User login
Prescribing antipsychotics in geriatric patients: Focus on schizophrenia and bipolar disorder
Antipsychotics are FDA-approved as a primary treatment for schizophrenia and bipolar disorder and as adjunctive therapy for major depressive disorder. In the United States, approximately 26% of antipsychotic prescriptions written for these indications are for individuals age >65.1 Additionally, antipsychotics are widely used to treat behavioral symptoms associated with dementia.1 The rapid expansion of the use of second-generation antipsychotics (SGAs), in particular, has been driven in part by their lower risk for extrapyramidal symptoms (EPS) compared with first-generation antipsychotics (FGAs).1 However, a growing body of data indicates that all antipsychotics have a range of adverse effects in older patients. This focus is critical in light of demographic trends—in the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.2
In this context, psychiatrists need information on the relative risks of antipsychotics for older patients. This 3-part series summarizes findings and recommendations on safety and tolerability when prescribing antipsychotics in older individuals with chronic psychotic disorders, such as schizophrenia, bipolar disorder, depression, and dementia. This review aims to:
- briefly summarize the major studies and analyses relevant to older patients with these diagnoses
- provide a summative opinion on safety and tolerability issues in these older adults
- highlight the gaps in the evidence base and areas that need additional research.
Part 1 focuses on older adults with schizophrenia or bipolar disorder. Subsequent articles will focus on prescribing antipsychotics to older adults with depression and those with dementia.
Schizophrenia
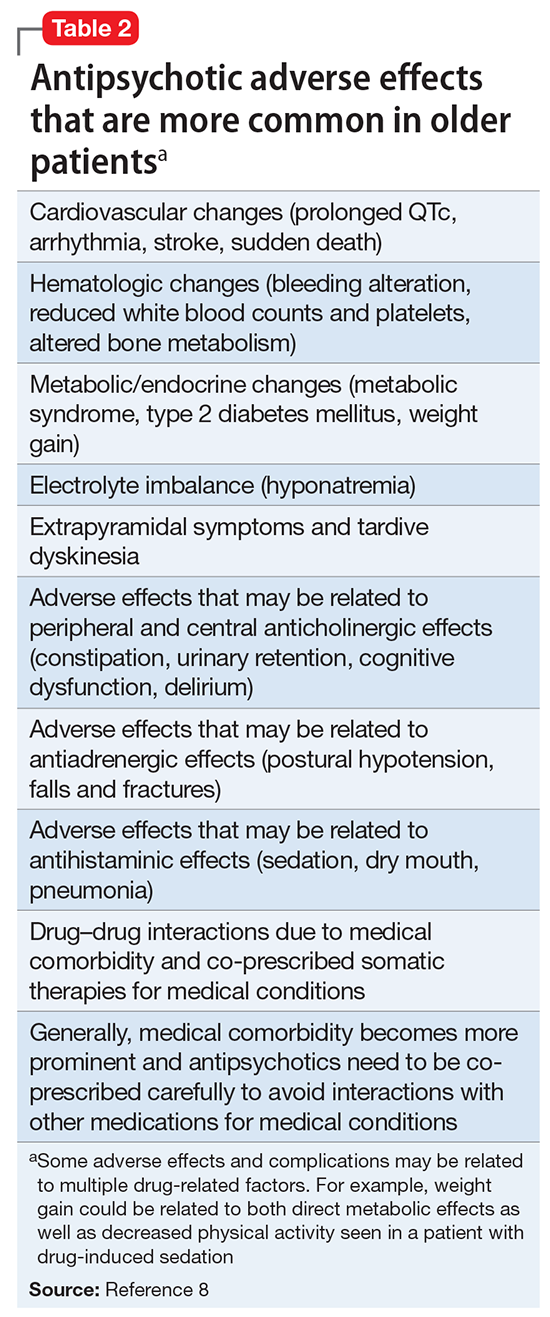
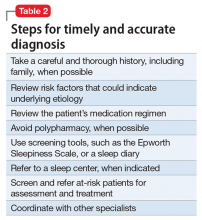
Summary of benefits, place in treatment armamentarium. Individuals with schizophrenia have a shorter life expectancy than that of the general population mostly as a result of suicide and comorbid physical illnesses,3 but the number of patients with schizophrenia age >55 will double over the next 2 decades.4 With aging, both positive and negative symptoms may be a focus of treatment (Table 1).5,6 Antipsychotics are a first-line treatment for older patients with schizophrenia with few medication alternatives.7 Safety risks associated with antipsychotics in older people span a broad spectrum (Table 2).8
A 6-week prospective RCT evaluated paliperidone extended-release vs placebo in 114 older adults (age ≥65 years; mean age, 70 years) with schizophrenia.14 There was an optional 24-week extension of open-label treatment with paliperidone. Mean daily dose of paliperidone was 8.4 mg. Efficacy measures did not show consistent statistically significant differences between treatment groups. Discontinuation rates were similar between paliperidone (7%) vs placebo (8%). Serious adverse events occurred in 3% of paliperidone-treated vs 8% of placebo-treated patients. Elevated prolactin levels occurred in one-half of paliperidone-treated patients. There were no prolactin or glucose treatment-related adverse events or significant mean changes in body weight for either paliperidone-treated or placebo-treated patients. Safety findings in the 24-week, open-label extension group were consistent with the RCT results.
Howanitz et al15 conducted a 12-week, prospective RCT that compared clozapine (mean dose, 300 mg/d) with chlorpromazine (mean dose, 600 mg/d) in 42 older adults (mean age, 67 years) with schizophrenia. Drop-out rate prior to 5 weeks was 19% and similar between groups. Common adverse effects included sialorrhea, hematologic abnormalities, sedation, tachycardia, EPS, and weight gain. Although both drugs were effective, more patients taking clozapine had tachycardia and weight gain, while more chlorpromazine patients reported sedation.
There have been other, less rigorous studies.7,8 Most of these studies evaluated risperidone and olanzapine, and most were conducted in “younger” geriatric patients (age <75 years). Although patients who participate in clinical trials may be healthier than “typical” patients, adverse effects such as EPS, sedation, and weight gain were still relatively common in these studies.
Other clinical data. A major consideration in treating older adults with schizophrenia is balancing the need to administer an antipsychotic dose high enough to alleviate psychotic symptoms while minimizing dose-dependent adverse effects. There is a U-shaped relationship between age and vulnerability to antipsychotic adverse effects,16,17 wherein adverse effects are highest at younger and older ages. Evidence supports using the lowest effective antipsychotic dose for geriatric patients with schizophrenia. Positive emission tomography (PET) studies suggest that older patients develop EPS with lower doses despite lower receptor occupancy.17,18 A recent study of 35 older patients (mean age, 60.1 years) with schizophrenia obtained PET, clinical measures, and blood pharmacokinetic measures before and after reduction of risperidone or olanzapine doses.18 A ≥40% reduction in dose was associated with reduced adverse effects, particularly EPS and elevation of prolactin levels. Moreover, the therapeutic window of striatal D2/D3 receptor occupancy appeared to be 50% to 60% in these older patients, compared with 65% to 80% in younger patients.
Long-term risks of antipsychotic treatment across the lifespan are less clear, with evidence suggesting both lower and higher mortality risk.19,20 It is difficult to fully disentangle the long-term risks of antipsychotics from the cumulative effects of lifestyle and comorbidity among individuals who have lived with schizophrenia for decades. Large naturalistic studies that include substantial numbers of older people with schizophrenia might be a way to elicit more information on long-term safety. The Schizophrenia Outpatient Health Outcome (SOHO) study was a large naturalistic trial that recruited >10,000 individuals with schizophrenia in 10 European countries.21 Although the SOHO study found differences between antipsychotics and adverse effects, such as EPS, weight gain, and sexual dysfunction, because the mean age of these patients was approximately 40 years and the follow-up period was only 3 years, it is difficult to draw conclusions that could be relevant to older individuals who have had schizophrenia for decades.
Bipolar Disorder
Clinical trials: Bipolar depression. A post hoc, secondary analysis of two 8-week, double-blind, randomized, placebo-controlled studies in bipolar depression compared 2 dosages of quetiapine (300 mg/d and 600 mg/d) with placebo in mixed-age patients.31 In a subgroup of 72 patients, ages 55 to 65, remission occurred more often with quetiapine than with placebo. Study discontinuation rates were similar between older people and younger people (age <55 years): quetiapine, 300 mg/d, 29.2%; quetiapine, 600 mg/d, 48.1%; and placebo, 29.6% in older adults, compared with 37.1%, 45.8%, and 38.1%, respectively, in younger adults. In all patients, the most common reason for discontinuation was adverse events with quetiapine and lack of efficacy for placebo. Adverse event rates were similar in older and younger adults. Dry mouth and dizziness were more common in older adults. Proportions of adults experiencing clinically significant weight gain (≥7% of body weight) were 5.3%, 8.3%, and 0% in older adults receiving quetiapine, 300 mg/d, quetiapine, 600 mg/d, and placebo, respectively, compared with 7.2%, 10.1%, and 2.6% in younger adults. EPS and treatment-emergent mania were minimal.
A secondary analysis of mixed-age, RCTs examined response in older adults (age ≥55 years) with bipolar I depression who received lurasidone as monotherapy or adjunctive therapy.32 In the monotherapy study, these patients were randomized to 6 weeks of lurasidone 20 to 60 mg/d, lurasidone 80 to 120 mg/d, or placebo. In the adjunctive therapy study, they were randomized to lurasidone 20 to 120 mg/d or placebo with either lithium or valproate. There were 83 older adults (17.1% of the sample) in the monotherapy study and 53 (15.6%) in the adjunctive therapy study. Mean improvement in depression was significantly higher for both doses of lurasidone monotherapy than placebo. Adjunctive lurasidone was not associated with statistically significant improvement vs placebo. The most frequent adverse events in older patients on lurasidone monotherapy 20 to 60 mg/d or 80 to 120 mg/d were nausea (18.5% and 9.7%, respectively) and somnolence (11.1% and 0%, respectively). Akathisia (9.7%) and insomnia (9.7%) were the most common adverse events in the group receiving 80 to 120 mg/d, with the rate of akathisia exhibiting a dose-related increase. Weight change with lurasidone was similar to placebo, and there were no clinically meaningful group changes in vital signs, electrocardiography, or laboratory parameters.
A small (N = 20) open study found improvement in older adults with bipolar depression with aripiprazole (mean dose, 10.3 mg/d).33 Adverse effects included restlessness and weight gain (n = 3, 9% each), sedation (n = 2, 10%), and drooling and diarrhea/loose stools (n = 1, 5% each). In another small study (N = 15) using asenapine (mean dose, 11.2 mg/d) in mainly older bipolar patients with depression, the most common adverse effects were gastrointestinal (GI) discomfort (n = 5, 33%) and restlessness, tremors, cognitive difficulties, and sluggishness (n = 2, 13% each).34
Clinical trials: Bipolar mania. Researchers conducted a pooled analysis of two 12-week randomized trials comparing quetiapine with placebo in a mixed-age sample with bipolar mania.35 In a subgroup of 59 older patients (mean age, 62.9 years), manic symptoms improved significantly more with quetiapine (modal dose, 550 mg/d) than with placebo. Adverse effects reported by >10% of older patients were dry mouth, somnolence, postural hypotension, insomnia, weight gain, and dizziness. Insomnia was reported by >10% of patients receiving placebo.
In a case series of 11 elderly patients with mania receiving asenapine, Baruch et al36 reported a 63% remission rate. One patient discontinued the study because of a new rash, 1 discontinued after developing peripheral edema, and 3 patients reported mild sedation.
Beyer et al37 reported on a post hoc analysis of 94 older adults (mean age, 57.1 years; range, 50.1 to 74.8 years) with acute bipolar mania receiving olanzapine (n = 47), divalproex (n = 31), or placebo (n = 16) in a pooled olanzapine clinical trials database. Patients receiving olanzapine or divalproex had improvement in mania; those receiving placebo did not improve. Safety findings were comparable with reports in younger patients with mania.
Other clinical data. Adverse effects found in mixed-age samples using secondary analyses of clinical trials need to be interpreted with caution because these types of studies usually exclude individuals with significant medical comorbidity. Medical burden, cognitive impairment, or concomitant medications generally necessitate slower drug titration and lower total daily dosing. For example, a secondary analysis of the U.S. National Institute of Health-funded Systematic Treatment Enhancement Program for Bipolar Disorder study, which had broader inclusion criteria than most clinical trials, reported that, although recovery rates in older adults with bipolar disorder were fairly good (78.5%), lower doses of risperidone were used in older vs younger patients.38
Clinical considerations
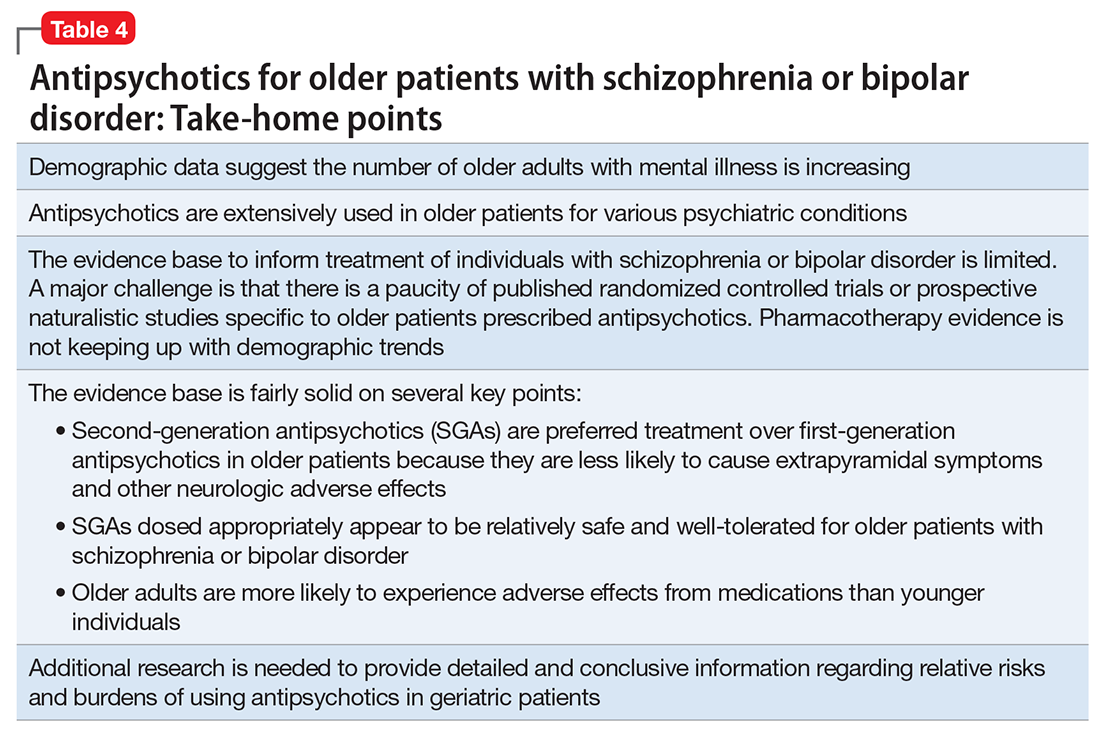
Interpretation of the relative risks of antipsychotics in older people must be tempered by the caveat that there is limited high-quality data (Table 4). Antipsychotics are the first-line therapy for older patients with schizophrenia, although their use is supported by a small number of prospective RCTs. SGAs are preferred because of their lower propensity to cause EPS and other motor adverse effects. Older persons with schizophrenia have an EPS threshold lower than younger patients and determining the lowest effective dosage may minimize EPS and cognitive adverse effects. As individuals with long-standing schizophrenia get older, their antipsychotic dosages may need to be reduced, and clinicians need to monitor for adverse effects that are more common among older people, such as tardive dyskinesia and metabolic abnormalities. In healthy, “younger” geriatric patients, monitoring for adverse effects may be similar to monitoring of younger patients. Patients who are older or frail may need more frequent assessment.
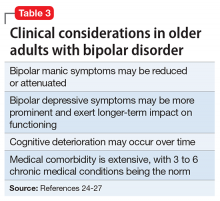
Like older adults with schizophrenia, geriatric patients with bipolar disorder have reduced drug tolerability and experience more adverse effects than younger patients. There are no prospective controlled studies that evaluated using antipsychotics in older patients with bipolar disorder. In older bipolar patients, the most problematic adverse effects of antipsychotics are akathisia, parkinsonism, other EPS, sedation and dizziness (which may increase fall risk), and GI discomfort. A key tolerability and safety consideration when treating older adults with bipolar disorder is the role of antipsychotics in relation to the use of lithium and mood stabilizers. Some studies have suggested that lithium has neuroprotective effects when used long-term; however, at least 1 report suggested that long-term antipsychotic treatment may be associated with neurodegeneration.39
The literature does not provide strong evidence on the many clinical variations that we see in routine practice settings, such as combinations of drug treatments or drugs prescribed to patients with specific comorbid conditions. There is a need for large cohort studies that monitor treatment course, medical comorbidity, and prognosis. Additionally, well-designed clinical trials such as the DART-AD, which investigated longer-term trajectories of people with dementia taking antipsychotics, should serve as a model for the type of research that is needed to better understand outcome variability among older people with chronic psychotic or bipolar disorders.40
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. United Nations, Department of Economic and Social Affairs, Population Division. World population ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Accessed September 1, 2017.
3. Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55(12):752-760.
4. Cohen CI, Vahia I, Reyes P, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. 2008;59(3):232-234.
5. Jeste DV, Barak Y, Madhusoodanan S, et al. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):638-647.
6. Kalache SM, Mulsant BH, Davies SJ, et al. The impact of aging, cognition, and symptoms on functional competence in individuals with schizophrenia across the lifespan. Schizophr Bull. 2015;41(2):374-381.
7. Suzuki T, Remington G, Uchida H, et al. Management of schizophrenia in late life with antipsychotic medications: a qualitative review. Drugs Aging. 2011;28(12):961-980.
8. Mulsant BH, Pollock BG. Psychopharmacology. In: David C. Steffens DC, Blazer DG, Thakur ME (eds). The American Psychiatric Publishing Textbook of Geriatric Psychiatry, 5th Edition. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
9. Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2(4):340-350.
10. Marriott RG, Neil W, Waddingham S. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database Syst Rev. 2006;(1):CD005580.
11. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012(2):CD004162.
12. Scott J, Greenwald BS, Kramer E, et al. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr. 2011;23(5):742-748.
13. Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783-791.
14. Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry. 2008;16(1):31-43.
15. Howanitz E, Pardo M, Smelson DA, et al. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. J Clin Psychiatry. 1999;60(1):41-44.
16. Sproule BA, Lake J, Mamo DC, et al. Are antipsychotic prescribing patterns different in older and younger adults?: a survey of 1357 psychiatric inpatients in Toronto. Can J Psychiatry. 2010;55(4):248-254.
17. Uchida H, Suzuki T, Mamo DC, et al. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry. 2008;16(7):584-593.
18. Graff-Guerrero A, Rajji TK, Mulsant BH, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 occupancy study. JAMA Psychiatry. 2015;72(9):927-934.
19. Khan A, Schwartz K, Stern C, et al. Mortality risk in patients with schizophrenia participating in premarketing atypical antipsychotic clinical trials. J Clin Psychiatry. 2007;68(12):1828-1833.
20. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: a systematic review. Schizophr Res. 2009;113(1):1-11.
21. Novick D, Haro JM, Perrin E, et al. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Eur Neuropsychopharmacol. 2009;19(8):542-550.
22. Sajatovic M, Blow FC, Ignacio RV, et al. Age-related modifiers of clinical presentation and health service use among veterans with bipolar disorder. Psychiatr Serv. 2004;55(9):1014-1021.
23. Jeste DV, Alexopoulos GS, Bartels SJ, et al. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry. 1999;56(9):848-853.
24. Sajatovic M, Chen P. Geriatric bipolar disorder. Psychiatr Clin North Am. 2011;34(2):319-333,vii.
25. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
26. Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol. 2012;25(1):20-25.
27. Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
28. Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63(1):83-86.
29. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26(5):603-617.
30. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
31. Sajatovic M, Paulsson B. Quetiapine for the treatment of depressive episodes in adults aged 55 to 65 years with bipolar disorder. Paper presented at: American Association of Geriatric Psychiatry Annual Meeting; 2007; New Orleans, LA.
32. Sajatovic M, Forester B, Tsai J, et al. Efficacy and safety of lurasidone in older adults with bipolar depression: analysis of two double-blind, placebo-controlled studies. Paper presented at: American College of Neuropsychopharmacology (ACNP) 53rd Annual Meeting; 2014; Phoenix, AZ.
33. Sajatovic M, Coconcea N, Ignacio RV, et al. Aripiprazole therapy in 20 older adults with bipolar disorder: a 12-week, open-label trial. J Clin Psychiatry. 2008;69(1):41-46.
34. Sajatovic M, Dines P, Fuentes-Casiano E, et al. Asenapine in the treatment of older adults with bipolar disorder. Int J Geriatr Psychiatry. 2015;30(7):710-719.
35. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
36. Baruch Y, Tadger S, Plopski I, et al. Asenapine for elderly bipolar manic patients. J Affect Disord. 2013;145(1):130-132.
37. Beyer JL, Siegal A, Kennedy JS. Olanzapine, divalproex and placebo treatment, non-head to head comparisons of older adults acute mania. Paper presented at: 10th Congress of the International Psychogeriatric Association; 2001; Nice, France.
38. Al Jurdi RK, Marangell LB, Petersen NJ, et al. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry. 2008;16(11):922-933.
39. Gildengers AG, Chung KH, Huang SH, et al. Neuroprogressive effects of lifetime illness duration in older adults with bipolar disorder. Bipolar Disord. 2014;16(6):617-623.
40. Ballard C, Lana MM, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76.
Antipsychotics are FDA-approved as a primary treatment for schizophrenia and bipolar disorder and as adjunctive therapy for major depressive disorder. In the United States, approximately 26% of antipsychotic prescriptions written for these indications are for individuals age >65.1 Additionally, antipsychotics are widely used to treat behavioral symptoms associated with dementia.1 The rapid expansion of the use of second-generation antipsychotics (SGAs), in particular, has been driven in part by their lower risk for extrapyramidal symptoms (EPS) compared with first-generation antipsychotics (FGAs).1 However, a growing body of data indicates that all antipsychotics have a range of adverse effects in older patients. This focus is critical in light of demographic trends—in the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.2
In this context, psychiatrists need information on the relative risks of antipsychotics for older patients. This 3-part series summarizes findings and recommendations on safety and tolerability when prescribing antipsychotics in older individuals with chronic psychotic disorders, such as schizophrenia, bipolar disorder, depression, and dementia. This review aims to:
- briefly summarize the major studies and analyses relevant to older patients with these diagnoses
- provide a summative opinion on safety and tolerability issues in these older adults
- highlight the gaps in the evidence base and areas that need additional research.
Part 1 focuses on older adults with schizophrenia or bipolar disorder. Subsequent articles will focus on prescribing antipsychotics to older adults with depression and those with dementia.
Schizophrenia
Summary of benefits, place in treatment armamentarium. Individuals with schizophrenia have a shorter life expectancy than that of the general population mostly as a result of suicide and comorbid physical illnesses,3 but the number of patients with schizophrenia age >55 will double over the next 2 decades.4 With aging, both positive and negative symptoms may be a focus of treatment (Table 1).5,6 Antipsychotics are a first-line treatment for older patients with schizophrenia with few medication alternatives.7 Safety risks associated with antipsychotics in older people span a broad spectrum (Table 2).8
A 6-week prospective RCT evaluated paliperidone extended-release vs placebo in 114 older adults (age ≥65 years; mean age, 70 years) with schizophrenia.14 There was an optional 24-week extension of open-label treatment with paliperidone. Mean daily dose of paliperidone was 8.4 mg. Efficacy measures did not show consistent statistically significant differences between treatment groups. Discontinuation rates were similar between paliperidone (7%) vs placebo (8%). Serious adverse events occurred in 3% of paliperidone-treated vs 8% of placebo-treated patients. Elevated prolactin levels occurred in one-half of paliperidone-treated patients. There were no prolactin or glucose treatment-related adverse events or significant mean changes in body weight for either paliperidone-treated or placebo-treated patients. Safety findings in the 24-week, open-label extension group were consistent with the RCT results.
Howanitz et al15 conducted a 12-week, prospective RCT that compared clozapine (mean dose, 300 mg/d) with chlorpromazine (mean dose, 600 mg/d) in 42 older adults (mean age, 67 years) with schizophrenia. Drop-out rate prior to 5 weeks was 19% and similar between groups. Common adverse effects included sialorrhea, hematologic abnormalities, sedation, tachycardia, EPS, and weight gain. Although both drugs were effective, more patients taking clozapine had tachycardia and weight gain, while more chlorpromazine patients reported sedation.
There have been other, less rigorous studies.7,8 Most of these studies evaluated risperidone and olanzapine, and most were conducted in “younger” geriatric patients (age <75 years). Although patients who participate in clinical trials may be healthier than “typical” patients, adverse effects such as EPS, sedation, and weight gain were still relatively common in these studies.
Other clinical data. A major consideration in treating older adults with schizophrenia is balancing the need to administer an antipsychotic dose high enough to alleviate psychotic symptoms while minimizing dose-dependent adverse effects. There is a U-shaped relationship between age and vulnerability to antipsychotic adverse effects,16,17 wherein adverse effects are highest at younger and older ages. Evidence supports using the lowest effective antipsychotic dose for geriatric patients with schizophrenia. Positive emission tomography (PET) studies suggest that older patients develop EPS with lower doses despite lower receptor occupancy.17,18 A recent study of 35 older patients (mean age, 60.1 years) with schizophrenia obtained PET, clinical measures, and blood pharmacokinetic measures before and after reduction of risperidone or olanzapine doses.18 A ≥40% reduction in dose was associated with reduced adverse effects, particularly EPS and elevation of prolactin levels. Moreover, the therapeutic window of striatal D2/D3 receptor occupancy appeared to be 50% to 60% in these older patients, compared with 65% to 80% in younger patients.
Long-term risks of antipsychotic treatment across the lifespan are less clear, with evidence suggesting both lower and higher mortality risk.19,20 It is difficult to fully disentangle the long-term risks of antipsychotics from the cumulative effects of lifestyle and comorbidity among individuals who have lived with schizophrenia for decades. Large naturalistic studies that include substantial numbers of older people with schizophrenia might be a way to elicit more information on long-term safety. The Schizophrenia Outpatient Health Outcome (SOHO) study was a large naturalistic trial that recruited >10,000 individuals with schizophrenia in 10 European countries.21 Although the SOHO study found differences between antipsychotics and adverse effects, such as EPS, weight gain, and sexual dysfunction, because the mean age of these patients was approximately 40 years and the follow-up period was only 3 years, it is difficult to draw conclusions that could be relevant to older individuals who have had schizophrenia for decades.
Bipolar Disorder
Clinical trials: Bipolar depression. A post hoc, secondary analysis of two 8-week, double-blind, randomized, placebo-controlled studies in bipolar depression compared 2 dosages of quetiapine (300 mg/d and 600 mg/d) with placebo in mixed-age patients.31 In a subgroup of 72 patients, ages 55 to 65, remission occurred more often with quetiapine than with placebo. Study discontinuation rates were similar between older people and younger people (age <55 years): quetiapine, 300 mg/d, 29.2%; quetiapine, 600 mg/d, 48.1%; and placebo, 29.6% in older adults, compared with 37.1%, 45.8%, and 38.1%, respectively, in younger adults. In all patients, the most common reason for discontinuation was adverse events with quetiapine and lack of efficacy for placebo. Adverse event rates were similar in older and younger adults. Dry mouth and dizziness were more common in older adults. Proportions of adults experiencing clinically significant weight gain (≥7% of body weight) were 5.3%, 8.3%, and 0% in older adults receiving quetiapine, 300 mg/d, quetiapine, 600 mg/d, and placebo, respectively, compared with 7.2%, 10.1%, and 2.6% in younger adults. EPS and treatment-emergent mania were minimal.
A secondary analysis of mixed-age, RCTs examined response in older adults (age ≥55 years) with bipolar I depression who received lurasidone as monotherapy or adjunctive therapy.32 In the monotherapy study, these patients were randomized to 6 weeks of lurasidone 20 to 60 mg/d, lurasidone 80 to 120 mg/d, or placebo. In the adjunctive therapy study, they were randomized to lurasidone 20 to 120 mg/d or placebo with either lithium or valproate. There were 83 older adults (17.1% of the sample) in the monotherapy study and 53 (15.6%) in the adjunctive therapy study. Mean improvement in depression was significantly higher for both doses of lurasidone monotherapy than placebo. Adjunctive lurasidone was not associated with statistically significant improvement vs placebo. The most frequent adverse events in older patients on lurasidone monotherapy 20 to 60 mg/d or 80 to 120 mg/d were nausea (18.5% and 9.7%, respectively) and somnolence (11.1% and 0%, respectively). Akathisia (9.7%) and insomnia (9.7%) were the most common adverse events in the group receiving 80 to 120 mg/d, with the rate of akathisia exhibiting a dose-related increase. Weight change with lurasidone was similar to placebo, and there were no clinically meaningful group changes in vital signs, electrocardiography, or laboratory parameters.
A small (N = 20) open study found improvement in older adults with bipolar depression with aripiprazole (mean dose, 10.3 mg/d).33 Adverse effects included restlessness and weight gain (n = 3, 9% each), sedation (n = 2, 10%), and drooling and diarrhea/loose stools (n = 1, 5% each). In another small study (N = 15) using asenapine (mean dose, 11.2 mg/d) in mainly older bipolar patients with depression, the most common adverse effects were gastrointestinal (GI) discomfort (n = 5, 33%) and restlessness, tremors, cognitive difficulties, and sluggishness (n = 2, 13% each).34
Clinical trials: Bipolar mania. Researchers conducted a pooled analysis of two 12-week randomized trials comparing quetiapine with placebo in a mixed-age sample with bipolar mania.35 In a subgroup of 59 older patients (mean age, 62.9 years), manic symptoms improved significantly more with quetiapine (modal dose, 550 mg/d) than with placebo. Adverse effects reported by >10% of older patients were dry mouth, somnolence, postural hypotension, insomnia, weight gain, and dizziness. Insomnia was reported by >10% of patients receiving placebo.
In a case series of 11 elderly patients with mania receiving asenapine, Baruch et al36 reported a 63% remission rate. One patient discontinued the study because of a new rash, 1 discontinued after developing peripheral edema, and 3 patients reported mild sedation.
