User login
Fine-needle aspiration alternative allows closer look at pancreatic cystic lesions
Endoscopic ultrasound (EUS)–guided through-the-needle biopsies (TTNBs) of pancreatic cystic lesions are sufficient for accurate molecular analysis, which offers a superior alternative to cyst fluid obtained via fine-needle aspiration, based on a prospective study.
For highest diagnostic clarity, next-generation sequencing (NGS) of TTNBs can be paired with histology, lead author Charlotte Vestrup Rift, MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“The diagnostic algorithm for the management of [pancreatic cystic lesions] includes endoscopic ultrasound examination with aspiration of cyst fluid for cytology,” the investigators wrote in Gastrointestinal Endoscopy. “However, the reported sensitivity of cytology is low [at 54%]. A new microforceps, introduced through a 19-gauge needle, has proven useful for procurement of [TTNBs] that represent both the epithelial and stromal component of the cyst wall. TTNBs have a high sensitivity of 86% for the diagnosis of mucinous cysts.”
Dr. Rift and colleagues evaluated the impact of introducing NGS to the diagnostic process. They noted that concomitant mutations in GNAS and KRAS are diagnostic for intraductal papillary mucinous neoplasms (IPMNs), while other mutations have been linked with progression to cancer.
The study involved 101 patients with pancreatic cystic lesions larger than 15 mm in diameter, mean age of 68 years, among whom 91 had residual TTNBs available after microscopic analysis. These samples underwent a 51-gene NGS panel that included the “most prevalent hot-spot mutations.” Diagnoses were sorted into four categories: neoplastic cyst, mucinous cyst, IPMN, or serous cystic neoplasm.
The primary endpoint was diagnostic yield, both for molecular analysis of TTNBs and for molecular analysis plus histopathology of TTNBs. Sensitivity and specificity of NGS were also determined using histopathology as the gold standard.
Relying on NGS alone, diagnostic yields were 44.5% and 27.7% for detecting a mucinous cyst and determining type of cyst, respectively. These yields rose to 73.3% and 70.3%, respectively, when NGS was used with microscopic evaluation. Continuing with this combined approach, sensitivity and specificity were 83.7% and 81.8%, respectively, for the diagnosis of a mucinous cyst. Sensitivity and specificity were higher still, at 87.2% and 84.6%, respectively, for identifying IPMNs.
The adverse-event rate was 9.9%, with a risk of postprocedure acute pancreatitis of 8.9 % and procedure-associated intracystic bleeding of 3%, according to the authors.
Limitations of the study include the relatively small sample size and the single-center design.
“TTNB-NGS is not sufficient as a stand-alone diagnostic tool as of yet but has a high diagnostic yield when combined with microscopic evaluation and subtyping by immunohistochemistry,” the investigators concluded. “The advantage of EUS-TTNB over EUS–[fine-needle aspiration] is the ability to perform detailed cyst subtyping and the high technical success rate of the procedure. ... However, the procedure comes with a risk of adverse events and thus should be offered to patients where the value of an exact diagnosis outweighs the risks.”
“Molecular subtyping is emerging as a useful clinical test for diagnosing pancreatic cysts,” said Margaret Geraldine Keane, MBBS, MSc, of Johns Hopkins Medicine, Baltimore, although she noted that NGS remains expensive and sporadically available, “which limits its clinical utility and incorporation into diagnostic algorithms for pancreatic cysts. In the future, as the cost of sequencing reduces, and availability improves, this may change.”
For now, Dr. Keane advised physicians to reserve molecular subtyping for cases in which “accurate cyst subtyping will change management ... or when other tests have not provided a clear diagnosis.”
She said the present study is valuable because better diagnostic tests are badly needed for patients with pancreatic cysts, considering the high rate of surgical overtreatment.
“Having more diagnostic tests, such as those described in this publication [to be used on their own or in combination] to decide which patients need surgery, is important,” Dr. Keane said who was not involved in the study.
Better diagnostic tests could also improve outcomes for patients with pancreatic cancer, she said, noting a 5-year survival rate of 10%.
“This outcome is in large part attributable to the late stage at which the majority of patients are diagnosed,” Dr. Keane said. “If patients can be diagnosed earlier, survival dramatically improves. Improvements in diagnostic tests for premalignant pancreatic cystic lesions are therefore vital.”
The study was supported by Rigshospitalets Research Foundation, The Novo Nordisk Foundation, The Danish Cancer Society, and others, although they did not have a role in conducting the study or preparing the manuscript. One investigator disclosed a relationship with MediGlobe. The other investigators reported no conflicts of interest. Dr. Keane disclosed no conflicts of interest.
Endoscopic ultrasound (EUS)–guided through-the-needle biopsies (TTNBs) of pancreatic cystic lesions are sufficient for accurate molecular analysis, which offers a superior alternative to cyst fluid obtained via fine-needle aspiration, based on a prospective study.
For highest diagnostic clarity, next-generation sequencing (NGS) of TTNBs can be paired with histology, lead author Charlotte Vestrup Rift, MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“The diagnostic algorithm for the management of [pancreatic cystic lesions] includes endoscopic ultrasound examination with aspiration of cyst fluid for cytology,” the investigators wrote in Gastrointestinal Endoscopy. “However, the reported sensitivity of cytology is low [at 54%]. A new microforceps, introduced through a 19-gauge needle, has proven useful for procurement of [TTNBs] that represent both the epithelial and stromal component of the cyst wall. TTNBs have a high sensitivity of 86% for the diagnosis of mucinous cysts.”
Dr. Rift and colleagues evaluated the impact of introducing NGS to the diagnostic process. They noted that concomitant mutations in GNAS and KRAS are diagnostic for intraductal papillary mucinous neoplasms (IPMNs), while other mutations have been linked with progression to cancer.
The study involved 101 patients with pancreatic cystic lesions larger than 15 mm in diameter, mean age of 68 years, among whom 91 had residual TTNBs available after microscopic analysis. These samples underwent a 51-gene NGS panel that included the “most prevalent hot-spot mutations.” Diagnoses were sorted into four categories: neoplastic cyst, mucinous cyst, IPMN, or serous cystic neoplasm.
The primary endpoint was diagnostic yield, both for molecular analysis of TTNBs and for molecular analysis plus histopathology of TTNBs. Sensitivity and specificity of NGS were also determined using histopathology as the gold standard.
Relying on NGS alone, diagnostic yields were 44.5% and 27.7% for detecting a mucinous cyst and determining type of cyst, respectively. These yields rose to 73.3% and 70.3%, respectively, when NGS was used with microscopic evaluation. Continuing with this combined approach, sensitivity and specificity were 83.7% and 81.8%, respectively, for the diagnosis of a mucinous cyst. Sensitivity and specificity were higher still, at 87.2% and 84.6%, respectively, for identifying IPMNs.
The adverse-event rate was 9.9%, with a risk of postprocedure acute pancreatitis of 8.9 % and procedure-associated intracystic bleeding of 3%, according to the authors.
Limitations of the study include the relatively small sample size and the single-center design.
“TTNB-NGS is not sufficient as a stand-alone diagnostic tool as of yet but has a high diagnostic yield when combined with microscopic evaluation and subtyping by immunohistochemistry,” the investigators concluded. “The advantage of EUS-TTNB over EUS–[fine-needle aspiration] is the ability to perform detailed cyst subtyping and the high technical success rate of the procedure. ... However, the procedure comes with a risk of adverse events and thus should be offered to patients where the value of an exact diagnosis outweighs the risks.”
“Molecular subtyping is emerging as a useful clinical test for diagnosing pancreatic cysts,” said Margaret Geraldine Keane, MBBS, MSc, of Johns Hopkins Medicine, Baltimore, although she noted that NGS remains expensive and sporadically available, “which limits its clinical utility and incorporation into diagnostic algorithms for pancreatic cysts. In the future, as the cost of sequencing reduces, and availability improves, this may change.”
For now, Dr. Keane advised physicians to reserve molecular subtyping for cases in which “accurate cyst subtyping will change management ... or when other tests have not provided a clear diagnosis.”
She said the present study is valuable because better diagnostic tests are badly needed for patients with pancreatic cysts, considering the high rate of surgical overtreatment.
“Having more diagnostic tests, such as those described in this publication [to be used on their own or in combination] to decide which patients need surgery, is important,” Dr. Keane said who was not involved in the study.
Better diagnostic tests could also improve outcomes for patients with pancreatic cancer, she said, noting a 5-year survival rate of 10%.
“This outcome is in large part attributable to the late stage at which the majority of patients are diagnosed,” Dr. Keane said. “If patients can be diagnosed earlier, survival dramatically improves. Improvements in diagnostic tests for premalignant pancreatic cystic lesions are therefore vital.”
The study was supported by Rigshospitalets Research Foundation, The Novo Nordisk Foundation, The Danish Cancer Society, and others, although they did not have a role in conducting the study or preparing the manuscript. One investigator disclosed a relationship with MediGlobe. The other investigators reported no conflicts of interest. Dr. Keane disclosed no conflicts of interest.
Endoscopic ultrasound (EUS)–guided through-the-needle biopsies (TTNBs) of pancreatic cystic lesions are sufficient for accurate molecular analysis, which offers a superior alternative to cyst fluid obtained via fine-needle aspiration, based on a prospective study.
For highest diagnostic clarity, next-generation sequencing (NGS) of TTNBs can be paired with histology, lead author Charlotte Vestrup Rift, MD, PhD, of Copenhagen University Hospital, and colleagues reported.
“The diagnostic algorithm for the management of [pancreatic cystic lesions] includes endoscopic ultrasound examination with aspiration of cyst fluid for cytology,” the investigators wrote in Gastrointestinal Endoscopy. “However, the reported sensitivity of cytology is low [at 54%]. A new microforceps, introduced through a 19-gauge needle, has proven useful for procurement of [TTNBs] that represent both the epithelial and stromal component of the cyst wall. TTNBs have a high sensitivity of 86% for the diagnosis of mucinous cysts.”
Dr. Rift and colleagues evaluated the impact of introducing NGS to the diagnostic process. They noted that concomitant mutations in GNAS and KRAS are diagnostic for intraductal papillary mucinous neoplasms (IPMNs), while other mutations have been linked with progression to cancer.
The study involved 101 patients with pancreatic cystic lesions larger than 15 mm in diameter, mean age of 68 years, among whom 91 had residual TTNBs available after microscopic analysis. These samples underwent a 51-gene NGS panel that included the “most prevalent hot-spot mutations.” Diagnoses were sorted into four categories: neoplastic cyst, mucinous cyst, IPMN, or serous cystic neoplasm.
The primary endpoint was diagnostic yield, both for molecular analysis of TTNBs and for molecular analysis plus histopathology of TTNBs. Sensitivity and specificity of NGS were also determined using histopathology as the gold standard.
Relying on NGS alone, diagnostic yields were 44.5% and 27.7% for detecting a mucinous cyst and determining type of cyst, respectively. These yields rose to 73.3% and 70.3%, respectively, when NGS was used with microscopic evaluation. Continuing with this combined approach, sensitivity and specificity were 83.7% and 81.8%, respectively, for the diagnosis of a mucinous cyst. Sensitivity and specificity were higher still, at 87.2% and 84.6%, respectively, for identifying IPMNs.
The adverse-event rate was 9.9%, with a risk of postprocedure acute pancreatitis of 8.9 % and procedure-associated intracystic bleeding of 3%, according to the authors.
Limitations of the study include the relatively small sample size and the single-center design.
