User login
Losing weight may bolster AFib ablation’s chances for success: LEAF interim results
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.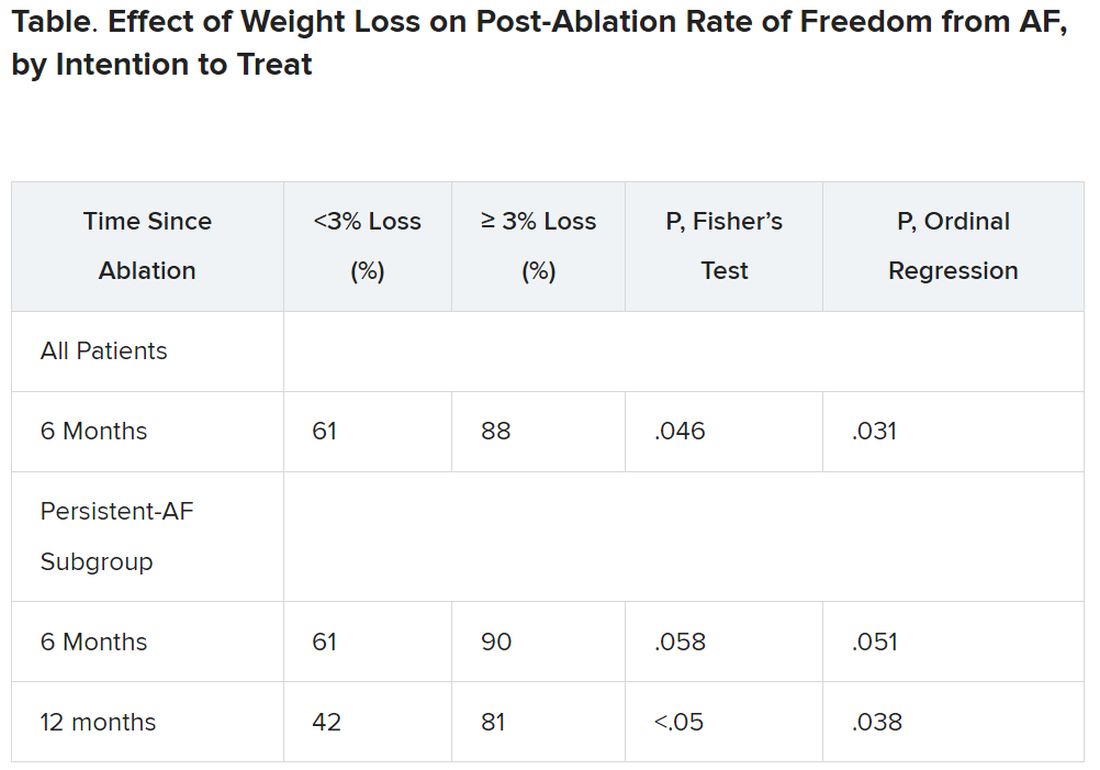
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HEART RHYTHM 2023
Safety remains top parent concern for HPV vaccine
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
FROM PEDIATRICS
AxSpA effects may be more severe for Black patients
CLEVELAND – Documenting the prevalence of axial spondyloarthritis (axSpA) among Black Americans has been difficult because of little published data, but new research suggests that when Black Americans do have the disease, it seems to be more severe.
Iman Abutineh, MD, of the University of Tennessee, Memphis, discussed her team’s work at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
A total of 244 patients with axSpA were identified, including 168 (69%) males, 78 (32%) Black patients, and 143 (59%) White patients.
Average age of onset for patients overall was 27.7 years, and age at diagnosis was 36.1 years with a 7-year delay in diagnosis. Sixty-six (27%) patients had nonradiographic axSpA, 83% were on tumor necrosis factor inhibitors, and 38% were prescribed glucocorticoids.
The researchers found several differences by race.
White patients were more likely to be HLA-B27 positive (77% vs. 59%; P = .010). White patients also had higher prevalence of psoriasis, coronary artery disease, and family history of SpA. White females had a higher prevalence of inflammatory bowel disease, fibromyalgia, depression, and lower grades of sacroiliitis.
Black patients had more hip involvement
A higher percentage of Black patients had elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and hip involvement. In comparing hip involvement, the researchers found that 39 (39%) White males had hip involvement as did 9 (21%) White females. In comparison, 22 (45%) Black men in the study and 14 (54%) Black women showed hip involvement (P = .035).
After adjustment for age, sex, HLA-B27, and insurance status, Black patients had higher grades of sacroiliitis with an odds ratio of 2.32 (95% confidence interval, 1.23-4.44). Black patients had a numerically longer delay in diagnosis, compared with Whites (7.93 vs. 6.64 years), but this did not achieve statistical significance (P = .454), the researchers wrote.
Study addresses racial disparities
“Traditionally we think of axial spondyloarthritis largely in Caucasian males who are HLA-B27 positive,” Dr. Abutineh said, “and we found that there is still a significant portion of patients who don’t meet the criteria that do have disease that is very significant.”
Although actual prevalence was not clear from this study, Dr. Abutineh said their data suggest a 3-to-1 ratio of White-to-Black patients with spondyloarthritis, “but of the Black patients who are diagnosed, their disease is almost always more severe. That points to why it’s important to have a high index of suspicion for this disease in that patient population because if you miss it, it could be detrimental to the patients.”
Swetha Alexander, MD, a rheumatology fellow at the University of Utah, Salt Lake City, who was not part of the study, said in an interview, “It is an excellent and timely study addressing the racial disparities and inequities surrounding axSpA diagnosis. It highlights the delay in diagnosis and increased burden of disease among Black Americans.”
She said the study may prompt a further look into barriers to care for Black Americans and their beliefs regarding seeking health care for their pain.
Higher rates of nonradiographic axSpA among Black patients
The rate of nonradiographic axSpA among Black Americans was more than twice that of their White counterparts, which, Dr. Alexander noted, could make it more difficult to diagnose axSpA in that population.
The odds ratio for Black patients having nonradiographic axSpA, compared with Whites, was 2.265 (95% CI, 1.082-4.999; P = .035), after adjustment for age, sex, and HLA-B27 status.
Adult patients with axSpA were identified from rheumatology clinics at four major hospital systems and one private clinic in Shelby County, Tenn., between 2011 and 2021. Patients met modified New York (mNY) or Assessment of Spondyloarthritis International Society (ASAS) criteria as assessed by reviewers.
The authors and Dr. Alexander reported no relevant financial relationships.
CLEVELAND – Documenting the prevalence of axial spondyloarthritis (axSpA) among Black Americans has been difficult because of little published data, but new research suggests that when Black Americans do have the disease, it seems to be more severe.
Iman Abutineh, MD, of the University of Tennessee, Memphis, discussed her team’s work at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
A total of 244 patients with axSpA were identified, including 168 (69%) males, 78 (32%) Black patients, and 143 (59%) White patients.
Average age of onset for patients overall was 27.7 years, and age at diagnosis was 36.1 years with a 7-year delay in diagnosis. Sixty-six (27%) patients had nonradiographic axSpA, 83% were on tumor necrosis factor inhibitors, and 38% were prescribed glucocorticoids.
The researchers found several differences by race.
White patients were more likely to be HLA-B27 positive (77% vs. 59%; P = .010). White patients also had higher prevalence of psoriasis, coronary artery disease, and family history of SpA. White females had a higher prevalence of inflammatory bowel disease, fibromyalgia, depression, and lower grades of sacroiliitis.
Black patients had more hip involvement
A higher percentage of Black patients had elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and hip involvement. In comparing hip involvement, the researchers found that 39 (39%) White males had hip involvement as did 9 (21%) White females. In comparison, 22 (45%) Black men in the study and 14 (54%) Black women showed hip involvement (P = .035).
After adjustment for age, sex, HLA-B27, and insurance status, Black patients had higher grades of sacroiliitis with an odds ratio of 2.32 (95% confidence interval, 1.23-4.44). Black patients had a numerically longer delay in diagnosis, compared with Whites (7.93 vs. 6.64 years), but this did not achieve statistical significance (P = .454), the researchers wrote.
Study addresses racial disparities
“Traditionally we think of axial spondyloarthritis largely in Caucasian males who are HLA-B27 positive,” Dr. Abutineh said, “and we found that there is still a significant portion of patients who don’t meet the criteria that do have disease that is very significant.”
Although actual prevalence was not clear from this study, Dr. Abutineh said their data suggest a 3-to-1 ratio of White-to-Black patients with spondyloarthritis, “but of the Black patients who are diagnosed, their disease is almost always more severe. That points to why it’s important to have a high index of suspicion for this disease in that patient population because if you miss it, it could be detrimental to the patients.”
Swetha Alexander, MD, a rheumatology fellow at the University of Utah, Salt Lake City, who was not part of the study, said in an interview, “It is an excellent and timely study addressing the racial disparities and inequities surrounding axSpA diagnosis. It highlights the delay in diagnosis and increased burden of disease among Black Americans.”
She said the study may prompt a further look into barriers to care for Black Americans and their beliefs regarding seeking health care for their pain.
Higher rates of nonradiographic axSpA among Black patients
The rate of nonradiographic axSpA among Black Americans was more than twice that of their White counterparts, which, Dr. Alexander noted, could make it more difficult to diagnose axSpA in that population.
The odds ratio for Black patients having nonradiographic axSpA, compared with Whites, was 2.265 (95% CI, 1.082-4.999; P = .035), after adjustment for age, sex, and HLA-B27 status.
Adult patients with axSpA were identified from rheumatology clinics at four major hospital systems and one private clinic in Shelby County, Tenn., between 2011 and 2021. Patients met modified New York (mNY) or Assessment of Spondyloarthritis International Society (ASAS) criteria as assessed by reviewers.
The authors and Dr. Alexander reported no relevant financial relationships.
CLEVELAND – Documenting the prevalence of axial spondyloarthritis (axSpA) among Black Americans has been difficult because of little published data, but new research suggests that when Black Americans do have the disease, it seems to be more severe.
Iman Abutineh, MD, of the University of Tennessee, Memphis, discussed her team’s work at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
A total of 244 patients with axSpA were identified, including 168 (69%) males, 78 (32%) Black patients, and 143 (59%) White patients.
Average age of onset for patients overall was 27.7 years, and age at diagnosis was 36.1 years with a 7-year delay in diagnosis. Sixty-six (27%) patients had nonradiographic axSpA, 83% were on tumor necrosis factor inhibitors, and 38% were prescribed glucocorticoids.
The researchers found several differences by race.
White patients were more likely to be HLA-B27 positive (77% vs. 59%; P = .010). White patients also had higher prevalence of psoriasis, coronary artery disease, and family history of SpA. White females had a higher prevalence of inflammatory bowel disease, fibromyalgia, depression, and lower grades of sacroiliitis.
