User login
Nutraceuticals for traumatic brain injury: Should you recommend their use?
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
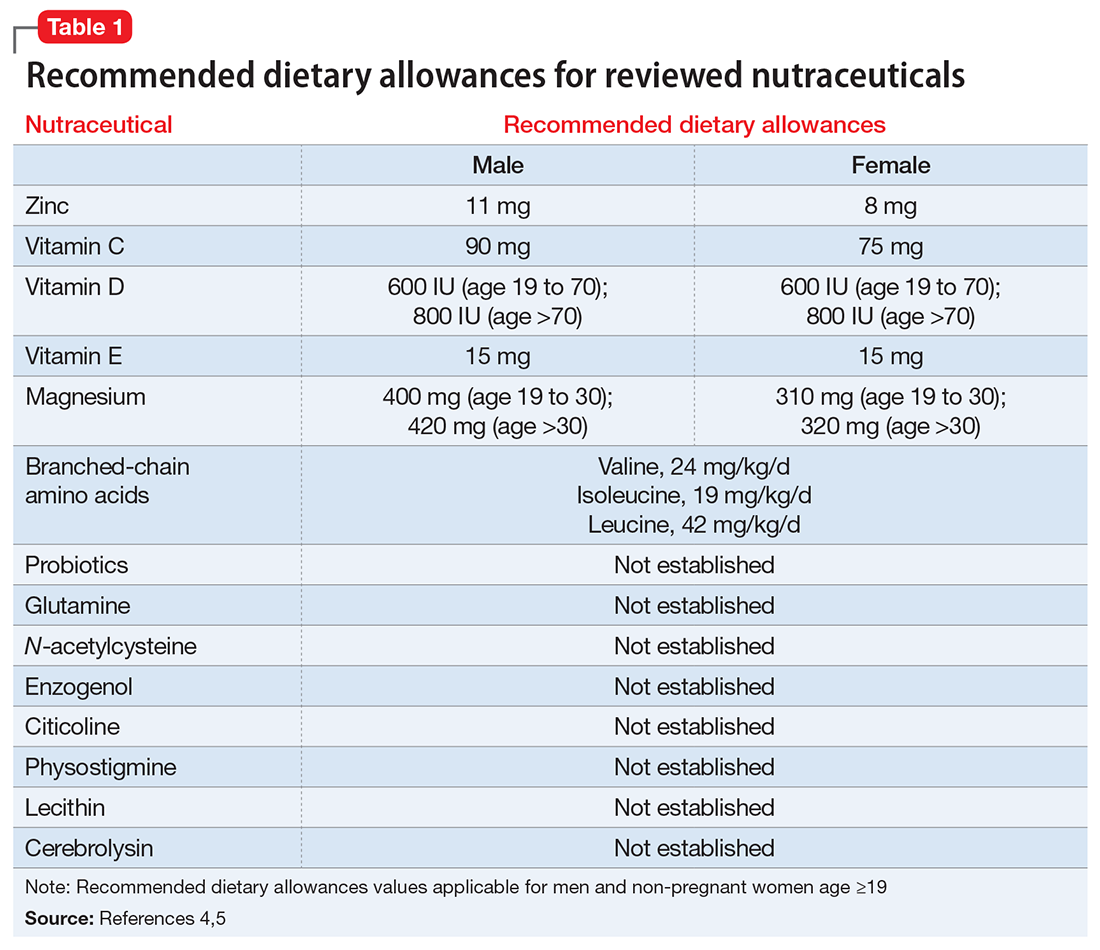
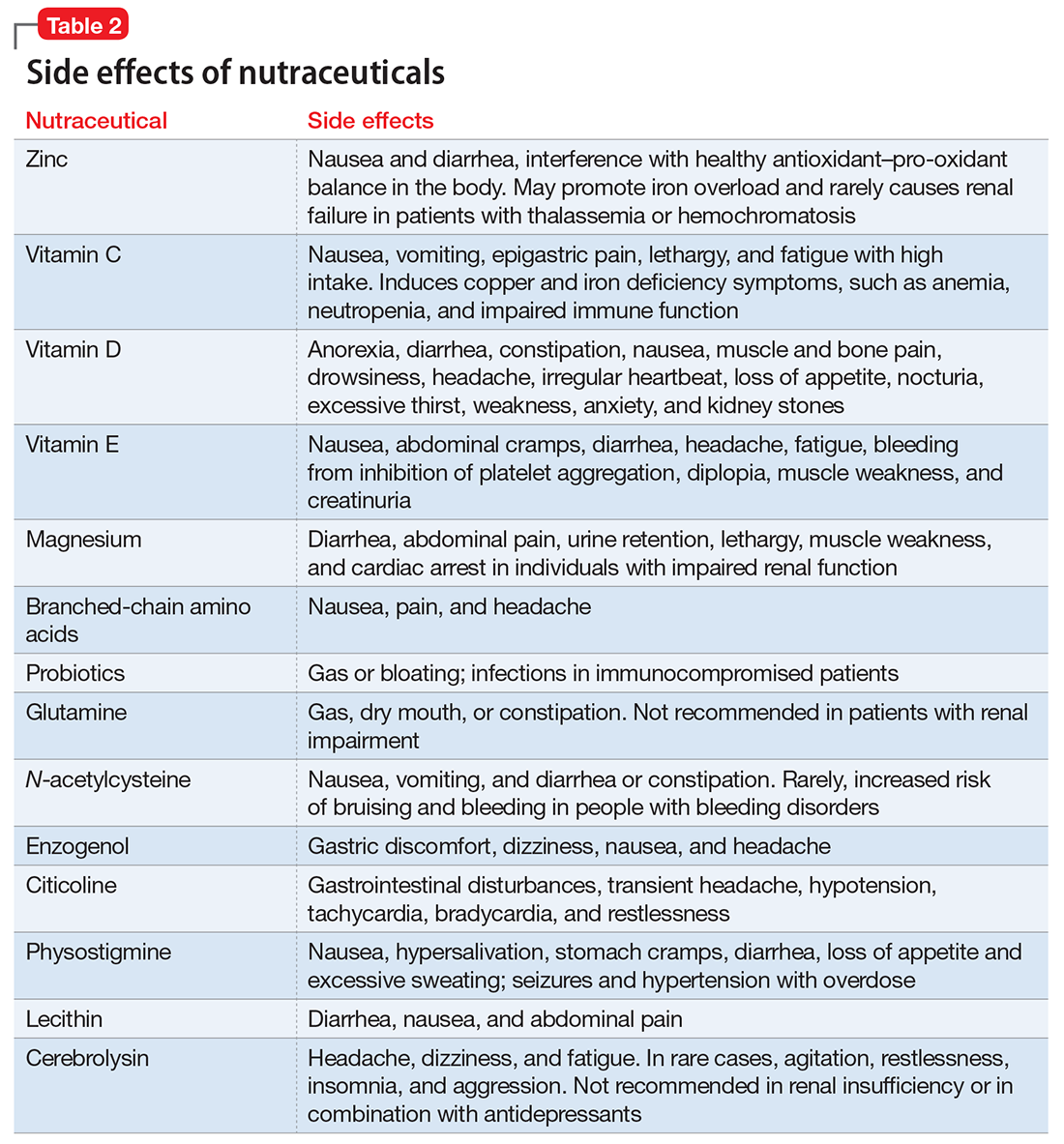
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.
Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10
What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
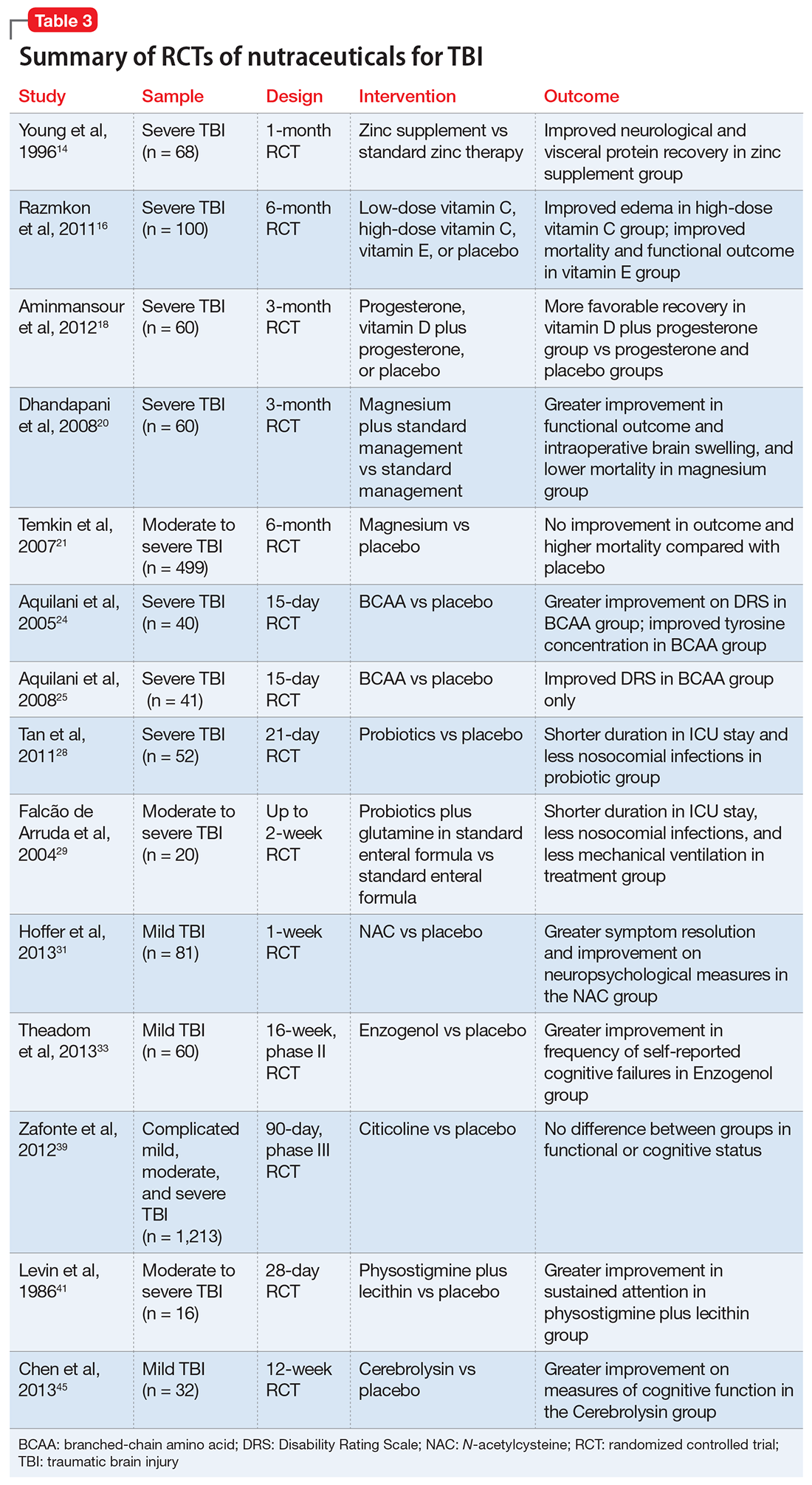
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.
Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.

Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10

What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.

Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
Traumatic brain injury (TBI) affects more than 2 million people in the United States each year.1 TBI can trigger a cascade of secondary injury mechanisms, such as inflammation, hypoxic/ischemic injury, excitotoxicity, and oxidative stress,2 that could contribute to cognitive and behavioral changes. Although neuropsychiatric symptoms might not be obvious after a TBI, they have a high prevalence in these patients, can last long term, and may be difficult to treat.3 Despite research advances in understanding the biological basis of TBI and identifying potential therapeutic targets, treatment options for individuals with TBI remain limited.
As a result, clinicians have turned to alternative treatments for TBI, including nutraceuticals. In this article, we will:
- provide an overview of nutraceuticals used in treating TBI, first exploring outcomes soon after TBI, then concentrating on neuropsychiatric outcomes
- evaluate the existing evidence, including recommended dietary allowances (Table 1)4,5 and side effects (Table 2)
- review recommendations for their clinical use.

Pharmacologic approaches are limited
Nutraceuticals have gained attention for managing TBI-associated neuropsychiatric disorders because of the limited evidence supporting current approaches. Existing strategies encompass pharmacologic and non-pharmacologic interventions, psychoeducation, supportive and behavioral psychotherapies, and cognitive rehabilitation.6
Many pharmacologic options exist for specific neurobehavioral symptoms, but the evidence for their use is based on small studies, case reports, and knowledge extrapolated from their use in idiopathic psychiatric disorders.7,8 No FDA-approved drugs have been effective for treating neuropsychiatric disturbances after a TBI. Off-label use of antidepressants, anticonvulsants, dopaminergic agents, and cholinesterase inhibitors in TBI has been associated with inadequate clinical response and/or intolerable side effects.9,10

What are nutraceuticals?
DeFelice11 introduced the term “nutraceutical” to refer to “any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease.” The term has been expanded to include dietary supplements, such as vitamins, minerals, amino acids, herbal or other botanicals, and food products that provide health benefits beyond what they normally provide in food form. The FDA does not regulate the marketing or manufacturing of nutraceuticals; therefore, their bioavailability and metabolism can vary.
Despite their widespread use, the evidence supporting the efficacy of nutraceuticals for patients with TBI is limited. Their effects might vary by population and depend on dose, timing, TBI severity, and whether taken alone or in combination with other nutraceutical or pharmaceutical agents. Fourteen randomized controlled trials (RCTs) have addressed the use of nutraceuticals in TBI (Table 3), but further research is needed to clarify for which conditions they provide maximum benefit.

Nutraceuticals and their potential use in TBI
Zinc is considered essential for optimal CNS functioning. Patients with TBI might be at risk for zinc deficiency, which has been associated with increased cell death and behavioral deficits.12,13 A randomized, prospective, double-blinded controlled trial examined the effects of supplemental zinc administration (12 mg for 15 days) compared with standard zinc therapy (2.5 mg for 15 days) over 1 month in 68 adults with acute severe closed head injury.14 The supplemental zinc group showed improved visceral protein levels, lower mortality, and more favorable neurologic recovery based on higher adjusted mean Glasgow Coma Scale score on day 28 and mean motor score on days 15 and 21.
Rodent studies have shown that zinc supplementation could reduce deficits in spatial learning and memory and depression-like behaviors and help decrease stress and anxiety,12 although no human clinical trials have been conducted. Despite the potential neuroprotective effects of zinc supplementation, evidence exists that endogenous zinc release and accumulation following TBI can trigger cellular changes that result in neuronal death.13
Vitamins C and E. Oxidative damage is believed to play a significant role in secondary injury in TBI, so research has focused on the role of antioxidants, such as vitamins C and E, to promote post-TBI recovery.15 One RCT16 of 100 adults with acute severe head injury reported that vitamin E administration was associated with reduced mortality and lower Glasgow Outcome Scale (GOS) scores, and vitamin C was associated with stabilized or reduced perilesional edema/infarct on CT scan.
Vitamin D. An animal study reported that vitamin D supplementation can help reduce inflammation, oxidative stress, and cell death in TBI, and that vitamin D deficiency has been associated with increased inflammation and behavioral deficits.17 Further evidence suggests that vitamin D may have a synergistic effect when used in combination with the hormone progesterone. A RCT of 60 patients with severe TBI reported that 60% of those who received progesterone plus vitamin D had GOS scores of 4 (good recovery) or 5 (moderate disability) vs 45% receiving progesterone alone or 25% receiving placebo.18
Magnesium, one of the most widely used nutraceuticals, is considered essential for CNS functioning, including the regulation of N-methyl-
A RCT evaluated the safety and efficacy of magnesium supplementation in 60 patients with severe closed TBI, with one-half randomized to standard care and the other also receiving magnesium sulfate (MgSO4; initiation dose of 4 g IV and 10 g IM, continuation dose of 5 g IM every 4 hours for 24 hours).20 After 3 months, more patients in the MgSO4 group had higher GOS scores than controls (73.3% vs 40%), lower 1-month mortality rates (13.3% vs 43.3%), and lower rates of intraoperative brain swelling (29.4% vs 73.3%).
However, a larger RCT of 499 patients with moderate or severe TBI randomized to high-dose (1.25 to 2.5 mmol/L) or low-dose (1.0 to 1.85 mmol/L) IV MgSO4 or placebo provided conflicting results.21 Participants received MgSO4 8 hours after injury and continued for 5 days. After 6 months, patients in the high-dose MgSO4 and placebo groups had similar composite primary outcome measures (eg, seizures, neuropsychological measures, functional status measures), although the high-dose group had a higher mortality rate than the placebo group. Patients who received low-dose MgSO4 showed worse outcomes than those assigned to placebo.
Amino acids. Branched-chain amino acids (BCAAs), including valine, isoleucine, and leucine, are essential in protein and neurotransmitter synthesis. Reduced levels of endogenous BCAAs have been reported in patients with mild or severe TBI.22 Preclinical studies suggest that BCAAs can improve hippocampal-dependent cognitive functioning following TBI.23
Two RCTs of BCAAs have been conducted in humans. One study randomized 40 men with severe TBI to IV BCAAs or placebo.24 After 15 days, the BCAA group showed greater improvement in Disability Rating Scale scores. The study also found that supplementation increased total BCAA levels without negatively affecting plasma levels of neurotransmitter precursors tyrosine and tryptophan. A second study found that 41 patients in a vegetative or minimally conscious state who received BCAA supplementation for 15 days had higher Disability Rating Scale scores than those receiving placebo.25
Probiotics and glutamine. Probiotics are non-pathogenic microorganisms that have been shown to modulate the host’s immune system.26 TBI is associated with immunological changes, including a shift from T-helper type 1 (TH1) cells to T-helper type 2 (TH2) cells that increase susceptibility to infection.27
A RCT of 52 patients with severe TBI suggested a correlation between probiotic administration-modulated cytokine levels and TH1/TH2 balance.28 A 3-times daily probiotic mix of Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus for 21 days led to shorter average ICU stays (6.8 vs 10.7 days, P = .034) and a decrease in nosocomial infections (34.6% vs 57.7%, P = .095) vs placebo, although the latter difference was not statistically significant.28
A prospective RCT of 20 patients with brain injury29 found a similar impact of early enteral nutrition supplemented with Lactobacillus johnsonii and glutamine, 30 g, vs a standard enteral nutrition formula. The treatment group experienced fewer nosocomial infections (50% vs 100%, P = .03), shorter ICU stays (10 vs 22 days, P < .01), and fewer days on mechanical ventilation (7 vs 14, P = .04). Despite these studies, evidence for the use of glutamine in patients with TBI is scarce and inconclusive.
N-acetylcysteine (NAC) comes from the amino acid L-cysteine. NAC is an effective scavenger of free radicals and improves cerebral microcirculatory blood flow and tissue oxygenation.30 A randomized, double-blind, placebo-controlled study of oral NAC supplementation in 81 active duty service members with mild TBI found NAC had a significant effect on outcomes.31 Oral NAC supplementation led to improved neuropsychological test results, number of mild TBI symptoms, complete symptom resolution by day 7 of treatment compared with placebo, and NAC was well tolerated. Lack of replication studies and generalizability of findings to civilian, moderate, or chronic TBI populations are key limitations of this study.
Proposed mechanisms for the neuroprotective benefit of NAC include its antioxidant and inflammatory activation of cysteine/glutamate exchange, metabotropic glutamate receptor modulation, and glutathione synthesis.32 NAC has poor blood–brain permeability, but the vascular disruption seen in acute TBI might facilitate its delivery to affected neural sites.31 As such, the benefits of NAC in subacute or chronic TBI are questionable.
Neuropsychiatric outcomes of nutraceuticals
Enzogenol. This flavonoid-rich extract from the bark of the Monterey pine tree (Pinus radiata), known by the trade name Enzogenol, reportedly has antioxidant and anti-inflammatory properties that may counter oxidative damage and neuroinflammation following TBI. A phase II trial randomized participants to Enzogenol, 1,000 mg/d, or placebo for 6 weeks, then all participants received Enzogenol for 6 weeks followed by placebo for 4 weeks.33 Enzogenol was well tolerated with few side effects.
Compared with placebo, participants receiving Enzogenol showed no significant change in mood, as measured by the Hospital Anxiety and Depression Scale, and greater improvement in overall cognition as assessed by the Cognitive Failures Questionnaire. However, measures of working memory (digit span, arithmetic, and letter–number sequencing subtests of the Wechsler Adult Intelligence Scale) and episodic memory (California Verbal Learning Test) showed no benefit from Enzogenol.
Citicoline (CDP-choline) is an endogenous compound widely available as a nutraceutical that has been approved for TBI therapy in 59 countries.34 Animal studies indicate that it could possess neuroprotective properties. Proposed mechanisms for such effects have included stabilizing cell membranes, reducing inflammation, reducing the presence of free radicals, or stimulating production of acetylcholine.35,36 A study in rats found that CDP-choline was associated with increased levels of acetylcholine in the hippocampus and neocortex, which may help reduce neurobehavioral deficits.37
A study of 14 adults with mild to moderate closed head injury38 found that patients who received CDP-choline showed a greater reduction in post-concussion symptoms and improvement in recognition memory than controls who received placebo. However, the Citicoline Brain Injury Treatment Trial, a large randomized trial of 1,213 adults with complicated mild, moderate, or severe TBI, reported that CDP-choline did not improve functional and cognitive status.39
Physostigmine and lecithin. The cholinergic system is a key modulatory neurotransmitter system of the brain that mediates conscious awareness, attention, learning, and working memory.40 A double-blind, placebo-controlled study of 16 patients with moderate to severe closed head injury provided inconsistent evidence for the efficacy of physostigmine and lecithin in the treatment of memory and attention disturbances.41The results showed no differences between the physostigmine–lecithin combination vs lecithin alone, although sustained attention on the Continuous Performance Test was more efficient with physostigmine than placebo when the drug condition occurred first in the crossover design. The lack of encouraging data and concerns about its cardiovascular and proconvulsant properties in patients with TBI may explain the dearth of studies with physostigmine.
Cerebrolysin. A peptide preparation produced from purified pig brain proteins, known by the trade name Cerebrolysin, is popular in Asia and Europe for its nootropic properties. Cerebrolysin may activate cerebral mechanisms related to attention and memory processes,42 and some data have shown efficacy in improving cognitive symptoms and daily activities in patients with Alzheimer’s disease43 and TBI.44
A blinded 12-week study of 32 participants with acute mild TBI reported that those randomized to Cerebrolysin showed improvement in cognitive functioning vs the placebo group.45 The authors concluded that Cerebrolysin provides an advantage for patients with mild TBI and brain contusion if treatment starts within 24 hours of mild TBI onset. Cerebrolysin was well tolerated. Major limitations of this study were small sample size, lack of information regarding comorbid neuropsychiatric conditions and treatments, and short treatment duration.
A recent Cochrane review of 6 RCTs with 1,501 participants found no clinical benefit of Cerebrolysin for treating acute ischemic stroke, and found moderate-quality evidence of an increase with non-fatal serious adverse events but not in total serious adverse events.46 We do not recommend Cerebrolysin use in patients with TBI at this time until additional efficacy and safety data are available.
Nutraceuticals used in other populations
Other nutraceuticals with preclinical evidence of possible benefit in TBI but lacking evidence from human clinical trials include omega-3 fatty acids,47 curcumin,48 and resveratrol,49 providing further proof that results from experimental studies do not necessarily extend to clinical trials.50
Studies of nutraceuticals in other neurological and psychiatric populations have yielded some promising results. Significant interest has focused on the association between vitamin D deficiency, dementia, and neurodegenerative conditions such as Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease.51 The role of vitamin D in regulation of calcium-mediated neuronal excitotoxicity and oxidative stress and in the induction of synaptic structural proteins, neurotrophic factors, and deficient neurotransmitters makes it an attractive candidate as a neuroprotective agent.52
RCTs of nutraceuticals also have reported positive findings for a variety of mood and anxiety disorders, such as St. John’s wort, S-adenosylmethionine, omega-3 fatty acids for major depression53 and bipolar depression,54 and kava for generalized anxiety disorder.55 More research, however, is needed in these areas.
The use of nonpharmacologic agents in TBI often relies on similar neuropsychiatric symptom profiles of idiopathic psychiatric disorders. Attention-deficit/hyperactivity disorder (ADHD) closely resembles TBI, but systemic reviews of studies of zinc, magnesium, and polyunsaturated fatty acids supplementation in ADHD provide no evidence of therapeutic benefit.56-58
Educate patients in role of nutraceuticals
Despite lack of FDA oversight and limited empirical support, nutraceuticals continue to be widely marketed and used for their putative health benefits59 and have gained increased attention among clinicians.60 Because nutritional deficiency may make the brain less able than other organs to recover from injury,61 supplementation is an option, especially in individuals who could be at greater risk of TBI (eg, athletes and military personnel).
Lacking robust scientific evidence to support the use of nutraceuticals either for enhancing TBI recovery or treating neuropsychiatric disturbances, clinicians must educate patients that these agents are not completely benign and can have significant side effects and drug interactions.62,63 Nutraceuticals may contain multiple ingredients, some of which can be toxic, particularly at higher doses. Many patients may not volunteer information about their nutraceutical use to their health care providers,64 so we must ask them about that and inform them of the potential for adverse events and drug interactions.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
1. Centers for Disease Control and Prevention. Report to Congress on traumatic brain injury in the United States: epidemiology and rehabilitation. https://www.cdc.gov/traumaticbraininjury/pubs/congress_epi_rehab.html. Updated January 22, 2016. Accessed June 5, 2017.
2. Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4-9.
3. Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: unraveling the silent epidemic. Psychosomatics. 2009;50(3):198-205.
4. National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheets. https://ods.od.nih.gov/factsheets/list-all. Accessed June 5, 2017.
5. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academy of Sciences; 2002.
6. Rao V, Koliatsos V, Ahmed F, et al. Neuropsychiatric disturbances associated with traumatic brain injury: a practical approach to evaluation and management. Semin Neurol. 2015;35(1):64-82.
7. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J Rehabil Res Dev. 2009;46(6):851-879.
8. Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70(6):1520-1533; discussion 1533.
9. Bengtsson M, Godbolt AK. Effects of acetylcholinesterase inhibitors on cognitive function in patients with chronic traumatic brain injury: a systematic review. J Rehabil Med. 2016;48(1):1-5.
10. Neurobehavioral Guidelines Working Group; Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468-1501.
11. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol. 1995;6(2):59-61.
12. Cope EC, Morris DR, Levenson CW. Improving treatments and outcomes: an emerging role for zinc in traumatic brain injury. Nutr Rev. 2012;70(7):410-413.
13. Morris DR, Levenson CW. Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care. 2013;16(6):708-711.
14. Young B, Ott L, Kasarskis E, et al. Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma. 1996;13(1):25-34.
15. Fernández-Gajardo R, Matamala JM, Carrasco R, et al. Novel therapeutic strategies for traumatic brain injury: acute antioxidant reinforcement. CNS Drugs. 2014;28(3):229-248.
16. Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, et al. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg. 2011;58:133-137.
17. Cekic M, Cutler SM, VanLandingham JW, et al. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864-874.
18. Aminmansour B, Nikbakht H, Ghorbani A, et al. Comparison of the administration of progesterone versus progesterone and vitamin D in improvement of outcomes in patients with traumatic brain injury: a randomized clinical trial with placebo group. Adv Biomed Res. 2012;1:58.
19. Cernak I, Savic VJ, Kotur J, et al. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J Neurotrauma. 2000;17(1):53-68.
20. Dhandapani SS, Gupta A, Vivekanandhan S, et al. Randomized controlled trial of magnesium sulphate in severe closed traumatic brain injury. The Indian Journal of Neurotrauma. 2008;5(1):27-33.
21. Temkin NR, Anderson GD, Winn HR, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29-38.
22. Jeter CB, Hergenroeder GW, Ward NH 3rd, et al. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J Neurotrauma. 2013;30(8):671-679.
23. Cole JT, Mitala CM, Kundu S, et al. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107(1):366-371.
24. Aquilani R, Iadarola P, Contardi A, et al. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2005;86(9):1729-1735.
25. Aquilani R, Boselli M, Boschi F, et al. Branched-chain amino acids may improve recovery from a vegetative or minimally conscious state in patients with traumatic brain injury: a pilot study. Arch Phys Med Rehabil. 2008;89(9):1642-1647.
26. Kang HJ, Im SH. Probiotics as an immune modulator. J Nutr Sci Vitaminol (Tokyo). 2015;61(suppl):S103-S105.
27. DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130(11):1159-1162; discussion 1162-1163.
28. Tan M, Zhu JC, Du J, et al. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
29. Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106(3):287-292.
30. Cuzzocrea S, Mazzon E, Costantino G, et al. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol. 2000;130(6):1219-1226.
31. Hoffer ME, Balaban C, Slade MD, et al. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One. 2013;8(1):e54163.
32. Eakin K, Baratz-Goldstein R, Pick CG, et al. Efficacy of N-acetyl cysteine in traumatic brain injury. PLoS One. 2014;9(4):e90617.
33. Theadom A, Mahon S, Barker-Collo S, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135-1144.
34. Arenth PM, Russell KC, Ricker JH, et al. CDP-choline as a biological supplement during neurorecovery: a focused review. PM R. 2011;3(6 suppl 1):S123-S131.
35. Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10(5):839-846.
36. Guseva MV, Hopkins DM, Scheff SW, et al. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25(8):975-983.
37. Dixon CE, Ma X, Marion DW. Effects of CDP-choline treatment on neurobehavioral deficits after TBI and on hippocampal and neocortical acetylcholine release. J Neurotrauma. 1997;14(3):161-169.
38. Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(suppl):S39-S42.
39. Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993-2000.
40. Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22(6):273-280.
41. Levin HS, Peters BH, Kalisky Z, et al. Effects of oral physostigmine and lecithin on memory and attention in closed head-injured patients. Cent Nerv Syst Trauma. 1986;3(4):333-342.
42. Alvarez XA, Lombardi VR, Corzo L, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. J Neural Transm Suppl. 2000;59:315-328.
43. Ruether E, Husmann R, Kinzler E, et al. A 28-week, double-blind, placebo-controlled study with cerebrolysin in patients with mild to moderate Alzheimer’s disease. Int Clin Psychopharmacol. 2001;16(5):253-263.
44. Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochir Suppl. 2005;95:59-60.
45. Chen CC, Wei ST, Tsaia SC, et al. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br J Neurosurg. 2013;27(6):803-807.
46. Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database Syst Rev. 2016;12:CD007026.
47. Barrett EC, McBurney MI, Ciappio ED. ω-3 fatty acid supplementation as a potential therapeutic aid for the recovery from mild traumatic brain injury/concussion. Adv Nutr. 2014;5(3):268-277.
48. Sharma S, Zhuang Y, Ying Z, et al. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161(4):1037-1044.
49. Gatson JW, Liu MM, Abdelfattah K, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470-475; discussion 474-475.
50. Grey A, Bolland M. Clinical trial evidence and use of fish oil supplements. JAMA Intern Med. 2014;174(3):460-462.
51. Mpandzou G, Aït Ben Haddou E, Regragui W, et al. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172(2):109-122.
52. Karakis I, Pase MP, Beiser A, et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The Framingham Heart Study. J Alzheimers Dis. 2016;51(2):451-461.
53. Sarris J, Papakostas GI, Vitolo O, et al. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76-81.
54. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
55. Sarris J, Stough C, Bousman C, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643-648.
56. Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: a systematic review on the recent literature. Int J Prev Med. 2015;6:83.
57. Gillies D, Sinn JKh, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986.
58. Ghanizadeh A, Berk M. Zinc for treating of children and adolescents with attention-deficit hyperactivity disorder: a systematic review of randomized controlled clinical trials. Eur J Clin Nutr. 2013;67(1):122-124.
59. U.S. Food and Drug Administration. Can a dietary supplement treat a concussion? No! http://www.fda.gov/forconsumers/consumerupdates/ucm378845.htm. Updated February 13, 2015. Accessed June 5, 2017.
60. Sarris J, Logan AC, Akbaraly TN, et al; International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2(3):271-274.
61. Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PLoS One. 2014;9(1):e86472.
62. Edie CF, Dewan N. Which psychotropics interact with four common supplements. Current Psychiatry. 2005;4(1):16-30.
63. Di Lorenzo C, Ceschi A, Kupferschmidt H, et al. Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br J Clin Pharmacol. 2015;79(4):578-592.
64. National Center for Complementary and Integrative Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers. https://nccih.nih.gov/research/statistics/2010. Published 2011. Accessed June 5, 2017.
Malingering in apparently psychotic patients: Detecting it and dealing with it
Imagine you’re on call in a busy emergency department (ED) overnight. Things are tough. The consults are piling up, no one is returning your calls for collateral information, and you’re dealing with a myriad of emergencies.
In walks Mr. D, age 45, complaining of hearing voices, feeling unsafe, and asking for admission. It’s now 2
Of course, like all qualified psychiatrists, you will dig a little deeper, and in doing so you learn that Mr. D has visited this hospital before and has been admitted to the psychiatry unit. Now you go from having a dearth of information to having more records than you can count.
You discover that Mr. D has a history of coming to the ED during precarious hours, with similar complaints, demanding admission.
Mr. D, you learn, is unemployed, single, and homeless. Your meticulous search through his hospital records and previous admission and discharge notes reveal that once he has slept for a night, eaten a hot meal, and received narcotics for his back pain and benzodiazepines for his “symptoms” he demands to leave the hospital. His psychotic symptoms disappear despite his consistent refusal to take antipsychotics throughout his stay.
Now, what would you do?
As earnest medical students and psychiatrists, we enjoy helping patients on their path toward recovery. We want to advocate for our patients and give them the benefit of the doubt. We’re taught in medical school to be non-judgmental and invite patients to share their narrative. But through experience, we start to become aware of malingering.
Suspecting malingering, diagnosed as a condition, often is avoided by psychiatrists.1 This makes sense—it goes against the essence of our training and imposes a pejorative label on someone who has reached out for help.
Often persons with mental illness will suffer for years until they to receive help.2 That’s exactly why, when patients like Mr. D come to the ED and report hearing voices, we’re not likely to shout, “Liar!” and invite them to leave.
However, malingering is a real problem, especially because the number of psychiatric hospital beds have dwindled to record lows, thereby overcrowding EDs. Resources are skimpy, and clinicians want to help those who need it the most and not waste resources on someone who is “faking it” for secondary gain.
To navigate this diagnostic challenge, psychiatrists need the skills to detect malingering and the confidence to deal with it appropriately. This article aims to:
- define psychosis and malingering
- review the prevalence and historical considerations of malingering
- offer practical strategies to deal with malingering.
Know the real thing
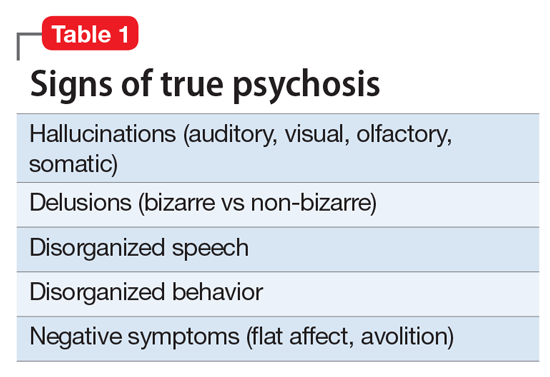
Clinicians first must have the clinical acumen and expertise to identify a true mental illness such as psychosis2 (Table 1). The differential diagnosis for psychotic symptoms is broad. The astute clinician might suspect that untreated bipolar disorder or depression led to the emergence of perceptual disturbances or disordered thinking. Transient psychotic symptoms can be associated with trauma disorders, borderline personality disorder, and acute intoxication. Psychotic spectrum disorders range from brief psychosis to schizophreniform to schizoaffective disorder or schizophrenia.
Malingering—which is a condition, not a diagnosis—is characterized by the intentional production of false or grossly exaggerated physical or psychological symptoms motivated by external incentives.3,4 The presence of external incentives differentiates malingering from true psychiatric disorders, including factitious disorder, somatoform disorder, and dissociative disorder, and specific medical conditions.1 In those disorders, there is no external incentive.
It is important to remember that malingering can coexist with a serious mental illness. For example, a truly psychotic person might malinger, feign, or exaggerate symptoms to try to receive much needed help. Individuals with true psychosis might have become disenchanted with the mental health system, and thereby have a tendency to over-report or exaggerate symptoms in an effort to obtain treatment. This also could explain why many clinicians intuitively are reluctant to make the determination that someone is malingering. Malingering also can be present in an individual who has antisocial personality disorder, factitious disorder, Ganser syndrome, and Munchausen syndrome.4 When symptoms or diseases that either are thought to be exaggerated or do not exist, consider a diagnosis of malingering.
A key challenge in any discussion of abnormal health care–seeking behavior is the extent to which a person’s reported symptoms are considered to be a product of choice, psychopathology beyond volitional control, or perhaps both. Clinical skills alone typically are not sufficient for diagnosing or detecting malingering. Medical education needs to provide doctors with the conceptual, developmental, and management frameworks to understand and manage patients whose symptoms appear to be simulated. Central to understanding factitious disorders and malingering are the explanatory models and beliefs used to provide meaning for both patients and doctors.7
When considering malingered psychosis, the suspecting physician must stay alert to possible motives. Also, the patient’s presentation might provide some clues when there is marked variability, such as discrepancies in the history, gross inconsistencies, or blatant contradictions. Hallucinations are a heterogeneous experience, and discerning between true vs feigned symptoms can be challenging for even the seasoned clinician. It can be helpful to study the phenomenology of typical vs atypical hallucinatory symptoms.8 Examples of atypical symptoms include:
- vague hallucinations
- experiencing hallucinations of only 1 sensory modality (such as voices alone, visual images in black and white only)
- delusions that have an abrupt onset
- bizarre content without disordered thinking.2,6,9,10
The truth about an untruthful condition
Although the exact prevalence of malingering varies by circumstance, Rissmiller et al12,13 demonstrated—and later replicated—a prevalence of approximately 10% among patients hospitalized for suicidal ideation or suicide attempts. Studies have demonstrated even higher prevalence within forensic populations, which seems reasonable because evading criminal responsibility is a large incentive to feign symptoms. Studies also have shown that 5% of military recruits will feign symptoms to avoid service. Moreover, 1% of psychiatric patients, such as Mr. D, feign symptoms for secondary gain.13
Although there are no psychometrically validated assessment tools to distinguish between real vs feigned hallucinations, several standardized tests can help tease out the truth.9 The preferred personality test used in forensic settings is the Minnesota Multiphasic Personality Inventory,14 which consists of 567 items, with 10 clinical scales and several validity scales. The F scale, “faking good” or “faking bad,” detects people who are answering questions with the goal of appearing better or worse than they actually are. In studies of patients hospitalized for being at risk for suicide who were administered tests of self-reported malingering, approximately 10% of people admitted to psychiatric units were “faking” their symptoms.14
It is important to identify malingering from a professional and public health standpoint. Society incurs incremental costs when a person uses dwindling mental health resources for their own reward, leaving others to suffer without treatment. The number of psychiatric hospital beds has fallen from half a million in the 1950s to approximately 100,000 today.15
Practical guidelines
Malingering presents specific challenges to clinicians, such as:
- diagnostic uncertainty
- inaccurately branding one a liar
- countertransference
- personal reactions.
Our ethical and fiduciary responsibility is to our patient. In examining the art in medicine, it has been suggested that malingering could be viewed as an immature or primitive defense.16
Although there often is suspicion that a person is malingering, a definitive statement of such must be confirmed. Without clarity, labeling an individual as a malingerer could have detrimental effects to his (her) future care, defames his character, and places a thoughtless examiner at risk of a lawsuit. Confirmation can be achieved by observation or psychological testing methods.
Observation. When in doubt of what to do with someone such as Mr. D, there is little harm in acting prudently by holding him in a controlled setting—whether keeping him overnight in an ED or admitting him for a brief psychiatric stay. By observing someone in a controlled environment, where there are multiple professional watchful eyes, inferences will be more accurate.1
Structured assessments have been developed to help detect malingering—one example is the Test of Memory Malingering—however, in daily practice, the physician generally should suspect malingering when there are tangible incentives and when reported symptoms do not match the physical examination or there is no organic basis for the physical complaints.17 Detecting illness deception relies on converging evidence sources, including detailed interview assessments, clinical notes, and consultations.7
When you feel certain that you are encountering someone who is malingering, the final step is to get a consult. Malingering is a serious label and warrants due diligence by the provider, rather than a haphazard guess that a patient is lying. Once you receive confirmatory opinions, great care should be taken in documenting a clear and accurate note that will benefit your clinical counterpart who might encounter a patient such as Mr. D when he (she) shows up again, and will go a long way toward appropriately directing his care.
1. LoPiccolo CJ, Goodkin K, Baldewicz TT. Current issues in the diagnosis and management of malingering. Ann Med. 1999;31(3):166-174.
2. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry. 2005;4(11):12-25.
3. Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 10th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007:887.
4. Gorman WF. Defining malingering. J Forensic Sci. 1982;27(2):401-407.
5. Mendelson G, Mendelson D. Malingering pain in the medicolegal context. Clin J Pain. 2004;20(6):423-432.
6. Resnick PJ. Malingered psychosis. In: Rogers R, ed. Clinical assessment of malingering and deception. 2nd ed. New York, NY: The Guilford Press; 1997:47-67.
7. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
8. McCarthy-Jones S, Resnick PJ. Listening to the voices: the use of phenomenology to differentiate malingered from genuine auditory verbal hallucinations. Int J Law Psychiatry. 2014;37(2):183-189.
9. Resnick PJ. Defrocking the fraud: the detection of malingering. Isr J Psychiatry Relat Sci. 1993;30(2):93-101.
10. Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med. 1996;26(1):177-189.
11. Pollock P. Feigning auditory hallucinations by offenders. Journal of Forensic Psychiatry. 1998;9(2)305-327.
12. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicide ideators and attempters. Crisis. 1998;19(2):62-66.
13. Rissmiller DA, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric patients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
14. Hathaway SR, McKinley JC. The Minnesota Multiphasic Personality Inventory-2. Minneapolis, MN: University of Minnesota Press; 1989.
15. Szabo L. Cost of not caring: Stigma set in stone. USA Today. http://www.usatoday.com/story/news/nation/2014/06/25/stigma-of-mental-illness/9875351. Published June 25, 2014. Accessed May 5, 2017.
16. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
17. McDermott BE, Feldman MD. Malingering in the medical setting. Psychiatr Clin North Am. 2007;30(4):645-662.
Imagine you’re on call in a busy emergency department (ED) overnight. Things are tough. The consults are piling up, no one is returning your calls for collateral information, and you’re dealing with a myriad of emergencies.
In walks Mr. D, age 45, complaining of hearing voices, feeling unsafe, and asking for admission. It’s now 2
Of course, like all qualified psychiatrists, you will dig a little deeper, and in doing so you learn that Mr. D has visited this hospital before and has been admitted to the psychiatry unit. Now you go from having a dearth of information to having more records than you can count.
You discover that Mr. D has a history of coming to the ED during precarious hours, with similar complaints, demanding admission.
Mr. D, you learn, is unemployed, single, and homeless. Your meticulous search through his hospital records and previous admission and discharge notes reveal that once he has slept for a night, eaten a hot meal, and received narcotics for his back pain and benzodiazepines for his “symptoms” he demands to leave the hospital. His psychotic symptoms disappear despite his consistent refusal to take antipsychotics throughout his stay.
Now, what would you do?
As earnest medical students and psychiatrists, we enjoy helping patients on their path toward recovery. We want to advocate for our patients and give them the benefit of the doubt. We’re taught in medical school to be non-judgmental and invite patients to share their narrative. But through experience, we start to become aware of malingering.
Suspecting malingering, diagnosed as a condition, often is avoided by psychiatrists.1 This makes sense—it goes against the essence of our training and imposes a pejorative label on someone who has reached out for help.
Often persons with mental illness will suffer for years until they to receive help.2 That’s exactly why, when patients like Mr. D come to the ED and report hearing voices, we’re not likely to shout, “Liar!” and invite them to leave.
However, malingering is a real problem, especially because the number of psychiatric hospital beds have dwindled to record lows, thereby overcrowding EDs. Resources are skimpy, and clinicians want to help those who need it the most and not waste resources on someone who is “faking it” for secondary gain.
To navigate this diagnostic challenge, psychiatrists need the skills to detect malingering and the confidence to deal with it appropriately. This article aims to:
- define psychosis and malingering
- review the prevalence and historical considerations of malingering
- offer practical strategies to deal with malingering.
Know the real thing