“TTNB-NGS is not sufficient as a stand-alone diagnostic tool as of yet but has a high diagnostic yield when combined with microscopic evaluation and subtyping by immunohistochemistry,” the investigators concluded. “The advantage of EUS-TTNB over EUS–[fine-needle aspiration] is the ability to perform detailed cyst subtyping and the high technical success rate of the procedure. ... However, the procedure comes with a risk of adverse events and thus should be offered to patients where the value of an exact diagnosis outweighs the risks.”
“Molecular subtyping is emerging as a useful clinical test for diagnosing pancreatic cysts,” said Margaret Geraldine Keane, MBBS, MSc, of Johns Hopkins Medicine, Baltimore, although she noted that NGS remains expensive and sporadically available, “which limits its clinical utility and incorporation into diagnostic algorithms for pancreatic cysts. In the future, as the cost of sequencing reduces, and availability improves, this may change.”
For now, Dr. Keane advised physicians to reserve molecular subtyping for cases in which “accurate cyst subtyping will change management ... or when other tests have not provided a clear diagnosis.”
She said the present study is valuable because better diagnostic tests are badly needed for patients with pancreatic cysts, considering the high rate of surgical overtreatment.
“Having more diagnostic tests, such as those described in this publication [to be used on their own or in combination] to decide which patients need surgery, is important,” Dr. Keane said who was not involved in the study.
Better diagnostic tests could also improve outcomes for patients with pancreatic cancer, she said, noting a 5-year survival rate of 10%.
“This outcome is in large part attributable to the late stage at which the majority of patients are diagnosed,” Dr. Keane said. “If patients can be diagnosed earlier, survival dramatically improves. Improvements in diagnostic tests for premalignant pancreatic cystic lesions are therefore vital.”
The study was supported by Rigshospitalets Research Foundation, The Novo Nordisk Foundation, The Danish Cancer Society, and others, although they did not have a role in conducting the study or preparing the manuscript. One investigator disclosed a relationship with MediGlobe. The other investigators reported no conflicts of interest. Dr. Keane disclosed no conflicts of interest.
FROM GASTROINTESTINAL ENDOSCOPY
How docs in firearm-friendly states talk gun safety
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
Samuel Mathis, MD, tries to cover a lot of ground during a wellness exam for his patients. Nutrition, immunizations, dental hygiene, and staying safe at school are a few of the topics on his list. And the Texas pediatrician asks one more question of children and their parents: “Are there any firearms in the house?”
If the answer is “yes,” Dr. Mathis discusses safety courses and other ideas with the families. “Rather than ask a bunch of questions, often I will say it’s recommended to keep them locked up and don’t forget toddlers can climb heights that you never would have envisioned,” said Dr. Mathis, an assistant professor at the University of Texas Medical Branch, Galveston.
Dr. Mathis said some of his physician colleagues are wary of bringing up the topic of guns in a state that leads the nation with more than 1 million registered firearms. “My discussion is more on firearm responsibility and just making sure they are taking extra steps to keep themselves and everyone around them safe. That works much better in these discussions.”
Gun safety: Public health concern, not politics
The statistics tell why:
- Unintentional shooting deaths by children rose by nearly one third in a 3-month period in 2020, compared with the same period in 2019.
- Of every 10 gun deaths in the United States, 6 are by suicide.
- As of July 28, 372 mass shootings have occured.
- Firearms now represent the leading cause of death among the nation’s youth.
In 2018, the editors of Annals of Internal Medicine urged physicians in the United States to sign a pledge to talk with their patients about guns in the home. To date, at least 3,664 have done so.
In 2019, the American Academy of Family Medicine, with other leading physician and public health organizations, issued a “call to action,” recommending ways to reduce firearm-related injury and death in the United States. Physicians can and should address the issue, it said, by counseling patients about firearm safety.
“This is just another part of healthcare,” said Sarah C. Nosal, MD, a member of the board of directors of the AAFP, who practices at the Urban Horizons Family Health Center, New York.
Dr. Nosal said she asks about firearms during every well-child visit. She also focuses on patients with a history of depression or suicide attempts and those who have experienced domestic violence.
Are physicians counseling patients about gun safety?
A 2018 survey of physicians found that 73% of the 71 who responded agreed to discuss gun safety with at-risk patients. But just 5% said they always talk to those at-risk patients, according to Melanie G. Hagen, MD, professor of internal medicine at the University of Florida, Gainesville, who led the study. While the overwhelming majority agreed that gun safety is a public health issue, only 55% said they felt comfortable initiating conversations about firearms with their patients.
Have things changed since then? “Probably not,” Dr. Hagen said in an interview. She cited some reasons, at least in her state.
One obstacle is that many people, including physicians, believe that Florida’s physician gag law, which prohibited physicians from asking about a patient’s firearm ownership, was still in effect. The law, passed in 2011, was overturned in 2017. In her survey, 76% said they were aware it had been overturned. But that awareness appears not to be universal, she said.
In a 2020 report about physician involvement in promoting gun safety, researchers noted four main challenges: lingering fears about the overturned law and potential liability from violating it, feeling unprepared, worry that patients don’t want to discuss the topic, and lack of time to talk about it during a rushed office visit.
But recent research suggests that patients are often open to talking about gun safety, and another study found that if physicians are given educational materials on firearm safety, more will counsel patients about gun safety.
Are patients and parents receptive?
Parents welcome discussion from health care providers about gun safety, according to a study from the University of Pennsylvania, Philadelphia.
Researchers asked roughly 100 parents to watch a short video about a firearm safety program designed to prevent accidents and suicides from guns. The program, still under study, involves a discussion between a parent and a pediatrician, with information given on secure storage of guns and the offering of a free cable lock.
The parents, about equally divided between gun owners and non–gun owners, said they were open to discussion about firearm safety, especially when the conversation involves their child’s pediatrician. Among the gun owners, only one in three said all their firearms were locked, unloaded, and stored properly. But after getting the safety information, 64% said they would change the way they stored their firearms.
A different program that offered pediatricians educational materials on firearm safety, as well as free firearm locks for distribution, increased the likelihood that the physicians would counsel patients on gun safety, other researchers reported.
Getting the conversation started
Some patients “bristle” when they’re asked about guns, Dr. Hagen said. Focusing on the “why” of the question can soften their response. One of her patients, a man in his 80s, had worked as a prison guard. After he was diagnosed with clinical depression, she asked him if he ever thought about ending his life. He said yes.
“And in Florida, I know a lot of people have guns,” she said. The state ranks second in the nation, with more than a half million registered weapons.
When Dr. Hagen asked him if he had firearms at home, he balked. Why did she need to know? “People do get defensive,” she said. “Luckily, I had a good relationship with this man, and he was willing to listen to me. If it’s someone I have a good relationship with, and I have this initial bristling, if I say: ‘I’m worried about you, I’m worried about your safety,’ that changes the entire conversation.”
She talked through the best plan for this patient, and he agreed to give his weapons to his son to keep.
Likewise, she talks with family members of dementia patients, urging them to be sure the weapons are stored and locked to prevent tragic accidents.
Dr. Nosal said reading the room is key. “Often, we are having the conversation with a parent with a child present,” she said. “Perhaps that is not the conversation the parent or guardian wanted to have with the child present.” In such a situation, she suggests asking the parent if they would talk about it solo.
“It can be a challenge to know the appropriate way to start the conversation,” Dr. Mathis said. The topic is not taught in medical school, although many experts think it should be. Dr. Hagen recently delivered a lecture to medical students about how to broach the topic with patients. She said she hopes it will become a regular event.
“It really comes down to being willing to be open and just ask that first question in a nonjudgmental way,” Dr. Mathis said. It helps, too, he said, for physicians to remember what he always tries to keep in mind: “My job isn’t politics, my job is health.”
Among the points Dr. Hagen makes in her lecture about talking to patients about guns are the following:
- Every day, more than 110 Americans are killed with guns.
- Gun violence accounts for just 1%-2% of those deaths, but mass shootings serve to shine a light on the issue of gun safety.
- 110,000 firearm injuries a year require medical or legal attention. Each year, more than 1,200 children in this country die from gun-related injuries.
- More than 33,000 people, on average, die in the United States each year from gun violence, including more than 21,000 from suicide.
- About 31% of all U.S. households have firearms; 22% of U.S. adults own one or more.
- Guns are 70% less likely to be stored locked and unloaded in homes where suicides or unintentional gun injuries occur.
- Action points: Identify risk, counsel patients at risk, act when someone is in imminent danger (such as unsafe practices or suicide threats).
- Focus on identifying adults who have a risk of inflicting violence on self or others.
- Focus on health and well-being with all; be conversational and educational.
- Clinicians should ask five crucial questions, all with an “L,” if firearms are in the home: Is it Loaded? Locked? Are Little children present? Is the owner feeling Low? Are they Learned [educated] in gun safety?
A version of this article first appeared on Medscape.com.
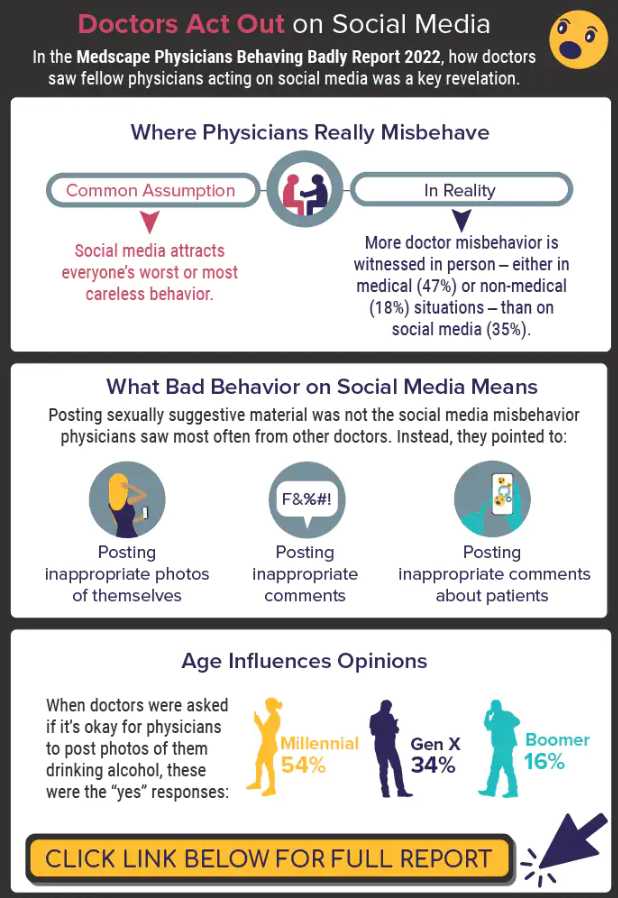
Infographic: Is physician behavior on social media really so bad?
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.
A version of this article first appeared on Medscape.com.
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.

A version of this article first appeared on Medscape.com.
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.

A version of this article first appeared on Medscape.com.
Home program improves some functional capacity in COPD
A home-based strength training program does not improve dyspnea in patients with chronic obstructive lung disease (COPD), but it does improve some functional capacity and helps patients feel better, a 12-month long HOMEX exercise program shows.
Anja Frei, PhD, University of Zurich, Switzerland, and colleagues reported.
“Our study showed that the HOMEX strength training program had no effect on dyspnea after 12 months in persons with COPD who completed PR, [but] the program improved functional exercise capacity ... and many participants reported having perceived positive effects that they attributed to the training,” investigators add.
The study was published online in the journal CHEST.
Intervention or controls
A total of 123 patients (mean age, 67 years) with COPD were randomly assigned to the intervention group or to the control group. The mean forced expiratory volume in 1 second (FEV1) was 39.3% of predicted. Three-quarters of participants had severe or very severe COPD.
