User login
Foodborne illnesses of foreign, domestic origin: On the rise?
Are foodborne illness outbreaks more common now, or are we simply better at detection? Have the foods and sources associated with foodborne illness changed? Two recent Centers for Disease Control & Prevention reports provide insight.1,2 In 2016, the Foodborne Diseases Active Surveillance Network (FoodNet) detected 24,029 infections, 5,212 hospitalizations, and 98 fatalities.1 FoodNet has 10 sites serving 49 million people (15% of the U.S. population). These 2016 numbers changed only modestly from the 3 prior years.
The big two
, detected by traditional cultures or culture-independent diagnostic tests (CIDTs). (See table.) CIDTs are relatively new molecular-based, mostly multiplex assays that test for more than a dozen pathogens in one assay.
Overall, Salmonella originated from diverse sources (eggs, poultry, meat, unpasteurized milk/juice/cheese, or raw fruits/vegetables/spices/nuts). But, in 2016, U.S. Salmonella outbreaks were from eggs, alfalfa sprouts, poultry, pistachios, and organic shake/meal products.
The runners-up
Most of the remainder of the 2016 foodborne illnesses were caused by Shigella, with nearly 3,000 cases; shigatoxin-producing Escherichia coli (STEC), with nearly 2,000 cases; and Cryptosporidium, also with nearly 2,000 cases. (See table.)
Hemolytic uremic syndrome (HUS)
HUS rates, mostly resulting from E. coli 0157 H7 in meat, did not vary from 2013 to 2016, with a total 62 pediatric HUS cases in FoodNet (0.56 /100,000 population). Slightly over half (56%) occurred in children under 5 years old at 1.18 per 100,000 population.
Does CIDT increase detection rates?
Detection of the “big two” did not change from 2013 to 2016 or over the past 2 decades. That said, Campylobacter detection was actually down 11% if considering only culture-confirmed cases. That is, if we do not count detections made exclusively by CIDT.
This is important because CIDT – now supplanting culture in many laboratories – identifies pathogens not likely detected by standard culture because culture is generally selective and CIDT is more sensitive. CIDT can increase detection rates (solo and multiple pathogens), even if illnesses do not really increase. The CDC suggested that this contributed to increased STEC and Yersinia detection in 2016. Some would not have been detected if only culture had been utilized.
Viable bacterial/viral isolates are not available from CIDT. A replicating pathogen is needed to characterize shifting/emerging pathogen strains (for example, analysis for mutations or new pathogens via sequencing or antimicrobial susceptibility testing).
To compensate, some CIDT-using laboratories perform “reflex cultures.” CIDT positive specimens also are cultured to provide viable isolates. However, this adds cost to an already costly CIDT test.
The role of imported food
Surveillance systems, such as the Foodborne Disease Outbreak Surveillance System, also track imported foodborne illness. Despite an approximately 50% decrease in overall U.S. foodborne outbreaks since 2000, imported food-related outbreaks increased to 195 during 2006-2014 from 54 during 1996-2004, with 10,685 illnesses, 1,017 hospitalizations, and 19 deaths since 2009. Also, imported food-related outbreaks rose from a mean 3 per year pre-2000 to a mean 18 per year during 2009-2014. Most imported food outbreaks (86% of total) had three causes: scombroid toxin (42% of total), Salmonella (33%), and hepatitis A virus (11%).

Most imported food illnesses were from Salmonella (4,421 from 52 outbreaks), Cyclospora (2,533 from 33 outbreaks), hepatitis A virus (1,150 from 11 outbreaks), and Shigella (625 from 6 outbreaks). While eggs, ice cream, and poultry are notorious origins for Salmonella in domestic food, most imported Salmonella were from produce: fruits (26%), seeded vegetables (20%), sprouts (11%), nuts/seeds (10%), spices (7%), and herbs (2%).
Seafood/fish caused 55% of outbreaks but few illnesses per outbreak (median 3 illnesses/outbreak), so only 11% of total illnesses were caused by seafood/fish. In contrast, fresh produce caused only 33% of outbreaks but 84% of illnesses (median 40 illnesses/outbreak).
Geographic source, outbreak locations
The origin was known in 91% of outbreaks. Latin America and the Caribbean were most common, followed by Asia.3 Main contributing countries were Mexico (42 outbreaks), Indonesia (17) and Canada (11).
Contaminated fish/shellfish originated from all regions except Europe, most commonly from Asia (the majority of fish/shellfish outbreaks were from Indonesia, Vietnam, China, Philippines, Taiwan, and Thailand) with smaller contributions from the Bahamas and Ecuador.
Contaminated produce originated from all regions, mostly (64%) from Mexico and the Americas (Chile, Guatemala, and Honduras). All but one dairy outbreak originated in Latin America/the Caribbean.3 Outbreaks occurred in 31 states, most commonly California (30), Florida (25), and New York (16). Additionally, 43 (22%) were multistate outbreaks.
Conclusions
Outbreaks from domestic foods decreased, but those from imported foods increased. This makes sense given recent increases in outbreak-prone food imports, such as seafood/fish and produce.
To reduce overall foodborne illness outbreaks, governmental agencies need to:
- Develop/enforce regulations that promote proper growing, handling, and processing of foods.
- Strengthen surveillance networks and share standard culture and molecular detection/characterization protocols to identify outbreaks as close to real time as possible.
- Ensure rapid traceability not only to country of origin but to an exact farm or seafood/fish harvesting entity.
- Provide rapid public knowledge of outbreaks and origins, plus outbreak-specific recommendations to control/minimize resultant illnesses.
Individuals can help protect themselves by avoiding inadequately washed or incompletely cooked foods or foods of uncertain origin.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2017 Apr 21;66(15):397-403.
2. Emerg Infect Dis. 2017 Mar;23(3):525-8.
3. Technical appendix in Emerg Infect Dis. 2017 Mar;23(3):525-8.
Are foodborne illness outbreaks more common now, or are we simply better at detection? Have the foods and sources associated with foodborne illness changed? Two recent Centers for Disease Control & Prevention reports provide insight.1,2 In 2016, the Foodborne Diseases Active Surveillance Network (FoodNet) detected 24,029 infections, 5,212 hospitalizations, and 98 fatalities.1 FoodNet has 10 sites serving 49 million people (15% of the U.S. population). These 2016 numbers changed only modestly from the 3 prior years.
The big two
, detected by traditional cultures or culture-independent diagnostic tests (CIDTs). (See table.) CIDTs are relatively new molecular-based, mostly multiplex assays that test for more than a dozen pathogens in one assay.
Overall, Salmonella originated from diverse sources (eggs, poultry, meat, unpasteurized milk/juice/cheese, or raw fruits/vegetables/spices/nuts). But, in 2016, U.S. Salmonella outbreaks were from eggs, alfalfa sprouts, poultry, pistachios, and organic shake/meal products.
The runners-up
Most of the remainder of the 2016 foodborne illnesses were caused by Shigella, with nearly 3,000 cases; shigatoxin-producing Escherichia coli (STEC), with nearly 2,000 cases; and Cryptosporidium, also with nearly 2,000 cases. (See table.)
Hemolytic uremic syndrome (HUS)
HUS rates, mostly resulting from E. coli 0157 H7 in meat, did not vary from 2013 to 2016, with a total 62 pediatric HUS cases in FoodNet (0.56 /100,000 population). Slightly over half (56%) occurred in children under 5 years old at 1.18 per 100,000 population.
Does CIDT increase detection rates?
Detection of the “big two” did not change from 2013 to 2016 or over the past 2 decades. That said, Campylobacter detection was actually down 11% if considering only culture-confirmed cases. That is, if we do not count detections made exclusively by CIDT.
This is important because CIDT – now supplanting culture in many laboratories – identifies pathogens not likely detected by standard culture because culture is generally selective and CIDT is more sensitive. CIDT can increase detection rates (solo and multiple pathogens), even if illnesses do not really increase. The CDC suggested that this contributed to increased STEC and Yersinia detection in 2016. Some would not have been detected if only culture had been utilized.
Viable bacterial/viral isolates are not available from CIDT. A replicating pathogen is needed to characterize shifting/emerging pathogen strains (for example, analysis for mutations or new pathogens via sequencing or antimicrobial susceptibility testing).
To compensate, some CIDT-using laboratories perform “reflex cultures.” CIDT positive specimens also are cultured to provide viable isolates. However, this adds cost to an already costly CIDT test.
The role of imported food
Surveillance systems, such as the Foodborne Disease Outbreak Surveillance System, also track imported foodborne illness. Despite an approximately 50% decrease in overall U.S. foodborne outbreaks since 2000, imported food-related outbreaks increased to 195 during 2006-2014 from 54 during 1996-2004, with 10,685 illnesses, 1,017 hospitalizations, and 19 deaths since 2009. Also, imported food-related outbreaks rose from a mean 3 per year pre-2000 to a mean 18 per year during 2009-2014. Most imported food outbreaks (86% of total) had three causes: scombroid toxin (42% of total), Salmonella (33%), and hepatitis A virus (11%).

Most imported food illnesses were from Salmonella (4,421 from 52 outbreaks), Cyclospora (2,533 from 33 outbreaks), hepatitis A virus (1,150 from 11 outbreaks), and Shigella (625 from 6 outbreaks). While eggs, ice cream, and poultry are notorious origins for Salmonella in domestic food, most imported Salmonella were from produce: fruits (26%), seeded vegetables (20%), sprouts (11%), nuts/seeds (10%), spices (7%), and herbs (2%).
Seafood/fish caused 55% of outbreaks but few illnesses per outbreak (median 3 illnesses/outbreak), so only 11% of total illnesses were caused by seafood/fish. In contrast, fresh produce caused only 33% of outbreaks but 84% of illnesses (median 40 illnesses/outbreak).
Geographic source, outbreak locations
The origin was known in 91% of outbreaks. Latin America and the Caribbean were most common, followed by Asia.3 Main contributing countries were Mexico (42 outbreaks), Indonesia (17) and Canada (11).
Contaminated fish/shellfish originated from all regions except Europe, most commonly from Asia (the majority of fish/shellfish outbreaks were from Indonesia, Vietnam, China, Philippines, Taiwan, and Thailand) with smaller contributions from the Bahamas and Ecuador.
Contaminated produce originated from all regions, mostly (64%) from Mexico and the Americas (Chile, Guatemala, and Honduras). All but one dairy outbreak originated in Latin America/the Caribbean.3 Outbreaks occurred in 31 states, most commonly California (30), Florida (25), and New York (16). Additionally, 43 (22%) were multistate outbreaks.
Conclusions
Outbreaks from domestic foods decreased, but those from imported foods increased. This makes sense given recent increases in outbreak-prone food imports, such as seafood/fish and produce.
To reduce overall foodborne illness outbreaks, governmental agencies need to:
- Develop/enforce regulations that promote proper growing, handling, and processing of foods.
- Strengthen surveillance networks and share standard culture and molecular detection/characterization protocols to identify outbreaks as close to real time as possible.
- Ensure rapid traceability not only to country of origin but to an exact farm or seafood/fish harvesting entity.
- Provide rapid public knowledge of outbreaks and origins, plus outbreak-specific recommendations to control/minimize resultant illnesses.
Individuals can help protect themselves by avoiding inadequately washed or incompletely cooked foods or foods of uncertain origin.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2017 Apr 21;66(15):397-403.
2. Emerg Infect Dis. 2017 Mar;23(3):525-8.
3. Technical appendix in Emerg Infect Dis. 2017 Mar;23(3):525-8.
Are foodborne illness outbreaks more common now, or are we simply better at detection? Have the foods and sources associated with foodborne illness changed? Two recent Centers for Disease Control & Prevention reports provide insight.1,2 In 2016, the Foodborne Diseases Active Surveillance Network (FoodNet) detected 24,029 infections, 5,212 hospitalizations, and 98 fatalities.1 FoodNet has 10 sites serving 49 million people (15% of the U.S. population). These 2016 numbers changed only modestly from the 3 prior years.
The big two
, detected by traditional cultures or culture-independent diagnostic tests (CIDTs). (See table.) CIDTs are relatively new molecular-based, mostly multiplex assays that test for more than a dozen pathogens in one assay.
Overall, Salmonella originated from diverse sources (eggs, poultry, meat, unpasteurized milk/juice/cheese, or raw fruits/vegetables/spices/nuts). But, in 2016, U.S. Salmonella outbreaks were from eggs, alfalfa sprouts, poultry, pistachios, and organic shake/meal products.
The runners-up
Most of the remainder of the 2016 foodborne illnesses were caused by Shigella, with nearly 3,000 cases; shigatoxin-producing Escherichia coli (STEC), with nearly 2,000 cases; and Cryptosporidium, also with nearly 2,000 cases. (See table.)
Hemolytic uremic syndrome (HUS)
HUS rates, mostly resulting from E. coli 0157 H7 in meat, did not vary from 2013 to 2016, with a total 62 pediatric HUS cases in FoodNet (0.56 /100,000 population). Slightly over half (56%) occurred in children under 5 years old at 1.18 per 100,000 population.
Does CIDT increase detection rates?
Detection of the “big two” did not change from 2013 to 2016 or over the past 2 decades. That said, Campylobacter detection was actually down 11% if considering only culture-confirmed cases. That is, if we do not count detections made exclusively by CIDT.
This is important because CIDT – now supplanting culture in many laboratories – identifies pathogens not likely detected by standard culture because culture is generally selective and CIDT is more sensitive. CIDT can increase detection rates (solo and multiple pathogens), even if illnesses do not really increase. The CDC suggested that this contributed to increased STEC and Yersinia detection in 2016. Some would not have been detected if only culture had been utilized.
Viable bacterial/viral isolates are not available from CIDT. A replicating pathogen is needed to characterize shifting/emerging pathogen strains (for example, analysis for mutations or new pathogens via sequencing or antimicrobial susceptibility testing).
To compensate, some CIDT-using laboratories perform “reflex cultures.” CIDT positive specimens also are cultured to provide viable isolates. However, this adds cost to an already costly CIDT test.
The role of imported food
Surveillance systems, such as the Foodborne Disease Outbreak Surveillance System, also track imported foodborne illness. Despite an approximately 50% decrease in overall U.S. foodborne outbreaks since 2000, imported food-related outbreaks increased to 195 during 2006-2014 from 54 during 1996-2004, with 10,685 illnesses, 1,017 hospitalizations, and 19 deaths since 2009. Also, imported food-related outbreaks rose from a mean 3 per year pre-2000 to a mean 18 per year during 2009-2014. Most imported food outbreaks (86% of total) had three causes: scombroid toxin (42% of total), Salmonella (33%), and hepatitis A virus (11%).

Most imported food illnesses were from Salmonella (4,421 from 52 outbreaks), Cyclospora (2,533 from 33 outbreaks), hepatitis A virus (1,150 from 11 outbreaks), and Shigella (625 from 6 outbreaks). While eggs, ice cream, and poultry are notorious origins for Salmonella in domestic food, most imported Salmonella were from produce: fruits (26%), seeded vegetables (20%), sprouts (11%), nuts/seeds (10%), spices (7%), and herbs (2%).
Seafood/fish caused 55% of outbreaks but few illnesses per outbreak (median 3 illnesses/outbreak), so only 11% of total illnesses were caused by seafood/fish. In contrast, fresh produce caused only 33% of outbreaks but 84% of illnesses (median 40 illnesses/outbreak).
Geographic source, outbreak locations
The origin was known in 91% of outbreaks. Latin America and the Caribbean were most common, followed by Asia.3 Main contributing countries were Mexico (42 outbreaks), Indonesia (17) and Canada (11).
Contaminated fish/shellfish originated from all regions except Europe, most commonly from Asia (the majority of fish/shellfish outbreaks were from Indonesia, Vietnam, China, Philippines, Taiwan, and Thailand) with smaller contributions from the Bahamas and Ecuador.
Contaminated produce originated from all regions, mostly (64%) from Mexico and the Americas (Chile, Guatemala, and Honduras). All but one dairy outbreak originated in Latin America/the Caribbean.3 Outbreaks occurred in 31 states, most commonly California (30), Florida (25), and New York (16). Additionally, 43 (22%) were multistate outbreaks.
Conclusions
Outbreaks from domestic foods decreased, but those from imported foods increased. This makes sense given recent increases in outbreak-prone food imports, such as seafood/fish and produce.
To reduce overall foodborne illness outbreaks, governmental agencies need to:
- Develop/enforce regulations that promote proper growing, handling, and processing of foods.
- Strengthen surveillance networks and share standard culture and molecular detection/characterization protocols to identify outbreaks as close to real time as possible.
- Ensure rapid traceability not only to country of origin but to an exact farm or seafood/fish harvesting entity.
- Provide rapid public knowledge of outbreaks and origins, plus outbreak-specific recommendations to control/minimize resultant illnesses.
Individuals can help protect themselves by avoiding inadequately washed or incompletely cooked foods or foods of uncertain origin.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. MMWR. 2017 Apr 21;66(15):397-403.
2. Emerg Infect Dis. 2017 Mar;23(3):525-8.
3. Technical appendix in Emerg Infect Dis. 2017 Mar;23(3):525-8.
International travel vaccination updates
There are several things you should know about necessary vaccinations, and sometimes potential supply problems, if your families will be traveling internationally.
Yellow fever and vaccine supply
Yellow fever is caused by a Flavivirus transmitted by the bite of an infected mosquito. It occurs in sub-Saharan Africa and in tropical areas in South America. Multiple factors determine a traveler’s risk for acquisition, including destination, season, duration of potential exposure, activities, and the local transmission rate. The majority of those infected are asymptomatic or have minimal clinical symptoms. The incubation period is 3-6 days, which is then followed by an influenza-like illness. Approximately 15% of infected individuals develop more serious symptoms including jaundice, hemorrhagic symptoms, shock, and, ultimately, multiorgan system failure with a fatality rate of 90%. There is no specific treatment.
Previously, vaccine boosters were required every 10 years. However, the duration of immunity was extensively reviewed by the World Health Organization and effective July 11, 2016, boosters are no longer required. A single dose of vaccine is now valid for the lifetime of the individual. This includes those persons vaccinated prior to July 11, 2016. Since it is a live vaccine, administration is contraindicated in certain individuals. Exemption letters are provided for those who have a medical contraindication.
Caution is advised in persons receiving their initial dose of YF-VAX who are older than 60 years of age because they have an increased risk of serious side effects. This is not a concern for the pediatrician. The vaccine can only be administered at state approved facilities. It is one vaccine that is not only recommended, but may be required for entry into certain countries. Go to www.cdc.gov/yellowfever for a complete list.
Sanofi Pasteur is the only U.S. manufacturer of YF-VAX. Production has ceased until mid-2018, when a new manufacturing facility will open. Current supplies are anticipated to be depleted by mid-2017, and orders have been limited to 5 doses per month. Sanofi Pasteur, in conjunction with the Food and Drug Administration, will make Stamaril – a yellow fever vaccine manufactured by the company in France and licensed in over 70 countries – available to U.S. travelers through an Expanded Access Investigational New Drug Application. Details on how and when this program will be operational are forthcoming. What is known is that, nationwide, there will be a limited number of sites administering Stamaril. Once finalized, a list of locations will be posted on the CDC Yellow Fever site.
