User login
Influenza: A vaccine we love to hate
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
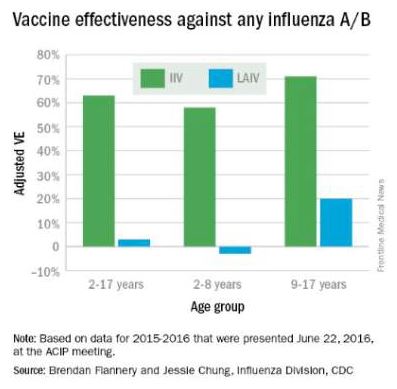
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.
ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at [email protected].
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.

ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at [email protected].
The Centers for Disease Control and Prevention, American Academy of Pediatrics, and American Academy of Family Physicians recommend that everyone 6 months of age and older get a seasonal flu vaccine. Emphasizing influenza vaccination in children recognizes the high burden of morbidity and significant mortality associated with influenza in young children as well as their role in transmission in the community.
In 2015-2016, the CDC reported 83 influenza deaths in children, and estimated the rate of hospitalization for children younger than 4 years of age to be 42/100,000 (at press time). In 2015-2016, the H1N1 strain was dominant in the community overall, with influenza B being most prevalent late in the season. The CDC estimates that nearly 75% of children less than 24 months and 68% between 2 and 4 years of age were immunized this year. Overall vaccine efficacy in children 6 months through 8 years was reported at 47% last season from a CDC study using a study design that compares vaccination odds among influenza reverse transcription polymerase chain reaction (RT-PCR)–positive cases and RT-PCR–negative controls.
Influenza virus vaccines are unique in that they are updated, often annually, to include the most current hemagglutinin (HA) antigens based on estimates from circulating strains. In the United States, influenza vaccine manufacturers submit a supplement to their license and obtain Food and Drug Administration approval. These applications require only a limited study of safety in approximately 300 adults, essentially to verify attenuation (Influenza Other Respir Viruses. 2016. doi: 10.111/irv.1283). They do not require clinical proof of efficacy or even a threshold of immunogenicity.
At the June 2016 CDC’s Advisory Committee on Immunization Practices (ACIP) meeting, data were presented comparing the efficacy of this season’s live attenuated influenza vaccine (LAIV) with inactivated influenza vaccine (IIV) by age and specific influenza type and subtype. Data from the U.S. Flu Vaccine Effectiveness (VE) Network, a consortium of five CDC-funded sites that conducts annual studies of influenza vaccine effectiveness, failed to demonstrate efficacy for LAIV in children aged 2-8 years. There was an absence of efficacy against the primary circulating strain, A(H1N1). This contrasted with the 62% efficacy report for IIV against A(H1N1).
The concern for efficacy for LAIV was not limited to 2015-2016; efficacy was poor in 2013-2014 during a year in which A(H1N1) was the dominant virus as well, and in 2014-2015 when the prevalent strain was a drifted A(H3N2). The lack of efficacy in 2015-2016 and 2013-2014 when A(H1N1) was the prevalent strain was especially enigmatic given its high efficacy against A(H1N1) between 2009 and 2011. Studies of LAIV from Astra Zeneca and the U.S. Department of Defense were consistent with those from the U.S. Flu VE Network; however, there were discordant data from Finland where vaccine efficacy was present. As a result of these studies, the ACIP voted that LAIV should not be used during the 2016-2017 flu season. This vote reinforces the importance of monitoring the effectiveness of annual flu vaccination and other public health interventions.

ACIP recommendations for 2016-2017
• Children younger than 2 years of age and those with chronic health problems such as asthma, diabetes, and disorders of the brain or nervous system are at especially high risk of developing serious flu complications.
• Annual influenza immunization, with either the IIV or recombinant influenza vaccine (RIV), for everyone 6 months and older, remains the only effective strategy for decreasing influenza disease in the community.
• LAIV should not be used during the 2016-2017 flu season.
ACIP recommendations must be reviewed and approved by the CDC’s director before becoming CDC policy. The final annual recommendations on the prevention and control of influenza with vaccines will be published in CDC Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports in late summer or early fall.
Flu vaccines available for children for 2016-2017
• The trivalent flu vaccine protects against three flu viruses; two influenza A viruses and an influenza B virus. Standard dose trivalent shots are manufactured with viruses grown in eggs. These are approved for children aged 6 months and older. There are different brands of this type of vaccine; each specific formulation has different age-based approvals.

• The quadrivalent flu vaccine protects against four flu viruses; two influenza A viruses and two influenza B viruses. A standard dose quadrivalent formulation is available for children; one brand is approved for children 6 months and older while others are approved for those 3 years and older.
• A cell-based vaccine, developed through a manufacturing process different from the traditional egg-based manufacturing process, was approved as a quadrivalent formulation for use in children 4 years of age and older.
Unanswered questions for the 2016-2017 influenza season
• Children 6 months to 8 years who are getting vaccinated for the first time need two doses. How should we consider influenza-naive children who received two doses of LAIV last year? The reason for the LAIV’s loss of efficacy in the years 2014 through 2016 is unknown, although it has been hypothesized that reduced immunogenicity is one possible cause for the lack of protection. Rather than speculate, we need to wait for ACIP to gather more data and then publish recommendations as to whether to consider such children vaccine naive (and therefore requiring two doses this season) or previously immunized (and therefore in need of only a single dose).
• Will supply be adequate this year? LAIV represents about 8% of the 171-176 million doses that were projected to be available during the 2016-2017 season; however, it represents nearly one-third of doses given to children. Thus, the potential for shortages in pediatric offices is real, and pediatricians and vaccine manufacturers need to work together to make sure sufficient pediatric formulation is available. The CDC is working with manufacturers to ensure there is sufficient supply to meet the demand.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He has received honoraria from Sanofi Pasteur and Seqirus for participation in vaccine advisory boards in the prior 12 months. Email him at [email protected].
Bacterial colonizer vs. pathogen
Although acute otitis media (AOM) has decreased in number, and especially the more severe difficult to treat versions, I was reminded that this still is a problem for young children based on personal experience with grandchildren. What can be baffling to some families is the fact that some strains of the same organism species cause AOM and some simply colonize the nasopharynx (NP) of children without causing any disease at all. These organisms include Streptococcus pneumoniae (SPN) and nontypeable Haemophilus influenzae (ntHi).
Recent studies have uncovered several molecular reasons for the pathogen vs. colonizer dichotomy:
• Strains within a species can have variants of a gene that make them more disease producing.
• Some usually colonizing strains produce disease after acquiring new genes.
• Some strains have native genes with on-off switches that convert them from a colonizing to disease producing under selected circumstances.
• Molecular targets in the respiratory tract increase, allowing more dense colonization that increases chances of AOM.
Variant gene
J.R. Gilsdorf, MD, and his group at the University of Michigan,1 Ann Arbor, recently showed that among the various high-molecular-weight molecules (HMW) produced by 170 ntHi from three different geographically diverse countries, one variant in particular (HMW-A) was more likely to be found in strains producing AOM than strains simply colonizing the nasopharynx. The protein product of this gene allows better adherence to respiratory epithelia. So more bacteria sticking in the NP near the eustachian tube opening make development of AOM more likely. Some call this the “more barbarians at the gate” phenomenon.
Gene acquisition
SPN inherently has a somewhat incomplete arginine synthesis pathway. Because arginine is essential for growth of SPN, S. pneumoniae utilizes some host factors to compensate; but this compensation is inefficient. However, SPN strains can acquire new genes – usually from other gram-positive organisms in their environment – by a process called conjugation.
One recently reported acquired gene set is that which completes functionality of SPN’s arginine synthesis pathway.2 Investigators showed that SPN that acquire these arginine synthesis genes replicate more readily in bodily fluids, such as serum or cerebrospinal fluid, making these strains more aggressive, more virulent, and more likely to produce disease. More efficient replication makes it very difficult for host immune responses to handle these SPN. This is not limited to AOM alone, but seems important in invasive disease (such as meningitis) from SPN type 7, which had recently become more frequent after introduction of pneumococcal conjugate vaccines.
On-off gene switches
Another group of investigators reported that a thing called “phasevarion,” which is fancy lingo for an on-off switch is at the root of more virulence in ntHi.3 It seems that some strains of ntHi have a version of the ModA2 gene, which is always turned off, while other strains have a gene that is always on. Then, there is a third version in which the gene is usually off, but turns on when in places like the middle ear. The ModA2 gene appears to affect several other downstream protein groups that include HMW-A, antibiotic susceptibility, and biofilm formation. When inoculated into the middle ear in a chinchilla AOM model, the ntHi strains that can turn on their ModA2 gene were much more likely to produce AOM than either version that could not change. Interestingly, the authors postulate that preventing the switch capability could be a novel way to prevent ntHi disease, such as pediatric AOM, acute bacterial sinusitis, or some bronchitis in adults.
Molecular environment becomes more favorable
Another group4 reported that adherence receptor for ntHi is intercellular adhesion molecule 1 (ICAM1), a molecule found in modest quantities on respiratory epithelium. You may know it as the attachment molecule for rhinovirus and enteroviruses. What makes this interesting is that adenovirus, respiratory syncytial virus, and exposure to cigarette smoke5 markedly increase expression of ICAM1 on respiratory epithelium, predisposing to more ntHi adhering and more likely to produce an inflammatory process, such as AOM. This is another version of the barbarians at the gate phenomenon.
So when families ask why SPN or ntHi sometimes exist quietly (colonize) the nasopharynx and sometimes they cause AOM or acute bacterial sinusitis, you can hopefully use these four examples as partial explanations of why the same bacterial species has strains that can be either colonizers or pathogens.
References
1. Infect Genet Evol. 2014 Dec;28:223-32
2. J Infect Dis. 2014 Jun 1;209:1781-91.
3. J Infect Dis. 2016 Jun 10. pii: jiw243. [Epub ahead of print]
4. Cell Microbiol. 2016 Feb 9. doi: 10.1111/cmi.12575. [Epub ahead of print]
5. Am J Respir Cell Mol Biol. 2003 Oct;29:472-82.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
Although acute otitis media (AOM) has decreased in number, and especially the more severe difficult to treat versions, I was reminded that this still is a problem for young children based on personal experience with grandchildren. What can be baffling to some families is the fact that some strains of the same organism species cause AOM and some simply colonize the nasopharynx (NP) of children without causing any disease at all. These organisms include Streptococcus pneumoniae (SPN) and nontypeable Haemophilus influenzae (ntHi).
Recent studies have uncovered several molecular reasons for the pathogen vs. colonizer dichotomy:
• Strains within a species can have variants of a gene that make them more disease producing.
• Some usually colonizing strains produce disease after acquiring new genes.
• Some strains have native genes with on-off switches that convert them from a colonizing to disease producing under selected circumstances.
• Molecular targets in the respiratory tract increase, allowing more dense colonization that increases chances of AOM.
Variant gene
J.R. Gilsdorf, MD, and his group at the University of Michigan,1 Ann Arbor, recently showed that among the various high-molecular-weight molecules (HMW) produced by 170 ntHi from three different geographically diverse countries, one variant in particular (HMW-A) was more likely to be found in strains producing AOM than strains simply colonizing the nasopharynx. The protein product of this gene allows better adherence to respiratory epithelia. So more bacteria sticking in the NP near the eustachian tube opening make development of AOM more likely. Some call this the “more barbarians at the gate” phenomenon.
Gene acquisition
SPN inherently has a somewhat incomplete arginine synthesis pathway. Because arginine is essential for growth of SPN, S. pneumoniae utilizes some host factors to compensate; but this compensation is inefficient. However, SPN strains can acquire new genes – usually from other gram-positive organisms in their environment – by a process called conjugation.
One recently reported acquired gene set is that which completes functionality of SPN’s arginine synthesis pathway.2 Investigators showed that SPN that acquire these arginine synthesis genes replicate more readily in bodily fluids, such as serum or cerebrospinal fluid, making these strains more aggressive, more virulent, and more likely to produce disease. More efficient replication makes it very difficult for host immune responses to handle these SPN. This is not limited to AOM alone, but seems important in invasive disease (such as meningitis) from SPN type 7, which had recently become more frequent after introduction of pneumococcal conjugate vaccines.
On-off gene switches
Another group of investigators reported that a thing called “phasevarion,” which is fancy lingo for an on-off switch is at the root of more virulence in ntHi.3 It seems that some strains of ntHi have a version of the ModA2 gene, which is always turned off, while other strains have a gene that is always on. Then, there is a third version in which the gene is usually off, but turns on when in places like the middle ear. The ModA2 gene appears to affect several other downstream protein groups that include HMW-A, antibiotic susceptibility, and biofilm formation. When inoculated into the middle ear in a chinchilla AOM model, the ntHi strains that can turn on their ModA2 gene were much more likely to produce AOM than either version that could not change. Interestingly, the authors postulate that preventing the switch capability could be a novel way to prevent ntHi disease, such as pediatric AOM, acute bacterial sinusitis, or some bronchitis in adults.
Molecular environment becomes more favorable
Another group4 reported that adherence receptor for ntHi is intercellular adhesion molecule 1 (ICAM1), a molecule found in modest quantities on respiratory epithelium. You may know it as the attachment molecule for rhinovirus and enteroviruses. What makes this interesting is that adenovirus, respiratory syncytial virus, and exposure to cigarette smoke5 markedly increase expression of ICAM1 on respiratory epithelium, predisposing to more ntHi adhering and more likely to produce an inflammatory process, such as AOM. This is another version of the barbarians at the gate phenomenon.
So when families ask why SPN or ntHi sometimes exist quietly (colonize) the nasopharynx and sometimes they cause AOM or acute bacterial sinusitis, you can hopefully use these four examples as partial explanations of why the same bacterial species has strains that can be either colonizers or pathogens.
References
1. Infect Genet Evol. 2014 Dec;28:223-32
2. J Infect Dis. 2014 Jun 1;209:1781-91.
3. J Infect Dis. 2016 Jun 10. pii: jiw243. [Epub ahead of print]
4. Cell Microbiol. 2016 Feb 9. doi: 10.1111/cmi.12575. [Epub ahead of print]
5. Am J Respir Cell Mol Biol. 2003 Oct;29:472-82.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
Although acute otitis media (AOM) has decreased in number, and especially the more severe difficult to treat versions, I was reminded that this still is a problem for young children based on personal experience with grandchildren. What can be baffling to some families is the fact that some strains of the same organism species cause AOM and some simply colonize the nasopharynx (NP) of children without causing any disease at all. These organisms include Streptococcus pneumoniae (SPN) and nontypeable Haemophilus influenzae (ntHi).
Recent studies have uncovered several molecular reasons for the pathogen vs. colonizer dichotomy:
• Strains within a species can have variants of a gene that make them more disease producing.
• Some usually colonizing strains produce disease after acquiring new genes.
• Some strains have native genes with on-off switches that convert them from a colonizing to disease producing under selected circumstances.
• Molecular targets in the respiratory tract increase, allowing more dense colonization that increases chances of AOM.
Variant gene
J.R. Gilsdorf, MD, and his group at the University of Michigan,1 Ann Arbor, recently showed that among the various high-molecular-weight molecules (HMW) produced by 170 ntHi from three different geographically diverse countries, one variant in particular (HMW-A) was more likely to be found in strains producing AOM than strains simply colonizing the nasopharynx. The protein product of this gene allows better adherence to respiratory epithelia. So more bacteria sticking in the NP near the eustachian tube opening make development of AOM more likely. Some call this the “more barbarians at the gate” phenomenon.
Gene acquisition
SPN inherently has a somewhat incomplete arginine synthesis pathway. Because arginine is essential for growth of SPN, S. pneumoniae utilizes some host factors to compensate; but this compensation is inefficient. However, SPN strains can acquire new genes – usually from other gram-positive organisms in their environment – by a process called conjugation.