A total of 104 patients completed the 12-month study. “The primary outcome was change in dyspnea (Chronic Respiratory Questionnaire, CRQ) from baseline to 12 months,” investigators note. Secondary outcomes included change in exercise capacity as assessed by the 1-minute-sit-to-stand test (1-min-STST); the 6-minute walk test (6MWT); health-related quality of life, exacerbations, and symptoms.
The HOMEX program was a structured, home-based strength training program developed for patients with COPD that could be done following the pulmonary rehabilitation program, with the intention of maintaining the training benefits gained during pulmonary rehabilitation.
“We deliberately focused on the strength component of exercise training due to the fact that skeletal muscle dysfunction is prevalent in COPD and [is] associated with lower daily physical activity and poor prognosis,” the authors explain. Patients had completed pulmonary rehabilitation no longer than 1 month prior to starting the training program. The program required a chair and a set of resistance bands and consisted of trunk, upper limb, and lower limb exercises done at different intensity levels.
Participants were instructed to do the exercises 6 days per week for about 20 minutes per day over the 12-month study interval. The dyspnea score dropped from 4.65 to 4.42 from baseline to 12 months in the intervention group, compared with a drop from 4.61 to 4.06 in the control group, the investigators reported. “There was no evidence for a difference between the two groups in change in the 6MWT distance after 12 months ... but moderate evidence for a between-group difference in the change of repetitions in the 1-min-STST favoring the IG (intervention group),” they also noted, at an adjusted mean difference of 2.6 (95% confidence interval, 0.22-5.03, P = .033).
In all other outcomes, no differences were observed between the two groups. Importantly, 70% of participants carried on with the HOMEX training program until study endpoint and at least 79% of them persevered for at least 10 months. Based on results from a satisfaction survey, 81% of participants randomly assigned to the intervention group indicated that they “liked” or “very much liked” participating in the program, and 79% of them reported that they experienced positive effects that they felt were attributed to the training.
“The program was safe and the majority of the multimorbid and severely ill study participants adhered to the training during the study year,” the authors write. And while the program had no effect on functional exercise capacity as measured by the 6MWT, it did improve the strength and intramuscular coordination of the lower leg muscles because the program had repetitive sit-to-stand exercises as a component of the training. “Adherence to this long-term training program was surprisingly high,” the authors say. “It was well accepted by COPD patients and may facilitate continued training at home.”
One limitation of the study was that some participants did not travel to the rehabilitation clinic for a follow-up assessment.
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A home-based strength training program does not improve dyspnea in patients with chronic obstructive lung disease (COPD), but it does improve some functional capacity and helps patients feel better, a 12-month long HOMEX exercise program shows.
Anja Frei, PhD, University of Zurich, Switzerland, and colleagues reported.
“Our study showed that the HOMEX strength training program had no effect on dyspnea after 12 months in persons with COPD who completed PR, [but] the program improved functional exercise capacity ... and many participants reported having perceived positive effects that they attributed to the training,” investigators add.
The study was published online in the journal CHEST.
Intervention or controls
A total of 123 patients (mean age, 67 years) with COPD were randomly assigned to the intervention group or to the control group. The mean forced expiratory volume in 1 second (FEV1) was 39.3% of predicted. Three-quarters of participants had severe or very severe COPD.
A total of 104 patients completed the 12-month study. “The primary outcome was change in dyspnea (Chronic Respiratory Questionnaire, CRQ) from baseline to 12 months,” investigators note. Secondary outcomes included change in exercise capacity as assessed by the 1-minute-sit-to-stand test (1-min-STST); the 6-minute walk test (6MWT); health-related quality of life, exacerbations, and symptoms.
The HOMEX program was a structured, home-based strength training program developed for patients with COPD that could be done following the pulmonary rehabilitation program, with the intention of maintaining the training benefits gained during pulmonary rehabilitation.
“We deliberately focused on the strength component of exercise training due to the fact that skeletal muscle dysfunction is prevalent in COPD and [is] associated with lower daily physical activity and poor prognosis,” the authors explain. Patients had completed pulmonary rehabilitation no longer than 1 month prior to starting the training program. The program required a chair and a set of resistance bands and consisted of trunk, upper limb, and lower limb exercises done at different intensity levels.
Participants were instructed to do the exercises 6 days per week for about 20 minutes per day over the 12-month study interval. The dyspnea score dropped from 4.65 to 4.42 from baseline to 12 months in the intervention group, compared with a drop from 4.61 to 4.06 in the control group, the investigators reported. “There was no evidence for a difference between the two groups in change in the 6MWT distance after 12 months ... but moderate evidence for a between-group difference in the change of repetitions in the 1-min-STST favoring the IG (intervention group),” they also noted, at an adjusted mean difference of 2.6 (95% confidence interval, 0.22-5.03, P = .033).
In all other outcomes, no differences were observed between the two groups. Importantly, 70% of participants carried on with the HOMEX training program until study endpoint and at least 79% of them persevered for at least 10 months. Based on results from a satisfaction survey, 81% of participants randomly assigned to the intervention group indicated that they “liked” or “very much liked” participating in the program, and 79% of them reported that they experienced positive effects that they felt were attributed to the training.
“The program was safe and the majority of the multimorbid and severely ill study participants adhered to the training during the study year,” the authors write. And while the program had no effect on functional exercise capacity as measured by the 6MWT, it did improve the strength and intramuscular coordination of the lower leg muscles because the program had repetitive sit-to-stand exercises as a component of the training. “Adherence to this long-term training program was surprisingly high,” the authors say. “It was well accepted by COPD patients and may facilitate continued training at home.”
One limitation of the study was that some participants did not travel to the rehabilitation clinic for a follow-up assessment.
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A home-based strength training program does not improve dyspnea in patients with chronic obstructive lung disease (COPD), but it does improve some functional capacity and helps patients feel better, a 12-month long HOMEX exercise program shows.
Anja Frei, PhD, University of Zurich, Switzerland, and colleagues reported.
“Our study showed that the HOMEX strength training program had no effect on dyspnea after 12 months in persons with COPD who completed PR, [but] the program improved functional exercise capacity ... and many participants reported having perceived positive effects that they attributed to the training,” investigators add.
The study was published online in the journal CHEST.
Intervention or controls
A total of 123 patients (mean age, 67 years) with COPD were randomly assigned to the intervention group or to the control group. The mean forced expiratory volume in 1 second (FEV1) was 39.3% of predicted. Three-quarters of participants had severe or very severe COPD.
A total of 104 patients completed the 12-month study. “The primary outcome was change in dyspnea (Chronic Respiratory Questionnaire, CRQ) from baseline to 12 months,” investigators note. Secondary outcomes included change in exercise capacity as assessed by the 1-minute-sit-to-stand test (1-min-STST); the 6-minute walk test (6MWT); health-related quality of life, exacerbations, and symptoms.
The HOMEX program was a structured, home-based strength training program developed for patients with COPD that could be done following the pulmonary rehabilitation program, with the intention of maintaining the training benefits gained during pulmonary rehabilitation.
“We deliberately focused on the strength component of exercise training due to the fact that skeletal muscle dysfunction is prevalent in COPD and [is] associated with lower daily physical activity and poor prognosis,” the authors explain. Patients had completed pulmonary rehabilitation no longer than 1 month prior to starting the training program. The program required a chair and a set of resistance bands and consisted of trunk, upper limb, and lower limb exercises done at different intensity levels.
Participants were instructed to do the exercises 6 days per week for about 20 minutes per day over the 12-month study interval. The dyspnea score dropped from 4.65 to 4.42 from baseline to 12 months in the intervention group, compared with a drop from 4.61 to 4.06 in the control group, the investigators reported. “There was no evidence for a difference between the two groups in change in the 6MWT distance after 12 months ... but moderate evidence for a between-group difference in the change of repetitions in the 1-min-STST favoring the IG (intervention group),” they also noted, at an adjusted mean difference of 2.6 (95% confidence interval, 0.22-5.03, P = .033).
In all other outcomes, no differences were observed between the two groups. Importantly, 70% of participants carried on with the HOMEX training program until study endpoint and at least 79% of them persevered for at least 10 months. Based on results from a satisfaction survey, 81% of participants randomly assigned to the intervention group indicated that they “liked” or “very much liked” participating in the program, and 79% of them reported that they experienced positive effects that they felt were attributed to the training.
“The program was safe and the majority of the multimorbid and severely ill study participants adhered to the training during the study year,” the authors write. And while the program had no effect on functional exercise capacity as measured by the 6MWT, it did improve the strength and intramuscular coordination of the lower leg muscles because the program had repetitive sit-to-stand exercises as a component of the training. “Adherence to this long-term training program was surprisingly high,” the authors say. “It was well accepted by COPD patients and may facilitate continued training at home.”
One limitation of the study was that some participants did not travel to the rehabilitation clinic for a follow-up assessment.
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Molecular map’ of CLL yields fresh genetic insights
Released in a report in Nature Genetics, the map has doubled the number of genetic traits linked the disease from around 100 to 202, lead author Binyamin A. Knisbacher, PhD, a postdoctoral fellow at the Broad Institute of MIT and Harvard Medical Schoo, Boston, said in an interview.
“It also delineated the molecular landscape of the two immunoglobulin gene (IGHV) subtypes, refined CLL subtyping, and built richer genetic prognostic models,” he said.
According to Dr. Knisbacher, CLL “has been at the forefront of genomic discovery,” and research has shown that there’s a wide variety of somatic mutations that drive CLL initiation across the patient population. However, as many as 10% of cases don’t appear to be driven by any known genetic variation, he said, and there’s a need to identify more subtypes and “build richer prognostic models of patient survival” based on genetics and multiomics such as genomics, transcriptomics, and epigenomics.
For the new study, researchers analyzed RNA and DNA from 1,095 patients with CLL and 54 patients with monoclonal B cell lymphocytosis and built what they say is the largest CLL dataset in existence. It’s twice the size of previous datasets, Dr. Knisbacher said.
“We found that RNA expression data was extremely informative for characterizing CLL,” Dr. Knisbacher said. “The RNA expression subtypes refined the ‘classic’ two IGHV subtypes. It is well documented that patients with U-CLL (IGHV-unmutated CLL) have substantially worse clinical outcome in comparison to M-CLL patients (IGHV-mutated CLLs). We found that M-CLLs that have RNA expression profiles similar to U-CLLs have worse survival than M-CLLs with a typical expression profile. Failure-free survival was 50% shorter – 5.3 versus 10.7 years median failure-free survival.”
In addition, he said, “U-CLLs with expression similar to M-CLLs had better survival than U-CLLs with an RNA expression profile typical to U-CLLs.”
The researchers have made their molecular map publicly available at https://cllmap.org/. Researchers can use it “to discover more about each subtype of CLL, and these future studies can help to improve clinical prognosis for the benefit of the patient,” Dr. Knisbacher said.
The study authors added that “this molecular foundation may allow for better prediction of response to therapy or provide the basis for rational combination of novel agents.”
Lee Greenberger, PhD, chief science officer of the Leukemia & Lymphoma Society, said in an interview that the study “provides foundational data further subtyping CLL patients and outcomes. It identifies new targets for therapy or diagnostic predictions in the future. This type of foundational work has proven invaluable in the development of new medicines for cancer in general.”
While there are many medications that have improved therapeutic outcomes in CLL, he added, “cures – or life-long disease control –remain elusive for many patients. Therefore, new molecular insights are needed that could personalize therapies or even lead to entirely new therapies.”
