User login
Technology for primary care — terrific, terrifying, or both?
We have all been using technology in our primary care practices for a long time but newer formats have been emerging so fast that our minds, much less our staff’s minds, may be spinning.
Our old friend the telephone, a time-soaking nemesis for scheduling, checking coverage, questions calls, prescribing, quick consults, and follow-up is being replaced by EHR portals and SMS for messaging (e.g. DoctorConnect, SimplePractice), drop-in televisits and patient education links on our websites (e.g. Schmitt Pediatric Care, Remedy Connect), and chatbots for scheduling (e.g. CHEC-UP). While time is saved, what is lost may be hearing the subtext of anxiety or misperceptions in parents’ voices that would change our advice and the empathetic human connection in conversations with our patients. A hybrid approach may be better.
The paper appointment book has been replaced by scheduling systems sometimes lacking in flexibility for double booking, sibling visits, and variable length or extremely valuable multi-professional visits. Allowing patients to book their own visits may place complex problems in inappropriate slots, so only allowing online requests for visits is safer. On the other hand, many of us can now squeeze in “same day” televisits (e.g. Blueberry Pediatrics), sometimes from outside our practice (e.g., zocdoc), to increase payments and even entice new patients to enroll.
Amazing advances in technology are being made in specialty care such as genetic modifications (CRISPR), immunotherapies (mRNA vaccines and AI drug design), robot-assisted surgery, and 3-D printing of body parts and prosthetics. Technology as treatment such as transcranial magnetic stimulation and vagal stimulation are finding value in psychiatry.
But beside being aware of and able to order such specialty technologies, innovations are now extending our senses in primary care such as amplified or visual stethoscopes, bedside ultrasound (e.g. Butterfly), remote visualization (oto-, endo-)scopes, photographic vision screens (e.g. iScreen) for skin lesion (VisualDx) and genetic syndrome facial recognition. We need to be sure that technologies are tested and calibrated for children and different racial groups and genders to provide safe and equitable care. Early adoption may not always be the best approach. Costs of technology, as usual, may limit access to these advanced care aids especially, as usual, in practices serving low income and rural communities.
Patients, especially younger parents and youth, now expect to participate and can directly benefit from technology as part of their health care. Validated parent or self-report screens (e.g. EHRs, Phreesia) can detect important issues early for more effective intervention. Such questionnaires typically provide a pass/fail result or score, but other delivery systems (e.g. CHADIS) include interpretation, assist patients/parents in setting visit priorities and health goals, and even chain results of one questionnaire to secondary screens to hone in on problems, sometimes obviating a time-consuming second visit. Patient-completed comprehensive questionnaires (e.g. Well Visit Planner, CHADIS) allow us time to use our skills to focus on concerns, education, and management rather than asking myriad routine questions. Some (e.g. CHADIS) even create visit documentation reducing our “pajama time” write ups (and burnout); automate repeated online measures to track progress; and use questionnaire results to trigger related patient-specific education and resources rather than the often-ignored generic EHR handouts.
Digital therapeutics such as apps for anxiety (e.g. Calm), depression (e.g. SparkRx, Cass), weight control (e.g. Noom, Lose it), fitness, or sleep tracking (e.g. Whoop) help educate and, in some cases, provide real-time feedback to personalize discovery of contributing factors in order to maintain motivation for positive health behavior change. Some video games improve ADHD symptoms (e.g. EndeavorRX). Virtual reality scenarios have been shown to desensitize those with PTSD and social anxiety or teach social skills to children with autism.
Systems that trigger resource listings (including apps) from screen results can help, but now with over 10,000 apps for mental health, knowing what to recommend for what conditions is a challenge for which ratings (e.g. MINDapps.org) can help. With few product reps visiting to tell us what’s new, we need to read critically about innovations, search the web, subscribe to the AAP SOAPM LISTSERV, visit exhibitors at professional meetings, and talk with peers.
All the digital data collected from health care technology, if assembled with privacy constraints and analyzed with advanced statistical methods, have the possibility, with or without inclusion of genomic data, to allow for more accurate diagnostic and treatment decision support. While AI can search widely for patterns, it needs to be “trained” on appropriate data to make correct conclusions. We are all aware that the history determines 85% of both diagnosis and treatment decisions, particularly in primary care where x-rays or lab tests are not often needed.
But history in EHR notes is often idiosyncratic, entered hours after the visit by the clinician, and does not include the information needed to define diagnostic or guideline criteria, even if the clinician knows and considered those criteria. EHR templates are presented blank and are onerous and time consuming for clinicians. In addition, individual patient barriers to care, preferences, and environmental or subjective concerns are infrequently documented even though they may make the biggest difference to adherence and/or outcomes.
Notes made from voice to text digital AI translation of the encounter (e.g. Nuance DAX) are even less likely to include diagnostic criteria as it would be inappropriate to speak these. To use EHR history data to train AI and to test efficacy of care using variations of guidelines, guideline-related data is needed from online patient entries in questionnaires that are transformed to fill in templates along with some structured choices for clinician entries forming visit notes (e.g. CHADIS). New apps to facilitate clinician documentation of guidelines (e.g. AvoMD) could streamline visits as well as help document guideline criteria. The resulting combination of guideline-relevant patient histories and objective data to test and iteratively refine guidelines will allow a process known as a “Learning Health System.”
Technology to collect this kind of data can allow for the aspirational American Academy of Pediatrics CHILD Registry to approach this goal. Population-level data can provide surveillance for illness, toxins, effects of climate change, social drivers of health, and even effects of technologies themselves such as social media and remote learning so that we can attempt to make the best choices for the future.
Clinicians, staff, and patients will need to develop trust in technology as it infiltrates all aspects of health care. Professionals need both evidence and experience to trust a technology, which takes time and effort. Disinformation in the media may reduce trust or evoke unwarranted trust, as we have all seen regarding vaccines. Clear and coherent public health messaging can help but is no longer a panacea for developing trust in health care. Our nonjudgmental listening and informed opinions are needed more than ever.
The biggest issues for new technology are likely to be the need for workflow adjustments, changing our habit patterns, training, and cost/benefit analyses. With today’s high staff churn, confusion and even chaos can ensue when adopting new technology.
Staff need to be part of the selection process, if at all possible, and discuss how roles and flow will need to change. Having one staff member be a champion and expert for new tech can move adoption to a shared process rather than imposing “one more thing.” It is crucial to discuss the benefits for patients and staff even if the change is required. Sometimes cost savings can include a bonus for staff or free group lunches. Providing a certificate of achievement or title promotion for mastering new tech may be appropriate. Giving some time off from other tasks to learn new workflows can reduce resistance rather than just adding it on to a regular workload. Office “huddles” going forward can include examples of benefits staff have observed or heard about from the adoption. There are quality improvement processes that engage the team — some that earn MOC-4 or CEU credits — that apply to making workflow changes and measuring them iteratively.
If technology takes over important aspects of the work of medical professionals, even if it is faster and/or more accurate, it may degrade clinical observational, interactional, and decision-making skills through lack of use. It may also remove the sense of self-efficacy that motivates professionals to endure onerous training and desire to enter the field. Using technology may reduce empathetic interactions that are basic to humanistic motivation, work satisfaction, and even community respect. Moral injury is already rampant in medicine from restrictions on freedom to do what we see as important for our patients. Technology has great potential and already is enhancing our ability to provide the best care for patients but the risks need to be watched for and ameliorated.
When technology automates comprehensive visit documentation that highlights priority and risk areas from patient input and individualizes decision support, it can facilitate the personalized care that we and our patients want to experience. We must not be so awed, intrigued, or wary of new technology to miss its benefits nor give up our good clinical judgment about the technology or about our patients.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
We have all been using technology in our primary care practices for a long time but newer formats have been emerging so fast that our minds, much less our staff’s minds, may be spinning.
Our old friend the telephone, a time-soaking nemesis for scheduling, checking coverage, questions calls, prescribing, quick consults, and follow-up is being replaced by EHR portals and SMS for messaging (e.g. DoctorConnect, SimplePractice), drop-in televisits and patient education links on our websites (e.g. Schmitt Pediatric Care, Remedy Connect), and chatbots for scheduling (e.g. CHEC-UP). While time is saved, what is lost may be hearing the subtext of anxiety or misperceptions in parents’ voices that would change our advice and the empathetic human connection in conversations with our patients. A hybrid approach may be better.
The paper appointment book has been replaced by scheduling systems sometimes lacking in flexibility for double booking, sibling visits, and variable length or extremely valuable multi-professional visits. Allowing patients to book their own visits may place complex problems in inappropriate slots, so only allowing online requests for visits is safer. On the other hand, many of us can now squeeze in “same day” televisits (e.g. Blueberry Pediatrics), sometimes from outside our practice (e.g., zocdoc), to increase payments and even entice new patients to enroll.
Amazing advances in technology are being made in specialty care such as genetic modifications (CRISPR), immunotherapies (mRNA vaccines and AI drug design), robot-assisted surgery, and 3-D printing of body parts and prosthetics. Technology as treatment such as transcranial magnetic stimulation and vagal stimulation are finding value in psychiatry.
But beside being aware of and able to order such specialty technologies, innovations are now extending our senses in primary care such as amplified or visual stethoscopes, bedside ultrasound (e.g. Butterfly), remote visualization (oto-, endo-)scopes, photographic vision screens (e.g. iScreen) for skin lesion (VisualDx) and genetic syndrome facial recognition. We need to be sure that technologies are tested and calibrated for children and different racial groups and genders to provide safe and equitable care. Early adoption may not always be the best approach. Costs of technology, as usual, may limit access to these advanced care aids especially, as usual, in practices serving low income and rural communities.
Patients, especially younger parents and youth, now expect to participate and can directly benefit from technology as part of their health care. Validated parent or self-report screens (e.g. EHRs, Phreesia) can detect important issues early for more effective intervention. Such questionnaires typically provide a pass/fail result or score, but other delivery systems (e.g. CHADIS) include interpretation, assist patients/parents in setting visit priorities and health goals, and even chain results of one questionnaire to secondary screens to hone in on problems, sometimes obviating a time-consuming second visit. Patient-completed comprehensive questionnaires (e.g. Well Visit Planner, CHADIS) allow us time to use our skills to focus on concerns, education, and management rather than asking myriad routine questions. Some (e.g. CHADIS) even create visit documentation reducing our “pajama time” write ups (and burnout); automate repeated online measures to track progress; and use questionnaire results to trigger related patient-specific education and resources rather than the often-ignored generic EHR handouts.
Digital therapeutics such as apps for anxiety (e.g. Calm), depression (e.g. SparkRx, Cass), weight control (e.g. Noom, Lose it), fitness, or sleep tracking (e.g. Whoop) help educate and, in some cases, provide real-time feedback to personalize discovery of contributing factors in order to maintain motivation for positive health behavior change. Some video games improve ADHD symptoms (e.g. EndeavorRX). Virtual reality scenarios have been shown to desensitize those with PTSD and social anxiety or teach social skills to children with autism.
Systems that trigger resource listings (including apps) from screen results can help, but now with over 10,000 apps for mental health, knowing what to recommend for what conditions is a challenge for which ratings (e.g. MINDapps.org) can help. With few product reps visiting to tell us what’s new, we need to read critically about innovations, search the web, subscribe to the AAP SOAPM LISTSERV, visit exhibitors at professional meetings, and talk with peers.
All the digital data collected from health care technology, if assembled with privacy constraints and analyzed with advanced statistical methods, have the possibility, with or without inclusion of genomic data, to allow for more accurate diagnostic and treatment decision support. While AI can search widely for patterns, it needs to be “trained” on appropriate data to make correct conclusions. We are all aware that the history determines 85% of both diagnosis and treatment decisions, particularly in primary care where x-rays or lab tests are not often needed.
But history in EHR notes is often idiosyncratic, entered hours after the visit by the clinician, and does not include the information needed to define diagnostic or guideline criteria, even if the clinician knows and considered those criteria. EHR templates are presented blank and are onerous and time consuming for clinicians. In addition, individual patient barriers to care, preferences, and environmental or subjective concerns are infrequently documented even though they may make the biggest difference to adherence and/or outcomes.
Notes made from voice to text digital AI translation of the encounter (e.g. Nuance DAX) are even less likely to include diagnostic criteria as it would be inappropriate to speak these. To use EHR history data to train AI and to test efficacy of care using variations of guidelines, guideline-related data is needed from online patient entries in questionnaires that are transformed to fill in templates along with some structured choices for clinician entries forming visit notes (e.g. CHADIS). New apps to facilitate clinician documentation of guidelines (e.g. AvoMD) could streamline visits as well as help document guideline criteria. The resulting combination of guideline-relevant patient histories and objective data to test and iteratively refine guidelines will allow a process known as a “Learning Health System.”
Technology to collect this kind of data can allow for the aspirational American Academy of Pediatrics CHILD Registry to approach this goal. Population-level data can provide surveillance for illness, toxins, effects of climate change, social drivers of health, and even effects of technologies themselves such as social media and remote learning so that we can attempt to make the best choices for the future.
Clinicians, staff, and patients will need to develop trust in technology as it infiltrates all aspects of health care. Professionals need both evidence and experience to trust a technology, which takes time and effort. Disinformation in the media may reduce trust or evoke unwarranted trust, as we have all seen regarding vaccines. Clear and coherent public health messaging can help but is no longer a panacea for developing trust in health care. Our nonjudgmental listening and informed opinions are needed more than ever.
The biggest issues for new technology are likely to be the need for workflow adjustments, changing our habit patterns, training, and cost/benefit analyses. With today’s high staff churn, confusion and even chaos can ensue when adopting new technology.
Staff need to be part of the selection process, if at all possible, and discuss how roles and flow will need to change. Having one staff member be a champion and expert for new tech can move adoption to a shared process rather than imposing “one more thing.” It is crucial to discuss the benefits for patients and staff even if the change is required. Sometimes cost savings can include a bonus for staff or free group lunches. Providing a certificate of achievement or title promotion for mastering new tech may be appropriate. Giving some time off from other tasks to learn new workflows can reduce resistance rather than just adding it on to a regular workload. Office “huddles” going forward can include examples of benefits staff have observed or heard about from the adoption. There are quality improvement processes that engage the team — some that earn MOC-4 or CEU credits — that apply to making workflow changes and measuring them iteratively.
