User login
50 years of ob.gyn.: Has practice changed for the better?
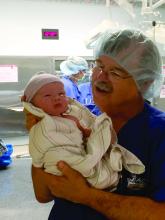
As a practicing obstetrician-gynecologist for nearly 40 years, Leonard Brabson, MD, has watched his specialty transform in ways both large and small.
For starters, his regular work attire in 1977 – a shirt and tie – is now scrubs. The paper charts that once filled his office shelves have been replaced with electronic records. And the cumbersome machines that once took blurry, still pictures of a fetus have advanced by leaps and bounds and become a staple of prenatal care.
“Back then, we were expected to be generalists. If [a patient] had a cancer, you took care of it. If [she] had a fertility problem or problem with endocrinology, you took care of it. Of course, now if I have a female cancer, we refer them to the gyn-oncologist. But when you go back 40 years and beyond, we did mostly everything.”
The ob.gyns. of today are practicing in a vastly different environment than their predecessors, and while many of the differences have improved patient care and enhanced efficiency, physicians also note that some changes have harmed the doctor-patient relationship and created career dissatisfaction.
“Certainly, the advances of modern medicine have enabled the current physician to provide the patient a level of care unparalleled in history,” said Charles E. Miller, MD, a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.
More volume, less time
Most long-time physicians agree that higher patient volumes and increasing administrative burdens have diminished the time they are able to spend with patients.
Rising clinical documentation and coding are the top administrative tasks taking away from one-on-one patient care, said Kristen Zeligs, MD, chair of the American Congress of Obstetricians and Gynecologists’ Junior Fellow Congress Advisory Council and a gynecologic oncology fellow at Walter Reed National Military Medical Center in Bethesda, Md.
But there are other factors straining the doctor-patient relationship. Decades ago, first-time mothers often stayed with one doctor for a lifetime, Dr. Brabson recalled, having all of her babies delivered by a single ob.gyn. The same can’t be said for today, where insurance changes, job relocations, and a lack of connections often lead patients to switch physicians frequently.
“Now a lot of patients [move on] from one year to the next,” Dr. Brabson said. “There’s not that same loyalty.”
Doctors, too, traditionally stuck with patients over the long haul, he added. In the past, if an ob.gyn treated a patient during pregnancy, that same physician was present during the delivery, even if it meant leaving a vacation early or coming in on a night off.
“Now the younger docs, especially those that have families with small children, when it’s not their night on call and it’s 5 o’clock, they’re checking out,” Dr. Brabson said. “One of my partners right now, she started inducing someone yesterday. Well today’s her day off, so I’m going to do the delivery. That’s been a big change.”
Highs and lows of liability premiums
Another pressure on the specialty over the last couple of decades has been the high cost of liability insurance.
“2003 was really the peak of the liability crisis,” Dr. Montgomery said. “Hospitals were closing. Doctors were giving up practice. It’s a little better now than it was.”
While ob.gyns. still pay higher premiums than many other specialties, the legal climate has improved in recent years, said Paul Greve Jr., executive vice president and senior consultant for the Willis Healthcare Practice, and author of the 2016 Medical Liability Monitor, an annual report that surveys medical liability premiums.
“The best way to characterize the overall environment for medical professional liability is stable,” he said. “In the area of obstetrics, for the first time ever in recent years, we are seeing lower frequency and lower severity [of lawsuits].”
The lower number of filings are largely due to patient safety initiatives among ob.gyn. programs and tort reforms – many of which have been upheld by courts in the last decade, Mr. Greve said.
Of course, the rate of premiums greatly differs depending on location, with ob.gyns. in Eastern New York paying a high of $214,999 and ob.gyns. in Minnesota paying a low of $16,449 in 2016, according to the Medical Liability Monitor.
“The environment is specific to the region,” Mr. Greve said. “New York City is still very problematic. Chicago is still very problematic. There are some pockets around the country where there’s no damage caps, and it’s really tough to defend claims.”
Technology ups and downs
Another fairly new pressure on ob.gyns. is the integration of the electronic health record and the federal reporting requirements that go along with it.
“Most practicing ob.gyns. are really fed up with the computerization of medicine and the tasking and the charting,” Dr. Montgomery said. “For every hour you spend seeing patients, you spend 1 or 2 hours doing computer chart work and paperwork. Most doctors don’t go into medicine so they can type; they go into medicine to take care of patients.”
The Internet age also poses challenges when it comes to patients conducting their own “research,” said Megan Evans, MD, an ob.gyn. at Tufts Medical Center, Boston.
Protecting the security of patients’ medical records in the digital age is another worry, she said.
But for Dr. Evans, who completed her residency training in 2015, having dozens of digital tools at her disposal as she treats patients is definitely an upside to today’s practice environment.
“I can review a practice bulletin, look up the latest treatment regimens, and contact my colleagues with a quick question – all on my iPhone,” she said. “I also believe there is so much potential for electronic medical records and how they communicate with each other.”
Advancements in ultrasound, fetal monitoring, and other medical technologies have also allowed ob.gyns. to intervene earlier and save lives.
Dr. Brabson recalled the helplessness he and other physicians felt in the 1970s when it came to delivering extremely premature babies.
“We didn’t really feel like you could save a baby under 2 pounds,” he said. “When I was a medical student, if you had a baby under 2 pounds, very commonly what they would do is lay the baby up on the table and watch and see how vigorous it was going to be, and if it really did breathe and carry on for awhile, then you might take it to the nursery. The equipment that we have to save babies with today, compared to 40 years ago, that’s a dramatic change.”
A changing focus for the future
If current trends continue, Dr. Brabson’s early experience of being a generalist ob.gyn. won’t be the norm. Instead, more ob.gyns. will choose to subspecialize. Whether this change is positive or negative for the specialty depends on who you ask.
“You could argue the pros and cons for both sides,” Dr. Zeligs said. “For me, it takes away from what drew me to the specialty – the breadth that ob.gyn. offers, both as a primary care specialty and as a surgical subspecialty.”
However, choosing one focus may offer some doctors a way to capture that elusive professional and personal balance, she added.
Despite the changing landscape of clinical duties and business operations, some parts of ob.gyn. practice have remained intact, according to Dr. Brabson. “The most rewarding and enjoyable part of the job is developing a relationship of mutual trust and respect,” he said. “As a result of developing such a relationship, both the patient and the doctor come away with positive feelings. This has not changed.”
[email protected]
On Twitter @legal_med
As a practicing obstetrician-gynecologist for nearly 40 years, Leonard Brabson, MD, has watched his specialty transform in ways both large and small.
For starters, his regular work attire in 1977 – a shirt and tie – is now scrubs. The paper charts that once filled his office shelves have been replaced with electronic records. And the cumbersome machines that once took blurry, still pictures of a fetus have advanced by leaps and bounds and become a staple of prenatal care.
“Back then, we were expected to be generalists. If [a patient] had a cancer, you took care of it. If [she] had a fertility problem or problem with endocrinology, you took care of it. Of course, now if I have a female cancer, we refer them to the gyn-oncologist. But when you go back 40 years and beyond, we did mostly everything.”
The ob.gyns. of today are practicing in a vastly different environment than their predecessors, and while many of the differences have improved patient care and enhanced efficiency, physicians also note that some changes have harmed the doctor-patient relationship and created career dissatisfaction.
“Certainly, the advances of modern medicine have enabled the current physician to provide the patient a level of care unparalleled in history,” said Charles E. Miller, MD, a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.
More volume, less time
Most long-time physicians agree that higher patient volumes and increasing administrative burdens have diminished the time they are able to spend with patients.
Rising clinical documentation and coding are the top administrative tasks taking away from one-on-one patient care, said Kristen Zeligs, MD, chair of the American Congress of Obstetricians and Gynecologists’ Junior Fellow Congress Advisory Council and a gynecologic oncology fellow at Walter Reed National Military Medical Center in Bethesda, Md.
But there are other factors straining the doctor-patient relationship. Decades ago, first-time mothers often stayed with one doctor for a lifetime, Dr. Brabson recalled, having all of her babies delivered by a single ob.gyn. The same can’t be said for today, where insurance changes, job relocations, and a lack of connections often lead patients to switch physicians frequently.
“Now a lot of patients [move on] from one year to the next,” Dr. Brabson said. “There’s not that same loyalty.”
Doctors, too, traditionally stuck with patients over the long haul, he added. In the past, if an ob.gyn treated a patient during pregnancy, that same physician was present during the delivery, even if it meant leaving a vacation early or coming in on a night off.
“Now the younger docs, especially those that have families with small children, when it’s not their night on call and it’s 5 o’clock, they’re checking out,” Dr. Brabson said. “One of my partners right now, she started inducing someone yesterday. Well today’s her day off, so I’m going to do the delivery. That’s been a big change.”
Highs and lows of liability premiums
Another pressure on the specialty over the last couple of decades has been the high cost of liability insurance.
“2003 was really the peak of the liability crisis,” Dr. Montgomery said. “Hospitals were closing. Doctors were giving up practice. It’s a little better now than it was.”
While ob.gyns. still pay higher premiums than many other specialties, the legal climate has improved in recent years, said Paul Greve Jr., executive vice president and senior consultant for the Willis Healthcare Practice, and author of the 2016 Medical Liability Monitor, an annual report that surveys medical liability premiums.
“The best way to characterize the overall environment for medical professional liability is stable,” he said. “In the area of obstetrics, for the first time ever in recent years, we are seeing lower frequency and lower severity [of lawsuits].”
The lower number of filings are largely due to patient safety initiatives among ob.gyn. programs and tort reforms – many of which have been upheld by courts in the last decade, Mr. Greve said.
Of course, the rate of premiums greatly differs depending on location, with ob.gyns. in Eastern New York paying a high of $214,999 and ob.gyns. in Minnesota paying a low of $16,449 in 2016, according to the Medical Liability Monitor.
“The environment is specific to the region,” Mr. Greve said. “New York City is still very problematic. Chicago is still very problematic. There are some pockets around the country where there’s no damage caps, and it’s really tough to defend claims.”
Technology ups and downs
Another fairly new pressure on ob.gyns. is the integration of the electronic health record and the federal reporting requirements that go along with it.
“Most practicing ob.gyns. are really fed up with the computerization of medicine and the tasking and the charting,” Dr. Montgomery said. “For every hour you spend seeing patients, you spend 1 or 2 hours doing computer chart work and paperwork. Most doctors don’t go into medicine so they can type; they go into medicine to take care of patients.”
The Internet age also poses challenges when it comes to patients conducting their own “research,” said Megan Evans, MD, an ob.gyn. at Tufts Medical Center, Boston.
Protecting the security of patients’ medical records in the digital age is another worry, she said.
But for Dr. Evans, who completed her residency training in 2015, having dozens of digital tools at her disposal as she treats patients is definitely an upside to today’s practice environment.
“I can review a practice bulletin, look up the latest treatment regimens, and contact my colleagues with a quick question – all on my iPhone,” she said. “I also believe there is so much potential for electronic medical records and how they communicate with each other.”
Advancements in ultrasound, fetal monitoring, and other medical technologies have also allowed ob.gyns. to intervene earlier and save lives.
Dr. Brabson recalled the helplessness he and other physicians felt in the 1970s when it came to delivering extremely premature babies.
“We didn’t really feel like you could save a baby under 2 pounds,” he said. “When I was a medical student, if you had a baby under 2 pounds, very commonly what they would do is lay the baby up on the table and watch and see how vigorous it was going to be, and if it really did breathe and carry on for awhile, then you might take it to the nursery. The equipment that we have to save babies with today, compared to 40 years ago, that’s a dramatic change.”
A changing focus for the future
If current trends continue, Dr. Brabson’s early experience of being a generalist ob.gyn. won’t be the norm. Instead, more ob.gyns. will choose to subspecialize. Whether this change is positive or negative for the specialty depends on who you ask.
“You could argue the pros and cons for both sides,” Dr. Zeligs said. “For me, it takes away from what drew me to the specialty – the breadth that ob.gyn. offers, both as a primary care specialty and as a surgical subspecialty.”
However, choosing one focus may offer some doctors a way to capture that elusive professional and personal balance, she added.
Despite the changing landscape of clinical duties and business operations, some parts of ob.gyn. practice have remained intact, according to Dr. Brabson. “The most rewarding and enjoyable part of the job is developing a relationship of mutual trust and respect,” he said. “As a result of developing such a relationship, both the patient and the doctor come away with positive feelings. This has not changed.”
[email protected]
On Twitter @legal_med
As a practicing obstetrician-gynecologist for nearly 40 years, Leonard Brabson, MD, has watched his specialty transform in ways both large and small.
For starters, his regular work attire in 1977 – a shirt and tie – is now scrubs. The paper charts that once filled his office shelves have been replaced with electronic records. And the cumbersome machines that once took blurry, still pictures of a fetus have advanced by leaps and bounds and become a staple of prenatal care.
“Back then, we were expected to be generalists. If [a patient] had a cancer, you took care of it. If [she] had a fertility problem or problem with endocrinology, you took care of it. Of course, now if I have a female cancer, we refer them to the gyn-oncologist. But when you go back 40 years and beyond, we did mostly everything.”
The ob.gyns. of today are practicing in a vastly different environment than their predecessors, and while many of the differences have improved patient care and enhanced efficiency, physicians also note that some changes have harmed the doctor-patient relationship and created career dissatisfaction.
“Certainly, the advances of modern medicine have enabled the current physician to provide the patient a level of care unparalleled in history,” said Charles E. Miller, MD, a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.
More volume, less time
Most long-time physicians agree that higher patient volumes and increasing administrative burdens have diminished the time they are able to spend with patients.
Rising clinical documentation and coding are the top administrative tasks taking away from one-on-one patient care, said Kristen Zeligs, MD, chair of the American Congress of Obstetricians and Gynecologists’ Junior Fellow Congress Advisory Council and a gynecologic oncology fellow at Walter Reed National Military Medical Center in Bethesda, Md.
But there are other factors straining the doctor-patient relationship. Decades ago, first-time mothers often stayed with one doctor for a lifetime, Dr. Brabson recalled, having all of her babies delivered by a single ob.gyn. The same can’t be said for today, where insurance changes, job relocations, and a lack of connections often lead patients to switch physicians frequently.
“Now a lot of patients [move on] from one year to the next,” Dr. Brabson said. “There’s not that same loyalty.”
Doctors, too, traditionally stuck with patients over the long haul, he added. In the past, if an ob.gyn treated a patient during pregnancy, that same physician was present during the delivery, even if it meant leaving a vacation early or coming in on a night off.
“Now the younger docs, especially those that have families with small children, when it’s not their night on call and it’s 5 o’clock, they’re checking out,” Dr. Brabson said. “One of my partners right now, she started inducing someone yesterday. Well today’s her day off, so I’m going to do the delivery. That’s been a big change.”
Highs and lows of liability premiums
Another pressure on the specialty over the last couple of decades has been the high cost of liability insurance.
“2003 was really the peak of the liability crisis,” Dr. Montgomery said. “Hospitals were closing. Doctors were giving up practice. It’s a little better now than it was.”
While ob.gyns. still pay higher premiums than many other specialties, the legal climate has improved in recent years, said Paul Greve Jr., executive vice president and senior consultant for the Willis Healthcare Practice, and author of the 2016 Medical Liability Monitor, an annual report that surveys medical liability premiums.
“The best way to characterize the overall environment for medical professional liability is stable,” he said. “In the area of obstetrics, for the first time ever in recent years, we are seeing lower frequency and lower severity [of lawsuits].”
The lower number of filings are largely due to patient safety initiatives among ob.gyn. programs and tort reforms – many of which have been upheld by courts in the last decade, Mr. Greve said.
Of course, the rate of premiums greatly differs depending on location, with ob.gyns. in Eastern New York paying a high of $214,999 and ob.gyns. in Minnesota paying a low of $16,449 in 2016, according to the Medical Liability Monitor.
“The environment is specific to the region,” Mr. Greve said. “New York City is still very problematic. Chicago is still very problematic. There are some pockets around the country where there’s no damage caps, and it’s really tough to defend claims.”
Technology ups and downs
Another fairly new pressure on ob.gyns. is the integration of the electronic health record and the federal reporting requirements that go along with it.
“Most practicing ob.gyns. are really fed up with the computerization of medicine and the tasking and the charting,” Dr. Montgomery said. “For every hour you spend seeing patients, you spend 1 or 2 hours doing computer chart work and paperwork. Most doctors don’t go into medicine so they can type; they go into medicine to take care of patients.”
The Internet age also poses challenges when it comes to patients conducting their own “research,” said Megan Evans, MD, an ob.gyn. at Tufts Medical Center, Boston.
Protecting the security of patients’ medical records in the digital age is another worry, she said.
But for Dr. Evans, who completed her residency training in 2015, having dozens of digital tools at her disposal as she treats patients is definitely an upside to today’s practice environment.
“I can review a practice bulletin, look up the latest treatment regimens, and contact my colleagues with a quick question – all on my iPhone,” she said. “I also believe there is so much potential for electronic medical records and how they communicate with each other.”
Advancements in ultrasound, fetal monitoring, and other medical technologies have also allowed ob.gyns. to intervene earlier and save lives.
Dr. Brabson recalled the helplessness he and other physicians felt in the 1970s when it came to delivering extremely premature babies.
