User login
MDedge conference coverage features onsite reporting of the latest study results and expert perspectives from leading researchers.
Organs of Metastasis Predominate with Age in Non-Small Cell Lung Cancer Subtypes: National Cancer Database Analysis
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Background
Patients diagnosed with lung cancer are predominantly non-small cell lung cancer (NSCLC), a leading cause of cancer-related deaths. Thus, it is imperative to investigate and distinguish the differences present at diagnosis to possibly improve survival outcomes. NSCLC commonly metastasizes within older patients near the mean age of 71 years, but also in early onset patients which represents the patients younger than the earliest lung cancer screening age of 50.
Objective
To reveal differences in ratios of metastasis locations in squamous cell carcinoma (SCC), adenocarcinoma (ACC), and adenosquamous carcinoma (ASC).
Methods
The National Cancer Database (NCDB) was utilized to identify patients diagnosed with SCC, ACC, and ASC using the histology codes 8070, 8140, and 8560 from the ICD-O-3.2 from 2004 to 2022. Age groups were 70 years. Metastases located to the brain, liver, bone, and lung were included. Chi-Square tests were performed. The data was analyzed using R version 4.4.2 and statistical significance was set to α = 0.05.
Results
In this study, 1,445,119 patients were analyzed. Chi-Square tests identified significant differences in the ratios of organ metastasis locations between age groups in each subtype (p < 0.001). SCC in each age group similarly metastasized most to bone (36.3%, 34.7%, 34.5%), but notably more local lung metastasis was observed in the oldest group (33.6%). In ACC and ASC, the oldest group also had greater ratios of spread within the lungs (28.0%, 27.2%). Overall, the younger the age group, distant spread to the brain increased (ex. 29.0%, 24.4%, 17.5%). This suggests a widely heterogenous distribution of metastases at diagnosis of NSCLC subtypes and patient age.
Conclusions
This study demonstrated that patients with SCC, ACC, or ASC subtypes of NSCLC share similar predominant locations based in part on patient age, irrespective of cancer origin. NSCLC may more distantly metastasize in younger patients to the brain, while older patients may have locally metastatic cancer. Further analysis of key demographic variables as well as common undertaken treatment options may prove informative and reveal existing differences in survival outcomes.
Shifting Demographics: A Temporal Analysis of the Alarming Rise in Rectal Adenocarcinoma Among Young Adults
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
Background
Rectal adenocarcinoma has long been associated with older adults, with routine screening typically beginning at age 45 or older. However, recent data reveal a concerning rise in rectal cancer incidence among adults under 40. These early-onset cases often present at later stages and may have distinct biological features. While some research attributes this trend to genetic or environmental factors, the contribution of socioeconomic disparities and healthcare access has not been fully explored. Identifying these influences is essential to shaping targeted prevention and early detection strategies for younger populations.
Objective
To evaluate temporal trends in rectal adenocarcinoma among young adults and assess demographic and socioeconomic predictors of early-onset diagnosis.
Methods
Data were drawn from the National Cancer Database (NCDB) for patients diagnosed with rectal adenocarcinoma from 2004 to 2022. Among 440,316 cases, 17,842 (4.1%) occurred in individuals under 40. Linear regression assessed temporal trends, while logistic regression evaluated associations between early-onset diagnosis and variables including sex, race, insurance status, income level, Charlson-Deyo comorbidity score, and tumor stage. Statistical significance was defined as α = 0.05.
Results
The number of young adults diagnosed rose from 424 in 2004 to 937 in 2022—an increase of over 120%. Each year was associated with a 1.7% rise in odds of early diagnosis (OR = 1.017, p < 0.001). Male patients had 24.7% higher odds (OR = 1.247, p < 0.001), and Black patients had 59.3% higher odds compared to White patients (OR = 1.593, p < 0.001). Non-private insurance was linked to a 41.6% decrease in early diagnosis (OR = 0.584, p < 0.001). Income level was not significant (p = 0.426). Lower Charlson-Deyo scores and higher tumor stages were also associated with early-onset cases.
Conclusions
Rectal adenocarcinoma is increasingly affecting younger adults, with significant associations across demographic and insurance variables. These findings call for improved awareness, early diagnostic strategies, and further research into underlying causes to mitigate this growing public health concern.
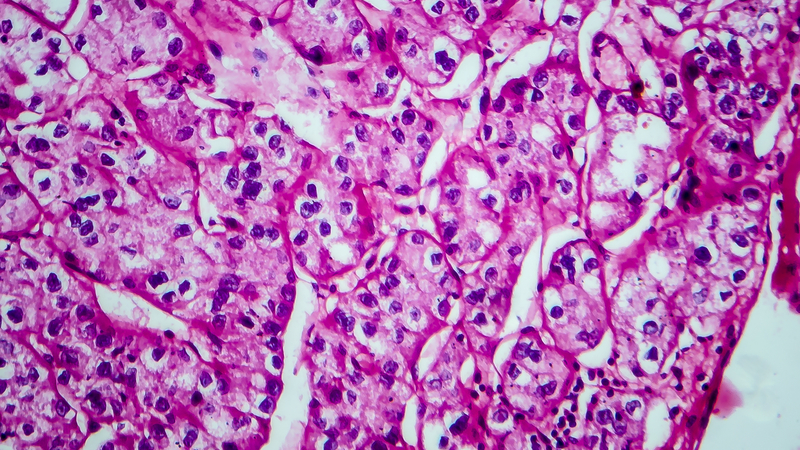
Epidemiology and Survival of Parotid Gland Malignancies With Brain Metastases: A Population- Based Study
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Background
Parotid gland malignancies are a rare subset of salivary gland tumors, comprising approximately 1–3% of all head and neck cancers. While distant metastases commonly involve the lungs, brain metastases are exceedingly rare and remain poorly characterized. Management typically includes stereotactic radiosurgery or whole-brain radiation. This study evaluates the incidence, clinicopathologic features, and survival outcomes of patients with parotid gland tumors and brain metastases using data from Surveillance, Epidemiology, and End Results (SEER) database.
Methods
SEER database (2010–2022) was queried for patients diagnosed with primary malignant neoplasms of the parotid gland (ICD-O-3 site code C07.9). Cases of brain metastases were identified using SEER metastatic site variables. Age-adjusted incidence rates (IR) per 100,000 population were calculated using SEER*Stat 8.4.5. Kaplan-Meier survival analyses were conducted using GraphPad Prism, and survival differences were assessed using the log-rank test.
Results
Among 12,951 patients diagnosed with parotid malignancy, 47 (0.36%) had brain metastases. The median age at diagnosis was 67 years, and 77.5% were male. The overall incidence rate (IR) of brain metastases was 0.00235 per 100,000 population, with a significantly higher rate observed in males compared to females (p < 0.0001). The most common histologic subtype associated with brain involvement was squamous cell carcinoma (SCC, n=10), followed by adenocarcinoma. Median overall survival (mOS) for patients with brain metastases was 2 months (hazard ratio [HR] 6.28; 95% CI: 2.71–14.55), compared to 131 months for those without brain involvement (p < 0.001). 1-year cancer-specific survival for patients with brain metastases was 38%. Among patients with parotid SCC and brain metastases, mOS was 3 months, compared to 39 months in those without brain involvement (HR 5.70; 95% CI: 1.09–29.68; p < 0.0001).
Conclusions
Brain metastases from parotid gland cancers, though rare, are associated with markedly poor outcomes. This highlights the importance of early neurologic assessment and brain imaging in high-risk patients, particularly with SCC histology. Prior studies have shown that TP53 mutations are common in parotid SCC, but their role in CNS spread remains unclear. Future research should explore molecular pathways underlying neurotropism in parotid cancers and investigate targeted systemic therapies with CNS penetration to improve outcomes.
Augmenting DNA Damage by Chemotherapy With CDK7 Inhibition to Disrupt PARP Expression in Cholangiocarcinoma
Papillary Cystadenocarcinoma: NCDB Insights on Outcomes and Socioeconomic Disparities
Background
Papillary cystadenocarcinoma is a rare, aggressive malignancy typically arising in the ovaries, often following malignant transformation of benign precursors. Characterized by local invasion and recurrence, it lacks standardized treatment protocols and comprehensive epidemiological data. Existing literature is limited to case reports and small series, leaving gaps in population-level data to guide clinical decision-making. This study uses the National Cancer Database (NCDB) to assess demographic, socioeconomic, and treatment patterns to identify disparities and inform management.
Methods
A retrospective cohort analysis of 345 patients with histologically confirmed papillary cystadenocarcinoma (ICD-O-3 code 8450) was conducted using the 2004–2020 NCDB. Demographic, treatment, and survival data were described; incidence trends were assessed via linear regression; and survival was analyzed using Kaplan-Meier curves.
Results
The cohort was predominantly female (97.1%), mean age 62.1 years (SD = 14.0), and 87.2% White. Most had private insurance (44.9%) or Medicare (40.9%). Over half (51.9%) resided in metropolitan areas >1 million. Primary tumor sites were ovarian (80.0%) and endometrial (5.2%), with 39.7% presenting at Stage III. Surgery was performed in 90.4% of cases, with 51.9% achieving negative margins. Most were treated at comprehensive community (41.0%) or academic/research programs (28.7%). Primary therapies included chemotherapy (62.3%), radiation (6.4%), and hormone therapy (1.7%). Thirty-day mortality was 1.9%, and 90-day mortality was 5.4%. Survival was 97.7% at 2 years, 94.2% at 5 years, and 88.6% at 10 years. Mean survival was 97.5 months (95% CI: 88.2–106.7).
