User login
Fighting to Serve: Women in Military Medicine
Let the generations know that women in uniform also guaranteed their freedom.
Mary Walker, MD
Hoping to make a career in nursing, my mother, a newly graduated registered nurse, enlisted in the US Army Nurse Corps shortly after the United States entered World War II. When she married my father, a US Army doctor, in 1942, she was summarily discharged (the Army Nurse Corp changed its policy and permitted married nurses to serve later that year), while my father went on to decades of distinguished service in military medicine.1 My mother always regretted being unable to advance through the ranks of the US Army as other woman nurses did in her training class.
March is Women’s History Month. My personal narrative of discrimination against women in military medicine is a footnote in a long volume of inequitable treatment. This column will examine a few of the most famous—or rather from a justice perspective, infamous—chapters in that story to illustrate how for centuries women heroically fought for the right to serve.
A theme of the early epochs of the American military is that women were forced to come to the difficult realization that the only way to serve was to conceal their identity. In 1776, Margaret Cochran Corbin felt called as her husband did to defend the new nation. She dressed as a man and joined him at the ramparts, helping load his cannon until he was killed, and took over firing at the enemy. Even after being shot, she remained in the ranks, entering the Invalid Regiment at West Point, New York, dedicated to caring for other injured soldiers. As recognition of her exemplary service and battlefield injury Corbin became the first US woman to receive a military pension. The Veterans Affairs New York Harbor Healthcare System Manhattan campus is named in her honor.2
The hypocrisy of the military’s gender politics was nowhere more evident than in the case of Mary Walker, MD, and the Congressional Medal of Honor. Walker graduated from Syracuse Medical College in 1855. At the beginning of the Civil War, Walker’s request to enlist as a surgeon was refused on the grounds of her gender. She declined to be a nurse, and instead volunteered for the Army where she cared for the wounded in various hospitals. Her medical degree was accepted in 1863, enabling her to become a paid surgical officer in the War Department, including 4 months as a prisoner of war.
An early and avid feminist, Walker wore men’s clothing and when she was arrested on the charge of impersonating a male, declared the government had given her permission to dress as a man to facilitate her surgical work. Walker separated from the military in 1865 and President Andrew Johnson awarded her the Congressional Medal of Honor that year. After Walker’s death in 1917, the Medal of Honor was rescinded on the grounds that she had never actually been commissioned and the medal could not be awarded to a civilian. It took 60 years of lobbying before President Jimmy Carter restored her award in 1977.3 That millions of women have served in the military since the Civil War, and Walker remains the only woman among the 3517 service members to have won the nation’s highest military honor, underscores the ongoing injustice.4
February commemorated Black History Month and a second theme that emerges from the study of the history of women in military medicine is intersectionality: How race, gender, sexual orientation, and other identities overlap and interact to generate distinctive forms of discrimination. Ethicists have applied the concept of intersectionality to health care and there are a plethora of examples in military medicine.5 Despite a dire need for nurses in the first and second world wars, and a track record of their exemplary service in prior conflicts, the government repeatedly set up arbitrary obstacles barring highly-qualified Black nurses from enlisting.6 Technically allowed to join the Army Nurse Corps in 1941, Black nurses confronted bureaucratic barriers that restricted them to only caring for Black servicemen and prisoners of war, and racial quotas that resulted in 500 Black nurses vs 59,000 White nurses that served during World War II. Black nurses and their supporters in government and society persisted, and once in uniform, broke through barriers to achieve administrative and clinical excellence.7
My mother’s experience mirrors that of thousands of women whose dreams for a career in military medicine were shattered or who enlisted only to find their aspirations for advancement in the service thwarted. Medical historians remind us that due to bias, much of the book of women healer’s accomplishments remains unwritten, itself a testimony to the pervasive and enduring marginalization of women in Western society. Yet, as this brief glimpse of women in military medicine shows, there is sufficient evidence for us to appreciate their impressive contributions.8
Reflecting on this sketch of women’s struggle for acceptance in military medicine in March 2024, we may presume that the fight for equity has been continuously trending upward.8 President Joseph R. Biden appointed, and even more surprisingly, the US Congress confirmed Rachel Levine, MD, as US Department of Health and Human Services Assistant Under Secretary for Health in 2021, making Levine the highest ranking openly transgender health official in the history of the US government.9 Levine also has the distinction of being the first 4-star admiral in the Commissioned Corps of the US Public Health Service and the only transgender person to achieve this rank in any branch of the US uniformed services.10
However, research suggests that the history of women in the military is far more like an undulating curve. A 2019 study of academic military surgery found evidence of gender disparity even greater than that of the civilian sector.11 True and lasting equity in federal health care practice will require all of us to follow the inspiring examples of so many women known and unknown who fought the military establishment within for the right to heal those wounded fighting the enemy without.
1. Treadwell ME. The Women’s Army Corps. US Army Center of Military History; 1991: Chap 25. Accessed February 20, 2024. https://history.army.mil/books/wwii/Wac/ch25.htm
2. Hayes P. Meet five inspiring women veterans. Published November 10, 2022. Accessed February 20, 2024. https://news.va.gov/110571/meet-five-inspiring-women-veterans/
3. Lange K. Meet Dr. Mary Walker: the only female recipient of the Medical of Honor recipient. Published March 7, 2017. Accessed February 20, 2024. https://www.army.mil/article/183800/meet_dr_mary_walker_the_only_female_medal_of_honor_recipient
4. The National Medal of Honor Museum. Accessed February 20, 2024. https://mohmuseum.org/the-medal
5. Wilson Y, White A, Jefferson A, Danis M. Intersectionality in Clinical Medicine: The Need for a Conceptual Framework. Am J Bioeth. 2019;19(2):8-19. doi:10.1080/15265161.2018.1557275
6. National Women’s History Museum. African American Nurses in World War II. Published July 8, 2019. Accessed February 20, 2024. https://www.womenshistory.org/articles/african-american-nurses-world-war-ii
7. O’Gan P. Smithsonian National Museum of African American History and Culture. Victory at Home and Abroad: African American Army Nurses in World War II. Published May 8, 2023. Accessed February 20, 2024. https://nmaahc.si.edu/explore/stories/nurses-WWII
8. Neve M. Conclusion. In Conrad LI, Neve M, Nutton V, Porter R, and Wear A, eds. The Western Medical Tradition 800 BC to AD 1800. Cambridge University Press; 1995:477-494.
9. Stolberg SG. ‘This is politics’: Dr. Rachel Levine’s rise as transgender issues gain prominence. The New York Times. Updated May 10, 2021. Accessed February 20, 2024. https://www.nytimes.com/2021/05/08/us/politics/rachel-levine-transgender.html
10. Franklin J. Dr. Rachel Levine is sworn in as the nation’s first transgender four-star officer. October 19, 2021. Accessed February 20, 2024. https://www.npr.org/2021/10/19/1047423156/rachel-levine-first-transgender-four-star-officer
11. Herrick-Reynolds K, Brooks D, Wind G, Jackson P, Latham K. Military medicine and the academic surgery gender gap. Mil Med. 2019;184(9-10):383-387. doi:10.1093/milmed/usz083
Let the generations know that women in uniform also guaranteed their freedom.
Mary Walker, MD
Hoping to make a career in nursing, my mother, a newly graduated registered nurse, enlisted in the US Army Nurse Corps shortly after the United States entered World War II. When she married my father, a US Army doctor, in 1942, she was summarily discharged (the Army Nurse Corp changed its policy and permitted married nurses to serve later that year), while my father went on to decades of distinguished service in military medicine.1 My mother always regretted being unable to advance through the ranks of the US Army as other woman nurses did in her training class.
March is Women’s History Month. My personal narrative of discrimination against women in military medicine is a footnote in a long volume of inequitable treatment. This column will examine a few of the most famous—or rather from a justice perspective, infamous—chapters in that story to illustrate how for centuries women heroically fought for the right to serve.
A theme of the early epochs of the American military is that women were forced to come to the difficult realization that the only way to serve was to conceal their identity. In 1776, Margaret Cochran Corbin felt called as her husband did to defend the new nation. She dressed as a man and joined him at the ramparts, helping load his cannon until he was killed, and took over firing at the enemy. Even after being shot, she remained in the ranks, entering the Invalid Regiment at West Point, New York, dedicated to caring for other injured soldiers. As recognition of her exemplary service and battlefield injury Corbin became the first US woman to receive a military pension. The Veterans Affairs New York Harbor Healthcare System Manhattan campus is named in her honor.2
The hypocrisy of the military’s gender politics was nowhere more evident than in the case of Mary Walker, MD, and the Congressional Medal of Honor. Walker graduated from Syracuse Medical College in 1855. At the beginning of the Civil War, Walker’s request to enlist as a surgeon was refused on the grounds of her gender. She declined to be a nurse, and instead volunteered for the Army where she cared for the wounded in various hospitals. Her medical degree was accepted in 1863, enabling her to become a paid surgical officer in the War Department, including 4 months as a prisoner of war.
An early and avid feminist, Walker wore men’s clothing and when she was arrested on the charge of impersonating a male, declared the government had given her permission to dress as a man to facilitate her surgical work. Walker separated from the military in 1865 and President Andrew Johnson awarded her the Congressional Medal of Honor that year. After Walker’s death in 1917, the Medal of Honor was rescinded on the grounds that she had never actually been commissioned and the medal could not be awarded to a civilian. It took 60 years of lobbying before President Jimmy Carter restored her award in 1977.3 That millions of women have served in the military since the Civil War, and Walker remains the only woman among the 3517 service members to have won the nation’s highest military honor, underscores the ongoing injustice.4
February commemorated Black History Month and a second theme that emerges from the study of the history of women in military medicine is intersectionality: How race, gender, sexual orientation, and other identities overlap and interact to generate distinctive forms of discrimination. Ethicists have applied the concept of intersectionality to health care and there are a plethora of examples in military medicine.5 Despite a dire need for nurses in the first and second world wars, and a track record of their exemplary service in prior conflicts, the government repeatedly set up arbitrary obstacles barring highly-qualified Black nurses from enlisting.6 Technically allowed to join the Army Nurse Corps in 1941, Black nurses confronted bureaucratic barriers that restricted them to only caring for Black servicemen and prisoners of war, and racial quotas that resulted in 500 Black nurses vs 59,000 White nurses that served during World War II. Black nurses and their supporters in government and society persisted, and once in uniform, broke through barriers to achieve administrative and clinical excellence.7
My mother’s experience mirrors that of thousands of women whose dreams for a career in military medicine were shattered or who enlisted only to find their aspirations for advancement in the service thwarted. Medical historians remind us that due to bias, much of the book of women healer’s accomplishments remains unwritten, itself a testimony to the pervasive and enduring marginalization of women in Western society. Yet, as this brief glimpse of women in military medicine shows, there is sufficient evidence for us to appreciate their impressive contributions.8
Reflecting on this sketch of women’s struggle for acceptance in military medicine in March 2024, we may presume that the fight for equity has been continuously trending upward.8 President Joseph R. Biden appointed, and even more surprisingly, the US Congress confirmed Rachel Levine, MD, as US Department of Health and Human Services Assistant Under Secretary for Health in 2021, making Levine the highest ranking openly transgender health official in the history of the US government.9 Levine also has the distinction of being the first 4-star admiral in the Commissioned Corps of the US Public Health Service and the only transgender person to achieve this rank in any branch of the US uniformed services.10
However, research suggests that the history of women in the military is far more like an undulating curve. A 2019 study of academic military surgery found evidence of gender disparity even greater than that of the civilian sector.11 True and lasting equity in federal health care practice will require all of us to follow the inspiring examples of so many women known and unknown who fought the military establishment within for the right to heal those wounded fighting the enemy without.
Let the generations know that women in uniform also guaranteed their freedom.
Mary Walker, MD
Hoping to make a career in nursing, my mother, a newly graduated registered nurse, enlisted in the US Army Nurse Corps shortly after the United States entered World War II. When she married my father, a US Army doctor, in 1942, she was summarily discharged (the Army Nurse Corp changed its policy and permitted married nurses to serve later that year), while my father went on to decades of distinguished service in military medicine.1 My mother always regretted being unable to advance through the ranks of the US Army as other woman nurses did in her training class.
March is Women’s History Month. My personal narrative of discrimination against women in military medicine is a footnote in a long volume of inequitable treatment. This column will examine a few of the most famous—or rather from a justice perspective, infamous—chapters in that story to illustrate how for centuries women heroically fought for the right to serve.
A theme of the early epochs of the American military is that women were forced to come to the difficult realization that the only way to serve was to conceal their identity. In 1776, Margaret Cochran Corbin felt called as her husband did to defend the new nation. She dressed as a man and joined him at the ramparts, helping load his cannon until he was killed, and took over firing at the enemy. Even after being shot, she remained in the ranks, entering the Invalid Regiment at West Point, New York, dedicated to caring for other injured soldiers. As recognition of her exemplary service and battlefield injury Corbin became the first US woman to receive a military pension. The Veterans Affairs New York Harbor Healthcare System Manhattan campus is named in her honor.2
The hypocrisy of the military’s gender politics was nowhere more evident than in the case of Mary Walker, MD, and the Congressional Medal of Honor. Walker graduated from Syracuse Medical College in 1855. At the beginning of the Civil War, Walker’s request to enlist as a surgeon was refused on the grounds of her gender. She declined to be a nurse, and instead volunteered for the Army where she cared for the wounded in various hospitals. Her medical degree was accepted in 1863, enabling her to become a paid surgical officer in the War Department, including 4 months as a prisoner of war.
