User login
Be vigilant about suspected cases of measles, expert advises
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
EoE: One-food elimination works as well as six-food elimination
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
FROM THE LANCET GASTROENTEROLOGY AND HEPATOLOGY
The SHOW UP Act Threatens VA Telehealth
In February, the US House of Representatives hurriedly passed the Stopping Home Office Work’s Unproductive Problems (SHOW UP) Act, H.R. 139, a bill that calls into question the contributions of federal employees allowed to work from home and resets telework policies to those in place in 2019. Its author, House Oversight Committee Chairman James Comer (R, Kentucky) claimed that this change was necessary because the expansion of federal telework during the COVID-19 pandemic “has crippled the ability of agencies to get their jobs done and created backlogs.” His targets included the US Department of Veterans Affairs (VA), where, he charged, “veterans have been unable…to obtain care they have earned.” He added, “it’s hard to argue that teleworking has helped the VA.”
While oversight of government programs is an authority of Congress, the SHOW UP Act is based on unsubstantiated assumptions of dereliction. It also disregards the devastating impact the proposed changes will have on veterans’ ability to receive care and inaccurately implies improving it. As the Senate considers the bill, they should take heed of these and other facts involving this often misunderstood form of labor.
COVID-19 irrevocably transformed the use of virtual care within the VA and across the world. Even as the pandemic subsides, public and private health care systems have continued to use telework-centered telehealth far above prepandemic levels, especially for mental health and primary care. Employers, including the VA, capitalize on telework for its benefits to both consumers and the workforce. For consumers, research supports the clinical effectiveness of telemental health service, as well as its cost-effectiveness and consumer satisfaction. On the workforce side, research has documented heightened productivity, lower distractibility, and higher job satisfaction among counselors who shifted to remote work.
Remote work also serves as a key tool in attracting and retaining a qualified workforce. As one VA service chief explained, “I am having enough trouble competing with the private sector, where extensive telework is now the norm. If telework options were rolled back, the private sector will have a field day picking off my best staff.” These comments are consistent with the data. McKinsey’s American Opportunity Survey shows that Americans have embraced remote work and want more of it. Recent data from Gallup show that 6 of 10 currently exclusively remote employees would be extremely likely to change companies if they lost their remote flexibility. Further, Gallup data show that when an employee’s location preference does not match their current work location, burnout rises, and engagement drops.
Between 2019 and 2023, the VA’s telework expansion is what has enabled it to meet the growing demand for mental health services. VA is keeping pace by having 2 or more clinicians rotate between home and a shared VA office. Forcing these hybrid practitioners to work full time at VA facilities would drastically reduce the number of patients they can care for. There simply are not enough offices on crammed VA grounds to house staff who telework today. The net result would be that fewer appointments would be available, creating longer wait times. And that is just for existing patients. It does not factor in the expected influx due to new veteran eligibility made possible by the toxic exposures PACT Act.
Here is another good example of crucial VA telework: With the advent of the 988 Suicide & Crisis Lifeline, VA is adding more than 1000 new Veterans Crisis Line responders. All these new positions are remote. The SHOW UP Act would inhibit this expansion of lifesaving programs.
Veterans want more, not fewer, telehealth options. At a House Committee on Veterans’ Affairs hearing this past September, the VA reported that most veterans would prefer to receive mental health services virtually than to have to commute to a VA medical center or clinic. Telehealth benefits veterans in meaningful ways, including that it reduces their travel time, travel expense, depletion of sick leave, and need for childcare. Veterans with posttraumatic stress disorder, military sexual trauma, those with mobility issues, or those who struggle with the stigma of mental health treatment may prefer the familiarity of their own homes for care. Virtual options also relieve a patient’s need to enter a hospital and be unnecessarily exposed to contagious viruses. That’s safer not only for veterans but also for VA staff.
Finally, virtual care improves treatment. Research has revealed that the likelihood of missing telehealth appointments is lower than for in-person appointments. When patients miss appointments, continuity of care is disrupted, and health care outcomes are diminished.
The pandemic is receding, but the advantages of telework-centered virtual care are greater than ever. Political representatives who want to show up for veterans should do everything in their power to expand—not cut—VA’s ability to authorize working from home.
In February, the US House of Representatives hurriedly passed the Stopping Home Office Work’s Unproductive Problems (SHOW UP) Act, H.R. 139, a bill that calls into question the contributions of federal employees allowed to work from home and resets telework policies to those in place in 2019. Its author, House Oversight Committee Chairman James Comer (R, Kentucky) claimed that this change was necessary because the expansion of federal telework during the COVID-19 pandemic “has crippled the ability of agencies to get their jobs done and created backlogs.” His targets included the US Department of Veterans Affairs (VA), where, he charged, “veterans have been unable…to obtain care they have earned.” He added, “it’s hard to argue that teleworking has helped the VA.”
While oversight of government programs is an authority of Congress, the SHOW UP Act is based on unsubstantiated assumptions of dereliction. It also disregards the devastating impact the proposed changes will have on veterans’ ability to receive care and inaccurately implies improving it. As the Senate considers the bill, they should take heed of these and other facts involving this often misunderstood form of labor.
COVID-19 irrevocably transformed the use of virtual care within the VA and across the world. Even as the pandemic subsides, public and private health care systems have continued to use telework-centered telehealth far above prepandemic levels, especially for mental health and primary care. Employers, including the VA, capitalize on telework for its benefits to both consumers and the workforce. For consumers, research supports the clinical effectiveness of telemental health service, as well as its cost-effectiveness and consumer satisfaction. On the workforce side, research has documented heightened productivity, lower distractibility, and higher job satisfaction among counselors who shifted to remote work.
Remote work also serves as a key tool in attracting and retaining a qualified workforce. As one VA service chief explained, “I am having enough trouble competing with the private sector, where extensive telework is now the norm. If telework options were rolled back, the private sector will have a field day picking off my best staff.” These comments are consistent with the data. McKinsey’s American Opportunity Survey shows that Americans have embraced remote work and want more of it. Recent data from Gallup show that 6 of 10 currently exclusively remote employees would be extremely likely to change companies if they lost their remote flexibility. Further, Gallup data show that when an employee’s location preference does not match their current work location, burnout rises, and engagement drops.
Between 2019 and 2023, the VA’s telework expansion is what has enabled it to meet the growing demand for mental health services. VA is keeping pace by having 2 or more clinicians rotate between home and a shared VA office. Forcing these hybrid practitioners to work full time at VA facilities would drastically reduce the number of patients they can care for. There simply are not enough offices on crammed VA grounds to house staff who telework today. The net result would be that fewer appointments would be available, creating longer wait times. And that is just for existing patients. It does not factor in the expected influx due to new veteran eligibility made possible by the toxic exposures PACT Act.
Here is another good example of crucial VA telework: With the advent of the 988 Suicide & Crisis Lifeline, VA is adding more than 1000 new Veterans Crisis Line responders. All these new positions are remote. The SHOW UP Act would inhibit this expansion of lifesaving programs.
Veterans want more, not fewer, telehealth options. At a House Committee on Veterans’ Affairs hearing this past September, the VA reported that most veterans would prefer to receive mental health services virtually than to have to commute to a VA medical center or clinic. Telehealth benefits veterans in meaningful ways, including that it reduces their travel time, travel expense, depletion of sick leave, and need for childcare. Veterans with posttraumatic stress disorder, military sexual trauma, those with mobility issues, or those who struggle with the stigma of mental health treatment may prefer the familiarity of their own homes for care. Virtual options also relieve a patient’s need to enter a hospital and be unnecessarily exposed to contagious viruses. That’s safer not only for veterans but also for VA staff.
Finally, virtual care improves treatment. Research has revealed that the likelihood of missing telehealth appointments is lower than for in-person appointments. When patients miss appointments, continuity of care is disrupted, and health care outcomes are diminished.
The pandemic is receding, but the advantages of telework-centered virtual care are greater than ever. Political representatives who want to show up for veterans should do everything in their power to expand—not cut—VA’s ability to authorize working from home.
In February, the US House of Representatives hurriedly passed the Stopping Home Office Work’s Unproductive Problems (SHOW UP) Act, H.R. 139, a bill that calls into question the contributions of federal employees allowed to work from home and resets telework policies to those in place in 2019. Its author, House Oversight Committee Chairman James Comer (R, Kentucky) claimed that this change was necessary because the expansion of federal telework during the COVID-19 pandemic “has crippled the ability of agencies to get their jobs done and created backlogs.” His targets included the US Department of Veterans Affairs (VA), where, he charged, “veterans have been unable…to obtain care they have earned.” He added, “it’s hard to argue that teleworking has helped the VA.”