Conclusions
This is the first NCDB-based analysis of papillary cystadenocarcinoma, offering insight into its clinical characteristics. Ovarian and endometrial origins were most common, reinforcing its gynecologic profile. High surgical rates and margin negativity suggest aggressive local treatment is central to management. Disparities emerged: patients were more likely to live in urban areas, hold private insurance, and receive care at community programs. These findings highlight the need for further investigation into socioeconomic inequities and may inform future guidelines to improve equitable care delivery across health systems, including community-based programs such as the VHA.
Background
Papillary cystadenocarcinoma is a rare, aggressive malignancy typically arising in the ovaries, often following malignant transformation of benign precursors. Characterized by local invasion and recurrence, it lacks standardized treatment protocols and comprehensive epidemiological data. Existing literature is limited to case reports and small series, leaving gaps in population-level data to guide clinical decision-making. This study uses the National Cancer Database (NCDB) to assess demographic, socioeconomic, and treatment patterns to identify disparities and inform management.
Methods
A retrospective cohort analysis of 345 patients with histologically confirmed papillary cystadenocarcinoma (ICD-O-3 code 8450) was conducted using the 2004–2020 NCDB. Demographic, treatment, and survival data were described; incidence trends were assessed via linear regression; and survival was analyzed using Kaplan-Meier curves.
Results
The cohort was predominantly female (97.1%), mean age 62.1 years (SD = 14.0), and 87.2% White. Most had private insurance (44.9%) or Medicare (40.9%). Over half (51.9%) resided in metropolitan areas >1 million. Primary tumor sites were ovarian (80.0%) and endometrial (5.2%), with 39.7% presenting at Stage III. Surgery was performed in 90.4% of cases, with 51.9% achieving negative margins. Most were treated at comprehensive community (41.0%) or academic/research programs (28.7%). Primary therapies included chemotherapy (62.3%), radiation (6.4%), and hormone therapy (1.7%). Thirty-day mortality was 1.9%, and 90-day mortality was 5.4%. Survival was 97.7% at 2 years, 94.2% at 5 years, and 88.6% at 10 years. Mean survival was 97.5 months (95% CI: 88.2–106.7).
Conclusions
This is the first NCDB-based analysis of papillary cystadenocarcinoma, offering insight into its clinical characteristics. Ovarian and endometrial origins were most common, reinforcing its gynecologic profile. High surgical rates and margin negativity suggest aggressive local treatment is central to management. Disparities emerged: patients were more likely to live in urban areas, hold private insurance, and receive care at community programs. These findings highlight the need for further investigation into socioeconomic inequities and may inform future guidelines to improve equitable care delivery across health systems, including community-based programs such as the VHA.
Background
Papillary cystadenocarcinoma is a rare, aggressive malignancy typically arising in the ovaries, often following malignant transformation of benign precursors. Characterized by local invasion and recurrence, it lacks standardized treatment protocols and comprehensive epidemiological data. Existing literature is limited to case reports and small series, leaving gaps in population-level data to guide clinical decision-making. This study uses the National Cancer Database (NCDB) to assess demographic, socioeconomic, and treatment patterns to identify disparities and inform management.
Methods
A retrospective cohort analysis of 345 patients with histologically confirmed papillary cystadenocarcinoma (ICD-O-3 code 8450) was conducted using the 2004–2020 NCDB. Demographic, treatment, and survival data were described; incidence trends were assessed via linear regression; and survival was analyzed using Kaplan-Meier curves.
Results
The cohort was predominantly female (97.1%), mean age 62.1 years (SD = 14.0), and 87.2% White. Most had private insurance (44.9%) or Medicare (40.9%). Over half (51.9%) resided in metropolitan areas >1 million. Primary tumor sites were ovarian (80.0%) and endometrial (5.2%), with 39.7% presenting at Stage III. Surgery was performed in 90.4% of cases, with 51.9% achieving negative margins. Most were treated at comprehensive community (41.0%) or academic/research programs (28.7%). Primary therapies included chemotherapy (62.3%), radiation (6.4%), and hormone therapy (1.7%). Thirty-day mortality was 1.9%, and 90-day mortality was 5.4%. Survival was 97.7% at 2 years, 94.2% at 5 years, and 88.6% at 10 years. Mean survival was 97.5 months (95% CI: 88.2–106.7).
Conclusions
This is the first NCDB-based analysis of papillary cystadenocarcinoma, offering insight into its clinical characteristics. Ovarian and endometrial origins were most common, reinforcing its gynecologic profile. High surgical rates and margin negativity suggest aggressive local treatment is central to management. Disparities emerged: patients were more likely to live in urban areas, hold private insurance, and receive care at community programs. These findings highlight the need for further investigation into socioeconomic inequities and may inform future guidelines to improve equitable care delivery across health systems, including community-based programs such as the VHA.
Assessing Geographical Trends in End-of-Life Cancer Care Using CDC WONDER’s Place of Death Data
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Background
19.8% of all deaths in the US in 2023 were due to cancer. Despite its prevalence, there is minimal literature analyzing geographical trends in end-of-life care in cancer patients. This study aims to assess the evolution of end-of-life preferences in cancer patients, particularly during the COVID-19 pandemic, and account for geographical disparities to optimize palliative care delivery.
Methods
The CDC WONDER database was used to collect data on place of death (home, hospice, medical facilities, nursing homes) in patients over 25 years old that died with malignant neoplasms (ICD 10: C00- C97) in the US from 2003-2023. Deaths were stratified by region and urbanization. Proportional mortality was calculated, and statistically significant trends in mortality over time were identified using Joinpoint regression.
Results
There were 13,654,631 total deaths from malignant neoplasms over the study period. Home (40.3%) was the most common place of death followed by medical facilities (30.4%), nursing homes (14.3%), and hospice (8.9%). In 2020, all places experienced a decreased in proportion except for home which rose 7.0% from 41.7% to 48.7%. The South had the highest hospice rates (11.3%); 5.0% greater than the next highest region (Northeast; 8.3%). The West had the highest home rates (47.1%); 6.2% greater than the next closest region (South; 40.9%). The Northeast had the highest medical facility rates (36.0%); 5.5% higher than the next highest region (South, 30.5%). Nonmetro areas (< 50,000 population) had the lowest hospice (4.9%) and highest nursing home rates (15.8%). They also saw a substantial jump (+15.4%) in home deaths from 2019-21. All urbanizations saw a drop in medical facility deaths in 2020 but all have since climbed to surpass their 2019 rates except for nonmetro areas which have dropped 7.3% from 2020-2023.
Conclusion
Hospice and home deaths have increased in frequency with home deaths spiking during the COVID-19 pandemic. Geographical disparities persist in end-of-life care, particularly in nonmetro areas. This highlights the need to increase education and access to palliative care. Further research should aim at why the rural populations have failed to revert to pre-COVID trends like the other urbanization groups.
Hypothyroidism Linked to Gut Microbiome Disturbances
, according to results of a study.
“[The research] supports the idea that improving gut health could have far-reaching effects beyond digestion, possibly even helping to prevent autoimmune diseases, such as Hashimoto thyroiditis,” said senior author Ruchi Mathur, MD, director of the Diabetes Outpatient Treatment and Education Center and director of Clinical Operations of Medically Associated Science and Technology, at Cedars-Sinai in Los Angeles, in a press statement for the study, which was presented at ENDO 2025: The Endocrine Society Annual Meeting.
“These findings open the door to new screening and prevention strategies,” Mathur added. “For example, doctors may begin to monitor thyroid health more closely in patients with SIBO, and vice versa.”
With some small studies previously suggesting an association between the gut microbiome and hypothyroidism, Mathur and colleagues further explored the relationship in two analyses.
Assessing the Role of the Small Bowel
For the first, they evaluated data on 49 patients with Hashimoto thyroiditis (HT) and 323 controls without the condition from their REIMAGINE trial, which included small bowel fluid samples from upper endoscopies and DNA sequencing.
In the study, all patients with HT were treated with thyroid replacement (levothyroxine), hence, there were notably no significant differences between the two groups in terms of thyroid stimulating hormone (TSH) levels.
Despite the lack of those differences, patients with HT had a prevalence of SIBO more than twice that of the control group, independent of gender (33% vs 15%; odds ratio, 2.71; P = .005).
When the two groups were further subdivided into two groups each — those with and without SIBO — significant further variations of microbial diversity were observed between those with and without HT, Mathur told GI & Hepatology News.
“Interestingly, we saw the small bowel microbiome was not only different in SIBO-positive patients, including higher gram negatives, which is to be expected, but that the presence or absence of hypothyroidism itself was associated with specific patterns of these gram-negative bacteria,” she explained.
“In addition, when we looked at hypothyroidism without SIBO present, there were also changes between groups, such as higher Neisseria in the hypothyroid group.”
“All these findings are novel as this is the first paper to look specifically at the small bowel,” she added, noting that previous smaller studies have focused more on evaluation of stool samples.
“We believe the small bowel is the most metabolically active area of the intestine and plays an important role in metabolism,” Mathur noted. “Thus, the microbial changes here are likely more physiologically significant than the patterns seen in stool.”
Further Findings from a Large Population
In a separate analysis, the team evaluated data from the TriNetX database on the 10-year incidence of developing SIBO among 1.1 million subjects with hypothyroidism in the US compared with 1 million controls.
They found that people with hypothyroidism were approximately twice as likely to develop SIBO compared with those without hypothyroidism (relative risk [RR], 2.20).
Furthermore, those with HT, in particular, had an even higher risk, at 2.4 times the controls (RR, 2.40).
Treatment with levothyroxine decreased the risk of developing SIBO in hypothyroidism (RR, 0.33) and HT (RR, 0.78) vs those who did not receive treatment.
Mechanisms?
However, the fact that differences in SIBO were observed even between people who were treated for HT and those without the condition in the first analysis, and hence had similar TSH levels, was notable, Mathur said.