An early and avid feminist, Walker wore men’s clothing and when she was arrested on the charge of impersonating a male, declared the government had given her permission to dress as a man to facilitate her surgical work. Walker separated from the military in 1865 and President Andrew Johnson awarded her the Congressional Medal of Honor that year. After Walker’s death in 1917, the Medal of Honor was rescinded on the grounds that she had never actually been commissioned and the medal could not be awarded to a civilian. It took 60 years of lobbying before President Jimmy Carter restored her award in 1977.3 That millions of women have served in the military since the Civil War, and Walker remains the only woman among the 3517 service members to have won the nation’s highest military honor, underscores the ongoing injustice.4
February commemorated Black History Month and a second theme that emerges from the study of the history of women in military medicine is intersectionality: How race, gender, sexual orientation, and other identities overlap and interact to generate distinctive forms of discrimination. Ethicists have applied the concept of intersectionality to health care and there are a plethora of examples in military medicine.5 Despite a dire need for nurses in the first and second world wars, and a track record of their exemplary service in prior conflicts, the government repeatedly set up arbitrary obstacles barring highly-qualified Black nurses from enlisting.6 Technically allowed to join the Army Nurse Corps in 1941, Black nurses confronted bureaucratic barriers that restricted them to only caring for Black servicemen and prisoners of war, and racial quotas that resulted in 500 Black nurses vs 59,000 White nurses that served during World War II. Black nurses and their supporters in government and society persisted, and once in uniform, broke through barriers to achieve administrative and clinical excellence.7
My mother’s experience mirrors that of thousands of women whose dreams for a career in military medicine were shattered or who enlisted only to find their aspirations for advancement in the service thwarted. Medical historians remind us that due to bias, much of the book of women healer’s accomplishments remains unwritten, itself a testimony to the pervasive and enduring marginalization of women in Western society. Yet, as this brief glimpse of women in military medicine shows, there is sufficient evidence for us to appreciate their impressive contributions.8
Reflecting on this sketch of women’s struggle for acceptance in military medicine in March 2024, we may presume that the fight for equity has been continuously trending upward.8 President Joseph R. Biden appointed, and even more surprisingly, the US Congress confirmed Rachel Levine, MD, as US Department of Health and Human Services Assistant Under Secretary for Health in 2021, making Levine the highest ranking openly transgender health official in the history of the US government.9 Levine also has the distinction of being the first 4-star admiral in the Commissioned Corps of the US Public Health Service and the only transgender person to achieve this rank in any branch of the US uniformed services.10
However, research suggests that the history of women in the military is far more like an undulating curve. A 2019 study of academic military surgery found evidence of gender disparity even greater than that of the civilian sector.11 True and lasting equity in federal health care practice will require all of us to follow the inspiring examples of so many women known and unknown who fought the military establishment within for the right to heal those wounded fighting the enemy without.
1. Treadwell ME. The Women’s Army Corps. US Army Center of Military History; 1991: Chap 25. Accessed February 20, 2024. https://history.army.mil/books/wwii/Wac/ch25.htm
2. Hayes P. Meet five inspiring women veterans. Published November 10, 2022. Accessed February 20, 2024. https://news.va.gov/110571/meet-five-inspiring-women-veterans/
3. Lange K. Meet Dr. Mary Walker: the only female recipient of the Medical of Honor recipient. Published March 7, 2017. Accessed February 20, 2024. https://www.army.mil/article/183800/meet_dr_mary_walker_the_only_female_medal_of_honor_recipient
4. The National Medal of Honor Museum. Accessed February 20, 2024. https://mohmuseum.org/the-medal
5. Wilson Y, White A, Jefferson A, Danis M. Intersectionality in Clinical Medicine: The Need for a Conceptual Framework. Am J Bioeth. 2019;19(2):8-19. doi:10.1080/15265161.2018.1557275
6. National Women’s History Museum. African American Nurses in World War II. Published July 8, 2019. Accessed February 20, 2024. https://www.womenshistory.org/articles/african-american-nurses-world-war-ii
7. O’Gan P. Smithsonian National Museum of African American History and Culture. Victory at Home and Abroad: African American Army Nurses in World War II. Published May 8, 2023. Accessed February 20, 2024. https://nmaahc.si.edu/explore/stories/nurses-WWII
8. Neve M. Conclusion. In Conrad LI, Neve M, Nutton V, Porter R, and Wear A, eds. The Western Medical Tradition 800 BC to AD 1800. Cambridge University Press; 1995:477-494.
9. Stolberg SG. ‘This is politics’: Dr. Rachel Levine’s rise as transgender issues gain prominence. The New York Times. Updated May 10, 2021. Accessed February 20, 2024. https://www.nytimes.com/2021/05/08/us/politics/rachel-levine-transgender.html
10. Franklin J. Dr. Rachel Levine is sworn in as the nation’s first transgender four-star officer. October 19, 2021. Accessed February 20, 2024. https://www.npr.org/2021/10/19/1047423156/rachel-levine-first-transgender-four-star-officer
11. Herrick-Reynolds K, Brooks D, Wind G, Jackson P, Latham K. Military medicine and the academic surgery gender gap. Mil Med. 2019;184(9-10):383-387. doi:10.1093/milmed/usz083
1. Treadwell ME. The Women’s Army Corps. US Army Center of Military History; 1991: Chap 25. Accessed February 20, 2024. https://history.army.mil/books/wwii/Wac/ch25.htm
2. Hayes P. Meet five inspiring women veterans. Published November 10, 2022. Accessed February 20, 2024. https://news.va.gov/110571/meet-five-inspiring-women-veterans/
3. Lange K. Meet Dr. Mary Walker: the only female recipient of the Medical of Honor recipient. Published March 7, 2017. Accessed February 20, 2024. https://www.army.mil/article/183800/meet_dr_mary_walker_the_only_female_medal_of_honor_recipient
4. The National Medal of Honor Museum. Accessed February 20, 2024. https://mohmuseum.org/the-medal
5. Wilson Y, White A, Jefferson A, Danis M. Intersectionality in Clinical Medicine: The Need for a Conceptual Framework. Am J Bioeth. 2019;19(2):8-19. doi:10.1080/15265161.2018.1557275
6. National Women’s History Museum. African American Nurses in World War II. Published July 8, 2019. Accessed February 20, 2024. https://www.womenshistory.org/articles/african-american-nurses-world-war-ii
7. O’Gan P. Smithsonian National Museum of African American History and Culture. Victory at Home and Abroad: African American Army Nurses in World War II. Published May 8, 2023. Accessed February 20, 2024. https://nmaahc.si.edu/explore/stories/nurses-WWII
8. Neve M. Conclusion. In Conrad LI, Neve M, Nutton V, Porter R, and Wear A, eds. The Western Medical Tradition 800 BC to AD 1800. Cambridge University Press; 1995:477-494.
9. Stolberg SG. ‘This is politics’: Dr. Rachel Levine’s rise as transgender issues gain prominence. The New York Times. Updated May 10, 2021. Accessed February 20, 2024. https://www.nytimes.com/2021/05/08/us/politics/rachel-levine-transgender.html
10. Franklin J. Dr. Rachel Levine is sworn in as the nation’s first transgender four-star officer. October 19, 2021. Accessed February 20, 2024. https://www.npr.org/2021/10/19/1047423156/rachel-levine-first-transgender-four-star-officer
11. Herrick-Reynolds K, Brooks D, Wind G, Jackson P, Latham K. Military medicine and the academic surgery gender gap. Mil Med. 2019;184(9-10):383-387. doi:10.1093/milmed/usz083
5 Interesting Neurology Studies
This transcript has been edited for clarity.
Dear colleagues, I’m Christoph Diener from the medical faculty of University Duisburg-Essen in Germany. Today I would like to tell you about five interesting studies that were published in January 2024.
Long COVID
I would like to start with long COVID. There is an ongoing discussion about whether this condition — which means symptoms like dizziness, vertigo, fatigue, headache, and cognitive impairment that persist for more than 6 months — is either a consequence of the infection, functional symptoms, psychosomatic disease, or a depression.
There is an important paper that came out in Science. The group investigated 39 controls and 113 patients who had COVID-19. At 6 months, 40 of them had long COVID. The researchers repeatedly measured more than 6500 proteins in serum. The patients with long COVID had a significant increase in complement activation, which persisted even beyond 6 months. These patients also showed increased tissue lesion markers in the blood and activation of the endothelium.
Also, they had increased platelet activation and autoantibodies with increased anti-cytomegalovirus and anti-Epstein-Barr virus immunoglobulins. These are very strong indicators that COVID-19 leads to long-term changes in our immune system, and different activations of complement factors could explain the variety of symptoms that these patients display. Whether this has consequences for treatment is unclear at the moment.
Parkinson’s Classification
Let me come to another issue, which is the future treatment of Parkinson’s disease, covered in a paper in The Lancet Neurology. I think you are all aware that once patients display symptoms like rigidity, bradykinesia, or tremor, it’s most probably too late for neuroprotective therapy because 70% of the dopaminergic neurons are already dead.
The authors propose a new biological diagnosis of the disease in the preclinical state. This early preclinical diagnosis has three components. One is to show the presence of synuclein either in skin biopsy or in serum. The second is proof of neurodegeneration either by MRI or by PET imaging. The third involves genetic markers.
On top of this, we know that we have preclinical manifestations of Parkinson’s disease, like REM sleep disorders, autonomic disturbances, and cognitive impairment. With this new classification, we should be able to identify the preclinical phase of Parkinson’s disease and include these patients in future trials for neuroprotection.
Niemann-Pick Disease
My third study, in The New England Journal of Medicine, deals with Niemann-Pick disease type C (Trial of N-Acetyl-l-Leucine in Niemann–Pick Disease Type C. This is a rare autosomal recessive disorder that involves impaired lysosomal storage. This disease, which manifests usually in childhood, goes along with systemic, psychiatric, and neurologic abnormalities, and in particular, ataxia. Until now, there has been only one therapy, with miglustat. which has many side effects.
The group of authors found a new therapeutic approach with N-acetyl-L-leucine, which primarily increases mitochondrial energy production. This was a small, placebo-controlled, crossover trial with 2 x 12 weeks of treatment. This new compound showed efficacy and was very well tolerated. This shows that we definitely need long-term studies with this new, well-tolerated drug in this rare disease.
Anticoagulation in Subclinical AF
My fourth study comes from the stroke-prevention field, published in The New England Journal of Medicine. I think you are aware of subclinical atrail fibrillation. These are high-frequency episodes in ECG, usually identified by pacemakers or ECG monitoring systems. The international ARTESIA study included more than 4000 patients randomized either to apixaban 5 mg twice daily or aspirin 81 mg.
After 3.5 years, the investigators showed a small but significant decrease in the rate of stroke, with a relative risk reduction of 37%, but also, unfortunately, a significantly increased risk for major bleeding with apixaban. This means that we need a careful discussion with the patient, the family, and the GP to decide whether these patients should be anticoagulated or not.
Migraine and Depression
My final study, published in the European Journal of Neurology, deals with the comorbidity of depression and migraine. This study in the Netherlands included 108 patients treated with erenumab and 90 with fremanezumab; 68 were controls.
They used two depression scales. They showed that treatment with the monoclonal antibodies improved at least one of the two depression scales. I think this is an important study because it indicates that you can improve comorbid depression in people with severe migraine, even if this study did not show a correlation between the reduction in monthly migraine days and the improvement of depression.
What we learned for clinical practice is that we have to identify depression in people with migraine and we have to deal with it. Whether it’s with the treatment of monoclonal antibodies or antidepressant therapy doesn’t really matter.
Dear colleagues, we had interesting studies this month. I think the most spectacular one was published in Science on long COVID. Thank you very much for listening and watching. I’m Christoph Diener from University Duisburg-Essen.
Dr. Diener is Professor, Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen, Essen, Germany. He disclosed ties with Abbott; Addex Pharma; Alder; Allergan; Almirall; Amgen; Autonomic Technology; AstraZeneca; Bayer Vital; Berlin Chemie; Bristol-Myers Squibb; Boehringer Ingelheim; Chordate; CoAxia; Corimmun; Covidien; Coherex; CoLucid; Daiichi-Sankyo; D-Pharml Electrocore; Fresenius; GlaxoSmithKline; Grunenthal; Janssen-Cilag; Labrys Biologics Lilly; La Roche; 3M Medica; MSD; Medtronic; Menarini; MindFrame; Minster; Neuroscore; Neurobiological Technologies; Novartis; Novo-Nordisk; Johnson & Johnson; Knoll; Paion; Parke-Davis; Pierre Fabre; Pfizer Inc; Schaper and Brummer; sanofi-aventis; Schering-Plough; Servier; Solvay; Syngis; St. Jude; Talecris; Thrombogenics; WebMD Global; Weber and Weber; Wyeth; and Yamanouchi.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I’m Christoph Diener from the medical faculty of University Duisburg-Essen in Germany. Today I would like to tell you about five interesting studies that were published in January 2024.
Long COVID
I would like to start with long COVID. There is an ongoing discussion about whether this condition — which means symptoms like dizziness, vertigo, fatigue, headache, and cognitive impairment that persist for more than 6 months — is either a consequence of the infection, functional symptoms, psychosomatic disease, or a depression.
There is an important paper that came out in Science. The group investigated 39 controls and 113 patients who had COVID-19. At 6 months, 40 of them had long COVID. The researchers repeatedly measured more than 6500 proteins in serum. The patients with long COVID had a significant increase in complement activation, which persisted even beyond 6 months. These patients also showed increased tissue lesion markers in the blood and activation of the endothelium.
Also, they had increased platelet activation and autoantibodies with increased anti-cytomegalovirus and anti-Epstein-Barr virus immunoglobulins. These are very strong indicators that COVID-19 leads to long-term changes in our immune system, and different activations of complement factors could explain the variety of symptoms that these patients display. Whether this has consequences for treatment is unclear at the moment.