“We didn’t really feel like you could save a baby under 2 pounds,” he said. “When I was a medical student, if you had a baby under 2 pounds, very commonly what they would do is lay the baby up on the table and watch and see how vigorous it was going to be, and if it really did breathe and carry on for awhile, then you might take it to the nursery. The equipment that we have to save babies with today, compared to 40 years ago, that’s a dramatic change.”
A changing focus for the future
If current trends continue, Dr. Brabson’s early experience of being a generalist ob.gyn. won’t be the norm. Instead, more ob.gyns. will choose to subspecialize. Whether this change is positive or negative for the specialty depends on who you ask.
“You could argue the pros and cons for both sides,” Dr. Zeligs said. “For me, it takes away from what drew me to the specialty – the breadth that ob.gyn. offers, both as a primary care specialty and as a surgical subspecialty.”
However, choosing one focus may offer some doctors a way to capture that elusive professional and personal balance, she added.
Despite the changing landscape of clinical duties and business operations, some parts of ob.gyn. practice have remained intact, according to Dr. Brabson. “The most rewarding and enjoyable part of the job is developing a relationship of mutual trust and respect,” he said. “As a result of developing such a relationship, both the patient and the doctor come away with positive feelings. This has not changed.”
[email protected]
On Twitter @legal_med
Are you neuroprotecting your patients? 10 Adjunctive therapies to consider
Are you ‘neuroprotecting’ your patients?
Fortunately, many studies have demonstrated that in addition to controlling clinical symptoms, antidepressants, mood stabilizers, and atypical antipsychotics all have neuroprotective effects, including stimulating neurogenesis, preventing apoptosis, and increasing neurotrophins. However, more needs to be done to protect the brain’s gray and white matter and to prevent negative neuroplasticity and disconnectivity of brain circuits that often are documented in patients with psychotic and mood disorders.
There are, in fact, many off-label supplements with strong neuroprotective effects that psychiatrists can use as an adjunct to standard evidence-based pharmacotherapy. These agents generally are safe and well tolerated and often are sold over the counter, but are not covered by insurance. However, considering the disability that often is associated with schizophrenia, treatment-resistant depression, or psychotic mania, it is reasonable to consider using these agents, many of which are supported by studies published in peer-reviewed journals. However, because they are widely available and not proprietary and large, expensive registration trials such as the ones conducted by the pharmaceutical industry are not done, none is likely to receive FDA approval. Therefore, it is up to psychiatrists and nurse practitioners to use them judiciously in patients at risk for neurotoxicity.
Here are 10 agents with neuroprotective effects supported by published data that can be considered as add-on to the standard treatments in an effort to mitigate neurotoxicity and protect the brain from the destructive processes that accompany acute episodes of psychosis, mania, and depression.
Omega-3 fatty acids have been shown in several studies to help reduce psychopathology of psychosis, mania, or depression when used as an adjunctive agent.1 It appears to be more effective in the early stages of psychiatric disorders than in the chronic phase. It has anti-inflammatory, anti-oxidant, and anti-apoptotic effects; activates cell-signaling pathways; and prevents synaptic loss as well as neuronal and glial death.2
N-acetylcysteine (NAC) is a powerful antioxidant that increases glutathione, which is produced in the mitochondria. Schizophrenia is associated with mitochondrial dysfunction with low levels of glutathione, which puts the brain at risk for neurodegeneration caused by high levels of free radicals produced during psychosis. Adding NAC to antipsychotics during acute psychotic episodes—especially the first episode—can significantly reduce the neurotoxic effects of reactive oxygen and nitrogen species, also known as free radicals.3 In studies of traumatic brain injury in rats, NAC reduced brain edema, neuroinflammation, blood–brain barrier permeability, and apoptosis.4
Minocycline. This antibiotic has been studied extensively as an adjunctive treatment in schizophrenia and has proven to have several neuroprotective effects including anti-inflammatory, anti-oxidant, and anti-apoptotic, and reduces glutamate excitotoxicity.5 Several studies have documented its usefulness in acute psychotic episodes.
Vitamin D. Because of its vital role in neurodevelopment (neuronal differentiation, axonal connectivity), vitamin D deficiency has been associated with several psychiatric disorders including autism, schizophrenia, depression, and Alzheimer’s disease.6 Measure serum levels of vitamin D in patients with psychotic and mood disorders and implement supplementation if it is low—and it often is in these patients.
Nicotine is neuroprotective against glutamate excitotoxicity and it also inhibits apoptosis.7 However, it should never be administered via cigarettes, which are loaded with hundreds of toxic substances! It can be administered via patches or nicotine gum, which are usually used to help in smoking cessation. Nicotine also also can have a pro-cognitive effect.
Melatonin. Many people associate melatonin with sleep. However, it has multiple neuroprotective effects by being an antioxidant, protecting mitochondrial integrity, and modulating the immune system, as well as attenuating microglial activation, which triggers neuroinflammation and oxidative stress. It also protects against cellular senescence, which is due to inflammation and reactive oxygen species. Furthermore, melatonin is useful in ameliorating the metabolic syndrome, which is associated with neurotoxic effects on brain tissue caused by the pro-inflammatory effects of peri-omental fat in obesity.8 Adjunctive melatonin could be helpful in patients with schizophrenia or depression who suffer from metabolic syndrome.
Erythropoietin is a hormone produced by the kidneys to promote the formation of red blood cells. It is a potent neuroprotective cytokine that promotes neuronal survival via anti-apoptotic effects. It protects against glutamate and nitrous oxide toxicity9 and haloperidol-induced neuronal death.10 It is clinically used (since FDA approval in 1989) in severe anemia due to chronic kidney disease or chemotherapy, as well as in inflammatory bowel disease. It does have some “black-box” warnings so its use should be limited.
Cox-2 inhibitors. This is a well-known class of anti-inflammatory drugs, which are FDA-approved for pain and inflammation. Studies of adjunctive use of cox-2 inhibitors in acute psychosis show that these drugs accentuate the efficacy of antipsychotic medications.11 The reason is that acute psychosis is associated with neuro-inflammation, which leads to neurotoxicity.
Lithium. Dosages to treat mania are usually 900 to 1500 mg/d. However, in minute (homeopathic) dosages as low as 1 mg/d, lithium has been shown to prevent progression of amnestic mild cognitive impairment to full dementia.12 This interesting observation suggests that lithium not only induces neurogenesis and increases gray matter volume,13 but may be neuroprotective against amyloid neurotoxicity. The effects of very low doses of lithium in depression and schizophrenia have not been studied yet.
Caffeine. Yes, the good old brew people seek all day is neuroprotective and prevents mood and memory dysfunction caused by stress.14 Caffeine should be avoided in patients with anxiety disorders, but it may be helpful for the brains of patients with mood or psychotic disorders. Caffeine reverses synaptic dysfunction in the circuits of the hippocampus caused by chronic unpredictable stress (quite common among our psychiatric patients).
The above interventions may be helpful for some patients but not others. Practitioners should consider using 1 or more of those adjunctive neuroprotective agents in patients who are at risk for neurodegenerative changes secondary to recurrences of acute and severe psychosis or mood episodes. Although clinicians cannot monitor brain structural integrity, they can assess the rate of symptomatic improvement and degree of functional restoration in their patients. Until a cure is found, these little steps—taken cautiously and judiciously—could help alleviate our patients’ suffering and the risk of neurotoxicity associated with their serious psychiatric disorder.
1. Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: possible stage-specific effects. Ann Clin Psychiatry. 2015;27(4):289-296.
2. Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids. 2007;7(5-6):287-293.
3. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
4. Chen G, Shi J, Hu Z, et al. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008;2008:716458. doi: 10.1155/2008/716458
5. Dean OM, Data-Franco J, Giorlando F, et al. Minocycline: therapeutic potential in psychiatry. CNS Drugs. 2012;26(5):391-401.
6. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47-64.
7. Akaike A, Tkada-Takatori Y, Kume T, et al. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010;40(1-2):211-216.
8. Cardinali DP, Hardeland R. Inflammaging, metabolic syndrome and melatonin: a call for treatment studies [published online May 11, 2016]. Neuroendocrinology. doi:10.1159/000446543.
9. Yamasaki M, Mishima HK, Yamashita H, et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050(1-2):15-26.
10. Pilllai A, Dhandapani KM, Pillai BA, et al. Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology. 2008;33(8):1942-1951.
11. Müller N, Myint AM, Weidinger E, et al. Anti-inflammatory treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:146-153.
12. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
13. Dong BT, Tu GJ, Han YX, et al. Lithium enhanced cell proliferation and differentiation of mesenchymal stem cells to neural cells in rat spinal cord. Int J Clin Exp Pathol. 2015;8(3):2473-2483.
14. Kaster MP, Machado NJ, Silva HB, et al. Caffeine acts through neuronal adenosine A24 receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A. 2015;112(25):7833-7838.
Are you ‘neuroprotecting’ your patients?
Fortunately, many studies have demonstrated that in addition to controlling clinical symptoms, antidepressants, mood stabilizers, and atypical antipsychotics all have neuroprotective effects, including stimulating neurogenesis, preventing apoptosis, and increasing neurotrophins. However, more needs to be done to protect the brain’s gray and white matter and to prevent negative neuroplasticity and disconnectivity of brain circuits that often are documented in patients with psychotic and mood disorders.
There are, in fact, many off-label supplements with strong neuroprotective effects that psychiatrists can use as an adjunct to standard evidence-based pharmacotherapy. These agents generally are safe and well tolerated and often are sold over the counter, but are not covered by insurance. However, considering the disability that often is associated with schizophrenia, treatment-resistant depression, or psychotic mania, it is reasonable to consider using these agents, many of which are supported by studies published in peer-reviewed journals. However, because they are widely available and not proprietary and large, expensive registration trials such as the ones conducted by the pharmaceutical industry are not done, none is likely to receive FDA approval. Therefore, it is up to psychiatrists and nurse practitioners to use them judiciously in patients at risk for neurotoxicity.
Here are 10 agents with neuroprotective effects supported by published data that can be considered as add-on to the standard treatments in an effort to mitigate neurotoxicity and protect the brain from the destructive processes that accompany acute episodes of psychosis, mania, and depression.
Omega-3 fatty acids have been shown in several studies to help reduce psychopathology of psychosis, mania, or depression when used as an adjunctive agent.1 It appears to be more effective in the early stages of psychiatric disorders than in the chronic phase. It has anti-inflammatory, anti-oxidant, and anti-apoptotic effects; activates cell-signaling pathways; and prevents synaptic loss as well as neuronal and glial death.2
N-acetylcysteine (NAC) is a powerful antioxidant that increases glutathione, which is produced in the mitochondria. Schizophrenia is associated with mitochondrial dysfunction with low levels of glutathione, which puts the brain at risk for neurodegeneration caused by high levels of free radicals produced during psychosis. Adding NAC to antipsychotics during acute psychotic episodes—especially the first episode—can significantly reduce the neurotoxic effects of reactive oxygen and nitrogen species, also known as free radicals.3 In studies of traumatic brain injury in rats, NAC reduced brain edema, neuroinflammation, blood–brain barrier permeability, and apoptosis.4
Minocycline. This antibiotic has been studied extensively as an adjunctive treatment in schizophrenia and has proven to have several neuroprotective effects including anti-inflammatory, anti-oxidant, and anti-apoptotic, and reduces glutamate excitotoxicity.5 Several studies have documented its usefulness in acute psychotic episodes.
Vitamin D. Because of its vital role in neurodevelopment (neuronal differentiation, axonal connectivity), vitamin D deficiency has been associated with several psychiatric disorders including autism, schizophrenia, depression, and Alzheimer’s disease.6 Measure serum levels of vitamin D in patients with psychotic and mood disorders and implement supplementation if it is low—and it often is in these patients.
Nicotine is neuroprotective against glutamate excitotoxicity and it also inhibits apoptosis.7 However, it should never be administered via cigarettes, which are loaded with hundreds of toxic substances! It can be administered via patches or nicotine gum, which are usually used to help in smoking cessation. Nicotine also also can have a pro-cognitive effect.
Melatonin. Many people associate melatonin with sleep. However, it has multiple neuroprotective effects by being an antioxidant, protecting mitochondrial integrity, and modulating the immune system, as well as attenuating microglial activation, which triggers neuroinflammation and oxidative stress. It also protects against cellular senescence, which is due to inflammation and reactive oxygen species. Furthermore, melatonin is useful in ameliorating the metabolic syndrome, which is associated with neurotoxic effects on brain tissue caused by the pro-inflammatory effects of peri-omental fat in obesity.8 Adjunctive melatonin could be helpful in patients with schizophrenia or depression who suffer from metabolic syndrome.
Erythropoietin is a hormone produced by the kidneys to promote the formation of red blood cells. It is a potent neuroprotective cytokine that promotes neuronal survival via anti-apoptotic effects. It protects against glutamate and nitrous oxide toxicity9 and haloperidol-induced neuronal death.10 It is clinically used (since FDA approval in 1989) in severe anemia due to chronic kidney disease or chemotherapy, as well as in inflammatory bowel disease. It does have some “black-box” warnings so its use should be limited.
Cox-2 inhibitors. This is a well-known class of anti-inflammatory drugs, which are FDA-approved for pain and inflammation. Studies of adjunctive use of cox-2 inhibitors in acute psychosis show that these drugs accentuate the efficacy of antipsychotic medications.11 The reason is that acute psychosis is associated with neuro-inflammation, which leads to neurotoxicity.
Lithium. Dosages to treat mania are usually 900 to 1500 mg/d. However, in minute (homeopathic) dosages as low as 1 mg/d, lithium has been shown to prevent progression of amnestic mild cognitive impairment to full dementia.12 This interesting observation suggests that lithium not only induces neurogenesis and increases gray matter volume,13 but may be neuroprotective against amyloid neurotoxicity. The effects of very low doses of lithium in depression and schizophrenia have not been studied yet.
Caffeine. Yes, the good old brew people seek all day is neuroprotective and prevents mood and memory dysfunction caused by stress.14 Caffeine should be avoided in patients with anxiety disorders, but it may be helpful for the brains of patients with mood or psychotic disorders. Caffeine reverses synaptic dysfunction in the circuits of the hippocampus caused by chronic unpredictable stress (quite common among our psychiatric patients).
The above interventions may be helpful for some patients but not others. Practitioners should consider using 1 or more of those adjunctive neuroprotective agents in patients who are at risk for neurodegenerative changes secondary to recurrences of acute and severe psychosis or mood episodes. Although clinicians cannot monitor brain structural integrity, they can assess the rate of symptomatic improvement and degree of functional restoration in their patients. Until a cure is found, these little steps—taken cautiously and judiciously—could help alleviate our patients’ suffering and the risk of neurotoxicity associated with their serious psychiatric disorder.
Are you ‘neuroprotecting’ your patients?
Fortunately, many studies have demonstrated that in addition to controlling clinical symptoms, antidepressants, mood stabilizers, and atypical antipsychotics all have neuroprotective effects, including stimulating neurogenesis, preventing apoptosis, and increasing neurotrophins. However, more needs to be done to protect the brain’s gray and white matter and to prevent negative neuroplasticity and disconnectivity of brain circuits that often are documented in patients with psychotic and mood disorders.
There are, in fact, many off-label supplements with strong neuroprotective effects that psychiatrists can use as an adjunct to standard evidence-based pharmacotherapy. These agents generally are safe and well tolerated and often are sold over the counter, but are not covered by insurance. However, considering the disability that often is associated with schizophrenia, treatment-resistant depression, or psychotic mania, it is reasonable to consider using these agents, many of which are supported by studies published in peer-reviewed journals. However, because they are widely available and not proprietary and large, expensive registration trials such as the ones conducted by the pharmaceutical industry are not done, none is likely to receive FDA approval. Therefore, it is up to psychiatrists and nurse practitioners to use them judiciously in patients at risk for neurotoxicity.
Here are 10 agents with neuroprotective effects supported by published data that can be considered as add-on to the standard treatments in an effort to mitigate neurotoxicity and protect the brain from the destructive processes that accompany acute episodes of psychosis, mania, and depression.
Omega-3 fatty acids have been shown in several studies to help reduce psychopathology of psychosis, mania, or depression when used as an adjunctive agent.1 It appears to be more effective in the early stages of psychiatric disorders than in the chronic phase. It has anti-inflammatory, anti-oxidant, and anti-apoptotic effects; activates cell-signaling pathways; and prevents synaptic loss as well as neuronal and glial death.2
N-acetylcysteine (NAC) is a powerful antioxidant that increases glutathione, which is produced in the mitochondria. Schizophrenia is associated with mitochondrial dysfunction with low levels of glutathione, which puts the brain at risk for neurodegeneration caused by high levels of free radicals produced during psychosis. Adding NAC to antipsychotics during acute psychotic episodes—especially the first episode—can significantly reduce the neurotoxic effects of reactive oxygen and nitrogen species, also known as free radicals.3 In studies of traumatic brain injury in rats, NAC reduced brain edema, neuroinflammation, blood–brain barrier permeability, and apoptosis.4
Minocycline. This antibiotic has been studied extensively as an adjunctive treatment in schizophrenia and has proven to have several neuroprotective effects including anti-inflammatory, anti-oxidant, and anti-apoptotic, and reduces glutamate excitotoxicity.5 Several studies have documented its usefulness in acute psychotic episodes.
