User login
PTSD, anxiety linked to out-of-hospital cardiac arrest
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.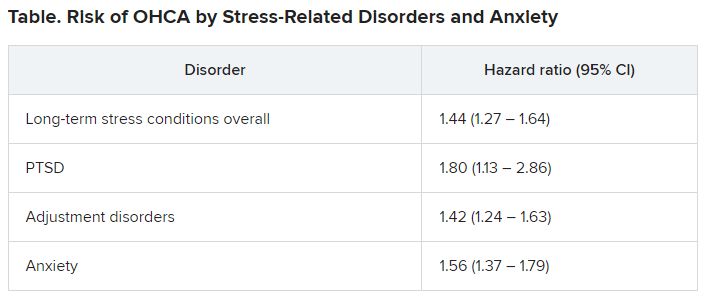
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN HEART
Can a saliva test predict the best way to manage obesity?
It sounds like a simple solution to a complicated problem: Find out what kind of obesity someone has based on a one-time genetic saliva test. Then patients and their doctor can get a better idea if antiobesity drugs or other treatments are more likely to work for them.
It’s what Mayo Clinic researchers had in mind when they created four phenotypes of obesity.
Obesity experts not affiliated with the research have some concerns and say independent studies are needed to verify the potential of this strategy.
This research could help predict who will respond best to popular antiobesity medications, said Andres Acosta, MD, PhD, cofounder of Phenomix Sciences, the company behind the tests. These medications include glucagonlike peptide–1 (GLP-1) receptor agonists like liraglutide (Saxenda, Victoza) and semaglutide (Ozempic, Wegovy).
“We know that not everyone on a GLP-1 will respond. In reality, about a third of the patients don’t do well with GLP-1s,” said Dr. Acosta, an assistant professor of medicine and researcher in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
Furthest along in development is the “My Phenome Hungry Gut” test for predicting GLP-1 response. People in this Hungry Gut group tend to empty their stomach after a meal faster and are more likely to feel hungry again a short time later, as explained on the company’s website.
A pilot study to test how well it works started in April at three primary care practices. Plans are to expand real-world testing for this and other obesity types later in 2023.
The other obesity categories are:
- “Hungry brain,” where the brain does not recognize signals that the stomach is full
- “Emotional hunger,” where cravings to eat are driven by emotions, anxiety, and negative feelings
- “Slow burn,” where people have a slow metabolism and low energy level
People in these categories might be more likely to benefit from other obesity management strategies, like changes to their diet or placement of an intragastric balloon.
Some things to consider
While applauding their efforts to be more precise in treating people with obesity, not all experts are convinced this saliva test will be the answer. The company’s research might look promising, but verification of results is warranted.
“Can we get better outcomes with things like this? Well, that’s the hope,” said Jaime Almandoz, MD, medical director of weight wellness at the University of Texas Southwestern Medical Center, Dallas.
“We still don’t have randomized trials where we’re looking at obesity phenotyping yet,” said Dr. Almandoz, who is also a spokesperson for the Obesity Society, a professional group of clinicians, researchers, educators, and others focused on obesity science, treatment, and prevention.
There is always concern when a diagnostic test is being developed for commercial use, said Daniel Bessesen, MD, a professor of medicine–endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora. “What they’re talking about doing is super important. But this is a company. This is a company that is, I think, selling a product.”
In an online search, Dr. Bessesen did not find any external studies that showed how well the saliva testing worked. But referring to work by Dr. Acosta and Michael Camilleri, MD, the other cofounder of Phenomix, he said, “I found some papers that they did that I hadn’t read before that are good.”
“These guys are smart guys. And they’ve done a lot of work on [the movement of food through the gut] and how that correlates with obesity and response to some therapies,” said Dr. Bessesen, who is also a spokesperson for the Obesity Society. “So their scientific work does line up with this area.”
Validation of any research is important because the obesity industry has been known for a lot of lose-weight-quick strategies, some with little or no science behind them, he said.
It is also essential, he said, because “anytime you do something commercial in the area of obesity, you have to acknowledge that people with obesity are a vulnerable population. These people face stigma and bias all the time.”
Removing the stigma
If knowing your obesity type ends up making a difference, it could change the conversation people have with their medical provider, Dr. Acosta said. It could also help remove some of the stigma around obesity.
“We’re going to change the conversation because now we can say: ‘Hey, you have obesity because you have ‘Hungry Gut’ phenotype. And because of that, you’re going to respond to this medication,” Dr. Acosta said. The phenotyping suggests a strong genetic tendency – a biologic basis for obesity.
“So it’s not only a way of taking the blame out, but it’s also way of explaining that there’s a reason why you have obesity,” Dr. Acosta said. It tells people: “You’re not a failure.”
More cost-effective treatment?
Targeting obesity treatment could also save on overall health care costs, Dr. Almandoz said. He estimated a cost of $1,400 per month “for forever and ever semaglutide” or at least $1,400 a month for a 3-month trial to see if this medication works in a particular person with obesity.
“That’s a lot of money when you extrapolate that out over the number of people who probably meet the criteria for treatment,” he said. A total 42% of Americans meet the Centers for Disease Control and Prevention definition for obesity.
“You can imagine the potential cost if we were to provide antiobesity therapies to everybody and we were to use what is the most effective class of medication, which is more than a thousand dollars per month, indefinitely,” Dr. Almandoz said. “Not that we should not treat everybody. That’s not the message I’m saying. But if we’re looking at yield or value in terms of treating obesity in a setting with limited resources, it may be best to start with who is most likely to benefit.”
How they created four obesity types
Starting in 2015, Dr. Acosta and colleagues started comparing tests in people with normal weight versus obesity. They used artificial intelligence and machine learning to classify obesity into 11 types at first. They realized this many obesity types were not practical for doctors and people with obesity, so they combined them into four phenotypes.
“The AI machine learning was followed by, as I like to call, HI, or human intelligence,” he said.
The saliva test checks for about 6,000 relevant genetic single-nucleotide polymorphisms. Six thousand genetic changes may sound like a large number to check; however, the average individual carries 5 million and 6 million SNPs in their DNA.
The results are translated to a score that yields a low risk or high risk for Hungry Gut or other types of obesity. “You can have all six thousand genetic mutations, or you can have zero,” Dr. Acosta said.
Moving forward
After the soft launch of Hungry Gut testing in April, Phenomix plans to continue studying their saliva test on other obesity types.
Dr. Acosta is not aware of any direct competitors to Phenomix, although that could change. “I think we’re the only diagnostic company in the space right now. But if it’s really a $14.8 billion market, we’re going to see a lot of diagnostic companies trying to do what we’re doing – if we’re successful,” he said.
An October 2022 report from Polaris Market Research estimates that the global market for obesity treatment – medications, surgery, and all others – was about $14 billion in 2021. The same report predicts the market will grow to $32 billion by 2030.
A version of this article first appeared on WebMD.com.
It sounds like a simple solution to a complicated problem: Find out what kind of obesity someone has based on a one-time genetic saliva test. Then patients and their doctor can get a better idea if antiobesity drugs or other treatments are more likely to work for them.
It’s what Mayo Clinic researchers had in mind when they created four phenotypes of obesity.
Obesity experts not affiliated with the research have some concerns and say independent studies are needed to verify the potential of this strategy.
This research could help predict who will respond best to popular antiobesity medications, said Andres Acosta, MD, PhD, cofounder of Phenomix Sciences, the company behind the tests. These medications include glucagonlike peptide–1 (GLP-1) receptor agonists like liraglutide (Saxenda, Victoza) and semaglutide (Ozempic, Wegovy).
“We know that not everyone on a GLP-1 will respond. In reality, about a third of the patients don’t do well with GLP-1s,” said Dr. Acosta, an assistant professor of medicine and researcher in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
Furthest along in development is the “My Phenome Hungry Gut” test for predicting GLP-1 response. People in this Hungry Gut group tend to empty their stomach after a meal faster and are more likely to feel hungry again a short time later, as explained on the company’s website.
A pilot study to test how well it works started in April at three primary care practices. Plans are to expand real-world testing for this and other obesity types later in 2023.