If technology takes over important aspects of the work of medical professionals, even if it is faster and/or more accurate, it may degrade clinical observational, interactional, and decision-making skills through lack of use. It may also remove the sense of self-efficacy that motivates professionals to endure onerous training and desire to enter the field. Using technology may reduce empathetic interactions that are basic to humanistic motivation, work satisfaction, and even community respect. Moral injury is already rampant in medicine from restrictions on freedom to do what we see as important for our patients. Technology has great potential and already is enhancing our ability to provide the best care for patients but the risks need to be watched for and ameliorated.
When technology automates comprehensive visit documentation that highlights priority and risk areas from patient input and individualizes decision support, it can facilitate the personalized care that we and our patients want to experience. We must not be so awed, intrigued, or wary of new technology to miss its benefits nor give up our good clinical judgment about the technology or about our patients.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
We have all been using technology in our primary care practices for a long time but newer formats have been emerging so fast that our minds, much less our staff’s minds, may be spinning.
Our old friend the telephone, a time-soaking nemesis for scheduling, checking coverage, questions calls, prescribing, quick consults, and follow-up is being replaced by EHR portals and SMS for messaging (e.g. DoctorConnect, SimplePractice), drop-in televisits and patient education links on our websites (e.g. Schmitt Pediatric Care, Remedy Connect), and chatbots for scheduling (e.g. CHEC-UP). While time is saved, what is lost may be hearing the subtext of anxiety or misperceptions in parents’ voices that would change our advice and the empathetic human connection in conversations with our patients. A hybrid approach may be better.
The paper appointment book has been replaced by scheduling systems sometimes lacking in flexibility for double booking, sibling visits, and variable length or extremely valuable multi-professional visits. Allowing patients to book their own visits may place complex problems in inappropriate slots, so only allowing online requests for visits is safer. On the other hand, many of us can now squeeze in “same day” televisits (e.g. Blueberry Pediatrics), sometimes from outside our practice (e.g., zocdoc), to increase payments and even entice new patients to enroll.
Amazing advances in technology are being made in specialty care such as genetic modifications (CRISPR), immunotherapies (mRNA vaccines and AI drug design), robot-assisted surgery, and 3-D printing of body parts and prosthetics. Technology as treatment such as transcranial magnetic stimulation and vagal stimulation are finding value in psychiatry.
But beside being aware of and able to order such specialty technologies, innovations are now extending our senses in primary care such as amplified or visual stethoscopes, bedside ultrasound (e.g. Butterfly), remote visualization (oto-, endo-)scopes, photographic vision screens (e.g. iScreen) for skin lesion (VisualDx) and genetic syndrome facial recognition. We need to be sure that technologies are tested and calibrated for children and different racial groups and genders to provide safe and equitable care. Early adoption may not always be the best approach. Costs of technology, as usual, may limit access to these advanced care aids especially, as usual, in practices serving low income and rural communities.
Patients, especially younger parents and youth, now expect to participate and can directly benefit from technology as part of their health care. Validated parent or self-report screens (e.g. EHRs, Phreesia) can detect important issues early for more effective intervention. Such questionnaires typically provide a pass/fail result or score, but other delivery systems (e.g. CHADIS) include interpretation, assist patients/parents in setting visit priorities and health goals, and even chain results of one questionnaire to secondary screens to hone in on problems, sometimes obviating a time-consuming second visit. Patient-completed comprehensive questionnaires (e.g. Well Visit Planner, CHADIS) allow us time to use our skills to focus on concerns, education, and management rather than asking myriad routine questions. Some (e.g. CHADIS) even create visit documentation reducing our “pajama time” write ups (and burnout); automate repeated online measures to track progress; and use questionnaire results to trigger related patient-specific education and resources rather than the often-ignored generic EHR handouts.
Digital therapeutics such as apps for anxiety (e.g. Calm), depression (e.g. SparkRx, Cass), weight control (e.g. Noom, Lose it), fitness, or sleep tracking (e.g. Whoop) help educate and, in some cases, provide real-time feedback to personalize discovery of contributing factors in order to maintain motivation for positive health behavior change. Some video games improve ADHD symptoms (e.g. EndeavorRX). Virtual reality scenarios have been shown to desensitize those with PTSD and social anxiety or teach social skills to children with autism.
Systems that trigger resource listings (including apps) from screen results can help, but now with over 10,000 apps for mental health, knowing what to recommend for what conditions is a challenge for which ratings (e.g. MINDapps.org) can help. With few product reps visiting to tell us what’s new, we need to read critically about innovations, search the web, subscribe to the AAP SOAPM LISTSERV, visit exhibitors at professional meetings, and talk with peers.
All the digital data collected from health care technology, if assembled with privacy constraints and analyzed with advanced statistical methods, have the possibility, with or without inclusion of genomic data, to allow for more accurate diagnostic and treatment decision support. While AI can search widely for patterns, it needs to be “trained” on appropriate data to make correct conclusions. We are all aware that the history determines 85% of both diagnosis and treatment decisions, particularly in primary care where x-rays or lab tests are not often needed.
But history in EHR notes is often idiosyncratic, entered hours after the visit by the clinician, and does not include the information needed to define diagnostic or guideline criteria, even if the clinician knows and considered those criteria. EHR templates are presented blank and are onerous and time consuming for clinicians. In addition, individual patient barriers to care, preferences, and environmental or subjective concerns are infrequently documented even though they may make the biggest difference to adherence and/or outcomes.
Notes made from voice to text digital AI translation of the encounter (e.g. Nuance DAX) are even less likely to include diagnostic criteria as it would be inappropriate to speak these. To use EHR history data to train AI and to test efficacy of care using variations of guidelines, guideline-related data is needed from online patient entries in questionnaires that are transformed to fill in templates along with some structured choices for clinician entries forming visit notes (e.g. CHADIS). New apps to facilitate clinician documentation of guidelines (e.g. AvoMD) could streamline visits as well as help document guideline criteria. The resulting combination of guideline-relevant patient histories and objective data to test and iteratively refine guidelines will allow a process known as a “Learning Health System.”
Technology to collect this kind of data can allow for the aspirational American Academy of Pediatrics CHILD Registry to approach this goal. Population-level data can provide surveillance for illness, toxins, effects of climate change, social drivers of health, and even effects of technologies themselves such as social media and remote learning so that we can attempt to make the best choices for the future.
Clinicians, staff, and patients will need to develop trust in technology as it infiltrates all aspects of health care. Professionals need both evidence and experience to trust a technology, which takes time and effort. Disinformation in the media may reduce trust or evoke unwarranted trust, as we have all seen regarding vaccines. Clear and coherent public health messaging can help but is no longer a panacea for developing trust in health care. Our nonjudgmental listening and informed opinions are needed more than ever.
The biggest issues for new technology are likely to be the need for workflow adjustments, changing our habit patterns, training, and cost/benefit analyses. With today’s high staff churn, confusion and even chaos can ensue when adopting new technology.
Staff need to be part of the selection process, if at all possible, and discuss how roles and flow will need to change. Having one staff member be a champion and expert for new tech can move adoption to a shared process rather than imposing “one more thing.” It is crucial to discuss the benefits for patients and staff even if the change is required. Sometimes cost savings can include a bonus for staff or free group lunches. Providing a certificate of achievement or title promotion for mastering new tech may be appropriate. Giving some time off from other tasks to learn new workflows can reduce resistance rather than just adding it on to a regular workload. Office “huddles” going forward can include examples of benefits staff have observed or heard about from the adoption. There are quality improvement processes that engage the team — some that earn MOC-4 or CEU credits — that apply to making workflow changes and measuring them iteratively.
If technology takes over important aspects of the work of medical professionals, even if it is faster and/or more accurate, it may degrade clinical observational, interactional, and decision-making skills through lack of use. It may also remove the sense of self-efficacy that motivates professionals to endure onerous training and desire to enter the field. Using technology may reduce empathetic interactions that are basic to humanistic motivation, work satisfaction, and even community respect. Moral injury is already rampant in medicine from restrictions on freedom to do what we see as important for our patients. Technology has great potential and already is enhancing our ability to provide the best care for patients but the risks need to be watched for and ameliorated.
When technology automates comprehensive visit documentation that highlights priority and risk areas from patient input and individualizes decision support, it can facilitate the personalized care that we and our patients want to experience. We must not be so awed, intrigued, or wary of new technology to miss its benefits nor give up our good clinical judgment about the technology or about our patients.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Why Are Prion Diseases on the Rise?
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 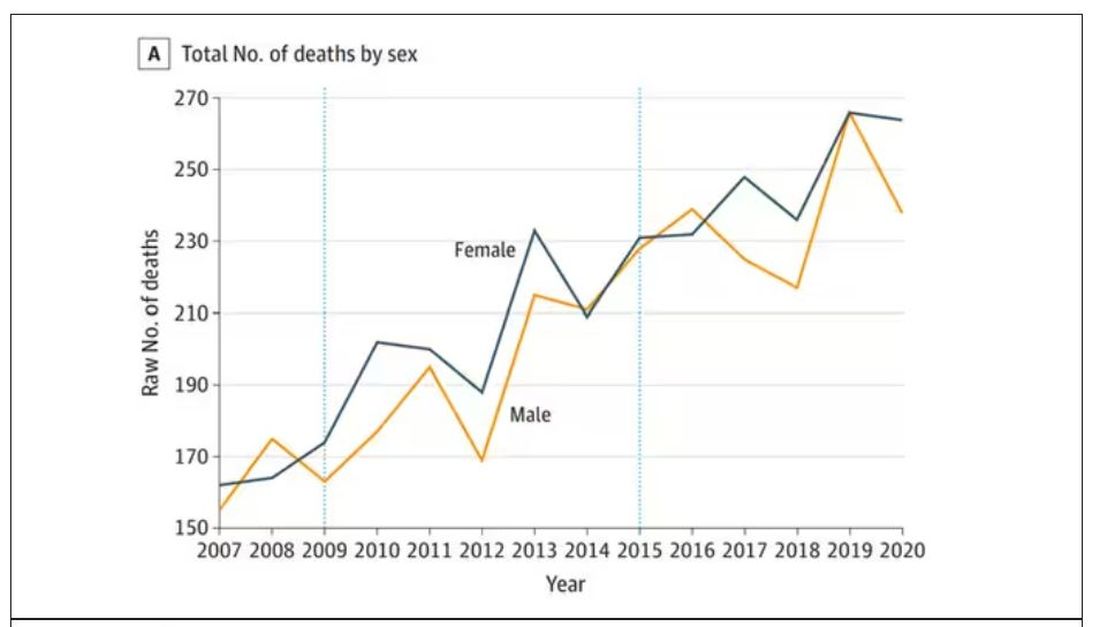
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.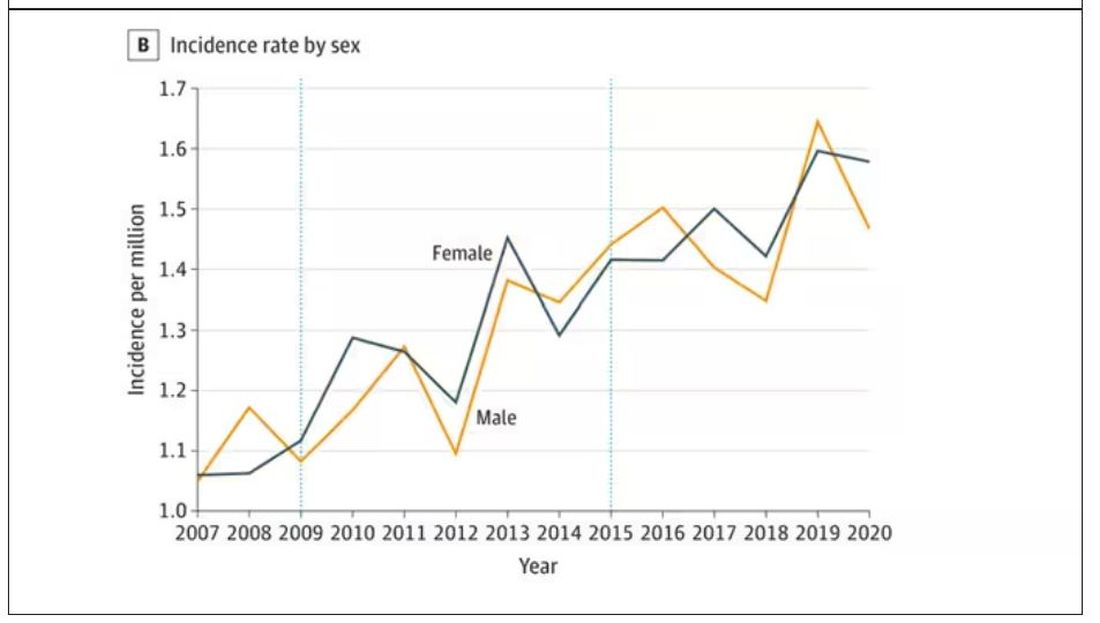
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Can AI enhance mental health treatment?
Three questions for clinicians
Artificial intelligence (AI) is already impacting the mental health care space, with several new tools available to both clinicians and patients. While this technology could be a game-changer amid a mental health crisis and clinician shortage, there are important ethical and efficacy concerns clinicians should be aware of.
Current use cases illustrate both the potential and risks of AI. On one hand, AI has the potential to improve patient care with tools that can support diagnoses and inform treatment decisions at scale. The UK’s National Health Service is using an AI-powered diagnostic tool to help clinicians diagnose mental health disorders and determine the severity of a patient’s needs. Other tools leverage AI to analyze a patient’s voice for signs of depression or anxiety.
On the other hand, there are serious potential risks involving privacy, bias, and misinformation. One chatbot tool designed to counsel patients through disordered eating was shut down after giving problematic weight-loss advice.
The number of AI tools in the healthcare space is expected to increase fivefold by 2035. Keeping up with these advances is just as important for clinicians as keeping up with the latest medication and treatment options. That means being aware of both the limitations and the potential of AI. Here are three questions clinicians can ask as they explore ways to integrate these tools into their practice while navigating the risks.
• How can AI augment, not replace, the work of my staff?
For example, documentation and the use of electronic health records have consistently been linked to clinician burnout. Using AI to cut down on documentation would leave clinicians with more time and energy to focus on patient care.
One study from the National Library of Medicine found that physicians who did not have enough time to complete documentation were nearly three times more likely to report burnout. In some cases, clinic schedules were deliberately shortened to allow time for documentation.
New tools are emerging that use audio recording, transcription services, and large language models to generate clinical summaries and other documentation support. Amazon and 3M have partnered to solve documentation challenges using AI. This is an area I’ll definitely be keeping an eye on as it develops.
