User login
Moroccan Health Care: A Link to Radicalization and Proposed Solution
The relationship between the Kingdom of Morocco and the US began just after the US declared its own independence. It is one of the oldest of US partnerships with a foreign country, and since the end of the First Barbary War in 1805 it has remained one of the most stable. The Utah National Guard (UTNG) has an active state partnership program (SPP) with Morocco, which helps maintain that stability and fosters the relationship. The SPP provides the Kingdom of Morocco assistance in the areas of disaster medicine, prehospital medicine, and rural access to health care.
The objective of this review is to highlight the role the SPP plays in ensuring Morocco’s continued stability, enhancing its role as a leader among African nations, aiding its medically vulnerable rural populations to prevent recruitment by terrorist organizations, and maintaining its long-term relationship with the US.
Background
The Kingdom of Morocco resides in a geologically and politically unstable part of the world, yet it has been a stable constitutional monarchy. Like California, Morocco has a long coastline of more than 1,000 miles. It sits along an active earthquake fault line with a disaster response program that is only in its infancy. The Kingdom has a high youth unemployment rate and lacks adequate public education opportunities, which exacerbate feelings of government indifference. Morocco’s medical system is highly centralized, and large parts of the rural population lack access to basic medical care—potentially alienating the population. The Moroccan current disaster plan ORSEC (plan d’ Organization des Secours) was established in 1966 and updated in 2005 but does not provide a comprehensive, unified disaster response. The ORSEC plan is of French derivation and is not a list of actions but a general plan of organization and supply. 1
When governments fail to provide basic services—health care being just one—those services may be filled by groups seeking to influence the government and population by threatening acts of violence to achieve political, religious, and ideologic gain; for example, the Taliban in Afghanistan, the Muslim brotherhood in Egypt and in the West Bank, and the Islamic State in Iraq and Syria (ISIS) in Syria.2-5 These groups gain a foothold and legitimacy by providing mosques, youth groups, clinics, hospitals, and schools. 2-5
Identified Needs
Morocco is at risk of experiencing an earthquake and possible subsequent tsunami. In 1755, Morocco was impacted by the Great Lisbon earthquake and tsunami. Witnesses reported 15-meter waves with 24-meter crests.6 Building codes and architecture laws have changed little since the 1960 Agadir earthquake, which killed 12,000 people. The disaster response program—although improved since the 1960s—is still in the early stages of development, and another earthquake and possible subsequent tsunami would result in a disaster that could overwhelm the medical community of Morocco.
Perceived Government Indifference
The Moroccan constitutional monarchy is more stable than are the governments of its North African neighbors. King Mohammed VI presides over the government, and regular elections are held for members of Parliament, which names a prime minister. However, in August 2019, overall unemployment was at 8.5%, and youth unemployment was 22.3%.7 A United Nations report in August 2019 stated that literacy rates for Morocco were 71.7%. These data were from a 2015 census, the last year data were collected.8 These deficits in employment and education can foster anger toward the Moroccan government for not adequately providing these services and possibly introduce radicalization as a result of the population’s perceived government indifference and lack of economic mobility.
Access to Medical Care
Morocco has a 2-tiered medical system for providing services: urban and rural. In 2018 the Legatum Prosperity Index ranked Morocco 103 of 149 countries in health care. The prosperity index measures health variables, which include but were not limited to basic physical and mental health, health infrastructure, and preventive care.9 Outside the metropolitan areas, emergency medical care is nonexistent, primary care is sporadic, and there is little modern technology available.
Despite humanitarian efforts over many years, there is little to no medical care in the rural “medical desert.” A 2017 study from the University of Washington Institute for Health Metrics and Evaluation compared the global burden of disease in similar countries. The study found that Morocco was significantly higher than the mean in the prevalence of ischemic heart disease and Alzheimer disease, lower than the mean in the areas of neonatal disorders, lower respiratory infections, and tuberculosis, and statistically indistinct from the mean in stroke, congenital defects, road injuries, diabetes mellitus, and hypertensive heart disease compared with the disease prevalence of other countries of similar size and economic measures.10 The study also found a particularly acute disparity in access to health care in rural areas. In 2016, the Oxford Business Group reported staff shortages and disproportionate distribution of resources in the Moroccan health care system.11
Additionally, the lack of trained health care personnel has added to an already overstressed health care system. A chief stressor in a health care system is an insufficient replacement rate. Health employees working for the Moroccan Ministry of Health retire at a rate of 1,500 per year.10,11 These shortages may serve to further the feelings of frustration and government indifference. This frustration is momentarily decreased by humanitarian efforts that have taken place in the African continent in the past decades, but this band-aid approach to assisting the population that is medically underserved has done little to alleviate the long-term problem of access to care. And feelings of government abandonment can sow the seeds of discontent in the rural population, creating fertile ground for recruitment by terrorist organizations.2,3
Lack of Health Care and Radicalism
It has been postulated that there is a link between radicalization and lack of medical care. Depression and perceived government indifference are considered contributors to radicalization.12-16 In 2005, Victoroff suggested that there are certain psychological traits characteristic of “typical" terrorists: these include high affective valence regarding an ideologic issue, a personal stake (perceived oppression, persecution or humiliation, need for identity, glory, or vengeance), low cognitive ability, low tolerance for ambiguity, and a capacity to suppress instinctive and learned moral constraints against harming innocents.15 In 2009, Lafree and Ackerman suggested that terrorism feeds on the ability of groups to portray governments and their agents as illegitimate.16 It is possible that part of the illegitimacy campaign of radicalization and terrorist recruitment may be identification of the lack of health care by the government thus magnifying feelings of government abandonment in a vulnerable population.
In 2011, the new Moroccan constitution identified access to basic health care as a right of the Moroccan people.17 Additionally, in 2013, a government white paper was produced outlining the need to increase access to health care, particularly in rural areas, including a focus on infant and maternal mortality, diabetes mellitus (DM), heart disease, and respiratory problems.17,18
Proposed Solutions, A Beginning
A health outreach program with a regional health professional training center in a relatively stable country within the African Union (AU) would be a step toward delivering health care to Morocco and interested AU members. Interested nations have been and will continue to be invited to train at the Moroccan center and return to their countries and start training programs. This idea was echoed by the World Bank in a 2015 loan proposal to Morocco, which suggested that addressing disparities in access to health care is a social justice issue, with other benefits such as increased productivity, employment, lower out-of-pocket expenditures, and promotion of good governance.17
In 2012, Buhi reported that a positive regard for authorities and healthier influences seemed to be a protective factor against radicalization. He also suggested a public health approach to understanding and preventing violent radicalization.19 The solutions are complex, especially in rural areas and in vulnerable nations common to Africa.
Medical training efforts by the US Department of Defense (DoD), Medical Readiness Training Institute (DMRT), and international health specialists working with the military and civilian entities in neighboring African countries have improved response to regional disasters. However, to address the broader issues, a more permanent, cooperative possible solution may begin with the establishment in Morocco of a regional education center for disaster preparedness and for health care providers (HCPs). This would serve as a training program for disaster first responders. Graduates of the program would receive additional training to become HCPs similar to physician assistant (PA) and nurse practitioner (NP) programs in the US. Morocco is uniquely positioned to accomplish this due to its location, political stability, and ties with other African nations.
The goal of the Moroccan regional education center (within the King Mohammed V Hospital) is to bring together global health experts and increase the intellectual infrastructure of not only Morocco, but also offer this training program to interested countries within the AU. Advancement of the regional education center will require legislative changes to expand prescriptive privileges and scope of practice within each country. The medical element of the SPP as presently constituted without the regional education center will continue its humanitarian goals, but the proposed creation of the regional education center will educate participants to serve the rural communities within each participating country. Eventually the entire educational program will be the responsibility of the Moroccan military and the AU participants. This will require reprioritizing resources from the provision of humanitarian health care services to an HCP education approach.
Disaster Response
Deficits in disaster response capabilities have been identified by members of the Moroccan military with the assistance of the UTNG. The most glaring deficit identified was the disparity in training between military and civilian first responders. Thus, a training program was initiated by the Moroccan military and the UTNG that combined internationally recognized, durable, robust emergency training programs. These programs consisted of, but were not limited to, parts or entire programs of the following: basic disaster life support, advanced disaster life support, disaster casualty care, and advanced trauma life support. The goal of this training was to improve communication, reduce mortality, and create strike teams, which can quickly provide health care independent of a hospital during a disaster.
Patients can overwhelm hospitals in a disaster when need exceeds resources. In 1996, Mallonee reported that at least 67% of the patients who sought care at a hospital during the Oklahoma City bombing disaster did not need advanced medical treatment.20 Such patients could be seen at an identified casualty collection point by a strike team and treated and released rather than traveling to the hospital and using staff and resources that could be used more judiciously for the more seriously injured.21 These teams consist of trained first responders with an experienced HCP (physician, PA, NP) and a nurse and are trained to operate for up to 72 hours in a predetermined location and serve as a “filter” for the hospital. Their role is to treat and release the less severely injured and refer only the more severely injured to the hospital after basic stabilization, thus preserving precious resources necessary for the more seriously injured.
This disaster response training program was offered to the Moroccan military, ministry of health and ministry of tourism, and quickly turned into an Africa-wide interest. A regional training center was proposed. This was assisted with the cooperation of Weber State University in Ogden Utah, Utah Valley University in Orem, and private interests in a public/private/military state partnership. Program supplies and didactic instruction were and will be provided by the UTNG and supplemented through the DoD Africa command. Instruction will be a cooperative effort agreed on between the UTNG and the Moroccan military medical specialists within their specific area of expertise.
Underserved Communities
Finally, from this pool of interested strike team members, a health care provider school will be formed to educate, certify, and service the needs of the underserved communities in Morocco and interested AU countries. This program will be similar to the PA and NP programs in the US and will be geared to those graduates from the previous programs with intense classroom instruction for one year followed by a year of one-on-one preceptorship with an experienced physician. The goal of the program is to prepare individuals with patient care experience to fulfill a bigger role in health care in an underserved (usually austere, rural) area that currently has minimal health care presence. This fills a need identified by the World Bank in 2015 that the Moroccan government needs to respond to the demand for improved access to and quality of health care services—particularly to the rural poor.17
The Moroccan military has a presence in many medically underserved areas. The logical fit for the HCP program will be drawn from a pool of active-duty military individuals who express an interest and qualify through attendance in all phases of the training.
Conclusion
This program of disaster medical education, strike teams, and HCPs is currently training more than 200 students a year throughout Morocco. The proposed direction of this cooperative program to produce HCPs in rural areas will increase access to health care for the Moroccan people who are now underserved. Morocco, as a health care training hub in Africa, will increase access to health care for interested African countries. The goal politically will be to reduce feelings of government indifference in vulnerable populations and reduce recruitment into radical ideologies.
1. Nahon M, Michaloux M. L’organisation de la réponse de la sécurité civile: le dispositif ORSEC Organisation of civilian emergency services: The ORSEC plan. https://www.sciencedirect.com/science/article/pii/S2211423816300499#! Published July 2016. Accessed October 7, 2019.
2. Berman E. Hamas, Taliban and the Jewish underground: an economist's view of radical religious militias. NBER Working Paper No. w10004. https://ssrn.com/abstract=450885. Published September 2003. Accessed October 7, 2019.
3. Jordan J. Attacking the leader. Missing the mark; why terrorist groups survive decapitation strikes. Int Secur. 2014;38(4):7-38.
4. Grynkewich A. Welfare as warfare: how violent non-state groups use social services to attack the state. Stud Conflict Terrorism. 2008;31(4):350-370.
5. Marin M, Solomon H. Islamic State: understanding the nature of the beast and its funding. Contemp Rev Middle East. 2017;4(1):18-49.
6. Bressan D. November 1, 1755: the earthquake of Lisbon: wrath of god or natural disaster? Scientific American, History of Geology. https://blogs.scientificamerican.com/history-of-geology/november-1-1755-the-earthquake-of-lisbon-wraith-of-god-or-natural-disaster. Published November 2011. Accessed October 7, 2019.
7. Trading Economics. Morocco unemployment rate. Second quarter statistics. August 2019. https://tradingeconomics.com/morocco/unemployment-rate. Accessed October 7, 2019.