The other obesity categories are:
- “Hungry brain,” where the brain does not recognize signals that the stomach is full
- “Emotional hunger,” where cravings to eat are driven by emotions, anxiety, and negative feelings
- “Slow burn,” where people have a slow metabolism and low energy level
People in these categories might be more likely to benefit from other obesity management strategies, like changes to their diet or placement of an intragastric balloon.
Some things to consider
While applauding their efforts to be more precise in treating people with obesity, not all experts are convinced this saliva test will be the answer. The company’s research might look promising, but verification of results is warranted.
“Can we get better outcomes with things like this? Well, that’s the hope,” said Jaime Almandoz, MD, medical director of weight wellness at the University of Texas Southwestern Medical Center, Dallas.
“We still don’t have randomized trials where we’re looking at obesity phenotyping yet,” said Dr. Almandoz, who is also a spokesperson for the Obesity Society, a professional group of clinicians, researchers, educators, and others focused on obesity science, treatment, and prevention.
There is always concern when a diagnostic test is being developed for commercial use, said Daniel Bessesen, MD, a professor of medicine–endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora. “What they’re talking about doing is super important. But this is a company. This is a company that is, I think, selling a product.”
In an online search, Dr. Bessesen did not find any external studies that showed how well the saliva testing worked. But referring to work by Dr. Acosta and Michael Camilleri, MD, the other cofounder of Phenomix, he said, “I found some papers that they did that I hadn’t read before that are good.”
“These guys are smart guys. And they’ve done a lot of work on [the movement of food through the gut] and how that correlates with obesity and response to some therapies,” said Dr. Bessesen, who is also a spokesperson for the Obesity Society. “So their scientific work does line up with this area.”
Validation of any research is important because the obesity industry has been known for a lot of lose-weight-quick strategies, some with little or no science behind them, he said.
It is also essential, he said, because “anytime you do something commercial in the area of obesity, you have to acknowledge that people with obesity are a vulnerable population. These people face stigma and bias all the time.”
Removing the stigma
If knowing your obesity type ends up making a difference, it could change the conversation people have with their medical provider, Dr. Acosta said. It could also help remove some of the stigma around obesity.
“We’re going to change the conversation because now we can say: ‘Hey, you have obesity because you have ‘Hungry Gut’ phenotype. And because of that, you’re going to respond to this medication,” Dr. Acosta said. The phenotyping suggests a strong genetic tendency – a biologic basis for obesity.
“So it’s not only a way of taking the blame out, but it’s also way of explaining that there’s a reason why you have obesity,” Dr. Acosta said. It tells people: “You’re not a failure.”
More cost-effective treatment?
Targeting obesity treatment could also save on overall health care costs, Dr. Almandoz said. He estimated a cost of $1,400 per month “for forever and ever semaglutide” or at least $1,400 a month for a 3-month trial to see if this medication works in a particular person with obesity.
“That’s a lot of money when you extrapolate that out over the number of people who probably meet the criteria for treatment,” he said. A total 42% of Americans meet the Centers for Disease Control and Prevention definition for obesity.
“You can imagine the potential cost if we were to provide antiobesity therapies to everybody and we were to use what is the most effective class of medication, which is more than a thousand dollars per month, indefinitely,” Dr. Almandoz said. “Not that we should not treat everybody. That’s not the message I’m saying. But if we’re looking at yield or value in terms of treating obesity in a setting with limited resources, it may be best to start with who is most likely to benefit.”
How they created four obesity types
Starting in 2015, Dr. Acosta and colleagues started comparing tests in people with normal weight versus obesity. They used artificial intelligence and machine learning to classify obesity into 11 types at first. They realized this many obesity types were not practical for doctors and people with obesity, so they combined them into four phenotypes.
“The AI machine learning was followed by, as I like to call, HI, or human intelligence,” he said.
The saliva test checks for about 6,000 relevant genetic single-nucleotide polymorphisms. Six thousand genetic changes may sound like a large number to check; however, the average individual carries 5 million and 6 million SNPs in their DNA.
The results are translated to a score that yields a low risk or high risk for Hungry Gut or other types of obesity. “You can have all six thousand genetic mutations, or you can have zero,” Dr. Acosta said.
Moving forward
After the soft launch of Hungry Gut testing in April, Phenomix plans to continue studying their saliva test on other obesity types.
Dr. Acosta is not aware of any direct competitors to Phenomix, although that could change. “I think we’re the only diagnostic company in the space right now. But if it’s really a $14.8 billion market, we’re going to see a lot of diagnostic companies trying to do what we’re doing – if we’re successful,” he said.
An October 2022 report from Polaris Market Research estimates that the global market for obesity treatment – medications, surgery, and all others – was about $14 billion in 2021. The same report predicts the market will grow to $32 billion by 2030.
A version of this article first appeared on WebMD.com.
It sounds like a simple solution to a complicated problem: Find out what kind of obesity someone has based on a one-time genetic saliva test. Then patients and their doctor can get a better idea if antiobesity drugs or other treatments are more likely to work for them.
It’s what Mayo Clinic researchers had in mind when they created four phenotypes of obesity.
Obesity experts not affiliated with the research have some concerns and say independent studies are needed to verify the potential of this strategy.
This research could help predict who will respond best to popular antiobesity medications, said Andres Acosta, MD, PhD, cofounder of Phenomix Sciences, the company behind the tests. These medications include glucagonlike peptide–1 (GLP-1) receptor agonists like liraglutide (Saxenda, Victoza) and semaglutide (Ozempic, Wegovy).
“We know that not everyone on a GLP-1 will respond. In reality, about a third of the patients don’t do well with GLP-1s,” said Dr. Acosta, an assistant professor of medicine and researcher in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
Furthest along in development is the “My Phenome Hungry Gut” test for predicting GLP-1 response. People in this Hungry Gut group tend to empty their stomach after a meal faster and are more likely to feel hungry again a short time later, as explained on the company’s website.
A pilot study to test how well it works started in April at three primary care practices. Plans are to expand real-world testing for this and other obesity types later in 2023.
The other obesity categories are:
- “Hungry brain,” where the brain does not recognize signals that the stomach is full
- “Emotional hunger,” where cravings to eat are driven by emotions, anxiety, and negative feelings
- “Slow burn,” where people have a slow metabolism and low energy level
People in these categories might be more likely to benefit from other obesity management strategies, like changes to their diet or placement of an intragastric balloon.
Some things to consider
While applauding their efforts to be more precise in treating people with obesity, not all experts are convinced this saliva test will be the answer. The company’s research might look promising, but verification of results is warranted.
“Can we get better outcomes with things like this? Well, that’s the hope,” said Jaime Almandoz, MD, medical director of weight wellness at the University of Texas Southwestern Medical Center, Dallas.
“We still don’t have randomized trials where we’re looking at obesity phenotyping yet,” said Dr. Almandoz, who is also a spokesperson for the Obesity Society, a professional group of clinicians, researchers, educators, and others focused on obesity science, treatment, and prevention.
There is always concern when a diagnostic test is being developed for commercial use, said Daniel Bessesen, MD, a professor of medicine–endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora. “What they’re talking about doing is super important. But this is a company. This is a company that is, I think, selling a product.”
In an online search, Dr. Bessesen did not find any external studies that showed how well the saliva testing worked. But referring to work by Dr. Acosta and Michael Camilleri, MD, the other cofounder of Phenomix, he said, “I found some papers that they did that I hadn’t read before that are good.”
“These guys are smart guys. And they’ve done a lot of work on [the movement of food through the gut] and how that correlates with obesity and response to some therapies,” said Dr. Bessesen, who is also a spokesperson for the Obesity Society. “So their scientific work does line up with this area.”
Validation of any research is important because the obesity industry has been known for a lot of lose-weight-quick strategies, some with little or no science behind them, he said.
It is also essential, he said, because “anytime you do something commercial in the area of obesity, you have to acknowledge that people with obesity are a vulnerable population. These people face stigma and bias all the time.”
Removing the stigma
If knowing your obesity type ends up making a difference, it could change the conversation people have with their medical provider, Dr. Acosta said. It could also help remove some of the stigma around obesity.
