User login
Infection prevention
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
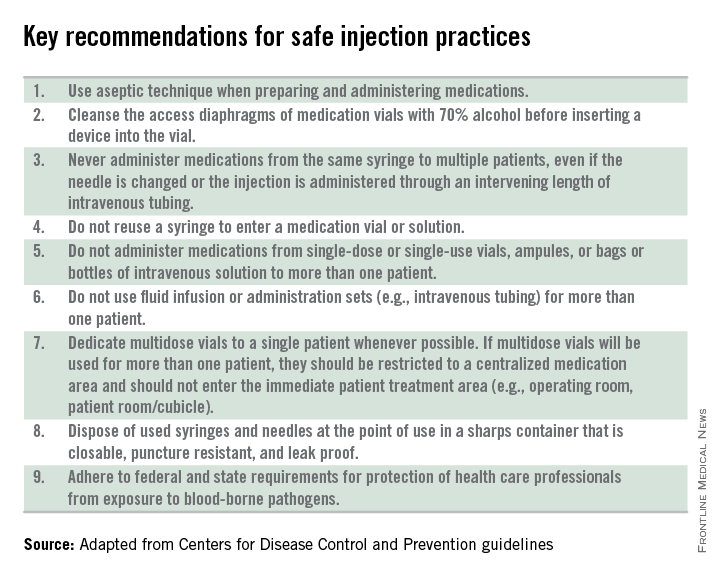
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.
Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.

Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Not a long ago, I received a call from a friend working in a local pediatric clinic. One of her partners had just seen a young child with an unusual rash. The diagnosis? Crusted scabies.
Sarcoptes scabiei var. hominis, the mite that causes typical scabies, also causes crusted or Norwegian scabies. These terms refer to severe infestations that occur in individuals who are immune compromised or debilitated. The rash is characterized by vesicles and thick crusts and may or may not be itchy. Because patients with crusted scabies can be infested with as many as 2 million mites, transmission from very brief skin-to-skin contact is possible, and outbreaks have occurred in health care facilities and other institutional settings.
That was the reason for my friend’s call. “What do we do for the doctors and nurses in the clinic who saw the patient?” she wanted to know.
“Everyone wore gloves, right?” I asked. There was silence on the other end of the phone.
After a quick consultation with our health department, every health care provider (HCP) who touched the patient without gloves was treated preemptively with topical permethrin. None went on to develop scabies. The experience prompted me to think about the challenges of infection prevention in ambulatory care.
Both the American Academy of Pediatrics (AAP Committee on Infectious Diseases, “Infection prevention and control in pediatric ambulatory settings,” Pediatrics 2007;20[3]:650-65) and the Centers for Disease Control and Prevention (Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care) have published recommendations for infection prevention in outpatient settings. Both organizations emphasize the importance of standard precautions. According to the CDC, standard precautions “are the minimum infection prevention practices that apply to all patient care, regardless of suspected or confirmed infection status of the patient, in any setting where health care is delivered.” They are designed to protect HCPs, as well as prevent us from spreading infections among patients. Standard precautions include:
• Hand hygiene.
• Use of personal protective equipment (gloves, gowns, masks).
• Safe injection practices.
• Safe handling of potentially contaminated equipment or surfaces in the patient environment.
• Respiratory hygiene/cough etiquette.
Some of these elements are likely second nature to office-based pediatricians. Hands must be cleaned before and after every patient encounter or an encounter with the patient’s immediate environment. “Cover your cough” signs have become ubiquitous in ambulatory care waiting rooms, even as we acknowledge the difficulties associated with expecting toddlers to wear masks or use a tissue to contain their coughs and sneezes.
Other elements of standard precautions may receive increased attention because the consequences of noncompliance are perceived to be dangerous or severe. For example, we know that failure to reliably employ safe injection practices (see table) has resulted in transmission of blood-borne pathogens, including hepatitis B and C, in ambulatory settings.
In my experience, the use of personal protective equipment (PPE) in the ambulatory setting is the element of standard precautions that is the least understood and perhaps the most underutilized. It’s certainly easier in the inpatient setting, where we use transmission-based precautions, and colorful isolation signs instruct us to put on gown and gloves when we visit the patient with viral gastroenteritis, or gown, gloves, and mask for the child with acute viral respiratory tract infection. In the office, we expect the HCP to anticipate what kind of contact with blood or body fluids is likely and choose PPE accordingly.

Of course, anticipation can be tricky. Gowns, for example, are only required during procedures or activities when contact with blood and body fluids is likely. In routine office-based care, these sorts of procedures are uncommon. Incision and drainage of an abscess is one example of a procedure that might warrant protection of one’s clothing with a gown. Conversely, the need for a mask might arise several times a day, as these are worn to protect the mouth, nose, and eyes “during procedures that are likely to generate splashes or sprays of blood or other body fluids.” Examination of a coughing patient is a common “procedure” likely to results in sprays of saliva. Use of a mask can protect the examiner from potential exposures to Bordetella pertussis, Mycoplasma pneumoniae, and a host of respiratory viruses.
While the AAP has been careful to point out that gloves are not needed for the routine care of well children, they should be used when “there is the potential to contact blood, body fluids, mucous membranes, nonintact skin, or potentially infectious material.” In our world, potentially infectious material might include a cluster of vesicles thought to be herpes simplex, the honey-crusted lesions of impetigo, or the weeping, crusted rash of Norwegian scabies.
My own office had a powerful reminder about the importance of standard precautions last year when we were referred a young infant with recurrent fevers and a mostly dry, peeling rash. As we learned in medical school, the mucocutanous lesions of congenital syphilis can be highly contagious. In accordance with AAP recommendations, all HCPs who examined this child without the protection of gloves underwent serologic testing for syphilis. Fortunately, there were no transmissions!
Published data about infectious disease exposures and the transmission of infectious diseases in the outpatient setting, either from patients to health care workers or among patients, are largely limited to outbreak or case reports. A 1991 review identified 53 reports of infectious disease transmission in outpatient settings between 1961 and 1990 (JAMA 1991;265(18): 2377-81). Transmission occurred in medical and dental offices, clinics, emergency departments, ophthalmology offices, and alternative care settings that included chiropractic clinics and an acupuncture practice. A variety of pathogens were involved, including measles, adenovirus, hepatitis B, atypical mycobacteria, and Streptococcus pyogenes. The authors concluded that many of the outbreaks and episodes of transmission could have been prevented “if existing infection control guidelines,” including what we now consider standard precautions, had been utilized. Many reports published in the intervening 25 years have come to similar conclusions.
So why don’t HCPs yet follow standard precautions, including appropriate use of PPE? The reasons are complex and multifactorial. We’re all busy and lack of time is a common complaint. Gowns, gloves, masks, and alcohol hand gel aren’t always readily available. Some HCPs may not be knowledgeable about the elements of standard precautions while others may not understand the risks to themselves and their patients associated with nonadherence. Finally, some organizations have not established clear expectations related to infection prevention and compliance with AAP and CDC recommendations.
Several years ago, at the very beginning of the H1N1 influenza epidemic, a colleague of mine working in a pediatric practice saw a patient complaining of fever, lethargy, and myalgia. Not surprisingly, the patient’s rapid influenza test was positive. My colleague recalls that she was handed the result before she ever walked into the room – without any PPE – to see the patient.
“This was different than my usual routine at the hospital,” she told me. The expectation at the hospital was gown, gloves, and masks for any patient with influenza or influenzalike illness. At the office though, there was no such expectation, and providers did not routinely wear masks, even when seeing patients with respiratory symptoms. My colleague wasn’t reckless or rebellious. She was simply conforming to the culture in that office, and following the behavioral cues of more senior physicians in the practice. Subsequently, she developed severe influenza infection requiring a prolonged hospital stay.
It’s time to change the culture. As a first step, perform a quick audit in the office, using the AAP’s “Infection prevention and control in pediatric ambulatory settings” as a guide.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Protecting pregnant women, infants from infections
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.
However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
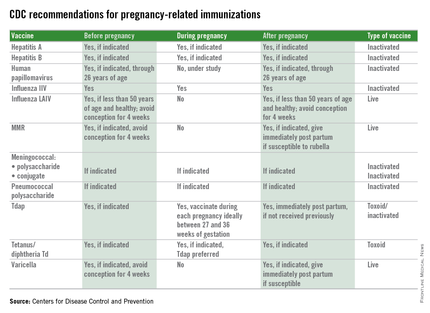
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at [email protected].
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.

However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at [email protected].
Infectious disease morbidity and mortality continue to disproportionately impact pregnant women and young infants.
In California, the incidence of pertussis approximates 100 cases per 100,000 in infants less than 5 months of age; a rate threefold greater than any other age group. Seven of nine (77%) deaths in 2013/2014 occurred in infants less than 3 months of age (California Department of Public Health Pertussis Report, Aug. 3, 2015).
Influenza severity and mortality is increased in pregnant women, and there is a greater risk of fetal morbidity and wastage. In the 2009 H1N1 pandemic, there was a 20% case fatality rate in women sick enough to be admitted to the ICU. The incidence of low birth weight also was increased among pregnant women delivering while hospitalized for influenza-related illness. These examples highlight the burden of vaccine-preventable disease in two vulnerable populations, pregnant women and infants too young to be protected by vaccines mandated by the U.S.immunization program.
The American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and many other national and state organizations endorse immunization of pregnant women to improve women’s and infants’ outcomes. Recent studies demonstrate that infants born to women vaccinated with influenza are 45%-48% less likely to be hospitalized for culture-proven influenza.
Benowitz et al. reported a 91.5% effectiveness for maternal influenza vaccination for prevention of hospitalization of infants caused by influenza in the first 6 months of life. The presumed mechanisms of protection are both the transplacental transfer of protective antibody as well as indirect protection from disease prevention in the mother (Clin Infect Dis. 2010 Dec 15;51(12):1355-61). The recommendation is that inactivated influenza vaccine can be given at any time during pregnancy; however, live attenuated influenza vaccine (LAIV; FluMist) is contraindicated, as are all live-virus vaccines. In contrast, Tdap is recommended for use either during pregnancy or post partum.

However, Healy et al. (Pediatr Infect Dis J. 2015;34(1):22-60) failed to demonstrate a benefit to postpartum immunization and cocooning for reducing pertussis illness in infants 6 months of age or younger. The likely explanation for this failure is revealed in a recent study in infant baboons where immunization with Tdap failed to decrease colonization or transmission of Bordetella pertussis, compared with natural disease or whole-cell pertussis. Thus, even though protective against disease, Tdap failure to prevent transmission within the community still occurs. The current Advisory Committee on Immunization Practices recommendation, immunization between 27 and 36 weeks, is designed to ensure high antibody concentrations in both mother and newborn at the time of birth and bridge the time period until infant immunization can elicit protective antibody.
The benefits achieved with maternal immunization must be weighed against potential for adverse events. There is no evidence of risk to either mother or infant from inactivated vaccines administered during pregnancy. Still, the recommendations for influenza and Tdap vaccine incorporate the high likelihood of exposure, the risk of morbidity or mortality from the infectious agent, and the likelihood of harm. During the H1N1 epidemic, a cohort study by Chambers et al. of H1N1 vaccine in exposed and unexposed pregnant women concluded that there was no increase in risk for major congenital defects, spontaneous abortion, or small for gestational age (Vaccine. 2013 Oct 17;31(44):5026-32). There was a signal for increase in prematurity, but the difference between H1N1-vaccinated and unvaccinated pregnancies was 3 days. In addition, a review of 11 studies, including one of 10,428 pregnant women, concluded there were no harmful maternal or fetal effects.
Additionally, no adverse risks have been identified in women who were inadvertently vaccinated during pregnancy with live-attenuated rubella, influenza, and yellow fever vaccines. Tetanus vaccination has been administered safely to several millions of pregnant women without documented serious adverse outcomes. Ongoing postmarketing surveillance continues as an important tool for identification of potential adverse effects.
One potential limitation is the blunting of infant immune responses to vaccination due to high serum antibody concentrations at the time of primary immunizations. Some studies have found lower antibody concentrations prior to booster vaccinations at 1 year of age. However, as morbidity and mortality is greater in the first months of life for many infectious diseases, this may be an acceptable trade off if high morbidity and mortality can be reduced in the first months of life.
Immunization during pregnancy represents only one aspect of prevention of vaccine preventable diseases. Preconception, prenatal, and postpartum visits with health care professionals represents an opportune time to discuss the benefits of immunization and their contribution to a healthy pregnancy outcome. Inactivated vaccines are safe for administration during pregnancy, live virus vaccines, despite being attenuated, are a theoretical risk if spread to the fetus occurs and therefore are contraindicated and should be administered during preconception counseling if indicated. The table below outlines vaccines that can be administered before, during, and after pregnancy.
Although once considered potentially contraindicated in pregnant women, evidence now supports specific vaccines as both safe for a pregnant woman and her fetus and effective for preventing serious disease in both. Universal immunization with influenza vaccine and Tdap, as recommended by multiple national professional medical organizations, will improve the outcome of pregnancy by prevention of morbidity and mortality from common community pathogens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. E-mail him at [email protected].
Water woes: Recognizing and treating recreational water illness
Most of our patients have been or will be exposed to water in a recreational setting this summer. As health care providers, we might not routinely consider illnesses associated with recreational water exposure or discuss preventive strategies; however, the Centers for Disease Control and Prevention has been actively promoting awareness about recreational water illnesses for years. May 18-24, 2015, was the 11th annual observance of Healthy and Safe Swimming Week, formerly known as Recreational Illness and Injury Prevention Week. The focus for 2015 was promoting the role of swimmers, residential pool owners, public health officials, and beach staff in the prevention of drownings, chemical injuries, and outbreaks of illness. One goal was for the swimmer to take a more active role in protecting themselves and preventing the spread of infections to others. For our colleagues, that means educating both parents and children.
To begin our discussion, let’s define recreational water illnesses (RWI). RWIs are caused by a variety of infectious pathogens transmitted by ingestion, inhalation of aerosols or mists, or having contact with contaminated water from both treated (swimming pools, hot tubs, water parks, and fountains) and untreated (lakes, rivers, and oceans) sources of water in recreational venues. RWIs also can be caused by chemicals that have evaporated from water leading to poor indoor air quality. However, I am focusing on the infectious etiologies.
A broad spectrum of infections are associated with RWIs, including infections of the gastrointestinal tract, ear, skin, eye, central nervous system, and wounds. Diarrhea is the most common infection. Implicated pathogens include Giardia, Shigella, norovirus, and Escherichia coli O157:H7, but it is Cryptosporidium that has emerged as the pathogen implicated most often in swimming pool–related outbreaks. Recently published data from the CDC revealed that in 2011-2012, there were 90 recreational-associated outbreaks reported from 32 states and Puerto Rico resulting in 1,788 infections, with 69 outbreaks occurring in treated water venues. Of these, 36 (51%) were caused by Cryptosporidium. Among 21 outbreaks occurring in untreated recreational water, E. coli was responsible for 7 (33%) (MMWR Morb. Mortal. Wkly Rep. 2015;64:668-72)
It’s no surprise diarrhea is the most common illness. Infection can easily occur after swallowing contaminated water. Many erroneously think chlorine kills all pathogens. Cryptosporidium is chlorine tolerant and can persist in treated water with the current recommended levels of chlorine for more than 10 days (J. Water Health 2008;6:513-20). For chlorine-sensitive pathogens, maintenance of the disinfection process must remain intact. What role do swimmers play? Most people have about 0.4 g of feces on their bottoms that can contaminate water when rinsed off. How many people enter a pool with a diarrheal illness? How many may go swimming after having recently recovered from a diarrheal illness and may have asymptomatic shedding? We all have cringed when we see a diapered child in the water. All of these are potential ways for the swimmer to contaminate an adequately treated pool. Additionally, while Cryptosporidium infections are usually self-limited, some individuals, including the immunocompromised host and especially those with advanced HIV and those who are solid organ transplant recipients, may have a protracted course of profuse diarrhea if infected.
While diarrhea maybe the most common RWI, it is not the only one. Acute otitis externa (AOE), more commonly known as “swimmer’s ear,” is one of the most frequent reasons for summer health care encounters. It has been estimated that in the United States in 2007, 2.4 million health care visits resulted in the diagnosis of AOE (MMWR Morb. Mortal. Wkly. Rep. 2011;60:605-9). Visits were highest among children aged 5-9 years; however, adults accounted for 53% of the encounters. Inflammation and infection of the external auditory canal is usually caused by bacteria. Pseudomonas aeruginosa or Staphylococcus aureus are the two most common etiologies. Water is easily introduced into the external auditory canal with recreational water activities, leading to maceration and subsequent infection of the canal. Simply reminding parents to thoroughly dry their child’s ears after water exposure can help prevent AOE.
P. aeruginosa also is the agent causing the self-limiting conditions hot tub folliculitis and hot-foot syndrome. Hot tub folliculitis is characterized by the development of tender, pruritic papules and papulopustules on the hips, buttocks, and axillae, usually developing 8-48 hours after exposure to water that has been contaminated because of inadequate chlorination. Hot-foot syndrome is characterized by painful planter nodules (N. Engl. J. Med. 2001;345:335).
Serious diseases are encountered infrequently, but there are some that require more urgent interventions. Primary amebic meningoencephalitis (PAM) is an extremely rare, progressive, and almost always fatal infection of the brain caused by Naegleria fowleri. The pathogen is found in warm freshwater including lakes, rivers, streams, and hot springs. It enters the body through the nose and travels via the olfactory nerve to the brain. Infection usually occurs when individuals swim or dive in warm freshwater. Most cases have been reported in children from Southern states. In 2010, the first case in a northern state was reported from Minnesota, and three additional cases have since been reported in Kansas and Indiana (J. Ped. Infect. Dis. 2014 [doi: 10.1093/jpids/piu103]). Cases also have been reported in two individuals who were regular users of neti pots for sinus irrigation because the irrigating solution was prepared with contaminated tap water (Clin. Infect. Dis. 2012;55:e79-85). Clinical presentation is similar to bacterial meningitis. Helpful diagnostic clues may come from obtaining a history of swimming in freshwater within the 2 weeks prior to presentation, especially during the summer, or the use of nasal or sinus irrigation with untreated tap water. Consultation with an infectious disease specialist is recommended.
Acanthamoeba keratitis is a potentially blinding infection of the cornea that primarily occurs in individuals who wear contact lenses. Risk factors for the infection include swimming, showering, and use of hot tubs while wearing contact lenses. Improper storage and cleansing contacts with tap water are other risk factors. Anyone with corneal trauma and similar water exposures also would be at risk. Clinically, the history combined with a foreign-body sensation, pain, and decreased visual acuity should make one include this infection in the differential diagnosis. Referral to an ophthalmologist is required.
Finally, swimming with an open wound is a portal of entry for Vibrio vulnificus. It usually is associated with consumption of contaminated seafood, especially oysters. In immunocompromised individuals, especially those with chronic liver disease, this bacteria can cause a life-threatening illness leading to bacteremia, septic shock, and development of blistering skin lesions. Infections are fatal in approximately 50% of cases.
The goal of this brief review was not to discourage swimming, but to make your patients and their families healthy swimmers. Here are a few things the CDC is recommending to help them achieve that goal:
• Shower prior to going swimming.
• Do not swallow or drink pool water.
• Take bathroom breaks every hour and rinse off before going back into the water.
• Do not swim if you have diarrhea.
• Wait at least 2 weeks to go swimming if you have had diarrhea.
• Change swim diapers frequently and away from the water.
• Suggest patients download the free CDC app Healthy Swimming for more detailed information and suggest they visit cdc.gov/healthywater/swimming.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Most of our patients have been or will be exposed to water in a recreational setting this summer. As health care providers, we might not routinely consider illnesses associated with recreational water exposure or discuss preventive strategies; however, the Centers for Disease Control and Prevention has been actively promoting awareness about recreational water illnesses for years. May 18-24, 2015, was the 11th annual observance of Healthy and Safe Swimming Week, formerly known as Recreational Illness and Injury Prevention Week. The focus for 2015 was promoting the role of swimmers, residential pool owners, public health officials, and beach staff in the prevention of drownings, chemical injuries, and outbreaks of illness. One goal was for the swimmer to take a more active role in protecting themselves and preventing the spread of infections to others. For our colleagues, that means educating both parents and children.
To begin our discussion, let’s define recreational water illnesses (RWI). RWIs are caused by a variety of infectious pathogens transmitted by ingestion, inhalation of aerosols or mists, or having contact with contaminated water from both treated (swimming pools, hot tubs, water parks, and fountains) and untreated (lakes, rivers, and oceans) sources of water in recreational venues. RWIs also can be caused by chemicals that have evaporated from water leading to poor indoor air quality. However, I am focusing on the infectious etiologies.
A broad spectrum of infections are associated with RWIs, including infections of the gastrointestinal tract, ear, skin, eye, central nervous system, and wounds. Diarrhea is the most common infection. Implicated pathogens include Giardia, Shigella, norovirus, and Escherichia coli O157:H7, but it is Cryptosporidium that has emerged as the pathogen implicated most often in swimming pool–related outbreaks. Recently published data from the CDC revealed that in 2011-2012, there were 90 recreational-associated outbreaks reported from 32 states and Puerto Rico resulting in 1,788 infections, with 69 outbreaks occurring in treated water venues. Of these, 36 (51%) were caused by Cryptosporidium. Among 21 outbreaks occurring in untreated recreational water, E. coli was responsible for 7 (33%) (MMWR Morb. Mortal. Wkly Rep. 2015;64:668-72)
It’s no surprise diarrhea is the most common illness. Infection can easily occur after swallowing contaminated water. Many erroneously think chlorine kills all pathogens. Cryptosporidium is chlorine tolerant and can persist in treated water with the current recommended levels of chlorine for more than 10 days (J. Water Health 2008;6:513-20). For chlorine-sensitive pathogens, maintenance of the disinfection process must remain intact. What role do swimmers play? Most people have about 0.4 g of feces on their bottoms that can contaminate water when rinsed off. How many people enter a pool with a diarrheal illness? How many may go swimming after having recently recovered from a diarrheal illness and may have asymptomatic shedding? We all have cringed when we see a diapered child in the water. All of these are potential ways for the swimmer to contaminate an adequately treated pool. Additionally, while Cryptosporidium infections are usually self-limited, some individuals, including the immunocompromised host and especially those with advanced HIV and those who are solid organ transplant recipients, may have a protracted course of profuse diarrhea if infected.
