User login
Personalizing treatment plans for older patients with T2D
In the United States, type 2 diabetes (T2D) more commonly affects people older than 40 years, but it is most prevalent among adults over age 65, affecting more than 29% of this population. The heterogeneity in the health and functional status of older adults presents a challenge in the management and treatment of older patients with T2D. Moreover, there is an increased risk for health-related comorbidities and complications from diabetes treatment (for example, hypoglycemia) in older adults. Physiologic changes, such as decreased renal function, cognitive decline, and sarcopenia, may lead to an increased risk for adverse reactions to medications and require an individualized treatment approach. Although there have been a limited number of randomized controlled studies targeting older adults with multiple comorbidities and poor health status, subanalyses of diabetes trials with a subpopulation of older adults have provided additional evidence to better guide therapeutic approaches in caring for older patients with T2D.
Here’s a guide to developing personalized therapeutic regimens for older patients with T2D using lifestyle interventions, pharmacotherapy, and diabetes technology.
Determining an optimal glycemic target
An important first step in diabetes treatment is to determine the optimal glycemic target for patients. Although data support intensive glycemic control (hemoglobin A1c < 7%) to prevent complications from diabetes in younger patients with recently diagnosed disease, the data are less compelling in trials involving older populations with longer durations of T2D. One observational study with 71,092 older adults over age 60 reported a U-shaped correlation between A1c and mortality, with higher risks for mortality in those with A1c levels < 6% and ≥ 11%, compared with those with A1c levels of 6%-9%. Risks for any diabetes complications were higher at an A1c level ≥ 8%. Another observational study reported a U-shaped association between A1c and mortality, with the lowest hazard ratio for mortality at an A1c level of about 7.5%. Similarly, the ACCORD trial, which included older and middle-aged patients with T2D who had or were at risk for atherosclerotic cardiovascular disease, found that mortality followed a U-shaped curve at the low (A1c < 7%) and high (A1c > 8%) ends in patients who were given standard glycemic therapy. Hence, there has been a general trend to recommend less strict glycemic control in older adults.
However, it is important to remember that older patients with T2D are a heterogeneous group. The spectrum includes adults with recent-onset diabetes with no or few complications, those with long-standing diabetes and many complications, and frail older adults with multiple comorbidities and complications. Determining the optimal glycemic target for an older patient with T2D requires assessment not only of the patient’s medical status and comorbidities but also functional status, cognitive and psychological health, social situation, individual preferences, and life expectancy. The American Diabetes Association Standards of Medical Care in Diabetes provides the following guidance in determining the optimal glycemic control for older adults:
- Healthy adults with few coexisting chronic illnesses and intact cognitive and functional status should have an A1c level < 7.0%-7.5%.
- Adults with complex or intermediate comorbidities (multiple coexisting chronic illnesses, or two or more instrumental activities of daily living impairments, or mild to moderate cognitive impairment) should have an A1c level < 8.0%.
- Patients with poor health (long-term care or end-stage chronic illnesses or moderate to severe cognitive impairment or two or more activities of daily living impairments) should avoid reliance on A1c, and the goal is to avoid hypoglycemia and symptomatic hyperglycemia.
Because older patients are at a higher risk for complications and adverse effects from polypharmacy, regular assessments are recommended and treatment plans should be routinely reviewed and modified to avoid overtreatment.
Lifestyle interventions and pharmacotherapy
Lifestyle interventions, such as exercise, optimal nutrition, and protein intake, are integral in treating older patients with T2D. Older adults should engage in regular exercise (that is, aerobic activity, weight-bearing exercise, or resistance training), and the activity should be customized to frailty status. Regular exercise improves insulin sensitivity and glucose control, enhances functional status, and provides cardiometabolic benefits. Optimal nutrition and adequate protein intake are also important to prevent the development or worsening of sarcopenia and frailty.
Several factors must be considered when choosing pharmacotherapy for T2D treatment in older adults. These patients are at higher risk for adverse reactions to medications that can trigger hypoglycemia and serious cardiovascular events, and worsen cognitive function. Therefore, side effects should always be reviewed when choosing antidiabetic drugs. The complexity of treatment plans needs to be matched with the patients’ self-management abilities and available social support. Medication costs and insurance coverage should be considered because many older adults live on a fixed income. Although limited, data exist on the safety and efficacy of some glucose-lowering agents in older adults, which can provide guidance for choosing the optimal therapy for these patients.
Among the insulin sensitizers, metformin is most commonly used because of its efficacy, low risk for hypoglycemia, and affordability. Metformin can be safely used in the setting of reduced renal function down to the estimated glomerular filtration rate ≥ 30 mL/min per 1.73 m2. However, metformin should be avoided in patients with more advanced renal disease, liver failure, or heart failure. In older patients with T2D, potential concerns of metformin include gastrointestinal side effects, leading to reduced appetite, mild weight loss, and risk for vitamin B12 deficiency.
Pioglitazone, an oral antidiabetic in the thiazolidinedione (TZD) class, also targets insulin resistance and may provide some cardiovascular benefits. However, these agents are not commonly used in treating older patients with T2D owing to associated risk for edema, heart failure, osteoporosis/fractures, and bladder cancer.
Sulfonylureas and meglitinides are insulin secretagogues, which can promote insulin release independent of glucose levels. Sulfonylureas are typically avoided in older patients because they are associated with high risk for hypoglycemia. Meglitinides have a lower hypoglycemia risk than sulfonylureas because of their short duration of action; however, they are more expensive and require multiple daily administration, which can lead to issues with adherence.
Since 2008, there have been numerous cardiovascular outcomes trials assessing the safety and efficacy of T2D therapies that included a subpopulation of older patients either with cardiovascular disease or at high risk for cardiovascular disease. Post hoc analysis of data from these trials and smaller studies dedicated to older adults demonstrated the safety and efficacy of most incretin-based therapies and sodium-glucose cotransporter 2 (SGLT2) inhibitors in these patients. These newer medications have low hypoglycemia risk if not used in combination with insulin or insulin secretagogues.
Dipeptidyl peptidase 4 (DPP-4) inhibitors have the mildest side effect profile. However, they can be expensive and not reduce major adverse cardiovascular outcomes, and one agent, saxagliptin, has been associated with increased risk for heart failure hospitalization. Some glucagon-like peptide 1 (GLP-1) receptor agonists are effective in reducing major adverse cardiovascular events (cardiovascular deaths, stroke, and myocardial infarction) in patients older and younger than age 65. However, the gastrointestinal side effects and weight loss associated with this medication can be problematic for older patients. Most of the GLP-1 receptor agonists are injectables, which require good visual, motor, and cognitive skills for administration. SGLT2 inhibitors offer benefits for patients with T2D who have established cardiovascular disease, heart failure, and chronic kidney disease, with possible greater cardiovascular benefits in older adults. Adverse effects associated with SGLT2 inhibitors, such as weight loss, volume depletion, urinary incontinence, and genitourinary infections, may be a concern in older patients with T2D who are using these medications.
Because the insulin-secreting capacity of the pancreas declines with age, insulin therapy may be required for treatment of T2D in older patients. Insulin therapy can be complex and consideration must be given to patients’ social circumstances, as well as their physical and cognitive abilities. Older adults may need adaptive strategies, such as additional lighting, magnification glass, and premixed syringes. Simplification of complex insulin therapy (discontinuation of prandial insulin or sliding scale, changing timing of basal insulin) and use of insulin analogs with lower hypoglycemia risks should be considered. Weight gain as a result of insulin therapy may be beneficial in older adults with sarcopenia or frailty.
T2D technology for glycemic improvement
There have been major technological advancements in diabetes therapy. Continuous glucose monitors (CGMs) and automated insulin delivery systems can improve glycemic control, decrease the rate of hypoglycemia, and enhance the quality of life of older patients. Most of the studies evaluating the use of automated insulin delivery systems in older patients have focused on those with type 1 diabetes and demonstrated improvement in glycemic control and/or reduced hypoglycemia. The DIAMOND trial demonstrated improved A1c and reduced glycemic variability with the use of CGM in adults older than 60 years with either type 1 or type 2 diabetes on multiple daily injections. Bluetooth-enabled “smart” insulin pens, which record the time and dose of insulin administrations, can also be a great asset in caring for older patients, especially those with cognitive impairment. With better insurance coverage, diabetes technologies may become more accessible and an asset in treating older patients with T2D.
In conclusion, management of T2D in older adults requires an individualized approach because of the heterogeneity in their health and functional status. Because cardiovascular disease is the leading cause of mortality in older patients with T2D, treatment plans should also address frequently coexisting cardiovascular risk factors, such as hypertension and hyperlipidemia. Clinicians should consider patients’ overall health, comorbidities, cognitive and functional status, social support systems, preferences, and life expectancy when developing individualized therapeutic plans.
Dr. Gunawan is an assistant professor in the department of internal medicine at UT Southwestern Medical Center, Dallas. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In the United States, type 2 diabetes (T2D) more commonly affects people older than 40 years, but it is most prevalent among adults over age 65, affecting more than 29% of this population. The heterogeneity in the health and functional status of older adults presents a challenge in the management and treatment of older patients with T2D. Moreover, there is an increased risk for health-related comorbidities and complications from diabetes treatment (for example, hypoglycemia) in older adults. Physiologic changes, such as decreased renal function, cognitive decline, and sarcopenia, may lead to an increased risk for adverse reactions to medications and require an individualized treatment approach. Although there have been a limited number of randomized controlled studies targeting older adults with multiple comorbidities and poor health status, subanalyses of diabetes trials with a subpopulation of older adults have provided additional evidence to better guide therapeutic approaches in caring for older patients with T2D.
Here’s a guide to developing personalized therapeutic regimens for older patients with T2D using lifestyle interventions, pharmacotherapy, and diabetes technology.
Determining an optimal glycemic target
An important first step in diabetes treatment is to determine the optimal glycemic target for patients. Although data support intensive glycemic control (hemoglobin A1c < 7%) to prevent complications from diabetes in younger patients with recently diagnosed disease, the data are less compelling in trials involving older populations with longer durations of T2D. One observational study with 71,092 older adults over age 60 reported a U-shaped correlation between A1c and mortality, with higher risks for mortality in those with A1c levels < 6% and ≥ 11%, compared with those with A1c levels of 6%-9%. Risks for any diabetes complications were higher at an A1c level ≥ 8%. Another observational study reported a U-shaped association between A1c and mortality, with the lowest hazard ratio for mortality at an A1c level of about 7.5%. Similarly, the ACCORD trial, which included older and middle-aged patients with T2D who had or were at risk for atherosclerotic cardiovascular disease, found that mortality followed a U-shaped curve at the low (A1c < 7%) and high (A1c > 8%) ends in patients who were given standard glycemic therapy. Hence, there has been a general trend to recommend less strict glycemic control in older adults.
However, it is important to remember that older patients with T2D are a heterogeneous group. The spectrum includes adults with recent-onset diabetes with no or few complications, those with long-standing diabetes and many complications, and frail older adults with multiple comorbidities and complications. Determining the optimal glycemic target for an older patient with T2D requires assessment not only of the patient’s medical status and comorbidities but also functional status, cognitive and psychological health, social situation, individual preferences, and life expectancy. The American Diabetes Association Standards of Medical Care in Diabetes provides the following guidance in determining the optimal glycemic control for older adults:
- Healthy adults with few coexisting chronic illnesses and intact cognitive and functional status should have an A1c level < 7.0%-7.5%.
- Adults with complex or intermediate comorbidities (multiple coexisting chronic illnesses, or two or more instrumental activities of daily living impairments, or mild to moderate cognitive impairment) should have an A1c level < 8.0%.
- Patients with poor health (long-term care or end-stage chronic illnesses or moderate to severe cognitive impairment or two or more activities of daily living impairments) should avoid reliance on A1c, and the goal is to avoid hypoglycemia and symptomatic hyperglycemia.
Because older patients are at a higher risk for complications and adverse effects from polypharmacy, regular assessments are recommended and treatment plans should be routinely reviewed and modified to avoid overtreatment.
Lifestyle interventions and pharmacotherapy
Lifestyle interventions, such as exercise, optimal nutrition, and protein intake, are integral in treating older patients with T2D. Older adults should engage in regular exercise (that is, aerobic activity, weight-bearing exercise, or resistance training), and the activity should be customized to frailty status. Regular exercise improves insulin sensitivity and glucose control, enhances functional status, and provides cardiometabolic benefits. Optimal nutrition and adequate protein intake are also important to prevent the development or worsening of sarcopenia and frailty.
Several factors must be considered when choosing pharmacotherapy for T2D treatment in older adults. These patients are at higher risk for adverse reactions to medications that can trigger hypoglycemia and serious cardiovascular events, and worsen cognitive function. Therefore, side effects should always be reviewed when choosing antidiabetic drugs. The complexity of treatment plans needs to be matched with the patients’ self-management abilities and available social support. Medication costs and insurance coverage should be considered because many older adults live on a fixed income. Although limited, data exist on the safety and efficacy of some glucose-lowering agents in older adults, which can provide guidance for choosing the optimal therapy for these patients.
Among the insulin sensitizers, metformin is most commonly used because of its efficacy, low risk for hypoglycemia, and affordability. Metformin can be safely used in the setting of reduced renal function down to the estimated glomerular filtration rate ≥ 30 mL/min per 1.73 m2. However, metformin should be avoided in patients with more advanced renal disease, liver failure, or heart failure. In older patients with T2D, potential concerns of metformin include gastrointestinal side effects, leading to reduced appetite, mild weight loss, and risk for vitamin B12 deficiency.
Pioglitazone, an oral antidiabetic in the thiazolidinedione (TZD) class, also targets insulin resistance and may provide some cardiovascular benefits. However, these agents are not commonly used in treating older patients with T2D owing to associated risk for edema, heart failure, osteoporosis/fractures, and bladder cancer.
Sulfonylureas and meglitinides are insulin secretagogues, which can promote insulin release independent of glucose levels. Sulfonylureas are typically avoided in older patients because they are associated with high risk for hypoglycemia. Meglitinides have a lower hypoglycemia risk than sulfonylureas because of their short duration of action; however, they are more expensive and require multiple daily administration, which can lead to issues with adherence.
Since 2008, there have been numerous cardiovascular outcomes trials assessing the safety and efficacy of T2D therapies that included a subpopulation of older patients either with cardiovascular disease or at high risk for cardiovascular disease. Post hoc analysis of data from these trials and smaller studies dedicated to older adults demonstrated the safety and efficacy of most incretin-based therapies and sodium-glucose cotransporter 2 (SGLT2) inhibitors in these patients. These newer medications have low hypoglycemia risk if not used in combination with insulin or insulin secretagogues.
Dipeptidyl peptidase 4 (DPP-4) inhibitors have the mildest side effect profile. However, they can be expensive and not reduce major adverse cardiovascular outcomes, and one agent, saxagliptin, has been associated with increased risk for heart failure hospitalization. Some glucagon-like peptide 1 (GLP-1) receptor agonists are effective in reducing major adverse cardiovascular events (cardiovascular deaths, stroke, and myocardial infarction) in patients older and younger than age 65. However, the gastrointestinal side effects and weight loss associated with this medication can be problematic for older patients. Most of the GLP-1 receptor agonists are injectables, which require good visual, motor, and cognitive skills for administration. SGLT2 inhibitors offer benefits for patients with T2D who have established cardiovascular disease, heart failure, and chronic kidney disease, with possible greater cardiovascular benefits in older adults. Adverse effects associated with SGLT2 inhibitors, such as weight loss, volume depletion, urinary incontinence, and genitourinary infections, may be a concern in older patients with T2D who are using these medications.
Because the insulin-secreting capacity of the pancreas declines with age, insulin therapy may be required for treatment of T2D in older patients. Insulin therapy can be complex and consideration must be given to patients’ social circumstances, as well as their physical and cognitive abilities. Older adults may need adaptive strategies, such as additional lighting, magnification glass, and premixed syringes. Simplification of complex insulin therapy (discontinuation of prandial insulin or sliding scale, changing timing of basal insulin) and use of insulin analogs with lower hypoglycemia risks should be considered. Weight gain as a result of insulin therapy may be beneficial in older adults with sarcopenia or frailty.
T2D technology for glycemic improvement
There have been major technological advancements in diabetes therapy. Continuous glucose monitors (CGMs) and automated insulin delivery systems can improve glycemic control, decrease the rate of hypoglycemia, and enhance the quality of life of older patients. Most of the studies evaluating the use of automated insulin delivery systems in older patients have focused on those with type 1 diabetes and demonstrated improvement in glycemic control and/or reduced hypoglycemia. The DIAMOND trial demonstrated improved A1c and reduced glycemic variability with the use of CGM in adults older than 60 years with either type 1 or type 2 diabetes on multiple daily injections. Bluetooth-enabled “smart” insulin pens, which record the time and dose of insulin administrations, can also be a great asset in caring for older patients, especially those with cognitive impairment. With better insurance coverage, diabetes technologies may become more accessible and an asset in treating older patients with T2D.
In conclusion, management of T2D in older adults requires an individualized approach because of the heterogeneity in their health and functional status. Because cardiovascular disease is the leading cause of mortality in older patients with T2D, treatment plans should also address frequently coexisting cardiovascular risk factors, such as hypertension and hyperlipidemia. Clinicians should consider patients’ overall health, comorbidities, cognitive and functional status, social support systems, preferences, and life expectancy when developing individualized therapeutic plans.
Dr. Gunawan is an assistant professor in the department of internal medicine at UT Southwestern Medical Center, Dallas. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In the United States, type 2 diabetes (T2D) more commonly affects people older than 40 years, but it is most prevalent among adults over age 65, affecting more than 29% of this population. The heterogeneity in the health and functional status of older adults presents a challenge in the management and treatment of older patients with T2D. Moreover, there is an increased risk for health-related comorbidities and complications from diabetes treatment (for example, hypoglycemia) in older adults. Physiologic changes, such as decreased renal function, cognitive decline, and sarcopenia, may lead to an increased risk for adverse reactions to medications and require an individualized treatment approach. Although there have been a limited number of randomized controlled studies targeting older adults with multiple comorbidities and poor health status, subanalyses of diabetes trials with a subpopulation of older adults have provided additional evidence to better guide therapeutic approaches in caring for older patients with T2D.
Here’s a guide to developing personalized therapeutic regimens for older patients with T2D using lifestyle interventions, pharmacotherapy, and diabetes technology.
Determining an optimal glycemic target
An important first step in diabetes treatment is to determine the optimal glycemic target for patients. Although data support intensive glycemic control (hemoglobin A1c < 7%) to prevent complications from diabetes in younger patients with recently diagnosed disease, the data are less compelling in trials involving older populations with longer durations of T2D. One observational study with 71,092 older adults over age 60 reported a U-shaped correlation between A1c and mortality, with higher risks for mortality in those with A1c levels < 6% and ≥ 11%, compared with those with A1c levels of 6%-9%. Risks for any diabetes complications were higher at an A1c level ≥ 8%. Another observational study reported a U-shaped association between A1c and mortality, with the lowest hazard ratio for mortality at an A1c level of about 7.5%. Similarly, the ACCORD trial, which included older and middle-aged patients with T2D who had or were at risk for atherosclerotic cardiovascular disease, found that mortality followed a U-shaped curve at the low (A1c < 7%) and high (A1c > 8%) ends in patients who were given standard glycemic therapy. Hence, there has been a general trend to recommend less strict glycemic control in older adults.