In addition, he said, although prevention of CLL still remains elusive, “it is conceivable that some of the mutations found in this paper occur early in the CLL trajectory, perhaps even before the disease is presented clinically.”
The study was funded by the National Institutes of Health and the Broad/IBM Cancer Resistance Research Project. Dr. Knisbacher and several other authors disclose that they are inventors on a patent related to CLL. Several authors report various relationships with industry. Dr. Greenberger has no disclosures.
Released in a report in Nature Genetics, the map has doubled the number of genetic traits linked the disease from around 100 to 202, lead author Binyamin A. Knisbacher, PhD, a postdoctoral fellow at the Broad Institute of MIT and Harvard Medical Schoo, Boston, said in an interview.
“It also delineated the molecular landscape of the two immunoglobulin gene (IGHV) subtypes, refined CLL subtyping, and built richer genetic prognostic models,” he said.
According to Dr. Knisbacher, CLL “has been at the forefront of genomic discovery,” and research has shown that there’s a wide variety of somatic mutations that drive CLL initiation across the patient population. However, as many as 10% of cases don’t appear to be driven by any known genetic variation, he said, and there’s a need to identify more subtypes and “build richer prognostic models of patient survival” based on genetics and multiomics such as genomics, transcriptomics, and epigenomics.
For the new study, researchers analyzed RNA and DNA from 1,095 patients with CLL and 54 patients with monoclonal B cell lymphocytosis and built what they say is the largest CLL dataset in existence. It’s twice the size of previous datasets, Dr. Knisbacher said.
“We found that RNA expression data was extremely informative for characterizing CLL,” Dr. Knisbacher said. “The RNA expression subtypes refined the ‘classic’ two IGHV subtypes. It is well documented that patients with U-CLL (IGHV-unmutated CLL) have substantially worse clinical outcome in comparison to M-CLL patients (IGHV-mutated CLLs). We found that M-CLLs that have RNA expression profiles similar to U-CLLs have worse survival than M-CLLs with a typical expression profile. Failure-free survival was 50% shorter – 5.3 versus 10.7 years median failure-free survival.”
In addition, he said, “U-CLLs with expression similar to M-CLLs had better survival than U-CLLs with an RNA expression profile typical to U-CLLs.”
The researchers have made their molecular map publicly available at https://cllmap.org/. Researchers can use it “to discover more about each subtype of CLL, and these future studies can help to improve clinical prognosis for the benefit of the patient,” Dr. Knisbacher said.
The study authors added that “this molecular foundation may allow for better prediction of response to therapy or provide the basis for rational combination of novel agents.”
Lee Greenberger, PhD, chief science officer of the Leukemia & Lymphoma Society, said in an interview that the study “provides foundational data further subtyping CLL patients and outcomes. It identifies new targets for therapy or diagnostic predictions in the future. This type of foundational work has proven invaluable in the development of new medicines for cancer in general.”
While there are many medications that have improved therapeutic outcomes in CLL, he added, “cures – or life-long disease control –remain elusive for many patients. Therefore, new molecular insights are needed that could personalize therapies or even lead to entirely new therapies.”
In addition, he said, although prevention of CLL still remains elusive, “it is conceivable that some of the mutations found in this paper occur early in the CLL trajectory, perhaps even before the disease is presented clinically.”
The study was funded by the National Institutes of Health and the Broad/IBM Cancer Resistance Research Project. Dr. Knisbacher and several other authors disclose that they are inventors on a patent related to CLL. Several authors report various relationships with industry. Dr. Greenberger has no disclosures.
Released in a report in Nature Genetics, the map has doubled the number of genetic traits linked the disease from around 100 to 202, lead author Binyamin A. Knisbacher, PhD, a postdoctoral fellow at the Broad Institute of MIT and Harvard Medical Schoo, Boston, said in an interview.
“It also delineated the molecular landscape of the two immunoglobulin gene (IGHV) subtypes, refined CLL subtyping, and built richer genetic prognostic models,” he said.
According to Dr. Knisbacher, CLL “has been at the forefront of genomic discovery,” and research has shown that there’s a wide variety of somatic mutations that drive CLL initiation across the patient population. However, as many as 10% of cases don’t appear to be driven by any known genetic variation, he said, and there’s a need to identify more subtypes and “build richer prognostic models of patient survival” based on genetics and multiomics such as genomics, transcriptomics, and epigenomics.
For the new study, researchers analyzed RNA and DNA from 1,095 patients with CLL and 54 patients with monoclonal B cell lymphocytosis and built what they say is the largest CLL dataset in existence. It’s twice the size of previous datasets, Dr. Knisbacher said.
“We found that RNA expression data was extremely informative for characterizing CLL,” Dr. Knisbacher said. “The RNA expression subtypes refined the ‘classic’ two IGHV subtypes. It is well documented that patients with U-CLL (IGHV-unmutated CLL) have substantially worse clinical outcome in comparison to M-CLL patients (IGHV-mutated CLLs). We found that M-CLLs that have RNA expression profiles similar to U-CLLs have worse survival than M-CLLs with a typical expression profile. Failure-free survival was 50% shorter – 5.3 versus 10.7 years median failure-free survival.”
In addition, he said, “U-CLLs with expression similar to M-CLLs had better survival than U-CLLs with an RNA expression profile typical to U-CLLs.”
The researchers have made their molecular map publicly available at https://cllmap.org/. Researchers can use it “to discover more about each subtype of CLL, and these future studies can help to improve clinical prognosis for the benefit of the patient,” Dr. Knisbacher said.
The study authors added that “this molecular foundation may allow for better prediction of response to therapy or provide the basis for rational combination of novel agents.”
Lee Greenberger, PhD, chief science officer of the Leukemia & Lymphoma Society, said in an interview that the study “provides foundational data further subtyping CLL patients and outcomes. It identifies new targets for therapy or diagnostic predictions in the future. This type of foundational work has proven invaluable in the development of new medicines for cancer in general.”
While there are many medications that have improved therapeutic outcomes in CLL, he added, “cures – or life-long disease control –remain elusive for many patients. Therefore, new molecular insights are needed that could personalize therapies or even lead to entirely new therapies.”
In addition, he said, although prevention of CLL still remains elusive, “it is conceivable that some of the mutations found in this paper occur early in the CLL trajectory, perhaps even before the disease is presented clinically.”
The study was funded by the National Institutes of Health and the Broad/IBM Cancer Resistance Research Project. Dr. Knisbacher and several other authors disclose that they are inventors on a patent related to CLL. Several authors report various relationships with industry. Dr. Greenberger has no disclosures.
FROM NATURE GENETICS
COVID-19 vaccine safe in patients with heart failure
Patients with heart failure (HF) who received two doses of COVID mRNA vaccines were not more likely to have worsening disease, venous thromboembolism, or myocarditis within 90 days than similar unvaccinated patients, in a case-control study in Denmark.
Moreover, in the 90 days after receiving the second shot, vaccinated patients were less likely to die of any cause, compared with unvaccinated patients during a similar 90-day period.
Caroline Sindet-Pedersen, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and colleagues presented these findings at the annual congress of the European Society of Cardiology.
Major risk is not receiving vaccine
These results “confirm that the major risk for patients with HF is not receiving vaccination for COVID-19,” Marco Metra, MD, who was not involved with this research, said in an interview.
Dr. Metra was coauthor of an ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic, published online ahead of print November 2021 in the European Heart Journal.
The guidance explains that patients with HF are at increased risk for hospitalization, need for mechanical ventilation, and death because of COVID-19, and that vaccination reduces the risk for serious illness from COVID-19, Dr. Sindet-Pedersen and colleagues explained in a press release from the ESC.
However, “concerns remain,” they added, “about the safety of the SARS-CoV-2 mRNA vaccines in heart failure patients, due to a perceived increased risk of cardiovascular side effects.”
The study findings suggest that “there should be no concern about cardiovascular side effects from mRNA vaccines in heart failure patients,” Dr. Sindet-Pedersen and colleagues summarized.
The results also “point to a beneficial effect of vaccination on mortality” and “indicate that patients with HF should be prioritized for COVID-19 vaccinations and boosters,” they added.
“There are ongoing concerns about the safety of COVID-19 vaccination in fragile patients and patients with heart failure,” said Dr. Metra, professor of cardiology and director of the Institute of Cardiology of the Civil Hospital and University of Brescia (Italy).
“These concerns are not based on evidence but just on reports of rare side effects (namely, myocarditis and pericarditis) in vaccinated people,” he added.
Dr. Metra also coauthored a position paper on COVID-19 vaccination in patients with HF from the Heart Failure Association of the ESC, which was published online October 2021 in the European Journal of Heart Failure.
“The current study,” he summarized, “shows a lower risk of mortality among patients vaccinated, compared with those not vaccinated.
“It has limitations,” he cautioned, “as it is not a prospective randomized study, but [rather] an observational one with comparison between vaccinated and not vaccinated patients with similar characteristics.
“However, it was done in a large population,” he noted, “and its results confirm that the major risk for patients with HF is not receiving vaccination for COVID-19.”
95% of patients with HF in Denmark double vaccinated
The group did not analyze the types of all-cause death in their study, Dr. Sindet-Pedersen clarified in an interview.
Other studies have shown that vaccines are associated with improved survival, she noted. For example, bacillus Calmette-Guérin vaccines and the measles vaccines have been linked with a decreased risk for nonspecific mortality in children, and influenza vaccines are associated with decreased all-cause mortality in patients with HF.
The rates of vaccination in this study were much higher than those for patients with HF in the United States.
In a study of 7,094 patients with HF seen at the Mount Sinai Health System between January 2021 and January 2022, 31% of patients were fully vaccinated with two doses and 14.8% had also received a booster, as per Centers for Disease Control and Prevention guidance. However, another 9.1% of patients were only partially vaccinated with one dose, and 45% remained unvaccinated by January 2022,
In the current study, “the uptake was very high,” Dr. Sindet-Pedersen noted, that is, “95% of the prevalent heart failure patients in 2021 received a vaccine.”
“It might be that the last 5% of the patients that did not receive a vaccine were too ill [terminal] to receive the vaccine,” she speculated, “or that was due to personal reasons.”
The researchers identified 50,893 patients with HF who were double vaccinated in 2021 and they matched them with 50,893 unvaccinated patients with HF in 2019 (prepandemic), with the same age, sex, HF duration, use of HF medications, ischemic heart disease, cancer, diabetes, atrial fibrillation, and admission with HF within 90 days.
Almost all patients in the vaccinated group received the Pfizer/BioNTech mRNA vaccine (92%) and the rest received the Moderna mRNA vaccine (8%), in 2021.
The patients had a mean age of 74, and 64% were men. They had HF for a median of 4.1 years.
During the 90-day follow-up, 1,311 patients in the unvaccinated cohort (2.56%) and 1,113 patients in the vaccinated cohort (2.23%) died; there was a significantly lower risk for all-cause death in the vaccinated cohort versus the unvaccinated cohort (–0.33 percentage points; 95% CI, –0.52 to –0.15 percentage points).
The risk for worsening heart failure was 1.1% in each group; myocarditis and venous thromboembolism were extremely rare, and risks for these conditions were not significantly different in the two groups.
The researchers and Dr. Metra declared they have no relevant financial disclosures. Dr. Metra is editor-in-chief of the European Journal of Heart Failure and senior consulting editor of the European Heart Journal.
A version of this article first appeared on Medscape.com.
Patients with heart failure (HF) who received two doses of COVID mRNA vaccines were not more likely to have worsening disease, venous thromboembolism, or myocarditis within 90 days than similar unvaccinated patients, in a case-control study in Denmark.