One recently reported acquired gene set is that which completes functionality of SPN’s arginine synthesis pathway.2 Investigators showed that SPN that acquire these arginine synthesis genes replicate more readily in bodily fluids, such as serum or cerebrospinal fluid, making these strains more aggressive, more virulent, and more likely to produce disease. More efficient replication makes it very difficult for host immune responses to handle these SPN. This is not limited to AOM alone, but seems important in invasive disease (such as meningitis) from SPN type 7, which had recently become more frequent after introduction of pneumococcal conjugate vaccines.
On-off gene switches
Another group of investigators reported that a thing called “phasevarion,” which is fancy lingo for an on-off switch is at the root of more virulence in ntHi.3 It seems that some strains of ntHi have a version of the ModA2 gene, which is always turned off, while other strains have a gene that is always on. Then, there is a third version in which the gene is usually off, but turns on when in places like the middle ear. The ModA2 gene appears to affect several other downstream protein groups that include HMW-A, antibiotic susceptibility, and biofilm formation. When inoculated into the middle ear in a chinchilla AOM model, the ntHi strains that can turn on their ModA2 gene were much more likely to produce AOM than either version that could not change. Interestingly, the authors postulate that preventing the switch capability could be a novel way to prevent ntHi disease, such as pediatric AOM, acute bacterial sinusitis, or some bronchitis in adults.
Molecular environment becomes more favorable
Another group4 reported that adherence receptor for ntHi is intercellular adhesion molecule 1 (ICAM1), a molecule found in modest quantities on respiratory epithelium. You may know it as the attachment molecule for rhinovirus and enteroviruses. What makes this interesting is that adenovirus, respiratory syncytial virus, and exposure to cigarette smoke5 markedly increase expression of ICAM1 on respiratory epithelium, predisposing to more ntHi adhering and more likely to produce an inflammatory process, such as AOM. This is another version of the barbarians at the gate phenomenon.
So when families ask why SPN or ntHi sometimes exist quietly (colonize) the nasopharynx and sometimes they cause AOM or acute bacterial sinusitis, you can hopefully use these four examples as partial explanations of why the same bacterial species has strains that can be either colonizers or pathogens.
References
1. Infect Genet Evol. 2014 Dec;28:223-32
2. J Infect Dis. 2014 Jun 1;209:1781-91.
3. J Infect Dis. 2016 Jun 10. pii: jiw243. [Epub ahead of print]
4. Cell Microbiol. 2016 Feb 9. doi: 10.1111/cmi.12575. [Epub ahead of print]
5. Am J Respir Cell Mol Biol. 2003 Oct;29:472-82.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
Summer colds
Most viral infections in summer months are caused by enteroviruses. We studied illnesses in about 400 kids aged 4-18 years seen in private pediatric practice and were surprised by what we found.
Our impression was that summer colds lasted for a shorter time span than winter colds. What we found was that the median duration of illness was about 8 days. Among the various syndromes, the most common was stomatitis (viral blisters in the throat), accounting for 58% of all cases seen. A flulike illness with fever, myalgias, and malaise was second most common (28% of cases), followed by hand/foot/mouth syndrome (8%), pleurodynia (3%), fever with viral rash (3%), and aseptic meningitis (1%). Most of the cases occurred among children 4-12 years old.
The most prevalent symptoms were fever, headache, sore throat, tiredness, muscle aches, and crankiness. Fever was present in about 85% of cases of children with stomatitis, in 95% of cases with myalgias and malaise, but in only 50% of cases of hand/foot/mouth. Headache was very common as well, occurring in about 40% of children with stomatitis, 70% of children with myalgias and malaise, and in 30% of children with hand/foot/mouth.
Illness within a household was quite common. About 50% of the children who came for care had a sibling or parent ill with a summer cold. However, while the symptoms of the family members often were the same as the child who presented for care, that was not always the case. As anticipated, most illness within a household occurred within a 2-week time span. Hand/foot/mouth was most easily recognized by parents to have spread among their children. When a parent became ill, it was almost always the mother because she was almost always the primary parent caretaker.
Summer colds took a toll on families in terms of loss of work by parents. Most of the children were ill enough to stay out of day care or school for about 2-4 days. Virtually all the children with hand/foot/mouth and stomatitis with classic viral blister lesions had a single visit to the pediatric practice, and very limited or no tests done or medications prescribed other than acetaminophen or ibuprofen. But for the children with higher fevers without hand/foot/mouth or stomatitis, the costs of care escalated as tests were much more often performed (CBC, chest x-ray), and medications prescribed (antibiotics for uncertain diagnosis in the context of high fever), and occasional referrals made to the emergency department for further work-up (100% of cases of aseptic meningitis and 50% of cases of pleurodynia).
Overall, summer colds are not so insignificant as presumed at first glance. What interests me now is why summer colds so infrequently are followed by an acute otitis media or sinusitis, whereas winter colds caused by respiratory syncytial virus, influenza, and rhinoviruses are followed by an acute otitis media in about one-third of cases. A new study is underway!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Most viral infections in summer months are caused by enteroviruses. We studied illnesses in about 400 kids aged 4-18 years seen in private pediatric practice and were surprised by what we found.
Our impression was that summer colds lasted for a shorter time span than winter colds. What we found was that the median duration of illness was about 8 days. Among the various syndromes, the most common was stomatitis (viral blisters in the throat), accounting for 58% of all cases seen. A flulike illness with fever, myalgias, and malaise was second most common (28% of cases), followed by hand/foot/mouth syndrome (8%), pleurodynia (3%), fever with viral rash (3%), and aseptic meningitis (1%). Most of the cases occurred among children 4-12 years old.
The most prevalent symptoms were fever, headache, sore throat, tiredness, muscle aches, and crankiness. Fever was present in about 85% of cases of children with stomatitis, in 95% of cases with myalgias and malaise, but in only 50% of cases of hand/foot/mouth. Headache was very common as well, occurring in about 40% of children with stomatitis, 70% of children with myalgias and malaise, and in 30% of children with hand/foot/mouth.
Illness within a household was quite common. About 50% of the children who came for care had a sibling or parent ill with a summer cold. However, while the symptoms of the family members often were the same as the child who presented for care, that was not always the case. As anticipated, most illness within a household occurred within a 2-week time span. Hand/foot/mouth was most easily recognized by parents to have spread among their children. When a parent became ill, it was almost always the mother because she was almost always the primary parent caretaker.
Summer colds took a toll on families in terms of loss of work by parents. Most of the children were ill enough to stay out of day care or school for about 2-4 days. Virtually all the children with hand/foot/mouth and stomatitis with classic viral blister lesions had a single visit to the pediatric practice, and very limited or no tests done or medications prescribed other than acetaminophen or ibuprofen. But for the children with higher fevers without hand/foot/mouth or stomatitis, the costs of care escalated as tests were much more often performed (CBC, chest x-ray), and medications prescribed (antibiotics for uncertain diagnosis in the context of high fever), and occasional referrals made to the emergency department for further work-up (100% of cases of aseptic meningitis and 50% of cases of pleurodynia).
Overall, summer colds are not so insignificant as presumed at first glance. What interests me now is why summer colds so infrequently are followed by an acute otitis media or sinusitis, whereas winter colds caused by respiratory syncytial virus, influenza, and rhinoviruses are followed by an acute otitis media in about one-third of cases. A new study is underway!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Most viral infections in summer months are caused by enteroviruses. We studied illnesses in about 400 kids aged 4-18 years seen in private pediatric practice and were surprised by what we found.
Our impression was that summer colds lasted for a shorter time span than winter colds. What we found was that the median duration of illness was about 8 days. Among the various syndromes, the most common was stomatitis (viral blisters in the throat), accounting for 58% of all cases seen. A flulike illness with fever, myalgias, and malaise was second most common (28% of cases), followed by hand/foot/mouth syndrome (8%), pleurodynia (3%), fever with viral rash (3%), and aseptic meningitis (1%). Most of the cases occurred among children 4-12 years old.
The most prevalent symptoms were fever, headache, sore throat, tiredness, muscle aches, and crankiness. Fever was present in about 85% of cases of children with stomatitis, in 95% of cases with myalgias and malaise, but in only 50% of cases of hand/foot/mouth. Headache was very common as well, occurring in about 40% of children with stomatitis, 70% of children with myalgias and malaise, and in 30% of children with hand/foot/mouth.
Illness within a household was quite common. About 50% of the children who came for care had a sibling or parent ill with a summer cold. However, while the symptoms of the family members often were the same as the child who presented for care, that was not always the case. As anticipated, most illness within a household occurred within a 2-week time span. Hand/foot/mouth was most easily recognized by parents to have spread among their children. When a parent became ill, it was almost always the mother because she was almost always the primary parent caretaker.
Summer colds took a toll on families in terms of loss of work by parents. Most of the children were ill enough to stay out of day care or school for about 2-4 days. Virtually all the children with hand/foot/mouth and stomatitis with classic viral blister lesions had a single visit to the pediatric practice, and very limited or no tests done or medications prescribed other than acetaminophen or ibuprofen. But for the children with higher fevers without hand/foot/mouth or stomatitis, the costs of care escalated as tests were much more often performed (CBC, chest x-ray), and medications prescribed (antibiotics for uncertain diagnosis in the context of high fever), and occasional referrals made to the emergency department for further work-up (100% of cases of aseptic meningitis and 50% of cases of pleurodynia).
Overall, summer colds are not so insignificant as presumed at first glance. What interests me now is why summer colds so infrequently are followed by an acute otitis media or sinusitis, whereas winter colds caused by respiratory syncytial virus, influenza, and rhinoviruses are followed by an acute otitis media in about one-third of cases. A new study is underway!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Arboviral and other vector-borne diseases
May has arrived, and for the majority of your patients it signals the end of the school year and the beginning of summer vacation. Zika virus is on the minds of most people since its arrival to the Western Hemisphere in March 2015. With the fluidity of this outbreak and almost daily news updates and recommendations, many parents have voiced or will be voicing concerns regarding summer travel destinations.
Many concerns about Zika virus have been previously addressed in this column (“Zika virus: More questions than answers?” by Dr. Kristina Bryant). However, if the decision is to avoid international travel because of the ongoing Zika outbreak, it doesn’t mean your patients get a free pass and will not have to be concerned about acquiring any infectious diseases. They still need to be vigilant about avoiding those pesky vectors that transmit arboviruses and other vector-borne diseases that occur in the United States.
Arboviruses are transmitted by mosquitoes, ticks, or fleas. Most infections are subclinical. If symptoms develop, they are manifested by a generalized febrile illness including fever, headache, myalgia, arthralgia, and rash. Hemorrhagic fever (dengue) or neuroinvasive disease can include aseptic meningitis, encephalitis, or acute flaccid paralysis. Neuroinvasive disease rarely occurs with dengue, Colorado tick fever, and chikungunya infections.
While more than 100 arboviruses can cause infection, some of the more common arboviruses associated with human disease include West Nile, first detected in the United States in 1999 and chikungunya, first reported in the Americas in 2013 with local transmission documented in Florida, Puerto Rico, and the U.S. Virgin Islands in 2014. It is estimated that dengue causes over 100 million cases worldwide annually. Almost 40% of the world’s inhabitants live in endemic areas. The majority of cases on the U.S. mainland are imported. However, it is endemic in all U.S. territories including Guam, American Samoa, the U.S. Virgin Islands, and Puerto Rico. Between September 2015 and March 2016, Hawaii experienced a dengue outbreak involving 264 individuals including 46 children. As of April 16, 2016, there were no infectious individuals on the island.
Other domestic arboviruses causing disease include St. Louis, Eastern, and Western Equine encephalitis, La Crosse encephalitis, Colorado tick fever, and Powassan virus. All are transmitted by mosquitoes with the exception of Powassan and Colorado tick fever, which are transmitted by ticks. The numbers of cases nationally are much lower for these diseases, compared with West Nile, dengue, and chikungunya. National and state-specific information is available for domestic arboviruses at diseasemaps.usgs.gov/mapviewer. Data is compiled by ArboNET, a national arboviral surveillance system that is managed by the Centers for Disease Control and Prevention (CDC) in conjunction with state health departments. Not only is human disease monitored, but it also maintains data on viremic blood donors, dead birds, mosquitoes, veterinary disease cases, and sentinel animals.
Spring and summer are the most active seasons for ticks. Bacterial and spirochetal diseases transmitted by them include rickettsial diseases such as Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis. Tularemia in addition to Lyme and tick-borne relapsing fever are also transmitted by ticks. Babesiosis, which is due to a parasite, and southern tick-associated rash illness (STARI), whose causative agent is yet to be determined, are two additional tick-related diagnoses.
Of note, dengue, chikungunya, and Zika are all transmitted by infected Aedes mosquitoes. There is no enzootic cycle. Just human-mosquito-human transmission. In contrast, West Nile virus is transmitted by Culex mosquitoes in an enzootic cycle between an avian reservoir and humans.
Treatment
There is no specific treatment for arboviral infections. The primary goal is relief of symptoms with fluids, bed rest, and analgesics. For bacterial vector-borne diseases, antibiotic therapy is indicated and is based on the specific pathogen. Doxycycline is the drug of choice for treatment of suspected and confirmed Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis even in children less than 8 years of age. Delay in initiation of antimicrobial therapy pending definitive diagnosis may lead to an adverse outcome. It is also the drug of choice for tick-borne relapsing fever.
Lyme disease is also responsive to antibiotic treatment. Therapy is based on the disease category. (Lyme disease in “Red Book: 2015 Report of the Committee on Infectious Diseases,” [Elk Grove Village, Ill.: American Academy of Pediatrics, 2015, pp. 516-25]).
STARI clinically presents with a lesion that resembles erythema migrans in southern and southeastern states. However, it has not been associated with any of the complications reported with disseminated Lyme disease. Treatment is not recommended.
Tularemia and babesiosis are both responsive to antimicrobial therapy and would best be managed in consultation with an infectious disease physician.
A handy, concise, up to date reference guide about all of the tick-borne diseases including photographs is available at the App Store. The Tickborne Diseases App was developed by the CDC and it is free!
Prevention
The cornerstone of disease prevention is avoidance of mosquito and tick bites, in addition to eliminating mosquito breeding sites. Ticks are generally found near the ground, in brushy or wooded areas. They usually wait for a potential host to brush against them. When this happens, they climb onto the host and find a site to attach.
Is there a role for antimicrobial prophylaxis once a tick has been discovered? There is no data to support antimicrobial prophylaxis to prevent Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis. Prophylaxis with doxycycline or ciprofloxacin is recommended for children and adults after exposure to an intentional release of tularemia and for laboratory workers after inadvertent exposure. For prevention of Lyme disease, a single dose of doxycycline (4 mg/kg, max dose 200 mg) may be offered under limited conditions: The patient is at least 8 years of age, resides in an area where Lyme is highly endemic, the tick removed was engorged, therapy can be initiated within 72 hours after tick removal, and the estimated time of attachment was at least 36 hours. There is inadequate data on the use of amoxicillin.
Remember, not all mosquitoes are alike. Those that transmit chikungunya, dengue, and Zika (Aedes mosquitoes) are primarily daytime mosquitoes, but also can bite at night. West Nile is transmitted by Culex mosquitoes, which feed from dusk to dawn.
Here are some tips to share with your patients that should decrease their chances of acquiring a mosquito or tick-borne disease:
• Apply mosquito repellent only to intact exposed skin when outdoors. Most repellents can be safely used on children at least 2 months of age and older. Avoid applying repellent directly on the child’s hand. Use at least a 20% DEET (N,N-diethyl-meta-toluamide) containing product. Other Environmental Protection Agency–registered repellents are an alternative (Additional information is available at http://www2.epa.gov/insect-repellents). Products containing oil of lemon eucalyptus (OLE) or p-Menthane-3,8-diol (PMD) should not be used on children under 3 years of age.