How does this affect your patients? If travel to a yellow fever risk area is anticipated, they should not delay in seeking pretravel advice and immunizations until the last minute. Individual clinic inventories will not be stable. Postponing a trip or changing destinations is preferred if the vaccine is not available. Yellow fever exemption letters are only provided for those persons who have a medical contraindication to receive YF-VAX.
Zika, dengue, and chikungunya
These three Flaviviruses all are transmitted by mosquitoes and can present with fever, rash, and headache. Their distribution is overlapping in several parts of the world. Most infected people are asymptomatic. If symptoms develop, they usually are self-limited. Disease prevention is by mosquito avoidance. There are no preventive vaccines.
Zika virus is the only one associated with a congenital syndrome. It is characterized by brain abnormalities with or without microcephaly, neural tube defects, and ocular abnormalities.
Guidelines for the evaluation and management of Zika virus–exposed infants were initially published in January, 2016, with the most recent update published in August 2016 (MMWR Morb Mortal Wkly Rep. 2016 Aug 26;65[33]:870-8).
Preliminary data from the U.S. Zika pregnancy registry of 442 completed pregnancies between Jan. 15 to Sept. 22, 2016, identified birth defects in 26 fetuses/ infants (6%). There were 21 infants with birth defects among 395 live births and 5 fetuses with birth defects among 47 pregnancy losses. Birth defects were reported for 16 of 271 (6%) asymptomatic and 10 of 167 (6%) symptomatic women. There were no birth defects in infants when exposure occurred after the first trimester. Of the 26 affected infants, 4 had microcephaly and no neuroimaging and 3 (12%) had no fetal or infant testing. Approximately 41% (82/442) of infants did not have Zika virus testing (JAMA. 2017 Jan 3;317[1]:59-68).
It is unclear why testing was not performed. One concern is that the pediatrician may not have been aware of the maternal Zika virus exposure or test results. It may behoove us to begin asking questions about parental international travel to provide optimal management for our patients. We also should be familiar with the current guidelines for evaluating any potentially exposed infants, which include postnatal neuroimaging, Zika virus testing, a comprehensive newborn examination including neurologic exam, and a standard newborn hearing screen prior to hospital discharge.
Regardless of maternal Zika virus test results, infants with any clinical findings suggestive of congenital Zika virus syndrome and possible maternal exposure based on epidemiologic link also should be tested. Zika virus travel alerts and the most up to date information can be found on the Centers for Disease Control and Prevention website (www. cdc.gov/Zika).
Measles
Although endemic measles was eliminated in the United States in 2000, it is still common in many countries in Europe, Africa, and the Pacific. Most cases in the United States occur in unvaccinated individuals, with 78 cases reported in 2016. As of March 25, 2017, 28 cases have been reported. At least 10 countries – including Belgium, France, Italy, Germany, Portugal, and Thailand – have reported outbreaks of measles since April 2017. As reminder, all children aged 6-11 months should receive one dose of MMR and those 12 months or older should receive two doses of MMR at least 28 days apart if international travel is planned. Adults born after 1956 also should have received two doses of MMR prior to international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported having no relevant financial disclosures.
There are several things you should know about necessary vaccinations, and sometimes potential supply problems, if your families will be traveling internationally.
Yellow fever and vaccine supply
Yellow fever is caused by a Flavivirus transmitted by the bite of an infected mosquito. It occurs in sub-Saharan Africa and in tropical areas in South America. Multiple factors determine a traveler’s risk for acquisition, including destination, season, duration of potential exposure, activities, and the local transmission rate. The majority of those infected are asymptomatic or have minimal clinical symptoms. The incubation period is 3-6 days, which is then followed by an influenza-like illness. Approximately 15% of infected individuals develop more serious symptoms including jaundice, hemorrhagic symptoms, shock, and, ultimately, multiorgan system failure with a fatality rate of 90%. There is no specific treatment.
Previously, vaccine boosters were required every 10 years. However, the duration of immunity was extensively reviewed by the World Health Organization and effective July 11, 2016, boosters are no longer required. A single dose of vaccine is now valid for the lifetime of the individual. This includes those persons vaccinated prior to July 11, 2016. Since it is a live vaccine, administration is contraindicated in certain individuals. Exemption letters are provided for those who have a medical contraindication.
Caution is advised in persons receiving their initial dose of YF-VAX who are older than 60 years of age because they have an increased risk of serious side effects. This is not a concern for the pediatrician. The vaccine can only be administered at state approved facilities. It is one vaccine that is not only recommended, but may be required for entry into certain countries. Go to www.cdc.gov/yellowfever for a complete list.
Sanofi Pasteur is the only U.S. manufacturer of YF-VAX. Production has ceased until mid-2018, when a new manufacturing facility will open. Current supplies are anticipated to be depleted by mid-2017, and orders have been limited to 5 doses per month. Sanofi Pasteur, in conjunction with the Food and Drug Administration, will make Stamaril – a yellow fever vaccine manufactured by the company in France and licensed in over 70 countries – available to U.S. travelers through an Expanded Access Investigational New Drug Application. Details on how and when this program will be operational are forthcoming. What is known is that, nationwide, there will be a limited number of sites administering Stamaril. Once finalized, a list of locations will be posted on the CDC Yellow Fever site.
How does this affect your patients? If travel to a yellow fever risk area is anticipated, they should not delay in seeking pretravel advice and immunizations until the last minute. Individual clinic inventories will not be stable. Postponing a trip or changing destinations is preferred if the vaccine is not available. Yellow fever exemption letters are only provided for those persons who have a medical contraindication to receive YF-VAX.
Zika, dengue, and chikungunya
These three Flaviviruses all are transmitted by mosquitoes and can present with fever, rash, and headache. Their distribution is overlapping in several parts of the world. Most infected people are asymptomatic. If symptoms develop, they usually are self-limited. Disease prevention is by mosquito avoidance. There are no preventive vaccines.
Zika virus is the only one associated with a congenital syndrome. It is characterized by brain abnormalities with or without microcephaly, neural tube defects, and ocular abnormalities.
Guidelines for the evaluation and management of Zika virus–exposed infants were initially published in January, 2016, with the most recent update published in August 2016 (MMWR Morb Mortal Wkly Rep. 2016 Aug 26;65[33]:870-8).
Preliminary data from the U.S. Zika pregnancy registry of 442 completed pregnancies between Jan. 15 to Sept. 22, 2016, identified birth defects in 26 fetuses/ infants (6%). There were 21 infants with birth defects among 395 live births and 5 fetuses with birth defects among 47 pregnancy losses. Birth defects were reported for 16 of 271 (6%) asymptomatic and 10 of 167 (6%) symptomatic women. There were no birth defects in infants when exposure occurred after the first trimester. Of the 26 affected infants, 4 had microcephaly and no neuroimaging and 3 (12%) had no fetal or infant testing. Approximately 41% (82/442) of infants did not have Zika virus testing (JAMA. 2017 Jan 3;317[1]:59-68).
It is unclear why testing was not performed. One concern is that the pediatrician may not have been aware of the maternal Zika virus exposure or test results. It may behoove us to begin asking questions about parental international travel to provide optimal management for our patients. We also should be familiar with the current guidelines for evaluating any potentially exposed infants, which include postnatal neuroimaging, Zika virus testing, a comprehensive newborn examination including neurologic exam, and a standard newborn hearing screen prior to hospital discharge.
Regardless of maternal Zika virus test results, infants with any clinical findings suggestive of congenital Zika virus syndrome and possible maternal exposure based on epidemiologic link also should be tested. Zika virus travel alerts and the most up to date information can be found on the Centers for Disease Control and Prevention website (www. cdc.gov/Zika).
Measles
Although endemic measles was eliminated in the United States in 2000, it is still common in many countries in Europe, Africa, and the Pacific. Most cases in the United States occur in unvaccinated individuals, with 78 cases reported in 2016. As of March 25, 2017, 28 cases have been reported. At least 10 countries – including Belgium, France, Italy, Germany, Portugal, and Thailand – have reported outbreaks of measles since April 2017. As reminder, all children aged 6-11 months should receive one dose of MMR and those 12 months or older should receive two doses of MMR at least 28 days apart if international travel is planned. Adults born after 1956 also should have received two doses of MMR prior to international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported having no relevant financial disclosures.
There are several things you should know about necessary vaccinations, and sometimes potential supply problems, if your families will be traveling internationally.
Yellow fever and vaccine supply
Yellow fever is caused by a Flavivirus transmitted by the bite of an infected mosquito. It occurs in sub-Saharan Africa and in tropical areas in South America. Multiple factors determine a traveler’s risk for acquisition, including destination, season, duration of potential exposure, activities, and the local transmission rate. The majority of those infected are asymptomatic or have minimal clinical symptoms. The incubation period is 3-6 days, which is then followed by an influenza-like illness. Approximately 15% of infected individuals develop more serious symptoms including jaundice, hemorrhagic symptoms, shock, and, ultimately, multiorgan system failure with a fatality rate of 90%. There is no specific treatment.
Previously, vaccine boosters were required every 10 years. However, the duration of immunity was extensively reviewed by the World Health Organization and effective July 11, 2016, boosters are no longer required. A single dose of vaccine is now valid for the lifetime of the individual. This includes those persons vaccinated prior to July 11, 2016. Since it is a live vaccine, administration is contraindicated in certain individuals. Exemption letters are provided for those who have a medical contraindication.
Caution is advised in persons receiving their initial dose of YF-VAX who are older than 60 years of age because they have an increased risk of serious side effects. This is not a concern for the pediatrician. The vaccine can only be administered at state approved facilities. It is one vaccine that is not only recommended, but may be required for entry into certain countries. Go to www.cdc.gov/yellowfever for a complete list.
Sanofi Pasteur is the only U.S. manufacturer of YF-VAX. Production has ceased until mid-2018, when a new manufacturing facility will open. Current supplies are anticipated to be depleted by mid-2017, and orders have been limited to 5 doses per month. Sanofi Pasteur, in conjunction with the Food and Drug Administration, will make Stamaril – a yellow fever vaccine manufactured by the company in France and licensed in over 70 countries – available to U.S. travelers through an Expanded Access Investigational New Drug Application. Details on how and when this program will be operational are forthcoming. What is known is that, nationwide, there will be a limited number of sites administering Stamaril. Once finalized, a list of locations will be posted on the CDC Yellow Fever site.
How does this affect your patients? If travel to a yellow fever risk area is anticipated, they should not delay in seeking pretravel advice and immunizations until the last minute. Individual clinic inventories will not be stable. Postponing a trip or changing destinations is preferred if the vaccine is not available. Yellow fever exemption letters are only provided for those persons who have a medical contraindication to receive YF-VAX.
Zika, dengue, and chikungunya
These three Flaviviruses all are transmitted by mosquitoes and can present with fever, rash, and headache. Their distribution is overlapping in several parts of the world. Most infected people are asymptomatic. If symptoms develop, they usually are self-limited. Disease prevention is by mosquito avoidance. There are no preventive vaccines.
Zika virus is the only one associated with a congenital syndrome. It is characterized by brain abnormalities with or without microcephaly, neural tube defects, and ocular abnormalities.
Guidelines for the evaluation and management of Zika virus–exposed infants were initially published in January, 2016, with the most recent update published in August 2016 (MMWR Morb Mortal Wkly Rep. 2016 Aug 26;65[33]:870-8).
Preliminary data from the U.S. Zika pregnancy registry of 442 completed pregnancies between Jan. 15 to Sept. 22, 2016, identified birth defects in 26 fetuses/ infants (6%). There were 21 infants with birth defects among 395 live births and 5 fetuses with birth defects among 47 pregnancy losses. Birth defects were reported for 16 of 271 (6%) asymptomatic and 10 of 167 (6%) symptomatic women. There were no birth defects in infants when exposure occurred after the first trimester. Of the 26 affected infants, 4 had microcephaly and no neuroimaging and 3 (12%) had no fetal or infant testing. Approximately 41% (82/442) of infants did not have Zika virus testing (JAMA. 2017 Jan 3;317[1]:59-68).
It is unclear why testing was not performed. One concern is that the pediatrician may not have been aware of the maternal Zika virus exposure or test results. It may behoove us to begin asking questions about parental international travel to provide optimal management for our patients. We also should be familiar with the current guidelines for evaluating any potentially exposed infants, which include postnatal neuroimaging, Zika virus testing, a comprehensive newborn examination including neurologic exam, and a standard newborn hearing screen prior to hospital discharge.
Regardless of maternal Zika virus test results, infants with any clinical findings suggestive of congenital Zika virus syndrome and possible maternal exposure based on epidemiologic link also should be tested. Zika virus travel alerts and the most up to date information can be found on the Centers for Disease Control and Prevention website (www. cdc.gov/Zika).
Measles
Although endemic measles was eliminated in the United States in 2000, it is still common in many countries in Europe, Africa, and the Pacific. Most cases in the United States occur in unvaccinated individuals, with 78 cases reported in 2016. As of March 25, 2017, 28 cases have been reported. At least 10 countries – including Belgium, France, Italy, Germany, Portugal, and Thailand – have reported outbreaks of measles since April 2017. As reminder, all children aged 6-11 months should receive one dose of MMR and those 12 months or older should receive two doses of MMR at least 28 days apart if international travel is planned. Adults born after 1956 also should have received two doses of MMR prior to international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported having no relevant financial disclosures.
Five-day treatment of ear infections
In December 2016, the results of a randomized, controlled trial of 5-day vs. 10-day amoxicillin/clavulanate treatment of acute otitis media (AOM) in children aged 6-23 months was reported by Hoberman et al. in the New England Journal of Medicine (NEJM).1 Predefined criteria for clinical failure were used that considered both symptoms and signs of AOM, assessed on days 12-14 after start of treatment with 5 vs. 10 days of treatment with the antibiotic. The conclusion reached was clear: The clinical failure rate for the 5-day regimen was 34% vs. 16% in the 10-day group, supporting a preference for the 10-day treatment.
I was surprised. The clinical failure rate for the 5-day regimen seemed very high for treatment with amoxicillin/clavulanate. If it is 34% with amoxicillin/clavulanate, then what would it have been with amoxicillin, as recommended by the American Academy of Pediatrics?
So, why did the systematic review conclude that there was a minimal difference between shortened treatments and the standard 10-day when the NEJM study reported such a striking difference?
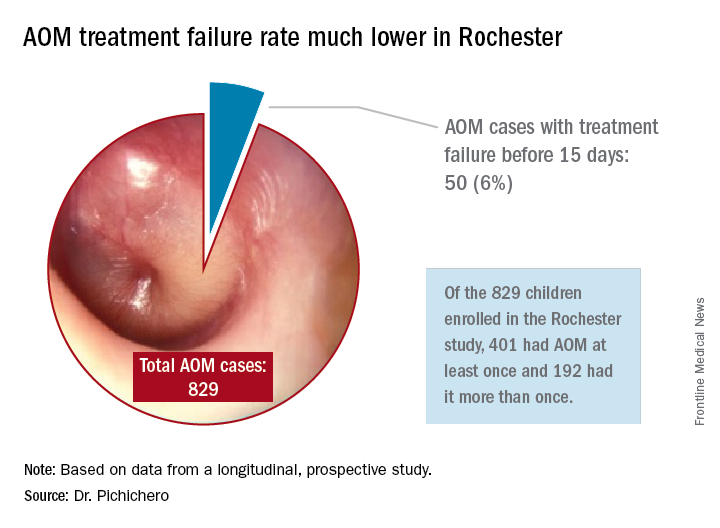
In Rochester, N.Y., we have been conducting a longitudinal, prospective study of AOM that is NIH-sponsored to better understand the immune response to AOM, especially in otitis-prone children.3,4 In that study we are treating all children aged 6-23 months with amoxicillin/clavulanate using the same dose as used in the study by Hoberman et al. We have two exceptions: If the child has a second AOM within 30 days of a prior episode or they have an eardrum rupture, we treat for 10 days.5 Our clinical failure rate is 6%. Why is the failure rate in Rochester so much lower than that in Pittsburgh and Bardstown, Ky., where the Hoberman et al. study was done?
One possibility is an important difference in our study design, compared with that of the NEJM study. All the children in our prospective study have a tympanocentesis to confirm the clinical diagnosis, and our research has shown that tympanocentesis results in immediate relief of ear pain and reduces the frequency of antibiotic treatment failure about twofold, compared with children diagnosed and treated by the same physicians in the same clinic practice.6 So, if the tympanocentesis is factored out of the equation, the Rochester clinical failure comes out to 14% for 5-day treatment. Why would the children in Rochester not getting a tympanocentesis, being treated with the same antibiotic, same dose, and same definition of clinical failure, during the same time frame, and having the same bacteria with the same antibiotic resistance rates have a clinical failure rate of 14%, compared with the 34% in the NEJM study?
Next question: How does a clinical failure rate of 34% fit according to past studies of shortened course antibiotic treatment of AOM? Besides the systematic review and meta-analysis noted above, in many countries outside the United States the 5-day regimen is standard, so, if health care providers were seeing a 34% failure rate, that would have been noticeable for sure.8 So, if health care providers were seeing a 34% failure rate, would that not have been noticeable? And would not a 16% failure rate, nearly 1 of 5 cases, be noticeable for children treated for 10 days?
Was there something different about the children who were in the Hoberman et al. study and the children treated in countries outside the United States and in our practice in Rochester? My group has collaborated and published on studies of AOM with the Pittsburgh and Kentucky groups, and we have not found significant site to site differences in outcomes, demonstrating that a population difference is unlikely.9-11
Next question: How does a clinical failure rate of 16% fit according to past studies of 10 days’ antibiotic treatment of AOM? It is on target with the meta-analysis and two other recent studies in the NEJM.12,13 However, if the failure rate was 16% with amoxicillin/clavulanate (which is effective against beta-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, whereas amoxicillin is not), then the predicted failure rate with amoxicillin for 10 days should be double (34%) or triple (51%) had amoxicillin been used as recommended by the AAP in light of the bacterial resistance of otopathogens. That calculation is based on the prevalence of beta-lactamase–producing H. influenzae and M. catarrhalis in the Pittsburgh and Kentucky populations, the same prevalence seen in the Rochester population.” 14
So, I conclude that this wonderful study does not convince me to change my practice from standard use of 5-day amoxicillin/clavulanate treatment of AOM. Besides, outside of a study setting, most parents don’t give the full 10-day treatment. They stop when their child seems normal (a few days after starting treatment) and save the remainder of the medicine in the refrigerator for the next illness to save a trip to the doctor. Plus, in this column, I did not even get into the issue of disturbing the microbiome with longer courses of antibiotic treatment, a topic for a future discussion.
References
1. N Engl J Med. 2016 Dec 22;375(25):2446-56.
2. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD001095.