8. Knoema World Data Atlas 2015. Morocco adult literacy rates. https://knoema.com/atlas/Morocco/topics/Education/Literacy/Adult-literacy-rate. Accessed October 4, 2019.
9. The Legatum Prosperity Index 2018. Morocco. https://www.prosperity.com/globe/morocco. Accessed October 7, 2019.
10. University of Washington, Institute for Health Metrics and Evaluation. Morocco. http://www.healthdata.org/morocco. Published 2018. Accessed October 7, 2019.
11. Oxford Business Group. Access to health care broadens in Morocco. https://oxfordbusinessgroup.com/overview/forward-steps-access-care-has-broadened-and-infrastructure-improved-challenges-remain. Accessed September 12. 2019.
12. Wright NMJ, Hankins FM. Preventing radicalization and terrorism: Is there a GP response? Br J Gen Pract. 2016;66(647):288-289.
13. Buhi K, Everitt K, Jones E. Might depression psychosocial adversity, and limited social assets explain vulnerability to and resistance against violent radicalization? PlosOne. 2014;9(9):e105918.
14. DeAngelis T. Understanding terrorism. apa.org/monitor/2009/11/terrorism. Published November 2009. Accessed October 14, 2019.
15. Victoroff J. The mind of the terrorist: a review and critique of psychological approaches. J Conflict Resolut. 2005;49(1):3-42.
16. Lafree G, Ackerman G. The empirical study of terrorism: social and legal research. Ann Rev Law Soc Sci. 2009;5:347-374.
17. World Bank. Morocco—improving primary health in rural areas program-for-results project (English). http://documents.worldbank.org/curated/en/716821468274482723/Morocco-Improving-Primary-Health-in-Rural-Areas-Program-for-Results-Project. Published 2015. Accessed September 16, 2019.
18. Royaume du Maroc, Ministère de la Santé. Livre blanc: pour une nouvelle gouvernance du secteur de la santé. Paper presented at: 2nd National Health Conference; July 1-3, 2013; Marrakesh, Morocco.
19. Buhi K, Hicks MH, Lashley M, Jones E. A public health approach to understanding and preventing violent radicalization. BMC Med. 2012;10:16.
20. Mallonee S, Sahriat S, Stennies G, Waxweiler R, Hogan D, Jordan F. Physical injuries and fatalities resulting from the Oklahoma City bombing. JAMA. 1996;276(5):382-387.
21. Ushizawa H, Foxwell AR, Bice S, et al. Needs for disaster medicine: lessons from the field of the Great East Japan Earthquake. Western Pac Surveil Response J. 2013;4(1):51-55.
The relationship between the Kingdom of Morocco and the US began just after the US declared its own independence. It is one of the oldest of US partnerships with a foreign country, and since the end of the First Barbary War in 1805 it has remained one of the most stable. The Utah National Guard (UTNG) has an active state partnership program (SPP) with Morocco, which helps maintain that stability and fosters the relationship. The SPP provides the Kingdom of Morocco assistance in the areas of disaster medicine, prehospital medicine, and rural access to health care.
The objective of this review is to highlight the role the SPP plays in ensuring Morocco’s continued stability, enhancing its role as a leader among African nations, aiding its medically vulnerable rural populations to prevent recruitment by terrorist organizations, and maintaining its long-term relationship with the US.
Background
The Kingdom of Morocco resides in a geologically and politically unstable part of the world, yet it has been a stable constitutional monarchy. Like California, Morocco has a long coastline of more than 1,000 miles. It sits along an active earthquake fault line with a disaster response program that is only in its infancy. The Kingdom has a high youth unemployment rate and lacks adequate public education opportunities, which exacerbate feelings of government indifference. Morocco’s medical system is highly centralized, and large parts of the rural population lack access to basic medical care—potentially alienating the population. The Moroccan current disaster plan ORSEC (plan d’ Organization des Secours) was established in 1966 and updated in 2005 but does not provide a comprehensive, unified disaster response. The ORSEC plan is of French derivation and is not a list of actions but a general plan of organization and supply. 1
When governments fail to provide basic services—health care being just one—those services may be filled by groups seeking to influence the government and population by threatening acts of violence to achieve political, religious, and ideologic gain; for example, the Taliban in Afghanistan, the Muslim brotherhood in Egypt and in the West Bank, and the Islamic State in Iraq and Syria (ISIS) in Syria.2-5 These groups gain a foothold and legitimacy by providing mosques, youth groups, clinics, hospitals, and schools. 2-5
Identified Needs
Morocco is at risk of experiencing an earthquake and possible subsequent tsunami. In 1755, Morocco was impacted by the Great Lisbon earthquake and tsunami. Witnesses reported 15-meter waves with 24-meter crests.6 Building codes and architecture laws have changed little since the 1960 Agadir earthquake, which killed 12,000 people. The disaster response program—although improved since the 1960s—is still in the early stages of development, and another earthquake and possible subsequent tsunami would result in a disaster that could overwhelm the medical community of Morocco.
Perceived Government Indifference
The Moroccan constitutional monarchy is more stable than are the governments of its North African neighbors. King Mohammed VI presides over the government, and regular elections are held for members of Parliament, which names a prime minister. However, in August 2019, overall unemployment was at 8.5%, and youth unemployment was 22.3%.7 A United Nations report in August 2019 stated that literacy rates for Morocco were 71.7%. These data were from a 2015 census, the last year data were collected.8 These deficits in employment and education can foster anger toward the Moroccan government for not adequately providing these services and possibly introduce radicalization as a result of the population’s perceived government indifference and lack of economic mobility.
Access to Medical Care
Morocco has a 2-tiered medical system for providing services: urban and rural. In 2018 the Legatum Prosperity Index ranked Morocco 103 of 149 countries in health care. The prosperity index measures health variables, which include but were not limited to basic physical and mental health, health infrastructure, and preventive care.9 Outside the metropolitan areas, emergency medical care is nonexistent, primary care is sporadic, and there is little modern technology available.
Despite humanitarian efforts over many years, there is little to no medical care in the rural “medical desert.” A 2017 study from the University of Washington Institute for Health Metrics and Evaluation compared the global burden of disease in similar countries. The study found that Morocco was significantly higher than the mean in the prevalence of ischemic heart disease and Alzheimer disease, lower than the mean in the areas of neonatal disorders, lower respiratory infections, and tuberculosis, and statistically indistinct from the mean in stroke, congenital defects, road injuries, diabetes mellitus, and hypertensive heart disease compared with the disease prevalence of other countries of similar size and economic measures.10 The study also found a particularly acute disparity in access to health care in rural areas. In 2016, the Oxford Business Group reported staff shortages and disproportionate distribution of resources in the Moroccan health care system.11
Additionally, the lack of trained health care personnel has added to an already overstressed health care system. A chief stressor in a health care system is an insufficient replacement rate. Health employees working for the Moroccan Ministry of Health retire at a rate of 1,500 per year.10,11 These shortages may serve to further the feelings of frustration and government indifference. This frustration is momentarily decreased by humanitarian efforts that have taken place in the African continent in the past decades, but this band-aid approach to assisting the population that is medically underserved has done little to alleviate the long-term problem of access to care. And feelings of government abandonment can sow the seeds of discontent in the rural population, creating fertile ground for recruitment by terrorist organizations.2,3
Lack of Health Care and Radicalism
It has been postulated that there is a link between radicalization and lack of medical care. Depression and perceived government indifference are considered contributors to radicalization.12-16 In 2005, Victoroff suggested that there are certain psychological traits characteristic of “typical" terrorists: these include high affective valence regarding an ideologic issue, a personal stake (perceived oppression, persecution or humiliation, need for identity, glory, or vengeance), low cognitive ability, low tolerance for ambiguity, and a capacity to suppress instinctive and learned moral constraints against harming innocents.15 In 2009, Lafree and Ackerman suggested that terrorism feeds on the ability of groups to portray governments and their agents as illegitimate.16 It is possible that part of the illegitimacy campaign of radicalization and terrorist recruitment may be identification of the lack of health care by the government thus magnifying feelings of government abandonment in a vulnerable population.
In 2011, the new Moroccan constitution identified access to basic health care as a right of the Moroccan people.17 Additionally, in 2013, a government white paper was produced outlining the need to increase access to health care, particularly in rural areas, including a focus on infant and maternal mortality, diabetes mellitus (DM), heart disease, and respiratory problems.17,18
Proposed Solutions, A Beginning
A health outreach program with a regional health professional training center in a relatively stable country within the African Union (AU) would be a step toward delivering health care to Morocco and interested AU members. Interested nations have been and will continue to be invited to train at the Moroccan center and return to their countries and start training programs. This idea was echoed by the World Bank in a 2015 loan proposal to Morocco, which suggested that addressing disparities in access to health care is a social justice issue, with other benefits such as increased productivity, employment, lower out-of-pocket expenditures, and promotion of good governance.17
In 2012, Buhi reported that a positive regard for authorities and healthier influences seemed to be a protective factor against radicalization. He also suggested a public health approach to understanding and preventing violent radicalization.19 The solutions are complex, especially in rural areas and in vulnerable nations common to Africa.
Medical training efforts by the US Department of Defense (DoD), Medical Readiness Training Institute (DMRT), and international health specialists working with the military and civilian entities in neighboring African countries have improved response to regional disasters. However, to address the broader issues, a more permanent, cooperative possible solution may begin with the establishment in Morocco of a regional education center for disaster preparedness and for health care providers (HCPs). This would serve as a training program for disaster first responders. Graduates of the program would receive additional training to become HCPs similar to physician assistant (PA) and nurse practitioner (NP) programs in the US. Morocco is uniquely positioned to accomplish this due to its location, political stability, and ties with other African nations.
The goal of the Moroccan regional education center (within the King Mohammed V Hospital) is to bring together global health experts and increase the intellectual infrastructure of not only Morocco, but also offer this training program to interested countries within the AU. Advancement of the regional education center will require legislative changes to expand prescriptive privileges and scope of practice within each country. The medical element of the SPP as presently constituted without the regional education center will continue its humanitarian goals, but the proposed creation of the regional education center will educate participants to serve the rural communities within each participating country. Eventually the entire educational program will be the responsibility of the Moroccan military and the AU participants. This will require reprioritizing resources from the provision of humanitarian health care services to an HCP education approach.
Disaster Response
Deficits in disaster response capabilities have been identified by members of the Moroccan military with the assistance of the UTNG. The most glaring deficit identified was the disparity in training between military and civilian first responders. Thus, a training program was initiated by the Moroccan military and the UTNG that combined internationally recognized, durable, robust emergency training programs. These programs consisted of, but were not limited to, parts or entire programs of the following: basic disaster life support, advanced disaster life support, disaster casualty care, and advanced trauma life support. The goal of this training was to improve communication, reduce mortality, and create strike teams, which can quickly provide health care independent of a hospital during a disaster.
Patients can overwhelm hospitals in a disaster when need exceeds resources. In 1996, Mallonee reported that at least 67% of the patients who sought care at a hospital during the Oklahoma City bombing disaster did not need advanced medical treatment.20 Such patients could be seen at an identified casualty collection point by a strike team and treated and released rather than traveling to the hospital and using staff and resources that could be used more judiciously for the more seriously injured.21 These teams consist of trained first responders with an experienced HCP (physician, PA, NP) and a nurse and are trained to operate for up to 72 hours in a predetermined location and serve as a “filter” for the hospital. Their role is to treat and release the less severely injured and refer only the more severely injured to the hospital after basic stabilization, thus preserving precious resources necessary for the more seriously injured.
This disaster response training program was offered to the Moroccan military, ministry of health and ministry of tourism, and quickly turned into an Africa-wide interest. A regional training center was proposed. This was assisted with the cooperation of Weber State University in Ogden Utah, Utah Valley University in Orem, and private interests in a public/private/military state partnership. Program supplies and didactic instruction were and will be provided by the UTNG and supplemented through the DoD Africa command. Instruction will be a cooperative effort agreed on between the UTNG and the Moroccan military medical specialists within their specific area of expertise.