Vitamin D. Because of its vital role in neurodevelopment (neuronal differentiation, axonal connectivity), vitamin D deficiency has been associated with several psychiatric disorders including autism, schizophrenia, depression, and Alzheimer’s disease.6 Measure serum levels of vitamin D in patients with psychotic and mood disorders and implement supplementation if it is low—and it often is in these patients.
Nicotine is neuroprotective against glutamate excitotoxicity and it also inhibits apoptosis.7 However, it should never be administered via cigarettes, which are loaded with hundreds of toxic substances! It can be administered via patches or nicotine gum, which are usually used to help in smoking cessation. Nicotine also also can have a pro-cognitive effect.
Melatonin. Many people associate melatonin with sleep. However, it has multiple neuroprotective effects by being an antioxidant, protecting mitochondrial integrity, and modulating the immune system, as well as attenuating microglial activation, which triggers neuroinflammation and oxidative stress. It also protects against cellular senescence, which is due to inflammation and reactive oxygen species. Furthermore, melatonin is useful in ameliorating the metabolic syndrome, which is associated with neurotoxic effects on brain tissue caused by the pro-inflammatory effects of peri-omental fat in obesity.8 Adjunctive melatonin could be helpful in patients with schizophrenia or depression who suffer from metabolic syndrome.
Erythropoietin is a hormone produced by the kidneys to promote the formation of red blood cells. It is a potent neuroprotective cytokine that promotes neuronal survival via anti-apoptotic effects. It protects against glutamate and nitrous oxide toxicity9 and haloperidol-induced neuronal death.10 It is clinically used (since FDA approval in 1989) in severe anemia due to chronic kidney disease or chemotherapy, as well as in inflammatory bowel disease. It does have some “black-box” warnings so its use should be limited.
Cox-2 inhibitors. This is a well-known class of anti-inflammatory drugs, which are FDA-approved for pain and inflammation. Studies of adjunctive use of cox-2 inhibitors in acute psychosis show that these drugs accentuate the efficacy of antipsychotic medications.11 The reason is that acute psychosis is associated with neuro-inflammation, which leads to neurotoxicity.
Lithium. Dosages to treat mania are usually 900 to 1500 mg/d. However, in minute (homeopathic) dosages as low as 1 mg/d, lithium has been shown to prevent progression of amnestic mild cognitive impairment to full dementia.12 This interesting observation suggests that lithium not only induces neurogenesis and increases gray matter volume,13 but may be neuroprotective against amyloid neurotoxicity. The effects of very low doses of lithium in depression and schizophrenia have not been studied yet.
Caffeine. Yes, the good old brew people seek all day is neuroprotective and prevents mood and memory dysfunction caused by stress.14 Caffeine should be avoided in patients with anxiety disorders, but it may be helpful for the brains of patients with mood or psychotic disorders. Caffeine reverses synaptic dysfunction in the circuits of the hippocampus caused by chronic unpredictable stress (quite common among our psychiatric patients).
The above interventions may be helpful for some patients but not others. Practitioners should consider using 1 or more of those adjunctive neuroprotective agents in patients who are at risk for neurodegenerative changes secondary to recurrences of acute and severe psychosis or mood episodes. Although clinicians cannot monitor brain structural integrity, they can assess the rate of symptomatic improvement and degree of functional restoration in their patients. Until a cure is found, these little steps—taken cautiously and judiciously—could help alleviate our patients’ suffering and the risk of neurotoxicity associated with their serious psychiatric disorder.
1. Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: possible stage-specific effects. Ann Clin Psychiatry. 2015;27(4):289-296.
2. Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids. 2007;7(5-6):287-293.
3. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
4. Chen G, Shi J, Hu Z, et al. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008;2008:716458. doi: 10.1155/2008/716458
5. Dean OM, Data-Franco J, Giorlando F, et al. Minocycline: therapeutic potential in psychiatry. CNS Drugs. 2012;26(5):391-401.
6. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47-64.
7. Akaike A, Tkada-Takatori Y, Kume T, et al. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010;40(1-2):211-216.
8. Cardinali DP, Hardeland R. Inflammaging, metabolic syndrome and melatonin: a call for treatment studies [published online May 11, 2016]. Neuroendocrinology. doi:10.1159/000446543.
9. Yamasaki M, Mishima HK, Yamashita H, et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050(1-2):15-26.
10. Pilllai A, Dhandapani KM, Pillai BA, et al. Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology. 2008;33(8):1942-1951.
11. Müller N, Myint AM, Weidinger E, et al. Anti-inflammatory treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:146-153.
12. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
13. Dong BT, Tu GJ, Han YX, et al. Lithium enhanced cell proliferation and differentiation of mesenchymal stem cells to neural cells in rat spinal cord. Int J Clin Exp Pathol. 2015;8(3):2473-2483.
14. Kaster MP, Machado NJ, Silva HB, et al. Caffeine acts through neuronal adenosine A24 receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A. 2015;112(25):7833-7838.
1. Chen AT, Chibnall JT, Nasrallah HA. A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: possible stage-specific effects. Ann Clin Psychiatry. 2015;27(4):289-296.
2. Calon F, Cole G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids. 2007;7(5-6):287-293.
3. Chen AT, Chibnall JT, Nasrallah HA. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
4. Chen G, Shi J, Hu Z, et al. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008;2008:716458. doi: 10.1155/2008/716458
5. Dean OM, Data-Franco J, Giorlando F, et al. Minocycline: therapeutic potential in psychiatry. CNS Drugs. 2012;26(5):391-401.
6. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47-64.
7. Akaike A, Tkada-Takatori Y, Kume T, et al. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010;40(1-2):211-216.
8. Cardinali DP, Hardeland R. Inflammaging, metabolic syndrome and melatonin: a call for treatment studies [published online May 11, 2016]. Neuroendocrinology. doi:10.1159/000446543.
9. Yamasaki M, Mishima HK, Yamashita H, et al. Neuroprotective effects of erythropoietin on glutamate and nitric oxide toxicity in primary cultured retinal ganglion cells. Brain Res. 2005;1050(1-2):15-26.
10. Pilllai A, Dhandapani KM, Pillai BA, et al. Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology. 2008;33(8):1942-1951.
11. Müller N, Myint AM, Weidinger E, et al. Anti-inflammatory treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:146-153.
12. Forlenza OV, Diniz BS, Radanovic M, et al. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351-356.
13. Dong BT, Tu GJ, Han YX, et al. Lithium enhanced cell proliferation and differentiation of mesenchymal stem cells to neural cells in rat spinal cord. Int J Clin Exp Pathol. 2015;8(3):2473-2483.
14. Kaster MP, Machado NJ, Silva HB, et al. Caffeine acts through neuronal adenosine A24 receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A. 2015;112(25):7833-7838.
Acne and Antiaging: Is There a Connection?
As a chronic inflammatory skin disease well known for its poor cosmesis including scarring, residual macular erythema, and postinflammatory pigment alteration, acne vulgaris may, according to recent research, confer some antiaging benefits to affected patients. In a research letter published online on September 27 in the Journal of Investigative Dermatology, Ribero et al analyzed white blood cells and found that women who said they had acne had longer telomeres (the "caps" at the end of chromosomes that protect them from deteriorating following repeated cell replication). Telomere length, or rather shortening, has been correlated with age-related degenerative change, according to Saum et al (Exp Gerontol. 2014;58:250-255), and therefore the thinking is that in women with acne, something is going on that maintains the length of the cellular guardians. Let's clarify a couple things to help us all understand the why and what.
The impetus of this study, according to Ribero et al, was the observation that women with acne show signs of aging later than those who have never had acne. I personally have not witnessed this finding in my patients, and given that acne in its essence is a disease of chronic inflammation resulting from, for example, persistent activation of toll-like receptor 2 (TLR2) and NOD-like receptor family pyrin domain containing 3 (NLRP3) pattern recognition receptors, one would think the skin damage accrued would make these individuals look older, right? Last I checked, pitted scarring does not make one immediately think of the fountain of youth.
The results from the study show that there is a link between acne and longer telomeres, but the study did not show that telomere length is a cause of acne, that women with longer telomeres had fewer signs of skin aging, or that women with acne lived longer.
Given these points, Ribero et al concluded that "delayed skin aging may be due to reduced senescence," which means that skin aging may be delayed because the longer telomeres in the cells protect them from deterioration. They did find that the expression of one gene in particular was reduced in women with acne--the regulatory gene zinc finger protein 420, ZNF420--suggesting that those without acne may produce more of a particular protein linked to that gene, though the significance is unclear.
What's the issue?
This study is interesting, but it is important not to make any broad conclusions, such as those who get acne will live longer or look younger longer regardless of other factors such as acne treatment, comorbidities, or even environmental factors. This study may give more support for the genetic contribution of acne, but much more work is needed to determine the clinical relevance. For starters, what about men?
Would you assure your acne patients that their disease may be for their own cosmetic good?
As a chronic inflammatory skin disease well known for its poor cosmesis including scarring, residual macular erythema, and postinflammatory pigment alteration, acne vulgaris may, according to recent research, confer some antiaging benefits to affected patients. In a research letter published online on September 27 in the Journal of Investigative Dermatology, Ribero et al analyzed white blood cells and found that women who said they had acne had longer telomeres (the "caps" at the end of chromosomes that protect them from deteriorating following repeated cell replication). Telomere length, or rather shortening, has been correlated with age-related degenerative change, according to Saum et al (Exp Gerontol. 2014;58:250-255), and therefore the thinking is that in women with acne, something is going on that maintains the length of the cellular guardians. Let's clarify a couple things to help us all understand the why and what.
The impetus of this study, according to Ribero et al, was the observation that women with acne show signs of aging later than those who have never had acne. I personally have not witnessed this finding in my patients, and given that acne in its essence is a disease of chronic inflammation resulting from, for example, persistent activation of toll-like receptor 2 (TLR2) and NOD-like receptor family pyrin domain containing 3 (NLRP3) pattern recognition receptors, one would think the skin damage accrued would make these individuals look older, right? Last I checked, pitted scarring does not make one immediately think of the fountain of youth.
The results from the study show that there is a link between acne and longer telomeres, but the study did not show that telomere length is a cause of acne, that women with longer telomeres had fewer signs of skin aging, or that women with acne lived longer.
Given these points, Ribero et al concluded that "delayed skin aging may be due to reduced senescence," which means that skin aging may be delayed because the longer telomeres in the cells protect them from deterioration. They did find that the expression of one gene in particular was reduced in women with acne--the regulatory gene zinc finger protein 420, ZNF420--suggesting that those without acne may produce more of a particular protein linked to that gene, though the significance is unclear.
What's the issue?
This study is interesting, but it is important not to make any broad conclusions, such as those who get acne will live longer or look younger longer regardless of other factors such as acne treatment, comorbidities, or even environmental factors. This study may give more support for the genetic contribution of acne, but much more work is needed to determine the clinical relevance. For starters, what about men?
Would you assure your acne patients that their disease may be for their own cosmetic good?
As a chronic inflammatory skin disease well known for its poor cosmesis including scarring, residual macular erythema, and postinflammatory pigment alteration, acne vulgaris may, according to recent research, confer some antiaging benefits to affected patients. In a research letter published online on September 27 in the Journal of Investigative Dermatology, Ribero et al analyzed white blood cells and found that women who said they had acne had longer telomeres (the "caps" at the end of chromosomes that protect them from deteriorating following repeated cell replication). Telomere length, or rather shortening, has been correlated with age-related degenerative change, according to Saum et al (Exp Gerontol. 2014;58:250-255), and therefore the thinking is that in women with acne, something is going on that maintains the length of the cellular guardians. Let's clarify a couple things to help us all understand the why and what.
The impetus of this study, according to Ribero et al, was the observation that women with acne show signs of aging later than those who have never had acne. I personally have not witnessed this finding in my patients, and given that acne in its essence is a disease of chronic inflammation resulting from, for example, persistent activation of toll-like receptor 2 (TLR2) and NOD-like receptor family pyrin domain containing 3 (NLRP3) pattern recognition receptors, one would think the skin damage accrued would make these individuals look older, right? Last I checked, pitted scarring does not make one immediately think of the fountain of youth.
The results from the study show that there is a link between acne and longer telomeres, but the study did not show that telomere length is a cause of acne, that women with longer telomeres had fewer signs of skin aging, or that women with acne lived longer.
Given these points, Ribero et al concluded that "delayed skin aging may be due to reduced senescence," which means that skin aging may be delayed because the longer telomeres in the cells protect them from deterioration. They did find that the expression of one gene in particular was reduced in women with acne--the regulatory gene zinc finger protein 420, ZNF420--suggesting that those without acne may produce more of a particular protein linked to that gene, though the significance is unclear.
What's the issue?
This study is interesting, but it is important not to make any broad conclusions, such as those who get acne will live longer or look younger longer regardless of other factors such as acne treatment, comorbidities, or even environmental factors. This study may give more support for the genetic contribution of acne, but much more work is needed to determine the clinical relevance. For starters, what about men?
Would you assure your acne patients that their disease may be for their own cosmetic good?
Sunscreen and Sperm: Can Chemical UV Filters Alter Sperm Function?

In an article published online on September 1 in Endocrinology, Rehfeld et al discussed their results after testing 29 UV filters. They found that 13 of 29 filters tested had in vitro effects on Ca2+: 4-methylbenzylidene camphor, 3-benzylidene camphor, menthyl anthranilate, isoamyl p-methoxycinnamate, ethylhexyl salicylate, benzylidene camphor sulfonic acid, homosalate, ethylhexyl methoxycinnamate, octcrylene, butyl methoxydibenzoylmethane, and diethylamino hydroxybenzoyl hexyl benzoate.
This study was prompted by a prior study by Schiffer et al (EMBO Rep. 2014;15:758-765) on multiple endocrine disrupting chemicals of which 33 of 96 tested chemicals induced Ca2+ signals in human sperm cells in vitro. Of these previously tested chemicals, some of the chemical sunscreen filters were the most potent, leading to the current study.
Rehfeld et al sought to determine how the UV filters affected calcium signaling, which is a pathway that is essential for sperm cells to be able to swim healthily. These calcium-signaling pathways usually are triggered by progesterone, but the authors showed that 13 of 29 UV filters (45%) also commenced calcium signaling. This effect began at low doses of the chemicals, below the levels of some UV filters found in people after whole-body application of sunscreens.
What’s the issue?
Are these chemical UV filters mimicking progesterone in vivo and could it be interfering with sperm motility? A suboptimal progesterone-induced Ca2+ influx has been associated with reduced male fertility and CatSper (cation channel of sperm) is essential for male fertility (Hum Reprod. 1995;10:120-124).
The UV filters tested are widely available in Europe and the United States. Although this study was in vitro, the in vivo effects will need to be explored. It has been reported by Chivsvert et al (Anal Chim Acta. 2012;752:11-29) that some UV filters can be transcutaneously absorbed into bodily tissues, which could be potentially important for men trying to conceive or for reproductively challenged couples.
What do you discuss with your patients regarding sunscreen safety?

In an article published online on September 1 in Endocrinology, Rehfeld et al discussed their results after testing 29 UV filters. They found that 13 of 29 filters tested had in vitro effects on Ca2+: 4-methylbenzylidene camphor, 3-benzylidene camphor, menthyl anthranilate, isoamyl p-methoxycinnamate, ethylhexyl salicylate, benzylidene camphor sulfonic acid, homosalate, ethylhexyl methoxycinnamate, octcrylene, butyl methoxydibenzoylmethane, and diethylamino hydroxybenzoyl hexyl benzoate.
This study was prompted by a prior study by Schiffer et al (EMBO Rep. 2014;15:758-765) on multiple endocrine disrupting chemicals of which 33 of 96 tested chemicals induced Ca2+ signals in human sperm cells in vitro. Of these previously tested chemicals, some of the chemical sunscreen filters were the most potent, leading to the current study.
Rehfeld et al sought to determine how the UV filters affected calcium signaling, which is a pathway that is essential for sperm cells to be able to swim healthily. These calcium-signaling pathways usually are triggered by progesterone, but the authors showed that 13 of 29 UV filters (45%) also commenced calcium signaling. This effect began at low doses of the chemicals, below the levels of some UV filters found in people after whole-body application of sunscreens.
What’s the issue?
Are these chemical UV filters mimicking progesterone in vivo and could it be interfering with sperm motility? A suboptimal progesterone-induced Ca2+ influx has been associated with reduced male fertility and CatSper (cation channel of sperm) is essential for male fertility (Hum Reprod. 1995;10:120-124).
The UV filters tested are widely available in Europe and the United States. Although this study was in vitro, the in vivo effects will need to be explored. It has been reported by Chivsvert et al (Anal Chim Acta. 2012;752:11-29) that some UV filters can be transcutaneously absorbed into bodily tissues, which could be potentially important for men trying to conceive or for reproductively challenged couples.
What do you discuss with your patients regarding sunscreen safety?

