User login
Sublingual immunotherapy stops onset and worsening of asthma
PARIS – The EfficAPSI study showed with real-world data that sublingual immunotherapy (SLIT) reduces the risks for asthma onset and the worsening of asthma symptoms for patients with allergic rhinitis. The research was presented at the 18th French-language allergy conference.
These results confirm that allergen immunotherapy, or “desensitization,” is indeed an etiologic treatment of this allergic condition.
SLIT encompasses personalized solutions created for an individual specifically for allergies to dust mites, grass, birch, cats, and so on. These preparations are commonly used by allergy specialists when establishing an AIT treatment plan.
In 2017, the French Health Authority published a report indicating that there was insufficient clinical proof regarding the efficacy of SLIT. It subsequently removed injectable forms of these allergen extracts from the list of drugs reimbursed by the state and reduced state reimbursement of sublingual SLIT preparations from 30% to 15%, a step it confirmed in March 2018 and that led to outrage from allergy specialists. The chair of the French allergy society at the time, Jocelyne Just, MD, PhD, argued that conducting double-blind, placebo-controlled studies for all types (grass pollen, birch pollen, dust mites, asthma, allergic rhinitis, subcutaneous injections, sublingual treatments, tablets, liquid preparations) would take decades. Furthermore, meta-analyses on the subject, despite being heterogeneous and unable to answer all questions, are indeed pointing to the effectiveness of SLIT. To supplement existing data and to answer the queries raised by the HAS, several studies have been launched, including EfficAPSI.
The pharmacoepidemiologic EfficAPSI study is the largest retrospective, real-world, longitudinal cohort study ever carried out regarding liquid SLIT using data stored in the French National Health Data System (SNDS). The primary objective of the study was to evaluate the real-world impact of liquid SLIT on the onset and worsening of asthma for patients with allergic rhinitis and to evaluate the impact of sublingual treatments on public health.
A cohort analysis of patients treated with SLIT and control patients treated for allergic rhinitis with or without treatment for asthma was carried out. The patients treated with SLIT for at least 2 consecutive years were anonymously selected from the SNDS using the Stallergenes Greer prescription database.
In all, 99,538 patients who received SLIT were compared with 333,082 control patients (those who had received treatment for allergic rhinitis without taking SLIT). Participants were stratified according to their treatment history for asthma and were paired using a propensity score to minimize comparison bias.
The main definition of the onset of asthma included the first prescription of an asthma medication, hospital admission for asthma, or a diagnosis of chronic asthma. The secondary definition omitted the prescription of any treatment, and the third (sensitive and specific) took into consideration an initial prescription of omalizumab or a prescription of three inhaled corticosteroids (ICSs) associated with or without a long-acting beta-2 agonist (LABA) for a period of 1 year, admission to the hospital, or chronic asthma.
Asthma risk reduced
Among patients with allergic rhinitis without preexisting asthma, liquid SLIT was associated with a significantly lower risk of asthma onset in comparison with the control group (primary hazard ratio: 0.77; secondary HR: 0.66; and tertiary HR: 0.62).
The risk reductions were significant and were consistent regardless of the allergens analyzed (tertiary HR, dust mites: 0.57; grass: 0.52) for all age groups. These new results that were based on the tertiary definition corroborate the results from the primary and secondary definitions.
said study co-author Philippe Devillier, MD, PhD, research director at the respiratory tract diseases center of Foch Hospital, Paris. “These results are consistent with previous studies in the same French health care database, as well as in a German database with SLIT preparations in tablet form. This not only confirms the soundness of the methodology but also the benefit of liquid SLIT as an etiological treatment of respiratory allergies.”
Risk for worsening
Furthermore, in the same study, liquid SLIT treatment was associated with a 27% reduced risk for worsening asthma and a 36% reduced risk for severe asthma. Among patients with allergic rhinitis and preexisting asthma, liquid SLIT was associated with a significantly lower risk for worsening of asthma, compared with the control group (primary HR: 0.73; secondary HR: 0.61; and tertiary HR: 0.64). The primary definition was an initial prescription of an ICS-LABA combination in a patient treated with ICS alone, severe exacerbation of asthma symptoms, hospital admission, or a diagnosis of chronic asthma.
“The risk reductions were significant and consistent for the allergens analyzed,” said study co-author Pascal Demoly, MD, PhD, head of pulmonology at Montpellier University Hospital, France (tertiary HR, dust mites: 0.66; grass: 0.59; birch: 0.34; and cats: 0.77). “This was across all age groups,” he added.
“The results of the EfficAPSI real-world study on health data from the SNDS are consistent with outcomes from clinical trials, suggestive of a reduced risk of asthma onset in patients with allergic rhinitis receiving liquid SLIT, as well as a reduced risk of worsening of preexisting asthma,” said Devillier. “SLIT, in this case in the form of a liquid, thus appears to be an effective etiological treatment, since the use of symptomatic drugs, in particular preventer inhalers, but also reliever inhalers, is lower in patients treated with SLIT over at least two consecutive years, compared with paired control subjects. And it’s the same for the risk of treating asthma in nonasthmatic patients at the start of the study. EfficAPSI is the largest study using data from a comprehensive state drug reimbursement database, allowing us to assess the impact of liquid SLIT on public health. These results, also obtained with other allergen preparations, particularly in tablet form in French and German studies using data from health care databases, demonstrate the consistency of the data regarding the efficacy of SLIT.”
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
PARIS – The EfficAPSI study showed with real-world data that sublingual immunotherapy (SLIT) reduces the risks for asthma onset and the worsening of asthma symptoms for patients with allergic rhinitis. The research was presented at the 18th French-language allergy conference.
These results confirm that allergen immunotherapy, or “desensitization,” is indeed an etiologic treatment of this allergic condition.
SLIT encompasses personalized solutions created for an individual specifically for allergies to dust mites, grass, birch, cats, and so on. These preparations are commonly used by allergy specialists when establishing an AIT treatment plan.
In 2017, the French Health Authority published a report indicating that there was insufficient clinical proof regarding the efficacy of SLIT. It subsequently removed injectable forms of these allergen extracts from the list of drugs reimbursed by the state and reduced state reimbursement of sublingual SLIT preparations from 30% to 15%, a step it confirmed in March 2018 and that led to outrage from allergy specialists. The chair of the French allergy society at the time, Jocelyne Just, MD, PhD, argued that conducting double-blind, placebo-controlled studies for all types (grass pollen, birch pollen, dust mites, asthma, allergic rhinitis, subcutaneous injections, sublingual treatments, tablets, liquid preparations) would take decades. Furthermore, meta-analyses on the subject, despite being heterogeneous and unable to answer all questions, are indeed pointing to the effectiveness of SLIT. To supplement existing data and to answer the queries raised by the HAS, several studies have been launched, including EfficAPSI.
The pharmacoepidemiologic EfficAPSI study is the largest retrospective, real-world, longitudinal cohort study ever carried out regarding liquid SLIT using data stored in the French National Health Data System (SNDS). The primary objective of the study was to evaluate the real-world impact of liquid SLIT on the onset and worsening of asthma for patients with allergic rhinitis and to evaluate the impact of sublingual treatments on public health.
A cohort analysis of patients treated with SLIT and control patients treated for allergic rhinitis with or without treatment for asthma was carried out. The patients treated with SLIT for at least 2 consecutive years were anonymously selected from the SNDS using the Stallergenes Greer prescription database.
In all, 99,538 patients who received SLIT were compared with 333,082 control patients (those who had received treatment for allergic rhinitis without taking SLIT). Participants were stratified according to their treatment history for asthma and were paired using a propensity score to minimize comparison bias.
The main definition of the onset of asthma included the first prescription of an asthma medication, hospital admission for asthma, or a diagnosis of chronic asthma. The secondary definition omitted the prescription of any treatment, and the third (sensitive and specific) took into consideration an initial prescription of omalizumab or a prescription of three inhaled corticosteroids (ICSs) associated with or without a long-acting beta-2 agonist (LABA) for a period of 1 year, admission to the hospital, or chronic asthma.
Asthma risk reduced
Among patients with allergic rhinitis without preexisting asthma, liquid SLIT was associated with a significantly lower risk of asthma onset in comparison with the control group (primary hazard ratio: 0.77; secondary HR: 0.66; and tertiary HR: 0.62).
The risk reductions were significant and were consistent regardless of the allergens analyzed (tertiary HR, dust mites: 0.57; grass: 0.52) for all age groups. These new results that were based on the tertiary definition corroborate the results from the primary and secondary definitions.
said study co-author Philippe Devillier, MD, PhD, research director at the respiratory tract diseases center of Foch Hospital, Paris. “These results are consistent with previous studies in the same French health care database, as well as in a German database with SLIT preparations in tablet form. This not only confirms the soundness of the methodology but also the benefit of liquid SLIT as an etiological treatment of respiratory allergies.”
Risk for worsening
Furthermore, in the same study, liquid SLIT treatment was associated with a 27% reduced risk for worsening asthma and a 36% reduced risk for severe asthma. Among patients with allergic rhinitis and preexisting asthma, liquid SLIT was associated with a significantly lower risk for worsening of asthma, compared with the control group (primary HR: 0.73; secondary HR: 0.61; and tertiary HR: 0.64). The primary definition was an initial prescription of an ICS-LABA combination in a patient treated with ICS alone, severe exacerbation of asthma symptoms, hospital admission, or a diagnosis of chronic asthma.
“The risk reductions were significant and consistent for the allergens analyzed,” said study co-author Pascal Demoly, MD, PhD, head of pulmonology at Montpellier University Hospital, France (tertiary HR, dust mites: 0.66; grass: 0.59; birch: 0.34; and cats: 0.77). “This was across all age groups,” he added.
“The results of the EfficAPSI real-world study on health data from the SNDS are consistent with outcomes from clinical trials, suggestive of a reduced risk of asthma onset in patients with allergic rhinitis receiving liquid SLIT, as well as a reduced risk of worsening of preexisting asthma,” said Devillier. “SLIT, in this case in the form of a liquid, thus appears to be an effective etiological treatment, since the use of symptomatic drugs, in particular preventer inhalers, but also reliever inhalers, is lower in patients treated with SLIT over at least two consecutive years, compared with paired control subjects. And it’s the same for the risk of treating asthma in nonasthmatic patients at the start of the study. EfficAPSI is the largest study using data from a comprehensive state drug reimbursement database, allowing us to assess the impact of liquid SLIT on public health. These results, also obtained with other allergen preparations, particularly in tablet form in French and German studies using data from health care databases, demonstrate the consistency of the data regarding the efficacy of SLIT.”
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
PARIS – The EfficAPSI study showed with real-world data that sublingual immunotherapy (SLIT) reduces the risks for asthma onset and the worsening of asthma symptoms for patients with allergic rhinitis. The research was presented at the 18th French-language allergy conference.
These results confirm that allergen immunotherapy, or “desensitization,” is indeed an etiologic treatment of this allergic condition.
SLIT encompasses personalized solutions created for an individual specifically for allergies to dust mites, grass, birch, cats, and so on. These preparations are commonly used by allergy specialists when establishing an AIT treatment plan.
In 2017, the French Health Authority published a report indicating that there was insufficient clinical proof regarding the efficacy of SLIT. It subsequently removed injectable forms of these allergen extracts from the list of drugs reimbursed by the state and reduced state reimbursement of sublingual SLIT preparations from 30% to 15%, a step it confirmed in March 2018 and that led to outrage from allergy specialists. The chair of the French allergy society at the time, Jocelyne Just, MD, PhD, argued that conducting double-blind, placebo-controlled studies for all types (grass pollen, birch pollen, dust mites, asthma, allergic rhinitis, subcutaneous injections, sublingual treatments, tablets, liquid preparations) would take decades. Furthermore, meta-analyses on the subject, despite being heterogeneous and unable to answer all questions, are indeed pointing to the effectiveness of SLIT. To supplement existing data and to answer the queries raised by the HAS, several studies have been launched, including EfficAPSI.
The pharmacoepidemiologic EfficAPSI study is the largest retrospective, real-world, longitudinal cohort study ever carried out regarding liquid SLIT using data stored in the French National Health Data System (SNDS). The primary objective of the study was to evaluate the real-world impact of liquid SLIT on the onset and worsening of asthma for patients with allergic rhinitis and to evaluate the impact of sublingual treatments on public health.
A cohort analysis of patients treated with SLIT and control patients treated for allergic rhinitis with or without treatment for asthma was carried out. The patients treated with SLIT for at least 2 consecutive years were anonymously selected from the SNDS using the Stallergenes Greer prescription database.
In all, 99,538 patients who received SLIT were compared with 333,082 control patients (those who had received treatment for allergic rhinitis without taking SLIT). Participants were stratified according to their treatment history for asthma and were paired using a propensity score to minimize comparison bias.
The main definition of the onset of asthma included the first prescription of an asthma medication, hospital admission for asthma, or a diagnosis of chronic asthma. The secondary definition omitted the prescription of any treatment, and the third (sensitive and specific) took into consideration an initial prescription of omalizumab or a prescription of three inhaled corticosteroids (ICSs) associated with or without a long-acting beta-2 agonist (LABA) for a period of 1 year, admission to the hospital, or chronic asthma.
Asthma risk reduced
Among patients with allergic rhinitis without preexisting asthma, liquid SLIT was associated with a significantly lower risk of asthma onset in comparison with the control group (primary hazard ratio: 0.77; secondary HR: 0.66; and tertiary HR: 0.62).
The risk reductions were significant and were consistent regardless of the allergens analyzed (tertiary HR, dust mites: 0.57; grass: 0.52) for all age groups. These new results that were based on the tertiary definition corroborate the results from the primary and secondary definitions.
said study co-author Philippe Devillier, MD, PhD, research director at the respiratory tract diseases center of Foch Hospital, Paris. “These results are consistent with previous studies in the same French health care database, as well as in a German database with SLIT preparations in tablet form. This not only confirms the soundness of the methodology but also the benefit of liquid SLIT as an etiological treatment of respiratory allergies.”
Risk for worsening
Furthermore, in the same study, liquid SLIT treatment was associated with a 27% reduced risk for worsening asthma and a 36% reduced risk for severe asthma. Among patients with allergic rhinitis and preexisting asthma, liquid SLIT was associated with a significantly lower risk for worsening of asthma, compared with the control group (primary HR: 0.73; secondary HR: 0.61; and tertiary HR: 0.64). The primary definition was an initial prescription of an ICS-LABA combination in a patient treated with ICS alone, severe exacerbation of asthma symptoms, hospital admission, or a diagnosis of chronic asthma.
“The risk reductions were significant and consistent for the allergens analyzed,” said study co-author Pascal Demoly, MD, PhD, head of pulmonology at Montpellier University Hospital, France (tertiary HR, dust mites: 0.66; grass: 0.59; birch: 0.34; and cats: 0.77). “This was across all age groups,” he added.
“The results of the EfficAPSI real-world study on health data from the SNDS are consistent with outcomes from clinical trials, suggestive of a reduced risk of asthma onset in patients with allergic rhinitis receiving liquid SLIT, as well as a reduced risk of worsening of preexisting asthma,” said Devillier. “SLIT, in this case in the form of a liquid, thus appears to be an effective etiological treatment, since the use of symptomatic drugs, in particular preventer inhalers, but also reliever inhalers, is lower in patients treated with SLIT over at least two consecutive years, compared with paired control subjects. And it’s the same for the risk of treating asthma in nonasthmatic patients at the start of the study. EfficAPSI is the largest study using data from a comprehensive state drug reimbursement database, allowing us to assess the impact of liquid SLIT on public health. These results, also obtained with other allergen preparations, particularly in tablet form in French and German studies using data from health care databases, demonstrate the consistency of the data regarding the efficacy of SLIT.”
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Circulating tumor DNA may predict poor prognosis in breast cancer
a new meta-analysis and systematic review found.
“Circulating tumor DNA (ctDNA) has been extensively studied as a prognostic biomarker in early breast cancer. However, there is a significant heterogeneity in the study results, which is probably related to the fact that each individual study included different patient populations, collected blood at different time points, and used different methods (assays) for ctDNA analysis,” said Guilherme Nader Marta, MD, of the Institut Jules Bordet, Anderlecht, Belgium, in an interview.
“The aim of our study was to summarize the available evidence that has been presented so far on this topic by performing a systematic review and meta-analysis including studies that reported the association between ctDNA detection and long-term outcomes,” said Dr. Nader Marta, who coauthored the new research, which was presented as a poster (Poster 26P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Methods and results
The authors identified 57 studies including data from 5,729 individuals with early breast cancer. The 44.5% for whom stages were reported consisted of 18.3% with stage I disease, 60.0% with stage II, and 21.5% with stage III. Patients’ ctDNA collection was divided into three groups: baseline, after neoadjuvant therapy (End-of-NAT), and during follow-up care; ctDNA assays were classified as tumor-informed or non–tumor-informed.
The detection of ctDNA at any time point during diagnosis and treatment was associated with worse disease-free survival (DFS) and overall survival (OS), compared with no ctDNA. The association was stronger in tumor-informed assays, the researchers said.
For disease-free survival, the overall multivariate hazard ratios were 2.5, 5.5, and 7.2 for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
For overall survival, the overall multivariate hazard ratios were 3.0, 12.9, and 5.6, for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
The pooled hazard ratios were numerically higher for both DFS and OS when ctDNA was detected at either End-of-NAT or follow-up.
In addition, detection of ctDNA was associated with a high degree of specificity (from 0.7 to 1.0) for breast cancer relapse; sensitivity ranged from 0.31 to 1.0, the researchers noted. The mean lead time from ctDNA detection to breast cancer recurrence in these cases was approximately 10 months.
Results show ctDNA detection is associated with worse survival
“Our study results demonstrate that ctDNA detection is associated with worse disease-free survival and overall survival in patients with early breast cancer, particularly when measured after treatment with tumor-informed assays,” Dr. Nader Marta said in an interview.
“As next steps, we need to build on this evidence to bring the potential benefits of this powerful prognostic tool to our patients,” said Dr. Nader Marta. “Ongoing studies exploring different management strategies based on serial ctDNA assessments will help us understand the exact role of this technology in our clinical practice.”
The study received no outside funding. Dr. Nader Marta disclosed relationships with companies including Roche and Bayer.
a new meta-analysis and systematic review found.
“Circulating tumor DNA (ctDNA) has been extensively studied as a prognostic biomarker in early breast cancer. However, there is a significant heterogeneity in the study results, which is probably related to the fact that each individual study included different patient populations, collected blood at different time points, and used different methods (assays) for ctDNA analysis,” said Guilherme Nader Marta, MD, of the Institut Jules Bordet, Anderlecht, Belgium, in an interview.