While oversight of government programs is an authority of Congress, the SHOW UP Act is based on unsubstantiated assumptions of dereliction. It also disregards the devastating impact the proposed changes will have on veterans’ ability to receive care and inaccurately implies improving it. As the Senate considers the bill, they should take heed of these and other facts involving this often misunderstood form of labor.
COVID-19 irrevocably transformed the use of virtual care within the VA and across the world. Even as the pandemic subsides, public and private health care systems have continued to use telework-centered telehealth far above prepandemic levels, especially for mental health and primary care. Employers, including the VA, capitalize on telework for its benefits to both consumers and the workforce. For consumers, research supports the clinical effectiveness of telemental health service, as well as its cost-effectiveness and consumer satisfaction. On the workforce side, research has documented heightened productivity, lower distractibility, and higher job satisfaction among counselors who shifted to remote work.
Remote work also serves as a key tool in attracting and retaining a qualified workforce. As one VA service chief explained, “I am having enough trouble competing with the private sector, where extensive telework is now the norm. If telework options were rolled back, the private sector will have a field day picking off my best staff.” These comments are consistent with the data. McKinsey’s American Opportunity Survey shows that Americans have embraced remote work and want more of it. Recent data from Gallup show that 6 of 10 currently exclusively remote employees would be extremely likely to change companies if they lost their remote flexibility. Further, Gallup data show that when an employee’s location preference does not match their current work location, burnout rises, and engagement drops.
Between 2019 and 2023, the VA’s telework expansion is what has enabled it to meet the growing demand for mental health services. VA is keeping pace by having 2 or more clinicians rotate between home and a shared VA office. Forcing these hybrid practitioners to work full time at VA facilities would drastically reduce the number of patients they can care for. There simply are not enough offices on crammed VA grounds to house staff who telework today. The net result would be that fewer appointments would be available, creating longer wait times. And that is just for existing patients. It does not factor in the expected influx due to new veteran eligibility made possible by the toxic exposures PACT Act.
Here is another good example of crucial VA telework: With the advent of the 988 Suicide & Crisis Lifeline, VA is adding more than 1000 new Veterans Crisis Line responders. All these new positions are remote. The SHOW UP Act would inhibit this expansion of lifesaving programs.
Veterans want more, not fewer, telehealth options. At a House Committee on Veterans’ Affairs hearing this past September, the VA reported that most veterans would prefer to receive mental health services virtually than to have to commute to a VA medical center or clinic. Telehealth benefits veterans in meaningful ways, including that it reduces their travel time, travel expense, depletion of sick leave, and need for childcare. Veterans with posttraumatic stress disorder, military sexual trauma, those with mobility issues, or those who struggle with the stigma of mental health treatment may prefer the familiarity of their own homes for care. Virtual options also relieve a patient’s need to enter a hospital and be unnecessarily exposed to contagious viruses. That’s safer not only for veterans but also for VA staff.
Finally, virtual care improves treatment. Research has revealed that the likelihood of missing telehealth appointments is lower than for in-person appointments. When patients miss appointments, continuity of care is disrupted, and health care outcomes are diminished.
The pandemic is receding, but the advantages of telework-centered virtual care are greater than ever. Political representatives who want to show up for veterans should do everything in their power to expand—not cut—VA’s ability to authorize working from home.
COORDINATEd effort boosts optimal therapy in patients with T2D and ASCVD
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
AT ACC 2023
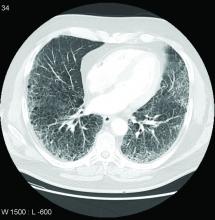
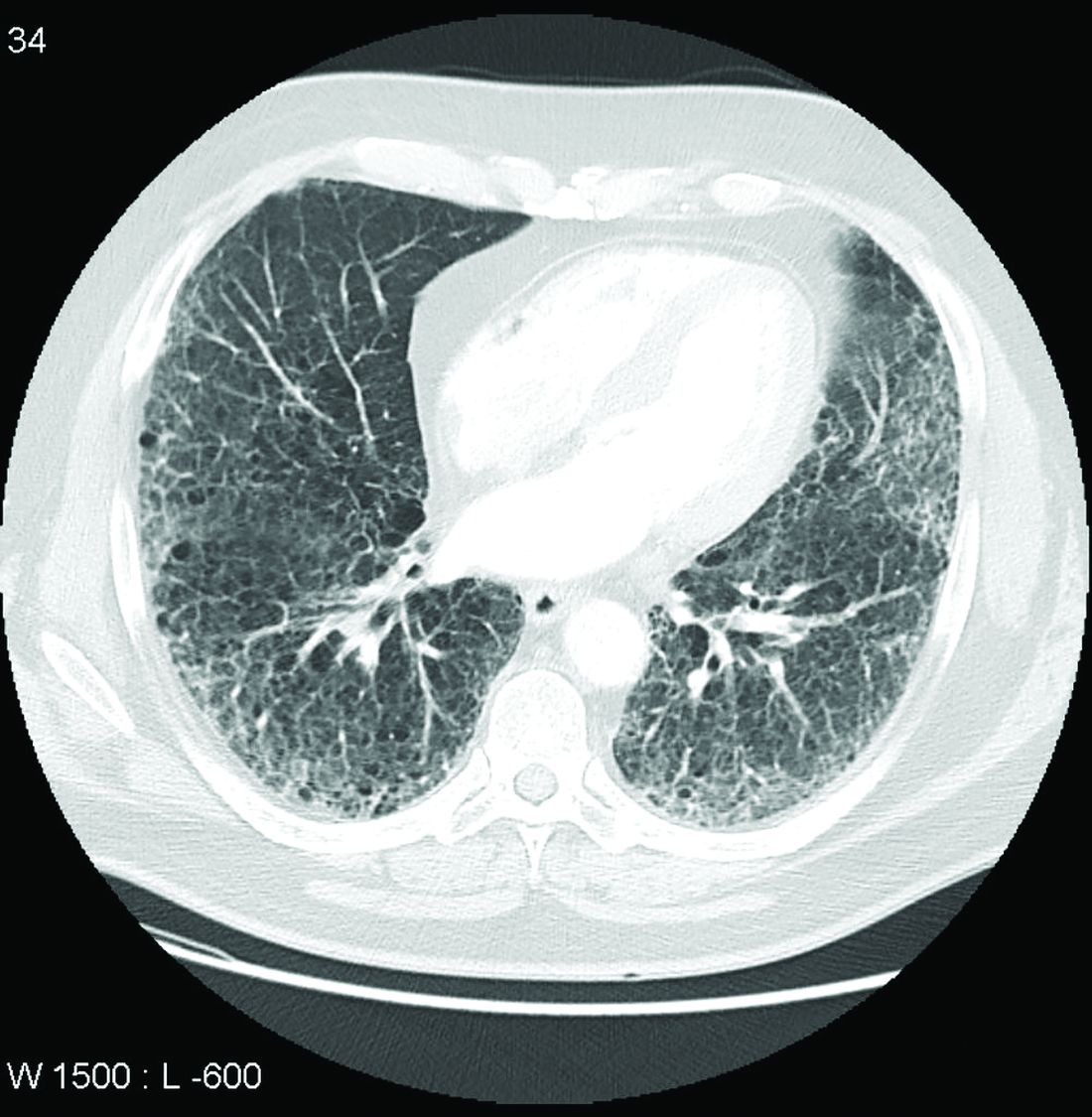
Call it preclinical or subclinical, ILD in RA needs to be tracked
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CLL treatment: More infections among real-world patients
For example, “the rate of severe infection for ibrutinib in clinical trials ranged from 12.8% to 45% with median follow-up ranging from 27 to 65 months. In our study, the rate of severe infection was 45.3% within a shorter median follow-up period of 23.3 months,” said study lead author Amanda Tey, MPharm, a hematology pharmacist with Monash Health in Clayton, Australia, in an interview.
The results suggest that “real-world severe infection risk is higher than previously appreciated,” said Ms. Tey, whose findings were published in the European Journal of Hematology. “Poor performance status and a high comorbidity burden further increase this risk.”
According to the study, there are limited data about real-world infection rates for patients with CLL or B-cell lymphoma who take the three drugs.
Both the underlying blood cancer and the drugs themselves may disrupt the immune system in these patients, Ms. Tey noted. “Ibrutinib inhibits interleukin-2-inducible T-cell kinase, which has a role in T-cell maturation. Idelalisib reduces regulatory T-cell activity and natural killer cell and neutrophil inflammatory responses. Venetoclax is associated with a high rate of neutropenia.”
For the new retrospective, single-center study, researchers tracked adult patients who’d received the drugs from 2014 to 2021 in a hospital network serving 1.5 million people in the Australian state of Victoria. The primary outcome was severe infection of grade 3 or higher. Patients were excluded for such factors as having been primarily treated at other facilities, receiving less than 30 days of treatment, or having been treated for other indications such as primary central nervous system lymphoma.