• Do I have patient consent to use this tool?
Since most AI tools remain relatively new, there is a gap in the legal and regulatory framework needed to ensure patient privacy and data protection. Clinicians should draw on existing guardrails and best practices to protect patient privacy and prioritize informed consent. The bottom line: Patients need to know how their data will be used and agree to it.
In the example above regarding documentation, a clinician should obtain patient consent before using technology that records or transcribes sessions. This extends to disclosing the use of AI chat tools and other touch points that occur between sessions. One mental health nonprofit has come under fire for using ChatGPT to provide mental health counseling to thousands of patients who weren’t aware the responses were generated by AI.
Beyond disclosing the use of these tools, clinicians should sufficiently explain how they work to ensure patients understand what they’re consenting to. Some technology companies offer guidance on how informed consent applies to their products and even offer template consent forms to support clinicians. Ultimately, accountability for maintaining patient privacy rests with the clinician, not the company behind the AI tool.
• Where is there a risk of bias?
There has been much discussion around the issue of bias within large language models in particular, since these programs will inherit any bias from the data points or text used to train them. However, there is often little to no visibility into how these models are trained, the algorithms they rely on, and how efficacy is measured.
This is especially concerning within the mental health care space, where bias can contribute to lower-quality care based on a patient’s race, gender or other characteristics. One systemic review published in JAMA Network Open found that most of the AI models used for psychiatric diagnoses that have been studied had a high overall risk of bias — which can lead to outputs that are misleading or incorrect, which can be dangerous in the healthcare field.
It’s important to keep the risk of bias top-of-mind when exploring AI tools and consider whether a tool would pose any direct harm to patients. Clinicians should have active oversight with any use of AI and, ultimately, consider an AI tool’s outputs alongside their own insights, expertise, and instincts.
Clinicians have the power to shape AI’s impact
While there is plenty to be excited about as these new tools develop, clinicians should explore AI with an eye toward the risks as well as the rewards. Practitioners have a significant opportunity to help shape how this technology develops by making informed decisions about which products to invest in and holding tech companies accountable. By educating patients, prioritizing informed consent, and seeking ways to augment their work that ultimately improve quality and scale of care, clinicians can help ensure positive outcomes while minimizing unintended consequences.
Dr. Patel-Dunn is a psychiatrist and chief medical officer at Lifestance Health, Scottsdale, Ariz.
Three questions for clinicians
Three questions for clinicians
Artificial intelligence (AI) is already impacting the mental health care space, with several new tools available to both clinicians and patients. While this technology could be a game-changer amid a mental health crisis and clinician shortage, there are important ethical and efficacy concerns clinicians should be aware of.
Current use cases illustrate both the potential and risks of AI. On one hand, AI has the potential to improve patient care with tools that can support diagnoses and inform treatment decisions at scale. The UK’s National Health Service is using an AI-powered diagnostic tool to help clinicians diagnose mental health disorders and determine the severity of a patient’s needs. Other tools leverage AI to analyze a patient’s voice for signs of depression or anxiety.
On the other hand, there are serious potential risks involving privacy, bias, and misinformation. One chatbot tool designed to counsel patients through disordered eating was shut down after giving problematic weight-loss advice.
The number of AI tools in the healthcare space is expected to increase fivefold by 2035. Keeping up with these advances is just as important for clinicians as keeping up with the latest medication and treatment options. That means being aware of both the limitations and the potential of AI. Here are three questions clinicians can ask as they explore ways to integrate these tools into their practice while navigating the risks.
• How can AI augment, not replace, the work of my staff?
For example, documentation and the use of electronic health records have consistently been linked to clinician burnout. Using AI to cut down on documentation would leave clinicians with more time and energy to focus on patient care.
One study from the National Library of Medicine found that physicians who did not have enough time to complete documentation were nearly three times more likely to report burnout. In some cases, clinic schedules were deliberately shortened to allow time for documentation.
New tools are emerging that use audio recording, transcription services, and large language models to generate clinical summaries and other documentation support. Amazon and 3M have partnered to solve documentation challenges using AI. This is an area I’ll definitely be keeping an eye on as it develops.
• Do I have patient consent to use this tool?
Since most AI tools remain relatively new, there is a gap in the legal and regulatory framework needed to ensure patient privacy and data protection. Clinicians should draw on existing guardrails and best practices to protect patient privacy and prioritize informed consent. The bottom line: Patients need to know how their data will be used and agree to it.
In the example above regarding documentation, a clinician should obtain patient consent before using technology that records or transcribes sessions. This extends to disclosing the use of AI chat tools and other touch points that occur between sessions. One mental health nonprofit has come under fire for using ChatGPT to provide mental health counseling to thousands of patients who weren’t aware the responses were generated by AI.
Beyond disclosing the use of these tools, clinicians should sufficiently explain how they work to ensure patients understand what they’re consenting to. Some technology companies offer guidance on how informed consent applies to their products and even offer template consent forms to support clinicians. Ultimately, accountability for maintaining patient privacy rests with the clinician, not the company behind the AI tool.
• Where is there a risk of bias?
There has been much discussion around the issue of bias within large language models in particular, since these programs will inherit any bias from the data points or text used to train them. However, there is often little to no visibility into how these models are trained, the algorithms they rely on, and how efficacy is measured.
This is especially concerning within the mental health care space, where bias can contribute to lower-quality care based on a patient’s race, gender or other characteristics. One systemic review published in JAMA Network Open found that most of the AI models used for psychiatric diagnoses that have been studied had a high overall risk of bias — which can lead to outputs that are misleading or incorrect, which can be dangerous in the healthcare field.
It’s important to keep the risk of bias top-of-mind when exploring AI tools and consider whether a tool would pose any direct harm to patients. Clinicians should have active oversight with any use of AI and, ultimately, consider an AI tool’s outputs alongside their own insights, expertise, and instincts.
Clinicians have the power to shape AI’s impact
While there is plenty to be excited about as these new tools develop, clinicians should explore AI with an eye toward the risks as well as the rewards. Practitioners have a significant opportunity to help shape how this technology develops by making informed decisions about which products to invest in and holding tech companies accountable. By educating patients, prioritizing informed consent, and seeking ways to augment their work that ultimately improve quality and scale of care, clinicians can help ensure positive outcomes while minimizing unintended consequences.
Dr. Patel-Dunn is a psychiatrist and chief medical officer at Lifestance Health, Scottsdale, Ariz.
Artificial intelligence (AI) is already impacting the mental health care space, with several new tools available to both clinicians and patients. While this technology could be a game-changer amid a mental health crisis and clinician shortage, there are important ethical and efficacy concerns clinicians should be aware of.
Current use cases illustrate both the potential and risks of AI. On one hand, AI has the potential to improve patient care with tools that can support diagnoses and inform treatment decisions at scale. The UK’s National Health Service is using an AI-powered diagnostic tool to help clinicians diagnose mental health disorders and determine the severity of a patient’s needs. Other tools leverage AI to analyze a patient’s voice for signs of depression or anxiety.
On the other hand, there are serious potential risks involving privacy, bias, and misinformation. One chatbot tool designed to counsel patients through disordered eating was shut down after giving problematic weight-loss advice.
The number of AI tools in the healthcare space is expected to increase fivefold by 2035. Keeping up with these advances is just as important for clinicians as keeping up with the latest medication and treatment options. That means being aware of both the limitations and the potential of AI. Here are three questions clinicians can ask as they explore ways to integrate these tools into their practice while navigating the risks.
• How can AI augment, not replace, the work of my staff?
For example, documentation and the use of electronic health records have consistently been linked to clinician burnout. Using AI to cut down on documentation would leave clinicians with more time and energy to focus on patient care.
One study from the National Library of Medicine found that physicians who did not have enough time to complete documentation were nearly three times more likely to report burnout. In some cases, clinic schedules were deliberately shortened to allow time for documentation.
New tools are emerging that use audio recording, transcription services, and large language models to generate clinical summaries and other documentation support. Amazon and 3M have partnered to solve documentation challenges using AI. This is an area I’ll definitely be keeping an eye on as it develops.
• Do I have patient consent to use this tool?
Since most AI tools remain relatively new, there is a gap in the legal and regulatory framework needed to ensure patient privacy and data protection. Clinicians should draw on existing guardrails and best practices to protect patient privacy and prioritize informed consent. The bottom line: Patients need to know how their data will be used and agree to it.
In the example above regarding documentation, a clinician should obtain patient consent before using technology that records or transcribes sessions. This extends to disclosing the use of AI chat tools and other touch points that occur between sessions. One mental health nonprofit has come under fire for using ChatGPT to provide mental health counseling to thousands of patients who weren’t aware the responses were generated by AI.
Beyond disclosing the use of these tools, clinicians should sufficiently explain how they work to ensure patients understand what they’re consenting to. Some technology companies offer guidance on how informed consent applies to their products and even offer template consent forms to support clinicians. Ultimately, accountability for maintaining patient privacy rests with the clinician, not the company behind the AI tool.
• Where is there a risk of bias?
There has been much discussion around the issue of bias within large language models in particular, since these programs will inherit any bias from the data points or text used to train them. However, there is often little to no visibility into how these models are trained, the algorithms they rely on, and how efficacy is measured.
This is especially concerning within the mental health care space, where bias can contribute to lower-quality care based on a patient’s race, gender or other characteristics. One systemic review published in JAMA Network Open found that most of the AI models used for psychiatric diagnoses that have been studied had a high overall risk of bias — which can lead to outputs that are misleading or incorrect, which can be dangerous in the healthcare field.
It’s important to keep the risk of bias top-of-mind when exploring AI tools and consider whether a tool would pose any direct harm to patients. Clinicians should have active oversight with any use of AI and, ultimately, consider an AI tool’s outputs alongside their own insights, expertise, and instincts.
Clinicians have the power to shape AI’s impact
While there is plenty to be excited about as these new tools develop, clinicians should explore AI with an eye toward the risks as well as the rewards. Practitioners have a significant opportunity to help shape how this technology develops by making informed decisions about which products to invest in and holding tech companies accountable. By educating patients, prioritizing informed consent, and seeking ways to augment their work that ultimately improve quality and scale of care, clinicians can help ensure positive outcomes while minimizing unintended consequences.
Dr. Patel-Dunn is a psychiatrist and chief medical officer at Lifestance Health, Scottsdale, Ariz.
Clinician responsibilities during times of geopolitical conflict
In the realm of clinical psychology and psychiatry, our primary duty and commitment is (and should be) to the well-being of our patients. Yet, as we find ourselves in an era marked by escalating geopolitical conflict, such as the Israel-Hamas war, probably more aptly titled the Israeli-Hamas-Hezbollah-Houthi war (a clarification that elucidates a later point), clinicians are increasingly confronted with ethical dilemmas that extend far beyond what is outlined in our code of ethics.
These challenges are not only impacting us on a personal level but are also spilling over into our professional lives, creating a divisive and non-collegial environment within the healthcare community. We commit to “do no harm” when delivering care and yet we are doing harm to one another as colleagues.
We are no strangers to the complexities of human behavior and the intricate tapestry of emotions that are involved with our professional work. However, the current geopolitical landscape has added an extra layer of difficulty to our already taxing professional lives. We are, after all, human first with unconscious drives that govern how we negotiate cognitive dissonance and our need for the illusion of absolute justice as Yuval Noah Harari explains in a recent podcast.
Humans are notoriously bad at holding the multiplicity of experience in mind and various (often competing narratives) that impede the capacity for nuanced thinking. We would like to believe we are better and more capable than the average person in doing so, but divisiveness in our profession has become disturbingly pronounced, making it essential for us to carve out reflective space, more than ever.
The personal and professional divide
Geopolitical conflicts like the current war have a unique capacity to ignite strong emotions and deeply held convictions. It’s not hard to quickly become embroiled in passionate and engaged debate.
While discussion and discourse are healthy, these are bleeding into professional spheres, creating rifts within our clinical communities and contributing to a culture where not everyone feels safe. Look at any professional listserv in medicine or psychology and you will find the evidence. It should be an immediate call to action that we need to be fostering a different type of environment.
The impact of divisiveness is profound, hindering opportunities for collaboration, mentorship, and the free exchange of ideas among clinicians. It may lead to misunderstandings, mistrust, and an erosion of the support systems we rely on, ultimately diverting energy away from the pursuit of providing quality patient-care.
Balancing obligations and limits
Because of the inherent power differential that accompanies being in a provider role (physician and psychologist alike), we have a social and moral responsibility to be mindful of what we share – for the sake of humanity. There is an implicit assumption that a provider’s guidance should be adhered to and respected. In other words, words carry tremendous weight and deeply matter, and people in the general public ascribe significant meaning to messages put out by professionals.
When providers steer from their lanes of professional expertise to provide the general public with opinions or recommendations on nonmedical topics, problematic precedents can be set. We may be doing people a disservice.
Unfortunately, I have heard several anecdotes about clinicians who spend their patient’s time in session pushing their own ideological agendas. The patient-provider relationship is founded on principles of trust, empathy, and collaboration, with the primary goal of improving overall well-being and addressing a specific presenting problem. Of course, issues emerge that need to be addressed outside of the initial scope of treatment, an inherent part of the process. However, a grave concern emerges when clinicians initiate dialogue that is not meaningful to a patient, disclose and discuss their personal ideologies, or put pressure on patients to explain their beliefs in an attempt to change the patients’ minds.
Clinicians pushing their own agenda during patient sessions is antithetical to the objectives of psychotherapy and compromises the therapeutic alliance by diverting the focus of care in a way that serves the clinician rather than the client. It is quite the opposite of the patient-centered care that we strive for in training and practice.
Even within one’s theoretical professional scope of competence, I have seen the impact of emotions running high during this conflict, and have witnessed trained professionals making light of, or even mocking, hostages and their behavior upon release. These are care providers who could elucidate the complexities of captor-captive dynamics and the impact of trauma for the general public, yet they are contributing to dangerous perceptions and divisiveness.
I have also seen providers justify sexual violence, diminishing survivor and witness testimony due to ideological differences and strong personal beliefs. This is harmful to those impacted and does a disservice to our profession at large. In a helping profession we should strive to support and advocate for anyone who has been maltreated or experienced any form of victimization, violence, or abuse. This should be a professional standard.
As clinicians, we have an ethical obligation to uphold the well-being, autonomy, and dignity of our patients — and humanity. It is crucial to recognize the limits of our expertise and the ethical concerns that can arise in light of geopolitical conflict. How can we balance our duty to provide psychological support while also being cautious about delving into the realms of political analysis, foreign policy, or international relations?