Clinicians first must have the clinical acumen and expertise to identify a true mental illness such as psychosis2 (Table 1). The differential diagnosis for psychotic symptoms is broad. The astute clinician might suspect that untreated bipolar disorder or depression led to the emergence of perceptual disturbances or disordered thinking. Transient psychotic symptoms can be associated with trauma disorders, borderline personality disorder, and acute intoxication. Psychotic spectrum disorders range from brief psychosis to schizophreniform to schizoaffective disorder or schizophrenia.
Malingering—which is a condition, not a diagnosis—is characterized by the intentional production of false or grossly exaggerated physical or psychological symptoms motivated by external incentives.3,4 The presence of external incentives differentiates malingering from true psychiatric disorders, including factitious disorder, somatoform disorder, and dissociative disorder, and specific medical conditions.1 In those disorders, there is no external incentive.
It is important to remember that malingering can coexist with a serious mental illness. For example, a truly psychotic person might malinger, feign, or exaggerate symptoms to try to receive much needed help. Individuals with true psychosis might have become disenchanted with the mental health system, and thereby have a tendency to over-report or exaggerate symptoms in an effort to obtain treatment. This also could explain why many clinicians intuitively are reluctant to make the determination that someone is malingering. Malingering also can be present in an individual who has antisocial personality disorder, factitious disorder, Ganser syndrome, and Munchausen syndrome.4 When symptoms or diseases that either are thought to be exaggerated or do not exist, consider a diagnosis of malingering.
A key challenge in any discussion of abnormal health care–seeking behavior is the extent to which a person’s reported symptoms are considered to be a product of choice, psychopathology beyond volitional control, or perhaps both. Clinical skills alone typically are not sufficient for diagnosing or detecting malingering. Medical education needs to provide doctors with the conceptual, developmental, and management frameworks to understand and manage patients whose symptoms appear to be simulated. Central to understanding factitious disorders and malingering are the explanatory models and beliefs used to provide meaning for both patients and doctors.7
When considering malingered psychosis, the suspecting physician must stay alert to possible motives. Also, the patient’s presentation might provide some clues when there is marked variability, such as discrepancies in the history, gross inconsistencies, or blatant contradictions. Hallucinations are a heterogeneous experience, and discerning between true vs feigned symptoms can be challenging for even the seasoned clinician. It can be helpful to study the phenomenology of typical vs atypical hallucinatory symptoms.8 Examples of atypical symptoms include:
- vague hallucinations
- experiencing hallucinations of only 1 sensory modality (such as voices alone, visual images in black and white only)
- delusions that have an abrupt onset
- bizarre content without disordered thinking.2,6,9,10
The truth about an untruthful condition
Although the exact prevalence of malingering varies by circumstance, Rissmiller et al12,13 demonstrated—and later replicated—a prevalence of approximately 10% among patients hospitalized for suicidal ideation or suicide attempts. Studies have demonstrated even higher prevalence within forensic populations, which seems reasonable because evading criminal responsibility is a large incentive to feign symptoms. Studies also have shown that 5% of military recruits will feign symptoms to avoid service. Moreover, 1% of psychiatric patients, such as Mr. D, feign symptoms for secondary gain.13
Although there are no psychometrically validated assessment tools to distinguish between real vs feigned hallucinations, several standardized tests can help tease out the truth.9 The preferred personality test used in forensic settings is the Minnesota Multiphasic Personality Inventory,14 which consists of 567 items, with 10 clinical scales and several validity scales. The F scale, “faking good” or “faking bad,” detects people who are answering questions with the goal of appearing better or worse than they actually are. In studies of patients hospitalized for being at risk for suicide who were administered tests of self-reported malingering, approximately 10% of people admitted to psychiatric units were “faking” their symptoms.14
It is important to identify malingering from a professional and public health standpoint. Society incurs incremental costs when a person uses dwindling mental health resources for their own reward, leaving others to suffer without treatment. The number of psychiatric hospital beds has fallen from half a million in the 1950s to approximately 100,000 today.15
Practical guidelines
Malingering presents specific challenges to clinicians, such as:
- diagnostic uncertainty
- inaccurately branding one a liar
- countertransference
- personal reactions.
Our ethical and fiduciary responsibility is to our patient. In examining the art in medicine, it has been suggested that malingering could be viewed as an immature or primitive defense.16
Although there often is suspicion that a person is malingering, a definitive statement of such must be confirmed. Without clarity, labeling an individual as a malingerer could have detrimental effects to his (her) future care, defames his character, and places a thoughtless examiner at risk of a lawsuit. Confirmation can be achieved by observation or psychological testing methods.
Observation. When in doubt of what to do with someone such as Mr. D, there is little harm in acting prudently by holding him in a controlled setting—whether keeping him overnight in an ED or admitting him for a brief psychiatric stay. By observing someone in a controlled environment, where there are multiple professional watchful eyes, inferences will be more accurate.1
Structured assessments have been developed to help detect malingering—one example is the Test of Memory Malingering—however, in daily practice, the physician generally should suspect malingering when there are tangible incentives and when reported symptoms do not match the physical examination or there is no organic basis for the physical complaints.17 Detecting illness deception relies on converging evidence sources, including detailed interview assessments, clinical notes, and consultations.7
When you feel certain that you are encountering someone who is malingering, the final step is to get a consult. Malingering is a serious label and warrants due diligence by the provider, rather than a haphazard guess that a patient is lying. Once you receive confirmatory opinions, great care should be taken in documenting a clear and accurate note that will benefit your clinical counterpart who might encounter a patient such as Mr. D when he (she) shows up again, and will go a long way toward appropriately directing his care.
Imagine you’re on call in a busy emergency department (ED) overnight. Things are tough. The consults are piling up, no one is returning your calls for collateral information, and you’re dealing with a myriad of emergencies.
In walks Mr. D, age 45, complaining of hearing voices, feeling unsafe, and asking for admission. It’s now 2
Of course, like all qualified psychiatrists, you will dig a little deeper, and in doing so you learn that Mr. D has visited this hospital before and has been admitted to the psychiatry unit. Now you go from having a dearth of information to having more records than you can count.
You discover that Mr. D has a history of coming to the ED during precarious hours, with similar complaints, demanding admission.
Mr. D, you learn, is unemployed, single, and homeless. Your meticulous search through his hospital records and previous admission and discharge notes reveal that once he has slept for a night, eaten a hot meal, and received narcotics for his back pain and benzodiazepines for his “symptoms” he demands to leave the hospital. His psychotic symptoms disappear despite his consistent refusal to take antipsychotics throughout his stay.
Now, what would you do?
As earnest medical students and psychiatrists, we enjoy helping patients on their path toward recovery. We want to advocate for our patients and give them the benefit of the doubt. We’re taught in medical school to be non-judgmental and invite patients to share their narrative. But through experience, we start to become aware of malingering.
Suspecting malingering, diagnosed as a condition, often is avoided by psychiatrists.1 This makes sense—it goes against the essence of our training and imposes a pejorative label on someone who has reached out for help.
Often persons with mental illness will suffer for years until they to receive help.2 That’s exactly why, when patients like Mr. D come to the ED and report hearing voices, we’re not likely to shout, “Liar!” and invite them to leave.
However, malingering is a real problem, especially because the number of psychiatric hospital beds have dwindled to record lows, thereby overcrowding EDs. Resources are skimpy, and clinicians want to help those who need it the most and not waste resources on someone who is “faking it” for secondary gain.
To navigate this diagnostic challenge, psychiatrists need the skills to detect malingering and the confidence to deal with it appropriately. This article aims to:
- define psychosis and malingering
- review the prevalence and historical considerations of malingering
- offer practical strategies to deal with malingering.
Know the real thing