3. Pediatr Infect Dis J. 2016 Sep;35(9):1027-32.
4. Pediatr Infect Dis J. 2016 Sep;35(9):1033-9.
5. Otolaryngol Head Neck Surg. 2001 Apr;124(4):381-7.
6. Pediatr Infect Dis J. 2013 May;32(5):473-8.
7. Pediatr Infect Dis J. 2006 Mar;25(3):211-8.
8. Pediatr Infect Dis J. 2000 Sep;19(9):929-37.
9. Pediatr Infect Dis J. 1999 Aug;18(8):741-4.
10. Clin Pediatr (Phila). 2008 Nov;47(9):901-6.
11. Drugs. 2012 Oct 22;72(15):1991-7.
12. N Engl J Med. 2011 Jan 13;364(2):105-15.
13. N Engl J Med. 2011 Jan 13;364(2):116-26.
14. Pediatr Infect Dis J. 2016 Aug;35(8):901-6.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
In December 2016, the results of a randomized, controlled trial of 5-day vs. 10-day amoxicillin/clavulanate treatment of acute otitis media (AOM) in children aged 6-23 months was reported by Hoberman et al. in the New England Journal of Medicine (NEJM).1 Predefined criteria for clinical failure were used that considered both symptoms and signs of AOM, assessed on days 12-14 after start of treatment with 5 vs. 10 days of treatment with the antibiotic. The conclusion reached was clear: The clinical failure rate for the 5-day regimen was 34% vs. 16% in the 10-day group, supporting a preference for the 10-day treatment.
I was surprised. The clinical failure rate for the 5-day regimen seemed very high for treatment with amoxicillin/clavulanate. If it is 34% with amoxicillin/clavulanate, then what would it have been with amoxicillin, as recommended by the American Academy of Pediatrics?
So, why did the systematic review conclude that there was a minimal difference between shortened treatments and the standard 10-day when the NEJM study reported such a striking difference?
In Rochester, N.Y., we have been conducting a longitudinal, prospective study of AOM that is NIH-sponsored to better understand the immune response to AOM, especially in otitis-prone children.3,4 In that study we are treating all children aged 6-23 months with amoxicillin/clavulanate using the same dose as used in the study by Hoberman et al. We have two exceptions: If the child has a second AOM within 30 days of a prior episode or they have an eardrum rupture, we treat for 10 days.5 Our clinical failure rate is 6%. Why is the failure rate in Rochester so much lower than that in Pittsburgh and Bardstown, Ky., where the Hoberman et al. study was done?
One possibility is an important difference in our study design, compared with that of the NEJM study. All the children in our prospective study have a tympanocentesis to confirm the clinical diagnosis, and our research has shown that tympanocentesis results in immediate relief of ear pain and reduces the frequency of antibiotic treatment failure about twofold, compared with children diagnosed and treated by the same physicians in the same clinic practice.6 So, if the tympanocentesis is factored out of the equation, the Rochester clinical failure comes out to 14% for 5-day treatment. Why would the children in Rochester not getting a tympanocentesis, being treated with the same antibiotic, same dose, and same definition of clinical failure, during the same time frame, and having the same bacteria with the same antibiotic resistance rates have a clinical failure rate of 14%, compared with the 34% in the NEJM study?

Next question: How does a clinical failure rate of 34% fit according to past studies of shortened course antibiotic treatment of AOM? Besides the systematic review and meta-analysis noted above, in many countries outside the United States the 5-day regimen is standard, so, if health care providers were seeing a 34% failure rate, that would have been noticeable for sure.8 So, if health care providers were seeing a 34% failure rate, would that not have been noticeable? And would not a 16% failure rate, nearly 1 of 5 cases, be noticeable for children treated for 10 days?
Was there something different about the children who were in the Hoberman et al. study and the children treated in countries outside the United States and in our practice in Rochester? My group has collaborated and published on studies of AOM with the Pittsburgh and Kentucky groups, and we have not found significant site to site differences in outcomes, demonstrating that a population difference is unlikely.9-11
Next question: How does a clinical failure rate of 16% fit according to past studies of 10 days’ antibiotic treatment of AOM? It is on target with the meta-analysis and two other recent studies in the NEJM.12,13 However, if the failure rate was 16% with amoxicillin/clavulanate (which is effective against beta-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, whereas amoxicillin is not), then the predicted failure rate with amoxicillin for 10 days should be double (34%) or triple (51%) had amoxicillin been used as recommended by the AAP in light of the bacterial resistance of otopathogens. That calculation is based on the prevalence of beta-lactamase–producing H. influenzae and M. catarrhalis in the Pittsburgh and Kentucky populations, the same prevalence seen in the Rochester population.” 14
So, I conclude that this wonderful study does not convince me to change my practice from standard use of 5-day amoxicillin/clavulanate treatment of AOM. Besides, outside of a study setting, most parents don’t give the full 10-day treatment. They stop when their child seems normal (a few days after starting treatment) and save the remainder of the medicine in the refrigerator for the next illness to save a trip to the doctor. Plus, in this column, I did not even get into the issue of disturbing the microbiome with longer courses of antibiotic treatment, a topic for a future discussion.
References
1. N Engl J Med. 2016 Dec 22;375(25):2446-56.
2. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD001095.
3. Pediatr Infect Dis J. 2016 Sep;35(9):1027-32.
4. Pediatr Infect Dis J. 2016 Sep;35(9):1033-9.
5. Otolaryngol Head Neck Surg. 2001 Apr;124(4):381-7.
6. Pediatr Infect Dis J. 2013 May;32(5):473-8.
7. Pediatr Infect Dis J. 2006 Mar;25(3):211-8.
8. Pediatr Infect Dis J. 2000 Sep;19(9):929-37.
9. Pediatr Infect Dis J. 1999 Aug;18(8):741-4.
10. Clin Pediatr (Phila). 2008 Nov;47(9):901-6.
11. Drugs. 2012 Oct 22;72(15):1991-7.
12. N Engl J Med. 2011 Jan 13;364(2):105-15.
13. N Engl J Med. 2011 Jan 13;364(2):116-26.
14. Pediatr Infect Dis J. 2016 Aug;35(8):901-6.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
In December 2016, the results of a randomized, controlled trial of 5-day vs. 10-day amoxicillin/clavulanate treatment of acute otitis media (AOM) in children aged 6-23 months was reported by Hoberman et al. in the New England Journal of Medicine (NEJM).1 Predefined criteria for clinical failure were used that considered both symptoms and signs of AOM, assessed on days 12-14 after start of treatment with 5 vs. 10 days of treatment with the antibiotic. The conclusion reached was clear: The clinical failure rate for the 5-day regimen was 34% vs. 16% in the 10-day group, supporting a preference for the 10-day treatment.
I was surprised. The clinical failure rate for the 5-day regimen seemed very high for treatment with amoxicillin/clavulanate. If it is 34% with amoxicillin/clavulanate, then what would it have been with amoxicillin, as recommended by the American Academy of Pediatrics?
So, why did the systematic review conclude that there was a minimal difference between shortened treatments and the standard 10-day when the NEJM study reported such a striking difference?
In Rochester, N.Y., we have been conducting a longitudinal, prospective study of AOM that is NIH-sponsored to better understand the immune response to AOM, especially in otitis-prone children.3,4 In that study we are treating all children aged 6-23 months with amoxicillin/clavulanate using the same dose as used in the study by Hoberman et al. We have two exceptions: If the child has a second AOM within 30 days of a prior episode or they have an eardrum rupture, we treat for 10 days.5 Our clinical failure rate is 6%. Why is the failure rate in Rochester so much lower than that in Pittsburgh and Bardstown, Ky., where the Hoberman et al. study was done?
One possibility is an important difference in our study design, compared with that of the NEJM study. All the children in our prospective study have a tympanocentesis to confirm the clinical diagnosis, and our research has shown that tympanocentesis results in immediate relief of ear pain and reduces the frequency of antibiotic treatment failure about twofold, compared with children diagnosed and treated by the same physicians in the same clinic practice.6 So, if the tympanocentesis is factored out of the equation, the Rochester clinical failure comes out to 14% for 5-day treatment. Why would the children in Rochester not getting a tympanocentesis, being treated with the same antibiotic, same dose, and same definition of clinical failure, during the same time frame, and having the same bacteria with the same antibiotic resistance rates have a clinical failure rate of 14%, compared with the 34% in the NEJM study?

Next question: How does a clinical failure rate of 34% fit according to past studies of shortened course antibiotic treatment of AOM? Besides the systematic review and meta-analysis noted above, in many countries outside the United States the 5-day regimen is standard, so, if health care providers were seeing a 34% failure rate, that would have been noticeable for sure.8 So, if health care providers were seeing a 34% failure rate, would that not have been noticeable? And would not a 16% failure rate, nearly 1 of 5 cases, be noticeable for children treated for 10 days?
Was there something different about the children who were in the Hoberman et al. study and the children treated in countries outside the United States and in our practice in Rochester? My group has collaborated and published on studies of AOM with the Pittsburgh and Kentucky groups, and we have not found significant site to site differences in outcomes, demonstrating that a population difference is unlikely.9-11
Next question: How does a clinical failure rate of 16% fit according to past studies of 10 days’ antibiotic treatment of AOM? It is on target with the meta-analysis and two other recent studies in the NEJM.12,13 However, if the failure rate was 16% with amoxicillin/clavulanate (which is effective against beta-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, whereas amoxicillin is not), then the predicted failure rate with amoxicillin for 10 days should be double (34%) or triple (51%) had amoxicillin been used as recommended by the AAP in light of the bacterial resistance of otopathogens. That calculation is based on the prevalence of beta-lactamase–producing H. influenzae and M. catarrhalis in the Pittsburgh and Kentucky populations, the same prevalence seen in the Rochester population.” 14
So, I conclude that this wonderful study does not convince me to change my practice from standard use of 5-day amoxicillin/clavulanate treatment of AOM. Besides, outside of a study setting, most parents don’t give the full 10-day treatment. They stop when their child seems normal (a few days after starting treatment) and save the remainder of the medicine in the refrigerator for the next illness to save a trip to the doctor. Plus, in this column, I did not even get into the issue of disturbing the microbiome with longer courses of antibiotic treatment, a topic for a future discussion.
References
1. N Engl J Med. 2016 Dec 22;375(25):2446-56.
2. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD001095.
3. Pediatr Infect Dis J. 2016 Sep;35(9):1027-32.
4. Pediatr Infect Dis J. 2016 Sep;35(9):1033-9.
5. Otolaryngol Head Neck Surg. 2001 Apr;124(4):381-7.
6. Pediatr Infect Dis J. 2013 May;32(5):473-8.
7. Pediatr Infect Dis J. 2006 Mar;25(3):211-8.
8. Pediatr Infect Dis J. 2000 Sep;19(9):929-37.
9. Pediatr Infect Dis J. 1999 Aug;18(8):741-4.
10. Clin Pediatr (Phila). 2008 Nov;47(9):901-6.
11. Drugs. 2012 Oct 22;72(15):1991-7.
12. N Engl J Med. 2011 Jan 13;364(2):105-15.
13. N Engl J Med. 2011 Jan 13;364(2):116-26.
14. Pediatr Infect Dis J. 2016 Aug;35(8):901-6.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Mycobacterium tuberculosis: Overcoming one obstacle on the road to elimination
March 24 is World TB Day. It was on this date in 1882 that physician Robert Koch announced the discovery of Mycobacterium tuberculosis, the causative agent of tuberculosis. Worldwide, activities are planned to raise awareness of TB and to support initiatives for prevention, better control, and ultimately the elimination of this disease.
Globally in 2015, the World Health Organization estimated there were 10.4 million new cases of TB, including 1 million in children. Data from the United States reveal that after 20 years of annual decline, the incidence of TB has plateaued. In 2015, 9,563 cases of TB disease were reported, including 440 cases in children less than 15 years of age. While the overall incidence was 3 cases per 100,000, the incidence among foreign-born persons was 15.1 cases per 100,000. There were 3,201 cases (33.5%) among U.S.-born individuals. Foreign-born persons accounted for 66.2% of cases; however, the majority of those cases were diagnosed several years after their arrival in the United States. The top five countries of origin of these individuals were China, India, Mexico, the Philippines, and Vietnam. In contrast, only one-quarter of all pediatric cases occurred in foreign-born children. Four states (California, Florida, New York, and Texas) reported more than 500 cases each in 2015, as they have for the last 7 consecutive years. In 2015, these states accounted for slightly more than half (4,839) of all cases (MMWR 2016 Mar 25;65[11]:273-8).
Why as pediatricians should we be concerned? TB in a child is a sentinel event and represents recent or ongoing transmission. Young children who are infected are more likely to progress to TB disease and develop severe manifestations such as miliary TB or meningitis. Children less than 4 years old and those with certain underlying disorders, including those with an immunodeficiency or who are receiving immunosuppressive agents, also are at greater risk for progression from infection to disease. Other predictors of disease progression include diagnosis of the infection within the past 2 years, use of chemotherapy and high-dose corticosteroids, as well as certain cancers, diabetes, and chronic renal failure.
Once infected, most children and adolescents remain asymptomatic. If disease occurs, symptoms develop 1-6 months after infection and include fever, cough, weight loss or failure to thrive, night sweats, and chills. Chest radiographic findings are nonspecific. Infiltrates and intrathoracic lymph node enlargement may or may not be present. However, our goal is to diagnose at-risk children with infection, treat them, and avoid their progression to TB disease.
Screening tests
The interferon-gamma release assay is a blood test that has a greater specificity than TST and requires only one visit. A positive test is seen in both latent TB infection and TB disease. There is no cross-reaction with BCG. This is the ideal test for prior BCG recipients and others who are unlikely to return for TST readings and are at least 5 years of age.
A chest radiograph is required to differentiate latent TB infection from TB disease. Latent TB infection is diagnosed when there is an absence of parenchymal disease, opacification, or intrathoracic adenopathy.
Treatment of latent TB infection versus TB disease is beyond the scope of this article. Consultation with an infectious disease expert is recommended.
For additional information and resources, go to www.cdc.gov/tb, and for a sample TB risk assessment tool, go to www.cdc.gov/tb/publications/ltbi/appendixa.htm.
As we mark the passing of another World TB Day, we have one goal – to identify, screen, and treat children and adolescents at risk for latent TB infection and help eliminate future cases of TB disease.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
March 24 is World TB Day. It was on this date in 1882 that physician Robert Koch announced the discovery of Mycobacterium tuberculosis, the causative agent of tuberculosis. Worldwide, activities are planned to raise awareness of TB and to support initiatives for prevention, better control, and ultimately the elimination of this disease.
Globally in 2015, the World Health Organization estimated there were 10.4 million new cases of TB, including 1 million in children. Data from the United States reveal that after 20 years of annual decline, the incidence of TB has plateaued. In 2015, 9,563 cases of TB disease were reported, including 440 cases in children less than 15 years of age. While the overall incidence was 3 cases per 100,000, the incidence among foreign-born persons was 15.1 cases per 100,000. There were 3,201 cases (33.5%) among U.S.-born individuals. Foreign-born persons accounted for 66.2% of cases; however, the majority of those cases were diagnosed several years after their arrival in the United States. The top five countries of origin of these individuals were China, India, Mexico, the Philippines, and Vietnam. In contrast, only one-quarter of all pediatric cases occurred in foreign-born children. Four states (California, Florida, New York, and Texas) reported more than 500 cases each in 2015, as they have for the last 7 consecutive years. In 2015, these states accounted for slightly more than half (4,839) of all cases (MMWR 2016 Mar 25;65[11]:273-8).
Why as pediatricians should we be concerned? TB in a child is a sentinel event and represents recent or ongoing transmission. Young children who are infected are more likely to progress to TB disease and develop severe manifestations such as miliary TB or meningitis. Children less than 4 years old and those with certain underlying disorders, including those with an immunodeficiency or who are receiving immunosuppressive agents, also are at greater risk for progression from infection to disease. Other predictors of disease progression include diagnosis of the infection within the past 2 years, use of chemotherapy and high-dose corticosteroids, as well as certain cancers, diabetes, and chronic renal failure.
Once infected, most children and adolescents remain asymptomatic. If disease occurs, symptoms develop 1-6 months after infection and include fever, cough, weight loss or failure to thrive, night sweats, and chills. Chest radiographic findings are nonspecific. Infiltrates and intrathoracic lymph node enlargement may or may not be present. However, our goal is to diagnose at-risk children with infection, treat them, and avoid their progression to TB disease.
Screening tests
The interferon-gamma release assay is a blood test that has a greater specificity than TST and requires only one visit. A positive test is seen in both latent TB infection and TB disease. There is no cross-reaction with BCG. This is the ideal test for prior BCG recipients and others who are unlikely to return for TST readings and are at least 5 years of age.
A chest radiograph is required to differentiate latent TB infection from TB disease. Latent TB infection is diagnosed when there is an absence of parenchymal disease, opacification, or intrathoracic adenopathy.
Treatment of latent TB infection versus TB disease is beyond the scope of this article. Consultation with an infectious disease expert is recommended.
For additional information and resources, go to www.cdc.gov/tb, and for a sample TB risk assessment tool, go to www.cdc.gov/tb/publications/ltbi/appendixa.htm.
As we mark the passing of another World TB Day, we have one goal – to identify, screen, and treat children and adolescents at risk for latent TB infection and help eliminate future cases of TB disease.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
March 24 is World TB Day. It was on this date in 1882 that physician Robert Koch announced the discovery of Mycobacterium tuberculosis, the causative agent of tuberculosis. Worldwide, activities are planned to raise awareness of TB and to support initiatives for prevention, better control, and ultimately the elimination of this disease.
Globally in 2015, the World Health Organization estimated there were 10.4 million new cases of TB, including 1 million in children. Data from the United States reveal that after 20 years of annual decline, the incidence of TB has plateaued. In 2015, 9,563 cases of TB disease were reported, including 440 cases in children less than 15 years of age. While the overall incidence was 3 cases per 100,000, the incidence among foreign-born persons was 15.1 cases per 100,000. There were 3,201 cases (33.5%) among U.S.-born individuals. Foreign-born persons accounted for 66.2% of cases; however, the majority of those cases were diagnosed several years after their arrival in the United States. The top five countries of origin of these individuals were China, India, Mexico, the Philippines, and Vietnam. In contrast, only one-quarter of all pediatric cases occurred in foreign-born children. Four states (California, Florida, New York, and Texas) reported more than 500 cases each in 2015, as they have for the last 7 consecutive years. In 2015, these states accounted for slightly more than half (4,839) of all cases (MMWR 2016 Mar 25;65[11]:273-8).
Why as pediatricians should we be concerned? TB in a child is a sentinel event and represents recent or ongoing transmission. Young children who are infected are more likely to progress to TB disease and develop severe manifestations such as miliary TB or meningitis. Children less than 4 years old and those with certain underlying disorders, including those with an immunodeficiency or who are receiving immunosuppressive agents, also are at greater risk for progression from infection to disease. Other predictors of disease progression include diagnosis of the infection within the past 2 years, use of chemotherapy and high-dose corticosteroids, as well as certain cancers, diabetes, and chronic renal failure.
Once infected, most children and adolescents remain asymptomatic. If disease occurs, symptoms develop 1-6 months after infection and include fever, cough, weight loss or failure to thrive, night sweats, and chills. Chest radiographic findings are nonspecific. Infiltrates and intrathoracic lymph node enlargement may or may not be present. However, our goal is to diagnose at-risk children with infection, treat them, and avoid their progression to TB disease.
Screening tests
The interferon-gamma release assay is a blood test that has a greater specificity than TST and requires only one visit. A positive test is seen in both latent TB infection and TB disease. There is no cross-reaction with BCG. This is the ideal test for prior BCG recipients and others who are unlikely to return for TST readings and are at least 5 years of age.