While diarrhea maybe the most common RWI, it is not the only one. Acute otitis externa (AOE), more commonly known as “swimmer’s ear,” is one of the most frequent reasons for summer health care encounters. It has been estimated that in the United States in 2007, 2.4 million health care visits resulted in the diagnosis of AOE (MMWR Morb. Mortal. Wkly. Rep. 2011;60:605-9). Visits were highest among children aged 5-9 years; however, adults accounted for 53% of the encounters. Inflammation and infection of the external auditory canal is usually caused by bacteria. Pseudomonas aeruginosa or Staphylococcus aureus are the two most common etiologies. Water is easily introduced into the external auditory canal with recreational water activities, leading to maceration and subsequent infection of the canal. Simply reminding parents to thoroughly dry their child’s ears after water exposure can help prevent AOE.
P. aeruginosa also is the agent causing the self-limiting conditions hot tub folliculitis and hot-foot syndrome. Hot tub folliculitis is characterized by the development of tender, pruritic papules and papulopustules on the hips, buttocks, and axillae, usually developing 8-48 hours after exposure to water that has been contaminated because of inadequate chlorination. Hot-foot syndrome is characterized by painful planter nodules (N. Engl. J. Med. 2001;345:335).
Serious diseases are encountered infrequently, but there are some that require more urgent interventions. Primary amebic meningoencephalitis (PAM) is an extremely rare, progressive, and almost always fatal infection of the brain caused by Naegleria fowleri. The pathogen is found in warm freshwater including lakes, rivers, streams, and hot springs. It enters the body through the nose and travels via the olfactory nerve to the brain. Infection usually occurs when individuals swim or dive in warm freshwater. Most cases have been reported in children from Southern states. In 2010, the first case in a northern state was reported from Minnesota, and three additional cases have since been reported in Kansas and Indiana (J. Ped. Infect. Dis. 2014 [doi: 10.1093/jpids/piu103]). Cases also have been reported in two individuals who were regular users of neti pots for sinus irrigation because the irrigating solution was prepared with contaminated tap water (Clin. Infect. Dis. 2012;55:e79-85). Clinical presentation is similar to bacterial meningitis. Helpful diagnostic clues may come from obtaining a history of swimming in freshwater within the 2 weeks prior to presentation, especially during the summer, or the use of nasal or sinus irrigation with untreated tap water. Consultation with an infectious disease specialist is recommended.
Acanthamoeba keratitis is a potentially blinding infection of the cornea that primarily occurs in individuals who wear contact lenses. Risk factors for the infection include swimming, showering, and use of hot tubs while wearing contact lenses. Improper storage and cleansing contacts with tap water are other risk factors. Anyone with corneal trauma and similar water exposures also would be at risk. Clinically, the history combined with a foreign-body sensation, pain, and decreased visual acuity should make one include this infection in the differential diagnosis. Referral to an ophthalmologist is required.
Finally, swimming with an open wound is a portal of entry for Vibrio vulnificus. It usually is associated with consumption of contaminated seafood, especially oysters. In immunocompromised individuals, especially those with chronic liver disease, this bacteria can cause a life-threatening illness leading to bacteremia, septic shock, and development of blistering skin lesions. Infections are fatal in approximately 50% of cases.
The goal of this brief review was not to discourage swimming, but to make your patients and their families healthy swimmers. Here are a few things the CDC is recommending to help them achieve that goal:
• Shower prior to going swimming.
• Do not swallow or drink pool water.
• Take bathroom breaks every hour and rinse off before going back into the water.
• Do not swim if you have diarrhea.
• Wait at least 2 weeks to go swimming if you have had diarrhea.
• Change swim diapers frequently and away from the water.
• Suggest patients download the free CDC app Healthy Swimming for more detailed information and suggest they visit cdc.gov/healthywater/swimming.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Most of our patients have been or will be exposed to water in a recreational setting this summer. As health care providers, we might not routinely consider illnesses associated with recreational water exposure or discuss preventive strategies; however, the Centers for Disease Control and Prevention has been actively promoting awareness about recreational water illnesses for years. May 18-24, 2015, was the 11th annual observance of Healthy and Safe Swimming Week, formerly known as Recreational Illness and Injury Prevention Week. The focus for 2015 was promoting the role of swimmers, residential pool owners, public health officials, and beach staff in the prevention of drownings, chemical injuries, and outbreaks of illness. One goal was for the swimmer to take a more active role in protecting themselves and preventing the spread of infections to others. For our colleagues, that means educating both parents and children.
To begin our discussion, let’s define recreational water illnesses (RWI). RWIs are caused by a variety of infectious pathogens transmitted by ingestion, inhalation of aerosols or mists, or having contact with contaminated water from both treated (swimming pools, hot tubs, water parks, and fountains) and untreated (lakes, rivers, and oceans) sources of water in recreational venues. RWIs also can be caused by chemicals that have evaporated from water leading to poor indoor air quality. However, I am focusing on the infectious etiologies.
A broad spectrum of infections are associated with RWIs, including infections of the gastrointestinal tract, ear, skin, eye, central nervous system, and wounds. Diarrhea is the most common infection. Implicated pathogens include Giardia, Shigella, norovirus, and Escherichia coli O157:H7, but it is Cryptosporidium that has emerged as the pathogen implicated most often in swimming pool–related outbreaks. Recently published data from the CDC revealed that in 2011-2012, there were 90 recreational-associated outbreaks reported from 32 states and Puerto Rico resulting in 1,788 infections, with 69 outbreaks occurring in treated water venues. Of these, 36 (51%) were caused by Cryptosporidium. Among 21 outbreaks occurring in untreated recreational water, E. coli was responsible for 7 (33%) (MMWR Morb. Mortal. Wkly Rep. 2015;64:668-72)
It’s no surprise diarrhea is the most common illness. Infection can easily occur after swallowing contaminated water. Many erroneously think chlorine kills all pathogens. Cryptosporidium is chlorine tolerant and can persist in treated water with the current recommended levels of chlorine for more than 10 days (J. Water Health 2008;6:513-20). For chlorine-sensitive pathogens, maintenance of the disinfection process must remain intact. What role do swimmers play? Most people have about 0.4 g of feces on their bottoms that can contaminate water when rinsed off. How many people enter a pool with a diarrheal illness? How many may go swimming after having recently recovered from a diarrheal illness and may have asymptomatic shedding? We all have cringed when we see a diapered child in the water. All of these are potential ways for the swimmer to contaminate an adequately treated pool. Additionally, while Cryptosporidium infections are usually self-limited, some individuals, including the immunocompromised host and especially those with advanced HIV and those who are solid organ transplant recipients, may have a protracted course of profuse diarrhea if infected.
While diarrhea maybe the most common RWI, it is not the only one. Acute otitis externa (AOE), more commonly known as “swimmer’s ear,” is one of the most frequent reasons for summer health care encounters. It has been estimated that in the United States in 2007, 2.4 million health care visits resulted in the diagnosis of AOE (MMWR Morb. Mortal. Wkly. Rep. 2011;60:605-9). Visits were highest among children aged 5-9 years; however, adults accounted for 53% of the encounters. Inflammation and infection of the external auditory canal is usually caused by bacteria. Pseudomonas aeruginosa or Staphylococcus aureus are the two most common etiologies. Water is easily introduced into the external auditory canal with recreational water activities, leading to maceration and subsequent infection of the canal. Simply reminding parents to thoroughly dry their child’s ears after water exposure can help prevent AOE.
P. aeruginosa also is the agent causing the self-limiting conditions hot tub folliculitis and hot-foot syndrome. Hot tub folliculitis is characterized by the development of tender, pruritic papules and papulopustules on the hips, buttocks, and axillae, usually developing 8-48 hours after exposure to water that has been contaminated because of inadequate chlorination. Hot-foot syndrome is characterized by painful planter nodules (N. Engl. J. Med. 2001;345:335).
Serious diseases are encountered infrequently, but there are some that require more urgent interventions. Primary amebic meningoencephalitis (PAM) is an extremely rare, progressive, and almost always fatal infection of the brain caused by Naegleria fowleri. The pathogen is found in warm freshwater including lakes, rivers, streams, and hot springs. It enters the body through the nose and travels via the olfactory nerve to the brain. Infection usually occurs when individuals swim or dive in warm freshwater. Most cases have been reported in children from Southern states. In 2010, the first case in a northern state was reported from Minnesota, and three additional cases have since been reported in Kansas and Indiana (J. Ped. Infect. Dis. 2014 [doi: 10.1093/jpids/piu103]). Cases also have been reported in two individuals who were regular users of neti pots for sinus irrigation because the irrigating solution was prepared with contaminated tap water (Clin. Infect. Dis. 2012;55:e79-85). Clinical presentation is similar to bacterial meningitis. Helpful diagnostic clues may come from obtaining a history of swimming in freshwater within the 2 weeks prior to presentation, especially during the summer, or the use of nasal or sinus irrigation with untreated tap water. Consultation with an infectious disease specialist is recommended.
Acanthamoeba keratitis is a potentially blinding infection of the cornea that primarily occurs in individuals who wear contact lenses. Risk factors for the infection include swimming, showering, and use of hot tubs while wearing contact lenses. Improper storage and cleansing contacts with tap water are other risk factors. Anyone with corneal trauma and similar water exposures also would be at risk. Clinically, the history combined with a foreign-body sensation, pain, and decreased visual acuity should make one include this infection in the differential diagnosis. Referral to an ophthalmologist is required.
Finally, swimming with an open wound is a portal of entry for Vibrio vulnificus. It usually is associated with consumption of contaminated seafood, especially oysters. In immunocompromised individuals, especially those with chronic liver disease, this bacteria can cause a life-threatening illness leading to bacteremia, septic shock, and development of blistering skin lesions. Infections are fatal in approximately 50% of cases.
The goal of this brief review was not to discourage swimming, but to make your patients and their families healthy swimmers. Here are a few things the CDC is recommending to help them achieve that goal:
• Shower prior to going swimming.
• Do not swallow or drink pool water.
• Take bathroom breaks every hour and rinse off before going back into the water.
• Do not swim if you have diarrhea.
• Wait at least 2 weeks to go swimming if you have had diarrhea.
• Change swim diapers frequently and away from the water.
• Suggest patients download the free CDC app Healthy Swimming for more detailed information and suggest they visit cdc.gov/healthywater/swimming.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
The importance of UA in diagnosing UTIs in infants under 2 months
A 28-day-old uncircumcised male infant presents to the emergency department with fever of 38.9° C, decreased feeding, and irritability. The physical examination is normal with the exception of the irritability and your assessment of mild dehydration. The infant undergoes a sepsis work-up. The CBC is remarkable for a WBC of 16,500/mm3 with 44% neutrophils, 52% lymphocytes, and 4% monocytes. Platelet count is normal. Cerebrospinal fluid (CSF) shows no white or red blood cells with normal glucose and protein. The urinalysis (UA) has a positive 1+ leukocyte esterase (LE) with 10 WBC per high-power field (HPF), but negative nitrite and 1+ bacteria microscopically. The child is admitted to the hospital for empiric antibiotics pending blood, urine, and CSF cultures. What are the chances that a urinary tract infection (UTI) is the origin of the febrile presentation?
UTIs are currently the most common serious bacterial infection (SBI) in < 2-year-old febrile children without an apparent source of fever (Pediatrics 2011;128:595-610). Since 2000, the prevalence of UTIs in all febrile infants and young children without an apparent source is unchanged, being approximately 5%. The rate of UTIs in fever-without-apparent-source presentations at < 90 days of age is higher, ranging from 6%-15% in different studies.
Meanwhile bacteremia, sepsis, meningitis, and other previously common SBIs, mostly caused by Haemophilus influenzae type b (Hib) or pneumococcus, have decreased. We recognize these reductions as effects of universal implementation of Hib (mid-1990s) and pneumococcal (2000 and 2010) conjugate vaccines.
Given the case above, other pertinent facts are that uncircumcised males have more UTIs in the first months of life (J. Pediatr. 1996;128:23-7) and approximately 5% of young infants with UTIs also are concurrently bacteremic (Pediatrics 1999;104:79-86;J. Pediatr. 1994;124:513-9)
The elephant in the room is the fact that we also need to be cognizant of asymptomatic bacteriuria (AB). AB is colonization of the lower urinary tract without infection. Patients with AB may meet culture criteria for UTI (whether we consider > 50,000 or > 100,000 colony-forming units/mL), but there is no evidence of true infection, that is no inflammation or mucosal injury. So children with AB are not at risk for renal injury or later renal damage and do not require antibiotic treatment.
But when AB patients develop fever, for example with an enterovirus infection, their urine cultures (together with the fever) can do a good imitation of a UTI, unless we focus on the UA results. It not only remains critical to detect true UTIs in infants < 90 days old, such as the one in our case above, but also to distinguish UTI from AB.
The 1999 American Academy of Pediatrics’ UTI guidelines (Pediatrics 1999;103:843-52) included UA results as suggestive of UTI. They stated that a positive LE or nitrite test or > 5 WBC/HPF in a spun urine, or bacteria visualized in unspun gram-stained specimen suggest, but cannot be diagnostic of a UTI. Recommendation five in the guidelines states that UTI diagnosis required 100,000 CFU/mL in culture of sterilely obtained catheterized urine as the threshold criterion (strength of evidence: strong). However, AB was not fully considered because, in part, data defining AB was incomplete in 1999.
The 1999 guidelines also stated, “The urinalysis … can be valuable in selecting individuals for prompt initiation of treatment while waiting for the results of the urine culture.” So, UA was considered adjunctive. UA’s main function was to allow empiric therapy of sufficiently ill children, given positive results for LE, nitrites, or microscopic visualization of > 5 WBC/HPF or bacteria in the spun urine.
In the 2011 AAP guidelines for UTI, things have changed (Pediatrics 2011;128:595-610). The third action statement tells us that both the UA and culture taken together are necessary for UTI diagnosis. To paraphrase: The diagnosis of UTI requires urinalysis results suggesting infection (pyuria or microscopic bacteriuria) plus > 50,000 CFU/mL of a uropathogen in urine from catheterization or suprapubic aspiration. But remember that these guidelines do not apply specifically to the youngest of infants, that is < 2 months old.
Both of these criteria were changes from the 1999 UTI guidelines. Previously pyuria or microscopic bacteriuria were not considered necessary to diagnose UTI, and >100,000 CFU/mL rather than > 50,000 CFU/mL of a single pathogen species was the critical diagnostic result for catheterized urine. For suprapubic aspiration urine samples, > 10,000 CFU/HPF were considered adequate for UTI diagnoses in 1999.
Now, a recent study of children < 90 days of age (including those < 2 months of age) reports that pyuria (> 3 WBC/HPF) plus > 50,000 CFU/mL are the keys to diagnosing UTI (Pediatrics 2015;135:965-71). One caveat is that the study population was febrile infants < 90 days old with concurrent bacteremia (bacteremic UTI). Bacteremic UTI was studied to reduce as much as possible the chance that AB patients might be inadvertently included in the study. One other conclusion of this new study is that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
These data in an overall younger population than that covered by the 2011 guidelines adds evidence that pyuria (but not microscopic bacteriuria) is critical to diagnosing UTI. Pyuria plus positive culture has been a combination for the pediatric infectious diseases practitioner’s toolkit for decades. Likewise, it seems to me that primary care pediatric clinicians also often decide whether to undertake the expense of culture based on UA results. For example, a completely normal UA may obviate need for culture except in selected unusual cases.
Requiring UA evidence of inflammation to diagnose UTI (per the 2011 guidelines and the recommendations of the authors of the recent 2015 study) makes sense because most UTIs in otherwise healthy children are caused by gram-negative organisms (> 90% from Escherichia coli) (J. Pediatr. 1994;124:513-9). Why are UA results so important?
A positive nitrite test strongly suggests UTI because nitrites in the urine indicate viable gram-negative organisms also are present in the urine. Nitrates in the urine are converted to nitrites by metabolic activity of gram-negative pathogens. For WBCs or LE in the urine, their presence indicates inflammation in the urinary tract, Consider that lipopolysaccharide (LPS), also known as gram-negative endotoxin, is a major component of the cell membrane of > 90% of uropathogens like E. coli. Moreover, LPS elicits about the strongest innate immune response via toll-like receptor 4 (TLR4) from monocytes/macrophages, inducing a large pro-inflammatory and chemotactic response – interleukin-6, interleukin-8, tetrahydrofuran-alpha. Remember that LPS is also a major cause of fever and of shock during gram-negative sepsis.
So a UTI diagnosis based on a “positive” culture without evidence of metabolic products of gram negatives (nitrites) or without inflammation (no pyuria or negative LE) should be questioned. The combination of > 50,000 CFU/mL with no detectable LE or < 3-5 WBC/HPF in a febrile child is most likely evidence for AB in a child with the fever caused by some non-UTI process.
In contrast, selected SBIs may occur when the culture is “positive” without inflammation or nitrites. The first of three examples is a renal parenchymal abscess, where bacteria enter the urine sporadically in only small numbers, and do not actually infect the urinary tract mucosa. The scenario of no inflammation but “positive” culture also may occur when a large bacteremic load causes results in organisms filtering through the kidney into the urine, again without urinary mucosal infection, such as Staphylococcus aureus, group A streptococcus, or group B streptococcus bacteremia/sepsis. The third scenario with a “positive” culture and no pyuria can be with organisms that have blunted abilities to induce inflammation, such as enterococcus. Enterococcal cell components have weak inflammatory and chemotactic capability. So a urinary mucosal infection in the collecting system or bladder may occur without much if any pyuria. In fact, the patients from the recent study with insufficient evidence of pyuria/inflammation were those who had either gram-positive organisms or considerably less than 50,000 CFU/mL of gram-negative organisms.
The sensitivity and specificity of the LE or pyuria was higher in the recent study (Pediatrics 2015;135:965-71) than any prior study. The authors comment that they had not expected such a high sensitivity of 97.6% (94.5-99.2) for LE in confirmed bacteremic UTI, nor did they expect the high specificity of 93.9% (87.9-97.5). The presence of microscopic pyuria defined as > 3 WBC/HPF was nearly as sensitive, 96%, and specific, 91.3%. Disappointingly, positive nitrite testing was only 39.5% sensitive, but it was 100% specific. This likely reflects the short time that urine resides in the bladder of infants < 90 days of age, so there is insufficient time for the pathogens to metabolically convert the nitrates to nitrites.
So how would the UA help with our example case? There is microscopic bacteriuria, pyuria, and positive LE, but negative nitrites. Using the suggestions of the authors of the recent report (Pediatrics 2015;135:965-71) and those of another report on the utility of UA results (Acta Paediatr. 2010;99:581-4), the UA in our case indicates that we should be highly suspicious of a UTI in this child < 2 months old for whom the 2011 guidelines do not directly apply. But remember that these impressive sensitivity and specificity values relate to bacteremic UTI. Whether they apply to nonbacteremic UTI is not known. Likewise, the authors caution that their study design did not allow calculation of positive or negative predictive values – aspects that would clarify things even further.
So we still cannot be more than highly suspicious. Without a positive predictive value, we do not know the odds of this case having a UTI with mathematical precision. The authors do point out that only one of their subjects had a completely normal UA and actually had a bacteremic UTI. If you guessed that it was a gram-positive pathogen, you win the prize. So it seems reasonable to predict that a normal UA has a high specificity for not being a UTI (87.8%), but a positive UA remains only highly suggestive. It is still not clear if a negative UA statistically justifies not submitting the culture of the sterilely obtained urine because we still don’t have a negative predictive value.
Bottom line: The 2011 UTI guidelines provide good advice on diagnosing UTIs.
1. We have more data that evidence of inflammation is essential for diagnosing gram-negative UTIs.
2. We also have more evidence that 50,000 CFU/mL is a good threshold for diagnosing UTIs.
3. It appears that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
4. And we now have more evidence that these criteria also apply to infants < 2 months of age.
To close the loop on our case, the child’s CSF and blood cultures were negative, but the urine culture revealed > 100,000 CFU/mL of E. coli susceptible to second- and third-generation cephalosporins, ciprofloxacin, and nitrofurantoin, but resistant to trimethoprim-sulfamethoxazole.
Have a great summer and watch for UTIs in your young patients < 90 days old and fever without apparent focus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. E-mail Dr. Harrison at [email protected].
A 28-day-old uncircumcised male infant presents to the emergency department with fever of 38.9° C, decreased feeding, and irritability. The physical examination is normal with the exception of the irritability and your assessment of mild dehydration. The infant undergoes a sepsis work-up. The CBC is remarkable for a WBC of 16,500/mm3 with 44% neutrophils, 52% lymphocytes, and 4% monocytes. Platelet count is normal. Cerebrospinal fluid (CSF) shows no white or red blood cells with normal glucose and protein. The urinalysis (UA) has a positive 1+ leukocyte esterase (LE) with 10 WBC per high-power field (HPF), but negative nitrite and 1+ bacteria microscopically. The child is admitted to the hospital for empiric antibiotics pending blood, urine, and CSF cultures. What are the chances that a urinary tract infection (UTI) is the origin of the febrile presentation?
UTIs are currently the most common serious bacterial infection (SBI) in < 2-year-old febrile children without an apparent source of fever (Pediatrics 2011;128:595-610). Since 2000, the prevalence of UTIs in all febrile infants and young children without an apparent source is unchanged, being approximately 5%. The rate of UTIs in fever-without-apparent-source presentations at < 90 days of age is higher, ranging from 6%-15% in different studies.
