User login
Legal Issues for the Gastroenterologist: Part II
In the previous issue of The New Gastroenterologist, we discussed statistics and the basis on which most gastroenterologists are sued as well as what you can do to minimize this risk. In this second article, we discuss steps to assist in your defense in the event you have been sued. The following suggestions are based on our experience as defense attorneys who practice in the arena of medical malpractice.
Do not, under any circumstances, add or alter the plaintiff’s medical records. Although you have continued access to electronic medical records, accessing or altering these documents leaves an electronic trail. Attorneys are now frequently requesting an “audit trail” during discovery, which shows who and when someone accessed or altered relevant medical records. Additionally, it is likely that the plaintiff’s counsel has already obtained and reviewed records for their client. As such, counsel will notice any alterations and will require an explanation as to the same. If you did alter any medical records, it is important that you notify your attorney about the specifics of such.
After you have secured an attorney, it is critical that you arrange a meeting to develop a positive relationship early in the litigation process. This is important for many reasons. A medical malpractice case can be a long and arduous process which requires that you be involved with your attorney during the course of the litigation. For the attorney-client relationship to be successful, it is imperative that you know and feel comfortable with your attorney and develop confidence and trust in her. Without this trust, it will be difficult for you to accept various decisions or suggestions that the attorney believes are in your best interest. Conversely, the attorney should get to know you and understand your background, as this will assist in your representation.
A good relationship with you will also aid your attorney in educating herself on medical concepts relating to your case. Remember, your attorney most likely has not attended medical school and many of the medical concepts will initially be new to her. By the time trial arrives, however, your attorney will be very familiar with the medical issues in your case. This learning process can be expedited with your assistance and research.
Your deposition
At some point during the lawsuit, the plaintiff’s attorney will take your deposition. The plaintiff’s attorney will strive to obtain concessions that establish the standard of care, breach of the standard, causation, and damages. Your deposition is not the time for you to provide explanations. It is the time for you to concisely answer specific questions posed by counsel without volunteering any additional information. Ultimately, trials build on what occurs during depositions. Preparation is key. Be open to advice or criticisms from your lawyer. Try to eliminate any quirks or habits that interfere with the substance of your testimony or perceived credibility. A deposition is not a casual conversation, nor is it a test of your memory. Limit your answers to personal knowledge; never guess or speculate. If you do not know the answer to a question, or do not remember something, it is perfectly acceptable for you to say so. Only answer questions that you understand. You are allowed to ask the plaintiff’s counsel to repeat or rephrase questions.
Finally, and most importantly, always tell the truth. Discuss any anticipated issues or concerns with your lawyer before your deposition.
Preparing for trial
Conclusion
In summary, remember that there are things you can do both before and after you are sued to minimize litigation and its impact. As mentioned previously, before a lawsuit, and as a regular part of your practice, it is important that you stay current with medical advances, that you take the time to create a relationship with your patients involving quality communication, and that you thoroughly and legibly document all aspects of care provided. After a suit is filed against you, make sure you notify your insurer immediately, do not alter any records or discuss the case with anyone other than your lawyer or spouse, and do all you can to create a productive and honest relationship with your lawyer. This relationship will be invaluable as you do the difficult and time-consuming work of preparing for your deposition and trial, and it can help you endure and successfully navigate the litigation process.
The Importance of Follow-Up: Further Advice on How to Decrease the Risk of Being Sued
A common basis for establishing a malpractice liability claim against a physician is the failure to follow-up or track a patient’s test results. In today’s world, there is an increasing number of moving parts involved in any given patient’s care. A particular patient may treat with numerous physicians, all of whom use different record systems. Electronic medical record systems have made records more accessible and easier to track, but they also present a new set of challenges.
Every physician needs to determine how they plan to track test results. The ideal system would allow a physician to quickly get back any lab or diagnostic test that he or she orders. All staff members should know how the physician’s system works. Otherwise, test results might accidentally be filed before the physician reviews them or a miscommunication could prevent test results from being delivered. Whatever choice of system, it is key to follow and effectively use the program every time.
Additionally, it can be beneficial to let the patient know when he or she can expect to hear about their results, as failure to keep the patient reasonably informed can create a new set of patient concerns and anxiety. Ultimately, establishing a well-defined system for record-tracking can help physicians avoid malpractice liability claims because of a failure to follow-up.
In the previous issue of The New Gastroenterologist, we discussed statistics and the basis on which most gastroenterologists are sued as well as what you can do to minimize this risk. In this second article, we discuss steps to assist in your defense in the event you have been sued. The following suggestions are based on our experience as defense attorneys who practice in the arena of medical malpractice.
Do not, under any circumstances, add or alter the plaintiff’s medical records. Although you have continued access to electronic medical records, accessing or altering these documents leaves an electronic trail. Attorneys are now frequently requesting an “audit trail” during discovery, which shows who and when someone accessed or altered relevant medical records. Additionally, it is likely that the plaintiff’s counsel has already obtained and reviewed records for their client. As such, counsel will notice any alterations and will require an explanation as to the same. If you did alter any medical records, it is important that you notify your attorney about the specifics of such.
After you have secured an attorney, it is critical that you arrange a meeting to develop a positive relationship early in the litigation process. This is important for many reasons. A medical malpractice case can be a long and arduous process which requires that you be involved with your attorney during the course of the litigation. For the attorney-client relationship to be successful, it is imperative that you know and feel comfortable with your attorney and develop confidence and trust in her. Without this trust, it will be difficult for you to accept various decisions or suggestions that the attorney believes are in your best interest. Conversely, the attorney should get to know you and understand your background, as this will assist in your representation.
A good relationship with you will also aid your attorney in educating herself on medical concepts relating to your case. Remember, your attorney most likely has not attended medical school and many of the medical concepts will initially be new to her. By the time trial arrives, however, your attorney will be very familiar with the medical issues in your case. This learning process can be expedited with your assistance and research.
Your deposition
At some point during the lawsuit, the plaintiff’s attorney will take your deposition. The plaintiff’s attorney will strive to obtain concessions that establish the standard of care, breach of the standard, causation, and damages. Your deposition is not the time for you to provide explanations. It is the time for you to concisely answer specific questions posed by counsel without volunteering any additional information. Ultimately, trials build on what occurs during depositions. Preparation is key. Be open to advice or criticisms from your lawyer. Try to eliminate any quirks or habits that interfere with the substance of your testimony or perceived credibility. A deposition is not a casual conversation, nor is it a test of your memory. Limit your answers to personal knowledge; never guess or speculate. If you do not know the answer to a question, or do not remember something, it is perfectly acceptable for you to say so. Only answer questions that you understand. You are allowed to ask the plaintiff’s counsel to repeat or rephrase questions.
Finally, and most importantly, always tell the truth. Discuss any anticipated issues or concerns with your lawyer before your deposition.
Preparing for trial
Conclusion
In summary, remember that there are things you can do both before and after you are sued to minimize litigation and its impact. As mentioned previously, before a lawsuit, and as a regular part of your practice, it is important that you stay current with medical advances, that you take the time to create a relationship with your patients involving quality communication, and that you thoroughly and legibly document all aspects of care provided. After a suit is filed against you, make sure you notify your insurer immediately, do not alter any records or discuss the case with anyone other than your lawyer or spouse, and do all you can to create a productive and honest relationship with your lawyer. This relationship will be invaluable as you do the difficult and time-consuming work of preparing for your deposition and trial, and it can help you endure and successfully navigate the litigation process.
The Importance of Follow-Up: Further Advice on How to Decrease the Risk of Being Sued
A common basis for establishing a malpractice liability claim against a physician is the failure to follow-up or track a patient’s test results. In today’s world, there is an increasing number of moving parts involved in any given patient’s care. A particular patient may treat with numerous physicians, all of whom use different record systems. Electronic medical record systems have made records more accessible and easier to track, but they also present a new set of challenges.
Every physician needs to determine how they plan to track test results. The ideal system would allow a physician to quickly get back any lab or diagnostic test that he or she orders. All staff members should know how the physician’s system works. Otherwise, test results might accidentally be filed before the physician reviews them or a miscommunication could prevent test results from being delivered. Whatever choice of system, it is key to follow and effectively use the program every time.
Additionally, it can be beneficial to let the patient know when he or she can expect to hear about their results, as failure to keep the patient reasonably informed can create a new set of patient concerns and anxiety. Ultimately, establishing a well-defined system for record-tracking can help physicians avoid malpractice liability claims because of a failure to follow-up.
In the previous issue of The New Gastroenterologist, we discussed statistics and the basis on which most gastroenterologists are sued as well as what you can do to minimize this risk. In this second article, we discuss steps to assist in your defense in the event you have been sued. The following suggestions are based on our experience as defense attorneys who practice in the arena of medical malpractice.
Do not, under any circumstances, add or alter the plaintiff’s medical records. Although you have continued access to electronic medical records, accessing or altering these documents leaves an electronic trail. Attorneys are now frequently requesting an “audit trail” during discovery, which shows who and when someone accessed or altered relevant medical records. Additionally, it is likely that the plaintiff’s counsel has already obtained and reviewed records for their client. As such, counsel will notice any alterations and will require an explanation as to the same. If you did alter any medical records, it is important that you notify your attorney about the specifics of such.
After you have secured an attorney, it is critical that you arrange a meeting to develop a positive relationship early in the litigation process. This is important for many reasons. A medical malpractice case can be a long and arduous process which requires that you be involved with your attorney during the course of the litigation. For the attorney-client relationship to be successful, it is imperative that you know and feel comfortable with your attorney and develop confidence and trust in her. Without this trust, it will be difficult for you to accept various decisions or suggestions that the attorney believes are in your best interest. Conversely, the attorney should get to know you and understand your background, as this will assist in your representation.
A good relationship with you will also aid your attorney in educating herself on medical concepts relating to your case. Remember, your attorney most likely has not attended medical school and many of the medical concepts will initially be new to her. By the time trial arrives, however, your attorney will be very familiar with the medical issues in your case. This learning process can be expedited with your assistance and research.
Your deposition
At some point during the lawsuit, the plaintiff’s attorney will take your deposition. The plaintiff’s attorney will strive to obtain concessions that establish the standard of care, breach of the standard, causation, and damages. Your deposition is not the time for you to provide explanations. It is the time for you to concisely answer specific questions posed by counsel without volunteering any additional information. Ultimately, trials build on what occurs during depositions. Preparation is key. Be open to advice or criticisms from your lawyer. Try to eliminate any quirks or habits that interfere with the substance of your testimony or perceived credibility. A deposition is not a casual conversation, nor is it a test of your memory. Limit your answers to personal knowledge; never guess or speculate. If you do not know the answer to a question, or do not remember something, it is perfectly acceptable for you to say so. Only answer questions that you understand. You are allowed to ask the plaintiff’s counsel to repeat or rephrase questions.
Finally, and most importantly, always tell the truth. Discuss any anticipated issues or concerns with your lawyer before your deposition.
Preparing for trial
Conclusion
In summary, remember that there are things you can do both before and after you are sued to minimize litigation and its impact. As mentioned previously, before a lawsuit, and as a regular part of your practice, it is important that you stay current with medical advances, that you take the time to create a relationship with your patients involving quality communication, and that you thoroughly and legibly document all aspects of care provided. After a suit is filed against you, make sure you notify your insurer immediately, do not alter any records or discuss the case with anyone other than your lawyer or spouse, and do all you can to create a productive and honest relationship with your lawyer. This relationship will be invaluable as you do the difficult and time-consuming work of preparing for your deposition and trial, and it can help you endure and successfully navigate the litigation process.
The Importance of Follow-Up: Further Advice on How to Decrease the Risk of Being Sued
A common basis for establishing a malpractice liability claim against a physician is the failure to follow-up or track a patient’s test results. In today’s world, there is an increasing number of moving parts involved in any given patient’s care. A particular patient may treat with numerous physicians, all of whom use different record systems. Electronic medical record systems have made records more accessible and easier to track, but they also present a new set of challenges.
Every physician needs to determine how they plan to track test results. The ideal system would allow a physician to quickly get back any lab or diagnostic test that he or she orders. All staff members should know how the physician’s system works. Otherwise, test results might accidentally be filed before the physician reviews them or a miscommunication could prevent test results from being delivered. Whatever choice of system, it is key to follow and effectively use the program every time.
Additionally, it can be beneficial to let the patient know when he or she can expect to hear about their results, as failure to keep the patient reasonably informed can create a new set of patient concerns and anxiety. Ultimately, establishing a well-defined system for record-tracking can help physicians avoid malpractice liability claims because of a failure to follow-up.
The Light at the End of the Tunnel: Recent Advances in Endoscopic Retrograde Cholangiopancreatograpy
Introduction
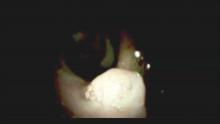
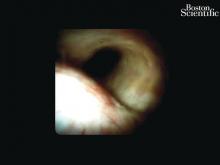
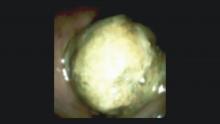
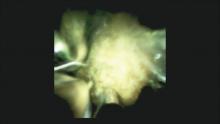
Direct visualization of the biliary ductal system is quickly gaining importance among gastroenterologists. Since the inception of cholangioscopy in the 1970s, the technology has progressed, allowing for ease of use, better visualization, and a growing number of indications. Conventional endoscopic retrograde cholangiopancreatography (ERCP) is successful for removal of bile duct stones (with success rates over 90%);1 however, its use in the evaluation of potential biliary neoplasia has been somewhat disappointing. The diagnostic yield of ERCP-guided biliary brushings can range from 30% to 40%.2-4 An alternative to ERCP-guided biliary brushings for biliary strictures is endoscopic ultrasound (EUS)-directed fine needle aspiration (FNA), but the reported sensitivity remains poor, ranging from 43% to 77% with negative predictive values of less than 30%.5-7 These results leave much to be desired for diagnostic yield.
Clinical indications
DSOCP appears to have improved accuracy over fiberoptic equipment. In a recent multicenter observational study in patients undergoing digital cholangioscopy, the guided biopsies resulted in adequate tissue for histologic evaluation in 98% of patients. In addition, the sensitivity and specificity of digital cholangioscope-guided biopsies for diagnosis of malignancy was 85% and 100%, respectively.11
Other less common diagnostic indications for DSOCP include evaluation of cystic lesions of the biliary tract, verifying clearance of bile duct stones, bile duct ischemia evaluation after liver transplantation, hemobilia evaluation, removal of a bile duct foreign body, and evaluation of bile duct involvement in the presence of an ampullary adenoma.3,14,15,20,26,27
Risks and complications
Conclusions
Direct visualization of the biliary and pancreatic ductal system with fiber-optic and now digital-based platforms have greatly expanded the diagnostic and therapeutic capabilities available to gastroenterologists in the diagnosis and management of biliary and pancreatic disorders. The digital single-operator cholangiopancreatascope system offers greater diagnostic yield of pancreaticobiliary disorders over conventional diagnostic sampling techniques. In addition, direct visualization has expanded our therapeutic ability in complex stone disease allowing laser-based therapies that are not available with traditional fluoroscopic based techniques. Cholangiopancreatoscopic techniques and indications are rapidly expanding and will continue to expand the diagnostic and therapeutic armamentarium available to gastroenterologists.
Dr. Sonnier is a general gastroenterology fellow, division of gastroenterology, University of South Alabama. Dr. Mizrahi is director of advanced endoscopy, division of gastroenterology, University of South Alabama. Dr. Pleskow, is clinical chief, department of gastroenterology, Beth Israel Deaconess Medical Center, and associate professor of medicine, Harvard Medical School, Boston. Dr. Sonnier and Dr. Mizrahi have no conflicts of interest. Dr. Pleskow serves as a consultant to Boston Scientific.