Black patients had more hip involvement
A higher percentage of Black patients had elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and hip involvement. In comparing hip involvement, the researchers found that 39 (39%) White males had hip involvement as did 9 (21%) White females. In comparison, 22 (45%) Black men in the study and 14 (54%) Black women showed hip involvement (P = .035).
After adjustment for age, sex, HLA-B27, and insurance status, Black patients had higher grades of sacroiliitis with an odds ratio of 2.32 (95% confidence interval, 1.23-4.44). Black patients had a numerically longer delay in diagnosis, compared with Whites (7.93 vs. 6.64 years), but this did not achieve statistical significance (P = .454), the researchers wrote.
Study addresses racial disparities
“Traditionally we think of axial spondyloarthritis largely in Caucasian males who are HLA-B27 positive,” Dr. Abutineh said, “and we found that there is still a significant portion of patients who don’t meet the criteria that do have disease that is very significant.”
Although actual prevalence was not clear from this study, Dr. Abutineh said their data suggest a 3-to-1 ratio of White-to-Black patients with spondyloarthritis, “but of the Black patients who are diagnosed, their disease is almost always more severe. That points to why it’s important to have a high index of suspicion for this disease in that patient population because if you miss it, it could be detrimental to the patients.”
Swetha Alexander, MD, a rheumatology fellow at the University of Utah, Salt Lake City, who was not part of the study, said in an interview, “It is an excellent and timely study addressing the racial disparities and inequities surrounding axSpA diagnosis. It highlights the delay in diagnosis and increased burden of disease among Black Americans.”
She said the study may prompt a further look into barriers to care for Black Americans and their beliefs regarding seeking health care for their pain.
Higher rates of nonradiographic axSpA among Black patients
The rate of nonradiographic axSpA among Black Americans was more than twice that of their White counterparts, which, Dr. Alexander noted, could make it more difficult to diagnose axSpA in that population.
The odds ratio for Black patients having nonradiographic axSpA, compared with Whites, was 2.265 (95% CI, 1.082-4.999; P = .035), after adjustment for age, sex, and HLA-B27 status.
Adult patients with axSpA were identified from rheumatology clinics at four major hospital systems and one private clinic in Shelby County, Tenn., between 2011 and 2021. Patients met modified New York (mNY) or Assessment of Spondyloarthritis International Society (ASAS) criteria as assessed by reviewers.
The authors and Dr. Alexander reported no relevant financial relationships.
AT SPARTAN 2023
Novel antibody safe, promising for ATTR in phase 1 trial
, a new study suggests.
Currently, the only drug approved to treat ATTR is tafamidis, which improves survival and reduces hospitalizations, but does not reverse disease symptoms, the authors noted.
NI006 is a recombinant human anti-ATTR antibody given by infusion that was developed to trigger removal of ATTR by the body’s phagocytic immune cells.
Use of the drug was not associated with serious drug-related adverse events, though mild and moderate adverse events did occur.
Median N-terminal pro–B-type natriuretic peptide (NT-proBNP) and troponin T levels also seemed to be reduced over the study period.
Given the success of the antibody in this initial 40-patient trial, a larger phase-3 placebo-controlled trial is planned and expected to launch in the second half of 2023, said lead author Pablo Garcia-Pavia, MD, of Hospital Universitario Puerta de Hierro and the Spanish National Cardiovascular Research Institute, Madrid.
However, “The design of appropriate phase-3 trials to demonstrate efficacy of drugs for ATTR-CM is becoming more complicated and challenging,” he said.
“Increased awareness of the disease and advances in cardiac imaging techniques have led to recognition of a larger number of patients with ATTR-CM who have a different clinical profile and a different prognosis than the patients who were diagnosed in previous years and were enrolled in the initial trials of stabilizers,” Dr. Garcia-Pavia added.
“Moreover, the availability of tafamidis, and hopefully soon other medications to treat ATTR-CM has complicated the design of new clinical trials because of the heterogenicity of treatments that patients might receive,” he said. “Therefore, it is critical to plan the design very well.”
Dr. Garcia-Pavia presented the findings on NI006 at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2023 sessions. The study was published simultaneously in the New England Journal of Medicine.
No serious adverse events
For the phase-1, double-blind, multicenter study, the investigators randomly assigned (2:1 ratio) 40 patients (median age, 72 years; 98% men) with wild-type or variant ATTR cardiomyopathy and chronic heart failure to receive IV infusions of either NI006, at one of six doses ranging from 0.3 mg/kg to 60 mg/kg of body weight, or placebo every 4 weeks for 4 months.
After the four infusions, participants were enrolled in an open-label extension phase in which they received eight NI006 infusions with stepwise increases in the dose.
Participants had a confirmed diagnosis of ATTR-CM; left ventricular wall thickness of at least 14 mm; left ventricular ejection fraction of at least 40%; New York Heart Association class I, II, or III; estimated glomerular filtration rate of more than 30 mL/min per 1.73 m2; and an NT-proBNP level of 600 to 6,000 pg/mL.
Most (36) were receiving tafamidis, with a median treatment duration of 7 months; other ATTR-specific drugs were not permitted. Patients randomly assigned to receive NI006 seemed to have more advanced disease compared with those assigned to placebo.
Adherence to the trial protocol was high: Thirty-four patients received the four scheduled infusions during the ascending-dose phase, and 34 of 35 patients who completed this phase subsequently enrolled in the open-label extension.
No apparent drug-related serious adverse events were reported. However, during the ascending-dose phase, 38 patients had at least one adverse event, most of which were mild or moderate; of the 191 total events, 124 were grade 1 and 60 were grade 2 (most commonly heart failure and arrhythmias). Three patients had cytokine release syndrome; all three completed treatment through the extension phase.
Musculoskeletal events increased with ascending doses of NI006, which led two patients to withdraw from the trial.
At doses of at least 10 mg/kg, cardiac tracer uptake on scintigraphy and extracellular volume on cardiac MRI, both of which are imaging-based surrogate markers of cardiac amyloid load, appeared to be reduced over 12 months.
Because NI006 stimulates the patient’s own immune system to eliminate cardiac amyloid fibrils, one session chair at the meeting wondered whether NI006 represented the “rise of immunology in cardiology,” and whether biologics might follow.
Another questioned how removing amyloid might affect cardiac function. The echocardiographic findings gathered so far don’t indicate dysfunction, “but this is a small trial, and we need more data,” Dr. Garcia-Pavia said.
Tempered excitement
In a comment, Ronald Witteles, MD, professor of cardiovascular medicine, Stanford (Calif.) University, and founder/codirector of the Stanford Amyloid Center, said that “antibody-based amyloid removal strategies are not currently clinically available and represent a fundamentally different mechanism to treat the disease from what we currently have.
“While the data are encouraging and will generate excitement for later-phase studies, we’re talking about small numbers of patients and nothing definitive should be drawn from this data,” said Dr. Witteles, deputy editor of JACC: CardioOncology.
“The biggest caveat is that similar approaches of antibody removal of amyloid deposits for other forms of amyloidosis — most notably AL amyloidosis (amyloid light chain or primary amyloidosis) – have failed in late-phase trials. Although there is reason to believe that ATTR amyloidosis may be more amenable to improvements with amyloid fibril removal than AL amyloidosis, the unimpressive results in other forms of amyloidosis still do temper the excitement to a degree.”
Like Dr. Garcia-Pavia, Dr. Witteles said, “Ultimately, we are going to need to see a phase 3 clinical trial which shows that NI006 – on top of standard-of-care treatment – improves hard outcomes in the disease. As treatment options likely expand in the coming years, that is likely to be a harder and harder bar to reach.”
Furthermore, although the safety profile was favorable overall, it “wasn’t entirely clean,” given cytokine release syndrome in three patients, a lowering of platelet counts in a couple of patients, and musculoskeletal side effects that triggered two to withdraw from the study. “Unless that changes,” he said, “that will be a barrier for some patients.”
Overall, he noted, “With the vast majority of patients being able to be diagnosed noninvasively, and with treatment options now available, we have seen a true explosion in the number of patients being diagnosed.
“But we also know that the large majority ... are still not getting diagnosed or are having huge delays in diagnosis. As such, the biggest thing we can do for patients with the disease is to continue to educate people about it,” Dr. Witteles concluded.
The study was funded by Neurimmune. Dr. Garcia-Pavia disclosed ties to Alexion, Alnylam Pharmaceuticals, AstraZeneca, Attralus, BridgeBio, General Electric, Intellia, Ionis Pharmaceuticals, Neurimmune, Novo Nordisk, and Pfizer. Dr. Witteles reported ties to Alexion, Alnylam, AstraZeneca, BridgeBio, Intellia, Ionis, Janssen, Novo Nordisk, and Pfizer.
A version of this article first appeared on Medscape.com.
, a new study suggests.
Currently, the only drug approved to treat ATTR is tafamidis, which improves survival and reduces hospitalizations, but does not reverse disease symptoms, the authors noted.
NI006 is a recombinant human anti-ATTR antibody given by infusion that was developed to trigger removal of ATTR by the body’s phagocytic immune cells.
Use of the drug was not associated with serious drug-related adverse events, though mild and moderate adverse events did occur.
Median N-terminal pro–B-type natriuretic peptide (NT-proBNP) and troponin T levels also seemed to be reduced over the study period.
Given the success of the antibody in this initial 40-patient trial, a larger phase-3 placebo-controlled trial is planned and expected to launch in the second half of 2023, said lead author Pablo Garcia-Pavia, MD, of Hospital Universitario Puerta de Hierro and the Spanish National Cardiovascular Research Institute, Madrid.
However, “The design of appropriate phase-3 trials to demonstrate efficacy of drugs for ATTR-CM is becoming more complicated and challenging,” he said.
“Increased awareness of the disease and advances in cardiac imaging techniques have led to recognition of a larger number of patients with ATTR-CM who have a different clinical profile and a different prognosis than the patients who were diagnosed in previous years and were enrolled in the initial trials of stabilizers,” Dr. Garcia-Pavia added.
“Moreover, the availability of tafamidis, and hopefully soon other medications to treat ATTR-CM has complicated the design of new clinical trials because of the heterogenicity of treatments that patients might receive,” he said. “Therefore, it is critical to plan the design very well.”
Dr. Garcia-Pavia presented the findings on NI006 at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2023 sessions. The study was published simultaneously in the New England Journal of Medicine.
No serious adverse events
For the phase-1, double-blind, multicenter study, the investigators randomly assigned (2:1 ratio) 40 patients (median age, 72 years; 98% men) with wild-type or variant ATTR cardiomyopathy and chronic heart failure to receive IV infusions of either NI006, at one of six doses ranging from 0.3 mg/kg to 60 mg/kg of body weight, or placebo every 4 weeks for 4 months.