Clinicians first must have the clinical acumen and expertise to identify a true mental illness such as psychosis2 (Table 1). The differential diagnosis for psychotic symptoms is broad. The astute clinician might suspect that untreated bipolar disorder or depression led to the emergence of perceptual disturbances or disordered thinking. Transient psychotic symptoms can be associated with trauma disorders, borderline personality disorder, and acute intoxication. Psychotic spectrum disorders range from brief psychosis to schizophreniform to schizoaffective disorder or schizophrenia.
Malingering—which is a condition, not a diagnosis—is characterized by the intentional production of false or grossly exaggerated physical or psychological symptoms motivated by external incentives.3,4 The presence of external incentives differentiates malingering from true psychiatric disorders, including factitious disorder, somatoform disorder, and dissociative disorder, and specific medical conditions.1 In those disorders, there is no external incentive.
It is important to remember that malingering can coexist with a serious mental illness. For example, a truly psychotic person might malinger, feign, or exaggerate symptoms to try to receive much needed help. Individuals with true psychosis might have become disenchanted with the mental health system, and thereby have a tendency to over-report or exaggerate symptoms in an effort to obtain treatment. This also could explain why many clinicians intuitively are reluctant to make the determination that someone is malingering. Malingering also can be present in an individual who has antisocial personality disorder, factitious disorder, Ganser syndrome, and Munchausen syndrome.4 When symptoms or diseases that either are thought to be exaggerated or do not exist, consider a diagnosis of malingering.
A key challenge in any discussion of abnormal health care–seeking behavior is the extent to which a person’s reported symptoms are considered to be a product of choice, psychopathology beyond volitional control, or perhaps both. Clinical skills alone typically are not sufficient for diagnosing or detecting malingering. Medical education needs to provide doctors with the conceptual, developmental, and management frameworks to understand and manage patients whose symptoms appear to be simulated. Central to understanding factitious disorders and malingering are the explanatory models and beliefs used to provide meaning for both patients and doctors.7
When considering malingered psychosis, the suspecting physician must stay alert to possible motives. Also, the patient’s presentation might provide some clues when there is marked variability, such as discrepancies in the history, gross inconsistencies, or blatant contradictions. Hallucinations are a heterogeneous experience, and discerning between true vs feigned symptoms can be challenging for even the seasoned clinician. It can be helpful to study the phenomenology of typical vs atypical hallucinatory symptoms.8 Examples of atypical symptoms include:
- vague hallucinations
- experiencing hallucinations of only 1 sensory modality (such as voices alone, visual images in black and white only)
- delusions that have an abrupt onset
- bizarre content without disordered thinking.2,6,9,10
The truth about an untruthful condition
Although the exact prevalence of malingering varies by circumstance, Rissmiller et al12,13 demonstrated—and later replicated—a prevalence of approximately 10% among patients hospitalized for suicidal ideation or suicide attempts. Studies have demonstrated even higher prevalence within forensic populations, which seems reasonable because evading criminal responsibility is a large incentive to feign symptoms. Studies also have shown that 5% of military recruits will feign symptoms to avoid service. Moreover, 1% of psychiatric patients, such as Mr. D, feign symptoms for secondary gain.13
Although there are no psychometrically validated assessment tools to distinguish between real vs feigned hallucinations, several standardized tests can help tease out the truth.9 The preferred personality test used in forensic settings is the Minnesota Multiphasic Personality Inventory,14 which consists of 567 items, with 10 clinical scales and several validity scales. The F scale, “faking good” or “faking bad,” detects people who are answering questions with the goal of appearing better or worse than they actually are. In studies of patients hospitalized for being at risk for suicide who were administered tests of self-reported malingering, approximately 10% of people admitted to psychiatric units were “faking” their symptoms.14
It is important to identify malingering from a professional and public health standpoint. Society incurs incremental costs when a person uses dwindling mental health resources for their own reward, leaving others to suffer without treatment. The number of psychiatric hospital beds has fallen from half a million in the 1950s to approximately 100,000 today.15
Practical guidelines
Malingering presents specific challenges to clinicians, such as:
- diagnostic uncertainty
- inaccurately branding one a liar
- countertransference
- personal reactions.
Our ethical and fiduciary responsibility is to our patient. In examining the art in medicine, it has been suggested that malingering could be viewed as an immature or primitive defense.16
Although there often is suspicion that a person is malingering, a definitive statement of such must be confirmed. Without clarity, labeling an individual as a malingerer could have detrimental effects to his (her) future care, defames his character, and places a thoughtless examiner at risk of a lawsuit. Confirmation can be achieved by observation or psychological testing methods.
Observation. When in doubt of what to do with someone such as Mr. D, there is little harm in acting prudently by holding him in a controlled setting—whether keeping him overnight in an ED or admitting him for a brief psychiatric stay. By observing someone in a controlled environment, where there are multiple professional watchful eyes, inferences will be more accurate.1
Structured assessments have been developed to help detect malingering—one example is the Test of Memory Malingering—however, in daily practice, the physician generally should suspect malingering when there are tangible incentives and when reported symptoms do not match the physical examination or there is no organic basis for the physical complaints.17 Detecting illness deception relies on converging evidence sources, including detailed interview assessments, clinical notes, and consultations.7
When you feel certain that you are encountering someone who is malingering, the final step is to get a consult. Malingering is a serious label and warrants due diligence by the provider, rather than a haphazard guess that a patient is lying. Once you receive confirmatory opinions, great care should be taken in documenting a clear and accurate note that will benefit your clinical counterpart who might encounter a patient such as Mr. D when he (she) shows up again, and will go a long way toward appropriately directing his care.
1. LoPiccolo CJ, Goodkin K, Baldewicz TT. Current issues in the diagnosis and management of malingering. Ann Med. 1999;31(3):166-174.
2. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry. 2005;4(11):12-25.
3. Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 10th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007:887.
4. Gorman WF. Defining malingering. J Forensic Sci. 1982;27(2):401-407.
5. Mendelson G, Mendelson D. Malingering pain in the medicolegal context. Clin J Pain. 2004;20(6):423-432.
6. Resnick PJ. Malingered psychosis. In: Rogers R, ed. Clinical assessment of malingering and deception. 2nd ed. New York, NY: The Guilford Press; 1997:47-67.
7. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
8. McCarthy-Jones S, Resnick PJ. Listening to the voices: the use of phenomenology to differentiate malingered from genuine auditory verbal hallucinations. Int J Law Psychiatry. 2014;37(2):183-189.
9. Resnick PJ. Defrocking the fraud: the detection of malingering. Isr J Psychiatry Relat Sci. 1993;30(2):93-101.
10. Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med. 1996;26(1):177-189.
11. Pollock P. Feigning auditory hallucinations by offenders. Journal of Forensic Psychiatry. 1998;9(2)305-327.
12. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicide ideators and attempters. Crisis. 1998;19(2):62-66.
13. Rissmiller DA, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric patients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
14. Hathaway SR, McKinley JC. The Minnesota Multiphasic Personality Inventory-2. Minneapolis, MN: University of Minnesota Press; 1989.
15. Szabo L. Cost of not caring: Stigma set in stone. USA Today. http://www.usatoday.com/story/news/nation/2014/06/25/stigma-of-mental-illness/9875351. Published June 25, 2014. Accessed May 5, 2017.
16. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
17. McDermott BE, Feldman MD. Malingering in the medical setting. Psychiatr Clin North Am. 2007;30(4):645-662.
1. LoPiccolo CJ, Goodkin K, Baldewicz TT. Current issues in the diagnosis and management of malingering. Ann Med. 1999;31(3):166-174.
2. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry. 2005;4(11):12-25.
3. Sadock VA. Kaplan and Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry. 10th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2007:887.
4. Gorman WF. Defining malingering. J Forensic Sci. 1982;27(2):401-407.
5. Mendelson G, Mendelson D. Malingering pain in the medicolegal context. Clin J Pain. 2004;20(6):423-432.
6. Resnick PJ. Malingered psychosis. In: Rogers R, ed. Clinical assessment of malingering and deception. 2nd ed. New York, NY: The Guilford Press; 1997:47-67.
7. Bass C, Halligan P. Factitious disorders and malingering: challenges for clinical assessment and management. Lancet. 2014;383(9926):1422-1432.
8. McCarthy-Jones S, Resnick PJ. Listening to the voices: the use of phenomenology to differentiate malingered from genuine auditory verbal hallucinations. Int J Law Psychiatry. 2014;37(2):183-189.
9. Resnick PJ. Defrocking the fraud: the detection of malingering. Isr J Psychiatry Relat Sci. 1993;30(2):93-101.
10. Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med. 1996;26(1):177-189.
11. Pollock P. Feigning auditory hallucinations by offenders. Journal of Forensic Psychiatry. 1998;9(2)305-327.
12. Rissmiller DJ, Wayslow A, Madison H, et al. Prevalence of malingering in inpatient suicide ideators and attempters. Crisis. 1998;19(2):62-66.
13. Rissmiller DA, Steer RA, Friedman M, et al. Prevalence of malingering in suicidal psychiatric patients: a replication. Psychol Rep. 1999;84(3 pt 1):726-730.
14. Hathaway SR, McKinley JC. The Minnesota Multiphasic Personality Inventory-2. Minneapolis, MN: University of Minnesota Press; 1989.
15. Szabo L. Cost of not caring: Stigma set in stone. USA Today. http://www.usatoday.com/story/news/nation/2014/06/25/stigma-of-mental-illness/9875351. Published June 25, 2014. Accessed May 5, 2017.
16. Malone RD, Lange CL. A clinical approach to the malingering patient. J Am Acad Psychoanal Dyn Psychiatry. 2007;35(1):13-21.
17. McDermott BE, Feldman MD. Malingering in the medical setting. Psychiatr Clin North Am. 2007;30(4):645-662.
E-cigarettes and vapes: Do they work for smoking cessation and should we be recommending their use?
The popularity of electronic cigarettes (E-cigs) and “vapes” has grown dramatically, spawning a new industry of electronic nicotine delivery systems (ENDS). With the increasing use of E-cigs not only for smoking cessation, but also as a primary nicotine source, it is important for mental health professionals to be prepared to discuss use of these devices with patients. In this article, we will describe:
- the composition of E-cigs and their current use
- evidence for their use for smoking cessation
- adverse health effects
- recommendations of major regulatory agencies.
Finally, we will provide recommendations for E-cig use in clinical populations.
What is an electronic nicotine delivery system?
ENDS produce an aerosol with or without nicotine that is inhaled and is thought to mimic the use of combustible cigarettes. ENDS evolved from basic E-cigs into a less “cigarette-like” and more customizable product (Figure 1). ENDS include a range of designs and go by various names, including “personal vaporizers,” “e-cigars,” and “e-hookahs” (in this article, we will use the term “ENDS” to refer to these devices).
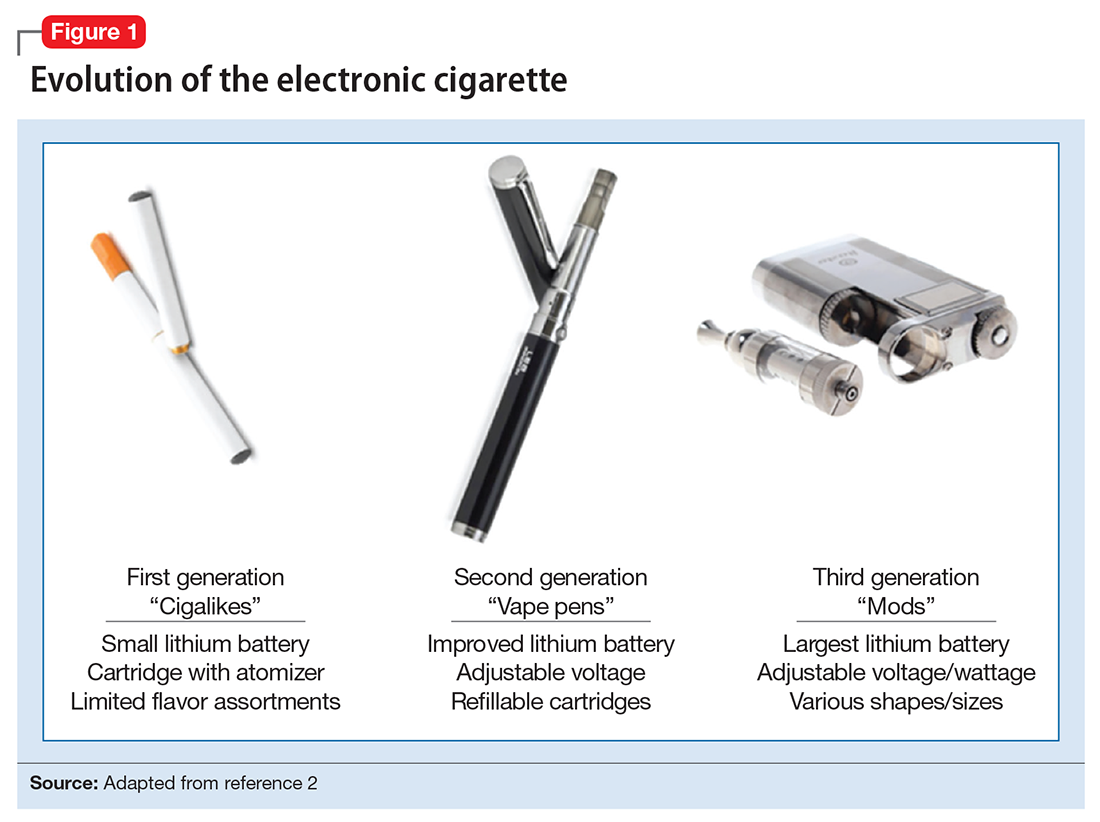
The general design of ENDS is a plastic tubing system that contains a mouthpiece, battery, electronic heating element (“vaporizer”), and a cartridge with liquid solvent with or without nicotine or flavoring (Figure 2). One draw on the mouthpiece or press of a button activates the device, heats the solution, and delivers a vapor in a similar manner to taking a puff of a cigarette. Although studies have shown that ENDS result in significant increases in plasma nicotine concentrations in 5 minutes,1 the plasma nicotine levels obtained with the first-generation “cigarette-like” ENDS are much lower than those caused by inhaling tobacco smoke.2 Over time nicotine delivery capability has improved as ENDS have evolved such that the rate of nicotine delivery and peak concentration obtained with newer models more closely mirror tobacco cigarettes.3 Whether the rapid delivery of larger amounts of nicotine helps or hinders one’s efforts to break nicotine addiction remains to be determined because of the reinforcing properties of the drug.
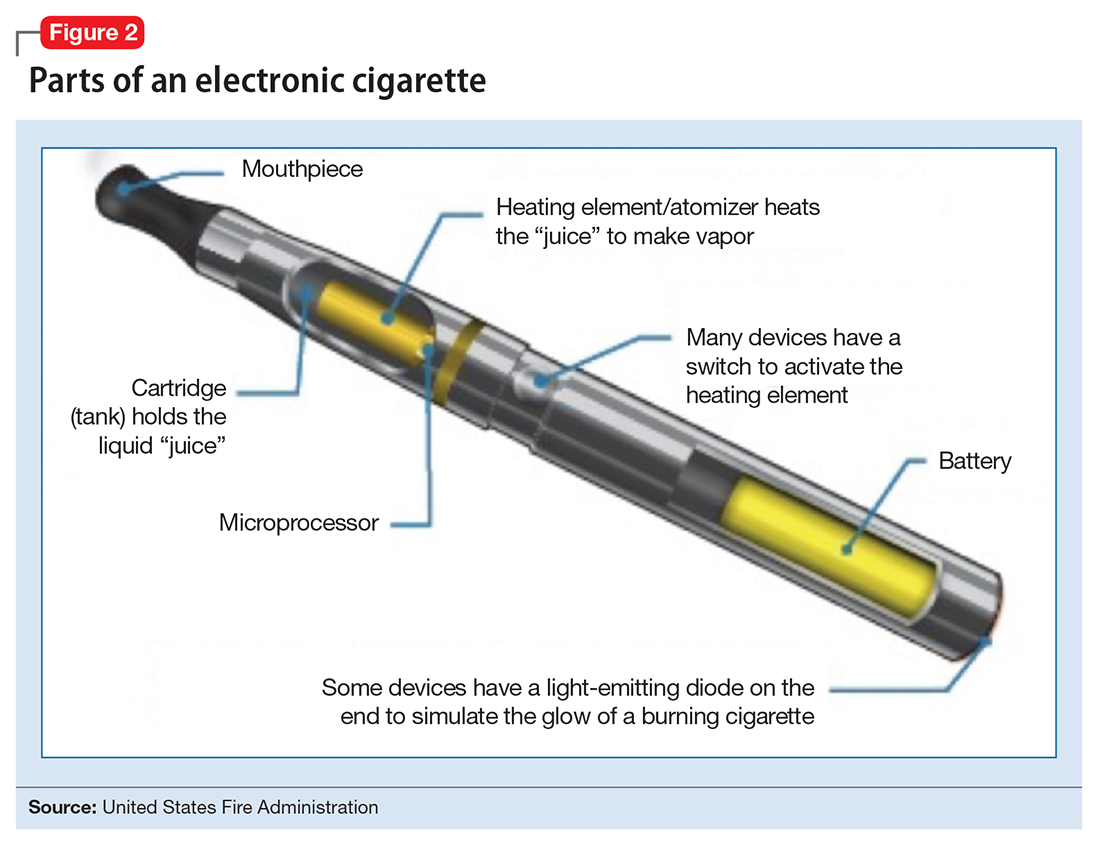
The liquid in the E-cig cartridge typically contains not only nicotine but a number of chemical compounds with potentially deleterious or unknown health risks. The 3 main ingredients include:
- a solvent of glycerin and/or propylene glycol
- nicotine in various concentrations
- flavorings.
The glycerin or propylene glycol forms the basis for the aerosol. Nicotine concentrations vary from 0 (denicotinized) to 35 mcg per puff.4 A study reported 7,700 unique flavors available for vaping liquid.5 The liquid also contains impurities, such as anabasine, which has effects on the α-7 nicotinic acetylcholine receptor and its principal use is as an insecticide and β-nicotyrine, which inhibits cytochrome P450 2A.
Epidemiology and end-user perspectives
In 2014, 12.4% of U.S. adults classified themselves as “ever users” of ENDS (used at least once) and 3.7% of adults classified themselves as current users, according to the National Health Interview Study.6 Importantly, among E-cig users who had not used combustible cigarettes, young adults (age 18 to 24) were more likely to have tried ENDS than older adults. ENDS are becoming more popular across the globe. A study in the European Union found that ever users of ENDS most commonly were current cigarette smokers (31%) followed by former (10.8%) and never smokers (2.3%).7
ENDS use is relevant for mental health professionals because of the high rate of comorbid tobacco use disorder in individuals with psychiatric conditions. For example, 2 U.S. population surveys8,9 revealed those with mental health conditions were 1.5 to 2 times more likely to have tried ENDS and 2 to 3 times more likely to be current users. Those with psychiatric illness reported similar reasons for ENDS use as other individuals, including “just because,” use as a smoking cessation aid, ease of use, and perceived safety vs combustible cigarettes.
A recent review that included 9 studies focusing on ENDS use in those with mental illness reported mixed findings on the utility of these devices to reduce or stop use of combustible cigarettes.10 Additionally, it is important to monitor the use of cigarettes and ENDS in patients with psychiatric illness because the byproducts of tobacco smoke can affect the metabolism of some psychotropic medications.11 Although reduced use of combustible cigarettes could lead to lower dosing of some psychotropics, an unreported decrease in combustible cigarette use could lead to supratherapeutic drug levels. There are no data on the effect of ENDS on the metabolism of psychotropics.
ENDS are increasingly popular among adolescents. In 2015, there were an estimated 4.6 million current tobacco users among middle/high school youths in the United States and 3 million current ENDS users, according to the National Youth Tobacco Surveys.12 The shift from combustible cigarettes to ENDS is notable, with an increase in the percentage of current E-cig users and a decrease in the percentage of exclusive combustible cigarette users. In addition, there has been no change in the prevalence of lifetime tobacco users.12 This is a global issue, as reports of ever use of ENDS by adolescents range from 6.5% to 31% in the United States, 14.6% in Canada, and 4.7% to 38.5% in Europe.13 Based on these trends, the U.S. Surgeon General released a statement warning against the use of ENDS in youth because of the lack of safety data and strong association with use of tobacco products.14
There are a number of possible reasons for the increasing popularity of ENDS, including the product’s novelty, lack of regulations regarding their sale, availability of flavorings, and the perception that ENDS are safe alternatives to cigarettes. E-cig–using youths have described ENDS as “not at all harmful” and “not at all addictive” and believe that ENDS with flavoring are less harmful than those without.15 Although studies in adults show some users reporting that ENDS are less satisfying, they are seen as useful in decreasing craving and a safer alternative to cigarettes.16,17
Are ENDS effective for smoking cessation?
The evidence for ENDS as aids to smoking cessation remains murky (Table 118-22). There is a paucity of randomized controlled clinical trials (RCTs) investigating ENDS for smoking cessation or reduction, and it is difficult to quantify the amount of nicotine used in ENDS because of the variety of delivery systems and cartridges. In a recent Cochrane review, those using ENDS to quit smoking were more likely to be abstinent from combustible cigarettes at 6 months vs those using nicotine-free ENDS (relative risk = 2.29; 95% CI, 1.05 to 4.96), but there was no significant difference in quit rates compared with nicotine patches.23 However, the confidence in this finding was rated as low because of the limited number of RCTs. Of note, the authors found 15 ongoing RCTs at the time of publication that might be eligible for later evaluation.
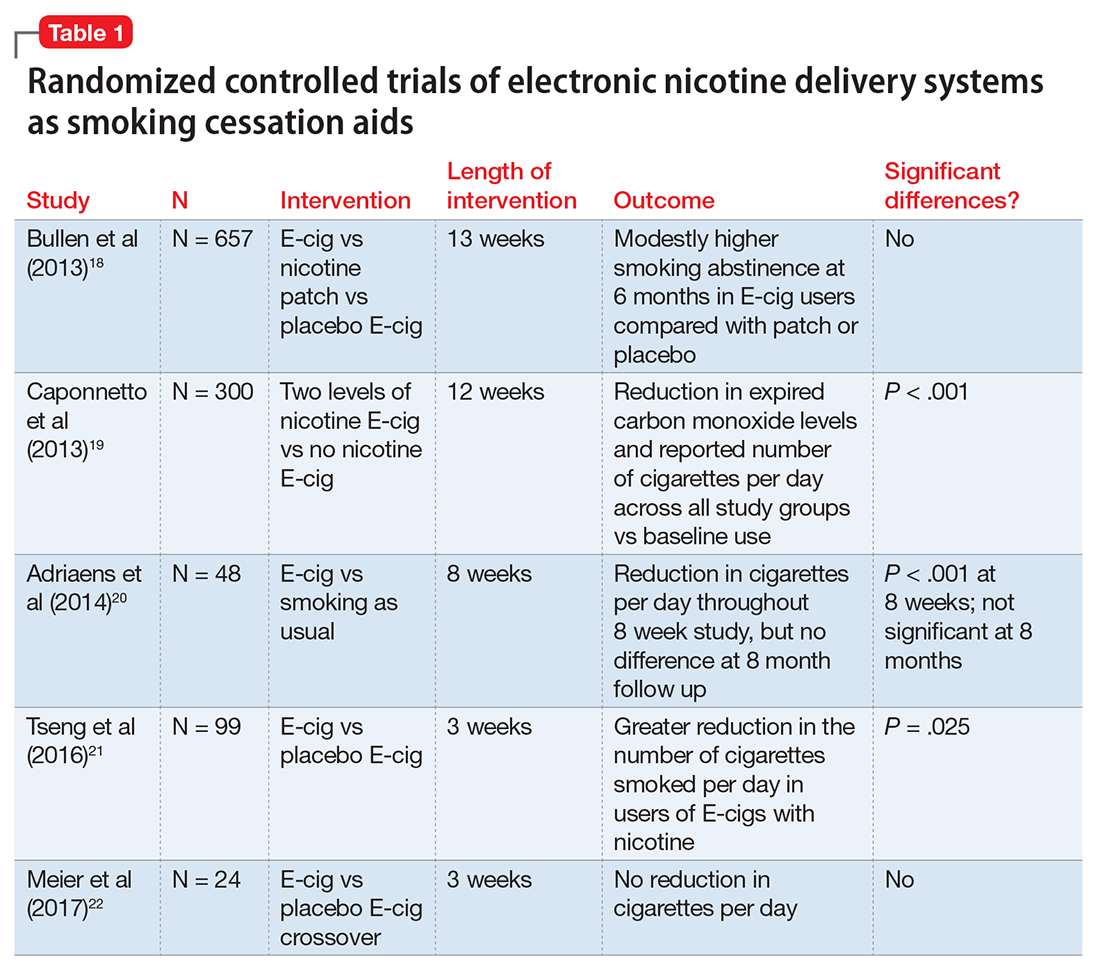
Non-RCTs reveal mixed data. Positive results include 1 study with an odds ratio of 6.07 to quit for intensive ENDS users vs non-users,24 and another with dual users of combustible and electronic cigarettes having a 46% quit rate at 1 year.25 Additionally, in a pilot study providing ENDS to 14 patients with schizophrenia who had no previous desire to quit smoking, authors noted a reduction in the number of cigarettes smoked per day by 50% in one-half of participants and abstinence in 14% of participants at 52 weeks.26 Studies with neutral or negative results include those showing ENDS users to be current combustible tobacco smokers, and use of ENDS not predicting smoking cessation.4,27 Data also are mixed regarding the use of ENDS as a harm reduction strategy. One study found that ENDS decreased cigarette consumption, but did not increase the likelihood of quitting,28 while another reported that daily use of ENDS increased the odds of reducing smoking by as much as 2.5 times compared with non-use of such aids.29 In a 24-month prospective cohort study following tobacco users, there was no difference in the number of cigarettes smoked per day in those who started the trial as users of combustible cigarettes alone vs combustible cigarettes plus ENDS users.30 Interestingly, those who started the study as combustible cigarette users and switched to ENDS and those who had continued dual use throughout the 24 months smoked fewer combustible cigarettes per day than those who never tried ENDS or quit during the study period.
Health effects


To better understand the adverse health effects of ENDS, one must consider potential short- and long-term consequences (Table 2). In the short-term, ENDS have been found to increase markers of inflammation and oxidative stress acutely as evidenced by in vivo laboratory studies.31,32 ENDS also have been linked to upper respiratory irritation, in part, because of the transformation of glycerin in the nicotine cartridge to acrolein upon combustion.33 Even 5 minutes of ad lib E-cig use has been found to significantly increase airflow resistance during pulmonary function tests34—changes that have been shown to precede more persistent alterations in peak expiratory flow, such as those seen in chronic obstructive pulmonary disease. The more common patient-reported side effects include:
- daytime cough (27%)
- phlegm production (25%)
- headache (21%)
- dry mouth/throat (20%)
- vertigo, headache, or nausea (9%).35,36
A RCT investigating efficacy of E-cigs vs nicotine patches vs denicotinized E-cigs found no difference among the groups in the number of reported adverse events.18 Interestingly, another RCT found a decrease in adverse events, such as dry cough, mouth irritation, throat irritation, shortness of breath, and headache, compared with baseline in combustible cigarette smokers who used regular or denicotinized E-cigs.19
Although no studies have directly investigated long-term health consequences of ENDS because of their relative novelty, one can extrapolate potential harmful long-term effects based on knowledge of the products’ chemical constituents. For example, propylene glycol can degrade into propylene oxide, a class 2B carcinogen.37 Other potential carcinogens in the aerosol include formaldehyde and acetaldehyde. On a broader scale, many of the particulates have been shown to cause systemic inflammation, which is thought to increase cardiovascular and respiratory disease and death.38 Flavorings in ENDS include a variety of components including, but not limited to, aldehydes, which are irritants, and other additives that have been associated with respiratory disease.39
Second-hand exposure. There are no long-term studies of second-hand vapor exposure, but similar to long-term health on primary users, one can glean some observations from the literature. It is promising that compared with cigarettes, ENDS lack sidestream smoke and the vapor has not been found to contain carbon monoxide.40 Some research has demonstrated that the size and spray of fine particles in the aerosol is as large or larger than combustible cigarettes.41 Formaldehyde, acetaldehyde, isoprene, and acetic acid have been found in ENDS vapor.40 Interestingly, a simulated café study found elevated nicotine, glycerine, hydrocarbon, and other materials classified as carcinogens in the air.42
Although it is popularly thought that ENDS are less toxic than tobacco cigarettes, there is not enough evidence to estimate precisely as to how much less toxic or the consequences of use. ENDS are increasingly popular and are being used by never smokers who should be educated on the potential harm that ENDS pose.
Recommendations from agencies and medical organizations
The World Health Organization (WHO) recommended prohibiting the use of ENDS in indoor spaces to minimize potential health risks to users and non-users. The WHO also aims to prevent dissemination of unproven health claims, including claims that ENDS are effective—or not—or that the devices are innocuous.36 In the United States, the FDA has stated that ENDS are not recommended for safe quitting (2009). In August 2016, the FDA introduced regulations banning the sale of ENDS to individuals age <18 and required manufacturers to submit documents detailing all ingredients for review and possible approval.
The American Lung Association has stated its concerns about the use of ENDS but has not made any direct recommendations. The American Heart Association reports a potential negative public health impact and provides clinical guideline recommendations.43 Prominent psychiatric organizations such as the American Psychiatric Association, American Academy of Addiction Psychiatry (AAAP), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institute of Drug Abuse do not have official statements supporting or rejecting the use of ENDS. However, they do note the potential harm and lack of substantial evidence for efficacy of ENDS as a smoking cessation tool, and the AAAP and SAMHSA state that they will work with regulatory agencies to reduce the use of toxic products with addictive potential including ENDS.44-46
Clinical recommendations
We do not recommend ENDS as a first-line treatment for smoking cessation because there is no evidence they are superior to the FDA-approved nicotine replacement therapies (NRTs), the paucity of research into the potential short- and long-term health risks of ENDS, and the fact that these products are not regulated for use as smoking cessation aids. It is, however, advisable to discuss ENDS use with patients by:
- asking if they are using the products
- assessing whether the user also is a smoker
- advising the patient to quit.
It also is important to assess the patient’s knowledge and attitudes regarding ENDS use and provide education about the products. Some patients firmly believe that ENDS are the lesser of 2 evils, and they are decreasing the harms of smoking by using these devices. While the debate over a potential harm reduction strategy unfolds,47 we think that because of the state of the evidence it is prudent to adopt a more precautionary stance and recommend that patients work toward abstinence from nicotine in any form.
For dual tobacco/ENDS users and for patients using ENDS who want to quit smoking, we recommend treatment with an approved pharmacotherapy (ie, NRTs, bupropion, and varenicline) combined with counseling. A 2013 Cochrane Review found that all pharamacotherapy options are more effective than placebo, and combination NRT and varenicline are superior to single NRT or bupropion (Box).23,48
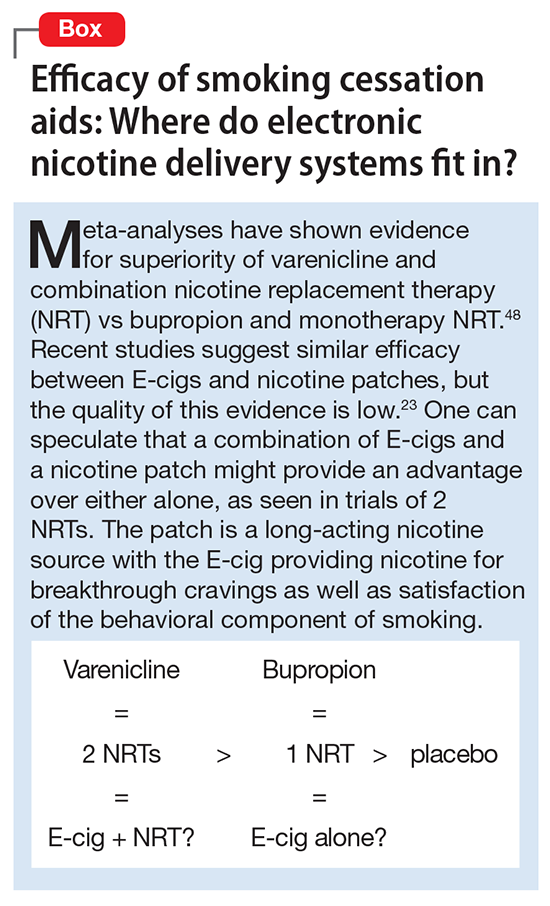
1. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175-179.
2. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86.
3. St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535-544.
4. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-1986.
5. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3-iii9. doi: 10.1136/tobaccocontrol-2014-051670.
6. Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015;(217):1-8.
7. Farsalinos KE, Poulas K, Voudris V, et al. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction. 2016;111(11):2032-2040.
8. Cummins SE, Zhu SH, Tedeschi GJ, et al. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2015;23(suppl 3):iii48-iii53. doi: 10.1136/tobaccocontrol-2013-051511.
9. Spears CA, Jones DM, Weaver SR, et al. Use of electronic nicotine delivery systems among adults with mental health conditions, 2015. Int J Environ Res Public Heal. 2017;14(1):10.
10. Hefner K, Valentine G, Sofuoglu M. Electronic cigarettes and mental illness: reviewing the evidence for help and harm among those with psychiatric and substance use disorders [published online February 2, 2017]. Am J Addict. doi: 10.1111/ajad.12504.
11. Anthenelli R. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
12. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361-367.
13. Greenhill R, Dawkins L, Notley C, et al. Adolescent awareness and use of electronic cigarettes: a review of emerging trends and findings. J Adolesc Heal. 2016;59(6):612-619.
14. U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016.
15. Cooper M, Harrell MB, Pérez A, et al. Flavorings and perceived harm and addictiveness of e-cigarettes among youth. Tob Regul Sci. 2016;2(3):278-289.
16. Kim H, Davis AH, Dohack JL, et al. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob Res. 2017;19(2):190-196.
17. Czoli CD, Fong GT, Mays D, et al. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control. 2017;26(e1):e49-e58.
18. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629-1637.
19. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317.
20. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220-11248.
21. Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937-1943.
22. Meier E, Wahlquist AE, Heckman BW, et al. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine Tob Res. 2017;19(2):176-182.
23. Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation [published online September 14, 2016]. Cochrane Database Syst Rev. 2016;9:CD010216.
24. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2014;17(2):127-133.
25. Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491-494.
26. Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446-461.
27. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923-930.
28. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: International Tobacco Control Four-Country Survey. Am J Prev Med. 2013;44(3):207-215.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160-1168.
30. Manzoli L, Flacco ME, Ferrante M, et al; ISLESE Working Group. Cohort study of electronic cigarette use: effectiveness and safety at 24 months [published online June 6, 2016]. Tob Control. doi: 10.1136/tobaccocontrol-2015-052822.
31. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732.
32. Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861.
33. US Environmental Protection Agency. Acrolein. https://www.epa.gov/sites/production/files/2016-08/documents/acrolein.pdf. Updated September 2009. Accessed April 7, 2017.
34. Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400-1406.
35. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231.
36. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32(2):133-140.
37. Laino T, Tuma C, Moor P, et al. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116(18):4602-4609.
38. Brook RD, Rajagopalan S, Pope CA 3rd, et al; American Heart Association Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331-2378.
39. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493-2494.
40. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25-31.
41. Fuoco FC, Buonanno G, Stabile L, et al. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523-529.
42. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628-637.
43. Bhatnagar A, Whitsel L, Ribisl K, et al; American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418-1436.
44. E-cigarettes pose risks. SAMHSA News. https://www.samhsa.gov/samhsaNewsLetter/Volume_22_Number_3/e_cigarettes. Published 2014. Accessed April 7, 2017.
45. National Institute on Drug Abuse. Electronic cigarettes (e-cigarettes). https://www.drugabuse.gov/publications/drugfacts/electronic-cigarettes-e-cigarettes. Revised May 2016. Accessed April 7, 2017.
46. American Academy of Addiction Psychiatry. Nicotine dependence. East Providence, RI: American Academy of Addition Psychiatry; 2015.
47. Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes — will England reframe the debate. N Engl J Med. 2016;374(14):1301-1303.
48. Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193-194.
The popularity of electronic cigarettes (E-cigs) and “vapes” has grown dramatically, spawning a new industry of electronic nicotine delivery systems (ENDS). With the increasing use of E-cigs not only for smoking cessation, but also as a primary nicotine source, it is important for mental health professionals to be prepared to discuss use of these devices with patients. In this article, we will describe:
- the composition of E-cigs and their current use
- evidence for their use for smoking cessation
- adverse health effects
- recommendations of major regulatory agencies.
Finally, we will provide recommendations for E-cig use in clinical populations.
What is an electronic nicotine delivery system?
ENDS produce an aerosol with or without nicotine that is inhaled and is thought to mimic the use of combustible cigarettes. ENDS evolved from basic E-cigs into a less “cigarette-like” and more customizable product (Figure 1). ENDS include a range of designs and go by various names, including “personal vaporizers,” “e-cigars,” and “e-hookahs” (in this article, we will use the term “ENDS” to refer to these devices).