References
1. Cohen S., et al. Gastrointest Endosc. 2002;56:803–9
2. Lee J.G., et al. Am J Gastroenterol. 1995;90:722-6.
3. De Bellis M., et al. Gastrointest Endosc. 2003;58:176-82
4. Fritcher E.G., et al. Gastroenterology. 2009;136:2180-6.
5. Rosch T., et al. Gastrointest Endosc. 2004;60:390-6.
6. Byrne M.F., et al. Endoscopy. 2004;36:715-9.
7. DeWitt J., et al. Gastrointest Endosc. 2006;64:325-33.
8. Rosch W., Endoscopy. 1976;8:172-5.
9. Takekoshi T., Takagi K. Gastrointest Endosc. 1975;17:678-83.
10. Chen Y.K. Gastrointest Endosc 2007;65:303-11.
11. Navaneethan U., et al. Gastrointest Endosc 2016;84:649-55.
12. Navaneethan U., et al. Gastrointest Endosc 2015;82: 608-14.
13. Chen Y.K., Pleskow DK. Gastrointest Endosc. 2007;65:832-41.
14. Draganov P.V., et al. Gastrointest Endosc. 2011;73:971-9.
15. Ramchandani M., et al. Gastrointest Endosc. 2011;74:511-9.
16. Chen Y.K., et al. Gastrointest Endosc. 2011;74:805-14.
17. Draganov P.V., et al. Gastrointest Endosc. 2012;75:347-53.
18. Classen M., et al. Endoscopy 1988;20:21-6.
19. Parsi M.A., et al. Gastrointest Endosc 2008;67:AB102.
20. Fishman D.S., et al. World J Gastroenterol. 2009;15:1353-8.
21. Maydeo A., et al. Gastrointest Endosc. 2011;74:1308-14.
22. Yamao K., et al. Gastrointest Endosc 2003;57:205-9.
23. Hara T., et al. Gastroenterology 2002;122:34-43.
24. Rösch T., et al. Endoscopy. 2002;34:765–71.
25. Bekkali N.L., et al. Pancreas. 2017;46:528-30.
26. Adwan H., et al. Dig Endosc. 2011;23:199-200.
27. Ransibrahmanakul K., et al. Clin Gastroenterol Hepatol. 2010;8:e9.
28. Pereira P., et al. J Gastrointestin Liver Dis, June 2017;Vol. 26(No 2):165-70.
29. Kawakubo K., et al. Endoscopy 2011;43:E241-2.
Introduction
Direct visualization of the biliary ductal system is quickly gaining importance among gastroenterologists. Since the inception of cholangioscopy in the 1970s, the technology has progressed, allowing for ease of use, better visualization, and a growing number of indications. Conventional endoscopic retrograde cholangiopancreatography (ERCP) is successful for removal of bile duct stones (with success rates over 90%);1 however, its use in the evaluation of potential biliary neoplasia has been somewhat disappointing. The diagnostic yield of ERCP-guided biliary brushings can range from 30% to 40%.2-4 An alternative to ERCP-guided biliary brushings for biliary strictures is endoscopic ultrasound (EUS)-directed fine needle aspiration (FNA), but the reported sensitivity remains poor, ranging from 43% to 77% with negative predictive values of less than 30%.5-7 These results leave much to be desired for diagnostic yield.
Clinical indications
DSOCP appears to have improved accuracy over fiberoptic equipment. In a recent multicenter observational study in patients undergoing digital cholangioscopy, the guided biopsies resulted in adequate tissue for histologic evaluation in 98% of patients. In addition, the sensitivity and specificity of digital cholangioscope-guided biopsies for diagnosis of malignancy was 85% and 100%, respectively.11
Other less common diagnostic indications for DSOCP include evaluation of cystic lesions of the biliary tract, verifying clearance of bile duct stones, bile duct ischemia evaluation after liver transplantation, hemobilia evaluation, removal of a bile duct foreign body, and evaluation of bile duct involvement in the presence of an ampullary adenoma.3,14,15,20,26,27
Risks and complications
Conclusions
Direct visualization of the biliary and pancreatic ductal system with fiber-optic and now digital-based platforms have greatly expanded the diagnostic and therapeutic capabilities available to gastroenterologists in the diagnosis and management of biliary and pancreatic disorders. The digital single-operator cholangiopancreatascope system offers greater diagnostic yield of pancreaticobiliary disorders over conventional diagnostic sampling techniques. In addition, direct visualization has expanded our therapeutic ability in complex stone disease allowing laser-based therapies that are not available with traditional fluoroscopic based techniques. Cholangiopancreatoscopic techniques and indications are rapidly expanding and will continue to expand the diagnostic and therapeutic armamentarium available to gastroenterologists.
Dr. Sonnier is a general gastroenterology fellow, division of gastroenterology, University of South Alabama. Dr. Mizrahi is director of advanced endoscopy, division of gastroenterology, University of South Alabama. Dr. Pleskow, is clinical chief, department of gastroenterology, Beth Israel Deaconess Medical Center, and associate professor of medicine, Harvard Medical School, Boston. Dr. Sonnier and Dr. Mizrahi have no conflicts of interest. Dr. Pleskow serves as a consultant to Boston Scientific.
References
1. Cohen S., et al. Gastrointest Endosc. 2002;56:803–9
2. Lee J.G., et al. Am J Gastroenterol. 1995;90:722-6.
3. De Bellis M., et al. Gastrointest Endosc. 2003;58:176-82
4. Fritcher E.G., et al. Gastroenterology. 2009;136:2180-6.
5. Rosch T., et al. Gastrointest Endosc. 2004;60:390-6.
6. Byrne M.F., et al. Endoscopy. 2004;36:715-9.
7. DeWitt J., et al. Gastrointest Endosc. 2006;64:325-33.
8. Rosch W., Endoscopy. 1976;8:172-5.
9. Takekoshi T., Takagi K. Gastrointest Endosc. 1975;17:678-83.
10. Chen Y.K. Gastrointest Endosc 2007;65:303-11.
11. Navaneethan U., et al. Gastrointest Endosc 2016;84:649-55.
12. Navaneethan U., et al. Gastrointest Endosc 2015;82: 608-14.
13. Chen Y.K., Pleskow DK. Gastrointest Endosc. 2007;65:832-41.
14. Draganov P.V., et al. Gastrointest Endosc. 2011;73:971-9.
15. Ramchandani M., et al. Gastrointest Endosc. 2011;74:511-9.
16. Chen Y.K., et al. Gastrointest Endosc. 2011;74:805-14.
17. Draganov P.V., et al. Gastrointest Endosc. 2012;75:347-53.
18. Classen M., et al. Endoscopy 1988;20:21-6.
19. Parsi M.A., et al. Gastrointest Endosc 2008;67:AB102.
20. Fishman D.S., et al. World J Gastroenterol. 2009;15:1353-8.
21. Maydeo A., et al. Gastrointest Endosc. 2011;74:1308-14.
22. Yamao K., et al. Gastrointest Endosc 2003;57:205-9.
23. Hara T., et al. Gastroenterology 2002;122:34-43.
24. Rösch T., et al. Endoscopy. 2002;34:765–71.
25. Bekkali N.L., et al. Pancreas. 2017;46:528-30.
26. Adwan H., et al. Dig Endosc. 2011;23:199-200.
27. Ransibrahmanakul K., et al. Clin Gastroenterol Hepatol. 2010;8:e9.
28. Pereira P., et al. J Gastrointestin Liver Dis, June 2017;Vol. 26(No 2):165-70.
29. Kawakubo K., et al. Endoscopy 2011;43:E241-2.
Introduction
Direct visualization of the biliary ductal system is quickly gaining importance among gastroenterologists. Since the inception of cholangioscopy in the 1970s, the technology has progressed, allowing for ease of use, better visualization, and a growing number of indications. Conventional endoscopic retrograde cholangiopancreatography (ERCP) is successful for removal of bile duct stones (with success rates over 90%);1 however, its use in the evaluation of potential biliary neoplasia has been somewhat disappointing. The diagnostic yield of ERCP-guided biliary brushings can range from 30% to 40%.2-4 An alternative to ERCP-guided biliary brushings for biliary strictures is endoscopic ultrasound (EUS)-directed fine needle aspiration (FNA), but the reported sensitivity remains poor, ranging from 43% to 77% with negative predictive values of less than 30%.5-7 These results leave much to be desired for diagnostic yield.
Clinical indications
DSOCP appears to have improved accuracy over fiberoptic equipment. In a recent multicenter observational study in patients undergoing digital cholangioscopy, the guided biopsies resulted in adequate tissue for histologic evaluation in 98% of patients. In addition, the sensitivity and specificity of digital cholangioscope-guided biopsies for diagnosis of malignancy was 85% and 100%, respectively.11
Other less common diagnostic indications for DSOCP include evaluation of cystic lesions of the biliary tract, verifying clearance of bile duct stones, bile duct ischemia evaluation after liver transplantation, hemobilia evaluation, removal of a bile duct foreign body, and evaluation of bile duct involvement in the presence of an ampullary adenoma.3,14,15,20,26,27
Risks and complications
Conclusions
Direct visualization of the biliary and pancreatic ductal system with fiber-optic and now digital-based platforms have greatly expanded the diagnostic and therapeutic capabilities available to gastroenterologists in the diagnosis and management of biliary and pancreatic disorders. The digital single-operator cholangiopancreatascope system offers greater diagnostic yield of pancreaticobiliary disorders over conventional diagnostic sampling techniques. In addition, direct visualization has expanded our therapeutic ability in complex stone disease allowing laser-based therapies that are not available with traditional fluoroscopic based techniques. Cholangiopancreatoscopic techniques and indications are rapidly expanding and will continue to expand the diagnostic and therapeutic armamentarium available to gastroenterologists.
Dr. Sonnier is a general gastroenterology fellow, division of gastroenterology, University of South Alabama. Dr. Mizrahi is director of advanced endoscopy, division of gastroenterology, University of South Alabama. Dr. Pleskow, is clinical chief, department of gastroenterology, Beth Israel Deaconess Medical Center, and associate professor of medicine, Harvard Medical School, Boston. Dr. Sonnier and Dr. Mizrahi have no conflicts of interest. Dr. Pleskow serves as a consultant to Boston Scientific.
References
1. Cohen S., et al. Gastrointest Endosc. 2002;56:803–9
2. Lee J.G., et al. Am J Gastroenterol. 1995;90:722-6.
3. De Bellis M., et al. Gastrointest Endosc. 2003;58:176-82
4. Fritcher E.G., et al. Gastroenterology. 2009;136:2180-6.
5. Rosch T., et al. Gastrointest Endosc. 2004;60:390-6.
6. Byrne M.F., et al. Endoscopy. 2004;36:715-9.
7. DeWitt J., et al. Gastrointest Endosc. 2006;64:325-33.
8. Rosch W., Endoscopy. 1976;8:172-5.
9. Takekoshi T., Takagi K. Gastrointest Endosc. 1975;17:678-83.
10. Chen Y.K. Gastrointest Endosc 2007;65:303-11.
11. Navaneethan U., et al. Gastrointest Endosc 2016;84:649-55.
12. Navaneethan U., et al. Gastrointest Endosc 2015;82: 608-14.
13. Chen Y.K., Pleskow DK. Gastrointest Endosc. 2007;65:832-41.
14. Draganov P.V., et al. Gastrointest Endosc. 2011;73:971-9.
15. Ramchandani M., et al. Gastrointest Endosc. 2011;74:511-9.
16. Chen Y.K., et al. Gastrointest Endosc. 2011;74:805-14.
17. Draganov P.V., et al. Gastrointest Endosc. 2012;75:347-53.
18. Classen M., et al. Endoscopy 1988;20:21-6.
19. Parsi M.A., et al. Gastrointest Endosc 2008;67:AB102.
20. Fishman D.S., et al. World J Gastroenterol. 2009;15:1353-8.
21. Maydeo A., et al. Gastrointest Endosc. 2011;74:1308-14.
22. Yamao K., et al. Gastrointest Endosc 2003;57:205-9.
23. Hara T., et al. Gastroenterology 2002;122:34-43.
24. Rösch T., et al. Endoscopy. 2002;34:765–71.
25. Bekkali N.L., et al. Pancreas. 2017;46:528-30.
26. Adwan H., et al. Dig Endosc. 2011;23:199-200.
27. Ransibrahmanakul K., et al. Clin Gastroenterol Hepatol. 2010;8:e9.
28. Pereira P., et al. J Gastrointestin Liver Dis, June 2017;Vol. 26(No 2):165-70.
29. Kawakubo K., et al. Endoscopy 2011;43:E241-2.
An Unusual Cause of Recurrent Severe Abdominal Colic
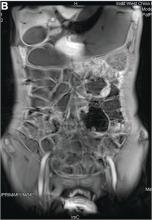
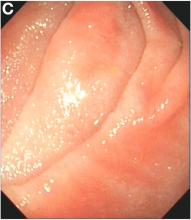
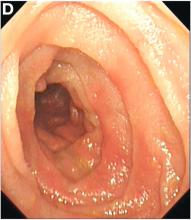
We carefully reviewed the patient’s history and found that he had been using jineijin, a traditional Chinese medicine (TCM) drug, which is made with dried endothelium corneum gigeriae galli (Figure E), at about 500 g/month and squama mantis (a TCM drug, at less than 5 g/month) as dietary supplements for 3 years.
Acknowledgments
We thank Linshen Xie, MD, department of environmental health and occupational diseases, No. 4 West China Teaching Hospital, Sichuan University, for offering some clinical data. We thank the patient for giving permission to share his information.
References
1. National Research Council (US). Safe Drinking Water Committee. Drinking water and health. National Academy Press. Washington, D.C. 1977;1:309.
2. State Administration of Traditional Chinese Medicine. Advanced Textbook on Traditional Chinese Medicine and Pharmacology. New World Press, Beijing. 1995. (vol. 2).
3. Hui Hu, Q.J., Kavan, P. A study of heavy metal pollution in China: Current status, pollution-control policies and countermeasures. Sustainability. 2014;6:5820-38.
This article has an accompanying continuing medical education activity, also eligible for MOC credit, Learning objective: Upon completion of this examination, successful learners will be able to identify the features of lead poisoning.
We carefully reviewed the patient’s history and found that he had been using jineijin, a traditional Chinese medicine (TCM) drug, which is made with dried endothelium corneum gigeriae galli (Figure E), at about 500 g/month and squama mantis (a TCM drug, at less than 5 g/month) as dietary supplements for 3 years.
Acknowledgments
We thank Linshen Xie, MD, department of environmental health and occupational diseases, No. 4 West China Teaching Hospital, Sichuan University, for offering some clinical data. We thank the patient for giving permission to share his information.
References
1. National Research Council (US). Safe Drinking Water Committee. Drinking water and health. National Academy Press. Washington, D.C. 1977;1:309.
2. State Administration of Traditional Chinese Medicine. Advanced Textbook on Traditional Chinese Medicine and Pharmacology. New World Press, Beijing. 1995. (vol. 2).
3. Hui Hu, Q.J., Kavan, P. A study of heavy metal pollution in China: Current status, pollution-control policies and countermeasures. Sustainability. 2014;6:5820-38.
This article has an accompanying continuing medical education activity, also eligible for MOC credit, Learning objective: Upon completion of this examination, successful learners will be able to identify the features of lead poisoning.
We carefully reviewed the patient’s history and found that he had been using jineijin, a traditional Chinese medicine (TCM) drug, which is made with dried endothelium corneum gigeriae galli (Figure E), at about 500 g/month and squama mantis (a TCM drug, at less than 5 g/month) as dietary supplements for 3 years.
Acknowledgments
We thank Linshen Xie, MD, department of environmental health and occupational diseases, No. 4 West China Teaching Hospital, Sichuan University, for offering some clinical data. We thank the patient for giving permission to share his information.