“This suggests that perhaps there are other factors aside from TSH levels and free T4 that are at play here,” she said. “Some people have theorized that perhaps delayed gut motility in hypothyroidism promotes the development of SIBO; however, there are many other factors within this complex interplay between the microbiome and the thyroid that could also be playing a role.”
“For example, SIBO leads to inflammation and weakening of the gut barrier,” Mathur explained.
Furthermore, “levothyroxine absorption and cycling of the thyroid hormone occurs predominantly in the small bowel, [while the] microbiome plays a key role in the absorption of iron, selenium, iodine, and zinc, which are critical for thyroid function.”
Overall, “further research is needed to understand how the mechanisms are affected during the development of SIBO and hypothyroidism,” Mathur said.
Assessment of Changes Over Time Anticipated
Commenting on the research, Gregory A. Brent, MD, senior executive academic vice-chair of the Department of Medicine and professor of medicine and physiology at the David Geffen School of Medicine at University of California Los Angeles said the study is indeed novel.
“This, to my knowledge, is the first investigation to link characteristics of the small bowel microbiome with hypothyroidism,” Brent told GI & Hepatology News.
While any clinical significance has yet to be determined, “the association of these small bowel microbiome changes with hypothyroidism may have implications for contributing to the onset of autoimmune hypothyroidism in susceptible populations as well as influences on levothyroxine absorption in hypothyroid patients on levothyroxine therapy,” Brent said.
With the SIBO differences observed even among treated patients with vs without HT, “it seems less likely that the microbiome changes are the result of reduced thyroid hormone signaling,” Brent noted.
Furthermore, a key piece of the puzzle will be to observe the microbiome changes over time, he added.
“These studies were at a single time point [and] longitudinal studies will be especially important to see how the association changes over time and are influenced by the treatment of hypothyroidism and of SIBO,” Brent said.
The authors and Brent had no disclosures to report.
A version of this article appeared on Medscape.com.
, according to results of a study.
“[The research] supports the idea that improving gut health could have far-reaching effects beyond digestion, possibly even helping to prevent autoimmune diseases, such as Hashimoto thyroiditis,” said senior author Ruchi Mathur, MD, director of the Diabetes Outpatient Treatment and Education Center and director of Clinical Operations of Medically Associated Science and Technology, at Cedars-Sinai in Los Angeles, in a press statement for the study, which was presented at ENDO 2025: The Endocrine Society Annual Meeting.
“These findings open the door to new screening and prevention strategies,” Mathur added. “For example, doctors may begin to monitor thyroid health more closely in patients with SIBO, and vice versa.”
With some small studies previously suggesting an association between the gut microbiome and hypothyroidism, Mathur and colleagues further explored the relationship in two analyses.
Assessing the Role of the Small Bowel
For the first, they evaluated data on 49 patients with Hashimoto thyroiditis (HT) and 323 controls without the condition from their REIMAGINE trial, which included small bowel fluid samples from upper endoscopies and DNA sequencing.
In the study, all patients with HT were treated with thyroid replacement (levothyroxine), hence, there were notably no significant differences between the two groups in terms of thyroid stimulating hormone (TSH) levels.
Despite the lack of those differences, patients with HT had a prevalence of SIBO more than twice that of the control group, independent of gender (33% vs 15%; odds ratio, 2.71; P = .005).
When the two groups were further subdivided into two groups each — those with and without SIBO — significant further variations of microbial diversity were observed between those with and without HT, Mathur told GI & Hepatology News.
“Interestingly, we saw the small bowel microbiome was not only different in SIBO-positive patients, including higher gram negatives, which is to be expected, but that the presence or absence of hypothyroidism itself was associated with specific patterns of these gram-negative bacteria,” she explained.
“In addition, when we looked at hypothyroidism without SIBO present, there were also changes between groups, such as higher Neisseria in the hypothyroid group.”
“All these findings are novel as this is the first paper to look specifically at the small bowel,” she added, noting that previous smaller studies have focused more on evaluation of stool samples.
“We believe the small bowel is the most metabolically active area of the intestine and plays an important role in metabolism,” Mathur noted. “Thus, the microbial changes here are likely more physiologically significant than the patterns seen in stool.”
Further Findings from a Large Population
In a separate analysis, the team evaluated data from the TriNetX database on the 10-year incidence of developing SIBO among 1.1 million subjects with hypothyroidism in the US compared with 1 million controls.
They found that people with hypothyroidism were approximately twice as likely to develop SIBO compared with those without hypothyroidism (relative risk [RR], 2.20).
Furthermore, those with HT, in particular, had an even higher risk, at 2.4 times the controls (RR, 2.40).
Treatment with levothyroxine decreased the risk of developing SIBO in hypothyroidism (RR, 0.33) and HT (RR, 0.78) vs those who did not receive treatment.
Mechanisms?
However, the fact that differences in SIBO were observed even between people who were treated for HT and those without the condition in the first analysis, and hence had similar TSH levels, was notable, Mathur said.
“This suggests that perhaps there are other factors aside from TSH levels and free T4 that are at play here,” she said. “Some people have theorized that perhaps delayed gut motility in hypothyroidism promotes the development of SIBO; however, there are many other factors within this complex interplay between the microbiome and the thyroid that could also be playing a role.”
“For example, SIBO leads to inflammation and weakening of the gut barrier,” Mathur explained.
Furthermore, “levothyroxine absorption and cycling of the thyroid hormone occurs predominantly in the small bowel, [while the] microbiome plays a key role in the absorption of iron, selenium, iodine, and zinc, which are critical for thyroid function.”
Overall, “further research is needed to understand how the mechanisms are affected during the development of SIBO and hypothyroidism,” Mathur said.
Assessment of Changes Over Time Anticipated
Commenting on the research, Gregory A. Brent, MD, senior executive academic vice-chair of the Department of Medicine and professor of medicine and physiology at the David Geffen School of Medicine at University of California Los Angeles said the study is indeed novel.
“This, to my knowledge, is the first investigation to link characteristics of the small bowel microbiome with hypothyroidism,” Brent told GI & Hepatology News.
While any clinical significance has yet to be determined, “the association of these small bowel microbiome changes with hypothyroidism may have implications for contributing to the onset of autoimmune hypothyroidism in susceptible populations as well as influences on levothyroxine absorption in hypothyroid patients on levothyroxine therapy,” Brent said.
With the SIBO differences observed even among treated patients with vs without HT, “it seems less likely that the microbiome changes are the result of reduced thyroid hormone signaling,” Brent noted.
Furthermore, a key piece of the puzzle will be to observe the microbiome changes over time, he added.
“These studies were at a single time point [and] longitudinal studies will be especially important to see how the association changes over time and are influenced by the treatment of hypothyroidism and of SIBO,” Brent said.
The authors and Brent had no disclosures to report.
A version of this article appeared on Medscape.com.
, according to results of a study.
“[The research] supports the idea that improving gut health could have far-reaching effects beyond digestion, possibly even helping to prevent autoimmune diseases, such as Hashimoto thyroiditis,” said senior author Ruchi Mathur, MD, director of the Diabetes Outpatient Treatment and Education Center and director of Clinical Operations of Medically Associated Science and Technology, at Cedars-Sinai in Los Angeles, in a press statement for the study, which was presented at ENDO 2025: The Endocrine Society Annual Meeting.
“These findings open the door to new screening and prevention strategies,” Mathur added. “For example, doctors may begin to monitor thyroid health more closely in patients with SIBO, and vice versa.”
With some small studies previously suggesting an association between the gut microbiome and hypothyroidism, Mathur and colleagues further explored the relationship in two analyses.
Assessing the Role of the Small Bowel
For the first, they evaluated data on 49 patients with Hashimoto thyroiditis (HT) and 323 controls without the condition from their REIMAGINE trial, which included small bowel fluid samples from upper endoscopies and DNA sequencing.
In the study, all patients with HT were treated with thyroid replacement (levothyroxine), hence, there were notably no significant differences between the two groups in terms of thyroid stimulating hormone (TSH) levels.
Despite the lack of those differences, patients with HT had a prevalence of SIBO more than twice that of the control group, independent of gender (33% vs 15%; odds ratio, 2.71; P = .005).
When the two groups were further subdivided into two groups each — those with and without SIBO — significant further variations of microbial diversity were observed between those with and without HT, Mathur told GI & Hepatology News.
“Interestingly, we saw the small bowel microbiome was not only different in SIBO-positive patients, including higher gram negatives, which is to be expected, but that the presence or absence of hypothyroidism itself was associated with specific patterns of these gram-negative bacteria,” she explained.
“In addition, when we looked at hypothyroidism without SIBO present, there were also changes between groups, such as higher Neisseria in the hypothyroid group.”
“All these findings are novel as this is the first paper to look specifically at the small bowel,” she added, noting that previous smaller studies have focused more on evaluation of stool samples.
“We believe the small bowel is the most metabolically active area of the intestine and plays an important role in metabolism,” Mathur noted. “Thus, the microbial changes here are likely more physiologically significant than the patterns seen in stool.”
Further Findings from a Large Population
In a separate analysis, the team evaluated data from the TriNetX database on the 10-year incidence of developing SIBO among 1.1 million subjects with hypothyroidism in the US compared with 1 million controls.
They found that people with hypothyroidism were approximately twice as likely to develop SIBO compared with those without hypothyroidism (relative risk [RR], 2.20).
Furthermore, those with HT, in particular, had an even higher risk, at 2.4 times the controls (RR, 2.40).
Treatment with levothyroxine decreased the risk of developing SIBO in hypothyroidism (RR, 0.33) and HT (RR, 0.78) vs those who did not receive treatment.
Mechanisms?
However, the fact that differences in SIBO were observed even between people who were treated for HT and those without the condition in the first analysis, and hence had similar TSH levels, was notable, Mathur said.