Parkinson’s Classification
Let me come to another issue, which is the future treatment of Parkinson’s disease, covered in a paper in The Lancet Neurology. I think you are all aware that once patients display symptoms like rigidity, bradykinesia, or tremor, it’s most probably too late for neuroprotective therapy because 70% of the dopaminergic neurons are already dead.
The authors propose a new biological diagnosis of the disease in the preclinical state. This early preclinical diagnosis has three components. One is to show the presence of synuclein either in skin biopsy or in serum. The second is proof of neurodegeneration either by MRI or by PET imaging. The third involves genetic markers.
On top of this, we know that we have preclinical manifestations of Parkinson’s disease, like REM sleep disorders, autonomic disturbances, and cognitive impairment. With this new classification, we should be able to identify the preclinical phase of Parkinson’s disease and include these patients in future trials for neuroprotection.
Niemann-Pick Disease
My third study, in The New England Journal of Medicine, deals with Niemann-Pick disease type C (Trial of N-Acetyl-l-Leucine in Niemann–Pick Disease Type C. This is a rare autosomal recessive disorder that involves impaired lysosomal storage. This disease, which manifests usually in childhood, goes along with systemic, psychiatric, and neurologic abnormalities, and in particular, ataxia. Until now, there has been only one therapy, with miglustat. which has many side effects.
The group of authors found a new therapeutic approach with N-acetyl-L-leucine, which primarily increases mitochondrial energy production. This was a small, placebo-controlled, crossover trial with 2 x 12 weeks of treatment. This new compound showed efficacy and was very well tolerated. This shows that we definitely need long-term studies with this new, well-tolerated drug in this rare disease.
Anticoagulation in Subclinical AF
My fourth study comes from the stroke-prevention field, published in The New England Journal of Medicine. I think you are aware of subclinical atrail fibrillation. These are high-frequency episodes in ECG, usually identified by pacemakers or ECG monitoring systems. The international ARTESIA study included more than 4000 patients randomized either to apixaban 5 mg twice daily or aspirin 81 mg.
After 3.5 years, the investigators showed a small but significant decrease in the rate of stroke, with a relative risk reduction of 37%, but also, unfortunately, a significantly increased risk for major bleeding with apixaban. This means that we need a careful discussion with the patient, the family, and the GP to decide whether these patients should be anticoagulated or not.
Migraine and Depression
My final study, published in the European Journal of Neurology, deals with the comorbidity of depression and migraine. This study in the Netherlands included 108 patients treated with erenumab and 90 with fremanezumab; 68 were controls.
They used two depression scales. They showed that treatment with the monoclonal antibodies improved at least one of the two depression scales. I think this is an important study because it indicates that you can improve comorbid depression in people with severe migraine, even if this study did not show a correlation between the reduction in monthly migraine days and the improvement of depression.
What we learned for clinical practice is that we have to identify depression in people with migraine and we have to deal with it. Whether it’s with the treatment of monoclonal antibodies or antidepressant therapy doesn’t really matter.
Dear colleagues, we had interesting studies this month. I think the most spectacular one was published in Science on long COVID. Thank you very much for listening and watching. I’m Christoph Diener from University Duisburg-Essen.
Dr. Diener is Professor, Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen, Essen, Germany. He disclosed ties with Abbott; Addex Pharma; Alder; Allergan; Almirall; Amgen; Autonomic Technology; AstraZeneca; Bayer Vital; Berlin Chemie; Bristol-Myers Squibb; Boehringer Ingelheim; Chordate; CoAxia; Corimmun; Covidien; Coherex; CoLucid; Daiichi-Sankyo; D-Pharml Electrocore; Fresenius; GlaxoSmithKline; Grunenthal; Janssen-Cilag; Labrys Biologics Lilly; La Roche; 3M Medica; MSD; Medtronic; Menarini; MindFrame; Minster; Neuroscore; Neurobiological Technologies; Novartis; Novo-Nordisk; Johnson & Johnson; Knoll; Paion; Parke-Davis; Pierre Fabre; Pfizer Inc; Schaper and Brummer; sanofi-aventis; Schering-Plough; Servier; Solvay; Syngis; St. Jude; Talecris; Thrombogenics; WebMD Global; Weber and Weber; Wyeth; and Yamanouchi.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I’m Christoph Diener from the medical faculty of University Duisburg-Essen in Germany. Today I would like to tell you about five interesting studies that were published in January 2024.
Long COVID
I would like to start with long COVID. There is an ongoing discussion about whether this condition — which means symptoms like dizziness, vertigo, fatigue, headache, and cognitive impairment that persist for more than 6 months — is either a consequence of the infection, functional symptoms, psychosomatic disease, or a depression.
There is an important paper that came out in Science. The group investigated 39 controls and 113 patients who had COVID-19. At 6 months, 40 of them had long COVID. The researchers repeatedly measured more than 6500 proteins in serum. The patients with long COVID had a significant increase in complement activation, which persisted even beyond 6 months. These patients also showed increased tissue lesion markers in the blood and activation of the endothelium.
Also, they had increased platelet activation and autoantibodies with increased anti-cytomegalovirus and anti-Epstein-Barr virus immunoglobulins. These are very strong indicators that COVID-19 leads to long-term changes in our immune system, and different activations of complement factors could explain the variety of symptoms that these patients display. Whether this has consequences for treatment is unclear at the moment.
Parkinson’s Classification
Let me come to another issue, which is the future treatment of Parkinson’s disease, covered in a paper in The Lancet Neurology. I think you are all aware that once patients display symptoms like rigidity, bradykinesia, or tremor, it’s most probably too late for neuroprotective therapy because 70% of the dopaminergic neurons are already dead.
The authors propose a new biological diagnosis of the disease in the preclinical state. This early preclinical diagnosis has three components. One is to show the presence of synuclein either in skin biopsy or in serum. The second is proof of neurodegeneration either by MRI or by PET imaging. The third involves genetic markers.
On top of this, we know that we have preclinical manifestations of Parkinson’s disease, like REM sleep disorders, autonomic disturbances, and cognitive impairment. With this new classification, we should be able to identify the preclinical phase of Parkinson’s disease and include these patients in future trials for neuroprotection.
Niemann-Pick Disease
My third study, in The New England Journal of Medicine, deals with Niemann-Pick disease type C (Trial of N-Acetyl-l-Leucine in Niemann–Pick Disease Type C. This is a rare autosomal recessive disorder that involves impaired lysosomal storage. This disease, which manifests usually in childhood, goes along with systemic, psychiatric, and neurologic abnormalities, and in particular, ataxia. Until now, there has been only one therapy, with miglustat. which has many side effects.
The group of authors found a new therapeutic approach with N-acetyl-L-leucine, which primarily increases mitochondrial energy production. This was a small, placebo-controlled, crossover trial with 2 x 12 weeks of treatment. This new compound showed efficacy and was very well tolerated. This shows that we definitely need long-term studies with this new, well-tolerated drug in this rare disease.
Anticoagulation in Subclinical AF
My fourth study comes from the stroke-prevention field, published in The New England Journal of Medicine. I think you are aware of subclinical atrail fibrillation. These are high-frequency episodes in ECG, usually identified by pacemakers or ECG monitoring systems. The international ARTESIA study included more than 4000 patients randomized either to apixaban 5 mg twice daily or aspirin 81 mg.
After 3.5 years, the investigators showed a small but significant decrease in the rate of stroke, with a relative risk reduction of 37%, but also, unfortunately, a significantly increased risk for major bleeding with apixaban. This means that we need a careful discussion with the patient, the family, and the GP to decide whether these patients should be anticoagulated or not.
Migraine and Depression
My final study, published in the European Journal of Neurology, deals with the comorbidity of depression and migraine. This study in the Netherlands included 108 patients treated with erenumab and 90 with fremanezumab; 68 were controls.
They used two depression scales. They showed that treatment with the monoclonal antibodies improved at least one of the two depression scales. I think this is an important study because it indicates that you can improve comorbid depression in people with severe migraine, even if this study did not show a correlation between the reduction in monthly migraine days and the improvement of depression.
What we learned for clinical practice is that we have to identify depression in people with migraine and we have to deal with it. Whether it’s with the treatment of monoclonal antibodies or antidepressant therapy doesn’t really matter.
Dear colleagues, we had interesting studies this month. I think the most spectacular one was published in Science on long COVID. Thank you very much for listening and watching. I’m Christoph Diener from University Duisburg-Essen.
Dr. Diener is Professor, Department of Neurology, Stroke Center-Headache Center, University Duisburg-Essen, Essen, Germany. He disclosed ties with Abbott; Addex Pharma; Alder; Allergan; Almirall; Amgen; Autonomic Technology; AstraZeneca; Bayer Vital; Berlin Chemie; Bristol-Myers Squibb; Boehringer Ingelheim; Chordate; CoAxia; Corimmun; Covidien; Coherex; CoLucid; Daiichi-Sankyo; D-Pharml Electrocore; Fresenius; GlaxoSmithKline; Grunenthal; Janssen-Cilag; Labrys Biologics Lilly; La Roche; 3M Medica; MSD; Medtronic; Menarini; MindFrame; Minster; Neuroscore; Neurobiological Technologies; Novartis; Novo-Nordisk; Johnson & Johnson; Knoll; Paion; Parke-Davis; Pierre Fabre; Pfizer Inc; Schaper and Brummer; sanofi-aventis; Schering-Plough; Servier; Solvay; Syngis; St. Jude; Talecris; Thrombogenics; WebMD Global; Weber and Weber; Wyeth; and Yamanouchi.
A version of this article appeared on Medscape.com.
Unexpectedly Helpful Effects of Drugs Used For Other Reasons
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
A 73-year-old man with hypertension is evaluated for right great toe pain. A tap of the toe reveals uric acid crystals. He has a history of hypertension and hyperlipidemia. His current medications are hydrochlorothiazide, amlodipine, and atorvastatin.
Which blood pressure medication would you recommend to replace his hydrochlorothiazide?
A. Furosemide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
Losartan
Diuretics should be avoided if possible in a patient with gout, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects — a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.1,2 The uric acid lowering appears to be a probenecid-like effect.
Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Matsumura and colleagues looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.3 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Ferreira and colleagues looked at the difference in uric acid lowering between high-dose (150 mg/day) vs low-dose losartan (50 mg/day).4 Compared with low-dose, high-dose losartan reduced serum uric acid by 0.27 (0.34 to 0.21) mg/dL, P < .001.
SGLT2 inhibitors
SGLT2 inhibitors also lower uric acid. Suijik and colleagues conducted an analysis of two randomized trials of SGLT2 inhibitors (empagliflozin and dapagliflozin), and concluded that SGLT2 inhibitors induce uric acid excretion, which is strongly linked to urinary glucose excretion.5
Metformin
Metformin is used as a firstline drug for the treatment of diabetes. It also has evidence for decreasing colonic polyps. Cho and colleagues looked at over 12,000 patients with diabetes over a 12-year period; 3775 underwent colonoscopies.6 They compared frequency of polyps in patients who were using metformin with those who were not treated with metformin. The polyp detection rate was lower in the metformin group than in the no metformin group (39.4% vs. 62.4%, P < .01).
Higurashi and colleagues performed a double-blind, placebo-controlled trial of metformin in nondiabetic patients for the prevention of colon polyps.7 The dose of metformin used in this study was very low (250 mg/day). There were significantly fewer adenomas in the metformin group (22 of 71 patients) than in the placebo group (32 of 62) (relative risk, 0.60; 95% confidence interval, 0.39-0.92, P = .016).
Thiazide diuretics
Thiazide diuretics have long been used to help prevent kidney stones in addition to treating hypertension. They decrease urinary calcium excretion, which may reduce kidney stones. Could this reduction in calcium excretion be good for bones?
Xiao and colleagues did a meta-analysis of 11 prospective studies involving 2,193,160 participants.8 Thiazide diuretic users had a significant 14% reduction in the risk of all fractures (RR, 0.86; 95% CI, 0.80-0.93; P = .009) and an 18% reduction in the risk of hip fracture (RR, 0.82; 95% CI, 0.80-0.93; P = .009). Kruse and colleagues found that long duration and continuity of thiazide exposure seemed to be important to obtain this protective effect on fracture risk.9
Pearls:
- Losartan, but not other ARBs, lowers uric acid levels and may be helpful in managing hypertension in gout patients; higher doses lower uric acid more.
- Metformin use appears to decrease colon polyp formation.
- Thiazide diuretics may reduce fracture risk while patients are taking them.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
2. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
3. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: The COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
4. Ferreira JP et al. High- versus low-dose losartan and uric acid: An analysis from HEAAL. J Cardiol. 2023 Jul;82(1):57-61.
5. Suijk DLS et al. SGLT2 inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Soc Nephrol. 2022 May;17(5):663-71.
6. Youn Hee Cho et al. Does metformin affect the incidence of colonic polyps and adenomas in patients with type 2 diabetes mellitus? Intestinal Res. 2014 Apr;12(2):139-45.
7. Higurashi T et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: A multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83.
8. Xiao X et al. Thiazide diuretic usage and risk of fracture: a meta-analysis of cohort studies. Osteoporos Int. 2018 Jul;29(7):1515-24.
9. Kruse C et al. Continuous and long-term treatment is more important than dosage for the protective effect of thiazide use on bone metabolism and fracture risk. J Intern Med. 2016 Jan;279(1):110-22.