References
1. National Research Council (US). Safe Drinking Water Committee. Drinking water and health. National Academy Press. Washington, D.C. 1977;1:309.
2. State Administration of Traditional Chinese Medicine. Advanced Textbook on Traditional Chinese Medicine and Pharmacology. New World Press, Beijing. 1995. (vol. 2).
3. Hui Hu, Q.J., Kavan, P. A study of heavy metal pollution in China: Current status, pollution-control policies and countermeasures. Sustainability. 2014;6:5820-38.
This article has an accompanying continuing medical education activity, also eligible for MOC credit, Learning objective: Upon completion of this examination, successful learners will be able to identify the features of lead poisoning.
Published previously in Gastroenterology (2016;151:819-21)
Dr. Deng, Dr. Hu, and Dr. Zhang are in the department of gastroenterology, West China Hospital, Sichuan University, Sichuan Province, China.
News from AGA
Advice on Achieving Work-Life Balance
Successfully maintaining a balance between your personal and professional lives is a difficult concept to grasp and practice to enforce. Is this thing called “work-life balance” within reach or just some elusive circumstance people talk about? The AGA Community Early Career Group was the hub for discussions on ways early-career gastroenterologists can modify their day-to-day approach to help prevent burnout.
We consolidated the advice and tips shared into a series of articles and resources to help students, trainees, and early career members get a little closer to balancing their work and professional lives. Here are some highlights:
Choose work-life “integration”
If your career and your personal life were a successful relationship, remember that it’s not always 50/50, and be sure to allow forgiveness and reparation when needed.
Maternity leave
When it comes to starting a family, think about your current training or career climate and how you can make it work. Be transparent with your supervisor so there aren’t any surprises, and plans can be made in advance to cover for your time away. Prepare to be flexible from the beginning.
Learn when to say “no”
Saying “yes” to too many things not only leads to overextending yourself beyond your capabilities, but you could also be losing time on what is important to you. Choose one night a week when you can work late – pack a snack, and give yourself a hard stop the rest of the week. Keep patient documentation as a daytime/work task.
Communication is key
When your partner or spouse is just as busy, it’s important to keep a joint calendar up to date and make plans far in advance. Also, create a routine: Try making time once a month to discuss calendars and anticipated events, face-to-face. When life throws a divot in your path, don’t lose sight of your priorities.
Make time for family and friends
Your career can take over as much of your life as you will allow. Making time for family and friends is rewarding and vacations, staycations, long weekends or even day trips can be great “resets.”
View the tip sheet and other work-life balance resources in the AGA Community Early Career Group library at http://community.gastro.org/WorkLife.
New Clinical Guidelines and Practice Updates
The latest AGA Clinical Practice Guideline, published in Gastroenterology, is on the role of therapeutic drug monitoring (TDM) in the management of IBD. It focuses on the application of TDM for biologic therapy, specifically anti-tumor necrosis factor-α (TNF) agents, and for thiopurines, and addresses questions on the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes.
View the full guideline, technical review, and patient guide at www.gastro.org/guidelines.
In addition to guidelines, please check out the most recent Clinical Practice Updates (CPU) in Gastroenterology and Clinical Gastroenterology and Hepatology (CGH), which are often accompanied by a practice quiz from one of the authors, via the AGA Community. Visit http://community.gastro.org/guidelinecpu to test your knowledge. The most recent CPU, published in the September issue of CGH, focuses on GI side effects related to opioid medications.
Be Part of the Meeting to Transform IBD
If you treat patients with inflammatory bowel disease, conduct IBD research, or plan to pursue a career in IBD, join us for the inaugural Crohn’s & Colitis Congress™, taking place Jan. 18-20, 2018, in Las Vegas, NV. The Crohn’s & Colitis Foundation (formerly CCFA) and AGA have joined together to develop a must-attend program for the entire IBD care team. Expand your knowledge, network with your peers as well as IBD leaders across multiple disciplines, and get inspired to improve care for patients with Crohn’s disease and ulcerative colitis.
You may also be interested in the free precongress workshop – The Lloyd Mayer, MD, Young IBD Investigators Clinical, Basic, and Translational Research Workshop. This half-day precongress workshop is targeted to early-career clinical, basic, and translational researchers as well as senior researchers and will feature a mix of research presentations by young investigator colleagues, keynote presentations, and panel discussion, featuring established IBD researchers. The theme this year is focused around grant proposals and will include two mock grant review sessions.
Learn more about the Crohn’s & Colitis Congress and register: http://crohnscolitiscongress.org.
Advice on Achieving Work-Life Balance
Successfully maintaining a balance between your personal and professional lives is a difficult concept to grasp and practice to enforce. Is this thing called “work-life balance” within reach or just some elusive circumstance people talk about? The AGA Community Early Career Group was the hub for discussions on ways early-career gastroenterologists can modify their day-to-day approach to help prevent burnout.
We consolidated the advice and tips shared into a series of articles and resources to help students, trainees, and early career members get a little closer to balancing their work and professional lives. Here are some highlights:
Choose work-life “integration”
If your career and your personal life were a successful relationship, remember that it’s not always 50/50, and be sure to allow forgiveness and reparation when needed.
Maternity leave
When it comes to starting a family, think about your current training or career climate and how you can make it work. Be transparent with your supervisor so there aren’t any surprises, and plans can be made in advance to cover for your time away. Prepare to be flexible from the beginning.
Learn when to say “no”
Saying “yes” to too many things not only leads to overextending yourself beyond your capabilities, but you could also be losing time on what is important to you. Choose one night a week when you can work late – pack a snack, and give yourself a hard stop the rest of the week. Keep patient documentation as a daytime/work task.
Communication is key
When your partner or spouse is just as busy, it’s important to keep a joint calendar up to date and make plans far in advance. Also, create a routine: Try making time once a month to discuss calendars and anticipated events, face-to-face. When life throws a divot in your path, don’t lose sight of your priorities.
Make time for family and friends
Your career can take over as much of your life as you will allow. Making time for family and friends is rewarding and vacations, staycations, long weekends or even day trips can be great “resets.”
View the tip sheet and other work-life balance resources in the AGA Community Early Career Group library at http://community.gastro.org/WorkLife.
New Clinical Guidelines and Practice Updates
The latest AGA Clinical Practice Guideline, published in Gastroenterology, is on the role of therapeutic drug monitoring (TDM) in the management of IBD. It focuses on the application of TDM for biologic therapy, specifically anti-tumor necrosis factor-α (TNF) agents, and for thiopurines, and addresses questions on the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes.
View the full guideline, technical review, and patient guide at www.gastro.org/guidelines.
In addition to guidelines, please check out the most recent Clinical Practice Updates (CPU) in Gastroenterology and Clinical Gastroenterology and Hepatology (CGH), which are often accompanied by a practice quiz from one of the authors, via the AGA Community. Visit http://community.gastro.org/guidelinecpu to test your knowledge. The most recent CPU, published in the September issue of CGH, focuses on GI side effects related to opioid medications.
Be Part of the Meeting to Transform IBD
If you treat patients with inflammatory bowel disease, conduct IBD research, or plan to pursue a career in IBD, join us for the inaugural Crohn’s & Colitis Congress™, taking place Jan. 18-20, 2018, in Las Vegas, NV. The Crohn’s & Colitis Foundation (formerly CCFA) and AGA have joined together to develop a must-attend program for the entire IBD care team. Expand your knowledge, network with your peers as well as IBD leaders across multiple disciplines, and get inspired to improve care for patients with Crohn’s disease and ulcerative colitis.
You may also be interested in the free precongress workshop – The Lloyd Mayer, MD, Young IBD Investigators Clinical, Basic, and Translational Research Workshop. This half-day precongress workshop is targeted to early-career clinical, basic, and translational researchers as well as senior researchers and will feature a mix of research presentations by young investigator colleagues, keynote presentations, and panel discussion, featuring established IBD researchers. The theme this year is focused around grant proposals and will include two mock grant review sessions.
Learn more about the Crohn’s & Colitis Congress and register: http://crohnscolitiscongress.org.
Advice on Achieving Work-Life Balance
Successfully maintaining a balance between your personal and professional lives is a difficult concept to grasp and practice to enforce. Is this thing called “work-life balance” within reach or just some elusive circumstance people talk about? The AGA Community Early Career Group was the hub for discussions on ways early-career gastroenterologists can modify their day-to-day approach to help prevent burnout.
We consolidated the advice and tips shared into a series of articles and resources to help students, trainees, and early career members get a little closer to balancing their work and professional lives. Here are some highlights:
Choose work-life “integration”
If your career and your personal life were a successful relationship, remember that it’s not always 50/50, and be sure to allow forgiveness and reparation when needed.
Maternity leave
When it comes to starting a family, think about your current training or career climate and how you can make it work. Be transparent with your supervisor so there aren’t any surprises, and plans can be made in advance to cover for your time away. Prepare to be flexible from the beginning.
Learn when to say “no”
Saying “yes” to too many things not only leads to overextending yourself beyond your capabilities, but you could also be losing time on what is important to you. Choose one night a week when you can work late – pack a snack, and give yourself a hard stop the rest of the week. Keep patient documentation as a daytime/work task.
Communication is key
When your partner or spouse is just as busy, it’s important to keep a joint calendar up to date and make plans far in advance. Also, create a routine: Try making time once a month to discuss calendars and anticipated events, face-to-face. When life throws a divot in your path, don’t lose sight of your priorities.
Make time for family and friends
Your career can take over as much of your life as you will allow. Making time for family and friends is rewarding and vacations, staycations, long weekends or even day trips can be great “resets.”
View the tip sheet and other work-life balance resources in the AGA Community Early Career Group library at http://community.gastro.org/WorkLife.
New Clinical Guidelines and Practice Updates
The latest AGA Clinical Practice Guideline, published in Gastroenterology, is on the role of therapeutic drug monitoring (TDM) in the management of IBD. It focuses on the application of TDM for biologic therapy, specifically anti-tumor necrosis factor-α (TNF) agents, and for thiopurines, and addresses questions on the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes.
View the full guideline, technical review, and patient guide at www.gastro.org/guidelines.
In addition to guidelines, please check out the most recent Clinical Practice Updates (CPU) in Gastroenterology and Clinical Gastroenterology and Hepatology (CGH), which are often accompanied by a practice quiz from one of the authors, via the AGA Community. Visit http://community.gastro.org/guidelinecpu to test your knowledge. The most recent CPU, published in the September issue of CGH, focuses on GI side effects related to opioid medications.
Be Part of the Meeting to Transform IBD
If you treat patients with inflammatory bowel disease, conduct IBD research, or plan to pursue a career in IBD, join us for the inaugural Crohn’s & Colitis Congress™, taking place Jan. 18-20, 2018, in Las Vegas, NV. The Crohn’s & Colitis Foundation (formerly CCFA) and AGA have joined together to develop a must-attend program for the entire IBD care team. Expand your knowledge, network with your peers as well as IBD leaders across multiple disciplines, and get inspired to improve care for patients with Crohn’s disease and ulcerative colitis.
You may also be interested in the free precongress workshop – The Lloyd Mayer, MD, Young IBD Investigators Clinical, Basic, and Translational Research Workshop. This half-day precongress workshop is targeted to early-career clinical, basic, and translational researchers as well as senior researchers and will feature a mix of research presentations by young investigator colleagues, keynote presentations, and panel discussion, featuring established IBD researchers. The theme this year is focused around grant proposals and will include two mock grant review sessions.
Learn more about the Crohn’s & Colitis Congress and register: http://crohnscolitiscongress.org.
Cholangiopancreatoscopy
Dear Colleagues,
In this issue of The New Gastroenterologist, the feature article examines recent advances in the field of cholangiopancreatoscopy. In this article, William Sonnier, Meir Mizrahi (University of South Alabama), and Douglas Pleskow (Beth Israel Deaconess) provide a fantastic overview of the technologic advances in the field of cholangiopancreatoscopy as well as the clinical indications for this procedure and the risks involved. Also in this issue, Deborah Fisher (Duke University) and Darrell Gray (Ohio State University) provide advice about how to appropriately and responsibly handle social media. This is an incredibly important topic, given the increasing pervasiveness of social media in many aspects of our personal and professional lives.
Finally, in this issue is the second part in a series on legal issues for gastroenterologists. In this article, which is again authored by a very experienced group of attorneys, many important issues are covered, including what steps should be taken if you are sued, what you should and should not do after being sued, as well as tips on how to best prepare for both deposition and trial.
If there are topics that you would be interested in writing or hearing about in The New Gastroenterologist, please let us know. You can contact me ([email protected]) or the Managing Editor of The New Gastroenterologist, Ryan Farrell ([email protected]).
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Bryson W. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania.
Dear Colleagues,
In this issue of The New Gastroenterologist, the feature article examines recent advances in the field of cholangiopancreatoscopy. In this article, William Sonnier, Meir Mizrahi (University of South Alabama), and Douglas Pleskow (Beth Israel Deaconess) provide a fantastic overview of the technologic advances in the field of cholangiopancreatoscopy as well as the clinical indications for this procedure and the risks involved. Also in this issue, Deborah Fisher (Duke University) and Darrell Gray (Ohio State University) provide advice about how to appropriately and responsibly handle social media. This is an incredibly important topic, given the increasing pervasiveness of social media in many aspects of our personal and professional lives.
Finally, in this issue is the second part in a series on legal issues for gastroenterologists. In this article, which is again authored by a very experienced group of attorneys, many important issues are covered, including what steps should be taken if you are sued, what you should and should not do after being sued, as well as tips on how to best prepare for both deposition and trial.
If there are topics that you would be interested in writing or hearing about in The New Gastroenterologist, please let us know. You can contact me ([email protected]) or the Managing Editor of The New Gastroenterologist, Ryan Farrell ([email protected]).
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Bryson W. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania.
Dear Colleagues,
In this issue of The New Gastroenterologist, the feature article examines recent advances in the field of cholangiopancreatoscopy. In this article, William Sonnier, Meir Mizrahi (University of South Alabama), and Douglas Pleskow (Beth Israel Deaconess) provide a fantastic overview of the technologic advances in the field of cholangiopancreatoscopy as well as the clinical indications for this procedure and the risks involved. Also in this issue, Deborah Fisher (Duke University) and Darrell Gray (Ohio State University) provide advice about how to appropriately and responsibly handle social media. This is an incredibly important topic, given the increasing pervasiveness of social media in many aspects of our personal and professional lives.
Finally, in this issue is the second part in a series on legal issues for gastroenterologists. In this article, which is again authored by a very experienced group of attorneys, many important issues are covered, including what steps should be taken if you are sued, what you should and should not do after being sued, as well as tips on how to best prepare for both deposition and trial.
If there are topics that you would be interested in writing or hearing about in The New Gastroenterologist, please let us know. You can contact me ([email protected]) or the Managing Editor of The New Gastroenterologist, Ryan Farrell ([email protected]).
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Bryson W. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania.
Trustworthy Recommendations: A Closer Look Inside the AGA’s Clinical Guideline Development Process
The AGA understands how important it is for busy physicians to have access to the most trustworthy, actionable, and evidence-based guidelines in order to achieve the highest possible quality of patient care. According to a 2016 survey, AGA members ranked guidelines as the most important of all AGA-specific benefits, giving guidelines an average of 4.61 out of 5 (where 5 was defined as “extremely important”). The AGA’s guidelines landing page (www.gastro.org/guidelines) has long been the most frequently accessed page on the AGA website.
The life cycle of an AGA guideline
In 2010, the AGA Institute officially adopted the GRADE (Grades of Recommendation Assessment, Development, and Evaluation) methodology for the development of all future guidelines. Since the publication of our first GRADE-based guideline in 2013, the AGA has developed and published 12 guidelines with an additional 11 more to be published by 2019. Based on the systematic rigor of the GRADE approach, the AGA’s guideline development process was created to result in clinical recommendations that are not only evidence based but actionable and responsive to varying patient needs and preferences at the point of care.
