User login
Idarucizumab reversed dabigatran completely and rapidly in study
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
FROM 2017 ISTH CONGRESS
Key clinical point:
Major finding: Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group, 197 patients were able to undergo urgent procedures after a median of 1.6 hours.
Data source: A multicenter, prospective, open-label study of 503 patients (RE-VERSE AD).
Disclosures: Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, BMS/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
Ceftaroline shortens duration of MRSA bacteremia
NEW ORLEANS – Ceftaroline fosamil reduced the median duration of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia by 2 days in Veterans Administration patients, a retrospective study showed.
Investigators identified 219 patients with MRSA within the Veterans Affairs (VA) medical system nationwide from 2011 to 2015. All patients received at least 48 hours of ceftaroline fosamil (Teflaro) therapy to treat MRSA bacteremia. “We know it has good activity against MRSA in vitro. We use it in bacteremia, but we don’t have a lot of clinical data to support or refute its use,” said Nicholas S. Britt, PharmD, a PGY2 infectious diseases resident at Barnes-Jewish Hospital in St. Louis.
“Ceftaroline was primarily used as second-line or salvage therapy … which is basically what we expected, based on how it’s used in clinical practice,” Dr. Britt said.
Treatment failures
A total of 88 of the 219 (40%) patients experienced treatment failure. This rate “seems kind of high, but, if you look at some of the other MRSA agents for bacteremia (vancomycin, for example), it usually has a treatment failure rate around 60%,” Dr. Britt said. “The outcomes were not as poor as I would expect with [patients] using it for second- and third-line therapy.”
Hospital-acquired infection (odds ratio, 2.11; P = .013), ICU admission (OR, 3.95; P less than .001) and infective endocarditis (OR, 4.77; P = .002) were significantly associated with treatment failure in a univariate analysis. “Admissions to the ICU and endocarditis were the big ones, factors you would associate with failure for most antibiotics,” Dr. Britt said. In a multivariate analysis, only ICU admission remained significantly associated with treatment failure (adjusted OR, 2.24; P = .028).
The investigators also looked at treatment failure with ceftaroline monotherapy, compared with its use in combination. There is in vitro data showing synergy when you add ceftaroline to daptomycin, vancomycin, or some of these other agents,” Dr. Britt said. However, he added, “We didn’t find any significant difference in outcomes when you added another agent.” Treatment failure with monotherapy was 35%, versus 46%, with combination treatment (P = .107).
“This could be because the sicker patients are the ones getting combination therapy.”
No observed differences by dosing
Dr. Britt and his colleagues also looked for any differences by dosing interval, “which hasn’t been evaluated extensively.”
The Food and Drug Administration labeled it for use every 12 hours, but treatment of MRSA bacteremia is an off-label use, Dr. Britt explained. Dosing every 8 hours instead improves the achievement of pharmacokinetic and pharmacodynamic parameters in in vitro studies. “Clinically, we’re almost always using it q8. They’re sick patients, so you don’t want to under-dose them. And ceftaroline is pretty well tolerated overall.”
“But, we didn’t really see any difference between the q8 and the q12” in terms of treatment failure. The rates were 36% and 42%, respectively, and not significantly different (P = .440). “Granted, patients who are sicker are probably going to get treated more aggressively,” Dr. Britt added.
The current research only focused on outcomes associated with ceftaroline. Going forward, Dr. Britt said, “We’re hoping to use this data to compare ceftaroline to other agents as well, probably as second-line therapy, since that’s how it’s used most often.”
Dr. Britt had no relevant financial disclosures.
NEW ORLEANS – Ceftaroline fosamil reduced the median duration of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia by 2 days in Veterans Administration patients, a retrospective study showed.
Investigators identified 219 patients with MRSA within the Veterans Affairs (VA) medical system nationwide from 2011 to 2015. All patients received at least 48 hours of ceftaroline fosamil (Teflaro) therapy to treat MRSA bacteremia. “We know it has good activity against MRSA in vitro. We use it in bacteremia, but we don’t have a lot of clinical data to support or refute its use,” said Nicholas S. Britt, PharmD, a PGY2 infectious diseases resident at Barnes-Jewish Hospital in St. Louis.
“Ceftaroline was primarily used as second-line or salvage therapy … which is basically what we expected, based on how it’s used in clinical practice,” Dr. Britt said.
Treatment failures
A total of 88 of the 219 (40%) patients experienced treatment failure. This rate “seems kind of high, but, if you look at some of the other MRSA agents for bacteremia (vancomycin, for example), it usually has a treatment failure rate around 60%,” Dr. Britt said. “The outcomes were not as poor as I would expect with [patients] using it for second- and third-line therapy.”
Hospital-acquired infection (odds ratio, 2.11; P = .013), ICU admission (OR, 3.95; P less than .001) and infective endocarditis (OR, 4.77; P = .002) were significantly associated with treatment failure in a univariate analysis. “Admissions to the ICU and endocarditis were the big ones, factors you would associate with failure for most antibiotics,” Dr. Britt said. In a multivariate analysis, only ICU admission remained significantly associated with treatment failure (adjusted OR, 2.24; P = .028).
The investigators also looked at treatment failure with ceftaroline monotherapy, compared with its use in combination. There is in vitro data showing synergy when you add ceftaroline to daptomycin, vancomycin, or some of these other agents,” Dr. Britt said. However, he added, “We didn’t find any significant difference in outcomes when you added another agent.” Treatment failure with monotherapy was 35%, versus 46%, with combination treatment (P = .107).
“This could be because the sicker patients are the ones getting combination therapy.”
No observed differences by dosing
Dr. Britt and his colleagues also looked for any differences by dosing interval, “which hasn’t been evaluated extensively.”
The Food and Drug Administration labeled it for use every 12 hours, but treatment of MRSA bacteremia is an off-label use, Dr. Britt explained. Dosing every 8 hours instead improves the achievement of pharmacokinetic and pharmacodynamic parameters in in vitro studies. “Clinically, we’re almost always using it q8. They’re sick patients, so you don’t want to under-dose them. And ceftaroline is pretty well tolerated overall.”
“But, we didn’t really see any difference between the q8 and the q12” in terms of treatment failure. The rates were 36% and 42%, respectively, and not significantly different (P = .440). “Granted, patients who are sicker are probably going to get treated more aggressively,” Dr. Britt added.
The current research only focused on outcomes associated with ceftaroline. Going forward, Dr. Britt said, “We’re hoping to use this data to compare ceftaroline to other agents as well, probably as second-line therapy, since that’s how it’s used most often.”
Dr. Britt had no relevant financial disclosures.
NEW ORLEANS – Ceftaroline fosamil reduced the median duration of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia by 2 days in Veterans Administration patients, a retrospective study showed.
Investigators identified 219 patients with MRSA within the Veterans Affairs (VA) medical system nationwide from 2011 to 2015. All patients received at least 48 hours of ceftaroline fosamil (Teflaro) therapy to treat MRSA bacteremia. “We know it has good activity against MRSA in vitro. We use it in bacteremia, but we don’t have a lot of clinical data to support or refute its use,” said Nicholas S. Britt, PharmD, a PGY2 infectious diseases resident at Barnes-Jewish Hospital in St. Louis.
“Ceftaroline was primarily used as second-line or salvage therapy … which is basically what we expected, based on how it’s used in clinical practice,” Dr. Britt said.
Treatment failures
A total of 88 of the 219 (40%) patients experienced treatment failure. This rate “seems kind of high, but, if you look at some of the other MRSA agents for bacteremia (vancomycin, for example), it usually has a treatment failure rate around 60%,” Dr. Britt said. “The outcomes were not as poor as I would expect with [patients] using it for second- and third-line therapy.”
Hospital-acquired infection (odds ratio, 2.11; P = .013), ICU admission (OR, 3.95; P less than .001) and infective endocarditis (OR, 4.77; P = .002) were significantly associated with treatment failure in a univariate analysis. “Admissions to the ICU and endocarditis were the big ones, factors you would associate with failure for most antibiotics,” Dr. Britt said. In a multivariate analysis, only ICU admission remained significantly associated with treatment failure (adjusted OR, 2.24; P = .028).
The investigators also looked at treatment failure with ceftaroline monotherapy, compared with its use in combination. There is in vitro data showing synergy when you add ceftaroline to daptomycin, vancomycin, or some of these other agents,” Dr. Britt said. However, he added, “We didn’t find any significant difference in outcomes when you added another agent.” Treatment failure with monotherapy was 35%, versus 46%, with combination treatment (P = .107).
“This could be because the sicker patients are the ones getting combination therapy.”
No observed differences by dosing
Dr. Britt and his colleagues also looked for any differences by dosing interval, “which hasn’t been evaluated extensively.”
The Food and Drug Administration labeled it for use every 12 hours, but treatment of MRSA bacteremia is an off-label use, Dr. Britt explained. Dosing every 8 hours instead improves the achievement of pharmacokinetic and pharmacodynamic parameters in in vitro studies. “Clinically, we’re almost always using it q8. They’re sick patients, so you don’t want to under-dose them. And ceftaroline is pretty well tolerated overall.”
“But, we didn’t really see any difference between the q8 and the q12” in terms of treatment failure. The rates were 36% and 42%, respectively, and not significantly different (P = .440). “Granted, patients who are sicker are probably going to get treated more aggressively,” Dr. Britt added.
The current research only focused on outcomes associated with ceftaroline. Going forward, Dr. Britt said, “We’re hoping to use this data to compare ceftaroline to other agents as well, probably as second-line therapy, since that’s how it’s used most often.”
Dr. Britt had no relevant financial disclosures.
AT ASM MICROBE 2017
Key clinical point:
Major finding: Median duration of MRSA bacteremia dropped from 2.79 days before to 1.18 days after initiation of ceftaroline (P less than .001).
Data source: A retrospective study of 219 hospitalized VA patients initiating ceftaroline for MRSA bacteremia.