“We’re going to change the conversation because now we can say: ‘Hey, you have obesity because you have ‘Hungry Gut’ phenotype. And because of that, you’re going to respond to this medication,” Dr. Acosta said. The phenotyping suggests a strong genetic tendency – a biologic basis for obesity.
“So it’s not only a way of taking the blame out, but it’s also way of explaining that there’s a reason why you have obesity,” Dr. Acosta said. It tells people: “You’re not a failure.”
More cost-effective treatment?
Targeting obesity treatment could also save on overall health care costs, Dr. Almandoz said. He estimated a cost of $1,400 per month “for forever and ever semaglutide” or at least $1,400 a month for a 3-month trial to see if this medication works in a particular person with obesity.
“That’s a lot of money when you extrapolate that out over the number of people who probably meet the criteria for treatment,” he said. A total 42% of Americans meet the Centers for Disease Control and Prevention definition for obesity.
“You can imagine the potential cost if we were to provide antiobesity therapies to everybody and we were to use what is the most effective class of medication, which is more than a thousand dollars per month, indefinitely,” Dr. Almandoz said. “Not that we should not treat everybody. That’s not the message I’m saying. But if we’re looking at yield or value in terms of treating obesity in a setting with limited resources, it may be best to start with who is most likely to benefit.”
How they created four obesity types
Starting in 2015, Dr. Acosta and colleagues started comparing tests in people with normal weight versus obesity. They used artificial intelligence and machine learning to classify obesity into 11 types at first. They realized this many obesity types were not practical for doctors and people with obesity, so they combined them into four phenotypes.
“The AI machine learning was followed by, as I like to call, HI, or human intelligence,” he said.
The saliva test checks for about 6,000 relevant genetic single-nucleotide polymorphisms. Six thousand genetic changes may sound like a large number to check; however, the average individual carries 5 million and 6 million SNPs in their DNA.
The results are translated to a score that yields a low risk or high risk for Hungry Gut or other types of obesity. “You can have all six thousand genetic mutations, or you can have zero,” Dr. Acosta said.
Moving forward
After the soft launch of Hungry Gut testing in April, Phenomix plans to continue studying their saliva test on other obesity types.
Dr. Acosta is not aware of any direct competitors to Phenomix, although that could change. “I think we’re the only diagnostic company in the space right now. But if it’s really a $14.8 billion market, we’re going to see a lot of diagnostic companies trying to do what we’re doing – if we’re successful,” he said.
An October 2022 report from Polaris Market Research estimates that the global market for obesity treatment – medications, surgery, and all others – was about $14 billion in 2021. The same report predicts the market will grow to $32 billion by 2030.
A version of this article first appeared on WebMD.com.
FDA approves new drug, sotagliflozin, for heart failure
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Plant-based diet tied to healthier blood lipid levels
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN HEART JOURNAL
Ticagrelor, DAPT equal in preventing repeat revascularization
PHOENIX – Post hoc analysis of the randomized TWILIGHT trial comparing ticagrelor alone with ticagrelor plus aspirin in high-risk patients after percutaneous coronary intervention (PCI) shows both regimens were similarly effective in preventing repeat revascularization after 1 year.
In TWILIGHT, the main findings of which were previously published in the New England Journal of Medicine, 7,119 high-risk PCI patients on standard dual antiplatelet therapy (DAPT) of ticagrelor plus aspirin for 3 months were randomized to continuation of DAPT or to ticagrelor plus placebo for 12 months.
The new post hoc analysis included 6,759 patients and shows the rates of clinically driven revascularization were similar between the two groups: 7.1% and 6.6% for the ticagrelor monotherapy and ticagrelor-based DAPT groups, respectively (P = .363).
The findings were presented at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
Three key findings come from the post hoc analysis, Usman Baber, MD, director of the cardiac catheterization lab and associate professor at the University of Oklahoma Health Sciences Center, Oklahoma City, who presented the findings, said in an interview.
“The first is that, over the 1-year follow-up of our trial, we found that a repeat revascularization event occurred in 6.7% of patients,” he said. “We found that a slight majority of these repeat revascularization events were due to events at the target lesion or target vessel; and we found that most of the repeat revascularization events actually occurred in patients without a concomitant acute coronary syndrome. In other words, these were essentially stable patients when they were getting their repeat revascularization.”
The second major finding was that these high-risk patients who had repeat revascularization were at three times greater risk for major adverse cardiac and cerebrovascular events (MACCE), based on a multivariable adjusted model, Dr. Baber said.
“And then third is that he said.
Repeat revascularization
The goal of the analysis was to focus on clinically driven repeat revascularization as an outcome, Dr. Baber said. The analysis also aimed to understand the association between repeat revascularization and subsequent risk.
Secondary endpoints included target lesion revascularization (TLR); target vessel revascularization (TVR); MACCE, including clinically driven revascularization; and net adverse clinical events (NACE), a composite of MACCE or Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding.
The outcomes of all those endpoints, except for NACE, were similar, Dr. Baber said. “Overall, ticagrelor monotherapy, as expected, reduced rates of bleeding as compared with ticagrelor plus aspirin,” he said. The rates of NACE were 12.2% versus 14.6%, respectively (P = .004). For BARC 2,3, or 5 bleeding, the rates were 3.4% versus 7.1% (P < .001).
The findings validated repeat revascularization as a meaningful endpoint, Dr. Baber said. “Certainly, we don’t elevate repeat revascularization as an endpoint to the same level as death or stroke, but certainly this analysis and some others prior to it highlight the fact that when these patients come back for repeat revascularization, even if they’re stable, they clearly are at elevated risk for future ischemic events,” he said.
One limitation of the analysis is that the data are from a clinical trial, “which renders the findings not as generalizable to the broader patients in a clinical practice,” he said. However, the TWILIGHT data are validated and adjusted for multiple risk factors.
“When patients come in and they have a repeat revascularization, should there be a consideration to placing them on more intensive antithrombotic therapy?” he asked. “Right now, if patients have a repeat revascularization event and they’re stable, guidelines and clinical practice usually calls for continuing clopidogrel, but again our study and others like it indicate these patients are at a higher thrombotic risk, so maybe there’s a rationale for at least a short course of a more potent antiplatelet agent in such patients.”
The post hoc findings confirm those of the primary TWILIGHT trial, Lorenzo Azzalini, MD, PhD, MSc, director of interventional cardiology research at the University of Washington Medical Center, Seattle, said in an interview.
“It’s not surprising to find no difference between the two therapies with regard to unplanned revascularization,” he said. “It’s considered that only stent thrombosis can only actually be mitigated by the drugs being investigated in the trial; all the other ischemic endpoints reflect more chronic ischemia—TLR or known TVR—upon which ticagrelor and aspirin do not play any role.”
However, he added, “I still think this study provides useful information to the community in a period of intense scrutiny on the relative benefits and merits of PCI versus CABG [coronary artery bypass graft], and this study confirms that shortening DAPT to 3 months and then continuing with just ticagrelor does not bring any penalty in terms of ischemic events or repeat revascularization.”
TWILIGHT enrolled high-risk patients, but not “very-high-risk” patients, Dr. Azzalini noted. The enrollment criteria excluded patients on chronic anticoagulation, who had a prior stroke or liver sclerosis, or were on dialysis.
“Future trials should focus more on very-high-risk patients because these are the patients that we deal with on a daily basis in our clinical practice and we need data to inform our decisions,” he said. “I’m not sure I could use the science contained in this study and extrapolate them to patients on dialysis because these patients really have a high risk of restenosis on follow-up.”
Dr. Baber disclosed relationships with Amgen and Abbott. Dr. Azzalini had no relevant disclosures.
PHOENIX – Post hoc analysis of the randomized TWILIGHT trial comparing ticagrelor alone with ticagrelor plus aspirin in high-risk patients after percutaneous coronary intervention (PCI) shows both regimens were similarly effective in preventing repeat revascularization after 1 year.
In TWILIGHT, the main findings of which were previously published in the New England Journal of Medicine, 7,119 high-risk PCI patients on standard dual antiplatelet therapy (DAPT) of ticagrelor plus aspirin for 3 months were randomized to continuation of DAPT or to ticagrelor plus placebo for 12 months.