• Apply permethrin to clothing, hats, boots, and so on. It is designed to repel mosquitoes and ticks. It can last for several washings. It is ideal to spray over nets covering carriers in children younger than 2 months of age.
• Wear long-sleeved shirts and long pants tucked inside of socks when hiking.
• Check for ticks daily, especially under the arms, behind the ears, around the waist, behind the knees, and inside belly buttons after outdoor activities.
• Have your patients learn how to effectively remove a tick. With a fine tipped tweezer, grasp the tick as close to the skin as possible and pull straight up with even pressure. Do not twist or jerk the tick. Do not squash the tick. Place it in a bag and dispose of it. Clean the site after removal with alcohol, iodine, or soap and water.
• Encourage families to mosquito proof their home by using screens on windows and doors, and using air conditioning when available.
• Empty and scrub all items that contain water such as birdbaths, planters, or wading pools around the outside of the home at least weekly because mosquitoes lay eggs in or near free standing water.
• Dogs and cats should be treated for ticks as recommended by the veterinarian.
The impact of the ongoing Zika virus outbreak is uncertain. While it may have an impact on those planning international travel now and in the near future, several arboviral and vector-borne diseases currently exist in the United States. Encouraging our patients to practice interventions to prevent mosquito and tick bites now will also serve to protect them if Zika virus becomes established in the Aedes mosquitoes here in the future and/or if they have plans for international travel. For up to date information on Zika virus for yourself and your patients, visit www.cdc.gov/zika.
Bonnie M. Word, M.D., is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email Dr. Word at [email protected].
May has arrived, and for the majority of your patients it signals the end of the school year and the beginning of summer vacation. Zika virus is on the minds of most people since its arrival to the Western Hemisphere in March 2015. With the fluidity of this outbreak and almost daily news updates and recommendations, many parents have voiced or will be voicing concerns regarding summer travel destinations.
Many concerns about Zika virus have been previously addressed in this column (“Zika virus: More questions than answers?” by Dr. Kristina Bryant). However, if the decision is to avoid international travel because of the ongoing Zika outbreak, it doesn’t mean your patients get a free pass and will not have to be concerned about acquiring any infectious diseases. They still need to be vigilant about avoiding those pesky vectors that transmit arboviruses and other vector-borne diseases that occur in the United States.
Arboviruses are transmitted by mosquitoes, ticks, or fleas. Most infections are subclinical. If symptoms develop, they are manifested by a generalized febrile illness including fever, headache, myalgia, arthralgia, and rash. Hemorrhagic fever (dengue) or neuroinvasive disease can include aseptic meningitis, encephalitis, or acute flaccid paralysis. Neuroinvasive disease rarely occurs with dengue, Colorado tick fever, and chikungunya infections.
While more than 100 arboviruses can cause infection, some of the more common arboviruses associated with human disease include West Nile, first detected in the United States in 1999 and chikungunya, first reported in the Americas in 2013 with local transmission documented in Florida, Puerto Rico, and the U.S. Virgin Islands in 2014. It is estimated that dengue causes over 100 million cases worldwide annually. Almost 40% of the world’s inhabitants live in endemic areas. The majority of cases on the U.S. mainland are imported. However, it is endemic in all U.S. territories including Guam, American Samoa, the U.S. Virgin Islands, and Puerto Rico. Between September 2015 and March 2016, Hawaii experienced a dengue outbreak involving 264 individuals including 46 children. As of April 16, 2016, there were no infectious individuals on the island.
Other domestic arboviruses causing disease include St. Louis, Eastern, and Western Equine encephalitis, La Crosse encephalitis, Colorado tick fever, and Powassan virus. All are transmitted by mosquitoes with the exception of Powassan and Colorado tick fever, which are transmitted by ticks. The numbers of cases nationally are much lower for these diseases, compared with West Nile, dengue, and chikungunya. National and state-specific information is available for domestic arboviruses at diseasemaps.usgs.gov/mapviewer. Data is compiled by ArboNET, a national arboviral surveillance system that is managed by the Centers for Disease Control and Prevention (CDC) in conjunction with state health departments. Not only is human disease monitored, but it also maintains data on viremic blood donors, dead birds, mosquitoes, veterinary disease cases, and sentinel animals.
Spring and summer are the most active seasons for ticks. Bacterial and spirochetal diseases transmitted by them include rickettsial diseases such as Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis. Tularemia in addition to Lyme and tick-borne relapsing fever are also transmitted by ticks. Babesiosis, which is due to a parasite, and southern tick-associated rash illness (STARI), whose causative agent is yet to be determined, are two additional tick-related diagnoses.
Of note, dengue, chikungunya, and Zika are all transmitted by infected Aedes mosquitoes. There is no enzootic cycle. Just human-mosquito-human transmission. In contrast, West Nile virus is transmitted by Culex mosquitoes in an enzootic cycle between an avian reservoir and humans.
Treatment
There is no specific treatment for arboviral infections. The primary goal is relief of symptoms with fluids, bed rest, and analgesics. For bacterial vector-borne diseases, antibiotic therapy is indicated and is based on the specific pathogen. Doxycycline is the drug of choice for treatment of suspected and confirmed Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis even in children less than 8 years of age. Delay in initiation of antimicrobial therapy pending definitive diagnosis may lead to an adverse outcome. It is also the drug of choice for tick-borne relapsing fever.
Lyme disease is also responsive to antibiotic treatment. Therapy is based on the disease category. (Lyme disease in “Red Book: 2015 Report of the Committee on Infectious Diseases,” [Elk Grove Village, Ill.: American Academy of Pediatrics, 2015, pp. 516-25]).
STARI clinically presents with a lesion that resembles erythema migrans in southern and southeastern states. However, it has not been associated with any of the complications reported with disseminated Lyme disease. Treatment is not recommended.
Tularemia and babesiosis are both responsive to antimicrobial therapy and would best be managed in consultation with an infectious disease physician.
A handy, concise, up to date reference guide about all of the tick-borne diseases including photographs is available at the App Store. The Tickborne Diseases App was developed by the CDC and it is free!
Prevention
The cornerstone of disease prevention is avoidance of mosquito and tick bites, in addition to eliminating mosquito breeding sites. Ticks are generally found near the ground, in brushy or wooded areas. They usually wait for a potential host to brush against them. When this happens, they climb onto the host and find a site to attach.
Is there a role for antimicrobial prophylaxis once a tick has been discovered? There is no data to support antimicrobial prophylaxis to prevent Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis. Prophylaxis with doxycycline or ciprofloxacin is recommended for children and adults after exposure to an intentional release of tularemia and for laboratory workers after inadvertent exposure. For prevention of Lyme disease, a single dose of doxycycline (4 mg/kg, max dose 200 mg) may be offered under limited conditions: The patient is at least 8 years of age, resides in an area where Lyme is highly endemic, the tick removed was engorged, therapy can be initiated within 72 hours after tick removal, and the estimated time of attachment was at least 36 hours. There is inadequate data on the use of amoxicillin.
Remember, not all mosquitoes are alike. Those that transmit chikungunya, dengue, and Zika (Aedes mosquitoes) are primarily daytime mosquitoes, but also can bite at night. West Nile is transmitted by Culex mosquitoes, which feed from dusk to dawn.
Here are some tips to share with your patients that should decrease their chances of acquiring a mosquito or tick-borne disease:
• Apply mosquito repellent only to intact exposed skin when outdoors. Most repellents can be safely used on children at least 2 months of age and older. Avoid applying repellent directly on the child’s hand. Use at least a 20% DEET (N,N-diethyl-meta-toluamide) containing product. Other Environmental Protection Agency–registered repellents are an alternative (Additional information is available at http://www2.epa.gov/insect-repellents). Products containing oil of lemon eucalyptus (OLE) or p-Menthane-3,8-diol (PMD) should not be used on children under 3 years of age.
• Apply permethrin to clothing, hats, boots, and so on. It is designed to repel mosquitoes and ticks. It can last for several washings. It is ideal to spray over nets covering carriers in children younger than 2 months of age.
• Wear long-sleeved shirts and long pants tucked inside of socks when hiking.
• Check for ticks daily, especially under the arms, behind the ears, around the waist, behind the knees, and inside belly buttons after outdoor activities.
• Have your patients learn how to effectively remove a tick. With a fine tipped tweezer, grasp the tick as close to the skin as possible and pull straight up with even pressure. Do not twist or jerk the tick. Do not squash the tick. Place it in a bag and dispose of it. Clean the site after removal with alcohol, iodine, or soap and water.
• Encourage families to mosquito proof their home by using screens on windows and doors, and using air conditioning when available.
• Empty and scrub all items that contain water such as birdbaths, planters, or wading pools around the outside of the home at least weekly because mosquitoes lay eggs in or near free standing water.
• Dogs and cats should be treated for ticks as recommended by the veterinarian.
The impact of the ongoing Zika virus outbreak is uncertain. While it may have an impact on those planning international travel now and in the near future, several arboviral and vector-borne diseases currently exist in the United States. Encouraging our patients to practice interventions to prevent mosquito and tick bites now will also serve to protect them if Zika virus becomes established in the Aedes mosquitoes here in the future and/or if they have plans for international travel. For up to date information on Zika virus for yourself and your patients, visit www.cdc.gov/zika.
Bonnie M. Word, M.D., is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email Dr. Word at [email protected].
May has arrived, and for the majority of your patients it signals the end of the school year and the beginning of summer vacation. Zika virus is on the minds of most people since its arrival to the Western Hemisphere in March 2015. With the fluidity of this outbreak and almost daily news updates and recommendations, many parents have voiced or will be voicing concerns regarding summer travel destinations.
Many concerns about Zika virus have been previously addressed in this column (“Zika virus: More questions than answers?” by Dr. Kristina Bryant). However, if the decision is to avoid international travel because of the ongoing Zika outbreak, it doesn’t mean your patients get a free pass and will not have to be concerned about acquiring any infectious diseases. They still need to be vigilant about avoiding those pesky vectors that transmit arboviruses and other vector-borne diseases that occur in the United States.
Arboviruses are transmitted by mosquitoes, ticks, or fleas. Most infections are subclinical. If symptoms develop, they are manifested by a generalized febrile illness including fever, headache, myalgia, arthralgia, and rash. Hemorrhagic fever (dengue) or neuroinvasive disease can include aseptic meningitis, encephalitis, or acute flaccid paralysis. Neuroinvasive disease rarely occurs with dengue, Colorado tick fever, and chikungunya infections.
While more than 100 arboviruses can cause infection, some of the more common arboviruses associated with human disease include West Nile, first detected in the United States in 1999 and chikungunya, first reported in the Americas in 2013 with local transmission documented in Florida, Puerto Rico, and the U.S. Virgin Islands in 2014. It is estimated that dengue causes over 100 million cases worldwide annually. Almost 40% of the world’s inhabitants live in endemic areas. The majority of cases on the U.S. mainland are imported. However, it is endemic in all U.S. territories including Guam, American Samoa, the U.S. Virgin Islands, and Puerto Rico. Between September 2015 and March 2016, Hawaii experienced a dengue outbreak involving 264 individuals including 46 children. As of April 16, 2016, there were no infectious individuals on the island.
Other domestic arboviruses causing disease include St. Louis, Eastern, and Western Equine encephalitis, La Crosse encephalitis, Colorado tick fever, and Powassan virus. All are transmitted by mosquitoes with the exception of Powassan and Colorado tick fever, which are transmitted by ticks. The numbers of cases nationally are much lower for these diseases, compared with West Nile, dengue, and chikungunya. National and state-specific information is available for domestic arboviruses at diseasemaps.usgs.gov/mapviewer. Data is compiled by ArboNET, a national arboviral surveillance system that is managed by the Centers for Disease Control and Prevention (CDC) in conjunction with state health departments. Not only is human disease monitored, but it also maintains data on viremic blood donors, dead birds, mosquitoes, veterinary disease cases, and sentinel animals.
Spring and summer are the most active seasons for ticks. Bacterial and spirochetal diseases transmitted by them include rickettsial diseases such as Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis. Tularemia in addition to Lyme and tick-borne relapsing fever are also transmitted by ticks. Babesiosis, which is due to a parasite, and southern tick-associated rash illness (STARI), whose causative agent is yet to be determined, are two additional tick-related diagnoses.
Of note, dengue, chikungunya, and Zika are all transmitted by infected Aedes mosquitoes. There is no enzootic cycle. Just human-mosquito-human transmission. In contrast, West Nile virus is transmitted by Culex mosquitoes in an enzootic cycle between an avian reservoir and humans.
Treatment
There is no specific treatment for arboviral infections. The primary goal is relief of symptoms with fluids, bed rest, and analgesics. For bacterial vector-borne diseases, antibiotic therapy is indicated and is based on the specific pathogen. Doxycycline is the drug of choice for treatment of suspected and confirmed Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis even in children less than 8 years of age. Delay in initiation of antimicrobial therapy pending definitive diagnosis may lead to an adverse outcome. It is also the drug of choice for tick-borne relapsing fever.
Lyme disease is also responsive to antibiotic treatment. Therapy is based on the disease category. (Lyme disease in “Red Book: 2015 Report of the Committee on Infectious Diseases,” [Elk Grove Village, Ill.: American Academy of Pediatrics, 2015, pp. 516-25]).
STARI clinically presents with a lesion that resembles erythema migrans in southern and southeastern states. However, it has not been associated with any of the complications reported with disseminated Lyme disease. Treatment is not recommended.
Tularemia and babesiosis are both responsive to antimicrobial therapy and would best be managed in consultation with an infectious disease physician.
A handy, concise, up to date reference guide about all of the tick-borne diseases including photographs is available at the App Store. The Tickborne Diseases App was developed by the CDC and it is free!
Prevention
The cornerstone of disease prevention is avoidance of mosquito and tick bites, in addition to eliminating mosquito breeding sites. Ticks are generally found near the ground, in brushy or wooded areas. They usually wait for a potential host to brush against them. When this happens, they climb onto the host and find a site to attach.
Is there a role for antimicrobial prophylaxis once a tick has been discovered? There is no data to support antimicrobial prophylaxis to prevent Rocky Mountain spotted fever, ehrlichiosis, and anaplasmosis. Prophylaxis with doxycycline or ciprofloxacin is recommended for children and adults after exposure to an intentional release of tularemia and for laboratory workers after inadvertent exposure. For prevention of Lyme disease, a single dose of doxycycline (4 mg/kg, max dose 200 mg) may be offered under limited conditions: The patient is at least 8 years of age, resides in an area where Lyme is highly endemic, the tick removed was engorged, therapy can be initiated within 72 hours after tick removal, and the estimated time of attachment was at least 36 hours. There is inadequate data on the use of amoxicillin.
Remember, not all mosquitoes are alike. Those that transmit chikungunya, dengue, and Zika (Aedes mosquitoes) are primarily daytime mosquitoes, but also can bite at night. West Nile is transmitted by Culex mosquitoes, which feed from dusk to dawn.
Here are some tips to share with your patients that should decrease their chances of acquiring a mosquito or tick-borne disease:
• Apply mosquito repellent only to intact exposed skin when outdoors. Most repellents can be safely used on children at least 2 months of age and older. Avoid applying repellent directly on the child’s hand. Use at least a 20% DEET (N,N-diethyl-meta-toluamide) containing product. Other Environmental Protection Agency–registered repellents are an alternative (Additional information is available at http://www2.epa.gov/insect-repellents). Products containing oil of lemon eucalyptus (OLE) or p-Menthane-3,8-diol (PMD) should not be used on children under 3 years of age.
• Apply permethrin to clothing, hats, boots, and so on. It is designed to repel mosquitoes and ticks. It can last for several washings. It is ideal to spray over nets covering carriers in children younger than 2 months of age.