In an article published online on September 1 in Endocrinology, Rehfeld et al discussed their results after testing 29 UV filters. They found that 13 of 29 filters tested had in vitro effects on Ca2+: 4-methylbenzylidene camphor, 3-benzylidene camphor, menthyl anthranilate, isoamyl p-methoxycinnamate, ethylhexyl salicylate, benzylidene camphor sulfonic acid, homosalate, ethylhexyl methoxycinnamate, octcrylene, butyl methoxydibenzoylmethane, and diethylamino hydroxybenzoyl hexyl benzoate.
This study was prompted by a prior study by Schiffer et al (EMBO Rep. 2014;15:758-765) on multiple endocrine disrupting chemicals of which 33 of 96 tested chemicals induced Ca2+ signals in human sperm cells in vitro. Of these previously tested chemicals, some of the chemical sunscreen filters were the most potent, leading to the current study.
Rehfeld et al sought to determine how the UV filters affected calcium signaling, which is a pathway that is essential for sperm cells to be able to swim healthily. These calcium-signaling pathways usually are triggered by progesterone, but the authors showed that 13 of 29 UV filters (45%) also commenced calcium signaling. This effect began at low doses of the chemicals, below the levels of some UV filters found in people after whole-body application of sunscreens.
What’s the issue?
Are these chemical UV filters mimicking progesterone in vivo and could it be interfering with sperm motility? A suboptimal progesterone-induced Ca2+ influx has been associated with reduced male fertility and CatSper (cation channel of sperm) is essential for male fertility (Hum Reprod. 1995;10:120-124).
The UV filters tested are widely available in Europe and the United States. Although this study was in vitro, the in vivo effects will need to be explored. It has been reported by Chivsvert et al (Anal Chim Acta. 2012;752:11-29) that some UV filters can be transcutaneously absorbed into bodily tissues, which could be potentially important for men trying to conceive or for reproductively challenged couples.
What do you discuss with your patients regarding sunscreen safety?
The 50-year quest for better pregnancy data
Editor’s note: As Ob.Gyn. News celebrates its 50th anniversary, we wanted to know how far the medical community has come in identifying and mitigating drug risks during pregnancy and in the postpartum period. In this article, our four expert columnists share their experiences trying to find and interpret critical pregnancy data, as well as how they weigh the potential risks and benefits for their patients.
The search for information
The biggest advance in the past 50 years is the availability of information, even though limited, relating to the effects of drugs in pregnancy and lactation. In the first few years of this period, it was a daunting task to obtain this information. I can recall spending hours in the hospital’s medical library going through huge volumes of Index Medicus to obtain references that the library could order for me. The appearance of Thomas H. Shepard’s first edition (Catalog of Teratogenic Agents) in 1973 was a step forward and in 1977, O.P. Heinonen and colleagues’ book (Birth Defects and Drugs in Pregnancy) was helpful.
Although all of the above sources were helpful, any book in an evolving field will not have the newest information. Two important services, TERIS and Reprotox, were started to allow clinicians to contact them for up-to-date data. Nevertheless, the biggest change was the availability of current information from the U.S. National Library of Medicine via Toxnet, PubMed, and LactMed, relating to the effects of drugs in pregnancy and lactation.
My method is to ask three questions. First, are there other drugs with a similar mechanism of action that have some human data? In most cases, the answer to this question is no, but even when there are data, it is typically very limited. Second, does the drug cross the human placenta? The answer is typically based on the molecular weight. Any drug with a molecular weight less than 1,000 daltons probably crosses. In the second half of pregnancy, especially in the third trimester, almost every drug crosses. Third, do the animal pregnancy data predict embryo/fetal risk? It was thought that it could if the dose causing harm was less than or equal to 10 times the human dose based on BSA or AUC and there were no signs of maternal toxicity. However, using data from my 10th edition, I and eight coauthors, all of whom are knowledgeable on the effects of drugs in pregnancy, found that the animal data for 311 drugs raised the possibility of human embryo-fetal harm that current data confirmed in only 75 (24%) of the drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5).
The system needs to be fixed. One method is to give the Food and Drug Administration the authority to require manufacturers of drugs likely to be used in pregnancy to gather and publish data on their use in pregnancy. That sounds reasonable, but will it ever occur?
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Learning the lessons of the past
During the last 50 years, two of the most potent known human teratogens, thalidomide and isotretinoin, became available for prescription in the United States. Thanks to the efforts of Frances Kelsey, MD, PhD, at the FDA, the initial application for approval of thalidomide in the United States was denied in the early 1960s. Subsequently, based on evidence from other countries where thalidomide was marketed that the drug can cause a pattern of serious birth defects, a very strict pregnancy prevention program was implemented when the drug was finally approved in the United States in 2006.
Over the last 50 years, we have also seen an important evolution in our ability to conduct pregnancy exposure safety studies. Though we still have limited ability to conduct clinical trials in pregnant women, the need for good quality observational studies has become more widely accepted. The Centers for Disease Control and Prevention’s National Birth Defects Prevention Study (now in its most recent iteration known as BD STEPS) has been one very important source of data on the safety of a wide variety of medications. Using a case-control study design, women who have delivered infants with specific birth defects and comparison women who have delivered non-malformed infants are interviewed about their exposures in pregnancy. These data have been extremely helpful in generating new hypotheses, confirming or refuting findings from other studies, and in testing hypotheses regarding the safety of medications widely used in women of reproductive age. These analyses, for example, have contributed to the large body of literature now available on the safety of antidepressant medications in pregnancy.
At the same time, in the last 30 years, we have seen a tremendous increase in the number of pregnancy registries required or recommended upon approval of a new drug in the United States. These registry studies, while challenging to complete in a timely manner, have steadily improved in terms of rigor, and several disease-based pregnancy exposure studies have been implemented, which have allowed us to better understand the comparative risks or safety of anticonvulsants and antiretroviral drugs, to name a few.
It is important to note that with all these advances in the last 50 years, we still have a huge gap in knowledge about medication safety in pregnancy and lactation. Recent reviews suggest that more than 80% of drugs currently marketed have insufficient or no data available. If we include over-the-counter medications, the knowledge gap grows larger. With the 2014 approval of the long-awaited Pregnancy and Lactation Labeling Rule, clinicians are now beginning to experience the elimination of the old A-B-C-D-X category system for pregnancy safety. In its place, data-driven product labels are required. These are expected to provide the clinician with a clear summary of the relevant studies for a given medication, and to place these in the context of the background risks for the underlying maternal disease being treated, as well as the population risks. However, it is painfully clear that we have a long way to go to generate the needed, high-quality data, to populate those labels.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, San Diego, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures.
Moving toward personalized medicine
Nowhere is a lack of actionable data more pronounced than in the impact of mental health drugs in pregnancy.
As Dr. Briggs and Dr. Chambers have outlined, the quality of data regarding the reproductive safety of medications across the therapeutic spectrum has historically been fair at best. The methodology and the rigor has been sparse and to a large extent, in psychiatry, we were only able to look for signals of concern. Prior to the late 1980s and early 1990s, there was little to guide clinicians on the safety of even very commonly used psychiatric medications during pregnancy. The health implications for women of reproductive age are extraordinary and yet that urgency was not matched by the level of investigation until more recently.
In psychiatry, we have rapidly improving data informing women about the risk for major congenital malformations. The clinical dilemma of weighing the necessity to stay on a medication to prevent relapse of a psychiatric disorder with the potential risk of malformation in the fetus is a wrenching one for the mother-to-be. Only good information can help patients, together with their physician, make collaborative decisions that make sense for them. Given the same information and the same severity of illness, women will make different decisions, and that’s a good thing. The calculus couples use to make these private decisions is unique to those involved. But they are able to move through the process because they have a platform of high-quality information.
So where do we go in the future? We need to get beyond the question of risk of major malformations and move toward understanding the long-term neurodevelopmental implications of prenatal exposures – whether such exposures confer risk or are even potentially salutary. One needs only look at the vast body of literature regarding fetal exposure to selective serotonin reuptake inhibitors (SSRIs) to observe the realization of this trend. When it comes to SSRIs, a fairly clear picture has emerged that they pose little absolute risk in terms of congenital malformations. What is missing is how SSRIs impact a child’s learning and development at age 3, 5, and 10. There have been a few studies in this area, but not a single, large prospective study that accurately quantifies both exposure to SSRIs and maternal psychiatric illness during pregnancy.
I expect that the future will also bring a greater understanding of the impact of untreated mental illness on the risk for obstetrical, neonatal, and longer-term neurodevelopmental outcomes. Most of the safety concerns have centered around the effect of fetal exposure to medications, but we also need to better understand how untreated psychiatric disorders impact the spectrum of relevant outcomes.
Getting back to the dilemma faced by pregnant women who really need medication to sustain emotional well-being, there simply is no perfect answer. No decision is perfect or risk free. What we can hope is that we’ll have personalized approaches that take into account the best available data and the patient’s individual situation and wishes. We’ve already come a long way toward meeting that goal, and I’m optimistic about where we’re going.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Perception of risk
Every year, numerous new medicines are approved by the FDA without data in pregnancy. Animal studies may show a problem that doesn’t appear in humans, or as was the case with thalidomide, the problem may not be apparent in animals and show up later in humans. There are many drugs that are safe in pregnancy, but women are understandably afraid of the potential impact on fetal development.
While my colleagues have presented the advances we’ve made in understanding the actual risks of medications during the prenatal period, it’s also important to focus on the perception of risk and to recognize that the reality and the perception can be vastly different.
At the same time, we began to ask women, using a visual analog scale, what would be their trend toward continuing or terminating pregnancy? Over several studies, we found that the likelihood of termination was high, and certainly much higher than was supported by the evidence of actual harm to the fetus. Specifically, if a woman received information about the safety of the drug and she still gave more than a 50% probability of terminating the pregnancy when surveyed, there was a good chance that she would terminate the pregnancy.
When you consider that most of the drugs that women are commonly prescribed in pregnancy – from most painkillers to antidepressants – are not known to cause malformations in pregnancy, you begin to see how problematic an inflated perception of risk can become.
But we see different trends in women with serious and chronic health problems, such as lupus or epilepsy. These women are typically under the care of a subspecialist, who in many cases has developed a significant knowledge base and comfort level around prescribing the drugs in this area and is able to communicate more clearly to patients both the risks to the fetus and the consequences of failure to treat their condition.
So clearly, the role of the physician and the ob.gyn. in particular is critical. It’s no secret that physicians face a negative legal climate that encourages defensive medicine and that they are often hesitant to tell women, without reservation, that it is okay to take a drug. But we must all remember that it is very easy to cause a woman not to take a medication in pregnancy and often that’s not what’s best for her health. Many women now postpone the age of starting a family and more have chronic conditions that require treatment. The idea of not treating certain conditions for the length of a pregnancy is not always a viable option. Yet there are quite a few women who would consider termination “just to be on the safe side.” That must be taken very seriously by the medical profession.
Dr. Koren is a professor of physiology/pharmacology at Western University, London, Ont., and a professor of medicine at Tel Aviv University. He is the founder of the Motherisk Program. He reported being a paid consultant for Duchesnay and Novartis.
Editor’s note: As Ob.Gyn. News celebrates its 50th anniversary, we wanted to know how far the medical community has come in identifying and mitigating drug risks during pregnancy and in the postpartum period. In this article, our four expert columnists share their experiences trying to find and interpret critical pregnancy data, as well as how they weigh the potential risks and benefits for their patients.
The search for information
The biggest advance in the past 50 years is the availability of information, even though limited, relating to the effects of drugs in pregnancy and lactation. In the first few years of this period, it was a daunting task to obtain this information. I can recall spending hours in the hospital’s medical library going through huge volumes of Index Medicus to obtain references that the library could order for me. The appearance of Thomas H. Shepard’s first edition (Catalog of Teratogenic Agents) in 1973 was a step forward and in 1977, O.P. Heinonen and colleagues’ book (Birth Defects and Drugs in Pregnancy) was helpful.
Although all of the above sources were helpful, any book in an evolving field will not have the newest information. Two important services, TERIS and Reprotox, were started to allow clinicians to contact them for up-to-date data. Nevertheless, the biggest change was the availability of current information from the U.S. National Library of Medicine via Toxnet, PubMed, and LactMed, relating to the effects of drugs in pregnancy and lactation.
My method is to ask three questions. First, are there other drugs with a similar mechanism of action that have some human data? In most cases, the answer to this question is no, but even when there are data, it is typically very limited. Second, does the drug cross the human placenta? The answer is typically based on the molecular weight. Any drug with a molecular weight less than 1,000 daltons probably crosses. In the second half of pregnancy, especially in the third trimester, almost every drug crosses. Third, do the animal pregnancy data predict embryo/fetal risk? It was thought that it could if the dose causing harm was less than or equal to 10 times the human dose based on BSA or AUC and there were no signs of maternal toxicity. However, using data from my 10th edition, I and eight coauthors, all of whom are knowledgeable on the effects of drugs in pregnancy, found that the animal data for 311 drugs raised the possibility of human embryo-fetal harm that current data confirmed in only 75 (24%) of the drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5).
The system needs to be fixed. One method is to give the Food and Drug Administration the authority to require manufacturers of drugs likely to be used in pregnancy to gather and publish data on their use in pregnancy. That sounds reasonable, but will it ever occur?
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Learning the lessons of the past
During the last 50 years, two of the most potent known human teratogens, thalidomide and isotretinoin, became available for prescription in the United States. Thanks to the efforts of Frances Kelsey, MD, PhD, at the FDA, the initial application for approval of thalidomide in the United States was denied in the early 1960s. Subsequently, based on evidence from other countries where thalidomide was marketed that the drug can cause a pattern of serious birth defects, a very strict pregnancy prevention program was implemented when the drug was finally approved in the United States in 2006.
Over the last 50 years, we have also seen an important evolution in our ability to conduct pregnancy exposure safety studies. Though we still have limited ability to conduct clinical trials in pregnant women, the need for good quality observational studies has become more widely accepted. The Centers for Disease Control and Prevention’s National Birth Defects Prevention Study (now in its most recent iteration known as BD STEPS) has been one very important source of data on the safety of a wide variety of medications. Using a case-control study design, women who have delivered infants with specific birth defects and comparison women who have delivered non-malformed infants are interviewed about their exposures in pregnancy. These data have been extremely helpful in generating new hypotheses, confirming or refuting findings from other studies, and in testing hypotheses regarding the safety of medications widely used in women of reproductive age. These analyses, for example, have contributed to the large body of literature now available on the safety of antidepressant medications in pregnancy.
At the same time, in the last 30 years, we have seen a tremendous increase in the number of pregnancy registries required or recommended upon approval of a new drug in the United States. These registry studies, while challenging to complete in a timely manner, have steadily improved in terms of rigor, and several disease-based pregnancy exposure studies have been implemented, which have allowed us to better understand the comparative risks or safety of anticonvulsants and antiretroviral drugs, to name a few.
It is important to note that with all these advances in the last 50 years, we still have a huge gap in knowledge about medication safety in pregnancy and lactation. Recent reviews suggest that more than 80% of drugs currently marketed have insufficient or no data available. If we include over-the-counter medications, the knowledge gap grows larger. With the 2014 approval of the long-awaited Pregnancy and Lactation Labeling Rule, clinicians are now beginning to experience the elimination of the old A-B-C-D-X category system for pregnancy safety. In its place, data-driven product labels are required. These are expected to provide the clinician with a clear summary of the relevant studies for a given medication, and to place these in the context of the background risks for the underlying maternal disease being treated, as well as the population risks. However, it is painfully clear that we have a long way to go to generate the needed, high-quality data, to populate those labels.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, San Diego, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures.
Moving toward personalized medicine
Nowhere is a lack of actionable data more pronounced than in the impact of mental health drugs in pregnancy.
As Dr. Briggs and Dr. Chambers have outlined, the quality of data regarding the reproductive safety of medications across the therapeutic spectrum has historically been fair at best. The methodology and the rigor has been sparse and to a large extent, in psychiatry, we were only able to look for signals of concern. Prior to the late 1980s and early 1990s, there was little to guide clinicians on the safety of even very commonly used psychiatric medications during pregnancy. The health implications for women of reproductive age are extraordinary and yet that urgency was not matched by the level of investigation until more recently.
In psychiatry, we have rapidly improving data informing women about the risk for major congenital malformations. The clinical dilemma of weighing the necessity to stay on a medication to prevent relapse of a psychiatric disorder with the potential risk of malformation in the fetus is a wrenching one for the mother-to-be. Only good information can help patients, together with their physician, make collaborative decisions that make sense for them. Given the same information and the same severity of illness, women will make different decisions, and that’s a good thing. The calculus couples use to make these private decisions is unique to those involved. But they are able to move through the process because they have a platform of high-quality information.
So where do we go in the future? We need to get beyond the question of risk of major malformations and move toward understanding the long-term neurodevelopmental implications of prenatal exposures – whether such exposures confer risk or are even potentially salutary. One needs only look at the vast body of literature regarding fetal exposure to selective serotonin reuptake inhibitors (SSRIs) to observe the realization of this trend. When it comes to SSRIs, a fairly clear picture has emerged that they pose little absolute risk in terms of congenital malformations. What is missing is how SSRIs impact a child’s learning and development at age 3, 5, and 10. There have been a few studies in this area, but not a single, large prospective study that accurately quantifies both exposure to SSRIs and maternal psychiatric illness during pregnancy.