Beyer et al37 reported on a post hoc analysis of 94 older adults (mean age, 57.1 years; range, 50.1 to 74.8 years) with acute bipolar mania receiving olanzapine (n = 47), divalproex (n = 31), or placebo (n = 16) in a pooled olanzapine clinical trials database. Patients receiving olanzapine or divalproex had improvement in mania; those receiving placebo did not improve. Safety findings were comparable with reports in younger patients with mania.
Other clinical data. Adverse effects found in mixed-age samples using secondary analyses of clinical trials need to be interpreted with caution because these types of studies usually exclude individuals with significant medical comorbidity. Medical burden, cognitive impairment, or concomitant medications generally necessitate slower drug titration and lower total daily dosing. For example, a secondary analysis of the U.S. National Institute of Health-funded Systematic Treatment Enhancement Program for Bipolar Disorder study, which had broader inclusion criteria than most clinical trials, reported that, although recovery rates in older adults with bipolar disorder were fairly good (78.5%), lower doses of risperidone were used in older vs younger patients.38
Clinical considerations
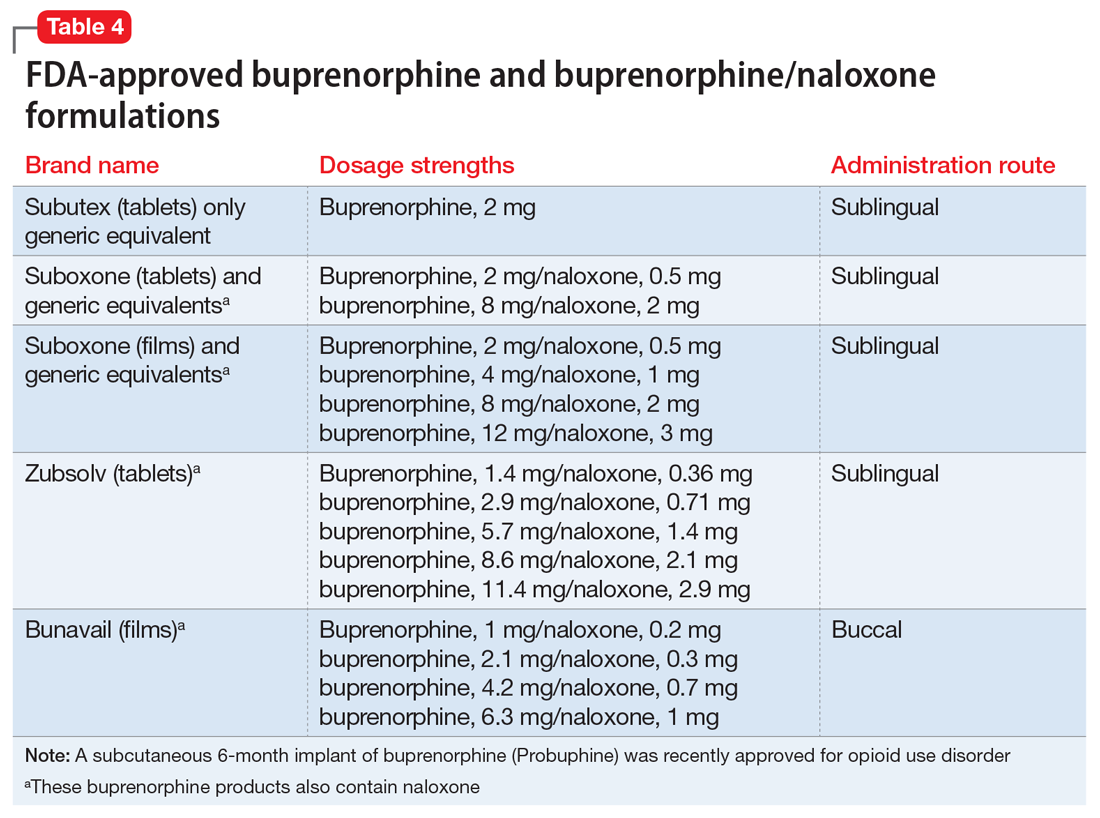
Interpretation of the relative risks of antipsychotics in older people must be tempered by the caveat that there is limited high-quality data (Table 4). Antipsychotics are the first-line therapy for older patients with schizophrenia, although their use is supported by a small number of prospective RCTs. SGAs are preferred because of their lower propensity to cause EPS and other motor adverse effects. Older persons with schizophrenia have an EPS threshold lower than younger patients and determining the lowest effective dosage may minimize EPS and cognitive adverse effects. As individuals with long-standing schizophrenia get older, their antipsychotic dosages may need to be reduced, and clinicians need to monitor for adverse effects that are more common among older people, such as tardive dyskinesia and metabolic abnormalities. In healthy, “younger” geriatric patients, monitoring for adverse effects may be similar to monitoring of younger patients. Patients who are older or frail may need more frequent assessment.
Like older adults with schizophrenia, geriatric patients with bipolar disorder have reduced drug tolerability and experience more adverse effects than younger patients. There are no prospective controlled studies that evaluated using antipsychotics in older patients with bipolar disorder. In older bipolar patients, the most problematic adverse effects of antipsychotics are akathisia, parkinsonism, other EPS, sedation and dizziness (which may increase fall risk), and GI discomfort. A key tolerability and safety consideration when treating older adults with bipolar disorder is the role of antipsychotics in relation to the use of lithium and mood stabilizers. Some studies have suggested that lithium has neuroprotective effects when used long-term; however, at least 1 report suggested that long-term antipsychotic treatment may be associated with neurodegeneration.39
The literature does not provide strong evidence on the many clinical variations that we see in routine practice settings, such as combinations of drug treatments or drugs prescribed to patients with specific comorbid conditions. There is a need for large cohort studies that monitor treatment course, medical comorbidity, and prognosis. Additionally, well-designed clinical trials such as the DART-AD, which investigated longer-term trajectories of people with dementia taking antipsychotics, should serve as a model for the type of research that is needed to better understand outcome variability among older people with chronic psychotic or bipolar disorders.40
Antipsychotics are FDA-approved as a primary treatment for schizophrenia and bipolar disorder and as adjunctive therapy for major depressive disorder. In the United States, approximately 26% of antipsychotic prescriptions written for these indications are for individuals age >65.1 Additionally, antipsychotics are widely used to treat behavioral symptoms associated with dementia.1 The rapid expansion of the use of second-generation antipsychotics (SGAs), in particular, has been driven in part by their lower risk for extrapyramidal symptoms (EPS) compared with first-generation antipsychotics (FGAs).1 However, a growing body of data indicates that all antipsychotics have a range of adverse effects in older patients. This focus is critical in light of demographic trends—in the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.2
In this context, psychiatrists need information on the relative risks of antipsychotics for older patients. This 3-part series summarizes findings and recommendations on safety and tolerability when prescribing antipsychotics in older individuals with chronic psychotic disorders, such as schizophrenia, bipolar disorder, depression, and dementia. This review aims to:
- briefly summarize the major studies and analyses relevant to older patients with these diagnoses
- provide a summative opinion on safety and tolerability issues in these older adults
- highlight the gaps in the evidence base and areas that need additional research.
Part 1 focuses on older adults with schizophrenia or bipolar disorder. Subsequent articles will focus on prescribing antipsychotics to older adults with depression and those with dementia.
Schizophrenia
Summary of benefits, place in treatment armamentarium. Individuals with schizophrenia have a shorter life expectancy than that of the general population mostly as a result of suicide and comorbid physical illnesses,3 but the number of patients with schizophrenia age >55 will double over the next 2 decades.4 With aging, both positive and negative symptoms may be a focus of treatment (Table 1).5,6 Antipsychotics are a first-line treatment for older patients with schizophrenia with few medication alternatives.7 Safety risks associated with antipsychotics in older people span a broad spectrum (Table 2).8
A 6-week prospective RCT evaluated paliperidone extended-release vs placebo in 114 older adults (age ≥65 years; mean age, 70 years) with schizophrenia.14 There was an optional 24-week extension of open-label treatment with paliperidone. Mean daily dose of paliperidone was 8.4 mg. Efficacy measures did not show consistent statistically significant differences between treatment groups. Discontinuation rates were similar between paliperidone (7%) vs placebo (8%). Serious adverse events occurred in 3% of paliperidone-treated vs 8% of placebo-treated patients. Elevated prolactin levels occurred in one-half of paliperidone-treated patients. There were no prolactin or glucose treatment-related adverse events or significant mean changes in body weight for either paliperidone-treated or placebo-treated patients. Safety findings in the 24-week, open-label extension group were consistent with the RCT results.
Howanitz et al15 conducted a 12-week, prospective RCT that compared clozapine (mean dose, 300 mg/d) with chlorpromazine (mean dose, 600 mg/d) in 42 older adults (mean age, 67 years) with schizophrenia. Drop-out rate prior to 5 weeks was 19% and similar between groups. Common adverse effects included sialorrhea, hematologic abnormalities, sedation, tachycardia, EPS, and weight gain. Although both drugs were effective, more patients taking clozapine had tachycardia and weight gain, while more chlorpromazine patients reported sedation.
There have been other, less rigorous studies.7,8 Most of these studies evaluated risperidone and olanzapine, and most were conducted in “younger” geriatric patients (age <75 years). Although patients who participate in clinical trials may be healthier than “typical” patients, adverse effects such as EPS, sedation, and weight gain were still relatively common in these studies.
Other clinical data. A major consideration in treating older adults with schizophrenia is balancing the need to administer an antipsychotic dose high enough to alleviate psychotic symptoms while minimizing dose-dependent adverse effects. There is a U-shaped relationship between age and vulnerability to antipsychotic adverse effects,16,17 wherein adverse effects are highest at younger and older ages. Evidence supports using the lowest effective antipsychotic dose for geriatric patients with schizophrenia. Positive emission tomography (PET) studies suggest that older patients develop EPS with lower doses despite lower receptor occupancy.17,18 A recent study of 35 older patients (mean age, 60.1 years) with schizophrenia obtained PET, clinical measures, and blood pharmacokinetic measures before and after reduction of risperidone or olanzapine doses.18 A ≥40% reduction in dose was associated with reduced adverse effects, particularly EPS and elevation of prolactin levels. Moreover, the therapeutic window of striatal D2/D3 receptor occupancy appeared to be 50% to 60% in these older patients, compared with 65% to 80% in younger patients.
Long-term risks of antipsychotic treatment across the lifespan are less clear, with evidence suggesting both lower and higher mortality risk.19,20 It is difficult to fully disentangle the long-term risks of antipsychotics from the cumulative effects of lifestyle and comorbidity among individuals who have lived with schizophrenia for decades. Large naturalistic studies that include substantial numbers of older people with schizophrenia might be a way to elicit more information on long-term safety. The Schizophrenia Outpatient Health Outcome (SOHO) study was a large naturalistic trial that recruited >10,000 individuals with schizophrenia in 10 European countries.21 Although the SOHO study found differences between antipsychotics and adverse effects, such as EPS, weight gain, and sexual dysfunction, because the mean age of these patients was approximately 40 years and the follow-up period was only 3 years, it is difficult to draw conclusions that could be relevant to older individuals who have had schizophrenia for decades.
Bipolar Disorder
Clinical trials: Bipolar depression. A post hoc, secondary analysis of two 8-week, double-blind, randomized, placebo-controlled studies in bipolar depression compared 2 dosages of quetiapine (300 mg/d and 600 mg/d) with placebo in mixed-age patients.31 In a subgroup of 72 patients, ages 55 to 65, remission occurred more often with quetiapine than with placebo. Study discontinuation rates were similar between older people and younger people (age <55 years): quetiapine, 300 mg/d, 29.2%; quetiapine, 600 mg/d, 48.1%; and placebo, 29.6% in older adults, compared with 37.1%, 45.8%, and 38.1%, respectively, in younger adults. In all patients, the most common reason for discontinuation was adverse events with quetiapine and lack of efficacy for placebo. Adverse event rates were similar in older and younger adults. Dry mouth and dizziness were more common in older adults. Proportions of adults experiencing clinically significant weight gain (≥7% of body weight) were 5.3%, 8.3%, and 0% in older adults receiving quetiapine, 300 mg/d, quetiapine, 600 mg/d, and placebo, respectively, compared with 7.2%, 10.1%, and 2.6% in younger adults. EPS and treatment-emergent mania were minimal.
A secondary analysis of mixed-age, RCTs examined response in older adults (age ≥55 years) with bipolar I depression who received lurasidone as monotherapy or adjunctive therapy.32 In the monotherapy study, these patients were randomized to 6 weeks of lurasidone 20 to 60 mg/d, lurasidone 80 to 120 mg/d, or placebo. In the adjunctive therapy study, they were randomized to lurasidone 20 to 120 mg/d or placebo with either lithium or valproate. There were 83 older adults (17.1% of the sample) in the monotherapy study and 53 (15.6%) in the adjunctive therapy study. Mean improvement in depression was significantly higher for both doses of lurasidone monotherapy than placebo. Adjunctive lurasidone was not associated with statistically significant improvement vs placebo. The most frequent adverse events in older patients on lurasidone monotherapy 20 to 60 mg/d or 80 to 120 mg/d were nausea (18.5% and 9.7%, respectively) and somnolence (11.1% and 0%, respectively). Akathisia (9.7%) and insomnia (9.7%) were the most common adverse events in the group receiving 80 to 120 mg/d, with the rate of akathisia exhibiting a dose-related increase. Weight change with lurasidone was similar to placebo, and there were no clinically meaningful group changes in vital signs, electrocardiography, or laboratory parameters.
A small (N = 20) open study found improvement in older adults with bipolar depression with aripiprazole (mean dose, 10.3 mg/d).33 Adverse effects included restlessness and weight gain (n = 3, 9% each), sedation (n = 2, 10%), and drooling and diarrhea/loose stools (n = 1, 5% each). In another small study (N = 15) using asenapine (mean dose, 11.2 mg/d) in mainly older bipolar patients with depression, the most common adverse effects were gastrointestinal (GI) discomfort (n = 5, 33%) and restlessness, tremors, cognitive difficulties, and sluggishness (n = 2, 13% each).34
Clinical trials: Bipolar mania. Researchers conducted a pooled analysis of two 12-week randomized trials comparing quetiapine with placebo in a mixed-age sample with bipolar mania.35 In a subgroup of 59 older patients (mean age, 62.9 years), manic symptoms improved significantly more with quetiapine (modal dose, 550 mg/d) than with placebo. Adverse effects reported by >10% of older patients were dry mouth, somnolence, postural hypotension, insomnia, weight gain, and dizziness. Insomnia was reported by >10% of patients receiving placebo.
In a case series of 11 elderly patients with mania receiving asenapine, Baruch et al36 reported a 63% remission rate. One patient discontinued the study because of a new rash, 1 discontinued after developing peripheral edema, and 3 patients reported mild sedation.
Beyer et al37 reported on a post hoc analysis of 94 older adults (mean age, 57.1 years; range, 50.1 to 74.8 years) with acute bipolar mania receiving olanzapine (n = 47), divalproex (n = 31), or placebo (n = 16) in a pooled olanzapine clinical trials database. Patients receiving olanzapine or divalproex had improvement in mania; those receiving placebo did not improve. Safety findings were comparable with reports in younger patients with mania.
Other clinical data. Adverse effects found in mixed-age samples using secondary analyses of clinical trials need to be interpreted with caution because these types of studies usually exclude individuals with significant medical comorbidity. Medical burden, cognitive impairment, or concomitant medications generally necessitate slower drug titration and lower total daily dosing. For example, a secondary analysis of the U.S. National Institute of Health-funded Systematic Treatment Enhancement Program for Bipolar Disorder study, which had broader inclusion criteria than most clinical trials, reported that, although recovery rates in older adults with bipolar disorder were fairly good (78.5%), lower doses of risperidone were used in older vs younger patients.38
Clinical considerations
Interpretation of the relative risks of antipsychotics in older people must be tempered by the caveat that there is limited high-quality data (Table 4). Antipsychotics are the first-line therapy for older patients with schizophrenia, although their use is supported by a small number of prospective RCTs. SGAs are preferred because of their lower propensity to cause EPS and other motor adverse effects. Older persons with schizophrenia have an EPS threshold lower than younger patients and determining the lowest effective dosage may minimize EPS and cognitive adverse effects. As individuals with long-standing schizophrenia get older, their antipsychotic dosages may need to be reduced, and clinicians need to monitor for adverse effects that are more common among older people, such as tardive dyskinesia and metabolic abnormalities. In healthy, “younger” geriatric patients, monitoring for adverse effects may be similar to monitoring of younger patients. Patients who are older or frail may need more frequent assessment.
Like older adults with schizophrenia, geriatric patients with bipolar disorder have reduced drug tolerability and experience more adverse effects than younger patients. There are no prospective controlled studies that evaluated using antipsychotics in older patients with bipolar disorder. In older bipolar patients, the most problematic adverse effects of antipsychotics are akathisia, parkinsonism, other EPS, sedation and dizziness (which may increase fall risk), and GI discomfort. A key tolerability and safety consideration when treating older adults with bipolar disorder is the role of antipsychotics in relation to the use of lithium and mood stabilizers. Some studies have suggested that lithium has neuroprotective effects when used long-term; however, at least 1 report suggested that long-term antipsychotic treatment may be associated with neurodegeneration.39
The literature does not provide strong evidence on the many clinical variations that we see in routine practice settings, such as combinations of drug treatments or drugs prescribed to patients with specific comorbid conditions. There is a need for large cohort studies that monitor treatment course, medical comorbidity, and prognosis. Additionally, well-designed clinical trials such as the DART-AD, which investigated longer-term trajectories of people with dementia taking antipsychotics, should serve as a model for the type of research that is needed to better understand outcome variability among older people with chronic psychotic or bipolar disorders.40
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. United Nations, Department of Economic and Social Affairs, Population Division. World population ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Accessed September 1, 2017.
3. Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55(12):752-760.
4. Cohen CI, Vahia I, Reyes P, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. 2008;59(3):232-234.
5. Jeste DV, Barak Y, Madhusoodanan S, et al. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):638-647.
6. Kalache SM, Mulsant BH, Davies SJ, et al. The impact of aging, cognition, and symptoms on functional competence in individuals with schizophrenia across the lifespan. Schizophr Bull. 2015;41(2):374-381.
7. Suzuki T, Remington G, Uchida H, et al. Management of schizophrenia in late life with antipsychotic medications: a qualitative review. Drugs Aging. 2011;28(12):961-980.
8. Mulsant BH, Pollock BG. Psychopharmacology. In: David C. Steffens DC, Blazer DG, Thakur ME (eds). The American Psychiatric Publishing Textbook of Geriatric Psychiatry, 5th Edition. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
9. Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2(4):340-350.
10. Marriott RG, Neil W, Waddingham S. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database Syst Rev. 2006;(1):CD005580.
11. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012(2):CD004162.
12. Scott J, Greenwald BS, Kramer E, et al. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr. 2011;23(5):742-748.
13. Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783-791.
14. Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry. 2008;16(1):31-43.
15. Howanitz E, Pardo M, Smelson DA, et al. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. J Clin Psychiatry. 1999;60(1):41-44.
16. Sproule BA, Lake J, Mamo DC, et al. Are antipsychotic prescribing patterns different in older and younger adults?: a survey of 1357 psychiatric inpatients in Toronto. Can J Psychiatry. 2010;55(4):248-254.
17. Uchida H, Suzuki T, Mamo DC, et al. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry. 2008;16(7):584-593.
18. Graff-Guerrero A, Rajji TK, Mulsant BH, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 occupancy study. JAMA Psychiatry. 2015;72(9):927-934.
19. Khan A, Schwartz K, Stern C, et al. Mortality risk in patients with schizophrenia participating in premarketing atypical antipsychotic clinical trials. J Clin Psychiatry. 2007;68(12):1828-1833.
20. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: a systematic review. Schizophr Res. 2009;113(1):1-11.
21. Novick D, Haro JM, Perrin E, et al. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Eur Neuropsychopharmacol. 2009;19(8):542-550.
22. Sajatovic M, Blow FC, Ignacio RV, et al. Age-related modifiers of clinical presentation and health service use among veterans with bipolar disorder. Psychiatr Serv. 2004;55(9):1014-1021.
23. Jeste DV, Alexopoulos GS, Bartels SJ, et al. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry. 1999;56(9):848-853.
24. Sajatovic M, Chen P. Geriatric bipolar disorder. Psychiatr Clin North Am. 2011;34(2):319-333,vii.
25. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
26. Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol. 2012;25(1):20-25.
27. Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
28. Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63(1):83-86.
29. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26(5):603-617.
30. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
31. Sajatovic M, Paulsson B. Quetiapine for the treatment of depressive episodes in adults aged 55 to 65 years with bipolar disorder. Paper presented at: American Association of Geriatric Psychiatry Annual Meeting; 2007; New Orleans, LA.
32. Sajatovic M, Forester B, Tsai J, et al. Efficacy and safety of lurasidone in older adults with bipolar depression: analysis of two double-blind, placebo-controlled studies. Paper presented at: American College of Neuropsychopharmacology (ACNP) 53rd Annual Meeting; 2014; Phoenix, AZ.
33. Sajatovic M, Coconcea N, Ignacio RV, et al. Aripiprazole therapy in 20 older adults with bipolar disorder: a 12-week, open-label trial. J Clin Psychiatry. 2008;69(1):41-46.
34. Sajatovic M, Dines P, Fuentes-Casiano E, et al. Asenapine in the treatment of older adults with bipolar disorder. Int J Geriatr Psychiatry. 2015;30(7):710-719.
35. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
36. Baruch Y, Tadger S, Plopski I, et al. Asenapine for elderly bipolar manic patients. J Affect Disord. 2013;145(1):130-132.
37. Beyer JL, Siegal A, Kennedy JS. Olanzapine, divalproex and placebo treatment, non-head to head comparisons of older adults acute mania. Paper presented at: 10th Congress of the International Psychogeriatric Association; 2001; Nice, France.
38. Al Jurdi RK, Marangell LB, Petersen NJ, et al. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry. 2008;16(11):922-933.
39. Gildengers AG, Chung KH, Huang SH, et al. Neuroprogressive effects of lifetime illness duration in older adults with bipolar disorder. Bipolar Disord. 2014;16(6):617-623.
40. Ballard C, Lana MM, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76.
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. United Nations, Department of Economic and Social Affairs, Population Division. World population ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Accessed September 1, 2017.
3. Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55(12):752-760.
4. Cohen CI, Vahia I, Reyes P, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. 2008;59(3):232-234.
5. Jeste DV, Barak Y, Madhusoodanan S, et al. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):638-647.
6. Kalache SM, Mulsant BH, Davies SJ, et al. The impact of aging, cognition, and symptoms on functional competence in individuals with schizophrenia across the lifespan. Schizophr Bull. 2015;41(2):374-381.
7. Suzuki T, Remington G, Uchida H, et al. Management of schizophrenia in late life with antipsychotic medications: a qualitative review. Drugs Aging. 2011;28(12):961-980.
8. Mulsant BH, Pollock BG. Psychopharmacology. In: David C. Steffens DC, Blazer DG, Thakur ME (eds). The American Psychiatric Publishing Textbook of Geriatric Psychiatry, 5th Edition. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
9. Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2(4):340-350.
10. Marriott RG, Neil W, Waddingham S. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database Syst Rev. 2006;(1):CD005580.
11. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012(2):CD004162.
12. Scott J, Greenwald BS, Kramer E, et al. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr. 2011;23(5):742-748.
13. Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783-791.
14. Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry. 2008;16(1):31-43.
15. Howanitz E, Pardo M, Smelson DA, et al. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. J Clin Psychiatry. 1999;60(1):41-44.
16. Sproule BA, Lake J, Mamo DC, et al. Are antipsychotic prescribing patterns different in older and younger adults?: a survey of 1357 psychiatric inpatients in Toronto. Can J Psychiatry. 2010;55(4):248-254.
17. Uchida H, Suzuki T, Mamo DC, et al. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry. 2008;16(7):584-593.
18. Graff-Guerrero A, Rajji TK, Mulsant BH, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 occupancy study. JAMA Psychiatry. 2015;72(9):927-934.
19. Khan A, Schwartz K, Stern C, et al. Mortality risk in patients with schizophrenia participating in premarketing atypical antipsychotic clinical trials. J Clin Psychiatry. 2007;68(12):1828-1833.
20. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: a systematic review. Schizophr Res. 2009;113(1):1-11.
21. Novick D, Haro JM, Perrin E, et al. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Eur Neuropsychopharmacol. 2009;19(8):542-550.
22. Sajatovic M, Blow FC, Ignacio RV, et al. Age-related modifiers of clinical presentation and health service use among veterans with bipolar disorder. Psychiatr Serv. 2004;55(9):1014-1021.
23. Jeste DV, Alexopoulos GS, Bartels SJ, et al. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry. 1999;56(9):848-853.
24. Sajatovic M, Chen P. Geriatric bipolar disorder. Psychiatr Clin North Am. 2011;34(2):319-333,vii.
25. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
26. Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol. 2012;25(1):20-25.
27. Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
28. Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63(1):83-86.
29. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26(5):603-617.
30. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
31. Sajatovic M, Paulsson B. Quetiapine for the treatment of depressive episodes in adults aged 55 to 65 years with bipolar disorder. Paper presented at: American Association of Geriatric Psychiatry Annual Meeting; 2007; New Orleans, LA.
32. Sajatovic M, Forester B, Tsai J, et al. Efficacy and safety of lurasidone in older adults with bipolar depression: analysis of two double-blind, placebo-controlled studies. Paper presented at: American College of Neuropsychopharmacology (ACNP) 53rd Annual Meeting; 2014; Phoenix, AZ.
33. Sajatovic M, Coconcea N, Ignacio RV, et al. Aripiprazole therapy in 20 older adults with bipolar disorder: a 12-week, open-label trial. J Clin Psychiatry. 2008;69(1):41-46.
34. Sajatovic M, Dines P, Fuentes-Casiano E, et al. Asenapine in the treatment of older adults with bipolar disorder. Int J Geriatr Psychiatry. 2015;30(7):710-719.
35. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
36. Baruch Y, Tadger S, Plopski I, et al. Asenapine for elderly bipolar manic patients. J Affect Disord. 2013;145(1):130-132.
37. Beyer JL, Siegal A, Kennedy JS. Olanzapine, divalproex and placebo treatment, non-head to head comparisons of older adults acute mania. Paper presented at: 10th Congress of the International Psychogeriatric Association; 2001; Nice, France.
38. Al Jurdi RK, Marangell LB, Petersen NJ, et al. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry. 2008;16(11):922-933.
39. Gildengers AG, Chung KH, Huang SH, et al. Neuroprogressive effects of lifetime illness duration in older adults with bipolar disorder. Bipolar Disord. 2014;16(6):617-623.
40. Ballard C, Lana MM, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76.
Improving the recognition of borderline personality disorder
Borderline personality disorder (BPD) is associated with impaired psychosocial functioning,1-4 reduced health-related quality of life,5 high utilization of services,6,7 and excess mortality.8-10 Although BPD occurs in up to 40% of psychiatric inpatients11 and 10% of outpatients,12 it is underrecognized.13-15 Often, patients with BPD do not receive an accurate diagnosis until ≥10 years after initially seeking treatment.16 The treatment and clinical implications of failing to recognize BPD include overprescribing medication and underutilizing empirically effective psychotherapies.14
This review summarizes studies of the underdiagnosis of BPD in routine clinical practice, describes which patients should be screened, and reviews alternative approaches to screening.
Underrecognition of BPD
The Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project is an ongoing clinical research study involving the integration of research assessment methods into routine clinical practice.17 In an early report from the MIDAS project, BPD diagnoses derived from structured and unstructured clinical interviews were compared between 2 groups of psychiatric outpatients in the same practice.15 Individuals in the structured interview cohort were 35 times more often diagnosed with BPD than individuals evaluated with an unstructured clinical interview. Importantly, when the information from the structured interview was presented to the clinicians, BPD was more likely to be diagnosed clinically.
Other studies13,16 also found that the rate of diagnosing BPD was higher when the diagnosis was based on a semi-structured diagnostic interview compared with an unstructured clinical interview, and that clinicians were reluctant to diagnose BPD during their routine intake diagnostic evaluation.
Clinicians, however, do not use semi-structured interviews in their practice, and they also do not tend to diagnose personality disorders (PDs) based on direct questioning, as they typically would when assessing a symptom-based disorder such as depression or anxiety. Rather, clinicians report that they rely on longitudinal observations to diagnose PDs.18 However, the results from the MIDAS project were inconsistent with clinicians’ reports. When clinicians were presented with the results of the semi-structured interview, they usually would diagnose BPD, even though it was the initial evaluation. If clinicians actually relied on longitudinal observations and considered information based on the direct question approach of research interviews to be irrelevant or invalid, then the results from the semi-structured interview should not have influenced the rate at which they diagnosed BPD. This suggests that the primary issue in diagnosing PDs is not the need for longitudinal observation but rather the need for more information, and that there is a role for screening questionnaires.
One potential criticism of studies demonstrating underrecognition of BPD in clinical practice is that patients typically were interviewed when they presented for treatment, when most were depressed or anxious. The possible pathologizing effects of psychiatric state on personality have been known for years.19 However, a large body of literature examining the treatment, prognostic, familial, and biological correlates of PDs supports the validity of diagnosing PDs in this manner. Moreover, from a clinical perspective, the sooner a clinician is aware of the presence of BPD, the more likely this information can be used for treatment planning.
Who should be screened for BPD?
BPD is underrecognized and underdiagnosed because patients with BPD often also have comorbid mood, anxiety, or substance use disorders.20,21 The symptoms associated with these disorders are typically the chief concern of patients with undiagnosed BPD who present for treatment. Patients with BPD rarely present for an intake evaluation and state that they are struggling with abandonment fears, chronic feelings of emptiness, or an identity disturbance. If patients identified these problems as their chief concerns, BPD would be easier to recognize.