All told, a single AGA guideline costs around $45,000 and takes approximately 24 months to complete and publish. Currently, the AGA is working to pilot new methods of shortening the time to publication through the development of rapid reviews within a focused topic (e.g., opioid-induced constipation).1 The development of each guideline requires a team of one or more specially trained GRADE methodologists, two or more content experts, a medical librarian, a panel of three or more guideline authors, two AGA staff members, and the Clinical Guidelines Committee Chair.
Determining the focused questions. First, the entire team of physician-authors determines a list of focused questions that the guideline will address. This list of focused questions is translated into a table of Population, Intervention, Comparison, Outcomes (PICOs) that operationalize the general questions into search terms utilized by the medical librarian to run the systematic search as well as define the final scope of the guideline. The focused questions and related PICOs are sent to the Governing Board for review and approval.
Developing the technical review. Over the next several months, the methodologist and content experts meet on a weekly basis to review the search results question-by-question and develop the technical review of evidence that will form the basis of the clinical recommendations. For each PICO, the technical review assesses the entire body of evidence and rates the overall quality of evidence gathered for each outcome related to the PICOs (from “very low” to “low” to “moderate” to “high”).
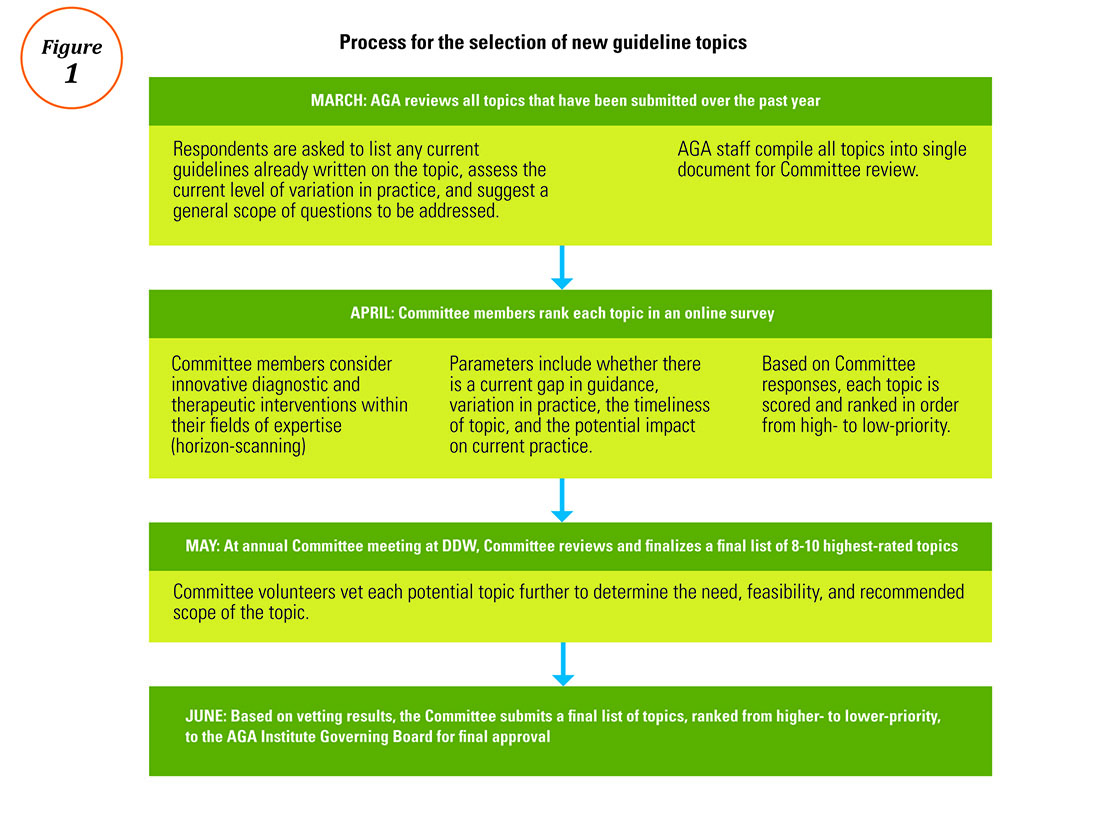
Drafting the clinical recommendations. The technical review presents the findings of the literature along with the authors’ assessment of the evidence quality. At a face-to-face meeting, these results are presented by the technical review authors to the guideline panel, who are responsible for developing the official guideline document. The role of the guideline panel is to understand the quality of evidence and determine an ultimate list of clinical recommendations and assign a strength (strong or conditional) to each recommendation, all while considering important factors such as the balance between benefits and downsides, potential variability in patients’ values and preferences, and impact on resource utilization. Oftentimes, but not always, recommendations based on higher-quality evidence for which most patients would request the recommended course of action translate into strong recommendations. Recommendations based on lower-quality evidence and those for which there is a higher variability in patient values or issues surrounding resource utilization are more likely to be conditional.
In addition to the guideline document, the guideline panel also drafts a Clinical Decision Support Tool, which illustrates the clinical recommendations within a visual algorithm. At the same time, AGA staff draft a patient summary that explains the recommendations in plain language. This summary can be used by physicians to improve clinical communication and shared decision making with their patients.2
Revising the guideline. Each AGA technical review goes through two layers of review: once by an anonymous peer-review panel of three content experts, and again during a 30-day public comment period in which both the technical review and guideline are posted for public input. The authors take all input into consideration while finalizing the documents, which are sent to the Governing Board for final approval. Once approved by the Board, the technical review, guideline, and all related materials are submitted for publication in Gastroenterology. In addition to print publication, each guideline is disseminated on the AGA website and through the official Clinical Guidelines mobile app (available via the App Store and Google Play), which includes interactive versions of the Clinical Decision Support Tools and plain-language summaries that can be sent via e-mail to patients at the point of care. The AGA is currently pursuing future directions for the dissemination and implementation of our guidelines, such as the seamless integration of clinical recommendations into electronic health records to further improve decision making and facilitate quality measurement and improvement.
Conclusion
Not all clinical guidelines are created with equal rigor. Clinicians should examine guidelines closely and consider whether or not they follow the Institute of Medicine’s standards for trustworthy clinical guidelines: Is the focus on transparency? Is a rigorous conflict of interest system in place that eliminates major sources of financial and intellectual conflict? Was an unconflicted GRADE-trained methodologist involved in ensuring that a systematic review process is followed and the method of rating the quality of evidence and strength of recommendation follows published principles? Are the recommendations clear and actionable?3 AGA Institute guidelines are developed with the goal of striking a balance between presenting the highest ideals of evidence-based medicine while remaining responsive to the needs of everyday practitioners dealing with real patients in real clinical settings.
Ms. Siedler is the director of clinical practice at the AGA Institute national office in Bethesda, Md.; Dr. Falck-Ytter is a professor of medicine at Case-Western Reserve University, Cleveland, chair of the AGA Institute Clinical Guidelines Committee, and chief of the division of gastroenterology at the Louis Stokes VA Medical Center in Cleveland. The authors disclose no conflicts of interest.
References
1. Hanson B., Siedler M., Falck-Ytter Y., Sultan S. Introducing the rapid review: How the AGA is working to get trustworthy clinical guidelines to practitioners in less time. AGA Perspectives. 2017; in press.
2. Siedler M., Allen J., Falck-Ytter Y., Weinberg D. AGA clinical practice guidelines: Robust, evidence-based tools for guiding clinical care decisions. Gastroenterology. 2015;149:493-5.
3. Institute of Medicine: Standards for developing trustworthy clinical practice guidelines. Available at http://www.nationalacademies.org/hmd/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust/Standards.aspx. Last accessed May 2017.Process for the selection of new guideline topics
The AGA understands how important it is for busy physicians to have access to the most trustworthy, actionable, and evidence-based guidelines in order to achieve the highest possible quality of patient care. According to a 2016 survey, AGA members ranked guidelines as the most important of all AGA-specific benefits, giving guidelines an average of 4.61 out of 5 (where 5 was defined as “extremely important”). The AGA’s guidelines landing page (www.gastro.org/guidelines) has long been the most frequently accessed page on the AGA website.
The life cycle of an AGA guideline
In 2010, the AGA Institute officially adopted the GRADE (Grades of Recommendation Assessment, Development, and Evaluation) methodology for the development of all future guidelines. Since the publication of our first GRADE-based guideline in 2013, the AGA has developed and published 12 guidelines with an additional 11 more to be published by 2019. Based on the systematic rigor of the GRADE approach, the AGA’s guideline development process was created to result in clinical recommendations that are not only evidence based but actionable and responsive to varying patient needs and preferences at the point of care.
All told, a single AGA guideline costs around $45,000 and takes approximately 24 months to complete and publish. Currently, the AGA is working to pilot new methods of shortening the time to publication through the development of rapid reviews within a focused topic (e.g., opioid-induced constipation).1 The development of each guideline requires a team of one or more specially trained GRADE methodologists, two or more content experts, a medical librarian, a panel of three or more guideline authors, two AGA staff members, and the Clinical Guidelines Committee Chair.
Determining the focused questions. First, the entire team of physician-authors determines a list of focused questions that the guideline will address. This list of focused questions is translated into a table of Population, Intervention, Comparison, Outcomes (PICOs) that operationalize the general questions into search terms utilized by the medical librarian to run the systematic search as well as define the final scope of the guideline. The focused questions and related PICOs are sent to the Governing Board for review and approval.
Developing the technical review. Over the next several months, the methodologist and content experts meet on a weekly basis to review the search results question-by-question and develop the technical review of evidence that will form the basis of the clinical recommendations. For each PICO, the technical review assesses the entire body of evidence and rates the overall quality of evidence gathered for each outcome related to the PICOs (from “very low” to “low” to “moderate” to “high”).

Drafting the clinical recommendations. The technical review presents the findings of the literature along with the authors’ assessment of the evidence quality. At a face-to-face meeting, these results are presented by the technical review authors to the guideline panel, who are responsible for developing the official guideline document. The role of the guideline panel is to understand the quality of evidence and determine an ultimate list of clinical recommendations and assign a strength (strong or conditional) to each recommendation, all while considering important factors such as the balance between benefits and downsides, potential variability in patients’ values and preferences, and impact on resource utilization. Oftentimes, but not always, recommendations based on higher-quality evidence for which most patients would request the recommended course of action translate into strong recommendations. Recommendations based on lower-quality evidence and those for which there is a higher variability in patient values or issues surrounding resource utilization are more likely to be conditional.
In addition to the guideline document, the guideline panel also drafts a Clinical Decision Support Tool, which illustrates the clinical recommendations within a visual algorithm. At the same time, AGA staff draft a patient summary that explains the recommendations in plain language. This summary can be used by physicians to improve clinical communication and shared decision making with their patients.2
Revising the guideline. Each AGA technical review goes through two layers of review: once by an anonymous peer-review panel of three content experts, and again during a 30-day public comment period in which both the technical review and guideline are posted for public input. The authors take all input into consideration while finalizing the documents, which are sent to the Governing Board for final approval. Once approved by the Board, the technical review, guideline, and all related materials are submitted for publication in Gastroenterology. In addition to print publication, each guideline is disseminated on the AGA website and through the official Clinical Guidelines mobile app (available via the App Store and Google Play), which includes interactive versions of the Clinical Decision Support Tools and plain-language summaries that can be sent via e-mail to patients at the point of care. The AGA is currently pursuing future directions for the dissemination and implementation of our guidelines, such as the seamless integration of clinical recommendations into electronic health records to further improve decision making and facilitate quality measurement and improvement.
Conclusion
Not all clinical guidelines are created with equal rigor. Clinicians should examine guidelines closely and consider whether or not they follow the Institute of Medicine’s standards for trustworthy clinical guidelines: Is the focus on transparency? Is a rigorous conflict of interest system in place that eliminates major sources of financial and intellectual conflict? Was an unconflicted GRADE-trained methodologist involved in ensuring that a systematic review process is followed and the method of rating the quality of evidence and strength of recommendation follows published principles? Are the recommendations clear and actionable?3 AGA Institute guidelines are developed with the goal of striking a balance between presenting the highest ideals of evidence-based medicine while remaining responsive to the needs of everyday practitioners dealing with real patients in real clinical settings.
Ms. Siedler is the director of clinical practice at the AGA Institute national office in Bethesda, Md.; Dr. Falck-Ytter is a professor of medicine at Case-Western Reserve University, Cleveland, chair of the AGA Institute Clinical Guidelines Committee, and chief of the division of gastroenterology at the Louis Stokes VA Medical Center in Cleveland. The authors disclose no conflicts of interest.
References
1. Hanson B., Siedler M., Falck-Ytter Y., Sultan S. Introducing the rapid review: How the AGA is working to get trustworthy clinical guidelines to practitioners in less time. AGA Perspectives. 2017; in press.
2. Siedler M., Allen J., Falck-Ytter Y., Weinberg D. AGA clinical practice guidelines: Robust, evidence-based tools for guiding clinical care decisions. Gastroenterology. 2015;149:493-5.
3. Institute of Medicine: Standards for developing trustworthy clinical practice guidelines. Available at http://www.nationalacademies.org/hmd/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust/Standards.aspx. Last accessed May 2017.Process for the selection of new guideline topics
The AGA understands how important it is for busy physicians to have access to the most trustworthy, actionable, and evidence-based guidelines in order to achieve the highest possible quality of patient care. According to a 2016 survey, AGA members ranked guidelines as the most important of all AGA-specific benefits, giving guidelines an average of 4.61 out of 5 (where 5 was defined as “extremely important”). The AGA’s guidelines landing page (www.gastro.org/guidelines) has long been the most frequently accessed page on the AGA website.
The life cycle of an AGA guideline
In 2010, the AGA Institute officially adopted the GRADE (Grades of Recommendation Assessment, Development, and Evaluation) methodology for the development of all future guidelines. Since the publication of our first GRADE-based guideline in 2013, the AGA has developed and published 12 guidelines with an additional 11 more to be published by 2019. Based on the systematic rigor of the GRADE approach, the AGA’s guideline development process was created to result in clinical recommendations that are not only evidence based but actionable and responsive to varying patient needs and preferences at the point of care.
All told, a single AGA guideline costs around $45,000 and takes approximately 24 months to complete and publish. Currently, the AGA is working to pilot new methods of shortening the time to publication through the development of rapid reviews within a focused topic (e.g., opioid-induced constipation).1 The development of each guideline requires a team of one or more specially trained GRADE methodologists, two or more content experts, a medical librarian, a panel of three or more guideline authors, two AGA staff members, and the Clinical Guidelines Committee Chair.
Determining the focused questions. First, the entire team of physician-authors determines a list of focused questions that the guideline will address. This list of focused questions is translated into a table of Population, Intervention, Comparison, Outcomes (PICOs) that operationalize the general questions into search terms utilized by the medical librarian to run the systematic search as well as define the final scope of the guideline. The focused questions and related PICOs are sent to the Governing Board for review and approval.
Developing the technical review. Over the next several months, the methodologist and content experts meet on a weekly basis to review the search results question-by-question and develop the technical review of evidence that will form the basis of the clinical recommendations. For each PICO, the technical review assesses the entire body of evidence and rates the overall quality of evidence gathered for each outcome related to the PICOs (from “very low” to “low” to “moderate” to “high”).

Drafting the clinical recommendations. The technical review presents the findings of the literature along with the authors’ assessment of the evidence quality. At a face-to-face meeting, these results are presented by the technical review authors to the guideline panel, who are responsible for developing the official guideline document. The role of the guideline panel is to understand the quality of evidence and determine an ultimate list of clinical recommendations and assign a strength (strong or conditional) to each recommendation, all while considering important factors such as the balance between benefits and downsides, potential variability in patients’ values and preferences, and impact on resource utilization. Oftentimes, but not always, recommendations based on higher-quality evidence for which most patients would request the recommended course of action translate into strong recommendations. Recommendations based on lower-quality evidence and those for which there is a higher variability in patient values or issues surrounding resource utilization are more likely to be conditional.