However, it is important to remember that older patients with T2D are a heterogeneous group. The spectrum includes adults with recent-onset diabetes with no or few complications, those with long-standing diabetes and many complications, and frail older adults with multiple comorbidities and complications. Determining the optimal glycemic target for an older patient with T2D requires assessment not only of the patient’s medical status and comorbidities but also functional status, cognitive and psychological health, social situation, individual preferences, and life expectancy. The American Diabetes Association Standards of Medical Care in Diabetes provides the following guidance in determining the optimal glycemic control for older adults:
- Healthy adults with few coexisting chronic illnesses and intact cognitive and functional status should have an A1c level < 7.0%-7.5%.
- Adults with complex or intermediate comorbidities (multiple coexisting chronic illnesses, or two or more instrumental activities of daily living impairments, or mild to moderate cognitive impairment) should have an A1c level < 8.0%.
- Patients with poor health (long-term care or end-stage chronic illnesses or moderate to severe cognitive impairment or two or more activities of daily living impairments) should avoid reliance on A1c, and the goal is to avoid hypoglycemia and symptomatic hyperglycemia.
Because older patients are at a higher risk for complications and adverse effects from polypharmacy, regular assessments are recommended and treatment plans should be routinely reviewed and modified to avoid overtreatment.
Lifestyle interventions and pharmacotherapy
Lifestyle interventions, such as exercise, optimal nutrition, and protein intake, are integral in treating older patients with T2D. Older adults should engage in regular exercise (that is, aerobic activity, weight-bearing exercise, or resistance training), and the activity should be customized to frailty status. Regular exercise improves insulin sensitivity and glucose control, enhances functional status, and provides cardiometabolic benefits. Optimal nutrition and adequate protein intake are also important to prevent the development or worsening of sarcopenia and frailty.
Several factors must be considered when choosing pharmacotherapy for T2D treatment in older adults. These patients are at higher risk for adverse reactions to medications that can trigger hypoglycemia and serious cardiovascular events, and worsen cognitive function. Therefore, side effects should always be reviewed when choosing antidiabetic drugs. The complexity of treatment plans needs to be matched with the patients’ self-management abilities and available social support. Medication costs and insurance coverage should be considered because many older adults live on a fixed income. Although limited, data exist on the safety and efficacy of some glucose-lowering agents in older adults, which can provide guidance for choosing the optimal therapy for these patients.
Among the insulin sensitizers, metformin is most commonly used because of its efficacy, low risk for hypoglycemia, and affordability. Metformin can be safely used in the setting of reduced renal function down to the estimated glomerular filtration rate ≥ 30 mL/min per 1.73 m2. However, metformin should be avoided in patients with more advanced renal disease, liver failure, or heart failure. In older patients with T2D, potential concerns of metformin include gastrointestinal side effects, leading to reduced appetite, mild weight loss, and risk for vitamin B12 deficiency.
Pioglitazone, an oral antidiabetic in the thiazolidinedione (TZD) class, also targets insulin resistance and may provide some cardiovascular benefits. However, these agents are not commonly used in treating older patients with T2D owing to associated risk for edema, heart failure, osteoporosis/fractures, and bladder cancer.
Sulfonylureas and meglitinides are insulin secretagogues, which can promote insulin release independent of glucose levels. Sulfonylureas are typically avoided in older patients because they are associated with high risk for hypoglycemia. Meglitinides have a lower hypoglycemia risk than sulfonylureas because of their short duration of action; however, they are more expensive and require multiple daily administration, which can lead to issues with adherence.
Since 2008, there have been numerous cardiovascular outcomes trials assessing the safety and efficacy of T2D therapies that included a subpopulation of older patients either with cardiovascular disease or at high risk for cardiovascular disease. Post hoc analysis of data from these trials and smaller studies dedicated to older adults demonstrated the safety and efficacy of most incretin-based therapies and sodium-glucose cotransporter 2 (SGLT2) inhibitors in these patients. These newer medications have low hypoglycemia risk if not used in combination with insulin or insulin secretagogues.
Dipeptidyl peptidase 4 (DPP-4) inhibitors have the mildest side effect profile. However, they can be expensive and not reduce major adverse cardiovascular outcomes, and one agent, saxagliptin, has been associated with increased risk for heart failure hospitalization. Some glucagon-like peptide 1 (GLP-1) receptor agonists are effective in reducing major adverse cardiovascular events (cardiovascular deaths, stroke, and myocardial infarction) in patients older and younger than age 65. However, the gastrointestinal side effects and weight loss associated with this medication can be problematic for older patients. Most of the GLP-1 receptor agonists are injectables, which require good visual, motor, and cognitive skills for administration. SGLT2 inhibitors offer benefits for patients with T2D who have established cardiovascular disease, heart failure, and chronic kidney disease, with possible greater cardiovascular benefits in older adults. Adverse effects associated with SGLT2 inhibitors, such as weight loss, volume depletion, urinary incontinence, and genitourinary infections, may be a concern in older patients with T2D who are using these medications.
Because the insulin-secreting capacity of the pancreas declines with age, insulin therapy may be required for treatment of T2D in older patients. Insulin therapy can be complex and consideration must be given to patients’ social circumstances, as well as their physical and cognitive abilities. Older adults may need adaptive strategies, such as additional lighting, magnification glass, and premixed syringes. Simplification of complex insulin therapy (discontinuation of prandial insulin or sliding scale, changing timing of basal insulin) and use of insulin analogs with lower hypoglycemia risks should be considered. Weight gain as a result of insulin therapy may be beneficial in older adults with sarcopenia or frailty.
T2D technology for glycemic improvement
There have been major technological advancements in diabetes therapy. Continuous glucose monitors (CGMs) and automated insulin delivery systems can improve glycemic control, decrease the rate of hypoglycemia, and enhance the quality of life of older patients. Most of the studies evaluating the use of automated insulin delivery systems in older patients have focused on those with type 1 diabetes and demonstrated improvement in glycemic control and/or reduced hypoglycemia. The DIAMOND trial demonstrated improved A1c and reduced glycemic variability with the use of CGM in adults older than 60 years with either type 1 or type 2 diabetes on multiple daily injections. Bluetooth-enabled “smart” insulin pens, which record the time and dose of insulin administrations, can also be a great asset in caring for older patients, especially those with cognitive impairment. With better insurance coverage, diabetes technologies may become more accessible and an asset in treating older patients with T2D.
In conclusion, management of T2D in older adults requires an individualized approach because of the heterogeneity in their health and functional status. Because cardiovascular disease is the leading cause of mortality in older patients with T2D, treatment plans should also address frequently coexisting cardiovascular risk factors, such as hypertension and hyperlipidemia. Clinicians should consider patients’ overall health, comorbidities, cognitive and functional status, social support systems, preferences, and life expectancy when developing individualized therapeutic plans.
Dr. Gunawan is an assistant professor in the department of internal medicine at UT Southwestern Medical Center, Dallas. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Should youth with type 1 diabetes use closed-loop systems?
Would closed-loop systems be a good option for young patients with type 1 diabetes?
International and French recommendations on closed-loop systems state that the use of an “artificial pancreas” should be reserved for adults who are fully engaged with their treatment. This means that young patients, especially adolescents, who are less likely to comply with treatment and are more likely to experience suboptimal blood glucose control, are often excluded from the use of such systems for managing their diabetes.
Several recent studies seem to call this approach into question.
One such study, which was presented at a Francophone Diabetes Society conference and was published in Nature Communications, showed that adolescents with poorly controlled diabetes who were equipped with closed-loop systems gained IQ points and reasoning capacity and experienced a reduction in edematous tissue in the brain cortex. Furthermore, with the closed-loop system, patients spent 13% more time in a target range, and there was a significant reduction in time spent in hyperglycemia.
In the same vein, a small prospective study published in Diabetes Care showed that the closed-loop system with the Minimed 780G pump improved glycemic control for 20 young patients with type 1 diabetes aged 13-25 years whose diabetes was poorly controlled (hemoglobin A1c ≥ 8.5%). At the end of the 3-month study period, the average A1c had decreased from 10.5% (±2.1%) to 7.6% (±1.1%), an average decrease of 2.9%. The time spent in target A1c, which was set from 0.70 g/L to 1.80 g/L, was increased by almost 40%.
With respect to very young children, a study published in The New England Journal of Medicine also showed a favorable risk-benefit ratio for closed-loop systems. The trial, which enrolled 102 children aged 2 years to less than 6 years who had type 1 diabetes, showed that the amount of time that the glucose level was within the target range during the 13-week study period was higher (+3 hours) for those who had been randomly assigned to receive the hybrid closed-loop system (n = 68) than for those who had received the standard treatment (n = 34), either with an insulin pump or multiple daily injections or a Dexcom G6 continuous glucose monitoring device.
A previous study carried out by the Paris Public Hospital System had already shown that the French Diabeloop system could reduce episodes of hypoglycemia and achieve good glycemic control for prepubescent children (n = 21; aged 6-12 years) with type 1 diabetes in real-life conditions.
Eric Renard, MD, PhD, head of the department of endocrinology and diabetes at Lapeyronie Hospital in Montpellier, France, was not surprised at the findings from the study, especially in adolescents with poorly controlled diabetes.
“We have already seen studies in which those patients who had the most poorly controlled diabetes at the start were the ones who improved the most with the closed-loop system, by at least 20% in terms of time in target. These findings resonate with what I see in my clinic,” said Dr. Renard in an interview.
“In my experience, these young adolescents, who neglected their diabetes when they had no devices to help control it, when they had to inject themselves, et cetera ... well, they’re just not the same people when they’re put on a closed-loop system,” he added. “They rise to the challenge, and for the first time, they succeed without making a huge effort, since the algorithm does what they weren’t doing. It’s astonishing to see near-total engagement in these young people when explaining the technology to them and saying, ‘Let’s give it a go.’ These are the very same youngsters who didn’t want to hear about their diabetes in the past. They are delighted and once again involved in managing their condition.”
That’s why Dr. Renard recommends keeping an open mind when considering treatment options for young patients with poorly controlled type 1 diabetes.
“When young people have very poorly controlled diabetes, they risk having cardiovascular complications and damaging their retinas and kidneys,” he said. “If we can get them from 25% to 45% time in target, even if that hasn’t been easy to achieve, this will help save their blood vessels! The only thing we have to be careful of is that we don’t set up a closed-loop system in someone who doesn’t want one. But, if it can manage to spark the interest of a young patient, in most cases, it’s beneficial.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Would closed-loop systems be a good option for young patients with type 1 diabetes?
International and French recommendations on closed-loop systems state that the use of an “artificial pancreas” should be reserved for adults who are fully engaged with their treatment. This means that young patients, especially adolescents, who are less likely to comply with treatment and are more likely to experience suboptimal blood glucose control, are often excluded from the use of such systems for managing their diabetes.
Several recent studies seem to call this approach into question.
One such study, which was presented at a Francophone Diabetes Society conference and was published in Nature Communications, showed that adolescents with poorly controlled diabetes who were equipped with closed-loop systems gained IQ points and reasoning capacity and experienced a reduction in edematous tissue in the brain cortex. Furthermore, with the closed-loop system, patients spent 13% more time in a target range, and there was a significant reduction in time spent in hyperglycemia.
In the same vein, a small prospective study published in Diabetes Care showed that the closed-loop system with the Minimed 780G pump improved glycemic control for 20 young patients with type 1 diabetes aged 13-25 years whose diabetes was poorly controlled (hemoglobin A1c ≥ 8.5%). At the end of the 3-month study period, the average A1c had decreased from 10.5% (±2.1%) to 7.6% (±1.1%), an average decrease of 2.9%. The time spent in target A1c, which was set from 0.70 g/L to 1.80 g/L, was increased by almost 40%.
With respect to very young children, a study published in The New England Journal of Medicine also showed a favorable risk-benefit ratio for closed-loop systems. The trial, which enrolled 102 children aged 2 years to less than 6 years who had type 1 diabetes, showed that the amount of time that the glucose level was within the target range during the 13-week study period was higher (+3 hours) for those who had been randomly assigned to receive the hybrid closed-loop system (n = 68) than for those who had received the standard treatment (n = 34), either with an insulin pump or multiple daily injections or a Dexcom G6 continuous glucose monitoring device.
A previous study carried out by the Paris Public Hospital System had already shown that the French Diabeloop system could reduce episodes of hypoglycemia and achieve good glycemic control for prepubescent children (n = 21; aged 6-12 years) with type 1 diabetes in real-life conditions.
Eric Renard, MD, PhD, head of the department of endocrinology and diabetes at Lapeyronie Hospital in Montpellier, France, was not surprised at the findings from the study, especially in adolescents with poorly controlled diabetes.
“We have already seen studies in which those patients who had the most poorly controlled diabetes at the start were the ones who improved the most with the closed-loop system, by at least 20% in terms of time in target. These findings resonate with what I see in my clinic,” said Dr. Renard in an interview.
“In my experience, these young adolescents, who neglected their diabetes when they had no devices to help control it, when they had to inject themselves, et cetera ... well, they’re just not the same people when they’re put on a closed-loop system,” he added. “They rise to the challenge, and for the first time, they succeed without making a huge effort, since the algorithm does what they weren’t doing. It’s astonishing to see near-total engagement in these young people when explaining the technology to them and saying, ‘Let’s give it a go.’ These are the very same youngsters who didn’t want to hear about their diabetes in the past. They are delighted and once again involved in managing their condition.”
That’s why Dr. Renard recommends keeping an open mind when considering treatment options for young patients with poorly controlled type 1 diabetes.
“When young people have very poorly controlled diabetes, they risk having cardiovascular complications and damaging their retinas and kidneys,” he said. “If we can get them from 25% to 45% time in target, even if that hasn’t been easy to achieve, this will help save their blood vessels! The only thing we have to be careful of is that we don’t set up a closed-loop system in someone who doesn’t want one. But, if it can manage to spark the interest of a young patient, in most cases, it’s beneficial.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Would closed-loop systems be a good option for young patients with type 1 diabetes?
International and French recommendations on closed-loop systems state that the use of an “artificial pancreas” should be reserved for adults who are fully engaged with their treatment. This means that young patients, especially adolescents, who are less likely to comply with treatment and are more likely to experience suboptimal blood glucose control, are often excluded from the use of such systems for managing their diabetes.
Several recent studies seem to call this approach into question.
One such study, which was presented at a Francophone Diabetes Society conference and was published in Nature Communications, showed that adolescents with poorly controlled diabetes who were equipped with closed-loop systems gained IQ points and reasoning capacity and experienced a reduction in edematous tissue in the brain cortex. Furthermore, with the closed-loop system, patients spent 13% more time in a target range, and there was a significant reduction in time spent in hyperglycemia.
In the same vein, a small prospective study published in Diabetes Care showed that the closed-loop system with the Minimed 780G pump improved glycemic control for 20 young patients with type 1 diabetes aged 13-25 years whose diabetes was poorly controlled (hemoglobin A1c ≥ 8.5%). At the end of the 3-month study period, the average A1c had decreased from 10.5% (±2.1%) to 7.6% (±1.1%), an average decrease of 2.9%. The time spent in target A1c, which was set from 0.70 g/L to 1.80 g/L, was increased by almost 40%.
With respect to very young children, a study published in The New England Journal of Medicine also showed a favorable risk-benefit ratio for closed-loop systems. The trial, which enrolled 102 children aged 2 years to less than 6 years who had type 1 diabetes, showed that the amount of time that the glucose level was within the target range during the 13-week study period was higher (+3 hours) for those who had been randomly assigned to receive the hybrid closed-loop system (n = 68) than for those who had received the standard treatment (n = 34), either with an insulin pump or multiple daily injections or a Dexcom G6 continuous glucose monitoring device.
A previous study carried out by the Paris Public Hospital System had already shown that the French Diabeloop system could reduce episodes of hypoglycemia and achieve good glycemic control for prepubescent children (n = 21; aged 6-12 years) with type 1 diabetes in real-life conditions.
Eric Renard, MD, PhD, head of the department of endocrinology and diabetes at Lapeyronie Hospital in Montpellier, France, was not surprised at the findings from the study, especially in adolescents with poorly controlled diabetes.
“We have already seen studies in which those patients who had the most poorly controlled diabetes at the start were the ones who improved the most with the closed-loop system, by at least 20% in terms of time in target. These findings resonate with what I see in my clinic,” said Dr. Renard in an interview.
“In my experience, these young adolescents, who neglected their diabetes when they had no devices to help control it, when they had to inject themselves, et cetera ... well, they’re just not the same people when they’re put on a closed-loop system,” he added. “They rise to the challenge, and for the first time, they succeed without making a huge effort, since the algorithm does what they weren’t doing. It’s astonishing to see near-total engagement in these young people when explaining the technology to them and saying, ‘Let’s give it a go.’ These are the very same youngsters who didn’t want to hear about their diabetes in the past. They are delighted and once again involved in managing their condition.”
That’s why Dr. Renard recommends keeping an open mind when considering treatment options for young patients with poorly controlled type 1 diabetes.
“When young people have very poorly controlled diabetes, they risk having cardiovascular complications and damaging their retinas and kidneys,” he said. “If we can get them from 25% to 45% time in target, even if that hasn’t been easy to achieve, this will help save their blood vessels! The only thing we have to be careful of is that we don’t set up a closed-loop system in someone who doesn’t want one. But, if it can manage to spark the interest of a young patient, in most cases, it’s beneficial.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Obesity drugs overpriced, change needed to tackle issue
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).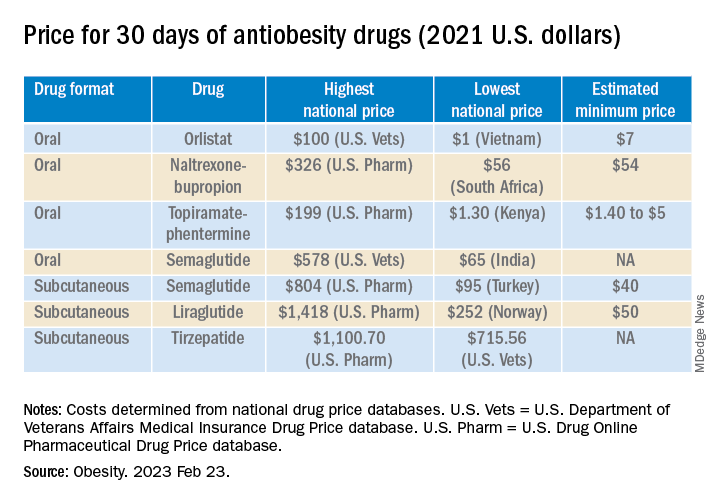
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
Tirzepatide scores win in second obesity trial, SURMOUNT-2
The “twincretin” tirzepatide (Mounjaro) has proven successful in SURMOUNT-2, the second pivotal trial for the drug as an antiobesity agent, according to top-line results reported April 27 by tirzepatide’s manufacturer, Lilly, in a press release. The company reveals that tirzepatide achieved both of its primary endpoints in the trial, as well as all its key secondary endpoints.
The findings pave the way for tirzepatide to likely receive Food and Drug Administration approval as a treatment for obesity, perhaps before the end of 2023.
Tirzepatide received FDA approval in May 2022 for the treatment of type 2 diabetes in adults, under the brand name Mounjaro, and some people have already been using it off-label to treat obesity.