“The aim of our study was to summarize the available evidence that has been presented so far on this topic by performing a systematic review and meta-analysis including studies that reported the association between ctDNA detection and long-term outcomes,” said Dr. Nader Marta, who coauthored the new research, which was presented as a poster (Poster 26P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Methods and results
The authors identified 57 studies including data from 5,729 individuals with early breast cancer. The 44.5% for whom stages were reported consisted of 18.3% with stage I disease, 60.0% with stage II, and 21.5% with stage III. Patients’ ctDNA collection was divided into three groups: baseline, after neoadjuvant therapy (End-of-NAT), and during follow-up care; ctDNA assays were classified as tumor-informed or non–tumor-informed.
The detection of ctDNA at any time point during diagnosis and treatment was associated with worse disease-free survival (DFS) and overall survival (OS), compared with no ctDNA. The association was stronger in tumor-informed assays, the researchers said.
For disease-free survival, the overall multivariate hazard ratios were 2.5, 5.5, and 7.2 for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
For overall survival, the overall multivariate hazard ratios were 3.0, 12.9, and 5.6, for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
The pooled hazard ratios were numerically higher for both DFS and OS when ctDNA was detected at either End-of-NAT or follow-up.
In addition, detection of ctDNA was associated with a high degree of specificity (from 0.7 to 1.0) for breast cancer relapse; sensitivity ranged from 0.31 to 1.0, the researchers noted. The mean lead time from ctDNA detection to breast cancer recurrence in these cases was approximately 10 months.
Results show ctDNA detection is associated with worse survival
“Our study results demonstrate that ctDNA detection is associated with worse disease-free survival and overall survival in patients with early breast cancer, particularly when measured after treatment with tumor-informed assays,” Dr. Nader Marta said in an interview.
“As next steps, we need to build on this evidence to bring the potential benefits of this powerful prognostic tool to our patients,” said Dr. Nader Marta. “Ongoing studies exploring different management strategies based on serial ctDNA assessments will help us understand the exact role of this technology in our clinical practice.”
The study received no outside funding. Dr. Nader Marta disclosed relationships with companies including Roche and Bayer.
a new meta-analysis and systematic review found.
“Circulating tumor DNA (ctDNA) has been extensively studied as a prognostic biomarker in early breast cancer. However, there is a significant heterogeneity in the study results, which is probably related to the fact that each individual study included different patient populations, collected blood at different time points, and used different methods (assays) for ctDNA analysis,” said Guilherme Nader Marta, MD, of the Institut Jules Bordet, Anderlecht, Belgium, in an interview.
“The aim of our study was to summarize the available evidence that has been presented so far on this topic by performing a systematic review and meta-analysis including studies that reported the association between ctDNA detection and long-term outcomes,” said Dr. Nader Marta, who coauthored the new research, which was presented as a poster (Poster 26P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Methods and results
The authors identified 57 studies including data from 5,729 individuals with early breast cancer. The 44.5% for whom stages were reported consisted of 18.3% with stage I disease, 60.0% with stage II, and 21.5% with stage III. Patients’ ctDNA collection was divided into three groups: baseline, after neoadjuvant therapy (End-of-NAT), and during follow-up care; ctDNA assays were classified as tumor-informed or non–tumor-informed.
The detection of ctDNA at any time point during diagnosis and treatment was associated with worse disease-free survival (DFS) and overall survival (OS), compared with no ctDNA. The association was stronger in tumor-informed assays, the researchers said.
For disease-free survival, the overall multivariate hazard ratios were 2.5, 5.5, and 7.2 for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
For overall survival, the overall multivariate hazard ratios were 3.0, 12.9, and 5.6, for ctDNA detection at baseline, End-of-NAT, and follow-up, respectively.
The pooled hazard ratios were numerically higher for both DFS and OS when ctDNA was detected at either End-of-NAT or follow-up.
In addition, detection of ctDNA was associated with a high degree of specificity (from 0.7 to 1.0) for breast cancer relapse; sensitivity ranged from 0.31 to 1.0, the researchers noted. The mean lead time from ctDNA detection to breast cancer recurrence in these cases was approximately 10 months.
Results show ctDNA detection is associated with worse survival
“Our study results demonstrate that ctDNA detection is associated with worse disease-free survival and overall survival in patients with early breast cancer, particularly when measured after treatment with tumor-informed assays,” Dr. Nader Marta said in an interview.
“As next steps, we need to build on this evidence to bring the potential benefits of this powerful prognostic tool to our patients,” said Dr. Nader Marta. “Ongoing studies exploring different management strategies based on serial ctDNA assessments will help us understand the exact role of this technology in our clinical practice.”
The study received no outside funding. Dr. Nader Marta disclosed relationships with companies including Roche and Bayer.
ESMO BREAST CANCER 2023
Breast cancer outcomes are worse for Black men
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
A new study finds that racial disparities in male breast cancer are persisting in the United States.
From 2000 to 2019, Black men were diagnosed at later ages than White males (median ages, 69 and 63 years, respectively) and were more likely to die from the disease (22.4% vs. 16.8%, respectively). Male breast cancer (MBC) was more likely to kill Black men in rural vs. urban areas (hazard ratio = 1.4; 95% confidence interval, 1.0-2.1; P less than .05). Among White males, in contrast, there was no difference on that front, according to the research, which was presented in a poster (Abstract No. 87P) at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
It’s not clear why the disparities exist, said lead author Lekha Yadukumar, MBBS, an internal medicine resident at the Wright Center for Graduate Medical Education in Scranton, Penn., in an interview.
“Several potential factors may contribute to the higher rate of breast cancer diagnosis in older [Black] men, including the pathology of the disease, limited awareness about breast cancer, and potential barriers to accessibility,” she said. “The increased mortality among [Black men] may be linked to variations in tumor pathology and molecular biology. Social factors may also potentially impact survival rates, including [having] limited access to health care in rural areas and inadequate social support.”
Male breast cancer is rare, accounting for less than 1% of all breast cancer cases in the United States, according to the Breast Cancer Research Foundation. An estimated 2,700 men are diagnosed each year, and about 530 will die. Previous research has suggested Black men have worse outcomes than White men, but the data covered earlier years than the new study.
Methods and results
Dr. Yadukumar and colleagues retrospectively analyzed statistics from the Surveillance, Epidemiology, and End Results database for patients diagnosed with primary male breast cancer from 2000 to 2019 (n = 8,373; Black men, 1,111 [13.26%]; White men, 6,817 [81.41%]).
Median income didn’t affect mortality, whereas men in both racial groups were less likely to die if they were married vs. single/divorced (hazard ratio = 0.6; P less than .05).
Other studies have shown that “[Black American] men diagnosed with breast cancer experience longer time intervals before receiving treatment, encounter more severe disease manifestations, and exhibit lower rates of survivorship,” Dr. Yadukumar said. “Despite these findings, there remains a scarcity of genetic studies aimed at comprehending the underlying causes of these disparities. Moreover, there is a dearth of research investigating other factors that may influence survival outcomes among men with breast cancer.”
Findings reflect the disparities in female breast cancer
In an interview, Duke University, Durham, N.C., oncologist Arif Kamal, MD, MBA, MHS, the chief patient officer at the American Cancer Society, said the study is impressive since the number of patients is large for a rare cancer and the population is diverse. Plus, the findings reflect the disparities in female breast cancer, he noted.
“We know that Black women’s mortality is worse vs. White women in breast cancer, and we believe that most of that has nothing to do with cancer screening,” said Dr. Kamal, who was not involved in the new study. “When the clock starts from diagnosis onwards, you start to see less introduction to clinical trials and standard care medications and more time to treatment, surgery, and radiation,” he said.
“You see similar disparities as related to mortality in Black vs. White men,” he noted.
The new findings about higher death rates for Black men, especially in rural areas, suggest that “distance matters, and race matters,” he said. In rural areas, it can be hard to access pathologists, radiologists, and surgeons with more experience with breast cancer, he said.
But, he noted, the study finds that income doesn’t appear to be a factor.
In the big picture, he said, the results suggest that when it comes to barriers to better outcomes, “things that are systemic don’t make exceptions because you are a man vs. a woman.”
No study funding was reported. The study authors and Dr. Kamal have no relevant financial disclosures.
FROM ESMO BREAST CANCER 2023
Eating disorder apps fall short when it comes to privacy
SAN FRANCISCO –
Federal laws require those handling sensitive health information to have policies and security safeguards in place to protect such information, whether it’s stored on paper or electronically.
“As it stands right now, there’s not enough evidence to support using these apps as an adjunct to clinical care,” study author Theodora O’Leary, a 4th-year medical student at Tufts University, Boston, said in an interview. “We need more research on the efficacy of these apps because right now not enough of them meet HIPAA [standards] and don’t have privacy and security measures.”
The findings were presented at the annual meeting of the American Psychiatric Association.
Eating disorders (EDs) are a common mental health condition affecting almost 1 in 10 Americans over their lifetime. Yet only about a third of patients with an eating disorder receive adequate treatment.
The pandemic saw a rise in eating disorders and in the use of mental health apps “to kind of fill the gap because people couldn’t be seen in person,” said Ms. O’Leary.
Inexpensive and accessible
Smartphone apps have a lot of advantages for patients with an ED. For one thing, they’re relatively inexpensive and accessible; most Americans already own one or more devices on which these apps can be used.
They’re also a feasible means of delivering psychological interventions, which are often recommended for EDs. Among these interventions, cognitive-behavioral therapy (CBT) has the largest evidence base for this condition.
Also, as many individuals with an ED may be reluctant to seek treatment because of stigma and shame, the anonymity afforded by an app could increase access to the help they seek.
But Ms. O’Leary warned the Food and Drug Administration does not regulate these apps, and people are sharing their personal health information on them.
The researchers conducted a review of commercially available eating disorder apps by searching the Apple and Google play stores using key phrases such as “eating disorder,” “anorexia,” and “binge eating disorder.”
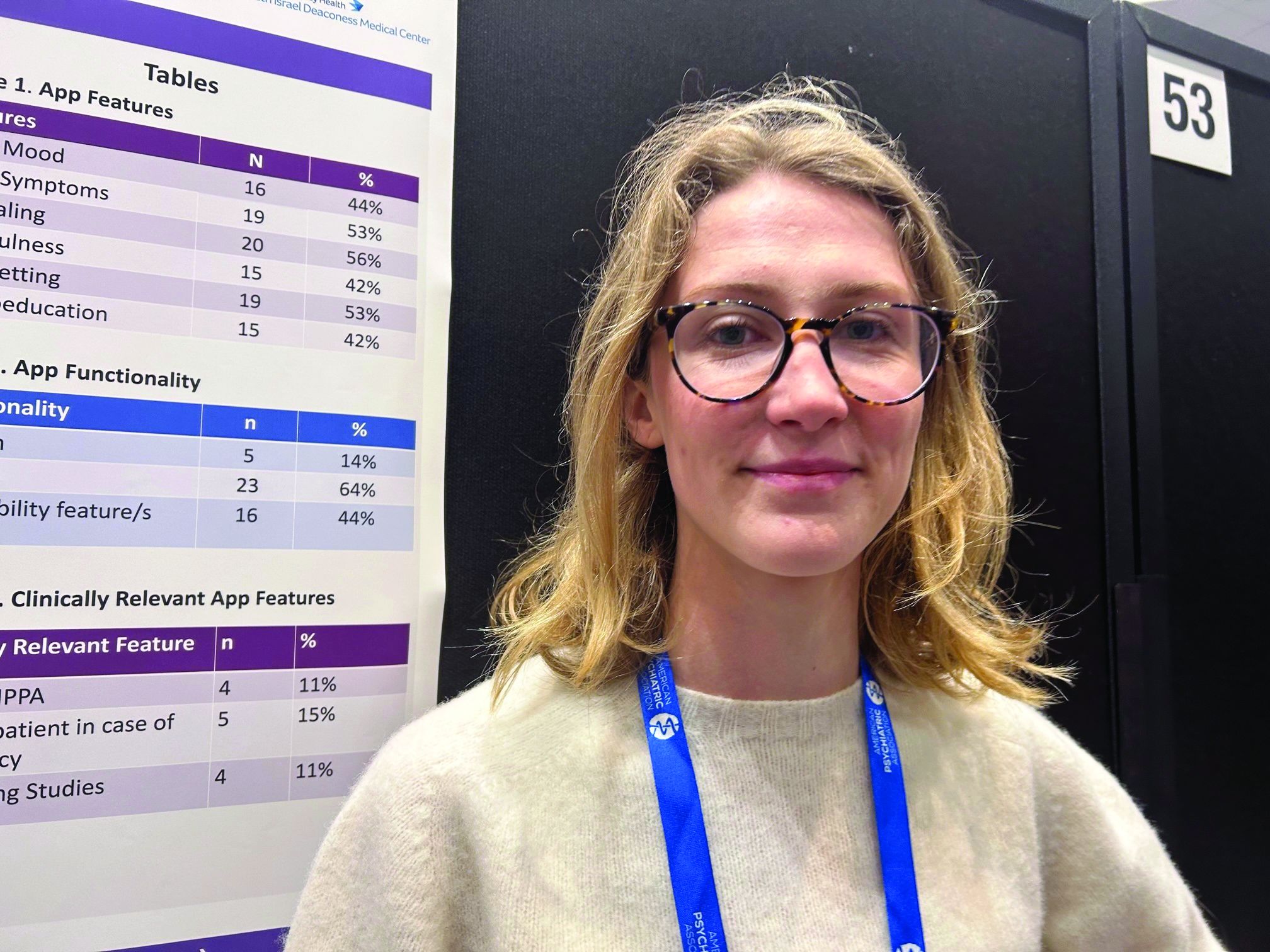
They found 16 relevant apps that they added to the publicly available apps already in a database at Tufts, for a total of about 36 that were evaluated in the study (the number fluctuates as apps are deleted.)
They then reviewed the apps using the 105 questions based on the APA’s app evaluation model, which covers categories such as efficacy, privacy, accessibility, and clinical applicability. And they used filters to group apps by characteristics such as function, cost, and features.
The vast majority were self-help apps, which include things like journaling, meditation, and information on CBT. Others were reference apps that provide related definitions and sometimes include surveys to determine, for example, if the user has an eating disorder.
About 44% of the apps track mood, and 53% track symptoms. Some 56% include journaling, 42% mindfulness, 53% goal setting, and 42% psychoeducation.
Hybrid care
Only 5% of apps allow for “hybrid” care. This, explained Ms. O’Leary, is when clinicians use their own app to access patients’ apps, allowing them to track food restrictions and therapies, and make comments.
“The hybrid is viewed as the best form of app”, she said. “It’s almost like an adjunct to clinical care.”
Hybrid apps also tend to have patient safety features, she added. And these apps meet HIPAA standards, which only 11% of the apps in the study did.
Only 15% of apps advised patients to take steps in case of an emergency, and 11% had supporting studies. And where there was supporting research, much of it was funded by the app creators, said Ms. O’Leary.
For example, an app provided by Noom (the weight loss program that promises to help change habits and mindsets around food) “has a bunch of feasibility studies but they’re all funded by Noom”, which can introduce bias, she explained.
None of the apps were created by an accredited health care institution, she noted. “And I think only one app out of all the eating disorder apps we looked at was from a nonprofit.”
About 17% of the apps offer help with a “coach” or “expert”. However, these apps often fail to disclose the definition of a coach or state they’re not a replacement for medical care.
Coaching apps more expensive
Additionally, these coaching apps are often some of the most expensive, said Ms. O’Leary.
Daniel E. Gih, MD, associate professor at the University of Nebraska Medical Center, Omaha, helped start an eating disorders program at the University of Michigan and continues to treat patients with eating disorders. He said the increase in the use of eating disorder apps isn’t surprising as the incidence of these disorders increased in the wake of pandemic restrictions, especially among young people.
“Patients are likely doing more research on their medical conditions and trying to crowdsource information or self-treat,” Dr. Gih said.
It’s unclear whether “shame or just the general lack of specialized eating disorder professionals,” including physicians, is driving some of the interest in these apps, he added.
Dr. Gih stressed eating disorder apps should not only include screening for suicidality, but also explicitly tell patients to seek immediate attention if they show certain signs – for example, fainting, chest pain, or blood in emesis.
“The apps may be giving patients false hope or delaying medical care,” he said. “Apps are likely not sufficient enough to replace a multidisciplinary team with experience and expertise in eating disorders.”
Ms. O’Leary and Dr. Gih report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO –
Federal laws require those handling sensitive health information to have policies and security safeguards in place to protect such information, whether it’s stored on paper or electronically.
“As it stands right now, there’s not enough evidence to support using these apps as an adjunct to clinical care,” study author Theodora O’Leary, a 4th-year medical student at Tufts University, Boston, said in an interview. “We need more research on the efficacy of these apps because right now not enough of them meet HIPAA [standards] and don’t have privacy and security measures.”
The findings were presented at the annual meeting of the American Psychiatric Association.
Eating disorders (EDs) are a common mental health condition affecting almost 1 in 10 Americans over their lifetime. Yet only about a third of patients with an eating disorder receive adequate treatment.
The pandemic saw a rise in eating disorders and in the use of mental health apps “to kind of fill the gap because people couldn’t be seen in person,” said Ms. O’Leary.
Inexpensive and accessible
Smartphone apps have a lot of advantages for patients with an ED. For one thing, they’re relatively inexpensive and accessible; most Americans already own one or more devices on which these apps can be used.
They’re also a feasible means of delivering psychological interventions, which are often recommended for EDs. Among these interventions, cognitive-behavioral therapy (CBT) has the largest evidence base for this condition.
Also, as many individuals with an ED may be reluctant to seek treatment because of stigma and shame, the anonymity afforded by an app could increase access to the help they seek.
But Ms. O’Leary warned the Food and Drug Administration does not regulate these apps, and people are sharing their personal health information on them.
The researchers conducted a review of commercially available eating disorder apps by searching the Apple and Google play stores using key phrases such as “eating disorder,” “anorexia,” and “binge eating disorder.”
They found 16 relevant apps that they added to the publicly available apps already in a database at Tufts, for a total of about 36 that were evaluated in the study (the number fluctuates as apps are deleted.)
They then reviewed the apps using the 105 questions based on the APA’s app evaluation model, which covers categories such as efficacy, privacy, accessibility, and clinical applicability. And they used filters to group apps by characteristics such as function, cost, and features.
The vast majority were self-help apps, which include things like journaling, meditation, and information on CBT. Others were reference apps that provide related definitions and sometimes include surveys to determine, for example, if the user has an eating disorder.
About 44% of the apps track mood, and 53% track symptoms. Some 56% include journaling, 42% mindfulness, 53% goal setting, and 42% psychoeducation.
Hybrid care
Only 5% of apps allow for “hybrid” care. This, explained Ms. O’Leary, is when clinicians use their own app to access patients’ apps, allowing them to track food restrictions and therapies, and make comments.
“The hybrid is viewed as the best form of app”, she said. “It’s almost like an adjunct to clinical care.”