Disclosures: Dr. Britt had no relevant financial disclosures.
Amplatzer devices outperform oral anticoagulation in atrial fib
PARIS – Percutaneous left atrial appendage closure with an Amplatzer device in patients with nonvalvular atrial fibrillation was associated with significantly lower rates of all-cause and cardiovascular mortality, compared with oral anticoagulation, in a large propensity score–matched observational registry study.
Left atrial appendage closure (LAAC) also bested oral anticoagulation (OAC) with warfarin or a novel oral anticoagulant (NOAC) in terms of net clinical benefit on the basis of the device therapy’s greater protection against stroke and systemic embolism coupled with a trend, albeit not statistically significant, for fewer bleeding events, Steffen Gloekler, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The Watchman LAAC device, commercially available both in Europe and the United States, has previously been shown to be superior to OAC in terms of efficacy and noninferior regarding safety. But there have been no randomized trials of an Amplatzer device versus OAC. This lack of data was the impetus for Dr. Gloekler and his coinvestigators to create a meticulously propensity-matched observational registry.
Five hundred consecutive patients with AF who received an Amplatzer Cardiac Plug or its second-generation version, the Amplatzer Amulet, during 2009-2014 were tightly matched to an equal number of AF patients on OAC based on age, sex, body mass index, left ventricular ejection fraction, renal function, coronary artery disease status, hemoglobin level, CHA2DS2-VASc score, and HAS-BLED score. During a mean 2.7 years, or 2,645 patient-years, of follow-up, the composite primary efficacy endpoint, composed of stroke, systemic embolism, and cardiovascular or unexplained death occurred in 5.6% of the LAAC group, compared with 7.8% of controls in the OAC arm, for a statistically significant 30% relative risk reduction. Disabling stroke occurred in 0.7% of Amplatzer patients versus 1.5% of controls. The ischemic stroke rate was 1.5% in the device therapy group and 2% in the OAC arm.
All-cause mortality occurred in 8.3% of Amplatzer patients and 11.6% of the OAC group, for a 28% relative risk reduction. The cardiovascular death rate was 4% in the Amplatzer group, compared with 6.5% of controls, for a 36% risk reduction.
The composite safety endpoint, comprising all major procedural adverse events and major or life-threatening bleeding during follow-up, occurred in 3.6% of the Amplatzer group and 4.6% of the OAC group, for a 20% relative risk reduction that is not significant at this point because of the low number of events. Major, life-threatening, or fatal bleeding occurred in 2% of Amplatzer recipients versus 5.5% of controls, added Dr. Gloekler of University Hospital in Bern, Switzerland.
The net clinical benefit, a composite of death, bleeding, or stroke, occurred in 8.1% of the Amplatzer group, compared with 10.9% of controls, for a significant 24% reduction in relative risk in favor of device therapy.
Of note, at 2.7 years of follow-up only 55% of the OAC group were still taking an anticoagulant: 38% of the original 500 patients were on warfarin, and 17% were taking a NOAC. At that point, 8% of the Amplatzer group were on any anticoagulation therapy.
Discussion of the study focused on that low rate of medication adherence in the OAC arm. Dr. Gloekler’s response was that, after looking at the literature, he was no longer surprised by the finding that only 55% of the control group were on OAC at follow-up.
“If you look in the literature, that’s exactly the real-world adherence for OACs. Even in all four certification trials for the NOACs, the rate of discontinuation was 30% after 2 years – and these were controlled studies. Ours was observational, and it depicts a good deal of the problem with any OAC in my eyes,” Dr. Gloekler said.
Patients on warfarin in the real-world Amplatzer registry study spent on average a mere 30% of time in the therapeutic international normalized ratio range of 2-3.
“That means 70% of the time patients are higher and have an increased bleeding risk or they are lower and don’t have adequate stroke protection,” he noted.
This prompted one observer to comment, “We either have to do a better job in our clinics with OAC or we have to occlude more appendages.”
A large pivotal U.S. trial aimed at winning FDA approval for the Amplatzer Amulet for LAAC is underway. Patients with AF are being randomized to the approved Watchman or investigational Amulet at roughly 100 U.S. and 50 foreign sites.
Dr. Gloekler reported receiving research funds for the registry from the Swiss Heart Foundation and Abbott.
PARIS – Percutaneous left atrial appendage closure with an Amplatzer device in patients with nonvalvular atrial fibrillation was associated with significantly lower rates of all-cause and cardiovascular mortality, compared with oral anticoagulation, in a large propensity score–matched observational registry study.
Left atrial appendage closure (LAAC) also bested oral anticoagulation (OAC) with warfarin or a novel oral anticoagulant (NOAC) in terms of net clinical benefit on the basis of the device therapy’s greater protection against stroke and systemic embolism coupled with a trend, albeit not statistically significant, for fewer bleeding events, Steffen Gloekler, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The Watchman LAAC device, commercially available both in Europe and the United States, has previously been shown to be superior to OAC in terms of efficacy and noninferior regarding safety. But there have been no randomized trials of an Amplatzer device versus OAC. This lack of data was the impetus for Dr. Gloekler and his coinvestigators to create a meticulously propensity-matched observational registry.
Five hundred consecutive patients with AF who received an Amplatzer Cardiac Plug or its second-generation version, the Amplatzer Amulet, during 2009-2014 were tightly matched to an equal number of AF patients on OAC based on age, sex, body mass index, left ventricular ejection fraction, renal function, coronary artery disease status, hemoglobin level, CHA2DS2-VASc score, and HAS-BLED score. During a mean 2.7 years, or 2,645 patient-years, of follow-up, the composite primary efficacy endpoint, composed of stroke, systemic embolism, and cardiovascular or unexplained death occurred in 5.6% of the LAAC group, compared with 7.8% of controls in the OAC arm, for a statistically significant 30% relative risk reduction. Disabling stroke occurred in 0.7% of Amplatzer patients versus 1.5% of controls. The ischemic stroke rate was 1.5% in the device therapy group and 2% in the OAC arm.
All-cause mortality occurred in 8.3% of Amplatzer patients and 11.6% of the OAC group, for a 28% relative risk reduction. The cardiovascular death rate was 4% in the Amplatzer group, compared with 6.5% of controls, for a 36% risk reduction.
The composite safety endpoint, comprising all major procedural adverse events and major or life-threatening bleeding during follow-up, occurred in 3.6% of the Amplatzer group and 4.6% of the OAC group, for a 20% relative risk reduction that is not significant at this point because of the low number of events. Major, life-threatening, or fatal bleeding occurred in 2% of Amplatzer recipients versus 5.5% of controls, added Dr. Gloekler of University Hospital in Bern, Switzerland.
The net clinical benefit, a composite of death, bleeding, or stroke, occurred in 8.1% of the Amplatzer group, compared with 10.9% of controls, for a significant 24% reduction in relative risk in favor of device therapy.
Of note, at 2.7 years of follow-up only 55% of the OAC group were still taking an anticoagulant: 38% of the original 500 patients were on warfarin, and 17% were taking a NOAC. At that point, 8% of the Amplatzer group were on any anticoagulation therapy.
Discussion of the study focused on that low rate of medication adherence in the OAC arm. Dr. Gloekler’s response was that, after looking at the literature, he was no longer surprised by the finding that only 55% of the control group were on OAC at follow-up.
“If you look in the literature, that’s exactly the real-world adherence for OACs. Even in all four certification trials for the NOACs, the rate of discontinuation was 30% after 2 years – and these were controlled studies. Ours was observational, and it depicts a good deal of the problem with any OAC in my eyes,” Dr. Gloekler said.
Patients on warfarin in the real-world Amplatzer registry study spent on average a mere 30% of time in the therapeutic international normalized ratio range of 2-3.
“That means 70% of the time patients are higher and have an increased bleeding risk or they are lower and don’t have adequate stroke protection,” he noted.
This prompted one observer to comment, “We either have to do a better job in our clinics with OAC or we have to occlude more appendages.”
A large pivotal U.S. trial aimed at winning FDA approval for the Amplatzer Amulet for LAAC is underway. Patients with AF are being randomized to the approved Watchman or investigational Amulet at roughly 100 U.S. and 50 foreign sites.
Dr. Gloekler reported receiving research funds for the registry from the Swiss Heart Foundation and Abbott.
PARIS – Percutaneous left atrial appendage closure with an Amplatzer device in patients with nonvalvular atrial fibrillation was associated with significantly lower rates of all-cause and cardiovascular mortality, compared with oral anticoagulation, in a large propensity score–matched observational registry study.
Left atrial appendage closure (LAAC) also bested oral anticoagulation (OAC) with warfarin or a novel oral anticoagulant (NOAC) in terms of net clinical benefit on the basis of the device therapy’s greater protection against stroke and systemic embolism coupled with a trend, albeit not statistically significant, for fewer bleeding events, Steffen Gloekler, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The Watchman LAAC device, commercially available both in Europe and the United States, has previously been shown to be superior to OAC in terms of efficacy and noninferior regarding safety. But there have been no randomized trials of an Amplatzer device versus OAC. This lack of data was the impetus for Dr. Gloekler and his coinvestigators to create a meticulously propensity-matched observational registry.
Five hundred consecutive patients with AF who received an Amplatzer Cardiac Plug or its second-generation version, the Amplatzer Amulet, during 2009-2014 were tightly matched to an equal number of AF patients on OAC based on age, sex, body mass index, left ventricular ejection fraction, renal function, coronary artery disease status, hemoglobin level, CHA2DS2-VASc score, and HAS-BLED score. During a mean 2.7 years, or 2,645 patient-years, of follow-up, the composite primary efficacy endpoint, composed of stroke, systemic embolism, and cardiovascular or unexplained death occurred in 5.6% of the LAAC group, compared with 7.8% of controls in the OAC arm, for a statistically significant 30% relative risk reduction. Disabling stroke occurred in 0.7% of Amplatzer patients versus 1.5% of controls. The ischemic stroke rate was 1.5% in the device therapy group and 2% in the OAC arm.
