User login
Design limitations may have compromised DVT intervention trial
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
WASHINGTON – On the basis of a large randomized trial called ATTRACT, many clinicians have concluded that pharmacomechanical intervention is ineffective for preventing postthrombotic syndrome (PTS) in patients with deep venous thrombosis (DVT). But weaknesses in the study design challenge this conclusion, according to several experts in a DVT symposium at the 2018 Cardiovascular Research Technologies (CRT) meeting.
“The diagnosis and evaluation of DVT must be performed with IVUS [intravascular ultrasound], not with venography,” said Peter A. Soukas, MD, director of vascular medicine at Miriam Hospital in Providence, R.I. “You cannot know whether you successfully treated the clot if you cannot see it.”
“There were lots of limitations to that study. Here are some,” said Dr. Soukas, who then listed on a list of several considerations, including the fact that venograms – rather than IVUS, which Dr. Soukas labeled the “current gold standard” – were taken to evaluate procedure success. Another was that only half of patients had a moderate to severe DVT based on a Villalta score.
“If you look at the subgroup with a Villalta score of 10 or greater, the benefit [of pharmacomechanical intervention] was statistically significant,” he said.
In addition, the study enrolled a substantial number of patients with femoral-popliteal DVTs even though iliofemoral DVTs pose the greatest risk of postthrombotic syndrome. Dr. Soukas suggested these would have been a more appropriate focus of a study exploring the benefits of an intervention.
The limitations of the ATTRACT trial, which was conceived more than 5 years ago, have arisen primarily from advances in the field rather than problems with the design, Dr. Soukas explained. IVUS was not the preferred method for deep vein thrombosis evaluation then as it is now, and there have been several advances in current models of pharmacomechanical devices, which involve catheter-directed delivery of fibrinolytic therapy into the thrombus along with mechanical destruction of the clot.
Although further steps beyond clot lysis, such as stenting, were encouraged in ATTRACT to maintain venous patency, Dr. Soukas questioned whether these were employed sufficiently. For example, the rate of stenting in the experimental arm was 28%, a rate that “is not what we currently do” for patients at high risk of PTS, Dr. Soukas said.
In ATTRACT, major bleeding events were significantly higher in the experimental group (1.7% vs. 0.3%; P = .049). The authors cited this finding when they concluded that the experimental intervention was ineffective. Dr. Soukas acknowledged that bleeding risk is an important factor to consider, but he also emphasized the serious risks for failing to treat patients at high risk for PTS.
“PTS is devastating for patients, both functionally and economically,” Dr. Soukas said. He called the morbidity of deep vein thrombosis “staggering,” with in-hospital mortality in some series exceeding 10% and a risk of late development of postthrombotic syndrome persisting for up to 5 years. For those with proximal iliofemoral DVT, the PTS rate can reach 90%, about 15% of which can develop claudication with ulcerations, according to Dr. Soukas.
A large trial that was published in a prominent journal, ATTRACT has the potential to dissuade clinicians from considering pharmacomechanical intervention in high-risk patients who could benefit, Dr. Soukas said. Others speaking during the same symposium about advances in this field, such as John Fritz Angle, MD, director of the division of vascular and interventional radiology at the University of Virginia, Charlottesville, agreed with this assessment. Although other studies underway will reexamine this issue, there was consensus from several speakers at the CRT symposium that the results of ATTRACT should not preclude intervention in patients at high risk of PTS.
“I believe there is a role for DVT intervention for symptomatic patients with an extensive [proximal iliofemoral] clot provided they have a low bleeding risk,” Dr. Soukas said.
Dr. Soukas reported no potential conflicts of interest.
EXPERT ANALYSIS FROM THE 2018 CRT MEETING
ESBL-resistant bacteria spread in hospital despite strict contact precautions
MADRID – even when staff employed an active surveillance screening protocol to identify every carrier at admission.
The failure of precautions may have root in two thorny issues, said Friederike Maechler, MD, who presented the data at the the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“Adherence to strict contact isolation and hand hygiene is never 100% in a real-life scenario,” said Dr. Maechler, of Charite University Hospital, Berlin. Also, she said, contact isolation can only be effective in a ward if all, or at least most, of the ESBL-E carriers are identified. “Even with an extensive surveillance screening program established, many carriers remained unknown to the health care staff.”
The 25-month study, dubbed R-Gnosis, was conducted in 20 Western European hospitals in Madrid, Berlin, Utrecht, and Geneva. It compared 12 months of contact precaution with standard precaution infection control strategies in medical and surgical non-ICUs.
The entire study hinged on a strict protocol to identify as many ESBL-E carriers as possible. This was done by screening upon admission to the unit, screening once per week during the hospital stay, and screening on discharge. Each patient underwent deep rectal swabs that were cultured on agar and screened for resistance.
The crossover design trial randomized each unit to either contact precautions or standard precautions for 12 months, followed by a 1-month washout period, after which they began the other protocol.
In all, 50,870 patients were entered into the study. By the end, Dr. Maechler had data on 11,367 patients with full screening and follow-up.
Standard precautions did not require a private bedroom, with gloves, gowns, and apron needed for direct contact to body fluids or wounds only, and consistent hand hygiene. Contact precautions required a private bedroom and strict hand hygiene, with gloves, gowns, and aprons used for any patient contact. Study staff monitored compliance with these procedures monthly.
The primary outcome was the ESBL-E acquisition rate per 1,000 patient days. This was defined as a new ESBL-E detection after the patient had a prior negative screen. Dr. Maechler noted that by epidemiological definition, acquisition does not necessarily imply cross-transmission from other patients.
Adherence to the study protocols was good, she said. Adherence to both contact and standard precautions was about 85%, while adherence to hand hygiene was less at around 62%.
Admission ESBL-E screenings revealed that about 12% of the study population was colonized with the strain at admission. The proportion was nearly identical in the contact and standard precaution groups (11.6%, 12.2%).
The incidence density of ward-acquired ESBL-E per 1,000 patient-days at risk was 4.6 in both intervention periods, regardless of the type of precaution taken. Contact precautions appeared to be slightly less effective for Escherichia coli (3.6 per 1,000 patient-days in contact precautions vs. 3.5 in standard), compared with Klebsiella pneumoniae (1.8 vs. 2.2).
A multivariate analysis controlled for screening compliance, colonization pressure, and length of stay, study site, and season of year. It showed that strict contact precautions did not reduce the risk of ward-acquired ESBL-E carriage.
Dr. Maechler had no financial disclosures. The R-Gnosis study was funded by the European Community’s Seventh Framework Programme.
SOURCE: Maechler F et al. ECCMID 2018, Oral Abstract O1130.
MADRID – even when staff employed an active surveillance screening protocol to identify every carrier at admission.
The failure of precautions may have root in two thorny issues, said Friederike Maechler, MD, who presented the data at the the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“Adherence to strict contact isolation and hand hygiene is never 100% in a real-life scenario,” said Dr. Maechler, of Charite University Hospital, Berlin. Also, she said, contact isolation can only be effective in a ward if all, or at least most, of the ESBL-E carriers are identified. “Even with an extensive surveillance screening program established, many carriers remained unknown to the health care staff.”
The 25-month study, dubbed R-Gnosis, was conducted in 20 Western European hospitals in Madrid, Berlin, Utrecht, and Geneva. It compared 12 months of contact precaution with standard precaution infection control strategies in medical and surgical non-ICUs.
The entire study hinged on a strict protocol to identify as many ESBL-E carriers as possible. This was done by screening upon admission to the unit, screening once per week during the hospital stay, and screening on discharge. Each patient underwent deep rectal swabs that were cultured on agar and screened for resistance.
The crossover design trial randomized each unit to either contact precautions or standard precautions for 12 months, followed by a 1-month washout period, after which they began the other protocol.
In all, 50,870 patients were entered into the study. By the end, Dr. Maechler had data on 11,367 patients with full screening and follow-up.
Standard precautions did not require a private bedroom, with gloves, gowns, and apron needed for direct contact to body fluids or wounds only, and consistent hand hygiene. Contact precautions required a private bedroom and strict hand hygiene, with gloves, gowns, and aprons used for any patient contact. Study staff monitored compliance with these procedures monthly.
The primary outcome was the ESBL-E acquisition rate per 1,000 patient days. This was defined as a new ESBL-E detection after the patient had a prior negative screen. Dr. Maechler noted that by epidemiological definition, acquisition does not necessarily imply cross-transmission from other patients.
Adherence to the study protocols was good, she said. Adherence to both contact and standard precautions was about 85%, while adherence to hand hygiene was less at around 62%.
Admission ESBL-E screenings revealed that about 12% of the study population was colonized with the strain at admission. The proportion was nearly identical in the contact and standard precaution groups (11.6%, 12.2%).
The incidence density of ward-acquired ESBL-E per 1,000 patient-days at risk was 4.6 in both intervention periods, regardless of the type of precaution taken. Contact precautions appeared to be slightly less effective for Escherichia coli (3.6 per 1,000 patient-days in contact precautions vs. 3.5 in standard), compared with Klebsiella pneumoniae (1.8 vs. 2.2).
A multivariate analysis controlled for screening compliance, colonization pressure, and length of stay, study site, and season of year. It showed that strict contact precautions did not reduce the risk of ward-acquired ESBL-E carriage.
Dr. Maechler had no financial disclosures. The R-Gnosis study was funded by the European Community’s Seventh Framework Programme.
SOURCE: Maechler F et al. ECCMID 2018, Oral Abstract O1130.
MADRID – even when staff employed an active surveillance screening protocol to identify every carrier at admission.
The failure of precautions may have root in two thorny issues, said Friederike Maechler, MD, who presented the data at the the European Society of Clinical Microbiology and Infectious Diseases annual congress.
“Adherence to strict contact isolation and hand hygiene is never 100% in a real-life scenario,” said Dr. Maechler, of Charite University Hospital, Berlin. Also, she said, contact isolation can only be effective in a ward if all, or at least most, of the ESBL-E carriers are identified. “Even with an extensive surveillance screening program established, many carriers remained unknown to the health care staff.”
The 25-month study, dubbed R-Gnosis, was conducted in 20 Western European hospitals in Madrid, Berlin, Utrecht, and Geneva. It compared 12 months of contact precaution with standard precaution infection control strategies in medical and surgical non-ICUs.
The entire study hinged on a strict protocol to identify as many ESBL-E carriers as possible. This was done by screening upon admission to the unit, screening once per week during the hospital stay, and screening on discharge. Each patient underwent deep rectal swabs that were cultured on agar and screened for resistance.
The crossover design trial randomized each unit to either contact precautions or standard precautions for 12 months, followed by a 1-month washout period, after which they began the other protocol.
In all, 50,870 patients were entered into the study. By the end, Dr. Maechler had data on 11,367 patients with full screening and follow-up.
Standard precautions did not require a private bedroom, with gloves, gowns, and apron needed for direct contact to body fluids or wounds only, and consistent hand hygiene. Contact precautions required a private bedroom and strict hand hygiene, with gloves, gowns, and aprons used for any patient contact. Study staff monitored compliance with these procedures monthly.