Private Equity in GI
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
Dear colleagues,
In this issue of Perspectives we will explore the business of medicine. With changes in reimbursement models and health care regulation over the past decades, private practice gastroenterology has evolved. Many gastroenterologists are now employed or are part of larger consolidated organizations. A key part of this evolution has been the influx of private equity in GI. The impact of private equity is still being written, and while many have embraced this business model, others have been critical of its influence.
In this issue, Dr. Paul J. Berggreen discusses his group’s experience with private equity and how it has helped improve the quality of patient care that they provide.
Dr. Michael L. Weinstein provides the counterpoint, discussing potential issues with the private equity model, and also highlighting an alternative path taken by his practice. An important topic for gastroenterologists of all ages. We welcome your experience with this issue. Please share with us on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
The Future of Medical Practice
BY DR. PAUL J. BERGGREEN
The future of medicine is being written as we speak. Trends that began in past decades have accelerated. Consolidation among massive hospital systems and health insurance conglomerates has gained momentum.
Physicians have been slow to organize and slower to mobilize. We spend our time caring for patients while national forces shape the future of our profession.
These trends have motivated many physicians to explore vehicles that allow them to remain independent. Creating business relationships with financial entities, including private equity, is one of those methods.
Before exploring those models, some background is instructive.
More than 100,000 doctors have left private practice and become employees of hospitals and other corporate entities since 2019. Today, approximately 75% of physicians are employees of larger health care entities – a record high.
This trend ought to alarm patients and policymakers. Research shows that independent medical practices often deliver better outcomes for patients than hospitals. Physician-owned practices also have lower per-patient costs, fewer preventable hospital admissions, and fewer re-admissions than their larger hospital-owned counterparts.
The business of medicine is very different than it was 40 years ago, when more than three in four doctors cared for patients in their own medical practices. The cost of managing a practice has surged. Labor, rent, and malpractice insurance have grown more expensive. Physicians have had to make significant investments in information technology and electronic health records.
Medicare’s reimbursement rates have not kept pace with these higher operational costs. In fact, Medicare payments to doctors have declined more than 25% in the last two decades after accounting for inflation.
By contrast, reimbursement for inpatient and outpatient hospital services as well as skilled nursing facilities has outpaced inflation since 2001.
Given these economic headwinds – and the mounting administrative and financial burdens that government regulation poses – many independent practitioners have concluded that they have little choice but to sell to larger entities like hospitals, health systems, or insurers.
If they do, they lose autonomy. Patients lose the personal touch an independent practice can offer.
To stay independent, many physicians are partnering with management services organizations (MSOs), which provide nonclinical services such as compliance, contracting, legal and IT support, cybersecurity, marketing, community outreach, recruiting assistance, billing, accounts payable, and guidance on the transition to value-based care.
MSOs are typically backed by investors: perhaps a public company, or a private equity firm. But it’s important to note that the clinical entity – the practice – remains separate from the MSO. Physicians retain control over clinical decision-making after partnering with an MSO.
Private equity is best viewed as a neutral financing mechanism that provides independent practices access to capital so they can build the business, clinical, and technological infrastructure to compete against the vertically integrated health systems that dominate medicine.
Private equity firms don’t “acquire” independent practices. A partnership with a private equity-backed MSO is often what empowers a practice to resist acquisition by a larger hospital or health care system.
The experience of my own practice, Arizona Digestive Health, is instructive. We partnered with GI Alliance – a private equity-backed, gastroenterologist-led MSO – in 2019.
My physician colleagues and I have retained complete clinical autonomy. But we now have the financial and operational support we need to remain independent – and deliver better care for our patients.
For example, we led the development of a GI-focused, population-based clinical dashboard that aggregates real-time data from almost 3 million patients across 16 states who are treated by practices affiliated with GI Alliance.
By drawing on that data, we’ve been able to implement comprehensive care-management programs. In the case of inflammatory bowel disease, for instance, we’ve been able to identify the highest-cost, most at-risk patients and implement more proactive treatment protocols, including dedicated care managers and hotlines. We’ve replicated this model in other disease states as well.
This kind of ongoing, high-touch intervention improves patient outcomes and reduces overall cost by minimizing unplanned episodes of care – like visits to the emergency room.
It’s not possible to provide this level of care in a smaller setting. I should know. I tried to implement a similar approach for the 55 doctors that comprise Arizona Digestive Health in Phoenix. We simply didn’t have the capital or resources to succeed.
Our experience at Arizona Digestive Health is not an outlier. I have seen numerous independent practices in gastroenterology and other specialties throughout the country leverage the resources of private equity-backed MSOs to enhance the level of care they provide – and improve patient outcomes and experiences.
In 2022, the physician leadership of GI Alliance spearheaded a transaction that resulted in the nearly 700 physicians whose independent gastroenterology practices were part of the alliance to grow their collective equity stake in the MSO to more than 85%. Our independent physicians now have voting control of the MSO board of directors.
This evolution of GI Alliance has enabled us to remain true to our mission of putting patients first while enhancing our ability to shape the business support our partnered gastroenterology practices need to expand access to the highest-quality, most affordable care in our communities.
Doctors caring for patients in their own practices used to be the foundation of the U.S. health care system – and for good reason. The model enables patients to receive more personalized care and build deeper, more longitudinal, more trusting relationships with their doctors. That remains the goal of physicians who value autonomy and independence.
Inaction will result in more of the same, with hospitals and insurance companies snapping up independent practices. It’s encouraging to see physicians take back control of their profession. But the climb remains steep.
The easiest way to predict the future is to invent it. Doing so in a patient-centric, physician-led, and physician-owned group is a great start to that journey.
Dr. Paul Berggreen is board chair and president of the American Independent Medical Practice Association. He is founder and president of Arizona Digestive Health, chief strategy officer for the GI Alliance, and chair of data analytics for the Digestive Health Physicians Association. He is also a consultant to Specialty Networks, which is not directly relevant to this article.
Thinking Strategically About Gastroenterology Practice
BY MICHAEL L. WEINSTEIN, MD
Whether you are a young gastroenterologist assessing your career opportunities, or a gastroenterology practice trying to assure your future success, you are likely considering how a private equity transaction might influence your options. In this column, I am going to share what I’ve learned and why my practice chose not to go the route of a private equity investment partner.
In 2018, Capital Digestive Care was an independent practice of 70 physicians centered around Washington, DC. Private equity firms were increasingly investing in health care, seeking to capitalize on the industry’s fragmentation, recession-proof business, and ability to leverage consolidation. Our leadership chose to spend a weekend on a strategic planning retreat to agree on our priorities and long-term goals. I highly recommend that you and/or your practice sit down to list your priorities as your first task.
After defining priorities, a SWOT analysis of your position today and what you project over the next decade will determine a strategy. There is a current shortage of more than 1,400 gastroenterologists in the United States. That gives us a pretty powerful “strength.” However, the consolidation of commercial payers and hospital systems is forcing physicians to accept low reimbursement and navigate a maze of administrative burdens. The mountain of regulatory, administrative, and financial functions can push physicians away from independent practice. Additionally, recruiting, training, and managing an office of medical personnel is not what most gastroenterologists want to do with their time.
The common denominator to achieve success with all of these practice management issues is size. So before providing thoughts about private equity, I recommend consolidation of medical practices as the strategy to achieving long-term goals. Practice size will allow physicians to spread out the administrative work, the cost of the business personnel, the IT systems, and the specialized resources. Purchasing power and negotiation relevance is achieved with size. Our priorities are taking care of our patients, our staff, and our practice colleagues. If we are providing high-value service and have a size relevant to the insurance companies, then we can negotiate value-based contracts, and at the end of the day, we will be financially well-off.
In contrast to the list of priorities a physician would create, the private equity fund manager’s goal is to generate wealth for themselves and their investors. Everything else, like innovation, enhanced service, employee satisfaction, and great quality, takes a back seat to accumulating profit. Their investments are made with a life-cycle of 4-6 years during which money is deployed by acquiring companies, improving the company bottom line profit through cost cutting or bolt-on acquisitions, increasing company profit distributions by adding leveraged debt to the corporate ledger, and then selling the companies often to another private equity fund. Physicians are trained to provide care to patients, and fund managers are trained to create wealth.
The medical practice as a business can grow over a career and provides physicians with top tier incomes. We are proud of the businesses we build and believe they are valuable. Private equity funds acquire medical practices for the future revenue and not the past results. They value a medical practice based on a multiple of the portion of future income the practice wants to sell. They ensure their future revenue through agreements that provide them management fees plus 25%-35% of future physician income for the current and all future physicians. The private equity company will say that the physicians are still independent but in reality all providers become employees of the company with wages defined by a formula. The private equity-owned Management Services Organization (MSO) controls decisions on carrier contracts, practice investments, purchasing, hiring, and the operations of the medical office. To get around corporate practice of medicine regulations, the ownership of the medical practice is placed in the hands of a single friendly physician who has a unique relationship to the MSO.
In my opinion, private equity is not the best strategy to achieve a successful medical practice, including acquiring the needed technology and human resources. It comes at a steep price, including loss of control and a permanent forfeiture of income (“the scrape”). The rhetoric professes that there will be income repair, monetization of practice value, and opportunity for a “second bite of the apple” when the private equity managers sell your practice to the next owner. Private equity’s main contribution for their outsized gains is the capital they bring to the practice. Everything else they bring can be found without selling the income of future partners to create a little more wealth for current partners.
The long-term results of private equity investment in gastroenterology practices has yet to be written. The experience in other specialties is partly documented in literature but the real stories are often hidden behind non-disparagement and non-disclosure clauses. Several investigations show that private equity ownership of health care providers leads to higher costs to patients and payers, employee dissatisfaction, diminished patient access, and worse health outcomes. The Federal Trade Commission and Department of Justice have vowed to scrutinize private equity deals because of mounting evidence that the motive for profit can conflict with maintaining quality.
In 2019, Capital Digestive Care chose Physicians Endoscopy as our strategic partner with the goal of separating and expanding our back-office functions into an MSO capable of providing business services to a larger practice and services to other practices outside of our own. Physicians Endoscopy has since been acquired by Optum/SCA. PE GI Solutions, the MSO, is now a partnership of CDC physician partners and Optum/SCA. Capital Digestive Care remains a practice owned 100% by the physicians. A Business Support Services Agreement defines the services CDC receives and the fees paid to the PE GI Solutions. We maintain MSO Board seats and have input into the operations of the MSO.
Consider your motivations and the degree of control you need. Do you recognize your gaps of knowledge and are you willing to hire people to advise you? Will your practice achieve a balance between the interests of older and younger physicians? Becoming an employed physician in a large practice is an option to manage the concerns about future career stability. Improved quality, expanded service offerings, clout to negotiate value-based payment deals with payers, and back-office business efficiency does not require selling yourself to a private equity fund.
Dr. Michael L. Weinstein is a founder and now chief executive officer of Capital Digestive Care. He is a founder and past president of the Digestive Health Physicians Association, previous counselor on the Governing Board of the American Gastroenterological Association. He reports no relevant conflicts.
References
The FTC and DOJ have vowed to scrutinize private equity deals. Here’s what it means for health care. FIERCE Healthcare, 2022, Oct. 21.
The Effect of Private Equity Investment in Health Care. Penn LDI. 2023 Mar. 10.
Olson, LK. Ethically challenged: Private equity storms US health care. Baltimore: Johns Hopkins University Press. 2022.
‘Miracle’ Drugs
We toss the word “miracle” around a lot — the ’69 Mets; the 1980 U.S. mens hockey team; Charlton Heston scowling into the wind, parting the waters of the Red Sea (or at least a Hollywood backlot).
We especially like to use it for medications, as in “miracle drug.”
Those of us who do this long enough know there ain’t no such thing, but the term keeps coming up — aspirin, penicillin, Botox ...
Recently, the popular press has hung the moniker on the GLP-1 drugs, like Ozempic, as “miracles.” While certainly useful, most of this comes from the drug’s reputation as the American dream of something that causes weight loss without the bother of diet and exercise. Of course, it’s also useful for diabetes, and is being investigated for numerous other conditions.
But the real truth is that it’s not a miracle (in fairness, none of the manufacturers of these drugs are making such a ridiculous claim). Nothing is. The word is tossed around for so many things that it’s almost become meaningless.
This isn’t a knock on the GLP-1 agents as much as it’s how people are. Of course, such a drug will never exist, in spite of all the claims on various Internet sites about miracle cures that Big Medicine is hiding from the public.
People are similar, but not the same, and too heterogeneous to know which drug will work/not work or cause a given side effect. We all deal with the uncertainties of medicine being a trial and error process. We try our best to communicate this to people, with varying degrees of success.
Unfortunately, human nature is such that we often hear only what we want to hear. You can run down the whole list of concerns, but some people stopped paying attention when they heard “weight loss” or “migraine relief” or whatever. Every physician ever has had to deal with this, as will those who follow us.
Medicine changes. People ... not so much.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
We toss the word “miracle” around a lot — the ’69 Mets; the 1980 U.S. mens hockey team; Charlton Heston scowling into the wind, parting the waters of the Red Sea (or at least a Hollywood backlot).
We especially like to use it for medications, as in “miracle drug.”
Those of us who do this long enough know there ain’t no such thing, but the term keeps coming up — aspirin, penicillin, Botox ...
Recently, the popular press has hung the moniker on the GLP-1 drugs, like Ozempic, as “miracles.” While certainly useful, most of this comes from the drug’s reputation as the American dream of something that causes weight loss without the bother of diet and exercise. Of course, it’s also useful for diabetes, and is being investigated for numerous other conditions.
But the real truth is that it’s not a miracle (in fairness, none of the manufacturers of these drugs are making such a ridiculous claim). Nothing is. The word is tossed around for so many things that it’s almost become meaningless.