All-cause mortality occurred in 8.3% of Amplatzer patients and 11.6% of the OAC group, for a 28% relative risk reduction. The cardiovascular death rate was 4% in the Amplatzer group, compared with 6.5% of controls, for a 36% risk reduction.
The composite safety endpoint, comprising all major procedural adverse events and major or life-threatening bleeding during follow-up, occurred in 3.6% of the Amplatzer group and 4.6% of the OAC group, for a 20% relative risk reduction that is not significant at this point because of the low number of events. Major, life-threatening, or fatal bleeding occurred in 2% of Amplatzer recipients versus 5.5% of controls, added Dr. Gloekler of University Hospital in Bern, Switzerland.
The net clinical benefit, a composite of death, bleeding, or stroke, occurred in 8.1% of the Amplatzer group, compared with 10.9% of controls, for a significant 24% reduction in relative risk in favor of device therapy.
Of note, at 2.7 years of follow-up only 55% of the OAC group were still taking an anticoagulant: 38% of the original 500 patients were on warfarin, and 17% were taking a NOAC. At that point, 8% of the Amplatzer group were on any anticoagulation therapy.
Discussion of the study focused on that low rate of medication adherence in the OAC arm. Dr. Gloekler’s response was that, after looking at the literature, he was no longer surprised by the finding that only 55% of the control group were on OAC at follow-up.
“If you look in the literature, that’s exactly the real-world adherence for OACs. Even in all four certification trials for the NOACs, the rate of discontinuation was 30% after 2 years – and these were controlled studies. Ours was observational, and it depicts a good deal of the problem with any OAC in my eyes,” Dr. Gloekler said.
Patients on warfarin in the real-world Amplatzer registry study spent on average a mere 30% of time in the therapeutic international normalized ratio range of 2-3.
“That means 70% of the time patients are higher and have an increased bleeding risk or they are lower and don’t have adequate stroke protection,” he noted.
This prompted one observer to comment, “We either have to do a better job in our clinics with OAC or we have to occlude more appendages.”
A large pivotal U.S. trial aimed at winning FDA approval for the Amplatzer Amulet for LAAC is underway. Patients with AF are being randomized to the approved Watchman or investigational Amulet at roughly 100 U.S. and 50 foreign sites.
Dr. Gloekler reported receiving research funds for the registry from the Swiss Heart Foundation and Abbott.
AT EUROPCR
Key clinical point:
Major finding: The primary composite efficacy endpoint of stroke, systemic embolism, or cardiovascular or unexplained death during a mean 2.7 years of follow-up occurred in 5.6% of Amplatzer device recipients, a 30% reduction, compared with the 7.8% rate in the oral anticoagulation group.
Data source: This observational registry included 500 patients with atrial fibrillation who received an Amplatzer left atrial appendage closure device and an equal number of carefully matched AF patients on oral anticoagulation.
Disclosures: The study presenter reported receiving research funds for the registry from the Swiss Heart Foundation and Abbott.
Opioid prescribing drops nationally, remains high in some counties
Opioid prescribing in the United States declined overall between 2010 and 2015, but remained stable or increased in some counties, according to a report from the Centers for Disease Control and Prevention. The findings were published online in the CDC’s Morbidity and Mortality Weekly Report.
“The bottom line remains: We have too many people getting too many prescriptions at too high a dose,” Anne Schuchat, MD, acting director of the CDC, said in a July 6 teleconference.
CDC researchers calculated prescribing rates from 2006 to 2015 by dividing the number of opioid prescriptions by the population estimates from the U.S. census for each year and created quartiles using morphine milligram equivalent per capita to analyze opioid distribution. Annual opioid prescribing rates increased from 72 to 81 prescriptions per 100 persons from 2006 to 2010 and remained relatively constant from 2010 to 2012 before showing a 13% decrease to 71 prescriptions per 100 persons from 2012 to 2015 (MMWR. 2017 Jul 7;66[26]:697-704. doi: 10.15585/mmwr.mm6626a4).
But despite these overall declines, “We are now experiencing the highest overdose death rates ever recorded in the United States,” Dr. Schuchat said. Quartiles were created using MME per capita to characterize the distribution of opioids prescribed.
In the report, areas associated with higher opioid prescribing rates on a county level included small cities or towns, areas that had a higher proportion of white residents, areas with more doctors and dentists, and areas with more cases of arthritis, diabetes, or other disabilities, she said.
The findings suggest a need for more consistency among health care providers about prescription opioids, Dr. Schuchat said. “Clinical practice is all over the place, which is a sign that you need better standards; we hope the 2016 guidelines are a turning point for better prescribing,” she said.
The CDC’s guidelines on opioid prescribing were released in 2016. The guidelines recommend alternatives when possible. Clinicians should instead consider nonopioid therapy, other types of pain medication, and nondrug pain relief options, such as physical therapy and cognitive-behavioral therapy. Other concerns include the length and strength of opioid prescriptions. Even taking opioids for a few months increases the risk for addiction, Dr. Schuchat said.
“Physicians must continue to lead efforts to reverse the epidemic by using prescription drug–monitoring programs, eliminating stigma, prescribing the overdose reversal drug naloxone, and enhancing their education about safe opioid prescribing and effective pain management,” Patrice A. Harris, MD, chair of the American Medical Association Opioid Task Force, said in a statement in response to the report. “Our country must do more to provide evidence-based, comprehensive treatment for pain and for substance use disorders,” she said.
“We really encourage clinicians to look to the guidelines and the tools that are available,” Dr. Schuchat said. “We do know that internists and other primary care physicians prescribe most of the opioids, so it is important for them to be aware.” The CDC has developed a checklist and a mobile app that have been downloaded by thousands of clinicians so far, she noted. Changes in annual prescribing hold promise that practices can improve, she said.
The researchers reported no conflicts of interest.
Opioid prescribing in the United States declined overall between 2010 and 2015, but remained stable or increased in some counties, according to a report from the Centers for Disease Control and Prevention. The findings were published online in the CDC’s Morbidity and Mortality Weekly Report.
“The bottom line remains: We have too many people getting too many prescriptions at too high a dose,” Anne Schuchat, MD, acting director of the CDC, said in a July 6 teleconference.
CDC researchers calculated prescribing rates from 2006 to 2015 by dividing the number of opioid prescriptions by the population estimates from the U.S. census for each year and created quartiles using morphine milligram equivalent per capita to analyze opioid distribution. Annual opioid prescribing rates increased from 72 to 81 prescriptions per 100 persons from 2006 to 2010 and remained relatively constant from 2010 to 2012 before showing a 13% decrease to 71 prescriptions per 100 persons from 2012 to 2015 (MMWR. 2017 Jul 7;66[26]:697-704. doi: 10.15585/mmwr.mm6626a4).
But despite these overall declines, “We are now experiencing the highest overdose death rates ever recorded in the United States,” Dr. Schuchat said. Quartiles were created using MME per capita to characterize the distribution of opioids prescribed.
In the report, areas associated with higher opioid prescribing rates on a county level included small cities or towns, areas that had a higher proportion of white residents, areas with more doctors and dentists, and areas with more cases of arthritis, diabetes, or other disabilities, she said.
The findings suggest a need for more consistency among health care providers about prescription opioids, Dr. Schuchat said. “Clinical practice is all over the place, which is a sign that you need better standards; we hope the 2016 guidelines are a turning point for better prescribing,” she said.
The CDC’s guidelines on opioid prescribing were released in 2016. The guidelines recommend alternatives when possible. Clinicians should instead consider nonopioid therapy, other types of pain medication, and nondrug pain relief options, such as physical therapy and cognitive-behavioral therapy. Other concerns include the length and strength of opioid prescriptions. Even taking opioids for a few months increases the risk for addiction, Dr. Schuchat said.
“Physicians must continue to lead efforts to reverse the epidemic by using prescription drug–monitoring programs, eliminating stigma, prescribing the overdose reversal drug naloxone, and enhancing their education about safe opioid prescribing and effective pain management,” Patrice A. Harris, MD, chair of the American Medical Association Opioid Task Force, said in a statement in response to the report. “Our country must do more to provide evidence-based, comprehensive treatment for pain and for substance use disorders,” she said.
“We really encourage clinicians to look to the guidelines and the tools that are available,” Dr. Schuchat said. “We do know that internists and other primary care physicians prescribe most of the opioids, so it is important for them to be aware.” The CDC has developed a checklist and a mobile app that have been downloaded by thousands of clinicians so far, she noted. Changes in annual prescribing hold promise that practices can improve, she said.
The researchers reported no conflicts of interest.
Opioid prescribing in the United States declined overall between 2010 and 2015, but remained stable or increased in some counties, according to a report from the Centers for Disease Control and Prevention. The findings were published online in the CDC’s Morbidity and Mortality Weekly Report.
“The bottom line remains: We have too many people getting too many prescriptions at too high a dose,” Anne Schuchat, MD, acting director of the CDC, said in a July 6 teleconference.
CDC researchers calculated prescribing rates from 2006 to 2015 by dividing the number of opioid prescriptions by the population estimates from the U.S. census for each year and created quartiles using morphine milligram equivalent per capita to analyze opioid distribution. Annual opioid prescribing rates increased from 72 to 81 prescriptions per 100 persons from 2006 to 2010 and remained relatively constant from 2010 to 2012 before showing a 13% decrease to 71 prescriptions per 100 persons from 2012 to 2015 (MMWR. 2017 Jul 7;66[26]:697-704. doi: 10.15585/mmwr.mm6626a4).
But despite these overall declines, “We are now experiencing the highest overdose death rates ever recorded in the United States,” Dr. Schuchat said. Quartiles were created using MME per capita to characterize the distribution of opioids prescribed.