The primary outcome was the ESBL-E acquisition rate per 1,000 patient days. This was defined as a new ESBL-E detection after the patient had a prior negative screen. Dr. Maechler noted that by epidemiological definition, acquisition does not necessarily imply cross-transmission from other patients.
Adherence to the study protocols was good, she said. Adherence to both contact and standard precautions was about 85%, while adherence to hand hygiene was less at around 62%.
Admission ESBL-E screenings revealed that about 12% of the study population was colonized with the strain at admission. The proportion was nearly identical in the contact and standard precaution groups (11.6%, 12.2%).
The incidence density of ward-acquired ESBL-E per 1,000 patient-days at risk was 4.6 in both intervention periods, regardless of the type of precaution taken. Contact precautions appeared to be slightly less effective for Escherichia coli (3.6 per 1,000 patient-days in contact precautions vs. 3.5 in standard), compared with Klebsiella pneumoniae (1.8 vs. 2.2).
A multivariate analysis controlled for screening compliance, colonization pressure, and length of stay, study site, and season of year. It showed that strict contact precautions did not reduce the risk of ward-acquired ESBL-E carriage.
Dr. Maechler had no financial disclosures. The R-Gnosis study was funded by the European Community’s Seventh Framework Programme.
SOURCE: Maechler F et al. ECCMID 2018, Oral Abstract O1130.
REPORTING FROM ECCMID 2018
Key clinical point: A protocol of strict contact precautions and hand hygiene was no better than standard contact precautions at preventing the spread of extended-spectrum, beta-lactamase–resistant Enterobacteriaceae.
Major finding: The incidence density of ward-acquired ESBL-E per 1,000 patient-days at risk was 4.6, regardless of precaution.
Study details: The 25-month crossover trial comprised more than 11,000 patients.
Disclosures: Dr. Maechler had no financial disclosures. The R-Gnosis study was funded by the European Community’s Seventh Framework Programme.
Source: Maechler F et al. ECCMID 2018, Oral Abstract O1130.
Multiple analgesia options for kids with acute pain
TORONTO – according to Naveen Poonai, MD, FRCPC.
“Too many times, nonpharmacologic therapies are relegated to the very last paragraph of recommendations or to the very bottom of a URL,” he said at the Pediatric Academic Societies meeting. “Nonpharmacologic therapies are things that our grandparents told us to do: common sense things that can be done at triage. They don’t require memorization of dosing, and most importantly, they don’t have side effects.”
When analgesia is indicated, clinicians can choose from a variety of agents in the postcodeine era. Dr. Poonai said that musculoskeletal injuries constitute 10-20% of pediatric emergency department visits, yet fewer than 60% of children receive adequate analgesia. “That’s what’s really important for patient and caregiver satisfaction,” he said.
Mounting evidence supports the use of ibuprofen as a go-to agent for mild to moderate pain in patients with musculoskeletal injuries, including results from a randomized, controlled multicenter trial of 500 youth (Canadian J Emerg Med. 2016:18:S29). “We know that ibuprofen is superior to acetaminophen or codeine and that it’s as good or better than oral opioids and with fewer side effects,” Dr. Poonai said, adding that it provides a 25 mm visual analog score (VAS) reduction in pain at 60 minutes. Another study that compared ibuprofen with codeine for acute pediatric arm fracture pain found that ibuprofen was associated with improved functioning and was at least as effective as acetaminophen plus codeine (Ann Emerg Med. 2009 Oct;54[4]:553-60).
A number of oral opioids have gained favor for use in children who present with acute pain. However, in a randomized trial, Dr. Poonai and his associates found no significant difference in analgesic efficacy between orally administered morphine and ibuprofen for the management of postfracture pain in 134 children (CMAJ. 2014 Dec 9;186[18]:1358-63). Oral morphine was also associated with more side effects. At the same time, tramadol and hydromorphone have not been well studied in children with musculoskeletal pain. “Currently, the use of hydromorphone is limited to children with sickle cell disease, but the use is branching out,” he said. “Oxycodone and oral morphine pose the greatest risk of side effects. The bottom line here is that opioids should be added to ibuprofen and acetaminophen rather than replacing them for mild to moderate pain.”
In 2014, a study from the Cochrane Database of Systematic Reviews concluded that intranasal fentanyl can be effective for the management of moderate to severe pain in children. A dose of 1.0-1.5 mcg/kg is associated with a 40-mm pain reduction in VAS at 10 minutes. “The benefits are that it is not an invasive approach, it’s been rigorously studied, and it is equivalent to IV morphine for moderate to severe pain,” said Dr. Poonai, who was not part of the Cochrane review. “It lasts about 60 minutes, with minimal side effects.”
A separate analysis found that intranasal fentanyl and ketamine were associated with similar pain reduction in children with moderate to severe pain from limb injury (Ann Emerg Med. 2015 Mar;65[3]:248-54.e1). Ketamine was associated with more minor adverse events. An intranasal dose of 1 mg/kg can cause a 40- to 45-mm reduction in VAS at 30 minutes.
Dr. Poonai went on to discuss treatment options for abdominal pain, noting that fewer than two-thirds of children with suspected appendicitis receive analgesia. “If they are receiving it, it’s often not until after the ultrasound is performed,” he said. “There is a still a reluctance toward providing opioid analgesia for a child with suspected appendicitis for fear of masking a diagnosis or leading to complications.” A systematic review led by Dr. Poonai found that the use of opioids in undifferentiated acute abdominal pain in children is associated with no difference in pain scores and an increased risk of mild side effects (Acad Emerg Med. 2014 21[11]:1183-92). However, there was no increased risk of perforation or abscess. “We found that single-dose IV opioids were actually beneficial,” he said.
Dr. Poonai characterized most of the current evidence on IV morphine for suspected appendicitis as being of low to moderate quality, “but they are generally favorable for the indication,” he said. “It is titratable to effect, and triage-initiated protocols improve timing and consistency of analgesia.” He reported having no financial disclosures.
TORONTO – according to Naveen Poonai, MD, FRCPC.
“Too many times, nonpharmacologic therapies are relegated to the very last paragraph of recommendations or to the very bottom of a URL,” he said at the Pediatric Academic Societies meeting. “Nonpharmacologic therapies are things that our grandparents told us to do: common sense things that can be done at triage. They don’t require memorization of dosing, and most importantly, they don’t have side effects.”
When analgesia is indicated, clinicians can choose from a variety of agents in the postcodeine era. Dr. Poonai said that musculoskeletal injuries constitute 10-20% of pediatric emergency department visits, yet fewer than 60% of children receive adequate analgesia. “That’s what’s really important for patient and caregiver satisfaction,” he said.
Mounting evidence supports the use of ibuprofen as a go-to agent for mild to moderate pain in patients with musculoskeletal injuries, including results from a randomized, controlled multicenter trial of 500 youth (Canadian J Emerg Med. 2016:18:S29). “We know that ibuprofen is superior to acetaminophen or codeine and that it’s as good or better than oral opioids and with fewer side effects,” Dr. Poonai said, adding that it provides a 25 mm visual analog score (VAS) reduction in pain at 60 minutes. Another study that compared ibuprofen with codeine for acute pediatric arm fracture pain found that ibuprofen was associated with improved functioning and was at least as effective as acetaminophen plus codeine (Ann Emerg Med. 2009 Oct;54[4]:553-60).
A number of oral opioids have gained favor for use in children who present with acute pain. However, in a randomized trial, Dr. Poonai and his associates found no significant difference in analgesic efficacy between orally administered morphine and ibuprofen for the management of postfracture pain in 134 children (CMAJ. 2014 Dec 9;186[18]:1358-63). Oral morphine was also associated with more side effects. At the same time, tramadol and hydromorphone have not been well studied in children with musculoskeletal pain. “Currently, the use of hydromorphone is limited to children with sickle cell disease, but the use is branching out,” he said. “Oxycodone and oral morphine pose the greatest risk of side effects. The bottom line here is that opioids should be added to ibuprofen and acetaminophen rather than replacing them for mild to moderate pain.”
In 2014, a study from the Cochrane Database of Systematic Reviews concluded that intranasal fentanyl can be effective for the management of moderate to severe pain in children. A dose of 1.0-1.5 mcg/kg is associated with a 40-mm pain reduction in VAS at 10 minutes. “The benefits are that it is not an invasive approach, it’s been rigorously studied, and it is equivalent to IV morphine for moderate to severe pain,” said Dr. Poonai, who was not part of the Cochrane review. “It lasts about 60 minutes, with minimal side effects.”
A separate analysis found that intranasal fentanyl and ketamine were associated with similar pain reduction in children with moderate to severe pain from limb injury (Ann Emerg Med. 2015 Mar;65[3]:248-54.e1). Ketamine was associated with more minor adverse events. An intranasal dose of 1 mg/kg can cause a 40- to 45-mm reduction in VAS at 30 minutes.
Dr. Poonai went on to discuss treatment options for abdominal pain, noting that fewer than two-thirds of children with suspected appendicitis receive analgesia. “If they are receiving it, it’s often not until after the ultrasound is performed,” he said. “There is a still a reluctance toward providing opioid analgesia for a child with suspected appendicitis for fear of masking a diagnosis or leading to complications.” A systematic review led by Dr. Poonai found that the use of opioids in undifferentiated acute abdominal pain in children is associated with no difference in pain scores and an increased risk of mild side effects (Acad Emerg Med. 2014 21[11]:1183-92). However, there was no increased risk of perforation or abscess. “We found that single-dose IV opioids were actually beneficial,” he said.
Dr. Poonai characterized most of the current evidence on IV morphine for suspected appendicitis as being of low to moderate quality, “but they are generally favorable for the indication,” he said. “It is titratable to effect, and triage-initiated protocols improve timing and consistency of analgesia.” He reported having no financial disclosures.
TORONTO – according to Naveen Poonai, MD, FRCPC.
“Too many times, nonpharmacologic therapies are relegated to the very last paragraph of recommendations or to the very bottom of a URL,” he said at the Pediatric Academic Societies meeting. “Nonpharmacologic therapies are things that our grandparents told us to do: common sense things that can be done at triage. They don’t require memorization of dosing, and most importantly, they don’t have side effects.”
When analgesia is indicated, clinicians can choose from a variety of agents in the postcodeine era. Dr. Poonai said that musculoskeletal injuries constitute 10-20% of pediatric emergency department visits, yet fewer than 60% of children receive adequate analgesia. “That’s what’s really important for patient and caregiver satisfaction,” he said.
Mounting evidence supports the use of ibuprofen as a go-to agent for mild to moderate pain in patients with musculoskeletal injuries, including results from a randomized, controlled multicenter trial of 500 youth (Canadian J Emerg Med. 2016:18:S29). “We know that ibuprofen is superior to acetaminophen or codeine and that it’s as good or better than oral opioids and with fewer side effects,” Dr. Poonai said, adding that it provides a 25 mm visual analog score (VAS) reduction in pain at 60 minutes. Another study that compared ibuprofen with codeine for acute pediatric arm fracture pain found that ibuprofen was associated with improved functioning and was at least as effective as acetaminophen plus codeine (Ann Emerg Med. 2009 Oct;54[4]:553-60).