• Wear long-sleeved shirts and long pants tucked inside of socks when hiking.
• Check for ticks daily, especially under the arms, behind the ears, around the waist, behind the knees, and inside belly buttons after outdoor activities.
• Have your patients learn how to effectively remove a tick. With a fine tipped tweezer, grasp the tick as close to the skin as possible and pull straight up with even pressure. Do not twist or jerk the tick. Do not squash the tick. Place it in a bag and dispose of it. Clean the site after removal with alcohol, iodine, or soap and water.
• Encourage families to mosquito proof their home by using screens on windows and doors, and using air conditioning when available.
• Empty and scrub all items that contain water such as birdbaths, planters, or wading pools around the outside of the home at least weekly because mosquitoes lay eggs in or near free standing water.
• Dogs and cats should be treated for ticks as recommended by the veterinarian.
The impact of the ongoing Zika virus outbreak is uncertain. While it may have an impact on those planning international travel now and in the near future, several arboviral and vector-borne diseases currently exist in the United States. Encouraging our patients to practice interventions to prevent mosquito and tick bites now will also serve to protect them if Zika virus becomes established in the Aedes mosquitoes here in the future and/or if they have plans for international travel. For up to date information on Zika virus for yourself and your patients, visit www.cdc.gov/zika.
Bonnie M. Word, M.D., is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email Dr. Word at [email protected].
Zika virus: More questions than answers?
With spring break in full swing and summer vacations right around the corner, pediatricians are increasingly fielding questions from families about Zika virus.
“There are a lot of resources available online, but they’re constantly being updated, and it’s difficult to stay current,” a friend and fellow pediatrician confided. “It seems like there’s new information every day, but still as many questions as answers.”
A quick PubMed search validated her concern: More than 200 articles have been published about Zika virus since the beginning of the year. The Centers for Disease Control and Prevention and the World Health Organization post new information to their Zika websites regularly, if not daily, and the WHO has released a Zika app for clinicians. Understanding that the busy pediatrician may not always have time to peruse these authoritative references during the course of a day in the office, I’ve compiled some common questions and answers.
“Is Zika really as serious as the media portrays it?” asked the mother of two children as she contemplated Caribbean vacation plans. In truth, most healthy people infected with Zika virus never develop symptoms. Illness, when it occurs, is most often mild and includes low-grade fever, headache, arthralgia, myalgia, nonpurulent conjunctivitis, and a maculopapular rash. Unlike dengue, another Flavivirus carried by Aedes mosquitoes, Zika does not cause hemorrhagic fever, and death appears to be rare.
An understanding of Zika infection and neurologic complications is a work in progress. A 20-fold increase in the incidence of Guillain-Barré (GBS) cases was noted in French Polynesia during a 2013-2014 outbreak of Zika virus.
In a case-control study involving 42 patients hospitalized with GBS, 98% had anti–Zika virus IgM or IgG, and all had neutralizing antibodies against Zika virus, compared with 56% of 98 control patients (P less than .0001 ) (Lancet. 2016 Feb 29. doi: 10.1016/S0140-6736(16)00562-6).
To date, 10 countries or territories have reported GBS cases with confirmed Zika virus infection. According to the World Health Organization, “Zika virus is highly likely to be a cause of the elevated incidence of GBS in countries and territories in the Western Pacific and Americas,” but further research is needed. Zika has recently been associated with other neurologic disorders, including myelitis, and the full spectrum of disease is likely not yet known.
Most Zika virus infections are transmitted from the bite of an Aedes mosquito. What we know about Zika transmission among humans continues to evolve. Viremia can persist for 14 or more days after the onset of symptoms, during which time blood is a potential source of infection. Two possible cases of transfusion-related viral transmission are under investigation in Brazil, and during the French Polynesia outbreak, 3% of samples from asymptomatic blood donors contained detectable Zika RNA. The U.S. Food and Drug Administration has recommended that individuals who have lived in or traveled to an area with active Zika virus transmission defer blood donation for 4 weeks after departure from the area .
Zika virus also has been detected in the urine and saliva of infected individuals, but these fluids have not been linked to transmission. Sexual transmission from infected men to their partners is well documented, but the period of risk remains undefined. The virus can persist in the semen long after viremia clears, and in one individual, Zika virus was detected in the semen 62 days after symptom onset.
Maternal-fetal transmission can occur as early as the first trimester and as late as at the time of delivery. Zika virus has been recovered from both amniotic fluid and placentas. The consequences of maternal-fetal transmission are less certain. Coincident with an epidemic of Zika in Brazil, that country has observed a marked increase in the incidence of microcephaly. Between Oct. 22, 2015, and March 12, 2016, 6,480 cases of microcephaly and/or central nervous system malformation were reported in Brazil, contrasting sharply with the average of 163 cases reported annually from 2001 to 2014. Zika virus has been linked to 863 cases of microcephaly investigated thus far. Proving causality takes time, but the World Health Organization says the link between microcephaly and Zika infection is “strongly suspected.”
Because of the association between Zika virus and birth defects, including abnormal brain development, eye abnormalities, and hearing deficits, the CDC currently recommends that pregnant women not travel to areas with Zika transmission, while men who have lived in or traveled to an area with Zika and who have a pregnant partner should either use condoms or not have sex for the duration of the pregnancy.
The good news for nonpregnant women who contract Zika infection is that the infection is not thought to pose any risk to future pregnancies. Currently, there is no evidence that a fetus conceived after maternal viremia has resolved would be at risk for infection. Still, many unanswered questions remain about Zika infection during pregnancy. For example, it’s currently unknown how often infection is transmitted from an infected mother to her fetus, or if infection is more severe at a particular point in gestation.
Although Zika virus has been isolated from breast milk, no infections have been linked to breastfeeding, and mothers are encouraged to continue to nurse, even in areas with widespread transmission. Infection with Zika at the time of birth or later in childhood has not been linked to microcephaly. Beyond that, the long-term health outcomes of infants and children with Zika virus infection are unknown.
“How far north do you think the virus will spread?” one mom asked me. “Do I need to be worried?”
For public health officials, that’s the sixty-four thousand dollar question. To date, there have been no cases acquired as a result of a mosquito bite in the United States, but the edge of the outbreak continues to creep north. Local transmission of the virus was reported in Cuba on March 14.
As of March 16, 2016, 258 travel-associated Zika virus cases have been diagnosed in the United States, including 18 in pregnant women. Six of these were sexually transmitted. Theoretically, “onward transmission” from one of these cases could occur if the right kind of mosquito bites an infected person during the period of active viremia and then bites someone else, transferring a tiny amount of the virus-contaminated blood.
According to CDC experts, “Texas, Florida, and Hawaii are likely to be the U.S. states with the highest risk of experiencing local transmission of Zika virus by mosquitoes.” Although this estimate is based on prior experience with similar viruses, the principal vector of Zika, Aedes aegypti, has been identified as far west as California and in a number of states across the South, including my home state of Kentucky. Aedes albopictus mosquitoes also have been proven competent vectors for Zika virus transmission and are more widely distributed throughout the continental United States.
In a thoughtful review published in JAMA Pediatrics, “What Pediatricians and Other Clinicians Should Know About Zika Virus,” Dr. Mark W. Kline and Dr. Gordon E. Schutze noted that up to two-thirds of the U.S. population live in an area where Aedes mosquitoes are present at least part of the year (JAMA Pediatr. 2016 Feb 18. doi: 10.1001/jamapediatrics.2016.0429). Fortunately, transmission of dengue and chikungunya, two other viruses carried by the same insect, is still very uncommon. Public health experts are urging individuals with Zika virus infection to avoid mosquito bites during the first week of illness, to protect others.
We should start now counseling our patients and families to avoid mosquito bites at home and abroad. Besides Zika virus, mosquitoes transmit several pathogens in the United States each year, including West Nile virus, LaCrosse encephalitis virus, St. Louis encephalitis virus, and dengue.
Any collections of standing water should be eliminated, as these can be mosquito breeding grounds. These include flower pots, buckets, barrels, and discarded tires. The water in bird baths and pet dishes should be changed at least weekly, and children’s wading pools should be drained and stored on their side after use.
To the extent practical, exposed skin should be covered with long-sleeved shirts, long pants, and socks when individuals are in areas with mosquito activity. To enhance protection, clothing can be treated with permethrin, or pretreated clothing can be worn. An FDA-registered insect repellent should be applied to exposed skin, especially during hours of highest mosquito activity. Zika-carrying mosquitoes bite during the day, or dawn to dusk. Effective repellents include DEET, picaridin, IR3535, and oil of lemon eucalyptus, although families should read labels carefully as instructions for use vary, as does the recommended time period of reapplication. Combination sunscreen/insect repellent products are not recommended as repellent usually does not need to be reapplied as often as sunscreen. Parents also should be reminded not to use oil of lemon eucalyptus–containing products on children under 3 years of age.
“We’re going to get a lot more questions as the weather turns warmer,” said a colleague of mine. “I’m just waiting for the first call about a child who develops fever and a rash after a mosquito bite. Parents will wonder if it could be Zika.”
It is going to be an interesting summer. Stay tuned.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
With spring break in full swing and summer vacations right around the corner, pediatricians are increasingly fielding questions from families about Zika virus.
“There are a lot of resources available online, but they’re constantly being updated, and it’s difficult to stay current,” a friend and fellow pediatrician confided. “It seems like there’s new information every day, but still as many questions as answers.”
A quick PubMed search validated her concern: More than 200 articles have been published about Zika virus since the beginning of the year. The Centers for Disease Control and Prevention and the World Health Organization post new information to their Zika websites regularly, if not daily, and the WHO has released a Zika app for clinicians. Understanding that the busy pediatrician may not always have time to peruse these authoritative references during the course of a day in the office, I’ve compiled some common questions and answers.
“Is Zika really as serious as the media portrays it?” asked the mother of two children as she contemplated Caribbean vacation plans. In truth, most healthy people infected with Zika virus never develop symptoms. Illness, when it occurs, is most often mild and includes low-grade fever, headache, arthralgia, myalgia, nonpurulent conjunctivitis, and a maculopapular rash. Unlike dengue, another Flavivirus carried by Aedes mosquitoes, Zika does not cause hemorrhagic fever, and death appears to be rare.
An understanding of Zika infection and neurologic complications is a work in progress. A 20-fold increase in the incidence of Guillain-Barré (GBS) cases was noted in French Polynesia during a 2013-2014 outbreak of Zika virus.
In a case-control study involving 42 patients hospitalized with GBS, 98% had anti–Zika virus IgM or IgG, and all had neutralizing antibodies against Zika virus, compared with 56% of 98 control patients (P less than .0001 ) (Lancet. 2016 Feb 29. doi: 10.1016/S0140-6736(16)00562-6).
To date, 10 countries or territories have reported GBS cases with confirmed Zika virus infection. According to the World Health Organization, “Zika virus is highly likely to be a cause of the elevated incidence of GBS in countries and territories in the Western Pacific and Americas,” but further research is needed. Zika has recently been associated with other neurologic disorders, including myelitis, and the full spectrum of disease is likely not yet known.
Most Zika virus infections are transmitted from the bite of an Aedes mosquito. What we know about Zika transmission among humans continues to evolve. Viremia can persist for 14 or more days after the onset of symptoms, during which time blood is a potential source of infection. Two possible cases of transfusion-related viral transmission are under investigation in Brazil, and during the French Polynesia outbreak, 3% of samples from asymptomatic blood donors contained detectable Zika RNA. The U.S. Food and Drug Administration has recommended that individuals who have lived in or traveled to an area with active Zika virus transmission defer blood donation for 4 weeks after departure from the area .
Zika virus also has been detected in the urine and saliva of infected individuals, but these fluids have not been linked to transmission. Sexual transmission from infected men to their partners is well documented, but the period of risk remains undefined. The virus can persist in the semen long after viremia clears, and in one individual, Zika virus was detected in the semen 62 days after symptom onset.
Maternal-fetal transmission can occur as early as the first trimester and as late as at the time of delivery. Zika virus has been recovered from both amniotic fluid and placentas. The consequences of maternal-fetal transmission are less certain. Coincident with an epidemic of Zika in Brazil, that country has observed a marked increase in the incidence of microcephaly. Between Oct. 22, 2015, and March 12, 2016, 6,480 cases of microcephaly and/or central nervous system malformation were reported in Brazil, contrasting sharply with the average of 163 cases reported annually from 2001 to 2014. Zika virus has been linked to 863 cases of microcephaly investigated thus far. Proving causality takes time, but the World Health Organization says the link between microcephaly and Zika infection is “strongly suspected.”
Because of the association between Zika virus and birth defects, including abnormal brain development, eye abnormalities, and hearing deficits, the CDC currently recommends that pregnant women not travel to areas with Zika transmission, while men who have lived in or traveled to an area with Zika and who have a pregnant partner should either use condoms or not have sex for the duration of the pregnancy.
The good news for nonpregnant women who contract Zika infection is that the infection is not thought to pose any risk to future pregnancies. Currently, there is no evidence that a fetus conceived after maternal viremia has resolved would be at risk for infection. Still, many unanswered questions remain about Zika infection during pregnancy. For example, it’s currently unknown how often infection is transmitted from an infected mother to her fetus, or if infection is more severe at a particular point in gestation.
Although Zika virus has been isolated from breast milk, no infections have been linked to breastfeeding, and mothers are encouraged to continue to nurse, even in areas with widespread transmission. Infection with Zika at the time of birth or later in childhood has not been linked to microcephaly. Beyond that, the long-term health outcomes of infants and children with Zika virus infection are unknown.
“How far north do you think the virus will spread?” one mom asked me. “Do I need to be worried?”
For public health officials, that’s the sixty-four thousand dollar question. To date, there have been no cases acquired as a result of a mosquito bite in the United States, but the edge of the outbreak continues to creep north. Local transmission of the virus was reported in Cuba on March 14.
As of March 16, 2016, 258 travel-associated Zika virus cases have been diagnosed in the United States, including 18 in pregnant women. Six of these were sexually transmitted. Theoretically, “onward transmission” from one of these cases could occur if the right kind of mosquito bites an infected person during the period of active viremia and then bites someone else, transferring a tiny amount of the virus-contaminated blood.
According to CDC experts, “Texas, Florida, and Hawaii are likely to be the U.S. states with the highest risk of experiencing local transmission of Zika virus by mosquitoes.” Although this estimate is based on prior experience with similar viruses, the principal vector of Zika, Aedes aegypti, has been identified as far west as California and in a number of states across the South, including my home state of Kentucky. Aedes albopictus mosquitoes also have been proven competent vectors for Zika virus transmission and are more widely distributed throughout the continental United States.
In a thoughtful review published in JAMA Pediatrics, “What Pediatricians and Other Clinicians Should Know About Zika Virus,” Dr. Mark W. Kline and Dr. Gordon E. Schutze noted that up to two-thirds of the U.S. population live in an area where Aedes mosquitoes are present at least part of the year (JAMA Pediatr. 2016 Feb 18. doi: 10.1001/jamapediatrics.2016.0429). Fortunately, transmission of dengue and chikungunya, two other viruses carried by the same insect, is still very uncommon. Public health experts are urging individuals with Zika virus infection to avoid mosquito bites during the first week of illness, to protect others.
We should start now counseling our patients and families to avoid mosquito bites at home and abroad. Besides Zika virus, mosquitoes transmit several pathogens in the United States each year, including West Nile virus, LaCrosse encephalitis virus, St. Louis encephalitis virus, and dengue.
Any collections of standing water should be eliminated, as these can be mosquito breeding grounds. These include flower pots, buckets, barrels, and discarded tires. The water in bird baths and pet dishes should be changed at least weekly, and children’s wading pools should be drained and stored on their side after use.