The new post hoc analysis included 6,759 patients and shows the rates of clinically driven revascularization were similar between the two groups: 7.1% and 6.6% for the ticagrelor monotherapy and ticagrelor-based DAPT groups, respectively (P = .363).
The findings were presented at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
Three key findings come from the post hoc analysis, Usman Baber, MD, director of the cardiac catheterization lab and associate professor at the University of Oklahoma Health Sciences Center, Oklahoma City, who presented the findings, said in an interview.
“The first is that, over the 1-year follow-up of our trial, we found that a repeat revascularization event occurred in 6.7% of patients,” he said. “We found that a slight majority of these repeat revascularization events were due to events at the target lesion or target vessel; and we found that most of the repeat revascularization events actually occurred in patients without a concomitant acute coronary syndrome. In other words, these were essentially stable patients when they were getting their repeat revascularization.”
The second major finding was that these high-risk patients who had repeat revascularization were at three times greater risk for major adverse cardiac and cerebrovascular events (MACCE), based on a multivariable adjusted model, Dr. Baber said.
“And then third is that he said.
Repeat revascularization
The goal of the analysis was to focus on clinically driven repeat revascularization as an outcome, Dr. Baber said. The analysis also aimed to understand the association between repeat revascularization and subsequent risk.
Secondary endpoints included target lesion revascularization (TLR); target vessel revascularization (TVR); MACCE, including clinically driven revascularization; and net adverse clinical events (NACE), a composite of MACCE or Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding.
The outcomes of all those endpoints, except for NACE, were similar, Dr. Baber said. “Overall, ticagrelor monotherapy, as expected, reduced rates of bleeding as compared with ticagrelor plus aspirin,” he said. The rates of NACE were 12.2% versus 14.6%, respectively (P = .004). For BARC 2,3, or 5 bleeding, the rates were 3.4% versus 7.1% (P < .001).
The findings validated repeat revascularization as a meaningful endpoint, Dr. Baber said. “Certainly, we don’t elevate repeat revascularization as an endpoint to the same level as death or stroke, but certainly this analysis and some others prior to it highlight the fact that when these patients come back for repeat revascularization, even if they’re stable, they clearly are at elevated risk for future ischemic events,” he said.
One limitation of the analysis is that the data are from a clinical trial, “which renders the findings not as generalizable to the broader patients in a clinical practice,” he said. However, the TWILIGHT data are validated and adjusted for multiple risk factors.
“When patients come in and they have a repeat revascularization, should there be a consideration to placing them on more intensive antithrombotic therapy?” he asked. “Right now, if patients have a repeat revascularization event and they’re stable, guidelines and clinical practice usually calls for continuing clopidogrel, but again our study and others like it indicate these patients are at a higher thrombotic risk, so maybe there’s a rationale for at least a short course of a more potent antiplatelet agent in such patients.”
The post hoc findings confirm those of the primary TWILIGHT trial, Lorenzo Azzalini, MD, PhD, MSc, director of interventional cardiology research at the University of Washington Medical Center, Seattle, said in an interview.
“It’s not surprising to find no difference between the two therapies with regard to unplanned revascularization,” he said. “It’s considered that only stent thrombosis can only actually be mitigated by the drugs being investigated in the trial; all the other ischemic endpoints reflect more chronic ischemia—TLR or known TVR—upon which ticagrelor and aspirin do not play any role.”
However, he added, “I still think this study provides useful information to the community in a period of intense scrutiny on the relative benefits and merits of PCI versus CABG [coronary artery bypass graft], and this study confirms that shortening DAPT to 3 months and then continuing with just ticagrelor does not bring any penalty in terms of ischemic events or repeat revascularization.”
TWILIGHT enrolled high-risk patients, but not “very-high-risk” patients, Dr. Azzalini noted. The enrollment criteria excluded patients on chronic anticoagulation, who had a prior stroke or liver sclerosis, or were on dialysis.
“Future trials should focus more on very-high-risk patients because these are the patients that we deal with on a daily basis in our clinical practice and we need data to inform our decisions,” he said. “I’m not sure I could use the science contained in this study and extrapolate them to patients on dialysis because these patients really have a high risk of restenosis on follow-up.”
Dr. Baber disclosed relationships with Amgen and Abbott. Dr. Azzalini had no relevant disclosures.
PHOENIX – Post hoc analysis of the randomized TWILIGHT trial comparing ticagrelor alone with ticagrelor plus aspirin in high-risk patients after percutaneous coronary intervention (PCI) shows both regimens were similarly effective in preventing repeat revascularization after 1 year.
In TWILIGHT, the main findings of which were previously published in the New England Journal of Medicine, 7,119 high-risk PCI patients on standard dual antiplatelet therapy (DAPT) of ticagrelor plus aspirin for 3 months were randomized to continuation of DAPT or to ticagrelor plus placebo for 12 months.
The new post hoc analysis included 6,759 patients and shows the rates of clinically driven revascularization were similar between the two groups: 7.1% and 6.6% for the ticagrelor monotherapy and ticagrelor-based DAPT groups, respectively (P = .363).
The findings were presented at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
Three key findings come from the post hoc analysis, Usman Baber, MD, director of the cardiac catheterization lab and associate professor at the University of Oklahoma Health Sciences Center, Oklahoma City, who presented the findings, said in an interview.
“The first is that, over the 1-year follow-up of our trial, we found that a repeat revascularization event occurred in 6.7% of patients,” he said. “We found that a slight majority of these repeat revascularization events were due to events at the target lesion or target vessel; and we found that most of the repeat revascularization events actually occurred in patients without a concomitant acute coronary syndrome. In other words, these were essentially stable patients when they were getting their repeat revascularization.”
The second major finding was that these high-risk patients who had repeat revascularization were at three times greater risk for major adverse cardiac and cerebrovascular events (MACCE), based on a multivariable adjusted model, Dr. Baber said.
“And then third is that he said.
Repeat revascularization
The goal of the analysis was to focus on clinically driven repeat revascularization as an outcome, Dr. Baber said. The analysis also aimed to understand the association between repeat revascularization and subsequent risk.
Secondary endpoints included target lesion revascularization (TLR); target vessel revascularization (TVR); MACCE, including clinically driven revascularization; and net adverse clinical events (NACE), a composite of MACCE or Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding.
The outcomes of all those endpoints, except for NACE, were similar, Dr. Baber said. “Overall, ticagrelor monotherapy, as expected, reduced rates of bleeding as compared with ticagrelor plus aspirin,” he said. The rates of NACE were 12.2% versus 14.6%, respectively (P = .004). For BARC 2,3, or 5 bleeding, the rates were 3.4% versus 7.1% (P < .001).
The findings validated repeat revascularization as a meaningful endpoint, Dr. Baber said. “Certainly, we don’t elevate repeat revascularization as an endpoint to the same level as death or stroke, but certainly this analysis and some others prior to it highlight the fact that when these patients come back for repeat revascularization, even if they’re stable, they clearly are at elevated risk for future ischemic events,” he said.
One limitation of the analysis is that the data are from a clinical trial, “which renders the findings not as generalizable to the broader patients in a clinical practice,” he said. However, the TWILIGHT data are validated and adjusted for multiple risk factors.
“When patients come in and they have a repeat revascularization, should there be a consideration to placing them on more intensive antithrombotic therapy?” he asked. “Right now, if patients have a repeat revascularization event and they’re stable, guidelines and clinical practice usually calls for continuing clopidogrel, but again our study and others like it indicate these patients are at a higher thrombotic risk, so maybe there’s a rationale for at least a short course of a more potent antiplatelet agent in such patients.”
The post hoc findings confirm those of the primary TWILIGHT trial, Lorenzo Azzalini, MD, PhD, MSc, director of interventional cardiology research at the University of Washington Medical Center, Seattle, said in an interview.
“It’s not surprising to find no difference between the two therapies with regard to unplanned revascularization,” he said. “It’s considered that only stent thrombosis can only actually be mitigated by the drugs being investigated in the trial; all the other ischemic endpoints reflect more chronic ischemia—TLR or known TVR—upon which ticagrelor and aspirin do not play any role.”