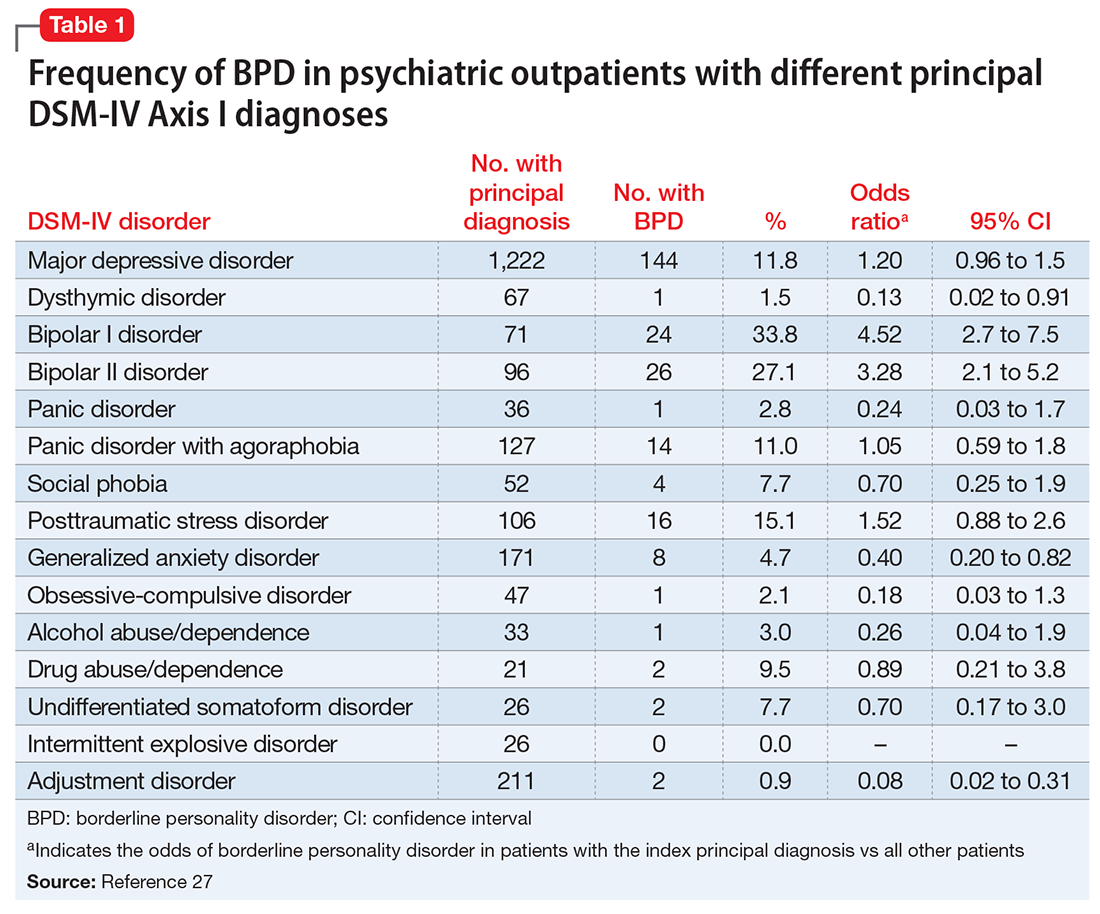
Although several studies have documented the frequency of BPD in patients with a specific psychiatric diagnosis such as major depressive disorder (MDD) or attention-deficit/hyperactivity disorder,22-26 the MIDAS project examined the frequency of BPD in patients with various diagnoses and evaluated which disorders were associated with a significantly increased rate of BPD.27 The highest rate of BPD was found in patients with bipolar disorder. Approximately 25% of patients with bipolar II disorder and one-third of those with bipolar I disorder were diagnosed with BPD; these rates were significantly higher than the rate of BPD in patients without these disorders (Table 127). The rate of BPD was second highest in patients with a principal diagnosis of posttraumatic stress disorder (PTSD) and MDD; however, the rate of BPD in these patients was not significantly elevated compared with patients who did not have these principal diagnoses. Three disorders were associated with a significantly lower rate of BPD: adjustment disorder, dysthymic disorder, and generalized anxiety disorder.
It would be easy to recommend screening for BPD in all psychiatric patients. However, that is not feasible or practical. In making screening recommendations, absolute risk should be considered more important than relative risk. Clinicians should screen for BPD in patients presenting to a general psychiatric outpatient practice with a principal diagnosis of MDD, bipolar disorder, PTSD, or panic disorder with agoraphobia. That is, I recommend screening for BPD in patients with a principal diagnosis in which the prevalence of BPD is ≥10% (Table 127).
A brief review of screening statistics
Screening tests for most psychiatric disorders are based on multi-item scales in which a total score is computed from a sum of item scores, and a cutoff point is established to determine who does and does not screen positive on the test. However, sensitivity, specificity, and positive and negative predictive values are not invariant properties of a screening test with a continuous score distribution. Rather, the performance statistics of a scale can be altered by changing the threshold score to distinguish cases from non-cases. When the screening threshold is lowered, sensitivity increases and specificity decreases.
For screening, a broad net needs to be cast so that all (or almost all) cases are included. Therefore, the cutoff score should be set low to prioritize the sensitivity of the instrument. A screening scale also should have high negative predictive value so that the clinician can be confident that patients who screen negative on the test do not have the disorder.
Screening questionnaires for BPD
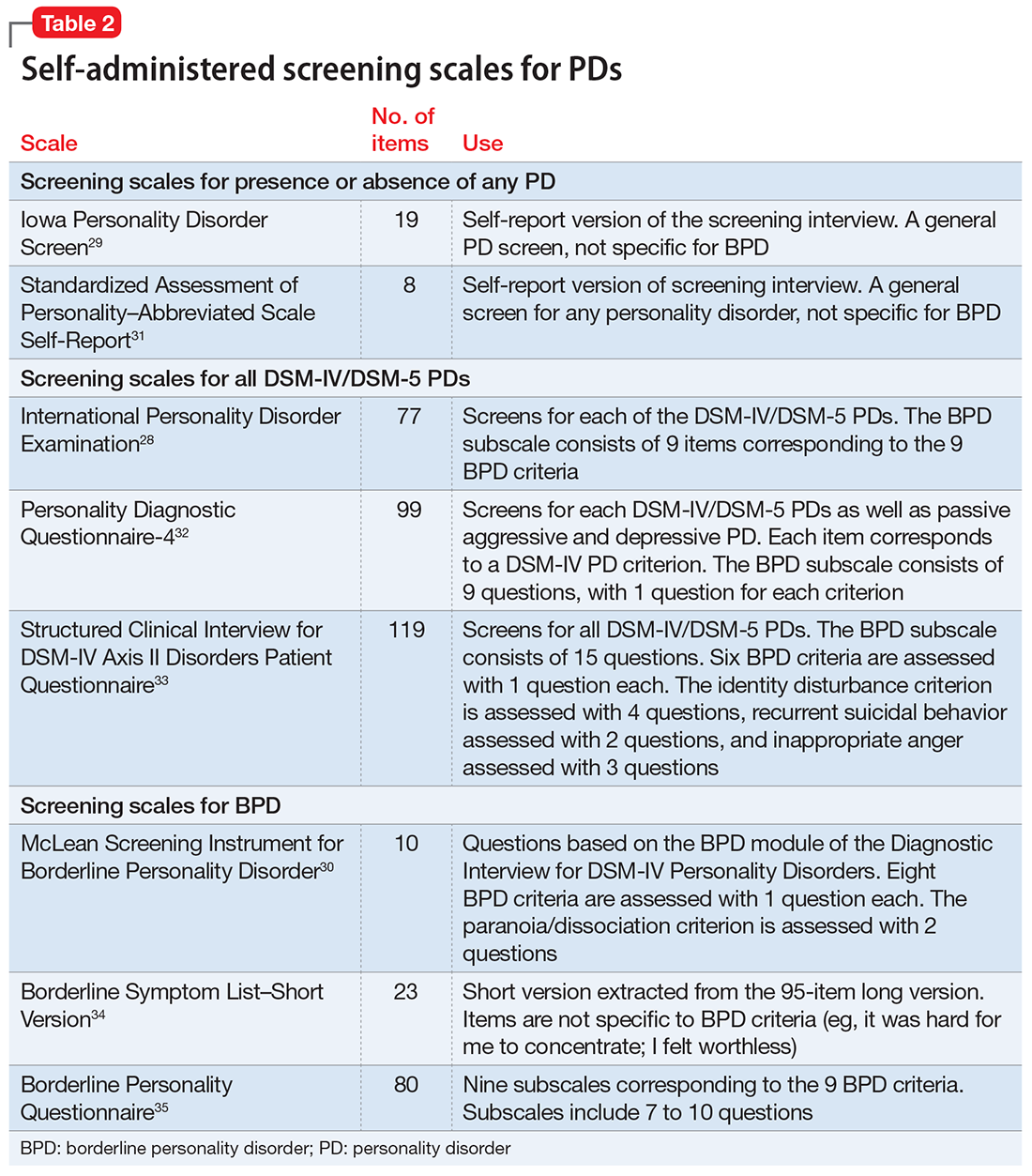
Several questionnaires have been developed to screen for PDs (Table 228-35). Some screen for each of the DSM PDs,28,36-42 and some screen more broadly for the presence or absence of any PD.29,43,44 The most commonly studied self-report scale for BPD is the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD),30 a 10-item self-report scale derived from a subset of questions from the BPD module of a semi-structured diagnostic interview.
The initial validation study30 found that the optimal cutoff score was 7, which resulted in a sensitivity of 81% and specificity of 89%. Three studies have evaluated the scale in adolescents and young adults,45-47 and 3 studies examined the scale in adult outpatients.48-50 Across all 6 studies, at the optimal cutoff scores determined in each study, the sensitivity of the MSI-BPD ranged from 68% to 94% (mean, 80%) and the specificity ranged from 66% to 80% (mean, 72%).
Problems with screening questionnaires. Although screening scales have been developed for many psychiatric disorders, they have not been widely used in mental health settings. In a previous commentary, I argued that the conceptual justification for using self-report screening scales for single disorders in psychiatric settings is questionable.51 Another problem with screening scales is their potential misuse as case-finding instruments. In the literature on bipolar disorder screening, several researchers misconstrued a positive screen to indicate caseness.51 Although this is not a problem with the screening measures or the selection of a cutoff score, caution must be taken to not confuse screening with diagnosis.52
Screening for BPD as part of your diagnostic interview
An alternative approach to using self-administered questionnaires for screening is for clinicians to include questions in their evaluation as part of a psychiatric review of systems. When conducting a diagnostic interview, clinicians typically screen for disorders that are comorbid with the principal diagnosis by asking about the comorbid disorders’ necessary features or “gate criteria.” For example, in a patient with a principal diagnosis of MDD, the clinician would inquire about the presence of panic attacks, excessive worry, or substance use to screen for the presence of panic disorder, generalized anxiety disorder, or a substance use disorder. In contrast, for polythetically defined disorders such as BPD, there is no single gate criterion, because the disorder is diagnosed based on the presence of at least 5 of 9 criteria and no single one of these criteria is required to be present to establish the diagnosis.
As part of the MIDAS project, the psychometric properties of the BPD criteria were examined to determine if it was possible to identify 1 or 2 criteria that could serve as gate criteria to screen for the disorder. If the sensitivity of 1 criterion or a combination of 2 BPD criteria was sufficiently high (ie, >90%), then the assessment of this criterion (or these criteria) could be included in a psychiatric review of systems, thus potentially improving the detection of BPD. Researchers hypothesized that affective instability, considered first by Linehan53 and later by other theorists54 to be of central importance to the clinical manifestations of BPD, could function as a gate criterion. In the sample of 3,674 psychiatric outpatients who were evaluated with a semi-structured interview, the sensitivity of the affective instability criterion was 92.8%, and the negative predictive value of the criterion was 99%.
Identifying a single BPD criterion that is present in the vast majority of patients diagnosed with BPD will allow clinicians to follow their usual clinical practice when conducting a psychiatric review of systems and inquire about the gate criteria of various disorders. Several studies have found that >90% of patients with BPD report affective instability. However, this does not mean that the diagnosis of BPD can be abbreviated to an assessment of the presence or absence of affective instability. Many patients who screen positive will not have BPD when a more definitive diagnostic evaluation is conducted. In the case of BPD, the more costly definitive diagnostic procedure simply entails inquiry of the other diagnostic criteria.
1. Bellino S, Patria L, Paradiso E, et al. Major depression in patients with borderline personality disorder: a clinical investigation. Can J Psychiatry. 2005;50(4):234-238.
2. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-283.
3. Gunderson JG, Stout RL, McGlashan TH, et al. Ten-year course of borderline personality disorder: psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Arch Gen Psychiatry. 2011;68(8):827-837.
4. Zanarini MC, Jacoby RJ, Frankenburg FR, et al. The 10-year course of social security disability income reported by patients with borderline personality disorder and axis II comparison subjects. J Pers Disord. 2009;23(4):346-356.
5. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
6. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
7. Zanarini MC, Frankenburg FR, Hennen J, et al. Mental health service utilization by borderline personality disorder patients and Axis II comparison subjects followed prospectively for 6 years. J Clin Psychiatry. 2004;65(1):28-36.
8. Pompili M, Girardi P, Ruberto A, et al. Suicide in borderline personality disorder: a meta-analysis. Nord J Psychiatry. 2005;59(5):319-324.
9. Oldham JM. Borderline personality disorder and suicidality. Am J Psychiatry. 2006;163(1):20-26.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Marinangeli M, Butti G, Scinto A, et al. Patterns of comorbidity among DSM-III-R personality disorders. Psychopathology. 2000;33(2):69-74.
12. Zimmerman M, Rothschild L, Chelminski I. The frequency of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918.
13. Comtois KA, Carmel A. Borderline personality disorder and high utilization of inpatient psychiatric hospitalization: concordance between research and clinical diagnosis. J Behav Health Servi Res. 2016;43(2):272-280.
14. Paris J, Black DW. Borderline personality disorder and bipolar disorder: what is the difference and why does it matter? J Nerv Ment Dis. 2015;203(1):3-7.
15. Zimmerman M, Mattia JI. Differences between clinical and research practice in diagnosing borderline personality disorder. Am J Psychiatry. 1999;156(10):1570-1574.
16. Magnavita JJ, Levy KN, Critchfield KL, et al. Ethical considerations in treatment of personality dysfunction: using evidence, principles, and clinical judgment. Professional Psychology: Research and Practice. 2010;41(1):64-74.
17. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
18. Westen D. Divergences between clinical and research methods for assessing personality disorders: implications for research and the evolution of axis II. Am J Psychiatry. 1997;154(7):895-903.
19. Zimmerman M. Diagnosing personality disorders: a review of issues and research methods. Arch Gen Psychiatry. 1994;51(3):225-245.
20. Zanarini MC, Gunderson JG, Frankenberg FR. Axis I phenomenology of borderline personality disorder. Compr Psychiatry. 1989;30(2):149-156.
21. Zimmerman M, Mattia JI. Axis I diagnostic comorbidity and borderline personality disorder. Compr Psychiatry. 1999;40(4):245-252.
22. Gunderson JG, Morey LC, Stout RL, et al. Major depressive disorder and borderline personality disorder revisited: longitudinal interactions. J Clin Psychiatry. 2004;65(8):1049-1056.
23. Bayes AJ, Parker GB. Clinical vs. DSM diagnosis of bipolar disorder, borderline personality disorder and their co-occurrence. Acta Psychiatr Scand. 2016;135(3):259-265.
24. Carpenter RW, Wood PK, Trull TJ. Comorbidity of borderline personality disorder and lifetime substance use disorders in a nationally representative sample. J Pers Disord. 2016;30(3):336-350.
25. Trull TJ, Sher KJ, Minks-Brown C, et al. Borderline personality disorder and substance use disorders: a review and integration. Clin Psychol Rev. 2000;20(2):235-253.
26. Matthies SD, Philipsen A. Common ground in attention deficit hyperactivity disorder (ADHD) and borderline personality disorder (BPD)-review of recent findings. Borderline Personal Disord Emot Dysregul. 2014;1:3.
27. Zimmerman M, Chelminski I, Dalrymple K, et al. Principal diagnoses in psychiatric outpatients with borderline personality disorder: implications for screening recommendations. Ann Clin Psychiatry. 2017;29(1):54-60.
28. Magallón-Neri EM, Forns M, Canalda G, et al. Usefulness of the International Personality Disorder Examination Screening Questionnaire for borderline and impulsive personality pathology in adolescents. Compr Psychiatry. 2013;54(3):301-308.
29. Germans S, Van Heck GL, Langbehn DR, et al. The Iowa Personality Disorder Screen. Eur J Psychol Assess. 2010;26(1):11-18.
30. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). J Pers Disord. 2003;17(6):568-573.
31. Germans S, Van Heck GL, Hodiamont PP. Results of the search for personality disorder screening tools: clinical implications. J Clin Psychiatry. 2012;73(2):165-173.
32. Hyler SE. Personality diagnostic questionnaire-4. New York, NY: New York State Psychiatric Institute; 1994.
33. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis II Disorders - Patient edition (SCID-I/P, version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995.
34. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
35. Poreh AM, Rawlings D, Claridge G, et al. The BPQ: a scale for the assessment of boderline personality based on DSM-IV criteria. J Pers Disord. 2006;20(3):247-260.
36. Ekselius L, Lindstrom E, von Knorring L, et al. SCID II interviews and the SCID Screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1994;90(2):120-123.
37. Hyler SE, Skodol AE, Oldham JM, et al. Validity of the Personality Diagnostic Questionnaire-Revised: a replication in an outpatient sample. Compr Psychiatry. 1992;33(2):73-77.
38. Davison S, Leese M, Taylor PJ. Examination of the screening properties of the personality diagnostic questionnaire 4+ (PDQ-4+) in a prison population. J Pers Disord. 2001;15(2):180-194.
39. Jacobsberg L, Perry S, Frances A. Diagnostic agreement between the SCID-II screening questionnaire and the Personality Disorder Examination. J Pers Assess. 1995;65(3):428-433.
40. Germans S, Van Heck GL, Masthoff ED, et al. Diagnostic efficiency among psychiatric outpatients of a self-report version of a subset of screen items of the Structured Clinical Interview for DSM-IV-TR Personality Disorders (SCID-II). Psychol Assess. 2010;22(4):945-952.
41. Lloyd C, Overall JE, Click M Jr. Screening for borderline personality disorders with the MMPI-168. J Clin Psychol. 1983;39(5):722-726.
42. Neal LA, Fox C, Carroll N, et al. Development and validation of a computerized screening test for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1997;95(4):351-356.
43. Germans S, Van Heck GL, Moran P, et al. The Self-Report Standardized Assessment of Personality-abbreviated Scale: preliminary results of a brief screening test for personality disorders. Pers Ment Health. 2008;2(2):70-76.
44. Moran P, Leese M, Lee T, et al. Standardized Assessment of Personality - Abbreviated Scale (SAPAS): preliminary validation of a brief screen for personality disorder. Br J Psychiatry. 2003;183:228-232.
45. Chanen AM, Jovev M, Djaja D, et al. Screening for borderline personality disorder in outpatient youth. J Pers Disord. 2008;22(4):353-364.
46. van Alebeek A, van der Heijden PT, Hessels C, et al. Comparison of three questionnaires to screen for borderline personality disorder in adolescents and young adults. Eur J Psychol Assess. 2017:33;123-128.
47. Noblin JL, Venta A, Sharp C. The validity of the MSI-BPD among inpatient adolescents. Assessment. 2014;21(2):210-217.
48. Kröger C, Vonau M, Kliem S, et al. Emotion dysregulation as a core feature of borderline personality disorder: comparison of the discriminatory ability of two self-rating measures. Psychopathology. 2011;44(4):253-260.
49. Soler J, Domínguez-Clav E, García-Rizo C, et al. Validation of the Spanish version of the McLean Screening Instrument for Borderline Personality Disorder. Rev Psiquiatr Salud Ment. 2016;9(4):195-202.
50. Melartin T, Häkkinen M, Koivisto M, et al. Screening of psychiatric outpatients for borderline personality disorder with the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). Nord J Psychiatry. 2009;63(6):475-479.
51. Zimmerman M. Misuse of the Mood Disorders Questionnaire as a case-finding measure and a critique of the concept of using a screening scale for bipolar disorder in psychiatric practice. Bipolar Disord. 2012;14(2):127-134.
52. Zimmerman M. Screening for bipolar disorder: confusion between case-finding and screening. Psychother Psychosom. 2014;83(5):259-262.
53. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York, NY: Guilford Press; 1993.
54. Koenigsberg HW, Harvey PD, Mitropoulou V, et al. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? J Pers Disord. 2001;15(4):358-370.
55. Grilo CM, Becker DF, Anez LM, et al. Diagnostic efficiency of DSM-IV criteria for borderline personality disorder: an evaluation in Hispanic men and women with substance use disorders. J Consult Clin Psychol. 2004;72(1):126-131.
56. Korfine L, Hooley JM. Detecting individuals with borderline personality disorder in the community: an ascertainment strategy and comparison with a hospital sample. J Pers Disord. 2009;23(1):62-75.
57. Leppänen V, Lindeman S, Arntz A, et al. Preliminary evaluation of psychometric properties of the Finnish Borderline Personality Disorder Severity Index: Oulu-BPD-Study. Nord J Psychiatry. 2013;67(5):312-319.
58. Pfohl B, Coryell W, Zimmerman M, et al. DSM-III personality disorders: diagnostic overlap and internal consistency of individual DSM-III criteria. Compr Psychiatry. 1986;27(1):22-34.
59. Reich J. Criteria for diagnosing DSM-III borderline personality disorder. Ann Clin Psychiatry. 1990;2(3):189-197.
60. Nurnberg HG, Raskin M, Levine PE, et al. Hierarchy of DSM-III-R criteria efficiency for the diagnosis of borderline personality disorder. J Pers Disord. 1991;5(3):211-224.
61. Farmer RF, Chapman AL. Evaluation of DSM-IV personality disorder criteria as assessed by the structured clinical interview for DSM-IV personality disorders. Compr Psychiatry. 2002;43(4):285-300.
62. Grilo CM, McGlashan TH, Morey LC, et al. Internal consistency, intercriterion overlap and diagnostic efficiency of criteria sets for DSM-IV schizotypal, borderline, avoidant and obsessive-compulsive personality disorders. Acta Psychiatr Scand. 2001;104(4):264-272.
63. Grilo CM, Sanislow CA, Skodol AE, et al. Longitudinal diagnostic efficiency of DSM-IV criteria for borderline personality disorder: a 2-year prospective study. Can J Psychiatry. 2007;52(6):357-362.
Borderline personality disorder (BPD) is associated with impaired psychosocial functioning,1-4 reduced health-related quality of life,5 high utilization of services,6,7 and excess mortality.8-10 Although BPD occurs in up to 40% of psychiatric inpatients11 and 10% of outpatients,12 it is underrecognized.13-15 Often, patients with BPD do not receive an accurate diagnosis until ≥10 years after initially seeking treatment.16 The treatment and clinical implications of failing to recognize BPD include overprescribing medication and underutilizing empirically effective psychotherapies.14
This review summarizes studies of the underdiagnosis of BPD in routine clinical practice, describes which patients should be screened, and reviews alternative approaches to screening.
Underrecognition of BPD
The Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project is an ongoing clinical research study involving the integration of research assessment methods into routine clinical practice.17 In an early report from the MIDAS project, BPD diagnoses derived from structured and unstructured clinical interviews were compared between 2 groups of psychiatric outpatients in the same practice.15 Individuals in the structured interview cohort were 35 times more often diagnosed with BPD than individuals evaluated with an unstructured clinical interview. Importantly, when the information from the structured interview was presented to the clinicians, BPD was more likely to be diagnosed clinically.
Other studies13,16 also found that the rate of diagnosing BPD was higher when the diagnosis was based on a semi-structured diagnostic interview compared with an unstructured clinical interview, and that clinicians were reluctant to diagnose BPD during their routine intake diagnostic evaluation.
Clinicians, however, do not use semi-structured interviews in their practice, and they also do not tend to diagnose personality disorders (PDs) based on direct questioning, as they typically would when assessing a symptom-based disorder such as depression or anxiety. Rather, clinicians report that they rely on longitudinal observations to diagnose PDs.18 However, the results from the MIDAS project were inconsistent with clinicians’ reports. When clinicians were presented with the results of the semi-structured interview, they usually would diagnose BPD, even though it was the initial evaluation. If clinicians actually relied on longitudinal observations and considered information based on the direct question approach of research interviews to be irrelevant or invalid, then the results from the semi-structured interview should not have influenced the rate at which they diagnosed BPD. This suggests that the primary issue in diagnosing PDs is not the need for longitudinal observation but rather the need for more information, and that there is a role for screening questionnaires.
One potential criticism of studies demonstrating underrecognition of BPD in clinical practice is that patients typically were interviewed when they presented for treatment, when most were depressed or anxious. The possible pathologizing effects of psychiatric state on personality have been known for years.19 However, a large body of literature examining the treatment, prognostic, familial, and biological correlates of PDs supports the validity of diagnosing PDs in this manner. Moreover, from a clinical perspective, the sooner a clinician is aware of the presence of BPD, the more likely this information can be used for treatment planning.
Who should be screened for BPD?
BPD is underrecognized and underdiagnosed because patients with BPD often also have comorbid mood, anxiety, or substance use disorders.20,21 The symptoms associated with these disorders are typically the chief concern of patients with undiagnosed BPD who present for treatment. Patients with BPD rarely present for an intake evaluation and state that they are struggling with abandonment fears, chronic feelings of emptiness, or an identity disturbance. If patients identified these problems as their chief concerns, BPD would be easier to recognize.
Although several studies have documented the frequency of BPD in patients with a specific psychiatric diagnosis such as major depressive disorder (MDD) or attention-deficit/hyperactivity disorder,22-26 the MIDAS project examined the frequency of BPD in patients with various diagnoses and evaluated which disorders were associated with a significantly increased rate of BPD.27 The highest rate of BPD was found in patients with bipolar disorder. Approximately 25% of patients with bipolar II disorder and one-third of those with bipolar I disorder were diagnosed with BPD; these rates were significantly higher than the rate of BPD in patients without these disorders (Table 127). The rate of BPD was second highest in patients with a principal diagnosis of posttraumatic stress disorder (PTSD) and MDD; however, the rate of BPD in these patients was not significantly elevated compared with patients who did not have these principal diagnoses. Three disorders were associated with a significantly lower rate of BPD: adjustment disorder, dysthymic disorder, and generalized anxiety disorder.
It would be easy to recommend screening for BPD in all psychiatric patients. However, that is not feasible or practical. In making screening recommendations, absolute risk should be considered more important than relative risk. Clinicians should screen for BPD in patients presenting to a general psychiatric outpatient practice with a principal diagnosis of MDD, bipolar disorder, PTSD, or panic disorder with agoraphobia. That is, I recommend screening for BPD in patients with a principal diagnosis in which the prevalence of BPD is ≥10% (Table 127).
A brief review of screening statistics
Screening tests for most psychiatric disorders are based on multi-item scales in which a total score is computed from a sum of item scores, and a cutoff point is established to determine who does and does not screen positive on the test. However, sensitivity, specificity, and positive and negative predictive values are not invariant properties of a screening test with a continuous score distribution. Rather, the performance statistics of a scale can be altered by changing the threshold score to distinguish cases from non-cases. When the screening threshold is lowered, sensitivity increases and specificity decreases.
For screening, a broad net needs to be cast so that all (or almost all) cases are included. Therefore, the cutoff score should be set low to prioritize the sensitivity of the instrument. A screening scale also should have high negative predictive value so that the clinician can be confident that patients who screen negative on the test do not have the disorder.
Screening questionnaires for BPD
Several questionnaires have been developed to screen for PDs (Table 228-35). Some screen for each of the DSM PDs,28,36-42 and some screen more broadly for the presence or absence of any PD.29,43,44 The most commonly studied self-report scale for BPD is the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD),30 a 10-item self-report scale derived from a subset of questions from the BPD module of a semi-structured diagnostic interview.
The initial validation study30 found that the optimal cutoff score was 7, which resulted in a sensitivity of 81% and specificity of 89%. Three studies have evaluated the scale in adolescents and young adults,45-47 and 3 studies examined the scale in adult outpatients.48-50 Across all 6 studies, at the optimal cutoff scores determined in each study, the sensitivity of the MSI-BPD ranged from 68% to 94% (mean, 80%) and the specificity ranged from 66% to 80% (mean, 72%).
Problems with screening questionnaires. Although screening scales have been developed for many psychiatric disorders, they have not been widely used in mental health settings. In a previous commentary, I argued that the conceptual justification for using self-report screening scales for single disorders in psychiatric settings is questionable.51 Another problem with screening scales is their potential misuse as case-finding instruments. In the literature on bipolar disorder screening, several researchers misconstrued a positive screen to indicate caseness.51 Although this is not a problem with the screening measures or the selection of a cutoff score, caution must be taken to not confuse screening with diagnosis.52
Screening for BPD as part of your diagnostic interview
An alternative approach to using self-administered questionnaires for screening is for clinicians to include questions in their evaluation as part of a psychiatric review of systems. When conducting a diagnostic interview, clinicians typically screen for disorders that are comorbid with the principal diagnosis by asking about the comorbid disorders’ necessary features or “gate criteria.” For example, in a patient with a principal diagnosis of MDD, the clinician would inquire about the presence of panic attacks, excessive worry, or substance use to screen for the presence of panic disorder, generalized anxiety disorder, or a substance use disorder. In contrast, for polythetically defined disorders such as BPD, there is no single gate criterion, because the disorder is diagnosed based on the presence of at least 5 of 9 criteria and no single one of these criteria is required to be present to establish the diagnosis.
As part of the MIDAS project, the psychometric properties of the BPD criteria were examined to determine if it was possible to identify 1 or 2 criteria that could serve as gate criteria to screen for the disorder. If the sensitivity of 1 criterion or a combination of 2 BPD criteria was sufficiently high (ie, >90%), then the assessment of this criterion (or these criteria) could be included in a psychiatric review of systems, thus potentially improving the detection of BPD. Researchers hypothesized that affective instability, considered first by Linehan53 and later by other theorists54 to be of central importance to the clinical manifestations of BPD, could function as a gate criterion. In the sample of 3,674 psychiatric outpatients who were evaluated with a semi-structured interview, the sensitivity of the affective instability criterion was 92.8%, and the negative predictive value of the criterion was 99%.
Identifying a single BPD criterion that is present in the vast majority of patients diagnosed with BPD will allow clinicians to follow their usual clinical practice when conducting a psychiatric review of systems and inquire about the gate criteria of various disorders. Several studies have found that >90% of patients with BPD report affective instability. However, this does not mean that the diagnosis of BPD can be abbreviated to an assessment of the presence or absence of affective instability. Many patients who screen positive will not have BPD when a more definitive diagnostic evaluation is conducted. In the case of BPD, the more costly definitive diagnostic procedure simply entails inquiry of the other diagnostic criteria.
Borderline personality disorder (BPD) is associated with impaired psychosocial functioning,1-4 reduced health-related quality of life,5 high utilization of services,6,7 and excess mortality.8-10 Although BPD occurs in up to 40% of psychiatric inpatients11 and 10% of outpatients,12 it is underrecognized.13-15 Often, patients with BPD do not receive an accurate diagnosis until ≥10 years after initially seeking treatment.16 The treatment and clinical implications of failing to recognize BPD include overprescribing medication and underutilizing empirically effective psychotherapies.14
This review summarizes studies of the underdiagnosis of BPD in routine clinical practice, describes which patients should be screened, and reviews alternative approaches to screening.