I expect that the future will also bring a greater understanding of the impact of untreated mental illness on the risk for obstetrical, neonatal, and longer-term neurodevelopmental outcomes. Most of the safety concerns have centered around the effect of fetal exposure to medications, but we also need to better understand how untreated psychiatric disorders impact the spectrum of relevant outcomes.
Getting back to the dilemma faced by pregnant women who really need medication to sustain emotional well-being, there simply is no perfect answer. No decision is perfect or risk free. What we can hope is that we’ll have personalized approaches that take into account the best available data and the patient’s individual situation and wishes. We’ve already come a long way toward meeting that goal, and I’m optimistic about where we’re going.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Perception of risk
Every year, numerous new medicines are approved by the FDA without data in pregnancy. Animal studies may show a problem that doesn’t appear in humans, or as was the case with thalidomide, the problem may not be apparent in animals and show up later in humans. There are many drugs that are safe in pregnancy, but women are understandably afraid of the potential impact on fetal development.
While my colleagues have presented the advances we’ve made in understanding the actual risks of medications during the prenatal period, it’s also important to focus on the perception of risk and to recognize that the reality and the perception can be vastly different.
At the same time, we began to ask women, using a visual analog scale, what would be their trend toward continuing or terminating pregnancy? Over several studies, we found that the likelihood of termination was high, and certainly much higher than was supported by the evidence of actual harm to the fetus. Specifically, if a woman received information about the safety of the drug and she still gave more than a 50% probability of terminating the pregnancy when surveyed, there was a good chance that she would terminate the pregnancy.
When you consider that most of the drugs that women are commonly prescribed in pregnancy – from most painkillers to antidepressants – are not known to cause malformations in pregnancy, you begin to see how problematic an inflated perception of risk can become.
But we see different trends in women with serious and chronic health problems, such as lupus or epilepsy. These women are typically under the care of a subspecialist, who in many cases has developed a significant knowledge base and comfort level around prescribing the drugs in this area and is able to communicate more clearly to patients both the risks to the fetus and the consequences of failure to treat their condition.
So clearly, the role of the physician and the ob.gyn. in particular is critical. It’s no secret that physicians face a negative legal climate that encourages defensive medicine and that they are often hesitant to tell women, without reservation, that it is okay to take a drug. But we must all remember that it is very easy to cause a woman not to take a medication in pregnancy and often that’s not what’s best for her health. Many women now postpone the age of starting a family and more have chronic conditions that require treatment. The idea of not treating certain conditions for the length of a pregnancy is not always a viable option. Yet there are quite a few women who would consider termination “just to be on the safe side.” That must be taken very seriously by the medical profession.
Dr. Koren is a professor of physiology/pharmacology at Western University, London, Ont., and a professor of medicine at Tel Aviv University. He is the founder of the Motherisk Program. He reported being a paid consultant for Duchesnay and Novartis.
Editor’s note: As Ob.Gyn. News celebrates its 50th anniversary, we wanted to know how far the medical community has come in identifying and mitigating drug risks during pregnancy and in the postpartum period. In this article, our four expert columnists share their experiences trying to find and interpret critical pregnancy data, as well as how they weigh the potential risks and benefits for their patients.
The search for information
The biggest advance in the past 50 years is the availability of information, even though limited, relating to the effects of drugs in pregnancy and lactation. In the first few years of this period, it was a daunting task to obtain this information. I can recall spending hours in the hospital’s medical library going through huge volumes of Index Medicus to obtain references that the library could order for me. The appearance of Thomas H. Shepard’s first edition (Catalog of Teratogenic Agents) in 1973 was a step forward and in 1977, O.P. Heinonen and colleagues’ book (Birth Defects and Drugs in Pregnancy) was helpful.
Although all of the above sources were helpful, any book in an evolving field will not have the newest information. Two important services, TERIS and Reprotox, were started to allow clinicians to contact them for up-to-date data. Nevertheless, the biggest change was the availability of current information from the U.S. National Library of Medicine via Toxnet, PubMed, and LactMed, relating to the effects of drugs in pregnancy and lactation.
My method is to ask three questions. First, are there other drugs with a similar mechanism of action that have some human data? In most cases, the answer to this question is no, but even when there are data, it is typically very limited. Second, does the drug cross the human placenta? The answer is typically based on the molecular weight. Any drug with a molecular weight less than 1,000 daltons probably crosses. In the second half of pregnancy, especially in the third trimester, almost every drug crosses. Third, do the animal pregnancy data predict embryo/fetal risk? It was thought that it could if the dose causing harm was less than or equal to 10 times the human dose based on BSA or AUC and there were no signs of maternal toxicity. However, using data from my 10th edition, I and eight coauthors, all of whom are knowledgeable on the effects of drugs in pregnancy, found that the animal data for 311 drugs raised the possibility of human embryo-fetal harm that current data confirmed in only 75 (24%) of the drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5).
The system needs to be fixed. One method is to give the Food and Drug Administration the authority to require manufacturers of drugs likely to be used in pregnancy to gather and publish data on their use in pregnancy. That sounds reasonable, but will it ever occur?
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Learning the lessons of the past
During the last 50 years, two of the most potent known human teratogens, thalidomide and isotretinoin, became available for prescription in the United States. Thanks to the efforts of Frances Kelsey, MD, PhD, at the FDA, the initial application for approval of thalidomide in the United States was denied in the early 1960s. Subsequently, based on evidence from other countries where thalidomide was marketed that the drug can cause a pattern of serious birth defects, a very strict pregnancy prevention program was implemented when the drug was finally approved in the United States in 2006.
Over the last 50 years, we have also seen an important evolution in our ability to conduct pregnancy exposure safety studies. Though we still have limited ability to conduct clinical trials in pregnant women, the need for good quality observational studies has become more widely accepted. The Centers for Disease Control and Prevention’s National Birth Defects Prevention Study (now in its most recent iteration known as BD STEPS) has been one very important source of data on the safety of a wide variety of medications. Using a case-control study design, women who have delivered infants with specific birth defects and comparison women who have delivered non-malformed infants are interviewed about their exposures in pregnancy. These data have been extremely helpful in generating new hypotheses, confirming or refuting findings from other studies, and in testing hypotheses regarding the safety of medications widely used in women of reproductive age. These analyses, for example, have contributed to the large body of literature now available on the safety of antidepressant medications in pregnancy.
At the same time, in the last 30 years, we have seen a tremendous increase in the number of pregnancy registries required or recommended upon approval of a new drug in the United States. These registry studies, while challenging to complete in a timely manner, have steadily improved in terms of rigor, and several disease-based pregnancy exposure studies have been implemented, which have allowed us to better understand the comparative risks or safety of anticonvulsants and antiretroviral drugs, to name a few.
It is important to note that with all these advances in the last 50 years, we still have a huge gap in knowledge about medication safety in pregnancy and lactation. Recent reviews suggest that more than 80% of drugs currently marketed have insufficient or no data available. If we include over-the-counter medications, the knowledge gap grows larger. With the 2014 approval of the long-awaited Pregnancy and Lactation Labeling Rule, clinicians are now beginning to experience the elimination of the old A-B-C-D-X category system for pregnancy safety. In its place, data-driven product labels are required. These are expected to provide the clinician with a clear summary of the relevant studies for a given medication, and to place these in the context of the background risks for the underlying maternal disease being treated, as well as the population risks. However, it is painfully clear that we have a long way to go to generate the needed, high-quality data, to populate those labels.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, San Diego, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures.
Moving toward personalized medicine
Nowhere is a lack of actionable data more pronounced than in the impact of mental health drugs in pregnancy.
As Dr. Briggs and Dr. Chambers have outlined, the quality of data regarding the reproductive safety of medications across the therapeutic spectrum has historically been fair at best. The methodology and the rigor has been sparse and to a large extent, in psychiatry, we were only able to look for signals of concern. Prior to the late 1980s and early 1990s, there was little to guide clinicians on the safety of even very commonly used psychiatric medications during pregnancy. The health implications for women of reproductive age are extraordinary and yet that urgency was not matched by the level of investigation until more recently.
In psychiatry, we have rapidly improving data informing women about the risk for major congenital malformations. The clinical dilemma of weighing the necessity to stay on a medication to prevent relapse of a psychiatric disorder with the potential risk of malformation in the fetus is a wrenching one for the mother-to-be. Only good information can help patients, together with their physician, make collaborative decisions that make sense for them. Given the same information and the same severity of illness, women will make different decisions, and that’s a good thing. The calculus couples use to make these private decisions is unique to those involved. But they are able to move through the process because they have a platform of high-quality information.
So where do we go in the future? We need to get beyond the question of risk of major malformations and move toward understanding the long-term neurodevelopmental implications of prenatal exposures – whether such exposures confer risk or are even potentially salutary. One needs only look at the vast body of literature regarding fetal exposure to selective serotonin reuptake inhibitors (SSRIs) to observe the realization of this trend. When it comes to SSRIs, a fairly clear picture has emerged that they pose little absolute risk in terms of congenital malformations. What is missing is how SSRIs impact a child’s learning and development at age 3, 5, and 10. There have been a few studies in this area, but not a single, large prospective study that accurately quantifies both exposure to SSRIs and maternal psychiatric illness during pregnancy.
I expect that the future will also bring a greater understanding of the impact of untreated mental illness on the risk for obstetrical, neonatal, and longer-term neurodevelopmental outcomes. Most of the safety concerns have centered around the effect of fetal exposure to medications, but we also need to better understand how untreated psychiatric disorders impact the spectrum of relevant outcomes.
Getting back to the dilemma faced by pregnant women who really need medication to sustain emotional well-being, there simply is no perfect answer. No decision is perfect or risk free. What we can hope is that we’ll have personalized approaches that take into account the best available data and the patient’s individual situation and wishes. We’ve already come a long way toward meeting that goal, and I’m optimistic about where we’re going.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
Perception of risk
Every year, numerous new medicines are approved by the FDA without data in pregnancy. Animal studies may show a problem that doesn’t appear in humans, or as was the case with thalidomide, the problem may not be apparent in animals and show up later in humans. There are many drugs that are safe in pregnancy, but women are understandably afraid of the potential impact on fetal development.
While my colleagues have presented the advances we’ve made in understanding the actual risks of medications during the prenatal period, it’s also important to focus on the perception of risk and to recognize that the reality and the perception can be vastly different.
At the same time, we began to ask women, using a visual analog scale, what would be their trend toward continuing or terminating pregnancy? Over several studies, we found that the likelihood of termination was high, and certainly much higher than was supported by the evidence of actual harm to the fetus. Specifically, if a woman received information about the safety of the drug and she still gave more than a 50% probability of terminating the pregnancy when surveyed, there was a good chance that she would terminate the pregnancy.
When you consider that most of the drugs that women are commonly prescribed in pregnancy – from most painkillers to antidepressants – are not known to cause malformations in pregnancy, you begin to see how problematic an inflated perception of risk can become.
But we see different trends in women with serious and chronic health problems, such as lupus or epilepsy. These women are typically under the care of a subspecialist, who in many cases has developed a significant knowledge base and comfort level around prescribing the drugs in this area and is able to communicate more clearly to patients both the risks to the fetus and the consequences of failure to treat their condition.
So clearly, the role of the physician and the ob.gyn. in particular is critical. It’s no secret that physicians face a negative legal climate that encourages defensive medicine and that they are often hesitant to tell women, without reservation, that it is okay to take a drug. But we must all remember that it is very easy to cause a woman not to take a medication in pregnancy and often that’s not what’s best for her health. Many women now postpone the age of starting a family and more have chronic conditions that require treatment. The idea of not treating certain conditions for the length of a pregnancy is not always a viable option. Yet there are quite a few women who would consider termination “just to be on the safe side.” That must be taken very seriously by the medical profession.
Dr. Koren is a professor of physiology/pharmacology at Western University, London, Ont., and a professor of medicine at Tel Aviv University. He is the founder of the Motherisk Program. He reported being a paid consultant for Duchesnay and Novartis.
Accelerated aging in schizophrenia: Shortened telomeres, mitochondrial dysfunction, inflammation, and oxidative stress
This implies early senescence, segmental aging, and, in young adult patients, premature onset of multi-system medical illnesses associated with aging, including cardiovascular disease, cancer, brain atrophy, and cognitive decline. This might be the real reason why persons with schizophrenia die 25 to 30 years too early, not only because of an unhealthy lifestyle and iatrogenic cardio-metabolic adverse effects.
One of the most consistent observations pointing to accelerated aging in schizophrenia is shortened telomeres.2,3 Telomeres are the terminal part of chromosomes (similar to the plastic aglets of shoelaces), which are known to shorten with each cell division because of “end replication losses.” Telomeres are measured in lymphocytes, which researchers regard as “windows to the brain” because they reflect brain aging.4 One study of lymphocytes in patients with schizophrenia found that they appear to be approximately 25 years older than the lymphocytes of healthy individuals!4
Possible causes of accelerated aging
Inflammation. The leading hypothesis for accelerated aging in schizophrenia is based on the inflammatory theory of aging. Schizophrenia has been strongly linked to immune dysregulation and neuroinflammation.5 Other components of the accelerated aging hypothesis include oxidative and nitrosative stress, which is associated with high levels of free radicals, and, importantly, mitochondrial dysfunction that fails to generate antioxidants (glutathione peroxidase, superoxide dismutase, and catalase) that can neutralize free radicals and reverse oxidative stress, as numerous studies have shown.
Clinically, and at a relatively young age, persons with schizophrenia show many physical features consistent with aging,6 including the following system changes:
- CNS: dilated ventricles, reduced brain volume and gray matter volume; hypofrontality, neurocognitive deficits such as executive functioning, working memory, and attention; neurophysiologic (low amplitudes on evoked potentials)
- Musculoskeletal system: abnormalities in muscle fibers; altered nerve conduction velocity; reduced bone density
- Skin: aging skin
- Eyes: increased rate of cataracts (not caused by medications); degradation in motion discrimination
- Endocrine system: abnormal gonadal hormones; low estrogen; low androgen; thyroid dysfunction, elevated cortisol
- Metabolism: increased rates of obesity; glucose dysregulation even before antipsychotic treatment; increased insulin resistance; abnormal glucose tolerance; reduced insulin-like growth factor-1 levels
- Immune system: increased pro-inflammatory cytokines (interleukin [IL]-1B, IL-6, IL-3, IL-4, IL-10, IL-13, tumor necrosis factor-Symbol Stdα) and decrease in anti-inflammatory cytokines (IL-2, interferon [INF]-Symbol Stdα, INF-Symbol Stdγ) and vitamin D
- Cardiovascular: systolic hypertension, increased pulse pressure
- Oxidative stress and mitochondrial dysfunction: increase in reactive oxygen species in brain tissue and increased DNA and RNA oxidation markers
- Telomere dynamics: significantly higher rates of telomere loss.
The mitochondrial theory of aging.7 The origin of this theory dates back to the landmark work of Denham Harman more than 2 decades ago in which he proposed a connection between free radicals and aging, which is associated with cell mutations and cancer.8 He suggested that because mitochondrial DNA is not protected by histones as DNA in the nucleus is, it might be the main target for free radicals, making the mitochondria more susceptible to oxidative damage. Therefore, it is possible that the high oxidative stress of schizophrenia could contribute to mitochondrial dysfunction, which leads to further telomere erosion.9 Perhaps reducing oxidative stress in schizophrenia with a powerful antioxidant, such as the supplement N-acetylcysteine,10 could help repair the dysfunctional mitochondria found in patients with schizophrenia and might mitigate accelerated aging.
I would like to propose a bolder, even radical, “out-of-the-box” therapeutic strategy for accelerated aging: mitochondrial transplantation. In fact, “mitochondrial donation” and transplant has been performed on fetuses with genetically defective mitochondria, which has prevented rapid death after birth.11
Similarities with progeria. The accumulating evidence for accelerated aging in schizophrenia has promoted some researchers to consider it a form of progeria12 because of the accelerated aging features that patients with schizophrenia manifest. Patients with schizophrenia share some risk factors with patients with progeria, including high paternal age, prenatal stress, prenatal famine, low birth weight, and premature cognitive decline. Both progeria and schizophrenia are associated with increased apoptosis and cell senescence, which could reduce the risk of cancer but result in premature aging along with age-related medical disorders that lead to mortality in the elderly.
This is why collaborative care between psychiatrists and primary care providers is so vital for patients with schizophrenia from the onset of the illness during the teens and young adulthood, not after years of treatment and unhealthy lifestyle habits (smoking, sedentary living, high-fat and high-calorie diet), which add insult to injury, culminating in loss of 25 to 30 years of potential life. Preventative medical care starting when schizophrenia is first diagnosed is vital, in addition to comprehensive psychiatric care, because premature mortality is the worst outcome in medicine.
Henry A. Nasrallah, MD
Editor-in-Chief
1. Kirkpatrick B, Messias E, Harvey PD, et al. Schizophrenia as a syndrome of accelerated aging? Schizophr Bull. 2005;34(6):1024-1032.
2. Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557-579.
3. Kao HT, Cawthon RM, Delisi LE, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13(2):118-119.
4. Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probes: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):559-576.