“This suggests that perhaps there are other factors aside from TSH levels and free T4 that are at play here,” she said. “Some people have theorized that perhaps delayed gut motility in hypothyroidism promotes the development of SIBO; however, there are many other factors within this complex interplay between the microbiome and the thyroid that could also be playing a role.”
“For example, SIBO leads to inflammation and weakening of the gut barrier,” Mathur explained.
Furthermore, “levothyroxine absorption and cycling of the thyroid hormone occurs predominantly in the small bowel, [while the] microbiome plays a key role in the absorption of iron, selenium, iodine, and zinc, which are critical for thyroid function.”
Overall, “further research is needed to understand how the mechanisms are affected during the development of SIBO and hypothyroidism,” Mathur said.
Assessment of Changes Over Time Anticipated
Commenting on the research, Gregory A. Brent, MD, senior executive academic vice-chair of the Department of Medicine and professor of medicine and physiology at the David Geffen School of Medicine at University of California Los Angeles said the study is indeed novel.
“This, to my knowledge, is the first investigation to link characteristics of the small bowel microbiome with hypothyroidism,” Brent told GI & Hepatology News.
While any clinical significance has yet to be determined, “the association of these small bowel microbiome changes with hypothyroidism may have implications for contributing to the onset of autoimmune hypothyroidism in susceptible populations as well as influences on levothyroxine absorption in hypothyroid patients on levothyroxine therapy,” Brent said.
With the SIBO differences observed even among treated patients with vs without HT, “it seems less likely that the microbiome changes are the result of reduced thyroid hormone signaling,” Brent noted.
Furthermore, a key piece of the puzzle will be to observe the microbiome changes over time, he added.
“These studies were at a single time point [and] longitudinal studies will be especially important to see how the association changes over time and are influenced by the treatment of hypothyroidism and of SIBO,” Brent said.
The authors and Brent had no disclosures to report.
A version of this article appeared on Medscape.com.
Dietary Trial Shows Benefits of a Low Emulsifier Diet for Crohn’s Disease
WASHINGTON, DC — involving 154 patients with mildly active disease living across the United Kingdom.
The findings were reported at Gut Microbiota for Health (GMFH) World Summit 2025 by Benoit Chassaing, PhD, of the Institut Pasteur, Paris, France, whose research leading up to the trial has demonstrated that food additive emulsifiers —ubiquitous in processed foods — alter microbiota composition and lead to microbiota encroachment into the mucus layer of the gut and subsequent chronic gut inflammation.
Patients in the ADDapt trial, which was also reported in an abstract earlier this year at the European Crohn’s and Colitis Organization (ECCO) 2025 Congress, had a Crohn’s disease activity index (CDAI) of 150-250 and evidence of inflammation (faecal calprotectin (FCP) ≥ 150 µg/g or endoscopy/radiology). All “had been exposed in their regular diets to emulsifiers,” said Chassaing, a co-investigator, during a GMFH session on “Dietary Drivers of Health and Disease.”
They were randomized to either a low-emulsifier diet or to a low-emulsifier diet followed by emulsifier “resupplementation” — a design meant to “account for the very strong placebo effect that is always observed with dietary studies,” he said.
All patients received dietary counseling, a smart phone app and barcode scan to support shopping, and weekly support. They also received supermarket foods for 25% of their needs that were either free of emulsifiers or contained emulsifiers, and they were provided three snacks per day that were emulsifier-free or contained carrageenan, carboxymethycellulse (CMC), and polysorbate-80 (P80) — dietary emulsifiers that are commonly added to processed foods to enhance texture and extend shelf-life.
In the intention-to-treat (ITT) analysis, 49% of patients in the intervention group reached the primary endpoint of a 70-point reduction or more in CDAI response after 8 weeks compared with 31% of those in the control group (P = .019), with an adjusted relative risk of response of 3.1 (P = .003), Chassaing shared at the GMFH meeting, convened by the American Gastroenterological Association and the European Society of Neurogastroenterology and Motility.
In the per-protocol analysis (n = 119), 61% and 47% of patients in the intervention and control groups, respectively, reached the primary outcome of CDAI response, with an adjusted relative risk of response of 3.0 (P = .018), he said.
Secondary endpoints included CDAI remission at 24 weeks, and according to the abstract for the ECCO Congress, in the ITT analysis, patients in the intervention group were more than twice as likely to experience remission.
Chassaing noted at the GMFH meeting that as part of the study, he and coinvestigators have been investigating the participants’ gut microbiota with metagenomic analyses. The study was led by Kevin Whelan, PhD, head of the Department of Nutritional Sciences at King’s College London, London, England.
Can Emulsifier-Sensitive Individuals Be Identified?
In murine model research 10 years ago, Chassaing showed that the administration of CMC and P80 results in microbiota encroachment into the mucus layer of the gut, alterations in microbiota composition — including an increase in bacteria that produce pro-inflammatory flagellin — and development of chronic inflammation.
Wild-type mice treated with these compounds developed metabolic disease, and mice that were modified to be predisposed to colitis had a higher incidence of robust colitis. Moreover, fecal transplantation from emulsifier-treated mice to germ-free mice reproduced these changes, “clearly suggesting that the microbiome itself is sufficient to drive chronic inflammation,” he said.
In recent years, in humans, analyses from the large French NutriNet-Sante prospective cohort study have shown associations between exposure to food additive emulsifiers and the risk for cardiovascular disease, the risk for cancer (overall, breast, and prostate), and the risk for type 2 diabetes.
But to explore causality and better understand the mechanisms of emulsifier-driven changes on the microbiota, Chassaing and his colleagues also launched the FRESH study (Functional Research on Emulsifier in Humans), a double-blind randomized controlled-feeding study of the emulsifier CMC. For 11 days, nine healthy patients consumed an emulsifier-free diet and 11 consumed an identical diet enriched with 15 g/d of CMC.
Patients on the CMC-containing diet had reduced microbiota diversity and depletions of an array of microbiota-related metabolites, but only a small subset had profound alterations in microbiota composition and increased microbiota encroachment into the mucus layer. “Some seemed to be resistant to CMC-induced microbiota encroachment, while some were highly susceptible,” Chassaing said.
The pilot study raised the question, he said, of whether there is an “infectivity component” — some kind of “sensitive” gut microbiota composition — that may be associated with dietary emulsifier-driven inflammation and disease.
In other murine research, Chassaing and his team found that germ-free mice colonized with Crohn’s disease-associated adherent-invasive E coli (AIEC) and subsequently given CMC or P80 developed chronic inflammation and metabolic dysregulation, “clearly demonstrating that you can convert resistant mice to sensitive mice just by adding one bacteria to the ecosystem,” he said. “The presence of AIEC alone was sufficient to drive the detrimental effects of dietary emulsifiers.”
(In vitro research with transcriptomic analysis then showed that the emulsifiers directly elicit AIEC virulence gene expression, Chassaing and his coauthors wrote in their 2020 paper, facilitating AIEC’s “penetration of the mucus layer and adherence to epithelial cells and resulting in activation of host pro-inflammatory signaling.”)
“We don’t think it’s solely the AIEC bacteria that will drive emulsifier sensitivity, though…we think it’s more complex,” Chassaing said at the meeting. Overall, the findings raise the question of whether emulsifier-sensitive individuals can be identified.
This, he said, is one of his most recent research questions. His lab has led the development of an in vitro microbiota model built to predict an individual’s sensitivity to emulsifiers. In a study published in April, the model recapitulated the differential CMC sensitivity observed in the earlier FRESH study, suggesting that an individual’s sensitivity to emulsifiers can indeed be predicted by examining their baseline microbiota.
Interpreting the Epidemiology
Chassaing’s research arch illustrates the synergy between epidemiological research, basic/translational research, and clinical interventional research that’s needed to understand the diet-microbiome intersection in inflammatory bowel disease, said Ashwin Ananthakrishnan, MBBS, MPH, AGAF, associate professor of medicine at Massachusetts General Hospital, Boston, in an interview at the meeting.
“It’s a good example of how to really span the spectrum, starting from the big picture and going deeper to understand mechanisms, and starting from mechanisms and expanding it out,” Ananthakrishnan said.
In his own talk about research on IBD, Ananthakrishnan said that epidemiological data have shown over the past 10-15 years that total dietary fiber is inversely associated with the risk for Crohn’s disease (with the strongest associations with fiber from fruits and vegetables). Studies have also shown that a higher intake of polyunsaturated fatty acids is associated with a lower risk for ulcerative colitis, whereas “an n-6-fatty acid-rich diet is associated with a higher risk of ulcerative colitis,” he said.
Dietary cohort studies, meanwhile, have shed light on the influence of dietary patterns — such as the Mediterranean diet and diets with high inflammatory potential—on IBD. A diet rich in ultra-processed foods has also been shown in a prospective cohort study to be associated with a higher risk for Crohn’s disease, with certain categories of ultra-processed foods (eg, breads and breakfast foods) having the strongest associations.
Such studies are limited in part, however, by inadequate assessment of potentially relevant variables such as emulsifiers, preservatives, and how the food is processed, he said.
And in interpreting the epidemiological research on fiber and IBD, for instance, one must appreciate that “there are a number of mechanisms by which fiber is impactful…there’s a big picture to look at,” Ananthakrishnan said. Fiber “can affect the microbiome, clearly, it can affect the gut barrier, and it can affect bile acids, and there are detailed translational studies in support of each of these.”
But there are other constituents of fruits and vegetables “that could potentially influence disease risk, such as AhR ligands and polyphenols,” he said. “And importantly, people not eating a lot of fiber may be eating a lot of ultra-processed foods.”
Most interventional studies of fiber have not shown a benefit of a high-fiber diet, Ananthakrishnan said, but there are multiple possible reasons and factors at play, including potential population differences (eg, in inflammatory status or baseline microbiota), shortcomings of the interventions, and potentially inaccurate outcomes.