A number of oral opioids have gained favor for use in children who present with acute pain. However, in a randomized trial, Dr. Poonai and his associates found no significant difference in analgesic efficacy between orally administered morphine and ibuprofen for the management of postfracture pain in 134 children (CMAJ. 2014 Dec 9;186[18]:1358-63). Oral morphine was also associated with more side effects. At the same time, tramadol and hydromorphone have not been well studied in children with musculoskeletal pain. “Currently, the use of hydromorphone is limited to children with sickle cell disease, but the use is branching out,” he said. “Oxycodone and oral morphine pose the greatest risk of side effects. The bottom line here is that opioids should be added to ibuprofen and acetaminophen rather than replacing them for mild to moderate pain.”
In 2014, a study from the Cochrane Database of Systematic Reviews concluded that intranasal fentanyl can be effective for the management of moderate to severe pain in children. A dose of 1.0-1.5 mcg/kg is associated with a 40-mm pain reduction in VAS at 10 minutes. “The benefits are that it is not an invasive approach, it’s been rigorously studied, and it is equivalent to IV morphine for moderate to severe pain,” said Dr. Poonai, who was not part of the Cochrane review. “It lasts about 60 minutes, with minimal side effects.”
A separate analysis found that intranasal fentanyl and ketamine were associated with similar pain reduction in children with moderate to severe pain from limb injury (Ann Emerg Med. 2015 Mar;65[3]:248-54.e1). Ketamine was associated with more minor adverse events. An intranasal dose of 1 mg/kg can cause a 40- to 45-mm reduction in VAS at 30 minutes.
Dr. Poonai went on to discuss treatment options for abdominal pain, noting that fewer than two-thirds of children with suspected appendicitis receive analgesia. “If they are receiving it, it’s often not until after the ultrasound is performed,” he said. “There is a still a reluctance toward providing opioid analgesia for a child with suspected appendicitis for fear of masking a diagnosis or leading to complications.” A systematic review led by Dr. Poonai found that the use of opioids in undifferentiated acute abdominal pain in children is associated with no difference in pain scores and an increased risk of mild side effects (Acad Emerg Med. 2014 21[11]:1183-92). However, there was no increased risk of perforation or abscess. “We found that single-dose IV opioids were actually beneficial,” he said.
Dr. Poonai characterized most of the current evidence on IV morphine for suspected appendicitis as being of low to moderate quality, “but they are generally favorable for the indication,” he said. “It is titratable to effect, and triage-initiated protocols improve timing and consistency of analgesia.” He reported having no financial disclosures.
EXPERT ANALYSIS FROM PAS 2018
CAZ-AVI appears safe, effective in pediatric complicated UTI, intra-abdominal infections
MADRID – Two randomized phase 2b trials show the combination of ceftazidime-avibactam (CAZ-AVI) is safe and effective in children with complicated intra-abdominal infections or complicated urinary tract infections (UTIs).
The combination already is approved for these conditions in adults, said John Bradley, MD, who presented the studies at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
However, Pfizer, which recently acquired the drug combination from AstraZeneca as part of its small-molecule anti-infectives sell-off, intends to go for a pediatric approval for these two indications. The studies, which had secondary efficacy endpoints, will be used as part of the application package to the Food and Drug Administration and the European Medicines Agency, said Dr. Bradley, professor of clinical pediatrics at the University of California, San Diego.
“For those of you who take care of adults and use these drugs, this seems like old news, but those of us who take care of children can rejoice, because these are the first pediatric data presented. And – no surprise – the combination appears to be as safe and effective in children as it is in adults.”
Both studies concluded in late 2017. “We have the data locked and it’s being cleaned and soon will be submitted to regulatory agencies,” Dr. Bradley said. “However, we do not yet have approval so if you do use it, it will still be considered an off-label use until regulatory agencies work with the sponsor to achieve approval.”
Both studies were international, conducted in the United States, Europe, Russia, South Korea, Taiwan, and Turkey.
The first study included 83 children, mean age 10 years, who had complicated intra-abdominal infections precipitated by ruptured appendicitis. About 90% already had been treated with other antibiotics. In this trial, the CAZ-AVI combination was augmented with metronidazole, and compared to meropenem, in 72-hour infusions. The microbiologic test of cure was conducted at 8-15 days with a late follow-up at 20-36 days after the last infusion.
Most of the patents (83%) had an infective organism identified; it was most often Escherichia coli or Pseudomonas aeruginosa. All pathogens were susceptible to the study drugs.
Five children in the combination group experienced a serious adverse event. These included one case each of ileus, intestinal obstruction, large intestine perforation, renal colic, and urethra meatus stenosis. There was one case of ileus in the meropenem group.
There was one case of diarrhea in the combination group. There were three allergic reactions in each group (cough, pruritus, and rash). The meropenem group also had two cases of anemia.
At the test-of-cure point, clinical response was similar in the combination and meropenem groups, both in clinical evidence (93% vs. 95%) and microbiological response (90% vs. 95%) At last follow-up, 100% of each group was clinically cured. The microbiological cure rates were 90% and 95%, respectively.
Success for complicated UTI
The complicated UTI study was likewise good news for CAZ-AVI, this time without metronidazole. This study included 95 children, mean age 6 years, in the same globally gathered cohorts. All of the children were hospitalized; they were randomized to CAZ-AVI at age-specific doses or cefepime, less than 2,000 mg/infusion for 72 hours. The test of cure was conducted at 8-15 days with a late follow-up at 20-36 days after the last infusion.
Most patients (83%) had acute pyelonephritis. About a quarter had at least one complicating factor, including obstructive uropathies due to functional or anatomic abnormalities of the urogenital tract, recurrent UTI, vesicoureteral reflex, or intermittent catheterization. About 20% of the group had a urological abnormality and 40% had been on a systemic antibiotic in the 2 weeks before study entry.
The most common infective organism was E. coli, (92%) followed by Klebsiella pneumoniae, Proteus mirabilis, and Enterobacter cloacae.
Again, about half of each group had at least one adverse event. Serious adverse events occurred in 12% of the combination group and 7% of the cefepime group. Three patients taking the combination discontinued because of the reaction.
There were eight serious events in the combination group, including abdominal pain, constipation, cystitis, acute pyelonephritis, UTI (not considered related to the study drug) viral infection, nervous system disorder, and nephrolithiasis. There were two serious adverse events in the cefepime group (cystitis and acute pyelonephritis).
Favorable clinical outcomes occurred in 89% of the combination group and 82.6% of the cefepime group. A microbiological cure was evident in 79.6% and 60.9%, respectively.
The combination was more effective than was cefepime at eradicating E. coli (79.6% vs. 59.1%), although no statistical analysis was presented. The other, less-frequent pathogens did not co-occur in both groups, so comparisons were not made. However, the combination eradicated P. mirabilis in both patients who had it, and 50% of K. pneumoniae infections.
A sustained clinical cure occurred in 81% of the combination group and 82.6% of the cefepime group.
Dr. Bradley said the University of California, San Diego, received fees from both Pfizer or AstraZeneca relating to the studies.
SOURCE: Bradley J et al. ECCMID 2018 oral abstracts O1123 and O1124.
MADRID – Two randomized phase 2b trials show the combination of ceftazidime-avibactam (CAZ-AVI) is safe and effective in children with complicated intra-abdominal infections or complicated urinary tract infections (UTIs).
The combination already is approved for these conditions in adults, said John Bradley, MD, who presented the studies at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
However, Pfizer, which recently acquired the drug combination from AstraZeneca as part of its small-molecule anti-infectives sell-off, intends to go for a pediatric approval for these two indications. The studies, which had secondary efficacy endpoints, will be used as part of the application package to the Food and Drug Administration and the European Medicines Agency, said Dr. Bradley, professor of clinical pediatrics at the University of California, San Diego.
“For those of you who take care of adults and use these drugs, this seems like old news, but those of us who take care of children can rejoice, because these are the first pediatric data presented. And – no surprise – the combination appears to be as safe and effective in children as it is in adults.”
Both studies concluded in late 2017. “We have the data locked and it’s being cleaned and soon will be submitted to regulatory agencies,” Dr. Bradley said. “However, we do not yet have approval so if you do use it, it will still be considered an off-label use until regulatory agencies work with the sponsor to achieve approval.”
Both studies were international, conducted in the United States, Europe, Russia, South Korea, Taiwan, and Turkey.
The first study included 83 children, mean age 10 years, who had complicated intra-abdominal infections precipitated by ruptured appendicitis. About 90% already had been treated with other antibiotics. In this trial, the CAZ-AVI combination was augmented with metronidazole, and compared to meropenem, in 72-hour infusions. The microbiologic test of cure was conducted at 8-15 days with a late follow-up at 20-36 days after the last infusion.
Most of the patents (83%) had an infective organism identified; it was most often Escherichia coli or Pseudomonas aeruginosa. All pathogens were susceptible to the study drugs.
Five children in the combination group experienced a serious adverse event. These included one case each of ileus, intestinal obstruction, large intestine perforation, renal colic, and urethra meatus stenosis. There was one case of ileus in the meropenem group.
There was one case of diarrhea in the combination group. There were three allergic reactions in each group (cough, pruritus, and rash). The meropenem group also had two cases of anemia.
At the test-of-cure point, clinical response was similar in the combination and meropenem groups, both in clinical evidence (93% vs. 95%) and microbiological response (90% vs. 95%) At last follow-up, 100% of each group was clinically cured. The microbiological cure rates were 90% and 95%, respectively.
Success for complicated UTI
The complicated UTI study was likewise good news for CAZ-AVI, this time without metronidazole. This study included 95 children, mean age 6 years, in the same globally gathered cohorts. All of the children were hospitalized; they were randomized to CAZ-AVI at age-specific doses or cefepime, less than 2,000 mg/infusion for 72 hours. The test of cure was conducted at 8-15 days with a late follow-up at 20-36 days after the last infusion.
Most patients (83%) had acute pyelonephritis. About a quarter had at least one complicating factor, including obstructive uropathies due to functional or anatomic abnormalities of the urogenital tract, recurrent UTI, vesicoureteral reflex, or intermittent catheterization. About 20% of the group had a urological abnormality and 40% had been on a systemic antibiotic in the 2 weeks before study entry.
The most common infective organism was E. coli, (92%) followed by Klebsiella pneumoniae, Proteus mirabilis, and Enterobacter cloacae.
Again, about half of each group had at least one adverse event. Serious adverse events occurred in 12% of the combination group and 7% of the cefepime group. Three patients taking the combination discontinued because of the reaction.
There were eight serious events in the combination group, including abdominal pain, constipation, cystitis, acute pyelonephritis, UTI (not considered related to the study drug) viral infection, nervous system disorder, and nephrolithiasis. There were two serious adverse events in the cefepime group (cystitis and acute pyelonephritis).
Favorable clinical outcomes occurred in 89% of the combination group and 82.6% of the cefepime group. A microbiological cure was evident in 79.6% and 60.9%, respectively.
The combination was more effective than was cefepime at eradicating E. coli (79.6% vs. 59.1%), although no statistical analysis was presented. The other, less-frequent pathogens did not co-occur in both groups, so comparisons were not made. However, the combination eradicated P. mirabilis in both patients who had it, and 50% of K. pneumoniae infections.
A sustained clinical cure occurred in 81% of the combination group and 82.6% of the cefepime group.