In addition to the guideline document, the guideline panel also drafts a Clinical Decision Support Tool, which illustrates the clinical recommendations within a visual algorithm. At the same time, AGA staff draft a patient summary that explains the recommendations in plain language. This summary can be used by physicians to improve clinical communication and shared decision making with their patients.2
Revising the guideline. Each AGA technical review goes through two layers of review: once by an anonymous peer-review panel of three content experts, and again during a 30-day public comment period in which both the technical review and guideline are posted for public input. The authors take all input into consideration while finalizing the documents, which are sent to the Governing Board for final approval. Once approved by the Board, the technical review, guideline, and all related materials are submitted for publication in Gastroenterology. In addition to print publication, each guideline is disseminated on the AGA website and through the official Clinical Guidelines mobile app (available via the App Store and Google Play), which includes interactive versions of the Clinical Decision Support Tools and plain-language summaries that can be sent via e-mail to patients at the point of care. The AGA is currently pursuing future directions for the dissemination and implementation of our guidelines, such as the seamless integration of clinical recommendations into electronic health records to further improve decision making and facilitate quality measurement and improvement.
Conclusion
Not all clinical guidelines are created with equal rigor. Clinicians should examine guidelines closely and consider whether or not they follow the Institute of Medicine’s standards for trustworthy clinical guidelines: Is the focus on transparency? Is a rigorous conflict of interest system in place that eliminates major sources of financial and intellectual conflict? Was an unconflicted GRADE-trained methodologist involved in ensuring that a systematic review process is followed and the method of rating the quality of evidence and strength of recommendation follows published principles? Are the recommendations clear and actionable?3 AGA Institute guidelines are developed with the goal of striking a balance between presenting the highest ideals of evidence-based medicine while remaining responsive to the needs of everyday practitioners dealing with real patients in real clinical settings.
Ms. Siedler is the director of clinical practice at the AGA Institute national office in Bethesda, Md.; Dr. Falck-Ytter is a professor of medicine at Case-Western Reserve University, Cleveland, chair of the AGA Institute Clinical Guidelines Committee, and chief of the division of gastroenterology at the Louis Stokes VA Medical Center in Cleveland. The authors disclose no conflicts of interest.
References
1. Hanson B., Siedler M., Falck-Ytter Y., Sultan S. Introducing the rapid review: How the AGA is working to get trustworthy clinical guidelines to practitioners in less time. AGA Perspectives. 2017; in press.
2. Siedler M., Allen J., Falck-Ytter Y., Weinberg D. AGA clinical practice guidelines: Robust, evidence-based tools for guiding clinical care decisions. Gastroenterology. 2015;149:493-5.
3. Institute of Medicine: Standards for developing trustworthy clinical practice guidelines. Available at http://www.nationalacademies.org/hmd/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust/Standards.aspx. Last accessed May 2017.Process for the selection of new guideline topics
Advice on Choosing Your GI Career Path
Mariam Naveed, MD, opened a discussion in the Early Career Group forum in AGA Community that invited GIs to share how or when they knew which career path was the best fit. Among those sharing their stories were Peter Liang, MD, MPH; Avinash Ketwaroo, MD; Maisa Abdalla, MD, MPH; Tara Altepeter, MD; Elliot Tapper, MD; and Brijen Shah, MD. Their expertise spans across the GI spectrum, including academia, research, drug development, and regulatory science.
For Dr. Liang, the key to succeeding on the research path is to be passionate about your topic(s), enjoy reading and writing, and be able to accept constructive criticism and rejection. Dr. Altepeter encourages all GIs early in their career to be open to exploring a variety of career options, as regulatory science was not a career path she was aware of at the beginning of training.
The conversation continued when trainee and early career members brought their career-specific questions, including the possibility of achieving tenure without publishing.
View a summary of advice shared at http://community.gastro.org/calling. The discussions around finding your GI calling are in the AGA Community Early Career Group, at http://community.gastro.org/EarlyCareerGroup.
Mariam Naveed, MD, opened a discussion in the Early Career Group forum in AGA Community that invited GIs to share how or when they knew which career path was the best fit. Among those sharing their stories were Peter Liang, MD, MPH; Avinash Ketwaroo, MD; Maisa Abdalla, MD, MPH; Tara Altepeter, MD; Elliot Tapper, MD; and Brijen Shah, MD. Their expertise spans across the GI spectrum, including academia, research, drug development, and regulatory science.
For Dr. Liang, the key to succeeding on the research path is to be passionate about your topic(s), enjoy reading and writing, and be able to accept constructive criticism and rejection. Dr. Altepeter encourages all GIs early in their career to be open to exploring a variety of career options, as regulatory science was not a career path she was aware of at the beginning of training.
The conversation continued when trainee and early career members brought their career-specific questions, including the possibility of achieving tenure without publishing.
View a summary of advice shared at http://community.gastro.org/calling. The discussions around finding your GI calling are in the AGA Community Early Career Group, at http://community.gastro.org/EarlyCareerGroup.
Mariam Naveed, MD, opened a discussion in the Early Career Group forum in AGA Community that invited GIs to share how or when they knew which career path was the best fit. Among those sharing their stories were Peter Liang, MD, MPH; Avinash Ketwaroo, MD; Maisa Abdalla, MD, MPH; Tara Altepeter, MD; Elliot Tapper, MD; and Brijen Shah, MD. Their expertise spans across the GI spectrum, including academia, research, drug development, and regulatory science.
For Dr. Liang, the key to succeeding on the research path is to be passionate about your topic(s), enjoy reading and writing, and be able to accept constructive criticism and rejection. Dr. Altepeter encourages all GIs early in their career to be open to exploring a variety of career options, as regulatory science was not a career path she was aware of at the beginning of training.
The conversation continued when trainee and early career members brought their career-specific questions, including the possibility of achieving tenure without publishing.
View a summary of advice shared at http://community.gastro.org/calling. The discussions around finding your GI calling are in the AGA Community Early Career Group, at http://community.gastro.org/EarlyCareerGroup.
Be Kind to Yourself: Preventing Burnout in New GIs Through Self-Compassion
Physician burnout is a growing epidemic, particularly in the early careers of gastroenterologists. Up to 50% of new physicians and trainees experience burnout with the first 3 years of independent practice.1 The negative consequences of burnout are well known – medical errors, depression, substance abuse, and even suicide.2,3 To meet criteria for burnout syndrome (Table 1), one must have two of three core symptoms, often experienced as phases: 1) physical and emotional exhaustion; 2) cynicism and detachment; and 3) feelings of ineffectiveness and lack of accomplishment.4
Emotional exhaustion, one of the earliest symptoms of burnout syndrome was reported to be as high as 63% among gastroenterologists in a survey study I conducted with colleagues a few years ago.5 Similar findings are noted amongst colorectal surgeons.6 We also noted in our study that burnout levels were highest in junior versus senior attendings, with junior attendings reporting more stress related to performing endoscopies and making split-second decisions. Interventional endoscopists may have been disproportionately affected by the latter, reporting that they were more likely to think about possible mistakes they made after work, have difficulty sleeping due to thinking about their day, and have difficulty separating work and personal life.5 Male and female physicians may progress through the phases of burnout differently, with men being more likely to experience cynicism and depersonalization first, followed by fatigue. Men may also not necessarily experience the third phase of feeling ineffective, which can be particularly dangerous because they will continue to push until there is a serious consequence. Women tend to go through all three phases of burnout beginning with emotional exhaustion, with a more rapid progression through the cynicism phase, and may end up spending the majority of their time feeling ineffective and limited in their accomplishments, a recipe for leaving medicine entirely.7
Prevention of burnout through self-compassion
Even though it may sometimes be easy to forget, most of us chose medicine as our profession because of our inherent compassion towards others and desire to care for those in need. But have we properly learned how to apply that same compassion to ourselves?
Self-compassion is one of the primary qualities of a happy, flourishing, resilient individual.8 Self-compassion is a psychological skill that can be applied to feelings of inadequacy, failure, or lack of control and includes: 1) self-kindness, 2) belief in a common humanity, and 3) mindfulness.8 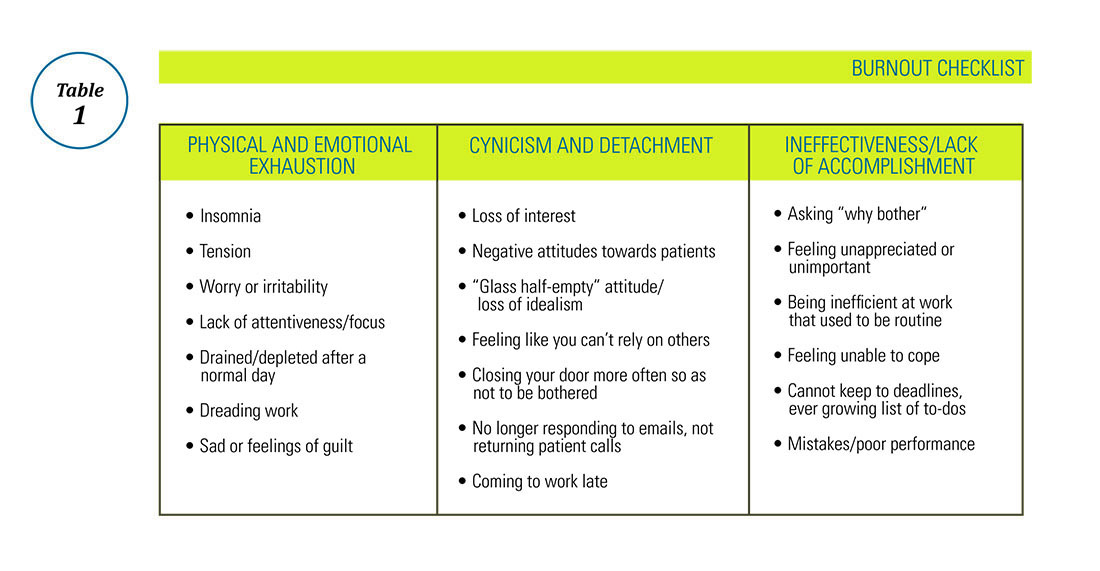
Are you self-compassionate? Take a quiz!
Self-kindness requires us to treat ourselves as kindly as we would a friend or patient in the same situation. We must consciously choose not to use harsh, self-critical language when we make mistakes. We are taught not to berate our trainees for mistakes in the clinical setting – we can be taught not to berate ourselves for shortcomings as well. Self-kindness also requires that we provide ourselves with sympathy when we experience disappointments through no fault of our own (e.g. despite all my best efforts, this clinical initiative failed) and give ourselves the opportunity to nurture and soothe ourselves when we experience pain.6 Belief in a common humanity fosters engagement with others, recognizing that nobody is perfect and that others suffer as well. Isolating ourselves because we feel ashamed, embarrassed, or “crazy” in our experience of a situation only increases our suffering. As we engage with others, we are able to view things from a different perspective and also recognize that others around us have problems too. Indeed, social support may be one of the best buffers against burnout, particularly cynicism.12 A recent meta-analysis concluded that a combination of institutional engagement techniques including reduced hours and support groups as well as access to individual behavioral techniques such as mindfulness could reduce or prevent burnout.13
I have previously commented on the practice of mindfulness in the AGA Community forums and, as a potentially stand-alone component of self-compassion training,14 recommend it here as well. In addition to traditional mindfulness-based stress-reduction courses and mindfulness meditation practice found in many hospitals and community centers, individual meditation focused on loving kindness or gratitude as well as mindful exercises such as writing a self-compassionate letter or statements to yourself can be used to offset burnout in daily life.15 From the perspective of reducing burnout, mindfulness allows us to look at our feelings of cynicism, exhaustion, and inadequacy without judgment, to view them as symptoms rather than ugly truths about ourselves and that rather than avoid or suppress these feelings, to be mindful and compassionate toward them.
Finally, in the spirit of self-compassion, we must not judge ourselves for needing the help of others to navigate adversity – whether that support comes from our personal or professional life, or is provided by a mental health professional, we deserve to be taken care of as much as our patients do.
For more information, please visit the following, helpful resources: www.CenterForMSC.org, www.Self-Compassion.org, and www.MindfulSelfCompassion.org.
Dr. Keefer is director, psychobehavioral research, Icahn School of Medicine at Mount Sinai, division of gastroenterology, New York, N.Y.
References
1. West C.P., Shanafelt T.D., Kolars J.C. JAMA. 2011;306[9]:952-60.
2. Maslach C., Leiter M.P. World Psychiatry. 2016;15[2]:103-11.
3. Ahola K., Honkonen T., Kivimaki M., et al. J Occup Environ Med. 2006;48[10]:1023-30.
4. Ahola K., Honkonen T., Isometsa E., et al. Soc Psychiatry Psychiatr Epidemiol. 2006;41[1]:11-7.
5. Farber B.A. J Clin Psychol. 2000;56[5]:589-94.
6. Keswani R.N., Taft T.H., Cote G.A., Keefer L. Am J Gastroenterol. 2011;106[10]:1734-40.
7. Sharma A., Sharp D.M., Walker L.G., Monson J.R. Psychooncology. 2008;17[6]:570-6.
8. Houkes I., Winants Y., Twellaar M., Verdonk P. BMC Public Health. 2011;11:240.
9. Neff K.D. Hum Dev. 2009;52[4]:211-4.
10. de Vente W., van Amsterdam J.G., Olff M., Kamphuis J.H., Emmelkamp P.M. Biomed Res Int. 2015;2015:431725.
11. Rockliff H., Karl A., McEwan K., Gilbert J., Matos M., Gilbert P. Effects of intranasal oxytocin on ‘compassion focused imagery’. Emotion. 2011;11[6]:1388-96.
12. Porges S.W. Biol Psychol. 2007;74[2]:301-7.
13. Breines J.G., Chen S. Pers Soc Psychol Bull. 2012;38[9]:1133-43.
14. Heffernan M., Quinn G.M.T., Sister R.M., Fitzpatrick JJ. Int J Nurs Pract. 2010;16[4]:366-73.
15. Crocker J., Canevello A. J Pers Soc Psychol. 2008;95[3]:555-75.
16. Thompson G., McBride R.B., Hosford C.C., Halaas G. Teach Learn Med. 2016;28[2]:174-82.
17. Nie Z., Jin Y., He L., et al. Int J Clin Exp Med. 2015;8[10]:19144-9.
18. West C.P., Dyrbye L.N., Erwin P.J., Shanafelt T.D. Lancet. 2016. Nov 5;388(10057)2272-81.
19. Luchterhand C., Rakel D., Haq C., et al. WMJ. 2015;114[3]:105-9.
20. Montero-Marin J., Tops M., Manzanera R, Piva Demarzo MM, Alvarez de Mon M, Garcia-Campayo J. Front Psychol. 2015;6:1895.