After the four infusions, participants were enrolled in an open-label extension phase in which they received eight NI006 infusions with stepwise increases in the dose.
Participants had a confirmed diagnosis of ATTR-CM; left ventricular wall thickness of at least 14 mm; left ventricular ejection fraction of at least 40%; New York Heart Association class I, II, or III; estimated glomerular filtration rate of more than 30 mL/min per 1.73 m2; and an NT-proBNP level of 600 to 6,000 pg/mL.
Most (36) were receiving tafamidis, with a median treatment duration of 7 months; other ATTR-specific drugs were not permitted. Patients randomly assigned to receive NI006 seemed to have more advanced disease compared with those assigned to placebo.
Adherence to the trial protocol was high: Thirty-four patients received the four scheduled infusions during the ascending-dose phase, and 34 of 35 patients who completed this phase subsequently enrolled in the open-label extension.
No apparent drug-related serious adverse events were reported. However, during the ascending-dose phase, 38 patients had at least one adverse event, most of which were mild or moderate; of the 191 total events, 124 were grade 1 and 60 were grade 2 (most commonly heart failure and arrhythmias). Three patients had cytokine release syndrome; all three completed treatment through the extension phase.
Musculoskeletal events increased with ascending doses of NI006, which led two patients to withdraw from the trial.
At doses of at least 10 mg/kg, cardiac tracer uptake on scintigraphy and extracellular volume on cardiac MRI, both of which are imaging-based surrogate markers of cardiac amyloid load, appeared to be reduced over 12 months.
Because NI006 stimulates the patient’s own immune system to eliminate cardiac amyloid fibrils, one session chair at the meeting wondered whether NI006 represented the “rise of immunology in cardiology,” and whether biologics might follow.
Another questioned how removing amyloid might affect cardiac function. The echocardiographic findings gathered so far don’t indicate dysfunction, “but this is a small trial, and we need more data,” Dr. Garcia-Pavia said.
Tempered excitement
In a comment, Ronald Witteles, MD, professor of cardiovascular medicine, Stanford (Calif.) University, and founder/codirector of the Stanford Amyloid Center, said that “antibody-based amyloid removal strategies are not currently clinically available and represent a fundamentally different mechanism to treat the disease from what we currently have.
“While the data are encouraging and will generate excitement for later-phase studies, we’re talking about small numbers of patients and nothing definitive should be drawn from this data,” said Dr. Witteles, deputy editor of JACC: CardioOncology.
“The biggest caveat is that similar approaches of antibody removal of amyloid deposits for other forms of amyloidosis — most notably AL amyloidosis (amyloid light chain or primary amyloidosis) – have failed in late-phase trials. Although there is reason to believe that ATTR amyloidosis may be more amenable to improvements with amyloid fibril removal than AL amyloidosis, the unimpressive results in other forms of amyloidosis still do temper the excitement to a degree.”
Like Dr. Garcia-Pavia, Dr. Witteles said, “Ultimately, we are going to need to see a phase 3 clinical trial which shows that NI006 – on top of standard-of-care treatment – improves hard outcomes in the disease. As treatment options likely expand in the coming years, that is likely to be a harder and harder bar to reach.”
Furthermore, although the safety profile was favorable overall, it “wasn’t entirely clean,” given cytokine release syndrome in three patients, a lowering of platelet counts in a couple of patients, and musculoskeletal side effects that triggered two to withdraw from the study. “Unless that changes,” he said, “that will be a barrier for some patients.”
Overall, he noted, “With the vast majority of patients being able to be diagnosed noninvasively, and with treatment options now available, we have seen a true explosion in the number of patients being diagnosed.
“But we also know that the large majority ... are still not getting diagnosed or are having huge delays in diagnosis. As such, the biggest thing we can do for patients with the disease is to continue to educate people about it,” Dr. Witteles concluded.
The study was funded by Neurimmune. Dr. Garcia-Pavia disclosed ties to Alexion, Alnylam Pharmaceuticals, AstraZeneca, Attralus, BridgeBio, General Electric, Intellia, Ionis Pharmaceuticals, Neurimmune, Novo Nordisk, and Pfizer. Dr. Witteles reported ties to Alexion, Alnylam, AstraZeneca, BridgeBio, Intellia, Ionis, Janssen, Novo Nordisk, and Pfizer.
A version of this article first appeared on Medscape.com.
, a new study suggests.
Currently, the only drug approved to treat ATTR is tafamidis, which improves survival and reduces hospitalizations, but does not reverse disease symptoms, the authors noted.
NI006 is a recombinant human anti-ATTR antibody given by infusion that was developed to trigger removal of ATTR by the body’s phagocytic immune cells.
Use of the drug was not associated with serious drug-related adverse events, though mild and moderate adverse events did occur.
Median N-terminal pro–B-type natriuretic peptide (NT-proBNP) and troponin T levels also seemed to be reduced over the study period.
Given the success of the antibody in this initial 40-patient trial, a larger phase-3 placebo-controlled trial is planned and expected to launch in the second half of 2023, said lead author Pablo Garcia-Pavia, MD, of Hospital Universitario Puerta de Hierro and the Spanish National Cardiovascular Research Institute, Madrid.
However, “The design of appropriate phase-3 trials to demonstrate efficacy of drugs for ATTR-CM is becoming more complicated and challenging,” he said.
“Increased awareness of the disease and advances in cardiac imaging techniques have led to recognition of a larger number of patients with ATTR-CM who have a different clinical profile and a different prognosis than the patients who were diagnosed in previous years and were enrolled in the initial trials of stabilizers,” Dr. Garcia-Pavia added.
“Moreover, the availability of tafamidis, and hopefully soon other medications to treat ATTR-CM has complicated the design of new clinical trials because of the heterogenicity of treatments that patients might receive,” he said. “Therefore, it is critical to plan the design very well.”
Dr. Garcia-Pavia presented the findings on NI006 at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2023 sessions. The study was published simultaneously in the New England Journal of Medicine.
No serious adverse events
For the phase-1, double-blind, multicenter study, the investigators randomly assigned (2:1 ratio) 40 patients (median age, 72 years; 98% men) with wild-type or variant ATTR cardiomyopathy and chronic heart failure to receive IV infusions of either NI006, at one of six doses ranging from 0.3 mg/kg to 60 mg/kg of body weight, or placebo every 4 weeks for 4 months.
After the four infusions, participants were enrolled in an open-label extension phase in which they received eight NI006 infusions with stepwise increases in the dose.
Participants had a confirmed diagnosis of ATTR-CM; left ventricular wall thickness of at least 14 mm; left ventricular ejection fraction of at least 40%; New York Heart Association class I, II, or III; estimated glomerular filtration rate of more than 30 mL/min per 1.73 m2; and an NT-proBNP level of 600 to 6,000 pg/mL.
Most (36) were receiving tafamidis, with a median treatment duration of 7 months; other ATTR-specific drugs were not permitted. Patients randomly assigned to receive NI006 seemed to have more advanced disease compared with those assigned to placebo.
Adherence to the trial protocol was high: Thirty-four patients received the four scheduled infusions during the ascending-dose phase, and 34 of 35 patients who completed this phase subsequently enrolled in the open-label extension.
No apparent drug-related serious adverse events were reported. However, during the ascending-dose phase, 38 patients had at least one adverse event, most of which were mild or moderate; of the 191 total events, 124 were grade 1 and 60 were grade 2 (most commonly heart failure and arrhythmias). Three patients had cytokine release syndrome; all three completed treatment through the extension phase.
Musculoskeletal events increased with ascending doses of NI006, which led two patients to withdraw from the trial.
At doses of at least 10 mg/kg, cardiac tracer uptake on scintigraphy and extracellular volume on cardiac MRI, both of which are imaging-based surrogate markers of cardiac amyloid load, appeared to be reduced over 12 months.
Because NI006 stimulates the patient’s own immune system to eliminate cardiac amyloid fibrils, one session chair at the meeting wondered whether NI006 represented the “rise of immunology in cardiology,” and whether biologics might follow.
Another questioned how removing amyloid might affect cardiac function. The echocardiographic findings gathered so far don’t indicate dysfunction, “but this is a small trial, and we need more data,” Dr. Garcia-Pavia said.
Tempered excitement
In a comment, Ronald Witteles, MD, professor of cardiovascular medicine, Stanford (Calif.) University, and founder/codirector of the Stanford Amyloid Center, said that “antibody-based amyloid removal strategies are not currently clinically available and represent a fundamentally different mechanism to treat the disease from what we currently have.
“While the data are encouraging and will generate excitement for later-phase studies, we’re talking about small numbers of patients and nothing definitive should be drawn from this data,” said Dr. Witteles, deputy editor of JACC: CardioOncology.
“The biggest caveat is that similar approaches of antibody removal of amyloid deposits for other forms of amyloidosis — most notably AL amyloidosis (amyloid light chain or primary amyloidosis) – have failed in late-phase trials. Although there is reason to believe that ATTR amyloidosis may be more amenable to improvements with amyloid fibril removal than AL amyloidosis, the unimpressive results in other forms of amyloidosis still do temper the excitement to a degree.”
Like Dr. Garcia-Pavia, Dr. Witteles said, “Ultimately, we are going to need to see a phase 3 clinical trial which shows that NI006 – on top of standard-of-care treatment – improves hard outcomes in the disease. As treatment options likely expand in the coming years, that is likely to be a harder and harder bar to reach.”
Furthermore, although the safety profile was favorable overall, it “wasn’t entirely clean,” given cytokine release syndrome in three patients, a lowering of platelet counts in a couple of patients, and musculoskeletal side effects that triggered two to withdraw from the study. “Unless that changes,” he said, “that will be a barrier for some patients.”
Overall, he noted, “With the vast majority of patients being able to be diagnosed noninvasively, and with treatment options now available, we have seen a true explosion in the number of patients being diagnosed.
“But we also know that the large majority ... are still not getting diagnosed or are having huge delays in diagnosis. As such, the biggest thing we can do for patients with the disease is to continue to educate people about it,” Dr. Witteles concluded.
The study was funded by Neurimmune. Dr. Garcia-Pavia disclosed ties to Alexion, Alnylam Pharmaceuticals, AstraZeneca, Attralus, BridgeBio, General Electric, Intellia, Ionis Pharmaceuticals, Neurimmune, Novo Nordisk, and Pfizer. Dr. Witteles reported ties to Alexion, Alnylam, AstraZeneca, BridgeBio, Intellia, Ionis, Janssen, Novo Nordisk, and Pfizer.
A version of this article first appeared on Medscape.com.