Abigail Johnson, PhD, RDN, associate director of the Nutrition Coordinating Center, University of Minnesota Twin Cities, which supports dietary analysis, said during the session that the focus of dietary research is “moving toward understanding overall dietary patterns” as opposed to focusing more narrowly on vitamins, minerals, and macronutrients such as proteins, fats, and carbohydrates.
This is an improvement, though “we still don’t have good approaches for understanding [the contributions of] things like additives and emulsifiers, food preparation and cooking, and food processing,” said Johnson, assistant professor in the Division of Epidemiology and Community Health at University of Minnesota Twin Cities. “Perhaps by looking at things at the food level we can overcome some of these limitations.”
Ananthakrishnan reported being a consultant for Geneoscopy and receiving a research grant from Takeda. Chassaing did not report any financial disclosures. Johnson reported that she had no financial disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON, DC — involving 154 patients with mildly active disease living across the United Kingdom.
The findings were reported at Gut Microbiota for Health (GMFH) World Summit 2025 by Benoit Chassaing, PhD, of the Institut Pasteur, Paris, France, whose research leading up to the trial has demonstrated that food additive emulsifiers —ubiquitous in processed foods — alter microbiota composition and lead to microbiota encroachment into the mucus layer of the gut and subsequent chronic gut inflammation.
Patients in the ADDapt trial, which was also reported in an abstract earlier this year at the European Crohn’s and Colitis Organization (ECCO) 2025 Congress, had a Crohn’s disease activity index (CDAI) of 150-250 and evidence of inflammation (faecal calprotectin (FCP) ≥ 150 µg/g or endoscopy/radiology). All “had been exposed in their regular diets to emulsifiers,” said Chassaing, a co-investigator, during a GMFH session on “Dietary Drivers of Health and Disease.”
They were randomized to either a low-emulsifier diet or to a low-emulsifier diet followed by emulsifier “resupplementation” — a design meant to “account for the very strong placebo effect that is always observed with dietary studies,” he said.
All patients received dietary counseling, a smart phone app and barcode scan to support shopping, and weekly support. They also received supermarket foods for 25% of their needs that were either free of emulsifiers or contained emulsifiers, and they were provided three snacks per day that were emulsifier-free or contained carrageenan, carboxymethycellulse (CMC), and polysorbate-80 (P80) — dietary emulsifiers that are commonly added to processed foods to enhance texture and extend shelf-life.
In the intention-to-treat (ITT) analysis, 49% of patients in the intervention group reached the primary endpoint of a 70-point reduction or more in CDAI response after 8 weeks compared with 31% of those in the control group (P = .019), with an adjusted relative risk of response of 3.1 (P = .003), Chassaing shared at the GMFH meeting, convened by the American Gastroenterological Association and the European Society of Neurogastroenterology and Motility.
In the per-protocol analysis (n = 119), 61% and 47% of patients in the intervention and control groups, respectively, reached the primary outcome of CDAI response, with an adjusted relative risk of response of 3.0 (P = .018), he said.
Secondary endpoints included CDAI remission at 24 weeks, and according to the abstract for the ECCO Congress, in the ITT analysis, patients in the intervention group were more than twice as likely to experience remission.
Chassaing noted at the GMFH meeting that as part of the study, he and coinvestigators have been investigating the participants’ gut microbiota with metagenomic analyses. The study was led by Kevin Whelan, PhD, head of the Department of Nutritional Sciences at King’s College London, London, England.
Can Emulsifier-Sensitive Individuals Be Identified?
In murine model research 10 years ago, Chassaing showed that the administration of CMC and P80 results in microbiota encroachment into the mucus layer of the gut, alterations in microbiota composition — including an increase in bacteria that produce pro-inflammatory flagellin — and development of chronic inflammation.
Wild-type mice treated with these compounds developed metabolic disease, and mice that were modified to be predisposed to colitis had a higher incidence of robust colitis. Moreover, fecal transplantation from emulsifier-treated mice to germ-free mice reproduced these changes, “clearly suggesting that the microbiome itself is sufficient to drive chronic inflammation,” he said.
In recent years, in humans, analyses from the large French NutriNet-Sante prospective cohort study have shown associations between exposure to food additive emulsifiers and the risk for cardiovascular disease, the risk for cancer (overall, breast, and prostate), and the risk for type 2 diabetes.
But to explore causality and better understand the mechanisms of emulsifier-driven changes on the microbiota, Chassaing and his colleagues also launched the FRESH study (Functional Research on Emulsifier in Humans), a double-blind randomized controlled-feeding study of the emulsifier CMC. For 11 days, nine healthy patients consumed an emulsifier-free diet and 11 consumed an identical diet enriched with 15 g/d of CMC.
Patients on the CMC-containing diet had reduced microbiota diversity and depletions of an array of microbiota-related metabolites, but only a small subset had profound alterations in microbiota composition and increased microbiota encroachment into the mucus layer. “Some seemed to be resistant to CMC-induced microbiota encroachment, while some were highly susceptible,” Chassaing said.
The pilot study raised the question, he said, of whether there is an “infectivity component” — some kind of “sensitive” gut microbiota composition — that may be associated with dietary emulsifier-driven inflammation and disease.
In other murine research, Chassaing and his team found that germ-free mice colonized with Crohn’s disease-associated adherent-invasive E coli (AIEC) and subsequently given CMC or P80 developed chronic inflammation and metabolic dysregulation, “clearly demonstrating that you can convert resistant mice to sensitive mice just by adding one bacteria to the ecosystem,” he said. “The presence of AIEC alone was sufficient to drive the detrimental effects of dietary emulsifiers.”
(In vitro research with transcriptomic analysis then showed that the emulsifiers directly elicit AIEC virulence gene expression, Chassaing and his coauthors wrote in their 2020 paper, facilitating AIEC’s “penetration of the mucus layer and adherence to epithelial cells and resulting in activation of host pro-inflammatory signaling.”)
“We don’t think it’s solely the AIEC bacteria that will drive emulsifier sensitivity, though…we think it’s more complex,” Chassaing said at the meeting. Overall, the findings raise the question of whether emulsifier-sensitive individuals can be identified.
This, he said, is one of his most recent research questions. His lab has led the development of an in vitro microbiota model built to predict an individual’s sensitivity to emulsifiers. In a study published in April, the model recapitulated the differential CMC sensitivity observed in the earlier FRESH study, suggesting that an individual’s sensitivity to emulsifiers can indeed be predicted by examining their baseline microbiota.
Interpreting the Epidemiology
Chassaing’s research arch illustrates the synergy between epidemiological research, basic/translational research, and clinical interventional research that’s needed to understand the diet-microbiome intersection in inflammatory bowel disease, said Ashwin Ananthakrishnan, MBBS, MPH, AGAF, associate professor of medicine at Massachusetts General Hospital, Boston, in an interview at the meeting.
“It’s a good example of how to really span the spectrum, starting from the big picture and going deeper to understand mechanisms, and starting from mechanisms and expanding it out,” Ananthakrishnan said.
In his own talk about research on IBD, Ananthakrishnan said that epidemiological data have shown over the past 10-15 years that total dietary fiber is inversely associated with the risk for Crohn’s disease (with the strongest associations with fiber from fruits and vegetables). Studies have also shown that a higher intake of polyunsaturated fatty acids is associated with a lower risk for ulcerative colitis, whereas “an n-6-fatty acid-rich diet is associated with a higher risk of ulcerative colitis,” he said.
Dietary cohort studies, meanwhile, have shed light on the influence of dietary patterns — such as the Mediterranean diet and diets with high inflammatory potential—on IBD. A diet rich in ultra-processed foods has also been shown in a prospective cohort study to be associated with a higher risk for Crohn’s disease, with certain categories of ultra-processed foods (eg, breads and breakfast foods) having the strongest associations.
Such studies are limited in part, however, by inadequate assessment of potentially relevant variables such as emulsifiers, preservatives, and how the food is processed, he said.
And in interpreting the epidemiological research on fiber and IBD, for instance, one must appreciate that “there are a number of mechanisms by which fiber is impactful…there’s a big picture to look at,” Ananthakrishnan said. Fiber “can affect the microbiome, clearly, it can affect the gut barrier, and it can affect bile acids, and there are detailed translational studies in support of each of these.”
But there are other constituents of fruits and vegetables “that could potentially influence disease risk, such as AhR ligands and polyphenols,” he said. “And importantly, people not eating a lot of fiber may be eating a lot of ultra-processed foods.”
Most interventional studies of fiber have not shown a benefit of a high-fiber diet, Ananthakrishnan said, but there are multiple possible reasons and factors at play, including potential population differences (eg, in inflammatory status or baseline microbiota), shortcomings of the interventions, and potentially inaccurate outcomes.
Abigail Johnson, PhD, RDN, associate director of the Nutrition Coordinating Center, University of Minnesota Twin Cities, which supports dietary analysis, said during the session that the focus of dietary research is “moving toward understanding overall dietary patterns” as opposed to focusing more narrowly on vitamins, minerals, and macronutrients such as proteins, fats, and carbohydrates.
This is an improvement, though “we still don’t have good approaches for understanding [the contributions of] things like additives and emulsifiers, food preparation and cooking, and food processing,” said Johnson, assistant professor in the Division of Epidemiology and Community Health at University of Minnesota Twin Cities. “Perhaps by looking at things at the food level we can overcome some of these limitations.”
Ananthakrishnan reported being a consultant for Geneoscopy and receiving a research grant from Takeda. Chassaing did not report any financial disclosures. Johnson reported that she had no financial disclosures.
A version of this article appeared on Medscape.com.
WASHINGTON, DC — involving 154 patients with mildly active disease living across the United Kingdom.