The general design of ENDS is a plastic tubing system that contains a mouthpiece, battery, electronic heating element (“vaporizer”), and a cartridge with liquid solvent with or without nicotine or flavoring (Figure 2). One draw on the mouthpiece or press of a button activates the device, heats the solution, and delivers a vapor in a similar manner to taking a puff of a cigarette. Although studies have shown that ENDS result in significant increases in plasma nicotine concentrations in 5 minutes,1 the plasma nicotine levels obtained with the first-generation “cigarette-like” ENDS are much lower than those caused by inhaling tobacco smoke.2 Over time nicotine delivery capability has improved as ENDS have evolved such that the rate of nicotine delivery and peak concentration obtained with newer models more closely mirror tobacco cigarettes.3 Whether the rapid delivery of larger amounts of nicotine helps or hinders one’s efforts to break nicotine addiction remains to be determined because of the reinforcing properties of the drug.

The liquid in the E-cig cartridge typically contains not only nicotine but a number of chemical compounds with potentially deleterious or unknown health risks. The 3 main ingredients include:
- a solvent of glycerin and/or propylene glycol
- nicotine in various concentrations
- flavorings.
The glycerin or propylene glycol forms the basis for the aerosol. Nicotine concentrations vary from 0 (denicotinized) to 35 mcg per puff.4 A study reported 7,700 unique flavors available for vaping liquid.5 The liquid also contains impurities, such as anabasine, which has effects on the α-7 nicotinic acetylcholine receptor and its principal use is as an insecticide and β-nicotyrine, which inhibits cytochrome P450 2A.
Epidemiology and end-user perspectives
In 2014, 12.4% of U.S. adults classified themselves as “ever users” of ENDS (used at least once) and 3.7% of adults classified themselves as current users, according to the National Health Interview Study.6 Importantly, among E-cig users who had not used combustible cigarettes, young adults (age 18 to 24) were more likely to have tried ENDS than older adults. ENDS are becoming more popular across the globe. A study in the European Union found that ever users of ENDS most commonly were current cigarette smokers (31%) followed by former (10.8%) and never smokers (2.3%).7
ENDS use is relevant for mental health professionals because of the high rate of comorbid tobacco use disorder in individuals with psychiatric conditions. For example, 2 U.S. population surveys8,9 revealed those with mental health conditions were 1.5 to 2 times more likely to have tried ENDS and 2 to 3 times more likely to be current users. Those with psychiatric illness reported similar reasons for ENDS use as other individuals, including “just because,” use as a smoking cessation aid, ease of use, and perceived safety vs combustible cigarettes.
A recent review that included 9 studies focusing on ENDS use in those with mental illness reported mixed findings on the utility of these devices to reduce or stop use of combustible cigarettes.10 Additionally, it is important to monitor the use of cigarettes and ENDS in patients with psychiatric illness because the byproducts of tobacco smoke can affect the metabolism of some psychotropic medications.11 Although reduced use of combustible cigarettes could lead to lower dosing of some psychotropics, an unreported decrease in combustible cigarette use could lead to supratherapeutic drug levels. There are no data on the effect of ENDS on the metabolism of psychotropics.
ENDS are increasingly popular among adolescents. In 2015, there were an estimated 4.6 million current tobacco users among middle/high school youths in the United States and 3 million current ENDS users, according to the National Youth Tobacco Surveys.12 The shift from combustible cigarettes to ENDS is notable, with an increase in the percentage of current E-cig users and a decrease in the percentage of exclusive combustible cigarette users. In addition, there has been no change in the prevalence of lifetime tobacco users.12 This is a global issue, as reports of ever use of ENDS by adolescents range from 6.5% to 31% in the United States, 14.6% in Canada, and 4.7% to 38.5% in Europe.13 Based on these trends, the U.S. Surgeon General released a statement warning against the use of ENDS in youth because of the lack of safety data and strong association with use of tobacco products.14
There are a number of possible reasons for the increasing popularity of ENDS, including the product’s novelty, lack of regulations regarding their sale, availability of flavorings, and the perception that ENDS are safe alternatives to cigarettes. E-cig–using youths have described ENDS as “not at all harmful” and “not at all addictive” and believe that ENDS with flavoring are less harmful than those without.15 Although studies in adults show some users reporting that ENDS are less satisfying, they are seen as useful in decreasing craving and a safer alternative to cigarettes.16,17
Are ENDS effective for smoking cessation?
The evidence for ENDS as aids to smoking cessation remains murky (Table 118-22). There is a paucity of randomized controlled clinical trials (RCTs) investigating ENDS for smoking cessation or reduction, and it is difficult to quantify the amount of nicotine used in ENDS because of the variety of delivery systems and cartridges. In a recent Cochrane review, those using ENDS to quit smoking were more likely to be abstinent from combustible cigarettes at 6 months vs those using nicotine-free ENDS (relative risk = 2.29; 95% CI, 1.05 to 4.96), but there was no significant difference in quit rates compared with nicotine patches.23 However, the confidence in this finding was rated as low because of the limited number of RCTs. Of note, the authors found 15 ongoing RCTs at the time of publication that might be eligible for later evaluation.

Non-RCTs reveal mixed data. Positive results include 1 study with an odds ratio of 6.07 to quit for intensive ENDS users vs non-users,24 and another with dual users of combustible and electronic cigarettes having a 46% quit rate at 1 year.25 Additionally, in a pilot study providing ENDS to 14 patients with schizophrenia who had no previous desire to quit smoking, authors noted a reduction in the number of cigarettes smoked per day by 50% in one-half of participants and abstinence in 14% of participants at 52 weeks.26 Studies with neutral or negative results include those showing ENDS users to be current combustible tobacco smokers, and use of ENDS not predicting smoking cessation.4,27 Data also are mixed regarding the use of ENDS as a harm reduction strategy. One study found that ENDS decreased cigarette consumption, but did not increase the likelihood of quitting,28 while another reported that daily use of ENDS increased the odds of reducing smoking by as much as 2.5 times compared with non-use of such aids.29 In a 24-month prospective cohort study following tobacco users, there was no difference in the number of cigarettes smoked per day in those who started the trial as users of combustible cigarettes alone vs combustible cigarettes plus ENDS users.30 Interestingly, those who started the study as combustible cigarette users and switched to ENDS and those who had continued dual use throughout the 24 months smoked fewer combustible cigarettes per day than those who never tried ENDS or quit during the study period.
Health effects


To better understand the adverse health effects of ENDS, one must consider potential short- and long-term consequences (Table 2). In the short-term, ENDS have been found to increase markers of inflammation and oxidative stress acutely as evidenced by in vivo laboratory studies.31,32 ENDS also have been linked to upper respiratory irritation, in part, because of the transformation of glycerin in the nicotine cartridge to acrolein upon combustion.33 Even 5 minutes of ad lib E-cig use has been found to significantly increase airflow resistance during pulmonary function tests34—changes that have been shown to precede more persistent alterations in peak expiratory flow, such as those seen in chronic obstructive pulmonary disease. The more common patient-reported side effects include:
- daytime cough (27%)
- phlegm production (25%)
- headache (21%)
- dry mouth/throat (20%)
- vertigo, headache, or nausea (9%).35,36
A RCT investigating efficacy of E-cigs vs nicotine patches vs denicotinized E-cigs found no difference among the groups in the number of reported adverse events.18 Interestingly, another RCT found a decrease in adverse events, such as dry cough, mouth irritation, throat irritation, shortness of breath, and headache, compared with baseline in combustible cigarette smokers who used regular or denicotinized E-cigs.19
Although no studies have directly investigated long-term health consequences of ENDS because of their relative novelty, one can extrapolate potential harmful long-term effects based on knowledge of the products’ chemical constituents. For example, propylene glycol can degrade into propylene oxide, a class 2B carcinogen.37 Other potential carcinogens in the aerosol include formaldehyde and acetaldehyde. On a broader scale, many of the particulates have been shown to cause systemic inflammation, which is thought to increase cardiovascular and respiratory disease and death.38 Flavorings in ENDS include a variety of components including, but not limited to, aldehydes, which are irritants, and other additives that have been associated with respiratory disease.39
Second-hand exposure. There are no long-term studies of second-hand vapor exposure, but similar to long-term health on primary users, one can glean some observations from the literature. It is promising that compared with cigarettes, ENDS lack sidestream smoke and the vapor has not been found to contain carbon monoxide.40 Some research has demonstrated that the size and spray of fine particles in the aerosol is as large or larger than combustible cigarettes.41 Formaldehyde, acetaldehyde, isoprene, and acetic acid have been found in ENDS vapor.40 Interestingly, a simulated café study found elevated nicotine, glycerine, hydrocarbon, and other materials classified as carcinogens in the air.42
Although it is popularly thought that ENDS are less toxic than tobacco cigarettes, there is not enough evidence to estimate precisely as to how much less toxic or the consequences of use. ENDS are increasingly popular and are being used by never smokers who should be educated on the potential harm that ENDS pose.
Recommendations from agencies and medical organizations
The World Health Organization (WHO) recommended prohibiting the use of ENDS in indoor spaces to minimize potential health risks to users and non-users. The WHO also aims to prevent dissemination of unproven health claims, including claims that ENDS are effective—or not—or that the devices are innocuous.36 In the United States, the FDA has stated that ENDS are not recommended for safe quitting (2009). In August 2016, the FDA introduced regulations banning the sale of ENDS to individuals age <18 and required manufacturers to submit documents detailing all ingredients for review and possible approval.
The American Lung Association has stated its concerns about the use of ENDS but has not made any direct recommendations. The American Heart Association reports a potential negative public health impact and provides clinical guideline recommendations.43 Prominent psychiatric organizations such as the American Psychiatric Association, American Academy of Addiction Psychiatry (AAAP), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institute of Drug Abuse do not have official statements supporting or rejecting the use of ENDS. However, they do note the potential harm and lack of substantial evidence for efficacy of ENDS as a smoking cessation tool, and the AAAP and SAMHSA state that they will work with regulatory agencies to reduce the use of toxic products with addictive potential including ENDS.44-46
Clinical recommendations
We do not recommend ENDS as a first-line treatment for smoking cessation because there is no evidence they are superior to the FDA-approved nicotine replacement therapies (NRTs), the paucity of research into the potential short- and long-term health risks of ENDS, and the fact that these products are not regulated for use as smoking cessation aids. It is, however, advisable to discuss ENDS use with patients by:
- asking if they are using the products
- assessing whether the user also is a smoker
- advising the patient to quit.
It also is important to assess the patient’s knowledge and attitudes regarding ENDS use and provide education about the products. Some patients firmly believe that ENDS are the lesser of 2 evils, and they are decreasing the harms of smoking by using these devices. While the debate over a potential harm reduction strategy unfolds,47 we think that because of the state of the evidence it is prudent to adopt a more precautionary stance and recommend that patients work toward abstinence from nicotine in any form.
For dual tobacco/ENDS users and for patients using ENDS who want to quit smoking, we recommend treatment with an approved pharmacotherapy (ie, NRTs, bupropion, and varenicline) combined with counseling. A 2013 Cochrane Review found that all pharamacotherapy options are more effective than placebo, and combination NRT and varenicline are superior to single NRT or bupropion (Box).23,48

The popularity of electronic cigarettes (E-cigs) and “vapes” has grown dramatically, spawning a new industry of electronic nicotine delivery systems (ENDS). With the increasing use of E-cigs not only for smoking cessation, but also as a primary nicotine source, it is important for mental health professionals to be prepared to discuss use of these devices with patients. In this article, we will describe:
- the composition of E-cigs and their current use
- evidence for their use for smoking cessation
- adverse health effects
- recommendations of major regulatory agencies.
Finally, we will provide recommendations for E-cig use in clinical populations.
What is an electronic nicotine delivery system?
ENDS produce an aerosol with or without nicotine that is inhaled and is thought to mimic the use of combustible cigarettes. ENDS evolved from basic E-cigs into a less “cigarette-like” and more customizable product (Figure 1). ENDS include a range of designs and go by various names, including “personal vaporizers,” “e-cigars,” and “e-hookahs” (in this article, we will use the term “ENDS” to refer to these devices).

The general design of ENDS is a plastic tubing system that contains a mouthpiece, battery, electronic heating element (“vaporizer”), and a cartridge with liquid solvent with or without nicotine or flavoring (Figure 2). One draw on the mouthpiece or press of a button activates the device, heats the solution, and delivers a vapor in a similar manner to taking a puff of a cigarette. Although studies have shown that ENDS result in significant increases in plasma nicotine concentrations in 5 minutes,1 the plasma nicotine levels obtained with the first-generation “cigarette-like” ENDS are much lower than those caused by inhaling tobacco smoke.2 Over time nicotine delivery capability has improved as ENDS have evolved such that the rate of nicotine delivery and peak concentration obtained with newer models more closely mirror tobacco cigarettes.3 Whether the rapid delivery of larger amounts of nicotine helps or hinders one’s efforts to break nicotine addiction remains to be determined because of the reinforcing properties of the drug.

The liquid in the E-cig cartridge typically contains not only nicotine but a number of chemical compounds with potentially deleterious or unknown health risks. The 3 main ingredients include:
- a solvent of glycerin and/or propylene glycol
- nicotine in various concentrations
- flavorings.
The glycerin or propylene glycol forms the basis for the aerosol. Nicotine concentrations vary from 0 (denicotinized) to 35 mcg per puff.4 A study reported 7,700 unique flavors available for vaping liquid.5 The liquid also contains impurities, such as anabasine, which has effects on the α-7 nicotinic acetylcholine receptor and its principal use is as an insecticide and β-nicotyrine, which inhibits cytochrome P450 2A.
Epidemiology and end-user perspectives
In 2014, 12.4% of U.S. adults classified themselves as “ever users” of ENDS (used at least once) and 3.7% of adults classified themselves as current users, according to the National Health Interview Study.6 Importantly, among E-cig users who had not used combustible cigarettes, young adults (age 18 to 24) were more likely to have tried ENDS than older adults. ENDS are becoming more popular across the globe. A study in the European Union found that ever users of ENDS most commonly were current cigarette smokers (31%) followed by former (10.8%) and never smokers (2.3%).7
ENDS use is relevant for mental health professionals because of the high rate of comorbid tobacco use disorder in individuals with psychiatric conditions. For example, 2 U.S. population surveys8,9 revealed those with mental health conditions were 1.5 to 2 times more likely to have tried ENDS and 2 to 3 times more likely to be current users. Those with psychiatric illness reported similar reasons for ENDS use as other individuals, including “just because,” use as a smoking cessation aid, ease of use, and perceived safety vs combustible cigarettes.
A recent review that included 9 studies focusing on ENDS use in those with mental illness reported mixed findings on the utility of these devices to reduce or stop use of combustible cigarettes.10 Additionally, it is important to monitor the use of cigarettes and ENDS in patients with psychiatric illness because the byproducts of tobacco smoke can affect the metabolism of some psychotropic medications.11 Although reduced use of combustible cigarettes could lead to lower dosing of some psychotropics, an unreported decrease in combustible cigarette use could lead to supratherapeutic drug levels. There are no data on the effect of ENDS on the metabolism of psychotropics.
ENDS are increasingly popular among adolescents. In 2015, there were an estimated 4.6 million current tobacco users among middle/high school youths in the United States and 3 million current ENDS users, according to the National Youth Tobacco Surveys.12 The shift from combustible cigarettes to ENDS is notable, with an increase in the percentage of current E-cig users and a decrease in the percentage of exclusive combustible cigarette users. In addition, there has been no change in the prevalence of lifetime tobacco users.12 This is a global issue, as reports of ever use of ENDS by adolescents range from 6.5% to 31% in the United States, 14.6% in Canada, and 4.7% to 38.5% in Europe.13 Based on these trends, the U.S. Surgeon General released a statement warning against the use of ENDS in youth because of the lack of safety data and strong association with use of tobacco products.14
There are a number of possible reasons for the increasing popularity of ENDS, including the product’s novelty, lack of regulations regarding their sale, availability of flavorings, and the perception that ENDS are safe alternatives to cigarettes. E-cig–using youths have described ENDS as “not at all harmful” and “not at all addictive” and believe that ENDS with flavoring are less harmful than those without.15 Although studies in adults show some users reporting that ENDS are less satisfying, they are seen as useful in decreasing craving and a safer alternative to cigarettes.16,17
Are ENDS effective for smoking cessation?
The evidence for ENDS as aids to smoking cessation remains murky (Table 118-22). There is a paucity of randomized controlled clinical trials (RCTs) investigating ENDS for smoking cessation or reduction, and it is difficult to quantify the amount of nicotine used in ENDS because of the variety of delivery systems and cartridges. In a recent Cochrane review, those using ENDS to quit smoking were more likely to be abstinent from combustible cigarettes at 6 months vs those using nicotine-free ENDS (relative risk = 2.29; 95% CI, 1.05 to 4.96), but there was no significant difference in quit rates compared with nicotine patches.23 However, the confidence in this finding was rated as low because of the limited number of RCTs. Of note, the authors found 15 ongoing RCTs at the time of publication that might be eligible for later evaluation.

Non-RCTs reveal mixed data. Positive results include 1 study with an odds ratio of 6.07 to quit for intensive ENDS users vs non-users,24 and another with dual users of combustible and electronic cigarettes having a 46% quit rate at 1 year.25 Additionally, in a pilot study providing ENDS to 14 patients with schizophrenia who had no previous desire to quit smoking, authors noted a reduction in the number of cigarettes smoked per day by 50% in one-half of participants and abstinence in 14% of participants at 52 weeks.26 Studies with neutral or negative results include those showing ENDS users to be current combustible tobacco smokers, and use of ENDS not predicting smoking cessation.4,27 Data also are mixed regarding the use of ENDS as a harm reduction strategy. One study found that ENDS decreased cigarette consumption, but did not increase the likelihood of quitting,28 while another reported that daily use of ENDS increased the odds of reducing smoking by as much as 2.5 times compared with non-use of such aids.29 In a 24-month prospective cohort study following tobacco users, there was no difference in the number of cigarettes smoked per day in those who started the trial as users of combustible cigarettes alone vs combustible cigarettes plus ENDS users.30 Interestingly, those who started the study as combustible cigarette users and switched to ENDS and those who had continued dual use throughout the 24 months smoked fewer combustible cigarettes per day than those who never tried ENDS or quit during the study period.
Health effects


To better understand the adverse health effects of ENDS, one must consider potential short- and long-term consequences (Table 2). In the short-term, ENDS have been found to increase markers of inflammation and oxidative stress acutely as evidenced by in vivo laboratory studies.31,32 ENDS also have been linked to upper respiratory irritation, in part, because of the transformation of glycerin in the nicotine cartridge to acrolein upon combustion.33 Even 5 minutes of ad lib E-cig use has been found to significantly increase airflow resistance during pulmonary function tests34—changes that have been shown to precede more persistent alterations in peak expiratory flow, such as those seen in chronic obstructive pulmonary disease. The more common patient-reported side effects include:
- daytime cough (27%)
- phlegm production (25%)
- headache (21%)
- dry mouth/throat (20%)
- vertigo, headache, or nausea (9%).35,36
A RCT investigating efficacy of E-cigs vs nicotine patches vs denicotinized E-cigs found no difference among the groups in the number of reported adverse events.18 Interestingly, another RCT found a decrease in adverse events, such as dry cough, mouth irritation, throat irritation, shortness of breath, and headache, compared with baseline in combustible cigarette smokers who used regular or denicotinized E-cigs.19
Although no studies have directly investigated long-term health consequences of ENDS because of their relative novelty, one can extrapolate potential harmful long-term effects based on knowledge of the products’ chemical constituents. For example, propylene glycol can degrade into propylene oxide, a class 2B carcinogen.37 Other potential carcinogens in the aerosol include formaldehyde and acetaldehyde. On a broader scale, many of the particulates have been shown to cause systemic inflammation, which is thought to increase cardiovascular and respiratory disease and death.38 Flavorings in ENDS include a variety of components including, but not limited to, aldehydes, which are irritants, and other additives that have been associated with respiratory disease.39
Second-hand exposure. There are no long-term studies of second-hand vapor exposure, but similar to long-term health on primary users, one can glean some observations from the literature. It is promising that compared with cigarettes, ENDS lack sidestream smoke and the vapor has not been found to contain carbon monoxide.40 Some research has demonstrated that the size and spray of fine particles in the aerosol is as large or larger than combustible cigarettes.41 Formaldehyde, acetaldehyde, isoprene, and acetic acid have been found in ENDS vapor.40 Interestingly, a simulated café study found elevated nicotine, glycerine, hydrocarbon, and other materials classified as carcinogens in the air.42
Although it is popularly thought that ENDS are less toxic than tobacco cigarettes, there is not enough evidence to estimate precisely as to how much less toxic or the consequences of use. ENDS are increasingly popular and are being used by never smokers who should be educated on the potential harm that ENDS pose.
Recommendations from agencies and medical organizations
The World Health Organization (WHO) recommended prohibiting the use of ENDS in indoor spaces to minimize potential health risks to users and non-users. The WHO also aims to prevent dissemination of unproven health claims, including claims that ENDS are effective—or not—or that the devices are innocuous.36 In the United States, the FDA has stated that ENDS are not recommended for safe quitting (2009). In August 2016, the FDA introduced regulations banning the sale of ENDS to individuals age <18 and required manufacturers to submit documents detailing all ingredients for review and possible approval.
The American Lung Association has stated its concerns about the use of ENDS but has not made any direct recommendations. The American Heart Association reports a potential negative public health impact and provides clinical guideline recommendations.43 Prominent psychiatric organizations such as the American Psychiatric Association, American Academy of Addiction Psychiatry (AAAP), the Substance Abuse and Mental Health Services Administration (SAMHSA), and the National Institute of Drug Abuse do not have official statements supporting or rejecting the use of ENDS. However, they do note the potential harm and lack of substantial evidence for efficacy of ENDS as a smoking cessation tool, and the AAAP and SAMHSA state that they will work with regulatory agencies to reduce the use of toxic products with addictive potential including ENDS.44-46
Clinical recommendations
We do not recommend ENDS as a first-line treatment for smoking cessation because there is no evidence they are superior to the FDA-approved nicotine replacement therapies (NRTs), the paucity of research into the potential short- and long-term health risks of ENDS, and the fact that these products are not regulated for use as smoking cessation aids. It is, however, advisable to discuss ENDS use with patients by:
- asking if they are using the products
- assessing whether the user also is a smoker
- advising the patient to quit.
It also is important to assess the patient’s knowledge and attitudes regarding ENDS use and provide education about the products. Some patients firmly believe that ENDS are the lesser of 2 evils, and they are decreasing the harms of smoking by using these devices. While the debate over a potential harm reduction strategy unfolds,47 we think that because of the state of the evidence it is prudent to adopt a more precautionary stance and recommend that patients work toward abstinence from nicotine in any form.
For dual tobacco/ENDS users and for patients using ENDS who want to quit smoking, we recommend treatment with an approved pharmacotherapy (ie, NRTs, bupropion, and varenicline) combined with counseling. A 2013 Cochrane Review found that all pharamacotherapy options are more effective than placebo, and combination NRT and varenicline are superior to single NRT or bupropion (Box).23,48

1. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175-179.
2. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86.
3. St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535-544.
4. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-1986.
5. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3-iii9. doi: 10.1136/tobaccocontrol-2014-051670.
6. Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015;(217):1-8.
7. Farsalinos KE, Poulas K, Voudris V, et al. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction. 2016;111(11):2032-2040.
8. Cummins SE, Zhu SH, Tedeschi GJ, et al. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2015;23(suppl 3):iii48-iii53. doi: 10.1136/tobaccocontrol-2013-051511.
9. Spears CA, Jones DM, Weaver SR, et al. Use of electronic nicotine delivery systems among adults with mental health conditions, 2015. Int J Environ Res Public Heal. 2017;14(1):10.
10. Hefner K, Valentine G, Sofuoglu M. Electronic cigarettes and mental illness: reviewing the evidence for help and harm among those with psychiatric and substance use disorders [published online February 2, 2017]. Am J Addict. doi: 10.1111/ajad.12504.
11. Anthenelli R. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
12. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361-367.
13. Greenhill R, Dawkins L, Notley C, et al. Adolescent awareness and use of electronic cigarettes: a review of emerging trends and findings. J Adolesc Heal. 2016;59(6):612-619.
14. U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016.
15. Cooper M, Harrell MB, Pérez A, et al. Flavorings and perceived harm and addictiveness of e-cigarettes among youth. Tob Regul Sci. 2016;2(3):278-289.
16. Kim H, Davis AH, Dohack JL, et al. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob Res. 2017;19(2):190-196.
17. Czoli CD, Fong GT, Mays D, et al. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control. 2017;26(e1):e49-e58.
18. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629-1637.
19. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317.
20. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220-11248.
21. Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937-1943.
22. Meier E, Wahlquist AE, Heckman BW, et al. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine Tob Res. 2017;19(2):176-182.
23. Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation [published online September 14, 2016]. Cochrane Database Syst Rev. 2016;9:CD010216.
24. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2014;17(2):127-133.
25. Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491-494.
26. Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446-461.
27. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923-930.
28. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: International Tobacco Control Four-Country Survey. Am J Prev Med. 2013;44(3):207-215.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160-1168.
30. Manzoli L, Flacco ME, Ferrante M, et al; ISLESE Working Group. Cohort study of electronic cigarette use: effectiveness and safety at 24 months [published online June 6, 2016]. Tob Control. doi: 10.1136/tobaccocontrol-2015-052822.
31. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732.
32. Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861.
33. US Environmental Protection Agency. Acrolein. https://www.epa.gov/sites/production/files/2016-08/documents/acrolein.pdf. Updated September 2009. Accessed April 7, 2017.
34. Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400-1406.
35. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231.
36. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32(2):133-140.
37. Laino T, Tuma C, Moor P, et al. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116(18):4602-4609.
38. Brook RD, Rajagopalan S, Pope CA 3rd, et al; American Heart Association Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331-2378.
39. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493-2494.
40. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25-31.
41. Fuoco FC, Buonanno G, Stabile L, et al. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523-529.
42. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628-637.
43. Bhatnagar A, Whitsel L, Ribisl K, et al; American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418-1436.
44. E-cigarettes pose risks. SAMHSA News. https://www.samhsa.gov/samhsaNewsLetter/Volume_22_Number_3/e_cigarettes. Published 2014. Accessed April 7, 2017.
45. National Institute on Drug Abuse. Electronic cigarettes (e-cigarettes). https://www.drugabuse.gov/publications/drugfacts/electronic-cigarettes-e-cigarettes. Revised May 2016. Accessed April 7, 2017.
46. American Academy of Addiction Psychiatry. Nicotine dependence. East Providence, RI: American Academy of Addition Psychiatry; 2015.
47. Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes — will England reframe the debate. N Engl J Med. 2016;374(14):1301-1303.
48. Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193-194.
1. Hajek P, Goniewicz ML, Phillips A, et al. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res. 2015;17(2):175-179.
2. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67-86.
3. St Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535-544.
4. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-1986.
5. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(suppl 3):iii3-iii9. doi: 10.1136/tobaccocontrol-2014-051670.
6. Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief. 2015;(217):1-8.
7. Farsalinos KE, Poulas K, Voudris V, et al. Electronic cigarette use in the European Union: analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction. 2016;111(11):2032-2040.
8. Cummins SE, Zhu SH, Tedeschi GJ, et al. Use of e-cigarettes by individuals with mental health conditions. Tob Control. 2015;23(suppl 3):iii48-iii53. doi: 10.1136/tobaccocontrol-2013-051511.
9. Spears CA, Jones DM, Weaver SR, et al. Use of electronic nicotine delivery systems among adults with mental health conditions, 2015. Int J Environ Res Public Heal. 2017;14(1):10.
10. Hefner K, Valentine G, Sofuoglu M. Electronic cigarettes and mental illness: reviewing the evidence for help and harm among those with psychiatric and substance use disorders [published online February 2, 2017]. Am J Addict. doi: 10.1111/ajad.12504.
11. Anthenelli R. How—and why—to help psychiatric patients stop smoking. Current Psychiatry. 2005;4(1):77-87.
12. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011-2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361-367.
13. Greenhill R, Dawkins L, Notley C, et al. Adolescent awareness and use of electronic cigarettes: a review of emerging trends and findings. J Adolesc Heal. 2016;59(6):612-619.
14. U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2016.
15. Cooper M, Harrell MB, Pérez A, et al. Flavorings and perceived harm and addictiveness of e-cigarettes among youth. Tob Regul Sci. 2016;2(3):278-289.
16. Kim H, Davis AH, Dohack JL, et al. E-cigarettes use behavior and experience of adults: qualitative research findings to inform e-cigarette use measure development. Nicotine Tob Res. 2017;19(2):190-196.
17. Czoli CD, Fong GT, Mays D, et al. How do consumers perceive differences in risk across nicotine products? A review of relative risk perceptions across smokeless tobacco, e-cigarettes, nicotine replacement therapy and combustible cigarettes. Tob Control. 2017;26(e1):e49-e58.
18. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629-1637.
19. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317.
20. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220-11248.
21. Tseng TY, Ostroff JS, Campo A, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tob Res. 2016;18(10):1937-1943.
22. Meier E, Wahlquist AE, Heckman BW, et al. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine Tob Res. 2017;19(2):176-182.
23. Hartmann-Boyce J, McRobbie H, Bullen C, et al. Electronic cigarettes for smoking cessation [published online September 14, 2016]. Cochrane Database Syst Rev. 2016;9:CD010216.
24. Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2014;17(2):127-133.
25. Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491-494.
26. Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446-461.
27. Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923-930.
28. Adkison SE, O’Connor RJ, Bansal-Travers M, et al. Electronic nicotine delivery systems: International Tobacco Control Four-Country Survey. Am J Prev Med. 2013;44(3):207-215.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160-1168.
30. Manzoli L, Flacco ME, Ferrante M, et al; ISLESE Working Group. Cohort study of electronic cigarette use: effectiveness and safety at 24 months [published online June 6, 2016]. Tob Control. doi: 10.1136/tobaccocontrol-2015-052822.
31. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and E-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732. doi: 10.1371/journal.pone.0116732.
32. Sussan TE, Gajghate S, Thimmulappa RK, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10(2):e0116861. doi: 10.1371/journal.pone.0116861.
33. US Environmental Protection Agency. Acrolein. https://www.epa.gov/sites/production/files/2016-08/documents/acrolein.pdf. Updated September 2009. Accessed April 7, 2017.
34. Vardavas CI, Anagnostopoulos N, Kougias M, et al. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400-1406.
35. Etter JF. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231.
36. Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an internet survey. Drug Alcohol Rev. 2013;32(2):133-140.
37. Laino T, Tuma C, Moor P, et al. Mechanisms of propylene glycol and triacetin pyrolysis. J Phys Chem A. 2012;116(18):4602-4609.
38. Brook RD, Rajagopalan S, Pope CA 3rd, et al; American Heart Association Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331-2378.
39. Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493-2494.
40. Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air. 2013;23(1):25-31.
41. Fuoco FC, Buonanno G, Stabile L, et al. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523-529.
42. Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628-637.
43. Bhatnagar A, Whitsel L, Ribisl K, et al; American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130(16):1418-1436.
44. E-cigarettes pose risks. SAMHSA News. https://www.samhsa.gov/samhsaNewsLetter/Volume_22_Number_3/e_cigarettes. Published 2014. Accessed April 7, 2017.
45. National Institute on Drug Abuse. Electronic cigarettes (e-cigarettes). https://www.drugabuse.gov/publications/drugfacts/electronic-cigarettes-e-cigarettes. Revised May 2016. Accessed April 7, 2017.
46. American Academy of Addiction Psychiatry. Nicotine dependence. East Providence, RI: American Academy of Addition Psychiatry; 2015.
47. Green SH, Bayer R, Fairchild AL. Evidence, policy, and e-cigarettes — will England reframe the debate. N Engl J Med. 2016;374(14):1301-1303.
48. Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193-194.
Psychosis in borderline personality disorder: How assessment and treatment differs from a psychotic disorder
Psychotic symptoms in patients with borderline personality disorder (BPD) are common, distressing to patients, and challenging to treat. Issues of comorbidities and misdiagnoses in BPD patients further complicate matters and could lead to iatrogenic harm. The dissociation that patients with BPD experience could be confused with psychosis and exacerbate treatment and diagnostic confusion. Furthermore, BPD patients with unstable identity and who are sensitive to rejection could present in a bizarre, disorganized, or agitated manner when under stress.
Although pitfalls occur when managing psychotic symptoms in patients with BPD, there are trends and clues to help clinicians navigate diagnostic and treatment challenges. This article will review the literature, propose how to distinguish psychotic symptoms in BPD from those in primary psychotic disorders such as schizophrenia, and explore reasonable treatment options.
The scope of the problem
The DSM-5 criteria for BPD states that “during periods of extreme stress, transient paranoid ideation or dissociative symptoms may occur.”1 The term “borderline” originated from the idea that symptoms bordered on the intersection of neurosis and psychosis.2 However, psychotic symptoms in BPD are more varied and frequent than what DSM-5 criteria suggests.
The prevalence of psychotic symptoms in patients with BPD has been estimated between 20% to 50%.3 There also is evidence of frequent auditory and visual hallucinations in patients with BPD, and a recent study using structured psychiatric interviews demonstrated that most BPD patients report at least 1 symptom of psychosis.4 Considering that psychiatric comorbidities are the rule rather than the exception in BPD, the presence of psychotic symptoms further complicates the diagnostic picture. Recognizing the symptoms of BPD is essential for understanding the course of the symptoms and predicting response to treatment.5
Treatment of BPD is strikingly different than that of a primary psychotic disorder. There is some evidence that low-dosage antipsychotics could ease mood instability and perceptual disturbances in patients with BPD.6 Antipsychotic dosages used to treat hallucinations and delusions in a primary psychotic disorder are unlikely to be as effective for a patient with BPD, and are associated with significant adverse effects. Furthermore, these adverse effects—such as weight gain, hyperlipidemia, and diabetes—could become new sources of distress. Clinicians also might miss an opportunity to engage a BPD patient in psychotherapy if the focus is on the anticipated effect of a medication. The mainstay treatment of BPD is an evidence-based psychotherapy, such as dialectical behavioral therapy, transference-focused psychotherapy, mentalization-based therapy, or good psychiatric management.7
CASE Hallucinations during times of stress
Ms. K, a 20-year-old single college student, presents to the psychiatric emergency room with worsening mood swings, anxiety, and hallucinations. Her mood swings are brief and intense, lasting minutes to hours. Anxiety often is triggered by feelings of emptiness and fear of abandonment. She describes herself as a “social chameleon” and notes that she changes how she behaves depending on who she spends time with.
She often hears the voice of her ex-boyfriend instructing her to kill herself and saying that she is a “terrible person.” Their relationship was intense, with many break-ups and reunions. She also reports feeling disconnected from herself at times as though she is being controlled by an outside entity. To relieve her emotional suffering, she cuts herself superficially. Although she has no family history of psychiatric illness, she fears that she may have schizophrenia.
Ms. K’s outpatient psychiatrist prescribes antipsychotics at escalating dosages over a few months (she now takes olanzapine, 40 mg/d, aripiprazole, 30 mg/d, clonazepam, 3 mg/d, and escitalopram, 30 mg/d), but the hallucinations remain. These symptoms worsen during stressful situations, and she notices that they almost are constant as she studies for final exams, prompting her psychiatrist to discuss a clozapine trial. Ms. K is not in psychotherapy, and recognizes that she does not deal with stress well. Despite her symptoms, she is organized in her thought process, has excellent grooming and hygiene, has many social connections, and performs well in school.
How does one approach a patient such as Ms. K?
A chief concern of hallucinations, particularly in a young adult at an age when psychotic disorders such as schizophrenia often emerge, can contribute to a diagnostic quandary. What evidence can guide the clinician? There are some key features to consider:
- Her “mood swings” are notable in their intensity and brevity, making a primary mood disorder with psychotic features less likely.
- Hallucinations are present in the absence of a prodromal period of functional decline or negative symptoms, making a primary psychotic disorder less likely.
- She does not have a family history of psychiatric illness, particularly a primary psychotic disorder.
- She maintains social connections, although her relationships are intense and tumultuous.
- Psychotic symptoms have not changed with higher dosages of antipsychotics.
- Complaints of feeling “disconnected from herself” and “empty” are common symptoms of BPD and necessitate further exploration.
- Psychotic symptoms are largely transient and stress-related, with an overwhelmingly negative tone.
- Techniques that individuals with schizophrenia use, such as distraction or trying to tune out voices, are not being employed. Instead, Ms. K attends to the voices and is anxiously focused on them.
- The relationship of her symptoms to interpersonal stress is key.
When evaluating a patient such as Ms. K, it is important to explore both the nature and timing of the psychotic symptoms and any other related psychiatric symptoms. This helps to determine a less ambiguous diagnosis and clearer treatment plan. Understanding the patient’s perspective about the psychotic symptoms also is useful to gauge the patient’s level of distress and her impression of what the symptoms mean.
Diagnostic considerations
BPD is characterized by a chaotic emotional climate with impulsivity and instability of self-image, affect, and relationships. Most BPD symptoms, including psychosis, often are exacerbated by the perception of abandonment or rejection and other interpersonal stressors.1 Both BPD and schizophrenia are estimated to affect at least 1% of the general population.8,9 Patients with BPD frequently meet criteria for comorbid mental illnesses, including major depressive disorder, substance use disorder, posttraumatic stress disorder, anxiety, and eating disorders.10 Because psychotic symptoms can present in some of these disorders, the context and time course of these symptoms are crucial to consider.
Misdiagnosis is common with BPD, and patients can receive the wrong treatment for years before BPD is considered, likely because of the stigma surrounding the diagnosis.5 One also must keep in mind that, although rare, a patient can have both BPD and a primary psychotic disorder.11 Although a patient with schizophrenia could be prone to social isolation because of delusions or paranoia, BPD patients are more apt to experience intense interpersonal relationships driven by the need to avoid abandonment. Manipulation, anger, and neediness in relationships with both peers and health care providers are common—stark contrasts to typical negative symptoms, blunted affect, and a lack of social drive characteristic of schizophrenia.12
Distinguishing between psychosis in BPD and a psychotic disorder
Studies have sought to explore the quality of psychotic symptoms in BPD vs primary psychotic disorders, which can be challenging to differentiate (Table 1). Some have found that transient symptoms, such as non-delusional paranoia, are more prevalent in BPD, and “true” psychotic symptoms that are long-lasting and bizarre are indicative of schizophrenia.13,14 Also, there is evidence that the lower levels of interpersonal functioning often found in BPD are predictive of psychotic symptoms in that disorder but not in schizophrenia.15

Auditory hallucinations in patients with BPD predominantly are negative and critical in tone.4 However, there is no consistent evidence that the quality of auditory hallucinations in BPD vs schizophrenia is different in any meaningful way.16 Because of the frequency of dissociative symptoms in BPD, it is likely that clinicians could misinterpret these symptoms to indicate disorganized behavior associated with a primary psychotic disorder. In one study, 50% of individuals with BPD experienced auditory hallucinations.11 Differentiating between “internal” or “external” voices did not help to clarify the diagnosis, and paranoid delusions occurred in less than one-third of patients with BPD, but in approximately two-third of those with a diagnosis of schizophrenia.
The McLean Study of Adult Development, a longitudinal study of BPD patients, found that the prevalence of psychotic symptoms diminished over time. It is unclear whether this was due to the spontaneus remission rate of BPD symptoms in general or because of effective treatment.13
Psychotic symptoms in BPD seem to react to stress and increase in intensity when patients are in crisis.17 Nonetheless, because of the prevalence of psychosis in BPD patients and the distress it causes, clinicians should be cautioned against using terms that imply that the symptoms are not “true” or “real.”3
Treatment recommendations
When considering pharmacologic management of psychotic symptoms in BPD, aim to limit antipsychotic medications to low dosages because of adverse effects and the limited evidence that escalating dosages—and especially using >1 antipsychotic concurrently—are more effective.18 Educate patients that in BPD medications are, at best, considered adjunctive treatments. Blaming psychotic symptoms on a purely biological process in BPD, not only is harmful because medications are unlikely to significantly or consistently help, but also because they can undermine patient autonomy and reinforce the need for an outside entity (ie, medication) to fix their problems.
When treatment is ineffective and symptoms do not improve, a patient with BPD likely will experience mounting distress. This, in turn, could exacerbate impulsive, suicidal, and self-injurious behaviors. Emphasize psychotherapy, particularly for those whose psychotic symptoms are transient, stress-related, and present during acute crises (Table 2). With evidence-based psychotherapy, BPD patients can become active participants in treatment, coupling developing insight with concrete skills and teachable principles. This leads to increased interpersonal effectiveness and resilience during times of stress. Challenging the patient’s psychotic symptoms as false or “made up” rarely is helpful and usually harmful, leading to the possible severance of the therapeutic alliance.3

Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) could look similar to those in primary psychotic disorders. Factors suggesting BPD include a pattern of worsening psychotic symptoms during stress, long-term symptom instability, lack of delusions, presence of dissociation, and nonresponse to antipsychotics. Although low-dosage antipsychotics could provide some relief of psychotic symptoms in a patient with BPD, they often are not consistently effective and frequently lead to adverse effects. Emphasize evidence-based psychotherapies.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern A. Borderline group of neuroses. The Psychoanalytic Quarterly. 1938;7:467-489.
3. Schroeder K, Fisher HL, Schäfer I, et al. Psychotic symptoms in patients with borderline personality disorder: prevalence and clinical management. Curr Opin Psychiatry. 2013;26(1):113-119.
4. Pearse LJ, Dibben C, Ziauddeen H, et al. A study of psychotic symptoms in borderline personality disorder. J Nerv Ment Dis. 2014;202(5):368-371.
5. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-
6. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29(5):461-467.
7. National Education Alliance for Borderline Personality Disorder. Treatments for BPD. http://www.borderlinepersonalitydisorder.com/what-is-bpd/treating-bpd. Accessed September 1, 2016.
8. Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50(2):85-94.
9. Lenzenweger MF, Lane MC, Loranger AW, et al. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62(6):553-564.
10. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155(12):1733-1739.
11. Kingdon DG, Ashcroft K, Bhandari B, et al. Schizophrenia and borderline personality disorder: similarities and differences in the experience of auditory hallucinations, paranoia, and childhood trauma. J Nerv Ment Dis. 2010;198(6):399-403.
12. Gunderson JG. Borderline personality disorder. Washington, DC: American Psychiatric Press; 1984.
13. Zanarini MC, Frankenburg FR, Wedig MM, et al. Cognitive experiences reported by patients with borderline personality disorder and Axis II comparison subjects: a 16-year prospective follow-up study. Am J Psychiatry. 2013;170(6):671-679.
14. Tschoeke S, Steinert T, Flammer E, et al. Similarities and differences in borderline personality disorder and schizophrenia with voice hearing. J Nerv Ment Dis. 2014;202(7):544-549.
15. Oliva F, Dalmotto M, Pirfo E, et al. A comparison of thought and perception disorders in borderline personality disorder and schizophrenia: psychotic experiences as a reaction to impaired social functioning. BMC Psychiatry. 2014;14:239.
16. Merrett Z, Rossell SL, Castle DJ, et al. Comparing the experience of voices in borderline personality disorder with the experience of voices in a psychotic disorder: a systematic review. Aust N Z J Psychiatry. 2016;50(7):640-648.
17. Glaser JP, Van Os J, Thewissen V, et al. Psychotic reactivity in borderline personality disorder. Acta Psychiatr Scand. 2010;121(2):125-134.
18. Rosenbluth M, Sinyor M. Off-label use of atypical antipsychotics in personality disorders. Expert Opin Pharmacother. 2012;13(11):1575-1585.
Psychotic symptoms in patients with borderline personality disorder (BPD) are common, distressing to patients, and challenging to treat. Issues of comorbidities and misdiagnoses in BPD patients further complicate matters and could lead to iatrogenic harm. The dissociation that patients with BPD experience could be confused with psychosis and exacerbate treatment and diagnostic confusion. Furthermore, BPD patients with unstable identity and who are sensitive to rejection could present in a bizarre, disorganized, or agitated manner when under stress.
Although pitfalls occur when managing psychotic symptoms in patients with BPD, there are trends and clues to help clinicians navigate diagnostic and treatment challenges. This article will review the literature, propose how to distinguish psychotic symptoms in BPD from those in primary psychotic disorders such as schizophrenia, and explore reasonable treatment options.
The scope of the problem
The DSM-5 criteria for BPD states that “during periods of extreme stress, transient paranoid ideation or dissociative symptoms may occur.”1 The term “borderline” originated from the idea that symptoms bordered on the intersection of neurosis and psychosis.2 However, psychotic symptoms in BPD are more varied and frequent than what DSM-5 criteria suggests.
The prevalence of psychotic symptoms in patients with BPD has been estimated between 20% to 50%.3 There also is evidence of frequent auditory and visual hallucinations in patients with BPD, and a recent study using structured psychiatric interviews demonstrated that most BPD patients report at least 1 symptom of psychosis.4 Considering that psychiatric comorbidities are the rule rather than the exception in BPD, the presence of psychotic symptoms further complicates the diagnostic picture. Recognizing the symptoms of BPD is essential for understanding the course of the symptoms and predicting response to treatment.5
Treatment of BPD is strikingly different than that of a primary psychotic disorder. There is some evidence that low-dosage antipsychotics could ease mood instability and perceptual disturbances in patients with BPD.6 Antipsychotic dosages used to treat hallucinations and delusions in a primary psychotic disorder are unlikely to be as effective for a patient with BPD, and are associated with significant adverse effects. Furthermore, these adverse effects—such as weight gain, hyperlipidemia, and diabetes—could become new sources of distress. Clinicians also might miss an opportunity to engage a BPD patient in psychotherapy if the focus is on the anticipated effect of a medication. The mainstay treatment of BPD is an evidence-based psychotherapy, such as dialectical behavioral therapy, transference-focused psychotherapy, mentalization-based therapy, or good psychiatric management.7
CASE Hallucinations during times of stress
Ms. K, a 20-year-old single college student, presents to the psychiatric emergency room with worsening mood swings, anxiety, and hallucinations. Her mood swings are brief and intense, lasting minutes to hours. Anxiety often is triggered by feelings of emptiness and fear of abandonment. She describes herself as a “social chameleon” and notes that she changes how she behaves depending on who she spends time with.
She often hears the voice of her ex-boyfriend instructing her to kill herself and saying that she is a “terrible person.” Their relationship was intense, with many break-ups and reunions. She also reports feeling disconnected from herself at times as though she is being controlled by an outside entity. To relieve her emotional suffering, she cuts herself superficially. Although she has no family history of psychiatric illness, she fears that she may have schizophrenia.
Ms. K’s outpatient psychiatrist prescribes antipsychotics at escalating dosages over a few months (she now takes olanzapine, 40 mg/d, aripiprazole, 30 mg/d, clonazepam, 3 mg/d, and escitalopram, 30 mg/d), but the hallucinations remain. These symptoms worsen during stressful situations, and she notices that they almost are constant as she studies for final exams, prompting her psychiatrist to discuss a clozapine trial. Ms. K is not in psychotherapy, and recognizes that she does not deal with stress well. Despite her symptoms, she is organized in her thought process, has excellent grooming and hygiene, has many social connections, and performs well in school.
How does one approach a patient such as Ms. K?
A chief concern of hallucinations, particularly in a young adult at an age when psychotic disorders such as schizophrenia often emerge, can contribute to a diagnostic quandary. What evidence can guide the clinician? There are some key features to consider:
- Her “mood swings” are notable in their intensity and brevity, making a primary mood disorder with psychotic features less likely.
- Hallucinations are present in the absence of a prodromal period of functional decline or negative symptoms, making a primary psychotic disorder less likely.
- She does not have a family history of psychiatric illness, particularly a primary psychotic disorder.
- She maintains social connections, although her relationships are intense and tumultuous.
- Psychotic symptoms have not changed with higher dosages of antipsychotics.
- Complaints of feeling “disconnected from herself” and “empty” are common symptoms of BPD and necessitate further exploration.
- Psychotic symptoms are largely transient and stress-related, with an overwhelmingly negative tone.
- Techniques that individuals with schizophrenia use, such as distraction or trying to tune out voices, are not being employed. Instead, Ms. K attends to the voices and is anxiously focused on them.
- The relationship of her symptoms to interpersonal stress is key.
When evaluating a patient such as Ms. K, it is important to explore both the nature and timing of the psychotic symptoms and any other related psychiatric symptoms. This helps to determine a less ambiguous diagnosis and clearer treatment plan. Understanding the patient’s perspective about the psychotic symptoms also is useful to gauge the patient’s level of distress and her impression of what the symptoms mean.
Diagnostic considerations
BPD is characterized by a chaotic emotional climate with impulsivity and instability of self-image, affect, and relationships. Most BPD symptoms, including psychosis, often are exacerbated by the perception of abandonment or rejection and other interpersonal stressors.1 Both BPD and schizophrenia are estimated to affect at least 1% of the general population.8,9 Patients with BPD frequently meet criteria for comorbid mental illnesses, including major depressive disorder, substance use disorder, posttraumatic stress disorder, anxiety, and eating disorders.10 Because psychotic symptoms can present in some of these disorders, the context and time course of these symptoms are crucial to consider.
Misdiagnosis is common with BPD, and patients can receive the wrong treatment for years before BPD is considered, likely because of the stigma surrounding the diagnosis.5 One also must keep in mind that, although rare, a patient can have both BPD and a primary psychotic disorder.11 Although a patient with schizophrenia could be prone to social isolation because of delusions or paranoia, BPD patients are more apt to experience intense interpersonal relationships driven by the need to avoid abandonment. Manipulation, anger, and neediness in relationships with both peers and health care providers are common—stark contrasts to typical negative symptoms, blunted affect, and a lack of social drive characteristic of schizophrenia.12
Distinguishing between psychosis in BPD and a psychotic disorder
Studies have sought to explore the quality of psychotic symptoms in BPD vs primary psychotic disorders, which can be challenging to differentiate (Table 1). Some have found that transient symptoms, such as non-delusional paranoia, are more prevalent in BPD, and “true” psychotic symptoms that are long-lasting and bizarre are indicative of schizophrenia.13,14 Also, there is evidence that the lower levels of interpersonal functioning often found in BPD are predictive of psychotic symptoms in that disorder but not in schizophrenia.15

Auditory hallucinations in patients with BPD predominantly are negative and critical in tone.4 However, there is no consistent evidence that the quality of auditory hallucinations in BPD vs schizophrenia is different in any meaningful way.16 Because of the frequency of dissociative symptoms in BPD, it is likely that clinicians could misinterpret these symptoms to indicate disorganized behavior associated with a primary psychotic disorder. In one study, 50% of individuals with BPD experienced auditory hallucinations.11 Differentiating between “internal” or “external” voices did not help to clarify the diagnosis, and paranoid delusions occurred in less than one-third of patients with BPD, but in approximately two-third of those with a diagnosis of schizophrenia.
The McLean Study of Adult Development, a longitudinal study of BPD patients, found that the prevalence of psychotic symptoms diminished over time. It is unclear whether this was due to the spontaneus remission rate of BPD symptoms in general or because of effective treatment.13
Psychotic symptoms in BPD seem to react to stress and increase in intensity when patients are in crisis.17 Nonetheless, because of the prevalence of psychosis in BPD patients and the distress it causes, clinicians should be cautioned against using terms that imply that the symptoms are not “true” or “real.”3
Treatment recommendations
When considering pharmacologic management of psychotic symptoms in BPD, aim to limit antipsychotic medications to low dosages because of adverse effects and the limited evidence that escalating dosages—and especially using >1 antipsychotic concurrently—are more effective.18 Educate patients that in BPD medications are, at best, considered adjunctive treatments. Blaming psychotic symptoms on a purely biological process in BPD, not only is harmful because medications are unlikely to significantly or consistently help, but also because they can undermine patient autonomy and reinforce the need for an outside entity (ie, medication) to fix their problems.
When treatment is ineffective and symptoms do not improve, a patient with BPD likely will experience mounting distress. This, in turn, could exacerbate impulsive, suicidal, and self-injurious behaviors. Emphasize psychotherapy, particularly for those whose psychotic symptoms are transient, stress-related, and present during acute crises (Table 2). With evidence-based psychotherapy, BPD patients can become active participants in treatment, coupling developing insight with concrete skills and teachable principles. This leads to increased interpersonal effectiveness and resilience during times of stress. Challenging the patient’s psychotic symptoms as false or “made up” rarely is helpful and usually harmful, leading to the possible severance of the therapeutic alliance.3

Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) could look similar to those in primary psychotic disorders. Factors suggesting BPD include a pattern of worsening psychotic symptoms during stress, long-term symptom instability, lack of delusions, presence of dissociation, and nonresponse to antipsychotics. Although low-dosage antipsychotics could provide some relief of psychotic symptoms in a patient with BPD, they often are not consistently effective and frequently lead to adverse effects. Emphasize evidence-based psychotherapies.
Psychotic symptoms in patients with borderline personality disorder (BPD) are common, distressing to patients, and challenging to treat. Issues of comorbidities and misdiagnoses in BPD patients further complicate matters and could lead to iatrogenic harm. The dissociation that patients with BPD experience could be confused with psychosis and exacerbate treatment and diagnostic confusion. Furthermore, BPD patients with unstable identity and who are sensitive to rejection could present in a bizarre, disorganized, or agitated manner when under stress.
Although pitfalls occur when managing psychotic symptoms in patients with BPD, there are trends and clues to help clinicians navigate diagnostic and treatment challenges. This article will review the literature, propose how to distinguish psychotic symptoms in BPD from those in primary psychotic disorders such as schizophrenia, and explore reasonable treatment options.
The scope of the problem
The DSM-5 criteria for BPD states that “during periods of extreme stress, transient paranoid ideation or dissociative symptoms may occur.”1 The term “borderline” originated from the idea that symptoms bordered on the intersection of neurosis and psychosis.2 However, psychotic symptoms in BPD are more varied and frequent than what DSM-5 criteria suggests.
The prevalence of psychotic symptoms in patients with BPD has been estimated between 20% to 50%.3 There also is evidence of frequent auditory and visual hallucinations in patients with BPD, and a recent study using structured psychiatric interviews demonstrated that most BPD patients report at least 1 symptom of psychosis.4 Considering that psychiatric comorbidities are the rule rather than the exception in BPD, the presence of psychotic symptoms further complicates the diagnostic picture. Recognizing the symptoms of BPD is essential for understanding the course of the symptoms and predicting response to treatment.5
Treatment of BPD is strikingly different than that of a primary psychotic disorder. There is some evidence that low-dosage antipsychotics could ease mood instability and perceptual disturbances in patients with BPD.6 Antipsychotic dosages used to treat hallucinations and delusions in a primary psychotic disorder are unlikely to be as effective for a patient with BPD, and are associated with significant adverse effects. Furthermore, these adverse effects—such as weight gain, hyperlipidemia, and diabetes—could become new sources of distress. Clinicians also might miss an opportunity to engage a BPD patient in psychotherapy if the focus is on the anticipated effect of a medication. The mainstay treatment of BPD is an evidence-based psychotherapy, such as dialectical behavioral therapy, transference-focused psychotherapy, mentalization-based therapy, or good psychiatric management.7
CASE Hallucinations during times of stress
Ms. K, a 20-year-old single college student, presents to the psychiatric emergency room with worsening mood swings, anxiety, and hallucinations. Her mood swings are brief and intense, lasting minutes to hours. Anxiety often is triggered by feelings of emptiness and fear of abandonment. She describes herself as a “social chameleon” and notes that she changes how she behaves depending on who she spends time with.
She often hears the voice of her ex-boyfriend instructing her to kill herself and saying that she is a “terrible person.” Their relationship was intense, with many break-ups and reunions. She also reports feeling disconnected from herself at times as though she is being controlled by an outside entity. To relieve her emotional suffering, she cuts herself superficially. Although she has no family history of psychiatric illness, she fears that she may have schizophrenia.
Ms. K’s outpatient psychiatrist prescribes antipsychotics at escalating dosages over a few months (she now takes olanzapine, 40 mg/d, aripiprazole, 30 mg/d, clonazepam, 3 mg/d, and escitalopram, 30 mg/d), but the hallucinations remain. These symptoms worsen during stressful situations, and she notices that they almost are constant as she studies for final exams, prompting her psychiatrist to discuss a clozapine trial. Ms. K is not in psychotherapy, and recognizes that she does not deal with stress well. Despite her symptoms, she is organized in her thought process, has excellent grooming and hygiene, has many social connections, and performs well in school.
How does one approach a patient such as Ms. K?
A chief concern of hallucinations, particularly in a young adult at an age when psychotic disorders such as schizophrenia often emerge, can contribute to a diagnostic quandary. What evidence can guide the clinician? There are some key features to consider:
- Her “mood swings” are notable in their intensity and brevity, making a primary mood disorder with psychotic features less likely.
- Hallucinations are present in the absence of a prodromal period of functional decline or negative symptoms, making a primary psychotic disorder less likely.
- She does not have a family history of psychiatric illness, particularly a primary psychotic disorder.
- She maintains social connections, although her relationships are intense and tumultuous.
- Psychotic symptoms have not changed with higher dosages of antipsychotics.
- Complaints of feeling “disconnected from herself” and “empty” are common symptoms of BPD and necessitate further exploration.
- Psychotic symptoms are largely transient and stress-related, with an overwhelmingly negative tone.
- Techniques that individuals with schizophrenia use, such as distraction or trying to tune out voices, are not being employed. Instead, Ms. K attends to the voices and is anxiously focused on them.
- The relationship of her symptoms to interpersonal stress is key.
When evaluating a patient such as Ms. K, it is important to explore both the nature and timing of the psychotic symptoms and any other related psychiatric symptoms. This helps to determine a less ambiguous diagnosis and clearer treatment plan. Understanding the patient’s perspective about the psychotic symptoms also is useful to gauge the patient’s level of distress and her impression of what the symptoms mean.
Diagnostic considerations
BPD is characterized by a chaotic emotional climate with impulsivity and instability of self-image, affect, and relationships. Most BPD symptoms, including psychosis, often are exacerbated by the perception of abandonment or rejection and other interpersonal stressors.1 Both BPD and schizophrenia are estimated to affect at least 1% of the general population.8,9 Patients with BPD frequently meet criteria for comorbid mental illnesses, including major depressive disorder, substance use disorder, posttraumatic stress disorder, anxiety, and eating disorders.10 Because psychotic symptoms can present in some of these disorders, the context and time course of these symptoms are crucial to consider.
Misdiagnosis is common with BPD, and patients can receive the wrong treatment for years before BPD is considered, likely because of the stigma surrounding the diagnosis.5 One also must keep in mind that, although rare, a patient can have both BPD and a primary psychotic disorder.11 Although a patient with schizophrenia could be prone to social isolation because of delusions or paranoia, BPD patients are more apt to experience intense interpersonal relationships driven by the need to avoid abandonment. Manipulation, anger, and neediness in relationships with both peers and health care providers are common—stark contrasts to typical negative symptoms, blunted affect, and a lack of social drive characteristic of schizophrenia.12
Distinguishing between psychosis in BPD and a psychotic disorder
Studies have sought to explore the quality of psychotic symptoms in BPD vs primary psychotic disorders, which can be challenging to differentiate (Table 1). Some have found that transient symptoms, such as non-delusional paranoia, are more prevalent in BPD, and “true” psychotic symptoms that are long-lasting and bizarre are indicative of schizophrenia.13,14 Also, there is evidence that the lower levels of interpersonal functioning often found in BPD are predictive of psychotic symptoms in that disorder but not in schizophrenia.15

