of disempowerment and lack of control over their life.
Information. Providing referral information or hotline numbers to community domestic violence programs is helpful. However, not all women feel safe or comfortable taking printed brochures or written information with them because their abusive partner may find it. Thus, clinicians should ask if the patient can take the information safely or offer to write the numbers down without labeling them if she is afraid. The National Domestic Violence hotline is a 24-hour toll-free resource that will help women locate and contact shelters and other support services in their own community. The number, 1-800-799-SAFE (7233), is easy to memorize and thus is an easy resource to pass along quickly and safely. Other national organizations such as Futures Without Violence (formerly known as the Family Violence Prevention Fund) and the National Coalition Against Violence also provide links to local resources (Table 1).
Safety planning. Discussing the need for a safety plan will help the patient prepare for future abusive episodes. Even women who report that they are no longer in an abusive relationship should be asked about their current safety needs and concerns because they often remain in contact with abusive partners even after a relationship has ended.
When discussing safety planning, clinicians should ask the woman if she is currently safe or if she needs shelter. If she intends to return to or is still in contact with her batterer, ask if she has a plan for what to do or how to escape if the violence occurs again. Advise the patient to:
- Hide money so that she can leave quickly
- Make copies of birth certificates, immunization records, Social Security Number, and other important documents and keep them hidden and accessible
- Make a spare car key
- Have a list of hotline numbers
- Develop a code with friends, family, and neighbors that will let them know she needs immediate help.
Studies show that discussing safety-promoting behaviors increases the number of them that are used by IPV victims.41 A detailed list that can be shared with patients is provided in the patient information page that accompanies this article.42 Examples of personalized safety plans are available from the National Center on Domestic and Sexual Violence at its website, www.ncdsv.org/images/NCDSV_DVSafetyPlan_updated2013.pdf.
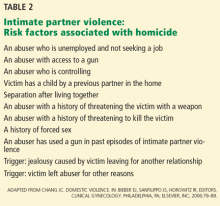
Danger assessment. Several researchers have examined potential risk factors associated with increased risk of homicide.43 Table 2 lists some of the characteristics associated with an increased risk of homicide in IPV situations.42 From this work, a danger assessment tool and scoring system has been developed. This tool and training on how to use it are available for free at www.dangerassessment.org. Although there are currently no outcome data on the benefits or risks of using this instrument, its objective is to increase women’s awareness of their danger level and individualize their safety counseling.
Proper documentation. Victim advocates and lawyers working on behalf of IPV victims emphasize that documentation by a medical provider can help a woman with her legal case. This documentation should be clear, legible, and as detailed as possible. These details should include a patient’s own words set off by quotation marks, a description or body map illustrating associated injuries or physical signs corroborating the violence, and a description of the patient’s demeanor or signs of emotion. Clinicians should avoid legal terms such as “alleges” or “alleged perpetrator” and should either define or avoid abbreviations that may be considered ambiguous in a legal proceeding (eg, clinicians should write out the words “domestic violence” or “intimate partner violence” rather than using “DV” or “IPV”).44 Most states have passed laws that prevent insurance companies from discriminating against IPV victims; insurance companies can no longer deny women coverage for seeking care related to IPV.45
A nonthreatening physical examination. Women who have experienced physical or sexual violence may feel anxious or experience added trauma during certain portions of the physical examination. Women who have been raped or otherwise sexually abused may have difficulty tolerating a pelvic examination, for example. Others who have been choked or grabbed by the neck may experience distress during palpation of the thyroid or cervical lymph nodes. The oropharyngeal examination may be challenging for women who have experienced forced oral sex or who may have had objects such as the barrel of a gun forced into their mouth. Asking women who have experienced IPV what aspects of the physical examination they may find difficult and how this could be made easier allows the patient and clinician to work together to develop strategies for or alternatives to necessary examinations or testing.
Before starting an examination, clinicians should explain what will happen and then ask the patient for her permission before moving on to the next step. Doing so creates a sense of control in an otherwise challenging and potentially traumatizing examination.
Disclosure of mandatory IPV reporting. Many states require health care providers to report any injuries caused by a weapon or criminal act to police or other official institutions.45,46 Several of these states specify that this reporting should occur during an IPV evaluation.
Mandatory reporting of IPV to police is highly controversial.47–49 Opponents of these laws argue that they do not benefit victims because they do not respect autonomy, they violate patient-clinician confidentiality, and they violate informed consent.47 Female IPV victims indicate that they would be less likely to disclose IPV and seek help if they knew it had to be reported.50–53 In a survey of California physicians, 59% stated that they might not comply with reporting laws if the patient objected.54
In states with mandatory reporting laws, clinicians can advocate for their patients by disclosing whether IPV must be reported before beginning their examination. The Michigan Coalition Against Domestic Violence devised this sample statement:
“I do have to let you know that under state law, I am obligated to make a police report to (name police agency with jurisdiction over the facility), if I/we are treating someone for any injury sustained by means of violence (tailor this information to the specifics of your state law). So, if you would have any concerns about that, I would encourage you to speak with one of the domestic violence advocates/counselors at (DV agency), who are able to provide confidential services and help without being required to make a police report.”55
The Compendium of State Statutes and Polices on Domestic Violence and Health Care by Futures Without Violence is an excellent reference that describes current state laws and policies regarding domestic violence and includes descriptions of the specific characteristics of each state’s mandatory reporting laws and their implications for health care providers. A copy can be downloaded from www.futureswithout-violence.org/content/features/detail/1584/.
What not to do
Clinicians should not ignore or minimize the extent of IPV, make excuses for the batterer, or blame the woman.32,56,57 They also should not inadvertently blame or criticize the woman by asking her, “What did you do to deserve this?” or “Why don’t you just leave?”57
While leaving a violent relationship is an obvious solution, many women experiencing IPV are either unwilling or unable to do so. In fact, leaving the batterer can be just as dangerous as staying. In a North Carolina study, half of all women killed by their partner had divorced or broken up with the partner immediately before the murder.4
What to do when a woman does not disclose IPV
Women who have experienced IPV often deny the violence to others out of fear. So a denial of violence, even in highly suspicious cases, can be expected. Abused patients often want help but are confused and afraid to ask for it.58,59 Providing easy and anonymous access to IPV information and resources through posters, flyers, brochures, and booklets will allow any woman—regardless of disclosure—to obtain help.36
In one IPV trial, all women in a family planning clinic who were in the intervention group were given information about IPV and how it can affect sexual and reproductive health, whether or not they disclosed. Compared with women in the control group, women who received the intervention—both those who did and did not experience IPV—were 63% more likely than those in the control group to end a relationship because they perceived it to be unhealthy or unsafe. In this case, all women—not just those who were victims—benefited from receiving information about IPV.60
PROVIDERS’ SUPPORT MAKES A DIFFERENCE, IMPROVES OUTCOMES
Patients may seek help for and find safety from IPV in stages or steps, and it may take them several months or even years to do so.61–63 In this sense, then, IPV is an issue that is not likely to be “cured” or changed in a single medical visit. In addition, a single visit may not be associated with direct health and safety outcomes. However, a patient is more likely to make changes if she has supportive and informative interaction with a caring, nonjudgmental clinician who can increase her awareness of IPV and IPV resources and promote an improved sense of self-efficacy or perceived power.40
For example, in a study in which health clinicians expressed concern about potential IPV health effects and offered support services such as counseling, 67% of women used one of those resources at least once.40 In another study, women who talked to a health care provider about their abuse were almost four times more likely to use an IPV intervention (eg, advocacy, shelter, restraining order) than women who did not talk to their provider. Those who used an IPV intervention were 2.6 times more likely to leave their abusive relationship, and those who left reported improved physical health.64
Thus, health care providers can have a positive effect by addressing this complex and difficult issue. The power and influence of a provider’s kindness, empathy, and support cannot be overestimated in their ability to improve the safety and well-being of women dealing with IPV.
- Saltzman LE, Fanslow JL, McMahon PM, Shelley GA; National Center for Injury Prevention and Control; Centers for Disease Control and Prevention. Intimate partner violence surveillance: uniform definitions and recommended data elements, Version 1.0. Atlanta, GA; 1999. www.cdc.gov/ncipc/pub-res/ipv_surveillance/intimate%20partner%20violence.pdf. Accessed June 3, 2014.
- Black MC, Basile KC, Breiding MJ, et al; Center for Injury Prevention and Control; Centers for Disease Control and Prevention. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed June 3, 2014.
- Kellermann AL, Mercy JA. Men, women, and murder: gender-specific differences in rates of fatal violence and victimization. J Trauma 1992; 33:1–5.
- Moracco KE, Runywan CW, Butts JD. Femicide in North Carolina, 1991–1993: a statewide study of patterns and precursors. Homicide Studies 1998; 4:422–446.
- Wadman MC, Muelleman RL. Domestic violence homicides: ED use before victimization. Am J Emerg Med 1999; 17:689–691.
- Catalano SM; United States Bureau of Justice Statistics. Intimate partner violence in the United States: 2007. http://bjs.ojp.usdoj.gov/index.cfm?ty=pbdetail&iid=1000. Accessed February 19, 2014.
- Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med 1995; 123:782–794.
- Scholle SH, Rost KM, Golding JM. Physical abuse among depressed women. J Gen Intern Med 1998; 13:607–613.
- Walling MK, O’Hara MW, Reiter RC, Milburn AK, Lilly G, Vincent SD. Abuse history and chronic pain in women: II. A multivariate analysis of abuse and psychological morbidity. Obstet Gynecol 1994; 84:200–206.
- McCauley J, Kern DE, Kolodner K, Derogatis LR, Bass EB. Relation of low-severity violence to women’s health. J Gen Intern Med 1998; 13:687–691.
- Wingood GM, DiClemente RJ. The effects of an abusive primary partner on the condom use and sexual negotiation practices of African-American women. Am J Public Health 1997; 87:1016–1018.
- Sales JM, Salazar LF, Wingood GM, DiClemente RJ, Rose E, Crosby RA. The mediating role of partner communication skills on HIV/STD-associated risk behaviors in young African American females with a history of sexual violence. Arch Pediatr Adolesc Med 2008; 162:432–438.
- Davila YR, Brackley MH. Mexican and Mexican American women in a battered women’s shelter: barriers to condom negotiation for HIV/AIDS prevention. Issues Ment Health Nurs 1999; 20:333–355.
- Tjaden P, Thoennes N; National Institute of Justice; Centers for Disease Control and Prevention. Prevalence, incidence and consequences of violence against women: findings from the national violence against women survey. Washington, DC; 1998. https://www.ncjrs.gov/pdffiles/172837.pdf. Accessed June 3, 2014.
- Masho SW, Odor RK, Adera T. Sexual assault in Virginia: a population-based study. Womens Health Issues 2005; 15:157–166.
- Miller E, Decker MR, McCauley HL, et al. Pregnancy coercion, intimate partner violence and unintended pregnancy. Contraception 2010; 81:316–322.
- Bonomi AE, Anderson ML, Rivara FP, Thompson RS. Health care utilization and costs associated with physical and nonphysical-only intimate partner violence. Health Serv Res 2009; 44:1052–1067.
- Rivara FP, Anderson ML, Fishman P, et al. Healthcare utilization and costs for women with a history of intimate partner violence. Am J Prev Med 2007; 32:89–96.
- American Nurses Association. Position statement on physical violence against women. Washington, DC; 1991.
- Physicians and domestic violence. Ethical considerations. Council on Ethical and Judicial Affairs, American Medical Association. JAMA 1992; 267:3190–3193.
- The American College of Obstetricians and Gynecologists. Screening tools: domestic violence. http://www.acog.org/About_ACOG/ACOG_Departments/Violence_Against_Women/Screening_Tools__Domestic_Violence. Accessed June 3, 2014.
- Lee D, James L, Sawires P; The Family Violence Prevention Fund. Preventing domestic violence: clinical guidelines on routine screening. San Francisco, CA; 1999. http://new.vawnet.org/Assoc_Files_VAWnet/screpol.pdf. Accessed June 3, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation manual for hospitals. Chicago, IL; 1992.
- US Department of Health and Human Services. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC; 1996.
- Ramsay J, Richardson J, Carter YH, Davidson LL, Feder G. Should health professionals screen women for domestic violence? Systematic review. BMJ 2002; 325:314.
- Nelson HD, Nygren P, McInerney Y, Klein J; US Preventive Services Task Force. Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med 2004; 140:387–396.
- Chamberlain L. The USPSTF recommendation on intimate partner violence: what we can learn from it and what we can do about it. Fam Viol Prev Health Pract 2005; 1:1–24.
- Moyer VA; US Preventive Services Task Force. Screening for intimate partner violence and abuse of elderly and vulnerable adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:478–486.
- Taft A, O’Doherty L, Hegarty K, Ramsay J, Davidson L, Feder G. Screening women for intimate partner violence in healthcare settings. Cochrane Database Syst Rev 2013; 4:CD007007.
- McNutt LA, Carlson BE, Gagen D, Winterbauer N. Reproductive violence screening in primary care: perspectives and experiences of patients and battered women. J Am Med Womens Assoc 1999; 54:85–90.
- Friedman LS, Samet JH, Roberts MS, Hudlin M, Hans P. Inquiry about victimization experiences. A survey of patient p and physician practices. Arch Intern Med 1992; 152:1186–1190.
- Hamberger LK, Ambuel B, Marbella A, Donze J. Physician interaction with battered women: the women’s perspective. Arch Fam Med 1998; 7:575–582.
- Rodriguez MA, Bauer HM, Flores-Ortiz Y, Szkupinski-Quiroga S. Factors affecting patient-physician communication for abused Latina and Asian immigrant women. J Fam Pract 1998; 47:309–311.
- Rhodes KV, Frankel RM, Levinthal N, Prenoveau E, Bailey J, Levinson W. “You’re not a victim of domestic violence, are you?” Provider patient communication about domestic violence”. Ann Intern Med 2007; 147:620–627.
- Chang JC, Decker M, Moracco KE, Martin SL, Petersen R, Frasier PY. What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors. J Am Med Womens Assoc 2003; 58:76–81.
- Chang JC, Decker MR, Moracco KE, Martin SL, Petersen R, Frasier PY. Asking about intimate partner violence: advice from female survivors to health care providers. Patient Educ Couns 2005; 59:141–147.
- Hathaway JE, Willis G, Zimmer B. Listening to survivors’ voices: addressing partner abuse in the health care setting. Violence Against Women 2002; 8:687–716.
- Gerbert B, Caspers N, Bronstone A, Moe J, Abercrombie P. A qualitative analysis of how physicians with expertise in domestic violence approach the identification of victims. Ann Intern Med 1999; 131:578–584.
- Gerbert B, Caspers N, Milliken N, Berlin M, Bronstone A, Moe J. Interventions that help victims of domestic violence. A qualitative analysis of physicians’ experiences. J Fam Pract 2000; 49:889–895.
- McCaw B, Bauer HM, Berman WH, Mooney L, Holmberg M, Hunkeler E. Women referred for on-site domestic violence services in a managed care organization. Women Health 2002; 35:23–40.
- McFarlane J, Malecha A, Gist J, et al. An intervention to increase safety behaviors of abused women: results of a randomized clinical trial. Nurs Res 2002; 51:347–354.
- Chang JC. Domestic violence. In:Bieber EJ, Sanfilippo JS, Horowitz IR, editors. Clinical Gynecology. Philadelphia, PA; Elsevier, Inc; 2006:79–89.
- Campbell JC, Webster D, Koziol-McLain J, et al. Risk factors for femicide in abusive relationships: results from a multisite case control study. Am J Public Health 2003; 93:1089–1097.
- Isaac NE, Enos V; National Institute of Justice. Documenting domestic violence: how health care providers can help victims. Research in Brief, 2001. https://www.ncjrs.gov/pdffiles1/nij/188564.pdf. Accessed June 3, 2014.
- Durborow N, Lizdas KC, O’Flaherty A, Marjavi A; Futures Without Violence. Compendium of state and US terrritory statutes and policies on domestic violence and health care, 2010. www.futureswithoutviolence.org/content/features/detail/1584/. Accessed June 3, 2014.
- Hyman A, Schillinger D, Lo B. Laws mandating reporting of domestic violence. Do they promote patient well-being? JAMA 1995; 273:1781–1787.
- Hyman A, Chez RA. Mandatory reporting of domestic violence by health care providers: a misguided approach. Womens Health Issues 1995; 5:208–213.
- Knight MA. Ethical debate: should doctors be more proactive as advocates for victims of violence? The police surgeon’s view: medical paternalism is unacceptable. BMJ 1995; 311:1620–1621.
- Hyman A; Futures Without Violence. Mandatory reporting of domestic violence by healthcare providers: a policy paper. San Francisco, CA; 1997. www.futureswithoutviolence.org/userfiles/file/HealthCare/mandatory_policypaper.pdf. Accessed June 3, 2014.
- Rodriguez MA, Craig AM, Mooney DR, Bauer HM. Patient attitudes about mandatory reporting of domestic violence. Implications for health care professionals. West J Med 1998; 169:337–341.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Sachs CJ, Koziol-McLain J, Glass N, Webster D, Campbell J. A population-based survey assessing support for mandatory domestic violence reporting by health care personnel. Women Health 2002; 35:121–133.
- Sullivan CM, Hagen LA. Survivors’ opinions about mandatory reporting of domestic violence and sexual assault by medical professionals. Affilia 2005; 20:1–16.
- Rodriguez MA, McLoughlin E, Bauer HM, Paredes V, Grumbach K. Mandatory reporting of intimate partner violence to police: views of physicians in California. Am J Public Health 1999; 89:575–578.
- Futures Without Violence. Mandatory reporting of domestic violence to law enforcement by health care providers: a guide for advocates working to respond to or amend reporting laws related to domestic violence. www.healthcaresaboutipv.org/wp-content/blogs.dir/3/files/2012/09/Mandatory_Reporting_of_DV_to_Law-Enforcement_by_HCP.pdf. Accessed June 3, 2014.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Gerbert B, Johnston K, Caspers N, Bleecker T, Woods A, Rosenbaum A. Experiences of battered women in health care settings: a qualitative study. Women Health 1996; 24:1–17.
- Chang JC, Cluss PA, Ranieri L, et al. Health care interventions for intimate partner violence: what women want. Womens Health Issues 2005; 15:21–30.
- Rodriguez MA, Quiroga SS, Bauer HM. Breaking the silence. Battered women’s perspectives on medical care. Arch Fam Med 1996; 5:153–158.
- Miller E, Decker MR, McCauley HL, et al. A family planning clinic partner violence intervention to reduce risk associated with reproductive coercion. Contraception 2011; 83:274–280.
- Landenburger K. A process of entrapment in and recovery from an abusive relationship. Issues Ment Health Nurs 1989; 10:209–227.
- Cluss PA, Chang JC, Hawker L, et al. The process of change for victims of intimate partner violence: support for a psychosocial readiness model. Womens Health Issues 2006; 16:262–274.
- Gerbert B, Abercrombie P, Caspers N, Love C, Bronstone A. How health care providers help battered women: the survivor’s perspective. Women Health 1999; 29:115–135.
- McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep 2006; 121:435–444.
Also known as “domestic violence” and “spouse abuse,” intimate partner violence (IPV) is now the term defined by the US Centers for Disease Control and Prevention to include physical violence, sexual violence, threats of physical or sexual violence, and psychological or emotional abuse by a current or former spouse, common-law spouse, nonmarital dating partner, or boyfriend or girlfriend of the same or opposite sex.1 Although IPV is often hidden or kept secret by those affected, it is a highly prevalent issue, especially in women. Knowing how to broach the subject and provide appropriate support in a caring and nonjudgmental manner are the keys to helping a woman move forward in her readiness and ability to improve her situation.
See related patient information
ONE IN THREE WOMEN EXPERIENCES IPV IN HER LIFE
As clinicians, we have all seen patients who have been affected by IPV—even if we did not realize it at the time. Indeed, 36% of women in the United States (approximately 42.4 million) have experienced rape, physical violence, or stalking by an intimate partner in their lifetime, and 6% (approximately 7 million) have experienced these forms of IPV within the past 12 months.2
ASSOCIATION WITH MURDER
From 30% to 70% of women who are murdered are killed by a current or former intimate partner.3,4 Of those killed by their partner, two-thirds had previously reported physical assault, and 83% had been threatened by the man who eventually killed them.4 In another study, 44% of IPV murder victims had presented to an emergency department within 2 years of their murder.5
PHYSICAL EFFECTS NOT ALWAYS APPARENT
Although 41% of women who experience IPV suffer physical injury from their attacks, only 28% of those who are injured seek medical care.6 Because injuries are often absent or no longer apparent when an IPV victim decides to get help, it is important to be aware of the clinical signs associated with IPV:
- Gastrointestinal disorders7
- Depression8
- Anxiety
- Chronic pain syndromes9
- Substance abuse
- Suicidal ideation.10
In women of childbearing age, IPV is associated with unintended pregnancy, sexually transmitted infections, condom non-use,11,12 inconsistent condom use,13 and fear of talking about condom use.11,12 Coerced sexual experiences (eg, sexual intercourse that was not wanted or consented to) are common, with 28% to 42% of college women reporting at least one such experience. In more than three quarters of women who have been sexually assaulted, the first experience occurred before age 25.14,15
One-quarter of women ages 16 to 29 have experienced reproductive coercion, which includes birth control sabotage or pregnancy coercion by the active male partner.16 Among women reporting birth control sabotage, 79% had also been victims of physical or sexual IPV.16
The cost of providing health care to women experiencing IPV is 1.4 to 2.5 times higher than that of the nonabused population. Studies have shown that female victims of both physical and nonphysical (eg, emotional or verbal) IPV are more likely to use emergency, mental health, and outpatient health care services. The economic toll of IPV, including health care and costs from lost productivity and premature death, ranges from $2.3 to $8.3 billion per year.17,18
ASK FEMALE PATIENTS ABOUT IPV
In the early 1990s, various medical organizations began issuing policy statements that endorsed screening for IPV.19–22 Since 1992, the Joint Commission on Accreditation of Healthcare Organizations has required hospitals and clinics to provide assistance to those experiencing IPV.23 Although the United States Preventive Services Task Force initially found insufficient evidence to support regular IPV screening in health care settings,24–27 the group reversed its position in 2012 after a review of more recent studies. The group now recommends that clinicians address IPV with all women of childbearing age.28
A Cochrane review found that IPV screening increased identification of IPV survivors.29 Female participants in many studies wanted clinicians to ask routinely about violence and to provide information on community and legal resources.30,31
How should we ask about IPV?
Although various sets of screening questions and tools are available, no one instrument is considered better than the others. However, women experiencing IPV have specific preferences regarding how they want clinicians to ask and talk about the topic. In one survey, women who had experienced IPV preferred that clinicians ask about it as part of the complete medical history, as long as it did not create “an atmosphere of interrogation.”32
The style in which a clinician asks about IPV may make a difference as well. In focus groups, immigrant Latina and Asian women who had experienced IPV stated that clinicians could facilitate open communication by initiating the discussion and exhibiting compassionate and supportive behavior during the visit.33 Being able to see the same clinician at each visit also enhanced clinician-patient communication.33
In a study of IPV screening in emergency room settings, most clinicians asked about IPV in a perfunctory, direct manner—generally some variant of, “Are you a victim of domestic violence?” In this study, patient IPV disclosure occurred more often when clinicians used an open-ended approach such as, “Tell me what happened,” or when clinicians probed for possible IPV (eg, “What do you think may be causing some of this stress?”).34
In a focus group, female IPV survivors described feeling stigmatized or invalidated when clinicians were condescending, judgmental, or dismissive.35 Nonjudgmental and supportive communication decreased the women’s sense of isolation and led to positive outcomes such as increased awareness of IPV as a problem, decreased isolation, and feeling that the clinician cared.35
When addressing IPV, clinicians should explain why they are asking about it because it allows the woman to understand the context of the inquiry and to feel more comfortable about disclosing IPV. If the query is a regular part of a general screening or history-taking, for example, they should frame the question to make that point apparent. For example, “Because we know that many women in the United States experience physical, sexual or emotional violence from their romantic partners, I like to ask all of my patients whether they have been hurt or have felt threatened or afraid in a current or past relationship.”
In situations in which clinicians are concerned about IPV with a particular patient, they should explicitly share their concerns and desire to help the patient. One IPV survivor offered this advice: “Just look at the patient like she is your friend. Call her by her name. For instance, say ‘Sally, is he hurting you? Are you having problems? If you need help, I have some [phone] numbers.’ Personalize the encounter.”
It is also important to address IPV in a manner that ensures the patient’s safety, confidentiality, and dignity. When having this type of sensitive conversation, the patient should ideally be clothed and alone—without others present, particularly her partner. Professional interpreters should be available to women who do not speak English. The clinician should maintain eye contact, smile to communicate friendliness, and use a supportive tone.36
Just asking may be an intervention
Qualitative studies have suggested that just the act of asking about IPV in a nonjudgmental and compassionate manner is helpful to women experiencing IPV.35,37 Doing so not only helps women recognize the abuse, but also begins to decrease their sense of isolation and increase their awareness of helpful resources. It also gives the patient a sense that the clinician cares about her situation.35 As a result, experts have begun to recommend that health clinicians view asking about IPV not merely as a screening tool, but as a potentially therapeutic intervention in and of itself.35,37,38
HOW TO HELP
What to do when a woman discloses IPV
Female survivors, advocates, and health care clinicians who care for abused women suggest responding to a positive disclosure of IPV by providing the following:
Validation. The IPV perpetrator will often attempt to justify the violence and abuse by shifting some of the blame or responsibility for the violence onto the victim. This “brainwashing” leads to self-blame and a diminished sense of self-worth. However, clinicians can help reverse this mindset by acknowledging the woman’s disclosure and emphasizing that she did not deserve the abuse or violence. An example of such a statement is, “I am so very sorry that you went through that with your partner. You definitely did not deserve that. No one should ever be hurt by or afraid of the people who are supposed to love them.” Providing validation helps women recognize that the violence was a problem they did not deserve.38,39
Support. Women who have experienced IPV appreciate feeling supported and cared for by their health care clinician. Even if the patient is not ready to take any definitive action regarding her relationship or situation, knowing that her clinician and the health care setting are resources and sources of support is both comforting and empowering.35,40 Clinicians can communicate this support by stating, “I want you to know that whatever happens and whatever you decide, we are here for you.”
Respect for autonomy. Women experiencing IPV best understand their own situation and its various complexities and so they know best what they can do, cannot do, or need to do. As such, clinicians must respect a woman’s autonomy and preserve her ability to express her own needs and desires and make her own decisions. Prescribing a plan of action or giving commands to IPV victims could further perpetuate their sense of disempowerment and lack of control over their life.
Information. Providing referral information or hotline numbers to community domestic violence programs is helpful. However, not all women feel safe or comfortable taking printed brochures or written information with them because their abusive partner may find it. Thus, clinicians should ask if the patient can take the information safely or offer to write the numbers down without labeling them if she is afraid. The National Domestic Violence hotline is a 24-hour toll-free resource that will help women locate and contact shelters and other support services in their own community. The number, 1-800-799-SAFE (7233), is easy to memorize and thus is an easy resource to pass along quickly and safely. Other national organizations such as Futures Without Violence (formerly known as the Family Violence Prevention Fund) and the National Coalition Against Violence also provide links to local resources (Table 1).
Safety planning. Discussing the need for a safety plan will help the patient prepare for future abusive episodes. Even women who report that they are no longer in an abusive relationship should be asked about their current safety needs and concerns because they often remain in contact with abusive partners even after a relationship has ended.
When discussing safety planning, clinicians should ask the woman if she is currently safe or if she needs shelter. If she intends to return to or is still in contact with her batterer, ask if she has a plan for what to do or how to escape if the violence occurs again. Advise the patient to:
- Hide money so that she can leave quickly
- Make copies of birth certificates, immunization records, Social Security Number, and other important documents and keep them hidden and accessible
- Make a spare car key
- Have a list of hotline numbers
- Develop a code with friends, family, and neighbors that will let them know she needs immediate help.
Studies show that discussing safety-promoting behaviors increases the number of them that are used by IPV victims.41 A detailed list that can be shared with patients is provided in the patient information page that accompanies this article.42 Examples of personalized safety plans are available from the National Center on Domestic and Sexual Violence at its website, www.ncdsv.org/images/NCDSV_DVSafetyPlan_updated2013.pdf.
Danger assessment. Several researchers have examined potential risk factors associated with increased risk of homicide.43 Table 2 lists some of the characteristics associated with an increased risk of homicide in IPV situations.42 From this work, a danger assessment tool and scoring system has been developed. This tool and training on how to use it are available for free at www.dangerassessment.org. Although there are currently no outcome data on the benefits or risks of using this instrument, its objective is to increase women’s awareness of their danger level and individualize their safety counseling.
Proper documentation. Victim advocates and lawyers working on behalf of IPV victims emphasize that documentation by a medical provider can help a woman with her legal case. This documentation should be clear, legible, and as detailed as possible. These details should include a patient’s own words set off by quotation marks, a description or body map illustrating associated injuries or physical signs corroborating the violence, and a description of the patient’s demeanor or signs of emotion. Clinicians should avoid legal terms such as “alleges” or “alleged perpetrator” and should either define or avoid abbreviations that may be considered ambiguous in a legal proceeding (eg, clinicians should write out the words “domestic violence” or “intimate partner violence” rather than using “DV” or “IPV”).44 Most states have passed laws that prevent insurance companies from discriminating against IPV victims; insurance companies can no longer deny women coverage for seeking care related to IPV.45
A nonthreatening physical examination. Women who have experienced physical or sexual violence may feel anxious or experience added trauma during certain portions of the physical examination. Women who have been raped or otherwise sexually abused may have difficulty tolerating a pelvic examination, for example. Others who have been choked or grabbed by the neck may experience distress during palpation of the thyroid or cervical lymph nodes. The oropharyngeal examination may be challenging for women who have experienced forced oral sex or who may have had objects such as the barrel of a gun forced into their mouth. Asking women who have experienced IPV what aspects of the physical examination they may find difficult and how this could be made easier allows the patient and clinician to work together to develop strategies for or alternatives to necessary examinations or testing.
Before starting an examination, clinicians should explain what will happen and then ask the patient for her permission before moving on to the next step. Doing so creates a sense of control in an otherwise challenging and potentially traumatizing examination.
Disclosure of mandatory IPV reporting. Many states require health care providers to report any injuries caused by a weapon or criminal act to police or other official institutions.45,46 Several of these states specify that this reporting should occur during an IPV evaluation.
Mandatory reporting of IPV to police is highly controversial.47–49 Opponents of these laws argue that they do not benefit victims because they do not respect autonomy, they violate patient-clinician confidentiality, and they violate informed consent.47 Female IPV victims indicate that they would be less likely to disclose IPV and seek help if they knew it had to be reported.50–53 In a survey of California physicians, 59% stated that they might not comply with reporting laws if the patient objected.54
In states with mandatory reporting laws, clinicians can advocate for their patients by disclosing whether IPV must be reported before beginning their examination. The Michigan Coalition Against Domestic Violence devised this sample statement:
“I do have to let you know that under state law, I am obligated to make a police report to (name police agency with jurisdiction over the facility), if I/we are treating someone for any injury sustained by means of violence (tailor this information to the specifics of your state law). So, if you would have any concerns about that, I would encourage you to speak with one of the domestic violence advocates/counselors at (DV agency), who are able to provide confidential services and help without being required to make a police report.”55
The Compendium of State Statutes and Polices on Domestic Violence and Health Care by Futures Without Violence is an excellent reference that describes current state laws and policies regarding domestic violence and includes descriptions of the specific characteristics of each state’s mandatory reporting laws and their implications for health care providers. A copy can be downloaded from www.futureswithout-violence.org/content/features/detail/1584/.