Dr. Bradley said the University of California, San Diego, received fees from both Pfizer or AstraZeneca relating to the studies.
SOURCE: Bradley J et al. ECCMID 2018 oral abstracts O1123 and O1124.
MADRID – Two randomized phase 2b trials show the combination of ceftazidime-avibactam (CAZ-AVI) is safe and effective in children with complicated intra-abdominal infections or complicated urinary tract infections (UTIs).
The combination already is approved for these conditions in adults, said John Bradley, MD, who presented the studies at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
However, Pfizer, which recently acquired the drug combination from AstraZeneca as part of its small-molecule anti-infectives sell-off, intends to go for a pediatric approval for these two indications. The studies, which had secondary efficacy endpoints, will be used as part of the application package to the Food and Drug Administration and the European Medicines Agency, said Dr. Bradley, professor of clinical pediatrics at the University of California, San Diego.
“For those of you who take care of adults and use these drugs, this seems like old news, but those of us who take care of children can rejoice, because these are the first pediatric data presented. And – no surprise – the combination appears to be as safe and effective in children as it is in adults.”
Both studies concluded in late 2017. “We have the data locked and it’s being cleaned and soon will be submitted to regulatory agencies,” Dr. Bradley said. “However, we do not yet have approval so if you do use it, it will still be considered an off-label use until regulatory agencies work with the sponsor to achieve approval.”
Both studies were international, conducted in the United States, Europe, Russia, South Korea, Taiwan, and Turkey.
The first study included 83 children, mean age 10 years, who had complicated intra-abdominal infections precipitated by ruptured appendicitis. About 90% already had been treated with other antibiotics. In this trial, the CAZ-AVI combination was augmented with metronidazole, and compared to meropenem, in 72-hour infusions. The microbiologic test of cure was conducted at 8-15 days with a late follow-up at 20-36 days after the last infusion.
Most of the patents (83%) had an infective organism identified; it was most often Escherichia coli or Pseudomonas aeruginosa. All pathogens were susceptible to the study drugs.
Five children in the combination group experienced a serious adverse event. These included one case each of ileus, intestinal obstruction, large intestine perforation, renal colic, and urethra meatus stenosis. There was one case of ileus in the meropenem group.
There was one case of diarrhea in the combination group. There were three allergic reactions in each group (cough, pruritus, and rash). The meropenem group also had two cases of anemia.
At the test-of-cure point, clinical response was similar in the combination and meropenem groups, both in clinical evidence (93% vs. 95%) and microbiological response (90% vs. 95%) At last follow-up, 100% of each group was clinically cured. The microbiological cure rates were 90% and 95%, respectively.
Success for complicated UTI
The complicated UTI study was likewise good news for CAZ-AVI, this time without metronidazole. This study included 95 children, mean age 6 years, in the same globally gathered cohorts. All of the children were hospitalized; they were randomized to CAZ-AVI at age-specific doses or cefepime, less than 2,000 mg/infusion for 72 hours. The test of cure was conducted at 8-15 days with a late follow-up at 20-36 days after the last infusion.
Most patients (83%) had acute pyelonephritis. About a quarter had at least one complicating factor, including obstructive uropathies due to functional or anatomic abnormalities of the urogenital tract, recurrent UTI, vesicoureteral reflex, or intermittent catheterization. About 20% of the group had a urological abnormality and 40% had been on a systemic antibiotic in the 2 weeks before study entry.
The most common infective organism was E. coli, (92%) followed by Klebsiella pneumoniae, Proteus mirabilis, and Enterobacter cloacae.
Again, about half of each group had at least one adverse event. Serious adverse events occurred in 12% of the combination group and 7% of the cefepime group. Three patients taking the combination discontinued because of the reaction.
There were eight serious events in the combination group, including abdominal pain, constipation, cystitis, acute pyelonephritis, UTI (not considered related to the study drug) viral infection, nervous system disorder, and nephrolithiasis. There were two serious adverse events in the cefepime group (cystitis and acute pyelonephritis).
Favorable clinical outcomes occurred in 89% of the combination group and 82.6% of the cefepime group. A microbiological cure was evident in 79.6% and 60.9%, respectively.
The combination was more effective than was cefepime at eradicating E. coli (79.6% vs. 59.1%), although no statistical analysis was presented. The other, less-frequent pathogens did not co-occur in both groups, so comparisons were not made. However, the combination eradicated P. mirabilis in both patients who had it, and 50% of K. pneumoniae infections.
A sustained clinical cure occurred in 81% of the combination group and 82.6% of the cefepime group.
Dr. Bradley said the University of California, San Diego, received fees from both Pfizer or AstraZeneca relating to the studies.
SOURCE: Bradley J et al. ECCMID 2018 oral abstracts O1123 and O1124.
REPORTING FROM ECCMID 2018
Key clinical point: The CAZ-AVI combination was as good as the standard comparator drug in both studies.
Major finding: The combination cured close to 90% of infections in both studies.
Study details: Together, the phase 2b studies comprised 178 children.
Disclosures: Pfizer sponsored the studies.
Source: Bradley J et al. ECCMID 2018 oral abstracts O1123 and O1124.
Thirty-second atrial fib threshold may drive overdiagnosis
BOSTON – The standard definition of an episode of atrial fibrillation is a fibrillation event that lasts at least 30 seconds, but a new analysis of monitoring data collected from 615 patients showed that this threshold can label many patients as having atrial fibrillation despite an extremely low disease burden.
A more clinically relevant definition of atrial fibrillation (AF) might be a patient with at least one episode that persists for at least 3.8 hours, because this threshold identified people with a median AF burden of just under 10%, Jonathan S. Steinberg, MD, said while presenting a poster at the annual scientific sessions of the Heart Rhythm Society.
The 30-second threshold for defining an AF episode dates from the early days of atrial ablation treatment, when researchers tracked ablated patients for signs of AF recurrence. But this definition that clinicians devised for a very select subgroup of AF patients subsequently “metastasized” to define AF in all settings, he noted. As one recent example, the 2017 consensus document on screening for AF in asymptomatic people defined asymptomatic patients as having AF if they had at least one 30-second event picked up on an ECG recording (EP Europace. 2017 Oct 1;19[10]:1589-623).
“How we define AF is very important as we look for it in asymptomatic people,” Dr. Steinberg said in an interview.
A better definition of AF might depend on total AF burden, which is the percentage of time the patient’s atrium spends fibrillating. But it’s impossible to directly measure AF burden over a reasonably representative period of time without having an implanted device. If AF is monitored with an external device, the sampling time will be relatively brief, and so the AF assessment needs to rely on a surrogate for AF burden: the longest duration of any measured AF episode.
“No prior AF database has been analyzed like we have,” to correlate AF burden with the duration of the longest AF episode, Dr. Steinberg said.
He and his associates used data collected by Medtronic from 1,040 patients enrolled in a company registry during 2005-2016 with an implanted dual-chamber pacemaker able to detect atrial arrhythmias. The researchers focused on the 615 patients who had AF detected during at least 30 days of monitoring. These 615 patients averaged 72 years of age, 54% were men, and 599 had at least one AF episode of at least 30 seconds duration. Each patient had an average 3.7-year accumulated archive of atrial rhythm data.
The analysis showed a close association between the longest AF episode detected and overall AF burden. Among patients with a longest episode of 30-119 seconds, the median burden was 0.1%. Among patients with a maximum duration of anywhere from 30 seconds to 3.7 hours, the median burden was 0.2%. But among people with a longest episode of 3.8 hours to 5.4 hours, the median burden was 1.2%. In those with a longest episode of at least 24 hours, the median burden was 25%. Finally, in those who had a longest AF episode that lasted at least 3.8 hours, the median AF burden was 9.5%.
Dr. Steinberg acknowledged that a very important additional step needed in this analysis is examining the correlations among AF burden, longest AF episode, and stroke incidence, something he and his associates are now doing. He expressed hope that these data will spur the cardiac electrophysiology community to rethink its AF definition.
The study was funded by Medtronic. Dr. Steinberg has been a consultant and/or has received research funding from Medtronic, AliveCor, Allergen, Atricure, Biosense Webster, G Medical, and National Cardiac. Several of his coauthors were Medtronic employees.
SOURCE: Steinberg J et al. Heart Rhythm Society scientific sessions, B-P001-062.
BOSTON – The standard definition of an episode of atrial fibrillation is a fibrillation event that lasts at least 30 seconds, but a new analysis of monitoring data collected from 615 patients showed that this threshold can label many patients as having atrial fibrillation despite an extremely low disease burden.
A more clinically relevant definition of atrial fibrillation (AF) might be a patient with at least one episode that persists for at least 3.8 hours, because this threshold identified people with a median AF burden of just under 10%, Jonathan S. Steinberg, MD, said while presenting a poster at the annual scientific sessions of the Heart Rhythm Society.
The 30-second threshold for defining an AF episode dates from the early days of atrial ablation treatment, when researchers tracked ablated patients for signs of AF recurrence. But this definition that clinicians devised for a very select subgroup of AF patients subsequently “metastasized” to define AF in all settings, he noted. As one recent example, the 2017 consensus document on screening for AF in asymptomatic people defined asymptomatic patients as having AF if they had at least one 30-second event picked up on an ECG recording (EP Europace. 2017 Oct 1;19[10]:1589-623).
“How we define AF is very important as we look for it in asymptomatic people,” Dr. Steinberg said in an interview.
A better definition of AF might depend on total AF burden, which is the percentage of time the patient’s atrium spends fibrillating. But it’s impossible to directly measure AF burden over a reasonably representative period of time without having an implanted device. If AF is monitored with an external device, the sampling time will be relatively brief, and so the AF assessment needs to rely on a surrogate for AF burden: the longest duration of any measured AF episode.
“No prior AF database has been analyzed like we have,” to correlate AF burden with the duration of the longest AF episode, Dr. Steinberg said.
He and his associates used data collected by Medtronic from 1,040 patients enrolled in a company registry during 2005-2016 with an implanted dual-chamber pacemaker able to detect atrial arrhythmias. The researchers focused on the 615 patients who had AF detected during at least 30 days of monitoring. These 615 patients averaged 72 years of age, 54% were men, and 599 had at least one AF episode of at least 30 seconds duration. Each patient had an average 3.7-year accumulated archive of atrial rhythm data.
The analysis showed a close association between the longest AF episode detected and overall AF burden. Among patients with a longest episode of 30-119 seconds, the median burden was 0.1%. Among patients with a maximum duration of anywhere from 30 seconds to 3.7 hours, the median burden was 0.2%. But among people with a longest episode of 3.8 hours to 5.4 hours, the median burden was 1.2%. In those with a longest episode of at least 24 hours, the median burden was 25%. Finally, in those who had a longest AF episode that lasted at least 3.8 hours, the median AF burden was 9.5%.
Dr. Steinberg acknowledged that a very important additional step needed in this analysis is examining the correlations among AF burden, longest AF episode, and stroke incidence, something he and his associates are now doing. He expressed hope that these data will spur the cardiac electrophysiology community to rethink its AF definition.