Tirzepatide is a dual glucagonlike peptide–1 (GLP-1) agonist and glucose-dependent insulinotropic polypeptide agonist. Several GLP-1 receptor agonists are already approved in the United States, including semaglutide, a once-weekly injection, which is approved as Wegovy for patients with obesity and as Ozempic for treatment of type 2 diabetes.
These agents have been incredibly popular among celebrity influencers, and with use of the #Ozempic hashtag and others on social media, this has led to unprecedented use of these products for weight loss, often among those who do not even have obesity or type 2 diabetes. Subsequently, patients with type 2 diabetes and obesity who need them have often struggled to obtain them, owing to shortages following this phenomenon.
SURMOUNT-2: Weight loss around 15%, less than seen in SURMOUNT-1
SURMOUNT-2 enrolled 938 adults with overweight or obesity and type 2 diabetes and had dual primary endpoints that both focused on weight loss, compared with placebo.
The first completed pivotal trial of tirzepatide for weight loss, SURMOUNT-1, enrolled people with overweight or obesity but no diabetes and had its main results reported in 2022. At the time, the weight loss achieved with tirzepatide, was described as “unprecedented,” with those given the highest dose in that trial (15 mg subcutaneously per week) losing an average of 20%-22% of body weight over 72 weeks, depending on the specific statistical analysis used.
For SURMOUNT-2’s first primary endpoint, 72 weeks of weekly subcutaneous injections with tirzepatide at dosages of 10 mg or 15 mg led to an average weight loss from baseline of 13.4% and 15.7%, respectively, compared with an average loss of 3.3% from baseline in the placebo-treated control arm.
For the second primary endpoint, 81.6% of people on the 10-mg dose and 86.4% on the 15-mg dose achieved at least 5% weight loss from baseline, compared with 30.5% of controls who had at least 5% weight loss from baseline.
In one key secondary endpoint, tirzepatide at dosages of 10 mg or 15 mg weekly produced at least a 15% cut in weight from baseline in 41.4% and 51.8% of participants, respectively, compared with a 2.6% rate of this endpoint in the placebo controls.
So the extent of weight loss seen in in SURMOUNT-2 was somewhat less than was reported in SURMOUNT-1, a finding consistent with many prior studies of incretin-based weight-loss agents, which seem to pack a more potent weight-loss punch in people without type 2 diabetes.
Lilly did not specifically report the treatment effect of tirzepatide on hemoglobin A1c in SURMOUNT-2, only saying that the effect was similar to what had been seen in the series of five SURPASS trials that led to the approval of tirzepatide for type 2 diabetes.
Lilly also reported that the safety profile of tirzepatide in SURMOUNT-2 generally matched what was seen in SURMOUNT-1 as well as in the SURPASS trials. The most common adverse events in SURMOUNT-2 involved gastrointestinal symptoms, such as nausea, diarrhea, and vomiting; these were generally mild to moderate in severity and clustered during the dose-escalation phase at the start of treatment. Treatment discontinuations caused by adverse effects were 3.8% on the 10-mg dosage, 7.4% on the 15-mg dosage, and 3.8% on placebo.
SURMOUNT-2 enrolled patients in the United States, Puerto Rico, and five other countries. All participants also received interventions designed to reduce their calorie intake and increase their physical activity.
More SURMOUNT-2 results at ADA in June
Lilly also announced that researchers would report more complete results from SURMOUNT-2 at the 2023 scientific sessions of the American Diabetes Association, being held in San Diego in late June, and publish the findings in a major medical journal.
Results from two additional phase 3 trials of tirzepatide in people with overweight or obesity, SURMOUNT-3 and SURMOUNT-4, are expected later in 2023.
Lilly started an application to the FDA for an indication for weight loss in October 2022 under a fast track designation by the agency, and the data collected in SURMOUNT-2 are expected to complete this application, which would then be subject to an FDA decision within about 6 months. Lilly said in its April 27 press release that it anticipates an FDA decision on this application may occur before the end of 2023.
SURMOUNT-2 and all of the other tirzepatide trials were sponsored by Lilly.
A version of this article first appeared on Medscape.com.
The “twincretin” tirzepatide (Mounjaro) has proven successful in SURMOUNT-2, the second pivotal trial for the drug as an antiobesity agent, according to top-line results reported April 27 by tirzepatide’s manufacturer, Lilly, in a press release. The company reveals that tirzepatide achieved both of its primary endpoints in the trial, as well as all its key secondary endpoints.
The findings pave the way for tirzepatide to likely receive Food and Drug Administration approval as a treatment for obesity, perhaps before the end of 2023.
Tirzepatide received FDA approval in May 2022 for the treatment of type 2 diabetes in adults, under the brand name Mounjaro, and some people have already been using it off-label to treat obesity.
Tirzepatide is a dual glucagonlike peptide–1 (GLP-1) agonist and glucose-dependent insulinotropic polypeptide agonist. Several GLP-1 receptor agonists are already approved in the United States, including semaglutide, a once-weekly injection, which is approved as Wegovy for patients with obesity and as Ozempic for treatment of type 2 diabetes.
These agents have been incredibly popular among celebrity influencers, and with use of the #Ozempic hashtag and others on social media, this has led to unprecedented use of these products for weight loss, often among those who do not even have obesity or type 2 diabetes. Subsequently, patients with type 2 diabetes and obesity who need them have often struggled to obtain them, owing to shortages following this phenomenon.
SURMOUNT-2: Weight loss around 15%, less than seen in SURMOUNT-1
SURMOUNT-2 enrolled 938 adults with overweight or obesity and type 2 diabetes and had dual primary endpoints that both focused on weight loss, compared with placebo.
The first completed pivotal trial of tirzepatide for weight loss, SURMOUNT-1, enrolled people with overweight or obesity but no diabetes and had its main results reported in 2022. At the time, the weight loss achieved with tirzepatide, was described as “unprecedented,” with those given the highest dose in that trial (15 mg subcutaneously per week) losing an average of 20%-22% of body weight over 72 weeks, depending on the specific statistical analysis used.
For SURMOUNT-2’s first primary endpoint, 72 weeks of weekly subcutaneous injections with tirzepatide at dosages of 10 mg or 15 mg led to an average weight loss from baseline of 13.4% and 15.7%, respectively, compared with an average loss of 3.3% from baseline in the placebo-treated control arm.
For the second primary endpoint, 81.6% of people on the 10-mg dose and 86.4% on the 15-mg dose achieved at least 5% weight loss from baseline, compared with 30.5% of controls who had at least 5% weight loss from baseline.
In one key secondary endpoint, tirzepatide at dosages of 10 mg or 15 mg weekly produced at least a 15% cut in weight from baseline in 41.4% and 51.8% of participants, respectively, compared with a 2.6% rate of this endpoint in the placebo controls.
So the extent of weight loss seen in in SURMOUNT-2 was somewhat less than was reported in SURMOUNT-1, a finding consistent with many prior studies of incretin-based weight-loss agents, which seem to pack a more potent weight-loss punch in people without type 2 diabetes.
Lilly did not specifically report the treatment effect of tirzepatide on hemoglobin A1c in SURMOUNT-2, only saying that the effect was similar to what had been seen in the series of five SURPASS trials that led to the approval of tirzepatide for type 2 diabetes.
Lilly also reported that the safety profile of tirzepatide in SURMOUNT-2 generally matched what was seen in SURMOUNT-1 as well as in the SURPASS trials. The most common adverse events in SURMOUNT-2 involved gastrointestinal symptoms, such as nausea, diarrhea, and vomiting; these were generally mild to moderate in severity and clustered during the dose-escalation phase at the start of treatment. Treatment discontinuations caused by adverse effects were 3.8% on the 10-mg dosage, 7.4% on the 15-mg dosage, and 3.8% on placebo.
SURMOUNT-2 enrolled patients in the United States, Puerto Rico, and five other countries. All participants also received interventions designed to reduce their calorie intake and increase their physical activity.
More SURMOUNT-2 results at ADA in June
Lilly also announced that researchers would report more complete results from SURMOUNT-2 at the 2023 scientific sessions of the American Diabetes Association, being held in San Diego in late June, and publish the findings in a major medical journal.
Results from two additional phase 3 trials of tirzepatide in people with overweight or obesity, SURMOUNT-3 and SURMOUNT-4, are expected later in 2023.
Lilly started an application to the FDA for an indication for weight loss in October 2022 under a fast track designation by the agency, and the data collected in SURMOUNT-2 are expected to complete this application, which would then be subject to an FDA decision within about 6 months. Lilly said in its April 27 press release that it anticipates an FDA decision on this application may occur before the end of 2023.
SURMOUNT-2 and all of the other tirzepatide trials were sponsored by Lilly.
A version of this article first appeared on Medscape.com.
The “twincretin” tirzepatide (Mounjaro) has proven successful in SURMOUNT-2, the second pivotal trial for the drug as an antiobesity agent, according to top-line results reported April 27 by tirzepatide’s manufacturer, Lilly, in a press release. The company reveals that tirzepatide achieved both of its primary endpoints in the trial, as well as all its key secondary endpoints.
The findings pave the way for tirzepatide to likely receive Food and Drug Administration approval as a treatment for obesity, perhaps before the end of 2023.
Tirzepatide received FDA approval in May 2022 for the treatment of type 2 diabetes in adults, under the brand name Mounjaro, and some people have already been using it off-label to treat obesity.
Tirzepatide is a dual glucagonlike peptide–1 (GLP-1) agonist and glucose-dependent insulinotropic polypeptide agonist. Several GLP-1 receptor agonists are already approved in the United States, including semaglutide, a once-weekly injection, which is approved as Wegovy for patients with obesity and as Ozempic for treatment of type 2 diabetes.
These agents have been incredibly popular among celebrity influencers, and with use of the #Ozempic hashtag and others on social media, this has led to unprecedented use of these products for weight loss, often among those who do not even have obesity or type 2 diabetes. Subsequently, patients with type 2 diabetes and obesity who need them have often struggled to obtain them, owing to shortages following this phenomenon.
SURMOUNT-2: Weight loss around 15%, less than seen in SURMOUNT-1
SURMOUNT-2 enrolled 938 adults with overweight or obesity and type 2 diabetes and had dual primary endpoints that both focused on weight loss, compared with placebo.
The first completed pivotal trial of tirzepatide for weight loss, SURMOUNT-1, enrolled people with overweight or obesity but no diabetes and had its main results reported in 2022. At the time, the weight loss achieved with tirzepatide, was described as “unprecedented,” with those given the highest dose in that trial (15 mg subcutaneously per week) losing an average of 20%-22% of body weight over 72 weeks, depending on the specific statistical analysis used.
For SURMOUNT-2’s first primary endpoint, 72 weeks of weekly subcutaneous injections with tirzepatide at dosages of 10 mg or 15 mg led to an average weight loss from baseline of 13.4% and 15.7%, respectively, compared with an average loss of 3.3% from baseline in the placebo-treated control arm.
For the second primary endpoint, 81.6% of people on the 10-mg dose and 86.4% on the 15-mg dose achieved at least 5% weight loss from baseline, compared with 30.5% of controls who had at least 5% weight loss from baseline.
In one key secondary endpoint, tirzepatide at dosages of 10 mg or 15 mg weekly produced at least a 15% cut in weight from baseline in 41.4% and 51.8% of participants, respectively, compared with a 2.6% rate of this endpoint in the placebo controls.
So the extent of weight loss seen in in SURMOUNT-2 was somewhat less than was reported in SURMOUNT-1, a finding consistent with many prior studies of incretin-based weight-loss agents, which seem to pack a more potent weight-loss punch in people without type 2 diabetes.
Lilly did not specifically report the treatment effect of tirzepatide on hemoglobin A1c in SURMOUNT-2, only saying that the effect was similar to what had been seen in the series of five SURPASS trials that led to the approval of tirzepatide for type 2 diabetes.
Lilly also reported that the safety profile of tirzepatide in SURMOUNT-2 generally matched what was seen in SURMOUNT-1 as well as in the SURPASS trials. The most common adverse events in SURMOUNT-2 involved gastrointestinal symptoms, such as nausea, diarrhea, and vomiting; these were generally mild to moderate in severity and clustered during the dose-escalation phase at the start of treatment. Treatment discontinuations caused by adverse effects were 3.8% on the 10-mg dosage, 7.4% on the 15-mg dosage, and 3.8% on placebo.
SURMOUNT-2 enrolled patients in the United States, Puerto Rico, and five other countries. All participants also received interventions designed to reduce their calorie intake and increase their physical activity.
More SURMOUNT-2 results at ADA in June
Lilly also announced that researchers would report more complete results from SURMOUNT-2 at the 2023 scientific sessions of the American Diabetes Association, being held in San Diego in late June, and publish the findings in a major medical journal.
Results from two additional phase 3 trials of tirzepatide in people with overweight or obesity, SURMOUNT-3 and SURMOUNT-4, are expected later in 2023.
Lilly started an application to the FDA for an indication for weight loss in October 2022 under a fast track designation by the agency, and the data collected in SURMOUNT-2 are expected to complete this application, which would then be subject to an FDA decision within about 6 months. Lilly said in its April 27 press release that it anticipates an FDA decision on this application may occur before the end of 2023.
SURMOUNT-2 and all of the other tirzepatide trials were sponsored by Lilly.
A version of this article first appeared on Medscape.com.
Drive, chip, and putt your way to osteoarthritis relief
Taking a swing against arthritis
Osteoarthritis is a tough disease to manage. Exercise helps ease the stiffness and pain of the joints, but at the same time, the disease makes it difficult to do that beneficial exercise. Even a relatively simple activity like jogging can hurt more than it helps. If only there were a low-impact exercise that was incredibly popular among the generally older population who are likely to have arthritis.
We love a good golf study here at LOTME, and a group of Australian and U.K. researchers have provided. Osteoarthritis affects 2 million people in the land down under, making it the most common source of disability there. In that population, only 64% reported their physical health to be good, very good, or excellent. Among the 459 golfers with OA that the study authors surveyed, however, the percentage reporting good health rose to more than 90%.
A similar story emerged when they looked at mental health. Nearly a quarter of nongolfers with OA reported high or very high levels of psychological distress, compared with just 8% of golfers. This pattern of improved physical and mental health remained when the researchers looked at the general, non-OA population.
This isn’t the first time golf’s been connected with improved health, and previous studies have shown golf to reduce the risks of cardiovascular disease, diabetes, and obesity, among other things. Just walking one 18-hole round significantly exceeds the CDC’s recommended 150 minutes of physical activity per week. Go out multiple times a week – leaving the cart and beer at home, American golfers – and you’ll be fit for a lifetime.
The golfers on our staff, however, are still waiting for those mental health benefits to kick in. Because when we’re adding up our scorecard after that string of four double bogeys to end the round, we’re most definitely thinking: “Yes, this sport is reducing my psychological distress. I am having fun right now.”
Battle of the sexes’ intestines
There are, we’re sure you’ve noticed, some differences between males and females. Females, for one thing, have longer small intestines than males. Everybody knows that, right? You didn’t know? Really? … Really?
Well, then, we’re guessing you haven’t read “Hidden diversity: Comparative functional morphology of humans and other species” by Erin A. McKenney, PhD, of North Carolina State University, Raleigh, and associates, which just appeared in PeerJ. We couldn’t put it down, even in the shower – a real page-turner/scroller. (It’s a great way to clean a phone, for those who also like to scroll, text, or talk on the toilet.)
The researchers got out their rulers, calipers, and string and took many measurements of the digestive systems of 45 human cadavers (21 female and 24 male), which were compared with data from 10 rats, 10 pigs, and 10 bullfrogs, which had been collected (the measurements, not the animals) by undergraduate students enrolled in a comparative anatomy laboratory course at the university.
There was little intestinal-length variation among the four-legged subjects, but when it comes to humans, females have “consistently and significantly longer small intestines than males,” the investigators noted.
The women’s small intestines, almost 14 feet long on average, were about a foot longer than the men’s, which suggests that women are better able to extract nutrients from food and “supports the canalization hypothesis, which posits that women are better able to survive during periods of stress,” coauthor Amanda Hale said in a written statement from the school. The way to a man’s heart may be through his stomach, but the way to a woman’s heart is through her duodenum, it seems.
Fascinating stuff, to be sure, but the thing that really caught our eye in the PeerJ article was the authors’ suggestion “that organs behave independently of one another, both within and across species.” Organs behaving independently? A somewhat ominous concept, no doubt, but it does explain a lot of the sounds we hear coming from our guts, which can get pretty frightening, especially on chili night.
Dog walking is dangerous business
Yes, you did read that right. A lot of strange things can send you to the emergency department. Go ahead and add dog walking onto that list.
Investigators from Johns Hopkins University estimate that over 422,000 adults presented to U.S. emergency departments with leash-dependent dog walking-related injuries between 2001 and 2020.
With almost 53% of U.S. households owning at least one dog in 2021-2022 in the wake of the COVID pet boom, this kind of occurrence is becoming more common than you think. The annual number of dog-walking injuries more than quadrupled from 7,300 to 32,000 over the course of the study, and the researchers link that spike to the promotion of dog walking for fitness, along with the boost of ownership itself.
The most common injuries listed in the National Electronic Injury Surveillance System database were finger fracture, traumatic brain injury, and shoulder sprain or strain. These mostly involved falls from being pulled, tripped, or tangled up in the leash while walking. For those aged 65 years and older, traumatic brain injury and hip fracture were the most common.
Women were 50% more likely to sustain a fracture than were men, and dog owners aged 65 and older were three times as likely to fall, twice as likely to get a fracture, and 60% more likely to have brain injury than were younger people. Now, that’s not to say younger people don’t also get hurt. After all, dogs aren’t ageists. The researchers have that data but it’s coming out later.
Meanwhile, the pitfalls involved with just trying to get our daily steps in while letting Muffin do her business have us on the lookout for random squirrels.
Taking a swing against arthritis
Osteoarthritis is a tough disease to manage. Exercise helps ease the stiffness and pain of the joints, but at the same time, the disease makes it difficult to do that beneficial exercise. Even a relatively simple activity like jogging can hurt more than it helps. If only there were a low-impact exercise that was incredibly popular among the generally older population who are likely to have arthritis.
We love a good golf study here at LOTME, and a group of Australian and U.K. researchers have provided. Osteoarthritis affects 2 million people in the land down under, making it the most common source of disability there. In that population, only 64% reported their physical health to be good, very good, or excellent. Among the 459 golfers with OA that the study authors surveyed, however, the percentage reporting good health rose to more than 90%.
A similar story emerged when they looked at mental health. Nearly a quarter of nongolfers with OA reported high or very high levels of psychological distress, compared with just 8% of golfers. This pattern of improved physical and mental health remained when the researchers looked at the general, non-OA population.
This isn’t the first time golf’s been connected with improved health, and previous studies have shown golf to reduce the risks of cardiovascular disease, diabetes, and obesity, among other things. Just walking one 18-hole round significantly exceeds the CDC’s recommended 150 minutes of physical activity per week. Go out multiple times a week – leaving the cart and beer at home, American golfers – and you’ll be fit for a lifetime.
The golfers on our staff, however, are still waiting for those mental health benefits to kick in. Because when we’re adding up our scorecard after that string of four double bogeys to end the round, we’re most definitely thinking: “Yes, this sport is reducing my psychological distress. I am having fun right now.”
Battle of the sexes’ intestines
There are, we’re sure you’ve noticed, some differences between males and females. Females, for one thing, have longer small intestines than males. Everybody knows that, right? You didn’t know? Really? … Really?