Of the 67 patients in the study, the numbers taking the drugs were 53 (ibrutinib), 8 (idelalisib), and 6 (venetoclax). Eleven patients took more than one drug. Median age was 73 years, and 73% of patients were male.
Patients spent a median 23.3, 4.8, and 3.5 months taking ibrutinib, idelalisib, and venetoclax, respectively, before treatment stopped or data were collected. Patients were commonly prescribed antimicrobials to prevent pneumocystis jirovecii pneumonia and herpes simplex virus (HSV)/varicella zoster virus (VZV) infection.
Researchers found that 48% of the patients had at least one serious infection: 45% of those on ibrutinib, 63% of those on idelalisib, and 50% of those on venetoclax. Seven patients died of infections.
In comparison, the researchers reported, a systematic review of idelalisib in blood cancer clinical trials reported an overall infection rate of 28%, while clinical trials reported an infection rate of 17.5%-22% in patients taking venetoclax for CLL.
Poor performance status and higher levels of comorbidity were linked to higher risk of infection, and infections occurred at a median of 5.4 months.
Lead author Ms. Tey highlighted the fact that most of the patients in the new study had relapsed/refractory disease. The infection risk in the real-world first-line setting is unknown, she said. “Furthermore, due to the size of our study and high uptake of antimicrobial prophylaxis, the optimal prophylaxis strategy for these patients remains unclear.”
In an interview, infectious disease physician Gemma Reynolds, MChD, MPH, of Austin Health and Peter MacCallum Cancer Center in Melbourne, said the study findings reflect “a lot of what we know from other observational studies and clinical practice. There is a risk of infection, and serious infection, associated with these agents. Sometimes the pathogen is classically opportunistic, but often it is bacterial, and respiratory sites are common. Infections often occur early into a course of therapy.”
Dr. Reynolds, who didn’t take part in the study, urged colleagues to cast a wide net if a patient appears to have an infection but doesn’t respond to conventional therapies such as antibiotics. “Unusual infections are possible,” she said, and aggressive early workups may be advisable via blood cultures, viral swabs, sputum culture, early imaging, bronchoscopy, and preemptive monitoring in patients with a prior infection history with a disease such as CMV.
Alessandra Ferrajoli, MD, a hematologist/oncologist at MD Anderson Cancer Center who also didn’t take part in the study, agreed in an interview that the findings reflect those found in other reports. “It should be highlighted that the population studied is at particular high risk for infections given the high proportion of patients with recurrent disease (85%), many patients with concurrent hypogammaglobulinemia (64%), and the patient median age of 73 years and a high comorbidities burden,” she said. “In my view, this explains the higher rate of infections reported in this study, when compared to other case series.”
Dr. Ferrajoli added that there’s no standard antimicrobial prophylaxis for patients with B-cell malignancies receiving targeted therapies. “Anti-HSV/VZV prophylaxis is commonly implemented. Additional antiviral, antimicrobial, and antifungal prophylaxis should be used based on patients’ absolute neutrophil and T-cell count and individual risk factors, including prior history of infections such as CMV, prior splenectomy, and history of invasive fungal infections.”
The study was funded by Monash Health, the National Health and Medical Research Council (Australia), and the Society of Hospital Pharmacists of Australia. Ms. Tey reported no disclosures. Some of the study authors reported multiple disclosures. Dr. Reynolds discloses a PhD scholarship from the National Health and Medical Research Council. Dr. Ferrajoli reported no disclosures.
For example, “the rate of severe infection for ibrutinib in clinical trials ranged from 12.8% to 45% with median follow-up ranging from 27 to 65 months. In our study, the rate of severe infection was 45.3% within a shorter median follow-up period of 23.3 months,” said study lead author Amanda Tey, MPharm, a hematology pharmacist with Monash Health in Clayton, Australia, in an interview.
The results suggest that “real-world severe infection risk is higher than previously appreciated,” said Ms. Tey, whose findings were published in the European Journal of Hematology. “Poor performance status and a high comorbidity burden further increase this risk.”
According to the study, there are limited data about real-world infection rates for patients with CLL or B-cell lymphoma who take the three drugs.
Both the underlying blood cancer and the drugs themselves may disrupt the immune system in these patients, Ms. Tey noted. “Ibrutinib inhibits interleukin-2-inducible T-cell kinase, which has a role in T-cell maturation. Idelalisib reduces regulatory T-cell activity and natural killer cell and neutrophil inflammatory responses. Venetoclax is associated with a high rate of neutropenia.”
For the new retrospective, single-center study, researchers tracked adult patients who’d received the drugs from 2014 to 2021 in a hospital network serving 1.5 million people in the Australian state of Victoria. The primary outcome was severe infection of grade 3 or higher. Patients were excluded for such factors as having been primarily treated at other facilities, receiving less than 30 days of treatment, or having been treated for other indications such as primary central nervous system lymphoma.
Of the 67 patients in the study, the numbers taking the drugs were 53 (ibrutinib), 8 (idelalisib), and 6 (venetoclax). Eleven patients took more than one drug. Median age was 73 years, and 73% of patients were male.
Patients spent a median 23.3, 4.8, and 3.5 months taking ibrutinib, idelalisib, and venetoclax, respectively, before treatment stopped or data were collected. Patients were commonly prescribed antimicrobials to prevent pneumocystis jirovecii pneumonia and herpes simplex virus (HSV)/varicella zoster virus (VZV) infection.
Researchers found that 48% of the patients had at least one serious infection: 45% of those on ibrutinib, 63% of those on idelalisib, and 50% of those on venetoclax. Seven patients died of infections.
In comparison, the researchers reported, a systematic review of idelalisib in blood cancer clinical trials reported an overall infection rate of 28%, while clinical trials reported an infection rate of 17.5%-22% in patients taking venetoclax for CLL.
Poor performance status and higher levels of comorbidity were linked to higher risk of infection, and infections occurred at a median of 5.4 months.
Lead author Ms. Tey highlighted the fact that most of the patients in the new study had relapsed/refractory disease. The infection risk in the real-world first-line setting is unknown, she said. “Furthermore, due to the size of our study and high uptake of antimicrobial prophylaxis, the optimal prophylaxis strategy for these patients remains unclear.”
In an interview, infectious disease physician Gemma Reynolds, MChD, MPH, of Austin Health and Peter MacCallum Cancer Center in Melbourne, said the study findings reflect “a lot of what we know from other observational studies and clinical practice. There is a risk of infection, and serious infection, associated with these agents. Sometimes the pathogen is classically opportunistic, but often it is bacterial, and respiratory sites are common. Infections often occur early into a course of therapy.”
Dr. Reynolds, who didn’t take part in the study, urged colleagues to cast a wide net if a patient appears to have an infection but doesn’t respond to conventional therapies such as antibiotics. “Unusual infections are possible,” she said, and aggressive early workups may be advisable via blood cultures, viral swabs, sputum culture, early imaging, bronchoscopy, and preemptive monitoring in patients with a prior infection history with a disease such as CMV.
Alessandra Ferrajoli, MD, a hematologist/oncologist at MD Anderson Cancer Center who also didn’t take part in the study, agreed in an interview that the findings reflect those found in other reports. “It should be highlighted that the population studied is at particular high risk for infections given the high proportion of patients with recurrent disease (85%), many patients with concurrent hypogammaglobulinemia (64%), and the patient median age of 73 years and a high comorbidities burden,” she said. “In my view, this explains the higher rate of infections reported in this study, when compared to other case series.”
Dr. Ferrajoli added that there’s no standard antimicrobial prophylaxis for patients with B-cell malignancies receiving targeted therapies. “Anti-HSV/VZV prophylaxis is commonly implemented. Additional antiviral, antimicrobial, and antifungal prophylaxis should be used based on patients’ absolute neutrophil and T-cell count and individual risk factors, including prior history of infections such as CMV, prior splenectomy, and history of invasive fungal infections.”
The study was funded by Monash Health, the National Health and Medical Research Council (Australia), and the Society of Hospital Pharmacists of Australia. Ms. Tey reported no disclosures. Some of the study authors reported multiple disclosures. Dr. Reynolds discloses a PhD scholarship from the National Health and Medical Research Council. Dr. Ferrajoli reported no disclosures.
For example, “the rate of severe infection for ibrutinib in clinical trials ranged from 12.8% to 45% with median follow-up ranging from 27 to 65 months. In our study, the rate of severe infection was 45.3% within a shorter median follow-up period of 23.3 months,” said study lead author Amanda Tey, MPharm, a hematology pharmacist with Monash Health in Clayton, Australia, in an interview.