The study was funded by Medtronic. Dr. Steinberg has been a consultant and/or has received research funding from Medtronic, AliveCor, Allergen, Atricure, Biosense Webster, G Medical, and National Cardiac. Several of his coauthors were Medtronic employees.
SOURCE: Steinberg J et al. Heart Rhythm Society scientific sessions, B-P001-062.
BOSTON – The standard definition of an episode of atrial fibrillation is a fibrillation event that lasts at least 30 seconds, but a new analysis of monitoring data collected from 615 patients showed that this threshold can label many patients as having atrial fibrillation despite an extremely low disease burden.
A more clinically relevant definition of atrial fibrillation (AF) might be a patient with at least one episode that persists for at least 3.8 hours, because this threshold identified people with a median AF burden of just under 10%, Jonathan S. Steinberg, MD, said while presenting a poster at the annual scientific sessions of the Heart Rhythm Society.
The 30-second threshold for defining an AF episode dates from the early days of atrial ablation treatment, when researchers tracked ablated patients for signs of AF recurrence. But this definition that clinicians devised for a very select subgroup of AF patients subsequently “metastasized” to define AF in all settings, he noted. As one recent example, the 2017 consensus document on screening for AF in asymptomatic people defined asymptomatic patients as having AF if they had at least one 30-second event picked up on an ECG recording (EP Europace. 2017 Oct 1;19[10]:1589-623).
“How we define AF is very important as we look for it in asymptomatic people,” Dr. Steinberg said in an interview.
A better definition of AF might depend on total AF burden, which is the percentage of time the patient’s atrium spends fibrillating. But it’s impossible to directly measure AF burden over a reasonably representative period of time without having an implanted device. If AF is monitored with an external device, the sampling time will be relatively brief, and so the AF assessment needs to rely on a surrogate for AF burden: the longest duration of any measured AF episode.
“No prior AF database has been analyzed like we have,” to correlate AF burden with the duration of the longest AF episode, Dr. Steinberg said.
He and his associates used data collected by Medtronic from 1,040 patients enrolled in a company registry during 2005-2016 with an implanted dual-chamber pacemaker able to detect atrial arrhythmias. The researchers focused on the 615 patients who had AF detected during at least 30 days of monitoring. These 615 patients averaged 72 years of age, 54% were men, and 599 had at least one AF episode of at least 30 seconds duration. Each patient had an average 3.7-year accumulated archive of atrial rhythm data.
The analysis showed a close association between the longest AF episode detected and overall AF burden. Among patients with a longest episode of 30-119 seconds, the median burden was 0.1%. Among patients with a maximum duration of anywhere from 30 seconds to 3.7 hours, the median burden was 0.2%. But among people with a longest episode of 3.8 hours to 5.4 hours, the median burden was 1.2%. In those with a longest episode of at least 24 hours, the median burden was 25%. Finally, in those who had a longest AF episode that lasted at least 3.8 hours, the median AF burden was 9.5%.
Dr. Steinberg acknowledged that a very important additional step needed in this analysis is examining the correlations among AF burden, longest AF episode, and stroke incidence, something he and his associates are now doing. He expressed hope that these data will spur the cardiac electrophysiology community to rethink its AF definition.
The study was funded by Medtronic. Dr. Steinberg has been a consultant and/or has received research funding from Medtronic, AliveCor, Allergen, Atricure, Biosense Webster, G Medical, and National Cardiac. Several of his coauthors were Medtronic employees.
SOURCE: Steinberg J et al. Heart Rhythm Society scientific sessions, B-P001-062.
REPORTING FROM HEART RHYTHM 2018
Key clinical point:
Major finding: The median atrial fibrillation burden was 0.1% when the longest AF episode was 30-119 seconds.
Study details: Review of data from 615 patients with AF events in a Medtronic registry.
Disclosures: Medtronic funded the study. Dr. Steinberg has been a consultant and/or has received research funding from Medtronic, AliveCor, Allergen, Atricure, Biosense Webster, G Medical, and National Cardiac. Several of his coauthors were Medtronic employees.
Source: Steinberg J et al. Heart Rhythm Society scientific sessions B-P001-062.
Zika topped Lyme in 2016
Ticks are the arthropod ride of choice for vector-borne diseases in the United States, but the Zika virus and its mosquito minions gave the ticks and their bacterial passengers a run for their money in 2016, according to the Centers for Disease Control and Prevention.
There were 41,680 cases of Zika virus that year, more than any other vector-borne disease, including Lyme disease, which had been the most common transmissible pathogen going back to at least 2004, when arthropod-borne viral diseases became nationally notifiable, said Ronald Rosenberg, ScD, and his associates at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases in Fort Collins, Colo.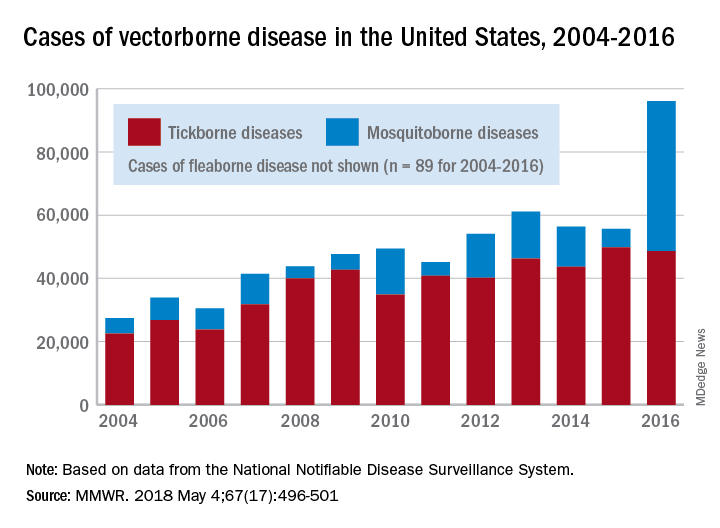
Since 2004, there have been 643,000 reported cases of vector-borne disease in the United States: 492,000 cases of tick-borne disease, of which over 402,000 were Lyme disease; 151,000 cases of mosquito-borne disease; and 89 cases of plague carried by the third type of vector, fleas, Dr. Rosenberg and his associates said based on data from the National Notifiable Disease Surveillance System.
In 2004, there were 22,527 cases of tick-borne disease and 4,858 cases of mosquito-borne disease, and the increases since then reflect the dynamics of the pathogens and vectors involved. Growth of tick-borne disease has been gradual: “Tick-borne pathogens rarely cause sudden epidemics because humans are typically incidental hosts who do not transmit further, and tick mobility is mostly limited to that of its animal hosts,” the researchers explained.
The number of mosquito-borne disease cases, on the other hand, varies considerably from year to year: There were 5,800 cases in 2015, almost 15,000 in 2013, and only 4,400 in 2011. Unlike ticks, which may feed on blood only once in a year, the more mobile mosquitoes feed every 48-72 hours and transmit their pathogens “directly between humans … resulting in explosive epidemics,” the investigators wrote.
SOURCE: Rosenberg R et al. MMWR 2018 May 4;67(17):496-501.
Ticks are the arthropod ride of choice for vector-borne diseases in the United States, but the Zika virus and its mosquito minions gave the ticks and their bacterial passengers a run for their money in 2016, according to the Centers for Disease Control and Prevention.
There were 41,680 cases of Zika virus that year, more than any other vector-borne disease, including Lyme disease, which had been the most common transmissible pathogen going back to at least 2004, when arthropod-borne viral diseases became nationally notifiable, said Ronald Rosenberg, ScD, and his associates at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases in Fort Collins, Colo.
Since 2004, there have been 643,000 reported cases of vector-borne disease in the United States: 492,000 cases of tick-borne disease, of which over 402,000 were Lyme disease; 151,000 cases of mosquito-borne disease; and 89 cases of plague carried by the third type of vector, fleas, Dr. Rosenberg and his associates said based on data from the National Notifiable Disease Surveillance System.
In 2004, there were 22,527 cases of tick-borne disease and 4,858 cases of mosquito-borne disease, and the increases since then reflect the dynamics of the pathogens and vectors involved. Growth of tick-borne disease has been gradual: “Tick-borne pathogens rarely cause sudden epidemics because humans are typically incidental hosts who do not transmit further, and tick mobility is mostly limited to that of its animal hosts,” the researchers explained.
The number of mosquito-borne disease cases, on the other hand, varies considerably from year to year: There were 5,800 cases in 2015, almost 15,000 in 2013, and only 4,400 in 2011. Unlike ticks, which may feed on blood only once in a year, the more mobile mosquitoes feed every 48-72 hours and transmit their pathogens “directly between humans … resulting in explosive epidemics,” the investigators wrote.
SOURCE: Rosenberg R et al. MMWR 2018 May 4;67(17):496-501.
Ticks are the arthropod ride of choice for vector-borne diseases in the United States, but the Zika virus and its mosquito minions gave the ticks and their bacterial passengers a run for their money in 2016, according to the Centers for Disease Control and Prevention.
There were 41,680 cases of Zika virus that year, more than any other vector-borne disease, including Lyme disease, which had been the most common transmissible pathogen going back to at least 2004, when arthropod-borne viral diseases became nationally notifiable, said Ronald Rosenberg, ScD, and his associates at the CDC’s National Center for Emerging and Zoonotic Infectious Diseases in Fort Collins, Colo.
Since 2004, there have been 643,000 reported cases of vector-borne disease in the United States: 492,000 cases of tick-borne disease, of which over 402,000 were Lyme disease; 151,000 cases of mosquito-borne disease; and 89 cases of plague carried by the third type of vector, fleas, Dr. Rosenberg and his associates said based on data from the National Notifiable Disease Surveillance System.
In 2004, there were 22,527 cases of tick-borne disease and 4,858 cases of mosquito-borne disease, and the increases since then reflect the dynamics of the pathogens and vectors involved. Growth of tick-borne disease has been gradual: “Tick-borne pathogens rarely cause sudden epidemics because humans are typically incidental hosts who do not transmit further, and tick mobility is mostly limited to that of its animal hosts,” the researchers explained.
The number of mosquito-borne disease cases, on the other hand, varies considerably from year to year: There were 5,800 cases in 2015, almost 15,000 in 2013, and only 4,400 in 2011. Unlike ticks, which may feed on blood only once in a year, the more mobile mosquitoes feed every 48-72 hours and transmit their pathogens “directly between humans … resulting in explosive epidemics,” the investigators wrote.
SOURCE: Rosenberg R et al. MMWR 2018 May 4;67(17):496-501.
FROM MMWR
Don’t shorten therapy for older, sicker cellulitis patients
MADRID – An attempt to balance effective treatment with good antibiotic stewardship fell short when patients with cellulitis who got 6 days of flucloxacillin relapsed significantly sooner and more frequently than did those who received the standard 12 days of treatment.
While cellulitis cure rates at 14 and 28 days were similar between the two groups, 90-day relapse rates were significantly higher for those who took the 6-day course (23.5% vs. 6%), Duncan R. Cranendonk, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual conference. The cohort demographics perhaps played into this finding: Most of the group was elderly, hospitalized, and had comorbid conditions.
“However, this is the population clinicians are most likely to see,” said Dr Cranendonk of the University of Amsterdam. “It appears that therapy cannot be safely shortened in this population.”