A chest radiograph is required to differentiate latent TB infection from TB disease. Latent TB infection is diagnosed when there is an absence of parenchymal disease, opacification, or intrathoracic adenopathy.
Treatment of latent TB infection versus TB disease is beyond the scope of this article. Consultation with an infectious disease expert is recommended.
For additional information and resources, go to www.cdc.gov/tb, and for a sample TB risk assessment tool, go to www.cdc.gov/tb/publications/ltbi/appendixa.htm.
As we mark the passing of another World TB Day, we have one goal – to identify, screen, and treat children and adolescents at risk for latent TB infection and help eliminate future cases of TB disease.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It isn’t over until it’s over
Pediatricians take heart.
Yes, I know it is discouraging when families occasionally ignore our advice and refuse vaccines for their children. It is even worse when political leaders who ought to know better question the safety and value of vaccines.
But let’s not lose perspective. Let me share a quick reminder of why vaccines are (almost) universally considered one of the greatest public health achievements of the 20th century.
Not long ago, I reviewed a clinical case with students as part of a medical microbiology course. A 6-year-old girl presented with fever, headache, and flaccid paralysis of the right arm with areflexia. With little prompting, the students generated a short differential diagnosis. Enterovirus. West Nile virus. “I guess we should include polio,” one student offered. “But who gets that anymore?”
A mere 120 years changes everything. At the dawn of the 20th century, we didn’t even know with certainty what caused polio, although infection was suspected.
On Sept. 9, 1954, the Courier-Journal, a newspaper in my hometown of Louisville, Ky., carried a story about the annual number of polio cases in Jefferson County, noting that they had reached 198 and General Hospital had opened a polio ward usually reserved for epidemics. Concerns about the infection were rippling throughout the state, and the paper reported that at least one high school marching band had elected to withdraw from annual Kentucky State Fair competition because of concerns about infection.
My mom was 10 years old in the summer of 1954, and she recalls that it was a “scary” time. Swimming pools closed. Parents refused to allow their children to go to movie theaters or the local amusement park because of fear that they might come into contact with the virus. My mom said, “Then one of my friends was diagnosed with polio. We had played together the week before she got sick. We worried that we were going to get sick, too. And once you got sick, you didn’t necessarily get better.”
I probably don’t need to remind you that both Dr. Sabin and Dr. Salk did develop successful poliovirus vaccines. Dr. Enders, along with junior colleagues Fred C. Robbins, MD, and Thomas H. Weller, MD, developed the techniques to grow poliovirus and other viruses in culture, making the work of Dr. Sabin and Dr. Salk possible. For this, Dr. Enders, Dr. Robbins, and Dr. Weller received the Nobel Prize in 1954.
Regarding the prediction of long-term protection, I’d say we’re there. According to the Centers for Disease Control and Prevention, wild poliovirus cases have declined more than 99.9% since 1988. According to the Global Polio Eradication Initiative, that means that there are approximately 10 million people walking today who would have otherwise been paralyzed by the disease.
In 2015, there were only 74 cases identified in the world, and these were localized to two countries. Even better, a global commission announced that wild poliovirus type 2 had been eradicated from the world. Eradicated. The last known transmission occurred in India in 1999.
Type 3 poliovirus may not be far behind. The last known case of wildtype poliovirus 3 was detected in 2012.
The complete story of poliovirus eradication efforts could read like a suspense novel: There have been twists and turns, some missed deadlines, and now a bit of irony. Success, in large part, has hinged on the use of trivalent, live attenuated oral poliovirus vaccine (tOPV) throughout much of the world. Now eradication of all polio disease is going to require withdrawal of OPV in countries that still use it.
Rarely, the live attenuated vaccine viruses contained in OPV can cause polio, and since 2012, vaccine-derived cases have exceeded wild poliovirus cases. Vaccine-derived cases include vaccine-associated paralytic polio (VAPP) – paralysis occurs in a vaccine recipient or a close contact – as well as cases of circulating vaccine-derived polioviruses (cVDPVs). Remember that vaccine viruses are shed in the stool, and in communities with low immunization rates, they circulate and acquire mutations that confer the transmissibility and neurovirulence properties of wild viruses. Ultimately, cVDPVs lead to outbreaks.
In 2013, the Global Polio Eradication Initiative published a new “endgame plan” for polio that outlined a stepwise approach for removing OPV from immunization programs. First, it called on all countries to introduce at least one dose of inactivated poliovirus vaccine by the third quarter of 2015, immunizing infants at 14 weeks or at first contact thereafter. Second, it called for all countries to replace tOPV with a bivalent vaccine containing only types 1 and 3 by 2016. Given the eradication of wild poliovirus type 2, keeping type 2 in the oral vaccine just creates risk. An estimated 40% of VAPP cases and 98% of cVDPVs detected since 2012 were caused by poliovirus type 2. The type 2 component of tOPV also interferes with the immune response to the other types. Once poliovirus eradication has been achieved and certified, hopefully no later than 2019, all OPV will be withdrawn.
What’s the role of pediatricians in the United States in polio eradication? For now, our job is to continue to protect all children in the United States against all three types of poliovirus. Current Advisory Committee on Immunization Practices (ACIP) recommendations specify 4 doses of trivalent inactivated poliovirus vaccine (IPV) at ages 2 months, 4 months, 6-18 months, and 4-6 years. Children vaccinated outside the United States with bivalent vaccine, including immigrants and refugees, will need to be revaccinated. Those without appropriate documentation of vaccine (written, dated records that specify trivalent vaccine) also should be revaccinated.
Serologic testing for immunity is no longer recommended. In the past, children without documentation of vaccines could be tested for neutralizing antibodies to poliovirus types 1, 2, and 3. Moving forward, serologic testing for antibodies to poliovirus type 2 won’t be available because it requires live virus, and in accordance with World Health Organization recommendations, laboratories have been destroying supplies of poliovirus type 2.
We also need to make sure that our patients who are traveling internationally receive all recommended vaccines, including a dose of IPV when appropriate. Specific recommendations can be found on the CDC’s pages for travelers.
A 2015 statement from the American Academy of Pediatrics called on pediatricians to consider polio as a potential diagnosis of any child presenting with fever and acute flaccid paralysis (Pediatrics. 2015 Jan;135[1]:196-202). When polio is suspected, public health authorities should be notified and two stool samples collected 24 hours apart, and within 14 days of the onset of paralysis, sent for testing. According to lead author Walter A. Orenstein, MD, “because most polio infections are silent, a case of paralytic polio in the United States may have been acquired from an asymptomatic individual, so a history of travel to a polio-infected area may be absent in the case of paralysis.”
I’ll second what my mom said. Scary.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Pediatricians take heart.
Yes, I know it is discouraging when families occasionally ignore our advice and refuse vaccines for their children. It is even worse when political leaders who ought to know better question the safety and value of vaccines.
But let’s not lose perspective. Let me share a quick reminder of why vaccines are (almost) universally considered one of the greatest public health achievements of the 20th century.
Not long ago, I reviewed a clinical case with students as part of a medical microbiology course. A 6-year-old girl presented with fever, headache, and flaccid paralysis of the right arm with areflexia. With little prompting, the students generated a short differential diagnosis. Enterovirus. West Nile virus. “I guess we should include polio,” one student offered. “But who gets that anymore?”
A mere 120 years changes everything. At the dawn of the 20th century, we didn’t even know with certainty what caused polio, although infection was suspected.
On Sept. 9, 1954, the Courier-Journal, a newspaper in my hometown of Louisville, Ky., carried a story about the annual number of polio cases in Jefferson County, noting that they had reached 198 and General Hospital had opened a polio ward usually reserved for epidemics. Concerns about the infection were rippling throughout the state, and the paper reported that at least one high school marching band had elected to withdraw from annual Kentucky State Fair competition because of concerns about infection.
My mom was 10 years old in the summer of 1954, and she recalls that it was a “scary” time. Swimming pools closed. Parents refused to allow their children to go to movie theaters or the local amusement park because of fear that they might come into contact with the virus. My mom said, “Then one of my friends was diagnosed with polio. We had played together the week before she got sick. We worried that we were going to get sick, too. And once you got sick, you didn’t necessarily get better.”
I probably don’t need to remind you that both Dr. Sabin and Dr. Salk did develop successful poliovirus vaccines. Dr. Enders, along with junior colleagues Fred C. Robbins, MD, and Thomas H. Weller, MD, developed the techniques to grow poliovirus and other viruses in culture, making the work of Dr. Sabin and Dr. Salk possible. For this, Dr. Enders, Dr. Robbins, and Dr. Weller received the Nobel Prize in 1954.
Regarding the prediction of long-term protection, I’d say we’re there. According to the Centers for Disease Control and Prevention, wild poliovirus cases have declined more than 99.9% since 1988. According to the Global Polio Eradication Initiative, that means that there are approximately 10 million people walking today who would have otherwise been paralyzed by the disease.
In 2015, there were only 74 cases identified in the world, and these were localized to two countries. Even better, a global commission announced that wild poliovirus type 2 had been eradicated from the world. Eradicated. The last known transmission occurred in India in 1999.
Type 3 poliovirus may not be far behind. The last known case of wildtype poliovirus 3 was detected in 2012.
The complete story of poliovirus eradication efforts could read like a suspense novel: There have been twists and turns, some missed deadlines, and now a bit of irony. Success, in large part, has hinged on the use of trivalent, live attenuated oral poliovirus vaccine (tOPV) throughout much of the world. Now eradication of all polio disease is going to require withdrawal of OPV in countries that still use it.
Rarely, the live attenuated vaccine viruses contained in OPV can cause polio, and since 2012, vaccine-derived cases have exceeded wild poliovirus cases. Vaccine-derived cases include vaccine-associated paralytic polio (VAPP) – paralysis occurs in a vaccine recipient or a close contact – as well as cases of circulating vaccine-derived polioviruses (cVDPVs). Remember that vaccine viruses are shed in the stool, and in communities with low immunization rates, they circulate and acquire mutations that confer the transmissibility and neurovirulence properties of wild viruses. Ultimately, cVDPVs lead to outbreaks.
In 2013, the Global Polio Eradication Initiative published a new “endgame plan” for polio that outlined a stepwise approach for removing OPV from immunization programs. First, it called on all countries to introduce at least one dose of inactivated poliovirus vaccine by the third quarter of 2015, immunizing infants at 14 weeks or at first contact thereafter. Second, it called for all countries to replace tOPV with a bivalent vaccine containing only types 1 and 3 by 2016. Given the eradication of wild poliovirus type 2, keeping type 2 in the oral vaccine just creates risk. An estimated 40% of VAPP cases and 98% of cVDPVs detected since 2012 were caused by poliovirus type 2. The type 2 component of tOPV also interferes with the immune response to the other types. Once poliovirus eradication has been achieved and certified, hopefully no later than 2019, all OPV will be withdrawn.
What’s the role of pediatricians in the United States in polio eradication? For now, our job is to continue to protect all children in the United States against all three types of poliovirus. Current Advisory Committee on Immunization Practices (ACIP) recommendations specify 4 doses of trivalent inactivated poliovirus vaccine (IPV) at ages 2 months, 4 months, 6-18 months, and 4-6 years. Children vaccinated outside the United States with bivalent vaccine, including immigrants and refugees, will need to be revaccinated. Those without appropriate documentation of vaccine (written, dated records that specify trivalent vaccine) also should be revaccinated.
Serologic testing for immunity is no longer recommended. In the past, children without documentation of vaccines could be tested for neutralizing antibodies to poliovirus types 1, 2, and 3. Moving forward, serologic testing for antibodies to poliovirus type 2 won’t be available because it requires live virus, and in accordance with World Health Organization recommendations, laboratories have been destroying supplies of poliovirus type 2.
We also need to make sure that our patients who are traveling internationally receive all recommended vaccines, including a dose of IPV when appropriate. Specific recommendations can be found on the CDC’s pages for travelers.
A 2015 statement from the American Academy of Pediatrics called on pediatricians to consider polio as a potential diagnosis of any child presenting with fever and acute flaccid paralysis (Pediatrics. 2015 Jan;135[1]:196-202). When polio is suspected, public health authorities should be notified and two stool samples collected 24 hours apart, and within 14 days of the onset of paralysis, sent for testing. According to lead author Walter A. Orenstein, MD, “because most polio infections are silent, a case of paralytic polio in the United States may have been acquired from an asymptomatic individual, so a history of travel to a polio-infected area may be absent in the case of paralysis.”
I’ll second what my mom said. Scary.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Pediatricians take heart.
Yes, I know it is discouraging when families occasionally ignore our advice and refuse vaccines for their children. It is even worse when political leaders who ought to know better question the safety and value of vaccines.
But let’s not lose perspective. Let me share a quick reminder of why vaccines are (almost) universally considered one of the greatest public health achievements of the 20th century.
Not long ago, I reviewed a clinical case with students as part of a medical microbiology course. A 6-year-old girl presented with fever, headache, and flaccid paralysis of the right arm with areflexia. With little prompting, the students generated a short differential diagnosis. Enterovirus. West Nile virus. “I guess we should include polio,” one student offered. “But who gets that anymore?”
A mere 120 years changes everything. At the dawn of the 20th century, we didn’t even know with certainty what caused polio, although infection was suspected.
On Sept. 9, 1954, the Courier-Journal, a newspaper in my hometown of Louisville, Ky., carried a story about the annual number of polio cases in Jefferson County, noting that they had reached 198 and General Hospital had opened a polio ward usually reserved for epidemics. Concerns about the infection were rippling throughout the state, and the paper reported that at least one high school marching band had elected to withdraw from annual Kentucky State Fair competition because of concerns about infection.
My mom was 10 years old in the summer of 1954, and she recalls that it was a “scary” time. Swimming pools closed. Parents refused to allow their children to go to movie theaters or the local amusement park because of fear that they might come into contact with the virus. My mom said, “Then one of my friends was diagnosed with polio. We had played together the week before she got sick. We worried that we were going to get sick, too. And once you got sick, you didn’t necessarily get better.”
I probably don’t need to remind you that both Dr. Sabin and Dr. Salk did develop successful poliovirus vaccines. Dr. Enders, along with junior colleagues Fred C. Robbins, MD, and Thomas H. Weller, MD, developed the techniques to grow poliovirus and other viruses in culture, making the work of Dr. Sabin and Dr. Salk possible. For this, Dr. Enders, Dr. Robbins, and Dr. Weller received the Nobel Prize in 1954.
Regarding the prediction of long-term protection, I’d say we’re there. According to the Centers for Disease Control and Prevention, wild poliovirus cases have declined more than 99.9% since 1988. According to the Global Polio Eradication Initiative, that means that there are approximately 10 million people walking today who would have otherwise been paralyzed by the disease.
In 2015, there were only 74 cases identified in the world, and these were localized to two countries. Even better, a global commission announced that wild poliovirus type 2 had been eradicated from the world. Eradicated. The last known transmission occurred in India in 1999.
Type 3 poliovirus may not be far behind. The last known case of wildtype poliovirus 3 was detected in 2012.
The complete story of poliovirus eradication efforts could read like a suspense novel: There have been twists and turns, some missed deadlines, and now a bit of irony. Success, in large part, has hinged on the use of trivalent, live attenuated oral poliovirus vaccine (tOPV) throughout much of the world. Now eradication of all polio disease is going to require withdrawal of OPV in countries that still use it.
Rarely, the live attenuated vaccine viruses contained in OPV can cause polio, and since 2012, vaccine-derived cases have exceeded wild poliovirus cases. Vaccine-derived cases include vaccine-associated paralytic polio (VAPP) – paralysis occurs in a vaccine recipient or a close contact – as well as cases of circulating vaccine-derived polioviruses (cVDPVs). Remember that vaccine viruses are shed in the stool, and in communities with low immunization rates, they circulate and acquire mutations that confer the transmissibility and neurovirulence properties of wild viruses. Ultimately, cVDPVs lead to outbreaks.
In 2013, the Global Polio Eradication Initiative published a new “endgame plan” for polio that outlined a stepwise approach for removing OPV from immunization programs. First, it called on all countries to introduce at least one dose of inactivated poliovirus vaccine by the third quarter of 2015, immunizing infants at 14 weeks or at first contact thereafter. Second, it called for all countries to replace tOPV with a bivalent vaccine containing only types 1 and 3 by 2016. Given the eradication of wild poliovirus type 2, keeping type 2 in the oral vaccine just creates risk. An estimated 40% of VAPP cases and 98% of cVDPVs detected since 2012 were caused by poliovirus type 2. The type 2 component of tOPV also interferes with the immune response to the other types. Once poliovirus eradication has been achieved and certified, hopefully no later than 2019, all OPV will be withdrawn.
What’s the role of pediatricians in the United States in polio eradication? For now, our job is to continue to protect all children in the United States against all three types of poliovirus. Current Advisory Committee on Immunization Practices (ACIP) recommendations specify 4 doses of trivalent inactivated poliovirus vaccine (IPV) at ages 2 months, 4 months, 6-18 months, and 4-6 years. Children vaccinated outside the United States with bivalent vaccine, including immigrants and refugees, will need to be revaccinated. Those without appropriate documentation of vaccine (written, dated records that specify trivalent vaccine) also should be revaccinated.
Serologic testing for immunity is no longer recommended. In the past, children without documentation of vaccines could be tested for neutralizing antibodies to poliovirus types 1, 2, and 3. Moving forward, serologic testing for antibodies to poliovirus type 2 won’t be available because it requires live virus, and in accordance with World Health Organization recommendations, laboratories have been destroying supplies of poliovirus type 2.
We also need to make sure that our patients who are traveling internationally receive all recommended vaccines, including a dose of IPV when appropriate. Specific recommendations can be found on the CDC’s pages for travelers.
A 2015 statement from the American Academy of Pediatrics called on pediatricians to consider polio as a potential diagnosis of any child presenting with fever and acute flaccid paralysis (Pediatrics. 2015 Jan;135[1]:196-202). When polio is suspected, public health authorities should be notified and two stool samples collected 24 hours apart, and within 14 days of the onset of paralysis, sent for testing. According to lead author Walter A. Orenstein, MD, “because most polio infections are silent, a case of paralytic polio in the United States may have been acquired from an asymptomatic individual, so a history of travel to a polio-infected area may be absent in the case of paralysis.”
I’ll second what my mom said. Scary.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Enterovirus D68 – An emerging threat to child health
In August 2014, we first heard of increased pediatric cases of severe respiratory tract disease, many requiring management in the ICU, and of acute flaccid myelitis/paralysis (AFM) of unknown etiology from many states across the United States. Concurrently with this outbreak in the United States, similar clinical cases were reported in Canada and Europe. Subsequently, enterovirus D68 was confirmed in some, but not all, of the paralyzed children. Although new to many of us, enterovirus D68 was already known as an atypical enterovirus sharing many of its structural and chemical properties with rhinovirus. For example, it most often was reported from respiratory samples and less common from stool samples. It also had been associated with clusters of respiratory disease since 2000 and a 2008 case of fatal AFM.