To the extent practical, exposed skin should be covered with long-sleeved shirts, long pants, and socks when individuals are in areas with mosquito activity. To enhance protection, clothing can be treated with permethrin, or pretreated clothing can be worn. An FDA-registered insect repellent should be applied to exposed skin, especially during hours of highest mosquito activity. Zika-carrying mosquitoes bite during the day, or dawn to dusk. Effective repellents include DEET, picaridin, IR3535, and oil of lemon eucalyptus, although families should read labels carefully as instructions for use vary, as does the recommended time period of reapplication. Combination sunscreen/insect repellent products are not recommended as repellent usually does not need to be reapplied as often as sunscreen. Parents also should be reminded not to use oil of lemon eucalyptus–containing products on children under 3 years of age.
“We’re going to get a lot more questions as the weather turns warmer,” said a colleague of mine. “I’m just waiting for the first call about a child who develops fever and a rash after a mosquito bite. Parents will wonder if it could be Zika.”
It is going to be an interesting summer. Stay tuned.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
With spring break in full swing and summer vacations right around the corner, pediatricians are increasingly fielding questions from families about Zika virus.
“There are a lot of resources available online, but they’re constantly being updated, and it’s difficult to stay current,” a friend and fellow pediatrician confided. “It seems like there’s new information every day, but still as many questions as answers.”
A quick PubMed search validated her concern: More than 200 articles have been published about Zika virus since the beginning of the year. The Centers for Disease Control and Prevention and the World Health Organization post new information to their Zika websites regularly, if not daily, and the WHO has released a Zika app for clinicians. Understanding that the busy pediatrician may not always have time to peruse these authoritative references during the course of a day in the office, I’ve compiled some common questions and answers.
“Is Zika really as serious as the media portrays it?” asked the mother of two children as she contemplated Caribbean vacation plans. In truth, most healthy people infected with Zika virus never develop symptoms. Illness, when it occurs, is most often mild and includes low-grade fever, headache, arthralgia, myalgia, nonpurulent conjunctivitis, and a maculopapular rash. Unlike dengue, another Flavivirus carried by Aedes mosquitoes, Zika does not cause hemorrhagic fever, and death appears to be rare.
An understanding of Zika infection and neurologic complications is a work in progress. A 20-fold increase in the incidence of Guillain-Barré (GBS) cases was noted in French Polynesia during a 2013-2014 outbreak of Zika virus.
In a case-control study involving 42 patients hospitalized with GBS, 98% had anti–Zika virus IgM or IgG, and all had neutralizing antibodies against Zika virus, compared with 56% of 98 control patients (P less than .0001 ) (Lancet. 2016 Feb 29. doi: 10.1016/S0140-6736(16)00562-6).
To date, 10 countries or territories have reported GBS cases with confirmed Zika virus infection. According to the World Health Organization, “Zika virus is highly likely to be a cause of the elevated incidence of GBS in countries and territories in the Western Pacific and Americas,” but further research is needed. Zika has recently been associated with other neurologic disorders, including myelitis, and the full spectrum of disease is likely not yet known.
Most Zika virus infections are transmitted from the bite of an Aedes mosquito. What we know about Zika transmission among humans continues to evolve. Viremia can persist for 14 or more days after the onset of symptoms, during which time blood is a potential source of infection. Two possible cases of transfusion-related viral transmission are under investigation in Brazil, and during the French Polynesia outbreak, 3% of samples from asymptomatic blood donors contained detectable Zika RNA. The U.S. Food and Drug Administration has recommended that individuals who have lived in or traveled to an area with active Zika virus transmission defer blood donation for 4 weeks after departure from the area .
Zika virus also has been detected in the urine and saliva of infected individuals, but these fluids have not been linked to transmission. Sexual transmission from infected men to their partners is well documented, but the period of risk remains undefined. The virus can persist in the semen long after viremia clears, and in one individual, Zika virus was detected in the semen 62 days after symptom onset.
Maternal-fetal transmission can occur as early as the first trimester and as late as at the time of delivery. Zika virus has been recovered from both amniotic fluid and placentas. The consequences of maternal-fetal transmission are less certain. Coincident with an epidemic of Zika in Brazil, that country has observed a marked increase in the incidence of microcephaly. Between Oct. 22, 2015, and March 12, 2016, 6,480 cases of microcephaly and/or central nervous system malformation were reported in Brazil, contrasting sharply with the average of 163 cases reported annually from 2001 to 2014. Zika virus has been linked to 863 cases of microcephaly investigated thus far. Proving causality takes time, but the World Health Organization says the link between microcephaly and Zika infection is “strongly suspected.”
Because of the association between Zika virus and birth defects, including abnormal brain development, eye abnormalities, and hearing deficits, the CDC currently recommends that pregnant women not travel to areas with Zika transmission, while men who have lived in or traveled to an area with Zika and who have a pregnant partner should either use condoms or not have sex for the duration of the pregnancy.
The good news for nonpregnant women who contract Zika infection is that the infection is not thought to pose any risk to future pregnancies. Currently, there is no evidence that a fetus conceived after maternal viremia has resolved would be at risk for infection. Still, many unanswered questions remain about Zika infection during pregnancy. For example, it’s currently unknown how often infection is transmitted from an infected mother to her fetus, or if infection is more severe at a particular point in gestation.
Although Zika virus has been isolated from breast milk, no infections have been linked to breastfeeding, and mothers are encouraged to continue to nurse, even in areas with widespread transmission. Infection with Zika at the time of birth or later in childhood has not been linked to microcephaly. Beyond that, the long-term health outcomes of infants and children with Zika virus infection are unknown.
“How far north do you think the virus will spread?” one mom asked me. “Do I need to be worried?”
For public health officials, that’s the sixty-four thousand dollar question. To date, there have been no cases acquired as a result of a mosquito bite in the United States, but the edge of the outbreak continues to creep north. Local transmission of the virus was reported in Cuba on March 14.
As of March 16, 2016, 258 travel-associated Zika virus cases have been diagnosed in the United States, including 18 in pregnant women. Six of these were sexually transmitted. Theoretically, “onward transmission” from one of these cases could occur if the right kind of mosquito bites an infected person during the period of active viremia and then bites someone else, transferring a tiny amount of the virus-contaminated blood.
According to CDC experts, “Texas, Florida, and Hawaii are likely to be the U.S. states with the highest risk of experiencing local transmission of Zika virus by mosquitoes.” Although this estimate is based on prior experience with similar viruses, the principal vector of Zika, Aedes aegypti, has been identified as far west as California and in a number of states across the South, including my home state of Kentucky. Aedes albopictus mosquitoes also have been proven competent vectors for Zika virus transmission and are more widely distributed throughout the continental United States.
In a thoughtful review published in JAMA Pediatrics, “What Pediatricians and Other Clinicians Should Know About Zika Virus,” Dr. Mark W. Kline and Dr. Gordon E. Schutze noted that up to two-thirds of the U.S. population live in an area where Aedes mosquitoes are present at least part of the year (JAMA Pediatr. 2016 Feb 18. doi: 10.1001/jamapediatrics.2016.0429). Fortunately, transmission of dengue and chikungunya, two other viruses carried by the same insect, is still very uncommon. Public health experts are urging individuals with Zika virus infection to avoid mosquito bites during the first week of illness, to protect others.
We should start now counseling our patients and families to avoid mosquito bites at home and abroad. Besides Zika virus, mosquitoes transmit several pathogens in the United States each year, including West Nile virus, LaCrosse encephalitis virus, St. Louis encephalitis virus, and dengue.
Any collections of standing water should be eliminated, as these can be mosquito breeding grounds. These include flower pots, buckets, barrels, and discarded tires. The water in bird baths and pet dishes should be changed at least weekly, and children’s wading pools should be drained and stored on their side after use.
To the extent practical, exposed skin should be covered with long-sleeved shirts, long pants, and socks when individuals are in areas with mosquito activity. To enhance protection, clothing can be treated with permethrin, or pretreated clothing can be worn. An FDA-registered insect repellent should be applied to exposed skin, especially during hours of highest mosquito activity. Zika-carrying mosquitoes bite during the day, or dawn to dusk. Effective repellents include DEET, picaridin, IR3535, and oil of lemon eucalyptus, although families should read labels carefully as instructions for use vary, as does the recommended time period of reapplication. Combination sunscreen/insect repellent products are not recommended as repellent usually does not need to be reapplied as often as sunscreen. Parents also should be reminded not to use oil of lemon eucalyptus–containing products on children under 3 years of age.
“We’re going to get a lot more questions as the weather turns warmer,” said a colleague of mine. “I’m just waiting for the first call about a child who develops fever and a rash after a mosquito bite. Parents will wonder if it could be Zika.”
It is going to be an interesting summer. Stay tuned.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Why so many pertussis outbreaks despite acellular pertussis vaccine? A call to action
There has been a justified re-examination of acellular pertussis vaccine (aP)1,2 in light of the multiple large outbreaks of pertussis since 2000, particularly the two large California outbreaks in 2010 and 2014.
Lessons learned: aP protection is less durable than originally thought, and much pertussis is not in infants, but in the school-age and adolescent populations.
aP appears to produce reasonable protection (approximately 84% overall) for infants and preschool children, plus a much improved adverse effect profile, compared with whole cell pertussis vaccine (WCP), which provided approximately 94% protection.1 This 10% difference in aP versus WCP, however, means that herd immunity is more difficult to attain. The accepted pertussis immunization rate needed to provide herd immunity is 92%-94%. Because our current tools (DTaP and Tdap) provide only 84% protection at least in infants and preschoolers, even 100% uptake may leave us 6% to 8% short of the threshold for complete herd immunity.
The California outbreak data from school-age and teenage populations show protection rates drop each year post aP booster. That means that by the fourth year after the last dose, protection is less than 10%. So despite a Tdap dose at 11- to 12-years-of-age, protection gaps occur in 8-to 10-year-olds and 14- to 18-year-olds. These vulnerable periods in older children add to aP’s 84% vs. WCP’s 94% protection for those greater than 3 years of age, explaining more frequent pertussis outbreaks as the pool of WCP-immunized children among older populations decreased.
But before we place all blame on switching to aP, consider that we can now confirm more pertussis infections with molecular assays than was possible with culture and fluorescent assay testing in the WCP era. So improved testing sensitivity means more reports of minimally symptomatic cases that may have been missed before. So WCP, if still used today, might not show 94% protection either.
Additionally, aPs rely heavily on pertactin as a target antigen,3 likely less than WCP, given that WCP contained all pertussis antigens rather than just the 3-5 purified antigens in aPs. So the emergence of pertactin-altered pertussis strains could disproportionately affect protection from aP, compared with WCP.
There seem to be no quick fixes to preventing outbreaks using aPs as our vaccine. One suggestion by the authors of the California outbreak report is to use aP mostly to terminate outbreaks rather than routinely in late childhood. My concern is that if we do not continue routine use in 4-to 6-year-olds, 10-to 11-year-olds, and in early adulthood, the vulnerable proportion of the population during outbreaks would be larger, making outbreaks more difficult to terminate. So continuing to produce some protection, albeit short-lived, with current schedules of aP vaccines seems important.
Also remember that T cells, particularly TH 17 pertussis-specific cells, may be as important as pertussis antibody. Therefore, crafting pertussis vaccines that yield improved antibody plus T cell responses is the current goal. Disease and WCP seem to elicit more T-17 response than aP. One method to craft a better vaccine is to use antigen blends that differ from those in the current vaccines, such as antigens derived from circulating pertussis strains instead of the standard laboratory strain. Another option is to use current antigens but with more potent adjuvants. Such vaccines are likely 5 years away.
But we need to have reasonable expectations for pertussis vaccines. Pertussis infection begins in respiratory epithelium. Many of the most obvious signs and symptoms are due to destruction of ciliated respiratory epithelium plus increased tenacity/volume of secretions. Can a parenterally administered vaccine that induces mostly serum antibody protect against infection of epithelium where antibody concentrations are likely 10% or less than in serum? The short answer is – likely not. We should expect neither aP nor WCP to consistently protect against pertussis infection, but it does seem reasonable to expect aP to reduce disease severity. Preventing infections awaits a vaccine that induces surface IgA. Mucosally administered vaccines produce surface IgA – for example, rotavirus vaccine – but no mucosal pertussis vaccine appears imminent.
A key question is whether our most vulnerable populations, young children, have increased morbidity and mortality. Data from the California suggest an increase but mostly in infants under 6 months of age, the group not old enough to benefit from even the most effective of infant vaccines. Protecting young infants depends on vaccine administered prenatally to mothers. The over-representation of the Hispanic infants among fatalities shows a population on which to focus with maternal immunization. Hopefully, the recent universal TdaP recommendation in pregnancy will help when maternal immunization is higher than current approximately 50% rates.4
Despite the problems, it seems clear that we must continue to use current aP vaccines according to the current schedules, attempting to get as close to 100% uptake as possible. While the current, nearly 10% unimmunized rates add to the likelihood that we are losing complete herd immunity, partial herd immunity is better than no herd immunity.
Expectations: There will be ongoing outbreaks. Continue to be alert for signs of pertussis. They are often less obvious in older patients, and may be as subtle as more than 2 weeks of persistent cough. During outbreaks, we may be called upon to give aP doses at intervals shorter than the usual schedule.
Our responsibility: Do not become discouraged or lose enthusiasm for aP, but explain to parents that because aP is less reactogenic, it produces less protection and is less durable, particularly in school-age children. But please emphasize that modest protection is best in the youngest and modest protection of older children is better than none. Emphasize that the adverse effect profile of current aPs puts the harm/benefit balance heavily in favor of aP.
Bottom line: We can hopefully do better than the current 88% to 92% rate of aP vaccine uptake. We need to get as close to 100% uptake as possible until new vaccines or new strategies become available.
1. Clin Infect Dis. 2016 Feb 7; doi: 10.1093/cid/ciw051.
2. Pediatrics. 2016 Feb 5; doi: 10.1542/peds.2015-3326.
3. Expert Rev Vaccines. 2007 Feb;6(1):47-56.
4. Vaccine. 2016 Feb 10;34(7):968-73.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He disclosed that his institution received grant support for a study on hexavalent infant vaccine containing pertussis from GlaxoSmithKline, and he was the local primary investigator.*
*Correction, 2/17/2016: An earlier version of this article incompletely stated Dr. Harrison's disclosure information.
There has been a justified re-examination of acellular pertussis vaccine (aP)1,2 in light of the multiple large outbreaks of pertussis since 2000, particularly the two large California outbreaks in 2010 and 2014.
Lessons learned: aP protection is less durable than originally thought, and much pertussis is not in infants, but in the school-age and adolescent populations.
aP appears to produce reasonable protection (approximately 84% overall) for infants and preschool children, plus a much improved adverse effect profile, compared with whole cell pertussis vaccine (WCP), which provided approximately 94% protection.1 This 10% difference in aP versus WCP, however, means that herd immunity is more difficult to attain. The accepted pertussis immunization rate needed to provide herd immunity is 92%-94%. Because our current tools (DTaP and Tdap) provide only 84% protection at least in infants and preschoolers, even 100% uptake may leave us 6% to 8% short of the threshold for complete herd immunity.
The California outbreak data from school-age and teenage populations show protection rates drop each year post aP booster. That means that by the fourth year after the last dose, protection is less than 10%. So despite a Tdap dose at 11- to 12-years-of-age, protection gaps occur in 8-to 10-year-olds and 14- to 18-year-olds. These vulnerable periods in older children add to aP’s 84% vs. WCP’s 94% protection for those greater than 3 years of age, explaining more frequent pertussis outbreaks as the pool of WCP-immunized children among older populations decreased.