Meanwhile bacteremia, sepsis, meningitis, and other previously common SBIs, mostly caused by Haemophilus influenzae type b (Hib) or pneumococcus, have decreased. We recognize these reductions as effects of universal implementation of Hib (mid-1990s) and pneumococcal (2000 and 2010) conjugate vaccines.
Given the case above, other pertinent facts are that uncircumcised males have more UTIs in the first months of life (J. Pediatr. 1996;128:23-7) and approximately 5% of young infants with UTIs also are concurrently bacteremic (Pediatrics 1999;104:79-86;J. Pediatr. 1994;124:513-9)
The elephant in the room is the fact that we also need to be cognizant of asymptomatic bacteriuria (AB). AB is colonization of the lower urinary tract without infection. Patients with AB may meet culture criteria for UTI (whether we consider > 50,000 or > 100,000 colony-forming units/mL), but there is no evidence of true infection, that is no inflammation or mucosal injury. So children with AB are not at risk for renal injury or later renal damage and do not require antibiotic treatment.
But when AB patients develop fever, for example with an enterovirus infection, their urine cultures (together with the fever) can do a good imitation of a UTI, unless we focus on the UA results. It not only remains critical to detect true UTIs in infants < 90 days old, such as the one in our case above, but also to distinguish UTI from AB.
The 1999 American Academy of Pediatrics’ UTI guidelines (Pediatrics 1999;103:843-52) included UA results as suggestive of UTI. They stated that a positive LE or nitrite test or > 5 WBC/HPF in a spun urine, or bacteria visualized in unspun gram-stained specimen suggest, but cannot be diagnostic of a UTI. Recommendation five in the guidelines states that UTI diagnosis required 100,000 CFU/mL in culture of sterilely obtained catheterized urine as the threshold criterion (strength of evidence: strong). However, AB was not fully considered because, in part, data defining AB was incomplete in 1999.
The 1999 guidelines also stated, “The urinalysis … can be valuable in selecting individuals for prompt initiation of treatment while waiting for the results of the urine culture.” So, UA was considered adjunctive. UA’s main function was to allow empiric therapy of sufficiently ill children, given positive results for LE, nitrites, or microscopic visualization of > 5 WBC/HPF or bacteria in the spun urine.
In the 2011 AAP guidelines for UTI, things have changed (Pediatrics 2011;128:595-610). The third action statement tells us that both the UA and culture taken together are necessary for UTI diagnosis. To paraphrase: The diagnosis of UTI requires urinalysis results suggesting infection (pyuria or microscopic bacteriuria) plus > 50,000 CFU/mL of a uropathogen in urine from catheterization or suprapubic aspiration. But remember that these guidelines do not apply specifically to the youngest of infants, that is < 2 months old.
Both of these criteria were changes from the 1999 UTI guidelines. Previously pyuria or microscopic bacteriuria were not considered necessary to diagnose UTI, and >100,000 CFU/mL rather than > 50,000 CFU/mL of a single pathogen species was the critical diagnostic result for catheterized urine. For suprapubic aspiration urine samples, > 10,000 CFU/HPF were considered adequate for UTI diagnoses in 1999.
Now, a recent study of children < 90 days of age (including those < 2 months of age) reports that pyuria (> 3 WBC/HPF) plus > 50,000 CFU/mL are the keys to diagnosing UTI (Pediatrics 2015;135:965-71). One caveat is that the study population was febrile infants < 90 days old with concurrent bacteremia (bacteremic UTI). Bacteremic UTI was studied to reduce as much as possible the chance that AB patients might be inadvertently included in the study. One other conclusion of this new study is that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
These data in an overall younger population than that covered by the 2011 guidelines adds evidence that pyuria (but not microscopic bacteriuria) is critical to diagnosing UTI. Pyuria plus positive culture has been a combination for the pediatric infectious diseases practitioner’s toolkit for decades. Likewise, it seems to me that primary care pediatric clinicians also often decide whether to undertake the expense of culture based on UA results. For example, a completely normal UA may obviate need for culture except in selected unusual cases.
Requiring UA evidence of inflammation to diagnose UTI (per the 2011 guidelines and the recommendations of the authors of the recent 2015 study) makes sense because most UTIs in otherwise healthy children are caused by gram-negative organisms (> 90% from Escherichia coli) (J. Pediatr. 1994;124:513-9). Why are UA results so important?
A positive nitrite test strongly suggests UTI because nitrites in the urine indicate viable gram-negative organisms also are present in the urine. Nitrates in the urine are converted to nitrites by metabolic activity of gram-negative pathogens. For WBCs or LE in the urine, their presence indicates inflammation in the urinary tract, Consider that lipopolysaccharide (LPS), also known as gram-negative endotoxin, is a major component of the cell membrane of > 90% of uropathogens like E. coli. Moreover, LPS elicits about the strongest innate immune response via toll-like receptor 4 (TLR4) from monocytes/macrophages, inducing a large pro-inflammatory and chemotactic response – interleukin-6, interleukin-8, tetrahydrofuran-alpha. Remember that LPS is also a major cause of fever and of shock during gram-negative sepsis.
So a UTI diagnosis based on a “positive” culture without evidence of metabolic products of gram negatives (nitrites) or without inflammation (no pyuria or negative LE) should be questioned. The combination of > 50,000 CFU/mL with no detectable LE or < 3-5 WBC/HPF in a febrile child is most likely evidence for AB in a child with the fever caused by some non-UTI process.
In contrast, selected SBIs may occur when the culture is “positive” without inflammation or nitrites. The first of three examples is a renal parenchymal abscess, where bacteria enter the urine sporadically in only small numbers, and do not actually infect the urinary tract mucosa. The scenario of no inflammation but “positive” culture also may occur when a large bacteremic load causes results in organisms filtering through the kidney into the urine, again without urinary mucosal infection, such as Staphylococcus aureus, group A streptococcus, or group B streptococcus bacteremia/sepsis. The third scenario with a “positive” culture and no pyuria can be with organisms that have blunted abilities to induce inflammation, such as enterococcus. Enterococcal cell components have weak inflammatory and chemotactic capability. So a urinary mucosal infection in the collecting system or bladder may occur without much if any pyuria. In fact, the patients from the recent study with insufficient evidence of pyuria/inflammation were those who had either gram-positive organisms or considerably less than 50,000 CFU/mL of gram-negative organisms.
The sensitivity and specificity of the LE or pyuria was higher in the recent study (Pediatrics 2015;135:965-71) than any prior study. The authors comment that they had not expected such a high sensitivity of 97.6% (94.5-99.2) for LE in confirmed bacteremic UTI, nor did they expect the high specificity of 93.9% (87.9-97.5). The presence of microscopic pyuria defined as > 3 WBC/HPF was nearly as sensitive, 96%, and specific, 91.3%. Disappointingly, positive nitrite testing was only 39.5% sensitive, but it was 100% specific. This likely reflects the short time that urine resides in the bladder of infants < 90 days of age, so there is insufficient time for the pathogens to metabolically convert the nitrates to nitrites.
So how would the UA help with our example case? There is microscopic bacteriuria, pyuria, and positive LE, but negative nitrites. Using the suggestions of the authors of the recent report (Pediatrics 2015;135:965-71) and those of another report on the utility of UA results (Acta Paediatr. 2010;99:581-4), the UA in our case indicates that we should be highly suspicious of a UTI in this child < 2 months old for whom the 2011 guidelines do not directly apply. But remember that these impressive sensitivity and specificity values relate to bacteremic UTI. Whether they apply to nonbacteremic UTI is not known. Likewise, the authors caution that their study design did not allow calculation of positive or negative predictive values – aspects that would clarify things even further.
So we still cannot be more than highly suspicious. Without a positive predictive value, we do not know the odds of this case having a UTI with mathematical precision. The authors do point out that only one of their subjects had a completely normal UA and actually had a bacteremic UTI. If you guessed that it was a gram-positive pathogen, you win the prize. So it seems reasonable to predict that a normal UA has a high specificity for not being a UTI (87.8%), but a positive UA remains only highly suggestive. It is still not clear if a negative UA statistically justifies not submitting the culture of the sterilely obtained urine because we still don’t have a negative predictive value.
Bottom line: The 2011 UTI guidelines provide good advice on diagnosing UTIs.
1. We have more data that evidence of inflammation is essential for diagnosing gram-negative UTIs.
2. We also have more evidence that 50,000 CFU/mL is a good threshold for diagnosing UTIs.
3. It appears that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
4. And we now have more evidence that these criteria also apply to infants < 2 months of age.
To close the loop on our case, the child’s CSF and blood cultures were negative, but the urine culture revealed > 100,000 CFU/mL of E. coli susceptible to second- and third-generation cephalosporins, ciprofloxacin, and nitrofurantoin, but resistant to trimethoprim-sulfamethoxazole.
Have a great summer and watch for UTIs in your young patients < 90 days old and fever without apparent focus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. E-mail Dr. Harrison at [email protected].
A 28-day-old uncircumcised male infant presents to the emergency department with fever of 38.9° C, decreased feeding, and irritability. The physical examination is normal with the exception of the irritability and your assessment of mild dehydration. The infant undergoes a sepsis work-up. The CBC is remarkable for a WBC of 16,500/mm3 with 44% neutrophils, 52% lymphocytes, and 4% monocytes. Platelet count is normal. Cerebrospinal fluid (CSF) shows no white or red blood cells with normal glucose and protein. The urinalysis (UA) has a positive 1+ leukocyte esterase (LE) with 10 WBC per high-power field (HPF), but negative nitrite and 1+ bacteria microscopically. The child is admitted to the hospital for empiric antibiotics pending blood, urine, and CSF cultures. What are the chances that a urinary tract infection (UTI) is the origin of the febrile presentation?
UTIs are currently the most common serious bacterial infection (SBI) in < 2-year-old febrile children without an apparent source of fever (Pediatrics 2011;128:595-610). Since 2000, the prevalence of UTIs in all febrile infants and young children without an apparent source is unchanged, being approximately 5%. The rate of UTIs in fever-without-apparent-source presentations at < 90 days of age is higher, ranging from 6%-15% in different studies.
Meanwhile bacteremia, sepsis, meningitis, and other previously common SBIs, mostly caused by Haemophilus influenzae type b (Hib) or pneumococcus, have decreased. We recognize these reductions as effects of universal implementation of Hib (mid-1990s) and pneumococcal (2000 and 2010) conjugate vaccines.
Given the case above, other pertinent facts are that uncircumcised males have more UTIs in the first months of life (J. Pediatr. 1996;128:23-7) and approximately 5% of young infants with UTIs also are concurrently bacteremic (Pediatrics 1999;104:79-86;J. Pediatr. 1994;124:513-9)
The elephant in the room is the fact that we also need to be cognizant of asymptomatic bacteriuria (AB). AB is colonization of the lower urinary tract without infection. Patients with AB may meet culture criteria for UTI (whether we consider > 50,000 or > 100,000 colony-forming units/mL), but there is no evidence of true infection, that is no inflammation or mucosal injury. So children with AB are not at risk for renal injury or later renal damage and do not require antibiotic treatment.
But when AB patients develop fever, for example with an enterovirus infection, their urine cultures (together with the fever) can do a good imitation of a UTI, unless we focus on the UA results. It not only remains critical to detect true UTIs in infants < 90 days old, such as the one in our case above, but also to distinguish UTI from AB.
The 1999 American Academy of Pediatrics’ UTI guidelines (Pediatrics 1999;103:843-52) included UA results as suggestive of UTI. They stated that a positive LE or nitrite test or > 5 WBC/HPF in a spun urine, or bacteria visualized in unspun gram-stained specimen suggest, but cannot be diagnostic of a UTI. Recommendation five in the guidelines states that UTI diagnosis required 100,000 CFU/mL in culture of sterilely obtained catheterized urine as the threshold criterion (strength of evidence: strong). However, AB was not fully considered because, in part, data defining AB was incomplete in 1999.
The 1999 guidelines also stated, “The urinalysis … can be valuable in selecting individuals for prompt initiation of treatment while waiting for the results of the urine culture.” So, UA was considered adjunctive. UA’s main function was to allow empiric therapy of sufficiently ill children, given positive results for LE, nitrites, or microscopic visualization of > 5 WBC/HPF or bacteria in the spun urine.
In the 2011 AAP guidelines for UTI, things have changed (Pediatrics 2011;128:595-610). The third action statement tells us that both the UA and culture taken together are necessary for UTI diagnosis. To paraphrase: The diagnosis of UTI requires urinalysis results suggesting infection (pyuria or microscopic bacteriuria) plus > 50,000 CFU/mL of a uropathogen in urine from catheterization or suprapubic aspiration. But remember that these guidelines do not apply specifically to the youngest of infants, that is < 2 months old.
Both of these criteria were changes from the 1999 UTI guidelines. Previously pyuria or microscopic bacteriuria were not considered necessary to diagnose UTI, and >100,000 CFU/mL rather than > 50,000 CFU/mL of a single pathogen species was the critical diagnostic result for catheterized urine. For suprapubic aspiration urine samples, > 10,000 CFU/HPF were considered adequate for UTI diagnoses in 1999.
Now, a recent study of children < 90 days of age (including those < 2 months of age) reports that pyuria (> 3 WBC/HPF) plus > 50,000 CFU/mL are the keys to diagnosing UTI (Pediatrics 2015;135:965-71). One caveat is that the study population was febrile infants < 90 days old with concurrent bacteremia (bacteremic UTI). Bacteremic UTI was studied to reduce as much as possible the chance that AB patients might be inadvertently included in the study. One other conclusion of this new study is that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
These data in an overall younger population than that covered by the 2011 guidelines adds evidence that pyuria (but not microscopic bacteriuria) is critical to diagnosing UTI. Pyuria plus positive culture has been a combination for the pediatric infectious diseases practitioner’s toolkit for decades. Likewise, it seems to me that primary care pediatric clinicians also often decide whether to undertake the expense of culture based on UA results. For example, a completely normal UA may obviate need for culture except in selected unusual cases.
Requiring UA evidence of inflammation to diagnose UTI (per the 2011 guidelines and the recommendations of the authors of the recent 2015 study) makes sense because most UTIs in otherwise healthy children are caused by gram-negative organisms (> 90% from Escherichia coli) (J. Pediatr. 1994;124:513-9). Why are UA results so important?
A positive nitrite test strongly suggests UTI because nitrites in the urine indicate viable gram-negative organisms also are present in the urine. Nitrates in the urine are converted to nitrites by metabolic activity of gram-negative pathogens. For WBCs or LE in the urine, their presence indicates inflammation in the urinary tract, Consider that lipopolysaccharide (LPS), also known as gram-negative endotoxin, is a major component of the cell membrane of > 90% of uropathogens like E. coli. Moreover, LPS elicits about the strongest innate immune response via toll-like receptor 4 (TLR4) from monocytes/macrophages, inducing a large pro-inflammatory and chemotactic response – interleukin-6, interleukin-8, tetrahydrofuran-alpha. Remember that LPS is also a major cause of fever and of shock during gram-negative sepsis.
So a UTI diagnosis based on a “positive” culture without evidence of metabolic products of gram negatives (nitrites) or without inflammation (no pyuria or negative LE) should be questioned. The combination of > 50,000 CFU/mL with no detectable LE or < 3-5 WBC/HPF in a febrile child is most likely evidence for AB in a child with the fever caused by some non-UTI process.
In contrast, selected SBIs may occur when the culture is “positive” without inflammation or nitrites. The first of three examples is a renal parenchymal abscess, where bacteria enter the urine sporadically in only small numbers, and do not actually infect the urinary tract mucosa. The scenario of no inflammation but “positive” culture also may occur when a large bacteremic load causes results in organisms filtering through the kidney into the urine, again without urinary mucosal infection, such as Staphylococcus aureus, group A streptococcus, or group B streptococcus bacteremia/sepsis. The third scenario with a “positive” culture and no pyuria can be with organisms that have blunted abilities to induce inflammation, such as enterococcus. Enterococcal cell components have weak inflammatory and chemotactic capability. So a urinary mucosal infection in the collecting system or bladder may occur without much if any pyuria. In fact, the patients from the recent study with insufficient evidence of pyuria/inflammation were those who had either gram-positive organisms or considerably less than 50,000 CFU/mL of gram-negative organisms.
The sensitivity and specificity of the LE or pyuria was higher in the recent study (Pediatrics 2015;135:965-71) than any prior study. The authors comment that they had not expected such a high sensitivity of 97.6% (94.5-99.2) for LE in confirmed bacteremic UTI, nor did they expect the high specificity of 93.9% (87.9-97.5). The presence of microscopic pyuria defined as > 3 WBC/HPF was nearly as sensitive, 96%, and specific, 91.3%. Disappointingly, positive nitrite testing was only 39.5% sensitive, but it was 100% specific. This likely reflects the short time that urine resides in the bladder of infants < 90 days of age, so there is insufficient time for the pathogens to metabolically convert the nitrates to nitrites.
So how would the UA help with our example case? There is microscopic bacteriuria, pyuria, and positive LE, but negative nitrites. Using the suggestions of the authors of the recent report (Pediatrics 2015;135:965-71) and those of another report on the utility of UA results (Acta Paediatr. 2010;99:581-4), the UA in our case indicates that we should be highly suspicious of a UTI in this child < 2 months old for whom the 2011 guidelines do not directly apply. But remember that these impressive sensitivity and specificity values relate to bacteremic UTI. Whether they apply to nonbacteremic UTI is not known. Likewise, the authors caution that their study design did not allow calculation of positive or negative predictive values – aspects that would clarify things even further.
So we still cannot be more than highly suspicious. Without a positive predictive value, we do not know the odds of this case having a UTI with mathematical precision. The authors do point out that only one of their subjects had a completely normal UA and actually had a bacteremic UTI. If you guessed that it was a gram-positive pathogen, you win the prize. So it seems reasonable to predict that a normal UA has a high specificity for not being a UTI (87.8%), but a positive UA remains only highly suggestive. It is still not clear if a negative UA statistically justifies not submitting the culture of the sterilely obtained urine because we still don’t have a negative predictive value.
Bottom line: The 2011 UTI guidelines provide good advice on diagnosing UTIs.
1. We have more data that evidence of inflammation is essential for diagnosing gram-negative UTIs.
2. We also have more evidence that 50,000 CFU/mL is a good threshold for diagnosing UTIs.
3. It appears that microscopic bacteriuria did not add significantly to the either sensitivity or specificity.
4. And we now have more evidence that these criteria also apply to infants < 2 months of age.
To close the loop on our case, the child’s CSF and blood cultures were negative, but the urine culture revealed > 100,000 CFU/mL of E. coli susceptible to second- and third-generation cephalosporins, ciprofloxacin, and nitrofurantoin, but resistant to trimethoprim-sulfamethoxazole.
Have a great summer and watch for UTIs in your young patients < 90 days old and fever without apparent focus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. E-mail Dr. Harrison at [email protected].
Food recalls highlight risk of listeriosis
Recently, after a long day at the hospital, I stopped at the grocery store to pick up something for a quick dinner. I drifted to the frozen food case in the organic food section, but pulled up short when I saw empty shelves. A paper sign announced that Amy’s Kitchen, a manufacturer of organic and natural frozen foods, had become the latest company to recall its products because of concern about Listeria monocytogenes contamination.
According to information posted on the Food and Drug Administration website, this facultative, anaerobic gram-positive bacillus has been the impetus behind 10 national recalls of food products between April 1 and May 8, 2015 alone. Implicated food products have ranged from gourmet ice cream to soybean sprouts to frozen vegetables. Unlike some other bacterial causes of food-borne illness, Listeria organisms can thrive at cold temperatures. Historically, outbreaks of disease have been linked to a variety of foods, including raw produce, contaminated ready-to-eat foods such as deli meats and prepared salads, and unpasteurized milk and milk products.
Clinical manifestations of listeriosis range from febrile gastroenteritis to bacteremia and meningitis, with severe disease seen primarily in immunocompromised individuals and adults 65 and older.
Pregnant women are especially susceptible, with incidence rates 13 times higher than in the general population. Probably as a result of food choices, Hispanic women are disproportionately affected, with rates up to 24 times higher. Maternal infection may be asymptomatic or may manifest with flulike symptoms that include fever, myalgias, headache, and backache, with or without a preceding diarrhea illness. Even mild maternal illness may result in adverse pregnancy outcomes such as fetal loss, premature labor, and severe neonatal infection.
While medical students and residents are still taught to think of Listeria infection as one of the “big three” causes of neonatal sepsis along with group B streptococcus and Escherichia coli, many pediatricians have never seen a case of this rare, but potentially devastating disease. As with group B streptococcus, both early-onset and late-onset disease occur. Sepsis is the most common presentation of disease in the first week of life, while meningitis predominates in late-onset disease. Pneumonia and myocarditis are occasionally seen. Granulomatosis infantisepticum is an uncommon manifestation of severe, disseminated Listeria infection. Granuloma can occur in nearly every organ, although involvement of the liver and skin is most common.
In 2002, investigators from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists surveyed more than 400 pregnant women from across the United States about their knowledge of the transmission, risk factors, symptoms, and prevention of listeriosis (Infect. Dis. Obstet. Gyn. 2005;13:11-15). A year later, the Minnesota Department of Health surveyed an additional 286 pregnant women from their state using the same survey instrument.
More than 80% of survey respondents had never heard of the disease, and knowledge about prevention strategies was therefore predictably limited. Only 33% of respondents in the national survey and 17% of respondents in the Minnesota survey knew, for example, that infection could be prevented by avoiding delicatessen meats and soft cheeses. Investigators concluded that “timely and appropriate education” of pregnant women about listeriosis could reduce cases of perinatal infection.
Data from the CDC suggest we have more work to do. The Listeria Initiative is an enhanced national surveillance system that collects laboratory, clinical, and food exposure data about listeriosis cases in the United States. Between 2009 and 2011, 14% of the 1,651 invasive Listeria infections reported were classified as pregnancy associated. Morbidity and mortality were significant, with 40 fetal losses and 6 neonatal deaths (MMWR 2013;62:448-52).