Physician burnout is a growing epidemic, particularly in the early careers of gastroenterologists. Up to 50% of new physicians and trainees experience burnout with the first 3 years of independent practice.1 The negative consequences of burnout are well known – medical errors, depression, substance abuse, and even suicide.2,3 To meet criteria for burnout syndrome (Table 1), one must have two of three core symptoms, often experienced as phases: 1) physical and emotional exhaustion; 2) cynicism and detachment; and 3) feelings of ineffectiveness and lack of accomplishment.4
Emotional exhaustion, one of the earliest symptoms of burnout syndrome was reported to be as high as 63% among gastroenterologists in a survey study I conducted with colleagues a few years ago.5 Similar findings are noted amongst colorectal surgeons.6 We also noted in our study that burnout levels were highest in junior versus senior attendings, with junior attendings reporting more stress related to performing endoscopies and making split-second decisions. Interventional endoscopists may have been disproportionately affected by the latter, reporting that they were more likely to think about possible mistakes they made after work, have difficulty sleeping due to thinking about their day, and have difficulty separating work and personal life.5 Male and female physicians may progress through the phases of burnout differently, with men being more likely to experience cynicism and depersonalization first, followed by fatigue. Men may also not necessarily experience the third phase of feeling ineffective, which can be particularly dangerous because they will continue to push until there is a serious consequence. Women tend to go through all three phases of burnout beginning with emotional exhaustion, with a more rapid progression through the cynicism phase, and may end up spending the majority of their time feeling ineffective and limited in their accomplishments, a recipe for leaving medicine entirely.7
Prevention of burnout through self-compassion
Even though it may sometimes be easy to forget, most of us chose medicine as our profession because of our inherent compassion towards others and desire to care for those in need. But have we properly learned how to apply that same compassion to ourselves?
Self-compassion is one of the primary qualities of a happy, flourishing, resilient individual.8 Self-compassion is a psychological skill that can be applied to feelings of inadequacy, failure, or lack of control and includes: 1) self-kindness, 2) belief in a common humanity, and 3) mindfulness.8 
Are you self-compassionate? Take a quiz!
Self-kindness requires us to treat ourselves as kindly as we would a friend or patient in the same situation. We must consciously choose not to use harsh, self-critical language when we make mistakes. We are taught not to berate our trainees for mistakes in the clinical setting – we can be taught not to berate ourselves for shortcomings as well. Self-kindness also requires that we provide ourselves with sympathy when we experience disappointments through no fault of our own (e.g. despite all my best efforts, this clinical initiative failed) and give ourselves the opportunity to nurture and soothe ourselves when we experience pain.6 Belief in a common humanity fosters engagement with others, recognizing that nobody is perfect and that others suffer as well. Isolating ourselves because we feel ashamed, embarrassed, or “crazy” in our experience of a situation only increases our suffering. As we engage with others, we are able to view things from a different perspective and also recognize that others around us have problems too. Indeed, social support may be one of the best buffers against burnout, particularly cynicism.12 A recent meta-analysis concluded that a combination of institutional engagement techniques including reduced hours and support groups as well as access to individual behavioral techniques such as mindfulness could reduce or prevent burnout.13
I have previously commented on the practice of mindfulness in the AGA Community forums and, as a potentially stand-alone component of self-compassion training,14 recommend it here as well. In addition to traditional mindfulness-based stress-reduction courses and mindfulness meditation practice found in many hospitals and community centers, individual meditation focused on loving kindness or gratitude as well as mindful exercises such as writing a self-compassionate letter or statements to yourself can be used to offset burnout in daily life.15 From the perspective of reducing burnout, mindfulness allows us to look at our feelings of cynicism, exhaustion, and inadequacy without judgment, to view them as symptoms rather than ugly truths about ourselves and that rather than avoid or suppress these feelings, to be mindful and compassionate toward them.
Finally, in the spirit of self-compassion, we must not judge ourselves for needing the help of others to navigate adversity – whether that support comes from our personal or professional life, or is provided by a mental health professional, we deserve to be taken care of as much as our patients do.
For more information, please visit the following, helpful resources: www.CenterForMSC.org, www.Self-Compassion.org, and www.MindfulSelfCompassion.org.
Dr. Keefer is director, psychobehavioral research, Icahn School of Medicine at Mount Sinai, division of gastroenterology, New York, N.Y.
References
1. West C.P., Shanafelt T.D., Kolars J.C. JAMA. 2011;306[9]:952-60.
2. Maslach C., Leiter M.P. World Psychiatry. 2016;15[2]:103-11.
3. Ahola K., Honkonen T., Kivimaki M., et al. J Occup Environ Med. 2006;48[10]:1023-30.
4. Ahola K., Honkonen T., Isometsa E., et al. Soc Psychiatry Psychiatr Epidemiol. 2006;41[1]:11-7.
5. Farber B.A. J Clin Psychol. 2000;56[5]:589-94.
6. Keswani R.N., Taft T.H., Cote G.A., Keefer L. Am J Gastroenterol. 2011;106[10]:1734-40.
7. Sharma A., Sharp D.M., Walker L.G., Monson J.R. Psychooncology. 2008;17[6]:570-6.
8. Houkes I., Winants Y., Twellaar M., Verdonk P. BMC Public Health. 2011;11:240.
9. Neff K.D. Hum Dev. 2009;52[4]:211-4.
10. de Vente W., van Amsterdam J.G., Olff M., Kamphuis J.H., Emmelkamp P.M. Biomed Res Int. 2015;2015:431725.
11. Rockliff H., Karl A., McEwan K., Gilbert J., Matos M., Gilbert P. Effects of intranasal oxytocin on ‘compassion focused imagery’. Emotion. 2011;11[6]:1388-96.
12. Porges S.W. Biol Psychol. 2007;74[2]:301-7.
13. Breines J.G., Chen S. Pers Soc Psychol Bull. 2012;38[9]:1133-43.
14. Heffernan M., Quinn G.M.T., Sister R.M., Fitzpatrick JJ. Int J Nurs Pract. 2010;16[4]:366-73.
15. Crocker J., Canevello A. J Pers Soc Psychol. 2008;95[3]:555-75.
16. Thompson G., McBride R.B., Hosford C.C., Halaas G. Teach Learn Med. 2016;28[2]:174-82.
17. Nie Z., Jin Y., He L., et al. Int J Clin Exp Med. 2015;8[10]:19144-9.
18. West C.P., Dyrbye L.N., Erwin P.J., Shanafelt T.D. Lancet. 2016. Nov 5;388(10057)2272-81.
19. Luchterhand C., Rakel D., Haq C., et al. WMJ. 2015;114[3]:105-9.
20. Montero-Marin J., Tops M., Manzanera R, Piva Demarzo MM, Alvarez de Mon M, Garcia-Campayo J. Front Psychol. 2015;6:1895.
Physician burnout is a growing epidemic, particularly in the early careers of gastroenterologists. Up to 50% of new physicians and trainees experience burnout with the first 3 years of independent practice.1 The negative consequences of burnout are well known – medical errors, depression, substance abuse, and even suicide.2,3 To meet criteria for burnout syndrome (Table 1), one must have two of three core symptoms, often experienced as phases: 1) physical and emotional exhaustion; 2) cynicism and detachment; and 3) feelings of ineffectiveness and lack of accomplishment.4
Emotional exhaustion, one of the earliest symptoms of burnout syndrome was reported to be as high as 63% among gastroenterologists in a survey study I conducted with colleagues a few years ago.5 Similar findings are noted amongst colorectal surgeons.6 We also noted in our study that burnout levels were highest in junior versus senior attendings, with junior attendings reporting more stress related to performing endoscopies and making split-second decisions. Interventional endoscopists may have been disproportionately affected by the latter, reporting that they were more likely to think about possible mistakes they made after work, have difficulty sleeping due to thinking about their day, and have difficulty separating work and personal life.5 Male and female physicians may progress through the phases of burnout differently, with men being more likely to experience cynicism and depersonalization first, followed by fatigue. Men may also not necessarily experience the third phase of feeling ineffective, which can be particularly dangerous because they will continue to push until there is a serious consequence. Women tend to go through all three phases of burnout beginning with emotional exhaustion, with a more rapid progression through the cynicism phase, and may end up spending the majority of their time feeling ineffective and limited in their accomplishments, a recipe for leaving medicine entirely.7
Prevention of burnout through self-compassion
Even though it may sometimes be easy to forget, most of us chose medicine as our profession because of our inherent compassion towards others and desire to care for those in need. But have we properly learned how to apply that same compassion to ourselves?
Self-compassion is one of the primary qualities of a happy, flourishing, resilient individual.8 Self-compassion is a psychological skill that can be applied to feelings of inadequacy, failure, or lack of control and includes: 1) self-kindness, 2) belief in a common humanity, and 3) mindfulness.8 
Are you self-compassionate? Take a quiz!
Self-kindness requires us to treat ourselves as kindly as we would a friend or patient in the same situation. We must consciously choose not to use harsh, self-critical language when we make mistakes. We are taught not to berate our trainees for mistakes in the clinical setting – we can be taught not to berate ourselves for shortcomings as well. Self-kindness also requires that we provide ourselves with sympathy when we experience disappointments through no fault of our own (e.g. despite all my best efforts, this clinical initiative failed) and give ourselves the opportunity to nurture and soothe ourselves when we experience pain.6 Belief in a common humanity fosters engagement with others, recognizing that nobody is perfect and that others suffer as well. Isolating ourselves because we feel ashamed, embarrassed, or “crazy” in our experience of a situation only increases our suffering. As we engage with others, we are able to view things from a different perspective and also recognize that others around us have problems too. Indeed, social support may be one of the best buffers against burnout, particularly cynicism.12 A recent meta-analysis concluded that a combination of institutional engagement techniques including reduced hours and support groups as well as access to individual behavioral techniques such as mindfulness could reduce or prevent burnout.13
I have previously commented on the practice of mindfulness in the AGA Community forums and, as a potentially stand-alone component of self-compassion training,14 recommend it here as well. In addition to traditional mindfulness-based stress-reduction courses and mindfulness meditation practice found in many hospitals and community centers, individual meditation focused on loving kindness or gratitude as well as mindful exercises such as writing a self-compassionate letter or statements to yourself can be used to offset burnout in daily life.15 From the perspective of reducing burnout, mindfulness allows us to look at our feelings of cynicism, exhaustion, and inadequacy without judgment, to view them as symptoms rather than ugly truths about ourselves and that rather than avoid or suppress these feelings, to be mindful and compassionate toward them.
Finally, in the spirit of self-compassion, we must not judge ourselves for needing the help of others to navigate adversity – whether that support comes from our personal or professional life, or is provided by a mental health professional, we deserve to be taken care of as much as our patients do.
For more information, please visit the following, helpful resources: www.CenterForMSC.org, www.Self-Compassion.org, and www.MindfulSelfCompassion.org.
Dr. Keefer is director, psychobehavioral research, Icahn School of Medicine at Mount Sinai, division of gastroenterology, New York, N.Y.
References
1. West C.P., Shanafelt T.D., Kolars J.C. JAMA. 2011;306[9]:952-60.
2. Maslach C., Leiter M.P. World Psychiatry. 2016;15[2]:103-11.
3. Ahola K., Honkonen T., Kivimaki M., et al. J Occup Environ Med. 2006;48[10]:1023-30.
4. Ahola K., Honkonen T., Isometsa E., et al. Soc Psychiatry Psychiatr Epidemiol. 2006;41[1]:11-7.
5. Farber B.A. J Clin Psychol. 2000;56[5]:589-94.
6. Keswani R.N., Taft T.H., Cote G.A., Keefer L. Am J Gastroenterol. 2011;106[10]:1734-40.
7. Sharma A., Sharp D.M., Walker L.G., Monson J.R. Psychooncology. 2008;17[6]:570-6.
8. Houkes I., Winants Y., Twellaar M., Verdonk P. BMC Public Health. 2011;11:240.
9. Neff K.D. Hum Dev. 2009;52[4]:211-4.
10. de Vente W., van Amsterdam J.G., Olff M., Kamphuis J.H., Emmelkamp P.M. Biomed Res Int. 2015;2015:431725.
11. Rockliff H., Karl A., McEwan K., Gilbert J., Matos M., Gilbert P. Effects of intranasal oxytocin on ‘compassion focused imagery’. Emotion. 2011;11[6]:1388-96.
12. Porges S.W. Biol Psychol. 2007;74[2]:301-7.
13. Breines J.G., Chen S. Pers Soc Psychol Bull. 2012;38[9]:1133-43.
14. Heffernan M., Quinn G.M.T., Sister R.M., Fitzpatrick JJ. Int J Nurs Pract. 2010;16[4]:366-73.
15. Crocker J., Canevello A. J Pers Soc Psychol. 2008;95[3]:555-75.
16. Thompson G., McBride R.B., Hosford C.C., Halaas G. Teach Learn Med. 2016;28[2]:174-82.
17. Nie Z., Jin Y., He L., et al. Int J Clin Exp Med. 2015;8[10]:19144-9.
18. West C.P., Dyrbye L.N., Erwin P.J., Shanafelt T.D. Lancet. 2016. Nov 5;388(10057)2272-81.
19. Luchterhand C., Rakel D., Haq C., et al. WMJ. 2015;114[3]:105-9.
20. Montero-Marin J., Tops M., Manzanera R, Piva Demarzo MM, Alvarez de Mon M, Garcia-Campayo J. Front Psychol. 2015;6:1895.
Diversity in GI Training: A Timely Goal
There is no denying that practicing medicine calls us to serve a population that is diverse in many aspects. We live and work in a world that is evolving so quickly that medical workforce demographics fail to keep pace. In the U.S. in particular, racial and ethnic diversity has already exceeded many previous forecasts and will likely continue to do so.