What not to do
Clinicians should not ignore or minimize the extent of IPV, make excuses for the batterer, or blame the woman.32,56,57 They also should not inadvertently blame or criticize the woman by asking her, “What did you do to deserve this?” or “Why don’t you just leave?”57
While leaving a violent relationship is an obvious solution, many women experiencing IPV are either unwilling or unable to do so. In fact, leaving the batterer can be just as dangerous as staying. In a North Carolina study, half of all women killed by their partner had divorced or broken up with the partner immediately before the murder.4
What to do when a woman does not disclose IPV
Women who have experienced IPV often deny the violence to others out of fear. So a denial of violence, even in highly suspicious cases, can be expected. Abused patients often want help but are confused and afraid to ask for it.58,59 Providing easy and anonymous access to IPV information and resources through posters, flyers, brochures, and booklets will allow any woman—regardless of disclosure—to obtain help.36
In one IPV trial, all women in a family planning clinic who were in the intervention group were given information about IPV and how it can affect sexual and reproductive health, whether or not they disclosed. Compared with women in the control group, women who received the intervention—both those who did and did not experience IPV—were 63% more likely than those in the control group to end a relationship because they perceived it to be unhealthy or unsafe. In this case, all women—not just those who were victims—benefited from receiving information about IPV.60
PROVIDERS’ SUPPORT MAKES A DIFFERENCE, IMPROVES OUTCOMES
Patients may seek help for and find safety from IPV in stages or steps, and it may take them several months or even years to do so.61–63 In this sense, then, IPV is an issue that is not likely to be “cured” or changed in a single medical visit. In addition, a single visit may not be associated with direct health and safety outcomes. However, a patient is more likely to make changes if she has supportive and informative interaction with a caring, nonjudgmental clinician who can increase her awareness of IPV and IPV resources and promote an improved sense of self-efficacy or perceived power.40
For example, in a study in which health clinicians expressed concern about potential IPV health effects and offered support services such as counseling, 67% of women used one of those resources at least once.40 In another study, women who talked to a health care provider about their abuse were almost four times more likely to use an IPV intervention (eg, advocacy, shelter, restraining order) than women who did not talk to their provider. Those who used an IPV intervention were 2.6 times more likely to leave their abusive relationship, and those who left reported improved physical health.64
Thus, health care providers can have a positive effect by addressing this complex and difficult issue. The power and influence of a provider’s kindness, empathy, and support cannot be overestimated in their ability to improve the safety and well-being of women dealing with IPV.
Also known as “domestic violence” and “spouse abuse,” intimate partner violence (IPV) is now the term defined by the US Centers for Disease Control and Prevention to include physical violence, sexual violence, threats of physical or sexual violence, and psychological or emotional abuse by a current or former spouse, common-law spouse, nonmarital dating partner, or boyfriend or girlfriend of the same or opposite sex.1 Although IPV is often hidden or kept secret by those affected, it is a highly prevalent issue, especially in women. Knowing how to broach the subject and provide appropriate support in a caring and nonjudgmental manner are the keys to helping a woman move forward in her readiness and ability to improve her situation.
See related patient information
ONE IN THREE WOMEN EXPERIENCES IPV IN HER LIFE
As clinicians, we have all seen patients who have been affected by IPV—even if we did not realize it at the time. Indeed, 36% of women in the United States (approximately 42.4 million) have experienced rape, physical violence, or stalking by an intimate partner in their lifetime, and 6% (approximately 7 million) have experienced these forms of IPV within the past 12 months.2
ASSOCIATION WITH MURDER
From 30% to 70% of women who are murdered are killed by a current or former intimate partner.3,4 Of those killed by their partner, two-thirds had previously reported physical assault, and 83% had been threatened by the man who eventually killed them.4 In another study, 44% of IPV murder victims had presented to an emergency department within 2 years of their murder.5
PHYSICAL EFFECTS NOT ALWAYS APPARENT
Although 41% of women who experience IPV suffer physical injury from their attacks, only 28% of those who are injured seek medical care.6 Because injuries are often absent or no longer apparent when an IPV victim decides to get help, it is important to be aware of the clinical signs associated with IPV:
- Gastrointestinal disorders7
- Depression8
- Anxiety
- Chronic pain syndromes9
- Substance abuse
- Suicidal ideation.10
In women of childbearing age, IPV is associated with unintended pregnancy, sexually transmitted infections, condom non-use,11,12 inconsistent condom use,13 and fear of talking about condom use.11,12 Coerced sexual experiences (eg, sexual intercourse that was not wanted or consented to) are common, with 28% to 42% of college women reporting at least one such experience. In more than three quarters of women who have been sexually assaulted, the first experience occurred before age 25.14,15
One-quarter of women ages 16 to 29 have experienced reproductive coercion, which includes birth control sabotage or pregnancy coercion by the active male partner.16 Among women reporting birth control sabotage, 79% had also been victims of physical or sexual IPV.16
The cost of providing health care to women experiencing IPV is 1.4 to 2.5 times higher than that of the nonabused population. Studies have shown that female victims of both physical and nonphysical (eg, emotional or verbal) IPV are more likely to use emergency, mental health, and outpatient health care services. The economic toll of IPV, including health care and costs from lost productivity and premature death, ranges from $2.3 to $8.3 billion per year.17,18
ASK FEMALE PATIENTS ABOUT IPV
In the early 1990s, various medical organizations began issuing policy statements that endorsed screening for IPV.19–22 Since 1992, the Joint Commission on Accreditation of Healthcare Organizations has required hospitals and clinics to provide assistance to those experiencing IPV.23 Although the United States Preventive Services Task Force initially found insufficient evidence to support regular IPV screening in health care settings,24–27 the group reversed its position in 2012 after a review of more recent studies. The group now recommends that clinicians address IPV with all women of childbearing age.28
A Cochrane review found that IPV screening increased identification of IPV survivors.29 Female participants in many studies wanted clinicians to ask routinely about violence and to provide information on community and legal resources.30,31
How should we ask about IPV?
Although various sets of screening questions and tools are available, no one instrument is considered better than the others. However, women experiencing IPV have specific preferences regarding how they want clinicians to ask and talk about the topic. In one survey, women who had experienced IPV preferred that clinicians ask about it as part of the complete medical history, as long as it did not create “an atmosphere of interrogation.”32
The style in which a clinician asks about IPV may make a difference as well. In focus groups, immigrant Latina and Asian women who had experienced IPV stated that clinicians could facilitate open communication by initiating the discussion and exhibiting compassionate and supportive behavior during the visit.33 Being able to see the same clinician at each visit also enhanced clinician-patient communication.33
In a study of IPV screening in emergency room settings, most clinicians asked about IPV in a perfunctory, direct manner—generally some variant of, “Are you a victim of domestic violence?” In this study, patient IPV disclosure occurred more often when clinicians used an open-ended approach such as, “Tell me what happened,” or when clinicians probed for possible IPV (eg, “What do you think may be causing some of this stress?”).34
In a focus group, female IPV survivors described feeling stigmatized or invalidated when clinicians were condescending, judgmental, or dismissive.35 Nonjudgmental and supportive communication decreased the women’s sense of isolation and led to positive outcomes such as increased awareness of IPV as a problem, decreased isolation, and feeling that the clinician cared.35
When addressing IPV, clinicians should explain why they are asking about it because it allows the woman to understand the context of the inquiry and to feel more comfortable about disclosing IPV. If the query is a regular part of a general screening or history-taking, for example, they should frame the question to make that point apparent. For example, “Because we know that many women in the United States experience physical, sexual or emotional violence from their romantic partners, I like to ask all of my patients whether they have been hurt or have felt threatened or afraid in a current or past relationship.”
In situations in which clinicians are concerned about IPV with a particular patient, they should explicitly share their concerns and desire to help the patient. One IPV survivor offered this advice: “Just look at the patient like she is your friend. Call her by her name. For instance, say ‘Sally, is he hurting you? Are you having problems? If you need help, I have some [phone] numbers.’ Personalize the encounter.”
It is also important to address IPV in a manner that ensures the patient’s safety, confidentiality, and dignity. When having this type of sensitive conversation, the patient should ideally be clothed and alone—without others present, particularly her partner. Professional interpreters should be available to women who do not speak English. The clinician should maintain eye contact, smile to communicate friendliness, and use a supportive tone.36
Just asking may be an intervention
Qualitative studies have suggested that just the act of asking about IPV in a nonjudgmental and compassionate manner is helpful to women experiencing IPV.35,37 Doing so not only helps women recognize the abuse, but also begins to decrease their sense of isolation and increase their awareness of helpful resources. It also gives the patient a sense that the clinician cares about her situation.35 As a result, experts have begun to recommend that health clinicians view asking about IPV not merely as a screening tool, but as a potentially therapeutic intervention in and of itself.35,37,38
HOW TO HELP
What to do when a woman discloses IPV
Female survivors, advocates, and health care clinicians who care for abused women suggest responding to a positive disclosure of IPV by providing the following:
Validation. The IPV perpetrator will often attempt to justify the violence and abuse by shifting some of the blame or responsibility for the violence onto the victim. This “brainwashing” leads to self-blame and a diminished sense of self-worth. However, clinicians can help reverse this mindset by acknowledging the woman’s disclosure and emphasizing that she did not deserve the abuse or violence. An example of such a statement is, “I am so very sorry that you went through that with your partner. You definitely did not deserve that. No one should ever be hurt by or afraid of the people who are supposed to love them.” Providing validation helps women recognize that the violence was a problem they did not deserve.38,39
Support. Women who have experienced IPV appreciate feeling supported and cared for by their health care clinician. Even if the patient is not ready to take any definitive action regarding her relationship or situation, knowing that her clinician and the health care setting are resources and sources of support is both comforting and empowering.35,40 Clinicians can communicate this support by stating, “I want you to know that whatever happens and whatever you decide, we are here for you.”
Respect for autonomy. Women experiencing IPV best understand their own situation and its various complexities and so they know best what they can do, cannot do, or need to do. As such, clinicians must respect a woman’s autonomy and preserve her ability to express her own needs and desires and make her own decisions. Prescribing a plan of action or giving commands to IPV victims could further perpetuate their sense of disempowerment and lack of control over their life.
Information. Providing referral information or hotline numbers to community domestic violence programs is helpful. However, not all women feel safe or comfortable taking printed brochures or written information with them because their abusive partner may find it. Thus, clinicians should ask if the patient can take the information safely or offer to write the numbers down without labeling them if she is afraid. The National Domestic Violence hotline is a 24-hour toll-free resource that will help women locate and contact shelters and other support services in their own community. The number, 1-800-799-SAFE (7233), is easy to memorize and thus is an easy resource to pass along quickly and safely. Other national organizations such as Futures Without Violence (formerly known as the Family Violence Prevention Fund) and the National Coalition Against Violence also provide links to local resources (Table 1).
Safety planning. Discussing the need for a safety plan will help the patient prepare for future abusive episodes. Even women who report that they are no longer in an abusive relationship should be asked about their current safety needs and concerns because they often remain in contact with abusive partners even after a relationship has ended.
When discussing safety planning, clinicians should ask the woman if she is currently safe or if she needs shelter. If she intends to return to or is still in contact with her batterer, ask if she has a plan for what to do or how to escape if the violence occurs again. Advise the patient to:
- Hide money so that she can leave quickly
- Make copies of birth certificates, immunization records, Social Security Number, and other important documents and keep them hidden and accessible
- Make a spare car key
- Have a list of hotline numbers
- Develop a code with friends, family, and neighbors that will let them know she needs immediate help.
Studies show that discussing safety-promoting behaviors increases the number of them that are used by IPV victims.41 A detailed list that can be shared with patients is provided in the patient information page that accompanies this article.42 Examples of personalized safety plans are available from the National Center on Domestic and Sexual Violence at its website, www.ncdsv.org/images/NCDSV_DVSafetyPlan_updated2013.pdf.
Danger assessment. Several researchers have examined potential risk factors associated with increased risk of homicide.43 Table 2 lists some of the characteristics associated with an increased risk of homicide in IPV situations.42 From this work, a danger assessment tool and scoring system has been developed. This tool and training on how to use it are available for free at www.dangerassessment.org. Although there are currently no outcome data on the benefits or risks of using this instrument, its objective is to increase women’s awareness of their danger level and individualize their safety counseling.
Proper documentation. Victim advocates and lawyers working on behalf of IPV victims emphasize that documentation by a medical provider can help a woman with her legal case. This documentation should be clear, legible, and as detailed as possible. These details should include a patient’s own words set off by quotation marks, a description or body map illustrating associated injuries or physical signs corroborating the violence, and a description of the patient’s demeanor or signs of emotion. Clinicians should avoid legal terms such as “alleges” or “alleged perpetrator” and should either define or avoid abbreviations that may be considered ambiguous in a legal proceeding (eg, clinicians should write out the words “domestic violence” or “intimate partner violence” rather than using “DV” or “IPV”).44 Most states have passed laws that prevent insurance companies from discriminating against IPV victims; insurance companies can no longer deny women coverage for seeking care related to IPV.45
A nonthreatening physical examination. Women who have experienced physical or sexual violence may feel anxious or experience added trauma during certain portions of the physical examination. Women who have been raped or otherwise sexually abused may have difficulty tolerating a pelvic examination, for example. Others who have been choked or grabbed by the neck may experience distress during palpation of the thyroid or cervical lymph nodes. The oropharyngeal examination may be challenging for women who have experienced forced oral sex or who may have had objects such as the barrel of a gun forced into their mouth. Asking women who have experienced IPV what aspects of the physical examination they may find difficult and how this could be made easier allows the patient and clinician to work together to develop strategies for or alternatives to necessary examinations or testing.
Before starting an examination, clinicians should explain what will happen and then ask the patient for her permission before moving on to the next step. Doing so creates a sense of control in an otherwise challenging and potentially traumatizing examination.
Disclosure of mandatory IPV reporting. Many states require health care providers to report any injuries caused by a weapon or criminal act to police or other official institutions.45,46 Several of these states specify that this reporting should occur during an IPV evaluation.
Mandatory reporting of IPV to police is highly controversial.47–49 Opponents of these laws argue that they do not benefit victims because they do not respect autonomy, they violate patient-clinician confidentiality, and they violate informed consent.47 Female IPV victims indicate that they would be less likely to disclose IPV and seek help if they knew it had to be reported.50–53 In a survey of California physicians, 59% stated that they might not comply with reporting laws if the patient objected.54
In states with mandatory reporting laws, clinicians can advocate for their patients by disclosing whether IPV must be reported before beginning their examination. The Michigan Coalition Against Domestic Violence devised this sample statement:
“I do have to let you know that under state law, I am obligated to make a police report to (name police agency with jurisdiction over the facility), if I/we are treating someone for any injury sustained by means of violence (tailor this information to the specifics of your state law). So, if you would have any concerns about that, I would encourage you to speak with one of the domestic violence advocates/counselors at (DV agency), who are able to provide confidential services and help without being required to make a police report.”55
The Compendium of State Statutes and Polices on Domestic Violence and Health Care by Futures Without Violence is an excellent reference that describes current state laws and policies regarding domestic violence and includes descriptions of the specific characteristics of each state’s mandatory reporting laws and their implications for health care providers. A copy can be downloaded from www.futureswithout-violence.org/content/features/detail/1584/.
What not to do
Clinicians should not ignore or minimize the extent of IPV, make excuses for the batterer, or blame the woman.32,56,57 They also should not inadvertently blame or criticize the woman by asking her, “What did you do to deserve this?” or “Why don’t you just leave?”57
While leaving a violent relationship is an obvious solution, many women experiencing IPV are either unwilling or unable to do so. In fact, leaving the batterer can be just as dangerous as staying. In a North Carolina study, half of all women killed by their partner had divorced or broken up with the partner immediately before the murder.4
What to do when a woman does not disclose IPV
Women who have experienced IPV often deny the violence to others out of fear. So a denial of violence, even in highly suspicious cases, can be expected. Abused patients often want help but are confused and afraid to ask for it.58,59 Providing easy and anonymous access to IPV information and resources through posters, flyers, brochures, and booklets will allow any woman—regardless of disclosure—to obtain help.36
In one IPV trial, all women in a family planning clinic who were in the intervention group were given information about IPV and how it can affect sexual and reproductive health, whether or not they disclosed. Compared with women in the control group, women who received the intervention—both those who did and did not experience IPV—were 63% more likely than those in the control group to end a relationship because they perceived it to be unhealthy or unsafe. In this case, all women—not just those who were victims—benefited from receiving information about IPV.60
PROVIDERS’ SUPPORT MAKES A DIFFERENCE, IMPROVES OUTCOMES
Patients may seek help for and find safety from IPV in stages or steps, and it may take them several months or even years to do so.61–63 In this sense, then, IPV is an issue that is not likely to be “cured” or changed in a single medical visit. In addition, a single visit may not be associated with direct health and safety outcomes. However, a patient is more likely to make changes if she has supportive and informative interaction with a caring, nonjudgmental clinician who can increase her awareness of IPV and IPV resources and promote an improved sense of self-efficacy or perceived power.40
For example, in a study in which health clinicians expressed concern about potential IPV health effects and offered support services such as counseling, 67% of women used one of those resources at least once.40 In another study, women who talked to a health care provider about their abuse were almost four times more likely to use an IPV intervention (eg, advocacy, shelter, restraining order) than women who did not talk to their provider. Those who used an IPV intervention were 2.6 times more likely to leave their abusive relationship, and those who left reported improved physical health.64
Thus, health care providers can have a positive effect by addressing this complex and difficult issue. The power and influence of a provider’s kindness, empathy, and support cannot be overestimated in their ability to improve the safety and well-being of women dealing with IPV.
- Saltzman LE, Fanslow JL, McMahon PM, Shelley GA; National Center for Injury Prevention and Control; Centers for Disease Control and Prevention. Intimate partner violence surveillance: uniform definitions and recommended data elements, Version 1.0. Atlanta, GA; 1999. www.cdc.gov/ncipc/pub-res/ipv_surveillance/intimate%20partner%20violence.pdf. Accessed June 3, 2014.
- Black MC, Basile KC, Breiding MJ, et al; Center for Injury Prevention and Control; Centers for Disease Control and Prevention. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed June 3, 2014.
- Kellermann AL, Mercy JA. Men, women, and murder: gender-specific differences in rates of fatal violence and victimization. J Trauma 1992; 33:1–5.
- Moracco KE, Runywan CW, Butts JD. Femicide in North Carolina, 1991–1993: a statewide study of patterns and precursors. Homicide Studies 1998; 4:422–446.
- Wadman MC, Muelleman RL. Domestic violence homicides: ED use before victimization. Am J Emerg Med 1999; 17:689–691.
- Catalano SM; United States Bureau of Justice Statistics. Intimate partner violence in the United States: 2007. http://bjs.ojp.usdoj.gov/index.cfm?ty=pbdetail&iid=1000. Accessed February 19, 2014.
- Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med 1995; 123:782–794.
- Scholle SH, Rost KM, Golding JM. Physical abuse among depressed women. J Gen Intern Med 1998; 13:607–613.
- Walling MK, O’Hara MW, Reiter RC, Milburn AK, Lilly G, Vincent SD. Abuse history and chronic pain in women: II. A multivariate analysis of abuse and psychological morbidity. Obstet Gynecol 1994; 84:200–206.
- McCauley J, Kern DE, Kolodner K, Derogatis LR, Bass EB. Relation of low-severity violence to women’s health. J Gen Intern Med 1998; 13:687–691.
- Wingood GM, DiClemente RJ. The effects of an abusive primary partner on the condom use and sexual negotiation practices of African-American women. Am J Public Health 1997; 87:1016–1018.
- Sales JM, Salazar LF, Wingood GM, DiClemente RJ, Rose E, Crosby RA. The mediating role of partner communication skills on HIV/STD-associated risk behaviors in young African American females with a history of sexual violence. Arch Pediatr Adolesc Med 2008; 162:432–438.
- Davila YR, Brackley MH. Mexican and Mexican American women in a battered women’s shelter: barriers to condom negotiation for HIV/AIDS prevention. Issues Ment Health Nurs 1999; 20:333–355.
- Tjaden P, Thoennes N; National Institute of Justice; Centers for Disease Control and Prevention. Prevalence, incidence and consequences of violence against women: findings from the national violence against women survey. Washington, DC; 1998. https://www.ncjrs.gov/pdffiles/172837.pdf. Accessed June 3, 2014.
- Masho SW, Odor RK, Adera T. Sexual assault in Virginia: a population-based study. Womens Health Issues 2005; 15:157–166.
- Miller E, Decker MR, McCauley HL, et al. Pregnancy coercion, intimate partner violence and unintended pregnancy. Contraception 2010; 81:316–322.
- Bonomi AE, Anderson ML, Rivara FP, Thompson RS. Health care utilization and costs associated with physical and nonphysical-only intimate partner violence. Health Serv Res 2009; 44:1052–1067.
- Rivara FP, Anderson ML, Fishman P, et al. Healthcare utilization and costs for women with a history of intimate partner violence. Am J Prev Med 2007; 32:89–96.
- American Nurses Association. Position statement on physical violence against women. Washington, DC; 1991.
- Physicians and domestic violence. Ethical considerations. Council on Ethical and Judicial Affairs, American Medical Association. JAMA 1992; 267:3190–3193.
- The American College of Obstetricians and Gynecologists. Screening tools: domestic violence. http://www.acog.org/About_ACOG/ACOG_Departments/Violence_Against_Women/Screening_Tools__Domestic_Violence. Accessed June 3, 2014.
- Lee D, James L, Sawires P; The Family Violence Prevention Fund. Preventing domestic violence: clinical guidelines on routine screening. San Francisco, CA; 1999. http://new.vawnet.org/Assoc_Files_VAWnet/screpol.pdf. Accessed June 3, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation manual for hospitals. Chicago, IL; 1992.
- US Department of Health and Human Services. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC; 1996.
- Ramsay J, Richardson J, Carter YH, Davidson LL, Feder G. Should health professionals screen women for domestic violence? Systematic review. BMJ 2002; 325:314.
- Nelson HD, Nygren P, McInerney Y, Klein J; US Preventive Services Task Force. Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med 2004; 140:387–396.
- Chamberlain L. The USPSTF recommendation on intimate partner violence: what we can learn from it and what we can do about it. Fam Viol Prev Health Pract 2005; 1:1–24.
- Moyer VA; US Preventive Services Task Force. Screening for intimate partner violence and abuse of elderly and vulnerable adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:478–486.
- Taft A, O’Doherty L, Hegarty K, Ramsay J, Davidson L, Feder G. Screening women for intimate partner violence in healthcare settings. Cochrane Database Syst Rev 2013; 4:CD007007.
- McNutt LA, Carlson BE, Gagen D, Winterbauer N. Reproductive violence screening in primary care: perspectives and experiences of patients and battered women. J Am Med Womens Assoc 1999; 54:85–90.
- Friedman LS, Samet JH, Roberts MS, Hudlin M, Hans P. Inquiry about victimization experiences. A survey of patient p and physician practices. Arch Intern Med 1992; 152:1186–1190.
- Hamberger LK, Ambuel B, Marbella A, Donze J. Physician interaction with battered women: the women’s perspective. Arch Fam Med 1998; 7:575–582.
- Rodriguez MA, Bauer HM, Flores-Ortiz Y, Szkupinski-Quiroga S. Factors affecting patient-physician communication for abused Latina and Asian immigrant women. J Fam Pract 1998; 47:309–311.
- Rhodes KV, Frankel RM, Levinthal N, Prenoveau E, Bailey J, Levinson W. “You’re not a victim of domestic violence, are you?” Provider patient communication about domestic violence”. Ann Intern Med 2007; 147:620–627.
- Chang JC, Decker M, Moracco KE, Martin SL, Petersen R, Frasier PY. What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors. J Am Med Womens Assoc 2003; 58:76–81.
- Chang JC, Decker MR, Moracco KE, Martin SL, Petersen R, Frasier PY. Asking about intimate partner violence: advice from female survivors to health care providers. Patient Educ Couns 2005; 59:141–147.
- Hathaway JE, Willis G, Zimmer B. Listening to survivors’ voices: addressing partner abuse in the health care setting. Violence Against Women 2002; 8:687–716.
- Gerbert B, Caspers N, Bronstone A, Moe J, Abercrombie P. A qualitative analysis of how physicians with expertise in domestic violence approach the identification of victims. Ann Intern Med 1999; 131:578–584.
- Gerbert B, Caspers N, Milliken N, Berlin M, Bronstone A, Moe J. Interventions that help victims of domestic violence. A qualitative analysis of physicians’ experiences. J Fam Pract 2000; 49:889–895.
- McCaw B, Bauer HM, Berman WH, Mooney L, Holmberg M, Hunkeler E. Women referred for on-site domestic violence services in a managed care organization. Women Health 2002; 35:23–40.
- McFarlane J, Malecha A, Gist J, et al. An intervention to increase safety behaviors of abused women: results of a randomized clinical trial. Nurs Res 2002; 51:347–354.
- Chang JC. Domestic violence. In:Bieber EJ, Sanfilippo JS, Horowitz IR, editors. Clinical Gynecology. Philadelphia, PA; Elsevier, Inc; 2006:79–89.
- Campbell JC, Webster D, Koziol-McLain J, et al. Risk factors for femicide in abusive relationships: results from a multisite case control study. Am J Public Health 2003; 93:1089–1097.
- Isaac NE, Enos V; National Institute of Justice. Documenting domestic violence: how health care providers can help victims. Research in Brief, 2001. https://www.ncjrs.gov/pdffiles1/nij/188564.pdf. Accessed June 3, 2014.
- Durborow N, Lizdas KC, O’Flaherty A, Marjavi A; Futures Without Violence. Compendium of state and US terrritory statutes and policies on domestic violence and health care, 2010. www.futureswithoutviolence.org/content/features/detail/1584/. Accessed June 3, 2014.
- Hyman A, Schillinger D, Lo B. Laws mandating reporting of domestic violence. Do they promote patient well-being? JAMA 1995; 273:1781–1787.
- Hyman A, Chez RA. Mandatory reporting of domestic violence by health care providers: a misguided approach. Womens Health Issues 1995; 5:208–213.
- Knight MA. Ethical debate: should doctors be more proactive as advocates for victims of violence? The police surgeon’s view: medical paternalism is unacceptable. BMJ 1995; 311:1620–1621.
- Hyman A; Futures Without Violence. Mandatory reporting of domestic violence by healthcare providers: a policy paper. San Francisco, CA; 1997. www.futureswithoutviolence.org/userfiles/file/HealthCare/mandatory_policypaper.pdf. Accessed June 3, 2014.
- Rodriguez MA, Craig AM, Mooney DR, Bauer HM. Patient attitudes about mandatory reporting of domestic violence. Implications for health care professionals. West J Med 1998; 169:337–341.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Sachs CJ, Koziol-McLain J, Glass N, Webster D, Campbell J. A population-based survey assessing support for mandatory domestic violence reporting by health care personnel. Women Health 2002; 35:121–133.
- Sullivan CM, Hagen LA. Survivors’ opinions about mandatory reporting of domestic violence and sexual assault by medical professionals. Affilia 2005; 20:1–16.
- Rodriguez MA, McLoughlin E, Bauer HM, Paredes V, Grumbach K. Mandatory reporting of intimate partner violence to police: views of physicians in California. Am J Public Health 1999; 89:575–578.
- Futures Without Violence. Mandatory reporting of domestic violence to law enforcement by health care providers: a guide for advocates working to respond to or amend reporting laws related to domestic violence. www.healthcaresaboutipv.org/wp-content/blogs.dir/3/files/2012/09/Mandatory_Reporting_of_DV_to_Law-Enforcement_by_HCP.pdf. Accessed June 3, 2014.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Gerbert B, Johnston K, Caspers N, Bleecker T, Woods A, Rosenbaum A. Experiences of battered women in health care settings: a qualitative study. Women Health 1996; 24:1–17.
- Chang JC, Cluss PA, Ranieri L, et al. Health care interventions for intimate partner violence: what women want. Womens Health Issues 2005; 15:21–30.
- Rodriguez MA, Quiroga SS, Bauer HM. Breaking the silence. Battered women’s perspectives on medical care. Arch Fam Med 1996; 5:153–158.
- Miller E, Decker MR, McCauley HL, et al. A family planning clinic partner violence intervention to reduce risk associated with reproductive coercion. Contraception 2011; 83:274–280.
- Landenburger K. A process of entrapment in and recovery from an abusive relationship. Issues Ment Health Nurs 1989; 10:209–227.
- Cluss PA, Chang JC, Hawker L, et al. The process of change for victims of intimate partner violence: support for a psychosocial readiness model. Womens Health Issues 2006; 16:262–274.
- Gerbert B, Abercrombie P, Caspers N, Love C, Bronstone A. How health care providers help battered women: the survivor’s perspective. Women Health 1999; 29:115–135.
- McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep 2006; 121:435–444.
- Saltzman LE, Fanslow JL, McMahon PM, Shelley GA; National Center for Injury Prevention and Control; Centers for Disease Control and Prevention. Intimate partner violence surveillance: uniform definitions and recommended data elements, Version 1.0. Atlanta, GA; 1999. www.cdc.gov/ncipc/pub-res/ipv_surveillance/intimate%20partner%20violence.pdf. Accessed June 3, 2014.
- Black MC, Basile KC, Breiding MJ, et al; Center for Injury Prevention and Control; Centers for Disease Control and Prevention. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf. Accessed June 3, 2014.
- Kellermann AL, Mercy JA. Men, women, and murder: gender-specific differences in rates of fatal violence and victimization. J Trauma 1992; 33:1–5.
- Moracco KE, Runywan CW, Butts JD. Femicide in North Carolina, 1991–1993: a statewide study of patterns and precursors. Homicide Studies 1998; 4:422–446.
- Wadman MC, Muelleman RL. Domestic violence homicides: ED use before victimization. Am J Emerg Med 1999; 17:689–691.
- Catalano SM; United States Bureau of Justice Statistics. Intimate partner violence in the United States: 2007. http://bjs.ojp.usdoj.gov/index.cfm?ty=pbdetail&iid=1000. Accessed February 19, 2014.
- Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med 1995; 123:782–794.
- Scholle SH, Rost KM, Golding JM. Physical abuse among depressed women. J Gen Intern Med 1998; 13:607–613.
- Walling MK, O’Hara MW, Reiter RC, Milburn AK, Lilly G, Vincent SD. Abuse history and chronic pain in women: II. A multivariate analysis of abuse and psychological morbidity. Obstet Gynecol 1994; 84:200–206.
- McCauley J, Kern DE, Kolodner K, Derogatis LR, Bass EB. Relation of low-severity violence to women’s health. J Gen Intern Med 1998; 13:687–691.
- Wingood GM, DiClemente RJ. The effects of an abusive primary partner on the condom use and sexual negotiation practices of African-American women. Am J Public Health 1997; 87:1016–1018.
- Sales JM, Salazar LF, Wingood GM, DiClemente RJ, Rose E, Crosby RA. The mediating role of partner communication skills on HIV/STD-associated risk behaviors in young African American females with a history of sexual violence. Arch Pediatr Adolesc Med 2008; 162:432–438.
- Davila YR, Brackley MH. Mexican and Mexican American women in a battered women’s shelter: barriers to condom negotiation for HIV/AIDS prevention. Issues Ment Health Nurs 1999; 20:333–355.
- Tjaden P, Thoennes N; National Institute of Justice; Centers for Disease Control and Prevention. Prevalence, incidence and consequences of violence against women: findings from the national violence against women survey. Washington, DC; 1998. https://www.ncjrs.gov/pdffiles/172837.pdf. Accessed June 3, 2014.
- Masho SW, Odor RK, Adera T. Sexual assault in Virginia: a population-based study. Womens Health Issues 2005; 15:157–166.
- Miller E, Decker MR, McCauley HL, et al. Pregnancy coercion, intimate partner violence and unintended pregnancy. Contraception 2010; 81:316–322.
- Bonomi AE, Anderson ML, Rivara FP, Thompson RS. Health care utilization and costs associated with physical and nonphysical-only intimate partner violence. Health Serv Res 2009; 44:1052–1067.
- Rivara FP, Anderson ML, Fishman P, et al. Healthcare utilization and costs for women with a history of intimate partner violence. Am J Prev Med 2007; 32:89–96.
- American Nurses Association. Position statement on physical violence against women. Washington, DC; 1991.
- Physicians and domestic violence. Ethical considerations. Council on Ethical and Judicial Affairs, American Medical Association. JAMA 1992; 267:3190–3193.
- The American College of Obstetricians and Gynecologists. Screening tools: domestic violence. http://www.acog.org/About_ACOG/ACOG_Departments/Violence_Against_Women/Screening_Tools__Domestic_Violence. Accessed June 3, 2014.