The results suggest that “real-world severe infection risk is higher than previously appreciated,” said Ms. Tey, whose findings were published in the European Journal of Hematology. “Poor performance status and a high comorbidity burden further increase this risk.”
According to the study, there are limited data about real-world infection rates for patients with CLL or B-cell lymphoma who take the three drugs.
Both the underlying blood cancer and the drugs themselves may disrupt the immune system in these patients, Ms. Tey noted. “Ibrutinib inhibits interleukin-2-inducible T-cell kinase, which has a role in T-cell maturation. Idelalisib reduces regulatory T-cell activity and natural killer cell and neutrophil inflammatory responses. Venetoclax is associated with a high rate of neutropenia.”
For the new retrospective, single-center study, researchers tracked adult patients who’d received the drugs from 2014 to 2021 in a hospital network serving 1.5 million people in the Australian state of Victoria. The primary outcome was severe infection of grade 3 or higher. Patients were excluded for such factors as having been primarily treated at other facilities, receiving less than 30 days of treatment, or having been treated for other indications such as primary central nervous system lymphoma.
Of the 67 patients in the study, the numbers taking the drugs were 53 (ibrutinib), 8 (idelalisib), and 6 (venetoclax). Eleven patients took more than one drug. Median age was 73 years, and 73% of patients were male.
Patients spent a median 23.3, 4.8, and 3.5 months taking ibrutinib, idelalisib, and venetoclax, respectively, before treatment stopped or data were collected. Patients were commonly prescribed antimicrobials to prevent pneumocystis jirovecii pneumonia and herpes simplex virus (HSV)/varicella zoster virus (VZV) infection.
Researchers found that 48% of the patients had at least one serious infection: 45% of those on ibrutinib, 63% of those on idelalisib, and 50% of those on venetoclax. Seven patients died of infections.
In comparison, the researchers reported, a systematic review of idelalisib in blood cancer clinical trials reported an overall infection rate of 28%, while clinical trials reported an infection rate of 17.5%-22% in patients taking venetoclax for CLL.
Poor performance status and higher levels of comorbidity were linked to higher risk of infection, and infections occurred at a median of 5.4 months.
Lead author Ms. Tey highlighted the fact that most of the patients in the new study had relapsed/refractory disease. The infection risk in the real-world first-line setting is unknown, she said. “Furthermore, due to the size of our study and high uptake of antimicrobial prophylaxis, the optimal prophylaxis strategy for these patients remains unclear.”
In an interview, infectious disease physician Gemma Reynolds, MChD, MPH, of Austin Health and Peter MacCallum Cancer Center in Melbourne, said the study findings reflect “a lot of what we know from other observational studies and clinical practice. There is a risk of infection, and serious infection, associated with these agents. Sometimes the pathogen is classically opportunistic, but often it is bacterial, and respiratory sites are common. Infections often occur early into a course of therapy.”
Dr. Reynolds, who didn’t take part in the study, urged colleagues to cast a wide net if a patient appears to have an infection but doesn’t respond to conventional therapies such as antibiotics. “Unusual infections are possible,” she said, and aggressive early workups may be advisable via blood cultures, viral swabs, sputum culture, early imaging, bronchoscopy, and preemptive monitoring in patients with a prior infection history with a disease such as CMV.
Alessandra Ferrajoli, MD, a hematologist/oncologist at MD Anderson Cancer Center who also didn’t take part in the study, agreed in an interview that the findings reflect those found in other reports. “It should be highlighted that the population studied is at particular high risk for infections given the high proportion of patients with recurrent disease (85%), many patients with concurrent hypogammaglobulinemia (64%), and the patient median age of 73 years and a high comorbidities burden,” she said. “In my view, this explains the higher rate of infections reported in this study, when compared to other case series.”
Dr. Ferrajoli added that there’s no standard antimicrobial prophylaxis for patients with B-cell malignancies receiving targeted therapies. “Anti-HSV/VZV prophylaxis is commonly implemented. Additional antiviral, antimicrobial, and antifungal prophylaxis should be used based on patients’ absolute neutrophil and T-cell count and individual risk factors, including prior history of infections such as CMV, prior splenectomy, and history of invasive fungal infections.”
The study was funded by Monash Health, the National Health and Medical Research Council (Australia), and the Society of Hospital Pharmacists of Australia. Ms. Tey reported no disclosures. Some of the study authors reported multiple disclosures. Dr. Reynolds discloses a PhD scholarship from the National Health and Medical Research Council. Dr. Ferrajoli reported no disclosures.
FROM THE EUROPEAN JOURNAL OF HEMATOLOGY
Med center and top cardio surgeon must pay $8.5 million for fraud, concurrent surgeries
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
What happens if we sit for more than 8 hours per day?
according to a recent Latin American study published in BMC Public Health.
These data come from almost 8,000 people aged 20-65 years (half of whom are women) who participated in the Latin American Study on Nutrition and Health (ELANS). The cross-sectional survey included representative samples from urban populations in Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Peru, and Venezuela. The average time spent sitting was 420 min/d. Ecuador had the lowest time (300 min/day), and Argentina and Peru had the highest (480 min/day).
No amount of sitting time has been associated with a greater health risk, but the World Health Organization recommends that sitting time be minimal.
“We used to believe that any intense physical exercise could compensate for a sedentary life. But now we know that a sedentary lifestyle in general and sitting time in particular have a direct effect and are an independent risk factor for chronic diseases,” said study author Irina Kovalskys, PhD, a pediatric specialist in nutrition and a professor of nutrition at the Catholic University of Argentina, Buenos Aires, and a principal investigator of ELANS.
Dr. Kovalskys stated that the 420-min average sitting time is worrying in a population such as the one studied, in which 60% of adults are obese and there are high rates of cardiometabolic risk factors. She affirmed that it is important to raise awareness among the population and focus on adolescents.
Felipe Lobelo, PhD, is a Colombian physician, an associate professor of global health at Emory University and director of epidemiology at Kaiser Permanente Georgia, both in Atlanta. He did not participate in this study but promotes the concept of exercise in medicine. The activity of the patient must be included in a clinical setting, and improving the level of physical activity can have a positive impact on health prognosis, he said.
“To make public health recommendations or even advise patients, a cutoff point is needed. Guidelines recommend 150 minutes per week of moderate to vigorous physical activity, and some countries have started to indicate that we should be concerned about people’s sitting time. There is still no equivalent to the 150 minutes, therefore, these studies are important, especially in the Latin American population,” said Dr. Lobelo.
He explained that the concept of an increased risk of death or chronic disease because of a lack of physical activity arose in the past 50 years, but only in the past 2 decades have we started thinking about sitting time.
“Spending more than 8 hours sitting per day clearly causes a much higher risk of chronic diseases, including obesity and diabetes. It may be a continuous and progressive association, and the point at which this increase becomes exponential is clearly between 6 and 8 hours of sitting time,” Dr. Lobelo added.
The authors expected to find a linear association with risk for being overweight or obese after 4 hours, but they did not find one. “This study has limitations. Among them was that other indicators were not considered, such as health indicators. Collaborations are starting with other research groups, and other studies are being designed,” said study author Gerson Ferrari, PhD, an associate professor at Santiago de Chile University.
Comparing indicators
The Latin American study tried to establish a sitting cutoff time after which the risk of becoming overweight or obese increases. It used three indicators of excess weight: body mass index (BMI), waist circumference, and neck circumference.
Sitting for more than 8 hours increased the chances of excess weight by 10% when measured by BMI and by 13% when neck circumference was used.
Dr. Ferrari stated that the result obtained measuring BMI is the one that should be considered, because it is used in public policy. Neck circumference is a more recent measurement of detection and it is less studied, but it is a valid indicator, with good sensitivity and advantages over others, such as ease of measurement and lack of variation over time.
According to the results of this study, measuring neck circumference may be the most sensitive method of the three. Neck circumference was proportionally greater in people who sat for at least 4, at least 6, and at least 8 hours/day than in those who sat for less than 4, less than 6, and less than 8 hours/day. This relationship was not observed with the other indicators.
Broaching the topic
“What is important is uninterrupted sitting time. The recommendation is to break up those sitting times with active periods. Health professionals have already incorporated the concept of moderate to vigorous physical exercise, but nonintense activities are sufficient to reduce sitting time. Yoga may not be vigorous, but it is valuable at reducing sitting time,” said Dr. Kovalskys.
Dr. Ferrari recommended giving patients concrete messages so that they spend as little time possible sitting. “It is better to stand on the bus or the subway even when there is a place to sit. Are you going to talk on the phone? It is better to do it while walking or at least standing instead of sitting.”
A recent literature review conducted by investigators of the University of Birmingham (England) studied the possible molecular and physiologic mechanisms of inactivity time, health consequences, and protection strategies. It offers an evaluation of interventions that can compensate for the immediate negative consequences of inactivity.