However, he added, “I still think this study provides useful information to the community in a period of intense scrutiny on the relative benefits and merits of PCI versus CABG [coronary artery bypass graft], and this study confirms that shortening DAPT to 3 months and then continuing with just ticagrelor does not bring any penalty in terms of ischemic events or repeat revascularization.”
TWILIGHT enrolled high-risk patients, but not “very-high-risk” patients, Dr. Azzalini noted. The enrollment criteria excluded patients on chronic anticoagulation, who had a prior stroke or liver sclerosis, or were on dialysis.
“Future trials should focus more on very-high-risk patients because these are the patients that we deal with on a daily basis in our clinical practice and we need data to inform our decisions,” he said. “I’m not sure I could use the science contained in this study and extrapolate them to patients on dialysis because these patients really have a high risk of restenosis on follow-up.”
Dr. Baber disclosed relationships with Amgen and Abbott. Dr. Azzalini had no relevant disclosures.
AT SCAI 2023
ECMO signals benefit for cardiogenic shock after MI in halted trial
Data support new randomized trial
At the time that it was halted, a multicenter randomized trial was associating venoarterial extracorporeal membrane oxygenation (VA-ECMO) with an intriguing signal of benefit for patients in cardiogenic shock undergoing percutaneous intervention (PCI) for acute myocardial infarction.
Stopped early because of the pandemic, the EURO SHOCK trial has data on only 35 patients, but all-cause mortality at 30 days was nearly 30% lower in the VA-ECMO arm than in the standard-therapy arm, reported Manel Sabate, MD, PhD, chief of the interventional cardiology unit, Clinic University Hospital, Barcelona.
When patients were followed out to 12 months, the numerical survival advantage appeared to persist.
Yet, because of the early trial termination, “there really are no definite conclusions to be drawn from these results,” acknowledged Dr. Sabate, who noted that less than 10% of the planned enrollment had been reached. In addition, the survival benefit in the VA-ECMO arm was achieved at the cost of a higher rate of complications.
Despite the small numbers, results from the halted trial were presented as a late-breaker at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions. They were also simultaneously published in EuroIntervention.
The interest is based on an important unmet need, said Dr. Sabate. Cardiogenic shock occurs in about 10% of acute MI patients. Of those that continue on to revascularization, the 30-day mortality can approach 50%.
Meanwhile, the potential of mechanical circulatory support to maintain perfusion during cardiogenic shock makes it one of the most attractive, if unproven, approaches for improving outcomes.
Major multicenter trial terminated
The EURO SHOCK trial had a planned enrollment of 428 patients when it was initiated; 15 centers in six European countries participated. Recruitment and the trial were brought to a halt by the COVID-19 pandemic.
When trial recruitment was stopped, 18 patients had been assigned to standard supportive care and 17 patients to VA-ECMO. The primary endpoint of the trial was all-cause mortality at 30 days. Mortality at 12 months along with bleeding complications, cerebrovascular events, and readmission for heart failure, were among secondary endpoints.
At 30 days, the mortality rate was 61.1% among patients randomized to standard care, versus 43.8% among patients randomized to VA ECMO (hazard ratio, 0.56; 95% confidence interval, 0.21-01.45; P = 0.22).
At 12 months, the numerical advantage of VA-ECMO persisted (81.5% vs. 51.8%) with a similar nonsignificant signal for potential benefit despite the small sample size (HR, 0.52; 95% CI, 0.21-1.26; P = 0.14).
There were also numerically lower rates of cardiovascular death, ischemic stroke, recurrent MI, and acute kidney injury among patients in the VA-ECMO group relative to those in the standard-care group, Dr. Sabate reported.
However, VA-ECMO was associated with more vascular complications (21.4% vs. 0%) and bleeding events (35.7% vs. 5.6%).
Furthermore, although quality of life data were limited, Dr. Sabate noted that about half of patients in the VA-ECMO group reported problems with mobility, self-care, or usual activities on the basis of the EQ-5D-3L questionnaire at 30 days. None of the patients in the standard-care group reported any such difficulties.
When standard care was compared with VA-ECMO, rates of readmission for heart failure over 12 months (8.0% vs. 6.9%) were not different.
To be enrolled in this study, patients being treated for MI had to be in cardiogenic shock for at least 30 minutes following primary PCI. The median time from onset of cardiogenic shock to VA-ECMO in the active treatment arm was 4.8 hours.
Patient enrollment was challenging
Even independent of the COVID-19 pandemic, enrolling patients proved to be difficult. The 35 patients enrolled represented about 10% of the 333 patients screened at the participating centers. Unwitnessed out-of-hospital cardiac arrest, cardiogenic shock from a cause other than MI, and recovery from cardiogenic shock after the PCI was performed were among reasons for the high rate of exclusions.
The difficulty of identifying and engaging appropriate candidates for VA-ECMO, along with a substantial crossover rate, should be among lessons for investigators planning the next trial, said Dr. Sabate, who pointed out that 5 of the 17 patients assigned to VA-ECMO were never treated due to complications or patient refusal.
Dr. Sabate said.
Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy), agreed.
“It was a good decision to publish these results,” he said. Noting that there were challenges in conducting the trial unrelated to COVID-19, Dr. Capodanno acknowledged the promise of mechanical ventilatory support for a relatively common and life-threatening complication.
“This study must be read for the lessons it will provide for future trials,” he said.
Dr. Sabate reported he has no potential conflicts of interest. Dr. Capodanno reported financial relationships with Amgen, Daiichi Sankyo, and Sanofi.
Data support new randomized trial
Data support new randomized trial
At the time that it was halted, a multicenter randomized trial was associating venoarterial extracorporeal membrane oxygenation (VA-ECMO) with an intriguing signal of benefit for patients in cardiogenic shock undergoing percutaneous intervention (PCI) for acute myocardial infarction.
Stopped early because of the pandemic, the EURO SHOCK trial has data on only 35 patients, but all-cause mortality at 30 days was nearly 30% lower in the VA-ECMO arm than in the standard-therapy arm, reported Manel Sabate, MD, PhD, chief of the interventional cardiology unit, Clinic University Hospital, Barcelona.
When patients were followed out to 12 months, the numerical survival advantage appeared to persist.
Yet, because of the early trial termination, “there really are no definite conclusions to be drawn from these results,” acknowledged Dr. Sabate, who noted that less than 10% of the planned enrollment had been reached. In addition, the survival benefit in the VA-ECMO arm was achieved at the cost of a higher rate of complications.
Despite the small numbers, results from the halted trial were presented as a late-breaker at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions. They were also simultaneously published in EuroIntervention.
The interest is based on an important unmet need, said Dr. Sabate. Cardiogenic shock occurs in about 10% of acute MI patients. Of those that continue on to revascularization, the 30-day mortality can approach 50%.
Meanwhile, the potential of mechanical circulatory support to maintain perfusion during cardiogenic shock makes it one of the most attractive, if unproven, approaches for improving outcomes.
Major multicenter trial terminated
The EURO SHOCK trial had a planned enrollment of 428 patients when it was initiated; 15 centers in six European countries participated. Recruitment and the trial were brought to a halt by the COVID-19 pandemic.
When trial recruitment was stopped, 18 patients had been assigned to standard supportive care and 17 patients to VA-ECMO. The primary endpoint of the trial was all-cause mortality at 30 days. Mortality at 12 months along with bleeding complications, cerebrovascular events, and readmission for heart failure, were among secondary endpoints.
At 30 days, the mortality rate was 61.1% among patients randomized to standard care, versus 43.8% among patients randomized to VA ECMO (hazard ratio, 0.56; 95% confidence interval, 0.21-01.45; P = 0.22).
At 12 months, the numerical advantage of VA-ECMO persisted (81.5% vs. 51.8%) with a similar nonsignificant signal for potential benefit despite the small sample size (HR, 0.52; 95% CI, 0.21-1.26; P = 0.14).
There were also numerically lower rates of cardiovascular death, ischemic stroke, recurrent MI, and acute kidney injury among patients in the VA-ECMO group relative to those in the standard-care group, Dr. Sabate reported.