- Lee D, James L, Sawires P; The Family Violence Prevention Fund. Preventing domestic violence: clinical guidelines on routine screening. San Francisco, CA; 1999. http://new.vawnet.org/Assoc_Files_VAWnet/screpol.pdf. Accessed June 3, 2014.
- Joint Commission on Accreditation of Healthcare Organizations. Accreditation manual for hospitals. Chicago, IL; 1992.
- US Department of Health and Human Services. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC; 1996.
- Ramsay J, Richardson J, Carter YH, Davidson LL, Feder G. Should health professionals screen women for domestic violence? Systematic review. BMJ 2002; 325:314.
- Nelson HD, Nygren P, McInerney Y, Klein J; US Preventive Services Task Force. Screening women and elderly adults for family and intimate partner violence: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med 2004; 140:387–396.
- Chamberlain L. The USPSTF recommendation on intimate partner violence: what we can learn from it and what we can do about it. Fam Viol Prev Health Pract 2005; 1:1–24.
- Moyer VA; US Preventive Services Task Force. Screening for intimate partner violence and abuse of elderly and vulnerable adults: US Preventive Services Task Force recommendation statement. Ann Intern Med 2013; 158:478–486.
- Taft A, O’Doherty L, Hegarty K, Ramsay J, Davidson L, Feder G. Screening women for intimate partner violence in healthcare settings. Cochrane Database Syst Rev 2013; 4:CD007007.
- McNutt LA, Carlson BE, Gagen D, Winterbauer N. Reproductive violence screening in primary care: perspectives and experiences of patients and battered women. J Am Med Womens Assoc 1999; 54:85–90.
- Friedman LS, Samet JH, Roberts MS, Hudlin M, Hans P. Inquiry about victimization experiences. A survey of patient p and physician practices. Arch Intern Med 1992; 152:1186–1190.
- Hamberger LK, Ambuel B, Marbella A, Donze J. Physician interaction with battered women: the women’s perspective. Arch Fam Med 1998; 7:575–582.
- Rodriguez MA, Bauer HM, Flores-Ortiz Y, Szkupinski-Quiroga S. Factors affecting patient-physician communication for abused Latina and Asian immigrant women. J Fam Pract 1998; 47:309–311.
- Rhodes KV, Frankel RM, Levinthal N, Prenoveau E, Bailey J, Levinson W. “You’re not a victim of domestic violence, are you?” Provider patient communication about domestic violence”. Ann Intern Med 2007; 147:620–627.
- Chang JC, Decker M, Moracco KE, Martin SL, Petersen R, Frasier PY. What happens when health care providers ask about intimate partner violence? A description of consequences from the perspectives of female survivors. J Am Med Womens Assoc 2003; 58:76–81.
- Chang JC, Decker MR, Moracco KE, Martin SL, Petersen R, Frasier PY. Asking about intimate partner violence: advice from female survivors to health care providers. Patient Educ Couns 2005; 59:141–147.
- Hathaway JE, Willis G, Zimmer B. Listening to survivors’ voices: addressing partner abuse in the health care setting. Violence Against Women 2002; 8:687–716.
- Gerbert B, Caspers N, Bronstone A, Moe J, Abercrombie P. A qualitative analysis of how physicians with expertise in domestic violence approach the identification of victims. Ann Intern Med 1999; 131:578–584.
- Gerbert B, Caspers N, Milliken N, Berlin M, Bronstone A, Moe J. Interventions that help victims of domestic violence. A qualitative analysis of physicians’ experiences. J Fam Pract 2000; 49:889–895.
- McCaw B, Bauer HM, Berman WH, Mooney L, Holmberg M, Hunkeler E. Women referred for on-site domestic violence services in a managed care organization. Women Health 2002; 35:23–40.
- McFarlane J, Malecha A, Gist J, et al. An intervention to increase safety behaviors of abused women: results of a randomized clinical trial. Nurs Res 2002; 51:347–354.
- Chang JC. Domestic violence. In:Bieber EJ, Sanfilippo JS, Horowitz IR, editors. Clinical Gynecology. Philadelphia, PA; Elsevier, Inc; 2006:79–89.
- Campbell JC, Webster D, Koziol-McLain J, et al. Risk factors for femicide in abusive relationships: results from a multisite case control study. Am J Public Health 2003; 93:1089–1097.
- Isaac NE, Enos V; National Institute of Justice. Documenting domestic violence: how health care providers can help victims. Research in Brief, 2001. https://www.ncjrs.gov/pdffiles1/nij/188564.pdf. Accessed June 3, 2014.
- Durborow N, Lizdas KC, O’Flaherty A, Marjavi A; Futures Without Violence. Compendium of state and US terrritory statutes and policies on domestic violence and health care, 2010. www.futureswithoutviolence.org/content/features/detail/1584/. Accessed June 3, 2014.
- Hyman A, Schillinger D, Lo B. Laws mandating reporting of domestic violence. Do they promote patient well-being? JAMA 1995; 273:1781–1787.
- Hyman A, Chez RA. Mandatory reporting of domestic violence by health care providers: a misguided approach. Womens Health Issues 1995; 5:208–213.
- Knight MA. Ethical debate: should doctors be more proactive as advocates for victims of violence? The police surgeon’s view: medical paternalism is unacceptable. BMJ 1995; 311:1620–1621.
- Hyman A; Futures Without Violence. Mandatory reporting of domestic violence by healthcare providers: a policy paper. San Francisco, CA; 1997. www.futureswithoutviolence.org/userfiles/file/HealthCare/mandatory_policypaper.pdf. Accessed June 3, 2014.
- Rodriguez MA, Craig AM, Mooney DR, Bauer HM. Patient attitudes about mandatory reporting of domestic violence. Implications for health care professionals. West J Med 1998; 169:337–341.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Sachs CJ, Koziol-McLain J, Glass N, Webster D, Campbell J. A population-based survey assessing support for mandatory domestic violence reporting by health care personnel. Women Health 2002; 35:121–133.
- Sullivan CM, Hagen LA. Survivors’ opinions about mandatory reporting of domestic violence and sexual assault by medical professionals. Affilia 2005; 20:1–16.
- Rodriguez MA, McLoughlin E, Bauer HM, Paredes V, Grumbach K. Mandatory reporting of intimate partner violence to police: views of physicians in California. Am J Public Health 1999; 89:575–578.
- Futures Without Violence. Mandatory reporting of domestic violence to law enforcement by health care providers: a guide for advocates working to respond to or amend reporting laws related to domestic violence. www.healthcaresaboutipv.org/wp-content/blogs.dir/3/files/2012/09/Mandatory_Reporting_of_DV_to_Law-Enforcement_by_HCP.pdf. Accessed June 3, 2014.
- Caralis PV, Musialowski R. Women’s experiences with domestic violence and their attitudes and expectations regarding medical care of abuse victims. South Med J 1997; 90:1075–1080.
- Gerbert B, Johnston K, Caspers N, Bleecker T, Woods A, Rosenbaum A. Experiences of battered women in health care settings: a qualitative study. Women Health 1996; 24:1–17.
- Chang JC, Cluss PA, Ranieri L, et al. Health care interventions for intimate partner violence: what women want. Womens Health Issues 2005; 15:21–30.
- Rodriguez MA, Quiroga SS, Bauer HM. Breaking the silence. Battered women’s perspectives on medical care. Arch Fam Med 1996; 5:153–158.
- Miller E, Decker MR, McCauley HL, et al. A family planning clinic partner violence intervention to reduce risk associated with reproductive coercion. Contraception 2011; 83:274–280.
- Landenburger K. A process of entrapment in and recovery from an abusive relationship. Issues Ment Health Nurs 1989; 10:209–227.
- Cluss PA, Chang JC, Hawker L, et al. The process of change for victims of intimate partner violence: support for a psychosocial readiness model. Womens Health Issues 2006; 16:262–274.
- Gerbert B, Abercrombie P, Caspers N, Love C, Bronstone A. How health care providers help battered women: the survivor’s perspective. Women Health 1999; 29:115–135.
- McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep 2006; 121:435–444.
KEY POINTS
- Many victims of IPV will not present with injuries but may have medical or mental health issues related to their IPV experiences.
- Addressing IPV with female patients not only results in increased identification of survivors but is also associated with cognitive and emotional benefits.
- IPV information and resources should be provided to all women, regardless of IPV disclosure.
- Clinicians should respond to a patient’s IPV disclosure with validation, support, respect, and information.
- Clinicians must respect patients’ autonomy, as they are the ones who best understand their situation and know what they need. In some cases, leaving an abusive relationship can be more dangerous than staying.
Promoting higher blood pressure targets for frail older adults: A consensus guideline from Canada
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
Frail older adults deserve guidelines that take frailty into account while assessing the potential benefit and risks of treatment.
Specifically, our group—the Dalhousie Academic Detailing Service (ADS) and the Palliative and Therapeutic Harmonization (PATH) program—recommends that physicians strive to achieve more liberal treatment targets for elderly frail patients who have high blood pressure,1 as evidence does not support an aggressive approach in the frail elderly and the potential exists for harm.
This article reviews the evidence and reasoning that were used to develop and promote a guideline for drug treatment of hypertension in frail older adults. Our recommendations differ from other guidelines in that they focus as much on stopping or decreasing therapy as on starting or increasing it.
FRAILTY INCREASES THE RISK OF ADVERSE EFFECTS
The word frail, applied to older adults, describes those who have complex medical illnesses severe enough to compromise their ability to live independently.2 Many have multiple coexisting medical problems for which they take numerous drugs, in addition to dementia, impaired mobility, compromised functional ability, or a history of falling.
Frailty denotes vulnerability; it increases the risk of adverse effects from medical and surgical procedures,3 complicates drug therapy,4 prolongs hospital length of stay,5 leads to functional and cognitive decline,6 increases the risk of institutionalization,7 and reduces life expectancy8—all of which affect the benefit and harm of medical treatments.
Guidelines for treating hypertension9–11 now acknowledge that little evidence exists to support starting treatment for systolic blood pressure between 140 and 160 mm Hg or aiming for a target of less than 140 mm Hg for “very old” adults, commonly defined as over the age of 80. New guidelines loosen the treatment targets for the very old, but they do not specify targets for the frail and do not describe how to recognize or measure frailty.
RECOGNIZING AND MEASURING FRAILTY
A number of tools are available to recognize and measure frailty.12
The Fried frailty assessment13 has five items:
- Unintentional weight loss
- Self-reported exhaustion
- Weakness in grip
- Slow walking speed
- Low physical activity and energy expenditure.
People are deemed frail if they have three or more of these five. However, experts disagree about whether this system is too sensitive14 or not sensitive enough.15,16
The FRAIL questionnaire17 also has five items:
- Fatigue
- Resistance (inability to climb stairs)
- Ambulation (inability to walk 1 city block)
- Illness (more than 5 major illnesses)
- Weight loss.
People are deemed frail if they have at least three of these five items, and “prefrail” if they have two.
These and other tools are limited by being dichotomous: they classify people as being either frail or not frail18–20 but do not define the spectrum of frailty.
Other frailty assessments such as the Frailty Index21 identify frailty based on the number of accumulated health deficits but take a long time to complete, making them difficult to use in busy clinical settings.22–24
The Clinical Frailty Scale7 is a validated scale that categorizes frailty based on physical and functional indicators of health, such as cognition, function, and mobility, with scores that range from 1 (very fit) to 9 (terminally ill).7,12
The Frailty Assessment for Care-planning Tool (FACT) uses scaling compatible with the Clinical Frailty Scale but has been developed for use as a practical and interpretable frailty screening tool for nonexperts (Table 1). The FACT assesses cognition, mobility, function, and the social situation, using a combination of caregiver report and objective measures. To assess cognition, a health care professional uses items from the Mini-Cog25 (ie, the ability to draw an analog clock face and then recall three unrelated items following the clock-drawing test) and the memory axis of the Brief Cognitive Rating Scale26 (ie, the ability to recall current events, the current US president, and the names of children or spouse). Mobility, function, and social circumstance scores are assigned according to the caregiver report of the patient’s baseline status.
The FACT can be completed in busy clinical settings. Once a caregiver is identified, it takes about 5 minutes to complete.
Our guideline27–31 is intended for those with a score of 7 or more on the Clinical Frailty Scale or FACT,7,12 a score we chose because it describes people who are severely frail with shortened life expectancy.8 At this level, people need help with all instrumental activities of daily living (eg, handling finances, medication management, household chores, and shopping) as well as with basic activities of daily living such as bathing or dressing.
REVIEWING THE LIMITED EVIDENCE
We found no studies that addressed the risks and benefits of treating hypertension in frail older adults; therefore, we concentrated on studies that enrolled individuals who were chronologically old but not frail. We reviewed prominent guidelines,9–11,32,33 the evidence base for these guidelines,34–44 and Cochrane reviews.45,46 A detailed description of the evidence used to build our recommendation can be found online.31
When we deliberated on treatment targets, we reviewed evidence from two types of randomized controlled trials47:
Drug treatment trials randomize patients to different treatments, such as placebo versus a drug or one drug compared with another drug. Patients in different treatment groups may achieve different blood pressures and clinical outcomes, and this information is then used to define optimal targets. However, it may be difficult to determine if the benefit came from lowering blood pressure or from some other effect of the drug, which can be independent of blood pressure lowering.
Treat-to-target trials randomize patients to different blood pressure goals, but the groups are treated with the same or similar drugs. Therefore, any identified benefit can be attributed to the differences in blood pressure rather than the medications used. Compared with a drug treatment trial, this type of trial provides stronger evidence about optimal targets.
We also considered the characteristics of frailty, the dilemma of polypharmacy, and the relevance of the available scientific evidence to those who are frail.
Drug treatment trials
A Cochrane review45 of 15 studies with approximately 24,000 elderly participants found that treating hypertension decreased the rates of cardiovascular morbidity and mortality as well as fatal and nonfatal stroke in the “elderly” (defined as age ≥ 60) and “very elderly” (age ≥ 80). However, in the very elderly, all-cause mortality rates were not statistically significantly different with treatment compared with placebo. The mean duration of treatment was 4.5 years in the elderly and 2.2 years in the very elderly (Table 2). Of importance, all the trials enrolled only those individuals whose systolic blood pressure was at least 160 mm Hg at baseline.
None of the studies were treat-to-target trials—patients were assigned either active medication or placebo. Thus, these trials provide evidence of benefit for treating hypertension in the elderly and very elderly but do not identify the optimal target. All of the drug treatment trials showed benefit, but none achieved a systolic pressure lower than 140 mm Hg with active treatment (Table 3). Therefore, these studies do not support a systolic target of less than 140 mm Hg in the elderly.
Treat-to-target trials: JATOS and VALISH
The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)42 and the Valsartan in Elderly Isolated Systolic Hypertension (VALISH) study43 each enrolled more than 3,000 people age 65 or older (mean age approximately 75). Patients were randomized to either a strict systolic target of less than 140 mm Hg or a higher (more permissive) target of 140 to 160 mm Hg in JATOS and 140 to 149 mm Hg in VALISH.
In both trials, the group with strict targets achieved a systolic pressure of approximately 136 mm Hg, while the group with higher blood pressure targets achieved a systolic pressure of 146 mm Hg in JATOS and 142 mm Hg in VALISH. Despite these differences, there was no statistically significant difference in the primary outcome.
Thus, treat-to-target studies also fail to support a systolic target of less than 140 mm Hg in the elderly, although it is important to recognize the limitations of the studies. Approximately 15% of the participants had cardiovascular disease, so the applicability of the findings to patients with target-organ damage is uncertain. In addition, there were fewer efficacy outcome events than expected, which suggests that the studies were underpowered.
When to start drug treatment
In each of the drug treatment and treat-to-target trials, the inclusion criterion for study entry was a systolic blood pressure above 160 mm Hg, with a mean blood pressure at entry into the drug treatment trials of 182/95 mm Hg.46 Thus, data support starting treatment if the systolic blood pressure is above 160 mm Hg, but not lower.
Notably, in all but one study,46 at least two-thirds of the participants took no more than two antihypertensive medications. Since adverse events become more common as the number of medications increases, the benefit of adding a third drug to lower blood pressure is uncertain.
Evidence in the ‘very elderly’: HYVET
With the exception of the Hypertension in the Very Elderly Trial (HYVET),44 the mean age of elderly patients in the reported studies was between 67 and 76.
HYVET patients were age 80 and older (mean age 84) and were randomized to receive either indapamide (with or without perindopril) or placebo. The trial was stopped early at 2 years because the mortality rate was lower in the treatment group (10.1%) than in the placebo group (12.3%) (number needed to treat 46, 95% confidence interval 24–637, P = .02). There was no significant difference in the primary outcome of fatal and nonfatal stroke.
Notably, trials that are stopped early may overestimate treatment benefit.48
Evidence in frail older adults
While the above studies provide some information about managing hypertension in the elderly, the participants were generally healthy. HYVET44 specifically excluded those with a standing systolic blood pressure of less than 140 mm Hg and enrolled few patients with orthostasis (7.9% in the placebo group and 8.8% in the treatment group), a condition commonly associated with frailty. As such, these studies may be less relevant to the frail elderly, who are at higher risk of adverse drug events and have competing risks for morbidity and mortality.
Observational studies, in fact, raise questions about whether tight blood pressure control improves clinical outcomes for the very elderly. In the Leiden 85-plus study, lower systolic blood pressure was associated with lower cognitive scores, worse functional ability,49,50 and a higher mortality rate51 compared with higher systolic pressure, although it is uncertain whether these outcomes were indicative of underlying disease that could result in lower blood pressure or an effect of blood pressure-lowering.
The National Health and Nutrition Examination Survey52 found an association between blood pressure and mortality rate that varied by walking speed. For slower walkers (based on the 6-minute walk test), higher systolic pressures were not associated with a higher risk of death, suggesting that when older adults are frail (as indicated by their slow walking speed) they are less likely to benefit from aggressive treatment of hypertension.
People at high risk because of stroke
Because the evidence is limited, it is even more difficult to judge whether lowering blood pressure below 140 mm Hg is beneficial for frail patients who have a history of stroke, compared with the possibility that medications will cause adverse effects such as weakness, orthostasis, and falls. When reviewing the evidence to answer this question, we especially looked at outcomes that affect quality of life, such as nonfatal stroke leading to disability. In contrast, because the frail elderly have competing causes of mortality, we could not assume that a mortality benefit shown in nonfrail populations could be applied to frail populations.
The PROGRESS trial (Perindopril Protection Against Recurrent Stroke Study)53 was in patients with a history of stroke or transient ischemic attack and a mean age of 64, who were treated with either perindopril (with or without indapamide) or placebo.
At almost 4 years, the rate of disabling stroke was 2.7% in the treatment group and 4.3% in the placebo group, a relative risk reduction of 38% and an absolute risk reduction of 1.64% (number needed to treat 61, 95% confidence interval 39–139). The relative risk reduction for all strokes (fatal and nonfatal) was similar across a range of baseline systolic pressures, but the absolute risk reduction was greater in the prespecified subgroup that had hypertension at baseline (mean blood pressure 159/94 mm Hg) than in the normotensive subgroup (mean blood pressure 136/79 mm Hg), suggesting that treatment is most beneficial for those with higher systolic blood pressures. Also, the benefit was only demonstrated in the subgroup that received two antihypertensive medications; those who received perindopril alone showed no benefit.
This study involved relatively young patients in relatively good health except for their strokes. The extent to which the results can be extrapolated to older, frail adults is uncertain because of the time needed to achieve benefit and because of the added vulnerability of frailty, which could make treatment with two antihypertensive medications riskier.
PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes),54 another study in patients with previous stroke (mean age 66) showed no benefit over 2.5 years in the primary outcome of stroke using telmesartan 80 mg daily compared with placebo. This result is concordant with that of PROGRESS,53 in which patients who took only one medication did not show a significant decrease in the rate of stroke.
A possible reason for the lack of benefit from monotherapy was that the differences in blood pressure between the placebo group and the treatment group on monotherapy were small in both studies (3.8/2.0 mm Hg in PRoFESS, 5/3 mm Hg in PROGRESS). In contrast, patients on dual therapy in PROGRESS decreased their blood pressure by 12/5 mm Hg compared with placebo.
CURRENT HYPERTENSION GUIDELINES
Current guidelines make reference to the elderly, but we found none that made specific recommendations for the frail elderly.
JNC 8
In December 2013, members of the Eighth Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8) released new recommendations.32 One significant revision was to support higher blood pressure targets for older adults (age 60 and older). Whereas JNC 7 stated that lowering blood pressure below 140/90 mm Hg reduced cardiovascular complications,33 JNC 8 now acknowledges that there is no strong evidence to support blood pressure targets below 150/90 mm Hg for hypertensive persons without kidney disease or diabetes age 60 and older. Thus, in the general population age 60 and older, JNC 8 recommends starting antihypertensive treatment when blood pressure is 150/90 mm Hg or higher, and treating to a goal blood pressure of less than 150/90 mm Hg. JNC 8 makes no recommendation about how to adjust blood pressure targets for frailty or how to measure blood pressure.
American College of Cardiology and American Heart Association
In 2011, the American College of Cardiology and American Heart Association published a consensus document on the management of hypertension in the elderly.9
They acknowledged that the generally recommended blood pressure goal of lower than 140/90 mm Hg in uncomplicated elderly patients is based on expert opinion rather than on data from randomized controlled trials, but nevertheless recommended a target systolic pressure lower than 140 mm Hg for older adults, except for octogenarians.
For those over age 80, systolic levels of 140 to 145 mm Hg can be acceptable if tolerated and if the patient does not experience orthostasis when standing. Systolic pressure lower than 130 mm Hg and diastolic pressures lower than 65 mm Hg should be avoided in this age group.
The document acknowledges that systolic pressure may have to remain above 150 mm Hg if there is no response to four “well-selected drugs” or if there are unacceptable side effects. In these cases, the lowest “safely achieved” systolic blood pressure should be the goal.
Canadian Hypertension Education Program
The 2014 Canadian Hypertension Education Program (CHEP) report makes several recommendations for the “very elderly,” a group they define as over the age of 80. The CHEP website and resources include the following recommendations10:
- For the very elderly without diabetes or target-organ damage, drug therapy should be initiated when systolic blood pressure is higher than 160 mm Hg to reach a systolic blood pressure target lower than 150 mm Hg. This is a grade C level recommendation, indicating that it is based on low-quality trials, unvalidated surrogate outcomes, or results from nonrandomized observational studies.
- For the very elderly with macrovascular target-organ damage, antihypertensive therapy should be considered if systolic blood pressure readings average 140 mm Hg or higher (grade D for 140 to 160 mm Hg; grade A for higher than 160 mm Hg), although caution should be exercised in elderly patients who are frail. (Grade D recommendations are the weakest, as they are based on low-powered, imprecise studies or expert opinion, whereas grade A recommendations are based on the strongest evidence from high-quality randomized clinical trials.)
- Decisions regarding initiating and intensifying pharmacotherapy in the very elderly should be based on an individualized risk-benefit analysis.
The European Society of Hypertension and European Society of Cardiology
The 2013 guidelines from the European Society of Hypertension and the European Society of Cardiology11 recommend that for elderly patients under age 80, antihypertensive treatment may be considered at systolic values higher than 140 mm Hg and aimed at values lower than 140 mm Hg if the patient is fit and treatment is well tolerated.
For those over age 80 with an initial systolic pressure of 160 mm Hg or higher, the guidelines recommend lowering systolic pressure to between 150 and 140 mm Hg, provided the patient is in good physical and mental condition. In frail elderly patients, they recommend leaving decisions on antihypertensive therapy to the treating physician, based on monitoring of the clinical effects of treatment.11
The ADS/PATH guidelines
When finalizing our recommendations,1 we considered the characteristics of frailty and the following key points from the evidence:
- Although evidence from drug treatment trials indicates that there is benefit in treating healthy older adults who have hypertension, the benefit of treating frail older adults is unknown.
- Major trials enrolled elderly patients only if they had systolic blood pressures of at least 160 mm Hg. Therefore, evidence supports initiating pharmacotherapy at a systolic pressure of 160 mm Hg or higher.
- No evidence from randomized controlled trials supports a systolic target lower than 140 mm Hg in the elderly, and there is some evidence that such a target does not benefit.
- The benefit of adding a third medication to lower blood pressure has not been studied.
- Frailty makes the potential benefits of strict blood pressure targets even less certain and increases the possibility of harm from adverse drug events.
- The only study of very old adults, HYVET,44 enrolled relatively healthy older adults and few with orthostasis, while excluding those with a standing systolic blood pressure lower than 140 mm Hg.
OUR RECOMMENDATIONS
Based on the above, we advise against unnecessarily strict targets and recommend stopping antihypertensive medications that are used for the sole purpose of keeping the systolic blood pressure below 140 mm Hg. Our guidelines are unique in that they focus equally on when to stop and when to start medications. We concluded that without evidence of definitive benefit, “less is more” with frailty.55 We believe that if physicians and health professionals understand the limitations of the evidence, they can be more confident in stopping medications that lower blood pressure to an unnecessarily low level.
We recommend the following (Table 4):
Before treating
- Carefully review the risks and the potential but unproven benefits of treatment.
- To avoid overtreatment, treatment decisions should be based on blood pressure measurements in the seated (not supine) position, while also considering the presence of orthostasis.
- To evaluate orthostasis, measure blood pressure in the supine position, then immediately on standing, and again after 2 minutes. Ask the patient if he or she feels light-headed or dizzy when standing.
Stop treatment
- If the seated systolic blood pressure is less than 140 mm Hg, medications can be tapered and discontinued to achieve the targets described below.
- Before discontinuation, consider whether the medications are treating additional conditions such as rate control for atrial fibrillation or symptomatic management of heart failure.
- It is uncertain whether to discontinue treatment when there is a history of stroke. Consider that treatment with two medications resulted in an absolute risk reduction for disabling stroke of 1.64% over approximately 4 years for adults with previous stroke and a mean age of 64,57 an effect that may be more prominent at higher systolic pressures.
Start treatment
- Consider starting treatment when systolic pressure is 160 mm Hg or higher.
- Aim for a seated systolic pressure between 140 and 160 mm Hg if there are no adverse effects from treatment that affect quality of life.
- If there is symptomatic orthostasis or if standing systolic pressure is lower than 140 mm Hg, the target seated systolic pressure can be adjusted upwards.
- In the severely frail nearing the end of life, a target systolic pressure of 160 to 190 mm Hg is reasonable.
- The blood pressure target is the same in people with diabetes.
- In general, use no more than two medications.
Dissemination and implementation
The ADS/PATH guideline is intended for use by physicians and other health professionals (eg, pharmacists and nurses) who care for frail older adults or who work in long-term care facilities. Since creating our guideline, we have disseminated it to physicians, pharmacists, and other health professionals through academic detailing, large conferences, and interactive webinars.
While we do not have objective evidence of practice change, our evaluation data found that 34% of 403 family physicians who received academic detailing indicated that the guideline would change their practice, while 36% stated that the guideline confirmed their practice, an indication that family physicians are sensitive to the needs of the frail elderly.
Because health professionals may be wary of stopping medications and not meeting recommended targets, there may be barriers to adopting this guideline. However, our experience with the PATH program indicates that these barriers can be overcome using effective communication strategies between health professionals and consumers.
AN APPROACH APPROPRIATE TO FRAILTY
There is no direct evidence for systolic blood pressure targets in the frail elderly, so we applied evidence from the nonfrail elderly. Our recommendations differ somewhat from those of other groups, which recommend targets below 140 to 150 mm Hg for older adults, although some do advise caution in the elderly for whom a substantial fall in blood pressure might be poorly tolerated. Despite these messages, we believe that clearer guidance is needed to direct health practitioners toward models that acknowledge that frail patients are in a precarious balance of health and may be harmed by treatments that strive to lower blood pressure to unproven targets. For this reason, our guideline clearly indicates when to decrease or stop drug treatment.
After physicians and health professionals examine the evidence and more fully understand the benefits and harms of treating frail older adults, we are confident that they will be more comfortable stopping medications that lower blood pressure to an unnecessarily low level and instead use an approach that is more appropriate to frailty. We hope clinicians can use this guideline with the same enthusiasm applied to other guidelines, and we welcome discussion.
Acknowledgments: We would like to thank and acknowledge Tanya MacLeod and Kathryn Yuill for their review of and advice about the manuscript.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
- Palliative and Therapeutic Harmonization program. Hypertension guidelines. Treating hypertension in frailty. http://pathclinic.ca/resources/hypertension/. Accessed May 2, 2014.
- Theou O, Rockwood MR, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 2012; 55:e1–e8.
- Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210:901–908.
- Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med 2004; 351:2870–2874.
- Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation 2011; 124:2397–2404.
- Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8:261–271.
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–495.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24.
- Aronow WS, Fleg JL, Pepine CJ, et al; ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011; 123:2434–2506.
- The Canadian Hypertension Education Program (CHEP). 2014 CHEP recommendations. www.hypertension.ca/en/. Accessed May 2, 2014.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–397.
- Fried LP, Tangen CM, Walston J, et al; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Ensrud KE, Ewing SK, Cawthon PM, et al; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc 2009; 57:492–498.
- Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc 2009; 57:453–461.
- Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci 2007; 62:731–737.
- Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16:601–608.
- Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci 1998; 53:S9–S16.
- Matthews M, Lucas A, Boland R, et al. Use of a questionnaire to screen for frailty in the elderly: an exploratory study. Aging Clin Exp Res 2004; 16:34–40.
- Salvi F, Morichi V, Grilli A, et al. Screening for frailty in elderly emergency department patients by using the Identification of Seniors At Risk (ISAR). J Nutr Health Aging 2012; 16:313–318.
- Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1:323–336.
- Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248.
- Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006; 35:526–529.
- Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing 2008; 37:129–131.
- Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 2000; 15:1021–1027.
- Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS). Psychopharmacol Bull 1988; 24:629–636.
- Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc 2012; 60:2326–2332.
- Palliative and Therapeutic Harmonization Clinic (PATH). www.pathclinic.ca. Accessed May 2, 2014.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. http://cme.medicine.dal.ca/ADS.htm. Accessed January 8, 2014.
- Mallery LH, Moorhouse P. Respecting frailty. J Med Ethics 2011; 37:126–128.
- Dalhousie University Faculty of Medicine: Continuing Medical Education. Issues in hypertension 2011. http://cme.medicine.dal.ca/files/Hypertension%20book.pdf. Accessed May 2, 2014.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Amery A, Birkenhäger W, Brixko P, et al. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet 1985; 1:1349–1354.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986; 293:1145–1151.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet 1991; 338:1281–1285.
- Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992; 304:405–412.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16:1823–1829.
- Lithell H, Hansson L, Skoog I, et al; SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens 2003; 21:875–886.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007; 370:221–229.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Musini VM, Tejani AM, Bassett K, Wright JM. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 2009;CD000028.
- He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2004;CD004937.
- Allen M, Kelly K, Fleming I. Hypertension in elderly patients: recommended systolic targets are not evidence based [in French]. Can Fam Physician 2013; 59:19–24.
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori VM. Problems of stopping trials early. BMJ 2012; 344:e3863.
- Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85-plus Study. J Am Geriatr Soc 2012; 60:2014–2019.
- Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke 2013; 44:15–20.
- Poortvliet RK, Blom JW, de Craen AJ, et al. Low blood pressure predicts increased mortality in very old age even without heart failure: the Leiden 85-plus Study. Eur J Heart Fail 2013; 15:528–533.
- Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med 2012; 172:1162–1168.
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001; 358:1033–1041.
- Yusuf S, Diener HC, Sacco RL, et al; PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359:1225–1237.
- Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med 2010; 170:1648–1654.
KEY POINTS
- For frail elderly patients, consider starting treatment if the systolic blood pressure is 160 mm Hg or higher.
- An appropriate target in this population is a seated systolic pressure between 140 and 160 mm Hg, as long as there is no orthostatic drop to less than 140 mm Hg upon standing from a lying position and treatment does not adversely affect quality of life.
- The blood pressure target does not need to be lower if the patient has diabetes. If the patient is severely frail and has a short life expectancy, a systolic target of 160 to 190 mm Hg may be reasonable.
- If the systolic pressure is below 140 mm Hg, antihypertensive medications can be reduced as long as they are not indicated for other conditions.
- In general, one should prescribe no more than two antihypertensive medications.