There were 120 cases of AFM, coinciding with the nationwide outbreak of enteroviral D68 disease, reported in 2014. The Centers for Disease Control and Prevention has evaluated the cerebrospinal fluid in many of these cases, and no pathogen has consistently been detected. The children were mostly school age, aged 7-11 years, presented with acute, febrile respiratory illness followed by acute onset of cranial nerve dysfunction or flaccid paralysis of one or more limbs. The CSF revealed mild pleocytosis, most often with mild elevation of protein and a normal glucose. However, the MRI was distinctly abnormal with focal lesion in the cranial nerve nuclei (in those with bulbar dysfunction) and/or in the anterior horn or spinal cord gray matter. Long-term prognosis is unknown, although most patients have persistent weakness, despite some improvement, to date.
In 2016, the CDC has reported an increase in cases after a decline in 2015 despite the absence of epidemic respiratory tract disease in the United States from enterovirus D68. In the Netherlands, an increase in respiratory disease from enterovirus D68 in children and adults also has been reported since June 2016. Respiratory disease has been observed in children as young as 3 months of age, and most of the children have underlying comorbidity, many with asthma or other pulmonary conditions. Thirteen of 17 (77%) cases in children have required ICU admission, while most of the adult cases were mild and influenzalike. One child developed bulbar dysfunction and limb weakness.
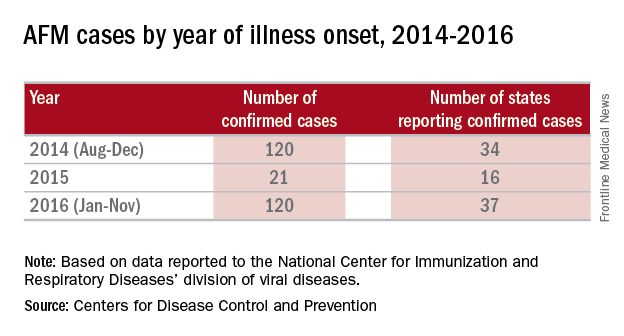
Enterovirus D68 infection should be suspected in children with moderate to severe respiratory tract infection or acute onset bulbar or flaccid paralysis of unknown etiology, especially in summer and fall. In such cases, respiratory specimens (nasopharyngeal or oral swabs or wash, tracheal secretions or bronchoalveolar lavage) should be obtained. Increasingly, hospitals and laboratories can perform multiplex polymerase chain reaction testing for enterovirus/rhinovirus. However, most do not determine the specific enterovirus. CDC and some state health departments use real-time reverse transcription polymerase chain reaction (rRT-PCR), which enables reporting of specific enterovirus species within days. CDC recommends that clinicians consider enterovirus D68 testing for children with unknown, severe respiratory illness or AFM. Details for sending specimens should be available from your state’s Department of Public Health website or the CDC.
Prevention strategies may be critical for limiting the spread of enterovirus D68 in the community. The CDC recommends:
- Wash your hands often with soap and water for 20 seconds.
- Avoid touching your eyes, nose and mouth with unwashed hands.
- Avoid close contact such as kissing, hugging, and sharing cups with people who are ill.
- Cover your coughs and sneezes with a tissue or shirt sleeve, not your hands.
- Clean and disinfect frequently touched surfaces, such as toys and doorknobs, especially if someone is sick.
- Stay home when you are ill.
In 2014, it was speculated that the epidemic might have been a one-time event. It now appears more likely that enterovirus D68 activity has been increasing since 2000, and that children and immunocompromised hosts will be at greatest risk because of a lack of neutralizing antibody. Ongoing enterovirus surveillance will be critical to understand the potential for severe respiratory disease as will the development of new and effective antivirals. A vaccine for enterovirus 71 recently demonstrated efficacy against hand, foot, and mouth disease in children and may provide insights into the development of vaccines against enterovirus D68.
References
Lancet Infect Dis. 2016 May;16(5):e64-75
Emerg Infect Dis. 2017 Jan;23(1):140-3.
J Med Virol. 2016 May;88(5):739-45
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
In August 2014, we first heard of increased pediatric cases of severe respiratory tract disease, many requiring management in the ICU, and of acute flaccid myelitis/paralysis (AFM) of unknown etiology from many states across the United States. Concurrently with this outbreak in the United States, similar clinical cases were reported in Canada and Europe. Subsequently, enterovirus D68 was confirmed in some, but not all, of the paralyzed children. Although new to many of us, enterovirus D68 was already known as an atypical enterovirus sharing many of its structural and chemical properties with rhinovirus. For example, it most often was reported from respiratory samples and less common from stool samples. It also had been associated with clusters of respiratory disease since 2000 and a 2008 case of fatal AFM.
There were 120 cases of AFM, coinciding with the nationwide outbreak of enteroviral D68 disease, reported in 2014. The Centers for Disease Control and Prevention has evaluated the cerebrospinal fluid in many of these cases, and no pathogen has consistently been detected. The children were mostly school age, aged 7-11 years, presented with acute, febrile respiratory illness followed by acute onset of cranial nerve dysfunction or flaccid paralysis of one or more limbs. The CSF revealed mild pleocytosis, most often with mild elevation of protein and a normal glucose. However, the MRI was distinctly abnormal with focal lesion in the cranial nerve nuclei (in those with bulbar dysfunction) and/or in the anterior horn or spinal cord gray matter. Long-term prognosis is unknown, although most patients have persistent weakness, despite some improvement, to date.
In 2016, the CDC has reported an increase in cases after a decline in 2015 despite the absence of epidemic respiratory tract disease in the United States from enterovirus D68. In the Netherlands, an increase in respiratory disease from enterovirus D68 in children and adults also has been reported since June 2016. Respiratory disease has been observed in children as young as 3 months of age, and most of the children have underlying comorbidity, many with asthma or other pulmonary conditions. Thirteen of 17 (77%) cases in children have required ICU admission, while most of the adult cases were mild and influenzalike. One child developed bulbar dysfunction and limb weakness.

Enterovirus D68 infection should be suspected in children with moderate to severe respiratory tract infection or acute onset bulbar or flaccid paralysis of unknown etiology, especially in summer and fall. In such cases, respiratory specimens (nasopharyngeal or oral swabs or wash, tracheal secretions or bronchoalveolar lavage) should be obtained. Increasingly, hospitals and laboratories can perform multiplex polymerase chain reaction testing for enterovirus/rhinovirus. However, most do not determine the specific enterovirus. CDC and some state health departments use real-time reverse transcription polymerase chain reaction (rRT-PCR), which enables reporting of specific enterovirus species within days. CDC recommends that clinicians consider enterovirus D68 testing for children with unknown, severe respiratory illness or AFM. Details for sending specimens should be available from your state’s Department of Public Health website or the CDC.
Prevention strategies may be critical for limiting the spread of enterovirus D68 in the community. The CDC recommends:
- Wash your hands often with soap and water for 20 seconds.
- Avoid touching your eyes, nose and mouth with unwashed hands.
- Avoid close contact such as kissing, hugging, and sharing cups with people who are ill.
- Cover your coughs and sneezes with a tissue or shirt sleeve, not your hands.
- Clean and disinfect frequently touched surfaces, such as toys and doorknobs, especially if someone is sick.
- Stay home when you are ill.
In 2014, it was speculated that the epidemic might have been a one-time event. It now appears more likely that enterovirus D68 activity has been increasing since 2000, and that children and immunocompromised hosts will be at greatest risk because of a lack of neutralizing antibody. Ongoing enterovirus surveillance will be critical to understand the potential for severe respiratory disease as will the development of new and effective antivirals. A vaccine for enterovirus 71 recently demonstrated efficacy against hand, foot, and mouth disease in children and may provide insights into the development of vaccines against enterovirus D68.
References
Lancet Infect Dis. 2016 May;16(5):e64-75
Emerg Infect Dis. 2017 Jan;23(1):140-3.
J Med Virol. 2016 May;88(5):739-45
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
In August 2014, we first heard of increased pediatric cases of severe respiratory tract disease, many requiring management in the ICU, and of acute flaccid myelitis/paralysis (AFM) of unknown etiology from many states across the United States. Concurrently with this outbreak in the United States, similar clinical cases were reported in Canada and Europe. Subsequently, enterovirus D68 was confirmed in some, but not all, of the paralyzed children. Although new to many of us, enterovirus D68 was already known as an atypical enterovirus sharing many of its structural and chemical properties with rhinovirus. For example, it most often was reported from respiratory samples and less common from stool samples. It also had been associated with clusters of respiratory disease since 2000 and a 2008 case of fatal AFM.
There were 120 cases of AFM, coinciding with the nationwide outbreak of enteroviral D68 disease, reported in 2014. The Centers for Disease Control and Prevention has evaluated the cerebrospinal fluid in many of these cases, and no pathogen has consistently been detected. The children were mostly school age, aged 7-11 years, presented with acute, febrile respiratory illness followed by acute onset of cranial nerve dysfunction or flaccid paralysis of one or more limbs. The CSF revealed mild pleocytosis, most often with mild elevation of protein and a normal glucose. However, the MRI was distinctly abnormal with focal lesion in the cranial nerve nuclei (in those with bulbar dysfunction) and/or in the anterior horn or spinal cord gray matter. Long-term prognosis is unknown, although most patients have persistent weakness, despite some improvement, to date.
In 2016, the CDC has reported an increase in cases after a decline in 2015 despite the absence of epidemic respiratory tract disease in the United States from enterovirus D68. In the Netherlands, an increase in respiratory disease from enterovirus D68 in children and adults also has been reported since June 2016. Respiratory disease has been observed in children as young as 3 months of age, and most of the children have underlying comorbidity, many with asthma or other pulmonary conditions. Thirteen of 17 (77%) cases in children have required ICU admission, while most of the adult cases were mild and influenzalike. One child developed bulbar dysfunction and limb weakness.

Enterovirus D68 infection should be suspected in children with moderate to severe respiratory tract infection or acute onset bulbar or flaccid paralysis of unknown etiology, especially in summer and fall. In such cases, respiratory specimens (nasopharyngeal or oral swabs or wash, tracheal secretions or bronchoalveolar lavage) should be obtained. Increasingly, hospitals and laboratories can perform multiplex polymerase chain reaction testing for enterovirus/rhinovirus. However, most do not determine the specific enterovirus. CDC and some state health departments use real-time reverse transcription polymerase chain reaction (rRT-PCR), which enables reporting of specific enterovirus species within days. CDC recommends that clinicians consider enterovirus D68 testing for children with unknown, severe respiratory illness or AFM. Details for sending specimens should be available from your state’s Department of Public Health website or the CDC.
Prevention strategies may be critical for limiting the spread of enterovirus D68 in the community. The CDC recommends:
- Wash your hands often with soap and water for 20 seconds.
- Avoid touching your eyes, nose and mouth with unwashed hands.
- Avoid close contact such as kissing, hugging, and sharing cups with people who are ill.
- Cover your coughs and sneezes with a tissue or shirt sleeve, not your hands.
- Clean and disinfect frequently touched surfaces, such as toys and doorknobs, especially if someone is sick.
- Stay home when you are ill.
In 2014, it was speculated that the epidemic might have been a one-time event. It now appears more likely that enterovirus D68 activity has been increasing since 2000, and that children and immunocompromised hosts will be at greatest risk because of a lack of neutralizing antibody. Ongoing enterovirus surveillance will be critical to understand the potential for severe respiratory disease as will the development of new and effective antivirals. A vaccine for enterovirus 71 recently demonstrated efficacy against hand, foot, and mouth disease in children and may provide insights into the development of vaccines against enterovirus D68.
References
Lancet Infect Dis. 2016 May;16(5):e64-75
Emerg Infect Dis. 2017 Jan;23(1):140-3.
J Med Virol. 2016 May;88(5):739-45
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
Make HIV testing of adolescents routine
Nearly 2 decades ago, I was a pediatric infectious diseases fellow fielding a call from a community pediatrician seeking advice on patient management. The patient in question was a 15-year-old male with fever, rash, and cervical adenopathy – a good clinical story for Epstein-Barr virus infection. A heterophile antibody test was negative, however, as were EBV titers.
We talked for a couple of minutes about the vagaries of EBV testing, as well as other organisms that could cause a mononucleosis-like illness. “Cytomegalovirus is a possibility, along with toxoplasmosis,” I told him. “I’d also test for HIV.”
There was a moment of silence and little throat-clearing. “I don’t think we need to that,” he finally responded. “I’ve known this boy since he was a baby, and I’m sure HIV’s not an issue. He’s not that kind of kid.”
Bear in mind that we lived in a Midwestern city with low rates of HIV, and I suspect this seasoned pediatrician had never seen a case. I argued (as only an impassioned trainee can) that every kid is the kind that could be at risk for HIV, and testing was ultimately done (and was negative).
A lot has changed in the intervening years. HIV infection, at least in adolescents and adults, can be controlled with a single pill taken once a day. Children infected perinatally can grow up and have (uninfected) children of their own. We have reasonably effective pre- and postexposure prophylaxis.
One thing that hasn’t changed, however, is the reluctance of some of us to test our patients for HIV. So what’s up with that?
It’s not because the virus has gone away. On Oct. 14, 2016, amid little fanfare, the Centers for Disease Control and Prevention released the United States Summary of Notifiable Infectious Diseases and Conditions for 2014. A total of 35,606 cases of HIV infection were diagnosed in the United States and reported to the CDC, and 7,723 were in individuals aged 15-24 years.
It is possible that the number of cases in adolescents is even higher. The CDC estimates as many as 60% of youth with HIV don’t know that they are infected, likely because they’ve never been tested. According to the 2015 Youth Risk Behavior Survey (YRBS), only 10% of United States high school students had ever been tested for HIV, and the number of teens tested has been dropping over time. In 2013, for example, the prevalence of having ever been tested for HIV was 13%.
It’s not because today’s teenagers lack risk factors, including sexual activity and drug use. Just over 30% of the U.S. students surveyed for the YRBS reported sexual intercourse with at least one person in the preceding 3 months, and more than 11% had had four or more lifetime partners. Among sexually active teenagers in the United States, only 57% reported that they or their partner used a condom during last sexual intercourse. Overall, 2% of those surveyed admitted a history of injecting an illegal drug.
It’s not because public health experts haven’t deemed testing a priority. The CDC recommends that everyone aged 13-64 years should get tested at least once. Annual testing is recommended for some individuals, including sexually active gay and bisexual males, those who have had more than one sexual partner since their last HIV test, and those who have another sexually transmitted disease. A 2011 American Academy of Pediatrics policy statement affirms the need for routine testing, calling for all adolescents living in geographic areas with an HIV prevalence greater than 0.1% to be offered routine HIV screening at least once by age 16-18 years. In communities with a lower prevalence, the AAP recommends routine HIV testing for sexually active adolescents as well as those with other risk factors, including substance use. Annual HIV testing is recommended for high-risk teenagers, and whenever testing for other sexually transmitted infections (STIs) is performed.
It’s probably not that most teenagers are being offered HIV tests and they’re declining. In 2008, the emergency department at Le Bonheur Children’s Hospital in Memphis, Tenn., implemented a protocol for routine, opt-out HIV screening for medically stable patients aged 13-18 years (Pediatrics. 2009 Oct;124:1076-84). Of the 2,002 patients approached for screening over an approximately 7-month period, only 267 (13%) opted out and of those, 73 had already been tested.
Yet many of us still are not testing. More recently, investigators in Philadelphia performed a retrospective, cross-sectional study of 1,000 randomly selected 13- to 19-year-old patients attending routine well visits conducted at 29 pediatric primary care practices to assess clinician documentation of sexual history and screening for STIs and HIV (J Pediatr. 2014 Aug;165[2]:343-7). Only 212 visits (21.2%) had a documented sexual history, and only 16 patients were tested for HIV (1.6%). HIV testing was more likely to be performed on older adolescents, those of non-Hispanic black race/ethnicity, and those with nonprivate insurance. Study authors called the results “concerning” and advocated for standardized protocols, documentation templates, and electronic decision support to facilitate improved sexual health assessments and screening.
I suspect we all can do better. I’m not a primary care provider, but I do see adolescents with a variety of complaints. I’m pretty diligent about testing teenagers admitted with unexplained fever, vague constitutional symptoms, and those with symptoms that suggest another STI. I’m less effective at discussing HIV testing with those being treated for a postop wound infection, or a routine community-acquired pneumonia.
December is a good time to reflect on practice and make resolutions for the new year. I resolve to talk to more of my adolescent patients about HIV. Who’s with me?
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Kosair Children’s Hospital, also in Louisville. Email her at [email protected].
Nearly 2 decades ago, I was a pediatric infectious diseases fellow fielding a call from a community pediatrician seeking advice on patient management. The patient in question was a 15-year-old male with fever, rash, and cervical adenopathy – a good clinical story for Epstein-Barr virus infection. A heterophile antibody test was negative, however, as were EBV titers.
We talked for a couple of minutes about the vagaries of EBV testing, as well as other organisms that could cause a mononucleosis-like illness. “Cytomegalovirus is a possibility, along with toxoplasmosis,” I told him. “I’d also test for HIV.”
There was a moment of silence and little throat-clearing. “I don’t think we need to that,” he finally responded. “I’ve known this boy since he was a baby, and I’m sure HIV’s not an issue. He’s not that kind of kid.”
Bear in mind that we lived in a Midwestern city with low rates of HIV, and I suspect this seasoned pediatrician had never seen a case. I argued (as only an impassioned trainee can) that every kid is the kind that could be at risk for HIV, and testing was ultimately done (and was negative).
A lot has changed in the intervening years. HIV infection, at least in adolescents and adults, can be controlled with a single pill taken once a day. Children infected perinatally can grow up and have (uninfected) children of their own. We have reasonably effective pre- and postexposure prophylaxis.
One thing that hasn’t changed, however, is the reluctance of some of us to test our patients for HIV. So what’s up with that?
It’s not because the virus has gone away. On Oct. 14, 2016, amid little fanfare, the Centers for Disease Control and Prevention released the United States Summary of Notifiable Infectious Diseases and Conditions for 2014. A total of 35,606 cases of HIV infection were diagnosed in the United States and reported to the CDC, and 7,723 were in individuals aged 15-24 years.
It is possible that the number of cases in adolescents is even higher. The CDC estimates as many as 60% of youth with HIV don’t know that they are infected, likely because they’ve never been tested. According to the 2015 Youth Risk Behavior Survey (YRBS), only 10% of United States high school students had ever been tested for HIV, and the number of teens tested has been dropping over time. In 2013, for example, the prevalence of having ever been tested for HIV was 13%.
It’s not because today’s teenagers lack risk factors, including sexual activity and drug use. Just over 30% of the U.S. students surveyed for the YRBS reported sexual intercourse with at least one person in the preceding 3 months, and more than 11% had had four or more lifetime partners. Among sexually active teenagers in the United States, only 57% reported that they or their partner used a condom during last sexual intercourse. Overall, 2% of those surveyed admitted a history of injecting an illegal drug.
It’s not because public health experts haven’t deemed testing a priority. The CDC recommends that everyone aged 13-64 years should get tested at least once. Annual testing is recommended for some individuals, including sexually active gay and bisexual males, those who have had more than one sexual partner since their last HIV test, and those who have another sexually transmitted disease. A 2011 American Academy of Pediatrics policy statement affirms the need for routine testing, calling for all adolescents living in geographic areas with an HIV prevalence greater than 0.1% to be offered routine HIV screening at least once by age 16-18 years. In communities with a lower prevalence, the AAP recommends routine HIV testing for sexually active adolescents as well as those with other risk factors, including substance use. Annual HIV testing is recommended for high-risk teenagers, and whenever testing for other sexually transmitted infections (STIs) is performed.
It’s probably not that most teenagers are being offered HIV tests and they’re declining. In 2008, the emergency department at Le Bonheur Children’s Hospital in Memphis, Tenn., implemented a protocol for routine, opt-out HIV screening for medically stable patients aged 13-18 years (Pediatrics. 2009 Oct;124:1076-84). Of the 2,002 patients approached for screening over an approximately 7-month period, only 267 (13%) opted out and of those, 73 had already been tested.
Yet many of us still are not testing. More recently, investigators in Philadelphia performed a retrospective, cross-sectional study of 1,000 randomly selected 13- to 19-year-old patients attending routine well visits conducted at 29 pediatric primary care practices to assess clinician documentation of sexual history and screening for STIs and HIV (J Pediatr. 2014 Aug;165[2]:343-7). Only 212 visits (21.2%) had a documented sexual history, and only 16 patients were tested for HIV (1.6%). HIV testing was more likely to be performed on older adolescents, those of non-Hispanic black race/ethnicity, and those with nonprivate insurance. Study authors called the results “concerning” and advocated for standardized protocols, documentation templates, and electronic decision support to facilitate improved sexual health assessments and screening.
I suspect we all can do better. I’m not a primary care provider, but I do see adolescents with a variety of complaints. I’m pretty diligent about testing teenagers admitted with unexplained fever, vague constitutional symptoms, and those with symptoms that suggest another STI. I’m less effective at discussing HIV testing with those being treated for a postop wound infection, or a routine community-acquired pneumonia.
December is a good time to reflect on practice and make resolutions for the new year. I resolve to talk to more of my adolescent patients about HIV. Who’s with me?
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Kosair Children’s Hospital, also in Louisville. Email her at [email protected].
Nearly 2 decades ago, I was a pediatric infectious diseases fellow fielding a call from a community pediatrician seeking advice on patient management. The patient in question was a 15-year-old male with fever, rash, and cervical adenopathy – a good clinical story for Epstein-Barr virus infection. A heterophile antibody test was negative, however, as were EBV titers.
We talked for a couple of minutes about the vagaries of EBV testing, as well as other organisms that could cause a mononucleosis-like illness. “Cytomegalovirus is a possibility, along with toxoplasmosis,” I told him. “I’d also test for HIV.”
There was a moment of silence and little throat-clearing. “I don’t think we need to that,” he finally responded. “I’ve known this boy since he was a baby, and I’m sure HIV’s not an issue. He’s not that kind of kid.”
Bear in mind that we lived in a Midwestern city with low rates of HIV, and I suspect this seasoned pediatrician had never seen a case. I argued (as only an impassioned trainee can) that every kid is the kind that could be at risk for HIV, and testing was ultimately done (and was negative).
A lot has changed in the intervening years. HIV infection, at least in adolescents and adults, can be controlled with a single pill taken once a day. Children infected perinatally can grow up and have (uninfected) children of their own. We have reasonably effective pre- and postexposure prophylaxis.
One thing that hasn’t changed, however, is the reluctance of some of us to test our patients for HIV. So what’s up with that?
It’s not because the virus has gone away. On Oct. 14, 2016, amid little fanfare, the Centers for Disease Control and Prevention released the United States Summary of Notifiable Infectious Diseases and Conditions for 2014. A total of 35,606 cases of HIV infection were diagnosed in the United States and reported to the CDC, and 7,723 were in individuals aged 15-24 years.
It is possible that the number of cases in adolescents is even higher. The CDC estimates as many as 60% of youth with HIV don’t know that they are infected, likely because they’ve never been tested. According to the 2015 Youth Risk Behavior Survey (YRBS), only 10% of United States high school students had ever been tested for HIV, and the number of teens tested has been dropping over time. In 2013, for example, the prevalence of having ever been tested for HIV was 13%.
It’s not because today’s teenagers lack risk factors, including sexual activity and drug use. Just over 30% of the U.S. students surveyed for the YRBS reported sexual intercourse with at least one person in the preceding 3 months, and more than 11% had had four or more lifetime partners. Among sexually active teenagers in the United States, only 57% reported that they or their partner used a condom during last sexual intercourse. Overall, 2% of those surveyed admitted a history of injecting an illegal drug.
It’s not because public health experts haven’t deemed testing a priority. The CDC recommends that everyone aged 13-64 years should get tested at least once. Annual testing is recommended for some individuals, including sexually active gay and bisexual males, those who have had more than one sexual partner since their last HIV test, and those who have another sexually transmitted disease. A 2011 American Academy of Pediatrics policy statement affirms the need for routine testing, calling for all adolescents living in geographic areas with an HIV prevalence greater than 0.1% to be offered routine HIV screening at least once by age 16-18 years. In communities with a lower prevalence, the AAP recommends routine HIV testing for sexually active adolescents as well as those with other risk factors, including substance use. Annual HIV testing is recommended for high-risk teenagers, and whenever testing for other sexually transmitted infections (STIs) is performed.
It’s probably not that most teenagers are being offered HIV tests and they’re declining. In 2008, the emergency department at Le Bonheur Children’s Hospital in Memphis, Tenn., implemented a protocol for routine, opt-out HIV screening for medically stable patients aged 13-18 years (Pediatrics. 2009 Oct;124:1076-84). Of the 2,002 patients approached for screening over an approximately 7-month period, only 267 (13%) opted out and of those, 73 had already been tested.
Yet many of us still are not testing. More recently, investigators in Philadelphia performed a retrospective, cross-sectional study of 1,000 randomly selected 13- to 19-year-old patients attending routine well visits conducted at 29 pediatric primary care practices to assess clinician documentation of sexual history and screening for STIs and HIV (J Pediatr. 2014 Aug;165[2]:343-7). Only 212 visits (21.2%) had a documented sexual history, and only 16 patients were tested for HIV (1.6%). HIV testing was more likely to be performed on older adolescents, those of non-Hispanic black race/ethnicity, and those with nonprivate insurance. Study authors called the results “concerning” and advocated for standardized protocols, documentation templates, and electronic decision support to facilitate improved sexual health assessments and screening.
I suspect we all can do better. I’m not a primary care provider, but I do see adolescents with a variety of complaints. I’m pretty diligent about testing teenagers admitted with unexplained fever, vague constitutional symptoms, and those with symptoms that suggest another STI. I’m less effective at discussing HIV testing with those being treated for a postop wound infection, or a routine community-acquired pneumonia.
December is a good time to reflect on practice and make resolutions for the new year. I resolve to talk to more of my adolescent patients about HIV. Who’s with me?
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Kosair Children’s Hospital, also in Louisville. Email her at [email protected].
It’s in the nose
There is a lot more going on in the nose besides air going in and out. The nose is where it all begins for pathogenesis for all respiratory infections. The interplay between the commensal microbes, the potential pathogens, innate immunity, and adaptive immunity is much more complex than was previously understood. So what is new?
In our research on acute otitis media, we swab and wash out noses of children aged 6-36 months to isolate the potential pathogens Streptococcus pneumoniae, nontypeable Haemophilus influenza, Moraxella catarrhalis, Staphylococcus aureus, and Group A streptococci. We isolate one or more of these bacteria from most of the children even though they are well. We observe perhaps a half-dozen other species of bacteria on the culture plate. Mostly, we isolate S. pneumoniae, nontypeable H. influenza, or M. catarrhalis and alpha-hemolytic streptococci and corynebacterium species.
We have recently begun to investigate the other microbiota in the nose and found they are indeed plentiful. In a recent screening of a half-dozen children, we found hundreds of different microbes in their noses, so cultures and standard molecular detection methods were just touching the surface. I was asked recently at a medical conference – the American Academy of Pediatrics– Orange County, California, annual CME course – at which I spoke on this topic what I thought would be the most-important area of research in the next decade. I responded, the microbiome. The microbiome is indeed a hot topic. Research over the last decade suggests that 90% of all diseases can be traced in some way to disturbances in the microbiome. What I mean by microbiome is “the totality of microorganisms and their collective genetic material present in or on the human body.” The term is often used interchangeably with “microbiota,” although the former refers to genes of microbes and the latter refers to the microbes themselves. What I mean by “disturbance” is excessive use of antibiotics.
How many microbes are in the nose? We don’t know. But if the gut is any indication, there are thousands of microbes in the nose because the gut has more than 10,000 different microbes. Recognizing that there are hundreds of microbes in the nose and from time to time children get colonized by potential pathogens that can cause otitis media, sinusitis, or pneumonia, how does pathogenesis get started? It starts with a respiratory virus infection. The bacteria need help from the viruses to cause disease. The viruses cause damage to the epithelial cells of the nose, and this gives the bacteria more places to attach when they divide so the amount of bacteria increases exponentially. As the viruses replicate, they more effectively slow down cilia beating, and the nasal mucus thickens. This, too, helps the bacteria and viruses attach to and penetrate epithelial cells in the nose and increase in density on the surface of the cells and inside the cells. The viruses divert and/or suppress the innate immune system, represented by neutrophils that migrate to the nose and discharge their intracellular contents to turn nasal mucus yellow and green. The viruses even down-modulate the adaptive immune system in clever ways that result in fewer potentially protective cytotoxic lymphocytes that kill viruses making their way to the nose, and fewer T cells that discharge cytokines that promote a necessary inflammatory response to clear both bacteria and viruses from the nose and fewer B cells that become plasma cells and release antibodies into the nose.
When the bacteria with potential to cause diseases reach a “pathogenic threshold,” they move, along with mucus, into the middle ear, the sinuses, or down the throat to the lungs, usually with the accompanying respiratory virus. There pathogenesis continues in the otherwise sterile and protected sanctuary of these interconnected respiratory sites. A few days later, we as clinicians observe the symptoms and signs of otitis media, sinusitis, or pneumonia.
What can we do to help the nose? Mostly, we should do no harm, and that has been our failing for decades since the introduction of antibiotics. The allure of antibiotics is great because they have indeed saved many lives and shortened many illnesses when appropriately used. However, too often clinicians have seen patients with yellow and green nasal mucus (or any increased nasal mucus) and diagnosed “a bacterial infection” and prescribed antibiotics. And too often clinicians have seen patients with an annoying cough (or any cough) and diagnosed “a bacterial chest infection” and prescribed antibiotics. The clinicians thought it was the right thing to do because they wanted to help their patient. And they did not want them to come back in a few days with persistence or worsening of symptoms, or worse, seek care from other health care providers elsewhere. So they gave antibiotics.
Well, the paradigm has changed. It is now clearly known that antibiotics can be harmful mainly by damaging the normal, healthy microbiome. The change in healthy homeostasis of the microbiome wrought by antibiotics is greatest in newborns, especially premature newborns, then next worst for infants, and then next worst for young children. These are the age groups where antibiotics are prescribed most frequently! And everyone needs to stop writing those prescriptions for runny noses, yellow and green mucus in the nose, and coughs. All of us need to prescribe antibiotics only when there is an accurate diagnosis of otitis media or sinusitis or bronchopneumonia or lobar pneumonia. And when we do prescribe the antibiotics ,we need to give them for as short a time as possible. But that is a topic for another column.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he has no relevant financial disclosures, and that his research is supported by a grant from the National Institutes of Health National Institute of Deafness and Communication Disorders. Email him at [email protected].
There is a lot more going on in the nose besides air going in and out. The nose is where it all begins for pathogenesis for all respiratory infections. The interplay between the commensal microbes, the potential pathogens, innate immunity, and adaptive immunity is much more complex than was previously understood. So what is new?
In our research on acute otitis media, we swab and wash out noses of children aged 6-36 months to isolate the potential pathogens Streptococcus pneumoniae, nontypeable Haemophilus influenza, Moraxella catarrhalis, Staphylococcus aureus, and Group A streptococci. We isolate one or more of these bacteria from most of the children even though they are well. We observe perhaps a half-dozen other species of bacteria on the culture plate. Mostly, we isolate S. pneumoniae, nontypeable H. influenza, or M. catarrhalis and alpha-hemolytic streptococci and corynebacterium species.
We have recently begun to investigate the other microbiota in the nose and found they are indeed plentiful. In a recent screening of a half-dozen children, we found hundreds of different microbes in their noses, so cultures and standard molecular detection methods were just touching the surface. I was asked recently at a medical conference – the American Academy of Pediatrics– Orange County, California, annual CME course – at which I spoke on this topic what I thought would be the most-important area of research in the next decade. I responded, the microbiome. The microbiome is indeed a hot topic. Research over the last decade suggests that 90% of all diseases can be traced in some way to disturbances in the microbiome. What I mean by microbiome is “the totality of microorganisms and their collective genetic material present in or on the human body.” The term is often used interchangeably with “microbiota,” although the former refers to genes of microbes and the latter refers to the microbes themselves. What I mean by “disturbance” is excessive use of antibiotics.
How many microbes are in the nose? We don’t know. But if the gut is any indication, there are thousands of microbes in the nose because the gut has more than 10,000 different microbes. Recognizing that there are hundreds of microbes in the nose and from time to time children get colonized by potential pathogens that can cause otitis media, sinusitis, or pneumonia, how does pathogenesis get started? It starts with a respiratory virus infection. The bacteria need help from the viruses to cause disease. The viruses cause damage to the epithelial cells of the nose, and this gives the bacteria more places to attach when they divide so the amount of bacteria increases exponentially. As the viruses replicate, they more effectively slow down cilia beating, and the nasal mucus thickens. This, too, helps the bacteria and viruses attach to and penetrate epithelial cells in the nose and increase in density on the surface of the cells and inside the cells. The viruses divert and/or suppress the innate immune system, represented by neutrophils that migrate to the nose and discharge their intracellular contents to turn nasal mucus yellow and green. The viruses even down-modulate the adaptive immune system in clever ways that result in fewer potentially protective cytotoxic lymphocytes that kill viruses making their way to the nose, and fewer T cells that discharge cytokines that promote a necessary inflammatory response to clear both bacteria and viruses from the nose and fewer B cells that become plasma cells and release antibodies into the nose.
When the bacteria with potential to cause diseases reach a “pathogenic threshold,” they move, along with mucus, into the middle ear, the sinuses, or down the throat to the lungs, usually with the accompanying respiratory virus. There pathogenesis continues in the otherwise sterile and protected sanctuary of these interconnected respiratory sites. A few days later, we as clinicians observe the symptoms and signs of otitis media, sinusitis, or pneumonia.
What can we do to help the nose? Mostly, we should do no harm, and that has been our failing for decades since the introduction of antibiotics. The allure of antibiotics is great because they have indeed saved many lives and shortened many illnesses when appropriately used. However, too often clinicians have seen patients with yellow and green nasal mucus (or any increased nasal mucus) and diagnosed “a bacterial infection” and prescribed antibiotics. And too often clinicians have seen patients with an annoying cough (or any cough) and diagnosed “a bacterial chest infection” and prescribed antibiotics. The clinicians thought it was the right thing to do because they wanted to help their patient. And they did not want them to come back in a few days with persistence or worsening of symptoms, or worse, seek care from other health care providers elsewhere. So they gave antibiotics.
Well, the paradigm has changed. It is now clearly known that antibiotics can be harmful mainly by damaging the normal, healthy microbiome. The change in healthy homeostasis of the microbiome wrought by antibiotics is greatest in newborns, especially premature newborns, then next worst for infants, and then next worst for young children. These are the age groups where antibiotics are prescribed most frequently! And everyone needs to stop writing those prescriptions for runny noses, yellow and green mucus in the nose, and coughs. All of us need to prescribe antibiotics only when there is an accurate diagnosis of otitis media or sinusitis or bronchopneumonia or lobar pneumonia. And when we do prescribe the antibiotics ,we need to give them for as short a time as possible. But that is a topic for another column.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he has no relevant financial disclosures, and that his research is supported by a grant from the National Institutes of Health National Institute of Deafness and Communication Disorders. Email him at [email protected].
There is a lot more going on in the nose besides air going in and out. The nose is where it all begins for pathogenesis for all respiratory infections. The interplay between the commensal microbes, the potential pathogens, innate immunity, and adaptive immunity is much more complex than was previously understood. So what is new?
In our research on acute otitis media, we swab and wash out noses of children aged 6-36 months to isolate the potential pathogens Streptococcus pneumoniae, nontypeable Haemophilus influenza, Moraxella catarrhalis, Staphylococcus aureus, and Group A streptococci. We isolate one or more of these bacteria from most of the children even though they are well. We observe perhaps a half-dozen other species of bacteria on the culture plate. Mostly, we isolate S. pneumoniae, nontypeable H. influenza, or M. catarrhalis and alpha-hemolytic streptococci and corynebacterium species.
We have recently begun to investigate the other microbiota in the nose and found they are indeed plentiful. In a recent screening of a half-dozen children, we found hundreds of different microbes in their noses, so cultures and standard molecular detection methods were just touching the surface. I was asked recently at a medical conference – the American Academy of Pediatrics– Orange County, California, annual CME course – at which I spoke on this topic what I thought would be the most-important area of research in the next decade. I responded, the microbiome. The microbiome is indeed a hot topic. Research over the last decade suggests that 90% of all diseases can be traced in some way to disturbances in the microbiome. What I mean by microbiome is “the totality of microorganisms and their collective genetic material present in or on the human body.” The term is often used interchangeably with “microbiota,” although the former refers to genes of microbes and the latter refers to the microbes themselves. What I mean by “disturbance” is excessive use of antibiotics.
How many microbes are in the nose? We don’t know. But if the gut is any indication, there are thousands of microbes in the nose because the gut has more than 10,000 different microbes. Recognizing that there are hundreds of microbes in the nose and from time to time children get colonized by potential pathogens that can cause otitis media, sinusitis, or pneumonia, how does pathogenesis get started? It starts with a respiratory virus infection. The bacteria need help from the viruses to cause disease. The viruses cause damage to the epithelial cells of the nose, and this gives the bacteria more places to attach when they divide so the amount of bacteria increases exponentially. As the viruses replicate, they more effectively slow down cilia beating, and the nasal mucus thickens. This, too, helps the bacteria and viruses attach to and penetrate epithelial cells in the nose and increase in density on the surface of the cells and inside the cells. The viruses divert and/or suppress the innate immune system, represented by neutrophils that migrate to the nose and discharge their intracellular contents to turn nasal mucus yellow and green. The viruses even down-modulate the adaptive immune system in clever ways that result in fewer potentially protective cytotoxic lymphocytes that kill viruses making their way to the nose, and fewer T cells that discharge cytokines that promote a necessary inflammatory response to clear both bacteria and viruses from the nose and fewer B cells that become plasma cells and release antibodies into the nose.
When the bacteria with potential to cause diseases reach a “pathogenic threshold,” they move, along with mucus, into the middle ear, the sinuses, or down the throat to the lungs, usually with the accompanying respiratory virus. There pathogenesis continues in the otherwise sterile and protected sanctuary of these interconnected respiratory sites. A few days later, we as clinicians observe the symptoms and signs of otitis media, sinusitis, or pneumonia.