Moreover, in the 90 days after receiving the second shot, vaccinated patients were less likely to die of any cause, compared with unvaccinated patients during a similar 90-day period.
Caroline Sindet-Pedersen, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and colleagues presented these findings at the annual congress of the European Society of Cardiology.
Major risk is not receiving vaccine
These results “confirm that the major risk for patients with HF is not receiving vaccination for COVID-19,” Marco Metra, MD, who was not involved with this research, said in an interview.
Dr. Metra was coauthor of an ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic, published online ahead of print November 2021 in the European Heart Journal.
The guidance explains that patients with HF are at increased risk for hospitalization, need for mechanical ventilation, and death because of COVID-19, and that vaccination reduces the risk for serious illness from COVID-19, Dr. Sindet-Pedersen and colleagues explained in a press release from the ESC.
However, “concerns remain,” they added, “about the safety of the SARS-CoV-2 mRNA vaccines in heart failure patients, due to a perceived increased risk of cardiovascular side effects.”
The study findings suggest that “there should be no concern about cardiovascular side effects from mRNA vaccines in heart failure patients,” Dr. Sindet-Pedersen and colleagues summarized.
The results also “point to a beneficial effect of vaccination on mortality” and “indicate that patients with HF should be prioritized for COVID-19 vaccinations and boosters,” they added.
“There are ongoing concerns about the safety of COVID-19 vaccination in fragile patients and patients with heart failure,” said Dr. Metra, professor of cardiology and director of the Institute of Cardiology of the Civil Hospital and University of Brescia (Italy).
“These concerns are not based on evidence but just on reports of rare side effects (namely, myocarditis and pericarditis) in vaccinated people,” he added.
Dr. Metra also coauthored a position paper on COVID-19 vaccination in patients with HF from the Heart Failure Association of the ESC, which was published online October 2021 in the European Journal of Heart Failure.
“The current study,” he summarized, “shows a lower risk of mortality among patients vaccinated, compared with those not vaccinated.
“It has limitations,” he cautioned, “as it is not a prospective randomized study, but [rather] an observational one with comparison between vaccinated and not vaccinated patients with similar characteristics.
“However, it was done in a large population,” he noted, “and its results confirm that the major risk for patients with HF is not receiving vaccination for COVID-19.”
95% of patients with HF in Denmark double vaccinated
The group did not analyze the types of all-cause death in their study, Dr. Sindet-Pedersen clarified in an interview.
Other studies have shown that vaccines are associated with improved survival, she noted. For example, bacillus Calmette-Guérin vaccines and the measles vaccines have been linked with a decreased risk for nonspecific mortality in children, and influenza vaccines are associated with decreased all-cause mortality in patients with HF.
The rates of vaccination in this study were much higher than those for patients with HF in the United States.
In a study of 7,094 patients with HF seen at the Mount Sinai Health System between January 2021 and January 2022, 31% of patients were fully vaccinated with two doses and 14.8% had also received a booster, as per Centers for Disease Control and Prevention guidance. However, another 9.1% of patients were only partially vaccinated with one dose, and 45% remained unvaccinated by January 2022,
In the current study, “the uptake was very high,” Dr. Sindet-Pedersen noted, that is, “95% of the prevalent heart failure patients in 2021 received a vaccine.”
“It might be that the last 5% of the patients that did not receive a vaccine were too ill [terminal] to receive the vaccine,” she speculated, “or that was due to personal reasons.”
The researchers identified 50,893 patients with HF who were double vaccinated in 2021 and they matched them with 50,893 unvaccinated patients with HF in 2019 (prepandemic), with the same age, sex, HF duration, use of HF medications, ischemic heart disease, cancer, diabetes, atrial fibrillation, and admission with HF within 90 days.
Almost all patients in the vaccinated group received the Pfizer/BioNTech mRNA vaccine (92%) and the rest received the Moderna mRNA vaccine (8%), in 2021.
The patients had a mean age of 74, and 64% were men. They had HF for a median of 4.1 years.
During the 90-day follow-up, 1,311 patients in the unvaccinated cohort (2.56%) and 1,113 patients in the vaccinated cohort (2.23%) died; there was a significantly lower risk for all-cause death in the vaccinated cohort versus the unvaccinated cohort (–0.33 percentage points; 95% CI, –0.52 to –0.15 percentage points).
The risk for worsening heart failure was 1.1% in each group; myocarditis and venous thromboembolism were extremely rare, and risks for these conditions were not significantly different in the two groups.
The researchers and Dr. Metra declared they have no relevant financial disclosures. Dr. Metra is editor-in-chief of the European Journal of Heart Failure and senior consulting editor of the European Heart Journal.
A version of this article first appeared on Medscape.com.
Patients with heart failure (HF) who received two doses of COVID mRNA vaccines were not more likely to have worsening disease, venous thromboembolism, or myocarditis within 90 days than similar unvaccinated patients, in a case-control study in Denmark.
Moreover, in the 90 days after receiving the second shot, vaccinated patients were less likely to die of any cause, compared with unvaccinated patients during a similar 90-day period.
Caroline Sindet-Pedersen, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and colleagues presented these findings at the annual congress of the European Society of Cardiology.
Major risk is not receiving vaccine
These results “confirm that the major risk for patients with HF is not receiving vaccination for COVID-19,” Marco Metra, MD, who was not involved with this research, said in an interview.
Dr. Metra was coauthor of an ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic, published online ahead of print November 2021 in the European Heart Journal.
The guidance explains that patients with HF are at increased risk for hospitalization, need for mechanical ventilation, and death because of COVID-19, and that vaccination reduces the risk for serious illness from COVID-19, Dr. Sindet-Pedersen and colleagues explained in a press release from the ESC.
However, “concerns remain,” they added, “about the safety of the SARS-CoV-2 mRNA vaccines in heart failure patients, due to a perceived increased risk of cardiovascular side effects.”
The study findings suggest that “there should be no concern about cardiovascular side effects from mRNA vaccines in heart failure patients,” Dr. Sindet-Pedersen and colleagues summarized.
The results also “point to a beneficial effect of vaccination on mortality” and “indicate that patients with HF should be prioritized for COVID-19 vaccinations and boosters,” they added.
“There are ongoing concerns about the safety of COVID-19 vaccination in fragile patients and patients with heart failure,” said Dr. Metra, professor of cardiology and director of the Institute of Cardiology of the Civil Hospital and University of Brescia (Italy).
“These concerns are not based on evidence but just on reports of rare side effects (namely, myocarditis and pericarditis) in vaccinated people,” he added.
Dr. Metra also coauthored a position paper on COVID-19 vaccination in patients with HF from the Heart Failure Association of the ESC, which was published online October 2021 in the European Journal of Heart Failure.
“The current study,” he summarized, “shows a lower risk of mortality among patients vaccinated, compared with those not vaccinated.
“It has limitations,” he cautioned, “as it is not a prospective randomized study, but [rather] an observational one with comparison between vaccinated and not vaccinated patients with similar characteristics.
“However, it was done in a large population,” he noted, “and its results confirm that the major risk for patients with HF is not receiving vaccination for COVID-19.”
95% of patients with HF in Denmark double vaccinated
The group did not analyze the types of all-cause death in their study, Dr. Sindet-Pedersen clarified in an interview.
Other studies have shown that vaccines are associated with improved survival, she noted. For example, bacillus Calmette-Guérin vaccines and the measles vaccines have been linked with a decreased risk for nonspecific mortality in children, and influenza vaccines are associated with decreased all-cause mortality in patients with HF.
The rates of vaccination in this study were much higher than those for patients with HF in the United States.
In a study of 7,094 patients with HF seen at the Mount Sinai Health System between January 2021 and January 2022, 31% of patients were fully vaccinated with two doses and 14.8% had also received a booster, as per Centers for Disease Control and Prevention guidance. However, another 9.1% of patients were only partially vaccinated with one dose, and 45% remained unvaccinated by January 2022,
In the current study, “the uptake was very high,” Dr. Sindet-Pedersen noted, that is, “95% of the prevalent heart failure patients in 2021 received a vaccine.”
“It might be that the last 5% of the patients that did not receive a vaccine were too ill [terminal] to receive the vaccine,” she speculated, “or that was due to personal reasons.”
The researchers identified 50,893 patients with HF who were double vaccinated in 2021 and they matched them with 50,893 unvaccinated patients with HF in 2019 (prepandemic), with the same age, sex, HF duration, use of HF medications, ischemic heart disease, cancer, diabetes, atrial fibrillation, and admission with HF within 90 days.
Almost all patients in the vaccinated group received the Pfizer/BioNTech mRNA vaccine (92%) and the rest received the Moderna mRNA vaccine (8%), in 2021.
The patients had a mean age of 74, and 64% were men. They had HF for a median of 4.1 years.
During the 90-day follow-up, 1,311 patients in the unvaccinated cohort (2.56%) and 1,113 patients in the vaccinated cohort (2.23%) died; there was a significantly lower risk for all-cause death in the vaccinated cohort versus the unvaccinated cohort (–0.33 percentage points; 95% CI, –0.52 to –0.15 percentage points).
The risk for worsening heart failure was 1.1% in each group; myocarditis and venous thromboembolism were extremely rare, and risks for these conditions were not significantly different in the two groups.
The researchers and Dr. Metra declared they have no relevant financial disclosures. Dr. Metra is editor-in-chief of the European Journal of Heart Failure and senior consulting editor of the European Heart Journal.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Former nurse charged with murder in death of 97-year-old war veteran
A former Kentucky nurse was charged with murder stemming from an incident in which she gave “something special” to a 97-year-old patient who died 5 days later, according to multiple sources, including police and nursing records.
Ms. Hunter allegedly gave lorazepam, typically used for anxiety, to Mr. Morris on April 30. He subsequently developed pneumonia and died on May 5.
Ms. Hunter “intentionally performed actions of medical maltreatment,” according to the Lexington Police Department’s report.
A Baptist Health Lexington spokeswoman told this news organization that the nurse who was charged hasn’t worked at the hospital since the April incident. “We have learned that a former nurse at our hospital has been arrested yesterday on criminal charges,” spokeswoman Ruth Ann Childers stated. “The hospital has fully cooperated with the police investigation. Patient care and safety are always our top priorities. Out of respect for the patient’s family and because this is criminal matter, we are not able to talk about the investigation.”
According to the Kentucky Board of Nursing, which suspended Ms. Hunter’s RN license on a temporary basis on Aug. 22, she allegedly asked the on-duty physician and a nurse practitioner separately for a medication order to calm Mr. Morris, who had become agitated and aggressive. They denied Ms. Hunter’s request, so she withdrew lorazepam intended for another patient and administered it to Mr. Morris, the nursing board suspension order states. “When asked what was administered, she replied ‘something special,’ “ the order states.
Another RN found the patient with labored breathing and “it was determined that respondent had disarmed/lowered the oxygen monitoring system several times as to not set off an alarm at the bedside,” the order continued. “The RN discussed with charge nurse that the patient had been given something intravenously that was causing his decline.”
When the charge nurse entered the room later, she found the patient in “respiratory distress with labored breathing and poor oxygen saturation. ... X-rays would show that the aspiration from the substances ingested by the patient while in his condition caused the patient to develop aspirational pneumonia,” the order continues.
“Despite the rapidly declining condition of the patient, respondent never called for rapid response nor acted with any sense of urgency. Respondent did however edit documentation of administration of Ativan on ‘patient B’ to state ‘not given.’ ”
Mr. Morris’ condition never improved. He was taken to hospice care on May 3 and died 2 days later, the order states.