Underrecognition of BPD
The Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project is an ongoing clinical research study involving the integration of research assessment methods into routine clinical practice.17 In an early report from the MIDAS project, BPD diagnoses derived from structured and unstructured clinical interviews were compared between 2 groups of psychiatric outpatients in the same practice.15 Individuals in the structured interview cohort were 35 times more often diagnosed with BPD than individuals evaluated with an unstructured clinical interview. Importantly, when the information from the structured interview was presented to the clinicians, BPD was more likely to be diagnosed clinically.
Other studies13,16 also found that the rate of diagnosing BPD was higher when the diagnosis was based on a semi-structured diagnostic interview compared with an unstructured clinical interview, and that clinicians were reluctant to diagnose BPD during their routine intake diagnostic evaluation.
Clinicians, however, do not use semi-structured interviews in their practice, and they also do not tend to diagnose personality disorders (PDs) based on direct questioning, as they typically would when assessing a symptom-based disorder such as depression or anxiety. Rather, clinicians report that they rely on longitudinal observations to diagnose PDs.18 However, the results from the MIDAS project were inconsistent with clinicians’ reports. When clinicians were presented with the results of the semi-structured interview, they usually would diagnose BPD, even though it was the initial evaluation. If clinicians actually relied on longitudinal observations and considered information based on the direct question approach of research interviews to be irrelevant or invalid, then the results from the semi-structured interview should not have influenced the rate at which they diagnosed BPD. This suggests that the primary issue in diagnosing PDs is not the need for longitudinal observation but rather the need for more information, and that there is a role for screening questionnaires.
One potential criticism of studies demonstrating underrecognition of BPD in clinical practice is that patients typically were interviewed when they presented for treatment, when most were depressed or anxious. The possible pathologizing effects of psychiatric state on personality have been known for years.19 However, a large body of literature examining the treatment, prognostic, familial, and biological correlates of PDs supports the validity of diagnosing PDs in this manner. Moreover, from a clinical perspective, the sooner a clinician is aware of the presence of BPD, the more likely this information can be used for treatment planning.
Who should be screened for BPD?
BPD is underrecognized and underdiagnosed because patients with BPD often also have comorbid mood, anxiety, or substance use disorders.20,21 The symptoms associated with these disorders are typically the chief concern of patients with undiagnosed BPD who present for treatment. Patients with BPD rarely present for an intake evaluation and state that they are struggling with abandonment fears, chronic feelings of emptiness, or an identity disturbance. If patients identified these problems as their chief concerns, BPD would be easier to recognize.
Although several studies have documented the frequency of BPD in patients with a specific psychiatric diagnosis such as major depressive disorder (MDD) or attention-deficit/hyperactivity disorder,22-26 the MIDAS project examined the frequency of BPD in patients with various diagnoses and evaluated which disorders were associated with a significantly increased rate of BPD.27 The highest rate of BPD was found in patients with bipolar disorder. Approximately 25% of patients with bipolar II disorder and one-third of those with bipolar I disorder were diagnosed with BPD; these rates were significantly higher than the rate of BPD in patients without these disorders (Table 127). The rate of BPD was second highest in patients with a principal diagnosis of posttraumatic stress disorder (PTSD) and MDD; however, the rate of BPD in these patients was not significantly elevated compared with patients who did not have these principal diagnoses. Three disorders were associated with a significantly lower rate of BPD: adjustment disorder, dysthymic disorder, and generalized anxiety disorder.
It would be easy to recommend screening for BPD in all psychiatric patients. However, that is not feasible or practical. In making screening recommendations, absolute risk should be considered more important than relative risk. Clinicians should screen for BPD in patients presenting to a general psychiatric outpatient practice with a principal diagnosis of MDD, bipolar disorder, PTSD, or panic disorder with agoraphobia. That is, I recommend screening for BPD in patients with a principal diagnosis in which the prevalence of BPD is ≥10% (Table 127).
A brief review of screening statistics
Screening tests for most psychiatric disorders are based on multi-item scales in which a total score is computed from a sum of item scores, and a cutoff point is established to determine who does and does not screen positive on the test. However, sensitivity, specificity, and positive and negative predictive values are not invariant properties of a screening test with a continuous score distribution. Rather, the performance statistics of a scale can be altered by changing the threshold score to distinguish cases from non-cases. When the screening threshold is lowered, sensitivity increases and specificity decreases.
For screening, a broad net needs to be cast so that all (or almost all) cases are included. Therefore, the cutoff score should be set low to prioritize the sensitivity of the instrument. A screening scale also should have high negative predictive value so that the clinician can be confident that patients who screen negative on the test do not have the disorder.
Screening questionnaires for BPD
Several questionnaires have been developed to screen for PDs (Table 228-35). Some screen for each of the DSM PDs,28,36-42 and some screen more broadly for the presence or absence of any PD.29,43,44 The most commonly studied self-report scale for BPD is the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD),30 a 10-item self-report scale derived from a subset of questions from the BPD module of a semi-structured diagnostic interview.
The initial validation study30 found that the optimal cutoff score was 7, which resulted in a sensitivity of 81% and specificity of 89%. Three studies have evaluated the scale in adolescents and young adults,45-47 and 3 studies examined the scale in adult outpatients.48-50 Across all 6 studies, at the optimal cutoff scores determined in each study, the sensitivity of the MSI-BPD ranged from 68% to 94% (mean, 80%) and the specificity ranged from 66% to 80% (mean, 72%).
Problems with screening questionnaires. Although screening scales have been developed for many psychiatric disorders, they have not been widely used in mental health settings. In a previous commentary, I argued that the conceptual justification for using self-report screening scales for single disorders in psychiatric settings is questionable.51 Another problem with screening scales is their potential misuse as case-finding instruments. In the literature on bipolar disorder screening, several researchers misconstrued a positive screen to indicate caseness.51 Although this is not a problem with the screening measures or the selection of a cutoff score, caution must be taken to not confuse screening with diagnosis.52
Screening for BPD as part of your diagnostic interview
An alternative approach to using self-administered questionnaires for screening is for clinicians to include questions in their evaluation as part of a psychiatric review of systems. When conducting a diagnostic interview, clinicians typically screen for disorders that are comorbid with the principal diagnosis by asking about the comorbid disorders’ necessary features or “gate criteria.” For example, in a patient with a principal diagnosis of MDD, the clinician would inquire about the presence of panic attacks, excessive worry, or substance use to screen for the presence of panic disorder, generalized anxiety disorder, or a substance use disorder. In contrast, for polythetically defined disorders such as BPD, there is no single gate criterion, because the disorder is diagnosed based on the presence of at least 5 of 9 criteria and no single one of these criteria is required to be present to establish the diagnosis.
As part of the MIDAS project, the psychometric properties of the BPD criteria were examined to determine if it was possible to identify 1 or 2 criteria that could serve as gate criteria to screen for the disorder. If the sensitivity of 1 criterion or a combination of 2 BPD criteria was sufficiently high (ie, >90%), then the assessment of this criterion (or these criteria) could be included in a psychiatric review of systems, thus potentially improving the detection of BPD. Researchers hypothesized that affective instability, considered first by Linehan53 and later by other theorists54 to be of central importance to the clinical manifestations of BPD, could function as a gate criterion. In the sample of 3,674 psychiatric outpatients who were evaluated with a semi-structured interview, the sensitivity of the affective instability criterion was 92.8%, and the negative predictive value of the criterion was 99%.
Identifying a single BPD criterion that is present in the vast majority of patients diagnosed with BPD will allow clinicians to follow their usual clinical practice when conducting a psychiatric review of systems and inquire about the gate criteria of various disorders. Several studies have found that >90% of patients with BPD report affective instability. However, this does not mean that the diagnosis of BPD can be abbreviated to an assessment of the presence or absence of affective instability. Many patients who screen positive will not have BPD when a more definitive diagnostic evaluation is conducted. In the case of BPD, the more costly definitive diagnostic procedure simply entails inquiry of the other diagnostic criteria.
1. Bellino S, Patria L, Paradiso E, et al. Major depression in patients with borderline personality disorder: a clinical investigation. Can J Psychiatry. 2005;50(4):234-238.
2. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-283.
3. Gunderson JG, Stout RL, McGlashan TH, et al. Ten-year course of borderline personality disorder: psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Arch Gen Psychiatry. 2011;68(8):827-837.
4. Zanarini MC, Jacoby RJ, Frankenburg FR, et al. The 10-year course of social security disability income reported by patients with borderline personality disorder and axis II comparison subjects. J Pers Disord. 2009;23(4):346-356.
5. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
6. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
7. Zanarini MC, Frankenburg FR, Hennen J, et al. Mental health service utilization by borderline personality disorder patients and Axis II comparison subjects followed prospectively for 6 years. J Clin Psychiatry. 2004;65(1):28-36.
8. Pompili M, Girardi P, Ruberto A, et al. Suicide in borderline personality disorder: a meta-analysis. Nord J Psychiatry. 2005;59(5):319-324.
9. Oldham JM. Borderline personality disorder and suicidality. Am J Psychiatry. 2006;163(1):20-26.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Marinangeli M, Butti G, Scinto A, et al. Patterns of comorbidity among DSM-III-R personality disorders. Psychopathology. 2000;33(2):69-74.
12. Zimmerman M, Rothschild L, Chelminski I. The frequency of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918.
13. Comtois KA, Carmel A. Borderline personality disorder and high utilization of inpatient psychiatric hospitalization: concordance between research and clinical diagnosis. J Behav Health Servi Res. 2016;43(2):272-280.
14. Paris J, Black DW. Borderline personality disorder and bipolar disorder: what is the difference and why does it matter? J Nerv Ment Dis. 2015;203(1):3-7.
15. Zimmerman M, Mattia JI. Differences between clinical and research practice in diagnosing borderline personality disorder. Am J Psychiatry. 1999;156(10):1570-1574.
16. Magnavita JJ, Levy KN, Critchfield KL, et al. Ethical considerations in treatment of personality dysfunction: using evidence, principles, and clinical judgment. Professional Psychology: Research and Practice. 2010;41(1):64-74.
17. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
18. Westen D. Divergences between clinical and research methods for assessing personality disorders: implications for research and the evolution of axis II. Am J Psychiatry. 1997;154(7):895-903.
19. Zimmerman M. Diagnosing personality disorders: a review of issues and research methods. Arch Gen Psychiatry. 1994;51(3):225-245.
20. Zanarini MC, Gunderson JG, Frankenberg FR. Axis I phenomenology of borderline personality disorder. Compr Psychiatry. 1989;30(2):149-156.
21. Zimmerman M, Mattia JI. Axis I diagnostic comorbidity and borderline personality disorder. Compr Psychiatry. 1999;40(4):245-252.
22. Gunderson JG, Morey LC, Stout RL, et al. Major depressive disorder and borderline personality disorder revisited: longitudinal interactions. J Clin Psychiatry. 2004;65(8):1049-1056.
23. Bayes AJ, Parker GB. Clinical vs. DSM diagnosis of bipolar disorder, borderline personality disorder and their co-occurrence. Acta Psychiatr Scand. 2016;135(3):259-265.
24. Carpenter RW, Wood PK, Trull TJ. Comorbidity of borderline personality disorder and lifetime substance use disorders in a nationally representative sample. J Pers Disord. 2016;30(3):336-350.
25. Trull TJ, Sher KJ, Minks-Brown C, et al. Borderline personality disorder and substance use disorders: a review and integration. Clin Psychol Rev. 2000;20(2):235-253.
26. Matthies SD, Philipsen A. Common ground in attention deficit hyperactivity disorder (ADHD) and borderline personality disorder (BPD)-review of recent findings. Borderline Personal Disord Emot Dysregul. 2014;1:3.
27. Zimmerman M, Chelminski I, Dalrymple K, et al. Principal diagnoses in psychiatric outpatients with borderline personality disorder: implications for screening recommendations. Ann Clin Psychiatry. 2017;29(1):54-60.
28. Magallón-Neri EM, Forns M, Canalda G, et al. Usefulness of the International Personality Disorder Examination Screening Questionnaire for borderline and impulsive personality pathology in adolescents. Compr Psychiatry. 2013;54(3):301-308.
29. Germans S, Van Heck GL, Langbehn DR, et al. The Iowa Personality Disorder Screen. Eur J Psychol Assess. 2010;26(1):11-18.
30. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). J Pers Disord. 2003;17(6):568-573.
31. Germans S, Van Heck GL, Hodiamont PP. Results of the search for personality disorder screening tools: clinical implications. J Clin Psychiatry. 2012;73(2):165-173.
32. Hyler SE. Personality diagnostic questionnaire-4. New York, NY: New York State Psychiatric Institute; 1994.
33. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis II Disorders - Patient edition (SCID-I/P, version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995.
34. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
35. Poreh AM, Rawlings D, Claridge G, et al. The BPQ: a scale for the assessment of boderline personality based on DSM-IV criteria. J Pers Disord. 2006;20(3):247-260.
36. Ekselius L, Lindstrom E, von Knorring L, et al. SCID II interviews and the SCID Screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1994;90(2):120-123.
37. Hyler SE, Skodol AE, Oldham JM, et al. Validity of the Personality Diagnostic Questionnaire-Revised: a replication in an outpatient sample. Compr Psychiatry. 1992;33(2):73-77.
38. Davison S, Leese M, Taylor PJ. Examination of the screening properties of the personality diagnostic questionnaire 4+ (PDQ-4+) in a prison population. J Pers Disord. 2001;15(2):180-194.
39. Jacobsberg L, Perry S, Frances A. Diagnostic agreement between the SCID-II screening questionnaire and the Personality Disorder Examination. J Pers Assess. 1995;65(3):428-433.
40. Germans S, Van Heck GL, Masthoff ED, et al. Diagnostic efficiency among psychiatric outpatients of a self-report version of a subset of screen items of the Structured Clinical Interview for DSM-IV-TR Personality Disorders (SCID-II). Psychol Assess. 2010;22(4):945-952.
41. Lloyd C, Overall JE, Click M Jr. Screening for borderline personality disorders with the MMPI-168. J Clin Psychol. 1983;39(5):722-726.
42. Neal LA, Fox C, Carroll N, et al. Development and validation of a computerized screening test for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1997;95(4):351-356.
43. Germans S, Van Heck GL, Moran P, et al. The Self-Report Standardized Assessment of Personality-abbreviated Scale: preliminary results of a brief screening test for personality disorders. Pers Ment Health. 2008;2(2):70-76.
44. Moran P, Leese M, Lee T, et al. Standardized Assessment of Personality - Abbreviated Scale (SAPAS): preliminary validation of a brief screen for personality disorder. Br J Psychiatry. 2003;183:228-232.
45. Chanen AM, Jovev M, Djaja D, et al. Screening for borderline personality disorder in outpatient youth. J Pers Disord. 2008;22(4):353-364.
46. van Alebeek A, van der Heijden PT, Hessels C, et al. Comparison of three questionnaires to screen for borderline personality disorder in adolescents and young adults. Eur J Psychol Assess. 2017:33;123-128.
47. Noblin JL, Venta A, Sharp C. The validity of the MSI-BPD among inpatient adolescents. Assessment. 2014;21(2):210-217.
48. Kröger C, Vonau M, Kliem S, et al. Emotion dysregulation as a core feature of borderline personality disorder: comparison of the discriminatory ability of two self-rating measures. Psychopathology. 2011;44(4):253-260.
49. Soler J, Domínguez-Clav E, García-Rizo C, et al. Validation of the Spanish version of the McLean Screening Instrument for Borderline Personality Disorder. Rev Psiquiatr Salud Ment. 2016;9(4):195-202.
50. Melartin T, Häkkinen M, Koivisto M, et al. Screening of psychiatric outpatients for borderline personality disorder with the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). Nord J Psychiatry. 2009;63(6):475-479.
51. Zimmerman M. Misuse of the Mood Disorders Questionnaire as a case-finding measure and a critique of the concept of using a screening scale for bipolar disorder in psychiatric practice. Bipolar Disord. 2012;14(2):127-134.
52. Zimmerman M. Screening for bipolar disorder: confusion between case-finding and screening. Psychother Psychosom. 2014;83(5):259-262.
53. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York, NY: Guilford Press; 1993.
54. Koenigsberg HW, Harvey PD, Mitropoulou V, et al. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? J Pers Disord. 2001;15(4):358-370.
55. Grilo CM, Becker DF, Anez LM, et al. Diagnostic efficiency of DSM-IV criteria for borderline personality disorder: an evaluation in Hispanic men and women with substance use disorders. J Consult Clin Psychol. 2004;72(1):126-131.
56. Korfine L, Hooley JM. Detecting individuals with borderline personality disorder in the community: an ascertainment strategy and comparison with a hospital sample. J Pers Disord. 2009;23(1):62-75.
57. Leppänen V, Lindeman S, Arntz A, et al. Preliminary evaluation of psychometric properties of the Finnish Borderline Personality Disorder Severity Index: Oulu-BPD-Study. Nord J Psychiatry. 2013;67(5):312-319.
58. Pfohl B, Coryell W, Zimmerman M, et al. DSM-III personality disorders: diagnostic overlap and internal consistency of individual DSM-III criteria. Compr Psychiatry. 1986;27(1):22-34.
59. Reich J. Criteria for diagnosing DSM-III borderline personality disorder. Ann Clin Psychiatry. 1990;2(3):189-197.
60. Nurnberg HG, Raskin M, Levine PE, et al. Hierarchy of DSM-III-R criteria efficiency for the diagnosis of borderline personality disorder. J Pers Disord. 1991;5(3):211-224.
61. Farmer RF, Chapman AL. Evaluation of DSM-IV personality disorder criteria as assessed by the structured clinical interview for DSM-IV personality disorders. Compr Psychiatry. 2002;43(4):285-300.
62. Grilo CM, McGlashan TH, Morey LC, et al. Internal consistency, intercriterion overlap and diagnostic efficiency of criteria sets for DSM-IV schizotypal, borderline, avoidant and obsessive-compulsive personality disorders. Acta Psychiatr Scand. 2001;104(4):264-272.
63. Grilo CM, Sanislow CA, Skodol AE, et al. Longitudinal diagnostic efficiency of DSM-IV criteria for borderline personality disorder: a 2-year prospective study. Can J Psychiatry. 2007;52(6):357-362.
1. Bellino S, Patria L, Paradiso E, et al. Major depression in patients with borderline personality disorder: a clinical investigation. Can J Psychiatry. 2005;50(4):234-238.
2. Skodol AE, Gunderson JG, McGlashan TH, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159(2):276-283.
3. Gunderson JG, Stout RL, McGlashan TH, et al. Ten-year course of borderline personality disorder: psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Arch Gen Psychiatry. 2011;68(8):827-837.
4. Zanarini MC, Jacoby RJ, Frankenburg FR, et al. The 10-year course of social security disability income reported by patients with borderline personality disorder and axis II comparison subjects. J Pers Disord. 2009;23(4):346-356.
5. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69(4):533-545.
6. Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry. 2001;158(2):295-302.
7. Zanarini MC, Frankenburg FR, Hennen J, et al. Mental health service utilization by borderline personality disorder patients and Axis II comparison subjects followed prospectively for 6 years. J Clin Psychiatry. 2004;65(1):28-36.
8. Pompili M, Girardi P, Ruberto A, et al. Suicide in borderline personality disorder: a meta-analysis. Nord J Psychiatry. 2005;59(5):319-324.
9. Oldham JM. Borderline personality disorder and suicidality. Am J Psychiatry. 2006;163(1):20-26.
10. Black DW, Blum N, Pfohl B, et al. Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord. 2004;18(3):226-239.
11. Marinangeli M, Butti G, Scinto A, et al. Patterns of comorbidity among DSM-III-R personality disorders. Psychopathology. 2000;33(2):69-74.
12. Zimmerman M, Rothschild L, Chelminski I. The frequency of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918.
13. Comtois KA, Carmel A. Borderline personality disorder and high utilization of inpatient psychiatric hospitalization: concordance between research and clinical diagnosis. J Behav Health Servi Res. 2016;43(2):272-280.
14. Paris J, Black DW. Borderline personality disorder and bipolar disorder: what is the difference and why does it matter? J Nerv Ment Dis. 2015;203(1):3-7.
15. Zimmerman M, Mattia JI. Differences between clinical and research practice in diagnosing borderline personality disorder. Am J Psychiatry. 1999;156(10):1570-1574.
16. Magnavita JJ, Levy KN, Critchfield KL, et al. Ethical considerations in treatment of personality dysfunction: using evidence, principles, and clinical judgment. Professional Psychology: Research and Practice. 2010;41(1):64-74.
17. Zimmerman M. A review of 20 years of research on overdiagnosis and underdiagnosis in the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) Project. Can J Psychiatry. 2016;61(2):71-79.
18. Westen D. Divergences between clinical and research methods for assessing personality disorders: implications for research and the evolution of axis II. Am J Psychiatry. 1997;154(7):895-903.
19. Zimmerman M. Diagnosing personality disorders: a review of issues and research methods. Arch Gen Psychiatry. 1994;51(3):225-245.
20. Zanarini MC, Gunderson JG, Frankenberg FR. Axis I phenomenology of borderline personality disorder. Compr Psychiatry. 1989;30(2):149-156.
21. Zimmerman M, Mattia JI. Axis I diagnostic comorbidity and borderline personality disorder. Compr Psychiatry. 1999;40(4):245-252.
22. Gunderson JG, Morey LC, Stout RL, et al. Major depressive disorder and borderline personality disorder revisited: longitudinal interactions. J Clin Psychiatry. 2004;65(8):1049-1056.
23. Bayes AJ, Parker GB. Clinical vs. DSM diagnosis of bipolar disorder, borderline personality disorder and their co-occurrence. Acta Psychiatr Scand. 2016;135(3):259-265.
24. Carpenter RW, Wood PK, Trull TJ. Comorbidity of borderline personality disorder and lifetime substance use disorders in a nationally representative sample. J Pers Disord. 2016;30(3):336-350.
25. Trull TJ, Sher KJ, Minks-Brown C, et al. Borderline personality disorder and substance use disorders: a review and integration. Clin Psychol Rev. 2000;20(2):235-253.
26. Matthies SD, Philipsen A. Common ground in attention deficit hyperactivity disorder (ADHD) and borderline personality disorder (BPD)-review of recent findings. Borderline Personal Disord Emot Dysregul. 2014;1:3.
27. Zimmerman M, Chelminski I, Dalrymple K, et al. Principal diagnoses in psychiatric outpatients with borderline personality disorder: implications for screening recommendations. Ann Clin Psychiatry. 2017;29(1):54-60.
28. Magallón-Neri EM, Forns M, Canalda G, et al. Usefulness of the International Personality Disorder Examination Screening Questionnaire for borderline and impulsive personality pathology in adolescents. Compr Psychiatry. 2013;54(3):301-308.
29. Germans S, Van Heck GL, Langbehn DR, et al. The Iowa Personality Disorder Screen. Eur J Psychol Assess. 2010;26(1):11-18.
30. Zanarini MC, Vujanovic AA, Parachini EA, et al. A screening measure for BPD: the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). J Pers Disord. 2003;17(6):568-573.
31. Germans S, Van Heck GL, Hodiamont PP. Results of the search for personality disorder screening tools: clinical implications. J Clin Psychiatry. 2012;73(2):165-173.
32. Hyler SE. Personality diagnostic questionnaire-4. New York, NY: New York State Psychiatric Institute; 1994.
33. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis II Disorders - Patient edition (SCID-I/P, version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995.
34. Bohus M, Kleindienst N, Limberger MF, et al. The short version of the Borderline Symptom List (BSL-23): development and initial data on psychometric properties. Psychopathology. 2009;42(1):32-39.
35. Poreh AM, Rawlings D, Claridge G, et al. The BPQ: a scale for the assessment of boderline personality based on DSM-IV criteria. J Pers Disord. 2006;20(3):247-260.
36. Ekselius L, Lindstrom E, von Knorring L, et al. SCID II interviews and the SCID Screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1994;90(2):120-123.
37. Hyler SE, Skodol AE, Oldham JM, et al. Validity of the Personality Diagnostic Questionnaire-Revised: a replication in an outpatient sample. Compr Psychiatry. 1992;33(2):73-77.
38. Davison S, Leese M, Taylor PJ. Examination of the screening properties of the personality diagnostic questionnaire 4+ (PDQ-4+) in a prison population. J Pers Disord. 2001;15(2):180-194.
39. Jacobsberg L, Perry S, Frances A. Diagnostic agreement between the SCID-II screening questionnaire and the Personality Disorder Examination. J Pers Assess. 1995;65(3):428-433.
40. Germans S, Van Heck GL, Masthoff ED, et al. Diagnostic efficiency among psychiatric outpatients of a self-report version of a subset of screen items of the Structured Clinical Interview for DSM-IV-TR Personality Disorders (SCID-II). Psychol Assess. 2010;22(4):945-952.
41. Lloyd C, Overall JE, Click M Jr. Screening for borderline personality disorders with the MMPI-168. J Clin Psychol. 1983;39(5):722-726.
42. Neal LA, Fox C, Carroll N, et al. Development and validation of a computerized screening test for personality disorders in DSM-III-R. Acta Psychiatr Scand. 1997;95(4):351-356.
43. Germans S, Van Heck GL, Moran P, et al. The Self-Report Standardized Assessment of Personality-abbreviated Scale: preliminary results of a brief screening test for personality disorders. Pers Ment Health. 2008;2(2):70-76.
44. Moran P, Leese M, Lee T, et al. Standardized Assessment of Personality - Abbreviated Scale (SAPAS): preliminary validation of a brief screen for personality disorder. Br J Psychiatry. 2003;183:228-232.
45. Chanen AM, Jovev M, Djaja D, et al. Screening for borderline personality disorder in outpatient youth. J Pers Disord. 2008;22(4):353-364.
46. van Alebeek A, van der Heijden PT, Hessels C, et al. Comparison of three questionnaires to screen for borderline personality disorder in adolescents and young adults. Eur J Psychol Assess. 2017:33;123-128.
47. Noblin JL, Venta A, Sharp C. The validity of the MSI-BPD among inpatient adolescents. Assessment. 2014;21(2):210-217.
48. Kröger C, Vonau M, Kliem S, et al. Emotion dysregulation as a core feature of borderline personality disorder: comparison of the discriminatory ability of two self-rating measures. Psychopathology. 2011;44(4):253-260.
49. Soler J, Domínguez-Clav E, García-Rizo C, et al. Validation of the Spanish version of the McLean Screening Instrument for Borderline Personality Disorder. Rev Psiquiatr Salud Ment. 2016;9(4):195-202.
50. Melartin T, Häkkinen M, Koivisto M, et al. Screening of psychiatric outpatients for borderline personality disorder with the McLean Screening Instrument for Borderline Personality Disorder (MSI-BPD). Nord J Psychiatry. 2009;63(6):475-479.
51. Zimmerman M. Misuse of the Mood Disorders Questionnaire as a case-finding measure and a critique of the concept of using a screening scale for bipolar disorder in psychiatric practice. Bipolar Disord. 2012;14(2):127-134.
52. Zimmerman M. Screening for bipolar disorder: confusion between case-finding and screening. Psychother Psychosom. 2014;83(5):259-262.
53. Linehan MM. Cognitive-behavioral treatment of borderline personality disorder. New York, NY: Guilford Press; 1993.
54. Koenigsberg HW, Harvey PD, Mitropoulou V, et al. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? J Pers Disord. 2001;15(4):358-370.
55. Grilo CM, Becker DF, Anez LM, et al. Diagnostic efficiency of DSM-IV criteria for borderline personality disorder: an evaluation in Hispanic men and women with substance use disorders. J Consult Clin Psychol. 2004;72(1):126-131.
56. Korfine L, Hooley JM. Detecting individuals with borderline personality disorder in the community: an ascertainment strategy and comparison with a hospital sample. J Pers Disord. 2009;23(1):62-75.
57. Leppänen V, Lindeman S, Arntz A, et al. Preliminary evaluation of psychometric properties of the Finnish Borderline Personality Disorder Severity Index: Oulu-BPD-Study. Nord J Psychiatry. 2013;67(5):312-319.
58. Pfohl B, Coryell W, Zimmerman M, et al. DSM-III personality disorders: diagnostic overlap and internal consistency of individual DSM-III criteria. Compr Psychiatry. 1986;27(1):22-34.
59. Reich J. Criteria for diagnosing DSM-III borderline personality disorder. Ann Clin Psychiatry. 1990;2(3):189-197.
60. Nurnberg HG, Raskin M, Levine PE, et al. Hierarchy of DSM-III-R criteria efficiency for the diagnosis of borderline personality disorder. J Pers Disord. 1991;5(3):211-224.
61. Farmer RF, Chapman AL. Evaluation of DSM-IV personality disorder criteria as assessed by the structured clinical interview for DSM-IV personality disorders. Compr Psychiatry. 2002;43(4):285-300.
62. Grilo CM, McGlashan TH, Morey LC, et al. Internal consistency, intercriterion overlap and diagnostic efficiency of criteria sets for DSM-IV schizotypal, borderline, avoidant and obsessive-compulsive personality disorders. Acta Psychiatr Scand. 2001;104(4):264-272.
63. Grilo CM, Sanislow CA, Skodol AE, et al. Longitudinal diagnostic efficiency of DSM-IV criteria for borderline personality disorder: a 2-year prospective study. Can J Psychiatry. 2007;52(6):357-362.
The etiology of premenstrual dysphoric disorder: 5 interwoven pieces
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3
The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.
Consider these 5 interwoven pieces
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21
Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3
The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.
Consider these 5 interwoven pieces
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21
Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
In an age when psychiatry strives to identify the biologic causes of disease, studying endocrine-related mood disorders is particularly intriguing. DSM-5 defines premenstrual dysphoric disorder (PMDD) as a depressive disorder, with a 12-month prevalence ranging from 1.8% to 5.8% among women who menstruate.1-3 Factors that differentiate PMDD from other affective disorders include etiology, duration, and temporal relationship with the menstrual cycle.
PMDD is a disorder of consistent yet intermittent change in mental health and functionality. Therefore, it may be underdiagnosed and consequently undertreated if a psychiatric evaluation does not coincide with symptom occurrence or if patients do not understand that intermittent symptoms are treatable.
This article summarizes what is known about the etiology of PMDD. Although there are several treatments for PMDD, many women experience adverse effects or incomplete effectiveness. Further understanding of this disorder may lead to more efficacious treatments. Additionally, understanding the pathophysiology of PMDD might shed a light on the etiology of other disorders that are temporally related to reproductive life changes, such as pregnancy-, postpartum-, or menopause-related affective dysregulation.