This isn’t a knock on the GLP-1 agents as much as it’s how people are. Of course, such a drug will never exist, in spite of all the claims on various Internet sites about miracle cures that Big Medicine is hiding from the public.
People are similar, but not the same, and too heterogeneous to know which drug will work/not work or cause a given side effect. We all deal with the uncertainties of medicine being a trial and error process. We try our best to communicate this to people, with varying degrees of success.
Unfortunately, human nature is such that we often hear only what we want to hear. You can run down the whole list of concerns, but some people stopped paying attention when they heard “weight loss” or “migraine relief” or whatever. Every physician ever has had to deal with this, as will those who follow us.
Medicine changes. People ... not so much.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
We toss the word “miracle” around a lot — the ’69 Mets; the 1980 U.S. mens hockey team; Charlton Heston scowling into the wind, parting the waters of the Red Sea (or at least a Hollywood backlot).
We especially like to use it for medications, as in “miracle drug.”
Those of us who do this long enough know there ain’t no such thing, but the term keeps coming up — aspirin, penicillin, Botox ...
Recently, the popular press has hung the moniker on the GLP-1 drugs, like Ozempic, as “miracles.” While certainly useful, most of this comes from the drug’s reputation as the American dream of something that causes weight loss without the bother of diet and exercise. Of course, it’s also useful for diabetes, and is being investigated for numerous other conditions.
But the real truth is that it’s not a miracle (in fairness, none of the manufacturers of these drugs are making such a ridiculous claim). Nothing is. The word is tossed around for so many things that it’s almost become meaningless.
This isn’t a knock on the GLP-1 agents as much as it’s how people are. Of course, such a drug will never exist, in spite of all the claims on various Internet sites about miracle cures that Big Medicine is hiding from the public.
People are similar, but not the same, and too heterogeneous to know which drug will work/not work or cause a given side effect. We all deal with the uncertainties of medicine being a trial and error process. We try our best to communicate this to people, with varying degrees of success.
Unfortunately, human nature is such that we often hear only what we want to hear. You can run down the whole list of concerns, but some people stopped paying attention when they heard “weight loss” or “migraine relief” or whatever. Every physician ever has had to deal with this, as will those who follow us.
Medicine changes. People ... not so much.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
SUID and a Cardboard Box
In this February’s issue of the journal Pediatrics there is an interesting paper that explores the demographics and sleep environments of the more than 8000 sudden unexpected infant death (SUID) victims who died in the United States between 2011 and 2020. The authors’ broad conclusion was “Most SUID, regardless of sleep location, had multiple unsafe sleep factors present demonstrating the need for comprehensive sleep counseling for every family at every encounter.”
From the perspective of a former busy primary care physician I shudder when I read a statement this broad because it doesn’t acknowledge the realities of my professional life. A sentence containing “comprehensive” and two “every’s” won’t be taken seriously by most of the practicing pediatricians I know. However, there is an abundance of information generated by these investigators that could help a pediatrician target his advice to the individual families in his practice without having to resort to time-consuming comprehensive counseling, which is likely to sound like just so much chatter to families overwhelmed by the new challenge of raising a child.
For example, nearly 60% of SUID cases were sharing a sleep surface when they died, and surface-sharing infants were more likely to not have a crib in their home. It seems to me that one should start with the simple question, “Do your have a crib in your home ... in all the homes where the baby will sleep?” And, it should be asked in the hospital prior to discharge.
In 1993, our second-born daughter went home from a teaching hospital in a cardboard box. So did all of her nursery mates. It was decorated with a stork motif and had served as her bassinet. Since early in the last century, families in Finland have been offered a similar box filled with baby supplies. Shrinkflation has caused a scale back in its contents, but the box itself has remained as a safe and inexpensive sleep surface for income-challenged families.
Many babies in this country are put to sleep in a variety of places as they are shuttled around to where the inexpensive childcare is available. Offering families as many boxes as they need may avoid the tragedy of their infant smothering on Aunt Louise’s couch or Cousin Martha’s bed littered with pillows. This is particularly important in a family with multiples (twins, triplets, etc.) who are overrepresented in the SUID population. The opening question about crib availability is likely to alert the healthcare provider to a social situation dominated by poverty that may include lower parental education and a higher likelihood of residential insecurity, all of which are associated with surface-sharing.
The authors observe “surface-sharing in and of itself may not be what caregiver education should focus on.” A simple cardboard box, however, is not a sleep surface likely to be shared with an adult.
For the families for whom surface-sharing is a choice and not a necessity, the investigators encourage us to engage families on their motivation for surface-sharing. This is a discussion that clinicians should be initiating, regardless of our concern of SUID prevention, by simply asking “How are you and your baby sleeping?” Is the baby being nursed to sleep? Where? While the authors acknowledge that their raw data did not allow them to make any observations on a relationship between surface-sharing and breastfeeding, my anecdotal observations have found an unfortunate number of mothers who have become human pacifiers for their babies and are co-sleeping. This association can result in a sleep-deprived parent(s) with unhealthy consequences. Although it can be difficult to uncouple breastfeeding from settling, early intervention triggered by asking a simple question can improve the chances of resolution.
Although I may quibble with the wording of authors’ final conclusion, this is an excellent paper worth looking at. SUID while infrequent and for the most part still mysterious is a tragedy that we should be able to prevent.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In this February’s issue of the journal Pediatrics there is an interesting paper that explores the demographics and sleep environments of the more than 8000 sudden unexpected infant death (SUID) victims who died in the United States between 2011 and 2020. The authors’ broad conclusion was “Most SUID, regardless of sleep location, had multiple unsafe sleep factors present demonstrating the need for comprehensive sleep counseling for every family at every encounter.”
From the perspective of a former busy primary care physician I shudder when I read a statement this broad because it doesn’t acknowledge the realities of my professional life. A sentence containing “comprehensive” and two “every’s” won’t be taken seriously by most of the practicing pediatricians I know. However, there is an abundance of information generated by these investigators that could help a pediatrician target his advice to the individual families in his practice without having to resort to time-consuming comprehensive counseling, which is likely to sound like just so much chatter to families overwhelmed by the new challenge of raising a child.
For example, nearly 60% of SUID cases were sharing a sleep surface when they died, and surface-sharing infants were more likely to not have a crib in their home. It seems to me that one should start with the simple question, “Do your have a crib in your home ... in all the homes where the baby will sleep?” And, it should be asked in the hospital prior to discharge.
In 1993, our second-born daughter went home from a teaching hospital in a cardboard box. So did all of her nursery mates. It was decorated with a stork motif and had served as her bassinet. Since early in the last century, families in Finland have been offered a similar box filled with baby supplies. Shrinkflation has caused a scale back in its contents, but the box itself has remained as a safe and inexpensive sleep surface for income-challenged families.
Many babies in this country are put to sleep in a variety of places as they are shuttled around to where the inexpensive childcare is available. Offering families as many boxes as they need may avoid the tragedy of their infant smothering on Aunt Louise’s couch or Cousin Martha’s bed littered with pillows. This is particularly important in a family with multiples (twins, triplets, etc.) who are overrepresented in the SUID population. The opening question about crib availability is likely to alert the healthcare provider to a social situation dominated by poverty that may include lower parental education and a higher likelihood of residential insecurity, all of which are associated with surface-sharing.
The authors observe “surface-sharing in and of itself may not be what caregiver education should focus on.” A simple cardboard box, however, is not a sleep surface likely to be shared with an adult.
For the families for whom surface-sharing is a choice and not a necessity, the investigators encourage us to engage families on their motivation for surface-sharing. This is a discussion that clinicians should be initiating, regardless of our concern of SUID prevention, by simply asking “How are you and your baby sleeping?” Is the baby being nursed to sleep? Where? While the authors acknowledge that their raw data did not allow them to make any observations on a relationship between surface-sharing and breastfeeding, my anecdotal observations have found an unfortunate number of mothers who have become human pacifiers for their babies and are co-sleeping. This association can result in a sleep-deprived parent(s) with unhealthy consequences. Although it can be difficult to uncouple breastfeeding from settling, early intervention triggered by asking a simple question can improve the chances of resolution.
Although I may quibble with the wording of authors’ final conclusion, this is an excellent paper worth looking at. SUID while infrequent and for the most part still mysterious is a tragedy that we should be able to prevent.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In this February’s issue of the journal Pediatrics there is an interesting paper that explores the demographics and sleep environments of the more than 8000 sudden unexpected infant death (SUID) victims who died in the United States between 2011 and 2020. The authors’ broad conclusion was “Most SUID, regardless of sleep location, had multiple unsafe sleep factors present demonstrating the need for comprehensive sleep counseling for every family at every encounter.”
From the perspective of a former busy primary care physician I shudder when I read a statement this broad because it doesn’t acknowledge the realities of my professional life. A sentence containing “comprehensive” and two “every’s” won’t be taken seriously by most of the practicing pediatricians I know. However, there is an abundance of information generated by these investigators that could help a pediatrician target his advice to the individual families in his practice without having to resort to time-consuming comprehensive counseling, which is likely to sound like just so much chatter to families overwhelmed by the new challenge of raising a child.
For example, nearly 60% of SUID cases were sharing a sleep surface when they died, and surface-sharing infants were more likely to not have a crib in their home. It seems to me that one should start with the simple question, “Do your have a crib in your home ... in all the homes where the baby will sleep?” And, it should be asked in the hospital prior to discharge.
In 1993, our second-born daughter went home from a teaching hospital in a cardboard box. So did all of her nursery mates. It was decorated with a stork motif and had served as her bassinet. Since early in the last century, families in Finland have been offered a similar box filled with baby supplies. Shrinkflation has caused a scale back in its contents, but the box itself has remained as a safe and inexpensive sleep surface for income-challenged families.
Many babies in this country are put to sleep in a variety of places as they are shuttled around to where the inexpensive childcare is available. Offering families as many boxes as they need may avoid the tragedy of their infant smothering on Aunt Louise’s couch or Cousin Martha’s bed littered with pillows. This is particularly important in a family with multiples (twins, triplets, etc.) who are overrepresented in the SUID population. The opening question about crib availability is likely to alert the healthcare provider to a social situation dominated by poverty that may include lower parental education and a higher likelihood of residential insecurity, all of which are associated with surface-sharing.
The authors observe “surface-sharing in and of itself may not be what caregiver education should focus on.” A simple cardboard box, however, is not a sleep surface likely to be shared with an adult.
For the families for whom surface-sharing is a choice and not a necessity, the investigators encourage us to engage families on their motivation for surface-sharing. This is a discussion that clinicians should be initiating, regardless of our concern of SUID prevention, by simply asking “How are you and your baby sleeping?” Is the baby being nursed to sleep? Where? While the authors acknowledge that their raw data did not allow them to make any observations on a relationship between surface-sharing and breastfeeding, my anecdotal observations have found an unfortunate number of mothers who have become human pacifiers for their babies and are co-sleeping. This association can result in a sleep-deprived parent(s) with unhealthy consequences. Although it can be difficult to uncouple breastfeeding from settling, early intervention triggered by asking a simple question can improve the chances of resolution.
Although I may quibble with the wording of authors’ final conclusion, this is an excellent paper worth looking at. SUID while infrequent and for the most part still mysterious is a tragedy that we should be able to prevent.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
It Sure Looks Like Cannabis Is Bad for the Heart, Doesn’t It?
This transcript has been edited for clarity.
If you’re an epidemiologist trying to explore whether some exposure is a risk factor for a disease, you can run into a tough problem when your exposure of interest is highly correlated with another risk factor for the disease. For decades, this stymied investigations into the link, if any, between marijuana use and cardiovascular disease because, for decades, most people who used marijuana in some way also smoked cigarettes — which is a very clear risk factor for heart disease.
But the times they are a-changing.
Thanks to the legalization of marijuana for recreational use in many states, and even broader social trends, there is now a large population of people who use marijuana but do not use cigarettes. That means we can start to determine whether marijuana use is an independent risk factor for heart disease.
And this week, we have the largest study yet to attempt to answer that question, though, as I’ll explain momentarily, the smoke hasn’t entirely cleared yet.
The centerpiece of the study we are discussing this week, “Association of Cannabis Use With Cardiovascular Outcomes Among US Adults,” which appeared in the Journal of the American Heart Association, is the Behavioral Risk Factor Surveillance System, an annual telephone survey conducted by the Centers for Disease Control and Prevention since 1984 that gathers data on all sorts of stuff that we do to ourselves: our drinking habits, our smoking habits, and, more recently, our marijuana habits.
The paper combines annual data from 2016 to 2020 representing 27 states and two US territories for a total sample size of more than 430,000 individuals. The key exposure? Marijuana use, which was coded as the number of days of marijuana use in the past 30 days. The key outcome? Coronary heart disease, collected through questions such as “Has a doctor, nurse, or other health professional ever told you that you had a heart attack?”
Right away you might detect a couple of problems here. But let me show you the results before we worry about what they mean.