Physical activity
Some studies suggest that more than 60 min/day of moderate-intensity exercise or more than 150 min/week of moderate to vigorous exercise may be effective at mitigating the increased risk for mortality associated with sitting time, but reduced intensity may not be enough.
Active pauses
Interrupting sitting every 30-60 min to walk or cycle (2-10 min), performing 3 minutes of simple resistance activities every 30 minutes, such as calf or knee lifts, performing intermittent leg movements (1 minute of activity for every 4 minutes of inactivity during a 3-hour protocol session), or pausing to climb stairs (5 minutes every hour) may be beneficial for vascular health. However, not all studies have demonstrated these positive effects, therefore, some populations may need exercise of greater intensity or duration to counteract the negative vascular effects of acute inactivity periods.
Standing workstations
Standing workstations are effective at reducing sitting time in offices but may be ineffective at reducing vascular alterations related to sitting time. Although some experimental studies indicate vascular benefits, epidemiologic studies suggest that long periods of standing can be harmful to vascular health, especially for venous diseases. Recommendations for use should be accompanied by specific regimens on the frequency and duration of the position to attain the maximum benefits and minimize other vascular complications.
One problem that Dr. Lobelo noted is that some doctors ask their patients how active they are, but they do so in a nonstandardized manner. This observation led him to publish, together with the American Heart Association, an article on the importance for health systems of considering physical activity as a vital sign and including it in records in a standardized manner.
He said that “one advantage of having physical activity as a vital sign in patient medical records is that it allows us to identify individuals who are at greater risk.”
Kaiser Permanente asks the following questions: how many minutes of physical activity do you perform regularly per week, and what is the average intensity of that activity? Patients can be classified into the following three groups: those who follow the recommendations, those with almost no activity, and those who perform some physical activity but do not meet the recommended 150 min/week of moderate to vigorous activity.
Recording sitting time is more difficult. Dr. Lobelo indicated that “it is easier for a person to remember how much time they spent running than how long they were sitting.” Regarding the use of technology, he commented that most watches provide a good estimate. Without technology, it can be estimated by asking how much time is spent in the car, on the bus, or in front of the computer or television and then adding up these times.
Dr. Lobelo emphasized that the two behaviors, lack of physical activity and excessive sitting time, have independent associations with health outcomes. But if both are combined, the risk of obesity, diabetes, and cardiovascular diseases is not just added but rather is multiplied. These behaviors contribute to the epidemic of obesity and diabetes, since most people do not follow either of the two recommendations.
“Studies show that of the two behaviors, the more negative for health would be not following the physical activity recommendations,” said Dr. Lobelo. “If the recommendation of 150 min/week of moderate to vigorous physical activity is followed, the associated risk of sitting too much declines by 80%-90%. Additionally, we can prevent, help to manage, and decrease the risk of complications in more than 100 diseases, including infections. During the pandemic, it was observed that more active people had a lower risk of dying or of being hospitalized due to COVID-19 than less active people, independently of other factors, such as hypertension, diabetes, and obesity.”
Moreover, Dr. Lobelo believes in “practicing what you preach” and advocates that doctors become healthy models.
Dr. Lobelo, Dr. Ferrari, and Dr. Kovalskys disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version appeared on Medscape.com.
according to a recent Latin American study published in BMC Public Health.
These data come from almost 8,000 people aged 20-65 years (half of whom are women) who participated in the Latin American Study on Nutrition and Health (ELANS). The cross-sectional survey included representative samples from urban populations in Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Peru, and Venezuela. The average time spent sitting was 420 min/d. Ecuador had the lowest time (300 min/day), and Argentina and Peru had the highest (480 min/day).
No amount of sitting time has been associated with a greater health risk, but the World Health Organization recommends that sitting time be minimal.
“We used to believe that any intense physical exercise could compensate for a sedentary life. But now we know that a sedentary lifestyle in general and sitting time in particular have a direct effect and are an independent risk factor for chronic diseases,” said study author Irina Kovalskys, PhD, a pediatric specialist in nutrition and a professor of nutrition at the Catholic University of Argentina, Buenos Aires, and a principal investigator of ELANS.
Dr. Kovalskys stated that the 420-min average sitting time is worrying in a population such as the one studied, in which 60% of adults are obese and there are high rates of cardiometabolic risk factors. She affirmed that it is important to raise awareness among the population and focus on adolescents.
Felipe Lobelo, PhD, is a Colombian physician, an associate professor of global health at Emory University and director of epidemiology at Kaiser Permanente Georgia, both in Atlanta. He did not participate in this study but promotes the concept of exercise in medicine. The activity of the patient must be included in a clinical setting, and improving the level of physical activity can have a positive impact on health prognosis, he said.
“To make public health recommendations or even advise patients, a cutoff point is needed. Guidelines recommend 150 minutes per week of moderate to vigorous physical activity, and some countries have started to indicate that we should be concerned about people’s sitting time. There is still no equivalent to the 150 minutes, therefore, these studies are important, especially in the Latin American population,” said Dr. Lobelo.
He explained that the concept of an increased risk of death or chronic disease because of a lack of physical activity arose in the past 50 years, but only in the past 2 decades have we started thinking about sitting time.
“Spending more than 8 hours sitting per day clearly causes a much higher risk of chronic diseases, including obesity and diabetes. It may be a continuous and progressive association, and the point at which this increase becomes exponential is clearly between 6 and 8 hours of sitting time,” Dr. Lobelo added.
The authors expected to find a linear association with risk for being overweight or obese after 4 hours, but they did not find one. “This study has limitations. Among them was that other indicators were not considered, such as health indicators. Collaborations are starting with other research groups, and other studies are being designed,” said study author Gerson Ferrari, PhD, an associate professor at Santiago de Chile University.
Comparing indicators
The Latin American study tried to establish a sitting cutoff time after which the risk of becoming overweight or obese increases. It used three indicators of excess weight: body mass index (BMI), waist circumference, and neck circumference.
Sitting for more than 8 hours increased the chances of excess weight by 10% when measured by BMI and by 13% when neck circumference was used.
Dr. Ferrari stated that the result obtained measuring BMI is the one that should be considered, because it is used in public policy. Neck circumference is a more recent measurement of detection and it is less studied, but it is a valid indicator, with good sensitivity and advantages over others, such as ease of measurement and lack of variation over time.
According to the results of this study, measuring neck circumference may be the most sensitive method of the three. Neck circumference was proportionally greater in people who sat for at least 4, at least 6, and at least 8 hours/day than in those who sat for less than 4, less than 6, and less than 8 hours/day. This relationship was not observed with the other indicators.
Broaching the topic
“What is important is uninterrupted sitting time. The recommendation is to break up those sitting times with active periods. Health professionals have already incorporated the concept of moderate to vigorous physical exercise, but nonintense activities are sufficient to reduce sitting time. Yoga may not be vigorous, but it is valuable at reducing sitting time,” said Dr. Kovalskys.
Dr. Ferrari recommended giving patients concrete messages so that they spend as little time possible sitting. “It is better to stand on the bus or the subway even when there is a place to sit. Are you going to talk on the phone? It is better to do it while walking or at least standing instead of sitting.”
A recent literature review conducted by investigators of the University of Birmingham (England) studied the possible molecular and physiologic mechanisms of inactivity time, health consequences, and protection strategies. It offers an evaluation of interventions that can compensate for the immediate negative consequences of inactivity.
Physical activity
Some studies suggest that more than 60 min/day of moderate-intensity exercise or more than 150 min/week of moderate to vigorous exercise may be effective at mitigating the increased risk for mortality associated with sitting time, but reduced intensity may not be enough.
Active pauses
Interrupting sitting every 30-60 min to walk or cycle (2-10 min), performing 3 minutes of simple resistance activities every 30 minutes, such as calf or knee lifts, performing intermittent leg movements (1 minute of activity for every 4 minutes of inactivity during a 3-hour protocol session), or pausing to climb stairs (5 minutes every hour) may be beneficial for vascular health. However, not all studies have demonstrated these positive effects, therefore, some populations may need exercise of greater intensity or duration to counteract the negative vascular effects of acute inactivity periods.
Standing workstations
Standing workstations are effective at reducing sitting time in offices but may be ineffective at reducing vascular alterations related to sitting time. Although some experimental studies indicate vascular benefits, epidemiologic studies suggest that long periods of standing can be harmful to vascular health, especially for venous diseases. Recommendations for use should be accompanied by specific regimens on the frequency and duration of the position to attain the maximum benefits and minimize other vascular complications.
One problem that Dr. Lobelo noted is that some doctors ask their patients how active they are, but they do so in a nonstandardized manner. This observation led him to publish, together with the American Heart Association, an article on the importance for health systems of considering physical activity as a vital sign and including it in records in a standardized manner.