What can we do to help the nose? Mostly, we should do no harm, and that has been our failing for decades since the introduction of antibiotics. The allure of antibiotics is great because they have indeed saved many lives and shortened many illnesses when appropriately used. However, too often clinicians have seen patients with yellow and green nasal mucus (or any increased nasal mucus) and diagnosed “a bacterial infection” and prescribed antibiotics. And too often clinicians have seen patients with an annoying cough (or any cough) and diagnosed “a bacterial chest infection” and prescribed antibiotics. The clinicians thought it was the right thing to do because they wanted to help their patient. And they did not want them to come back in a few days with persistence or worsening of symptoms, or worse, seek care from other health care providers elsewhere. So they gave antibiotics.
Well, the paradigm has changed. It is now clearly known that antibiotics can be harmful mainly by damaging the normal, healthy microbiome. The change in healthy homeostasis of the microbiome wrought by antibiotics is greatest in newborns, especially premature newborns, then next worst for infants, and then next worst for young children. These are the age groups where antibiotics are prescribed most frequently! And everyone needs to stop writing those prescriptions for runny noses, yellow and green mucus in the nose, and coughs. All of us need to prescribe antibiotics only when there is an accurate diagnosis of otitis media or sinusitis or bronchopneumonia or lobar pneumonia. And when we do prescribe the antibiotics ,we need to give them for as short a time as possible. But that is a topic for another column.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he has no relevant financial disclosures, and that his research is supported by a grant from the National Institutes of Health National Institute of Deafness and Communication Disorders. Email him at [email protected].
HPV vaccine and adolescents: What we say really does matter
It has been almost 10 years since the Advisory Committee on Immunization Practices (ACIP) recommended administration of human papillomavirus (HPV) vaccine for 11- to 12-year-old girls and young women up to 26 years of age. Routine administration in preteen boys and young adult males up to 21 years of age was recommended in 2011. An HPV series should be completed by 13 years. So how well are we protecting our patients?
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually among adolescents 13-17 years. Data are obtained from individuals from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR. 2016 Aug 26;65[33]:850-8).
HPV vaccination continues to lag behind Tdap and the meningococcal conjugate vaccine (MCV), although each one is recommended to be administered at the 11- to 12-year visit. In 2015, coverage for receiving at least one dose of HPV vaccine among females was almost 62.8 % and for at least three doses was 41.9%; among males, coverage with at least one dose was 49.8% and for at least three doses was 28.1%. Compared with 2014, coverage for at least one dose of HPV vaccine increased 2.8% in females and 8.1% in males. Males also had a 7.6% for receipt of at least two doses of HPV vaccine, compared with 2014. HPV vaccine coverage in females aged 13 and younger also was lower than for those aged 15 and older. Coverage did not differ for males based on age.
HPV vaccination coverage also differed by state. In 2015, 28 states reported increased coverage in males, but only 7 states had increased coverage in females. Among all adolescents, coverage with at least one dose of HPV vaccine was 56.1%, at least two doses was 45.4%, and at least three doses was 34.9%. In contrast, 86.4% of all adolescents received at least one dose of Tdap, and 81.3% received at least one dose of MCV.
HPV-associated cancers
HPV is the most common sexually transmitted infection in both men and women. It is estimated that 79 million Americans are infected and 14 million new infections occur annually, usually in teens and young adults. Although most infections are asymptomatic and clear spontaneously, persistent infection with oncogenic types can progress to cancer. Cervical and oropharyngeal cancer were the most common HPV-associated cancers in women and men, respectively, in 2008-2012 (MMWR 2016;65:661-6).
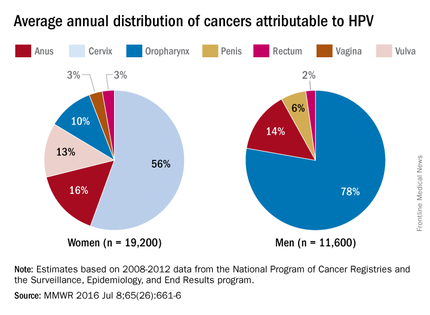
All three HPV vaccines protect against HPV types 16 and 18. These types are estimated to account for the majority of cervical and oropharyngeal cancers, 66% and 62%, respectively. The additional types in the 9-valent HPV will protect against HPV types that cause approximately 15% of cervical cancers.
The association between HPV and cancer is clear. So why isn’t this vaccine being embraced? HPV vaccine is all about cancer prevention. Isn’t it? What are the barriers to HPV vaccination? Are parental concerns the only barrier? Are we recommending this vaccine as strongly as others?
Vaccine safety and efficacy
Safety has been a concern voiced by some parents. Collectively, HPV vaccines were studied in more than 95,000 individuals prior to licensure. Almost 90 million doses of vaccine have been distributed in the United States and more than 183 million, worldwide. The federal government utilizes three systems to monitor vaccine safety once a vaccine is licensed: The Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Network. Ongoing safety studies also are conducted by vaccine manufacturers. Since licensure, no serious safety concerns have been identified. Postvaccination syncope, first identified in the VAERS database in 2006, has declined since observation post injection was recommended by ACIP. Multiple studies in the United States and abroad have not demonstrated a causal association with HPV vaccine and any autoimmune and/or neurologic condition or increased risk for thromboembolism.
Mélanie Drolet, PhD, and her colleagues reviewed 20 studies in nine countries with at least 50% coverage in female adolescents aged 13-19 years. There was a 68% reduction in the prevalence of HPV types 16 and 18 and a 61% reduction in anal warts in the postvaccine era (Lancet Infect Dis. 2015 May;15[5]:565-80). Studies also indicate there is no indication of waning immunity.
Parental perceptions
Some parents feel the vaccine is not necessary because their child is not sexually active and/or is not at risk for acquiring a sexually transmitted infection. Others opt to delay initiation. NHANES (National Health and Nutrition Examination Survey) data from 2011 to 2014 revealed that among females aged 14-26 years whose age was known at the time of their first dose of HPV vaccine, 43% had reported having sex before or in the same year that they received their first dose.
One consistent reason parents indicate for not vaccinating is the lack of recommendation from their child’s health provider. Differences in age and sex recommendations also are reported. NIS-Teen 2013 demonstrated that parents of girls were more likely than parents of boys to receive a provider recommendation (65% vs.42%.) Only 29% of female parents indicated they’d received a provider recommendation to have their child vaccinated with HPV by ages 11-12 years.
Mandy A. Allison, MD, and her colleagues reviewed primary care physician perspectives about HPV vaccine in a national survey among 364 pediatricians and 218 family physicians (FPs). Although 84% of pediatricians and 75% of FPs indicated they always discuss HPV vaccination, only 60% of pediatricians and 59% of FPs strongly recommend HPV vaccine for 11- to 12-year-old girls; for boys it was 52% and 41%. More than half reported parental deferral. For pediatricians who almost never discussed the topic, the reasons included that the patient was not sexually active (54%), the child was young (38%), and the patient was already receiving other vaccines (35%) (Pediatrics. 2016 Feb;137[2]:e20152488).
Providers can be influenced by their perceptions of what value parents place on vaccines. In one study, parents were asked to put a value on specific vaccines. Providers were then asked to estimate how parents ranked the vaccines on a scale of 0-10. Providers underestimated the value placed on HPV vaccine (9.3 vs 5.2) (Vaccine 2014;32:579-84).
Improving HPV coverage: Preventing future HPV-related cancers
HPV vaccine should be recommended with as much conviction as Tdap and MCV at the 11- to 12-year visit for both girls and boys. Administration of all three should occur on the same day. Clinician recommendation is the No. 1 reason parents decide to vaccinate. The mantra “same way, same day” should become synonymous with the 11- to 12-year visit. All who have contact with the patient, beginning with the front desk staff, should know the importance of HPV vaccine, and when and why it is recommended. Often, families spend more time with support staff and have discussions prior to interacting with you.
Anticipate questions about HPV. Why give the vaccine when the child is so young and not sexually active? Is my child really at risk? Is it safe? I read on the Internet. … Questions should be interpreted as a need for additional information and reassurance from you.
Remember to emphasize that HPV vaccine is important because it prevents cancer and it is most effective prior to exposure to HPV.
Additional resources to facilitate your discussions about HPV can be found at www.cdc.gov/hpv.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It has been almost 10 years since the Advisory Committee on Immunization Practices (ACIP) recommended administration of human papillomavirus (HPV) vaccine for 11- to 12-year-old girls and young women up to 26 years of age. Routine administration in preteen boys and young adult males up to 21 years of age was recommended in 2011. An HPV series should be completed by 13 years. So how well are we protecting our patients?
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually among adolescents 13-17 years. Data are obtained from individuals from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR. 2016 Aug 26;65[33]:850-8).
HPV vaccination continues to lag behind Tdap and the meningococcal conjugate vaccine (MCV), although each one is recommended to be administered at the 11- to 12-year visit. In 2015, coverage for receiving at least one dose of HPV vaccine among females was almost 62.8 % and for at least three doses was 41.9%; among males, coverage with at least one dose was 49.8% and for at least three doses was 28.1%. Compared with 2014, coverage for at least one dose of HPV vaccine increased 2.8% in females and 8.1% in males. Males also had a 7.6% for receipt of at least two doses of HPV vaccine, compared with 2014. HPV vaccine coverage in females aged 13 and younger also was lower than for those aged 15 and older. Coverage did not differ for males based on age.
HPV vaccination coverage also differed by state. In 2015, 28 states reported increased coverage in males, but only 7 states had increased coverage in females. Among all adolescents, coverage with at least one dose of HPV vaccine was 56.1%, at least two doses was 45.4%, and at least three doses was 34.9%. In contrast, 86.4% of all adolescents received at least one dose of Tdap, and 81.3% received at least one dose of MCV.
HPV-associated cancers
HPV is the most common sexually transmitted infection in both men and women. It is estimated that 79 million Americans are infected and 14 million new infections occur annually, usually in teens and young adults. Although most infections are asymptomatic and clear spontaneously, persistent infection with oncogenic types can progress to cancer. Cervical and oropharyngeal cancer were the most common HPV-associated cancers in women and men, respectively, in 2008-2012 (MMWR 2016;65:661-6).

All three HPV vaccines protect against HPV types 16 and 18. These types are estimated to account for the majority of cervical and oropharyngeal cancers, 66% and 62%, respectively. The additional types in the 9-valent HPV will protect against HPV types that cause approximately 15% of cervical cancers.
The association between HPV and cancer is clear. So why isn’t this vaccine being embraced? HPV vaccine is all about cancer prevention. Isn’t it? What are the barriers to HPV vaccination? Are parental concerns the only barrier? Are we recommending this vaccine as strongly as others?
Vaccine safety and efficacy
Safety has been a concern voiced by some parents. Collectively, HPV vaccines were studied in more than 95,000 individuals prior to licensure. Almost 90 million doses of vaccine have been distributed in the United States and more than 183 million, worldwide. The federal government utilizes three systems to monitor vaccine safety once a vaccine is licensed: The Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Network. Ongoing safety studies also are conducted by vaccine manufacturers. Since licensure, no serious safety concerns have been identified. Postvaccination syncope, first identified in the VAERS database in 2006, has declined since observation post injection was recommended by ACIP. Multiple studies in the United States and abroad have not demonstrated a causal association with HPV vaccine and any autoimmune and/or neurologic condition or increased risk for thromboembolism.
Mélanie Drolet, PhD, and her colleagues reviewed 20 studies in nine countries with at least 50% coverage in female adolescents aged 13-19 years. There was a 68% reduction in the prevalence of HPV types 16 and 18 and a 61% reduction in anal warts in the postvaccine era (Lancet Infect Dis. 2015 May;15[5]:565-80). Studies also indicate there is no indication of waning immunity.
Parental perceptions
Some parents feel the vaccine is not necessary because their child is not sexually active and/or is not at risk for acquiring a sexually transmitted infection. Others opt to delay initiation. NHANES (National Health and Nutrition Examination Survey) data from 2011 to 2014 revealed that among females aged 14-26 years whose age was known at the time of their first dose of HPV vaccine, 43% had reported having sex before or in the same year that they received their first dose.

One consistent reason parents indicate for not vaccinating is the lack of recommendation from their child’s health provider. Differences in age and sex recommendations also are reported. NIS-Teen 2013 demonstrated that parents of girls were more likely than parents of boys to receive a provider recommendation (65% vs.42%.) Only 29% of female parents indicated they’d received a provider recommendation to have their child vaccinated with HPV by ages 11-12 years.
Mandy A. Allison, MD, and her colleagues reviewed primary care physician perspectives about HPV vaccine in a national survey among 364 pediatricians and 218 family physicians (FPs). Although 84% of pediatricians and 75% of FPs indicated they always discuss HPV vaccination, only 60% of pediatricians and 59% of FPs strongly recommend HPV vaccine for 11- to 12-year-old girls; for boys it was 52% and 41%. More than half reported parental deferral. For pediatricians who almost never discussed the topic, the reasons included that the patient was not sexually active (54%), the child was young (38%), and the patient was already receiving other vaccines (35%) (Pediatrics. 2016 Feb;137[2]:e20152488).
Providers can be influenced by their perceptions of what value parents place on vaccines. In one study, parents were asked to put a value on specific vaccines. Providers were then asked to estimate how parents ranked the vaccines on a scale of 0-10. Providers underestimated the value placed on HPV vaccine (9.3 vs 5.2) (Vaccine 2014;32:579-84).
Improving HPV coverage: Preventing future HPV-related cancers
HPV vaccine should be recommended with as much conviction as Tdap and MCV at the 11- to 12-year visit for both girls and boys. Administration of all three should occur on the same day. Clinician recommendation is the No. 1 reason parents decide to vaccinate. The mantra “same way, same day” should become synonymous with the 11- to 12-year visit. All who have contact with the patient, beginning with the front desk staff, should know the importance of HPV vaccine, and when and why it is recommended. Often, families spend more time with support staff and have discussions prior to interacting with you.
Anticipate questions about HPV. Why give the vaccine when the child is so young and not sexually active? Is my child really at risk? Is it safe? I read on the Internet. … Questions should be interpreted as a need for additional information and reassurance from you.
Remember to emphasize that HPV vaccine is important because it prevents cancer and it is most effective prior to exposure to HPV.
Additional resources to facilitate your discussions about HPV can be found at www.cdc.gov/hpv.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
It has been almost 10 years since the Advisory Committee on Immunization Practices (ACIP) recommended administration of human papillomavirus (HPV) vaccine for 11- to 12-year-old girls and young women up to 26 years of age. Routine administration in preteen boys and young adult males up to 21 years of age was recommended in 2011. An HPV series should be completed by 13 years. So how well are we protecting our patients?
Vaccine coverage
The National Immunization Survey–Teen (NIS-Teen) monitors vaccine coverage annually among adolescents 13-17 years. Data are obtained from individuals from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR. 2016 Aug 26;65[33]:850-8).
HPV vaccination continues to lag behind Tdap and the meningococcal conjugate vaccine (MCV), although each one is recommended to be administered at the 11- to 12-year visit. In 2015, coverage for receiving at least one dose of HPV vaccine among females was almost 62.8 % and for at least three doses was 41.9%; among males, coverage with at least one dose was 49.8% and for at least three doses was 28.1%. Compared with 2014, coverage for at least one dose of HPV vaccine increased 2.8% in females and 8.1% in males. Males also had a 7.6% for receipt of at least two doses of HPV vaccine, compared with 2014. HPV vaccine coverage in females aged 13 and younger also was lower than for those aged 15 and older. Coverage did not differ for males based on age.
HPV vaccination coverage also differed by state. In 2015, 28 states reported increased coverage in males, but only 7 states had increased coverage in females. Among all adolescents, coverage with at least one dose of HPV vaccine was 56.1%, at least two doses was 45.4%, and at least three doses was 34.9%. In contrast, 86.4% of all adolescents received at least one dose of Tdap, and 81.3% received at least one dose of MCV.
HPV-associated cancers
HPV is the most common sexually transmitted infection in both men and women. It is estimated that 79 million Americans are infected and 14 million new infections occur annually, usually in teens and young adults. Although most infections are asymptomatic and clear spontaneously, persistent infection with oncogenic types can progress to cancer. Cervical and oropharyngeal cancer were the most common HPV-associated cancers in women and men, respectively, in 2008-2012 (MMWR 2016;65:661-6).

All three HPV vaccines protect against HPV types 16 and 18. These types are estimated to account for the majority of cervical and oropharyngeal cancers, 66% and 62%, respectively. The additional types in the 9-valent HPV will protect against HPV types that cause approximately 15% of cervical cancers.
The association between HPV and cancer is clear. So why isn’t this vaccine being embraced? HPV vaccine is all about cancer prevention. Isn’t it? What are the barriers to HPV vaccination? Are parental concerns the only barrier? Are we recommending this vaccine as strongly as others?
Vaccine safety and efficacy
Safety has been a concern voiced by some parents. Collectively, HPV vaccines were studied in more than 95,000 individuals prior to licensure. Almost 90 million doses of vaccine have been distributed in the United States and more than 183 million, worldwide. The federal government utilizes three systems to monitor vaccine safety once a vaccine is licensed: The Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment (CISA) Network. Ongoing safety studies also are conducted by vaccine manufacturers. Since licensure, no serious safety concerns have been identified. Postvaccination syncope, first identified in the VAERS database in 2006, has declined since observation post injection was recommended by ACIP. Multiple studies in the United States and abroad have not demonstrated a causal association with HPV vaccine and any autoimmune and/or neurologic condition or increased risk for thromboembolism.
Mélanie Drolet, PhD, and her colleagues reviewed 20 studies in nine countries with at least 50% coverage in female adolescents aged 13-19 years. There was a 68% reduction in the prevalence of HPV types 16 and 18 and a 61% reduction in anal warts in the postvaccine era (Lancet Infect Dis. 2015 May;15[5]:565-80). Studies also indicate there is no indication of waning immunity.
Parental perceptions
Some parents feel the vaccine is not necessary because their child is not sexually active and/or is not at risk for acquiring a sexually transmitted infection. Others opt to delay initiation. NHANES (National Health and Nutrition Examination Survey) data from 2011 to 2014 revealed that among females aged 14-26 years whose age was known at the time of their first dose of HPV vaccine, 43% had reported having sex before or in the same year that they received their first dose.

One consistent reason parents indicate for not vaccinating is the lack of recommendation from their child’s health provider. Differences in age and sex recommendations also are reported. NIS-Teen 2013 demonstrated that parents of girls were more likely than parents of boys to receive a provider recommendation (65% vs.42%.) Only 29% of female parents indicated they’d received a provider recommendation to have their child vaccinated with HPV by ages 11-12 years.
Mandy A. Allison, MD, and her colleagues reviewed primary care physician perspectives about HPV vaccine in a national survey among 364 pediatricians and 218 family physicians (FPs). Although 84% of pediatricians and 75% of FPs indicated they always discuss HPV vaccination, only 60% of pediatricians and 59% of FPs strongly recommend HPV vaccine for 11- to 12-year-old girls; for boys it was 52% and 41%. More than half reported parental deferral. For pediatricians who almost never discussed the topic, the reasons included that the patient was not sexually active (54%), the child was young (38%), and the patient was already receiving other vaccines (35%) (Pediatrics. 2016 Feb;137[2]:e20152488).