In the report, areas associated with higher opioid prescribing rates on a county level included small cities or towns, areas that had a higher proportion of white residents, areas with more doctors and dentists, and areas with more cases of arthritis, diabetes, or other disabilities, she said.
The findings suggest a need for more consistency among health care providers about prescription opioids, Dr. Schuchat said. “Clinical practice is all over the place, which is a sign that you need better standards; we hope the 2016 guidelines are a turning point for better prescribing,” she said.
The CDC’s guidelines on opioid prescribing were released in 2016. The guidelines recommend alternatives when possible. Clinicians should instead consider nonopioid therapy, other types of pain medication, and nondrug pain relief options, such as physical therapy and cognitive-behavioral therapy. Other concerns include the length and strength of opioid prescriptions. Even taking opioids for a few months increases the risk for addiction, Dr. Schuchat said.
“Physicians must continue to lead efforts to reverse the epidemic by using prescription drug–monitoring programs, eliminating stigma, prescribing the overdose reversal drug naloxone, and enhancing their education about safe opioid prescribing and effective pain management,” Patrice A. Harris, MD, chair of the American Medical Association Opioid Task Force, said in a statement in response to the report. “Our country must do more to provide evidence-based, comprehensive treatment for pain and for substance use disorders,” she said.
“We really encourage clinicians to look to the guidelines and the tools that are available,” Dr. Schuchat said. “We do know that internists and other primary care physicians prescribe most of the opioids, so it is important for them to be aware.” The CDC has developed a checklist and a mobile app that have been downloaded by thousands of clinicians so far, she noted. Changes in annual prescribing hold promise that practices can improve, she said.
The researchers reported no conflicts of interest.
FROM MMWR
Septicemia admissions almost tripled from 2005 to 2014
Admissions for septicemia nearly tripled from 2005 to 2014, as it became the third most common diagnosis for hospital stays, according to the Agency for Healthcare Research and Quality.
There were over 1.5 million hospital stays with a principal diagnosis of septicemia in 2014, an increase of 192% over the 518,000 stays in 2005. The only diagnoses with more admissions in 2014 were pregnancy/childbirth with 4.1 million stays and newborns/neonates at almost 4 million, although both were down from 2005. That year, septicemia did not even rank among the top 10 diagnoses, the AHRQ reported.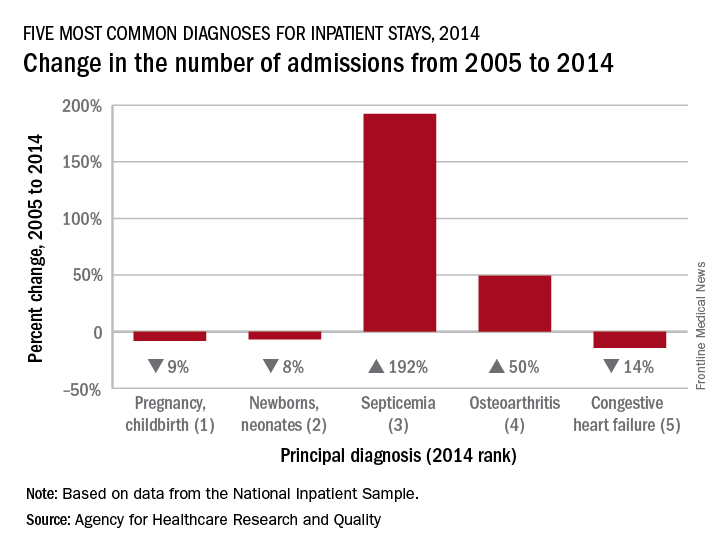
Pneumonia, which was the third most common diagnosis in 2005, dropped by 32% and ended up in sixth place in 2014, while admissions for coronary atherosclerosis, which was fourth in 2005, decreased by 63%, dropping out of the top 10, by 2014, the AHRQ said.
Septicemia was the most common diagnosis for inpatient stays among those aged 75 years and older and the second most common for those aged 65-74 and 45-64. The leading nonmaternal, nonneonatal diagnosis in the two youngest age groups, 0-17 and 18-44 years, was mood disorders, and the most common cause of admissions for those aged 45-64 and 65-74 was osteoarthritis, the AHRQ reported.
Admissions for septicemia nearly tripled from 2005 to 2014, as it became the third most common diagnosis for hospital stays, according to the Agency for Healthcare Research and Quality.
There were over 1.5 million hospital stays with a principal diagnosis of septicemia in 2014, an increase of 192% over the 518,000 stays in 2005. The only diagnoses with more admissions in 2014 were pregnancy/childbirth with 4.1 million stays and newborns/neonates at almost 4 million, although both were down from 2005. That year, septicemia did not even rank among the top 10 diagnoses, the AHRQ reported.
Pneumonia, which was the third most common diagnosis in 2005, dropped by 32% and ended up in sixth place in 2014, while admissions for coronary atherosclerosis, which was fourth in 2005, decreased by 63%, dropping out of the top 10, by 2014, the AHRQ said.
Septicemia was the most common diagnosis for inpatient stays among those aged 75 years and older and the second most common for those aged 65-74 and 45-64. The leading nonmaternal, nonneonatal diagnosis in the two youngest age groups, 0-17 and 18-44 years, was mood disorders, and the most common cause of admissions for those aged 45-64 and 65-74 was osteoarthritis, the AHRQ reported.
Admissions for septicemia nearly tripled from 2005 to 2014, as it became the third most common diagnosis for hospital stays, according to the Agency for Healthcare Research and Quality.
There were over 1.5 million hospital stays with a principal diagnosis of septicemia in 2014, an increase of 192% over the 518,000 stays in 2005. The only diagnoses with more admissions in 2014 were pregnancy/childbirth with 4.1 million stays and newborns/neonates at almost 4 million, although both were down from 2005. That year, septicemia did not even rank among the top 10 diagnoses, the AHRQ reported.
Pneumonia, which was the third most common diagnosis in 2005, dropped by 32% and ended up in sixth place in 2014, while admissions for coronary atherosclerosis, which was fourth in 2005, decreased by 63%, dropping out of the top 10, by 2014, the AHRQ said.
Septicemia was the most common diagnosis for inpatient stays among those aged 75 years and older and the second most common for those aged 65-74 and 45-64. The leading nonmaternal, nonneonatal diagnosis in the two youngest age groups, 0-17 and 18-44 years, was mood disorders, and the most common cause of admissions for those aged 45-64 and 65-74 was osteoarthritis, the AHRQ reported.
VIDEO: Meta-analysis favors anticoagulation for patients with cirrhosis and portal vein thrombosis
Patients with cirrhosis and portal vein thrombosis (PVT) who received anticoagulation therapy had nearly fivefold greater odds of recanalization compared with untreated patients, and were no more likely to experience major or minor bleeding, in a pooled analysis of eight studies published in the August issue of Gastroenterology (doi: 10.1053/j.gastro.2017.04.042).
Rates of any recanalization were 71% in treated patients and 42% in untreated patients (P less than .0001), wrote Lorenzo Loffredo, MD, of Sapienza University, Rome, and his coinvestigators. Rates of complete recanalization were 53% and 33%, respectively (P = .002), rates of spontaneous variceal bleeding were 2% and 12% (P = .04), and bleeding affected 11% of patients in each group. Together, the findings “show that anticoagulants are efficacious and safe for treatment of portal vein thrombosis in cirrhotic patients,” although larger, interventional clinical trials are needed to pinpoint the clinical role of anticoagulation in cirrhotic patients with PVT, the reviewers reported.
Source: American Gastroenterological Association
Bleeding from portal hypertension is a major complication in cirrhosis, but PVT affects about 20% of patients and predicts poor outcomes, they noted. Anticoagulation in this setting can be difficult because patients often have concurrent coagulopathies that are hard to assess with standard techniques, such as PT-INR (international normalized ratio). Although some studies support anticoagulating these patients, data are limited. Therefore, the reviewers searched PubMed, the ISI Web of Science, SCOPUS, and the Cochrane database through Feb. 14, 2017, for trials comparing anticoagulation with no treatment in patients with cirrhosis and PVT.
This search yielded eight trials of 353 patients who received low-molecular-weight heparin, warfarin, or no treatment for about 6 months, with a typical follow-up period of 2 years. The reviewers found no evidence of publication bias or significant heterogeneity among the trials. Six studies evaluated complete recanalization, another set of six studies tracked progression of PVT, a third set of six studies evaluated major or minor bleeding events, and four studies evaluated spontaneous variceal bleeding. Compared with no treatment, anticoagulation was tied to a significantly greater likelihood of complete recanalization (pooled odds ratio, 3.4; 95% confidence interval, 1.5-7.4; P = .002), a significantly lower chance of PVT progressing (9% vs. 33%; pooled odds ratio, 0.14; 95% CI, 0.06-0.31; P less than .0001), no difference in bleeding rates (11% in each pooled group), and a significantly lower risk of spontaneous variceal bleeding (OR, 0.23; 95% CI, 0.06-0.94; P = .04).
“Metaregression analysis showed that duration of anticoagulation did not influence outcomes,” the reviewers wrote. “Low-molecular-weight heparin, but not warfarin, was significantly associated with a complete PVT resolution as compared to untreated patients, while both low-molecular-weight heparin and warfarin were effective in reducing PVT progression.” That finding merits careful interpretation, however, because most studies on warfarin were retrospective and lacked data on the quality of anticoagulation, they added.
“It is a challenge to treat patients with cirrhosis using anticoagulants because of the perception that the coexistent coagulopathy could promote bleeding,” the researchers wrote. Nonetheless, their analysis suggests that anticoagulation has significant benefits and does not increase bleeding risk, regardless of the severity of liver failure, they concluded.
The reviewers reported having no funding sources or conflicts of interest.