Auditory hallucinations in patients with BPD predominantly are negative and critical in tone.4 However, there is no consistent evidence that the quality of auditory hallucinations in BPD vs schizophrenia is different in any meaningful way.16 Because of the frequency of dissociative symptoms in BPD, it is likely that clinicians could misinterpret these symptoms to indicate disorganized behavior associated with a primary psychotic disorder. In one study, 50% of individuals with BPD experienced auditory hallucinations.11 Differentiating between “internal” or “external” voices did not help to clarify the diagnosis, and paranoid delusions occurred in less than one-third of patients with BPD, but in approximately two-third of those with a diagnosis of schizophrenia.
The McLean Study of Adult Development, a longitudinal study of BPD patients, found that the prevalence of psychotic symptoms diminished over time. It is unclear whether this was due to the spontaneus remission rate of BPD symptoms in general or because of effective treatment.13
Psychotic symptoms in BPD seem to react to stress and increase in intensity when patients are in crisis.17 Nonetheless, because of the prevalence of psychosis in BPD patients and the distress it causes, clinicians should be cautioned against using terms that imply that the symptoms are not “true” or “real.”3
Treatment recommendations
When considering pharmacologic management of psychotic symptoms in BPD, aim to limit antipsychotic medications to low dosages because of adverse effects and the limited evidence that escalating dosages—and especially using >1 antipsychotic concurrently—are more effective.18 Educate patients that in BPD medications are, at best, considered adjunctive treatments. Blaming psychotic symptoms on a purely biological process in BPD, not only is harmful because medications are unlikely to significantly or consistently help, but also because they can undermine patient autonomy and reinforce the need for an outside entity (ie, medication) to fix their problems.
When treatment is ineffective and symptoms do not improve, a patient with BPD likely will experience mounting distress. This, in turn, could exacerbate impulsive, suicidal, and self-injurious behaviors. Emphasize psychotherapy, particularly for those whose psychotic symptoms are transient, stress-related, and present during acute crises (Table 2). With evidence-based psychotherapy, BPD patients can become active participants in treatment, coupling developing insight with concrete skills and teachable principles. This leads to increased interpersonal effectiveness and resilience during times of stress. Challenging the patient’s psychotic symptoms as false or “made up” rarely is helpful and usually harmful, leading to the possible severance of the therapeutic alliance.3

Bottom Line
Psychotic symptoms in patients with borderline personality disorder (BPD) could look similar to those in primary psychotic disorders. Factors suggesting BPD include a pattern of worsening psychotic symptoms during stress, long-term symptom instability, lack of delusions, presence of dissociation, and nonresponse to antipsychotics. Although low-dosage antipsychotics could provide some relief of psychotic symptoms in a patient with BPD, they often are not consistently effective and frequently lead to adverse effects. Emphasize evidence-based psychotherapies.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern A. Borderline group of neuroses. The Psychoanalytic Quarterly. 1938;7:467-489.
3. Schroeder K, Fisher HL, Schäfer I, et al. Psychotic symptoms in patients with borderline personality disorder: prevalence and clinical management. Curr Opin Psychiatry. 2013;26(1):113-119.
4. Pearse LJ, Dibben C, Ziauddeen H, et al. A study of psychotic symptoms in borderline personality disorder. J Nerv Ment Dis. 2014;202(5):368-371.
5. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-
6. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29(5):461-467.
7. National Education Alliance for Borderline Personality Disorder. Treatments for BPD. http://www.borderlinepersonalitydisorder.com/what-is-bpd/treating-bpd. Accessed September 1, 2016.
8. Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50(2):85-94.
9. Lenzenweger MF, Lane MC, Loranger AW, et al. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62(6):553-564.
10. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155(12):1733-1739.
11. Kingdon DG, Ashcroft K, Bhandari B, et al. Schizophrenia and borderline personality disorder: similarities and differences in the experience of auditory hallucinations, paranoia, and childhood trauma. J Nerv Ment Dis. 2010;198(6):399-403.
12. Gunderson JG. Borderline personality disorder. Washington, DC: American Psychiatric Press; 1984.
13. Zanarini MC, Frankenburg FR, Wedig MM, et al. Cognitive experiences reported by patients with borderline personality disorder and Axis II comparison subjects: a 16-year prospective follow-up study. Am J Psychiatry. 2013;170(6):671-679.
14. Tschoeke S, Steinert T, Flammer E, et al. Similarities and differences in borderline personality disorder and schizophrenia with voice hearing. J Nerv Ment Dis. 2014;202(7):544-549.
15. Oliva F, Dalmotto M, Pirfo E, et al. A comparison of thought and perception disorders in borderline personality disorder and schizophrenia: psychotic experiences as a reaction to impaired social functioning. BMC Psychiatry. 2014;14:239.
16. Merrett Z, Rossell SL, Castle DJ, et al. Comparing the experience of voices in borderline personality disorder with the experience of voices in a psychotic disorder: a systematic review. Aust N Z J Psychiatry. 2016;50(7):640-648.
17. Glaser JP, Van Os J, Thewissen V, et al. Psychotic reactivity in borderline personality disorder. Acta Psychiatr Scand. 2010;121(2):125-134.
18. Rosenbluth M, Sinyor M. Off-label use of atypical antipsychotics in personality disorders. Expert Opin Pharmacother. 2012;13(11):1575-1585.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern A. Borderline group of neuroses. The Psychoanalytic Quarterly. 1938;7:467-489.
3. Schroeder K, Fisher HL, Schäfer I, et al. Psychotic symptoms in patients with borderline personality disorder: prevalence and clinical management. Curr Opin Psychiatry. 2013;26(1):113-119.
4. Pearse LJ, Dibben C, Ziauddeen H, et al. A study of psychotic symptoms in borderline personality disorder. J Nerv Ment Dis. 2014;202(5):368-371.
5. Paris J. Why psychiatrists are reluctant to diagnose: borderline personality disorder. Psychiatry (Edgmont). 2007;4(1):35-
6. Saunders EF, Silk KR. Personality trait dimensions and the pharmacological treatment of borderline personality disorder. J Clin Psychopharmacol. 2009;29(5):461-467.
7. National Education Alliance for Borderline Personality Disorder. Treatments for BPD. http://www.borderlinepersonalitydisorder.com/what-is-bpd/treating-bpd. Accessed September 1, 2016.
8. Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50(2):85-94.
9. Lenzenweger MF, Lane MC, Loranger AW, et al. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62(6):553-564.
10. Zanarini MC, Frankenburg FR, Dubo ED, et al. Axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155(12):1733-1739.
11. Kingdon DG, Ashcroft K, Bhandari B, et al. Schizophrenia and borderline personality disorder: similarities and differences in the experience of auditory hallucinations, paranoia, and childhood trauma. J Nerv Ment Dis. 2010;198(6):399-403.
12. Gunderson JG. Borderline personality disorder. Washington, DC: American Psychiatric Press; 1984.
13. Zanarini MC, Frankenburg FR, Wedig MM, et al. Cognitive experiences reported by patients with borderline personality disorder and Axis II comparison subjects: a 16-year prospective follow-up study. Am J Psychiatry. 2013;170(6):671-679.
14. Tschoeke S, Steinert T, Flammer E, et al. Similarities and differences in borderline personality disorder and schizophrenia with voice hearing. J Nerv Ment Dis. 2014;202(7):544-549.
15. Oliva F, Dalmotto M, Pirfo E, et al. A comparison of thought and perception disorders in borderline personality disorder and schizophrenia: psychotic experiences as a reaction to impaired social functioning. BMC Psychiatry. 2014;14:239.
16. Merrett Z, Rossell SL, Castle DJ, et al. Comparing the experience of voices in borderline personality disorder with the experience of voices in a psychotic disorder: a systematic review. Aust N Z J Psychiatry. 2016;50(7):640-648.
17. Glaser JP, Van Os J, Thewissen V, et al. Psychotic reactivity in borderline personality disorder. Acta Psychiatr Scand. 2010;121(2):125-134.
18. Rosenbluth M, Sinyor M. Off-label use of atypical antipsychotics in personality disorders. Expert Opin Pharmacother. 2012;13(11):1575-1585.
Evaluating and monitoring drug and alcohol use during child custody disputes
Alcohol or drug use is frequently reported as a factor in divorce; 10.6% of divorcing couples list it as a precipitant for the marriage dissolution, surpassed by infidelity (21.6%) and incompatibility (19.2%).1 An effective drug and alcohol evaluation and monitoring plan during a child custody dispute safeguards the well-being of the minor children and protects—as much as possible—the parenting time of drug- or alcohol-involved parents. The evaluation maneuvers discussed in this article most likely will produce a complete, fair, and transparent evaluation and monitoring plan.
An evaluator—usually a clinician trained in diagnosing and treating a substance use disorder (SUD) and other psychiatric illnesses—performs a comprehensive alcohol/drug evaluation, prepares a monitoring program, or both. The evaluation and monitoring plan should be fair and transparent to all parties. Specific evaluation maneuvers, such as forensic-quality testing, detailed interviews with collateral informants, and ongoing collaboration with attorneys, are likely to yield a thorough evaluation and an effective and fair monitoring program. The evaluating clinician should strive for objectivity, accuracy, and practical workability when constructing these reports and monitoring plans. However, the evaluator should—in most cases—not provide treatment because this likely would represent a boundary violation between clinical treatment and forensic evaluation.
Addiction-specific evaluation maneuvers
As in all forensic matters, the evaluator’s report must answer the court’s “psycho-legal question as objectively as possible”2 rather than benefit the subject of that report. (Describing the individual being examined as the “subject” rather than “patient” emphasizes the forensic rather than clinical nature of the evaluation and the absence of a doctor–patient relationship.) Similarly, a monitoring program for drug/alcohol use should be designed to flag use of banned substances and protect the well-being of the minor child, not the parents.
Acting more as a detective than a clinician, the evaluator should maintain a skeptical—although not cynical or disrespectful—attitude when interviewing individuals who might have knowledge of the subject’s drug or alcohol use, including friends, co-workers, therapists, physicians, and even the soon-to-be-ex spouse. These collateral informants will have their own preferences or loyalties, and the examining clinician must consider these biases in the final report. A spouse often is biased and could exaggerate, emphasize, or invent addictive behaviors committed by the subject.
Collaboration among attorneys and evaluators/monitors
A strong collaboration between the judge and the attorney requesting a drug/alcohol evaluation or monitoring plan likely will result in a better outcome. This collaboration must begin with a clear delineation of the report’s purpose:
- Is the court appointing the evaluator to help gauge a drug/alcohol-involved parent’s ongoing ability to care for a child?
- Is an attorney looking for advice on how to best present the matter to the court?
- Is the evaluator expected to present and maintain a position in a court proceeding against another evaluator in a “battle of the experts?”
- Is the evaluator to consider only drug use? Only illicit drug use?
- Is the subject banned from using the substance at all times or just when she (he) is caring for the child?
A clear understanding of the evaluator’s mission is important, in part because the subject must fully comprehend the plan to consent to having the results disseminated.
To foster an effective collaboration with legal personnel the evaluator should frame the final report, testimony, and monitoring plan using clinical rather than colloquial language. To best describe the subject’s situation, diagnosis, and likely prognosis, these clinical terms often will require explanation or clarification. For example, urine drug screens (UDS) should be described as “positive for the cocaine metabolite benzoylecgonine” rather than “dirty,” and the subject might be described as “meeting criteria for alcohol use disorder” rather than an “alcoholic” or “abuser.” Using DSM-5 terminology allows for a respectful, reasonably reproducible diagnostic assessment that promotes civil discussion about disagreements, rather than name-calling in the courtroom. Professional third-party evaluation and monitoring programs in custody dispute proceedings can de-escalate the tension between the parents around issues of substance use. The conversation becomes professional, dispassionate, and focused on the best interests of the child.
Use of appropriate language allows the evaluator to expand the parameters of the report or recommend an expansion of it. If a drug/alcohol evaluation finds a relevant mental illness—in addition to a SUD—or finds another caregiver who seems incompetent, the evaluator might be professionally obligated to bring up these points, even if they are outside the purview of the requested report and monitoring plan.
Planning a monitoring program
If the evaluation determines a monitoring plan is indicated and the court orders a testing program, the evaluator must design a program that accomplishes the specific goals established by the court order. The evaluator might help the court draft that plan, but the evaluator must accommodate the final court order. Table 2 lists vital aspects of a monitoring program in a child custody dispute.
Describe goals. A court-ordered monitoring program includes:
- a clear description of goals
- what specific substances are being tested for
- how and when they are being tested for
- who pays for the testing
- what will happen after a positive or missed test.
The situation will determine whether random, scheduled, or for-cause testing is indicated.
A frequent sticking point is the decision as to whether an individual can use alcohol or other substances while he (she) is not caring for the child. A person who does not meet criteria for a SUD could argue that abstinence from alcohol or any sort of testing is unwarranted when another person is taking care of the child. The evaluator should provide input, but the court will determine the outcome.
Develop a testing program. The evaluator should develop a testing program that accomplishes the goals set out by the court, usually to protect the child from possible harm caused by a parent who uses alcohol or drugs. However, this narrow goal often is expanded to allow testing for drugs/alcohol at all times, because the parent’s slip or relapse could harm the child in the long or short term.
Describe consequences. A carefully structured definition of the consequences of a positive or missed test is an important aspect of the monitoring program. In protecting the best interests of the child, the consequences usually include the immediate transfer of the child to a safe environment. This often involves the person who receives the positive test result—usually with a physician monitoring the testing—notifying the other parent or the other parent’s attorney of the positive test result.
Testing
Although an important part of evaluation and monitoring, drug and alcohol testing alone does not diagnose a SUD or even misuse.3 Adults often use alcohol with no consequence to their children, and illicit drug use is not a prima facie bar to parenthood or taking care of a child. Also, the results of a thorough alcohol or drug evaluation cannot determine the ideal custody arrangement. The court’s final decision is based on a more wide-ranging evaluation of the family system as a whole, with the drug/alcohol issue as 1 component. In addition, the court could use the results of a forensic examiner’s assessment to advocate or mandate the appropriate treatment.
With that caveat, the specific tests used and the timing of those tests are important in the context of a child custody dispute. Once the parties have agreed on the time frame of the testing (ongoing or only during visits with the child), the specific substances that are tested for must be listed. Forensic quality testing—often called “employment testing” in clinical laboratories—decreases but does not eliminate the possibility of evasion of the test. Although addiction clinicians usually request a full screen for drugs of abuse for their patients, in the legal sphere, often only the problematic substances are tested for, which are listed in the court order.
UDS, the most common test, is non-invasive, although awkward and intrusive for the subject when done with the strictest “observed” protocol. Most testing protocols do not require a “directly observed” urine collection unless there is a suspicion that the testee has substituted her (his) urine for a sample from someone else. Breath testing, although similarly non-invasive, is only useful for alcohol testing and can detect use only several hours before the test.
The urine test for the alcohol metabolites ethyl glucuronide (EtG) and ethyl sulfate (EtS) points toward alcohol use in the previous 3 days, but the test is plagued with false-positives at the lower cutoff values.8 EtG can be accurately assayed in human hair.9
Other tests. Dried blood spot testing for phosphatidylethanol is accurate in finding moderate to heavy alcohol use up to 3 weeks before the test.10 Saliva tests also can be useful for point-of-service testing, but the dearth of studies for this methodology makes it less useful in a courtroom setting. Newer technologies using handheld breathalyzers connected to a device with facial recognition software11,12 allow for random and “for-cause” alcohol testing, and can be useful in child custody negotiations. Hair sample testing, which can detect drug use over the 3 months before the test, is becoming more acceptable in the legal setting. However, hair testing cannot identify drug use 7 to 10 days before the test and does not test for alcohol13; and some questions remain regarding its reliability for different ethnic groups.14
Table 4 summarizes some of the most productive testing methods for child custody disputes. Selecting the best tissue, method, and timing for testing will depend on the clinical scenario, as well as the court’s requirements. For example, negotiations between parties could result in a testing protocol that uses both random and for-cause testing of urine, breath, and hair to prove that the individual does not use any illicit substances. In a less serious clinical circumstance—or less contentious legal situation—the testing protocol may necessitate only occasional UDS to make sure that the subject is not using prohibited substances.
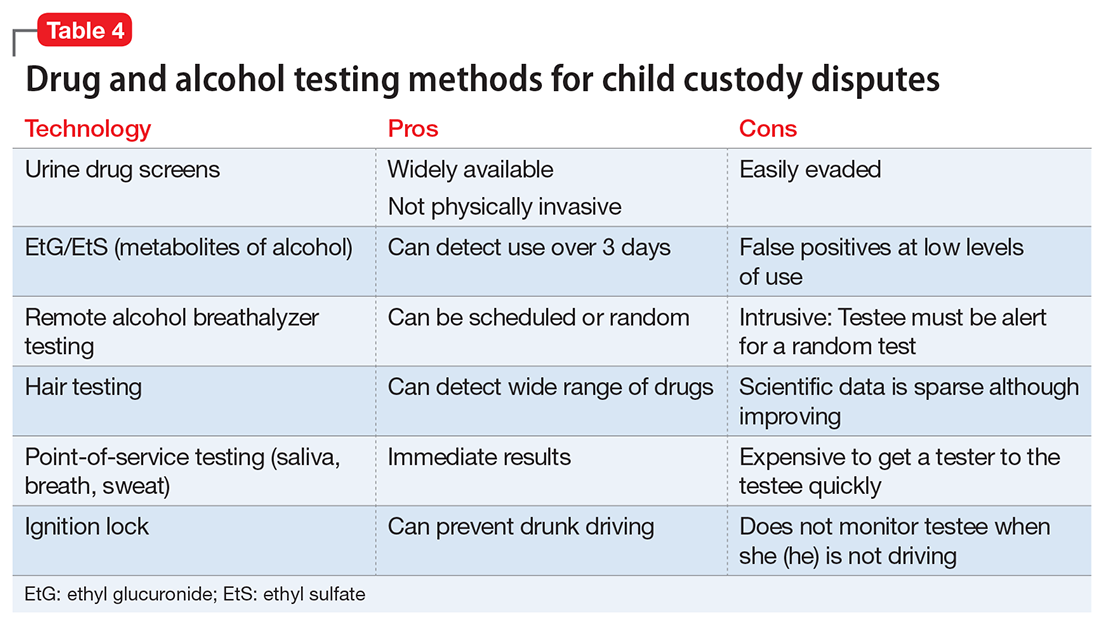
Practical considerations
It is important to remember that drug/alcohol evaluation and testing does not provide a clear-cut answer in child custody proceedings. Any drug or alcohol use must be evaluated under the standard used in child custody disputes: “the best interests of the child.” However, what is in the child’s best interests can be disputed in a courtroom. One California judge understood this as a process to identify the parent who can best provide the child with “… the ethical, emotional, and intellectual guidance the parent gives the child throughout his formative years, and beyond ….”15 However, in determining child custody the need for assuring the child’s physical and emotional safety overrules these long-term goals, and the parents’ emotional needs are disregarded. In a custody dispute, the conflict between parents vying for custody of their child is matched by a corresponding tension between the state’s interest in protecting a minor child while preserving an adult’s right to parent her child.
The Montana custody dispute described in Stout v Stout16 demonstrates some aspects typical of these cases. In deciding to grant custody of a then 3-year-old girl to the father, the presiding judge noted that, although the mother had completed an inpatient alcohol treatment program, her apparent unwillingness or inability to stop drinking or enroll in outpatient treatment, combined with a driving under the influence arrest while the child was in her care, were too worrisome to allow her to have physical custody of the child. The judge mentioned other factors that supported granting custody to the father, but a deciding factor was that “the evidence shows that her drinking adversely affects her parenting ability.” The judge’s ruling demonstrates his judgment in balancing the mother’s legal but harmful alcohol use with potential catastrophic effects for the child.
Although a thorough drug/alcohol evaluation, an evidence-based set of treatment recommendations, and a well-planned monitoring program all promote progress in a child custody dispute, the reality is that the clinical situation could change and all 3 aspects would have to be modified.
Manualized diagnostic rubrics and formal psychological testing, although often used in general forensic assessments, usually are not central to the drug/alcohol evaluation in a child custody dispute,17 because confirming a SUD diagnosis might not be relevant to the task of attending to the child’s best interest. Rather, the danger—or potential danger—of the subject’s substance use to the minor child is paramount, regardless of the diagnosis. Of course, an established diagnosis of a SUD might be relevant to the parent being examined, and might necessitate modifications in the testing protocol, the tissues examined, and the monitor’s overall level of skepticism about testing results.
The evaluator and monitor should be prepared to respond quickly to a slip or relapse, while remaining vigilant for exaggerated, inaccurate, or even deceitful accusations about the subject from the co-parent or others. The evaluator should assess all the relevant sources of information when performing an evaluation and use careful interviewing and testing techniques during the monitoring process. Even with this sort of deliberate evaluation and monitoring the evaluator should never assert that any testing regimen is incapable of error, and always keep in mind that the primary goal—and presumably the interest of all parties involved—is to protect the child’s well-being.
1. Amato PT, Previti D. People’s reasons for divorcing: gender, social class, the life course, and adjustment. J Fam Issues. 2003;24(5):602-606.
2. Glancy GD, Ash P, Bath EP, et al. AAPL practice guideline for the forensic assessment. J Am Acad Psychiatry Law. 2015;43(suppl 2):S3-S53.
3. Center for Substance Abuse Treatment. Drug testing in child welfare: practice and policy considerations. HHS Pub. No. (SMA) 10-4556. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
4. Macdonald DI, DuPont RL. The role of the medical review officer. In: Graham AW, Schultz TK, eds. Principles of addiction medicine, 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine; 1998:1259.
5. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015.
6. Marques PR, McKnight AS. Evaluating transdermal alcohol measuring devices. Calverton, MD: Pacific Institute for Research and Evaluation; 2007.
7. Steroidal.com. How steroid drug testing works. https://www.steroidal.com/steroid-detection-times. Accessed March 8, 2017.
8. Substance Abuse and Mental Health Services Administration. The role of biomarkers in the treatment of alcohol use disorders, 2012 revision. SAMHSA Advisory. 2012;11(2):1-8.
9. United States Drug Testing Laboratories, Inc. Detection of the direct alcohol biomarker ethyl glucuronide (EtG) in hair: an annotated bibliography. http://www.usdtl.com/media/white-papers/ETG_hair_annotated_bibliography_032014.pdf. Accessed March 8, 2017.
10. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788-14812.
11. SoberLink. https://www.soberlink.com. Accessed March 8, 2017.
12. Scram Systems. https://www.scramsystems.com/products/scram-continuous-alcohol-monitoring/?gclid=CIqUr8Kqx9ICFZmCswodI0QKPA. Accessed March 8, 2017.
13. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015:208.
14. Chamberlain RT. Legal review for testing of drugs in hair. Forensic Sci Rev. 2007;19(1-2):85-94.
15. Marriage of Carney, 24 Cal 3d725,157 Cal Rptr 383 (1979).
16. Marriage of Stout, 216 Mont 342 (Mont 1985).
17. Hynan DJ. Child custody evaluation, new theoretical applications and research. In: Hynan DJ. Difficult evaluation challenges: domestic violence, child abuse, substance abuse, and relocations. Springfield, IL: Charles C. Thomas Publisher; 2014:178-195.
Alcohol or drug use is frequently reported as a factor in divorce; 10.6% of divorcing couples list it as a precipitant for the marriage dissolution, surpassed by infidelity (21.6%) and incompatibility (19.2%).1 An effective drug and alcohol evaluation and monitoring plan during a child custody dispute safeguards the well-being of the minor children and protects—as much as possible—the parenting time of drug- or alcohol-involved parents. The evaluation maneuvers discussed in this article most likely will produce a complete, fair, and transparent evaluation and monitoring plan.
An evaluator—usually a clinician trained in diagnosing and treating a substance use disorder (SUD) and other psychiatric illnesses—performs a comprehensive alcohol/drug evaluation, prepares a monitoring program, or both. The evaluation and monitoring plan should be fair and transparent to all parties. Specific evaluation maneuvers, such as forensic-quality testing, detailed interviews with collateral informants, and ongoing collaboration with attorneys, are likely to yield a thorough evaluation and an effective and fair monitoring program. The evaluating clinician should strive for objectivity, accuracy, and practical workability when constructing these reports and monitoring plans. However, the evaluator should—in most cases—not provide treatment because this likely would represent a boundary violation between clinical treatment and forensic evaluation.
Addiction-specific evaluation maneuvers
As in all forensic matters, the evaluator’s report must answer the court’s “psycho-legal question as objectively as possible”2 rather than benefit the subject of that report. (Describing the individual being examined as the “subject” rather than “patient” emphasizes the forensic rather than clinical nature of the evaluation and the absence of a doctor–patient relationship.) Similarly, a monitoring program for drug/alcohol use should be designed to flag use of banned substances and protect the well-being of the minor child, not the parents.
Acting more as a detective than a clinician, the evaluator should maintain a skeptical—although not cynical or disrespectful—attitude when interviewing individuals who might have knowledge of the subject’s drug or alcohol use, including friends, co-workers, therapists, physicians, and even the soon-to-be-ex spouse. These collateral informants will have their own preferences or loyalties, and the examining clinician must consider these biases in the final report. A spouse often is biased and could exaggerate, emphasize, or invent addictive behaviors committed by the subject.
Collaboration among attorneys and evaluators/monitors
A strong collaboration between the judge and the attorney requesting a drug/alcohol evaluation or monitoring plan likely will result in a better outcome. This collaboration must begin with a clear delineation of the report’s purpose:
- Is the court appointing the evaluator to help gauge a drug/alcohol-involved parent’s ongoing ability to care for a child?
- Is an attorney looking for advice on how to best present the matter to the court?
- Is the evaluator expected to present and maintain a position in a court proceeding against another evaluator in a “battle of the experts?”
- Is the evaluator to consider only drug use? Only illicit drug use?
- Is the subject banned from using the substance at all times or just when she (he) is caring for the child?
A clear understanding of the evaluator’s mission is important, in part because the subject must fully comprehend the plan to consent to having the results disseminated.
To foster an effective collaboration with legal personnel the evaluator should frame the final report, testimony, and monitoring plan using clinical rather than colloquial language. To best describe the subject’s situation, diagnosis, and likely prognosis, these clinical terms often will require explanation or clarification. For example, urine drug screens (UDS) should be described as “positive for the cocaine metabolite benzoylecgonine” rather than “dirty,” and the subject might be described as “meeting criteria for alcohol use disorder” rather than an “alcoholic” or “abuser.” Using DSM-5 terminology allows for a respectful, reasonably reproducible diagnostic assessment that promotes civil discussion about disagreements, rather than name-calling in the courtroom. Professional third-party evaluation and monitoring programs in custody dispute proceedings can de-escalate the tension between the parents around issues of substance use. The conversation becomes professional, dispassionate, and focused on the best interests of the child.
Use of appropriate language allows the evaluator to expand the parameters of the report or recommend an expansion of it. If a drug/alcohol evaluation finds a relevant mental illness—in addition to a SUD—or finds another caregiver who seems incompetent, the evaluator might be professionally obligated to bring up these points, even if they are outside the purview of the requested report and monitoring plan.
Planning a monitoring program
If the evaluation determines a monitoring plan is indicated and the court orders a testing program, the evaluator must design a program that accomplishes the specific goals established by the court order. The evaluator might help the court draft that plan, but the evaluator must accommodate the final court order. Table 2 lists vital aspects of a monitoring program in a child custody dispute.
Describe goals. A court-ordered monitoring program includes:
- a clear description of goals
- what specific substances are being tested for
- how and when they are being tested for
- who pays for the testing
- what will happen after a positive or missed test.
The situation will determine whether random, scheduled, or for-cause testing is indicated.
A frequent sticking point is the decision as to whether an individual can use alcohol or other substances while he (she) is not caring for the child. A person who does not meet criteria for a SUD could argue that abstinence from alcohol or any sort of testing is unwarranted when another person is taking care of the child. The evaluator should provide input, but the court will determine the outcome.
Develop a testing program. The evaluator should develop a testing program that accomplishes the goals set out by the court, usually to protect the child from possible harm caused by a parent who uses alcohol or drugs. However, this narrow goal often is expanded to allow testing for drugs/alcohol at all times, because the parent’s slip or relapse could harm the child in the long or short term.
Describe consequences. A carefully structured definition of the consequences of a positive or missed test is an important aspect of the monitoring program. In protecting the best interests of the child, the consequences usually include the immediate transfer of the child to a safe environment. This often involves the person who receives the positive test result—usually with a physician monitoring the testing—notifying the other parent or the other parent’s attorney of the positive test result.
Testing
Although an important part of evaluation and monitoring, drug and alcohol testing alone does not diagnose a SUD or even misuse.3 Adults often use alcohol with no consequence to their children, and illicit drug use is not a prima facie bar to parenthood or taking care of a child. Also, the results of a thorough alcohol or drug evaluation cannot determine the ideal custody arrangement. The court’s final decision is based on a more wide-ranging evaluation of the family system as a whole, with the drug/alcohol issue as 1 component. In addition, the court could use the results of a forensic examiner’s assessment to advocate or mandate the appropriate treatment.
With that caveat, the specific tests used and the timing of those tests are important in the context of a child custody dispute. Once the parties have agreed on the time frame of the testing (ongoing or only during visits with the child), the specific substances that are tested for must be listed. Forensic quality testing—often called “employment testing” in clinical laboratories—decreases but does not eliminate the possibility of evasion of the test. Although addiction clinicians usually request a full screen for drugs of abuse for their patients, in the legal sphere, often only the problematic substances are tested for, which are listed in the court order.
UDS, the most common test, is non-invasive, although awkward and intrusive for the subject when done with the strictest “observed” protocol. Most testing protocols do not require a “directly observed” urine collection unless there is a suspicion that the testee has substituted her (his) urine for a sample from someone else. Breath testing, although similarly non-invasive, is only useful for alcohol testing and can detect use only several hours before the test.
The urine test for the alcohol metabolites ethyl glucuronide (EtG) and ethyl sulfate (EtS) points toward alcohol use in the previous 3 days, but the test is plagued with false-positives at the lower cutoff values.8 EtG can be accurately assayed in human hair.9
Other tests. Dried blood spot testing for phosphatidylethanol is accurate in finding moderate to heavy alcohol use up to 3 weeks before the test.10 Saliva tests also can be useful for point-of-service testing, but the dearth of studies for this methodology makes it less useful in a courtroom setting. Newer technologies using handheld breathalyzers connected to a device with facial recognition software11,12 allow for random and “for-cause” alcohol testing, and can be useful in child custody negotiations. Hair sample testing, which can detect drug use over the 3 months before the test, is becoming more acceptable in the legal setting. However, hair testing cannot identify drug use 7 to 10 days before the test and does not test for alcohol13; and some questions remain regarding its reliability for different ethnic groups.14
Table 4 summarizes some of the most productive testing methods for child custody disputes. Selecting the best tissue, method, and timing for testing will depend on the clinical scenario, as well as the court’s requirements. For example, negotiations between parties could result in a testing protocol that uses both random and for-cause testing of urine, breath, and hair to prove that the individual does not use any illicit substances. In a less serious clinical circumstance—or less contentious legal situation—the testing protocol may necessitate only occasional UDS to make sure that the subject is not using prohibited substances.