Underserved Communities
Finally, from this pool of interested strike team members, a health care provider school will be formed to educate, certify, and service the needs of the underserved communities in Morocco and interested AU countries. This program will be similar to the PA and NP programs in the US and will be geared to those graduates from the previous programs with intense classroom instruction for one year followed by a year of one-on-one preceptorship with an experienced physician. The goal of the program is to prepare individuals with patient care experience to fulfill a bigger role in health care in an underserved (usually austere, rural) area that currently has minimal health care presence. This fills a need identified by the World Bank in 2015 that the Moroccan government needs to respond to the demand for improved access to and quality of health care services—particularly to the rural poor.17
The Moroccan military has a presence in many medically underserved areas. The logical fit for the HCP program will be drawn from a pool of active-duty military individuals who express an interest and qualify through attendance in all phases of the training.
Conclusion
This program of disaster medical education, strike teams, and HCPs is currently training more than 200 students a year throughout Morocco. The proposed direction of this cooperative program to produce HCPs in rural areas will increase access to health care for the Moroccan people who are now underserved. Morocco, as a health care training hub in Africa, will increase access to health care for interested African countries. The goal politically will be to reduce feelings of government indifference in vulnerable populations and reduce recruitment into radical ideologies.
The relationship between the Kingdom of Morocco and the US began just after the US declared its own independence. It is one of the oldest of US partnerships with a foreign country, and since the end of the First Barbary War in 1805 it has remained one of the most stable. The Utah National Guard (UTNG) has an active state partnership program (SPP) with Morocco, which helps maintain that stability and fosters the relationship. The SPP provides the Kingdom of Morocco assistance in the areas of disaster medicine, prehospital medicine, and rural access to health care.
The objective of this review is to highlight the role the SPP plays in ensuring Morocco’s continued stability, enhancing its role as a leader among African nations, aiding its medically vulnerable rural populations to prevent recruitment by terrorist organizations, and maintaining its long-term relationship with the US.
Background
The Kingdom of Morocco resides in a geologically and politically unstable part of the world, yet it has been a stable constitutional monarchy. Like California, Morocco has a long coastline of more than 1,000 miles. It sits along an active earthquake fault line with a disaster response program that is only in its infancy. The Kingdom has a high youth unemployment rate and lacks adequate public education opportunities, which exacerbate feelings of government indifference. Morocco’s medical system is highly centralized, and large parts of the rural population lack access to basic medical care—potentially alienating the population. The Moroccan current disaster plan ORSEC (plan d’ Organization des Secours) was established in 1966 and updated in 2005 but does not provide a comprehensive, unified disaster response. The ORSEC plan is of French derivation and is not a list of actions but a general plan of organization and supply. 1
When governments fail to provide basic services—health care being just one—those services may be filled by groups seeking to influence the government and population by threatening acts of violence to achieve political, religious, and ideologic gain; for example, the Taliban in Afghanistan, the Muslim brotherhood in Egypt and in the West Bank, and the Islamic State in Iraq and Syria (ISIS) in Syria.2-5 These groups gain a foothold and legitimacy by providing mosques, youth groups, clinics, hospitals, and schools. 2-5
Identified Needs
Morocco is at risk of experiencing an earthquake and possible subsequent tsunami. In 1755, Morocco was impacted by the Great Lisbon earthquake and tsunami. Witnesses reported 15-meter waves with 24-meter crests.6 Building codes and architecture laws have changed little since the 1960 Agadir earthquake, which killed 12,000 people. The disaster response program—although improved since the 1960s—is still in the early stages of development, and another earthquake and possible subsequent tsunami would result in a disaster that could overwhelm the medical community of Morocco.
Perceived Government Indifference
The Moroccan constitutional monarchy is more stable than are the governments of its North African neighbors. King Mohammed VI presides over the government, and regular elections are held for members of Parliament, which names a prime minister. However, in August 2019, overall unemployment was at 8.5%, and youth unemployment was 22.3%.7 A United Nations report in August 2019 stated that literacy rates for Morocco were 71.7%. These data were from a 2015 census, the last year data were collected.8 These deficits in employment and education can foster anger toward the Moroccan government for not adequately providing these services and possibly introduce radicalization as a result of the population’s perceived government indifference and lack of economic mobility.
Access to Medical Care
Morocco has a 2-tiered medical system for providing services: urban and rural. In 2018 the Legatum Prosperity Index ranked Morocco 103 of 149 countries in health care. The prosperity index measures health variables, which include but were not limited to basic physical and mental health, health infrastructure, and preventive care.9 Outside the metropolitan areas, emergency medical care is nonexistent, primary care is sporadic, and there is little modern technology available.
Despite humanitarian efforts over many years, there is little to no medical care in the rural “medical desert.” A 2017 study from the University of Washington Institute for Health Metrics and Evaluation compared the global burden of disease in similar countries. The study found that Morocco was significantly higher than the mean in the prevalence of ischemic heart disease and Alzheimer disease, lower than the mean in the areas of neonatal disorders, lower respiratory infections, and tuberculosis, and statistically indistinct from the mean in stroke, congenital defects, road injuries, diabetes mellitus, and hypertensive heart disease compared with the disease prevalence of other countries of similar size and economic measures.10 The study also found a particularly acute disparity in access to health care in rural areas. In 2016, the Oxford Business Group reported staff shortages and disproportionate distribution of resources in the Moroccan health care system.11
Additionally, the lack of trained health care personnel has added to an already overstressed health care system. A chief stressor in a health care system is an insufficient replacement rate. Health employees working for the Moroccan Ministry of Health retire at a rate of 1,500 per year.10,11 These shortages may serve to further the feelings of frustration and government indifference. This frustration is momentarily decreased by humanitarian efforts that have taken place in the African continent in the past decades, but this band-aid approach to assisting the population that is medically underserved has done little to alleviate the long-term problem of access to care. And feelings of government abandonment can sow the seeds of discontent in the rural population, creating fertile ground for recruitment by terrorist organizations.2,3
Lack of Health Care and Radicalism
It has been postulated that there is a link between radicalization and lack of medical care. Depression and perceived government indifference are considered contributors to radicalization.12-16 In 2005, Victoroff suggested that there are certain psychological traits characteristic of “typical" terrorists: these include high affective valence regarding an ideologic issue, a personal stake (perceived oppression, persecution or humiliation, need for identity, glory, or vengeance), low cognitive ability, low tolerance for ambiguity, and a capacity to suppress instinctive and learned moral constraints against harming innocents.15 In 2009, Lafree and Ackerman suggested that terrorism feeds on the ability of groups to portray governments and their agents as illegitimate.16 It is possible that part of the illegitimacy campaign of radicalization and terrorist recruitment may be identification of the lack of health care by the government thus magnifying feelings of government abandonment in a vulnerable population.
In 2011, the new Moroccan constitution identified access to basic health care as a right of the Moroccan people.17 Additionally, in 2013, a government white paper was produced outlining the need to increase access to health care, particularly in rural areas, including a focus on infant and maternal mortality, diabetes mellitus (DM), heart disease, and respiratory problems.17,18
Proposed Solutions, A Beginning
A health outreach program with a regional health professional training center in a relatively stable country within the African Union (AU) would be a step toward delivering health care to Morocco and interested AU members. Interested nations have been and will continue to be invited to train at the Moroccan center and return to their countries and start training programs. This idea was echoed by the World Bank in a 2015 loan proposal to Morocco, which suggested that addressing disparities in access to health care is a social justice issue, with other benefits such as increased productivity, employment, lower out-of-pocket expenditures, and promotion of good governance.17
In 2012, Buhi reported that a positive regard for authorities and healthier influences seemed to be a protective factor against radicalization. He also suggested a public health approach to understanding and preventing violent radicalization.19 The solutions are complex, especially in rural areas and in vulnerable nations common to Africa.
Medical training efforts by the US Department of Defense (DoD), Medical Readiness Training Institute (DMRT), and international health specialists working with the military and civilian entities in neighboring African countries have improved response to regional disasters. However, to address the broader issues, a more permanent, cooperative possible solution may begin with the establishment in Morocco of a regional education center for disaster preparedness and for health care providers (HCPs). This would serve as a training program for disaster first responders. Graduates of the program would receive additional training to become HCPs similar to physician assistant (PA) and nurse practitioner (NP) programs in the US. Morocco is uniquely positioned to accomplish this due to its location, political stability, and ties with other African nations.
The goal of the Moroccan regional education center (within the King Mohammed V Hospital) is to bring together global health experts and increase the intellectual infrastructure of not only Morocco, but also offer this training program to interested countries within the AU. Advancement of the regional education center will require legislative changes to expand prescriptive privileges and scope of practice within each country. The medical element of the SPP as presently constituted without the regional education center will continue its humanitarian goals, but the proposed creation of the regional education center will educate participants to serve the rural communities within each participating country. Eventually the entire educational program will be the responsibility of the Moroccan military and the AU participants. This will require reprioritizing resources from the provision of humanitarian health care services to an HCP education approach.
Disaster Response
Deficits in disaster response capabilities have been identified by members of the Moroccan military with the assistance of the UTNG. The most glaring deficit identified was the disparity in training between military and civilian first responders. Thus, a training program was initiated by the Moroccan military and the UTNG that combined internationally recognized, durable, robust emergency training programs. These programs consisted of, but were not limited to, parts or entire programs of the following: basic disaster life support, advanced disaster life support, disaster casualty care, and advanced trauma life support. The goal of this training was to improve communication, reduce mortality, and create strike teams, which can quickly provide health care independent of a hospital during a disaster.
Patients can overwhelm hospitals in a disaster when need exceeds resources. In 1996, Mallonee reported that at least 67% of the patients who sought care at a hospital during the Oklahoma City bombing disaster did not need advanced medical treatment.20 Such patients could be seen at an identified casualty collection point by a strike team and treated and released rather than traveling to the hospital and using staff and resources that could be used more judiciously for the more seriously injured.21 These teams consist of trained first responders with an experienced HCP (physician, PA, NP) and a nurse and are trained to operate for up to 72 hours in a predetermined location and serve as a “filter” for the hospital. Their role is to treat and release the less severely injured and refer only the more severely injured to the hospital after basic stabilization, thus preserving precious resources necessary for the more seriously injured.
This disaster response training program was offered to the Moroccan military, ministry of health and ministry of tourism, and quickly turned into an Africa-wide interest. A regional training center was proposed. This was assisted with the cooperation of Weber State University in Ogden Utah, Utah Valley University in Orem, and private interests in a public/private/military state partnership. Program supplies and didactic instruction were and will be provided by the UTNG and supplemented through the DoD Africa command. Instruction will be a cooperative effort agreed on between the UTNG and the Moroccan military medical specialists within their specific area of expertise.
Underserved Communities
Finally, from this pool of interested strike team members, a health care provider school will be formed to educate, certify, and service the needs of the underserved communities in Morocco and interested AU countries. This program will be similar to the PA and NP programs in the US and will be geared to those graduates from the previous programs with intense classroom instruction for one year followed by a year of one-on-one preceptorship with an experienced physician. The goal of the program is to prepare individuals with patient care experience to fulfill a bigger role in health care in an underserved (usually austere, rural) area that currently has minimal health care presence. This fills a need identified by the World Bank in 2015 that the Moroccan government needs to respond to the demand for improved access to and quality of health care services—particularly to the rural poor.17
The Moroccan military has a presence in many medically underserved areas. The logical fit for the HCP program will be drawn from a pool of active-duty military individuals who express an interest and qualify through attendance in all phases of the training.
Conclusion
This program of disaster medical education, strike teams, and HCPs is currently training more than 200 students a year throughout Morocco. The proposed direction of this cooperative program to produce HCPs in rural areas will increase access to health care for the Moroccan people who are now underserved. Morocco, as a health care training hub in Africa, will increase access to health care for interested African countries. The goal politically will be to reduce feelings of government indifference in vulnerable populations and reduce recruitment into radical ideologies.
1. Nahon M, Michaloux M. L’organisation de la réponse de la sécurité civile: le dispositif ORSEC Organisation of civilian emergency services: The ORSEC plan. https://www.sciencedirect.com/science/article/pii/S2211423816300499#! Published July 2016. Accessed October 7, 2019.
2. Berman E. Hamas, Taliban and the Jewish underground: an economist's view of radical religious militias. NBER Working Paper No. w10004. https://ssrn.com/abstract=450885. Published September 2003. Accessed October 7, 2019.