But before we place all blame on switching to aP, consider that we can now confirm more pertussis infections with molecular assays than was possible with culture and fluorescent assay testing in the WCP era. So improved testing sensitivity means more reports of minimally symptomatic cases that may have been missed before. So WCP, if still used today, might not show 94% protection either.
Additionally, aPs rely heavily on pertactin as a target antigen,3 likely less than WCP, given that WCP contained all pertussis antigens rather than just the 3-5 purified antigens in aPs. So the emergence of pertactin-altered pertussis strains could disproportionately affect protection from aP, compared with WCP.
There seem to be no quick fixes to preventing outbreaks using aPs as our vaccine. One suggestion by the authors of the California outbreak report is to use aP mostly to terminate outbreaks rather than routinely in late childhood. My concern is that if we do not continue routine use in 4-to 6-year-olds, 10-to 11-year-olds, and in early adulthood, the vulnerable proportion of the population during outbreaks would be larger, making outbreaks more difficult to terminate. So continuing to produce some protection, albeit short-lived, with current schedules of aP vaccines seems important.
Also remember that T cells, particularly TH 17 pertussis-specific cells, may be as important as pertussis antibody. Therefore, crafting pertussis vaccines that yield improved antibody plus T cell responses is the current goal. Disease and WCP seem to elicit more T-17 response than aP. One method to craft a better vaccine is to use antigen blends that differ from those in the current vaccines, such as antigens derived from circulating pertussis strains instead of the standard laboratory strain. Another option is to use current antigens but with more potent adjuvants. Such vaccines are likely 5 years away.
But we need to have reasonable expectations for pertussis vaccines. Pertussis infection begins in respiratory epithelium. Many of the most obvious signs and symptoms are due to destruction of ciliated respiratory epithelium plus increased tenacity/volume of secretions. Can a parenterally administered vaccine that induces mostly serum antibody protect against infection of epithelium where antibody concentrations are likely 10% or less than in serum? The short answer is – likely not. We should expect neither aP nor WCP to consistently protect against pertussis infection, but it does seem reasonable to expect aP to reduce disease severity. Preventing infections awaits a vaccine that induces surface IgA. Mucosally administered vaccines produce surface IgA – for example, rotavirus vaccine – but no mucosal pertussis vaccine appears imminent.
A key question is whether our most vulnerable populations, young children, have increased morbidity and mortality. Data from the California suggest an increase but mostly in infants under 6 months of age, the group not old enough to benefit from even the most effective of infant vaccines. Protecting young infants depends on vaccine administered prenatally to mothers. The over-representation of the Hispanic infants among fatalities shows a population on which to focus with maternal immunization. Hopefully, the recent universal TdaP recommendation in pregnancy will help when maternal immunization is higher than current approximately 50% rates.4
Despite the problems, it seems clear that we must continue to use current aP vaccines according to the current schedules, attempting to get as close to 100% uptake as possible. While the current, nearly 10% unimmunized rates add to the likelihood that we are losing complete herd immunity, partial herd immunity is better than no herd immunity.
Expectations: There will be ongoing outbreaks. Continue to be alert for signs of pertussis. They are often less obvious in older patients, and may be as subtle as more than 2 weeks of persistent cough. During outbreaks, we may be called upon to give aP doses at intervals shorter than the usual schedule.
Our responsibility: Do not become discouraged or lose enthusiasm for aP, but explain to parents that because aP is less reactogenic, it produces less protection and is less durable, particularly in school-age children. But please emphasize that modest protection is best in the youngest and modest protection of older children is better than none. Emphasize that the adverse effect profile of current aPs puts the harm/benefit balance heavily in favor of aP.
Bottom line: We can hopefully do better than the current 88% to 92% rate of aP vaccine uptake. We need to get as close to 100% uptake as possible until new vaccines or new strategies become available.
1. Clin Infect Dis. 2016 Feb 7; doi: 10.1093/cid/ciw051.
2. Pediatrics. 2016 Feb 5; doi: 10.1542/peds.2015-3326.
3. Expert Rev Vaccines. 2007 Feb;6(1):47-56.
4. Vaccine. 2016 Feb 10;34(7):968-73.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He disclosed that his institution received grant support for a study on hexavalent infant vaccine containing pertussis from GlaxoSmithKline, and he was the local primary investigator.*
*Correction, 2/17/2016: An earlier version of this article incompletely stated Dr. Harrison's disclosure information.
There has been a justified re-examination of acellular pertussis vaccine (aP)1,2 in light of the multiple large outbreaks of pertussis since 2000, particularly the two large California outbreaks in 2010 and 2014.
Lessons learned: aP protection is less durable than originally thought, and much pertussis is not in infants, but in the school-age and adolescent populations.
aP appears to produce reasonable protection (approximately 84% overall) for infants and preschool children, plus a much improved adverse effect profile, compared with whole cell pertussis vaccine (WCP), which provided approximately 94% protection.1 This 10% difference in aP versus WCP, however, means that herd immunity is more difficult to attain. The accepted pertussis immunization rate needed to provide herd immunity is 92%-94%. Because our current tools (DTaP and Tdap) provide only 84% protection at least in infants and preschoolers, even 100% uptake may leave us 6% to 8% short of the threshold for complete herd immunity.
The California outbreak data from school-age and teenage populations show protection rates drop each year post aP booster. That means that by the fourth year after the last dose, protection is less than 10%. So despite a Tdap dose at 11- to 12-years-of-age, protection gaps occur in 8-to 10-year-olds and 14- to 18-year-olds. These vulnerable periods in older children add to aP’s 84% vs. WCP’s 94% protection for those greater than 3 years of age, explaining more frequent pertussis outbreaks as the pool of WCP-immunized children among older populations decreased.
But before we place all blame on switching to aP, consider that we can now confirm more pertussis infections with molecular assays than was possible with culture and fluorescent assay testing in the WCP era. So improved testing sensitivity means more reports of minimally symptomatic cases that may have been missed before. So WCP, if still used today, might not show 94% protection either.
Additionally, aPs rely heavily on pertactin as a target antigen,3 likely less than WCP, given that WCP contained all pertussis antigens rather than just the 3-5 purified antigens in aPs. So the emergence of pertactin-altered pertussis strains could disproportionately affect protection from aP, compared with WCP.
There seem to be no quick fixes to preventing outbreaks using aPs as our vaccine. One suggestion by the authors of the California outbreak report is to use aP mostly to terminate outbreaks rather than routinely in late childhood. My concern is that if we do not continue routine use in 4-to 6-year-olds, 10-to 11-year-olds, and in early adulthood, the vulnerable proportion of the population during outbreaks would be larger, making outbreaks more difficult to terminate. So continuing to produce some protection, albeit short-lived, with current schedules of aP vaccines seems important.
Also remember that T cells, particularly TH 17 pertussis-specific cells, may be as important as pertussis antibody. Therefore, crafting pertussis vaccines that yield improved antibody plus T cell responses is the current goal. Disease and WCP seem to elicit more T-17 response than aP. One method to craft a better vaccine is to use antigen blends that differ from those in the current vaccines, such as antigens derived from circulating pertussis strains instead of the standard laboratory strain. Another option is to use current antigens but with more potent adjuvants. Such vaccines are likely 5 years away.
But we need to have reasonable expectations for pertussis vaccines. Pertussis infection begins in respiratory epithelium. Many of the most obvious signs and symptoms are due to destruction of ciliated respiratory epithelium plus increased tenacity/volume of secretions. Can a parenterally administered vaccine that induces mostly serum antibody protect against infection of epithelium where antibody concentrations are likely 10% or less than in serum? The short answer is – likely not. We should expect neither aP nor WCP to consistently protect against pertussis infection, but it does seem reasonable to expect aP to reduce disease severity. Preventing infections awaits a vaccine that induces surface IgA. Mucosally administered vaccines produce surface IgA – for example, rotavirus vaccine – but no mucosal pertussis vaccine appears imminent.
A key question is whether our most vulnerable populations, young children, have increased morbidity and mortality. Data from the California suggest an increase but mostly in infants under 6 months of age, the group not old enough to benefit from even the most effective of infant vaccines. Protecting young infants depends on vaccine administered prenatally to mothers. The over-representation of the Hispanic infants among fatalities shows a population on which to focus with maternal immunization. Hopefully, the recent universal TdaP recommendation in pregnancy will help when maternal immunization is higher than current approximately 50% rates.4
Despite the problems, it seems clear that we must continue to use current aP vaccines according to the current schedules, attempting to get as close to 100% uptake as possible. While the current, nearly 10% unimmunized rates add to the likelihood that we are losing complete herd immunity, partial herd immunity is better than no herd immunity.
Expectations: There will be ongoing outbreaks. Continue to be alert for signs of pertussis. They are often less obvious in older patients, and may be as subtle as more than 2 weeks of persistent cough. During outbreaks, we may be called upon to give aP doses at intervals shorter than the usual schedule.
Our responsibility: Do not become discouraged or lose enthusiasm for aP, but explain to parents that because aP is less reactogenic, it produces less protection and is less durable, particularly in school-age children. But please emphasize that modest protection is best in the youngest and modest protection of older children is better than none. Emphasize that the adverse effect profile of current aPs puts the harm/benefit balance heavily in favor of aP.
Bottom line: We can hopefully do better than the current 88% to 92% rate of aP vaccine uptake. We need to get as close to 100% uptake as possible until new vaccines or new strategies become available.
1. Clin Infect Dis. 2016 Feb 7; doi: 10.1093/cid/ciw051.
2. Pediatrics. 2016 Feb 5; doi: 10.1542/peds.2015-3326.
3. Expert Rev Vaccines. 2007 Feb;6(1):47-56.
4. Vaccine. 2016 Feb 10;34(7):968-73.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He disclosed that his institution received grant support for a study on hexavalent infant vaccine containing pertussis from GlaxoSmithKline, and he was the local primary investigator.*
*Correction, 2/17/2016: An earlier version of this article incompletely stated Dr. Harrison's disclosure information.
Appendicitis, antibiotics, and surgery: An evolving trilogy
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Why 10 days of antibiotics for infections is not magic
In the United States, we treat almost all infections for 10 days. Why? In France, most infections are treated for 8 days. In the U.K., most infections are treated for 5 days. In many other countries, infections are treated until symptomatic improvement occurs. Can everyone outside the United States be wrong? What is the evidence base for the various recommended durations? Moreover, what is the harm in treating for longer than necessary?
The U.S. tradition of 10 days’ treatment for infections arose from the 1940 trials of injectable penicillin for prevention of acute rheumatic fever in military recruits who had group A streptococcal pharyngitis. Injections of penicillin G mixed in peanut oil produced therapeutic levels of penicillin for about 3 days. Soldiers who received three sequential injections had the lowest occurrence of rheumatic fever; two injections were not as good and four injections did not add to the prevention rate. So three injections meant 9 days’ treatment; 9 days was rounded up to 10 days, and there you have it.
We have come a long way since the 1940s. For strep throat, we now have three approved antibiotics for 5 days’ treatment: cefdinir, cefpodoxime proxetil, and azithromycin, all evidence based and U.S. Food and Drug Administration approved. One large study was done in the 1980s with cefadroxil for 5 days, and that duration was as effective in strep eradication as was 10 days, but the company never pursued the 5-day indication.
The optimal duration of antibiotic treatment is generally considered to be 10 days in the United States, however, there is scant evidence base for that recommendation. The recent American Academy of Pediatrics/American Academy of Family Physicians guidelines endorse 10 days of treatment duration as the standard for most acute otitis media (AOM) (Pediatrics 2013;131[3]:e964-99), but acknowledge that shorter treatment regimens may be as effective. Specifically, the guideline states: “A 7-day course of oral antibiotic appears to be equally effective in children 2- to 5 years of age with mild to moderate AOM. For children 6 years and older with mild to moderate AOM symptoms, a 5- to 7-day course is adequate treatment.” A systematic analysis and a meta-analysis have concluded that 5 days’ duration of antibiotics is as effective as 10 days’ treatment for all children over age 2 years and only marginally inferior to 10 days for children under the age of 2 years old (Cochrane Database Syst Rev. 2010;[9]:CD001095).
Thirty years ago, our group and others began to do studies involving “double tympanocentesis,” where an ear tap was done at time of diagnosis and again 3-5 days later to prove bacterial cure for various antibiotics that were in trials. We learned that if the organism was sensitive to the antibiotic chosen, then it was dead by days 3-5. Most of the failures were due to resistant bacteria. So treating longer was not going to help. It was time to change the antibiotic if clinical improvement had not occurred. Our group published a study 15 years ago of 2,172 children comparing 5-, 7-, and 10-days’ treatment of AOM, and concluded that 5 days’ treatment was equivalent to 7- and 10-days of treatment for all ages unless the child had a perforated tympanic membrane or the child had been treated for AOM within the preceding month since recently treated AOM was associated with more frequent causation of AOM by resistant bacteria and with a continued inflamed middle ear mucosa (Otolaryngol Head Neck Surg. 2001 Apr;124[4]:381-7). Since then we have treated all children with ear infections for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins unless the eardrum had perforated or the child had a recurrent AOM within the prior 30 days. That is a lot of patients in 15 years, and the results have been just as good as when we used 10 days as standard.
Acute sinusitis is another interesting story. The AAP guideline states: “The optimal duration of antimicrobial therapy for patients with acute bacterial sinusitis has not received systematic study. Recommendations based on clinical observation varied widely, from 1- to 28 days (Pediatrics. 2013 Jul;132[1]:e262-80). The prior AAP guideline endorsed “antibiotic therapy be continued for 7 days after the patient becomes free of symptoms and signs (Pediatrics. 2001 Sep;108[3]:798-808). Our group reasoned that the etiology and pathogenesis of sinusitis and AOM are identical, involving ascension of a bacterial inoculum from the nasopharynx via the osteomeatal complex to the sinuses just like ascension of infection via the eustachian tube to the middle ear. Therefore, beginning 25 years ago, we began to treat all children with sinus infections for 5 days, including amoxicillin and amoxicillin/clavulanate, as well as various cephalosporins. Again, that is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about community-acquired pneumonia? The Infectious Disease Society of America (IDSA) guideline states: “Treatment courses of 10 days [of antibiotics] have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis” (Clin Infect Dis. 2011 Oct;53[7]:617-30). Our group reasoned that antibiotics reach higher levels in the lungs than they do in the closed space of the middle ear or sinuses. Therefore, beginning 25 years ago, we began to treat all children with bronchopneumonia and lobar pneumonia for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins and azithromycin. That is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about skin and soft tissue infections? The IDSA guideline states that the duration of treatment for impetigo is 7 days, for cellulitis is 5 days, and for furuncles and carbuncles no duration is stated, but they allow no antibiotics be used at all if the patient is not febrile and white blood cell count is not elevated after incision and drainage (Clin Infect Dis. 2014 Jul 15;59[2]:e10-52).
So what is the harm to longer courses of antibiotics? As I have written in this column recently, we have learned a lot about the importance of our gut microbiome. The resident flora of our gut modulates our immune system favorably. Disturbing our gut flora with antibiotics is potentially harmful because the antibiotics often kill many species of healthy gut flora and cause disequilibrium of the flora, resulting in diminished innate immunity responses. Shorter treatment courses with antibiotics cause less disturbance of the healthy gut flora.
The rest of the world cannot all be wrong and the United States all right regarding the duration of antibiotic treatment for common infections. Moreover, in an era of evidence-based medicine, it is necessary to make changes from tradition. The evidence is there to recommend that 5 days’ treatment become the standard for treatment with selected cephalosporins as approved by the FDA – for AOM, for sinusitis, for community-acquired pneumonia, and for skin and soft tissue infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said that he had no relevant financial disclosures. Email him at [email protected].
In the United States, we treat almost all infections for 10 days. Why? In France, most infections are treated for 8 days. In the U.K., most infections are treated for 5 days. In many other countries, infections are treated until symptomatic improvement occurs. Can everyone outside the United States be wrong? What is the evidence base for the various recommended durations? Moreover, what is the harm in treating for longer than necessary?