The CDC offers some common sense tips for preventing listeriosis and other food-borne illness. Raw fruits and vegetables should be thoroughly rinsed with tap water and dried with a clean cloth or paper towel before being eaten or cooked. Even foods that are typically peeled first should be washed, and firm produce, such as cantaloupe, should be scrubbed with a produce brush to reduce surface contamination. Uncooked meats and poultry should never come in contact with other food. Hands, knives, cutting boards, and other food preparation surfaces should be washed thoroughly after uncooked food is handled.
Pregnant women and others at increased risk for listeriosis should not eat hot dogs or deli meats unless they are cooked to steaming. Soft cheeses, including feta, brie, Camembert, queso blanco, or anything blue veined, should be avoided unless the label clearly states that the product has been made with pasteurized milk. Even then, it might not be safe. Pasteurized Mexican-style cheeses, such as queso fresco, have been linked to Listeria infections, likely as a result of contamination during the cheese-making process.
Physicians should be prepared to field calls from concerned parents who believe their children may have consumed a product potentially contaminated with Listeria. In general, someone who has eaten a recalled food product but has no symptoms doesn’t need a laboratory evaluation or treatment. Screening blood cultures is not indicated, and routine tests such as a complete blood count are unlikely to be helpful. Instead, patients should be counseled about the symptoms of listeriosis and undergo prompt evaluation if any develop within 2 months of exposure. The typical interval between exposure and the development of symptoms is 1 day to 3 weeks, but may be as long as 70 days.
Although Listeria infection may result in gastrointestinal symptoms, stool cultures are not recommended for diagnosis. According to the CDC, ingestion of food contaminated with Listeria occurs frequently because the organisms are commonly found in the environment. Although uncommon, intermittent fecal carriage and shedding have been observed in asymptomatic individuals.
Back at the grocery, I sighed and resigned myself to a grilled cheese sandwich for dinner. I turned and saw another woman in the aisle stop and read the sign on the freezer case.
“It’s a little scary,” she said with a sigh. “It seems like there is another recall every week, and I’m wondering what’s safe to eat.”
The parents of our patients may have similar questions. Although the Food and Drug Administration offers detailed guidance for food manufacturers about reducing Listeria contamination, perfect compliance wouldn’t eliminate the risk for consumers because L. monocytogenes is widespread in the environment. The organisms are found in water, soil, sewage, and decaying vegetation, and can be isolated from a variety of animals. Fresh fruits and vegetables are “healthy” choices as long as they are handled and prepared appropriately. Conversely, unpasteurized milk and milk products can never be considered safe.
That’s food for thought.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Recently, after a long day at the hospital, I stopped at the grocery store to pick up something for a quick dinner. I drifted to the frozen food case in the organic food section, but pulled up short when I saw empty shelves. A paper sign announced that Amy’s Kitchen, a manufacturer of organic and natural frozen foods, had become the latest company to recall its products because of concern about Listeria monocytogenes contamination.
According to information posted on the Food and Drug Administration website, this facultative, anaerobic gram-positive bacillus has been the impetus behind 10 national recalls of food products between April 1 and May 8, 2015 alone. Implicated food products have ranged from gourmet ice cream to soybean sprouts to frozen vegetables. Unlike some other bacterial causes of food-borne illness, Listeria organisms can thrive at cold temperatures. Historically, outbreaks of disease have been linked to a variety of foods, including raw produce, contaminated ready-to-eat foods such as deli meats and prepared salads, and unpasteurized milk and milk products.
Clinical manifestations of listeriosis range from febrile gastroenteritis to bacteremia and meningitis, with severe disease seen primarily in immunocompromised individuals and adults 65 and older.
Pregnant women are especially susceptible, with incidence rates 13 times higher than in the general population. Probably as a result of food choices, Hispanic women are disproportionately affected, with rates up to 24 times higher. Maternal infection may be asymptomatic or may manifest with flulike symptoms that include fever, myalgias, headache, and backache, with or without a preceding diarrhea illness. Even mild maternal illness may result in adverse pregnancy outcomes such as fetal loss, premature labor, and severe neonatal infection.
While medical students and residents are still taught to think of Listeria infection as one of the “big three” causes of neonatal sepsis along with group B streptococcus and Escherichia coli, many pediatricians have never seen a case of this rare, but potentially devastating disease. As with group B streptococcus, both early-onset and late-onset disease occur. Sepsis is the most common presentation of disease in the first week of life, while meningitis predominates in late-onset disease. Pneumonia and myocarditis are occasionally seen. Granulomatosis infantisepticum is an uncommon manifestation of severe, disseminated Listeria infection. Granuloma can occur in nearly every organ, although involvement of the liver and skin is most common.
In 2002, investigators from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists surveyed more than 400 pregnant women from across the United States about their knowledge of the transmission, risk factors, symptoms, and prevention of listeriosis (Infect. Dis. Obstet. Gyn. 2005;13:11-15). A year later, the Minnesota Department of Health surveyed an additional 286 pregnant women from their state using the same survey instrument.
More than 80% of survey respondents had never heard of the disease, and knowledge about prevention strategies was therefore predictably limited. Only 33% of respondents in the national survey and 17% of respondents in the Minnesota survey knew, for example, that infection could be prevented by avoiding delicatessen meats and soft cheeses. Investigators concluded that “timely and appropriate education” of pregnant women about listeriosis could reduce cases of perinatal infection.
Data from the CDC suggest we have more work to do. The Listeria Initiative is an enhanced national surveillance system that collects laboratory, clinical, and food exposure data about listeriosis cases in the United States. Between 2009 and 2011, 14% of the 1,651 invasive Listeria infections reported were classified as pregnancy associated. Morbidity and mortality were significant, with 40 fetal losses and 6 neonatal deaths (MMWR 2013;62:448-52).
The CDC offers some common sense tips for preventing listeriosis and other food-borne illness. Raw fruits and vegetables should be thoroughly rinsed with tap water and dried with a clean cloth or paper towel before being eaten or cooked. Even foods that are typically peeled first should be washed, and firm produce, such as cantaloupe, should be scrubbed with a produce brush to reduce surface contamination. Uncooked meats and poultry should never come in contact with other food. Hands, knives, cutting boards, and other food preparation surfaces should be washed thoroughly after uncooked food is handled.
Pregnant women and others at increased risk for listeriosis should not eat hot dogs or deli meats unless they are cooked to steaming. Soft cheeses, including feta, brie, Camembert, queso blanco, or anything blue veined, should be avoided unless the label clearly states that the product has been made with pasteurized milk. Even then, it might not be safe. Pasteurized Mexican-style cheeses, such as queso fresco, have been linked to Listeria infections, likely as a result of contamination during the cheese-making process.
Physicians should be prepared to field calls from concerned parents who believe their children may have consumed a product potentially contaminated with Listeria. In general, someone who has eaten a recalled food product but has no symptoms doesn’t need a laboratory evaluation or treatment. Screening blood cultures is not indicated, and routine tests such as a complete blood count are unlikely to be helpful. Instead, patients should be counseled about the symptoms of listeriosis and undergo prompt evaluation if any develop within 2 months of exposure. The typical interval between exposure and the development of symptoms is 1 day to 3 weeks, but may be as long as 70 days.
Although Listeria infection may result in gastrointestinal symptoms, stool cultures are not recommended for diagnosis. According to the CDC, ingestion of food contaminated with Listeria occurs frequently because the organisms are commonly found in the environment. Although uncommon, intermittent fecal carriage and shedding have been observed in asymptomatic individuals.
Back at the grocery, I sighed and resigned myself to a grilled cheese sandwich for dinner. I turned and saw another woman in the aisle stop and read the sign on the freezer case.
“It’s a little scary,” she said with a sigh. “It seems like there is another recall every week, and I’m wondering what’s safe to eat.”
The parents of our patients may have similar questions. Although the Food and Drug Administration offers detailed guidance for food manufacturers about reducing Listeria contamination, perfect compliance wouldn’t eliminate the risk for consumers because L. monocytogenes is widespread in the environment. The organisms are found in water, soil, sewage, and decaying vegetation, and can be isolated from a variety of animals. Fresh fruits and vegetables are “healthy” choices as long as they are handled and prepared appropriately. Conversely, unpasteurized milk and milk products can never be considered safe.
That’s food for thought.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Recently, after a long day at the hospital, I stopped at the grocery store to pick up something for a quick dinner. I drifted to the frozen food case in the organic food section, but pulled up short when I saw empty shelves. A paper sign announced that Amy’s Kitchen, a manufacturer of organic and natural frozen foods, had become the latest company to recall its products because of concern about Listeria monocytogenes contamination.
According to information posted on the Food and Drug Administration website, this facultative, anaerobic gram-positive bacillus has been the impetus behind 10 national recalls of food products between April 1 and May 8, 2015 alone. Implicated food products have ranged from gourmet ice cream to soybean sprouts to frozen vegetables. Unlike some other bacterial causes of food-borne illness, Listeria organisms can thrive at cold temperatures. Historically, outbreaks of disease have been linked to a variety of foods, including raw produce, contaminated ready-to-eat foods such as deli meats and prepared salads, and unpasteurized milk and milk products.
Clinical manifestations of listeriosis range from febrile gastroenteritis to bacteremia and meningitis, with severe disease seen primarily in immunocompromised individuals and adults 65 and older.
Pregnant women are especially susceptible, with incidence rates 13 times higher than in the general population. Probably as a result of food choices, Hispanic women are disproportionately affected, with rates up to 24 times higher. Maternal infection may be asymptomatic or may manifest with flulike symptoms that include fever, myalgias, headache, and backache, with or without a preceding diarrhea illness. Even mild maternal illness may result in adverse pregnancy outcomes such as fetal loss, premature labor, and severe neonatal infection.
While medical students and residents are still taught to think of Listeria infection as one of the “big three” causes of neonatal sepsis along with group B streptococcus and Escherichia coli, many pediatricians have never seen a case of this rare, but potentially devastating disease. As with group B streptococcus, both early-onset and late-onset disease occur. Sepsis is the most common presentation of disease in the first week of life, while meningitis predominates in late-onset disease. Pneumonia and myocarditis are occasionally seen. Granulomatosis infantisepticum is an uncommon manifestation of severe, disseminated Listeria infection. Granuloma can occur in nearly every organ, although involvement of the liver and skin is most common.
In 2002, investigators from the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists surveyed more than 400 pregnant women from across the United States about their knowledge of the transmission, risk factors, symptoms, and prevention of listeriosis (Infect. Dis. Obstet. Gyn. 2005;13:11-15). A year later, the Minnesota Department of Health surveyed an additional 286 pregnant women from their state using the same survey instrument.
More than 80% of survey respondents had never heard of the disease, and knowledge about prevention strategies was therefore predictably limited. Only 33% of respondents in the national survey and 17% of respondents in the Minnesota survey knew, for example, that infection could be prevented by avoiding delicatessen meats and soft cheeses. Investigators concluded that “timely and appropriate education” of pregnant women about listeriosis could reduce cases of perinatal infection.
Data from the CDC suggest we have more work to do. The Listeria Initiative is an enhanced national surveillance system that collects laboratory, clinical, and food exposure data about listeriosis cases in the United States. Between 2009 and 2011, 14% of the 1,651 invasive Listeria infections reported were classified as pregnancy associated. Morbidity and mortality were significant, with 40 fetal losses and 6 neonatal deaths (MMWR 2013;62:448-52).
The CDC offers some common sense tips for preventing listeriosis and other food-borne illness. Raw fruits and vegetables should be thoroughly rinsed with tap water and dried with a clean cloth or paper towel before being eaten or cooked. Even foods that are typically peeled first should be washed, and firm produce, such as cantaloupe, should be scrubbed with a produce brush to reduce surface contamination. Uncooked meats and poultry should never come in contact with other food. Hands, knives, cutting boards, and other food preparation surfaces should be washed thoroughly after uncooked food is handled.
Pregnant women and others at increased risk for listeriosis should not eat hot dogs or deli meats unless they are cooked to steaming. Soft cheeses, including feta, brie, Camembert, queso blanco, or anything blue veined, should be avoided unless the label clearly states that the product has been made with pasteurized milk. Even then, it might not be safe. Pasteurized Mexican-style cheeses, such as queso fresco, have been linked to Listeria infections, likely as a result of contamination during the cheese-making process.
Physicians should be prepared to field calls from concerned parents who believe their children may have consumed a product potentially contaminated with Listeria. In general, someone who has eaten a recalled food product but has no symptoms doesn’t need a laboratory evaluation or treatment. Screening blood cultures is not indicated, and routine tests such as a complete blood count are unlikely to be helpful. Instead, patients should be counseled about the symptoms of listeriosis and undergo prompt evaluation if any develop within 2 months of exposure. The typical interval between exposure and the development of symptoms is 1 day to 3 weeks, but may be as long as 70 days.
Although Listeria infection may result in gastrointestinal symptoms, stool cultures are not recommended for diagnosis. According to the CDC, ingestion of food contaminated with Listeria occurs frequently because the organisms are commonly found in the environment. Although uncommon, intermittent fecal carriage and shedding have been observed in asymptomatic individuals.
Back at the grocery, I sighed and resigned myself to a grilled cheese sandwich for dinner. I turned and saw another woman in the aisle stop and read the sign on the freezer case.
“It’s a little scary,” she said with a sigh. “It seems like there is another recall every week, and I’m wondering what’s safe to eat.”
The parents of our patients may have similar questions. Although the Food and Drug Administration offers detailed guidance for food manufacturers about reducing Listeria contamination, perfect compliance wouldn’t eliminate the risk for consumers because L. monocytogenes is widespread in the environment. The organisms are found in water, soil, sewage, and decaying vegetation, and can be isolated from a variety of animals. Fresh fruits and vegetables are “healthy” choices as long as they are handled and prepared appropriately. Conversely, unpasteurized milk and milk products can never be considered safe.
That’s food for thought.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
Are your patients vaccinated for travel?
Graduation season is rapidly approaching, with high school graduations, followed by summer vacations. While searching for that unique gift and /or summer experience, many of your patients may choose an international destination. Not to be forgotten are those who might travel to resource-limited areas to visit relatives, volunteer, or have extended stays because of parental job relocation. More U.S. high school graduates are participating in gap year programs, many of which involve extensive travel while providing the participant the opportunity to immerse and to actively participate in other cultures. For many, it may be their first experience in a country with poor hygiene. This week alone, I’ve helped prepare travelers, including adolescents and children, for a safari and one for 4 weeks of volunteerism in Tanzania. Another young traveler’s destinations were Rwanda, Uganda, and Kenya, and a fourth is planning to explore and trek regions in the high elevations of Bolivia and Peru. The question is, Will you be ready to help prepare young travelers to stay healthy and return home without any unwanted souvenirs?
For many, health concerns often are not the top priority when they are planning vacations. However, the primary care physician will most likely will be their initial call and resource once they realize their potential to be exposed to diseases and/or conditions not routinely encountered in the United States. Even if you receive the call late, there are still interventions you can provide.
To avoid that last-minute call, develop strategies to identify international travelers in your practice. Many practices send out reminders yearly for influenza and well visits, so consider developing one for international travel. Text-message reminders have been shown to improve influenza vaccine administration rates and are another form of communication that can be considered. Frequently remind families that if planning international travel, they should seek pretravel advice in a timely manner: Ideally advice should be obtained 4-6 weeks in advance, and definitely at least 2 weeks prior to departure. Remind them that adequate time is needed for the vaccine to become effective. In addition, depending on the patients’ destination, trip duration, and type of activity, two vaccines (rabies and Japanese encephalitis) may be recommended and are administered over a 28-day period. Yellow fever vaccine, which is recommended or required for entry into some countries, can be obtained only at centers designated by each state health department. It should be administered at least 10 days prior to travel.
Vaccine interventions are based on the potential risk for disease exposure/acquisition. Factors to consider include the age of the travelers, their health and immunization status, in addition to their destination, duration of stay, accommodations, activities, and reason for travel (such as business or visiting friends and relatives). If you have a child with a chronic disorder or who is immunocompromised, comparable medical care may not be available at all international destinations. In addition, not everyone may be a candidate to receive some recommended or required vaccines. Involvement with a health professional prior to booking the trip would be advisable.
Identify a travel health specialist in your area as a local resource who can provide the most up-to-date information and recommendations. Ensure that individual is willing to see children of all ages.
Make sure routine immunizations are up to date for age. Measles is the one exception. I know you have heard it before, but outbreaks persist, even in the United States. Travelers 6- to 11-months-old should receive one MMR dose prior to international travel. This dose will not count, so these children should receive two additional doses of vaccine once they are at least 1 year old. Many children travel with adults. All travelers at least 12 months of age and born after 1956 should have two documented doses of MMR prior to international travel unless they have serologic evidence of immunity. The second dose can be given as early as 4 weeks after the first. If two doses at least 4 weeks apart are administered when a child is at least 12 months of age, no additional doses are necessary.
In 2014, there were 668 cases of measles from 27 states in the United States. The United States is still experiencing a multistate outbreak of measles at press time, which began December 2014. As of April 24, 2015, 166 cases have been reported from 19 states. The Centers for Disease Control and Prevention analyzed the virus type (B3). It is identical to the one responsible for the outbreak in the Philippines in 2014, and it has now been identified in 14 other countries.
Most U.S. measles cases occur in unvaccinated travelers who become ill after their return and spread the disease to susceptible individuals. Do you have patients who are unimmunized? Another point to consider when speaking with these parents about travel is the potential loss of the herd immunity afforded their children while living in the United States. This benefit may not exist when they are visiting and/or relocating to countries with lower immunization rates. Measles outbreaks are occurring in multiple countries and are not limited to underdeveloped countries. For the most up-to-date travel health-related information from the CDC, click here.
Travelers’ diarrhea (TD) occurs in up to 70 % of travelers to developing countries. The World Health Organization defines it as passage of at least three loose stools in a 24-hour period. Most often it is self-limited, with symptoms lasting a median of 3-4 days. Although TD can be caused by bacteria, protozoa, and viruses, bacteria are usually the etiology, with enterotoxigenic Escherichia coli being the most common pathogens. Other bacterial etiologies include Shigella and Campylobacter species. Two antimicrobials are frequently prescribed to travelers for self-treatment of TD: ciprofloxacin and azithromycin. Most young children are prescribed the latter; however, in older children, ciprofloxacin may be prescribed off label, as its use in persons younger than 18 years is not approved by the Food and Drug Administration.
In December 2014, PulseNet, the national molecular subtyping network for food-borne disease, detected a multistate cluster of ciprofloxacin-resistant Shigella sonnei. Between May 2014 and February 2015, 157 cases including 37 children were detected in 32 states and Puerto Rico. Nine of the cases identified by PulseNet, and an additional 76 cases, were associated with an outbreak of ciprofloxacin-resistant S. sonnei in San Francisco. Antibiotic susceptibility was available for 126 isolates, of which 109 (87%) were not ciprofloxacin susceptible. Travel history was available for 75 patients not associated with the San Francisco outbreak, and slightly more than half (40) were associated with international travel. The island of Hispaniola (Dominican Republic = 22 cases and Haiti = 4 cases) was the most common destination, followed by India (8 cases) and Morocco (3 cases). The remaining destinations were Asia and Europe (MMWR 2015;64:318-20) Travel history was available and positive for 23 of the 37 children (62%).
Why such a concern? International travelers are at risk of becoming colonized with drug-resistant bacteria and have the potential to spread them domestically. It has already begun. In 2012, the National Antimicrobial Resistance Monitoring System (NARMS) revealed that isolates of S. sonnei had the following resistance pattern: trimethoprim/sulfamethoxazole, 42%; ampicillin, 18%; and ciprofloxacin, 2.1%. During this outbreak, 19 of the 126 isolates were tested by NARMS with the following resistance patterns noted: trimethoprim/sulfamethoxazole, 84%; ampicillin, 5%; and ciprofloxacin, 32%.
More judicious use of antibiotics is necessary. As pediatricians, we are not immune to this issue. The challenge is when, if at all, antibiotics should be prescribed for TD, and under what conditions should patients be instructed to use them. I’m rethinking my own practice. TD is one of the most common illnesses travelers acquire and is easily treated, but at what cost? The one expression I keep hearing myself say is, First do no harm.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures.
Graduation season is rapidly approaching, with high school graduations, followed by summer vacations. While searching for that unique gift and /or summer experience, many of your patients may choose an international destination. Not to be forgotten are those who might travel to resource-limited areas to visit relatives, volunteer, or have extended stays because of parental job relocation. More U.S. high school graduates are participating in gap year programs, many of which involve extensive travel while providing the participant the opportunity to immerse and to actively participate in other cultures. For many, it may be their first experience in a country with poor hygiene. This week alone, I’ve helped prepare travelers, including adolescents and children, for a safari and one for 4 weeks of volunteerism in Tanzania. Another young traveler’s destinations were Rwanda, Uganda, and Kenya, and a fourth is planning to explore and trek regions in the high elevations of Bolivia and Peru. The question is, Will you be ready to help prepare young travelers to stay healthy and return home without any unwanted souvenirs?
For many, health concerns often are not the top priority when they are planning vacations. However, the primary care physician will most likely will be their initial call and resource once they realize their potential to be exposed to diseases and/or conditions not routinely encountered in the United States. Even if you receive the call late, there are still interventions you can provide.
To avoid that last-minute call, develop strategies to identify international travelers in your practice. Many practices send out reminders yearly for influenza and well visits, so consider developing one for international travel. Text-message reminders have been shown to improve influenza vaccine administration rates and are another form of communication that can be considered. Frequently remind families that if planning international travel, they should seek pretravel advice in a timely manner: Ideally advice should be obtained 4-6 weeks in advance, and definitely at least 2 weeks prior to departure. Remind them that adequate time is needed for the vaccine to become effective. In addition, depending on the patients’ destination, trip duration, and type of activity, two vaccines (rabies and Japanese encephalitis) may be recommended and are administered over a 28-day period. Yellow fever vaccine, which is recommended or required for entry into some countries, can be obtained only at centers designated by each state health department. It should be administered at least 10 days prior to travel.