5. Horváth S, Mirnics K. Immune system disturbance in schizophrenia. Biol Psychiatry. 2014;75(4):316-323.
6. Shirakumar V, Kalmady SV, Venkatasubramanian G, et al. Do schizophrenia patients age early? Asian J Psychiatr. 2014;10:3-9.
7. Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp Gerontol. 2005;40(6):466-472.
8. Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1992;20(4):145-147.
9. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2000;27(7):339-344.
10. Chen AT, Chibnall JT, Nasrallah HA. A systematic review of placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
11. Three’s company. The Economist. July 9, 2016:88.
12. Papanastasiov E, Gaughran F, Smith S. Schizophrenia as segmental progeria. J R Soc Med. 2011;104(11):475-484.
This implies early senescence, segmental aging, and, in young adult patients, premature onset of multi-system medical illnesses associated with aging, including cardiovascular disease, cancer, brain atrophy, and cognitive decline. This might be the real reason why persons with schizophrenia die 25 to 30 years too early, not only because of an unhealthy lifestyle and iatrogenic cardio-metabolic adverse effects.
One of the most consistent observations pointing to accelerated aging in schizophrenia is shortened telomeres.2,3 Telomeres are the terminal part of chromosomes (similar to the plastic aglets of shoelaces), which are known to shorten with each cell division because of “end replication losses.” Telomeres are measured in lymphocytes, which researchers regard as “windows to the brain” because they reflect brain aging.4 One study of lymphocytes in patients with schizophrenia found that they appear to be approximately 25 years older than the lymphocytes of healthy individuals!4
Possible causes of accelerated aging
Inflammation. The leading hypothesis for accelerated aging in schizophrenia is based on the inflammatory theory of aging. Schizophrenia has been strongly linked to immune dysregulation and neuroinflammation.5 Other components of the accelerated aging hypothesis include oxidative and nitrosative stress, which is associated with high levels of free radicals, and, importantly, mitochondrial dysfunction that fails to generate antioxidants (glutathione peroxidase, superoxide dismutase, and catalase) that can neutralize free radicals and reverse oxidative stress, as numerous studies have shown.
Clinically, and at a relatively young age, persons with schizophrenia show many physical features consistent with aging,6 including the following system changes:
- CNS: dilated ventricles, reduced brain volume and gray matter volume; hypofrontality, neurocognitive deficits such as executive functioning, working memory, and attention; neurophysiologic (low amplitudes on evoked potentials)
- Musculoskeletal system: abnormalities in muscle fibers; altered nerve conduction velocity; reduced bone density
- Skin: aging skin
- Eyes: increased rate of cataracts (not caused by medications); degradation in motion discrimination
- Endocrine system: abnormal gonadal hormones; low estrogen; low androgen; thyroid dysfunction, elevated cortisol
- Metabolism: increased rates of obesity; glucose dysregulation even before antipsychotic treatment; increased insulin resistance; abnormal glucose tolerance; reduced insulin-like growth factor-1 levels
- Immune system: increased pro-inflammatory cytokines (interleukin [IL]-1B, IL-6, IL-3, IL-4, IL-10, IL-13, tumor necrosis factor-Symbol Stdα) and decrease in anti-inflammatory cytokines (IL-2, interferon [INF]-Symbol Stdα, INF-Symbol Stdγ) and vitamin D
- Cardiovascular: systolic hypertension, increased pulse pressure
- Oxidative stress and mitochondrial dysfunction: increase in reactive oxygen species in brain tissue and increased DNA and RNA oxidation markers
- Telomere dynamics: significantly higher rates of telomere loss.
The mitochondrial theory of aging.7 The origin of this theory dates back to the landmark work of Denham Harman more than 2 decades ago in which he proposed a connection between free radicals and aging, which is associated with cell mutations and cancer.8 He suggested that because mitochondrial DNA is not protected by histones as DNA in the nucleus is, it might be the main target for free radicals, making the mitochondria more susceptible to oxidative damage. Therefore, it is possible that the high oxidative stress of schizophrenia could contribute to mitochondrial dysfunction, which leads to further telomere erosion.9 Perhaps reducing oxidative stress in schizophrenia with a powerful antioxidant, such as the supplement N-acetylcysteine,10 could help repair the dysfunctional mitochondria found in patients with schizophrenia and might mitigate accelerated aging.
I would like to propose a bolder, even radical, “out-of-the-box” therapeutic strategy for accelerated aging: mitochondrial transplantation. In fact, “mitochondrial donation” and transplant has been performed on fetuses with genetically defective mitochondria, which has prevented rapid death after birth.11
Similarities with progeria. The accumulating evidence for accelerated aging in schizophrenia has promoted some researchers to consider it a form of progeria12 because of the accelerated aging features that patients with schizophrenia manifest. Patients with schizophrenia share some risk factors with patients with progeria, including high paternal age, prenatal stress, prenatal famine, low birth weight, and premature cognitive decline. Both progeria and schizophrenia are associated with increased apoptosis and cell senescence, which could reduce the risk of cancer but result in premature aging along with age-related medical disorders that lead to mortality in the elderly.
This is why collaborative care between psychiatrists and primary care providers is so vital for patients with schizophrenia from the onset of the illness during the teens and young adulthood, not after years of treatment and unhealthy lifestyle habits (smoking, sedentary living, high-fat and high-calorie diet), which add insult to injury, culminating in loss of 25 to 30 years of potential life. Preventative medical care starting when schizophrenia is first diagnosed is vital, in addition to comprehensive psychiatric care, because premature mortality is the worst outcome in medicine.
Henry A. Nasrallah, MD
Editor-in-Chief
This implies early senescence, segmental aging, and, in young adult patients, premature onset of multi-system medical illnesses associated with aging, including cardiovascular disease, cancer, brain atrophy, and cognitive decline. This might be the real reason why persons with schizophrenia die 25 to 30 years too early, not only because of an unhealthy lifestyle and iatrogenic cardio-metabolic adverse effects.
One of the most consistent observations pointing to accelerated aging in schizophrenia is shortened telomeres.2,3 Telomeres are the terminal part of chromosomes (similar to the plastic aglets of shoelaces), which are known to shorten with each cell division because of “end replication losses.” Telomeres are measured in lymphocytes, which researchers regard as “windows to the brain” because they reflect brain aging.4 One study of lymphocytes in patients with schizophrenia found that they appear to be approximately 25 years older than the lymphocytes of healthy individuals!4
Possible causes of accelerated aging
Inflammation. The leading hypothesis for accelerated aging in schizophrenia is based on the inflammatory theory of aging. Schizophrenia has been strongly linked to immune dysregulation and neuroinflammation.5 Other components of the accelerated aging hypothesis include oxidative and nitrosative stress, which is associated with high levels of free radicals, and, importantly, mitochondrial dysfunction that fails to generate antioxidants (glutathione peroxidase, superoxide dismutase, and catalase) that can neutralize free radicals and reverse oxidative stress, as numerous studies have shown.
Clinically, and at a relatively young age, persons with schizophrenia show many physical features consistent with aging,6 including the following system changes:
- CNS: dilated ventricles, reduced brain volume and gray matter volume; hypofrontality, neurocognitive deficits such as executive functioning, working memory, and attention; neurophysiologic (low amplitudes on evoked potentials)
- Musculoskeletal system: abnormalities in muscle fibers; altered nerve conduction velocity; reduced bone density
- Skin: aging skin
- Eyes: increased rate of cataracts (not caused by medications); degradation in motion discrimination
- Endocrine system: abnormal gonadal hormones; low estrogen; low androgen; thyroid dysfunction, elevated cortisol
- Metabolism: increased rates of obesity; glucose dysregulation even before antipsychotic treatment; increased insulin resistance; abnormal glucose tolerance; reduced insulin-like growth factor-1 levels
- Immune system: increased pro-inflammatory cytokines (interleukin [IL]-1B, IL-6, IL-3, IL-4, IL-10, IL-13, tumor necrosis factor-Symbol Stdα) and decrease in anti-inflammatory cytokines (IL-2, interferon [INF]-Symbol Stdα, INF-Symbol Stdγ) and vitamin D
- Cardiovascular: systolic hypertension, increased pulse pressure
- Oxidative stress and mitochondrial dysfunction: increase in reactive oxygen species in brain tissue and increased DNA and RNA oxidation markers
- Telomere dynamics: significantly higher rates of telomere loss.
The mitochondrial theory of aging.7 The origin of this theory dates back to the landmark work of Denham Harman more than 2 decades ago in which he proposed a connection between free radicals and aging, which is associated with cell mutations and cancer.8 He suggested that because mitochondrial DNA is not protected by histones as DNA in the nucleus is, it might be the main target for free radicals, making the mitochondria more susceptible to oxidative damage. Therefore, it is possible that the high oxidative stress of schizophrenia could contribute to mitochondrial dysfunction, which leads to further telomere erosion.9 Perhaps reducing oxidative stress in schizophrenia with a powerful antioxidant, such as the supplement N-acetylcysteine,10 could help repair the dysfunctional mitochondria found in patients with schizophrenia and might mitigate accelerated aging.
I would like to propose a bolder, even radical, “out-of-the-box” therapeutic strategy for accelerated aging: mitochondrial transplantation. In fact, “mitochondrial donation” and transplant has been performed on fetuses with genetically defective mitochondria, which has prevented rapid death after birth.11
Similarities with progeria. The accumulating evidence for accelerated aging in schizophrenia has promoted some researchers to consider it a form of progeria12 because of the accelerated aging features that patients with schizophrenia manifest. Patients with schizophrenia share some risk factors with patients with progeria, including high paternal age, prenatal stress, prenatal famine, low birth weight, and premature cognitive decline. Both progeria and schizophrenia are associated with increased apoptosis and cell senescence, which could reduce the risk of cancer but result in premature aging along with age-related medical disorders that lead to mortality in the elderly.
This is why collaborative care between psychiatrists and primary care providers is so vital for patients with schizophrenia from the onset of the illness during the teens and young adulthood, not after years of treatment and unhealthy lifestyle habits (smoking, sedentary living, high-fat and high-calorie diet), which add insult to injury, culminating in loss of 25 to 30 years of potential life. Preventative medical care starting when schizophrenia is first diagnosed is vital, in addition to comprehensive psychiatric care, because premature mortality is the worst outcome in medicine.
Henry A. Nasrallah, MD
Editor-in-Chief
1. Kirkpatrick B, Messias E, Harvey PD, et al. Schizophrenia as a syndrome of accelerated aging? Schizophr Bull. 2005;34(6):1024-1032.
2. Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557-579.
3. Kao HT, Cawthon RM, Delisi LE, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13(2):118-119.
4. Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probes: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):559-576.
5. Horváth S, Mirnics K. Immune system disturbance in schizophrenia. Biol Psychiatry. 2014;75(4):316-323.
6. Shirakumar V, Kalmady SV, Venkatasubramanian G, et al. Do schizophrenia patients age early? Asian J Psychiatr. 2014;10:3-9.
7. Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp Gerontol. 2005;40(6):466-472.
8. Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1992;20(4):145-147.
9. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2000;27(7):339-344.
10. Chen AT, Chibnall JT, Nasrallah HA. A systematic review of placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
11. Three’s company. The Economist. July 9, 2016:88.
12. Papanastasiov E, Gaughran F, Smith S. Schizophrenia as segmental progeria. J R Soc Med. 2011;104(11):475-484.
1. Kirkpatrick B, Messias E, Harvey PD, et al. Schizophrenia as a syndrome of accelerated aging? Schizophr Bull. 2005;34(6):1024-1032.
2. Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557-579.
3. Kao HT, Cawthon RM, Delisi LE, et al. Rapid telomere erosion in schizophrenia. Mol Psychiatry. 2008;13(2):118-119.
4. Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probes: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):559-576.
5. Horváth S, Mirnics K. Immune system disturbance in schizophrenia. Biol Psychiatry. 2014;75(4):316-323.
6. Shirakumar V, Kalmady SV, Venkatasubramanian G, et al. Do schizophrenia patients age early? Asian J Psychiatr. 2014;10:3-9.
7. Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp Gerontol. 2005;40(6):466-472.
8. Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1992;20(4):145-147.
9. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2000;27(7):339-344.
10. Chen AT, Chibnall JT, Nasrallah HA. A systematic review of placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: a review. Ann Clin Psychiatry. 2016;28(3):190-196.
11. Three’s company. The Economist. July 9, 2016:88.
12. Papanastasiov E, Gaughran F, Smith S. Schizophrenia as segmental progeria. J R Soc Med. 2011;104(11):475-484.
‘Doc in a box’ vs.’tele-teaming’: Contending models of telepsychiatric care
As articulated by Steve Daviss, MD, DFAPA, in the inaugural column of Techiatry, the adoption and diffusion of telepsychiatry (live two-way interactive videoconferencing) have not been as rapid and universal as expected from those of us immersed in the field.
Despite having yet to achieve its full promise, telepsychiatry has reached maturity, and is being widely deployed and used across multiple systems, settings, and applications, albeit at times in an uneven, unsystematic manner. Emerging over the past decade of development are two distinct models/approaches to telepsychiatry, which I refer to as “doc in a box” and “tele-teaming.” Those models coexist, compete, and conflict within and across organizations. The dynamic between those two models highlights a larger emergent dialogue within psychiatry around psychiatrists’ core clinical roles and functions in our evolving health care systems.
The doc in a box model, as captured in early telepsychiatry services in the 1990s and early 2000s, focused on the virtual insertion of a solo psychiatrist into a distant setting. The services involved core psychiatric activities, such as diagnosis and assessment, with a heavy emphasis on pharmacologic management.
Not surprisingly, those services paralleled what was occurring for the rest of psychiatry at the time and were driven by the closer alignment of psychiatry with a biologic framework – and most importantly, reimbursement models that favored pharmacologic interventions and management. The subsequent rise of viable commercial telepsychiatry companies has continued offering this model driven by demands of the marketplace. While there is a legitimate place and need for such services, the phrase “doc in a box” narrows the scope of psychiatric practice, and reinforces current systems of health care structure and funding.
I proffer the phrase “tele-teaming” to denote telepsychiatric care that virtually embeds a psychiatrist as a member of a care team at a distant location. The use of telepsychiatry in integrated care is the clearest example of this. In integrated care, a psychiatrist works as part of a larger behavioral and medical team that may include case managers, social workers, psychologists, nurses, and family physicians to render care to patients in primary care clinics. The psychiatrist performs consultative, direct care, and supervisory roles in the context of the integrated care team, focusing on more holistic and population-based approaches to treatment.
Telepsychiatry, as well as other technologies (for example, electronic medical records, email, and patient registries), enables and enhances integrated care. Telepsychiatry allows smaller primary care practices, which on their own could not support a full-time psychiatrist, to create a full virtual team across multiple sites.
Tele-teaming as a concept is not limited to integrated care. Other notable examples include the use of telepsychiatry in substance rehabilitation facilities, long-term nursing homes, and psychiatric emergency services as a component of ERs. Tele-teaming and doc in a box models are not about the settings or populations to which they provide care, but the structure and philosophy of the psychiatric service.
As an illustration, imagine a rural community mental health center whose long-term psychiatric care provider retires. The center could set up a contract with a psychiatrist to provide medication management services for its patients through telepsychiatry. The psychiatrist, armed with a pen or eprescribing credentials, could provide several days a week of medication management. Treatment planning, therapy, and care coordination could be segmented off to other providers from the center (psychologists, social workers, and case managers). The psychiatrist’s time could be maximized by having the psychiatrist manage prescriptions, with communication between the patients’ various providers through a shared electronic medical record, as in the doc in a box model.
Alternatively, the center could set up a service where the psychiatrist became a virtual team member working to provide complete assessments, treatment planning, supervision, and psychiatric consultation, as well as pharmacologic management. Although in this scenario, the psychiatrist still could devote time to managing prescriptions, she/he also could spend time in team meetings, supervision, and seeing patients, often with her colleagues. In addition, the psychiatrist could work to coordinate care beyond the EMR, for example, through tele-teaming. Either of these scenarios can and do occur with in-person care as well, and the choice of which model to use would not be decided by the technology but by the underlying health care system in which it is being used.
Telepsychiatry begins to become transformative when it’s leveraged to shift, change, or innovate the model of care delivery. Historically, telepsychiatry has been thought of as a solution to patient access issues and as a way to address workforce shortages. The real promise of telepsychiatry in the form of tele-teaming is how it can begin to fundamentally change how care is delivered. The triple aim calls for decreased costs, improved health care for individuals, and a focus on population health.
We know that most Americans with major mental health issues will be seen in primary care, and that providing behavioral health care in primary care settings leads to lower overall health care costs, and improved behavioral and general health outcomes. Telepsychiatry will be essential in the further dissemination and expansion of integrated care, not only in rural and underserved areas, but across large health care systems. Tele-teaming will be essential in other arenas as well, as psychiatry faces the future challenges of an aging and decreasing (per capita) workforce. Team approaches help maximize psychiatrists’ role in the health care system, and help magnify the number of patients their skills and training can support.
Team approaches also help to broaden the scope of psychiatric practice and reclaim psychiatry’s place within the house of medicine. Telepsychiatry, if structured correctly, helps increase flexibility for psychiatrists. It expands the settings available to practice and provides more opportunities to engage in team-based care. Both the doc in a box and tele-teaming models have arisen from forces in the health care marketplace. The differing application of those two models across and within health care systems illustrates overall tensions within psychiatry about the current and future roles of psychiatrists.