The pitfalls of well-intentioned speaking out
In the age of social media and instant communication, a critical aspect to consider is the role of speaking out. The point I made above, in naming all partaking in the current conflict, speaks to this issue.
As providers and programs, we must be mindful of the inadvertent harm that can arise from making brief, underdeveloped, uninformed, or emotionally charged statements. Expressing opinions without a solid understanding of the historical, cultural, and political nuances of a conflict can contribute to misinformation and further polarization.
Anecdotally, there appears to be some significant degree of bias emerging within professional fields (e.g., psychology, medicine) and an innate calling for providers to “weigh in” as the war continues. Obviously, physicians and psychologists are trained to provide care and to be humanistic and empathic, but the majority do not have expertise in geopolitics or a nuanced awareness of the complexities of the conflict in the Middle East.
While hearts may be in the right place, issuing statements on complicated humanitarian/political situations can inadvertently have unintended and harmful consequences (in terms of antisemitism and islamophobia, increased incidence of hate crimes, and colleagues not feeling safe within professional societies or member organizations).
Unsophisticated, overly simplistic, and reductionistic statements that do not adequately convey nuance will not reflect the range of experience reflected by providers in the field (or the patients we treat). It is essential for clinicians and institutions putting out public statements to engage in deep reflection and utilize discernment. We must recognize that our words carry weight, given our position of influence as treatment providers. To minimize harm, we should seek to provide information that is fair, vetted, and balanced, and encourage open, respectful dialogue rather than asserting definitive positions.
Ultimately, as providers we must strive to seek unity and inclusivity amidst the current challenges. It is important for us to embody a spirit of collaboration during a time demarcated by deep fragmentation.
By acknowledging our limitations, promoting informed discussion, and avoiding the pitfalls of uninformed advocacy, we can contribute to a more compassionate and understanding world, even in the face of the most divisive geopolitical conflicts. We have an obligation to uphold when it comes to ourselves as professionals, and we need to foster healthy, respectful dialogue while maintaining an awareness of our blind spots.
Dr. Feldman is a licensed clinical psychologist in private practice in Miami. She is an adjunct professor in the College of Psychology at Nova Southeastern University, Fort Lauderdale, Fla., where she teaches clinical psychology doctoral students. She is an affiliate of Baptist West Kendall Hospital/FIU Family Medicine Residency Program and serves as president on the board of directors of The Southeast Florida Association for Psychoanalytic Psychology. The opinions expressed by Dr. Feldman are her own and do not represent the institutions with which she is affiliated. She has no disclosures.
In the realm of clinical psychology and psychiatry, our primary duty and commitment is (and should be) to the well-being of our patients. Yet, as we find ourselves in an era marked by escalating geopolitical conflict, such as the Israel-Hamas war, probably more aptly titled the Israeli-Hamas-Hezbollah-Houthi war (a clarification that elucidates a later point), clinicians are increasingly confronted with ethical dilemmas that extend far beyond what is outlined in our code of ethics.
These challenges are not only impacting us on a personal level but are also spilling over into our professional lives, creating a divisive and non-collegial environment within the healthcare community. We commit to “do no harm” when delivering care and yet we are doing harm to one another as colleagues.
We are no strangers to the complexities of human behavior and the intricate tapestry of emotions that are involved with our professional work. However, the current geopolitical landscape has added an extra layer of difficulty to our already taxing professional lives. We are, after all, human first with unconscious drives that govern how we negotiate cognitive dissonance and our need for the illusion of absolute justice as Yuval Noah Harari explains in a recent podcast.
Humans are notoriously bad at holding the multiplicity of experience in mind and various (often competing narratives) that impede the capacity for nuanced thinking. We would like to believe we are better and more capable than the average person in doing so, but divisiveness in our profession has become disturbingly pronounced, making it essential for us to carve out reflective space, more than ever.
The personal and professional divide
Geopolitical conflicts like the current war have a unique capacity to ignite strong emotions and deeply held convictions. It’s not hard to quickly become embroiled in passionate and engaged debate.
While discussion and discourse are healthy, these are bleeding into professional spheres, creating rifts within our clinical communities and contributing to a culture where not everyone feels safe. Look at any professional listserv in medicine or psychology and you will find the evidence. It should be an immediate call to action that we need to be fostering a different type of environment.
The impact of divisiveness is profound, hindering opportunities for collaboration, mentorship, and the free exchange of ideas among clinicians. It may lead to misunderstandings, mistrust, and an erosion of the support systems we rely on, ultimately diverting energy away from the pursuit of providing quality patient-care.
Balancing obligations and limits
Because of the inherent power differential that accompanies being in a provider role (physician and psychologist alike), we have a social and moral responsibility to be mindful of what we share – for the sake of humanity. There is an implicit assumption that a provider’s guidance should be adhered to and respected. In other words, words carry tremendous weight and deeply matter, and people in the general public ascribe significant meaning to messages put out by professionals.
When providers steer from their lanes of professional expertise to provide the general public with opinions or recommendations on nonmedical topics, problematic precedents can be set. We may be doing people a disservice.
Unfortunately, I have heard several anecdotes about clinicians who spend their patient’s time in session pushing their own ideological agendas. The patient-provider relationship is founded on principles of trust, empathy, and collaboration, with the primary goal of improving overall well-being and addressing a specific presenting problem. Of course, issues emerge that need to be addressed outside of the initial scope of treatment, an inherent part of the process. However, a grave concern emerges when clinicians initiate dialogue that is not meaningful to a patient, disclose and discuss their personal ideologies, or put pressure on patients to explain their beliefs in an attempt to change the patients’ minds.
Clinicians pushing their own agenda during patient sessions is antithetical to the objectives of psychotherapy and compromises the therapeutic alliance by diverting the focus of care in a way that serves the clinician rather than the client. It is quite the opposite of the patient-centered care that we strive for in training and practice.
Even within one’s theoretical professional scope of competence, I have seen the impact of emotions running high during this conflict, and have witnessed trained professionals making light of, or even mocking, hostages and their behavior upon release. These are care providers who could elucidate the complexities of captor-captive dynamics and the impact of trauma for the general public, yet they are contributing to dangerous perceptions and divisiveness.
I have also seen providers justify sexual violence, diminishing survivor and witness testimony due to ideological differences and strong personal beliefs. This is harmful to those impacted and does a disservice to our profession at large. In a helping profession we should strive to support and advocate for anyone who has been maltreated or experienced any form of victimization, violence, or abuse. This should be a professional standard.
As clinicians, we have an ethical obligation to uphold the well-being, autonomy, and dignity of our patients — and humanity. It is crucial to recognize the limits of our expertise and the ethical concerns that can arise in light of geopolitical conflict. How can we balance our duty to provide psychological support while also being cautious about delving into the realms of political analysis, foreign policy, or international relations?
The pitfalls of well-intentioned speaking out
In the age of social media and instant communication, a critical aspect to consider is the role of speaking out. The point I made above, in naming all partaking in the current conflict, speaks to this issue.
As providers and programs, we must be mindful of the inadvertent harm that can arise from making brief, underdeveloped, uninformed, or emotionally charged statements. Expressing opinions without a solid understanding of the historical, cultural, and political nuances of a conflict can contribute to misinformation and further polarization.
Anecdotally, there appears to be some significant degree of bias emerging within professional fields (e.g., psychology, medicine) and an innate calling for providers to “weigh in” as the war continues. Obviously, physicians and psychologists are trained to provide care and to be humanistic and empathic, but the majority do not have expertise in geopolitics or a nuanced awareness of the complexities of the conflict in the Middle East.
While hearts may be in the right place, issuing statements on complicated humanitarian/political situations can inadvertently have unintended and harmful consequences (in terms of antisemitism and islamophobia, increased incidence of hate crimes, and colleagues not feeling safe within professional societies or member organizations).
Unsophisticated, overly simplistic, and reductionistic statements that do not adequately convey nuance will not reflect the range of experience reflected by providers in the field (or the patients we treat). It is essential for clinicians and institutions putting out public statements to engage in deep reflection and utilize discernment. We must recognize that our words carry weight, given our position of influence as treatment providers. To minimize harm, we should seek to provide information that is fair, vetted, and balanced, and encourage open, respectful dialogue rather than asserting definitive positions.
Ultimately, as providers we must strive to seek unity and inclusivity amidst the current challenges. It is important for us to embody a spirit of collaboration during a time demarcated by deep fragmentation.
By acknowledging our limitations, promoting informed discussion, and avoiding the pitfalls of uninformed advocacy, we can contribute to a more compassionate and understanding world, even in the face of the most divisive geopolitical conflicts. We have an obligation to uphold when it comes to ourselves as professionals, and we need to foster healthy, respectful dialogue while maintaining an awareness of our blind spots.
Dr. Feldman is a licensed clinical psychologist in private practice in Miami. She is an adjunct professor in the College of Psychology at Nova Southeastern University, Fort Lauderdale, Fla., where she teaches clinical psychology doctoral students. She is an affiliate of Baptist West Kendall Hospital/FIU Family Medicine Residency Program and serves as president on the board of directors of The Southeast Florida Association for Psychoanalytic Psychology. The opinions expressed by Dr. Feldman are her own and do not represent the institutions with which she is affiliated. She has no disclosures.
In the realm of clinical psychology and psychiatry, our primary duty and commitment is (and should be) to the well-being of our patients. Yet, as we find ourselves in an era marked by escalating geopolitical conflict, such as the Israel-Hamas war, probably more aptly titled the Israeli-Hamas-Hezbollah-Houthi war (a clarification that elucidates a later point), clinicians are increasingly confronted with ethical dilemmas that extend far beyond what is outlined in our code of ethics.
These challenges are not only impacting us on a personal level but are also spilling over into our professional lives, creating a divisive and non-collegial environment within the healthcare community. We commit to “do no harm” when delivering care and yet we are doing harm to one another as colleagues.
We are no strangers to the complexities of human behavior and the intricate tapestry of emotions that are involved with our professional work. However, the current geopolitical landscape has added an extra layer of difficulty to our already taxing professional lives. We are, after all, human first with unconscious drives that govern how we negotiate cognitive dissonance and our need for the illusion of absolute justice as Yuval Noah Harari explains in a recent podcast.
Humans are notoriously bad at holding the multiplicity of experience in mind and various (often competing narratives) that impede the capacity for nuanced thinking. We would like to believe we are better and more capable than the average person in doing so, but divisiveness in our profession has become disturbingly pronounced, making it essential for us to carve out reflective space, more than ever.
The personal and professional divide
Geopolitical conflicts like the current war have a unique capacity to ignite strong emotions and deeply held convictions. It’s not hard to quickly become embroiled in passionate and engaged debate.
While discussion and discourse are healthy, these are bleeding into professional spheres, creating rifts within our clinical communities and contributing to a culture where not everyone feels safe. Look at any professional listserv in medicine or psychology and you will find the evidence. It should be an immediate call to action that we need to be fostering a different type of environment.
The impact of divisiveness is profound, hindering opportunities for collaboration, mentorship, and the free exchange of ideas among clinicians. It may lead to misunderstandings, mistrust, and an erosion of the support systems we rely on, ultimately diverting energy away from the pursuit of providing quality patient-care.
Balancing obligations and limits
Because of the inherent power differential that accompanies being in a provider role (physician and psychologist alike), we have a social and moral responsibility to be mindful of what we share – for the sake of humanity. There is an implicit assumption that a provider’s guidance should be adhered to and respected. In other words, words carry tremendous weight and deeply matter, and people in the general public ascribe significant meaning to messages put out by professionals.
When providers steer from their lanes of professional expertise to provide the general public with opinions or recommendations on nonmedical topics, problematic precedents can be set. We may be doing people a disservice.
Unfortunately, I have heard several anecdotes about clinicians who spend their patient’s time in session pushing their own ideological agendas. The patient-provider relationship is founded on principles of trust, empathy, and collaboration, with the primary goal of improving overall well-being and addressing a specific presenting problem. Of course, issues emerge that need to be addressed outside of the initial scope of treatment, an inherent part of the process. However, a grave concern emerges when clinicians initiate dialogue that is not meaningful to a patient, disclose and discuss their personal ideologies, or put pressure on patients to explain their beliefs in an attempt to change the patients’ minds.
Clinicians pushing their own agenda during patient sessions is antithetical to the objectives of psychotherapy and compromises the therapeutic alliance by diverting the focus of care in a way that serves the clinician rather than the client. It is quite the opposite of the patient-centered care that we strive for in training and practice.
Even within one’s theoretical professional scope of competence, I have seen the impact of emotions running high during this conflict, and have witnessed trained professionals making light of, or even mocking, hostages and their behavior upon release. These are care providers who could elucidate the complexities of captor-captive dynamics and the impact of trauma for the general public, yet they are contributing to dangerous perceptions and divisiveness.
I have also seen providers justify sexual violence, diminishing survivor and witness testimony due to ideological differences and strong personal beliefs. This is harmful to those impacted and does a disservice to our profession at large. In a helping profession we should strive to support and advocate for anyone who has been maltreated or experienced any form of victimization, violence, or abuse. This should be a professional standard.
As clinicians, we have an ethical obligation to uphold the well-being, autonomy, and dignity of our patients — and humanity. It is crucial to recognize the limits of our expertise and the ethical concerns that can arise in light of geopolitical conflict. How can we balance our duty to provide psychological support while also being cautious about delving into the realms of political analysis, foreign policy, or international relations?
The pitfalls of well-intentioned speaking out
In the age of social media and instant communication, a critical aspect to consider is the role of speaking out. The point I made above, in naming all partaking in the current conflict, speaks to this issue.
As providers and programs, we must be mindful of the inadvertent harm that can arise from making brief, underdeveloped, uninformed, or emotionally charged statements. Expressing opinions without a solid understanding of the historical, cultural, and political nuances of a conflict can contribute to misinformation and further polarization.
Anecdotally, there appears to be some significant degree of bias emerging within professional fields (e.g., psychology, medicine) and an innate calling for providers to “weigh in” as the war continues. Obviously, physicians and psychologists are trained to provide care and to be humanistic and empathic, but the majority do not have expertise in geopolitics or a nuanced awareness of the complexities of the conflict in the Middle East.
While hearts may be in the right place, issuing statements on complicated humanitarian/political situations can inadvertently have unintended and harmful consequences (in terms of antisemitism and islamophobia, increased incidence of hate crimes, and colleagues not feeling safe within professional societies or member organizations).