Hybrid apps also tend to have patient safety features, she added. And these apps meet HIPAA standards, which only 11% of the apps in the study did.
Only 15% of apps advised patients to take steps in case of an emergency, and 11% had supporting studies. And where there was supporting research, much of it was funded by the app creators, said Ms. O’Leary.
For example, an app provided by Noom (the weight loss program that promises to help change habits and mindsets around food) “has a bunch of feasibility studies but they’re all funded by Noom”, which can introduce bias, she explained.
None of the apps were created by an accredited health care institution, she noted. “And I think only one app out of all the eating disorder apps we looked at was from a nonprofit.”
About 17% of the apps offer help with a “coach” or “expert”. However, these apps often fail to disclose the definition of a coach or state they’re not a replacement for medical care.
Coaching apps more expensive
Additionally, these coaching apps are often some of the most expensive, said Ms. O’Leary.
Daniel E. Gih, MD, associate professor at the University of Nebraska Medical Center, Omaha, helped start an eating disorders program at the University of Michigan and continues to treat patients with eating disorders. He said the increase in the use of eating disorder apps isn’t surprising as the incidence of these disorders increased in the wake of pandemic restrictions, especially among young people.
“Patients are likely doing more research on their medical conditions and trying to crowdsource information or self-treat,” Dr. Gih said.
It’s unclear whether “shame or just the general lack of specialized eating disorder professionals,” including physicians, is driving some of the interest in these apps, he added.
Dr. Gih stressed eating disorder apps should not only include screening for suicidality, but also explicitly tell patients to seek immediate attention if they show certain signs – for example, fainting, chest pain, or blood in emesis.
“The apps may be giving patients false hope or delaying medical care,” he said. “Apps are likely not sufficient enough to replace a multidisciplinary team with experience and expertise in eating disorders.”
Ms. O’Leary and Dr. Gih report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO –
Federal laws require those handling sensitive health information to have policies and security safeguards in place to protect such information, whether it’s stored on paper or electronically.
“As it stands right now, there’s not enough evidence to support using these apps as an adjunct to clinical care,” study author Theodora O’Leary, a 4th-year medical student at Tufts University, Boston, said in an interview. “We need more research on the efficacy of these apps because right now not enough of them meet HIPAA [standards] and don’t have privacy and security measures.”
The findings were presented at the annual meeting of the American Psychiatric Association.
Eating disorders (EDs) are a common mental health condition affecting almost 1 in 10 Americans over their lifetime. Yet only about a third of patients with an eating disorder receive adequate treatment.
The pandemic saw a rise in eating disorders and in the use of mental health apps “to kind of fill the gap because people couldn’t be seen in person,” said Ms. O’Leary.
Inexpensive and accessible
Smartphone apps have a lot of advantages for patients with an ED. For one thing, they’re relatively inexpensive and accessible; most Americans already own one or more devices on which these apps can be used.
They’re also a feasible means of delivering psychological interventions, which are often recommended for EDs. Among these interventions, cognitive-behavioral therapy (CBT) has the largest evidence base for this condition.
Also, as many individuals with an ED may be reluctant to seek treatment because of stigma and shame, the anonymity afforded by an app could increase access to the help they seek.
But Ms. O’Leary warned the Food and Drug Administration does not regulate these apps, and people are sharing their personal health information on them.
The researchers conducted a review of commercially available eating disorder apps by searching the Apple and Google play stores using key phrases such as “eating disorder,” “anorexia,” and “binge eating disorder.”
They found 16 relevant apps that they added to the publicly available apps already in a database at Tufts, for a total of about 36 that were evaluated in the study (the number fluctuates as apps are deleted.)
They then reviewed the apps using the 105 questions based on the APA’s app evaluation model, which covers categories such as efficacy, privacy, accessibility, and clinical applicability. And they used filters to group apps by characteristics such as function, cost, and features.
The vast majority were self-help apps, which include things like journaling, meditation, and information on CBT. Others were reference apps that provide related definitions and sometimes include surveys to determine, for example, if the user has an eating disorder.
About 44% of the apps track mood, and 53% track symptoms. Some 56% include journaling, 42% mindfulness, 53% goal setting, and 42% psychoeducation.
Hybrid care
Only 5% of apps allow for “hybrid” care. This, explained Ms. O’Leary, is when clinicians use their own app to access patients’ apps, allowing them to track food restrictions and therapies, and make comments.
“The hybrid is viewed as the best form of app”, she said. “It’s almost like an adjunct to clinical care.”
Hybrid apps also tend to have patient safety features, she added. And these apps meet HIPAA standards, which only 11% of the apps in the study did.
Only 15% of apps advised patients to take steps in case of an emergency, and 11% had supporting studies. And where there was supporting research, much of it was funded by the app creators, said Ms. O’Leary.
For example, an app provided by Noom (the weight loss program that promises to help change habits and mindsets around food) “has a bunch of feasibility studies but they’re all funded by Noom”, which can introduce bias, she explained.
None of the apps were created by an accredited health care institution, she noted. “And I think only one app out of all the eating disorder apps we looked at was from a nonprofit.”
About 17% of the apps offer help with a “coach” or “expert”. However, these apps often fail to disclose the definition of a coach or state they’re not a replacement for medical care.
Coaching apps more expensive
Additionally, these coaching apps are often some of the most expensive, said Ms. O’Leary.
Daniel E. Gih, MD, associate professor at the University of Nebraska Medical Center, Omaha, helped start an eating disorders program at the University of Michigan and continues to treat patients with eating disorders. He said the increase in the use of eating disorder apps isn’t surprising as the incidence of these disorders increased in the wake of pandemic restrictions, especially among young people.
“Patients are likely doing more research on their medical conditions and trying to crowdsource information or self-treat,” Dr. Gih said.
It’s unclear whether “shame or just the general lack of specialized eating disorder professionals,” including physicians, is driving some of the interest in these apps, he added.
Dr. Gih stressed eating disorder apps should not only include screening for suicidality, but also explicitly tell patients to seek immediate attention if they show certain signs – for example, fainting, chest pain, or blood in emesis.
“The apps may be giving patients false hope or delaying medical care,” he said. “Apps are likely not sufficient enough to replace a multidisciplinary team with experience and expertise in eating disorders.”
Ms. O’Leary and Dr. Gih report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT APA 2023
Tweaking food delivery apps can lower calories purchased
DUBLIN – , show three new randomized trials from the United Kingdom.
The prominent positioning of low-calorie menu items, and restaurants with low-calorie main meals, on a food app emerged as the most promising approach to promote healthier eating, followed by preselecting smaller portions by default, and finally calorie labels, Anna Keleher, MPA, a behavioral scientist at Nesta, London, reported at the European Congress on Obesity (ECO) meeting.
“Many out-of-home meals have more calories than meals cooked in-home and using delivery apps is linked with a higher risk of becoming overweight or obese,” she remarked. “We’re interested in understanding more about delivery apps because they can be modified at scale easily and can reach millions of people with interventions to promote healthier and more nutritious options in these settings.”
Food delivery apps have surged in use in the United Kingdom with a 55% increase since 2015; examples include Uber Eats, Just Eat, and Deliveroo. “This trend is similar in the United States, with more and more consumers using delivery apps to buy food,” said Ms. Keleher, a senior adviser at the Behavioral Insights Team, New York.
Emma Boyland, PhD, an obesity psychologist from Liverpool (England) University, said: “Apps are an increasingly popular way for people to buy food and the virtual food environment is becoming as prominent as the physical food environment in how we go about obtaining meals.”
She highlighted the need to understand more about how food apps change the way we purchase and eat, but noted that “the work presented today” showed that “moving the position of food choices and information, as well as the brand name and imagery, influences what people end up buying and consuming.
“I think there’s a place for interventions that challenge these things and improve dietary health,” said Dr. Boyland, who chaired the session during which Ms. Keleher presented her results. “However, as we’ve seen with calorie labeling, they don’t always have the biggest effect on their own, so as is often the case, we need to take multiple actions, incorporating all the elements of the environment to make a meaningful difference.”
Three trials changing displays on simulated food delivery apps
“Delivery apps could reach millions of people and help us select healthier food options, and yet there is very little research looking at what works to promote healthier and more nutritious options in these settings,” Filippo Bianchi, MD, a colleague working with Ms. Keleher, said in a press release issued by ECO.
So the research team carried out a proof-of-concept testing of health-promoting interventions by developing a simulated food delivery app and asking 23,783 adults who typically use such services to choose a meal for themselves as if it were a real-life food delivery order.
“As a first step, we developed a simulated online food delivery platform to generate evidence on the effectiveness of our interventions,” Ms. Keleher explained, noting that the simulated platform included 21 restaurants and almost 600 food and drink items to choose from.
The research evaluated 14 interventions across three randomized controlled trials, displaying various food-ordering options that promoted lower-calorie options against a control. The trials investigated default choices (promoting the selection of small portion sizes through defaults, n = 6,000); positioning (promoting the selection of less calorie-dense options through positioning, n = 9,003); and labeling (promoting the selection of less calorific options through calorie labels, n = 8,780).
The primary outcome was the total number of calories in the basket at checkout. The results were adjusted for potentially confounding factors, such as body mass index, age, gender, and income.
For the trial that promoted smaller portions by default, “all of our interventions significantly reduced calorie purchases, with each additional intervention element increasing the effect sizes, which ranged from a 6% to 13% reduction in calories [–5.5% to –12.5% kcal/order; P < .05],” reported Ms. Keleher.
The second trial varied the position of both items on the menu and the order of restaurants – effectively, lower-calorie menu options were more prominent, and restaurant options with lower-calorie main meals were placed at the top of the restaurant selection page.
Ms. Keleher noted that there have been some concerns about whether this strategy would negatively affect restaurant business, so the research team counteracted this by also incorporating an option where low-calorie but high-price options were placed near the top of the display to promote healthier options but without loss of income for participating restaurants. This last intervention with low-calorie/high-price options placed near the top also led to reduced calorie intake.
“This showed that promoting low-calorie options does not necessarily mean damaging business revenue,” she said. “We hope that the industry can evolve to meet the widely recognized needs of society and consumers.”
Repositioning restaurants emerged as more effective than repositioning foods on the menu, while all interventions significantly reduced calorie purchases. “Effect sizes ranged from 6% to 15% reductions in calories purchased per order [P < .05],” reported Ms. Keleher.
The last trial tested seven calorie labels: four that changed the font size and location of the label, two that added a switch on/off filter for calorie label display, and one that was a calorie summary at checkout.
“All these standard calorie labels directionally reduced the number of excess calories with two [options] reaching statistical significance. Five out of seven labels significantly reduced calorie purchases with effect sizes ranging from 4.3% to –7.8% kcal/order (P < .05),” reported Ms. Keleher.
“This research is important for policymakers so they can understand the best way for companies to display calorie labels and what to include in regulations and guidelines,” she summarized.
Qualitative think-aloud study explored views around food delivery apps
Another piece of research, the think-aloud study, by the same authors, was presented at ECO, and explored how best to enhance the effectiveness and acceptability of calorie labels in food delivery apps in consultation with 20 adult delivery app users in the United Kingdom.
Researchers tried to document the range of views people have about calorie labels, including variation both between people and within an individual.
“For example, on a weekend, people might not want to engage with calories at all because they are more concerned to treat themselves, whereas at a mid-week lunch that same person might really want the ability to check the calorie content of their food,” Ms. Keleher reported.
She said that considerations varied significantly between people such that they described different ways in which calorie labeling impacted their food-ordering experience.
“Some people felt labels supported their existing intentions, whereas others felt labels built their knowledge. Still others felt calorie labels were insufficient to support their health and wanted more information, such as on macronutrients,” said Ms. Keleher, quoting one participant: “There’s no situation in which I would look at [calories]. I look at nutrients. I prefer the traffic light system [color-coding salt, fat, and sugar content],” she relayed.
The key recommendations based on the think-aloud study included providing a filter that allows users to switch calorie labels on and off; communicating recommended energy intake per meal (that is, 600 kcal) and not just per day (that is, 2,000 kcal); and avoiding framing calorie label messaging or formatting as judgmental (for example, red fonts).
“These studies provide encouraging proof-of-concept evidence that small tweaks in delivery apps could help many people to identify and select healthier foods. Testing similar initiatives with real restaurants and delivery apps will be important to assess the long-term impact of these interventions in the real world. Further research should also explore the best way to balance desired health impacts while minimizing effects on businesses and on cost-of-living concerns for consumers,” concluded Dr. Bianchi.
A version of this article first appeared on Medscape.com.
DUBLIN – , show three new randomized trials from the United Kingdom.
The prominent positioning of low-calorie menu items, and restaurants with low-calorie main meals, on a food app emerged as the most promising approach to promote healthier eating, followed by preselecting smaller portions by default, and finally calorie labels, Anna Keleher, MPA, a behavioral scientist at Nesta, London, reported at the European Congress on Obesity (ECO) meeting.
“Many out-of-home meals have more calories than meals cooked in-home and using delivery apps is linked with a higher risk of becoming overweight or obese,” she remarked. “We’re interested in understanding more about delivery apps because they can be modified at scale easily and can reach millions of people with interventions to promote healthier and more nutritious options in these settings.”
Food delivery apps have surged in use in the United Kingdom with a 55% increase since 2015; examples include Uber Eats, Just Eat, and Deliveroo. “This trend is similar in the United States, with more and more consumers using delivery apps to buy food,” said Ms. Keleher, a senior adviser at the Behavioral Insights Team, New York.
Emma Boyland, PhD, an obesity psychologist from Liverpool (England) University, said: “Apps are an increasingly popular way for people to buy food and the virtual food environment is becoming as prominent as the physical food environment in how we go about obtaining meals.”
She highlighted the need to understand more about how food apps change the way we purchase and eat, but noted that “the work presented today” showed that “moving the position of food choices and information, as well as the brand name and imagery, influences what people end up buying and consuming.
“I think there’s a place for interventions that challenge these things and improve dietary health,” said Dr. Boyland, who chaired the session during which Ms. Keleher presented her results. “However, as we’ve seen with calorie labeling, they don’t always have the biggest effect on their own, so as is often the case, we need to take multiple actions, incorporating all the elements of the environment to make a meaningful difference.”
Three trials changing displays on simulated food delivery apps
“Delivery apps could reach millions of people and help us select healthier food options, and yet there is very little research looking at what works to promote healthier and more nutritious options in these settings,” Filippo Bianchi, MD, a colleague working with Ms. Keleher, said in a press release issued by ECO.
So the research team carried out a proof-of-concept testing of health-promoting interventions by developing a simulated food delivery app and asking 23,783 adults who typically use such services to choose a meal for themselves as if it were a real-life food delivery order.
“As a first step, we developed a simulated online food delivery platform to generate evidence on the effectiveness of our interventions,” Ms. Keleher explained, noting that the simulated platform included 21 restaurants and almost 600 food and drink items to choose from.
The research evaluated 14 interventions across three randomized controlled trials, displaying various food-ordering options that promoted lower-calorie options against a control. The trials investigated default choices (promoting the selection of small portion sizes through defaults, n = 6,000); positioning (promoting the selection of less calorie-dense options through positioning, n = 9,003); and labeling (promoting the selection of less calorific options through calorie labels, n = 8,780).
The primary outcome was the total number of calories in the basket at checkout. The results were adjusted for potentially confounding factors, such as body mass index, age, gender, and income.
For the trial that promoted smaller portions by default, “all of our interventions significantly reduced calorie purchases, with each additional intervention element increasing the effect sizes, which ranged from a 6% to 13% reduction in calories [–5.5% to –12.5% kcal/order; P < .05],” reported Ms. Keleher.
The second trial varied the position of both items on the menu and the order of restaurants – effectively, lower-calorie menu options were more prominent, and restaurant options with lower-calorie main meals were placed at the top of the restaurant selection page.
Ms. Keleher noted that there have been some concerns about whether this strategy would negatively affect restaurant business, so the research team counteracted this by also incorporating an option where low-calorie but high-price options were placed near the top of the display to promote healthier options but without loss of income for participating restaurants. This last intervention with low-calorie/high-price options placed near the top also led to reduced calorie intake.
“This showed that promoting low-calorie options does not necessarily mean damaging business revenue,” she said. “We hope that the industry can evolve to meet the widely recognized needs of society and consumers.”
Repositioning restaurants emerged as more effective than repositioning foods on the menu, while all interventions significantly reduced calorie purchases. “Effect sizes ranged from 6% to 15% reductions in calories purchased per order [P < .05],” reported Ms. Keleher.
The last trial tested seven calorie labels: four that changed the font size and location of the label, two that added a switch on/off filter for calorie label display, and one that was a calorie summary at checkout.
“All these standard calorie labels directionally reduced the number of excess calories with two [options] reaching statistical significance. Five out of seven labels significantly reduced calorie purchases with effect sizes ranging from 4.3% to –7.8% kcal/order (P < .05),” reported Ms. Keleher.
“This research is important for policymakers so they can understand the best way for companies to display calorie labels and what to include in regulations and guidelines,” she summarized.
Qualitative think-aloud study explored views around food delivery apps
Another piece of research, the think-aloud study, by the same authors, was presented at ECO, and explored how best to enhance the effectiveness and acceptability of calorie labels in food delivery apps in consultation with 20 adult delivery app users in the United Kingdom.
Researchers tried to document the range of views people have about calorie labels, including variation both between people and within an individual.
“For example, on a weekend, people might not want to engage with calories at all because they are more concerned to treat themselves, whereas at a mid-week lunch that same person might really want the ability to check the calorie content of their food,” Ms. Keleher reported.
She said that considerations varied significantly between people such that they described different ways in which calorie labeling impacted their food-ordering experience.
“Some people felt labels supported their existing intentions, whereas others felt labels built their knowledge. Still others felt calorie labels were insufficient to support their health and wanted more information, such as on macronutrients,” said Ms. Keleher, quoting one participant: “There’s no situation in which I would look at [calories]. I look at nutrients. I prefer the traffic light system [color-coding salt, fat, and sugar content],” she relayed.
The key recommendations based on the think-aloud study included providing a filter that allows users to switch calorie labels on and off; communicating recommended energy intake per meal (that is, 600 kcal) and not just per day (that is, 2,000 kcal); and avoiding framing calorie label messaging or formatting as judgmental (for example, red fonts).
“These studies provide encouraging proof-of-concept evidence that small tweaks in delivery apps could help many people to identify and select healthier foods. Testing similar initiatives with real restaurants and delivery apps will be important to assess the long-term impact of these interventions in the real world. Further research should also explore the best way to balance desired health impacts while minimizing effects on businesses and on cost-of-living concerns for consumers,” concluded Dr. Bianchi.
A version of this article first appeared on Medscape.com.
DUBLIN – , show three new randomized trials from the United Kingdom.