In light of recent antibiotic trials showing that shorter courses can be as effective as prolonged treatment, Dr. Cranendonk and his colleagues conducted the DANCE (Duration of Antibiotic Therapy for Cellulitis) trial. The study investigated the efficacy of an abbreviated course of intravenous flucloxacillin among 248 patients with cellulitis admitted to 11 Dutch hospitals. At treatment day 6, those who had clinically improved after their initial treatment were randomized to 6 additional days of IV flucloxacillin or to placebo. The primary outcome was cure by day 14 without relapse by day 28.
A 2004 study successfully paved the way for DANCE, Dr. Cranendonk noted. That trial examined 5 versus 10 days of levofloxacin 500 mg for uncomplicated cellulitis in 87 patients. The outcome was positive: There was no significant difference in clinical outcome between the two arms, with a 98% cure rate in both groups.
However, Dr. Cranendonk noted, there were some important differences between the patients in that study and the DANCE cohort. They were, on the whole, younger and generally in better overall health. Also, only 15% of those patients were hospitalized for their infections, while all of the DANCE subjects were treated in the hospital.
Patients enrolled in DANCE were a mean of 62 years old, with a median 28 kg/m2 body mass index. About 40% had experienced cellulitis before, and 25% had diabetes. Most infections were on the leg (84%) and involved the lower leg or the lower leg and the foot. Fever was present in half of the group, lymphadenopathy in a third, and leukocytosis in 70%.
Upon enrollment, all 248 patients received 6 days of 1,000 mg/day IV flucloxacillin, with the option of a step-down to oral treatment (500 mg four times per day) at the treating physician’s discretion. At day 6, patients who were clinically improved (afebrile, no need to an antibiotic switch, no growth in blood culture, and improved symptoms of pain, ulceration, discharge, and fluctuance) were randomized to either another 6 days of flucloxacillin or placebo.
The primary endpoint was cure by day 14, with no relapse and no need for new antibiotics by day 28. The secondary endpoint was relapse by 90 days after initial cure.
After initial treatment, 151 patients entered the randomization phase. At 28 days, relapse-free cure rates were nearly identical: 49% of the 12-day group and 50% of the 6-day group. However, by 90 days, a significant difference became apparent: Patients who had received the 6-day course of flucloxacillin were significantly more likely to have experienced a relapse of cellulitis in the same region (23.5% vs. 6% in the 12-day group). A Kaplan-Meier analysis showed that these patients began to relapse as early as 35 days after the end of therapy. Most relapses occurred during days 60-90. The few relapses in the 12-day group occurred toward the end of the follow-up period, from day 75 onward.
Dr. Cranendonk said the investigation shows that older, less-healthy cellulitis patients can probably benefit from the longer course of antibiotics. “Short-term outcomes aren’t everything,” he noted.
He had no financial disclosures.
A video interview of Dr. Cranendock by ECCMID 2018 is available.
SOURCE: Cranendonk et al. ECCMID 2018, Abstract O1122
MADRID – An attempt to balance effective treatment with good antibiotic stewardship fell short when patients with cellulitis who got 6 days of flucloxacillin relapsed significantly sooner and more frequently than did those who received the standard 12 days of treatment.
While cellulitis cure rates at 14 and 28 days were similar between the two groups, 90-day relapse rates were significantly higher for those who took the 6-day course (23.5% vs. 6%), Duncan R. Cranendonk, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual conference. The cohort demographics perhaps played into this finding: Most of the group was elderly, hospitalized, and had comorbid conditions.
“However, this is the population clinicians are most likely to see,” said Dr Cranendonk of the University of Amsterdam. “It appears that therapy cannot be safely shortened in this population.”
In light of recent antibiotic trials showing that shorter courses can be as effective as prolonged treatment, Dr. Cranendonk and his colleagues conducted the DANCE (Duration of Antibiotic Therapy for Cellulitis) trial. The study investigated the efficacy of an abbreviated course of intravenous flucloxacillin among 248 patients with cellulitis admitted to 11 Dutch hospitals. At treatment day 6, those who had clinically improved after their initial treatment were randomized to 6 additional days of IV flucloxacillin or to placebo. The primary outcome was cure by day 14 without relapse by day 28.
A 2004 study successfully paved the way for DANCE, Dr. Cranendonk noted. That trial examined 5 versus 10 days of levofloxacin 500 mg for uncomplicated cellulitis in 87 patients. The outcome was positive: There was no significant difference in clinical outcome between the two arms, with a 98% cure rate in both groups.
However, Dr. Cranendonk noted, there were some important differences between the patients in that study and the DANCE cohort. They were, on the whole, younger and generally in better overall health. Also, only 15% of those patients were hospitalized for their infections, while all of the DANCE subjects were treated in the hospital.
Patients enrolled in DANCE were a mean of 62 years old, with a median 28 kg/m2 body mass index. About 40% had experienced cellulitis before, and 25% had diabetes. Most infections were on the leg (84%) and involved the lower leg or the lower leg and the foot. Fever was present in half of the group, lymphadenopathy in a third, and leukocytosis in 70%.
Upon enrollment, all 248 patients received 6 days of 1,000 mg/day IV flucloxacillin, with the option of a step-down to oral treatment (500 mg four times per day) at the treating physician’s discretion. At day 6, patients who were clinically improved (afebrile, no need to an antibiotic switch, no growth in blood culture, and improved symptoms of pain, ulceration, discharge, and fluctuance) were randomized to either another 6 days of flucloxacillin or placebo.
The primary endpoint was cure by day 14, with no relapse and no need for new antibiotics by day 28. The secondary endpoint was relapse by 90 days after initial cure.
After initial treatment, 151 patients entered the randomization phase. At 28 days, relapse-free cure rates were nearly identical: 49% of the 12-day group and 50% of the 6-day group. However, by 90 days, a significant difference became apparent: Patients who had received the 6-day course of flucloxacillin were significantly more likely to have experienced a relapse of cellulitis in the same region (23.5% vs. 6% in the 12-day group). A Kaplan-Meier analysis showed that these patients began to relapse as early as 35 days after the end of therapy. Most relapses occurred during days 60-90. The few relapses in the 12-day group occurred toward the end of the follow-up period, from day 75 onward.
Dr. Cranendonk said the investigation shows that older, less-healthy cellulitis patients can probably benefit from the longer course of antibiotics. “Short-term outcomes aren’t everything,” he noted.
He had no financial disclosures.
A video interview of Dr. Cranendock by ECCMID 2018 is available.
SOURCE: Cranendonk et al. ECCMID 2018, Abstract O1122
MADRID – An attempt to balance effective treatment with good antibiotic stewardship fell short when patients with cellulitis who got 6 days of flucloxacillin relapsed significantly sooner and more frequently than did those who received the standard 12 days of treatment.
While cellulitis cure rates at 14 and 28 days were similar between the two groups, 90-day relapse rates were significantly higher for those who took the 6-day course (23.5% vs. 6%), Duncan R. Cranendonk, MD, said at the European Congress of Clinical Microbiology and Infectious Diseases annual conference. The cohort demographics perhaps played into this finding: Most of the group was elderly, hospitalized, and had comorbid conditions.
“However, this is the population clinicians are most likely to see,” said Dr Cranendonk of the University of Amsterdam. “It appears that therapy cannot be safely shortened in this population.”
In light of recent antibiotic trials showing that shorter courses can be as effective as prolonged treatment, Dr. Cranendonk and his colleagues conducted the DANCE (Duration of Antibiotic Therapy for Cellulitis) trial. The study investigated the efficacy of an abbreviated course of intravenous flucloxacillin among 248 patients with cellulitis admitted to 11 Dutch hospitals. At treatment day 6, those who had clinically improved after their initial treatment were randomized to 6 additional days of IV flucloxacillin or to placebo. The primary outcome was cure by day 14 without relapse by day 28.
A 2004 study successfully paved the way for DANCE, Dr. Cranendonk noted. That trial examined 5 versus 10 days of levofloxacin 500 mg for uncomplicated cellulitis in 87 patients. The outcome was positive: There was no significant difference in clinical outcome between the two arms, with a 98% cure rate in both groups.
However, Dr. Cranendonk noted, there were some important differences between the patients in that study and the DANCE cohort. They were, on the whole, younger and generally in better overall health. Also, only 15% of those patients were hospitalized for their infections, while all of the DANCE subjects were treated in the hospital.
Patients enrolled in DANCE were a mean of 62 years old, with a median 28 kg/m2 body mass index. About 40% had experienced cellulitis before, and 25% had diabetes. Most infections were on the leg (84%) and involved the lower leg or the lower leg and the foot. Fever was present in half of the group, lymphadenopathy in a third, and leukocytosis in 70%.
Upon enrollment, all 248 patients received 6 days of 1,000 mg/day IV flucloxacillin, with the option of a step-down to oral treatment (500 mg four times per day) at the treating physician’s discretion. At day 6, patients who were clinically improved (afebrile, no need to an antibiotic switch, no growth in blood culture, and improved symptoms of pain, ulceration, discharge, and fluctuance) were randomized to either another 6 days of flucloxacillin or placebo.
The primary endpoint was cure by day 14, with no relapse and no need for new antibiotics by day 28. The secondary endpoint was relapse by 90 days after initial cure.
After initial treatment, 151 patients entered the randomization phase. At 28 days, relapse-free cure rates were nearly identical: 49% of the 12-day group and 50% of the 6-day group. However, by 90 days, a significant difference became apparent: Patients who had received the 6-day course of flucloxacillin were significantly more likely to have experienced a relapse of cellulitis in the same region (23.5% vs. 6% in the 12-day group). A Kaplan-Meier analysis showed that these patients began to relapse as early as 35 days after the end of therapy. Most relapses occurred during days 60-90. The few relapses in the 12-day group occurred toward the end of the follow-up period, from day 75 onward.
Dr. Cranendonk said the investigation shows that older, less-healthy cellulitis patients can probably benefit from the longer course of antibiotics. “Short-term outcomes aren’t everything,” he noted.
He had no financial disclosures.
A video interview of Dr. Cranendock by ECCMID 2018 is available.
SOURCE: Cranendonk et al. ECCMID 2018, Abstract O1122
REPORTING FROM ECCMID 2018
Key clinical point: Elderly patients with cellulitis and comorbid conditions probably need a full 12-day course of treatment.
Major finding: Three-month relapse rates were significantly higher in those who received 6 days of flucloxacillin than they were among those who received 12 days (23.5% vs. 6%).
Study details: Patients who improved on 6 days of treatment were randomized to either placebo or another 6 days of therapy.
Disclosures: Dr. Cranendonk had no financial disclosures.
Source: Cranendonk DR et al. ECCMID 2018, Abstract O1122
Class III obesity increases risk of acute on chronic liver failure in cirrhotic patients
Class III obesity was significantly, independently associated with acute on chronic liver failure (ACLF) in patients with decompensated cirrhosis, and patients with both class III obesity and acute on chronic liver failure also had a significant risk of renal failure, according to a recent retrospective analysis of two databases publised in the Journal of Hepatology.