However, VA-ECMO was associated with more vascular complications (21.4% vs. 0%) and bleeding events (35.7% vs. 5.6%).
Furthermore, although quality of life data were limited, Dr. Sabate noted that about half of patients in the VA-ECMO group reported problems with mobility, self-care, or usual activities on the basis of the EQ-5D-3L questionnaire at 30 days. None of the patients in the standard-care group reported any such difficulties.
When standard care was compared with VA-ECMO, rates of readmission for heart failure over 12 months (8.0% vs. 6.9%) were not different.
To be enrolled in this study, patients being treated for MI had to be in cardiogenic shock for at least 30 minutes following primary PCI. The median time from onset of cardiogenic shock to VA-ECMO in the active treatment arm was 4.8 hours.
Patient enrollment was challenging
Even independent of the COVID-19 pandemic, enrolling patients proved to be difficult. The 35 patients enrolled represented about 10% of the 333 patients screened at the participating centers. Unwitnessed out-of-hospital cardiac arrest, cardiogenic shock from a cause other than MI, and recovery from cardiogenic shock after the PCI was performed were among reasons for the high rate of exclusions.
The difficulty of identifying and engaging appropriate candidates for VA-ECMO, along with a substantial crossover rate, should be among lessons for investigators planning the next trial, said Dr. Sabate, who pointed out that 5 of the 17 patients assigned to VA-ECMO were never treated due to complications or patient refusal.
Dr. Sabate said.
Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy), agreed.
“It was a good decision to publish these results,” he said. Noting that there were challenges in conducting the trial unrelated to COVID-19, Dr. Capodanno acknowledged the promise of mechanical ventilatory support for a relatively common and life-threatening complication.
“This study must be read for the lessons it will provide for future trials,” he said.
Dr. Sabate reported he has no potential conflicts of interest. Dr. Capodanno reported financial relationships with Amgen, Daiichi Sankyo, and Sanofi.
At the time that it was halted, a multicenter randomized trial was associating venoarterial extracorporeal membrane oxygenation (VA-ECMO) with an intriguing signal of benefit for patients in cardiogenic shock undergoing percutaneous intervention (PCI) for acute myocardial infarction.
Stopped early because of the pandemic, the EURO SHOCK trial has data on only 35 patients, but all-cause mortality at 30 days was nearly 30% lower in the VA-ECMO arm than in the standard-therapy arm, reported Manel Sabate, MD, PhD, chief of the interventional cardiology unit, Clinic University Hospital, Barcelona.
When patients were followed out to 12 months, the numerical survival advantage appeared to persist.
Yet, because of the early trial termination, “there really are no definite conclusions to be drawn from these results,” acknowledged Dr. Sabate, who noted that less than 10% of the planned enrollment had been reached. In addition, the survival benefit in the VA-ECMO arm was achieved at the cost of a higher rate of complications.
Despite the small numbers, results from the halted trial were presented as a late-breaker at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions. They were also simultaneously published in EuroIntervention.
The interest is based on an important unmet need, said Dr. Sabate. Cardiogenic shock occurs in about 10% of acute MI patients. Of those that continue on to revascularization, the 30-day mortality can approach 50%.
Meanwhile, the potential of mechanical circulatory support to maintain perfusion during cardiogenic shock makes it one of the most attractive, if unproven, approaches for improving outcomes.
Major multicenter trial terminated
The EURO SHOCK trial had a planned enrollment of 428 patients when it was initiated; 15 centers in six European countries participated. Recruitment and the trial were brought to a halt by the COVID-19 pandemic.
When trial recruitment was stopped, 18 patients had been assigned to standard supportive care and 17 patients to VA-ECMO. The primary endpoint of the trial was all-cause mortality at 30 days. Mortality at 12 months along with bleeding complications, cerebrovascular events, and readmission for heart failure, were among secondary endpoints.
At 30 days, the mortality rate was 61.1% among patients randomized to standard care, versus 43.8% among patients randomized to VA ECMO (hazard ratio, 0.56; 95% confidence interval, 0.21-01.45; P = 0.22).
At 12 months, the numerical advantage of VA-ECMO persisted (81.5% vs. 51.8%) with a similar nonsignificant signal for potential benefit despite the small sample size (HR, 0.52; 95% CI, 0.21-1.26; P = 0.14).
There were also numerically lower rates of cardiovascular death, ischemic stroke, recurrent MI, and acute kidney injury among patients in the VA-ECMO group relative to those in the standard-care group, Dr. Sabate reported.
However, VA-ECMO was associated with more vascular complications (21.4% vs. 0%) and bleeding events (35.7% vs. 5.6%).
Furthermore, although quality of life data were limited, Dr. Sabate noted that about half of patients in the VA-ECMO group reported problems with mobility, self-care, or usual activities on the basis of the EQ-5D-3L questionnaire at 30 days. None of the patients in the standard-care group reported any such difficulties.
When standard care was compared with VA-ECMO, rates of readmission for heart failure over 12 months (8.0% vs. 6.9%) were not different.
To be enrolled in this study, patients being treated for MI had to be in cardiogenic shock for at least 30 minutes following primary PCI. The median time from onset of cardiogenic shock to VA-ECMO in the active treatment arm was 4.8 hours.
Patient enrollment was challenging
Even independent of the COVID-19 pandemic, enrolling patients proved to be difficult. The 35 patients enrolled represented about 10% of the 333 patients screened at the participating centers. Unwitnessed out-of-hospital cardiac arrest, cardiogenic shock from a cause other than MI, and recovery from cardiogenic shock after the PCI was performed were among reasons for the high rate of exclusions.
The difficulty of identifying and engaging appropriate candidates for VA-ECMO, along with a substantial crossover rate, should be among lessons for investigators planning the next trial, said Dr. Sabate, who pointed out that 5 of the 17 patients assigned to VA-ECMO were never treated due to complications or patient refusal.
Dr. Sabate said.
Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy), agreed.
“It was a good decision to publish these results,” he said. Noting that there were challenges in conducting the trial unrelated to COVID-19, Dr. Capodanno acknowledged the promise of mechanical ventilatory support for a relatively common and life-threatening complication.
“This study must be read for the lessons it will provide for future trials,” he said.
Dr. Sabate reported he has no potential conflicts of interest. Dr. Capodanno reported financial relationships with Amgen, Daiichi Sankyo, and Sanofi.
FROM EUROPCR 2023
JAK-inhibitor safety in adolescents with AD: Long-term analyses reported
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
AT RAD 2023
First prospective study finds pregnancies with Sjögren’s to be largely safe
Women with Sjögren’s syndrome have pregnancy outcomes similar to those of the general population, according to the first study to prospectively track pregnancy outcomes among people with the autoimmune condition.
“Most early studies of pregnancy in rheumatic disease patients were retrospective and included only small numbers, making it difficult to know how generalizable the reported results were,” said Lisa Sammaritano, MD, a rheumatologist at Hospital for Special Surgery in New York, in an email interview with this news organization. She was not involved with the research.
Most of these previous studies suggested an increased risk of adverse outcomes, such as miscarriages, preterm deliveries, and small-for-gestational-age birth weight. But in addition to small patient numbers, retrospective studies “are subject to greater reporting bias, which may predispose patients with negative outcomes being more likely to be included because they were followed more closely,” Dr. Sammaritano said.
“This prospective study has several advantages over the earlier retrospective reports: The same data were collected in the same way for all the patients, the patients were recruited at similar time points, and – due to the multicenter nature of the cohort – numbers are larger than in prior studies. All these factors make the results stronger and more generalizable to the Sjögren’s patients we see in our practices,” she added.
In the study, published May 8 in The Lancet Rheumatology, first author Grégoire Martin de Frémont, MD, of the rheumatology service at Bicêtre Hospital, Paris-Saclay University and colleagues used the GR2 registry, an observational database of pregnancies of women with systemic autoimmune diseases managed at 76 participating centers in France, to identify pregnant women with primary Sjögren’s syndrome. To avoid bias, only women who entered the database before 18 weeks’ gestation were included. The final cohort included 106 pregnancies in 96 women with primary Sjögren’s syndrome and 420 control pregnancies that were matched from the general population.