The prominent positioning of low-calorie menu items, and restaurants with low-calorie main meals, on a food app emerged as the most promising approach to promote healthier eating, followed by preselecting smaller portions by default, and finally calorie labels, Anna Keleher, MPA, a behavioral scientist at Nesta, London, reported at the European Congress on Obesity (ECO) meeting.
“Many out-of-home meals have more calories than meals cooked in-home and using delivery apps is linked with a higher risk of becoming overweight or obese,” she remarked. “We’re interested in understanding more about delivery apps because they can be modified at scale easily and can reach millions of people with interventions to promote healthier and more nutritious options in these settings.”
Food delivery apps have surged in use in the United Kingdom with a 55% increase since 2015; examples include Uber Eats, Just Eat, and Deliveroo. “This trend is similar in the United States, with more and more consumers using delivery apps to buy food,” said Ms. Keleher, a senior adviser at the Behavioral Insights Team, New York.
Emma Boyland, PhD, an obesity psychologist from Liverpool (England) University, said: “Apps are an increasingly popular way for people to buy food and the virtual food environment is becoming as prominent as the physical food environment in how we go about obtaining meals.”
She highlighted the need to understand more about how food apps change the way we purchase and eat, but noted that “the work presented today” showed that “moving the position of food choices and information, as well as the brand name and imagery, influences what people end up buying and consuming.
“I think there’s a place for interventions that challenge these things and improve dietary health,” said Dr. Boyland, who chaired the session during which Ms. Keleher presented her results. “However, as we’ve seen with calorie labeling, they don’t always have the biggest effect on their own, so as is often the case, we need to take multiple actions, incorporating all the elements of the environment to make a meaningful difference.”
Three trials changing displays on simulated food delivery apps
“Delivery apps could reach millions of people and help us select healthier food options, and yet there is very little research looking at what works to promote healthier and more nutritious options in these settings,” Filippo Bianchi, MD, a colleague working with Ms. Keleher, said in a press release issued by ECO.
So the research team carried out a proof-of-concept testing of health-promoting interventions by developing a simulated food delivery app and asking 23,783 adults who typically use such services to choose a meal for themselves as if it were a real-life food delivery order.
“As a first step, we developed a simulated online food delivery platform to generate evidence on the effectiveness of our interventions,” Ms. Keleher explained, noting that the simulated platform included 21 restaurants and almost 600 food and drink items to choose from.
The research evaluated 14 interventions across three randomized controlled trials, displaying various food-ordering options that promoted lower-calorie options against a control. The trials investigated default choices (promoting the selection of small portion sizes through defaults, n = 6,000); positioning (promoting the selection of less calorie-dense options through positioning, n = 9,003); and labeling (promoting the selection of less calorific options through calorie labels, n = 8,780).
The primary outcome was the total number of calories in the basket at checkout. The results were adjusted for potentially confounding factors, such as body mass index, age, gender, and income.
For the trial that promoted smaller portions by default, “all of our interventions significantly reduced calorie purchases, with each additional intervention element increasing the effect sizes, which ranged from a 6% to 13% reduction in calories [–5.5% to –12.5% kcal/order; P < .05],” reported Ms. Keleher.
The second trial varied the position of both items on the menu and the order of restaurants – effectively, lower-calorie menu options were more prominent, and restaurant options with lower-calorie main meals were placed at the top of the restaurant selection page.
Ms. Keleher noted that there have been some concerns about whether this strategy would negatively affect restaurant business, so the research team counteracted this by also incorporating an option where low-calorie but high-price options were placed near the top of the display to promote healthier options but without loss of income for participating restaurants. This last intervention with low-calorie/high-price options placed near the top also led to reduced calorie intake.
“This showed that promoting low-calorie options does not necessarily mean damaging business revenue,” she said. “We hope that the industry can evolve to meet the widely recognized needs of society and consumers.”
Repositioning restaurants emerged as more effective than repositioning foods on the menu, while all interventions significantly reduced calorie purchases. “Effect sizes ranged from 6% to 15% reductions in calories purchased per order [P < .05],” reported Ms. Keleher.
The last trial tested seven calorie labels: four that changed the font size and location of the label, two that added a switch on/off filter for calorie label display, and one that was a calorie summary at checkout.
“All these standard calorie labels directionally reduced the number of excess calories with two [options] reaching statistical significance. Five out of seven labels significantly reduced calorie purchases with effect sizes ranging from 4.3% to –7.8% kcal/order (P < .05),” reported Ms. Keleher.
“This research is important for policymakers so they can understand the best way for companies to display calorie labels and what to include in regulations and guidelines,” she summarized.
Qualitative think-aloud study explored views around food delivery apps
Another piece of research, the think-aloud study, by the same authors, was presented at ECO, and explored how best to enhance the effectiveness and acceptability of calorie labels in food delivery apps in consultation with 20 adult delivery app users in the United Kingdom.
Researchers tried to document the range of views people have about calorie labels, including variation both between people and within an individual.
“For example, on a weekend, people might not want to engage with calories at all because they are more concerned to treat themselves, whereas at a mid-week lunch that same person might really want the ability to check the calorie content of their food,” Ms. Keleher reported.
She said that considerations varied significantly between people such that they described different ways in which calorie labeling impacted their food-ordering experience.
“Some people felt labels supported their existing intentions, whereas others felt labels built their knowledge. Still others felt calorie labels were insufficient to support their health and wanted more information, such as on macronutrients,” said Ms. Keleher, quoting one participant: “There’s no situation in which I would look at [calories]. I look at nutrients. I prefer the traffic light system [color-coding salt, fat, and sugar content],” she relayed.
The key recommendations based on the think-aloud study included providing a filter that allows users to switch calorie labels on and off; communicating recommended energy intake per meal (that is, 600 kcal) and not just per day (that is, 2,000 kcal); and avoiding framing calorie label messaging or formatting as judgmental (for example, red fonts).
“These studies provide encouraging proof-of-concept evidence that small tweaks in delivery apps could help many people to identify and select healthier foods. Testing similar initiatives with real restaurants and delivery apps will be important to assess the long-term impact of these interventions in the real world. Further research should also explore the best way to balance desired health impacts while minimizing effects on businesses and on cost-of-living concerns for consumers,” concluded Dr. Bianchi.
A version of this article first appeared on Medscape.com.
AT ECO 2023
Lupus landmark study aims for personalized medicine goals
A new prospective, observational study from the Lupus Research Alliance (LRA) aims to enroll 3,500 patients in an effort to accelerate the development of personalized treatments for individuals living with systemic lupus erythematosus (SLE).
The LRA on May 23 announced the launch of the Lupus Landmark Study (LLS). The study will be conducted in partnership with Lupus Therapeutics, the clinical research affiliate of the LRA.
The study will be a key feature of the Lupus Nexus, a unique combination of lupus patient registry, biorepository, and portal for data sharing and analysis.
“The aim of the Lupus Nexus is to transform lupus research and drug development through unprecedented information exchange capabilities,” according to the LRA press release.
“SLE is a debilitating autoimmune disease that disproportionately impacts women and people from minority groups, but the cause of lupus is unknown, and no single laboratory test can definitively identify lupus,” lead investigator S. Sam Lim, MD, of Emory University, Atlanta, told this news organization.
“Nevertheless, early detection and treatment can often lessen the progression and severity of the disease. Although there are numerous contributing factors to the lag in research discoveries and new treatments for patients with lupus, limited access to standardized, high-quality biological samples and natural history data provides a significant roadblock to advancing lupus research,” Dr. Lim said.
“Existing registry and biorepository resources in the lupus field are largely siloed, mostly limited to relatively small or discrete patient populations, and frequently not designed for broad sharing across all stakeholders of the research community,” Dr. Lim said. The LRA and its affiliate Lupus Therapeutics are committed to developing Lupus Nexus, a first-of-its-kind registry and biorepository, to serve as a collaborative research platform for lupus and a leading source of prospective, longitudinal patient data and biological samples for the research community, Dr. Lim added.
“The Lupus Landmark Study will form the foundation of this registry and biorepository and will provide a critical resource to enable the understanding of lupus heterogeneity at the molecular level,” Dr. Lim said. The molecular data can be linked to clinical phenotypes, he explained, “while providing an opportunity to better understand the holistic experience of patients with lupus, thus helping patients address the daily life challenges they face.”
The Lupus Accelerating Breakthroughs Consortium (Lupus ABC) was announced earlier this spring by the LRA. It represents a collaboration between the U.S. Food and Drug Administration and the lupus community to improve and accelerate the development of safer and more effective treatments for people with lupus, Dr. Lim said. “Data and other results from the LLS will inform this collaboration,” he said.
“The LLS will provide greater insight into the pathogenesis and evolution of the condition, providing much needed information and guidance to clinicians so that the disease can be detected and treated earlier and with better precision,” Dr. Lim said. “The partnership with patients will ensure that advances will not only be meaningful to clinicians but their patients and caregivers as well,” he added.
Individuals living with lupus were essential to the development of the Lupus Nexus, and patients will continue to be engaged through participation in the LLS, which will not only generate data to promote patient-centered treatments but will also give participants more insight into their health data, according to the LRA press release.
The clinical coordinating center and biorepository elements of the Lupus Nexus will be managed by Embleema and Azenta Life Sciences, respectively, according the LRA.
Biomarker analysis will be conducted by DxTerity Diagnostics via the company’s proprietary DxCollection MicroCollection Device and Modular Immune Profile platform.
The LLS is scheduled to begin enrolling patients through select academic medical centers in the Lupus Therapeutics Lupus Clinical Investigators Network later in 2023, with an expanded roll-out in 2024, according to the press release. More information about the Lupus Landmark Study is available from Lupus Nexus at [email protected].
A version of this article first appeared on Medscape.com.
A new prospective, observational study from the Lupus Research Alliance (LRA) aims to enroll 3,500 patients in an effort to accelerate the development of personalized treatments for individuals living with systemic lupus erythematosus (SLE).
The LRA on May 23 announced the launch of the Lupus Landmark Study (LLS). The study will be conducted in partnership with Lupus Therapeutics, the clinical research affiliate of the LRA.
The study will be a key feature of the Lupus Nexus, a unique combination of lupus patient registry, biorepository, and portal for data sharing and analysis.
“The aim of the Lupus Nexus is to transform lupus research and drug development through unprecedented information exchange capabilities,” according to the LRA press release.
“SLE is a debilitating autoimmune disease that disproportionately impacts women and people from minority groups, but the cause of lupus is unknown, and no single laboratory test can definitively identify lupus,” lead investigator S. Sam Lim, MD, of Emory University, Atlanta, told this news organization.
“Nevertheless, early detection and treatment can often lessen the progression and severity of the disease. Although there are numerous contributing factors to the lag in research discoveries and new treatments for patients with lupus, limited access to standardized, high-quality biological samples and natural history data provides a significant roadblock to advancing lupus research,” Dr. Lim said.
“Existing registry and biorepository resources in the lupus field are largely siloed, mostly limited to relatively small or discrete patient populations, and frequently not designed for broad sharing across all stakeholders of the research community,” Dr. Lim said. The LRA and its affiliate Lupus Therapeutics are committed to developing Lupus Nexus, a first-of-its-kind registry and biorepository, to serve as a collaborative research platform for lupus and a leading source of prospective, longitudinal patient data and biological samples for the research community, Dr. Lim added.
“The Lupus Landmark Study will form the foundation of this registry and biorepository and will provide a critical resource to enable the understanding of lupus heterogeneity at the molecular level,” Dr. Lim said. The molecular data can be linked to clinical phenotypes, he explained, “while providing an opportunity to better understand the holistic experience of patients with lupus, thus helping patients address the daily life challenges they face.”
The Lupus Accelerating Breakthroughs Consortium (Lupus ABC) was announced earlier this spring by the LRA. It represents a collaboration between the U.S. Food and Drug Administration and the lupus community to improve and accelerate the development of safer and more effective treatments for people with lupus, Dr. Lim said. “Data and other results from the LLS will inform this collaboration,” he said.
“The LLS will provide greater insight into the pathogenesis and evolution of the condition, providing much needed information and guidance to clinicians so that the disease can be detected and treated earlier and with better precision,” Dr. Lim said. “The partnership with patients will ensure that advances will not only be meaningful to clinicians but their patients and caregivers as well,” he added.
Individuals living with lupus were essential to the development of the Lupus Nexus, and patients will continue to be engaged through participation in the LLS, which will not only generate data to promote patient-centered treatments but will also give participants more insight into their health data, according to the LRA press release.
The clinical coordinating center and biorepository elements of the Lupus Nexus will be managed by Embleema and Azenta Life Sciences, respectively, according the LRA.
Biomarker analysis will be conducted by DxTerity Diagnostics via the company’s proprietary DxCollection MicroCollection Device and Modular Immune Profile platform.
The LLS is scheduled to begin enrolling patients through select academic medical centers in the Lupus Therapeutics Lupus Clinical Investigators Network later in 2023, with an expanded roll-out in 2024, according to the press release. More information about the Lupus Landmark Study is available from Lupus Nexus at [email protected].
A version of this article first appeared on Medscape.com.
A new prospective, observational study from the Lupus Research Alliance (LRA) aims to enroll 3,500 patients in an effort to accelerate the development of personalized treatments for individuals living with systemic lupus erythematosus (SLE).
The LRA on May 23 announced the launch of the Lupus Landmark Study (LLS). The study will be conducted in partnership with Lupus Therapeutics, the clinical research affiliate of the LRA.
The study will be a key feature of the Lupus Nexus, a unique combination of lupus patient registry, biorepository, and portal for data sharing and analysis.
“The aim of the Lupus Nexus is to transform lupus research and drug development through unprecedented information exchange capabilities,” according to the LRA press release.
“SLE is a debilitating autoimmune disease that disproportionately impacts women and people from minority groups, but the cause of lupus is unknown, and no single laboratory test can definitively identify lupus,” lead investigator S. Sam Lim, MD, of Emory University, Atlanta, told this news organization.
“Nevertheless, early detection and treatment can often lessen the progression and severity of the disease. Although there are numerous contributing factors to the lag in research discoveries and new treatments for patients with lupus, limited access to standardized, high-quality biological samples and natural history data provides a significant roadblock to advancing lupus research,” Dr. Lim said.
“Existing registry and biorepository resources in the lupus field are largely siloed, mostly limited to relatively small or discrete patient populations, and frequently not designed for broad sharing across all stakeholders of the research community,” Dr. Lim said. The LRA and its affiliate Lupus Therapeutics are committed to developing Lupus Nexus, a first-of-its-kind registry and biorepository, to serve as a collaborative research platform for lupus and a leading source of prospective, longitudinal patient data and biological samples for the research community, Dr. Lim added.
“The Lupus Landmark Study will form the foundation of this registry and biorepository and will provide a critical resource to enable the understanding of lupus heterogeneity at the molecular level,” Dr. Lim said. The molecular data can be linked to clinical phenotypes, he explained, “while providing an opportunity to better understand the holistic experience of patients with lupus, thus helping patients address the daily life challenges they face.”
The Lupus Accelerating Breakthroughs Consortium (Lupus ABC) was announced earlier this spring by the LRA. It represents a collaboration between the U.S. Food and Drug Administration and the lupus community to improve and accelerate the development of safer and more effective treatments for people with lupus, Dr. Lim said. “Data and other results from the LLS will inform this collaboration,” he said.
“The LLS will provide greater insight into the pathogenesis and evolution of the condition, providing much needed information and guidance to clinicians so that the disease can be detected and treated earlier and with better precision,” Dr. Lim said. “The partnership with patients will ensure that advances will not only be meaningful to clinicians but their patients and caregivers as well,” he added.
Individuals living with lupus were essential to the development of the Lupus Nexus, and patients will continue to be engaged through participation in the LLS, which will not only generate data to promote patient-centered treatments but will also give participants more insight into their health data, according to the LRA press release.
The clinical coordinating center and biorepository elements of the Lupus Nexus will be managed by Embleema and Azenta Life Sciences, respectively, according the LRA.
Biomarker analysis will be conducted by DxTerity Diagnostics via the company’s proprietary DxCollection MicroCollection Device and Modular Immune Profile platform.
The LLS is scheduled to begin enrolling patients through select academic medical centers in the Lupus Therapeutics Lupus Clinical Investigators Network later in 2023, with an expanded roll-out in 2024, according to the press release. More information about the Lupus Landmark Study is available from Lupus Nexus at [email protected].
A version of this article first appeared on Medscape.com.
First in utero cerebrovascular surgery success
The team from Boston Children’s Hospital and Brigham and Women’s Hospital used ultrasound guidance to repair the vein of Galen malformation, which causes excessively high blood flow, resulting in both neurologic and cardiac complications.
The surgery was performed in a fetus at 34 weeks’ gestational age, with remarkable results. Since birth, the baby girl, who was identified in utero as being at high risk of suffering serious complications of the malformation, has required no medication to treat heart failure and no postnatal surgery.
Repeated echocardiograms after birth displayed marked improvement in cardiac output, and brain MRI showed no brain injury and a normal neurologic exam.
“This is incredibly exciting. The hope is that this baby, and others with this condition who receive this in utero surgery in future, will go on to have a normal life,” lead researcher Darren B. Orbach, MD, PhD, said in an interview.
“We were thrilled to see that the aggressive decline usually seen after birth simply did not appear. We are pleased to report that at 6 weeks, the infant is progressing remarkably well, on no medications, eating normally, gaining weight and is back home. There are no signs of any negative effects on the brain,” he added.
Dr. Orbach, codirector of the Cerebrovascular Surgery & Interventions Center at Boston Children’s Hospital, and colleagues described this first case report of the in utero vein of Galen malformation repair in a research letter, published online in the journal Stroke.
Vein of Galen malformation
Dr. Orbach explained that vein of Galen malformation, which occurs in around 1 in every 60,000 births, is a cerebrovascular anomaly in which the arterial system is directly connected to the venous system rather than to capillaries that are necessary to slow blood flow and deliver oxygen to surrounding brain tissue.
“The arterial and venous systems are fundamentally very different. The arterial system is high pressure, high flow; while the venous system is low pressure, low flow. They shouldn’t be directly connected,” he noted.
The vein of Galen malformation is the most extreme version of such an anomaly. Developing in early gestation, it is associated with a large increase in blood flow through the brain which grows over time and can sometimes result in twice the total cardiac output of the body or even more, Dr. Orbach said.
The placenta is believed to be protective as most babies don’t have overt physiologic problems in utero, but they can run into crisis after birth, with the abnormally high blood flow causing an immense stress to the heart.
Babies typically present with heart failure as their first major symptom soon after birth, Dr. Orbach said. “Although the anatomical problem is in the brain, the clinical manifestation is high-output heart failure. The heart is trying to do double its normal work, pumping the blood to the malformation and immediately back to the heart and that blood is not performing any useful function.
“These newborns can get very sick. They need multiple medications to support their cardiovascular system and we need to do procedures to try and reduce the blood flow,” he explained.