Practical considerations
It is important to remember that drug/alcohol evaluation and testing does not provide a clear-cut answer in child custody proceedings. Any drug or alcohol use must be evaluated under the standard used in child custody disputes: “the best interests of the child.” However, what is in the child’s best interests can be disputed in a courtroom. One California judge understood this as a process to identify the parent who can best provide the child with “… the ethical, emotional, and intellectual guidance the parent gives the child throughout his formative years, and beyond ….”15 However, in determining child custody the need for assuring the child’s physical and emotional safety overrules these long-term goals, and the parents’ emotional needs are disregarded. In a custody dispute, the conflict between parents vying for custody of their child is matched by a corresponding tension between the state’s interest in protecting a minor child while preserving an adult’s right to parent her child.
The Montana custody dispute described in Stout v Stout16 demonstrates some aspects typical of these cases. In deciding to grant custody of a then 3-year-old girl to the father, the presiding judge noted that, although the mother had completed an inpatient alcohol treatment program, her apparent unwillingness or inability to stop drinking or enroll in outpatient treatment, combined with a driving under the influence arrest while the child was in her care, were too worrisome to allow her to have physical custody of the child. The judge mentioned other factors that supported granting custody to the father, but a deciding factor was that “the evidence shows that her drinking adversely affects her parenting ability.” The judge’s ruling demonstrates his judgment in balancing the mother’s legal but harmful alcohol use with potential catastrophic effects for the child.
Although a thorough drug/alcohol evaluation, an evidence-based set of treatment recommendations, and a well-planned monitoring program all promote progress in a child custody dispute, the reality is that the clinical situation could change and all 3 aspects would have to be modified.
Manualized diagnostic rubrics and formal psychological testing, although often used in general forensic assessments, usually are not central to the drug/alcohol evaluation in a child custody dispute,17 because confirming a SUD diagnosis might not be relevant to the task of attending to the child’s best interest. Rather, the danger—or potential danger—of the subject’s substance use to the minor child is paramount, regardless of the diagnosis. Of course, an established diagnosis of a SUD might be relevant to the parent being examined, and might necessitate modifications in the testing protocol, the tissues examined, and the monitor’s overall level of skepticism about testing results.
The evaluator and monitor should be prepared to respond quickly to a slip or relapse, while remaining vigilant for exaggerated, inaccurate, or even deceitful accusations about the subject from the co-parent or others. The evaluator should assess all the relevant sources of information when performing an evaluation and use careful interviewing and testing techniques during the monitoring process. Even with this sort of deliberate evaluation and monitoring the evaluator should never assert that any testing regimen is incapable of error, and always keep in mind that the primary goal—and presumably the interest of all parties involved—is to protect the child’s well-being.
Alcohol or drug use is frequently reported as a factor in divorce; 10.6% of divorcing couples list it as a precipitant for the marriage dissolution, surpassed by infidelity (21.6%) and incompatibility (19.2%).1 An effective drug and alcohol evaluation and monitoring plan during a child custody dispute safeguards the well-being of the minor children and protects—as much as possible—the parenting time of drug- or alcohol-involved parents. The evaluation maneuvers discussed in this article most likely will produce a complete, fair, and transparent evaluation and monitoring plan.
An evaluator—usually a clinician trained in diagnosing and treating a substance use disorder (SUD) and other psychiatric illnesses—performs a comprehensive alcohol/drug evaluation, prepares a monitoring program, or both. The evaluation and monitoring plan should be fair and transparent to all parties. Specific evaluation maneuvers, such as forensic-quality testing, detailed interviews with collateral informants, and ongoing collaboration with attorneys, are likely to yield a thorough evaluation and an effective and fair monitoring program. The evaluating clinician should strive for objectivity, accuracy, and practical workability when constructing these reports and monitoring plans. However, the evaluator should—in most cases—not provide treatment because this likely would represent a boundary violation between clinical treatment and forensic evaluation.
Addiction-specific evaluation maneuvers
As in all forensic matters, the evaluator’s report must answer the court’s “psycho-legal question as objectively as possible”2 rather than benefit the subject of that report. (Describing the individual being examined as the “subject” rather than “patient” emphasizes the forensic rather than clinical nature of the evaluation and the absence of a doctor–patient relationship.) Similarly, a monitoring program for drug/alcohol use should be designed to flag use of banned substances and protect the well-being of the minor child, not the parents.
Acting more as a detective than a clinician, the evaluator should maintain a skeptical—although not cynical or disrespectful—attitude when interviewing individuals who might have knowledge of the subject’s drug or alcohol use, including friends, co-workers, therapists, physicians, and even the soon-to-be-ex spouse. These collateral informants will have their own preferences or loyalties, and the examining clinician must consider these biases in the final report. A spouse often is biased and could exaggerate, emphasize, or invent addictive behaviors committed by the subject.
Collaboration among attorneys and evaluators/monitors
A strong collaboration between the judge and the attorney requesting a drug/alcohol evaluation or monitoring plan likely will result in a better outcome. This collaboration must begin with a clear delineation of the report’s purpose:
- Is the court appointing the evaluator to help gauge a drug/alcohol-involved parent’s ongoing ability to care for a child?
- Is an attorney looking for advice on how to best present the matter to the court?
- Is the evaluator expected to present and maintain a position in a court proceeding against another evaluator in a “battle of the experts?”
- Is the evaluator to consider only drug use? Only illicit drug use?
- Is the subject banned from using the substance at all times or just when she (he) is caring for the child?
A clear understanding of the evaluator’s mission is important, in part because the subject must fully comprehend the plan to consent to having the results disseminated.
To foster an effective collaboration with legal personnel the evaluator should frame the final report, testimony, and monitoring plan using clinical rather than colloquial language. To best describe the subject’s situation, diagnosis, and likely prognosis, these clinical terms often will require explanation or clarification. For example, urine drug screens (UDS) should be described as “positive for the cocaine metabolite benzoylecgonine” rather than “dirty,” and the subject might be described as “meeting criteria for alcohol use disorder” rather than an “alcoholic” or “abuser.” Using DSM-5 terminology allows for a respectful, reasonably reproducible diagnostic assessment that promotes civil discussion about disagreements, rather than name-calling in the courtroom. Professional third-party evaluation and monitoring programs in custody dispute proceedings can de-escalate the tension between the parents around issues of substance use. The conversation becomes professional, dispassionate, and focused on the best interests of the child.
Use of appropriate language allows the evaluator to expand the parameters of the report or recommend an expansion of it. If a drug/alcohol evaluation finds a relevant mental illness—in addition to a SUD—or finds another caregiver who seems incompetent, the evaluator might be professionally obligated to bring up these points, even if they are outside the purview of the requested report and monitoring plan.
Planning a monitoring program
If the evaluation determines a monitoring plan is indicated and the court orders a testing program, the evaluator must design a program that accomplishes the specific goals established by the court order. The evaluator might help the court draft that plan, but the evaluator must accommodate the final court order. Table 2 lists vital aspects of a monitoring program in a child custody dispute.
Describe goals. A court-ordered monitoring program includes:
- a clear description of goals
- what specific substances are being tested for
- how and when they are being tested for
- who pays for the testing
- what will happen after a positive or missed test.
The situation will determine whether random, scheduled, or for-cause testing is indicated.
A frequent sticking point is the decision as to whether an individual can use alcohol or other substances while he (she) is not caring for the child. A person who does not meet criteria for a SUD could argue that abstinence from alcohol or any sort of testing is unwarranted when another person is taking care of the child. The evaluator should provide input, but the court will determine the outcome.
Develop a testing program. The evaluator should develop a testing program that accomplishes the goals set out by the court, usually to protect the child from possible harm caused by a parent who uses alcohol or drugs. However, this narrow goal often is expanded to allow testing for drugs/alcohol at all times, because the parent’s slip or relapse could harm the child in the long or short term.
Describe consequences. A carefully structured definition of the consequences of a positive or missed test is an important aspect of the monitoring program. In protecting the best interests of the child, the consequences usually include the immediate transfer of the child to a safe environment. This often involves the person who receives the positive test result—usually with a physician monitoring the testing—notifying the other parent or the other parent’s attorney of the positive test result.
Testing
Although an important part of evaluation and monitoring, drug and alcohol testing alone does not diagnose a SUD or even misuse.3 Adults often use alcohol with no consequence to their children, and illicit drug use is not a prima facie bar to parenthood or taking care of a child. Also, the results of a thorough alcohol or drug evaluation cannot determine the ideal custody arrangement. The court’s final decision is based on a more wide-ranging evaluation of the family system as a whole, with the drug/alcohol issue as 1 component. In addition, the court could use the results of a forensic examiner’s assessment to advocate or mandate the appropriate treatment.
With that caveat, the specific tests used and the timing of those tests are important in the context of a child custody dispute. Once the parties have agreed on the time frame of the testing (ongoing or only during visits with the child), the specific substances that are tested for must be listed. Forensic quality testing—often called “employment testing” in clinical laboratories—decreases but does not eliminate the possibility of evasion of the test. Although addiction clinicians usually request a full screen for drugs of abuse for their patients, in the legal sphere, often only the problematic substances are tested for, which are listed in the court order.
UDS, the most common test, is non-invasive, although awkward and intrusive for the subject when done with the strictest “observed” protocol. Most testing protocols do not require a “directly observed” urine collection unless there is a suspicion that the testee has substituted her (his) urine for a sample from someone else. Breath testing, although similarly non-invasive, is only useful for alcohol testing and can detect use only several hours before the test.
The urine test for the alcohol metabolites ethyl glucuronide (EtG) and ethyl sulfate (EtS) points toward alcohol use in the previous 3 days, but the test is plagued with false-positives at the lower cutoff values.8 EtG can be accurately assayed in human hair.9
Other tests. Dried blood spot testing for phosphatidylethanol is accurate in finding moderate to heavy alcohol use up to 3 weeks before the test.10 Saliva tests also can be useful for point-of-service testing, but the dearth of studies for this methodology makes it less useful in a courtroom setting. Newer technologies using handheld breathalyzers connected to a device with facial recognition software11,12 allow for random and “for-cause” alcohol testing, and can be useful in child custody negotiations. Hair sample testing, which can detect drug use over the 3 months before the test, is becoming more acceptable in the legal setting. However, hair testing cannot identify drug use 7 to 10 days before the test and does not test for alcohol13; and some questions remain regarding its reliability for different ethnic groups.14
Table 4 summarizes some of the most productive testing methods for child custody disputes. Selecting the best tissue, method, and timing for testing will depend on the clinical scenario, as well as the court’s requirements. For example, negotiations between parties could result in a testing protocol that uses both random and for-cause testing of urine, breath, and hair to prove that the individual does not use any illicit substances. In a less serious clinical circumstance—or less contentious legal situation—the testing protocol may necessitate only occasional UDS to make sure that the subject is not using prohibited substances.

Practical considerations
It is important to remember that drug/alcohol evaluation and testing does not provide a clear-cut answer in child custody proceedings. Any drug or alcohol use must be evaluated under the standard used in child custody disputes: “the best interests of the child.” However, what is in the child’s best interests can be disputed in a courtroom. One California judge understood this as a process to identify the parent who can best provide the child with “… the ethical, emotional, and intellectual guidance the parent gives the child throughout his formative years, and beyond ….”15 However, in determining child custody the need for assuring the child’s physical and emotional safety overrules these long-term goals, and the parents’ emotional needs are disregarded. In a custody dispute, the conflict between parents vying for custody of their child is matched by a corresponding tension between the state’s interest in protecting a minor child while preserving an adult’s right to parent her child.
The Montana custody dispute described in Stout v Stout16 demonstrates some aspects typical of these cases. In deciding to grant custody of a then 3-year-old girl to the father, the presiding judge noted that, although the mother had completed an inpatient alcohol treatment program, her apparent unwillingness or inability to stop drinking or enroll in outpatient treatment, combined with a driving under the influence arrest while the child was in her care, were too worrisome to allow her to have physical custody of the child. The judge mentioned other factors that supported granting custody to the father, but a deciding factor was that “the evidence shows that her drinking adversely affects her parenting ability.” The judge’s ruling demonstrates his judgment in balancing the mother’s legal but harmful alcohol use with potential catastrophic effects for the child.
Although a thorough drug/alcohol evaluation, an evidence-based set of treatment recommendations, and a well-planned monitoring program all promote progress in a child custody dispute, the reality is that the clinical situation could change and all 3 aspects would have to be modified.
Manualized diagnostic rubrics and formal psychological testing, although often used in general forensic assessments, usually are not central to the drug/alcohol evaluation in a child custody dispute,17 because confirming a SUD diagnosis might not be relevant to the task of attending to the child’s best interest. Rather, the danger—or potential danger—of the subject’s substance use to the minor child is paramount, regardless of the diagnosis. Of course, an established diagnosis of a SUD might be relevant to the parent being examined, and might necessitate modifications in the testing protocol, the tissues examined, and the monitor’s overall level of skepticism about testing results.
The evaluator and monitor should be prepared to respond quickly to a slip or relapse, while remaining vigilant for exaggerated, inaccurate, or even deceitful accusations about the subject from the co-parent or others. The evaluator should assess all the relevant sources of information when performing an evaluation and use careful interviewing and testing techniques during the monitoring process. Even with this sort of deliberate evaluation and monitoring the evaluator should never assert that any testing regimen is incapable of error, and always keep in mind that the primary goal—and presumably the interest of all parties involved—is to protect the child’s well-being.
1. Amato PT, Previti D. People’s reasons for divorcing: gender, social class, the life course, and adjustment. J Fam Issues. 2003;24(5):602-606.
2. Glancy GD, Ash P, Bath EP, et al. AAPL practice guideline for the forensic assessment. J Am Acad Psychiatry Law. 2015;43(suppl 2):S3-S53.
3. Center for Substance Abuse Treatment. Drug testing in child welfare: practice and policy considerations. HHS Pub. No. (SMA) 10-4556. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
4. Macdonald DI, DuPont RL. The role of the medical review officer. In: Graham AW, Schultz TK, eds. Principles of addiction medicine, 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine; 1998:1259.
5. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015.
6. Marques PR, McKnight AS. Evaluating transdermal alcohol measuring devices. Calverton, MD: Pacific Institute for Research and Evaluation; 2007.
7. Steroidal.com. How steroid drug testing works. https://www.steroidal.com/steroid-detection-times. Accessed March 8, 2017.
8. Substance Abuse and Mental Health Services Administration. The role of biomarkers in the treatment of alcohol use disorders, 2012 revision. SAMHSA Advisory. 2012;11(2):1-8.
9. United States Drug Testing Laboratories, Inc. Detection of the direct alcohol biomarker ethyl glucuronide (EtG) in hair: an annotated bibliography. http://www.usdtl.com/media/white-papers/ETG_hair_annotated_bibliography_032014.pdf. Accessed March 8, 2017.
10. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788-14812.
11. SoberLink. https://www.soberlink.com. Accessed March 8, 2017.
12. Scram Systems. https://www.scramsystems.com/products/scram-continuous-alcohol-monitoring/?gclid=CIqUr8Kqx9ICFZmCswodI0QKPA. Accessed March 8, 2017.
13. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015:208.
14. Chamberlain RT. Legal review for testing of drugs in hair. Forensic Sci Rev. 2007;19(1-2):85-94.
15. Marriage of Carney, 24 Cal 3d725,157 Cal Rptr 383 (1979).
16. Marriage of Stout, 216 Mont 342 (Mont 1985).
17. Hynan DJ. Child custody evaluation, new theoretical applications and research. In: Hynan DJ. Difficult evaluation challenges: domestic violence, child abuse, substance abuse, and relocations. Springfield, IL: Charles C. Thomas Publisher; 2014:178-195.
1. Amato PT, Previti D. People’s reasons for divorcing: gender, social class, the life course, and adjustment. J Fam Issues. 2003;24(5):602-606.
2. Glancy GD, Ash P, Bath EP, et al. AAPL practice guideline for the forensic assessment. J Am Acad Psychiatry Law. 2015;43(suppl 2):S3-S53.
3. Center for Substance Abuse Treatment. Drug testing in child welfare: practice and policy considerations. HHS Pub. No. (SMA) 10-4556. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2010.
4. Macdonald DI, DuPont RL. The role of the medical review officer. In: Graham AW, Schultz TK, eds. Principles of addiction medicine, 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine; 1998:1259.
5. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015.
6. Marques PR, McKnight AS. Evaluating transdermal alcohol measuring devices. Calverton, MD: Pacific Institute for Research and Evaluation; 2007.
7. Steroidal.com. How steroid drug testing works. https://www.steroidal.com/steroid-detection-times. Accessed March 8, 2017.
8. Substance Abuse and Mental Health Services Administration. The role of biomarkers in the treatment of alcohol use disorders, 2012 revision. SAMHSA Advisory. 2012;11(2):1-8.
9. United States Drug Testing Laboratories, Inc. Detection of the direct alcohol biomarker ethyl glucuronide (EtG) in hair: an annotated bibliography. http://www.usdtl.com/media/white-papers/ETG_hair_annotated_bibliography_032014.pdf. Accessed March 8, 2017.
10. Viel G, Boscolo-Berto R, Cecchetto G, et al. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13(11):14788-14812.
11. SoberLink. https://www.soberlink.com. Accessed March 8, 2017.
12. Scram Systems. https://www.scramsystems.com/products/scram-continuous-alcohol-monitoring/?gclid=CIqUr8Kqx9ICFZmCswodI0QKPA. Accessed March 8, 2017.
13. Swotinsky RB. The medical review officer’s manual: MROCC’s guide to drug testing. 5th ed. Beverly Farms, MA: OEM Health Information; 2015:208.
14. Chamberlain RT. Legal review for testing of drugs in hair. Forensic Sci Rev. 2007;19(1-2):85-94.
15. Marriage of Carney, 24 Cal 3d725,157 Cal Rptr 383 (1979).
16. Marriage of Stout, 216 Mont 342 (Mont 1985).
17. Hynan DJ. Child custody evaluation, new theoretical applications and research. In: Hynan DJ. Difficult evaluation challenges: domestic violence, child abuse, substance abuse, and relocations. Springfield, IL: Charles C. Thomas Publisher; 2014:178-195.
Residual symptoms of schizophrenia: What are realistic treatment goals?
The course of chronic psychiatric conditions, such as schizophrenia, differs from chronic medical conditions, such as diabetes. Some patients with chronic psychiatric conditions achieve remission and become symptom-free, while others continue to have lingering signs of disease for life.
Residual symptoms of schizophrenia are not fully defined in the literature, which poses a challenge because they are central in the overall treatment of schizophrenia spectrum disorders.1 During this phase of schizophrenia, patients continue to have symptoms after psychosis has subsided. These patients might continue to have negative symptoms such as social and emotional withdrawal and low energy. Although frank psychotic behavior has disappeared, the patient might continue to hold strange beliefs. Pharmacotherapy is the primary treatment option for psychiatric conditions, but the psychosocial aspect may have greater importance when treating residual symptoms and patients with chronic psychiatric illness.2
A naturalistic study in Germany evaluated the occurrence and characteristics of residual symptoms in patients with schizophrenia.3 The authors used a Positive and Negative Syndrome Scale symptom severity score >1 for those purposes, which is possibly a stringent criterion to define residual symptoms. This multicenter study enrolled 399 individuals age 18 to 65 with a DSM-IV-TR diagnosis of schizophrenia, schizophreniform disorder, delusional disorder, or schizoaffective disorder.3 Of the 236 patients achieving remission at discharge, 94% had at least 1 residual symptom and 69% had at least 4 residual symptoms. Therefore, residual symptoms were highly prevalent in remitted patients. The most frequent residual symptoms were:
- blunted affect
- conceptual disorganization
- passive or apathetic social withdrawal
- emotional withdrawal
- lack of judgment and insight
- poor attention
- somatic concern
- difficulty with abstract thinking
- anxiety
- poor rapport.3
Of note, positive symptoms, such as delusions and hallucinatory behavior, were found in remitted patients at discharge (17% and 10%, respectively). The study concluded that the severity of residual symptoms was associated with relapse risk and had an overall negative impact on the outcome of patients with schizophrenia.3 The study noted that residual symptoms may be greater in number or volume than negative symptoms and questioned the origins of residual symptoms because most were present at baseline in more than two-third of patients.
Patients with residual symptoms of schizophrenia usually are older and therefore present specific management challenges for clinicians. Changes associated with aging, such as medical problems, cognitive deficits, and lack of social support, could create new care needs for this patient population. Although the biopsychosocial model used to treat chronic psychiatric conditions, especially schizophrenia, is preferred, older schizophrenia patients with residual symptoms often need more psychosocial interventions compared with young adults with schizophrenia.
Managing residual symptoms in schizophrenia
Few studies are devoted to pharmacological treatment of older adults with schizophrenia, likely because pharmacotherapy for older patients with schizophrenia can be challenging. Evidence-based treatment is based primarily on findings from younger patients who survived into later life. Clinicians often use the adage of geriatric psychiatry, “start low, go slow,” because older patients are susceptible to adverse effects associated with psychiatric medications, including cardiovascular, metabolic, anticholinergic, and extrapyramidal effects, orthostasis, sedation, falls, and neuroleptic malignant syndrome.
Older patients with schizophrenia are at an increased risk for extrapyramidal symptoms (EPS) and anticholinergic adverse effects, perhaps because of degeneration of dopaminergic and cholinergic neurons.4 Lowering the anticholinergic load by discontinuing or reducing the dosage of medications with anticholinergic properties, when possible, is a key principle when treating these patients. This tactic could help improve cognition and quality of life by decreasing the risk of other anticholinergic adverse effects, including delirium, constipation, urinary retention, and blurred vision.
Patients treated with typical antipsychotics are nearly twice as likely to develop tardive dyskinesia compared with those receiving atypical antipsychotics.5 Sedation, orthostatic hypotension, and anticholinergic effects can cause cognitive clouding, worsen cognitive impairment, and increase the risk of falls, especially in older patients.6 Clozapine and olanzapine have the strongest association with clinically significant weight gain and treatment-induced type 2 diabetes mellitus.7
The appropriate starting dosage of antipsychotics in older patients with schizophrenia is one-fourth of the starting adult dosage. Total daily maintenance dosages may be one-third to one-half of the adult dosage.6 Consensus guidelines for dosing atypical antipsychotics for older patients with schizophrenia are as shown in Table 1.8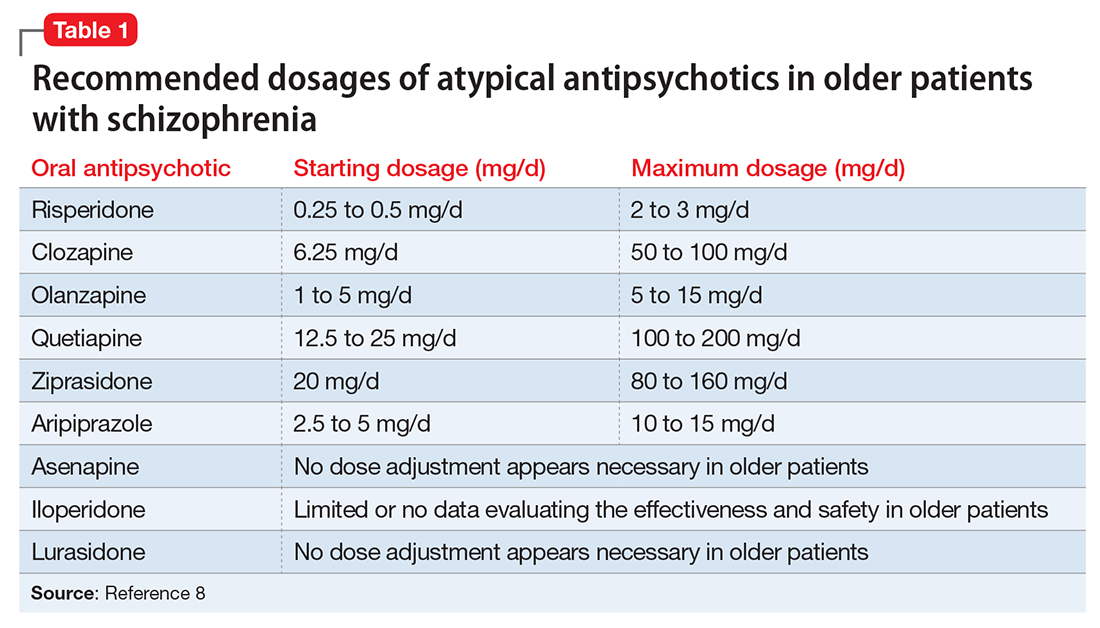
To ensure safety, patients should be regularly monitored with a complete blood count, comprehensive metabolic panel, lipid panel, hemoglobin A1C, electrocardiogram, orthostatic vital signs, Abnormal Involuntary Movement Scale, and weight check.7,9
When negative symptoms remain after a patient has achieved remission, it is important to evaluate whether the symptoms are related to adverse effects of medication (eg, parkinsonism syndrome), untreated depressive symptoms, or persistent positive symptoms, such as paranoia. Management of these symptoms consists of treating the cause, for example, using antipsychotics for primary positive symptoms, antidepressants for depression, anxiolytics for anxiety, and anti-parkinsonian agents or antipsychotic dosage reduction for EPS.
It is important to differentiate between negative symptoms of schizophrenia and depression in these patients. Negative symptoms of schizophrenia include affective flattening, alogia, avolition, and anhedonia. In depression, patients could have depressed mood, cognitive problems, sleep disturbances, and loss of appetite. Also, long-term symptoms are more consistent with negative symptomatology.
Keep in mind the potential for pharmacokinetic drug–drug interaction when using a combination of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, paroxetine, and fluvoxamine (to treat negative/depressive symptoms), because all are significant inhibitors of cytochrome P450 enzymes and increase antipsychotic plasma level. The Expert Treatment Guidelines for Patients with Schizophrenia recommends SSRIs, followed by venlafaxine then bupropion to treat depressive symptoms after optimizing second-generation antipsychotics.9
Another point to consider when treating residual symptoms in patients with schizophrenia is to not discontinue antipsychotic medications. Relapse rates for these patients can occur up to 5 times higher than for those who continue treatment.10 A way to address this problem could be the use of depot antipsychotic medications, but there are no set recommendations for the use of long-acting injectable antipsychotics in older patients. These medications should be used with caution and at lowest effective dosages to offset potential adverse effects.
With the introduction of typical and atypical antipsychotics, the use of electroconvulsive therapy in older patients with schizophrenia has declined. In a 2009 meta-analysis of studies that included patients with refractory schizophrenia and repetitive transcranial magnetic stimulation (rTMS), results revealed a mixed effect size for controlled and uncontrolled studies. The authors stated the need for further controlled trials, assessing the efficacy of rTMS on negative and positive symptoms of schizophrenia.11
Psychotherapy and psychosocial interventions
Patients with schizophrenia who have persistent psychotic symptoms while receiving adequate pharmacotherapy should be offered adjunctive cognitive, behaviorally oriented psychotherapy to reduce symptom severity. Cognitive-behavioral therapy (CBT) has been shown to help reduce relapse rates, reduce psychotic symptoms, and improve patients’ mental state.12 Amotivation and lack of insight can be particularly troublesome, which CBT can help address.12 Psychoeducation can:
- empower patients to understand their illness
- help them cope with their disease
- be aware of symptom relapse
- seek help sooner rather than later.
Also, counseling and supportive therapy are recommended by the American Psychiatric Association guidelines. Providers should involve family and loved ones in this discussion, so that they can help collaborate with care and provide a supportive and non-judgmental environment.
Older patients with residual symptoms of schizophrenia are less likely to have completed their education, pursued a career, or developed long-lasting relationships. Family members who were their support system earlier in life, such as parents, often are unable to provide care for them by the time patients with schizophrenia become older. These patients also are less likely to get married or have children, meaning that they are more likely to live alone. The advent of the interdisciplinary team, integration of several therapeutic modalities, the provision of case managers, and assertive community treatment (ACT) teams has provided help with social support, relapses, and hospitalizations, for older patients with schizophrenia.13 Key elements of ACT include:
- a multidisciplinary team, including a medication prescriber
- a shared caseload among team members
- direct service provision by team members
- frequent patient contact
- low patient to staff ratios
- outreach to patients in the community.
Medical care
Patients with schizophrenia are at higher risk for several comorbid medical conditions, such as diabetes, coronary artery disease, and digestive and liver disorders, compared with individuals without schizophrenia. This risk is associated with numerous factors, including sedentary lifestyle, high rates of lifetime cigarette use (70% to 80% of schizophrenia outpatients age <67 smoke), poor self-management skills, frequent homelessness, and unhealthy diet.
Although substantial attention is devoted to the psychiatric and behavioral management of patients with schizophrenia, many barriers impede the detection and treatment of their medical conditions. Patients with schizophrenia could experience delays in diagnosing a medical disorder, leading to more acute comorbidities at the time of diagnosis and premature mortality. Studies have confirmed that cardiovascular diseases are the leading cause of premature death among psychiatric patients in the United States.14 Key risk factors include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more common among patients with schizophrenia compared with the general population.15 In addition, antipsychotics are associated with adverse metabolic effects.16
What are realistic treatment goals to manage residual symptoms in schizophrenia?
We believe that because remission in schizophrenia has been defined consensually, the bar for treatment expectations is set higher than it was 20 years ago. There can be patient-, family-, and system-related variables affecting the feasibility of treating residual symptoms. Providers who treat patients with schizophrenia should consider the following treatment goals:
- Prevent relapse and acute psychiatric hospitalization
- Use evidence-based strategies to minimize or monitor adverse effects
- Monitor compliance and consider use of depot antipsychotics combined with patients’ preference
- Facilitate ongoing safety assessment, including suicide risk
- Monitor negative and cognitive symptoms in addition to positive symptoms, using evidence-based management
- Encourage collaboration of care with family, caretakers, and other members of the treatment team
- Empower patients by providing psychoeducation and social skills training and assisting in their vocational rehabilitation
- Educate the patient and family about healthy lifestyle interventions and medical comorbidities common with schizophrenia
- Perform baseline screening and follow-up for early detection and treatment of medical comorbidities in patients with schizophrenia
- Improve functional status and quality of life.
In addition to meeting these treatment goals, a measurement-based method can be implemented to monitor improvement and status of the independent treatment domains. A collection of rating instruments can be found in Table 2.17-30
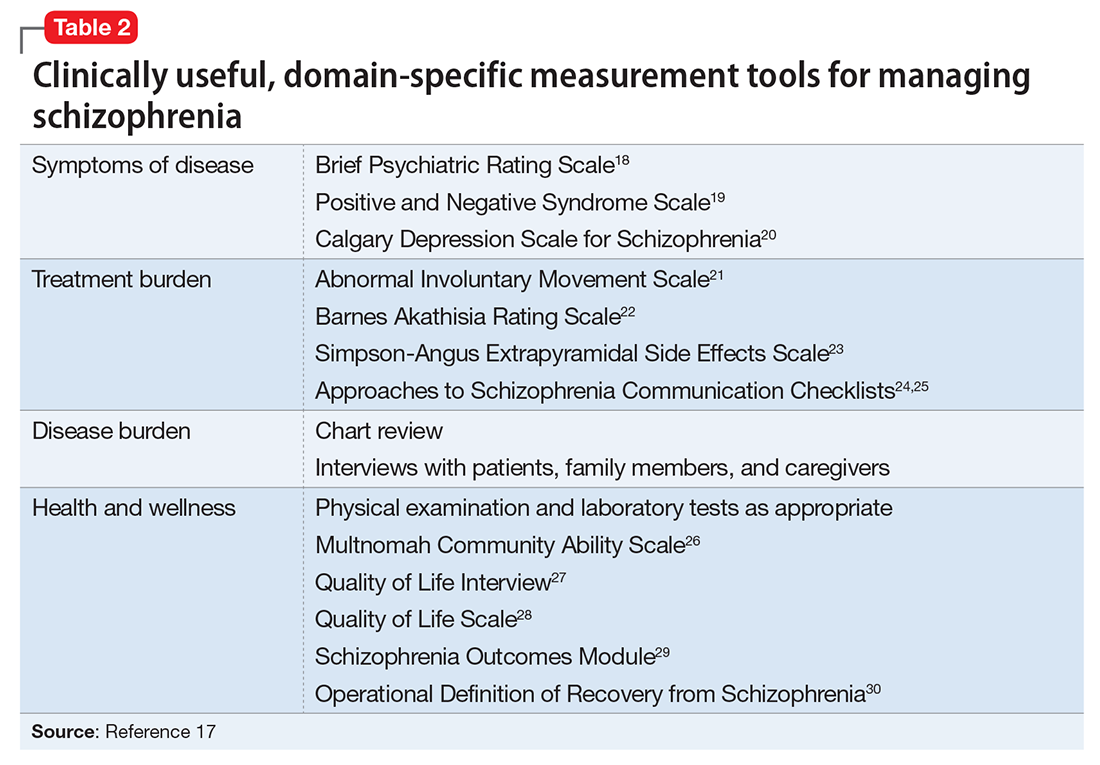
The clinical presentation of patients with residual symptoms of schizophrenia differs from that of other patients with schizophrenia. Our understanding of residual symptoms in schizophrenia has come a long way in the last decade; however, we are still far from pinning the complex nature of these symptoms, let alone their management. Given the risk of morbidity and disability, there clearly is a need for further investigation and investment of time and resources to support developing novel pharmacological treatment options to manage residual symptoms in patients with schizophrenia.
Because patients with residual symptoms of schizophrenia usually are older, psychiatrists should be responsible for implementing necessary screening assessments and should closely collaborate with primary care practitioners and other specialists, and when necessary, treat comorbid medical conditions. The importance of educating patients, their families, and the treatment team cannot be overlooked. Further, psychiatric treatment facilities should offer and promote healthy lifestyle interventions.
1. Kaiser S, Lyne J, Agartz I, et al. Individual negative symptoms and domains - relevance for assessment, pathomechanisms and treatment [published online July 21, 2016]. Schizophr Res. doi:10.1016/j.schres.2016.07.013.
2. Taylor M, Chaudhry I, Cross M, et al. Towards consensus in the long-term management of relapse prevention in schizophrenia. Hum Psychopharmacol. 2005;20(3):175-181.
3. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
4. Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging. 2000;17(5):363-384.
5. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
6. Sable JA, Jeste DV. Antipsychotic treatment for late-life schizophrenia. Curr Psychiatry Rep. 2002;4(4):299-306.
7. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
8. Khan AY, Redden W, Ovais M, et al. Current concepts in the diagnosis and treatment of schizophrenia in later life. Current Geriatric Reports. 2015;4(4):290-300.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1):64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
The course of chronic psychiatric conditions, such as schizophrenia, differs from chronic medical conditions, such as diabetes. Some patients with chronic psychiatric conditions achieve remission and become symptom-free, while others continue to have lingering signs of disease for life.
Residual symptoms of schizophrenia are not fully defined in the literature, which poses a challenge because they are central in the overall treatment of schizophrenia spectrum disorders.1 During this phase of schizophrenia, patients continue to have symptoms after psychosis has subsided. These patients might continue to have negative symptoms such as social and emotional withdrawal and low energy. Although frank psychotic behavior has disappeared, the patient might continue to hold strange beliefs. Pharmacotherapy is the primary treatment option for psychiatric conditions, but the psychosocial aspect may have greater importance when treating residual symptoms and patients with chronic psychiatric illness.2
A naturalistic study in Germany evaluated the occurrence and characteristics of residual symptoms in patients with schizophrenia.3 The authors used a Positive and Negative Syndrome Scale symptom severity score >1 for those purposes, which is possibly a stringent criterion to define residual symptoms. This multicenter study enrolled 399 individuals age 18 to 65 with a DSM-IV-TR diagnosis of schizophrenia, schizophreniform disorder, delusional disorder, or schizoaffective disorder.3 Of the 236 patients achieving remission at discharge, 94% had at least 1 residual symptom and 69% had at least 4 residual symptoms. Therefore, residual symptoms were highly prevalent in remitted patients. The most frequent residual symptoms were:
- blunted affect
- conceptual disorganization
- passive or apathetic social withdrawal
- emotional withdrawal
- lack of judgment and insight
- poor attention
- somatic concern
- difficulty with abstract thinking
- anxiety
- poor rapport.3
Of note, positive symptoms, such as delusions and hallucinatory behavior, were found in remitted patients at discharge (17% and 10%, respectively). The study concluded that the severity of residual symptoms was associated with relapse risk and had an overall negative impact on the outcome of patients with schizophrenia.3 The study noted that residual symptoms may be greater in number or volume than negative symptoms and questioned the origins of residual symptoms because most were present at baseline in more than two-third of patients.
Patients with residual symptoms of schizophrenia usually are older and therefore present specific management challenges for clinicians. Changes associated with aging, such as medical problems, cognitive deficits, and lack of social support, could create new care needs for this patient population. Although the biopsychosocial model used to treat chronic psychiatric conditions, especially schizophrenia, is preferred, older schizophrenia patients with residual symptoms often need more psychosocial interventions compared with young adults with schizophrenia.
Managing residual symptoms in schizophrenia
Few studies are devoted to pharmacological treatment of older adults with schizophrenia, likely because pharmacotherapy for older patients with schizophrenia can be challenging. Evidence-based treatment is based primarily on findings from younger patients who survived into later life. Clinicians often use the adage of geriatric psychiatry, “start low, go slow,” because older patients are susceptible to adverse effects associated with psychiatric medications, including cardiovascular, metabolic, anticholinergic, and extrapyramidal effects, orthostasis, sedation, falls, and neuroleptic malignant syndrome.
Older patients with schizophrenia are at an increased risk for extrapyramidal symptoms (EPS) and anticholinergic adverse effects, perhaps because of degeneration of dopaminergic and cholinergic neurons.4 Lowering the anticholinergic load by discontinuing or reducing the dosage of medications with anticholinergic properties, when possible, is a key principle when treating these patients. This tactic could help improve cognition and quality of life by decreasing the risk of other anticholinergic adverse effects, including delirium, constipation, urinary retention, and blurred vision.
Patients treated with typical antipsychotics are nearly twice as likely to develop tardive dyskinesia compared with those receiving atypical antipsychotics.5 Sedation, orthostatic hypotension, and anticholinergic effects can cause cognitive clouding, worsen cognitive impairment, and increase the risk of falls, especially in older patients.6 Clozapine and olanzapine have the strongest association with clinically significant weight gain and treatment-induced type 2 diabetes mellitus.7
The appropriate starting dosage of antipsychotics in older patients with schizophrenia is one-fourth of the starting adult dosage. Total daily maintenance dosages may be one-third to one-half of the adult dosage.6 Consensus guidelines for dosing atypical antipsychotics for older patients with schizophrenia are as shown in Table 1.8
To ensure safety, patients should be regularly monitored with a complete blood count, comprehensive metabolic panel, lipid panel, hemoglobin A1C, electrocardiogram, orthostatic vital signs, Abnormal Involuntary Movement Scale, and weight check.7,9
When negative symptoms remain after a patient has achieved remission, it is important to evaluate whether the symptoms are related to adverse effects of medication (eg, parkinsonism syndrome), untreated depressive symptoms, or persistent positive symptoms, such as paranoia. Management of these symptoms consists of treating the cause, for example, using antipsychotics for primary positive symptoms, antidepressants for depression, anxiolytics for anxiety, and anti-parkinsonian agents or antipsychotic dosage reduction for EPS.
It is important to differentiate between negative symptoms of schizophrenia and depression in these patients. Negative symptoms of schizophrenia include affective flattening, alogia, avolition, and anhedonia. In depression, patients could have depressed mood, cognitive problems, sleep disturbances, and loss of appetite. Also, long-term symptoms are more consistent with negative symptomatology.
Keep in mind the potential for pharmacokinetic drug–drug interaction when using a combination of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, paroxetine, and fluvoxamine (to treat negative/depressive symptoms), because all are significant inhibitors of cytochrome P450 enzymes and increase antipsychotic plasma level. The Expert Treatment Guidelines for Patients with Schizophrenia recommends SSRIs, followed by venlafaxine then bupropion to treat depressive symptoms after optimizing second-generation antipsychotics.9
Another point to consider when treating residual symptoms in patients with schizophrenia is to not discontinue antipsychotic medications. Relapse rates for these patients can occur up to 5 times higher than for those who continue treatment.10 A way to address this problem could be the use of depot antipsychotic medications, but there are no set recommendations for the use of long-acting injectable antipsychotics in older patients. These medications should be used with caution and at lowest effective dosages to offset potential adverse effects.
With the introduction of typical and atypical antipsychotics, the use of electroconvulsive therapy in older patients with schizophrenia has declined. In a 2009 meta-analysis of studies that included patients with refractory schizophrenia and repetitive transcranial magnetic stimulation (rTMS), results revealed a mixed effect size for controlled and uncontrolled studies. The authors stated the need for further controlled trials, assessing the efficacy of rTMS on negative and positive symptoms of schizophrenia.11
Psychotherapy and psychosocial interventions
Patients with schizophrenia who have persistent psychotic symptoms while receiving adequate pharmacotherapy should be offered adjunctive cognitive, behaviorally oriented psychotherapy to reduce symptom severity. Cognitive-behavioral therapy (CBT) has been shown to help reduce relapse rates, reduce psychotic symptoms, and improve patients’ mental state.12 Amotivation and lack of insight can be particularly troublesome, which CBT can help address.12 Psychoeducation can:
- empower patients to understand their illness
- help them cope with their disease
- be aware of symptom relapse
- seek help sooner rather than later.
Also, counseling and supportive therapy are recommended by the American Psychiatric Association guidelines. Providers should involve family and loved ones in this discussion, so that they can help collaborate with care and provide a supportive and non-judgmental environment.
Older patients with residual symptoms of schizophrenia are less likely to have completed their education, pursued a career, or developed long-lasting relationships. Family members who were their support system earlier in life, such as parents, often are unable to provide care for them by the time patients with schizophrenia become older. These patients also are less likely to get married or have children, meaning that they are more likely to live alone. The advent of the interdisciplinary team, integration of several therapeutic modalities, the provision of case managers, and assertive community treatment (ACT) teams has provided help with social support, relapses, and hospitalizations, for older patients with schizophrenia.13 Key elements of ACT include:
- a multidisciplinary team, including a medication prescriber
- a shared caseload among team members
- direct service provision by team members
- frequent patient contact
- low patient to staff ratios
- outreach to patients in the community.
Medical care
Patients with schizophrenia are at higher risk for several comorbid medical conditions, such as diabetes, coronary artery disease, and digestive and liver disorders, compared with individuals without schizophrenia. This risk is associated with numerous factors, including sedentary lifestyle, high rates of lifetime cigarette use (70% to 80% of schizophrenia outpatients age <67 smoke), poor self-management skills, frequent homelessness, and unhealthy diet.
Although substantial attention is devoted to the psychiatric and behavioral management of patients with schizophrenia, many barriers impede the detection and treatment of their medical conditions. Patients with schizophrenia could experience delays in diagnosing a medical disorder, leading to more acute comorbidities at the time of diagnosis and premature mortality. Studies have confirmed that cardiovascular diseases are the leading cause of premature death among psychiatric patients in the United States.14 Key risk factors include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more common among patients with schizophrenia compared with the general population.15 In addition, antipsychotics are associated with adverse metabolic effects.16
What are realistic treatment goals to manage residual symptoms in schizophrenia?
We believe that because remission in schizophrenia has been defined consensually, the bar for treatment expectations is set higher than it was 20 years ago. There can be patient-, family-, and system-related variables affecting the feasibility of treating residual symptoms. Providers who treat patients with schizophrenia should consider the following treatment goals:
- Prevent relapse and acute psychiatric hospitalization
- Use evidence-based strategies to minimize or monitor adverse effects
- Monitor compliance and consider use of depot antipsychotics combined with patients’ preference
- Facilitate ongoing safety assessment, including suicide risk
- Monitor negative and cognitive symptoms in addition to positive symptoms, using evidence-based management
- Encourage collaboration of care with family, caretakers, and other members of the treatment team
- Empower patients by providing psychoeducation and social skills training and assisting in their vocational rehabilitation
- Educate the patient and family about healthy lifestyle interventions and medical comorbidities common with schizophrenia
- Perform baseline screening and follow-up for early detection and treatment of medical comorbidities in patients with schizophrenia
- Improve functional status and quality of life.
In addition to meeting these treatment goals, a measurement-based method can be implemented to monitor improvement and status of the independent treatment domains. A collection of rating instruments can be found in Table 2.17-30