Adverse pregnancy outcomes, including preterm delivery (< 37 weeks of gestation), intrauterine growth retardation, and low birth weight occurred in nine pregnancies (9%) in the Sjögren’s syndrome group and in 28 pregnancies in the control group (7%). Adverse pregnancy outcomes were not significantly associated with Sjögren’s syndrome (P = .52). Researchers found that there were more adverse pregnancy outcomes among women with Sjögren’s syndrome with antiphospholipid (aPL) antibodies. Negative outcomes also increased among those with anti-RNP antibodies, but this association was not statistically significant.
“The main message – based on strong data from a well-designed study – is that patients with Sjögren’s overall do as well as the general population in terms of standard adverse pregnancy outcomes. The rate of flare of Sjögren’s disease was relatively low during the second and third trimesters, also reassuring,” Dr. Sammaritano said. She noted that the association between adverse pregnancy outcomes and aPL antibodies was not unexpected, given that they are a known risk factor.
The study authors recommend that patients with Sjögren’s syndrome be screened for aPL and anti-RNP antibodies prior to conception because of the potential increased risk for complications and that patients with positive screens be closely monitored during their pregnancy.
Dr. Sammaritano noted that there are other health problems to keep in mind. “It is important to remember that Sjögren’s patients – more than any other rheumatic disease patients – have the additional risk for neonatal lupus and complete heart block in their infant, since about two-thirds of Sjögren’s patients are positive for anti-Ro/SSA antibody,” she said. “This is a distinct issue related to the presence of this antibody alone and not specifically related to the underlying diagnosis. In clinical practice, positive anti-Ro/SSA antibody is often the main reason for counseling, monitoring, and even recommending therapy (hydroxychloroquine) in these patients.”
The study received funding from Lupus France, the France Association of Scleroderma, and the Association Gougerot Sjögren, among others. Dr. Sammaritano reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women with Sjögren’s syndrome have pregnancy outcomes similar to those of the general population, according to the first study to prospectively track pregnancy outcomes among people with the autoimmune condition.
“Most early studies of pregnancy in rheumatic disease patients were retrospective and included only small numbers, making it difficult to know how generalizable the reported results were,” said Lisa Sammaritano, MD, a rheumatologist at Hospital for Special Surgery in New York, in an email interview with this news organization. She was not involved with the research.
Most of these previous studies suggested an increased risk of adverse outcomes, such as miscarriages, preterm deliveries, and small-for-gestational-age birth weight. But in addition to small patient numbers, retrospective studies “are subject to greater reporting bias, which may predispose patients with negative outcomes being more likely to be included because they were followed more closely,” Dr. Sammaritano said.
“This prospective study has several advantages over the earlier retrospective reports: The same data were collected in the same way for all the patients, the patients were recruited at similar time points, and – due to the multicenter nature of the cohort – numbers are larger than in prior studies. All these factors make the results stronger and more generalizable to the Sjögren’s patients we see in our practices,” she added.
In the study, published May 8 in The Lancet Rheumatology, first author Grégoire Martin de Frémont, MD, of the rheumatology service at Bicêtre Hospital, Paris-Saclay University and colleagues used the GR2 registry, an observational database of pregnancies of women with systemic autoimmune diseases managed at 76 participating centers in France, to identify pregnant women with primary Sjögren’s syndrome. To avoid bias, only women who entered the database before 18 weeks’ gestation were included. The final cohort included 106 pregnancies in 96 women with primary Sjögren’s syndrome and 420 control pregnancies that were matched from the general population.
Adverse pregnancy outcomes, including preterm delivery (< 37 weeks of gestation), intrauterine growth retardation, and low birth weight occurred in nine pregnancies (9%) in the Sjögren’s syndrome group and in 28 pregnancies in the control group (7%). Adverse pregnancy outcomes were not significantly associated with Sjögren’s syndrome (P = .52). Researchers found that there were more adverse pregnancy outcomes among women with Sjögren’s syndrome with antiphospholipid (aPL) antibodies. Negative outcomes also increased among those with anti-RNP antibodies, but this association was not statistically significant.
“The main message – based on strong data from a well-designed study – is that patients with Sjögren’s overall do as well as the general population in terms of standard adverse pregnancy outcomes. The rate of flare of Sjögren’s disease was relatively low during the second and third trimesters, also reassuring,” Dr. Sammaritano said. She noted that the association between adverse pregnancy outcomes and aPL antibodies was not unexpected, given that they are a known risk factor.
The study authors recommend that patients with Sjögren’s syndrome be screened for aPL and anti-RNP antibodies prior to conception because of the potential increased risk for complications and that patients with positive screens be closely monitored during their pregnancy.
Dr. Sammaritano noted that there are other health problems to keep in mind. “It is important to remember that Sjögren’s patients – more than any other rheumatic disease patients – have the additional risk for neonatal lupus and complete heart block in their infant, since about two-thirds of Sjögren’s patients are positive for anti-Ro/SSA antibody,” she said. “This is a distinct issue related to the presence of this antibody alone and not specifically related to the underlying diagnosis. In clinical practice, positive anti-Ro/SSA antibody is often the main reason for counseling, monitoring, and even recommending therapy (hydroxychloroquine) in these patients.”
The study received funding from Lupus France, the France Association of Scleroderma, and the Association Gougerot Sjögren, among others. Dr. Sammaritano reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women with Sjögren’s syndrome have pregnancy outcomes similar to those of the general population, according to the first study to prospectively track pregnancy outcomes among people with the autoimmune condition.
“Most early studies of pregnancy in rheumatic disease patients were retrospective and included only small numbers, making it difficult to know how generalizable the reported results were,” said Lisa Sammaritano, MD, a rheumatologist at Hospital for Special Surgery in New York, in an email interview with this news organization. She was not involved with the research.
Most of these previous studies suggested an increased risk of adverse outcomes, such as miscarriages, preterm deliveries, and small-for-gestational-age birth weight. But in addition to small patient numbers, retrospective studies “are subject to greater reporting bias, which may predispose patients with negative outcomes being more likely to be included because they were followed more closely,” Dr. Sammaritano said.
“This prospective study has several advantages over the earlier retrospective reports: The same data were collected in the same way for all the patients, the patients were recruited at similar time points, and – due to the multicenter nature of the cohort – numbers are larger than in prior studies. All these factors make the results stronger and more generalizable to the Sjögren’s patients we see in our practices,” she added.
In the study, published May 8 in The Lancet Rheumatology, first author Grégoire Martin de Frémont, MD, of the rheumatology service at Bicêtre Hospital, Paris-Saclay University and colleagues used the GR2 registry, an observational database of pregnancies of women with systemic autoimmune diseases managed at 76 participating centers in France, to identify pregnant women with primary Sjögren’s syndrome. To avoid bias, only women who entered the database before 18 weeks’ gestation were included. The final cohort included 106 pregnancies in 96 women with primary Sjögren’s syndrome and 420 control pregnancies that were matched from the general population.
Adverse pregnancy outcomes, including preterm delivery (< 37 weeks of gestation), intrauterine growth retardation, and low birth weight occurred in nine pregnancies (9%) in the Sjögren’s syndrome group and in 28 pregnancies in the control group (7%). Adverse pregnancy outcomes were not significantly associated with Sjögren’s syndrome (P = .52). Researchers found that there were more adverse pregnancy outcomes among women with Sjögren’s syndrome with antiphospholipid (aPL) antibodies. Negative outcomes also increased among those with anti-RNP antibodies, but this association was not statistically significant.
“The main message – based on strong data from a well-designed study – is that patients with Sjögren’s overall do as well as the general population in terms of standard adverse pregnancy outcomes. The rate of flare of Sjögren’s disease was relatively low during the second and third trimesters, also reassuring,” Dr. Sammaritano said. She noted that the association between adverse pregnancy outcomes and aPL antibodies was not unexpected, given that they are a known risk factor.
The study authors recommend that patients with Sjögren’s syndrome be screened for aPL and anti-RNP antibodies prior to conception because of the potential increased risk for complications and that patients with positive screens be closely monitored during their pregnancy.