Vinay Sundaram, MD, from Cedars-Sinai Medical Center in Los Angeles, and his colleagues evaluated 387,884 patients who were in the United Network for Organ Sharing (UNOS) during 2005-2016; were class I or II obese (body mass index 30-39 kg/m2), class III obese (BMI greater than or equal to 40), or not obese (BMI less than 30); and were on a wait list for liver transplantation.
They used the definition of ACLF outlined in the CANONIC (Consortium Acute on Chronic Liver Failure in Cirrhosis) study, which defined it as having “a single hepatic decompensation, such as ascites, hepatic encephalopathy, variceal bleed, or bacterial infection, and one of the following organ failures: single renal failure, single nonrenal organ failure with renal dysfunction or hepatic encephalopathy, or two nonrenal organ failures,” and confirmed the results in the Nationwide Inpatient Sample (NIS) databases by using diagnostic coding algorithms to identify factors such as hepatic decompensation, obesity, and ACLF in that study population.
Dr. Sundarem and his colleagues identified 116,704 patients (30.1%) with acute on chronic liver failure in both the UNOS and NIS databases. At the time of liver transplantation, there was a significant association between ACLF and class I and class II obesity (hazard ratio, 1.12; 95% confidence interval, 1.05-1.19; P less than .001) and class III obesity (HR, 1.24; 95% CI, 1.09-1.41; P less than .001). Other predictors of ACLF in this population were increased age (HR, 1.01 per year; 95% CI, 1.00-1.01; P = .037), hepatitis C virus (HR, 1.25; 95% CI, 1.16-1.35; P less than .001) and hepatitis C combined with alcoholic liver disease (HR, 1.18; 95% CI, 1.06-1.30; P = .002). Regarding organ failure, “renal insufficiency was similar among the three groups,” with increasing obesity class associated with a greater prevalence of renal failure.
“Given the heightened risk of renal failure among obese patients with cirrhosis, we suggest particularly careful management of this fragile population regarding diuretic usage, avoidance of nephrotoxic agents, and administration of an adequate albumin challenge in the setting of acute kidney injury,” the researchers wrote.
The researchers encouraged “an even greater emphasis on weight reduction” for class III obese patients. They noted the association between class III obesity and ACLF is likely caused by an “obesity-related chronic inflammatory state” and said future prospective studies should seek to describe the inflammatory pathways for each condition to predict risk of ACLF in these patients.
The authors reported having no financial disclosures.
SOURCE: Sundarem V et al. J Hepatol. 2018 April 27. doi: 10.1016/j.jhep.2018.04.016.
Class III obesity was significantly, independently associated with acute on chronic liver failure (ACLF) in patients with decompensated cirrhosis, and patients with both class III obesity and acute on chronic liver failure also had a significant risk of renal failure, according to a recent retrospective analysis of two databases publised in the Journal of Hepatology.
Vinay Sundaram, MD, from Cedars-Sinai Medical Center in Los Angeles, and his colleagues evaluated 387,884 patients who were in the United Network for Organ Sharing (UNOS) during 2005-2016; were class I or II obese (body mass index 30-39 kg/m2), class III obese (BMI greater than or equal to 40), or not obese (BMI less than 30); and were on a wait list for liver transplantation.
They used the definition of ACLF outlined in the CANONIC (Consortium Acute on Chronic Liver Failure in Cirrhosis) study, which defined it as having “a single hepatic decompensation, such as ascites, hepatic encephalopathy, variceal bleed, or bacterial infection, and one of the following organ failures: single renal failure, single nonrenal organ failure with renal dysfunction or hepatic encephalopathy, or two nonrenal organ failures,” and confirmed the results in the Nationwide Inpatient Sample (NIS) databases by using diagnostic coding algorithms to identify factors such as hepatic decompensation, obesity, and ACLF in that study population.
Dr. Sundarem and his colleagues identified 116,704 patients (30.1%) with acute on chronic liver failure in both the UNOS and NIS databases. At the time of liver transplantation, there was a significant association between ACLF and class I and class II obesity (hazard ratio, 1.12; 95% confidence interval, 1.05-1.19; P less than .001) and class III obesity (HR, 1.24; 95% CI, 1.09-1.41; P less than .001). Other predictors of ACLF in this population were increased age (HR, 1.01 per year; 95% CI, 1.00-1.01; P = .037), hepatitis C virus (HR, 1.25; 95% CI, 1.16-1.35; P less than .001) and hepatitis C combined with alcoholic liver disease (HR, 1.18; 95% CI, 1.06-1.30; P = .002). Regarding organ failure, “renal insufficiency was similar among the three groups,” with increasing obesity class associated with a greater prevalence of renal failure.
“Given the heightened risk of renal failure among obese patients with cirrhosis, we suggest particularly careful management of this fragile population regarding diuretic usage, avoidance of nephrotoxic agents, and administration of an adequate albumin challenge in the setting of acute kidney injury,” the researchers wrote.
The researchers encouraged “an even greater emphasis on weight reduction” for class III obese patients. They noted the association between class III obesity and ACLF is likely caused by an “obesity-related chronic inflammatory state” and said future prospective studies should seek to describe the inflammatory pathways for each condition to predict risk of ACLF in these patients.
The authors reported having no financial disclosures.
SOURCE: Sundarem V et al. J Hepatol. 2018 April 27. doi: 10.1016/j.jhep.2018.04.016.
Class III obesity was significantly, independently associated with acute on chronic liver failure (ACLF) in patients with decompensated cirrhosis, and patients with both class III obesity and acute on chronic liver failure also had a significant risk of renal failure, according to a recent retrospective analysis of two databases publised in the Journal of Hepatology.
Vinay Sundaram, MD, from Cedars-Sinai Medical Center in Los Angeles, and his colleagues evaluated 387,884 patients who were in the United Network for Organ Sharing (UNOS) during 2005-2016; were class I or II obese (body mass index 30-39 kg/m2), class III obese (BMI greater than or equal to 40), or not obese (BMI less than 30); and were on a wait list for liver transplantation.
They used the definition of ACLF outlined in the CANONIC (Consortium Acute on Chronic Liver Failure in Cirrhosis) study, which defined it as having “a single hepatic decompensation, such as ascites, hepatic encephalopathy, variceal bleed, or bacterial infection, and one of the following organ failures: single renal failure, single nonrenal organ failure with renal dysfunction or hepatic encephalopathy, or two nonrenal organ failures,” and confirmed the results in the Nationwide Inpatient Sample (NIS) databases by using diagnostic coding algorithms to identify factors such as hepatic decompensation, obesity, and ACLF in that study population.
Dr. Sundarem and his colleagues identified 116,704 patients (30.1%) with acute on chronic liver failure in both the UNOS and NIS databases. At the time of liver transplantation, there was a significant association between ACLF and class I and class II obesity (hazard ratio, 1.12; 95% confidence interval, 1.05-1.19; P less than .001) and class III obesity (HR, 1.24; 95% CI, 1.09-1.41; P less than .001). Other predictors of ACLF in this population were increased age (HR, 1.01 per year; 95% CI, 1.00-1.01; P = .037), hepatitis C virus (HR, 1.25; 95% CI, 1.16-1.35; P less than .001) and hepatitis C combined with alcoholic liver disease (HR, 1.18; 95% CI, 1.06-1.30; P = .002). Regarding organ failure, “renal insufficiency was similar among the three groups,” with increasing obesity class associated with a greater prevalence of renal failure.
“Given the heightened risk of renal failure among obese patients with cirrhosis, we suggest particularly careful management of this fragile population regarding diuretic usage, avoidance of nephrotoxic agents, and administration of an adequate albumin challenge in the setting of acute kidney injury,” the researchers wrote.
The researchers encouraged “an even greater emphasis on weight reduction” for class III obese patients. They noted the association between class III obesity and ACLF is likely caused by an “obesity-related chronic inflammatory state” and said future prospective studies should seek to describe the inflammatory pathways for each condition to predict risk of ACLF in these patients.
The authors reported having no financial disclosures.
SOURCE: Sundarem V et al. J Hepatol. 2018 April 27. doi: 10.1016/j.jhep.2018.04.016.
FROM THE JOURNAL OF HEPATOLOGY
Key clinical point: Patients with a BMI greater than or equal to 40 kg/m2 with decompensated cirrhosis are at greater risk of developing acute on chronic liver failure.
Major finding: Class III obesity carried a hazard ratio of 1.24 in the UNOS database and an odds ratio of 1.30 in the NIS database at the time of liver transplantation.
Data source: A retrospective cohort database study of 116,704 patients with acute on chronic liver failure listed during 2005-2016.
Disclosures: The authors reported having no financial disclosures.
Source: Sundaram V et al. J Hepatol. 2018 Apr 27. doi: 10.1016/j.jhep.2018.04.016.
First reversal agent for apixaban and rivaroxaban gets fast-track approval
, according to a May 3 statement from Portola Pharmaceuticals.
It is approved for use in patients treated with these factor Xa inhibitors when reversal of anticoagulation is needed because of life-threatening or uncontrolled bleeding, according to the company.
Andexanet alfa (Andexxa, Portola) received both U.S. Orphan Drug and FDA Breakthrough Therapy designations and was approved under the FDA’s Accelerated Approval pathway.
“Today’s approval represents a significant step forward in patient care and one that the medical community has been eagerly anticipating,” said Stuart J. Connolly, MD, professor of medicine and an electrophysiologist at McMaster University in Hamilton, Ont., who is chair of the ANNEXA-4 executive committee. “Andexxa’s rapid reversal of the anticoagulating effects of rivaroxaban and apixaban will help clinicians treat life-threatening bleeds, where every minute counts,” he added in the statement.
The approval was supported by two phase 3 trials in the ANNEXA series, which showed acceptable change from baseline in anti-Factor Xa activity in healthy volunteers. But the strongest data came from interim results from ANNEXA-4, a single-arm cohort study with 227 patients who were receiving a factor Xa inhibitor and were experiencing an acute major bleeding event.
Clinicians administered andexanet alfa as a bolus followed by a 2-hour continuous infusion, with hemostatic efficacy assessed 12 hours after the start of treatment. The results showed that factor Xa inhibition fell by a median 90% for rivaroxaban and 93% for apixaban.
Andexanet alfa is a factor Xa “decoy” molecule that acts by latching onto the inhibitor molecules and thereby preventing them from interacting with actual factor Xa, but andexanet also has a short half life and hence the effect quickly reduces once treatment stops, Dr. Connelly reported at the American College of Cardiology annual meeting in March when presenting ANNEXA-4.
He noted at the time the results placed andexanet in the same ballpark for efficacy and safety as idarucizumab (Praxbind) approved in 2015 for reversing the anticoagulant dabigatran (Pradaxa)
“The expansion of available reversal agents for people prescribed newer oral anticoagulant therapies is crucial,” Randy Fenninger, chief executive officer of the National Blood Clot Alliance, said in the Portola statement. “The availability now of a reversal agent specific to rivaroxaban and apixaban expands choice and enables patients and providers to consider these treatment options with greater confidence.”
The prescribing information for andexanet states that treated patients should be monitored for signs and symptoms of arterial and venous thromboembolic events, ischemic events, and cardiac arrest. Further, anticoagulant therapy should be resumed as soon as medically appropriate following andexanet treatment to reduce thromboembolic risk.