FROM ESC HEART FAILURE 2023
FDA approves autoinjector pen for Humira biosimilar, Cyltezo
The U.S. Food and Drug Administration on May 22 approved a new autoinjection option for adalimumab-adbm (Cyltezo), a biosimilar to AbbVie’s adalimumab (Humira), ahead of Cyltezo’s commercial launch on July 1, 2023.
Cyltezo was approved by the FDA in 2017 as a prefilled syringe and was the first biosimilar deemed to be interchangeable with Humira in 2021. It is indicated to treat multiple chronic inflammatory conditions, including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and hidradenitis suppurativa. This new design, which features one-button, three-step activation, has been certified as an “Ease of Use” product by the Arthritis Foundation, Boehringer Ingelheim said in a press release. The 40-mg, prefilled Cyltezo Pen will be available in two-, four-, and six-pack options.
“The FDA approval of the Cyltezo Pen is great news for patients living with chronic inflammatory diseases who may prefer administering the medication needed to manage their conditions via an autoinjector,” said Stephen Pagnotta, the executive director and biosimilar commercial lead at Boehringer Ingelheim in a statement; “we’re excited to be able to offer the Cyltezo Pen as an additional option to patients at Cyltezo’s launch on July 1.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration on May 22 approved a new autoinjection option for adalimumab-adbm (Cyltezo), a biosimilar to AbbVie’s adalimumab (Humira), ahead of Cyltezo’s commercial launch on July 1, 2023.
Cyltezo was approved by the FDA in 2017 as a prefilled syringe and was the first biosimilar deemed to be interchangeable with Humira in 2021. It is indicated to treat multiple chronic inflammatory conditions, including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and hidradenitis suppurativa. This new design, which features one-button, three-step activation, has been certified as an “Ease of Use” product by the Arthritis Foundation, Boehringer Ingelheim said in a press release. The 40-mg, prefilled Cyltezo Pen will be available in two-, four-, and six-pack options.
“The FDA approval of the Cyltezo Pen is great news for patients living with chronic inflammatory diseases who may prefer administering the medication needed to manage their conditions via an autoinjector,” said Stephen Pagnotta, the executive director and biosimilar commercial lead at Boehringer Ingelheim in a statement; “we’re excited to be able to offer the Cyltezo Pen as an additional option to patients at Cyltezo’s launch on July 1.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration on May 22 approved a new autoinjection option for adalimumab-adbm (Cyltezo), a biosimilar to AbbVie’s adalimumab (Humira), ahead of Cyltezo’s commercial launch on July 1, 2023.
Cyltezo was approved by the FDA in 2017 as a prefilled syringe and was the first biosimilar deemed to be interchangeable with Humira in 2021. It is indicated to treat multiple chronic inflammatory conditions, including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and hidradenitis suppurativa. This new design, which features one-button, three-step activation, has been certified as an “Ease of Use” product by the Arthritis Foundation, Boehringer Ingelheim said in a press release. The 40-mg, prefilled Cyltezo Pen will be available in two-, four-, and six-pack options.
“The FDA approval of the Cyltezo Pen is great news for patients living with chronic inflammatory diseases who may prefer administering the medication needed to manage their conditions via an autoinjector,” said Stephen Pagnotta, the executive director and biosimilar commercial lead at Boehringer Ingelheim in a statement; “we’re excited to be able to offer the Cyltezo Pen as an additional option to patients at Cyltezo’s launch on July 1.”
A version of this article first appeared on Medscape.com.
Docs can help combat TikTok misinformation on rare psychiatric disorder
SAN FRANCISCO – , new research shows.
These findings, say investigators, underscore the need for mental health professionals to help counter the spread of false information by creating accurate content and posting it on the popular social media platform.
“Health care professionals need to make engaging content to post on social media platforms like YouTube and especially TikTok, to reach wider audiences and combat misinformation about DID,” study investigator Isreal Bladimir Munoz, a fourth-year medical student at University of Texas, Galveston, said in an interview.
The findings were presented at the annual meeting of the American Psychiatric Association.
Popularized by the media
A rare condition affecting less than 1% of the general population, DID involves two or more distinct personality states, along with changes in behavior and memory gaps.
The condition has been popularized in books and the media. Movies such as “Split,” “Psycho,” and “Fight Club” all feature characters with DID.
“These bring awareness about the condition, but also often sensationalize or stigmatize it and reinforce stereotypes,” said Mr. Munoz.
In recent years, social media has become an integral part of everyday life. An estimated 4.2 billion people actively frequent sites such as YouTube, TikTok, Twitter, and Meta.
Although social media allows for instant communication and the opportunity for self-expression, there are mounting concerns about the spread of misinformation and its potential impact on mental health and privacy, said Mr. Munoz.
To evaluate the quality and accuracy of information about DID on social media, investigators analyzed 60 YouTube and 97 TikTok videos on the condition.
To evaluate the reliability and the intent and reliability of videos, researchers used the modified DISCERN instrument and the Global Quality Scale, a five-point rating system.
Using these tools, the researchers determined whether the selected videos were useful, misleading, or neither. Mr. Munoz said videos were classified as useful if they contained accurate information about the condition and its pathogenesis, treatment, and management.
Researchers determined that for YouTube videos, 51.7% were useful, 6.6% were misleading, and 34.7% were neither. About 43.3% of these videos involved interviews, 21.7% were educational, 15% involved personal stories, 8.3% were films/TV programs, 5% were comedy skits, and 3.3% were another content type.
The highest quality YouTube videos were from educational organizations and health care professionals. The least accurate videos came from independent users and film/TV sources.
As for TikTok videos on DID, only 5.2% were useful, 10.3% were misleading, and 41.7% were neither.
The main sources for TikTok videos were independent organizations, whereas podcasts and film/TV were the least common sources.
The findings, said Mr. Munoz, underscore the need for medical professionals to develop high-quality content about DID and post it on TikTok to counter misinformation on social media.
Call to action
In a comment, Howard Y. Liu, MD, a child and adolescent psychiatrist and chair of the department of psychiatry, University of Nebraska, Omaha, described the study as “compelling.”
When it comes to public health messages, it’s important to know what social media people are using. Today’s parents are on Twitter and Facebook, whereas their children are more likely to be checking out YouTube and TikTok, said Dr. Liu, chair of the APA’s Council on Communications.
“TikTok is critical because that’s where all the youth eyeballs are,” he said.
The medical profession needs to engage with the platform to reach this next-generation audience and help stop the spread of misinformation about DID, said Dr. Liu. He noted that the APA is looking into undertaking such an initiative.
The study investigators report no relevant disclosures. Dr. Liu reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , new research shows.
These findings, say investigators, underscore the need for mental health professionals to help counter the spread of false information by creating accurate content and posting it on the popular social media platform.
“Health care professionals need to make engaging content to post on social media platforms like YouTube and especially TikTok, to reach wider audiences and combat misinformation about DID,” study investigator Isreal Bladimir Munoz, a fourth-year medical student at University of Texas, Galveston, said in an interview.
The findings were presented at the annual meeting of the American Psychiatric Association.
Popularized by the media
A rare condition affecting less than 1% of the general population, DID involves two or more distinct personality states, along with changes in behavior and memory gaps.
The condition has been popularized in books and the media. Movies such as “Split,” “Psycho,” and “Fight Club” all feature characters with DID.
“These bring awareness about the condition, but also often sensationalize or stigmatize it and reinforce stereotypes,” said Mr. Munoz.
In recent years, social media has become an integral part of everyday life. An estimated 4.2 billion people actively frequent sites such as YouTube, TikTok, Twitter, and Meta.
Although social media allows for instant communication and the opportunity for self-expression, there are mounting concerns about the spread of misinformation and its potential impact on mental health and privacy, said Mr. Munoz.
To evaluate the quality and accuracy of information about DID on social media, investigators analyzed 60 YouTube and 97 TikTok videos on the condition.
To evaluate the reliability and the intent and reliability of videos, researchers used the modified DISCERN instrument and the Global Quality Scale, a five-point rating system.
Using these tools, the researchers determined whether the selected videos were useful, misleading, or neither. Mr. Munoz said videos were classified as useful if they contained accurate information about the condition and its pathogenesis, treatment, and management.
Researchers determined that for YouTube videos, 51.7% were useful, 6.6% were misleading, and 34.7% were neither. About 43.3% of these videos involved interviews, 21.7% were educational, 15% involved personal stories, 8.3% were films/TV programs, 5% were comedy skits, and 3.3% were another content type.
The highest quality YouTube videos were from educational organizations and health care professionals. The least accurate videos came from independent users and film/TV sources.
As for TikTok videos on DID, only 5.2% were useful, 10.3% were misleading, and 41.7% were neither.
The main sources for TikTok videos were independent organizations, whereas podcasts and film/TV were the least common sources.
The findings, said Mr. Munoz, underscore the need for medical professionals to develop high-quality content about DID and post it on TikTok to counter misinformation on social media.
Call to action
In a comment, Howard Y. Liu, MD, a child and adolescent psychiatrist and chair of the department of psychiatry, University of Nebraska, Omaha, described the study as “compelling.”
When it comes to public health messages, it’s important to know what social media people are using. Today’s parents are on Twitter and Facebook, whereas their children are more likely to be checking out YouTube and TikTok, said Dr. Liu, chair of the APA’s Council on Communications.
“TikTok is critical because that’s where all the youth eyeballs are,” he said.
The medical profession needs to engage with the platform to reach this next-generation audience and help stop the spread of misinformation about DID, said Dr. Liu. He noted that the APA is looking into undertaking such an initiative.
The study investigators report no relevant disclosures. Dr. Liu reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , new research shows.
These findings, say investigators, underscore the need for mental health professionals to help counter the spread of false information by creating accurate content and posting it on the popular social media platform.
“Health care professionals need to make engaging content to post on social media platforms like YouTube and especially TikTok, to reach wider audiences and combat misinformation about DID,” study investigator Isreal Bladimir Munoz, a fourth-year medical student at University of Texas, Galveston, said in an interview.
The findings were presented at the annual meeting of the American Psychiatric Association.
Popularized by the media
A rare condition affecting less than 1% of the general population, DID involves two or more distinct personality states, along with changes in behavior and memory gaps.
The condition has been popularized in books and the media. Movies such as “Split,” “Psycho,” and “Fight Club” all feature characters with DID.
“These bring awareness about the condition, but also often sensationalize or stigmatize it and reinforce stereotypes,” said Mr. Munoz.
In recent years, social media has become an integral part of everyday life. An estimated 4.2 billion people actively frequent sites such as YouTube, TikTok, Twitter, and Meta.
Although social media allows for instant communication and the opportunity for self-expression, there are mounting concerns about the spread of misinformation and its potential impact on mental health and privacy, said Mr. Munoz.