3. Jordan J. Attacking the leader. Missing the mark; why terrorist groups survive decapitation strikes. Int Secur. 2014;38(4):7-38.
4. Grynkewich A. Welfare as warfare: how violent non-state groups use social services to attack the state. Stud Conflict Terrorism. 2008;31(4):350-370.
5. Marin M, Solomon H. Islamic State: understanding the nature of the beast and its funding. Contemp Rev Middle East. 2017;4(1):18-49.
6. Bressan D. November 1, 1755: the earthquake of Lisbon: wrath of god or natural disaster? Scientific American, History of Geology. https://blogs.scientificamerican.com/history-of-geology/november-1-1755-the-earthquake-of-lisbon-wraith-of-god-or-natural-disaster. Published November 2011. Accessed October 7, 2019.
7. Trading Economics. Morocco unemployment rate. Second quarter statistics. August 2019. https://tradingeconomics.com/morocco/unemployment-rate. Accessed October 7, 2019.
8. Knoema World Data Atlas 2015. Morocco adult literacy rates. https://knoema.com/atlas/Morocco/topics/Education/Literacy/Adult-literacy-rate. Accessed October 4, 2019.
9. The Legatum Prosperity Index 2018. Morocco. https://www.prosperity.com/globe/morocco. Accessed October 7, 2019.
10. University of Washington, Institute for Health Metrics and Evaluation. Morocco. http://www.healthdata.org/morocco. Published 2018. Accessed October 7, 2019.
11. Oxford Business Group. Access to health care broadens in Morocco. https://oxfordbusinessgroup.com/overview/forward-steps-access-care-has-broadened-and-infrastructure-improved-challenges-remain. Accessed September 12. 2019.
12. Wright NMJ, Hankins FM. Preventing radicalization and terrorism: Is there a GP response? Br J Gen Pract. 2016;66(647):288-289.
13. Buhi K, Everitt K, Jones E. Might depression psychosocial adversity, and limited social assets explain vulnerability to and resistance against violent radicalization? PlosOne. 2014;9(9):e105918.
14. DeAngelis T. Understanding terrorism. apa.org/monitor/2009/11/terrorism. Published November 2009. Accessed October 14, 2019.
15. Victoroff J. The mind of the terrorist: a review and critique of psychological approaches. J Conflict Resolut. 2005;49(1):3-42.
16. Lafree G, Ackerman G. The empirical study of terrorism: social and legal research. Ann Rev Law Soc Sci. 2009;5:347-374.
17. World Bank. Morocco—improving primary health in rural areas program-for-results project (English). http://documents.worldbank.org/curated/en/716821468274482723/Morocco-Improving-Primary-Health-in-Rural-Areas-Program-for-Results-Project. Published 2015. Accessed September 16, 2019.
18. Royaume du Maroc, Ministère de la Santé. Livre blanc: pour une nouvelle gouvernance du secteur de la santé. Paper presented at: 2nd National Health Conference; July 1-3, 2013; Marrakesh, Morocco.
19. Buhi K, Hicks MH, Lashley M, Jones E. A public health approach to understanding and preventing violent radicalization. BMC Med. 2012;10:16.
20. Mallonee S, Sahriat S, Stennies G, Waxweiler R, Hogan D, Jordan F. Physical injuries and fatalities resulting from the Oklahoma City bombing. JAMA. 1996;276(5):382-387.
21. Ushizawa H, Foxwell AR, Bice S, et al. Needs for disaster medicine: lessons from the field of the Great East Japan Earthquake. Western Pac Surveil Response J. 2013;4(1):51-55.
1. Nahon M, Michaloux M. L’organisation de la réponse de la sécurité civile: le dispositif ORSEC Organisation of civilian emergency services: The ORSEC plan. https://www.sciencedirect.com/science/article/pii/S2211423816300499#! Published July 2016. Accessed October 7, 2019.
2. Berman E. Hamas, Taliban and the Jewish underground: an economist's view of radical religious militias. NBER Working Paper No. w10004. https://ssrn.com/abstract=450885. Published September 2003. Accessed October 7, 2019.
3. Jordan J. Attacking the leader. Missing the mark; why terrorist groups survive decapitation strikes. Int Secur. 2014;38(4):7-38.
4. Grynkewich A. Welfare as warfare: how violent non-state groups use social services to attack the state. Stud Conflict Terrorism. 2008;31(4):350-370.
5. Marin M, Solomon H. Islamic State: understanding the nature of the beast and its funding. Contemp Rev Middle East. 2017;4(1):18-49.
6. Bressan D. November 1, 1755: the earthquake of Lisbon: wrath of god or natural disaster? Scientific American, History of Geology. https://blogs.scientificamerican.com/history-of-geology/november-1-1755-the-earthquake-of-lisbon-wraith-of-god-or-natural-disaster. Published November 2011. Accessed October 7, 2019.
7. Trading Economics. Morocco unemployment rate. Second quarter statistics. August 2019. https://tradingeconomics.com/morocco/unemployment-rate. Accessed October 7, 2019.
8. Knoema World Data Atlas 2015. Morocco adult literacy rates. https://knoema.com/atlas/Morocco/topics/Education/Literacy/Adult-literacy-rate. Accessed October 4, 2019.
9. The Legatum Prosperity Index 2018. Morocco. https://www.prosperity.com/globe/morocco. Accessed October 7, 2019.
10. University of Washington, Institute for Health Metrics and Evaluation. Morocco. http://www.healthdata.org/morocco. Published 2018. Accessed October 7, 2019.
11. Oxford Business Group. Access to health care broadens in Morocco. https://oxfordbusinessgroup.com/overview/forward-steps-access-care-has-broadened-and-infrastructure-improved-challenges-remain. Accessed September 12. 2019.
12. Wright NMJ, Hankins FM. Preventing radicalization and terrorism: Is there a GP response? Br J Gen Pract. 2016;66(647):288-289.
13. Buhi K, Everitt K, Jones E. Might depression psychosocial adversity, and limited social assets explain vulnerability to and resistance against violent radicalization? PlosOne. 2014;9(9):e105918.
14. DeAngelis T. Understanding terrorism. apa.org/monitor/2009/11/terrorism. Published November 2009. Accessed October 14, 2019.
15. Victoroff J. The mind of the terrorist: a review and critique of psychological approaches. J Conflict Resolut. 2005;49(1):3-42.
16. Lafree G, Ackerman G. The empirical study of terrorism: social and legal research. Ann Rev Law Soc Sci. 2009;5:347-374.
17. World Bank. Morocco—improving primary health in rural areas program-for-results project (English). http://documents.worldbank.org/curated/en/716821468274482723/Morocco-Improving-Primary-Health-in-Rural-Areas-Program-for-Results-Project. Published 2015. Accessed September 16, 2019.
18. Royaume du Maroc, Ministère de la Santé. Livre blanc: pour une nouvelle gouvernance du secteur de la santé. Paper presented at: 2nd National Health Conference; July 1-3, 2013; Marrakesh, Morocco.
19. Buhi K, Hicks MH, Lashley M, Jones E. A public health approach to understanding and preventing violent radicalization. BMC Med. 2012;10:16.
20. Mallonee S, Sahriat S, Stennies G, Waxweiler R, Hogan D, Jordan F. Physical injuries and fatalities resulting from the Oklahoma City bombing. JAMA. 1996;276(5):382-387.
21. Ushizawa H, Foxwell AR, Bice S, et al. Needs for disaster medicine: lessons from the field of the Great East Japan Earthquake. Western Pac Surveil Response J. 2013;4(1):51-55.
CDC, FDA in hot pursuit of source of vaping lung injuries
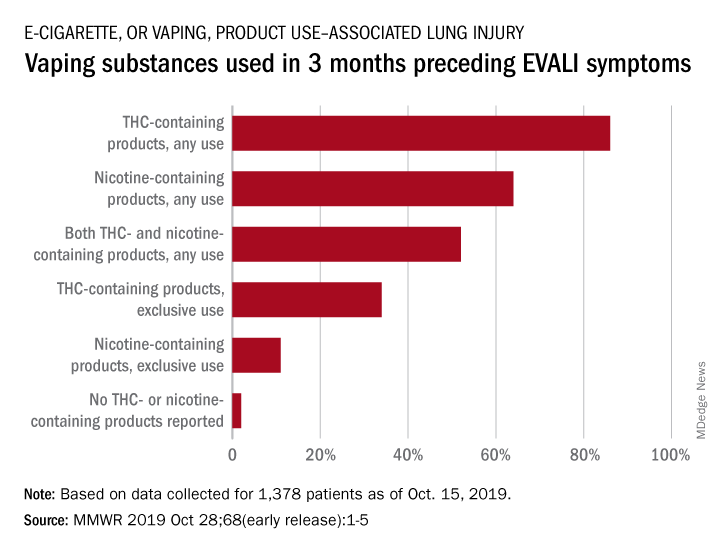
The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.

The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.

The Centers for Disease Control and Prevention is providing frequent updates of the wide-ranging and aggressive investigation of the cases and deaths linked to vaping, and although a definitive cause remains unknown, evidence is accumulating to implicate tetrahydrocannabinol (THC)-containing devices. The investigation is being conducted in concert with the Food and Drug Administration, many state and local health departments, and public health and clinical partners.
The acronym EVALI has been developed by CDC to refer to e-cigarette, or vaping products use–associated lung injury. In a report summarizing data up to Oct. 22, CDC reported 1,604 EVALI cases and 34 deaths. These cases have occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. The CDC also published a report in the Morbidity and Mortality Weekly report on characteristics of those patients who have died from EVALI-based symptoms as of Oct. 15, 2019.
With data available for more than 867 patients with EVALI, about 86% had a history of using e-cigarette or vaping products that contained THC in the previous 90 days; 64% reported using nicotine-containing products; 34% reported exclusive use of THC-containing products, and 11% reported exclusive use of nicotine-containing products; 52% reported use of both.
In a telebriefing on Oct. 25, Anne Schuchat, MD, CDC principal deputy director, said, “The data do continue to point towards THC-containing products as the source of the vast majority of individuals’ lung injury. There are continuing cases that do not report that history. But I’d like to stress that we don’t know what the risky material or substance is. THC may be a marker for a way that cartridges were prepared or the way that the devices are producing harm. Whether there are similar activities going on with cartridges that don’t contain THC, for instance, remains to be seen. So, I think we are seeing the THC as a marker for products that are risky.”
EVALI deaths
Among the 29 deaths reported as of Oct. 15, 59% (17) were male; the median age was 45 years (range, 17-75 years), 55 years (range, 17-71 years) among males, and 43 years (range, 27-75 years) among females; the age difference between males and females was not statistically significant. Patients who died tended to be older than patients who survived. Among 19 EVALI patients who died and for whom data on substance use was available, the use of any THC-containing products was reported by patients or proxies for 84% (16), including 63% (12) who exclusively used THC-containing products. Use of any nicotine-containing products was reported for 37% (7), including 16% (3) who exclusively used nicotine-containing products. Use of both THC- and nicotine-containing products was reported in four of those who died.
Investigation update
Mitch Zeller, JD, director, Center for Tobacco Products at the Food and Drug Administration, participated in the telebriefing and provided an update on the ongoing investigation. “State of the art methods are being used to assess the presence of a broad range of chemicals including nicotine, THC, and other cannabinoids, opioids, additives, pesticides, poisons and toxins,” he said. “FDA has received or collected over 900 samples from 25 states to date. Those numbers continue to increase. The samples [were] collected directly from consumers, hospitals, and from state offices include vaping devices and products that contain liquid as well as packaging and some nearly empty containers.” He cautioned that identifying the substance is “but one piece of the puzzle and will not necessarily answer questions about causality.” He also noted that the self-reports of THC and/or nicotine could mean that there is misreported data, because reports in many cases are coming from teens and from jurisdictions in which THC is not legal.
The issue of whether EVALI has been seen in recent years but not recognized or whether EVALI is a new phenomenon was raised by a caller at the telebriefing. Dr. Schuchat responded, “We are aware of older cases that look similar to what we are seeing now. But we do not believe that this outbreak or surge in cases is due to better recognition.” She suggested that some evidence points to cutting agents being introduced to increase profits of e-cigarettes and that risky and unknown substances have been introduced into the supply chain.