He said that “one advantage of having physical activity as a vital sign in patient medical records is that it allows us to identify individuals who are at greater risk.”
Kaiser Permanente asks the following questions: how many minutes of physical activity do you perform regularly per week, and what is the average intensity of that activity? Patients can be classified into the following three groups: those who follow the recommendations, those with almost no activity, and those who perform some physical activity but do not meet the recommended 150 min/week of moderate to vigorous activity.
Recording sitting time is more difficult. Dr. Lobelo indicated that “it is easier for a person to remember how much time they spent running than how long they were sitting.” Regarding the use of technology, he commented that most watches provide a good estimate. Without technology, it can be estimated by asking how much time is spent in the car, on the bus, or in front of the computer or television and then adding up these times.
Dr. Lobelo emphasized that the two behaviors, lack of physical activity and excessive sitting time, have independent associations with health outcomes. But if both are combined, the risk of obesity, diabetes, and cardiovascular diseases is not just added but rather is multiplied. These behaviors contribute to the epidemic of obesity and diabetes, since most people do not follow either of the two recommendations.
“Studies show that of the two behaviors, the more negative for health would be not following the physical activity recommendations,” said Dr. Lobelo. “If the recommendation of 150 min/week of moderate to vigorous physical activity is followed, the associated risk of sitting too much declines by 80%-90%. Additionally, we can prevent, help to manage, and decrease the risk of complications in more than 100 diseases, including infections. During the pandemic, it was observed that more active people had a lower risk of dying or of being hospitalized due to COVID-19 than less active people, independently of other factors, such as hypertension, diabetes, and obesity.”
Moreover, Dr. Lobelo believes in “practicing what you preach” and advocates that doctors become healthy models.
Dr. Lobelo, Dr. Ferrari, and Dr. Kovalskys disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version appeared on Medscape.com.
according to a recent Latin American study published in BMC Public Health.
These data come from almost 8,000 people aged 20-65 years (half of whom are women) who participated in the Latin American Study on Nutrition and Health (ELANS). The cross-sectional survey included representative samples from urban populations in Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Peru, and Venezuela. The average time spent sitting was 420 min/d. Ecuador had the lowest time (300 min/day), and Argentina and Peru had the highest (480 min/day).
No amount of sitting time has been associated with a greater health risk, but the World Health Organization recommends that sitting time be minimal.
“We used to believe that any intense physical exercise could compensate for a sedentary life. But now we know that a sedentary lifestyle in general and sitting time in particular have a direct effect and are an independent risk factor for chronic diseases,” said study author Irina Kovalskys, PhD, a pediatric specialist in nutrition and a professor of nutrition at the Catholic University of Argentina, Buenos Aires, and a principal investigator of ELANS.
Dr. Kovalskys stated that the 420-min average sitting time is worrying in a population such as the one studied, in which 60% of adults are obese and there are high rates of cardiometabolic risk factors. She affirmed that it is important to raise awareness among the population and focus on adolescents.
Felipe Lobelo, PhD, is a Colombian physician, an associate professor of global health at Emory University and director of epidemiology at Kaiser Permanente Georgia, both in Atlanta. He did not participate in this study but promotes the concept of exercise in medicine. The activity of the patient must be included in a clinical setting, and improving the level of physical activity can have a positive impact on health prognosis, he said.
“To make public health recommendations or even advise patients, a cutoff point is needed. Guidelines recommend 150 minutes per week of moderate to vigorous physical activity, and some countries have started to indicate that we should be concerned about people’s sitting time. There is still no equivalent to the 150 minutes, therefore, these studies are important, especially in the Latin American population,” said Dr. Lobelo.
He explained that the concept of an increased risk of death or chronic disease because of a lack of physical activity arose in the past 50 years, but only in the past 2 decades have we started thinking about sitting time.
“Spending more than 8 hours sitting per day clearly causes a much higher risk of chronic diseases, including obesity and diabetes. It may be a continuous and progressive association, and the point at which this increase becomes exponential is clearly between 6 and 8 hours of sitting time,” Dr. Lobelo added.
The authors expected to find a linear association with risk for being overweight or obese after 4 hours, but they did not find one. “This study has limitations. Among them was that other indicators were not considered, such as health indicators. Collaborations are starting with other research groups, and other studies are being designed,” said study author Gerson Ferrari, PhD, an associate professor at Santiago de Chile University.
Comparing indicators
The Latin American study tried to establish a sitting cutoff time after which the risk of becoming overweight or obese increases. It used three indicators of excess weight: body mass index (BMI), waist circumference, and neck circumference.
Sitting for more than 8 hours increased the chances of excess weight by 10% when measured by BMI and by 13% when neck circumference was used.
Dr. Ferrari stated that the result obtained measuring BMI is the one that should be considered, because it is used in public policy. Neck circumference is a more recent measurement of detection and it is less studied, but it is a valid indicator, with good sensitivity and advantages over others, such as ease of measurement and lack of variation over time.
According to the results of this study, measuring neck circumference may be the most sensitive method of the three. Neck circumference was proportionally greater in people who sat for at least 4, at least 6, and at least 8 hours/day than in those who sat for less than 4, less than 6, and less than 8 hours/day. This relationship was not observed with the other indicators.
Broaching the topic
“What is important is uninterrupted sitting time. The recommendation is to break up those sitting times with active periods. Health professionals have already incorporated the concept of moderate to vigorous physical exercise, but nonintense activities are sufficient to reduce sitting time. Yoga may not be vigorous, but it is valuable at reducing sitting time,” said Dr. Kovalskys.
Dr. Ferrari recommended giving patients concrete messages so that they spend as little time possible sitting. “It is better to stand on the bus or the subway even when there is a place to sit. Are you going to talk on the phone? It is better to do it while walking or at least standing instead of sitting.”
A recent literature review conducted by investigators of the University of Birmingham (England) studied the possible molecular and physiologic mechanisms of inactivity time, health consequences, and protection strategies. It offers an evaluation of interventions that can compensate for the immediate negative consequences of inactivity.
Physical activity
Some studies suggest that more than 60 min/day of moderate-intensity exercise or more than 150 min/week of moderate to vigorous exercise may be effective at mitigating the increased risk for mortality associated with sitting time, but reduced intensity may not be enough.
Active pauses
Interrupting sitting every 30-60 min to walk or cycle (2-10 min), performing 3 minutes of simple resistance activities every 30 minutes, such as calf or knee lifts, performing intermittent leg movements (1 minute of activity for every 4 minutes of inactivity during a 3-hour protocol session), or pausing to climb stairs (5 minutes every hour) may be beneficial for vascular health. However, not all studies have demonstrated these positive effects, therefore, some populations may need exercise of greater intensity or duration to counteract the negative vascular effects of acute inactivity periods.
Standing workstations
Standing workstations are effective at reducing sitting time in offices but may be ineffective at reducing vascular alterations related to sitting time. Although some experimental studies indicate vascular benefits, epidemiologic studies suggest that long periods of standing can be harmful to vascular health, especially for venous diseases. Recommendations for use should be accompanied by specific regimens on the frequency and duration of the position to attain the maximum benefits and minimize other vascular complications.
One problem that Dr. Lobelo noted is that some doctors ask their patients how active they are, but they do so in a nonstandardized manner. This observation led him to publish, together with the American Heart Association, an article on the importance for health systems of considering physical activity as a vital sign and including it in records in a standardized manner.
He said that “one advantage of having physical activity as a vital sign in patient medical records is that it allows us to identify individuals who are at greater risk.”
Kaiser Permanente asks the following questions: how many minutes of physical activity do you perform regularly per week, and what is the average intensity of that activity? Patients can be classified into the following three groups: those who follow the recommendations, those with almost no activity, and those who perform some physical activity but do not meet the recommended 150 min/week of moderate to vigorous activity.
Recording sitting time is more difficult. Dr. Lobelo indicated that “it is easier for a person to remember how much time they spent running than how long they were sitting.” Regarding the use of technology, he commented that most watches provide a good estimate. Without technology, it can be estimated by asking how much time is spent in the car, on the bus, or in front of the computer or television and then adding up these times.
Dr. Lobelo emphasized that the two behaviors, lack of physical activity and excessive sitting time, have independent associations with health outcomes. But if both are combined, the risk of obesity, diabetes, and cardiovascular diseases is not just added but rather is multiplied. These behaviors contribute to the epidemic of obesity and diabetes, since most people do not follow either of the two recommendations.