Telepsychiatry is a powerful tool that can be leveraged to shape models of psychiatric care delivery or reinforce our existing structures. Which models are embraced will affect how psychiatry continues to evolve and address the challenges before the specialty.
Dr. Shore chairs the American Psychiatric Association’s Committee on Telepsychiatry, and is director of telemedicine at the Helen & Arthur E. Johnson Depression Center and associate professor of psychiatry at the University of Colorado at Denver, Aurora.
As articulated by Steve Daviss, MD, DFAPA, in the inaugural column of Techiatry, the adoption and diffusion of telepsychiatry (live two-way interactive videoconferencing) have not been as rapid and universal as expected from those of us immersed in the field.
Despite having yet to achieve its full promise, telepsychiatry has reached maturity, and is being widely deployed and used across multiple systems, settings, and applications, albeit at times in an uneven, unsystematic manner. Emerging over the past decade of development are two distinct models/approaches to telepsychiatry, which I refer to as “doc in a box” and “tele-teaming.” Those models coexist, compete, and conflict within and across organizations. The dynamic between those two models highlights a larger emergent dialogue within psychiatry around psychiatrists’ core clinical roles and functions in our evolving health care systems.
The doc in a box model, as captured in early telepsychiatry services in the 1990s and early 2000s, focused on the virtual insertion of a solo psychiatrist into a distant setting. The services involved core psychiatric activities, such as diagnosis and assessment, with a heavy emphasis on pharmacologic management.
Not surprisingly, those services paralleled what was occurring for the rest of psychiatry at the time and were driven by the closer alignment of psychiatry with a biologic framework – and most importantly, reimbursement models that favored pharmacologic interventions and management. The subsequent rise of viable commercial telepsychiatry companies has continued offering this model driven by demands of the marketplace. While there is a legitimate place and need for such services, the phrase “doc in a box” narrows the scope of psychiatric practice, and reinforces current systems of health care structure and funding.
I proffer the phrase “tele-teaming” to denote telepsychiatric care that virtually embeds a psychiatrist as a member of a care team at a distant location. The use of telepsychiatry in integrated care is the clearest example of this. In integrated care, a psychiatrist works as part of a larger behavioral and medical team that may include case managers, social workers, psychologists, nurses, and family physicians to render care to patients in primary care clinics. The psychiatrist performs consultative, direct care, and supervisory roles in the context of the integrated care team, focusing on more holistic and population-based approaches to treatment.
Telepsychiatry, as well as other technologies (for example, electronic medical records, email, and patient registries), enables and enhances integrated care. Telepsychiatry allows smaller primary care practices, which on their own could not support a full-time psychiatrist, to create a full virtual team across multiple sites.
Tele-teaming as a concept is not limited to integrated care. Other notable examples include the use of telepsychiatry in substance rehabilitation facilities, long-term nursing homes, and psychiatric emergency services as a component of ERs. Tele-teaming and doc in a box models are not about the settings or populations to which they provide care, but the structure and philosophy of the psychiatric service.
As an illustration, imagine a rural community mental health center whose long-term psychiatric care provider retires. The center could set up a contract with a psychiatrist to provide medication management services for its patients through telepsychiatry. The psychiatrist, armed with a pen or eprescribing credentials, could provide several days a week of medication management. Treatment planning, therapy, and care coordination could be segmented off to other providers from the center (psychologists, social workers, and case managers). The psychiatrist’s time could be maximized by having the psychiatrist manage prescriptions, with communication between the patients’ various providers through a shared electronic medical record, as in the doc in a box model.
Alternatively, the center could set up a service where the psychiatrist became a virtual team member working to provide complete assessments, treatment planning, supervision, and psychiatric consultation, as well as pharmacologic management. Although in this scenario, the psychiatrist still could devote time to managing prescriptions, she/he also could spend time in team meetings, supervision, and seeing patients, often with her colleagues. In addition, the psychiatrist could work to coordinate care beyond the EMR, for example, through tele-teaming. Either of these scenarios can and do occur with in-person care as well, and the choice of which model to use would not be decided by the technology but by the underlying health care system in which it is being used.
Telepsychiatry begins to become transformative when it’s leveraged to shift, change, or innovate the model of care delivery. Historically, telepsychiatry has been thought of as a solution to patient access issues and as a way to address workforce shortages. The real promise of telepsychiatry in the form of tele-teaming is how it can begin to fundamentally change how care is delivered. The triple aim calls for decreased costs, improved health care for individuals, and a focus on population health.
We know that most Americans with major mental health issues will be seen in primary care, and that providing behavioral health care in primary care settings leads to lower overall health care costs, and improved behavioral and general health outcomes. Telepsychiatry will be essential in the further dissemination and expansion of integrated care, not only in rural and underserved areas, but across large health care systems. Tele-teaming will be essential in other arenas as well, as psychiatry faces the future challenges of an aging and decreasing (per capita) workforce. Team approaches help maximize psychiatrists’ role in the health care system, and help magnify the number of patients their skills and training can support.
Team approaches also help to broaden the scope of psychiatric practice and reclaim psychiatry’s place within the house of medicine. Telepsychiatry, if structured correctly, helps increase flexibility for psychiatrists. It expands the settings available to practice and provides more opportunities to engage in team-based care. Both the doc in a box and tele-teaming models have arisen from forces in the health care marketplace. The differing application of those two models across and within health care systems illustrates overall tensions within psychiatry about the current and future roles of psychiatrists.
Telepsychiatry is a powerful tool that can be leveraged to shape models of psychiatric care delivery or reinforce our existing structures. Which models are embraced will affect how psychiatry continues to evolve and address the challenges before the specialty.
Dr. Shore chairs the American Psychiatric Association’s Committee on Telepsychiatry, and is director of telemedicine at the Helen & Arthur E. Johnson Depression Center and associate professor of psychiatry at the University of Colorado at Denver, Aurora.
As articulated by Steve Daviss, MD, DFAPA, in the inaugural column of Techiatry, the adoption and diffusion of telepsychiatry (live two-way interactive videoconferencing) have not been as rapid and universal as expected from those of us immersed in the field.
Despite having yet to achieve its full promise, telepsychiatry has reached maturity, and is being widely deployed and used across multiple systems, settings, and applications, albeit at times in an uneven, unsystematic manner. Emerging over the past decade of development are two distinct models/approaches to telepsychiatry, which I refer to as “doc in a box” and “tele-teaming.” Those models coexist, compete, and conflict within and across organizations. The dynamic between those two models highlights a larger emergent dialogue within psychiatry around psychiatrists’ core clinical roles and functions in our evolving health care systems.
The doc in a box model, as captured in early telepsychiatry services in the 1990s and early 2000s, focused on the virtual insertion of a solo psychiatrist into a distant setting. The services involved core psychiatric activities, such as diagnosis and assessment, with a heavy emphasis on pharmacologic management.
Not surprisingly, those services paralleled what was occurring for the rest of psychiatry at the time and were driven by the closer alignment of psychiatry with a biologic framework – and most importantly, reimbursement models that favored pharmacologic interventions and management. The subsequent rise of viable commercial telepsychiatry companies has continued offering this model driven by demands of the marketplace. While there is a legitimate place and need for such services, the phrase “doc in a box” narrows the scope of psychiatric practice, and reinforces current systems of health care structure and funding.
I proffer the phrase “tele-teaming” to denote telepsychiatric care that virtually embeds a psychiatrist as a member of a care team at a distant location. The use of telepsychiatry in integrated care is the clearest example of this. In integrated care, a psychiatrist works as part of a larger behavioral and medical team that may include case managers, social workers, psychologists, nurses, and family physicians to render care to patients in primary care clinics. The psychiatrist performs consultative, direct care, and supervisory roles in the context of the integrated care team, focusing on more holistic and population-based approaches to treatment.
Telepsychiatry, as well as other technologies (for example, electronic medical records, email, and patient registries), enables and enhances integrated care. Telepsychiatry allows smaller primary care practices, which on their own could not support a full-time psychiatrist, to create a full virtual team across multiple sites.
Tele-teaming as a concept is not limited to integrated care. Other notable examples include the use of telepsychiatry in substance rehabilitation facilities, long-term nursing homes, and psychiatric emergency services as a component of ERs. Tele-teaming and doc in a box models are not about the settings or populations to which they provide care, but the structure and philosophy of the psychiatric service.
As an illustration, imagine a rural community mental health center whose long-term psychiatric care provider retires. The center could set up a contract with a psychiatrist to provide medication management services for its patients through telepsychiatry. The psychiatrist, armed with a pen or eprescribing credentials, could provide several days a week of medication management. Treatment planning, therapy, and care coordination could be segmented off to other providers from the center (psychologists, social workers, and case managers). The psychiatrist’s time could be maximized by having the psychiatrist manage prescriptions, with communication between the patients’ various providers through a shared electronic medical record, as in the doc in a box model.
Alternatively, the center could set up a service where the psychiatrist became a virtual team member working to provide complete assessments, treatment planning, supervision, and psychiatric consultation, as well as pharmacologic management. Although in this scenario, the psychiatrist still could devote time to managing prescriptions, she/he also could spend time in team meetings, supervision, and seeing patients, often with her colleagues. In addition, the psychiatrist could work to coordinate care beyond the EMR, for example, through tele-teaming. Either of these scenarios can and do occur with in-person care as well, and the choice of which model to use would not be decided by the technology but by the underlying health care system in which it is being used.
Telepsychiatry begins to become transformative when it’s leveraged to shift, change, or innovate the model of care delivery. Historically, telepsychiatry has been thought of as a solution to patient access issues and as a way to address workforce shortages. The real promise of telepsychiatry in the form of tele-teaming is how it can begin to fundamentally change how care is delivered. The triple aim calls for decreased costs, improved health care for individuals, and a focus on population health.
We know that most Americans with major mental health issues will be seen in primary care, and that providing behavioral health care in primary care settings leads to lower overall health care costs, and improved behavioral and general health outcomes. Telepsychiatry will be essential in the further dissemination and expansion of integrated care, not only in rural and underserved areas, but across large health care systems. Tele-teaming will be essential in other arenas as well, as psychiatry faces the future challenges of an aging and decreasing (per capita) workforce. Team approaches help maximize psychiatrists’ role in the health care system, and help magnify the number of patients their skills and training can support.
Team approaches also help to broaden the scope of psychiatric practice and reclaim psychiatry’s place within the house of medicine. Telepsychiatry, if structured correctly, helps increase flexibility for psychiatrists. It expands the settings available to practice and provides more opportunities to engage in team-based care. Both the doc in a box and tele-teaming models have arisen from forces in the health care marketplace. The differing application of those two models across and within health care systems illustrates overall tensions within psychiatry about the current and future roles of psychiatrists.
Telepsychiatry is a powerful tool that can be leveraged to shape models of psychiatric care delivery or reinforce our existing structures. Which models are embraced will affect how psychiatry continues to evolve and address the challenges before the specialty.
Dr. Shore chairs the American Psychiatric Association’s Committee on Telepsychiatry, and is director of telemedicine at the Helen & Arthur E. Johnson Depression Center and associate professor of psychiatry at the University of Colorado at Denver, Aurora.
Surgical Risks From Systemic Psoriasis Therapies

I am a coauthor on a recent literature review (J Am Acad Dermatol. 2016;75:798.e7-805.e7) that addressed a common question regarding the use of systemic agents: What should a clinician do if a patient on one of these therapies has an upcoming elective surgery?
Treatment with systemic immunomodulatory agents commonly is employed in patients with moderate to severe plaque psoriasis and psoriatic arthritis. In these individuals, the concern is that surgery may carry an increased risk for infectious or surgical complications. Based on the available literature, my coauthors and I sought to create recommendations for the perioperative management of systemic immunosuppressive therapies in patients with psoriasis and psoriatic arthritis. We conducted a literature review to examine studies that addressed the use of methotrexate, cyclosporine, and biologic agents in patients undergoing surgery. A total of 46 studies were examined, nearly all retrospective studies in patients with inflammatory bowel disease and rheumatoid arthritis.
Based on level III evidence, we concluded that infliximab, adalimumab, etanercept, methotrexate, and cyclosporine can be safely continued through low-risk operations in patients with psoriasis and psoriatic arthritis. For moderate- and high-risk surgeries, a case-by-case approach should be taken based on the patient’s individual risk factors and comorbidities.
What’s the issue?
This study does not provide specific guidelines because of limited and conflicting literature. However, it does provide general guidelines that hopefully will be augmented in the future. How will you handle this situation when it arises in your practice?

I am a coauthor on a recent literature review (J Am Acad Dermatol. 2016;75:798.e7-805.e7) that addressed a common question regarding the use of systemic agents: What should a clinician do if a patient on one of these therapies has an upcoming elective surgery?
Treatment with systemic immunomodulatory agents commonly is employed in patients with moderate to severe plaque psoriasis and psoriatic arthritis. In these individuals, the concern is that surgery may carry an increased risk for infectious or surgical complications. Based on the available literature, my coauthors and I sought to create recommendations for the perioperative management of systemic immunosuppressive therapies in patients with psoriasis and psoriatic arthritis. We conducted a literature review to examine studies that addressed the use of methotrexate, cyclosporine, and biologic agents in patients undergoing surgery. A total of 46 studies were examined, nearly all retrospective studies in patients with inflammatory bowel disease and rheumatoid arthritis.
Based on level III evidence, we concluded that infliximab, adalimumab, etanercept, methotrexate, and cyclosporine can be safely continued through low-risk operations in patients with psoriasis and psoriatic arthritis. For moderate- and high-risk surgeries, a case-by-case approach should be taken based on the patient’s individual risk factors and comorbidities.
What’s the issue?
This study does not provide specific guidelines because of limited and conflicting literature. However, it does provide general guidelines that hopefully will be augmented in the future. How will you handle this situation when it arises in your practice?

I am a coauthor on a recent literature review (J Am Acad Dermatol. 2016;75:798.e7-805.e7) that addressed a common question regarding the use of systemic agents: What should a clinician do if a patient on one of these therapies has an upcoming elective surgery?
Treatment with systemic immunomodulatory agents commonly is employed in patients with moderate to severe plaque psoriasis and psoriatic arthritis. In these individuals, the concern is that surgery may carry an increased risk for infectious or surgical complications. Based on the available literature, my coauthors and I sought to create recommendations for the perioperative management of systemic immunosuppressive therapies in patients with psoriasis and psoriatic arthritis. We conducted a literature review to examine studies that addressed the use of methotrexate, cyclosporine, and biologic agents in patients undergoing surgery. A total of 46 studies were examined, nearly all retrospective studies in patients with inflammatory bowel disease and rheumatoid arthritis.
Based on level III evidence, we concluded that infliximab, adalimumab, etanercept, methotrexate, and cyclosporine can be safely continued through low-risk operations in patients with psoriasis and psoriatic arthritis. For moderate- and high-risk surgeries, a case-by-case approach should be taken based on the patient’s individual risk factors and comorbidities.
What’s the issue?
This study does not provide specific guidelines because of limited and conflicting literature. However, it does provide general guidelines that hopefully will be augmented in the future. How will you handle this situation when it arises in your practice?
Scalp Psoriasis: Weighing Treatment Options

Scalp psoriasis often is the initial presentation of psoriasis, and it can be one of the most challenging aspects of the disease. It can be difficult to treat for several reasons. First, hair can interfere with topical therapy reaching its site of action on the scalp. Second, facial skin also can be exposed to these treatments with the associated risk for adverse events. Finally, compliance often is difficult.
An evidence-based review published online on September 21 in the American Journal of Clinical Dermatology examined treatments for scalp psoriasis, including newer systemic therapies. Of 475 studies initially identified from PubMed and 845 from Embase (up to May 2016), the review included 27 clinical trials, 4 papers reporting pooled analyses of other clinical trials, 10 open-label trials, 1 case series, and 2 case reports after excluding non-English literature.
Wang and Tsai noted that few randomized controlled trials have been performed specifically in scalp psoriasis. The authors found that topical corticosteroids provide good effects and are usually recommended as first-line treatment. Calcipotriol–betamethasone dipropionate is more highly effective than either of its individual components.
The analysis also suggested that localized phototherapy is better than generalized phototherapy on hair-bearing areas. Methotrexate, cyclosporine, fumaric acid esters, and acitretin are well-recognized agents in the treatment of psoriasis, but they located no published randomized controlled trials specifically evaluating these agents in scalp psoriasis. Wang and Tsai also commented that biologics and new small-molecule agents show excellent effects on scalp psoriasis, but the high cost of these treatments mean they may be limited to use in extensive scalp psoriasis. They suggested that more controlled studies are needed for an evidence-based approach to scalp psoriasis.
What’s the issue?
Scalp psoriasis can be an isolated condition or may occur in association with more extensive disease. There has been increased attention to its treatment over the last several years, with several new options. What is your preferred approach to scalp psoriasis?

Scalp psoriasis often is the initial presentation of psoriasis, and it can be one of the most challenging aspects of the disease. It can be difficult to treat for several reasons. First, hair can interfere with topical therapy reaching its site of action on the scalp. Second, facial skin also can be exposed to these treatments with the associated risk for adverse events. Finally, compliance often is difficult.