Unsophisticated, overly simplistic, and reductionistic statements that do not adequately convey nuance will not reflect the range of experience reflected by providers in the field (or the patients we treat). It is essential for clinicians and institutions putting out public statements to engage in deep reflection and utilize discernment. We must recognize that our words carry weight, given our position of influence as treatment providers. To minimize harm, we should seek to provide information that is fair, vetted, and balanced, and encourage open, respectful dialogue rather than asserting definitive positions.
Ultimately, as providers we must strive to seek unity and inclusivity amidst the current challenges. It is important for us to embody a spirit of collaboration during a time demarcated by deep fragmentation.
By acknowledging our limitations, promoting informed discussion, and avoiding the pitfalls of uninformed advocacy, we can contribute to a more compassionate and understanding world, even in the face of the most divisive geopolitical conflicts. We have an obligation to uphold when it comes to ourselves as professionals, and we need to foster healthy, respectful dialogue while maintaining an awareness of our blind spots.
Dr. Feldman is a licensed clinical psychologist in private practice in Miami. She is an adjunct professor in the College of Psychology at Nova Southeastern University, Fort Lauderdale, Fla., where she teaches clinical psychology doctoral students. She is an affiliate of Baptist West Kendall Hospital/FIU Family Medicine Residency Program and serves as president on the board of directors of The Southeast Florida Association for Psychoanalytic Psychology. The opinions expressed by Dr. Feldman are her own and do not represent the institutions with which she is affiliated. She has no disclosures.
EMRs: gumming up the works
I don’t like EMR systems, with all their requirements, click boxes, endless cut & paste abuse, and 20-page notes that say nothing.
But I am a fan of what computers have brought to medical charts.
When I started out in 2000, I had no patients, hence no charts. I had the advantage of being able to start from scratch — there was nothing to convert to digital. So, from the beginning, that’s how I went. Back then, of course, everything came to the office as paper. It had to be scanned in, then named, then placed in the right computer file.
But it was still easier than amassing paper records. At that time I subleased from a doc who’d been in practice for 15 years. His charts were all paper. Charts were neatly filed on shelves, everything was initialed, hole-punched, and put in the right section (which involved pulling out other stuff and putting it back). A few times a year, his staff would comb through the charts in front, and anyone who hadn’t been seen in 2 years would have their chart moved to a storage room in the back. Once a year they’d pull the charts of anyone not seen in 7 years and a company would come in and shred those records.
After 23 years, I still have it all. The whole thing takes up a little over 50 gigabytes on a hard drive, which realistically is nothing these days. Electrons don’t take up much space.
The majority of the charts — those that are more than 7 years old — I’ll probably never need to access, but it still happens sometimes. People call in and say they’ve moved back to Phoenix, or need to see a neurologist again, or need the records for insurance reasons, or whatever. My staff is also spared from moving charts to a storage room, then to shredding. Since they don’t take up any physical space, it’s no effort to keep everything.
And they aren’t just at my office. They’re at home, on my phone, wherever I am. If I get called from an ER, I can pull them up quickly. If I travel, they’re with me. My memory is good, but not that good, and I’d rather be able to look things up than guess.
This, at least to me, is the advantage of computers. Their data storage and retrieval advantages far exceed that of paper. In my opinion EMRs, while well-intentioned, have taken these benefits and twisted them into something cumbersome, geared more to meet nonmedical requirements and billing purposes.
In the process they’ve lost sight of our age-old job of caring for patients.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I don’t like EMR systems, with all their requirements, click boxes, endless cut & paste abuse, and 20-page notes that say nothing.
But I am a fan of what computers have brought to medical charts.
When I started out in 2000, I had no patients, hence no charts. I had the advantage of being able to start from scratch — there was nothing to convert to digital. So, from the beginning, that’s how I went. Back then, of course, everything came to the office as paper. It had to be scanned in, then named, then placed in the right computer file.
But it was still easier than amassing paper records. At that time I subleased from a doc who’d been in practice for 15 years. His charts were all paper. Charts were neatly filed on shelves, everything was initialed, hole-punched, and put in the right section (which involved pulling out other stuff and putting it back). A few times a year, his staff would comb through the charts in front, and anyone who hadn’t been seen in 2 years would have their chart moved to a storage room in the back. Once a year they’d pull the charts of anyone not seen in 7 years and a company would come in and shred those records.
After 23 years, I still have it all. The whole thing takes up a little over 50 gigabytes on a hard drive, which realistically is nothing these days. Electrons don’t take up much space.
The majority of the charts — those that are more than 7 years old — I’ll probably never need to access, but it still happens sometimes. People call in and say they’ve moved back to Phoenix, or need to see a neurologist again, or need the records for insurance reasons, or whatever. My staff is also spared from moving charts to a storage room, then to shredding. Since they don’t take up any physical space, it’s no effort to keep everything.
And they aren’t just at my office. They’re at home, on my phone, wherever I am. If I get called from an ER, I can pull them up quickly. If I travel, they’re with me. My memory is good, but not that good, and I’d rather be able to look things up than guess.
This, at least to me, is the advantage of computers. Their data storage and retrieval advantages far exceed that of paper. In my opinion EMRs, while well-intentioned, have taken these benefits and twisted them into something cumbersome, geared more to meet nonmedical requirements and billing purposes.
In the process they’ve lost sight of our age-old job of caring for patients.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I don’t like EMR systems, with all their requirements, click boxes, endless cut & paste abuse, and 20-page notes that say nothing.
But I am a fan of what computers have brought to medical charts.
When I started out in 2000, I had no patients, hence no charts. I had the advantage of being able to start from scratch — there was nothing to convert to digital. So, from the beginning, that’s how I went. Back then, of course, everything came to the office as paper. It had to be scanned in, then named, then placed in the right computer file.
But it was still easier than amassing paper records. At that time I subleased from a doc who’d been in practice for 15 years. His charts were all paper. Charts were neatly filed on shelves, everything was initialed, hole-punched, and put in the right section (which involved pulling out other stuff and putting it back). A few times a year, his staff would comb through the charts in front, and anyone who hadn’t been seen in 2 years would have their chart moved to a storage room in the back. Once a year they’d pull the charts of anyone not seen in 7 years and a company would come in and shred those records.
After 23 years, I still have it all. The whole thing takes up a little over 50 gigabytes on a hard drive, which realistically is nothing these days. Electrons don’t take up much space.
The majority of the charts — those that are more than 7 years old — I’ll probably never need to access, but it still happens sometimes. People call in and say they’ve moved back to Phoenix, or need to see a neurologist again, or need the records for insurance reasons, or whatever. My staff is also spared from moving charts to a storage room, then to shredding. Since they don’t take up any physical space, it’s no effort to keep everything.
And they aren’t just at my office. They’re at home, on my phone, wherever I am. If I get called from an ER, I can pull them up quickly. If I travel, they’re with me. My memory is good, but not that good, and I’d rather be able to look things up than guess.
This, at least to me, is the advantage of computers. Their data storage and retrieval advantages far exceed that of paper. In my opinion EMRs, while well-intentioned, have taken these benefits and twisted them into something cumbersome, geared more to meet nonmedical requirements and billing purposes.
In the process they’ve lost sight of our age-old job of caring for patients.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
An alternative to walking out
Organized labor seems to be experiencing a rebirth of sorts. In October 2022 a strike by railroad workers was averted when a tentative agreement about wages, working conditions, health insurance, and medical leave was hammered out. This past fall, strikes by auto workers that threatened to paralyze the big three manufacturers have now been resolved with agreements that meet many of the workers’ demands. The President even made an appearance on a picket line. Baristas at coffee shops, screenwriters, and actors have all been involved in work actions around the country.
While the health care industry has been relatively immune to threatened work stoppages, there are a growing number of hospitals and clinics where nurses and physicians are exploring the possibility of organizing to give themselves a stronger voice in how health care is being delivered. The realities that come when you transition from owner to employee are finally beginning to sink in for physicians, whether they are specialists or primary care providers.
One of the most significant efforts toward unionization recently occurred in Minnesota and Wisconsin. About 400 physicians and 150 physician’s assistants and nurse practitioners employed at Allina Health System voted to unionize and join the Doctors Council.
In an interview with Jacobin, a publication that offers a socialist perspective, three of the providers involved in the process that led to the vote shared their observations. The physicians claim that the first steps toward unionization came after multiple efforts to work with the Allina’s administration were rebuffed. As primary care physicians, their initial demands focused on getting help with hiring staffing and getting support with paperwork and administrative obligations.
The organizers complained that while Medicare hoped to bolster primary care by paying the providers more, the funds went to the companies, who then distributed them in a way that often did little to help the overworked providers. In addition to achieving a more equitable distribution of the monies, one of the organizers sees unionization as a way to provide a layer of protection when providers feel they must speak out about situations which clearly put quality of care at risk.
The organizers say the idea of unionization has been particularly appealing to the younger providers who are feeling threatened by burnout. When these new physicians look to their older coworkers for advice, they often find that the seasoned employees are as stressed as they are. Realizing that things aren’t going to improve with time, acting now to strengthen their voices sounds appealing.
With the vote for unionization behind them, the organizers are now ready to formulate a prioritized list of demands. Those of you who are regular readers of Letters from Maine know that I have been urging primary care physicians to find their voices. Unfortunately, unionization seems to be becoming a more common fall-back strategy when other avenues have failed to reach a sympathetic ear in the corporate boardrooms.
As more unions form, it will be interesting to see how the organizers structure their demands and job actions. While walkouts and strikes can certainly be effective in gaining attention, that attention can carry a risk of counter productivity sometimes by alienating patients, who should become allies.
Since an unsustainable burden of paperwork and administrative demands seems to be at the top of everyone’s priority list, it might make sense to adopt this message as a scaffolding on which to built a work action. Instead of walking off the job or marching on a picket line, why not stay in the hospital and continue to see patients but only for part of the work day. The remainder of the day would be spent doing all the clerical work that has become so onerous.
Providers would agree to see patients in the mornings, saving up the clerical work and administrative obligations for the afternoon. The definition of “morning” could vary depending on local conditions.
The important message to the public and the patients would be that the providers were not abandoning them by walking out. The patients’ access to face-to-face care was being limited not because the doctors didn’t want to see them but because the providers were being forced to accept other responsibilities by the administration. The physicians would always be on site in case of a crisis, but until reasonable demands for support from the company were met, a certain portion of the providers’ day would be spent doing things not directly related to face-to-face patient care. This burden of meaningless work is the reality as it stands already. Why not organize it in a way that makes it startlingly visible to the patients and the public.
There would be no video clips of physicians walking the picket lines carrying signs. Any images released to the media would be of empty waiting rooms while providers sat hunched over their computers or talking on the phone to insurance companies.
The strategy needs a catchy phrase like “a paperwork-in” but I’m still struggling with a name. Let me know if you have a better one or even a better strategy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Organized labor seems to be experiencing a rebirth of sorts. In October 2022 a strike by railroad workers was averted when a tentative agreement about wages, working conditions, health insurance, and medical leave was hammered out. This past fall, strikes by auto workers that threatened to paralyze the big three manufacturers have now been resolved with agreements that meet many of the workers’ demands. The President even made an appearance on a picket line. Baristas at coffee shops, screenwriters, and actors have all been involved in work actions around the country.
While the health care industry has been relatively immune to threatened work stoppages, there are a growing number of hospitals and clinics where nurses and physicians are exploring the possibility of organizing to give themselves a stronger voice in how health care is being delivered. The realities that come when you transition from owner to employee are finally beginning to sink in for physicians, whether they are specialists or primary care providers.
One of the most significant efforts toward unionization recently occurred in Minnesota and Wisconsin. About 400 physicians and 150 physician’s assistants and nurse practitioners employed at Allina Health System voted to unionize and join the Doctors Council.
In an interview with Jacobin, a publication that offers a socialist perspective, three of the providers involved in the process that led to the vote shared their observations. The physicians claim that the first steps toward unionization came after multiple efforts to work with the Allina’s administration were rebuffed. As primary care physicians, their initial demands focused on getting help with hiring staffing and getting support with paperwork and administrative obligations.
The organizers complained that while Medicare hoped to bolster primary care by paying the providers more, the funds went to the companies, who then distributed them in a way that often did little to help the overworked providers. In addition to achieving a more equitable distribution of the monies, one of the organizers sees unionization as a way to provide a layer of protection when providers feel they must speak out about situations which clearly put quality of care at risk.
The organizers say the idea of unionization has been particularly appealing to the younger providers who are feeling threatened by burnout. When these new physicians look to their older coworkers for advice, they often find that the seasoned employees are as stressed as they are. Realizing that things aren’t going to improve with time, acting now to strengthen their voices sounds appealing.
With the vote for unionization behind them, the organizers are now ready to formulate a prioritized list of demands. Those of you who are regular readers of Letters from Maine know that I have been urging primary care physicians to find their voices. Unfortunately, unionization seems to be becoming a more common fall-back strategy when other avenues have failed to reach a sympathetic ear in the corporate boardrooms.
As more unions form, it will be interesting to see how the organizers structure their demands and job actions. While walkouts and strikes can certainly be effective in gaining attention, that attention can carry a risk of counter productivity sometimes by alienating patients, who should become allies.
Since an unsustainable burden of paperwork and administrative demands seems to be at the top of everyone’s priority list, it might make sense to adopt this message as a scaffolding on which to built a work action. Instead of walking off the job or marching on a picket line, why not stay in the hospital and continue to see patients but only for part of the work day. The remainder of the day would be spent doing all the clerical work that has become so onerous.
Providers would agree to see patients in the mornings, saving up the clerical work and administrative obligations for the afternoon. The definition of “morning” could vary depending on local conditions.
The important message to the public and the patients would be that the providers were not abandoning them by walking out. The patients’ access to face-to-face care was being limited not because the doctors didn’t want to see them but because the providers were being forced to accept other responsibilities by the administration. The physicians would always be on site in case of a crisis, but until reasonable demands for support from the company were met, a certain portion of the providers’ day would be spent doing things not directly related to face-to-face patient care. This burden of meaningless work is the reality as it stands already. Why not organize it in a way that makes it startlingly visible to the patients and the public.
There would be no video clips of physicians walking the picket lines carrying signs. Any images released to the media would be of empty waiting rooms while providers sat hunched over their computers or talking on the phone to insurance companies.