Providers can be influenced by their perceptions of what value parents place on vaccines. In one study, parents were asked to put a value on specific vaccines. Providers were then asked to estimate how parents ranked the vaccines on a scale of 0-10. Providers underestimated the value placed on HPV vaccine (9.3 vs 5.2) (Vaccine 2014;32:579-84).
Improving HPV coverage: Preventing future HPV-related cancers
HPV vaccine should be recommended with as much conviction as Tdap and MCV at the 11- to 12-year visit for both girls and boys. Administration of all three should occur on the same day. Clinician recommendation is the No. 1 reason parents decide to vaccinate. The mantra “same way, same day” should become synonymous with the 11- to 12-year visit. All who have contact with the patient, beginning with the front desk staff, should know the importance of HPV vaccine, and when and why it is recommended. Often, families spend more time with support staff and have discussions prior to interacting with you.
Anticipate questions about HPV. Why give the vaccine when the child is so young and not sexually active? Is my child really at risk? Is it safe? I read on the Internet. … Questions should be interpreted as a need for additional information and reassurance from you.
Remember to emphasize that HPV vaccine is important because it prevents cancer and it is most effective prior to exposure to HPV.
Additional resources to facilitate your discussions about HPV can be found at www.cdc.gov/hpv.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Summer flu? Think variant swine influenza virus infection
Two children presented with influenza, and both recovered without the need for hospitalization. This scenario would fail to pique the interest of any pediatrician in January. But what about when it happens in July?
In early August, public health authorities in Ohio announced that two children had tested positive for the variant swine influenza virus H3N2v. Both children had direct contact with pigs at the Clark County Fair in late July. Along with a handful of cases diagnosed in Michigan, these represent the first H3N2v cases in the United States in 2016.
Influenza viruses that normally circulate in swine are designated as “variant” when they infect humans. According to the Centers for Disease Control and Prevention (CDC), human infections with H1N1v, H1N2v, and H3N2v have been identified in the United States. Influenza A H3N2v viruses carrying the matrix gene from the 2009 H1N1 pandemic virus were first detected in pigs in 2010, and in people in the summer of 2011. Since that time, 357 human cases have been reported from 14 states, with nearly 75% occurring in Indiana and Ohio. Most infections occurred after prolonged exposure to pigs at agricultural fairs.
Fortunately, most H3N2v infections have been mild: Since July 2012, only 21 individuals have required hospitalization and a single case resulted in death. Notably, though, many of the hospitalizations involved children.
On Aug. 15, the Centers for Disease Control and Prevention released Interim Guidance for Clinicians on Human Infections with Variant Influenza Viruses.
Because variant virus infection is indistinguishable from seasonal influenza or any other virus that cause influenzalike illness (think fever, cough, sore throat), physicians and other frontline providers need to maintain an index of suspicion. The key is eliciting a history of swine exposure in the week before illness onset. Practically, this means asking about direct contact with pigs, indirect contact with pigs, or close contact with an ill person who has had contact with pigs. Kudos to the astute clinicians in Ohio who thought to send the appropriate influenza testing in July.
When variant influenza virus is suspected, a nasopharyngeal swab or aspirate should be obtained for testing at a state public health lab or the CDC. Rapid antigen tests for influenza may be falsely negative in the setting of H3N2v infection, just as they may be with seasonal influenza infection. Molecular tests such as reverse transcription polymerase chain reaction (RT-PCR) are likely more sensitive, but cannot distinguish variant influenza A viruses from seasonal influenza A viruses.
The Kentucky State Fair opened on Aug. 18, making the CDC guidance especially timely for health care providers in my area. I called a friend who is a pediatric emergency medicine physician to ask if she and her colleagues were routinely screening patients for encounters of the porcine kind.
“For example, are you asking, ‘Have you been showing, raising or feeding swine? Have you been to the pig barn at the fair?’ ”
When my friend quit laughing, I confessed to her that I had not been doing that routinely either. The exposure history is often the most interesting part of the infectious disease evaluation and in the last month, I’ve asked about exposure to sheep (a risk factor for Q fever), exposure to chickens (a risk factor for Salmonella infections), and exposure to beaver dams (a risk factor for blastomycosis). But I’ve not asked about exposure to pigs.
“The emergency medicine approach is to avoid a lot of viral diagnostic testing unless it is going to impact management,” she said. “Tell me how this changes management of my patient.”
From the patient perspective, making a presumptive diagnosis of H3N2v infection would open the door to empiric treatment with antivirals, at least for individuals who are hospitalized, have severe or progressive disease, or who at high risk for complications of influenza. Neuraminidase inhibitors, including oral oseltamivir, inhaled zanamivir, and intravenous peramivir, can be used for treatment of H3N2v infections.
From a societal perspective, making the diagnosis gives public health experts the opportunity to investigate and potentially prevent further infections by isolating sick pigs. Human to human transmission of H3N2v is rare, but has occasionally occurred in households and in one instance, a child care setting. Careful surveillance of each swine flu case in a human is important to exclude the possibility that the virus has developed the ability to spread efficiently from person to person, creating the risk for an epidemic.
Seasonal influenza vaccine does not prevent infection with variant viruses, so avoidance is key. Those at high risk for complications from influenza infection, including children younger than 5 years of age and those with asthma, diabetes, heart disease, immunocompromised conditions, and neurologic or neurodevelopmental disorders, are urged to avoid pigs and swine barns when visiting fairs where the animals are present. Everyone else needs to follow common sense measures to prevent the spread of infection.
• Don’t take food or drink into pig areas; don’t eat, drink or put anything in your mouth in pig areas.
• Don’t take toys, pacifiers, cups, baby bottles, strollers, or similar items into pig areas.
• Wash your hands often with soap and running water before and after exposure to pigs. If soap and water are not available, use an alcohol-based hand rub.
• Avoid close contact with pigs that look or act ill.
• Take protective measures if you must come in contact with pigs that are known or suspected to be sick. This includes wearing personal protective equipment like protective clothing, gloves, and masks that cover your mouth and nose when contact is required.
• To further reduce the risk of infection, minimize contact with pigs in the pig barn and arenas.
It shouldn’t be surprising that flu viruses spread from pigs to people in the same way that regular seasonal influenza spread from person to person. An infected pig coughs or sneezes influenza-containing droplets, and these droplets are inhaled or swallowed by a susceptible human. That makes avoiding contact with pigs that look or act ill especially important. For the record, a pig with flu might have fever, depression, cough, nasal or eye discharge, eye redness, or a poor appetite.
On the bright side, you can’t get H3N2v or any other variant virus from eating properly prepared pork meat. Fairgoers can feel free to indulge in a deep-fried pork chop or one of this year’s featured food items: a basket of French fries topped with smoked pork, cheddar cheese sauce, red onions, jalapeño peppers and barbecue sauce.
Or maybe not. The CDC has a web page devoted to food safety at fairs and festivals. It notes that cases of foodborne illness increase during summer months, and usual safety controls “like monitoring of food temperatures, refrigeration, workers trained in food safety and washing facilities, may not be available when cooking and dining at fairs and festivals.”
The public is urged to seek out “healthy options” from fair vendors first. If healthy options aren’t available, we’re advised to consider bringing food from home to save money and calories.
Sigh. I remember when summer used to be more fun.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky. and Kosair Children’s Hospital, also in Louisville.
Two children presented with influenza, and both recovered without the need for hospitalization. This scenario would fail to pique the interest of any pediatrician in January. But what about when it happens in July?
In early August, public health authorities in Ohio announced that two children had tested positive for the variant swine influenza virus H3N2v. Both children had direct contact with pigs at the Clark County Fair in late July. Along with a handful of cases diagnosed in Michigan, these represent the first H3N2v cases in the United States in 2016.
Influenza viruses that normally circulate in swine are designated as “variant” when they infect humans. According to the Centers for Disease Control and Prevention (CDC), human infections with H1N1v, H1N2v, and H3N2v have been identified in the United States. Influenza A H3N2v viruses carrying the matrix gene from the 2009 H1N1 pandemic virus were first detected in pigs in 2010, and in people in the summer of 2011. Since that time, 357 human cases have been reported from 14 states, with nearly 75% occurring in Indiana and Ohio. Most infections occurred after prolonged exposure to pigs at agricultural fairs.
Fortunately, most H3N2v infections have been mild: Since July 2012, only 21 individuals have required hospitalization and a single case resulted in death. Notably, though, many of the hospitalizations involved children.
On Aug. 15, the Centers for Disease Control and Prevention released Interim Guidance for Clinicians on Human Infections with Variant Influenza Viruses.
Because variant virus infection is indistinguishable from seasonal influenza or any other virus that cause influenzalike illness (think fever, cough, sore throat), physicians and other frontline providers need to maintain an index of suspicion. The key is eliciting a history of swine exposure in the week before illness onset. Practically, this means asking about direct contact with pigs, indirect contact with pigs, or close contact with an ill person who has had contact with pigs. Kudos to the astute clinicians in Ohio who thought to send the appropriate influenza testing in July.
When variant influenza virus is suspected, a nasopharyngeal swab or aspirate should be obtained for testing at a state public health lab or the CDC. Rapid antigen tests for influenza may be falsely negative in the setting of H3N2v infection, just as they may be with seasonal influenza infection. Molecular tests such as reverse transcription polymerase chain reaction (RT-PCR) are likely more sensitive, but cannot distinguish variant influenza A viruses from seasonal influenza A viruses.
The Kentucky State Fair opened on Aug. 18, making the CDC guidance especially timely for health care providers in my area. I called a friend who is a pediatric emergency medicine physician to ask if she and her colleagues were routinely screening patients for encounters of the porcine kind.
“For example, are you asking, ‘Have you been showing, raising or feeding swine? Have you been to the pig barn at the fair?’ ”
When my friend quit laughing, I confessed to her that I had not been doing that routinely either. The exposure history is often the most interesting part of the infectious disease evaluation and in the last month, I’ve asked about exposure to sheep (a risk factor for Q fever), exposure to chickens (a risk factor for Salmonella infections), and exposure to beaver dams (a risk factor for blastomycosis). But I’ve not asked about exposure to pigs.
“The emergency medicine approach is to avoid a lot of viral diagnostic testing unless it is going to impact management,” she said. “Tell me how this changes management of my patient.”
From the patient perspective, making a presumptive diagnosis of H3N2v infection would open the door to empiric treatment with antivirals, at least for individuals who are hospitalized, have severe or progressive disease, or who at high risk for complications of influenza. Neuraminidase inhibitors, including oral oseltamivir, inhaled zanamivir, and intravenous peramivir, can be used for treatment of H3N2v infections.
From a societal perspective, making the diagnosis gives public health experts the opportunity to investigate and potentially prevent further infections by isolating sick pigs. Human to human transmission of H3N2v is rare, but has occasionally occurred in households and in one instance, a child care setting. Careful surveillance of each swine flu case in a human is important to exclude the possibility that the virus has developed the ability to spread efficiently from person to person, creating the risk for an epidemic.
Seasonal influenza vaccine does not prevent infection with variant viruses, so avoidance is key. Those at high risk for complications from influenza infection, including children younger than 5 years of age and those with asthma, diabetes, heart disease, immunocompromised conditions, and neurologic or neurodevelopmental disorders, are urged to avoid pigs and swine barns when visiting fairs where the animals are present. Everyone else needs to follow common sense measures to prevent the spread of infection.
• Don’t take food or drink into pig areas; don’t eat, drink or put anything in your mouth in pig areas.
• Don’t take toys, pacifiers, cups, baby bottles, strollers, or similar items into pig areas.
• Wash your hands often with soap and running water before and after exposure to pigs. If soap and water are not available, use an alcohol-based hand rub.
• Avoid close contact with pigs that look or act ill.
• Take protective measures if you must come in contact with pigs that are known or suspected to be sick. This includes wearing personal protective equipment like protective clothing, gloves, and masks that cover your mouth and nose when contact is required.
• To further reduce the risk of infection, minimize contact with pigs in the pig barn and arenas.
It shouldn’t be surprising that flu viruses spread from pigs to people in the same way that regular seasonal influenza spread from person to person. An infected pig coughs or sneezes influenza-containing droplets, and these droplets are inhaled or swallowed by a susceptible human. That makes avoiding contact with pigs that look or act ill especially important. For the record, a pig with flu might have fever, depression, cough, nasal or eye discharge, eye redness, or a poor appetite.
On the bright side, you can’t get H3N2v or any other variant virus from eating properly prepared pork meat. Fairgoers can feel free to indulge in a deep-fried pork chop or one of this year’s featured food items: a basket of French fries topped with smoked pork, cheddar cheese sauce, red onions, jalapeño peppers and barbecue sauce.
Or maybe not. The CDC has a web page devoted to food safety at fairs and festivals. It notes that cases of foodborne illness increase during summer months, and usual safety controls “like monitoring of food temperatures, refrigeration, workers trained in food safety and washing facilities, may not be available when cooking and dining at fairs and festivals.”
The public is urged to seek out “healthy options” from fair vendors first. If healthy options aren’t available, we’re advised to consider bringing food from home to save money and calories.
Sigh. I remember when summer used to be more fun.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky. and Kosair Children’s Hospital, also in Louisville.
Two children presented with influenza, and both recovered without the need for hospitalization. This scenario would fail to pique the interest of any pediatrician in January. But what about when it happens in July?
In early August, public health authorities in Ohio announced that two children had tested positive for the variant swine influenza virus H3N2v. Both children had direct contact with pigs at the Clark County Fair in late July. Along with a handful of cases diagnosed in Michigan, these represent the first H3N2v cases in the United States in 2016.
Influenza viruses that normally circulate in swine are designated as “variant” when they infect humans. According to the Centers for Disease Control and Prevention (CDC), human infections with H1N1v, H1N2v, and H3N2v have been identified in the United States. Influenza A H3N2v viruses carrying the matrix gene from the 2009 H1N1 pandemic virus were first detected in pigs in 2010, and in people in the summer of 2011. Since that time, 357 human cases have been reported from 14 states, with nearly 75% occurring in Indiana and Ohio. Most infections occurred after prolonged exposure to pigs at agricultural fairs.
Fortunately, most H3N2v infections have been mild: Since July 2012, only 21 individuals have required hospitalization and a single case resulted in death. Notably, though, many of the hospitalizations involved children.
On Aug. 15, the Centers for Disease Control and Prevention released Interim Guidance for Clinicians on Human Infections with Variant Influenza Viruses.
Because variant virus infection is indistinguishable from seasonal influenza or any other virus that cause influenzalike illness (think fever, cough, sore throat), physicians and other frontline providers need to maintain an index of suspicion. The key is eliciting a history of swine exposure in the week before illness onset. Practically, this means asking about direct contact with pigs, indirect contact with pigs, or close contact with an ill person who has had contact with pigs. Kudos to the astute clinicians in Ohio who thought to send the appropriate influenza testing in July.
When variant influenza virus is suspected, a nasopharyngeal swab or aspirate should be obtained for testing at a state public health lab or the CDC. Rapid antigen tests for influenza may be falsely negative in the setting of H3N2v infection, just as they may be with seasonal influenza infection. Molecular tests such as reverse transcription polymerase chain reaction (RT-PCR) are likely more sensitive, but cannot distinguish variant influenza A viruses from seasonal influenza A viruses.
The Kentucky State Fair opened on Aug. 18, making the CDC guidance especially timely for health care providers in my area. I called a friend who is a pediatric emergency medicine physician to ask if she and her colleagues were routinely screening patients for encounters of the porcine kind.
“For example, are you asking, ‘Have you been showing, raising or feeding swine? Have you been to the pig barn at the fair?’ ”
When my friend quit laughing, I confessed to her that I had not been doing that routinely either. The exposure history is often the most interesting part of the infectious disease evaluation and in the last month, I’ve asked about exposure to sheep (a risk factor for Q fever), exposure to chickens (a risk factor for Salmonella infections), and exposure to beaver dams (a risk factor for blastomycosis). But I’ve not asked about exposure to pigs.
“The emergency medicine approach is to avoid a lot of viral diagnostic testing unless it is going to impact management,” she said. “Tell me how this changes management of my patient.”
From the patient perspective, making a presumptive diagnosis of H3N2v infection would open the door to empiric treatment with antivirals, at least for individuals who are hospitalized, have severe or progressive disease, or who at high risk for complications of influenza. Neuraminidase inhibitors, including oral oseltamivir, inhaled zanamivir, and intravenous peramivir, can be used for treatment of H3N2v infections.
From a societal perspective, making the diagnosis gives public health experts the opportunity to investigate and potentially prevent further infections by isolating sick pigs. Human to human transmission of H3N2v is rare, but has occasionally occurred in households and in one instance, a child care setting. Careful surveillance of each swine flu case in a human is important to exclude the possibility that the virus has developed the ability to spread efficiently from person to person, creating the risk for an epidemic.
Seasonal influenza vaccine does not prevent infection with variant viruses, so avoidance is key. Those at high risk for complications from influenza infection, including children younger than 5 years of age and those with asthma, diabetes, heart disease, immunocompromised conditions, and neurologic or neurodevelopmental disorders, are urged to avoid pigs and swine barns when visiting fairs where the animals are present. Everyone else needs to follow common sense measures to prevent the spread of infection.
• Don’t take food or drink into pig areas; don’t eat, drink or put anything in your mouth in pig areas.
• Don’t take toys, pacifiers, cups, baby bottles, strollers, or similar items into pig areas.
• Wash your hands often with soap and running water before and after exposure to pigs. If soap and water are not available, use an alcohol-based hand rub.
• Avoid close contact with pigs that look or act ill.
• Take protective measures if you must come in contact with pigs that are known or suspected to be sick. This includes wearing personal protective equipment like protective clothing, gloves, and masks that cover your mouth and nose when contact is required.
• To further reduce the risk of infection, minimize contact with pigs in the pig barn and arenas.
It shouldn’t be surprising that flu viruses spread from pigs to people in the same way that regular seasonal influenza spread from person to person. An infected pig coughs or sneezes influenza-containing droplets, and these droplets are inhaled or swallowed by a susceptible human. That makes avoiding contact with pigs that look or act ill especially important. For the record, a pig with flu might have fever, depression, cough, nasal or eye discharge, eye redness, or a poor appetite.
On the bright side, you can’t get H3N2v or any other variant virus from eating properly prepared pork meat. Fairgoers can feel free to indulge in a deep-fried pork chop or one of this year’s featured food items: a basket of French fries topped with smoked pork, cheddar cheese sauce, red onions, jalapeño peppers and barbecue sauce.
Or maybe not. The CDC has a web page devoted to food safety at fairs and festivals. It notes that cases of foodborne illness increase during summer months, and usual safety controls “like monitoring of food temperatures, refrigeration, workers trained in food safety and washing facilities, may not be available when cooking and dining at fairs and festivals.”
The public is urged to seek out “healthy options” from fair vendors first. If healthy options aren’t available, we’re advised to consider bringing food from home to save money and calories.
Sigh. I remember when summer used to be more fun.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky. and Kosair Children’s Hospital, also in Louisville.