The findings were reported at Gut Microbiota for Health (GMFH) World Summit 2025 by Benoit Chassaing, PhD, of the Institut Pasteur, Paris, France, whose research leading up to the trial has demonstrated that food additive emulsifiers —ubiquitous in processed foods — alter microbiota composition and lead to microbiota encroachment into the mucus layer of the gut and subsequent chronic gut inflammation.
Patients in the ADDapt trial, which was also reported in an abstract earlier this year at the European Crohn’s and Colitis Organization (ECCO) 2025 Congress, had a Crohn’s disease activity index (CDAI) of 150-250 and evidence of inflammation (faecal calprotectin (FCP) ≥ 150 µg/g or endoscopy/radiology). All “had been exposed in their regular diets to emulsifiers,” said Chassaing, a co-investigator, during a GMFH session on “Dietary Drivers of Health and Disease.”
They were randomized to either a low-emulsifier diet or to a low-emulsifier diet followed by emulsifier “resupplementation” — a design meant to “account for the very strong placebo effect that is always observed with dietary studies,” he said.
All patients received dietary counseling, a smart phone app and barcode scan to support shopping, and weekly support. They also received supermarket foods for 25% of their needs that were either free of emulsifiers or contained emulsifiers, and they were provided three snacks per day that were emulsifier-free or contained carrageenan, carboxymethycellulse (CMC), and polysorbate-80 (P80) — dietary emulsifiers that are commonly added to processed foods to enhance texture and extend shelf-life.
In the intention-to-treat (ITT) analysis, 49% of patients in the intervention group reached the primary endpoint of a 70-point reduction or more in CDAI response after 8 weeks compared with 31% of those in the control group (P = .019), with an adjusted relative risk of response of 3.1 (P = .003), Chassaing shared at the GMFH meeting, convened by the American Gastroenterological Association and the European Society of Neurogastroenterology and Motility.
In the per-protocol analysis (n = 119), 61% and 47% of patients in the intervention and control groups, respectively, reached the primary outcome of CDAI response, with an adjusted relative risk of response of 3.0 (P = .018), he said.
Secondary endpoints included CDAI remission at 24 weeks, and according to the abstract for the ECCO Congress, in the ITT analysis, patients in the intervention group were more than twice as likely to experience remission.
Chassaing noted at the GMFH meeting that as part of the study, he and coinvestigators have been investigating the participants’ gut microbiota with metagenomic analyses. The study was led by Kevin Whelan, PhD, head of the Department of Nutritional Sciences at King’s College London, London, England.
Can Emulsifier-Sensitive Individuals Be Identified?
In murine model research 10 years ago, Chassaing showed that the administration of CMC and P80 results in microbiota encroachment into the mucus layer of the gut, alterations in microbiota composition — including an increase in bacteria that produce pro-inflammatory flagellin — and development of chronic inflammation.
Wild-type mice treated with these compounds developed metabolic disease, and mice that were modified to be predisposed to colitis had a higher incidence of robust colitis. Moreover, fecal transplantation from emulsifier-treated mice to germ-free mice reproduced these changes, “clearly suggesting that the microbiome itself is sufficient to drive chronic inflammation,” he said.
In recent years, in humans, analyses from the large French NutriNet-Sante prospective cohort study have shown associations between exposure to food additive emulsifiers and the risk for cardiovascular disease, the risk for cancer (overall, breast, and prostate), and the risk for type 2 diabetes.
But to explore causality and better understand the mechanisms of emulsifier-driven changes on the microbiota, Chassaing and his colleagues also launched the FRESH study (Functional Research on Emulsifier in Humans), a double-blind randomized controlled-feeding study of the emulsifier CMC. For 11 days, nine healthy patients consumed an emulsifier-free diet and 11 consumed an identical diet enriched with 15 g/d of CMC.
Patients on the CMC-containing diet had reduced microbiota diversity and depletions of an array of microbiota-related metabolites, but only a small subset had profound alterations in microbiota composition and increased microbiota encroachment into the mucus layer. “Some seemed to be resistant to CMC-induced microbiota encroachment, while some were highly susceptible,” Chassaing said.
The pilot study raised the question, he said, of whether there is an “infectivity component” — some kind of “sensitive” gut microbiota composition — that may be associated with dietary emulsifier-driven inflammation and disease.
In other murine research, Chassaing and his team found that germ-free mice colonized with Crohn’s disease-associated adherent-invasive E coli (AIEC) and subsequently given CMC or P80 developed chronic inflammation and metabolic dysregulation, “clearly demonstrating that you can convert resistant mice to sensitive mice just by adding one bacteria to the ecosystem,” he said. “The presence of AIEC alone was sufficient to drive the detrimental effects of dietary emulsifiers.”
(In vitro research with transcriptomic analysis then showed that the emulsifiers directly elicit AIEC virulence gene expression, Chassaing and his coauthors wrote in their 2020 paper, facilitating AIEC’s “penetration of the mucus layer and adherence to epithelial cells and resulting in activation of host pro-inflammatory signaling.”)
“We don’t think it’s solely the AIEC bacteria that will drive emulsifier sensitivity, though…we think it’s more complex,” Chassaing said at the meeting. Overall, the findings raise the question of whether emulsifier-sensitive individuals can be identified.
This, he said, is one of his most recent research questions. His lab has led the development of an in vitro microbiota model built to predict an individual’s sensitivity to emulsifiers. In a study published in April, the model recapitulated the differential CMC sensitivity observed in the earlier FRESH study, suggesting that an individual’s sensitivity to emulsifiers can indeed be predicted by examining their baseline microbiota.
Interpreting the Epidemiology
Chassaing’s research arch illustrates the synergy between epidemiological research, basic/translational research, and clinical interventional research that’s needed to understand the diet-microbiome intersection in inflammatory bowel disease, said Ashwin Ananthakrishnan, MBBS, MPH, AGAF, associate professor of medicine at Massachusetts General Hospital, Boston, in an interview at the meeting.
“It’s a good example of how to really span the spectrum, starting from the big picture and going deeper to understand mechanisms, and starting from mechanisms and expanding it out,” Ananthakrishnan said.
In his own talk about research on IBD, Ananthakrishnan said that epidemiological data have shown over the past 10-15 years that total dietary fiber is inversely associated with the risk for Crohn’s disease (with the strongest associations with fiber from fruits and vegetables). Studies have also shown that a higher intake of polyunsaturated fatty acids is associated with a lower risk for ulcerative colitis, whereas “an n-6-fatty acid-rich diet is associated with a higher risk of ulcerative colitis,” he said.
Dietary cohort studies, meanwhile, have shed light on the influence of dietary patterns — such as the Mediterranean diet and diets with high inflammatory potential—on IBD. A diet rich in ultra-processed foods has also been shown in a prospective cohort study to be associated with a higher risk for Crohn’s disease, with certain categories of ultra-processed foods (eg, breads and breakfast foods) having the strongest associations.
Such studies are limited in part, however, by inadequate assessment of potentially relevant variables such as emulsifiers, preservatives, and how the food is processed, he said.
And in interpreting the epidemiological research on fiber and IBD, for instance, one must appreciate that “there are a number of mechanisms by which fiber is impactful…there’s a big picture to look at,” Ananthakrishnan said. Fiber “can affect the microbiome, clearly, it can affect the gut barrier, and it can affect bile acids, and there are detailed translational studies in support of each of these.”
But there are other constituents of fruits and vegetables “that could potentially influence disease risk, such as AhR ligands and polyphenols,” he said. “And importantly, people not eating a lot of fiber may be eating a lot of ultra-processed foods.”
Most interventional studies of fiber have not shown a benefit of a high-fiber diet, Ananthakrishnan said, but there are multiple possible reasons and factors at play, including potential population differences (eg, in inflammatory status or baseline microbiota), shortcomings of the interventions, and potentially inaccurate outcomes.
Abigail Johnson, PhD, RDN, associate director of the Nutrition Coordinating Center, University of Minnesota Twin Cities, which supports dietary analysis, said during the session that the focus of dietary research is “moving toward understanding overall dietary patterns” as opposed to focusing more narrowly on vitamins, minerals, and macronutrients such as proteins, fats, and carbohydrates.
This is an improvement, though “we still don’t have good approaches for understanding [the contributions of] things like additives and emulsifiers, food preparation and cooking, and food processing,” said Johnson, assistant professor in the Division of Epidemiology and Community Health at University of Minnesota Twin Cities. “Perhaps by looking at things at the food level we can overcome some of these limitations.”
Ananthakrishnan reported being a consultant for Geneoscopy and receiving a research grant from Takeda. Chassaing did not report any financial disclosures. Johnson reported that she had no financial disclosures.
A version of this article appeared on Medscape.com.
FROM GMFH 2025
Treating Metastatic RCC: From Risk Assessment to Therapy Selection
Treating Metastatic RCC: From Risk Assessment to Therapy Selection
Treatment of metastatic renal cell carcinoma (RCC) is complex and requires careful analysis of risk and treatment options, an oncologist said at the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, regarding treating veterans with kidney cancer.
“We’ve come a long way in treating this disease, but individualizing therapy remains critical, especially in complex populations like our veterans,” said Matthew B. Rettig, MD, chief of Hematology-Oncology at the Veterans Affairs Greater Los Angeles Healthcare System and professor of Medicine and Urology at UCLA.
Rettig emphasized 2 critical early questions clinicians should consider when encountering metastatic RCC. First: Can the patient be treated with localized interventions such as metastasectomy, radiation therapy, or nephrectomy? These can be curative, Rettig said.
And second: Does the patient currently need systemic therapy? “[There are] a small subset of patients,” Rettig said, “who go into a durable, complete remission, dare I say ‘cure,’ with immunotherapeutic-based approaches.”
Rettig highlighted the International Metastatic Renal Cell Carcinoma Database Consortium criteria as a guide for clinicians as they determine the best strategy for treatment. The Database Consortium estimates survival in various lines of therapy by incorporating 6 prognostic factors: anemia, hypercalcemia, neutrophilia, thrombocytosis, performance status, and time from diagnosis to treatment.
These criteria classify patients into favorable, intermediate, or poor risk categories that can guide first-line systemic therapy. The criteria also provide estimates of median survival.
Rettig noted a “huge percentage” of veterans mirror the intermediate-risk demographics of clinical trial cohorts but often present with greater comorbidity burdens: “That plays into whether we treat and how we treat,” he said.
Rettig highlighted kidney cancer guidelines from the National Comprehensive Cancer Network and noted that several trials examined first-line use of combinations of vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors.
There’s a general theme in the findings, he said: “You have OS (overall survival) and PFS (progression-free survival) benefit in the intermediate/poor risk group, but only PFS benefit in the patients who have favorable-risk disease. And you see higher objective response rates with the combinations.
“If you have a patient who's highly symptomatic or has an organ system threatened by a metastasis, you'd want to use a combination that elicits a higher objective response rate,” Rettig added.
A TKI is going to be the most appropriate second-line therapy for patients who received a prior checkpoint inhibitor, Rettig said.
“Don't change to another checkpoint inhibitor,” he said. “We have enough phase 3 data that indicates checkpoint inhibitors are no longer really adding to benefit once they’ve had a checkpoint inhibitor.”
Rettig said to even consider checkpoint inhibitors for patients who are checkpoint inhibitor-naïve, especially given the potential for durable remissions. As for third-line therapy, he said, “we have both belzutifan and tivozanib, which have been shown to improve PFS. More studies are ongoing.”
There are many adverse events linked to TKIs, Rettig said, including cardiovascular problems, thrombosis, hypertension, heart failure, torsades de pointes, QT prolongation, and gastrointestinal toxicity. TKIs tend to be the major drivers of adverse events in combination therapy.
Rettig emphasized the shorter half-life of the TKI axitinib, which he said allows for easier management of toxicities: “That’s why it’s preferred in the VA RCC clinical pathway.”
Rettig discloses relationships with Ambrx, Amgen, AVEO, Bayer, INmune Bio, Johnson & Johnson Health Care Systems, Lantheus, Merck, Myovant, Novartis, ORIC, and Progenics.
Treatment of metastatic renal cell carcinoma (RCC) is complex and requires careful analysis of risk and treatment options, an oncologist said at the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, regarding treating veterans with kidney cancer.
“We’ve come a long way in treating this disease, but individualizing therapy remains critical, especially in complex populations like our veterans,” said Matthew B. Rettig, MD, chief of Hematology-Oncology at the Veterans Affairs Greater Los Angeles Healthcare System and professor of Medicine and Urology at UCLA.
Rettig emphasized 2 critical early questions clinicians should consider when encountering metastatic RCC. First: Can the patient be treated with localized interventions such as metastasectomy, radiation therapy, or nephrectomy? These can be curative, Rettig said.
And second: Does the patient currently need systemic therapy? “[There are] a small subset of patients,” Rettig said, “who go into a durable, complete remission, dare I say ‘cure,’ with immunotherapeutic-based approaches.”
Rettig highlighted the International Metastatic Renal Cell Carcinoma Database Consortium criteria as a guide for clinicians as they determine the best strategy for treatment. The Database Consortium estimates survival in various lines of therapy by incorporating 6 prognostic factors: anemia, hypercalcemia, neutrophilia, thrombocytosis, performance status, and time from diagnosis to treatment.
These criteria classify patients into favorable, intermediate, or poor risk categories that can guide first-line systemic therapy. The criteria also provide estimates of median survival.
Rettig noted a “huge percentage” of veterans mirror the intermediate-risk demographics of clinical trial cohorts but often present with greater comorbidity burdens: “That plays into whether we treat and how we treat,” he said.
Rettig highlighted kidney cancer guidelines from the National Comprehensive Cancer Network and noted that several trials examined first-line use of combinations of vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors.
There’s a general theme in the findings, he said: “You have OS (overall survival) and PFS (progression-free survival) benefit in the intermediate/poor risk group, but only PFS benefit in the patients who have favorable-risk disease. And you see higher objective response rates with the combinations.
“If you have a patient who's highly symptomatic or has an organ system threatened by a metastasis, you'd want to use a combination that elicits a higher objective response rate,” Rettig added.
A TKI is going to be the most appropriate second-line therapy for patients who received a prior checkpoint inhibitor, Rettig said.
“Don't change to another checkpoint inhibitor,” he said. “We have enough phase 3 data that indicates checkpoint inhibitors are no longer really adding to benefit once they’ve had a checkpoint inhibitor.”
Rettig said to even consider checkpoint inhibitors for patients who are checkpoint inhibitor-naïve, especially given the potential for durable remissions. As for third-line therapy, he said, “we have both belzutifan and tivozanib, which have been shown to improve PFS. More studies are ongoing.”
There are many adverse events linked to TKIs, Rettig said, including cardiovascular problems, thrombosis, hypertension, heart failure, torsades de pointes, QT prolongation, and gastrointestinal toxicity. TKIs tend to be the major drivers of adverse events in combination therapy.
Rettig emphasized the shorter half-life of the TKI axitinib, which he said allows for easier management of toxicities: “That’s why it’s preferred in the VA RCC clinical pathway.”
Rettig discloses relationships with Ambrx, Amgen, AVEO, Bayer, INmune Bio, Johnson & Johnson Health Care Systems, Lantheus, Merck, Myovant, Novartis, ORIC, and Progenics.
Treatment of metastatic renal cell carcinoma (RCC) is complex and requires careful analysis of risk and treatment options, an oncologist said at the July Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, regarding treating veterans with kidney cancer.
“We’ve come a long way in treating this disease, but individualizing therapy remains critical, especially in complex populations like our veterans,” said Matthew B. Rettig, MD, chief of Hematology-Oncology at the Veterans Affairs Greater Los Angeles Healthcare System and professor of Medicine and Urology at UCLA.
Rettig emphasized 2 critical early questions clinicians should consider when encountering metastatic RCC. First: Can the patient be treated with localized interventions such as metastasectomy, radiation therapy, or nephrectomy? These can be curative, Rettig said.
And second: Does the patient currently need systemic therapy? “[There are] a small subset of patients,” Rettig said, “who go into a durable, complete remission, dare I say ‘cure,’ with immunotherapeutic-based approaches.”
Rettig highlighted the International Metastatic Renal Cell Carcinoma Database Consortium criteria as a guide for clinicians as they determine the best strategy for treatment. The Database Consortium estimates survival in various lines of therapy by incorporating 6 prognostic factors: anemia, hypercalcemia, neutrophilia, thrombocytosis, performance status, and time from diagnosis to treatment.
These criteria classify patients into favorable, intermediate, or poor risk categories that can guide first-line systemic therapy. The criteria also provide estimates of median survival.
Rettig noted a “huge percentage” of veterans mirror the intermediate-risk demographics of clinical trial cohorts but often present with greater comorbidity burdens: “That plays into whether we treat and how we treat,” he said.
Rettig highlighted kidney cancer guidelines from the National Comprehensive Cancer Network and noted that several trials examined first-line use of combinations of vascular endothelial growth factor receptor tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors.
There’s a general theme in the findings, he said: “You have OS (overall survival) and PFS (progression-free survival) benefit in the intermediate/poor risk group, but only PFS benefit in the patients who have favorable-risk disease. And you see higher objective response rates with the combinations.
“If you have a patient who's highly symptomatic or has an organ system threatened by a metastasis, you'd want to use a combination that elicits a higher objective response rate,” Rettig added.
A TKI is going to be the most appropriate second-line therapy for patients who received a prior checkpoint inhibitor, Rettig said.
“Don't change to another checkpoint inhibitor,” he said. “We have enough phase 3 data that indicates checkpoint inhibitors are no longer really adding to benefit once they’ve had a checkpoint inhibitor.”
Rettig said to even consider checkpoint inhibitors for patients who are checkpoint inhibitor-naïve, especially given the potential for durable remissions. As for third-line therapy, he said, “we have both belzutifan and tivozanib, which have been shown to improve PFS. More studies are ongoing.”
There are many adverse events linked to TKIs, Rettig said, including cardiovascular problems, thrombosis, hypertension, heart failure, torsades de pointes, QT prolongation, and gastrointestinal toxicity. TKIs tend to be the major drivers of adverse events in combination therapy.
Rettig emphasized the shorter half-life of the TKI axitinib, which he said allows for easier management of toxicities: “That’s why it’s preferred in the VA RCC clinical pathway.”
Rettig discloses relationships with Ambrx, Amgen, AVEO, Bayer, INmune Bio, Johnson & Johnson Health Care Systems, Lantheus, Merck, Myovant, Novartis, ORIC, and Progenics.
Treating Metastatic RCC: From Risk Assessment to Therapy Selection
Treating Metastatic RCC: From Risk Assessment to Therapy Selection
Renal Cell Carcinoma: What You Need to Know About Hereditary Syndromes
Renal Cell Carcinoma: What You Need to Know About Hereditary Syndromes
The role of hereditary syndromes in renal cell carcinoma (RCC) might be easily missed, a kidney cancer specialist said during a recent Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, though careful clinical evaluation can uncover genetic traits that may affect treatment and familial risk.
“The importance of finding or identifying hereditary forms of kidney cancer really should not be underestimated,” said urologist Brian Shuch, MD, director of the UCLA Kidney Cancer Program, on treating veterans with kidney cancer.
According to Shuch, recent data suggest that about 4.5% of patients with RCC have a hereditary syndrome: “A lot of times, these hide in plain sight. You have to really look deep and try to figure things out and understand that maybe they have a hereditary form of kidney cancer.”
It is important to consider early genetic testing, Shuch said. Red flags for hereditary syndromes include early-onset RCC (age ≤ 45 years), multifocal tumors, bilateral tumors (especially in younger individuals), or a relevant family personal history, he said.
Unusual skin conditions are also potential signs, Shuch said. These can include leiomyomas, fibrofolliculomas, and angiofibromas: “Patients have lots of lumps or bumps.”
“When I look at a patient, I go head to toe and ask if there any issues with your vision, any issues with your hearing, any issues swallowing,” he explained at the meeting. “Do you have any problems with heart issues, adrenal issues? You’ve got to go through each organ, and it can lead you to different things.”
Shuch highlighted Von Hippel-Lindau (VHL) syndrome, which affects 1 in 25,000 people. About 80% to 90% of these patients have a family history, Shuch said.
But the others do not. “Unfortunately, some get diagnosed later in life because they don’t get cascade testing starting at aged 2, which is recommended. These are the patients who might be coming into the ER with a hemangioblastoma or picking up the phone and all of a sudden being deaf in one ear due to an endolymphatic sac tumor.
“We want to limit metastatic spread and preserve the kidneys,” Shuch said. “We don’t want to be doing radical nephrectomies. We want to avoid chronic kidney disease, prevent end-stage renal disease, and maximize quality of life.”
It’s a good idea to avoid surgical removal unless a patient’s tumor grows to be > 3 cm, a line that indicates risk of metastases, he said.
In terms of treatment, Shuch highlighted a 2021 study that showed benefit in VHL from belzutifan (Welireg), an oral HIF-2 α inhibitor approved by the US Food and Drug Administration. The medication significantly reduced the need for surgical intervention.
“Patients go on this drug, and surgeons are putting their scalpels down,” said Shuch, who worked on the 2021 study.
Other hereditary syndromes include the rare hereditary papillary RCC, and Birt-Hogg-Dubé syndrome, believed to affect 1 in 200,000 people but may be more common, he said.
Birt-Hogg-Dubé syndrome is linked to lung cysts, lung collapse, and skin manifestations. The 3 cm surgery rule is appropriate in these cases, Shuch said, and metastases are rare.
Another condition, hereditary leiomyomatosis and RCC, is the most dangerous hereditary form. Originally thought to affect 1 in 200,000 people, hereditary leiomyomatosis and RCC is similar to Birt-Hogg-Dubé syndrome in that it is believed to be more common.
“You will see this,” Shuch predicted.
Shuch advised colleagues to intervene early and take a large margin during surgery.
He also highlighted familial paraganglioma syndrome, which is associated with gastrointestinal stromal tumors, and Cowden syndrome, which is linked to skin manifestations and breast, thyroid, and endometrial cancer.
Shuch reported that he had no disclosures.
The role of hereditary syndromes in renal cell carcinoma (RCC) might be easily missed, a kidney cancer specialist said during a recent Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, though careful clinical evaluation can uncover genetic traits that may affect treatment and familial risk.
“The importance of finding or identifying hereditary forms of kidney cancer really should not be underestimated,” said urologist Brian Shuch, MD, director of the UCLA Kidney Cancer Program, on treating veterans with kidney cancer.
According to Shuch, recent data suggest that about 4.5% of patients with RCC have a hereditary syndrome: “A lot of times, these hide in plain sight. You have to really look deep and try to figure things out and understand that maybe they have a hereditary form of kidney cancer.”
It is important to consider early genetic testing, Shuch said. Red flags for hereditary syndromes include early-onset RCC (age ≤ 45 years), multifocal tumors, bilateral tumors (especially in younger individuals), or a relevant family personal history, he said.
Unusual skin conditions are also potential signs, Shuch said. These can include leiomyomas, fibrofolliculomas, and angiofibromas: “Patients have lots of lumps or bumps.”
“When I look at a patient, I go head to toe and ask if there any issues with your vision, any issues with your hearing, any issues swallowing,” he explained at the meeting. “Do you have any problems with heart issues, adrenal issues? You’ve got to go through each organ, and it can lead you to different things.”
Shuch highlighted Von Hippel-Lindau (VHL) syndrome, which affects 1 in 25,000 people. About 80% to 90% of these patients have a family history, Shuch said.
But the others do not. “Unfortunately, some get diagnosed later in life because they don’t get cascade testing starting at aged 2, which is recommended. These are the patients who might be coming into the ER with a hemangioblastoma or picking up the phone and all of a sudden being deaf in one ear due to an endolymphatic sac tumor.
“We want to limit metastatic spread and preserve the kidneys,” Shuch said. “We don’t want to be doing radical nephrectomies. We want to avoid chronic kidney disease, prevent end-stage renal disease, and maximize quality of life.”
It’s a good idea to avoid surgical removal unless a patient’s tumor grows to be > 3 cm, a line that indicates risk of metastases, he said.
In terms of treatment, Shuch highlighted a 2021 study that showed benefit in VHL from belzutifan (Welireg), an oral HIF-2 α inhibitor approved by the US Food and Drug Administration. The medication significantly reduced the need for surgical intervention.
“Patients go on this drug, and surgeons are putting their scalpels down,” said Shuch, who worked on the 2021 study.
Other hereditary syndromes include the rare hereditary papillary RCC, and Birt-Hogg-Dubé syndrome, believed to affect 1 in 200,000 people but may be more common, he said.
Birt-Hogg-Dubé syndrome is linked to lung cysts, lung collapse, and skin manifestations. The 3 cm surgery rule is appropriate in these cases, Shuch said, and metastases are rare.
Another condition, hereditary leiomyomatosis and RCC, is the most dangerous hereditary form. Originally thought to affect 1 in 200,000 people, hereditary leiomyomatosis and RCC is similar to Birt-Hogg-Dubé syndrome in that it is believed to be more common.
“You will see this,” Shuch predicted.
Shuch advised colleagues to intervene early and take a large margin during surgery.
He also highlighted familial paraganglioma syndrome, which is associated with gastrointestinal stromal tumors, and Cowden syndrome, which is linked to skin manifestations and breast, thyroid, and endometrial cancer.
Shuch reported that he had no disclosures.
The role of hereditary syndromes in renal cell carcinoma (RCC) might be easily missed, a kidney cancer specialist said during a recent Association of VA Hematology and Oncology (AVAHO) seminar in Long Beach, California, though careful clinical evaluation can uncover genetic traits that may affect treatment and familial risk.
“The importance of finding or identifying hereditary forms of kidney cancer really should not be underestimated,” said urologist Brian Shuch, MD, director of the UCLA Kidney Cancer Program, on treating veterans with kidney cancer.
According to Shuch, recent data suggest that about 4.5% of patients with RCC have a hereditary syndrome: “A lot of times, these hide in plain sight. You have to really look deep and try to figure things out and understand that maybe they have a hereditary form of kidney cancer.”
It is important to consider early genetic testing, Shuch said. Red flags for hereditary syndromes include early-onset RCC (age ≤ 45 years), multifocal tumors, bilateral tumors (especially in younger individuals), or a relevant family personal history, he said.
Unusual skin conditions are also potential signs, Shuch said. These can include leiomyomas, fibrofolliculomas, and angiofibromas: “Patients have lots of lumps or bumps.”
“When I look at a patient, I go head to toe and ask if there any issues with your vision, any issues with your hearing, any issues swallowing,” he explained at the meeting. “Do you have any problems with heart issues, adrenal issues? You’ve got to go through each organ, and it can lead you to different things.”
Shuch highlighted Von Hippel-Lindau (VHL) syndrome, which affects 1 in 25,000 people. About 80% to 90% of these patients have a family history, Shuch said.
But the others do not. “Unfortunately, some get diagnosed later in life because they don’t get cascade testing starting at aged 2, which is recommended. These are the patients who might be coming into the ER with a hemangioblastoma or picking up the phone and all of a sudden being deaf in one ear due to an endolymphatic sac tumor.
“We want to limit metastatic spread and preserve the kidneys,” Shuch said. “We don’t want to be doing radical nephrectomies. We want to avoid chronic kidney disease, prevent end-stage renal disease, and maximize quality of life.”
It’s a good idea to avoid surgical removal unless a patient’s tumor grows to be > 3 cm, a line that indicates risk of metastases, he said.
In terms of treatment, Shuch highlighted a 2021 study that showed benefit in VHL from belzutifan (Welireg), an oral HIF-2 α inhibitor approved by the US Food and Drug Administration. The medication significantly reduced the need for surgical intervention.
“Patients go on this drug, and surgeons are putting their scalpels down,” said Shuch, who worked on the 2021 study.
Other hereditary syndromes include the rare hereditary papillary RCC, and Birt-Hogg-Dubé syndrome, believed to affect 1 in 200,000 people but may be more common, he said.
Birt-Hogg-Dubé syndrome is linked to lung cysts, lung collapse, and skin manifestations. The 3 cm surgery rule is appropriate in these cases, Shuch said, and metastases are rare.
Another condition, hereditary leiomyomatosis and RCC, is the most dangerous hereditary form. Originally thought to affect 1 in 200,000 people, hereditary leiomyomatosis and RCC is similar to Birt-Hogg-Dubé syndrome in that it is believed to be more common.
“You will see this,” Shuch predicted.
Shuch advised colleagues to intervene early and take a large margin during surgery.
He also highlighted familial paraganglioma syndrome, which is associated with gastrointestinal stromal tumors, and Cowden syndrome, which is linked to skin manifestations and breast, thyroid, and endometrial cancer.
Shuch reported that he had no disclosures.
Renal Cell Carcinoma: What You Need to Know About Hereditary Syndromes
Renal Cell Carcinoma: What You Need to Know About Hereditary Syndromes