You can see the rates of the major cardiovascular outcomes here, stratified by daily use of marijuana, nondaily use, and no use. Broadly speaking, the risk was highest for daily users, lowest for occasional users, and in the middle for non-users.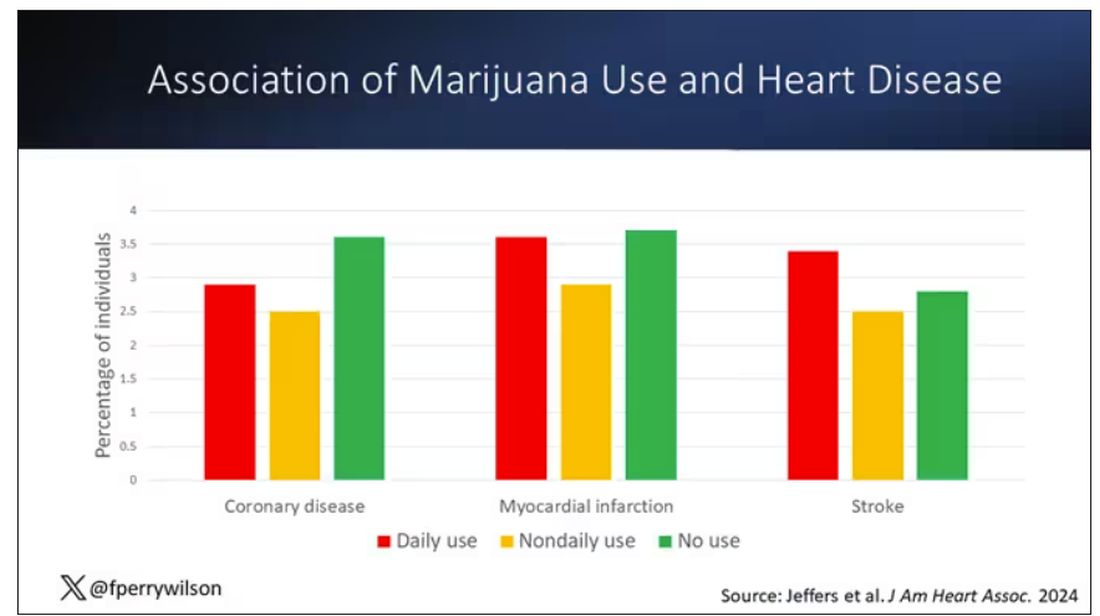
Of course, non-users and users are different in lots of other ways; non-users were quite a bit older, for example. Adjusting for all those factors showed that, independent of age, smoking status, the presence of diabetes, and so on, there was an independently increased risk for cardiovascular outcomes in people who used marijuana.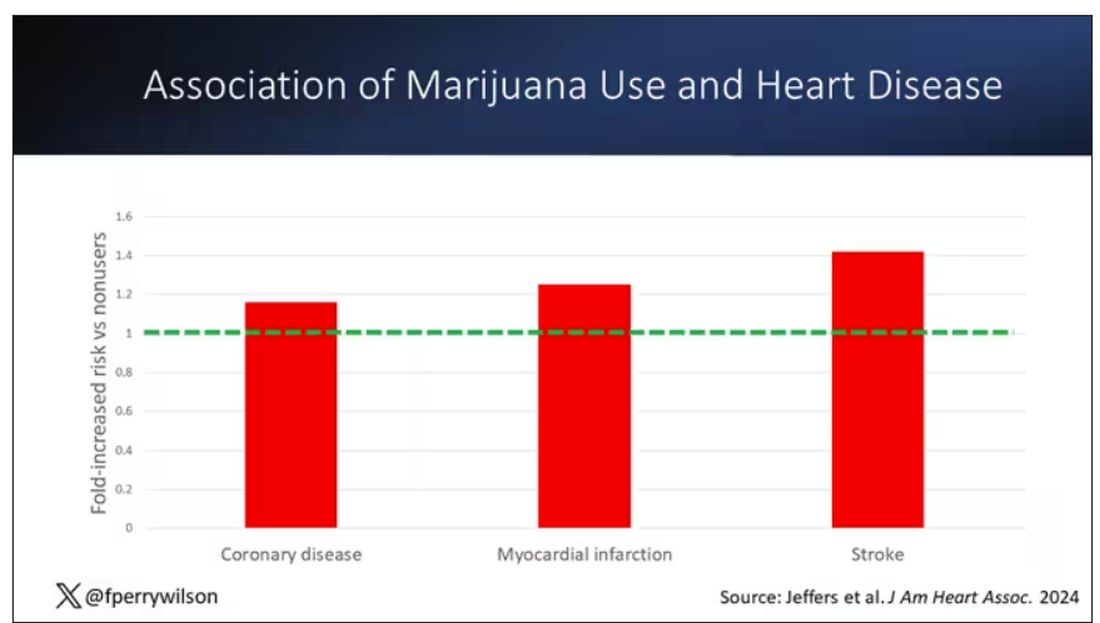
Importantly, 60% of people in this study were never smokers, and the results in that group looked pretty similar to the results overall.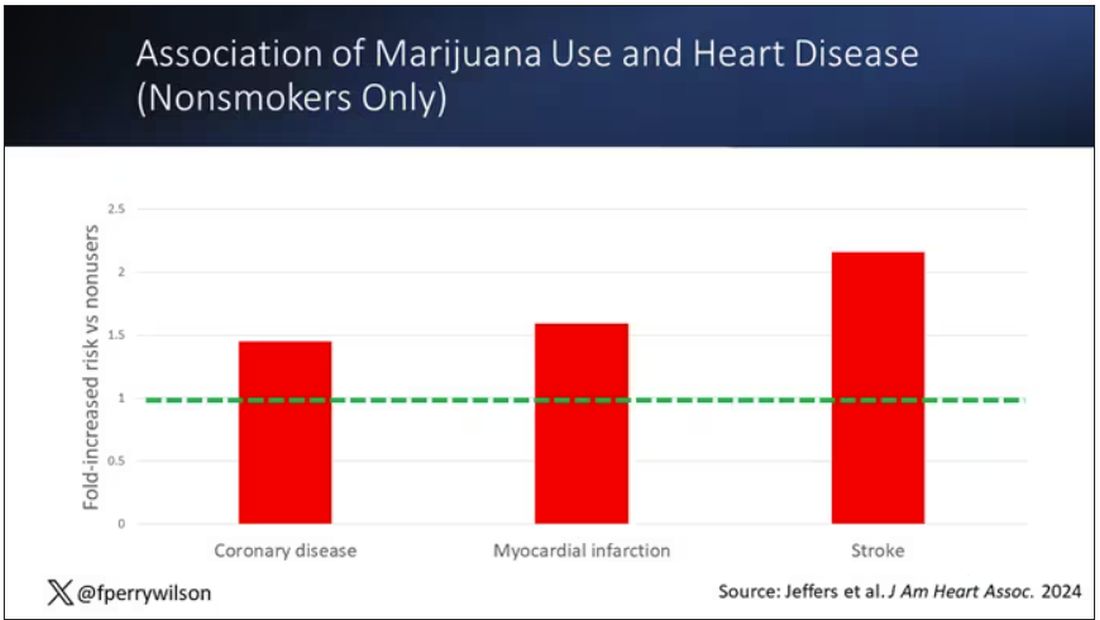
But I said there were a couple of problems, so let’s dig into those a bit.
First, like most survey studies, this one requires honest and accurate reporting from its subjects. There was no verification of heart disease using electronic health records or of marijuana usage based on biosamples. Broadly, miscategorization of exposure and outcomes in surveys tends to bias the results toward the null hypothesis, toward concluding that there is no link between exposure and outcome, so perhaps this is okay.
The bigger problem is the fact that this is a cross-sectional design. If you really wanted to know whether marijuana led to heart disease, you’d do a longitudinal study following users and non-users for some number of decades and see who developed heart disease and who didn’t. (For the pedants out there, I suppose you’d actually want to randomize people to use marijuana or not and then see who had a heart attack, but the IRB keeps rejecting my protocol when I submit it.)
Here, though, we literally can’t tell whether people who use marijuana have more heart attacks or whether people who have heart attacks use more marijuana. The authors argue that there are no data that show that people are more likely to use marijuana after a heart attack or stroke, but at the time the survey was conducted, they had already had their heart attack or stroke.
The authors also imply that they found a dose-response relationship between marijuana use and these cardiovascular outcomes. This is an important statement because dose response is one factor that we use to determine whether a risk factor may actually be causative as opposed to just correlative.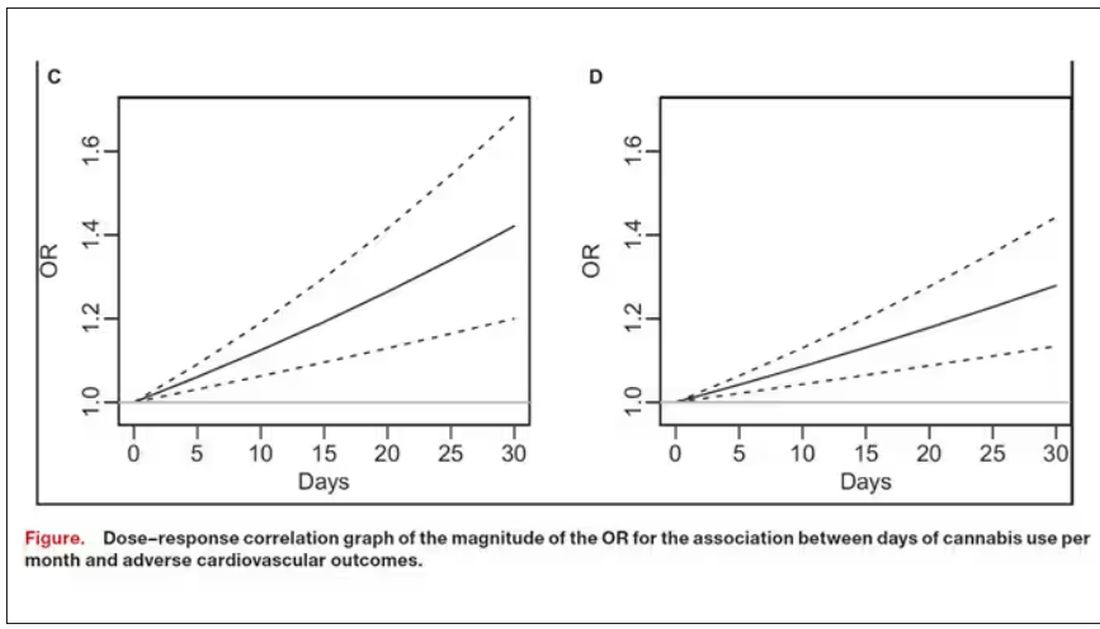
But I take issue with the dose-response language here. The model used to make these graphs classifies marijuana use as a single continuous variable ranging from 0 (no days of use in the past 30 days) to 1 (30 days of use in the past 30 days). The model is thus constrained to monotonically increase or decrease with respect to the outcome. To prove a dose response, you have to give the model the option to find something that isn’t a dose response — for example, by classifying marijuana use into discrete, independent categories rather than a single continuous number.
Am I arguing here that marijuana use is good for you? Of course not. Nor am I even arguing that it has no effect on the cardiovascular system. There are endocannabinoid receptors all over your vasculature. But a cross-sectional survey study, while a good start, is not quite the right way to answer the question. So, while the jury is still out, it’s high time for more research.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re an epidemiologist trying to explore whether some exposure is a risk factor for a disease, you can run into a tough problem when your exposure of interest is highly correlated with another risk factor for the disease. For decades, this stymied investigations into the link, if any, between marijuana use and cardiovascular disease because, for decades, most people who used marijuana in some way also smoked cigarettes — which is a very clear risk factor for heart disease.
But the times they are a-changing.
Thanks to the legalization of marijuana for recreational use in many states, and even broader social trends, there is now a large population of people who use marijuana but do not use cigarettes. That means we can start to determine whether marijuana use is an independent risk factor for heart disease.
And this week, we have the largest study yet to attempt to answer that question, though, as I’ll explain momentarily, the smoke hasn’t entirely cleared yet.
The centerpiece of the study we are discussing this week, “Association of Cannabis Use With Cardiovascular Outcomes Among US Adults,” which appeared in the Journal of the American Heart Association, is the Behavioral Risk Factor Surveillance System, an annual telephone survey conducted by the Centers for Disease Control and Prevention since 1984 that gathers data on all sorts of stuff that we do to ourselves: our drinking habits, our smoking habits, and, more recently, our marijuana habits.
The paper combines annual data from 2016 to 2020 representing 27 states and two US territories for a total sample size of more than 430,000 individuals. The key exposure? Marijuana use, which was coded as the number of days of marijuana use in the past 30 days. The key outcome? Coronary heart disease, collected through questions such as “Has a doctor, nurse, or other health professional ever told you that you had a heart attack?”
Right away you might detect a couple of problems here. But let me show you the results before we worry about what they mean.
You can see the rates of the major cardiovascular outcomes here, stratified by daily use of marijuana, nondaily use, and no use. Broadly speaking, the risk was highest for daily users, lowest for occasional users, and in the middle for non-users.
Of course, non-users and users are different in lots of other ways; non-users were quite a bit older, for example. Adjusting for all those factors showed that, independent of age, smoking status, the presence of diabetes, and so on, there was an independently increased risk for cardiovascular outcomes in people who used marijuana.
Importantly, 60% of people in this study were never smokers, and the results in that group looked pretty similar to the results overall.
But I said there were a couple of problems, so let’s dig into those a bit.
First, like most survey studies, this one requires honest and accurate reporting from its subjects. There was no verification of heart disease using electronic health records or of marijuana usage based on biosamples. Broadly, miscategorization of exposure and outcomes in surveys tends to bias the results toward the null hypothesis, toward concluding that there is no link between exposure and outcome, so perhaps this is okay.
The bigger problem is the fact that this is a cross-sectional design. If you really wanted to know whether marijuana led to heart disease, you’d do a longitudinal study following users and non-users for some number of decades and see who developed heart disease and who didn’t. (For the pedants out there, I suppose you’d actually want to randomize people to use marijuana or not and then see who had a heart attack, but the IRB keeps rejecting my protocol when I submit it.)
Here, though, we literally can’t tell whether people who use marijuana have more heart attacks or whether people who have heart attacks use more marijuana. The authors argue that there are no data that show that people are more likely to use marijuana after a heart attack or stroke, but at the time the survey was conducted, they had already had their heart attack or stroke.