Vaccine interventions are based on the potential risk for disease exposure/acquisition. Factors to consider include the age of the travelers, their health and immunization status, in addition to their destination, duration of stay, accommodations, activities, and reason for travel (such as business or visiting friends and relatives). If you have a child with a chronic disorder or who is immunocompromised, comparable medical care may not be available at all international destinations. In addition, not everyone may be a candidate to receive some recommended or required vaccines. Involvement with a health professional prior to booking the trip would be advisable.
Identify a travel health specialist in your area as a local resource who can provide the most up-to-date information and recommendations. Ensure that individual is willing to see children of all ages.
Make sure routine immunizations are up to date for age. Measles is the one exception. I know you have heard it before, but outbreaks persist, even in the United States. Travelers 6- to 11-months-old should receive one MMR dose prior to international travel. This dose will not count, so these children should receive two additional doses of vaccine once they are at least 1 year old. Many children travel with adults. All travelers at least 12 months of age and born after 1956 should have two documented doses of MMR prior to international travel unless they have serologic evidence of immunity. The second dose can be given as early as 4 weeks after the first. If two doses at least 4 weeks apart are administered when a child is at least 12 months of age, no additional doses are necessary.
In 2014, there were 668 cases of measles from 27 states in the United States. The United States is still experiencing a multistate outbreak of measles at press time, which began December 2014. As of April 24, 2015, 166 cases have been reported from 19 states. The Centers for Disease Control and Prevention analyzed the virus type (B3). It is identical to the one responsible for the outbreak in the Philippines in 2014, and it has now been identified in 14 other countries.
Most U.S. measles cases occur in unvaccinated travelers who become ill after their return and spread the disease to susceptible individuals. Do you have patients who are unimmunized? Another point to consider when speaking with these parents about travel is the potential loss of the herd immunity afforded their children while living in the United States. This benefit may not exist when they are visiting and/or relocating to countries with lower immunization rates. Measles outbreaks are occurring in multiple countries and are not limited to underdeveloped countries. For the most up-to-date travel health-related information from the CDC, click here.
Travelers’ diarrhea (TD) occurs in up to 70 % of travelers to developing countries. The World Health Organization defines it as passage of at least three loose stools in a 24-hour period. Most often it is self-limited, with symptoms lasting a median of 3-4 days. Although TD can be caused by bacteria, protozoa, and viruses, bacteria are usually the etiology, with enterotoxigenic Escherichia coli being the most common pathogens. Other bacterial etiologies include Shigella and Campylobacter species. Two antimicrobials are frequently prescribed to travelers for self-treatment of TD: ciprofloxacin and azithromycin. Most young children are prescribed the latter; however, in older children, ciprofloxacin may be prescribed off label, as its use in persons younger than 18 years is not approved by the Food and Drug Administration.
In December 2014, PulseNet, the national molecular subtyping network for food-borne disease, detected a multistate cluster of ciprofloxacin-resistant Shigella sonnei. Between May 2014 and February 2015, 157 cases including 37 children were detected in 32 states and Puerto Rico. Nine of the cases identified by PulseNet, and an additional 76 cases, were associated with an outbreak of ciprofloxacin-resistant S. sonnei in San Francisco. Antibiotic susceptibility was available for 126 isolates, of which 109 (87%) were not ciprofloxacin susceptible. Travel history was available for 75 patients not associated with the San Francisco outbreak, and slightly more than half (40) were associated with international travel. The island of Hispaniola (Dominican Republic = 22 cases and Haiti = 4 cases) was the most common destination, followed by India (8 cases) and Morocco (3 cases). The remaining destinations were Asia and Europe (MMWR 2015;64:318-20) Travel history was available and positive for 23 of the 37 children (62%).
Why such a concern? International travelers are at risk of becoming colonized with drug-resistant bacteria and have the potential to spread them domestically. It has already begun. In 2012, the National Antimicrobial Resistance Monitoring System (NARMS) revealed that isolates of S. sonnei had the following resistance pattern: trimethoprim/sulfamethoxazole, 42%; ampicillin, 18%; and ciprofloxacin, 2.1%. During this outbreak, 19 of the 126 isolates were tested by NARMS with the following resistance patterns noted: trimethoprim/sulfamethoxazole, 84%; ampicillin, 5%; and ciprofloxacin, 32%.
More judicious use of antibiotics is necessary. As pediatricians, we are not immune to this issue. The challenge is when, if at all, antibiotics should be prescribed for TD, and under what conditions should patients be instructed to use them. I’m rethinking my own practice. TD is one of the most common illnesses travelers acquire and is easily treated, but at what cost? The one expression I keep hearing myself say is, First do no harm.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures.
Graduation season is rapidly approaching, with high school graduations, followed by summer vacations. While searching for that unique gift and /or summer experience, many of your patients may choose an international destination. Not to be forgotten are those who might travel to resource-limited areas to visit relatives, volunteer, or have extended stays because of parental job relocation. More U.S. high school graduates are participating in gap year programs, many of which involve extensive travel while providing the participant the opportunity to immerse and to actively participate in other cultures. For many, it may be their first experience in a country with poor hygiene. This week alone, I’ve helped prepare travelers, including adolescents and children, for a safari and one for 4 weeks of volunteerism in Tanzania. Another young traveler’s destinations were Rwanda, Uganda, and Kenya, and a fourth is planning to explore and trek regions in the high elevations of Bolivia and Peru. The question is, Will you be ready to help prepare young travelers to stay healthy and return home without any unwanted souvenirs?
For many, health concerns often are not the top priority when they are planning vacations. However, the primary care physician will most likely will be their initial call and resource once they realize their potential to be exposed to diseases and/or conditions not routinely encountered in the United States. Even if you receive the call late, there are still interventions you can provide.
To avoid that last-minute call, develop strategies to identify international travelers in your practice. Many practices send out reminders yearly for influenza and well visits, so consider developing one for international travel. Text-message reminders have been shown to improve influenza vaccine administration rates and are another form of communication that can be considered. Frequently remind families that if planning international travel, they should seek pretravel advice in a timely manner: Ideally advice should be obtained 4-6 weeks in advance, and definitely at least 2 weeks prior to departure. Remind them that adequate time is needed for the vaccine to become effective. In addition, depending on the patients’ destination, trip duration, and type of activity, two vaccines (rabies and Japanese encephalitis) may be recommended and are administered over a 28-day period. Yellow fever vaccine, which is recommended or required for entry into some countries, can be obtained only at centers designated by each state health department. It should be administered at least 10 days prior to travel.
Vaccine interventions are based on the potential risk for disease exposure/acquisition. Factors to consider include the age of the travelers, their health and immunization status, in addition to their destination, duration of stay, accommodations, activities, and reason for travel (such as business or visiting friends and relatives). If you have a child with a chronic disorder or who is immunocompromised, comparable medical care may not be available at all international destinations. In addition, not everyone may be a candidate to receive some recommended or required vaccines. Involvement with a health professional prior to booking the trip would be advisable.
Identify a travel health specialist in your area as a local resource who can provide the most up-to-date information and recommendations. Ensure that individual is willing to see children of all ages.
Make sure routine immunizations are up to date for age. Measles is the one exception. I know you have heard it before, but outbreaks persist, even in the United States. Travelers 6- to 11-months-old should receive one MMR dose prior to international travel. This dose will not count, so these children should receive two additional doses of vaccine once they are at least 1 year old. Many children travel with adults. All travelers at least 12 months of age and born after 1956 should have two documented doses of MMR prior to international travel unless they have serologic evidence of immunity. The second dose can be given as early as 4 weeks after the first. If two doses at least 4 weeks apart are administered when a child is at least 12 months of age, no additional doses are necessary.
In 2014, there were 668 cases of measles from 27 states in the United States. The United States is still experiencing a multistate outbreak of measles at press time, which began December 2014. As of April 24, 2015, 166 cases have been reported from 19 states. The Centers for Disease Control and Prevention analyzed the virus type (B3). It is identical to the one responsible for the outbreak in the Philippines in 2014, and it has now been identified in 14 other countries.
Most U.S. measles cases occur in unvaccinated travelers who become ill after their return and spread the disease to susceptible individuals. Do you have patients who are unimmunized? Another point to consider when speaking with these parents about travel is the potential loss of the herd immunity afforded their children while living in the United States. This benefit may not exist when they are visiting and/or relocating to countries with lower immunization rates. Measles outbreaks are occurring in multiple countries and are not limited to underdeveloped countries. For the most up-to-date travel health-related information from the CDC, click here.
Travelers’ diarrhea (TD) occurs in up to 70 % of travelers to developing countries. The World Health Organization defines it as passage of at least three loose stools in a 24-hour period. Most often it is self-limited, with symptoms lasting a median of 3-4 days. Although TD can be caused by bacteria, protozoa, and viruses, bacteria are usually the etiology, with enterotoxigenic Escherichia coli being the most common pathogens. Other bacterial etiologies include Shigella and Campylobacter species. Two antimicrobials are frequently prescribed to travelers for self-treatment of TD: ciprofloxacin and azithromycin. Most young children are prescribed the latter; however, in older children, ciprofloxacin may be prescribed off label, as its use in persons younger than 18 years is not approved by the Food and Drug Administration.
In December 2014, PulseNet, the national molecular subtyping network for food-borne disease, detected a multistate cluster of ciprofloxacin-resistant Shigella sonnei. Between May 2014 and February 2015, 157 cases including 37 children were detected in 32 states and Puerto Rico. Nine of the cases identified by PulseNet, and an additional 76 cases, were associated with an outbreak of ciprofloxacin-resistant S. sonnei in San Francisco. Antibiotic susceptibility was available for 126 isolates, of which 109 (87%) were not ciprofloxacin susceptible. Travel history was available for 75 patients not associated with the San Francisco outbreak, and slightly more than half (40) were associated with international travel. The island of Hispaniola (Dominican Republic = 22 cases and Haiti = 4 cases) was the most common destination, followed by India (8 cases) and Morocco (3 cases). The remaining destinations were Asia and Europe (MMWR 2015;64:318-20) Travel history was available and positive for 23 of the 37 children (62%).
Why such a concern? International travelers are at risk of becoming colonized with drug-resistant bacteria and have the potential to spread them domestically. It has already begun. In 2012, the National Antimicrobial Resistance Monitoring System (NARMS) revealed that isolates of S. sonnei had the following resistance pattern: trimethoprim/sulfamethoxazole, 42%; ampicillin, 18%; and ciprofloxacin, 2.1%. During this outbreak, 19 of the 126 isolates were tested by NARMS with the following resistance patterns noted: trimethoprim/sulfamethoxazole, 84%; ampicillin, 5%; and ciprofloxacin, 32%.
More judicious use of antibiotics is necessary. As pediatricians, we are not immune to this issue. The challenge is when, if at all, antibiotics should be prescribed for TD, and under what conditions should patients be instructed to use them. I’m rethinking my own practice. TD is one of the most common illnesses travelers acquire and is easily treated, but at what cost? The one expression I keep hearing myself say is, First do no harm.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures.
Microbiome and innate immunity in the respiratory tract – a primer
The pathogenesis of respiratory infections such as acute otitis media (AOM), sinusitis, and pneumonia involves complex interactions among bacteria, respiratory viruses, and host immune responses.
My clinical and laboratory group and others have described respiratory infections as resulting from the growth of a single otopathogen, such as Streptococcus pneumoniae (Spn), nontypeable Haemophilus influenzae (NTHi), or Moraxella catarrhalis (Mcat) in the nasopharynx (NP) followed by ascension to the middle ear, sinuses, or descent to the lungs. Recent research from my group and others has resulted in a shift from a single pathogen focus toward consideration of respiratory infections as a polymicrobial disease. Bacterial and viral interactions are critical in respiratory infection pathogenesis. Commensal bacteria can alter virulence of bacterial pathogens and interfere with antibiotic treatment.
The traditional view of the immune system is that it is an assembly of human tissues, cells, and molecules that work to eliminate pathogens. Recent discoveries indicate that commensals play a central role in regulating human immune responses. Thus, the key questions in the field are:
1) How do members of the NP microbiome and innate immune responses maintain health in young children over time?
2) Do specific deleterious members of the NP microbiome alter host innate immune responses in a manner that predisposes to respiratory infections?
3) How does the microbiome and innate response in the NP differ when recovery, relapse of infection, or persistent infection occurs?
Virtually all young children are colonized by Spn, NTHi, or Mcat during the first 3 years of life. My group and others have shown that competitive interactions among bacteria influence whether these potential pathogens successfully colonize and cause respiratory infections. Recent studies have demonstrated that hundreds of different bacterial species colonize the upper respiratory tract. Diverse communities have been shown to be more stable and resistant to invasion by foreign species. Data from cross-sectional studies demonstrate that specific commensals, including Dolosigranulum, Corynebacterium, and Lactococcus, are associated with decreased risk of respiratory infections. Prior studies have been limited by the use of culture-based methods or have been cross sectional in design. Therefore, the optimal levels of diversity and NP commensals critical for maintaining health in the upper respiratory tract of children are currently unknown and under study by my group and others. Studies that utilize high-throughput culture-independent molecular detection methods are now used to identify optimal levels of diversity and commensal members of the microbiome critical for maintaining health homeostasis.
The innate immune system constitutes the first line of defense against respiratory pathogen colonization and respiratory virus infection. It relies on pattern recognition receptors on innate immune cells to detect evolutionarily conserved pathogen-associated molecular patterns expressed on pathogen surfaces. Toll-like receptors (TLRs) are crucial in the innate immune response; TLR 3, 7, and 8 recognize respiratory infection-associated viral pathogens. TLR2, 4, and 5 recognize respiratory infection-associated bacterial pathogens, and TLR9 and TLR13 recognize both viral and bacterial pathogens. The activation of TLRs triggers signaling cascades and regulates the expression of a wide range of cytokines leading to antimicrobial and inflammatory responses. Cytokines (there are dozens) associated with the pathogenesis, development, severity, and clinical outcomes of respiratory infections identify hypotheses that our group is exploring to expand our understanding of how innate responses might be manipulated to favor the child host. Importantly, it has already been shown that cytokine profiles differ in the NP depending on the number and type of bacteria and viruses involved.
My group recently has shown that serum IL-10 levels are significantly higher in AOM from Spn than are the levels associated with NTHi and Mcat, suggesting use of detection of this cytokine as a serum biomarker. Others have shown that the levels of IL-1-beta, TNF-alpha, IL-6, IL-8, IL-10, and IL-17a in middle ear fluids from children with recurrent AOM correlate significantly with higher bacterial load (and worse disease). Previous studies on cytokine responses associated with AOM have focused on limited numbers of cytokines and have not examined any relationship with commensals of the NP microbiome. Moreover, the subset of children who experience excessively frequent respiratory infections likely have disturbances in their microbiome (made worse with antibiotics) and innate immune response. Because of our growing knowledge about the microbiome and innate immune response, I see a compelling need to assess interactions of the NP microbiome and innate immune responses in children that are associated with sustained health and control of respiratory infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said the work was supported by a National Institutes of Health grant, and he had no relevant conflicts of interest. E-mail him at [email protected].
The pathogenesis of respiratory infections such as acute otitis media (AOM), sinusitis, and pneumonia involves complex interactions among bacteria, respiratory viruses, and host immune responses.
My clinical and laboratory group and others have described respiratory infections as resulting from the growth of a single otopathogen, such as Streptococcus pneumoniae (Spn), nontypeable Haemophilus influenzae (NTHi), or Moraxella catarrhalis (Mcat) in the nasopharynx (NP) followed by ascension to the middle ear, sinuses, or descent to the lungs. Recent research from my group and others has resulted in a shift from a single pathogen focus toward consideration of respiratory infections as a polymicrobial disease. Bacterial and viral interactions are critical in respiratory infection pathogenesis. Commensal bacteria can alter virulence of bacterial pathogens and interfere with antibiotic treatment.
The traditional view of the immune system is that it is an assembly of human tissues, cells, and molecules that work to eliminate pathogens. Recent discoveries indicate that commensals play a central role in regulating human immune responses. Thus, the key questions in the field are:
1) How do members of the NP microbiome and innate immune responses maintain health in young children over time?
2) Do specific deleterious members of the NP microbiome alter host innate immune responses in a manner that predisposes to respiratory infections?
3) How does the microbiome and innate response in the NP differ when recovery, relapse of infection, or persistent infection occurs?
Virtually all young children are colonized by Spn, NTHi, or Mcat during the first 3 years of life. My group and others have shown that competitive interactions among bacteria influence whether these potential pathogens successfully colonize and cause respiratory infections. Recent studies have demonstrated that hundreds of different bacterial species colonize the upper respiratory tract. Diverse communities have been shown to be more stable and resistant to invasion by foreign species. Data from cross-sectional studies demonstrate that specific commensals, including Dolosigranulum, Corynebacterium, and Lactococcus, are associated with decreased risk of respiratory infections. Prior studies have been limited by the use of culture-based methods or have been cross sectional in design. Therefore, the optimal levels of diversity and NP commensals critical for maintaining health in the upper respiratory tract of children are currently unknown and under study by my group and others. Studies that utilize high-throughput culture-independent molecular detection methods are now used to identify optimal levels of diversity and commensal members of the microbiome critical for maintaining health homeostasis.
The innate immune system constitutes the first line of defense against respiratory pathogen colonization and respiratory virus infection. It relies on pattern recognition receptors on innate immune cells to detect evolutionarily conserved pathogen-associated molecular patterns expressed on pathogen surfaces. Toll-like receptors (TLRs) are crucial in the innate immune response; TLR 3, 7, and 8 recognize respiratory infection-associated viral pathogens. TLR2, 4, and 5 recognize respiratory infection-associated bacterial pathogens, and TLR9 and TLR13 recognize both viral and bacterial pathogens. The activation of TLRs triggers signaling cascades and regulates the expression of a wide range of cytokines leading to antimicrobial and inflammatory responses. Cytokines (there are dozens) associated with the pathogenesis, development, severity, and clinical outcomes of respiratory infections identify hypotheses that our group is exploring to expand our understanding of how innate responses might be manipulated to favor the child host. Importantly, it has already been shown that cytokine profiles differ in the NP depending on the number and type of bacteria and viruses involved.
My group recently has shown that serum IL-10 levels are significantly higher in AOM from Spn than are the levels associated with NTHi and Mcat, suggesting use of detection of this cytokine as a serum biomarker. Others have shown that the levels of IL-1-beta, TNF-alpha, IL-6, IL-8, IL-10, and IL-17a in middle ear fluids from children with recurrent AOM correlate significantly with higher bacterial load (and worse disease). Previous studies on cytokine responses associated with AOM have focused on limited numbers of cytokines and have not examined any relationship with commensals of the NP microbiome. Moreover, the subset of children who experience excessively frequent respiratory infections likely have disturbances in their microbiome (made worse with antibiotics) and innate immune response. Because of our growing knowledge about the microbiome and innate immune response, I see a compelling need to assess interactions of the NP microbiome and innate immune responses in children that are associated with sustained health and control of respiratory infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said the work was supported by a National Institutes of Health grant, and he had no relevant conflicts of interest. E-mail him at [email protected].
The pathogenesis of respiratory infections such as acute otitis media (AOM), sinusitis, and pneumonia involves complex interactions among bacteria, respiratory viruses, and host immune responses.
My clinical and laboratory group and others have described respiratory infections as resulting from the growth of a single otopathogen, such as Streptococcus pneumoniae (Spn), nontypeable Haemophilus influenzae (NTHi), or Moraxella catarrhalis (Mcat) in the nasopharynx (NP) followed by ascension to the middle ear, sinuses, or descent to the lungs. Recent research from my group and others has resulted in a shift from a single pathogen focus toward consideration of respiratory infections as a polymicrobial disease. Bacterial and viral interactions are critical in respiratory infection pathogenesis. Commensal bacteria can alter virulence of bacterial pathogens and interfere with antibiotic treatment.
The traditional view of the immune system is that it is an assembly of human tissues, cells, and molecules that work to eliminate pathogens. Recent discoveries indicate that commensals play a central role in regulating human immune responses. Thus, the key questions in the field are:
1) How do members of the NP microbiome and innate immune responses maintain health in young children over time?
2) Do specific deleterious members of the NP microbiome alter host innate immune responses in a manner that predisposes to respiratory infections?
3) How does the microbiome and innate response in the NP differ when recovery, relapse of infection, or persistent infection occurs?
Virtually all young children are colonized by Spn, NTHi, or Mcat during the first 3 years of life. My group and others have shown that competitive interactions among bacteria influence whether these potential pathogens successfully colonize and cause respiratory infections. Recent studies have demonstrated that hundreds of different bacterial species colonize the upper respiratory tract. Diverse communities have been shown to be more stable and resistant to invasion by foreign species. Data from cross-sectional studies demonstrate that specific commensals, including Dolosigranulum, Corynebacterium, and Lactococcus, are associated with decreased risk of respiratory infections. Prior studies have been limited by the use of culture-based methods or have been cross sectional in design. Therefore, the optimal levels of diversity and NP commensals critical for maintaining health in the upper respiratory tract of children are currently unknown and under study by my group and others. Studies that utilize high-throughput culture-independent molecular detection methods are now used to identify optimal levels of diversity and commensal members of the microbiome critical for maintaining health homeostasis.
The innate immune system constitutes the first line of defense against respiratory pathogen colonization and respiratory virus infection. It relies on pattern recognition receptors on innate immune cells to detect evolutionarily conserved pathogen-associated molecular patterns expressed on pathogen surfaces. Toll-like receptors (TLRs) are crucial in the innate immune response; TLR 3, 7, and 8 recognize respiratory infection-associated viral pathogens. TLR2, 4, and 5 recognize respiratory infection-associated bacterial pathogens, and TLR9 and TLR13 recognize both viral and bacterial pathogens. The activation of TLRs triggers signaling cascades and regulates the expression of a wide range of cytokines leading to antimicrobial and inflammatory responses. Cytokines (there are dozens) associated with the pathogenesis, development, severity, and clinical outcomes of respiratory infections identify hypotheses that our group is exploring to expand our understanding of how innate responses might be manipulated to favor the child host. Importantly, it has already been shown that cytokine profiles differ in the NP depending on the number and type of bacteria and viruses involved.
My group recently has shown that serum IL-10 levels are significantly higher in AOM from Spn than are the levels associated with NTHi and Mcat, suggesting use of detection of this cytokine as a serum biomarker. Others have shown that the levels of IL-1-beta, TNF-alpha, IL-6, IL-8, IL-10, and IL-17a in middle ear fluids from children with recurrent AOM correlate significantly with higher bacterial load (and worse disease). Previous studies on cytokine responses associated with AOM have focused on limited numbers of cytokines and have not examined any relationship with commensals of the NP microbiome. Moreover, the subset of children who experience excessively frequent respiratory infections likely have disturbances in their microbiome (made worse with antibiotics) and innate immune response. Because of our growing knowledge about the microbiome and innate immune response, I see a compelling need to assess interactions of the NP microbiome and innate immune responses in children that are associated with sustained health and control of respiratory infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said the work was supported by a National Institutes of Health grant, and he had no relevant conflicts of interest. E-mail him at [email protected].
It’s time to take a stand against vaccine refusers
The challenges in primary care are many, and one of increasing importance is what to say to vaccine refusers. After much debate and thoughtful discussion, my medical partner, Dr. Janet Casey at Legacy Pediatrics, decided that the practice would refuse to care for the refusers.
Over the years, I have accepted such patients into my practice and worked with them to gain their confidence and debunk the many myths about the safety of vaccination that are so visible on the Internet. The approach worked well, and by the time the children were 1 year of age, I cannot remember but a handful of parents who did not come around to realize that it was best to vaccinate. However, with the recent measles outbreak at Disneyland in California, pertussis at epidemic proportions in pockets of the United States and elsewhere in the world, and the antivaccine voices gaining more and more attention, I agree, it is time to take a stand.
When a family brings their unvaccinated or undervaccinated child into the waiting room of a physician’s practice, that family is potentially exposing others in that waiting room to serious infectious diseases – that is not fair. In the waiting room may well be a patient who is on chemotherapy or immunotherapy or otherwise immunocompromised, and he relies on the “herd immunity” achieved by vaccinations of those who can safely be vaccinated for individual protection and public health. Those patients who have weakened immune systems did not choose to have their medical condition, whereas the vaccine refusers are choosing not to vaccinate their child (or typically themselves as well). And the reasons they are choosing not to vaccinate are based on misrepresentation of medical facts, fabrications of safety concerns, long ago disproven speculations by well-meaning and not so well-meaning physicians and scientists, pseudoscience published in pseudoscientific journals, and/or general distrust of the federal government that mandates vaccinations for the good of the public health.
My personal experience with vaccine scares dates back to a time when whole-cell pertussis vaccine was the only pertussis vaccine available. I was a medical student, resident, and then an infectious diseases fellow during the escalating debate about the significant side effects of vaccines. I joined in the chorus of voices questioning the need for clear data on the problem, and then the pursuit of a safer acellular pertussis vaccine. The physician community and the public were ready for change, and the National Institutes of Health took the lead in organizing multiple studies and clinical trials leading to eventual replacement of the whole cell pertussis vaccine with the current acellular vaccines.
Much more recently, at the request of National Institutes of Health, I led studies of the safety of thimerosal preservative in multidose vaccine vials that appeared in the Lancet (2002;360:1737-41); Pediatrics (2008;121:e208-14) and the Journal of Pediatrics (2009;155:495-9). Using the data from those three studies, the World Health Organization (WHO), the United Nations, the Institute of Medicine, and other organizations were able to see that the metabolism and elimination from the body of ethylmercury in thimerosal was dramatically faster, compared with methylmercury in fish. Therefore, the presumption of possible accumulation of mercury in the body of infants receiving vaccines from multidose vials when such vaccines were closely spaced was disproven by scientific data.
In plain language, there was never a known risk from thimerosal, but a premature, hurried decision was made to mandate removal of thimerosal from vaccines given to children in the United States and western Europe; thereby the myth lives on that thimerosal is not safe. Yet thimerosal is safe, and the WHO continues to advocate use of thimerosal in multidose vaccine vials. Nevertheless, I have been criticized personally on the Internet for this work. The accusation is that I, the rest of the scientists who participated in the study, and the NIH oversight were biased because our academic institutions had previously received funding from vaccine companies to perform clinical and translational research. I received many hate e-mails and even a death threat.
To close this column with a sense of humor, I suggest you Google the responses by U.S. presidential hopefuls on their stand with regard to vaccine refusers. The comments, then the reversal and “corrections” to their comments is amusing. The presidential hopefuls quickly recognized that the right to choose may not be the best policy for the public health of American citizens. Refusing to vaccinate a child potentially harms the child and may harm others!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures. E-mail him at [email protected].
The challenges in primary care are many, and one of increasing importance is what to say to vaccine refusers. After much debate and thoughtful discussion, my medical partner, Dr. Janet Casey at Legacy Pediatrics, decided that the practice would refuse to care for the refusers.
Over the years, I have accepted such patients into my practice and worked with them to gain their confidence and debunk the many myths about the safety of vaccination that are so visible on the Internet. The approach worked well, and by the time the children were 1 year of age, I cannot remember but a handful of parents who did not come around to realize that it was best to vaccinate. However, with the recent measles outbreak at Disneyland in California, pertussis at epidemic proportions in pockets of the United States and elsewhere in the world, and the antivaccine voices gaining more and more attention, I agree, it is time to take a stand.
When a family brings their unvaccinated or undervaccinated child into the waiting room of a physician’s practice, that family is potentially exposing others in that waiting room to serious infectious diseases – that is not fair. In the waiting room may well be a patient who is on chemotherapy or immunotherapy or otherwise immunocompromised, and he relies on the “herd immunity” achieved by vaccinations of those who can safely be vaccinated for individual protection and public health. Those patients who have weakened immune systems did not choose to have their medical condition, whereas the vaccine refusers are choosing not to vaccinate their child (or typically themselves as well). And the reasons they are choosing not to vaccinate are based on misrepresentation of medical facts, fabrications of safety concerns, long ago disproven speculations by well-meaning and not so well-meaning physicians and scientists, pseudoscience published in pseudoscientific journals, and/or general distrust of the federal government that mandates vaccinations for the good of the public health.
My personal experience with vaccine scares dates back to a time when whole-cell pertussis vaccine was the only pertussis vaccine available. I was a medical student, resident, and then an infectious diseases fellow during the escalating debate about the significant side effects of vaccines. I joined in the chorus of voices questioning the need for clear data on the problem, and then the pursuit of a safer acellular pertussis vaccine. The physician community and the public were ready for change, and the National Institutes of Health took the lead in organizing multiple studies and clinical trials leading to eventual replacement of the whole cell pertussis vaccine with the current acellular vaccines.
Much more recently, at the request of National Institutes of Health, I led studies of the safety of thimerosal preservative in multidose vaccine vials that appeared in the Lancet (2002;360:1737-41); Pediatrics (2008;121:e208-14) and the Journal of Pediatrics (2009;155:495-9). Using the data from those three studies, the World Health Organization (WHO), the United Nations, the Institute of Medicine, and other organizations were able to see that the metabolism and elimination from the body of ethylmercury in thimerosal was dramatically faster, compared with methylmercury in fish. Therefore, the presumption of possible accumulation of mercury in the body of infants receiving vaccines from multidose vials when such vaccines were closely spaced was disproven by scientific data.
In plain language, there was never a known risk from thimerosal, but a premature, hurried decision was made to mandate removal of thimerosal from vaccines given to children in the United States and western Europe; thereby the myth lives on that thimerosal is not safe. Yet thimerosal is safe, and the WHO continues to advocate use of thimerosal in multidose vaccine vials. Nevertheless, I have been criticized personally on the Internet for this work. The accusation is that I, the rest of the scientists who participated in the study, and the NIH oversight were biased because our academic institutions had previously received funding from vaccine companies to perform clinical and translational research. I received many hate e-mails and even a death threat.
To close this column with a sense of humor, I suggest you Google the responses by U.S. presidential hopefuls on their stand with regard to vaccine refusers. The comments, then the reversal and “corrections” to their comments is amusing. The presidential hopefuls quickly recognized that the right to choose may not be the best policy for the public health of American citizens. Refusing to vaccinate a child potentially harms the child and may harm others!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures. E-mail him at [email protected].
The challenges in primary care are many, and one of increasing importance is what to say to vaccine refusers. After much debate and thoughtful discussion, my medical partner, Dr. Janet Casey at Legacy Pediatrics, decided that the practice would refuse to care for the refusers.
Over the years, I have accepted such patients into my practice and worked with them to gain their confidence and debunk the many myths about the safety of vaccination that are so visible on the Internet. The approach worked well, and by the time the children were 1 year of age, I cannot remember but a handful of parents who did not come around to realize that it was best to vaccinate. However, with the recent measles outbreak at Disneyland in California, pertussis at epidemic proportions in pockets of the United States and elsewhere in the world, and the antivaccine voices gaining more and more attention, I agree, it is time to take a stand.
When a family brings their unvaccinated or undervaccinated child into the waiting room of a physician’s practice, that family is potentially exposing others in that waiting room to serious infectious diseases – that is not fair. In the waiting room may well be a patient who is on chemotherapy or immunotherapy or otherwise immunocompromised, and he relies on the “herd immunity” achieved by vaccinations of those who can safely be vaccinated for individual protection and public health. Those patients who have weakened immune systems did not choose to have their medical condition, whereas the vaccine refusers are choosing not to vaccinate their child (or typically themselves as well). And the reasons they are choosing not to vaccinate are based on misrepresentation of medical facts, fabrications of safety concerns, long ago disproven speculations by well-meaning and not so well-meaning physicians and scientists, pseudoscience published in pseudoscientific journals, and/or general distrust of the federal government that mandates vaccinations for the good of the public health.
My personal experience with vaccine scares dates back to a time when whole-cell pertussis vaccine was the only pertussis vaccine available. I was a medical student, resident, and then an infectious diseases fellow during the escalating debate about the significant side effects of vaccines. I joined in the chorus of voices questioning the need for clear data on the problem, and then the pursuit of a safer acellular pertussis vaccine. The physician community and the public were ready for change, and the National Institutes of Health took the lead in organizing multiple studies and clinical trials leading to eventual replacement of the whole cell pertussis vaccine with the current acellular vaccines.
Much more recently, at the request of National Institutes of Health, I led studies of the safety of thimerosal preservative in multidose vaccine vials that appeared in the Lancet (2002;360:1737-41); Pediatrics (2008;121:e208-14) and the Journal of Pediatrics (2009;155:495-9). Using the data from those three studies, the World Health Organization (WHO), the United Nations, the Institute of Medicine, and other organizations were able to see that the metabolism and elimination from the body of ethylmercury in thimerosal was dramatically faster, compared with methylmercury in fish. Therefore, the presumption of possible accumulation of mercury in the body of infants receiving vaccines from multidose vials when such vaccines were closely spaced was disproven by scientific data.
In plain language, there was never a known risk from thimerosal, but a premature, hurried decision was made to mandate removal of thimerosal from vaccines given to children in the United States and western Europe; thereby the myth lives on that thimerosal is not safe. Yet thimerosal is safe, and the WHO continues to advocate use of thimerosal in multidose vaccine vials. Nevertheless, I have been criticized personally on the Internet for this work. The accusation is that I, the rest of the scientists who participated in the study, and the NIH oversight were biased because our academic institutions had previously received funding from vaccine companies to perform clinical and translational research. I received many hate e-mails and even a death threat.
To close this column with a sense of humor, I suggest you Google the responses by U.S. presidential hopefuls on their stand with regard to vaccine refusers. The comments, then the reversal and “corrections” to their comments is amusing. The presidential hopefuls quickly recognized that the right to choose may not be the best policy for the public health of American citizens. Refusing to vaccinate a child potentially harms the child and may harm others!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures. E-mail him at [email protected].
Pertussis persists
The Centers for Disease Control and Prevention suggests that recurring pertussis outbreaks may be the “new normal.” Such outbreaks show that some of what we “know” about pertussis is still correct, but some things are evolving. So in this new year, what do we need to know about patient vulnerability post vaccine as well as the clinical course, diagnosis, and treatment of this stubborn persisting disease?
Vulnerability after acellular pertussis vaccine
The recent large 2014 California outbreak surpassed the record numbers for the previously highest incidence year, 2010 (MMWR 2014;63:1129-32). This is scary because more cases had been reported in California in 2010 than in any prior year since the 1940s. The overall 2014 California pertussis rate (26/100,000 population) was approximately 10 times the national average for the first 9 years of this century, Are there clues as to who is most vulnerable and why?
No age group was spared, but certain age groups did appear more vulnerable. Infants had a startling 174.6/100,000 incidence (six times the rate for the overall population). It is not surprising to any clinician that infants less than 1 year of age were hardest hit. Infants have the most evident symptoms with pertussis. Infants also have 5-7 months of their first year in which they are incompletely immunized. Therefore, many are not expected to be protected until about 7-9 months of age. This vulnerability could be partly remedied if all pregnant women got Tdap boosters as recommended during pregnancy.
Of note, 15-year-olds had an incidence similar to that of infants (137.8/100,000). Ethnically, non-Hispanic whites had the highest incidence among adolescents (166.2/100,000), compared with Hispanics (64.2/100,000), Asian/Pacific Islanders (43.9/100,000), and non-Hispanic blacks (23.7/100,000). Disturbingly, 87% of cases among 15-year-olds had received a prior Tdap booster dose (median time since booster Tdap = 3 years, range = 0-7 years). Previous data from the 2010 outbreak suggested that immunity to pertussis wanes 3-4 years after receipt of the last acellular pertussis (aP)–containing vaccine. This is likely part of the explanation in 2014 as well. However, waning immunity after the booster does not explain why non-Hispanic whites had two to six times the incidence of other ethnicities. Non-Hispanic whites are thought to be the demographic with the most vaccine refusal and vaccine delay in California, so this may partially explain excess cases. Racial differences in access to care or genetic differences in disease susceptibility also may play a role.
Why is this biphasic increase in incidence in California a microcosm of the new epidemiology of pertussis in the United States? A kinder, gentler DTaP vaccine replaced the whole-cell DTP in the late 1990s. This occurred in response to the public’s concern about potential central nervous system adverse effects associated with the whole-cell DTP vaccine. Immunogenicity studies seemed to show equivalent immune responses in infants and toddlers receiving DTaP, compared with those who received DTP. It has only been in the last 5 years that we now know that the new DTaP and Tdap are not working as well as we had hoped.
The two aspects to the lesser protection provided by aP vaccines are pertactin-deficient pertussis strains and quicker waning of aP vaccine–induced immunity. Antibody to pertactin appears to be important in protection against clinical pertussis. New circulating clinical strains of pertussis may not have pertactin (N. Engl. J. Med. 2013;368:583-4). The strains used in our current DTaP and Tdap were designed to protect against pertactin-containing strains and were tested for this. This means that a proportion of the antibodies induced by vaccine strains are not useful against pertactin-deficient strains. The aP vaccine still induces antibody to the pertussis toxin and other pertussis components in the vaccines, so they will likely still reduce the severity of disease. But the vaccines are not likely to prevent infections from pertactin-deficient strains. This is similar to the partial vaccine mismatch that we are seeing with the current seasonal H3N2 influenza vaccine strain.
The second aspect is that protection appears to wane approximately 3-5 years after the last dose of aP-containing vaccine. This contrasts sharply with expectations in the past of 7-10 years of protection from whole cell pertussis–containing vaccines. The less reactive aP vaccine produces fewer adverse effects by not producing as much inflammation as DPT. The problem is that part of the reason the DPT has such good protective responses is the amount of inflammation it produces. So with less aP vaccine–induced inflammation comes less robust antibody and T-cell responses.
Nevertheless, the current acellular pertussis vaccines remain the most effective available tools to reduce pertussis disease (Cochrane Database Syst. Rev. 2014;9:CD001478]). But until we have new versions of pertussis vaccines that address these two issues, we clinicians need to remain vigilant for signs and symptoms of pertussis.
Clinical course
Remember that a whoop is rarely seen in young children and often also not seen when older patients present. The many outbreaks over the last 10 years have confirmed that paroxysmal cough with/without apnea in an infant/toddler should raise our index of suspicion. Likewise, older children, adolescents, and adults with persistent cough beyond 2 weeks are potential pertussis cases. Once the diagnosis is made, treatment is not expected to have a major impact on the clinical course, in part because the diagnosis is usually delayed (more than 10 days into symptoms). This delay allows more injury to the respiratory mucosa and cilia so that healing can require 6-12 weeks after bacterial replication ceases. This prolonged healing process is what is mostly responsible for the syndrome known as the “100-day cough.” So the clinical course of pertussis has not changed in the last 10 years. However, there have been changes in the commonly used diagnostic approach.
Pertussis diagnosis and contagion
During the last 5 years, polymerase chain reaction (PCR) testing has become the preferred technology to detect pertussis. This is due to the sensitivity and quick turnaround time of the assay. The gold standard for pertussis diagnosis remains culture, but it is expensive, cumbersome, and slow (up to a week to provide results). An ongoing debate arose about how long PCR testing remains positive after the onset of symptoms or treatment. This was not the problem when culture was the diagnostic tool of choice. Data from the 1970s and 1980s indicated that cultures were rarely positive after the third week of symptoms even without treatment. Furthermore, macrolides eliminated both contagion and positive culture results of infected patients after 5 days of treatment.
So now that we use PCR most often for diagnosis, what is the outer limit of positivity? A recent prospective cohort study from Salt Lake City suggests that PCR may detect pertussis DNA way beyond 3 weeks after symptom onset (J. Ped. Infect. Dis. 2014;3:347-9). Among patients hospitalized with laboratory-confirmed Bordetella pertussis infection, half had persistently positive pertussis PCR testing more than 50 days after symptom onset, despite antibiotic treatment and clinical improvement. The median (range) for the last day for a positive test after symptom onset was 58 days (4-172 days).
This raises the question as to whether there are viable pertussis organisms in the respiratory tract beyond the traditional 3 weeks defined by culture data. It is likely that DNA persists in the thick mucus of the respiratory tract way beyond viability of the last pertussis organisms. Put another way, PCR likely detects bacterial corpses or components way beyond the time that the patient is contagious. Unfortunately, current PCR data do not tell us how long patients remain contagious with the current strains of pertussis as infecting agents. Some institutions appear to be extending the isolation time for patients treated for pertussis beyond the traditional 5 days post initiation of effective treatment. Until more data are available, we are somewhat in the dark. But I would take comfort in the fact that it is unlikely the “new” data will be much different from those derived from the traditional studies that use culture to define infectivity. The American Academy of Pediatrics Committee on Infectious Diseases Red Book appears to agree.
For hospitalized pertussis patients, the AAP Committee on Infectious Diseases Red Book recommends standard and droplet precautions for 5 days after starting effective therapy, or 3 weeks after cough onset if appropriate antimicrobial therapy has not been given.
In addition, the CDC states: “PCR has optimal sensitivity during the first 3 weeks of cough when bacterial DNA is still present in the nasopharynx. After the fourth week of cough, the amount of bacterial DNA rapidly diminishes, which increases the risk of obtaining falsely negative results.” Later in the same document, the CDC says: “PCR testing following antibiotic therapy also can result in falsely negative findings. The exact duration of positivity following antibiotic use is not well understood, but PCR testing after 5 days of antibiotic use is unlikely to be of benefit and is generally not recommended.”
So what do we know? Not all PCR assays use the same primers, so some variance from the usual experience of up to 4 weeks of positive PCR results may be due to differences in the assays. But this raises concern that the PCR that you order may be positive at times when the patient is no longer contagious.
Pertussis treatment
If strains of pertussis have changed their pertactin antigen, are they changing their antibiotic susceptibility patterns? While there have been reports of macrolide resistance in a few pertussis strains, these still remain rare. The most recent comprehensive review of treatment efficacy was a Cochrane review performed in 2005 and published in 2007 (Cochrane Database Syst. Rev. 2007;3:CD004404). They evaluated 10 trials from 1969 to 2004 in which microbiologic eradication was defined by negative results from repeat pertussis culture. While meta-analysis of microbiologic eradication was not possible because of differences in antibiotic use, the investigators did conclude that antibiotic treatment “is effective in eliminating B. pertussis from patients with the disease to render them noninfectious, but does not alter the subsequent clinical course of the illness.”
Further, they state that “the best regimens for microbiologic clearance, with fewer side effects,” are 3 days of azithromycin (a single 10-mg/kg dose on 3 consecutive days) or 7 days of clarithromycin (7.5-mg/kg dose twice daily).
Another effective regimen is 14 days of erythromycin ethylsuccinate (60 mg/kg per day in 3 divided doses) .
CDC treatment recommendations include azithromycin or erythromycin, with trimethoprim-sulfamethoxazole as a possibility for macrolide-intolerant patients, although there are less data and success rates may not be as high.
Conclusion
So what do we know now about pertussis?
• Outbreaks are ongoing and likely will continue until newer more effective vaccines are produced, including those that circumvent the problem of pertactin-deficient strains.
• Pertussis is likely contagious up to 5 days on effective therapy, and for as long as 3 weeks if effective therapy has not been administered.
• PCR is a sensitive test that may remain positive for many weeks beyond contagion.