The U.S. tradition of 10 days’ treatment for infections arose from the 1940 trials of injectable penicillin for prevention of acute rheumatic fever in military recruits who had group A streptococcal pharyngitis. Injections of penicillin G mixed in peanut oil produced therapeutic levels of penicillin for about 3 days. Soldiers who received three sequential injections had the lowest occurrence of rheumatic fever; two injections were not as good and four injections did not add to the prevention rate. So three injections meant 9 days’ treatment; 9 days was rounded up to 10 days, and there you have it.
We have come a long way since the 1940s. For strep throat, we now have three approved antibiotics for 5 days’ treatment: cefdinir, cefpodoxime proxetil, and azithromycin, all evidence based and U.S. Food and Drug Administration approved. One large study was done in the 1980s with cefadroxil for 5 days, and that duration was as effective in strep eradication as was 10 days, but the company never pursued the 5-day indication.
The optimal duration of antibiotic treatment is generally considered to be 10 days in the United States, however, there is scant evidence base for that recommendation. The recent American Academy of Pediatrics/American Academy of Family Physicians guidelines endorse 10 days of treatment duration as the standard for most acute otitis media (AOM) (Pediatrics 2013;131[3]:e964-99), but acknowledge that shorter treatment regimens may be as effective. Specifically, the guideline states: “A 7-day course of oral antibiotic appears to be equally effective in children 2- to 5 years of age with mild to moderate AOM. For children 6 years and older with mild to moderate AOM symptoms, a 5- to 7-day course is adequate treatment.” A systematic analysis and a meta-analysis have concluded that 5 days’ duration of antibiotics is as effective as 10 days’ treatment for all children over age 2 years and only marginally inferior to 10 days for children under the age of 2 years old (Cochrane Database Syst Rev. 2010;[9]:CD001095).
Thirty years ago, our group and others began to do studies involving “double tympanocentesis,” where an ear tap was done at time of diagnosis and again 3-5 days later to prove bacterial cure for various antibiotics that were in trials. We learned that if the organism was sensitive to the antibiotic chosen, then it was dead by days 3-5. Most of the failures were due to resistant bacteria. So treating longer was not going to help. It was time to change the antibiotic if clinical improvement had not occurred. Our group published a study 15 years ago of 2,172 children comparing 5-, 7-, and 10-days’ treatment of AOM, and concluded that 5 days’ treatment was equivalent to 7- and 10-days of treatment for all ages unless the child had a perforated tympanic membrane or the child had been treated for AOM within the preceding month since recently treated AOM was associated with more frequent causation of AOM by resistant bacteria and with a continued inflamed middle ear mucosa (Otolaryngol Head Neck Surg. 2001 Apr;124[4]:381-7). Since then we have treated all children with ear infections for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins unless the eardrum had perforated or the child had a recurrent AOM within the prior 30 days. That is a lot of patients in 15 years, and the results have been just as good as when we used 10 days as standard.
Acute sinusitis is another interesting story. The AAP guideline states: “The optimal duration of antimicrobial therapy for patients with acute bacterial sinusitis has not received systematic study. Recommendations based on clinical observation varied widely, from 1- to 28 days (Pediatrics. 2013 Jul;132[1]:e262-80). The prior AAP guideline endorsed “antibiotic therapy be continued for 7 days after the patient becomes free of symptoms and signs (Pediatrics. 2001 Sep;108[3]:798-808). Our group reasoned that the etiology and pathogenesis of sinusitis and AOM are identical, involving ascension of a bacterial inoculum from the nasopharynx via the osteomeatal complex to the sinuses just like ascension of infection via the eustachian tube to the middle ear. Therefore, beginning 25 years ago, we began to treat all children with sinus infections for 5 days, including amoxicillin and amoxicillin/clavulanate, as well as various cephalosporins. Again, that is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about community-acquired pneumonia? The Infectious Disease Society of America (IDSA) guideline states: “Treatment courses of 10 days [of antibiotics] have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis” (Clin Infect Dis. 2011 Oct;53[7]:617-30). Our group reasoned that antibiotics reach higher levels in the lungs than they do in the closed space of the middle ear or sinuses. Therefore, beginning 25 years ago, we began to treat all children with bronchopneumonia and lobar pneumonia for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins and azithromycin. That is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about skin and soft tissue infections? The IDSA guideline states that the duration of treatment for impetigo is 7 days, for cellulitis is 5 days, and for furuncles and carbuncles no duration is stated, but they allow no antibiotics be used at all if the patient is not febrile and white blood cell count is not elevated after incision and drainage (Clin Infect Dis. 2014 Jul 15;59[2]:e10-52).
So what is the harm to longer courses of antibiotics? As I have written in this column recently, we have learned a lot about the importance of our gut microbiome. The resident flora of our gut modulates our immune system favorably. Disturbing our gut flora with antibiotics is potentially harmful because the antibiotics often kill many species of healthy gut flora and cause disequilibrium of the flora, resulting in diminished innate immunity responses. Shorter treatment courses with antibiotics cause less disturbance of the healthy gut flora.
The rest of the world cannot all be wrong and the United States all right regarding the duration of antibiotic treatment for common infections. Moreover, in an era of evidence-based medicine, it is necessary to make changes from tradition. The evidence is there to recommend that 5 days’ treatment become the standard for treatment with selected cephalosporins as approved by the FDA – for AOM, for sinusitis, for community-acquired pneumonia, and for skin and soft tissue infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said that he had no relevant financial disclosures. Email him at [email protected].
In the United States, we treat almost all infections for 10 days. Why? In France, most infections are treated for 8 days. In the U.K., most infections are treated for 5 days. In many other countries, infections are treated until symptomatic improvement occurs. Can everyone outside the United States be wrong? What is the evidence base for the various recommended durations? Moreover, what is the harm in treating for longer than necessary?
The U.S. tradition of 10 days’ treatment for infections arose from the 1940 trials of injectable penicillin for prevention of acute rheumatic fever in military recruits who had group A streptococcal pharyngitis. Injections of penicillin G mixed in peanut oil produced therapeutic levels of penicillin for about 3 days. Soldiers who received three sequential injections had the lowest occurrence of rheumatic fever; two injections were not as good and four injections did not add to the prevention rate. So three injections meant 9 days’ treatment; 9 days was rounded up to 10 days, and there you have it.
We have come a long way since the 1940s. For strep throat, we now have three approved antibiotics for 5 days’ treatment: cefdinir, cefpodoxime proxetil, and azithromycin, all evidence based and U.S. Food and Drug Administration approved. One large study was done in the 1980s with cefadroxil for 5 days, and that duration was as effective in strep eradication as was 10 days, but the company never pursued the 5-day indication.
The optimal duration of antibiotic treatment is generally considered to be 10 days in the United States, however, there is scant evidence base for that recommendation. The recent American Academy of Pediatrics/American Academy of Family Physicians guidelines endorse 10 days of treatment duration as the standard for most acute otitis media (AOM) (Pediatrics 2013;131[3]:e964-99), but acknowledge that shorter treatment regimens may be as effective. Specifically, the guideline states: “A 7-day course of oral antibiotic appears to be equally effective in children 2- to 5 years of age with mild to moderate AOM. For children 6 years and older with mild to moderate AOM symptoms, a 5- to 7-day course is adequate treatment.” A systematic analysis and a meta-analysis have concluded that 5 days’ duration of antibiotics is as effective as 10 days’ treatment for all children over age 2 years and only marginally inferior to 10 days for children under the age of 2 years old (Cochrane Database Syst Rev. 2010;[9]:CD001095).
Thirty years ago, our group and others began to do studies involving “double tympanocentesis,” where an ear tap was done at time of diagnosis and again 3-5 days later to prove bacterial cure for various antibiotics that were in trials. We learned that if the organism was sensitive to the antibiotic chosen, then it was dead by days 3-5. Most of the failures were due to resistant bacteria. So treating longer was not going to help. It was time to change the antibiotic if clinical improvement had not occurred. Our group published a study 15 years ago of 2,172 children comparing 5-, 7-, and 10-days’ treatment of AOM, and concluded that 5 days’ treatment was equivalent to 7- and 10-days of treatment for all ages unless the child had a perforated tympanic membrane or the child had been treated for AOM within the preceding month since recently treated AOM was associated with more frequent causation of AOM by resistant bacteria and with a continued inflamed middle ear mucosa (Otolaryngol Head Neck Surg. 2001 Apr;124[4]:381-7). Since then we have treated all children with ear infections for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins unless the eardrum had perforated or the child had a recurrent AOM within the prior 30 days. That is a lot of patients in 15 years, and the results have been just as good as when we used 10 days as standard.
Acute sinusitis is another interesting story. The AAP guideline states: “The optimal duration of antimicrobial therapy for patients with acute bacterial sinusitis has not received systematic study. Recommendations based on clinical observation varied widely, from 1- to 28 days (Pediatrics. 2013 Jul;132[1]:e262-80). The prior AAP guideline endorsed “antibiotic therapy be continued for 7 days after the patient becomes free of symptoms and signs (Pediatrics. 2001 Sep;108[3]:798-808). Our group reasoned that the etiology and pathogenesis of sinusitis and AOM are identical, involving ascension of a bacterial inoculum from the nasopharynx via the osteomeatal complex to the sinuses just like ascension of infection via the eustachian tube to the middle ear. Therefore, beginning 25 years ago, we began to treat all children with sinus infections for 5 days, including amoxicillin and amoxicillin/clavulanate, as well as various cephalosporins. Again, that is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about community-acquired pneumonia? The Infectious Disease Society of America (IDSA) guideline states: “Treatment courses of 10 days [of antibiotics] have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis” (Clin Infect Dis. 2011 Oct;53[7]:617-30). Our group reasoned that antibiotics reach higher levels in the lungs than they do in the closed space of the middle ear or sinuses. Therefore, beginning 25 years ago, we began to treat all children with bronchopneumonia and lobar pneumonia for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins and azithromycin. That is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about skin and soft tissue infections? The IDSA guideline states that the duration of treatment for impetigo is 7 days, for cellulitis is 5 days, and for furuncles and carbuncles no duration is stated, but they allow no antibiotics be used at all if the patient is not febrile and white blood cell count is not elevated after incision and drainage (Clin Infect Dis. 2014 Jul 15;59[2]:e10-52).
So what is the harm to longer courses of antibiotics? As I have written in this column recently, we have learned a lot about the importance of our gut microbiome. The resident flora of our gut modulates our immune system favorably. Disturbing our gut flora with antibiotics is potentially harmful because the antibiotics often kill many species of healthy gut flora and cause disequilibrium of the flora, resulting in diminished innate immunity responses. Shorter treatment courses with antibiotics cause less disturbance of the healthy gut flora.
The rest of the world cannot all be wrong and the United States all right regarding the duration of antibiotic treatment for common infections. Moreover, in an era of evidence-based medicine, it is necessary to make changes from tradition. The evidence is there to recommend that 5 days’ treatment become the standard for treatment with selected cephalosporins as approved by the FDA – for AOM, for sinusitis, for community-acquired pneumonia, and for skin and soft tissue infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said that he had no relevant financial disclosures. Email him at [email protected].
Judicious antibiotic use key in ambulatory settings
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”
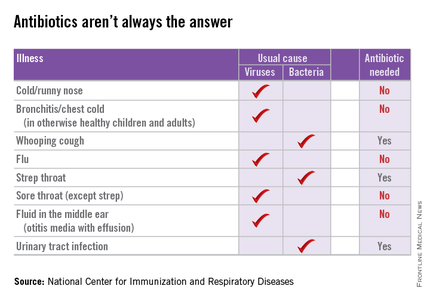
Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”

Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
I was recently asked to evaluate a young child with a urinary tract infection caused by an extended spectrum beta-lactamase (ESBL)–producing Escherichia coli.
I’d just broken the bad news to the mother: There was no oral medication available to treat the baby, so she’d have to stay in the hospital for a full intravenous course.
“Has your child been treated with antibiotics recently?” I asked the mother, wondering how the baby had come to have such a resistant infection.
“She had a couple days of runny nose and a low-grade fever a couple of weeks ago,” she told me. “Her doctor treated her for a sinus infection.”
In 2011, doctors in outpatient settings across the United States wrote 262.5 million prescriptions for antibiotics – 73.7 million for children – and according to the Centers for Disease Control and Prevention, about 50% of these were completely unnecessary because they were prescribed for viral respiratory tract infections (Clin Infect Dis. 2015 May 1;60[9]:1308-16).
Prescribing practices varied by region, with the highest rates in the South. Don’t think I’m judging. I live in Kentucky, the state with the highest rate of antibiotic prescribing at 1,281 prescriptions per 1,000 persons. Is it any wonder that we’re seeing kids with very resistant infections?
The CDC estimates that at least two million people in the United States are infected annually with antibiotic-resistant bacteria and at least 23,000 of them die as a result of these infections. It is estimated that prevention strategies that include better antibiotic prescribing could prevent as many as 619,000 infections and 37,000 deaths over 5 years. Fortunately, my little patient recovered fully, but it has made me think about antimicrobial stewardship, especially its role in the outpatient setting.
According the American Academy of Pediatrics, the goal of antimicrobial stewardship is “to optimize antimicrobial use, with the aim of decreasing inappropriate use that leads to unwarranted toxicity and to selection and spread of resistant organisms.”
Antimicrobial stewardship programs (ASPs) are increasingly common in inpatient settings and have been shown to reduce antibiotic use. These programs can take many forms. The hospital where I work relies primarily on clinical guidelines emphasizing appropriate empiric therapy for a variety of common conditions. Other hospitals employ prospective audit and feedback, as well as a restricted formulary. Medicare and Medicaid Conditions of Participation will soon require hospitals that receive funds from the Centers for Medicare and Medicaid Services have an ASP.
Comparatively little has been published about ASPs in the outpatient setting. The American Academy of Pediatrics suggests that effective strategies include patient education, provider education, provider audit and feedback, and clinical decision support. We have at least some data that these work, at least in a research setting.
From 2000 to 2003, a controlled, cluster-randomized trial in 16 Massachusetts communities demonstrated that a 3-year, multifaceted, community-level intervention was “modestly successful” in reducing antibiotic use (Pediatrics. 2008 Jan;121[1]:e15-23). As a part of this intervention, parents received education via direct mail and in primary care settings, pharmacies, and child care centers while physicians received small-group education, frequent updates and educational materials, and prescribing feedback. Antibiotic prescribing was measured via health insurance claims data from all children who were 6 years of age or younger and resided in study communities, and were insured by one of four participating health plans. Coincident with the intervention, there was 4.2% decrease in antibiotic prescribing among children aged 24 to <48 months and a 6.7% decrease among those aged 48-72 months. The effect was greatest among Medicaid-insured children.
More recently, 18 primary care practices in Pennsylvania and New Jersey were randomized to an intervention that consisted of a 1-hour, on-site education session followed by 1 year of personalized, quarterly audit and feedback of prescribing for bacterial and viral acute respiratory tract infections (ARTIs), or usual practice (JAMA. 2013 Jun 12;309[22]:2345-52). The prescribing practices of 162 clinicians were included in the analysis.
Broad spectrum–antibiotic prescribing decreased in intervention practices, compared with controls (26.8% to 14.3% among intervention practices vs. 28.4% to 22.6% in controls), as did “off-guideline” prescribing for pneumonia and acute sinusitis. Antibiotic prescribing for viral infections was relatively low at baseline and did not change. The authors concluded that “extending antimicrobial stewardship to the ambulatory setting, where such programs have generally not been implemented, may have important health benefits.” Unfortunately, the positive effect in these practices was not sustained after the audit and feedback stopped (JAMA. 2014 Dec 17;312[23]:2569-70).