To evaluate the quality and accuracy of information about DID on social media, investigators analyzed 60 YouTube and 97 TikTok videos on the condition.
To evaluate the reliability and the intent and reliability of videos, researchers used the modified DISCERN instrument and the Global Quality Scale, a five-point rating system.
Using these tools, the researchers determined whether the selected videos were useful, misleading, or neither. Mr. Munoz said videos were classified as useful if they contained accurate information about the condition and its pathogenesis, treatment, and management.
Researchers determined that for YouTube videos, 51.7% were useful, 6.6% were misleading, and 34.7% were neither. About 43.3% of these videos involved interviews, 21.7% were educational, 15% involved personal stories, 8.3% were films/TV programs, 5% were comedy skits, and 3.3% were another content type.
The highest quality YouTube videos were from educational organizations and health care professionals. The least accurate videos came from independent users and film/TV sources.
As for TikTok videos on DID, only 5.2% were useful, 10.3% were misleading, and 41.7% were neither.
The main sources for TikTok videos were independent organizations, whereas podcasts and film/TV were the least common sources.
The findings, said Mr. Munoz, underscore the need for medical professionals to develop high-quality content about DID and post it on TikTok to counter misinformation on social media.
Call to action
In a comment, Howard Y. Liu, MD, a child and adolescent psychiatrist and chair of the department of psychiatry, University of Nebraska, Omaha, described the study as “compelling.”
When it comes to public health messages, it’s important to know what social media people are using. Today’s parents are on Twitter and Facebook, whereas their children are more likely to be checking out YouTube and TikTok, said Dr. Liu, chair of the APA’s Council on Communications.
“TikTok is critical because that’s where all the youth eyeballs are,” he said.
The medical profession needs to engage with the platform to reach this next-generation audience and help stop the spread of misinformation about DID, said Dr. Liu. He noted that the APA is looking into undertaking such an initiative.
The study investigators report no relevant disclosures. Dr. Liu reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
AT APA 2023
The family firearm often used in youth suicide
SAN FRANCISCO – , according to results of a novel “psychological autopsy study” of loved ones of youth who died by gun-related suicide.
Yet, families don’t always recognize the danger firearms pose to a young person with suicide risk factors, even when there is a young person in the house with a mental health condition, the data show.
Perhaps most importantly, many parents indicated that they would have removed firearms from the home if it had been suggested by their health care professionals.
The study was presented at the American Psychiatric Association annual meeting.
The message is very clear: Clinicians need to ask about guns and gun safety with patients and families, said study investigator Paul Nestadt, MD, of Johns Hopkins Bloomberg School of Public Health in Baltimore.
“It’s never illegal to ask about gun access and it’s medically relevant. Just do it,” he said during a briefing with reporters.
Grim statistics
Suicide rates have been climbing in the United States for the majority of the past 20 years. Suicide is the second most common cause of death among youth.
Dr. Nestadt noted that overall about 8% of suicide attempts result in death, but when an attempt involves a firearm the percentage jumps astronomically to 90%.
Research has shown that for every 10% increase in household firearms in a given community there is a 27% increase in youth suicide deaths.
“In the world of public health and mental health, we think about having access to firearms as an important risk factor for completed suicide. But in the United States, guns have become an important part of how many Americans see themselves,” Dr. Nestadt told reporters.
Research has shown that half of gun owners say owning a gun is central to their identity and three quarters say it’s essential to their freedom, he noted.
To explore these attitudes further, Dr. Nestadt and colleagues did 11 “psychological autopsy interviews” with the loved ones of nine young people aged 17-21 who died by gun-related suicide. They interviewed six mothers, three fathers, one sibling, and one close friend.
Most of the families had some level of “familial engagement” with firearms, Dr. Nestadt reported.
In more than two-thirds of the families, the youth used a family-owned firearm to commit suicide.
Notably, more than three-quarters of the youth had received mental health care before taking their lives, with many receiving care in the weeks prior to their suicide; 44% had made a prior suicide attempt.
In many cases, parents shared that they had not considered their family-owned firearms to be sources of danger and indicated that had their clinicians expressed concern about the gun in the home, they may have acted to reduce the risk by removing it.
Several also shared that they would have considered using Maryland’s Extreme Risk Protective Order Law if it had existed at the time and they had been made aware of it.
Extreme risk protection order (ERPO) laws, or “red flag laws,” prohibit individuals at risk for harming themselves or others from purchasing or owning a firearm.
Dr. Nestadt said youth suicide interventions “must acknowledge culturally embedded roots of identity formation while rescripting firearms from expressions of family cohesion to instruments that may undermine that cohesion.”
‘Courageous study’
Dr. Nestadt noted that while this study was challenging on many fronts, it took no convincing to get these grieving families to participate.
“They wanted to talk to us, especially because they were hopeful that our work could help prevent future suicides, but also they wanted to talk about their loved ones,” he said.
“When you lose someone to cancer, people give you hugs and flowers. When you lose someone to suicide, people don’t discuss it. Suicide has a stigma to it.”
Briefing moderator Howard Liu, MD, MBA, chair of the department of psychiatry, University of Nebraska Medical Center, Omaha, praised the study team for a “courageous study that really required a tremendous amount of vulnerability from the research team and clearly from the survivors as well.”
This is an “important and timely public health discussion,” said Dr. Liu, chair of the APA Council on Communications.
“We’re all facing this challenge of how do we reduce suicide across all ages, from youth to adults as well. This is a really vital discussion and such an important clue about access and trying to reduce access in a moment of impulsivity,” he added.
The study had no commercial funding. Dr. Nestadt and Dr. Liu report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , according to results of a novel “psychological autopsy study” of loved ones of youth who died by gun-related suicide.
Yet, families don’t always recognize the danger firearms pose to a young person with suicide risk factors, even when there is a young person in the house with a mental health condition, the data show.
Perhaps most importantly, many parents indicated that they would have removed firearms from the home if it had been suggested by their health care professionals.
The study was presented at the American Psychiatric Association annual meeting.
The message is very clear: Clinicians need to ask about guns and gun safety with patients and families, said study investigator Paul Nestadt, MD, of Johns Hopkins Bloomberg School of Public Health in Baltimore.
“It’s never illegal to ask about gun access and it’s medically relevant. Just do it,” he said during a briefing with reporters.
Grim statistics
Suicide rates have been climbing in the United States for the majority of the past 20 years. Suicide is the second most common cause of death among youth.
Dr. Nestadt noted that overall about 8% of suicide attempts result in death, but when an attempt involves a firearm the percentage jumps astronomically to 90%.
Research has shown that for every 10% increase in household firearms in a given community there is a 27% increase in youth suicide deaths.
“In the world of public health and mental health, we think about having access to firearms as an important risk factor for completed suicide. But in the United States, guns have become an important part of how many Americans see themselves,” Dr. Nestadt told reporters.
Research has shown that half of gun owners say owning a gun is central to their identity and three quarters say it’s essential to their freedom, he noted.
To explore these attitudes further, Dr. Nestadt and colleagues did 11 “psychological autopsy interviews” with the loved ones of nine young people aged 17-21 who died by gun-related suicide. They interviewed six mothers, three fathers, one sibling, and one close friend.
Most of the families had some level of “familial engagement” with firearms, Dr. Nestadt reported.
In more than two-thirds of the families, the youth used a family-owned firearm to commit suicide.
Notably, more than three-quarters of the youth had received mental health care before taking their lives, with many receiving care in the weeks prior to their suicide; 44% had made a prior suicide attempt.
In many cases, parents shared that they had not considered their family-owned firearms to be sources of danger and indicated that had their clinicians expressed concern about the gun in the home, they may have acted to reduce the risk by removing it.
Several also shared that they would have considered using Maryland’s Extreme Risk Protective Order Law if it had existed at the time and they had been made aware of it.
Extreme risk protection order (ERPO) laws, or “red flag laws,” prohibit individuals at risk for harming themselves or others from purchasing or owning a firearm.
Dr. Nestadt said youth suicide interventions “must acknowledge culturally embedded roots of identity formation while rescripting firearms from expressions of family cohesion to instruments that may undermine that cohesion.”
‘Courageous study’
Dr. Nestadt noted that while this study was challenging on many fronts, it took no convincing to get these grieving families to participate.
“They wanted to talk to us, especially because they were hopeful that our work could help prevent future suicides, but also they wanted to talk about their loved ones,” he said.
“When you lose someone to cancer, people give you hugs and flowers. When you lose someone to suicide, people don’t discuss it. Suicide has a stigma to it.”
Briefing moderator Howard Liu, MD, MBA, chair of the department of psychiatry, University of Nebraska Medical Center, Omaha, praised the study team for a “courageous study that really required a tremendous amount of vulnerability from the research team and clearly from the survivors as well.”
This is an “important and timely public health discussion,” said Dr. Liu, chair of the APA Council on Communications.
“We’re all facing this challenge of how do we reduce suicide across all ages, from youth to adults as well. This is a really vital discussion and such an important clue about access and trying to reduce access in a moment of impulsivity,” he added.
The study had no commercial funding. Dr. Nestadt and Dr. Liu report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , according to results of a novel “psychological autopsy study” of loved ones of youth who died by gun-related suicide.
Yet, families don’t always recognize the danger firearms pose to a young person with suicide risk factors, even when there is a young person in the house with a mental health condition, the data show.
Perhaps most importantly, many parents indicated that they would have removed firearms from the home if it had been suggested by their health care professionals.
The study was presented at the American Psychiatric Association annual meeting.
The message is very clear: Clinicians need to ask about guns and gun safety with patients and families, said study investigator Paul Nestadt, MD, of Johns Hopkins Bloomberg School of Public Health in Baltimore.
“It’s never illegal to ask about gun access and it’s medically relevant. Just do it,” he said during a briefing with reporters.
Grim statistics
Suicide rates have been climbing in the United States for the majority of the past 20 years. Suicide is the second most common cause of death among youth.
Dr. Nestadt noted that overall about 8% of suicide attempts result in death, but when an attempt involves a firearm the percentage jumps astronomically to 90%.
Research has shown that for every 10% increase in household firearms in a given community there is a 27% increase in youth suicide deaths.
“In the world of public health and mental health, we think about having access to firearms as an important risk factor for completed suicide. But in the United States, guns have become an important part of how many Americans see themselves,” Dr. Nestadt told reporters.
Research has shown that half of gun owners say owning a gun is central to their identity and three quarters say it’s essential to their freedom, he noted.
To explore these attitudes further, Dr. Nestadt and colleagues did 11 “psychological autopsy interviews” with the loved ones of nine young people aged 17-21 who died by gun-related suicide. They interviewed six mothers, three fathers, one sibling, and one close friend.