Well, then, we’re guessing you haven’t read “Hidden diversity: Comparative functional morphology of humans and other species” by Erin A. McKenney, PhD, of North Carolina State University, Raleigh, and associates, which just appeared in PeerJ. We couldn’t put it down, even in the shower – a real page-turner/scroller. (It’s a great way to clean a phone, for those who also like to scroll, text, or talk on the toilet.)
The researchers got out their rulers, calipers, and string and took many measurements of the digestive systems of 45 human cadavers (21 female and 24 male), which were compared with data from 10 rats, 10 pigs, and 10 bullfrogs, which had been collected (the measurements, not the animals) by undergraduate students enrolled in a comparative anatomy laboratory course at the university.
There was little intestinal-length variation among the four-legged subjects, but when it comes to humans, females have “consistently and significantly longer small intestines than males,” the investigators noted.
The women’s small intestines, almost 14 feet long on average, were about a foot longer than the men’s, which suggests that women are better able to extract nutrients from food and “supports the canalization hypothesis, which posits that women are better able to survive during periods of stress,” coauthor Amanda Hale said in a written statement from the school. The way to a man’s heart may be through his stomach, but the way to a woman’s heart is through her duodenum, it seems.
Fascinating stuff, to be sure, but the thing that really caught our eye in the PeerJ article was the authors’ suggestion “that organs behave independently of one another, both within and across species.” Organs behaving independently? A somewhat ominous concept, no doubt, but it does explain a lot of the sounds we hear coming from our guts, which can get pretty frightening, especially on chili night.
Dog walking is dangerous business
Yes, you did read that right. A lot of strange things can send you to the emergency department. Go ahead and add dog walking onto that list.
Investigators from Johns Hopkins University estimate that over 422,000 adults presented to U.S. emergency departments with leash-dependent dog walking-related injuries between 2001 and 2020.
With almost 53% of U.S. households owning at least one dog in 2021-2022 in the wake of the COVID pet boom, this kind of occurrence is becoming more common than you think. The annual number of dog-walking injuries more than quadrupled from 7,300 to 32,000 over the course of the study, and the researchers link that spike to the promotion of dog walking for fitness, along with the boost of ownership itself.
The most common injuries listed in the National Electronic Injury Surveillance System database were finger fracture, traumatic brain injury, and shoulder sprain or strain. These mostly involved falls from being pulled, tripped, or tangled up in the leash while walking. For those aged 65 years and older, traumatic brain injury and hip fracture were the most common.
Women were 50% more likely to sustain a fracture than were men, and dog owners aged 65 and older were three times as likely to fall, twice as likely to get a fracture, and 60% more likely to have brain injury than were younger people. Now, that’s not to say younger people don’t also get hurt. After all, dogs aren’t ageists. The researchers have that data but it’s coming out later.
Meanwhile, the pitfalls involved with just trying to get our daily steps in while letting Muffin do her business have us on the lookout for random squirrels.
Taking a swing against arthritis
Osteoarthritis is a tough disease to manage. Exercise helps ease the stiffness and pain of the joints, but at the same time, the disease makes it difficult to do that beneficial exercise. Even a relatively simple activity like jogging can hurt more than it helps. If only there were a low-impact exercise that was incredibly popular among the generally older population who are likely to have arthritis.
We love a good golf study here at LOTME, and a group of Australian and U.K. researchers have provided. Osteoarthritis affects 2 million people in the land down under, making it the most common source of disability there. In that population, only 64% reported their physical health to be good, very good, or excellent. Among the 459 golfers with OA that the study authors surveyed, however, the percentage reporting good health rose to more than 90%.
A similar story emerged when they looked at mental health. Nearly a quarter of nongolfers with OA reported high or very high levels of psychological distress, compared with just 8% of golfers. This pattern of improved physical and mental health remained when the researchers looked at the general, non-OA population.
This isn’t the first time golf’s been connected with improved health, and previous studies have shown golf to reduce the risks of cardiovascular disease, diabetes, and obesity, among other things. Just walking one 18-hole round significantly exceeds the CDC’s recommended 150 minutes of physical activity per week. Go out multiple times a week – leaving the cart and beer at home, American golfers – and you’ll be fit for a lifetime.
The golfers on our staff, however, are still waiting for those mental health benefits to kick in. Because when we’re adding up our scorecard after that string of four double bogeys to end the round, we’re most definitely thinking: “Yes, this sport is reducing my psychological distress. I am having fun right now.”
Battle of the sexes’ intestines
There are, we’re sure you’ve noticed, some differences between males and females. Females, for one thing, have longer small intestines than males. Everybody knows that, right? You didn’t know? Really? … Really?
Well, then, we’re guessing you haven’t read “Hidden diversity: Comparative functional morphology of humans and other species” by Erin A. McKenney, PhD, of North Carolina State University, Raleigh, and associates, which just appeared in PeerJ. We couldn’t put it down, even in the shower – a real page-turner/scroller. (It’s a great way to clean a phone, for those who also like to scroll, text, or talk on the toilet.)
The researchers got out their rulers, calipers, and string and took many measurements of the digestive systems of 45 human cadavers (21 female and 24 male), which were compared with data from 10 rats, 10 pigs, and 10 bullfrogs, which had been collected (the measurements, not the animals) by undergraduate students enrolled in a comparative anatomy laboratory course at the university.
There was little intestinal-length variation among the four-legged subjects, but when it comes to humans, females have “consistently and significantly longer small intestines than males,” the investigators noted.
The women’s small intestines, almost 14 feet long on average, were about a foot longer than the men’s, which suggests that women are better able to extract nutrients from food and “supports the canalization hypothesis, which posits that women are better able to survive during periods of stress,” coauthor Amanda Hale said in a written statement from the school. The way to a man’s heart may be through his stomach, but the way to a woman’s heart is through her duodenum, it seems.
Fascinating stuff, to be sure, but the thing that really caught our eye in the PeerJ article was the authors’ suggestion “that organs behave independently of one another, both within and across species.” Organs behaving independently? A somewhat ominous concept, no doubt, but it does explain a lot of the sounds we hear coming from our guts, which can get pretty frightening, especially on chili night.
Dog walking is dangerous business
Yes, you did read that right. A lot of strange things can send you to the emergency department. Go ahead and add dog walking onto that list.
Investigators from Johns Hopkins University estimate that over 422,000 adults presented to U.S. emergency departments with leash-dependent dog walking-related injuries between 2001 and 2020.
With almost 53% of U.S. households owning at least one dog in 2021-2022 in the wake of the COVID pet boom, this kind of occurrence is becoming more common than you think. The annual number of dog-walking injuries more than quadrupled from 7,300 to 32,000 over the course of the study, and the researchers link that spike to the promotion of dog walking for fitness, along with the boost of ownership itself.
The most common injuries listed in the National Electronic Injury Surveillance System database were finger fracture, traumatic brain injury, and shoulder sprain or strain. These mostly involved falls from being pulled, tripped, or tangled up in the leash while walking. For those aged 65 years and older, traumatic brain injury and hip fracture were the most common.
Women were 50% more likely to sustain a fracture than were men, and dog owners aged 65 and older were three times as likely to fall, twice as likely to get a fracture, and 60% more likely to have brain injury than were younger people. Now, that’s not to say younger people don’t also get hurt. After all, dogs aren’t ageists. The researchers have that data but it’s coming out later.
Meanwhile, the pitfalls involved with just trying to get our daily steps in while letting Muffin do her business have us on the lookout for random squirrels.
BMI is a flawed measure of obesity. What are alternatives?
“BMI is trash. Full stop.” This controversial tweet, which received thousands of likes and retweets, was cited in a recent article by one doctor on when physicians might stop using body mass index (BMI) to diagnose obesity.
BMI has for years been the consensus default method for assessing whether a person is overweight or has obesity, and is still widely used as the gatekeeper metric for treatment eligibility for certain weight-loss agents and bariatric surgery.
an important determinant of the cardiometabolic consequences of fat.
Alternative metrics include waist circumference and/or waist-to-height ratio (WHtR); imaging methods such as CT, MRI, and dual-energy x-ray absorptiometry (DXA); and bioelectrical impedance to assess fat volume and location. All have made some inroads on the tight grip BMI has had on obesity assessment.
Chances are, however, that BMI will not fade away anytime soon given how entrenched it has become in clinical practice and for insurance coverage, as well as its relative simplicity and precision.
“BMI is embedded in a wide range of guidelines on the use of medications and surgery. It’s embedded in Food and Drug Administration regulations and for billing and insurance coverage. It would take extremely strong data and years of work to undo the infrastructure built around BMI and replace it with something else. I don’t see that happening [anytime soon],” commented Daniel H. Bessesen, MD, a professor at the University of Colorado at Denver, Aurora, and chief of endocrinology for Denver Health.
“It would be almost impossible to replace all the studies that have used BMI with investigations using some other measure,” he said.
BMI Is ‘imperfect’
The entrenched position of BMI as the go-to metric doesn’t keep detractors from weighing in. As noted in a commentary on current clinical challenges surrounding obesity recently published in Annals of Internal Medicine, the journal’s editor-in-chief, Christine Laine, MD, and senior deputy editor Christina C. Wee, MD, listed six top issues clinicians must deal with, one of which, they say, is the need for a better measure of obesity than BMI.
“Unfortunately, BMI is an imperfect measure of body composition that differs with ethnicity, sex, body frame, and muscle mass,” noted Dr. Laine and Dr. Wee.
BMI is based on a person’s weight in kilograms divided by the square of their height in meters. A “healthy” BMI is between 18.5 and 24.9 kg/m2, overweight is 25-29.9, and 30 or greater is considered to represent obesity. However, certain ethnic groups have lower cutoffs for overweight or obesity because of evidence that such individuals can be at higher risk of obesity-related comorbidities at lower BMIs.
“BMI was chosen as the initial screening tool [for obesity] not because anyone thought it was perfect or the best measure but because of its simplicity. All you need is height, weight, and a calculator,” Dr. Wee said in an interview.
Numerous online calculators are available, including one from the Centers for Disease Control and Prevention where height in feet and inches and weight in pounds can be entered to generate the BMI.
BMI is also inherently limited by being “a proxy for adiposity” and not a direct measure, added Dr. Wee, who is also director of the Obesity Research Program of Beth Israel Deaconess Medical Center, Boston.
As such, BMI can’t distinguish between fat and muscle because it relies on weight only to gauge adiposity, noted Tiffany Powell-Wiley, MD, an obesity researcher at the National Heart, Lung, and Blood Institute in Bethesda, Md. Another shortcoming of BMI is that it “is good for distinguishing population-level risk for cardiovascular disease and other chronic diseases, but it does not help as much for distinguishing risk at an individual level,” she said in an interview.
These and other drawbacks have prompted researchers to look for other useful metrics. WHtR, for example, has recently made headway as a potential BMI alternative or complement.
The case for WHtR
Concern about overreliance on BMI despite its limitations is not new. In 2015, an American Heart Association scientific statement from the group’s Obesity Committee concluded that “BMI alone, even with lower thresholds, is a useful but not an ideal tool for identification of obesity or assessment of cardiovascular risk,” especially for people from Asian, Black, Hispanic, and Pacific Islander populations.
The writing panel also recommended that clinicians measure waist circumference annually and use that information along with BMI “to better gauge cardiovascular risk in diverse populations.”
Momentum for moving beyond BMI alone has continued to build following the AHA statement.
In September 2022, the National Institute for Health and Care Excellence, which sets policies for the United Kingdom’s National Health Service, revised its guidancefor assessment and management of people with obesity. The updated guidance recommends that when clinicians assess “adults with BMI below 35 kg/m2, measure and use their WHtR, as well as their BMI, as a practical estimate of central adiposity and use these measurements to help to assess and predict health risks.”
NICE released an extensive literature review with the revision, and based on the evidence, said that “using waist-to-height ratio as well as BMI would help give a practical estimate of central adiposity in adults with BMI under 35 kg/m2. This would in turn help professionals assess and predict health risks.”
However, the review added that, “because people with a BMI over 35 kg/m2 are always likely to have a high WHtR, the committee recognized that it may not be a useful addition for predicting health risks in this group.” The 2022 NICE review also said that it is “important to estimate central adiposity when assessing future health risks, including for people whose BMI is in the healthy-weight category.”
This new emphasis by NICE on measuring and using WHtR as part of obesity assessment “represents an important change in population health policy,” commented Dr. Powell-Wiley. “I expect more professional organizations will endorse use of waist circumference or waist-to-height ratio now that NICE has taken this step,” she predicted.
Waist circumference and WHtR may become standard measures of adiposity in clinical practice over the next 5-10 years.
The recent move by NICE to highlight a complementary role for WHtR “is another acknowledgment that BMI is an imperfect tool for stratifying cardiometabolic risk in a diverse population, especially in people with lower BMIs” because of its variability, commented Jamie Almandoz, MD, medical director of the weight wellness program at UT Southwestern Medical Center, Dallas.
WHtR vs. BMI
Another recent step forward for WHtR came with the publication of a post hoc analysis of data collected in the PARADIGM-HF trial, a study that had the primary purpose of comparing two medications for improving outcomes in more than 8,000 patients with heart failure with reduced ejection fraction.
The new analysis showed that “two indices that incorporate waist circumference and height, but not weight, showed a clearer association between greater adiposity and a higher risk of heart failure hospitalization,” compared with BMI.
WHtR was one of the two indices identified as being a better correlate for the adverse effect of excess adiposity compared with BMI.
The authors of the post hoc analysis did not design their analysis to compare WHtR with BMI. Instead, their goal was to better understand what’s known as the “obesity paradox” in people with heart failure with reduced ejection fraction: The recurring observation that, when these patients with heart failure have lower BMIs they fare worse, with higher rates of mortality and adverse cardiovascular outcomes, compared with patients with higher BMIs.
The new analysis showed that this paradox disappeared when WHtR was substituted for BMI as the obesity metric.
This “provides meaningful data about the superiority of WHtR, compared with BMI, for predicting heart failure outcomes,” said Dr. Powell-Wiley, although she cautioned that the analysis was limited by scant data in diverse populations and did not look at other important cardiovascular disease outcomes. While Dr. Powell-Wiley does not think that WHtR needs assessment in a prospective, controlled trial, she called for analysis of pooled prospective studies with more diverse populations to better document the advantages of WHtR over BMI.
The PARADIGM-HF post hoc analysis shows again how flawed BMI is for health assessment and the relative importance of an individualized understanding of a person’s body composition, Dr. Almandoz said in an interview. “As we collect more data, there is increasing awareness of how imperfect BMI is.”
Measuring waist circumference is tricky
Although WHtR looks promising as a substitute for or add-on to BMI, it has its own limitations, particularly the challenge of accurately measuring waist circumference.
Measuring waist circumference “not only takes more time but requires the assessor to be well trained about where to put the tape measure and making sure it’s measured at the same place each time,” even when different people take serial measurements from individual patients, noted Dr. Wee. Determining waist circumference can also be technically difficult when done on larger people, she added, and collectively these challenges make waist circumference “less reproducible from measurement to measurement.”
“It’s relatively clear how to standardize measurement of weight and height, but there is a huge amount of variability when the waist is measured,” agreed Dr. Almandoz. “And waist circumference also differs by ethnicity, race, sex, and body frame. There are significant differences in waist circumference levels that associate with increased health risks” between, for example, White and South Asian people.
Another limitation of waist circumference and WHtR is that they “cannot differentiate between visceral and abdominal subcutaneous adipose tissue, which are vastly different regarding cardiometabolic risk, commented Ian Neeland, MD, director of cardiovascular prevention at the University Hospitals Harrington Heart & Vascular Institute, Cleveland.
The imaging option
“Waist-to-height ratio is not the ultimate answer,” Dr. Neeland said in an interview. He instead endorsed “advanced imaging for body fat distribution,” such as CT or MRI scans, as his pick for what should be the standard obesity metric, “given that it is much more specific and actionable for both risk assessment and response to therapy. I expect slow but steady advancements that move away from BMI cutoffs, for example for bariatric surgery, given that BMI is an imprecise and crude tool.”
But although imaging with methods like CT and MRI may provide the best accuracy and precision for tracking the volume of a person’s cardiometabolically dangerous fat, they are also hampered by relatively high cost and, for CT and DXA, the issue of radiation exposure.
“CT, MRI, and DXA scans give more in-depth assessment of body composition, but should we expose people to the radiation and the cost?” Dr. Almandoz wondered.
“Height, weight, and waist circumference cost nothing to obtain,” creating a big relative disadvantage for imaging, said Naveed Sattar, MD, professor of metabolic medicine at the University of Glasgow.
“Data would need to show that imaging gives clinicians substantially more information about future risk” to justify its price, Dr. Sattar emphasized.
BMI’s limits mean adding on
Regardless of whichever alternatives to BMI end up getting used most, experts generally agree that BMI alone is looking increasingly inadequate.
“Over the next 5 years, BMI will come to be seen as a screening tool that categorizes people into general risk groups” that also needs “other metrics and variables, such as age, race, ethnicity, family history, blood glucose, and blood pressure to better describe health risk in an individual,” predicted Dr. Bessesen.
The endorsement of WHtR by NICE “will lead to more research into how to incorporate WHtR into routine practice. We need more evidence to translate what NICE said into practice,” said Dr. Sattar. “I don’t think we’ll see a shift away from BMI, but we’ll add alternative measures that are particularly useful in certain patients.”
“Because we live in diverse societies, we need to individualize risk assessment and couple that with technology that makes analysis of body composition more accessible,” agreed Dr. Almandoz. He noted that the UT Southwestern weight wellness program where he practices has, for about the past decade, routinely collected waist circumference and bioelectrical impedance data as well as BMI on all people seen in the practice for obesity concerns. Making these additional measurements on a routine basis also helps strengthen patient engagement.
“We get into trouble when we make rigid health policy and clinical decisions based on BMI alone without looking at the patient holistically,” said Dr. Wee. “Patients are more than arbitrary numbers, and clinicians should make clinical decisions based on the totality of evidence for each individual patient.”
Dr. Bessesen, Dr. Wee, Dr. Powell-Wiley, and Dr. Almandoz reported no relevant financial relationships. Dr. Neeland has reported being a consultant for Merck. Dr. Sattar has reported being a consultant or speaker for Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi.
A version of this article originally appeared on Medscape.com.
“BMI is trash. Full stop.” This controversial tweet, which received thousands of likes and retweets, was cited in a recent article by one doctor on when physicians might stop using body mass index (BMI) to diagnose obesity.
BMI has for years been the consensus default method for assessing whether a person is overweight or has obesity, and is still widely used as the gatekeeper metric for treatment eligibility for certain weight-loss agents and bariatric surgery.
an important determinant of the cardiometabolic consequences of fat.
Alternative metrics include waist circumference and/or waist-to-height ratio (WHtR); imaging methods such as CT, MRI, and dual-energy x-ray absorptiometry (DXA); and bioelectrical impedance to assess fat volume and location. All have made some inroads on the tight grip BMI has had on obesity assessment.
Chances are, however, that BMI will not fade away anytime soon given how entrenched it has become in clinical practice and for insurance coverage, as well as its relative simplicity and precision.
“BMI is embedded in a wide range of guidelines on the use of medications and surgery. It’s embedded in Food and Drug Administration regulations and for billing and insurance coverage. It would take extremely strong data and years of work to undo the infrastructure built around BMI and replace it with something else. I don’t see that happening [anytime soon],” commented Daniel H. Bessesen, MD, a professor at the University of Colorado at Denver, Aurora, and chief of endocrinology for Denver Health.