“Studies show that of the two behaviors, the more negative for health would be not following the physical activity recommendations,” said Dr. Lobelo. “If the recommendation of 150 min/week of moderate to vigorous physical activity is followed, the associated risk of sitting too much declines by 80%-90%. Additionally, we can prevent, help to manage, and decrease the risk of complications in more than 100 diseases, including infections. During the pandemic, it was observed that more active people had a lower risk of dying or of being hospitalized due to COVID-19 than less active people, independently of other factors, such as hypertension, diabetes, and obesity.”
Moreover, Dr. Lobelo believes in “practicing what you preach” and advocates that doctors become healthy models.
Dr. Lobelo, Dr. Ferrari, and Dr. Kovalskys disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version appeared on Medscape.com.
FROM BMC PUBLIC HEALTH
How to help pediatricians apply peanut allergy guidelines
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
FROM AAAAI 2023
Distinct suicidal thought patterns flag those at highest risk
Long-term assessment of suicide risk and ideation in older adults may help identify distinct ideation patterns and predict potential future suicidal behavior, new research suggests.
Investigators studied over 300 older adults, assessing suicidal ideation and behavior for up to 14 years at least once annually. They then identified four suicidal ideation profiles.
They found that In turn, fast-remitting ideators were at higher risk in comparison to low/nonideators with no attempts or suicide.
Chronic severe ideators also showed the most severe levels of dysfunction across personality, social characteristics, and impulsivity measures, while highly variable and fast-remitting ideators displayed more specific deficits.
“We identified longitudinal ideation profiles that convey differential risk of future suicidal behavior to help clinicians recognize high suicide risk patients for preventing suicide,” said lead author Hanga Galfalvy, PhD, associate professor, department of psychiatry, Columbia University Irving Medical Center, New York.
“Clinicians should repeatedly assess suicidal ideation and ask not only about current ideation but also about the worst ideation since the last visit [because] similar levels of ideation during a single assessment can belong to very different risk profiles,” said Dr. Galfalvy, also a professor of biostatistics and a coinvestigator in the Conte Center for Suicide Prevention at Columbia University.
The study was published online in the Journal of Clinical Psychiatry.
Vulnerable population
“Older adults in most countries, including the U.S., are at the highest risk of dying of suicide out of all age groups,” said Dr. Galfalvy. “A significant number of depressed older adults experience thoughts of killing themselves, but fortunately, only a few transition from suicidal thoughts to behavior.”
Senior author Katalin Szanto, MD, professor of psychiatry, University of Pittsburgh, said in an interview that currently established clinical and psychosocial suicide risk factors have “low predictive value and provide little insight into the high suicide rate in the elderly.”
These traditional risk factors “poorly distinguish between suicide ideators and suicide attempters and do not take into consideration the heterogeneity of suicidal behavior,” said Dr. Szanto, principal investigator at the University of Pittsburgh’s Longitudinal research Program in Late-Life Suicide, where the study was conducted.
“Suicidal ideation measured at one time point – current or lifetime – may not be enough to accurately predict suicide risk,” the investigators wrote.
The current study, a collaboration between investigators from the Longitudinal Research Program in Late-Life Suicide and the Conte Center for Suicide Prevention, investigates “profiles of suicidal thoughts and behavior in patients with late-life depression over a longer period of time,” Dr. Galfalvy said.
The researchers used latent profile analysis (LPA) in a cohort of adults with nonpsychotic unipolar depression (aged 50-93 years; n = 337; mean age, 65.12 years) to “identify distinct ideation profiles and their clinical correlates” and to “test the profiles’ association with the risk of suicidal behavior before and during follow-up.”
LPA is “a data-driven method of grouping individuals into subgroups, based on quantitative characteristics,” Dr. Galfalvy explained.
The LPA yielded four profiles of ideation.
At baseline, the researchers assessed the presence or absence of suicidal behavior history and the number and lethality of attempts. They prospectively assessed suicidal ideation and attempts at least once annually thereafter over a period ranging from 3 months to 14 years (median, 3 years; IQR, 1.6-4 years).
At baseline and at follow-ups, they assessed ideation severity.
They also assessed depression severity, impulsivity, and personality measures, as well as perception of social support, social problem solving, cognitive performance, and physical comorbidities.
Personalized prevention
Of the original cohort, 92 patients died during the follow-up period, with 13 dying of suicide (or suspected suicide).
Over half (60%) of the chronic severe as well as the highly variable groups and almost half (48%) of the fast-remitting group had a history of past suicide attempt – all significantly higher than the low-nonideators (0%).
Despite comparable current ideation severity at baseline, the risk of suicide attempt/death was greater for chronic severe ideators versus fast-remitting ideators, but not greater than for highly variable ideators. On the other hand, highly variable ideators were at greater risk, compared with fast-remitting ideators.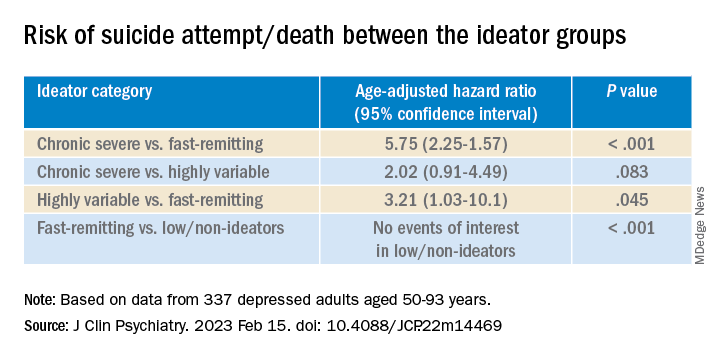
Cognitive factors “did not significantly discriminate between the ideation profiles, although ... lower global cognitive performance predicted suicidal behavior during follow-up,” the authors wrote.
This finding “aligns with prior studies indicating that late-life suicidal behavior but not ideation may be related to cognition ... and instead, ideation and cognition may act as independent risk factors for suicidal behavior,” they added.
“Patients in the fluctuating ideator group generally had moderate or high levels of worst suicidal ideation between visits, but not when asked about current ideation levels at the time of the follow-up assessment,” Dr. Galfalvy noted. “For them, the time frame of the question made a difference as to the level of ideation reported.”
The study “identified several clinical differences among these subgroups which could lead to more personalized suicide prevention efforts and further research into the heterogeneity of suicidal behavior,” she suggested.
New insight
Commenting on the study, Ari Cuperfain, MD, of the University of Toronto said the study “adds to the nuanced understanding of how changes in suicidal ideation over time can lead to suicidal actions and behavior.”
The study “sheds light on the notion of how older adults who die by suicide can demonstrate a greater degree of premeditated intent relative to younger cohorts, with chronic severe ideators portending the highest risk for suicide in this sample,” added Dr. Cuperfain, who was not involved with the current research.
“Overall, the paper highlights the importance of both screening for current levels of suicidal ideation in addition to the evolution of suicidal ideation in developing a risk assessment and in finding interventions to reduce this risk when it is most prominent,” he stated.
The research was supported by the National Institutes of Health. The authors and Dr. Cuperfain disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term assessment of suicide risk and ideation in older adults may help identify distinct ideation patterns and predict potential future suicidal behavior, new research suggests.
Investigators studied over 300 older adults, assessing suicidal ideation and behavior for up to 14 years at least once annually. They then identified four suicidal ideation profiles.
They found that In turn, fast-remitting ideators were at higher risk in comparison to low/nonideators with no attempts or suicide.
Chronic severe ideators also showed the most severe levels of dysfunction across personality, social characteristics, and impulsivity measures, while highly variable and fast-remitting ideators displayed more specific deficits.
“We identified longitudinal ideation profiles that convey differential risk of future suicidal behavior to help clinicians recognize high suicide risk patients for preventing suicide,” said lead author Hanga Galfalvy, PhD, associate professor, department of psychiatry, Columbia University Irving Medical Center, New York.
“Clinicians should repeatedly assess suicidal ideation and ask not only about current ideation but also about the worst ideation since the last visit [because] similar levels of ideation during a single assessment can belong to very different risk profiles,” said Dr. Galfalvy, also a professor of biostatistics and a coinvestigator in the Conte Center for Suicide Prevention at Columbia University.
The study was published online in the Journal of Clinical Psychiatry.
Vulnerable population
“Older adults in most countries, including the U.S., are at the highest risk of dying of suicide out of all age groups,” said Dr. Galfalvy. “A significant number of depressed older adults experience thoughts of killing themselves, but fortunately, only a few transition from suicidal thoughts to behavior.”
Senior author Katalin Szanto, MD, professor of psychiatry, University of Pittsburgh, said in an interview that currently established clinical and psychosocial suicide risk factors have “low predictive value and provide little insight into the high suicide rate in the elderly.”
These traditional risk factors “poorly distinguish between suicide ideators and suicide attempters and do not take into consideration the heterogeneity of suicidal behavior,” said Dr. Szanto, principal investigator at the University of Pittsburgh’s Longitudinal research Program in Late-Life Suicide, where the study was conducted.