Brain injury is also a common problem. “The brain circulation is very abnormal. The blood is being shunted through the malformation rather than circulating through the brain tissue which can become ischemic,” Dr. Orbach commented.
“The babies who get sick would have a very high mortality (up to 90%) without expert care. Even those who do receive expert care at a specialty center have a mortality rate of 30% to 40% and those who survive have a high risk of neurologic and cognitive impairment,” he added.
The current treatment for babies born with the condition involves transarterial embolization, by which a catheter is inserted into the arterial system to enable the malformation to be occluded by various techniques.
But Dr. Orbach pointed out that some babies are born too sick to have the postnatal intervention. “The heart failure and brain injury is so overwhelming that no matter what we do, we cannot reverse it, and these babies normally do not survive. What we are doing with the fetal surgery is trying to help those babies who cannot be treated with the current postnatal approach,” he said.
The first stage of this research involved trying to identify these very-high-risk babies in utero, and the researchers found that on fetal MRI a particular measurement of one of the venous sinuses that drains the main malformation was a good predictor of how the baby would fare after birth. The babies predicted to do poorly from this test are the targets for the fetal surgery.
The technique used for the postnatal intervention is too technically challenging to perform in utero. “So we have developed a different approach for the in utero surgery that involves navigating into the accepting vein in the malformation with a needle under ultrasound guidance, and then packing the vein with metal coils to dramatically reduce the blood flow,” Dr. Orbach explained.
This procedure was performed in this first patient on March 15. The surgery was part of a clinical trial that is planned to include 20 cases in total.
“The immediate goal is to see whether we can transform those fetuses who are at very high risk of getting sick after birth into babies who do well in the [neonatal] ICU and are able to be sent home for elective treatment at a few months of age,” Dr. Orbach noted. “The study is continuing as it is vital that we continue and show efficacy and safety in other patients as well,” he added.
Dr. Orbach said the results of this first case were extremely encouraging. “Each stage was exciting – the technical success of the procedure, and then seeing the [blood] flow diminish on the ultrasound right there during the procedure; then the next day we did a fetal echocardiogram, and we could see that the abnormal cardiac output was dramatically reduced, and a fetal MRI scan also showed the malformation was already coming down in size.”
The baby was born prematurely 2 days after the procedure because of ruptured membranes with a birth weight of 1.9 kg (4.2 lb). She has not required any cardiovascular support or postnatal embolization.
“We were waiting with bated breath until the baby was born to see how she did clinically. I was trying to be conservative in my expectations, but it was quickly apparent that she was going to do great,” he said. Now at home, she has some oxygen treatment for the first few weeks, “but right now her neurological status is completely intact and essentially she looks like any other baby,” Dr. Orbach commented.
It is not yet known whether the infant will need any additional procedures. “We will follow her closely and make a decision on whether further treatment is needed based on whether the malformation is growing or not,” Dr. Orbach said. Longer term follow-up will also assess secondary problems sometimes seen, such as learning problems and seizures.
Although other fetal surgeries are now routinely performed, this is believed to be the first in utero surgery aimed at the cerebrovascular system.
“There were a lot of uncertainties,” Dr. Orbach said. “We didn’t even know if we would be able to see our instruments on ultrasound.” To model the procedure, the researchers had a phantom fetal skull and brain constructed with a vein of Galen malformation, which was key to obtaining Food and Drug Administration approval for the study.
If the study shows success in the other patients too, the technique could be rolled out to other centers. “There definitely needs to be fetal surgery and neurointerventional teams familiar with vein of Galen malformation in place, and ready to manage complications after delivery regardless of outcome. But we are not the only center with those capabilities, so if our trial pans out, yes, the hope is that other teams in specialist children’s hospitals around the world could do this too,” he added.
Pioneering work
Commenting on the case report in an American Heart Association press release, Colin Derdeyn, MD, a neurointerventional radiologist at University of Iowa Health Care, Iowa City, who performs vein of Galen malformation embolizations on neonates, said: “The key advance here is to intervene before the physiologic events of birth can cause life-threatening heart failure.”
Dr. Derdeyn, who is a past chair of the American Heart Association’s Stroke Council, cautioned that one successful case is not enough experience to conclude that the risks of this procedure are worth the benefits.
But, he added: “The positive hemodynamic changes that they observed in utero and after birth – reduction in flow, reduction in size of the draining vein, reversal of the abnormal reversed flow in the aorta – are really encouraging. These are some of the most exciting and surprising aspects of this case report. This is pioneering work being done in a very careful and responsible way.”
The study was funded by a grant from the Sage Schermerhorn Chair for Image-Guided Therapy.
A version of this article first appeared on Medscape.com.
The team from Boston Children’s Hospital and Brigham and Women’s Hospital used ultrasound guidance to repair the vein of Galen malformation, which causes excessively high blood flow, resulting in both neurologic and cardiac complications.
The surgery was performed in a fetus at 34 weeks’ gestational age, with remarkable results. Since birth, the baby girl, who was identified in utero as being at high risk of suffering serious complications of the malformation, has required no medication to treat heart failure and no postnatal surgery.
Repeated echocardiograms after birth displayed marked improvement in cardiac output, and brain MRI showed no brain injury and a normal neurologic exam.
“This is incredibly exciting. The hope is that this baby, and others with this condition who receive this in utero surgery in future, will go on to have a normal life,” lead researcher Darren B. Orbach, MD, PhD, said in an interview.
“We were thrilled to see that the aggressive decline usually seen after birth simply did not appear. We are pleased to report that at 6 weeks, the infant is progressing remarkably well, on no medications, eating normally, gaining weight and is back home. There are no signs of any negative effects on the brain,” he added.
Dr. Orbach, codirector of the Cerebrovascular Surgery & Interventions Center at Boston Children’s Hospital, and colleagues described this first case report of the in utero vein of Galen malformation repair in a research letter, published online in the journal Stroke.
Vein of Galen malformation
Dr. Orbach explained that vein of Galen malformation, which occurs in around 1 in every 60,000 births, is a cerebrovascular anomaly in which the arterial system is directly connected to the venous system rather than to capillaries that are necessary to slow blood flow and deliver oxygen to surrounding brain tissue.
“The arterial and venous systems are fundamentally very different. The arterial system is high pressure, high flow; while the venous system is low pressure, low flow. They shouldn’t be directly connected,” he noted.
The vein of Galen malformation is the most extreme version of such an anomaly. Developing in early gestation, it is associated with a large increase in blood flow through the brain which grows over time and can sometimes result in twice the total cardiac output of the body or even more, Dr. Orbach said.
The placenta is believed to be protective as most babies don’t have overt physiologic problems in utero, but they can run into crisis after birth, with the abnormally high blood flow causing an immense stress to the heart.
Babies typically present with heart failure as their first major symptom soon after birth, Dr. Orbach said. “Although the anatomical problem is in the brain, the clinical manifestation is high-output heart failure. The heart is trying to do double its normal work, pumping the blood to the malformation and immediately back to the heart and that blood is not performing any useful function.
“These newborns can get very sick. They need multiple medications to support their cardiovascular system and we need to do procedures to try and reduce the blood flow,” he explained.
Brain injury is also a common problem. “The brain circulation is very abnormal. The blood is being shunted through the malformation rather than circulating through the brain tissue which can become ischemic,” Dr. Orbach commented.
“The babies who get sick would have a very high mortality (up to 90%) without expert care. Even those who do receive expert care at a specialty center have a mortality rate of 30% to 40% and those who survive have a high risk of neurologic and cognitive impairment,” he added.
The current treatment for babies born with the condition involves transarterial embolization, by which a catheter is inserted into the arterial system to enable the malformation to be occluded by various techniques.
But Dr. Orbach pointed out that some babies are born too sick to have the postnatal intervention. “The heart failure and brain injury is so overwhelming that no matter what we do, we cannot reverse it, and these babies normally do not survive. What we are doing with the fetal surgery is trying to help those babies who cannot be treated with the current postnatal approach,” he said.
The first stage of this research involved trying to identify these very-high-risk babies in utero, and the researchers found that on fetal MRI a particular measurement of one of the venous sinuses that drains the main malformation was a good predictor of how the baby would fare after birth. The babies predicted to do poorly from this test are the targets for the fetal surgery.
The technique used for the postnatal intervention is too technically challenging to perform in utero. “So we have developed a different approach for the in utero surgery that involves navigating into the accepting vein in the malformation with a needle under ultrasound guidance, and then packing the vein with metal coils to dramatically reduce the blood flow,” Dr. Orbach explained.
This procedure was performed in this first patient on March 15. The surgery was part of a clinical trial that is planned to include 20 cases in total.
“The immediate goal is to see whether we can transform those fetuses who are at very high risk of getting sick after birth into babies who do well in the [neonatal] ICU and are able to be sent home for elective treatment at a few months of age,” Dr. Orbach noted. “The study is continuing as it is vital that we continue and show efficacy and safety in other patients as well,” he added.
Dr. Orbach said the results of this first case were extremely encouraging. “Each stage was exciting – the technical success of the procedure, and then seeing the [blood] flow diminish on the ultrasound right there during the procedure; then the next day we did a fetal echocardiogram, and we could see that the abnormal cardiac output was dramatically reduced, and a fetal MRI scan also showed the malformation was already coming down in size.”
The baby was born prematurely 2 days after the procedure because of ruptured membranes with a birth weight of 1.9 kg (4.2 lb). She has not required any cardiovascular support or postnatal embolization.
“We were waiting with bated breath until the baby was born to see how she did clinically. I was trying to be conservative in my expectations, but it was quickly apparent that she was going to do great,” he said. Now at home, she has some oxygen treatment for the first few weeks, “but right now her neurological status is completely intact and essentially she looks like any other baby,” Dr. Orbach commented.
It is not yet known whether the infant will need any additional procedures. “We will follow her closely and make a decision on whether further treatment is needed based on whether the malformation is growing or not,” Dr. Orbach said. Longer term follow-up will also assess secondary problems sometimes seen, such as learning problems and seizures.
Although other fetal surgeries are now routinely performed, this is believed to be the first in utero surgery aimed at the cerebrovascular system.
“There were a lot of uncertainties,” Dr. Orbach said. “We didn’t even know if we would be able to see our instruments on ultrasound.” To model the procedure, the researchers had a phantom fetal skull and brain constructed with a vein of Galen malformation, which was key to obtaining Food and Drug Administration approval for the study.
If the study shows success in the other patients too, the technique could be rolled out to other centers. “There definitely needs to be fetal surgery and neurointerventional teams familiar with vein of Galen malformation in place, and ready to manage complications after delivery regardless of outcome. But we are not the only center with those capabilities, so if our trial pans out, yes, the hope is that other teams in specialist children’s hospitals around the world could do this too,” he added.
Pioneering work
Commenting on the case report in an American Heart Association press release, Colin Derdeyn, MD, a neurointerventional radiologist at University of Iowa Health Care, Iowa City, who performs vein of Galen malformation embolizations on neonates, said: “The key advance here is to intervene before the physiologic events of birth can cause life-threatening heart failure.”
Dr. Derdeyn, who is a past chair of the American Heart Association’s Stroke Council, cautioned that one successful case is not enough experience to conclude that the risks of this procedure are worth the benefits.
But, he added: “The positive hemodynamic changes that they observed in utero and after birth – reduction in flow, reduction in size of the draining vein, reversal of the abnormal reversed flow in the aorta – are really encouraging. These are some of the most exciting and surprising aspects of this case report. This is pioneering work being done in a very careful and responsible way.”
The study was funded by a grant from the Sage Schermerhorn Chair for Image-Guided Therapy.
A version of this article first appeared on Medscape.com.
The team from Boston Children’s Hospital and Brigham and Women’s Hospital used ultrasound guidance to repair the vein of Galen malformation, which causes excessively high blood flow, resulting in both neurologic and cardiac complications.
The surgery was performed in a fetus at 34 weeks’ gestational age, with remarkable results. Since birth, the baby girl, who was identified in utero as being at high risk of suffering serious complications of the malformation, has required no medication to treat heart failure and no postnatal surgery.
Repeated echocardiograms after birth displayed marked improvement in cardiac output, and brain MRI showed no brain injury and a normal neurologic exam.
“This is incredibly exciting. The hope is that this baby, and others with this condition who receive this in utero surgery in future, will go on to have a normal life,” lead researcher Darren B. Orbach, MD, PhD, said in an interview.
“We were thrilled to see that the aggressive decline usually seen after birth simply did not appear. We are pleased to report that at 6 weeks, the infant is progressing remarkably well, on no medications, eating normally, gaining weight and is back home. There are no signs of any negative effects on the brain,” he added.
Dr. Orbach, codirector of the Cerebrovascular Surgery & Interventions Center at Boston Children’s Hospital, and colleagues described this first case report of the in utero vein of Galen malformation repair in a research letter, published online in the journal Stroke.
Vein of Galen malformation
Dr. Orbach explained that vein of Galen malformation, which occurs in around 1 in every 60,000 births, is a cerebrovascular anomaly in which the arterial system is directly connected to the venous system rather than to capillaries that are necessary to slow blood flow and deliver oxygen to surrounding brain tissue.
“The arterial and venous systems are fundamentally very different. The arterial system is high pressure, high flow; while the venous system is low pressure, low flow. They shouldn’t be directly connected,” he noted.
The vein of Galen malformation is the most extreme version of such an anomaly. Developing in early gestation, it is associated with a large increase in blood flow through the brain which grows over time and can sometimes result in twice the total cardiac output of the body or even more, Dr. Orbach said.
The placenta is believed to be protective as most babies don’t have overt physiologic problems in utero, but they can run into crisis after birth, with the abnormally high blood flow causing an immense stress to the heart.
Babies typically present with heart failure as their first major symptom soon after birth, Dr. Orbach said. “Although the anatomical problem is in the brain, the clinical manifestation is high-output heart failure. The heart is trying to do double its normal work, pumping the blood to the malformation and immediately back to the heart and that blood is not performing any useful function.
“These newborns can get very sick. They need multiple medications to support their cardiovascular system and we need to do procedures to try and reduce the blood flow,” he explained.
Brain injury is also a common problem. “The brain circulation is very abnormal. The blood is being shunted through the malformation rather than circulating through the brain tissue which can become ischemic,” Dr. Orbach commented.
“The babies who get sick would have a very high mortality (up to 90%) without expert care. Even those who do receive expert care at a specialty center have a mortality rate of 30% to 40% and those who survive have a high risk of neurologic and cognitive impairment,” he added.
The current treatment for babies born with the condition involves transarterial embolization, by which a catheter is inserted into the arterial system to enable the malformation to be occluded by various techniques.
But Dr. Orbach pointed out that some babies are born too sick to have the postnatal intervention. “The heart failure and brain injury is so overwhelming that no matter what we do, we cannot reverse it, and these babies normally do not survive. What we are doing with the fetal surgery is trying to help those babies who cannot be treated with the current postnatal approach,” he said.
The first stage of this research involved trying to identify these very-high-risk babies in utero, and the researchers found that on fetal MRI a particular measurement of one of the venous sinuses that drains the main malformation was a good predictor of how the baby would fare after birth. The babies predicted to do poorly from this test are the targets for the fetal surgery.
The technique used for the postnatal intervention is too technically challenging to perform in utero. “So we have developed a different approach for the in utero surgery that involves navigating into the accepting vein in the malformation with a needle under ultrasound guidance, and then packing the vein with metal coils to dramatically reduce the blood flow,” Dr. Orbach explained.
This procedure was performed in this first patient on March 15. The surgery was part of a clinical trial that is planned to include 20 cases in total.
“The immediate goal is to see whether we can transform those fetuses who are at very high risk of getting sick after birth into babies who do well in the [neonatal] ICU and are able to be sent home for elective treatment at a few months of age,” Dr. Orbach noted. “The study is continuing as it is vital that we continue and show efficacy and safety in other patients as well,” he added.
Dr. Orbach said the results of this first case were extremely encouraging. “Each stage was exciting – the technical success of the procedure, and then seeing the [blood] flow diminish on the ultrasound right there during the procedure; then the next day we did a fetal echocardiogram, and we could see that the abnormal cardiac output was dramatically reduced, and a fetal MRI scan also showed the malformation was already coming down in size.”
The baby was born prematurely 2 days after the procedure because of ruptured membranes with a birth weight of 1.9 kg (4.2 lb). She has not required any cardiovascular support or postnatal embolization.
“We were waiting with bated breath until the baby was born to see how she did clinically. I was trying to be conservative in my expectations, but it was quickly apparent that she was going to do great,” he said. Now at home, she has some oxygen treatment for the first few weeks, “but right now her neurological status is completely intact and essentially she looks like any other baby,” Dr. Orbach commented.
It is not yet known whether the infant will need any additional procedures. “We will follow her closely and make a decision on whether further treatment is needed based on whether the malformation is growing or not,” Dr. Orbach said. Longer term follow-up will also assess secondary problems sometimes seen, such as learning problems and seizures.
Although other fetal surgeries are now routinely performed, this is believed to be the first in utero surgery aimed at the cerebrovascular system.
“There were a lot of uncertainties,” Dr. Orbach said. “We didn’t even know if we would be able to see our instruments on ultrasound.” To model the procedure, the researchers had a phantom fetal skull and brain constructed with a vein of Galen malformation, which was key to obtaining Food and Drug Administration approval for the study.
If the study shows success in the other patients too, the technique could be rolled out to other centers. “There definitely needs to be fetal surgery and neurointerventional teams familiar with vein of Galen malformation in place, and ready to manage complications after delivery regardless of outcome. But we are not the only center with those capabilities, so if our trial pans out, yes, the hope is that other teams in specialist children’s hospitals around the world could do this too,” he added.
Pioneering work
Commenting on the case report in an American Heart Association press release, Colin Derdeyn, MD, a neurointerventional radiologist at University of Iowa Health Care, Iowa City, who performs vein of Galen malformation embolizations on neonates, said: “The key advance here is to intervene before the physiologic events of birth can cause life-threatening heart failure.”
Dr. Derdeyn, who is a past chair of the American Heart Association’s Stroke Council, cautioned that one successful case is not enough experience to conclude that the risks of this procedure are worth the benefits.
But, he added: “The positive hemodynamic changes that they observed in utero and after birth – reduction in flow, reduction in size of the draining vein, reversal of the abnormal reversed flow in the aorta – are really encouraging. These are some of the most exciting and surprising aspects of this case report. This is pioneering work being done in a very careful and responsible way.”
The study was funded by a grant from the Sage Schermerhorn Chair for Image-Guided Therapy.
A version of this article first appeared on Medscape.com.
FROM STROKE
Urology groups endorse two prostate biopsy approaches
CHICAGO - , endorsing both transperineal and transrectal biopsy instead of choosing one over the other.
The new guidelines, issued at the annual meeting of the American Urological Association, contrast with 2021 recommendations from the European Association of Urologists (EAU), which regard the transperineal approach as superior to and safer than the transrectal approach.
The new guidelines state: “Clinicians may use either a transrectal or transperineal biopsy route when performing a biopsy. (Conditional Recommendation; Evidence Level: Grade C).” Grade C is the lowest grade of acceptance the guideline committee could issue, according to Daniel Lin, MD, vice-chair of the AUA guideline panel.
“The AUA looked at all the higher-level data comparing the two procedures. There was a lack of that data,” Dr. Lin, chief of urologic oncology at the University of Washington, Seattle, said in an interview. He said the literature consists mainly of systematic single-center reviews, rather than multicenter randomized trials.
But Hendrik Van Poppel, MD, policy chief for the EAU, said that in Europe, transrectal biopsies are now considered “medical malpractice.”
Philip Cornford, MD, associate professor of urology at the University of Liverpool, England, and chair of the prostate biopsy guidelines panel for the EAU, said the society in 2021 concluded that the transperineal approach is the preferred one.
The EAU stated that transperineal prostate biopsies should be performed “due to the lower risk of infectious complications.” The EAU described the evidence as strong: A meta-analysis of seven studies that included 1,330 patients showed that for patients undergoing transperineal biopsy, infectious complications were significantly reduced.
Dr. Cornford said in essence, the EAU made its decision out of concern about infections, whereas the AUA and SUO based their decision on the ability of the methods to detect cancer.
Advocates for transperineal procedures cite several studies that show that the rate of infection, including sepsis, with such biopsies is virtually zero.
However, Dr. Lin noted that the committee said existing data on infection did not support this position. He also cited a “a fairly compelling” single-center randomized study with 750 patients that showed no difference in infection rates. The study was presented at the AUA meeting.
Agents of death and destruction?
Badar Mian, MD, professor of surgery at Albany (N.Y.) Medical College, who led the study, told an AUA session that urology has been trapped in an “echo chamber” regarding the relative safety of biopsies.
Clinicians hear “loud proclamations, which get repeated and magnified, that there is a real zero risk of complications after transperineal biopsies as compared to the horrendous 5% to 10% or higher rate of transrectal biopsy complications and that you, with your transrectal biopsies, are the cause of death and destruction all around,” Dr. Mian said. “Well, if you step out of the echo chamber, what you’ll find is that the accurate complications amongst the two procedures are not that dramatically different, much less dramatic than what you’ve been told to believe.”
The campaign to end transrectal biopsies in Europe started in 2018 with the death of a Norwegian man who experienced an infection after the procedure. Truls Bjerklund Johansen, MD, who’d performed the biopsy on the patient and who worked with the man’s daughter to change national practice, persuaded the EAU to look at the issue.
Advocates also say transperineal biopsies are better at detecting anterior and apical cancers.
“I would agree the data on cancer detection is less convincing, but that is not the basis of the EAU recommendation,” Dr. Cornford said.
Arvin George, MD, leads the transperineal biopsy program at the University of Michigan, Ann Arbor, and directs the transperineal training program at the AUA’s annual meeting. He said his course was sold out early and included about 60 trainees.
Dr. George said the new guideline statement “is not an unequivocal endorsement for transperineal biopsy as the preferred approach for diagnostic sampling but rather an acknowledgment of this approach as an alternative option.”
He said that although the new position statement should increase awareness of the transperineal approach in the United States, “without a strong recommendation, the guideline statement is unlikely to spark a large switch to the transperineal biopsy but rather supports the continued slow and steady adoption.”
Matthew Allaway, DO, founder of Perineologic, developer of the PrecisionPoint Transperineal Access System, said industry figures show that about 10% of the 1.5 million prostate biopsies performed in the United States annually are performed transperineally, a doubling in 2 years.
Jeremy Grummet, MD, clinical professor of urology at Monash University, Melbourne, and leader of the TREXIT (Transperineal Exit) movement to abandon transrectal procedures, said the AUA guidelines are biased toward “physician convenience.”
Lack of training
The AUA said another reason it did not endorse the transperineal approach was that currently, American urologists lack training and experience with transperineal procedures.
Dr. Grummet blamed major medical centers for any gap in the familiarity of clinicians with transperineal biopsies, which have been available for more than a decade.
“It is incumbent on the leaders of urology departments globally to ensure that their colleagues are trained in transperineal biopsy and have access to the appropriate equipment,” he said in an interview. “Lack of training didn’t seem to prevent the rapid uptake of robotic prostatectomy – a far more complex procedure.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO - , endorsing both transperineal and transrectal biopsy instead of choosing one over the other.
The new guidelines, issued at the annual meeting of the American Urological Association, contrast with 2021 recommendations from the European Association of Urologists (EAU), which regard the transperineal approach as superior to and safer than the transrectal approach.
The new guidelines state: “Clinicians may use either a transrectal or transperineal biopsy route when performing a biopsy. (Conditional Recommendation; Evidence Level: Grade C).” Grade C is the lowest grade of acceptance the guideline committee could issue, according to Daniel Lin, MD, vice-chair of the AUA guideline panel.
“The AUA looked at all the higher-level data comparing the two procedures. There was a lack of that data,” Dr. Lin, chief of urologic oncology at the University of Washington, Seattle, said in an interview. He said the literature consists mainly of systematic single-center reviews, rather than multicenter randomized trials.
But Hendrik Van Poppel, MD, policy chief for the EAU, said that in Europe, transrectal biopsies are now considered “medical malpractice.”
Philip Cornford, MD, associate professor of urology at the University of Liverpool, England, and chair of the prostate biopsy guidelines panel for the EAU, said the society in 2021 concluded that the transperineal approach is the preferred one.
The EAU stated that transperineal prostate biopsies should be performed “due to the lower risk of infectious complications.” The EAU described the evidence as strong: A meta-analysis of seven studies that included 1,330 patients showed that for patients undergoing transperineal biopsy, infectious complications were significantly reduced.
Dr. Cornford said in essence, the EAU made its decision out of concern about infections, whereas the AUA and SUO based their decision on the ability of the methods to detect cancer.
Advocates for transperineal procedures cite several studies that show that the rate of infection, including sepsis, with such biopsies is virtually zero.
However, Dr. Lin noted that the committee said existing data on infection did not support this position. He also cited a “a fairly compelling” single-center randomized study with 750 patients that showed no difference in infection rates. The study was presented at the AUA meeting.
Agents of death and destruction?
Badar Mian, MD, professor of surgery at Albany (N.Y.) Medical College, who led the study, told an AUA session that urology has been trapped in an “echo chamber” regarding the relative safety of biopsies.
Clinicians hear “loud proclamations, which get repeated and magnified, that there is a real zero risk of complications after transperineal biopsies as compared to the horrendous 5% to 10% or higher rate of transrectal biopsy complications and that you, with your transrectal biopsies, are the cause of death and destruction all around,” Dr. Mian said. “Well, if you step out of the echo chamber, what you’ll find is that the accurate complications amongst the two procedures are not that dramatically different, much less dramatic than what you’ve been told to believe.”
The campaign to end transrectal biopsies in Europe started in 2018 with the death of a Norwegian man who experienced an infection after the procedure. Truls Bjerklund Johansen, MD, who’d performed the biopsy on the patient and who worked with the man’s daughter to change national practice, persuaded the EAU to look at the issue.
Advocates also say transperineal biopsies are better at detecting anterior and apical cancers.
“I would agree the data on cancer detection is less convincing, but that is not the basis of the EAU recommendation,” Dr. Cornford said.
Arvin George, MD, leads the transperineal biopsy program at the University of Michigan, Ann Arbor, and directs the transperineal training program at the AUA’s annual meeting. He said his course was sold out early and included about 60 trainees.
Dr. George said the new guideline statement “is not an unequivocal endorsement for transperineal biopsy as the preferred approach for diagnostic sampling but rather an acknowledgment of this approach as an alternative option.”
He said that although the new position statement should increase awareness of the transperineal approach in the United States, “without a strong recommendation, the guideline statement is unlikely to spark a large switch to the transperineal biopsy but rather supports the continued slow and steady adoption.”
Matthew Allaway, DO, founder of Perineologic, developer of the PrecisionPoint Transperineal Access System, said industry figures show that about 10% of the 1.5 million prostate biopsies performed in the United States annually are performed transperineally, a doubling in 2 years.
Jeremy Grummet, MD, clinical professor of urology at Monash University, Melbourne, and leader of the TREXIT (Transperineal Exit) movement to abandon transrectal procedures, said the AUA guidelines are biased toward “physician convenience.”
Lack of training
The AUA said another reason it did not endorse the transperineal approach was that currently, American urologists lack training and experience with transperineal procedures.
Dr. Grummet blamed major medical centers for any gap in the familiarity of clinicians with transperineal biopsies, which have been available for more than a decade.
“It is incumbent on the leaders of urology departments globally to ensure that their colleagues are trained in transperineal biopsy and have access to the appropriate equipment,” he said in an interview. “Lack of training didn’t seem to prevent the rapid uptake of robotic prostatectomy – a far more complex procedure.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO - , endorsing both transperineal and transrectal biopsy instead of choosing one over the other.
The new guidelines, issued at the annual meeting of the American Urological Association, contrast with 2021 recommendations from the European Association of Urologists (EAU), which regard the transperineal approach as superior to and safer than the transrectal approach.
The new guidelines state: “Clinicians may use either a transrectal or transperineal biopsy route when performing a biopsy. (Conditional Recommendation; Evidence Level: Grade C).” Grade C is the lowest grade of acceptance the guideline committee could issue, according to Daniel Lin, MD, vice-chair of the AUA guideline panel.
“The AUA looked at all the higher-level data comparing the two procedures. There was a lack of that data,” Dr. Lin, chief of urologic oncology at the University of Washington, Seattle, said in an interview. He said the literature consists mainly of systematic single-center reviews, rather than multicenter randomized trials.
But Hendrik Van Poppel, MD, policy chief for the EAU, said that in Europe, transrectal biopsies are now considered “medical malpractice.”
Philip Cornford, MD, associate professor of urology at the University of Liverpool, England, and chair of the prostate biopsy guidelines panel for the EAU, said the society in 2021 concluded that the transperineal approach is the preferred one.
The EAU stated that transperineal prostate biopsies should be performed “due to the lower risk of infectious complications.” The EAU described the evidence as strong: A meta-analysis of seven studies that included 1,330 patients showed that for patients undergoing transperineal biopsy, infectious complications were significantly reduced.
Dr. Cornford said in essence, the EAU made its decision out of concern about infections, whereas the AUA and SUO based their decision on the ability of the methods to detect cancer.
Advocates for transperineal procedures cite several studies that show that the rate of infection, including sepsis, with such biopsies is virtually zero.
However, Dr. Lin noted that the committee said existing data on infection did not support this position. He also cited a “a fairly compelling” single-center randomized study with 750 patients that showed no difference in infection rates. The study was presented at the AUA meeting.
Agents of death and destruction?
Badar Mian, MD, professor of surgery at Albany (N.Y.) Medical College, who led the study, told an AUA session that urology has been trapped in an “echo chamber” regarding the relative safety of biopsies.
Clinicians hear “loud proclamations, which get repeated and magnified, that there is a real zero risk of complications after transperineal biopsies as compared to the horrendous 5% to 10% or higher rate of transrectal biopsy complications and that you, with your transrectal biopsies, are the cause of death and destruction all around,” Dr. Mian said. “Well, if you step out of the echo chamber, what you’ll find is that the accurate complications amongst the two procedures are not that dramatically different, much less dramatic than what you’ve been told to believe.”
The campaign to end transrectal biopsies in Europe started in 2018 with the death of a Norwegian man who experienced an infection after the procedure. Truls Bjerklund Johansen, MD, who’d performed the biopsy on the patient and who worked with the man’s daughter to change national practice, persuaded the EAU to look at the issue.
Advocates also say transperineal biopsies are better at detecting anterior and apical cancers.
“I would agree the data on cancer detection is less convincing, but that is not the basis of the EAU recommendation,” Dr. Cornford said.
Arvin George, MD, leads the transperineal biopsy program at the University of Michigan, Ann Arbor, and directs the transperineal training program at the AUA’s annual meeting. He said his course was sold out early and included about 60 trainees.
Dr. George said the new guideline statement “is not an unequivocal endorsement for transperineal biopsy as the preferred approach for diagnostic sampling but rather an acknowledgment of this approach as an alternative option.”
He said that although the new position statement should increase awareness of the transperineal approach in the United States, “without a strong recommendation, the guideline statement is unlikely to spark a large switch to the transperineal biopsy but rather supports the continued slow and steady adoption.”
Matthew Allaway, DO, founder of Perineologic, developer of the PrecisionPoint Transperineal Access System, said industry figures show that about 10% of the 1.5 million prostate biopsies performed in the United States annually are performed transperineally, a doubling in 2 years.
Jeremy Grummet, MD, clinical professor of urology at Monash University, Melbourne, and leader of the TREXIT (Transperineal Exit) movement to abandon transrectal procedures, said the AUA guidelines are biased toward “physician convenience.”
Lack of training
The AUA said another reason it did not endorse the transperineal approach was that currently, American urologists lack training and experience with transperineal procedures.
Dr. Grummet blamed major medical centers for any gap in the familiarity of clinicians with transperineal biopsies, which have been available for more than a decade.
“It is incumbent on the leaders of urology departments globally to ensure that their colleagues are trained in transperineal biopsy and have access to the appropriate equipment,” he said in an interview. “Lack of training didn’t seem to prevent the rapid uptake of robotic prostatectomy – a far more complex procedure.”
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AUA 2023
Doc accused of impairment wins $3.7M for unproven complaint
A jury on May 2 awarded John M. Farmer, MD, $3.7 million for emotional distress and contract damages against Baptist Health Madisonville and Baptist Health Medical Group for a series of actions they took against Dr. Farmer after the impairment complaint.
“It’s been the worst thing that I’ve ever gone through in my entire life,” Dr. Farmer said in an interview. “My career was disrupted, because I couldn’t finish residency on time, and I had difficulty finding full-time employment comparable to what I expected to obtain immediately following residency. It continues to significantly impact my life and my job, because I remain subject to random drug testing at any time and must check in every day to see whether I have to get drug tested.”
Dr. Farmer was in his third year of residency at the hospital when the mother of two young patients accused the doctor of being “on something” during a visit with her children, said Kathleen DeLaney, an Indianapolis-based attorney who represented Dr. Farmer in the case.
According to the lawsuit, the hospital violated its fitness for duty and drug testing policy by not immediately notifying Dr. Farmer of the complaint nor immediately testing him to prove whether or not there was a factual basis for the allegation. Repercussions from the unproven complaint damaged Dr. Farmer’s personal and professional reputation. It severely limited his job prospects and earning potential, the suit alleged.
Baptist Health spokeswoman Rebecca Towles Brown said Baptist Health is exploring its legal options after the jury’s decision. “We strongly disagree with the allegations made against Baptist Health in this case and are disappointed in the jury’s verdict. Baptist Health followed its medical staff policies and appropriately responded to concerns raised about Dr. Farmer’s well-being and behavior on the date in question. We are evaluating our postverdict options, as we believe the facts as they occurred do not support the verdict. Our primary focus remains providing high-quality care to our patients and families.”
What sparked the complaint?
On Nov. 4, 2019, Dr. Farmer worked a full day in the clinic at Baptist Health, visiting and treating patients and interacting with colleagues, according to court documents. In the late afternoon, he conducted a routine appointment with two children while their mother, her boyfriend, and a medical student were present.
Following the afternoon appointment, the mother issued a complaint to an office manager that Dr. Farmer was impaired, noting that he was “touching his nose a lot,” according to the lawsuit.
The next morning, hospital administrators met with Dr. Farmer and asked whether he was impaired the day before, to which he replied, “Absolutely not,” court documents state. Dr. Farmer asked to be given a urine drug screen immediately, but administrators allegedly said he needed to be tested at the Kentucky Physicians Health Foundation in Louisville.
Dr. Farmer immediately made the 3-hour drive to the facility, and Baptist Health placed him on leave, pending the evaluation. The health foundation sent Dr. Farmer to a third-party vendor to complete a urine drug screen, which returned a result of “dilute.” (A “dilute” result occurs when the urine concentration is weak because of too much water in the urine and testers are unable to detect whether alcohol or drugs are present.)
He was then instructed to go to a separate alcohol treatment facility for a 96-hour evaluation, where he was ultimately diagnosed with mild alcohol use disorder, according to Ms. DeLaney. The facility did not recommend that he receive any inpatient care.
Hospital administrators later sent a letter to the Kentucky Board of Medical Licensure alerting them of the patient’s complaint. The board opened an investigation, and Farmer was required to sign an interim order in which he agreed not to practice medicine until approved by the board, according to court documents. The order was reported to the National Practitioner’s Data Bank.
To maintain his employment and complete his residency, Dr. Farmer was ultimately required to sign a 2-year agreement with Kentucky Physicians Health Foundation, which included regular testing, monitoring, and therapy. The board later extended the agreement to 5 years and made Dr. Farmer’s compliance a condition of retaining his medical license, according to legal records.
Dr. Farmer sued Baptist Health Medical Group and Baptist Heath Madisonville in 2021, alleging breach of contract and tortious interference with prospective business advantage.
At trial, coworkers, including Farmer’s attending physicians, testified that Dr. Farmer was not impaired on the date in question, Ms. DeLaney said. A key fact highlighted at trial is that Dr. Farmer has ADHD.
“My client has ADHD, so he’s normally a twitchy person,” she said. “There was lots of testimony about how he moves a lot and that he’s fidgety and doesn’t stand still. The two attending doctors that were supervising him at clinic that afternoon both said 100% he was not impaired; he was his usual self. They told the residency director that right after the incident. They both testified at trial they thought that would be the end of the matter.”
Baptist Health would not comment about whether it followed its fitness for duty and drug testing policy or whether leaders spoke with other medical professionals who worked with Dr. Farmer on the day of the complaint.
Dr. Farmer said he feels vindicated by the verdict and grateful to the jury.
“I intend to continue practicing as a family medicine doctor and hope to continue to grow and advance in my career,” he said.
Have you been falsely accused? Here’s what to do
Dr. Farmer is not alone in fighting back against allegations by hospitals regarding conduct associated with impairment.
In 2020, an ob.gyn. who had been accused of being under the influence while working won $4.75 million in fraud and defamation damages against St. Vincent Carmel (Ind.) Hospital and St. Vincent Carmel Medical Group for its treatment following an impairment complaint by a nurse.
It’s unclear how prevalent such scenarios are because frequently, physicians are embarrassed and keep quiet about the situation and how they were treated, said Louise B. Andrew, MD, JD, an emergency physician/internist and attorney who consults on physician health and wellness, litigation stress, and disability discrimination.
“Physicians are unlikely to reveal that it’s happened to them unless they happen to have had a good outcome” she said. “All we know is that we’re hearing more and more about it, and that might be because people are becoming more open and outraged when it happens. It’s quite easy for anyone in a hospital environment or in an office environment, for a competitor, a coworker, or even a disgruntled patient to allege a physician has ‘glazed eyes’ or ‘alcohol on the breath,’ and that’s all it takes to start the ball rolling.”
If you are falsely accused of being impaired at work or are suddenly confronted with a complaint, the first step is to remain calm, said Kernan Manion, MD, executive director for the Center for Physician Rights, a nonprofit organization that assists physicians who have been subject to unfair medical board, health program, or peer review processes.
“The first thing is to keep your wits about you,” he said, “because often, docs get frightened or angry, and they overact. You have to gain your composure and ask for documentation about the nature of the allegation.”
Obtain in writing any and all information that supports the allegation. Physicians who are asked to report to a physician health program should ask the reason they are being referred and whether it is for a medical evaluation or another type of evaluation, he said. If it’s a medical reason, the process needs to follow medical parameters in terms of confidentiality.
“The bottom line is that a doctor should not take everything at face value and follow the organization’s orders unquestioning,” Dr. Manion said. “They have a right to get their concerns addressed.”
Physicians who are accused of using substances on duty or being under the influence while working have to right to undergo testing immediately, Dr. Andrew said.
“If you’re told on the spot: ‘You need to submit to testing,’ then you should do it, but make sure it’s done properly,” she said. “Ensure that forensically, you give two samples and that they are sealed and the chain of evidence is maintained. The reason for that is if one of them is a false positive, the second one can be reviewed separately.”
If administrators do not allow for prompt testing, get yourself tested immediately on your own, she said.
As far as leaves of absence are concerned, ensure you know what type of leave is being executed, Dr. Manion said. Ask the nature of the leave and whether the leave counts as a suspension that will go against your medical license and be reportable to the NPDB. In such cases, the only reason to suspend a doctor’s privileges is because they are considered a danger to others, or, in other words, there’s been an allegation of unsafe care.
“If there is an allegation of unsafe care, the physician should ask for documentation of the patient safety issues in question and why they are being deemed unsafe to practice,” he said.
Ms. DeLaney recommended physicians not report to or communicate with any state medical association, physician health foundation, or licensing authority without first getting legal advice.
In addition, doctors will likely be tested for acute and long-term drug and alcohol use, so it’s a good idea to avoid any activity or substances that could result in a dilute sample or a positive result on a drug or alcohol test, she said.
As for broader solutions, it’s important that more physicians come out of the shadows and tell their stories when these injustices take place, said Dr. Andrew.
“Doctors need to be more open about this when it happens, which is not easy,” she said. “More need to be suing, which is certainly not cheap. Also, when they do come to settlements, they should not sign nondisclosure agreements so that they can talk about what happened and it can be publicized. This way, more doctors are aware of the types of tactics used against physicians and what other doctors have done that can help.”
A version of this article first appeared on Medscape.com.
A jury on May 2 awarded John M. Farmer, MD, $3.7 million for emotional distress and contract damages against Baptist Health Madisonville and Baptist Health Medical Group for a series of actions they took against Dr. Farmer after the impairment complaint.
“It’s been the worst thing that I’ve ever gone through in my entire life,” Dr. Farmer said in an interview. “My career was disrupted, because I couldn’t finish residency on time, and I had difficulty finding full-time employment comparable to what I expected to obtain immediately following residency. It continues to significantly impact my life and my job, because I remain subject to random drug testing at any time and must check in every day to see whether I have to get drug tested.”
Dr. Farmer was in his third year of residency at the hospital when the mother of two young patients accused the doctor of being “on something” during a visit with her children, said Kathleen DeLaney, an Indianapolis-based attorney who represented Dr. Farmer in the case.
According to the lawsuit, the hospital violated its fitness for duty and drug testing policy by not immediately notifying Dr. Farmer of the complaint nor immediately testing him to prove whether or not there was a factual basis for the allegation. Repercussions from the unproven complaint damaged Dr. Farmer’s personal and professional reputation. It severely limited his job prospects and earning potential, the suit alleged.
Baptist Health spokeswoman Rebecca Towles Brown said Baptist Health is exploring its legal options after the jury’s decision. “We strongly disagree with the allegations made against Baptist Health in this case and are disappointed in the jury’s verdict. Baptist Health followed its medical staff policies and appropriately responded to concerns raised about Dr. Farmer’s well-being and behavior on the date in question. We are evaluating our postverdict options, as we believe the facts as they occurred do not support the verdict. Our primary focus remains providing high-quality care to our patients and families.”
What sparked the complaint?
On Nov. 4, 2019, Dr. Farmer worked a full day in the clinic at Baptist Health, visiting and treating patients and interacting with colleagues, according to court documents. In the late afternoon, he conducted a routine appointment with two children while their mother, her boyfriend, and a medical student were present.
Following the afternoon appointment, the mother issued a complaint to an office manager that Dr. Farmer was impaired, noting that he was “touching his nose a lot,” according to the lawsuit.
The next morning, hospital administrators met with Dr. Farmer and asked whether he was impaired the day before, to which he replied, “Absolutely not,” court documents state. Dr. Farmer asked to be given a urine drug screen immediately, but administrators allegedly said he needed to be tested at the Kentucky Physicians Health Foundation in Louisville.
Dr. Farmer immediately made the 3-hour drive to the facility, and Baptist Health placed him on leave, pending the evaluation. The health foundation sent Dr. Farmer to a third-party vendor to complete a urine drug screen, which returned a result of “dilute.” (A “dilute” result occurs when the urine concentration is weak because of too much water in the urine and testers are unable to detect whether alcohol or drugs are present.)
He was then instructed to go to a separate alcohol treatment facility for a 96-hour evaluation, where he was ultimately diagnosed with mild alcohol use disorder, according to Ms. DeLaney. The facility did not recommend that he receive any inpatient care.
Hospital administrators later sent a letter to the Kentucky Board of Medical Licensure alerting them of the patient’s complaint. The board opened an investigation, and Farmer was required to sign an interim order in which he agreed not to practice medicine until approved by the board, according to court documents. The order was reported to the National Practitioner’s Data Bank.
To maintain his employment and complete his residency, Dr. Farmer was ultimately required to sign a 2-year agreement with Kentucky Physicians Health Foundation, which included regular testing, monitoring, and therapy. The board later extended the agreement to 5 years and made Dr. Farmer’s compliance a condition of retaining his medical license, according to legal records.
Dr. Farmer sued Baptist Health Medical Group and Baptist Heath Madisonville in 2021, alleging breach of contract and tortious interference with prospective business advantage.
At trial, coworkers, including Farmer’s attending physicians, testified that Dr. Farmer was not impaired on the date in question, Ms. DeLaney said. A key fact highlighted at trial is that Dr. Farmer has ADHD.
“My client has ADHD, so he’s normally a twitchy person,” she said. “There was lots of testimony about how he moves a lot and that he’s fidgety and doesn’t stand still. The two attending doctors that were supervising him at clinic that afternoon both said 100% he was not impaired; he was his usual self. They told the residency director that right after the incident. They both testified at trial they thought that would be the end of the matter.”
Baptist Health would not comment about whether it followed its fitness for duty and drug testing policy or whether leaders spoke with other medical professionals who worked with Dr. Farmer on the day of the complaint.
Dr. Farmer said he feels vindicated by the verdict and grateful to the jury.
“I intend to continue practicing as a family medicine doctor and hope to continue to grow and advance in my career,” he said.
Have you been falsely accused? Here’s what to do
Dr. Farmer is not alone in fighting back against allegations by hospitals regarding conduct associated with impairment.
In 2020, an ob.gyn. who had been accused of being under the influence while working won $4.75 million in fraud and defamation damages against St. Vincent Carmel (Ind.) Hospital and St. Vincent Carmel Medical Group for its treatment following an impairment complaint by a nurse.
It’s unclear how prevalent such scenarios are because frequently, physicians are embarrassed and keep quiet about the situation and how they were treated, said Louise B. Andrew, MD, JD, an emergency physician/internist and attorney who consults on physician health and wellness, litigation stress, and disability discrimination.
“Physicians are unlikely to reveal that it’s happened to them unless they happen to have had a good outcome” she said. “All we know is that we’re hearing more and more about it, and that might be because people are becoming more open and outraged when it happens. It’s quite easy for anyone in a hospital environment or in an office environment, for a competitor, a coworker, or even a disgruntled patient to allege a physician has ‘glazed eyes’ or ‘alcohol on the breath,’ and that’s all it takes to start the ball rolling.”
If you are falsely accused of being impaired at work or are suddenly confronted with a complaint, the first step is to remain calm, said Kernan Manion, MD, executive director for the Center for Physician Rights, a nonprofit organization that assists physicians who have been subject to unfair medical board, health program, or peer review processes.
“The first thing is to keep your wits about you,” he said, “because often, docs get frightened or angry, and they overact. You have to gain your composure and ask for documentation about the nature of the allegation.”
Obtain in writing any and all information that supports the allegation. Physicians who are asked to report to a physician health program should ask the reason they are being referred and whether it is for a medical evaluation or another type of evaluation, he said. If it’s a medical reason, the process needs to follow medical parameters in terms of confidentiality.
“The bottom line is that a doctor should not take everything at face value and follow the organization’s orders unquestioning,” Dr. Manion said. “They have a right to get their concerns addressed.”
Physicians who are accused of using substances on duty or being under the influence while working have to right to undergo testing immediately, Dr. Andrew said.
“If you’re told on the spot: ‘You need to submit to testing,’ then you should do it, but make sure it’s done properly,” she said. “Ensure that forensically, you give two samples and that they are sealed and the chain of evidence is maintained. The reason for that is if one of them is a false positive, the second one can be reviewed separately.”
If administrators do not allow for prompt testing, get yourself tested immediately on your own, she said.
As far as leaves of absence are concerned, ensure you know what type of leave is being executed, Dr. Manion said. Ask the nature of the leave and whether the leave counts as a suspension that will go against your medical license and be reportable to the NPDB. In such cases, the only reason to suspend a doctor’s privileges is because they are considered a danger to others, or, in other words, there’s been an allegation of unsafe care.
“If there is an allegation of unsafe care, the physician should ask for documentation of the patient safety issues in question and why they are being deemed unsafe to practice,” he said.
Ms. DeLaney recommended physicians not report to or communicate with any state medical association, physician health foundation, or licensing authority without first getting legal advice.
In addition, doctors will likely be tested for acute and long-term drug and alcohol use, so it’s a good idea to avoid any activity or substances that could result in a dilute sample or a positive result on a drug or alcohol test, she said.
As for broader solutions, it’s important that more physicians come out of the shadows and tell their stories when these injustices take place, said Dr. Andrew.
“Doctors need to be more open about this when it happens, which is not easy,” she said. “More need to be suing, which is certainly not cheap. Also, when they do come to settlements, they should not sign nondisclosure agreements so that they can talk about what happened and it can be publicized. This way, more doctors are aware of the types of tactics used against physicians and what other doctors have done that can help.”
A version of this article first appeared on Medscape.com.
A jury on May 2 awarded John M. Farmer, MD, $3.7 million for emotional distress and contract damages against Baptist Health Madisonville and Baptist Health Medical Group for a series of actions they took against Dr. Farmer after the impairment complaint.
“It’s been the worst thing that I’ve ever gone through in my entire life,” Dr. Farmer said in an interview. “My career was disrupted, because I couldn’t finish residency on time, and I had difficulty finding full-time employment comparable to what I expected to obtain immediately following residency. It continues to significantly impact my life and my job, because I remain subject to random drug testing at any time and must check in every day to see whether I have to get drug tested.”
Dr. Farmer was in his third year of residency at the hospital when the mother of two young patients accused the doctor of being “on something” during a visit with her children, said Kathleen DeLaney, an Indianapolis-based attorney who represented Dr. Farmer in the case.
According to the lawsuit, the hospital violated its fitness for duty and drug testing policy by not immediately notifying Dr. Farmer of the complaint nor immediately testing him to prove whether or not there was a factual basis for the allegation. Repercussions from the unproven complaint damaged Dr. Farmer’s personal and professional reputation. It severely limited his job prospects and earning potential, the suit alleged.
Baptist Health spokeswoman Rebecca Towles Brown said Baptist Health is exploring its legal options after the jury’s decision. “We strongly disagree with the allegations made against Baptist Health in this case and are disappointed in the jury’s verdict. Baptist Health followed its medical staff policies and appropriately responded to concerns raised about Dr. Farmer’s well-being and behavior on the date in question. We are evaluating our postverdict options, as we believe the facts as they occurred do not support the verdict. Our primary focus remains providing high-quality care to our patients and families.”
What sparked the complaint?
On Nov. 4, 2019, Dr. Farmer worked a full day in the clinic at Baptist Health, visiting and treating patients and interacting with colleagues, according to court documents. In the late afternoon, he conducted a routine appointment with two children while their mother, her boyfriend, and a medical student were present.
Following the afternoon appointment, the mother issued a complaint to an office manager that Dr. Farmer was impaired, noting that he was “touching his nose a lot,” according to the lawsuit.
The next morning, hospital administrators met with Dr. Farmer and asked whether he was impaired the day before, to which he replied, “Absolutely not,” court documents state. Dr. Farmer asked to be given a urine drug screen immediately, but administrators allegedly said he needed to be tested at the Kentucky Physicians Health Foundation in Louisville.
Dr. Farmer immediately made the 3-hour drive to the facility, and Baptist Health placed him on leave, pending the evaluation. The health foundation sent Dr. Farmer to a third-party vendor to complete a urine drug screen, which returned a result of “dilute.” (A “dilute” result occurs when the urine concentration is weak because of too much water in the urine and testers are unable to detect whether alcohol or drugs are present.)
He was then instructed to go to a separate alcohol treatment facility for a 96-hour evaluation, where he was ultimately diagnosed with mild alcohol use disorder, according to Ms. DeLaney. The facility did not recommend that he receive any inpatient care.
Hospital administrators later sent a letter to the Kentucky Board of Medical Licensure alerting them of the patient’s complaint. The board opened an investigation, and Farmer was required to sign an interim order in which he agreed not to practice medicine until approved by the board, according to court documents. The order was reported to the National Practitioner’s Data Bank.
To maintain his employment and complete his residency, Dr. Farmer was ultimately required to sign a 2-year agreement with Kentucky Physicians Health Foundation, which included regular testing, monitoring, and therapy. The board later extended the agreement to 5 years and made Dr. Farmer’s compliance a condition of retaining his medical license, according to legal records.
Dr. Farmer sued Baptist Health Medical Group and Baptist Heath Madisonville in 2021, alleging breach of contract and tortious interference with prospective business advantage.
At trial, coworkers, including Farmer’s attending physicians, testified that Dr. Farmer was not impaired on the date in question, Ms. DeLaney said. A key fact highlighted at trial is that Dr. Farmer has ADHD.
“My client has ADHD, so he’s normally a twitchy person,” she said. “There was lots of testimony about how he moves a lot and that he’s fidgety and doesn’t stand still. The two attending doctors that were supervising him at clinic that afternoon both said 100% he was not impaired; he was his usual self. They told the residency director that right after the incident. They both testified at trial they thought that would be the end of the matter.”
Baptist Health would not comment about whether it followed its fitness for duty and drug testing policy or whether leaders spoke with other medical professionals who worked with Dr. Farmer on the day of the complaint.
Dr. Farmer said he feels vindicated by the verdict and grateful to the jury.
“I intend to continue practicing as a family medicine doctor and hope to continue to grow and advance in my career,” he said.
Have you been falsely accused? Here’s what to do
Dr. Farmer is not alone in fighting back against allegations by hospitals regarding conduct associated with impairment.
In 2020, an ob.gyn. who had been accused of being under the influence while working won $4.75 million in fraud and defamation damages against St. Vincent Carmel (Ind.) Hospital and St. Vincent Carmel Medical Group for its treatment following an impairment complaint by a nurse.
It’s unclear how prevalent such scenarios are because frequently, physicians are embarrassed and keep quiet about the situation and how they were treated, said Louise B. Andrew, MD, JD, an emergency physician/internist and attorney who consults on physician health and wellness, litigation stress, and disability discrimination.
“Physicians are unlikely to reveal that it’s happened to them unless they happen to have had a good outcome” she said. “All we know is that we’re hearing more and more about it, and that might be because people are becoming more open and outraged when it happens. It’s quite easy for anyone in a hospital environment or in an office environment, for a competitor, a coworker, or even a disgruntled patient to allege a physician has ‘glazed eyes’ or ‘alcohol on the breath,’ and that’s all it takes to start the ball rolling.”
If you are falsely accused of being impaired at work or are suddenly confronted with a complaint, the first step is to remain calm, said Kernan Manion, MD, executive director for the Center for Physician Rights, a nonprofit organization that assists physicians who have been subject to unfair medical board, health program, or peer review processes.
“The first thing is to keep your wits about you,” he said, “because often, docs get frightened or angry, and they overact. You have to gain your composure and ask for documentation about the nature of the allegation.”
Obtain in writing any and all information that supports the allegation. Physicians who are asked to report to a physician health program should ask the reason they are being referred and whether it is for a medical evaluation or another type of evaluation, he said. If it’s a medical reason, the process needs to follow medical parameters in terms of confidentiality.
“The bottom line is that a doctor should not take everything at face value and follow the organization’s orders unquestioning,” Dr. Manion said. “They have a right to get their concerns addressed.”
Physicians who are accused of using substances on duty or being under the influence while working have to right to undergo testing immediately, Dr. Andrew said.
“If you’re told on the spot: ‘You need to submit to testing,’ then you should do it, but make sure it’s done properly,” she said. “Ensure that forensically, you give two samples and that they are sealed and the chain of evidence is maintained. The reason for that is if one of them is a false positive, the second one can be reviewed separately.”
If administrators do not allow for prompt testing, get yourself tested immediately on your own, she said.
As far as leaves of absence are concerned, ensure you know what type of leave is being executed, Dr. Manion said. Ask the nature of the leave and whether the leave counts as a suspension that will go against your medical license and be reportable to the NPDB. In such cases, the only reason to suspend a doctor’s privileges is because they are considered a danger to others, or, in other words, there’s been an allegation of unsafe care.
“If there is an allegation of unsafe care, the physician should ask for documentation of the patient safety issues in question and why they are being deemed unsafe to practice,” he said.
Ms. DeLaney recommended physicians not report to or communicate with any state medical association, physician health foundation, or licensing authority without first getting legal advice.
In addition, doctors will likely be tested for acute and long-term drug and alcohol use, so it’s a good idea to avoid any activity or substances that could result in a dilute sample or a positive result on a drug or alcohol test, she said.
As for broader solutions, it’s important that more physicians come out of the shadows and tell their stories when these injustices take place, said Dr. Andrew.
“Doctors need to be more open about this when it happens, which is not easy,” she said. “More need to be suing, which is certainly not cheap. Also, when they do come to settlements, they should not sign nondisclosure agreements so that they can talk about what happened and it can be publicized. This way, more doctors are aware of the types of tactics used against physicians and what other doctors have done that can help.”
A version of this article first appeared on Medscape.com.
CardioMEMS boosts QoL, curbs HF hospitalizations: MONITOR-HF
In the first randomized clinical trial of remote pulmonary artery pressure–guided monitoring and management of chronic heart failure (HF) in Europe, the intervention “substantially” improved quality of life (QoL) and reduced HF hospitalizations, new data show.
The CardioMEMS-HF system (Abbot Laboratories) used in the trial, called MONITOR-HF, remotely monitors changes in pulmonary artery pressure and provides an early warning of worsening HF.
Jasper Brugts, MD, PhD, of Erasmus MC University Medical Centre, Rotterdam, the Netherlands, said in an interview, “The concordance on outcomes of the three CardioMEMS trials across different eras, evolving GDMT [guideline-directed medical therapy], different conditions (pandemic), and different health care systems is reassuring and supportive of technologies such as CardioMEMS to improve patient monitoring to prevent HF hospitalizations and improve QoL.”
Dr. Brugts presented the study at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2023 sessions.
(11 vs. 17), in comparison with standard of care, Dr. Brugts told meeting attendees.
Furthermore, CardioMEMS monitors hypervolemia as well as hypovolemia, enabling “fine-tuning of diuretics.”
The presentation drew such applause that one chairperson described it as “close to a standing ovation.” The study was published simultaneously in The Lancet.
Aggregate evidence
Early clinical evidence of the benefits of remote monitoring with the CardioMEMS-HF system was provided by the CHAMPION trial, which included patients with New York Heart Association (NYHA) class III heart failure.
Results of the subsequent GUIDE-HF trial, which aimed to test a broader population of patients with NYHA class II–IV heart failure and either increased N-terminal-pro-B-type natriuretic peptide (NT-proBNP) concentrations or hospitalization, were inconclusive.
However, a pre–COVID-19 impact analysis of GUIDE-HF indicated a possible benefit, which was primarily driven by a lower HF hospitalization rate, compared with the control group. That finding was the basis for an expanded indication for the system from the U.S. Food and Drug Administration.
The 2022 FDA indication permits the use of CardioMEMS for patients with NYHA class II HF and for those with worsening HF, as assessed by elevated natriuretic peptide levels.
From United States to Europe
Aware that most CardioMEMS data came from U.S. trials, the investigators embarked on the current trial, MONITOR-HF, an open-label, randomized trial in 25 centers in the Netherlands. Eligible patients had chronic NYHA class III HF, irrespective of ejection fraction, and had previously undergone hospitalization for HF.
A total of 348 patients were randomly assigned to either CardioMEMS-HF or standard of care (SoC) between 2019 and 2022.The median age of the patients was 69 years, and the median ejection fraction was 30%.
All patients were scheduled to be seen by their clinician at 3 months, 6 months, and every 6 months thereafter for up to 48 months.
The primary endpoint was the mean difference in the Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score at 12 months
That difference between groups was 7.13 (+7.05 in the CardioMEMS group and –0.08 in the SoC group).
In the responder analysis, the odds ratio of an improvement of at least 5 points in the KCCQ overall summary score was 1.69 in the CardioMEMS group vs. the SoC group; the OR of a deterioration of at least 5 points was 0.45.
Subgroup analyses showed no relevant heterogeneity in the treatment effect on total HF hospitalizations and, notably, no significant interaction in patients with an EF below 40% and an EF above 40%.
There was a significant reduction in the median NT-proBNP change from baseline only in the remote monitoring group (800 pg/mL) and a smaller, nonsignificant difference with SoC.
Both groups received highly appropriate background guideline–directed medical therapy throughout the study. There were no significant between-group differences at 12 months.
Freedom from device-related or system-related complications and sensor failure were 97.7% and 98.8%, respectively.
Two sensor failures occurred during a mean follow-up 1.8 years. The percentage of failures was comparable to CHAMPION and GUIDE-HF trials.
The trial was not powered to assess a mortality benefit.
Pick the right patients
“As in the U.S. trials, there will be side effects, so select the right patients, because [remote monitoring] is not without risk,” Dr. Brugts told meeting attendees.
That point also was made by Christiane E. Angermann of University and University Hospital Würzburg, Germany, in a related editorial in The Lancet.
“To reproduce these results on a large scale in real-life health care, diligent patient selection should identify those at high risk of heart failure–related hospitalization who agree with the concept of daily data collection and are able and motivated to comply with treatment recommendations even if asymptomatic,” Dr. Angermann writes.
“Without direct interaction between health care providers and patients, and timely treatment modification triggered by abnormal monitoring results, the care cycle might break and the potential benefits from early detection of decompensation would be lost.”
Val Rakita, MD, assistant professor of medicine at Temple University, Philadelphia, a specialist in advanced heart failure and main implanter of the CardioMEMS device at Temple University Hospital, commented on the study for this article.
“This study confirms the previous data that the device is very safe and effective in preventing HF hospitalizations and improving patients’ quality of life, even in a different population with more modern background guideline-directed medical therapy.”
Nevertheless, he noted, “Studies have yet to confirm a mortality benefit, despite logic telling us that preventing heart failure hospitalizations should also improve patient survival. More studies are needed to see if a survival benefit can be proven over a longer follow-up period.”
Overall, he said, “Remote monitoring allows more precise management of medications, prevention of hospitalizations, and improvement in quality of life, and I am an advocate for it in my practice.”
Not everyone is an advocate, however. In a commentary published last year, John M. Mandrola, MD, a cardiac electrophysiologist at Baptist Medical Associates in Louisville, Ky., said the expanded FDA indication for the device is the result of “dubious trial analysis, spin, lax regulation, and the growth of low-value care.”
Others also have questioned the device’s value in the clinic.
But at least for now, as Dr. Angermann writes, “Scientific evidence supports the use of the CardioMEMS-HF system to enhance remote patient management in heart failure care. For more widespread application, technological advancements are desirable to provide more comfort for patients and reusable external device components, thereby improving care experience and saving resources.”
The MONITOR-HF trial is funded by the Dutch Ministry of Health and Health Care institute. Dr. Brugts has an independent research grant from Abbott (investigator-sponsored study) and has had speaker engagements or has participated in advisory boards for Abbott and other pharmaceutical companies. Dr. Angermann has received personal fees from Abbott for serving as chair of the steering committee for the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF) and consulting fees, honoraria, and travel costs from Abbott. Dr. Rakita has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the first randomized clinical trial of remote pulmonary artery pressure–guided monitoring and management of chronic heart failure (HF) in Europe, the intervention “substantially” improved quality of life (QoL) and reduced HF hospitalizations, new data show.
The CardioMEMS-HF system (Abbot Laboratories) used in the trial, called MONITOR-HF, remotely monitors changes in pulmonary artery pressure and provides an early warning of worsening HF.
Jasper Brugts, MD, PhD, of Erasmus MC University Medical Centre, Rotterdam, the Netherlands, said in an interview, “The concordance on outcomes of the three CardioMEMS trials across different eras, evolving GDMT [guideline-directed medical therapy], different conditions (pandemic), and different health care systems is reassuring and supportive of technologies such as CardioMEMS to improve patient monitoring to prevent HF hospitalizations and improve QoL.”
Dr. Brugts presented the study at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2023 sessions.
(11 vs. 17), in comparison with standard of care, Dr. Brugts told meeting attendees.
Furthermore, CardioMEMS monitors hypervolemia as well as hypovolemia, enabling “fine-tuning of diuretics.”
The presentation drew such applause that one chairperson described it as “close to a standing ovation.” The study was published simultaneously in The Lancet.
Aggregate evidence
Early clinical evidence of the benefits of remote monitoring with the CardioMEMS-HF system was provided by the CHAMPION trial, which included patients with New York Heart Association (NYHA) class III heart failure.
Results of the subsequent GUIDE-HF trial, which aimed to test a broader population of patients with NYHA class II–IV heart failure and either increased N-terminal-pro-B-type natriuretic peptide (NT-proBNP) concentrations or hospitalization, were inconclusive.
However, a pre–COVID-19 impact analysis of GUIDE-HF indicated a possible benefit, which was primarily driven by a lower HF hospitalization rate, compared with the control group. That finding was the basis for an expanded indication for the system from the U.S. Food and Drug Administration.
The 2022 FDA indication permits the use of CardioMEMS for patients with NYHA class II HF and for those with worsening HF, as assessed by elevated natriuretic peptide levels.
From United States to Europe
Aware that most CardioMEMS data came from U.S. trials, the investigators embarked on the current trial, MONITOR-HF, an open-label, randomized trial in 25 centers in the Netherlands. Eligible patients had chronic NYHA class III HF, irrespective of ejection fraction, and had previously undergone hospitalization for HF.
A total of 348 patients were randomly assigned to either CardioMEMS-HF or standard of care (SoC) between 2019 and 2022.The median age of the patients was 69 years, and the median ejection fraction was 30%.
All patients were scheduled to be seen by their clinician at 3 months, 6 months, and every 6 months thereafter for up to 48 months.
The primary endpoint was the mean difference in the Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score at 12 months
That difference between groups was 7.13 (+7.05 in the CardioMEMS group and –0.08 in the SoC group).
In the responder analysis, the odds ratio of an improvement of at least 5 points in the KCCQ overall summary score was 1.69 in the CardioMEMS group vs. the SoC group; the OR of a deterioration of at least 5 points was 0.45.
Subgroup analyses showed no relevant heterogeneity in the treatment effect on total HF hospitalizations and, notably, no significant interaction in patients with an EF below 40% and an EF above 40%.
There was a significant reduction in the median NT-proBNP change from baseline only in the remote monitoring group (800 pg/mL) and a smaller, nonsignificant difference with SoC.
Both groups received highly appropriate background guideline–directed medical therapy throughout the study. There were no significant between-group differences at 12 months.
Freedom from device-related or system-related complications and sensor failure were 97.7% and 98.8%, respectively.
Two sensor failures occurred during a mean follow-up 1.8 years. The percentage of failures was comparable to CHAMPION and GUIDE-HF trials.
The trial was not powered to assess a mortality benefit.
Pick the right patients
“As in the U.S. trials, there will be side effects, so select the right patients, because [remote monitoring] is not without risk,” Dr. Brugts told meeting attendees.
That point also was made by Christiane E. Angermann of University and University Hospital Würzburg, Germany, in a related editorial in The Lancet.
“To reproduce these results on a large scale in real-life health care, diligent patient selection should identify those at high risk of heart failure–related hospitalization who agree with the concept of daily data collection and are able and motivated to comply with treatment recommendations even if asymptomatic,” Dr. Angermann writes.
“Without direct interaction between health care providers and patients, and timely treatment modification triggered by abnormal monitoring results, the care cycle might break and the potential benefits from early detection of decompensation would be lost.”
Val Rakita, MD, assistant professor of medicine at Temple University, Philadelphia, a specialist in advanced heart failure and main implanter of the CardioMEMS device at Temple University Hospital, commented on the study for this article.
“This study confirms the previous data that the device is very safe and effective in preventing HF hospitalizations and improving patients’ quality of life, even in a different population with more modern background guideline-directed medical therapy.”
Nevertheless, he noted, “Studies have yet to confirm a mortality benefit, despite logic telling us that preventing heart failure hospitalizations should also improve patient survival. More studies are needed to see if a survival benefit can be proven over a longer follow-up period.”
Overall, he said, “Remote monitoring allows more precise management of medications, prevention of hospitalizations, and improvement in quality of life, and I am an advocate for it in my practice.”
Not everyone is an advocate, however. In a commentary published last year, John M. Mandrola, MD, a cardiac electrophysiologist at Baptist Medical Associates in Louisville, Ky., said the expanded FDA indication for the device is the result of “dubious trial analysis, spin, lax regulation, and the growth of low-value care.”
Others also have questioned the device’s value in the clinic.
But at least for now, as Dr. Angermann writes, “Scientific evidence supports the use of the CardioMEMS-HF system to enhance remote patient management in heart failure care. For more widespread application, technological advancements are desirable to provide more comfort for patients and reusable external device components, thereby improving care experience and saving resources.”
The MONITOR-HF trial is funded by the Dutch Ministry of Health and Health Care institute. Dr. Brugts has an independent research grant from Abbott (investigator-sponsored study) and has had speaker engagements or has participated in advisory boards for Abbott and other pharmaceutical companies. Dr. Angermann has received personal fees from Abbott for serving as chair of the steering committee for the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF) and consulting fees, honoraria, and travel costs from Abbott. Dr. Rakita has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the first randomized clinical trial of remote pulmonary artery pressure–guided monitoring and management of chronic heart failure (HF) in Europe, the intervention “substantially” improved quality of life (QoL) and reduced HF hospitalizations, new data show.
The CardioMEMS-HF system (Abbot Laboratories) used in the trial, called MONITOR-HF, remotely monitors changes in pulmonary artery pressure and provides an early warning of worsening HF.
Jasper Brugts, MD, PhD, of Erasmus MC University Medical Centre, Rotterdam, the Netherlands, said in an interview, “The concordance on outcomes of the three CardioMEMS trials across different eras, evolving GDMT [guideline-directed medical therapy], different conditions (pandemic), and different health care systems is reassuring and supportive of technologies such as CardioMEMS to improve patient monitoring to prevent HF hospitalizations and improve QoL.”
Dr. Brugts presented the study at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2023 sessions.
(11 vs. 17), in comparison with standard of care, Dr. Brugts told meeting attendees.
Furthermore, CardioMEMS monitors hypervolemia as well as hypovolemia, enabling “fine-tuning of diuretics.”
The presentation drew such applause that one chairperson described it as “close to a standing ovation.” The study was published simultaneously in The Lancet.
Aggregate evidence
Early clinical evidence of the benefits of remote monitoring with the CardioMEMS-HF system was provided by the CHAMPION trial, which included patients with New York Heart Association (NYHA) class III heart failure.
Results of the subsequent GUIDE-HF trial, which aimed to test a broader population of patients with NYHA class II–IV heart failure and either increased N-terminal-pro-B-type natriuretic peptide (NT-proBNP) concentrations or hospitalization, were inconclusive.
However, a pre–COVID-19 impact analysis of GUIDE-HF indicated a possible benefit, which was primarily driven by a lower HF hospitalization rate, compared with the control group. That finding was the basis for an expanded indication for the system from the U.S. Food and Drug Administration.
The 2022 FDA indication permits the use of CardioMEMS for patients with NYHA class II HF and for those with worsening HF, as assessed by elevated natriuretic peptide levels.
From United States to Europe
Aware that most CardioMEMS data came from U.S. trials, the investigators embarked on the current trial, MONITOR-HF, an open-label, randomized trial in 25 centers in the Netherlands. Eligible patients had chronic NYHA class III HF, irrespective of ejection fraction, and had previously undergone hospitalization for HF.
A total of 348 patients were randomly assigned to either CardioMEMS-HF or standard of care (SoC) between 2019 and 2022.The median age of the patients was 69 years, and the median ejection fraction was 30%.
All patients were scheduled to be seen by their clinician at 3 months, 6 months, and every 6 months thereafter for up to 48 months.
The primary endpoint was the mean difference in the Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score at 12 months
That difference between groups was 7.13 (+7.05 in the CardioMEMS group and –0.08 in the SoC group).
In the responder analysis, the odds ratio of an improvement of at least 5 points in the KCCQ overall summary score was 1.69 in the CardioMEMS group vs. the SoC group; the OR of a deterioration of at least 5 points was 0.45.
Subgroup analyses showed no relevant heterogeneity in the treatment effect on total HF hospitalizations and, notably, no significant interaction in patients with an EF below 40% and an EF above 40%.
There was a significant reduction in the median NT-proBNP change from baseline only in the remote monitoring group (800 pg/mL) and a smaller, nonsignificant difference with SoC.
Both groups received highly appropriate background guideline–directed medical therapy throughout the study. There were no significant between-group differences at 12 months.
Freedom from device-related or system-related complications and sensor failure were 97.7% and 98.8%, respectively.
Two sensor failures occurred during a mean follow-up 1.8 years. The percentage of failures was comparable to CHAMPION and GUIDE-HF trials.
The trial was not powered to assess a mortality benefit.
Pick the right patients
“As in the U.S. trials, there will be side effects, so select the right patients, because [remote monitoring] is not without risk,” Dr. Brugts told meeting attendees.
That point also was made by Christiane E. Angermann of University and University Hospital Würzburg, Germany, in a related editorial in The Lancet.
“To reproduce these results on a large scale in real-life health care, diligent patient selection should identify those at high risk of heart failure–related hospitalization who agree with the concept of daily data collection and are able and motivated to comply with treatment recommendations even if asymptomatic,” Dr. Angermann writes.
“Without direct interaction between health care providers and patients, and timely treatment modification triggered by abnormal monitoring results, the care cycle might break and the potential benefits from early detection of decompensation would be lost.”
Val Rakita, MD, assistant professor of medicine at Temple University, Philadelphia, a specialist in advanced heart failure and main implanter of the CardioMEMS device at Temple University Hospital, commented on the study for this article.
“This study confirms the previous data that the device is very safe and effective in preventing HF hospitalizations and improving patients’ quality of life, even in a different population with more modern background guideline-directed medical therapy.”
Nevertheless, he noted, “Studies have yet to confirm a mortality benefit, despite logic telling us that preventing heart failure hospitalizations should also improve patient survival. More studies are needed to see if a survival benefit can be proven over a longer follow-up period.”
Overall, he said, “Remote monitoring allows more precise management of medications, prevention of hospitalizations, and improvement in quality of life, and I am an advocate for it in my practice.”
Not everyone is an advocate, however. In a commentary published last year, John M. Mandrola, MD, a cardiac electrophysiologist at Baptist Medical Associates in Louisville, Ky., said the expanded FDA indication for the device is the result of “dubious trial analysis, spin, lax regulation, and the growth of low-value care.”
Others also have questioned the device’s value in the clinic.
But at least for now, as Dr. Angermann writes, “Scientific evidence supports the use of the CardioMEMS-HF system to enhance remote patient management in heart failure care. For more widespread application, technological advancements are desirable to provide more comfort for patients and reusable external device components, thereby improving care experience and saving resources.”
The MONITOR-HF trial is funded by the Dutch Ministry of Health and Health Care institute. Dr. Brugts has an independent research grant from Abbott (investigator-sponsored study) and has had speaker engagements or has participated in advisory boards for Abbott and other pharmaceutical companies. Dr. Angermann has received personal fees from Abbott for serving as chair of the steering committee for the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF) and consulting fees, honoraria, and travel costs from Abbott. Dr. Rakita has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ESC HEART FAILURE 2023