An evidence-based review published online on September 21 in the American Journal of Clinical Dermatology examined treatments for scalp psoriasis, including newer systemic therapies. Of 475 studies initially identified from PubMed and 845 from Embase (up to May 2016), the review included 27 clinical trials, 4 papers reporting pooled analyses of other clinical trials, 10 open-label trials, 1 case series, and 2 case reports after excluding non-English literature.
Wang and Tsai noted that few randomized controlled trials have been performed specifically in scalp psoriasis. The authors found that topical corticosteroids provide good effects and are usually recommended as first-line treatment. Calcipotriol–betamethasone dipropionate is more highly effective than either of its individual components.
The analysis also suggested that localized phototherapy is better than generalized phototherapy on hair-bearing areas. Methotrexate, cyclosporine, fumaric acid esters, and acitretin are well-recognized agents in the treatment of psoriasis, but they located no published randomized controlled trials specifically evaluating these agents in scalp psoriasis. Wang and Tsai also commented that biologics and new small-molecule agents show excellent effects on scalp psoriasis, but the high cost of these treatments mean they may be limited to use in extensive scalp psoriasis. They suggested that more controlled studies are needed for an evidence-based approach to scalp psoriasis.
What’s the issue?
Scalp psoriasis can be an isolated condition or may occur in association with more extensive disease. There has been increased attention to its treatment over the last several years, with several new options. What is your preferred approach to scalp psoriasis?

Scalp psoriasis often is the initial presentation of psoriasis, and it can be one of the most challenging aspects of the disease. It can be difficult to treat for several reasons. First, hair can interfere with topical therapy reaching its site of action on the scalp. Second, facial skin also can be exposed to these treatments with the associated risk for adverse events. Finally, compliance often is difficult.
An evidence-based review published online on September 21 in the American Journal of Clinical Dermatology examined treatments for scalp psoriasis, including newer systemic therapies. Of 475 studies initially identified from PubMed and 845 from Embase (up to May 2016), the review included 27 clinical trials, 4 papers reporting pooled analyses of other clinical trials, 10 open-label trials, 1 case series, and 2 case reports after excluding non-English literature.
Wang and Tsai noted that few randomized controlled trials have been performed specifically in scalp psoriasis. The authors found that topical corticosteroids provide good effects and are usually recommended as first-line treatment. Calcipotriol–betamethasone dipropionate is more highly effective than either of its individual components.
The analysis also suggested that localized phototherapy is better than generalized phototherapy on hair-bearing areas. Methotrexate, cyclosporine, fumaric acid esters, and acitretin are well-recognized agents in the treatment of psoriasis, but they located no published randomized controlled trials specifically evaluating these agents in scalp psoriasis. Wang and Tsai also commented that biologics and new small-molecule agents show excellent effects on scalp psoriasis, but the high cost of these treatments mean they may be limited to use in extensive scalp psoriasis. They suggested that more controlled studies are needed for an evidence-based approach to scalp psoriasis.
What’s the issue?
Scalp psoriasis can be an isolated condition or may occur in association with more extensive disease. There has been increased attention to its treatment over the last several years, with several new options. What is your preferred approach to scalp psoriasis?
Technology underused in psychiatry, but changes are ahead
Editors’ Note: The intent of this new column is to discuss topics at the intersection of technology and psychiatry – “Techiatry.” We’ve enlisted two leaders in this field to write for the column. Steven R. Daviss, MD, DFAPA (@HITshrink), is the chief medical informatics officer at M3 Information and chairs the American Psychiatric Association’s Committee on Mental Health Information Technology. James (Jay) H. Shore, MD, MPH, chairs the APA Committee on Telepsychiatry, is director of telemedicine at the Helen & Arthur E. Johnson Depression Center, and an associate professor of psychiatry at the University of Colorado at Denver, Aurora. Email them at [email protected].
Medicine is late to the game when it comes to technology, specifically information technology. And psychiatry, even more so. Jay will talk in future columns about early use of telepsychiatry in the 1960s and since. But here in 2016, a surprisingly low percentage of us are using it to deliver care, despite the fact that half of the counties in the United States lack psychiatrists – and telemedicine has been shown to improve access to care.
Nonetheless, telemedicine and other uses of technology across all specialties is growing quickly, as usability, mobile technology, economics, and policy-making all converge. The integration of mental health care (including addiction treatment) with primary care is one of the driving forces in expanding access to the expertise that physicians trained in psychiatry possess. The collaborative care model of integrated care has the most evidence, making regular access to psychiatric consultants a weekly event.
This exchange of information and knowledge between primary care and psychiatry is being formally incentivized by the Centers for Medicare & Medicaid Services (CMS) with proposed new codes to pay for this exchange, while the American Psychiatric Association has received a large grant from CMS to train 10% of its members in this care model.
Information technology is fundamental to this care model, because the efficient exchange of clinical information is important to optimize the capabilities and comprehensiveness of the clinical decision support provided by the psychiatrist to the primary care team.
As the team members learn what questions are asked and how the consultant arrives at her recommendations, they will become better at making these decisions on their own. They will learn how psychiatrists think and make decisions, weighing other medical, personal, social, family, and logistical aspects to guide the decision making process with the patient.
While this model of care is certainly helpful in expanding access to psychiatric expertise, there are other ways to achieve this access to expert knowledge. One of them is through electronic clinical decision support (CDS) tools. These are tools that apply clinical rules, algorithms, and other knowledge discovery processes to the information within the electronic health record (EHR) about a patient, with the goal of assessing and filling gaps in available patient information so that a set of possible recommendations can be delivered to the clinician.
Knowledge-based CDS tools apply clinical knowledge that comes from practice guidelines, textbooks, and the medical literature to what is known about the patient. The simplest CDS tool might be a rule that says, “IF patient is on lithium for bipolar disorder AND patient has current mood symptoms AND has not had a recent lithium level, THEN check a lithium level.” Applying and coding this rule into an EHR is fairly straightforward. A much more complex CDS tool could help the clinician think through the question, “What should I do next for this 32yo woman with hypertension and moderate depression who is symptomatic?”
Non–knowledge-based CDS tools use machine learning techniques, like neural networks, genetic algorithms, and natural language processing, to “learn” new clinical rules by going through a training process that inputs a large amount of clinical data and uses experts to “train” the system. Such a system was recently developed by IBM Watson and Memorial Sloan-Kettering Cancer Center to aid in developing recommendations for treating oncology patients.
The APA recently formed the CDS Product Workgroup (which I chair) to explore the feasibility of developing an electronic clinical decision support (CDS) tool that leverages the information and knowledge within the APA’s series of Practice Guidelines, DSM library, and other reference materials. This group will consider the necessary important clinical information sources – such as EHRs, personal health records, health information exchanges, claims and utilization data, patient generated data, mobile health apps, and clinical registries – from which to analyze patient-specific data and produce a set of ranked, evidence-based, annotated clinical suggestions.
The goal is to develop a CDS tool that is designed in a manner that ultimately benefits patients being treated by primary care practitioners, emergency practitioners, psychiatrists, and other medical specialists who treat patients with mental health and substance use disorders. Tools like this are being developed now in many specialties. Given the vast amount of psychiatric expertise within the APA, as well as the trove of content that exists within the publishing arm of the APA, the opportunity to make this more broadly available to medical practitioners is one that demands consideration.
Such an undertaking would require substantial time and commitment of resources, thus the task of the workgroup is to understand the pros and cons of developing this tool, and to explore its feasibility, including various business models to ensure that this CDS tool becomes a maintainable and sustainable product.
Bringing our expertise to the primary care settings, where most of our patients are treated, should greatly benefit the care of our patients, whether this is through collaborative care, clinical decision support, or telepsychiatry. In the same way that many people with diabetes do not require an endocrinologist, many with mental health conditions do not require a psychiatrist. Yet, primary care practitioners would certainly benefit from more help from us.
I will update readers of this column on our workgroup’s progress at the end of the year.
Editors’ Note: The intent of this new column is to discuss topics at the intersection of technology and psychiatry – “Techiatry.” We’ve enlisted two leaders in this field to write for the column. Steven R. Daviss, MD, DFAPA (@HITshrink), is the chief medical informatics officer at M3 Information and chairs the American Psychiatric Association’s Committee on Mental Health Information Technology. James (Jay) H. Shore, MD, MPH, chairs the APA Committee on Telepsychiatry, is director of telemedicine at the Helen & Arthur E. Johnson Depression Center, and an associate professor of psychiatry at the University of Colorado at Denver, Aurora. Email them at [email protected].
Medicine is late to the game when it comes to technology, specifically information technology. And psychiatry, even more so. Jay will talk in future columns about early use of telepsychiatry in the 1960s and since. But here in 2016, a surprisingly low percentage of us are using it to deliver care, despite the fact that half of the counties in the United States lack psychiatrists – and telemedicine has been shown to improve access to care.
Nonetheless, telemedicine and other uses of technology across all specialties is growing quickly, as usability, mobile technology, economics, and policy-making all converge. The integration of mental health care (including addiction treatment) with primary care is one of the driving forces in expanding access to the expertise that physicians trained in psychiatry possess. The collaborative care model of integrated care has the most evidence, making regular access to psychiatric consultants a weekly event.
This exchange of information and knowledge between primary care and psychiatry is being formally incentivized by the Centers for Medicare & Medicaid Services (CMS) with proposed new codes to pay for this exchange, while the American Psychiatric Association has received a large grant from CMS to train 10% of its members in this care model.
Information technology is fundamental to this care model, because the efficient exchange of clinical information is important to optimize the capabilities and comprehensiveness of the clinical decision support provided by the psychiatrist to the primary care team.
As the team members learn what questions are asked and how the consultant arrives at her recommendations, they will become better at making these decisions on their own. They will learn how psychiatrists think and make decisions, weighing other medical, personal, social, family, and logistical aspects to guide the decision making process with the patient.
While this model of care is certainly helpful in expanding access to psychiatric expertise, there are other ways to achieve this access to expert knowledge. One of them is through electronic clinical decision support (CDS) tools. These are tools that apply clinical rules, algorithms, and other knowledge discovery processes to the information within the electronic health record (EHR) about a patient, with the goal of assessing and filling gaps in available patient information so that a set of possible recommendations can be delivered to the clinician.
Knowledge-based CDS tools apply clinical knowledge that comes from practice guidelines, textbooks, and the medical literature to what is known about the patient. The simplest CDS tool might be a rule that says, “IF patient is on lithium for bipolar disorder AND patient has current mood symptoms AND has not had a recent lithium level, THEN check a lithium level.” Applying and coding this rule into an EHR is fairly straightforward. A much more complex CDS tool could help the clinician think through the question, “What should I do next for this 32yo woman with hypertension and moderate depression who is symptomatic?”
Non–knowledge-based CDS tools use machine learning techniques, like neural networks, genetic algorithms, and natural language processing, to “learn” new clinical rules by going through a training process that inputs a large amount of clinical data and uses experts to “train” the system. Such a system was recently developed by IBM Watson and Memorial Sloan-Kettering Cancer Center to aid in developing recommendations for treating oncology patients.
The APA recently formed the CDS Product Workgroup (which I chair) to explore the feasibility of developing an electronic clinical decision support (CDS) tool that leverages the information and knowledge within the APA’s series of Practice Guidelines, DSM library, and other reference materials. This group will consider the necessary important clinical information sources – such as EHRs, personal health records, health information exchanges, claims and utilization data, patient generated data, mobile health apps, and clinical registries – from which to analyze patient-specific data and produce a set of ranked, evidence-based, annotated clinical suggestions.
The goal is to develop a CDS tool that is designed in a manner that ultimately benefits patients being treated by primary care practitioners, emergency practitioners, psychiatrists, and other medical specialists who treat patients with mental health and substance use disorders. Tools like this are being developed now in many specialties. Given the vast amount of psychiatric expertise within the APA, as well as the trove of content that exists within the publishing arm of the APA, the opportunity to make this more broadly available to medical practitioners is one that demands consideration.
Such an undertaking would require substantial time and commitment of resources, thus the task of the workgroup is to understand the pros and cons of developing this tool, and to explore its feasibility, including various business models to ensure that this CDS tool becomes a maintainable and sustainable product.
Bringing our expertise to the primary care settings, where most of our patients are treated, should greatly benefit the care of our patients, whether this is through collaborative care, clinical decision support, or telepsychiatry. In the same way that many people with diabetes do not require an endocrinologist, many with mental health conditions do not require a psychiatrist. Yet, primary care practitioners would certainly benefit from more help from us.
I will update readers of this column on our workgroup’s progress at the end of the year.
Editors’ Note: The intent of this new column is to discuss topics at the intersection of technology and psychiatry – “Techiatry.” We’ve enlisted two leaders in this field to write for the column. Steven R. Daviss, MD, DFAPA (@HITshrink), is the chief medical informatics officer at M3 Information and chairs the American Psychiatric Association’s Committee on Mental Health Information Technology. James (Jay) H. Shore, MD, MPH, chairs the APA Committee on Telepsychiatry, is director of telemedicine at the Helen & Arthur E. Johnson Depression Center, and an associate professor of psychiatry at the University of Colorado at Denver, Aurora. Email them at [email protected].
Medicine is late to the game when it comes to technology, specifically information technology. And psychiatry, even more so. Jay will talk in future columns about early use of telepsychiatry in the 1960s and since. But here in 2016, a surprisingly low percentage of us are using it to deliver care, despite the fact that half of the counties in the United States lack psychiatrists – and telemedicine has been shown to improve access to care.
Nonetheless, telemedicine and other uses of technology across all specialties is growing quickly, as usability, mobile technology, economics, and policy-making all converge. The integration of mental health care (including addiction treatment) with primary care is one of the driving forces in expanding access to the expertise that physicians trained in psychiatry possess. The collaborative care model of integrated care has the most evidence, making regular access to psychiatric consultants a weekly event.
This exchange of information and knowledge between primary care and psychiatry is being formally incentivized by the Centers for Medicare & Medicaid Services (CMS) with proposed new codes to pay for this exchange, while the American Psychiatric Association has received a large grant from CMS to train 10% of its members in this care model.
Information technology is fundamental to this care model, because the efficient exchange of clinical information is important to optimize the capabilities and comprehensiveness of the clinical decision support provided by the psychiatrist to the primary care team.
As the team members learn what questions are asked and how the consultant arrives at her recommendations, they will become better at making these decisions on their own. They will learn how psychiatrists think and make decisions, weighing other medical, personal, social, family, and logistical aspects to guide the decision making process with the patient.
While this model of care is certainly helpful in expanding access to psychiatric expertise, there are other ways to achieve this access to expert knowledge. One of them is through electronic clinical decision support (CDS) tools. These are tools that apply clinical rules, algorithms, and other knowledge discovery processes to the information within the electronic health record (EHR) about a patient, with the goal of assessing and filling gaps in available patient information so that a set of possible recommendations can be delivered to the clinician.
Knowledge-based CDS tools apply clinical knowledge that comes from practice guidelines, textbooks, and the medical literature to what is known about the patient. The simplest CDS tool might be a rule that says, “IF patient is on lithium for bipolar disorder AND patient has current mood symptoms AND has not had a recent lithium level, THEN check a lithium level.” Applying and coding this rule into an EHR is fairly straightforward. A much more complex CDS tool could help the clinician think through the question, “What should I do next for this 32yo woman with hypertension and moderate depression who is symptomatic?”
Non–knowledge-based CDS tools use machine learning techniques, like neural networks, genetic algorithms, and natural language processing, to “learn” new clinical rules by going through a training process that inputs a large amount of clinical data and uses experts to “train” the system. Such a system was recently developed by IBM Watson and Memorial Sloan-Kettering Cancer Center to aid in developing recommendations for treating oncology patients.
The APA recently formed the CDS Product Workgroup (which I chair) to explore the feasibility of developing an electronic clinical decision support (CDS) tool that leverages the information and knowledge within the APA’s series of Practice Guidelines, DSM library, and other reference materials. This group will consider the necessary important clinical information sources – such as EHRs, personal health records, health information exchanges, claims and utilization data, patient generated data, mobile health apps, and clinical registries – from which to analyze patient-specific data and produce a set of ranked, evidence-based, annotated clinical suggestions.
The goal is to develop a CDS tool that is designed in a manner that ultimately benefits patients being treated by primary care practitioners, emergency practitioners, psychiatrists, and other medical specialists who treat patients with mental health and substance use disorders. Tools like this are being developed now in many specialties. Given the vast amount of psychiatric expertise within the APA, as well as the trove of content that exists within the publishing arm of the APA, the opportunity to make this more broadly available to medical practitioners is one that demands consideration.
Such an undertaking would require substantial time and commitment of resources, thus the task of the workgroup is to understand the pros and cons of developing this tool, and to explore its feasibility, including various business models to ensure that this CDS tool becomes a maintainable and sustainable product.
Bringing our expertise to the primary care settings, where most of our patients are treated, should greatly benefit the care of our patients, whether this is through collaborative care, clinical decision support, or telepsychiatry. In the same way that many people with diabetes do not require an endocrinologist, many with mental health conditions do not require a psychiatrist. Yet, primary care practitioners would certainly benefit from more help from us.
I will update readers of this column on our workgroup’s progress at the end of the year.