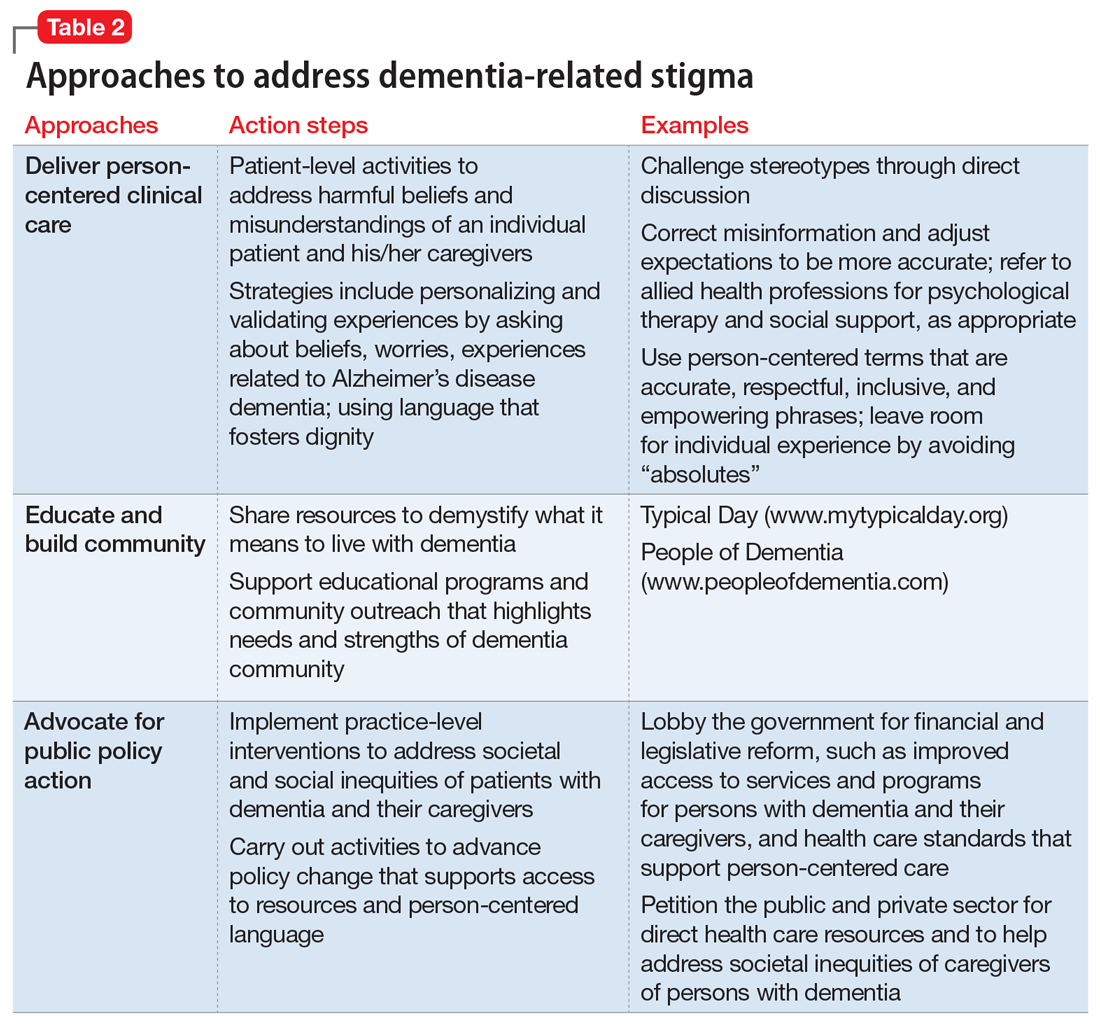
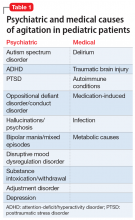
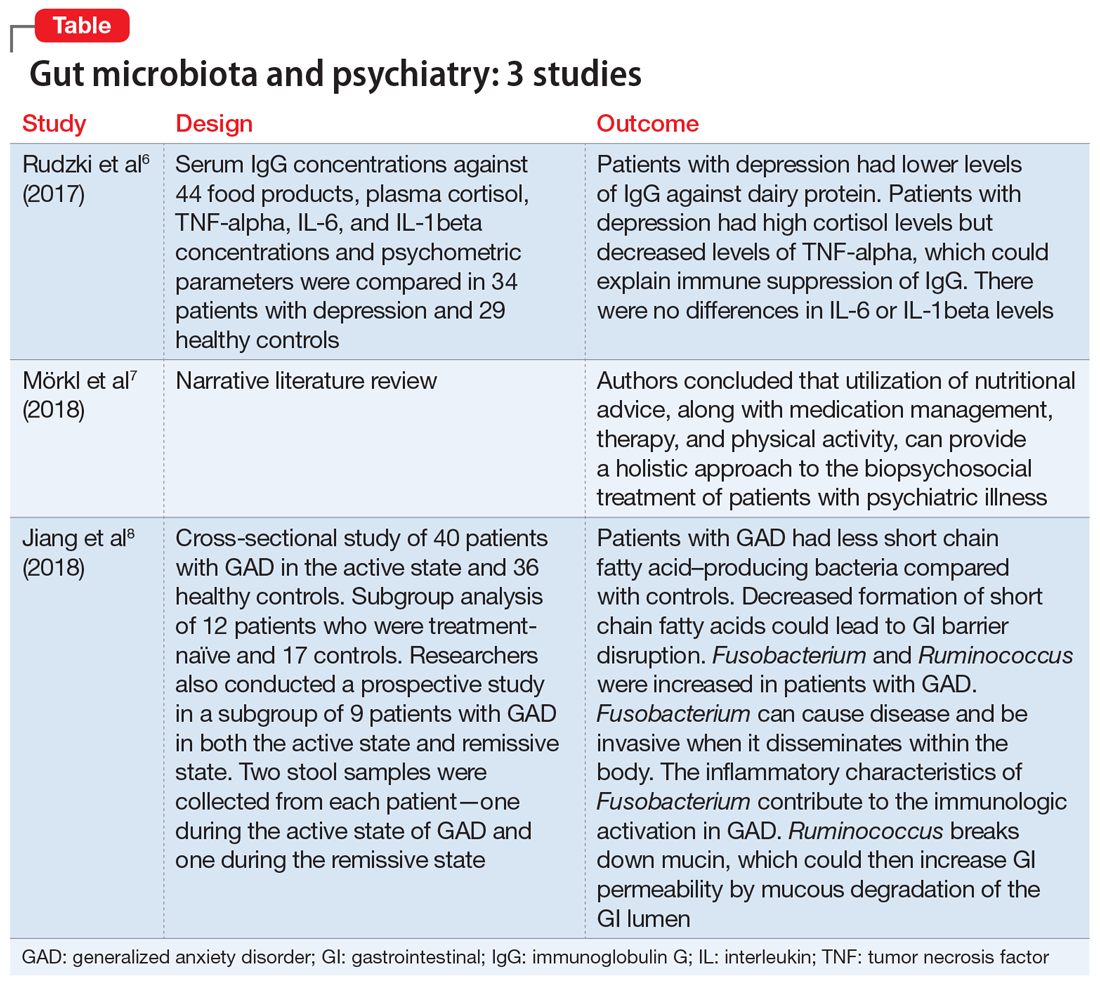
User login
Treatment of delirium: A review of 3 studies
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
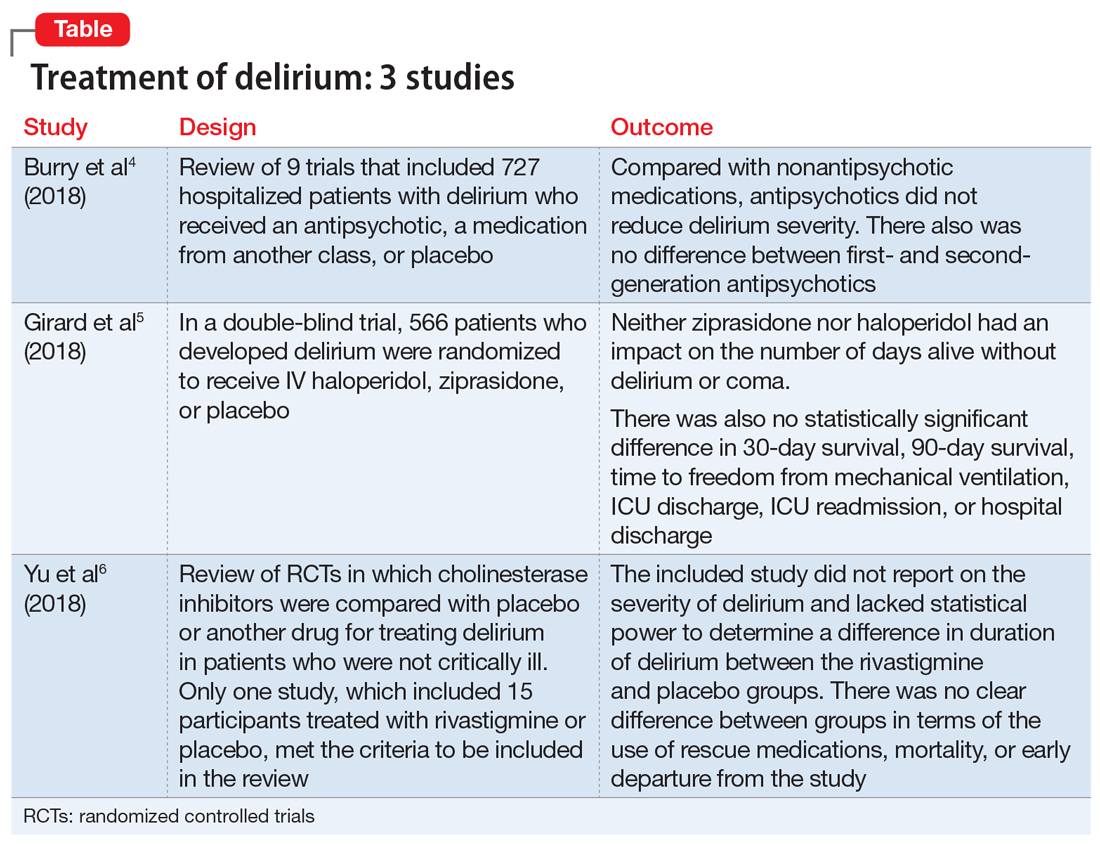
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Nothing to sneeze at: Upper respiratory infections and mood disorders
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
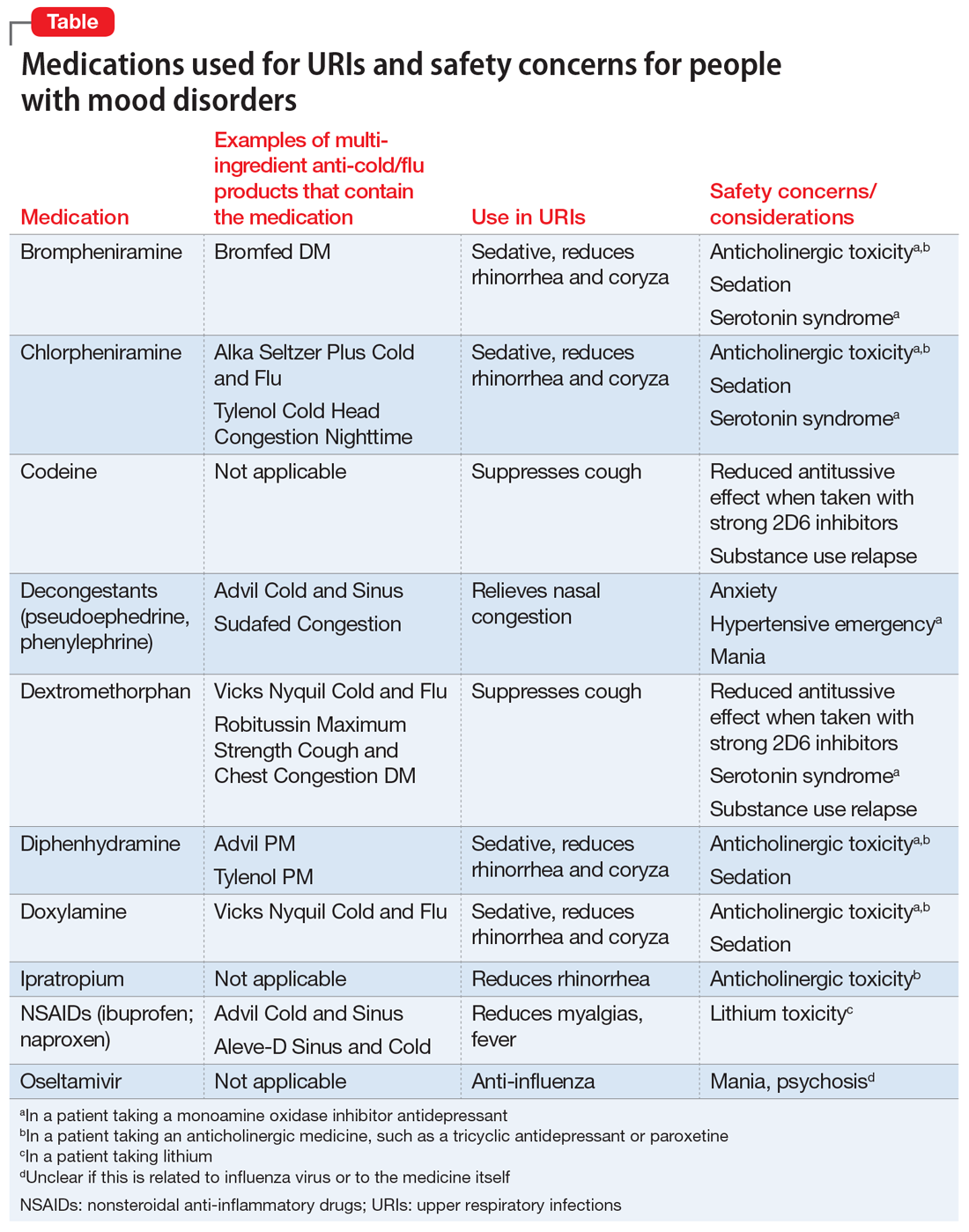
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Stigma in dementia: It’s time to talk about it
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).
Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).
Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).
Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Caring for patients on probation or parole
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
Mr. A, age 35, presents to your outpatient community mental health practice. He has a history of psychosis that began in his late teens. Since then, his symptoms have included derogatory auditory hallucinations, a recurrent persecutory delusion that governmental agencies are tracking his movements, and intermittent disorganized speech. At age 30, Mr. A assaulted a stranger out of fear that the individual was a government agent. He was arrested and experienced a severe psychotic decompensation while awaiting trial. He was found incompetent to stand trial and sent to a state hospital for restoration.
After 6 months of treatment and observation, Mr. A was deemed competent to proceed and returned to jail. He was subsequently convicted of assault and sentenced to 7 years in prison. While in prison, he received regular mental health care with infrequent recurrence of minor psychotic symptoms. He was released on parole due to his good behavior, but as part of his conditions of parole, he was mandated to follow up with an outpatient mental health clinician.
After telling you the story of how he ended up in your office, Mr. A says he needs you to speak regularly with his parole officer to verify his attendance at appointments and to discuss any mental health concerns you may have. Since you have not worked with a patient on parole before, your mind is full of questions: What are the expectations regarding your communication with his parole officer? Could Mr. A return to prison if you express concerns about his mental health? What can you do to improve his chances of success in the community?
Given the high rates of mental illness among individuals incarcerated in the United States, it shouldn’t be surprising that there are similarly high rates of mental illness among those on supervised release from jails and prisons. Clinicians who work with patients on community release need to understand basic concepts related to probation and parole, and how to promote patients’ stability in the community to reduce recidivism and re-incarceration. The court may require individuals on probation or parole to adhere to certain conditions of release, which could include seeing a psychiatrist or psychotherapist, participating in substance abuse treatment, and/or taking psychotropic medication. The court usually closely monitors the probationer or parolee’s adherence, and noncompliance can be grounds for probation or parole violation and revocation.
This article reviews the concepts of probation and parole (Box1,2), describes the prevalence of mental illness among probationers and parolees, and discusses the unique challenges and opportunities psychiatrists and other mental health professionals face when working with individuals on community supervision.
Box
The US Bureau of Justice Statistics (BJS) defines probation as a “court-ordered period of correctional supervision in the community, generally as an alternative to incarceration.” Probation allows individuals to be released from jail to community supervision, with the potential for dismissal or lowering of charges if they adhere to the conditions of probation. Conditions of probation may include participating in substance abuse or mental health treatment programs, abstaining from drugs and alcohol, and avoiding contact with known felons. Failure to comply with conditions of probation can lead to re-incarceration and probation revocation.1 If probation is revoked, a probationer may be sentenced, potentially to prison, depending on the severity of the original offense.2
The BJS defines parole as “a period of conditional supervised release in the community following a term in state or federal prison.”2 Parole allows for the community supervision of individuals who have already been convicted of and sentenced to prison for a crime. Individuals may be released on parole if they demonstrate good behavior while incarcerated. Similar to probationers, parolees must adhere to the conditions of parole, and violation of these may lead to re-incarceration.1
As of December 31, 2016, there were more than 4.5 million adults on community supervision in the United States, representing 1 out of every 55 adults in the US population. Individuals on probation accounted for 81% of adults on community supervision. The number of people on community supervision has dropped continuously over the last decade, a trend driven by 2% annual decreases in the probation population. In contrast, the parolee population has continued to grow over time and was approximately 900,000 individuals at the end of 2016.2
Mental illness among probationers and parolees
Research on mental illness in people involved in the criminal justice system has largely focused on those who are incarcerated. Studies have documented high rates of severe mental illness (SMI), such as schizophrenia and bipolar disorder, among those who are incarcerated; some estimate the rates to be 3 times as high as those of community samples.3,4 In addition to SMI, substance use disorders and personality disorders (in particular, antisocial personality disorder) are common among people who are incarcerated.5,6
Comparatively little is known about mental illness among probationers and parolees, although presumably there would be a similarly high prevalence of SMI, substance use disorders, and other psychiatric disorders among this population. A 1997 Bureau of Justice Statistics (BJS) survey of approximately 3.4 million probationers found that 13.8% self-reported a mental or emotional condition and 8.2% self-reported a history of an “overnight stay in a mental hospital.”7 The BJS estimated that there were approximately 550,000 probationers with mental illness in the United States. The study’s author noted that probationers with mental illness were more likely to have a history of prior offenses and more likely to be violent recidivists. In terms of substance use, compared with other probationers, those with mental illness were more likely to report using drugs in the month before their most recent offense and at the time of the offense.7
Continue to: More recent research...
More recent research, although limited, has shed some light on the role of mental health services for individuals on probation and parole. In 2009, Crilly et al8 reported that 23% of probationers reported accessing mental health services within the past year. Other studies have found that probationer and parolee engagement in mental health care reduces the risk of recidivism.9,10 A 2011 study evaluated 100 individuals on probation and parole in 2 counties in a southeastern state. The authors found that 75% of participants reported that they needed counseling for a mental health concern in the past year, but that only approximately 30% of them actually sought help. Individuals reporting higher levels of posttraumatic stress disorder symptomatology or greater drug use before being on probation or parole were more likely to seek counseling in the past year.11
An alternative: Problem-solving courts
Problem-solving courts (PSCs) offer an alternative to standard probation and/or sentencing. Problem-solving courts are founded on the concept of therapeutic jurisprudence, which seeks to change “the behavior of litigants and [ensure] the future well-being of communities.”12 Types of PSCs include drug court (the most common type in the United States), domestic violence court, veterans court, and mental health court (MHC), among others.
An individual may choose a PSC over standard probation because participants usually receive more assistance in obtaining treatment and closer supervision with an emphasis on rehabilitation rather than incapacitation or retribution. The success of PSCs relies heavily on the judge, as he/she plays a pivotal role in developing relationships with the participants, considering therapeutic alternatives to “bad” behaviors, determining sanctions, and relying on community mental health partners to assist participants in complying with conditions of the court.13-15
Psychiatrists and other mental health clinicians should be aware of MHCs, which are a type of PSC that provides for the community supervision of individuals with mental illness. Mental health courts vary in terms of eligibility criteria. Some accept individuals who merely report a history of mental illness, whereas others have specific diagnostic requirements.16 Some accept individuals accused of minor violations such as ordinance violations or misdemeanor offenses, while others accept individuals accused of felonies. Like other PSCs, participation in an MHC is voluntary, and most require a participant to enter a guilty plea upon entry.17 Participants may choose to enter an MHC to avoid prison time or to reduce or expunge charges after completing the program. Many MHCs also assign a probation officer to follow the participant in the community, similar to a standard probation model. Participants are usually expected to engage in psychiatric treatment, including psychotherapy, substance abuse counseling, medication management, and other services. If they do not comply with these conditions, they face sanctions that could include jail “shock” time, enhanced supervision, or an increase in psychiatric services.
Outpatient mental health professionals play an integral role in MHCs. Depending on the model, he/she may be asked to communicate treatment recommendations, attend weekly meetings at the court, and provide suggestions for interventions when the participant relapses, recidivates, and/or decompensates psychiatrically. This collaborative model can work well and allow the clinician unique opportunities to educate the court and advocate for his/her patient. However, clinicians who participate in an MHC need to remain aware of the potential to become a de facto probation officer, and need to maintain appropriate boundaries and roles. They should ensure that the patient provides initial and ongoing consent for them to communicate with the court, and share their programmatic recommendations with the patient to preserve the therapeutic alliance.
Continue to: Challenges upon re-entering the community
Challenges upon re-entering the community
Individuals recently released from jail or prison face unique challenges when re-entering the community. An individual who has been incarcerated, particularly for months to years, has likely lost his/her job, housing, health insurance, and access to primary supports. People with mental illness with a history of incarceration have higher rates of homelessness, substance use disorders, and unemployment than those with no history of incarceration.7,18 For individuals with mental illness, these additional stressors lead to further psychiatric decompensation, recidivism, and overutilization of emergency and crisis services upon release from prison or jail. The loss of health insurance presents great challenges: when someone is incarcerated, his/her Medicaid is suspended or terminated.19 This can happen at any point during incarceration. In states that terminate rather than suspend Medicaid, former prisoners face even longer waits to re-establish access to needed health care.
The period immediately after release is a critical time for individuals to be linked with substance and mental health treatment. Binswanger et al20 found former prisoners were at highest risk of mortality in the 2 weeks following release from prison; the highest rates of death were from drug overdose, cardiovascular disease, homicide, and suicide. A subsequent study found that women were at increased risk of drug overdose and opioid-related deaths.21 One explanation for the increase in drug-related deaths is the loss of physiologic tolerance while incarcerated; however, a lack of treatment while incarcerated, high levels of stress upon re-entry, and poor linkage to aftercare also may be contributing factors. Among prisoners recently released from New York City jails, Lim et al22 found that those with a history of homelessness and previous incarceration had the highest rates of drug-related deaths and homicides in the first 2 weeks after release. Non-Hispanic white men had the highest risk of drug-related deaths and suicides. While the risk of death is greatest immediately after release, former prisoners face increased mortality from multiple causes for multiple years after release.20-22
Clinicians who work with recently released prisoners should be aware of these individuals’ risks and actively work with them and other members of the mental health team to ensure these patients have access to social services, employment training, housing, and substance use resources, including medication-assisted treatment. Patients with SMI should be considered for more intensive services, such as assertive community treatment (ACT) or even forensic ACT (FACT) services, given that FACTs have a modest impact in reducing recidivism.23
Knowing whether the patient is on probation or parole and the terms of his/her supervision can also be useful in creating and executing a collaborative treatment plan. The clinician can assist the patient in meeting conditions of probation/parole such as:
- creating a stable home plan with a permanent address
- planning routine check-ins with probation/parole officers, and
- keeping documentation of ongoing mental health and substance use treatment.
Being aware of other terms of supervision, such as abstaining from alcohol and drugs, or remaining in one’s jurisdiction, also can help the patient avoid technical violations and a return to jail or prison.
Continue to: How to best help patients on community supervision
How to best help patients on community supervision
There are some clinical recommendations when working with patients on community supervision. First, do not assume that someone who has been incarcerated has antisocial personality disorder. Behaviors primarily related to seeking or using drugs or survival-type crimes should not be considered “antisocial” without additional evidence of pervasive and persistent conduct demonstrating impulsivity, lack of empathy, dishonesty, or repeated disregard for social norms and others’ rights. To meet criteria for antisocial personality disorder, these behaviors must have begun during childhood or adolescence.
If a patient does meet criteria for antisocial personality disorder, remember that he/she may also have a psychotic, mood, substance use, or other disorder that could lead to a greater likelihood of violence, recidivism, or other poor outcomes if left untreated. Treating any co-occurring disorders could enhance the patient’s engagement with treatment. There is some evidence that certain psychotropic medications, su
In addition to promoting patients’ mental health, such efforts can prevent re-arrest and re-incarceration and make a lasting positive impact on patients’ lives.
CASE CONTINUED
Mr. A signs a release-of-information form and you call his parole officer. His parole officer states that he would like to speak with you every few months to check on Mr. A’s treatment adherence. Within a few months, you transition Mr. A from an oral antipsychotic medication to a long-acting injectable antipsychotic medication to manage his psychotic disorder. He presents on time each month to your clinic to receive the injection.
Five months later, Mr. A receives 2 weeks of “shock time” at the local county jail for “dropping a dirty urine” that was positive for cannabinoids at a meeting with his parole officer. During his time in jail, he receives no treatment and he misses his monthly long-acting injectable dose.
Continue to: Upon release...
Upon release, he demonstrates the recurrence of some mild persecutory fears and hallucinations, but you resume him on his prior treatment regimen, and he recovers.
You encourage the parole officer to notify you if Mr. A violates parole and is incarcerated so that you can speak with clinicians in the jail to ensure that Mr. A remains adequately treated while incarcerated.
In the coming years, you continue to work with Mr. A and his parole officer to manage his mental health condition and to navigate his parole requirements in order to reduce his risk of relapse and recidivism. After Mr. A completes his time on parole, you continue to see him for outpatient follow-up.
Bottom Line
Clinicians may provide psychiatric care to probationers and parolees in traditional outpatient settings or in collaboration with a mental health court (MHC) or forensic assertive community treatment team. It is crucial to be aware of the legal expectations of individuals on community supervision, as well as the unique mental health risks and challenges they face. You can help reduce probationers’ and parolees’ risk of relapse and recidivism and support their recovery in the community by engaging in collaborative treatment planning involving the patient, the court, and/or MHCs.
Related Resources
- Lamb HR. Weinberger LE. Understanding and treating offenders with serious mental illness in public sector mental health. Behav Sci Law. 2017;35(4):303-318.
- Worcester S. Mental illness and the criminal justice system: Reducing the risks. Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/173208/schizophrenia-other-psychotic-disorders/mental-illness-and-criminal. Published August 22, 2018.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
1. Bureau of Justice Statistics. FAQ detail: What is the difference between probation and parole? U.S. Department of Justice. https://www.bjs.gov/index.cfm?ty=qa&iid=324. Accessed November 17, 2018.
2. Kaeble D. Probation and parole in the United States, 2016. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/ppus16.pdf. Published April 2018. Accessed April 23, 2019.
3. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
4. Diamond, P.M., et al., The prevalence of mental illness in prison. Adm Policy Ment Health. 2001;29(1):21-40.
5. MacDonald R, Kaba F, Rosner Z, et al. The Rikers Island hot spotters: defining the needs of the most frequently incarcerated. Am J Public Health. 2015;105(11):2262-2268.
6. Trestman RL, Ford J, Zhang W, et al. Current and lifetime psychiatric illness among inmates not identified as acutely mentally ill at intake in Connecticut’s jails. J Am Acad Psychiatry Law. 2007;35(4):490-500.
7. Ditton PM. Bureau of Justice Statistics special report: mental health and treatment of inmates and probationers. U.S. Department of Justice. https://www.bjs.gov/content/pub/pdf/mhtip.pdf. Published July 1999. Accessed April 24, 2019.
8. Crilly JF, Caine ED, Lamberti JS, et al. Mental health services use and symptom prevalence in a cohort of adults on probation. Psychiatr Serv. 2009;60(4):542-544.
9. Herinckx HA, Swart SC, Ama SM, et al. Rearrest and linkage to mental health services among clients of the Clark County mental health court program. Psychiatr Serv. 2005;56(7):853-857.
10. Solomon P, Draine J, Marcus SC. Predicting incarceration of clients of a psychiatric probation and parole service. Psychiatr Serv. 2002;53(1):50-56.
11. Owens GP, Rogers SM, Whitesell AA. Use of mental health services and barriers to care for individuals on probation or parole. J Offender Rehabil. 2011;50(1):35-47.
12. Berman G, Feinblatt J. Problem‐solving courts: a brief primer. Law and Policy. 2001;23(2):126.
13. The Council of State Governments Justice Center. Mental health courts: a guide to research-informed policy and practice. U.S. Department of Justice. https://www.bja.gov/Publications/CSG_MHC_Research.pdf. Published 2009. Accessed November 22, 2018.
14. Landess J, Holoyda B. Mental health courts and forensic assertive community treatment teams as correctional diversion programs. Behav Sci Law. 2017;35(5-6):501-511.
15. Sammon KC. Therapeutic jurisprudence: an examination of problem‐solving justice in New York. Journal of Civil Rights and Economic Development. 2008;23:923.
16. Sarteschi CM, Vaughn MG, Kim, K. Assessing the effectiveness of mental health courts: a quantitative review. Journal of Criminal Justice. 2011;39(1):12-20.
17. Strong SM, Rantala RR. Census of problem-solving courts, 2012. U.S. Department of Justice, Bureau of Justice Assistance. http://www.bjs.gov/content/pub/pdf/cpsc12.pdf. Revised October 12, 2016. Accessed April 24, 2019.
18. McGuire JF, Rosenheck RA. Criminal history as a prognostic indicator in the treatment of homeless people with severe mental illness. Psychiatr Serv. 2004;55(1):42-48.
19. Families USA. Medicaid suspension policies for incarcerated people: a 50-state map. Families USA. https://familiesusa.org/product/medicaid-suspension-policies-incarcerated-people-50-state-map. Published July 2016. Accessed December 7, 2018.
20. Binswanger IA, Stern MF, Deyo RA, et al. Release from prison—a high risk of death for former inmates. N Engl J Med. 2007;356(2):157-165.
21. Binswanger IA, Blatchford PJ, Mueller SR, et al. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159(9):592-600.
22. Lim S, Seligson AL, Parvez FM, et al. Risks of drug-related death, suicide, and homicide during the immediate post-release period among people released from New York City Jails, 2001-2005. Am J Epidemiol. 2012;175(6):519-526.
23. Cusack KJ, Morrissey JP, Cuddeback GS, et al. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Ment Health J. 2010;46(4):356-363.
24. Felthous AR, Stanford MS. A proposed algorithm for the pharmacotherapy of impulsive aggression. J Am Acad Psychiatry Law. 2015:43(4);456-467.
Agitation in children and adolescents: Diagnostic and treatment considerations
Managing agitation—verbal and/or motor restlessness that often is accompanied by irritability and a predisposition to aggression or violence—can be challenging in any patient, but particularly so in children and adolescents. In the United States, the prevalence of children and adolescents presenting to an emergency department (ED) for treatment of psychiatric symptoms, including agitation, has been on the rise.1,2
Similar to the multitude of causes of fever, agitation among children and adolescents has many possible causes.3 Because agitation can pose a risk for harm to others and/or self, it is important to manage it proactively. Other than studies that focus on agitation in pediatric anesthesia, there is a dearth of studies examining agitation and its treatment in children and adolescents. There is also a scarcity of training in the management of acute agitation in children and adolescents. In a 2017 survey of pediatric hospitalists and consultation-liaison psychiatrists at 38 academic children’s hospitals in North America, approximately 60% of respondents indicated that they had received no training in the evaluation or management of pediatric acute agitation.4 In addition, approximately 54% of participants said they did not screen for risk factors for pediatric agitation, even though 84% encountered the condition at least once a month, and as often as weekly.4
This article reviews evidence on the causes and treatments of agitation in children and adolescents. For the purposes of this review, child refers to a patient age 6 to 12, and adolescent refers to a patient age 13 to 17.
Identifying the cause
Addressing the underlying cause of agitation is essential. It’s also important to manage acute agitation while the underlying cause is being investigated in a way that does not jeopardize the patient’s emotional or physical safety.
Agitation in children or teens can be due to psychiatric causes such as autism, attention-deficit/hyperactivity disorder (ADHD), or posttraumatic stress disorder (PTSD), or due to medical conditions such as delirium, traumatic brain injury, or other conditions (Table 1).
In a 2005 study of 194 children with agitation in a pediatric post-anesthesia care unit, pain (27%) and anxiety (25%) were found to be the most common causes of agitation.3 Anesthesia-related agitation was a less common cause (11%). Physiologic anomalies were found to be the underlying cause of agitation in only 3 children in this study, but were undiagnosed for a prolonged period in 2 of these 3 children, which highlights the importance of a thorough differential diagnosis in the management of agitation in children.3
Assessment of an agitated child should include a comprehensive history, physical exam, and laboratory testing as indicated. When a pediatric patient comes to the ED with a chief presentation of agitation, a thorough medical and psychiatric assessment should be performed. For patients with a history of psychiatric diagnoses, do not assume that the cause of agitation is psychiatric.
Continue to: Psychiatric causes
Psychiatric causes
Autism spectrum disorder. Children and teens with autism often feel overwhelmed due to transitions, changes, and/or sensory overload. This sensory overload may be in response to relatively subtle sensory stimuli, so it may not always be apparent to parents or others around them.
Research suggests that in general, the ability to cope effectively with emotions is difficult without optimal language development. Due to cognitive/language delays and a related lack of emotional attunement and limited skills in recognizing, expressing, or coping with emotions, difficult emotions in children and adolescents with autism can manifest as agitation.
Attention-deficit/hyperactivity disorder. Children with ADHD may be at a higher risk for agitation, in part due to poor impulse control and limited coping skills. In addition, chronic negative feedback (from parents, teachers, or both) may contribute to low self-esteem, mood symptoms, defiance, and/or other behavioral difficulties. In addition to standard pharmacotherapy for ADHD, treatment involves parent behavior modification training. Setting firm yet empathic limits, “picking battles,” and implementing a developmentally appropriate behavioral plan to manage disruptive behavior in children or adolescents with ADHD can go a long way in helping to prevent the emergence of agitation.
Posttraumatic stress disorder. In some young children, new-onset, unexplained agitation may be the only sign of abuse or trauma. Children who have undergone trauma tend to experience confusion and distress. This may manifest as agitation or aggression, or other symptoms such as increased anxiety or nightmares.5 Trauma may be in the form of witnessing violence (domestic or other); experiencing physical, sexual, and/or emotional abuse; or witnessing/experiencing other significant threats to the safety of self and/or loved ones. Re-establishing (or establishing) a sense of psychological and physical safety is paramount in such patients.6 Psychotherapy is the first-line modality of treatment in children and adolescents with PTSD.6 In general, there is a scarcity of research on medication treatments for PTSD symptoms among children and adolescents.6
Oppositional defiant disorder/conduct disorder. Oppositional defiant disorder (ODD) can be comorbid with ADHD. The diagnosis of ODD requires a pervasive pattern of anger, defiance, vindictiveness, and hostility, particularly towards authority figures. However, these symptoms need to be differentiated from the normal range of childhood behavior. Occasionally, children learn to cope maladaptively through disruptive behavior or agitation. Although a parent or caregiver may see this behavior as intentionally malevolent, in a child with limited coping skills (whether due to young age, developmental/cognitive/language/learning delays, or social communication deficits) or one who has witnessed frequent agitation or aggression in the family environment, agitation and disruptive behavior may be a maladaptive form of coping. Thus, diligence needs to be exercised in the diagnosis of ODD and in understanding the psychosocial factors affecting the child, particularly because impulsiveness and uncooperativeness on their own have been found to be linked to greater likelihood of prescription of psychotropic medications from multiple classes.7 Family-based interventions, particularly parent training, family therapy, and age-appropriate child skills training, are of prime importance in managing this condition.8 Research shows that a shortage of resources, system issues, and cultural roadblocks in implementing family-based psychosocial interventions also can contribute to the increased use of psychotropic medications for aggression in children and teens with ODD, conduct disorder, or ADHD.8 The astute clinician needs to be cognizant of this before prescribing.
Continue to: Hallucinations/psychosis
Hallucinations/psychosis. Hallucinations (whether from psychiatric or medical causes) are significantly associated with agitation.9 In particular, auditory command hallucinations have been linked to agitation. Command hallucinations in children and adolescents may be secondary to early-onset schizophrenia; however, this diagnosis is rare.10 Hallucinations can also be an adverse effect of amphetamine-based stimulant medications in children and adolescents. Visual hallucinations are most often a sign of an underlying medical disorder such as delirium, occipital lobe mass/infection, or drug intoxication or withdrawal. Hallucinations need to be distinguished from the normal, imaginative play of a young child.10
Bipolar mania. In adults, bipolar disorder is a primary psychiatric cause of agitation. In children and adolescents, the diagnosis of bipolar disorder can be complex and requires careful and nuanced history-taking. The risks of agitation are greater with bipolar disorder than with unipolar depression.11,12
Disruptive mood dysregulation disorder. Prior to DSM-5, many children and adolescents with chronic, non-episodic irritability and severe outbursts out of proportion to the situation or stimuli were given a diagnosis of bipolar disorder. These symptoms, in combination with other symptoms, are now considered part of disruptive mood dysregulation disorder when severe outbursts in a child or adolescent occur 3 to 4 times a week consistently, for at least 1 year. The diagnosis of disruptive mood dysregulation disorder requires ruling out other psychiatric and medical conditions, particularly ADHD.13
Substance intoxication/withdrawal. Intoxication or withdrawal from substances such as alcohol, stimulant medications, opioids, methamphetamines, and other agents can lead to agitation. This is more likely to occur among adolescents than children.14
Adjustment disorder. Parental divorce, especially if it is conflictual, or other life stressors, such as experiencing a move or frequent moves, may contribute to the development of agitation in children and adolescents.
Continue to: Depression
Depression. In children and adolescents, depression can manifest as anger or irritability, and occasionally as agitation.
Medical causes
Delirium. Refractory agitation is often a manifestation of delirium in children and adolescents.15 If unrecognized and untreated, delirium can be fatal.16 Therefore, it is imperative that clinicians routinely assess for delirium in any patient who presents with agitation.
Because a patient with delirium often presents with agitation and visual or auditory hallucinations, the medical team may tend to assume these symptoms are secondary to a psychiatric disorder. In this case, the role of the consultation-liaison psychiatrist is critical for guiding the medical team, particularly to continue a thorough exploration of underlying causes while avoiding polypharmacy. Noise, bright lights, frequent changes in nursing staff or caregivers, anticholinergic or benzodiazepine medications, and frequent changes in schedules should be avoided to prevent delirium from occurring or getting worse.17 A multidisciplinary team approach is key in identifying the underlying cause and managing delirium in pediatric patients.
Traumatic brain injury. Agitation may be a presenting symptom in youth with traumatic brain injury (TBI).18 Agitation may present often in the acute recovery phase.19 There is limited evidence on the efficacy and safety of pharmacotherapy for agitation in pediatric patients with TBI.18
Autoimmune conditions. In a study of 27 patients with
Continue to: Medication-induced/iatrogenic
Medication-induced/iatrogenic. Agitation can be an adverse effect of medications such as amantadine (often used for TBI),18 atypical antipsychotics,21 selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors.
Infection. Agitation can be a result of encephalitis, meningitis, or other infectious processes.22
Metabolic conditions. Hepatic or renal failure, diabetic ketoacidosis, and thyroid toxicosis may cause agitation in children or adolescents.22
Start with nonpharmacologic interventions
Few studies have examined de-escalation techniques in agitated children and adolescents. However, verbal de-escalation is generally viewed as the first-line technique for managing agitation in children and adolescents. When feasible, teaching and modeling developmentally appropriate stress management skills for children and teens can be a beneficial preventative strategy to reduce the incidence and worsening of agitation.23
Clinicians should refrain from using coercion.24 Coercion could harm the therapeutic alliance, thereby impeding assessment of the underlying causes of agitation, and can be particularly harmful for patients who have a history of trauma or abuse. Even in pediatric patients with no such history, coercion is discouraged due to its punitive connotations and potential to adversely impact a vulnerable child or teen.
Continue to: Establishing a therapeutic rapport...
Establishing a therapeutic rapport with the patient, when feasible, can facilitate smoother de-escalation by offering the patient an outlet to air his/her frustrations and emotions, and by helping the patient feel understood.24 To facilitate this, ensure that the patient’s basic comforts and needs are met, such as access to a warm bed, food, and safety.25
The psychiatrist’s role is to help uncover and address the underlying reason for the patient’s agony or distress. Once the child or adolescent has calmed, explore potential triggers or causes of the agitation.
There has been a significant move away from the use of restraints for managing agitation in children and adolescents.26 Restraints have a psychologically traumatizing effect,27 and have been linked to life-threatening injuries and death in children.24
Pharmacotherapy: Proceed with caution
There are no FDA-approved medications for the treatment of agitation in the general pediatric population, and any medication use in this population is off-label. There is also a dearth of research examining the safety and efficacy of using psychotropic medications for agitation in pediatric patients. Because children and adolescents are more susceptible to adverse effects and risks associated with the use of psychotropic medications, special caution is warranted. In general, pharmacologic interventions are not recommended without the use of psychotherapy-based modalities.
In the past, the aim of using medications to treat patients with agitation was to put the patient to sleep.25 This practice did not help clinicians to assess for underlying causes, and was often accompanied by a greater risk of adverse effects and reactions.24 Therefore, the goal of medication treatment for agitation is to help calm the patient instead of inducing sleep.25
Continue to: Pharmacotherapy should...
Pharmacotherapy should be used only when behavioral interventions have been unsuccessful. Key considerations for using psychotropic medications to address agitation in children and adolescents are summarized in Table 2.25
Antipsychotics, particularly second-generation antipsychotics (SGAs), have been commonly used to manage acute agitation in children and adolescents, and there has been an upswing in the use of these medications in the United States in the last several years.28 Research indicates that males, children and adolescents in foster care, and those with Medicaid have been the more frequent youth recipients of SGAs.29 Of particular concern is the prevalence of antipsychotic use among children younger than age 6. In the last few decades, there has been an increase in the prescription of antipsychotics for children younger than age 6, particularly for disruptive behavior and aggression.30 In a study of preschool-age Medicaid patients in Kentucky, 70,777 prescriptions for SGAs were given to 6,915 children <6 years of age; 73% of these prescriptions were for male patients.30 Because there is a lack of controlled studies examining the safety and efficacy of SGAs among children and adolescents, especially with long-term use, further research is needed and caution is warranted.28
The FDA has approved
Externalizing disorders among children and adolescents tend to get treated with antipsychotics.28 A Canadian study examining records of 6,916 children found that most children who had been prescribed risperidone received it for ADHD or conduct disorder, and most patients had not received laboratory testing for monitoring the antipsychotic medication they were taking.31 In a 2018 study examining medical records of 120 pediatric patients who presented to an ED in British Columbia with agitation, antipsychotics were the most commonly used medications for patients with autism spectrum disorder; most patients received at least 1 dose.14
For children and adolescents with agitation or aggression who were admitted to inpatient units, IM
Continue to: In case reports...
In case reports, a combination of olanzapine with CNS-suppressing agents has resulted in death. Therefore, do not combine olanzapine with agents such as benzodiazepines.25 In a patient with a likely medical source of agitation, insufficient evidence exists to support the use of olanzapine, and additional research is needed.25
Low-dose haloperidol has been found to be effective for delirium-related agitation in pediatric studies.15 Before initiating an antipsychotic for any child or adolescent, review the patient’s family history for reports of early cardiac death and the patient’s own history of cardiac symptoms, palpitations, syncope, or prolonged QT interval. Monitor for QT prolongation. Among commonly used antipsychotics, the risk of QT prolongation is higher with IV administration of haloperidol and with ziprasidone. Studies show that compared with oral or IM
A few studies have found risperidone to be efficacious for treating ODD and conduct disorder; however, this use is off-label, and its considerable adverse effect and risk profile needs to be weighed against the potential benefit.8
Antipsychotic polypharmacy should be avoided because of the higher risk of adverse effects and interactions, and a lack of robust, controlled studies evaluating the safety of using antipsychotics for non-FDA-approved indications in children and adolescents.7 All patients who receive antipsychotics require monitoring for extrapyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, orthostatic hypotension, sedation, metabolic syndrome, and other potential adverse effects. Patients receiving risperidone need to have their prolactin levels monitored periodically, and their parents should be made aware of the potential for hyperprolactinemia and other adverse effects. Aripiprazole and quetiapine may increase the risk of suicidality.
Antiepileptics. A meta-analysis of 7 randomized controlled trials examining the use of antiepileptic medications (
Continue to: In a retrospective case series...
In a retrospective case series of 30 pediatric patients with autism spectrum disorder who were given oxcarbazepine, Douglas et al35 found that 47% of participants experienced significant improvement in irritability/agitation. However, 23% of patients reported significant adverse effects leading to discontinuation. Insufficient evidence exists for the safety and efficacy of oxcarbazepine in this population.35
Benzodiazepines. The use of benzodiazepines in pediatric patients has been associated with paradoxical disinhibition reactions, particularly in children with autism and other developmental or cognitive disabilities or delays.21 There is a lack of data on the safety and efficacy of long-term use of benzodiazepines in children, especially in light of these patients’ developing brains, the risk of cognitive impairment, and the potential for dependence with long-term use. Despite this, some studies show that the use of benzodiazepines is fairly common among pediatric patients who present to the ED with agitation.14 In a recent retrospective study, Kendrick et al14 found that among pediatric patients with agitation who were brought to the ED, benzodiazepines were the most commonly prescribed medications.
Other medications. Clonidine and
Diphenhydramine, in both oral and IM forms, has been used to treat agitation in children,32 but has also been associated with a paradoxical disinhibition reaction in pediatric patients21 and therefore should be used only sparingly and with caution. Diphenhydramine has anticholinergic properties, and may worsen delirium.15 Stimulant medications can help aggressive behavior in children and adolescents with ADHD.37
Bottom Line
Agitation among children and adolescents has many possible causes. A combination of a comprehensive assessment and evidence-based, judicious treatment interventions can help prevent and manage agitation in this vulnerable population.
Related Resources
- Baker M, Carlson GA. What do we really know about PRN use in agitated children with mental health conditions: a clinical review. Evid Based Ment Health. 2018;21(4):166-170.
- Gerson R, Malas N, Mroczkowski MM. Crisis in the emergency department: the evaluation and management of acute agitation in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2018;27(3):367-386.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Clonidine • Catapres
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra, Spritam
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Quetiapine • Seroquel
Topiramate • Topamax
Risperidone • Risperdal
Valproate • Depakene
Ziprasidone • Geodon
1. Frosch E, Kelly P. Issues in pediatric psychiatric emergency care. In: Emergency psychiatry. Cambridge, UK: Cambridge University Press; 2011:185-199.
2. American College of Emergency Physicians. Pediatric mental health emergencies in the emergency department. https://www.acep.org/patient-care/policy-statements/pediatric-mental-health-emergencies-in-the-emergency-medical-services-system/. Revised September 2018. Accessed February 23, 2019.
3. Voepel-Lewis, T, Burke C, Hadden S, et al. Nurses’ diagnoses and treatment decisions regarding care of the agitated child. J Perianesth Nurs. 2005;20(4):239-248.
4. Malas N, Spital L, Fischer J, et al. National survey on pediatric acute agitation and behavioral escalation in academic inpatient pediatric care settings. Psychosomatics. 2017;58(3):299-306.
5. Famularo R, Kinscherff R, Fenton T. Symptom differences in acute and chronic presentation of childhood post-traumatic stress disorder. Child Abuse Negl. 1990;14(3):439-444.
6. Kaminer D, Seedat S, Stein DJ. Post-traumatic stress disorder in children. World Psychiatry. 2005;4(2):121-125.
7. Ninan A, Stewart SL, Theall LA, et al. Adverse effects of psychotropic medications in children: predictive factors. J Can Acad Child Adolesc Psychiatry. 2014;23(3):218-225.
8. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52-61.
9. Vareilles D, Bréhin C, Cortey C, et al. Hallucinations: Etiological analysis of children admitted to a pediatric emergency department. Arch Pediatr. 2017;24(5):445-452.
10. Bartlett J. Childhood-onset schizophrenia: what do we really know? Health Psychol Behav Med. 2014;2(1):735-747.
11. Diler RS, Goldstein TR, Hafeman D, et al. Distinguishing bipolar depression from unipolar depression in youth: Preliminary findings. J Child Adolesc Psychopharmacol. 2017;27(4):310-319.
12. Dervic K, Garcia-Amador M, Sudol K, et al. Bipolar I and II versus unipolar depression: clinical differences and impulsivity/aggression traits. Eur Psychiatry. 2015;30(1):106-113.
13. Masi L, Gignac M ADHD and DMDD comorbidities, similarities and distinctions. J Child Adolesc Behav2016;4:325.
14. Kendrick JG, Goldman RD, Carr RR. Pharmacologic management of agitation and aggression in a pediatric emergency department - a retrospective cohort study. J Pediatr Pharmacol Ther. 2018;23(6):455-459.
15. Schieveld JN, Staal M, Voogd L, et al. Refractory agitation as a marker for pediatric delirium in very young infants at a pediatric intensive care unit. Intensive Care Med. 2010;36(11):1982-1983.
16. Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med. 2017;45(5):891-898.
17. Bettencourt A, Mullen JE. Delirium in children: identification, prevention, and management. Crit Care Nurse. 2017;37(3):e9-e18.
18. Suskauer SJ, Trovato MK. Update on pharmaceutical intervention for disorders of consciousness and agitation after traumatic brain injury in children. PM R. 2013;5(2):142-147.
19. Nowicki M, Pearlman L, Campbell C, et al. Agitated behavior scale in pediatric traumatic brain injury. Brain Inj. 2019. doi: 10.1080/02699052.2019.1565893.
20. Mohammad SS, Jones H, Hong M, et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev Med Child Neurol. 2016;58(4):376-384.
21. Sonnier L, Barzman D. Pharmacologic management of acutely agitated pediatric patients. Pediatr Drugs. 2011;13(1):1-10.
22. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
23. Masters KJ, Bellonci C, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):4S-25S.
24. Croce ND, Mantovani C. Using de-escalation techniques to prevent violent behavior in pediatric psychiatric emergencies: It is possible. Pediatric Dimensions, 2017;2(1):1-2.
25. Marzullo LR. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269-275.
26. Caldwell B, Albert C, Azeem MW, et al. Successful seclusion and restraint prevention effort in child and adolescent programs. J Psychosoc Nurs Ment Health Serv. 2014;52(11):30-38.
27. De Hert M, Dirix N, Demunter H, et al. Prevalence and correlates of seclusion and restraint use in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2011;20(5):221-230.
28. Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770-w781.
29. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of atypical antipsychotic medication in children and adolescents. https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf. Accessed March 4, 2019.
30. Lohr WD, Chowning RT, Stevenson MD, et al. Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol. 2015;25(5):440-443.
31. Chen W, Cepoiu-Martin M, Stang A, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449-455.
32. Deshmukh P, Kulkarni G, Barzman D. Recommendations for pharmacological management of inpatient aggression in children and adolescents. Psychiatry (Edgmont). 2010;7(2):32-40.
33. Haldol [package insert]. Beerse, Belgium: Janssen Pharmaceutica NV; 2005.
34. Hirota T, Veenstra-Vanderweele J, Hollander E, et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
35. Douglas JF, Sanders KB, Benneyworth MH, et al. Brief report: retrospective case series of oxcarbazepine for irritability/agitation symptoms in autism spectrum disorder. J Autism Dev Disord. 2013;43(5):1243-1247.
36. Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad
37. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: Psychostimulants, alpha-2 Agonists, and atomoxetine. Can J Psychiatry. 2015;60(2):42-51
Managing agitation—verbal and/or motor restlessness that often is accompanied by irritability and a predisposition to aggression or violence—can be challenging in any patient, but particularly so in children and adolescents. In the United States, the prevalence of children and adolescents presenting to an emergency department (ED) for treatment of psychiatric symptoms, including agitation, has been on the rise.1,2
Similar to the multitude of causes of fever, agitation among children and adolescents has many possible causes.3 Because agitation can pose a risk for harm to others and/or self, it is important to manage it proactively. Other than studies that focus on agitation in pediatric anesthesia, there is a dearth of studies examining agitation and its treatment in children and adolescents. There is also a scarcity of training in the management of acute agitation in children and adolescents. In a 2017 survey of pediatric hospitalists and consultation-liaison psychiatrists at 38 academic children’s hospitals in North America, approximately 60% of respondents indicated that they had received no training in the evaluation or management of pediatric acute agitation.4 In addition, approximately 54% of participants said they did not screen for risk factors for pediatric agitation, even though 84% encountered the condition at least once a month, and as often as weekly.4
This article reviews evidence on the causes and treatments of agitation in children and adolescents. For the purposes of this review, child refers to a patient age 6 to 12, and adolescent refers to a patient age 13 to 17.
Identifying the cause
Addressing the underlying cause of agitation is essential. It’s also important to manage acute agitation while the underlying cause is being investigated in a way that does not jeopardize the patient’s emotional or physical safety.
Agitation in children or teens can be due to psychiatric causes such as autism, attention-deficit/hyperactivity disorder (ADHD), or posttraumatic stress disorder (PTSD), or due to medical conditions such as delirium, traumatic brain injury, or other conditions (Table 1).
In a 2005 study of 194 children with agitation in a pediatric post-anesthesia care unit, pain (27%) and anxiety (25%) were found to be the most common causes of agitation.3 Anesthesia-related agitation was a less common cause (11%). Physiologic anomalies were found to be the underlying cause of agitation in only 3 children in this study, but were undiagnosed for a prolonged period in 2 of these 3 children, which highlights the importance of a thorough differential diagnosis in the management of agitation in children.3
Assessment of an agitated child should include a comprehensive history, physical exam, and laboratory testing as indicated. When a pediatric patient comes to the ED with a chief presentation of agitation, a thorough medical and psychiatric assessment should be performed. For patients with a history of psychiatric diagnoses, do not assume that the cause of agitation is psychiatric.
Continue to: Psychiatric causes
Psychiatric causes
Autism spectrum disorder. Children and teens with autism often feel overwhelmed due to transitions, changes, and/or sensory overload. This sensory overload may be in response to relatively subtle sensory stimuli, so it may not always be apparent to parents or others around them.
Research suggests that in general, the ability to cope effectively with emotions is difficult without optimal language development. Due to cognitive/language delays and a related lack of emotional attunement and limited skills in recognizing, expressing, or coping with emotions, difficult emotions in children and adolescents with autism can manifest as agitation.
Attention-deficit/hyperactivity disorder. Children with ADHD may be at a higher risk for agitation, in part due to poor impulse control and limited coping skills. In addition, chronic negative feedback (from parents, teachers, or both) may contribute to low self-esteem, mood symptoms, defiance, and/or other behavioral difficulties. In addition to standard pharmacotherapy for ADHD, treatment involves parent behavior modification training. Setting firm yet empathic limits, “picking battles,” and implementing a developmentally appropriate behavioral plan to manage disruptive behavior in children or adolescents with ADHD can go a long way in helping to prevent the emergence of agitation.
Posttraumatic stress disorder. In some young children, new-onset, unexplained agitation may be the only sign of abuse or trauma. Children who have undergone trauma tend to experience confusion and distress. This may manifest as agitation or aggression, or other symptoms such as increased anxiety or nightmares.5 Trauma may be in the form of witnessing violence (domestic or other); experiencing physical, sexual, and/or emotional abuse; or witnessing/experiencing other significant threats to the safety of self and/or loved ones. Re-establishing (or establishing) a sense of psychological and physical safety is paramount in such patients.6 Psychotherapy is the first-line modality of treatment in children and adolescents with PTSD.6 In general, there is a scarcity of research on medication treatments for PTSD symptoms among children and adolescents.6
Oppositional defiant disorder/conduct disorder. Oppositional defiant disorder (ODD) can be comorbid with ADHD. The diagnosis of ODD requires a pervasive pattern of anger, defiance, vindictiveness, and hostility, particularly towards authority figures. However, these symptoms need to be differentiated from the normal range of childhood behavior. Occasionally, children learn to cope maladaptively through disruptive behavior or agitation. Although a parent or caregiver may see this behavior as intentionally malevolent, in a child with limited coping skills (whether due to young age, developmental/cognitive/language/learning delays, or social communication deficits) or one who has witnessed frequent agitation or aggression in the family environment, agitation and disruptive behavior may be a maladaptive form of coping. Thus, diligence needs to be exercised in the diagnosis of ODD and in understanding the psychosocial factors affecting the child, particularly because impulsiveness and uncooperativeness on their own have been found to be linked to greater likelihood of prescription of psychotropic medications from multiple classes.7 Family-based interventions, particularly parent training, family therapy, and age-appropriate child skills training, are of prime importance in managing this condition.8 Research shows that a shortage of resources, system issues, and cultural roadblocks in implementing family-based psychosocial interventions also can contribute to the increased use of psychotropic medications for aggression in children and teens with ODD, conduct disorder, or ADHD.8 The astute clinician needs to be cognizant of this before prescribing.
Continue to: Hallucinations/psychosis
Hallucinations/psychosis. Hallucinations (whether from psychiatric or medical causes) are significantly associated with agitation.9 In particular, auditory command hallucinations have been linked to agitation. Command hallucinations in children and adolescents may be secondary to early-onset schizophrenia; however, this diagnosis is rare.10 Hallucinations can also be an adverse effect of amphetamine-based stimulant medications in children and adolescents. Visual hallucinations are most often a sign of an underlying medical disorder such as delirium, occipital lobe mass/infection, or drug intoxication or withdrawal. Hallucinations need to be distinguished from the normal, imaginative play of a young child.10
Bipolar mania. In adults, bipolar disorder is a primary psychiatric cause of agitation. In children and adolescents, the diagnosis of bipolar disorder can be complex and requires careful and nuanced history-taking. The risks of agitation are greater with bipolar disorder than with unipolar depression.11,12
Disruptive mood dysregulation disorder. Prior to DSM-5, many children and adolescents with chronic, non-episodic irritability and severe outbursts out of proportion to the situation or stimuli were given a diagnosis of bipolar disorder. These symptoms, in combination with other symptoms, are now considered part of disruptive mood dysregulation disorder when severe outbursts in a child or adolescent occur 3 to 4 times a week consistently, for at least 1 year. The diagnosis of disruptive mood dysregulation disorder requires ruling out other psychiatric and medical conditions, particularly ADHD.13
Substance intoxication/withdrawal. Intoxication or withdrawal from substances such as alcohol, stimulant medications, opioids, methamphetamines, and other agents can lead to agitation. This is more likely to occur among adolescents than children.14
Adjustment disorder. Parental divorce, especially if it is conflictual, or other life stressors, such as experiencing a move or frequent moves, may contribute to the development of agitation in children and adolescents.
Continue to: Depression
Depression. In children and adolescents, depression can manifest as anger or irritability, and occasionally as agitation.
Medical causes
Delirium. Refractory agitation is often a manifestation of delirium in children and adolescents.15 If unrecognized and untreated, delirium can be fatal.16 Therefore, it is imperative that clinicians routinely assess for delirium in any patient who presents with agitation.
Because a patient with delirium often presents with agitation and visual or auditory hallucinations, the medical team may tend to assume these symptoms are secondary to a psychiatric disorder. In this case, the role of the consultation-liaison psychiatrist is critical for guiding the medical team, particularly to continue a thorough exploration of underlying causes while avoiding polypharmacy. Noise, bright lights, frequent changes in nursing staff or caregivers, anticholinergic or benzodiazepine medications, and frequent changes in schedules should be avoided to prevent delirium from occurring or getting worse.17 A multidisciplinary team approach is key in identifying the underlying cause and managing delirium in pediatric patients.
Traumatic brain injury. Agitation may be a presenting symptom in youth with traumatic brain injury (TBI).18 Agitation may present often in the acute recovery phase.19 There is limited evidence on the efficacy and safety of pharmacotherapy for agitation in pediatric patients with TBI.18
Autoimmune conditions. In a study of 27 patients with
Continue to: Medication-induced/iatrogenic
Medication-induced/iatrogenic. Agitation can be an adverse effect of medications such as amantadine (often used for TBI),18 atypical antipsychotics,21 selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors.
Infection. Agitation can be a result of encephalitis, meningitis, or other infectious processes.22
Metabolic conditions. Hepatic or renal failure, diabetic ketoacidosis, and thyroid toxicosis may cause agitation in children or adolescents.22
Start with nonpharmacologic interventions
Few studies have examined de-escalation techniques in agitated children and adolescents. However, verbal de-escalation is generally viewed as the first-line technique for managing agitation in children and adolescents. When feasible, teaching and modeling developmentally appropriate stress management skills for children and teens can be a beneficial preventative strategy to reduce the incidence and worsening of agitation.23
Clinicians should refrain from using coercion.24 Coercion could harm the therapeutic alliance, thereby impeding assessment of the underlying causes of agitation, and can be particularly harmful for patients who have a history of trauma or abuse. Even in pediatric patients with no such history, coercion is discouraged due to its punitive connotations and potential to adversely impact a vulnerable child or teen.
Continue to: Establishing a therapeutic rapport...
Establishing a therapeutic rapport with the patient, when feasible, can facilitate smoother de-escalation by offering the patient an outlet to air his/her frustrations and emotions, and by helping the patient feel understood.24 To facilitate this, ensure that the patient’s basic comforts and needs are met, such as access to a warm bed, food, and safety.25
The psychiatrist’s role is to help uncover and address the underlying reason for the patient’s agony or distress. Once the child or adolescent has calmed, explore potential triggers or causes of the agitation.
There has been a significant move away from the use of restraints for managing agitation in children and adolescents.26 Restraints have a psychologically traumatizing effect,27 and have been linked to life-threatening injuries and death in children.24
Pharmacotherapy: Proceed with caution
There are no FDA-approved medications for the treatment of agitation in the general pediatric population, and any medication use in this population is off-label. There is also a dearth of research examining the safety and efficacy of using psychotropic medications for agitation in pediatric patients. Because children and adolescents are more susceptible to adverse effects and risks associated with the use of psychotropic medications, special caution is warranted. In general, pharmacologic interventions are not recommended without the use of psychotherapy-based modalities.
In the past, the aim of using medications to treat patients with agitation was to put the patient to sleep.25 This practice did not help clinicians to assess for underlying causes, and was often accompanied by a greater risk of adverse effects and reactions.24 Therefore, the goal of medication treatment for agitation is to help calm the patient instead of inducing sleep.25
Continue to: Pharmacotherapy should...
Pharmacotherapy should be used only when behavioral interventions have been unsuccessful. Key considerations for using psychotropic medications to address agitation in children and adolescents are summarized in Table 2.25
Antipsychotics, particularly second-generation antipsychotics (SGAs), have been commonly used to manage acute agitation in children and adolescents, and there has been an upswing in the use of these medications in the United States in the last several years.28 Research indicates that males, children and adolescents in foster care, and those with Medicaid have been the more frequent youth recipients of SGAs.29 Of particular concern is the prevalence of antipsychotic use among children younger than age 6. In the last few decades, there has been an increase in the prescription of antipsychotics for children younger than age 6, particularly for disruptive behavior and aggression.30 In a study of preschool-age Medicaid patients in Kentucky, 70,777 prescriptions for SGAs were given to 6,915 children <6 years of age; 73% of these prescriptions were for male patients.30 Because there is a lack of controlled studies examining the safety and efficacy of SGAs among children and adolescents, especially with long-term use, further research is needed and caution is warranted.28
The FDA has approved
Externalizing disorders among children and adolescents tend to get treated with antipsychotics.28 A Canadian study examining records of 6,916 children found that most children who had been prescribed risperidone received it for ADHD or conduct disorder, and most patients had not received laboratory testing for monitoring the antipsychotic medication they were taking.31 In a 2018 study examining medical records of 120 pediatric patients who presented to an ED in British Columbia with agitation, antipsychotics were the most commonly used medications for patients with autism spectrum disorder; most patients received at least 1 dose.14
For children and adolescents with agitation or aggression who were admitted to inpatient units, IM
Continue to: In case reports...
In case reports, a combination of olanzapine with CNS-suppressing agents has resulted in death. Therefore, do not combine olanzapine with agents such as benzodiazepines.25 In a patient with a likely medical source of agitation, insufficient evidence exists to support the use of olanzapine, and additional research is needed.25
Low-dose haloperidol has been found to be effective for delirium-related agitation in pediatric studies.15 Before initiating an antipsychotic for any child or adolescent, review the patient’s family history for reports of early cardiac death and the patient’s own history of cardiac symptoms, palpitations, syncope, or prolonged QT interval. Monitor for QT prolongation. Among commonly used antipsychotics, the risk of QT prolongation is higher with IV administration of haloperidol and with ziprasidone. Studies show that compared with oral or IM
A few studies have found risperidone to be efficacious for treating ODD and conduct disorder; however, this use is off-label, and its considerable adverse effect and risk profile needs to be weighed against the potential benefit.8
Antipsychotic polypharmacy should be avoided because of the higher risk of adverse effects and interactions, and a lack of robust, controlled studies evaluating the safety of using antipsychotics for non-FDA-approved indications in children and adolescents.7 All patients who receive antipsychotics require monitoring for extrapyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, orthostatic hypotension, sedation, metabolic syndrome, and other potential adverse effects. Patients receiving risperidone need to have their prolactin levels monitored periodically, and their parents should be made aware of the potential for hyperprolactinemia and other adverse effects. Aripiprazole and quetiapine may increase the risk of suicidality.
Antiepileptics. A meta-analysis of 7 randomized controlled trials examining the use of antiepileptic medications (
Continue to: In a retrospective case series...
In a retrospective case series of 30 pediatric patients with autism spectrum disorder who were given oxcarbazepine, Douglas et al35 found that 47% of participants experienced significant improvement in irritability/agitation. However, 23% of patients reported significant adverse effects leading to discontinuation. Insufficient evidence exists for the safety and efficacy of oxcarbazepine in this population.35
Benzodiazepines. The use of benzodiazepines in pediatric patients has been associated with paradoxical disinhibition reactions, particularly in children with autism and other developmental or cognitive disabilities or delays.21 There is a lack of data on the safety and efficacy of long-term use of benzodiazepines in children, especially in light of these patients’ developing brains, the risk of cognitive impairment, and the potential for dependence with long-term use. Despite this, some studies show that the use of benzodiazepines is fairly common among pediatric patients who present to the ED with agitation.14 In a recent retrospective study, Kendrick et al14 found that among pediatric patients with agitation who were brought to the ED, benzodiazepines were the most commonly prescribed medications.
Other medications. Clonidine and
Diphenhydramine, in both oral and IM forms, has been used to treat agitation in children,32 but has also been associated with a paradoxical disinhibition reaction in pediatric patients21 and therefore should be used only sparingly and with caution. Diphenhydramine has anticholinergic properties, and may worsen delirium.15 Stimulant medications can help aggressive behavior in children and adolescents with ADHD.37
Bottom Line
Agitation among children and adolescents has many possible causes. A combination of a comprehensive assessment and evidence-based, judicious treatment interventions can help prevent and manage agitation in this vulnerable population.
Related Resources
- Baker M, Carlson GA. What do we really know about PRN use in agitated children with mental health conditions: a clinical review. Evid Based Ment Health. 2018;21(4):166-170.
- Gerson R, Malas N, Mroczkowski MM. Crisis in the emergency department: the evaluation and management of acute agitation in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2018;27(3):367-386.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Clonidine • Catapres
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra, Spritam
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Quetiapine • Seroquel
Topiramate • Topamax
Risperidone • Risperdal
Valproate • Depakene
Ziprasidone • Geodon
Managing agitation—verbal and/or motor restlessness that often is accompanied by irritability and a predisposition to aggression or violence—can be challenging in any patient, but particularly so in children and adolescents. In the United States, the prevalence of children and adolescents presenting to an emergency department (ED) for treatment of psychiatric symptoms, including agitation, has been on the rise.1,2
Similar to the multitude of causes of fever, agitation among children and adolescents has many possible causes.3 Because agitation can pose a risk for harm to others and/or self, it is important to manage it proactively. Other than studies that focus on agitation in pediatric anesthesia, there is a dearth of studies examining agitation and its treatment in children and adolescents. There is also a scarcity of training in the management of acute agitation in children and adolescents. In a 2017 survey of pediatric hospitalists and consultation-liaison psychiatrists at 38 academic children’s hospitals in North America, approximately 60% of respondents indicated that they had received no training in the evaluation or management of pediatric acute agitation.4 In addition, approximately 54% of participants said they did not screen for risk factors for pediatric agitation, even though 84% encountered the condition at least once a month, and as often as weekly.4
This article reviews evidence on the causes and treatments of agitation in children and adolescents. For the purposes of this review, child refers to a patient age 6 to 12, and adolescent refers to a patient age 13 to 17.
Identifying the cause
Addressing the underlying cause of agitation is essential. It’s also important to manage acute agitation while the underlying cause is being investigated in a way that does not jeopardize the patient’s emotional or physical safety.
Agitation in children or teens can be due to psychiatric causes such as autism, attention-deficit/hyperactivity disorder (ADHD), or posttraumatic stress disorder (PTSD), or due to medical conditions such as delirium, traumatic brain injury, or other conditions (Table 1).
In a 2005 study of 194 children with agitation in a pediatric post-anesthesia care unit, pain (27%) and anxiety (25%) were found to be the most common causes of agitation.3 Anesthesia-related agitation was a less common cause (11%). Physiologic anomalies were found to be the underlying cause of agitation in only 3 children in this study, but were undiagnosed for a prolonged period in 2 of these 3 children, which highlights the importance of a thorough differential diagnosis in the management of agitation in children.3
Assessment of an agitated child should include a comprehensive history, physical exam, and laboratory testing as indicated. When a pediatric patient comes to the ED with a chief presentation of agitation, a thorough medical and psychiatric assessment should be performed. For patients with a history of psychiatric diagnoses, do not assume that the cause of agitation is psychiatric.
Continue to: Psychiatric causes
Psychiatric causes
Autism spectrum disorder. Children and teens with autism often feel overwhelmed due to transitions, changes, and/or sensory overload. This sensory overload may be in response to relatively subtle sensory stimuli, so it may not always be apparent to parents or others around them.
Research suggests that in general, the ability to cope effectively with emotions is difficult without optimal language development. Due to cognitive/language delays and a related lack of emotional attunement and limited skills in recognizing, expressing, or coping with emotions, difficult emotions in children and adolescents with autism can manifest as agitation.
Attention-deficit/hyperactivity disorder. Children with ADHD may be at a higher risk for agitation, in part due to poor impulse control and limited coping skills. In addition, chronic negative feedback (from parents, teachers, or both) may contribute to low self-esteem, mood symptoms, defiance, and/or other behavioral difficulties. In addition to standard pharmacotherapy for ADHD, treatment involves parent behavior modification training. Setting firm yet empathic limits, “picking battles,” and implementing a developmentally appropriate behavioral plan to manage disruptive behavior in children or adolescents with ADHD can go a long way in helping to prevent the emergence of agitation.
Posttraumatic stress disorder. In some young children, new-onset, unexplained agitation may be the only sign of abuse or trauma. Children who have undergone trauma tend to experience confusion and distress. This may manifest as agitation or aggression, or other symptoms such as increased anxiety or nightmares.5 Trauma may be in the form of witnessing violence (domestic or other); experiencing physical, sexual, and/or emotional abuse; or witnessing/experiencing other significant threats to the safety of self and/or loved ones. Re-establishing (or establishing) a sense of psychological and physical safety is paramount in such patients.6 Psychotherapy is the first-line modality of treatment in children and adolescents with PTSD.6 In general, there is a scarcity of research on medication treatments for PTSD symptoms among children and adolescents.6
Oppositional defiant disorder/conduct disorder. Oppositional defiant disorder (ODD) can be comorbid with ADHD. The diagnosis of ODD requires a pervasive pattern of anger, defiance, vindictiveness, and hostility, particularly towards authority figures. However, these symptoms need to be differentiated from the normal range of childhood behavior. Occasionally, children learn to cope maladaptively through disruptive behavior or agitation. Although a parent or caregiver may see this behavior as intentionally malevolent, in a child with limited coping skills (whether due to young age, developmental/cognitive/language/learning delays, or social communication deficits) or one who has witnessed frequent agitation or aggression in the family environment, agitation and disruptive behavior may be a maladaptive form of coping. Thus, diligence needs to be exercised in the diagnosis of ODD and in understanding the psychosocial factors affecting the child, particularly because impulsiveness and uncooperativeness on their own have been found to be linked to greater likelihood of prescription of psychotropic medications from multiple classes.7 Family-based interventions, particularly parent training, family therapy, and age-appropriate child skills training, are of prime importance in managing this condition.8 Research shows that a shortage of resources, system issues, and cultural roadblocks in implementing family-based psychosocial interventions also can contribute to the increased use of psychotropic medications for aggression in children and teens with ODD, conduct disorder, or ADHD.8 The astute clinician needs to be cognizant of this before prescribing.
Continue to: Hallucinations/psychosis
Hallucinations/psychosis. Hallucinations (whether from psychiatric or medical causes) are significantly associated with agitation.9 In particular, auditory command hallucinations have been linked to agitation. Command hallucinations in children and adolescents may be secondary to early-onset schizophrenia; however, this diagnosis is rare.10 Hallucinations can also be an adverse effect of amphetamine-based stimulant medications in children and adolescents. Visual hallucinations are most often a sign of an underlying medical disorder such as delirium, occipital lobe mass/infection, or drug intoxication or withdrawal. Hallucinations need to be distinguished from the normal, imaginative play of a young child.10
Bipolar mania. In adults, bipolar disorder is a primary psychiatric cause of agitation. In children and adolescents, the diagnosis of bipolar disorder can be complex and requires careful and nuanced history-taking. The risks of agitation are greater with bipolar disorder than with unipolar depression.11,12
Disruptive mood dysregulation disorder. Prior to DSM-5, many children and adolescents with chronic, non-episodic irritability and severe outbursts out of proportion to the situation or stimuli were given a diagnosis of bipolar disorder. These symptoms, in combination with other symptoms, are now considered part of disruptive mood dysregulation disorder when severe outbursts in a child or adolescent occur 3 to 4 times a week consistently, for at least 1 year. The diagnosis of disruptive mood dysregulation disorder requires ruling out other psychiatric and medical conditions, particularly ADHD.13
Substance intoxication/withdrawal. Intoxication or withdrawal from substances such as alcohol, stimulant medications, opioids, methamphetamines, and other agents can lead to agitation. This is more likely to occur among adolescents than children.14
Adjustment disorder. Parental divorce, especially if it is conflictual, or other life stressors, such as experiencing a move or frequent moves, may contribute to the development of agitation in children and adolescents.
Continue to: Depression
Depression. In children and adolescents, depression can manifest as anger or irritability, and occasionally as agitation.
Medical causes
Delirium. Refractory agitation is often a manifestation of delirium in children and adolescents.15 If unrecognized and untreated, delirium can be fatal.16 Therefore, it is imperative that clinicians routinely assess for delirium in any patient who presents with agitation.
Because a patient with delirium often presents with agitation and visual or auditory hallucinations, the medical team may tend to assume these symptoms are secondary to a psychiatric disorder. In this case, the role of the consultation-liaison psychiatrist is critical for guiding the medical team, particularly to continue a thorough exploration of underlying causes while avoiding polypharmacy. Noise, bright lights, frequent changes in nursing staff or caregivers, anticholinergic or benzodiazepine medications, and frequent changes in schedules should be avoided to prevent delirium from occurring or getting worse.17 A multidisciplinary team approach is key in identifying the underlying cause and managing delirium in pediatric patients.
Traumatic brain injury. Agitation may be a presenting symptom in youth with traumatic brain injury (TBI).18 Agitation may present often in the acute recovery phase.19 There is limited evidence on the efficacy and safety of pharmacotherapy for agitation in pediatric patients with TBI.18
Autoimmune conditions. In a study of 27 patients with
Continue to: Medication-induced/iatrogenic
Medication-induced/iatrogenic. Agitation can be an adverse effect of medications such as amantadine (often used for TBI),18 atypical antipsychotics,21 selective serotonin reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors.
Infection. Agitation can be a result of encephalitis, meningitis, or other infectious processes.22
Metabolic conditions. Hepatic or renal failure, diabetic ketoacidosis, and thyroid toxicosis may cause agitation in children or adolescents.22
Start with nonpharmacologic interventions
Few studies have examined de-escalation techniques in agitated children and adolescents. However, verbal de-escalation is generally viewed as the first-line technique for managing agitation in children and adolescents. When feasible, teaching and modeling developmentally appropriate stress management skills for children and teens can be a beneficial preventative strategy to reduce the incidence and worsening of agitation.23
Clinicians should refrain from using coercion.24 Coercion could harm the therapeutic alliance, thereby impeding assessment of the underlying causes of agitation, and can be particularly harmful for patients who have a history of trauma or abuse. Even in pediatric patients with no such history, coercion is discouraged due to its punitive connotations and potential to adversely impact a vulnerable child or teen.
Continue to: Establishing a therapeutic rapport...
Establishing a therapeutic rapport with the patient, when feasible, can facilitate smoother de-escalation by offering the patient an outlet to air his/her frustrations and emotions, and by helping the patient feel understood.24 To facilitate this, ensure that the patient’s basic comforts and needs are met, such as access to a warm bed, food, and safety.25
The psychiatrist’s role is to help uncover and address the underlying reason for the patient’s agony or distress. Once the child or adolescent has calmed, explore potential triggers or causes of the agitation.
There has been a significant move away from the use of restraints for managing agitation in children and adolescents.26 Restraints have a psychologically traumatizing effect,27 and have been linked to life-threatening injuries and death in children.24
Pharmacotherapy: Proceed with caution
There are no FDA-approved medications for the treatment of agitation in the general pediatric population, and any medication use in this population is off-label. There is also a dearth of research examining the safety and efficacy of using psychotropic medications for agitation in pediatric patients. Because children and adolescents are more susceptible to adverse effects and risks associated with the use of psychotropic medications, special caution is warranted. In general, pharmacologic interventions are not recommended without the use of psychotherapy-based modalities.
In the past, the aim of using medications to treat patients with agitation was to put the patient to sleep.25 This practice did not help clinicians to assess for underlying causes, and was often accompanied by a greater risk of adverse effects and reactions.24 Therefore, the goal of medication treatment for agitation is to help calm the patient instead of inducing sleep.25
Continue to: Pharmacotherapy should...
Pharmacotherapy should be used only when behavioral interventions have been unsuccessful. Key considerations for using psychotropic medications to address agitation in children and adolescents are summarized in Table 2.25
Antipsychotics, particularly second-generation antipsychotics (SGAs), have been commonly used to manage acute agitation in children and adolescents, and there has been an upswing in the use of these medications in the United States in the last several years.28 Research indicates that males, children and adolescents in foster care, and those with Medicaid have been the more frequent youth recipients of SGAs.29 Of particular concern is the prevalence of antipsychotic use among children younger than age 6. In the last few decades, there has been an increase in the prescription of antipsychotics for children younger than age 6, particularly for disruptive behavior and aggression.30 In a study of preschool-age Medicaid patients in Kentucky, 70,777 prescriptions for SGAs were given to 6,915 children <6 years of age; 73% of these prescriptions were for male patients.30 Because there is a lack of controlled studies examining the safety and efficacy of SGAs among children and adolescents, especially with long-term use, further research is needed and caution is warranted.28
The FDA has approved
Externalizing disorders among children and adolescents tend to get treated with antipsychotics.28 A Canadian study examining records of 6,916 children found that most children who had been prescribed risperidone received it for ADHD or conduct disorder, and most patients had not received laboratory testing for monitoring the antipsychotic medication they were taking.31 In a 2018 study examining medical records of 120 pediatric patients who presented to an ED in British Columbia with agitation, antipsychotics were the most commonly used medications for patients with autism spectrum disorder; most patients received at least 1 dose.14
For children and adolescents with agitation or aggression who were admitted to inpatient units, IM
Continue to: In case reports...
In case reports, a combination of olanzapine with CNS-suppressing agents has resulted in death. Therefore, do not combine olanzapine with agents such as benzodiazepines.25 In a patient with a likely medical source of agitation, insufficient evidence exists to support the use of olanzapine, and additional research is needed.25
Low-dose haloperidol has been found to be effective for delirium-related agitation in pediatric studies.15 Before initiating an antipsychotic for any child or adolescent, review the patient’s family history for reports of early cardiac death and the patient’s own history of cardiac symptoms, palpitations, syncope, or prolonged QT interval. Monitor for QT prolongation. Among commonly used antipsychotics, the risk of QT prolongation is higher with IV administration of haloperidol and with ziprasidone. Studies show that compared with oral or IM
A few studies have found risperidone to be efficacious for treating ODD and conduct disorder; however, this use is off-label, and its considerable adverse effect and risk profile needs to be weighed against the potential benefit.8
Antipsychotic polypharmacy should be avoided because of the higher risk of adverse effects and interactions, and a lack of robust, controlled studies evaluating the safety of using antipsychotics for non-FDA-approved indications in children and adolescents.7 All patients who receive antipsychotics require monitoring for extrapyramidal symptoms, tardive dyskinesia, neuroleptic malignant syndrome, orthostatic hypotension, sedation, metabolic syndrome, and other potential adverse effects. Patients receiving risperidone need to have their prolactin levels monitored periodically, and their parents should be made aware of the potential for hyperprolactinemia and other adverse effects. Aripiprazole and quetiapine may increase the risk of suicidality.
Antiepileptics. A meta-analysis of 7 randomized controlled trials examining the use of antiepileptic medications (
Continue to: In a retrospective case series...
In a retrospective case series of 30 pediatric patients with autism spectrum disorder who were given oxcarbazepine, Douglas et al35 found that 47% of participants experienced significant improvement in irritability/agitation. However, 23% of patients reported significant adverse effects leading to discontinuation. Insufficient evidence exists for the safety and efficacy of oxcarbazepine in this population.35
Benzodiazepines. The use of benzodiazepines in pediatric patients has been associated with paradoxical disinhibition reactions, particularly in children with autism and other developmental or cognitive disabilities or delays.21 There is a lack of data on the safety and efficacy of long-term use of benzodiazepines in children, especially in light of these patients’ developing brains, the risk of cognitive impairment, and the potential for dependence with long-term use. Despite this, some studies show that the use of benzodiazepines is fairly common among pediatric patients who present to the ED with agitation.14 In a recent retrospective study, Kendrick et al14 found that among pediatric patients with agitation who were brought to the ED, benzodiazepines were the most commonly prescribed medications.
Other medications. Clonidine and
Diphenhydramine, in both oral and IM forms, has been used to treat agitation in children,32 but has also been associated with a paradoxical disinhibition reaction in pediatric patients21 and therefore should be used only sparingly and with caution. Diphenhydramine has anticholinergic properties, and may worsen delirium.15 Stimulant medications can help aggressive behavior in children and adolescents with ADHD.37
Bottom Line
Agitation among children and adolescents has many possible causes. A combination of a comprehensive assessment and evidence-based, judicious treatment interventions can help prevent and manage agitation in this vulnerable population.
Related Resources
- Baker M, Carlson GA. What do we really know about PRN use in agitated children with mental health conditions: a clinical review. Evid Based Ment Health. 2018;21(4):166-170.
- Gerson R, Malas N, Mroczkowski MM. Crisis in the emergency department: the evaluation and management of acute agitation in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2018;27(3):367-386.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Clonidine • Catapres
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra, Spritam
Olanzapine • Zyprexa
Oxcarbazepine • Trileptal
Quetiapine • Seroquel
Topiramate • Topamax
Risperidone • Risperdal
Valproate • Depakene
Ziprasidone • Geodon
1. Frosch E, Kelly P. Issues in pediatric psychiatric emergency care. In: Emergency psychiatry. Cambridge, UK: Cambridge University Press; 2011:185-199.
2. American College of Emergency Physicians. Pediatric mental health emergencies in the emergency department. https://www.acep.org/patient-care/policy-statements/pediatric-mental-health-emergencies-in-the-emergency-medical-services-system/. Revised September 2018. Accessed February 23, 2019.
3. Voepel-Lewis, T, Burke C, Hadden S, et al. Nurses’ diagnoses and treatment decisions regarding care of the agitated child. J Perianesth Nurs. 2005;20(4):239-248.
4. Malas N, Spital L, Fischer J, et al. National survey on pediatric acute agitation and behavioral escalation in academic inpatient pediatric care settings. Psychosomatics. 2017;58(3):299-306.
5. Famularo R, Kinscherff R, Fenton T. Symptom differences in acute and chronic presentation of childhood post-traumatic stress disorder. Child Abuse Negl. 1990;14(3):439-444.
6. Kaminer D, Seedat S, Stein DJ. Post-traumatic stress disorder in children. World Psychiatry. 2005;4(2):121-125.
7. Ninan A, Stewart SL, Theall LA, et al. Adverse effects of psychotropic medications in children: predictive factors. J Can Acad Child Adolesc Psychiatry. 2014;23(3):218-225.
8. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52-61.
9. Vareilles D, Bréhin C, Cortey C, et al. Hallucinations: Etiological analysis of children admitted to a pediatric emergency department. Arch Pediatr. 2017;24(5):445-452.
10. Bartlett J. Childhood-onset schizophrenia: what do we really know? Health Psychol Behav Med. 2014;2(1):735-747.
11. Diler RS, Goldstein TR, Hafeman D, et al. Distinguishing bipolar depression from unipolar depression in youth: Preliminary findings. J Child Adolesc Psychopharmacol. 2017;27(4):310-319.
12. Dervic K, Garcia-Amador M, Sudol K, et al. Bipolar I and II versus unipolar depression: clinical differences and impulsivity/aggression traits. Eur Psychiatry. 2015;30(1):106-113.
13. Masi L, Gignac M ADHD and DMDD comorbidities, similarities and distinctions. J Child Adolesc Behav2016;4:325.
14. Kendrick JG, Goldman RD, Carr RR. Pharmacologic management of agitation and aggression in a pediatric emergency department - a retrospective cohort study. J Pediatr Pharmacol Ther. 2018;23(6):455-459.
15. Schieveld JN, Staal M, Voogd L, et al. Refractory agitation as a marker for pediatric delirium in very young infants at a pediatric intensive care unit. Intensive Care Med. 2010;36(11):1982-1983.
16. Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med. 2017;45(5):891-898.
17. Bettencourt A, Mullen JE. Delirium in children: identification, prevention, and management. Crit Care Nurse. 2017;37(3):e9-e18.
18. Suskauer SJ, Trovato MK. Update on pharmaceutical intervention for disorders of consciousness and agitation after traumatic brain injury in children. PM R. 2013;5(2):142-147.
19. Nowicki M, Pearlman L, Campbell C, et al. Agitated behavior scale in pediatric traumatic brain injury. Brain Inj. 2019. doi: 10.1080/02699052.2019.1565893.
20. Mohammad SS, Jones H, Hong M, et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev Med Child Neurol. 2016;58(4):376-384.
21. Sonnier L, Barzman D. Pharmacologic management of acutely agitated pediatric patients. Pediatr Drugs. 2011;13(1):1-10.
22. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
23. Masters KJ, Bellonci C, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):4S-25S.
24. Croce ND, Mantovani C. Using de-escalation techniques to prevent violent behavior in pediatric psychiatric emergencies: It is possible. Pediatric Dimensions, 2017;2(1):1-2.
25. Marzullo LR. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269-275.
26. Caldwell B, Albert C, Azeem MW, et al. Successful seclusion and restraint prevention effort in child and adolescent programs. J Psychosoc Nurs Ment Health Serv. 2014;52(11):30-38.
27. De Hert M, Dirix N, Demunter H, et al. Prevalence and correlates of seclusion and restraint use in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2011;20(5):221-230.
28. Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770-w781.
29. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of atypical antipsychotic medication in children and adolescents. https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf. Accessed March 4, 2019.
30. Lohr WD, Chowning RT, Stevenson MD, et al. Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol. 2015;25(5):440-443.
31. Chen W, Cepoiu-Martin M, Stang A, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449-455.
32. Deshmukh P, Kulkarni G, Barzman D. Recommendations for pharmacological management of inpatient aggression in children and adolescents. Psychiatry (Edgmont). 2010;7(2):32-40.
33. Haldol [package insert]. Beerse, Belgium: Janssen Pharmaceutica NV; 2005.
34. Hirota T, Veenstra-Vanderweele J, Hollander E, et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
35. Douglas JF, Sanders KB, Benneyworth MH, et al. Brief report: retrospective case series of oxcarbazepine for irritability/agitation symptoms in autism spectrum disorder. J Autism Dev Disord. 2013;43(5):1243-1247.
36. Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad
37. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: Psychostimulants, alpha-2 Agonists, and atomoxetine. Can J Psychiatry. 2015;60(2):42-51
1. Frosch E, Kelly P. Issues in pediatric psychiatric emergency care. In: Emergency psychiatry. Cambridge, UK: Cambridge University Press; 2011:185-199.
2. American College of Emergency Physicians. Pediatric mental health emergencies in the emergency department. https://www.acep.org/patient-care/policy-statements/pediatric-mental-health-emergencies-in-the-emergency-medical-services-system/. Revised September 2018. Accessed February 23, 2019.
3. Voepel-Lewis, T, Burke C, Hadden S, et al. Nurses’ diagnoses and treatment decisions regarding care of the agitated child. J Perianesth Nurs. 2005;20(4):239-248.
4. Malas N, Spital L, Fischer J, et al. National survey on pediatric acute agitation and behavioral escalation in academic inpatient pediatric care settings. Psychosomatics. 2017;58(3):299-306.
5. Famularo R, Kinscherff R, Fenton T. Symptom differences in acute and chronic presentation of childhood post-traumatic stress disorder. Child Abuse Negl. 1990;14(3):439-444.
6. Kaminer D, Seedat S, Stein DJ. Post-traumatic stress disorder in children. World Psychiatry. 2005;4(2):121-125.
7. Ninan A, Stewart SL, Theall LA, et al. Adverse effects of psychotropic medications in children: predictive factors. J Can Acad Child Adolesc Psychiatry. 2014;23(3):218-225.
8. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 2: antipsychotics and traditional mood stabilizers. Can J Psychiatry. 2015;60(2):52-61.
9. Vareilles D, Bréhin C, Cortey C, et al. Hallucinations: Etiological analysis of children admitted to a pediatric emergency department. Arch Pediatr. 2017;24(5):445-452.
10. Bartlett J. Childhood-onset schizophrenia: what do we really know? Health Psychol Behav Med. 2014;2(1):735-747.
11. Diler RS, Goldstein TR, Hafeman D, et al. Distinguishing bipolar depression from unipolar depression in youth: Preliminary findings. J Child Adolesc Psychopharmacol. 2017;27(4):310-319.
12. Dervic K, Garcia-Amador M, Sudol K, et al. Bipolar I and II versus unipolar depression: clinical differences and impulsivity/aggression traits. Eur Psychiatry. 2015;30(1):106-113.
13. Masi L, Gignac M ADHD and DMDD comorbidities, similarities and distinctions. J Child Adolesc Behav2016;4:325.
14. Kendrick JG, Goldman RD, Carr RR. Pharmacologic management of agitation and aggression in a pediatric emergency department - a retrospective cohort study. J Pediatr Pharmacol Ther. 2018;23(6):455-459.
15. Schieveld JN, Staal M, Voogd L, et al. Refractory agitation as a marker for pediatric delirium in very young infants at a pediatric intensive care unit. Intensive Care Med. 2010;36(11):1982-1983.
16. Traube C, Silver G, Gerber LM, et al. Delirium and mortality in critically ill children: epidemiology and outcomes of pediatric delirium. Crit Care Med. 2017;45(5):891-898.
17. Bettencourt A, Mullen JE. Delirium in children: identification, prevention, and management. Crit Care Nurse. 2017;37(3):e9-e18.
18. Suskauer SJ, Trovato MK. Update on pharmaceutical intervention for disorders of consciousness and agitation after traumatic brain injury in children. PM R. 2013;5(2):142-147.
19. Nowicki M, Pearlman L, Campbell C, et al. Agitated behavior scale in pediatric traumatic brain injury. Brain Inj. 2019. doi: 10.1080/02699052.2019.1565893.
20. Mohammad SS, Jones H, Hong M, et al. Symptomatic treatment of children with anti-NMDAR encephalitis. Dev Med Child Neurol. 2016;58(4):376-384.
21. Sonnier L, Barzman D. Pharmacologic management of acutely agitated pediatric patients. Pediatr Drugs. 2011;13(1):1-10.
22. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the american association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3-10.
23. Masters KJ, Bellonci C, Bernet W, et al; American Academy of Child and Adolescent Psychiatry. Practice parameter for the prevention and management of aggressive behavior in child and adolescent psychiatric institutions, with special reference to seclusion and restraint. J Am Acad Child Adolesc Psychiatry. 2002;41(2 suppl):4S-25S.
24. Croce ND, Mantovani C. Using de-escalation techniques to prevent violent behavior in pediatric psychiatric emergencies: It is possible. Pediatric Dimensions, 2017;2(1):1-2.
25. Marzullo LR. Pharmacologic management of the agitated child. Pediatr Emerg Care. 2014;30(4):269-275.
26. Caldwell B, Albert C, Azeem MW, et al. Successful seclusion and restraint prevention effort in child and adolescent programs. J Psychosoc Nurs Ment Health Serv. 2014;52(11):30-38.
27. De Hert M, Dirix N, Demunter H, et al. Prevalence and correlates of seclusion and restraint use in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry. 2011;20(5):221-230.
28. Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770-w781.
29. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of atypical antipsychotic medication in children and adolescents. https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf. Accessed March 4, 2019.
30. Lohr WD, Chowning RT, Stevenson MD, et al. Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol. 2015;25(5):440-443.
31. Chen W, Cepoiu-Martin M, Stang A, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. 2018;38(5):449-455.
32. Deshmukh P, Kulkarni G, Barzman D. Recommendations for pharmacological management of inpatient aggression in children and adolescents. Psychiatry (Edgmont). 2010;7(2):32-40.
33. Haldol [package insert]. Beerse, Belgium: Janssen Pharmaceutica NV; 2005.
34. Hirota T, Veenstra-Vanderweele J, Hollander E, et al. Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2014;44(4):948-957.
35. Douglas JF, Sanders KB, Benneyworth MH, et al. Brief report: retrospective case series of oxcarbazepine for irritability/agitation symptoms in autism spectrum disorder. J Autism Dev Disord. 2013;43(5):1243-1247.
36. Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad
37. Pringsheim T, Hirsch L, Gardner D, et al. The pharmacological management of oppositional behaviour, conduct problems, and aggression in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder: a systematic review and meta-analysis. Part 1: Psychostimulants, alpha-2 Agonists, and atomoxetine. Can J Psychiatry. 2015;60(2):42-51
Unipolar vs bipolar depression: A clinician’s perspective
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
Gut microbiota and its implications for psychiatry: A review of 3 studies
The “human microbiota” describes all microorganisms within the human body, including bacteria, viruses, and eukaryotes. The related term “microbiome” refers to the complete catalog of these microbes and their genes.1 There is a growing awareness that the human microbiota plays an important role in maintaining mental health, and that a disruption in its composition can contribute to manifestations of psychiatric disorders. A growing body of evidence has also linked mental health outcomes to the gut microbiome, suggesting that the gut microbiota can modulate the gut-brain axis.2
Numerous neurotransmitters, including dopamine, serotonin, gamma-aminobutyric acid, and acetylcholine, are produced in the gastrointestinal (GI) tract, and our diet is vital in sustaining and replenishing them. At the same time, our brain regulates our GI tract by secretion of hormones such as oxytocin, leptin, ghrelin, neuropeptide Y, corticotrophin-releasing factor, and a plethora of others. Dysregulation of this microbiome can lead to both physical and mental illnesses. Symptoms of psychiatric disorders, such as depression, psychosis, anxiety, and autism, can be a consequence of this dysregulation.2
Our diet can also modify the gut microorganisms and therefore many of its metabolic pathways. More attention has been given to pre- and probiotics and their effects on DNA by epigenetic changes. One can quickly start to appreciate how this intricate crosstalk can lead to a variety of pathologic and psychiatric problems that have an adverse effect on autoimmune, inflammatory, metabolic, cognitive, and behavioral processes.2,3
Thus far, links have mostly been reported in animal models, and human studies are limited.4 Researchers are just beginning to elucidate how the microbiota affect gut-brain signaling in humans. Such mechanisms may include alterations in microbial composition, immune activation, vagus nerve signaling, alterations in tryptophan metabolism, production of specific microbial neuroactive metabolites, and bacterial cell wall sugars.5 The microbiota-gut-brain axis plays a part in regulating/programming the hypothalamic-pituitary-adrenal (HPA) axis throughout the life span.3 The interactions between the gut microbiome, the immune system, and the CNS are regulated through pathways that involve endocrine functions (HPA axis), the immune system, and metabolic factors.3,4 Recent research focusing on the gut microbiome has also given rise to international projects such as the Human Microbiome Project (Human Microbiome Project Consortium, 2012).3
Several studies have looked into psychiatry and inflammatory/immune pathways. Here we review 3 recent studies that have focused on the gut-brain axis (Table6-8).
1. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
The aim of this study was to evaluate immunoglobulin G (IgG) response against 40 food products in patients with depression vs those in a control group, along with changes in inflammatory markers, psychological stress, and dietary variables.6
Study design
- N = 63, IgG levels against 44 food products, cortisol levels, tumor necrosis factor (TNF)-alpha, interleukin 6 (IL-6), and IL-1 beta levels were recorded. The psychological parameters of 34 participants with depression and 29 controls were compared using the Hamilton Depression Rating scale, (HAM-D-17), Perceived Stress scale, and Symptom Checklist scale. The study was conducted in Poland.
Continue to: Outcomes
Outcomes
- Patients who were depressed had lower IgG levels against dairy products compared to controls when there was high dairy consumption. However, there was no overall difference between patients and controls in mean IgG concentration against food products.
- Patients who were depressed had higher levels of cortisol. Levels of cortisol had a positive correlation with HAM-D-17 score. Patients with depression had lower levels of TNF-alpha.
Conclusion
- Patients with depression had lower levels of IgG against dairy protein. Patients with depression had high cortisol levels but decreased levels of TNF-alpha, which could explain an immune suppression of IgG in these patients. There were no differences in IL-6 or IL-1beta levels.
Hypercortisolemia is present in approximately 60% of patients with depression. Elevated cortisol levels have a negative effect on lymphocyte function. B-lymphocytes (CD 10+ and CD 19+) are sensitive to glucocorticoids. Studies in mice have demonstrated that elevated glucocorticoid levels are associated with a 50% decrease in serum B-lymphocytes, and this can be explained by downregulation of c-myc protein, which plays a role in cell proliferation and cell survival. Glucocorticoids also decrease levels of protein kinases that are vital for the cell cycle to continue, and they upregulate p27 and p21, which are cell cycle inhibitors. Therefore, if high cortisol suppresses B-lymphocyte production, this can explain how patients with depression have low IgG levels, since B-lymphocytes differentiate into plasma cells that will produce antibodies.6
Depression can trigger an inflammatory response by increasing levels of inflammatory cytokines, acute phase reactants, and oxidative molecules. The inflammatory response can lead to intestinal wall disruption, and therefore bacteria can migrate across the GI barrier, along with food antigens, which could then lead to food antigen hypersensitivity.6
The significance of diet
Many studies have looked into specific types of diets, such as the Mediterranean diet, the ketogenic diet, and the addition of supplements such as probiotics, omega-3 fatty acids, zinc, and multivitamins.7 The Mediterranean diet is high in fiber, nuts, legumes, and fish.7 The ketogenic diet includes a controlled amount of fat, but is low in protein and carbohydrates.7 The main point is that a balanced diet can have a positive effect on mental health.7 The Mediterranean diet has shown to decrease the incidence of cardiovascular disease and lower the risk of depression.7 In animal studies, the ketogenic diet has improved anxiety, depression, and autism.7 Diet clearly affects gut microbiota and, as a consequence, the body’s level of inflammation.7
Continue to: The following review...
The following review highlighted the significance of diet on gut microbiome and mental health.7
2. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut- brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17: 1-9.
Study design
- These researchers provided a narrative review of the significance of a healthy diet and nutritional supplements on the gut microbiome and the treatment of patients with psychiatric illness.
Outcomes
- This review suggested dietary coaching as a nonpharmacologic treatment for patients with psychiatric illness.
Conclusion
- The utilization of nutritional advice, along with medication management, therapy, and physical activity, can provide a holistic approach to the biopsychosocial treatment of patients with psychiatric illness.
This review also emphasized the poor dietary trends of Westernized countries, which include calorie-dense, genetically altered, processed meals. As Mörkl et al7 noted, we are overfed but undernourished. Mörkl et al7 reviewed studies that involve dietary coaching as part of the treatment plan of patients with mental illness. In one of these studies, patients who received nutritional advice and coaching over 6 weeks had a 40% to 50% decrease in depressive symptoms. These effects persisted for 2 more years. Mörkl et al7 also reviewed an Italian study that found that providing nutritional advice in patients with affective disorders and psychosis helped improve symptom severity and sleep.7
Continue to: Mörkl et al...
Mörkl et al7 also reviewed dietary supplements. Some studies have linked use of omega-3 fatty acids with improvement in affective disorders, Alzheimer’s disease, and posttraumatic stress disorder, as well as cardiovascular conditions. Omega-3 fatty acids may exert beneficial effects by enhancing brain-derived neurotrophic factor and neurogenesis as well as by decreasing inflammation.7
Zinc supplementation can also improve depression, as it has been linked to cytokine variation and hippocampal neuronal growth. Vitamin B9 deficiency and vitamin D deficiency also have been associated with depression. Mörkl et al7 emphasized that a balanced diet that incorporates a variety of nutrients is more beneficial than supplementation of any individual vitamin alone.
Researchers have long emphasized the importance of a healthy balanced diet when treating patients with medical conditions such as cardiovascular or cerebrovascular diseases. Based on the studies Mörkl et al7 reviewed, the same emphasis should be communicated to our patients who suffer from psychiatric conditions.
The gut and anxiety
The gut microbiome has also been an area of research when studying generalized anxiety disorder (GAD).8
3. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
The aim of the study was to determine if there were changes in the composition of the gut microbiome in patients with GAD compared with healthy controls.8
Continue to: Study design
Study design
- A cross-sectional study of 76 patients in Zhejiang, China. Forty patients with GAD in the active state and 36 healthy controls were compared in terms of composition of GI microbacterial flora.
- Researchers also examined a subgroup of 12 patients who were treatment-naïve and 17 controls. Stool samples were collected from the 12 patients who were treatment-naïve before initiating medication.
- Researchers also conducted a prospective study in a subgroup of 9 patients with GAD in both the active state and remissive state. Two stool samples were collected from each patient—one during the active state of GAD and one during the remissive state—for a total of 18 samples. Stool samples analyzed with the use of polymerase chain reaction and microbial analysis.
- Patients completed the Hamilton Anxiety Rating (HAM-A) scale and were classified into groups. Those with HAM-A scores >14 were classified as being in the active state of GAD, and those with scores <7 were classified as being in the remissive state.
Outcomes
- Among the samples collected, 8 bacterial taxa were found in different amounts in patients with GAD and healthy controls. Bacteroidetes, Ruminococcus gnavus, and Fusobacterium were increased in patients with GAD compared with controls, while Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus were increased in healthy controls.
- Bacterial variety was notably lower in the 12 patients who were treatment-naïve compared with the control group.
- There was no notable difference in microbial composition between patients in the active vs remissive state.
Conclusion
- Patients with GAD had less short chain fatty acid–producing bacteria (Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus) compared with controls. Decreased formation of short chain fatty acids could lead to GI barrier disruption. Fusobacterium and Ruminococcus were increased in patients with GAD. Fusobacterium can cause disease and be invasive when it disseminates within the body. The inflammatory characteristics of Fusobacterium contribute to the immunologic activation in GAD. Ruminococcus breaks down mucin, which could then increase GI permeability by mucous degradation of the GI lumen.
Changes in food processing and manufacturing have led to changes in our diets. Changes in our normal GI microbacterial flora could lead to increased gut permeability, bacterial dissemination, and subsequent systemic inflammation. Research has shown that the composition of the microbiota changes across the life span.9 A balanced intake of nutrients is important for both our physical and mental health and safeguards the basis of gut microbiome regulation. A well-regulated gut microbiome ensures low levels of inflammation in the brain and body. Lifestyle modifications and dietary coaching could be practical interventions for patients with psychiatric conditions.5 Current advances in technology now offer precise analyses of thousands of metabolites, enabling metabolomics to offer the promise of discovering new drug targets and biomarkers that may help pave a way to precision medicine.
1. Dave M, Higgins PD, Middha S, et al. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160:246-257.
2. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
3. Malan-Muller S, Valles-Colomer M, Raes J, et al. The gut microbiome and mental health: implications for anxiety-and trauma-related disorders. OMICS. 2018;22(2):90-107.
4. Du Toit A. The gut microbiome and mental health. Nat Rev Microbiol. 2019;17(4):196.
5. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
6. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
7. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17:1-9.
8. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
9. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167(4):374-379.
The “human microbiota” describes all microorganisms within the human body, including bacteria, viruses, and eukaryotes. The related term “microbiome” refers to the complete catalog of these microbes and their genes.1 There is a growing awareness that the human microbiota plays an important role in maintaining mental health, and that a disruption in its composition can contribute to manifestations of psychiatric disorders. A growing body of evidence has also linked mental health outcomes to the gut microbiome, suggesting that the gut microbiota can modulate the gut-brain axis.2
Numerous neurotransmitters, including dopamine, serotonin, gamma-aminobutyric acid, and acetylcholine, are produced in the gastrointestinal (GI) tract, and our diet is vital in sustaining and replenishing them. At the same time, our brain regulates our GI tract by secretion of hormones such as oxytocin, leptin, ghrelin, neuropeptide Y, corticotrophin-releasing factor, and a plethora of others. Dysregulation of this microbiome can lead to both physical and mental illnesses. Symptoms of psychiatric disorders, such as depression, psychosis, anxiety, and autism, can be a consequence of this dysregulation.2
Our diet can also modify the gut microorganisms and therefore many of its metabolic pathways. More attention has been given to pre- and probiotics and their effects on DNA by epigenetic changes. One can quickly start to appreciate how this intricate crosstalk can lead to a variety of pathologic and psychiatric problems that have an adverse effect on autoimmune, inflammatory, metabolic, cognitive, and behavioral processes.2,3
Thus far, links have mostly been reported in animal models, and human studies are limited.4 Researchers are just beginning to elucidate how the microbiota affect gut-brain signaling in humans. Such mechanisms may include alterations in microbial composition, immune activation, vagus nerve signaling, alterations in tryptophan metabolism, production of specific microbial neuroactive metabolites, and bacterial cell wall sugars.5 The microbiota-gut-brain axis plays a part in regulating/programming the hypothalamic-pituitary-adrenal (HPA) axis throughout the life span.3 The interactions between the gut microbiome, the immune system, and the CNS are regulated through pathways that involve endocrine functions (HPA axis), the immune system, and metabolic factors.3,4 Recent research focusing on the gut microbiome has also given rise to international projects such as the Human Microbiome Project (Human Microbiome Project Consortium, 2012).3
Several studies have looked into psychiatry and inflammatory/immune pathways. Here we review 3 recent studies that have focused on the gut-brain axis (Table6-8).
1. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
The aim of this study was to evaluate immunoglobulin G (IgG) response against 40 food products in patients with depression vs those in a control group, along with changes in inflammatory markers, psychological stress, and dietary variables.6
Study design
- N = 63, IgG levels against 44 food products, cortisol levels, tumor necrosis factor (TNF)-alpha, interleukin 6 (IL-6), and IL-1 beta levels were recorded. The psychological parameters of 34 participants with depression and 29 controls were compared using the Hamilton Depression Rating scale, (HAM-D-17), Perceived Stress scale, and Symptom Checklist scale. The study was conducted in Poland.
Continue to: Outcomes
Outcomes
- Patients who were depressed had lower IgG levels against dairy products compared to controls when there was high dairy consumption. However, there was no overall difference between patients and controls in mean IgG concentration against food products.
- Patients who were depressed had higher levels of cortisol. Levels of cortisol had a positive correlation with HAM-D-17 score. Patients with depression had lower levels of TNF-alpha.
Conclusion
- Patients with depression had lower levels of IgG against dairy protein. Patients with depression had high cortisol levels but decreased levels of TNF-alpha, which could explain an immune suppression of IgG in these patients. There were no differences in IL-6 or IL-1beta levels.
Hypercortisolemia is present in approximately 60% of patients with depression. Elevated cortisol levels have a negative effect on lymphocyte function. B-lymphocytes (CD 10+ and CD 19+) are sensitive to glucocorticoids. Studies in mice have demonstrated that elevated glucocorticoid levels are associated with a 50% decrease in serum B-lymphocytes, and this can be explained by downregulation of c-myc protein, which plays a role in cell proliferation and cell survival. Glucocorticoids also decrease levels of protein kinases that are vital for the cell cycle to continue, and they upregulate p27 and p21, which are cell cycle inhibitors. Therefore, if high cortisol suppresses B-lymphocyte production, this can explain how patients with depression have low IgG levels, since B-lymphocytes differentiate into plasma cells that will produce antibodies.6
Depression can trigger an inflammatory response by increasing levels of inflammatory cytokines, acute phase reactants, and oxidative molecules. The inflammatory response can lead to intestinal wall disruption, and therefore bacteria can migrate across the GI barrier, along with food antigens, which could then lead to food antigen hypersensitivity.6
The significance of diet
Many studies have looked into specific types of diets, such as the Mediterranean diet, the ketogenic diet, and the addition of supplements such as probiotics, omega-3 fatty acids, zinc, and multivitamins.7 The Mediterranean diet is high in fiber, nuts, legumes, and fish.7 The ketogenic diet includes a controlled amount of fat, but is low in protein and carbohydrates.7 The main point is that a balanced diet can have a positive effect on mental health.7 The Mediterranean diet has shown to decrease the incidence of cardiovascular disease and lower the risk of depression.7 In animal studies, the ketogenic diet has improved anxiety, depression, and autism.7 Diet clearly affects gut microbiota and, as a consequence, the body’s level of inflammation.7
Continue to: The following review...
The following review highlighted the significance of diet on gut microbiome and mental health.7
2. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut- brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17: 1-9.
Study design
- These researchers provided a narrative review of the significance of a healthy diet and nutritional supplements on the gut microbiome and the treatment of patients with psychiatric illness.
Outcomes
- This review suggested dietary coaching as a nonpharmacologic treatment for patients with psychiatric illness.
Conclusion
- The utilization of nutritional advice, along with medication management, therapy, and physical activity, can provide a holistic approach to the biopsychosocial treatment of patients with psychiatric illness.
This review also emphasized the poor dietary trends of Westernized countries, which include calorie-dense, genetically altered, processed meals. As Mörkl et al7 noted, we are overfed but undernourished. Mörkl et al7 reviewed studies that involve dietary coaching as part of the treatment plan of patients with mental illness. In one of these studies, patients who received nutritional advice and coaching over 6 weeks had a 40% to 50% decrease in depressive symptoms. These effects persisted for 2 more years. Mörkl et al7 also reviewed an Italian study that found that providing nutritional advice in patients with affective disorders and psychosis helped improve symptom severity and sleep.7
Continue to: Mörkl et al...
Mörkl et al7 also reviewed dietary supplements. Some studies have linked use of omega-3 fatty acids with improvement in affective disorders, Alzheimer’s disease, and posttraumatic stress disorder, as well as cardiovascular conditions. Omega-3 fatty acids may exert beneficial effects by enhancing brain-derived neurotrophic factor and neurogenesis as well as by decreasing inflammation.7
Zinc supplementation can also improve depression, as it has been linked to cytokine variation and hippocampal neuronal growth. Vitamin B9 deficiency and vitamin D deficiency also have been associated with depression. Mörkl et al7 emphasized that a balanced diet that incorporates a variety of nutrients is more beneficial than supplementation of any individual vitamin alone.
Researchers have long emphasized the importance of a healthy balanced diet when treating patients with medical conditions such as cardiovascular or cerebrovascular diseases. Based on the studies Mörkl et al7 reviewed, the same emphasis should be communicated to our patients who suffer from psychiatric conditions.
The gut and anxiety
The gut microbiome has also been an area of research when studying generalized anxiety disorder (GAD).8
3. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
The aim of the study was to determine if there were changes in the composition of the gut microbiome in patients with GAD compared with healthy controls.8
Continue to: Study design
Study design
- A cross-sectional study of 76 patients in Zhejiang, China. Forty patients with GAD in the active state and 36 healthy controls were compared in terms of composition of GI microbacterial flora.
- Researchers also examined a subgroup of 12 patients who were treatment-naïve and 17 controls. Stool samples were collected from the 12 patients who were treatment-naïve before initiating medication.
- Researchers also conducted a prospective study in a subgroup of 9 patients with GAD in both the active state and remissive state. Two stool samples were collected from each patient—one during the active state of GAD and one during the remissive state—for a total of 18 samples. Stool samples analyzed with the use of polymerase chain reaction and microbial analysis.
- Patients completed the Hamilton Anxiety Rating (HAM-A) scale and were classified into groups. Those with HAM-A scores >14 were classified as being in the active state of GAD, and those with scores <7 were classified as being in the remissive state.
Outcomes
- Among the samples collected, 8 bacterial taxa were found in different amounts in patients with GAD and healthy controls. Bacteroidetes, Ruminococcus gnavus, and Fusobacterium were increased in patients with GAD compared with controls, while Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus were increased in healthy controls.
- Bacterial variety was notably lower in the 12 patients who were treatment-naïve compared with the control group.
- There was no notable difference in microbial composition between patients in the active vs remissive state.
Conclusion
- Patients with GAD had less short chain fatty acid–producing bacteria (Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus) compared with controls. Decreased formation of short chain fatty acids could lead to GI barrier disruption. Fusobacterium and Ruminococcus were increased in patients with GAD. Fusobacterium can cause disease and be invasive when it disseminates within the body. The inflammatory characteristics of Fusobacterium contribute to the immunologic activation in GAD. Ruminococcus breaks down mucin, which could then increase GI permeability by mucous degradation of the GI lumen.
Changes in food processing and manufacturing have led to changes in our diets. Changes in our normal GI microbacterial flora could lead to increased gut permeability, bacterial dissemination, and subsequent systemic inflammation. Research has shown that the composition of the microbiota changes across the life span.9 A balanced intake of nutrients is important for both our physical and mental health and safeguards the basis of gut microbiome regulation. A well-regulated gut microbiome ensures low levels of inflammation in the brain and body. Lifestyle modifications and dietary coaching could be practical interventions for patients with psychiatric conditions.5 Current advances in technology now offer precise analyses of thousands of metabolites, enabling metabolomics to offer the promise of discovering new drug targets and biomarkers that may help pave a way to precision medicine.
The “human microbiota” describes all microorganisms within the human body, including bacteria, viruses, and eukaryotes. The related term “microbiome” refers to the complete catalog of these microbes and their genes.1 There is a growing awareness that the human microbiota plays an important role in maintaining mental health, and that a disruption in its composition can contribute to manifestations of psychiatric disorders. A growing body of evidence has also linked mental health outcomes to the gut microbiome, suggesting that the gut microbiota can modulate the gut-brain axis.2
Numerous neurotransmitters, including dopamine, serotonin, gamma-aminobutyric acid, and acetylcholine, are produced in the gastrointestinal (GI) tract, and our diet is vital in sustaining and replenishing them. At the same time, our brain regulates our GI tract by secretion of hormones such as oxytocin, leptin, ghrelin, neuropeptide Y, corticotrophin-releasing factor, and a plethora of others. Dysregulation of this microbiome can lead to both physical and mental illnesses. Symptoms of psychiatric disorders, such as depression, psychosis, anxiety, and autism, can be a consequence of this dysregulation.2
Our diet can also modify the gut microorganisms and therefore many of its metabolic pathways. More attention has been given to pre- and probiotics and their effects on DNA by epigenetic changes. One can quickly start to appreciate how this intricate crosstalk can lead to a variety of pathologic and psychiatric problems that have an adverse effect on autoimmune, inflammatory, metabolic, cognitive, and behavioral processes.2,3
Thus far, links have mostly been reported in animal models, and human studies are limited.4 Researchers are just beginning to elucidate how the microbiota affect gut-brain signaling in humans. Such mechanisms may include alterations in microbial composition, immune activation, vagus nerve signaling, alterations in tryptophan metabolism, production of specific microbial neuroactive metabolites, and bacterial cell wall sugars.5 The microbiota-gut-brain axis plays a part in regulating/programming the hypothalamic-pituitary-adrenal (HPA) axis throughout the life span.3 The interactions between the gut microbiome, the immune system, and the CNS are regulated through pathways that involve endocrine functions (HPA axis), the immune system, and metabolic factors.3,4 Recent research focusing on the gut microbiome has also given rise to international projects such as the Human Microbiome Project (Human Microbiome Project Consortium, 2012).3
Several studies have looked into psychiatry and inflammatory/immune pathways. Here we review 3 recent studies that have focused on the gut-brain axis (Table6-8).
1. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
The aim of this study was to evaluate immunoglobulin G (IgG) response against 40 food products in patients with depression vs those in a control group, along with changes in inflammatory markers, psychological stress, and dietary variables.6
Study design
- N = 63, IgG levels against 44 food products, cortisol levels, tumor necrosis factor (TNF)-alpha, interleukin 6 (IL-6), and IL-1 beta levels were recorded. The psychological parameters of 34 participants with depression and 29 controls were compared using the Hamilton Depression Rating scale, (HAM-D-17), Perceived Stress scale, and Symptom Checklist scale. The study was conducted in Poland.
Continue to: Outcomes
Outcomes
- Patients who were depressed had lower IgG levels against dairy products compared to controls when there was high dairy consumption. However, there was no overall difference between patients and controls in mean IgG concentration against food products.
- Patients who were depressed had higher levels of cortisol. Levels of cortisol had a positive correlation with HAM-D-17 score. Patients with depression had lower levels of TNF-alpha.
Conclusion
- Patients with depression had lower levels of IgG against dairy protein. Patients with depression had high cortisol levels but decreased levels of TNF-alpha, which could explain an immune suppression of IgG in these patients. There were no differences in IL-6 or IL-1beta levels.
Hypercortisolemia is present in approximately 60% of patients with depression. Elevated cortisol levels have a negative effect on lymphocyte function. B-lymphocytes (CD 10+ and CD 19+) are sensitive to glucocorticoids. Studies in mice have demonstrated that elevated glucocorticoid levels are associated with a 50% decrease in serum B-lymphocytes, and this can be explained by downregulation of c-myc protein, which plays a role in cell proliferation and cell survival. Glucocorticoids also decrease levels of protein kinases that are vital for the cell cycle to continue, and they upregulate p27 and p21, which are cell cycle inhibitors. Therefore, if high cortisol suppresses B-lymphocyte production, this can explain how patients with depression have low IgG levels, since B-lymphocytes differentiate into plasma cells that will produce antibodies.6
Depression can trigger an inflammatory response by increasing levels of inflammatory cytokines, acute phase reactants, and oxidative molecules. The inflammatory response can lead to intestinal wall disruption, and therefore bacteria can migrate across the GI barrier, along with food antigens, which could then lead to food antigen hypersensitivity.6
The significance of diet
Many studies have looked into specific types of diets, such as the Mediterranean diet, the ketogenic diet, and the addition of supplements such as probiotics, omega-3 fatty acids, zinc, and multivitamins.7 The Mediterranean diet is high in fiber, nuts, legumes, and fish.7 The ketogenic diet includes a controlled amount of fat, but is low in protein and carbohydrates.7 The main point is that a balanced diet can have a positive effect on mental health.7 The Mediterranean diet has shown to decrease the incidence of cardiovascular disease and lower the risk of depression.7 In animal studies, the ketogenic diet has improved anxiety, depression, and autism.7 Diet clearly affects gut microbiota and, as a consequence, the body’s level of inflammation.7
Continue to: The following review...
The following review highlighted the significance of diet on gut microbiome and mental health.7
2. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut- brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17: 1-9.
Study design
- These researchers provided a narrative review of the significance of a healthy diet and nutritional supplements on the gut microbiome and the treatment of patients with psychiatric illness.
Outcomes
- This review suggested dietary coaching as a nonpharmacologic treatment for patients with psychiatric illness.
Conclusion
- The utilization of nutritional advice, along with medication management, therapy, and physical activity, can provide a holistic approach to the biopsychosocial treatment of patients with psychiatric illness.
This review also emphasized the poor dietary trends of Westernized countries, which include calorie-dense, genetically altered, processed meals. As Mörkl et al7 noted, we are overfed but undernourished. Mörkl et al7 reviewed studies that involve dietary coaching as part of the treatment plan of patients with mental illness. In one of these studies, patients who received nutritional advice and coaching over 6 weeks had a 40% to 50% decrease in depressive symptoms. These effects persisted for 2 more years. Mörkl et al7 also reviewed an Italian study that found that providing nutritional advice in patients with affective disorders and psychosis helped improve symptom severity and sleep.7
Continue to: Mörkl et al...
Mörkl et al7 also reviewed dietary supplements. Some studies have linked use of omega-3 fatty acids with improvement in affective disorders, Alzheimer’s disease, and posttraumatic stress disorder, as well as cardiovascular conditions. Omega-3 fatty acids may exert beneficial effects by enhancing brain-derived neurotrophic factor and neurogenesis as well as by decreasing inflammation.7
Zinc supplementation can also improve depression, as it has been linked to cytokine variation and hippocampal neuronal growth. Vitamin B9 deficiency and vitamin D deficiency also have been associated with depression. Mörkl et al7 emphasized that a balanced diet that incorporates a variety of nutrients is more beneficial than supplementation of any individual vitamin alone.
Researchers have long emphasized the importance of a healthy balanced diet when treating patients with medical conditions such as cardiovascular or cerebrovascular diseases. Based on the studies Mörkl et al7 reviewed, the same emphasis should be communicated to our patients who suffer from psychiatric conditions.
The gut and anxiety
The gut microbiome has also been an area of research when studying generalized anxiety disorder (GAD).8
3. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
The aim of the study was to determine if there were changes in the composition of the gut microbiome in patients with GAD compared with healthy controls.8
Continue to: Study design
Study design
- A cross-sectional study of 76 patients in Zhejiang, China. Forty patients with GAD in the active state and 36 healthy controls were compared in terms of composition of GI microbacterial flora.
- Researchers also examined a subgroup of 12 patients who were treatment-naïve and 17 controls. Stool samples were collected from the 12 patients who were treatment-naïve before initiating medication.
- Researchers also conducted a prospective study in a subgroup of 9 patients with GAD in both the active state and remissive state. Two stool samples were collected from each patient—one during the active state of GAD and one during the remissive state—for a total of 18 samples. Stool samples analyzed with the use of polymerase chain reaction and microbial analysis.
- Patients completed the Hamilton Anxiety Rating (HAM-A) scale and were classified into groups. Those with HAM-A scores >14 were classified as being in the active state of GAD, and those with scores <7 were classified as being in the remissive state.
Outcomes
- Among the samples collected, 8 bacterial taxa were found in different amounts in patients with GAD and healthy controls. Bacteroidetes, Ruminococcus gnavus, and Fusobacterium were increased in patients with GAD compared with controls, while Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus were increased in healthy controls.
- Bacterial variety was notably lower in the 12 patients who were treatment-naïve compared with the control group.
- There was no notable difference in microbial composition between patients in the active vs remissive state.
Conclusion
- Patients with GAD had less short chain fatty acid–producing bacteria (Faecalibacterium, Eubacterium rectale, Sutterella, Lachnospira, and Butyricicoccus) compared with controls. Decreased formation of short chain fatty acids could lead to GI barrier disruption. Fusobacterium and Ruminococcus were increased in patients with GAD. Fusobacterium can cause disease and be invasive when it disseminates within the body. The inflammatory characteristics of Fusobacterium contribute to the immunologic activation in GAD. Ruminococcus breaks down mucin, which could then increase GI permeability by mucous degradation of the GI lumen.
Changes in food processing and manufacturing have led to changes in our diets. Changes in our normal GI microbacterial flora could lead to increased gut permeability, bacterial dissemination, and subsequent systemic inflammation. Research has shown that the composition of the microbiota changes across the life span.9 A balanced intake of nutrients is important for both our physical and mental health and safeguards the basis of gut microbiome regulation. A well-regulated gut microbiome ensures low levels of inflammation in the brain and body. Lifestyle modifications and dietary coaching could be practical interventions for patients with psychiatric conditions.5 Current advances in technology now offer precise analyses of thousands of metabolites, enabling metabolomics to offer the promise of discovering new drug targets and biomarkers that may help pave a way to precision medicine.
1. Dave M, Higgins PD, Middha S, et al. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160:246-257.
2. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
3. Malan-Muller S, Valles-Colomer M, Raes J, et al. The gut microbiome and mental health: implications for anxiety-and trauma-related disorders. OMICS. 2018;22(2):90-107.
4. Du Toit A. The gut microbiome and mental health. Nat Rev Microbiol. 2019;17(4):196.
5. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
6. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
7. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17:1-9.
8. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
9. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167(4):374-379.
1. Dave M, Higgins PD, Middha S, et al. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160:246-257.
2. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
3. Malan-Muller S, Valles-Colomer M, Raes J, et al. The gut microbiome and mental health: implications for anxiety-and trauma-related disorders. OMICS. 2018;22(2):90-107.
4. Du Toit A. The gut microbiome and mental health. Nat Rev Microbiol. 2019;17(4):196.
5. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701-712.
6. Rudzki L, Pawlak D, Pawlak K, et al. Immune suppression of IgG response against dairy proteins in major depression. BMC Psychiatry. 2017;17(1):268.
7. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology. 2018;17:1-9.
8. Jiang HY, Zhang X, Yu ZH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130-136.
9. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167(4):374-379.
When a disaster disrupts access to psychiatric medications
In recent decades, disasters such as storms, earthquakes, and terrorism have occurred with increasing frequency. Disaster planners assess the needs and vulnerabilities of communities in order to save lives during these events. They focus on providing electricity and clean water and addressing other public health measures. What is not adequately planned for, in our opinion, is a disruption in the pharmaceutical supply chain, particularly supplies of psychiatric medications.
There is now a rich literature on disaster psychiatry.1-4 However, there’s been a lack of information about disrupted access to psychiatric medications. Disruptive behavior after Hurricanes Katrina, Maria, Rita, and others were a consequence of a lack of medications or difficulty obtaining medications following these disasters.5-7
This article discusses the pharmaceutical supply chain, the lack of stockpiles of psychiatric medications, and how clinicians can prepare themselves and their patients in the event a disaster strikes.
Supply chains
Each day, nearly 12 million prescriptions are filled in the United States, with gratifying swiftness, efficiency, and accuracy. Our confidence in the nation’s pharmaceutical dependability, however, rests squarely upon the strength and resilience of vast, interconnected supply chains that involve the myriad aspects of private industry—from manufacturing to shipping and transport to last-mile delivery from pharmacy to patient. The failure of any one of the links in any of these supply chains can result in the instant unavailability of critical medications.
Supply chains are fundamental to modern life and must fluctuate to address disruptions; however, common supplemental and gap-filling functions that address minor changes may be insufficient to mitigate supply chain disruptions during a disaster. While supply chains can be extremely complex and can vary significantly from product to product, all supply chains can generally be presented through the components found in Table 1.
All components within a supply chain, such as the transportation mechanisms between nodes, facilities, people, and communication networks, can affect a supply chain’s resilience. For a supply chain to be resilient, key players—in this case, psychiatrists and associated medical professionals—must be acutely aware of the supply chain elements within their vision and reasonable anticipation: known nodes and links, their potential vulnerabilities, and ways and means to mitigate expected disruption.
Recent natural disasters, especially Hurricanes Katrina, Sandy, Harvey, and Maria, have given both government emergency management (at all levels) and clinicians the opportunity to understand the full effects of broken pharmaceutical supply chains under varying and extreme circumstances.
Continue to: As stated in a...
As stated in a recent Department of Homeland Security health care supply chain report, “Pharmaceuticals are one of the top concerns for healthcare providers in terms of supply chain disruptions. They are prone to various supply chain problems, including limited sources, lack of alternatives, time sensitivity, frequent shortages, and minimal on-site inventories. Each stakeholder along the pharmaceutical supply chain faces challenges with understanding and planning for possible disruptions emerging further up the chain. The rapidly expanding use of just-in-time inventory practices by distributors and healthcare customers is creating an increasingly fragile supply-demand balance that could be highly disrupted by a major event either further up the supply chain or within the last mile of delivery.”8,9
No national stockpiles of psychiatric medications
The CDC maintains stockpiles of emergency medications, but these supplies focus on medications to combat infection. In these caches, there are no psychiatric medications other than diazepam, which is stocked for its ability to combat the effects of nerve agents.
In major storm-related events, such as Hurricane Katrina in New Orleans in 2005, the disruptions in all supply chains included psychiatric medications. In the aftermath, many people with addictions and/or severe mental illnesses did not receive either their drugs of choice and/or antimanic and antipsychotic medications. As a result, disruptive behavior became common, especially in the shelters.5-7
During a widespread public emergency, police and emergency services are often stretched very thin. In calmer times, police or emergency services may take a person with disruptive and aggressive behavior to a local emergency department. However, in times of chaos, such as during Hurricane Katrina, patients with aggressive or disruptive behaviors were forcefully incapacitated (ie, “tased”) or shot.
Withdrawal from antidepressants, opiates, alcohol, and benzodiazepines has its own risks. Withdrawal from alcohol or benzodiazepines can be life-threatening. Therefore, it is critically important that clinicians think about how to ensure their patients have a supply of their medications. This may imply stockpiling on a personal or community basis.
Continue to: What to consider before disruption
What to consider before disruption
Many psychiatrists, especially those who have not practiced through a local disaster, may have never contemplated how they would support their patients during a disruptive event. Psychiatrists should carefully consider the questions outlined in Table 2 before a disaster strikes.
Medication-specific issues
During major disasters, patients may not have access to their medications, or the medications may not be able to be fed into the health care system for dispersion. Other issues include closed pharmacies, expired medications as a result of limited refrigeration service, inability to deliver medications to an affected area, and the inability of manufacturing plants to produce medications. For example, after Hurricane Maria, sterile water was in short supply.
After a major disaster, clinicians often leave their communities because they cannot support themselves or their practices. Thus, clinicians may not be available to prescribe needed medications. Available clinicians—often primary care physicians—may not be aware of a patient’s medication history, or they may be uncomfortable prescribing psychiatric medications, especially antipsychotics.
Abrupt discontinuation of psychiatric medications can have severe consequences. Patients may experience withdrawal symptoms, worsening psychiatric symptoms, new-onset psychiatric symptoms, thoughts of harm to self or others, psychosis, or cravings. These issues may be particularly problematic for patients receiving antidepressants, antipsychotics, benzodiazepines, or medication-assisted treatment for opioid use disorder.
Antidepressants. Patients experiencing antidepressant withdrawal, particularly withdrawal from selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, may exhibit severe symptoms. In addition to the potential recurrence of depressive or anxiety symptoms and suicidal thoughts, patients may experience irritability, insomnia, headache, nausea, and electric shock–like sensations. Prescribing an antidepressant with a longer half-life could potentially prevent an abrupt withdrawal in the event a disaster occurs.
Continue to: Antipsychotics
Antipsychotics. Rapid or abrupt withdrawal of antipsychotics could lead to an increase in psychosis, paranoia, hallucinations, or delusions. Withdrawal of antipsychotics could also lead to agitation, restlessness, insomnia, paresthesia, and anxiety. If a known disaster is likely to occur, such as in the case of a hurricane forecast, clinicians may consider switching a patient a long-acting injectable antipsychotic to minimize the risk of withdrawal and symptom exacerbation.
Benzodiazepines. The abrupt withdrawal of benzodiazepines could result in symptoms that include rebound anxiety, insomnia, restlessness, muscle tension, irritability, nausea, malaise, blurred vision, diaphoresis, nightmares, and seizures. Additionally, many people use benzodiazepines recreationally, and their illicit supply may run out during disasters, which could lead to untreated withdrawal and violence in the community.
Clinicians need to develop action plans for any patients who are receiving scheduled benzodiazepine dosing in order to prevent abrupt withdrawal if a disaster occurs.
Opioids. Opioid cravings and withdrawal are also a major concern during times of disrupted supply. Patients receiving chronic opioid therapy may not be able to receive their maintenance medications, which could lead to withdrawal. Additionally, patients taking illicit opioids may also be at risk of withdrawal.
Early symptoms of opioid withdrawal include watery eyes, runny nose, sweating, anxiety and irritability, poor sleep, and muscle pain. Later symptoms could include cramping, diarrhea, vomiting, increased heart rate and blood pressure, restlessness, shakiness, chills, sweating, and dilated pupils.
Continue to: Contingency planning...
Contingency planning should be a part of the treatment plan for every patient receiving chronic opioid therapy who lives in an area where major disasters are likely to occur.
Medication-assisted treatment for opioid use disorder. Patients receiving treatment for opioid use disorder may be prescribed the partial opioid agonist buprenorphine, either by itself or in combination with the opioid antagonist naloxone. This could be particularly problematic to continue in a major disaster due to the lack of credentialed clinicians, limited supplies, and patients only receiving small amounts of the medication at a time due to the risk of diversion.
Symptoms of buprenorphine withdrawal are similar to those associated with opioid withdrawal. Developing a thoughtful plan in case of a disaster should be part of all buprenorphine prescribing. Patients should be aware of withdrawal symptoms and what to do if they run out of medication.
Additionally, emergency clinicians should have access to buprenorphine and buprenorphine/naloxone and the ability to prescribe them in disaster situations. As with all aspects of disaster response, it is wise to work out issues in advance.
Help your patients get ready
Advise your patients to prepare emergency kits that contain their psychiatric medications that they could quickly grab and go if needed. Because there may be times when it is not possible to gather all necessary medications, having even a small supply ready to go at a moment’s notice would be beneficial. If permitted, patients should also consider keeping medications in multiple locations, including at their place of work, home, or a family member’s home.
Continue to: Additionally, instruct patients...
Additionally, instruct patients to always carry a list of all medications they currently take. Ideally, this list should also include past medications and responses, allergies, and provider contact information. During a disaster, this information could prove vital to an emergency clinician. At a minimum, verify that your patient maintains a list of current medications.
Clinicians should develop emergency plans for all psychiatric medications they prescribe. Document and discuss with your patients any necessary considerations for patients who take medications that require more intensive monitoring, such as lithium or clozapine.
Clinicians, patients, emergency responders, and health care workers need to work together to prepare for major disasters to avoid withdrawal and other consequences of disrupted access to psychiatric medications.
Bottom Line
Consult with local public health officials to determine and develop contingency plans to provide psychiatric medications to your patients in the event of a disaster. Discuss treatment plans and contingency planning with patients, particularly those in regions most likely to be affected by a disaster. Instruct patients to refill medications prior to a foreseeable disaster and to maintain a personal stockpile of medications when appropriate.
Related Resources
- Ochi S, Hodgson S, Landeg O, et al. Disaster-driven evacuation and medication loss: A systematic literature review. PLoS Curr. 2014;6.b. doi: 10.1371/currents.dis.fa417630b566a0c7dfdbf945910edd96.
- Pate JE, Fisher JW. Disaster ethics: What are the ground rules? Current Psychiatry. 2007;6(6):69-78.
Drug Brand Names
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone
Clozapine • Clozaril
Diazepam • Valium
Lithium • Eskalith, Lithobid
1. National Institute of Mental Health. Mental health and mass violence: evidence based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. https://cpa.ca/docs/File/Emergencies/massviolence.pdf. Published 2002. Accessed March 11, 2019.
2. Ritchie EC, Friedman M, Watson P. Interventions following mass violence and disasters: strategies for mental health practice. New York, NY: Guilford Press; 2006.
3. Ritchie EC, O’Brien K, Grant M, et al. Disaster psychiatry. In: Stern TA, Rosenbaum JF, Fava M, et al. The Massachusetts General Hospital textbook of comprehensive clinical psychiatry, 2nd edition. Philadelphia, PA: Mosby/Elsevier; 2016:968-974.
4. Ritchie EC, Hamilton S. Early interventions and risk assessment following disaster. Psychiatric Annals. 2004;34(8):605-610.
5. Kessler RC, Galea S, Gruber MJ, et al. Trends in mental illness and suicidality after Hurricane Katrina. Mol Psychiatry. 2008;13(4):374-384.
6. Weisler RH, Barbee JG IV, Townsend MH. Mental health and recovery in the Gulf Coast after Hurricanes Katrina and Rita. JAMA. 2006;296(5):585-588.
7. Galea S, Brewin CR, Gruber M, et al. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64(12).1427-1434.
8. Federal Emergency Management Agency. Supply Chain Resilience Guide Department of Homeland Security. https://www.fema.gov/media-library-data/1544795397837-767851ba177c7097bf8672aadf8a93c9/NE_DRAFT_Supply_Chain_Resilience.pdf. Published December 17, 2018. Accessed January 2, 2019.
9. Durkin J, Telab M, Fitzmaurice P, et al. Only as strong as its weakest link: resilience of the healthcare supply chain in New York. https://www.hstoday.us/subject-matter-areas/emergency-preparedness/only-as-strong-as-its-weakest-link-the-resilience-of-the-healthcare-supply-chain-in-new-york/. Published October 26, 2018. Accessed February 14, 2019.
In recent decades, disasters such as storms, earthquakes, and terrorism have occurred with increasing frequency. Disaster planners assess the needs and vulnerabilities of communities in order to save lives during these events. They focus on providing electricity and clean water and addressing other public health measures. What is not adequately planned for, in our opinion, is a disruption in the pharmaceutical supply chain, particularly supplies of psychiatric medications.
There is now a rich literature on disaster psychiatry.1-4 However, there’s been a lack of information about disrupted access to psychiatric medications. Disruptive behavior after Hurricanes Katrina, Maria, Rita, and others were a consequence of a lack of medications or difficulty obtaining medications following these disasters.5-7
This article discusses the pharmaceutical supply chain, the lack of stockpiles of psychiatric medications, and how clinicians can prepare themselves and their patients in the event a disaster strikes.
Supply chains
Each day, nearly 12 million prescriptions are filled in the United States, with gratifying swiftness, efficiency, and accuracy. Our confidence in the nation’s pharmaceutical dependability, however, rests squarely upon the strength and resilience of vast, interconnected supply chains that involve the myriad aspects of private industry—from manufacturing to shipping and transport to last-mile delivery from pharmacy to patient. The failure of any one of the links in any of these supply chains can result in the instant unavailability of critical medications.
Supply chains are fundamental to modern life and must fluctuate to address disruptions; however, common supplemental and gap-filling functions that address minor changes may be insufficient to mitigate supply chain disruptions during a disaster. While supply chains can be extremely complex and can vary significantly from product to product, all supply chains can generally be presented through the components found in Table 1.
All components within a supply chain, such as the transportation mechanisms between nodes, facilities, people, and communication networks, can affect a supply chain’s resilience. For a supply chain to be resilient, key players—in this case, psychiatrists and associated medical professionals—must be acutely aware of the supply chain elements within their vision and reasonable anticipation: known nodes and links, their potential vulnerabilities, and ways and means to mitigate expected disruption.
Recent natural disasters, especially Hurricanes Katrina, Sandy, Harvey, and Maria, have given both government emergency management (at all levels) and clinicians the opportunity to understand the full effects of broken pharmaceutical supply chains under varying and extreme circumstances.
Continue to: As stated in a...
As stated in a recent Department of Homeland Security health care supply chain report, “Pharmaceuticals are one of the top concerns for healthcare providers in terms of supply chain disruptions. They are prone to various supply chain problems, including limited sources, lack of alternatives, time sensitivity, frequent shortages, and minimal on-site inventories. Each stakeholder along the pharmaceutical supply chain faces challenges with understanding and planning for possible disruptions emerging further up the chain. The rapidly expanding use of just-in-time inventory practices by distributors and healthcare customers is creating an increasingly fragile supply-demand balance that could be highly disrupted by a major event either further up the supply chain or within the last mile of delivery.”8,9
No national stockpiles of psychiatric medications
The CDC maintains stockpiles of emergency medications, but these supplies focus on medications to combat infection. In these caches, there are no psychiatric medications other than diazepam, which is stocked for its ability to combat the effects of nerve agents.
In major storm-related events, such as Hurricane Katrina in New Orleans in 2005, the disruptions in all supply chains included psychiatric medications. In the aftermath, many people with addictions and/or severe mental illnesses did not receive either their drugs of choice and/or antimanic and antipsychotic medications. As a result, disruptive behavior became common, especially in the shelters.5-7
During a widespread public emergency, police and emergency services are often stretched very thin. In calmer times, police or emergency services may take a person with disruptive and aggressive behavior to a local emergency department. However, in times of chaos, such as during Hurricane Katrina, patients with aggressive or disruptive behaviors were forcefully incapacitated (ie, “tased”) or shot.
Withdrawal from antidepressants, opiates, alcohol, and benzodiazepines has its own risks. Withdrawal from alcohol or benzodiazepines can be life-threatening. Therefore, it is critically important that clinicians think about how to ensure their patients have a supply of their medications. This may imply stockpiling on a personal or community basis.
Continue to: What to consider before disruption
What to consider before disruption
Many psychiatrists, especially those who have not practiced through a local disaster, may have never contemplated how they would support their patients during a disruptive event. Psychiatrists should carefully consider the questions outlined in Table 2 before a disaster strikes.
Medication-specific issues
During major disasters, patients may not have access to their medications, or the medications may not be able to be fed into the health care system for dispersion. Other issues include closed pharmacies, expired medications as a result of limited refrigeration service, inability to deliver medications to an affected area, and the inability of manufacturing plants to produce medications. For example, after Hurricane Maria, sterile water was in short supply.
After a major disaster, clinicians often leave their communities because they cannot support themselves or their practices. Thus, clinicians may not be available to prescribe needed medications. Available clinicians—often primary care physicians—may not be aware of a patient’s medication history, or they may be uncomfortable prescribing psychiatric medications, especially antipsychotics.
Abrupt discontinuation of psychiatric medications can have severe consequences. Patients may experience withdrawal symptoms, worsening psychiatric symptoms, new-onset psychiatric symptoms, thoughts of harm to self or others, psychosis, or cravings. These issues may be particularly problematic for patients receiving antidepressants, antipsychotics, benzodiazepines, or medication-assisted treatment for opioid use disorder.
Antidepressants. Patients experiencing antidepressant withdrawal, particularly withdrawal from selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, may exhibit severe symptoms. In addition to the potential recurrence of depressive or anxiety symptoms and suicidal thoughts, patients may experience irritability, insomnia, headache, nausea, and electric shock–like sensations. Prescribing an antidepressant with a longer half-life could potentially prevent an abrupt withdrawal in the event a disaster occurs.
Continue to: Antipsychotics
Antipsychotics. Rapid or abrupt withdrawal of antipsychotics could lead to an increase in psychosis, paranoia, hallucinations, or delusions. Withdrawal of antipsychotics could also lead to agitation, restlessness, insomnia, paresthesia, and anxiety. If a known disaster is likely to occur, such as in the case of a hurricane forecast, clinicians may consider switching a patient a long-acting injectable antipsychotic to minimize the risk of withdrawal and symptom exacerbation.
Benzodiazepines. The abrupt withdrawal of benzodiazepines could result in symptoms that include rebound anxiety, insomnia, restlessness, muscle tension, irritability, nausea, malaise, blurred vision, diaphoresis, nightmares, and seizures. Additionally, many people use benzodiazepines recreationally, and their illicit supply may run out during disasters, which could lead to untreated withdrawal and violence in the community.
Clinicians need to develop action plans for any patients who are receiving scheduled benzodiazepine dosing in order to prevent abrupt withdrawal if a disaster occurs.
Opioids. Opioid cravings and withdrawal are also a major concern during times of disrupted supply. Patients receiving chronic opioid therapy may not be able to receive their maintenance medications, which could lead to withdrawal. Additionally, patients taking illicit opioids may also be at risk of withdrawal.
Early symptoms of opioid withdrawal include watery eyes, runny nose, sweating, anxiety and irritability, poor sleep, and muscle pain. Later symptoms could include cramping, diarrhea, vomiting, increased heart rate and blood pressure, restlessness, shakiness, chills, sweating, and dilated pupils.
Continue to: Contingency planning...
Contingency planning should be a part of the treatment plan for every patient receiving chronic opioid therapy who lives in an area where major disasters are likely to occur.
Medication-assisted treatment for opioid use disorder. Patients receiving treatment for opioid use disorder may be prescribed the partial opioid agonist buprenorphine, either by itself or in combination with the opioid antagonist naloxone. This could be particularly problematic to continue in a major disaster due to the lack of credentialed clinicians, limited supplies, and patients only receiving small amounts of the medication at a time due to the risk of diversion.
Symptoms of buprenorphine withdrawal are similar to those associated with opioid withdrawal. Developing a thoughtful plan in case of a disaster should be part of all buprenorphine prescribing. Patients should be aware of withdrawal symptoms and what to do if they run out of medication.
Additionally, emergency clinicians should have access to buprenorphine and buprenorphine/naloxone and the ability to prescribe them in disaster situations. As with all aspects of disaster response, it is wise to work out issues in advance.
Help your patients get ready
Advise your patients to prepare emergency kits that contain their psychiatric medications that they could quickly grab and go if needed. Because there may be times when it is not possible to gather all necessary medications, having even a small supply ready to go at a moment’s notice would be beneficial. If permitted, patients should also consider keeping medications in multiple locations, including at their place of work, home, or a family member’s home.
Continue to: Additionally, instruct patients...
Additionally, instruct patients to always carry a list of all medications they currently take. Ideally, this list should also include past medications and responses, allergies, and provider contact information. During a disaster, this information could prove vital to an emergency clinician. At a minimum, verify that your patient maintains a list of current medications.
Clinicians should develop emergency plans for all psychiatric medications they prescribe. Document and discuss with your patients any necessary considerations for patients who take medications that require more intensive monitoring, such as lithium or clozapine.
Clinicians, patients, emergency responders, and health care workers need to work together to prepare for major disasters to avoid withdrawal and other consequences of disrupted access to psychiatric medications.
Bottom Line
Consult with local public health officials to determine and develop contingency plans to provide psychiatric medications to your patients in the event of a disaster. Discuss treatment plans and contingency planning with patients, particularly those in regions most likely to be affected by a disaster. Instruct patients to refill medications prior to a foreseeable disaster and to maintain a personal stockpile of medications when appropriate.
Related Resources
- Ochi S, Hodgson S, Landeg O, et al. Disaster-driven evacuation and medication loss: A systematic literature review. PLoS Curr. 2014;6.b. doi: 10.1371/currents.dis.fa417630b566a0c7dfdbf945910edd96.
- Pate JE, Fisher JW. Disaster ethics: What are the ground rules? Current Psychiatry. 2007;6(6):69-78.
Drug Brand Names
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone
Clozapine • Clozaril
Diazepam • Valium
Lithium • Eskalith, Lithobid
In recent decades, disasters such as storms, earthquakes, and terrorism have occurred with increasing frequency. Disaster planners assess the needs and vulnerabilities of communities in order to save lives during these events. They focus on providing electricity and clean water and addressing other public health measures. What is not adequately planned for, in our opinion, is a disruption in the pharmaceutical supply chain, particularly supplies of psychiatric medications.
There is now a rich literature on disaster psychiatry.1-4 However, there’s been a lack of information about disrupted access to psychiatric medications. Disruptive behavior after Hurricanes Katrina, Maria, Rita, and others were a consequence of a lack of medications or difficulty obtaining medications following these disasters.5-7
This article discusses the pharmaceutical supply chain, the lack of stockpiles of psychiatric medications, and how clinicians can prepare themselves and their patients in the event a disaster strikes.
Supply chains
Each day, nearly 12 million prescriptions are filled in the United States, with gratifying swiftness, efficiency, and accuracy. Our confidence in the nation’s pharmaceutical dependability, however, rests squarely upon the strength and resilience of vast, interconnected supply chains that involve the myriad aspects of private industry—from manufacturing to shipping and transport to last-mile delivery from pharmacy to patient. The failure of any one of the links in any of these supply chains can result in the instant unavailability of critical medications.
Supply chains are fundamental to modern life and must fluctuate to address disruptions; however, common supplemental and gap-filling functions that address minor changes may be insufficient to mitigate supply chain disruptions during a disaster. While supply chains can be extremely complex and can vary significantly from product to product, all supply chains can generally be presented through the components found in Table 1.
All components within a supply chain, such as the transportation mechanisms between nodes, facilities, people, and communication networks, can affect a supply chain’s resilience. For a supply chain to be resilient, key players—in this case, psychiatrists and associated medical professionals—must be acutely aware of the supply chain elements within their vision and reasonable anticipation: known nodes and links, their potential vulnerabilities, and ways and means to mitigate expected disruption.
Recent natural disasters, especially Hurricanes Katrina, Sandy, Harvey, and Maria, have given both government emergency management (at all levels) and clinicians the opportunity to understand the full effects of broken pharmaceutical supply chains under varying and extreme circumstances.
Continue to: As stated in a...
As stated in a recent Department of Homeland Security health care supply chain report, “Pharmaceuticals are one of the top concerns for healthcare providers in terms of supply chain disruptions. They are prone to various supply chain problems, including limited sources, lack of alternatives, time sensitivity, frequent shortages, and minimal on-site inventories. Each stakeholder along the pharmaceutical supply chain faces challenges with understanding and planning for possible disruptions emerging further up the chain. The rapidly expanding use of just-in-time inventory practices by distributors and healthcare customers is creating an increasingly fragile supply-demand balance that could be highly disrupted by a major event either further up the supply chain or within the last mile of delivery.”8,9
No national stockpiles of psychiatric medications
The CDC maintains stockpiles of emergency medications, but these supplies focus on medications to combat infection. In these caches, there are no psychiatric medications other than diazepam, which is stocked for its ability to combat the effects of nerve agents.
In major storm-related events, such as Hurricane Katrina in New Orleans in 2005, the disruptions in all supply chains included psychiatric medications. In the aftermath, many people with addictions and/or severe mental illnesses did not receive either their drugs of choice and/or antimanic and antipsychotic medications. As a result, disruptive behavior became common, especially in the shelters.5-7
During a widespread public emergency, police and emergency services are often stretched very thin. In calmer times, police or emergency services may take a person with disruptive and aggressive behavior to a local emergency department. However, in times of chaos, such as during Hurricane Katrina, patients with aggressive or disruptive behaviors were forcefully incapacitated (ie, “tased”) or shot.
Withdrawal from antidepressants, opiates, alcohol, and benzodiazepines has its own risks. Withdrawal from alcohol or benzodiazepines can be life-threatening. Therefore, it is critically important that clinicians think about how to ensure their patients have a supply of their medications. This may imply stockpiling on a personal or community basis.
Continue to: What to consider before disruption
What to consider before disruption
Many psychiatrists, especially those who have not practiced through a local disaster, may have never contemplated how they would support their patients during a disruptive event. Psychiatrists should carefully consider the questions outlined in Table 2 before a disaster strikes.
Medication-specific issues
During major disasters, patients may not have access to their medications, or the medications may not be able to be fed into the health care system for dispersion. Other issues include closed pharmacies, expired medications as a result of limited refrigeration service, inability to deliver medications to an affected area, and the inability of manufacturing plants to produce medications. For example, after Hurricane Maria, sterile water was in short supply.
After a major disaster, clinicians often leave their communities because they cannot support themselves or their practices. Thus, clinicians may not be available to prescribe needed medications. Available clinicians—often primary care physicians—may not be aware of a patient’s medication history, or they may be uncomfortable prescribing psychiatric medications, especially antipsychotics.
Abrupt discontinuation of psychiatric medications can have severe consequences. Patients may experience withdrawal symptoms, worsening psychiatric symptoms, new-onset psychiatric symptoms, thoughts of harm to self or others, psychosis, or cravings. These issues may be particularly problematic for patients receiving antidepressants, antipsychotics, benzodiazepines, or medication-assisted treatment for opioid use disorder.
Antidepressants. Patients experiencing antidepressant withdrawal, particularly withdrawal from selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, may exhibit severe symptoms. In addition to the potential recurrence of depressive or anxiety symptoms and suicidal thoughts, patients may experience irritability, insomnia, headache, nausea, and electric shock–like sensations. Prescribing an antidepressant with a longer half-life could potentially prevent an abrupt withdrawal in the event a disaster occurs.
Continue to: Antipsychotics
Antipsychotics. Rapid or abrupt withdrawal of antipsychotics could lead to an increase in psychosis, paranoia, hallucinations, or delusions. Withdrawal of antipsychotics could also lead to agitation, restlessness, insomnia, paresthesia, and anxiety. If a known disaster is likely to occur, such as in the case of a hurricane forecast, clinicians may consider switching a patient a long-acting injectable antipsychotic to minimize the risk of withdrawal and symptom exacerbation.
Benzodiazepines. The abrupt withdrawal of benzodiazepines could result in symptoms that include rebound anxiety, insomnia, restlessness, muscle tension, irritability, nausea, malaise, blurred vision, diaphoresis, nightmares, and seizures. Additionally, many people use benzodiazepines recreationally, and their illicit supply may run out during disasters, which could lead to untreated withdrawal and violence in the community.
Clinicians need to develop action plans for any patients who are receiving scheduled benzodiazepine dosing in order to prevent abrupt withdrawal if a disaster occurs.
Opioids. Opioid cravings and withdrawal are also a major concern during times of disrupted supply. Patients receiving chronic opioid therapy may not be able to receive their maintenance medications, which could lead to withdrawal. Additionally, patients taking illicit opioids may also be at risk of withdrawal.
Early symptoms of opioid withdrawal include watery eyes, runny nose, sweating, anxiety and irritability, poor sleep, and muscle pain. Later symptoms could include cramping, diarrhea, vomiting, increased heart rate and blood pressure, restlessness, shakiness, chills, sweating, and dilated pupils.
Continue to: Contingency planning...
Contingency planning should be a part of the treatment plan for every patient receiving chronic opioid therapy who lives in an area where major disasters are likely to occur.
Medication-assisted treatment for opioid use disorder. Patients receiving treatment for opioid use disorder may be prescribed the partial opioid agonist buprenorphine, either by itself or in combination with the opioid antagonist naloxone. This could be particularly problematic to continue in a major disaster due to the lack of credentialed clinicians, limited supplies, and patients only receiving small amounts of the medication at a time due to the risk of diversion.
Symptoms of buprenorphine withdrawal are similar to those associated with opioid withdrawal. Developing a thoughtful plan in case of a disaster should be part of all buprenorphine prescribing. Patients should be aware of withdrawal symptoms and what to do if they run out of medication.
Additionally, emergency clinicians should have access to buprenorphine and buprenorphine/naloxone and the ability to prescribe them in disaster situations. As with all aspects of disaster response, it is wise to work out issues in advance.
Help your patients get ready
Advise your patients to prepare emergency kits that contain their psychiatric medications that they could quickly grab and go if needed. Because there may be times when it is not possible to gather all necessary medications, having even a small supply ready to go at a moment’s notice would be beneficial. If permitted, patients should also consider keeping medications in multiple locations, including at their place of work, home, or a family member’s home.
Continue to: Additionally, instruct patients...
Additionally, instruct patients to always carry a list of all medications they currently take. Ideally, this list should also include past medications and responses, allergies, and provider contact information. During a disaster, this information could prove vital to an emergency clinician. At a minimum, verify that your patient maintains a list of current medications.
Clinicians should develop emergency plans for all psychiatric medications they prescribe. Document and discuss with your patients any necessary considerations for patients who take medications that require more intensive monitoring, such as lithium or clozapine.
Clinicians, patients, emergency responders, and health care workers need to work together to prepare for major disasters to avoid withdrawal and other consequences of disrupted access to psychiatric medications.
Bottom Line
Consult with local public health officials to determine and develop contingency plans to provide psychiatric medications to your patients in the event of a disaster. Discuss treatment plans and contingency planning with patients, particularly those in regions most likely to be affected by a disaster. Instruct patients to refill medications prior to a foreseeable disaster and to maintain a personal stockpile of medications when appropriate.
Related Resources
- Ochi S, Hodgson S, Landeg O, et al. Disaster-driven evacuation and medication loss: A systematic literature review. PLoS Curr. 2014;6.b. doi: 10.1371/currents.dis.fa417630b566a0c7dfdbf945910edd96.
- Pate JE, Fisher JW. Disaster ethics: What are the ground rules? Current Psychiatry. 2007;6(6):69-78.
Drug Brand Names
Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone
Clozapine • Clozaril
Diazepam • Valium
Lithium • Eskalith, Lithobid
1. National Institute of Mental Health. Mental health and mass violence: evidence based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. https://cpa.ca/docs/File/Emergencies/massviolence.pdf. Published 2002. Accessed March 11, 2019.
2. Ritchie EC, Friedman M, Watson P. Interventions following mass violence and disasters: strategies for mental health practice. New York, NY: Guilford Press; 2006.
3. Ritchie EC, O’Brien K, Grant M, et al. Disaster psychiatry. In: Stern TA, Rosenbaum JF, Fava M, et al. The Massachusetts General Hospital textbook of comprehensive clinical psychiatry, 2nd edition. Philadelphia, PA: Mosby/Elsevier; 2016:968-974.
4. Ritchie EC, Hamilton S. Early interventions and risk assessment following disaster. Psychiatric Annals. 2004;34(8):605-610.
5. Kessler RC, Galea S, Gruber MJ, et al. Trends in mental illness and suicidality after Hurricane Katrina. Mol Psychiatry. 2008;13(4):374-384.
6. Weisler RH, Barbee JG IV, Townsend MH. Mental health and recovery in the Gulf Coast after Hurricanes Katrina and Rita. JAMA. 2006;296(5):585-588.
7. Galea S, Brewin CR, Gruber M, et al. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64(12).1427-1434.
8. Federal Emergency Management Agency. Supply Chain Resilience Guide Department of Homeland Security. https://www.fema.gov/media-library-data/1544795397837-767851ba177c7097bf8672aadf8a93c9/NE_DRAFT_Supply_Chain_Resilience.pdf. Published December 17, 2018. Accessed January 2, 2019.
9. Durkin J, Telab M, Fitzmaurice P, et al. Only as strong as its weakest link: resilience of the healthcare supply chain in New York. https://www.hstoday.us/subject-matter-areas/emergency-preparedness/only-as-strong-as-its-weakest-link-the-resilience-of-the-healthcare-supply-chain-in-new-york/. Published October 26, 2018. Accessed February 14, 2019.
1. National Institute of Mental Health. Mental health and mass violence: evidence based early psychological intervention for victims/survivors of mass violence. A workshop to reach consensus on best practices. https://cpa.ca/docs/File/Emergencies/massviolence.pdf. Published 2002. Accessed March 11, 2019.
2. Ritchie EC, Friedman M, Watson P. Interventions following mass violence and disasters: strategies for mental health practice. New York, NY: Guilford Press; 2006.
3. Ritchie EC, O’Brien K, Grant M, et al. Disaster psychiatry. In: Stern TA, Rosenbaum JF, Fava M, et al. The Massachusetts General Hospital textbook of comprehensive clinical psychiatry, 2nd edition. Philadelphia, PA: Mosby/Elsevier; 2016:968-974.
4. Ritchie EC, Hamilton S. Early interventions and risk assessment following disaster. Psychiatric Annals. 2004;34(8):605-610.
5. Kessler RC, Galea S, Gruber MJ, et al. Trends in mental illness and suicidality after Hurricane Katrina. Mol Psychiatry. 2008;13(4):374-384.
6. Weisler RH, Barbee JG IV, Townsend MH. Mental health and recovery in the Gulf Coast after Hurricanes Katrina and Rita. JAMA. 2006;296(5):585-588.
7. Galea S, Brewin CR, Gruber M, et al. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64(12).1427-1434.
8. Federal Emergency Management Agency. Supply Chain Resilience Guide Department of Homeland Security. https://www.fema.gov/media-library-data/1544795397837-767851ba177c7097bf8672aadf8a93c9/NE_DRAFT_Supply_Chain_Resilience.pdf. Published December 17, 2018. Accessed January 2, 2019.
9. Durkin J, Telab M, Fitzmaurice P, et al. Only as strong as its weakest link: resilience of the healthcare supply chain in New York. https://www.hstoday.us/subject-matter-areas/emergency-preparedness/only-as-strong-as-its-weakest-link-the-resilience-of-the-healthcare-supply-chain-in-new-york/. Published October 26, 2018. Accessed February 14, 2019.
Cannabidiol (CBD) for schizophrenia: Promise or pipe dream?
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.
Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.
Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
Over the past few decades, it has become increasingly clear that cannabis use can increase the risk of developing a psychotic disorder and worsen the course of existing schizophrenia in a dose-dependent fashion.1-3 Beyond psychosis, although many patients with mental illness use cannabis for recreational purposes or as purported “self-medication,” currently available evidence suggests that marijuana is more likely to represent a harm than a benefit for psychiatric disorders4 (Box4-8). Our current state of knowledge therefore suggests that psychiatrists should caution their patients against using cannabis and prioritize interventions to reduce or discontinue use, especially among those with psychotic disorders.
Box
Data from California in 2006—a decade after the state’s legalization of “medical marijuana”—revealed that 23% of patients in a sample enrolled in medical marijuana clinics were receiving cannabis to treat a mental disorder.5 That was a striking statistic given the dearth of evidence to support a benefit of cannabis for psychiatric conditions at the time, leaving clinicians who provided the necessary recommendations to obtain medical marijuana largely unable to give informed consent about the risks and benefits, much less recommendations about specific products, routes of administration, or dosing. In 2019, we know considerably more about the interaction between cannabinoids and mental health, but research findings thus far warrant more caution than enthusiasm, with one recent review concluding that “whenever an association is observed between cannabis use and psychiatric disorders, the relationship is generally an adverse one.”4
Some critics have argued that the medical marijuana industry represents little more than a front for recreational use. In California and other states that have legalized recreational use, that claim has been rendered all but moot, although the public remains curious about the potential health benefits of cannabinoids and will likely continue to look to clinicians for advice. For those seeking guidance from evidence-based research, the existing state of knowledge can seem like a “Wild West” of anecdotal subjective reports, biased opinions, and uncontrolled clinical studies. Cannabis remains a Schedule I drug at the federal level, and quality clinical research has been limited to a relatively modest number of randomized controlled trials (RCTs), mostly involving FDA-approved cannabinoids rather than smoked cannabis. Randomized controlled trials that have involved smoked marijuana have generally involved low-potency delta-9-tetrahydrocannabinol (THC) cannabis that may not reflect the same therapeutic and adverse effects of the increasingly high potency cannabis now available on the street and in dispensaries.
In psychiatry, a few RCTs are underway exploring cannabis as a viable treatment for mental disorders (eg, posttraumatic stress disorder), but none have yet been completed or published. At best, retrospective studies to date have failed to support a consistent benefit of cannabis for any psychiatric disorder and at worst increasingly suggest a negative impact on psychotic, mood, and anxiety disorders.4,6 Meanwhile, synthetic cannabinoid receptor agonists (eg, “Spice” products) have come to represent a clear public health risk, with both medical and psychiatric toxicity.7
A more cautiously optimistic case for the therapeutic potential of cannabinoids in psychiatry could be made for cannabidiol (CBD), which may possess anxiolytic, antipsychotic, and neuroprotective properties.8 Based on its purported health benefits, it is possible that CBD may even gain widespread popularity as a food supplement. Because a pharmaceutically-manufactured form of CBD was recently FDA-approved for the treatment of seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, off-label prescribing of CBD for psychiatric disorders can be anticipated. While there is not yet sufficient evidence about risks and benefits to justify CBD being recommended broadly in psychiatry, that same informational vacuum has not stopped eager patients from seeking approval for cannabis, and some physicians from providing it.
Despite that conclusion, because cannabis is classified as a Schedule I drug by the US Drug Enforcement Agency, clinical research investigating the risks and benefits of cannabis has been limited. It therefore remains possible that cannabis, or individual cannabinoids such as cannabidiol (CBD), may yet find a therapeutic niche in psychiatry. This article reviews evidence on CBD for the treatment of schizophrenia.
Cannabinergic drugs as potential antipsychotics
Although the bulk of evidence indicates a harmful effect of cannabis in individuals with or at risk for psychosis, there have been a few published cases of schizophrenia improving with dronabinol, an FDA-approved, synthetic form of delta-9-tetrahydrocannabinol (THC).9,10 THC is the constituent of cannabis that produces euphoric effects. These provocative findings have not been replicated in controlled clinical trials, but suggest at least the theoretical possibility of idiosyncratic benefits from THC for some individuals within the psychotic spectrum.
Still, given that most available evidence supports that THC has a harmful effect on psychosis and psychosis risk, researchers have instead performed randomized controlled trials (RCTs) to investigate a possible therapeutic role for medications that oppose the agonist effects of THC at cannabinoid type 1 (CB1) receptors. To date, 2 RCTs comparing rimonabant, a CB1 inverse agonist, with placebo (PLB) in patients with schizophrenia have failed to demonstrate any benefit for psychotic symptoms or cognitive deficits.11,12 A third trial examining rimonabant for people diagnosed with schizophrenia who were overweight found significant benefits for anxiety and depressive symptoms, but none for positive symptoms or the primary outcome of weight loss.13 While these results are discouraging, the role of THC in precipitating psychosis suggests that novel agents opposing the actions of THC on the cannabinoid system could have antipsychotic properties.14
Cannabidiol: An antipsychotic medication?
In contrast to THC, CBD has minimal euphorigenic properties and has recently been heralded in the popular press as a “miracle drug” with benefits for medical and psychiatric disorders alike.15 It has even been speculated that it could become a popular food supplement.16 In 2018, the FDA gave full approval to a pharmaceutically manufactured form of CBD (brand name: Epidiolex) as a novel treatment for 2 rare and severe forms of pediatric epilepsy, Lennox-Gastaut syndrome and Dravet syndrome,17 based on RCTs supporting its efficacy for these often refractory and life-threatening conditions.18-20
In psychiatry, there have not yet been enough robust clinical studies to support broad therapeutic claims for CBD as a treatment for any mental disorder.21 However, there is growing evidence that CBD has potential as an antipsychotic medication. In 1995, the first case report was published describing the efficacy of CBD, 1,500 mg/d, as standalone therapy in a single individual with schizophrenia.22 In 2006, the same research group followed up with a case series in which only 1 out of 3 patients with treatment-refractory schizophrenia improved with flexible dosing of CBD to a maximum dose of 1,280 mg/d.23
There have been 3 published RCTs exploring the efficacy of CBD in schizophrenia (Table24-26). The first study, published in 2012, included 39 adults with schizophrenia who were randomized to 800 mg/d of CBD or amisulpride (AMS), a second-generation antipsychotic that is popular in Europe but is not available in the United States.24 Over 4 weeks of randomized treatment, CBD resulted in as much improvement in overall symptoms and positive symptoms as AMS, and improvement of negative symptoms was significantly greater with CBD. Compared with patients treated with antipsychotic medication, patients who were treated with CBD had fewer extrapyramidal symptoms, less weight gain, and less prolactin elevation. This initial trial suggests that CBD might be as efficacious in schizophrenia as antipsychotic medication, without its burdensome adverse effects. However, this is the only RCT of CBD monotherapy published to date.
Continue to: Two other recently published RCTs...
Two other recently published RCTs compared CBD with PLB as add-on therapy to antipsychotics. McGuire et al25 compared CBD, 1,000 mg/d, to PLB over 6 weeks in 88 patients with schizophrenia. Positive symptom improvement was statistically greater with CBD than with PLB, although the magnitude of clinical change was modest (using the Positive and Negative Syndrome Scale [PANSS] positive symptom subscale: −3.2 points for CBD vs −1.7 points for PLB). Changes in PANSS total score and subscales for general and negative symptoms were not significantly different between treatment groups. There was also no significant difference in overall change in neurocognitive symptoms, although post-hoc analysis revealed significantly greater improvement in motor speed for patients treated with CBD. More than twice the number of patients treated with CBD were rated as “much improved” by the Clinical Global Impressions scale compared with patients treated with PLB, but this was not a statistically significant finding, and most patients experienced only “minimal” or “no improvement.” In terms of adverse events, there were no significant differences between patients in the CBD and PLB groups. Although this study is technically “positive” for CBD and suggests minimal adverse effects, it is not clear whether the statistically significant positive symptom improvements (+1.5 PANSS points for CBD over PLB) were clinically significant.
The most recently published placebo-controlled RCT of CBD as add-on therapy to antipsychotic medication included 36 patients with schizophrenia treated over 6 weeks.26 In this study, there was no benefit of CBD, 600 mg/d, on any PANSS score outcome (total, general, positive, or negative symptoms). For the primary outcome of the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery, there were no significant drug × time effects, and post-hoc analyses showed that only patients treated with PLB improved with time. Sedation was more common among patients treated with CBD compared with PLB.
Making sense of the data
There have been mixed results from the few case reports and 3 RCTs of patients with schizophrenia who were treated with CBD. How can we resolve these disparate findings? A few possible interpretations of the data that warrant clarification through additional research include:
Dosing. In the first case report with positive results, CBD was dosed at 1,500 mg/d,22 whereas in the subsequent case series with mixed results, the maximum allowable dose of CBD was 1,280 mg/d.23 Likewise, in the RCTs, positive results were found when CBD was dosed at 800 to 1,000 mg/d,24,25 but not at 600 mg/d.26 The efficacy of CBD for schizophrenia might depend on higher doses.
Treatment resistance. In the second case series in which only 1 out of 3 patients responded to treatment with CBD,23 the patients had demonstrated previous nonresponse to at least 2 first-generation antipsychotics (FGAs) and risperidone, 6 mg/d. In the RCTs, all patients were antipsychotic-responsive.24-26 Cannabidiol may not be as effective for patients with treatment-refractory schizophrenia as it is for patients with schizophrenia who respond to antipsychotics.
Continue to: Clinical stability
Clinical stability. Within the RCTs, the greatest response was observed in the study that enrolled patients who were hospitalized with acute symptoms of schizophrenia.23 In the 2 studies that found either modest or no benefit with CBD, the patients had been stabilized on antipsychotic medications prior to randomization. Cannabidiol may offer limited benefit as add-on therapy to patients who have already responded to antipsychotic treatment, where there is “less room” for additional improvement.
Monotherapy. Both the case reports22,23 and the RCT with the most robust positive findings24 involved treatment with CBD as monotherapy. For some patients with schizophrenia, CBD might be effective as standalone therapy as an alternative to antipsychotics that is better tolerated. Adding CBD to antipsychotic therapy might be redundant and therefore less effective.
Answering questions about CBD
Cannabidiol is becoming increasingly popular for its purported health benefits. The mixed results of the few studies published on CBD for schizophrenia place clinicians in a difficult position when attempting to answer questions about how cannabinoids might fit into treatment of patients with psychosis. Consider the following:
Is cannabis helpful for patients with schizophrenia? No. Aside from the few case reports suggesting that FDA-approved THC (dronabinol) can improve symptoms in some patients,9,10 most of the evidence from anecdotal reports and both experimental and observational studies indicate that cannabis, THC, and synthetic cannabinoids have a harmful effect in patients with or at risk for psychosis.1-3
If you are considering recommending some form of cannabis to patients with schizophrenia, what kind should you recommend? Recommending or encouraging cannabis use for patients with psychosis is ill-advised. Although certain types of cannabis might contain more THC (eg, Cannabis indica vs Cannabis sativa) or variable amounts of CBD, in general the amount of CBD in whole leaf cannabis is minimal, with the ratio of THC to CBD increasingly significantly over the past decade.3,27 Most forms of cannabis should therefore be avoided by individuals with or at risk for psychotic disorders.
Continue to: What about CBD oil and other CBD products sold in dispensaries?
What about CBD oil and other CBD products sold in dispensaries? Cannabidiol is increasingly available in various forms based on its ability to be designated as a legal hemp product (containing <0.3% THC) at the federal level or as a cannabinoid in states where cannabis is legal. However, several studies have now shown that cannabis products sold online or in dispensaries are often labeled inaccurately, with both under- and over-reporting of THC and CBD content.28-30 Some CBD products have been found to have almost no CBD at all.29,30 The unreliability of product labeling makes it difficult to predict the effects of CBD products that are not subject to FDA purity standards for medications or dietary supplements. It also raises questions about the sources of CBD and the reliability of dosing in the studies discussed above.
Why might CBD work as an antipsychotic? Although CBD has minimal affinity for cannabinoid receptors, it appears to act as a partial agonist of dopamine D2 receptors and an agonist at 5-HT1A receptors, with overall effects that decrease mesolimbic dopamine activity.31,32 In addition, CBD increases the availability of the endogenous cannabinoid anandamide, which may have antipsychotic properties.14,33
Now that the FDA has approved CBD manufactured by a pharmaceutical company, should it be prescribed “off-label” for patients with schizophrenia? This is the “million dollar question,” with insufficient evidence to provide a clear answer. It should now be possible to prescribe FDA-approved CBD for off-label purposes, including the treatment of schizophrenia and other psychiatric disorders. No doubt, some clinicians are already doing so. This will predictably yield more anecdotal evidence about efficacy and adverse effects in the future, but there is not yet adequate evidence to support an FDA indication for CBD in schizophrenia. Additional studies of CBD for schizophrenia are ongoing.
Bottom Line
Cannabidiol (CBD) is becoming increasingly popular based on its purported health benefits, but the evidence supporting a therapeutic role in psychiatry is preliminary at best. Although CBD is now available by prescription as an FDA-approved drug for the treatment of 2 rare forms of epilepsy, its benefits in patients with schizophrenia are uncertain based on mixed results in clinical trials.
Related Resources
- Clinicaltrials.gov. Studies of “cannabidiol” and “schizophrenia.” U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Schizophrenia&term=cannabidiol.
- Grinspoon P. Cannabidiol (CBD) – what we know and what we don’t. Harvard Health Blog. https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-wedont-2018082414476. Published August 24, 2018.
Drug Brand Names
Cannabidiol • Epidiolex
Dronabinol • Marinol
Risperidone • Risperdal
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
1. Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: what the evidence says. Current Psychiatry. 2011;10(9):49-58.
2. Radhakrishan R, Wilkinson ST, D’Souza DC. Gone to pot – a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54.
3. Pierre JM. Risks of increasingly potent cannabis: joint effects of potency and frequency. Current Psychiatry. 2016;16(2):14-20.
4. Hanna RC, Perez JM, Ghose S. Cannabis and development of dual diagnoses: a literature review. Am J Drug Alcohol Abuse. 2017;43(4):442-255.
5. Nunberg H, Kilmer B, Pacula RL, et al. An analysis of applicants presenting to a medical marijuana specialty practice in California. J Drug Policy Anal. 2011;4(1):1.
6. Wilkinson ST, Radhakrishnan, D’Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064.
7. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: Update 2015. Subst Abus. 2016;38(3):344-366.
8. Crippa JA, Guimarães FS, Campos A, et al. Translational investigation of the therapeutic potential of cannabidiol (CBD): toward a new age. Front Immunol. 2018;9:2009.
9. Schwarz G, Karajgi B. Improvement in refractory psychosis with dronabinol: four case reports. J Clin Psychiatry. 2010;71(11):1552-1553.
10. Schwarz G, Karajgi B, McCarthy R. Synthetic delta-9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258.
11. Meltzer HY, Arvanitis L, Bauer D, et al. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161(6):975-984.
12. Boggs DL, Kelly DL, McMahon RP, et al. Rimonabant for neurocognition in schizophrenia: a 16-week double blind placebo controlled trial. Schizophr Res. 2012;134(2-3):207-210.
13. Kelly DL, Gorelick DA, Conley RR, et al. Effects of cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia: a randomized, double-blind, pilot study. J Clin Psychopharmacol. 2011;31(1):86-91.
14. Leweke FM, Mueller JK, Lange B, et al. Therapeutic potential of cannabinoids in psychosis. Biol Psychiatry. 2016;79(7):604-612.
15. Halperin A. What is CBD? The ‘miracle’ cannabis compound that doesn’t get you high. The Guardian. https://www.theguardian.com/society/2018/may/28/what-is-cbd-cannabidiol-cannabis-medical-uses. Published May 28, 2018. Accessed April 3, 2019.
16. Pierre J. Coca, cola, and cannabis: psychoactive drugs as beverages. Psychology Today (blog) Psych Unseen. https://www.psychologytoday.com/us/blog/psych-unseen/201810/coca-cola-and-cannabis-psychoactive-drugs-beverages. Published October 1, 2018. Accessed April 3, 2019.
17. U.S. Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. FDA News Release. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Published June 25, 2018. Accessed April 3, 2019.
18. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376:2011-2020.
19. Thiele EA, March ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085-1096.
20. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378:1888-1897.
21. Khoury JM, Neves MCLD, Rogue MAV, et al. Is there a role of cannabidiol in psychiatry? World J Biol Psychiatry. 2017:1-16.
22. Zuardi AW, Morais SL, Guimares FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
23. Zuardi AW, Hallak JEC, Dursun SM. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
24. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. doi: 10.1038/tp.2012.15.
25. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
26. Boggs DL, Surti I, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacol. 2018;235(7):1923-1932.
27. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016; 79(7):613-619.
28. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491-2492.
29. Ruth AC, Gryniewicz-Ruzicka CM, Trehy ML, et al. Consistency of label claims of internet-purchased hemp oil and cannabis products as determined using IMS and LC-MS: a marketplace study. J Reg Sci. 2016;3:1-6.
30. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
31. Seeman P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl Psychiatry. 2016;6(10):e920. doi: 10.1038/tp.2016.195.
32. Renard J, Norris C, Rushlow W, et al. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: implications for novel schizophrenia treatments. Neurosci Biobehav Rev. 2017;157-165.
33. Gururajan A, Malone DT. Does cannabidiol have a role in the treatment of schizophrenia? Schizophr Res. 2016;176(2-3):281-290.
Postpartum psychosis: Protecting mother and infant
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
A new mother drowned her 6-month-old daughter in the bathtub. The married woman, who had a history of schizoaffective disorder, had been high functioning and worked in a managerial role prior to giving birth. However, within a day of delivery, her mental state deteriorated. She quickly became convinced that her daughter had a genetic disorder such as achondroplasia. Physical examinations, genetic testing, and x-rays all failed to alleviate her concerns. Examination of her computer revealed thousands of searches for various medical conditions and surgical treatments. After the baby’s death, the mother was admitted to a psychiatric hospital. She eventually pled guilty to manslaughter.1
Mothers with postpartum psychosis (PPP) typically present fulminantly within days to weeks of giving birth. Symptoms of PPP may include not only psychosis, but also confusion and dysphoric mania. These symptoms often wax and wane, which can make it challenging to establish the diagnosis. In addition, many mothers hide their symptoms due to poor insight, delusions, or fear of loss of custody of their infant. In the vast majority of cases, psychiatric hospitalization is required to protect both mother and baby; untreated, there is an elevated risk of both maternal suicide and infanticide. This article discusses the presentation of PPP, its differential diagnosis, risk factors for developing PPP, suicide and infanticide risk assessment, treatment (including during breastfeeding), and prevention.
The bipolar connection
While multiple factors may increase the risk of PPP (Table 12), women with bipolar disorder have a particularly elevated risk. After experiencing incipient postpartum affective psychosis, a woman has a 50% to 80% chance of having another psychiatric episode, usually within the bipolar spectrum.2 Of all women with PPP, 70% to 90% have bipolar illness or schizoaffective disorder, while approximately 12% have schizophrenia.3,4Women with bipolar disorder are more likely to experience a postpartum psychiatric admission than mothers with any other psychiatric diagnosis5 and have an increased risk of PPP by a factor of 100 over the general population.2
For women with bipolar disorder, PPP should be understood as a recurrence of the chronic disease. Recent evidence does suggest, however, that a significant minority of women progress to experience mood and psychotic symptoms only in the postpartum period.6,7 It is hypothesized that this subgroup of women has a biologic vulnerability to affective psychosis that is limited to the postpartum period. Clinically, understanding a woman’s disease course is important because it may guide decision-making about prophylactic medications during or after pregnancy.
A rapid, delirium-like presentation
Postpartum psychosis is a rare disorder, with a prevalence of 1 to 2 cases per 1,000 childbirths.3 While symptoms may begin days to weeks postpartum, the typical time of onset is between 3 to 10 days after birth, occurring after a woman has been discharged from the hospital and during a time of change and uncertainty. This can make the presentation of PPP a confusing and distressing experience for both the new mother and the family, resulting in delays in seeking care.
Subtle prodromal symptoms may include insomnia, mood fluctuation, and irritability. As symptoms progress, PPP is notable for a rapid onset and a delirium-like appearance that may include waxing and waning cognitive symptoms such as disorientation and confusion.8 Grossly disorganized behaviors and rapid mood fluctuations are typical. Distinct from mood episodes outside the peripartum period, women with PPP often experience mood-incongruent delusions and obsessive thoughts, often focused on their child.9 Women with PPP appear less likely to experience thought insertion or withdrawal or auditory hallucinations that give a running commentary.2
Differential diagnosis includes depression, OCD
When evaluating a woman with possible postpartum psychotic symptoms or delirium, it is important to include a thorough history, physical examination, and relevant laboratory and/or imaging investigations to assess for organic causes or contributors (Table 22,6,10-12 and Table 32,6,10-12). A detailed psychiatric history should establish whether the patient is presenting with new-onset psychosis or has had previous mood or psychotic episodes that may have gone undetected. Important perinatal psychiatric differential diagnoses should include “baby blues,” postpartum depression (PPD), and obsessive-compulsive disorder (OCD).
Continue to: PPP vs "baby blues."
PPP vs “baby blues.” “Baby blues” is not an official DSM-5 diagnosis but rather a normative postpartum experience that affects 50% to 80% of postpartum women. A woman with the “baby blues” may feel weepy or have mild mood lability, irritability, or anxiety; however, these symptoms do not significantly impair function. Peak symptoms typically occur between 2 to 5 days postpartum and generally resolve within 2 weeks. Women who have the “baby blues” are at an increased risk for PPD and should be monitored over time.13,14
PPP vs PPD. Postpartum depression affects approximately 10% to 15% of new mothers.15 Women with PPD may experience feelings of persistent and severe sadness, feelings of detachment, insomnia, and fatigue. Symptoms of PPD can interfere with a mother’s interest in caring for her baby and present a barrier to maternal bonding.16,17
As the awareness of PPD has increased in recent years, screening for depressive symptoms during and after pregnancy has increasingly become the standard of care.18 When evaluating a postpartum woman for PPD, it is important to consider PPP in the differential. Women with severe or persistent depressive symptoms may also develop psychotic symptoms. Furthermore, suicidal thoughts or thoughts of harming the infant may be present in either PPD or PPP. One study found that 41% of mothers with depression endorsed thoughts of harming their infants.19
PPP vs postpartum OCD. Postpartum obsessive-compulsive symptoms commonly occur comorbidly with PPD,9 and OCD often presents for the first time in the postpartum period.20 Obsessive-compulsive disorder affects between 2% to 9% of new mothers.21,22 It is critical to properly differentiate PPP from postpartum OCD. Clinical questions should be posed with a non-judgmental stance. Just as delusions in PPP are often focused on the infant, for women with OCD, obsessive thoughts may center on worries about the infant’s safety. Distressing obsessions about violence are common in OCD.23 Mothers with OCD may experience intrusive thinking about accidentally or purposefully harming their infant. For example, they may intrusively worry that they will accidentally put the baby in the microwave or oven, leave the baby in a hot car, or throw the baby down the stairs. However, a postpartum woman with OCD may be reluctant to share her ego-dystonic thoughts of infant harm. Mothers with OCD are not out of touch with reality; instead, their intrusive thoughts are ego-dystonic and distressing. These are thoughts and fears that they focus on and try to avoid, rather than plan. The psychiatrist must carefully differentiate between ego-syntonic and ego-dystonic thoughts. These patients often avoid seeking treatment because of their shame and guilt.23 Clinicians often under-recognize OCD and risk inappropriate hospitalization, treatment, and inappropriate referral to Child Protective Services (CPS).23
Perinatal psychiatric risk assessment
When a mother develops PPP, consider the risks of suicide, child harm, and infanticide. Although suicide risk is generally lower in the postpartum period, suicide is the cause of 20% of postpartum deaths.24,25 When PPP is untreated, suicide risk is elevated. A careful suicide risk assessment should be completed.
Continue to: Particularly in PPP...
Particularly in PPP, a mother may be at risk of child neglect or abuse due to her confused or delusional thinking and mood state.26 For example, one mother heated empty bottles and gave them to her baby, and then became frustrated when the baby continued to cry.
The risk of infanticide is also elevated in untreated PPP, with approximately 4% of these women committing infanticide.9 There are 5 motives for infanticide (Table 427). Altruistic and acutely psychotic motives are more likely to be related to PPP, while fatal maltreatment, unwanted child, and partner revenge motives are less likely to be related to PPP. Among mothers who kill both their child and themselves (filicide-suicide), altruistic motives were the most common.28 Mothers in psychiatric samples who kill their children have often experienced psychosis, suicidality, depression, and significant life stresses.27 Both infanticidal ideas and behaviors have been associated with psychotic thinking about the infant,29 so it is critical to ascertain whether the mother’s delusions or hallucinations involve the infant.30 In contrast, neonaticide (murder in the first day of life) is rarely related to PPP because PPP typically has a later onset.31
Treating acute PPP
The fulminant nature of PPP can make its treatment difficult. Thinking through the case in an organized fashion is critical (Table 5).
Hospitalization. Postpartum psychosis is a psychiatric emergency with a rapid onset of symptoms. Hospitalization is required in almost all cases for diagnostic evaluation, assessment and management of safety, and initiation of treatment. While maternal-infant bonding in the perinatal period is important, infant safety is critical and usually requires maternal psychiatric hospitalization.
The specialized mother-baby psychiatric unit (MBU) is a model of care first developed in the United Kingdom and is now available in many European countries as well as in New Zealand and Australia. Mother-baby psychiatric units admit the mother and the baby together and provide dyadic treatment to allow for enhanced bonding and parenting support, and often to encourage breastfeeding.30 In the United States, there has been growing interest in specialized inpatient settings that acknowledge the importance of maternal-infant attachment in the treatment of perinatal disorders and provide care with a dyadic focus; however, differences in the health care payer system have been a barrier to full-scale MBUs. The Perinatal Psychiatry Inpatient Unit at University of North Carolina-Chapel Hill is among the first of such a model in the United States.32
Continue to: Although this specialized treatment setting...
Although this specialized treatment setting is unlikely to be available in most American cities, treatment should still consider the maternal role. When possible, the infant should stay with the father or family members during the mother’s hospitalization, and supervised visits should be arranged when appropriate. If the mother is breastfeeding, or plans to breastfeed after the hospitalization, the treatment team may consider providing supervised use of a breast pump and making arrangements for breast milk storage. During the mother’s hospitalization, staff should provide psychoeducation and convey hopefulness and support.
Medication management. Mood stabilizers and second-generation antipsychotics (SGAs) are often used for acute management of PPP. The choice of medication is determined by individual symptoms, severity of presentation, previous response to medication, and maternal adverse effects.30 In a naturalistic study of 64 women admitted for new-onset PPP, sequential administration of benzodiazepines, antipsychotics, and lithium was found to be effective in achieving remission for 99% of patients, with 80% sustaining remission at 9 months postpartum.6 Second-generation antipsychotics such as
Breastfeeding. It is important to discuss breastfeeding with the mother and her partner or family. The patient’s preference, the maternal and infant benefits of breastfeeding, the potential for sleep disruption, and the safety profile of needed medications should all be considered. Because sleep loss is a modifiable risk factor in PPP, the benefits of breastfeeding may be outweighed by the risks for some patients.9 For others, breastfeeding during the day and bottle-feeding at night may be preferred.
What to consider during discharge planning
Discharge arrangements require careful consideration (Table 6). Meet with the family prior to discharge to provide psychoeducation and to underscore the importance of family involvement with both mother and infant. It is important to ensure adequate support at home, including at night, since sleep is critical to improved stability. Encourage the patient and her family to monitor for early warning signs of relapse, which might include refractory insomnia, mood instability, poor judgment, or hypomanic symptoms.35 She should be followed closely as an outpatient. Having her partner (or another close family member) and infant present during appointments can help in obtaining collateral information and assessing mother-infant bonding. The clinician should also consider whether it is necessary to contact CPS. Many mothers with mental illness appropriately parent their child, but CPS should be alerted when there is a reasonable concern about safe parenting—abuse, neglect, or significant risk.36
Take steps for prevention
An important part of managing PPP is prevention. This involves providing preconception counseling to the woman and her partner.30 Preconception advice should be individualized and include discussion of:
- risks of relapse in pregnancy and the postpartum period
- optimal physical and mental health
- potential risks and benefits of medication options in pregnancy
- potential effects of untreated illness for the fetus, infant, and family
- a strategy outlining whether medication is continued in pregnancy or started in the postpartum period.
Continue to: For women at risk of PPP...
For women at risk of PPP, the risks of medications need to be balanced with the risks of untreated illness. To reduce the risk of PPP relapse, guidelines recommend a robust antenatal care plan that should include37,38:
- close monitoring of a woman’s mental state for early warning signs of PPP, with active participation from the woman’s partner and family
- ongoing discussion of the risks and benefits of pharmacotherapy (and, for women who prefer to not take medication in the first trimester, a plan for when medications will be restarted)
- collaboration with other professionals involved in care during pregnancy and postpartum (eg, obstetricians, midwives, family practitioners, pediatricians)
- planning to minimize risk factors associated with relapse (eg, sleep deprivation, lack of social supports, domestic violence, and substance abuse).
Evidence clearly suggests that women with bipolar disorder are at increased risk for illness recurrence without continued maintenance medication.39 A subgroup of women with PPP go on to have psychosis limited to the postpartum period, and reinstating prophylactic medication in late pregnancy (preferably) or immediately after birth should be discussed.2 The choice of prophylactic medication should be determined by the woman’s previous response.
Regarding prophylaxis, the most evidence exists for lithium.6 Lithium use during the first trimester carries a risk of Ebstein’s anomaly. However, a recent systematic review and meta-analysis have concluded that the teratogenic risks of lithium have been overestimated.40,41
Lamotrigine is an alternative mood stabilizer with a favorable safety profile in pregnancy. In a small naturalistic study in which lamotrigine was continued in pregnancy in women with bipolar disorder, the medication was effective in preventing relapse in pregnancy and postpartum.42 A small population-based cohort study found lamotrigine was as effective as lithium in preventing severe postpartum relapse in women with bipolar disorder,43 although this study was limited by its observational design. Recently published studies have found no significant association between
Box
It is essential to consider the patient’s individual symptoms and treatment history when making pharmacologic recommendations during pregnancy. Discussion with the patient about the risks and benefits of lithium is recommended. For women who continue to use lithium during pregnancy, ongoing pharmacokinetic changes warrant more frequent monitoring (some experts advise monthly monitoring throughout pregnancy, moving to more frequent monitoring at 36 weeks).47 During labor, the team might consider temporary cessation of lithium and particular attention to hydration status.30 In the postpartum period, there is a quick return to baseline glomerular filtration rate and a rapid decrease in vascular volume, so it is advisable to restart the patient at her pre-pregnancy lithium dosage. It is recommended to check lithium levels within 24 hours of delivery.47 While lithium is not an absolute contraindication to breastfeeding, there is particular concern in situations of prematurity or neonatal dehydration. Collaboration with and close monitoring by the pediatrician is essential to determine an infant monitoring plan.48
If lamotrigine is used during pregnancy, be aware that pregnancy-related pharmacokinetic changes result in increased lamotrigine clearance, which will vary in magnitude among individuals. Faster clearance may necessitate dose increases during pregnancy and a taper back to pre-pregnancy dose in the postpartum period. Dosing should always take clinical symptoms into account.
Pharmacotherapy can reduce relapse risk
To prevent relapse in the postpartum period, consider initiating treatment with mood stabilizers and/or SGAs, particularly for women with bipolar disorder who do not take medication during pregnancy. A recent meta-analysis found a high postpartum relapse rate (66%) in women with bipolar disorder who did not take prophylactic medication, compared with a relapse rate of 23% for women who did take such medication. In women with psychosis limited to the postpartum period, prophylaxis with lithium or antipsychotics in the immediate postpartum can prevent relapse.39 The SGAs olanzapine and quetiapine are often used to manage acute symptoms because they are considered acceptable during breastfeeding.33 The use of lithium when breastfeeding is complex to manage48 and may require advice to not breastfeed, which can be an important consideration for patients and their families.
Bottom Line
Postpartum psychosis (PPP) typically presents with a rapid onset of hallucinations, delusions, confusion, and mood swings within days to weeks of giving birth. Mothers with PPP almost always require hospitalization for the safety of their infants and themselves. Mood stabilizers and second-generation antipsychotics are used for acute management.
Related Resources
- Clark CT, Wisner KL. Treatment of peripartum bipolar disorder. Obstet Gynecol Clin N Am. 2018;45:403-417.
- Massachusetts General Hospital Center for Women’s Mental Health. https://womensmentalhealth.org/. 2018.
- Postpartum Support International. Postpartum psychosis. http://www.postpartum.net/learn-more/postpartumpsychosis/. 2019.
Drug Brand Names
Bromocriptine • Cycloset, Parlodel
Cabergoline • Dostinex
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Quetiapine • Seroquel
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.
1. Hall L. Mother who killed baby believing she was a dwarf should not be jailed, court told. The Sydney Morning Herald. https://www.smh.com.au/national/nsw/mother-who-killed-baby-believing-she-was-a-dwarf-should-not-be-jailed-court-told-20170428-gvud4d.html. Published April 28, 2017. Accessed March 12, 2019.
2. Bergink V, Rasgon N, Wisner KL. Postpartum psychosis: madness, mania, and melancholia in motherhood. Am J Psychiatry. 2016;173(12):1179-1188.
3. Sit D, Rothschild AJ, Wisner KL. A review of postpartum psychosis. J Womens Health (Larchmt). 2006;15(4):352-368.
4. Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150(5):662-673.
5. Munk-Olsen T, Laursen TM, Mendelson T, et al. Risks and predictors of readmission for a mental disorder during the postpartum period. Arch Gen Psychiatry. 2009;66(2):189-195.
6. Bergink V, Burgerhout KM, Koorengevel KM, et al. Treatment of psychosis and mania in the postpartum period. Am J Psychiatry. 2015;172(2):115-123.
7. Wesseloo R, Kamperman AM, Munk-Olsen T, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2015;173(2):117-127.
8. Wisner KL, Peindl K, Hanusa BH. Symptomatology of affective and psychotic illnesses related to childbearing. J Affect Disord. 1994;30(2):77-87.
9. Spinelli MG. Postpartum psychosis: detection of risk and management. Am J Psychiatry. 2009;166(4):405-408.
10. Fassier T, Guffon N, Acquaviva C, et al. Misdiagnosed postpartum psychosis revealing a late-onset urea cycle disorder. Am J Psychiatry. 2011;168(6):576-580.
11. Yu AYX, Moore FG. Paraneoplastic encephalitis presenting as postpartum psychosis. Psychosomatics. 2011;52(6):568-570.
12. Patil NJ, Yadav SS, Gokhale YA, et al. Primary hypoparathyroidism: psychosis in postpartum period. J Assoc Physicians India. 2010;58:506-508.
13. O’Hara MW, Schlechte JA, Lewis DA, et al. Prospective study of postpartum blues: biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801-806.
14. Burt VK, Hendrick VC. Clinical manual of women’s mental health. Washington, DC. American Psychiatric Association Publishing; 2007:79-80.
15. Melzer-Brody S. Postpartum depression: what to tell patients who breast-feed. Current Psychiatry. 2008;7(5):87-95.
16. Alhusen JL, Gross D, Hayat MJ, et al. The role of mental health on maternal‐fetal attachment in low‐income women. J Obstet Gynecol Neonatal Nurs. 2012;41(6):E71-E81.
17. McLearn KT, Minkovitz CS, Strobino DM, et al. Maternal depressive symptoms at 2 to 4 months postpartum and early parenting practices. Arch Pediatr Adolesc Med. 2006;160(3):279-284.
18. Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268-1271.
19. Jennings KD, Ross S, Popper S. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
20. Miller ES, Hoxha D, Wisner KL, et al. Obsessions and compulsions in postpartum women without obsessive compulsive disorder. J Womens Health. 2015;24(10):825-830.
21. Russell EJ, Fawcett JM, Mazmanian D. Risk of obsessive-compulsive disorder in pregnant and postpartum women: a meta-analysis. J Clin Psychiatry. 2013;74(4):377-385.
22. Zambaldi CF, Cantilino A, Montenegro AC, et al. Postpartum obsessive-compulsive disorder: prevalence and clinical characteristics. Compr Psychiatry. 2009;50(6):503-509.
23. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
24. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77-87.
25. Samandari G, Martin SL, Kupper LL, et al. Are pregnant and postpartum women: at increased risk for violent death? Suicide and homicide findings from North Carolina. Matern Child Health J. 2011;15(5):660-669.
26. Friedman SH, Sorrentino R. Commentary: postpartum psychosis, infanticide, and insanity—implications for forensic psychiatry. J Am Acad Psychiatry Law. 2012;40(3):326-332.
27. Friedman SH, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
28. Friedman SH, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
29. Chandra PS, Venkatasubramanian G, Thomas T. Infanticidal ideas and infanticidal behavior in Indian women with severe postpartum psychiatric disorders. J Nerv Ment Dis. 2002;190(7):457-461.
30. Jones I, Chandra PS, Dazzan P, et al. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384(9956):1789-1799.
31. Friedman SH. Neonaticide. In: Friedman SH. Family murder: pathologies of love and hate. Washington, DC: American Psychiatric Association Publishing; 2018:53-67.
32. Meltzer-Brody S, Brandon AR, Pearson B, et al. Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health. 2014;17(2):107-113.
33. Klinger G, Stahl B, Fusar-Poli P, et al. Antipsychotic drugs and breastfeeding. Pediatri Endocrinol Rev. 2013;10(3):308-317.
34. Focht A, Kellner CH. Electroconvulsive therapy (ECT) in the treatment of postpartum psychosis. J ECT. 2012;28(1):31-33.
35. Heron J, McGuinness M, Blackmore ER, et al. Early postpartum symptoms in puerperal psychosis. BJOG. 2008;115(3):348-353.
36. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
37. Centre of Perinatal Excellence. National Perinatal Mental Health Guideline. http://cope.org.au/about/review-of-new-perinatal-mental-health-guidelines/. Published October 27, 2017. Accessed November 22, 2018.
38. National Institute for Health and Care Excellence. Antenatal and postnatal mental health overview. https://pathways.nice.org.uk/pathways/antenatal-and-postnatal-mental-health. 2017. Accessed November 22, 2018.
39. Wesseloo R, Kamperman AM, Olsen TM, et al. Risk of postpartum relapse in bipolar disorder and postpartum psychosis: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(2):117-127.
40. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
41. Munk-Olsen T, Liu X, Viktorin A, et al. Maternal and infant outcomes associated with lithium use in pregnancy: an international collaborative meta-analysis of six cohort studies. Lancet Psychiatry. 2018;5(8):644-652.
42. Prakash C, Friedman SH, Moller-Olsen C, et al. Maternal and fetal outcomes after lamotrigine use in pregnancy: a retrospective analysis from an urban maternal mental health centre in New Zealand. Psychopharmacology Bull. 2016;46(2):63-69.
43. Wesseloo R, Liu X, Clark CT, et al. Risk of postpartum episodes in women with bipolar disorder after lamotrigine or lithium use in pregnancy: a population-based cohort study. J Affect Disord. 2017;218:394-397.
44. Dolk H, Wang H, Loane M, et al. Lamotrigine use in pregnancy and risk of orofacial cleft and other congenital anomalies. Neurology. 2016;86(18):1716-1725.
45. Diav-Citrin O, Shechtman S, Zvi N, et al. Is it safe to use lamotrigine during pregnancy? A prospective comparative observational study. Birth Defects Res. 2017;109(15):1196-1203.
46. Kong L, Zhou T, Wang B, et al. The risks associated with the use of lamotrigine during pregnancy. Int J Psychiatry Clin Pract. 2018;22(1):2-5.
47. Deligiannidis KM, Byatt N, Freeman MP. Pharmacotherapy for mood disorders in pregnancy: a review of pharmacokinetic changes and clinical recommendations for therapeutic drug monitoring. J Clin Psychopharmacol. 2014;34(2):244.
48. Bogen DL, Sit D, Genovese A, et al. Three cases of lithium exposure and exclusive breastfeeding. Arch Womens Ment Health. 2012;15(1):69-72.