Most of the families had some level of “familial engagement” with firearms, Dr. Nestadt reported.
In more than two-thirds of the families, the youth used a family-owned firearm to commit suicide.
Notably, more than three-quarters of the youth had received mental health care before taking their lives, with many receiving care in the weeks prior to their suicide; 44% had made a prior suicide attempt.
In many cases, parents shared that they had not considered their family-owned firearms to be sources of danger and indicated that had their clinicians expressed concern about the gun in the home, they may have acted to reduce the risk by removing it.
Several also shared that they would have considered using Maryland’s Extreme Risk Protective Order Law if it had existed at the time and they had been made aware of it.
Extreme risk protection order (ERPO) laws, or “red flag laws,” prohibit individuals at risk for harming themselves or others from purchasing or owning a firearm.
Dr. Nestadt said youth suicide interventions “must acknowledge culturally embedded roots of identity formation while rescripting firearms from expressions of family cohesion to instruments that may undermine that cohesion.”
‘Courageous study’
Dr. Nestadt noted that while this study was challenging on many fronts, it took no convincing to get these grieving families to participate.
“They wanted to talk to us, especially because they were hopeful that our work could help prevent future suicides, but also they wanted to talk about their loved ones,” he said.
“When you lose someone to cancer, people give you hugs and flowers. When you lose someone to suicide, people don’t discuss it. Suicide has a stigma to it.”
Briefing moderator Howard Liu, MD, MBA, chair of the department of psychiatry, University of Nebraska Medical Center, Omaha, praised the study team for a “courageous study that really required a tremendous amount of vulnerability from the research team and clearly from the survivors as well.”
This is an “important and timely public health discussion,” said Dr. Liu, chair of the APA Council on Communications.
“We’re all facing this challenge of how do we reduce suicide across all ages, from youth to adults as well. This is a really vital discussion and such an important clue about access and trying to reduce access in a moment of impulsivity,” he added.
The study had no commercial funding. Dr. Nestadt and Dr. Liu report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT APA 2023
Beta-blocker gel shows promise for diabetic foot ulcers
say Indian researchers.
Esmolol is a short-acting beta-adrenergic receptor blocker that is currently approved by the Food and Drug Administration for cardiac indications such as short-term use for supraventricular tachycardia.
As a gel, esmolol hydrochloride is administered topically to stimulate wound healing via mechanisms such as the migration of keratinocytes, fibroblasts, and endothelial cells into wound tissue.
The current trial enrolled patients with type 1 or 2 diabetes, finding that, among 140 assessed, target ulcer closure within 12 weeks was more than twice as likely in those assigned esmolol gel plus standard of care than those given standard of care alone.
The impact of adding esmolol gel to standard of care was even greater in patients with a body mass index (BMI) over 25 kg/m2 and in those who weighed more than 80 kg (176 lb).
“The use of esmolol in the treatment of diabetic foot ulcers in addition to standard of care may be an important addition to the endeavor of healing diabetic foot ulcers,” wrote Ashu Rastogi, MD, DM, department of endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India, and colleagues, in their article recently published in JAMA Network Open.
Dr. Rastogi first presented the findings at the 2022 annual meeting of the European Association for the Study of Diabetes. The results were well received, with one clinician describing them as “astounding.”
However, Andrew Boulton, MD, PhD, said in an interview that, although the final published data are “interesting,” they “need further confirmation” because “there are one or two unusual features” about the study. Dr. Boulton is a professor of medicine, division of diabetes, endocrinology & gastroenterology, at the University of Manchester (England).
He highlighted that the study was of “basically neuropathic ulcers, many of which were plantar and should be able to heal without any specific additional therapy.”
In addition, the inclusion criteria state that the ulcers could be below the malleoli or 5 cm above them, which Dr. Boulton explained is “very unusual and would therefore include some atypical and not truly diabetic ‘foot’ ulcers.”
And Frances Game, MBBCh, department of diabetes and endocrinology, University Hospitals of Derby (England) and Burton NHS Foundation Trust, added that there are questions about the study methodology.
She said in an interview that although it is a “fascinating study,” the main comparison group did not receive vehicle, or placebo, gel in addition to standard of care. “How were they blinded [to treatment]?”
The “biggest problem” with the study, however, is that the primary outcome was reported as a per-protocol endpoint, not as a standard intention-to-treat analysis, which allowed the researchers to exclude patients whose ulcers increased in size by over 30% on two consecutive visits.
“That kind of makes [esmolol gel] look better than it is because they’ve taken out the ones who got worse,” Dr. Game noted. However, the findings, while not conclusive, do warrant further study of esmolol gel.
The authors noted that diabetic foot ulcers are a severe complication of diabetes, with a prevalence of 1.3%-12.0% across various countries, And the complication contributes to patient morbidity and mortality, with a 5-year mortality that is substantially higher than that of many cancers.
Moreover, “even with the best therapy,” such as advanced moist wound therapy, bioengineered tissue or skin substitutes, peptides, growth factors, electric stimulation, and negative-pressure wound therapy, just 30% of wounds linked to diabetes heal and recurrence is as high as 70%.
Against this backdrop, topical esmolol 14% gel was shown in a phase 1/2 study to be associated with ulcer area reduction and earlier wound closure versus standard of care plus a control vehicle gel.
The current phase 3, randomized, controlled trial involved individuals aged 18-75 years with type 1 or type 2 diabetes and noninfected diabetic foot ulcers classified as grade 1A and 1C on the University of Texas Wound Classification System, which had been open for at least 6 weeks and had an area of 2-25 cm2.
Patients from 27 tertiary care centers across India were enrolled in 2018-2020. They were randomized in a 3:3:1 ratio to one of three groups: esmolol 14% gel plus standard of care, standard of care only, or vehicle plus standard of care.
The study lasted 25 weeks and included a 1-week screening phase, during which all patients received standard of care, a 12-week treatment phase, and a 12-week follow-up phase. The latter included a closure confirmation period of 4 weeks and an observation period of 8 weeks.
Patients were assessed once a week during the treatment phase, and then at weeks 14, 16, 20, and 24.
In all, 176 patients were enrolled. Participants were a mean age of 56.4 years and 69.3% were men. Average hemoglobin A1c was 8.6%. Mean diabetic foot ulcer area was 4.7 cm2 and the average ulcer duration was 49.8 weeks.
The primary outcome was the proportion of patients who achieved target ulcer closure during the 12-week treatment phase and was assessed in 140 patients.
Overall, 60.3% of patients treated with esmolol gel plus standard of care achieved target ulcer closure versus 41.7% of those in the standard of care alone group (odds ratio, 2.13; P = .03).
The secondary outcome was the proportion of patients with target ulcer closure by the study end and was assessed in 120 patients.
In total, 77.2% of patients in the esmolol gel plus standard of care group met the secondary endpoint, compared with 55.6% of those receiving standard of care alone (OR, 1.72; P = .01).
Further analysis suggested the benefit seen with esmolol gel plus standard of care was greater in patients with a weight greater than 80 kg versus standard of care alone (OR, 4.04; P = .04), and in those with a BMI greater than 25 (OR, 2.72; P = .03).
Treatment-emergent adverse events were reported by 33 (18.8%) participants, with 12 events deemed serious. “However, none of the serious adverse events were considered as drug-related by the investigators,” concluded the researchers.
The study was partly funded by NovaLead Pharma and the Biotechnology Industry Research Assistance Council, New Delhi, set up by the Department of Biotechnology, Government of India. Dr. Rastogi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
say Indian researchers.
Esmolol is a short-acting beta-adrenergic receptor blocker that is currently approved by the Food and Drug Administration for cardiac indications such as short-term use for supraventricular tachycardia.
As a gel, esmolol hydrochloride is administered topically to stimulate wound healing via mechanisms such as the migration of keratinocytes, fibroblasts, and endothelial cells into wound tissue.
The current trial enrolled patients with type 1 or 2 diabetes, finding that, among 140 assessed, target ulcer closure within 12 weeks was more than twice as likely in those assigned esmolol gel plus standard of care than those given standard of care alone.
The impact of adding esmolol gel to standard of care was even greater in patients with a body mass index (BMI) over 25 kg/m2 and in those who weighed more than 80 kg (176 lb).
“The use of esmolol in the treatment of diabetic foot ulcers in addition to standard of care may be an important addition to the endeavor of healing diabetic foot ulcers,” wrote Ashu Rastogi, MD, DM, department of endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India, and colleagues, in their article recently published in JAMA Network Open.
Dr. Rastogi first presented the findings at the 2022 annual meeting of the European Association for the Study of Diabetes. The results were well received, with one clinician describing them as “astounding.”
However, Andrew Boulton, MD, PhD, said in an interview that, although the final published data are “interesting,” they “need further confirmation” because “there are one or two unusual features” about the study. Dr. Boulton is a professor of medicine, division of diabetes, endocrinology & gastroenterology, at the University of Manchester (England).
He highlighted that the study was of “basically neuropathic ulcers, many of which were plantar and should be able to heal without any specific additional therapy.”
In addition, the inclusion criteria state that the ulcers could be below the malleoli or 5 cm above them, which Dr. Boulton explained is “very unusual and would therefore include some atypical and not truly diabetic ‘foot’ ulcers.”
And Frances Game, MBBCh, department of diabetes and endocrinology, University Hospitals of Derby (England) and Burton NHS Foundation Trust, added that there are questions about the study methodology.
She said in an interview that although it is a “fascinating study,” the main comparison group did not receive vehicle, or placebo, gel in addition to standard of care. “How were they blinded [to treatment]?”
The “biggest problem” with the study, however, is that the primary outcome was reported as a per-protocol endpoint, not as a standard intention-to-treat analysis, which allowed the researchers to exclude patients whose ulcers increased in size by over 30% on two consecutive visits.
“That kind of makes [esmolol gel] look better than it is because they’ve taken out the ones who got worse,” Dr. Game noted. However, the findings, while not conclusive, do warrant further study of esmolol gel.
The authors noted that diabetic foot ulcers are a severe complication of diabetes, with a prevalence of 1.3%-12.0% across various countries, And the complication contributes to patient morbidity and mortality, with a 5-year mortality that is substantially higher than that of many cancers.
Moreover, “even with the best therapy,” such as advanced moist wound therapy, bioengineered tissue or skin substitutes, peptides, growth factors, electric stimulation, and negative-pressure wound therapy, just 30% of wounds linked to diabetes heal and recurrence is as high as 70%.
Against this backdrop, topical esmolol 14% gel was shown in a phase 1/2 study to be associated with ulcer area reduction and earlier wound closure versus standard of care plus a control vehicle gel.