Not all antimicrobial stewardship interventions need to be time- and resource-intensive. Investigators in California found that providers who publicly pledged to reducing inappropriate antibiotic use for ARTIs by signing and posting a commitment letter in exam rooms actually prescribed fewer inappropriate antibiotic courses for their adult patients (JAMA Intern Med. 2014 Mar;174[3]:425-31).
“When you have a cough, sore throat, or other illness, your doctor will help you select the best possible treatments. If an antibiotic would do more harm than good, your doctor will explain this to you, and may offer other treatments that are better for you,” the letter read in part. There was a 19.7 absolute percentage reduction in inappropriate antibiotic prescribing for ARTIs among clinicians randomized to the commitment letter invention relative to controls.
Can antimicrobial strategies work in the “real” world, in a busy pediatrician’s office? According to Dr. Patricia Purcell, a physician with East Louisville Pediatrics in Louisville, Ky., the answer is “yes.”
“We actually start with education in the newborn period,” Dr. Purcell said. “We let parents know that we are not going to call in antibiotics over the phone, and we’re not going to prescribe them for an upper respiratory tract infection.”

Dr. Purcell and her partners have committed to following evidence-based guidelines for antibiotic practices, such as the AAP’s guidelines for otitis media and sinusitis. She also noted that at least one major insurance company is starting to provide the group feedback about their antibiotic-prescribing practices. “They want to make sure we are not prescribing antibiotics for viruses,” she said.
Still, the message that antibiotics are not always the answer can be a bitter pill for some parents to swallow. A pediatrician friend in Alabama notes: “I have these conversations every day, and a lot of parents are mad at me for not prescribing antibiotics for their child’s ‘terrible cold.’” Another friend notes that watchful waiting can be a burden for parents who have high copays or difficulties with transportation.
Still, many parents would welcome a frank discussion about the risks and benefits of antibiotics. After I shared some of the CDC information for parents with a nursing colleague, she told me that her daughter recently had a febrile illness and was diagnosed with otitis media. “I don’t like giving my kids meds they don’t need,” she told me. “However, if the doc says they need antibiotics and they prescribe them, I give them. I never say, ‘Do we really need antibiotics for that?’”
Now she is rethinking that approach. “Was 10 days of amoxicillin necessary for a ‘red’ eardrum?! I’m just a mom. ... I don’t know the answer to that! Was her ear red because she had been crying or because of her fever? Did she get ‘treatment’ she did not need? Did the doctor give me antibiotics without education because she assumed that is why I brought her in?”
This year’s “Get Smart About Antibiotics Week” was Nov. 16-22. This annual 1-week observance is intended to raise awareness of the threat of antibiotic resistance and the importance of appropriate prescribing and use. Kudos if you celebrated this in your office. If you missed it, it’s not too late to check out some of the activities suggested by the CDC, and try one or two in your own practice. Email me with your ideas about stewardship in the outpatient setting, and I’ll try to feature at least some of them in a future column.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. Dr. Bryant disclosed that she has been an investigator for clinical trials funded by Pfizer for the past 2 years. Email her at [email protected].
Fewer doses of PCV13 could save money – but at what cost?
Streptococcus pneumoniae is the most common bacterial cause of pneumonia, sinusitis, and acute otitis media (AOM). It also causes invasive pneumococcal disease (IPD), such as bacteremia and meningitis, and it is the leading cause of vaccine-preventable death in children younger than 5 years of age. Pneumococcal conjugate vaccines (PCVs) are effective in infants and young children against IPD, non-IPD, and the acquisition of vaccine serotype nasopharyngeal carriage (contagion). PCV7 was licensed and introduced in 2000 on a schedule that matched the schedule of other routine infant immunizations of three primary doses at 2, 4, and 6 months, and a booster at 12-15 months. Later in 2010, PCV13 was licensed on that same “3+1” schedule. Different pneumococcal vaccination schedules are recommended across Europe and other countries, after consideration of the epidemiology, disease burden, immunogenicity of the vaccine, its compatibility with other vaccines, and its cost. The World Health Organization recently updated its PCV policy to support the use of three doses on either 3+0 or 2+1 schedules. Most European countries have adopted the 2+1 schedule used for routine infant immunizations.
In light of the escalating costs of providing current vaccines, and the anticipated need for additional vaccines, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) has convened a working group to evaluate the transition from a 3+1 to a 2+1 schedule for PCV administration to infants and children. This is not a trivial decision. In the United States, cost must be considered in the context of an additional focus on non-IPD disease prevention, especially AOM, where serotypes and immune protection levels differ from IPD. A 2+1 schedule may be effective to prevent IPD, compared with a 3+1 schedule, but its impact on non-IPD may be compromised, especially for AOM, for some serotypes of pneumococci, and for control of nasopharyngeal carriage.
Immunogenicity studies show that antibody responses from a vaccine regimen consisting of two doses in the primary series are less immunogenic, compared with those for a three-dose regimen, yet both regimens are effective for the prevention of IPD. Immunogenicity data that support the use of reduced-dose schedules for most, but not all, vaccine serotypes, were based on IPD. The degree to which higher antibody concentrations are important for protecting against nonbacteremic pneumonia, sinusitis, and AOM, and for preventing nasopharyngeal carriage, is not established.
However, clinical outcomes since the introduction of PCVs indicate that the true threshold will vary by serotype and host and disease condition, with higher concentrations required for certain serotypes, in immunologically less mature hosts, and in mucosal infections like nonbacteremic pneumonia, sinusitis, and AOM, compared with IPD. Also, higher IgG levels clearly are important in protecting against nasopharyngeal colonization, thereby conferring herd immunity, prolonging individual protection, and possibly correlating at the individual level with disease protection. Studies that evaluated the correlation of antibody concentration and protection against nasopharyngeal colonization have shown that a greater than 10-fold higher antibody concentration is needed, compared with levels in blood, to protect against IPD. Similarly protection against AOM require higher levels of antibody than are needed to protect against IPD, as evidenced by the lower efficacy of PCVs against AOM, compared with IPD.
Epidemiology and risk factors differ among countries of the world. Therefore, even among developed countries, there is a need for caution in accepting that what works in one country will work as well in another. For example, attendance at day care is the highest risk factor for both IPD and non-IPD. In the United States, we have many types of day care, including relatively large day care centers, and many infants enter day care at 2 months of age. In other developed countries, the size of day care centers is much smaller, and children may not enter day care until 1 or even 2 years of age. Those differences may have implications for protective efficacy with a reduced-dose vaccine schedule.
Siblings under the age of 8 years are also at significant risk. Again, the family size may differ among developed countries. Breastfeeding is protective for pneumococcal infections. Breastfeeding duration may differ among countries. The theme of this concern is apparent: Even evidence of adequate protection with a reduced-dose schedule in Finland, France, Germany, the United Kingdom, or elsewhere should not be interpreted to be completely applicable to the United States.
Whether reduced-dose schedules can provide equivalent protection against vaccine type IPD equivalent to a 3+1 schedule for all serotypes and for non-IPD when introduced into a national immunization program is unclear. Do we have enough data to inform the decision process, and specifically do we have a clear understanding of the full impact of reduced-dose schedules on non-IPD relative to 3+1? How would you vote?
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Pfizer, which makes PCV vaccine, has funded an investigator-initiated grant and a postmarketing study to Dr. Pichichero’s institution, and he is the primary investigator of both grants.
Streptococcus pneumoniae is the most common bacterial cause of pneumonia, sinusitis, and acute otitis media (AOM). It also causes invasive pneumococcal disease (IPD), such as bacteremia and meningitis, and it is the leading cause of vaccine-preventable death in children younger than 5 years of age. Pneumococcal conjugate vaccines (PCVs) are effective in infants and young children against IPD, non-IPD, and the acquisition of vaccine serotype nasopharyngeal carriage (contagion). PCV7 was licensed and introduced in 2000 on a schedule that matched the schedule of other routine infant immunizations of three primary doses at 2, 4, and 6 months, and a booster at 12-15 months. Later in 2010, PCV13 was licensed on that same “3+1” schedule. Different pneumococcal vaccination schedules are recommended across Europe and other countries, after consideration of the epidemiology, disease burden, immunogenicity of the vaccine, its compatibility with other vaccines, and its cost. The World Health Organization recently updated its PCV policy to support the use of three doses on either 3+0 or 2+1 schedules. Most European countries have adopted the 2+1 schedule used for routine infant immunizations.
In light of the escalating costs of providing current vaccines, and the anticipated need for additional vaccines, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) has convened a working group to evaluate the transition from a 3+1 to a 2+1 schedule for PCV administration to infants and children. This is not a trivial decision. In the United States, cost must be considered in the context of an additional focus on non-IPD disease prevention, especially AOM, where serotypes and immune protection levels differ from IPD. A 2+1 schedule may be effective to prevent IPD, compared with a 3+1 schedule, but its impact on non-IPD may be compromised, especially for AOM, for some serotypes of pneumococci, and for control of nasopharyngeal carriage.
Immunogenicity studies show that antibody responses from a vaccine regimen consisting of two doses in the primary series are less immunogenic, compared with those for a three-dose regimen, yet both regimens are effective for the prevention of IPD. Immunogenicity data that support the use of reduced-dose schedules for most, but not all, vaccine serotypes, were based on IPD. The degree to which higher antibody concentrations are important for protecting against nonbacteremic pneumonia, sinusitis, and AOM, and for preventing nasopharyngeal carriage, is not established.
However, clinical outcomes since the introduction of PCVs indicate that the true threshold will vary by serotype and host and disease condition, with higher concentrations required for certain serotypes, in immunologically less mature hosts, and in mucosal infections like nonbacteremic pneumonia, sinusitis, and AOM, compared with IPD. Also, higher IgG levels clearly are important in protecting against nasopharyngeal colonization, thereby conferring herd immunity, prolonging individual protection, and possibly correlating at the individual level with disease protection. Studies that evaluated the correlation of antibody concentration and protection against nasopharyngeal colonization have shown that a greater than 10-fold higher antibody concentration is needed, compared with levels in blood, to protect against IPD. Similarly protection against AOM require higher levels of antibody than are needed to protect against IPD, as evidenced by the lower efficacy of PCVs against AOM, compared with IPD.
Epidemiology and risk factors differ among countries of the world. Therefore, even among developed countries, there is a need for caution in accepting that what works in one country will work as well in another. For example, attendance at day care is the highest risk factor for both IPD and non-IPD. In the United States, we have many types of day care, including relatively large day care centers, and many infants enter day care at 2 months of age. In other developed countries, the size of day care centers is much smaller, and children may not enter day care until 1 or even 2 years of age. Those differences may have implications for protective efficacy with a reduced-dose vaccine schedule.
Siblings under the age of 8 years are also at significant risk. Again, the family size may differ among developed countries. Breastfeeding is protective for pneumococcal infections. Breastfeeding duration may differ among countries. The theme of this concern is apparent: Even evidence of adequate protection with a reduced-dose schedule in Finland, France, Germany, the United Kingdom, or elsewhere should not be interpreted to be completely applicable to the United States.
Whether reduced-dose schedules can provide equivalent protection against vaccine type IPD equivalent to a 3+1 schedule for all serotypes and for non-IPD when introduced into a national immunization program is unclear. Do we have enough data to inform the decision process, and specifically do we have a clear understanding of the full impact of reduced-dose schedules on non-IPD relative to 3+1? How would you vote?
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Pfizer, which makes PCV vaccine, has funded an investigator-initiated grant and a postmarketing study to Dr. Pichichero’s institution, and he is the primary investigator of both grants.
Streptococcus pneumoniae is the most common bacterial cause of pneumonia, sinusitis, and acute otitis media (AOM). It also causes invasive pneumococcal disease (IPD), such as bacteremia and meningitis, and it is the leading cause of vaccine-preventable death in children younger than 5 years of age. Pneumococcal conjugate vaccines (PCVs) are effective in infants and young children against IPD, non-IPD, and the acquisition of vaccine serotype nasopharyngeal carriage (contagion). PCV7 was licensed and introduced in 2000 on a schedule that matched the schedule of other routine infant immunizations of three primary doses at 2, 4, and 6 months, and a booster at 12-15 months. Later in 2010, PCV13 was licensed on that same “3+1” schedule. Different pneumococcal vaccination schedules are recommended across Europe and other countries, after consideration of the epidemiology, disease burden, immunogenicity of the vaccine, its compatibility with other vaccines, and its cost. The World Health Organization recently updated its PCV policy to support the use of three doses on either 3+0 or 2+1 schedules. Most European countries have adopted the 2+1 schedule used for routine infant immunizations.
In light of the escalating costs of providing current vaccines, and the anticipated need for additional vaccines, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) has convened a working group to evaluate the transition from a 3+1 to a 2+1 schedule for PCV administration to infants and children. This is not a trivial decision. In the United States, cost must be considered in the context of an additional focus on non-IPD disease prevention, especially AOM, where serotypes and immune protection levels differ from IPD. A 2+1 schedule may be effective to prevent IPD, compared with a 3+1 schedule, but its impact on non-IPD may be compromised, especially for AOM, for some serotypes of pneumococci, and for control of nasopharyngeal carriage.
Immunogenicity studies show that antibody responses from a vaccine regimen consisting of two doses in the primary series are less immunogenic, compared with those for a three-dose regimen, yet both regimens are effective for the prevention of IPD. Immunogenicity data that support the use of reduced-dose schedules for most, but not all, vaccine serotypes, were based on IPD. The degree to which higher antibody concentrations are important for protecting against nonbacteremic pneumonia, sinusitis, and AOM, and for preventing nasopharyngeal carriage, is not established.
However, clinical outcomes since the introduction of PCVs indicate that the true threshold will vary by serotype and host and disease condition, with higher concentrations required for certain serotypes, in immunologically less mature hosts, and in mucosal infections like nonbacteremic pneumonia, sinusitis, and AOM, compared with IPD. Also, higher IgG levels clearly are important in protecting against nasopharyngeal colonization, thereby conferring herd immunity, prolonging individual protection, and possibly correlating at the individual level with disease protection. Studies that evaluated the correlation of antibody concentration and protection against nasopharyngeal colonization have shown that a greater than 10-fold higher antibody concentration is needed, compared with levels in blood, to protect against IPD. Similarly protection against AOM require higher levels of antibody than are needed to protect against IPD, as evidenced by the lower efficacy of PCVs against AOM, compared with IPD.
Epidemiology and risk factors differ among countries of the world. Therefore, even among developed countries, there is a need for caution in accepting that what works in one country will work as well in another. For example, attendance at day care is the highest risk factor for both IPD and non-IPD. In the United States, we have many types of day care, including relatively large day care centers, and many infants enter day care at 2 months of age. In other developed countries, the size of day care centers is much smaller, and children may not enter day care until 1 or even 2 years of age. Those differences may have implications for protective efficacy with a reduced-dose vaccine schedule.
Siblings under the age of 8 years are also at significant risk. Again, the family size may differ among developed countries. Breastfeeding is protective for pneumococcal infections. Breastfeeding duration may differ among countries. The theme of this concern is apparent: Even evidence of adequate protection with a reduced-dose schedule in Finland, France, Germany, the United Kingdom, or elsewhere should not be interpreted to be completely applicable to the United States.
Whether reduced-dose schedules can provide equivalent protection against vaccine type IPD equivalent to a 3+1 schedule for all serotypes and for non-IPD when introduced into a national immunization program is unclear. Do we have enough data to inform the decision process, and specifically do we have a clear understanding of the full impact of reduced-dose schedules on non-IPD relative to 3+1? How would you vote?
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Pfizer, which makes PCV vaccine, has funded an investigator-initiated grant and a postmarketing study to Dr. Pichichero’s institution, and he is the primary investigator of both grants.