Ms. Hunter was being held in the Lexington Jail on $100,000 bond, according to jail records.
A version of this article first appeared on Medscape.com.
A former Kentucky nurse was charged with murder stemming from an incident in which she gave “something special” to a 97-year-old patient who died 5 days later, according to multiple sources, including police and nursing records.
Ms. Hunter allegedly gave lorazepam, typically used for anxiety, to Mr. Morris on April 30. He subsequently developed pneumonia and died on May 5.
Ms. Hunter “intentionally performed actions of medical maltreatment,” according to the Lexington Police Department’s report.
A Baptist Health Lexington spokeswoman told this news organization that the nurse who was charged hasn’t worked at the hospital since the April incident. “We have learned that a former nurse at our hospital has been arrested yesterday on criminal charges,” spokeswoman Ruth Ann Childers stated. “The hospital has fully cooperated with the police investigation. Patient care and safety are always our top priorities. Out of respect for the patient’s family and because this is criminal matter, we are not able to talk about the investigation.”
According to the Kentucky Board of Nursing, which suspended Ms. Hunter’s RN license on a temporary basis on Aug. 22, she allegedly asked the on-duty physician and a nurse practitioner separately for a medication order to calm Mr. Morris, who had become agitated and aggressive. They denied Ms. Hunter’s request, so she withdrew lorazepam intended for another patient and administered it to Mr. Morris, the nursing board suspension order states. “When asked what was administered, she replied ‘something special,’ “ the order states.
Another RN found the patient with labored breathing and “it was determined that respondent had disarmed/lowered the oxygen monitoring system several times as to not set off an alarm at the bedside,” the order continued. “The RN discussed with charge nurse that the patient had been given something intravenously that was causing his decline.”
When the charge nurse entered the room later, she found the patient in “respiratory distress with labored breathing and poor oxygen saturation. ... X-rays would show that the aspiration from the substances ingested by the patient while in his condition caused the patient to develop aspirational pneumonia,” the order continues.
“Despite the rapidly declining condition of the patient, respondent never called for rapid response nor acted with any sense of urgency. Respondent did however edit documentation of administration of Ativan on ‘patient B’ to state ‘not given.’ ”
Mr. Morris’ condition never improved. He was taken to hospice care on May 3 and died 2 days later, the order states.
Ms. Hunter was being held in the Lexington Jail on $100,000 bond, according to jail records.
A version of this article first appeared on Medscape.com.
A former Kentucky nurse was charged with murder stemming from an incident in which she gave “something special” to a 97-year-old patient who died 5 days later, according to multiple sources, including police and nursing records.
Ms. Hunter allegedly gave lorazepam, typically used for anxiety, to Mr. Morris on April 30. He subsequently developed pneumonia and died on May 5.
Ms. Hunter “intentionally performed actions of medical maltreatment,” according to the Lexington Police Department’s report.
A Baptist Health Lexington spokeswoman told this news organization that the nurse who was charged hasn’t worked at the hospital since the April incident. “We have learned that a former nurse at our hospital has been arrested yesterday on criminal charges,” spokeswoman Ruth Ann Childers stated. “The hospital has fully cooperated with the police investigation. Patient care and safety are always our top priorities. Out of respect for the patient’s family and because this is criminal matter, we are not able to talk about the investigation.”
According to the Kentucky Board of Nursing, which suspended Ms. Hunter’s RN license on a temporary basis on Aug. 22, she allegedly asked the on-duty physician and a nurse practitioner separately for a medication order to calm Mr. Morris, who had become agitated and aggressive. They denied Ms. Hunter’s request, so she withdrew lorazepam intended for another patient and administered it to Mr. Morris, the nursing board suspension order states. “When asked what was administered, she replied ‘something special,’ “ the order states.
Another RN found the patient with labored breathing and “it was determined that respondent had disarmed/lowered the oxygen monitoring system several times as to not set off an alarm at the bedside,” the order continued. “The RN discussed with charge nurse that the patient had been given something intravenously that was causing his decline.”
When the charge nurse entered the room later, she found the patient in “respiratory distress with labored breathing and poor oxygen saturation. ... X-rays would show that the aspiration from the substances ingested by the patient while in his condition caused the patient to develop aspirational pneumonia,” the order continues.
“Despite the rapidly declining condition of the patient, respondent never called for rapid response nor acted with any sense of urgency. Respondent did however edit documentation of administration of Ativan on ‘patient B’ to state ‘not given.’ ”
Mr. Morris’ condition never improved. He was taken to hospice care on May 3 and died 2 days later, the order states.
Ms. Hunter was being held in the Lexington Jail on $100,000 bond, according to jail records.
A version of this article first appeared on Medscape.com.
Metformin fails as early COVID-19 treatment but shows potential
Neither metformin, ivermectin, or fluvoxamine had any impact on reducing disease severity, hospitalization, or death from COVID-19, according to results from more than 1,000 overweight or obese adult patients in the COVID-OUT randomized trial.
However, metformin showed some potential in a secondary analysis.
Early treatment to prevent severe disease remains a goal in managing the ongoing COVID-19 pandemic, and biophysical modeling suggested that metformin, ivermectin, and fluvoxamine may serve as antivirals to help reduce severe disease in COVID-19 patients, Carolyn T. Bramante, MD, of the University of Minnesota, Minneapolis, and colleagues wrote.
“We started enrolling patients at the end of December 2020,” Dr. Bramante said in an interview. “At that time, even though vaccine data were coming out, we thought it was important to test early outpatient treatment with widely available safe medications with no interactions, because the virus would evolve and vaccine availability may be limited.”
In a study published in the New England Journal of Medicine, the researchers used a two-by-three factorial design to test the ability of metformin, ivermectin, and fluvoxamine to prevent severe COVID-19 infection in nonhospitalized adults aged 30-85 years. A total of 1,431 patients at six U.S. sites were enrolled within 3 days of a confirmed infection and less than 7 days after the start of symptoms, then randomized to one of six groups: metformin plus fluvoxamine; metformin plus ivermectin; metformin plus placebo; placebo plus fluvoxamine; placebo plus ivermectin; and placebo plus placebo.
A total of 1,323 patients were included in the primary analysis. The median age of the patients was 46 years, 56% were female (of whom 6% were pregnant), and all individuals met criteria for overweight or obesity. About half (52%) of the patients had been vaccinated against COVID-19.
The primary endpoint was a composite of hypoxemia, ED visit, hospitalization, or death. The analyses were adjusted for COVID-19 vaccination and other trial medications. Overall, the adjusted odds ratios of any primary event, compared with placebo, was 0.84 for metformin (P = .19), 1.05 for ivermectin (P = .78), and 0.94 for fluvoxamine (P = .75).
The researchers also conducted a prespecified secondary analysis of components of the primary endpoint. In this analysis, the aORs for an ED visit, hospitalization, or death was 0.58 for metformin, 1.39 for ivermectin, and 1.17 for fluvoxamine. The aORs for hospitalization or death were 0.47, 0.73, and 1.11 for metformin, ivermectin, and fluvoxamine, respectively. No medication-related serious adverse events were reported with any of the drugs during the study period.
The possible benefit for prevention of severe COVID-19 with metformin was a prespecified secondary endpoint, and therefore not definitive until more research has been completed, the researchers said. Metformin has demonstrated anti-inflammatory actions in previous studies, and has shown protective effects against COVID-19 lung injury in animal studies.
Previous observational studies also have shown an association between metformin use and less severe COVID-19 in patients already taking metformin. “The proposed mechanisms of action against COVID-19 for metformin include anti-inflammatory and antiviral activity and the prevention of hyperglycemia during acute illness,” they added.
The study findings were limited by several factors including the population age range and focus on overweight and obese patients, which may limit generalizability, the researchers noted. Other limitations include the disproportionately small percentage of Black and Latino patients and the potential lack of accuracy in identifying hypoxemia via home oxygen monitors.
However, the results demonstrate that none of the three repurposed drugs – metformin, ivermectin, and fluvoxamine – prevented primary events or reduced symptom severity in COVID-19, compared with placebos, the researchers concluded.
“Metformin had several streams of evidence supporting its use: in vitro, in silico [computer modeled], observational, and in tissue. We were not surprised to see that it reduced emergency department visits, hospitalization, and death,” Dr. Bramante said in an interview.
The take-home message for clinicians is to continue to look to guideline committees for direction on COVID-19 treatments, but to continue to consider metformin along with other treatments, she said.
“All research should be replicated, whether the primary outcome is positive or negative,” Dr. Bramante emphasized. “In this case, when our positive outcome was negative and secondary outcome was positive, a confirmatory trial for metformin is particularly important.”
Ineffective drugs are inefficient use of resources
“The results of the COVID-OUT trial provide persuasive additional data that increase the confidence and degree of certainty that fluvoxamine and ivermectin are not effective in preventing progression to severe disease,” wrote Salim S. Abdool Karim, MB, and Nikita Devnarain, PhD, of the Centre for the AIDS Programme of Research in South Africa, Durban, in an accompanying editorial.
At the start of the study, in 2020, data on the use of the three drugs to prevent severe COVID-19 were “either unavailable or equivocal,” they said. Since then, accumulating data support the current study findings of the nonefficacy of ivermectin and fluvoxamine, and the World Health Organization has advised against their use for COVID-19, although the WHO has not provided guidance for the use of metformin.
The authors called on clinicians to stop using ivermectin and fluvoxamine to treat COVID-19 patients.
“With respect to clinical decisions about COVID-19 treatment, some drug choices, especially those that have negative [World Health Organization] recommendations, are clearly wrong,” they wrote. “In keeping with evidence-based medical practice, patients with COVID-19 must be treated with efficacious medications; they deserve nothing less.”
The study was supported by the Parsemus Foundation, Rainwater Charitable Foundation, Fast Grants, and UnitedHealth Group Foundation. The fluvoxamine placebo tablets were donated by Apotex Pharmaceuticals. The ivermectin placebo and active tablets were donated by Edenbridge Pharmaceuticals. Lead author Dr. Bramante was supported the National Center for Advancing Translational Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers had no financial conflicts to disclose. Dr. Abdool Karim serves as a member of the World Health Organization Science Council. Dr. Devnarain had no financial conflicts to disclose.
Neither metformin, ivermectin, or fluvoxamine had any impact on reducing disease severity, hospitalization, or death from COVID-19, according to results from more than 1,000 overweight or obese adult patients in the COVID-OUT randomized trial.
However, metformin showed some potential in a secondary analysis.
Early treatment to prevent severe disease remains a goal in managing the ongoing COVID-19 pandemic, and biophysical modeling suggested that metformin, ivermectin, and fluvoxamine may serve as antivirals to help reduce severe disease in COVID-19 patients, Carolyn T. Bramante, MD, of the University of Minnesota, Minneapolis, and colleagues wrote.
“We started enrolling patients at the end of December 2020,” Dr. Bramante said in an interview. “At that time, even though vaccine data were coming out, we thought it was important to test early outpatient treatment with widely available safe medications with no interactions, because the virus would evolve and vaccine availability may be limited.”
In a study published in the New England Journal of Medicine, the researchers used a two-by-three factorial design to test the ability of metformin, ivermectin, and fluvoxamine to prevent severe COVID-19 infection in nonhospitalized adults aged 30-85 years. A total of 1,431 patients at six U.S. sites were enrolled within 3 days of a confirmed infection and less than 7 days after the start of symptoms, then randomized to one of six groups: metformin plus fluvoxamine; metformin plus ivermectin; metformin plus placebo; placebo plus fluvoxamine; placebo plus ivermectin; and placebo plus placebo.