Patent foramen ovale and cryptogenic stroke: Many unanswered questions
Your patient has had an ischemic stroke, and so far you have found no obvious cause such as atrial fibrillation or carotid disease. Should you look for a patent foramen ovale (PFO)? And if you find it, what should you do?
This scenario continues to challenge primary care physicians and subspecialists and requires an understanding of the relationship between PFO and cryptogenic stroke, as well as familiarity with current data on the safety and effectiveness of the management options. PFO is known to be associated with cryptogenic stroke, but many questions remain, including:
- How can we tell if PFO is a culprit (“pathologic”) or an innocent bystander (“incidental”) in a patient who has had a cryptogenic stroke?
- Should stroke patients receive different medical therapy if they have a PFO? In particular, should they receive warfarin in addition to aspirin? And what about the novel oral anticoagulants?
- Which patients should undergo percutaneous closure of the PFO?
- Should we even be looking for PFO in stroke patients at this point, if we cannot say with certainty what we should do if we find it?
WHY IS THIS IMPORTANT?
Cerebrovascular disease is common and costly. The estimated yearly incidence of stroke in the United States is 795,000 events, at a cost of nearly $30 billion.1 The incidence of stroke in Europe is more than 1 million annually.2
During the diagnostic evaluation of stroke or transient ischemic attack (TIA), PFO is occasionally discovered incidentally by echocardiography. The management decisions that follow often fall to the primary care physician, who must decipher the conflicting data currently available and explain the options to the patient.
Although reviews have been published on this subject,3 several newer key trials and data on risk stratification warrant consideration.
DEFINITIONS
PFO is the failure of the septum primum to fuse with the septum secundum, so that a communication remains between the atria (Figure 1). The diagnosis is commonly made by echocardiography, when agitated saline is injected into the venous system and bubbles can be seen in the left atrium within three to five cardiac cycles (see video).
Atrial septal aneurysm is loosely defined as a septal excursion or bulging of at least 10 to 15 mm into the left and right atria during the cardiac cycle (Figure 2). The combination of PFO and atrial septal aneurysm may be more of a risk factor for stroke than PFO alone (see discussion below).
Cryptogenic stroke. The diagnostic workup of stroke fails to elucidate a clear cause in up to 40% of cases, which are thus called cryptogenic.4 The workup varies, but typically includes a search for a cardioembolic source and for atherosclerotic disease. Embolic sources are evaluated for by electrocardiography, transthoracic echocardiography, and possibly imaging of the aortic arch. Evaluation for atherosclerotic disease of the intracranial and extracranial arteries includes magnetic resonance angiography or, if that is unavailable, computed tomographic angiography or carotid Doppler ultrasonography. If no source is found, long-term cardiac monitoring may be used to detect paroxysmal atrial fibrillation, which may be more common than previously thought.
PFO AND CRYPTOGENIC STROKE ARE COMMON
As noted, there are approximately 800,000 strokes every year in the United States. If 25% to 40% of them are cryptogenic (the true prevalence warrants more evaluation),4,5 then 200,000 to 320,000 strokes are cryptogenic.
Autopsy studies indicate that 25% of the general population have a PFO, and if the prevalence is the same in people with cryptogenic stroke, that would equal 80,000 people with both cryptogenic stroke and PFO every year. However, the prevalence of PFO in patients with cryptogenic stroke appears to be significantly higher than in the general population.6 Although these numbers are crude estimates, they provide some insight into the prevalence of this clinical presentation.
HOW ARE CRYPTOGENIC STROKE AND PFO RELATED?
The exact relationship between PFO and cryptogenic stroke is unknown, although cases have been reported of thrombus in transit through a PFO, supporting paradoxical embolism as the plausible cause in stroke patients with PFO.7–9
There is clear evidence that the two conditions are associated by more than chance. Homma and Sacco6 reported that, in several studies, 93 (46%) of 202 patients under age 55 with cryptogenic stroke had PFOs, compared with 29 (11%) of 271 controls (P < .05 in all studies).6
In their evaluation of 23 case-control studies, Alsheikh-Ali et al10 found that the summary odds ratio (OR) for PFO in cryptogenic stroke vs PFO in control patients was 2.9 (95% confidence interval [CI] 2.1–4), largely driven by an OR of 5.1 (3.3–7.8) in those under age 55. Through Bayesian probability theory, this correlated with only a 33% probability that PFO in a patient with cryptogenic stroke was an innocent bystander rather than the culprit.10
IS PFO A RISK FACTOR FOR STROKE?
One of the more puzzling aspects of the relationship of PFO to cryptogenic stroke is that despite a clear association, there is little evidence that the relationship is causal.
Di Tullio et al11 followed 1,100 people who had no history of stroke and found that the risk of a first stroke in those with a PFO was not significantly higher than in those without a PFO, regardless of age, sex, or ethnic or racial group. At 80 months, the hazard ratio of stroke in people who had a PFO was 1.64 (95% CI 0.87–3.09).11 The findings were similar at 11 years, with a hazard ratio of 1.10 (95% CI 0.64–1.91).12
A prospective study of 585 patients found a similar risk of stroke in those with and without a PFO, with a hazard ratio of 1.46 (95% CI 0.74–2.88; P = .28).13
These prospective trials suggest that although previous studies have found a higher prevalence of PFOs in patients with cryptogenic stroke than in patients without stroke, there appears to be very little if any increased risk from baseline for a first stroke or TIA.
The lack of statistical significance in these trials should be interpreted with some caution, as a small increased risk is difficult to show if the event rate is low (approximately 10% of patients had events over 11 years in the study by Di Tullio et al12).
HOW DO WE KNOW IF A PFO IS A CULPRIT OR BYSTANDER?
Unfortunately, this is largely unanswered, though experts have suggested that echocardiographic features of the PFO, radiographic characteristics of the stroke, and clinical features of the patient may provide useful information.
‘High-risk’ features on echocardiography
Certain features of PFO may portend a high risk of cerebrovascular events. Both right-to-left shunting at rest and septal hypermobility were found in one study14 to be more common in patients with a PFO who had a stroke or TIA than in patients with a PFO but no cerebrovascular events. Also, patients who had these features and had a stroke had a higher risk of recurrence than stroke patients without these features (12.5% vs 4.3%, P = .05).14
Septal hypermobility and shunting at rest are easily diagnosed by echocardiography, and detecting these “high-risk” features would be useful if they could identify patients who would benefit from special therapy, such as percutaneous closure of the PFO.
Unfortunately, when investigators looked at these features in subgroup analysis of the major randomized controlled trials of percutaneous closure vs medical therapy, the results were mixed.
CLOSURE 1 (the Evaluation of the STARFlex Septal Closure System in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale)15 found percutaneous closure to be no better than medical therapy, regardless of shunt size or the presence of atrial septal aneurysm.
Similarly, the PC trial (Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder With Medical Treatment in Patients With Cryptogenic Embolism)16 found no statistically significant benefit of closure in those with atrial septal aneurysm.
In contrast, the RESPECT trial (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment)17 showed percutaneous closure to be beneficial in patients with atrial septal aneurysm or large shunt.
Radiographic characteristics of the stroke
Another area of interest in trying to identify culprit PFOs is the radiographic characteristics of the stroke.
In a study comparing patients with stroke related to atrial fibrillation vs patients with cryptogenic stroke and a known PFO, those in the latter group were more likely to have a single cortical infarction (34.2% vs 3.1%; P < .001) or multiple small scattered lesions (23.1% vs 5.9%; P < .01).18 Similarly, in a large database of patients with cryptogenic stroke and known PFO status, a superficially located stroke was associated with the presence of PFO (OR 1.54; P < .0001).19
Although these findings do not tell us with certainty that a patient’s PFO was the cause of his or her stroke, they provide guidance when dealing with the uncertainty of how to manage a patient with PFO. They may be useful in clinical practice, for example, when discussing treatment options with a young patient with cryptogenic stroke who has no risk factors and a superficial single infarct and who is found to have a PFO with a right-to-left shunting at rest.
Patient characteristics
Kent et al20 developed a 10-point index (the RoPE score) in an attempt to assign a probability to whether a stroke was PFO-related. Points were assigned for patients who were younger, who had a cortical stroke on neuroimaging, and who did not have diabetes, hypertension, smoking, or prior stroke or TIA. Patients with cryptogenic stroke with a higher RoPE score were more likely to have a PFO and thus had a higher likelihood that the index event was related to PFO. Of note, the patients with the highest likelihood of PFO-related stroke were the least likely to have a recurrence (RoPE score of 9 to 10; PFO-attributable fraction 88%; estimated 2-year recurrence rate 2%; 95% CI 0%–4%), whereas those with a low RoPE score have more traditional risk factors for stroke and thus are more likely to have a recurrence (RoPE 0 to 3; estimated 2-year recurrence rate 20%; 95% CI 12%–28%).20
Again, this sheds light on a difficulty faced by randomized controlled trials: the patients who may benefit from closure of a PFO may very well be those with the lowest recurrence rates without intervention.
The RoPE index was examined in an attempt to validate previously described morphologic criteria of “high-risk” PFO,21 though none of the previously described “high-risk” echocardiographic features (large physiologic size, hypermobile septum, shunt at rest) were more common in the group with presumed PFO-attributable stroke (RoPE score > 6). This underscores the difficulty of distinguishing pathologic PFO from incidental PFO.
KEY TREATMENT CONSIDERATIONS FOR SECONDARY PREVENTION
Given the complicated relationship between PFO and cryptogenic stroke, there has been much debate over management strategies. The three options are surgical closure, percutaneous closure with a device, and medical therapy. The goal of all three is to prevent the recurrence of stroke or TIA.
Surgical closure has largely been supplanted by percutaneous closure, but is still done in specific situations such as when a PFO is found incidentally on transesophageal echocardiography during surgery for another cardiac condition. The data on such cases22 tend to support the argument that asymptomatic PFOs in the general population have a relatively benign natural history.
Thus, the two key questions about management that warrant discussion are: is anticoagulation superior to antiplatelet therapy? And is percutaneous closure superior to medical management?
Anticoagulant vs antiplatelet therapy
Whether to treat with aspirin or with a vitamin K antagonist has been a subject of debate, although there is no strong evidence to suggest that anticoagulation is superior to antiplatelet therapy.
The concern that aspirin alone is insufficient in some patients stems from a study by Mas et al,23 who followed 581 patients with cryptogenic stroke who had a PFO alone, a PFO with an atrial septal aneurysm, or neither. The rate of stroke recurrence at 4 years on aspirin therapy was 2.3% in those with a PFO alone, 15.2% in those with a PFO with an atrial septal aneurysm, and 4.2% in those with neither.
Many have concluded that aspirin therapy does not sufficiently protect those with both PFO and atrial septal aneurysm, given the high recurrence rate in this group. This might lead to the suggestion that anticoagulation could be of benefit in these patients.
However, the Patent Foramen Ovale in Cryptogenic Stroke Study (PiCSS)24 and the Spanish Multicenter Study Into Right-to-Left Shunt in Cryptogenic Stroke (CODICIA)25 found similar recurrence rates in patients with PFO and atrial septal aneurysm compared with those with only PFO. In these two studies, recurrence rates were similar regardless of whether patients were taking aspirin or warfarin.
In a study that followed 140 consecutive patients with both stroke and PFO, those treated in a nonrandomized fashion with antiplatelet agents had no difference in the recurrence rate compared with those treated with anticoagulation.26
Although uncertainty remains because no head-to-head randomized controlled trial has been done, some patients with PFO have other indications for anticoagulation, most commonly atrial fibrillation and venous thromboembolic disease.
There are currently no data on the use of novel oral anticoagulants in this setting.
Is percutaneous closure better than medical therapy?
When cryptogenic stroke is treated with antiplatelet therapy or anticoagulation therapy, the recurrence rate is the same whether or not the patient has a PFO.23–25 The belief that medical therapy offers adequate secondary protection is supported by a meta-analysis of 15 studies that found no increased risk of recurrent ischemic events in those with a PFO on medical therapy (antiplatelet or anticoagulant) vs those without a PFO (relative risk 1.1, 95% CI 0.8–1.5).27
Despite the conflicting evidence, percutaneous closure of PFO is still performed, mostly on a case-by-case basis. This has been supported by an apparent benefit in observational studies.
A systematic review of 52 single-arm studies and 7 comparative nonrandomized studies of patients with PFO and cryptogenic stroke found the rate of recurrent stroke to be 0.36 per 100 person-years with percutaneous closure vs 2.53 per 100 person-years with medical therapy.28 However, three long-awaited randomized controlled trials (CLOSURE 1, the PC trial, and RESPECT) failed to show a significant reduction in primary end points with percutaneous closure vs standard medical therapy.15–17
These trials had several limitations: event rates were low, medical therapy varied by provider, and enrollment was slowed by out-of-study percutaneous closure in patients perceived to be at high risk (though, as discussed above, high risk is difficult to determine).
Intention-to-treat analysis in RESPECT showed no benefit from percutaneous closure, but a favorable outcome was noted with closure in as-treated analysis (HR 0.27; 95% CI 0.1–0.75; P = .007) and per-protocol analysis (HR 0.37; 95% CI 0.14–0.96; P = .03) of the 980 randomized patients.17 This suggests some benefit, as does the CLOSURE 1 trial, in which 3 of the 12 recurrent strokes in the percutaneous closure group occurred before the device was implanted.15
The low event rates in these studies prompted several meta-analyses.29–35 However, only two suggested a benefit of percutaneous closure over medical therapy. In one recent meta-analysis,29 observational study data suggested benefit from percutaneous closure, whereas three randomized controlled trials failed to show a statistically significant benefit.
The conclusions of the meta-analyses must be interpreted with caution because of inherent differences in the randomized controlled trials, including the closure device used, inclusion criteria, study end points, and variations in medical therapy.
Devices differ
A meta-analysis by Khan et al35 showed a benefit of percutaneous closure when evaluating only studies using the Amplatzer PFO occluder (AGA Medical), as in RESPECT and the PC trial.35 As data accumulate, it is important to remember that there are differences between devices. Ongoing trials continue to investigate the Amplatzer device (NCT01550588) and the GORE HELEX Septal Occluder/GORE Septal Occluder (Gore Medical) (NCT00738894).
In another meta-analysis, Pineda et al31 found a benefit with closure in the as-treated analysis using data from all three randomized controlled trials (OR 0.62; 95% CI 0.41–0.94; P = .02).31 Although paradoxical embolism through the PFO as the mechanism of stroke has been questioned, this finding suggests that actual closure of a PFO may protect against further events, presumably by preventing paradoxical embolism.
Different closure devices have different side effects. The incidence of atrial fibrillation with the CardioSEAL STARFlex device (NMT Medical) is higher than with medical therapy (used in the CLOSURE trial15), whereas this risk was not statistically significantly increased in the PC trial16 and RESPECT,17 which used the Amplatzer device.
Benefit in those with atrial septal aneurysm?
Percutaneous closure has been shown to be safe and effective in patients with PFO and atrial septal aneurysm.36 There was some benefit of closure over medical therapy in a subgroup analysis from RESPECT in these patients, with a HR of 0.19 (95% CI 0.04–0.87, P = .02),17 although this was not seen in either CLOSURE 1 or the PC trial.
WHAT ARE THE RISKS OF PERCUTANEOUS CLOSURE?
Minor complications of percutaneous closure include bleeding, atrial arrhythmias, device embolization and fracture, and complications related to vascular access. Major complications include hemorrhage requiring transfusion, need for surgery, cardiac tamponade, pulmonary embolism, and death.
The cumulative rate of major complications in 10 observational studies was 1.5%, and the rate of minor complications was 7.9%.37 The RESPECT investigators reported a serious adverse event in 4.2% of patients (ranging in severity from chest tightness to cardiac tamponade).17
Another possible consequence of percutaneous closure is the need for chronic anticoagulation because of the increased risk of postprocedural atrial fibrillation seen in meta-analyses,29,31,32 though this may be device-specific.32
Percutaneous closure was considered successful—ie, to have nearly or completely eliminated shunting of blood through the defect—at 6 months of follow-up in 95.9% of patients in the PC trial, 93.5% in RESPECT, and 86.1% in CLOSURE 1.15–17
WHAT SHOULD WE BE DOING IN DAILY PRACTICE?
Give aspirin. Aspirin is effective in secondary stroke prevention, and data suggest that patients with PFO and cryptogenic stroke who receive aspirin therapy alone have a similar risk of recurrent events as patients without PFO.
Give warfarin if indicated. Evidence is insufficient to recommend vitamin K antagonist therapy in all patients with PFO and cryptogenic stroke. However, coexisting conditions that warrant anticoagulation must be taken into account.
Individualize. Given the lack of evidence to definitively guide management of patients with cryptogenic stroke and PFO, we need to individualize our approach, taking into account patient preferences, bleeding risk, ability to tolerate procedures, and the likelihood that the PFO is at fault.
No definitive answer on PFO closure. The most recent data suggest that closure may be beneficial, but key questions remain: Who will benefit? And what is the ideal medical therapy? Optimal management will only be established by the continued enrollment of appropriate patients into ongoing clinical trials.
Another question is whether it is possible to perform a randomized controlled trial with enough patients to definitively prove whether percutaneous closure is superior to medical therapy. Recent experience would suggest not.
In the meantime, we have some guidance from the American Heart Association and the American Stroke Association Council on Stroke38 based on the limited evidence available.
Consider patient preference. The physician should present the options to the patient in a balanced manner to enable him or her to make an informed decision. Patients can also be encouraged to seek additional information at websites such as www.stroke.org and www.nlm.nih.gov.
Referral to an interventional cardiologist for evaluation for closure is reasonable in patients with recurrent stroke, medication failure, complicated atrial septal anatomy such as PFO with aneurysm or large shunt, concurrent thromboembolic disease, or contraindications to anticoagulation.
MORE WORK NEEDED
Areas for further study include further identifying the characteristics of patients with PFO and cryptogenic stroke that might indicate who would benefit from percutaneous closure, elucidating the mechanism of stroke in these patients, and determining whether routine stroke evaluation should include echocardiography with a bubble study if there is no change in management based on the finding of PFO.39
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- Truelsen T, Piechowski-JóŸwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol 2006; 13:581–598.
- Furlan AJ. Patent foramen ovale and stroke: to close or not to close? Cleve Clin J Med 2007; 74(suppl 1):S118–S120.
- Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol 1989; 25:382–390.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001; 32:2559–2566.
- Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation 2005; 112:1063–1072.
- Sattiraju S, Masri SC, Liao K, Missov E. Three-dimensional transesophageal echocardiography of a thrombus entrapped by a patent foramen ovale. Ann Thorac Surg 2012; 94:e101–e102.
- Schreiter SW, Phillips JH. Thromboembolus traversing a patent foramen ovale: resolution with anticoagulation. J Am Soc Echocardiogr 1994; 7:659–662.
- Hust MH, Staiger M, Braun B. Migration of paradoxic embolus through a patent foramen ovale diagnosed by echocardiography: successful thrombolysis. Am Heart J 1995; 129:620–622.
- Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009; 40:2349–2355.
- Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol 2007; 49:797–802.
- Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
- Meissner I, Khandheria BK, Heit JA, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol 2006; 47:440–445.
- De Castro S, Cartoni D, Fiorelli M, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke 2000; 31:2407–2413.
- Furlan AJ, Reisman M, Massaro J, et al; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012; 366:991–999.
- Meier B, Kalesan B, Mattle HP, et al; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013; 368:1083–1091.
- Carroll JD, Saver JL, Thaler DE, et al; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013; 368:1092–1100.
- Kim BJ, Sohn H, Sun BJ, et al. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke 2013; 44:3350–3356.
- Thaler DE, Ruthazer R, Di Angelantonio E, et al. Neuroimaging findings in cryptogenic stroke patients with and without patent foramen ovale. Stroke 2013; 44:675–680.
- Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013; 81:619–625.
- Wessler BS, Thaler DE, Ruthazer R, et al. Transesophageal echocardiography in cryptogenic stroke and patent foramen ovale: analysis of putative high-risk features from the risk of paradoxical embolism database. Circ Cardiovasc Imaging 2014; 7:125–131.
- Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of intraoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA 2009; 302:290–297.
- Mas JL, Arquizan C, Lamy C, et al; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001; 345:1740–1746.
- Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP; PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002; 105:2625–2631.
- Serena J, Marti-Fàbregas J, Santamarina E, et al; CODICIA, Right-to-Left Shunt in Cryptogenic Stroke Study; Stroke Project of the Cerebrovascular Diseases Study Group, Spanish Society of Neurology. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke 2008; 39:3131–3136.
- Bogousslavsky J, Garazi S, Jeanrenaud X, Aebischer N, Van Melle G. Stroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study Group. Neurology 1996; 46:1301–1305.
- Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology 2009; 73:89–97.
- Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke 2012; 43:422–431.
- Wolfrum M, Froehlich GM, Knapp G, et al. Stroke prevention by percutaneous closure of patent foramen ovale: a systematic review and meta-analysis. Heart 2014; 100:389–395.
- Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2013; 34:3342–3352.
- Pineda AM, Nascimento FO, Yang SC, Kirtane AJ, Sommer RJ, Beohar N. A meta-analysis of transcatheter closure of patent foramen ovale versus medical therapy for prevention of recurrent thromboembolic events in patients with cryptogenic cerebrovascular events. Catheter Cardiovasc Interv 2013; 82:968–975.
- Kwong JS, Lam YY, Yu CM. Percutaneous closure of patent foramen ovale for cryptogenic stroke: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 168:4132–4148.
- Ntaios G, Papavasileiou V, Makaritsis K, Michel P. PFO closure vs medical therapy in cryptogenic stroke or transient ischemic attack: a systematic review and meta-analysis. Int J Cardiol 2013; 169:101–105.
- Nagaraja V, Raval J, Eslick GD, Burgess D, Denniss AR. Is transcatheter closure better than medical therapy for cryptogenic stroke with patent foramen ovale? A meta-analysis of randomised trials. Heart Lung Circ 2013; 22:903–909.
- Khan AR, Bin Abdulhak AA, Sheikh MA, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: a systematic review and meta-analysis. JACC Cardiovasc Interv 2013; 6:1316–1323.
- Wahl A, Krumsdorf U, Meier B, et al. Transcatheter treatment of atrial septal aneurysm associated with patent foramen ovale for prevention of recurrent paradoxical embolism in high-risk patients. J Am Coll Cardiol 2005; 45:377–380.
- Khairy P, O’Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med 2003; 139:753–760.
- Sacco RL, Adams R, Albers G, et al; American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37:577–617.
- Rana BS, Thomas MR, Calvert PA, Monaghan MJ, Hildick-Smith D. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging 2010; 3:749–760.
Your patient has had an ischemic stroke, and so far you have found no obvious cause such as atrial fibrillation or carotid disease. Should you look for a patent foramen ovale (PFO)? And if you find it, what should you do?
This scenario continues to challenge primary care physicians and subspecialists and requires an understanding of the relationship between PFO and cryptogenic stroke, as well as familiarity with current data on the safety and effectiveness of the management options. PFO is known to be associated with cryptogenic stroke, but many questions remain, including:
- How can we tell if PFO is a culprit (“pathologic”) or an innocent bystander (“incidental”) in a patient who has had a cryptogenic stroke?
- Should stroke patients receive different medical therapy if they have a PFO? In particular, should they receive warfarin in addition to aspirin? And what about the novel oral anticoagulants?
- Which patients should undergo percutaneous closure of the PFO?
- Should we even be looking for PFO in stroke patients at this point, if we cannot say with certainty what we should do if we find it?
WHY IS THIS IMPORTANT?
Cerebrovascular disease is common and costly. The estimated yearly incidence of stroke in the United States is 795,000 events, at a cost of nearly $30 billion.1 The incidence of stroke in Europe is more than 1 million annually.2
During the diagnostic evaluation of stroke or transient ischemic attack (TIA), PFO is occasionally discovered incidentally by echocardiography. The management decisions that follow often fall to the primary care physician, who must decipher the conflicting data currently available and explain the options to the patient.
Although reviews have been published on this subject,3 several newer key trials and data on risk stratification warrant consideration.
DEFINITIONS
PFO is the failure of the septum primum to fuse with the septum secundum, so that a communication remains between the atria (Figure 1). The diagnosis is commonly made by echocardiography, when agitated saline is injected into the venous system and bubbles can be seen in the left atrium within three to five cardiac cycles (see video).
Atrial septal aneurysm is loosely defined as a septal excursion or bulging of at least 10 to 15 mm into the left and right atria during the cardiac cycle (Figure 2). The combination of PFO and atrial septal aneurysm may be more of a risk factor for stroke than PFO alone (see discussion below).
Cryptogenic stroke. The diagnostic workup of stroke fails to elucidate a clear cause in up to 40% of cases, which are thus called cryptogenic.4 The workup varies, but typically includes a search for a cardioembolic source and for atherosclerotic disease. Embolic sources are evaluated for by electrocardiography, transthoracic echocardiography, and possibly imaging of the aortic arch. Evaluation for atherosclerotic disease of the intracranial and extracranial arteries includes magnetic resonance angiography or, if that is unavailable, computed tomographic angiography or carotid Doppler ultrasonography. If no source is found, long-term cardiac monitoring may be used to detect paroxysmal atrial fibrillation, which may be more common than previously thought.
PFO AND CRYPTOGENIC STROKE ARE COMMON
As noted, there are approximately 800,000 strokes every year in the United States. If 25% to 40% of them are cryptogenic (the true prevalence warrants more evaluation),4,5 then 200,000 to 320,000 strokes are cryptogenic.
Autopsy studies indicate that 25% of the general population have a PFO, and if the prevalence is the same in people with cryptogenic stroke, that would equal 80,000 people with both cryptogenic stroke and PFO every year. However, the prevalence of PFO in patients with cryptogenic stroke appears to be significantly higher than in the general population.6 Although these numbers are crude estimates, they provide some insight into the prevalence of this clinical presentation.
HOW ARE CRYPTOGENIC STROKE AND PFO RELATED?
The exact relationship between PFO and cryptogenic stroke is unknown, although cases have been reported of thrombus in transit through a PFO, supporting paradoxical embolism as the plausible cause in stroke patients with PFO.7–9
There is clear evidence that the two conditions are associated by more than chance. Homma and Sacco6 reported that, in several studies, 93 (46%) of 202 patients under age 55 with cryptogenic stroke had PFOs, compared with 29 (11%) of 271 controls (P < .05 in all studies).6
In their evaluation of 23 case-control studies, Alsheikh-Ali et al10 found that the summary odds ratio (OR) for PFO in cryptogenic stroke vs PFO in control patients was 2.9 (95% confidence interval [CI] 2.1–4), largely driven by an OR of 5.1 (3.3–7.8) in those under age 55. Through Bayesian probability theory, this correlated with only a 33% probability that PFO in a patient with cryptogenic stroke was an innocent bystander rather than the culprit.10
IS PFO A RISK FACTOR FOR STROKE?
One of the more puzzling aspects of the relationship of PFO to cryptogenic stroke is that despite a clear association, there is little evidence that the relationship is causal.
Di Tullio et al11 followed 1,100 people who had no history of stroke and found that the risk of a first stroke in those with a PFO was not significantly higher than in those without a PFO, regardless of age, sex, or ethnic or racial group. At 80 months, the hazard ratio of stroke in people who had a PFO was 1.64 (95% CI 0.87–3.09).11 The findings were similar at 11 years, with a hazard ratio of 1.10 (95% CI 0.64–1.91).12
A prospective study of 585 patients found a similar risk of stroke in those with and without a PFO, with a hazard ratio of 1.46 (95% CI 0.74–2.88; P = .28).13
These prospective trials suggest that although previous studies have found a higher prevalence of PFOs in patients with cryptogenic stroke than in patients without stroke, there appears to be very little if any increased risk from baseline for a first stroke or TIA.
The lack of statistical significance in these trials should be interpreted with some caution, as a small increased risk is difficult to show if the event rate is low (approximately 10% of patients had events over 11 years in the study by Di Tullio et al12).
HOW DO WE KNOW IF A PFO IS A CULPRIT OR BYSTANDER?
Unfortunately, this is largely unanswered, though experts have suggested that echocardiographic features of the PFO, radiographic characteristics of the stroke, and clinical features of the patient may provide useful information.
‘High-risk’ features on echocardiography
Certain features of PFO may portend a high risk of cerebrovascular events. Both right-to-left shunting at rest and septal hypermobility were found in one study14 to be more common in patients with a PFO who had a stroke or TIA than in patients with a PFO but no cerebrovascular events. Also, patients who had these features and had a stroke had a higher risk of recurrence than stroke patients without these features (12.5% vs 4.3%, P = .05).14
Septal hypermobility and shunting at rest are easily diagnosed by echocardiography, and detecting these “high-risk” features would be useful if they could identify patients who would benefit from special therapy, such as percutaneous closure of the PFO.
Unfortunately, when investigators looked at these features in subgroup analysis of the major randomized controlled trials of percutaneous closure vs medical therapy, the results were mixed.
CLOSURE 1 (the Evaluation of the STARFlex Septal Closure System in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale)15 found percutaneous closure to be no better than medical therapy, regardless of shunt size or the presence of atrial septal aneurysm.
Similarly, the PC trial (Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder With Medical Treatment in Patients With Cryptogenic Embolism)16 found no statistically significant benefit of closure in those with atrial septal aneurysm.
In contrast, the RESPECT trial (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment)17 showed percutaneous closure to be beneficial in patients with atrial septal aneurysm or large shunt.
Radiographic characteristics of the stroke
Another area of interest in trying to identify culprit PFOs is the radiographic characteristics of the stroke.
In a study comparing patients with stroke related to atrial fibrillation vs patients with cryptogenic stroke and a known PFO, those in the latter group were more likely to have a single cortical infarction (34.2% vs 3.1%; P < .001) or multiple small scattered lesions (23.1% vs 5.9%; P < .01).18 Similarly, in a large database of patients with cryptogenic stroke and known PFO status, a superficially located stroke was associated with the presence of PFO (OR 1.54; P < .0001).19
Although these findings do not tell us with certainty that a patient’s PFO was the cause of his or her stroke, they provide guidance when dealing with the uncertainty of how to manage a patient with PFO. They may be useful in clinical practice, for example, when discussing treatment options with a young patient with cryptogenic stroke who has no risk factors and a superficial single infarct and who is found to have a PFO with a right-to-left shunting at rest.
Patient characteristics
Kent et al20 developed a 10-point index (the RoPE score) in an attempt to assign a probability to whether a stroke was PFO-related. Points were assigned for patients who were younger, who had a cortical stroke on neuroimaging, and who did not have diabetes, hypertension, smoking, or prior stroke or TIA. Patients with cryptogenic stroke with a higher RoPE score were more likely to have a PFO and thus had a higher likelihood that the index event was related to PFO. Of note, the patients with the highest likelihood of PFO-related stroke were the least likely to have a recurrence (RoPE score of 9 to 10; PFO-attributable fraction 88%; estimated 2-year recurrence rate 2%; 95% CI 0%–4%), whereas those with a low RoPE score have more traditional risk factors for stroke and thus are more likely to have a recurrence (RoPE 0 to 3; estimated 2-year recurrence rate 20%; 95% CI 12%–28%).20
Again, this sheds light on a difficulty faced by randomized controlled trials: the patients who may benefit from closure of a PFO may very well be those with the lowest recurrence rates without intervention.
The RoPE index was examined in an attempt to validate previously described morphologic criteria of “high-risk” PFO,21 though none of the previously described “high-risk” echocardiographic features (large physiologic size, hypermobile septum, shunt at rest) were more common in the group with presumed PFO-attributable stroke (RoPE score > 6). This underscores the difficulty of distinguishing pathologic PFO from incidental PFO.
KEY TREATMENT CONSIDERATIONS FOR SECONDARY PREVENTION
Given the complicated relationship between PFO and cryptogenic stroke, there has been much debate over management strategies. The three options are surgical closure, percutaneous closure with a device, and medical therapy. The goal of all three is to prevent the recurrence of stroke or TIA.
Surgical closure has largely been supplanted by percutaneous closure, but is still done in specific situations such as when a PFO is found incidentally on transesophageal echocardiography during surgery for another cardiac condition. The data on such cases22 tend to support the argument that asymptomatic PFOs in the general population have a relatively benign natural history.