“It would be almost impossible to replace all the studies that have used BMI with investigations using some other measure,” he said.
BMI Is ‘imperfect’
The entrenched position of BMI as the go-to metric doesn’t keep detractors from weighing in. As noted in a commentary on current clinical challenges surrounding obesity recently published in Annals of Internal Medicine, the journal’s editor-in-chief, Christine Laine, MD, and senior deputy editor Christina C. Wee, MD, listed six top issues clinicians must deal with, one of which, they say, is the need for a better measure of obesity than BMI.
“Unfortunately, BMI is an imperfect measure of body composition that differs with ethnicity, sex, body frame, and muscle mass,” noted Dr. Laine and Dr. Wee.
BMI is based on a person’s weight in kilograms divided by the square of their height in meters. A “healthy” BMI is between 18.5 and 24.9 kg/m2, overweight is 25-29.9, and 30 or greater is considered to represent obesity. However, certain ethnic groups have lower cutoffs for overweight or obesity because of evidence that such individuals can be at higher risk of obesity-related comorbidities at lower BMIs.
“BMI was chosen as the initial screening tool [for obesity] not because anyone thought it was perfect or the best measure but because of its simplicity. All you need is height, weight, and a calculator,” Dr. Wee said in an interview.
Numerous online calculators are available, including one from the Centers for Disease Control and Prevention where height in feet and inches and weight in pounds can be entered to generate the BMI.
BMI is also inherently limited by being “a proxy for adiposity” and not a direct measure, added Dr. Wee, who is also director of the Obesity Research Program of Beth Israel Deaconess Medical Center, Boston.
As such, BMI can’t distinguish between fat and muscle because it relies on weight only to gauge adiposity, noted Tiffany Powell-Wiley, MD, an obesity researcher at the National Heart, Lung, and Blood Institute in Bethesda, Md. Another shortcoming of BMI is that it “is good for distinguishing population-level risk for cardiovascular disease and other chronic diseases, but it does not help as much for distinguishing risk at an individual level,” she said in an interview.
These and other drawbacks have prompted researchers to look for other useful metrics. WHtR, for example, has recently made headway as a potential BMI alternative or complement.
The case for WHtR
Concern about overreliance on BMI despite its limitations is not new. In 2015, an American Heart Association scientific statement from the group’s Obesity Committee concluded that “BMI alone, even with lower thresholds, is a useful but not an ideal tool for identification of obesity or assessment of cardiovascular risk,” especially for people from Asian, Black, Hispanic, and Pacific Islander populations.
The writing panel also recommended that clinicians measure waist circumference annually and use that information along with BMI “to better gauge cardiovascular risk in diverse populations.”
Momentum for moving beyond BMI alone has continued to build following the AHA statement.
In September 2022, the National Institute for Health and Care Excellence, which sets policies for the United Kingdom’s National Health Service, revised its guidancefor assessment and management of people with obesity. The updated guidance recommends that when clinicians assess “adults with BMI below 35 kg/m2, measure and use their WHtR, as well as their BMI, as a practical estimate of central adiposity and use these measurements to help to assess and predict health risks.”
NICE released an extensive literature review with the revision, and based on the evidence, said that “using waist-to-height ratio as well as BMI would help give a practical estimate of central adiposity in adults with BMI under 35 kg/m2. This would in turn help professionals assess and predict health risks.”
However, the review added that, “because people with a BMI over 35 kg/m2 are always likely to have a high WHtR, the committee recognized that it may not be a useful addition for predicting health risks in this group.” The 2022 NICE review also said that it is “important to estimate central adiposity when assessing future health risks, including for people whose BMI is in the healthy-weight category.”
This new emphasis by NICE on measuring and using WHtR as part of obesity assessment “represents an important change in population health policy,” commented Dr. Powell-Wiley. “I expect more professional organizations will endorse use of waist circumference or waist-to-height ratio now that NICE has taken this step,” she predicted.
Waist circumference and WHtR may become standard measures of adiposity in clinical practice over the next 5-10 years.
The recent move by NICE to highlight a complementary role for WHtR “is another acknowledgment that BMI is an imperfect tool for stratifying cardiometabolic risk in a diverse population, especially in people with lower BMIs” because of its variability, commented Jamie Almandoz, MD, medical director of the weight wellness program at UT Southwestern Medical Center, Dallas.
WHtR vs. BMI
Another recent step forward for WHtR came with the publication of a post hoc analysis of data collected in the PARADIGM-HF trial, a study that had the primary purpose of comparing two medications for improving outcomes in more than 8,000 patients with heart failure with reduced ejection fraction.
The new analysis showed that “two indices that incorporate waist circumference and height, but not weight, showed a clearer association between greater adiposity and a higher risk of heart failure hospitalization,” compared with BMI.
WHtR was one of the two indices identified as being a better correlate for the adverse effect of excess adiposity compared with BMI.
The authors of the post hoc analysis did not design their analysis to compare WHtR with BMI. Instead, their goal was to better understand what’s known as the “obesity paradox” in people with heart failure with reduced ejection fraction: The recurring observation that, when these patients with heart failure have lower BMIs they fare worse, with higher rates of mortality and adverse cardiovascular outcomes, compared with patients with higher BMIs.
The new analysis showed that this paradox disappeared when WHtR was substituted for BMI as the obesity metric.
This “provides meaningful data about the superiority of WHtR, compared with BMI, for predicting heart failure outcomes,” said Dr. Powell-Wiley, although she cautioned that the analysis was limited by scant data in diverse populations and did not look at other important cardiovascular disease outcomes. While Dr. Powell-Wiley does not think that WHtR needs assessment in a prospective, controlled trial, she called for analysis of pooled prospective studies with more diverse populations to better document the advantages of WHtR over BMI.
The PARADIGM-HF post hoc analysis shows again how flawed BMI is for health assessment and the relative importance of an individualized understanding of a person’s body composition, Dr. Almandoz said in an interview. “As we collect more data, there is increasing awareness of how imperfect BMI is.”
Measuring waist circumference is tricky
Although WHtR looks promising as a substitute for or add-on to BMI, it has its own limitations, particularly the challenge of accurately measuring waist circumference.
Measuring waist circumference “not only takes more time but requires the assessor to be well trained about where to put the tape measure and making sure it’s measured at the same place each time,” even when different people take serial measurements from individual patients, noted Dr. Wee. Determining waist circumference can also be technically difficult when done on larger people, she added, and collectively these challenges make waist circumference “less reproducible from measurement to measurement.”
“It’s relatively clear how to standardize measurement of weight and height, but there is a huge amount of variability when the waist is measured,” agreed Dr. Almandoz. “And waist circumference also differs by ethnicity, race, sex, and body frame. There are significant differences in waist circumference levels that associate with increased health risks” between, for example, White and South Asian people.
Another limitation of waist circumference and WHtR is that they “cannot differentiate between visceral and abdominal subcutaneous adipose tissue, which are vastly different regarding cardiometabolic risk, commented Ian Neeland, MD, director of cardiovascular prevention at the University Hospitals Harrington Heart & Vascular Institute, Cleveland.
The imaging option
“Waist-to-height ratio is not the ultimate answer,” Dr. Neeland said in an interview. He instead endorsed “advanced imaging for body fat distribution,” such as CT or MRI scans, as his pick for what should be the standard obesity metric, “given that it is much more specific and actionable for both risk assessment and response to therapy. I expect slow but steady advancements that move away from BMI cutoffs, for example for bariatric surgery, given that BMI is an imprecise and crude tool.”
But although imaging with methods like CT and MRI may provide the best accuracy and precision for tracking the volume of a person’s cardiometabolically dangerous fat, they are also hampered by relatively high cost and, for CT and DXA, the issue of radiation exposure.
“CT, MRI, and DXA scans give more in-depth assessment of body composition, but should we expose people to the radiation and the cost?” Dr. Almandoz wondered.
“Height, weight, and waist circumference cost nothing to obtain,” creating a big relative disadvantage for imaging, said Naveed Sattar, MD, professor of metabolic medicine at the University of Glasgow.
“Data would need to show that imaging gives clinicians substantially more information about future risk” to justify its price, Dr. Sattar emphasized.
BMI’s limits mean adding on
Regardless of whichever alternatives to BMI end up getting used most, experts generally agree that BMI alone is looking increasingly inadequate.
“Over the next 5 years, BMI will come to be seen as a screening tool that categorizes people into general risk groups” that also needs “other metrics and variables, such as age, race, ethnicity, family history, blood glucose, and blood pressure to better describe health risk in an individual,” predicted Dr. Bessesen.
The endorsement of WHtR by NICE “will lead to more research into how to incorporate WHtR into routine practice. We need more evidence to translate what NICE said into practice,” said Dr. Sattar. “I don’t think we’ll see a shift away from BMI, but we’ll add alternative measures that are particularly useful in certain patients.”
“Because we live in diverse societies, we need to individualize risk assessment and couple that with technology that makes analysis of body composition more accessible,” agreed Dr. Almandoz. He noted that the UT Southwestern weight wellness program where he practices has, for about the past decade, routinely collected waist circumference and bioelectrical impedance data as well as BMI on all people seen in the practice for obesity concerns. Making these additional measurements on a routine basis also helps strengthen patient engagement.
“We get into trouble when we make rigid health policy and clinical decisions based on BMI alone without looking at the patient holistically,” said Dr. Wee. “Patients are more than arbitrary numbers, and clinicians should make clinical decisions based on the totality of evidence for each individual patient.”
Dr. Bessesen, Dr. Wee, Dr. Powell-Wiley, and Dr. Almandoz reported no relevant financial relationships. Dr. Neeland has reported being a consultant for Merck. Dr. Sattar has reported being a consultant or speaker for Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi.
A version of this article originally appeared on Medscape.com.
“BMI is trash. Full stop.” This controversial tweet, which received thousands of likes and retweets, was cited in a recent article by one doctor on when physicians might stop using body mass index (BMI) to diagnose obesity.
BMI has for years been the consensus default method for assessing whether a person is overweight or has obesity, and is still widely used as the gatekeeper metric for treatment eligibility for certain weight-loss agents and bariatric surgery.
an important determinant of the cardiometabolic consequences of fat.
Alternative metrics include waist circumference and/or waist-to-height ratio (WHtR); imaging methods such as CT, MRI, and dual-energy x-ray absorptiometry (DXA); and bioelectrical impedance to assess fat volume and location. All have made some inroads on the tight grip BMI has had on obesity assessment.
Chances are, however, that BMI will not fade away anytime soon given how entrenched it has become in clinical practice and for insurance coverage, as well as its relative simplicity and precision.
“BMI is embedded in a wide range of guidelines on the use of medications and surgery. It’s embedded in Food and Drug Administration regulations and for billing and insurance coverage. It would take extremely strong data and years of work to undo the infrastructure built around BMI and replace it with something else. I don’t see that happening [anytime soon],” commented Daniel H. Bessesen, MD, a professor at the University of Colorado at Denver, Aurora, and chief of endocrinology for Denver Health.
“It would be almost impossible to replace all the studies that have used BMI with investigations using some other measure,” he said.
BMI Is ‘imperfect’
The entrenched position of BMI as the go-to metric doesn’t keep detractors from weighing in. As noted in a commentary on current clinical challenges surrounding obesity recently published in Annals of Internal Medicine, the journal’s editor-in-chief, Christine Laine, MD, and senior deputy editor Christina C. Wee, MD, listed six top issues clinicians must deal with, one of which, they say, is the need for a better measure of obesity than BMI.
“Unfortunately, BMI is an imperfect measure of body composition that differs with ethnicity, sex, body frame, and muscle mass,” noted Dr. Laine and Dr. Wee.
BMI is based on a person’s weight in kilograms divided by the square of their height in meters. A “healthy” BMI is between 18.5 and 24.9 kg/m2, overweight is 25-29.9, and 30 or greater is considered to represent obesity. However, certain ethnic groups have lower cutoffs for overweight or obesity because of evidence that such individuals can be at higher risk of obesity-related comorbidities at lower BMIs.
“BMI was chosen as the initial screening tool [for obesity] not because anyone thought it was perfect or the best measure but because of its simplicity. All you need is height, weight, and a calculator,” Dr. Wee said in an interview.
Numerous online calculators are available, including one from the Centers for Disease Control and Prevention where height in feet and inches and weight in pounds can be entered to generate the BMI.
BMI is also inherently limited by being “a proxy for adiposity” and not a direct measure, added Dr. Wee, who is also director of the Obesity Research Program of Beth Israel Deaconess Medical Center, Boston.
As such, BMI can’t distinguish between fat and muscle because it relies on weight only to gauge adiposity, noted Tiffany Powell-Wiley, MD, an obesity researcher at the National Heart, Lung, and Blood Institute in Bethesda, Md. Another shortcoming of BMI is that it “is good for distinguishing population-level risk for cardiovascular disease and other chronic diseases, but it does not help as much for distinguishing risk at an individual level,” she said in an interview.
These and other drawbacks have prompted researchers to look for other useful metrics. WHtR, for example, has recently made headway as a potential BMI alternative or complement.
The case for WHtR
Concern about overreliance on BMI despite its limitations is not new. In 2015, an American Heart Association scientific statement from the group’s Obesity Committee concluded that “BMI alone, even with lower thresholds, is a useful but not an ideal tool for identification of obesity or assessment of cardiovascular risk,” especially for people from Asian, Black, Hispanic, and Pacific Islander populations.
The writing panel also recommended that clinicians measure waist circumference annually and use that information along with BMI “to better gauge cardiovascular risk in diverse populations.”
Momentum for moving beyond BMI alone has continued to build following the AHA statement.
In September 2022, the National Institute for Health and Care Excellence, which sets policies for the United Kingdom’s National Health Service, revised its guidancefor assessment and management of people with obesity. The updated guidance recommends that when clinicians assess “adults with BMI below 35 kg/m2, measure and use their WHtR, as well as their BMI, as a practical estimate of central adiposity and use these measurements to help to assess and predict health risks.”
NICE released an extensive literature review with the revision, and based on the evidence, said that “using waist-to-height ratio as well as BMI would help give a practical estimate of central adiposity in adults with BMI under 35 kg/m2. This would in turn help professionals assess and predict health risks.”
However, the review added that, “because people with a BMI over 35 kg/m2 are always likely to have a high WHtR, the committee recognized that it may not be a useful addition for predicting health risks in this group.” The 2022 NICE review also said that it is “important to estimate central adiposity when assessing future health risks, including for people whose BMI is in the healthy-weight category.”
This new emphasis by NICE on measuring and using WHtR as part of obesity assessment “represents an important change in population health policy,” commented Dr. Powell-Wiley. “I expect more professional organizations will endorse use of waist circumference or waist-to-height ratio now that NICE has taken this step,” she predicted.
Waist circumference and WHtR may become standard measures of adiposity in clinical practice over the next 5-10 years.
The recent move by NICE to highlight a complementary role for WHtR “is another acknowledgment that BMI is an imperfect tool for stratifying cardiometabolic risk in a diverse population, especially in people with lower BMIs” because of its variability, commented Jamie Almandoz, MD, medical director of the weight wellness program at UT Southwestern Medical Center, Dallas.
WHtR vs. BMI
Another recent step forward for WHtR came with the publication of a post hoc analysis of data collected in the PARADIGM-HF trial, a study that had the primary purpose of comparing two medications for improving outcomes in more than 8,000 patients with heart failure with reduced ejection fraction.
The new analysis showed that “two indices that incorporate waist circumference and height, but not weight, showed a clearer association between greater adiposity and a higher risk of heart failure hospitalization,” compared with BMI.
WHtR was one of the two indices identified as being a better correlate for the adverse effect of excess adiposity compared with BMI.
The authors of the post hoc analysis did not design their analysis to compare WHtR with BMI. Instead, their goal was to better understand what’s known as the “obesity paradox” in people with heart failure with reduced ejection fraction: The recurring observation that, when these patients with heart failure have lower BMIs they fare worse, with higher rates of mortality and adverse cardiovascular outcomes, compared with patients with higher BMIs.
The new analysis showed that this paradox disappeared when WHtR was substituted for BMI as the obesity metric.
This “provides meaningful data about the superiority of WHtR, compared with BMI, for predicting heart failure outcomes,” said Dr. Powell-Wiley, although she cautioned that the analysis was limited by scant data in diverse populations and did not look at other important cardiovascular disease outcomes. While Dr. Powell-Wiley does not think that WHtR needs assessment in a prospective, controlled trial, she called for analysis of pooled prospective studies with more diverse populations to better document the advantages of WHtR over BMI.
The PARADIGM-HF post hoc analysis shows again how flawed BMI is for health assessment and the relative importance of an individualized understanding of a person’s body composition, Dr. Almandoz said in an interview. “As we collect more data, there is increasing awareness of how imperfect BMI is.”
Measuring waist circumference is tricky
Although WHtR looks promising as a substitute for or add-on to BMI, it has its own limitations, particularly the challenge of accurately measuring waist circumference.
Measuring waist circumference “not only takes more time but requires the assessor to be well trained about where to put the tape measure and making sure it’s measured at the same place each time,” even when different people take serial measurements from individual patients, noted Dr. Wee. Determining waist circumference can also be technically difficult when done on larger people, she added, and collectively these challenges make waist circumference “less reproducible from measurement to measurement.”
“It’s relatively clear how to standardize measurement of weight and height, but there is a huge amount of variability when the waist is measured,” agreed Dr. Almandoz. “And waist circumference also differs by ethnicity, race, sex, and body frame. There are significant differences in waist circumference levels that associate with increased health risks” between, for example, White and South Asian people.
Another limitation of waist circumference and WHtR is that they “cannot differentiate between visceral and abdominal subcutaneous adipose tissue, which are vastly different regarding cardiometabolic risk, commented Ian Neeland, MD, director of cardiovascular prevention at the University Hospitals Harrington Heart & Vascular Institute, Cleveland.
The imaging option
“Waist-to-height ratio is not the ultimate answer,” Dr. Neeland said in an interview. He instead endorsed “advanced imaging for body fat distribution,” such as CT or MRI scans, as his pick for what should be the standard obesity metric, “given that it is much more specific and actionable for both risk assessment and response to therapy. I expect slow but steady advancements that move away from BMI cutoffs, for example for bariatric surgery, given that BMI is an imprecise and crude tool.”
But although imaging with methods like CT and MRI may provide the best accuracy and precision for tracking the volume of a person’s cardiometabolically dangerous fat, they are also hampered by relatively high cost and, for CT and DXA, the issue of radiation exposure.
“CT, MRI, and DXA scans give more in-depth assessment of body composition, but should we expose people to the radiation and the cost?” Dr. Almandoz wondered.
“Height, weight, and waist circumference cost nothing to obtain,” creating a big relative disadvantage for imaging, said Naveed Sattar, MD, professor of metabolic medicine at the University of Glasgow.
“Data would need to show that imaging gives clinicians substantially more information about future risk” to justify its price, Dr. Sattar emphasized.
BMI’s limits mean adding on
Regardless of whichever alternatives to BMI end up getting used most, experts generally agree that BMI alone is looking increasingly inadequate.
“Over the next 5 years, BMI will come to be seen as a screening tool that categorizes people into general risk groups” that also needs “other metrics and variables, such as age, race, ethnicity, family history, blood glucose, and blood pressure to better describe health risk in an individual,” predicted Dr. Bessesen.