A “handful” of cases of readmission have been reported, and the CDC is currently investigating whether these cases included patients who took up vaping again or had some other possible contributing factor. Dr. Schuchat cautioned recovering patients not to resume vaping because of the risk of readmission and the probability that their lungs will remain in a weakened state.
Clinical guidance update
The CDC provided detailed interim clinical guidance on evaluating and caring for patients with EVALI. The recommendations focus on patient history, lab testing, criteria for hospitalization, and follow-up for these patients.
Obtaining a detailed history of patients presenting with suspected EVALI is especially important for this patient population, given the many unknowns surrounding this condition, according to the CDC. The updated guidance states, “All health care providers evaluating patients for EVALI should ask about the use of e-cigarette or vaping products, and ideally should ask about types of substances used (e.g.,THC, cannabis [oil, dabs], nicotine, modified products or the addition of substances not intended by the manufacturer); product source, specific product brand and name; duration and frequency of use, time of last use; product delivery system and method of use (aerosolization, dabbing, or dripping).” The approach recommended for soliciting accurate information is “empathetic, nonjudgmental” and, the guidelines say, patients should be questioned in private regarding sensitive information to assure confidentiality.
A respiratory virus panel is recommended for all suspected EVALI patients, although at this time, these tests cannot be used to distinguish EVALI from infectious etiologies. All patients should be considered for urine toxicology testing, including testing for THC.
Imaging guidance for suspected EVALI patients includes chest x-ray, with additional CT scan when the x-ray result does not correlate with clinical findings or to evaluate severe or worsening disease.
Recommended criteria for hospitalization of patients with suspected EVALI are those patients with decreased O2 saturation (less than 95%) on room air, in respiratory distress, or with comorbidities that compromise pulmonary reserve. As of Oct. 8, 96% of patients with suspected EVALI reported to the CDC have been hospitalized.
As for medical treatment of these patients, corticosteroids have been found to be helpful. The statement noted, “Among 140 cases reported nationally to CDC that received corticosteroids, 82% of patients improved.”
The natural progression of this injury is not known, however, and it is possible that patients might recover without corticosteroids. Given the unknown etiology of the disease and “because the diagnosis remains one of exclusion, aggressive empiric therapy with corticosteroids, antimicrobial, and antiviral therapy might be warranted for patients with severe illness. A range of corticosteroid doses, durations, and taper plans might be considered on a case-by-case basis.”
The report concluded with a strong recommendation that patients hospitalized with EVALI are followed closely with a visit 1-2 weeks after discharge and again with additional testing 1-2 months later. Health care providers are also advised to consult medical specialists, in particular pulmonologists, who can offer further evaluation, recommend empiric treatment, and review indications for bronchoscopy.
CPT coding for EVALI
CDC has issued coding guidance to help track EVALI. The document was posted on the CDC website. The coding guidance is consistent with current clinical knowledge about EVALI-related disorders and is intended for use in conjunction with current ICD-10-CM classifications.
The following conditions associated with EVALI are covered in the new coding guidance:
- Bronchitis and pneumonitis caused by chemicals, gases, and fumes; including chemical pneumonitis; J68.0.
- Pneumonitis caused by inhalation of oils and essences; including lipoid pneumonia; J69.1.
- Acute respiratory distress syndrome; J80.
- Pulmonary eosinophilia, not elsewhere classified; J82.
- Acute interstitial pneumonitis; J84.114.
The document notes that the coding guidance has been approved by the National Center for Health Statistics, the American Health Information Management Association, the American Hospital Association, and the Centers for Medicare & Medicaid Services.
Investigation continues
Mr. Zeller cautioned that this investigation will not be concluded in the near future. He noted, “We are committed to working to [solve the mystery] just as quickly as we can, but we also recognize that it will likely take some time. Importantly, the diversity of the patients and the products or substances they have reported using and the samples being tested may mean ultimately that there are multiple causes of these injuries.”
Richard Franki and Gregory Twachtman contributed to this story.
Pelosi drug pricing bill passes Ways and Means on party line vote
The House Ways and Means Committee is the latest to pass H.R. 3, a bill aimed at driving the price of prescription drugs down.
During an Oct. 22, 2019, markup of the bill, Republican members criticized committee leadership for abandoning bipartisan efforts to reign in drug prices in favor of a partisan bill that so far gained no support from the minority party. H.R. 3 was passed by the Ways and Means Committee on a 24-17 party line vote.
Both “Democrats and Republicans support lowering drug prices, cracking down on overpriced drugs, giving patients more power to choose affordable medicines, and removing the wrong incentives in federal health programs that reward bad actors for raising prices,” Committee Ranking Member Kevin Brady (R-Tex.) said in his opening statement. In fact, at the request of Committee Chairman Richard Neal (D-Mass.), “both parties in this committee were working together toward that important goal. At least until Speaker Nancy Pelosi (D-Calif.) trashed the bipartisan work and forced through a secretly written, deeply controversial, and highly partisan drug bill to cure political illnesses rather than real ones.”
H.R. 3, recently renamed the Elijah E. Cummings Lower Drug Costs Now Act of 2019, would give the secretary of the Department of Health & Human Services the ability to negotiate drug prices for Medicare Part D (something explicitly banned under current law), implement an excise tax on drugs that see price hikes above the rate of inflation, cap out-of-pocket expenditures annually for Medicare Part D beneficiaries at $2,000, and use an international pricing index to help bring prices for drugs sold in the United States more in line with the lower prices in foreign countries.
But panel Democrats praised the bill as a step forward in helping to lower the cost of prescription drugs.
“H.R. 3 levels the playing field for U.S. consumers who, on average, pay four times more than patients in other countries for the exact same drugs,” Chairman Neal said in a statement following the passage.
He highlighted specifically the provision that caps out-of-pocket expenses in Part D and the HHS’ negotiating power, noting that “more people will be able to afford the drugs they need that they may have previously forgone due to high costs. With more Americans taking the medicines they’re prescribed, families will be healthier, and premiums will go down.”
Republican committee members argued that these same provisions would stifle innovation and ultimately would reduce access to medicine. Most attempts at altering the provisions through amendments were met with strict party line rejection.
The House Ways and Means Committee is the latest to pass H.R. 3, a bill aimed at driving the price of prescription drugs down.
During an Oct. 22, 2019, markup of the bill, Republican members criticized committee leadership for abandoning bipartisan efforts to reign in drug prices in favor of a partisan bill that so far gained no support from the minority party. H.R. 3 was passed by the Ways and Means Committee on a 24-17 party line vote.
Both “Democrats and Republicans support lowering drug prices, cracking down on overpriced drugs, giving patients more power to choose affordable medicines, and removing the wrong incentives in federal health programs that reward bad actors for raising prices,” Committee Ranking Member Kevin Brady (R-Tex.) said in his opening statement. In fact, at the request of Committee Chairman Richard Neal (D-Mass.), “both parties in this committee were working together toward that important goal. At least until Speaker Nancy Pelosi (D-Calif.) trashed the bipartisan work and forced through a secretly written, deeply controversial, and highly partisan drug bill to cure political illnesses rather than real ones.”
H.R. 3, recently renamed the Elijah E. Cummings Lower Drug Costs Now Act of 2019, would give the secretary of the Department of Health & Human Services the ability to negotiate drug prices for Medicare Part D (something explicitly banned under current law), implement an excise tax on drugs that see price hikes above the rate of inflation, cap out-of-pocket expenditures annually for Medicare Part D beneficiaries at $2,000, and use an international pricing index to help bring prices for drugs sold in the United States more in line with the lower prices in foreign countries.
But panel Democrats praised the bill as a step forward in helping to lower the cost of prescription drugs.
“H.R. 3 levels the playing field for U.S. consumers who, on average, pay four times more than patients in other countries for the exact same drugs,” Chairman Neal said in a statement following the passage.
He highlighted specifically the provision that caps out-of-pocket expenses in Part D and the HHS’ negotiating power, noting that “more people will be able to afford the drugs they need that they may have previously forgone due to high costs. With more Americans taking the medicines they’re prescribed, families will be healthier, and premiums will go down.”
Republican committee members argued that these same provisions would stifle innovation and ultimately would reduce access to medicine. Most attempts at altering the provisions through amendments were met with strict party line rejection.
The House Ways and Means Committee is the latest to pass H.R. 3, a bill aimed at driving the price of prescription drugs down.
During an Oct. 22, 2019, markup of the bill, Republican members criticized committee leadership for abandoning bipartisan efforts to reign in drug prices in favor of a partisan bill that so far gained no support from the minority party. H.R. 3 was passed by the Ways and Means Committee on a 24-17 party line vote.
Both “Democrats and Republicans support lowering drug prices, cracking down on overpriced drugs, giving patients more power to choose affordable medicines, and removing the wrong incentives in federal health programs that reward bad actors for raising prices,” Committee Ranking Member Kevin Brady (R-Tex.) said in his opening statement. In fact, at the request of Committee Chairman Richard Neal (D-Mass.), “both parties in this committee were working together toward that important goal. At least until Speaker Nancy Pelosi (D-Calif.) trashed the bipartisan work and forced through a secretly written, deeply controversial, and highly partisan drug bill to cure political illnesses rather than real ones.”
H.R. 3, recently renamed the Elijah E. Cummings Lower Drug Costs Now Act of 2019, would give the secretary of the Department of Health & Human Services the ability to negotiate drug prices for Medicare Part D (something explicitly banned under current law), implement an excise tax on drugs that see price hikes above the rate of inflation, cap out-of-pocket expenditures annually for Medicare Part D beneficiaries at $2,000, and use an international pricing index to help bring prices for drugs sold in the United States more in line with the lower prices in foreign countries.
But panel Democrats praised the bill as a step forward in helping to lower the cost of prescription drugs.
“H.R. 3 levels the playing field for U.S. consumers who, on average, pay four times more than patients in other countries for the exact same drugs,” Chairman Neal said in a statement following the passage.
He highlighted specifically the provision that caps out-of-pocket expenses in Part D and the HHS’ negotiating power, noting that “more people will be able to afford the drugs they need that they may have previously forgone due to high costs. With more Americans taking the medicines they’re prescribed, families will be healthier, and premiums will go down.”
Republican committee members argued that these same provisions would stifle innovation and ultimately would reduce access to medicine. Most attempts at altering the provisions through amendments were met with strict party line rejection.
Inspector General: NIH must improve conflict of interest reviews
the Department of Health & Human Services’ Office of Inspector General reported.
In highlighting the improvement in a September 2019 report, “NIH has made strides in reviewing financial conflicts of interest in extramural research, but could do more,” the OIG noted that, in the past 10 years, “NIH has strengthened its reporting requirements and developed an online system for collecting, reviewing, and storing financial conflicts of interest (FCOIs) that institutions report. These changes resulted in improvements in how NIH tracks and reviews FCOIs that institutions report.”
That being said, OIG also highlighted some ongoing issues with NIH’s FCOI oversight.
“Across the three NIH Institutes and Centers (ICs) that we reviewed, staff differed in the level of scrutiny they applied to their review of FCOIs,” the report states.
For example, the report notes that 15 of the 25 ICs have written procedures related to FCOI reviews and the documentation shared by the three ICs showed different levels of detail and instruction.
“Only one of the three guidance documents provided IC staff with specific criteria aimed at standardizing the review of FCOIs,” the report stated. Two of the three ICs also reported using external resources to aid in the review.
Review times also varied significantly, with two of the three ICs reporting that they spend generally 5-30 minutes per review, while the third said staff spends several hours on reviews.
The OIG also reported that “NIH lacks quality assurance procedures in its review process. Specifically, NIH central management and the three ICs that we reviewed do not perform any systematic analyses or even ad hoc checks to determine whether staff accurately and consistently review reported FCOIs, and OIG found a small number of inconsistencies in the FCOI data that institutions reported, which might highlight the need for more oversight of the review process.”
The report notes that there is a process in place to provide oversight of ICs’ review of reported FCOIs, but there is no longer sufficient staff to continue this oversight.
The “OER [Office of Extramural Research] now relies on IC staff to seek guidance when needed and does not conduct regular oversight of the ICs. Similarly, none of the three ICs we reviewed perform quality checks to ensure the thoroughness or consistency of review by program officials. Staff members from one IC stated that while they do not conduct quality checks, the IC provides new program officials more guidance during their first few reviews.”