Dr. Sammaritano noted that there are other health problems to keep in mind. “It is important to remember that Sjögren’s patients – more than any other rheumatic disease patients – have the additional risk for neonatal lupus and complete heart block in their infant, since about two-thirds of Sjögren’s patients are positive for anti-Ro/SSA antibody,” she said. “This is a distinct issue related to the presence of this antibody alone and not specifically related to the underlying diagnosis. In clinical practice, positive anti-Ro/SSA antibody is often the main reason for counseling, monitoring, and even recommending therapy (hydroxychloroquine) in these patients.”
The study received funding from Lupus France, the France Association of Scleroderma, and the Association Gougerot Sjögren, among others. Dr. Sammaritano reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Endobronchial valves: Sustained improvement in emphysema
WASHINGTON – based on data from 174 individuals.
One-way endobronchial valves demonstrated benefits for patients with severe emphysema over a 12-month period in the EMPROVE trial, according to Gerard J. Criner, MD, of Temple University, Philadelphia, and colleagues.
Five-year results from the EMPROVE study were presented in a poster session at the American Thoracic Society’s international conference.
The initial EMPROVE trial demonstrated safety and efficacy of the Spiration Valve System (SVS) over 12 months. However, data on the long-term benefits of one-way endobronchial values are limited, the researchers wrote.
The valve was designed for use in selected areas of the bronchial airways and features a flexible umbrella that allows air and mucus to clear from treated airways while blocking inspired air flow to areas of the lungs affected by disease, the researchers explained in the poster.
Dr. Criner and colleagues assessed 172 patients who were randomly assigned to treatment with a one-way valve system (113 patients) or a control group (59 patients).
Participants were evaluated at 1, 3, 6, and 12 months, then annually for 5 years.
The primary efficacy outcome was lung function, measured by forced expiratory volume per second (FEV1). At five years, the FEV1 values improved by 0.1098 liters in the treatment group (P < .001). Treated patients and controls experienced decreased FEV1 at a rate of 0.0440 liters per year from baseline, a significant difference (P < .001). Assuming a steady rate of disease progression, “the treatment group gained approximately 2.5 years of FEV1 improvement immediately following SVS treatment, which was maintained, compared to controls,” the researchers noted in their abstract.
Serious adverse events were assessed from 6 months to 5 years (352.7 patient-years) for treated patients and from 6 months to 2 years (72.9 patient-years) for controls.
Overall, 210 SAEs occurred in the treatment group and 35 occurred in controls, for rates of 0.60 and 0.48, respectively (P = .201). The most common SAEs in the treatment and control groups were COPD exacerbations, pneumothorax, and death.
The results suggest that the FEV1 improvements seen in patients with severe emphysema after one-way endobronchial value placement compared with usual care are enduring after 5 years, with no significant changes in safety, the researchers concluded.
The original EMPROVE study was supported by Olympus Respiratory America, a part of Olympus Corporation and the developer of the Spiration Valve System. Results of the original study were published in the American Journal of Respiratory and Critical Care Medicine. Dr. Criner is associate editor of the American Journal of Respiratory and Critical Care Medicine. His participation complies with American Thoracic Society requirements for recusal from review and decisions for authored works.
A version of this article first appeared on Medscape.com.
WASHINGTON – based on data from 174 individuals.
One-way endobronchial valves demonstrated benefits for patients with severe emphysema over a 12-month period in the EMPROVE trial, according to Gerard J. Criner, MD, of Temple University, Philadelphia, and colleagues.
Five-year results from the EMPROVE study were presented in a poster session at the American Thoracic Society’s international conference.
The initial EMPROVE trial demonstrated safety and efficacy of the Spiration Valve System (SVS) over 12 months. However, data on the long-term benefits of one-way endobronchial values are limited, the researchers wrote.
The valve was designed for use in selected areas of the bronchial airways and features a flexible umbrella that allows air and mucus to clear from treated airways while blocking inspired air flow to areas of the lungs affected by disease, the researchers explained in the poster.
Dr. Criner and colleagues assessed 172 patients who were randomly assigned to treatment with a one-way valve system (113 patients) or a control group (59 patients).
Participants were evaluated at 1, 3, 6, and 12 months, then annually for 5 years.
The primary efficacy outcome was lung function, measured by forced expiratory volume per second (FEV1). At five years, the FEV1 values improved by 0.1098 liters in the treatment group (P < .001). Treated patients and controls experienced decreased FEV1 at a rate of 0.0440 liters per year from baseline, a significant difference (P < .001). Assuming a steady rate of disease progression, “the treatment group gained approximately 2.5 years of FEV1 improvement immediately following SVS treatment, which was maintained, compared to controls,” the researchers noted in their abstract.
Serious adverse events were assessed from 6 months to 5 years (352.7 patient-years) for treated patients and from 6 months to 2 years (72.9 patient-years) for controls.
Overall, 210 SAEs occurred in the treatment group and 35 occurred in controls, for rates of 0.60 and 0.48, respectively (P = .201). The most common SAEs in the treatment and control groups were COPD exacerbations, pneumothorax, and death.
The results suggest that the FEV1 improvements seen in patients with severe emphysema after one-way endobronchial value placement compared with usual care are enduring after 5 years, with no significant changes in safety, the researchers concluded.
The original EMPROVE study was supported by Olympus Respiratory America, a part of Olympus Corporation and the developer of the Spiration Valve System. Results of the original study were published in the American Journal of Respiratory and Critical Care Medicine. Dr. Criner is associate editor of the American Journal of Respiratory and Critical Care Medicine. His participation complies with American Thoracic Society requirements for recusal from review and decisions for authored works.
A version of this article first appeared on Medscape.com.
WASHINGTON – based on data from 174 individuals.
One-way endobronchial valves demonstrated benefits for patients with severe emphysema over a 12-month period in the EMPROVE trial, according to Gerard J. Criner, MD, of Temple University, Philadelphia, and colleagues.
Five-year results from the EMPROVE study were presented in a poster session at the American Thoracic Society’s international conference.
The initial EMPROVE trial demonstrated safety and efficacy of the Spiration Valve System (SVS) over 12 months. However, data on the long-term benefits of one-way endobronchial values are limited, the researchers wrote.
The valve was designed for use in selected areas of the bronchial airways and features a flexible umbrella that allows air and mucus to clear from treated airways while blocking inspired air flow to areas of the lungs affected by disease, the researchers explained in the poster.
Dr. Criner and colleagues assessed 172 patients who were randomly assigned to treatment with a one-way valve system (113 patients) or a control group (59 patients).
Participants were evaluated at 1, 3, 6, and 12 months, then annually for 5 years.
The primary efficacy outcome was lung function, measured by forced expiratory volume per second (FEV1). At five years, the FEV1 values improved by 0.1098 liters in the treatment group (P < .001). Treated patients and controls experienced decreased FEV1 at a rate of 0.0440 liters per year from baseline, a significant difference (P < .001). Assuming a steady rate of disease progression, “the treatment group gained approximately 2.5 years of FEV1 improvement immediately following SVS treatment, which was maintained, compared to controls,” the researchers noted in their abstract.
Serious adverse events were assessed from 6 months to 5 years (352.7 patient-years) for treated patients and from 6 months to 2 years (72.9 patient-years) for controls.
Overall, 210 SAEs occurred in the treatment group and 35 occurred in controls, for rates of 0.60 and 0.48, respectively (P = .201). The most common SAEs in the treatment and control groups were COPD exacerbations, pneumothorax, and death.
The results suggest that the FEV1 improvements seen in patients with severe emphysema after one-way endobronchial value placement compared with usual care are enduring after 5 years, with no significant changes in safety, the researchers concluded.
The original EMPROVE study was supported by Olympus Respiratory America, a part of Olympus Corporation and the developer of the Spiration Valve System. Results of the original study were published in the American Journal of Respiratory and Critical Care Medicine. Dr. Criner is associate editor of the American Journal of Respiratory and Critical Care Medicine. His participation complies with American Thoracic Society requirements for recusal from review and decisions for authored works.
A version of this article first appeared on Medscape.com.
AT ATS 2023
Standard measure may underestimate OSA in Black patients
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
FROM ATS 2023


