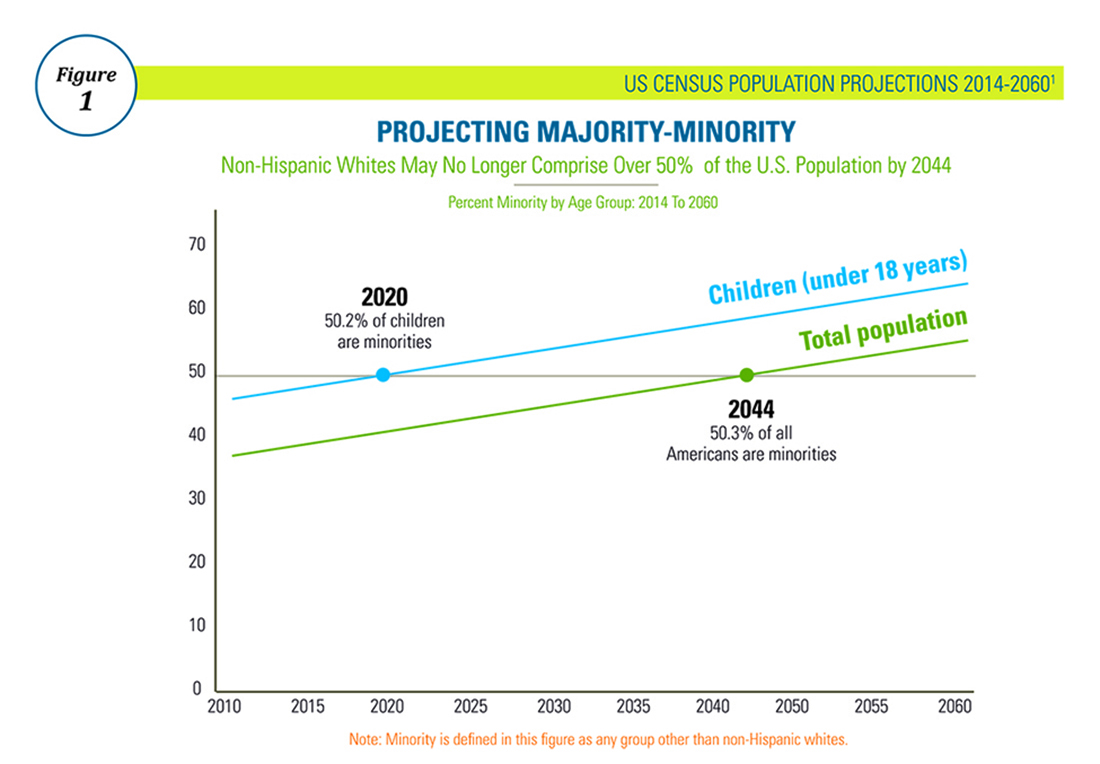
The American Gastroenterological Association (AGA) recognizes that broader representation in the GI workforce requires increasing diversity at the trainee level and values this change for reasons beyond diversity for diversity’s sake. Based on education research, improving diversity at the trainee level helps learners thrive through the sharing of varied perspectives and enhancement of complex, critical thinking5. Moreover, diverse learning environments promote a culture of tolerance and understanding, tools needed to prepare trainees for future patient interactions. Diversity also translates into better patient satisfaction, as several studies have shown that physician-patient concordance on race, ethnicity, and gender result in higher patient satisfaction scores6. Additionally, minority physicians are more likely to practice in underserved areas and to conduct research addressing health care disparities, an area that will require an even greater investment as the U.S. population demographic continues to evolve3,7.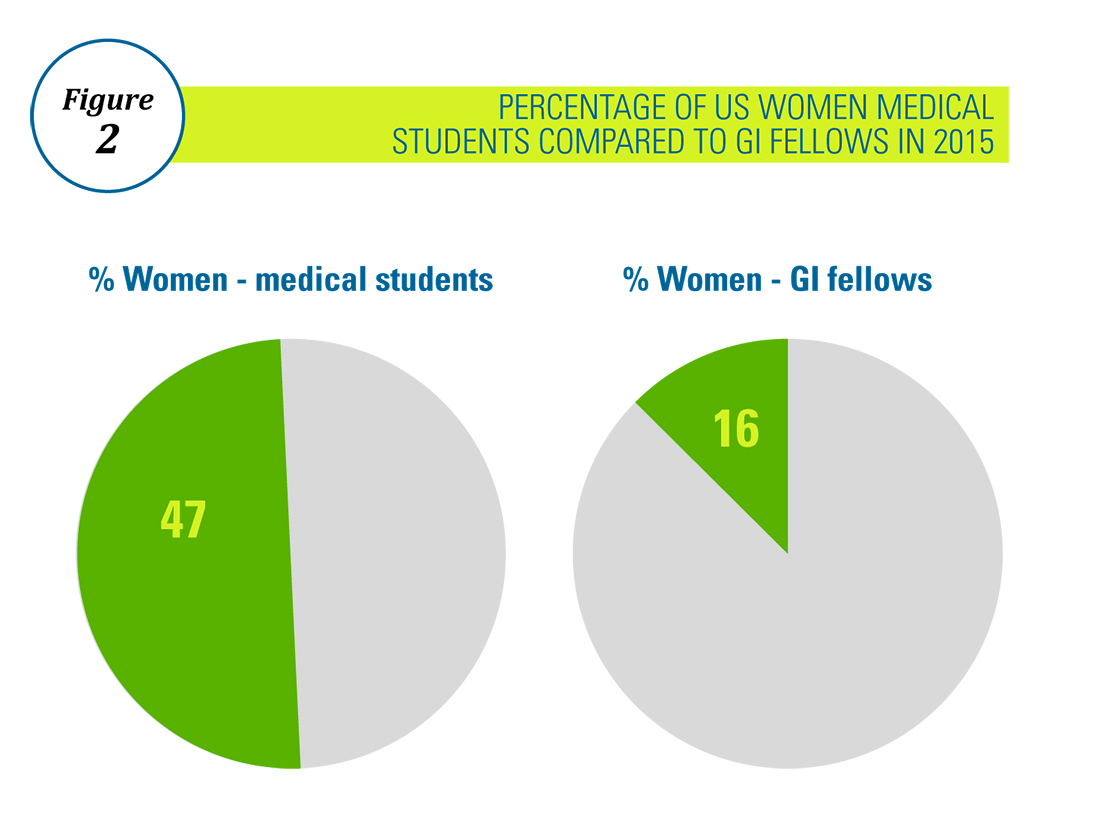
The AGA is committed to diversity, which is an inclusive concept that encompasses race, ethnicity, national origin, religion, gender, age, sexual orientation, and disability. We strive to cultivate diversity within the organization at all levels, including governance, committee structure, staffing, and program and policy development. We are committed to the following goals intended to reflect the interests of the diverse patient population we serve:
1) Promotion of diversity within the practice of gastroenterology and in the individual care of patients of all backgrounds.
2) Recruitment and retention of GI providers and researchers from diverse backgrounds and the support of the advancement of their careers.
3) Elimination of disparities in GI diseases through community engagement, research, and advocacy.
Gastroenterology has been the most competitive fellowship specialty for the past 4 consecutive years, above pediatric surgery and cardiology8. We are privileged to practice an exciting, fascinating specialty that demands diversity of skill, acuity of care, and knowledge of pathophysiology. Increased diversity among those who research, teach, and practice in this wonderful field will only enhance it, and being mindful of this goal in our recruitment and retention efforts will help us achieve it.
For more information on the AGA Institute Diversity Committee and its ongoing initiatives, please visit http://www.gastro.org/about/people/committees/diversity-committee. Additionally, any specific enquiries should be addressed to Taylor Monson ([email protected]).
Dr. Quezeda is assistant dean for admissions, assistant professor of medicine, division of gastroenterology and hepatology, University of Maryland School of Medicine, Baltimore, and a member of the AGA Institute Diversity Committee.
On behalf of the AGA Institute Diversity Committee: Rotonya M. Carr, MD (Chair, AGA Diversity Committee; assistant professor of medicine, division of gastroenterology, University of Pennsylvania, Philadelphia), Karen A. Chachu, MD, PhD (assistant professor of medicine, Duke University, Durham, N.C.), Elizabeth Coss, MD (clinical assistant professor, University of Texas Health Science Center at San Antonio), Maria Cruz-Correa, MD PhD (associate professor of medicine, biochemistry and surgery, University of Puerto Rico Comprehensive Cancer Center), Lukejohn Day, MD (associate clinical professor, University of California, San Francisco), Darrell M. Gray II, MD, MPH (assistant professor of medicine, The Ohio State University Wexner Medical Center), Esi Lamouse-Smith, MD, PhD (assistant professor of pediatrics, Columbia University, New York), Antonio Mendoza Ladd, MD (assistant professor, Texas Tech University Health Sciences Center, El Paso), and Celena NuQuay (AGA staff liaison).
References
1. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 Population Estimates and Projections Current Population Reports. Colby S, Ortman JM. Issued March 2015.
2. Association of American Medical Colleges 2016 Physician Specialty Databook, https://www.aamc.org/data/workforce/reports/457712/2016-specialty-databook.html.
3. Association of American Medical Colleges Diversity in the Physician Workforce: Facts and Figures 2010.
4. Deville C, Hwang WT, Burgos R. Diversity in Graduate Medical Education in the United States by Race, Ethnicity, and Sex, 2012. JAMA Intern Med. 2015;175(10):1706-8.
5. Wells AS, Fox L, Cordova-Cobo D. How Racially Diverse Schools and Classrooms Can Benefit All Students. The Century Foundation, Feb 2016. https://tcf.org/content/report/how-racially-diverse-schools-and-classrooms-can-benefit-all-students/.
6. Johnson RL, Saha S, Arbelaez JJ et al. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004 Feb;19(2):101-10.
7. Saha S, Guiton G, Wimmers PF et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008 Sep 10;300(10):1135-45.
8. Association of American Medical Colleges, ERAS Data. https://www.aamc.org/services/eras/stats/359278/stats.html.
There is no denying that practicing medicine calls us to serve a population that is diverse in many aspects. We live and work in a world that is evolving so quickly that medical workforce demographics fail to keep pace. In the U.S. in particular, racial and ethnic diversity has already exceeded many previous forecasts and will likely continue to do so.

The American Gastroenterological Association (AGA) recognizes that broader representation in the GI workforce requires increasing diversity at the trainee level and values this change for reasons beyond diversity for diversity’s sake. Based on education research, improving diversity at the trainee level helps learners thrive through the sharing of varied perspectives and enhancement of complex, critical thinking5. Moreover, diverse learning environments promote a culture of tolerance and understanding, tools needed to prepare trainees for future patient interactions. Diversity also translates into better patient satisfaction, as several studies have shown that physician-patient concordance on race, ethnicity, and gender result in higher patient satisfaction scores6. Additionally, minority physicians are more likely to practice in underserved areas and to conduct research addressing health care disparities, an area that will require an even greater investment as the U.S. population demographic continues to evolve3,7.
The AGA is committed to diversity, which is an inclusive concept that encompasses race, ethnicity, national origin, religion, gender, age, sexual orientation, and disability. We strive to cultivate diversity within the organization at all levels, including governance, committee structure, staffing, and program and policy development. We are committed to the following goals intended to reflect the interests of the diverse patient population we serve:
1) Promotion of diversity within the practice of gastroenterology and in the individual care of patients of all backgrounds.
2) Recruitment and retention of GI providers and researchers from diverse backgrounds and the support of the advancement of their careers.
3) Elimination of disparities in GI diseases through community engagement, research, and advocacy.
Gastroenterology has been the most competitive fellowship specialty for the past 4 consecutive years, above pediatric surgery and cardiology8. We are privileged to practice an exciting, fascinating specialty that demands diversity of skill, acuity of care, and knowledge of pathophysiology. Increased diversity among those who research, teach, and practice in this wonderful field will only enhance it, and being mindful of this goal in our recruitment and retention efforts will help us achieve it.
For more information on the AGA Institute Diversity Committee and its ongoing initiatives, please visit http://www.gastro.org/about/people/committees/diversity-committee. Additionally, any specific enquiries should be addressed to Taylor Monson ([email protected]).
Dr. Quezeda is assistant dean for admissions, assistant professor of medicine, division of gastroenterology and hepatology, University of Maryland School of Medicine, Baltimore, and a member of the AGA Institute Diversity Committee.
On behalf of the AGA Institute Diversity Committee: Rotonya M. Carr, MD (Chair, AGA Diversity Committee; assistant professor of medicine, division of gastroenterology, University of Pennsylvania, Philadelphia), Karen A. Chachu, MD, PhD (assistant professor of medicine, Duke University, Durham, N.C.), Elizabeth Coss, MD (clinical assistant professor, University of Texas Health Science Center at San Antonio), Maria Cruz-Correa, MD PhD (associate professor of medicine, biochemistry and surgery, University of Puerto Rico Comprehensive Cancer Center), Lukejohn Day, MD (associate clinical professor, University of California, San Francisco), Darrell M. Gray II, MD, MPH (assistant professor of medicine, The Ohio State University Wexner Medical Center), Esi Lamouse-Smith, MD, PhD (assistant professor of pediatrics, Columbia University, New York), Antonio Mendoza Ladd, MD (assistant professor, Texas Tech University Health Sciences Center, El Paso), and Celena NuQuay (AGA staff liaison).
References
1. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 Population Estimates and Projections Current Population Reports. Colby S, Ortman JM. Issued March 2015.
2. Association of American Medical Colleges 2016 Physician Specialty Databook, https://www.aamc.org/data/workforce/reports/457712/2016-specialty-databook.html.
3. Association of American Medical Colleges Diversity in the Physician Workforce: Facts and Figures 2010.
4. Deville C, Hwang WT, Burgos R. Diversity in Graduate Medical Education in the United States by Race, Ethnicity, and Sex, 2012. JAMA Intern Med. 2015;175(10):1706-8.
5. Wells AS, Fox L, Cordova-Cobo D. How Racially Diverse Schools and Classrooms Can Benefit All Students. The Century Foundation, Feb 2016. https://tcf.org/content/report/how-racially-diverse-schools-and-classrooms-can-benefit-all-students/.
6. Johnson RL, Saha S, Arbelaez JJ et al. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004 Feb;19(2):101-10.
7. Saha S, Guiton G, Wimmers PF et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008 Sep 10;300(10):1135-45.
8. Association of American Medical Colleges, ERAS Data. https://www.aamc.org/services/eras/stats/359278/stats.html.
There is no denying that practicing medicine calls us to serve a population that is diverse in many aspects. We live and work in a world that is evolving so quickly that medical workforce demographics fail to keep pace. In the U.S. in particular, racial and ethnic diversity has already exceeded many previous forecasts and will likely continue to do so.

The American Gastroenterological Association (AGA) recognizes that broader representation in the GI workforce requires increasing diversity at the trainee level and values this change for reasons beyond diversity for diversity’s sake. Based on education research, improving diversity at the trainee level helps learners thrive through the sharing of varied perspectives and enhancement of complex, critical thinking5. Moreover, diverse learning environments promote a culture of tolerance and understanding, tools needed to prepare trainees for future patient interactions. Diversity also translates into better patient satisfaction, as several studies have shown that physician-patient concordance on race, ethnicity, and gender result in higher patient satisfaction scores6. Additionally, minority physicians are more likely to practice in underserved areas and to conduct research addressing health care disparities, an area that will require an even greater investment as the U.S. population demographic continues to evolve3,7.
The AGA is committed to diversity, which is an inclusive concept that encompasses race, ethnicity, national origin, religion, gender, age, sexual orientation, and disability. We strive to cultivate diversity within the organization at all levels, including governance, committee structure, staffing, and program and policy development. We are committed to the following goals intended to reflect the interests of the diverse patient population we serve:
1) Promotion of diversity within the practice of gastroenterology and in the individual care of patients of all backgrounds.
2) Recruitment and retention of GI providers and researchers from diverse backgrounds and the support of the advancement of their careers.
3) Elimination of disparities in GI diseases through community engagement, research, and advocacy.
Gastroenterology has been the most competitive fellowship specialty for the past 4 consecutive years, above pediatric surgery and cardiology8. We are privileged to practice an exciting, fascinating specialty that demands diversity of skill, acuity of care, and knowledge of pathophysiology. Increased diversity among those who research, teach, and practice in this wonderful field will only enhance it, and being mindful of this goal in our recruitment and retention efforts will help us achieve it.
For more information on the AGA Institute Diversity Committee and its ongoing initiatives, please visit http://www.gastro.org/about/people/committees/diversity-committee. Additionally, any specific enquiries should be addressed to Taylor Monson ([email protected]).
Dr. Quezeda is assistant dean for admissions, assistant professor of medicine, division of gastroenterology and hepatology, University of Maryland School of Medicine, Baltimore, and a member of the AGA Institute Diversity Committee.
On behalf of the AGA Institute Diversity Committee: Rotonya M. Carr, MD (Chair, AGA Diversity Committee; assistant professor of medicine, division of gastroenterology, University of Pennsylvania, Philadelphia), Karen A. Chachu, MD, PhD (assistant professor of medicine, Duke University, Durham, N.C.), Elizabeth Coss, MD (clinical assistant professor, University of Texas Health Science Center at San Antonio), Maria Cruz-Correa, MD PhD (associate professor of medicine, biochemistry and surgery, University of Puerto Rico Comprehensive Cancer Center), Lukejohn Day, MD (associate clinical professor, University of California, San Francisco), Darrell M. Gray II, MD, MPH (assistant professor of medicine, The Ohio State University Wexner Medical Center), Esi Lamouse-Smith, MD, PhD (assistant professor of pediatrics, Columbia University, New York), Antonio Mendoza Ladd, MD (assistant professor, Texas Tech University Health Sciences Center, El Paso), and Celena NuQuay (AGA staff liaison).
References
1. Projections of the Size and Composition of the U.S. Population: 2014 to 2060 Population Estimates and Projections Current Population Reports. Colby S, Ortman JM. Issued March 2015.
2. Association of American Medical Colleges 2016 Physician Specialty Databook, https://www.aamc.org/data/workforce/reports/457712/2016-specialty-databook.html.
3. Association of American Medical Colleges Diversity in the Physician Workforce: Facts and Figures 2010.
4. Deville C, Hwang WT, Burgos R. Diversity in Graduate Medical Education in the United States by Race, Ethnicity, and Sex, 2012. JAMA Intern Med. 2015;175(10):1706-8.
5. Wells AS, Fox L, Cordova-Cobo D. How Racially Diverse Schools and Classrooms Can Benefit All Students. The Century Foundation, Feb 2016. https://tcf.org/content/report/how-racially-diverse-schools-and-classrooms-can-benefit-all-students/.