Patients with cirrhosis and portal vein thrombosis (PVT) who received anticoagulation therapy had nearly fivefold greater odds of recanalization compared with untreated patients, and were no more likely to experience major or minor bleeding, in a pooled analysis of eight studies published in the August issue of Gastroenterology (doi: 10.1053/j.gastro.2017.04.042).
Rates of any recanalization were 71% in treated patients and 42% in untreated patients (P less than .0001), wrote Lorenzo Loffredo, MD, of Sapienza University, Rome, and his coinvestigators. Rates of complete recanalization were 53% and 33%, respectively (P = .002), rates of spontaneous variceal bleeding were 2% and 12% (P = .04), and bleeding affected 11% of patients in each group. Together, the findings “show that anticoagulants are efficacious and safe for treatment of portal vein thrombosis in cirrhotic patients,” although larger, interventional clinical trials are needed to pinpoint the clinical role of anticoagulation in cirrhotic patients with PVT, the reviewers reported.
Source: American Gastroenterological Association
Bleeding from portal hypertension is a major complication in cirrhosis, but PVT affects about 20% of patients and predicts poor outcomes, they noted. Anticoagulation in this setting can be difficult because patients often have concurrent coagulopathies that are hard to assess with standard techniques, such as PT-INR (international normalized ratio). Although some studies support anticoagulating these patients, data are limited. Therefore, the reviewers searched PubMed, the ISI Web of Science, SCOPUS, and the Cochrane database through Feb. 14, 2017, for trials comparing anticoagulation with no treatment in patients with cirrhosis and PVT.
This search yielded eight trials of 353 patients who received low-molecular-weight heparin, warfarin, or no treatment for about 6 months, with a typical follow-up period of 2 years. The reviewers found no evidence of publication bias or significant heterogeneity among the trials. Six studies evaluated complete recanalization, another set of six studies tracked progression of PVT, a third set of six studies evaluated major or minor bleeding events, and four studies evaluated spontaneous variceal bleeding. Compared with no treatment, anticoagulation was tied to a significantly greater likelihood of complete recanalization (pooled odds ratio, 3.4; 95% confidence interval, 1.5-7.4; P = .002), a significantly lower chance of PVT progressing (9% vs. 33%; pooled odds ratio, 0.14; 95% CI, 0.06-0.31; P less than .0001), no difference in bleeding rates (11% in each pooled group), and a significantly lower risk of spontaneous variceal bleeding (OR, 0.23; 95% CI, 0.06-0.94; P = .04).
“Metaregression analysis showed that duration of anticoagulation did not influence outcomes,” the reviewers wrote. “Low-molecular-weight heparin, but not warfarin, was significantly associated with a complete PVT resolution as compared to untreated patients, while both low-molecular-weight heparin and warfarin were effective in reducing PVT progression.” That finding merits careful interpretation, however, because most studies on warfarin were retrospective and lacked data on the quality of anticoagulation, they added.
“It is a challenge to treat patients with cirrhosis using anticoagulants because of the perception that the coexistent coagulopathy could promote bleeding,” the researchers wrote. Nonetheless, their analysis suggests that anticoagulation has significant benefits and does not increase bleeding risk, regardless of the severity of liver failure, they concluded.
The reviewers reported having no funding sources or conflicts of interest.
Patients with cirrhosis and portal vein thrombosis (PVT) who received anticoagulation therapy had nearly fivefold greater odds of recanalization compared with untreated patients, and were no more likely to experience major or minor bleeding, in a pooled analysis of eight studies published in the August issue of Gastroenterology (doi: 10.1053/j.gastro.2017.04.042).
Rates of any recanalization were 71% in treated patients and 42% in untreated patients (P less than .0001), wrote Lorenzo Loffredo, MD, of Sapienza University, Rome, and his coinvestigators. Rates of complete recanalization were 53% and 33%, respectively (P = .002), rates of spontaneous variceal bleeding were 2% and 12% (P = .04), and bleeding affected 11% of patients in each group. Together, the findings “show that anticoagulants are efficacious and safe for treatment of portal vein thrombosis in cirrhotic patients,” although larger, interventional clinical trials are needed to pinpoint the clinical role of anticoagulation in cirrhotic patients with PVT, the reviewers reported.
Source: American Gastroenterological Association
Bleeding from portal hypertension is a major complication in cirrhosis, but PVT affects about 20% of patients and predicts poor outcomes, they noted. Anticoagulation in this setting can be difficult because patients often have concurrent coagulopathies that are hard to assess with standard techniques, such as PT-INR (international normalized ratio). Although some studies support anticoagulating these patients, data are limited. Therefore, the reviewers searched PubMed, the ISI Web of Science, SCOPUS, and the Cochrane database through Feb. 14, 2017, for trials comparing anticoagulation with no treatment in patients with cirrhosis and PVT.
This search yielded eight trials of 353 patients who received low-molecular-weight heparin, warfarin, or no treatment for about 6 months, with a typical follow-up period of 2 years. The reviewers found no evidence of publication bias or significant heterogeneity among the trials. Six studies evaluated complete recanalization, another set of six studies tracked progression of PVT, a third set of six studies evaluated major or minor bleeding events, and four studies evaluated spontaneous variceal bleeding. Compared with no treatment, anticoagulation was tied to a significantly greater likelihood of complete recanalization (pooled odds ratio, 3.4; 95% confidence interval, 1.5-7.4; P = .002), a significantly lower chance of PVT progressing (9% vs. 33%; pooled odds ratio, 0.14; 95% CI, 0.06-0.31; P less than .0001), no difference in bleeding rates (11% in each pooled group), and a significantly lower risk of spontaneous variceal bleeding (OR, 0.23; 95% CI, 0.06-0.94; P = .04).
“Metaregression analysis showed that duration of anticoagulation did not influence outcomes,” the reviewers wrote. “Low-molecular-weight heparin, but not warfarin, was significantly associated with a complete PVT resolution as compared to untreated patients, while both low-molecular-weight heparin and warfarin were effective in reducing PVT progression.” That finding merits careful interpretation, however, because most studies on warfarin were retrospective and lacked data on the quality of anticoagulation, they added.
“It is a challenge to treat patients with cirrhosis using anticoagulants because of the perception that the coexistent coagulopathy could promote bleeding,” the researchers wrote. Nonetheless, their analysis suggests that anticoagulation has significant benefits and does not increase bleeding risk, regardless of the severity of liver failure, they concluded.
The reviewers reported having no funding sources or conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Anticoagulation produced favorable outcomes with no increase in bleeding risk in patients with cirrhosis and portal vein thrombosis.
Major finding: Rates of any recanalization were 71% in treated patients and 42% in untreated patients (P less than .0001); rates of complete recanalization were 53% and 33%, respectively (P = .002), rates of spontaneous variceal bleeding were 2% and 12% (P = .04), and bleeding affected 11% of patients in each group.
Data source: A systematic review and meta-analysis of eight studies of 353 patients with cirrhosis and portal vein thrombosis.
Disclosures: The reviewers reported having no funding sources or conflicts of interest.
New drug choices emerging to battle antibiotic resistance
SAN FRANCISCO – When the Infectious Diseases Society of America released the “Bad Bugs, No Drugs” report in 2004, its authors warned that effective antibiotics may not be available to treat seriously ill patients in the near future.
It also proposed legislative, regulatory, and funding solutions with a goal of developing and licensing 10 new antibiotics by the year 2020.
One such advancement was the Generating Antibiotics Incentives Now Act, which was signed into law in 2012 and created a designation for new antibiotics that are used to treat serious and/or life-threatening diseases due to certain pathogens. It also extends the patent life of these antibiotics and allows for fast-track Food and Drug Administration approval.
According to Dr. Erlich, chief of staff and medical director of infection control and antibiotic stewardship at Mills Peninsula Medical Center, Burlingame, Calif., increasingly common antibiotic-resistant pathogens besides MRSA and VRE include penicillin-resistant Streptococcus pneumoniae, extended-spectrum beta-lactamase–producing gram-negative rods, carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Mycobacterium tuberculosis, Salmonella enterica serotype Typhimurium DT 104, and drug-resistant Candida species.
Since 2010, several new antibiotics have been introduced to the market, including three second-generation lipoglycopeptide antibiotics with gram-positive coverage that are approved primarily for skin and soft tissue infections: dalbavancin (Dalvance), telavancin (Vibativ), and oritavancin (Orbactiv).
Compared with vancomycin, these new agents have more convenient dosing and a longer half life, “but they’re also more expensive,” said Dr. Erlich. Dalbavancin can be dosed once a week intravenously, telavancin can be dosed once daily intravenously, and oritavancin requires just one dose.
Another new agent is tedizolid phosphate (Sivextro), a second-generation oxazolidinone that is in the same drug class as linezolid (Zyvox). Tedizolid phosphate has gram-positive coverage including MRSA, but it is not approved for VRE. “It’s FDA approved for skin and soft-tissue infections (SSTI) but can be used for other locations as well,” Dr. Erlich said. “It features once-daily dosing IV or PO.”
Ceftaroline fosamil (Teflaro), ceftolozane/tazobactam (Zerbaxa), and ceftazidime/avibactam (Avycaz) are broad-spectrum cephalosporins with or without beta-lactamase inhibitors resulting in extended gram-negative coverage. FDA-approved indications include complicated urinary tract infections, complicated abdominal infections, SSTI, and pneumonia.
The primary advantage of these drugs, compared with other agents, is for multidrug-resistant gram-negative bacteria such as extended-spectrum beta-lactamase producers and CRE. “We’re not using a lot of these drugs in clinical practice, but they are available for patients with multidrug-resistant gram-negative rods who have no other options,” Dr. Erlich said.
Practical ways that clinicians can prevent antibiotic resistance include prescribing antibiotics only when necessary. “Be aware of local resistance patterns, avoid antibiotics for probable viral infections, use narrow-spectrum choices when possible, use shorter durations when appropriate, and consult published guidelines for optimal empiric antibiotic therapy,” Dr. Erlich advised.