A total of 1,323 patients were included in the primary analysis. The median age of the patients was 46 years, 56% were female (of whom 6% were pregnant), and all individuals met criteria for overweight or obesity. About half (52%) of the patients had been vaccinated against COVID-19.
The primary endpoint was a composite of hypoxemia, ED visit, hospitalization, or death. The analyses were adjusted for COVID-19 vaccination and other trial medications. Overall, the adjusted odds ratios of any primary event, compared with placebo, was 0.84 for metformin (P = .19), 1.05 for ivermectin (P = .78), and 0.94 for fluvoxamine (P = .75).
The researchers also conducted a prespecified secondary analysis of components of the primary endpoint. In this analysis, the aORs for an ED visit, hospitalization, or death was 0.58 for metformin, 1.39 for ivermectin, and 1.17 for fluvoxamine. The aORs for hospitalization or death were 0.47, 0.73, and 1.11 for metformin, ivermectin, and fluvoxamine, respectively. No medication-related serious adverse events were reported with any of the drugs during the study period.
The possible benefit for prevention of severe COVID-19 with metformin was a prespecified secondary endpoint, and therefore not definitive until more research has been completed, the researchers said. Metformin has demonstrated anti-inflammatory actions in previous studies, and has shown protective effects against COVID-19 lung injury in animal studies.
Previous observational studies also have shown an association between metformin use and less severe COVID-19 in patients already taking metformin. “The proposed mechanisms of action against COVID-19 for metformin include anti-inflammatory and antiviral activity and the prevention of hyperglycemia during acute illness,” they added.
The study findings were limited by several factors including the population age range and focus on overweight and obese patients, which may limit generalizability, the researchers noted. Other limitations include the disproportionately small percentage of Black and Latino patients and the potential lack of accuracy in identifying hypoxemia via home oxygen monitors.
However, the results demonstrate that none of the three repurposed drugs – metformin, ivermectin, and fluvoxamine – prevented primary events or reduced symptom severity in COVID-19, compared with placebos, the researchers concluded.
“Metformin had several streams of evidence supporting its use: in vitro, in silico [computer modeled], observational, and in tissue. We were not surprised to see that it reduced emergency department visits, hospitalization, and death,” Dr. Bramante said in an interview.
The take-home message for clinicians is to continue to look to guideline committees for direction on COVID-19 treatments, but to continue to consider metformin along with other treatments, she said.
“All research should be replicated, whether the primary outcome is positive or negative,” Dr. Bramante emphasized. “In this case, when our positive outcome was negative and secondary outcome was positive, a confirmatory trial for metformin is particularly important.”
Ineffective drugs are inefficient use of resources
“The results of the COVID-OUT trial provide persuasive additional data that increase the confidence and degree of certainty that fluvoxamine and ivermectin are not effective in preventing progression to severe disease,” wrote Salim S. Abdool Karim, MB, and Nikita Devnarain, PhD, of the Centre for the AIDS Programme of Research in South Africa, Durban, in an accompanying editorial.
At the start of the study, in 2020, data on the use of the three drugs to prevent severe COVID-19 were “either unavailable or equivocal,” they said. Since then, accumulating data support the current study findings of the nonefficacy of ivermectin and fluvoxamine, and the World Health Organization has advised against their use for COVID-19, although the WHO has not provided guidance for the use of metformin.
The authors called on clinicians to stop using ivermectin and fluvoxamine to treat COVID-19 patients.
“With respect to clinical decisions about COVID-19 treatment, some drug choices, especially those that have negative [World Health Organization] recommendations, are clearly wrong,” they wrote. “In keeping with evidence-based medical practice, patients with COVID-19 must be treated with efficacious medications; they deserve nothing less.”
The study was supported by the Parsemus Foundation, Rainwater Charitable Foundation, Fast Grants, and UnitedHealth Group Foundation. The fluvoxamine placebo tablets were donated by Apotex Pharmaceuticals. The ivermectin placebo and active tablets were donated by Edenbridge Pharmaceuticals. Lead author Dr. Bramante was supported the National Center for Advancing Translational Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers had no financial conflicts to disclose. Dr. Abdool Karim serves as a member of the World Health Organization Science Council. Dr. Devnarain had no financial conflicts to disclose.
Neither metformin, ivermectin, or fluvoxamine had any impact on reducing disease severity, hospitalization, or death from COVID-19, according to results from more than 1,000 overweight or obese adult patients in the COVID-OUT randomized trial.
However, metformin showed some potential in a secondary analysis.
Early treatment to prevent severe disease remains a goal in managing the ongoing COVID-19 pandemic, and biophysical modeling suggested that metformin, ivermectin, and fluvoxamine may serve as antivirals to help reduce severe disease in COVID-19 patients, Carolyn T. Bramante, MD, of the University of Minnesota, Minneapolis, and colleagues wrote.
“We started enrolling patients at the end of December 2020,” Dr. Bramante said in an interview. “At that time, even though vaccine data were coming out, we thought it was important to test early outpatient treatment with widely available safe medications with no interactions, because the virus would evolve and vaccine availability may be limited.”
In a study published in the New England Journal of Medicine, the researchers used a two-by-three factorial design to test the ability of metformin, ivermectin, and fluvoxamine to prevent severe COVID-19 infection in nonhospitalized adults aged 30-85 years. A total of 1,431 patients at six U.S. sites were enrolled within 3 days of a confirmed infection and less than 7 days after the start of symptoms, then randomized to one of six groups: metformin plus fluvoxamine; metformin plus ivermectin; metformin plus placebo; placebo plus fluvoxamine; placebo plus ivermectin; and placebo plus placebo.
A total of 1,323 patients were included in the primary analysis. The median age of the patients was 46 years, 56% were female (of whom 6% were pregnant), and all individuals met criteria for overweight or obesity. About half (52%) of the patients had been vaccinated against COVID-19.
The primary endpoint was a composite of hypoxemia, ED visit, hospitalization, or death. The analyses were adjusted for COVID-19 vaccination and other trial medications. Overall, the adjusted odds ratios of any primary event, compared with placebo, was 0.84 for metformin (P = .19), 1.05 for ivermectin (P = .78), and 0.94 for fluvoxamine (P = .75).
The researchers also conducted a prespecified secondary analysis of components of the primary endpoint. In this analysis, the aORs for an ED visit, hospitalization, or death was 0.58 for metformin, 1.39 for ivermectin, and 1.17 for fluvoxamine. The aORs for hospitalization or death were 0.47, 0.73, and 1.11 for metformin, ivermectin, and fluvoxamine, respectively. No medication-related serious adverse events were reported with any of the drugs during the study period.
The possible benefit for prevention of severe COVID-19 with metformin was a prespecified secondary endpoint, and therefore not definitive until more research has been completed, the researchers said. Metformin has demonstrated anti-inflammatory actions in previous studies, and has shown protective effects against COVID-19 lung injury in animal studies.
Previous observational studies also have shown an association between metformin use and less severe COVID-19 in patients already taking metformin. “The proposed mechanisms of action against COVID-19 for metformin include anti-inflammatory and antiviral activity and the prevention of hyperglycemia during acute illness,” they added.
The study findings were limited by several factors including the population age range and focus on overweight and obese patients, which may limit generalizability, the researchers noted. Other limitations include the disproportionately small percentage of Black and Latino patients and the potential lack of accuracy in identifying hypoxemia via home oxygen monitors.
However, the results demonstrate that none of the three repurposed drugs – metformin, ivermectin, and fluvoxamine – prevented primary events or reduced symptom severity in COVID-19, compared with placebos, the researchers concluded.
“Metformin had several streams of evidence supporting its use: in vitro, in silico [computer modeled], observational, and in tissue. We were not surprised to see that it reduced emergency department visits, hospitalization, and death,” Dr. Bramante said in an interview.
The take-home message for clinicians is to continue to look to guideline committees for direction on COVID-19 treatments, but to continue to consider metformin along with other treatments, she said.
“All research should be replicated, whether the primary outcome is positive or negative,” Dr. Bramante emphasized. “In this case, when our positive outcome was negative and secondary outcome was positive, a confirmatory trial for metformin is particularly important.”
Ineffective drugs are inefficient use of resources
“The results of the COVID-OUT trial provide persuasive additional data that increase the confidence and degree of certainty that fluvoxamine and ivermectin are not effective in preventing progression to severe disease,” wrote Salim S. Abdool Karim, MB, and Nikita Devnarain, PhD, of the Centre for the AIDS Programme of Research in South Africa, Durban, in an accompanying editorial.
At the start of the study, in 2020, data on the use of the three drugs to prevent severe COVID-19 were “either unavailable or equivocal,” they said. Since then, accumulating data support the current study findings of the nonefficacy of ivermectin and fluvoxamine, and the World Health Organization has advised against their use for COVID-19, although the WHO has not provided guidance for the use of metformin.
The authors called on clinicians to stop using ivermectin and fluvoxamine to treat COVID-19 patients.
“With respect to clinical decisions about COVID-19 treatment, some drug choices, especially those that have negative [World Health Organization] recommendations, are clearly wrong,” they wrote. “In keeping with evidence-based medical practice, patients with COVID-19 must be treated with efficacious medications; they deserve nothing less.”
The study was supported by the Parsemus Foundation, Rainwater Charitable Foundation, Fast Grants, and UnitedHealth Group Foundation. The fluvoxamine placebo tablets were donated by Apotex Pharmaceuticals. The ivermectin placebo and active tablets were donated by Edenbridge Pharmaceuticals. Lead author Dr. Bramante was supported the National Center for Advancing Translational Sciences and the National Institute of Diabetes and Digestive and Kidney Diseases. The researchers had no financial conflicts to disclose. Dr. Abdool Karim serves as a member of the World Health Organization Science Council. Dr. Devnarain had no financial conflicts to disclose.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Call for Neurology Papers
Federal Practitioner invites VA, DoD, and PHS health care professionals and researchers to contribute to a future special issue on neurology. Topics of interest include epilepsy, headache and migraine, COVID-19 and neurology, Alzheimer and dementia, MS, and other neurological disorders.
Interested authors should submit an abstract to [email protected] with the subject line “Neurology Special Issue” for consideration. Once the editorial team confirms the article is eligible for consideration, authors will be asked to submit their manuscript in full through Editorial Manager.
Federal Practitioner never charges authors or readers. All submissions undergo a double-blinded peer review before publication. Accepted manuscripts are always available for free online at www.mdedge.com/fedprac and on PubMed Central.
Federal Practitioner welcomes original research, commentaries, clinical reviews, program profiles, case reports, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
Federal Practitioner invites VA, DoD, and PHS health care professionals and researchers to contribute to a future special issue on neurology. Topics of interest include epilepsy, headache and migraine, COVID-19 and neurology, Alzheimer and dementia, MS, and other neurological disorders.
Interested authors should submit an abstract to [email protected] with the subject line “Neurology Special Issue” for consideration. Once the editorial team confirms the article is eligible for consideration, authors will be asked to submit their manuscript in full through Editorial Manager.
Federal Practitioner never charges authors or readers. All submissions undergo a double-blinded peer review before publication. Accepted manuscripts are always available for free online at www.mdedge.com/fedprac and on PubMed Central.
Federal Practitioner welcomes original research, commentaries, clinical reviews, program profiles, case reports, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
Federal Practitioner invites VA, DoD, and PHS health care professionals and researchers to contribute to a future special issue on neurology. Topics of interest include epilepsy, headache and migraine, COVID-19 and neurology, Alzheimer and dementia, MS, and other neurological disorders.