The clinical presentation of patients with residual symptoms of schizophrenia differs from that of other patients with schizophrenia. Our understanding of residual symptoms in schizophrenia has come a long way in the last decade; however, we are still far from pinning the complex nature of these symptoms, let alone their management. Given the risk of morbidity and disability, there clearly is a need for further investigation and investment of time and resources to support developing novel pharmacological treatment options to manage residual symptoms in patients with schizophrenia.
Because patients with residual symptoms of schizophrenia usually are older, psychiatrists should be responsible for implementing necessary screening assessments and should closely collaborate with primary care practitioners and other specialists, and when necessary, treat comorbid medical conditions. The importance of educating patients, their families, and the treatment team cannot be overlooked. Further, psychiatric treatment facilities should offer and promote healthy lifestyle interventions.
The course of chronic psychiatric conditions, such as schizophrenia, differs from chronic medical conditions, such as diabetes. Some patients with chronic psychiatric conditions achieve remission and become symptom-free, while others continue to have lingering signs of disease for life.
Residual symptoms of schizophrenia are not fully defined in the literature, which poses a challenge because they are central in the overall treatment of schizophrenia spectrum disorders.1 During this phase of schizophrenia, patients continue to have symptoms after psychosis has subsided. These patients might continue to have negative symptoms such as social and emotional withdrawal and low energy. Although frank psychotic behavior has disappeared, the patient might continue to hold strange beliefs. Pharmacotherapy is the primary treatment option for psychiatric conditions, but the psychosocial aspect may have greater importance when treating residual symptoms and patients with chronic psychiatric illness.2
A naturalistic study in Germany evaluated the occurrence and characteristics of residual symptoms in patients with schizophrenia.3 The authors used a Positive and Negative Syndrome Scale symptom severity score >1 for those purposes, which is possibly a stringent criterion to define residual symptoms. This multicenter study enrolled 399 individuals age 18 to 65 with a DSM-IV-TR diagnosis of schizophrenia, schizophreniform disorder, delusional disorder, or schizoaffective disorder.3 Of the 236 patients achieving remission at discharge, 94% had at least 1 residual symptom and 69% had at least 4 residual symptoms. Therefore, residual symptoms were highly prevalent in remitted patients. The most frequent residual symptoms were:
- blunted affect
- conceptual disorganization
- passive or apathetic social withdrawal
- emotional withdrawal
- lack of judgment and insight
- poor attention
- somatic concern
- difficulty with abstract thinking
- anxiety
- poor rapport.3
Of note, positive symptoms, such as delusions and hallucinatory behavior, were found in remitted patients at discharge (17% and 10%, respectively). The study concluded that the severity of residual symptoms was associated with relapse risk and had an overall negative impact on the outcome of patients with schizophrenia.3 The study noted that residual symptoms may be greater in number or volume than negative symptoms and questioned the origins of residual symptoms because most were present at baseline in more than two-third of patients.
Patients with residual symptoms of schizophrenia usually are older and therefore present specific management challenges for clinicians. Changes associated with aging, such as medical problems, cognitive deficits, and lack of social support, could create new care needs for this patient population. Although the biopsychosocial model used to treat chronic psychiatric conditions, especially schizophrenia, is preferred, older schizophrenia patients with residual symptoms often need more psychosocial interventions compared with young adults with schizophrenia.
Managing residual symptoms in schizophrenia
Few studies are devoted to pharmacological treatment of older adults with schizophrenia, likely because pharmacotherapy for older patients with schizophrenia can be challenging. Evidence-based treatment is based primarily on findings from younger patients who survived into later life. Clinicians often use the adage of geriatric psychiatry, “start low, go slow,” because older patients are susceptible to adverse effects associated with psychiatric medications, including cardiovascular, metabolic, anticholinergic, and extrapyramidal effects, orthostasis, sedation, falls, and neuroleptic malignant syndrome.
Older patients with schizophrenia are at an increased risk for extrapyramidal symptoms (EPS) and anticholinergic adverse effects, perhaps because of degeneration of dopaminergic and cholinergic neurons.4 Lowering the anticholinergic load by discontinuing or reducing the dosage of medications with anticholinergic properties, when possible, is a key principle when treating these patients. This tactic could help improve cognition and quality of life by decreasing the risk of other anticholinergic adverse effects, including delirium, constipation, urinary retention, and blurred vision.
Patients treated with typical antipsychotics are nearly twice as likely to develop tardive dyskinesia compared with those receiving atypical antipsychotics.5 Sedation, orthostatic hypotension, and anticholinergic effects can cause cognitive clouding, worsen cognitive impairment, and increase the risk of falls, especially in older patients.6 Clozapine and olanzapine have the strongest association with clinically significant weight gain and treatment-induced type 2 diabetes mellitus.7
The appropriate starting dosage of antipsychotics in older patients with schizophrenia is one-fourth of the starting adult dosage. Total daily maintenance dosages may be one-third to one-half of the adult dosage.6 Consensus guidelines for dosing atypical antipsychotics for older patients with schizophrenia are as shown in Table 1.8
To ensure safety, patients should be regularly monitored with a complete blood count, comprehensive metabolic panel, lipid panel, hemoglobin A1C, electrocardiogram, orthostatic vital signs, Abnormal Involuntary Movement Scale, and weight check.7,9
When negative symptoms remain after a patient has achieved remission, it is important to evaluate whether the symptoms are related to adverse effects of medication (eg, parkinsonism syndrome), untreated depressive symptoms, or persistent positive symptoms, such as paranoia. Management of these symptoms consists of treating the cause, for example, using antipsychotics for primary positive symptoms, antidepressants for depression, anxiolytics for anxiety, and anti-parkinsonian agents or antipsychotic dosage reduction for EPS.
It is important to differentiate between negative symptoms of schizophrenia and depression in these patients. Negative symptoms of schizophrenia include affective flattening, alogia, avolition, and anhedonia. In depression, patients could have depressed mood, cognitive problems, sleep disturbances, and loss of appetite. Also, long-term symptoms are more consistent with negative symptomatology.
Keep in mind the potential for pharmacokinetic drug–drug interaction when using a combination of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, paroxetine, and fluvoxamine (to treat negative/depressive symptoms), because all are significant inhibitors of cytochrome P450 enzymes and increase antipsychotic plasma level. The Expert Treatment Guidelines for Patients with Schizophrenia recommends SSRIs, followed by venlafaxine then bupropion to treat depressive symptoms after optimizing second-generation antipsychotics.9
Another point to consider when treating residual symptoms in patients with schizophrenia is to not discontinue antipsychotic medications. Relapse rates for these patients can occur up to 5 times higher than for those who continue treatment.10 A way to address this problem could be the use of depot antipsychotic medications, but there are no set recommendations for the use of long-acting injectable antipsychotics in older patients. These medications should be used with caution and at lowest effective dosages to offset potential adverse effects.
With the introduction of typical and atypical antipsychotics, the use of electroconvulsive therapy in older patients with schizophrenia has declined. In a 2009 meta-analysis of studies that included patients with refractory schizophrenia and repetitive transcranial magnetic stimulation (rTMS), results revealed a mixed effect size for controlled and uncontrolled studies. The authors stated the need for further controlled trials, assessing the efficacy of rTMS on negative and positive symptoms of schizophrenia.11
Psychotherapy and psychosocial interventions
Patients with schizophrenia who have persistent psychotic symptoms while receiving adequate pharmacotherapy should be offered adjunctive cognitive, behaviorally oriented psychotherapy to reduce symptom severity. Cognitive-behavioral therapy (CBT) has been shown to help reduce relapse rates, reduce psychotic symptoms, and improve patients’ mental state.12 Amotivation and lack of insight can be particularly troublesome, which CBT can help address.12 Psychoeducation can:
- empower patients to understand their illness
- help them cope with their disease
- be aware of symptom relapse
- seek help sooner rather than later.
Also, counseling and supportive therapy are recommended by the American Psychiatric Association guidelines. Providers should involve family and loved ones in this discussion, so that they can help collaborate with care and provide a supportive and non-judgmental environment.
Older patients with residual symptoms of schizophrenia are less likely to have completed their education, pursued a career, or developed long-lasting relationships. Family members who were their support system earlier in life, such as parents, often are unable to provide care for them by the time patients with schizophrenia become older. These patients also are less likely to get married or have children, meaning that they are more likely to live alone. The advent of the interdisciplinary team, integration of several therapeutic modalities, the provision of case managers, and assertive community treatment (ACT) teams has provided help with social support, relapses, and hospitalizations, for older patients with schizophrenia.13 Key elements of ACT include:
- a multidisciplinary team, including a medication prescriber
- a shared caseload among team members
- direct service provision by team members
- frequent patient contact
- low patient to staff ratios
- outreach to patients in the community.
Medical care
Patients with schizophrenia are at higher risk for several comorbid medical conditions, such as diabetes, coronary artery disease, and digestive and liver disorders, compared with individuals without schizophrenia. This risk is associated with numerous factors, including sedentary lifestyle, high rates of lifetime cigarette use (70% to 80% of schizophrenia outpatients age <67 smoke), poor self-management skills, frequent homelessness, and unhealthy diet.
Although substantial attention is devoted to the psychiatric and behavioral management of patients with schizophrenia, many barriers impede the detection and treatment of their medical conditions. Patients with schizophrenia could experience delays in diagnosing a medical disorder, leading to more acute comorbidities at the time of diagnosis and premature mortality. Studies have confirmed that cardiovascular diseases are the leading cause of premature death among psychiatric patients in the United States.14 Key risk factors include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more common among patients with schizophrenia compared with the general population.15 In addition, antipsychotics are associated with adverse metabolic effects.16
What are realistic treatment goals to manage residual symptoms in schizophrenia?
We believe that because remission in schizophrenia has been defined consensually, the bar for treatment expectations is set higher than it was 20 years ago. There can be patient-, family-, and system-related variables affecting the feasibility of treating residual symptoms. Providers who treat patients with schizophrenia should consider the following treatment goals:
- Prevent relapse and acute psychiatric hospitalization
- Use evidence-based strategies to minimize or monitor adverse effects
- Monitor compliance and consider use of depot antipsychotics combined with patients’ preference
- Facilitate ongoing safety assessment, including suicide risk
- Monitor negative and cognitive symptoms in addition to positive symptoms, using evidence-based management
- Encourage collaboration of care with family, caretakers, and other members of the treatment team
- Empower patients by providing psychoeducation and social skills training and assisting in their vocational rehabilitation
- Educate the patient and family about healthy lifestyle interventions and medical comorbidities common with schizophrenia
- Perform baseline screening and follow-up for early detection and treatment of medical comorbidities in patients with schizophrenia
- Improve functional status and quality of life.
In addition to meeting these treatment goals, a measurement-based method can be implemented to monitor improvement and status of the independent treatment domains. A collection of rating instruments can be found in Table 2.17-30

The clinical presentation of patients with residual symptoms of schizophrenia differs from that of other patients with schizophrenia. Our understanding of residual symptoms in schizophrenia has come a long way in the last decade; however, we are still far from pinning the complex nature of these symptoms, let alone their management. Given the risk of morbidity and disability, there clearly is a need for further investigation and investment of time and resources to support developing novel pharmacological treatment options to manage residual symptoms in patients with schizophrenia.
Because patients with residual symptoms of schizophrenia usually are older, psychiatrists should be responsible for implementing necessary screening assessments and should closely collaborate with primary care practitioners and other specialists, and when necessary, treat comorbid medical conditions. The importance of educating patients, their families, and the treatment team cannot be overlooked. Further, psychiatric treatment facilities should offer and promote healthy lifestyle interventions.
1. Kaiser S, Lyne J, Agartz I, et al. Individual negative symptoms and domains - relevance for assessment, pathomechanisms and treatment [published online July 21, 2016]. Schizophr Res. doi:10.1016/j.schres.2016.07.013.
2. Taylor M, Chaudhry I, Cross M, et al. Towards consensus in the long-term management of relapse prevention in schizophrenia. Hum Psychopharmacol. 2005;20(3):175-181.
3. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
4. Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging. 2000;17(5):363-384.
5. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
6. Sable JA, Jeste DV. Antipsychotic treatment for late-life schizophrenia. Curr Psychiatry Rep. 2002;4(4):299-306.
7. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
8. Khan AY, Redden W, Ovais M, et al. Current concepts in the diagnosis and treatment of schizophrenia in later life. Current Geriatric Reports. 2015;4(4):290-300.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1):64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
1. Kaiser S, Lyne J, Agartz I, et al. Individual negative symptoms and domains - relevance for assessment, pathomechanisms and treatment [published online July 21, 2016]. Schizophr Res. doi:10.1016/j.schres.2016.07.013.
2. Taylor M, Chaudhry I, Cross M, et al. Towards consensus in the long-term management of relapse prevention in schizophrenia. Hum Psychopharmacol. 2005;20(3):175-181.
3. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
4. Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging. 2000;17(5):363-384.
5. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
6. Sable JA, Jeste DV. Antipsychotic treatment for late-life schizophrenia. Curr Psychiatry Rep. 2002;4(4):299-306.
7. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
8. Khan AY, Redden W, Ovais M, et al. Current concepts in the diagnosis and treatment of schizophrenia in later life. Current Geriatric Reports. 2015;4(4):290-300.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1):64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.