“Suicidal ideation measured at one time point – current or lifetime – may not be enough to accurately predict suicide risk,” the investigators wrote.
The current study, a collaboration between investigators from the Longitudinal Research Program in Late-Life Suicide and the Conte Center for Suicide Prevention, investigates “profiles of suicidal thoughts and behavior in patients with late-life depression over a longer period of time,” Dr. Galfalvy said.
The researchers used latent profile analysis (LPA) in a cohort of adults with nonpsychotic unipolar depression (aged 50-93 years; n = 337; mean age, 65.12 years) to “identify distinct ideation profiles and their clinical correlates” and to “test the profiles’ association with the risk of suicidal behavior before and during follow-up.”
LPA is “a data-driven method of grouping individuals into subgroups, based on quantitative characteristics,” Dr. Galfalvy explained.
The LPA yielded four profiles of ideation.
At baseline, the researchers assessed the presence or absence of suicidal behavior history and the number and lethality of attempts. They prospectively assessed suicidal ideation and attempts at least once annually thereafter over a period ranging from 3 months to 14 years (median, 3 years; IQR, 1.6-4 years).
At baseline and at follow-ups, they assessed ideation severity.
They also assessed depression severity, impulsivity, and personality measures, as well as perception of social support, social problem solving, cognitive performance, and physical comorbidities.
Personalized prevention
Of the original cohort, 92 patients died during the follow-up period, with 13 dying of suicide (or suspected suicide).
Over half (60%) of the chronic severe as well as the highly variable groups and almost half (48%) of the fast-remitting group had a history of past suicide attempt – all significantly higher than the low-nonideators (0%).
Despite comparable current ideation severity at baseline, the risk of suicide attempt/death was greater for chronic severe ideators versus fast-remitting ideators, but not greater than for highly variable ideators. On the other hand, highly variable ideators were at greater risk, compared with fast-remitting ideators.
Cognitive factors “did not significantly discriminate between the ideation profiles, although ... lower global cognitive performance predicted suicidal behavior during follow-up,” the authors wrote.
This finding “aligns with prior studies indicating that late-life suicidal behavior but not ideation may be related to cognition ... and instead, ideation and cognition may act as independent risk factors for suicidal behavior,” they added.
“Patients in the fluctuating ideator group generally had moderate or high levels of worst suicidal ideation between visits, but not when asked about current ideation levels at the time of the follow-up assessment,” Dr. Galfalvy noted. “For them, the time frame of the question made a difference as to the level of ideation reported.”
The study “identified several clinical differences among these subgroups which could lead to more personalized suicide prevention efforts and further research into the heterogeneity of suicidal behavior,” she suggested.
New insight
Commenting on the study, Ari Cuperfain, MD, of the University of Toronto said the study “adds to the nuanced understanding of how changes in suicidal ideation over time can lead to suicidal actions and behavior.”
The study “sheds light on the notion of how older adults who die by suicide can demonstrate a greater degree of premeditated intent relative to younger cohorts, with chronic severe ideators portending the highest risk for suicide in this sample,” added Dr. Cuperfain, who was not involved with the current research.
“Overall, the paper highlights the importance of both screening for current levels of suicidal ideation in addition to the evolution of suicidal ideation in developing a risk assessment and in finding interventions to reduce this risk when it is most prominent,” he stated.
The research was supported by the National Institutes of Health. The authors and Dr. Cuperfain disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term assessment of suicide risk and ideation in older adults may help identify distinct ideation patterns and predict potential future suicidal behavior, new research suggests.
Investigators studied over 300 older adults, assessing suicidal ideation and behavior for up to 14 years at least once annually. They then identified four suicidal ideation profiles.
They found that In turn, fast-remitting ideators were at higher risk in comparison to low/nonideators with no attempts or suicide.
Chronic severe ideators also showed the most severe levels of dysfunction across personality, social characteristics, and impulsivity measures, while highly variable and fast-remitting ideators displayed more specific deficits.
“We identified longitudinal ideation profiles that convey differential risk of future suicidal behavior to help clinicians recognize high suicide risk patients for preventing suicide,” said lead author Hanga Galfalvy, PhD, associate professor, department of psychiatry, Columbia University Irving Medical Center, New York.
“Clinicians should repeatedly assess suicidal ideation and ask not only about current ideation but also about the worst ideation since the last visit [because] similar levels of ideation during a single assessment can belong to very different risk profiles,” said Dr. Galfalvy, also a professor of biostatistics and a coinvestigator in the Conte Center for Suicide Prevention at Columbia University.
The study was published online in the Journal of Clinical Psychiatry.
Vulnerable population
“Older adults in most countries, including the U.S., are at the highest risk of dying of suicide out of all age groups,” said Dr. Galfalvy. “A significant number of depressed older adults experience thoughts of killing themselves, but fortunately, only a few transition from suicidal thoughts to behavior.”
Senior author Katalin Szanto, MD, professor of psychiatry, University of Pittsburgh, said in an interview that currently established clinical and psychosocial suicide risk factors have “low predictive value and provide little insight into the high suicide rate in the elderly.”
These traditional risk factors “poorly distinguish between suicide ideators and suicide attempters and do not take into consideration the heterogeneity of suicidal behavior,” said Dr. Szanto, principal investigator at the University of Pittsburgh’s Longitudinal research Program in Late-Life Suicide, where the study was conducted.
“Suicidal ideation measured at one time point – current or lifetime – may not be enough to accurately predict suicide risk,” the investigators wrote.
The current study, a collaboration between investigators from the Longitudinal Research Program in Late-Life Suicide and the Conte Center for Suicide Prevention, investigates “profiles of suicidal thoughts and behavior in patients with late-life depression over a longer period of time,” Dr. Galfalvy said.
The researchers used latent profile analysis (LPA) in a cohort of adults with nonpsychotic unipolar depression (aged 50-93 years; n = 337; mean age, 65.12 years) to “identify distinct ideation profiles and their clinical correlates” and to “test the profiles’ association with the risk of suicidal behavior before and during follow-up.”
LPA is “a data-driven method of grouping individuals into subgroups, based on quantitative characteristics,” Dr. Galfalvy explained.
The LPA yielded four profiles of ideation.
At baseline, the researchers assessed the presence or absence of suicidal behavior history and the number and lethality of attempts. They prospectively assessed suicidal ideation and attempts at least once annually thereafter over a period ranging from 3 months to 14 years (median, 3 years; IQR, 1.6-4 years).
At baseline and at follow-ups, they assessed ideation severity.
They also assessed depression severity, impulsivity, and personality measures, as well as perception of social support, social problem solving, cognitive performance, and physical comorbidities.
Personalized prevention
Of the original cohort, 92 patients died during the follow-up period, with 13 dying of suicide (or suspected suicide).
Over half (60%) of the chronic severe as well as the highly variable groups and almost half (48%) of the fast-remitting group had a history of past suicide attempt – all significantly higher than the low-nonideators (0%).
Despite comparable current ideation severity at baseline, the risk of suicide attempt/death was greater for chronic severe ideators versus fast-remitting ideators, but not greater than for highly variable ideators. On the other hand, highly variable ideators were at greater risk, compared with fast-remitting ideators.
Cognitive factors “did not significantly discriminate between the ideation profiles, although ... lower global cognitive performance predicted suicidal behavior during follow-up,” the authors wrote.
This finding “aligns with prior studies indicating that late-life suicidal behavior but not ideation may be related to cognition ... and instead, ideation and cognition may act as independent risk factors for suicidal behavior,” they added.
“Patients in the fluctuating ideator group generally had moderate or high levels of worst suicidal ideation between visits, but not when asked about current ideation levels at the time of the follow-up assessment,” Dr. Galfalvy noted. “For them, the time frame of the question made a difference as to the level of ideation reported.”
The study “identified several clinical differences among these subgroups which could lead to more personalized suicide prevention efforts and further research into the heterogeneity of suicidal behavior,” she suggested.
New insight
Commenting on the study, Ari Cuperfain, MD, of the University of Toronto said the study “adds to the nuanced understanding of how changes in suicidal ideation over time can lead to suicidal actions and behavior.”
The study “sheds light on the notion of how older adults who die by suicide can demonstrate a greater degree of premeditated intent relative to younger cohorts, with chronic severe ideators portending the highest risk for suicide in this sample,” added Dr. Cuperfain, who was not involved with the current research.
“Overall, the paper highlights the importance of both screening for current levels of suicidal ideation in addition to the evolution of suicidal ideation in developing a risk assessment and in finding interventions to reduce this risk when it is most prominent,” he stated.
The research was supported by the National Institutes of Health. The authors and Dr. Cuperfain disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY