User login
Adult ADHD: Pharmacologic treatment in the DSM-5 era
Attention-deficit/hyperactivity disorder (ADHD) is common; it affects 5% to 7% of children1,2 and 4% to 5% of all adults.3,4 Pediatric ADHD often persists into adulthood, as 65% of individuals diagnosed as children retain impairing symptoms by age 25.4
The prevalence of ADHD in childhood is 2 to 3 times greater among boys than girls, but more comparable between the sexes in adulthood.2 Symptoms could be more easily overlooked in women because of the greater prominence of hyperactivity and impulsivity-type symptoms in men.5
Untreated ADHD is associated with significant costs. Adults with ADHD have increased unemployment rates, poor work performance, and comparatively lower educational performance.6,7 Compared with non-ADHD adults, those with ADHD have:
- more traffic violations and accidents and a higher rate of criminal convictions and incarcerations8,9
- a mortality rate almost 2 times higher, with the greatest differences seen in deaths by suicide and accidents.10,11
Adults with ADHD also are more likely to have a comorbid psychiatric disorder—in particular, substance use11—and often are in treatment for other mental or substance use disorders. Among adults who meet diagnostic criteria for ADHD, approximately only 10% are receiving treatment for ADHD symptoms.3,12
Changes in DSM-5
Revisions within DSM-5 simplify ADHD’s diagnosis—and make it more difficult to ignore in
DSM-5 also provides examples of behaviors more commonly found in adults, such as “feelings of restlessness,” compared with DSM-IV’s “often runs about or climbs excessively in situations in which it is inappropriate.” Finally, ADHD now may be diagnosed in a person with an autism spectrum disorder who meets diagnostic criteria for both disorders.13,14
Identifying ADHD in adults
ADHD diagnosis in adults is made through careful clinical interviewing. For example, ask about what factors motivated an individual to seek evaluation for ADHD. Often, patients present after a change in responsibility at work or at home, such as a promotion or birth/adoption of a new child.
Consider incorporating a brief screen for adult ADHD in all new outpatient evaluations (Table 2).15 Screen for other psychiatric disorders as well; comorbidity with ADHD is high, and hyperactivity and inattention symptoms may result from anxiety, depression, or substance use.
Screen for learning disorders, which can present with ADHD symptoms (such as poor concentration) when the individual attempts difficult tasks. Evaluate for risk factors associated with ADHD medications, such as a history of cardiac problems, hypertension, or tachycardia. A family history of ADHD is found in approximately 80% of cases.16,17 Determine the presence of ADHD symptoms in childhood. A careful review of the educational history often reveals long-term underachievement and struggles in school. Patients may report a chronic history of poor attention or feelings of restlessness in school. Sometimes problems do not become apparent until high school or college; some individuals, especially those with high intelligence, compensate for deficits and show fewer overt symptoms of impairment until later in their education.18Occupational history also may be revealing:
- How are they performing at work?
- Have they changed jobs multiple times in a short period?
- Do they have difficulty organizing tasks?
Subtle ADHD signs include time of arrival to appointments (eg, late or extremely early), missing data on intake paperwork, and a history of losing keys or phones.
Neuropsychological testing. Some clinicians routinely include neuropsychological testing in an adult ADHD evaluation, but these studies have shown inconsistent cognitive deficits in people with ADHD.19,20 No distinct psychometric cognitive test or profile is diagnostic of ADHD or its subtypes.21
Treatment and follow-up care
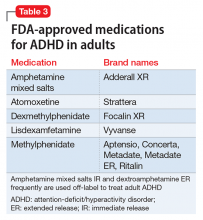
Four general categories of medications are used to treat ADHD in children and adults:
After starting a patient on medication, at each follow-up appointment ask about new cardiac symptoms or diagnoses, new family history of cardiac problems, or new medications. Measure pulse and blood pressure every 1 to 3 months. Measure vital signs more frequently during titration and weaning periods.23
Stimulant medications
Amphetamines have dual action: they block the reuptake of dopamine and noradrenaline by competitive inhibition of the transporters and promote the release of dopamine and noradrenaline by competitive inhibition of the intraneuronal vesicular monoamine transporter.24
For most amphetamine products, including dextroamphetamine and amphetamine mixed salts, the target dosage is approximately 0.5 mg/kg. Start at a lower dosage, however, and rapidly titrate weekly so patients can adjust to the medication while not becoming frustrated with a lack of efficacy. Some patients may require short-acting forms with dosing 3 times per day, and twice daily dosing is not uncommon with extended-release (ER) formulations.
Metabolism of most amphetamine products—with the exception of lisdexamfetamine—involves the cytochrome P450 (CYP) enzyme CYP2D6, leading to the formation of the metabolite 4-hydroxyamphetamine.25 The pharmacokinetics of lisdexamfetamine in slow or ultra-rapid CYP2D6 metabolizers has not been evaluated (Shire US Inc., written communication, July 2014).
Agents that alter urinary pH can affect blood levels of amphetamine. Acidifying agents decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels.26
Lisdexamfetamine contains L-lysine, an essential amino acid, covalently bound to d-amphetamine via an amide linking group.27 After absorption, lisdexamfetamine is metabolized by rate-limited, enzymatic hydrolysis to yield d-amphetamine and L-lysine.24,28,29 A starting dose of 40 mg is advised; twice-daily dosing rarely is required.
A meta-analysis of 5 randomized, controlled trials in the treatment of adult ADHD showed a response rate of 70% for lisdexamfetamine compared with 37% for placebo. Trial duration ranged from 4 to 14 weeks, with dosages of 30 to 70 mg/d.30 Another analysis of data from lisdexamfetamine trials predicted an effect size of 1.07 for European adults, which is larger than the 0.8 threshold for large effect sizes.31
Methylphenidate products. Methylphenidate’s main action is through enhancement of dopamine signaling by blockade of the dopamine transporter, leading to increases in extracellular dopamine as well as norepinephrine.22,32 Optimized dosing is generally 1 mg/kg per day, and dosing up to 80 to 120 mg/d is not unusual.33
Dexmethylphenidate is the more pharmacologically active enantiomer of racemic methylphenidate and is twice as potent.34-36 Target dosing of dexmethylphenidate should be one-half as much (ie, 0.5 mg/kg per day) as other methylphenidate products.37
Managing stimulants’ side effects
Amphetamines’ side effects may include insomnia, dry mouth, decreased appetite, weight loss, headaches, and anxiety. To help minimize sleep problems, advise patients to take a second immediate-release dose at noon, rather than later in the afternoon. The longer-acting formulation taken once per day in the morning may be offered as an alternative. Some patients may experience improved sleep because of diminished bedtime ruminations.
Oral rinses, such as Biotène, could help reduce discomfort associated with dry mouth. Pilocarpine, which stimulates saliva production, is another option if rinses are not effective. To address decreased appetite, advise patients to take their medication after they eat. Switching from an immediate-release amphetamine to a longer-acting formulation also may lessen symptoms. Lisdexamfetamine might be a good choice for adults with ADHD who have undergone bariatric surgeries because it is absorbed in the small bowel.38
Methylphenidate has no interactions with CYP enzymes, making it an attractive option for patients taking CYP inhibiting or stimulating medications.39 The most common side effects of methylphenidate products include appetite loss, insomnia, irritability, and tachycardia. Some side effects will abate after 1 to 2 weeks of treatment, but persistence of insomnia and appetite loss may require a decrease in dosage. In rare cases, methylphenidate may produce tics, exacerbate an existing tic disorder, or produce mania or psychosis.40,41 Methylphenidate inhibits the metabolism of tricyclic antidepressants; use methylphenidate with caution in patients taking monoamine oxidase inhibitors.42,43Cardiovascular risks. Possible cardiovascular risks associated with stimulant use have gained widespread attention, although research has not demonstrated an increased risk of serious cardiovascular events in young and middle-aged adults receiving stimulant medications for ADHD.44 Nonetheless, obtain a thorough medical history in adult patients, including cardiac history, family history of cardiac disease, history of any cardiac symptoms, and a medication history. Baseline ECG is not required.45
Screen for a family history of sudden death in a young person, sudden death during exercise, cardiac arrhythmia, cardiomyopathies (including hypertrophic cardiomyopathy, dilated cardiomyopathy, and right ventricular cardiomyopathy), prolonged QT interval, short QT syndrome, Brugada syndrome, Wolff-Parkinson-White syndrome, Marfan syndrome, and an event requiring resuscitation in a family member younger than 35, including syncope requiring rescuscitation.23 If fainting spells, palpitations, chest pain, or other symptoms suggest preexisting cardiovascular disease, refer the patient promptly to a cardiologist.
Peripheral vasculopathy, including Raynaud’s phenomenon, is a lesser known side effect associated with stimulants.46 Symptoms are usually mild, but in rare instances stimulants are associated with digital ulceration or soft tissue breakdown.47 Advise patients to tell you if they experience any new symptoms of numbness, pain, skin color changes, or sensitivity to temperature in fingers and toes. Signs and symptoms generally improve after dosage reduction or discontinuation of the stimulant medication.46 Referral to a rheumatologist might be appropriate if symptoms persist.
A noradrenergic medication
Atomoxetine is a potent, selective inhibitor of the presynaptic noradrenaline transporter that increases the availability of extracellular noradrenaline in the prefrontal cortex.48,49 Atomoxetine may be a good alternative for adult patients with ADHD and comorbid anxiety.50
For adults, the optimal starting dosage is 40 mg in the morning for 1 week, followed by an increase to 80 mg. Insufficient dosing is common with atomoxetine, and the dosage could be increased to 100 mg/d.51 Dosing twice per day may be associated with higher rates of insomnia.
Atomoxetine’s efficacy for managing ADHD in adults has been consistently demonstrated by 6 placebo-controlled trials of 10 to 16 weeks, 3 placebo-controlled 6-month trials, and a 1-year maintenance-of-response trial.52 Atomoxetine was found to have an effect size of 0.45 (medium) (number needed to treat [NNT] = 5).53-55The most common adverse effects include nausea, dry mouth, insomnia, and erectile dysfunction. Small increases in heart rate and blood pressure have been reported, so use this medication with caution in patients for whom this might be problematic. Atomoxetine is metabolized by CYP2D6; 7% of white individuals have a genotype corresponding to a nonfunctional CYP2D6 enzyme.56-58
Alpha-2 adrenergic agonists
Clonidine and guanfacine are antihypertensive drugs that induce peripheral sympathoinhibition via the stimulation of receptors. Clonidine binds equally to adrenergic receptor subtypes α-2A, α-2B, and α-2C (as well as to α-1 and β subtypes, histamine receptors, and possibly dopamine receptors).59,60 Guanfacine binds preferentially to postsynaptic α-2A adrenoceptors in the prefrontal cortex, which have been implicated in attentional and organizational functions.61,62
ER guanfacine and ER clonidine are FDA-approved as monotherapy for ADHD in children and adolescents.
Efficacy in adults. A small (N = 17), double-blind, placebo-controlled, crossover study comparing immediate-release guanfacine and dextroamphetamine found that both medications significantly reduced adult ADHD symptoms, as measured with the DSM-IV Adult Behavior Checklist for Adults.63
No trials have been published regarding the efficacy of ER clonidine in adults with ADHD; adverse effects including sedation, bradycardia, and hypotension may limit its use. One study compared the supplemental use of ER guanfacine (1 to 6 mg/d) or a matching placebo in 26 adults with ADHD who had suboptimal response to stimulant-only treatment. After 10 weeks, both the guanfacine ER and placebo groups showed statistically significant improvements in ADHD symptoms and general functioning. The treatments did not differ in efficacy, safety, or tolerability.64
Adverse events. Compared with clonidine, guanfacine has less CNS depressant and hypotensive activity.58 A phase I trial of ER guanfacine in healthy adults found its single-dose pharmacokinetic properties in 1-, 2-, and 4-mg tablets appeared to be statistically linear. Somnolence—the most common treatment-emergent adverse effect—occurred in 33 of 52 participants (63.5%). All mean vital-sign measurements and ECG parameters remained within normal limits after dosing, and no marked changes from baseline measurements were noted.65
Antidepressants
Antidepressants used in ADHD treatment include bupropion and tricyclic antidepressants.
Bupropion is a noradrenaline and dopamine reuptake inhibitor and is considered to be a mild psychostimulant because of its amphetamine-derived chemical structure.66,67 It generally is considered a third-line medication when stimulants have not improved ADHD symptoms or are not tolerated.
A 2011 meta-analysis examined 5 randomized, controlled trials including 175 adults treated with bupropion for ADHD. Bupropion was found to be more effective than placebo (NNT = 5), although bupropion’s therapeutic benefits were not observed until weeks 5 and 6. Its effects were less pronounced than those of methylphenidate. Mean daily dosages were 362 mg for the bupropion SR trials and 393 mg for the bupropion XL trial.68
Tricyclics. Desipramine and nortriptyline have been found to be efficacious in childhood ADHD,69,70 although cardiovascular risk and toxicity in overdose limit their use.71
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systemic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723.
4. Faraone S, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165.
5. Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143-154.
6. Halmøy A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187.
7. Kuriyan AB, Pelham WE Jr, Molina BS, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27-41.
8. Murphy K, Barkley RA. Attention deficit hyperactivity disorder in adults: comorbidities and adaptive impairment. Compr Psychiatry. 1996;37(6):393-401.
9. Mannuzza S, Klein RG, Mouton JL 3rd. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res. 2008;160(3):237-246.
10. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
11. Barbaresi WJ, Colligan RC, Weaver AL, et al. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637-644.
12. Babcock T, Ornstein CS. Comorbidity and its impact in adult patients with attention-deficit/hyperactivity disorder: a primary care perspective. Postgrad Med. 2009;121(3):73-82.
13. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-66.
14. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:78-85.
15. Kooij JJS. Adult ADHD: diagnostic assessment and treatment. 3rd ed. Amsterdam, Netherlands: Springer; 2013:34.
16. Faraone SV, Khan SA. Candidate gene studies of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):13-20.
17. Neale BM, Medland SE, Ripke S, et al; Psychiatric GWAS Consortium: ADHD Subgroup. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884-897.
18. Milioni AL, Chaim TM, Cavallet M, et al. High IQ may “mask” the diagnosis of ADHD by compensating for deficits in executive functions in treatment-naïve adults with ADHD [published online October 30, 2014]. J Atten Disord. pii: 1087054714554933.
19. Rapport MD, Chung KM, Shore G, et al. Upgrading the science and technology of assessment and diagnosis: laboratory and clinic-based assessment of children with ADHD. J Clin Child Psychol. 2000;29(4):555-568.
20. Woods SP, Lovejoy DW, Ball JD. Neuropsychological characteristics of adults with ADHD: a comprehensive review of initial studies. Clin Neuropsychol. 2002;16(1):12-34.
21. Lange KW, Hauser J, Lange KM, et al. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2014;6(4):241-248.
22. Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3(4):200-211.
23. Vetter VL Elia J, Erickson, C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [Erratum in: Circulation. 2009;120(7):e55-e59]. Circulation. 2008;117(18):2407-2423.
24. Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639-677.
25. Wu D, Otton SV, Inaba T, et al. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol. 1997;53(11):1605-1612.
26. Vyvanse [package insert]. Lexington, MA: Shire Pharmaceuticals; 2015.
27. Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317-327.
28. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
29. Krishnan SM, Pennick M, Stark JG. Metabolism, distribution and elimination of lisdexamfetamine dimesylate: open-label, single-centre, phase I study in healthy adult volunteers. Clin Drug Invest. 2008;28(12):745-755.
30. Maneeton N, Maneeton B, Suttajit S, et al. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685-1693.
31. Fridman M, Hodgkins P, Kahle JS, et al. Predicted effect size of lisdexamfetamine treatment of attention deficit/hyperactivity disorder (ADHD) in European adults: estimates based on indirect analysis using a systematic review and meta-regression analysis. Eur Psychiatry. 2015;30(4):521-527.
32. Markowitz JS, DeVane CL, Pestreich L, et al. Session 1-87-differentiation of d-, L- and dl-methylphenidate through in vitro pharmacological screening. In: Abstracts: Oral and Poster Presentations of the NCDEU 45th Annual Meeting; June 6-9, 2005; Boca Raton, FL:186.
33. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
34. Teo SK, Stirling DI, Thomas SD, et al. Neurobehavioral effects of racemic threo-methylphenidate and its D and L enantiomers in rats. Pharmacol Biochem Behav. 2003;74(3):747-754.
35. Ding YS, Fowler JS, Volkow ND, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacol (Berl). 1997;131(1):71-78.
36. Davids E, Zhang K, Tarazi FI, et al. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacol (Berl). 2002;160(1):92-98.
37. Srinivas NR, Hubbard JW, Quinn D, et al. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52(5):561-568.
38. Ermer JC, Haffey MB, Doll WJ, et al. Pharmacokinetics of lisdexamfetamine dimesylate after targeted gastrointestinal release or oral administration in healthy adults. Drug Metab Dispos. 2012;40(2):290-297.
39. DeVane CL, Markowitz JS, Carson SW, et al. Single-dose pharmacokinetics of methylphenidate in CYP2D6 extensive and poor metabolizers. J Clin Psychopharmacol. 2000;20(3):347-349.
40. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237.
41. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(7):1149-1152.
42. Shelton Clauson A, Elliott ES, Watson BD, et al. Coadministration of phenelzine and methylphenidate for treatment-resistant depression. Ann Pharmacother. 2004;38(3):508.
43. Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
44. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
45. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20(1):17-37.
46. Goldman W, Seltzer R, Reuman P. Association between treatment with central nervous system stimulants and Raynaud’s syndrome in children: a retrospective case-control study of rheumatology patients. Arthritis Rheum. 2008;58(2):563-566.
47. Syed RH, Moore TL. Methylphenidate and dextroamphetamine-induced peripheral vasculopathy. J Clin Rheum. 2008;14(1):30-33.
48. Wilens TE. Mechanism of action of agents in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):32-38.
49. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
50. Adler LA, Liebowitz M, Kronenberger W, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26(3):212-221.
51. Clemow DB. Suboptimal dosing of Strattera (atomoxetine) for ADHD patients. Postgrad Med. 2014;126(5):196-198.
52. Camporeale A, Porsdal V, De Bruyckere K, et al. Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol. 2015;29(1):3-14.
53. Young JL, Sarkis E, Qiao M, et al. Once-daily treatment with atomoxetine in adults with attention-deficit/hyperactivity disorder: a 24-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2011;34(2):51-60.
54. Bitter I, Angyalosi A, Czobor P. Pharmacological treatment of adult ADHD. Curr Opin Psychiatry. 2012;25(6):529-534.
55. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
56. Ring BJ, Gillespie JS, Eckstein JA, et al. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30(3):319-323.
57. Farid NA, Bergstrom RF, Ziege EA, et al. Single-dose and steady state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol. 1985;25(4):296-301.
58. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35(2-3):99-106.
59. Jasper JR, Lesnick JD, Chang LK, et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol. 1998;55(7):1035-1043.
60. Ruggiero S, Clavenna A, Reale L, et al. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(10):1578-1590.
61. Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99(2):211-216.
62. Uhlén S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202(2):235-243.
63. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21(2):223-228.
64. Butterfield ME, Saal J, Young B, et al. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res. 2016;236:136-141.
65. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29(4):617-625.
66. Cooper BR, Wang CM, Cox RF. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11(2):133-141.
67. Reimherr FW, Hedges DW, Strong RE, et al. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245-251.
68. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
69. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
70. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
71. Bond DJ, Hadjipavlou G, Lam RW, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2012;24(1):23-37.
Attention-deficit/hyperactivity disorder (ADHD) is common; it affects 5% to 7% of children1,2 and 4% to 5% of all adults.3,4 Pediatric ADHD often persists into adulthood, as 65% of individuals diagnosed as children retain impairing symptoms by age 25.4
The prevalence of ADHD in childhood is 2 to 3 times greater among boys than girls, but more comparable between the sexes in adulthood.2 Symptoms could be more easily overlooked in women because of the greater prominence of hyperactivity and impulsivity-type symptoms in men.5
Untreated ADHD is associated with significant costs. Adults with ADHD have increased unemployment rates, poor work performance, and comparatively lower educational performance.6,7 Compared with non-ADHD adults, those with ADHD have:
- more traffic violations and accidents and a higher rate of criminal convictions and incarcerations8,9
- a mortality rate almost 2 times higher, with the greatest differences seen in deaths by suicide and accidents.10,11
Adults with ADHD also are more likely to have a comorbid psychiatric disorder—in particular, substance use11—and often are in treatment for other mental or substance use disorders. Among adults who meet diagnostic criteria for ADHD, approximately only 10% are receiving treatment for ADHD symptoms.3,12
Changes in DSM-5
Revisions within DSM-5 simplify ADHD’s diagnosis—and make it more difficult to ignore in
DSM-5 also provides examples of behaviors more commonly found in adults, such as “feelings of restlessness,” compared with DSM-IV’s “often runs about or climbs excessively in situations in which it is inappropriate.” Finally, ADHD now may be diagnosed in a person with an autism spectrum disorder who meets diagnostic criteria for both disorders.13,14
Identifying ADHD in adults
ADHD diagnosis in adults is made through careful clinical interviewing. For example, ask about what factors motivated an individual to seek evaluation for ADHD. Often, patients present after a change in responsibility at work or at home, such as a promotion or birth/adoption of a new child.
Consider incorporating a brief screen for adult ADHD in all new outpatient evaluations (Table 2).15 Screen for other psychiatric disorders as well; comorbidity with ADHD is high, and hyperactivity and inattention symptoms may result from anxiety, depression, or substance use.
Screen for learning disorders, which can present with ADHD symptoms (such as poor concentration) when the individual attempts difficult tasks. Evaluate for risk factors associated with ADHD medications, such as a history of cardiac problems, hypertension, or tachycardia. A family history of ADHD is found in approximately 80% of cases.16,17 Determine the presence of ADHD symptoms in childhood. A careful review of the educational history often reveals long-term underachievement and struggles in school. Patients may report a chronic history of poor attention or feelings of restlessness in school. Sometimes problems do not become apparent until high school or college; some individuals, especially those with high intelligence, compensate for deficits and show fewer overt symptoms of impairment until later in their education.18Occupational history also may be revealing:
- How are they performing at work?
- Have they changed jobs multiple times in a short period?
- Do they have difficulty organizing tasks?
Subtle ADHD signs include time of arrival to appointments (eg, late or extremely early), missing data on intake paperwork, and a history of losing keys or phones.
Neuropsychological testing. Some clinicians routinely include neuropsychological testing in an adult ADHD evaluation, but these studies have shown inconsistent cognitive deficits in people with ADHD.19,20 No distinct psychometric cognitive test or profile is diagnostic of ADHD or its subtypes.21
Treatment and follow-up care
Four general categories of medications are used to treat ADHD in children and adults:
After starting a patient on medication, at each follow-up appointment ask about new cardiac symptoms or diagnoses, new family history of cardiac problems, or new medications. Measure pulse and blood pressure every 1 to 3 months. Measure vital signs more frequently during titration and weaning periods.23
Stimulant medications
Amphetamines have dual action: they block the reuptake of dopamine and noradrenaline by competitive inhibition of the transporters and promote the release of dopamine and noradrenaline by competitive inhibition of the intraneuronal vesicular monoamine transporter.24
For most amphetamine products, including dextroamphetamine and amphetamine mixed salts, the target dosage is approximately 0.5 mg/kg. Start at a lower dosage, however, and rapidly titrate weekly so patients can adjust to the medication while not becoming frustrated with a lack of efficacy. Some patients may require short-acting forms with dosing 3 times per day, and twice daily dosing is not uncommon with extended-release (ER) formulations.
Metabolism of most amphetamine products—with the exception of lisdexamfetamine—involves the cytochrome P450 (CYP) enzyme CYP2D6, leading to the formation of the metabolite 4-hydroxyamphetamine.25 The pharmacokinetics of lisdexamfetamine in slow or ultra-rapid CYP2D6 metabolizers has not been evaluated (Shire US Inc., written communication, July 2014).
Agents that alter urinary pH can affect blood levels of amphetamine. Acidifying agents decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels.26
Lisdexamfetamine contains L-lysine, an essential amino acid, covalently bound to d-amphetamine via an amide linking group.27 After absorption, lisdexamfetamine is metabolized by rate-limited, enzymatic hydrolysis to yield d-amphetamine and L-lysine.24,28,29 A starting dose of 40 mg is advised; twice-daily dosing rarely is required.
A meta-analysis of 5 randomized, controlled trials in the treatment of adult ADHD showed a response rate of 70% for lisdexamfetamine compared with 37% for placebo. Trial duration ranged from 4 to 14 weeks, with dosages of 30 to 70 mg/d.30 Another analysis of data from lisdexamfetamine trials predicted an effect size of 1.07 for European adults, which is larger than the 0.8 threshold for large effect sizes.31
Methylphenidate products. Methylphenidate’s main action is through enhancement of dopamine signaling by blockade of the dopamine transporter, leading to increases in extracellular dopamine as well as norepinephrine.22,32 Optimized dosing is generally 1 mg/kg per day, and dosing up to 80 to 120 mg/d is not unusual.33
Dexmethylphenidate is the more pharmacologically active enantiomer of racemic methylphenidate and is twice as potent.34-36 Target dosing of dexmethylphenidate should be one-half as much (ie, 0.5 mg/kg per day) as other methylphenidate products.37
Managing stimulants’ side effects
Amphetamines’ side effects may include insomnia, dry mouth, decreased appetite, weight loss, headaches, and anxiety. To help minimize sleep problems, advise patients to take a second immediate-release dose at noon, rather than later in the afternoon. The longer-acting formulation taken once per day in the morning may be offered as an alternative. Some patients may experience improved sleep because of diminished bedtime ruminations.
Oral rinses, such as Biotène, could help reduce discomfort associated with dry mouth. Pilocarpine, which stimulates saliva production, is another option if rinses are not effective. To address decreased appetite, advise patients to take their medication after they eat. Switching from an immediate-release amphetamine to a longer-acting formulation also may lessen symptoms. Lisdexamfetamine might be a good choice for adults with ADHD who have undergone bariatric surgeries because it is absorbed in the small bowel.38
Methylphenidate has no interactions with CYP enzymes, making it an attractive option for patients taking CYP inhibiting or stimulating medications.39 The most common side effects of methylphenidate products include appetite loss, insomnia, irritability, and tachycardia. Some side effects will abate after 1 to 2 weeks of treatment, but persistence of insomnia and appetite loss may require a decrease in dosage. In rare cases, methylphenidate may produce tics, exacerbate an existing tic disorder, or produce mania or psychosis.40,41 Methylphenidate inhibits the metabolism of tricyclic antidepressants; use methylphenidate with caution in patients taking monoamine oxidase inhibitors.42,43Cardiovascular risks. Possible cardiovascular risks associated with stimulant use have gained widespread attention, although research has not demonstrated an increased risk of serious cardiovascular events in young and middle-aged adults receiving stimulant medications for ADHD.44 Nonetheless, obtain a thorough medical history in adult patients, including cardiac history, family history of cardiac disease, history of any cardiac symptoms, and a medication history. Baseline ECG is not required.45
Screen for a family history of sudden death in a young person, sudden death during exercise, cardiac arrhythmia, cardiomyopathies (including hypertrophic cardiomyopathy, dilated cardiomyopathy, and right ventricular cardiomyopathy), prolonged QT interval, short QT syndrome, Brugada syndrome, Wolff-Parkinson-White syndrome, Marfan syndrome, and an event requiring resuscitation in a family member younger than 35, including syncope requiring rescuscitation.23 If fainting spells, palpitations, chest pain, or other symptoms suggest preexisting cardiovascular disease, refer the patient promptly to a cardiologist.
Peripheral vasculopathy, including Raynaud’s phenomenon, is a lesser known side effect associated with stimulants.46 Symptoms are usually mild, but in rare instances stimulants are associated with digital ulceration or soft tissue breakdown.47 Advise patients to tell you if they experience any new symptoms of numbness, pain, skin color changes, or sensitivity to temperature in fingers and toes. Signs and symptoms generally improve after dosage reduction or discontinuation of the stimulant medication.46 Referral to a rheumatologist might be appropriate if symptoms persist.
A noradrenergic medication
Atomoxetine is a potent, selective inhibitor of the presynaptic noradrenaline transporter that increases the availability of extracellular noradrenaline in the prefrontal cortex.48,49 Atomoxetine may be a good alternative for adult patients with ADHD and comorbid anxiety.50
For adults, the optimal starting dosage is 40 mg in the morning for 1 week, followed by an increase to 80 mg. Insufficient dosing is common with atomoxetine, and the dosage could be increased to 100 mg/d.51 Dosing twice per day may be associated with higher rates of insomnia.
Atomoxetine’s efficacy for managing ADHD in adults has been consistently demonstrated by 6 placebo-controlled trials of 10 to 16 weeks, 3 placebo-controlled 6-month trials, and a 1-year maintenance-of-response trial.52 Atomoxetine was found to have an effect size of 0.45 (medium) (number needed to treat [NNT] = 5).53-55The most common adverse effects include nausea, dry mouth, insomnia, and erectile dysfunction. Small increases in heart rate and blood pressure have been reported, so use this medication with caution in patients for whom this might be problematic. Atomoxetine is metabolized by CYP2D6; 7% of white individuals have a genotype corresponding to a nonfunctional CYP2D6 enzyme.56-58
Alpha-2 adrenergic agonists
Clonidine and guanfacine are antihypertensive drugs that induce peripheral sympathoinhibition via the stimulation of receptors. Clonidine binds equally to adrenergic receptor subtypes α-2A, α-2B, and α-2C (as well as to α-1 and β subtypes, histamine receptors, and possibly dopamine receptors).59,60 Guanfacine binds preferentially to postsynaptic α-2A adrenoceptors in the prefrontal cortex, which have been implicated in attentional and organizational functions.61,62
ER guanfacine and ER clonidine are FDA-approved as monotherapy for ADHD in children and adolescents.
Efficacy in adults. A small (N = 17), double-blind, placebo-controlled, crossover study comparing immediate-release guanfacine and dextroamphetamine found that both medications significantly reduced adult ADHD symptoms, as measured with the DSM-IV Adult Behavior Checklist for Adults.63
No trials have been published regarding the efficacy of ER clonidine in adults with ADHD; adverse effects including sedation, bradycardia, and hypotension may limit its use. One study compared the supplemental use of ER guanfacine (1 to 6 mg/d) or a matching placebo in 26 adults with ADHD who had suboptimal response to stimulant-only treatment. After 10 weeks, both the guanfacine ER and placebo groups showed statistically significant improvements in ADHD symptoms and general functioning. The treatments did not differ in efficacy, safety, or tolerability.64
Adverse events. Compared with clonidine, guanfacine has less CNS depressant and hypotensive activity.58 A phase I trial of ER guanfacine in healthy adults found its single-dose pharmacokinetic properties in 1-, 2-, and 4-mg tablets appeared to be statistically linear. Somnolence—the most common treatment-emergent adverse effect—occurred in 33 of 52 participants (63.5%). All mean vital-sign measurements and ECG parameters remained within normal limits after dosing, and no marked changes from baseline measurements were noted.65
Antidepressants
Antidepressants used in ADHD treatment include bupropion and tricyclic antidepressants.
Bupropion is a noradrenaline and dopamine reuptake inhibitor and is considered to be a mild psychostimulant because of its amphetamine-derived chemical structure.66,67 It generally is considered a third-line medication when stimulants have not improved ADHD symptoms or are not tolerated.
A 2011 meta-analysis examined 5 randomized, controlled trials including 175 adults treated with bupropion for ADHD. Bupropion was found to be more effective than placebo (NNT = 5), although bupropion’s therapeutic benefits were not observed until weeks 5 and 6. Its effects were less pronounced than those of methylphenidate. Mean daily dosages were 362 mg for the bupropion SR trials and 393 mg for the bupropion XL trial.68
Tricyclics. Desipramine and nortriptyline have been found to be efficacious in childhood ADHD,69,70 although cardiovascular risk and toxicity in overdose limit their use.71
Attention-deficit/hyperactivity disorder (ADHD) is common; it affects 5% to 7% of children1,2 and 4% to 5% of all adults.3,4 Pediatric ADHD often persists into adulthood, as 65% of individuals diagnosed as children retain impairing symptoms by age 25.4
The prevalence of ADHD in childhood is 2 to 3 times greater among boys than girls, but more comparable between the sexes in adulthood.2 Symptoms could be more easily overlooked in women because of the greater prominence of hyperactivity and impulsivity-type symptoms in men.5
Untreated ADHD is associated with significant costs. Adults with ADHD have increased unemployment rates, poor work performance, and comparatively lower educational performance.6,7 Compared with non-ADHD adults, those with ADHD have:
- more traffic violations and accidents and a higher rate of criminal convictions and incarcerations8,9
- a mortality rate almost 2 times higher, with the greatest differences seen in deaths by suicide and accidents.10,11
Adults with ADHD also are more likely to have a comorbid psychiatric disorder—in particular, substance use11—and often are in treatment for other mental or substance use disorders. Among adults who meet diagnostic criteria for ADHD, approximately only 10% are receiving treatment for ADHD symptoms.3,12
Changes in DSM-5
Revisions within DSM-5 simplify ADHD’s diagnosis—and make it more difficult to ignore in
DSM-5 also provides examples of behaviors more commonly found in adults, such as “feelings of restlessness,” compared with DSM-IV’s “often runs about or climbs excessively in situations in which it is inappropriate.” Finally, ADHD now may be diagnosed in a person with an autism spectrum disorder who meets diagnostic criteria for both disorders.13,14
Identifying ADHD in adults
ADHD diagnosis in adults is made through careful clinical interviewing. For example, ask about what factors motivated an individual to seek evaluation for ADHD. Often, patients present after a change in responsibility at work or at home, such as a promotion or birth/adoption of a new child.
Consider incorporating a brief screen for adult ADHD in all new outpatient evaluations (Table 2).15 Screen for other psychiatric disorders as well; comorbidity with ADHD is high, and hyperactivity and inattention symptoms may result from anxiety, depression, or substance use.
Screen for learning disorders, which can present with ADHD symptoms (such as poor concentration) when the individual attempts difficult tasks. Evaluate for risk factors associated with ADHD medications, such as a history of cardiac problems, hypertension, or tachycardia. A family history of ADHD is found in approximately 80% of cases.16,17 Determine the presence of ADHD symptoms in childhood. A careful review of the educational history often reveals long-term underachievement and struggles in school. Patients may report a chronic history of poor attention or feelings of restlessness in school. Sometimes problems do not become apparent until high school or college; some individuals, especially those with high intelligence, compensate for deficits and show fewer overt symptoms of impairment until later in their education.18Occupational history also may be revealing:
- How are they performing at work?
- Have they changed jobs multiple times in a short period?
- Do they have difficulty organizing tasks?
Subtle ADHD signs include time of arrival to appointments (eg, late or extremely early), missing data on intake paperwork, and a history of losing keys or phones.
Neuropsychological testing. Some clinicians routinely include neuropsychological testing in an adult ADHD evaluation, but these studies have shown inconsistent cognitive deficits in people with ADHD.19,20 No distinct psychometric cognitive test or profile is diagnostic of ADHD or its subtypes.21
Treatment and follow-up care
Four general categories of medications are used to treat ADHD in children and adults:
After starting a patient on medication, at each follow-up appointment ask about new cardiac symptoms or diagnoses, new family history of cardiac problems, or new medications. Measure pulse and blood pressure every 1 to 3 months. Measure vital signs more frequently during titration and weaning periods.23
Stimulant medications
Amphetamines have dual action: they block the reuptake of dopamine and noradrenaline by competitive inhibition of the transporters and promote the release of dopamine and noradrenaline by competitive inhibition of the intraneuronal vesicular monoamine transporter.24
For most amphetamine products, including dextroamphetamine and amphetamine mixed salts, the target dosage is approximately 0.5 mg/kg. Start at a lower dosage, however, and rapidly titrate weekly so patients can adjust to the medication while not becoming frustrated with a lack of efficacy. Some patients may require short-acting forms with dosing 3 times per day, and twice daily dosing is not uncommon with extended-release (ER) formulations.
Metabolism of most amphetamine products—with the exception of lisdexamfetamine—involves the cytochrome P450 (CYP) enzyme CYP2D6, leading to the formation of the metabolite 4-hydroxyamphetamine.25 The pharmacokinetics of lisdexamfetamine in slow or ultra-rapid CYP2D6 metabolizers has not been evaluated (Shire US Inc., written communication, July 2014).
Agents that alter urinary pH can affect blood levels of amphetamine. Acidifying agents decrease amphetamine blood levels, while alkalinizing agents increase amphetamine blood levels.26
Lisdexamfetamine contains L-lysine, an essential amino acid, covalently bound to d-amphetamine via an amide linking group.27 After absorption, lisdexamfetamine is metabolized by rate-limited, enzymatic hydrolysis to yield d-amphetamine and L-lysine.24,28,29 A starting dose of 40 mg is advised; twice-daily dosing rarely is required.
A meta-analysis of 5 randomized, controlled trials in the treatment of adult ADHD showed a response rate of 70% for lisdexamfetamine compared with 37% for placebo. Trial duration ranged from 4 to 14 weeks, with dosages of 30 to 70 mg/d.30 Another analysis of data from lisdexamfetamine trials predicted an effect size of 1.07 for European adults, which is larger than the 0.8 threshold for large effect sizes.31
Methylphenidate products. Methylphenidate’s main action is through enhancement of dopamine signaling by blockade of the dopamine transporter, leading to increases in extracellular dopamine as well as norepinephrine.22,32 Optimized dosing is generally 1 mg/kg per day, and dosing up to 80 to 120 mg/d is not unusual.33
Dexmethylphenidate is the more pharmacologically active enantiomer of racemic methylphenidate and is twice as potent.34-36 Target dosing of dexmethylphenidate should be one-half as much (ie, 0.5 mg/kg per day) as other methylphenidate products.37
Managing stimulants’ side effects
Amphetamines’ side effects may include insomnia, dry mouth, decreased appetite, weight loss, headaches, and anxiety. To help minimize sleep problems, advise patients to take a second immediate-release dose at noon, rather than later in the afternoon. The longer-acting formulation taken once per day in the morning may be offered as an alternative. Some patients may experience improved sleep because of diminished bedtime ruminations.
Oral rinses, such as Biotène, could help reduce discomfort associated with dry mouth. Pilocarpine, which stimulates saliva production, is another option if rinses are not effective. To address decreased appetite, advise patients to take their medication after they eat. Switching from an immediate-release amphetamine to a longer-acting formulation also may lessen symptoms. Lisdexamfetamine might be a good choice for adults with ADHD who have undergone bariatric surgeries because it is absorbed in the small bowel.38
Methylphenidate has no interactions with CYP enzymes, making it an attractive option for patients taking CYP inhibiting or stimulating medications.39 The most common side effects of methylphenidate products include appetite loss, insomnia, irritability, and tachycardia. Some side effects will abate after 1 to 2 weeks of treatment, but persistence of insomnia and appetite loss may require a decrease in dosage. In rare cases, methylphenidate may produce tics, exacerbate an existing tic disorder, or produce mania or psychosis.40,41 Methylphenidate inhibits the metabolism of tricyclic antidepressants; use methylphenidate with caution in patients taking monoamine oxidase inhibitors.42,43Cardiovascular risks. Possible cardiovascular risks associated with stimulant use have gained widespread attention, although research has not demonstrated an increased risk of serious cardiovascular events in young and middle-aged adults receiving stimulant medications for ADHD.44 Nonetheless, obtain a thorough medical history in adult patients, including cardiac history, family history of cardiac disease, history of any cardiac symptoms, and a medication history. Baseline ECG is not required.45
Screen for a family history of sudden death in a young person, sudden death during exercise, cardiac arrhythmia, cardiomyopathies (including hypertrophic cardiomyopathy, dilated cardiomyopathy, and right ventricular cardiomyopathy), prolonged QT interval, short QT syndrome, Brugada syndrome, Wolff-Parkinson-White syndrome, Marfan syndrome, and an event requiring resuscitation in a family member younger than 35, including syncope requiring rescuscitation.23 If fainting spells, palpitations, chest pain, or other symptoms suggest preexisting cardiovascular disease, refer the patient promptly to a cardiologist.
Peripheral vasculopathy, including Raynaud’s phenomenon, is a lesser known side effect associated with stimulants.46 Symptoms are usually mild, but in rare instances stimulants are associated with digital ulceration or soft tissue breakdown.47 Advise patients to tell you if they experience any new symptoms of numbness, pain, skin color changes, or sensitivity to temperature in fingers and toes. Signs and symptoms generally improve after dosage reduction or discontinuation of the stimulant medication.46 Referral to a rheumatologist might be appropriate if symptoms persist.
A noradrenergic medication
Atomoxetine is a potent, selective inhibitor of the presynaptic noradrenaline transporter that increases the availability of extracellular noradrenaline in the prefrontal cortex.48,49 Atomoxetine may be a good alternative for adult patients with ADHD and comorbid anxiety.50
For adults, the optimal starting dosage is 40 mg in the morning for 1 week, followed by an increase to 80 mg. Insufficient dosing is common with atomoxetine, and the dosage could be increased to 100 mg/d.51 Dosing twice per day may be associated with higher rates of insomnia.
Atomoxetine’s efficacy for managing ADHD in adults has been consistently demonstrated by 6 placebo-controlled trials of 10 to 16 weeks, 3 placebo-controlled 6-month trials, and a 1-year maintenance-of-response trial.52 Atomoxetine was found to have an effect size of 0.45 (medium) (number needed to treat [NNT] = 5).53-55The most common adverse effects include nausea, dry mouth, insomnia, and erectile dysfunction. Small increases in heart rate and blood pressure have been reported, so use this medication with caution in patients for whom this might be problematic. Atomoxetine is metabolized by CYP2D6; 7% of white individuals have a genotype corresponding to a nonfunctional CYP2D6 enzyme.56-58
Alpha-2 adrenergic agonists
Clonidine and guanfacine are antihypertensive drugs that induce peripheral sympathoinhibition via the stimulation of receptors. Clonidine binds equally to adrenergic receptor subtypes α-2A, α-2B, and α-2C (as well as to α-1 and β subtypes, histamine receptors, and possibly dopamine receptors).59,60 Guanfacine binds preferentially to postsynaptic α-2A adrenoceptors in the prefrontal cortex, which have been implicated in attentional and organizational functions.61,62
ER guanfacine and ER clonidine are FDA-approved as monotherapy for ADHD in children and adolescents.
Efficacy in adults. A small (N = 17), double-blind, placebo-controlled, crossover study comparing immediate-release guanfacine and dextroamphetamine found that both medications significantly reduced adult ADHD symptoms, as measured with the DSM-IV Adult Behavior Checklist for Adults.63
No trials have been published regarding the efficacy of ER clonidine in adults with ADHD; adverse effects including sedation, bradycardia, and hypotension may limit its use. One study compared the supplemental use of ER guanfacine (1 to 6 mg/d) or a matching placebo in 26 adults with ADHD who had suboptimal response to stimulant-only treatment. After 10 weeks, both the guanfacine ER and placebo groups showed statistically significant improvements in ADHD symptoms and general functioning. The treatments did not differ in efficacy, safety, or tolerability.64
Adverse events. Compared with clonidine, guanfacine has less CNS depressant and hypotensive activity.58 A phase I trial of ER guanfacine in healthy adults found its single-dose pharmacokinetic properties in 1-, 2-, and 4-mg tablets appeared to be statistically linear. Somnolence—the most common treatment-emergent adverse effect—occurred in 33 of 52 participants (63.5%). All mean vital-sign measurements and ECG parameters remained within normal limits after dosing, and no marked changes from baseline measurements were noted.65
Antidepressants
Antidepressants used in ADHD treatment include bupropion and tricyclic antidepressants.
Bupropion is a noradrenaline and dopamine reuptake inhibitor and is considered to be a mild psychostimulant because of its amphetamine-derived chemical structure.66,67 It generally is considered a third-line medication when stimulants have not improved ADHD symptoms or are not tolerated.
A 2011 meta-analysis examined 5 randomized, controlled trials including 175 adults treated with bupropion for ADHD. Bupropion was found to be more effective than placebo (NNT = 5), although bupropion’s therapeutic benefits were not observed until weeks 5 and 6. Its effects were less pronounced than those of methylphenidate. Mean daily dosages were 362 mg for the bupropion SR trials and 393 mg for the bupropion XL trial.68
Tricyclics. Desipramine and nortriptyline have been found to be efficacious in childhood ADHD,69,70 although cardiovascular risk and toxicity in overdose limit their use.71
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systemic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723.
4. Faraone S, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165.
5. Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143-154.
6. Halmøy A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187.
7. Kuriyan AB, Pelham WE Jr, Molina BS, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27-41.
8. Murphy K, Barkley RA. Attention deficit hyperactivity disorder in adults: comorbidities and adaptive impairment. Compr Psychiatry. 1996;37(6):393-401.
9. Mannuzza S, Klein RG, Mouton JL 3rd. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res. 2008;160(3):237-246.
10. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
11. Barbaresi WJ, Colligan RC, Weaver AL, et al. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637-644.
12. Babcock T, Ornstein CS. Comorbidity and its impact in adult patients with attention-deficit/hyperactivity disorder: a primary care perspective. Postgrad Med. 2009;121(3):73-82.
13. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-66.
14. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:78-85.
15. Kooij JJS. Adult ADHD: diagnostic assessment and treatment. 3rd ed. Amsterdam, Netherlands: Springer; 2013:34.
16. Faraone SV, Khan SA. Candidate gene studies of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):13-20.
17. Neale BM, Medland SE, Ripke S, et al; Psychiatric GWAS Consortium: ADHD Subgroup. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884-897.
18. Milioni AL, Chaim TM, Cavallet M, et al. High IQ may “mask” the diagnosis of ADHD by compensating for deficits in executive functions in treatment-naïve adults with ADHD [published online October 30, 2014]. J Atten Disord. pii: 1087054714554933.
19. Rapport MD, Chung KM, Shore G, et al. Upgrading the science and technology of assessment and diagnosis: laboratory and clinic-based assessment of children with ADHD. J Clin Child Psychol. 2000;29(4):555-568.
20. Woods SP, Lovejoy DW, Ball JD. Neuropsychological characteristics of adults with ADHD: a comprehensive review of initial studies. Clin Neuropsychol. 2002;16(1):12-34.
21. Lange KW, Hauser J, Lange KM, et al. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2014;6(4):241-248.
22. Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3(4):200-211.
23. Vetter VL Elia J, Erickson, C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [Erratum in: Circulation. 2009;120(7):e55-e59]. Circulation. 2008;117(18):2407-2423.
24. Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639-677.
25. Wu D, Otton SV, Inaba T, et al. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol. 1997;53(11):1605-1612.
26. Vyvanse [package insert]. Lexington, MA: Shire Pharmaceuticals; 2015.
27. Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317-327.
28. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
29. Krishnan SM, Pennick M, Stark JG. Metabolism, distribution and elimination of lisdexamfetamine dimesylate: open-label, single-centre, phase I study in healthy adult volunteers. Clin Drug Invest. 2008;28(12):745-755.
30. Maneeton N, Maneeton B, Suttajit S, et al. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685-1693.
31. Fridman M, Hodgkins P, Kahle JS, et al. Predicted effect size of lisdexamfetamine treatment of attention deficit/hyperactivity disorder (ADHD) in European adults: estimates based on indirect analysis using a systematic review and meta-regression analysis. Eur Psychiatry. 2015;30(4):521-527.
32. Markowitz JS, DeVane CL, Pestreich L, et al. Session 1-87-differentiation of d-, L- and dl-methylphenidate through in vitro pharmacological screening. In: Abstracts: Oral and Poster Presentations of the NCDEU 45th Annual Meeting; June 6-9, 2005; Boca Raton, FL:186.
33. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
34. Teo SK, Stirling DI, Thomas SD, et al. Neurobehavioral effects of racemic threo-methylphenidate and its D and L enantiomers in rats. Pharmacol Biochem Behav. 2003;74(3):747-754.
35. Ding YS, Fowler JS, Volkow ND, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacol (Berl). 1997;131(1):71-78.
36. Davids E, Zhang K, Tarazi FI, et al. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacol (Berl). 2002;160(1):92-98.
37. Srinivas NR, Hubbard JW, Quinn D, et al. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52(5):561-568.
38. Ermer JC, Haffey MB, Doll WJ, et al. Pharmacokinetics of lisdexamfetamine dimesylate after targeted gastrointestinal release or oral administration in healthy adults. Drug Metab Dispos. 2012;40(2):290-297.
39. DeVane CL, Markowitz JS, Carson SW, et al. Single-dose pharmacokinetics of methylphenidate in CYP2D6 extensive and poor metabolizers. J Clin Psychopharmacol. 2000;20(3):347-349.
40. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237.
41. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(7):1149-1152.
42. Shelton Clauson A, Elliott ES, Watson BD, et al. Coadministration of phenelzine and methylphenidate for treatment-resistant depression. Ann Pharmacother. 2004;38(3):508.
43. Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
44. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
45. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20(1):17-37.
46. Goldman W, Seltzer R, Reuman P. Association between treatment with central nervous system stimulants and Raynaud’s syndrome in children: a retrospective case-control study of rheumatology patients. Arthritis Rheum. 2008;58(2):563-566.
47. Syed RH, Moore TL. Methylphenidate and dextroamphetamine-induced peripheral vasculopathy. J Clin Rheum. 2008;14(1):30-33.
48. Wilens TE. Mechanism of action of agents in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):32-38.
49. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
50. Adler LA, Liebowitz M, Kronenberger W, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26(3):212-221.
51. Clemow DB. Suboptimal dosing of Strattera (atomoxetine) for ADHD patients. Postgrad Med. 2014;126(5):196-198.
52. Camporeale A, Porsdal V, De Bruyckere K, et al. Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol. 2015;29(1):3-14.
53. Young JL, Sarkis E, Qiao M, et al. Once-daily treatment with atomoxetine in adults with attention-deficit/hyperactivity disorder: a 24-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2011;34(2):51-60.
54. Bitter I, Angyalosi A, Czobor P. Pharmacological treatment of adult ADHD. Curr Opin Psychiatry. 2012;25(6):529-534.
55. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
56. Ring BJ, Gillespie JS, Eckstein JA, et al. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30(3):319-323.
57. Farid NA, Bergstrom RF, Ziege EA, et al. Single-dose and steady state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol. 1985;25(4):296-301.
58. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35(2-3):99-106.
59. Jasper JR, Lesnick JD, Chang LK, et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol. 1998;55(7):1035-1043.
60. Ruggiero S, Clavenna A, Reale L, et al. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(10):1578-1590.
61. Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99(2):211-216.
62. Uhlén S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202(2):235-243.
63. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21(2):223-228.
64. Butterfield ME, Saal J, Young B, et al. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res. 2016;236:136-141.
65. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29(4):617-625.
66. Cooper BR, Wang CM, Cox RF. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11(2):133-141.
67. Reimherr FW, Hedges DW, Strong RE, et al. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245-251.
68. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
69. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
70. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
71. Bond DJ, Hadjipavlou G, Lam RW, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2012;24(1):23-37.
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systemic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716-723.
4. Faraone S, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159-165.
5. Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5(3):143-154.
6. Halmøy A, Fasmer OB, Gillberg C, et al. Occupational outcome in adult ADHD: impact of symptom profile, comorbid psychiatric problems, and treatment: a cross-sectional study of 414 clinically diagnosed adult ADHD patients. J Atten Disord. 2009;13(2):175-187.
7. Kuriyan AB, Pelham WE Jr, Molina BS, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27-41.
8. Murphy K, Barkley RA. Attention deficit hyperactivity disorder in adults: comorbidities and adaptive impairment. Compr Psychiatry. 1996;37(6):393-401.
9. Mannuzza S, Klein RG, Mouton JL 3rd. Lifetime criminality among boys with attention deficit hyperactivity disorder: a prospective follow-up study into adulthood using official arrest records. Psychiatry Res. 2008;160(3):237-246.
10. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
11. Barbaresi WJ, Colligan RC, Weaver AL, et al. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131(4):637-644.
12. Babcock T, Ornstein CS. Comorbidity and its impact in adult patients with attention-deficit/hyperactivity disorder: a primary care perspective. Postgrad Med. 2009;121(3):73-82.
13. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013:59-66.
14. Attention-deficit/hyperactivity disorder. In: Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000:78-85.
15. Kooij JJS. Adult ADHD: diagnostic assessment and treatment. 3rd ed. Amsterdam, Netherlands: Springer; 2013:34.
16. Faraone SV, Khan SA. Candidate gene studies of attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):13-20.
17. Neale BM, Medland SE, Ripke S, et al; Psychiatric GWAS Consortium: ADHD Subgroup. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884-897.
18. Milioni AL, Chaim TM, Cavallet M, et al. High IQ may “mask” the diagnosis of ADHD by compensating for deficits in executive functions in treatment-naïve adults with ADHD [published online October 30, 2014]. J Atten Disord. pii: 1087054714554933.
19. Rapport MD, Chung KM, Shore G, et al. Upgrading the science and technology of assessment and diagnosis: laboratory and clinic-based assessment of children with ADHD. J Clin Child Psychol. 2000;29(4):555-568.
20. Woods SP, Lovejoy DW, Ball JD. Neuropsychological characteristics of adults with ADHD: a comprehensive review of initial studies. Clin Neuropsychol. 2002;16(1):12-34.
21. Lange KW, Hauser J, Lange KM, et al. Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2014;6(4):241-248.
22. Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord. 2000;3(4):200-211.
23. Vetter VL Elia J, Erickson, C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing [Erratum in: Circulation. 2009;120(7):e55-e59]. Circulation. 2008;117(18):2407-2423.
24. Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639-677.
25. Wu D, Otton SV, Inaba T, et al. Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol. 1997;53(11):1605-1612.
26. Vyvanse [package insert]. Lexington, MA: Shire Pharmaceuticals; 2015.
27. Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317-327.
28. Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present—a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479-496.
29. Krishnan SM, Pennick M, Stark JG. Metabolism, distribution and elimination of lisdexamfetamine dimesylate: open-label, single-centre, phase I study in healthy adult volunteers. Clin Drug Invest. 2008;28(12):745-755.
30. Maneeton N, Maneeton B, Suttajit S, et al. Exploratory meta-analysis on lisdexamfetamine versus placebo in adult ADHD. Drug Des Devel Ther. 2014;8:1685-1693.
31. Fridman M, Hodgkins P, Kahle JS, et al. Predicted effect size of lisdexamfetamine treatment of attention deficit/hyperactivity disorder (ADHD) in European adults: estimates based on indirect analysis using a systematic review and meta-regression analysis. Eur Psychiatry. 2015;30(4):521-527.
32. Markowitz JS, DeVane CL, Pestreich L, et al. Session 1-87-differentiation of d-, L- and dl-methylphenidate through in vitro pharmacological screening. In: Abstracts: Oral and Poster Presentations of the NCDEU 45th Annual Meeting; June 6-9, 2005; Boca Raton, FL:186.
33. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
34. Teo SK, Stirling DI, Thomas SD, et al. Neurobehavioral effects of racemic threo-methylphenidate and its D and L enantiomers in rats. Pharmacol Biochem Behav. 2003;74(3):747-754.
35. Ding YS, Fowler JS, Volkow ND, et al. Chiral drugs: comparison of the pharmacokinetics of [11C]d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacol (Berl). 1997;131(1):71-78.
36. Davids E, Zhang K, Tarazi FI, et al. Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning. Psychopharmacol (Berl). 2002;160(1):92-98.
37. Srinivas NR, Hubbard JW, Quinn D, et al. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther. 1992;52(5):561-568.
38. Ermer JC, Haffey MB, Doll WJ, et al. Pharmacokinetics of lisdexamfetamine dimesylate after targeted gastrointestinal release or oral administration in healthy adults. Drug Metab Dispos. 2012;40(2):290-297.
39. DeVane CL, Markowitz JS, Carson SW, et al. Single-dose pharmacokinetics of methylphenidate in CYP2D6 extensive and poor metabolizers. J Clin Psychopharmacol. 2000;20(3):347-349.
40. Graham J, Coghill D. Adverse effects of pharmacotherapies for attention-deficit hyperactivity disorder: epidemiology, prevention and management. CNS Drugs. 2008;22(3):213-237.
41. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(7):1149-1152.
42. Shelton Clauson A, Elliott ES, Watson BD, et al. Coadministration of phenelzine and methylphenidate for treatment-resistant depression. Ann Pharmacother. 2004;38(3):508.
43. Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
44. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
45. Graham J, Banaschewski T, Buitelaar J, et al; European Guidelines Group. European guidelines on managing adverse effects of medication for ADHD. Eur Child Adolesc Psychiatry. 2011;20(1):17-37.
46. Goldman W, Seltzer R, Reuman P. Association between treatment with central nervous system stimulants and Raynaud’s syndrome in children: a retrospective case-control study of rheumatology patients. Arthritis Rheum. 2008;58(2):563-566.
47. Syed RH, Moore TL. Methylphenidate and dextroamphetamine-induced peripheral vasculopathy. J Clin Rheum. 2008;14(1):30-33.
48. Wilens TE. Mechanism of action of agents in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(suppl 8):32-38.
49. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
50. Adler LA, Liebowitz M, Kronenberger W, et al. Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety. 2009;26(3):212-221.
51. Clemow DB. Suboptimal dosing of Strattera (atomoxetine) for ADHD patients. Postgrad Med. 2014;126(5):196-198.
52. Camporeale A, Porsdal V, De Bruyckere K, et al. Safety and tolerability of atomoxetine in treatment of attention deficit hyperactivity disorder in adult patients: an integrated analysis of 15 clinical trials. J Psychopharmacol. 2015;29(1):3-14.
53. Young JL, Sarkis E, Qiao M, et al. Once-daily treatment with atomoxetine in adults with attention-deficit/hyperactivity disorder: a 24-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2011;34(2):51-60.
54. Bitter I, Angyalosi A, Czobor P. Pharmacological treatment of adult ADHD. Curr Opin Psychiatry. 2012;25(6):529-534.
55. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
56. Ring BJ, Gillespie JS, Eckstein JA, et al. Identification of the human cytochromes P450 responsible for atomoxetine metabolism. Drug Metab Dispos. 2002;30(3):319-323.
57. Farid NA, Bergstrom RF, Ziege EA, et al. Single-dose and steady state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol. 1985;25(4):296-301.
58. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003;35(2-3):99-106.
59. Jasper JR, Lesnick JD, Chang LK, et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol. 1998;55(7):1035-1043.
60. Ruggiero S, Clavenna A, Reale L, et al. Guanfacine for attention deficit and hyperactivity disorder in pediatrics: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(10):1578-1590.
61. Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99(2):211-216.
62. Uhlén S, Wikberg JE. Delineation of rat kidney alpha 2A- and alpha 2B-adrenoceptors with [3H]RX821002 radioligand binding: computer modelling reveals that guanfacine is an alpha 2A-selective compound. Eur J Pharmacol. 1991;202(2):235-243.
63. Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21(2):223-228.
64. Butterfield ME, Saal J, Young B, et al. Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res. 2016;236:136-141.
65. Swearingen D, Pennick M, Shojaei A, et al. A phase I, randomized, open-label, crossover study of the single-dose pharmacokinetic properties of guanfacine extended-release 1-, 2-, and 4-mg tablets in healthy adults. Clin Ther. 2007;29(4):617-625.
66. Cooper BR, Wang CM, Cox RF. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11(2):133-141.
67. Reimherr FW, Hedges DW, Strong RE, et al. Bupropion SR in adults with ADHD: a short-term, placebo-controlled trial. Neuropsychiatr Dis Treat. 2005;1(3):245-251.
68. Maneeton N, Maneeton B, Srisurapanont M, et al. Bupropion for adults with attention-deficit hyperactivity disorder: meta-analysis of randomized, placebo-controlled trials. Psychiatry Clin Neurosci. 2011;65(7):611-617.
69. Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo controlled study of desipramine in the treatment of ADD: I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28(5):777-784.
70. Spencer T, Biederman J, Wilens T, et al. Nortriptyline treatment of children with attention-deficit hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32(1):205-210.
71. Bond DJ, Hadjipavlou G, Lam RW, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2012;24(1):23-37.
Risks of increasingly potent Cannabis: The joint effects of potency and frequency
In the United States, the average potency of Cannabis has increased significantly over the past few decades in response to consumer demand and policies in some states that have legalized marijuana for medicinal and recreational purposes.1 Whereas the delta-9-tetrahydrocannabinol (THC) content of “street” marijuana was <1% in the 1970s and 4% in the 1990s, by 2012, analyses of Cannabis samples seized by law enforcement agencies documented a rise in average THC potency to >12%.1-3
Although this increase in potency has been overstated in the media because studies did not control for the effects of changes in sampling methods on freshness, it is estimated that Cannabis potency increased 7-fold from 1970 to 2010.3 Also, Cannabis preparations such as hashish and hash oil extracts containing THC well above average—from 35% to 90% THC—are now more widely available. In states where marijuana has been legalized, high-potency Cannabis (HPC) in the form of “edibles” (eg, marijuana added to baked goods, candy, or drinks) and hash oil extracts (Table 1)4-13 can be readily obtained from dispensaries or even at local farmers’ markets.
The potency of Cannabis, typically defined as the percentage of THC, its chief psychoactive component, varies depending on the genetic strain of the plant, cultivation techniques, and methods of processing and storage. For example, relative to “average marijuana,” hemp (Cannabis bred for industrial purposes) has very little THC, while sinsemilla (flowering buds from unpollinated female plants), hashish (Cannabis resin), and extracted hash oil contain increasing amounts of THC (Table 2).1,2
As THC levels in Cannabis have risen over time, cannabidiol (CBD) levels have dropped to <0.2%.2 Although THC appears to be largely responsible for the psychiatric morbidity associated with Cannabis, CBD may have neuroprotective and antipsychotic properties.14,15 The sharp spike in the THC:CBD ratio in recent years therefore raises the possibility that Cannabis use today might carry a much greater risk of psychiatric sequelae than it did in previous generations.
This article reviews the evidence for an increased risk of psychiatric morbidity with increasing Cannabis potency.
Cannabis use disorder
Recent data indicate that the prevalence of Cannabis use disorders (eg, abuse and dependence) in the United States is approximately 3% among the general population and >30% among Cannabis users.16 The availability of increasingly potent forms of Cannabis has been cited as a possible explanation for this rise, despite no change in the prevalence of overall marijuana use between 1991 to 1992 and 2001 to 2002.17 However, while the prevalence of marijuana use disorders has continued to rise—nearly doubling from 2001 to 2002 to 2012 to 2013—this latest increase occurred with a significant increase in overall marijuana use, such that the actual rate of Cannabis use disorders among users seems to have plateaued, despite the continued rise in marijuana potency.16 This discrepancy could be explained if Cannabis users cut back past a specific threshold of increasing potency. However, 2 studies have called into question how effective such titration efforts might be in practice. In one study, Cannabis users who preferred more potent Cannabis inhaled lower volumes of smoke, but did not fully compensate for the increased potency, such that use of HPC still resulted in greater THC exposure.18 Another study found that HPC users rolled less marijuana into their joints but not enough to mitigate the impact of greater potency.19 Therefore, it appears that HPC users typically expose themselves to greater amounts of THC, which could place them at higher risk of addiction.
Although a causal association between increasing Cannabis potency and the rate of substance use disorders among users remains unclear based on epidemiologic studies from the United States, a recent study from the United Kingdom examined the impact of Cannabis potency on dependence.20 This cross-sectional survey found that, although HPC was preferred by users and was rated as offering the “best high,” its use was associated with increasing severity of dependence, especially among young people. The limited available evidence supports a greater risk of Cannabis use disorders with increasing potency.
Psychosis
Based on longitudinal studies published over the past 30 years, it is clear that using Cannabis at a young age (age <15 to 18) increases the risk of developing a psychotic disorder.21 This association appears to be dose-dependent, with studies consistently demonstrating that psychosis risk increases with greater frequency of Cannabis use.22 The accumulated evidence to date is strong enough to view the psychotic potential of Cannabis as a significant public health concern.21
If risk of psychosis is proportional to the amount of Cannabis used as measured by frequency, it follows that this risk might be affected similarly by Cannabis potency. In another paper, I discussed the potential for greater risk of psychosis in the context of medical marijuana and synthetic cannabinoids.23 My colleagues and I also have published case reports describing emerging psychosis among regular Cannabis users after escalating to higher potency medical marijuana24 and a hyperconcentrated form of hash oil known as Cannabis “wax” or “dabs” that contains as much as 90% THC.4 Preliminary anecdotal evidence supports the plausibility of HPC being more psychotoxic than less potent forms.
Several studies from a research group in the United Kingdom, where sinsemilla has increasingly dominated the drug market, likewise have reported that the use of HPC is associated with a greater risk of psychosis. The first of these studies, published in 2009, found that adults hospitalized for first-episode psychosis were more likely to have used HPC than healthy controls.25 Among Cannabis users, HPC use was associated with a 7-fold increased risk of psychosis, with daily HPC use associated with a 12-fold increased risk.
Based on a larger dataset, a second study reported that high-potency, but not low-potency, Cannabis increased the risk of first-episode psychosis with increasing frequency of use.26 Daily users of HPC had a 5-fold higher risk of psychosis compared with those that had never used Cannabis. A third study reported that HPC use and daily Cannabis use were independently associated with an earlier onset of first-episode psychosis, with daily HPC users developing first-episode psychosis an average of 6 years earlier than non-Cannabis users.27 Finally, a prospective study following patients with first-episode psychosis over 2 years found that the greatest risk of relapse—defined by hospital admission caused by exacerbation of psychotic symptoms—was found among self-reported daily users of HPC, while the lowest risk was among those who stopped using Cannabis after their initial psychotic episode.28
The findings from these 4 studies suggest that the increased risk of psychosis with Cannabis is proportional to overall exposure, determined by both frequency of use and Cannabis potency.
Cognition
There is little doubt that using Cannabis can impair cognition acutely, “after all, this is the basic reason for its recreational use,” as one author wrote.29 As with psychosis, the available evidence indicates that the degree of cognitive impairment is related to the frequency and duration of Cannabis use as well as age of onset of use.30,31
Few studies have assessed cognitive functioning in relation to Cannabis potency with most only examining the effects of relatively low-potency Cannabis with inconsistent results. For example, 2 studies compared cognitive performance in individuals smoking Cannabis with 1.8% and 3.9% THC. One study found that using higher potency Cannabis resulted in prolonged time needed to complete certain cognitive tasks,32 whereas the other found greater impairment in performance on a decision-making task at both potencies compared with non-users but no differences between the 2 dosages.33 Detecting significant differences may be difficult within the narrow range of low Cannabis potency studied where any findings have limited applicability in the context of today’s Cannabis with much higher THC content.
To date, only 1 study has assessed cognition at higher Cannabis potencies, comparing Cannabis with 4% THC to 13% THC.34 Cognitive impairments increased with higher potency, especially in tasks that measured motor control and executive functioning. Therefore it appears that higher potency Cannabis use is associated with greater acute cognitive impairment.
The longer-term effects on cognition are less clear, with conflicting evidence about whether Cannabis use can result in residual cognitive impairment despite abstinence.30,35 A recent review concluded that “the magnitude of neuropsychological impairment and the extent to which it persists after abstinence may depend on the frequency and the duration of Cannabis use, length of abstinence, and age at onset of use.”31 The effects of HPC on long-term cognitive deficits have not been studied.
Structural brain changes
A number of studies have determined an association between Cannabis use and brain changes involving structures governing memory and emotional processing, including reduced volume of the hippocampus,36 temporal cortex, insula, and orbitofrontal cortex.37 Although many of these changes appear to be dose-related, some morphologic changes have been reported among young recreational users without Cannabis dependence.38 This has resulted in an understandable concern about the effects of Cannabis on the brains of young people with limited exposure; however, it is not yet clear to what extent detected brain changes are pathological and reflect functional deficits.
Recent research using newer neuroimaging modalities provides preliminary support of Cannabis use associated with white matter changes that, in turn, are correlated with cognitive impairment.39 One study comparing low-potency Cannabis and HPC users with and without first-episode psychosis found a significant effect of Cannabis potency on disturbances in white matter microstructural organization in the corpus callosum.40 These findings provide sufficient cause for concern that structural brain changes associated with cognitive impairment are more likely to occur with HPC use.
Recommendations for clinicians
Similar to any drug, the effects of THC and its psychiatric sequelae can be expected to increase with dosage. To date, much of the information about psychiatric risks has been based on studies of low- and moderate-potency Cannabis rather than the much higher potency Cannabis products, such as hyper-concentrated “wax dabs,” that are available today. Data from social media suggest that these products may be associated with novel patterns of use, such as with the intention of “passing out.”41 It is likely that clinicians will encounter greater psychiatric morbidity associated with HPC use.
Although clinicians may be accustomed to asking about the frequency and duration of Cannabis use, it is now prudent also to ask patients about Cannabis potency to better assess the potential risks of use. The potency of different marijuana products is openly advertised within some “medical marijuana” dispensaries, although the accuracy of information in products such as “edibles” has been called into question.5
Physicians are increasingly asked to provide recommendations on “medical marijuana” use. A recent paper outlined characteristics of appropriate candidates for “medical marijuana” including:
- having a debilitating condition that might benefit from Cannabis
- multiple failed trials of conventional pharmacotherapies including FDA-approved cannabinoids
- lack of substance use disorders, psychosis, or unstable mood or anxiety disorders
- residence in a state where “medical marijuana” is legal.42
As part of the informed consent process, physicians providing recommendations for “medical marijuana” now must consider the effects of HPC when weighing potential risks against any benefits of Cannabis use. Those monitoring patients using Cannabis should be aware of the potential for greater psychiatric morbidity with HPC and should educate patients about that risk. Failure to adequately warn patients about such morbidity or to screen for risk factors such as psychosis could leave physicians vulnerable to malpractice litigation.
In the United States, the average potency of Cannabis has increased significantly over the past few decades in response to consumer demand and policies in some states that have legalized marijuana for medicinal and recreational purposes.1 Whereas the delta-9-tetrahydrocannabinol (THC) content of “street” marijuana was <1% in the 1970s and 4% in the 1990s, by 2012, analyses of Cannabis samples seized by law enforcement agencies documented a rise in average THC potency to >12%.1-3
Although this increase in potency has been overstated in the media because studies did not control for the effects of changes in sampling methods on freshness, it is estimated that Cannabis potency increased 7-fold from 1970 to 2010.3 Also, Cannabis preparations such as hashish and hash oil extracts containing THC well above average—from 35% to 90% THC—are now more widely available. In states where marijuana has been legalized, high-potency Cannabis (HPC) in the form of “edibles” (eg, marijuana added to baked goods, candy, or drinks) and hash oil extracts (Table 1)4-13 can be readily obtained from dispensaries or even at local farmers’ markets.