In addition, “advocate infection control measures to keep patients from developing infections, including proper wound care, hand washing, respiratory etiquette, vaccinations, and social isolation for symptomatic individuals,” he noted.
Dr. Erlich reported having no relevant financial disclosures.
SAN FRANCISCO – When the Infectious Diseases Society of America released the “Bad Bugs, No Drugs” report in 2004, its authors warned that effective antibiotics may not be available to treat seriously ill patients in the near future.
It also proposed legislative, regulatory, and funding solutions with a goal of developing and licensing 10 new antibiotics by the year 2020.
One such advancement was the Generating Antibiotics Incentives Now Act, which was signed into law in 2012 and created a designation for new antibiotics that are used to treat serious and/or life-threatening diseases due to certain pathogens. It also extends the patent life of these antibiotics and allows for fast-track Food and Drug Administration approval.
According to Dr. Erlich, chief of staff and medical director of infection control and antibiotic stewardship at Mills Peninsula Medical Center, Burlingame, Calif., increasingly common antibiotic-resistant pathogens besides MRSA and VRE include penicillin-resistant Streptococcus pneumoniae, extended-spectrum beta-lactamase–producing gram-negative rods, carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Mycobacterium tuberculosis, Salmonella enterica serotype Typhimurium DT 104, and drug-resistant Candida species.
Since 2010, several new antibiotics have been introduced to the market, including three second-generation lipoglycopeptide antibiotics with gram-positive coverage that are approved primarily for skin and soft tissue infections: dalbavancin (Dalvance), telavancin (Vibativ), and oritavancin (Orbactiv).
Compared with vancomycin, these new agents have more convenient dosing and a longer half life, “but they’re also more expensive,” said Dr. Erlich. Dalbavancin can be dosed once a week intravenously, telavancin can be dosed once daily intravenously, and oritavancin requires just one dose.
Another new agent is tedizolid phosphate (Sivextro), a second-generation oxazolidinone that is in the same drug class as linezolid (Zyvox). Tedizolid phosphate has gram-positive coverage including MRSA, but it is not approved for VRE. “It’s FDA approved for skin and soft-tissue infections (SSTI) but can be used for other locations as well,” Dr. Erlich said. “It features once-daily dosing IV or PO.”
Ceftaroline fosamil (Teflaro), ceftolozane/tazobactam (Zerbaxa), and ceftazidime/avibactam (Avycaz) are broad-spectrum cephalosporins with or without beta-lactamase inhibitors resulting in extended gram-negative coverage. FDA-approved indications include complicated urinary tract infections, complicated abdominal infections, SSTI, and pneumonia.
The primary advantage of these drugs, compared with other agents, is for multidrug-resistant gram-negative bacteria such as extended-spectrum beta-lactamase producers and CRE. “We’re not using a lot of these drugs in clinical practice, but they are available for patients with multidrug-resistant gram-negative rods who have no other options,” Dr. Erlich said.
Practical ways that clinicians can prevent antibiotic resistance include prescribing antibiotics only when necessary. “Be aware of local resistance patterns, avoid antibiotics for probable viral infections, use narrow-spectrum choices when possible, use shorter durations when appropriate, and consult published guidelines for optimal empiric antibiotic therapy,” Dr. Erlich advised.
In addition, “advocate infection control measures to keep patients from developing infections, including proper wound care, hand washing, respiratory etiquette, vaccinations, and social isolation for symptomatic individuals,” he noted.
Dr. Erlich reported having no relevant financial disclosures.
SAN FRANCISCO – When the Infectious Diseases Society of America released the “Bad Bugs, No Drugs” report in 2004, its authors warned that effective antibiotics may not be available to treat seriously ill patients in the near future.
It also proposed legislative, regulatory, and funding solutions with a goal of developing and licensing 10 new antibiotics by the year 2020.
One such advancement was the Generating Antibiotics Incentives Now Act, which was signed into law in 2012 and created a designation for new antibiotics that are used to treat serious and/or life-threatening diseases due to certain pathogens. It also extends the patent life of these antibiotics and allows for fast-track Food and Drug Administration approval.
According to Dr. Erlich, chief of staff and medical director of infection control and antibiotic stewardship at Mills Peninsula Medical Center, Burlingame, Calif., increasingly common antibiotic-resistant pathogens besides MRSA and VRE include penicillin-resistant Streptococcus pneumoniae, extended-spectrum beta-lactamase–producing gram-negative rods, carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Mycobacterium tuberculosis, Salmonella enterica serotype Typhimurium DT 104, and drug-resistant Candida species.
Since 2010, several new antibiotics have been introduced to the market, including three second-generation lipoglycopeptide antibiotics with gram-positive coverage that are approved primarily for skin and soft tissue infections: dalbavancin (Dalvance), telavancin (Vibativ), and oritavancin (Orbactiv).
Compared with vancomycin, these new agents have more convenient dosing and a longer half life, “but they’re also more expensive,” said Dr. Erlich. Dalbavancin can be dosed once a week intravenously, telavancin can be dosed once daily intravenously, and oritavancin requires just one dose.
Another new agent is tedizolid phosphate (Sivextro), a second-generation oxazolidinone that is in the same drug class as linezolid (Zyvox). Tedizolid phosphate has gram-positive coverage including MRSA, but it is not approved for VRE. “It’s FDA approved for skin and soft-tissue infections (SSTI) but can be used for other locations as well,” Dr. Erlich said. “It features once-daily dosing IV or PO.”
Ceftaroline fosamil (Teflaro), ceftolozane/tazobactam (Zerbaxa), and ceftazidime/avibactam (Avycaz) are broad-spectrum cephalosporins with or without beta-lactamase inhibitors resulting in extended gram-negative coverage. FDA-approved indications include complicated urinary tract infections, complicated abdominal infections, SSTI, and pneumonia.
The primary advantage of these drugs, compared with other agents, is for multidrug-resistant gram-negative bacteria such as extended-spectrum beta-lactamase producers and CRE. “We’re not using a lot of these drugs in clinical practice, but they are available for patients with multidrug-resistant gram-negative rods who have no other options,” Dr. Erlich said.
Practical ways that clinicians can prevent antibiotic resistance include prescribing antibiotics only when necessary. “Be aware of local resistance patterns, avoid antibiotics for probable viral infections, use narrow-spectrum choices when possible, use shorter durations when appropriate, and consult published guidelines for optimal empiric antibiotic therapy,” Dr. Erlich advised.
In addition, “advocate infection control measures to keep patients from developing infections, including proper wound care, hand washing, respiratory etiquette, vaccinations, and social isolation for symptomatic individuals,” he noted.
Dr. Erlich reported having no relevant financial disclosures.
AT THE ANNUAL ADVANCES IN INTERNAL MEDICINE
Study shows that 20% of inpatients given antibiotics develop adverse reactions
Twenty percent of hospitalized adults given antibiotics develop adverse drug events, including GI, nephrotoxic, hematologic, cardiac, and neurotoxic effects, according to a report in JAMA Internal Medicine.
This high frequency of adverse reactions “may not be recognized by clinicians because [these events] have varied manifestations, clinicians may be unaware of the risks associated with specific antibiotic agents, or because they occur after patients are discharged from the hospital,” said Pranita D. Tamma, MD, of the division of pediatric infectious diseases, Johns Hopkins University, Baltimore, and her associates.
They assessed antibiotic-associated adverse drug events in all 1,488 adults admitted to four general medicine services at a single medical center during a 9-month period and given at least 24 hours of any antibiotic therapy. The most common indications for antibiotics were urinary tract infections (12%), skin and soft-tissue infections (8%), and community-acquired pneumonia (7%).
Perhaps as important, the researchers noted, 19% of these adverse drug events were attributed to unnecessary antibiotics – drugs given for conditions for which they were not clinically indicated according to the hospital’s own antibiotic guidelines. These included asymptomatic bacteriuria, aspiration pneumonitis, and heart failure (JAMA Intern. Med. 2017 June 12. doi: 10.1001/jamainternmed.2017.1938).
The most common adverse reactions that developed within 30 days were GI, renal, and hematologic abnormalities. Neurotoxic effects included encephalopathy and seizures; cardiotoxic effects included QTc prolongation. Less frequent adverse events included anaphylaxis, daptomycin-associated myositis, trimethoprim/sulfamethoxazole-associated pancreatitis, linezolid-associated neuropathy, and ciprofloxacin-related tendinitis. The most common adverse reactions that developed within 90 days were C. difficile infections and infections involving multidrug-resistant organisms.
“Our findings underscore the importance of avoiding unnecessary antibiotic prescribing to reduce the harm that can result from antibiotic-associated adverse drug events,” Dr. Tamma and her associates said.
Twenty percent of hospitalized adults given antibiotics develop adverse drug events, including GI, nephrotoxic, hematologic, cardiac, and neurotoxic effects, according to a report in JAMA Internal Medicine.
This high frequency of adverse reactions “may not be recognized by clinicians because [these events] have varied manifestations, clinicians may be unaware of the risks associated with specific antibiotic agents, or because they occur after patients are discharged from the hospital,” said Pranita D. Tamma, MD, of the division of pediatric infectious diseases, Johns Hopkins University, Baltimore, and her associates.
They assessed antibiotic-associated adverse drug events in all 1,488 adults admitted to four general medicine services at a single medical center during a 9-month period and given at least 24 hours of any antibiotic therapy. The most common indications for antibiotics were urinary tract infections (12%), skin and soft-tissue infections (8%), and community-acquired pneumonia (7%).
Perhaps as important, the researchers noted, 19% of these adverse drug events were attributed to unnecessary antibiotics – drugs given for conditions for which they were not clinically indicated according to the hospital’s own antibiotic guidelines. These included asymptomatic bacteriuria, aspiration pneumonitis, and heart failure (JAMA Intern. Med. 2017 June 12. doi: 10.1001/jamainternmed.2017.1938).