Making the diagnosis
The diagnosis of PMDD is made when a patient has at least 5 of 11 specific symptoms that occur during the week before onset of menses, improve within a few days after the onset of menses (shown as the “PMDD Hazard Zone” in Figure 1), and are minimal or absent post-menses.3 Symptoms should be tracked prospectively for at least 2 menstrual cycles in order to confirm the diagnosis (one must be an affective symptom and another must be a behavioral/cognitive symptom).3
The affective symptoms are:
- lability of affect (eg, sudden sadness, tearfulness, or sensitivity to rejection)
- irritability, anger, or increased interpersonal conflicts
- depressed mood, hopelessness, or self- deprecating thoughts
- anxiety or tension, feeling “keyed up” or “on edge.”
The behavioral/cognitive symptoms are:
- decreased interest in usual activities (eg, work, hobbies, friends, school)
- difficulty concentrating
- lethargy, low energy, easy fatigability
- change in appetite, overeating, food cravings
- hypersomnia or insomnia
- feeling overwhelmed or out of control
- physical symptoms (breast tenderness or swelling, headache, joint or muscle pain, bloating, weight gain).
Ruling out premenstrual exacerbation (PME). Perhaps the most common cause for misdiagnosis of PMDD is failing to rule out PME of another underlying or comorbid condition (Figure 2). In many women who have a primary mood or anxiety disorder, the late luteal phase is a vulnerable time. A patient might be coping with untreated anxiety, for example, but the symptoms become unbearable the week before menstruation begins, which is likely when she seeks help. At this stage, a diagnosis of PMDD should be provisional at best. Often, PME is treated by treating the underlying condition. Therefore, a full diagnostic psychiatric interview is important to first rule out other underlying psychiatric disorders. PMDD is diagnosed if the premenstrual symptoms persist for 2 consecutive months after treating the suspected mood or anxiety disorder. Patients can use one of many PMDD daily symptom charts available online. Alternatively, they can use a cycle-tracking mobile phone application to correlate their symptoms with their cycle and share this information with their providers.
Consider these 5 interwoven pieces
The many variables that contribute to the pathophysiology of PMDD overlap and should be considered connecting pieces in the puzzle that is the etiology of this disorder (Figure 3). In reviewing the literature, we have identified 5 topics likely to be major contributors to this disorder:
- genetic susceptibility
- progesterone and allopregnanolone (ALLO)
- estrogen, serotonin, and brain-derived neurotrophic factor (BDNF)
- putative brain structural and functional differences
- further involvement of the hypothalamic–pituitary–adrenal (HPA) axis and hypothalamic–pituitary–gonadal (HPG) axis: trauma, resiliency, and inflammation.
Genetic susceptibility. PMDD is thought to have a heritability range between 30% to 80%.3 This is demonstrated by family and twin studies4-7 and specific genetic studies.8 The involvement of genetics means an underlying neurobiologic pathophysiology is in place.
Estrogen receptor alpha (ESR1) gene. Huo et al8 found an associated variation in ESR1 in women with PMDD compared with controls. They speculated that because ESR1 is important for arousal, if dysfunctional, this gene could be implicated in somatic as well as affective and cognitive deficits in PMDD patients. In another study, investigators reported a relationship between PMDD and heritable personality traits, as well as a link between these traits and ESR1 polymorphic variants.1 They suggested that personality traits (independent of affective state) might be used to distinguish patients with PMDD from controls.1
Studies on serotonin gene polymorphism and serotonin transporter genotype. Although a study of serotonin gene polymorphism did not find an association between serotonin1A gene polymorphism and PMDD, it did show that the presence of at least 1 C allele was associated with a 2.5-fold increased risk of PMDD.9 Another study did not find an association between the serotonin transporter genotype 5-HTTLPR and PMDD.10 However, it showed lower frontocingulate cortex activation during the luteal phase of PMDD patients compared with controls, suggesting that PMDD is linked to impaired frontocingulate cortex activation induced by emotions during the luteal phase.10
Seasonal affective disorder (SAD) and PMDD have shared clinical features. A polymorphism in the serotonin transporter promoter gene 5-HTTLPR has been associated with SAD. One study found that patients with comorbid SAD and PMDD are genetically more vulnerable to comorbid affective disorders compared with patients who have SAD only.11
Progesterone and ALLO. Chronic exposure to progesterone and ALLO (a main progesterone metabolite) and rapid withdrawal from ovarian hormones may play a role in the etiology of PMDD. Much like alcohol or benzodiazepines, ALLO is a potent positive allosteric modulator of GABAA receptors and has sedative, anesthetic, and anxiolytic properties. In times of acute stress, increased ALLO is known to provide relief.12,13 However, in women with PMDD, this typical ALLO increase might not occur.14
Patients with PMDD have been reported to have decreased levels of ALLO in the luteal phase.15-17 In one study, women with highly symptomatic PMDD had lower levels of ALLO compared with women with less symptomatic PMDD.14 A gonadotropin-releasing hormone challenge study showed the increase in ALLO response was less in PMDD patients compared with controls.17 Luteal-phase ALLO concentrations are reported to be lower in women with premenstrual syndrome (PMS), a milder form of PMDD.14,17
The efficacy of selective serotonin reuptake inhibitors (SSRIs) for treating PMDD could be the result of the interaction of these medications with neuroactive steroids,18 possibly because SSRIs enhance the sensitivity of GABAA receptors or promote the formation of more ALLO (Figure 4).19-21
Estrogen, serotonin, and BDNF. Estrogen affects multiple neurotransmitter systems that regulate mood, cognition, sleep, and eating.22 Studying estrogen in context of PMDD is important because women with PMDD can have low mood, specific food cravings, and impaired cognitive function.
Estrogen–serotonin interactions are thought to be involved in hormone-related mood disorders such as perimenopausal depression and PMDD.23,24 However, the nature of their relationship is not yet fully understood. Ovariectomized animals have shown estrogen-induced changes related to serotonin metabolism, binding, and transmission in the regions of the brain involved in regulation of affect and cognition. Research in menopausal women also has provided some support for this interaction.24
Positron emission tomography studies in humans have found increased cortical serotonin binding modulated by levels of estrogen, similar to those previously seen in rat studies.24-27 One study showed an increased binding potential of serotonin in the cerebral cortex with estrogen treatment. This study further showed an even greater binding potential with estrogen plus progesterone, signaling a synergistic effect of the 2 hormones.28
SSRIs are an effective treatment for the irritability, anxiety, and mood swings of PMDD.29-30 Although the exact mechanism of action is unknown, the serotonergic properties are certainly of primary attention. For some PMDD patients, SSRIs work within hours to days, as opposed to days or weeks for patients with depression or anxiety, which suggests a separate or co-occurring mechanism of action is in place. In a double-blind, placebo-controlled crossover study, researchers administered the serotonin receptor antagonist metergoline to women with PMDD whose symptoms had remitted during treatment with fluoxetine and a group of healthy controls who were not receiving any medication.31 The women with PMDD experienced a return of symptoms 24 hours after treatment with metergoline but not with placebo; the controls experienced no mood changes.31
BDNF is a neurotransmitter linked to estrogen and likely related to PMDD. BDNF is critical for neurogenesis and is expressed in brain regions involved in learning and memory and also affects regulation.32 BDNF levels are increased by serotonergic antidepressants, affected by estradiol, and have cyclicity throughout the menstrual cycle.33-35
Putative brain structural and functional differences. Imaging studies have suggested differences in brain structure in women with PMDD, with a focus on the amygdala and the prefrontal cortex. Women with PMDD have greater gray matter volume in the posterior cerebellum,36 greater gray matter density of hippocampal cortex, and lower gray matter density in the parahippocampal cortex.37
Some studies have shown a functional variability of the amygdala’s response to stress in women with PMDD vs healthy controls.38,39 A proton magnetic resonance spectroscopy (1H-MRS) study of the displays the possibility of an altered GABAergic function in patients with PMDD.40
Patients will PMDD have enhanced dorsolateral prefrontal cortex reactivity when anticipating negative stimuli (but not to the actual exposure) during the luteal phase. A positive correlation between this reactivity and progesterone levels also was observed.41 Some researchers have suggested that prefrontal cortex dysfunction may be a risk factor for PMDD.42
HPA axis and HPG axis: Trauma, resiliency, inflammation. Altered cortisol levels (higher during the luteal phase43 and lower during times of stress14,44) suggest a possibly altered HPA axis in some women with PMDD. However, studies on this topic have been few and inconsistent.
Dysregulation of the HPG axis could cause vasomotor symptoms, sleep dysregulation, and mood symptoms during menopause; women with PMDD can also experience these symptoms. The influence of estrogen and progesterone on mood is also highly dependent on this axis.
Ultimately, the interplay between the HPA axis and the HPG axis is important. One study found that women with PMDD who had high serum ALLO levels (HPG-related) had blunted nocturnal cortisol levels (HPA-related) compared with healthy controls who had low ALLO levels.45
Significant stress and trauma exposure have been associated with PMDD. A study of 3,968 women found a history of trauma and PTSD were independently associated with PMDD.46 Another study of approximately 3,000 women found a strong correlation between abuse and PMS.47 However, a third study found no correlations between PMDD and trauma.48
Patients with a predisposition to PMDD may be more vulnerable to develop a posttraumatic stress-related disorder, perhaps due to decreased biologic resiliency. For example, the startle response (hypervigilance) has been shown to be different in women with PMDD. One study suggested that suboptimal production of premenstrual ALLO may lead to increased arousal and increased stress reactivity to psychosocial or environmental triggers.49
The possible role of inflammation in PMDD deserves further investigation. The luteal phase entails an increase in the production of proinflammatory markers.50,51 A 10-fold increase in progesterone is correlated with a 20% to 23% increase in C-reactive protein levels.52,53 Women with inflammatory diseases (eg, gingivitis or irritable bowel syndrome) show worsening of symptoms prior to menstruation.54-56 One study found increased levels of proinflammatory markers in women with PMDD compared with controls.57
Putting together the 5 pieces of the puzzle
Because PMDD is heritable, it must have an underlying neurobiologic pathophysiology. Brain imaging studies show differences in structure and function in women with PMDD across the menstrual cycle. Conversion of progesterone to ALLO and the GABAergic influence of this metabolite is a topic of interest in current research. Similarly, the role of estrogen and its connection to serotonin and other neurotransmitters such as BDNF have been implicated.
The link between a history of stress, trauma, and PMDD raises the question of biologic resiliency and illness in these patients, as it connects to the HPA and HPG axis and production of inflammatory stress hormones and steroid hormones and their metabolites. PMDD can be conceptualized as variable sensitivity to hormonal response to stress,58 thus contextualizing biochemical and psychological resiliency.
Further research is needed to clarify the possibility of a shared pathophysiology between endocrine-related mood disorders such as postpartum depression (PPD) and PMDD because current research is controversial.59,60 In PPD, women who are exposed to high levels of progesterone and estrogen during pregnancy (just like in the mid-luteal phase) have a sudden drop in these hormones postpartum.
The ‘withdrawal theory.’ The affective symptoms of PMDD resolve almost instantaneously after the start of menstruation. Perhaps this type of immediate relief is akin to substance use disorders and symptoms of withdrawal. It could be that reinstatement of a certain amount of gonadal steroids in the follicular phase of the cycle diminishes a withdrawal-like response to these steroids.
Currently, the main leading theory is that PMDD is a result of “an abnormal response to normal hormonal changes.”61 A new study also has shown that the change in estradiol/progesterone levels (vs the steady state) was associated with PMDD symptoms.62 Thinking of PMDD as a disorder of withdrawal offers an alternative (yet complementary) perspective to the current theory: PMDD may be caused by the absence or diminishing of the above-named hormones and their metabolites in the late luteal phase (in the context of developed “tolerance” during the early- to mid-luteal phase).
Considering the interplay between neurotransmitters and neurosteroids, both a “serotonin withdrawal theory” (caused by a drop in steroid hormones) and a “GABAergic withdrawal theory” (due to the decline in progesterone) could be proposed. This theory would be supported by the fact that SSRIs seem to mitigate symptoms of PMDD as well as the genetic association between PMDD and ESR1. It is more than likely that the “withdrawal” is caused by the interactions between estrogen-serotonin, progesterone-ALLO, and GABA receptors, and the complementary fashion in which progesterone and estrogen influence each other.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
1. Miller A, Vo H, Huo L, et al. Estrogen receptor alpha (ESR-1) associations with psychological traits in women with PMDD and controls. J Psychiatr Res. 2010;44(12):788-794.
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Wilson CA, Turner CW, Keye WR Jr. Firstborn adolescent daughters and mothers with and without premenstrual syndrome: a comparison. J Adolesc Health. 1991;12(2):130-137.
5. Kendler KS, Silberg JL, Neale MC, et al. Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med. 1992;22(1):85-100.
6. Condon JT. The premenstrual syndrome: a twin study. Br J Psychiatry. 1993;162:481-486.
7. Kendler KS, Karkowski LM, Corey LA, et al. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155(9):1234-1240.
8. Huo L, Straub RE, Roca C, et al. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62(8):925-933.
9. Dhingra V, Magnay JL, O’Brien PM, et al. Serotonin receptor 1A C(-1019)G polymorphism associated with premenstrual dysphoric disorder. Obstet Gynecol. 2007;110(4):788-792.
10. Comasco E, Hahn A, Ganger S, et al. Emotional fronto-cingulate cortex activation and brain derived neurotrophic factor polymorphism in premenstrual dysphoric disorder. Hum Brain Mapp. 2014;35(9):4450-4458.
11. Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol. 2002;12(2):129-134.
12. Klatzkin RR, Morrow AL, Light KC, et al. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006;31(10):1208-1219.
13. Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl). 2014;231(17):3619-3634.
14. Girdler SS, Straneva PA, Light KC, et al. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49(9):788-797.
15. Rapkin AJ, Morgan M, Goldman L, et al. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90(5):709-714.
16. Bicíková M, Dibbelt L, Hill M, et al. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30(4):227-230.
17. Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142(3):269-273.
18. Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995;332(23):1529-1534.
19. Sundström I, Bäckström T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23(1):73-88.
20. Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci U S A. 1999;96(23):13512-13517.
21. Trauger JW, Jiang A, Stearns BA, et al. Kinetics of allopregnanolone formation catalyzed by human 3 alpha-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry. 2002;41(45):13451-13459.
22. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865.
23. Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839-850.
24. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4(1):43-58.
25. Cyr M, Bossé R, Di Paolo T. Gonadal hormones modulate 5-hydroxytryptamine2A receptors: emphasis on the rat frontal cortex. Neuroscience. 1998;83(3):829-836.
26. Fink G, Sumner BE, Rosie R, et al. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16(3):325-344.
27. Sumner BE, Grant KE, Rosie R, et al. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res Mol Brain Res. 1999;73(1-2):119-128.
28. Moses-Kolko EL, Berga SL, Greer PJ, et al. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80(3):554-559.
29. Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology. 1997;16(5):346-356.
30. Steinberg EM, Cardoso GM, Martinez PE, et al. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 2012;29(6):531-540.
31. Roca CA, Schmidt PJ, Smith MJ, et al. Effects of metergoline on symptoms in women with premenstrual dysphoric disorder. Am J Psychiatry. 2002;159(11):1876-1881.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214-227.
33. Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295-303.
34. Pilar-Cuéllar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, β-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165(4b):1046-1057.
35. Deuschle M, Gilles M, Scharnholz B, et al. Changes of serum concentrations of brain-derived neurotrophic factor (BDNF) during treatment with venlafaxine and mirtazapine: role of medication and response to treatment. Pharmacopsychiatry. 2013;46(2):54-58.
36. Berman SM, London ED, Morgan M, et al. Elevated gray matter volume of the emotional cerebellum in women with premenstrual dysphoric disorder. J Affect Disord. 2013;146(2):266-271.
37. Jeong HG, Ham BJ, Yeo HB, et al. Gray matter abnormalities in patients with premenstrual dysphoric disorder: an optimized voxel-based morphometry. J Affect Disord. 2012;140(3):260-267.
38. Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108(1-2):87-94.
39. Gingnell M, Morell A, Bannbers E, et al. Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm Behav. 2012;62(4):400-406.
40. Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851-858.
41. Gingnell M, Bannbers E, Wikström J, et al. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. 2013;23(11):1474-1483.
42. Baller EB, Wei SM, Kohn PD, et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am J Psychiatry. 2013;170(3):305-314.
43. Rasgon N, McGuire M, Tanavoli S, et al. Neuroendocrine response to an intravenous L-tryptophan challenge in women with premenstrual syndrome. Fertil Steril. 2000;73(1):144-149.
44. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
45. Segebladh B, Bannbers E, Moby L, et al. Allopregnanolone serum concentrations and diurnal cortisol secretion in women with premenstrual dysphoric disorder. Arch Womens Ment Health. 2013;16(2):131-137.
46. Pilver CE, Levy BR, Libby DJ, et al. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch Womens Ment Health. 2011;14(5):383-393.
47. Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J Womens Health (Larchmt). 2014;23(9):729-739.
48. Segebladh B, Bannbers E, Kask K, et al. Prevalence of violence exposure in women with premenstrual dysphoric disorder in comparison with other gynecological patients and asymptomatic controls. Acta Obstet Gynecol Scand. 2011;90(7):746-752.
49. Kask K, Gulinello M, Bäckström T, et al. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33(9):2283-2290.
50. O’Brien SM, Fitzgerald P, Scully P, et al. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14(2):84-90.
51. Northoff H, Symons S, Zieker D, et al. Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev. 2008;14:86-103.
52. Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am J Epidemiol. 2012;175(5):423-431.
53. Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136(2):138-146.
54. Jane ZY, Chang CC, Lin HK, et al. The association between the exacerbation of irritable bowel syndrome and menstrual symptoms in young Taiwanese women. Gastroenterol Nurs. 2011;34(4):277-286.
55. Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93(10):1867-1872.
56. Shourie V, Dwarakanath CD, Prashanth GV, et al. The effect of menstrual cycle on periodontal health - a clinical and microbiological study. Oral Health Prev Dent. 2012;10(2):185-192.
57. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
58. Maeng LY, Milad MR. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 2015;76:106-117.
59. Lee YJ, Yi SW, Ju DH, et al. Correlation between postpartum depression and premenstrual dysphoric disorder: single center study. Obstet Gynecol Sci. 2015;58(5):353-358.
60. Kepple AL, Lee EE, Haq N, et al. History of postpartum depression in a clinic-based sample of women with premenstrual dysphoric disorder. J Clin Psychiatry. 2016;77(4):e415-e420.
61. Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209-216.
62. Schmidt PJ, Martinez PE, Nieman LK, et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am J Psychiatry. [published online April 21, 2017]. doi: 10.1176/appi.ajp.2017.16101113.
How to preserve your own well-being in a challenging medical environment
Like all physicians, psychiatrists practice in an increasingly complex health care environment, with escalating demands for productivity, rising threats of malpractice, expanding clinical oversight, and growing concerns about income. Additionally, psychiatric practice presents its own challenges, including limited resources and concerns about patient violence and suicide. These concerns can make it difficult to establish a healthy work–life balance.
Physicians, including psychiatrists, are at risk for alcohol or substance abuse/dependency, burnout, and suicide. As psychiatrists, we need to attend to our own personal and professional health so that we can best help our patients. This review focuses on the challenges psychiatrists face that can adversely affect their well-being and offers strategies to reduce the risk of burnout and enhance wellness.
The challenges of medicine and their impact on psychiatrists
The practice of medicine is inherently challenging. It requires hard work, discipline, dedication, and faithfulness to high ethical standards. Additional challenges include declining autonomy and opportunities for social support, increasing accountability, and a growing interest in reducing the cost of care by employing more non-physician health professionals—which in psychiatry typically include psychologists, nurse practitioners, and social workers. The uncertainty of the Affordable Care Act, declining income, and concerns about the nature of future medical practice are also stressors.1,2
Factors that contribute to psychiatrists’ stress include:
- limited resources
- concerns about patient violence and suicide
- crowded inpatient units
- changing culture in mental health services
- high work demands
- poorly defined roles of consultants
- declining authority
- frustration with the inability to impact systemic change
- conflict between responsibility toward employers vs the patient
- isolation.3
Concern about patient suicide is a significant stressor.4,5 Some evidence suggests that the impact of a patient’s suicide on a physician is more severe when it occurs during training than after graduation and is inversely correlated with the clinician’s perceived social integration into their professional network.5
Impediments to a physician’s well-being
Alcohol abuse/dependence. Approximately 13% of male physicians and 21% of female physicians meet Alcohol Use Disorders Identification Test Version C criteria for alcohol abuse or dependence, according to a study of approximately 7,300 U.S. physicians from all specialties.6 (In this study, prescription drug abuse and use of illicit drugs were rare.) Age, hours worked, male sex, being married or partnered, having children, and being in a specialty other than internal medicine were independently associated with alcohol abuse or dependence.
Fortunately, psychiatrists were among the specialties with below average likelihood to meet diagnostic criteria for alcohol abuse/dependency.6 However, alcohol abuse or dependency was associated with burnout, depression, suicidal ideation, lower quality of life, lower career satisfaction, and medical errors.
Burnout is a long-term stress reaction consisting of:
- physical and emotional exhaustion (feeling depleted)
- depersonalization (cynicism, lack of engagement with or negative attitudes toward patients)
- reduced sense of personal accomplishment (lack of a sense of purpose).7
In a 2017 survey of >14,000 U.S. physicians from 27 specialties, 42% of psychiatrists reported burnout.8 In another survey of approximately 300 resident physicians across all specialties in a tertiary academic hospital, 69% met criteria for burnout.9 This condition affects resident physicians as well as those in practice. Residents and program directors cited a lack of work–life balance and feeling unappreciated as factors contributing to burnout.
Among physicians, factors that contribute to burnout include loss of autonomy, diminished status as physicians, and increased work pressures. Burnout has a negative impact on both patients and health care systems. It is associated with an increased risk of depression and can contribute to:
- broken relationships
- alcohol abuse
- physician suicide
- decreased quality of care, including patient safety and satisfaction
- increased risk of malpractice suits
- reduced patient adherence to medical recommendations.5,10-12
Physicians who embrace medicine as a calling (ie, committing one’s life to personally meaningful work that serves a prosocial purpose) experience less burnout. According to a survey of approximately 900 primary care physicians and 300 psychiatrists, 42% of psychiatrists strongly agreed that medicine is a calling.13 Overall, physicians with a high sense of calling reported less burnout than those with a lower sense of calling (17% vs 31%, respectively).13
Depression and suicide. Gold et al12 analyzed a database that included information on approximately 31,600 adult suicide victims, and 203 of these victims were physicians. Compared with others, physicians were more likely to have a diagnosed mental illness or an occupation-related problem that contributed to suicide. Toxicology results also showed that physician suicide victims were significantly more likely than non-physician victims to test positive for benzodiazepines and barbiturates, but not antidepressants, which suggests that physicians with depression may not have been receiving adequate treatment.12
Although occupation-related stress and inadequate mental health treatment may be modifiable risk factors to reduce suicide deaths among physicians, stigma and fear of medical staff and licensure issues may deter physicians from seeking treatment.14
Steps to avoid burnout
Evidence-based interventions. There is limited evidence-based data regarding specific interventions for preventing burnout and reducing stress among physicians, particularly among psychiatrists.4
A randomized controlled trial of 74 practicing physicians at the Mayo Clinic in Rochester, Minnesota, evaluated the effectiveness of 19 biweekly physician-facilitated discussion groups.15 The groups covered topics such as elements of mindfulness, reflection, shared experience, and small-group learning. The institution provided 1 hour of paid time every other week for physicians to participate in this program. Physicians in the control group could schedule and use this time as they chose. Researchers also collected data on 350 non-trial participants.
The proportion of participants who strongly agreed that their work was meaningful increased 6.3% in the intervention group but decreased 6.3% in the control group and 13.4% among non-trial participants (P = .04).15 Rates of depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention group, decreased slightly in the control group, and increased in the non-trial cohort. Results were sustained at 12 months after the study. There were no statistically significant differences in stress, symptoms of depression, overall quality of life, or job satisfaction.15
Preliminary evidence suggests that residents and fellows would find a wellness or suicide prevention program helpful. One study found that the use of one such program, which provided individual counseling, psychiatric evaluation, and wellness workshops for residents, fellows, and faculty in an academic health center, increased from 5% to 25% of eligible participants, and participants reported high levels of satisfaction with the program.16 Such programs would require institutional support for space and clinical staff.15
Empathy. As psychiatrists, we are taught to be empathetic. Yet, with the numerous challenges we face, it is not always easy. Stressors such as an increased workload or burnout can adversely affect a psychiatrist’s ability to provide empathetic care.17 However, empathetic treatment has clear benefits for both physicians and patients. Empathic skills can lead to more professional satisfaction and outcomes, which are important components of accountability, and can:
- promote patient satisfaction
- establish trust
- reduce anxiety
- increase adherence to treatment regimens
- improve health outcomes
- decrease the likelihood of malpractice suits.17
Mindfulness is a “flexible state of mind in which we are actively engaged in the present, noticing new things and sensitive to context.”18,19 It may sound mundane to cling to phrases such as “living in the present,” but mindfulness can be a valuable tool for psychiatrists who struggle to maintain well-being in medicine’s challenging milieu. The process of mindfulness—actively drawing distinctions and noticing new things, “seeing the familiar in the novel and the novel in the familiar”—can ensure that we have active minds, that we are involved, and that we are capturing the joy of living in the stimulating present.18
Focus on issues you can control
Many of the factors that negatively influence professional satisfaction and well-being, such as loss of autonomy, demand for increased patient care volume, and increasing scrutiny on the quality of care, are beyond a psychiatrist’s control. Medical administrators can help reduce some of these issues by increasing physician autonomy, offering physicians the opportunity to work part-time, offering medical staff workshops to enhance positive communication, or addressing leadership problems. However, psychiatrists may benefit most by identifying modifiable issues under their own control, such as prioritizing a work–life balance, applying the fundamentals of a health prevention strategy to their own lives (Box20,21), approaching medicine as a calling, embracing an empathetic approach to patient care, and bringing mindfulness to medical practice.
1. Goitein L. Physician well-being: addressing downstream effects, but looking upstream. JAMA Intern Med. 2014;174(4):533-534.
2. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
3. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
4. Fothergill A, Edwards D, Burnard P. Stress, burnout, coping and stress management in psychiatrists: findings from a systematic review. Int J Soc Psychiatry. 2004;50(1):54-65.
5. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
6. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38.
7. Maslach C, Jackson SE. The measurement of experienced burnout. J Occup Behav. 1981;2:99-113.
8. Peckham C. Medscape Psychiatrist Lifestyle Report 2017: race and ethnicity, bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1. Published January 11, 2017. Accessed July 25, 2017.
9. Holmes EG, Connolly A, Putnam KT, et al. Taking care of our own: a multispecialty study of resident and program director perspectives on contributors to burnout and potential interventions. Acad Psychiatry. 2017;41(2):159-166.
10. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
11. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
12. Gold MS, Frost-Pineda K, Melker RJ. Physician suicide and drug abuse. Am J Psychiatry. 2005;162:1390; author reply 1390.
13. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41(2):167-173.
14. Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
15. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
16. Ey S, Moffit M, Kinzie JM, et al. Feasibility of a comprehensive wellness and suicide prevention program: a decade of caring for physicians in training and practice. J Grad Med Educ. 2016;8(5):747-753.
17. Newton BW. Walking a fine line: is it possible to remain an empathic physician and have a hardened heart? Front Hum Neurosci. 2013;7:233.
18. Langer EJ. Mindful learning: current directions in psychological science. Am Psychological Society. 2000(6);9:220-223.
19. Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18(2):165-171.
20. U.S. Department of Health & Human Services, Office of the Surgeon General. National Prevention Strategy. https://www.surgeongeneral.gov/priorities/prevention/strategy/report.pdf. Published June 2011. Accessed July 26, 2017.
21. Benjamin RM. The national prevention strategy: shifting the nation’s health-care system. Public Health Rep. 2011;126(6):774-776.
Like all physicians, psychiatrists practice in an increasingly complex health care environment, with escalating demands for productivity, rising threats of malpractice, expanding clinical oversight, and growing concerns about income. Additionally, psychiatric practice presents its own challenges, including limited resources and concerns about patient violence and suicide. These concerns can make it difficult to establish a healthy work–life balance.
Physicians, including psychiatrists, are at risk for alcohol or substance abuse/dependency, burnout, and suicide. As psychiatrists, we need to attend to our own personal and professional health so that we can best help our patients. This review focuses on the challenges psychiatrists face that can adversely affect their well-being and offers strategies to reduce the risk of burnout and enhance wellness.
The challenges of medicine and their impact on psychiatrists
The practice of medicine is inherently challenging. It requires hard work, discipline, dedication, and faithfulness to high ethical standards. Additional challenges include declining autonomy and opportunities for social support, increasing accountability, and a growing interest in reducing the cost of care by employing more non-physician health professionals—which in psychiatry typically include psychologists, nurse practitioners, and social workers. The uncertainty of the Affordable Care Act, declining income, and concerns about the nature of future medical practice are also stressors.1,2
Factors that contribute to psychiatrists’ stress include:
- limited resources
- concerns about patient violence and suicide
- crowded inpatient units
- changing culture in mental health services
- high work demands
- poorly defined roles of consultants
- declining authority
- frustration with the inability to impact systemic change
- conflict between responsibility toward employers vs the patient
- isolation.3
Concern about patient suicide is a significant stressor.4,5 Some evidence suggests that the impact of a patient’s suicide on a physician is more severe when it occurs during training than after graduation and is inversely correlated with the clinician’s perceived social integration into their professional network.5
Impediments to a physician’s well-being
Alcohol abuse/dependence. Approximately 13% of male physicians and 21% of female physicians meet Alcohol Use Disorders Identification Test Version C criteria for alcohol abuse or dependence, according to a study of approximately 7,300 U.S. physicians from all specialties.6 (In this study, prescription drug abuse and use of illicit drugs were rare.) Age, hours worked, male sex, being married or partnered, having children, and being in a specialty other than internal medicine were independently associated with alcohol abuse or dependence.
Fortunately, psychiatrists were among the specialties with below average likelihood to meet diagnostic criteria for alcohol abuse/dependency.6 However, alcohol abuse or dependency was associated with burnout, depression, suicidal ideation, lower quality of life, lower career satisfaction, and medical errors.
Burnout is a long-term stress reaction consisting of:
- physical and emotional exhaustion (feeling depleted)
- depersonalization (cynicism, lack of engagement with or negative attitudes toward patients)
- reduced sense of personal accomplishment (lack of a sense of purpose).7
In a 2017 survey of >14,000 U.S. physicians from 27 specialties, 42% of psychiatrists reported burnout.8 In another survey of approximately 300 resident physicians across all specialties in a tertiary academic hospital, 69% met criteria for burnout.9 This condition affects resident physicians as well as those in practice. Residents and program directors cited a lack of work–life balance and feeling unappreciated as factors contributing to burnout.