• Treatment with macrolides appears to be the most effective way to eradicate replicating pertussis pathogens.
• Treatment is not likely to have a major impact on the clinical course of disease because most of the damage to the respiratory tract is done prior to diagnosis and treatment. Treatment does reduce infectivity and subsequent cases.
• Current aP vaccines currently are our best preventative tools – including use in pregnant women to protect young infants.
As clinicians, our best course is to continue to immunize with the current vaccines, and remain vigilant for symptoms and signs of pertussis infection of patients so that early diagnosis and treatment can prevent further spread.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospitals receives funds from GlaxoSmithKline for Dr. Harrison being principal investigator on a multicenter research study of a hexavalent pertussis-containing infant vaccine. E-mail Dr. Harrison at [email protected].
The Centers for Disease Control and Prevention suggests that recurring pertussis outbreaks may be the “new normal.” Such outbreaks show that some of what we “know” about pertussis is still correct, but some things are evolving. So in this new year, what do we need to know about patient vulnerability post vaccine as well as the clinical course, diagnosis, and treatment of this stubborn persisting disease?
Vulnerability after acellular pertussis vaccine
The recent large 2014 California outbreak surpassed the record numbers for the previously highest incidence year, 2010 (MMWR 2014;63:1129-32). This is scary because more cases had been reported in California in 2010 than in any prior year since the 1940s. The overall 2014 California pertussis rate (26/100,000 population) was approximately 10 times the national average for the first 9 years of this century, Are there clues as to who is most vulnerable and why?
No age group was spared, but certain age groups did appear more vulnerable. Infants had a startling 174.6/100,000 incidence (six times the rate for the overall population). It is not surprising to any clinician that infants less than 1 year of age were hardest hit. Infants have the most evident symptoms with pertussis. Infants also have 5-7 months of their first year in which they are incompletely immunized. Therefore, many are not expected to be protected until about 7-9 months of age. This vulnerability could be partly remedied if all pregnant women got Tdap boosters as recommended during pregnancy.
Of note, 15-year-olds had an incidence similar to that of infants (137.8/100,000). Ethnically, non-Hispanic whites had the highest incidence among adolescents (166.2/100,000), compared with Hispanics (64.2/100,000), Asian/Pacific Islanders (43.9/100,000), and non-Hispanic blacks (23.7/100,000). Disturbingly, 87% of cases among 15-year-olds had received a prior Tdap booster dose (median time since booster Tdap = 3 years, range = 0-7 years). Previous data from the 2010 outbreak suggested that immunity to pertussis wanes 3-4 years after receipt of the last acellular pertussis (aP)–containing vaccine. This is likely part of the explanation in 2014 as well. However, waning immunity after the booster does not explain why non-Hispanic whites had two to six times the incidence of other ethnicities. Non-Hispanic whites are thought to be the demographic with the most vaccine refusal and vaccine delay in California, so this may partially explain excess cases. Racial differences in access to care or genetic differences in disease susceptibility also may play a role.
Why is this biphasic increase in incidence in California a microcosm of the new epidemiology of pertussis in the United States? A kinder, gentler DTaP vaccine replaced the whole-cell DTP in the late 1990s. This occurred in response to the public’s concern about potential central nervous system adverse effects associated with the whole-cell DTP vaccine. Immunogenicity studies seemed to show equivalent immune responses in infants and toddlers receiving DTaP, compared with those who received DTP. It has only been in the last 5 years that we now know that the new DTaP and Tdap are not working as well as we had hoped.
The two aspects to the lesser protection provided by aP vaccines are pertactin-deficient pertussis strains and quicker waning of aP vaccine–induced immunity. Antibody to pertactin appears to be important in protection against clinical pertussis. New circulating clinical strains of pertussis may not have pertactin (N. Engl. J. Med. 2013;368:583-4). The strains used in our current DTaP and Tdap were designed to protect against pertactin-containing strains and were tested for this. This means that a proportion of the antibodies induced by vaccine strains are not useful against pertactin-deficient strains. The aP vaccine still induces antibody to the pertussis toxin and other pertussis components in the vaccines, so they will likely still reduce the severity of disease. But the vaccines are not likely to prevent infections from pertactin-deficient strains. This is similar to the partial vaccine mismatch that we are seeing with the current seasonal H3N2 influenza vaccine strain.
The second aspect is that protection appears to wane approximately 3-5 years after the last dose of aP-containing vaccine. This contrasts sharply with expectations in the past of 7-10 years of protection from whole cell pertussis–containing vaccines. The less reactive aP vaccine produces fewer adverse effects by not producing as much inflammation as DPT. The problem is that part of the reason the DPT has such good protective responses is the amount of inflammation it produces. So with less aP vaccine–induced inflammation comes less robust antibody and T-cell responses.
Nevertheless, the current acellular pertussis vaccines remain the most effective available tools to reduce pertussis disease (Cochrane Database Syst. Rev. 2014;9:CD001478]). But until we have new versions of pertussis vaccines that address these two issues, we clinicians need to remain vigilant for signs and symptoms of pertussis.
Clinical course
Remember that a whoop is rarely seen in young children and often also not seen when older patients present. The many outbreaks over the last 10 years have confirmed that paroxysmal cough with/without apnea in an infant/toddler should raise our index of suspicion. Likewise, older children, adolescents, and adults with persistent cough beyond 2 weeks are potential pertussis cases. Once the diagnosis is made, treatment is not expected to have a major impact on the clinical course, in part because the diagnosis is usually delayed (more than 10 days into symptoms). This delay allows more injury to the respiratory mucosa and cilia so that healing can require 6-12 weeks after bacterial replication ceases. This prolonged healing process is what is mostly responsible for the syndrome known as the “100-day cough.” So the clinical course of pertussis has not changed in the last 10 years. However, there have been changes in the commonly used diagnostic approach.
Pertussis diagnosis and contagion
During the last 5 years, polymerase chain reaction (PCR) testing has become the preferred technology to detect pertussis. This is due to the sensitivity and quick turnaround time of the assay. The gold standard for pertussis diagnosis remains culture, but it is expensive, cumbersome, and slow (up to a week to provide results). An ongoing debate arose about how long PCR testing remains positive after the onset of symptoms or treatment. This was not the problem when culture was the diagnostic tool of choice. Data from the 1970s and 1980s indicated that cultures were rarely positive after the third week of symptoms even without treatment. Furthermore, macrolides eliminated both contagion and positive culture results of infected patients after 5 days of treatment.
So now that we use PCR most often for diagnosis, what is the outer limit of positivity? A recent prospective cohort study from Salt Lake City suggests that PCR may detect pertussis DNA way beyond 3 weeks after symptom onset (J. Ped. Infect. Dis. 2014;3:347-9). Among patients hospitalized with laboratory-confirmed Bordetella pertussis infection, half had persistently positive pertussis PCR testing more than 50 days after symptom onset, despite antibiotic treatment and clinical improvement. The median (range) for the last day for a positive test after symptom onset was 58 days (4-172 days).
This raises the question as to whether there are viable pertussis organisms in the respiratory tract beyond the traditional 3 weeks defined by culture data. It is likely that DNA persists in the thick mucus of the respiratory tract way beyond viability of the last pertussis organisms. Put another way, PCR likely detects bacterial corpses or components way beyond the time that the patient is contagious. Unfortunately, current PCR data do not tell us how long patients remain contagious with the current strains of pertussis as infecting agents. Some institutions appear to be extending the isolation time for patients treated for pertussis beyond the traditional 5 days post initiation of effective treatment. Until more data are available, we are somewhat in the dark. But I would take comfort in the fact that it is unlikely the “new” data will be much different from those derived from the traditional studies that use culture to define infectivity. The American Academy of Pediatrics Committee on Infectious Diseases Red Book appears to agree.
For hospitalized pertussis patients, the AAP Committee on Infectious Diseases Red Book recommends standard and droplet precautions for 5 days after starting effective therapy, or 3 weeks after cough onset if appropriate antimicrobial therapy has not been given.
In addition, the CDC states: “PCR has optimal sensitivity during the first 3 weeks of cough when bacterial DNA is still present in the nasopharynx. After the fourth week of cough, the amount of bacterial DNA rapidly diminishes, which increases the risk of obtaining falsely negative results.” Later in the same document, the CDC says: “PCR testing following antibiotic therapy also can result in falsely negative findings. The exact duration of positivity following antibiotic use is not well understood, but PCR testing after 5 days of antibiotic use is unlikely to be of benefit and is generally not recommended.”
So what do we know? Not all PCR assays use the same primers, so some variance from the usual experience of up to 4 weeks of positive PCR results may be due to differences in the assays. But this raises concern that the PCR that you order may be positive at times when the patient is no longer contagious.
Pertussis treatment
If strains of pertussis have changed their pertactin antigen, are they changing their antibiotic susceptibility patterns? While there have been reports of macrolide resistance in a few pertussis strains, these still remain rare. The most recent comprehensive review of treatment efficacy was a Cochrane review performed in 2005 and published in 2007 (Cochrane Database Syst. Rev. 2007;3:CD004404). They evaluated 10 trials from 1969 to 2004 in which microbiologic eradication was defined by negative results from repeat pertussis culture. While meta-analysis of microbiologic eradication was not possible because of differences in antibiotic use, the investigators did conclude that antibiotic treatment “is effective in eliminating B. pertussis from patients with the disease to render them noninfectious, but does not alter the subsequent clinical course of the illness.”
Further, they state that “the best regimens for microbiologic clearance, with fewer side effects,” are 3 days of azithromycin (a single 10-mg/kg dose on 3 consecutive days) or 7 days of clarithromycin (7.5-mg/kg dose twice daily).
Another effective regimen is 14 days of erythromycin ethylsuccinate (60 mg/kg per day in 3 divided doses) .
CDC treatment recommendations include azithromycin or erythromycin, with trimethoprim-sulfamethoxazole as a possibility for macrolide-intolerant patients, although there are less data and success rates may not be as high.
Conclusion
So what do we know now about pertussis?
• Outbreaks are ongoing and likely will continue until newer more effective vaccines are produced, including those that circumvent the problem of pertactin-deficient strains.
• Pertussis is likely contagious up to 5 days on effective therapy, and for as long as 3 weeks if effective therapy has not been administered.
• PCR is a sensitive test that may remain positive for many weeks beyond contagion.
• Treatment with macrolides appears to be the most effective way to eradicate replicating pertussis pathogens.
• Treatment is not likely to have a major impact on the clinical course of disease because most of the damage to the respiratory tract is done prior to diagnosis and treatment. Treatment does reduce infectivity and subsequent cases.
• Current aP vaccines currently are our best preventative tools – including use in pregnant women to protect young infants.
As clinicians, our best course is to continue to immunize with the current vaccines, and remain vigilant for symptoms and signs of pertussis infection of patients so that early diagnosis and treatment can prevent further spread.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospitals receives funds from GlaxoSmithKline for Dr. Harrison being principal investigator on a multicenter research study of a hexavalent pertussis-containing infant vaccine. E-mail Dr. Harrison at [email protected].
The Centers for Disease Control and Prevention suggests that recurring pertussis outbreaks may be the “new normal.” Such outbreaks show that some of what we “know” about pertussis is still correct, but some things are evolving. So in this new year, what do we need to know about patient vulnerability post vaccine as well as the clinical course, diagnosis, and treatment of this stubborn persisting disease?
Vulnerability after acellular pertussis vaccine
The recent large 2014 California outbreak surpassed the record numbers for the previously highest incidence year, 2010 (MMWR 2014;63:1129-32). This is scary because more cases had been reported in California in 2010 than in any prior year since the 1940s. The overall 2014 California pertussis rate (26/100,000 population) was approximately 10 times the national average for the first 9 years of this century, Are there clues as to who is most vulnerable and why?
No age group was spared, but certain age groups did appear more vulnerable. Infants had a startling 174.6/100,000 incidence (six times the rate for the overall population). It is not surprising to any clinician that infants less than 1 year of age were hardest hit. Infants have the most evident symptoms with pertussis. Infants also have 5-7 months of their first year in which they are incompletely immunized. Therefore, many are not expected to be protected until about 7-9 months of age. This vulnerability could be partly remedied if all pregnant women got Tdap boosters as recommended during pregnancy.
Of note, 15-year-olds had an incidence similar to that of infants (137.8/100,000). Ethnically, non-Hispanic whites had the highest incidence among adolescents (166.2/100,000), compared with Hispanics (64.2/100,000), Asian/Pacific Islanders (43.9/100,000), and non-Hispanic blacks (23.7/100,000). Disturbingly, 87% of cases among 15-year-olds had received a prior Tdap booster dose (median time since booster Tdap = 3 years, range = 0-7 years). Previous data from the 2010 outbreak suggested that immunity to pertussis wanes 3-4 years after receipt of the last acellular pertussis (aP)–containing vaccine. This is likely part of the explanation in 2014 as well. However, waning immunity after the booster does not explain why non-Hispanic whites had two to six times the incidence of other ethnicities. Non-Hispanic whites are thought to be the demographic with the most vaccine refusal and vaccine delay in California, so this may partially explain excess cases. Racial differences in access to care or genetic differences in disease susceptibility also may play a role.
Why is this biphasic increase in incidence in California a microcosm of the new epidemiology of pertussis in the United States? A kinder, gentler DTaP vaccine replaced the whole-cell DTP in the late 1990s. This occurred in response to the public’s concern about potential central nervous system adverse effects associated with the whole-cell DTP vaccine. Immunogenicity studies seemed to show equivalent immune responses in infants and toddlers receiving DTaP, compared with those who received DTP. It has only been in the last 5 years that we now know that the new DTaP and Tdap are not working as well as we had hoped.
The two aspects to the lesser protection provided by aP vaccines are pertactin-deficient pertussis strains and quicker waning of aP vaccine–induced immunity. Antibody to pertactin appears to be important in protection against clinical pertussis. New circulating clinical strains of pertussis may not have pertactin (N. Engl. J. Med. 2013;368:583-4). The strains used in our current DTaP and Tdap were designed to protect against pertactin-containing strains and were tested for this. This means that a proportion of the antibodies induced by vaccine strains are not useful against pertactin-deficient strains. The aP vaccine still induces antibody to the pertussis toxin and other pertussis components in the vaccines, so they will likely still reduce the severity of disease. But the vaccines are not likely to prevent infections from pertactin-deficient strains. This is similar to the partial vaccine mismatch that we are seeing with the current seasonal H3N2 influenza vaccine strain.
The second aspect is that protection appears to wane approximately 3-5 years after the last dose of aP-containing vaccine. This contrasts sharply with expectations in the past of 7-10 years of protection from whole cell pertussis–containing vaccines. The less reactive aP vaccine produces fewer adverse effects by not producing as much inflammation as DPT. The problem is that part of the reason the DPT has such good protective responses is the amount of inflammation it produces. So with less aP vaccine–induced inflammation comes less robust antibody and T-cell responses.
Nevertheless, the current acellular pertussis vaccines remain the most effective available tools to reduce pertussis disease (Cochrane Database Syst. Rev. 2014;9:CD001478]). But until we have new versions of pertussis vaccines that address these two issues, we clinicians need to remain vigilant for signs and symptoms of pertussis.
Clinical course
Remember that a whoop is rarely seen in young children and often also not seen when older patients present. The many outbreaks over the last 10 years have confirmed that paroxysmal cough with/without apnea in an infant/toddler should raise our index of suspicion. Likewise, older children, adolescents, and adults with persistent cough beyond 2 weeks are potential pertussis cases. Once the diagnosis is made, treatment is not expected to have a major impact on the clinical course, in part because the diagnosis is usually delayed (more than 10 days into symptoms). This delay allows more injury to the respiratory mucosa and cilia so that healing can require 6-12 weeks after bacterial replication ceases. This prolonged healing process is what is mostly responsible for the syndrome known as the “100-day cough.” So the clinical course of pertussis has not changed in the last 10 years. However, there have been changes in the commonly used diagnostic approach.
Pertussis diagnosis and contagion
During the last 5 years, polymerase chain reaction (PCR) testing has become the preferred technology to detect pertussis. This is due to the sensitivity and quick turnaround time of the assay. The gold standard for pertussis diagnosis remains culture, but it is expensive, cumbersome, and slow (up to a week to provide results). An ongoing debate arose about how long PCR testing remains positive after the onset of symptoms or treatment. This was not the problem when culture was the diagnostic tool of choice. Data from the 1970s and 1980s indicated that cultures were rarely positive after the third week of symptoms even without treatment. Furthermore, macrolides eliminated both contagion and positive culture results of infected patients after 5 days of treatment.
So now that we use PCR most often for diagnosis, what is the outer limit of positivity? A recent prospective cohort study from Salt Lake City suggests that PCR may detect pertussis DNA way beyond 3 weeks after symptom onset (J. Ped. Infect. Dis. 2014;3:347-9). Among patients hospitalized with laboratory-confirmed Bordetella pertussis infection, half had persistently positive pertussis PCR testing more than 50 days after symptom onset, despite antibiotic treatment and clinical improvement. The median (range) for the last day for a positive test after symptom onset was 58 days (4-172 days).
This raises the question as to whether there are viable pertussis organisms in the respiratory tract beyond the traditional 3 weeks defined by culture data. It is likely that DNA persists in the thick mucus of the respiratory tract way beyond viability of the last pertussis organisms. Put another way, PCR likely detects bacterial corpses or components way beyond the time that the patient is contagious. Unfortunately, current PCR data do not tell us how long patients remain contagious with the current strains of pertussis as infecting agents. Some institutions appear to be extending the isolation time for patients treated for pertussis beyond the traditional 5 days post initiation of effective treatment. Until more data are available, we are somewhat in the dark. But I would take comfort in the fact that it is unlikely the “new” data will be much different from those derived from the traditional studies that use culture to define infectivity. The American Academy of Pediatrics Committee on Infectious Diseases Red Book appears to agree.
For hospitalized pertussis patients, the AAP Committee on Infectious Diseases Red Book recommends standard and droplet precautions for 5 days after starting effective therapy, or 3 weeks after cough onset if appropriate antimicrobial therapy has not been given.
In addition, the CDC states: “PCR has optimal sensitivity during the first 3 weeks of cough when bacterial DNA is still present in the nasopharynx. After the fourth week of cough, the amount of bacterial DNA rapidly diminishes, which increases the risk of obtaining falsely negative results.” Later in the same document, the CDC says: “PCR testing following antibiotic therapy also can result in falsely negative findings. The exact duration of positivity following antibiotic use is not well understood, but PCR testing after 5 days of antibiotic use is unlikely to be of benefit and is generally not recommended.”
So what do we know? Not all PCR assays use the same primers, so some variance from the usual experience of up to 4 weeks of positive PCR results may be due to differences in the assays. But this raises concern that the PCR that you order may be positive at times when the patient is no longer contagious.
Pertussis treatment
If strains of pertussis have changed their pertactin antigen, are they changing their antibiotic susceptibility patterns? While there have been reports of macrolide resistance in a few pertussis strains, these still remain rare. The most recent comprehensive review of treatment efficacy was a Cochrane review performed in 2005 and published in 2007 (Cochrane Database Syst. Rev. 2007;3:CD004404). They evaluated 10 trials from 1969 to 2004 in which microbiologic eradication was defined by negative results from repeat pertussis culture. While meta-analysis of microbiologic eradication was not possible because of differences in antibiotic use, the investigators did conclude that antibiotic treatment “is effective in eliminating B. pertussis from patients with the disease to render them noninfectious, but does not alter the subsequent clinical course of the illness.”
Further, they state that “the best regimens for microbiologic clearance, with fewer side effects,” are 3 days of azithromycin (a single 10-mg/kg dose on 3 consecutive days) or 7 days of clarithromycin (7.5-mg/kg dose twice daily).
Another effective regimen is 14 days of erythromycin ethylsuccinate (60 mg/kg per day in 3 divided doses) .
CDC treatment recommendations include azithromycin or erythromycin, with trimethoprim-sulfamethoxazole as a possibility for macrolide-intolerant patients, although there are less data and success rates may not be as high.
Conclusion
So what do we know now about pertussis?
• Outbreaks are ongoing and likely will continue until newer more effective vaccines are produced, including those that circumvent the problem of pertactin-deficient strains.
• Pertussis is likely contagious up to 5 days on effective therapy, and for as long as 3 weeks if effective therapy has not been administered.
• PCR is a sensitive test that may remain positive for many weeks beyond contagion.
• Treatment with macrolides appears to be the most effective way to eradicate replicating pertussis pathogens.
• Treatment is not likely to have a major impact on the clinical course of disease because most of the damage to the respiratory tract is done prior to diagnosis and treatment. Treatment does reduce infectivity and subsequent cases.
• Current aP vaccines currently are our best preventative tools – including use in pregnant women to protect young infants.
As clinicians, our best course is to continue to immunize with the current vaccines, and remain vigilant for symptoms and signs of pertussis infection of patients so that early diagnosis and treatment can prevent further spread.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Children’s Mercy Hospitals receives funds from GlaxoSmithKline for Dr. Harrison being principal investigator on a multicenter research study of a hexavalent pertussis-containing infant vaccine. E-mail Dr. Harrison at [email protected].
Predictions for 2015
Last year, there were five predictions made that appeared to be on the money – but there is more to the story!
1. The approach to diagnosis and treatment of influenza was essential knowledge for clinicians. Last year, we started seeing influenza activity early – with disease confirmed in mid-November, peaking during the week ending December 28, 2013 and trending downward in early January 2014. Hospitalizations were most common in young and middle aged adults and the 2009 H1N1 virus predominated.
This year, again we are seeing influenza early – with nearly all states reporting at least sporadic and local activity, and several states (Alaska, Florida, Louisiana, Massachusetts, and Texas) reporting regional activity as of the week ending November 24, 2014. At my institution, we’ve already tested over 500 children and over 100 were positive – influenza A (H3N2) strains are predominating. That may be important for the two reasons you’ll read below.