The most common adverse reactions that developed within 30 days were GI, renal, and hematologic abnormalities. Neurotoxic effects included encephalopathy and seizures; cardiotoxic effects included QTc prolongation. Less frequent adverse events included anaphylaxis, daptomycin-associated myositis, trimethoprim/sulfamethoxazole-associated pancreatitis, linezolid-associated neuropathy, and ciprofloxacin-related tendinitis. The most common adverse reactions that developed within 90 days were C. difficile infections and infections involving multidrug-resistant organisms.
“Our findings underscore the importance of avoiding unnecessary antibiotic prescribing to reduce the harm that can result from antibiotic-associated adverse drug events,” Dr. Tamma and her associates said.
Twenty percent of hospitalized adults given antibiotics develop adverse drug events, including GI, nephrotoxic, hematologic, cardiac, and neurotoxic effects, according to a report in JAMA Internal Medicine.
This high frequency of adverse reactions “may not be recognized by clinicians because [these events] have varied manifestations, clinicians may be unaware of the risks associated with specific antibiotic agents, or because they occur after patients are discharged from the hospital,” said Pranita D. Tamma, MD, of the division of pediatric infectious diseases, Johns Hopkins University, Baltimore, and her associates.
They assessed antibiotic-associated adverse drug events in all 1,488 adults admitted to four general medicine services at a single medical center during a 9-month period and given at least 24 hours of any antibiotic therapy. The most common indications for antibiotics were urinary tract infections (12%), skin and soft-tissue infections (8%), and community-acquired pneumonia (7%).
Perhaps as important, the researchers noted, 19% of these adverse drug events were attributed to unnecessary antibiotics – drugs given for conditions for which they were not clinically indicated according to the hospital’s own antibiotic guidelines. These included asymptomatic bacteriuria, aspiration pneumonitis, and heart failure (JAMA Intern. Med. 2017 June 12. doi: 10.1001/jamainternmed.2017.1938).
The most common adverse reactions that developed within 30 days were GI, renal, and hematologic abnormalities. Neurotoxic effects included encephalopathy and seizures; cardiotoxic effects included QTc prolongation. Less frequent adverse events included anaphylaxis, daptomycin-associated myositis, trimethoprim/sulfamethoxazole-associated pancreatitis, linezolid-associated neuropathy, and ciprofloxacin-related tendinitis. The most common adverse reactions that developed within 90 days were C. difficile infections and infections involving multidrug-resistant organisms.
“Our findings underscore the importance of avoiding unnecessary antibiotic prescribing to reduce the harm that can result from antibiotic-associated adverse drug events,” Dr. Tamma and her associates said.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Among hospitalized adults given antibiotics, 20% develop adverse reactions, including GI, nephrotoxic, hematologic, cardiac, and neurotoxic effects.
Major finding: Among 1,488 patients, 298 (20%) developed 324 adverse reactions to antibiotics – 73% during hospitalization and 27% after discharge – requiring prolonged hospitalization; a subsequent hospitalization; an ED visit; or additional lab tests, ECGs, or imaging studies.
Data source: A single-center retrospective cohort study involving all 1,488 general medicine inpatients admitted during a 9-month period who received any antibiotic for at least 24 hours.
Disclosures: This study was supported by Pfizer Independent Grants for Learning and Change and the Joint Commission. Dr. Tamma and her associates reported having no relevant financial disclosures.
FDA approves betrixaban for VTE prophylaxis
Betrixaban, a factor Xa inhibitor, has been approved for the prophylaxis of venous thromboembolism (VTE) in at-risk adult patients hospitalized with an acute illness, according to an announcement from the Food and Drug Administration.
Approval was based on results from a randomized, double-blind clinical trial in which over 7,000 hospitalized patients at risk for VTE received either extended-duration betrixaban (35-42 days) or short duration enoxaparin (6-14 days), a low molecular weight heparin administered subcutaneously. The rate of deep vein thrombosis, nonfatal pulmonary embolism, or VTE-related death was 4.4% among patients receiving betrixaban and 6% among patients receiving enoxaparin (relative risk, 0.75; 95% confidence interval: 0.61, 0.91).
The recommended dosage for betrixaban is 80 mg per day for 35-42 days at the same time every day with food, after a dose of 160 mg on the first day of treatment.
Betrixaban will be marketed as Bevyxxa by Portola.
Find the full FDA announcement and prescribing information on the FDA website.
Betrixaban, a factor Xa inhibitor, has been approved for the prophylaxis of venous thromboembolism (VTE) in at-risk adult patients hospitalized with an acute illness, according to an announcement from the Food and Drug Administration.
Approval was based on results from a randomized, double-blind clinical trial in which over 7,000 hospitalized patients at risk for VTE received either extended-duration betrixaban (35-42 days) or short duration enoxaparin (6-14 days), a low molecular weight heparin administered subcutaneously. The rate of deep vein thrombosis, nonfatal pulmonary embolism, or VTE-related death was 4.4% among patients receiving betrixaban and 6% among patients receiving enoxaparin (relative risk, 0.75; 95% confidence interval: 0.61, 0.91).
The recommended dosage for betrixaban is 80 mg per day for 35-42 days at the same time every day with food, after a dose of 160 mg on the first day of treatment.
Betrixaban will be marketed as Bevyxxa by Portola.
Find the full FDA announcement and prescribing information on the FDA website.
Betrixaban, a factor Xa inhibitor, has been approved for the prophylaxis of venous thromboembolism (VTE) in at-risk adult patients hospitalized with an acute illness, according to an announcement from the Food and Drug Administration.
Approval was based on results from a randomized, double-blind clinical trial in which over 7,000 hospitalized patients at risk for VTE received either extended-duration betrixaban (35-42 days) or short duration enoxaparin (6-14 days), a low molecular weight heparin administered subcutaneously. The rate of deep vein thrombosis, nonfatal pulmonary embolism, or VTE-related death was 4.4% among patients receiving betrixaban and 6% among patients receiving enoxaparin (relative risk, 0.75; 95% confidence interval: 0.61, 0.91).
The recommended dosage for betrixaban is 80 mg per day for 35-42 days at the same time every day with food, after a dose of 160 mg on the first day of treatment.
Betrixaban will be marketed as Bevyxxa by Portola.
Find the full FDA announcement and prescribing information on the FDA website.
Follow five tips to mitigate opioid prescribing risks
CHICAGO – As the epidemic of opioid addiction and overdose deaths continues to surge, state and federal authorities are keeping a close eye on physicians who prescribe controlled substances.
Experts offer the following guidance on how well-meaning doctors can avoid coming under scrutiny for prescribing opioids and successfully manage investigations and audits.
1. Know who’s on the radar: The Drug Enforcement Agency (DEA) compiles a “black list” yearly of physicians and health care providers they plan to target for audits, said Natalia Mazina, a San Francisco–based attorney who specializes in health and pharmacy law. For 2017, the list includes physicians who have prior noncompliance records, providers who specialize in pain management, and those who dispense or administer large quantities of controlled substances.
In addition, family physicians, psychiatrists, and other specialists who come under investigation by a state medical board because of suspected inappropriate prescribing or reporting violations may also come under the purview of federal authorities, Ms. Mazina said.
“If they come on the medical board radar, they may come on the [DEA’s] radar as well,” she said during the interview. “They just have to watch how many prescriptions they write for controlled substances and make sure they are legitimate prescriptions.”
2. Maintain proper records: Poor record keeping is a top reason that the DEA investigates health care providers for potential prescribing violations, said Dennis A. Wichern, a DEA agent with the Chicago Field Division. Federal law requires that registered practitioners who store or dispense controlled substances keep records of controlled substances coming in and out of the practice. That includes physicians who hand out samples of controlled substances to patients and also pertains to samples provided to doctors by pharmaceutical companies.
Records should include whether the inventory was taken at the beginning or close of business, names of controlled substances, each finished form of the substances, the number of dosage units of each finished form in the commercial container, the number of commercial containers of each finished form, and disposition of the controlled substances.
Law requires that physicians take a new inventory of all controlled substances on hand every 2 years. Doctors are not required to keep records of controlled substances that are merely prescribed, unless such substances are prescribed in the course of maintenance or detoxification treatment.
Ms. Mazina notes that there are many software platforms that can assist practices with proper inventory and record keeping for opioids and other drugs.
3. Check the state database: Before prescribing opioids, check your state’s prescription drug monitoring program (PDMP) database, advises Ms. Mazina. At least 37 states have operational PDMPs that receive and distribute controlled substance prescription information to authorized users. About 11 states have enacted legislation to establish a PDMP, but some databases are not fully operational.
A state’s PDMP can reveal whether patients may be obtaining multiple controlled substance prescriptions from different doctors or doctor-shopping, Ms. Mazina said. Such due diligence helps inform treatment decisions and can assist a doctor’s case if a medical board or DEA investigation later arises.
“Even if your state law does not require you to check a patient’s history prior to prescribing, you have to check it to protect yourself,” she said. “If you want to avoid controlled substances problems, PDMP is the way to go.”
4. Establish an audit response plan: Have an audit response plan ready to roll should an inquiry arise, experts advise. The policies ensure that only approved information is released to authorities, and that all staff members are on the same page about how to react to audits, Ms. Mazina said.
Plans should clearly state what information can be collected and what data should be kept confidential. Financial information, for example, should be off limits, she said. Government agents are entitled to inventory, dispensary data, and records of receipts.
“Agents very often do the mirror image of the database, and they get too much information,” she said. “You don’t want to [allow] that.”
Train staff members how to respond to government authorities seeking audit information, and explain they have the right to refuse being interviewed, Ms. Mazina said.