The current phase 3, randomized, controlled trial involved individuals aged 18-75 years with type 1 or type 2 diabetes and noninfected diabetic foot ulcers classified as grade 1A and 1C on the University of Texas Wound Classification System, which had been open for at least 6 weeks and had an area of 2-25 cm2.
Patients from 27 tertiary care centers across India were enrolled in 2018-2020. They were randomized in a 3:3:1 ratio to one of three groups: esmolol 14% gel plus standard of care, standard of care only, or vehicle plus standard of care.
The study lasted 25 weeks and included a 1-week screening phase, during which all patients received standard of care, a 12-week treatment phase, and a 12-week follow-up phase. The latter included a closure confirmation period of 4 weeks and an observation period of 8 weeks.
Patients were assessed once a week during the treatment phase, and then at weeks 14, 16, 20, and 24.
In all, 176 patients were enrolled. Participants were a mean age of 56.4 years and 69.3% were men. Average hemoglobin A1c was 8.6%. Mean diabetic foot ulcer area was 4.7 cm2 and the average ulcer duration was 49.8 weeks.
The primary outcome was the proportion of patients who achieved target ulcer closure during the 12-week treatment phase and was assessed in 140 patients.
Overall, 60.3% of patients treated with esmolol gel plus standard of care achieved target ulcer closure versus 41.7% of those in the standard of care alone group (odds ratio, 2.13; P = .03).
The secondary outcome was the proportion of patients with target ulcer closure by the study end and was assessed in 120 patients.
In total, 77.2% of patients in the esmolol gel plus standard of care group met the secondary endpoint, compared with 55.6% of those receiving standard of care alone (OR, 1.72; P = .01).
Further analysis suggested the benefit seen with esmolol gel plus standard of care was greater in patients with a weight greater than 80 kg versus standard of care alone (OR, 4.04; P = .04), and in those with a BMI greater than 25 (OR, 2.72; P = .03).
Treatment-emergent adverse events were reported by 33 (18.8%) participants, with 12 events deemed serious. “However, none of the serious adverse events were considered as drug-related by the investigators,” concluded the researchers.
The study was partly funded by NovaLead Pharma and the Biotechnology Industry Research Assistance Council, New Delhi, set up by the Department of Biotechnology, Government of India. Dr. Rastogi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
say Indian researchers.
Esmolol is a short-acting beta-adrenergic receptor blocker that is currently approved by the Food and Drug Administration for cardiac indications such as short-term use for supraventricular tachycardia.
As a gel, esmolol hydrochloride is administered topically to stimulate wound healing via mechanisms such as the migration of keratinocytes, fibroblasts, and endothelial cells into wound tissue.
The current trial enrolled patients with type 1 or 2 diabetes, finding that, among 140 assessed, target ulcer closure within 12 weeks was more than twice as likely in those assigned esmolol gel plus standard of care than those given standard of care alone.
The impact of adding esmolol gel to standard of care was even greater in patients with a body mass index (BMI) over 25 kg/m2 and in those who weighed more than 80 kg (176 lb).
“The use of esmolol in the treatment of diabetic foot ulcers in addition to standard of care may be an important addition to the endeavor of healing diabetic foot ulcers,” wrote Ashu Rastogi, MD, DM, department of endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India, and colleagues, in their article recently published in JAMA Network Open.
Dr. Rastogi first presented the findings at the 2022 annual meeting of the European Association for the Study of Diabetes. The results were well received, with one clinician describing them as “astounding.”
However, Andrew Boulton, MD, PhD, said in an interview that, although the final published data are “interesting,” they “need further confirmation” because “there are one or two unusual features” about the study. Dr. Boulton is a professor of medicine, division of diabetes, endocrinology & gastroenterology, at the University of Manchester (England).
He highlighted that the study was of “basically neuropathic ulcers, many of which were plantar and should be able to heal without any specific additional therapy.”
In addition, the inclusion criteria state that the ulcers could be below the malleoli or 5 cm above them, which Dr. Boulton explained is “very unusual and would therefore include some atypical and not truly diabetic ‘foot’ ulcers.”
And Frances Game, MBBCh, department of diabetes and endocrinology, University Hospitals of Derby (England) and Burton NHS Foundation Trust, added that there are questions about the study methodology.
She said in an interview that although it is a “fascinating study,” the main comparison group did not receive vehicle, or placebo, gel in addition to standard of care. “How were they blinded [to treatment]?”
The “biggest problem” with the study, however, is that the primary outcome was reported as a per-protocol endpoint, not as a standard intention-to-treat analysis, which allowed the researchers to exclude patients whose ulcers increased in size by over 30% on two consecutive visits.
“That kind of makes [esmolol gel] look better than it is because they’ve taken out the ones who got worse,” Dr. Game noted. However, the findings, while not conclusive, do warrant further study of esmolol gel.
The authors noted that diabetic foot ulcers are a severe complication of diabetes, with a prevalence of 1.3%-12.0% across various countries, And the complication contributes to patient morbidity and mortality, with a 5-year mortality that is substantially higher than that of many cancers.
Moreover, “even with the best therapy,” such as advanced moist wound therapy, bioengineered tissue or skin substitutes, peptides, growth factors, electric stimulation, and negative-pressure wound therapy, just 30% of wounds linked to diabetes heal and recurrence is as high as 70%.
Against this backdrop, topical esmolol 14% gel was shown in a phase 1/2 study to be associated with ulcer area reduction and earlier wound closure versus standard of care plus a control vehicle gel.
The current phase 3, randomized, controlled trial involved individuals aged 18-75 years with type 1 or type 2 diabetes and noninfected diabetic foot ulcers classified as grade 1A and 1C on the University of Texas Wound Classification System, which had been open for at least 6 weeks and had an area of 2-25 cm2.
Patients from 27 tertiary care centers across India were enrolled in 2018-2020. They were randomized in a 3:3:1 ratio to one of three groups: esmolol 14% gel plus standard of care, standard of care only, or vehicle plus standard of care.
The study lasted 25 weeks and included a 1-week screening phase, during which all patients received standard of care, a 12-week treatment phase, and a 12-week follow-up phase. The latter included a closure confirmation period of 4 weeks and an observation period of 8 weeks.
Patients were assessed once a week during the treatment phase, and then at weeks 14, 16, 20, and 24.
In all, 176 patients were enrolled. Participants were a mean age of 56.4 years and 69.3% were men. Average hemoglobin A1c was 8.6%. Mean diabetic foot ulcer area was 4.7 cm2 and the average ulcer duration was 49.8 weeks.
The primary outcome was the proportion of patients who achieved target ulcer closure during the 12-week treatment phase and was assessed in 140 patients.
Overall, 60.3% of patients treated with esmolol gel plus standard of care achieved target ulcer closure versus 41.7% of those in the standard of care alone group (odds ratio, 2.13; P = .03).
The secondary outcome was the proportion of patients with target ulcer closure by the study end and was assessed in 120 patients.
In total, 77.2% of patients in the esmolol gel plus standard of care group met the secondary endpoint, compared with 55.6% of those receiving standard of care alone (OR, 1.72; P = .01).
Further analysis suggested the benefit seen with esmolol gel plus standard of care was greater in patients with a weight greater than 80 kg versus standard of care alone (OR, 4.04; P = .04), and in those with a BMI greater than 25 (OR, 2.72; P = .03).
Treatment-emergent adverse events were reported by 33 (18.8%) participants, with 12 events deemed serious. “However, none of the serious adverse events were considered as drug-related by the investigators,” concluded the researchers.
The study was partly funded by NovaLead Pharma and the Biotechnology Industry Research Assistance Council, New Delhi, set up by the Department of Biotechnology, Government of India. Dr. Rastogi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
FDA approves new indication for avapritinib
Avapritinib, a selective KIT mutation-targeted tyrosine kinase inhibitor, was approved in 2021 to treat advanced systemic mastocytosis, a rare and potentially fatal hematologic disorder. Nonadvanced forms include indolent or smoldering disease; advanced disease can progress to leukemia. The expanded approval now covers patients with indolent disease, which represents the majority of patients with systemic mastocytosis.
The drug is also approved for adults with unresectable or metastatic GIST that harbors a platelet-derived growth factor receptor alpha exon 18 mutation.
The approval is based on data from the phase 2 PIONEER trial. In the trial, 222 patients with moderate to severe indolent, systemic mastocytosis* were randomly assigned in a 2:1 ratio to receive either avapritinib 25 mg once daily plus best supportive care or placebo plus best supportive care.
The findings, published in February, revealed that patients who received avapritinib experienced significantly greater improvements in total symptom scores at 24 weeks (–15.6 vs. –9.2 for control patients). Significantly more patients in the avapritinib arm achieved greater than or equal to 50% reductions in serum tryptase (54% vs. 0%), bone marrow mast cell aggregates (53% vs. 23%), and KIT D816V variant allele fraction (68% vs. 6%).
Most adverse reactions were mild to moderate in severity and included eye edema, dizziness, peripheral edema, and flushing. Fewer than 1% of patients discontinued treatment because of serious adverse reactions.
“People with indolent systemic mastocytosis are significantly impacted by their disease symptoms, and many individuals self-isolate at home to protect against unpredictable external triggers,” Judith Kain Emmel, board chair of the Mast Cell Disease Society, said in the company press release. “Today’s approval is a historic moment for the [systemic mastocytosis] community and offers new hope for patients and their families.
A version of this article first appeared on Medscape.com.
Correction, 5/23/23: An earlier version of this article mischaracterized these patients' conditions. They had moderate to severe indolent, systemic mastocytosis.
Avapritinib, a selective KIT mutation-targeted tyrosine kinase inhibitor, was approved in 2021 to treat advanced systemic mastocytosis, a rare and potentially fatal hematologic disorder. Nonadvanced forms include indolent or smoldering disease; advanced disease can progress to leukemia. The expanded approval now covers patients with indolent disease, which represents the majority of patients with systemic mastocytosis.
The drug is also approved for adults with unresectable or metastatic GIST that harbors a platelet-derived growth factor receptor alpha exon 18 mutation.
The approval is based on data from the phase 2 PIONEER trial. In the trial, 222 patients with moderate to severe indolent, systemic mastocytosis* were randomly assigned in a 2:1 ratio to receive either avapritinib 25 mg once daily plus best supportive care or placebo plus best supportive care.
The findings, published in February, revealed that patients who received avapritinib experienced significantly greater improvements in total symptom scores at 24 weeks (–15.6 vs. –9.2 for control patients). Significantly more patients in the avapritinib arm achieved greater than or equal to 50% reductions in serum tryptase (54% vs. 0%), bone marrow mast cell aggregates (53% vs. 23%), and KIT D816V variant allele fraction (68% vs. 6%).