The strategy needs a catchy phrase like “a paperwork-in” but I’m still struggling with a name. Let me know if you have a better one or even a better strategy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Organized labor seems to be experiencing a rebirth of sorts. In October 2022 a strike by railroad workers was averted when a tentative agreement about wages, working conditions, health insurance, and medical leave was hammered out. This past fall, strikes by auto workers that threatened to paralyze the big three manufacturers have now been resolved with agreements that meet many of the workers’ demands. The President even made an appearance on a picket line. Baristas at coffee shops, screenwriters, and actors have all been involved in work actions around the country.
While the health care industry has been relatively immune to threatened work stoppages, there are a growing number of hospitals and clinics where nurses and physicians are exploring the possibility of organizing to give themselves a stronger voice in how health care is being delivered. The realities that come when you transition from owner to employee are finally beginning to sink in for physicians, whether they are specialists or primary care providers.
One of the most significant efforts toward unionization recently occurred in Minnesota and Wisconsin. About 400 physicians and 150 physician’s assistants and nurse practitioners employed at Allina Health System voted to unionize and join the Doctors Council.
In an interview with Jacobin, a publication that offers a socialist perspective, three of the providers involved in the process that led to the vote shared their observations. The physicians claim that the first steps toward unionization came after multiple efforts to work with the Allina’s administration were rebuffed. As primary care physicians, their initial demands focused on getting help with hiring staffing and getting support with paperwork and administrative obligations.
The organizers complained that while Medicare hoped to bolster primary care by paying the providers more, the funds went to the companies, who then distributed them in a way that often did little to help the overworked providers. In addition to achieving a more equitable distribution of the monies, one of the organizers sees unionization as a way to provide a layer of protection when providers feel they must speak out about situations which clearly put quality of care at risk.
The organizers say the idea of unionization has been particularly appealing to the younger providers who are feeling threatened by burnout. When these new physicians look to their older coworkers for advice, they often find that the seasoned employees are as stressed as they are. Realizing that things aren’t going to improve with time, acting now to strengthen their voices sounds appealing.
With the vote for unionization behind them, the organizers are now ready to formulate a prioritized list of demands. Those of you who are regular readers of Letters from Maine know that I have been urging primary care physicians to find their voices. Unfortunately, unionization seems to be becoming a more common fall-back strategy when other avenues have failed to reach a sympathetic ear in the corporate boardrooms.
As more unions form, it will be interesting to see how the organizers structure their demands and job actions. While walkouts and strikes can certainly be effective in gaining attention, that attention can carry a risk of counter productivity sometimes by alienating patients, who should become allies.
Since an unsustainable burden of paperwork and administrative demands seems to be at the top of everyone’s priority list, it might make sense to adopt this message as a scaffolding on which to built a work action. Instead of walking off the job or marching on a picket line, why not stay in the hospital and continue to see patients but only for part of the work day. The remainder of the day would be spent doing all the clerical work that has become so onerous.
Providers would agree to see patients in the mornings, saving up the clerical work and administrative obligations for the afternoon. The definition of “morning” could vary depending on local conditions.
The important message to the public and the patients would be that the providers were not abandoning them by walking out. The patients’ access to face-to-face care was being limited not because the doctors didn’t want to see them but because the providers were being forced to accept other responsibilities by the administration. The physicians would always be on site in case of a crisis, but until reasonable demands for support from the company were met, a certain portion of the providers’ day would be spent doing things not directly related to face-to-face patient care. This burden of meaningless work is the reality as it stands already. Why not organize it in a way that makes it startlingly visible to the patients and the public.
There would be no video clips of physicians walking the picket lines carrying signs. Any images released to the media would be of empty waiting rooms while providers sat hunched over their computers or talking on the phone to insurance companies.
The strategy needs a catchy phrase like “a paperwork-in” but I’m still struggling with a name. Let me know if you have a better one or even a better strategy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Mass shooters and mental illness: Reexamining the connection
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Equity and Inclusion in Military Recruitment: The Case for Neurodiversity in Uniform
The willingness with which our young people are likely to serve in any war, no matter how justified, shall be directly proportional to how they perceive how the veterans of earlier wars were treated and appreciated by their nation.
George Washington? 1
This editorial is the second of the 2-part series on the recruitment crisis currently confronting the Army, Navy, and Air Force. Part 1 focused on rationales for the lack of interest or motivation among those potentially eligible to join the military. This column looks at individuals eager to serve who do not meet eligibility requirements. A 2022 article examining the 2020 Qualified Military Available Study found that without a waiver 77% of Americans in the prime recruiting age group 17 to 24 years would be ineligible for the military due to weight, substance use, or mental and physical health conditions. Most young adults met several ineligibility criteria.2
Obesity and substance use are the most common disqualifiers, mirroring the culture at large. Scores of other physical and mental health conditions render an applicant ineligible for military service or require a waiver. The justification of all eligibility criteria is to: (1) ensure that service members can safely and effectively deploy; and (2) reduce the attrition rate. Both are essential to the mission readiness of the military. In 2022, the military gave 1 in 6 of those seeking enlistment an accession waiver.3 About 4% of waivers issued were for mental health conditions, such as autism and attention-deficit hyperactivity disorder (ADHD). The response to the recruiting crisis resulted in the largest number of waivers granted in a decade. Military Times noted that exact numbers are hard to obtain, interfering with the transparency of public policy as well as high-quality research on waivers’ impact on recruits and the service.3
The War Horse reported that the current waiver process is riddled with procedural injustice and inequity in implementation.4 Each service sets its eligibility requirements: the rationale being that the respective branches have distinct roles necessitating distinguishing qualifications. What is far more difficult to defend is that wide variation exists in the application of the criteria. Similar cases are judged differently, depending on nonmaterial factors, such as geographic location and unwritten policies of recruiting offices. Waiver approval rates for mental health conditions range from 35% for the Army to 71% for the Marines. The prospective recruit, not the military service, bears the burden of demonstrating that their condition does not impair their fitness for duty; hence, thousands have been disqualified based on their diagnosis.4 This comes at a time when the US Department of Veterans Affairs (VA) and the US Department of Defense (DoD) have been battling a suicide epidemic for years. Current qualifying standards send a strong stigmatizing message to those who want to enlist and those already in the ranks at a time the DoD and VA are launching campaigns to persuade active-duty members and veterans to seek mental health treatment.5
The recruiting crisis brought into stark relief more fundamental questions about the clinical and ethical aspects of eligibility criteria that either disqualify outright or require a waiver process for many young Americans with mental health conditions who want to serve their country. One of the most clinically perplexing standards is that applicants with ADHD are disqualified if they have taken medications in the past 12 to 24 months, depending on the service.6 Despite this policy, the Army acknowledges that stimulant medications may improve the function of individuals with ADHD and reduce the rates of substance use and behavior disturbances, the real concerns for recruiters and commanders.7
Requirements like these place otherwise high-functioning individuals whose professional goal is to serve in the military in a double bind. The military’s studies show that recruits’ persistent nondisclosure of their diagnoses results in poorer performance and higher attrition rates of those who have enlisted, even when treated.8 If potential recruits disclose their psychiatric history, they may well be disqualified and/or denied a waiver. This is even more true for service members already in the military who may believe they have one of the conditions but fear that being diagnosed will negatively impact their career. Not disclosing their condition prevents service members from obtaining the clinical care and support they need to succeed and also limits the ability of commanders to make decisions about deployment that ensure maximal unit performance and the safety of the service member.9 However, ADHD is one of 38 diagnoses that the DoD is considering for possible removal or modification of the waiver for some subset of applicants.10
The final irony is that medicine and warfare have changed dramatically and rapidly since the initial determination that diagnoses like ADHD and autism disqualify individuals from serving. A Rand Corporation study found that individuals who are neurodivergent—the name collectively assigned to individuals with diagnoses like autism and ADHD—may have unique abilities that enable them to outperform neurotypical persons in areas like pattern recognition, attention to detail, repetitive tasks, and memory, among others. These highly technical skills are essential to intelligence analysis and cybersecurity domains that are increasingly crucial to both national defense and victory on the battlefield.11 Even congressional representatives who just a few years ago criticized waivers for mental health conditions as “lowering the standards” are now pushing for more moderate policies, especially for those who have received and responded to treatment for their mental health disorders.12
The epigraph has been widely and persistently misattributed to the country’s first commander in chief, George Washington, because it captures a salient sentiment directly bearing on the question of who is fit for duty.1 History has shown that discrimination in enlistment only weakens the fighting force, whereas diversity, including neurodiversity, in the military as in society is a source of strength. Equitable inclusion of those who have the discipline, desire, and dedication to serve their country may be the most positive response to the recruitment crisis.
1. George Washington’s Mount Vernon Washington Library. Accessed November 13, 2023. https://www.mountvernon.org/library/digitalhistory/digital-encyclopedia/article/spurious-quotations/
2. Novelty T. Even more young Americans are unfit to serve, a new study finds, here’s why. Accessed November 20, 2023. https://www.military.com/daily-news/2022/09/28/new-pentagon-study-shows-77-of-young-americans-are-ineligible-military-service.html
3. Cohen RS. Need for accession waivers soars amid historic recruiting challenges. Accessed November 20, 2023. https://www.militarytimes.com/news/your-air-force/2023/04/10/need-for-accession-waivers-soars-amid-historic-recruiting-challenges
4. Barnhill J. The military is missing recruitment goals. Are thousands being disqualified. The War Horse. Accessed November 20, 2023. https://thewarhorse.org/us-military-recruitment-crisis-may-hinge-on-medical-waivers
5. Hauschild V. Army experts: mixed messages can fuel stigma, prevent soldiers from accessing behavioral healthcare. Accessed November 20, 2023. https://www.army.mil/article/262525/army_experts_mixed_messages_can_fuel_stigma_prevent_soldiers_from_accessing_behavioral_healthcare
6. US Department of Defense. DoD Instructions 6130.03 Volume 1. Section 6, Medical Standards for Military Service: Appointment, Enlistment, or Induction. Updated November 16, 2022. Accessed November 20, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF
7. Sayers D, Hu Z, Clark LL. Attrition rates and incidence of mental health disorders in an attention-deficit hyperactivity disorder (ADHD) cohort, active component, U.S. Armed Forces, 2014-2018. MSMR. 2021;28(1):2-8.
8. Woods J. Serving with ADHD. Accessed November 20, 2023. https://www.armyupress.army.mil/Journals/NCO-Journal/Archives/2022/February/Serving-with-ADHD
9. Thayer RL. Pentagon reviews whether 38 medical conditions should remain as disqualifiers for military service. Accessed November 20, 2023. https://www.stripes.com/theaters/us/2023-03-07/military-medical-waivers-recruitment-9417905.html
10. Weinbaum C. An autistic soldier wants you to read this. Accessed November 20, 2023. https://mwi.usma.edu/an-autistic-soldier-wants-you-to-read-this
11. Weinbaum C, Khan O, Thomas TD, Stein BD. Neurodiversity and national security. Accessed November 20, 2023. https://www.rand.org/pubs/research_reports/RRA1875-1.html
12. Myers M. Senators push DoD to approve recruits who have sought mental health care. Accessed November 20, 2023. https://www.militarytimes.com/news/your-military/2023/03/16/senators-push-dod-to-approve-recruits-whove-sought-mental-health-care
The willingness with which our young people are likely to serve in any war, no matter how justified, shall be directly proportional to how they perceive how the veterans of earlier wars were treated and appreciated by their nation.
George Washington? 1
This editorial is the second of the 2-part series on the recruitment crisis currently confronting the Army, Navy, and Air Force. Part 1 focused on rationales for the lack of interest or motivation among those potentially eligible to join the military. This column looks at individuals eager to serve who do not meet eligibility requirements. A 2022 article examining the 2020 Qualified Military Available Study found that without a waiver 77% of Americans in the prime recruiting age group 17 to 24 years would be ineligible for the military due to weight, substance use, or mental and physical health conditions. Most young adults met several ineligibility criteria.2
Obesity and substance use are the most common disqualifiers, mirroring the culture at large. Scores of other physical and mental health conditions render an applicant ineligible for military service or require a waiver. The justification of all eligibility criteria is to: (1) ensure that service members can safely and effectively deploy; and (2) reduce the attrition rate. Both are essential to the mission readiness of the military. In 2022, the military gave 1 in 6 of those seeking enlistment an accession waiver.3 About 4% of waivers issued were for mental health conditions, such as autism and attention-deficit hyperactivity disorder (ADHD). The response to the recruiting crisis resulted in the largest number of waivers granted in a decade. Military Times noted that exact numbers are hard to obtain, interfering with the transparency of public policy as well as high-quality research on waivers’ impact on recruits and the service.3
The War Horse reported that the current waiver process is riddled with procedural injustice and inequity in implementation.4 Each service sets its eligibility requirements: the rationale being that the respective branches have distinct roles necessitating distinguishing qualifications. What is far more difficult to defend is that wide variation exists in the application of the criteria. Similar cases are judged differently, depending on nonmaterial factors, such as geographic location and unwritten policies of recruiting offices. Waiver approval rates for mental health conditions range from 35% for the Army to 71% for the Marines. The prospective recruit, not the military service, bears the burden of demonstrating that their condition does not impair their fitness for duty; hence, thousands have been disqualified based on their diagnosis.4 This comes at a time when the US Department of Veterans Affairs (VA) and the US Department of Defense (DoD) have been battling a suicide epidemic for years. Current qualifying standards send a strong stigmatizing message to those who want to enlist and those already in the ranks at a time the DoD and VA are launching campaigns to persuade active-duty members and veterans to seek mental health treatment.5
The recruiting crisis brought into stark relief more fundamental questions about the clinical and ethical aspects of eligibility criteria that either disqualify outright or require a waiver process for many young Americans with mental health conditions who want to serve their country. One of the most clinically perplexing standards is that applicants with ADHD are disqualified if they have taken medications in the past 12 to 24 months, depending on the service.6 Despite this policy, the Army acknowledges that stimulant medications may improve the function of individuals with ADHD and reduce the rates of substance use and behavior disturbances, the real concerns for recruiters and commanders.7
Requirements like these place otherwise high-functioning individuals whose professional goal is to serve in the military in a double bind. The military’s studies show that recruits’ persistent nondisclosure of their diagnoses results in poorer performance and higher attrition rates of those who have enlisted, even when treated.8 If potential recruits disclose their psychiatric history, they may well be disqualified and/or denied a waiver. This is even more true for service members already in the military who may believe they have one of the conditions but fear that being diagnosed will negatively impact their career. Not disclosing their condition prevents service members from obtaining the clinical care and support they need to succeed and also limits the ability of commanders to make decisions about deployment that ensure maximal unit performance and the safety of the service member.9 However, ADHD is one of 38 diagnoses that the DoD is considering for possible removal or modification of the waiver for some subset of applicants.10
The final irony is that medicine and warfare have changed dramatically and rapidly since the initial determination that diagnoses like ADHD and autism disqualify individuals from serving. A Rand Corporation study found that individuals who are neurodivergent—the name collectively assigned to individuals with diagnoses like autism and ADHD—may have unique abilities that enable them to outperform neurotypical persons in areas like pattern recognition, attention to detail, repetitive tasks, and memory, among others. These highly technical skills are essential to intelligence analysis and cybersecurity domains that are increasingly crucial to both national defense and victory on the battlefield.11 Even congressional representatives who just a few years ago criticized waivers for mental health conditions as “lowering the standards” are now pushing for more moderate policies, especially for those who have received and responded to treatment for their mental health disorders.12
The epigraph has been widely and persistently misattributed to the country’s first commander in chief, George Washington, because it captures a salient sentiment directly bearing on the question of who is fit for duty.1 History has shown that discrimination in enlistment only weakens the fighting force, whereas diversity, including neurodiversity, in the military as in society is a source of strength. Equitable inclusion of those who have the discipline, desire, and dedication to serve their country may be the most positive response to the recruitment crisis.
The willingness with which our young people are likely to serve in any war, no matter how justified, shall be directly proportional to how they perceive how the veterans of earlier wars were treated and appreciated by their nation.
George Washington? 1
This editorial is the second of the 2-part series on the recruitment crisis currently confronting the Army, Navy, and Air Force. Part 1 focused on rationales for the lack of interest or motivation among those potentially eligible to join the military. This column looks at individuals eager to serve who do not meet eligibility requirements. A 2022 article examining the 2020 Qualified Military Available Study found that without a waiver 77% of Americans in the prime recruiting age group 17 to 24 years would be ineligible for the military due to weight, substance use, or mental and physical health conditions. Most young adults met several ineligibility criteria.2
Obesity and substance use are the most common disqualifiers, mirroring the culture at large. Scores of other physical and mental health conditions render an applicant ineligible for military service or require a waiver. The justification of all eligibility criteria is to: (1) ensure that service members can safely and effectively deploy; and (2) reduce the attrition rate. Both are essential to the mission readiness of the military. In 2022, the military gave 1 in 6 of those seeking enlistment an accession waiver.3 About 4% of waivers issued were for mental health conditions, such as autism and attention-deficit hyperactivity disorder (ADHD). The response to the recruiting crisis resulted in the largest number of waivers granted in a decade. Military Times noted that exact numbers are hard to obtain, interfering with the transparency of public policy as well as high-quality research on waivers’ impact on recruits and the service.3
The War Horse reported that the current waiver process is riddled with procedural injustice and inequity in implementation.4 Each service sets its eligibility requirements: the rationale being that the respective branches have distinct roles necessitating distinguishing qualifications. What is far more difficult to defend is that wide variation exists in the application of the criteria. Similar cases are judged differently, depending on nonmaterial factors, such as geographic location and unwritten policies of recruiting offices. Waiver approval rates for mental health conditions range from 35% for the Army to 71% for the Marines. The prospective recruit, not the military service, bears the burden of demonstrating that their condition does not impair their fitness for duty; hence, thousands have been disqualified based on their diagnosis.4 This comes at a time when the US Department of Veterans Affairs (VA) and the US Department of Defense (DoD) have been battling a suicide epidemic for years. Current qualifying standards send a strong stigmatizing message to those who want to enlist and those already in the ranks at a time the DoD and VA are launching campaigns to persuade active-duty members and veterans to seek mental health treatment.5
The recruiting crisis brought into stark relief more fundamental questions about the clinical and ethical aspects of eligibility criteria that either disqualify outright or require a waiver process for many young Americans with mental health conditions who want to serve their country. One of the most clinically perplexing standards is that applicants with ADHD are disqualified if they have taken medications in the past 12 to 24 months, depending on the service.6 Despite this policy, the Army acknowledges that stimulant medications may improve the function of individuals with ADHD and reduce the rates of substance use and behavior disturbances, the real concerns for recruiters and commanders.7
Requirements like these place otherwise high-functioning individuals whose professional goal is to serve in the military in a double bind. The military’s studies show that recruits’ persistent nondisclosure of their diagnoses results in poorer performance and higher attrition rates of those who have enlisted, even when treated.8 If potential recruits disclose their psychiatric history, they may well be disqualified and/or denied a waiver. This is even more true for service members already in the military who may believe they have one of the conditions but fear that being diagnosed will negatively impact their career. Not disclosing their condition prevents service members from obtaining the clinical care and support they need to succeed and also limits the ability of commanders to make decisions about deployment that ensure maximal unit performance and the safety of the service member.9 However, ADHD is one of 38 diagnoses that the DoD is considering for possible removal or modification of the waiver for some subset of applicants.10
The final irony is that medicine and warfare have changed dramatically and rapidly since the initial determination that diagnoses like ADHD and autism disqualify individuals from serving. A Rand Corporation study found that individuals who are neurodivergent—the name collectively assigned to individuals with diagnoses like autism and ADHD—may have unique abilities that enable them to outperform neurotypical persons in areas like pattern recognition, attention to detail, repetitive tasks, and memory, among others. These highly technical skills are essential to intelligence analysis and cybersecurity domains that are increasingly crucial to both national defense and victory on the battlefield.11 Even congressional representatives who just a few years ago criticized waivers for mental health conditions as “lowering the standards” are now pushing for more moderate policies, especially for those who have received and responded to treatment for their mental health disorders.12
The epigraph has been widely and persistently misattributed to the country’s first commander in chief, George Washington, because it captures a salient sentiment directly bearing on the question of who is fit for duty.1 History has shown that discrimination in enlistment only weakens the fighting force, whereas diversity, including neurodiversity, in the military as in society is a source of strength. Equitable inclusion of those who have the discipline, desire, and dedication to serve their country may be the most positive response to the recruitment crisis.
1. George Washington’s Mount Vernon Washington Library. Accessed November 13, 2023. https://www.mountvernon.org/library/digitalhistory/digital-encyclopedia/article/spurious-quotations/
2. Novelty T. Even more young Americans are unfit to serve, a new study finds, here’s why. Accessed November 20, 2023. https://www.military.com/daily-news/2022/09/28/new-pentagon-study-shows-77-of-young-americans-are-ineligible-military-service.html
3. Cohen RS. Need for accession waivers soars amid historic recruiting challenges. Accessed November 20, 2023. https://www.militarytimes.com/news/your-air-force/2023/04/10/need-for-accession-waivers-soars-amid-historic-recruiting-challenges
4. Barnhill J. The military is missing recruitment goals. Are thousands being disqualified. The War Horse. Accessed November 20, 2023. https://thewarhorse.org/us-military-recruitment-crisis-may-hinge-on-medical-waivers
5. Hauschild V. Army experts: mixed messages can fuel stigma, prevent soldiers from accessing behavioral healthcare. Accessed November 20, 2023. https://www.army.mil/article/262525/army_experts_mixed_messages_can_fuel_stigma_prevent_soldiers_from_accessing_behavioral_healthcare
6. US Department of Defense. DoD Instructions 6130.03 Volume 1. Section 6, Medical Standards for Military Service: Appointment, Enlistment, or Induction. Updated November 16, 2022. Accessed November 20, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF
7. Sayers D, Hu Z, Clark LL. Attrition rates and incidence of mental health disorders in an attention-deficit hyperactivity disorder (ADHD) cohort, active component, U.S. Armed Forces, 2014-2018. MSMR. 2021;28(1):2-8.
8. Woods J. Serving with ADHD. Accessed November 20, 2023. https://www.armyupress.army.mil/Journals/NCO-Journal/Archives/2022/February/Serving-with-ADHD
9. Thayer RL. Pentagon reviews whether 38 medical conditions should remain as disqualifiers for military service. Accessed November 20, 2023. https://www.stripes.com/theaters/us/2023-03-07/military-medical-waivers-recruitment-9417905.html
10. Weinbaum C. An autistic soldier wants you to read this. Accessed November 20, 2023. https://mwi.usma.edu/an-autistic-soldier-wants-you-to-read-this
11. Weinbaum C, Khan O, Thomas TD, Stein BD. Neurodiversity and national security. Accessed November 20, 2023. https://www.rand.org/pubs/research_reports/RRA1875-1.html
12. Myers M. Senators push DoD to approve recruits who have sought mental health care. Accessed November 20, 2023. https://www.militarytimes.com/news/your-military/2023/03/16/senators-push-dod-to-approve-recruits-whove-sought-mental-health-care
1. George Washington’s Mount Vernon Washington Library. Accessed November 13, 2023. https://www.mountvernon.org/library/digitalhistory/digital-encyclopedia/article/spurious-quotations/
2. Novelty T. Even more young Americans are unfit to serve, a new study finds, here’s why. Accessed November 20, 2023. https://www.military.com/daily-news/2022/09/28/new-pentagon-study-shows-77-of-young-americans-are-ineligible-military-service.html
3. Cohen RS. Need for accession waivers soars amid historic recruiting challenges. Accessed November 20, 2023. https://www.militarytimes.com/news/your-air-force/2023/04/10/need-for-accession-waivers-soars-amid-historic-recruiting-challenges
4. Barnhill J. The military is missing recruitment goals. Are thousands being disqualified. The War Horse. Accessed November 20, 2023. https://thewarhorse.org/us-military-recruitment-crisis-may-hinge-on-medical-waivers
5. Hauschild V. Army experts: mixed messages can fuel stigma, prevent soldiers from accessing behavioral healthcare. Accessed November 20, 2023. https://www.army.mil/article/262525/army_experts_mixed_messages_can_fuel_stigma_prevent_soldiers_from_accessing_behavioral_healthcare
6. US Department of Defense. DoD Instructions 6130.03 Volume 1. Section 6, Medical Standards for Military Service: Appointment, Enlistment, or Induction. Updated November 16, 2022. Accessed November 20, 2023. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_vol1.PDF
7. Sayers D, Hu Z, Clark LL. Attrition rates and incidence of mental health disorders in an attention-deficit hyperactivity disorder (ADHD) cohort, active component, U.S. Armed Forces, 2014-2018. MSMR. 2021;28(1):2-8.
8. Woods J. Serving with ADHD. Accessed November 20, 2023. https://www.armyupress.army.mil/Journals/NCO-Journal/Archives/2022/February/Serving-with-ADHD
9. Thayer RL. Pentagon reviews whether 38 medical conditions should remain as disqualifiers for military service. Accessed November 20, 2023. https://www.stripes.com/theaters/us/2023-03-07/military-medical-waivers-recruitment-9417905.html
10. Weinbaum C. An autistic soldier wants you to read this. Accessed November 20, 2023. https://mwi.usma.edu/an-autistic-soldier-wants-you-to-read-this
11. Weinbaum C, Khan O, Thomas TD, Stein BD. Neurodiversity and national security. Accessed November 20, 2023. https://www.rand.org/pubs/research_reports/RRA1875-1.html
12. Myers M. Senators push DoD to approve recruits who have sought mental health care. Accessed November 20, 2023. https://www.militarytimes.com/news/your-military/2023/03/16/senators-push-dod-to-approve-recruits-whove-sought-mental-health-care
In general, I’m happy
I’m a general neurologist. I consider myself a jack of all (or at least most) trades in my field, and a master of none.
In the April 2023 issue of JAMA Neurology there was an editorial about neurology training, with general neurology being renamed “comprehensive neurology” and a fellowship offered in practicing general neurology.
This seems rather silly to me. If 4 years of residency (1 of internship and 3 of neurology) don’t prepare you to practice general neurology, then what’s the point of residency at all? For that matter, what difference will renaming it do?
Imagine completing a 3-year internal medicine residency, then being told you need to do a fellowship in “comprehensive medicine” in order to practice. Or at least so you can add the word “comprehensive” to your shingle.
The authors bemoan the increasing number of neurology residents wanting to do fellowships and subspecialize, a situation that mirrors the general trend of people away from general medicine toward specialties.
While I agree we do need subspecialists in neurology (and currently there are at least 31 recognized, which is way more than I would have guessed), the fact is that patients, and sometimes their internists, aren’t going to be the best judge of who does or doesn’t need to see one, compared with a general neurologist.
Most of us general people can handle straightforward Parkinson’s disease, epilepsy, migraines, etc. Certainly, there are times where the condition is refractory to our care, or there’s something unusual about the case, that leads us to refer them to someone with more expertise. But isn’t that how it’s supposed to work? Like medicine in general, we need more general people than subspecialists.
Honestly, I can’t claim to be any different. Twenty-six years ago, when I finished residency, I did a clinical neurophysiology fellowship. From a practical view it was an epilepsy fellowship at my program. Some of this was an interest at the time in subspecializing, some of it was me putting off joining the “real world” of having to find a job for a year.
When I hung up my own shingle, my business card listed a subspecialty in epilepsy. Looking back years later, this wasn’t the best move. In solo practice I had no access to an epilepsy monitoring unit, vagus nerve stimulation capabilities, or epilepsy surgery at the hospital I rounded at. Not only that, I discovered it put me at a disadvantage, as internists were referring only epilepsy patients to me, and all the other stuff (which is the majority of patients) to the general (or comprehensive) neurologists around me. Which, financially, wasn’t a good thing when you’re young and starting out.
Not only that, but I discovered that I didn’t like only seeing one thing. I found it boring, and not for me.
So after a year or so, I took the word “epilepsy” off my card, left it at “general neurology,” and sent out letters reminding my referral base that I was willing to see the majority of things in my field (rare diseases, even today, I won’t attempt to handle).
So now my days are a mix of things, which I like. Neurology is enough of a specialty for me without going further up the pyramid. Having sub (and even sub-sub) specialists is important to maintain medical excellence, but we still need people willing to do general neurology, and I’m happy there.
Changing my title to “comprehensive” is unnecessary. I’m happy with what I am.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m a general neurologist. I consider myself a jack of all (or at least most) trades in my field, and a master of none.
In the April 2023 issue of JAMA Neurology there was an editorial about neurology training, with general neurology being renamed “comprehensive neurology” and a fellowship offered in practicing general neurology.