The authors also imply that they found a dose-response relationship between marijuana use and these cardiovascular outcomes. This is an important statement because dose response is one factor that we use to determine whether a risk factor may actually be causative as opposed to just correlative.
But I take issue with the dose-response language here. The model used to make these graphs classifies marijuana use as a single continuous variable ranging from 0 (no days of use in the past 30 days) to 1 (30 days of use in the past 30 days). The model is thus constrained to monotonically increase or decrease with respect to the outcome. To prove a dose response, you have to give the model the option to find something that isn’t a dose response — for example, by classifying marijuana use into discrete, independent categories rather than a single continuous number.
Am I arguing here that marijuana use is good for you? Of course not. Nor am I even arguing that it has no effect on the cardiovascular system. There are endocannabinoid receptors all over your vasculature. But a cross-sectional survey study, while a good start, is not quite the right way to answer the question. So, while the jury is still out, it’s high time for more research.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re an epidemiologist trying to explore whether some exposure is a risk factor for a disease, you can run into a tough problem when your exposure of interest is highly correlated with another risk factor for the disease. For decades, this stymied investigations into the link, if any, between marijuana use and cardiovascular disease because, for decades, most people who used marijuana in some way also smoked cigarettes — which is a very clear risk factor for heart disease.
But the times they are a-changing.
Thanks to the legalization of marijuana for recreational use in many states, and even broader social trends, there is now a large population of people who use marijuana but do not use cigarettes. That means we can start to determine whether marijuana use is an independent risk factor for heart disease.
And this week, we have the largest study yet to attempt to answer that question, though, as I’ll explain momentarily, the smoke hasn’t entirely cleared yet.
The centerpiece of the study we are discussing this week, “Association of Cannabis Use With Cardiovascular Outcomes Among US Adults,” which appeared in the Journal of the American Heart Association, is the Behavioral Risk Factor Surveillance System, an annual telephone survey conducted by the Centers for Disease Control and Prevention since 1984 that gathers data on all sorts of stuff that we do to ourselves: our drinking habits, our smoking habits, and, more recently, our marijuana habits.
The paper combines annual data from 2016 to 2020 representing 27 states and two US territories for a total sample size of more than 430,000 individuals. The key exposure? Marijuana use, which was coded as the number of days of marijuana use in the past 30 days. The key outcome? Coronary heart disease, collected through questions such as “Has a doctor, nurse, or other health professional ever told you that you had a heart attack?”
Right away you might detect a couple of problems here. But let me show you the results before we worry about what they mean.
You can see the rates of the major cardiovascular outcomes here, stratified by daily use of marijuana, nondaily use, and no use. Broadly speaking, the risk was highest for daily users, lowest for occasional users, and in the middle for non-users.
Of course, non-users and users are different in lots of other ways; non-users were quite a bit older, for example. Adjusting for all those factors showed that, independent of age, smoking status, the presence of diabetes, and so on, there was an independently increased risk for cardiovascular outcomes in people who used marijuana.
Importantly, 60% of people in this study were never smokers, and the results in that group looked pretty similar to the results overall.
But I said there were a couple of problems, so let’s dig into those a bit.
First, like most survey studies, this one requires honest and accurate reporting from its subjects. There was no verification of heart disease using electronic health records or of marijuana usage based on biosamples. Broadly, miscategorization of exposure and outcomes in surveys tends to bias the results toward the null hypothesis, toward concluding that there is no link between exposure and outcome, so perhaps this is okay.
The bigger problem is the fact that this is a cross-sectional design. If you really wanted to know whether marijuana led to heart disease, you’d do a longitudinal study following users and non-users for some number of decades and see who developed heart disease and who didn’t. (For the pedants out there, I suppose you’d actually want to randomize people to use marijuana or not and then see who had a heart attack, but the IRB keeps rejecting my protocol when I submit it.)
Here, though, we literally can’t tell whether people who use marijuana have more heart attacks or whether people who have heart attacks use more marijuana. The authors argue that there are no data that show that people are more likely to use marijuana after a heart attack or stroke, but at the time the survey was conducted, they had already had their heart attack or stroke.
The authors also imply that they found a dose-response relationship between marijuana use and these cardiovascular outcomes. This is an important statement because dose response is one factor that we use to determine whether a risk factor may actually be causative as opposed to just correlative.
But I take issue with the dose-response language here. The model used to make these graphs classifies marijuana use as a single continuous variable ranging from 0 (no days of use in the past 30 days) to 1 (30 days of use in the past 30 days). The model is thus constrained to monotonically increase or decrease with respect to the outcome. To prove a dose response, you have to give the model the option to find something that isn’t a dose response — for example, by classifying marijuana use into discrete, independent categories rather than a single continuous number.
Am I arguing here that marijuana use is good for you? Of course not. Nor am I even arguing that it has no effect on the cardiovascular system. There are endocannabinoid receptors all over your vasculature. But a cross-sectional survey study, while a good start, is not quite the right way to answer the question. So, while the jury is still out, it’s high time for more research.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Different cultures, same wiring
Some things are universal, or at least worldwide.
She didn’t speak a word of English, but I don’t speak any Mandarin. Fortunately, her concerned son was fluent in both.
A nice lady in her 60s, here from China to visit her son and his family for a month. The visit was going fine until she abruptly developed double vision. Through the modern miracle of email she contacted her doctor in Beijing, who told her to find a neurologist here or go to an ER.
I’d had a last minute cancellation a few minutes before her son called and so was able to see her that afternoon. Both were scared that I was going to admit her to a hospital.
Fortunately, people are wired the same no matter where they’re from. The electrical fibers of the nervous system are predictable across international borders, as are the maladies.
A history and exam made the diagnosis of a diabetic cranial nerve palsy most likely, and I was able to reassure them. I ordered the usual imaging studies (fortunately she’d bought travelers’ insurance in advance). As anticipated, they were normal.
Her son and I spoke by phone to close things out, with her in the background and him translating between us. By the time she left 2 weeks later the symptoms were resolving. I made sure she went home with copies of my notes and the MRI reports, figuring someone there would be able to translate them for her physician.
These sorts of encounters are uncommon in my little solo practice, but still drive home the point that people around the world have more in common than not.
Not to mention families. The mother traveling around the world to see her son and grandchildren. The child concerned for the welfare of his parent and helping her get care. These, too, are human universals, regardless of the language spoken. There isn’t a culture on Earth that doesn’t value family connections, nor is there one that didn’t develop (albeit in different forms) doctors.
The human population is 8 billion. Everyone is different, and yet everyone, overall, is the same. Fellow travelers on a small planet.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Some things are universal, or at least worldwide.
She didn’t speak a word of English, but I don’t speak any Mandarin. Fortunately, her concerned son was fluent in both.
A nice lady in her 60s, here from China to visit her son and his family for a month. The visit was going fine until she abruptly developed double vision. Through the modern miracle of email she contacted her doctor in Beijing, who told her to find a neurologist here or go to an ER.
I’d had a last minute cancellation a few minutes before her son called and so was able to see her that afternoon. Both were scared that I was going to admit her to a hospital.
Fortunately, people are wired the same no matter where they’re from. The electrical fibers of the nervous system are predictable across international borders, as are the maladies.
A history and exam made the diagnosis of a diabetic cranial nerve palsy most likely, and I was able to reassure them. I ordered the usual imaging studies (fortunately she’d bought travelers’ insurance in advance). As anticipated, they were normal.
Her son and I spoke by phone to close things out, with her in the background and him translating between us. By the time she left 2 weeks later the symptoms were resolving. I made sure she went home with copies of my notes and the MRI reports, figuring someone there would be able to translate them for her physician.
These sorts of encounters are uncommon in my little solo practice, but still drive home the point that people around the world have more in common than not.
Not to mention families. The mother traveling around the world to see her son and grandchildren. The child concerned for the welfare of his parent and helping her get care. These, too, are human universals, regardless of the language spoken. There isn’t a culture on Earth that doesn’t value family connections, nor is there one that didn’t develop (albeit in different forms) doctors.
The human population is 8 billion. Everyone is different, and yet everyone, overall, is the same. Fellow travelers on a small planet.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Some things are universal, or at least worldwide.
She didn’t speak a word of English, but I don’t speak any Mandarin. Fortunately, her concerned son was fluent in both.
A nice lady in her 60s, here from China to visit her son and his family for a month. The visit was going fine until she abruptly developed double vision. Through the modern miracle of email she contacted her doctor in Beijing, who told her to find a neurologist here or go to an ER.
I’d had a last minute cancellation a few minutes before her son called and so was able to see her that afternoon. Both were scared that I was going to admit her to a hospital.
Fortunately, people are wired the same no matter where they’re from. The electrical fibers of the nervous system are predictable across international borders, as are the maladies.
A history and exam made the diagnosis of a diabetic cranial nerve palsy most likely, and I was able to reassure them. I ordered the usual imaging studies (fortunately she’d bought travelers’ insurance in advance). As anticipated, they were normal.
Her son and I spoke by phone to close things out, with her in the background and him translating between us. By the time she left 2 weeks later the symptoms were resolving. I made sure she went home with copies of my notes and the MRI reports, figuring someone there would be able to translate them for her physician.
These sorts of encounters are uncommon in my little solo practice, but still drive home the point that people around the world have more in common than not.
Not to mention families. The mother traveling around the world to see her son and grandchildren. The child concerned for the welfare of his parent and helping her get care. These, too, are human universals, regardless of the language spoken. There isn’t a culture on Earth that doesn’t value family connections, nor is there one that didn’t develop (albeit in different forms) doctors.
The human population is 8 billion. Everyone is different, and yet everyone, overall, is the same. Fellow travelers on a small planet.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Oxaliplatin in Older Adults With Resected Colorectal Cancer: Is There a Benefit?
This transcript has been edited for clarity.
Our group, the QUASAR Group, made a very significant contribution in terms of trials and knowledge in this field. One of the things that we still need to discuss and think about in our wider community is the impact of adjuvant chemotherapy in older patients.
Colorectal cancer is a disease of the elderly, with the median age of presentation around 72. We know that, at presentation, more than 50% of patients are aged 65 or over and one third of patients are 75 or over. It’s a disease predominantly of the elderly. Are we justified in giving combination chemotherapy with oxaliplatin to high-risk resected colorectal cancer patients?
There’s a very nice report of a meta-analysis by Dottorini and colleagues that came out recently in the Journal of Clinical Oncology. It’s an excellent group. They did their meta-analytical work according to a strict, rational protocol. Their statistical analyses were on point, and they collected data from all the relevant trials.
According to the results of their study, it could be concluded that the addition of oxaliplatin to adjuvant therapy for resected high-risk colorectal cancer in older patients — patients older than 70 — doesn’t result in any statistically significant gain in terms of preventing recurrences or saving lives.
When we did our QUASAR trials, initially we were looking at control vs fluoropyrimidine chemotherapy. Although there was an overall impact on survival of the whole trial group (the 5000 patients in our study), when we looked by decile, there was a significant diminution of benefit even to fluoropyrimidine therapy in our trial in patients aged 70 or above. I think this careful meta-analysis must make us question the use of oxaliplatin in elderly patients.
What could the explanation be? Why could the well-known and described benefits of oxaliplatin, particularly for stage III disease, attenuate in older people? It may be to do with reduction in dose intensity. Older people have more side effects; therefore, the chemotherapy isn’t completed as planned. Although, increasingly these days, we tend only to be giving 3 months of treatment.
Is there something biologically in terms of the biology or the somatic mutational landscape of the tumor in older people? I don’t think so. Certainly, in terms of their capacity, in terms of stem cell reserve to be as resistant to the side effects of chemotherapy as younger people, we know that does attenuate with age.
Food for thought: The majority of patients I see in the clinic for the adjuvant treatment are elderly. The majority are coming these days with high-risk stage II or stage III disease. There is a real question mark about whether we should be using oxaliplatin at all.
Clearly, one would say that we need more trials of chemotherapy in older folk to see if the addition of drugs like oxaliplatin to a fluoropyrimidine backbone really does make a difference. I’ve said many times before that we, the medical community recommending adjuvant treatment, need to have better risk stratifiers. We need to have better prognostic markers. We need to have better indices that would allow us to perhaps consider these combination treatments in a more focused group of patients who may have a higher risk for recurrence.
Have a look at the paper and see what you think. I think it’s well done. It’s certainly given me pause for thought about the treatment that we will offer our elderly patients.
Have a think about it. Let me know if there are any comments that you’d like to make. For the time being, over and out.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom. He disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our group, the QUASAR Group, made a very significant contribution in terms of trials and knowledge in this field. One of the things that we still need to discuss and think about in our wider community is the impact of adjuvant chemotherapy in older patients.
Colorectal cancer is a disease of the elderly, with the median age of presentation around 72. We know that, at presentation, more than 50% of patients are aged 65 or over and one third of patients are 75 or over. It’s a disease predominantly of the elderly. Are we justified in giving combination chemotherapy with oxaliplatin to high-risk resected colorectal cancer patients?
There’s a very nice report of a meta-analysis by Dottorini and colleagues that came out recently in the Journal of Clinical Oncology. It’s an excellent group. They did their meta-analytical work according to a strict, rational protocol. Their statistical analyses were on point, and they collected data from all the relevant trials.
According to the results of their study, it could be concluded that the addition of oxaliplatin to adjuvant therapy for resected high-risk colorectal cancer in older patients — patients older than 70 — doesn’t result in any statistically significant gain in terms of preventing recurrences or saving lives.