The potency of Cannabis, typically defined as the percentage of THC, its chief psychoactive component, varies depending on the genetic strain of the plant, cultivation techniques, and methods of processing and storage. For example, relative to “average marijuana,” hemp (Cannabis bred for industrial purposes) has very little THC, while sinsemilla (flowering buds from unpollinated female plants), hashish (Cannabis resin), and extracted hash oil contain increasing amounts of THC (Table 2).1,2
As THC levels in Cannabis have risen over time, cannabidiol (CBD) levels have dropped to <0.2%.2 Although THC appears to be largely responsible for the psychiatric morbidity associated with Cannabis, CBD may have neuroprotective and antipsychotic properties.14,15 The sharp spike in the THC:CBD ratio in recent years therefore raises the possibility that Cannabis use today might carry a much greater risk of psychiatric sequelae than it did in previous generations.
This article reviews the evidence for an increased risk of psychiatric morbidity with increasing Cannabis potency.
Cannabis use disorder
Recent data indicate that the prevalence of Cannabis use disorders (eg, abuse and dependence) in the United States is approximately 3% among the general population and >30% among Cannabis users.16 The availability of increasingly potent forms of Cannabis has been cited as a possible explanation for this rise, despite no change in the prevalence of overall marijuana use between 1991 to 1992 and 2001 to 2002.17 However, while the prevalence of marijuana use disorders has continued to rise—nearly doubling from 2001 to 2002 to 2012 to 2013—this latest increase occurred with a significant increase in overall marijuana use, such that the actual rate of Cannabis use disorders among users seems to have plateaued, despite the continued rise in marijuana potency.16 This discrepancy could be explained if Cannabis users cut back past a specific threshold of increasing potency. However, 2 studies have called into question how effective such titration efforts might be in practice. In one study, Cannabis users who preferred more potent Cannabis inhaled lower volumes of smoke, but did not fully compensate for the increased potency, such that use of HPC still resulted in greater THC exposure.18 Another study found that HPC users rolled less marijuana into their joints but not enough to mitigate the impact of greater potency.19 Therefore, it appears that HPC users typically expose themselves to greater amounts of THC, which could place them at higher risk of addiction.
Although a causal association between increasing Cannabis potency and the rate of substance use disorders among users remains unclear based on epidemiologic studies from the United States, a recent study from the United Kingdom examined the impact of Cannabis potency on dependence.20 This cross-sectional survey found that, although HPC was preferred by users and was rated as offering the “best high,” its use was associated with increasing severity of dependence, especially among young people. The limited available evidence supports a greater risk of Cannabis use disorders with increasing potency.
Psychosis
Based on longitudinal studies published over the past 30 years, it is clear that using Cannabis at a young age (age <15 to 18) increases the risk of developing a psychotic disorder.21 This association appears to be dose-dependent, with studies consistently demonstrating that psychosis risk increases with greater frequency of Cannabis use.22 The accumulated evidence to date is strong enough to view the psychotic potential of Cannabis as a significant public health concern.21
If risk of psychosis is proportional to the amount of Cannabis used as measured by frequency, it follows that this risk might be affected similarly by Cannabis potency. In another paper, I discussed the potential for greater risk of psychosis in the context of medical marijuana and synthetic cannabinoids.23 My colleagues and I also have published case reports describing emerging psychosis among regular Cannabis users after escalating to higher potency medical marijuana24 and a hyperconcentrated form of hash oil known as Cannabis “wax” or “dabs” that contains as much as 90% THC.4 Preliminary anecdotal evidence supports the plausibility of HPC being more psychotoxic than less potent forms.
Several studies from a research group in the United Kingdom, where sinsemilla has increasingly dominated the drug market, likewise have reported that the use of HPC is associated with a greater risk of psychosis. The first of these studies, published in 2009, found that adults hospitalized for first-episode psychosis were more likely to have used HPC than healthy controls.25 Among Cannabis users, HPC use was associated with a 7-fold increased risk of psychosis, with daily HPC use associated with a 12-fold increased risk.
Based on a larger dataset, a second study reported that high-potency, but not low-potency, Cannabis increased the risk of first-episode psychosis with increasing frequency of use.26 Daily users of HPC had a 5-fold higher risk of psychosis compared with those that had never used Cannabis. A third study reported that HPC use and daily Cannabis use were independently associated with an earlier onset of first-episode psychosis, with daily HPC users developing first-episode psychosis an average of 6 years earlier than non-Cannabis users.27 Finally, a prospective study following patients with first-episode psychosis over 2 years found that the greatest risk of relapse—defined by hospital admission caused by exacerbation of psychotic symptoms—was found among self-reported daily users of HPC, while the lowest risk was among those who stopped using Cannabis after their initial psychotic episode.28
The findings from these 4 studies suggest that the increased risk of psychosis with Cannabis is proportional to overall exposure, determined by both frequency of use and Cannabis potency.
Cognition
There is little doubt that using Cannabis can impair cognition acutely, “after all, this is the basic reason for its recreational use,” as one author wrote.29 As with psychosis, the available evidence indicates that the degree of cognitive impairment is related to the frequency and duration of Cannabis use as well as age of onset of use.30,31
Few studies have assessed cognitive functioning in relation to Cannabis potency with most only examining the effects of relatively low-potency Cannabis with inconsistent results. For example, 2 studies compared cognitive performance in individuals smoking Cannabis with 1.8% and 3.9% THC. One study found that using higher potency Cannabis resulted in prolonged time needed to complete certain cognitive tasks,32 whereas the other found greater impairment in performance on a decision-making task at both potencies compared with non-users but no differences between the 2 dosages.33 Detecting significant differences may be difficult within the narrow range of low Cannabis potency studied where any findings have limited applicability in the context of today’s Cannabis with much higher THC content.
To date, only 1 study has assessed cognition at higher Cannabis potencies, comparing Cannabis with 4% THC to 13% THC.34 Cognitive impairments increased with higher potency, especially in tasks that measured motor control and executive functioning. Therefore it appears that higher potency Cannabis use is associated with greater acute cognitive impairment.
The longer-term effects on cognition are less clear, with conflicting evidence about whether Cannabis use can result in residual cognitive impairment despite abstinence.30,35 A recent review concluded that “the magnitude of neuropsychological impairment and the extent to which it persists after abstinence may depend on the frequency and the duration of Cannabis use, length of abstinence, and age at onset of use.”31 The effects of HPC on long-term cognitive deficits have not been studied.
Structural brain changes
A number of studies have determined an association between Cannabis use and brain changes involving structures governing memory and emotional processing, including reduced volume of the hippocampus,36 temporal cortex, insula, and orbitofrontal cortex.37 Although many of these changes appear to be dose-related, some morphologic changes have been reported among young recreational users without Cannabis dependence.38 This has resulted in an understandable concern about the effects of Cannabis on the brains of young people with limited exposure; however, it is not yet clear to what extent detected brain changes are pathological and reflect functional deficits.
Recent research using newer neuroimaging modalities provides preliminary support of Cannabis use associated with white matter changes that, in turn, are correlated with cognitive impairment.39 One study comparing low-potency Cannabis and HPC users with and without first-episode psychosis found a significant effect of Cannabis potency on disturbances in white matter microstructural organization in the corpus callosum.40 These findings provide sufficient cause for concern that structural brain changes associated with cognitive impairment are more likely to occur with HPC use.
Recommendations for clinicians
Similar to any drug, the effects of THC and its psychiatric sequelae can be expected to increase with dosage. To date, much of the information about psychiatric risks has been based on studies of low- and moderate-potency Cannabis rather than the much higher potency Cannabis products, such as hyper-concentrated “wax dabs,” that are available today. Data from social media suggest that these products may be associated with novel patterns of use, such as with the intention of “passing out.”41 It is likely that clinicians will encounter greater psychiatric morbidity associated with HPC use.
Although clinicians may be accustomed to asking about the frequency and duration of Cannabis use, it is now prudent also to ask patients about Cannabis potency to better assess the potential risks of use. The potency of different marijuana products is openly advertised within some “medical marijuana” dispensaries, although the accuracy of information in products such as “edibles” has been called into question.5
Physicians are increasingly asked to provide recommendations on “medical marijuana” use. A recent paper outlined characteristics of appropriate candidates for “medical marijuana” including:
- having a debilitating condition that might benefit from Cannabis
- multiple failed trials of conventional pharmacotherapies including FDA-approved cannabinoids
- lack of substance use disorders, psychosis, or unstable mood or anxiety disorders
- residence in a state where “medical marijuana” is legal.42
As part of the informed consent process, physicians providing recommendations for “medical marijuana” now must consider the effects of HPC when weighing potential risks against any benefits of Cannabis use. Those monitoring patients using Cannabis should be aware of the potential for greater psychiatric morbidity with HPC and should educate patients about that risk. Failure to adequately warn patients about such morbidity or to screen for risk factors such as psychosis could leave physicians vulnerable to malpractice litigation.
In the United States, the average potency of Cannabis has increased significantly over the past few decades in response to consumer demand and policies in some states that have legalized marijuana for medicinal and recreational purposes.1 Whereas the delta-9-tetrahydrocannabinol (THC) content of “street” marijuana was <1% in the 1970s and 4% in the 1990s, by 2012, analyses of Cannabis samples seized by law enforcement agencies documented a rise in average THC potency to >12%.1-3
Although this increase in potency has been overstated in the media because studies did not control for the effects of changes in sampling methods on freshness, it is estimated that Cannabis potency increased 7-fold from 1970 to 2010.3 Also, Cannabis preparations such as hashish and hash oil extracts containing THC well above average—from 35% to 90% THC—are now more widely available. In states where marijuana has been legalized, high-potency Cannabis (HPC) in the form of “edibles” (eg, marijuana added to baked goods, candy, or drinks) and hash oil extracts (Table 1)4-13 can be readily obtained from dispensaries or even at local farmers’ markets.

The potency of Cannabis, typically defined as the percentage of THC, its chief psychoactive component, varies depending on the genetic strain of the plant, cultivation techniques, and methods of processing and storage. For example, relative to “average marijuana,” hemp (Cannabis bred for industrial purposes) has very little THC, while sinsemilla (flowering buds from unpollinated female plants), hashish (Cannabis resin), and extracted hash oil contain increasing amounts of THC (Table 2).1,2
As THC levels in Cannabis have risen over time, cannabidiol (CBD) levels have dropped to <0.2%.2 Although THC appears to be largely responsible for the psychiatric morbidity associated with Cannabis, CBD may have neuroprotective and antipsychotic properties.14,15 The sharp spike in the THC:CBD ratio in recent years therefore raises the possibility that Cannabis use today might carry a much greater risk of psychiatric sequelae than it did in previous generations.
This article reviews the evidence for an increased risk of psychiatric morbidity with increasing Cannabis potency.
Cannabis use disorder
Recent data indicate that the prevalence of Cannabis use disorders (eg, abuse and dependence) in the United States is approximately 3% among the general population and >30% among Cannabis users.16 The availability of increasingly potent forms of Cannabis has been cited as a possible explanation for this rise, despite no change in the prevalence of overall marijuana use between 1991 to 1992 and 2001 to 2002.17 However, while the prevalence of marijuana use disorders has continued to rise—nearly doubling from 2001 to 2002 to 2012 to 2013—this latest increase occurred with a significant increase in overall marijuana use, such that the actual rate of Cannabis use disorders among users seems to have plateaued, despite the continued rise in marijuana potency.16 This discrepancy could be explained if Cannabis users cut back past a specific threshold of increasing potency. However, 2 studies have called into question how effective such titration efforts might be in practice. In one study, Cannabis users who preferred more potent Cannabis inhaled lower volumes of smoke, but did not fully compensate for the increased potency, such that use of HPC still resulted in greater THC exposure.18 Another study found that HPC users rolled less marijuana into their joints but not enough to mitigate the impact of greater potency.19 Therefore, it appears that HPC users typically expose themselves to greater amounts of THC, which could place them at higher risk of addiction.
Although a causal association between increasing Cannabis potency and the rate of substance use disorders among users remains unclear based on epidemiologic studies from the United States, a recent study from the United Kingdom examined the impact of Cannabis potency on dependence.20 This cross-sectional survey found that, although HPC was preferred by users and was rated as offering the “best high,” its use was associated with increasing severity of dependence, especially among young people. The limited available evidence supports a greater risk of Cannabis use disorders with increasing potency.
Psychosis
Based on longitudinal studies published over the past 30 years, it is clear that using Cannabis at a young age (age <15 to 18) increases the risk of developing a psychotic disorder.21 This association appears to be dose-dependent, with studies consistently demonstrating that psychosis risk increases with greater frequency of Cannabis use.22 The accumulated evidence to date is strong enough to view the psychotic potential of Cannabis as a significant public health concern.21
If risk of psychosis is proportional to the amount of Cannabis used as measured by frequency, it follows that this risk might be affected similarly by Cannabis potency. In another paper, I discussed the potential for greater risk of psychosis in the context of medical marijuana and synthetic cannabinoids.23 My colleagues and I also have published case reports describing emerging psychosis among regular Cannabis users after escalating to higher potency medical marijuana24 and a hyperconcentrated form of hash oil known as Cannabis “wax” or “dabs” that contains as much as 90% THC.4 Preliminary anecdotal evidence supports the plausibility of HPC being more psychotoxic than less potent forms.
Several studies from a research group in the United Kingdom, where sinsemilla has increasingly dominated the drug market, likewise have reported that the use of HPC is associated with a greater risk of psychosis. The first of these studies, published in 2009, found that adults hospitalized for first-episode psychosis were more likely to have used HPC than healthy controls.25 Among Cannabis users, HPC use was associated with a 7-fold increased risk of psychosis, with daily HPC use associated with a 12-fold increased risk.
Based on a larger dataset, a second study reported that high-potency, but not low-potency, Cannabis increased the risk of first-episode psychosis with increasing frequency of use.26 Daily users of HPC had a 5-fold higher risk of psychosis compared with those that had never used Cannabis. A third study reported that HPC use and daily Cannabis use were independently associated with an earlier onset of first-episode psychosis, with daily HPC users developing first-episode psychosis an average of 6 years earlier than non-Cannabis users.27 Finally, a prospective study following patients with first-episode psychosis over 2 years found that the greatest risk of relapse—defined by hospital admission caused by exacerbation of psychotic symptoms—was found among self-reported daily users of HPC, while the lowest risk was among those who stopped using Cannabis after their initial psychotic episode.28
The findings from these 4 studies suggest that the increased risk of psychosis with Cannabis is proportional to overall exposure, determined by both frequency of use and Cannabis potency.
Cognition
There is little doubt that using Cannabis can impair cognition acutely, “after all, this is the basic reason for its recreational use,” as one author wrote.29 As with psychosis, the available evidence indicates that the degree of cognitive impairment is related to the frequency and duration of Cannabis use as well as age of onset of use.30,31
Few studies have assessed cognitive functioning in relation to Cannabis potency with most only examining the effects of relatively low-potency Cannabis with inconsistent results. For example, 2 studies compared cognitive performance in individuals smoking Cannabis with 1.8% and 3.9% THC. One study found that using higher potency Cannabis resulted in prolonged time needed to complete certain cognitive tasks,32 whereas the other found greater impairment in performance on a decision-making task at both potencies compared with non-users but no differences between the 2 dosages.33 Detecting significant differences may be difficult within the narrow range of low Cannabis potency studied where any findings have limited applicability in the context of today’s Cannabis with much higher THC content.
To date, only 1 study has assessed cognition at higher Cannabis potencies, comparing Cannabis with 4% THC to 13% THC.34 Cognitive impairments increased with higher potency, especially in tasks that measured motor control and executive functioning. Therefore it appears that higher potency Cannabis use is associated with greater acute cognitive impairment.
The longer-term effects on cognition are less clear, with conflicting evidence about whether Cannabis use can result in residual cognitive impairment despite abstinence.30,35 A recent review concluded that “the magnitude of neuropsychological impairment and the extent to which it persists after abstinence may depend on the frequency and the duration of Cannabis use, length of abstinence, and age at onset of use.”31 The effects of HPC on long-term cognitive deficits have not been studied.
Structural brain changes
A number of studies have determined an association between Cannabis use and brain changes involving structures governing memory and emotional processing, including reduced volume of the hippocampus,36 temporal cortex, insula, and orbitofrontal cortex.37 Although many of these changes appear to be dose-related, some morphologic changes have been reported among young recreational users without Cannabis dependence.38 This has resulted in an understandable concern about the effects of Cannabis on the brains of young people with limited exposure; however, it is not yet clear to what extent detected brain changes are pathological and reflect functional deficits.
Recent research using newer neuroimaging modalities provides preliminary support of Cannabis use associated with white matter changes that, in turn, are correlated with cognitive impairment.39 One study comparing low-potency Cannabis and HPC users with and without first-episode psychosis found a significant effect of Cannabis potency on disturbances in white matter microstructural organization in the corpus callosum.40 These findings provide sufficient cause for concern that structural brain changes associated with cognitive impairment are more likely to occur with HPC use.
Recommendations for clinicians
Similar to any drug, the effects of THC and its psychiatric sequelae can be expected to increase with dosage. To date, much of the information about psychiatric risks has been based on studies of low- and moderate-potency Cannabis rather than the much higher potency Cannabis products, such as hyper-concentrated “wax dabs,” that are available today. Data from social media suggest that these products may be associated with novel patterns of use, such as with the intention of “passing out.”41 It is likely that clinicians will encounter greater psychiatric morbidity associated with HPC use.
Although clinicians may be accustomed to asking about the frequency and duration of Cannabis use, it is now prudent also to ask patients about Cannabis potency to better assess the potential risks of use. The potency of different marijuana products is openly advertised within some “medical marijuana” dispensaries, although the accuracy of information in products such as “edibles” has been called into question.5
Physicians are increasingly asked to provide recommendations on “medical marijuana” use. A recent paper outlined characteristics of appropriate candidates for “medical marijuana” including:
- having a debilitating condition that might benefit from Cannabis
- multiple failed trials of conventional pharmacotherapies including FDA-approved cannabinoids
- lack of substance use disorders, psychosis, or unstable mood or anxiety disorders
- residence in a state where “medical marijuana” is legal.42
As part of the informed consent process, physicians providing recommendations for “medical marijuana” now must consider the effects of HPC when weighing potential risks against any benefits of Cannabis use. Those monitoring patients using Cannabis should be aware of the potential for greater psychiatric morbidity with HPC and should educate patients about that risk. Failure to adequately warn patients about such morbidity or to screen for risk factors such as psychosis could leave physicians vulnerable to malpractice litigation.
Using rating scales in a clinical setting: A guide for psychiatrists
In the current health care environment, there is an increasing demand for objective assessment of disease states.1 This is particularly apparent in psychiatry, where documentation of outcomes lags that of other areas of medicine.
In 2012, the additional health care costs incurred by persons with mental health diagnoses were estimated to be $293 billion among commercially insured, Medicaid, and Medicare beneficiaries in the United States—a figure that is 273% higher than the cost for those without psychiatric diagnoses.2 Psychiatric and medical illnesses can be so tightly linked that accurate diagnosis and treatment of psychiatric disorders becomes essential to control medical illnesses. It is not surprising that there is increased scrutiny to the ways in which psychiatric care can be objectively assessed and monitored, and payers such as Centers for Medicare and Medicaid Services (CMS) increasingly require objective documentation of disease state improvement for payment.3
Support for objective assessment of disease derives from the collaborative care model. This model is designed to better integrate psychiatric and primary care by (among other practices) establishing the Patient-Centered Medical Home and emphasizing screening and monitoring patient-reported outcomes over time to assess treatment response.4 This approach, which is endorsed by the American Psychiatric Association, is associated with significant improvements in outcomes compared with usual care.5 It tracks a patient’s progress using validated clinical rating scales and other screening tools (eg, Patient Health Questionnaire [PHQ-9] for depression), an approach that is analogous to how patients with type 2 diabetes mellitus are monitored by hemoglobin A1c laboratory tests.6 An increasingly extensive body of research supports the impact of this approach on treatment. A 2012 Cochrane Review associated collaborative care with significant improvements in depression and anxiety outcomes compared with usual treatment.7
Despite these findings, a recent Kennedy Forum brief asserts that behavioral health is characterized by a “lack of systematic measurement to determine whether patients are responding to treatment.”8 That same brief points to the many easy-to-administer and validated rating scales and other screening tools that can reliably measure the frequency and severity of psychiatric symptoms over time, and likens the lack of their use as “equivalent to treating high blood pressure without using a blood pressure cuff to measure if a patient’s blood pressure is improving.”8 It is estimated that only 18% of psychiatrists and 11% of psychologists administer them routinely.9,10 This lack of use denies clinicians important information that can help detect deterioration or lack of improvement in their patients.
Psychiatry is replete with rating scales and screening tools, and the number of competing scales can make choosing a measure difficult.1 Nonetheless, not all scales are appropriate for clinical use; many are designed for research, for instance, and are lengthy and difficult to administer.
This article reviews a number of rating scales that are brief, useful, and easy to administer. A framework for the screening tools addressed in this article is available on the federally funded Center for Integrated Health Systems Web site (www.integration.samhsa.gov). This site promotes the use of tools designed to assist in screening and monitoring for depression, anxiety, bipolar disorder, substance use, and suicidality.11
Quality criteria for rating scales
The quality of a rating scale is determined by the following attributes12:
- Objectivity. The ability of a scale to obtain the same results, regardless of who administers, analyzes, or interprets it.
- Reliability. The ability of a scale to convey consistent and reproducible information across time, patients, and raters.
- Validity. The degree to which the scale measures what it is supposed to measure (eg, depressive symptoms). Sensitivity and specificity are measures of validity and provide additional information about the rating scale; namely, whether the scale can detect the presence of a disease (sensitivity) and whether it detects only that disease or condition and not another (specificity).
- Establishment of norms. Whether a scale provides reference values for different clinical groups.
- Practicability. The resources required to administer the assessment instrument in terms of time, staff, and material.
In addition to meeting these quality criteria, selection of a scale can be based on whether it is self-rated or observer-rated. Advantages to self-rated scales, such as the PHQ-9, Mood Disorder Questionnaire (MDQ), and Generalized Anxiety Disorder 7-item (GAD-7) scale, are their practicability—they are easy to administer and don’t require clinician or staff time—and their use in evaluating and raising awareness of subjective states.
However, reliability may be a concern, as some patients either may lack insight or exaggerate or mask symptoms when completing such scales.13 Both observer and self-rated scales can be used together to minimize bias, identify symptoms that might have been missed/not addressed in the clinical interview, and drive clinical decision-making. Both also can help patients communicate with their providers and make them feel more involved in clinical decision-making.8
The following scales have met many of the quality criteria described here and are endorsed by the government payer system. They can easily be incorporated into clinical practice and will provide useful clinical information that can assist in diagnosis and monitoring patient outcomes.
Patient Health Questionnaire
PHQ-9 is a 9-item self-report questionnaire that can help to detect the presence of depression and supplement a thorough psychiatric and mental health interview. It scores the 9 DSM-IV criteria for depression on a scale of 0 (not at all) to 3 (nearly every day). It is a public resource that is easy to find online, available without cost in several languages, and takes just a few minutes to complete.14
PHQ-9 has shown excellent test–retest reliability in screening for depression, and normative data on the instrument’s use are available in various clinical populations.15 Research has shown that as PHQ-9 depression scores increase, functional status decrease, while depressive symptoms, sick days, and health care utilization increase.15 In one study, a PHQ-9 score of ≥10 had 88% sensitivity and specificity for detecting depression, with scores of 5, 10, 15, and 20 indicating mild, moderate, moderately severe, and severe depression, respectively.16 In addition to its use as a screening tool, PHQ-9 is a responsive and reliable measure of depression treatment outcomes.17
Mood Disorder Questionnaire
MDQ is another brief, self-report questionnaire that is available online. It is designed to identify and monitor patients who are likely to meet diagnostic criteria for bipolar disorder.18,19
The first question on the MDQ asks if the patient has experienced any of 13 common mood and behavior symptoms. The second question asks if these symptoms have ever occurred at the same time, and the third asks the degree to which the patient finds the symptoms to be problematic. The remaining 2 questions provide additional, clinical information, because they address family history of manic–depressive illness or bipolar disorder and whether a diagnosis of either disorder has been made.
The MDQ has shown validity in assessing bipolar disorder symptoms in a general population,20 although recent research suggests that imprecise recall bias may limit its reliability in detecting hypomanic episodes earlier in life.21 Nonetheless, its specificity of >97% means that it will effectively screen out just about all true negatives.18
Generalized Anxiety Disorder 7-item scale
GAD-7 scale is a brief, self-administered questionnaire for screening and measuring severity of GAD.22 It asks patients to rate 7 items that represent problems with general anxiety and scores each item on a scale of 0 (not at all) to 3 (nearly every day). Similar to the other measures, it is easily accessible online.
Research evidence supports the reliability and validity of GAD-7 as a measure of anxiety in the general population. Sensitivity and specificity are 89% and 82%, respectively. Normative data for age and sex specific subgroups support its use across age groups and in both males and females.23 The GAD-7 performs well for detecting and monitoring not only GAD but also panic disorder, social anxiety disorder, and posttraumatic stress disorder.24
CAGE questionnaire for detection of substance use
The CAGE questionnaire is a widely-used screening tool that was originally developed to detect alcohol abuse, but has been adapted to assess other substance abuse.25,26 The omission of substance abuse from diagnostic consideration can have a major effect on quality of care, because substance abuse can be the underlying cause of other diseases. Therefore, routine administration of this instrument in clinical practice can lead to better understanding and monitoring of patient health.27
Similar to other instruments, CAGE is free and available online.27 It contains 4 simple questions, with 1 point is assigned to each positive answer.
Have you ever:
1. Felt the need to cut down on your drinking or drug use?
2. Have people annoyed you by criticizing your drinking or drug use?
3. Have you felt bad or guilty about your drinking or drug use?
4. Have you ever had a drink or used drugs first thing in the morning to steady your nerves or to get rid of a hangover (eye-opener)?
The simple mnemonic CAGE makes the questions easy to remember and to administer in a clinical setting. CAGE has demonstrated validity, with one study determining that CAGE scores ≥2 had a specificity and sensitivity of 76% and 93%, respectively, for identifying excessive drinking, and a specificity and sensitivity of 77% and 91%, respectively, for identifying alcohol abuse.28
Columbia Suicide Severity Rating Scale (C-SSRS)
C-SSRS was developed by researchers at Columbia University to assess the severity of and track changes over time in suicidal ideation and behavior. C-SSRS is 2 pages and takes only a few minutes to administer; however, it also may be completed as a self-report measure. The questions are phrased for use in an interview format, and clinicians are encouraged to receive training prior to its administration, although specific training in mental health is not required.
The “Lifetime/Recent” version allows practitioners to gather lifetime history of suicidality as well as any recent suicidal ideation and/or behavior, whereas the “Since Last Visit” version of the scale assesses suicidality in patients who have completed at least 1 Lifetime/Recent C-SSRS assessment. A truncated, 6-item “Screener” version is typically used in emergency situations. A risk assessment can be added to either the Full or Screener version to summarize the answers from C-SSRS and document risk and protective factors.29
Several studies have found C-SSRS to be reliable and valid for identifying suicide risk in children and adults.30,31USA Today reported that an individual exhibiting even a single behavior identified by the scale is 8 to 10 times more likely to complete suicide.32 In addition, the C-SSRS has helped reduce the suicide rate 65% in one of the largest providers of community-based behavioral health care in the United States.32
Using scales to augment care
Each of the scales described in this article can easily be incorporated into clinical practice and offers psychiatrists important clinical information that may have been missed or not addressed in the initial clinical interview. This information can be used to follow progression of symptoms and effectiveness of treatment. Although rating scales should never be used alone to establish a diagnosis or clinical treatment plan, they can and should be used to augment information from the clinician’s assessment and follow-up interviews.5
1. McDowell I. Measuring health: a guide to rating scales and questionnaires. 3rd ed. New York, NY: Oxford University Press; 2006.
2. Kennedy Forum. Fixing behavioral health care in America: a national call for integrating and coordinating specialty behavioral health care with the medical system. http://thekennedyforum-dot-org.s3.amazonaws.com/documents/KennedyForum-BehavioralHealth_FINAL_3.pdf. Published 2015. Accessed January 13, 2017.
3. The Office of the National Coordinator for Health Information Technology. Behavioral health (BH) Clinical Quality Measures (CQMs) Program initiatives. https://www.healthit.gov/sites/default/files/pdf/2012-09-27-behavioral-health-clinical-quality-measures-program-initiatives-public-forum.pdf. Published September 27, 2012. Accessed January 13, 2017.
4. Unutzer J, Harbin H, Schoenbaum M. The collaborative care model: an approach for integrating physical and mental health care in Medicaid health homes. https://www.medicaid.gov/State-Resource-Center/Medicaid-State-Technical-Assistance/Health-Homes-Technical-Assistance/Downloads/HH-IRC-Collaborative-5-13.pdf. Published May 2013. Accessed January 13, 2016.
5. World Group On Psychiatric Evaluation; American Psychiatric Association Steering Committee On Practice Guidelines. Practice guideline for the psychiatric evaluation of adults. 2nd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/psychevaladults.pdf. Published June 2006. Accessed January 13, 2016.
6. Melek S, Norris D, Paulus J. Economic impact of integrated medical-behavioral healthcare: implications for psychiatry. Denver, CO: Milliman, Inc; 2014.
7. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2.
8. Kennedy P. Forum. Fixing behavioral health care in America: a national call for measurement-based care. https://www.thekennedyforum.org/news/measurement-based-care-issue-brief. Published December 10, 2015. Accessed January 13, 2017.
9. Zimmerman M, McGlinchey JB. Why don’t psychiatrists use scales to measure outcome when treating depressed patients? J Clin Psychiatry. 2008;69(12):1916-1919.
10. Hatfield D, McCullough L, Frantz SH, et al. Do we know when our clients get worse? An investigation of therapists’ ability to detect negative client change. Clin Psychol Psychother. 2010;17(1):25-32.
11. SAMHSA-HRSA Center for Integrated Solutions. Screening tools. http://www.integration.samhsa.gov/clinical-practice/screening-tools. Accessed January 14, 2016.
12. Moller HJ. Standardised rating scales in psychiatry: methodological basis, their possibilities and limitations and descriptions of important rating scales. World J Biol Psychiatry. 2009;10(1):6-26.
13. Sajatovic M, Ramirez LF. Rating scales in mental health. 2nd ed. Hudson, OH: Lexi-Comp; 2003.
14. Patient Health Questionnaire-9 (PHQ-9). http://www.agencymeddirectors.wa.gov/files/AssessmentTools/14-PHQ-9%20overview.pdf. Accessed February 16, 2016.
15. Patient Health Questionnaire-9 (PHQ-9). Rehab Measures Web site. http://www.rehabmeasures.org/Lists/RehabMeasures/DispForm.aspx?ID=954. Updated August 28, 2014. Accessed February 16, 2016.
16. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
17. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
18. Ketter TA. Strategies for monitoring outcomes in patients with bipolar disorder. Prim Care Companion J Clin Psychiatry. 2010;12(suppl 1):10-16.
19. The Mood Disorder Questionnaire. University of Texas Medical Branch. http://www.dbsalliance.org/pdfs/MDQ.pdf. Published 2000. Accessed March 1, 2016.
20. Hirschfeld RM, Holzer C, Calabrese JR, et al. Validity of the mood disorder questionnaire: a general population study. Am J Psychiatry. 2003;160(1):178-180.
21. Boschloo L, Nolen WA, Spijker AT, et al. The Mood Disorder Questionnaire (MDQ) for detecting (hypo)manic episodes: its validity and impact of recall bias. J Affect Disord. 2013;151(1):203-208.
22. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
23. Lowe B, Decker O, Müller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266-274.
24. Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317-325.
25. Ewing JA. Detecting alcoholism. The CAGE Questionnaire. JAMA. 1984;252(14):1905-1907.
26. CAGE substance abuse screening tool. Johns Hopkins Medicine. http://www.hopkinsmedicine.org/johns_hopkins_healthcare/downloads/CAGE%20Substance%20Screening%20Tool.pdf. Accessed January 13, 2017.
27. O’Brien CP. The CAGE questionnaire for detection of alcoholism: a remarkably useful but simple tool. JAMA. 2008;300(17):2054-2056.
28. Bernadt MW, Mumford J, Taylor C, et al. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet. 1982;1(8267):325-328.
29. Columbia Suicide-Severity Rating Scale (CS-SRS). http://cssrs.columbia.edu/the-columbia-scale-c-ssrs/cssrs-for-communities-and-healthcare/#filter=.general-use.english. Accessed March 6, 2016.
30. Mundt JC, Greist JH, Jefferson JW, et al. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic Columbia-Suicide Severity Rating Scale. J Clin Psychiatry. 2013;74(9):887-893.
31. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277.
32. Esposito L. Suicide Checklist Spots People at Highest Risk. USA Today. http://usatoday30.usatoday.com/news/health/story/health/story/2011-11-09/Suicide-checklist-spots-people-at-highest-risk/51135944/1. Published November 9, 2011. Accessed March 6, 2016.
In the current health care environment, there is an increasing demand for objective assessment of disease states.1 This is particularly apparent in psychiatry, where documentation of outcomes lags that of other areas of medicine.
In 2012, the additional health care costs incurred by persons with mental health diagnoses were estimated to be $293 billion among commercially insured, Medicaid, and Medicare beneficiaries in the United States—a figure that is 273% higher than the cost for those without psychiatric diagnoses.2 Psychiatric and medical illnesses can be so tightly linked that accurate diagnosis and treatment of psychiatric disorders becomes essential to control medical illnesses. It is not surprising that there is increased scrutiny to the ways in which psychiatric care can be objectively assessed and monitored, and payers such as Centers for Medicare and Medicaid Services (CMS) increasingly require objective documentation of disease state improvement for payment.3
Support for objective assessment of disease derives from the collaborative care model. This model is designed to better integrate psychiatric and primary care by (among other practices) establishing the Patient-Centered Medical Home and emphasizing screening and monitoring patient-reported outcomes over time to assess treatment response.4 This approach, which is endorsed by the American Psychiatric Association, is associated with significant improvements in outcomes compared with usual care.5 It tracks a patient’s progress using validated clinical rating scales and other screening tools (eg, Patient Health Questionnaire [PHQ-9] for depression), an approach that is analogous to how patients with type 2 diabetes mellitus are monitored by hemoglobin A1c laboratory tests.6 An increasingly extensive body of research supports the impact of this approach on treatment. A 2012 Cochrane Review associated collaborative care with significant improvements in depression and anxiety outcomes compared with usual treatment.7
Despite these findings, a recent Kennedy Forum brief asserts that behavioral health is characterized by a “lack of systematic measurement to determine whether patients are responding to treatment.”8 That same brief points to the many easy-to-administer and validated rating scales and other screening tools that can reliably measure the frequency and severity of psychiatric symptoms over time, and likens the lack of their use as “equivalent to treating high blood pressure without using a blood pressure cuff to measure if a patient’s blood pressure is improving.”8 It is estimated that only 18% of psychiatrists and 11% of psychologists administer them routinely.9,10 This lack of use denies clinicians important information that can help detect deterioration or lack of improvement in their patients.
Psychiatry is replete with rating scales and screening tools, and the number of competing scales can make choosing a measure difficult.1 Nonetheless, not all scales are appropriate for clinical use; many are designed for research, for instance, and are lengthy and difficult to administer.
This article reviews a number of rating scales that are brief, useful, and easy to administer. A framework for the screening tools addressed in this article is available on the federally funded Center for Integrated Health Systems Web site (www.integration.samhsa.gov). This site promotes the use of tools designed to assist in screening and monitoring for depression, anxiety, bipolar disorder, substance use, and suicidality.11
Quality criteria for rating scales
The quality of a rating scale is determined by the following attributes12:
- Objectivity. The ability of a scale to obtain the same results, regardless of who administers, analyzes, or interprets it.
- Reliability. The ability of a scale to convey consistent and reproducible information across time, patients, and raters.
- Validity. The degree to which the scale measures what it is supposed to measure (eg, depressive symptoms). Sensitivity and specificity are measures of validity and provide additional information about the rating scale; namely, whether the scale can detect the presence of a disease (sensitivity) and whether it detects only that disease or condition and not another (specificity).
- Establishment of norms. Whether a scale provides reference values for different clinical groups.
- Practicability. The resources required to administer the assessment instrument in terms of time, staff, and material.
In addition to meeting these quality criteria, selection of a scale can be based on whether it is self-rated or observer-rated. Advantages to self-rated scales, such as the PHQ-9, Mood Disorder Questionnaire (MDQ), and Generalized Anxiety Disorder 7-item (GAD-7) scale, are their practicability—they are easy to administer and don’t require clinician or staff time—and their use in evaluating and raising awareness of subjective states.
However, reliability may be a concern, as some patients either may lack insight or exaggerate or mask symptoms when completing such scales.13 Both observer and self-rated scales can be used together to minimize bias, identify symptoms that might have been missed/not addressed in the clinical interview, and drive clinical decision-making. Both also can help patients communicate with their providers and make them feel more involved in clinical decision-making.8
The following scales have met many of the quality criteria described here and are endorsed by the government payer system. They can easily be incorporated into clinical practice and will provide useful clinical information that can assist in diagnosis and monitoring patient outcomes.
Patient Health Questionnaire
PHQ-9 is a 9-item self-report questionnaire that can help to detect the presence of depression and supplement a thorough psychiatric and mental health interview. It scores the 9 DSM-IV criteria for depression on a scale of 0 (not at all) to 3 (nearly every day). It is a public resource that is easy to find online, available without cost in several languages, and takes just a few minutes to complete.14
PHQ-9 has shown excellent test–retest reliability in screening for depression, and normative data on the instrument’s use are available in various clinical populations.15 Research has shown that as PHQ-9 depression scores increase, functional status decrease, while depressive symptoms, sick days, and health care utilization increase.15 In one study, a PHQ-9 score of ≥10 had 88% sensitivity and specificity for detecting depression, with scores of 5, 10, 15, and 20 indicating mild, moderate, moderately severe, and severe depression, respectively.16 In addition to its use as a screening tool, PHQ-9 is a responsive and reliable measure of depression treatment outcomes.17
Mood Disorder Questionnaire
MDQ is another brief, self-report questionnaire that is available online. It is designed to identify and monitor patients who are likely to meet diagnostic criteria for bipolar disorder.18,19
The first question on the MDQ asks if the patient has experienced any of 13 common mood and behavior symptoms. The second question asks if these symptoms have ever occurred at the same time, and the third asks the degree to which the patient finds the symptoms to be problematic. The remaining 2 questions provide additional, clinical information, because they address family history of manic–depressive illness or bipolar disorder and whether a diagnosis of either disorder has been made.
The MDQ has shown validity in assessing bipolar disorder symptoms in a general population,20 although recent research suggests that imprecise recall bias may limit its reliability in detecting hypomanic episodes earlier in life.21 Nonetheless, its specificity of >97% means that it will effectively screen out just about all true negatives.18
Generalized Anxiety Disorder 7-item scale
GAD-7 scale is a brief, self-administered questionnaire for screening and measuring severity of GAD.22 It asks patients to rate 7 items that represent problems with general anxiety and scores each item on a scale of 0 (not at all) to 3 (nearly every day). Similar to the other measures, it is easily accessible online.
Research evidence supports the reliability and validity of GAD-7 as a measure of anxiety in the general population. Sensitivity and specificity are 89% and 82%, respectively. Normative data for age and sex specific subgroups support its use across age groups and in both males and females.23 The GAD-7 performs well for detecting and monitoring not only GAD but also panic disorder, social anxiety disorder, and posttraumatic stress disorder.24
CAGE questionnaire for detection of substance use
The CAGE questionnaire is a widely-used screening tool that was originally developed to detect alcohol abuse, but has been adapted to assess other substance abuse.25,26 The omission of substance abuse from diagnostic consideration can have a major effect on quality of care, because substance abuse can be the underlying cause of other diseases. Therefore, routine administration of this instrument in clinical practice can lead to better understanding and monitoring of patient health.27
Similar to other instruments, CAGE is free and available online.27 It contains 4 simple questions, with 1 point is assigned to each positive answer.
Have you ever:
1. Felt the need to cut down on your drinking or drug use?
2. Have people annoyed you by criticizing your drinking or drug use?
3. Have you felt bad or guilty about your drinking or drug use?
4. Have you ever had a drink or used drugs first thing in the morning to steady your nerves or to get rid of a hangover (eye-opener)?
The simple mnemonic CAGE makes the questions easy to remember and to administer in a clinical setting. CAGE has demonstrated validity, with one study determining that CAGE scores ≥2 had a specificity and sensitivity of 76% and 93%, respectively, for identifying excessive drinking, and a specificity and sensitivity of 77% and 91%, respectively, for identifying alcohol abuse.28
Columbia Suicide Severity Rating Scale (C-SSRS)
C-SSRS was developed by researchers at Columbia University to assess the severity of and track changes over time in suicidal ideation and behavior. C-SSRS is 2 pages and takes only a few minutes to administer; however, it also may be completed as a self-report measure. The questions are phrased for use in an interview format, and clinicians are encouraged to receive training prior to its administration, although specific training in mental health is not required.
The “Lifetime/Recent” version allows practitioners to gather lifetime history of suicidality as well as any recent suicidal ideation and/or behavior, whereas the “Since Last Visit” version of the scale assesses suicidality in patients who have completed at least 1 Lifetime/Recent C-SSRS assessment. A truncated, 6-item “Screener” version is typically used in emergency situations. A risk assessment can be added to either the Full or Screener version to summarize the answers from C-SSRS and document risk and protective factors.29
Several studies have found C-SSRS to be reliable and valid for identifying suicide risk in children and adults.30,31USA Today reported that an individual exhibiting even a single behavior identified by the scale is 8 to 10 times more likely to complete suicide.32 In addition, the C-SSRS has helped reduce the suicide rate 65% in one of the largest providers of community-based behavioral health care in the United States.32
Using scales to augment care
Each of the scales described in this article can easily be incorporated into clinical practice and offers psychiatrists important clinical information that may have been missed or not addressed in the initial clinical interview. This information can be used to follow progression of symptoms and effectiveness of treatment. Although rating scales should never be used alone to establish a diagnosis or clinical treatment plan, they can and should be used to augment information from the clinician’s assessment and follow-up interviews.5
In the current health care environment, there is an increasing demand for objective assessment of disease states.1 This is particularly apparent in psychiatry, where documentation of outcomes lags that of other areas of medicine.
In 2012, the additional health care costs incurred by persons with mental health diagnoses were estimated to be $293 billion among commercially insured, Medicaid, and Medicare beneficiaries in the United States—a figure that is 273% higher than the cost for those without psychiatric diagnoses.2 Psychiatric and medical illnesses can be so tightly linked that accurate diagnosis and treatment of psychiatric disorders becomes essential to control medical illnesses. It is not surprising that there is increased scrutiny to the ways in which psychiatric care can be objectively assessed and monitored, and payers such as Centers for Medicare and Medicaid Services (CMS) increasingly require objective documentation of disease state improvement for payment.3
Support for objective assessment of disease derives from the collaborative care model. This model is designed to better integrate psychiatric and primary care by (among other practices) establishing the Patient-Centered Medical Home and emphasizing screening and monitoring patient-reported outcomes over time to assess treatment response.4 This approach, which is endorsed by the American Psychiatric Association, is associated with significant improvements in outcomes compared with usual care.5 It tracks a patient’s progress using validated clinical rating scales and other screening tools (eg, Patient Health Questionnaire [PHQ-9] for depression), an approach that is analogous to how patients with type 2 diabetes mellitus are monitored by hemoglobin A1c laboratory tests.6 An increasingly extensive body of research supports the impact of this approach on treatment. A 2012 Cochrane Review associated collaborative care with significant improvements in depression and anxiety outcomes compared with usual treatment.7
Despite these findings, a recent Kennedy Forum brief asserts that behavioral health is characterized by a “lack of systematic measurement to determine whether patients are responding to treatment.”8 That same brief points to the many easy-to-administer and validated rating scales and other screening tools that can reliably measure the frequency and severity of psychiatric symptoms over time, and likens the lack of their use as “equivalent to treating high blood pressure without using a blood pressure cuff to measure if a patient’s blood pressure is improving.”8 It is estimated that only 18% of psychiatrists and 11% of psychologists administer them routinely.9,10 This lack of use denies clinicians important information that can help detect deterioration or lack of improvement in their patients.
Psychiatry is replete with rating scales and screening tools, and the number of competing scales can make choosing a measure difficult.1 Nonetheless, not all scales are appropriate for clinical use; many are designed for research, for instance, and are lengthy and difficult to administer.
This article reviews a number of rating scales that are brief, useful, and easy to administer. A framework for the screening tools addressed in this article is available on the federally funded Center for Integrated Health Systems Web site (www.integration.samhsa.gov). This site promotes the use of tools designed to assist in screening and monitoring for depression, anxiety, bipolar disorder, substance use, and suicidality.11
Quality criteria for rating scales
The quality of a rating scale is determined by the following attributes12:
- Objectivity. The ability of a scale to obtain the same results, regardless of who administers, analyzes, or interprets it.
- Reliability. The ability of a scale to convey consistent and reproducible information across time, patients, and raters.
- Validity. The degree to which the scale measures what it is supposed to measure (eg, depressive symptoms). Sensitivity and specificity are measures of validity and provide additional information about the rating scale; namely, whether the scale can detect the presence of a disease (sensitivity) and whether it detects only that disease or condition and not another (specificity).
- Establishment of norms. Whether a scale provides reference values for different clinical groups.
- Practicability. The resources required to administer the assessment instrument in terms of time, staff, and material.
In addition to meeting these quality criteria, selection of a scale can be based on whether it is self-rated or observer-rated. Advantages to self-rated scales, such as the PHQ-9, Mood Disorder Questionnaire (MDQ), and Generalized Anxiety Disorder 7-item (GAD-7) scale, are their practicability—they are easy to administer and don’t require clinician or staff time—and their use in evaluating and raising awareness of subjective states.
However, reliability may be a concern, as some patients either may lack insight or exaggerate or mask symptoms when completing such scales.13 Both observer and self-rated scales can be used together to minimize bias, identify symptoms that might have been missed/not addressed in the clinical interview, and drive clinical decision-making. Both also can help patients communicate with their providers and make them feel more involved in clinical decision-making.8
The following scales have met many of the quality criteria described here and are endorsed by the government payer system. They can easily be incorporated into clinical practice and will provide useful clinical information that can assist in diagnosis and monitoring patient outcomes.
Patient Health Questionnaire
PHQ-9 is a 9-item self-report questionnaire that can help to detect the presence of depression and supplement a thorough psychiatric and mental health interview. It scores the 9 DSM-IV criteria for depression on a scale of 0 (not at all) to 3 (nearly every day). It is a public resource that is easy to find online, available without cost in several languages, and takes just a few minutes to complete.14
PHQ-9 has shown excellent test–retest reliability in screening for depression, and normative data on the instrument’s use are available in various clinical populations.15 Research has shown that as PHQ-9 depression scores increase, functional status decrease, while depressive symptoms, sick days, and health care utilization increase.15 In one study, a PHQ-9 score of ≥10 had 88% sensitivity and specificity for detecting depression, with scores of 5, 10, 15, and 20 indicating mild, moderate, moderately severe, and severe depression, respectively.16 In addition to its use as a screening tool, PHQ-9 is a responsive and reliable measure of depression treatment outcomes.17
Mood Disorder Questionnaire
MDQ is another brief, self-report questionnaire that is available online. It is designed to identify and monitor patients who are likely to meet diagnostic criteria for bipolar disorder.18,19
The first question on the MDQ asks if the patient has experienced any of 13 common mood and behavior symptoms. The second question asks if these symptoms have ever occurred at the same time, and the third asks the degree to which the patient finds the symptoms to be problematic. The remaining 2 questions provide additional, clinical information, because they address family history of manic–depressive illness or bipolar disorder and whether a diagnosis of either disorder has been made.
The MDQ has shown validity in assessing bipolar disorder symptoms in a general population,20 although recent research suggests that imprecise recall bias may limit its reliability in detecting hypomanic episodes earlier in life.21 Nonetheless, its specificity of >97% means that it will effectively screen out just about all true negatives.18
Generalized Anxiety Disorder 7-item scale
GAD-7 scale is a brief, self-administered questionnaire for screening and measuring severity of GAD.22 It asks patients to rate 7 items that represent problems with general anxiety and scores each item on a scale of 0 (not at all) to 3 (nearly every day). Similar to the other measures, it is easily accessible online.
Research evidence supports the reliability and validity of GAD-7 as a measure of anxiety in the general population. Sensitivity and specificity are 89% and 82%, respectively. Normative data for age and sex specific subgroups support its use across age groups and in both males and females.23 The GAD-7 performs well for detecting and monitoring not only GAD but also panic disorder, social anxiety disorder, and posttraumatic stress disorder.24
CAGE questionnaire for detection of substance use
The CAGE questionnaire is a widely-used screening tool that was originally developed to detect alcohol abuse, but has been adapted to assess other substance abuse.25,26 The omission of substance abuse from diagnostic consideration can have a major effect on quality of care, because substance abuse can be the underlying cause of other diseases. Therefore, routine administration of this instrument in clinical practice can lead to better understanding and monitoring of patient health.27
Similar to other instruments, CAGE is free and available online.27 It contains 4 simple questions, with 1 point is assigned to each positive answer.
Have you ever:
1. Felt the need to cut down on your drinking or drug use?
2. Have people annoyed you by criticizing your drinking or drug use?
3. Have you felt bad or guilty about your drinking or drug use?
4. Have you ever had a drink or used drugs first thing in the morning to steady your nerves or to get rid of a hangover (eye-opener)?
The simple mnemonic CAGE makes the questions easy to remember and to administer in a clinical setting. CAGE has demonstrated validity, with one study determining that CAGE scores ≥2 had a specificity and sensitivity of 76% and 93%, respectively, for identifying excessive drinking, and a specificity and sensitivity of 77% and 91%, respectively, for identifying alcohol abuse.28
Columbia Suicide Severity Rating Scale (C-SSRS)
C-SSRS was developed by researchers at Columbia University to assess the severity of and track changes over time in suicidal ideation and behavior. C-SSRS is 2 pages and takes only a few minutes to administer; however, it also may be completed as a self-report measure. The questions are phrased for use in an interview format, and clinicians are encouraged to receive training prior to its administration, although specific training in mental health is not required.
The “Lifetime/Recent” version allows practitioners to gather lifetime history of suicidality as well as any recent suicidal ideation and/or behavior, whereas the “Since Last Visit” version of the scale assesses suicidality in patients who have completed at least 1 Lifetime/Recent C-SSRS assessment. A truncated, 6-item “Screener” version is typically used in emergency situations. A risk assessment can be added to either the Full or Screener version to summarize the answers from C-SSRS and document risk and protective factors.29
Several studies have found C-SSRS to be reliable and valid for identifying suicide risk in children and adults.30,31USA Today reported that an individual exhibiting even a single behavior identified by the scale is 8 to 10 times more likely to complete suicide.32 In addition, the C-SSRS has helped reduce the suicide rate 65% in one of the largest providers of community-based behavioral health care in the United States.32
Using scales to augment care
Each of the scales described in this article can easily be incorporated into clinical practice and offers psychiatrists important clinical information that may have been missed or not addressed in the initial clinical interview. This information can be used to follow progression of symptoms and effectiveness of treatment. Although rating scales should never be used alone to establish a diagnosis or clinical treatment plan, they can and should be used to augment information from the clinician’s assessment and follow-up interviews.5
1. McDowell I. Measuring health: a guide to rating scales and questionnaires. 3rd ed. New York, NY: Oxford University Press; 2006.
2. Kennedy Forum. Fixing behavioral health care in America: a national call for integrating and coordinating specialty behavioral health care with the medical system. http://thekennedyforum-dot-org.s3.amazonaws.com/documents/KennedyForum-BehavioralHealth_FINAL_3.pdf. Published 2015. Accessed January 13, 2017.
3. The Office of the National Coordinator for Health Information Technology. Behavioral health (BH) Clinical Quality Measures (CQMs) Program initiatives. https://www.healthit.gov/sites/default/files/pdf/2012-09-27-behavioral-health-clinical-quality-measures-program-initiatives-public-forum.pdf. Published September 27, 2012. Accessed January 13, 2017.
4. Unutzer J, Harbin H, Schoenbaum M. The collaborative care model: an approach for integrating physical and mental health care in Medicaid health homes. https://www.medicaid.gov/State-Resource-Center/Medicaid-State-Technical-Assistance/Health-Homes-Technical-Assistance/Downloads/HH-IRC-Collaborative-5-13.pdf. Published May 2013. Accessed January 13, 2016.
5. World Group On Psychiatric Evaluation; American Psychiatric Association Steering Committee On Practice Guidelines. Practice guideline for the psychiatric evaluation of adults. 2nd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/psychevaladults.pdf. Published June 2006. Accessed January 13, 2016.
6. Melek S, Norris D, Paulus J. Economic impact of integrated medical-behavioral healthcare: implications for psychiatry. Denver, CO: Milliman, Inc; 2014.
7. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2.
8. Kennedy P. Forum. Fixing behavioral health care in America: a national call for measurement-based care. https://www.thekennedyforum.org/news/measurement-based-care-issue-brief. Published December 10, 2015. Accessed January 13, 2017.
9. Zimmerman M, McGlinchey JB. Why don’t psychiatrists use scales to measure outcome when treating depressed patients? J Clin Psychiatry. 2008;69(12):1916-1919.
10. Hatfield D, McCullough L, Frantz SH, et al. Do we know when our clients get worse? An investigation of therapists’ ability to detect negative client change. Clin Psychol Psychother. 2010;17(1):25-32.
11. SAMHSA-HRSA Center for Integrated Solutions. Screening tools. http://www.integration.samhsa.gov/clinical-practice/screening-tools. Accessed January 14, 2016.
12. Moller HJ. Standardised rating scales in psychiatry: methodological basis, their possibilities and limitations and descriptions of important rating scales. World J Biol Psychiatry. 2009;10(1):6-26.
13. Sajatovic M, Ramirez LF. Rating scales in mental health. 2nd ed. Hudson, OH: Lexi-Comp; 2003.
14. Patient Health Questionnaire-9 (PHQ-9). http://www.agencymeddirectors.wa.gov/files/AssessmentTools/14-PHQ-9%20overview.pdf. Accessed February 16, 2016.
15. Patient Health Questionnaire-9 (PHQ-9). Rehab Measures Web site. http://www.rehabmeasures.org/Lists/RehabMeasures/DispForm.aspx?ID=954. Updated August 28, 2014. Accessed February 16, 2016.
16. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
17. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
18. Ketter TA. Strategies for monitoring outcomes in patients with bipolar disorder. Prim Care Companion J Clin Psychiatry. 2010;12(suppl 1):10-16.
19. The Mood Disorder Questionnaire. University of Texas Medical Branch. http://www.dbsalliance.org/pdfs/MDQ.pdf. Published 2000. Accessed March 1, 2016.
20. Hirschfeld RM, Holzer C, Calabrese JR, et al. Validity of the mood disorder questionnaire: a general population study. Am J Psychiatry. 2003;160(1):178-180.
21. Boschloo L, Nolen WA, Spijker AT, et al. The Mood Disorder Questionnaire (MDQ) for detecting (hypo)manic episodes: its validity and impact of recall bias. J Affect Disord. 2013;151(1):203-208.
22. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
23. Lowe B, Decker O, Müller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266-274.
24. Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317-325.
25. Ewing JA. Detecting alcoholism. The CAGE Questionnaire. JAMA. 1984;252(14):1905-1907.
26. CAGE substance abuse screening tool. Johns Hopkins Medicine. http://www.hopkinsmedicine.org/johns_hopkins_healthcare/downloads/CAGE%20Substance%20Screening%20Tool.pdf. Accessed January 13, 2017.
27. O’Brien CP. The CAGE questionnaire for detection of alcoholism: a remarkably useful but simple tool. JAMA. 2008;300(17):2054-2056.
28. Bernadt MW, Mumford J, Taylor C, et al. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet. 1982;1(8267):325-328.
29. Columbia Suicide-Severity Rating Scale (CS-SRS). http://cssrs.columbia.edu/the-columbia-scale-c-ssrs/cssrs-for-communities-and-healthcare/#filter=.general-use.english. Accessed March 6, 2016.
30. Mundt JC, Greist JH, Jefferson JW, et al. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic Columbia-Suicide Severity Rating Scale. J Clin Psychiatry. 2013;74(9):887-893.
31. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277.
32. Esposito L. Suicide Checklist Spots People at Highest Risk. USA Today. http://usatoday30.usatoday.com/news/health/story/health/story/2011-11-09/Suicide-checklist-spots-people-at-highest-risk/51135944/1. Published November 9, 2011. Accessed March 6, 2016.
1. McDowell I. Measuring health: a guide to rating scales and questionnaires. 3rd ed. New York, NY: Oxford University Press; 2006.
2. Kennedy Forum. Fixing behavioral health care in America: a national call for integrating and coordinating specialty behavioral health care with the medical system. http://thekennedyforum-dot-org.s3.amazonaws.com/documents/KennedyForum-BehavioralHealth_FINAL_3.pdf. Published 2015. Accessed January 13, 2017.
3. The Office of the National Coordinator for Health Information Technology. Behavioral health (BH) Clinical Quality Measures (CQMs) Program initiatives. https://www.healthit.gov/sites/default/files/pdf/2012-09-27-behavioral-health-clinical-quality-measures-program-initiatives-public-forum.pdf. Published September 27, 2012. Accessed January 13, 2017.
4. Unutzer J, Harbin H, Schoenbaum M. The collaborative care model: an approach for integrating physical and mental health care in Medicaid health homes. https://www.medicaid.gov/State-Resource-Center/Medicaid-State-Technical-Assistance/Health-Homes-Technical-Assistance/Downloads/HH-IRC-Collaborative-5-13.pdf. Published May 2013. Accessed January 13, 2016.
5. World Group On Psychiatric Evaluation; American Psychiatric Association Steering Committee On Practice Guidelines. Practice guideline for the psychiatric evaluation of adults. 2nd ed. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/psychevaladults.pdf. Published June 2006. Accessed January 13, 2016.
6. Melek S, Norris D, Paulus J. Economic impact of integrated medical-behavioral healthcare: implications for psychiatry. Denver, CO: Milliman, Inc; 2014.
7. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525. doi: 10.1002/14651858.CD006525.pub2.
8. Kennedy P. Forum. Fixing behavioral health care in America: a national call for measurement-based care. https://www.thekennedyforum.org/news/measurement-based-care-issue-brief. Published December 10, 2015. Accessed January 13, 2017.
9. Zimmerman M, McGlinchey JB. Why don’t psychiatrists use scales to measure outcome when treating depressed patients? J Clin Psychiatry. 2008;69(12):1916-1919.
10. Hatfield D, McCullough L, Frantz SH, et al. Do we know when our clients get worse? An investigation of therapists’ ability to detect negative client change. Clin Psychol Psychother. 2010;17(1):25-32.
11. SAMHSA-HRSA Center for Integrated Solutions. Screening tools. http://www.integration.samhsa.gov/clinical-practice/screening-tools. Accessed January 14, 2016.
12. Moller HJ. Standardised rating scales in psychiatry: methodological basis, their possibilities and limitations and descriptions of important rating scales. World J Biol Psychiatry. 2009;10(1):6-26.
13. Sajatovic M, Ramirez LF. Rating scales in mental health. 2nd ed. Hudson, OH: Lexi-Comp; 2003.
14. Patient Health Questionnaire-9 (PHQ-9). http://www.agencymeddirectors.wa.gov/files/AssessmentTools/14-PHQ-9%20overview.pdf. Accessed February 16, 2016.
15. Patient Health Questionnaire-9 (PHQ-9). Rehab Measures Web site. http://www.rehabmeasures.org/Lists/RehabMeasures/DispForm.aspx?ID=954. Updated August 28, 2014. Accessed February 16, 2016.
16. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
17. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
18. Ketter TA. Strategies for monitoring outcomes in patients with bipolar disorder. Prim Care Companion J Clin Psychiatry. 2010;12(suppl 1):10-16.
19. The Mood Disorder Questionnaire. University of Texas Medical Branch. http://www.dbsalliance.org/pdfs/MDQ.pdf. Published 2000. Accessed March 1, 2016.
20. Hirschfeld RM, Holzer C, Calabrese JR, et al. Validity of the mood disorder questionnaire: a general population study. Am J Psychiatry. 2003;160(1):178-180.
21. Boschloo L, Nolen WA, Spijker AT, et al. The Mood Disorder Questionnaire (MDQ) for detecting (hypo)manic episodes: its validity and impact of recall bias. J Affect Disord. 2013;151(1):203-208.
22. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
23. Lowe B, Decker O, Müller S, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. 2008;46(3):266-274.
24. Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317-325.
25. Ewing JA. Detecting alcoholism. The CAGE Questionnaire. JAMA. 1984;252(14):1905-1907.
26. CAGE substance abuse screening tool. Johns Hopkins Medicine. http://www.hopkinsmedicine.org/johns_hopkins_healthcare/downloads/CAGE%20Substance%20Screening%20Tool.pdf. Accessed January 13, 2017.
27. O’Brien CP. The CAGE questionnaire for detection of alcoholism: a remarkably useful but simple tool. JAMA. 2008;300(17):2054-2056.
28. Bernadt MW, Mumford J, Taylor C, et al. Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet. 1982;1(8267):325-328.
29. Columbia Suicide-Severity Rating Scale (CS-SRS). http://cssrs.columbia.edu/the-columbia-scale-c-ssrs/cssrs-for-communities-and-healthcare/#filter=.general-use.english. Accessed March 6, 2016.
30. Mundt JC, Greist JH, Jefferson JW, et al. Prediction of suicidal behavior in clinical research by lifetime suicidal ideation and behavior ascertained by the electronic Columbia-Suicide Severity Rating Scale. J Clin Psychiatry. 2013;74(9):887-893.
31. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277.
32. Esposito L. Suicide Checklist Spots People at Highest Risk. USA Today. http://usatoday30.usatoday.com/news/health/story/health/story/2011-11-09/Suicide-checklist-spots-people-at-highest-risk/51135944/1. Published November 9, 2011. Accessed March 6, 2016.
Alcohol-use disorders after bariatric surgery: The case for targeted group therapy
Maladaptive alcohol use has emerged as a risk for a subset of individuals who have undergone weight loss surgery (WLS); studies report they are vulnerable to consuming alcohol in greater quantities or more frequently.1,2 Estimates of the prevalence of “high-risk” or “hazardous” alcohol use after WLS range from 4% to 28%,3,4 while the prevalence of alcohol use meeting DSM-IV-TR5 criteria for alcohol use disorders (AUDs) hovers around 10%.6
Heavy alcohol users or patients who have active AUD at the time of WLS are at greater risk for continuation of these problems after surgery.2,6 For patients with a long-remitted history of AUD, the evidence regarding risk for post-WLS relapse is lacking, and some evidence suggests they may have better weight loss outcomes after WLS.7
However, approximately two-third of cases of post-WLS alcohol problems occur in patients who have had no history of such problems before surgery.5,8,9 Reported prevalence rates of new-onset alcohol problems range from 3% to 18%,6,9 with the modal finding being approximately 7% to 8%. New-onset alcohol problems appear to occur at a considerable latency after surgery. One study found little risk at 1 year post-surgery, but a significant increase in AUD symptoms at 2 years.6 Another study identified 3 years post-surgery as a high-risk time point,8 and yet another reported a linear increase in the risk for developing alcohol problems for at least 10 years after WLS.10
This article describes a group treatment protocol developed specifically for patients with post-WLS substance use disorder (SUD), and explores:
- risk factors and causal mechanisms of post-WLS AUDs
- weight stigma and emotional stressors
- the role of specialized treatment
- group treatment based on the Health at Every Size® (HAES)-oriented, trauma-informed and fat acceptance framework.
Post-WLS patients with alcohol problems may be a distinct phenotype among people with substance abuse issues. For this reason, they may have a need to address their experiences and issues specific to WLS as part of their alcohol treatment.
Etiology
Risk factors. Empirical findings have identified few predictors or risk factors for post-WLS SUD. These patients are more likely to be male and of a younger age.6 Notably, the vast majority of individuals reporting post-WLS alcohol problems have undergone Roux-en-Y gastric bypass (RYGB), rather than other WLS procedures, such as the laparoscopic adjustable gastric band,6,11 suggesting some physiological mechanism specific to RYGB.
Other potential predictors of postoperative alcohol problems include a pre-operative history of depression, generalized anxiety disorder, smoking, and/or recreational drug use.3,6 Likewise, patients with depression or anxiety disorder symptoms after surgery also may be at higher risk for postoperative alcohol problems.4 The evidence of an association between postoperative weight outcomes and post-WLS alcohol problems is mixed.3,12 Interestingly, patients who had no personal history of substance abuse but who have a family history may have a higher risk of new-onset alcohol problems after surgery.9,12
Causal mechanisms. The etiology of post-WLS alcohol problems is not well understood. If anything, epidemiological data suggest that larger-bodied individuals tend to consume lower levels of alcohol and have lower rates of AUD than individuals in the general population with thinner bodies.13 However, an association has been found between a family history of SUD, but not a personal history, and being large.14 This suggests a shared etiological pathway between addiction and being “overweight,” of which the onset of AUD after RYGB may be a manifestation.
Human and animal studies have shown that WLS may affect alcohol use differently in specific subgroups. Studies have shown that wild-type rats greatly increase their consumption of, or operant responding for, alcohol after RYGB,15 while genetically “alcohol-preferring” rats decrease consumption of, or responding for, alcohol after RYGB.16 A human study likewise found some patients decreased alcohol use or experienced improvement of or remission of AUD symptoms after WLS.4 Combined with the finding that a family history of substance abuse is related to risk for post-operative AUD, these data suggest a potential genetic vulnerability or protection in some individuals.
Turning to potential psychosocial explanations, the lay media has popularized the concepts of “addiction transfer,” or “transfer addiction,”12 with the implication that some patients, who had a preoperative history of “food addiction,” transfer that “addiction” after surgery to substances of abuse.
However, the “addiction transfer” model has a number of flaws:
- it is stigmatizing, because it assumes the patient possesses an innate, chronic, and inalterable pathology
- it relies upon the validity of the controversial construct of “food addiction,” a construct of mixed scientific evidence.17
Further, our knowledge of post-WLS SUD argues against “addiction transfer.” As noted, postoperative alcohol problems are more likely to develop years after surgery, rather than in the first few months afterward when eating is most significantly curtailed. Additionally, post-WLS alcohol problems are significantly more likely to occur after RYGB than other procedures, whereas the “addiction transfer” model would hypothesize that all WLS patients would be at equal risk for postoperative “addiction transfer,” because their eating is similarly affected after surgery.
Links to RYGB. Some clues to physiological mechanisms underlying alcohol problems after RYGB have been identified. After surgery, many RYGB patients report a quicker effect from a smaller amount of alcohol than was the case pre-surgery.18 Studies have demonstrated a number of changes in the pharmacodynamics of alcohol after RYGB not seen in other WLS procedures19:
- a much faster time to peak blood (or breath) alcohol content (BAC)
- significantly higher peak BAC
- a precipitous initial decline in perceived intoxication.18,20
Anatomical features of RYGB may explain such changes.8 However, an increased response to both IV alcohol and IV morphine after RYGB21,22 in rodents suggests that gastrointestinal tract changes are not solely responsible for changes in alcohol use. Emerging research reports that WLS has been found to cause alterations in brain reward pathways,23 which may be an additional contributor to changes in alcohol misuse after surgery.
However, even combined, pharmacokinetic and neurobiological factors cannot entirely explain new-onset alcohol problems after WLS; if they could, one would expect to see a much higher prevalence of this complication. Some psychosocial factors are likely involved as well.
Emotional stressors. One possibility involves a mismatch between post-WLS stressors and coping skills. After WLS, these patients face a multitude of challenges inherent in adjusting to changes in lifestyle, weight, body image, and social functioning, which most individuals would find daunting. These challenges become even more acute in the absence of appropriate psychoeducation, preparation, and intervention from qualified professionals. Individuals who lack effective and adaptive coping skills and supports may have a particularly heightened vulnerability to increased alcohol use in the setting of post-surgery changes in brain reward circuits and pharmacodynamics in alcohol metabolism. For example, one patient reported that her spouse’s pressure to “do something about her weight” was a significant factor in her decision to undergo surgery, but that her spouse was blaming and unsupportive when post-WLS complications developed. The patient believed that these experiences helped fuel development of her post-RYGB alcohol abuse.
Specialized treatment
The number of patients experiencing post-WLS alcohol problems likely will continue to grow, given that the risk of onset of has been shown increase over years. Already, post-WLS patients are proportionally overrepresented among substance abuse treatment populations.24 Empirically, however, we do not know yet if these patients need a different type of addiction treatment than patients who have not had WLS.
Some evidence suggests that post-WLS patients with alcohol problems may be a distinct phenotype within the general population with alcohol problems, as their presentations differ in several ways, including their demographics, alcohol use patterns, and premorbid functioning. A number of studies have found that, despite their increased pharmacodynamic sensitivity to alcohol, people with post-WLS AUDs actually consume a larger amount of alcohol on both typical and maximum drinking days than other individuals with AUDs.24 Additionally, although the median age of onset for AUD is around age 20,25 patients presenting with new-onset, post-WLS alcohol problems are usually in their late 30s, or even 40s or 50s. Further, many of these patients were quite high functioning before their alcohol problems, and are unlikely to identify with the cultural stereotype of a person with AUD (eg, homeless, unemployed), which may hamper or delay their own willingness to accept that they have a problem. These phenotypic differences suggest that post-WLS patients may require substance abuse treatment approaches tailored to their unique presentation. There are additional factors specific to the experiences of being larger-bodied and WLS that also may need to be addressed in specialized treatment for post-WLS addiction patients.
Weight stigma. By definition, patients who have undergone WLS have spent a significant portion of their lives inhabiting larger bodies, an experience that, in our culture, can produce adverse psychosocial effects. Compared with the general population, patients seeking WLS exhibit psychological distress equivalent to psychiatric patients.26 Weight stigma or weight bias—negative judgments directed toward people in larger bodies—is pervasive and continues to increase.27 Further, evidence suggests that, unlike almost all other stigmatized groups, people in larger bodies tend to internalize this stigma, holding an unfavorable attitude toward their own social group.28 Weight stigma impacts the well-being of people all along the weight spectrum, affecting many domains including educational achievements and classroom experiences, job opportunities, salaries, and medical care.27 Weight stigma increases the likelihood of bullying, teasing, and harassment for both adults and children.27 Weight bias has been associated with any number of adverse psychosocial effects, including symptoms of depression, anxiety, and eating pathology; poor body image; and a decrease in healthy self-care behaviors.29-33
Weight stigma makes it more difficult for people to enjoy physical activities, nourish their bodies, and manage stress, which contributes to poorer health outcomes and lower quality of life.33,34 For example, one study showed that, regardless of actual body mass index, people experiencing weight stigma have significantly increased risk of developing an illness or dying.35
Factors specific to WLS. WLS may lead to significant changes in eating habits, and some patients experience a sense of loss, particularly if eating represented one of their primary coping strategies—this may represent a heightened emotional vulnerability for developing AUD.
The fairly rapid and substantial weight loss that WLS produces can lead to sweeping changes in lifestyle, body image, and functional factors for many individuals. Patients often report profound changes, both positive and negative, in their relationships and interactions not only with people in their support network, but also with strangers.36
After the first year or 2 post-WLS, it is fairly common for patients to regain some weight, sometimes in significant amounts.37 This can lead to a sense of “failure.” Life stressors, including difficulties in important relationships, can further add to patients’ vulnerability. For example, one patient noticed that when she was at her thinnest after WLS, drivers were more likely to stop for her when she crossed the street, which pleased but also angered her because they hadn’t extended the same courtesy before WLS. After she regained a significant amount of weight, she began to notice drivers stopping for her less and less frequently. This took her back to her previous feelings of being ignored but now with the certainty that she would be treated better if she were thinner.
Patients also may experience ambivalence about changes in their body size. One might expect that body image would improve after weight loss, but the evidence is mixed.38 Although there is some evidence that body image improves in the short term after WLS,38 other research indicates that body image does not improve with weight loss.39 However, the evidence is clear that the appearance of excess skin after weight loss worsens some patients’ body image.40
To date, there has been no research examining treatment modalities for this population. Because experiences common to individuals who have had WLS could play a role in the development of AUD after surgery, it is intuitive that it would be important to address these factors when designing a treatment plan for post-WLS substance abuse.
Group treatment approach
In 2013, in response to the increase in rates of post-WLS addictions presenting to West End Clinic, an outpatient dual-diagnosis (addiction and psychiatry) service at Massachusetts General Hospital, a specialized treatment group was developed. Nine patients have enrolled since October 2013.
The Post-WLS Addictions Group (PWAG) was designed to be HAES-oriented, trauma-informed, and run within a fat acceptance framework. The HAES model prioritizes a weight-neutral approach that sees health and well-being as multifaceted. This approach directs both patient and clinician to focus on improving health behaviors and reducing internalized weight bias, while building a supportive community that buffers against external cultural weight bias.41
Trauma-informed care42 emphasizes the principles of safety, trustworthiness, and transparency; peer support; collaboration and mutuality; empowerment; and awareness of cultural, historical, and gender issues. In the context of PWAG, weight stigma is conceptualized as a traumatic experience.43 The fat acceptance approach promotes a culture that accepts people of every size with dignity and equality in all aspects of life.44
Self-care emphasis. The HAES model encourages patients to allow their bodies to determine what weight to settle at, and to focus on sustainable health-enhancing behaviors rather than weight loss. Patients who asked about the PWAG were told that this group would not explicitly support, or even encourage, continued pursuit of weight loss per se, but instead would assist patients with relapse prevention, mindful eating, improving self-care, and ongoing stress management. Moving away from a focus on weight loss and toward improvement of self-care skills allowed patients to focus on behaviors and outcomes over which they had more direct control and were more likely to yield immediate benefits.
All of the PWAG group members were in early recovery from an SUD, with a minimum of 4 weeks of abstinence; all had at least 1 co-occurring mental health diagnosis. A licensed independent clinical social worker (LICSW) and a physician familiar with bariatric surgery ran the sessions. The group met weekly for 1 hour. The 8 weekly sessions included both psychoeducation and discussion, with each session covering different topics (Table). The first 20 minutes of each session were devoted to an educational presentation; the remaining 40 minutes for reflection and discussion. In sessions 2 through 8, participants were asked about any recent use or cravings, and problem-solving techniques were employed as needed.
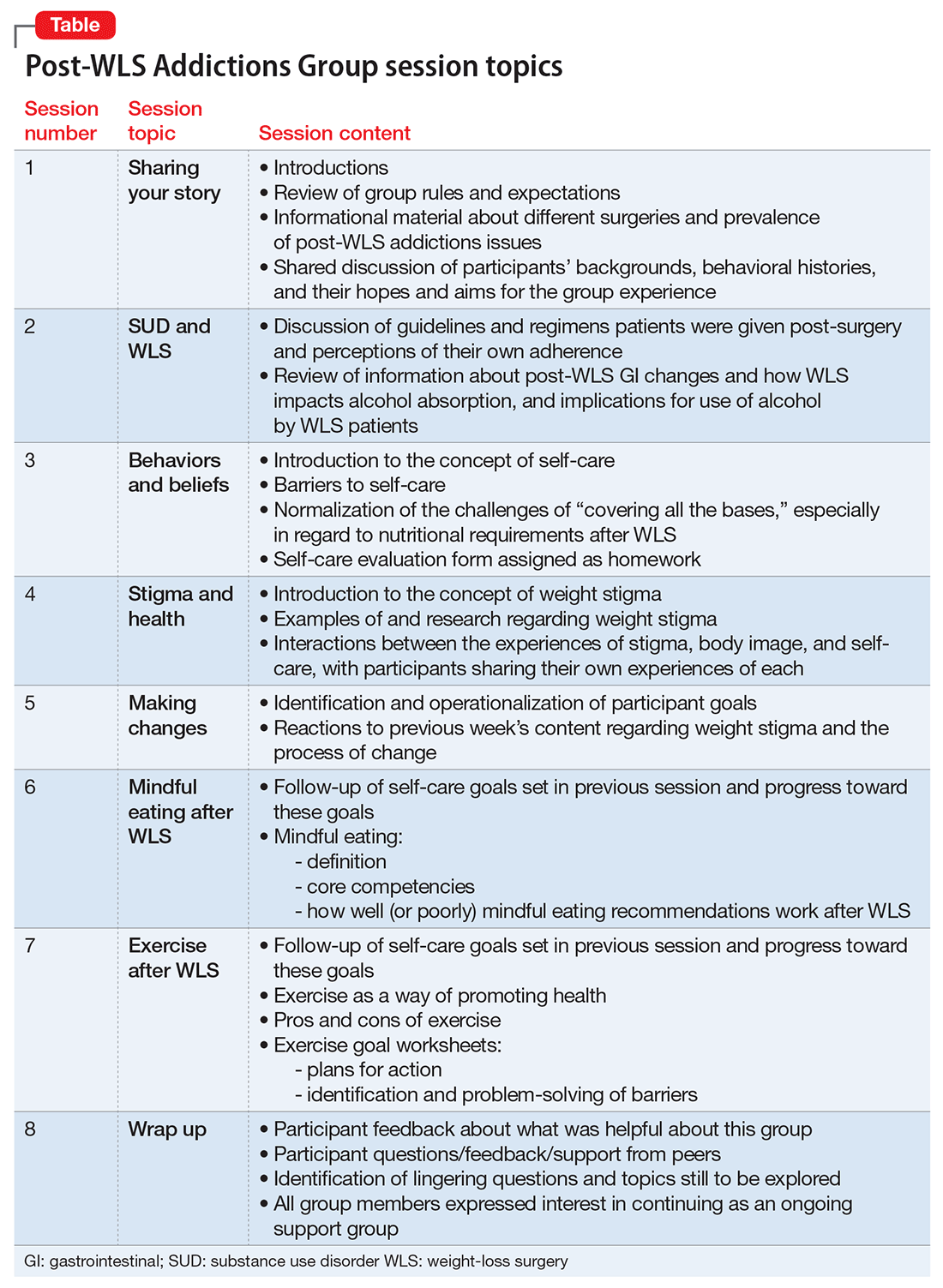
The PWAG group leader herself is a large person who modeled fat acceptance and follows the HAES approach; she led the group using both this experience and her specialized clinical training. As is the case with other addictions recovery treatment modalities, clinicians with lived experience may add a valuable component to both the program design and patient experience.
After the first 8 sessions, all members expressed interest in continuing as an ongoing relapse prevention and HAES support group, and they reported that meeting regularly was very helpful. The group continued with the LICSW alone, who continued to share HAES-oriented and fat acceptance information and resources that group members requested specifically. Over time, new members joined following an individual orientation session with the group leader, and the group has revisited each of the psychoeducational topics repeatedly, though not in a formally structured way.
Process and observations. Participants described high levels of excitement and hopefulness about being in a group with other WLS patients who had developed SUDs. They had a particular interest in reviewing medical/anatomical information about WLS and understanding more about the potential reasons for the elevated risk for developing SUD following WLS. Discussions regarding weight stigma proved to be quite emotional; most participants reported that this material readily related to their own experiences with weight stigma, but they had never discussed these ideas before.
Participants explored the role that grief, loss, guilt, and shame had in the decision to have WLS, the development of SUDs, weight regain or medical complications from the surgery or from substance abuse, career and relationship changes, and worsened body image. Another theme that emerged was the various reasons that prompted the members have WLS that they may not have been conscious of, or willing to discuss with others, such as pressure from a spouse, fears of remaining single due to their size, and a desire to finally “fit in.”
Repeatedly, group members expressed how satisfied and emotionally validated they felt being with people with similar experiences. Most of them had felt alone. They reported a belief that “everyone else” who had WLS was doing well, and that they were the exceptions. Such beliefs and emotions increased the risk of relapse and decreased participants’ ability to develop more positive coping strategies and self-care skills.
Participants reported that feeling less alone, understanding how stigma impacts health and well-being, and focusing on the general benefits of good self-care rather than the pursuit of weight loss were particularly helpful. The HAES and fat acceptance approaches have given group members new ways to think about their bodies and decreased shame. Several group members reported that if they had learned about the HAES approach prior to having a WLS, they might have made a different decision about having surgery, or at least might have been better prepared to handle the emotional and psychological challenges after WLS.
Although evidence for post-WLS addictions is fairly robust, causal mechanisms are not well understood, and research identifying specific risk factors is lacking. Because post-WLS patients with addictions seem to represent a specific phenotype, specialized treatment might be indicated. Future research will be needed to determine optimal treatment approaches for post-WLS addictions. However, a number of aspects are likely to be important. For example, it is likely that unaddressed experiences of weight stigma contribute to challenges, including substance abuse, after WLS; therefore, clinicians involved in the care of individuals presenting with post-WLS SUD should be knowledgeable about weight stigma and how to address it. Because of the specific nature of post-WLS addictions, patients often feel alone and isolated, and seem to benefit from the specialized group setting. We note that the PWAG group leader is herself a large person who models fat acceptance and follows the HAES approach, and therefore led the group using this experience and her specialized clinical training. As with other addiction recovery treatment modalities, clinicians who have lived the experience can add a valuable component to the program design and patient experience.
2. Lent MR, Hayes SM, Wood GC, et al. Smoking and alcohol use in gastric bypass patients. Eat Behav. 2013;14(4):460-463.
4. Wee CC, Mukamal KJ, Huskey KW, et al. High-risk alcohol use after weight loss surgery. Surg Obes Relat Dis. 2014;10(3):508-513.
5. Diagnostic and statistical manual of mental disorders, 4th, text rev. Washington, DC: American Psychiatric Association; 2000.
6. King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516-2525.
9. Ivezaj V, Saules KK, Schuh LM. New-onset substance use disorder after gastric bypass surgery: rates and associated characteristics. Obes Surg. 2014;24(11):1975-1980.
10. Svensson PA, Anveden Å, Romeo S, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity. 2013;21(12):2444-2451.
11. Ostlund MP, Backman O, Marsk R, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148(4):374-377.
13. Gearhardt AN, Corbin WR. Body mass index and alcohol consumption: family history of alcoholism as a moderator. Psychol Addict Behav. 2009;23(2):216-225.
14. Grucza RA, Krueger RF, Racette SB, et al. The emerging link between alcoholism risk and obesity in the United States. Arch Gen Psychiatry. 2010;67(12):1301-1308.
15. Davis JF, Tracy AL, Schurdak JD, et al. Roux en y gastric bypass increases ethanol intake in the rat. Obes Surg. 2013;23(7):920-930.
16. Davis JF, Schurdak JD, Magrisso IJ, et al. Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats. Biol Psychiatry. 2012;72(5):354-360.
18. Pepino MY, Okunade AL, Eagon JC, et al. Effect of Roux-en-Y gastric bypass surgery: converting 2 alcoholic drinks to 4. JAMA Surg. 2015
19. Changchien EM, Woodard GA, Hernandez-Boussard T, et al. Normal alcohol metabolism after gastric banding and sleeve gastrectomy: a case-cross-over trial. J Am Coll Surg. 2012;215(4):475-479.
22. Polston JE, Pritchett CE, Tomasko JM, et al. Roux-en-Y gastric bypass increases intravenous ethanol self-administration in dietary obese rats. PLoS ONE. 2013;8(12):e83741. doi: 10.1371/journal.pone.0083741.
26. Higgs ML, Wade T, Cescato M, et al. Differences between treatment seekers in an obese population: medical intervention vs. dietary restriction. J Behav Med. 1997;20(4):391-405.
35. Sutin AR, Stephan Y, Terracciano A. Weight discrimination and risk of mortality. Psychol Sci. 2015;26(11):1803-1811.
36. Sogg S, Gorman MJ. Interpersonal changes and challenges after weight loss surgery. Prim Psychiatry. 2008;15(8):61-66.
37. Yanos BR, Saules KK, Schuh LM, et al. Predictors of lowest weight and long-term weight regain among Roux-en-Y gastric bypass patients. Obes Surg. 2015;25(8):1364-1370.
40. van der Beek E, Te Riele W, Specken TF, et al. The impact of reconstructive procedures following bariatric surgery on patient well-being and quality of life. Obes Surg. 2010;20(1):36-41.
Maladaptive alcohol use has emerged as a risk for a subset of individuals who have undergone weight loss surgery (WLS); studies report they are vulnerable to consuming alcohol in greater quantities or more frequently.1,2 Estimates of the prevalence of “high-risk” or “hazardous” alcohol use after WLS range from 4% to 28%,3,4 while the prevalence of alcohol use meeting DSM-IV-TR5 criteria for alcohol use disorders (AUDs) hovers around 10%.6
Heavy alcohol users or patients who have active AUD at the time of WLS are at greater risk for continuation of these problems after surgery.2,6 For patients with a long-remitted history of AUD, the evidence regarding risk for post-WLS relapse is lacking, and some evidence suggests they may have better weight loss outcomes after WLS.7
However, approximately two-third of cases of post-WLS alcohol problems occur in patients who have had no history of such problems before surgery.5,8,9 Reported prevalence rates of new-onset alcohol problems range from 3% to 18%,6,9 with the modal finding being approximately 7% to 8%. New-onset alcohol problems appear to occur at a considerable latency after surgery. One study found little risk at 1 year post-surgery, but a significant increase in AUD symptoms at 2 years.6 Another study identified 3 years post-surgery as a high-risk time point,8 and yet another reported a linear increase in the risk for developing alcohol problems for at least 10 years after WLS.10
This article describes a group treatment protocol developed specifically for patients with post-WLS substance use disorder (SUD), and explores:
- risk factors and causal mechanisms of post-WLS AUDs
- weight stigma and emotional stressors
- the role of specialized treatment
- group treatment based on the Health at Every Size® (HAES)-oriented, trauma-informed and fat acceptance framework.
Post-WLS patients with alcohol problems may be a distinct phenotype among people with substance abuse issues. For this reason, they may have a need to address their experiences and issues specific to WLS as part of their alcohol treatment.
Etiology
Risk factors. Empirical findings have identified few predictors or risk factors for post-WLS SUD. These patients are more likely to be male and of a younger age.6 Notably, the vast majority of individuals reporting post-WLS alcohol problems have undergone Roux-en-Y gastric bypass (RYGB), rather than other WLS procedures, such as the laparoscopic adjustable gastric band,6,11 suggesting some physiological mechanism specific to RYGB.
Other potential predictors of postoperative alcohol problems include a pre-operative history of depression, generalized anxiety disorder, smoking, and/or recreational drug use.3,6 Likewise, patients with depression or anxiety disorder symptoms after surgery also may be at higher risk for postoperative alcohol problems.4 The evidence of an association between postoperative weight outcomes and post-WLS alcohol problems is mixed.3,12 Interestingly, patients who had no personal history of substance abuse but who have a family history may have a higher risk of new-onset alcohol problems after surgery.9,12
Causal mechanisms. The etiology of post-WLS alcohol problems is not well understood. If anything, epidemiological data suggest that larger-bodied individuals tend to consume lower levels of alcohol and have lower rates of AUD than individuals in the general population with thinner bodies.13 However, an association has been found between a family history of SUD, but not a personal history, and being large.14 This suggests a shared etiological pathway between addiction and being “overweight,” of which the onset of AUD after RYGB may be a manifestation.
Human and animal studies have shown that WLS may affect alcohol use differently in specific subgroups. Studies have shown that wild-type rats greatly increase their consumption of, or operant responding for, alcohol after RYGB,15 while genetically “alcohol-preferring” rats decrease consumption of, or responding for, alcohol after RYGB.16 A human study likewise found some patients decreased alcohol use or experienced improvement of or remission of AUD symptoms after WLS.4 Combined with the finding that a family history of substance abuse is related to risk for post-operative AUD, these data suggest a potential genetic vulnerability or protection in some individuals.
Turning to potential psychosocial explanations, the lay media has popularized the concepts of “addiction transfer,” or “transfer addiction,”12 with the implication that some patients, who had a preoperative history of “food addiction,” transfer that “addiction” after surgery to substances of abuse.
However, the “addiction transfer” model has a number of flaws:
- it is stigmatizing, because it assumes the patient possesses an innate, chronic, and inalterable pathology
- it relies upon the validity of the controversial construct of “food addiction,” a construct of mixed scientific evidence.17
Further, our knowledge of post-WLS SUD argues against “addiction transfer.” As noted, postoperative alcohol problems are more likely to develop years after surgery, rather than in the first few months afterward when eating is most significantly curtailed. Additionally, post-WLS alcohol problems are significantly more likely to occur after RYGB than other procedures, whereas the “addiction transfer” model would hypothesize that all WLS patients would be at equal risk for postoperative “addiction transfer,” because their eating is similarly affected after surgery.
Links to RYGB. Some clues to physiological mechanisms underlying alcohol problems after RYGB have been identified. After surgery, many RYGB patients report a quicker effect from a smaller amount of alcohol than was the case pre-surgery.18 Studies have demonstrated a number of changes in the pharmacodynamics of alcohol after RYGB not seen in other WLS procedures19:
- a much faster time to peak blood (or breath) alcohol content (BAC)
- significantly higher peak BAC
- a precipitous initial decline in perceived intoxication.18,20
Anatomical features of RYGB may explain such changes.8 However, an increased response to both IV alcohol and IV morphine after RYGB21,22 in rodents suggests that gastrointestinal tract changes are not solely responsible for changes in alcohol use. Emerging research reports that WLS has been found to cause alterations in brain reward pathways,23 which may be an additional contributor to changes in alcohol misuse after surgery.
However, even combined, pharmacokinetic and neurobiological factors cannot entirely explain new-onset alcohol problems after WLS; if they could, one would expect to see a much higher prevalence of this complication. Some psychosocial factors are likely involved as well.
Emotional stressors. One possibility involves a mismatch between post-WLS stressors and coping skills. After WLS, these patients face a multitude of challenges inherent in adjusting to changes in lifestyle, weight, body image, and social functioning, which most individuals would find daunting. These challenges become even more acute in the absence of appropriate psychoeducation, preparation, and intervention from qualified professionals. Individuals who lack effective and adaptive coping skills and supports may have a particularly heightened vulnerability to increased alcohol use in the setting of post-surgery changes in brain reward circuits and pharmacodynamics in alcohol metabolism. For example, one patient reported that her spouse’s pressure to “do something about her weight” was a significant factor in her decision to undergo surgery, but that her spouse was blaming and unsupportive when post-WLS complications developed. The patient believed that these experiences helped fuel development of her post-RYGB alcohol abuse.
Specialized treatment
The number of patients experiencing post-WLS alcohol problems likely will continue to grow, given that the risk of onset of has been shown increase over years. Already, post-WLS patients are proportionally overrepresented among substance abuse treatment populations.24 Empirically, however, we do not know yet if these patients need a different type of addiction treatment than patients who have not had WLS.
Some evidence suggests that post-WLS patients with alcohol problems may be a distinct phenotype within the general population with alcohol problems, as their presentations differ in several ways, including their demographics, alcohol use patterns, and premorbid functioning. A number of studies have found that, despite their increased pharmacodynamic sensitivity to alcohol, people with post-WLS AUDs actually consume a larger amount of alcohol on both typical and maximum drinking days than other individuals with AUDs.24 Additionally, although the median age of onset for AUD is around age 20,25 patients presenting with new-onset, post-WLS alcohol problems are usually in their late 30s, or even 40s or 50s. Further, many of these patients were quite high functioning before their alcohol problems, and are unlikely to identify with the cultural stereotype of a person with AUD (eg, homeless, unemployed), which may hamper or delay their own willingness to accept that they have a problem. These phenotypic differences suggest that post-WLS patients may require substance abuse treatment approaches tailored to their unique presentation. There are additional factors specific to the experiences of being larger-bodied and WLS that also may need to be addressed in specialized treatment for post-WLS addiction patients.
Weight stigma. By definition, patients who have undergone WLS have spent a significant portion of their lives inhabiting larger bodies, an experience that, in our culture, can produce adverse psychosocial effects. Compared with the general population, patients seeking WLS exhibit psychological distress equivalent to psychiatric patients.26 Weight stigma or weight bias—negative judgments directed toward people in larger bodies—is pervasive and continues to increase.27 Further, evidence suggests that, unlike almost all other stigmatized groups, people in larger bodies tend to internalize this stigma, holding an unfavorable attitude toward their own social group.28 Weight stigma impacts the well-being of people all along the weight spectrum, affecting many domains including educational achievements and classroom experiences, job opportunities, salaries, and medical care.27 Weight stigma increases the likelihood of bullying, teasing, and harassment for both adults and children.27 Weight bias has been associated with any number of adverse psychosocial effects, including symptoms of depression, anxiety, and eating pathology; poor body image; and a decrease in healthy self-care behaviors.29-33
Weight stigma makes it more difficult for people to enjoy physical activities, nourish their bodies, and manage stress, which contributes to poorer health outcomes and lower quality of life.33,34 For example, one study showed that, regardless of actual body mass index, people experiencing weight stigma have significantly increased risk of developing an illness or dying.35
Factors specific to WLS. WLS may lead to significant changes in eating habits, and some patients experience a sense of loss, particularly if eating represented one of their primary coping strategies—this may represent a heightened emotional vulnerability for developing AUD.
The fairly rapid and substantial weight loss that WLS produces can lead to sweeping changes in lifestyle, body image, and functional factors for many individuals. Patients often report profound changes, both positive and negative, in their relationships and interactions not only with people in their support network, but also with strangers.36
After the first year or 2 post-WLS, it is fairly common for patients to regain some weight, sometimes in significant amounts.37 This can lead to a sense of “failure.” Life stressors, including difficulties in important relationships, can further add to patients’ vulnerability. For example, one patient noticed that when she was at her thinnest after WLS, drivers were more likely to stop for her when she crossed the street, which pleased but also angered her because they hadn’t extended the same courtesy before WLS. After she regained a significant amount of weight, she began to notice drivers stopping for her less and less frequently. This took her back to her previous feelings of being ignored but now with the certainty that she would be treated better if she were thinner.
Patients also may experience ambivalence about changes in their body size. One might expect that body image would improve after weight loss, but the evidence is mixed.38 Although there is some evidence that body image improves in the short term after WLS,38 other research indicates that body image does not improve with weight loss.39 However, the evidence is clear that the appearance of excess skin after weight loss worsens some patients’ body image.40
To date, there has been no research examining treatment modalities for this population. Because experiences common to individuals who have had WLS could play a role in the development of AUD after surgery, it is intuitive that it would be important to address these factors when designing a treatment plan for post-WLS substance abuse.
Group treatment approach
In 2013, in response to the increase in rates of post-WLS addictions presenting to West End Clinic, an outpatient dual-diagnosis (addiction and psychiatry) service at Massachusetts General Hospital, a specialized treatment group was developed. Nine patients have enrolled since October 2013.
The Post-WLS Addictions Group (PWAG) was designed to be HAES-oriented, trauma-informed, and run within a fat acceptance framework. The HAES model prioritizes a weight-neutral approach that sees health and well-being as multifaceted. This approach directs both patient and clinician to focus on improving health behaviors and reducing internalized weight bias, while building a supportive community that buffers against external cultural weight bias.41
Trauma-informed care42 emphasizes the principles of safety, trustworthiness, and transparency; peer support; collaboration and mutuality; empowerment; and awareness of cultural, historical, and gender issues. In the context of PWAG, weight stigma is conceptualized as a traumatic experience.43 The fat acceptance approach promotes a culture that accepts people of every size with dignity and equality in all aspects of life.44
Self-care emphasis. The HAES model encourages patients to allow their bodies to determine what weight to settle at, and to focus on sustainable health-enhancing behaviors rather than weight loss. Patients who asked about the PWAG were told that this group would not explicitly support, or even encourage, continued pursuit of weight loss per se, but instead would assist patients with relapse prevention, mindful eating, improving self-care, and ongoing stress management. Moving away from a focus on weight loss and toward improvement of self-care skills allowed patients to focus on behaviors and outcomes over which they had more direct control and were more likely to yield immediate benefits.
All of the PWAG group members were in early recovery from an SUD, with a minimum of 4 weeks of abstinence; all had at least 1 co-occurring mental health diagnosis. A licensed independent clinical social worker (LICSW) and a physician familiar with bariatric surgery ran the sessions. The group met weekly for 1 hour. The 8 weekly sessions included both psychoeducation and discussion, with each session covering different topics (Table). The first 20 minutes of each session were devoted to an educational presentation; the remaining 40 minutes for reflection and discussion. In sessions 2 through 8, participants were asked about any recent use or cravings, and problem-solving techniques were employed as needed.

The PWAG group leader herself is a large person who modeled fat acceptance and follows the HAES approach; she led the group using both this experience and her specialized clinical training. As is the case with other addictions recovery treatment modalities, clinicians with lived experience may add a valuable component to both the program design and patient experience.
After the first 8 sessions, all members expressed interest in continuing as an ongoing relapse prevention and HAES support group, and they reported that meeting regularly was very helpful. The group continued with the LICSW alone, who continued to share HAES-oriented and fat acceptance information and resources that group members requested specifically. Over time, new members joined following an individual orientation session with the group leader, and the group has revisited each of the psychoeducational topics repeatedly, though not in a formally structured way.
Process and observations. Participants described high levels of excitement and hopefulness about being in a group with other WLS patients who had developed SUDs. They had a particular interest in reviewing medical/anatomical information about WLS and understanding more about the potential reasons for the elevated risk for developing SUD following WLS. Discussions regarding weight stigma proved to be quite emotional; most participants reported that this material readily related to their own experiences with weight stigma, but they had never discussed these ideas before.
Participants explored the role that grief, loss, guilt, and shame had in the decision to have WLS, the development of SUDs, weight regain or medical complications from the surgery or from substance abuse, career and relationship changes, and worsened body image. Another theme that emerged was the various reasons that prompted the members have WLS that they may not have been conscious of, or willing to discuss with others, such as pressure from a spouse, fears of remaining single due to their size, and a desire to finally “fit in.”
Repeatedly, group members expressed how satisfied and emotionally validated they felt being with people with similar experiences. Most of them had felt alone. They reported a belief that “everyone else” who had WLS was doing well, and that they were the exceptions. Such beliefs and emotions increased the risk of relapse and decreased participants’ ability to develop more positive coping strategies and self-care skills.
Participants reported that feeling less alone, understanding how stigma impacts health and well-being, and focusing on the general benefits of good self-care rather than the pursuit of weight loss were particularly helpful. The HAES and fat acceptance approaches have given group members new ways to think about their bodies and decreased shame. Several group members reported that if they had learned about the HAES approach prior to having a WLS, they might have made a different decision about having surgery, or at least might have been better prepared to handle the emotional and psychological challenges after WLS.
Although evidence for post-WLS addictions is fairly robust, causal mechanisms are not well understood, and research identifying specific risk factors is lacking. Because post-WLS patients with addictions seem to represent a specific phenotype, specialized treatment might be indicated. Future research will be needed to determine optimal treatment approaches for post-WLS addictions. However, a number of aspects are likely to be important. For example, it is likely that unaddressed experiences of weight stigma contribute to challenges, including substance abuse, after WLS; therefore, clinicians involved in the care of individuals presenting with post-WLS SUD should be knowledgeable about weight stigma and how to address it. Because of the specific nature of post-WLS addictions, patients often feel alone and isolated, and seem to benefit from the specialized group setting. We note that the PWAG group leader is herself a large person who models fat acceptance and follows the HAES approach, and therefore led the group using this experience and her specialized clinical training. As with other addiction recovery treatment modalities, clinicians who have lived the experience can add a valuable component to the program design and patient experience.
Maladaptive alcohol use has emerged as a risk for a subset of individuals who have undergone weight loss surgery (WLS); studies report they are vulnerable to consuming alcohol in greater quantities or more frequently.1,2 Estimates of the prevalence of “high-risk” or “hazardous” alcohol use after WLS range from 4% to 28%,3,4 while the prevalence of alcohol use meeting DSM-IV-TR5 criteria for alcohol use disorders (AUDs) hovers around 10%.6
Heavy alcohol users or patients who have active AUD at the time of WLS are at greater risk for continuation of these problems after surgery.2,6 For patients with a long-remitted history of AUD, the evidence regarding risk for post-WLS relapse is lacking, and some evidence suggests they may have better weight loss outcomes after WLS.7
However, approximately two-third of cases of post-WLS alcohol problems occur in patients who have had no history of such problems before surgery.5,8,9 Reported prevalence rates of new-onset alcohol problems range from 3% to 18%,6,9 with the modal finding being approximately 7% to 8%. New-onset alcohol problems appear to occur at a considerable latency after surgery. One study found little risk at 1 year post-surgery, but a significant increase in AUD symptoms at 2 years.6 Another study identified 3 years post-surgery as a high-risk time point,8 and yet another reported a linear increase in the risk for developing alcohol problems for at least 10 years after WLS.10
This article describes a group treatment protocol developed specifically for patients with post-WLS substance use disorder (SUD), and explores:
- risk factors and causal mechanisms of post-WLS AUDs
- weight stigma and emotional stressors
- the role of specialized treatment
- group treatment based on the Health at Every Size® (HAES)-oriented, trauma-informed and fat acceptance framework.
Post-WLS patients with alcohol problems may be a distinct phenotype among people with substance abuse issues. For this reason, they may have a need to address their experiences and issues specific to WLS as part of their alcohol treatment.
Etiology
Risk factors. Empirical findings have identified few predictors or risk factors for post-WLS SUD. These patients are more likely to be male and of a younger age.6 Notably, the vast majority of individuals reporting post-WLS alcohol problems have undergone Roux-en-Y gastric bypass (RYGB), rather than other WLS procedures, such as the laparoscopic adjustable gastric band,6,11 suggesting some physiological mechanism specific to RYGB.
Other potential predictors of postoperative alcohol problems include a pre-operative history of depression, generalized anxiety disorder, smoking, and/or recreational drug use.3,6 Likewise, patients with depression or anxiety disorder symptoms after surgery also may be at higher risk for postoperative alcohol problems.4 The evidence of an association between postoperative weight outcomes and post-WLS alcohol problems is mixed.3,12 Interestingly, patients who had no personal history of substance abuse but who have a family history may have a higher risk of new-onset alcohol problems after surgery.9,12
Causal mechanisms. The etiology of post-WLS alcohol problems is not well understood. If anything, epidemiological data suggest that larger-bodied individuals tend to consume lower levels of alcohol and have lower rates of AUD than individuals in the general population with thinner bodies.13 However, an association has been found between a family history of SUD, but not a personal history, and being large.14 This suggests a shared etiological pathway between addiction and being “overweight,” of which the onset of AUD after RYGB may be a manifestation.
Human and animal studies have shown that WLS may affect alcohol use differently in specific subgroups. Studies have shown that wild-type rats greatly increase their consumption of, or operant responding for, alcohol after RYGB,15 while genetically “alcohol-preferring” rats decrease consumption of, or responding for, alcohol after RYGB.16 A human study likewise found some patients decreased alcohol use or experienced improvement of or remission of AUD symptoms after WLS.4 Combined with the finding that a family history of substance abuse is related to risk for post-operative AUD, these data suggest a potential genetic vulnerability or protection in some individuals.
Turning to potential psychosocial explanations, the lay media has popularized the concepts of “addiction transfer,” or “transfer addiction,”12 with the implication that some patients, who had a preoperative history of “food addiction,” transfer that “addiction” after surgery to substances of abuse.
However, the “addiction transfer” model has a number of flaws:
- it is stigmatizing, because it assumes the patient possesses an innate, chronic, and inalterable pathology
- it relies upon the validity of the controversial construct of “food addiction,” a construct of mixed scientific evidence.17
Further, our knowledge of post-WLS SUD argues against “addiction transfer.” As noted, postoperative alcohol problems are more likely to develop years after surgery, rather than in the first few months afterward when eating is most significantly curtailed. Additionally, post-WLS alcohol problems are significantly more likely to occur after RYGB than other procedures, whereas the “addiction transfer” model would hypothesize that all WLS patients would be at equal risk for postoperative “addiction transfer,” because their eating is similarly affected after surgery.
Links to RYGB. Some clues to physiological mechanisms underlying alcohol problems after RYGB have been identified. After surgery, many RYGB patients report a quicker effect from a smaller amount of alcohol than was the case pre-surgery.18 Studies have demonstrated a number of changes in the pharmacodynamics of alcohol after RYGB not seen in other WLS procedures19:
- a much faster time to peak blood (or breath) alcohol content (BAC)
- significantly higher peak BAC
- a precipitous initial decline in perceived intoxication.18,20
Anatomical features of RYGB may explain such changes.8 However, an increased response to both IV alcohol and IV morphine after RYGB21,22 in rodents suggests that gastrointestinal tract changes are not solely responsible for changes in alcohol use. Emerging research reports that WLS has been found to cause alterations in brain reward pathways,23 which may be an additional contributor to changes in alcohol misuse after surgery.
However, even combined, pharmacokinetic and neurobiological factors cannot entirely explain new-onset alcohol problems after WLS; if they could, one would expect to see a much higher prevalence of this complication. Some psychosocial factors are likely involved as well.
Emotional stressors. One possibility involves a mismatch between post-WLS stressors and coping skills. After WLS, these patients face a multitude of challenges inherent in adjusting to changes in lifestyle, weight, body image, and social functioning, which most individuals would find daunting. These challenges become even more acute in the absence of appropriate psychoeducation, preparation, and intervention from qualified professionals. Individuals who lack effective and adaptive coping skills and supports may have a particularly heightened vulnerability to increased alcohol use in the setting of post-surgery changes in brain reward circuits and pharmacodynamics in alcohol metabolism. For example, one patient reported that her spouse’s pressure to “do something about her weight” was a significant factor in her decision to undergo surgery, but that her spouse was blaming and unsupportive when post-WLS complications developed. The patient believed that these experiences helped fuel development of her post-RYGB alcohol abuse.
Specialized treatment
The number of patients experiencing post-WLS alcohol problems likely will continue to grow, given that the risk of onset of has been shown increase over years. Already, post-WLS patients are proportionally overrepresented among substance abuse treatment populations.24 Empirically, however, we do not know yet if these patients need a different type of addiction treatment than patients who have not had WLS.
Some evidence suggests that post-WLS patients with alcohol problems may be a distinct phenotype within the general population with alcohol problems, as their presentations differ in several ways, including their demographics, alcohol use patterns, and premorbid functioning. A number of studies have found that, despite their increased pharmacodynamic sensitivity to alcohol, people with post-WLS AUDs actually consume a larger amount of alcohol on both typical and maximum drinking days than other individuals with AUDs.24 Additionally, although the median age of onset for AUD is around age 20,25 patients presenting with new-onset, post-WLS alcohol problems are usually in their late 30s, or even 40s or 50s. Further, many of these patients were quite high functioning before their alcohol problems, and are unlikely to identify with the cultural stereotype of a person with AUD (eg, homeless, unemployed), which may hamper or delay their own willingness to accept that they have a problem. These phenotypic differences suggest that post-WLS patients may require substance abuse treatment approaches tailored to their unique presentation. There are additional factors specific to the experiences of being larger-bodied and WLS that also may need to be addressed in specialized treatment for post-WLS addiction patients.
Weight stigma. By definition, patients who have undergone WLS have spent a significant portion of their lives inhabiting larger bodies, an experience that, in our culture, can produce adverse psychosocial effects. Compared with the general population, patients seeking WLS exhibit psychological distress equivalent to psychiatric patients.26 Weight stigma or weight bias—negative judgments directed toward people in larger bodies—is pervasive and continues to increase.27 Further, evidence suggests that, unlike almost all other stigmatized groups, people in larger bodies tend to internalize this stigma, holding an unfavorable attitude toward their own social group.28 Weight stigma impacts the well-being of people all along the weight spectrum, affecting many domains including educational achievements and classroom experiences, job opportunities, salaries, and medical care.27 Weight stigma increases the likelihood of bullying, teasing, and harassment for both adults and children.27 Weight bias has been associated with any number of adverse psychosocial effects, including symptoms of depression, anxiety, and eating pathology; poor body image; and a decrease in healthy self-care behaviors.29-33
Weight stigma makes it more difficult for people to enjoy physical activities, nourish their bodies, and manage stress, which contributes to poorer health outcomes and lower quality of life.33,34 For example, one study showed that, regardless of actual body mass index, people experiencing weight stigma have significantly increased risk of developing an illness or dying.35
Factors specific to WLS. WLS may lead to significant changes in eating habits, and some patients experience a sense of loss, particularly if eating represented one of their primary coping strategies—this may represent a heightened emotional vulnerability for developing AUD.
The fairly rapid and substantial weight loss that WLS produces can lead to sweeping changes in lifestyle, body image, and functional factors for many individuals. Patients often report profound changes, both positive and negative, in their relationships and interactions not only with people in their support network, but also with strangers.36
After the first year or 2 post-WLS, it is fairly common for patients to regain some weight, sometimes in significant amounts.37 This can lead to a sense of “failure.” Life stressors, including difficulties in important relationships, can further add to patients’ vulnerability. For example, one patient noticed that when she was at her thinnest after WLS, drivers were more likely to stop for her when she crossed the street, which pleased but also angered her because they hadn’t extended the same courtesy before WLS. After she regained a significant amount of weight, she began to notice drivers stopping for her less and less frequently. This took her back to her previous feelings of being ignored but now with the certainty that she would be treated better if she were thinner.
Patients also may experience ambivalence about changes in their body size. One might expect that body image would improve after weight loss, but the evidence is mixed.38 Although there is some evidence that body image improves in the short term after WLS,38 other research indicates that body image does not improve with weight loss.39 However, the evidence is clear that the appearance of excess skin after weight loss worsens some patients’ body image.40
To date, there has been no research examining treatment modalities for this population. Because experiences common to individuals who have had WLS could play a role in the development of AUD after surgery, it is intuitive that it would be important to address these factors when designing a treatment plan for post-WLS substance abuse.
Group treatment approach
In 2013, in response to the increase in rates of post-WLS addictions presenting to West End Clinic, an outpatient dual-diagnosis (addiction and psychiatry) service at Massachusetts General Hospital, a specialized treatment group was developed. Nine patients have enrolled since October 2013.
The Post-WLS Addictions Group (PWAG) was designed to be HAES-oriented, trauma-informed, and run within a fat acceptance framework. The HAES model prioritizes a weight-neutral approach that sees health and well-being as multifaceted. This approach directs both patient and clinician to focus on improving health behaviors and reducing internalized weight bias, while building a supportive community that buffers against external cultural weight bias.41
Trauma-informed care42 emphasizes the principles of safety, trustworthiness, and transparency; peer support; collaboration and mutuality; empowerment; and awareness of cultural, historical, and gender issues. In the context of PWAG, weight stigma is conceptualized as a traumatic experience.43 The fat acceptance approach promotes a culture that accepts people of every size with dignity and equality in all aspects of life.44
Self-care emphasis. The HAES model encourages patients to allow their bodies to determine what weight to settle at, and to focus on sustainable health-enhancing behaviors rather than weight loss. Patients who asked about the PWAG were told that this group would not explicitly support, or even encourage, continued pursuit of weight loss per se, but instead would assist patients with relapse prevention, mindful eating, improving self-care, and ongoing stress management. Moving away from a focus on weight loss and toward improvement of self-care skills allowed patients to focus on behaviors and outcomes over which they had more direct control and were more likely to yield immediate benefits.
All of the PWAG group members were in early recovery from an SUD, with a minimum of 4 weeks of abstinence; all had at least 1 co-occurring mental health diagnosis. A licensed independent clinical social worker (LICSW) and a physician familiar with bariatric surgery ran the sessions. The group met weekly for 1 hour. The 8 weekly sessions included both psychoeducation and discussion, with each session covering different topics (Table). The first 20 minutes of each session were devoted to an educational presentation; the remaining 40 minutes for reflection and discussion. In sessions 2 through 8, participants were asked about any recent use or cravings, and problem-solving techniques were employed as needed.

The PWAG group leader herself is a large person who modeled fat acceptance and follows the HAES approach; she led the group using both this experience and her specialized clinical training. As is the case with other addictions recovery treatment modalities, clinicians with lived experience may add a valuable component to both the program design and patient experience.
After the first 8 sessions, all members expressed interest in continuing as an ongoing relapse prevention and HAES support group, and they reported that meeting regularly was very helpful. The group continued with the LICSW alone, who continued to share HAES-oriented and fat acceptance information and resources that group members requested specifically. Over time, new members joined following an individual orientation session with the group leader, and the group has revisited each of the psychoeducational topics repeatedly, though not in a formally structured way.
Process and observations. Participants described high levels of excitement and hopefulness about being in a group with other WLS patients who had developed SUDs. They had a particular interest in reviewing medical/anatomical information about WLS and understanding more about the potential reasons for the elevated risk for developing SUD following WLS. Discussions regarding weight stigma proved to be quite emotional; most participants reported that this material readily related to their own experiences with weight stigma, but they had never discussed these ideas before.
Participants explored the role that grief, loss, guilt, and shame had in the decision to have WLS, the development of SUDs, weight regain or medical complications from the surgery or from substance abuse, career and relationship changes, and worsened body image. Another theme that emerged was the various reasons that prompted the members have WLS that they may not have been conscious of, or willing to discuss with others, such as pressure from a spouse, fears of remaining single due to their size, and a desire to finally “fit in.”
Repeatedly, group members expressed how satisfied and emotionally validated they felt being with people with similar experiences. Most of them had felt alone. They reported a belief that “everyone else” who had WLS was doing well, and that they were the exceptions. Such beliefs and emotions increased the risk of relapse and decreased participants’ ability to develop more positive coping strategies and self-care skills.
Participants reported that feeling less alone, understanding how stigma impacts health and well-being, and focusing on the general benefits of good self-care rather than the pursuit of weight loss were particularly helpful. The HAES and fat acceptance approaches have given group members new ways to think about their bodies and decreased shame. Several group members reported that if they had learned about the HAES approach prior to having a WLS, they might have made a different decision about having surgery, or at least might have been better prepared to handle the emotional and psychological challenges after WLS.
Although evidence for post-WLS addictions is fairly robust, causal mechanisms are not well understood, and research identifying specific risk factors is lacking. Because post-WLS patients with addictions seem to represent a specific phenotype, specialized treatment might be indicated. Future research will be needed to determine optimal treatment approaches for post-WLS addictions. However, a number of aspects are likely to be important. For example, it is likely that unaddressed experiences of weight stigma contribute to challenges, including substance abuse, after WLS; therefore, clinicians involved in the care of individuals presenting with post-WLS SUD should be knowledgeable about weight stigma and how to address it. Because of the specific nature of post-WLS addictions, patients often feel alone and isolated, and seem to benefit from the specialized group setting. We note that the PWAG group leader is herself a large person who models fat acceptance and follows the HAES approach, and therefore led the group using this experience and her specialized clinical training. As with other addiction recovery treatment modalities, clinicians who have lived the experience can add a valuable component to the program design and patient experience.
2. Lent MR, Hayes SM, Wood GC, et al. Smoking and alcohol use in gastric bypass patients. Eat Behav. 2013;14(4):460-463.
4. Wee CC, Mukamal KJ, Huskey KW, et al. High-risk alcohol use after weight loss surgery. Surg Obes Relat Dis. 2014;10(3):508-513.
5. Diagnostic and statistical manual of mental disorders, 4th, text rev. Washington, DC: American Psychiatric Association; 2000.
6. King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516-2525.
9. Ivezaj V, Saules KK, Schuh LM. New-onset substance use disorder after gastric bypass surgery: rates and associated characteristics. Obes Surg. 2014;24(11):1975-1980.
10. Svensson PA, Anveden Å, Romeo S, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity. 2013;21(12):2444-2451.
11. Ostlund MP, Backman O, Marsk R, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148(4):374-377.
13. Gearhardt AN, Corbin WR. Body mass index and alcohol consumption: family history of alcoholism as a moderator. Psychol Addict Behav. 2009;23(2):216-225.
14. Grucza RA, Krueger RF, Racette SB, et al. The emerging link between alcoholism risk and obesity in the United States. Arch Gen Psychiatry. 2010;67(12):1301-1308.
15. Davis JF, Tracy AL, Schurdak JD, et al. Roux en y gastric bypass increases ethanol intake in the rat. Obes Surg. 2013;23(7):920-930.
16. Davis JF, Schurdak JD, Magrisso IJ, et al. Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats. Biol Psychiatry. 2012;72(5):354-360.
18. Pepino MY, Okunade AL, Eagon JC, et al. Effect of Roux-en-Y gastric bypass surgery: converting 2 alcoholic drinks to 4. JAMA Surg. 2015
19. Changchien EM, Woodard GA, Hernandez-Boussard T, et al. Normal alcohol metabolism after gastric banding and sleeve gastrectomy: a case-cross-over trial. J Am Coll Surg. 2012;215(4):475-479.
22. Polston JE, Pritchett CE, Tomasko JM, et al. Roux-en-Y gastric bypass increases intravenous ethanol self-administration in dietary obese rats. PLoS ONE. 2013;8(12):e83741. doi: 10.1371/journal.pone.0083741.
26. Higgs ML, Wade T, Cescato M, et al. Differences between treatment seekers in an obese population: medical intervention vs. dietary restriction. J Behav Med. 1997;20(4):391-405.
35. Sutin AR, Stephan Y, Terracciano A. Weight discrimination and risk of mortality. Psychol Sci. 2015;26(11):1803-1811.
36. Sogg S, Gorman MJ. Interpersonal changes and challenges after weight loss surgery. Prim Psychiatry. 2008;15(8):61-66.
37. Yanos BR, Saules KK, Schuh LM, et al. Predictors of lowest weight and long-term weight regain among Roux-en-Y gastric bypass patients. Obes Surg. 2015;25(8):1364-1370.
40. van der Beek E, Te Riele W, Specken TF, et al. The impact of reconstructive procedures following bariatric surgery on patient well-being and quality of life. Obes Surg. 2010;20(1):36-41.
2. Lent MR, Hayes SM, Wood GC, et al. Smoking and alcohol use in gastric bypass patients. Eat Behav. 2013;14(4):460-463.
4. Wee CC, Mukamal KJ, Huskey KW, et al. High-risk alcohol use after weight loss surgery. Surg Obes Relat Dis. 2014;10(3):508-513.
5. Diagnostic and statistical manual of mental disorders, 4th, text rev. Washington, DC: American Psychiatric Association; 2000.
6. King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516-2525.
9. Ivezaj V, Saules KK, Schuh LM. New-onset substance use disorder after gastric bypass surgery: rates and associated characteristics. Obes Surg. 2014;24(11):1975-1980.
10. Svensson PA, Anveden Å, Romeo S, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity. 2013;21(12):2444-2451.
11. Ostlund MP, Backman O, Marsk R, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148(4):374-377.
13. Gearhardt AN, Corbin WR. Body mass index and alcohol consumption: family history of alcoholism as a moderator. Psychol Addict Behav. 2009;23(2):216-225.
14. Grucza RA, Krueger RF, Racette SB, et al. The emerging link between alcoholism risk and obesity in the United States. Arch Gen Psychiatry. 2010;67(12):1301-1308.
15. Davis JF, Tracy AL, Schurdak JD, et al. Roux en y gastric bypass increases ethanol intake in the rat. Obes Surg. 2013;23(7):920-930.
16. Davis JF, Schurdak JD, Magrisso IJ, et al. Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats. Biol Psychiatry. 2012;72(5):354-360.
18. Pepino MY, Okunade AL, Eagon JC, et al. Effect of Roux-en-Y gastric bypass surgery: converting 2 alcoholic drinks to 4. JAMA Surg. 2015
19. Changchien EM, Woodard GA, Hernandez-Boussard T, et al. Normal alcohol metabolism after gastric banding and sleeve gastrectomy: a case-cross-over trial. J Am Coll Surg. 2012;215(4):475-479.
22. Polston JE, Pritchett CE, Tomasko JM, et al. Roux-en-Y gastric bypass increases intravenous ethanol self-administration in dietary obese rats. PLoS ONE. 2013;8(12):e83741. doi: 10.1371/journal.pone.0083741.
26. Higgs ML, Wade T, Cescato M, et al. Differences between treatment seekers in an obese population: medical intervention vs. dietary restriction. J Behav Med. 1997;20(4):391-405.
35. Sutin AR, Stephan Y, Terracciano A. Weight discrimination and risk of mortality. Psychol Sci. 2015;26(11):1803-1811.
36. Sogg S, Gorman MJ. Interpersonal changes and challenges after weight loss surgery. Prim Psychiatry. 2008;15(8):61-66.
37. Yanos BR, Saules KK, Schuh LM, et al. Predictors of lowest weight and long-term weight regain among Roux-en-Y gastric bypass patients. Obes Surg. 2015;25(8):1364-1370.
40. van der Beek E, Te Riele W, Specken TF, et al. The impact of reconstructive procedures following bariatric surgery on patient well-being and quality of life. Obes Surg. 2010;20(1):36-41.
Self-criticism and self-compassion: Risk and resilience
Once thought to only be associated with depression, self-criticism is a transdiagnostic risk factor for diverse forms of psychopathology.1,2 However, research has shown that self-compassion is a robust resilience factor when faced with feelings of personal inadequacy.3,4
Self-critical individuals experience feelings of unworthiness, inferiority, failure, and guilt. They engage in constant and harsh self-scrutiny and evaluation, and fear being disapproved and criticized and losing the approval and acceptance of others.5 Self-compassion involves treating oneself with care and concern when confronted with personal inadequacies, mistakes, failures, and painful life situations.6,7
Although self-criticism is destructive across clinical disorders and interpersonal relationships, self-compassion is associated with healthy relationships, emotional well-being, and better treatment outcomes.
Recent research shows how clinicians can teach their patients how to be less self-critical and more self-compassionate. Neff6,7 proposes that self-compassion involves treating yourself with care and concern when being confronted with personal inadequacies, mistakes, failures, and painful life situations. It consists of 3 interacting components, each of which has a positive and negative pole:
- self-kindness vs self-judgment
- a sense of common humanity vs isolation
- mindfulness vs over-identification.
Self-kindness refers to being caring and understanding with oneself rather than harshly judgmental. Instead of attacking and berating oneself for personal shortcomings, the self is offered warmth and unconditional acceptance.
Humanity involves recognizing that humans are imperfect, that all people fail, make mistakes, and have serious life challenges. By remembering that imperfection is part of life, we feel less isolated when we are in pain.
Mindfulness in the context of self-compassion involves being aware of one’s painful experiences in a balanced way that neither ignores and avoids nor exaggerates painful thoughts and emotions.
Self-compassion is more than the absence of self-judgment, although a defining feature of self-compassion is the lack of self-judgment, and self-judgment overlaps with self-criticism. Rather, self-compassion provides several access points for reducing self-criticism. For example, being kind and understanding when confronting personal inadequacies (eg, “it’s okay not to be perfect”) can counter harsh self-talk (eg, “I’m not defective”). Mindfulness of emotional pain (eg, “this is hard”) can facilitate a kind and warm response (eg, “what can I do to take care of myself right now?”) and therefore lessen self-blame (eg, “blaming myself is just causing me more suffering”). Similarly, remembering that failure is part of the human experience (eg, “it’s normal to mess up sometimes”) can lessen egocentric feelings of isolation (eg, “it’s not just me”) and over-identification (eg, “it’s not the end of the world”), resulting in lessened self-criticism (eg, “maybe it’s not just because I’m a bad person”).
Depression
Several studies have found that self-criticism predicts depression. In 3 epidemiological studies, “feeling worthless” was among the top 2 symptoms predicting a depression diagnosis and later depressive episodes.10 Self-criticism in fourth-year medical students predicted depression 2 years later, and—in males—10 years later in their medical careers better than a history of depression.11 Self-critical perfectionism also is associated with suicidal ideation and lethality of suicide attempts.12
Self-criticism has been shown to predict depressive relapse and residual self-devaluative symptoms in recovered depressed patients.13 In one study, currently depressed and remitted depressed patients had higher self-criticism and lower self-compassion compared with healthy controls. Both factors were more strongly associated with depression status than higher perfectionistic beliefs and cognitions, rumination, and maladaptive emotional regulation.14
Self-criticism and response to treatment. In the National Institute of Mental Health Treatment of Depression Collaborative Research Program,15 self-critical perfectionism predicted a poorer outcome across all 4 treatments (cognitive-behavioral therapy [CBT], interpersonal psychotherapy [IPT], pharmacotherapy plus clinical management, and placebo plus clinical management). Subsequent studies found that self-criticism predicted poorer response to CBT16 and IPT.17 The authors suggest that self-criticism could interfere with treatment because self-critical patients might have difficulty developing a strong therapeutic alliance.18,19
Anxiety disorders
Self-criticism is common across psychiatric disorders. In a study of 5,877 respondents in the National Comorbidity Survey (NCS), self-criticism was associated with social phobia, findings that were significant after controlling for current emotional distress, neuroticism, and lifetime history of mood, anxiety, and substance use disorders.20 Further, in a CBT treatment study, baseline self-criticism was associated with severity of social phobia and changes in self-criticism predicted treatment outcome.21 Self-criticism might be an important core psychological process in the development, maintenance, and course of social phobia. Patients with social anxiety disorder have less self-compassion than healthy controls and greater fear of negative evaluation.
In the NCS, self-criticism was associated with posttraumatic stress disorder (PTSD) even after controlling for lifetime history of affective and anxiety disorders.20 Self-criticism predicted greater severity of combat-related PTSD in hospitalized male veterans,22 and those with PTSD had higher scores on self-criticism scales than those with major depressive disorder.23 In a study of Holocaust survivors, those with PTSD scored higher on self-criticism than survivors without PTSD.24 Self-criticism also distinguished between female victims of domestic violence with and without PTSD.25
Self-compassion could be a protective factor for posttraumatic stress.26 Combat veterans with higher levels of self-compassion showed lower levels of psychopathology, better functioning in daily life, and fewer symptoms of posttraumatic stress.27 In fact, self-compassion has been found to be a stronger predictor of PTSD than level of combat exposure.28
In an early study, self-criticism scores were higher in patients with panic disorder than in healthy controls, but lower than in patients with depression.29 In a study of a mixed sample of anxiety disorder patients, symptoms of generalized anxiety disorder were associated with shame proneness.30 Consistent with these results, Hoge et al31 found that self-compassion was lower in generalized anxiety disorder patients compared with healthy controls with elevated stress. Low self-compassion has been associated with severity of obsessive-compulsive disorder.32
Eating disorders
Self-criticism is correlated with eating disorder severity.33 In a study of patients with binge eating disorder, Dunkley and Grilo34 found that self-criticism was associated with the over-evaluation of shape and weight independently of self-esteem and depression. Self-criticism also is associated with body dissatisfaction, independent of self-esteem and depression. Dunkley et al35 found that self-criticism, but not global self-esteem, in patients with binge eating disorder mediated the relationship between childhood abuse and body dissatisfaction and depression. Numerous studies have shown that shame is associated with more severe eating disorder pathology.33
Self-compassion seems to buffer against body image concerns. It is associated with less body dissatisfaction, body preoccupation, and weight worries,36 greater body appreciation37 and less disordered eating.37-39 Early decreases in shame during eating disorder treatment was associated with more rapid reduction in eating disorder symptoms.40
Interpersonal relationships
Several studies have shown that self-criticism has negative effects on interpersonal relationships throughout life.5,41,42
- Self-criticism at age 12 predicted less involvement in high school activities and, at age 31, personal and social maladjustment.43
- High school students with high self-criticism reported more interpersonal problems.44
- Self-criticism was associated with loneliness, depression, and lack of intimacy with opposite sex friends or partners during the transition to college.45
- In a study of college roommates,46 self-criticism was associated with increased likelihood of rejection.
- Whiffen and Aube47 found that self-criticism was associated with marital dissatisfaction and depression.
- Self-critical mothers with postpartum depression were less satisfied with social support and were more vulnerable to depression.48
Self-compassion appears to enhance interpersonal relationships. In a study of heterosexual couples,49 self-compassionate individuals were described by their partners as being more emotionally connected, as well as accepting and supporting autonomy, while being less detached, controlling, and verbally or physically aggressive than those lacking self-compassion. Because self-compassionate people give themselves care and support, they seem to have more emotional resources available to give to others.
See the Box examining the evidence on the role of self-compassion in borderline personality disorder and non-suicidal self-injury.
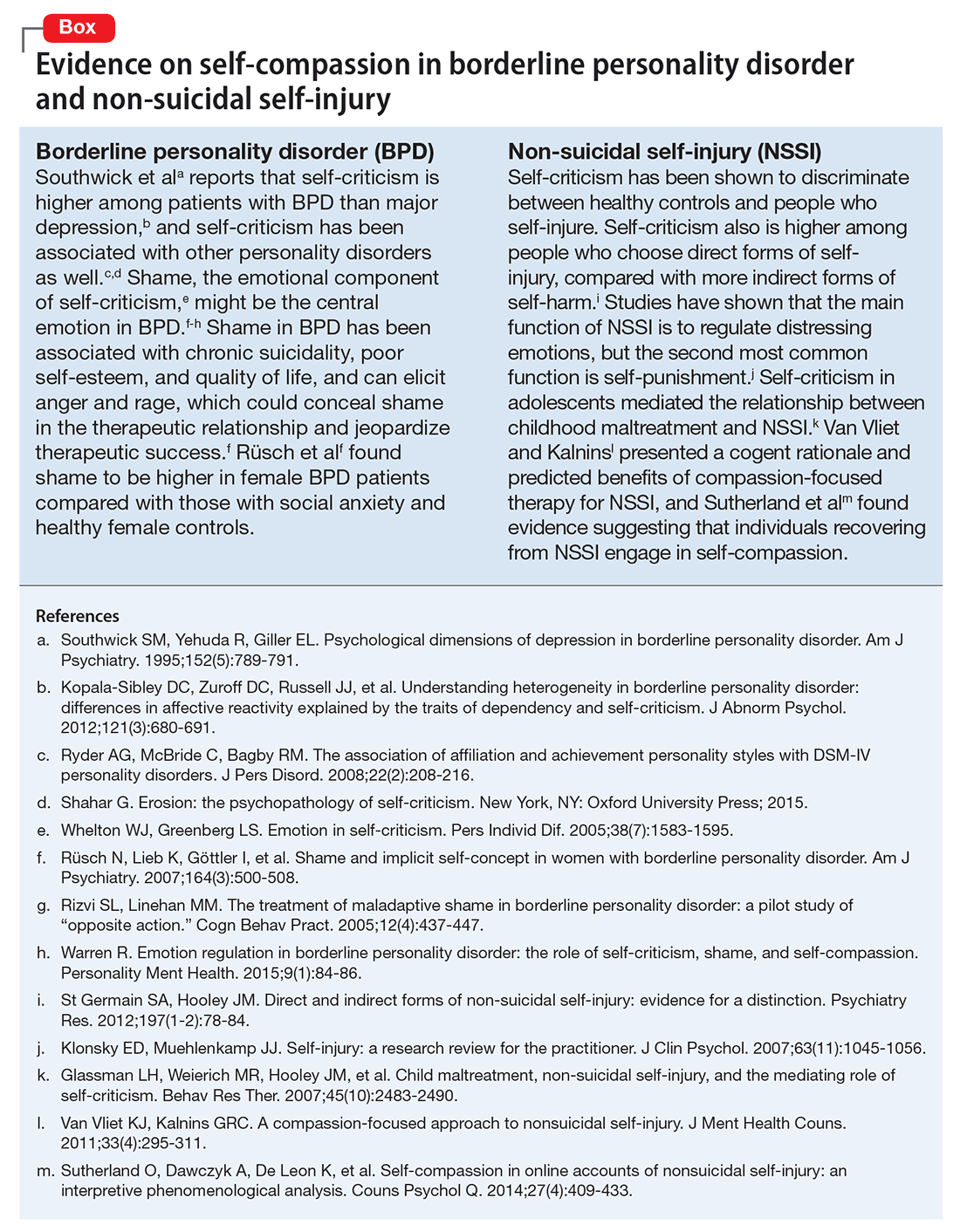
Achieving goals
Powers et al50 suggest that self-critics approach goals based on motivation to avoid failure and disapproval, rather than on intrinsic interest and personal meaning. In studies of college students pursuing academic, social, or weight loss goals, self-criticism was associated with less progress to that goal. Self-criticism was associated with rumination and procrastination, which the authors suggest might have focused the self-critic on potential failure, negative evaluation from others, and loss of self-esteem. Additional studies showed the deleterious effects of self-criticism on college students’ progress on obtaining academic or music performance goals and on community residents’ weight loss goals.51
Not surprisingly, self-compassion is associated with successful goal pursuit and resilience when goals are not met52 and less procrastination and academic worry.53 Self-compassion also is associated with intrinsic motivation, goals based on mastery rather than performance, and less fear of academic failure.54
How self-criticism and self-compassion develop
Studies have explored the impact of early relationships with parents and development of self-criticism. Parental overcontrol and restrictiveness and lack of warmth consistently have been identified as parenting styles associated with development of self-criticism in children.55 One study found that self-criticism fully mediated the relationship between childhood verbal abuse from parents and depression and anxiety in adulthood.56 Reports from parents on their current parenting styles are consistent with these studies.57 Amitay et al57 states that “[s]elf-critics’ negative childhood experiences thus seem to contribute to a pattern of entering, creating, or manipulating subsequent interpersonal environments in ways that perpetuate their negative self-image and increase vulnerability to depression.” Not surprisingly, self-criticism is associated with a fearful avoidant attachment style.58 Review of the developmental origins of self-criticism confirms these factors and presents findings that peer relationships also are important factors in the development of self-criticism.59,60
Early positive relationships with caregivers are associated with self-compassion. Recollections of maternal support are correlated with self-compassion and secure attachment styles in adolescents and adults.61 Pepping et al62 found that retrospective reports of parental rejection, overprotection, and low parental warmth was associated with low self-compassion.
Benefits of self-compassion
A growing body of research suggests that self-compassion is strongly linked to mental health. Greater self-compassion consistently has been associated with lower levels of depression and anxiety,3 with a large effect size.4 Of course, central to self-compassion is the lack of self-criticism, but self-compassion still protects against anxiety and depression when controlling for self-criticism and negative affect.6,63 Self-compassion is a strong predictor of symptom severity and quality of life among individuals with anxious distress.64
The benefits of self-compassion stem partly from a greater ability to cope with negative emotions.6,63,65 Self-compassionate people are less likely to ruminate on their negative thoughts and emotions or suppress them,6,66 which helps to explain why self-compassion is a negative predictor of depression.67
Self-compassion also enhances positive mind states. A number of studies have found links between self-compassion and positive psychological qualities, such as happiness, optimism, wisdom, curiosity, and exploration, and personal initiative.63,65,68,69 By embracing one’s suffering with compassion, negative states are ameliorated when positive emotions of kindness, connectedness, and mindful presence are generated.
Misconceptions about self-compassion
A common misconception is that abandoning self-criticism in favor of self-compassion will undermine motivation70; however, research indicates the opposite. Although self-compassion is negatively associated with maladaptive perfectionism, it is not correlated with self-adopted performance standards.6 Self-compassionate people have less fear of failure54 and, when they do fail, they are more likely to try again.71 Breines and Chen72 found in a series of experimental studies that engendering feelings of self-compassion for personal weaknesses, failures, and past transgressions resulted in more motivation to change, to try harder to learn, and to avoid repeating past mistakes.
Another common misunderstanding is that self-compassion is a weakness. In fact, research suggests that self-compassion is a powerful way to cope with life challenges.73
Although some fear that self-compassion leads to self-indulgence, there is evidence that self-compassion promotes health-related behaviors. Self-compassionate individuals are more likely to seek medical treatment when needed,74 exercise for intrinsic reasons,75 and drink less alcohol.76 Inducing self-compassion has been found to help people stick to their diets77 and quit smoking.78
Self-compassion interventions
Individuals can develop self-compassion. Shapira and Mongrain79 found that adults who wrote a compassionate letter to themselves once a day for a week about the distressing events they were experiencing showed significant reductions in depression up to 3 months and significant increases in happiness up to 6 months compared with a control group who wrote about early memories. Albertson et al80 found that, compared with a wait-list control group, 3 weeks of self-compassion meditation training improved body dissatisfaction, body shame, and body appreciation among women with body image concerns. Similarly, Smeets et al81 found that 3 weeks of self-compassion training for female college students led to significantly greater increases in mindfulness, optimism, and self-efficacy, as well as greater decreases in rumination compared with a time management control group.
The Box6,70,82-86 describes rating scales that can measure self-compassion and self-criticism.

Mindful self-compassion (MSC), developed by Neff and Germer,87 is an 8-week group intervention designed to teach people how to be more self-compassionate through meditation and informal practices in daily life. Results of a randomized controlled trial found that, compared with a wait-list control group, participants using MSC reported significantly greater increases in self-compassion, compassion for others, mindfulness, and life satisfaction, and greater decreases in depression, anxiety, stress, and emotional avoidance, with large effect sizes indicated. These results were maintained up to 1 year.
Compassion-focused therapy (CFT) is designed to enhance self-compassion in clinical populations.88 The approach uses a number of imagery and experiential exercises to enhance patients’ abilities to extend feelings of reassurance, safeness, and understanding toward themselves. CFT has shown promise in treating a diverse group of clinical disorders such as depression and shame,8,89 social anxiety and shame,90 eating disorders,91 psychosis,92 and patients with acquired brain injury.93 A group-based CFT intervention with a heterogeneous group of community mental health patients led to significant reductions in depression, anxiety, stress, and self-criticism.94 See Leaviss and Utley95 for a review of the benefits of CFT.
Fears of developing self-compassion
It is important to note that some people can access self-compassion more easily than others. Highly self-critical patients could feel anxious when learning to be compassionate to themselves, a phenomenon known as “fear of compassion”96 or “backdraft.”97 Backdraft occurs when a firefighter opens a door with a hot fire behind it. Oxygen rushes in, causing a burst of flame. Similarly, when the door of the heart is opened with compassion, intense pain could be released. Unconditional love reveals the conditions under which we were unloved in the past. Some individuals, especially those with a history of childhood abuse or neglect, are fearful of compassion because it activates grief associated with feelings of wanting, but not receiving, affection and care from significant others in childhood.
Clinicians should be aware that anxiety could arise and should help patients learn how to go slowly and stabilize themselves if overwhelming emotions occur as a part of self-compassion practice. Both CFT and MSC have processes to deal with fear of compassion in their protocols,98,99 with the focus on explaining to individuals that although such fears may occur, they are a normal and necessary part of the healing process. Individuals also are taught to focus on the breath, feeling the sensations in the soles of their feet, or other mindfulness practices to ground and stabilize attention when overwhelming feelings arise.
Clinical interventions
Self-compassion interventions that I (R.W.) find most helpful, in the order I administer them, are:
- exploring perceived advantages and disadvantages of self-criticism
- presenting self-compassion as a way to get the perceived advantages of self-criticism without the disadvantages
- discussing what it means to be compassionate for someone else who is suffering, and then asking what it would be like if they treated themselves with the same compassion
- exploring patients’ misconceptions and fears of self-compassion
- directing patients to the self-compassion Web site to get an understanding of what self-compassion is and how it differs from self-esteem
- taking an example of a recent situation in which the patient was self-critical and exploring how a self-compassionate response would differ.
Asking what they would say to a friend often is an effective way to get at this. In a later therapy session, self-compassionate imagery is a useful way to get the patient to experience self-compassion on an emotional level. See Neff100 and Gilbert98 for other techniques to enhance self-compassion.
1. Shahar B, Doron G, Ohad S. Childhood maltreatment, shame-proneness and self-criticism in social anxiety disorder: a sequential mediational model. Clin Psychol Psychother. 2015:22(6):570-579.
2. Kannan D, Levitt HM. A review of client self-criticism in psychotherapy. J Psychother Integr. 2013;23(2):166-178.
3. Barnard LK, Curry JF. Self-compassion: conceptualizations, correlates, and interventions. Rev Gen Psychol. 2011;15(4):289-303.
4. MacBeth A, Gumley A. Exploring compassion: a meta-analysis of the association between self-compassion and psychopathology. Clin Psychol Rev. 2012;32(6):545-552
5. Blatt SJ, Zuroff DC. Interpersonal relatedness and self-definition: two prototypes for depression. Clin Psychol Rev. 1992;12(5):527-562.
6. Neff KD. The development and validation of a scale to measure self-compassion. Self Identity. 2003;2(2):223-250.
7. Neff KD. Self-compassion: an alternative conceptualization of a healthy attitude toward oneself. Self Identity. 2003;(2)2:85-101.
8. Gilbert P, Procter S. Compassionate mind training for people with high shame and self-criticism: overview and pilot study of a group therapy approach. Clin Psychol Psychother. 2006;13(6):353-379.
9. Dunkley DM, Zuroff DC, Blankstein KR. Specific perfectionism components versus self-criticism in predicting maladjustment. Pers Individ Dif. 2006;40(4):665-676.
10. Murphy JM, Nierenberg AA, Monson RR, et al. Self-disparagement as feature and forerunner of depression: Mindfindings from the Stirling County Study. Compr Psychiatry. 2002;43(1):13-21.
11. Brewin CR, Firth-Cozens J. Dependency and self-criticism as predictors of depression in young doctors. J Occup Health Psychol. 1997;2(3):242-246.
12. Fazaa N, Page S. Dependency and self-criticism as predictors of suicidal behavior. Suicide Life Threat Behav. 2003;33(2):172-185.
13. Teasdale JD, Cox SG. Dysphoria: self-devaluative and affective components in recovered depressed patients and never depressed controls. Psychol Med. 2001;31(7):1311-1316.
14. Ehret AM, Joormann J, Berking M. Examining risk and resilience factors for depression: the role of self-criticism and self-compassion. Cogn Emot. 2015;29(8):1496-1504.
15. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
16. Rector NA, Bagby RM, Segal ZV, et al. Self-criticism and dependency in depressed patients treated with cognitive therapy or pharmacotherapy. Cognit Ther Res. 2000;24(5):571-584.
17. Marshall MB, Zuroff DC, McBride C, et al. Self-criticism predicts differential response to treatment for major depression. J Clin Psychol. 2008;64(3):231-244.
18. Zuroff DC, Blatt SJ, Sotsky SM, et al. Relation of therapeutic alliance and perfectionism to outcome in brief outpatient treatment of depression. J Consult Clin Psychol. 2000;68(1):114-124.
19. Whelton WJ, Greenberg LS. Emotion in self-criticism. Pers Individ Dif. 2005;38(7):1583-1595.
20. Cox BJ, Fleet C, Stein MB. Self-criticism and social phobia in the US national comorbidity survey. J Affect Disord. 2004;82(2):227-234.
21. Cox BJ, Walker JR, Enns MW, et al. Self-criticism in generalized social phobia and response to cognitive-behavioral treatment. Behav Ther. 2002;33(4):479-491.
22. McCranie EW, Hyer LA. Self-critical depressive experience in posttraumatic stress disorder. Psychol Rep. 1995;77(3 pt 1):880-882.
23. Southwick SM, Yehuda R, Giller EL Jr. Characterization of depression in war-related posttraumatic stress disorder. Am J Psychiatry. 1991;148(2):179-183.
24. Yehuda R, Kahana B, Southwick SM, et al. Depressive features in Holocaust survivors with post-traumatic stress disorder. J Traumatic Stress. 1994;7(4):699-704.
25. Sharhabani-Arzy R, Amir M, Swisa A. Self-criticism, dependency and posttraumatic stress disorder among a female group of help-seeking victims of domestic violence in Israel. Pers Individ Dif. 2005;38(5):1231-1240.
26. Beaumont E, Galpin A, Jenkins P. ‘Being kinder to myself’: a prospective comparative study, exploring post-trauma therapy outcome measures, for two groups of clients, receiving either cognitive behaviour therapy or cognitive behaviour therapy and compassionate mind training. Counselling Psychol Rev. 2012;27(1):31-43.
27. Dahm KA. Mindfulness and self-compassion as predictors of functional outcomes and psychopathology in OEF/OIF veterans exposed to trauma. https://repositories.lib.utexas.edu/handle/2152/21635. Published August 2013. Accessed November 8, 2016.
28. Hiraoka R, Meyer EC, Kimbrel NA, et al. Self-compassion as a prospective predictor of PTSD symptom severity among trauma-exposed US Iraq and Afghanistan war veterans. J Trauma Stress. 2015;28(2):127-133.
29. Bagby RM, Cox BJ, Schuller DR, et al. Diagnostic specificity of the dependent and self-critical personality dimensions in major depression. J Affect Disord. 1992;26(1):59-63.
30. Hedman E, Ström P, Stünkel A, et al. Shame and guilt in social anxiety disorder: effects of cognitive behavior therapy and association with social anxiety and depressive symptoms. PLoS One. 2013;8(4):e61713. doi: 10.1371/journal.pone.0061713.
31. Hoge EA, Hölzel BK, Marques L, et al. Mindfulness and self-compassion in generalized anxiety disorder: examining predictors of disability. Evid Based Complement Alternat Med. 2013;2013:576258. doi: 10.1155/2013/576258.
32. Wetterneck CT, Lee EB, Smith AH, et al. Courage, self-compassion, and values in obsessive-compulsive disorder. J Contextual Behav Sci. 2013;2(3-4):68-73.
33. Kelly AC, Carter JC. Why self-critical patients present with more severe eating disorder pathology: The mediating role of shame. Br J Clin Psychol. 2013;52(2):148-161.
34. Dunkley DM, Grilo CM. Self-criticism, low self-esteem, depressive symptoms, and over-evaluation of shape and weight in binge eating disorder patients. Behav Res Ther. 2007;45(1):139-149.
35. Dunkley DM, Masheb RM, Grilo CM. Childhood maltreatment, depressive symptoms, and body dissatisfaction in patients with binge eating disorder: the mediating role of self-criticism. Int J Eat Disord. 2010;43(3):274-281.
36. Wasylkiw L, MacKinnon AL, MacLellan AM. Exploring the link between self-compassion and body image in university women. Body Image. 2012;9(2):236-245.
37. Ferreira C, Pinto-Gouveia J, Duarte C. Self-compassion in the face of shame and body image dissatisfaction: implications for eating disorders. Eat Behavs. 2013;14(2):207-210.
38. Kelly AC, Carter JC, Zuroff DC, et al. Self-compassion and fear of self-compassion interact to predict response to eating disorders treatment: a preliminary investigation. Psychother Res. 2013;23(3):252-264.
39. Webb JB, Forman MJ. Evaluating the indirect effect of self-compassion on binge eating severity through cognitive-affective self-regulatory pathways. Eat Behavs. 2013;14(2):224-228.
40. Kelly AC, Carter JC, Borairi S. Are improvements in shame and self-compassion early in eating disorders treatment associated with better patient outcomes? Int J Eat Disord. 2014;47(1):54-64.
41. Wiseman H, Raz A, Sharabany R. Depressive personality styles and interpersonal problems in young adults with difficulties in establishing long-term romantic relationships. Isr J Psychiatry Rel Sci. 2007;44(4):280-291.
42. Besser A, Priel B. A multisource approach to self-critical vulnerability to depression: the moderating role of attachment. J Pers. 2003;71(4):515-555.
43. Zuroff DC, Koestner R, Powers TA. Self-criticism at age 12: a longitudinal-study of adjustment. Cognit Ther Res. 1994;18(4):367-385.
44. Fichman L, Koestner R, Zuroff DC. Depressive styles in adolescence: Assessment, relation to social functioning, and developmental trends. J Youth Adolesc. 1994;23(3):315-330.
45. Wiseman H. Interpersonal relatedness and self-definition in the experience of loneliness during the transition to university. Personal Relationships. 1997;4(3):285-299.
46. Mongrain M, Lubbers R, Struthers W. The power of love: mediation of rejection in roommate relationships of dependents and self-critics. Pers Soc Psychol Bull. 2004;30(1):94-105.
47. Whiffen VE, Aube JA. Personality, interpersonal context and depression in couples. J Soc Pers Relat. 1999;16(3):369-383.
48. Priel B, Besser A. Dependency and self-criticism among first-time mothers: the roles of global and specific support. J Soc Clin Psychol. 2000;19(4):437-450.
49. Neff KD, Beretvas SN. The role of self-compassion in romantic relationships. Self Identity. 2013;12(1):78-98.
50. Powers TA, Koestner R, Zuroff DC. Self-criticism, goal motivation, and goal progress. J Soc Clin Psychol. 2007;26(7):826-840.
51. Powers TA, Koestner R, Zuroff DC, et al. The effects of self-criticism and self-oriented perfectionism on goal pursuit. Pers Soc Psychol Bull. 2011;37(7):964-975.
52. Hope N, Koestner R, Milyavskaya M. The role of self-compassion in goal pursuit and well-being among university freshmen. Self Identity. 2014;13(5):579-593.
53. Williams JG, Stark SK, Foster EE. Start today or the very last day? The relationships among self-compassion, motivation, and procrastination. Am J Psychol Res. 2008;4(1):37-44.
54. Neff KD, Hseih Y, Dejittherat K. Self-compassion, achievement goals, and coping with academic failure. Self Identity. 2005;4(3):263-287.
55. Campos RC, Besser A, Blatt SJ. The mediating role of self-criticism and dependency in the association between perceptions of maternal caring and depressive symptoms. Depress Anxiety. 2010;27(12):1149-1157.
56. Sachs-Ericsson N, Verona E, Joiner T, et al. Parental verbal abuse and the mediating role of self-criticism in adult internalizing disorders. J Affect Disord. 2006;93(1-3):71-78.
57. Amitay OA, Mongrain M, Fazaa N. Love and control: self-criticism in parents and daughters and perceptions of relationship partners. Pers Individ Dif. 2008;44(1):75-85.
58. Zuroff DC, Fitzpatrick DK. Depressive personality styles: implications for adult attachment. Pers Individ Dif. 1995;18(2):253-265.
59. Kopala-Sibley DC, Zuroff DC. The developmental origins of personality factors from the self-definitional and relatedness domains: a review of theory and research. Rev Gen Psychol. 2014;18(3):137-155.
60. Kopala-Sibley DC, Zuroff DC, Leybman MJ, et al. Recalled peer relationship experiences and current levels of self-criticism and self-reassurance. Psychol Psychother. 2013;86(1):33-51.
61. Neff KD, McGehee P. Self-compassion and psychological resilience among adolescents and young adults. Self Identity. 2010;9(3):225-240.
62. Pepping CA, Davis PJ, O’Donovan A, et al. Individual differences in self-compassion: the role of attachment and experiences of parenting in childhood. Self Identity. 2015;14(1):104-117.
63. Neff KD, Rude SS, Kirkpatrick KL. An examination of self-compassion in relation to positive psychological functioning and personality traits. J Res Pers. 2007;41(4):908-916.
64. Van Dam NT, Sheppard SC, Forsyth JP, et al. Self-compassion is a better predictor than mindfulness of symptom severity and quality of life in mixed anxiety and depression. J Anxiety Disord. 2011;25(1):123-130.
65. Heffernan M, Quinn MT, McNulty SR, et al. Self-compassion and emotional intelligence in nurses. Int J Nursing Practice. 2010;16(4):366-373.
66. Neff KD, Kirkpatrick KL, Rude SS. Self-compassion and adaptive psychological functioning. J Res Pers. 2007;41(1):139-154.
67. Krieger T, Altenstein D, Baettig I, et al. Self-compassion in depression: associations with depressive symptoms, rumination, and avoidance in depressed outpatients. Behav Ther. 2013;44(3):501-513.
68. Breen WE, Kashdan TB, Lenser ML, et al. Gratitude and forgiveness: convergence and divergence on self-report and informant ratings. Pers Individ Dif. 2010;49(8):932-937.
69. Hollis-Walker L, Colosimo K. Mindfulness, self-compassion, and happiness in non-meditators: A theoretical and empirical examination. Pers Individ Dif. 2011;50(2):222-227.
70. Gilbert P, McEwan K, Matos M, et al. Fears of compassion: development of three self-report measures. Psychol Psychother. 2011;84(3):239-255.
71. Neely ME, Schallert DL, Mohammed SS, et al. Self-kindness when facing stress: the role of self-compassion, goal regulation, and support in college students’ well-being. Motiv Emot. 2009;33(1):88-97.
72. Breines JG, Chen S. Self-compassion increases self-improvement motivation. Pers Soc Psychol Bull. 2012;38(9):1133-1143.
73. Allen AB, Leary MR. Self-compassion, stress, and coping. Soc Pers Psychol Compass. 2010;4(2):107-118.
74. Terry ML, Leary MR. Self-compassion, self-regulation, and health. Self Identity. 2011;10(3):352-362.
75. Magnus CMR, Kowalski KC, McHugh TF. The role of self-compassion in women’s self-determined motives to exercise and exercise-related outcomes. Self Identity. 2010;9(4):363-382.
76. Brooks M, Kay-Lambkin F, Bowman J, et al. Self-compassion amongst clients with problematic alcohol use. Mindfulness. 2012;3(4):308-317.
77. Adams CE, Leary MR. Promoting self-compassionate attitudes toward eating among restrictive and guilty eaters. J Soc Clin Psychol. 2007;26(10):1120-1144.
78. Kelly AC, Zuroff DC, Foa CL, et al. Who benefits from training in self-compassionate self-regulation? A study of smoking reduction. J Soc Clin Psychol. 2010;29(7):727-755.
79. Shapira LB, Mongrain M. The benefits of self-compassion and optimism exercises for individuals vulnerable to depression. J Posit Psychol. 2010;5(5):377-389.
80. Albertson ER, Neff KD, Dill-Shackleford KE. Self-compassion and body dissatisfaction in women: a randomized controlled trial of a brief meditation intervention. Mindfulness. 2015;6(3):444-454.
81. Smeets E, Neff K, Alberts H, et al. Meeting suffering with kindness: effects of a brief self-compassion intervention for female college students. J Clinical Psychol. 2014;70(9):794-807.
82. Blatt SJ, D’Afflitti JP, Quinlan DM. Depressive experiences questionnaire. New Haven, CT: Yale University Press; 1976.
83. Weissman AN, Beck AT. Development and validation of the dysfunctional attitude scale: a preliminary investigation. Paper presented at: 62nd Annual Meeting of the Association for Advanced Behavior Therapy; March 27-31, 1978; Toronto, Ontario, Canada.
84. Gilbert P, Clarke M, Hempel S, et al. Criticizing and reassuring oneself: an exploration of forms, styles and reasons in female students. Br J Clin Psychol. 2004;43(pt 1):31-50.
85. Baião R, Gilbert P, McEwan K, et al. Forms of self-criticising/attacking & self-reassuring scale: psychometric properties and normative study. Psychol Psychother. 2015;88(4):438-452.
86. Neff KD. The self-compassion scale is a valid and theoretically coherent measure of self-compassion. Mindfulness. 2016;7(1):264-274.
87. Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clinical Psychol. 2013;69(1):28-44.
88. Gilbert P. Introducing compassion-focused therapy. Adv Psychiatr Treat. 2009;15(3):199-208.
89. Kelly AC, Zuroff DC, Shapira LB. Soothing oneself and resisting self-attacks: the treatment of two intrapersonal deficits in depression vulnerability. Cognit Ther Res. 2009;33(3):301-313.
90. Boersma K, Hakanson A, Salomonsson E, et al. Compassion focused therapy to counteract shame, self-criticism and isolation. A replicated single case experimental study of individuals with social anxiety. J Contemp Psychother. 2015;45(2):89-98.
91. Gale C, Gilbert P, Read N, et al. An evaluation of the impact of introducing compassion focused therapy to a standard treatment programme for people with eating disorders. Clin Psychol Psychother. 2014;21(1):1-12.
92. Braehler C, Gumley A, Harper J, et al. Exploring change processes in compassion focused therapy in psychosis: results of a feasibility randomized controlled trial. Br J Clin Psychol. 2013;52(2):199-214.
93. Ashworth F, Clarke A, Jones L, et al. An exploration of compassion focused therapy following acquired brain injury. Psychol Psychother. 2014;88(2):143-162.
94. Judge L, Cleghorn A, McEwan K, et al. An exploration of group-based compassion focused therapy for a heterogeneous range of clients presenting to a community mental health team. Int J Cogn Ther. 2012;5(4):420-429.
95. Leaviss J, Utley L. Psychotherapeutic benefits of compassion-focused therapy: an early systematic review. Psychol Med. 2015;45(5):927-945.
96. Gilbert P, McEwan K, Gibbons L, et al. Fears of compassion and happiness in relation to alexithymia, mindfulness, and self‐criticism. Psychol Psychother. 2012;85(4):374-390.
97. Germer CK, Neff KD. Cultivating self-compassion in trauma survivors. In: Follette VM, Briere J, Rozelle D, et al, eds. Mindfulness-oriented interventions for trauma: integrating contemplative practices. New York, NY: Guilford Press; 2015:43-58.
98. Gilbert P. Compassion focused therapy: the CBT distinctive features series. London, United Kingdom: Routledge; 2010.
99. Germer C, Neff K. The mindful self-compassion training program. In: Singer T, Bolz M, eds. Compassion: bridging theory and practice: a multimedia book. Leipzig, Germany: Max-Planck Institute; 2013:365-396.
100. Neff K. Self-compassion: the proven power of being kind to yourself. New York, NY: HarperCollins; 2015.
Once thought to only be associated with depression, self-criticism is a transdiagnostic risk factor for diverse forms of psychopathology.1,2 However, research has shown that self-compassion is a robust resilience factor when faced with feelings of personal inadequacy.3,4
Self-critical individuals experience feelings of unworthiness, inferiority, failure, and guilt. They engage in constant and harsh self-scrutiny and evaluation, and fear being disapproved and criticized and losing the approval and acceptance of others.5 Self-compassion involves treating oneself with care and concern when confronted with personal inadequacies, mistakes, failures, and painful life situations.6,7
Although self-criticism is destructive across clinical disorders and interpersonal relationships, self-compassion is associated with healthy relationships, emotional well-being, and better treatment outcomes.
Recent research shows how clinicians can teach their patients how to be less self-critical and more self-compassionate. Neff6,7 proposes that self-compassion involves treating yourself with care and concern when being confronted with personal inadequacies, mistakes, failures, and painful life situations. It consists of 3 interacting components, each of which has a positive and negative pole:
- self-kindness vs self-judgment
- a sense of common humanity vs isolation
- mindfulness vs over-identification.
Self-kindness refers to being caring and understanding with oneself rather than harshly judgmental. Instead of attacking and berating oneself for personal shortcomings, the self is offered warmth and unconditional acceptance.
Humanity involves recognizing that humans are imperfect, that all people fail, make mistakes, and have serious life challenges. By remembering that imperfection is part of life, we feel less isolated when we are in pain.
Mindfulness in the context of self-compassion involves being aware of one’s painful experiences in a balanced way that neither ignores and avoids nor exaggerates painful thoughts and emotions.
Self-compassion is more than the absence of self-judgment, although a defining feature of self-compassion is the lack of self-judgment, and self-judgment overlaps with self-criticism. Rather, self-compassion provides several access points for reducing self-criticism. For example, being kind and understanding when confronting personal inadequacies (eg, “it’s okay not to be perfect”) can counter harsh self-talk (eg, “I’m not defective”). Mindfulness of emotional pain (eg, “this is hard”) can facilitate a kind and warm response (eg, “what can I do to take care of myself right now?”) and therefore lessen self-blame (eg, “blaming myself is just causing me more suffering”). Similarly, remembering that failure is part of the human experience (eg, “it’s normal to mess up sometimes”) can lessen egocentric feelings of isolation (eg, “it’s not just me”) and over-identification (eg, “it’s not the end of the world”), resulting in lessened self-criticism (eg, “maybe it’s not just because I’m a bad person”).
Depression
Several studies have found that self-criticism predicts depression. In 3 epidemiological studies, “feeling worthless” was among the top 2 symptoms predicting a depression diagnosis and later depressive episodes.10 Self-criticism in fourth-year medical students predicted depression 2 years later, and—in males—10 years later in their medical careers better than a history of depression.11 Self-critical perfectionism also is associated with suicidal ideation and lethality of suicide attempts.12
Self-criticism has been shown to predict depressive relapse and residual self-devaluative symptoms in recovered depressed patients.13 In one study, currently depressed and remitted depressed patients had higher self-criticism and lower self-compassion compared with healthy controls. Both factors were more strongly associated with depression status than higher perfectionistic beliefs and cognitions, rumination, and maladaptive emotional regulation.14
Self-criticism and response to treatment. In the National Institute of Mental Health Treatment of Depression Collaborative Research Program,15 self-critical perfectionism predicted a poorer outcome across all 4 treatments (cognitive-behavioral therapy [CBT], interpersonal psychotherapy [IPT], pharmacotherapy plus clinical management, and placebo plus clinical management). Subsequent studies found that self-criticism predicted poorer response to CBT16 and IPT.17 The authors suggest that self-criticism could interfere with treatment because self-critical patients might have difficulty developing a strong therapeutic alliance.18,19
Anxiety disorders
Self-criticism is common across psychiatric disorders. In a study of 5,877 respondents in the National Comorbidity Survey (NCS), self-criticism was associated with social phobia, findings that were significant after controlling for current emotional distress, neuroticism, and lifetime history of mood, anxiety, and substance use disorders.20 Further, in a CBT treatment study, baseline self-criticism was associated with severity of social phobia and changes in self-criticism predicted treatment outcome.21 Self-criticism might be an important core psychological process in the development, maintenance, and course of social phobia. Patients with social anxiety disorder have less self-compassion than healthy controls and greater fear of negative evaluation.
In the NCS, self-criticism was associated with posttraumatic stress disorder (PTSD) even after controlling for lifetime history of affective and anxiety disorders.20 Self-criticism predicted greater severity of combat-related PTSD in hospitalized male veterans,22 and those with PTSD had higher scores on self-criticism scales than those with major depressive disorder.23 In a study of Holocaust survivors, those with PTSD scored higher on self-criticism than survivors without PTSD.24 Self-criticism also distinguished between female victims of domestic violence with and without PTSD.25
Self-compassion could be a protective factor for posttraumatic stress.26 Combat veterans with higher levels of self-compassion showed lower levels of psychopathology, better functioning in daily life, and fewer symptoms of posttraumatic stress.27 In fact, self-compassion has been found to be a stronger predictor of PTSD than level of combat exposure.28
In an early study, self-criticism scores were higher in patients with panic disorder than in healthy controls, but lower than in patients with depression.29 In a study of a mixed sample of anxiety disorder patients, symptoms of generalized anxiety disorder were associated with shame proneness.30 Consistent with these results, Hoge et al31 found that self-compassion was lower in generalized anxiety disorder patients compared with healthy controls with elevated stress. Low self-compassion has been associated with severity of obsessive-compulsive disorder.32
Eating disorders
Self-criticism is correlated with eating disorder severity.33 In a study of patients with binge eating disorder, Dunkley and Grilo34 found that self-criticism was associated with the over-evaluation of shape and weight independently of self-esteem and depression. Self-criticism also is associated with body dissatisfaction, independent of self-esteem and depression. Dunkley et al35 found that self-criticism, but not global self-esteem, in patients with binge eating disorder mediated the relationship between childhood abuse and body dissatisfaction and depression. Numerous studies have shown that shame is associated with more severe eating disorder pathology.33
Self-compassion seems to buffer against body image concerns. It is associated with less body dissatisfaction, body preoccupation, and weight worries,36 greater body appreciation37 and less disordered eating.37-39 Early decreases in shame during eating disorder treatment was associated with more rapid reduction in eating disorder symptoms.40
Interpersonal relationships
Several studies have shown that self-criticism has negative effects on interpersonal relationships throughout life.5,41,42
- Self-criticism at age 12 predicted less involvement in high school activities and, at age 31, personal and social maladjustment.43
- High school students with high self-criticism reported more interpersonal problems.44
- Self-criticism was associated with loneliness, depression, and lack of intimacy with opposite sex friends or partners during the transition to college.45
- In a study of college roommates,46 self-criticism was associated with increased likelihood of rejection.
- Whiffen and Aube47 found that self-criticism was associated with marital dissatisfaction and depression.
- Self-critical mothers with postpartum depression were less satisfied with social support and were more vulnerable to depression.48
Self-compassion appears to enhance interpersonal relationships. In a study of heterosexual couples,49 self-compassionate individuals were described by their partners as being more emotionally connected, as well as accepting and supporting autonomy, while being less detached, controlling, and verbally or physically aggressive than those lacking self-compassion. Because self-compassionate people give themselves care and support, they seem to have more emotional resources available to give to others.
See the Box examining the evidence on the role of self-compassion in borderline personality disorder and non-suicidal self-injury.

Achieving goals
Powers et al50 suggest that self-critics approach goals based on motivation to avoid failure and disapproval, rather than on intrinsic interest and personal meaning. In studies of college students pursuing academic, social, or weight loss goals, self-criticism was associated with less progress to that goal. Self-criticism was associated with rumination and procrastination, which the authors suggest might have focused the self-critic on potential failure, negative evaluation from others, and loss of self-esteem. Additional studies showed the deleterious effects of self-criticism on college students’ progress on obtaining academic or music performance goals and on community residents’ weight loss goals.51
Not surprisingly, self-compassion is associated with successful goal pursuit and resilience when goals are not met52 and less procrastination and academic worry.53 Self-compassion also is associated with intrinsic motivation, goals based on mastery rather than performance, and less fear of academic failure.54
How self-criticism and self-compassion develop
Studies have explored the impact of early relationships with parents and development of self-criticism. Parental overcontrol and restrictiveness and lack of warmth consistently have been identified as parenting styles associated with development of self-criticism in children.55 One study found that self-criticism fully mediated the relationship between childhood verbal abuse from parents and depression and anxiety in adulthood.56 Reports from parents on their current parenting styles are consistent with these studies.57 Amitay et al57 states that “[s]elf-critics’ negative childhood experiences thus seem to contribute to a pattern of entering, creating, or manipulating subsequent interpersonal environments in ways that perpetuate their negative self-image and increase vulnerability to depression.” Not surprisingly, self-criticism is associated with a fearful avoidant attachment style.58 Review of the developmental origins of self-criticism confirms these factors and presents findings that peer relationships also are important factors in the development of self-criticism.59,60
Early positive relationships with caregivers are associated with self-compassion. Recollections of maternal support are correlated with self-compassion and secure attachment styles in adolescents and adults.61 Pepping et al62 found that retrospective reports of parental rejection, overprotection, and low parental warmth was associated with low self-compassion.
Benefits of self-compassion
A growing body of research suggests that self-compassion is strongly linked to mental health. Greater self-compassion consistently has been associated with lower levels of depression and anxiety,3 with a large effect size.4 Of course, central to self-compassion is the lack of self-criticism, but self-compassion still protects against anxiety and depression when controlling for self-criticism and negative affect.6,63 Self-compassion is a strong predictor of symptom severity and quality of life among individuals with anxious distress.64
The benefits of self-compassion stem partly from a greater ability to cope with negative emotions.6,63,65 Self-compassionate people are less likely to ruminate on their negative thoughts and emotions or suppress them,6,66 which helps to explain why self-compassion is a negative predictor of depression.67
Self-compassion also enhances positive mind states. A number of studies have found links between self-compassion and positive psychological qualities, such as happiness, optimism, wisdom, curiosity, and exploration, and personal initiative.63,65,68,69 By embracing one’s suffering with compassion, negative states are ameliorated when positive emotions of kindness, connectedness, and mindful presence are generated.
Misconceptions about self-compassion
A common misconception is that abandoning self-criticism in favor of self-compassion will undermine motivation70; however, research indicates the opposite. Although self-compassion is negatively associated with maladaptive perfectionism, it is not correlated with self-adopted performance standards.6 Self-compassionate people have less fear of failure54 and, when they do fail, they are more likely to try again.71 Breines and Chen72 found in a series of experimental studies that engendering feelings of self-compassion for personal weaknesses, failures, and past transgressions resulted in more motivation to change, to try harder to learn, and to avoid repeating past mistakes.
Another common misunderstanding is that self-compassion is a weakness. In fact, research suggests that self-compassion is a powerful way to cope with life challenges.73
Although some fear that self-compassion leads to self-indulgence, there is evidence that self-compassion promotes health-related behaviors. Self-compassionate individuals are more likely to seek medical treatment when needed,74 exercise for intrinsic reasons,75 and drink less alcohol.76 Inducing self-compassion has been found to help people stick to their diets77 and quit smoking.78
Self-compassion interventions
Individuals can develop self-compassion. Shapira and Mongrain79 found that adults who wrote a compassionate letter to themselves once a day for a week about the distressing events they were experiencing showed significant reductions in depression up to 3 months and significant increases in happiness up to 6 months compared with a control group who wrote about early memories. Albertson et al80 found that, compared with a wait-list control group, 3 weeks of self-compassion meditation training improved body dissatisfaction, body shame, and body appreciation among women with body image concerns. Similarly, Smeets et al81 found that 3 weeks of self-compassion training for female college students led to significantly greater increases in mindfulness, optimism, and self-efficacy, as well as greater decreases in rumination compared with a time management control group.
The Box6,70,82-86 describes rating scales that can measure self-compassion and self-criticism.

Mindful self-compassion (MSC), developed by Neff and Germer,87 is an 8-week group intervention designed to teach people how to be more self-compassionate through meditation and informal practices in daily life. Results of a randomized controlled trial found that, compared with a wait-list control group, participants using MSC reported significantly greater increases in self-compassion, compassion for others, mindfulness, and life satisfaction, and greater decreases in depression, anxiety, stress, and emotional avoidance, with large effect sizes indicated. These results were maintained up to 1 year.
Compassion-focused therapy (CFT) is designed to enhance self-compassion in clinical populations.88 The approach uses a number of imagery and experiential exercises to enhance patients’ abilities to extend feelings of reassurance, safeness, and understanding toward themselves. CFT has shown promise in treating a diverse group of clinical disorders such as depression and shame,8,89 social anxiety and shame,90 eating disorders,91 psychosis,92 and patients with acquired brain injury.93 A group-based CFT intervention with a heterogeneous group of community mental health patients led to significant reductions in depression, anxiety, stress, and self-criticism.94 See Leaviss and Utley95 for a review of the benefits of CFT.
Fears of developing self-compassion
It is important to note that some people can access self-compassion more easily than others. Highly self-critical patients could feel anxious when learning to be compassionate to themselves, a phenomenon known as “fear of compassion”96 or “backdraft.”97 Backdraft occurs when a firefighter opens a door with a hot fire behind it. Oxygen rushes in, causing a burst of flame. Similarly, when the door of the heart is opened with compassion, intense pain could be released. Unconditional love reveals the conditions under which we were unloved in the past. Some individuals, especially those with a history of childhood abuse or neglect, are fearful of compassion because it activates grief associated with feelings of wanting, but not receiving, affection and care from significant others in childhood.
Clinicians should be aware that anxiety could arise and should help patients learn how to go slowly and stabilize themselves if overwhelming emotions occur as a part of self-compassion practice. Both CFT and MSC have processes to deal with fear of compassion in their protocols,98,99 with the focus on explaining to individuals that although such fears may occur, they are a normal and necessary part of the healing process. Individuals also are taught to focus on the breath, feeling the sensations in the soles of their feet, or other mindfulness practices to ground and stabilize attention when overwhelming feelings arise.
Clinical interventions
Self-compassion interventions that I (R.W.) find most helpful, in the order I administer them, are:
- exploring perceived advantages and disadvantages of self-criticism
- presenting self-compassion as a way to get the perceived advantages of self-criticism without the disadvantages
- discussing what it means to be compassionate for someone else who is suffering, and then asking what it would be like if they treated themselves with the same compassion
- exploring patients’ misconceptions and fears of self-compassion
- directing patients to the self-compassion Web site to get an understanding of what self-compassion is and how it differs from self-esteem
- taking an example of a recent situation in which the patient was self-critical and exploring how a self-compassionate response would differ.
Asking what they would say to a friend often is an effective way to get at this. In a later therapy session, self-compassionate imagery is a useful way to get the patient to experience self-compassion on an emotional level. See Neff100 and Gilbert98 for other techniques to enhance self-compassion.
Once thought to only be associated with depression, self-criticism is a transdiagnostic risk factor for diverse forms of psychopathology.1,2 However, research has shown that self-compassion is a robust resilience factor when faced with feelings of personal inadequacy.3,4
Self-critical individuals experience feelings of unworthiness, inferiority, failure, and guilt. They engage in constant and harsh self-scrutiny and evaluation, and fear being disapproved and criticized and losing the approval and acceptance of others.5 Self-compassion involves treating oneself with care and concern when confronted with personal inadequacies, mistakes, failures, and painful life situations.6,7
Although self-criticism is destructive across clinical disorders and interpersonal relationships, self-compassion is associated with healthy relationships, emotional well-being, and better treatment outcomes.
Recent research shows how clinicians can teach their patients how to be less self-critical and more self-compassionate. Neff6,7 proposes that self-compassion involves treating yourself with care and concern when being confronted with personal inadequacies, mistakes, failures, and painful life situations. It consists of 3 interacting components, each of which has a positive and negative pole:
- self-kindness vs self-judgment
- a sense of common humanity vs isolation
- mindfulness vs over-identification.
Self-kindness refers to being caring and understanding with oneself rather than harshly judgmental. Instead of attacking and berating oneself for personal shortcomings, the self is offered warmth and unconditional acceptance.
Humanity involves recognizing that humans are imperfect, that all people fail, make mistakes, and have serious life challenges. By remembering that imperfection is part of life, we feel less isolated when we are in pain.
Mindfulness in the context of self-compassion involves being aware of one’s painful experiences in a balanced way that neither ignores and avoids nor exaggerates painful thoughts and emotions.
Self-compassion is more than the absence of self-judgment, although a defining feature of self-compassion is the lack of self-judgment, and self-judgment overlaps with self-criticism. Rather, self-compassion provides several access points for reducing self-criticism. For example, being kind and understanding when confronting personal inadequacies (eg, “it’s okay not to be perfect”) can counter harsh self-talk (eg, “I’m not defective”). Mindfulness of emotional pain (eg, “this is hard”) can facilitate a kind and warm response (eg, “what can I do to take care of myself right now?”) and therefore lessen self-blame (eg, “blaming myself is just causing me more suffering”). Similarly, remembering that failure is part of the human experience (eg, “it’s normal to mess up sometimes”) can lessen egocentric feelings of isolation (eg, “it’s not just me”) and over-identification (eg, “it’s not the end of the world”), resulting in lessened self-criticism (eg, “maybe it’s not just because I’m a bad person”).
Depression
Several studies have found that self-criticism predicts depression. In 3 epidemiological studies, “feeling worthless” was among the top 2 symptoms predicting a depression diagnosis and later depressive episodes.10 Self-criticism in fourth-year medical students predicted depression 2 years later, and—in males—10 years later in their medical careers better than a history of depression.11 Self-critical perfectionism also is associated with suicidal ideation and lethality of suicide attempts.12
Self-criticism has been shown to predict depressive relapse and residual self-devaluative symptoms in recovered depressed patients.13 In one study, currently depressed and remitted depressed patients had higher self-criticism and lower self-compassion compared with healthy controls. Both factors were more strongly associated with depression status than higher perfectionistic beliefs and cognitions, rumination, and maladaptive emotional regulation.14
Self-criticism and response to treatment. In the National Institute of Mental Health Treatment of Depression Collaborative Research Program,15 self-critical perfectionism predicted a poorer outcome across all 4 treatments (cognitive-behavioral therapy [CBT], interpersonal psychotherapy [IPT], pharmacotherapy plus clinical management, and placebo plus clinical management). Subsequent studies found that self-criticism predicted poorer response to CBT16 and IPT.17 The authors suggest that self-criticism could interfere with treatment because self-critical patients might have difficulty developing a strong therapeutic alliance.18,19
Anxiety disorders
Self-criticism is common across psychiatric disorders. In a study of 5,877 respondents in the National Comorbidity Survey (NCS), self-criticism was associated with social phobia, findings that were significant after controlling for current emotional distress, neuroticism, and lifetime history of mood, anxiety, and substance use disorders.20 Further, in a CBT treatment study, baseline self-criticism was associated with severity of social phobia and changes in self-criticism predicted treatment outcome.21 Self-criticism might be an important core psychological process in the development, maintenance, and course of social phobia. Patients with social anxiety disorder have less self-compassion than healthy controls and greater fear of negative evaluation.
In the NCS, self-criticism was associated with posttraumatic stress disorder (PTSD) even after controlling for lifetime history of affective and anxiety disorders.20 Self-criticism predicted greater severity of combat-related PTSD in hospitalized male veterans,22 and those with PTSD had higher scores on self-criticism scales than those with major depressive disorder.23 In a study of Holocaust survivors, those with PTSD scored higher on self-criticism than survivors without PTSD.24 Self-criticism also distinguished between female victims of domestic violence with and without PTSD.25
Self-compassion could be a protective factor for posttraumatic stress.26 Combat veterans with higher levels of self-compassion showed lower levels of psychopathology, better functioning in daily life, and fewer symptoms of posttraumatic stress.27 In fact, self-compassion has been found to be a stronger predictor of PTSD than level of combat exposure.28
In an early study, self-criticism scores were higher in patients with panic disorder than in healthy controls, but lower than in patients with depression.29 In a study of a mixed sample of anxiety disorder patients, symptoms of generalized anxiety disorder were associated with shame proneness.30 Consistent with these results, Hoge et al31 found that self-compassion was lower in generalized anxiety disorder patients compared with healthy controls with elevated stress. Low self-compassion has been associated with severity of obsessive-compulsive disorder.32
Eating disorders
Self-criticism is correlated with eating disorder severity.33 In a study of patients with binge eating disorder, Dunkley and Grilo34 found that self-criticism was associated with the over-evaluation of shape and weight independently of self-esteem and depression. Self-criticism also is associated with body dissatisfaction, independent of self-esteem and depression. Dunkley et al35 found that self-criticism, but not global self-esteem, in patients with binge eating disorder mediated the relationship between childhood abuse and body dissatisfaction and depression. Numerous studies have shown that shame is associated with more severe eating disorder pathology.33
Self-compassion seems to buffer against body image concerns. It is associated with less body dissatisfaction, body preoccupation, and weight worries,36 greater body appreciation37 and less disordered eating.37-39 Early decreases in shame during eating disorder treatment was associated with more rapid reduction in eating disorder symptoms.40
Interpersonal relationships
Several studies have shown that self-criticism has negative effects on interpersonal relationships throughout life.5,41,42
- Self-criticism at age 12 predicted less involvement in high school activities and, at age 31, personal and social maladjustment.43
- High school students with high self-criticism reported more interpersonal problems.44
- Self-criticism was associated with loneliness, depression, and lack of intimacy with opposite sex friends or partners during the transition to college.45
- In a study of college roommates,46 self-criticism was associated with increased likelihood of rejection.
- Whiffen and Aube47 found that self-criticism was associated with marital dissatisfaction and depression.
- Self-critical mothers with postpartum depression were less satisfied with social support and were more vulnerable to depression.48
Self-compassion appears to enhance interpersonal relationships. In a study of heterosexual couples,49 self-compassionate individuals were described by their partners as being more emotionally connected, as well as accepting and supporting autonomy, while being less detached, controlling, and verbally or physically aggressive than those lacking self-compassion. Because self-compassionate people give themselves care and support, they seem to have more emotional resources available to give to others.
See the Box examining the evidence on the role of self-compassion in borderline personality disorder and non-suicidal self-injury.

Achieving goals
Powers et al50 suggest that self-critics approach goals based on motivation to avoid failure and disapproval, rather than on intrinsic interest and personal meaning. In studies of college students pursuing academic, social, or weight loss goals, self-criticism was associated with less progress to that goal. Self-criticism was associated with rumination and procrastination, which the authors suggest might have focused the self-critic on potential failure, negative evaluation from others, and loss of self-esteem. Additional studies showed the deleterious effects of self-criticism on college students’ progress on obtaining academic or music performance goals and on community residents’ weight loss goals.51
Not surprisingly, self-compassion is associated with successful goal pursuit and resilience when goals are not met52 and less procrastination and academic worry.53 Self-compassion also is associated with intrinsic motivation, goals based on mastery rather than performance, and less fear of academic failure.54
How self-criticism and self-compassion develop
Studies have explored the impact of early relationships with parents and development of self-criticism. Parental overcontrol and restrictiveness and lack of warmth consistently have been identified as parenting styles associated with development of self-criticism in children.55 One study found that self-criticism fully mediated the relationship between childhood verbal abuse from parents and depression and anxiety in adulthood.56 Reports from parents on their current parenting styles are consistent with these studies.57 Amitay et al57 states that “[s]elf-critics’ negative childhood experiences thus seem to contribute to a pattern of entering, creating, or manipulating subsequent interpersonal environments in ways that perpetuate their negative self-image and increase vulnerability to depression.” Not surprisingly, self-criticism is associated with a fearful avoidant attachment style.58 Review of the developmental origins of self-criticism confirms these factors and presents findings that peer relationships also are important factors in the development of self-criticism.59,60
Early positive relationships with caregivers are associated with self-compassion. Recollections of maternal support are correlated with self-compassion and secure attachment styles in adolescents and adults.61 Pepping et al62 found that retrospective reports of parental rejection, overprotection, and low parental warmth was associated with low self-compassion.
Benefits of self-compassion
A growing body of research suggests that self-compassion is strongly linked to mental health. Greater self-compassion consistently has been associated with lower levels of depression and anxiety,3 with a large effect size.4 Of course, central to self-compassion is the lack of self-criticism, but self-compassion still protects against anxiety and depression when controlling for self-criticism and negative affect.6,63 Self-compassion is a strong predictor of symptom severity and quality of life among individuals with anxious distress.64
The benefits of self-compassion stem partly from a greater ability to cope with negative emotions.6,63,65 Self-compassionate people are less likely to ruminate on their negative thoughts and emotions or suppress them,6,66 which helps to explain why self-compassion is a negative predictor of depression.67
Self-compassion also enhances positive mind states. A number of studies have found links between self-compassion and positive psychological qualities, such as happiness, optimism, wisdom, curiosity, and exploration, and personal initiative.63,65,68,69 By embracing one’s suffering with compassion, negative states are ameliorated when positive emotions of kindness, connectedness, and mindful presence are generated.
Misconceptions about self-compassion
A common misconception is that abandoning self-criticism in favor of self-compassion will undermine motivation70; however, research indicates the opposite. Although self-compassion is negatively associated with maladaptive perfectionism, it is not correlated with self-adopted performance standards.6 Self-compassionate people have less fear of failure54 and, when they do fail, they are more likely to try again.71 Breines and Chen72 found in a series of experimental studies that engendering feelings of self-compassion for personal weaknesses, failures, and past transgressions resulted in more motivation to change, to try harder to learn, and to avoid repeating past mistakes.
Another common misunderstanding is that self-compassion is a weakness. In fact, research suggests that self-compassion is a powerful way to cope with life challenges.73
Although some fear that self-compassion leads to self-indulgence, there is evidence that self-compassion promotes health-related behaviors. Self-compassionate individuals are more likely to seek medical treatment when needed,74 exercise for intrinsic reasons,75 and drink less alcohol.76 Inducing self-compassion has been found to help people stick to their diets77 and quit smoking.78
Self-compassion interventions
Individuals can develop self-compassion. Shapira and Mongrain79 found that adults who wrote a compassionate letter to themselves once a day for a week about the distressing events they were experiencing showed significant reductions in depression up to 3 months and significant increases in happiness up to 6 months compared with a control group who wrote about early memories. Albertson et al80 found that, compared with a wait-list control group, 3 weeks of self-compassion meditation training improved body dissatisfaction, body shame, and body appreciation among women with body image concerns. Similarly, Smeets et al81 found that 3 weeks of self-compassion training for female college students led to significantly greater increases in mindfulness, optimism, and self-efficacy, as well as greater decreases in rumination compared with a time management control group.
The Box6,70,82-86 describes rating scales that can measure self-compassion and self-criticism.

Mindful self-compassion (MSC), developed by Neff and Germer,87 is an 8-week group intervention designed to teach people how to be more self-compassionate through meditation and informal practices in daily life. Results of a randomized controlled trial found that, compared with a wait-list control group, participants using MSC reported significantly greater increases in self-compassion, compassion for others, mindfulness, and life satisfaction, and greater decreases in depression, anxiety, stress, and emotional avoidance, with large effect sizes indicated. These results were maintained up to 1 year.
Compassion-focused therapy (CFT) is designed to enhance self-compassion in clinical populations.88 The approach uses a number of imagery and experiential exercises to enhance patients’ abilities to extend feelings of reassurance, safeness, and understanding toward themselves. CFT has shown promise in treating a diverse group of clinical disorders such as depression and shame,8,89 social anxiety and shame,90 eating disorders,91 psychosis,92 and patients with acquired brain injury.93 A group-based CFT intervention with a heterogeneous group of community mental health patients led to significant reductions in depression, anxiety, stress, and self-criticism.94 See Leaviss and Utley95 for a review of the benefits of CFT.
Fears of developing self-compassion
It is important to note that some people can access self-compassion more easily than others. Highly self-critical patients could feel anxious when learning to be compassionate to themselves, a phenomenon known as “fear of compassion”96 or “backdraft.”97 Backdraft occurs when a firefighter opens a door with a hot fire behind it. Oxygen rushes in, causing a burst of flame. Similarly, when the door of the heart is opened with compassion, intense pain could be released. Unconditional love reveals the conditions under which we were unloved in the past. Some individuals, especially those with a history of childhood abuse or neglect, are fearful of compassion because it activates grief associated with feelings of wanting, but not receiving, affection and care from significant others in childhood.
Clinicians should be aware that anxiety could arise and should help patients learn how to go slowly and stabilize themselves if overwhelming emotions occur as a part of self-compassion practice. Both CFT and MSC have processes to deal with fear of compassion in their protocols,98,99 with the focus on explaining to individuals that although such fears may occur, they are a normal and necessary part of the healing process. Individuals also are taught to focus on the breath, feeling the sensations in the soles of their feet, or other mindfulness practices to ground and stabilize attention when overwhelming feelings arise.
Clinical interventions
Self-compassion interventions that I (R.W.) find most helpful, in the order I administer them, are:
- exploring perceived advantages and disadvantages of self-criticism
- presenting self-compassion as a way to get the perceived advantages of self-criticism without the disadvantages
- discussing what it means to be compassionate for someone else who is suffering, and then asking what it would be like if they treated themselves with the same compassion
- exploring patients’ misconceptions and fears of self-compassion
- directing patients to the self-compassion Web site to get an understanding of what self-compassion is and how it differs from self-esteem
- taking an example of a recent situation in which the patient was self-critical and exploring how a self-compassionate response would differ.
Asking what they would say to a friend often is an effective way to get at this. In a later therapy session, self-compassionate imagery is a useful way to get the patient to experience self-compassion on an emotional level. See Neff100 and Gilbert98 for other techniques to enhance self-compassion.
1. Shahar B, Doron G, Ohad S. Childhood maltreatment, shame-proneness and self-criticism in social anxiety disorder: a sequential mediational model. Clin Psychol Psychother. 2015:22(6):570-579.
2. Kannan D, Levitt HM. A review of client self-criticism in psychotherapy. J Psychother Integr. 2013;23(2):166-178.
3. Barnard LK, Curry JF. Self-compassion: conceptualizations, correlates, and interventions. Rev Gen Psychol. 2011;15(4):289-303.
4. MacBeth A, Gumley A. Exploring compassion: a meta-analysis of the association between self-compassion and psychopathology. Clin Psychol Rev. 2012;32(6):545-552
5. Blatt SJ, Zuroff DC. Interpersonal relatedness and self-definition: two prototypes for depression. Clin Psychol Rev. 1992;12(5):527-562.
6. Neff KD. The development and validation of a scale to measure self-compassion. Self Identity. 2003;2(2):223-250.
7. Neff KD. Self-compassion: an alternative conceptualization of a healthy attitude toward oneself. Self Identity. 2003;(2)2:85-101.
8. Gilbert P, Procter S. Compassionate mind training for people with high shame and self-criticism: overview and pilot study of a group therapy approach. Clin Psychol Psychother. 2006;13(6):353-379.
9. Dunkley DM, Zuroff DC, Blankstein KR. Specific perfectionism components versus self-criticism in predicting maladjustment. Pers Individ Dif. 2006;40(4):665-676.
10. Murphy JM, Nierenberg AA, Monson RR, et al. Self-disparagement as feature and forerunner of depression: Mindfindings from the Stirling County Study. Compr Psychiatry. 2002;43(1):13-21.
11. Brewin CR, Firth-Cozens J. Dependency and self-criticism as predictors of depression in young doctors. J Occup Health Psychol. 1997;2(3):242-246.
12. Fazaa N, Page S. Dependency and self-criticism as predictors of suicidal behavior. Suicide Life Threat Behav. 2003;33(2):172-185.
13. Teasdale JD, Cox SG. Dysphoria: self-devaluative and affective components in recovered depressed patients and never depressed controls. Psychol Med. 2001;31(7):1311-1316.
14. Ehret AM, Joormann J, Berking M. Examining risk and resilience factors for depression: the role of self-criticism and self-compassion. Cogn Emot. 2015;29(8):1496-1504.
15. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
16. Rector NA, Bagby RM, Segal ZV, et al. Self-criticism and dependency in depressed patients treated with cognitive therapy or pharmacotherapy. Cognit Ther Res. 2000;24(5):571-584.
17. Marshall MB, Zuroff DC, McBride C, et al. Self-criticism predicts differential response to treatment for major depression. J Clin Psychol. 2008;64(3):231-244.
18. Zuroff DC, Blatt SJ, Sotsky SM, et al. Relation of therapeutic alliance and perfectionism to outcome in brief outpatient treatment of depression. J Consult Clin Psychol. 2000;68(1):114-124.
19. Whelton WJ, Greenberg LS. Emotion in self-criticism. Pers Individ Dif. 2005;38(7):1583-1595.
20. Cox BJ, Fleet C, Stein MB. Self-criticism and social phobia in the US national comorbidity survey. J Affect Disord. 2004;82(2):227-234.
21. Cox BJ, Walker JR, Enns MW, et al. Self-criticism in generalized social phobia and response to cognitive-behavioral treatment. Behav Ther. 2002;33(4):479-491.
22. McCranie EW, Hyer LA. Self-critical depressive experience in posttraumatic stress disorder. Psychol Rep. 1995;77(3 pt 1):880-882.
23. Southwick SM, Yehuda R, Giller EL Jr. Characterization of depression in war-related posttraumatic stress disorder. Am J Psychiatry. 1991;148(2):179-183.
24. Yehuda R, Kahana B, Southwick SM, et al. Depressive features in Holocaust survivors with post-traumatic stress disorder. J Traumatic Stress. 1994;7(4):699-704.
25. Sharhabani-Arzy R, Amir M, Swisa A. Self-criticism, dependency and posttraumatic stress disorder among a female group of help-seeking victims of domestic violence in Israel. Pers Individ Dif. 2005;38(5):1231-1240.
26. Beaumont E, Galpin A, Jenkins P. ‘Being kinder to myself’: a prospective comparative study, exploring post-trauma therapy outcome measures, for two groups of clients, receiving either cognitive behaviour therapy or cognitive behaviour therapy and compassionate mind training. Counselling Psychol Rev. 2012;27(1):31-43.
27. Dahm KA. Mindfulness and self-compassion as predictors of functional outcomes and psychopathology in OEF/OIF veterans exposed to trauma. https://repositories.lib.utexas.edu/handle/2152/21635. Published August 2013. Accessed November 8, 2016.
28. Hiraoka R, Meyer EC, Kimbrel NA, et al. Self-compassion as a prospective predictor of PTSD symptom severity among trauma-exposed US Iraq and Afghanistan war veterans. J Trauma Stress. 2015;28(2):127-133.
29. Bagby RM, Cox BJ, Schuller DR, et al. Diagnostic specificity of the dependent and self-critical personality dimensions in major depression. J Affect Disord. 1992;26(1):59-63.
30. Hedman E, Ström P, Stünkel A, et al. Shame and guilt in social anxiety disorder: effects of cognitive behavior therapy and association with social anxiety and depressive symptoms. PLoS One. 2013;8(4):e61713. doi: 10.1371/journal.pone.0061713.
31. Hoge EA, Hölzel BK, Marques L, et al. Mindfulness and self-compassion in generalized anxiety disorder: examining predictors of disability. Evid Based Complement Alternat Med. 2013;2013:576258. doi: 10.1155/2013/576258.
32. Wetterneck CT, Lee EB, Smith AH, et al. Courage, self-compassion, and values in obsessive-compulsive disorder. J Contextual Behav Sci. 2013;2(3-4):68-73.
33. Kelly AC, Carter JC. Why self-critical patients present with more severe eating disorder pathology: The mediating role of shame. Br J Clin Psychol. 2013;52(2):148-161.
34. Dunkley DM, Grilo CM. Self-criticism, low self-esteem, depressive symptoms, and over-evaluation of shape and weight in binge eating disorder patients. Behav Res Ther. 2007;45(1):139-149.
35. Dunkley DM, Masheb RM, Grilo CM. Childhood maltreatment, depressive symptoms, and body dissatisfaction in patients with binge eating disorder: the mediating role of self-criticism. Int J Eat Disord. 2010;43(3):274-281.
36. Wasylkiw L, MacKinnon AL, MacLellan AM. Exploring the link between self-compassion and body image in university women. Body Image. 2012;9(2):236-245.
37. Ferreira C, Pinto-Gouveia J, Duarte C. Self-compassion in the face of shame and body image dissatisfaction: implications for eating disorders. Eat Behavs. 2013;14(2):207-210.
38. Kelly AC, Carter JC, Zuroff DC, et al. Self-compassion and fear of self-compassion interact to predict response to eating disorders treatment: a preliminary investigation. Psychother Res. 2013;23(3):252-264.
39. Webb JB, Forman MJ. Evaluating the indirect effect of self-compassion on binge eating severity through cognitive-affective self-regulatory pathways. Eat Behavs. 2013;14(2):224-228.
40. Kelly AC, Carter JC, Borairi S. Are improvements in shame and self-compassion early in eating disorders treatment associated with better patient outcomes? Int J Eat Disord. 2014;47(1):54-64.
41. Wiseman H, Raz A, Sharabany R. Depressive personality styles and interpersonal problems in young adults with difficulties in establishing long-term romantic relationships. Isr J Psychiatry Rel Sci. 2007;44(4):280-291.
42. Besser A, Priel B. A multisource approach to self-critical vulnerability to depression: the moderating role of attachment. J Pers. 2003;71(4):515-555.
43. Zuroff DC, Koestner R, Powers TA. Self-criticism at age 12: a longitudinal-study of adjustment. Cognit Ther Res. 1994;18(4):367-385.
44. Fichman L, Koestner R, Zuroff DC. Depressive styles in adolescence: Assessment, relation to social functioning, and developmental trends. J Youth Adolesc. 1994;23(3):315-330.
45. Wiseman H. Interpersonal relatedness and self-definition in the experience of loneliness during the transition to university. Personal Relationships. 1997;4(3):285-299.
46. Mongrain M, Lubbers R, Struthers W. The power of love: mediation of rejection in roommate relationships of dependents and self-critics. Pers Soc Psychol Bull. 2004;30(1):94-105.
47. Whiffen VE, Aube JA. Personality, interpersonal context and depression in couples. J Soc Pers Relat. 1999;16(3):369-383.
48. Priel B, Besser A. Dependency and self-criticism among first-time mothers: the roles of global and specific support. J Soc Clin Psychol. 2000;19(4):437-450.
49. Neff KD, Beretvas SN. The role of self-compassion in romantic relationships. Self Identity. 2013;12(1):78-98.
50. Powers TA, Koestner R, Zuroff DC. Self-criticism, goal motivation, and goal progress. J Soc Clin Psychol. 2007;26(7):826-840.
51. Powers TA, Koestner R, Zuroff DC, et al. The effects of self-criticism and self-oriented perfectionism on goal pursuit. Pers Soc Psychol Bull. 2011;37(7):964-975.
52. Hope N, Koestner R, Milyavskaya M. The role of self-compassion in goal pursuit and well-being among university freshmen. Self Identity. 2014;13(5):579-593.
53. Williams JG, Stark SK, Foster EE. Start today or the very last day? The relationships among self-compassion, motivation, and procrastination. Am J Psychol Res. 2008;4(1):37-44.
54. Neff KD, Hseih Y, Dejittherat K. Self-compassion, achievement goals, and coping with academic failure. Self Identity. 2005;4(3):263-287.
55. Campos RC, Besser A, Blatt SJ. The mediating role of self-criticism and dependency in the association between perceptions of maternal caring and depressive symptoms. Depress Anxiety. 2010;27(12):1149-1157.
56. Sachs-Ericsson N, Verona E, Joiner T, et al. Parental verbal abuse and the mediating role of self-criticism in adult internalizing disorders. J Affect Disord. 2006;93(1-3):71-78.
57. Amitay OA, Mongrain M, Fazaa N. Love and control: self-criticism in parents and daughters and perceptions of relationship partners. Pers Individ Dif. 2008;44(1):75-85.
58. Zuroff DC, Fitzpatrick DK. Depressive personality styles: implications for adult attachment. Pers Individ Dif. 1995;18(2):253-265.
59. Kopala-Sibley DC, Zuroff DC. The developmental origins of personality factors from the self-definitional and relatedness domains: a review of theory and research. Rev Gen Psychol. 2014;18(3):137-155.
60. Kopala-Sibley DC, Zuroff DC, Leybman MJ, et al. Recalled peer relationship experiences and current levels of self-criticism and self-reassurance. Psychol Psychother. 2013;86(1):33-51.
61. Neff KD, McGehee P. Self-compassion and psychological resilience among adolescents and young adults. Self Identity. 2010;9(3):225-240.
62. Pepping CA, Davis PJ, O’Donovan A, et al. Individual differences in self-compassion: the role of attachment and experiences of parenting in childhood. Self Identity. 2015;14(1):104-117.
63. Neff KD, Rude SS, Kirkpatrick KL. An examination of self-compassion in relation to positive psychological functioning and personality traits. J Res Pers. 2007;41(4):908-916.
64. Van Dam NT, Sheppard SC, Forsyth JP, et al. Self-compassion is a better predictor than mindfulness of symptom severity and quality of life in mixed anxiety and depression. J Anxiety Disord. 2011;25(1):123-130.
65. Heffernan M, Quinn MT, McNulty SR, et al. Self-compassion and emotional intelligence in nurses. Int J Nursing Practice. 2010;16(4):366-373.
66. Neff KD, Kirkpatrick KL, Rude SS. Self-compassion and adaptive psychological functioning. J Res Pers. 2007;41(1):139-154.
67. Krieger T, Altenstein D, Baettig I, et al. Self-compassion in depression: associations with depressive symptoms, rumination, and avoidance in depressed outpatients. Behav Ther. 2013;44(3):501-513.
68. Breen WE, Kashdan TB, Lenser ML, et al. Gratitude and forgiveness: convergence and divergence on self-report and informant ratings. Pers Individ Dif. 2010;49(8):932-937.
69. Hollis-Walker L, Colosimo K. Mindfulness, self-compassion, and happiness in non-meditators: A theoretical and empirical examination. Pers Individ Dif. 2011;50(2):222-227.
70. Gilbert P, McEwan K, Matos M, et al. Fears of compassion: development of three self-report measures. Psychol Psychother. 2011;84(3):239-255.
71. Neely ME, Schallert DL, Mohammed SS, et al. Self-kindness when facing stress: the role of self-compassion, goal regulation, and support in college students’ well-being. Motiv Emot. 2009;33(1):88-97.
72. Breines JG, Chen S. Self-compassion increases self-improvement motivation. Pers Soc Psychol Bull. 2012;38(9):1133-1143.
73. Allen AB, Leary MR. Self-compassion, stress, and coping. Soc Pers Psychol Compass. 2010;4(2):107-118.
74. Terry ML, Leary MR. Self-compassion, self-regulation, and health. Self Identity. 2011;10(3):352-362.
75. Magnus CMR, Kowalski KC, McHugh TF. The role of self-compassion in women’s self-determined motives to exercise and exercise-related outcomes. Self Identity. 2010;9(4):363-382.
76. Brooks M, Kay-Lambkin F, Bowman J, et al. Self-compassion amongst clients with problematic alcohol use. Mindfulness. 2012;3(4):308-317.
77. Adams CE, Leary MR. Promoting self-compassionate attitudes toward eating among restrictive and guilty eaters. J Soc Clin Psychol. 2007;26(10):1120-1144.
78. Kelly AC, Zuroff DC, Foa CL, et al. Who benefits from training in self-compassionate self-regulation? A study of smoking reduction. J Soc Clin Psychol. 2010;29(7):727-755.
79. Shapira LB, Mongrain M. The benefits of self-compassion and optimism exercises for individuals vulnerable to depression. J Posit Psychol. 2010;5(5):377-389.
80. Albertson ER, Neff KD, Dill-Shackleford KE. Self-compassion and body dissatisfaction in women: a randomized controlled trial of a brief meditation intervention. Mindfulness. 2015;6(3):444-454.
81. Smeets E, Neff K, Alberts H, et al. Meeting suffering with kindness: effects of a brief self-compassion intervention for female college students. J Clinical Psychol. 2014;70(9):794-807.
82. Blatt SJ, D’Afflitti JP, Quinlan DM. Depressive experiences questionnaire. New Haven, CT: Yale University Press; 1976.
83. Weissman AN, Beck AT. Development and validation of the dysfunctional attitude scale: a preliminary investigation. Paper presented at: 62nd Annual Meeting of the Association for Advanced Behavior Therapy; March 27-31, 1978; Toronto, Ontario, Canada.
84. Gilbert P, Clarke M, Hempel S, et al. Criticizing and reassuring oneself: an exploration of forms, styles and reasons in female students. Br J Clin Psychol. 2004;43(pt 1):31-50.
85. Baião R, Gilbert P, McEwan K, et al. Forms of self-criticising/attacking & self-reassuring scale: psychometric properties and normative study. Psychol Psychother. 2015;88(4):438-452.
86. Neff KD. The self-compassion scale is a valid and theoretically coherent measure of self-compassion. Mindfulness. 2016;7(1):264-274.
87. Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clinical Psychol. 2013;69(1):28-44.
88. Gilbert P. Introducing compassion-focused therapy. Adv Psychiatr Treat. 2009;15(3):199-208.
89. Kelly AC, Zuroff DC, Shapira LB. Soothing oneself and resisting self-attacks: the treatment of two intrapersonal deficits in depression vulnerability. Cognit Ther Res. 2009;33(3):301-313.
90. Boersma K, Hakanson A, Salomonsson E, et al. Compassion focused therapy to counteract shame, self-criticism and isolation. A replicated single case experimental study of individuals with social anxiety. J Contemp Psychother. 2015;45(2):89-98.
91. Gale C, Gilbert P, Read N, et al. An evaluation of the impact of introducing compassion focused therapy to a standard treatment programme for people with eating disorders. Clin Psychol Psychother. 2014;21(1):1-12.
92. Braehler C, Gumley A, Harper J, et al. Exploring change processes in compassion focused therapy in psychosis: results of a feasibility randomized controlled trial. Br J Clin Psychol. 2013;52(2):199-214.
93. Ashworth F, Clarke A, Jones L, et al. An exploration of compassion focused therapy following acquired brain injury. Psychol Psychother. 2014;88(2):143-162.
94. Judge L, Cleghorn A, McEwan K, et al. An exploration of group-based compassion focused therapy for a heterogeneous range of clients presenting to a community mental health team. Int J Cogn Ther. 2012;5(4):420-429.
95. Leaviss J, Utley L. Psychotherapeutic benefits of compassion-focused therapy: an early systematic review. Psychol Med. 2015;45(5):927-945.
96. Gilbert P, McEwan K, Gibbons L, et al. Fears of compassion and happiness in relation to alexithymia, mindfulness, and self‐criticism. Psychol Psychother. 2012;85(4):374-390.
97. Germer CK, Neff KD. Cultivating self-compassion in trauma survivors. In: Follette VM, Briere J, Rozelle D, et al, eds. Mindfulness-oriented interventions for trauma: integrating contemplative practices. New York, NY: Guilford Press; 2015:43-58.
98. Gilbert P. Compassion focused therapy: the CBT distinctive features series. London, United Kingdom: Routledge; 2010.
99. Germer C, Neff K. The mindful self-compassion training program. In: Singer T, Bolz M, eds. Compassion: bridging theory and practice: a multimedia book. Leipzig, Germany: Max-Planck Institute; 2013:365-396.
100. Neff K. Self-compassion: the proven power of being kind to yourself. New York, NY: HarperCollins; 2015.
1. Shahar B, Doron G, Ohad S. Childhood maltreatment, shame-proneness and self-criticism in social anxiety disorder: a sequential mediational model. Clin Psychol Psychother. 2015:22(6):570-579.
2. Kannan D, Levitt HM. A review of client self-criticism in psychotherapy. J Psychother Integr. 2013;23(2):166-178.
3. Barnard LK, Curry JF. Self-compassion: conceptualizations, correlates, and interventions. Rev Gen Psychol. 2011;15(4):289-303.
4. MacBeth A, Gumley A. Exploring compassion: a meta-analysis of the association between self-compassion and psychopathology. Clin Psychol Rev. 2012;32(6):545-552
5. Blatt SJ, Zuroff DC. Interpersonal relatedness and self-definition: two prototypes for depression. Clin Psychol Rev. 1992;12(5):527-562.
6. Neff KD. The development and validation of a scale to measure self-compassion. Self Identity. 2003;2(2):223-250.
7. Neff KD. Self-compassion: an alternative conceptualization of a healthy attitude toward oneself. Self Identity. 2003;(2)2:85-101.
8. Gilbert P, Procter S. Compassionate mind training for people with high shame and self-criticism: overview and pilot study of a group therapy approach. Clin Psychol Psychother. 2006;13(6):353-379.
9. Dunkley DM, Zuroff DC, Blankstein KR. Specific perfectionism components versus self-criticism in predicting maladjustment. Pers Individ Dif. 2006;40(4):665-676.
10. Murphy JM, Nierenberg AA, Monson RR, et al. Self-disparagement as feature and forerunner of depression: Mindfindings from the Stirling County Study. Compr Psychiatry. 2002;43(1):13-21.
11. Brewin CR, Firth-Cozens J. Dependency and self-criticism as predictors of depression in young doctors. J Occup Health Psychol. 1997;2(3):242-246.
12. Fazaa N, Page S. Dependency and self-criticism as predictors of suicidal behavior. Suicide Life Threat Behav. 2003;33(2):172-185.
13. Teasdale JD, Cox SG. Dysphoria: self-devaluative and affective components in recovered depressed patients and never depressed controls. Psychol Med. 2001;31(7):1311-1316.
14. Ehret AM, Joormann J, Berking M. Examining risk and resilience factors for depression: the role of self-criticism and self-compassion. Cogn Emot. 2015;29(8):1496-1504.
15. Elkin I, Shea MT, Watkins JT, et al. National Institute of Mental Health Treatment of Depression Collaborative Research Program. General effectiveness of treatments. Arch Gen Psychiatry. 1989;46(11):971-982; discussion 983.
16. Rector NA, Bagby RM, Segal ZV, et al. Self-criticism and dependency in depressed patients treated with cognitive therapy or pharmacotherapy. Cognit Ther Res. 2000;24(5):571-584.
17. Marshall MB, Zuroff DC, McBride C, et al. Self-criticism predicts differential response to treatment for major depression. J Clin Psychol. 2008;64(3):231-244.
18. Zuroff DC, Blatt SJ, Sotsky SM, et al. Relation of therapeutic alliance and perfectionism to outcome in brief outpatient treatment of depression. J Consult Clin Psychol. 2000;68(1):114-124.
19. Whelton WJ, Greenberg LS. Emotion in self-criticism. Pers Individ Dif. 2005;38(7):1583-1595.
20. Cox BJ, Fleet C, Stein MB. Self-criticism and social phobia in the US national comorbidity survey. J Affect Disord. 2004;82(2):227-234.
21. Cox BJ, Walker JR, Enns MW, et al. Self-criticism in generalized social phobia and response to cognitive-behavioral treatment. Behav Ther. 2002;33(4):479-491.
22. McCranie EW, Hyer LA. Self-critical depressive experience in posttraumatic stress disorder. Psychol Rep. 1995;77(3 pt 1):880-882.
23. Southwick SM, Yehuda R, Giller EL Jr. Characterization of depression in war-related posttraumatic stress disorder. Am J Psychiatry. 1991;148(2):179-183.
24. Yehuda R, Kahana B, Southwick SM, et al. Depressive features in Holocaust survivors with post-traumatic stress disorder. J Traumatic Stress. 1994;7(4):699-704.
25. Sharhabani-Arzy R, Amir M, Swisa A. Self-criticism, dependency and posttraumatic stress disorder among a female group of help-seeking victims of domestic violence in Israel. Pers Individ Dif. 2005;38(5):1231-1240.
26. Beaumont E, Galpin A, Jenkins P. ‘Being kinder to myself’: a prospective comparative study, exploring post-trauma therapy outcome measures, for two groups of clients, receiving either cognitive behaviour therapy or cognitive behaviour therapy and compassionate mind training. Counselling Psychol Rev. 2012;27(1):31-43.
27. Dahm KA. Mindfulness and self-compassion as predictors of functional outcomes and psychopathology in OEF/OIF veterans exposed to trauma. https://repositories.lib.utexas.edu/handle/2152/21635. Published August 2013. Accessed November 8, 2016.
28. Hiraoka R, Meyer EC, Kimbrel NA, et al. Self-compassion as a prospective predictor of PTSD symptom severity among trauma-exposed US Iraq and Afghanistan war veterans. J Trauma Stress. 2015;28(2):127-133.
29. Bagby RM, Cox BJ, Schuller DR, et al. Diagnostic specificity of the dependent and self-critical personality dimensions in major depression. J Affect Disord. 1992;26(1):59-63.
30. Hedman E, Ström P, Stünkel A, et al. Shame and guilt in social anxiety disorder: effects of cognitive behavior therapy and association with social anxiety and depressive symptoms. PLoS One. 2013;8(4):e61713. doi: 10.1371/journal.pone.0061713.
31. Hoge EA, Hölzel BK, Marques L, et al. Mindfulness and self-compassion in generalized anxiety disorder: examining predictors of disability. Evid Based Complement Alternat Med. 2013;2013:576258. doi: 10.1155/2013/576258.
32. Wetterneck CT, Lee EB, Smith AH, et al. Courage, self-compassion, and values in obsessive-compulsive disorder. J Contextual Behav Sci. 2013;2(3-4):68-73.
33. Kelly AC, Carter JC. Why self-critical patients present with more severe eating disorder pathology: The mediating role of shame. Br J Clin Psychol. 2013;52(2):148-161.
34. Dunkley DM, Grilo CM. Self-criticism, low self-esteem, depressive symptoms, and over-evaluation of shape and weight in binge eating disorder patients. Behav Res Ther. 2007;45(1):139-149.
35. Dunkley DM, Masheb RM, Grilo CM. Childhood maltreatment, depressive symptoms, and body dissatisfaction in patients with binge eating disorder: the mediating role of self-criticism. Int J Eat Disord. 2010;43(3):274-281.
36. Wasylkiw L, MacKinnon AL, MacLellan AM. Exploring the link between self-compassion and body image in university women. Body Image. 2012;9(2):236-245.
37. Ferreira C, Pinto-Gouveia J, Duarte C. Self-compassion in the face of shame and body image dissatisfaction: implications for eating disorders. Eat Behavs. 2013;14(2):207-210.
38. Kelly AC, Carter JC, Zuroff DC, et al. Self-compassion and fear of self-compassion interact to predict response to eating disorders treatment: a preliminary investigation. Psychother Res. 2013;23(3):252-264.
39. Webb JB, Forman MJ. Evaluating the indirect effect of self-compassion on binge eating severity through cognitive-affective self-regulatory pathways. Eat Behavs. 2013;14(2):224-228.
40. Kelly AC, Carter JC, Borairi S. Are improvements in shame and self-compassion early in eating disorders treatment associated with better patient outcomes? Int J Eat Disord. 2014;47(1):54-64.
41. Wiseman H, Raz A, Sharabany R. Depressive personality styles and interpersonal problems in young adults with difficulties in establishing long-term romantic relationships. Isr J Psychiatry Rel Sci. 2007;44(4):280-291.
42. Besser A, Priel B. A multisource approach to self-critical vulnerability to depression: the moderating role of attachment. J Pers. 2003;71(4):515-555.
43. Zuroff DC, Koestner R, Powers TA. Self-criticism at age 12: a longitudinal-study of adjustment. Cognit Ther Res. 1994;18(4):367-385.
44. Fichman L, Koestner R, Zuroff DC. Depressive styles in adolescence: Assessment, relation to social functioning, and developmental trends. J Youth Adolesc. 1994;23(3):315-330.
45. Wiseman H. Interpersonal relatedness and self-definition in the experience of loneliness during the transition to university. Personal Relationships. 1997;4(3):285-299.
46. Mongrain M, Lubbers R, Struthers W. The power of love: mediation of rejection in roommate relationships of dependents and self-critics. Pers Soc Psychol Bull. 2004;30(1):94-105.
47. Whiffen VE, Aube JA. Personality, interpersonal context and depression in couples. J Soc Pers Relat. 1999;16(3):369-383.
48. Priel B, Besser A. Dependency and self-criticism among first-time mothers: the roles of global and specific support. J Soc Clin Psychol. 2000;19(4):437-450.
49. Neff KD, Beretvas SN. The role of self-compassion in romantic relationships. Self Identity. 2013;12(1):78-98.
50. Powers TA, Koestner R, Zuroff DC. Self-criticism, goal motivation, and goal progress. J Soc Clin Psychol. 2007;26(7):826-840.
51. Powers TA, Koestner R, Zuroff DC, et al. The effects of self-criticism and self-oriented perfectionism on goal pursuit. Pers Soc Psychol Bull. 2011;37(7):964-975.
52. Hope N, Koestner R, Milyavskaya M. The role of self-compassion in goal pursuit and well-being among university freshmen. Self Identity. 2014;13(5):579-593.
53. Williams JG, Stark SK, Foster EE. Start today or the very last day? The relationships among self-compassion, motivation, and procrastination. Am J Psychol Res. 2008;4(1):37-44.
54. Neff KD, Hseih Y, Dejittherat K. Self-compassion, achievement goals, and coping with academic failure. Self Identity. 2005;4(3):263-287.
55. Campos RC, Besser A, Blatt SJ. The mediating role of self-criticism and dependency in the association between perceptions of maternal caring and depressive symptoms. Depress Anxiety. 2010;27(12):1149-1157.
56. Sachs-Ericsson N, Verona E, Joiner T, et al. Parental verbal abuse and the mediating role of self-criticism in adult internalizing disorders. J Affect Disord. 2006;93(1-3):71-78.
57. Amitay OA, Mongrain M, Fazaa N. Love and control: self-criticism in parents and daughters and perceptions of relationship partners. Pers Individ Dif. 2008;44(1):75-85.
58. Zuroff DC, Fitzpatrick DK. Depressive personality styles: implications for adult attachment. Pers Individ Dif. 1995;18(2):253-265.
59. Kopala-Sibley DC, Zuroff DC. The developmental origins of personality factors from the self-definitional and relatedness domains: a review of theory and research. Rev Gen Psychol. 2014;18(3):137-155.
60. Kopala-Sibley DC, Zuroff DC, Leybman MJ, et al. Recalled peer relationship experiences and current levels of self-criticism and self-reassurance. Psychol Psychother. 2013;86(1):33-51.
61. Neff KD, McGehee P. Self-compassion and psychological resilience among adolescents and young adults. Self Identity. 2010;9(3):225-240.
62. Pepping CA, Davis PJ, O’Donovan A, et al. Individual differences in self-compassion: the role of attachment and experiences of parenting in childhood. Self Identity. 2015;14(1):104-117.
63. Neff KD, Rude SS, Kirkpatrick KL. An examination of self-compassion in relation to positive psychological functioning and personality traits. J Res Pers. 2007;41(4):908-916.
64. Van Dam NT, Sheppard SC, Forsyth JP, et al. Self-compassion is a better predictor than mindfulness of symptom severity and quality of life in mixed anxiety and depression. J Anxiety Disord. 2011;25(1):123-130.
65. Heffernan M, Quinn MT, McNulty SR, et al. Self-compassion and emotional intelligence in nurses. Int J Nursing Practice. 2010;16(4):366-373.
66. Neff KD, Kirkpatrick KL, Rude SS. Self-compassion and adaptive psychological functioning. J Res Pers. 2007;41(1):139-154.
67. Krieger T, Altenstein D, Baettig I, et al. Self-compassion in depression: associations with depressive symptoms, rumination, and avoidance in depressed outpatients. Behav Ther. 2013;44(3):501-513.
68. Breen WE, Kashdan TB, Lenser ML, et al. Gratitude and forgiveness: convergence and divergence on self-report and informant ratings. Pers Individ Dif. 2010;49(8):932-937.
69. Hollis-Walker L, Colosimo K. Mindfulness, self-compassion, and happiness in non-meditators: A theoretical and empirical examination. Pers Individ Dif. 2011;50(2):222-227.
70. Gilbert P, McEwan K, Matos M, et al. Fears of compassion: development of three self-report measures. Psychol Psychother. 2011;84(3):239-255.
71. Neely ME, Schallert DL, Mohammed SS, et al. Self-kindness when facing stress: the role of self-compassion, goal regulation, and support in college students’ well-being. Motiv Emot. 2009;33(1):88-97.
72. Breines JG, Chen S. Self-compassion increases self-improvement motivation. Pers Soc Psychol Bull. 2012;38(9):1133-1143.
73. Allen AB, Leary MR. Self-compassion, stress, and coping. Soc Pers Psychol Compass. 2010;4(2):107-118.
74. Terry ML, Leary MR. Self-compassion, self-regulation, and health. Self Identity. 2011;10(3):352-362.
75. Magnus CMR, Kowalski KC, McHugh TF. The role of self-compassion in women’s self-determined motives to exercise and exercise-related outcomes. Self Identity. 2010;9(4):363-382.
76. Brooks M, Kay-Lambkin F, Bowman J, et al. Self-compassion amongst clients with problematic alcohol use. Mindfulness. 2012;3(4):308-317.
77. Adams CE, Leary MR. Promoting self-compassionate attitudes toward eating among restrictive and guilty eaters. J Soc Clin Psychol. 2007;26(10):1120-1144.
78. Kelly AC, Zuroff DC, Foa CL, et al. Who benefits from training in self-compassionate self-regulation? A study of smoking reduction. J Soc Clin Psychol. 2010;29(7):727-755.
79. Shapira LB, Mongrain M. The benefits of self-compassion and optimism exercises for individuals vulnerable to depression. J Posit Psychol. 2010;5(5):377-389.
80. Albertson ER, Neff KD, Dill-Shackleford KE. Self-compassion and body dissatisfaction in women: a randomized controlled trial of a brief meditation intervention. Mindfulness. 2015;6(3):444-454.
81. Smeets E, Neff K, Alberts H, et al. Meeting suffering with kindness: effects of a brief self-compassion intervention for female college students. J Clinical Psychol. 2014;70(9):794-807.
82. Blatt SJ, D’Afflitti JP, Quinlan DM. Depressive experiences questionnaire. New Haven, CT: Yale University Press; 1976.
83. Weissman AN, Beck AT. Development and validation of the dysfunctional attitude scale: a preliminary investigation. Paper presented at: 62nd Annual Meeting of the Association for Advanced Behavior Therapy; March 27-31, 1978; Toronto, Ontario, Canada.
84. Gilbert P, Clarke M, Hempel S, et al. Criticizing and reassuring oneself: an exploration of forms, styles and reasons in female students. Br J Clin Psychol. 2004;43(pt 1):31-50.
85. Baião R, Gilbert P, McEwan K, et al. Forms of self-criticising/attacking & self-reassuring scale: psychometric properties and normative study. Psychol Psychother. 2015;88(4):438-452.
86. Neff KD. The self-compassion scale is a valid and theoretically coherent measure of self-compassion. Mindfulness. 2016;7(1):264-274.
87. Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clinical Psychol. 2013;69(1):28-44.
88. Gilbert P. Introducing compassion-focused therapy. Adv Psychiatr Treat. 2009;15(3):199-208.
89. Kelly AC, Zuroff DC, Shapira LB. Soothing oneself and resisting self-attacks: the treatment of two intrapersonal deficits in depression vulnerability. Cognit Ther Res. 2009;33(3):301-313.
90. Boersma K, Hakanson A, Salomonsson E, et al. Compassion focused therapy to counteract shame, self-criticism and isolation. A replicated single case experimental study of individuals with social anxiety. J Contemp Psychother. 2015;45(2):89-98.
91. Gale C, Gilbert P, Read N, et al. An evaluation of the impact of introducing compassion focused therapy to a standard treatment programme for people with eating disorders. Clin Psychol Psychother. 2014;21(1):1-12.
92. Braehler C, Gumley A, Harper J, et al. Exploring change processes in compassion focused therapy in psychosis: results of a feasibility randomized controlled trial. Br J Clin Psychol. 2013;52(2):199-214.
93. Ashworth F, Clarke A, Jones L, et al. An exploration of compassion focused therapy following acquired brain injury. Psychol Psychother. 2014;88(2):143-162.
94. Judge L, Cleghorn A, McEwan K, et al. An exploration of group-based compassion focused therapy for a heterogeneous range of clients presenting to a community mental health team. Int J Cogn Ther. 2012;5(4):420-429.
95. Leaviss J, Utley L. Psychotherapeutic benefits of compassion-focused therapy: an early systematic review. Psychol Med. 2015;45(5):927-945.
96. Gilbert P, McEwan K, Gibbons L, et al. Fears of compassion and happiness in relation to alexithymia, mindfulness, and self‐criticism. Psychol Psychother. 2012;85(4):374-390.
97. Germer CK, Neff KD. Cultivating self-compassion in trauma survivors. In: Follette VM, Briere J, Rozelle D, et al, eds. Mindfulness-oriented interventions for trauma: integrating contemplative practices. New York, NY: Guilford Press; 2015:43-58.
98. Gilbert P. Compassion focused therapy: the CBT distinctive features series. London, United Kingdom: Routledge; 2010.
99. Germer C, Neff K. The mindful self-compassion training program. In: Singer T, Bolz M, eds. Compassion: bridging theory and practice: a multimedia book. Leipzig, Germany: Max-Planck Institute; 2013:365-396.
100. Neff K. Self-compassion: the proven power of being kind to yourself. New York, NY: HarperCollins; 2015.
Psychiatric consultations in long-term care: An evidence-based practical guide
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).
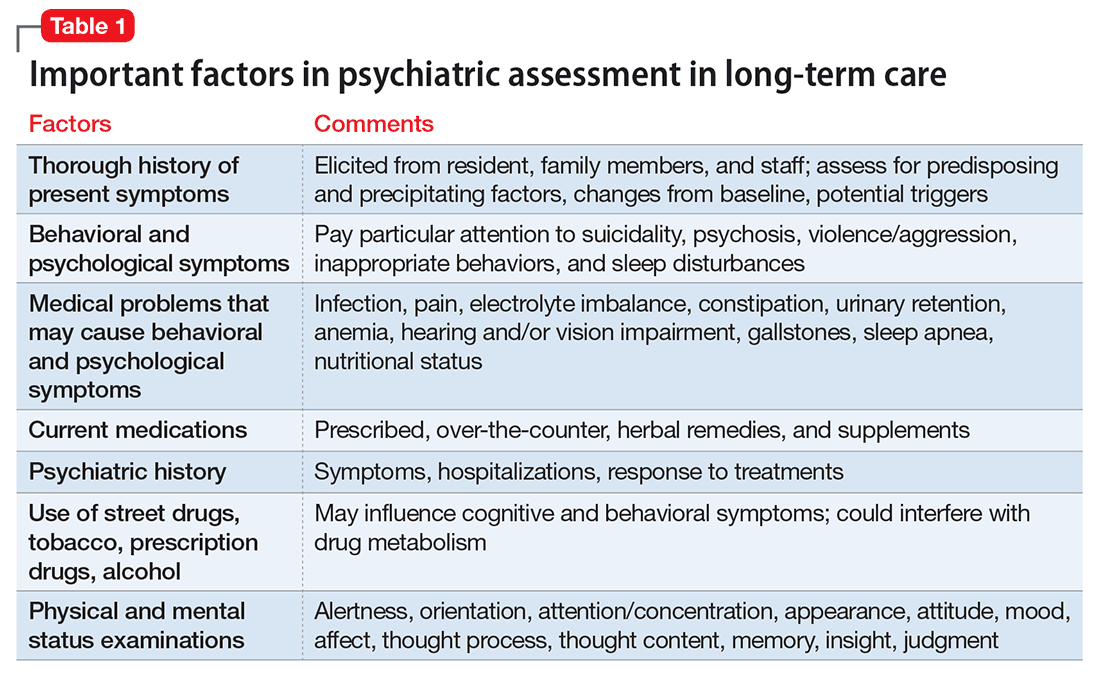
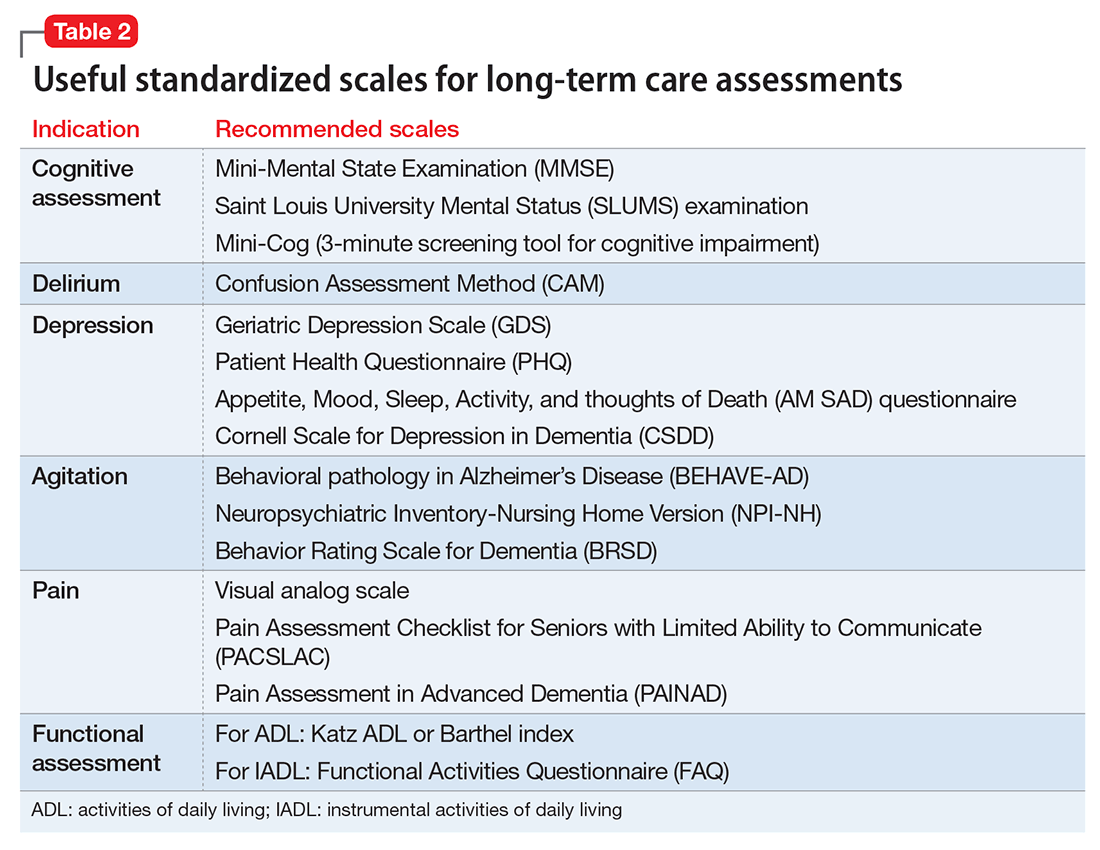
This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5
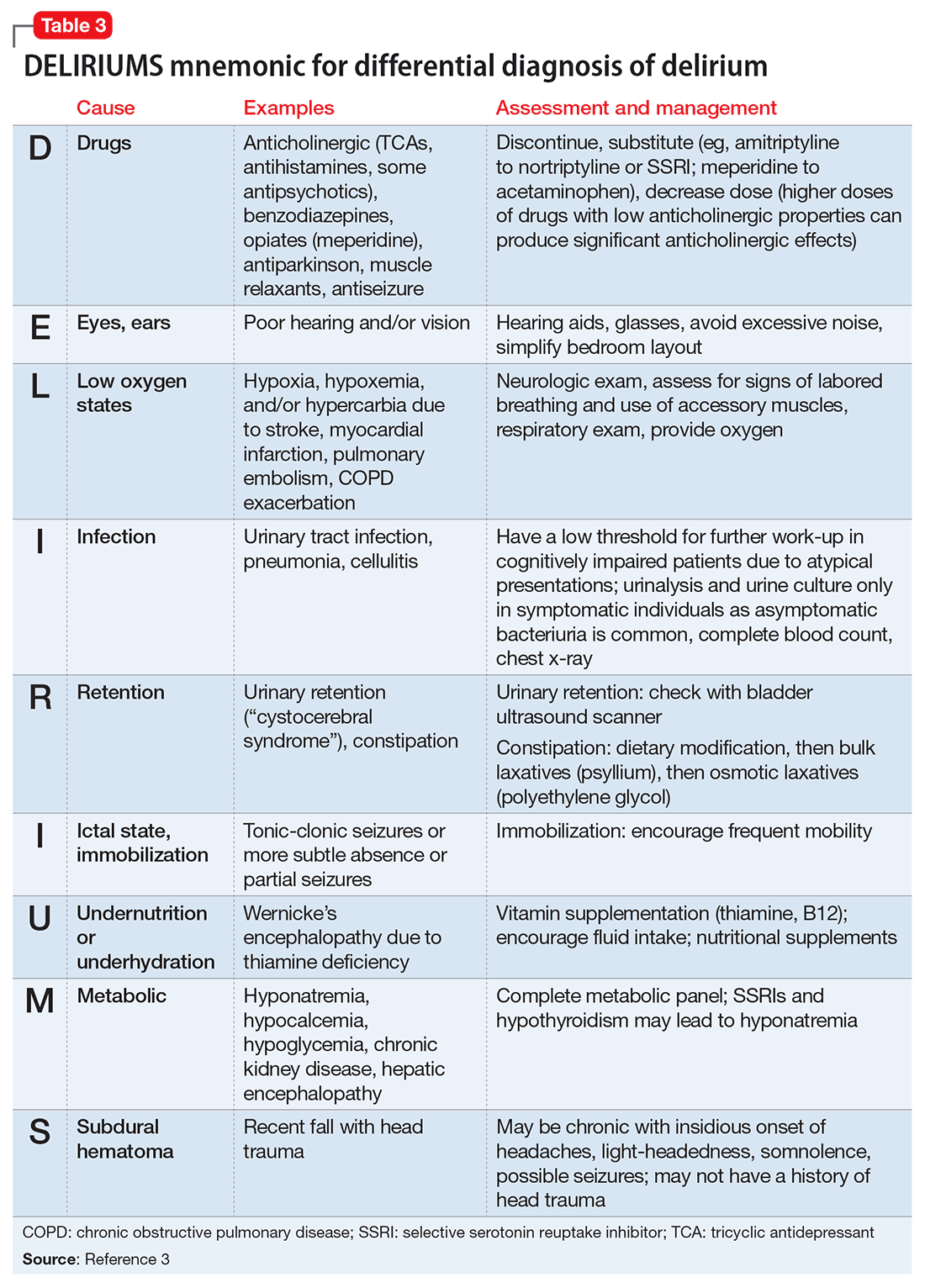
The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14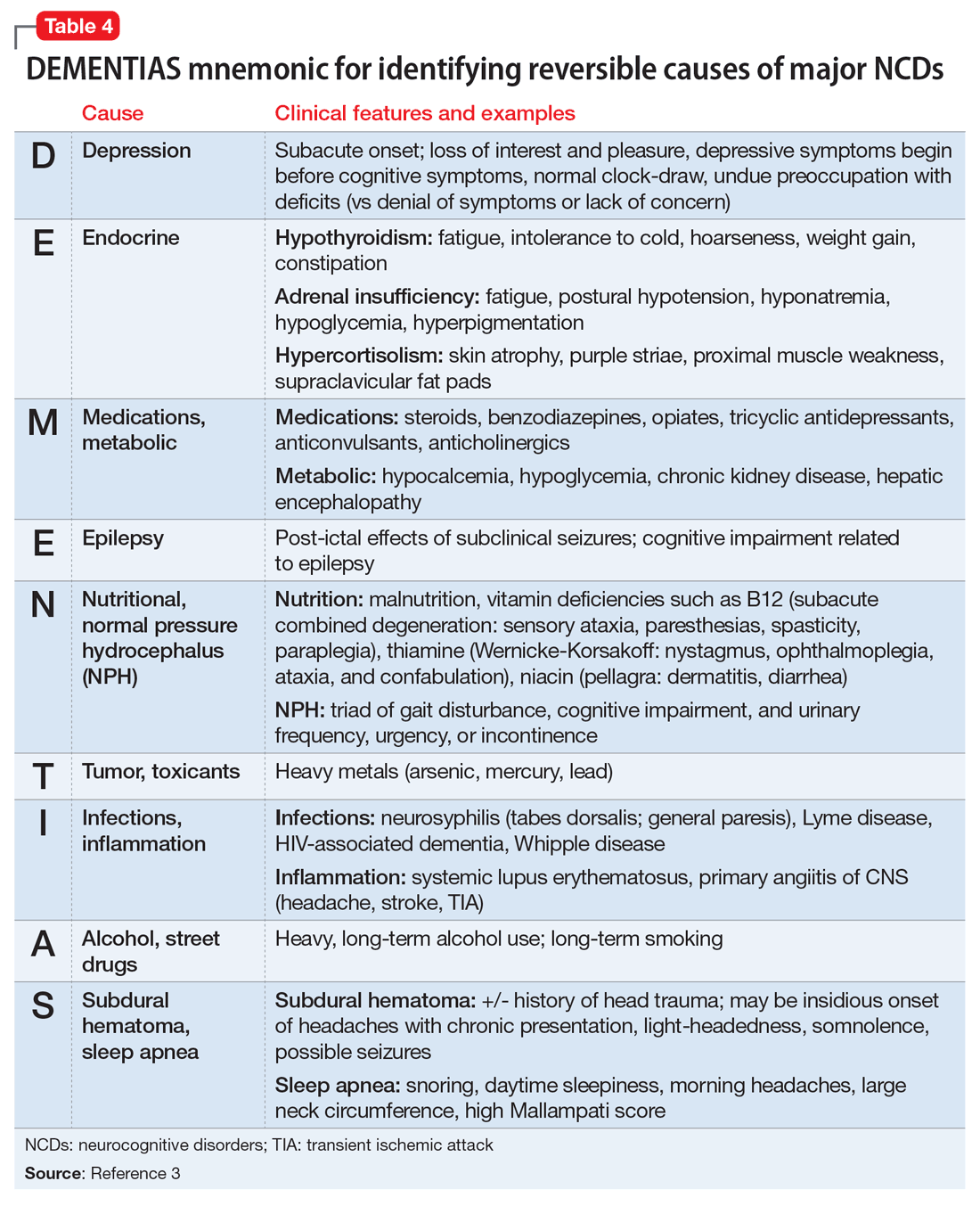
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).


This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5

The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
Long-term care (LTC) services provide health care to >8 million people in approximately 30,000 nursing homes and assisted living/residential care communities in the United States.1 One-half of older adults in LTC have neurocognitive disorders (NCDs), and one-third have depressive syndromes.2 Common reasons for psychiatric consultation include these 2 major diagnoses, as well as delirium, behavioral and psychological symptoms of dementia (BPSD), bipolar disorder, anxiety, sleep disorders, and pain management.
Psychiatric assessment of individuals in LTC can be challenging because of atypical presentations, cognitive impairment, and multiple comorbidities. Establishing a management plan involves eliciting a careful history from both the patient and caretakers, examining previous records and medications, and selecting appropriate screening tools and laboratory tests (Table 1 and Table 2).


This article offers a practical approach to assess and manage common psychiatric conditions in LTC. We include new evidence about:
- assessment tools for psychiatric symptoms in LTC
- potentially inappropriate medication use in older adults
- antipsychotic use for agitation and psychosis with dementia
- nonpharmacologic interventions to help prevent cognitive decline
- antipsychotic review in reducing antipsychotic use and mortality.
Delirium
Delirium is an important topic in LTC because it is highly prevalent, poorly recognized, and can be difficult to manage. Common causes of delirium in LTC include infection (often urinary), dehydration, medications, long-standing constipation, and urinary retention (Table 3).3 Early recognition is key because delirium has been associated with cognitive decline, decreased functional status, increased caregiver burden, and increased mortality.4,5

The Confusion Assessment Method (CAM) is a quick tool with 4 features to differentiate delirium from other forms of cognitive impairment.6 The 2 core features are an acute change or fluctuating course of mental status and inattention. Family members or caregivers can provide information about an acute change. To assess inattention, ask the patient to say the days of the week backward or spell the word “world” backward. The 2 other features of delirium—one of which must be present when using the CAM—are disorganized thinking and altered level of consciousness.
Individuals with delirium may present with hyperactive or hypoactive psychomotor activity. Hypoactive delirium’s features, such as sluggishness and lethargy, could be confused with depression.7 A careful history to determine symptom onset and fluctuation in course can help differentiate between the 2.
Management. Delirium management always should begin by addressing underlying causes and implementing psychosocial and environmental interventions. Pharmacologic interventions have not demonstrated consistent benefit for delirium in well-designed trials and are not recommended as first-line treatment.8 The American Geriatrics Society (AGS) Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommends avoiding benzodiazepines in this population.9 Antipsychotics could be used in patients with severe agitation who pose harm to themselves or others. Nonpharmacologic approaches to delirium in LTC include:
- frequent reorientation (clocks, daily schedule)
- one-on-one monitoring by staff or family members
- use of hearing aids and eye-glasses, if needed
- maintaining an appropriate sleep-wake cycle by encouraging exposure to bright light during the day and avoiding night-time interruptions.
Restraints should not be used; they appear to worsen delirium severity, and their removal does not increase the rate of falls or fall-related injury.10
Various methods for managing a patient with delirium have been proposed, such as the TADA approach (tolerate, anticipate, and don’t agitate).5,11,12 For example, if a patient’s agitation worsens with attempted reorientation, distraction or playing along with the disorientation could be more beneficial.12
Keep in mind delirium’s overlapping presentation with Lewy body dementia (LBD). Patients with LBD demonstrate a progressive decline in cognitive functioning associated with fluctuating cognition, visual hallucinations, and parkinsonism features. Consider LBD when no cause for delirium-like symptoms is found. These patients may show increased sensitivity to neuroleptics and extrapyramidal side effects.
Neurocognitive disorders
Reversible causes. Although most individuals with major NCDs are diagnosed before entering LTC, the consulting psychiatrist’s review of potentially reversible causes of neurocognitive symptoms can lead to dramatically different treatment regimens (Table 43). For example, anticholinergic medications can harm the aging brain and have been linked to delirium, increased brain atrophy, and lower scores on tests of cognitive functioning.13 Given the prevalence of polypharmacy in older adults, be aware of unexpected anticholinergic properties of many common drugs, as rated by the Aging Brain Care initiative.14
Mild cognitive impairment. Should patients showing signs of cognitive impairment or those at risk for major NCDs begin pharmacotherapy? The FDA has approved no medications for this indication, and clinical trials with agents such as cholinesterase inhibitors (ChEIs) have shown inconsistent results.
The randomized, double-blind Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability provides convincing data that a nonpharmacologic approach could benefit older adults at risk for a major NCD. A 2-year intervention of nutritional advice, aerobic and strength training, cognitive training, social activities, and blood pressure and weight monitoring was more effective in improving or maintaining cognitive function in individuals age 60 to 77, compared with general health advice given to a control group.15
Behavioral and psychological symptoms. Psychiatrists are likely to be consulted in LTC when a person with a major NCD presents with an acute episode of increased confusion and cognitive worsening, often accompanied by behavioral symptoms. BPSD may include agitation, aggression, apathy, depression, sleep problems, socially inappropriate behaviors, and psychosis. One study of patients with Alzheimer’s disease (AD) reported a cumulative 51% incidence of new-onset hallucinations and delusions at 4 years.16
Increased vulnerability to stressors, unmet needs, over- or under-stimulation, or lack of routines may predispose individuals with major NCDs to developing BPSD.17 Nonpharmacologic approaches usually are tried first, although supporting evidence is not substantial.18 Changes in environment, behavioral redirection, sensory interventions, or music therapy may reduce disruptive behaviors.19 Patients with increased confusion and agitation in late afternoon and evening (“sundowning”) may benefit from short naps after lunch, light therapy, calming activities in late afternoon, and reduced noise (such as from dishes, loud speakers, staff conversations).20
Antipsychotics. The drugs most commonly used to manage BPSD are antipsychotics, antidepressants, mood stabilizers/anticonvulsants, ChEIs, and the N-methyl-
When nonpharmacotherapeutic interventions are not successful, most guidelines agree that using an atypical antipsychotic is warranted in AD patients with severe agitation and/or psychosis that pose a risk to the patient or others or severely impair their quality of life.9,22,23
Antipsychotic review. Recent guidelines from the American Psychiatric Association (APA) recommend that attempts to taper and withdraw antipsychotic drugs be made within 4 months of initiating treatment in patients with dementia who display an adequate response.23 In a recent nursing home study, antipsychotic review was found to reduce antipsychotic use by 50% and, when combined with a social intervention, to reduce mortality compared with a group receiving neither intervention.24
Interestingly, patients receiving antipsychotic review alone showed an increase in overall neuropsychiatric symptoms.24 A previous study of patients with AD whose psychosis or agitation responded to risperidone also found an increased risk of relapse when risperidone was discontinued.25 These results highlight the importance of making patient-centered decisions, frequent re-assessments, and adding non-pharmacologic interventions (eg, positive social interactions or exercise) when attempting to discontinue antipsychotics.
Other treatment options. Because patients with LBD often display increased sensitivity to neuroleptics, agents such as quetiapine or aripiprazole (with a lower risk of EPS) are preferred when managing severe psychosis/aggression. ChEIs may show some benefit for behavioral disturbances in patients with LBD.26
In patients with AD, ChEIs have shown inconsistent results in benefiting neuropsychiatric symptoms. Preliminary data suggest some benefit with citalopram (also associated with prolonged QTc)27 and the dextromethorphan/quinidine combination FDA-approved for pseudobulbar affect, but more studies are needed.28 Pimavanserin, a 5-HT2A receptor inverse agonist, recently was approved for treating hallucinations and delusions associated with Parkinson’s disease psychosis and currently is in clinical trials for Alzheimer’s disease psychosis.
Electroconvulsive therapy (ECT) may be a therapeutic option for agitation and aggression in people with dementia.29 ECT has no absolute contraindications and can be safely performed in individuals with pacemakers or implantable cardioverter defibrillators. Common adverse effects include transient changes in blood pressure or heart rate, headache, and nausea. Cognitive adverse effects from ECT may include:
- anterograde amnesia, which typically resolves after a few weeks
- retrograde amnesia, which typically manifests as loss of impersonal memories occurring in the past few months.
Depression
The prevalence of depression in nursing home residents is an estimated 3 to 4 times that of community-dwelling older adults.30 Assessing for depression is particularly important in people with mild cognitive impairment, as depressive symptoms have been associated with progression to AD.31 Quick screening tools (Table 2) include short forms of the Patient Health Questionnaire (PHQ-2 or PHQ-9)32 or the Saint Louis University Appetite, Mood, Sleep, Activity, and thoughts of Death (SLU “AM SAD”) scale.33 The Cornell Scale for Depression in Dementia is useful for individuals with major NCDs because it relies on interviews with the patient and nursing staff or family.34
To test for other causes of depression, order a complete blood count for anemia, serum glucose, thyroid-stimulating hormone for hypothyroidism or hyperthyroidism, B12 and folate levels, and a cognitive screen such as the Saint Louis University Mental Status examination.35
Treatment. Antidepressants are generally considered effective in older patients with depression. Selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) are first-line treatments because of safety concerns with tricyclic antidepressants. All 3 classes have shown similar efficacy in comparison trials in geriatric populations.
When initiating these agents, take care in the first few days and weeks to monitor for potential serious adverse effects, such as nausea and vomiting, which may be associated with substantial morbidity in patients with comorbidities. For monitoring treatment response, the PHQ-9 can effectively distinguish patients with persistent major depression, partial remission, or full remission.36
The optimal duration of a short-term antidepressant trial before switching to a different agent is unclear, although a good therapeutic trial typically is 4 to 12 weeks. In one study of older adults with depression, 4 weeks was enough to reliably identify those likely to benefit from a change in treatment plan.37
Cognitive-behavioral therapy (CBT) can be used in older adults not wishing to pursue pharmacotherapy or as an adjunct to antidepressants. Randomized controlled trials have shown some benefit for those with depression, anxiety, and insomnia.38 Individuals with significant cognitive deficits or those not motivated to apply CBT strategies might not benefit.
ECT may be appropriate for treating depression in older adults with:
- urgent need of a therapeutic response (eg, suicidal ideation or nutritional compromise)
- lack of response to antidepressant medication
- major depressive disorder with psychotic or catatonic features.
Evidence regarding ECT’s efficacy for late-life depression is derived primarily from clinical experience and open-label trials.39
Bipolar disorder
Most individuals with bipolar disorder present before age 50, although 9% of first manic episodes occur after age 60.40 Earlier age of onset appears to predict poor outcomes, and early-onset bipolar disorder may worsen with advanced age related to increased comorbidities and difficulty in medical management.41 Compared with younger patients, features of bipolar disorder in older adults include increased prominence of rapid cycling, more time spent in a depressed state than in manic state, and less severe manic and psychotic symptoms.42
When older patients present with depression, always evaluate for clinical features more consistent with late-onset bipolar disorder than with major depressive disorder: hypomania, family history of bipolar disorder, higher number of prior depressive episodes, and higher levels of fear and inner tension.43 The differential diagnosis for new-onset manic symptoms in older adults includes:
- general medical conditions (stroke, brain tumors, hyperthyroidism, neurosyphilis)
- medications (corticosteroids, dopaminergic drugs, St. John’s wort)
- substance use.
Hyperthyroidism deserves special attention because it can present in older adults with either manic-like symptoms and hyperkinesis or features of apathy, depression, and somnolence. Given that older age and bipolar disorder both are associated with increased suicide risk, monitor these individuals for signs of hopelessness and statements of suicide.44
Treatment. Managing bipolar disorder in older adults often requires complex medication regimens. Acute treatment options for geriatric mania and hypomania with the most supporting evidence include lithium, valproate, quetiapine, and olanzapine.45-47 The therapeutic index of lithium is small, and older individuals are more vulnerable to adverse effects related to physiologic changes (eg, decreased glomerular filtration rate or low volume of distribution) that impair lithium clearance. Lithium also interacts with many drugs commonly used by older patients, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. Common adverse events associated with lithium include memory impairment, diarrhea, falls, and tremors.
Maintenance treatment for bipolar disorder is generally the same medication used to induce remission. The evidence for maintenance treatment of bipolar disorder in older adults is limited mostly to subgroup analyses. In one retrospective analysis of patients age ≥55 in 2 randomized trials, lamotrigine and lithium were effective and well-tolerated in delaying time to intervention.48
Anxiety disorders
Anxiety among LTC residents may manifest as irritability, insomnia, restlessness, and verbal and/or physical agitation/aggression.49 Typical causes include:
- primary anxiety disorders
- anxiety symptoms during depressive episodes or bereavement
- adverse effects of medications
- complications of major NCDs or delirium.
Anxiety disorders and subsyndromal anxiety have been associated with poorer quality of life, decreased sleep, and increased distress and impairment.50
Assessment begins with a self-report of symptoms, although this may be difficult in people with major NCDs. Factors that may differentiate true anxiety from major NCDs include restlessness, irritability, muscle tension, fears, and respiratory symptoms in addition to excessive anxiety and worry.51 The Geriatric Anxiety Inventory is a useful screening tool.52 The newer Brief Anxiety and Depression Scale may identify and differentiate patients with major depressive episodes and generalized anxiety disorder (GAD).53 Potential instruments for patients with comorbid anxiety and major NCDs include the Neuropsychiatric Inventory, Rating Anxiety in Dementia scale,54 and the Anxiety in Cognitive Impairment and Dementia scale.55 Because medications can cause akathisia that may mimic anxiety symptoms, screen for the recent addition of antidepressants, antipsychotics, sympathomimetics, thyroid supplements, and corticosteroids.
Treatment of anxiety disorders—such as panic disorder, social phobia, or GAD—generally starts with SSRIs or SNRIs. Although benzodiazepines are commonly used for anxiety in older adults,56 these drugs are associated with a high rate of adverse effects: increased risk of agitation, falls, impaired cognition, and possibly dementia.57 In general, reserve benzodiazepines for treating acute episodes of severe anxiety in this population.
A particularly prevalent source of anxiety in LTC is fear of falling, which may affect up to 50% of residents and cause them to restrict their activities.58 Interventions such as CBT, exercise, or tai chi may be beneficial, although supporting evidence is lacking.
Pain and sleep management
Addressing pain. Age-related changes in pain perception and difficulty in reporting pain likely contribute to under-recognition of pain in LTC residents. Two useful methods to recognize their pain are to:
- observe for pain behaviors, such as facial expressions (grimacing and brow lowering), vocalizations, and body movements (clenched fists)
- solicit reports from nurses and other caregivers.59
Self-report may be a reliable indicator of pain for individuals with mild-to-moderate NCDs. Observational pain scales, such as the Pain Assessment Checklist for Seniors with Limited Ability to Communicate, may be useful in severe NCDs.60
The AGS recommends acetaminophen as initial pharmacotherapy to manage persistent pain.61 NSAIDs may be another option, but caution is warranted for patients with acid-peptic disease or chronic kidney disease. Opioids may be considered for severe pain, but otherwise avoid using them.
Sleep disturbances are common in LTC because of physiologic changes associated with aging (altered circadian rhythm), comorbidities (depression), and environmental factors.62 A strong association appears to exist between insomnia and use of sedative-hypnotic drugs, and the AGS Beers Criteria recommend avoiding non-benzodiazepine receptor agonists and benzodiazepines when treating insomnia in older adults.9
Assess factors that may contribute to sleep disturbances, including medications and use of caffeine or alcohol. Have the resident or caregiver document sleep patterns in a sleep diary.
Consider administrating medications at different times (eg, switch donepezil from bedtime to morning) or replace with alternatives (switch from the more anticholinergic amitriptyline to nortriptyline). Ensure that residents engage in physical activity and have at least 30 minutes daily exposure to sunlight.
In addition to behavioral interventions and CBT, treatment in older adults can involve melatonin—which has mixed evidence—or sedating antidepressants, such as mirtazapine or trazodone, in patients with comorbid depression.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
1. Harris-Kojetin L, Sengupta M, Park-Lee E, et al. Long-term care services in the United States: 2013 overview. Vital Health Stat 3. 2013(37):1-107.
2. Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long-term care homes: a systematic review. Int Psychogeriatr. 2010;22(7):1025-1039.
3. Flaherty J, Tumosa N. Saint Louis University Geriatric Evaluation Mnemonics and Screening Tools. http://aging.slu.edu/uploads/pdf/Saint-Louis-University-Geriatric-Evaluation_2013.pdf. Accessed October 5, 2016.
4. Boockvar K, Signor D, Ramaswamy R, et al. Delirium during acute illness in nursing home residents. J Am Med Dir Assoc. 2013;14(9):656-660.
5. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
6. Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
7. Farrell KR, Ganzini L. Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med. 1995;155(22):2459-2464.
8. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269-S276.
9. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246.
10. Capezuti E, Strumpf NE, Evans LK, et al. The relationship between physical restraint removal and falls and injuries among nursing home residents. J Gerontol A Biol Sci Med Sci. 1998;53(1):M47-M52.
11. Flaherty JH, Morley JE. Delirium in the nursing home. J Am Med Dir Assoc. 2013;14(9):632-634.
12. Flaherty JH. The evaluation and management of delirium among older persons. Med Clin North Am. 2011;95(3):555-577, xi.
13. Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732.
14. Anticholinergic Cognitive Burden Scale. Aging Brain Care. http://agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf. Published 2012. Accessed October 5, 2016.
15. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263.
16. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
17. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
18. Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess. 2014;18(39):1-226, v-vi.
19. Kong EH, Evans LK, Guevara JP. Nonpharmacological intervention for agitation in dementia: a systematic review and meta-analysis. Aging Ment Health. 2009;13(4):512-520.
20. Khachiyants N, Trinkle D, Son SJ, et al. Sundown syndrome in persons with dementia: an update. Psychiatry Investig. 2011;8(4):275-287.
21. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
22. Jennings L, Grossberg GT. Antipsychotics continue to have a place in the management of difficult behavior problems in patients with dementia. J Am Med Dir Assoc. 2013;14(6):447-449.
23. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
24. Ballard C, Orrell M, YongZhong S, et al. Impact of antipsychotic review and nonpharmacological intervention on antipsychotic use, neuropsychiatric symptoms, and mortality in people with dementia living in nursing homes: a factorial cluster-randomized controlled trial by the Well-Being and Health for People With Dementia (WHELD) program. Am J Psychiatry. 2015;173(3):252-262.
25. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497-1507.
26. Matsunaga S, Kishi T, Yasue I, et al. Cholinesterase inhibitors for Lewy body disorders: a meta-analysis. Int J Neuropsychopharmacol. 2015;19(2). doi: 10.1093/ijnp/pyv086.
27. Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research Group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
28. Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
29. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
30. Jongenelis K, Pot AM, Eisses AM, et al. Prevalence and risk indicators of depression in elderly nursing home patients: the AGED study. J Affect Disord. 2004;83(2-3):135-142.
31. Van der Mussele S, Fransen E, Struyfs H, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer’s disease: a longitudinal study. J Alzheimers Dis. 2014;42(4):1239-1250.
32. Li C, Friedman B, Conwell Y, et al. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55(4):596-602.
33. Chakkamparambil B, Chibnall JT, Graypel EA, et al. Development of a brief validated geriatric depression screening tool: the SLU “AM SAD”. Am J Geriatr Psychiatry. 2015;23(8):780-783.
34. Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60(5):360-364.
35. Tariq SH, Tumosa N, Chibnall JT, et al. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900-910.
36. Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194-1201.
37. Mulsant BH, Houck PR, Gildengers AG, et al. What is the optimal duration of a short-term antidepressant trial when treating geriatric depression? J Clin Psychopharmacol. 2006;26(2):113-120.
38. Chand SP, Grossberg GT. How to adapt cognitive-behavioral therapy for older adults. Current Psychiatry. 2013;12(3):10-15.
39. Van der Wurff FB, Stek ML, Hoogendijk WL, et al. Electroconvulsive therapy for the depressed elderly. Cochrane Database Syst Rev. 2003;(2):CD003593.
40. Kennedy N, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results from a 35-year study. Psychol Med. 2005;35(6):855-863.
41. Carter TD, Mundo E, Parikh SV, et al. Early age at onset as a risk factor for poor outcome of bipolar disorder. J Psychiatr Res. 2003;37(4):297-303.
42. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
43. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163(2):225-231.
44. Aizenberg D, Olmer A, Barak Y. Suicide attempts amongst elderly bipolar patients. J Affect Disord. 2006;91(1):91-94.
45. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
46. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12(4):342-357.
47. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
48. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
49. Gum AM, King-Kallimanis B, Kohn R. Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the National Comorbidity Survey-Replication. Am J Geriatr Psychiatry. 2009;17(9):769-781.
50. Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychol Aging. 2003;18(3):622-627.
51. Starkstein SE, Jorge R, Petracca G, et al. The construct of generalized anxiety disorder in Alzheimer disease. Am J Geriatr Psychiatry. 2007;15(1):42-49.
52. Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the Geriatric Anxiety Inventory. Int Psychogeriatr. 2007;19(1):103-114.
53. Mansbach WE, Mace RA, Clark KM. The Brief Anxiety and Depression Scale (BADS): a new instrument for detecting anxiety and depression in long-term care residents. Int Psychogeriatr. 2015;27(4):673-681.
54. Seignourel PJ, Kunik ME, Snow L, et al. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28(7):1071-1082.
55. Gerolimatos LA, Ciliberti CM, Gregg JJ, et al. Development and preliminary evaluation of the Anxiety in Cognitive Impairment and Dementia (ACID) scales. Int Psychogeriatr. 2015;27(11):1825-1838.
56. Benitez CI, Smith K, Vasile RG, et al. Use of benzodiazepines and selective serotonin reuptake inhibitors in middle-aged and older adults with anxiety disorders: a longitudinal and prospective study. Am J Geriatr Psychiatry. 2008;16(1):5-13.
57. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case control study. BMJ. 2014;349:g5205.
58. Lach HW, Parsons JL. Impact of fear of falling in long term care: an integrative review. J Am Med Dir Assoc. 2013;14(8):573-577.
59. Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13(12):1216-1227.
60. Zwakhalen SM, Hamers JP, Abu-Saad HH, et al. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools [published online January 27, 2006]. BMC Geriatr. doi: 10.1186/1471-2318-6-3.
61. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Adults. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
62. Gindin J, Shochat T, Chetrit A, et al; SHELTER project. Insomnia in long-term care facilities: a comparison of seven European countries and Israel: the Services and Health for Elderly in Long TERm care study. J Am Geriatr Soc. 2014;62(11):2033-2039.
Rediscovering clozapine: Clinically relevant off-label uses
Clozapine has been available for decades, but relatively little has been published regarding its off-label uses. This data shortage likely is due in part to clozapine’s strict monitoring requirements, and we suspect off-label use is more commonplace than the literature reflects.
Refractory schizophrenia and reduction in suicidal behavior in schizophrenia or schizoaffective disorder are clozapine’s 2 FDA-approved indications. Clozapine also may be prescribed for other indications, and off-label uses have varying degrees of scientific support.
Our goal in “Rediscovering clozapine” has been to deepen clinicians’ appreciation for this unique medication and provide practical clinical guidance for its safe and effective use.1,2 This final segment reviews representative literature regarding clozapine’s off-label use for bipolar disorder and other indications (Table).
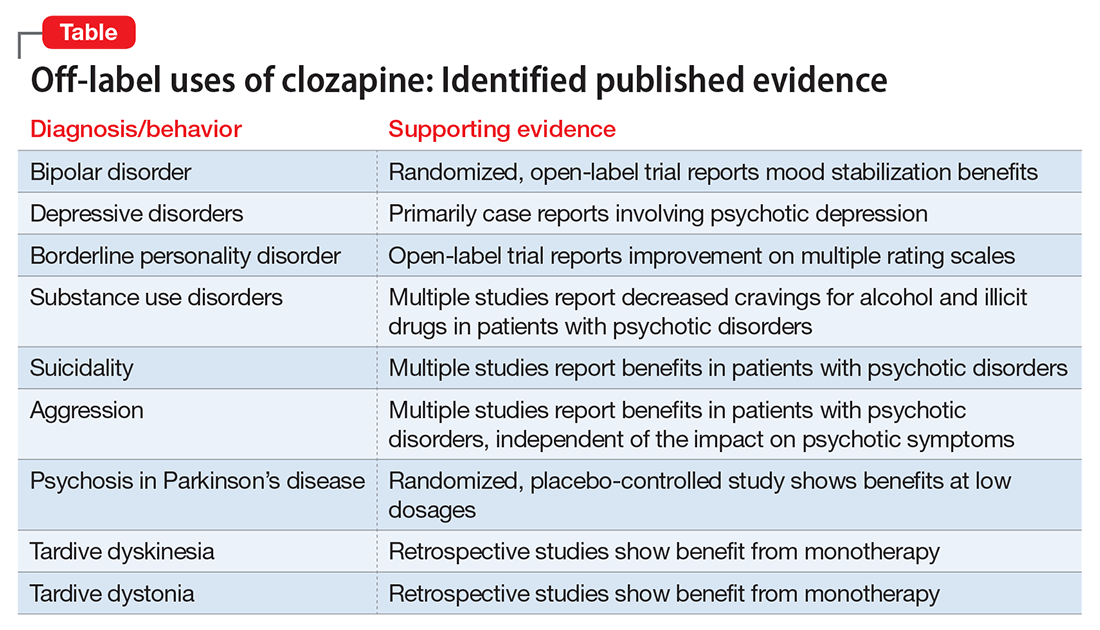
At this point, clozapine still is generally most appropriate for use in refractory cases, regardless of the primary condition being treated. We suggest, however, that physicians should at least consider, “Why is clozapine NOT appropriate for this refractory patient?”
7 Steps define off-label use
Seven steps are useful to consider when prescribing a medication off-label (Figure).3 Off-label prescribing is common in medicine and remains an important component of clinical practice. Sixty percent of antipsychotic prescriptions are written off-label,4 and physicians can prescribe any available medication to any patient for any purpose.
The FDA endorses off-label prescribing: “Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices according to their best knowledge and judgment.”5 Published case reports and case series provide guidance about the scientific support behind specific off-label indications.
Prescribing off-label based on clinical experience alone is legal, and 1 study reported that 73% of off-label prescriptions written by office-based physicians had little or no scientific support.6 From a medicolegal perspective, prescribing off-label with scientific support is preferred.
Bipolar disorder
Clozapine clearly is established as the most effective antipsychotic for treating refractory schizophrenia. A growing body of evidence supports the off-label use of clozapine for patients with bipolar disorder as well. This literature includes:
- a randomized, open-label trial of maintenance treatment of refractory bipolar disorder7
- 2 studies of treatment of acute mania8,9
- a case series of 3 patients with refractory bipolar disorder and psychotic features who were effectively treated during acute manic episodes with ultra-rapid dose titrations of clozapine.10
In China, clozapine commonly is used to treat bipolar disorder. Results have been positive, and some clinicians there consider clozapine a first-line treatment for this indication.11
In the largest published study of clozapine’s benefits for bipolar disorder, a Danish group presented a retrospective analysis of 326 patients with bipolar disorder (and no history of a schizophrenia-spectrum disorder) treated with clozapine between 1996 and 2007. The study group displayed a significant and clinically relevant reduction in psychiatric hospitalizations, polypharmacy, and self-harm. The authors concluded that clozapine appeared to be an appropriate choice for refractory bipolar disorder and encouraged future investigators to consider randomized controlled studies.12
Major depressive disorder
Published evidence supporting clozapine’s use for refractory unipolar depression is less robust than the evidence for refractory bipolar disorder. One retrospective analysis comparing clozapine treatment for bipolar disorder and unipolar depression concluded that patients with bipolar disorder responded better overall.13
Most case reports involve psychotic depression. One case series discussed clozapine treatment of 3 patients with psychotic depression and reported significant improvement in both depressive and psychotic symptoms.14 Other case reports also described patients with refractory psychotic depression.15,16
We located only 1 case report about using clozapine for depressive symptoms absent psychosis. This case involved a patient who developed recurrent depression, hypersomnia, and behavioral disturbances at age 13 after a viral febrile infection. At age 27, she was hospitalized during an episode and started on low-dose clozapine. After discharge, she remained symptom-free for 30 months on clozapine, 50 to 100 mg/d. Although her symptoms included recurrent depression, her overall clinical picture seemed most consistent with Kleine-Levin syndrome (also known as “Sleeping Beauty” syndrome) rather than a primary mood disorder.17
Borderline personality disorder
Psychotherapy is the mainstay for treating borderline personality disorder (BPD), with pharmacotherapy often added to target symptoms such as anger and impulsivity.18 Some small studies and case series have examined clozapine use for BPD.
An open-label study of 15 inpatients with BPD and psychotic disorder not otherwise specified showed improvement on multiple rating scales with clozapine dosages averaging 250 mg/d.19 In a case series of 22 female inpatients with a primary diagnosis of BPD, clozapine showed beneficial effects in several clinical domains, including symptom severity and frequency of aggressive incidents. The greatest improvement occurred within the first 6 months of treatment.20
Eight patients who continued clozapine after hospital discharge had fewer and shorter subsequent hospitalizations than others with BPD who were not prescribed clozapine at discharge.21 Individual case reports have discussed benefits of clozapine in challenging BPD cases.22-24
Substance use treatment
A growing body of literature suggests that clozapine may reduce cravings for alcohol and illicit drugs because of its unique receptor profile. Much of the data has been collected in dual diagnosis patients taking clozapine primarily to treat schizophrenia or schizoaffective disorder. Patients in 1 study showed a comparable response to clozapine therapy whether they had a history of substance abuse or not. The authors opined that their results demonstrated a more generalizable decrease in cravings and recommended further study.25
In a naturalistic study of 151 dual diagnosis patients with schizophrenia, alcohol use rates decreased significantly among those who received clozapine for psychiatric symptoms. After 3 years, 79% of patients treated with clozapine were in remission from alcohol use, compared with 33.7% of patients treated with other antipsychotics.26
Other studies have reported decreased alcohol and illicit drug use in patients with schizophrenia and concomitant substance use.27,28 Animal studies have displayed similar results, showing decreased alcohol intake with clozapine.29,30
Compelling results have been shown in patients with schizophrenia and Cannabis use disorder. A small randomized trial compared clozapine with other antipsychotics in individuals with schizophrenia and Cannabis use disorder. Clozapine was associated with significantly decreased Cannabis use, independent of overall symptom response or level of functioning.31 An animal study demonstrated an attenuated development of conditioned place preference (classical conditioning) to cocaine. The authors suggested that clozapine should be considered as a future pharmacotherapy to treat cocaine use.32
The literature does not support prescribing clozapine solely for alcohol or illicit drug use, but clozapine merits consideration in patients with schizophrenia and comorbid substance use. This approach may be most beneficial in controlled environments, such as inpatient or residential facilities.
Suicidality
The 2-year International Suicide Prevention Trial (InterSePT) was the first to support clozapine’s efficacy in reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder.33 InterSePT data were in line with earlier observations, including improvement in reported depression and hopelessness in patients with primary psychotic disorders.34,35 Clozapine’s action at serotonin receptors (in addition to dopamine receptors) may explain the benefits, based on the suspected link between suicide risk and serotonin.34,36
Most published reports regarding clozapine for suicidality involve patients with schizophrenia or schizoaffective disorder. We found only 1 published case report describing clozapine’s use for recurrent suicidality in a patient with bipolar disorder. The authors described a dramatic reduction in suicidal ideation, suicide attempts, and hospitalizations after other attempted interventions—including electroconvulsive therapy—had been ineffective.37
Aggression
In the absence of FDA-approved treatments for long-term management of aggression, many clinicians prescribe atypical antipsychotics. With the exception of clozapine, the demonstrated benefits of these medications for reducing aggression are equivocal. Clozapine is thought to be superior among atypical antipsychotics for addressing aggression because of its unique and broad combination of dopaminergic and serotonergic activity. Its effects on the D1-dopamine receptor likely target aggression, and its effects on the serotonin 2A receptor (5-HT2A) likely target the impulsivity commonly associated with aggression.38,39
Clozapine has been shown to reduce long-term aggression in patients with psychotic disorders.40-44 Most reports involve individuals with schizophrenia or schizoaffective disorder because this population is most commonly treated with clozapine. However, clozapine’s anti-aggressive benefits appear not to be solely related to sedation or improvement in psychosis.42,45
What is known about clozapine’s mechanism suggests that its anti-aggressive benefits would extend beyond patients with schizophrenia and schizoaffective disorder. In a case series of 7 nonpsychotic patients with antisocial personality disorder and psychopathic traits, all displayed benefits with clozapine—particularly in domains of impulsive behavioral dyscontrol and anger.46
Self-injurious behaviors (SIB) and aggression in 2 patients with profound mental retardation were reduced significantly after treatment was switched from risperidone to clozapine.47 In a similar case, SIB and aggression improved in a man with cognitive impairment.48 The case of Mr. C recounts our experience with using clozapine in a patient with cognitive impairment.
CASE REPORT
Daily assaults keep patient hospitalized
Mr. C, age 19 at the end of treatment, had moderate intellectual disability and an extensive history of violence. He grew up in group homes and long-term psychiatric facilities. Immediately after turning 18, he was transferred from an adolescent facility to an adult psychiatric hospital.
Our treatment team tried various combinations of benzodiazepines, mood stabilizers, and antipsychotics, but Mr. C consistently assaulted 1 or 2 peers daily without clear provocation. Eventually we started him on clozapine, which we titrated to an effective dose (based on a therapeutic serum level). We also added a therapeutic dosage of lithium to address his residual aggression. With the regimen of clozapine and lithium, Mr. C’s assaultive behavior improved dramatically. After going more than 1 year without assaulting a peer, he was placed in the community.
Movement disorders
Parkinson’s disease. The most extensive evidence for treating movement disorders with clozapine involves patients with Parkinson’s disease (PD). Geriatric psychiatrists commonly use clozapine, particularly at low doses, to treat psychotic symptoms in patients with PD. Because of a relatively low likelihood of extrapyramidal side effects, clozapine and quetiapine are the 2 antipsychotics most often used to treat dopamimetic psychosis in PD.49 In a randomized, placebo-controlled study, low-dose clozapine showed benefits in treating dopamimetic psychosis in PD, without worsening overall motor function.50 (The recent approval of pimavanserin for PD psychosis likely will impact off-label use of clozapine for this condition.)
A retrospective review of patients with PD and Lewy body dementia described benefits of treating psychosis with clozapine.51 Benefits also have been reported in using clozapine to address levodopa-induced dyskinesia (LID) absent psychotic symptoms. In an evidence-based review, the Movement Disorder Society described clozapine for LID as “efficacious and possibly useful.”52
Tardive syndromes. In a retrospective review of clozapine use for tardive dyskinesia, 43% of the 30 patients showed improvement, particularly those with concomitant dystonia.53 Another retrospective analysis reported similar outcomes for 48 patients with tardive dyskinesia treated with clozapine.54 Case series and case reports show support for clozapine as monotherapy for tardive dystonia.55
Huntington’s disease. A randomized, double-blind study found little benefit in using clozapine for patients with Huntington’s disease. The authors concluded that, although individual patients may be able to tolerate sufficiently high dosages to improve chorea, clinicians should use restraint when considering clozapine for this population.56
Precautions in older patients. Caution is advised when using clozapine for movement disorders in older individuals, particularly those with concurrent dementia. All antipsychotics, including clozapine,57 carry a “black-box” warning of increased mortality in older adults with dementia.
We hope that this series, “Rediscovering clozapine,” has helped you get reacquainted with this effective medication, employ appropriate caution, and explore off-label uses.
1. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
2. Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48-49.
3. Newman WJ, Xiong GL, Barnhorst AV. Beta-blockers: off-label use in psychiatric disorders. Psychopharm Review. 2013;48(10):73-80.
4. Stafford RS. Regulating off-label drug use—rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
5. U.S. Food and Drug Administration. “Off-label” and investigational use of marketed drugs, biologics, and medical devices—information sheet. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm. Updated January 25, 2016. Accessed November 24, 2015.
6. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026.
7. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164-1169.
8. Barbini B, Scherillo P, Benedetti F, et al. Response to clozapine in acute mania is more rapid than that of chlorpromazine. Int Clin Psychopharmacol. 1997;12(2):109-112.
9. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry. 2000;157(6):982-986.
10. Aksoy-Poyraz C, Turan ¸S, Demirel ÖF, et al. Effectiveness of ultra-rapid dose titration of clozapine for treatment-resistant bipolar mania: case series. Ther Adv Psychopharmacol. 2015;5(4):237-242.
11. Li XB, Tang YL, Wang CY, et al. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235-247.
12. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863-869.
13. Banov MD, Zarate CA Jr, Tohen M, et al. Clozapine therapy in refractory affective disorders: polarity predicts response in long-term follow-up. J Clin Psychiatry. 1994;55(7):295-300.
14. Ranjan R, Meltzer HY. Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiatry. 1996;40(4):253-258.
15. Dassa D, Kaladjian A, Azorin JM, et al. Clozapine in the treatment of psychotic refractory depression. Br J Psychiatry. 1993;163:822-824.
16. Jeyapaul P, Vieweg R. A case study evaluating the use of clozapine in depression with psychotic features. Ann Gen Psychiatry. 2006;5:20.
17. Havaki-Kontaxaki BJ, Ferentinos PP, Kontaxakis VP, et al. Low-dose clozapine monotherapy for recurring episodes of depression, hypersomnia and behavioural disturbances: a case report. Acta Neuropsychiatr. 2011;23(4):191-193.
18. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653. doi: 10.1002/14651858.CD005653.pub2.
19. Frankenburg FR, Zanarini MC. Clozapine treatment of borderline patients: a preliminary study. Compr Psychiatry. 1993;34(6):402-405.
20. Frogley C, Anagnostakis K, Mitchell S, et al. A case series of clozapine for borderline personality disorder. Ann Clin Psychiatry. 2013;25(2):125-134.
21. Parker GF. Clozapine and borderline personality disorder. Psychiatr Serv. 2002;53(3):348-349.
22. Chengappa KNR, Baker RW, Sirri C. The successful use of clozapine in ameliorating severe self mutilation in a patient with borderline personality disorder. J Pers Disord. 1995;9(1):76-82.
23. Rutledge E, O’Regan M, Mohan D. Borderline personality disorder and clozapine. Ir J Psychol Med. 2007;24(1):40-41.
24. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
25. Buckley P, Thompson P, Way L, et al. Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry. 1994;151(3):385-389.
26. Drake RE, Xie H, McHugo GJ, et al. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441-449.
27. Zimmet SV, Strous RD, Burgess ES, et al. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20(1):94-98.
28. Green AI, Noordsy DL, Brunette MF, et al. Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat. 2008;34(1):61-71.
29. Green AI, Chau DT, Keung WM, et al. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128(1):9-20.
30. Chau DT, Gulick D, Xie H, et al. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58(2):351-356.
31. Brunette MF, Dawson R, O’Keefe CD, et al. A randomized trial of clozapine vs. other antipsychotics for cannabis use disorder in patients with schizophrenia. J Dual Diagn. 2011;7(1-2):50-63.
32. Kosten TA, Nestler EJ. Clozapine attenuates cocaine conditioned place preference. Life Sci. 1994;55(1):9-14.
33. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91.
34. Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183-190.
35. Sernyak MJ, Desai R, Stolar M, et al. Impact of clozapine on completed suicide. Am J Psychiatry. 2001;158(6):931-937.
36. Nordström P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6(suppl 6):12-21.
37. Vangala VR, Brown ES, Suppes T. Clozapine associated with decreased suicidality in bipolar disorder: a case report. Bipolar Disord. 1999;1(2):123-124.
38. Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17(2):263-287.
39. Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl B):47-52.
40. Rabinowitz J, Avnon M, Rosenberg V. Effect of clozapine on physical and verbal aggression. Schizophr Res. 1996;22(3):249-255.
41. Spivak B, Roitman S, Vered Y, et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol. 1998;21(4):245-250.
42. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510-1514.
43. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225-228.
44. Krakowski MI, Czobar P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
45. Chiles JA, Davidson P, McBride D. Effects of clozapine on use of seclusion and restraint at a state hospital. Hosp Community Psychiatry. 1994;45(3):269-271.
46. Brown D, Larkin F, Sengupta S, et al. Clozapine: an effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectr. 2014;19(5):391-402.
47. Hammock R, Levine WR, Schroeder SR. Brief report: effects of clozapine on self-injurious behavior of two risperidone nonresponders with mental retardation. J Autism Dev Disord. 2001;31(1):109-113.
48. Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self-injurious behavior. J Autism Dev Disord. 1995;25(6):611-626.
49. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis [Erratum in: Clin Neuropharmacol. 2004;27(5):256]. Clin Neuropharmacol. 2004;27(4):153-156.
50. Pollak P, Tison F, Rascol O. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689-695.
51. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
52. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatment for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S2-S41.
53. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
54. Naber D, Leppig M, Grohmann R, et al. Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia—a retrospective study. Psychopharmacology (Berl). 1989;99(suppl):S73-S76.
55. Pinninti NR, Faden J, Adityanjee A. Are second-generation antipsychotics useful in tardive dystonia? Clin Neuropharmacol. 2015;38(5):183-197.
56. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35-39.
57. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed September 2, 2016.
Clozapine has been available for decades, but relatively little has been published regarding its off-label uses. This data shortage likely is due in part to clozapine’s strict monitoring requirements, and we suspect off-label use is more commonplace than the literature reflects.
Refractory schizophrenia and reduction in suicidal behavior in schizophrenia or schizoaffective disorder are clozapine’s 2 FDA-approved indications. Clozapine also may be prescribed for other indications, and off-label uses have varying degrees of scientific support.
Our goal in “Rediscovering clozapine” has been to deepen clinicians’ appreciation for this unique medication and provide practical clinical guidance for its safe and effective use.1,2 This final segment reviews representative literature regarding clozapine’s off-label use for bipolar disorder and other indications (Table).

At this point, clozapine still is generally most appropriate for use in refractory cases, regardless of the primary condition being treated. We suggest, however, that physicians should at least consider, “Why is clozapine NOT appropriate for this refractory patient?”
7 Steps define off-label use
Seven steps are useful to consider when prescribing a medication off-label (Figure).3 Off-label prescribing is common in medicine and remains an important component of clinical practice. Sixty percent of antipsychotic prescriptions are written off-label,4 and physicians can prescribe any available medication to any patient for any purpose.
The FDA endorses off-label prescribing: “Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices according to their best knowledge and judgment.”5 Published case reports and case series provide guidance about the scientific support behind specific off-label indications.
Prescribing off-label based on clinical experience alone is legal, and 1 study reported that 73% of off-label prescriptions written by office-based physicians had little or no scientific support.6 From a medicolegal perspective, prescribing off-label with scientific support is preferred.
Bipolar disorder
Clozapine clearly is established as the most effective antipsychotic for treating refractory schizophrenia. A growing body of evidence supports the off-label use of clozapine for patients with bipolar disorder as well. This literature includes:
- a randomized, open-label trial of maintenance treatment of refractory bipolar disorder7
- 2 studies of treatment of acute mania8,9
- a case series of 3 patients with refractory bipolar disorder and psychotic features who were effectively treated during acute manic episodes with ultra-rapid dose titrations of clozapine.10
In China, clozapine commonly is used to treat bipolar disorder. Results have been positive, and some clinicians there consider clozapine a first-line treatment for this indication.11
In the largest published study of clozapine’s benefits for bipolar disorder, a Danish group presented a retrospective analysis of 326 patients with bipolar disorder (and no history of a schizophrenia-spectrum disorder) treated with clozapine between 1996 and 2007. The study group displayed a significant and clinically relevant reduction in psychiatric hospitalizations, polypharmacy, and self-harm. The authors concluded that clozapine appeared to be an appropriate choice for refractory bipolar disorder and encouraged future investigators to consider randomized controlled studies.12
Major depressive disorder
Published evidence supporting clozapine’s use for refractory unipolar depression is less robust than the evidence for refractory bipolar disorder. One retrospective analysis comparing clozapine treatment for bipolar disorder and unipolar depression concluded that patients with bipolar disorder responded better overall.13
Most case reports involve psychotic depression. One case series discussed clozapine treatment of 3 patients with psychotic depression and reported significant improvement in both depressive and psychotic symptoms.14 Other case reports also described patients with refractory psychotic depression.15,16
We located only 1 case report about using clozapine for depressive symptoms absent psychosis. This case involved a patient who developed recurrent depression, hypersomnia, and behavioral disturbances at age 13 after a viral febrile infection. At age 27, she was hospitalized during an episode and started on low-dose clozapine. After discharge, she remained symptom-free for 30 months on clozapine, 50 to 100 mg/d. Although her symptoms included recurrent depression, her overall clinical picture seemed most consistent with Kleine-Levin syndrome (also known as “Sleeping Beauty” syndrome) rather than a primary mood disorder.17
Borderline personality disorder
Psychotherapy is the mainstay for treating borderline personality disorder (BPD), with pharmacotherapy often added to target symptoms such as anger and impulsivity.18 Some small studies and case series have examined clozapine use for BPD.
An open-label study of 15 inpatients with BPD and psychotic disorder not otherwise specified showed improvement on multiple rating scales with clozapine dosages averaging 250 mg/d.19 In a case series of 22 female inpatients with a primary diagnosis of BPD, clozapine showed beneficial effects in several clinical domains, including symptom severity and frequency of aggressive incidents. The greatest improvement occurred within the first 6 months of treatment.20
Eight patients who continued clozapine after hospital discharge had fewer and shorter subsequent hospitalizations than others with BPD who were not prescribed clozapine at discharge.21 Individual case reports have discussed benefits of clozapine in challenging BPD cases.22-24
Substance use treatment
A growing body of literature suggests that clozapine may reduce cravings for alcohol and illicit drugs because of its unique receptor profile. Much of the data has been collected in dual diagnosis patients taking clozapine primarily to treat schizophrenia or schizoaffective disorder. Patients in 1 study showed a comparable response to clozapine therapy whether they had a history of substance abuse or not. The authors opined that their results demonstrated a more generalizable decrease in cravings and recommended further study.25
In a naturalistic study of 151 dual diagnosis patients with schizophrenia, alcohol use rates decreased significantly among those who received clozapine for psychiatric symptoms. After 3 years, 79% of patients treated with clozapine were in remission from alcohol use, compared with 33.7% of patients treated with other antipsychotics.26
Other studies have reported decreased alcohol and illicit drug use in patients with schizophrenia and concomitant substance use.27,28 Animal studies have displayed similar results, showing decreased alcohol intake with clozapine.29,30
Compelling results have been shown in patients with schizophrenia and Cannabis use disorder. A small randomized trial compared clozapine with other antipsychotics in individuals with schizophrenia and Cannabis use disorder. Clozapine was associated with significantly decreased Cannabis use, independent of overall symptom response or level of functioning.31 An animal study demonstrated an attenuated development of conditioned place preference (classical conditioning) to cocaine. The authors suggested that clozapine should be considered as a future pharmacotherapy to treat cocaine use.32
The literature does not support prescribing clozapine solely for alcohol or illicit drug use, but clozapine merits consideration in patients with schizophrenia and comorbid substance use. This approach may be most beneficial in controlled environments, such as inpatient or residential facilities.
Suicidality
The 2-year International Suicide Prevention Trial (InterSePT) was the first to support clozapine’s efficacy in reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder.33 InterSePT data were in line with earlier observations, including improvement in reported depression and hopelessness in patients with primary psychotic disorders.34,35 Clozapine’s action at serotonin receptors (in addition to dopamine receptors) may explain the benefits, based on the suspected link between suicide risk and serotonin.34,36
Most published reports regarding clozapine for suicidality involve patients with schizophrenia or schizoaffective disorder. We found only 1 published case report describing clozapine’s use for recurrent suicidality in a patient with bipolar disorder. The authors described a dramatic reduction in suicidal ideation, suicide attempts, and hospitalizations after other attempted interventions—including electroconvulsive therapy—had been ineffective.37
Aggression
In the absence of FDA-approved treatments for long-term management of aggression, many clinicians prescribe atypical antipsychotics. With the exception of clozapine, the demonstrated benefits of these medications for reducing aggression are equivocal. Clozapine is thought to be superior among atypical antipsychotics for addressing aggression because of its unique and broad combination of dopaminergic and serotonergic activity. Its effects on the D1-dopamine receptor likely target aggression, and its effects on the serotonin 2A receptor (5-HT2A) likely target the impulsivity commonly associated with aggression.38,39
Clozapine has been shown to reduce long-term aggression in patients with psychotic disorders.40-44 Most reports involve individuals with schizophrenia or schizoaffective disorder because this population is most commonly treated with clozapine. However, clozapine’s anti-aggressive benefits appear not to be solely related to sedation or improvement in psychosis.42,45
What is known about clozapine’s mechanism suggests that its anti-aggressive benefits would extend beyond patients with schizophrenia and schizoaffective disorder. In a case series of 7 nonpsychotic patients with antisocial personality disorder and psychopathic traits, all displayed benefits with clozapine—particularly in domains of impulsive behavioral dyscontrol and anger.46
Self-injurious behaviors (SIB) and aggression in 2 patients with profound mental retardation were reduced significantly after treatment was switched from risperidone to clozapine.47 In a similar case, SIB and aggression improved in a man with cognitive impairment.48 The case of Mr. C recounts our experience with using clozapine in a patient with cognitive impairment.
CASE REPORT
Daily assaults keep patient hospitalized
Mr. C, age 19 at the end of treatment, had moderate intellectual disability and an extensive history of violence. He grew up in group homes and long-term psychiatric facilities. Immediately after turning 18, he was transferred from an adolescent facility to an adult psychiatric hospital.
Our treatment team tried various combinations of benzodiazepines, mood stabilizers, and antipsychotics, but Mr. C consistently assaulted 1 or 2 peers daily without clear provocation. Eventually we started him on clozapine, which we titrated to an effective dose (based on a therapeutic serum level). We also added a therapeutic dosage of lithium to address his residual aggression. With the regimen of clozapine and lithium, Mr. C’s assaultive behavior improved dramatically. After going more than 1 year without assaulting a peer, he was placed in the community.
Movement disorders
Parkinson’s disease. The most extensive evidence for treating movement disorders with clozapine involves patients with Parkinson’s disease (PD). Geriatric psychiatrists commonly use clozapine, particularly at low doses, to treat psychotic symptoms in patients with PD. Because of a relatively low likelihood of extrapyramidal side effects, clozapine and quetiapine are the 2 antipsychotics most often used to treat dopamimetic psychosis in PD.49 In a randomized, placebo-controlled study, low-dose clozapine showed benefits in treating dopamimetic psychosis in PD, without worsening overall motor function.50 (The recent approval of pimavanserin for PD psychosis likely will impact off-label use of clozapine for this condition.)
A retrospective review of patients with PD and Lewy body dementia described benefits of treating psychosis with clozapine.51 Benefits also have been reported in using clozapine to address levodopa-induced dyskinesia (LID) absent psychotic symptoms. In an evidence-based review, the Movement Disorder Society described clozapine for LID as “efficacious and possibly useful.”52
Tardive syndromes. In a retrospective review of clozapine use for tardive dyskinesia, 43% of the 30 patients showed improvement, particularly those with concomitant dystonia.53 Another retrospective analysis reported similar outcomes for 48 patients with tardive dyskinesia treated with clozapine.54 Case series and case reports show support for clozapine as monotherapy for tardive dystonia.55
Huntington’s disease. A randomized, double-blind study found little benefit in using clozapine for patients with Huntington’s disease. The authors concluded that, although individual patients may be able to tolerate sufficiently high dosages to improve chorea, clinicians should use restraint when considering clozapine for this population.56
Precautions in older patients. Caution is advised when using clozapine for movement disorders in older individuals, particularly those with concurrent dementia. All antipsychotics, including clozapine,57 carry a “black-box” warning of increased mortality in older adults with dementia.
We hope that this series, “Rediscovering clozapine,” has helped you get reacquainted with this effective medication, employ appropriate caution, and explore off-label uses.
Clozapine has been available for decades, but relatively little has been published regarding its off-label uses. This data shortage likely is due in part to clozapine’s strict monitoring requirements, and we suspect off-label use is more commonplace than the literature reflects.
Refractory schizophrenia and reduction in suicidal behavior in schizophrenia or schizoaffective disorder are clozapine’s 2 FDA-approved indications. Clozapine also may be prescribed for other indications, and off-label uses have varying degrees of scientific support.
Our goal in “Rediscovering clozapine” has been to deepen clinicians’ appreciation for this unique medication and provide practical clinical guidance for its safe and effective use.1,2 This final segment reviews representative literature regarding clozapine’s off-label use for bipolar disorder and other indications (Table).

At this point, clozapine still is generally most appropriate for use in refractory cases, regardless of the primary condition being treated. We suggest, however, that physicians should at least consider, “Why is clozapine NOT appropriate for this refractory patient?”
7 Steps define off-label use
Seven steps are useful to consider when prescribing a medication off-label (Figure).3 Off-label prescribing is common in medicine and remains an important component of clinical practice. Sixty percent of antipsychotic prescriptions are written off-label,4 and physicians can prescribe any available medication to any patient for any purpose.
The FDA endorses off-label prescribing: “Good medical practice and the best interests of the patient require that physicians use legally available drugs, biologics and devices according to their best knowledge and judgment.”5 Published case reports and case series provide guidance about the scientific support behind specific off-label indications.
Prescribing off-label based on clinical experience alone is legal, and 1 study reported that 73% of off-label prescriptions written by office-based physicians had little or no scientific support.6 From a medicolegal perspective, prescribing off-label with scientific support is preferred.
Bipolar disorder
Clozapine clearly is established as the most effective antipsychotic for treating refractory schizophrenia. A growing body of evidence supports the off-label use of clozapine for patients with bipolar disorder as well. This literature includes:
- a randomized, open-label trial of maintenance treatment of refractory bipolar disorder7
- 2 studies of treatment of acute mania8,9
- a case series of 3 patients with refractory bipolar disorder and psychotic features who were effectively treated during acute manic episodes with ultra-rapid dose titrations of clozapine.10
In China, clozapine commonly is used to treat bipolar disorder. Results have been positive, and some clinicians there consider clozapine a first-line treatment for this indication.11
In the largest published study of clozapine’s benefits for bipolar disorder, a Danish group presented a retrospective analysis of 326 patients with bipolar disorder (and no history of a schizophrenia-spectrum disorder) treated with clozapine between 1996 and 2007. The study group displayed a significant and clinically relevant reduction in psychiatric hospitalizations, polypharmacy, and self-harm. The authors concluded that clozapine appeared to be an appropriate choice for refractory bipolar disorder and encouraged future investigators to consider randomized controlled studies.12
Major depressive disorder
Published evidence supporting clozapine’s use for refractory unipolar depression is less robust than the evidence for refractory bipolar disorder. One retrospective analysis comparing clozapine treatment for bipolar disorder and unipolar depression concluded that patients with bipolar disorder responded better overall.13
Most case reports involve psychotic depression. One case series discussed clozapine treatment of 3 patients with psychotic depression and reported significant improvement in both depressive and psychotic symptoms.14 Other case reports also described patients with refractory psychotic depression.15,16
We located only 1 case report about using clozapine for depressive symptoms absent psychosis. This case involved a patient who developed recurrent depression, hypersomnia, and behavioral disturbances at age 13 after a viral febrile infection. At age 27, she was hospitalized during an episode and started on low-dose clozapine. After discharge, she remained symptom-free for 30 months on clozapine, 50 to 100 mg/d. Although her symptoms included recurrent depression, her overall clinical picture seemed most consistent with Kleine-Levin syndrome (also known as “Sleeping Beauty” syndrome) rather than a primary mood disorder.17
Borderline personality disorder
Psychotherapy is the mainstay for treating borderline personality disorder (BPD), with pharmacotherapy often added to target symptoms such as anger and impulsivity.18 Some small studies and case series have examined clozapine use for BPD.
An open-label study of 15 inpatients with BPD and psychotic disorder not otherwise specified showed improvement on multiple rating scales with clozapine dosages averaging 250 mg/d.19 In a case series of 22 female inpatients with a primary diagnosis of BPD, clozapine showed beneficial effects in several clinical domains, including symptom severity and frequency of aggressive incidents. The greatest improvement occurred within the first 6 months of treatment.20
Eight patients who continued clozapine after hospital discharge had fewer and shorter subsequent hospitalizations than others with BPD who were not prescribed clozapine at discharge.21 Individual case reports have discussed benefits of clozapine in challenging BPD cases.22-24
Substance use treatment
A growing body of literature suggests that clozapine may reduce cravings for alcohol and illicit drugs because of its unique receptor profile. Much of the data has been collected in dual diagnosis patients taking clozapine primarily to treat schizophrenia or schizoaffective disorder. Patients in 1 study showed a comparable response to clozapine therapy whether they had a history of substance abuse or not. The authors opined that their results demonstrated a more generalizable decrease in cravings and recommended further study.25
In a naturalistic study of 151 dual diagnosis patients with schizophrenia, alcohol use rates decreased significantly among those who received clozapine for psychiatric symptoms. After 3 years, 79% of patients treated with clozapine were in remission from alcohol use, compared with 33.7% of patients treated with other antipsychotics.26
Other studies have reported decreased alcohol and illicit drug use in patients with schizophrenia and concomitant substance use.27,28 Animal studies have displayed similar results, showing decreased alcohol intake with clozapine.29,30
Compelling results have been shown in patients with schizophrenia and Cannabis use disorder. A small randomized trial compared clozapine with other antipsychotics in individuals with schizophrenia and Cannabis use disorder. Clozapine was associated with significantly decreased Cannabis use, independent of overall symptom response or level of functioning.31 An animal study demonstrated an attenuated development of conditioned place preference (classical conditioning) to cocaine. The authors suggested that clozapine should be considered as a future pharmacotherapy to treat cocaine use.32
The literature does not support prescribing clozapine solely for alcohol or illicit drug use, but clozapine merits consideration in patients with schizophrenia and comorbid substance use. This approach may be most beneficial in controlled environments, such as inpatient or residential facilities.
Suicidality
The 2-year International Suicide Prevention Trial (InterSePT) was the first to support clozapine’s efficacy in reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder.33 InterSePT data were in line with earlier observations, including improvement in reported depression and hopelessness in patients with primary psychotic disorders.34,35 Clozapine’s action at serotonin receptors (in addition to dopamine receptors) may explain the benefits, based on the suspected link between suicide risk and serotonin.34,36
Most published reports regarding clozapine for suicidality involve patients with schizophrenia or schizoaffective disorder. We found only 1 published case report describing clozapine’s use for recurrent suicidality in a patient with bipolar disorder. The authors described a dramatic reduction in suicidal ideation, suicide attempts, and hospitalizations after other attempted interventions—including electroconvulsive therapy—had been ineffective.37
Aggression
In the absence of FDA-approved treatments for long-term management of aggression, many clinicians prescribe atypical antipsychotics. With the exception of clozapine, the demonstrated benefits of these medications for reducing aggression are equivocal. Clozapine is thought to be superior among atypical antipsychotics for addressing aggression because of its unique and broad combination of dopaminergic and serotonergic activity. Its effects on the D1-dopamine receptor likely target aggression, and its effects on the serotonin 2A receptor (5-HT2A) likely target the impulsivity commonly associated with aggression.38,39
Clozapine has been shown to reduce long-term aggression in patients with psychotic disorders.40-44 Most reports involve individuals with schizophrenia or schizoaffective disorder because this population is most commonly treated with clozapine. However, clozapine’s anti-aggressive benefits appear not to be solely related to sedation or improvement in psychosis.42,45
What is known about clozapine’s mechanism suggests that its anti-aggressive benefits would extend beyond patients with schizophrenia and schizoaffective disorder. In a case series of 7 nonpsychotic patients with antisocial personality disorder and psychopathic traits, all displayed benefits with clozapine—particularly in domains of impulsive behavioral dyscontrol and anger.46
Self-injurious behaviors (SIB) and aggression in 2 patients with profound mental retardation were reduced significantly after treatment was switched from risperidone to clozapine.47 In a similar case, SIB and aggression improved in a man with cognitive impairment.48 The case of Mr. C recounts our experience with using clozapine in a patient with cognitive impairment.
CASE REPORT
Daily assaults keep patient hospitalized
Mr. C, age 19 at the end of treatment, had moderate intellectual disability and an extensive history of violence. He grew up in group homes and long-term psychiatric facilities. Immediately after turning 18, he was transferred from an adolescent facility to an adult psychiatric hospital.
Our treatment team tried various combinations of benzodiazepines, mood stabilizers, and antipsychotics, but Mr. C consistently assaulted 1 or 2 peers daily without clear provocation. Eventually we started him on clozapine, which we titrated to an effective dose (based on a therapeutic serum level). We also added a therapeutic dosage of lithium to address his residual aggression. With the regimen of clozapine and lithium, Mr. C’s assaultive behavior improved dramatically. After going more than 1 year without assaulting a peer, he was placed in the community.
Movement disorders
Parkinson’s disease. The most extensive evidence for treating movement disorders with clozapine involves patients with Parkinson’s disease (PD). Geriatric psychiatrists commonly use clozapine, particularly at low doses, to treat psychotic symptoms in patients with PD. Because of a relatively low likelihood of extrapyramidal side effects, clozapine and quetiapine are the 2 antipsychotics most often used to treat dopamimetic psychosis in PD.49 In a randomized, placebo-controlled study, low-dose clozapine showed benefits in treating dopamimetic psychosis in PD, without worsening overall motor function.50 (The recent approval of pimavanserin for PD psychosis likely will impact off-label use of clozapine for this condition.)
A retrospective review of patients with PD and Lewy body dementia described benefits of treating psychosis with clozapine.51 Benefits also have been reported in using clozapine to address levodopa-induced dyskinesia (LID) absent psychotic symptoms. In an evidence-based review, the Movement Disorder Society described clozapine for LID as “efficacious and possibly useful.”52
Tardive syndromes. In a retrospective review of clozapine use for tardive dyskinesia, 43% of the 30 patients showed improvement, particularly those with concomitant dystonia.53 Another retrospective analysis reported similar outcomes for 48 patients with tardive dyskinesia treated with clozapine.54 Case series and case reports show support for clozapine as monotherapy for tardive dystonia.55
Huntington’s disease. A randomized, double-blind study found little benefit in using clozapine for patients with Huntington’s disease. The authors concluded that, although individual patients may be able to tolerate sufficiently high dosages to improve chorea, clinicians should use restraint when considering clozapine for this population.56
Precautions in older patients. Caution is advised when using clozapine for movement disorders in older individuals, particularly those with concurrent dementia. All antipsychotics, including clozapine,57 carry a “black-box” warning of increased mortality in older adults with dementia.
We hope that this series, “Rediscovering clozapine,” has helped you get reacquainted with this effective medication, employ appropriate caution, and explore off-label uses.
1. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
2. Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48-49.
3. Newman WJ, Xiong GL, Barnhorst AV. Beta-blockers: off-label use in psychiatric disorders. Psychopharm Review. 2013;48(10):73-80.
4. Stafford RS. Regulating off-label drug use—rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
5. U.S. Food and Drug Administration. “Off-label” and investigational use of marketed drugs, biologics, and medical devices—information sheet. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm. Updated January 25, 2016. Accessed November 24, 2015.
6. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026.
7. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164-1169.
8. Barbini B, Scherillo P, Benedetti F, et al. Response to clozapine in acute mania is more rapid than that of chlorpromazine. Int Clin Psychopharmacol. 1997;12(2):109-112.
9. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry. 2000;157(6):982-986.
10. Aksoy-Poyraz C, Turan ¸S, Demirel ÖF, et al. Effectiveness of ultra-rapid dose titration of clozapine for treatment-resistant bipolar mania: case series. Ther Adv Psychopharmacol. 2015;5(4):237-242.
11. Li XB, Tang YL, Wang CY, et al. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235-247.
12. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863-869.
13. Banov MD, Zarate CA Jr, Tohen M, et al. Clozapine therapy in refractory affective disorders: polarity predicts response in long-term follow-up. J Clin Psychiatry. 1994;55(7):295-300.
14. Ranjan R, Meltzer HY. Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiatry. 1996;40(4):253-258.
15. Dassa D, Kaladjian A, Azorin JM, et al. Clozapine in the treatment of psychotic refractory depression. Br J Psychiatry. 1993;163:822-824.
16. Jeyapaul P, Vieweg R. A case study evaluating the use of clozapine in depression with psychotic features. Ann Gen Psychiatry. 2006;5:20.
17. Havaki-Kontaxaki BJ, Ferentinos PP, Kontaxakis VP, et al. Low-dose clozapine monotherapy for recurring episodes of depression, hypersomnia and behavioural disturbances: a case report. Acta Neuropsychiatr. 2011;23(4):191-193.
18. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653. doi: 10.1002/14651858.CD005653.pub2.
19. Frankenburg FR, Zanarini MC. Clozapine treatment of borderline patients: a preliminary study. Compr Psychiatry. 1993;34(6):402-405.
20. Frogley C, Anagnostakis K, Mitchell S, et al. A case series of clozapine for borderline personality disorder. Ann Clin Psychiatry. 2013;25(2):125-134.
21. Parker GF. Clozapine and borderline personality disorder. Psychiatr Serv. 2002;53(3):348-349.
22. Chengappa KNR, Baker RW, Sirri C. The successful use of clozapine in ameliorating severe self mutilation in a patient with borderline personality disorder. J Pers Disord. 1995;9(1):76-82.
23. Rutledge E, O’Regan M, Mohan D. Borderline personality disorder and clozapine. Ir J Psychol Med. 2007;24(1):40-41.
24. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
25. Buckley P, Thompson P, Way L, et al. Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry. 1994;151(3):385-389.
26. Drake RE, Xie H, McHugo GJ, et al. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441-449.
27. Zimmet SV, Strous RD, Burgess ES, et al. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20(1):94-98.
28. Green AI, Noordsy DL, Brunette MF, et al. Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat. 2008;34(1):61-71.
29. Green AI, Chau DT, Keung WM, et al. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128(1):9-20.
30. Chau DT, Gulick D, Xie H, et al. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58(2):351-356.
31. Brunette MF, Dawson R, O’Keefe CD, et al. A randomized trial of clozapine vs. other antipsychotics for cannabis use disorder in patients with schizophrenia. J Dual Diagn. 2011;7(1-2):50-63.
32. Kosten TA, Nestler EJ. Clozapine attenuates cocaine conditioned place preference. Life Sci. 1994;55(1):9-14.
33. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91.
34. Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183-190.
35. Sernyak MJ, Desai R, Stolar M, et al. Impact of clozapine on completed suicide. Am J Psychiatry. 2001;158(6):931-937.
36. Nordström P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6(suppl 6):12-21.
37. Vangala VR, Brown ES, Suppes T. Clozapine associated with decreased suicidality in bipolar disorder: a case report. Bipolar Disord. 1999;1(2):123-124.
38. Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17(2):263-287.
39. Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl B):47-52.
40. Rabinowitz J, Avnon M, Rosenberg V. Effect of clozapine on physical and verbal aggression. Schizophr Res. 1996;22(3):249-255.
41. Spivak B, Roitman S, Vered Y, et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol. 1998;21(4):245-250.
42. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510-1514.
43. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225-228.
44. Krakowski MI, Czobar P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
45. Chiles JA, Davidson P, McBride D. Effects of clozapine on use of seclusion and restraint at a state hospital. Hosp Community Psychiatry. 1994;45(3):269-271.
46. Brown D, Larkin F, Sengupta S, et al. Clozapine: an effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectr. 2014;19(5):391-402.
47. Hammock R, Levine WR, Schroeder SR. Brief report: effects of clozapine on self-injurious behavior of two risperidone nonresponders with mental retardation. J Autism Dev Disord. 2001;31(1):109-113.
48. Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self-injurious behavior. J Autism Dev Disord. 1995;25(6):611-626.
49. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis [Erratum in: Clin Neuropharmacol. 2004;27(5):256]. Clin Neuropharmacol. 2004;27(4):153-156.
50. Pollak P, Tison F, Rascol O. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689-695.
51. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
52. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatment for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S2-S41.
53. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
54. Naber D, Leppig M, Grohmann R, et al. Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia—a retrospective study. Psychopharmacology (Berl). 1989;99(suppl):S73-S76.
55. Pinninti NR, Faden J, Adityanjee A. Are second-generation antipsychotics useful in tardive dystonia? Clin Neuropharmacol. 2015;38(5):183-197.
56. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35-39.
57. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed September 2, 2016.
1. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
2. Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop—what should you do now? Current Psychiatry. 2016;15(8):40-46,48-49.
3. Newman WJ, Xiong GL, Barnhorst AV. Beta-blockers: off-label use in psychiatric disorders. Psychopharm Review. 2013;48(10):73-80.
4. Stafford RS. Regulating off-label drug use—rethinking the role of the FDA. N Engl J Med. 2008;358(14):1427-1429.
5. U.S. Food and Drug Administration. “Off-label” and investigational use of marketed drugs, biologics, and medical devices—information sheet. http://www.fda.gov/RegulatoryInformation/Guidances/ucm126486.htm. Updated January 25, 2016. Accessed November 24, 2015.
6. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026.
7. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164-1169.
8. Barbini B, Scherillo P, Benedetti F, et al. Response to clozapine in acute mania is more rapid than that of chlorpromazine. Int Clin Psychopharmacol. 1997;12(2):109-112.
9. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry. 2000;157(6):982-986.
10. Aksoy-Poyraz C, Turan ¸S, Demirel ÖF, et al. Effectiveness of ultra-rapid dose titration of clozapine for treatment-resistant bipolar mania: case series. Ther Adv Psychopharmacol. 2015;5(4):237-242.
11. Li XB, Tang YL, Wang CY, et al. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235-247.
12. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863-869.
13. Banov MD, Zarate CA Jr, Tohen M, et al. Clozapine therapy in refractory affective disorders: polarity predicts response in long-term follow-up. J Clin Psychiatry. 1994;55(7):295-300.
14. Ranjan R, Meltzer HY. Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiatry. 1996;40(4):253-258.
15. Dassa D, Kaladjian A, Azorin JM, et al. Clozapine in the treatment of psychotic refractory depression. Br J Psychiatry. 1993;163:822-824.
16. Jeyapaul P, Vieweg R. A case study evaluating the use of clozapine in depression with psychotic features. Ann Gen Psychiatry. 2006;5:20.
17. Havaki-Kontaxaki BJ, Ferentinos PP, Kontaxakis VP, et al. Low-dose clozapine monotherapy for recurring episodes of depression, hypersomnia and behavioural disturbances: a case report. Acta Neuropsychiatr. 2011;23(4):191-193.
18. Stoffers J, Völlm BA, Rücker G, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. 2010;(6):CD005653. doi: 10.1002/14651858.CD005653.pub2.
19. Frankenburg FR, Zanarini MC. Clozapine treatment of borderline patients: a preliminary study. Compr Psychiatry. 1993;34(6):402-405.
20. Frogley C, Anagnostakis K, Mitchell S, et al. A case series of clozapine for borderline personality disorder. Ann Clin Psychiatry. 2013;25(2):125-134.
21. Parker GF. Clozapine and borderline personality disorder. Psychiatr Serv. 2002;53(3):348-349.
22. Chengappa KNR, Baker RW, Sirri C. The successful use of clozapine in ameliorating severe self mutilation in a patient with borderline personality disorder. J Pers Disord. 1995;9(1):76-82.
23. Rutledge E, O’Regan M, Mohan D. Borderline personality disorder and clozapine. Ir J Psychol Med. 2007;24(1):40-41.
24. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
25. Buckley P, Thompson P, Way L, et al. Substance abuse among patients with treatment-resistant schizophrenia: characteristics and implications for clozapine therapy. Am J Psychiatry. 1994;151(3):385-389.
26. Drake RE, Xie H, McHugo GJ, et al. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441-449.
27. Zimmet SV, Strous RD, Burgess ES, et al. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20(1):94-98.
28. Green AI, Noordsy DL, Brunette MF, et al. Substance abuse and schizophrenia: pharmacotherapeutic intervention. J Subst Abuse Treat. 2008;34(1):61-71.
29. Green AI, Chau DT, Keung WM, et al. Clozapine reduces alcohol drinking in Syrian golden hamsters. Psychiatry Res. 2004;128(1):9-20.
30. Chau DT, Gulick D, Xie H, et al. Clozapine chronically suppresses alcohol drinking in Syrian golden hamsters. Neuropharmacology. 2010;58(2):351-356.
31. Brunette MF, Dawson R, O’Keefe CD, et al. A randomized trial of clozapine vs. other antipsychotics for cannabis use disorder in patients with schizophrenia. J Dual Diagn. 2011;7(1-2):50-63.
32. Kosten TA, Nestler EJ. Clozapine attenuates cocaine conditioned place preference. Life Sci. 1994;55(1):9-14.
33. Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91.
34. Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183-190.
35. Sernyak MJ, Desai R, Stolar M, et al. Impact of clozapine on completed suicide. Am J Psychiatry. 2001;158(6):931-937.
36. Nordström P, Asberg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6(suppl 6):12-21.
37. Vangala VR, Brown ES, Suppes T. Clozapine associated with decreased suicidality in bipolar disorder: a case report. Bipolar Disord. 1999;1(2):123-124.
38. Meltzer HY. The mechanism of action of novel antipsychotic drugs. Schizophr Bull. 1991;17(2):263-287.
39. Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl B):47-52.
40. Rabinowitz J, Avnon M, Rosenberg V. Effect of clozapine on physical and verbal aggression. Schizophr Res. 1996;22(3):249-255.
41. Spivak B, Roitman S, Vered Y, et al. Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol. 1998;21(4):245-250.
42. Citrome L, Volavka J, Czobor P, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatr Serv. 2001;52(11):1510-1514.
43. Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225-228.
44. Krakowski MI, Czobar P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622-629.
45. Chiles JA, Davidson P, McBride D. Effects of clozapine on use of seclusion and restraint at a state hospital. Hosp Community Psychiatry. 1994;45(3):269-271.
46. Brown D, Larkin F, Sengupta S, et al. Clozapine: an effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectr. 2014;19(5):391-402.
47. Hammock R, Levine WR, Schroeder SR. Brief report: effects of clozapine on self-injurious behavior of two risperidone nonresponders with mental retardation. J Autism Dev Disord. 2001;31(1):109-113.
48. Hammock RG, Schroeder SR, Levine WR. The effect of clozapine on self-injurious behavior. J Autism Dev Disord. 1995;25(6):611-626.
49. Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis [Erratum in: Clin Neuropharmacol. 2004;27(5):256]. Clin Neuropharmacol. 2004;27(4):153-156.
50. Pollak P, Tison F, Rascol O. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689-695.
51. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
52. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatment for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S2-S41.
53. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
54. Naber D, Leppig M, Grohmann R, et al. Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia—a retrospective study. Psychopharmacology (Berl). 1989;99(suppl):S73-S76.
55. Pinninti NR, Faden J, Adityanjee A. Are second-generation antipsychotics useful in tardive dystonia? Clin Neuropharmacol. 2015;38(5):183-197.
56. van Vugt JP, Siesling S, Vergeer M, et al. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J Neurol Neurosurg Psychiatry. 1997;63(1):35-39.
57. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed September 2, 2016.