Thus, the two key questions about management that warrant discussion are: is anticoagulation superior to antiplatelet therapy? And is percutaneous closure superior to medical management?
Anticoagulant vs antiplatelet therapy
Whether to treat with aspirin or with a vitamin K antagonist has been a subject of debate, although there is no strong evidence to suggest that anticoagulation is superior to antiplatelet therapy.
The concern that aspirin alone is insufficient in some patients stems from a study by Mas et al,23 who followed 581 patients with cryptogenic stroke who had a PFO alone, a PFO with an atrial septal aneurysm, or neither. The rate of stroke recurrence at 4 years on aspirin therapy was 2.3% in those with a PFO alone, 15.2% in those with a PFO with an atrial septal aneurysm, and 4.2% in those with neither.
Many have concluded that aspirin therapy does not sufficiently protect those with both PFO and atrial septal aneurysm, given the high recurrence rate in this group. This might lead to the suggestion that anticoagulation could be of benefit in these patients.
However, the Patent Foramen Ovale in Cryptogenic Stroke Study (PiCSS)24 and the Spanish Multicenter Study Into Right-to-Left Shunt in Cryptogenic Stroke (CODICIA)25 found similar recurrence rates in patients with PFO and atrial septal aneurysm compared with those with only PFO. In these two studies, recurrence rates were similar regardless of whether patients were taking aspirin or warfarin.
In a study that followed 140 consecutive patients with both stroke and PFO, those treated in a nonrandomized fashion with antiplatelet agents had no difference in the recurrence rate compared with those treated with anticoagulation.26
Although uncertainty remains because no head-to-head randomized controlled trial has been done, some patients with PFO have other indications for anticoagulation, most commonly atrial fibrillation and venous thromboembolic disease.
There are currently no data on the use of novel oral anticoagulants in this setting.
Is percutaneous closure better than medical therapy?
When cryptogenic stroke is treated with antiplatelet therapy or anticoagulation therapy, the recurrence rate is the same whether or not the patient has a PFO.23–25 The belief that medical therapy offers adequate secondary protection is supported by a meta-analysis of 15 studies that found no increased risk of recurrent ischemic events in those with a PFO on medical therapy (antiplatelet or anticoagulant) vs those without a PFO (relative risk 1.1, 95% CI 0.8–1.5).27
Despite the conflicting evidence, percutaneous closure of PFO is still performed, mostly on a case-by-case basis. This has been supported by an apparent benefit in observational studies.
A systematic review of 52 single-arm studies and 7 comparative nonrandomized studies of patients with PFO and cryptogenic stroke found the rate of recurrent stroke to be 0.36 per 100 person-years with percutaneous closure vs 2.53 per 100 person-years with medical therapy.28 However, three long-awaited randomized controlled trials (CLOSURE 1, the PC trial, and RESPECT) failed to show a significant reduction in primary end points with percutaneous closure vs standard medical therapy.15–17
These trials had several limitations: event rates were low, medical therapy varied by provider, and enrollment was slowed by out-of-study percutaneous closure in patients perceived to be at high risk (though, as discussed above, high risk is difficult to determine).
Intention-to-treat analysis in RESPECT showed no benefit from percutaneous closure, but a favorable outcome was noted with closure in as-treated analysis (HR 0.27; 95% CI 0.1–0.75; P = .007) and per-protocol analysis (HR 0.37; 95% CI 0.14–0.96; P = .03) of the 980 randomized patients.17 This suggests some benefit, as does the CLOSURE 1 trial, in which 3 of the 12 recurrent strokes in the percutaneous closure group occurred before the device was implanted.15
The low event rates in these studies prompted several meta-analyses.29–35 However, only two suggested a benefit of percutaneous closure over medical therapy. In one recent meta-analysis,29 observational study data suggested benefit from percutaneous closure, whereas three randomized controlled trials failed to show a statistically significant benefit.
The conclusions of the meta-analyses must be interpreted with caution because of inherent differences in the randomized controlled trials, including the closure device used, inclusion criteria, study end points, and variations in medical therapy.
Devices differ
A meta-analysis by Khan et al35 showed a benefit of percutaneous closure when evaluating only studies using the Amplatzer PFO occluder (AGA Medical), as in RESPECT and the PC trial.35 As data accumulate, it is important to remember that there are differences between devices. Ongoing trials continue to investigate the Amplatzer device (NCT01550588) and the GORE HELEX Septal Occluder/GORE Septal Occluder (Gore Medical) (NCT00738894).
In another meta-analysis, Pineda et al31 found a benefit with closure in the as-treated analysis using data from all three randomized controlled trials (OR 0.62; 95% CI 0.41–0.94; P = .02).31 Although paradoxical embolism through the PFO as the mechanism of stroke has been questioned, this finding suggests that actual closure of a PFO may protect against further events, presumably by preventing paradoxical embolism.
Different closure devices have different side effects. The incidence of atrial fibrillation with the CardioSEAL STARFlex device (NMT Medical) is higher than with medical therapy (used in the CLOSURE trial15), whereas this risk was not statistically significantly increased in the PC trial16 and RESPECT,17 which used the Amplatzer device.
Benefit in those with atrial septal aneurysm?
Percutaneous closure has been shown to be safe and effective in patients with PFO and atrial septal aneurysm.36 There was some benefit of closure over medical therapy in a subgroup analysis from RESPECT in these patients, with a HR of 0.19 (95% CI 0.04–0.87, P = .02),17 although this was not seen in either CLOSURE 1 or the PC trial.
WHAT ARE THE RISKS OF PERCUTANEOUS CLOSURE?
Minor complications of percutaneous closure include bleeding, atrial arrhythmias, device embolization and fracture, and complications related to vascular access. Major complications include hemorrhage requiring transfusion, need for surgery, cardiac tamponade, pulmonary embolism, and death.
The cumulative rate of major complications in 10 observational studies was 1.5%, and the rate of minor complications was 7.9%.37 The RESPECT investigators reported a serious adverse event in 4.2% of patients (ranging in severity from chest tightness to cardiac tamponade).17
Another possible consequence of percutaneous closure is the need for chronic anticoagulation because of the increased risk of postprocedural atrial fibrillation seen in meta-analyses,29,31,32 though this may be device-specific.32
Percutaneous closure was considered successful—ie, to have nearly or completely eliminated shunting of blood through the defect—at 6 months of follow-up in 95.9% of patients in the PC trial, 93.5% in RESPECT, and 86.1% in CLOSURE 1.15–17
WHAT SHOULD WE BE DOING IN DAILY PRACTICE?
Give aspirin. Aspirin is effective in secondary stroke prevention, and data suggest that patients with PFO and cryptogenic stroke who receive aspirin therapy alone have a similar risk of recurrent events as patients without PFO.
Give warfarin if indicated. Evidence is insufficient to recommend vitamin K antagonist therapy in all patients with PFO and cryptogenic stroke. However, coexisting conditions that warrant anticoagulation must be taken into account.
Individualize. Given the lack of evidence to definitively guide management of patients with cryptogenic stroke and PFO, we need to individualize our approach, taking into account patient preferences, bleeding risk, ability to tolerate procedures, and the likelihood that the PFO is at fault.
No definitive answer on PFO closure. The most recent data suggest that closure may be beneficial, but key questions remain: Who will benefit? And what is the ideal medical therapy? Optimal management will only be established by the continued enrollment of appropriate patients into ongoing clinical trials.
Another question is whether it is possible to perform a randomized controlled trial with enough patients to definitively prove whether percutaneous closure is superior to medical therapy. Recent experience would suggest not.
In the meantime, we have some guidance from the American Heart Association and the American Stroke Association Council on Stroke38 based on the limited evidence available.
Consider patient preference. The physician should present the options to the patient in a balanced manner to enable him or her to make an informed decision. Patients can also be encouraged to seek additional information at websites such as www.stroke.org and www.nlm.nih.gov.
Referral to an interventional cardiologist for evaluation for closure is reasonable in patients with recurrent stroke, medication failure, complicated atrial septal anatomy such as PFO with aneurysm or large shunt, concurrent thromboembolic disease, or contraindications to anticoagulation.
MORE WORK NEEDED
Areas for further study include further identifying the characteristics of patients with PFO and cryptogenic stroke that might indicate who would benefit from percutaneous closure, elucidating the mechanism of stroke in these patients, and determining whether routine stroke evaluation should include echocardiography with a bubble study if there is no change in management based on the finding of PFO.39
Your patient has had an ischemic stroke, and so far you have found no obvious cause such as atrial fibrillation or carotid disease. Should you look for a patent foramen ovale (PFO)? And if you find it, what should you do?
This scenario continues to challenge primary care physicians and subspecialists and requires an understanding of the relationship between PFO and cryptogenic stroke, as well as familiarity with current data on the safety and effectiveness of the management options. PFO is known to be associated with cryptogenic stroke, but many questions remain, including:
- How can we tell if PFO is a culprit (“pathologic”) or an innocent bystander (“incidental”) in a patient who has had a cryptogenic stroke?
- Should stroke patients receive different medical therapy if they have a PFO? In particular, should they receive warfarin in addition to aspirin? And what about the novel oral anticoagulants?
- Which patients should undergo percutaneous closure of the PFO?
- Should we even be looking for PFO in stroke patients at this point, if we cannot say with certainty what we should do if we find it?
WHY IS THIS IMPORTANT?
Cerebrovascular disease is common and costly. The estimated yearly incidence of stroke in the United States is 795,000 events, at a cost of nearly $30 billion.1 The incidence of stroke in Europe is more than 1 million annually.2
During the diagnostic evaluation of stroke or transient ischemic attack (TIA), PFO is occasionally discovered incidentally by echocardiography. The management decisions that follow often fall to the primary care physician, who must decipher the conflicting data currently available and explain the options to the patient.
Although reviews have been published on this subject,3 several newer key trials and data on risk stratification warrant consideration.
DEFINITIONS
PFO is the failure of the septum primum to fuse with the septum secundum, so that a communication remains between the atria (Figure 1). The diagnosis is commonly made by echocardiography, when agitated saline is injected into the venous system and bubbles can be seen in the left atrium within three to five cardiac cycles (see video).
Atrial septal aneurysm is loosely defined as a septal excursion or bulging of at least 10 to 15 mm into the left and right atria during the cardiac cycle (Figure 2). The combination of PFO and atrial septal aneurysm may be more of a risk factor for stroke than PFO alone (see discussion below).
Cryptogenic stroke. The diagnostic workup of stroke fails to elucidate a clear cause in up to 40% of cases, which are thus called cryptogenic.4 The workup varies, but typically includes a search for a cardioembolic source and for atherosclerotic disease. Embolic sources are evaluated for by electrocardiography, transthoracic echocardiography, and possibly imaging of the aortic arch. Evaluation for atherosclerotic disease of the intracranial and extracranial arteries includes magnetic resonance angiography or, if that is unavailable, computed tomographic angiography or carotid Doppler ultrasonography. If no source is found, long-term cardiac monitoring may be used to detect paroxysmal atrial fibrillation, which may be more common than previously thought.
PFO AND CRYPTOGENIC STROKE ARE COMMON
As noted, there are approximately 800,000 strokes every year in the United States. If 25% to 40% of them are cryptogenic (the true prevalence warrants more evaluation),4,5 then 200,000 to 320,000 strokes are cryptogenic.
Autopsy studies indicate that 25% of the general population have a PFO, and if the prevalence is the same in people with cryptogenic stroke, that would equal 80,000 people with both cryptogenic stroke and PFO every year. However, the prevalence of PFO in patients with cryptogenic stroke appears to be significantly higher than in the general population.6 Although these numbers are crude estimates, they provide some insight into the prevalence of this clinical presentation.
HOW ARE CRYPTOGENIC STROKE AND PFO RELATED?
The exact relationship between PFO and cryptogenic stroke is unknown, although cases have been reported of thrombus in transit through a PFO, supporting paradoxical embolism as the plausible cause in stroke patients with PFO.7–9
There is clear evidence that the two conditions are associated by more than chance. Homma and Sacco6 reported that, in several studies, 93 (46%) of 202 patients under age 55 with cryptogenic stroke had PFOs, compared with 29 (11%) of 271 controls (P < .05 in all studies).6
In their evaluation of 23 case-control studies, Alsheikh-Ali et al10 found that the summary odds ratio (OR) for PFO in cryptogenic stroke vs PFO in control patients was 2.9 (95% confidence interval [CI] 2.1–4), largely driven by an OR of 5.1 (3.3–7.8) in those under age 55. Through Bayesian probability theory, this correlated with only a 33% probability that PFO in a patient with cryptogenic stroke was an innocent bystander rather than the culprit.10
IS PFO A RISK FACTOR FOR STROKE?
One of the more puzzling aspects of the relationship of PFO to cryptogenic stroke is that despite a clear association, there is little evidence that the relationship is causal.
Di Tullio et al11 followed 1,100 people who had no history of stroke and found that the risk of a first stroke in those with a PFO was not significantly higher than in those without a PFO, regardless of age, sex, or ethnic or racial group. At 80 months, the hazard ratio of stroke in people who had a PFO was 1.64 (95% CI 0.87–3.09).11 The findings were similar at 11 years, with a hazard ratio of 1.10 (95% CI 0.64–1.91).12
A prospective study of 585 patients found a similar risk of stroke in those with and without a PFO, with a hazard ratio of 1.46 (95% CI 0.74–2.88; P = .28).13
These prospective trials suggest that although previous studies have found a higher prevalence of PFOs in patients with cryptogenic stroke than in patients without stroke, there appears to be very little if any increased risk from baseline for a first stroke or TIA.
The lack of statistical significance in these trials should be interpreted with some caution, as a small increased risk is difficult to show if the event rate is low (approximately 10% of patients had events over 11 years in the study by Di Tullio et al12).
HOW DO WE KNOW IF A PFO IS A CULPRIT OR BYSTANDER?
Unfortunately, this is largely unanswered, though experts have suggested that echocardiographic features of the PFO, radiographic characteristics of the stroke, and clinical features of the patient may provide useful information.
‘High-risk’ features on echocardiography
Certain features of PFO may portend a high risk of cerebrovascular events. Both right-to-left shunting at rest and septal hypermobility were found in one study14 to be more common in patients with a PFO who had a stroke or TIA than in patients with a PFO but no cerebrovascular events. Also, patients who had these features and had a stroke had a higher risk of recurrence than stroke patients without these features (12.5% vs 4.3%, P = .05).14
Septal hypermobility and shunting at rest are easily diagnosed by echocardiography, and detecting these “high-risk” features would be useful if they could identify patients who would benefit from special therapy, such as percutaneous closure of the PFO.
Unfortunately, when investigators looked at these features in subgroup analysis of the major randomized controlled trials of percutaneous closure vs medical therapy, the results were mixed.
CLOSURE 1 (the Evaluation of the STARFlex Septal Closure System in Patients With a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through a Patent Foramen Ovale)15 found percutaneous closure to be no better than medical therapy, regardless of shunt size or the presence of atrial septal aneurysm.
Similarly, the PC trial (Clinical Trial Comparing Percutaneous Closure of Patent Foramen Ovale Using the Amplatzer PFO Occluder With Medical Treatment in Patients With Cryptogenic Embolism)16 found no statistically significant benefit of closure in those with atrial septal aneurysm.
In contrast, the RESPECT trial (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment)17 showed percutaneous closure to be beneficial in patients with atrial septal aneurysm or large shunt.
Radiographic characteristics of the stroke
Another area of interest in trying to identify culprit PFOs is the radiographic characteristics of the stroke.
In a study comparing patients with stroke related to atrial fibrillation vs patients with cryptogenic stroke and a known PFO, those in the latter group were more likely to have a single cortical infarction (34.2% vs 3.1%; P < .001) or multiple small scattered lesions (23.1% vs 5.9%; P < .01).18 Similarly, in a large database of patients with cryptogenic stroke and known PFO status, a superficially located stroke was associated with the presence of PFO (OR 1.54; P < .0001).19
Although these findings do not tell us with certainty that a patient’s PFO was the cause of his or her stroke, they provide guidance when dealing with the uncertainty of how to manage a patient with PFO. They may be useful in clinical practice, for example, when discussing treatment options with a young patient with cryptogenic stroke who has no risk factors and a superficial single infarct and who is found to have a PFO with a right-to-left shunting at rest.
Patient characteristics
Kent et al20 developed a 10-point index (the RoPE score) in an attempt to assign a probability to whether a stroke was PFO-related. Points were assigned for patients who were younger, who had a cortical stroke on neuroimaging, and who did not have diabetes, hypertension, smoking, or prior stroke or TIA. Patients with cryptogenic stroke with a higher RoPE score were more likely to have a PFO and thus had a higher likelihood that the index event was related to PFO. Of note, the patients with the highest likelihood of PFO-related stroke were the least likely to have a recurrence (RoPE score of 9 to 10; PFO-attributable fraction 88%; estimated 2-year recurrence rate 2%; 95% CI 0%–4%), whereas those with a low RoPE score have more traditional risk factors for stroke and thus are more likely to have a recurrence (RoPE 0 to 3; estimated 2-year recurrence rate 20%; 95% CI 12%–28%).20
Again, this sheds light on a difficulty faced by randomized controlled trials: the patients who may benefit from closure of a PFO may very well be those with the lowest recurrence rates without intervention.
The RoPE index was examined in an attempt to validate previously described morphologic criteria of “high-risk” PFO,21 though none of the previously described “high-risk” echocardiographic features (large physiologic size, hypermobile septum, shunt at rest) were more common in the group with presumed PFO-attributable stroke (RoPE score > 6). This underscores the difficulty of distinguishing pathologic PFO from incidental PFO.
KEY TREATMENT CONSIDERATIONS FOR SECONDARY PREVENTION
Given the complicated relationship between PFO and cryptogenic stroke, there has been much debate over management strategies. The three options are surgical closure, percutaneous closure with a device, and medical therapy. The goal of all three is to prevent the recurrence of stroke or TIA.
Surgical closure has largely been supplanted by percutaneous closure, but is still done in specific situations such as when a PFO is found incidentally on transesophageal echocardiography during surgery for another cardiac condition. The data on such cases22 tend to support the argument that asymptomatic PFOs in the general population have a relatively benign natural history.
Thus, the two key questions about management that warrant discussion are: is anticoagulation superior to antiplatelet therapy? And is percutaneous closure superior to medical management?
Anticoagulant vs antiplatelet therapy
Whether to treat with aspirin or with a vitamin K antagonist has been a subject of debate, although there is no strong evidence to suggest that anticoagulation is superior to antiplatelet therapy.
The concern that aspirin alone is insufficient in some patients stems from a study by Mas et al,23 who followed 581 patients with cryptogenic stroke who had a PFO alone, a PFO with an atrial septal aneurysm, or neither. The rate of stroke recurrence at 4 years on aspirin therapy was 2.3% in those with a PFO alone, 15.2% in those with a PFO with an atrial septal aneurysm, and 4.2% in those with neither.
Many have concluded that aspirin therapy does not sufficiently protect those with both PFO and atrial septal aneurysm, given the high recurrence rate in this group. This might lead to the suggestion that anticoagulation could be of benefit in these patients.
However, the Patent Foramen Ovale in Cryptogenic Stroke Study (PiCSS)24 and the Spanish Multicenter Study Into Right-to-Left Shunt in Cryptogenic Stroke (CODICIA)25 found similar recurrence rates in patients with PFO and atrial septal aneurysm compared with those with only PFO. In these two studies, recurrence rates were similar regardless of whether patients were taking aspirin or warfarin.
In a study that followed 140 consecutive patients with both stroke and PFO, those treated in a nonrandomized fashion with antiplatelet agents had no difference in the recurrence rate compared with those treated with anticoagulation.26
Although uncertainty remains because no head-to-head randomized controlled trial has been done, some patients with PFO have other indications for anticoagulation, most commonly atrial fibrillation and venous thromboembolic disease.
There are currently no data on the use of novel oral anticoagulants in this setting.
Is percutaneous closure better than medical therapy?
When cryptogenic stroke is treated with antiplatelet therapy or anticoagulation therapy, the recurrence rate is the same whether or not the patient has a PFO.23–25 The belief that medical therapy offers adequate secondary protection is supported by a meta-analysis of 15 studies that found no increased risk of recurrent ischemic events in those with a PFO on medical therapy (antiplatelet or anticoagulant) vs those without a PFO (relative risk 1.1, 95% CI 0.8–1.5).27
Despite the conflicting evidence, percutaneous closure of PFO is still performed, mostly on a case-by-case basis. This has been supported by an apparent benefit in observational studies.
A systematic review of 52 single-arm studies and 7 comparative nonrandomized studies of patients with PFO and cryptogenic stroke found the rate of recurrent stroke to be 0.36 per 100 person-years with percutaneous closure vs 2.53 per 100 person-years with medical therapy.28 However, three long-awaited randomized controlled trials (CLOSURE 1, the PC trial, and RESPECT) failed to show a significant reduction in primary end points with percutaneous closure vs standard medical therapy.15–17
These trials had several limitations: event rates were low, medical therapy varied by provider, and enrollment was slowed by out-of-study percutaneous closure in patients perceived to be at high risk (though, as discussed above, high risk is difficult to determine).
Intention-to-treat analysis in RESPECT showed no benefit from percutaneous closure, but a favorable outcome was noted with closure in as-treated analysis (HR 0.27; 95% CI 0.1–0.75; P = .007) and per-protocol analysis (HR 0.37; 95% CI 0.14–0.96; P = .03) of the 980 randomized patients.17 This suggests some benefit, as does the CLOSURE 1 trial, in which 3 of the 12 recurrent strokes in the percutaneous closure group occurred before the device was implanted.15
The low event rates in these studies prompted several meta-analyses.29–35 However, only two suggested a benefit of percutaneous closure over medical therapy. In one recent meta-analysis,29 observational study data suggested benefit from percutaneous closure, whereas three randomized controlled trials failed to show a statistically significant benefit.
The conclusions of the meta-analyses must be interpreted with caution because of inherent differences in the randomized controlled trials, including the closure device used, inclusion criteria, study end points, and variations in medical therapy.
Devices differ
A meta-analysis by Khan et al35 showed a benefit of percutaneous closure when evaluating only studies using the Amplatzer PFO occluder (AGA Medical), as in RESPECT and the PC trial.35 As data accumulate, it is important to remember that there are differences between devices. Ongoing trials continue to investigate the Amplatzer device (NCT01550588) and the GORE HELEX Septal Occluder/GORE Septal Occluder (Gore Medical) (NCT00738894).
In another meta-analysis, Pineda et al31 found a benefit with closure in the as-treated analysis using data from all three randomized controlled trials (OR 0.62; 95% CI 0.41–0.94; P = .02).31 Although paradoxical embolism through the PFO as the mechanism of stroke has been questioned, this finding suggests that actual closure of a PFO may protect against further events, presumably by preventing paradoxical embolism.
Different closure devices have different side effects. The incidence of atrial fibrillation with the CardioSEAL STARFlex device (NMT Medical) is higher than with medical therapy (used in the CLOSURE trial15), whereas this risk was not statistically significantly increased in the PC trial16 and RESPECT,17 which used the Amplatzer device.
Benefit in those with atrial septal aneurysm?
Percutaneous closure has been shown to be safe and effective in patients with PFO and atrial septal aneurysm.36 There was some benefit of closure over medical therapy in a subgroup analysis from RESPECT in these patients, with a HR of 0.19 (95% CI 0.04–0.87, P = .02),17 although this was not seen in either CLOSURE 1 or the PC trial.
WHAT ARE THE RISKS OF PERCUTANEOUS CLOSURE?
Minor complications of percutaneous closure include bleeding, atrial arrhythmias, device embolization and fracture, and complications related to vascular access. Major complications include hemorrhage requiring transfusion, need for surgery, cardiac tamponade, pulmonary embolism, and death.
The cumulative rate of major complications in 10 observational studies was 1.5%, and the rate of minor complications was 7.9%.37 The RESPECT investigators reported a serious adverse event in 4.2% of patients (ranging in severity from chest tightness to cardiac tamponade).17
Another possible consequence of percutaneous closure is the need for chronic anticoagulation because of the increased risk of postprocedural atrial fibrillation seen in meta-analyses,29,31,32 though this may be device-specific.32
Percutaneous closure was considered successful—ie, to have nearly or completely eliminated shunting of blood through the defect—at 6 months of follow-up in 95.9% of patients in the PC trial, 93.5% in RESPECT, and 86.1% in CLOSURE 1.15–17
WHAT SHOULD WE BE DOING IN DAILY PRACTICE?
Give aspirin. Aspirin is effective in secondary stroke prevention, and data suggest that patients with PFO and cryptogenic stroke who receive aspirin therapy alone have a similar risk of recurrent events as patients without PFO.
Give warfarin if indicated. Evidence is insufficient to recommend vitamin K antagonist therapy in all patients with PFO and cryptogenic stroke. However, coexisting conditions that warrant anticoagulation must be taken into account.
Individualize. Given the lack of evidence to definitively guide management of patients with cryptogenic stroke and PFO, we need to individualize our approach, taking into account patient preferences, bleeding risk, ability to tolerate procedures, and the likelihood that the PFO is at fault.
No definitive answer on PFO closure. The most recent data suggest that closure may be beneficial, but key questions remain: Who will benefit? And what is the ideal medical therapy? Optimal management will only be established by the continued enrollment of appropriate patients into ongoing clinical trials.
Another question is whether it is possible to perform a randomized controlled trial with enough patients to definitively prove whether percutaneous closure is superior to medical therapy. Recent experience would suggest not.
In the meantime, we have some guidance from the American Heart Association and the American Stroke Association Council on Stroke38 based on the limited evidence available.
Consider patient preference. The physician should present the options to the patient in a balanced manner to enable him or her to make an informed decision. Patients can also be encouraged to seek additional information at websites such as www.stroke.org and www.nlm.nih.gov.
Referral to an interventional cardiologist for evaluation for closure is reasonable in patients with recurrent stroke, medication failure, complicated atrial septal anatomy such as PFO with aneurysm or large shunt, concurrent thromboembolic disease, or contraindications to anticoagulation.
MORE WORK NEEDED
Areas for further study include further identifying the characteristics of patients with PFO and cryptogenic stroke that might indicate who would benefit from percutaneous closure, elucidating the mechanism of stroke in these patients, and determining whether routine stroke evaluation should include echocardiography with a bubble study if there is no change in management based on the finding of PFO.39
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- Truelsen T, Piechowski-JóŸwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol 2006; 13:581–598.
- Furlan AJ. Patent foramen ovale and stroke: to close or not to close? Cleve Clin J Med 2007; 74(suppl 1):S118–S120.
- Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol 1989; 25:382–390.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001; 32:2559–2566.
- Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation 2005; 112:1063–1072.
- Sattiraju S, Masri SC, Liao K, Missov E. Three-dimensional transesophageal echocardiography of a thrombus entrapped by a patent foramen ovale. Ann Thorac Surg 2012; 94:e101–e102.
- Schreiter SW, Phillips JH. Thromboembolus traversing a patent foramen ovale: resolution with anticoagulation. J Am Soc Echocardiogr 1994; 7:659–662.
- Hust MH, Staiger M, Braun B. Migration of paradoxic embolus through a patent foramen ovale diagnosed by echocardiography: successful thrombolysis. Am Heart J 1995; 129:620–622.
- Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009; 40:2349–2355.
- Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol 2007; 49:797–802.
- Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
- Meissner I, Khandheria BK, Heit JA, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol 2006; 47:440–445.
- De Castro S, Cartoni D, Fiorelli M, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke 2000; 31:2407–2413.
- Furlan AJ, Reisman M, Massaro J, et al; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012; 366:991–999.
- Meier B, Kalesan B, Mattle HP, et al; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013; 368:1083–1091.
- Carroll JD, Saver JL, Thaler DE, et al; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013; 368:1092–1100.
- Kim BJ, Sohn H, Sun BJ, et al. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke 2013; 44:3350–3356.
- Thaler DE, Ruthazer R, Di Angelantonio E, et al. Neuroimaging findings in cryptogenic stroke patients with and without patent foramen ovale. Stroke 2013; 44:675–680.
- Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013; 81:619–625.
- Wessler BS, Thaler DE, Ruthazer R, et al. Transesophageal echocardiography in cryptogenic stroke and patent foramen ovale: analysis of putative high-risk features from the risk of paradoxical embolism database. Circ Cardiovasc Imaging 2014; 7:125–131.
- Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of intraoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA 2009; 302:290–297.
- Mas JL, Arquizan C, Lamy C, et al; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001; 345:1740–1746.
- Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP; PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002; 105:2625–2631.
- Serena J, Marti-Fàbregas J, Santamarina E, et al; CODICIA, Right-to-Left Shunt in Cryptogenic Stroke Study; Stroke Project of the Cerebrovascular Diseases Study Group, Spanish Society of Neurology. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke 2008; 39:3131–3136.
- Bogousslavsky J, Garazi S, Jeanrenaud X, Aebischer N, Van Melle G. Stroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study Group. Neurology 1996; 46:1301–1305.
- Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology 2009; 73:89–97.
- Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke 2012; 43:422–431.
- Wolfrum M, Froehlich GM, Knapp G, et al. Stroke prevention by percutaneous closure of patent foramen ovale: a systematic review and meta-analysis. Heart 2014; 100:389–395.
- Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2013; 34:3342–3352.
- Pineda AM, Nascimento FO, Yang SC, Kirtane AJ, Sommer RJ, Beohar N. A meta-analysis of transcatheter closure of patent foramen ovale versus medical therapy for prevention of recurrent thromboembolic events in patients with cryptogenic cerebrovascular events. Catheter Cardiovasc Interv 2013; 82:968–975.
- Kwong JS, Lam YY, Yu CM. Percutaneous closure of patent foramen ovale for cryptogenic stroke: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 168:4132–4148.
- Ntaios G, Papavasileiou V, Makaritsis K, Michel P. PFO closure vs medical therapy in cryptogenic stroke or transient ischemic attack: a systematic review and meta-analysis. Int J Cardiol 2013; 169:101–105.
- Nagaraja V, Raval J, Eslick GD, Burgess D, Denniss AR. Is transcatheter closure better than medical therapy for cryptogenic stroke with patent foramen ovale? A meta-analysis of randomised trials. Heart Lung Circ 2013; 22:903–909.
- Khan AR, Bin Abdulhak AA, Sheikh MA, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: a systematic review and meta-analysis. JACC Cardiovasc Interv 2013; 6:1316–1323.
- Wahl A, Krumsdorf U, Meier B, et al. Transcatheter treatment of atrial septal aneurysm associated with patent foramen ovale for prevention of recurrent paradoxical embolism in high-risk patients. J Am Coll Cardiol 2005; 45:377–380.
- Khairy P, O’Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med 2003; 139:753–760.
- Sacco RL, Adams R, Albers G, et al; American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37:577–617.
- Rana BS, Thomas MR, Calvert PA, Monaghan MJ, Hildick-Smith D. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging 2010; 3:749–760.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- Truelsen T, Piechowski-JóŸwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol 2006; 13:581–598.
- Furlan AJ. Patent foramen ovale and stroke: to close or not to close? Cleve Clin J Med 2007; 74(suppl 1):S118–S120.
- Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol 1989; 25:382–390.
- Grau AJ, Weimar C, Buggle F, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001; 32:2559–2566.
- Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation 2005; 112:1063–1072.
- Sattiraju S, Masri SC, Liao K, Missov E. Three-dimensional transesophageal echocardiography of a thrombus entrapped by a patent foramen ovale. Ann Thorac Surg 2012; 94:e101–e102.
- Schreiter SW, Phillips JH. Thromboembolus traversing a patent foramen ovale: resolution with anticoagulation. J Am Soc Echocardiogr 1994; 7:659–662.
- Hust MH, Staiger M, Braun B. Migration of paradoxic embolus through a patent foramen ovale diagnosed by echocardiography: successful thrombolysis. Am Heart J 1995; 129:620–622.
- Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009; 40:2349–2355.
- Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol 2007; 49:797–802.
- Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
- Meissner I, Khandheria BK, Heit JA, et al. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol 2006; 47:440–445.
- De Castro S, Cartoni D, Fiorelli M, et al. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke 2000; 31:2407–2413.
- Furlan AJ, Reisman M, Massaro J, et al; CLOSURE I Investigators. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012; 366:991–999.