“Train your employees on what’s going to happen if the DEA comes in,” she said. “If I don’t have clear policies and procedures, and I’m not trained, I might disclose everything and blame someone. That puts everyone in a [bad] position, because [authorities] will record everything and use it against [the practice].”
5. Confer with the experts: It doesn’t hurt to consult with other medical professionals, such as emergency physicians or pain management specialists, for practical advice on inventory policies or software suggestions. But when it comes to staying updated on new drug laws and regulations, confer with a health law attorney or compliance officer, Ms. Mazina said. The DEA website also includes useful information about recent laws and rules pertaining to prescription drugs, as does the Centers for Disease Control and Prevention website.
If an investigation or audit emerges, work with an attorney as early as possible. Often, practices wait until too late after an investigation begins to contact legal counsel, Ms. Mazina noted. The earlier an attorney gets involved, the sooner that person can build a strong case for the practice and work toward the best resolution.
“Very often, the physician thinks they are right, and there’s nothing for them to fear,” she said. “There is something for you to fear. There’s a lot at stake.”
[email protected]
On Twitter @legal_med
CHICAGO – As the epidemic of opioid addiction and overdose deaths continues to surge, state and federal authorities are keeping a close eye on physicians who prescribe controlled substances.
Experts offer the following guidance on how well-meaning doctors can avoid coming under scrutiny for prescribing opioids and successfully manage investigations and audits.
1. Know who’s on the radar: The Drug Enforcement Agency (DEA) compiles a “black list” yearly of physicians and health care providers they plan to target for audits, said Natalia Mazina, a San Francisco–based attorney who specializes in health and pharmacy law. For 2017, the list includes physicians who have prior noncompliance records, providers who specialize in pain management, and those who dispense or administer large quantities of controlled substances.
In addition, family physicians, psychiatrists, and other specialists who come under investigation by a state medical board because of suspected inappropriate prescribing or reporting violations may also come under the purview of federal authorities, Ms. Mazina said.
“If they come on the medical board radar, they may come on the [DEA’s] radar as well,” she said during the interview. “They just have to watch how many prescriptions they write for controlled substances and make sure they are legitimate prescriptions.”
2. Maintain proper records: Poor record keeping is a top reason that the DEA investigates health care providers for potential prescribing violations, said Dennis A. Wichern, a DEA agent with the Chicago Field Division. Federal law requires that registered practitioners who store or dispense controlled substances keep records of controlled substances coming in and out of the practice. That includes physicians who hand out samples of controlled substances to patients and also pertains to samples provided to doctors by pharmaceutical companies.
Records should include whether the inventory was taken at the beginning or close of business, names of controlled substances, each finished form of the substances, the number of dosage units of each finished form in the commercial container, the number of commercial containers of each finished form, and disposition of the controlled substances.
Law requires that physicians take a new inventory of all controlled substances on hand every 2 years. Doctors are not required to keep records of controlled substances that are merely prescribed, unless such substances are prescribed in the course of maintenance or detoxification treatment.
Ms. Mazina notes that there are many software platforms that can assist practices with proper inventory and record keeping for opioids and other drugs.
3. Check the state database: Before prescribing opioids, check your state’s prescription drug monitoring program (PDMP) database, advises Ms. Mazina. At least 37 states have operational PDMPs that receive and distribute controlled substance prescription information to authorized users. About 11 states have enacted legislation to establish a PDMP, but some databases are not fully operational.
A state’s PDMP can reveal whether patients may be obtaining multiple controlled substance prescriptions from different doctors or doctor-shopping, Ms. Mazina said. Such due diligence helps inform treatment decisions and can assist a doctor’s case if a medical board or DEA investigation later arises.
“Even if your state law does not require you to check a patient’s history prior to prescribing, you have to check it to protect yourself,” she said. “If you want to avoid controlled substances problems, PDMP is the way to go.”
4. Establish an audit response plan: Have an audit response plan ready to roll should an inquiry arise, experts advise. The policies ensure that only approved information is released to authorities, and that all staff members are on the same page about how to react to audits, Ms. Mazina said.
Plans should clearly state what information can be collected and what data should be kept confidential. Financial information, for example, should be off limits, she said. Government agents are entitled to inventory, dispensary data, and records of receipts.
“Agents very often do the mirror image of the database, and they get too much information,” she said. “You don’t want to [allow] that.”
Train staff members how to respond to government authorities seeking audit information, and explain they have the right to refuse being interviewed, Ms. Mazina said.
“Train your employees on what’s going to happen if the DEA comes in,” she said. “If I don’t have clear policies and procedures, and I’m not trained, I might disclose everything and blame someone. That puts everyone in a [bad] position, because [authorities] will record everything and use it against [the practice].”
5. Confer with the experts: It doesn’t hurt to consult with other medical professionals, such as emergency physicians or pain management specialists, for practical advice on inventory policies or software suggestions. But when it comes to staying updated on new drug laws and regulations, confer with a health law attorney or compliance officer, Ms. Mazina said. The DEA website also includes useful information about recent laws and rules pertaining to prescription drugs, as does the Centers for Disease Control and Prevention website.
If an investigation or audit emerges, work with an attorney as early as possible. Often, practices wait until too late after an investigation begins to contact legal counsel, Ms. Mazina noted. The earlier an attorney gets involved, the sooner that person can build a strong case for the practice and work toward the best resolution.
“Very often, the physician thinks they are right, and there’s nothing for them to fear,” she said. “There is something for you to fear. There’s a lot at stake.”
[email protected]
On Twitter @legal_med
CHICAGO – As the epidemic of opioid addiction and overdose deaths continues to surge, state and federal authorities are keeping a close eye on physicians who prescribe controlled substances.
Experts offer the following guidance on how well-meaning doctors can avoid coming under scrutiny for prescribing opioids and successfully manage investigations and audits.
1. Know who’s on the radar: The Drug Enforcement Agency (DEA) compiles a “black list” yearly of physicians and health care providers they plan to target for audits, said Natalia Mazina, a San Francisco–based attorney who specializes in health and pharmacy law. For 2017, the list includes physicians who have prior noncompliance records, providers who specialize in pain management, and those who dispense or administer large quantities of controlled substances.
In addition, family physicians, psychiatrists, and other specialists who come under investigation by a state medical board because of suspected inappropriate prescribing or reporting violations may also come under the purview of federal authorities, Ms. Mazina said.
“If they come on the medical board radar, they may come on the [DEA’s] radar as well,” she said during the interview. “They just have to watch how many prescriptions they write for controlled substances and make sure they are legitimate prescriptions.”
2. Maintain proper records: Poor record keeping is a top reason that the DEA investigates health care providers for potential prescribing violations, said Dennis A. Wichern, a DEA agent with the Chicago Field Division. Federal law requires that registered practitioners who store or dispense controlled substances keep records of controlled substances coming in and out of the practice. That includes physicians who hand out samples of controlled substances to patients and also pertains to samples provided to doctors by pharmaceutical companies.
Records should include whether the inventory was taken at the beginning or close of business, names of controlled substances, each finished form of the substances, the number of dosage units of each finished form in the commercial container, the number of commercial containers of each finished form, and disposition of the controlled substances.
Law requires that physicians take a new inventory of all controlled substances on hand every 2 years. Doctors are not required to keep records of controlled substances that are merely prescribed, unless such substances are prescribed in the course of maintenance or detoxification treatment.
Ms. Mazina notes that there are many software platforms that can assist practices with proper inventory and record keeping for opioids and other drugs.
3. Check the state database: Before prescribing opioids, check your state’s prescription drug monitoring program (PDMP) database, advises Ms. Mazina. At least 37 states have operational PDMPs that receive and distribute controlled substance prescription information to authorized users. About 11 states have enacted legislation to establish a PDMP, but some databases are not fully operational.
A state’s PDMP can reveal whether patients may be obtaining multiple controlled substance prescriptions from different doctors or doctor-shopping, Ms. Mazina said. Such due diligence helps inform treatment decisions and can assist a doctor’s case if a medical board or DEA investigation later arises.
“Even if your state law does not require you to check a patient’s history prior to prescribing, you have to check it to protect yourself,” she said. “If you want to avoid controlled substances problems, PDMP is the way to go.”
4. Establish an audit response plan: Have an audit response plan ready to roll should an inquiry arise, experts advise. The policies ensure that only approved information is released to authorities, and that all staff members are on the same page about how to react to audits, Ms. Mazina said.
Plans should clearly state what information can be collected and what data should be kept confidential. Financial information, for example, should be off limits, she said. Government agents are entitled to inventory, dispensary data, and records of receipts.
“Agents very often do the mirror image of the database, and they get too much information,” she said. “You don’t want to [allow] that.”
Train staff members how to respond to government authorities seeking audit information, and explain they have the right to refuse being interviewed, Ms. Mazina said.
“Train your employees on what’s going to happen if the DEA comes in,” she said. “If I don’t have clear policies and procedures, and I’m not trained, I might disclose everything and blame someone. That puts everyone in a [bad] position, because [authorities] will record everything and use it against [the practice].”
5. Confer with the experts: It doesn’t hurt to consult with other medical professionals, such as emergency physicians or pain management specialists, for practical advice on inventory policies or software suggestions. But when it comes to staying updated on new drug laws and regulations, confer with a health law attorney or compliance officer, Ms. Mazina said. The DEA website also includes useful information about recent laws and rules pertaining to prescription drugs, as does the Centers for Disease Control and Prevention website.
If an investigation or audit emerges, work with an attorney as early as possible. Often, practices wait until too late after an investigation begins to contact legal counsel, Ms. Mazina noted. The earlier an attorney gets involved, the sooner that person can build a strong case for the practice and work toward the best resolution.
“Very often, the physician thinks they are right, and there’s nothing for them to fear,” she said. “There is something for you to fear. There’s a lot at stake.”
[email protected]
On Twitter @legal_med
AT THE PHYSICIANS LEGAL ISSUES CONFERENCE