This seems rather silly to me. If 4 years of residency (1 of internship and 3 of neurology) don’t prepare you to practice general neurology, then what’s the point of residency at all? For that matter, what difference will renaming it do?
Imagine completing a 3-year internal medicine residency, then being told you need to do a fellowship in “comprehensive medicine” in order to practice. Or at least so you can add the word “comprehensive” to your shingle.
The authors bemoan the increasing number of neurology residents wanting to do fellowships and subspecialize, a situation that mirrors the general trend of people away from general medicine toward specialties.
While I agree we do need subspecialists in neurology (and currently there are at least 31 recognized, which is way more than I would have guessed), the fact is that patients, and sometimes their internists, aren’t going to be the best judge of who does or doesn’t need to see one, compared with a general neurologist.
Most of us general people can handle straightforward Parkinson’s disease, epilepsy, migraines, etc. Certainly, there are times where the condition is refractory to our care, or there’s something unusual about the case, that leads us to refer them to someone with more expertise. But isn’t that how it’s supposed to work? Like medicine in general, we need more general people than subspecialists.
Honestly, I can’t claim to be any different. Twenty-six years ago, when I finished residency, I did a clinical neurophysiology fellowship. From a practical view it was an epilepsy fellowship at my program. Some of this was an interest at the time in subspecializing, some of it was me putting off joining the “real world” of having to find a job for a year.
When I hung up my own shingle, my business card listed a subspecialty in epilepsy. Looking back years later, this wasn’t the best move. In solo practice I had no access to an epilepsy monitoring unit, vagus nerve stimulation capabilities, or epilepsy surgery at the hospital I rounded at. Not only that, I discovered it put me at a disadvantage, as internists were referring only epilepsy patients to me, and all the other stuff (which is the majority of patients) to the general (or comprehensive) neurologists around me. Which, financially, wasn’t a good thing when you’re young and starting out.
Not only that, but I discovered that I didn’t like only seeing one thing. I found it boring, and not for me.
So after a year or so, I took the word “epilepsy” off my card, left it at “general neurology,” and sent out letters reminding my referral base that I was willing to see the majority of things in my field (rare diseases, even today, I won’t attempt to handle).
So now my days are a mix of things, which I like. Neurology is enough of a specialty for me without going further up the pyramid. Having sub (and even sub-sub) specialists is important to maintain medical excellence, but we still need people willing to do general neurology, and I’m happy there.
Changing my title to “comprehensive” is unnecessary. I’m happy with what I am.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m a general neurologist. I consider myself a jack of all (or at least most) trades in my field, and a master of none.
In the April 2023 issue of JAMA Neurology there was an editorial about neurology training, with general neurology being renamed “comprehensive neurology” and a fellowship offered in practicing general neurology.
This seems rather silly to me. If 4 years of residency (1 of internship and 3 of neurology) don’t prepare you to practice general neurology, then what’s the point of residency at all? For that matter, what difference will renaming it do?
Imagine completing a 3-year internal medicine residency, then being told you need to do a fellowship in “comprehensive medicine” in order to practice. Or at least so you can add the word “comprehensive” to your shingle.
The authors bemoan the increasing number of neurology residents wanting to do fellowships and subspecialize, a situation that mirrors the general trend of people away from general medicine toward specialties.
While I agree we do need subspecialists in neurology (and currently there are at least 31 recognized, which is way more than I would have guessed), the fact is that patients, and sometimes their internists, aren’t going to be the best judge of who does or doesn’t need to see one, compared with a general neurologist.
Most of us general people can handle straightforward Parkinson’s disease, epilepsy, migraines, etc. Certainly, there are times where the condition is refractory to our care, or there’s something unusual about the case, that leads us to refer them to someone with more expertise. But isn’t that how it’s supposed to work? Like medicine in general, we need more general people than subspecialists.
Honestly, I can’t claim to be any different. Twenty-six years ago, when I finished residency, I did a clinical neurophysiology fellowship. From a practical view it was an epilepsy fellowship at my program. Some of this was an interest at the time in subspecializing, some of it was me putting off joining the “real world” of having to find a job for a year.
When I hung up my own shingle, my business card listed a subspecialty in epilepsy. Looking back years later, this wasn’t the best move. In solo practice I had no access to an epilepsy monitoring unit, vagus nerve stimulation capabilities, or epilepsy surgery at the hospital I rounded at. Not only that, I discovered it put me at a disadvantage, as internists were referring only epilepsy patients to me, and all the other stuff (which is the majority of patients) to the general (or comprehensive) neurologists around me. Which, financially, wasn’t a good thing when you’re young and starting out.
Not only that, but I discovered that I didn’t like only seeing one thing. I found it boring, and not for me.
So after a year or so, I took the word “epilepsy” off my card, left it at “general neurology,” and sent out letters reminding my referral base that I was willing to see the majority of things in my field (rare diseases, even today, I won’t attempt to handle).
So now my days are a mix of things, which I like. Neurology is enough of a specialty for me without going further up the pyramid. Having sub (and even sub-sub) specialists is important to maintain medical excellence, but we still need people willing to do general neurology, and I’m happy there.
Changing my title to “comprehensive” is unnecessary. I’m happy with what I am.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is air filtration the best public health intervention against respiratory viruses?
This transcript has been edited for clarity.
When it comes to the public health fight against respiratory viruses – COVID, flu, RSV, and so on – it has always struck me as strange how staunchly basically any intervention is opposed. Masking was, of course, the prototypical entrenched warfare of opposing ideologies, with advocates pointing to studies suggesting the efficacy of masking to prevent transmission and advocating for broad masking recommendations, and detractors citing studies that suggested masks were ineffective and characterizing masking policies as fascist overreach. I’ll admit that I was always perplexed by this a bit, as that particular intervention seemed so benign – a bit annoying, I guess, but not crazy.
I have come to appreciate what I call status quo bias, which is the tendency to reject any policy, advice, or intervention that would force you, as an individual, to change your usual behavior. We just don’t like to do that. It has made me think that the most successful public health interventions might be the ones that take the individual out of the loop. And air quality control seems an ideal fit here. Here is a potential intervention where you, the individual, have to do precisely nothing. The status quo is preserved. We just, you know, have cleaner indoor air.
But even the suggestion of air treatment systems as a bulwark against respiratory virus transmission has been met with not just skepticism but cynicism, and perhaps even defeatism. It seems that there are those out there who think there really is nothing we can do. Sickness is interpreted in a Calvinistic framework: You become ill because it is your pre-destiny. But maybe air treatment could actually work. It seems like it might, if a new paper from PLOS One is to be believed.
What we’re talking about is a study titled “Bipolar Ionization Rapidly Inactivates Real-World, Airborne Concentrations of Infective Respiratory Viruses” – a highly controlled, laboratory-based analysis of a bipolar ionization system which seems to rapidly reduce viral counts in the air.
The proposed mechanism of action is pretty simple. The ionization system – which, don’t worry, has been shown not to produce ozone – spits out positively and negatively charged particles, which float around the test chamber, designed to look like a pretty standard room that you might find in an office or a school.
Virus is then injected into the chamber through an aerosolization machine, to achieve concentrations on the order of what you might get standing within 6 feet or so of someone actively infected with COVID while they are breathing and talking.
The idea is that those ions stick to the virus particles, similar to how a balloon sticks to the wall after you rub it on your hair, and that tends to cause them to clump together and settle on surfaces more rapidly, and thus get farther away from their ports of entry to the human system: nose, mouth, and eyes. But the ions may also interfere with viruses’ ability to bind to cellular receptors, even in the air.
To quantify viral infectivity, the researchers used a biological system. Basically, you take air samples and expose a petri dish of cells to them and see how many cells die. Fewer cells dying, less infective. Under control conditions, you can see that virus infectivity does decrease over time. Time zero here is the end of a SARS-CoV-2 aerosolization.
This may simply reflect the fact that virus particles settle out of the air. But As you can see, within about an hour, you have almost no infective virus detectable. That’s fairly impressive.
Now, I’m not saying that this is a panacea, but it is certainly worth considering the use of technologies like these if we are going to revamp the infrastructure of our offices and schools. And, of course, it would be nice to see this tested in a rigorous clinical trial with actual infected people, not cells, as the outcome. But I continue to be encouraged by interventions like this which, to be honest, ask very little of us as individuals. Maybe it’s time we accept the things, or people, that we cannot change.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When it comes to the public health fight against respiratory viruses – COVID, flu, RSV, and so on – it has always struck me as strange how staunchly basically any intervention is opposed. Masking was, of course, the prototypical entrenched warfare of opposing ideologies, with advocates pointing to studies suggesting the efficacy of masking to prevent transmission and advocating for broad masking recommendations, and detractors citing studies that suggested masks were ineffective and characterizing masking policies as fascist overreach. I’ll admit that I was always perplexed by this a bit, as that particular intervention seemed so benign – a bit annoying, I guess, but not crazy.
I have come to appreciate what I call status quo bias, which is the tendency to reject any policy, advice, or intervention that would force you, as an individual, to change your usual behavior. We just don’t like to do that. It has made me think that the most successful public health interventions might be the ones that take the individual out of the loop. And air quality control seems an ideal fit here. Here is a potential intervention where you, the individual, have to do precisely nothing. The status quo is preserved. We just, you know, have cleaner indoor air.
But even the suggestion of air treatment systems as a bulwark against respiratory virus transmission has been met with not just skepticism but cynicism, and perhaps even defeatism. It seems that there are those out there who think there really is nothing we can do. Sickness is interpreted in a Calvinistic framework: You become ill because it is your pre-destiny. But maybe air treatment could actually work. It seems like it might, if a new paper from PLOS One is to be believed.
What we’re talking about is a study titled “Bipolar Ionization Rapidly Inactivates Real-World, Airborne Concentrations of Infective Respiratory Viruses” – a highly controlled, laboratory-based analysis of a bipolar ionization system which seems to rapidly reduce viral counts in the air.
The proposed mechanism of action is pretty simple. The ionization system – which, don’t worry, has been shown not to produce ozone – spits out positively and negatively charged particles, which float around the test chamber, designed to look like a pretty standard room that you might find in an office or a school.
Virus is then injected into the chamber through an aerosolization machine, to achieve concentrations on the order of what you might get standing within 6 feet or so of someone actively infected with COVID while they are breathing and talking.
The idea is that those ions stick to the virus particles, similar to how a balloon sticks to the wall after you rub it on your hair, and that tends to cause them to clump together and settle on surfaces more rapidly, and thus get farther away from their ports of entry to the human system: nose, mouth, and eyes. But the ions may also interfere with viruses’ ability to bind to cellular receptors, even in the air.
To quantify viral infectivity, the researchers used a biological system. Basically, you take air samples and expose a petri dish of cells to them and see how many cells die. Fewer cells dying, less infective. Under control conditions, you can see that virus infectivity does decrease over time. Time zero here is the end of a SARS-CoV-2 aerosolization.
This may simply reflect the fact that virus particles settle out of the air. But As you can see, within about an hour, you have almost no infective virus detectable. That’s fairly impressive.
Now, I’m not saying that this is a panacea, but it is certainly worth considering the use of technologies like these if we are going to revamp the infrastructure of our offices and schools. And, of course, it would be nice to see this tested in a rigorous clinical trial with actual infected people, not cells, as the outcome. But I continue to be encouraged by interventions like this which, to be honest, ask very little of us as individuals. Maybe it’s time we accept the things, or people, that we cannot change.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
When it comes to the public health fight against respiratory viruses – COVID, flu, RSV, and so on – it has always struck me as strange how staunchly basically any intervention is opposed. Masking was, of course, the prototypical entrenched warfare of opposing ideologies, with advocates pointing to studies suggesting the efficacy of masking to prevent transmission and advocating for broad masking recommendations, and detractors citing studies that suggested masks were ineffective and characterizing masking policies as fascist overreach. I’ll admit that I was always perplexed by this a bit, as that particular intervention seemed so benign – a bit annoying, I guess, but not crazy.
I have come to appreciate what I call status quo bias, which is the tendency to reject any policy, advice, or intervention that would force you, as an individual, to change your usual behavior. We just don’t like to do that. It has made me think that the most successful public health interventions might be the ones that take the individual out of the loop. And air quality control seems an ideal fit here. Here is a potential intervention where you, the individual, have to do precisely nothing. The status quo is preserved. We just, you know, have cleaner indoor air.
But even the suggestion of air treatment systems as a bulwark against respiratory virus transmission has been met with not just skepticism but cynicism, and perhaps even defeatism. It seems that there are those out there who think there really is nothing we can do. Sickness is interpreted in a Calvinistic framework: You become ill because it is your pre-destiny. But maybe air treatment could actually work. It seems like it might, if a new paper from PLOS One is to be believed.
What we’re talking about is a study titled “Bipolar Ionization Rapidly Inactivates Real-World, Airborne Concentrations of Infective Respiratory Viruses” – a highly controlled, laboratory-based analysis of a bipolar ionization system which seems to rapidly reduce viral counts in the air.
The proposed mechanism of action is pretty simple. The ionization system – which, don’t worry, has been shown not to produce ozone – spits out positively and negatively charged particles, which float around the test chamber, designed to look like a pretty standard room that you might find in an office or a school.
Virus is then injected into the chamber through an aerosolization machine, to achieve concentrations on the order of what you might get standing within 6 feet or so of someone actively infected with COVID while they are breathing and talking.
The idea is that those ions stick to the virus particles, similar to how a balloon sticks to the wall after you rub it on your hair, and that tends to cause them to clump together and settle on surfaces more rapidly, and thus get farther away from their ports of entry to the human system: nose, mouth, and eyes. But the ions may also interfere with viruses’ ability to bind to cellular receptors, even in the air.
To quantify viral infectivity, the researchers used a biological system. Basically, you take air samples and expose a petri dish of cells to them and see how many cells die. Fewer cells dying, less infective. Under control conditions, you can see that virus infectivity does decrease over time. Time zero here is the end of a SARS-CoV-2 aerosolization.
This may simply reflect the fact that virus particles settle out of the air. But As you can see, within about an hour, you have almost no infective virus detectable. That’s fairly impressive.
Now, I’m not saying that this is a panacea, but it is certainly worth considering the use of technologies like these if we are going to revamp the infrastructure of our offices and schools. And, of course, it would be nice to see this tested in a rigorous clinical trial with actual infected people, not cells, as the outcome. But I continue to be encouraged by interventions like this which, to be honest, ask very little of us as individuals. Maybe it’s time we accept the things, or people, that we cannot change.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.