The endorsement of WHtR by NICE “will lead to more research into how to incorporate WHtR into routine practice. We need more evidence to translate what NICE said into practice,” said Dr. Sattar. “I don’t think we’ll see a shift away from BMI, but we’ll add alternative measures that are particularly useful in certain patients.”
“Because we live in diverse societies, we need to individualize risk assessment and couple that with technology that makes analysis of body composition more accessible,” agreed Dr. Almandoz. He noted that the UT Southwestern weight wellness program where he practices has, for about the past decade, routinely collected waist circumference and bioelectrical impedance data as well as BMI on all people seen in the practice for obesity concerns. Making these additional measurements on a routine basis also helps strengthen patient engagement.
“We get into trouble when we make rigid health policy and clinical decisions based on BMI alone without looking at the patient holistically,” said Dr. Wee. “Patients are more than arbitrary numbers, and clinicians should make clinical decisions based on the totality of evidence for each individual patient.”
Dr. Bessesen, Dr. Wee, Dr. Powell-Wiley, and Dr. Almandoz reported no relevant financial relationships. Dr. Neeland has reported being a consultant for Merck. Dr. Sattar has reported being a consultant or speaker for Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi.
A version of this article originally appeared on Medscape.com.
Long COVID mobile monitoring study hunts for answers
A new federal research project aims to answer lingering questions about long COVID using mobile monitoring devices to help track the condition.
The federally funded RECOVER Initiative expects to give out 10,000 sensors to people with long COVID to collect data in real time.
The hope is that researchers will be able to provide doctors and patients with a wealth of information to address gaps in knowledge about long COVID.
The project takes advantage of the approach other researchers have used to track patients’ health data on heart rate, exercise, and more using mobile monitoring devices such as Fitbits, smartwatches, and other remote sensors.
Researchers believe the initiative could be particularly useful for people with long COVID – whose symptoms come and go. They can use a wristband sensor to passively collect data in real time.
For a condition defined by its symptoms, that kind of data promises to be useful, experts said.
But not everyone has room in their budget for a smartwatch or a fitness tracker. Until recently, most clinical trials were BYOD: Bring your own device. At a time when researchers are trying to make sure that clinical trials reflect the diversity of the population, that leaves a lot of people out.
So, researchers are starting to supply subjects with their own monitors. The RECOVER Initiative expects to give out 10,000 sensors to people who are eligible based on race/ethnicity, income, and other demographic factors (rural residents for example). After 2 months, all people in the RECOVER study over the age of 13 will be eligible for the sensors.
The federal program builds on earlier research at places like The Scripps Institute, a center of research into remote monitoring. The institute supplied 7,000 monitors to people in an arm of the All of Us study, a 5-year-old multisite cohort that aims to collect medical information from 1 million people.
The devices went to people who have been historically underrepresented in biomedical research, said Scripps researchers, who plan to give out more this year.
In March of 2023, Scripps researchers published a study on the tracking data that found a significant post-COVID-19 drop in physical activity. But the data are incomplete because many people can’t always afford these devices. Most of the people in the study were “White, young, and active,” they wrote.
Researchers at an All of Us site at Vanderbilt University, which also used a BYOD approach, realized that they produced biased results. They reported their findings at the Pacific Symposium on Biocomputing in January.
“[The] majority of participants who provided Fitbit data reported being White and employed for wages,” they said. “However, these data represent participants who had their own Fitbit devices and consented to share EHR [electronic health record] data.”
Their solution: The program has begun providing Fitbit devices to all study participants who do not own one or cannot afford one.
Now, the web page for the All of Us study asks visitors to “Learn about the All of Us WEAR study. You could get a Fitbit at no cost! … As a part of the WEAR Study, you could receive a new Fitbit to wear at no cost to you. All of Us will be able to get the data the Fitbit collects. This data may help us understand how behavior impacts health.”
Jennifer Radin, PhD, an epidemiologist at Scripps Research Translational Institute, is heading up the DETECT study, which is a remote monitoring research project that has enrolled over 40,000 people who have their own sensors – be it a smartwatch or Fitbit. She was looking at remote monitoring for disease before COVID emerged.
Dr. Radin said she began researching remote sensing after working in public health and dealing with outdated data collection systems.
“They typically rely on case reports that are recorded by pen and paper and faxed or mailed in,” she said. “Then, they have to be entered into a database. “
In addition to offering objective data on a subject’s physical response to the infection, she said, the data collection can be long-term and continuous.
DETECT collects data on resting heart rate, which is unique to every person, and activity levels. Both measures are meaningful for those with long COVID. Her research found differences in sleep, heart rate, and activity between those with COVID and those without.
Joseph Kvedar, MD, is a Harvard Medical School researcher and the editor of NPJ Digital Medicine. He’s been studying digital health systems and called clinical research a “beachhead” for the use of data from monitors. But he also said problems remain that need to be worked out. The quality of the devices and their Bluetooth connections are better. But different devices measure different things, and a counted step can vary from person to person, he said. And the problems of the early days of electronic health records have not been fully resolved.
“We haven’t gotten to this universal language to connect all these things and make them relevant,” he said.
The All of Us researchers are working with the RECOVER project to address some of those issues. Usually not focused on a single condition, the All of Us researchers are testing a machine-learning approach for identifying long COVID.
A version of this article originally appeared on WebMD.com.
A new federal research project aims to answer lingering questions about long COVID using mobile monitoring devices to help track the condition.
The federally funded RECOVER Initiative expects to give out 10,000 sensors to people with long COVID to collect data in real time.
The hope is that researchers will be able to provide doctors and patients with a wealth of information to address gaps in knowledge about long COVID.
The project takes advantage of the approach other researchers have used to track patients’ health data on heart rate, exercise, and more using mobile monitoring devices such as Fitbits, smartwatches, and other remote sensors.
Researchers believe the initiative could be particularly useful for people with long COVID – whose symptoms come and go. They can use a wristband sensor to passively collect data in real time.
For a condition defined by its symptoms, that kind of data promises to be useful, experts said.
But not everyone has room in their budget for a smartwatch or a fitness tracker. Until recently, most clinical trials were BYOD: Bring your own device. At a time when researchers are trying to make sure that clinical trials reflect the diversity of the population, that leaves a lot of people out.
So, researchers are starting to supply subjects with their own monitors. The RECOVER Initiative expects to give out 10,000 sensors to people who are eligible based on race/ethnicity, income, and other demographic factors (rural residents for example). After 2 months, all people in the RECOVER study over the age of 13 will be eligible for the sensors.
The federal program builds on earlier research at places like The Scripps Institute, a center of research into remote monitoring. The institute supplied 7,000 monitors to people in an arm of the All of Us study, a 5-year-old multisite cohort that aims to collect medical information from 1 million people.
The devices went to people who have been historically underrepresented in biomedical research, said Scripps researchers, who plan to give out more this year.
In March of 2023, Scripps researchers published a study on the tracking data that found a significant post-COVID-19 drop in physical activity. But the data are incomplete because many people can’t always afford these devices. Most of the people in the study were “White, young, and active,” they wrote.
Researchers at an All of Us site at Vanderbilt University, which also used a BYOD approach, realized that they produced biased results. They reported their findings at the Pacific Symposium on Biocomputing in January.
“[The] majority of participants who provided Fitbit data reported being White and employed for wages,” they said. “However, these data represent participants who had their own Fitbit devices and consented to share EHR [electronic health record] data.”
Their solution: The program has begun providing Fitbit devices to all study participants who do not own one or cannot afford one.
Now, the web page for the All of Us study asks visitors to “Learn about the All of Us WEAR study. You could get a Fitbit at no cost! … As a part of the WEAR Study, you could receive a new Fitbit to wear at no cost to you. All of Us will be able to get the data the Fitbit collects. This data may help us understand how behavior impacts health.”
Jennifer Radin, PhD, an epidemiologist at Scripps Research Translational Institute, is heading up the DETECT study, which is a remote monitoring research project that has enrolled over 40,000 people who have their own sensors – be it a smartwatch or Fitbit. She was looking at remote monitoring for disease before COVID emerged.
Dr. Radin said she began researching remote sensing after working in public health and dealing with outdated data collection systems.
“They typically rely on case reports that are recorded by pen and paper and faxed or mailed in,” she said. “Then, they have to be entered into a database. “
In addition to offering objective data on a subject’s physical response to the infection, she said, the data collection can be long-term and continuous.
DETECT collects data on resting heart rate, which is unique to every person, and activity levels. Both measures are meaningful for those with long COVID. Her research found differences in sleep, heart rate, and activity between those with COVID and those without.
Joseph Kvedar, MD, is a Harvard Medical School researcher and the editor of NPJ Digital Medicine. He’s been studying digital health systems and called clinical research a “beachhead” for the use of data from monitors. But he also said problems remain that need to be worked out. The quality of the devices and their Bluetooth connections are better. But different devices measure different things, and a counted step can vary from person to person, he said. And the problems of the early days of electronic health records have not been fully resolved.
“We haven’t gotten to this universal language to connect all these things and make them relevant,” he said.
The All of Us researchers are working with the RECOVER project to address some of those issues. Usually not focused on a single condition, the All of Us researchers are testing a machine-learning approach for identifying long COVID.
A version of this article originally appeared on WebMD.com.
A new federal research project aims to answer lingering questions about long COVID using mobile monitoring devices to help track the condition.
The federally funded RECOVER Initiative expects to give out 10,000 sensors to people with long COVID to collect data in real time.
The hope is that researchers will be able to provide doctors and patients with a wealth of information to address gaps in knowledge about long COVID.
The project takes advantage of the approach other researchers have used to track patients’ health data on heart rate, exercise, and more using mobile monitoring devices such as Fitbits, smartwatches, and other remote sensors.
Researchers believe the initiative could be particularly useful for people with long COVID – whose symptoms come and go. They can use a wristband sensor to passively collect data in real time.
For a condition defined by its symptoms, that kind of data promises to be useful, experts said.
But not everyone has room in their budget for a smartwatch or a fitness tracker. Until recently, most clinical trials were BYOD: Bring your own device. At a time when researchers are trying to make sure that clinical trials reflect the diversity of the population, that leaves a lot of people out.
So, researchers are starting to supply subjects with their own monitors. The RECOVER Initiative expects to give out 10,000 sensors to people who are eligible based on race/ethnicity, income, and other demographic factors (rural residents for example). After 2 months, all people in the RECOVER study over the age of 13 will be eligible for the sensors.
The federal program builds on earlier research at places like The Scripps Institute, a center of research into remote monitoring. The institute supplied 7,000 monitors to people in an arm of the All of Us study, a 5-year-old multisite cohort that aims to collect medical information from 1 million people.
The devices went to people who have been historically underrepresented in biomedical research, said Scripps researchers, who plan to give out more this year.
In March of 2023, Scripps researchers published a study on the tracking data that found a significant post-COVID-19 drop in physical activity. But the data are incomplete because many people can’t always afford these devices. Most of the people in the study were “White, young, and active,” they wrote.
Researchers at an All of Us site at Vanderbilt University, which also used a BYOD approach, realized that they produced biased results. They reported their findings at the Pacific Symposium on Biocomputing in January.
“[The] majority of participants who provided Fitbit data reported being White and employed for wages,” they said. “However, these data represent participants who had their own Fitbit devices and consented to share EHR [electronic health record] data.”
Their solution: The program has begun providing Fitbit devices to all study participants who do not own one or cannot afford one.
Now, the web page for the All of Us study asks visitors to “Learn about the All of Us WEAR study. You could get a Fitbit at no cost! … As a part of the WEAR Study, you could receive a new Fitbit to wear at no cost to you. All of Us will be able to get the data the Fitbit collects. This data may help us understand how behavior impacts health.”
Jennifer Radin, PhD, an epidemiologist at Scripps Research Translational Institute, is heading up the DETECT study, which is a remote monitoring research project that has enrolled over 40,000 people who have their own sensors – be it a smartwatch or Fitbit. She was looking at remote monitoring for disease before COVID emerged.
Dr. Radin said she began researching remote sensing after working in public health and dealing with outdated data collection systems.
“They typically rely on case reports that are recorded by pen and paper and faxed or mailed in,” she said. “Then, they have to be entered into a database. “
In addition to offering objective data on a subject’s physical response to the infection, she said, the data collection can be long-term and continuous.
DETECT collects data on resting heart rate, which is unique to every person, and activity levels. Both measures are meaningful for those with long COVID. Her research found differences in sleep, heart rate, and activity between those with COVID and those without.
Joseph Kvedar, MD, is a Harvard Medical School researcher and the editor of NPJ Digital Medicine. He’s been studying digital health systems and called clinical research a “beachhead” for the use of data from monitors. But he also said problems remain that need to be worked out. The quality of the devices and their Bluetooth connections are better. But different devices measure different things, and a counted step can vary from person to person, he said. And the problems of the early days of electronic health records have not been fully resolved.
“We haven’t gotten to this universal language to connect all these things and make them relevant,” he said.
The All of Us researchers are working with the RECOVER project to address some of those issues. Usually not focused on a single condition, the All of Us researchers are testing a machine-learning approach for identifying long COVID.
A version of this article originally appeared on WebMD.com.
Five chronic mistakes that can sabotage your medical practice
A physician who in the past has led medical groups as both chief medical officer and president, Gerda Maissel, MD, president of My MD Advisor, a private patient advocacy group, has seen the good, bad, and ugly of practice administration. There’s a spectrum of infractions: Anything from doctors making inappropriate jokes with staff or patients, to failing to establish key relationships with other critical entities, says Dr. Maissel.
“Being a good physician who provides value is important in building a practice,” explained Dr. Maissel. “But it is not the be-all and end-all.”
While the number of physician-owned practices is declining, just under 50% are still in private practice, according to the American Medical Association’s 2020 survey. There’s also a continuing trend toward larger practices. Whatever the size, the physicians are responsible for strategy, marketing, building the practice, and maintaining profitability.
Catherine Lightfoot, CPA, CHBC, president of the National Society of Certified Healthcare Business Consultants (NSCHBC), has her finger on the pulse of what’s right and what’s wrong when it comes to running a medical practice. Although she says there are no hard and fast rules on how to run a thriving medical group, there are common mistakes that physicians often don’t recognize.
Here are the five key mistakes that commonly crop up, and the experts’ thoughts on how to prevent or fix them.
1. Failing to engage in outreach activities and community efforts to build your practice.
Yes, physicians earn good reputations through dedicated work, and that often precedes them when it comes to building a practice. But assuming that hanging a shingle backed by strong credentials is all it takes for success is akin to building a website and assuming people will find it organically. Maybe there was a time, in a small community, where this was good enough. But no longer.
It’s important to plan to get your practice and your name known to potential patients. “Most physicians think that means advertising, but that’s not the complete case,” Dr. Maissel said.
Much of the equation involves ensuring availability. This means setting office hours that work for your target audience of patients, and then ensuring you stick to those hours. This extends beyond scheduling your current patients and into referral patients, too. And it’s particularly true while in the building phase of a new practice.
“If one of your colleagues calls with a referral patient, and they consider the matter urgent, you need to heed that,” explained Dr. Maissel. “So have a breadth of availability for these referral cases.” Through word of mouth, you’ll get a good reputation for patient care and availability, and that will go a long way toward helping to grow your practice.
Establishing a culture that doesn’t involve canceling and rescheduling patients is part of the scheduling equation, too. “I’ve seen the full gamut of cancellation policies, ranging from a month’s notice on changes to 3 months’ notice,” said Dr. Maissel. “It all gets at the same issue, which is failing to set up a culture where doctors don’t change their schedules and leave patients hanging.”
In the end, wonky scheduling, cancellations, and a lack of respect for the urgency of referrals can cost a practice. Forge a reputation in reliability and word will get around, in all the right ways.
2. Not having enough oversight of your outsourced billing service
Billing is one of the biggest pieces of running a successful and profitable practice, yet too many practices ignore it once they’ve handed it off to a billing company. That can cost you in more ways than one, said Ms. Lightfoot. “Billing changes all the time, and if you’re not monitoring your billing partner, you don’t know what you’re getting,” she said.
Ms. Lightfoot said that a decade ago, billing was much more straightforward – essentially, you did the work and received payment. Today’s complex insurance, Medicare, and Medicaid environment have changed the landscape. “Now you have to fight for every dollar you’re billing,” said Ms. Lightfoot. “Rates get cut all the time, you might miss out on a claim, and the rules are constantly changing.”
The solution for many practices is to outsource billing, which Ms. Lightfoot supports. “They specialize in this, and that’s a great start,” she said. “But it’s not as simple as handing it off and forgetting it.”
Instead, ensure your internal staff is up to date on all things coding and billing so that they can catch what your outsourced billing partner doesn’t. Your internal staff should be prepared to carry out coding, check coding, and stay on top of the billing company if they aren’t processing claims quickly enough. For instance: If there’s a denial, how many times will the billing company go after that money?
Other questions to ask when entering a billing relationship: What does the billing company expect from your practice? Do they communicate what needs to be worked on or fixed? Are they providing you with monthly reports? “You want to make sure you’re getting those reports every month and reading them over carefully,” said Ms. Lightfoot.
This means that if you have a large practice, you should have a point person within your billing department to handle the relationship with your billing partner. If it’s a smaller practice, the task will likely fall to the office manager. The ‘who’ isn’t important, but having someone on the case is.
Another important aspect of this billing relationship is understanding what you’re receiving for your payment. “Sometimes going with the cheapest offer amounts to a billing partner who isn’t working on those claims and denials as much as they should,” said Ms. Lightfoot. “I’ve seen fees anywhere from 4% to 9%, and the lower end can mean you’ll need to chase down every penny.”
3. Neglecting to forge the right relationships in the community.
Another common mistake physicians make is failing to develop the professional relationships that will help you thrive. Successful practices need to establish relationships with the right people and organizations. While the occasional afternoon of golf used to serve this purpose, today outreach must go beyond that, said Dr. Maissel. “You need to create relationships with hospitals and hospital-based practices because you may have value to them,” she said. “You should also get into some sort of relationship with your local ACO (Accountable Care Organization) or PHO (Physician Hospital Organization). Identify the leaders there and let them know you exist.”
Establishing these relationships goes beyond that first step of introducing yourself, or you risk losing their benefits. You must also nurture and “fertilize” these relationships in an ongoing fashion. “For years, as the head of employee practice, I had a competitor who would go out of his way to invite me to lunch regularly,” said Dr. Maissel. “When there were opportunities for his group, I would connect him. I wouldn’t have done that had he not worked on our relationship over time.”
The adage of “it’s not what you know but who you know” holds up here. If you don’t do the reach out to the right people and organizations in your community, you will have a harder time succeeding as a practice.
4. Hiring the wrong person/a family member for the job.
When starting a new practice, or if you’re running a small practice, it can be tempting to look for affordable or reliable staffing from among family members or friends. That’s fine if your family member or friend is also qualified for the job. If they aren’t, however, you might be setting up for failure.
“When you hire someone without the right qualifications, you need to be willing to train them for the job,” said Ms. Lightfoot. “Doctors don’t have that kind of time.”
Too often, Ms. Lightfoot said, a doctor will have a position like officer manager open and fill it with an in-law, whether he or she is experienced or not. “Now you have someone in the role who is unqualified, and the rest of the office can’t speak up about that because it’s a relative to the lead physician,” she said. “That doesn’t create a good environment for anyone.”