2. Invasive staphylococcal disease caused by methicillin-susceptible Staphylococcus aureus (MSSA) was more common than methicillin-resistant Staphylococcus aureus (MRSA), as the national burden of MRSA disease decreased (JAMA 2014;311:1438-9). The rates of clindamycin resistance continue to be pretty steady at approximately 15%-18%, but higher for MSSA than for MRSA – a point that is important to consider when empirically treating suspected invasive staphylococcal infection.
3. Multidrug resistant uropathogens took an increasingly prominent role in 2014, requiring careful approach to diagnosis (every child treated for urinary tract infection should have an appropriately obtained urine culture with an identified pathogen) and treatment (the drug used should be based on antibiotic susceptibility testing results). Particularly concerning is the emergence of carbapenem-resistant Enterobacteriaceae, which cause infection more commonly in hospitalized patients, those with indwelling devices, and those who have received long courses of antibiotics.
4. It was an outbreak year for parechovirus (HPeV), a viral pathogen causing meningitis in very young infants. Such infants present with signs and symptoms of meningitis but rarely show CSF pleocytosis. Diagnosis relies on the detection of the virus by polymerase chain reaction testing in CSF – a test which is not routinely available in many laboratories. At my institution this season, we saw nearly as many cases of parechovirus meningitis (n = 43) as we saw cases of enterovirus meningitis (n = 63). The parechovirus virus we detected was HPeV type 3, which can cause particularly severe disease in neonates.
5. Data confirmed that making human papillomavirus (HPV) vaccine a standard recommendation increased vaccine uptake and coverage. In February of 2014, a “Dear Colleague” letter that was endorsed by six leading medical organizations encouraged providers to promote HPV vaccination by giving a strong recommendation, citing data based on research conducted by the Centers for Disease Control and Prevention. We still have a long way to go as HPV vaccine coverage for teens remains at 35% for the three-dose series while meningococcal and Tdap vaccine (both vaccines that generally receive a standard recommendation by physicians) coverage is at nearly 90%.
So for 2015, I’ll start the discussion by saying there are five major developments I did not see coming for this past year, but that will remain relevant for the year 2015!
1. In June of 2014, live attenuated influenza vaccine (LAIV) was announced by the Advisory Committee on Immunization Practices to be the preferred vaccine in children aged 2-8 years. The American Academy of Pediatrics followed with a recommendation that either inactivated influenza vaccine (IIV) or LAIV be used for children, including children aged 2-8 years – the key being to give the vaccine as soon as one had it available. What was not known then and I did not predict was that newer data would confirm that in children aged 2-8 years who received LAIV last year when 2009 H1N1 strains predominated, there was essentially no coverage against 2009 H1N1 virus. This was in contrast to data from the prior 2 years and is as yet unexplained. The AAP continues to recommend that either vaccine be given and all children be immunized. That may be especially important this year as the influenza season started early. Disease will likely have been widespread by Christmas in many parts of the United States, and it looks like influenza A H3N2 strains will be most commonly noted. So the good news for young children who received LAIV is that 2009 H1N1 strains so far have not been seen this year. The bad news is that there are two H3N2 strains circulating, and potentially only one will be covered by the 2014-2015 seasonal vaccine. Staffing your office and hospital for a likely high census respiratory viral season is going to be essential.
2. The largest U.S. outbreak ever of enterovirus (EV) D-68 respiratory infection occurred between August and October of 2014. This virus – which had been identified in 1962 but was rarely described over the next 36 years except in small clusters of disease – was reported in nearly every state and characterized by unusually severe respiratory tract infection. Many, but not all children, had a history of asthma or prior wheezing, and the clinical presentation was that of severe bronchospasm that was generally resistant to standard bronchodilator therapy. The spectrum of infection likely ranged from mild upper respiratory infection to severe bronchospasm with respiratory failure, and the burden of disease resulting in hospitalization was substantial at many children’s hospitals. The big question now is what will enterovirus season 2015 bring us? The good news here is that we now have a test to rapidly diagnose EV D-68, which will allow us to more clearly understand the burden of disease – and potentially to define antiviral treatment (none of the current antivirals is effective) and prevention (there is no vaccine against EV D-68).
3. The etiology of the neurologic illness, which appeared to mimic polio and presented during the same time frame during which EV D-68 was circulating, is as yet unknown. As of Nov. 26, 2014, the CDC has received reports of 90 children in 32 states who meet a case definition consistent with acute flaccid myelitis. While certain viruses – including West Nile virus, herpes virus, adenovirus, and certain enterovirus types (for example, enterovirus 71, and the classic being polio) – may cause acute flaccid paralysis and can be confirmed by detecting the virus in cerebrospinal fluid and stool, to date virus testing for all viruses, including EV D-68, has been negative in all of the patients reported. Hopefully, 2015 will be the year that will allow us to more clearly understand this neurologic illness – and this is important because so far most children have shown minimal recovery of function.
4. If you see a child (or adult) who recently traveled to the Caribbean and returns with fever, rash, and joint pain, especially with severe pain of the hands and feet, think chikungunya virus infection. As of the end of October 2014, local transmission had been identified in 37 countries or territories in the Caribbean (including Puerto Rico and the U.S. Virgin Islands), with a total of 780,206 suspected cases and over 15,000 confirmed cases reported from these areas. Consider this in contrast to the numbers from 2006 through 2011, when 117 cases of chikungunya fever were reported in returning travelers. As of Dec. 2, a total of 1,911 chikungunya virus disease cases have been reported to ArboNET from U.S. states. The mosquito that transmits chikungunya virus can bite in day and night, and prevention relies on appropriate use of mosquito repellents. Physicians should be prepared to discuss the risks of this virus with travelers who plan a trip to the Caribbean, especially those at high risk, including those with underlying medical conditions, preexisting arthritis diagnoses, and pregnant women (because of the potential risk to newborns whose mothers develop intrapartum infection).
5. And lastly, Ebola. While there were reports that Ebola virus disease had emerged in West Africa as early as December of 2013, the scope of the outbreak and extent of loss of human life has been unbelievably huge. Dr. Carrie Byington, who is the current chair of the AAP Committee on Infectious Diseases, wrote an article in AAP News in October 2014 describing the needs of children who have been impacted by Ebola virus disease (EVD). She noted that UNICEF estimated there were at that time, over 4,000 Ebola orphans in the countries most affected by EVD, including Sierra Leone, Liberia, and Guinea, and that these countries urgently needed medical infrastructure for treatment and prevention of this disease. It appears that at least two Ebola vaccines will be deployed in West Africa in 2015, and it is not a moment too soon. While cases in Liberia seemed to be decreasing, it looks like Sierra Leone cases continue to mount.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. Dr. Jackson was a member of the AAP Committee on Infectious Diseases who wrote the AAP clinical report entitled “Guidance on Management of Asymptomatic Neonates Born to Women With Active Genital Herpes Lesions,” but said she had no other conflicts of interest to disclose. E-mail her at [email protected].
Last year, there were five predictions made that appeared to be on the money – but there is more to the story!
1. The approach to diagnosis and treatment of influenza was essential knowledge for clinicians. Last year, we started seeing influenza activity early – with disease confirmed in mid-November, peaking during the week ending December 28, 2013 and trending downward in early January 2014. Hospitalizations were most common in young and middle aged adults and the 2009 H1N1 virus predominated.
This year, again we are seeing influenza early – with nearly all states reporting at least sporadic and local activity, and several states (Alaska, Florida, Louisiana, Massachusetts, and Texas) reporting regional activity as of the week ending November 24, 2014. At my institution, we’ve already tested over 500 children and over 100 were positive – influenza A (H3N2) strains are predominating. That may be important for the two reasons you’ll read below.
2. Invasive staphylococcal disease caused by methicillin-susceptible Staphylococcus aureus (MSSA) was more common than methicillin-resistant Staphylococcus aureus (MRSA), as the national burden of MRSA disease decreased (JAMA 2014;311:1438-9). The rates of clindamycin resistance continue to be pretty steady at approximately 15%-18%, but higher for MSSA than for MRSA – a point that is important to consider when empirically treating suspected invasive staphylococcal infection.
3. Multidrug resistant uropathogens took an increasingly prominent role in 2014, requiring careful approach to diagnosis (every child treated for urinary tract infection should have an appropriately obtained urine culture with an identified pathogen) and treatment (the drug used should be based on antibiotic susceptibility testing results). Particularly concerning is the emergence of carbapenem-resistant Enterobacteriaceae, which cause infection more commonly in hospitalized patients, those with indwelling devices, and those who have received long courses of antibiotics.
4. It was an outbreak year for parechovirus (HPeV), a viral pathogen causing meningitis in very young infants. Such infants present with signs and symptoms of meningitis but rarely show CSF pleocytosis. Diagnosis relies on the detection of the virus by polymerase chain reaction testing in CSF – a test which is not routinely available in many laboratories. At my institution this season, we saw nearly as many cases of parechovirus meningitis (n = 43) as we saw cases of enterovirus meningitis (n = 63). The parechovirus virus we detected was HPeV type 3, which can cause particularly severe disease in neonates.
5. Data confirmed that making human papillomavirus (HPV) vaccine a standard recommendation increased vaccine uptake and coverage. In February of 2014, a “Dear Colleague” letter that was endorsed by six leading medical organizations encouraged providers to promote HPV vaccination by giving a strong recommendation, citing data based on research conducted by the Centers for Disease Control and Prevention. We still have a long way to go as HPV vaccine coverage for teens remains at 35% for the three-dose series while meningococcal and Tdap vaccine (both vaccines that generally receive a standard recommendation by physicians) coverage is at nearly 90%.
So for 2015, I’ll start the discussion by saying there are five major developments I did not see coming for this past year, but that will remain relevant for the year 2015!
1. In June of 2014, live attenuated influenza vaccine (LAIV) was announced by the Advisory Committee on Immunization Practices to be the preferred vaccine in children aged 2-8 years. The American Academy of Pediatrics followed with a recommendation that either inactivated influenza vaccine (IIV) or LAIV be used for children, including children aged 2-8 years – the key being to give the vaccine as soon as one had it available. What was not known then and I did not predict was that newer data would confirm that in children aged 2-8 years who received LAIV last year when 2009 H1N1 strains predominated, there was essentially no coverage against 2009 H1N1 virus. This was in contrast to data from the prior 2 years and is as yet unexplained. The AAP continues to recommend that either vaccine be given and all children be immunized. That may be especially important this year as the influenza season started early. Disease will likely have been widespread by Christmas in many parts of the United States, and it looks like influenza A H3N2 strains will be most commonly noted. So the good news for young children who received LAIV is that 2009 H1N1 strains so far have not been seen this year. The bad news is that there are two H3N2 strains circulating, and potentially only one will be covered by the 2014-2015 seasonal vaccine. Staffing your office and hospital for a likely high census respiratory viral season is going to be essential.
2. The largest U.S. outbreak ever of enterovirus (EV) D-68 respiratory infection occurred between August and October of 2014. This virus – which had been identified in 1962 but was rarely described over the next 36 years except in small clusters of disease – was reported in nearly every state and characterized by unusually severe respiratory tract infection. Many, but not all children, had a history of asthma or prior wheezing, and the clinical presentation was that of severe bronchospasm that was generally resistant to standard bronchodilator therapy. The spectrum of infection likely ranged from mild upper respiratory infection to severe bronchospasm with respiratory failure, and the burden of disease resulting in hospitalization was substantial at many children’s hospitals. The big question now is what will enterovirus season 2015 bring us? The good news here is that we now have a test to rapidly diagnose EV D-68, which will allow us to more clearly understand the burden of disease – and potentially to define antiviral treatment (none of the current antivirals is effective) and prevention (there is no vaccine against EV D-68).
3. The etiology of the neurologic illness, which appeared to mimic polio and presented during the same time frame during which EV D-68 was circulating, is as yet unknown. As of Nov. 26, 2014, the CDC has received reports of 90 children in 32 states who meet a case definition consistent with acute flaccid myelitis. While certain viruses – including West Nile virus, herpes virus, adenovirus, and certain enterovirus types (for example, enterovirus 71, and the classic being polio) – may cause acute flaccid paralysis and can be confirmed by detecting the virus in cerebrospinal fluid and stool, to date virus testing for all viruses, including EV D-68, has been negative in all of the patients reported. Hopefully, 2015 will be the year that will allow us to more clearly understand this neurologic illness – and this is important because so far most children have shown minimal recovery of function.
4. If you see a child (or adult) who recently traveled to the Caribbean and returns with fever, rash, and joint pain, especially with severe pain of the hands and feet, think chikungunya virus infection. As of the end of October 2014, local transmission had been identified in 37 countries or territories in the Caribbean (including Puerto Rico and the U.S. Virgin Islands), with a total of 780,206 suspected cases and over 15,000 confirmed cases reported from these areas. Consider this in contrast to the numbers from 2006 through 2011, when 117 cases of chikungunya fever were reported in returning travelers. As of Dec. 2, a total of 1,911 chikungunya virus disease cases have been reported to ArboNET from U.S. states. The mosquito that transmits chikungunya virus can bite in day and night, and prevention relies on appropriate use of mosquito repellents. Physicians should be prepared to discuss the risks of this virus with travelers who plan a trip to the Caribbean, especially those at high risk, including those with underlying medical conditions, preexisting arthritis diagnoses, and pregnant women (because of the potential risk to newborns whose mothers develop intrapartum infection).
5. And lastly, Ebola. While there were reports that Ebola virus disease had emerged in West Africa as early as December of 2013, the scope of the outbreak and extent of loss of human life has been unbelievably huge. Dr. Carrie Byington, who is the current chair of the AAP Committee on Infectious Diseases, wrote an article in AAP News in October 2014 describing the needs of children who have been impacted by Ebola virus disease (EVD). She noted that UNICEF estimated there were at that time, over 4,000 Ebola orphans in the countries most affected by EVD, including Sierra Leone, Liberia, and Guinea, and that these countries urgently needed medical infrastructure for treatment and prevention of this disease. It appears that at least two Ebola vaccines will be deployed in West Africa in 2015, and it is not a moment too soon. While cases in Liberia seemed to be decreasing, it looks like Sierra Leone cases continue to mount.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. Dr. Jackson was a member of the AAP Committee on Infectious Diseases who wrote the AAP clinical report entitled “Guidance on Management of Asymptomatic Neonates Born to Women With Active Genital Herpes Lesions,” but said she had no other conflicts of interest to disclose. E-mail her at [email protected].
Last year, there were five predictions made that appeared to be on the money – but there is more to the story!
1. The approach to diagnosis and treatment of influenza was essential knowledge for clinicians. Last year, we started seeing influenza activity early – with disease confirmed in mid-November, peaking during the week ending December 28, 2013 and trending downward in early January 2014. Hospitalizations were most common in young and middle aged adults and the 2009 H1N1 virus predominated.
This year, again we are seeing influenza early – with nearly all states reporting at least sporadic and local activity, and several states (Alaska, Florida, Louisiana, Massachusetts, and Texas) reporting regional activity as of the week ending November 24, 2014. At my institution, we’ve already tested over 500 children and over 100 were positive – influenza A (H3N2) strains are predominating. That may be important for the two reasons you’ll read below.
2. Invasive staphylococcal disease caused by methicillin-susceptible Staphylococcus aureus (MSSA) was more common than methicillin-resistant Staphylococcus aureus (MRSA), as the national burden of MRSA disease decreased (JAMA 2014;311:1438-9). The rates of clindamycin resistance continue to be pretty steady at approximately 15%-18%, but higher for MSSA than for MRSA – a point that is important to consider when empirically treating suspected invasive staphylococcal infection.
3. Multidrug resistant uropathogens took an increasingly prominent role in 2014, requiring careful approach to diagnosis (every child treated for urinary tract infection should have an appropriately obtained urine culture with an identified pathogen) and treatment (the drug used should be based on antibiotic susceptibility testing results). Particularly concerning is the emergence of carbapenem-resistant Enterobacteriaceae, which cause infection more commonly in hospitalized patients, those with indwelling devices, and those who have received long courses of antibiotics.
4. It was an outbreak year for parechovirus (HPeV), a viral pathogen causing meningitis in very young infants. Such infants present with signs and symptoms of meningitis but rarely show CSF pleocytosis. Diagnosis relies on the detection of the virus by polymerase chain reaction testing in CSF – a test which is not routinely available in many laboratories. At my institution this season, we saw nearly as many cases of parechovirus meningitis (n = 43) as we saw cases of enterovirus meningitis (n = 63). The parechovirus virus we detected was HPeV type 3, which can cause particularly severe disease in neonates.
5. Data confirmed that making human papillomavirus (HPV) vaccine a standard recommendation increased vaccine uptake and coverage. In February of 2014, a “Dear Colleague” letter that was endorsed by six leading medical organizations encouraged providers to promote HPV vaccination by giving a strong recommendation, citing data based on research conducted by the Centers for Disease Control and Prevention. We still have a long way to go as HPV vaccine coverage for teens remains at 35% for the three-dose series while meningococcal and Tdap vaccine (both vaccines that generally receive a standard recommendation by physicians) coverage is at nearly 90%.
So for 2015, I’ll start the discussion by saying there are five major developments I did not see coming for this past year, but that will remain relevant for the year 2015!
1. In June of 2014, live attenuated influenza vaccine (LAIV) was announced by the Advisory Committee on Immunization Practices to be the preferred vaccine in children aged 2-8 years. The American Academy of Pediatrics followed with a recommendation that either inactivated influenza vaccine (IIV) or LAIV be used for children, including children aged 2-8 years – the key being to give the vaccine as soon as one had it available. What was not known then and I did not predict was that newer data would confirm that in children aged 2-8 years who received LAIV last year when 2009 H1N1 strains predominated, there was essentially no coverage against 2009 H1N1 virus. This was in contrast to data from the prior 2 years and is as yet unexplained. The AAP continues to recommend that either vaccine be given and all children be immunized. That may be especially important this year as the influenza season started early. Disease will likely have been widespread by Christmas in many parts of the United States, and it looks like influenza A H3N2 strains will be most commonly noted. So the good news for young children who received LAIV is that 2009 H1N1 strains so far have not been seen this year. The bad news is that there are two H3N2 strains circulating, and potentially only one will be covered by the 2014-2015 seasonal vaccine. Staffing your office and hospital for a likely high census respiratory viral season is going to be essential.
2. The largest U.S. outbreak ever of enterovirus (EV) D-68 respiratory infection occurred between August and October of 2014. This virus – which had been identified in 1962 but was rarely described over the next 36 years except in small clusters of disease – was reported in nearly every state and characterized by unusually severe respiratory tract infection. Many, but not all children, had a history of asthma or prior wheezing, and the clinical presentation was that of severe bronchospasm that was generally resistant to standard bronchodilator therapy. The spectrum of infection likely ranged from mild upper respiratory infection to severe bronchospasm with respiratory failure, and the burden of disease resulting in hospitalization was substantial at many children’s hospitals. The big question now is what will enterovirus season 2015 bring us? The good news here is that we now have a test to rapidly diagnose EV D-68, which will allow us to more clearly understand the burden of disease – and potentially to define antiviral treatment (none of the current antivirals is effective) and prevention (there is no vaccine against EV D-68).
3. The etiology of the neurologic illness, which appeared to mimic polio and presented during the same time frame during which EV D-68 was circulating, is as yet unknown. As of Nov. 26, 2014, the CDC has received reports of 90 children in 32 states who meet a case definition consistent with acute flaccid myelitis. While certain viruses – including West Nile virus, herpes virus, adenovirus, and certain enterovirus types (for example, enterovirus 71, and the classic being polio) – may cause acute flaccid paralysis and can be confirmed by detecting the virus in cerebrospinal fluid and stool, to date virus testing for all viruses, including EV D-68, has been negative in all of the patients reported. Hopefully, 2015 will be the year that will allow us to more clearly understand this neurologic illness – and this is important because so far most children have shown minimal recovery of function.
4. If you see a child (or adult) who recently traveled to the Caribbean and returns with fever, rash, and joint pain, especially with severe pain of the hands and feet, think chikungunya virus infection. As of the end of October 2014, local transmission had been identified in 37 countries or territories in the Caribbean (including Puerto Rico and the U.S. Virgin Islands), with a total of 780,206 suspected cases and over 15,000 confirmed cases reported from these areas. Consider this in contrast to the numbers from 2006 through 2011, when 117 cases of chikungunya fever were reported in returning travelers. As of Dec. 2, a total of 1,911 chikungunya virus disease cases have been reported to ArboNET from U.S. states. The mosquito that transmits chikungunya virus can bite in day and night, and prevention relies on appropriate use of mosquito repellents. Physicians should be prepared to discuss the risks of this virus with travelers who plan a trip to the Caribbean, especially those at high risk, including those with underlying medical conditions, preexisting arthritis diagnoses, and pregnant women (because of the potential risk to newborns whose mothers develop intrapartum infection).
5. And lastly, Ebola. While there were reports that Ebola virus disease had emerged in West Africa as early as December of 2013, the scope of the outbreak and extent of loss of human life has been unbelievably huge. Dr. Carrie Byington, who is the current chair of the AAP Committee on Infectious Diseases, wrote an article in AAP News in October 2014 describing the needs of children who have been impacted by Ebola virus disease (EVD). She noted that UNICEF estimated there were at that time, over 4,000 Ebola orphans in the countries most affected by EVD, including Sierra Leone, Liberia, and Guinea, and that these countries urgently needed medical infrastructure for treatment and prevention of this disease. It appears that at least two Ebola vaccines will be deployed in West Africa in 2015, and it is not a moment too soon. While cases in Liberia seemed to be decreasing, it looks like Sierra Leone cases continue to mount.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. Dr. Jackson was a member of the AAP Committee on Infectious Diseases who wrote the AAP clinical report entitled “Guidance on Management of Asymptomatic Neonates Born to Women With Active Genital Herpes Lesions,” but said she had no other conflicts of interest to disclose. E-mail her at [email protected].


