- Meier B, Kalesan B, Mattle HP, et al; PC Trial Investigators. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013; 368:1083–1091.
- Carroll JD, Saver JL, Thaler DE, et al; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013; 368:1092–1100.
- Kim BJ, Sohn H, Sun BJ, et al. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke 2013; 44:3350–3356.
- Thaler DE, Ruthazer R, Di Angelantonio E, et al. Neuroimaging findings in cryptogenic stroke patients with and without patent foramen ovale. Stroke 2013; 44:675–680.
- Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology 2013; 81:619–625.
- Wessler BS, Thaler DE, Ruthazer R, et al. Transesophageal echocardiography in cryptogenic stroke and patent foramen ovale: analysis of putative high-risk features from the risk of paradoxical embolism database. Circ Cardiovasc Imaging 2014; 7:125–131.
- Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of intraoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA 2009; 302:290–297.
- Mas JL, Arquizan C, Lamy C, et al; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001; 345:1740–1746.
- Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP; PFO in Cryptogenic Stroke Study (PICSS) Investigators. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002; 105:2625–2631.
- Serena J, Marti-Fàbregas J, Santamarina E, et al; CODICIA, Right-to-Left Shunt in Cryptogenic Stroke Study; Stroke Project of the Cerebrovascular Diseases Study Group, Spanish Society of Neurology. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke 2008; 39:3131–3136.
- Bogousslavsky J, Garazi S, Jeanrenaud X, Aebischer N, Van Melle G. Stroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study Group. Neurology 1996; 46:1301–1305.
- Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology 2009; 73:89–97.
- Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke 2012; 43:422–431.
- Wolfrum M, Froehlich GM, Knapp G, et al. Stroke prevention by percutaneous closure of patent foramen ovale: a systematic review and meta-analysis. Heart 2014; 100:389–395.
- Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2013; 34:3342–3352.
- Pineda AM, Nascimento FO, Yang SC, Kirtane AJ, Sommer RJ, Beohar N. A meta-analysis of transcatheter closure of patent foramen ovale versus medical therapy for prevention of recurrent thromboembolic events in patients with cryptogenic cerebrovascular events. Catheter Cardiovasc Interv 2013; 82:968–975.
- Kwong JS, Lam YY, Yu CM. Percutaneous closure of patent foramen ovale for cryptogenic stroke: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 168:4132–4148.
- Ntaios G, Papavasileiou V, Makaritsis K, Michel P. PFO closure vs medical therapy in cryptogenic stroke or transient ischemic attack: a systematic review and meta-analysis. Int J Cardiol 2013; 169:101–105.
- Nagaraja V, Raval J, Eslick GD, Burgess D, Denniss AR. Is transcatheter closure better than medical therapy for cryptogenic stroke with patent foramen ovale? A meta-analysis of randomised trials. Heart Lung Circ 2013; 22:903–909.
- Khan AR, Bin Abdulhak AA, Sheikh MA, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: a systematic review and meta-analysis. JACC Cardiovasc Interv 2013; 6:1316–1323.
- Wahl A, Krumsdorf U, Meier B, et al. Transcatheter treatment of atrial septal aneurysm associated with patent foramen ovale for prevention of recurrent paradoxical embolism in high-risk patients. J Am Coll Cardiol 2005; 45:377–380.
- Khairy P, O’Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med 2003; 139:753–760.
- Sacco RL, Adams R, Albers G, et al; American Heart Association; American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke 2006; 37:577–617.
- Rana BS, Thomas MR, Calvert PA, Monaghan MJ, Hildick-Smith D. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging 2010; 3:749–760.
KEY POINTS
- PFO is present in up to 25% of the general population, and it is even more common in young patients with cryptogenic stroke.
- PFO has not been shown to cause stroke or to significantly increase the risk of recurrent cerebrovascular events in patients treated with antiplatelet drugs.
- In patients with PFO, atrial septal aneurysm and large shunt size may confer increased risk of stroke.
- There is still no definitive evidence that closure of PFO is better than medical therapy in all patients with PFO and cryptogenic stroke.
Blue towel left in abdomen: $7.2M verdict
Blue towel left in abdomen: $7.2M verdict
When a 61-year-old woman underwent laparoscopic hysterectomy, her gynecologist, Dr. A, was assisted by another gynecologist (Dr. B), a nurse, and a technician. When Dr. A noted that the uterine artery had been injured, he converted to an open procedure, retracted the bowel, repaired the artery, and completed the operation.
Postdischarge, the patient was febrile and developed abdominal pain and an odorous vaginal discharge. A month later, exploratory surgery revealed a retained blue towel that had been used for bowel retraction. The patient required open healing of the surgical wound and a temporary colostomy. She developed an incisional hernia after colostomy reversal, and hernia repair required resection of a small portion of the bowel.
PATIENT’S CLAIM It was negligent to use a blue towel to retract the bowel. The towel should have been removed from her abdomen before closure.
DEFENDANTS’ DEFENSE The technician claimed that she did not provide the towel, did not see the towel used, and that she was not told that the towel had to be tracked. She noted that its color indicated that it lacked a radiopaque tag, and that hospital policy forbade use of untagged towels in an open wound.
Dr. A claimed that he specifically requested a blue towel because it was absorbent, that the technician provided the towel, and that the towel’s use prevented the patient from bleeding to death.
VERDICT A $7.2 million New York verdict was returned against both gynecologists and the hospital as the technician’s employer.
MISCARRIAGE AFTER D&C
A few days after a woman thought she miscarried, her family practi-tioner (FP) performed a dilation and curettage (D&C).
The patient was at work 12 days later when she expelled a fully formed 14-week fetus into a toilet. She was taken to the emergency department (ED), where the cord was cut. Later that day, she passed placental tissue; a repeat D&C was performed the next day.
PATIENT'S CLAIM The FP did not properly perform the first D&C. Although the pathology report was available to the FP prior to the patient’s postoperative visit, the FP failed to inform the patient that no fetal parts had been extracted.
PHYSICIAN’S DEFENSE Because the FP thought that the fetus had been passed prior to the D&C, she believed the pathology report was appropriate.
The patient had been informed of the possibility of retained products of conception after the D&C. The FP had ordered a blood pregnancy test that would have revealed the presence of retained products of conception, but the patient did not have the test. The patient did not contact the FP to report symptoms that felt like labor pains on the day that she passed the fetus.
VERDICT A bench trial resulted in a $51,000 California verdict.
PREGNANT WOMAN COMPLAINS OF LEG PAIN; DIES OF DVT
A 23-year-old woman went to the ED with pain and swelling in her lower left leg and calf. The symptoms were reported to her ObGyn, who examined and then discharged her within a few hours, with instructions to come for her regularly scheduled prenatal visit.
The patient died 2 weeks later. The cause of death was determined to be a pulmonary embolus from a thrombus of the left popliteal vein.
ESTATE’S CLAIM The ObGyn was negligent in failing to test the patient for thrombosis in her left leg when she was in the ED or several days later at the office, when she continued to report leg pain.
PHYSICIAN’S DEFENSE The patient did not have signs of thrombosis at the ED or at the subsequent office visit. The pathologist reported that the clot that caused the embolus appeared fresh. The ObGyn surmised that it had formed after the patient’s last appointment.
VERDICT A Texas defense verdict was returned.
Mother took topiramate; child born with cleft lip and palate: $3M verdict
When a woman learned she was pregnant in December 2007, she was taking topiramate (Topamax) to treat migraine headaches. She discussed tapering off but not discontinuing topiramate usage with her neurologist. The patient’s ObGyn told her that topiramate was safe to take during pregnancy. The child was born with a cleft lip and palate.
PARENTS’ CLAIM Janssen Pharmaceuticals, manufacturer of Topamax, failed to provide adequate warnings about the potential risks associated with Topamax until labeling was changed in March 2011. Janssen knew of potential birth defects associated with Topamax use during pregnancy more than a decade before the labeling change; Janssen’s associate director of regulatory affairs had testified in an earlier hearing that there was knowledge of related birth defects as early as 1996.
DEFENDANTS’ DEFENSE There is uncertainty as to whether exposure to Topamax during pregnancy causes birth defects. The neurologist had warned the patient of possible risks associated with taking Topamax during pregnancy, but the patient had refused to discontinue the drug.
VERDICT A $3 million Pennsylvania verdict was returned.
Related articles:
• Is it time to rethink the use of oral contraceptives in premenopausal women with migraine? Anne H. Calhoun, MD (Audiocast; October 2013)
• How to choose a contraceptive for a patient who has headaches. Kristina M. Tocce, MD; Stephanie B. Teal, MD, MPH (February 2011)
• The gynecologist’s role in managing menstrual migraine. Anne H. Calhoun, MD (April 2010)
WAS MOTHER’S HISTORY OF INCOMPETENT CERVIX IGNORED?
Early in her second pregnancy, a woman told her ObGyn that she had previously miscarried due to an incompetent cervix.
At 24 weeks’ gestation, the patient was admitted to the hospital with back and pelvic pain and vaginal bleeding. Shortly after admission, the ObGyn performed a vaginal examination and ordered ultrasonography (US), which showed that the fetus was in the transverse position and the membranes were bulging.
The ObGyn performed an emergency cesarean delivery, but the premature infant died within 2 hours.
PARENTS’ CLAIM The ObGyn should have performed a cervical cerclage because of the mother’s history of an incompetent cervix. The mother should have been placed on bed rest and monitored every 2 weeks for cervical dilation.
PHYSICIAN’S DEFENSE The patient underwent regular prenatal evaluations for an incompetent cervix, and the findings were always normal.
VERDICT A Florida defense verdict was returned.
Related article:
A stepwise approach to cervical cerclage. Katrin Karl, MD; Michael Katz, MD (Surgical Technique; June 2012)
ObGyn unresponsive to patient’s postsurgical phone calls
In 2009, a 50-year-old woman reported occasional right lower quadrant pain to her ObGyn. US results were normal. The menopausal patient’s history included three cesarean deliveries, a total abdominal hysterectomy, and a laparoscopic ovarian cystectomy.
When the patient saw her ObGyn in December 2010, she reported intermittent, progressive right lower quadrant pain that radiated down her right leg. She also reported urine loss with coughing or sneezing, and slight pain on intercourse. The ObGyn prescribed oxybutynin chloride (Ditropan) to treat the patient’s incontinence.
Three weeks later, the patient reported bilateral lower quadrant pain to her ObGyn, with minor improvement in incontinence.
The ObGyn performed bilateral salpingo-oophorectomy (BSO) in January 2011. Surgery took 3.5 hours due to extensive adhesiolysis.
After discharge, the patient felt ill and vomited. She attempted to reach the ObGyn by phone several times. That evening, the ObGyn prescribed a suppository to treat nausea and vomiting.
The patient went to the ED later that night and was found to have a perforated colon. Emergency surgery to repair the injury included creation of a colostomy, which was repaired 20 months later.
PATIENT’S CLAIM A proper workup of her symptoms was not performed; BSO was unnecessary. The ObGyn was negligent for failing to respond in a timely manner to her post-discharge phone calls, and did not properly evaluate her postoperative symptoms.
PHYSICIAN’S DEFENSE BSO was warranted. Colon injury is a known complication of the procedure.
VERDICT A $716,976 California verdict was returned, but was reduced to $591,967 under the state cap.
Who delayed delivery? $32.8M verdict for child with CP
An 18-year-old woman at 38 weeks’ gestation went to the hospital in labor. After 3.5 hours, the fetal heart rate dropped to 60 bpm. A nurse repositioned the patient, administered oxygen, and increased intravenous fluids. When the nurse rang the emergency call bell, a second nurse responded. Eighteen minutes after the fetal heart rate first dropped, a nurse rang the call bell again and the on-call ObGyn appeared.
The ObGyn performed a vaginal examination and repositioned the patient. She noted that the fetal heart-rate monitor was not working correctly, and called for an emergency cesarean delivery. The baby was born 42 minutes after the fetal heart rate initially dropped.
The child received a diagnosis of spastic-quadriplegia cerebral palsy (CP). She requires a wheelchair and has severe speech deficits and developmental delays.
PARENT’S CLAIM Cesarean delivery was not performed in a timely manner; the delivery delay was responsible for the injury that caused CP. The ObGyn was negligent in not responding to the initial emergency call. The nurses should have summoned the ObGyn earlier.
DEFENDANTS’ DEFENSE The hospital argued that the nurses followed proper protocol. Furthermore, the hospital noted that the ObGyn did not respond to the first call, and did not request a cesarean delivery for 17 minutes.
The ObGyn claimed that she made the decision to perform cesarean delivery within 5 minutes of her arrival, but it took another 15 minutes to gather the surgical team.
VERDICT A $32,882,860 Pennsylvania verdict was returned against the hospital. The ObGyn was vindicated.
DIFFICULT DELIVERY: ZAVANELLI MANEUVER
At 38 5/7 weeks’ gestation, a woman went to the hospital for induction of labor. Twenty-four hours later, she began to push. After an hour of pushing, the mother was exhausted and had a low-grade fever, and the fetal heart rate was slowing. Her ObGyn, Dr. A, attempted vacuum extraction and performed a midline episiotomy. Shoulder dystocia was encountered and maneuvers were used, but without success. Another ObGyn, Dr. B, arrived to assist and also attempted the maneuvers.
The physicians agreed to try the Zavanelli maneuver, which involves pushing the baby’s head back inside the vagina and performing a cesarean delivery.
The baby was sent to the neonatal intensive care unit, where her breathing quickly normalized without supplemental oxygen. The child has a brachial plexus injury.
PARENTS’ CLAIM Dr. A should have performed an earlier cesarean delivery. Excessive traction was used when shoulder dystocia maneuvers were attempted.
PHYSICIANS’ DEFENSE The ObGyns’ actions saved the baby’s life and prevented serious injury to both mother and baby.
VERDICT An Alabama defense verdict was returned.
Related article:
You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial; May 2013)
PLACENTA PREVIA FOUND EARLY, BUT FETUS DIES
A woman's first pregnancy was complicated by complete placenta previa. A cesarean delivery was scheduled at 36 weeks’ gestation. However, before that date, the mother developed vaginal bleeding and was taken to the ED. The covering ObGyn was notified of the mother’s arrival within 15 minutes, but did not come to the hospital for 2.5 hours. After examining her, the ObGyn ordered US evaluation and transferred the mother to the obstetric floor. Nursing notes indicate that the fetal heart rate was 120 bpm at that time.
There are no notes from the ObGyn between 5:30 am and mid-afternoon. There is no record of the fetal heart rate when the mother was taken for US in the afternoon, which revealed fetal demise and a large extraovular hematoma. A cesarean delivery was performed. It was determined that the fetus died from placental abruption.
PARENTS’ CLAIM The mother was not adequately evaluated and monitored, which led to fetal demise. Delivery could have proceeded while the fetus was still alive.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $495,000 Massachusetts settlement was reached.
Related articles:
• What is the optimal time to deliver a woman who has placenta previa? John T. Repke, MD (Examining the Evidence; April 2011)
• Act fast when confronted by a coagulopathy postpartum. Robert L. Barbieri, MD (Editorial; March 2012)
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
TELL US WHAT YOU THINK! Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state. Stay in touch! Your feedback is important to us!
Blue towel left in abdomen: $7.2M verdict
When a 61-year-old woman underwent laparoscopic hysterectomy, her gynecologist, Dr. A, was assisted by another gynecologist (Dr. B), a nurse, and a technician. When Dr. A noted that the uterine artery had been injured, he converted to an open procedure, retracted the bowel, repaired the artery, and completed the operation.
Postdischarge, the patient was febrile and developed abdominal pain and an odorous vaginal discharge. A month later, exploratory surgery revealed a retained blue towel that had been used for bowel retraction. The patient required open healing of the surgical wound and a temporary colostomy. She developed an incisional hernia after colostomy reversal, and hernia repair required resection of a small portion of the bowel.
PATIENT’S CLAIM It was negligent to use a blue towel to retract the bowel. The towel should have been removed from her abdomen before closure.
DEFENDANTS’ DEFENSE The technician claimed that she did not provide the towel, did not see the towel used, and that she was not told that the towel had to be tracked. She noted that its color indicated that it lacked a radiopaque tag, and that hospital policy forbade use of untagged towels in an open wound.
Dr. A claimed that he specifically requested a blue towel because it was absorbent, that the technician provided the towel, and that the towel’s use prevented the patient from bleeding to death.
VERDICT A $7.2 million New York verdict was returned against both gynecologists and the hospital as the technician’s employer.
MISCARRIAGE AFTER D&C
A few days after a woman thought she miscarried, her family practi-tioner (FP) performed a dilation and curettage (D&C).
The patient was at work 12 days later when she expelled a fully formed 14-week fetus into a toilet. She was taken to the emergency department (ED), where the cord was cut. Later that day, she passed placental tissue; a repeat D&C was performed the next day.
PATIENT'S CLAIM The FP did not properly perform the first D&C. Although the pathology report was available to the FP prior to the patient’s postoperative visit, the FP failed to inform the patient that no fetal parts had been extracted.
PHYSICIAN’S DEFENSE Because the FP thought that the fetus had been passed prior to the D&C, she believed the pathology report was appropriate.
The patient had been informed of the possibility of retained products of conception after the D&C. The FP had ordered a blood pregnancy test that would have revealed the presence of retained products of conception, but the patient did not have the test. The patient did not contact the FP to report symptoms that felt like labor pains on the day that she passed the fetus.
VERDICT A bench trial resulted in a $51,000 California verdict.
PREGNANT WOMAN COMPLAINS OF LEG PAIN; DIES OF DVT
A 23-year-old woman went to the ED with pain and swelling in her lower left leg and calf. The symptoms were reported to her ObGyn, who examined and then discharged her within a few hours, with instructions to come for her regularly scheduled prenatal visit.
The patient died 2 weeks later. The cause of death was determined to be a pulmonary embolus from a thrombus of the left popliteal vein.
ESTATE’S CLAIM The ObGyn was negligent in failing to test the patient for thrombosis in her left leg when she was in the ED or several days later at the office, when she continued to report leg pain.
PHYSICIAN’S DEFENSE The patient did not have signs of thrombosis at the ED or at the subsequent office visit. The pathologist reported that the clot that caused the embolus appeared fresh. The ObGyn surmised that it had formed after the patient’s last appointment.
VERDICT A Texas defense verdict was returned.
Mother took topiramate; child born with cleft lip and palate: $3M verdict
When a woman learned she was pregnant in December 2007, she was taking topiramate (Topamax) to treat migraine headaches. She discussed tapering off but not discontinuing topiramate usage with her neurologist. The patient’s ObGyn told her that topiramate was safe to take during pregnancy. The child was born with a cleft lip and palate.
PARENTS’ CLAIM Janssen Pharmaceuticals, manufacturer of Topamax, failed to provide adequate warnings about the potential risks associated with Topamax until labeling was changed in March 2011. Janssen knew of potential birth defects associated with Topamax use during pregnancy more than a decade before the labeling change; Janssen’s associate director of regulatory affairs had testified in an earlier hearing that there was knowledge of related birth defects as early as 1996.
DEFENDANTS’ DEFENSE There is uncertainty as to whether exposure to Topamax during pregnancy causes birth defects. The neurologist had warned the patient of possible risks associated with taking Topamax during pregnancy, but the patient had refused to discontinue the drug.
VERDICT A $3 million Pennsylvania verdict was returned.
Related articles:
• Is it time to rethink the use of oral contraceptives in premenopausal women with migraine? Anne H. Calhoun, MD (Audiocast; October 2013)
• How to choose a contraceptive for a patient who has headaches. Kristina M. Tocce, MD; Stephanie B. Teal, MD, MPH (February 2011)
• The gynecologist’s role in managing menstrual migraine. Anne H. Calhoun, MD (April 2010)
WAS MOTHER’S HISTORY OF INCOMPETENT CERVIX IGNORED?
Early in her second pregnancy, a woman told her ObGyn that she had previously miscarried due to an incompetent cervix.
At 24 weeks’ gestation, the patient was admitted to the hospital with back and pelvic pain and vaginal bleeding. Shortly after admission, the ObGyn performed a vaginal examination and ordered ultrasonography (US), which showed that the fetus was in the transverse position and the membranes were bulging.
The ObGyn performed an emergency cesarean delivery, but the premature infant died within 2 hours.
PARENTS’ CLAIM The ObGyn should have performed a cervical cerclage because of the mother’s history of an incompetent cervix. The mother should have been placed on bed rest and monitored every 2 weeks for cervical dilation.
PHYSICIAN’S DEFENSE The patient underwent regular prenatal evaluations for an incompetent cervix, and the findings were always normal.
VERDICT A Florida defense verdict was returned.
Related article:
A stepwise approach to cervical cerclage. Katrin Karl, MD; Michael Katz, MD (Surgical Technique; June 2012)
ObGyn unresponsive to patient’s postsurgical phone calls
In 2009, a 50-year-old woman reported occasional right lower quadrant pain to her ObGyn. US results were normal. The menopausal patient’s history included three cesarean deliveries, a total abdominal hysterectomy, and a laparoscopic ovarian cystectomy.
When the patient saw her ObGyn in December 2010, she reported intermittent, progressive right lower quadrant pain that radiated down her right leg. She also reported urine loss with coughing or sneezing, and slight pain on intercourse. The ObGyn prescribed oxybutynin chloride (Ditropan) to treat the patient’s incontinence.
Three weeks later, the patient reported bilateral lower quadrant pain to her ObGyn, with minor improvement in incontinence.
The ObGyn performed bilateral salpingo-oophorectomy (BSO) in January 2011. Surgery took 3.5 hours due to extensive adhesiolysis.
After discharge, the patient felt ill and vomited. She attempted to reach the ObGyn by phone several times. That evening, the ObGyn prescribed a suppository to treat nausea and vomiting.
The patient went to the ED later that night and was found to have a perforated colon. Emergency surgery to repair the injury included creation of a colostomy, which was repaired 20 months later.
PATIENT’S CLAIM A proper workup of her symptoms was not performed; BSO was unnecessary. The ObGyn was negligent for failing to respond in a timely manner to her post-discharge phone calls, and did not properly evaluate her postoperative symptoms.
PHYSICIAN’S DEFENSE BSO was warranted. Colon injury is a known complication of the procedure.
VERDICT A $716,976 California verdict was returned, but was reduced to $591,967 under the state cap.
Who delayed delivery? $32.8M verdict for child with CP
An 18-year-old woman at 38 weeks’ gestation went to the hospital in labor. After 3.5 hours, the fetal heart rate dropped to 60 bpm. A nurse repositioned the patient, administered oxygen, and increased intravenous fluids. When the nurse rang the emergency call bell, a second nurse responded. Eighteen minutes after the fetal heart rate first dropped, a nurse rang the call bell again and the on-call ObGyn appeared.
The ObGyn performed a vaginal examination and repositioned the patient. She noted that the fetal heart-rate monitor was not working correctly, and called for an emergency cesarean delivery. The baby was born 42 minutes after the fetal heart rate initially dropped.
The child received a diagnosis of spastic-quadriplegia cerebral palsy (CP). She requires a wheelchair and has severe speech deficits and developmental delays.
PARENT’S CLAIM Cesarean delivery was not performed in a timely manner; the delivery delay was responsible for the injury that caused CP. The ObGyn was negligent in not responding to the initial emergency call. The nurses should have summoned the ObGyn earlier.
DEFENDANTS’ DEFENSE The hospital argued that the nurses followed proper protocol. Furthermore, the hospital noted that the ObGyn did not respond to the first call, and did not request a cesarean delivery for 17 minutes.
The ObGyn claimed that she made the decision to perform cesarean delivery within 5 minutes of her arrival, but it took another 15 minutes to gather the surgical team.
VERDICT A $32,882,860 Pennsylvania verdict was returned against the hospital. The ObGyn was vindicated.
DIFFICULT DELIVERY: ZAVANELLI MANEUVER
At 38 5/7 weeks’ gestation, a woman went to the hospital for induction of labor. Twenty-four hours later, she began to push. After an hour of pushing, the mother was exhausted and had a low-grade fever, and the fetal heart rate was slowing. Her ObGyn, Dr. A, attempted vacuum extraction and performed a midline episiotomy. Shoulder dystocia was encountered and maneuvers were used, but without success. Another ObGyn, Dr. B, arrived to assist and also attempted the maneuvers.
The physicians agreed to try the Zavanelli maneuver, which involves pushing the baby’s head back inside the vagina and performing a cesarean delivery.
The baby was sent to the neonatal intensive care unit, where her breathing quickly normalized without supplemental oxygen. The child has a brachial plexus injury.
PARENTS’ CLAIM Dr. A should have performed an earlier cesarean delivery. Excessive traction was used when shoulder dystocia maneuvers were attempted.
PHYSICIANS’ DEFENSE The ObGyns’ actions saved the baby’s life and prevented serious injury to both mother and baby.
VERDICT An Alabama defense verdict was returned.
Related article:
You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial; May 2013)
PLACENTA PREVIA FOUND EARLY, BUT FETUS DIES
A woman's first pregnancy was complicated by complete placenta previa. A cesarean delivery was scheduled at 36 weeks’ gestation. However, before that date, the mother developed vaginal bleeding and was taken to the ED. The covering ObGyn was notified of the mother’s arrival within 15 minutes, but did not come to the hospital for 2.5 hours. After examining her, the ObGyn ordered US evaluation and transferred the mother to the obstetric floor. Nursing notes indicate that the fetal heart rate was 120 bpm at that time.
There are no notes from the ObGyn between 5:30 am and mid-afternoon. There is no record of the fetal heart rate when the mother was taken for US in the afternoon, which revealed fetal demise and a large extraovular hematoma. A cesarean delivery was performed. It was determined that the fetus died from placental abruption.
PARENTS’ CLAIM The mother was not adequately evaluated and monitored, which led to fetal demise. Delivery could have proceeded while the fetus was still alive.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $495,000 Massachusetts settlement was reached.
Related articles:
• What is the optimal time to deliver a woman who has placenta previa? John T. Repke, MD (Examining the Evidence; April 2011)
• Act fast when confronted by a coagulopathy postpartum. Robert L. Barbieri, MD (Editorial; March 2012)
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
TELL US WHAT YOU THINK! Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state. Stay in touch! Your feedback is important to us!
Blue towel left in abdomen: $7.2M verdict
When a 61-year-old woman underwent laparoscopic hysterectomy, her gynecologist, Dr. A, was assisted by another gynecologist (Dr. B), a nurse, and a technician. When Dr. A noted that the uterine artery had been injured, he converted to an open procedure, retracted the bowel, repaired the artery, and completed the operation.
Postdischarge, the patient was febrile and developed abdominal pain and an odorous vaginal discharge. A month later, exploratory surgery revealed a retained blue towel that had been used for bowel retraction. The patient required open healing of the surgical wound and a temporary colostomy. She developed an incisional hernia after colostomy reversal, and hernia repair required resection of a small portion of the bowel.
PATIENT’S CLAIM It was negligent to use a blue towel to retract the bowel. The towel should have been removed from her abdomen before closure.
DEFENDANTS’ DEFENSE The technician claimed that she did not provide the towel, did not see the towel used, and that she was not told that the towel had to be tracked. She noted that its color indicated that it lacked a radiopaque tag, and that hospital policy forbade use of untagged towels in an open wound.
Dr. A claimed that he specifically requested a blue towel because it was absorbent, that the technician provided the towel, and that the towel’s use prevented the patient from bleeding to death.
VERDICT A $7.2 million New York verdict was returned against both gynecologists and the hospital as the technician’s employer.
MISCARRIAGE AFTER D&C
A few days after a woman thought she miscarried, her family practi-tioner (FP) performed a dilation and curettage (D&C).
The patient was at work 12 days later when she expelled a fully formed 14-week fetus into a toilet. She was taken to the emergency department (ED), where the cord was cut. Later that day, she passed placental tissue; a repeat D&C was performed the next day.
PATIENT'S CLAIM The FP did not properly perform the first D&C. Although the pathology report was available to the FP prior to the patient’s postoperative visit, the FP failed to inform the patient that no fetal parts had been extracted.
PHYSICIAN’S DEFENSE Because the FP thought that the fetus had been passed prior to the D&C, she believed the pathology report was appropriate.
The patient had been informed of the possibility of retained products of conception after the D&C. The FP had ordered a blood pregnancy test that would have revealed the presence of retained products of conception, but the patient did not have the test. The patient did not contact the FP to report symptoms that felt like labor pains on the day that she passed the fetus.
VERDICT A bench trial resulted in a $51,000 California verdict.
PREGNANT WOMAN COMPLAINS OF LEG PAIN; DIES OF DVT
A 23-year-old woman went to the ED with pain and swelling in her lower left leg and calf. The symptoms were reported to her ObGyn, who examined and then discharged her within a few hours, with instructions to come for her regularly scheduled prenatal visit.
The patient died 2 weeks later. The cause of death was determined to be a pulmonary embolus from a thrombus of the left popliteal vein.
ESTATE’S CLAIM The ObGyn was negligent in failing to test the patient for thrombosis in her left leg when she was in the ED or several days later at the office, when she continued to report leg pain.
PHYSICIAN’S DEFENSE The patient did not have signs of thrombosis at the ED or at the subsequent office visit. The pathologist reported that the clot that caused the embolus appeared fresh. The ObGyn surmised that it had formed after the patient’s last appointment.
VERDICT A Texas defense verdict was returned.
Mother took topiramate; child born with cleft lip and palate: $3M verdict
When a woman learned she was pregnant in December 2007, she was taking topiramate (Topamax) to treat migraine headaches. She discussed tapering off but not discontinuing topiramate usage with her neurologist. The patient’s ObGyn told her that topiramate was safe to take during pregnancy. The child was born with a cleft lip and palate.
PARENTS’ CLAIM Janssen Pharmaceuticals, manufacturer of Topamax, failed to provide adequate warnings about the potential risks associated with Topamax until labeling was changed in March 2011. Janssen knew of potential birth defects associated with Topamax use during pregnancy more than a decade before the labeling change; Janssen’s associate director of regulatory affairs had testified in an earlier hearing that there was knowledge of related birth defects as early as 1996.
DEFENDANTS’ DEFENSE There is uncertainty as to whether exposure to Topamax during pregnancy causes birth defects. The neurologist had warned the patient of possible risks associated with taking Topamax during pregnancy, but the patient had refused to discontinue the drug.
VERDICT A $3 million Pennsylvania verdict was returned.
Related articles:
• Is it time to rethink the use of oral contraceptives in premenopausal women with migraine? Anne H. Calhoun, MD (Audiocast; October 2013)
• How to choose a contraceptive for a patient who has headaches. Kristina M. Tocce, MD; Stephanie B. Teal, MD, MPH (February 2011)
• The gynecologist’s role in managing menstrual migraine. Anne H. Calhoun, MD (April 2010)
WAS MOTHER’S HISTORY OF INCOMPETENT CERVIX IGNORED?
Early in her second pregnancy, a woman told her ObGyn that she had previously miscarried due to an incompetent cervix.
At 24 weeks’ gestation, the patient was admitted to the hospital with back and pelvic pain and vaginal bleeding. Shortly after admission, the ObGyn performed a vaginal examination and ordered ultrasonography (US), which showed that the fetus was in the transverse position and the membranes were bulging.
The ObGyn performed an emergency cesarean delivery, but the premature infant died within 2 hours.
PARENTS’ CLAIM The ObGyn should have performed a cervical cerclage because of the mother’s history of an incompetent cervix. The mother should have been placed on bed rest and monitored every 2 weeks for cervical dilation.
PHYSICIAN’S DEFENSE The patient underwent regular prenatal evaluations for an incompetent cervix, and the findings were always normal.
VERDICT A Florida defense verdict was returned.
Related article:
A stepwise approach to cervical cerclage. Katrin Karl, MD; Michael Katz, MD (Surgical Technique; June 2012)
ObGyn unresponsive to patient’s postsurgical phone calls
In 2009, a 50-year-old woman reported occasional right lower quadrant pain to her ObGyn. US results were normal. The menopausal patient’s history included three cesarean deliveries, a total abdominal hysterectomy, and a laparoscopic ovarian cystectomy.
When the patient saw her ObGyn in December 2010, she reported intermittent, progressive right lower quadrant pain that radiated down her right leg. She also reported urine loss with coughing or sneezing, and slight pain on intercourse. The ObGyn prescribed oxybutynin chloride (Ditropan) to treat the patient’s incontinence.