Also, a setup for failure is hiring someone who might be qualified, but not possessing the right personality for the role. A front desk position, for instance, should be held by someone who’s a bit upbeat and able to multitask. “You can’t put a shy, quiet person in that job,” said Ms. Lightfoot. “So, if you see a person with 10 years’ experience in a medical practice, but they’re reserved, what will happen? You must think about this when hiring.”
One PA recalled a small family practice in which the lead physician’s wife was the office manager. To save money, the wife removed lights from the staff restroom and staff lunchroom and declined staff requests for earned vacation. The staff felt unable to speak up, and they – and all new office staff members – ultimately left the practice.
5. Overlooking the importance of acting like a professional and respecting your staff.
This one might seem obvious, but many physicians get a bit too comfortable in the office environment, said Dr. Maissel. This can encompass a whole host of bad behaviors, from making inappropriate jokes to staff and patients, to trash-talking colleagues. None of this behavior is acceptable and can set you up for things to go wrong, especially when good labor is hard to come by. “Your staff is made up of people for whom 50 cents an hour is meaningful,” she said. “If they don’t have a warm, supportive office, they will look elsewhere.”
This is especially true of younger people now entering the workforce – they are less tolerant than generations past of egregious behavior. Try to establish a professional, yet nurturing environment for your staff. “Inquire about things that matter to them,” said Dr. Maissel. “Small talk can go a long way. See them as human beings, not cogs in the wheel.”
Inappropriate and uncaring behaviors will give physician leaders a reputation, one that sticks. “The medical community is pretty connected, and if you behave inappropriately enough times, it will circle back to you,” said Dr. Maissel.
Launching, and sustaining, a successful medical practice is never a given, but mistakes are. With the right approach, however, you can avoid these common – and impactful – errors and set your practice up for success.
A version of this article first appeared on Medscape.com.
A physician who in the past has led medical groups as both chief medical officer and president, Gerda Maissel, MD, president of My MD Advisor, a private patient advocacy group, has seen the good, bad, and ugly of practice administration. There’s a spectrum of infractions: Anything from doctors making inappropriate jokes with staff or patients, to failing to establish key relationships with other critical entities, says Dr. Maissel.
“Being a good physician who provides value is important in building a practice,” explained Dr. Maissel. “But it is not the be-all and end-all.”
While the number of physician-owned practices is declining, just under 50% are still in private practice, according to the American Medical Association’s 2020 survey. There’s also a continuing trend toward larger practices. Whatever the size, the physicians are responsible for strategy, marketing, building the practice, and maintaining profitability.
Catherine Lightfoot, CPA, CHBC, president of the National Society of Certified Healthcare Business Consultants (NSCHBC), has her finger on the pulse of what’s right and what’s wrong when it comes to running a medical practice. Although she says there are no hard and fast rules on how to run a thriving medical group, there are common mistakes that physicians often don’t recognize.
Here are the five key mistakes that commonly crop up, and the experts’ thoughts on how to prevent or fix them.
1. Failing to engage in outreach activities and community efforts to build your practice.
Yes, physicians earn good reputations through dedicated work, and that often precedes them when it comes to building a practice. But assuming that hanging a shingle backed by strong credentials is all it takes for success is akin to building a website and assuming people will find it organically. Maybe there was a time, in a small community, where this was good enough. But no longer.
It’s important to plan to get your practice and your name known to potential patients. “Most physicians think that means advertising, but that’s not the complete case,” Dr. Maissel said.
Much of the equation involves ensuring availability. This means setting office hours that work for your target audience of patients, and then ensuring you stick to those hours. This extends beyond scheduling your current patients and into referral patients, too. And it’s particularly true while in the building phase of a new practice.
“If one of your colleagues calls with a referral patient, and they consider the matter urgent, you need to heed that,” explained Dr. Maissel. “So have a breadth of availability for these referral cases.” Through word of mouth, you’ll get a good reputation for patient care and availability, and that will go a long way toward helping to grow your practice.
Establishing a culture that doesn’t involve canceling and rescheduling patients is part of the scheduling equation, too. “I’ve seen the full gamut of cancellation policies, ranging from a month’s notice on changes to 3 months’ notice,” said Dr. Maissel. “It all gets at the same issue, which is failing to set up a culture where doctors don’t change their schedules and leave patients hanging.”
In the end, wonky scheduling, cancellations, and a lack of respect for the urgency of referrals can cost a practice. Forge a reputation in reliability and word will get around, in all the right ways.
2. Not having enough oversight of your outsourced billing service
Billing is one of the biggest pieces of running a successful and profitable practice, yet too many practices ignore it once they’ve handed it off to a billing company. That can cost you in more ways than one, said Ms. Lightfoot. “Billing changes all the time, and if you’re not monitoring your billing partner, you don’t know what you’re getting,” she said.
Ms. Lightfoot said that a decade ago, billing was much more straightforward – essentially, you did the work and received payment. Today’s complex insurance, Medicare, and Medicaid environment have changed the landscape. “Now you have to fight for every dollar you’re billing,” said Ms. Lightfoot. “Rates get cut all the time, you might miss out on a claim, and the rules are constantly changing.”
The solution for many practices is to outsource billing, which Ms. Lightfoot supports. “They specialize in this, and that’s a great start,” she said. “But it’s not as simple as handing it off and forgetting it.”
Instead, ensure your internal staff is up to date on all things coding and billing so that they can catch what your outsourced billing partner doesn’t. Your internal staff should be prepared to carry out coding, check coding, and stay on top of the billing company if they aren’t processing claims quickly enough. For instance: If there’s a denial, how many times will the billing company go after that money?
Other questions to ask when entering a billing relationship: What does the billing company expect from your practice? Do they communicate what needs to be worked on or fixed? Are they providing you with monthly reports? “You want to make sure you’re getting those reports every month and reading them over carefully,” said Ms. Lightfoot.
This means that if you have a large practice, you should have a point person within your billing department to handle the relationship with your billing partner. If it’s a smaller practice, the task will likely fall to the office manager. The ‘who’ isn’t important, but having someone on the case is.
Another important aspect of this billing relationship is understanding what you’re receiving for your payment. “Sometimes going with the cheapest offer amounts to a billing partner who isn’t working on those claims and denials as much as they should,” said Ms. Lightfoot. “I’ve seen fees anywhere from 4% to 9%, and the lower end can mean you’ll need to chase down every penny.”
3. Neglecting to forge the right relationships in the community.
Another common mistake physicians make is failing to develop the professional relationships that will help you thrive. Successful practices need to establish relationships with the right people and organizations. While the occasional afternoon of golf used to serve this purpose, today outreach must go beyond that, said Dr. Maissel. “You need to create relationships with hospitals and hospital-based practices because you may have value to them,” she said. “You should also get into some sort of relationship with your local ACO (Accountable Care Organization) or PHO (Physician Hospital Organization). Identify the leaders there and let them know you exist.”
Establishing these relationships goes beyond that first step of introducing yourself, or you risk losing their benefits. You must also nurture and “fertilize” these relationships in an ongoing fashion. “For years, as the head of employee practice, I had a competitor who would go out of his way to invite me to lunch regularly,” said Dr. Maissel. “When there were opportunities for his group, I would connect him. I wouldn’t have done that had he not worked on our relationship over time.”
The adage of “it’s not what you know but who you know” holds up here. If you don’t do the reach out to the right people and organizations in your community, you will have a harder time succeeding as a practice.
4. Hiring the wrong person/a family member for the job.
When starting a new practice, or if you’re running a small practice, it can be tempting to look for affordable or reliable staffing from among family members or friends. That’s fine if your family member or friend is also qualified for the job. If they aren’t, however, you might be setting up for failure.
“When you hire someone without the right qualifications, you need to be willing to train them for the job,” said Ms. Lightfoot. “Doctors don’t have that kind of time.”
Too often, Ms. Lightfoot said, a doctor will have a position like officer manager open and fill it with an in-law, whether he or she is experienced or not. “Now you have someone in the role who is unqualified, and the rest of the office can’t speak up about that because it’s a relative to the lead physician,” she said. “That doesn’t create a good environment for anyone.”
Also, a setup for failure is hiring someone who might be qualified, but not possessing the right personality for the role. A front desk position, for instance, should be held by someone who’s a bit upbeat and able to multitask. “You can’t put a shy, quiet person in that job,” said Ms. Lightfoot. “So, if you see a person with 10 years’ experience in a medical practice, but they’re reserved, what will happen? You must think about this when hiring.”
One PA recalled a small family practice in which the lead physician’s wife was the office manager. To save money, the wife removed lights from the staff restroom and staff lunchroom and declined staff requests for earned vacation. The staff felt unable to speak up, and they – and all new office staff members – ultimately left the practice.
5. Overlooking the importance of acting like a professional and respecting your staff.
This one might seem obvious, but many physicians get a bit too comfortable in the office environment, said Dr. Maissel. This can encompass a whole host of bad behaviors, from making inappropriate jokes to staff and patients, to trash-talking colleagues. None of this behavior is acceptable and can set you up for things to go wrong, especially when good labor is hard to come by. “Your staff is made up of people for whom 50 cents an hour is meaningful,” she said. “If they don’t have a warm, supportive office, they will look elsewhere.”
This is especially true of younger people now entering the workforce – they are less tolerant than generations past of egregious behavior. Try to establish a professional, yet nurturing environment for your staff. “Inquire about things that matter to them,” said Dr. Maissel. “Small talk can go a long way. See them as human beings, not cogs in the wheel.”
Inappropriate and uncaring behaviors will give physician leaders a reputation, one that sticks. “The medical community is pretty connected, and if you behave inappropriately enough times, it will circle back to you,” said Dr. Maissel.
Launching, and sustaining, a successful medical practice is never a given, but mistakes are. With the right approach, however, you can avoid these common – and impactful – errors and set your practice up for success.
A version of this article first appeared on Medscape.com.
A physician who in the past has led medical groups as both chief medical officer and president, Gerda Maissel, MD, president of My MD Advisor, a private patient advocacy group, has seen the good, bad, and ugly of practice administration. There’s a spectrum of infractions: Anything from doctors making inappropriate jokes with staff or patients, to failing to establish key relationships with other critical entities, says Dr. Maissel.
“Being a good physician who provides value is important in building a practice,” explained Dr. Maissel. “But it is not the be-all and end-all.”
While the number of physician-owned practices is declining, just under 50% are still in private practice, according to the American Medical Association’s 2020 survey. There’s also a continuing trend toward larger practices. Whatever the size, the physicians are responsible for strategy, marketing, building the practice, and maintaining profitability.
Catherine Lightfoot, CPA, CHBC, president of the National Society of Certified Healthcare Business Consultants (NSCHBC), has her finger on the pulse of what’s right and what’s wrong when it comes to running a medical practice. Although she says there are no hard and fast rules on how to run a thriving medical group, there are common mistakes that physicians often don’t recognize.
Here are the five key mistakes that commonly crop up, and the experts’ thoughts on how to prevent or fix them.
1. Failing to engage in outreach activities and community efforts to build your practice.
Yes, physicians earn good reputations through dedicated work, and that often precedes them when it comes to building a practice. But assuming that hanging a shingle backed by strong credentials is all it takes for success is akin to building a website and assuming people will find it organically. Maybe there was a time, in a small community, where this was good enough. But no longer.
It’s important to plan to get your practice and your name known to potential patients. “Most physicians think that means advertising, but that’s not the complete case,” Dr. Maissel said.
Much of the equation involves ensuring availability. This means setting office hours that work for your target audience of patients, and then ensuring you stick to those hours. This extends beyond scheduling your current patients and into referral patients, too. And it’s particularly true while in the building phase of a new practice.
“If one of your colleagues calls with a referral patient, and they consider the matter urgent, you need to heed that,” explained Dr. Maissel. “So have a breadth of availability for these referral cases.” Through word of mouth, you’ll get a good reputation for patient care and availability, and that will go a long way toward helping to grow your practice.
Establishing a culture that doesn’t involve canceling and rescheduling patients is part of the scheduling equation, too. “I’ve seen the full gamut of cancellation policies, ranging from a month’s notice on changes to 3 months’ notice,” said Dr. Maissel. “It all gets at the same issue, which is failing to set up a culture where doctors don’t change their schedules and leave patients hanging.”
In the end, wonky scheduling, cancellations, and a lack of respect for the urgency of referrals can cost a practice. Forge a reputation in reliability and word will get around, in all the right ways.
2. Not having enough oversight of your outsourced billing service
Billing is one of the biggest pieces of running a successful and profitable practice, yet too many practices ignore it once they’ve handed it off to a billing company. That can cost you in more ways than one, said Ms. Lightfoot. “Billing changes all the time, and if you’re not monitoring your billing partner, you don’t know what you’re getting,” she said.
Ms. Lightfoot said that a decade ago, billing was much more straightforward – essentially, you did the work and received payment. Today’s complex insurance, Medicare, and Medicaid environment have changed the landscape. “Now you have to fight for every dollar you’re billing,” said Ms. Lightfoot. “Rates get cut all the time, you might miss out on a claim, and the rules are constantly changing.”
The solution for many practices is to outsource billing, which Ms. Lightfoot supports. “They specialize in this, and that’s a great start,” she said. “But it’s not as simple as handing it off and forgetting it.”
Instead, ensure your internal staff is up to date on all things coding and billing so that they can catch what your outsourced billing partner doesn’t. Your internal staff should be prepared to carry out coding, check coding, and stay on top of the billing company if they aren’t processing claims quickly enough. For instance: If there’s a denial, how many times will the billing company go after that money?
Other questions to ask when entering a billing relationship: What does the billing company expect from your practice? Do they communicate what needs to be worked on or fixed? Are they providing you with monthly reports? “You want to make sure you’re getting those reports every month and reading them over carefully,” said Ms. Lightfoot.
This means that if you have a large practice, you should have a point person within your billing department to handle the relationship with your billing partner. If it’s a smaller practice, the task will likely fall to the office manager. The ‘who’ isn’t important, but having someone on the case is.
Another important aspect of this billing relationship is understanding what you’re receiving for your payment. “Sometimes going with the cheapest offer amounts to a billing partner who isn’t working on those claims and denials as much as they should,” said Ms. Lightfoot. “I’ve seen fees anywhere from 4% to 9%, and the lower end can mean you’ll need to chase down every penny.”
3. Neglecting to forge the right relationships in the community.
Another common mistake physicians make is failing to develop the professional relationships that will help you thrive. Successful practices need to establish relationships with the right people and organizations. While the occasional afternoon of golf used to serve this purpose, today outreach must go beyond that, said Dr. Maissel. “You need to create relationships with hospitals and hospital-based practices because you may have value to them,” she said. “You should also get into some sort of relationship with your local ACO (Accountable Care Organization) or PHO (Physician Hospital Organization). Identify the leaders there and let them know you exist.”
Establishing these relationships goes beyond that first step of introducing yourself, or you risk losing their benefits. You must also nurture and “fertilize” these relationships in an ongoing fashion. “For years, as the head of employee practice, I had a competitor who would go out of his way to invite me to lunch regularly,” said Dr. Maissel. “When there were opportunities for his group, I would connect him. I wouldn’t have done that had he not worked on our relationship over time.”
The adage of “it’s not what you know but who you know” holds up here. If you don’t do the reach out to the right people and organizations in your community, you will have a harder time succeeding as a practice.
4. Hiring the wrong person/a family member for the job.
When starting a new practice, or if you’re running a small practice, it can be tempting to look for affordable or reliable staffing from among family members or friends. That’s fine if your family member or friend is also qualified for the job. If they aren’t, however, you might be setting up for failure.
“When you hire someone without the right qualifications, you need to be willing to train them for the job,” said Ms. Lightfoot. “Doctors don’t have that kind of time.”
Too often, Ms. Lightfoot said, a doctor will have a position like officer manager open and fill it with an in-law, whether he or she is experienced or not. “Now you have someone in the role who is unqualified, and the rest of the office can’t speak up about that because it’s a relative to the lead physician,” she said. “That doesn’t create a good environment for anyone.”
Also, a setup for failure is hiring someone who might be qualified, but not possessing the right personality for the role. A front desk position, for instance, should be held by someone who’s a bit upbeat and able to multitask. “You can’t put a shy, quiet person in that job,” said Ms. Lightfoot. “So, if you see a person with 10 years’ experience in a medical practice, but they’re reserved, what will happen? You must think about this when hiring.”
One PA recalled a small family practice in which the lead physician’s wife was the office manager. To save money, the wife removed lights from the staff restroom and staff lunchroom and declined staff requests for earned vacation. The staff felt unable to speak up, and they – and all new office staff members – ultimately left the practice.
5. Overlooking the importance of acting like a professional and respecting your staff.
This one might seem obvious, but many physicians get a bit too comfortable in the office environment, said Dr. Maissel. This can encompass a whole host of bad behaviors, from making inappropriate jokes to staff and patients, to trash-talking colleagues. None of this behavior is acceptable and can set you up for things to go wrong, especially when good labor is hard to come by. “Your staff is made up of people for whom 50 cents an hour is meaningful,” she said. “If they don’t have a warm, supportive office, they will look elsewhere.”
This is especially true of younger people now entering the workforce – they are less tolerant than generations past of egregious behavior. Try to establish a professional, yet nurturing environment for your staff. “Inquire about things that matter to them,” said Dr. Maissel. “Small talk can go a long way. See them as human beings, not cogs in the wheel.”
Inappropriate and uncaring behaviors will give physician leaders a reputation, one that sticks. “The medical community is pretty connected, and if you behave inappropriately enough times, it will circle back to you,” said Dr. Maissel.
Launching, and sustaining, a successful medical practice is never a given, but mistakes are. With the right approach, however, you can avoid these common – and impactful – errors and set your practice up for success.
A version of this article first appeared on Medscape.com.
A baby stops breathing at a grocery store – An ICU nurse steps in
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
My son needed a physical for his football team, and we couldn’t get an appointment. So, we went to the urgent care next to the H Mart in Cary, N.C. While I was waiting, I thought, let me go get a coffee or an iced tea at the H Mart. They have this French bakery in there.
I went in and ordered my drink, and I was waiting in line. I saw this woman pass me running with a baby. Another woman – I found out later it was her sister – was running after her, and she said: “Call 911!”
“I don’t have my phone,” I said. I left my phone with my son; he was using it.
I said: “Are you okay?” And she just handed me the baby. The baby was gray, and there was blood in her nose and mouth. The woman said: “She’s my baby. She’s 1 week old.”
I was trying to think very quickly. I didn’t see any bubbles in the blood around the baby’s nose or mouth to tell me if she was breathing. She was just limp. The mom was still screaming, but I couldn’t even hear her anymore. It was like I was having an out-of-body experience. All I could hear were my thoughts: “I need to put this baby down to start CPR. Someone was calling 911. I should go in the front of the store to save time, so EMS doesn’t have to look for me when they come.”
I started moving and trying to clean the blood from the baby’s face with her blanket. At the front of the store, I saw a display of rice bags. I put the baby on top of one of the bags. “Okay, where do I check for a pulse on a baby?” I took care of adults, never pediatric patients, never babies. She was so tiny. I put my hand on her chest and felt nothing. No heartbeat. She still wasn’t breathing.
People were around me, but I couldn’t see or hear anybody. All I was thinking was: “What can I do for this patient right now?” I started CPR with two fingers. Nothing was happening. It wasn’t that long, but it felt like forever for me. I couldn’t do mouth-to-mouth because there was so much blood on her face. I still don’t know what caused the bleeding.