When we did our QUASAR trials, initially we were looking at control vs fluoropyrimidine chemotherapy. Although there was an overall impact on survival of the whole trial group (the 5000 patients in our study), when we looked by decile, there was a significant diminution of benefit even to fluoropyrimidine therapy in our trial in patients aged 70 or above. I think this careful meta-analysis must make us question the use of oxaliplatin in elderly patients.
What could the explanation be? Why could the well-known and described benefits of oxaliplatin, particularly for stage III disease, attenuate in older people? It may be to do with reduction in dose intensity. Older people have more side effects; therefore, the chemotherapy isn’t completed as planned. Although, increasingly these days, we tend only to be giving 3 months of treatment.
Is there something biologically in terms of the biology or the somatic mutational landscape of the tumor in older people? I don’t think so. Certainly, in terms of their capacity, in terms of stem cell reserve to be as resistant to the side effects of chemotherapy as younger people, we know that does attenuate with age.
Food for thought: The majority of patients I see in the clinic for the adjuvant treatment are elderly. The majority are coming these days with high-risk stage II or stage III disease. There is a real question mark about whether we should be using oxaliplatin at all.
Clearly, one would say that we need more trials of chemotherapy in older folk to see if the addition of drugs like oxaliplatin to a fluoropyrimidine backbone really does make a difference. I’ve said many times before that we, the medical community recommending adjuvant treatment, need to have better risk stratifiers. We need to have better prognostic markers. We need to have better indices that would allow us to perhaps consider these combination treatments in a more focused group of patients who may have a higher risk for recurrence.
Have a look at the paper and see what you think. I think it’s well done. It’s certainly given me pause for thought about the treatment that we will offer our elderly patients.
Have a think about it. Let me know if there are any comments that you’d like to make. For the time being, over and out.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom. He disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Our group, the QUASAR Group, made a very significant contribution in terms of trials and knowledge in this field. One of the things that we still need to discuss and think about in our wider community is the impact of adjuvant chemotherapy in older patients.
Colorectal cancer is a disease of the elderly, with the median age of presentation around 72. We know that, at presentation, more than 50% of patients are aged 65 or over and one third of patients are 75 or over. It’s a disease predominantly of the elderly. Are we justified in giving combination chemotherapy with oxaliplatin to high-risk resected colorectal cancer patients?
There’s a very nice report of a meta-analysis by Dottorini and colleagues that came out recently in the Journal of Clinical Oncology. It’s an excellent group. They did their meta-analytical work according to a strict, rational protocol. Their statistical analyses were on point, and they collected data from all the relevant trials.
According to the results of their study, it could be concluded that the addition of oxaliplatin to adjuvant therapy for resected high-risk colorectal cancer in older patients — patients older than 70 — doesn’t result in any statistically significant gain in terms of preventing recurrences or saving lives.
When we did our QUASAR trials, initially we were looking at control vs fluoropyrimidine chemotherapy. Although there was an overall impact on survival of the whole trial group (the 5000 patients in our study), when we looked by decile, there was a significant diminution of benefit even to fluoropyrimidine therapy in our trial in patients aged 70 or above. I think this careful meta-analysis must make us question the use of oxaliplatin in elderly patients.
What could the explanation be? Why could the well-known and described benefits of oxaliplatin, particularly for stage III disease, attenuate in older people? It may be to do with reduction in dose intensity. Older people have more side effects; therefore, the chemotherapy isn’t completed as planned. Although, increasingly these days, we tend only to be giving 3 months of treatment.
Is there something biologically in terms of the biology or the somatic mutational landscape of the tumor in older people? I don’t think so. Certainly, in terms of their capacity, in terms of stem cell reserve to be as resistant to the side effects of chemotherapy as younger people, we know that does attenuate with age.
Food for thought: The majority of patients I see in the clinic for the adjuvant treatment are elderly. The majority are coming these days with high-risk stage II or stage III disease. There is a real question mark about whether we should be using oxaliplatin at all.
Clearly, one would say that we need more trials of chemotherapy in older folk to see if the addition of drugs like oxaliplatin to a fluoropyrimidine backbone really does make a difference. I’ve said many times before that we, the medical community recommending adjuvant treatment, need to have better risk stratifiers. We need to have better prognostic markers. We need to have better indices that would allow us to perhaps consider these combination treatments in a more focused group of patients who may have a higher risk for recurrence.
Have a look at the paper and see what you think. I think it’s well done. It’s certainly given me pause for thought about the treatment that we will offer our elderly patients.
Have a think about it. Let me know if there are any comments that you’d like to make. For the time being, over and out.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford; Professor of Cancer Medicine, Oxford Cancer Centre, Oxford, United Kingdom. He disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
Bent but Not Broken: The Truth About Penile Curvature
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. This is Sex Matters, and I’m here today with my friend and colleague, Dr. Matt Ziegelmann, who is the sexual medicine expert at the Mayo Clinic and who does all things men’s health, including penile curvature, testosterone, and sexual function.
Matthew J. Ziegelmann, MD: Penile curvature (often due to Peyronie’s disease) is actually incredibly common; as many as 10% of men have this condition. We need to normalize it and let men know that this is something we see often, and we have treatments for it. One of the biggest concerns these men have is cancer, but I have yet to see this as an indicator of cancer.
Penile curvature has a significant impact on affected men — their relationships, psychological well-being, sexual functioning, and overall health. We can provide treatment if they are interested. A small subset of men are born with natural penile curvature, which is different from Peyronie’s disease. Natural curvature can still affect their mental health, and we have treatment options.
Rubin: What happens when a patient is referred to urology? We want to tell our patients what to expect. What’s in our toolbox to help patients with penile curvature?
Dr. Ziegelmann: Many patient resources are available. For example, the Sexual Medicine Society of North America (SMSNA) has a patient-facing website on sexual health with lots of information about Peyronie’s disease and other aspects of sexual health.
Patients who are bothered by their penile curvature can be referred to us to find out about treatment options, or even just to get reassurance. It might be as simple as a conversation and a physical exam — that’s all we need to make the diagnosis. We can provide reassurance and get an idea of how bothered they are by this condition without doing anything invasive.
If they are considering definitive treatment, we would need to do more invasive testing. Sometimes we have the patient bring in photos of their erection to help establish the change they see in the shape of their penis.
Dr. Rubin: What about the patient who asks, “Doc, did I do this to myself? Did I break my penis? What do I do?”
Dr. Ziegelmann: That’s a common question — “How the heck did this happen?” No, you didn’t do this to yourself. We still have much to understand about why this happens to some men. Our approach is to acknowledge what we do and don’t know, and partner with the patient to discuss treatment.
Dr. Rubin: It’s very important to support your patient’s mental health, because this can be really devastating. So, what are the treatment options from conservative to invasive? What do you recommend for patients?
Dr. Ziegelmann: It can be as simple as observation if the patient is just seeking reassurance that it’s not cancer. This is a benign condition, but for men who are more bothered by their curvature, we’ll talk about options. We have oral medications that help improved the rigidity of the penis. Many men are also suffering from inadequate functioning. We can use devices called traction or vacuum to stretch the area of the penis that’s curved. We can use injections of medications, including FDA-approved agents that are injected into the penis in the outpatient setting. For men who are either later in the treatment protocol or want to resolve the problem right away, we can offer surgical intervention. We have a host of options, and a very individualized approach and a shared decision-making model. It’s not a one-size-fits-all problem.
Dr. Rubin: The real takeaway is that there is a lot of hope for this condition. Many doctors care deeply about these issues and are ready to partner with specialists to figure out the right treatment strategy. The SMSNA is a great place to find a provider like Dr Ziegelmann or myself, or any of our incredible colleagues throughout North America and the world. Thank you for joining us today.
Dr. Rubin is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. This is Sex Matters, and I’m here today with my friend and colleague, Dr. Matt Ziegelmann, who is the sexual medicine expert at the Mayo Clinic and who does all things men’s health, including penile curvature, testosterone, and sexual function.
Matthew J. Ziegelmann, MD: Penile curvature (often due to Peyronie’s disease) is actually incredibly common; as many as 10% of men have this condition. We need to normalize it and let men know that this is something we see often, and we have treatments for it. One of the biggest concerns these men have is cancer, but I have yet to see this as an indicator of cancer.
Penile curvature has a significant impact on affected men — their relationships, psychological well-being, sexual functioning, and overall health. We can provide treatment if they are interested. A small subset of men are born with natural penile curvature, which is different from Peyronie’s disease. Natural curvature can still affect their mental health, and we have treatment options.
Rubin: What happens when a patient is referred to urology? We want to tell our patients what to expect. What’s in our toolbox to help patients with penile curvature?
Dr. Ziegelmann: Many patient resources are available. For example, the Sexual Medicine Society of North America (SMSNA) has a patient-facing website on sexual health with lots of information about Peyronie’s disease and other aspects of sexual health.
Patients who are bothered by their penile curvature can be referred to us to find out about treatment options, or even just to get reassurance. It might be as simple as a conversation and a physical exam — that’s all we need to make the diagnosis. We can provide reassurance and get an idea of how bothered they are by this condition without doing anything invasive.
If they are considering definitive treatment, we would need to do more invasive testing. Sometimes we have the patient bring in photos of their erection to help establish the change they see in the shape of their penis.
Dr. Rubin: What about the patient who asks, “Doc, did I do this to myself? Did I break my penis? What do I do?”
Dr. Ziegelmann: That’s a common question — “How the heck did this happen?” No, you didn’t do this to yourself. We still have much to understand about why this happens to some men. Our approach is to acknowledge what we do and don’t know, and partner with the patient to discuss treatment.
Dr. Rubin: It’s very important to support your patient’s mental health, because this can be really devastating. So, what are the treatment options from conservative to invasive? What do you recommend for patients?
Dr. Ziegelmann: It can be as simple as observation if the patient is just seeking reassurance that it’s not cancer. This is a benign condition, but for men who are more bothered by their curvature, we’ll talk about options. We have oral medications that help improved the rigidity of the penis. Many men are also suffering from inadequate functioning. We can use devices called traction or vacuum to stretch the area of the penis that’s curved. We can use injections of medications, including FDA-approved agents that are injected into the penis in the outpatient setting. For men who are either later in the treatment protocol or want to resolve the problem right away, we can offer surgical intervention. We have a host of options, and a very individualized approach and a shared decision-making model. It’s not a one-size-fits-all problem.
Dr. Rubin: The real takeaway is that there is a lot of hope for this condition. Many doctors care deeply about these issues and are ready to partner with specialists to figure out the right treatment strategy. The SMSNA is a great place to find a provider like Dr Ziegelmann or myself, or any of our incredible colleagues throughout North America and the world. Thank you for joining us today.
Dr. Rubin is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I’m Dr Rachel Rubin, urologist and sexual medicine specialist in the Washington, DC, area. This is Sex Matters, and I’m here today with my friend and colleague, Dr. Matt Ziegelmann, who is the sexual medicine expert at the Mayo Clinic and who does all things men’s health, including penile curvature, testosterone, and sexual function.
Matthew J. Ziegelmann, MD: Penile curvature (often due to Peyronie’s disease) is actually incredibly common; as many as 10% of men have this condition. We need to normalize it and let men know that this is something we see often, and we have treatments for it. One of the biggest concerns these men have is cancer, but I have yet to see this as an indicator of cancer.
Penile curvature has a significant impact on affected men — their relationships, psychological well-being, sexual functioning, and overall health. We can provide treatment if they are interested. A small subset of men are born with natural penile curvature, which is different from Peyronie’s disease. Natural curvature can still affect their mental health, and we have treatment options.
Rubin: What happens when a patient is referred to urology? We want to tell our patients what to expect. What’s in our toolbox to help patients with penile curvature?
Dr. Ziegelmann: Many patient resources are available. For example, the Sexual Medicine Society of North America (SMSNA) has a patient-facing website on sexual health with lots of information about Peyronie’s disease and other aspects of sexual health.
Patients who are bothered by their penile curvature can be referred to us to find out about treatment options, or even just to get reassurance. It might be as simple as a conversation and a physical exam — that’s all we need to make the diagnosis. We can provide reassurance and get an idea of how bothered they are by this condition without doing anything invasive.
If they are considering definitive treatment, we would need to do more invasive testing. Sometimes we have the patient bring in photos of their erection to help establish the change they see in the shape of their penis.
Dr. Rubin: What about the patient who asks, “Doc, did I do this to myself? Did I break my penis? What do I do?”
Dr. Ziegelmann: That’s a common question — “How the heck did this happen?” No, you didn’t do this to yourself. We still have much to understand about why this happens to some men. Our approach is to acknowledge what we do and don’t know, and partner with the patient to discuss treatment.
Dr. Rubin: It’s very important to support your patient’s mental health, because this can be really devastating. So, what are the treatment options from conservative to invasive? What do you recommend for patients?
Dr. Ziegelmann: It can be as simple as observation if the patient is just seeking reassurance that it’s not cancer. This is a benign condition, but for men who are more bothered by their curvature, we’ll talk about options. We have oral medications that help improved the rigidity of the penis. Many men are also suffering from inadequate functioning. We can use devices called traction or vacuum to stretch the area of the penis that’s curved. We can use injections of medications, including FDA-approved agents that are injected into the penis in the outpatient setting. For men who are either later in the treatment protocol or want to resolve the problem right away, we can offer surgical intervention. We have a host of options, and a very individualized approach and a shared decision-making model. It’s not a one-size-fits-all problem.
Dr. Rubin: The real takeaway is that there is a lot of hope for this condition. Many doctors care deeply about these issues and are ready to partner with specialists to figure out the right treatment strategy. The SMSNA is a great place to find a provider like Dr Ziegelmann or myself, or any of our incredible colleagues throughout North America and the world. Thank you for joining us today.
Dr. Rubin is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.