Three weeks later, the patient reported bilateral lower quadrant pain to her ObGyn, with minor improvement in incontinence.
The ObGyn performed bilateral salpingo-oophorectomy (BSO) in January 2011. Surgery took 3.5 hours due to extensive adhesiolysis.
After discharge, the patient felt ill and vomited. She attempted to reach the ObGyn by phone several times. That evening, the ObGyn prescribed a suppository to treat nausea and vomiting.
The patient went to the ED later that night and was found to have a perforated colon. Emergency surgery to repair the injury included creation of a colostomy, which was repaired 20 months later.
PATIENT’S CLAIM A proper workup of her symptoms was not performed; BSO was unnecessary. The ObGyn was negligent for failing to respond in a timely manner to her post-discharge phone calls, and did not properly evaluate her postoperative symptoms.
PHYSICIAN’S DEFENSE BSO was warranted. Colon injury is a known complication of the procedure.
VERDICT A $716,976 California verdict was returned, but was reduced to $591,967 under the state cap.
Who delayed delivery? $32.8M verdict for child with CP
An 18-year-old woman at 38 weeks’ gestation went to the hospital in labor. After 3.5 hours, the fetal heart rate dropped to 60 bpm. A nurse repositioned the patient, administered oxygen, and increased intravenous fluids. When the nurse rang the emergency call bell, a second nurse responded. Eighteen minutes after the fetal heart rate first dropped, a nurse rang the call bell again and the on-call ObGyn appeared.
The ObGyn performed a vaginal examination and repositioned the patient. She noted that the fetal heart-rate monitor was not working correctly, and called for an emergency cesarean delivery. The baby was born 42 minutes after the fetal heart rate initially dropped.
The child received a diagnosis of spastic-quadriplegia cerebral palsy (CP). She requires a wheelchair and has severe speech deficits and developmental delays.
PARENT’S CLAIM Cesarean delivery was not performed in a timely manner; the delivery delay was responsible for the injury that caused CP. The ObGyn was negligent in not responding to the initial emergency call. The nurses should have summoned the ObGyn earlier.
DEFENDANTS’ DEFENSE The hospital argued that the nurses followed proper protocol. Furthermore, the hospital noted that the ObGyn did not respond to the first call, and did not request a cesarean delivery for 17 minutes.
The ObGyn claimed that she made the decision to perform cesarean delivery within 5 minutes of her arrival, but it took another 15 minutes to gather the surgical team.
VERDICT A $32,882,860 Pennsylvania verdict was returned against the hospital. The ObGyn was vindicated.
DIFFICULT DELIVERY: ZAVANELLI MANEUVER
At 38 5/7 weeks’ gestation, a woman went to the hospital for induction of labor. Twenty-four hours later, she began to push. After an hour of pushing, the mother was exhausted and had a low-grade fever, and the fetal heart rate was slowing. Her ObGyn, Dr. A, attempted vacuum extraction and performed a midline episiotomy. Shoulder dystocia was encountered and maneuvers were used, but without success. Another ObGyn, Dr. B, arrived to assist and also attempted the maneuvers.
The physicians agreed to try the Zavanelli maneuver, which involves pushing the baby’s head back inside the vagina and performing a cesarean delivery.
The baby was sent to the neonatal intensive care unit, where her breathing quickly normalized without supplemental oxygen. The child has a brachial plexus injury.
PARENTS’ CLAIM Dr. A should have performed an earlier cesarean delivery. Excessive traction was used when shoulder dystocia maneuvers were attempted.
PHYSICIANS’ DEFENSE The ObGyns’ actions saved the baby’s life and prevented serious injury to both mother and baby.
VERDICT An Alabama defense verdict was returned.
Related article:
You are the second responder to a shoulder dystocia emergency. What do you do first? Robert L. Barbieri, MD (Editorial; May 2013)
PLACENTA PREVIA FOUND EARLY, BUT FETUS DIES
A woman's first pregnancy was complicated by complete placenta previa. A cesarean delivery was scheduled at 36 weeks’ gestation. However, before that date, the mother developed vaginal bleeding and was taken to the ED. The covering ObGyn was notified of the mother’s arrival within 15 minutes, but did not come to the hospital for 2.5 hours. After examining her, the ObGyn ordered US evaluation and transferred the mother to the obstetric floor. Nursing notes indicate that the fetal heart rate was 120 bpm at that time.
There are no notes from the ObGyn between 5:30 am and mid-afternoon. There is no record of the fetal heart rate when the mother was taken for US in the afternoon, which revealed fetal demise and a large extraovular hematoma. A cesarean delivery was performed. It was determined that the fetus died from placental abruption.
PARENTS’ CLAIM The mother was not adequately evaluated and monitored, which led to fetal demise. Delivery could have proceeded while the fetus was still alive.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $495,000 Massachusetts settlement was reached.
Related articles:
• What is the optimal time to deliver a woman who has placenta previa? John T. Repke, MD (Examining the Evidence; April 2011)
• Act fast when confronted by a coagulopathy postpartum. Robert L. Barbieri, MD (Editorial; March 2012)
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
TELL US WHAT YOU THINK! Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, city and state. Stay in touch! Your feedback is important to us!
Contemporary management of small renal tumors
The incidence of kidney cancer in the United States is rising because the increased use of cross-sectional imaging is resulting in more tumors being detected and because the population is aging. In addition, a stage migration in kidney cancer has been observed, again because of improved detection, with an increase in stage T1 tumors and a concomitant decrease in the number of stage T2 to T4 tumors. Recent studies have shown that up to 80% of small renal tumors (SRTs) either have an indolent course or are histologically benign. These findings raise the question of what the optimal management of SRTs should be. Radical nephrectomy, the traditional, most aggressive, and still most frequently used extirpative surgery, has been shown to increase the risk of chronic kidney disease. Therefore, during the past 2 decades there has been a shift toward nephron-sparing surgery in carefully selected patients as such procedures have demonstrated equivalent oncologic outcomes with a decrease in long-term renal-induced morbidities.
Click on the PDF icon at the top of this introduction to read the full article.
The incidence of kidney cancer in the United States is rising because the increased use of cross-sectional imaging is resulting in more tumors being detected and because the population is aging. In addition, a stage migration in kidney cancer has been observed, again because of improved detection, with an increase in stage T1 tumors and a concomitant decrease in the number of stage T2 to T4 tumors. Recent studies have shown that up to 80% of small renal tumors (SRTs) either have an indolent course or are histologically benign. These findings raise the question of what the optimal management of SRTs should be. Radical nephrectomy, the traditional, most aggressive, and still most frequently used extirpative surgery, has been shown to increase the risk of chronic kidney disease. Therefore, during the past 2 decades there has been a shift toward nephron-sparing surgery in carefully selected patients as such procedures have demonstrated equivalent oncologic outcomes with a decrease in long-term renal-induced morbidities.
Click on the PDF icon at the top of this introduction to read the full article.
The incidence of kidney cancer in the United States is rising because the increased use of cross-sectional imaging is resulting in more tumors being detected and because the population is aging. In addition, a stage migration in kidney cancer has been observed, again because of improved detection, with an increase in stage T1 tumors and a concomitant decrease in the number of stage T2 to T4 tumors. Recent studies have shown that up to 80% of small renal tumors (SRTs) either have an indolent course or are histologically benign. These findings raise the question of what the optimal management of SRTs should be. Radical nephrectomy, the traditional, most aggressive, and still most frequently used extirpative surgery, has been shown to increase the risk of chronic kidney disease. Therefore, during the past 2 decades there has been a shift toward nephron-sparing surgery in carefully selected patients as such procedures have demonstrated equivalent oncologic outcomes with a decrease in long-term renal-induced morbidities.
Click on the PDF icon at the top of this introduction to read the full article.
Take caution: Look for DISTURBED behaviors when you assess violence risk
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impusivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be aware of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings—2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impusivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be aware of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings—2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
A common misconception is that persons who are mentally ill are inherently dangerous. However, there is, at most, a weak overall relationship between mental illness and violence. Increased violence is more likely to occur during periods of acute psychiatric symptoms.1 Because few patients evaluated in most clinical settings will commit a violent act, it is important to assess for specific risk factors for violence to guide clinical decision making.
The acronym DISTURBED can be a reminder about important patient-specific features that correlate with violence. There are several variables to consider when identifying persons who are more likely to commit acts of violence.2
Demographics. Young age, male sex, cognitive deficits, less formal education, unemployment, financial hardship, and homelessness are associated with an increased risk of violence. A person’s living environment and ongoing social circumstances are important considerations when assessing violence risk.
Impusivity. Persons who display impulsive behaviors generally are more likely to behave violently. This is particularly true in persons who have been given a diagnosis of antisocial personality disorder or borderline personality disorder. Impulsivity often can be treated with medication, behavioral therapy, and other psychotherapeutic modalities.
Substance use is associated with an increased risk of violence in people with and without other mental health issues. Alcohol can increase the likelihood of violence through intoxication, withdrawal, or brain changes related to chronic drinking. Some illicit drugs are associated with violence, including phencyclidine, cocaine, methamphetamine, inhalants, anabolic steroids, and so-called bath salts. Be cautious when treating a patient who is intoxicated with one or more of these substances.
Threats. Persons who express a threat are more likely to behave violently3; those who voice threats against an identified target should be taken seriously. The more specific the threat, the more consideration it should be given. In a clinical setting, the potential target should be informed as soon as possible about the threat. If a patient is voicing a threat against a person outside the clinical setting, you may have a duty to protect by reporting that threat to law enforcement.
Untreated psychosis. Be aware of patients who have untreated or undertreated symptoms, including psychosis and substance intoxication. Patients in a triage setting or who are newly admitted to an inpatient unit often present the greatest risk because their symptoms have not been treated. People with paranoid delusions are at a higher risk of assaulting their perceived persecutors. Those who are highly disorganized also are more prone to lash out and commit a violent act.4,5
Repeat violence. The best predictor of violence is a history of violence. The severity of the violent acts is an important consideration. Even a person who has only a single known) past violent act can pose a high risk if the act was murder, rape, or another highly violent assault. Learning details about past assaults, through reviewing available records or gathering collateral information, is important when assessing violence risk.
Behaviors. There are physical warning signs that often are observed immediately before a person commits a violent act. Potential warning signs include: punching a wall or breaking objects; tightening of facial muscles; clenching of fists; and pacing. These behaviors suggest a risk of imminent violence and should be closely monitored when assessing a patient who might be prone to violence. If a patient does not respond to redirection, he (she) may require staff intervention.
Eagerness. Much like when assessing the risk of suicide, intent is an important consideration in assessing the risk of violence. A person who is eager to commit an act of violence presents significant risk. Basic inquiries about homicidal ideation are insufficient; instead, explore potential responses to situations that might have a direct impact on the individual patient. For example, if the patient has had frequent disagreements with a family member, inquiring about hypothetical violent scenarios involving that family member would be valuable.
Distress. Persons who are concerned about safety often are inclined to lash out in perceived self-defense. For example, fear often is reported by psychiatric inpatients immediately before they commit an act of violence. In inpatient psychiatric units, providing a quiet room, or a similar amenity, can help prevent an assault by a patient who feels cornered or afraid. The staff can ease patients’ concerns by taking a calm and caring approach to addressing their needs.
Valuable tool for maintaining a safe environment
We recommend that clinicians—especially those who have little clinical experience (medical students, residents)—refer to this mnemonic before starting work in emergency and inpatient psychiatric settings—2 settings in which assessment of violence risk is common. The mnemonic will help when gathering information to assess important risk factors for violence.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
1. Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55(5):393-401.
2. Tardiff K. Clinical risk assessment of violence. In: Simon RI, Tardiff K, eds. Textbook of violence assessment and management. Arlington, VA: American Psychiatric Publishing, Inc; 2008:3-16.
3. Maier GJ. Managing threatening behavior. The role of talk down and talk up. J Psychosoc Nurs Ment Health Serv. 1996;34(6):25-30.
4. McNiel DE, Binder RL. The relationship between acute psychiatric symptoms, diagnosis, and short-term risk of violence. Hosp Community Psychiatry. 1994;45(2): 133-137.
5. Krakowski M, Czobor P, Chou JC. Course of violence in patients with schizophrenia: relationship to clinical symptoms. Schizophr Bull. 1999;25(3):505-517.
A diverted or stolen prescription has been signed in your name. What do you do now?
For a busy clinician, learning that a prescription pad has been stolen, sub-mitted with a counterfeit signature, and used to acquire a controlled substance comes as a shock. It evokes a sense of betrayal and raises a number of medico-legal issues that can be avoided if you know how to protect yourself.
Prescription pad security
One of the simplest ways to reduce prescription pad theft is to lock the pads in a secure location when the office is closed.1 Establish and maintain an inventory of prescription pads; you should number and count pads weekly. For Schedule-II controlled substance prescription pads, document the control number on each new pad.1 The best way to ensure that all pads are accounted for is by using sequential numbering similar to bank check numbers.
Do not allow staff to sign your prescription pad. Limit access to prescription pads to authorized personnel; be sure that they keep the prescription pad in their pocket, not on their desk or a counter, and not in examining rooms, where they could be stolen. For electronic prescribing, always lock the drawer where the computer prescription paper sits.1
Some physicians might find it helpful to invest in tamper-resistant prescription pads. As of April 2008, the Centers for Medicare and Medicaid Services mandates that for a prescription pad to be considered tamper-resistant it must include 1 or more industry-recognized features designed to prevent unauthorized copying, erasure, or modification of prescriptions.2
When you order prescription pads, do not print your Drug Enforcement Administration (DEA) number on the pads. Also, check that your printer maintains strict process controls over prescription pad production, storage, and delivery.1
Other ways to prevent fraudulent prescriptions include using a gel pen to write prescriptions, because these pens contain pigments that are quickly absorbed, preventing ink from being washed away with chemical solvents.1 Never leave blank space on a written prescription and do not sign blank prescription pads beforehand.3 Write instructions clearly on each prescription, informing pharmacists of ways to verify the prescription’s authenticity.
Legal responsibilities
In case your prescription pads are stolen, even after taking precautionary measures, make the following actions to report and record fraudulent charges:
• If your prescription pads for Schedule-II medications—known as “triplicates”— are missing, give the control number of the first and last prescription in the pad to your state’s pharmacy organization. Some states have an electronic alert system to aid with filing a fraud claim (eg, the Texas Pharmacy Association has a section on its Web site for reporting prescription fraud and theft).
• Immediately inform the local police department and local DEA office of the theft.3 Keep a copy of all communications for future reference.
• If a pharmacy alerts you that a fraudulent prescription has been filled using one of your pads, request a copy of each filled prescription. Keep these records and file a copy with the police department and DEA.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Seven helpful tips to improve prescription security in your medical practice. Standard Register Healthcare. http:// www.standardregister. com/securescrip/guide-to-prescription-pad-security. asp. Accessed June 6, 2012.
2. Guide to tamper-resistant Rx pads. Standard Register Healthcare. http://www. securescrip.com/guide-to-tamper-resistant-rx-pads. asp. Accessed June 6, 2012.
3. U.S. Department of Justice. Drug Enforcement Administration. Office of Diversion Control. Practitioner’s manual. Section III – security requirements. http://www. deadiversion.usdoj.gov/pubs/ manuals/pract/section3. htm. Accessed June 6, 2012.
For a busy clinician, learning that a prescription pad has been stolen, sub-mitted with a counterfeit signature, and used to acquire a controlled substance comes as a shock. It evokes a sense of betrayal and raises a number of medico-legal issues that can be avoided if you know how to protect yourself.
Prescription pad security
One of the simplest ways to reduce prescription pad theft is to lock the pads in a secure location when the office is closed.1 Establish and maintain an inventory of prescription pads; you should number and count pads weekly. For Schedule-II controlled substance prescription pads, document the control number on each new pad.1 The best way to ensure that all pads are accounted for is by using sequential numbering similar to bank check numbers.
Do not allow staff to sign your prescription pad. Limit access to prescription pads to authorized personnel; be sure that they keep the prescription pad in their pocket, not on their desk or a counter, and not in examining rooms, where they could be stolen. For electronic prescribing, always lock the drawer where the computer prescription paper sits.1
Some physicians might find it helpful to invest in tamper-resistant prescription pads. As of April 2008, the Centers for Medicare and Medicaid Services mandates that for a prescription pad to be considered tamper-resistant it must include 1 or more industry-recognized features designed to prevent unauthorized copying, erasure, or modification of prescriptions.2
When you order prescription pads, do not print your Drug Enforcement Administration (DEA) number on the pads. Also, check that your printer maintains strict process controls over prescription pad production, storage, and delivery.1
Other ways to prevent fraudulent prescriptions include using a gel pen to write prescriptions, because these pens contain pigments that are quickly absorbed, preventing ink from being washed away with chemical solvents.1 Never leave blank space on a written prescription and do not sign blank prescription pads beforehand.3 Write instructions clearly on each prescription, informing pharmacists of ways to verify the prescription’s authenticity.
Legal responsibilities
In case your prescription pads are stolen, even after taking precautionary measures, make the following actions to report and record fraudulent charges:
• If your prescription pads for Schedule-II medications—known as “triplicates”— are missing, give the control number of the first and last prescription in the pad to your state’s pharmacy organization. Some states have an electronic alert system to aid with filing a fraud claim (eg, the Texas Pharmacy Association has a section on its Web site for reporting prescription fraud and theft).
• Immediately inform the local police department and local DEA office of the theft.3 Keep a copy of all communications for future reference.
• If a pharmacy alerts you that a fraudulent prescription has been filled using one of your pads, request a copy of each filled prescription. Keep these records and file a copy with the police department and DEA.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
For a busy clinician, learning that a prescription pad has been stolen, sub-mitted with a counterfeit signature, and used to acquire a controlled substance comes as a shock. It evokes a sense of betrayal and raises a number of medico-legal issues that can be avoided if you know how to protect yourself.
Prescription pad security
One of the simplest ways to reduce prescription pad theft is to lock the pads in a secure location when the office is closed.1 Establish and maintain an inventory of prescription pads; you should number and count pads weekly. For Schedule-II controlled substance prescription pads, document the control number on each new pad.1 The best way to ensure that all pads are accounted for is by using sequential numbering similar to bank check numbers.
Do not allow staff to sign your prescription pad. Limit access to prescription pads to authorized personnel; be sure that they keep the prescription pad in their pocket, not on their desk or a counter, and not in examining rooms, where they could be stolen. For electronic prescribing, always lock the drawer where the computer prescription paper sits.1
Some physicians might find it helpful to invest in tamper-resistant prescription pads. As of April 2008, the Centers for Medicare and Medicaid Services mandates that for a prescription pad to be considered tamper-resistant it must include 1 or more industry-recognized features designed to prevent unauthorized copying, erasure, or modification of prescriptions.2
When you order prescription pads, do not print your Drug Enforcement Administration (DEA) number on the pads. Also, check that your printer maintains strict process controls over prescription pad production, storage, and delivery.1
Other ways to prevent fraudulent prescriptions include using a gel pen to write prescriptions, because these pens contain pigments that are quickly absorbed, preventing ink from being washed away with chemical solvents.1 Never leave blank space on a written prescription and do not sign blank prescription pads beforehand.3 Write instructions clearly on each prescription, informing pharmacists of ways to verify the prescription’s authenticity.
Legal responsibilities
In case your prescription pads are stolen, even after taking precautionary measures, make the following actions to report and record fraudulent charges:
• If your prescription pads for Schedule-II medications—known as “triplicates”— are missing, give the control number of the first and last prescription in the pad to your state’s pharmacy organization. Some states have an electronic alert system to aid with filing a fraud claim (eg, the Texas Pharmacy Association has a section on its Web site for reporting prescription fraud and theft).
• Immediately inform the local police department and local DEA office of the theft.3 Keep a copy of all communications for future reference.
• If a pharmacy alerts you that a fraudulent prescription has been filled using one of your pads, request a copy of each filled prescription. Keep these records and file a copy with the police department and DEA.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Seven helpful tips to improve prescription security in your medical practice. Standard Register Healthcare. http:// www.standardregister. com/securescrip/guide-to-prescription-pad-security. asp. Accessed June 6, 2012.
2. Guide to tamper-resistant Rx pads. Standard Register Healthcare. http://www. securescrip.com/guide-to-tamper-resistant-rx-pads. asp. Accessed June 6, 2012.
3. U.S. Department of Justice. Drug Enforcement Administration. Office of Diversion Control. Practitioner’s manual. Section III – security requirements. http://www. deadiversion.usdoj.gov/pubs/ manuals/pract/section3. htm. Accessed June 6, 2012.
1. Seven helpful tips to improve prescription security in your medical practice. Standard Register Healthcare. http:// www.standardregister. com/securescrip/guide-to-prescription-pad-security. asp. Accessed June 6, 2012.
2. Guide to tamper-resistant Rx pads. Standard Register Healthcare. http://www. securescrip.com/guide-to-tamper-resistant-rx-pads. asp. Accessed June 6, 2012.
3. U.S. Department of Justice. Drug Enforcement Administration. Office of Diversion Control. Practitioner’s manual. Section III – security requirements. http://www. deadiversion.usdoj.gov/pubs/ manuals/pract/section3. htm. Accessed June 6, 2012.
Opportunities to partner with clinical pharmacists in ambulatory psychiatry
In this article, we highlight key steps that were needed to integrate clinical pharmacy specialists at an academic ambulatory psychiatric and addiction treatment center that serves pediatric and adult populations. Academic stakeholders identified addition of pharmacy services as a strategic goal in an effort to maximize services offered by the center and increase patient access to care while aligning with the standards set out by the patient-centered medical home (PCMH) model.
We outline the role of clinical pharmacists in the care of adult patients in ambulatory psychiatry, illustrate opportunities to enhance patient care, point out possible challenges with implementation, and propose future initiatives to optimize the practitioner-pharmacist partnership.
Background: Role of ambulatory pharmacists in psychiatry
Clinical pharmacists’ role in the psychiatric ambulatory care setting generally is associated with positive outcomes. One study looking at a collaborative care model that utilized clinical pharmacist follow-up in managing major depressive disorder found that patients who received pharmacist intervention in the collaborative care model had, on average, a significantly higher adherence rate and patient satisfaction score than the “usual care” group.1 Within this study, patients in both groups experienced global clinical improvement with no significant difference; however, pharmacist interventions had a positive impact on several aspects of the care model, suggesting that pharmacists can be used effectively in ambulatory psychiatry.
Furthermore, a systematic study evaluating pharmacists’ impact on clinical and functional mental health outcomes identified 8 relevant studies conducted in the outpatient setting.2 Although interventions varied widely, most studies focused on pharmacists’ providing a combination of drug monitoring, treatment recommendations, and patient education. Outcomes were largely positive, including an overall reduction in number and dosage of psychiatric drugs, inferred cost savings, and significant improvements in the safe and efficacious use of antidepressant and antipsychotic medications.
These preliminary positive results require replication in larger, randomized cohorts. Additionally, the role of the pharmacist as medication manager in the collaborative care model requires further study. Results so far, however, indicate that pharmacists can have a positive impact on the care of ambulatory psychiatry patients. Nevertheless, there is still considerable need for ongoing exploration in this field.
Pre-implementation
The need for pharmacy services. Various initiatives and existing practices within our health care system have underscored the need for a psychiatric pharmacist in the outpatient setting (Table 1).
A board-certified psychiatric pharmacist (BCPP) possesses specialized knowledge about treating patients affected by psychiatric illnesses. BCPPs work with prescribers and members of other disciplines, such as nurses and social workers, to optimize drug treatment by making pharmacotherapeutic recommendations and providing appropriate monitoring to enhance patient satisfaction and quality of life.3,4
Existing relationship with pharmacy. Along with evidence to support the positive impact clinical pharmacists can have in caring for patients with mental illness in the outpatient setting, a strong existing relationship between the Department of Psychiatry and our adult inpatient psychiatric pharmacist helped make it possible to develop an ambulatory psychiatric pharmacist position.
Each day, the inpatient psychiatric pharmacist works closely with the attending psychiatrists and psychiatry residents to provide treatment recommendations and counseling services for patients on the unit. The psychiatry residents highly valued their experiences with the pharmacist in the inpatient setting and expressed disappointment that this collaborative relationship was no longer available after they transitioned into the ambulatory setting.
Further, by being involved in initiatives that were relevant to both inpatient and outpatient psychiatry, such as metabolic monitoring for patients taking atypical antipsychotics, the clinical pharmacist in inpatient psychiatry had the opportunity to interact with key stakeholders in both settings. As a result of these pre-existing collaborative relationships, many clinicians were eager to have pharmacists available as a resource for patient care in the outpatient setting.
Pharmacist perspective: Outreach to psychiatry leadershipRecognizing the incentives and opportunities inherent in our emerging health care system, pharmacists became integral members of the patient care team in the PCMH model. Thanks to this effort, we now have PCMH pharmacists at every primary care health center in our health system (14 sites), providing disease management programs and polypharmacy services.
PCMH pharmacists’ role in the primary care setting fueled interest from specialty services and created opportunities to extend our existing partnership in inpatient psychiatry. One such opportunity to demonstrate the expertise of a psychiatric pharmacist was fueled by the FDA’s citalopram dosing alert5 at a system-wide level. This warning emerged as a chance to showcase the skill set of psychiatric pharmacists and the pharmacists’ successes in our PCMH model. The partnership was extended to include the buy-in of ambulatory pharmacy leadership and key stakeholders in ambulatory psychiatry.
Initial meetings included ambulatory care site leadership in psychiatry to increase awareness and understanding of pharmacists’ potential role in direct patient care. Achieving site leadership support was critical to successful implementation of pharmacist services in psychiatry. We also obtained approval from the Chair of the Department of Psychiatry to elicit support from faculty group practice.
Psychiatry leadership perspective
As fiscal pressures intensify at academic health centers, it becomes increasingly important for resources to be used as efficiently and effectively as possible. As a greater percentage of mental health patients with more “straightforward,” less complex conditions are being managed by their primary care providers or nonprescribing psychotherapists, or both, the acuity and complexity of cases in patients who present to psychiatric clinics have intensified. This intensification of patient needs and clinical acuity is in heightened conflict with the ongoing demand for clinician productivity and efficiency.
Additionally, the need to provide care to a seemingly ever-growing number of moderately or severely ill patients during shorter, less frequent visits presents a daunting task for clinicians and clinical leaders. Collaborative care models appear to offer the best hope for managing the seemingly overwhelming demand for services.
In this model, the patient, who is the critical member of the team, is expected to become an “expert” on his or her illness and to partner with members of the multidisciplinary team; with this support, patients are encouraged to develop a broad range of self-management skills and strategies to manage their illness. We believe that clinical pharmacists can and should play a critical role, not only in delivering direct clinical services to patients but also in developing and devising the care models that will most effectively apply each team member’s unique set of knowledge, skills, and experience. Given the large percentage of our patients who have multiple medical comorbidities and who require complex medical and psychiatric medication regimens, the role of the pharmacist in reviewing, educating, and advising patients and other team members on these crucial pharmacy concerns will be paramount.
In light of these complex medication issues, pharmacists are uniquely positioned to serve as a liaison among the patient, the primary care provider, and other members of their treatment team. We anticipate that our ambulatory psychiatry pharmacists will greatly enhance the comfort and confidence of patients and their primary care providers during periods of care transition.
Potential roles for pharmacists in ambulatory psychiatry
One potential role for pharmacists in ambulatory psychiatry is to perform polypharmacy assessments of patients receiving complex medication regimens, prompted by physician referral. The poly pharmacy intake interview, performed to obtain an accurate medication list and to identify patients’ concerns about their medications, can be conducted in person or by telephone. Patients’ knowledge about medications and medication adherence are discussed, as are their perceptions of effectiveness and adverse effects.
After initial data gathering, pharmacists complete a review of the medications, identifying any problems associated with medication indication, efficacy, tolerance, or adverse effects, drug-drug interactions, drug-nutrient interactions, and nonadherence. Pharmacists work to reduce medication costs if that is a concern of the patient, because nonadherence can result. A medication care plan is then developed in consultation with the primary care provider; here, the medication list is reconciled, the electronic medical record is updated, and actions to address any medication-related problems are prioritized.
Other services that might be offered include:• group education classes, based on patient motivational interviewing strategies, to address therapeutic nonadherence and to improve understanding of their disease and treatment regimens• medication safety and monitoring• treatment intensification, as needed, following established protocols.
These are a few of the ways in which pharmacists can be relied on to expand and improve access to patient care services within ambulatory psychiatry. Key stakeholders anticipate development of newer ideas as the pharmacist’s role in ambulatory psychiatry is increasingly clarified.
Reimbursement model
In creating a role for pharmacy in ambulatory psychiatry, it was essential that the model be financially viable and appealing. Alongside its clinical model, our institution has developed a financial model to support the pharmacist’s role. The lump-sum payment to the health centers from Blue Cross Blue Shield of Michigan afforded the ambulatory care clinics an opportunity to invest in PCMH pharmacists. This funding, and the reimbursement based on T-code billing (face-to-face visits and phone consultation) for depression and other conditions requiring chronic care, provides ongoing support. From our experience, understanding physician reimbursement models and identifying relevant changes in health care reform are necessary to integrate new providers, including pharmacists, into a team-based care model.
Implementation
Promoting pharmacy services. To foster anticipated collaboration with clinical pharmacists, the medical director of outpatient psychiatry disseminated an announcement to all providers regarding the investiture of clinical pharmacists to support patient care activities, education, and research. Clinicians were educated about the pharmacists’ potential roles and about guidelines and methods for referral. Use of our electronic health record system enabled us to establish a relatively simple referral process involving sharing electronic messages with our pharmacists.
Further, as part of the planned integration of clinical pharmacists in the ambulatory psychiatry setting, pharmacists met strategically with members of various disciplines, clinical programs, specialty clinic programs, and teams throughout the center. In addition to answering questions about the referral process, they emphasized the role of pharmacy and opportunities for collaboration.
Collaborating with others. Because the involvement of clinical pharmacists is unfamiliar to some practitioners in outpatient psychiatry, it is important to develop services without infringing on the roles other disciplines play. Indeed, a survey by Wheeler et al6 identified many concerns and potential boundaries among pharmacists, other providers, and patients. Concerns included confusion of practitioner roles and boundaries, a too-traditional perception of the pharmacist, and demonstration of competence.6
Early on, we developed a structured forum to discuss ongoing challenges and address issues related to the rapidly changing clinical landscape. During these discussions we conveyed that adding pharmacists to psychiatric services would be collaborative in nature and intended to augment existing services. This communication was pivotal to maintain the psychiatrist’s role as the ultimate prescriber and authority in the care of their patients; however, the pharmacist’s expertise, when sought, would help spur clinical and academic discussion that will benefit the patient. These discussions are paramount to achieving a productive, team-based approach, to overcome challenges, and to identify opportunities of value to our providers and patients (Box).
Work in progress
Implementing change in any clinical setting invariably creates challenges, and our endeavors to integrate clinical pharmacists into ambulatory psychiatry are no exception. We have identified several factors that we believe will optimize successful collaboration between pharmacy and ambulatory psychiatry (Table 2). Our primary challenge has been changing clinician behavior. Clinical practitioners can become too comfortable, wedded to their routines, and often are understandably resistant to change. Additionally, clinical systems often are inadvertently designed to obstruct change in ways that are not readily apparent. Efforts must be focused on behaviors and practices the clinical culture should encourage.
Regarding specific initiatives, clinical pharmacists have successfully identified patients on higher than recommended dosages of citalopram; they are working alongside prescribers to recommend ways to minimize the risk of heart rhythm abnormalities in these patients. Numerous prescribers have sought clinical pharmacists’ input to manage pharmacotherapy in their patients and to respond to patients’ questions on drug information.
The prospect of access to clinical pharmacist expertise in the outpatient setting was heralded with excitement, but the flow of referrals and consultations has been uneven. However simple the path for referral is, clinicians’ use of the system has been inconsistent—perhaps because of referrals’ passive, clinician-dependent nature. Educational outreach efforts often prompt a brief spike in referrals, only to be followed over time by a slow, steady drop-off. More active strategies will be needed, such as embedding the pharmacists as regular, active, visible members of the various clinical teams, and implementing a system in which patient record reviews are assigned to the pharmacists according to agreed-upon clusters of clinical criteria.