The HHS watchdog also noted that NIH cannot identify whether FCOIs involve foreign entities even though investigators must disclose financial interests from foreign investments.
“The HHS regulations on FCOI do not require institutions to designate whether FCOIs involve foreign entities, and NIH reported that it has no plans to expand these regulations to include such a requirement,” the OIG reported.
The OIG recommended that NIH perform periodic quality assurance reviews of FCOI information to ensure adequacy of oversight and suggested it use “information regarding foreign affiliations and support that it collects during the pre-award process to decide whether to revise its FCOI review process to address concerns regarding foreign influence.”
SOURCE: Murrin S. Office of Inspector General. 2019 Sep 25. OEI-03-19-00150.
the Department of Health & Human Services’ Office of Inspector General reported.
In highlighting the improvement in a September 2019 report, “NIH has made strides in reviewing financial conflicts of interest in extramural research, but could do more,” the OIG noted that, in the past 10 years, “NIH has strengthened its reporting requirements and developed an online system for collecting, reviewing, and storing financial conflicts of interest (FCOIs) that institutions report. These changes resulted in improvements in how NIH tracks and reviews FCOIs that institutions report.”
That being said, OIG also highlighted some ongoing issues with NIH’s FCOI oversight.
“Across the three NIH Institutes and Centers (ICs) that we reviewed, staff differed in the level of scrutiny they applied to their review of FCOIs,” the report states.
For example, the report notes that 15 of the 25 ICs have written procedures related to FCOI reviews and the documentation shared by the three ICs showed different levels of detail and instruction.
“Only one of the three guidance documents provided IC staff with specific criteria aimed at standardizing the review of FCOIs,” the report stated. Two of the three ICs also reported using external resources to aid in the review.
Review times also varied significantly, with two of the three ICs reporting that they spend generally 5-30 minutes per review, while the third said staff spends several hours on reviews.
The OIG also reported that “NIH lacks quality assurance procedures in its review process. Specifically, NIH central management and the three ICs that we reviewed do not perform any systematic analyses or even ad hoc checks to determine whether staff accurately and consistently review reported FCOIs, and OIG found a small number of inconsistencies in the FCOI data that institutions reported, which might highlight the need for more oversight of the review process.”
The report notes that there is a process in place to provide oversight of ICs’ review of reported FCOIs, but there is no longer sufficient staff to continue this oversight.
The “OER [Office of Extramural Research] now relies on IC staff to seek guidance when needed and does not conduct regular oversight of the ICs. Similarly, none of the three ICs we reviewed perform quality checks to ensure the thoroughness or consistency of review by program officials. Staff members from one IC stated that while they do not conduct quality checks, the IC provides new program officials more guidance during their first few reviews.”
The HHS watchdog also noted that NIH cannot identify whether FCOIs involve foreign entities even though investigators must disclose financial interests from foreign investments.
“The HHS regulations on FCOI do not require institutions to designate whether FCOIs involve foreign entities, and NIH reported that it has no plans to expand these regulations to include such a requirement,” the OIG reported.
The OIG recommended that NIH perform periodic quality assurance reviews of FCOI information to ensure adequacy of oversight and suggested it use “information regarding foreign affiliations and support that it collects during the pre-award process to decide whether to revise its FCOI review process to address concerns regarding foreign influence.”
SOURCE: Murrin S. Office of Inspector General. 2019 Sep 25. OEI-03-19-00150.
the Department of Health & Human Services’ Office of Inspector General reported.
In highlighting the improvement in a September 2019 report, “NIH has made strides in reviewing financial conflicts of interest in extramural research, but could do more,” the OIG noted that, in the past 10 years, “NIH has strengthened its reporting requirements and developed an online system for collecting, reviewing, and storing financial conflicts of interest (FCOIs) that institutions report. These changes resulted in improvements in how NIH tracks and reviews FCOIs that institutions report.”
That being said, OIG also highlighted some ongoing issues with NIH’s FCOI oversight.
“Across the three NIH Institutes and Centers (ICs) that we reviewed, staff differed in the level of scrutiny they applied to their review of FCOIs,” the report states.
For example, the report notes that 15 of the 25 ICs have written procedures related to FCOI reviews and the documentation shared by the three ICs showed different levels of detail and instruction.
“Only one of the three guidance documents provided IC staff with specific criteria aimed at standardizing the review of FCOIs,” the report stated. Two of the three ICs also reported using external resources to aid in the review.
Review times also varied significantly, with two of the three ICs reporting that they spend generally 5-30 minutes per review, while the third said staff spends several hours on reviews.
The OIG also reported that “NIH lacks quality assurance procedures in its review process. Specifically, NIH central management and the three ICs that we reviewed do not perform any systematic analyses or even ad hoc checks to determine whether staff accurately and consistently review reported FCOIs, and OIG found a small number of inconsistencies in the FCOI data that institutions reported, which might highlight the need for more oversight of the review process.”
The report notes that there is a process in place to provide oversight of ICs’ review of reported FCOIs, but there is no longer sufficient staff to continue this oversight.
The “OER [Office of Extramural Research] now relies on IC staff to seek guidance when needed and does not conduct regular oversight of the ICs. Similarly, none of the three ICs we reviewed perform quality checks to ensure the thoroughness or consistency of review by program officials. Staff members from one IC stated that while they do not conduct quality checks, the IC provides new program officials more guidance during their first few reviews.”
The HHS watchdog also noted that NIH cannot identify whether FCOIs involve foreign entities even though investigators must disclose financial interests from foreign investments.
“The HHS regulations on FCOI do not require institutions to designate whether FCOIs involve foreign entities, and NIH reported that it has no plans to expand these regulations to include such a requirement,” the OIG reported.
The OIG recommended that NIH perform periodic quality assurance reviews of FCOI information to ensure adequacy of oversight and suggested it use “information regarding foreign affiliations and support that it collects during the pre-award process to decide whether to revise its FCOI review process to address concerns regarding foreign influence.”
SOURCE: Murrin S. Office of Inspector General. 2019 Sep 25. OEI-03-19-00150.
HHS floats Stark/anti-kickback revisions to support value-based care
The long-awaited reforms would create permanent exemptions and safe harbors to protect doctors participating in legitimate value-based arrangements. If finalized, the proposals also would offer flexibility for innovation and improved care coordination, while easing the compliance burden for health care professionals and maintaining safeguards against actual fraud and abuse, according to the U.S. Department of Health & Human Services.
The proposals acknowledge that the Stark Law has been an unintentional roadblock to value-based programs in part because it circumscribed parties’ exchanges of rewards for good behavior, said Donna K. Thiel, a Washington-based health law attorney.
“This should be helpful to doctors in that it removes some of the risk in such arrangements under the existing law,” she said in an interview. “If finalized, the new regulations will alleviate some roadblocks created by the Stark Law with respect to hospital-physician and other arrangements designed to enhance care coordination, improve quality, and reduce waste. Likewise, the changes to the [Anti-Kickback Statute] and Beneficiary Inducement laws loosen the reins on compensation arrangements that might be technical violations of those laws where the arrangement fosters [value-based payments] or efficiency, transparency, or innovation in the provision of health care.”
“These proposed rules would be a historic reform of how healthcare is regulated in America,” HHS Deputy Secretary Eric Hargan said in a statement. “They are part of a much broader effort to update, reform, and cut back our regulations to allow innovation toward a more affordable, higher quality, value-based health care system, while maintaining the important protections patients need.”
The two proposed measures – one rule by the Centers for Medicare and Medicaid Services and the other rule by the Office of Inspector General – include safe harbors for certain remuneration exchanged among participants in a value-based arrangement that fosters better coordinated and managed patient care. This includes care arrangements that improve quality, health outcomes, and efficiency, value-based arrangements with substantial downside financial risk, and value-based arrangements with full financial risk.
In addition, the proposals would protect certain tools and supports shared or delivered under patient engagement and support arrangements to improve quality, health outcomes, and efficiency. For example, a specialty physician practice could share data analytics services with a primary care physician practice in an effort to coordinate care and better manage shared patients, according to the HHS.
If finalized, the changes would modify existing safe harbor for personal services and management contracts to add flexibility with respect to outcomes-based payments and part-time arrangements, according to a fact sheet by the OIG. The rule would also modify existing safe harbors for local transportation to expand and modify mileage limits for rural areas and for transportation for discharged patients.
The proposals include guidance on several requirements that must be met for physicians and health care providers to comply with the Stark Law. For example, compensation provided to a doctor by another health care provider generally must be at fair-market value. As part of the proposals, the HHS offers guidance on how to determine if compensation meets this requirement and provides clarity on a range of other technical compliance requirements.
If the rules are approved, more physicians may be encouraged to become part of value-based arrangements, according to Anjali N.C. Downs, a health law attorney based in Washington.
“As stakeholders have long known, physicians are key components to achieving value-based health care delivery and payment systems,” Ms. Downs said in an interview. “The proposed rules remove regulatory barriers that chill physician’s willingness and ability to participate in or even consider participating in integrated care delivery models, alternative payment models, and incentive based arrangements based on outcomes and reductions in cost.”
However, Ms. Thiel noted the proposed rules do not scale back the affected laws as comprehensively as some stakeholders hoped.
“Some would like to see the Stark law repealed completely, opining that the Stark Law has become too complex, creating obstacles in the transition from the fee-for-service model,” Ms. Thiel said. “Because Stark is a strict liability law, meaning no proof of specific intent to violate is required, providers and doctors can violate Stark even when there is no corrupt intent involved. This new regulation purports to fix some of those issues, but others will remain. Some in the industry believe full repeal is necessary to allow the health industry to move forward with pay-for-performance initiatives.”
The agency is also proposing a safe harbor for donations of cybersecurity technology and services between aligned providers in both the fee-for-service and the value-based settings. For example, a local hospital looking to improve its cybersecurity and that of nearby providers could donate cybersecurity software to each physician that refers patients to its hospital, according to the HHS. In addition, the proposals would add protections for certain cybersecurity technology included as part of an electronic health records (EHR) arrangement.
Physician organizations expressed cautious optimism about the proposed changes.
“While the [American Medical Association] is assessing the full scope of today’s proposals, we are pleased to see that the administration has acknowledged a need for policy revisions in response to potential barriers that impede the delivery of patient-centric care,” AMA President Patrice A. Harris, MD, said in a statement. “Currently, the Stark Law and Anti-Kickback Statute can have a negative impact on the ability of physicians to assist with coordination because they inhibit collaborative partnerships, care continuity, and the engagement of patients in their care. These obstacles can hinder the health care system’s movement to value-based care.”
The proposed rules have been submitted to the Federal Registry and are not yet published. The HHS will accept mail and electronic comments about the proposals up to 75 days after publication in the registry.
The long-awaited reforms would create permanent exemptions and safe harbors to protect doctors participating in legitimate value-based arrangements. If finalized, the proposals also would offer flexibility for innovation and improved care coordination, while easing the compliance burden for health care professionals and maintaining safeguards against actual fraud and abuse, according to the U.S. Department of Health & Human Services.
The proposals acknowledge that the Stark Law has been an unintentional roadblock to value-based programs in part because it circumscribed parties’ exchanges of rewards for good behavior, said Donna K. Thiel, a Washington-based health law attorney.
“This should be helpful to doctors in that it removes some of the risk in such arrangements under the existing law,” she said in an interview. “If finalized, the new regulations will alleviate some roadblocks created by the Stark Law with respect to hospital-physician and other arrangements designed to enhance care coordination, improve quality, and reduce waste. Likewise, the changes to the [Anti-Kickback Statute] and Beneficiary Inducement laws loosen the reins on compensation arrangements that might be technical violations of those laws where the arrangement fosters [value-based payments] or efficiency, transparency, or innovation in the provision of health care.”
“These proposed rules would be a historic reform of how healthcare is regulated in America,” HHS Deputy Secretary Eric Hargan said in a statement. “They are part of a much broader effort to update, reform, and cut back our regulations to allow innovation toward a more affordable, higher quality, value-based health care system, while maintaining the important protections patients need.”
The two proposed measures – one rule by the Centers for Medicare and Medicaid Services and the other rule by the Office of Inspector General – include safe harbors for certain remuneration exchanged among participants in a value-based arrangement that fosters better coordinated and managed patient care. This includes care arrangements that improve quality, health outcomes, and efficiency, value-based arrangements with substantial downside financial risk, and value-based arrangements with full financial risk.