Among physicians, factors that contribute to burnout include loss of autonomy, diminished status as physicians, and increased work pressures. Burnout has a negative impact on both patients and health care systems. It is associated with an increased risk of depression and can contribute to:
- broken relationships
- alcohol abuse
- physician suicide
- decreased quality of care, including patient safety and satisfaction
- increased risk of malpractice suits
- reduced patient adherence to medical recommendations.5,10-12
Physicians who embrace medicine as a calling (ie, committing one’s life to personally meaningful work that serves a prosocial purpose) experience less burnout. According to a survey of approximately 900 primary care physicians and 300 psychiatrists, 42% of psychiatrists strongly agreed that medicine is a calling.13 Overall, physicians with a high sense of calling reported less burnout than those with a lower sense of calling (17% vs 31%, respectively).13
Depression and suicide. Gold et al12 analyzed a database that included information on approximately 31,600 adult suicide victims, and 203 of these victims were physicians. Compared with others, physicians were more likely to have a diagnosed mental illness or an occupation-related problem that contributed to suicide. Toxicology results also showed that physician suicide victims were significantly more likely than non-physician victims to test positive for benzodiazepines and barbiturates, but not antidepressants, which suggests that physicians with depression may not have been receiving adequate treatment.12
Although occupation-related stress and inadequate mental health treatment may be modifiable risk factors to reduce suicide deaths among physicians, stigma and fear of medical staff and licensure issues may deter physicians from seeking treatment.14
Steps to avoid burnout
Evidence-based interventions. There is limited evidence-based data regarding specific interventions for preventing burnout and reducing stress among physicians, particularly among psychiatrists.4
A randomized controlled trial of 74 practicing physicians at the Mayo Clinic in Rochester, Minnesota, evaluated the effectiveness of 19 biweekly physician-facilitated discussion groups.15 The groups covered topics such as elements of mindfulness, reflection, shared experience, and small-group learning. The institution provided 1 hour of paid time every other week for physicians to participate in this program. Physicians in the control group could schedule and use this time as they chose. Researchers also collected data on 350 non-trial participants.
The proportion of participants who strongly agreed that their work was meaningful increased 6.3% in the intervention group but decreased 6.3% in the control group and 13.4% among non-trial participants (P = .04).15 Rates of depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention group, decreased slightly in the control group, and increased in the non-trial cohort. Results were sustained at 12 months after the study. There were no statistically significant differences in stress, symptoms of depression, overall quality of life, or job satisfaction.15
Preliminary evidence suggests that residents and fellows would find a wellness or suicide prevention program helpful. One study found that the use of one such program, which provided individual counseling, psychiatric evaluation, and wellness workshops for residents, fellows, and faculty in an academic health center, increased from 5% to 25% of eligible participants, and participants reported high levels of satisfaction with the program.16 Such programs would require institutional support for space and clinical staff.15
Empathy. As psychiatrists, we are taught to be empathetic. Yet, with the numerous challenges we face, it is not always easy. Stressors such as an increased workload or burnout can adversely affect a psychiatrist’s ability to provide empathetic care.17 However, empathetic treatment has clear benefits for both physicians and patients. Empathic skills can lead to more professional satisfaction and outcomes, which are important components of accountability, and can:
- promote patient satisfaction
- establish trust
- reduce anxiety
- increase adherence to treatment regimens
- improve health outcomes
- decrease the likelihood of malpractice suits.17
Mindfulness is a “flexible state of mind in which we are actively engaged in the present, noticing new things and sensitive to context.”18,19 It may sound mundane to cling to phrases such as “living in the present,” but mindfulness can be a valuable tool for psychiatrists who struggle to maintain well-being in medicine’s challenging milieu. The process of mindfulness—actively drawing distinctions and noticing new things, “seeing the familiar in the novel and the novel in the familiar”—can ensure that we have active minds, that we are involved, and that we are capturing the joy of living in the stimulating present.18
Focus on issues you can control
Many of the factors that negatively influence professional satisfaction and well-being, such as loss of autonomy, demand for increased patient care volume, and increasing scrutiny on the quality of care, are beyond a psychiatrist’s control. Medical administrators can help reduce some of these issues by increasing physician autonomy, offering physicians the opportunity to work part-time, offering medical staff workshops to enhance positive communication, or addressing leadership problems. However, psychiatrists may benefit most by identifying modifiable issues under their own control, such as prioritizing a work–life balance, applying the fundamentals of a health prevention strategy to their own lives (Box20,21), approaching medicine as a calling, embracing an empathetic approach to patient care, and bringing mindfulness to medical practice.
Like all physicians, psychiatrists practice in an increasingly complex health care environment, with escalating demands for productivity, rising threats of malpractice, expanding clinical oversight, and growing concerns about income. Additionally, psychiatric practice presents its own challenges, including limited resources and concerns about patient violence and suicide. These concerns can make it difficult to establish a healthy work–life balance.
Physicians, including psychiatrists, are at risk for alcohol or substance abuse/dependency, burnout, and suicide. As psychiatrists, we need to attend to our own personal and professional health so that we can best help our patients. This review focuses on the challenges psychiatrists face that can adversely affect their well-being and offers strategies to reduce the risk of burnout and enhance wellness.
The challenges of medicine and their impact on psychiatrists
The practice of medicine is inherently challenging. It requires hard work, discipline, dedication, and faithfulness to high ethical standards. Additional challenges include declining autonomy and opportunities for social support, increasing accountability, and a growing interest in reducing the cost of care by employing more non-physician health professionals—which in psychiatry typically include psychologists, nurse practitioners, and social workers. The uncertainty of the Affordable Care Act, declining income, and concerns about the nature of future medical practice are also stressors.1,2
Factors that contribute to psychiatrists’ stress include:
- limited resources
- concerns about patient violence and suicide
- crowded inpatient units
- changing culture in mental health services
- high work demands
- poorly defined roles of consultants
- declining authority
- frustration with the inability to impact systemic change
- conflict between responsibility toward employers vs the patient
- isolation.3
Concern about patient suicide is a significant stressor.4,5 Some evidence suggests that the impact of a patient’s suicide on a physician is more severe when it occurs during training than after graduation and is inversely correlated with the clinician’s perceived social integration into their professional network.5
Impediments to a physician’s well-being
Alcohol abuse/dependence. Approximately 13% of male physicians and 21% of female physicians meet Alcohol Use Disorders Identification Test Version C criteria for alcohol abuse or dependence, according to a study of approximately 7,300 U.S. physicians from all specialties.6 (In this study, prescription drug abuse and use of illicit drugs were rare.) Age, hours worked, male sex, being married or partnered, having children, and being in a specialty other than internal medicine were independently associated with alcohol abuse or dependence.
Fortunately, psychiatrists were among the specialties with below average likelihood to meet diagnostic criteria for alcohol abuse/dependency.6 However, alcohol abuse or dependency was associated with burnout, depression, suicidal ideation, lower quality of life, lower career satisfaction, and medical errors.
Burnout is a long-term stress reaction consisting of:
- physical and emotional exhaustion (feeling depleted)
- depersonalization (cynicism, lack of engagement with or negative attitudes toward patients)
- reduced sense of personal accomplishment (lack of a sense of purpose).7
In a 2017 survey of >14,000 U.S. physicians from 27 specialties, 42% of psychiatrists reported burnout.8 In another survey of approximately 300 resident physicians across all specialties in a tertiary academic hospital, 69% met criteria for burnout.9 This condition affects resident physicians as well as those in practice. Residents and program directors cited a lack of work–life balance and feeling unappreciated as factors contributing to burnout.
Among physicians, factors that contribute to burnout include loss of autonomy, diminished status as physicians, and increased work pressures. Burnout has a negative impact on both patients and health care systems. It is associated with an increased risk of depression and can contribute to:
- broken relationships
- alcohol abuse
- physician suicide
- decreased quality of care, including patient safety and satisfaction
- increased risk of malpractice suits
- reduced patient adherence to medical recommendations.5,10-12
Physicians who embrace medicine as a calling (ie, committing one’s life to personally meaningful work that serves a prosocial purpose) experience less burnout. According to a survey of approximately 900 primary care physicians and 300 psychiatrists, 42% of psychiatrists strongly agreed that medicine is a calling.13 Overall, physicians with a high sense of calling reported less burnout than those with a lower sense of calling (17% vs 31%, respectively).13
Depression and suicide. Gold et al12 analyzed a database that included information on approximately 31,600 adult suicide victims, and 203 of these victims were physicians. Compared with others, physicians were more likely to have a diagnosed mental illness or an occupation-related problem that contributed to suicide. Toxicology results also showed that physician suicide victims were significantly more likely than non-physician victims to test positive for benzodiazepines and barbiturates, but not antidepressants, which suggests that physicians with depression may not have been receiving adequate treatment.12
Although occupation-related stress and inadequate mental health treatment may be modifiable risk factors to reduce suicide deaths among physicians, stigma and fear of medical staff and licensure issues may deter physicians from seeking treatment.14
Steps to avoid burnout
Evidence-based interventions. There is limited evidence-based data regarding specific interventions for preventing burnout and reducing stress among physicians, particularly among psychiatrists.4
A randomized controlled trial of 74 practicing physicians at the Mayo Clinic in Rochester, Minnesota, evaluated the effectiveness of 19 biweekly physician-facilitated discussion groups.15 The groups covered topics such as elements of mindfulness, reflection, shared experience, and small-group learning. The institution provided 1 hour of paid time every other week for physicians to participate in this program. Physicians in the control group could schedule and use this time as they chose. Researchers also collected data on 350 non-trial participants.
The proportion of participants who strongly agreed that their work was meaningful increased 6.3% in the intervention group but decreased 6.3% in the control group and 13.4% among non-trial participants (P = .04).15 Rates of depersonalization, emotional exhaustion, and overall burnout decreased substantially in the intervention group, decreased slightly in the control group, and increased in the non-trial cohort. Results were sustained at 12 months after the study. There were no statistically significant differences in stress, symptoms of depression, overall quality of life, or job satisfaction.15
Preliminary evidence suggests that residents and fellows would find a wellness or suicide prevention program helpful. One study found that the use of one such program, which provided individual counseling, psychiatric evaluation, and wellness workshops for residents, fellows, and faculty in an academic health center, increased from 5% to 25% of eligible participants, and participants reported high levels of satisfaction with the program.16 Such programs would require institutional support for space and clinical staff.15
Empathy. As psychiatrists, we are taught to be empathetic. Yet, with the numerous challenges we face, it is not always easy. Stressors such as an increased workload or burnout can adversely affect a psychiatrist’s ability to provide empathetic care.17 However, empathetic treatment has clear benefits for both physicians and patients. Empathic skills can lead to more professional satisfaction and outcomes, which are important components of accountability, and can:
- promote patient satisfaction
- establish trust
- reduce anxiety
- increase adherence to treatment regimens
- improve health outcomes
- decrease the likelihood of malpractice suits.17
Mindfulness is a “flexible state of mind in which we are actively engaged in the present, noticing new things and sensitive to context.”18,19 It may sound mundane to cling to phrases such as “living in the present,” but mindfulness can be a valuable tool for psychiatrists who struggle to maintain well-being in medicine’s challenging milieu. The process of mindfulness—actively drawing distinctions and noticing new things, “seeing the familiar in the novel and the novel in the familiar”—can ensure that we have active minds, that we are involved, and that we are capturing the joy of living in the stimulating present.18
Focus on issues you can control
Many of the factors that negatively influence professional satisfaction and well-being, such as loss of autonomy, demand for increased patient care volume, and increasing scrutiny on the quality of care, are beyond a psychiatrist’s control. Medical administrators can help reduce some of these issues by increasing physician autonomy, offering physicians the opportunity to work part-time, offering medical staff workshops to enhance positive communication, or addressing leadership problems. However, psychiatrists may benefit most by identifying modifiable issues under their own control, such as prioritizing a work–life balance, applying the fundamentals of a health prevention strategy to their own lives (Box20,21), approaching medicine as a calling, embracing an empathetic approach to patient care, and bringing mindfulness to medical practice.
1. Goitein L. Physician well-being: addressing downstream effects, but looking upstream. JAMA Intern Med. 2014;174(4):533-534.
2. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
3. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
4. Fothergill A, Edwards D, Burnard P. Stress, burnout, coping and stress management in psychiatrists: findings from a systematic review. Int J Soc Psychiatry. 2004;50(1):54-65.
5. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
6. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38.
7. Maslach C, Jackson SE. The measurement of experienced burnout. J Occup Behav. 1981;2:99-113.
8. Peckham C. Medscape Psychiatrist Lifestyle Report 2017: race and ethnicity, bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1. Published January 11, 2017. Accessed July 25, 2017.
9. Holmes EG, Connolly A, Putnam KT, et al. Taking care of our own: a multispecialty study of resident and program director perspectives on contributors to burnout and potential interventions. Acad Psychiatry. 2017;41(2):159-166.
10. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
11. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
12. Gold MS, Frost-Pineda K, Melker RJ. Physician suicide and drug abuse. Am J Psychiatry. 2005;162:1390; author reply 1390.
13. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41(2):167-173.
14. Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
15. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
16. Ey S, Moffit M, Kinzie JM, et al. Feasibility of a comprehensive wellness and suicide prevention program: a decade of caring for physicians in training and practice. J Grad Med Educ. 2016;8(5):747-753.
17. Newton BW. Walking a fine line: is it possible to remain an empathic physician and have a hardened heart? Front Hum Neurosci. 2013;7:233.
18. Langer EJ. Mindful learning: current directions in psychological science. Am Psychological Society. 2000(6);9:220-223.
19. Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18(2):165-171.
20. U.S. Department of Health & Human Services, Office of the Surgeon General. National Prevention Strategy. https://www.surgeongeneral.gov/priorities/prevention/strategy/report.pdf. Published June 2011. Accessed July 26, 2017.
21. Benjamin RM. The national prevention strategy: shifting the nation’s health-care system. Public Health Rep. 2011;126(6):774-776.
1. Goitein L. Physician well-being: addressing downstream effects, but looking upstream. JAMA Intern Med. 2014;174(4):533-534.
2. Dunn PM, Arnetz BB, Christensen JF, et al. Meeting the imperative to improve physician well-being: assessment of an innovative program. J Gen Intern Med. 2007;22(11):1544-1552.
3. Kumar S. Burnout in psychiatrists. World Psychiatry. 2007;6(3):186-189.
4. Fothergill A, Edwards D, Burnard P. Stress, burnout, coping and stress management in psychiatrists: findings from a systematic review. Int J Soc Psychiatry. 2004;50(1):54-65.
5. Ruskin R, Sakinofsky I, Bagby RM, et al. Impact of patient suicide on psychiatrists and psychiatric trainees. Acad Psychiatry. 2004;28(2):104-110.
6. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38.
7. Maslach C, Jackson SE. The measurement of experienced burnout. J Occup Behav. 1981;2:99-113.
8. Peckham C. Medscape Psychiatrist Lifestyle Report 2017: race and ethnicity, bias and burnout. http://www.medscape.com/features/slideshow/lifestyle/2017/psychiatry#page=1. Published January 11, 2017. Accessed July 25, 2017.
9. Holmes EG, Connolly A, Putnam KT, et al. Taking care of our own: a multispecialty study of resident and program director perspectives on contributors to burnout and potential interventions. Acad Psychiatry. 2017;41(2):159-166.
10. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-146.
11. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
12. Gold MS, Frost-Pineda K, Melker RJ. Physician suicide and drug abuse. Am J Psychiatry. 2005;162:1390; author reply 1390.
13. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41(2):167-173.
14. Gold KJ, Andrew LB, Goldman EB, et al. “I would never want to have a mental health diagnosis on my record”: a survey of female physicians on mental health diagnosis, treatment, and reporting. Gen Hosp Psychiatry. 2016;43:51-57.
15. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533.
16. Ey S, Moffit M, Kinzie JM, et al. Feasibility of a comprehensive wellness and suicide prevention program: a decade of caring for physicians in training and practice. J Grad Med Educ. 2016;8(5):747-753.
17. Newton BW. Walking a fine line: is it possible to remain an empathic physician and have a hardened heart? Front Hum Neurosci. 2013;7:233.
18. Langer EJ. Mindful learning: current directions in psychological science. Am Psychological Society. 2000(6);9:220-223.
19. Crum AJ, Langer EJ. Mind-set matters: exercise and the placebo effect. Psychol Sci. 2007;18(2):165-171.
20. U.S. Department of Health & Human Services, Office of the Surgeon General. National Prevention Strategy. https://www.surgeongeneral.gov/priorities/prevention/strategy/report.pdf. Published June 2011. Accessed July 26, 2017.
21. Benjamin RM. The national prevention strategy: shifting the nation’s health-care system. Public Health Rep. 2011;126(6):774-776.
Opioid abuse and overdose: Keep your patients safe
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5
Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13
Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28
MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5
Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13
Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28
MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
Opioid abuse and overdose are large and growing problems, and in recent years the numbers have been staggering. Overdose deaths related to opioids increased from 28,647 in 2014 to 33,091 in 2015 (Figure).1 More than 2 million individuals in the United States had opioid use disorder in 2015,2 and approximately 80% of them received no treatment,3 even though effective treatment could reduce the scope of abuse.4,5
Although psychiatrists typically are not the primary prescribers of opioid medications, they often treat psychiatric disorders in patients with chronic pain who take prescription opioids. A recent study found that, despite representing only 16% of the adult population, adults with mental health disorders receive more than one-half of all opioid prescriptions distributed each year in the United States.6 Therefore, psychiatrists must be aware of risk assessment strategies for patients receiving opioids.
In this article, we provide recommendations for managing individuals with opioid use disorder, including:
- how to identify risk factors for opioid use disorder and use screening tools
- how to evaluate a patient with suspected opioid use disorder and make the diagnosis
- how to treat a patient with opioid use disorder, including a review of approved pharmaceutical agents.
Risk factors for opioid abuse and overdose
Patients with a history of mental health and/or substance use disorders or at least 3 months of prescribed opioid treatment are at risk for opioid abuse. Those taking a high daily dose of opioids or who have a history of overdose are at risk for overdose from opioid abuse (Table 1).7-12 Standardized tools, such as the Opioid Risk Tool, can be used to screen to assess risk for opioid abuse among individuals prescribed opioids for treatment of chronic pain.12 However, clinicians must be aware that even patients without characteristic risk factors can become dependent on opioids and/or be at risk for an accidental or intentional overdose. For example, opioid therapy following surgical procedures, even in patients who do not have a history of opioid use, increases risk of developing opioid use disorder.13
Evaluation and diagnosis
DSM-5 criteria define 3 degrees of opioid use disorder, depending on how many of the following traits a patient exhibits (mild, 2 to 3; moderate, 4 to 5; and severe, ≥6 )14:
- taking more than the initially intended quantities of opioids or for a longer period of time than intended
- continuous attempts to reduce or otherwise manage opioid use or desires to do so
- a great deal of time using, recovering from, or acquiring opioids
- reports of strong cravings to use opioids
- failing to meet personal objectives at home, work, or school
- continued opioid use even though it causes recurrent social problems
- reduction or elimination of activities the patient once considered important due to opioid use
- opioid use in situations where it is physically dangerous
- continued opioid use despite persistent psychological or physiologic problems despite knowing that continued use is causing or worsening those problems
- tolerance to opioids (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision)
- withdrawal or use of opioids (or related substances) to prevent withdrawal (not consequential for the diagnosis if the patient is taking opioids under appropriate medical supervision).
Clinicians should be vigilant for symptoms of opioid use or withdrawal, such as needle marks and weight loss, during the interview (Table 2). High-risk populations that require regular screening include individuals with a history of opioid use disorder, patients taking chronic pain medication, and psychiatric patients.15 During the interview, clinicians should take an nonjudgmental approach and avoid “shame and blame.”
Patients often will withhold information about drug use for various reasons.16 Therefore, collateral information from the patient’s family, close friends, or a referral source is important.
Standardized scales. Various standardized scales can be used to evaluate patients for opioid withdrawal and risk for substance use disorder. Scales for assessing opioid withdrawal include:
- Clinical Opiate Withdrawal Scale
- Subjective Opiate Withdrawal Scale.
Substance use disorder screening tools include:
- Drug Abuse Screen Test-10
- Alcohol Use Disorders Identification Test
- National Institute on Drug Abuse (NIDA) Drug Screening Tool.17
Examination findings. A brief physical examination is necessary to document key findings (Table 2). Patients should undergo a urine drug screen; gas chromatography/mass spectroscopy can confirm positive results. During the examination, clinicians should look for signs and symptoms of co-occurring substance use (eg, benzodiazepines, marijuana, alcohol, cocaine) or mental disorders (mood, anxiety, attention-deficit).18-21 Because nonprescription opioid use is associated with increased risk of suicide attempts and ideation,22 a suicide risk assessment is necessary.
Managing opioid use disorder
Detoxification is a 3-tiered approach that requires judicious prescription of medication, psychosocial support, and supervision to relieve opioid withdrawal symptoms. In both inpatient and outpatient settings, medications used for opioid detoxification include buprenorphine, clonidine, and methadone administered in doses tapered over 5 to 7 days. Appropriate detoxification increases treatment retention for continuing care.23,24
Buprenorphine or buprenorphine/naloxone is the first-line option for outpatient and inpatient detoxification. Short-term detoxification schedules include starting doses between 4 and 16 mg/d, tapered over 5 to 7 days. Compared with methadone, buprenorphine has a lower risk of overdose25 and abuse potential and can be given in an office-based setting. Clonidine, 0.3 to 1.2 mg/d in divided doses, is an alternative to buprenorphine and can be used in inpatient settings.26
Clonidine is not as effective as buprenorphine for detoxification, but it may be used when buprenorphine is contraindicated. Clonidine may require adjuvant symptomatic treatment for insomnia (eg, trazodone, 100 mg at bedtime), anxiety (eg, hydroxyzine, 25 mg, twice a day), or diarrhea (loperamide, 2 mg/d). If a patient needs more structure and monitoring, he (she) should be referred for inpatient detoxification or to a methadone program.27
Medication-assisted therapies
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28
MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
1. Centers for Disease Control and Prevention. Opioid data analysis. http://www.cdc.gov/drugoverdose/data/analysis.html. Updated February 9, 2017. Accessed June 27, 2017.
2. Substance Abuse and Mental Health Services Administration. Results from the 2015 National Survey on Drug Use and Health: detailed tables. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment of opioid use disorder pocket guide. https://store.samhsa.gov/shin/content//SMA16-4892PG/SMA16-4892PG.pdf. Accessed June 29, 2017.
4. Mutlu C, Demirci AC, Yalcin O, et al. One-year follow-up of heroin-dependent adolescents treated with buprenorphine/naloxone for the first time in a substance treatment unit. J Subst Abuse Treat. 2016;67:1-8.
5. Sharma B, Bruner A, Barnett G, et al. Opioid use disorders. Child Adolesc Psychiatr Clin N Am. 2016;25(3):473-487.
6. Davis MA, Lin LA, Liu H, Sites BD. Prescription Opioid Use among Adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:42-47.
7. Icahn School of Medicine at Mount Sinai. Substance use: prescription drugs. http://www.mountsinai.org/patient-care/health-library/diseases-and-conditions/opioid-abuse#risk. Accessed June 27, 2017.
8. Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776-1782.
9. Edlund M, Steffick D, Hudson T, et al. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355-362.
10. Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103-107.
11. Bohnert AS, Valenstein M, Bair M, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432.
13. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
14. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
15. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Internal Med. 2010;152(11):712-720.
16. Substance Abuse and Mental Health Services Administration. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction: a treatment improvement protocol: TIP 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004.
17. NIDA drug screening tool: clinician’s screening tool for drug use in general medical settings. National Institutes of Health. https://www.drugabuse.gov/nmassist. Accessed June 27, 2017.
18. Fareed A, Eilender P, Haber M, et al. Comorbid posttraumatic stress disorder and opiate addiction: a literature review. J Addict Dis. 2013;32(2):168-179.
19. Rosen D, Smith ML, Reynolds CF 3rd. The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16(6):488-497.
20. Goldner EM, Lusted A, Roerecke M, et al. Prevalence of Axis-1 psychiatric (with focus on depression and anxiety) disorder and symptomatology among non-medical prescription opioid users in substance use treatment: systematic review and meta-analyses. Addict Behav. 2014;39(3):520-531.
21. Barry DT, Cutter CJ, Beitel M, et al. Psychiatric disorders among patients seeking treatment for co-occurring chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(10):1413-1419.
22. Kuramoto SJ, Chilcoat HD, Ko J, et al. Suicidal ideation and suicide attempt across stages of nonmedical prescription opioid use and presence of prescription opioid disorders among U.S. adults. J Stud Alcohol Drugs. 2012;73(2):178-184.
23. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4.
24. Evans E, Li L, Min J, et al. Mortality among individuals accessing pharmacological treatment for opioid dependence in California, 2006-10. Addiction. 2015;110(6):996-1005.
25. Marteu D, McDonald R, Patel K. The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales. BMJ Open. 2015;5(5):e007629. doi: 10.1136/bmjopen-2015-007629.
26. Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42(11):1063-1066.
27. Whelan PJ, Remski K. Buprenorphine vs methadone treatment: a review of evidence in both developed and developing worlds. J Neurosci Rural Pract. 2012;3(1):45-50.
28. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
29. Dutra L, Stathopoulou G, Basden SL, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179-187.
30. Brown HL, Britton KA, Mahaffey D, et al. Methadone maintenance in pregnancy: a reappraisal. Am J Obstet Gynecol. 1998;179(2):459-463.
31. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) 43.
32. Zimlich R. AAP recommends on medication assisted therapy for adolescent opioid addiction. Contemporary Pediatrics. http://contemporarypediatrics.modernmedicine.com/contemporary-pediatrics/news/aap-recommends-medication-assisted-therapy-adolescent-opioid-addiction. Published September 15, 2016. Accessed June 29, 2017.
33. Patkar A, Lee J, Burgess D. Opioid use disorder. BMJ Publishing Group. http://bestpractice.bmj.com/best-practice/monograph/200.html. Published 2015. Accessed July 6, 2017.
34. Alho H, Sinclair D, Vuori E, et al. Abuse liability of buprenorphine-naloxone tablets in untreated IV drug users. Drug Alcohol Depend. 2007;88(1):75-78.
35. Centers for Disease Control and Prevention (CDC). Vital signs: risk for overdose from methadone used for pain relief - United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61(26):493-497.
36. Soyka M. New developments in the management of opioid dependence: focus on sublingual buprenorphine-naloxone. Subst Abuse Rehabil. 2015;6:1-14.
37. Lee JD, Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders N Engl J Med. 2016;374(13):1232-1242.
38. Vo HT, Robbins E, Westwood M, et al. Relapse prevention medications in community treatment for young adults with opioid addiction. Subst Abus. 2016;37(3):392-397.
39. McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 2017;178:176-187.
40. Centers for Disease Control and Prevention. Overdose prevention. https://www.cdc.gov/drugoverdose/opioids/odprevention.html. Updated February 9, 2017. Accessed July 6, 2017.
A shot in the arm: Boost your knowledge about immunizations for psychiatric patients
Patients with chronic, severe mental illness live much shorter lives than the general population. The 25-year loss in life expectancy for people with chronic mental illness has been attributed to higher rates of cardiovascular disease driven by increased smoking, obesity, poverty, and poor nutrition.1 These individuals also face the added burden of struggling with a psychiatric condition that often interferes with their ability to make optimal preventative health decisions, including staying up to date on vaccinations.2 A recent review from Toronto, Canada, found that the influenza vaccination rates among homeless adults with mental illness—a population at high risk of respiratory illness—was only 6.7% compared with 31.1% for the general population of Ontario.3
Mental health professionals may serve as the only contacts to offer medical care to this vulnerable population, leading some psychiatric leaders to advocate that psychiatrists be considered primary care providers within accountable care organizations. Because most vaccines are easily available, mental health professionals should know about key immunizations to guide their patients accordingly.
In the United States, approximately 45,000 adults die annually from vaccine-preventable diseases, the majority from influenza.4 When combined with the most recent Adult Immunization Schedule and general recommendations adapted from the CDC,5,6 the mnemonic ARM SHOT allows for a quick assessment of risk factors to guide administration and education about most vaccinations (Table 1). ARM SHOT involves assessing the following components of an individual’s health status and living arrangements to determine one’s risk of contracting communicable diseases:
- Age
- Risk of exposure
- Medical conditions (comorbidities)
- Substance use history
- HIV status or other immunocompromised states
- Occupancy, or living arrangements
- Tobacco use.
We recommend keeping a copy of the Adult Immunization Schedule (age ≥19) and/or the immunization schedule for children and adolescents (age ≤18) close for quick reference. Here, we provide a case and then explore how each component of the ARM SHOT mnemonic applies in decision-making.
Case Evaluating risk, assess needs
Ms. W, age 24, has bipolar I disorder, most recently manic with psychotic features. She presents for follow-up in clinic after a 5-day hospitalization for mania and comorbid alcohol use disorder. Her medical comorbidities include asthma and active tobacco use. She is taking lurasidone, 20 mg/d, and lithium, 900 mg/d. Her case manager is working to place Ms. W in a residential substance use disorder treatment program. Ms. W is on a waiting list to establish care with a primary care physician and has a history of poor engagement with medical services in general; prior attempts to place her with a primary care physician failed.
In advance of Ms. W’s transfer to a residential treatment facility, you have been asked to place a Mantoux screening test for tuberculosis (purified protein derivative), which raises the important question about her susceptibility to infectious diseases in general. To protect Ms. W from preventable diseases for which vaccines are available, you review the ARM SHOT mnemonic to broadly assess her candidacy for vaccinations.
Age
Age may be the most important determinant of a patient’s need for vaccination (Table 2). The CDC immunization schedules account for age-specific risks for diseases, complications, and responses to vaccination (Figure 1).6
Influenza vaccination. Adults can have an intramuscular or intradermal inactivated influenza vaccination yearly in the fall or winter, unless they have an allergy to a vaccine component such as egg protein. Those with such an allergy can receive a recombinant influenza vaccine. Until the 2016 to 2017 flu season, an intranasal mist of live, attenuated influenza vaccine was available to healthy, non-pregnant women, ages 2 to 49, without high-risk medical conditions. However, the CDC dropped its recommendation for this vaccine because data showed it did not effectively prevent the flu.7 Individuals age ≥65 can receive either the standard- or high-dose inactivated influenza vaccination. The latter contains 4 times the amount of antigen with the intention of triggering a stronger immune response in older adults.