Interested authors should submit an abstract to [email protected] with the subject line “Neurology Special Issue” for consideration. Once the editorial team confirms the article is eligible for consideration, authors will be asked to submit their manuscript in full through Editorial Manager.
Federal Practitioner never charges authors or readers. All submissions undergo a double-blinded peer review before publication. Accepted manuscripts are always available for free online at www.mdedge.com/fedprac and on PubMed Central.
Federal Practitioner welcomes original research, commentaries, clinical reviews, program profiles, case reports, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
Blood biomarkers predict TBI disability and mortality
, new research suggests.
In new data from the TRACK-TBI study group, high levels of glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) proteins found in glial cells and neurons, respectively, correlated with death and severe injury. Investigators note that measuring these biomarkers may give a more accurate assessment of a patient’s prognosis following TBI.
This study is the “first report of the accuracy of a blood test that can be obtained rapidly on the day of injury to predict neurological recovery at 6 months after injury,” lead author Frederick Korley, MD, PhD, associate professor of emergency medicine at the University of Michigan, Ann Arbor, said in a news release.
The findings were published online in the Lancet Neurology.
Added value
The researchers measured GFAP and UCH-L1 in blood samples taken from 1,696 patients with TBI on the day of their injury, and they assessed patient recovery 6 months later.
The markers were measured using the i-STAT TBI Plasma test (Abbott Labs). The test was approved in 2021 by the U.S. Food and Drug Administration to determine which patients with mild TBI should undergo computed tomography scans.
About two-thirds of the study population were men, and the average age was 39 years. All patients were evaluated at Level I trauma centers for injuries caused primarily by traffic accidents or falls.
Six months following injury, 7% of the patients had died and 14% had an unfavorable outcome, ranging from vegetative state to severe disability requiring daily support. In addition, 67% had incomplete recovery, ranging from moderate disabilities requiring assistance outside of the home to minor disabling neurological or psychological deficits.
Day-of-injury GFAP and UCH-L1 levels had a high probability of predicting death (87% for GFAP and 89% for UCH-L1) and severe disability (86% for both GFAP and UCH-L1) at 6 months, the investigators reported.
The biomarkers were less accurate in predicting incomplete recovery (62% for GFAP and 61% for UCH-L1).
The researchers also assessed the added value of combining the blood biomarkers to current TBI prognostic models that take into account variables such as age, motor score, pupil reactivity, and CT characteristics.
In patients with a Glasgow Coma Scale (GCS) score of 3-12, adding GFAP and UCH-L1 alone or combined to each of the three International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) models significantly increased their accuracy for predicting death (range, 90%-94%) and unfavorable outcome (range, 83%-89%).
In patients with milder TBI (GCS score, 13-15), adding GFAP and UCH-L1 to the UPFRONT prognostic model modestly increased accuracy for predicting incomplete recovery (69%).
‘Important’ findings
Commenting on the study, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said this “critical” study shows that these biomarkers can “predict key outcomes,” including mortality and severe disability. “Thus, in conjunction with clinical evaluations and related data such as neuroimaging, these tests may warrant translation to broader clinical practice, particularly in acute settings,” said Dr. Raji, who was not involved in the research.
Also weighing in, Heidi Fusco, MD, assistant director of the traumatic brain injury program at NYU Langone Rusk Rehabilitation, said the findings are “important.”
“Prognosis after brain injury often is based on the initial presentation, ongoing clinical exams, and neuroimaging; and the addition of biomarkers would contribute to creating a more objective prognostic model,” Dr. Fusco said.
She noted “it’s unclear” whether clinical hospital laboratories would be able to accommodate this type of laboratory drawing.
“It is imperative that clinicians still use the patient history [and] clinical and radiological exam when making clinical decisions for a patient and not just lab values. It would be best to incorporate the GFAP and UCH-L1 into a preexisting prognostic model,” Dr. Fusco said.
The study was funded by the U.S. National Institutes of Health, the National Institute of Neurologic Disorders and Stroke, the U.S. Department of Defense, One Mind, and U.S. Army Medical Research and Development Command. Dr. Korley reported having previously consulted for Abbott Laboratories and has received research funding from Abbott Laboratories, which makes the assays used in the study. Dr. Raji is a consultant for Brainreader ApS and Neurevolution. Dr. Fusco has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In new data from the TRACK-TBI study group, high levels of glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) proteins found in glial cells and neurons, respectively, correlated with death and severe injury. Investigators note that measuring these biomarkers may give a more accurate assessment of a patient’s prognosis following TBI.
This study is the “first report of the accuracy of a blood test that can be obtained rapidly on the day of injury to predict neurological recovery at 6 months after injury,” lead author Frederick Korley, MD, PhD, associate professor of emergency medicine at the University of Michigan, Ann Arbor, said in a news release.
The findings were published online in the Lancet Neurology.
Added value
The researchers measured GFAP and UCH-L1 in blood samples taken from 1,696 patients with TBI on the day of their injury, and they assessed patient recovery 6 months later.
The markers were measured using the i-STAT TBI Plasma test (Abbott Labs). The test was approved in 2021 by the U.S. Food and Drug Administration to determine which patients with mild TBI should undergo computed tomography scans.
About two-thirds of the study population were men, and the average age was 39 years. All patients were evaluated at Level I trauma centers for injuries caused primarily by traffic accidents or falls.
Six months following injury, 7% of the patients had died and 14% had an unfavorable outcome, ranging from vegetative state to severe disability requiring daily support. In addition, 67% had incomplete recovery, ranging from moderate disabilities requiring assistance outside of the home to minor disabling neurological or psychological deficits.
Day-of-injury GFAP and UCH-L1 levels had a high probability of predicting death (87% for GFAP and 89% for UCH-L1) and severe disability (86% for both GFAP and UCH-L1) at 6 months, the investigators reported.
The biomarkers were less accurate in predicting incomplete recovery (62% for GFAP and 61% for UCH-L1).
The researchers also assessed the added value of combining the blood biomarkers to current TBI prognostic models that take into account variables such as age, motor score, pupil reactivity, and CT characteristics.
In patients with a Glasgow Coma Scale (GCS) score of 3-12, adding GFAP and UCH-L1 alone or combined to each of the three International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) models significantly increased their accuracy for predicting death (range, 90%-94%) and unfavorable outcome (range, 83%-89%).
In patients with milder TBI (GCS score, 13-15), adding GFAP and UCH-L1 to the UPFRONT prognostic model modestly increased accuracy for predicting incomplete recovery (69%).
‘Important’ findings
Commenting on the study, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said this “critical” study shows that these biomarkers can “predict key outcomes,” including mortality and severe disability. “Thus, in conjunction with clinical evaluations and related data such as neuroimaging, these tests may warrant translation to broader clinical practice, particularly in acute settings,” said Dr. Raji, who was not involved in the research.
Also weighing in, Heidi Fusco, MD, assistant director of the traumatic brain injury program at NYU Langone Rusk Rehabilitation, said the findings are “important.”
“Prognosis after brain injury often is based on the initial presentation, ongoing clinical exams, and neuroimaging; and the addition of biomarkers would contribute to creating a more objective prognostic model,” Dr. Fusco said.
She noted “it’s unclear” whether clinical hospital laboratories would be able to accommodate this type of laboratory drawing.
“It is imperative that clinicians still use the patient history [and] clinical and radiological exam when making clinical decisions for a patient and not just lab values. It would be best to incorporate the GFAP and UCH-L1 into a preexisting prognostic model,” Dr. Fusco said.
The study was funded by the U.S. National Institutes of Health, the National Institute of Neurologic Disorders and Stroke, the U.S. Department of Defense, One Mind, and U.S. Army Medical Research and Development Command. Dr. Korley reported having previously consulted for Abbott Laboratories and has received research funding from Abbott Laboratories, which makes the assays used in the study. Dr. Raji is a consultant for Brainreader ApS and Neurevolution. Dr. Fusco has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In new data from the TRACK-TBI study group, high levels of glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) proteins found in glial cells and neurons, respectively, correlated with death and severe injury. Investigators note that measuring these biomarkers may give a more accurate assessment of a patient’s prognosis following TBI.
This study is the “first report of the accuracy of a blood test that can be obtained rapidly on the day of injury to predict neurological recovery at 6 months after injury,” lead author Frederick Korley, MD, PhD, associate professor of emergency medicine at the University of Michigan, Ann Arbor, said in a news release.
The findings were published online in the Lancet Neurology.
Added value
The researchers measured GFAP and UCH-L1 in blood samples taken from 1,696 patients with TBI on the day of their injury, and they assessed patient recovery 6 months later.
The markers were measured using the i-STAT TBI Plasma test (Abbott Labs). The test was approved in 2021 by the U.S. Food and Drug Administration to determine which patients with mild TBI should undergo computed tomography scans.
About two-thirds of the study population were men, and the average age was 39 years. All patients were evaluated at Level I trauma centers for injuries caused primarily by traffic accidents or falls.
Six months following injury, 7% of the patients had died and 14% had an unfavorable outcome, ranging from vegetative state to severe disability requiring daily support. In addition, 67% had incomplete recovery, ranging from moderate disabilities requiring assistance outside of the home to minor disabling neurological or psychological deficits.
Day-of-injury GFAP and UCH-L1 levels had a high probability of predicting death (87% for GFAP and 89% for UCH-L1) and severe disability (86% for both GFAP and UCH-L1) at 6 months, the investigators reported.
The biomarkers were less accurate in predicting incomplete recovery (62% for GFAP and 61% for UCH-L1).
The researchers also assessed the added value of combining the blood biomarkers to current TBI prognostic models that take into account variables such as age, motor score, pupil reactivity, and CT characteristics.
In patients with a Glasgow Coma Scale (GCS) score of 3-12, adding GFAP and UCH-L1 alone or combined to each of the three International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) models significantly increased their accuracy for predicting death (range, 90%-94%) and unfavorable outcome (range, 83%-89%).
In patients with milder TBI (GCS score, 13-15), adding GFAP and UCH-L1 to the UPFRONT prognostic model modestly increased accuracy for predicting incomplete recovery (69%).
‘Important’ findings
Commenting on the study, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said this “critical” study shows that these biomarkers can “predict key outcomes,” including mortality and severe disability. “Thus, in conjunction with clinical evaluations and related data such as neuroimaging, these tests may warrant translation to broader clinical practice, particularly in acute settings,” said Dr. Raji, who was not involved in the research.
Also weighing in, Heidi Fusco, MD, assistant director of the traumatic brain injury program at NYU Langone Rusk Rehabilitation, said the findings are “important.”
“Prognosis after brain injury often is based on the initial presentation, ongoing clinical exams, and neuroimaging; and the addition of biomarkers would contribute to creating a more objective prognostic model,” Dr. Fusco said.
She noted “it’s unclear” whether clinical hospital laboratories would be able to accommodate this type of laboratory drawing.
“It is imperative that clinicians still use the patient history [and] clinical and radiological exam when making clinical decisions for a patient and not just lab values. It would be best to incorporate the GFAP and UCH-L1 into a preexisting prognostic model,” Dr. Fusco said.
The study was funded by the U.S. National Institutes of Health, the National Institute of Neurologic Disorders and Stroke, the U.S. Department of Defense, One Mind, and U.S. Army Medical Research and Development Command. Dr. Korley reported having previously consulted for Abbott Laboratories and has received research funding from Abbott Laboratories, which makes the assays used in the study. Dr. Raji is a consultant for Brainreader ApS and Neurevolution. Dr. Fusco has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET NEUROLOGY