In addition, the proposals would protect certain tools and supports shared or delivered under patient engagement and support arrangements to improve quality, health outcomes, and efficiency. For example, a specialty physician practice could share data analytics services with a primary care physician practice in an effort to coordinate care and better manage shared patients, according to the HHS.
If finalized, the changes would modify existing safe harbor for personal services and management contracts to add flexibility with respect to outcomes-based payments and part-time arrangements, according to a fact sheet by the OIG. The rule would also modify existing safe harbors for local transportation to expand and modify mileage limits for rural areas and for transportation for discharged patients.
The proposals include guidance on several requirements that must be met for physicians and health care providers to comply with the Stark Law. For example, compensation provided to a doctor by another health care provider generally must be at fair-market value. As part of the proposals, the HHS offers guidance on how to determine if compensation meets this requirement and provides clarity on a range of other technical compliance requirements.
If the rules are approved, more physicians may be encouraged to become part of value-based arrangements, according to Anjali N.C. Downs, a health law attorney based in Washington.
“As stakeholders have long known, physicians are key components to achieving value-based health care delivery and payment systems,” Ms. Downs said in an interview. “The proposed rules remove regulatory barriers that chill physician’s willingness and ability to participate in or even consider participating in integrated care delivery models, alternative payment models, and incentive based arrangements based on outcomes and reductions in cost.”
However, Ms. Thiel noted the proposed rules do not scale back the affected laws as comprehensively as some stakeholders hoped.
“Some would like to see the Stark law repealed completely, opining that the Stark Law has become too complex, creating obstacles in the transition from the fee-for-service model,” Ms. Thiel said. “Because Stark is a strict liability law, meaning no proof of specific intent to violate is required, providers and doctors can violate Stark even when there is no corrupt intent involved. This new regulation purports to fix some of those issues, but others will remain. Some in the industry believe full repeal is necessary to allow the health industry to move forward with pay-for-performance initiatives.”
The agency is also proposing a safe harbor for donations of cybersecurity technology and services between aligned providers in both the fee-for-service and the value-based settings. For example, a local hospital looking to improve its cybersecurity and that of nearby providers could donate cybersecurity software to each physician that refers patients to its hospital, according to the HHS. In addition, the proposals would add protections for certain cybersecurity technology included as part of an electronic health records (EHR) arrangement.
Physician organizations expressed cautious optimism about the proposed changes.
“While the [American Medical Association] is assessing the full scope of today’s proposals, we are pleased to see that the administration has acknowledged a need for policy revisions in response to potential barriers that impede the delivery of patient-centric care,” AMA President Patrice A. Harris, MD, said in a statement. “Currently, the Stark Law and Anti-Kickback Statute can have a negative impact on the ability of physicians to assist with coordination because they inhibit collaborative partnerships, care continuity, and the engagement of patients in their care. These obstacles can hinder the health care system’s movement to value-based care.”
The proposed rules have been submitted to the Federal Registry and are not yet published. The HHS will accept mail and electronic comments about the proposals up to 75 days after publication in the registry.
The long-awaited reforms would create permanent exemptions and safe harbors to protect doctors participating in legitimate value-based arrangements. If finalized, the proposals also would offer flexibility for innovation and improved care coordination, while easing the compliance burden for health care professionals and maintaining safeguards against actual fraud and abuse, according to the U.S. Department of Health & Human Services.
The proposals acknowledge that the Stark Law has been an unintentional roadblock to value-based programs in part because it circumscribed parties’ exchanges of rewards for good behavior, said Donna K. Thiel, a Washington-based health law attorney.
“This should be helpful to doctors in that it removes some of the risk in such arrangements under the existing law,” she said in an interview. “If finalized, the new regulations will alleviate some roadblocks created by the Stark Law with respect to hospital-physician and other arrangements designed to enhance care coordination, improve quality, and reduce waste. Likewise, the changes to the [Anti-Kickback Statute] and Beneficiary Inducement laws loosen the reins on compensation arrangements that might be technical violations of those laws where the arrangement fosters [value-based payments] or efficiency, transparency, or innovation in the provision of health care.”
“These proposed rules would be a historic reform of how healthcare is regulated in America,” HHS Deputy Secretary Eric Hargan said in a statement. “They are part of a much broader effort to update, reform, and cut back our regulations to allow innovation toward a more affordable, higher quality, value-based health care system, while maintaining the important protections patients need.”
The two proposed measures – one rule by the Centers for Medicare and Medicaid Services and the other rule by the Office of Inspector General – include safe harbors for certain remuneration exchanged among participants in a value-based arrangement that fosters better coordinated and managed patient care. This includes care arrangements that improve quality, health outcomes, and efficiency, value-based arrangements with substantial downside financial risk, and value-based arrangements with full financial risk.
In addition, the proposals would protect certain tools and supports shared or delivered under patient engagement and support arrangements to improve quality, health outcomes, and efficiency. For example, a specialty physician practice could share data analytics services with a primary care physician practice in an effort to coordinate care and better manage shared patients, according to the HHS.
If finalized, the changes would modify existing safe harbor for personal services and management contracts to add flexibility with respect to outcomes-based payments and part-time arrangements, according to a fact sheet by the OIG. The rule would also modify existing safe harbors for local transportation to expand and modify mileage limits for rural areas and for transportation for discharged patients.
The proposals include guidance on several requirements that must be met for physicians and health care providers to comply with the Stark Law. For example, compensation provided to a doctor by another health care provider generally must be at fair-market value. As part of the proposals, the HHS offers guidance on how to determine if compensation meets this requirement and provides clarity on a range of other technical compliance requirements.
If the rules are approved, more physicians may be encouraged to become part of value-based arrangements, according to Anjali N.C. Downs, a health law attorney based in Washington.
“As stakeholders have long known, physicians are key components to achieving value-based health care delivery and payment systems,” Ms. Downs said in an interview. “The proposed rules remove regulatory barriers that chill physician’s willingness and ability to participate in or even consider participating in integrated care delivery models, alternative payment models, and incentive based arrangements based on outcomes and reductions in cost.”
However, Ms. Thiel noted the proposed rules do not scale back the affected laws as comprehensively as some stakeholders hoped.
“Some would like to see the Stark law repealed completely, opining that the Stark Law has become too complex, creating obstacles in the transition from the fee-for-service model,” Ms. Thiel said. “Because Stark is a strict liability law, meaning no proof of specific intent to violate is required, providers and doctors can violate Stark even when there is no corrupt intent involved. This new regulation purports to fix some of those issues, but others will remain. Some in the industry believe full repeal is necessary to allow the health industry to move forward with pay-for-performance initiatives.”
The agency is also proposing a safe harbor for donations of cybersecurity technology and services between aligned providers in both the fee-for-service and the value-based settings. For example, a local hospital looking to improve its cybersecurity and that of nearby providers could donate cybersecurity software to each physician that refers patients to its hospital, according to the HHS. In addition, the proposals would add protections for certain cybersecurity technology included as part of an electronic health records (EHR) arrangement.
Physician organizations expressed cautious optimism about the proposed changes.
“While the [American Medical Association] is assessing the full scope of today’s proposals, we are pleased to see that the administration has acknowledged a need for policy revisions in response to potential barriers that impede the delivery of patient-centric care,” AMA President Patrice A. Harris, MD, said in a statement. “Currently, the Stark Law and Anti-Kickback Statute can have a negative impact on the ability of physicians to assist with coordination because they inhibit collaborative partnerships, care continuity, and the engagement of patients in their care. These obstacles can hinder the health care system’s movement to value-based care.”
The proposed rules have been submitted to the Federal Registry and are not yet published. The HHS will accept mail and electronic comments about the proposals up to 75 days after publication in the registry.
The VA Ketamine Controversies
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
CDC, SAMHSA commit $1.8 billion to combat opioid crisis
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
FCC backs designating 988 as suicide prevention hotline
A Federal Communications Commission report recommends that 988 be designated as a nationwide suicide and mental health crisis support hotline, because a three-digit number “could be more effective” than the current 10-digit number, according to an FCC release.
The report was prepared by the FCC’s Wireline Competition Bureau and Office of Economics and Analytics at the direction of the National Suicide Hotline Improvement Act of 2018. The current service, known as the National Suicide Prevention Lifeline, uses the 10-digit number of 800-273-8255 (TALK). The report noted that the hotline has proved effective: The service answered 2.2 million calls in 2018 and various assessments have shown significant reductions in hopelessness and suicidal ideation among callers.
However, based on data and conclusions from some of those assessments, the report determined “that the Lifeline could be more effective in preventing suicides and providing crisis intervention if it were accessible via a simple, easy-to-remember, 3-digit dialing code.” The report examined the feasibility of three-digit options, including N11 options such as 211 and 511, but based on an analysis by the North American Numbering Council, it found that “technical and operational concerns related to the 988 code could be more easily and quickly addressed and resolved than any re-education efforts related to repurposing a N11 code.”
Suicide and mental health crises have been on the rise, according to numbers from the Centers for Disease Control and Prevention cited by the FCC report. Among the 50 states, 49 saw increases during 1999-2016, and more than half saw increases greater than 20%. The report describes how some groups, such as veterans and LGBTQ youth, are at especially high risk:
A Federal Communications Commission report recommends that 988 be designated as a nationwide suicide and mental health crisis support hotline, because a three-digit number “could be more effective” than the current 10-digit number, according to an FCC release.
The report was prepared by the FCC’s Wireline Competition Bureau and Office of Economics and Analytics at the direction of the National Suicide Hotline Improvement Act of 2018. The current service, known as the National Suicide Prevention Lifeline, uses the 10-digit number of 800-273-8255 (TALK). The report noted that the hotline has proved effective: The service answered 2.2 million calls in 2018 and various assessments have shown significant reductions in hopelessness and suicidal ideation among callers.
However, based on data and conclusions from some of those assessments, the report determined “that the Lifeline could be more effective in preventing suicides and providing crisis intervention if it were accessible via a simple, easy-to-remember, 3-digit dialing code.” The report examined the feasibility of three-digit options, including N11 options such as 211 and 511, but based on an analysis by the North American Numbering Council, it found that “technical and operational concerns related to the 988 code could be more easily and quickly addressed and resolved than any re-education efforts related to repurposing a N11 code.”
Suicide and mental health crises have been on the rise, according to numbers from the Centers for Disease Control and Prevention cited by the FCC report. Among the 50 states, 49 saw increases during 1999-2016, and more than half saw increases greater than 20%. The report describes how some groups, such as veterans and LGBTQ youth, are at especially high risk:
A Federal Communications Commission report recommends that 988 be designated as a nationwide suicide and mental health crisis support hotline, because a three-digit number “could be more effective” than the current 10-digit number, according to an FCC release.
The report was prepared by the FCC’s Wireline Competition Bureau and Office of Economics and Analytics at the direction of the National Suicide Hotline Improvement Act of 2018. The current service, known as the National Suicide Prevention Lifeline, uses the 10-digit number of 800-273-8255 (TALK). The report noted that the hotline has proved effective: The service answered 2.2 million calls in 2018 and various assessments have shown significant reductions in hopelessness and suicidal ideation among callers.
However, based on data and conclusions from some of those assessments, the report determined “that the Lifeline could be more effective in preventing suicides and providing crisis intervention if it were accessible via a simple, easy-to-remember, 3-digit dialing code.” The report examined the feasibility of three-digit options, including N11 options such as 211 and 511, but based on an analysis by the North American Numbering Council, it found that “technical and operational concerns related to the 988 code could be more easily and quickly addressed and resolved than any re-education efforts related to repurposing a N11 code.”
Suicide and mental health crises have been on the rise, according to numbers from the Centers for Disease Control and Prevention cited by the FCC report. Among the 50 states, 49 saw increases during 1999-2016, and more than half saw increases greater than 20%. The report describes how some groups, such as veterans and LGBTQ youth, are at especially high risk:
Changing the VA’s OC Pill Dispensing Could Save Money—and Avoid Unwanted Pregnancy
The VA currently stipulates a 3-month maximum dispensing limit for all medications, including oral contraceptive pills (OCPs). But a 12-month cycle for OCPs improves adherence, reduces coverage gaps, and reduces unintended pregnancy, say researchers from University of Pittsburgh and the VA Pittsburgh Health Care System, both in Pennsylvania. Not only that, they add, the VA could save > $2 million a year.