Pneumonia immunization. All patients age ≥65 should receive vaccinations for Streptococcus pneumoniae and its variants in the form of one 13-valent pneumococcal conjugate vaccine and, at least 1 year later, one 23-valent pneumococcal polysaccharide vaccine (PPSV23). Immunization reduces the morbidity and mortality from pneumococcal illness by decreasing the burden of a pneumonia, bacteremia, or meningitis infection. Adults, ages 19 to 64, with a chronic disease (referred to as “special populations” in CDC tables), such as diabetes, heart or lung disease, alcoholism, or cirrhosis, or those who smoke cigarettes, should receive PPSV23 with a second dose administered at least 5 years after the first. The CDC recommends a 1-time re-vaccination at age 65 for patients if >5 years have passed since the last PPSV23 and if the patient was younger than age 65 at the time of primary vaccine for S. pneumoniae. This can be a rather tricky clinical situation; the health care provider should verify a patient’s immunization history to ensure that she (he) is receiving only necessary vaccines. However, when the history cannot be verified, err on the side of inclusion, because risks are minimal.
Shingles vaccination. Adults age ≥60 who are not immunocompromised should receive a single dose of live attenuated vaccine from varicella-zoster virus (VZV) to limit the risk of shingles from a prior chickenpox infection. The vaccine is approximately 66.5% effective at preventing postherpetic neuralgia for up to 4.9 years. Individuals as young as age 50 may have the vaccine because the risk of herpes zoster radically increases from then on,8 although most insurers only cover VZV vaccination after age 60.
Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. All adults should complete the 3-dose primary vaccination series for tetanus, diphtheria, and pertussis (also known as whooping cough) and this should include 1 dose of Tdap. Administration of the primary series is staged so that the second dose is given 4 weeks after the initial dose and the final dose 6 to 12 months after the first dose. After receiving the primary series, adults should receive a tetanus-diphtheria booster dose every 10 years. For adults ages 19 to 64, the Advisory Committee on Immunization Practices (ACIP) recommends 1 dose of Tdap in place of a booster vaccination to decrease the transmission risk of pertussis to vulnerable persons, especially children.
Human papillomavirus (HPV) immunization. The ACIP recommendation9 has been for children to receive routine vaccination for the 4 major strains of HPV—strains 6, 11, 16, and 18—starting at ages 11 to 12 to confer protection from HPV-associated diseases, such as genital warts, oropharyngeal cancer, and anal cancer; cancers of the cervix, vulva, and vagina in women; and penile cancer in men. Ideally, the vaccines are administered prior to HPV exposure from sexual contact. The quadrivalent HPV vaccine is safe and is administered as a 3-dose series, with the second and third doses given 2 and 6 months, respectively, after the initial dose. Adolescent girls also have the option of a bivalent HPV vaccine.
In 2016, the FDA approved a 9-valent HPV vaccine, a simpler 2-dose schedule for children ages 9 to 14 (2 doses at least 6 months apart). Leading cancer centers have endorsed this vaccine based on strong comparative data with the 3-dose regimen.10 For those not previously vaccinated, the HPV vaccine is available for women ages 13 to 26 and for men ages 13 to 21 (although men ages 22 to 26 can receive the vaccine, and it is recommended for men who have sex with men [MSM]). Women do not require Papanicolaou, serum pregnancy, HPV DNA, or HPV antibody tests prior to vaccination. If a woman becomes pregnant, remaining doses of the vaccine should be postponed until after delivery. Women still need to follow recommendations for cervical cancer screening because the HPV vaccine does not cover all genital strains of the virus. For sexually active individuals who might have HPV or genital warts, immunization has no clinical effect except to prevent other HPV strains.
Measles, mumps, and rubella (MMR) vaccine. All adults should receive, at minimum, 1 dose of MMR vaccination unless serological immunity can be verified or if contraindicated. Two doses of the vaccine are recommended for students attending post-high school institutions, health care personnel, and international travelers because they are at higher risk for exposure and transmission of measles and mumps. Individuals born before 1957 are considered immune to measles and mumps. A measles outbreak from December 2014 to February 201511 highlighted the importance of maintaining one’s immunity status for MMR.
Case continued
Based on Ms. W’s age, she should be offered vaccinations for influenza and opportunities to receive vaccinations for HPV, Tdap (the primary series, a Tdap or Td booster), and MMR, if appropriate and not completed previously.
Risk of exposure
Certain behaviors will increase the risk of exposure to and transmission of diseases communicable by blood and other bodily fluids (Table 3). These behaviors include needle injections (eg, during use of illicit drugs) and sexual activity with multiple partners, including MSM or promiscuity/impulsivity during a manic episode. A common consequence of risky behaviors is comorbid infection of HIV and viral hepatitis for those with substance use disorder or those who engage in high-risk sexual practices.12,13
Hepatitis B virus (HBV) immunization. Vaccination is one of the most effective ways to prevent HBV infection, which is why it is offered to all health care workers. HBV immunization is a 3-dose series in which the second and third doses are given 1 and 6 months after the initial doses, respectively. In addition to certain medical risk factors or conditions that indicate HBV vaccination, people should be offered the vaccine if they are in a higher risk occupation, travel, are of Asian or Pacific Islander ethnicity from an endemic area, or have any present or suspected sexually transmitted diseases.
Hepatitis A virus (HAV) vaccination. HAV is transmitted via fecal–oral routes, often from contaminated water or food, or through household or sexual contact with an infected person. Individuals should receive the HAV vaccine if they use illicit drugs by any route of administration, work with primates infected with HAV, travel to countries with unknown or high rates of HAV, or have chronic liver disease (ie, hepatitis, alcohol use disorder, or non-alcoholic fatty liver disease) or clotting deficiencies. The CDC Health Information for International Travel, commonly called the “Yellow Book,” publishes vaccination recommendations for those who plan travel to specific countries.14
Case continued
Ms. W’s history of mania (if such episodes included increased sexual activity) and substance use would make her a candidate for the HBV and HAV vaccinations and could also strengthen our previous recommendation that she receive the HPV vaccination.
Medical conditions
Patients with certain medical conditions may have difficulty fighting infections or become more susceptible to morbidity and mortality from coinfection with vaccine-preventable illnesses. Secondary effects of psychotropic medications that may carry implications for vaccine recommendations (eg, risk of agranulocytosis and impaired cell-medicated immunity with mirtazapine and clozapine or renal impairment from lithium use) are of particular concern in psychiatric patients.2
To help care for these patients, the CDC has developed a “medical conditions” schedule (Figure 2). This schedule makes vaccination recommendations for those with a weakened immune system, including patients with HIV, chronic obstructive pulmonary disease (COPD), diabetes, hepatitis, asplenia, end-stage renal disease, cardiac disease, and pregnancy.
Because patients with psychiatric illness face a greater risk of heart disease and diabetes, these conditions may warrant special reference on the schedule. The increased cardiometabolic risk factors in these patients may be due in part to genetics, socioeconomic status, lifestyle behaviors, and medications to treat their mental illness (eg, antipsychotics). Patients with bipolar disorder or schizophrenia in particular tend to have higher rates of COPD (mainly from chronic bronchitis) and asthma than the general population.12 Pay special attention to the indications schedule for those with chronic lung disease, especially patients who continue to smoke cigarettes.
Case continued
Because of Ms. W’s asthma, the CDC schedule recommends ensuring she is up to date on her influenza, pneumococcal, and Tdap vaccinations.
Substance use
Patients with combined psychiatric and substance use disorders (“dual diagnosis”) have lower rates of receiving preventive care than patients with either condition alone.15 Substance use can be behaviorally disinhibiting, leading to increased risk of exposures from sexual contact or other risky activities. The use of illicit substances can provide a nidus for infection depending on the route of administration and can result in negative effects on organ systems, compromising one’s ability to ward off infection.
Patients who use any illicit drugs, regardless of the method of delivery, should be recommended for HAV vaccination. For those with alcohol use disorder and/or chronic liver disease, and/or seeking treatment for substance use, hepatitis B screening and vaccination is recommended.
Case continued
From a substance use perspective, discussion of vaccination status for both hepatitis A and B would be important for Ms. W.
HIV or immunocompromised
Persons with severe mental illness have high rates of HIV, with almost 8 times the risk of exposure, compared with the general population due to myriad reasons, including greater rates of substance abuse, higher risk sexual behavior, and lack of awareness of HIV transmission.12,13 Patients with mental illness are also at risk of leukopenia and agranulocytosis from certain drugs used to treat their conditions, such as clozapine.
Pregnancy is a challenge for women with mental illness because of the pharmacologic risk and immune-system compromise to the mother and baby. A pregnant woman who has HIV with a CD4 count <200, or has a weakened immune system from an organ transplant or a similar condition, is a candidate for certain vaccines based on the Adult Immunization Schedule (Figure 2). However, these patients should avoid live vaccines, such as the intranasal mist of live influenza, MMR, VZV, and varicella, to avoid illness from these inoculations.
Case continued
Ms. W should undergo testing for pregnancy and HIV (and preferably other sexually transmitted infections per general preventive health guidelines) before receiving any live vaccinations.
Occupancy
Aside from direct transmission of bodily fluids, infectious diseases also can spread through droplets/secretions from the throat and respiratory tract. Close quarters or lengthy contact enhances communicability by droplets, and therefore people who reside in a communal living space (eg, individuals in substance use treatment facilities or those who reside in a nursing home) are most susceptible.
The bacterial disease Neisseria meningitidis (meningococcus) can spread through droplets and can cause pneumonia, bacteremia, and meningitis. Vaccination is indicated, and in some states is mandated, for college students who live in residence halls and missed routine vaccination by age 16. Meningococcus conjugate vaccine is administered in 2 doses; each dose may be given at least 2 months apart for those with HIV, asplenia, or persistent complement-related disorders. A single dose may be recommended for travelers to areas where meningococcal disease is hyperendemic or epidemic, military recruits, or microbiologists. For those age ≥55 and older, meningococcal polysaccharide vaccine is recommended over meningococcal conjugate vaccine.
Influenza, MMR, diphtheria, pertussis, and pneumococcus also spread through droplet contact.
Case continued
If Ms. W had not previously received the meningococcus vaccine as part of adolescent immunizations, she could benefit from this vaccine because she plans to enter a residential substance use disorder treatment program.
Tobacco use
Patients with psychiatric illness are twice as likely to smoke compared with the general population.16 Adult smokers, especially those with chronic lung disease, are at higher risk for influenza and pneumococcal-related illness; they should be vaccinated against these illnesses regardless of age (as discussed in the “Age” section).
Case continued
Because she smokes, Ms. W should receive counseling on vaccinations, such as influenza and pneumonia, to lessen her risk of respiratory illnesses and downstream sepsis.
Conclusion
Ms. W’s case represents an unfortunately all-too-common scenario where her multifaceted biopsychosocial circumstances place her at high risk for vaccine-preventable conditions. Her weight is recorded and laboratory work ordered to evaluate her pregnancy status, blood counts, lipids, complete metabolic panel, lithium level, and HIV status. Fortunately, she had received her series of MMR, meningococcal, and Tdap vaccinations when she was younger. Influenza, HPV, HAV, HBV, and pneumococcal vaccinations were all recommended to her, all of which can be given on the same day (HAV and HBV often are available as a combined vaccine). Ms. W receives a renewal of her psychiatric medications and counseling on healthy living habits (eg, diet and exercise, quitting tobacco and alcohol use, and safe sex practices) and the importance of immunizations.
Vaccination is 1 of the 10 great public health achievements of the 20th century when one considers how immunization of vaccine-preventable diseases has reduced morbidity, mortality, and health-associated costs.17 As mental health professionals, we can help pass on the direct and indirect benefits of immunizations to an often underserved and undertreated population to help improve their health outcomes and quality of life.
1. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
2. Raj YP, Lloyd L. Adult immunizations. In: McCarron RM, Xiong GL, Keenan GR, et al, eds. Preventive medical care in psychiatry. Arlington, VA: American Psychiatric Publishing. 2015;215-227.
3. Young S, Dosani N, Whisler A, et al. Influenza vaccination rates among homeless adults with mental illness in Toronto. J Prim Care Community Health. 2015;6(3):211-214.
4. Kroger AT, Atkinson WL, Marcues EK, et al; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1-48.
5. Centers for Disease Control and Prevention. Recommended Adult Immunization by Vaccine and Age Group. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated February 27, 2017. Accessed February 1, 2017.
6. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1-64.
7. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Updated June 22, 2016. Accessed February 1, 2017.
8. Hales CM, Harpaz, R, Ortega-Sanchez I, et al; Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729-731.
9. Petrosky E, Bocchini Jr JA, Hariri S, et al; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccine recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11)300-304.
10. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411-2421.
11. Zipprich J, Winter K, Hacker J, et al; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
14. Centers for Disease for Control and Prevention. CDC yellow book 2018: health information for international travel. New York, NY: Oxford University Press; 2017.
15. Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129-136.
16. Lasser K, Boyd J, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
17. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(19);619-623.
Patients with chronic, severe mental illness live much shorter lives than the general population. The 25-year loss in life expectancy for people with chronic mental illness has been attributed to higher rates of cardiovascular disease driven by increased smoking, obesity, poverty, and poor nutrition.1 These individuals also face the added burden of struggling with a psychiatric condition that often interferes with their ability to make optimal preventative health decisions, including staying up to date on vaccinations.2 A recent review from Toronto, Canada, found that the influenza vaccination rates among homeless adults with mental illness—a population at high risk of respiratory illness—was only 6.7% compared with 31.1% for the general population of Ontario.3
Mental health professionals may serve as the only contacts to offer medical care to this vulnerable population, leading some psychiatric leaders to advocate that psychiatrists be considered primary care providers within accountable care organizations. Because most vaccines are easily available, mental health professionals should know about key immunizations to guide their patients accordingly.
In the United States, approximately 45,000 adults die annually from vaccine-preventable diseases, the majority from influenza.4 When combined with the most recent Adult Immunization Schedule and general recommendations adapted from the CDC,5,6 the mnemonic ARM SHOT allows for a quick assessment of risk factors to guide administration and education about most vaccinations (Table 1). ARM SHOT involves assessing the following components of an individual’s health status and living arrangements to determine one’s risk of contracting communicable diseases:
- Age
- Risk of exposure
- Medical conditions (comorbidities)
- Substance use history
- HIV status or other immunocompromised states
- Occupancy, or living arrangements
- Tobacco use.
We recommend keeping a copy of the Adult Immunization Schedule (age ≥19) and/or the immunization schedule for children and adolescents (age ≤18) close for quick reference. Here, we provide a case and then explore how each component of the ARM SHOT mnemonic applies in decision-making.
Case Evaluating risk, assess needs
Ms. W, age 24, has bipolar I disorder, most recently manic with psychotic features. She presents for follow-up in clinic after a 5-day hospitalization for mania and comorbid alcohol use disorder. Her medical comorbidities include asthma and active tobacco use. She is taking lurasidone, 20 mg/d, and lithium, 900 mg/d. Her case manager is working to place Ms. W in a residential substance use disorder treatment program. Ms. W is on a waiting list to establish care with a primary care physician and has a history of poor engagement with medical services in general; prior attempts to place her with a primary care physician failed.
In advance of Ms. W’s transfer to a residential treatment facility, you have been asked to place a Mantoux screening test for tuberculosis (purified protein derivative), which raises the important question about her susceptibility to infectious diseases in general. To protect Ms. W from preventable diseases for which vaccines are available, you review the ARM SHOT mnemonic to broadly assess her candidacy for vaccinations.
Age
Age may be the most important determinant of a patient’s need for vaccination (Table 2). The CDC immunization schedules account for age-specific risks for diseases, complications, and responses to vaccination (Figure 1).6
Influenza vaccination. Adults can have an intramuscular or intradermal inactivated influenza vaccination yearly in the fall or winter, unless they have an allergy to a vaccine component such as egg protein. Those with such an allergy can receive a recombinant influenza vaccine. Until the 2016 to 2017 flu season, an intranasal mist of live, attenuated influenza vaccine was available to healthy, non-pregnant women, ages 2 to 49, without high-risk medical conditions. However, the CDC dropped its recommendation for this vaccine because data showed it did not effectively prevent the flu.7 Individuals age ≥65 can receive either the standard- or high-dose inactivated influenza vaccination. The latter contains 4 times the amount of antigen with the intention of triggering a stronger immune response in older adults.
Pneumonia immunization. All patients age ≥65 should receive vaccinations for Streptococcus pneumoniae and its variants in the form of one 13-valent pneumococcal conjugate vaccine and, at least 1 year later, one 23-valent pneumococcal polysaccharide vaccine (PPSV23). Immunization reduces the morbidity and mortality from pneumococcal illness by decreasing the burden of a pneumonia, bacteremia, or meningitis infection. Adults, ages 19 to 64, with a chronic disease (referred to as “special populations” in CDC tables), such as diabetes, heart or lung disease, alcoholism, or cirrhosis, or those who smoke cigarettes, should receive PPSV23 with a second dose administered at least 5 years after the first. The CDC recommends a 1-time re-vaccination at age 65 for patients if >5 years have passed since the last PPSV23 and if the patient was younger than age 65 at the time of primary vaccine for S. pneumoniae. This can be a rather tricky clinical situation; the health care provider should verify a patient’s immunization history to ensure that she (he) is receiving only necessary vaccines. However, when the history cannot be verified, err on the side of inclusion, because risks are minimal.
Shingles vaccination. Adults age ≥60 who are not immunocompromised should receive a single dose of live attenuated vaccine from varicella-zoster virus (VZV) to limit the risk of shingles from a prior chickenpox infection. The vaccine is approximately 66.5% effective at preventing postherpetic neuralgia for up to 4.9 years. Individuals as young as age 50 may have the vaccine because the risk of herpes zoster radically increases from then on,8 although most insurers only cover VZV vaccination after age 60.
Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. All adults should complete the 3-dose primary vaccination series for tetanus, diphtheria, and pertussis (also known as whooping cough) and this should include 1 dose of Tdap. Administration of the primary series is staged so that the second dose is given 4 weeks after the initial dose and the final dose 6 to 12 months after the first dose. After receiving the primary series, adults should receive a tetanus-diphtheria booster dose every 10 years. For adults ages 19 to 64, the Advisory Committee on Immunization Practices (ACIP) recommends 1 dose of Tdap in place of a booster vaccination to decrease the transmission risk of pertussis to vulnerable persons, especially children.
Human papillomavirus (HPV) immunization. The ACIP recommendation9 has been for children to receive routine vaccination for the 4 major strains of HPV—strains 6, 11, 16, and 18—starting at ages 11 to 12 to confer protection from HPV-associated diseases, such as genital warts, oropharyngeal cancer, and anal cancer; cancers of the cervix, vulva, and vagina in women; and penile cancer in men. Ideally, the vaccines are administered prior to HPV exposure from sexual contact. The quadrivalent HPV vaccine is safe and is administered as a 3-dose series, with the second and third doses given 2 and 6 months, respectively, after the initial dose. Adolescent girls also have the option of a bivalent HPV vaccine.
In 2016, the FDA approved a 9-valent HPV vaccine, a simpler 2-dose schedule for children ages 9 to 14 (2 doses at least 6 months apart). Leading cancer centers have endorsed this vaccine based on strong comparative data with the 3-dose regimen.10 For those not previously vaccinated, the HPV vaccine is available for women ages 13 to 26 and for men ages 13 to 21 (although men ages 22 to 26 can receive the vaccine, and it is recommended for men who have sex with men [MSM]). Women do not require Papanicolaou, serum pregnancy, HPV DNA, or HPV antibody tests prior to vaccination. If a woman becomes pregnant, remaining doses of the vaccine should be postponed until after delivery. Women still need to follow recommendations for cervical cancer screening because the HPV vaccine does not cover all genital strains of the virus. For sexually active individuals who might have HPV or genital warts, immunization has no clinical effect except to prevent other HPV strains.
Measles, mumps, and rubella (MMR) vaccine. All adults should receive, at minimum, 1 dose of MMR vaccination unless serological immunity can be verified or if contraindicated. Two doses of the vaccine are recommended for students attending post-high school institutions, health care personnel, and international travelers because they are at higher risk for exposure and transmission of measles and mumps. Individuals born before 1957 are considered immune to measles and mumps. A measles outbreak from December 2014 to February 201511 highlighted the importance of maintaining one’s immunity status for MMR.
Case continued
Based on Ms. W’s age, she should be offered vaccinations for influenza and opportunities to receive vaccinations for HPV, Tdap (the primary series, a Tdap or Td booster), and MMR, if appropriate and not completed previously.
Risk of exposure
Certain behaviors will increase the risk of exposure to and transmission of diseases communicable by blood and other bodily fluids (Table 3). These behaviors include needle injections (eg, during use of illicit drugs) and sexual activity with multiple partners, including MSM or promiscuity/impulsivity during a manic episode. A common consequence of risky behaviors is comorbid infection of HIV and viral hepatitis for those with substance use disorder or those who engage in high-risk sexual practices.12,13
Hepatitis B virus (HBV) immunization. Vaccination is one of the most effective ways to prevent HBV infection, which is why it is offered to all health care workers. HBV immunization is a 3-dose series in which the second and third doses are given 1 and 6 months after the initial doses, respectively. In addition to certain medical risk factors or conditions that indicate HBV vaccination, people should be offered the vaccine if they are in a higher risk occupation, travel, are of Asian or Pacific Islander ethnicity from an endemic area, or have any present or suspected sexually transmitted diseases.
Hepatitis A virus (HAV) vaccination. HAV is transmitted via fecal–oral routes, often from contaminated water or food, or through household or sexual contact with an infected person. Individuals should receive the HAV vaccine if they use illicit drugs by any route of administration, work with primates infected with HAV, travel to countries with unknown or high rates of HAV, or have chronic liver disease (ie, hepatitis, alcohol use disorder, or non-alcoholic fatty liver disease) or clotting deficiencies. The CDC Health Information for International Travel, commonly called the “Yellow Book,” publishes vaccination recommendations for those who plan travel to specific countries.14
Case continued
Ms. W’s history of mania (if such episodes included increased sexual activity) and substance use would make her a candidate for the HBV and HAV vaccinations and could also strengthen our previous recommendation that she receive the HPV vaccination.
Medical conditions
Patients with certain medical conditions may have difficulty fighting infections or become more susceptible to morbidity and mortality from coinfection with vaccine-preventable illnesses. Secondary effects of psychotropic medications that may carry implications for vaccine recommendations (eg, risk of agranulocytosis and impaired cell-medicated immunity with mirtazapine and clozapine or renal impairment from lithium use) are of particular concern in psychiatric patients.2
To help care for these patients, the CDC has developed a “medical conditions” schedule (Figure 2). This schedule makes vaccination recommendations for those with a weakened immune system, including patients with HIV, chronic obstructive pulmonary disease (COPD), diabetes, hepatitis, asplenia, end-stage renal disease, cardiac disease, and pregnancy.
Because patients with psychiatric illness face a greater risk of heart disease and diabetes, these conditions may warrant special reference on the schedule. The increased cardiometabolic risk factors in these patients may be due in part to genetics, socioeconomic status, lifestyle behaviors, and medications to treat their mental illness (eg, antipsychotics). Patients with bipolar disorder or schizophrenia in particular tend to have higher rates of COPD (mainly from chronic bronchitis) and asthma than the general population.12 Pay special attention to the indications schedule for those with chronic lung disease, especially patients who continue to smoke cigarettes.
Case continued
Because of Ms. W’s asthma, the CDC schedule recommends ensuring she is up to date on her influenza, pneumococcal, and Tdap vaccinations.
Substance use
Patients with combined psychiatric and substance use disorders (“dual diagnosis”) have lower rates of receiving preventive care than patients with either condition alone.15 Substance use can be behaviorally disinhibiting, leading to increased risk of exposures from sexual contact or other risky activities. The use of illicit substances can provide a nidus for infection depending on the route of administration and can result in negative effects on organ systems, compromising one’s ability to ward off infection.
Patients who use any illicit drugs, regardless of the method of delivery, should be recommended for HAV vaccination. For those with alcohol use disorder and/or chronic liver disease, and/or seeking treatment for substance use, hepatitis B screening and vaccination is recommended.
Case continued
From a substance use perspective, discussion of vaccination status for both hepatitis A and B would be important for Ms. W.
HIV or immunocompromised
Persons with severe mental illness have high rates of HIV, with almost 8 times the risk of exposure, compared with the general population due to myriad reasons, including greater rates of substance abuse, higher risk sexual behavior, and lack of awareness of HIV transmission.12,13 Patients with mental illness are also at risk of leukopenia and agranulocytosis from certain drugs used to treat their conditions, such as clozapine.
Pregnancy is a challenge for women with mental illness because of the pharmacologic risk and immune-system compromise to the mother and baby. A pregnant woman who has HIV with a CD4 count <200, or has a weakened immune system from an organ transplant or a similar condition, is a candidate for certain vaccines based on the Adult Immunization Schedule (Figure 2). However, these patients should avoid live vaccines, such as the intranasal mist of live influenza, MMR, VZV, and varicella, to avoid illness from these inoculations.
Case continued
Ms. W should undergo testing for pregnancy and HIV (and preferably other sexually transmitted infections per general preventive health guidelines) before receiving any live vaccinations.
Occupancy
Aside from direct transmission of bodily fluids, infectious diseases also can spread through droplets/secretions from the throat and respiratory tract. Close quarters or lengthy contact enhances communicability by droplets, and therefore people who reside in a communal living space (eg, individuals in substance use treatment facilities or those who reside in a nursing home) are most susceptible.
The bacterial disease Neisseria meningitidis (meningococcus) can spread through droplets and can cause pneumonia, bacteremia, and meningitis. Vaccination is indicated, and in some states is mandated, for college students who live in residence halls and missed routine vaccination by age 16. Meningococcus conjugate vaccine is administered in 2 doses; each dose may be given at least 2 months apart for those with HIV, asplenia, or persistent complement-related disorders. A single dose may be recommended for travelers to areas where meningococcal disease is hyperendemic or epidemic, military recruits, or microbiologists. For those age ≥55 and older, meningococcal polysaccharide vaccine is recommended over meningococcal conjugate vaccine.
Influenza, MMR, diphtheria, pertussis, and pneumococcus also spread through droplet contact.
Case continued
If Ms. W had not previously received the meningococcus vaccine as part of adolescent immunizations, she could benefit from this vaccine because she plans to enter a residential substance use disorder treatment program.
Tobacco use
Patients with psychiatric illness are twice as likely to smoke compared with the general population.16 Adult smokers, especially those with chronic lung disease, are at higher risk for influenza and pneumococcal-related illness; they should be vaccinated against these illnesses regardless of age (as discussed in the “Age” section).
Case continued
Because she smokes, Ms. W should receive counseling on vaccinations, such as influenza and pneumonia, to lessen her risk of respiratory illnesses and downstream sepsis.
Conclusion
Ms. W’s case represents an unfortunately all-too-common scenario where her multifaceted biopsychosocial circumstances place her at high risk for vaccine-preventable conditions. Her weight is recorded and laboratory work ordered to evaluate her pregnancy status, blood counts, lipids, complete metabolic panel, lithium level, and HIV status. Fortunately, she had received her series of MMR, meningococcal, and Tdap vaccinations when she was younger. Influenza, HPV, HAV, HBV, and pneumococcal vaccinations were all recommended to her, all of which can be given on the same day (HAV and HBV often are available as a combined vaccine). Ms. W receives a renewal of her psychiatric medications and counseling on healthy living habits (eg, diet and exercise, quitting tobacco and alcohol use, and safe sex practices) and the importance of immunizations.
Vaccination is 1 of the 10 great public health achievements of the 20th century when one considers how immunization of vaccine-preventable diseases has reduced morbidity, mortality, and health-associated costs.17 As mental health professionals, we can help pass on the direct and indirect benefits of immunizations to an often underserved and undertreated population to help improve their health outcomes and quality of life.
Patients with chronic, severe mental illness live much shorter lives than the general population. The 25-year loss in life expectancy for people with chronic mental illness has been attributed to higher rates of cardiovascular disease driven by increased smoking, obesity, poverty, and poor nutrition.1 These individuals also face the added burden of struggling with a psychiatric condition that often interferes with their ability to make optimal preventative health decisions, including staying up to date on vaccinations.2 A recent review from Toronto, Canada, found that the influenza vaccination rates among homeless adults with mental illness—a population at high risk of respiratory illness—was only 6.7% compared with 31.1% for the general population of Ontario.3
Mental health professionals may serve as the only contacts to offer medical care to this vulnerable population, leading some psychiatric leaders to advocate that psychiatrists be considered primary care providers within accountable care organizations. Because most vaccines are easily available, mental health professionals should know about key immunizations to guide their patients accordingly.
In the United States, approximately 45,000 adults die annually from vaccine-preventable diseases, the majority from influenza.4 When combined with the most recent Adult Immunization Schedule and general recommendations adapted from the CDC,5,6 the mnemonic ARM SHOT allows for a quick assessment of risk factors to guide administration and education about most vaccinations (Table 1). ARM SHOT involves assessing the following components of an individual’s health status and living arrangements to determine one’s risk of contracting communicable diseases:
- Age
- Risk of exposure
- Medical conditions (comorbidities)
- Substance use history
- HIV status or other immunocompromised states
- Occupancy, or living arrangements
- Tobacco use.
We recommend keeping a copy of the Adult Immunization Schedule (age ≥19) and/or the immunization schedule for children and adolescents (age ≤18) close for quick reference. Here, we provide a case and then explore how each component of the ARM SHOT mnemonic applies in decision-making.
Case Evaluating risk, assess needs
Ms. W, age 24, has bipolar I disorder, most recently manic with psychotic features. She presents for follow-up in clinic after a 5-day hospitalization for mania and comorbid alcohol use disorder. Her medical comorbidities include asthma and active tobacco use. She is taking lurasidone, 20 mg/d, and lithium, 900 mg/d. Her case manager is working to place Ms. W in a residential substance use disorder treatment program. Ms. W is on a waiting list to establish care with a primary care physician and has a history of poor engagement with medical services in general; prior attempts to place her with a primary care physician failed.
In advance of Ms. W’s transfer to a residential treatment facility, you have been asked to place a Mantoux screening test for tuberculosis (purified protein derivative), which raises the important question about her susceptibility to infectious diseases in general. To protect Ms. W from preventable diseases for which vaccines are available, you review the ARM SHOT mnemonic to broadly assess her candidacy for vaccinations.
Age
Age may be the most important determinant of a patient’s need for vaccination (Table 2). The CDC immunization schedules account for age-specific risks for diseases, complications, and responses to vaccination (Figure 1).6
Influenza vaccination. Adults can have an intramuscular or intradermal inactivated influenza vaccination yearly in the fall or winter, unless they have an allergy to a vaccine component such as egg protein. Those with such an allergy can receive a recombinant influenza vaccine. Until the 2016 to 2017 flu season, an intranasal mist of live, attenuated influenza vaccine was available to healthy, non-pregnant women, ages 2 to 49, without high-risk medical conditions. However, the CDC dropped its recommendation for this vaccine because data showed it did not effectively prevent the flu.7 Individuals age ≥65 can receive either the standard- or high-dose inactivated influenza vaccination. The latter contains 4 times the amount of antigen with the intention of triggering a stronger immune response in older adults.
Pneumonia immunization. All patients age ≥65 should receive vaccinations for Streptococcus pneumoniae and its variants in the form of one 13-valent pneumococcal conjugate vaccine and, at least 1 year later, one 23-valent pneumococcal polysaccharide vaccine (PPSV23). Immunization reduces the morbidity and mortality from pneumococcal illness by decreasing the burden of a pneumonia, bacteremia, or meningitis infection. Adults, ages 19 to 64, with a chronic disease (referred to as “special populations” in CDC tables), such as diabetes, heart or lung disease, alcoholism, or cirrhosis, or those who smoke cigarettes, should receive PPSV23 with a second dose administered at least 5 years after the first. The CDC recommends a 1-time re-vaccination at age 65 for patients if >5 years have passed since the last PPSV23 and if the patient was younger than age 65 at the time of primary vaccine for S. pneumoniae. This can be a rather tricky clinical situation; the health care provider should verify a patient’s immunization history to ensure that she (he) is receiving only necessary vaccines. However, when the history cannot be verified, err on the side of inclusion, because risks are minimal.
Shingles vaccination. Adults age ≥60 who are not immunocompromised should receive a single dose of live attenuated vaccine from varicella-zoster virus (VZV) to limit the risk of shingles from a prior chickenpox infection. The vaccine is approximately 66.5% effective at preventing postherpetic neuralgia for up to 4.9 years. Individuals as young as age 50 may have the vaccine because the risk of herpes zoster radically increases from then on,8 although most insurers only cover VZV vaccination after age 60.
Tetanus, diphtheria, and acellular pertussis (Tdap) vaccine. All adults should complete the 3-dose primary vaccination series for tetanus, diphtheria, and pertussis (also known as whooping cough) and this should include 1 dose of Tdap. Administration of the primary series is staged so that the second dose is given 4 weeks after the initial dose and the final dose 6 to 12 months after the first dose. After receiving the primary series, adults should receive a tetanus-diphtheria booster dose every 10 years. For adults ages 19 to 64, the Advisory Committee on Immunization Practices (ACIP) recommends 1 dose of Tdap in place of a booster vaccination to decrease the transmission risk of pertussis to vulnerable persons, especially children.
Human papillomavirus (HPV) immunization. The ACIP recommendation9 has been for children to receive routine vaccination for the 4 major strains of HPV—strains 6, 11, 16, and 18—starting at ages 11 to 12 to confer protection from HPV-associated diseases, such as genital warts, oropharyngeal cancer, and anal cancer; cancers of the cervix, vulva, and vagina in women; and penile cancer in men. Ideally, the vaccines are administered prior to HPV exposure from sexual contact. The quadrivalent HPV vaccine is safe and is administered as a 3-dose series, with the second and third doses given 2 and 6 months, respectively, after the initial dose. Adolescent girls also have the option of a bivalent HPV vaccine.
In 2016, the FDA approved a 9-valent HPV vaccine, a simpler 2-dose schedule for children ages 9 to 14 (2 doses at least 6 months apart). Leading cancer centers have endorsed this vaccine based on strong comparative data with the 3-dose regimen.10 For those not previously vaccinated, the HPV vaccine is available for women ages 13 to 26 and for men ages 13 to 21 (although men ages 22 to 26 can receive the vaccine, and it is recommended for men who have sex with men [MSM]). Women do not require Papanicolaou, serum pregnancy, HPV DNA, or HPV antibody tests prior to vaccination. If a woman becomes pregnant, remaining doses of the vaccine should be postponed until after delivery. Women still need to follow recommendations for cervical cancer screening because the HPV vaccine does not cover all genital strains of the virus. For sexually active individuals who might have HPV or genital warts, immunization has no clinical effect except to prevent other HPV strains.
Measles, mumps, and rubella (MMR) vaccine. All adults should receive, at minimum, 1 dose of MMR vaccination unless serological immunity can be verified or if contraindicated. Two doses of the vaccine are recommended for students attending post-high school institutions, health care personnel, and international travelers because they are at higher risk for exposure and transmission of measles and mumps. Individuals born before 1957 are considered immune to measles and mumps. A measles outbreak from December 2014 to February 201511 highlighted the importance of maintaining one’s immunity status for MMR.
Case continued
Based on Ms. W’s age, she should be offered vaccinations for influenza and opportunities to receive vaccinations for HPV, Tdap (the primary series, a Tdap or Td booster), and MMR, if appropriate and not completed previously.
Risk of exposure
Certain behaviors will increase the risk of exposure to and transmission of diseases communicable by blood and other bodily fluids (Table 3). These behaviors include needle injections (eg, during use of illicit drugs) and sexual activity with multiple partners, including MSM or promiscuity/impulsivity during a manic episode. A common consequence of risky behaviors is comorbid infection of HIV and viral hepatitis for those with substance use disorder or those who engage in high-risk sexual practices.12,13
Hepatitis B virus (HBV) immunization. Vaccination is one of the most effective ways to prevent HBV infection, which is why it is offered to all health care workers. HBV immunization is a 3-dose series in which the second and third doses are given 1 and 6 months after the initial doses, respectively. In addition to certain medical risk factors or conditions that indicate HBV vaccination, people should be offered the vaccine if they are in a higher risk occupation, travel, are of Asian or Pacific Islander ethnicity from an endemic area, or have any present or suspected sexually transmitted diseases.
Hepatitis A virus (HAV) vaccination. HAV is transmitted via fecal–oral routes, often from contaminated water or food, or through household or sexual contact with an infected person. Individuals should receive the HAV vaccine if they use illicit drugs by any route of administration, work with primates infected with HAV, travel to countries with unknown or high rates of HAV, or have chronic liver disease (ie, hepatitis, alcohol use disorder, or non-alcoholic fatty liver disease) or clotting deficiencies. The CDC Health Information for International Travel, commonly called the “Yellow Book,” publishes vaccination recommendations for those who plan travel to specific countries.14
Case continued
Ms. W’s history of mania (if such episodes included increased sexual activity) and substance use would make her a candidate for the HBV and HAV vaccinations and could also strengthen our previous recommendation that she receive the HPV vaccination.
Medical conditions
Patients with certain medical conditions may have difficulty fighting infections or become more susceptible to morbidity and mortality from coinfection with vaccine-preventable illnesses. Secondary effects of psychotropic medications that may carry implications for vaccine recommendations (eg, risk of agranulocytosis and impaired cell-medicated immunity with mirtazapine and clozapine or renal impairment from lithium use) are of particular concern in psychiatric patients.2
To help care for these patients, the CDC has developed a “medical conditions” schedule (Figure 2). This schedule makes vaccination recommendations for those with a weakened immune system, including patients with HIV, chronic obstructive pulmonary disease (COPD), diabetes, hepatitis, asplenia, end-stage renal disease, cardiac disease, and pregnancy.
Because patients with psychiatric illness face a greater risk of heart disease and diabetes, these conditions may warrant special reference on the schedule. The increased cardiometabolic risk factors in these patients may be due in part to genetics, socioeconomic status, lifestyle behaviors, and medications to treat their mental illness (eg, antipsychotics). Patients with bipolar disorder or schizophrenia in particular tend to have higher rates of COPD (mainly from chronic bronchitis) and asthma than the general population.12 Pay special attention to the indications schedule for those with chronic lung disease, especially patients who continue to smoke cigarettes.
Case continued
Because of Ms. W’s asthma, the CDC schedule recommends ensuring she is up to date on her influenza, pneumococcal, and Tdap vaccinations.
Substance use
Patients with combined psychiatric and substance use disorders (“dual diagnosis”) have lower rates of receiving preventive care than patients with either condition alone.15 Substance use can be behaviorally disinhibiting, leading to increased risk of exposures from sexual contact or other risky activities. The use of illicit substances can provide a nidus for infection depending on the route of administration and can result in negative effects on organ systems, compromising one’s ability to ward off infection.
Patients who use any illicit drugs, regardless of the method of delivery, should be recommended for HAV vaccination. For those with alcohol use disorder and/or chronic liver disease, and/or seeking treatment for substance use, hepatitis B screening and vaccination is recommended.
Case continued
From a substance use perspective, discussion of vaccination status for both hepatitis A and B would be important for Ms. W.
HIV or immunocompromised
Persons with severe mental illness have high rates of HIV, with almost 8 times the risk of exposure, compared with the general population due to myriad reasons, including greater rates of substance abuse, higher risk sexual behavior, and lack of awareness of HIV transmission.12,13 Patients with mental illness are also at risk of leukopenia and agranulocytosis from certain drugs used to treat their conditions, such as clozapine.
Pregnancy is a challenge for women with mental illness because of the pharmacologic risk and immune-system compromise to the mother and baby. A pregnant woman who has HIV with a CD4 count <200, or has a weakened immune system from an organ transplant or a similar condition, is a candidate for certain vaccines based on the Adult Immunization Schedule (Figure 2). However, these patients should avoid live vaccines, such as the intranasal mist of live influenza, MMR, VZV, and varicella, to avoid illness from these inoculations.
Case continued
Ms. W should undergo testing for pregnancy and HIV (and preferably other sexually transmitted infections per general preventive health guidelines) before receiving any live vaccinations.
Occupancy
Aside from direct transmission of bodily fluids, infectious diseases also can spread through droplets/secretions from the throat and respiratory tract. Close quarters or lengthy contact enhances communicability by droplets, and therefore people who reside in a communal living space (eg, individuals in substance use treatment facilities or those who reside in a nursing home) are most susceptible.
The bacterial disease Neisseria meningitidis (meningococcus) can spread through droplets and can cause pneumonia, bacteremia, and meningitis. Vaccination is indicated, and in some states is mandated, for college students who live in residence halls and missed routine vaccination by age 16. Meningococcus conjugate vaccine is administered in 2 doses; each dose may be given at least 2 months apart for those with HIV, asplenia, or persistent complement-related disorders. A single dose may be recommended for travelers to areas where meningococcal disease is hyperendemic or epidemic, military recruits, or microbiologists. For those age ≥55 and older, meningococcal polysaccharide vaccine is recommended over meningococcal conjugate vaccine.
Influenza, MMR, diphtheria, pertussis, and pneumococcus also spread through droplet contact.
Case continued
If Ms. W had not previously received the meningococcus vaccine as part of adolescent immunizations, she could benefit from this vaccine because she plans to enter a residential substance use disorder treatment program.
Tobacco use
Patients with psychiatric illness are twice as likely to smoke compared with the general population.16 Adult smokers, especially those with chronic lung disease, are at higher risk for influenza and pneumococcal-related illness; they should be vaccinated against these illnesses regardless of age (as discussed in the “Age” section).
Case continued
Because she smokes, Ms. W should receive counseling on vaccinations, such as influenza and pneumonia, to lessen her risk of respiratory illnesses and downstream sepsis.
Conclusion
Ms. W’s case represents an unfortunately all-too-common scenario where her multifaceted biopsychosocial circumstances place her at high risk for vaccine-preventable conditions. Her weight is recorded and laboratory work ordered to evaluate her pregnancy status, blood counts, lipids, complete metabolic panel, lithium level, and HIV status. Fortunately, she had received her series of MMR, meningococcal, and Tdap vaccinations when she was younger. Influenza, HPV, HAV, HBV, and pneumococcal vaccinations were all recommended to her, all of which can be given on the same day (HAV and HBV often are available as a combined vaccine). Ms. W receives a renewal of her psychiatric medications and counseling on healthy living habits (eg, diet and exercise, quitting tobacco and alcohol use, and safe sex practices) and the importance of immunizations.
Vaccination is 1 of the 10 great public health achievements of the 20th century when one considers how immunization of vaccine-preventable diseases has reduced morbidity, mortality, and health-associated costs.17 As mental health professionals, we can help pass on the direct and indirect benefits of immunizations to an often underserved and undertreated population to help improve their health outcomes and quality of life.
1. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
2. Raj YP, Lloyd L. Adult immunizations. In: McCarron RM, Xiong GL, Keenan GR, et al, eds. Preventive medical care in psychiatry. Arlington, VA: American Psychiatric Publishing. 2015;215-227.
3. Young S, Dosani N, Whisler A, et al. Influenza vaccination rates among homeless adults with mental illness in Toronto. J Prim Care Community Health. 2015;6(3):211-214.
4. Kroger AT, Atkinson WL, Marcues EK, et al; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1-48.
5. Centers for Disease Control and Prevention. Recommended Adult Immunization by Vaccine and Age Group. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated February 27, 2017. Accessed February 1, 2017.
6. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1-64.
7. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Updated June 22, 2016. Accessed February 1, 2017.
8. Hales CM, Harpaz, R, Ortega-Sanchez I, et al; Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729-731.
9. Petrosky E, Bocchini Jr JA, Hariri S, et al; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccine recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11)300-304.
10. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411-2421.
11. Zipprich J, Winter K, Hacker J, et al; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
14. Centers for Disease for Control and Prevention. CDC yellow book 2018: health information for international travel. New York, NY: Oxford University Press; 2017.
15. Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129-136.
16. Lasser K, Boyd J, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
17. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(19);619-623.
1. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
2. Raj YP, Lloyd L. Adult immunizations. In: McCarron RM, Xiong GL, Keenan GR, et al, eds. Preventive medical care in psychiatry. Arlington, VA: American Psychiatric Publishing. 2015;215-227.
3. Young S, Dosani N, Whisler A, et al. Influenza vaccination rates among homeless adults with mental illness in Toronto. J Prim Care Community Health. 2015;6(3):211-214.
4. Kroger AT, Atkinson WL, Marcues EK, et al; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). General recommendations on immunization: recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1-48.
5. Centers for Disease Control and Prevention. Recommended Adult Immunization by Vaccine and Age Group. http://www.cdc.gov/vaccines/schedules/hcp/adult.html. Updated February 27, 2017. Accessed February 1, 2017.
6. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations on the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1-64.
7. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Updated June 22, 2016. Accessed February 1, 2017.
8. Hales CM, Harpaz, R, Ortega-Sanchez I, et al; Centers for Disease Control and Prevention. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63(33):729-731.
9. Petrosky E, Bocchini Jr JA, Hariri S, et al; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccine recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11)300-304.
10. Iversen OE, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411-2421.
11. Zipprich J, Winter K, Hacker J, et al; Centers for Disease Control and Prevention (CDC). Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015;64(6):153-154.
12. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
13. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
14. Centers for Disease for Control and Prevention. CDC yellow book 2018: health information for international travel. New York, NY: Oxford University Press; 2017.
15. Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Med Care. 2002;40(2):129-136.
16. Lasser K, Boyd J, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606-2610.
17. Centers for Disease Control and Prevention (CDC). Ten great public health achievements—United States, 2001-2010. MMWR Morb Mortal Wkly Rep. 2011;60(19);619-623.
Dozing off: Examining excessive daytime sleepiness in psychiatric patients
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
Excessive daytime sleepiness (EDS) is “the inability to maintain wakefulness and alertness during the major waking periods of the day, with sleep occurring unintentionally or at inappropriate times, almost daily for at least 3 months,” according to the American Academy of Sleep Medicine.1 EDS is common, with a prevalence up to 25% to 30% in the general population.1-4 The prevalence rate varies in different studies, primarily because of inconsistent definitions of EDS, and therefore differences in diagnosis and assessment.1,2,4 In a study of 300 psychiatric outpatients, 34% had EDS.3 However, studies and evidence reviewing EDS in psychiatric patients are limited.
The causes of EDS are many and varied,1,8 including medical and psychiatric etiologies. A thorough history, screening at-risk patients, and timely sleep center referral are vital to detect and appropriately manage the cause of EDS.5
This article reviews the literature on EDS, with a focus on the risks of untreated EDS, common etiologies of the condition, as well as a brief description of screening and treatment strategies.
EDS vs fatigue
Many patients describe EDS as “fatigue”1; however, a patient’s report of fatigue could be mistaken for EDS.4 Although there is overlap, it is important for physicians to distinguish between these 2 entities for accurate identification and treatment.1,4
Risk of inadequate screening
A study of 117 patients with symptomatic coronary artery disease showed that EDS is associated with significantly greater incidence of cardiovascular adverse events at 16-month follow up.2 This study had limitations such as small sample size; therefore, more studies are needed. Because of these risks, timely and accurate diagnosis not only improves the patient’s quality of life and reduces polypharmacy but also can be life-saving.
Common causes of EDS in psychiatric patients
Because of the high prevalence and severity of impairments caused by EDS, it is essential for psychiatrists to be informed about causes of EDS and thoroughly assess for the potential underlying etiology before concluding that the sleep problem is a manifestation of the psychiatric disorder and prescribing psychotropic medication for it.
Some common causes of EDS in psychiatric patients include:
Sleep-disordered breathing.8 Obstructive sleep apnea (OSA) is often underdiagnosed,6,7 and considering how common it is,6 psychiatrists likely will see many patients with OSA in their practice.5 OSA has a higher prevalence among patients with psychiatric disorders such as depression6,9 and schizophrenia. Additionally, there is evidence suggesting that patients with OSA are more likely to suffer from depression and EDS than healthy controls6,9,10; some of the proposed mechanisms are sleep fragmentation and hypoxemia.6,9-11 OSA is the most common form of sleep-disordered breathing and is a common cause of EDS.1,2,12 Also, undiagnosed and untreated OSA in patients with depression could cause refractoriness to pharmacological treatment of depression.6,9,10
When unrecognized and untreated, OSA can be life-threatening. Despite this, OSA is not regularly screened for in clinical psychiatric practice.6,10 Therefore, it is imperative that psychiatrists be well-acquainted with measures to identify at-risk patients and refer to a sleep specialist when appropriate.
OSA is accompanied by irritability, cognitive difficulties, and poor sleep, creating an overlap with symptoms of depressive disorders.6,10 Use of sedative hypnotic medications, such as benzodiazepines, which further reduces muscle tone in the airway and suppresses respiratory effort, can worsen OSA symptoms5,6,10 and pose cerebrovascular, cardiovascular, and potentially life-threatening risks, and therefore is not indicated in this population.9,13
Obesity is a risk factor for OSA.6 Patients with mood disorders or schizophrenia or other psychotic disorders are at higher risk of obesity because of psychotropic-induced weight gain, stress-induced mechanisms, and/or lower levels of self-care. When these patients have unrecognized or untreated OSA and are prescribed sedative medications at night or stimulant medications during the day, they could be at increased cardiac or respiratory risks without resolving their underlying condition. A diligent psychiatrist can dramatically reduce the risks by referring a patient for nocturnal polysomnography,1 helping the patient implement lifestyle modifications (eg, exercise, weight loss, and healthy nutrition), prescribing judiciously, and monitoring closely for such risks. An accurate diagnosis of and treatment for OSA can improve sleep6 dramatically and help depressive symptoms through better sleep, more daytime energy and concentration, and adequate oxygenation of the brain while sleeping.
Psychiatrists can screen for OSA using the STOP-Bang (Snoring, Tired, Observed apnea, Pressure, Body mass index, Age, Neck circumference, Gender) Questionnaire, which is a quick, 8-item screening scale that helps to categorize OSA risk as mild, moderate, or severe.12 Hypertension, snoring, and/or gasping for breath (“observed apnea”)—a history which often is provided by spouses or significant others—daytime dozing and/or tiredness, having a large neck circumference or volume, body mass index, male sex, and age are items on the STOP-Bang Questionnaire and also are features that should raise high clinical suspicion of OSA.12 Referral for nocturnal polysomnography in at-risk patients should be the next step1,5 in any sleep-related breathing disorder.
Treatment for OSA involves continuous positive airway pressure (CPAP) therapy, which has been shown to relieve OSA and decrease related EDS.5,6 Other treatment modalities, such as oral appliances and surgery, may be used5 in some cases, but more studies are needed for conclusive results.
Several studies have shown improved depression, mood, and cognition after administering treatment such as CPAP6,9,14 in patients with OSA and depression. Considering the significant risks of cardiovascular,8 cerebrovascular,8 and overall morbidity and mortality associated with untreated OSA,12 it is important to routinely screen for sleep-disordered breathing in patients with depression9 or other psychiatric disorders and refer for specialized sleep evaluation and treatment, when indicated.
Medications. EDS can result from some prescription and over-the-counter medications.1,2,5,7 Sedating antidepressants, antihistamines, antipsychotics, anticonvulsants,1,8 and beta blockers2 could cause sedation, which can persist during daytime, although a few studies did not find an association between antipsychotic use and EDS.3 Benzodiazepines and other sedative-hypnotics,1,7 especially long-acting agents or higher dosages,5 can lead to EDS and decreased alertness. Non-psychotropics, such as opioid pain medications,1,7 antitussives, and skeletal muscle relaxants, also can contribute to or cause daytime sedation.7 When using these agents, psychiatrists should monitor and routinely assess patients while aiming for the lowest effective dosage when feasible.
This strategy creates a framework for psychiatrists to routinely educate patients about these commonly encountered side effects, reduce polypharmacy when possible, and help patients effectively manage or prevent these adverse effects.
Depression.1 Some studies found >45% patients with depression had EDS.3,13,15 Besides an association between depression and EDS,13,16 Chellappa and Araújo13 also found a significant association between EDS and suicidal ideation. The causes of EDS in patients with depression may be varied, ranging from restless legs syndrome, residual depressive symptoms,15 to OSA. Depression is often comorbid with OSA,6 with up to 20% of patients with depression suffering from OSA,10 creating higher risk for EDS. Depressive disorders are routinely assessed during an evaluation of OSA at sleep centers, but OSA often is not screened in psychiatric practice.10
There is a strong need for regular screening for OSA in patients with depression, particularly because most studies show a link between the 2 conditions.10 Both depression and OSA have some common risk factors, such as obesity, hypertension, and metabolic syndrome.10 Patients with these conditions are at greater risk for OSA, and therefore a psychiatrist should proactively screen and refer such patients for nocturnal polysomnography when they suspect OSA. Patients with OSA and depression often present to the psychiatrist with depressive symptoms that appear to be resistant to pharmacological treatment,10 therefore underscoring the importance of screening and ruling out OSA in patients with depression.
Circadian rhythm disorders, restless legs syndrome, alcohol and other substance use, and use of prescription sedative-hypnotics are more common in patients with depression; therefore, this population is at high risk for EDS.
Circadian rhythm disorders and insufficient sleep syndrome. Insufficient sleep syndrome1,2,8 frequently causes EDS and occurs more commonly in busy people who try to get by with less sleep.8 Over time, the effect of sleep loss is cumulative and can be accompanied by mood symptoms, such as irritability, fatigue, and problems with concentration.8 Shift workers1,8 commonly experience insufficient sleep as well as circadian rhythm disorders and EDS. Modafinil is FDA-approved for EDS in shift work sleep disorder.
Geriatric patients may experience advanced sleep phase syndrome involving early awakenings.8 Adolescents, on the other hand, often suffer from delayed sleep phase syndrome, which is a type of circadian rhythm disorder, related to increasing academic and social pressures, natural pubertal shift to later sleep onset, pervading technology use, and often nebulous bedtime routines. This can be a cause of sleep persisting into daytime.8 Taking a careful history and a sleep diary may be useful because this disorder might be confused for insomnia. Treatment involves gradual shifting of the time of sleep onset through bright light exposure and other modalities.8
Adolescents might not be forthcoming about the severity of their sleep problems; therefore, psychiatrists should screen proactively through clinical interviews of patients and parents and consider this possibility when encountering an adolescent with recent-onset attention or cognitive difficulties.
Treatment for circadian rhythm disorders usually includes planned or prescribed sleep scheduling, timed light exposure,8 and occasional use of melatonin or other sedative agents.17
Hypersomnia of central origin, which includes narcolepsy, idiopathic hypersomnia, and recurrent hypersomnia, can present with EDS.1,18,19 Narcolepsy is a rare, debilitating sleep disorder that manifests as EDS or sleep attacks, with or without cataplexy, and sleep paralysis.5,8,18,19 The Multiple Sleep Latency Test and polysomnography are used for diagnosis.1,5 Shortened REM latency is a classic finding often noted on polysomnography. Treatment involves pharmacologic and behavioral strategies and education.5,8 Modafinil is FDA-approved for EDS associated with narcolepsy. Stimulant medications have been used for narcolepsy in the past; further studies are needed to establish benefit–risk ratio of use in this population.18
Kleine-Levin syndrome is a form of recurrent hypersomnia, a less common sleep disorder, characterized by episodes of excessive sleepiness accompanied by hyperphagia and hypersexuality.5,18,19
Other medical conditions,1 such as the rare familial fatal insomnia, neurological conditions1 such as encephalitis,8 epilepsy,8 Alzheimer’s disease or other types of dementia,8 Parkinson’s disease,1 or multiple sclerosis,1,18 can cause excessive daytime fatigue by causing secondary insomnia or hypersomnia.
Treating the underlying disorder is an important first step in these cases. In addition, coordinating with neurologists or other specialists involved in caring for patients with these conditions is important. Regularly reviewing and simplifying the often complex medication regimen, when possible, can go a long way in mitigating EDS in this population.
Other disorders affecting sleep. Restless legs syndrome and periodic limb movement disorder are other causes of EDS.3 Treatment involves lifestyle modifications, iron supplementation in certain patients, and use of dopaminergic agents such as ropinirole, pramipexole, and other medications, depending on severity of the condition, comorbidities, and other factors.20
Alcohol or substance use. Substance use or withdrawal can be associated with sleep disorders, such as hypersomnia,19 insomnia,19 and related EDS.5 For example, alcohol use disorder affects REM sleep, and can cause EDS. Secondary central apnea can be the result of long-standing opioid use19 and can present like EDS.
Insomnia. Primary insomnia rarely causes EDS.5 Insomnia due to a medical or psychiatric condition may be an indirect cause of EDS by causing sleep deprivation.
Steps for timely and accurate diagnosis
Utilize the following steps for facilitating timely diagnosis and treatment of EDS:
Thorough history. Patients often describe “tiredness” instead of sleepiness.8 Therefore, the astute psychiatrist should explore further when patients are presenting with this concern, especially by asking more specific questions such as the tendency to doze off during daytime.8
Family members can be vital sources for obtaining a complete history,5 especially because patients might deny,8 minimize, or not be fully aware1 of the extent of their symptoms. Asking family members about patient’s snoring, irregular breathing, or gasping at night can be particularly valuable.5 Obtaining a family history of sleep disorders can be particularly important, especially in conditions such as OSA and narcolepsy.
Asking about any history of safety issues,8 including sleepiness during driving, cooking, or other activities, is also important.
Use of scales and other screening measures. Psychiatrists can use initial screening measures in the office setting. Epworth Sleepiness Scale15,21 is a validated,2 short, self-administered measure to assess the level of daytime sleepiness; however, it has some limitations such as not being able to measure changes in sleepiness from hour to hour or day to day. Because of its limitations, the Epworth Sleepiness Scale should not be used by itself as a diagnostic tool.3 It has been commonly used for detecting OSA2 and narcolepsy. The Stanford Sleepiness Scale is a self-rating scale that measures the subjective degree of sleepiness and alertness; it has limitations as well, such as having little correlation with chronic sleep loss.8 Other tools such as visual analogue scales also could be helpful.8 For more specialized testing, such as Multiple Sleep Latency Test or polysomnography, referral to a sleep specialist is ideal.8
Education. The assessment is an opportunity for the psychiatrist to educate patients about sleep hygiene, the importance of regular bedtimes, and getting adequate sleep to avoid accumulating a sleep deficit.
Urgent referral of at-risk populations. Prompt or urgent referral of at-risk populations, such as geriatric patients or those with a history of dozing off during driving, is invaluable in preventing morbidity and mortality from untreated sleep disorders.
Patients with severe daytime sleepiness should be advised to not drive or operate heavy machinery until this condition is adequately controlled.18
Bottom Line
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.
1. Chervin RD. Approach to the patient with excessive daytime sleepiness. http://www.uptodate.com/contents/approach-to-the-patient-with-excessive-daytime-sleepiness. Updated January 2016. Accessed June 5, 2017.
2. Lee CH, Ng WY, Hau W, et al. Excessive daytime sleepiness is associated with longer culprit lesion and adverse outcomes in patients with coronary artery disease. J Clin Sleep Med. 2013;9(12):1267-1272.
3. Hawley CJ, Gale TM, Sivakumaran T, et al. Excessive daytime sleepiness in psychiatric disorders: prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1-2):138-141.
4. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54(1):61-69.
5. Krahn LE. Excessive daytime sleepiness: diagnosing the causes. Current Psychiatry. 2002;1(1):49-57.
6. Ejaz SM, Khawaja IS, Bhatia S, et al. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8(8):17-25.
7. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
8. Guilleminault C, Brooks SN. Excessive daytime sleepiness: a challenge for the practising neurologist. Brain. 2001;124(pt 8):1482-1491.
9. Cheng P, Casement M, Chen CF, et al. Sleep disordered breathing in major depressive disorder. J Sleep Res. 2013;22(4):459-462.
10. Schröder CM, O’Hara R. Depression and obstructive sleep apnea (OSA). Ann Gen Psychiatry. 2005;4:13.
11. Bardwell WA, Berry CC, Ancoli-Israel S, et al. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47(6):583-596.
12. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631-638.
13. Chellappa SL, Araújo JF. Excessive daytime sleepiness in patients with depressive disorder. Rev Bras Psiquiatr. 2006;28(2):126-129.
14. Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11(6):552-557.
15. Lundt L. Use of the Epworth Sleepiness Scale to evaluate the symptom of excessive sleepiness in major depressive disorder. Gen Hosp Psychiatry. 2005;27(2):146-148.
16. Hawley CJ. Excessive daytime sleepiness in psychiatry: a relevant focus for clinical attention and treatment? Int J Psychiatry Clin Pract. 2006;10(2):117-123.
17. Dodson ER, Zee PC. Therapeutics for circadian rhythm sleep disorders. Sleep Med Clin. 2010;5(4):701-715.
18. Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705-1711.
19. Thorpy MJ. Classification of sleep disorders. Neurotherapeutics. 2012;9(4):687-701.
20. National Institute of Neurological Disorders and Stroke. Restless legs syndrome information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Restless-Legs-Syndrome-Information-Page. Accessed June 2, 2017.
21. Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376-381.