Most adverse reactions were mild to moderate in severity and included eye edema, dizziness, peripheral edema, and flushing. Fewer than 1% of patients discontinued treatment because of serious adverse reactions.
“People with indolent systemic mastocytosis are significantly impacted by their disease symptoms, and many individuals self-isolate at home to protect against unpredictable external triggers,” Judith Kain Emmel, board chair of the Mast Cell Disease Society, said in the company press release. “Today’s approval is a historic moment for the [systemic mastocytosis] community and offers new hope for patients and their families.
A version of this article first appeared on Medscape.com.
Correction, 5/23/23: An earlier version of this article mischaracterized these patients' conditions. They had moderate to severe indolent, systemic mastocytosis.
Avapritinib, a selective KIT mutation-targeted tyrosine kinase inhibitor, was approved in 2021 to treat advanced systemic mastocytosis, a rare and potentially fatal hematologic disorder. Nonadvanced forms include indolent or smoldering disease; advanced disease can progress to leukemia. The expanded approval now covers patients with indolent disease, which represents the majority of patients with systemic mastocytosis.
The drug is also approved for adults with unresectable or metastatic GIST that harbors a platelet-derived growth factor receptor alpha exon 18 mutation.
The approval is based on data from the phase 2 PIONEER trial. In the trial, 222 patients with moderate to severe indolent, systemic mastocytosis* were randomly assigned in a 2:1 ratio to receive either avapritinib 25 mg once daily plus best supportive care or placebo plus best supportive care.
The findings, published in February, revealed that patients who received avapritinib experienced significantly greater improvements in total symptom scores at 24 weeks (–15.6 vs. –9.2 for control patients). Significantly more patients in the avapritinib arm achieved greater than or equal to 50% reductions in serum tryptase (54% vs. 0%), bone marrow mast cell aggregates (53% vs. 23%), and KIT D816V variant allele fraction (68% vs. 6%).
Most adverse reactions were mild to moderate in severity and included eye edema, dizziness, peripheral edema, and flushing. Fewer than 1% of patients discontinued treatment because of serious adverse reactions.
“People with indolent systemic mastocytosis are significantly impacted by their disease symptoms, and many individuals self-isolate at home to protect against unpredictable external triggers,” Judith Kain Emmel, board chair of the Mast Cell Disease Society, said in the company press release. “Today’s approval is a historic moment for the [systemic mastocytosis] community and offers new hope for patients and their families.
A version of this article first appeared on Medscape.com.
Correction, 5/23/23: An earlier version of this article mischaracterized these patients' conditions. They had moderate to severe indolent, systemic mastocytosis.
Which interventions could lessen the burden of dementia?
Using a microsimulation algorithm that accounts for the effect on mortality, a team from Marseille, France, has shown that interventions targeting the three main vascular risk factors for dementia – hypertension, diabetes, and physical inactivity – could significantly reduce the burden of dementia by 2040.
Although these modeling results could appear too optimistic, since total disappearance of the risk factors was assumed, the authors say the results do show that targeted interventions for these factors could be effective in reducing the future burden of dementia.
Increasing prevalence
According to the World Alzheimer Report 2018, 50 million people around the world were living with dementia; a population roughly around the size of South Korea or Spain. That community is likely to rise to about 152 million people by 2050, which is similar to the size of Russia or Bangladesh, the result of an aging population.
Among modifiable risk factors, many studies support a deleterious effect of hypertension, diabetes, and physical inactivity on the risk of dementia. However, since the distribution of these risk factors could have a direct impact on mortality, reducing it should increase life expectancy and the number of cases of dementia.
The team, headed by Hélène Jacqmin-Gadda, PhD, research director at the University of Bordeaux (France), has developed a microsimulation model capable of predicting the burden of dementia while accounting for the impact on mortality. The team used this approach to assess the impact of interventions targeting these three main risk factors on the burden of dementia in France by 2040.
Removing risk factors
The researchers estimated the incidence of dementia for men and women using data from the 2020 PAQUID cohort, and these data were combined with the projections forecast by the French National Institute of Statistics and Economic Studies to account for mortality with and without dementia.
Without intervention, the prevalence rate of dementia in 2040 would be 9.6% among men and 14% among women older than 65 years.
These figures would decrease to 6.4% (−33%) and 10.4% (−26%), respectively, under the intervention scenario whereby the three modifiable vascular risk factors (hypertension, diabetes, and physical inactivity) would be removed simultaneously beginning in 2020. The prevalence rates are significantly reduced for men and women from age 75 years. In this scenario, life expectancy without dementia would increase by 3.4 years in men and 2.6 years in women, the result of men being more exposed to these three risk factors.
Other scenarios have estimated dementia prevalence with the disappearance of just one of these risk factors. For example, the disappearance of hypertension alone from 2020 could decrease dementia prevalence by 21% in men and 16% in women (because this risk factor is less common in women than in men) by 2040. This reduction would be associated with a decrease in the lifelong probability of dementia among men and women and a gain in life expectancy without dementia of 2 years in men and 1.4 years in women.
Among the three factors, hypertension has the largest impact on dementia burden in the French population, since this is, by far, the most prevalent (69% in men and 49% in women), while intervention targeting only diabetes or physical inactivity would lead to a reduction in dementia prevalence of only 4%-7%.
The authors reported no conflicts of interest.
This article was translated from Univadis France. A version appeared on Medscape.com.
Using a microsimulation algorithm that accounts for the effect on mortality, a team from Marseille, France, has shown that interventions targeting the three main vascular risk factors for dementia – hypertension, diabetes, and physical inactivity – could significantly reduce the burden of dementia by 2040.
Although these modeling results could appear too optimistic, since total disappearance of the risk factors was assumed, the authors say the results do show that targeted interventions for these factors could be effective in reducing the future burden of dementia.
Increasing prevalence
According to the World Alzheimer Report 2018, 50 million people around the world were living with dementia; a population roughly around the size of South Korea or Spain. That community is likely to rise to about 152 million people by 2050, which is similar to the size of Russia or Bangladesh, the result of an aging population.
Among modifiable risk factors, many studies support a deleterious effect of hypertension, diabetes, and physical inactivity on the risk of dementia. However, since the distribution of these risk factors could have a direct impact on mortality, reducing it should increase life expectancy and the number of cases of dementia.
The team, headed by Hélène Jacqmin-Gadda, PhD, research director at the University of Bordeaux (France), has developed a microsimulation model capable of predicting the burden of dementia while accounting for the impact on mortality. The team used this approach to assess the impact of interventions targeting these three main risk factors on the burden of dementia in France by 2040.
Removing risk factors
The researchers estimated the incidence of dementia for men and women using data from the 2020 PAQUID cohort, and these data were combined with the projections forecast by the French National Institute of Statistics and Economic Studies to account for mortality with and without dementia.
Without intervention, the prevalence rate of dementia in 2040 would be 9.6% among men and 14% among women older than 65 years.
These figures would decrease to 6.4% (−33%) and 10.4% (−26%), respectively, under the intervention scenario whereby the three modifiable vascular risk factors (hypertension, diabetes, and physical inactivity) would be removed simultaneously beginning in 2020. The prevalence rates are significantly reduced for men and women from age 75 years. In this scenario, life expectancy without dementia would increase by 3.4 years in men and 2.6 years in women, the result of men being more exposed to these three risk factors.
Other scenarios have estimated dementia prevalence with the disappearance of just one of these risk factors. For example, the disappearance of hypertension alone from 2020 could decrease dementia prevalence by 21% in men and 16% in women (because this risk factor is less common in women than in men) by 2040. This reduction would be associated with a decrease in the lifelong probability of dementia among men and women and a gain in life expectancy without dementia of 2 years in men and 1.4 years in women.
Among the three factors, hypertension has the largest impact on dementia burden in the French population, since this is, by far, the most prevalent (69% in men and 49% in women), while intervention targeting only diabetes or physical inactivity would lead to a reduction in dementia prevalence of only 4%-7%.
The authors reported no conflicts of interest.
This article was translated from Univadis France. A version appeared on Medscape.com.
Using a microsimulation algorithm that accounts for the effect on mortality, a team from Marseille, France, has shown that interventions targeting the three main vascular risk factors for dementia – hypertension, diabetes, and physical inactivity – could significantly reduce the burden of dementia by 2040.
Although these modeling results could appear too optimistic, since total disappearance of the risk factors was assumed, the authors say the results do show that targeted interventions for these factors could be effective in reducing the future burden of dementia.
Increasing prevalence
According to the World Alzheimer Report 2018, 50 million people around the world were living with dementia; a population roughly around the size of South Korea or Spain. That community is likely to rise to about 152 million people by 2050, which is similar to the size of Russia or Bangladesh, the result of an aging population.
Among modifiable risk factors, many studies support a deleterious effect of hypertension, diabetes, and physical inactivity on the risk of dementia. However, since the distribution of these risk factors could have a direct impact on mortality, reducing it should increase life expectancy and the number of cases of dementia.
The team, headed by Hélène Jacqmin-Gadda, PhD, research director at the University of Bordeaux (France), has developed a microsimulation model capable of predicting the burden of dementia while accounting for the impact on mortality. The team used this approach to assess the impact of interventions targeting these three main risk factors on the burden of dementia in France by 2040.
Removing risk factors
The researchers estimated the incidence of dementia for men and women using data from the 2020 PAQUID cohort, and these data were combined with the projections forecast by the French National Institute of Statistics and Economic Studies to account for mortality with and without dementia.
Without intervention, the prevalence rate of dementia in 2040 would be 9.6% among men and 14% among women older than 65 years.
These figures would decrease to 6.4% (−33%) and 10.4% (−26%), respectively, under the intervention scenario whereby the three modifiable vascular risk factors (hypertension, diabetes, and physical inactivity) would be removed simultaneously beginning in 2020. The prevalence rates are significantly reduced for men and women from age 75 years. In this scenario, life expectancy without dementia would increase by 3.4 years in men and 2.6 years in women, the result of men being more exposed to these three risk factors.
Other scenarios have estimated dementia prevalence with the disappearance of just one of these risk factors. For example, the disappearance of hypertension alone from 2020 could decrease dementia prevalence by 21% in men and 16% in women (because this risk factor is less common in women than in men) by 2040. This reduction would be associated with a decrease in the lifelong probability of dementia among men and women and a gain in life expectancy without dementia of 2 years in men and 1.4 years in women.
Among the three factors, hypertension has the largest impact on dementia burden in the French population, since this is, by far, the most prevalent (69% in men and 49% in women), while intervention targeting only diabetes or physical inactivity would lead to a reduction in dementia prevalence of only 4%-7%.
The authors reported no conflicts of interest.
This article was translated from Univadis France. A version appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF EPIDEMIOLOGY