One of these tactics has, in the short term, showed success. Embedded in one of our newer clinics, which were designed to bridge primary and psychiatric care, clinical pharmacists are helping manage medically complicated patients. They assist with medication selection in light of drug interactions and medical comorbidities, conduct detailed medication histories, schedule follow-up visits to assess medication adherence and tolerability, and counsel patients experiencing insurance changes that make their medications less affordable. Integrating pharmacists in the new clinics has resulted in a steady flow of patient referrals and collaborative care work.
Clinical pharmacists are brainstorming with outpatient psychiatry leadership to build on these early successes. Ongoing communication and enhanced collaboration are essential, and can only improve the lives of our psychiatric patients.
For the future
Our partnership in ambulatory psychiatry was timed to occur during implementation of our health system’s new electronic health record initiative. Clinical pharmacists can play a key role in demonstrating use of the system to provide consistently accurate drug information to patients and to monitor patients receiving specific medications.
Development of ambulatory patient medication education groups, which has proved useful on the inpatient side, is another endeavor in the works. Integrating the clinical pharmacist with psychiatrists, psychologists, nurse practitioners, social workers, and trainees on specific teams devoted to depression, bipolar disorder, anxiety, perinatal mental health, and personality disorders also might prove to be a wide-ranging and promising strategy.
Enhancing the education and training experiences of residents, fellows, medical students, pharmacy students, and allied health professional learners present in our clinics is another exciting prospect. This cross-disciplinary training will yield a new generation of providers who will be more comfortable collaborating with colleagues from other disciplines, all intent on providing high-quality, efficient care. We hope that, as these initiatives take root, we will recognize many opportunities to disseminate our collaborative efforts in scholarly venues, documenting and sharing the positive impact of our partnership.
Bottom Line
Because psychiatric outpatients present with challenging medical comorbidities and increasingly complex medication regimens, specialized clinical pharmacists can enrich the management team by offering essential monitoring and polypharmacy services, patient education and counseling, and cross-discipline training. At one academic treatment center, psychiatric and non-psychiatric practitioners are gradually buying in to these promising collaborative efforts.
Related Resources
• Board of Pharmacy Specialties. www.bpsweb.org/specialties/psychiatric.cfm.
• Abramowitz P. Ambulatory care pharmacy practice: The future is now. www.connect.ashp.org/blogs/paul-abramowitz/2014/05/14/ambulatory-care-pharmacy-practice-the-future-is-now.
Drug Brand Name
Citalopram • Celexa
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Finley PR, Rens HR, Pont JT, et al. Impact of a collaborative care model on depression in a primary care setting: a randomized controlled trial. Pharmacotherapy. 2003;23(9):1175-1185.
2. Finley PR, Crismon ML, Rush AJ. Evaluating the impact of pharmacists in mental health: a systematic review. Pharmacotherapy. 2003;23(12):1634-1644.
3. Board of Pharmacy Specialties. http://www.bpsweb. org. Accessed June 4, 2014.
4. Cohen LJ. The role of neuropsychiatric pharmacists. J Clin Psychiatry. 1999;60(suppl 19):54-57.
5. U.S. Food and Drug Administration. FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). http://www.fda.gov/Drugs/DrugSafety/ucm297391. htm. Accessed June 4, 2014.
6. Wheeler A, Crump K, Lee M, et al. Collaborative prescribing: a qualitative exploration of a role for pharmacists in mental health. Res Social Adm Pharm. 2012;8(3):179-192.
In this article, we highlight key steps that were needed to integrate clinical pharmacy specialists at an academic ambulatory psychiatric and addiction treatment center that serves pediatric and adult populations. Academic stakeholders identified addition of pharmacy services as a strategic goal in an effort to maximize services offered by the center and increase patient access to care while aligning with the standards set out by the patient-centered medical home (PCMH) model.
We outline the role of clinical pharmacists in the care of adult patients in ambulatory psychiatry, illustrate opportunities to enhance patient care, point out possible challenges with implementation, and propose future initiatives to optimize the practitioner-pharmacist partnership.
Background: Role of ambulatory pharmacists in psychiatry
Clinical pharmacists’ role in the psychiatric ambulatory care setting generally is associated with positive outcomes. One study looking at a collaborative care model that utilized clinical pharmacist follow-up in managing major depressive disorder found that patients who received pharmacist intervention in the collaborative care model had, on average, a significantly higher adherence rate and patient satisfaction score than the “usual care” group.1 Within this study, patients in both groups experienced global clinical improvement with no significant difference; however, pharmacist interventions had a positive impact on several aspects of the care model, suggesting that pharmacists can be used effectively in ambulatory psychiatry.
Furthermore, a systematic study evaluating pharmacists’ impact on clinical and functional mental health outcomes identified 8 relevant studies conducted in the outpatient setting.2 Although interventions varied widely, most studies focused on pharmacists’ providing a combination of drug monitoring, treatment recommendations, and patient education. Outcomes were largely positive, including an overall reduction in number and dosage of psychiatric drugs, inferred cost savings, and significant improvements in the safe and efficacious use of antidepressant and antipsychotic medications.
These preliminary positive results require replication in larger, randomized cohorts. Additionally, the role of the pharmacist as medication manager in the collaborative care model requires further study. Results so far, however, indicate that pharmacists can have a positive impact on the care of ambulatory psychiatry patients. Nevertheless, there is still considerable need for ongoing exploration in this field.
Pre-implementation
The need for pharmacy services. Various initiatives and existing practices within our health care system have underscored the need for a psychiatric pharmacist in the outpatient setting (Table 1).
A board-certified psychiatric pharmacist (BCPP) possesses specialized knowledge about treating patients affected by psychiatric illnesses. BCPPs work with prescribers and members of other disciplines, such as nurses and social workers, to optimize drug treatment by making pharmacotherapeutic recommendations and providing appropriate monitoring to enhance patient satisfaction and quality of life.3,4
Existing relationship with pharmacy. Along with evidence to support the positive impact clinical pharmacists can have in caring for patients with mental illness in the outpatient setting, a strong existing relationship between the Department of Psychiatry and our adult inpatient psychiatric pharmacist helped make it possible to develop an ambulatory psychiatric pharmacist position.
Each day, the inpatient psychiatric pharmacist works closely with the attending psychiatrists and psychiatry residents to provide treatment recommendations and counseling services for patients on the unit. The psychiatry residents highly valued their experiences with the pharmacist in the inpatient setting and expressed disappointment that this collaborative relationship was no longer available after they transitioned into the ambulatory setting.
Further, by being involved in initiatives that were relevant to both inpatient and outpatient psychiatry, such as metabolic monitoring for patients taking atypical antipsychotics, the clinical pharmacist in inpatient psychiatry had the opportunity to interact with key stakeholders in both settings. As a result of these pre-existing collaborative relationships, many clinicians were eager to have pharmacists available as a resource for patient care in the outpatient setting.
Pharmacist perspective: Outreach to psychiatry leadershipRecognizing the incentives and opportunities inherent in our emerging health care system, pharmacists became integral members of the patient care team in the PCMH model. Thanks to this effort, we now have PCMH pharmacists at every primary care health center in our health system (14 sites), providing disease management programs and polypharmacy services.
PCMH pharmacists’ role in the primary care setting fueled interest from specialty services and created opportunities to extend our existing partnership in inpatient psychiatry. One such opportunity to demonstrate the expertise of a psychiatric pharmacist was fueled by the FDA’s citalopram dosing alert5 at a system-wide level. This warning emerged as a chance to showcase the skill set of psychiatric pharmacists and the pharmacists’ successes in our PCMH model. The partnership was extended to include the buy-in of ambulatory pharmacy leadership and key stakeholders in ambulatory psychiatry.
Initial meetings included ambulatory care site leadership in psychiatry to increase awareness and understanding of pharmacists’ potential role in direct patient care. Achieving site leadership support was critical to successful implementation of pharmacist services in psychiatry. We also obtained approval from the Chair of the Department of Psychiatry to elicit support from faculty group practice.
Psychiatry leadership perspective
As fiscal pressures intensify at academic health centers, it becomes increasingly important for resources to be used as efficiently and effectively as possible. As a greater percentage of mental health patients with more “straightforward,” less complex conditions are being managed by their primary care providers or nonprescribing psychotherapists, or both, the acuity and complexity of cases in patients who present to psychiatric clinics have intensified. This intensification of patient needs and clinical acuity is in heightened conflict with the ongoing demand for clinician productivity and efficiency.
Additionally, the need to provide care to a seemingly ever-growing number of moderately or severely ill patients during shorter, less frequent visits presents a daunting task for clinicians and clinical leaders. Collaborative care models appear to offer the best hope for managing the seemingly overwhelming demand for services.
In this model, the patient, who is the critical member of the team, is expected to become an “expert” on his or her illness and to partner with members of the multidisciplinary team; with this support, patients are encouraged to develop a broad range of self-management skills and strategies to manage their illness. We believe that clinical pharmacists can and should play a critical role, not only in delivering direct clinical services to patients but also in developing and devising the care models that will most effectively apply each team member’s unique set of knowledge, skills, and experience. Given the large percentage of our patients who have multiple medical comorbidities and who require complex medical and psychiatric medication regimens, the role of the pharmacist in reviewing, educating, and advising patients and other team members on these crucial pharmacy concerns will be paramount.
In light of these complex medication issues, pharmacists are uniquely positioned to serve as a liaison among the patient, the primary care provider, and other members of their treatment team. We anticipate that our ambulatory psychiatry pharmacists will greatly enhance the comfort and confidence of patients and their primary care providers during periods of care transition.
Potential roles for pharmacists in ambulatory psychiatry
One potential role for pharmacists in ambulatory psychiatry is to perform polypharmacy assessments of patients receiving complex medication regimens, prompted by physician referral. The poly pharmacy intake interview, performed to obtain an accurate medication list and to identify patients’ concerns about their medications, can be conducted in person or by telephone. Patients’ knowledge about medications and medication adherence are discussed, as are their perceptions of effectiveness and adverse effects.
After initial data gathering, pharmacists complete a review of the medications, identifying any problems associated with medication indication, efficacy, tolerance, or adverse effects, drug-drug interactions, drug-nutrient interactions, and nonadherence. Pharmacists work to reduce medication costs if that is a concern of the patient, because nonadherence can result. A medication care plan is then developed in consultation with the primary care provider; here, the medication list is reconciled, the electronic medical record is updated, and actions to address any medication-related problems are prioritized.
Other services that might be offered include:• group education classes, based on patient motivational interviewing strategies, to address therapeutic nonadherence and to improve understanding of their disease and treatment regimens• medication safety and monitoring• treatment intensification, as needed, following established protocols.
These are a few of the ways in which pharmacists can be relied on to expand and improve access to patient care services within ambulatory psychiatry. Key stakeholders anticipate development of newer ideas as the pharmacist’s role in ambulatory psychiatry is increasingly clarified.
Reimbursement model
In creating a role for pharmacy in ambulatory psychiatry, it was essential that the model be financially viable and appealing. Alongside its clinical model, our institution has developed a financial model to support the pharmacist’s role. The lump-sum payment to the health centers from Blue Cross Blue Shield of Michigan afforded the ambulatory care clinics an opportunity to invest in PCMH pharmacists. This funding, and the reimbursement based on T-code billing (face-to-face visits and phone consultation) for depression and other conditions requiring chronic care, provides ongoing support. From our experience, understanding physician reimbursement models and identifying relevant changes in health care reform are necessary to integrate new providers, including pharmacists, into a team-based care model.
Implementation
Promoting pharmacy services. To foster anticipated collaboration with clinical pharmacists, the medical director of outpatient psychiatry disseminated an announcement to all providers regarding the investiture of clinical pharmacists to support patient care activities, education, and research. Clinicians were educated about the pharmacists’ potential roles and about guidelines and methods for referral. Use of our electronic health record system enabled us to establish a relatively simple referral process involving sharing electronic messages with our pharmacists.
Further, as part of the planned integration of clinical pharmacists in the ambulatory psychiatry setting, pharmacists met strategically with members of various disciplines, clinical programs, specialty clinic programs, and teams throughout the center. In addition to answering questions about the referral process, they emphasized the role of pharmacy and opportunities for collaboration.
Collaborating with others. Because the involvement of clinical pharmacists is unfamiliar to some practitioners in outpatient psychiatry, it is important to develop services without infringing on the roles other disciplines play. Indeed, a survey by Wheeler et al6 identified many concerns and potential boundaries among pharmacists, other providers, and patients. Concerns included confusion of practitioner roles and boundaries, a too-traditional perception of the pharmacist, and demonstration of competence.6
Early on, we developed a structured forum to discuss ongoing challenges and address issues related to the rapidly changing clinical landscape. During these discussions we conveyed that adding pharmacists to psychiatric services would be collaborative in nature and intended to augment existing services. This communication was pivotal to maintain the psychiatrist’s role as the ultimate prescriber and authority in the care of their patients; however, the pharmacist’s expertise, when sought, would help spur clinical and academic discussion that will benefit the patient. These discussions are paramount to achieving a productive, team-based approach, to overcome challenges, and to identify opportunities of value to our providers and patients (Box).
Work in progress
Implementing change in any clinical setting invariably creates challenges, and our endeavors to integrate clinical pharmacists into ambulatory psychiatry are no exception. We have identified several factors that we believe will optimize successful collaboration between pharmacy and ambulatory psychiatry (Table 2). Our primary challenge has been changing clinician behavior. Clinical practitioners can become too comfortable, wedded to their routines, and often are understandably resistant to change. Additionally, clinical systems often are inadvertently designed to obstruct change in ways that are not readily apparent. Efforts must be focused on behaviors and practices the clinical culture should encourage.
Regarding specific initiatives, clinical pharmacists have successfully identified patients on higher than recommended dosages of citalopram; they are working alongside prescribers to recommend ways to minimize the risk of heart rhythm abnormalities in these patients. Numerous prescribers have sought clinical pharmacists’ input to manage pharmacotherapy in their patients and to respond to patients’ questions on drug information.
The prospect of access to clinical pharmacist expertise in the outpatient setting was heralded with excitement, but the flow of referrals and consultations has been uneven. However simple the path for referral is, clinicians’ use of the system has been inconsistent—perhaps because of referrals’ passive, clinician-dependent nature. Educational outreach efforts often prompt a brief spike in referrals, only to be followed over time by a slow, steady drop-off. More active strategies will be needed, such as embedding the pharmacists as regular, active, visible members of the various clinical teams, and implementing a system in which patient record reviews are assigned to the pharmacists according to agreed-upon clusters of clinical criteria.
One of these tactics has, in the short term, showed success. Embedded in one of our newer clinics, which were designed to bridge primary and psychiatric care, clinical pharmacists are helping manage medically complicated patients. They assist with medication selection in light of drug interactions and medical comorbidities, conduct detailed medication histories, schedule follow-up visits to assess medication adherence and tolerability, and counsel patients experiencing insurance changes that make their medications less affordable. Integrating pharmacists in the new clinics has resulted in a steady flow of patient referrals and collaborative care work.
Clinical pharmacists are brainstorming with outpatient psychiatry leadership to build on these early successes. Ongoing communication and enhanced collaboration are essential, and can only improve the lives of our psychiatric patients.
For the future
Our partnership in ambulatory psychiatry was timed to occur during implementation of our health system’s new electronic health record initiative. Clinical pharmacists can play a key role in demonstrating use of the system to provide consistently accurate drug information to patients and to monitor patients receiving specific medications.
Development of ambulatory patient medication education groups, which has proved useful on the inpatient side, is another endeavor in the works. Integrating the clinical pharmacist with psychiatrists, psychologists, nurse practitioners, social workers, and trainees on specific teams devoted to depression, bipolar disorder, anxiety, perinatal mental health, and personality disorders also might prove to be a wide-ranging and promising strategy.
Enhancing the education and training experiences of residents, fellows, medical students, pharmacy students, and allied health professional learners present in our clinics is another exciting prospect. This cross-disciplinary training will yield a new generation of providers who will be more comfortable collaborating with colleagues from other disciplines, all intent on providing high-quality, efficient care. We hope that, as these initiatives take root, we will recognize many opportunities to disseminate our collaborative efforts in scholarly venues, documenting and sharing the positive impact of our partnership.
Bottom Line
Because psychiatric outpatients present with challenging medical comorbidities and increasingly complex medication regimens, specialized clinical pharmacists can enrich the management team by offering essential monitoring and polypharmacy services, patient education and counseling, and cross-discipline training. At one academic treatment center, psychiatric and non-psychiatric practitioners are gradually buying in to these promising collaborative efforts.
Related Resources
• Board of Pharmacy Specialties. www.bpsweb.org/specialties/psychiatric.cfm.
• Abramowitz P. Ambulatory care pharmacy practice: The future is now. www.connect.ashp.org/blogs/paul-abramowitz/2014/05/14/ambulatory-care-pharmacy-practice-the-future-is-now.
Drug Brand Name
Citalopram • Celexa
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In this article, we highlight key steps that were needed to integrate clinical pharmacy specialists at an academic ambulatory psychiatric and addiction treatment center that serves pediatric and adult populations. Academic stakeholders identified addition of pharmacy services as a strategic goal in an effort to maximize services offered by the center and increase patient access to care while aligning with the standards set out by the patient-centered medical home (PCMH) model.
We outline the role of clinical pharmacists in the care of adult patients in ambulatory psychiatry, illustrate opportunities to enhance patient care, point out possible challenges with implementation, and propose future initiatives to optimize the practitioner-pharmacist partnership.
Background: Role of ambulatory pharmacists in psychiatry
Clinical pharmacists’ role in the psychiatric ambulatory care setting generally is associated with positive outcomes. One study looking at a collaborative care model that utilized clinical pharmacist follow-up in managing major depressive disorder found that patients who received pharmacist intervention in the collaborative care model had, on average, a significantly higher adherence rate and patient satisfaction score than the “usual care” group.1 Within this study, patients in both groups experienced global clinical improvement with no significant difference; however, pharmacist interventions had a positive impact on several aspects of the care model, suggesting that pharmacists can be used effectively in ambulatory psychiatry.
Furthermore, a systematic study evaluating pharmacists’ impact on clinical and functional mental health outcomes identified 8 relevant studies conducted in the outpatient setting.2 Although interventions varied widely, most studies focused on pharmacists’ providing a combination of drug monitoring, treatment recommendations, and patient education. Outcomes were largely positive, including an overall reduction in number and dosage of psychiatric drugs, inferred cost savings, and significant improvements in the safe and efficacious use of antidepressant and antipsychotic medications.
These preliminary positive results require replication in larger, randomized cohorts. Additionally, the role of the pharmacist as medication manager in the collaborative care model requires further study. Results so far, however, indicate that pharmacists can have a positive impact on the care of ambulatory psychiatry patients. Nevertheless, there is still considerable need for ongoing exploration in this field.
Pre-implementation
The need for pharmacy services. Various initiatives and existing practices within our health care system have underscored the need for a psychiatric pharmacist in the outpatient setting (Table 1).
A board-certified psychiatric pharmacist (BCPP) possesses specialized knowledge about treating patients affected by psychiatric illnesses. BCPPs work with prescribers and members of other disciplines, such as nurses and social workers, to optimize drug treatment by making pharmacotherapeutic recommendations and providing appropriate monitoring to enhance patient satisfaction and quality of life.3,4
Existing relationship with pharmacy. Along with evidence to support the positive impact clinical pharmacists can have in caring for patients with mental illness in the outpatient setting, a strong existing relationship between the Department of Psychiatry and our adult inpatient psychiatric pharmacist helped make it possible to develop an ambulatory psychiatric pharmacist position.
Each day, the inpatient psychiatric pharmacist works closely with the attending psychiatrists and psychiatry residents to provide treatment recommendations and counseling services for patients on the unit. The psychiatry residents highly valued their experiences with the pharmacist in the inpatient setting and expressed disappointment that this collaborative relationship was no longer available after they transitioned into the ambulatory setting.
Further, by being involved in initiatives that were relevant to both inpatient and outpatient psychiatry, such as metabolic monitoring for patients taking atypical antipsychotics, the clinical pharmacist in inpatient psychiatry had the opportunity to interact with key stakeholders in both settings. As a result of these pre-existing collaborative relationships, many clinicians were eager to have pharmacists available as a resource for patient care in the outpatient setting.
Pharmacist perspective: Outreach to psychiatry leadershipRecognizing the incentives and opportunities inherent in our emerging health care system, pharmacists became integral members of the patient care team in the PCMH model. Thanks to this effort, we now have PCMH pharmacists at every primary care health center in our health system (14 sites), providing disease management programs and polypharmacy services.
PCMH pharmacists’ role in the primary care setting fueled interest from specialty services and created opportunities to extend our existing partnership in inpatient psychiatry. One such opportunity to demonstrate the expertise of a psychiatric pharmacist was fueled by the FDA’s citalopram dosing alert5 at a system-wide level. This warning emerged as a chance to showcase the skill set of psychiatric pharmacists and the pharmacists’ successes in our PCMH model. The partnership was extended to include the buy-in of ambulatory pharmacy leadership and key stakeholders in ambulatory psychiatry.
Initial meetings included ambulatory care site leadership in psychiatry to increase awareness and understanding of pharmacists’ potential role in direct patient care. Achieving site leadership support was critical to successful implementation of pharmacist services in psychiatry. We also obtained approval from the Chair of the Department of Psychiatry to elicit support from faculty group practice.
Psychiatry leadership perspective
As fiscal pressures intensify at academic health centers, it becomes increasingly important for resources to be used as efficiently and effectively as possible. As a greater percentage of mental health patients with more “straightforward,” less complex conditions are being managed by their primary care providers or nonprescribing psychotherapists, or both, the acuity and complexity of cases in patients who present to psychiatric clinics have intensified. This intensification of patient needs and clinical acuity is in heightened conflict with the ongoing demand for clinician productivity and efficiency.
Additionally, the need to provide care to a seemingly ever-growing number of moderately or severely ill patients during shorter, less frequent visits presents a daunting task for clinicians and clinical leaders. Collaborative care models appear to offer the best hope for managing the seemingly overwhelming demand for services.
In this model, the patient, who is the critical member of the team, is expected to become an “expert” on his or her illness and to partner with members of the multidisciplinary team; with this support, patients are encouraged to develop a broad range of self-management skills and strategies to manage their illness. We believe that clinical pharmacists can and should play a critical role, not only in delivering direct clinical services to patients but also in developing and devising the care models that will most effectively apply each team member’s unique set of knowledge, skills, and experience. Given the large percentage of our patients who have multiple medical comorbidities and who require complex medical and psychiatric medication regimens, the role of the pharmacist in reviewing, educating, and advising patients and other team members on these crucial pharmacy concerns will be paramount.
In light of these complex medication issues, pharmacists are uniquely positioned to serve as a liaison among the patient, the primary care provider, and other members of their treatment team. We anticipate that our ambulatory psychiatry pharmacists will greatly enhance the comfort and confidence of patients and their primary care providers during periods of care transition.
Potential roles for pharmacists in ambulatory psychiatry
One potential role for pharmacists in ambulatory psychiatry is to perform polypharmacy assessments of patients receiving complex medication regimens, prompted by physician referral. The poly pharmacy intake interview, performed to obtain an accurate medication list and to identify patients’ concerns about their medications, can be conducted in person or by telephone. Patients’ knowledge about medications and medication adherence are discussed, as are their perceptions of effectiveness and adverse effects.
After initial data gathering, pharmacists complete a review of the medications, identifying any problems associated with medication indication, efficacy, tolerance, or adverse effects, drug-drug interactions, drug-nutrient interactions, and nonadherence. Pharmacists work to reduce medication costs if that is a concern of the patient, because nonadherence can result. A medication care plan is then developed in consultation with the primary care provider; here, the medication list is reconciled, the electronic medical record is updated, and actions to address any medication-related problems are prioritized.
Other services that might be offered include:• group education classes, based on patient motivational interviewing strategies, to address therapeutic nonadherence and to improve understanding of their disease and treatment regimens• medication safety and monitoring• treatment intensification, as needed, following established protocols.
These are a few of the ways in which pharmacists can be relied on to expand and improve access to patient care services within ambulatory psychiatry. Key stakeholders anticipate development of newer ideas as the pharmacist’s role in ambulatory psychiatry is increasingly clarified.
Reimbursement model
In creating a role for pharmacy in ambulatory psychiatry, it was essential that the model be financially viable and appealing. Alongside its clinical model, our institution has developed a financial model to support the pharmacist’s role. The lump-sum payment to the health centers from Blue Cross Blue Shield of Michigan afforded the ambulatory care clinics an opportunity to invest in PCMH pharmacists. This funding, and the reimbursement based on T-code billing (face-to-face visits and phone consultation) for depression and other conditions requiring chronic care, provides ongoing support. From our experience, understanding physician reimbursement models and identifying relevant changes in health care reform are necessary to integrate new providers, including pharmacists, into a team-based care model.
Implementation
Promoting pharmacy services. To foster anticipated collaboration with clinical pharmacists, the medical director of outpatient psychiatry disseminated an announcement to all providers regarding the investiture of clinical pharmacists to support patient care activities, education, and research. Clinicians were educated about the pharmacists’ potential roles and about guidelines and methods for referral. Use of our electronic health record system enabled us to establish a relatively simple referral process involving sharing electronic messages with our pharmacists.
Further, as part of the planned integration of clinical pharmacists in the ambulatory psychiatry setting, pharmacists met strategically with members of various disciplines, clinical programs, specialty clinic programs, and teams throughout the center. In addition to answering questions about the referral process, they emphasized the role of pharmacy and opportunities for collaboration.
Collaborating with others. Because the involvement of clinical pharmacists is unfamiliar to some practitioners in outpatient psychiatry, it is important to develop services without infringing on the roles other disciplines play. Indeed, a survey by Wheeler et al6 identified many concerns and potential boundaries among pharmacists, other providers, and patients. Concerns included confusion of practitioner roles and boundaries, a too-traditional perception of the pharmacist, and demonstration of competence.6
Early on, we developed a structured forum to discuss ongoing challenges and address issues related to the rapidly changing clinical landscape. During these discussions we conveyed that adding pharmacists to psychiatric services would be collaborative in nature and intended to augment existing services. This communication was pivotal to maintain the psychiatrist’s role as the ultimate prescriber and authority in the care of their patients; however, the pharmacist’s expertise, when sought, would help spur clinical and academic discussion that will benefit the patient. These discussions are paramount to achieving a productive, team-based approach, to overcome challenges, and to identify opportunities of value to our providers and patients (Box).
Work in progress
Implementing change in any clinical setting invariably creates challenges, and our endeavors to integrate clinical pharmacists into ambulatory psychiatry are no exception. We have identified several factors that we believe will optimize successful collaboration between pharmacy and ambulatory psychiatry (Table 2). Our primary challenge has been changing clinician behavior. Clinical practitioners can become too comfortable, wedded to their routines, and often are understandably resistant to change. Additionally, clinical systems often are inadvertently designed to obstruct change in ways that are not readily apparent. Efforts must be focused on behaviors and practices the clinical culture should encourage.
Regarding specific initiatives, clinical pharmacists have successfully identified patients on higher than recommended dosages of citalopram; they are working alongside prescribers to recommend ways to minimize the risk of heart rhythm abnormalities in these patients. Numerous prescribers have sought clinical pharmacists’ input to manage pharmacotherapy in their patients and to respond to patients’ questions on drug information.
The prospect of access to clinical pharmacist expertise in the outpatient setting was heralded with excitement, but the flow of referrals and consultations has been uneven. However simple the path for referral is, clinicians’ use of the system has been inconsistent—perhaps because of referrals’ passive, clinician-dependent nature. Educational outreach efforts often prompt a brief spike in referrals, only to be followed over time by a slow, steady drop-off. More active strategies will be needed, such as embedding the pharmacists as regular, active, visible members of the various clinical teams, and implementing a system in which patient record reviews are assigned to the pharmacists according to agreed-upon clusters of clinical criteria.
One of these tactics has, in the short term, showed success. Embedded in one of our newer clinics, which were designed to bridge primary and psychiatric care, clinical pharmacists are helping manage medically complicated patients. They assist with medication selection in light of drug interactions and medical comorbidities, conduct detailed medication histories, schedule follow-up visits to assess medication adherence and tolerability, and counsel patients experiencing insurance changes that make their medications less affordable. Integrating pharmacists in the new clinics has resulted in a steady flow of patient referrals and collaborative care work.
Clinical pharmacists are brainstorming with outpatient psychiatry leadership to build on these early successes. Ongoing communication and enhanced collaboration are essential, and can only improve the lives of our psychiatric patients.
For the future
Our partnership in ambulatory psychiatry was timed to occur during implementation of our health system’s new electronic health record initiative. Clinical pharmacists can play a key role in demonstrating use of the system to provide consistently accurate drug information to patients and to monitor patients receiving specific medications.
Development of ambulatory patient medication education groups, which has proved useful on the inpatient side, is another endeavor in the works. Integrating the clinical pharmacist with psychiatrists, psychologists, nurse practitioners, social workers, and trainees on specific teams devoted to depression, bipolar disorder, anxiety, perinatal mental health, and personality disorders also might prove to be a wide-ranging and promising strategy.
Enhancing the education and training experiences of residents, fellows, medical students, pharmacy students, and allied health professional learners present in our clinics is another exciting prospect. This cross-disciplinary training will yield a new generation of providers who will be more comfortable collaborating with colleagues from other disciplines, all intent on providing high-quality, efficient care. We hope that, as these initiatives take root, we will recognize many opportunities to disseminate our collaborative efforts in scholarly venues, documenting and sharing the positive impact of our partnership.
Bottom Line
Because psychiatric outpatients present with challenging medical comorbidities and increasingly complex medication regimens, specialized clinical pharmacists can enrich the management team by offering essential monitoring and polypharmacy services, patient education and counseling, and cross-discipline training. At one academic treatment center, psychiatric and non-psychiatric practitioners are gradually buying in to these promising collaborative efforts.
Related Resources
• Board of Pharmacy Specialties. www.bpsweb.org/specialties/psychiatric.cfm.
• Abramowitz P. Ambulatory care pharmacy practice: The future is now. www.connect.ashp.org/blogs/paul-abramowitz/2014/05/14/ambulatory-care-pharmacy-practice-the-future-is-now.
Drug Brand Name
Citalopram • Celexa
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Finley PR, Rens HR, Pont JT, et al. Impact of a collaborative care model on depression in a primary care setting: a randomized controlled trial. Pharmacotherapy. 2003;23(9):1175-1185.
2. Finley PR, Crismon ML, Rush AJ. Evaluating the impact of pharmacists in mental health: a systematic review. Pharmacotherapy. 2003;23(12):1634-1644.
3. Board of Pharmacy Specialties. http://www.bpsweb. org. Accessed June 4, 2014.
4. Cohen LJ. The role of neuropsychiatric pharmacists. J Clin Psychiatry. 1999;60(suppl 19):54-57.
5. U.S. Food and Drug Administration. FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). http://www.fda.gov/Drugs/DrugSafety/ucm297391. htm. Accessed June 4, 2014.
6. Wheeler A, Crump K, Lee M, et al. Collaborative prescribing: a qualitative exploration of a role for pharmacists in mental health. Res Social Adm Pharm. 2012;8(3):179-192.
1. Finley PR, Rens HR, Pont JT, et al. Impact of a collaborative care model on depression in a primary care setting: a randomized controlled trial. Pharmacotherapy. 2003;23(9):1175-1185.
2. Finley PR, Crismon ML, Rush AJ. Evaluating the impact of pharmacists in mental health: a systematic review. Pharmacotherapy. 2003;23(12):1634-1644.
3. Board of Pharmacy Specialties. http://www.bpsweb. org. Accessed June 4, 2014.
4. Cohen LJ. The role of neuropsychiatric pharmacists. J Clin Psychiatry. 1999;60(suppl 19):54-57.
5. U.S. Food and Drug Administration. FDA Drug Safety Communication: Abnormal heart rhythms associated with high doses of Celexa (citalopram hydrobromide). http://www.fda.gov/Drugs/DrugSafety/ucm297391. htm. Accessed June 4, 2014.
6. Wheeler A, Crump K, Lee M, et al. Collaborative prescribing: a qualitative exploration of a role for pharmacists in mental health. Res Social Adm Pharm. 2012;8(3):179-192.