It was COVID time, so I had my mask on. I was, like: “You know what? Screw this. She’s a 1-week-old baby. Her lungs are tiny. Maybe I don’t have to do mouth-to-mouth. I can just blow in her mouth.” I took off my mask and opened her mouth. I took a deep breath and blew a little bit of air in her mouth. I continued CPR for maybe 5 or 10 seconds.
And then she gasped! She opened her eyes, but they were rolled up. I was still doing CPR, and maybe 2 second after that, I could feel under my hand a very rapid heart rate. I took my hand away and lifted her up.
Just then the EMS got there. I gave them the baby and said: “I did CPR. I don’t know how long it lasted.” The EMS person looked at me, said: “Thank you for what you did. Now we need you to help us with mom.” I said, “okay.”
I turned around, and the mom was still screaming and crying. I asked one of the ladies that worked there, “Can you get me water?” She brought it, and I gave some to the mom, and she started talking to EMS.
People were asking me: “What happened? What happened?” It’s funny, I guess the nurse in me didn’t want to give out information. And I didn’t want to ask for information. I was thinking about privacy. I said, “I don’t know,” and walked away.
The mom’s sister came and hugged me and said thank you. I was still in this out-of-body zone, and I just wanted to get the hell out of there. So, I left. I went to my car and when I got in it, I started shaking and sweating and crying.
I had been so calm in the moment, not thinking about if the baby was going to survive or not. I didn’t know how long she was without oxygen, if she would have some anoxic brain injury or stroke. I’m a mom, too. I would have been just as terrified as that mom. I just hoped there was a chance that she could take her baby home.
I went back to the urgent care, and my son was, like, “are you okay?” I said: “You will not believe this. I just did CPR on a baby.” He said: “Oh. Okay.” I don’t think he even knew what that meant.
I’ve been an ICU nurse since 2008. I’ve been in very critical moments with patients, life or death situations. I help save people all the time at the hospital. Most of the time, you know what you’re getting. You can prepare. You have everything you need, and everyone knows what to do. You know what the worst will look like. You know the outcome.
But this was something else. You read about things like this. You hear about them. But you never think it’ll happen to you – until it happens.
I couldn’t stop thinking about the baby. So, 2 days later, I posted on Next Door to see if somebody would read it and say, “hey, the baby survived.” I was amazed at how many people responded, but no one knew the family.
The local news got hold of me and asked me to do a story. I told them, “the only way I can do a story is if the baby survived. I’m not going to do a story about a dead baby, and the mom has to live through it again.”
The reporter called me later on that day and said she had talked to the police. They said the family was visiting from out of state. The baby went to the hospital and was discharged home 2 days later. I said, “okay, then I can talk.”
When the news story came out, I started getting texts from people at work the same night. So many people were reaching out. Even people from out of state. But I never heard from the family. No one knew how to reach them.
Since I was very young, I wanted to work in a hospital, to help people. It really brings me joy, seeing somebody go home, knowing, yes, we did this. It’s a great feeling. I love this job. I wouldn’t trade it for anything.
I just wish I had asked the mom’s name. Because I always think about that baby. I always wonder, what did she become? I hope somebody reads this who might know that little girl. It would be so nice to meet her one day.
Ms. Diallo is an ICU nurse and now works as nurse care coordinator at the University of North Carolina’s Children’s Neurology Clinic in Chapel Hill.
A version of this article first appeared on Medscape.com.
From Beirut to frontline hematology research
“If we start treatment earlier, in the smoldering phase, maybe there is a chance of actually curing the disease and completely getting rid of it,” said Dr. Mouhieddine, 31, a research fellow at the Icahn School of Medicine at Mount Sinai, in New York. “We haven’t proven that yet, and it’s going to take years before we’re able to prove it. I’m hoping to be one of those spearheading the initiative.”
As he develops clinical trials, the young physician scientist has another focus: A deeply personal connection to the very disease he’s trying to cure. Last year Dr. Mouhieddine diagnosed his aunt back in Lebanon with multiple myeloma. “I have always been close to her, and I’m like her son,” he said, and her situation is especially scary because she lives in a country where treatment options are limited.
Dr. Mouhieddine was born and raised in Beirut, the son of a sports journalist father and a mother who worked in a bank. Lebanon’s civil war ended in 1990, shortly before his birth, but political instability returned when he was a child.
“Everything was a disaster,” he recalled. “There was a period of time when there were bombs throughout the city because certain politicians were being targeted. I remember when groups of people would have gunfights in the street.”
Dr. Mouhieddine attended the American University of Beirut, then after college and medical school there, he headed to the United States.
“I wanted to make a difference in medicine. And I knew that if I stayed back home, I wouldn’t be able to,” he said. Fortunately, “everybody has made me feel that I really belong here, and I’ve never felt like I’m an outsider.”
Early on, as he went through fellowships and residency, he developed an interest in multiple myeloma.
Ajai Chari, MD, a colleague of Dr. Mouhieddine’s at Icahn School of Medicine, said in an interview, “I remember meeting him at a conference before he had even started an internal medicine residency, let alone a hematology oncology fellowship. He was already certain he wanted to work in multiple myeloma, due to his work at Dana-Farber Cancer Institute.”
Myeloma was especially intriguing to Dr. Mouhieddine because of the rapid rate of progress in treating the disease. “Over the past 10 years, the myeloma field has advanced at such an extremely fast pace, more than any other cancer,” he said. “Maybe 15 years ago, you would tell someone with newly diagnosed myeloma that they had a chance for an average of another 2 years. Now, we tell patients they have 10 years to live on average, which means you could live 15 or 20 years. That alone was astounding to me and piqued my interest in myeloma.”
At the same time, smoldering myeloma – which can be discovered during routine blood work – remains little understood. As the National Cancer Institute explains, “smoldering myeloma is a precancerous condition that alters certain proteins in blood and/or increases plasma cells in bone marrow, but it does not cause symptoms of disease. About half of those diagnosed with the condition, however, will develop multiple myeloma within 5 years.”
“If we understand what drives smoldering myeloma, we may be able to prevent it from progressing to its active form,” said hematologist oncologist Samir Parekh, MD, who works with Dr. Mouhieddine at Icahn School of Medicine. “Or at the minimum, we could better predict who will progress so we can tailor therapy for high-risk patients and minimize toxicity by not overtreating patients who may not need therapy.”
Dr. Mouhieddine’s current work is focusing on developing clinical trials to test whether immunotherapy can snuff out myeloma when it’s at the smoldering stage, “before anything bad happens.
“If a myeloma patient comes in with renal failure, and we treat the myeloma at that stage, it doesn’t mean that the patient’s kidneys are gonna go back to normal. A lot of the damage can be permanent,” he said. “Even when you treat multiple myeloma, and it goes into remission, it ends up coming back. And you just have to go from one therapy to the other.”
In contrast, a successful treatment for smoldering myeloma would prevent progression to the full disease. In other words, it would be a cure – which is now elusive.
Specifically, Dr. Mouhieddine hopes to test whether bispecific antibodies, a type of immunotherapy that enlists the body’s T cells to kill myeloma cells, will be effective in the smoldering phase. Bispecific antibodies are now being explored as treatments for full multiple myeloma when the immune system is weaker, he said, and they may be even more effective earlier, when the body is better equipped to fight off the disease.
Dr. Mouhieddine hopes better treatments for multiple myeloma itself will help save his 64-year-old aunt Hassana, back in Beirut. He diagnosed her in 2022 after she told him that she felt tired all the time and underwent various tests. The woman he calls his “second mom” is doing well, despite struggles to buy medication due to the lack of access to bank funds in Lebanon.
“I’m always going to be afraid that the disease is going to progress or come back at some point,” he said. “Lebanon doesn’t have as many options as people in the U.S. do. Once you exhaust your first option, and maybe your second option, then you don’t have any other options. Here, we have outpatients who exhaust option number 15 and go to option number 16. That’s definitely not the case over there.”
For now, Dr. Mouhieddine is treating patients and working to launch clinical trials into smoldering myeloma. “His work ethic is incredible,” said his colleague, Dr. Chari. “He has seen multiple projects to publication, and he develops deep connections with his patients and follows up on their care whether or not he is in clinic on a particular day.”
Dr. Parekh, another colleague, said Dr. Mouhieddine can even be a role model. “Other trainees may benefit from thinking about their career early on and exploring both lab and clinical research projects, so that they can develop the necessary experience to be competitive in academia later on.”
His workload can a burden for Dr. Mouhieddine, who is Muslim. He expressed regret that his busy schedule does not always permit him to fast during Ramadan. On a nonmedical front, his recent efforts have paid off. In March 2023 Dr. Mouhieddine became a U.S. citizen.
“It’s surreal,” he said, “but also a dream come true. I feel very grateful, like it’s like an appreciation of who I am, what I’ve done, and what I can do for this country.”
“If we start treatment earlier, in the smoldering phase, maybe there is a chance of actually curing the disease and completely getting rid of it,” said Dr. Mouhieddine, 31, a research fellow at the Icahn School of Medicine at Mount Sinai, in New York. “We haven’t proven that yet, and it’s going to take years before we’re able to prove it. I’m hoping to be one of those spearheading the initiative.”
As he develops clinical trials, the young physician scientist has another focus: A deeply personal connection to the very disease he’s trying to cure. Last year Dr. Mouhieddine diagnosed his aunt back in Lebanon with multiple myeloma. “I have always been close to her, and I’m like her son,” he said, and her situation is especially scary because she lives in a country where treatment options are limited.
Dr. Mouhieddine was born and raised in Beirut, the son of a sports journalist father and a mother who worked in a bank. Lebanon’s civil war ended in 1990, shortly before his birth, but political instability returned when he was a child.
“Everything was a disaster,” he recalled. “There was a period of time when there were bombs throughout the city because certain politicians were being targeted. I remember when groups of people would have gunfights in the street.”
Dr. Mouhieddine attended the American University of Beirut, then after college and medical school there, he headed to the United States.
“I wanted to make a difference in medicine. And I knew that if I stayed back home, I wouldn’t be able to,” he said. Fortunately, “everybody has made me feel that I really belong here, and I’ve never felt like I’m an outsider.”
Early on, as he went through fellowships and residency, he developed an interest in multiple myeloma.
Ajai Chari, MD, a colleague of Dr. Mouhieddine’s at Icahn School of Medicine, said in an interview, “I remember meeting him at a conference before he had even started an internal medicine residency, let alone a hematology oncology fellowship. He was already certain he wanted to work in multiple myeloma, due to his work at Dana-Farber Cancer Institute.”
Myeloma was especially intriguing to Dr. Mouhieddine because of the rapid rate of progress in treating the disease. “Over the past 10 years, the myeloma field has advanced at such an extremely fast pace, more than any other cancer,” he said. “Maybe 15 years ago, you would tell someone with newly diagnosed myeloma that they had a chance for an average of another 2 years. Now, we tell patients they have 10 years to live on average, which means you could live 15 or 20 years. That alone was astounding to me and piqued my interest in myeloma.”
At the same time, smoldering myeloma – which can be discovered during routine blood work – remains little understood. As the National Cancer Institute explains, “smoldering myeloma is a precancerous condition that alters certain proteins in blood and/or increases plasma cells in bone marrow, but it does not cause symptoms of disease. About half of those diagnosed with the condition, however, will develop multiple myeloma within 5 years.”
“If we understand what drives smoldering myeloma, we may be able to prevent it from progressing to its active form,” said hematologist oncologist Samir Parekh, MD, who works with Dr. Mouhieddine at Icahn School of Medicine. “Or at the minimum, we could better predict who will progress so we can tailor therapy for high-risk patients and minimize toxicity by not overtreating patients who may not need therapy.”
Dr. Mouhieddine’s current work is focusing on developing clinical trials to test whether immunotherapy can snuff out myeloma when it’s at the smoldering stage, “before anything bad happens.
“If a myeloma patient comes in with renal failure, and we treat the myeloma at that stage, it doesn’t mean that the patient’s kidneys are gonna go back to normal. A lot of the damage can be permanent,” he said. “Even when you treat multiple myeloma, and it goes into remission, it ends up coming back. And you just have to go from one therapy to the other.”
In contrast, a successful treatment for smoldering myeloma would prevent progression to the full disease. In other words, it would be a cure – which is now elusive.
Specifically, Dr. Mouhieddine hopes to test whether bispecific antibodies, a type of immunotherapy that enlists the body’s T cells to kill myeloma cells, will be effective in the smoldering phase. Bispecific antibodies are now being explored as treatments for full multiple myeloma when the immune system is weaker, he said, and they may be even more effective earlier, when the body is better equipped to fight off the disease.
Dr. Mouhieddine hopes better treatments for multiple myeloma itself will help save his 64-year-old aunt Hassana, back in Beirut. He diagnosed her in 2022 after she told him that she felt tired all the time and underwent various tests. The woman he calls his “second mom” is doing well, despite struggles to buy medication due to the lack of access to bank funds in Lebanon.
“I’m always going to be afraid that the disease is going to progress or come back at some point,” he said. “Lebanon doesn’t have as many options as people in the U.S. do. Once you exhaust your first option, and maybe your second option, then you don’t have any other options. Here, we have outpatients who exhaust option number 15 and go to option number 16. That’s definitely not the case over there.”
For now, Dr. Mouhieddine is treating patients and working to launch clinical trials into smoldering myeloma. “His work ethic is incredible,” said his colleague, Dr. Chari. “He has seen multiple projects to publication, and he develops deep connections with his patients and follows up on their care whether or not he is in clinic on a particular day.”
Dr. Parekh, another colleague, said Dr. Mouhieddine can even be a role model. “Other trainees may benefit from thinking about their career early on and exploring both lab and clinical research projects, so that they can develop the necessary experience to be competitive in academia later on.”
His workload can a burden for Dr. Mouhieddine, who is Muslim. He expressed regret that his busy schedule does not always permit him to fast during Ramadan. On a nonmedical front, his recent efforts have paid off. In March 2023 Dr. Mouhieddine became a U.S. citizen.
“It’s surreal,” he said, “but also a dream come true. I feel very grateful, like it’s like an appreciation of who I am, what I’ve done, and what I can do for this country.”
“If we start treatment earlier, in the smoldering phase, maybe there is a chance of actually curing the disease and completely getting rid of it,” said Dr. Mouhieddine, 31, a research fellow at the Icahn School of Medicine at Mount Sinai, in New York. “We haven’t proven that yet, and it’s going to take years before we’re able to prove it. I’m hoping to be one of those spearheading the initiative.”
As he develops clinical trials, the young physician scientist has another focus: A deeply personal connection to the very disease he’s trying to cure. Last year Dr. Mouhieddine diagnosed his aunt back in Lebanon with multiple myeloma. “I have always been close to her, and I’m like her son,” he said, and her situation is especially scary because she lives in a country where treatment options are limited.
Dr. Mouhieddine was born and raised in Beirut, the son of a sports journalist father and a mother who worked in a bank. Lebanon’s civil war ended in 1990, shortly before his birth, but political instability returned when he was a child.
“Everything was a disaster,” he recalled. “There was a period of time when there were bombs throughout the city because certain politicians were being targeted. I remember when groups of people would have gunfights in the street.”
Dr. Mouhieddine attended the American University of Beirut, then after college and medical school there, he headed to the United States.
“I wanted to make a difference in medicine. And I knew that if I stayed back home, I wouldn’t be able to,” he said. Fortunately, “everybody has made me feel that I really belong here, and I’ve never felt like I’m an outsider.”
Early on, as he went through fellowships and residency, he developed an interest in multiple myeloma.
Ajai Chari, MD, a colleague of Dr. Mouhieddine’s at Icahn School of Medicine, said in an interview, “I remember meeting him at a conference before he had even started an internal medicine residency, let alone a hematology oncology fellowship. He was already certain he wanted to work in multiple myeloma, due to his work at Dana-Farber Cancer Institute.”
Myeloma was especially intriguing to Dr. Mouhieddine because of the rapid rate of progress in treating the disease. “Over the past 10 years, the myeloma field has advanced at such an extremely fast pace, more than any other cancer,” he said. “Maybe 15 years ago, you would tell someone with newly diagnosed myeloma that they had a chance for an average of another 2 years. Now, we tell patients they have 10 years to live on average, which means you could live 15 or 20 years. That alone was astounding to me and piqued my interest in myeloma.”
At the same time, smoldering myeloma – which can be discovered during routine blood work – remains little understood. As the National Cancer Institute explains, “smoldering myeloma is a precancerous condition that alters certain proteins in blood and/or increases plasma cells in bone marrow, but it does not cause symptoms of disease. About half of those diagnosed with the condition, however, will develop multiple myeloma within 5 years.”
“If we understand what drives smoldering myeloma, we may be able to prevent it from progressing to its active form,” said hematologist oncologist Samir Parekh, MD, who works with Dr. Mouhieddine at Icahn School of Medicine. “Or at the minimum, we could better predict who will progress so we can tailor therapy for high-risk patients and minimize toxicity by not overtreating patients who may not need therapy.”
Dr. Mouhieddine’s current work is focusing on developing clinical trials to test whether immunotherapy can snuff out myeloma when it’s at the smoldering stage, “before anything bad happens.
“If a myeloma patient comes in with renal failure, and we treat the myeloma at that stage, it doesn’t mean that the patient’s kidneys are gonna go back to normal. A lot of the damage can be permanent,” he said. “Even when you treat multiple myeloma, and it goes into remission, it ends up coming back. And you just have to go from one therapy to the other.”
In contrast, a successful treatment for smoldering myeloma would prevent progression to the full disease. In other words, it would be a cure – which is now elusive.
Specifically, Dr. Mouhieddine hopes to test whether bispecific antibodies, a type of immunotherapy that enlists the body’s T cells to kill myeloma cells, will be effective in the smoldering phase. Bispecific antibodies are now being explored as treatments for full multiple myeloma when the immune system is weaker, he said, and they may be even more effective earlier, when the body is better equipped to fight off the disease.
Dr. Mouhieddine hopes better treatments for multiple myeloma itself will help save his 64-year-old aunt Hassana, back in Beirut. He diagnosed her in 2022 after she told him that she felt tired all the time and underwent various tests. The woman he calls his “second mom” is doing well, despite struggles to buy medication due to the lack of access to bank funds in Lebanon.
“I’m always going to be afraid that the disease is going to progress or come back at some point,” he said. “Lebanon doesn’t have as many options as people in the U.S. do. Once you exhaust your first option, and maybe your second option, then you don’t have any other options. Here, we have outpatients who exhaust option number 15 and go to option number 16. That’s definitely not the case over there.”
For now, Dr. Mouhieddine is treating patients and working to launch clinical trials into smoldering myeloma. “His work ethic is incredible,” said his colleague, Dr. Chari. “He has seen multiple projects to publication, and he develops deep connections with his patients and follows up on their care whether or not he is in clinic on a particular day.”
Dr. Parekh, another colleague, said Dr. Mouhieddine can even be a role model. “Other trainees may benefit from thinking about their career early on and exploring both lab and clinical research projects, so that they can develop the necessary experience to be competitive in academia later on.”
His workload can a burden for Dr. Mouhieddine, who is Muslim. He expressed regret that his busy schedule does not always permit him to fast during Ramadan. On a nonmedical front, his recent efforts have paid off. In March 2023 Dr. Mouhieddine became a U.S. citizen.
“It’s surreal,” he said, “but also a dream come true. I feel very grateful, like it’s like an appreciation of who I am, what I’ve done, and what I can do for this country.”