6. Johnson RL, Saha S, Arbelaez JJ et al. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004 Feb;19(2):101-10.
7. Saha S, Guiton G, Wimmers PF et al. Student body racial and ethnic composition and diversity-related outcomes in US medical schools. JAMA. 2008 Sep 10;300(10):1135-45.
8. Association of American Medical Colleges, ERAS Data. https://www.aamc.org/services/eras/stats/359278/stats.html.
Ten Financial Tips for a Worry-Free Retirement
As a contract and tax attorney for physicians for over 30 years, I have reviewed many asset summaries of late-career physicians. Although most have historically strong annual incomes of $200,000 to $400,000, accumulated wealth varies tremendously. Some physicians in their 60s have a home, a small retirement plan, and little else. Others have cash equivalents of $5,000,000 or more, no debt, real estate, and other assets. In my experience, this variance usually does not relate primarily to income differences but rather spending control and financial knowledge. If you are interested in having the opportunity to retire and not worry about finding an “early bird” special at your favorite restaurant, this article provides ten tips to help you achieve that dream.
2. Contribute to an employer retirement plan. Contribute to your employer’s Roth 401-K or regular 401-K. Add money starting the first day you are eligible at the rate of at least 5% of your compensation. By age 35, contribute no less than 10% of your compensation up to the legal maximum. In a Roth 401-K, you will have decades of tax-free accumulation. You may also enjoy the employer matching contribution, which varies from job to job. Do not take loans on 401-K plans. If you borrow and then terminate employment before completing repayment, the borrowed funds are treated as a plan distribution, subjecting them to taxation and possibly a penalty if you are under age 59.5. If switching jobs, move your 401-K retirement plan account into an IRA; do not cash it out. If necessary, you usually can withdraw funds to make a down payment on a home or for an emergency, but plan contributions should be viewed as “tomorrow” money. You can borrow to purchase a home and to finance your children’s educations but you cannot borrow to retire.
3. Be debt-free. It is easier to accumulate wealth if you are debt-free. Mortgages, student loans, and car payments should be minimized and eliminated as quickly as possible so that available net income is used to invest both through retirement plans and on an after-tax basis. Cars should be purchased, not leased as the “tax benefit” of leasing is a myth. Leasing a car is an expensive way of borrowing money, as you are effectively purchasing only the most expensive depreciating years of the car’s useful life (the initial few years). You should also not have credit card debt at any time as credit card debt means you are spending money before you earn it. Borrowing for clothing or a vacation reflects the inability to control one’s spending.
4. Use tax-advantaged investment vehicles. Interest income on your investments is taxed at ordinary income rates, perhaps 30% or more, but dividends issued from stock or stock mutual funds are taxed at lower long-term capital gains rates. Similarly, when you sell a stock or a stock mutual fund, the appreciation is taxed at long-term capital gains rates under most circumstances. As you are able to set funds aside, make sure that you are using tax-advantaged investment vehicles.
5. Consider no-load mutual funds. When investing in the stock market or otherwise, consider no-load mutual funds such as those offered by Vanguard that do not require an “investment advisor.” Such funds do not have sales charges and save you money. The greatest chance you have of underperforming the market relates to the expenses associated with investment, more so than the particular investments selected. Since almost all advisors underperform the market, you should consider investing on your own, minimizing costs, and watching your funds grow. As a younger physician with many high-income years in front of you, a good portion of your investments should be in equities to enjoy their appreciation over decades. With bank interest rates being minuscule, there is no reasonable alternative.
6. Develop a budget. If you or your spouse has an issue with shopping or overspending, it is imperative that you develop a budget: first allocating funds to long-term savings such as a retirement plan, next to short-term savings, then to unavoidable recurring costs such as rent or mortgage, student loans, food, and discretionary expenditures. The perfect time to put this in place is when you go from the salary of a resident or fellow into a full-time job and your pay increases by multifold. Read the book The Millionaire Next Door: The Surprising Secrets of America’s Wealthy by Thomas J. Stanley and gain control, as it is easy to do otherwise with an unprecedented and significant salary jump. If you start to live on your new salary, you will never be in a position to amass wealth and retire comfortably.
7. Send your kids to public, not private school. For each of your children, would you rather pay astronomic tuition bills for 4-8 years of college or 16-20 years counting grades 1-12 in private school? When you have children approaching school age, choose an A+ school district and send your kids to public school, not private school – they will still get into competitive colleges. This can save hundreds of thousands of dollars per child.
8. Fund a 529 plan. Whether or not you currently have children, you can fund a 529 plan to enjoy tax-free growth and plan for education expenses of children or future children. If you do not have children yet, you can name yourself or a different party as the beneficiary and then change it after children are born. If you do not have children, you can either use the 529 for someone else or cash the investment and recover the money including growth/loss thereon. Trying to fund college educations out of current income is difficult and it is better to prefund than to pay back student loans over many years.
9. Draft a will. If you are married or have children or both, it is imperative that you have wills drafted so that your wishes are implemented upon your passing. Many tax advantages are available without using complicated trusts and it is important that you maintain up-to-date wills should the unforeseen occur.
10. Purchase disability and life insurance. Your most valuable financial asset is your income stream over the coming years. Protect it with adequate private disability and life insurance policies. Policies provided by your employer typically end upon termination of employment and having a portable policy is important.
These tips will help you maximize your financial position over your work life and through retirement. The best time to get on the right track is yesterday; the second best time is today. Staying in shape financially is easier than messing up and then attempting to fix it.
Mr. Schiller is a physician contract and tax attorney and has practiced in Norristown, Penn. for the past 30 years. He can be contacted at 610-277-5900 or www.schillerlawassociates.com or [email protected].
As a contract and tax attorney for physicians for over 30 years, I have reviewed many asset summaries of late-career physicians. Although most have historically strong annual incomes of $200,000 to $400,000, accumulated wealth varies tremendously. Some physicians in their 60s have a home, a small retirement plan, and little else. Others have cash equivalents of $5,000,000 or more, no debt, real estate, and other assets. In my experience, this variance usually does not relate primarily to income differences but rather spending control and financial knowledge. If you are interested in having the opportunity to retire and not worry about finding an “early bird” special at your favorite restaurant, this article provides ten tips to help you achieve that dream.
2. Contribute to an employer retirement plan. Contribute to your employer’s Roth 401-K or regular 401-K. Add money starting the first day you are eligible at the rate of at least 5% of your compensation. By age 35, contribute no less than 10% of your compensation up to the legal maximum. In a Roth 401-K, you will have decades of tax-free accumulation. You may also enjoy the employer matching contribution, which varies from job to job. Do not take loans on 401-K plans. If you borrow and then terminate employment before completing repayment, the borrowed funds are treated as a plan distribution, subjecting them to taxation and possibly a penalty if you are under age 59.5. If switching jobs, move your 401-K retirement plan account into an IRA; do not cash it out. If necessary, you usually can withdraw funds to make a down payment on a home or for an emergency, but plan contributions should be viewed as “tomorrow” money. You can borrow to purchase a home and to finance your children’s educations but you cannot borrow to retire.
3. Be debt-free. It is easier to accumulate wealth if you are debt-free. Mortgages, student loans, and car payments should be minimized and eliminated as quickly as possible so that available net income is used to invest both through retirement plans and on an after-tax basis. Cars should be purchased, not leased as the “tax benefit” of leasing is a myth. Leasing a car is an expensive way of borrowing money, as you are effectively purchasing only the most expensive depreciating years of the car’s useful life (the initial few years). You should also not have credit card debt at any time as credit card debt means you are spending money before you earn it. Borrowing for clothing or a vacation reflects the inability to control one’s spending.
4. Use tax-advantaged investment vehicles. Interest income on your investments is taxed at ordinary income rates, perhaps 30% or more, but dividends issued from stock or stock mutual funds are taxed at lower long-term capital gains rates. Similarly, when you sell a stock or a stock mutual fund, the appreciation is taxed at long-term capital gains rates under most circumstances. As you are able to set funds aside, make sure that you are using tax-advantaged investment vehicles.
5. Consider no-load mutual funds. When investing in the stock market or otherwise, consider no-load mutual funds such as those offered by Vanguard that do not require an “investment advisor.” Such funds do not have sales charges and save you money. The greatest chance you have of underperforming the market relates to the expenses associated with investment, more so than the particular investments selected. Since almost all advisors underperform the market, you should consider investing on your own, minimizing costs, and watching your funds grow. As a younger physician with many high-income years in front of you, a good portion of your investments should be in equities to enjoy their appreciation over decades. With bank interest rates being minuscule, there is no reasonable alternative.
6. Develop a budget. If you or your spouse has an issue with shopping or overspending, it is imperative that you develop a budget: first allocating funds to long-term savings such as a retirement plan, next to short-term savings, then to unavoidable recurring costs such as rent or mortgage, student loans, food, and discretionary expenditures. The perfect time to put this in place is when you go from the salary of a resident or fellow into a full-time job and your pay increases by multifold. Read the book The Millionaire Next Door: The Surprising Secrets of America’s Wealthy by Thomas J. Stanley and gain control, as it is easy to do otherwise with an unprecedented and significant salary jump. If you start to live on your new salary, you will never be in a position to amass wealth and retire comfortably.
7. Send your kids to public, not private school. For each of your children, would you rather pay astronomic tuition bills for 4-8 years of college or 16-20 years counting grades 1-12 in private school? When you have children approaching school age, choose an A+ school district and send your kids to public school, not private school – they will still get into competitive colleges. This can save hundreds of thousands of dollars per child.
8. Fund a 529 plan. Whether or not you currently have children, you can fund a 529 plan to enjoy tax-free growth and plan for education expenses of children or future children. If you do not have children yet, you can name yourself or a different party as the beneficiary and then change it after children are born. If you do not have children, you can either use the 529 for someone else or cash the investment and recover the money including growth/loss thereon. Trying to fund college educations out of current income is difficult and it is better to prefund than to pay back student loans over many years.
9. Draft a will. If you are married or have children or both, it is imperative that you have wills drafted so that your wishes are implemented upon your passing. Many tax advantages are available without using complicated trusts and it is important that you maintain up-to-date wills should the unforeseen occur.
10. Purchase disability and life insurance. Your most valuable financial asset is your income stream over the coming years. Protect it with adequate private disability and life insurance policies. Policies provided by your employer typically end upon termination of employment and having a portable policy is important.
These tips will help you maximize your financial position over your work life and through retirement. The best time to get on the right track is yesterday; the second best time is today. Staying in shape financially is easier than messing up and then attempting to fix it.
Mr. Schiller is a physician contract and tax attorney and has practiced in Norristown, Penn. for the past 30 years. He can be contacted at 610-277-5900 or www.schillerlawassociates.com or [email protected].
As a contract and tax attorney for physicians for over 30 years, I have reviewed many asset summaries of late-career physicians. Although most have historically strong annual incomes of $200,000 to $400,000, accumulated wealth varies tremendously. Some physicians in their 60s have a home, a small retirement plan, and little else. Others have cash equivalents of $5,000,000 or more, no debt, real estate, and other assets. In my experience, this variance usually does not relate primarily to income differences but rather spending control and financial knowledge. If you are interested in having the opportunity to retire and not worry about finding an “early bird” special at your favorite restaurant, this article provides ten tips to help you achieve that dream.
2. Contribute to an employer retirement plan. Contribute to your employer’s Roth 401-K or regular 401-K. Add money starting the first day you are eligible at the rate of at least 5% of your compensation. By age 35, contribute no less than 10% of your compensation up to the legal maximum. In a Roth 401-K, you will have decades of tax-free accumulation. You may also enjoy the employer matching contribution, which varies from job to job. Do not take loans on 401-K plans. If you borrow and then terminate employment before completing repayment, the borrowed funds are treated as a plan distribution, subjecting them to taxation and possibly a penalty if you are under age 59.5. If switching jobs, move your 401-K retirement plan account into an IRA; do not cash it out. If necessary, you usually can withdraw funds to make a down payment on a home or for an emergency, but plan contributions should be viewed as “tomorrow” money. You can borrow to purchase a home and to finance your children’s educations but you cannot borrow to retire.
3. Be debt-free. It is easier to accumulate wealth if you are debt-free. Mortgages, student loans, and car payments should be minimized and eliminated as quickly as possible so that available net income is used to invest both through retirement plans and on an after-tax basis. Cars should be purchased, not leased as the “tax benefit” of leasing is a myth. Leasing a car is an expensive way of borrowing money, as you are effectively purchasing only the most expensive depreciating years of the car’s useful life (the initial few years). You should also not have credit card debt at any time as credit card debt means you are spending money before you earn it. Borrowing for clothing or a vacation reflects the inability to control one’s spending.
4. Use tax-advantaged investment vehicles. Interest income on your investments is taxed at ordinary income rates, perhaps 30% or more, but dividends issued from stock or stock mutual funds are taxed at lower long-term capital gains rates. Similarly, when you sell a stock or a stock mutual fund, the appreciation is taxed at long-term capital gains rates under most circumstances. As you are able to set funds aside, make sure that you are using tax-advantaged investment vehicles.
5. Consider no-load mutual funds. When investing in the stock market or otherwise, consider no-load mutual funds such as those offered by Vanguard that do not require an “investment advisor.” Such funds do not have sales charges and save you money. The greatest chance you have of underperforming the market relates to the expenses associated with investment, more so than the particular investments selected. Since almost all advisors underperform the market, you should consider investing on your own, minimizing costs, and watching your funds grow. As a younger physician with many high-income years in front of you, a good portion of your investments should be in equities to enjoy their appreciation over decades. With bank interest rates being minuscule, there is no reasonable alternative.
6. Develop a budget. If you or your spouse has an issue with shopping or overspending, it is imperative that you develop a budget: first allocating funds to long-term savings such as a retirement plan, next to short-term savings, then to unavoidable recurring costs such as rent or mortgage, student loans, food, and discretionary expenditures. The perfect time to put this in place is when you go from the salary of a resident or fellow into a full-time job and your pay increases by multifold. Read the book The Millionaire Next Door: The Surprising Secrets of America’s Wealthy by Thomas J. Stanley and gain control, as it is easy to do otherwise with an unprecedented and significant salary jump. If you start to live on your new salary, you will never be in a position to amass wealth and retire comfortably.
7. Send your kids to public, not private school. For each of your children, would you rather pay astronomic tuition bills for 4-8 years of college or 16-20 years counting grades 1-12 in private school? When you have children approaching school age, choose an A+ school district and send your kids to public school, not private school – they will still get into competitive colleges. This can save hundreds of thousands of dollars per child.
8. Fund a 529 plan. Whether or not you currently have children, you can fund a 529 plan to enjoy tax-free growth and plan for education expenses of children or future children. If you do not have children yet, you can name yourself or a different party as the beneficiary and then change it after children are born. If you do not have children, you can either use the 529 for someone else or cash the investment and recover the money including growth/loss thereon. Trying to fund college educations out of current income is difficult and it is better to prefund than to pay back student loans over many years.
9. Draft a will. If you are married or have children or both, it is imperative that you have wills drafted so that your wishes are implemented upon your passing. Many tax advantages are available without using complicated trusts and it is important that you maintain up-to-date wills should the unforeseen occur.
10. Purchase disability and life insurance. Your most valuable financial asset is your income stream over the coming years. Protect it with adequate private disability and life insurance policies. Policies provided by your employer typically end upon termination of employment and having a portable policy is important.
These tips will help you maximize your financial position over your work life and through retirement. The best time to get on the right track is yesterday; the second best time is today. Staying in shape financially is easier than messing up and then attempting to fix it.
Mr. Schiller is a physician contract and tax attorney and has practiced in Norristown, Penn. for the past 30 years. He can be contacted at 610-277-5900 or www.schillerlawassociates.com or [email protected].