OCPs are among the most commonly used methods of contraception among women veterans. VA data indicate that 43% of women dispensed 3-month supplies experience ≤ 1 gap of ≤ 7 days between refills during a year of use. Citing research that has found women on 12-month dispensing cycles have fewer gaps, which leads to fewer unintended pregnancies and abortions. US guidelines now recommend routine initial dispensing of up to 1-year supplies of hormonal contraception.
However, the financial consequences for such a switch in the VA were unclear, the researchers say. To find out, they developed a decision analysis model from the VA perspective to compare incremental costs of a 12-month supply vs a 3-month supply dispensed quarterly. Basing their model on a cohort of 24,309 women, the researchers looked at the effects of each strategy on resulting coverage gaps, discontinuation of OCPs, pregnancy, birth, miscarriage, and abortion.
The model projected that the 12-month system would reduce unintended pregnancies by 14%, or 583 unintended pregnancies averted annually—a conservative estimate, the researchers say.
Overall, the model estimated total savings of > $2 million annually.
Their results suggest obvious financial benefits for the VA—for example, less money spent on intrapartum care, the researchers say. But they add, “it is vital that contraceptive policies serve first and foremost to augment women’s reproductive outcomes and autonomy.” They highlight the potential financial gains as a “secondary benefit to the more important and evidence-based goal of improving contraceptive access.”
The VA currently stipulates a 3-month maximum dispensing limit for all medications, including oral contraceptive pills (OCPs). But a 12-month cycle for OCPs improves adherence, reduces coverage gaps, and reduces unintended pregnancy, say researchers from University of Pittsburgh and the VA Pittsburgh Health Care System, both in Pennsylvania. Not only that, they add, the VA could save > $2 million a year.
OCPs are among the most commonly used methods of contraception among women veterans. VA data indicate that 43% of women dispensed 3-month supplies experience ≤ 1 gap of ≤ 7 days between refills during a year of use. Citing research that has found women on 12-month dispensing cycles have fewer gaps, which leads to fewer unintended pregnancies and abortions. US guidelines now recommend routine initial dispensing of up to 1-year supplies of hormonal contraception.
However, the financial consequences for such a switch in the VA were unclear, the researchers say. To find out, they developed a decision analysis model from the VA perspective to compare incremental costs of a 12-month supply vs a 3-month supply dispensed quarterly. Basing their model on a cohort of 24,309 women, the researchers looked at the effects of each strategy on resulting coverage gaps, discontinuation of OCPs, pregnancy, birth, miscarriage, and abortion.
The model projected that the 12-month system would reduce unintended pregnancies by 14%, or 583 unintended pregnancies averted annually—a conservative estimate, the researchers say.
Overall, the model estimated total savings of > $2 million annually.
Their results suggest obvious financial benefits for the VA—for example, less money spent on intrapartum care, the researchers say. But they add, “it is vital that contraceptive policies serve first and foremost to augment women’s reproductive outcomes and autonomy.” They highlight the potential financial gains as a “secondary benefit to the more important and evidence-based goal of improving contraceptive access.”
The VA currently stipulates a 3-month maximum dispensing limit for all medications, including oral contraceptive pills (OCPs). But a 12-month cycle for OCPs improves adherence, reduces coverage gaps, and reduces unintended pregnancy, say researchers from University of Pittsburgh and the VA Pittsburgh Health Care System, both in Pennsylvania. Not only that, they add, the VA could save > $2 million a year.
OCPs are among the most commonly used methods of contraception among women veterans. VA data indicate that 43% of women dispensed 3-month supplies experience ≤ 1 gap of ≤ 7 days between refills during a year of use. Citing research that has found women on 12-month dispensing cycles have fewer gaps, which leads to fewer unintended pregnancies and abortions. US guidelines now recommend routine initial dispensing of up to 1-year supplies of hormonal contraception.
However, the financial consequences for such a switch in the VA were unclear, the researchers say. To find out, they developed a decision analysis model from the VA perspective to compare incremental costs of a 12-month supply vs a 3-month supply dispensed quarterly. Basing their model on a cohort of 24,309 women, the researchers looked at the effects of each strategy on resulting coverage gaps, discontinuation of OCPs, pregnancy, birth, miscarriage, and abortion.
The model projected that the 12-month system would reduce unintended pregnancies by 14%, or 583 unintended pregnancies averted annually—a conservative estimate, the researchers say.
Overall, the model estimated total savings of > $2 million annually.
Their results suggest obvious financial benefits for the VA—for example, less money spent on intrapartum care, the researchers say. But they add, “it is vital that contraceptive policies serve first and foremost to augment women’s reproductive outcomes and autonomy.” They highlight the potential financial gains as a “secondary benefit to the more important and evidence-based goal of improving contraceptive access.”
Burn-Pit Research Gets Renewed Focus
During Operations Iraqi and Enduring Freedom, everything from old uniforms to plastic, aerosol cans, electronic equipment, human waste, tires, and batteries were thrown into open pits, often doused with jet fuel, and set on fire.
Many deployed soldiers were exposed to smoke from these open-air burn pits, putting them at risk for cancer, neurologic effects, reproductive effects, respiratory toxicity, and cardiovascular toxicity. Veterans who were close to burn pits have reported eye irritation, itching, rashes, and respiratory problems, such as bronchitis, asthma, and emphysema.
In May 2019, the VA redesignated the Airborne Hazards Center of Excellence (AHCE), established in 2013, as the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). The redesignation was a consequence of the Helping Veterans Exposed to Burn Pits Act, which stemmed from an 18-month bipartisan effort to prevent burn pits from becoming “the Agent Orange of this generation of soldiers.” Senator Amy Klobuchar (D-MN), who cosponsored the legislation with Thom Tillis (R-NC) said, “After the Vietnam War, it took the US government years to recognize that there was a link between Agent Orange and its devastating health effects on our soldiers. … [W]e can’t make that same tragic mistake again by failing to identify the devastating health effects associated with burn pits.”
The AHCE was responsible for assessing veterans’ cardiopulmonary function, military/ nonmilitary exposures, and health-related symptoms for those with airborne hazard concerns. The AHBPCE will specialize in clinical and transitional research, focusing on expanding understanding of health outcomes and treatments for burn pit–related issues.
VA providers can consult with the AHBPCE about assessment and treatment. When appropriate, veterans may be invited for a comprehensive, multiday health evaluation from a specialized team. The examination includes state-of-the-art assessments of lung function and exercise capacity. The findings are used to develop recommendations, which are shared with the veteran and referring provider for follow-up care. The findings also are used by researchers at the center and throughout the VA to develop research questions to investigate and potentially improve clinical practice.
Veterans (including those who receive VA-authorized care in the community) with complex clinical presentations who are unable to be diagnosed locally may be referred for consultation or examination.
AHBPCE, which is located at the New Jersey War Related Illness and Injury Study Center (WRIISC), also provides the AHBPCE-WRIISC Airborne hazards Registry (AWARE) program, designed for veterans who complete the Airborne Hazards and Open Burn Pit Registry online questionnaire, report chronic respiratory symptoms, and meet other eligibility criteria. AHBPCE’s mandate also includes analyzing registry data to monitor the VA’s overall clinical response to exposure concerns.
During Operations Iraqi and Enduring Freedom, everything from old uniforms to plastic, aerosol cans, electronic equipment, human waste, tires, and batteries were thrown into open pits, often doused with jet fuel, and set on fire.
Many deployed soldiers were exposed to smoke from these open-air burn pits, putting them at risk for cancer, neurologic effects, reproductive effects, respiratory toxicity, and cardiovascular toxicity. Veterans who were close to burn pits have reported eye irritation, itching, rashes, and respiratory problems, such as bronchitis, asthma, and emphysema.
In May 2019, the VA redesignated the Airborne Hazards Center of Excellence (AHCE), established in 2013, as the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). The redesignation was a consequence of the Helping Veterans Exposed to Burn Pits Act, which stemmed from an 18-month bipartisan effort to prevent burn pits from becoming “the Agent Orange of this generation of soldiers.” Senator Amy Klobuchar (D-MN), who cosponsored the legislation with Thom Tillis (R-NC) said, “After the Vietnam War, it took the US government years to recognize that there was a link between Agent Orange and its devastating health effects on our soldiers. … [W]e can’t make that same tragic mistake again by failing to identify the devastating health effects associated with burn pits.”
The AHCE was responsible for assessing veterans’ cardiopulmonary function, military/ nonmilitary exposures, and health-related symptoms for those with airborne hazard concerns. The AHBPCE will specialize in clinical and transitional research, focusing on expanding understanding of health outcomes and treatments for burn pit–related issues.
VA providers can consult with the AHBPCE about assessment and treatment. When appropriate, veterans may be invited for a comprehensive, multiday health evaluation from a specialized team. The examination includes state-of-the-art assessments of lung function and exercise capacity. The findings are used to develop recommendations, which are shared with the veteran and referring provider for follow-up care. The findings also are used by researchers at the center and throughout the VA to develop research questions to investigate and potentially improve clinical practice.
Veterans (including those who receive VA-authorized care in the community) with complex clinical presentations who are unable to be diagnosed locally may be referred for consultation or examination.
AHBPCE, which is located at the New Jersey War Related Illness and Injury Study Center (WRIISC), also provides the AHBPCE-WRIISC Airborne hazards Registry (AWARE) program, designed for veterans who complete the Airborne Hazards and Open Burn Pit Registry online questionnaire, report chronic respiratory symptoms, and meet other eligibility criteria. AHBPCE’s mandate also includes analyzing registry data to monitor the VA’s overall clinical response to exposure concerns.
During Operations Iraqi and Enduring Freedom, everything from old uniforms to plastic, aerosol cans, electronic equipment, human waste, tires, and batteries were thrown into open pits, often doused with jet fuel, and set on fire.
Many deployed soldiers were exposed to smoke from these open-air burn pits, putting them at risk for cancer, neurologic effects, reproductive effects, respiratory toxicity, and cardiovascular toxicity. Veterans who were close to burn pits have reported eye irritation, itching, rashes, and respiratory problems, such as bronchitis, asthma, and emphysema.
In May 2019, the VA redesignated the Airborne Hazards Center of Excellence (AHCE), established in 2013, as the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). The redesignation was a consequence of the Helping Veterans Exposed to Burn Pits Act, which stemmed from an 18-month bipartisan effort to prevent burn pits from becoming “the Agent Orange of this generation of soldiers.” Senator Amy Klobuchar (D-MN), who cosponsored the legislation with Thom Tillis (R-NC) said, “After the Vietnam War, it took the US government years to recognize that there was a link between Agent Orange and its devastating health effects on our soldiers. … [W]e can’t make that same tragic mistake again by failing to identify the devastating health effects associated with burn pits.”
The AHCE was responsible for assessing veterans’ cardiopulmonary function, military/ nonmilitary exposures, and health-related symptoms for those with airborne hazard concerns. The AHBPCE will specialize in clinical and transitional research, focusing on expanding understanding of health outcomes and treatments for burn pit–related issues.
VA providers can consult with the AHBPCE about assessment and treatment. When appropriate, veterans may be invited for a comprehensive, multiday health evaluation from a specialized team. The examination includes state-of-the-art assessments of lung function and exercise capacity. The findings are used to develop recommendations, which are shared with the veteran and referring provider for follow-up care. The findings also are used by researchers at the center and throughout the VA to develop research questions to investigate and potentially improve clinical practice.
Veterans (including those who receive VA-authorized care in the community) with complex clinical presentations who are unable to be diagnosed locally may be referred for consultation or examination.
AHBPCE, which is located at the New Jersey War Related Illness and Injury Study Center (WRIISC), also provides the AHBPCE-WRIISC Airborne hazards Registry (AWARE) program, designed for veterans who complete the Airborne Hazards and Open Burn Pit Registry online questionnaire, report chronic respiratory symptoms, and meet other eligibility criteria. AHBPCE’s mandate also includes analyzing registry data to monitor the VA’s overall clinical response to exposure concerns.