The most common adverse reactions, occurring in at least 5% of patients, were urinary tract infections and pneumonia.
Portola intends to bring Andexxa to limited markets in early June; a broader commercial launch is anticipated in early 2019.*
The FDA is requiring a postmarketing clinical trial that randomizes patients to either andexanet or usual care. The study is scheduled to begin in 2019 and report outcomes in 2023.
*This article was updated on May 7, 2018.
, according to a May 3 statement from Portola Pharmaceuticals.
It is approved for use in patients treated with these factor Xa inhibitors when reversal of anticoagulation is needed because of life-threatening or uncontrolled bleeding, according to the company.
Andexanet alfa (Andexxa, Portola) received both U.S. Orphan Drug and FDA Breakthrough Therapy designations and was approved under the FDA’s Accelerated Approval pathway.
“Today’s approval represents a significant step forward in patient care and one that the medical community has been eagerly anticipating,” said Stuart J. Connolly, MD, professor of medicine and an electrophysiologist at McMaster University in Hamilton, Ont., who is chair of the ANNEXA-4 executive committee. “Andexxa’s rapid reversal of the anticoagulating effects of rivaroxaban and apixaban will help clinicians treat life-threatening bleeds, where every minute counts,” he added in the statement.
The approval was supported by two phase 3 trials in the ANNEXA series, which showed acceptable change from baseline in anti-Factor Xa activity in healthy volunteers. But the strongest data came from interim results from ANNEXA-4, a single-arm cohort study with 227 patients who were receiving a factor Xa inhibitor and were experiencing an acute major bleeding event.
Clinicians administered andexanet alfa as a bolus followed by a 2-hour continuous infusion, with hemostatic efficacy assessed 12 hours after the start of treatment. The results showed that factor Xa inhibition fell by a median 90% for rivaroxaban and 93% for apixaban.
Andexanet alfa is a factor Xa “decoy” molecule that acts by latching onto the inhibitor molecules and thereby preventing them from interacting with actual factor Xa, but andexanet also has a short half life and hence the effect quickly reduces once treatment stops, Dr. Connelly reported at the American College of Cardiology annual meeting in March when presenting ANNEXA-4.
He noted at the time the results placed andexanet in the same ballpark for efficacy and safety as idarucizumab (Praxbind) approved in 2015 for reversing the anticoagulant dabigatran (Pradaxa)
“The expansion of available reversal agents for people prescribed newer oral anticoagulant therapies is crucial,” Randy Fenninger, chief executive officer of the National Blood Clot Alliance, said in the Portola statement. “The availability now of a reversal agent specific to rivaroxaban and apixaban expands choice and enables patients and providers to consider these treatment options with greater confidence.”
The prescribing information for andexanet states that treated patients should be monitored for signs and symptoms of arterial and venous thromboembolic events, ischemic events, and cardiac arrest. Further, anticoagulant therapy should be resumed as soon as medically appropriate following andexanet treatment to reduce thromboembolic risk.
The most common adverse reactions, occurring in at least 5% of patients, were urinary tract infections and pneumonia.
Portola intends to bring Andexxa to limited markets in early June; a broader commercial launch is anticipated in early 2019.*
The FDA is requiring a postmarketing clinical trial that randomizes patients to either andexanet or usual care. The study is scheduled to begin in 2019 and report outcomes in 2023.
*This article was updated on May 7, 2018.
, according to a May 3 statement from Portola Pharmaceuticals.
It is approved for use in patients treated with these factor Xa inhibitors when reversal of anticoagulation is needed because of life-threatening or uncontrolled bleeding, according to the company.
Andexanet alfa (Andexxa, Portola) received both U.S. Orphan Drug and FDA Breakthrough Therapy designations and was approved under the FDA’s Accelerated Approval pathway.
“Today’s approval represents a significant step forward in patient care and one that the medical community has been eagerly anticipating,” said Stuart J. Connolly, MD, professor of medicine and an electrophysiologist at McMaster University in Hamilton, Ont., who is chair of the ANNEXA-4 executive committee. “Andexxa’s rapid reversal of the anticoagulating effects of rivaroxaban and apixaban will help clinicians treat life-threatening bleeds, where every minute counts,” he added in the statement.
The approval was supported by two phase 3 trials in the ANNEXA series, which showed acceptable change from baseline in anti-Factor Xa activity in healthy volunteers. But the strongest data came from interim results from ANNEXA-4, a single-arm cohort study with 227 patients who were receiving a factor Xa inhibitor and were experiencing an acute major bleeding event.
Clinicians administered andexanet alfa as a bolus followed by a 2-hour continuous infusion, with hemostatic efficacy assessed 12 hours after the start of treatment. The results showed that factor Xa inhibition fell by a median 90% for rivaroxaban and 93% for apixaban.
Andexanet alfa is a factor Xa “decoy” molecule that acts by latching onto the inhibitor molecules and thereby preventing them from interacting with actual factor Xa, but andexanet also has a short half life and hence the effect quickly reduces once treatment stops, Dr. Connelly reported at the American College of Cardiology annual meeting in March when presenting ANNEXA-4.
He noted at the time the results placed andexanet in the same ballpark for efficacy and safety as idarucizumab (Praxbind) approved in 2015 for reversing the anticoagulant dabigatran (Pradaxa)
“The expansion of available reversal agents for people prescribed newer oral anticoagulant therapies is crucial,” Randy Fenninger, chief executive officer of the National Blood Clot Alliance, said in the Portola statement. “The availability now of a reversal agent specific to rivaroxaban and apixaban expands choice and enables patients and providers to consider these treatment options with greater confidence.”
The prescribing information for andexanet states that treated patients should be monitored for signs and symptoms of arterial and venous thromboembolic events, ischemic events, and cardiac arrest. Further, anticoagulant therapy should be resumed as soon as medically appropriate following andexanet treatment to reduce thromboembolic risk.
The most common adverse reactions, occurring in at least 5% of patients, were urinary tract infections and pneumonia.
Portola intends to bring Andexxa to limited markets in early June; a broader commercial launch is anticipated in early 2019.*
The FDA is requiring a postmarketing clinical trial that randomizes patients to either andexanet or usual care. The study is scheduled to begin in 2019 and report outcomes in 2023.
*This article was updated on May 7, 2018.
Lower glucose target linked to improved mortality in critically ill
compared with patients with a target of 90-140 mg/dL, according to results of a retrospective cohort analysis.
With the computerized intravenous insulin protocol used in the study, the strict target could be achieved with a low rate of hypoglycemia, the authors wrote. The analysis was published in the journal CHEST®.
However, it does raise the possibility that earlier investigations finding an association between intensive insulin therapy and excess mortality “may have been accurate only in the setting of technologies which led to high rates of severe hypoglycemia,” they wrote.
The retrospective cohort analysis by Dr. Hersh and his colleagues included 1,809 adult patients treated at three different ICUs in two hospitals between January 2010 and December 2015. Treatment was delivered with a computerized ICU insulin infusion protocol that allows clinicians to choose between two blood glucose targets: 80-110 mg/dL or 90-140 mg/dL. The lower target was chosen for 951 patients, and the moderate target for 858 patients.
The most common primary admission diagnoses in the cohort included chest pain or acute coronary syndrome in 43.3%, cardiothoracic surgery in 31.9%, heart failure (including cardiogenic shock) in 6.8%, and vascular surgery in 6.0%.
While patients in the low blood glucose target group had a higher rate of moderate hypoglycemia, both groups had a low rate of severe hypoglycemia, at 1.16% in the low target group and 0.35% in the moderate target group (P = .051).
Unadjusted 30-day mortality was significantly lower in the 80-110–mg/dL group compared with the 90-140–mg/dL group (4.3% vs. 9.2%, respectively; P less than .001), according to the investigators.
Furthermore, logistic regression analysis showed that patients treated with a target of 80-110 mg/dL had a lower risk of 30-day mortality compared with patients with a target of 90-140 mg/dL (odds ratio 0.65; 95% confidence interval, 0.43-0.98; P = .04).
These results advance the debate over appropriate blood glucose targets in critically ill patients, as they suggest that the effects of targeting blood glucose and the effects of severe hypoglycemia “can be separated,” the investigators wrote.
Current guidelines on intensive insulin therapy are based in part on findings of the NICE-SUGAR trial, which found that among adults treated in the ICU, intensive glucose control increased mortality. However, a post hoc analysis suggested the mortality increase in NICE-SUGAR was “largely driven by a significant incidence of moderate hypoglycemia, and to a greater degree severe hypoglycemia,” Dr. Hersh and his coauthors noted in their report.
“Given improvements in insulin delivery and glucose monitoring, a reassessment of potential benefits of [intensive insulin therapy] should once again be evaluated in a prospective randomized trial,” they wrote.
Dr. Hersh and his coauthors declared no financial or nonfinancial disclosures related to the study.
SOURCE: Hersh AM et al. CHEST 2018. doi: 10.1016/j.chest.2018.04.025.
compared with patients with a target of 90-140 mg/dL, according to results of a retrospective cohort analysis.
With the computerized intravenous insulin protocol used in the study, the strict target could be achieved with a low rate of hypoglycemia, the authors wrote. The analysis was published in the journal CHEST®.
However, it does raise the possibility that earlier investigations finding an association between intensive insulin therapy and excess mortality “may have been accurate only in the setting of technologies which led to high rates of severe hypoglycemia,” they wrote.
The retrospective cohort analysis by Dr. Hersh and his colleagues included 1,809 adult patients treated at three different ICUs in two hospitals between January 2010 and December 2015. Treatment was delivered with a computerized ICU insulin infusion protocol that allows clinicians to choose between two blood glucose targets: 80-110 mg/dL or 90-140 mg/dL. The lower target was chosen for 951 patients, and the moderate target for 858 patients.
The most common primary admission diagnoses in the cohort included chest pain or acute coronary syndrome in 43.3%, cardiothoracic surgery in 31.9%, heart failure (including cardiogenic shock) in 6.8%, and vascular surgery in 6.0%.
While patients in the low blood glucose target group had a higher rate of moderate hypoglycemia, both groups had a low rate of severe hypoglycemia, at 1.16% in the low target group and 0.35% in the moderate target group (P = .051).
Unadjusted 30-day mortality was significantly lower in the 80-110–mg/dL group compared with the 90-140–mg/dL group (4.3% vs. 9.2%, respectively; P less than .001), according to the investigators.
Furthermore, logistic regression analysis showed that patients treated with a target of 80-110 mg/dL had a lower risk of 30-day mortality compared with patients with a target of 90-140 mg/dL (odds ratio 0.65; 95% confidence interval, 0.43-0.98; P = .04).
These results advance the debate over appropriate blood glucose targets in critically ill patients, as they suggest that the effects of targeting blood glucose and the effects of severe hypoglycemia “can be separated,” the investigators wrote.
Current guidelines on intensive insulin therapy are based in part on findings of the NICE-SUGAR trial, which found that among adults treated in the ICU, intensive glucose control increased mortality. However, a post hoc analysis suggested the mortality increase in NICE-SUGAR was “largely driven by a significant incidence of moderate hypoglycemia, and to a greater degree severe hypoglycemia,” Dr. Hersh and his coauthors noted in their report.
“Given improvements in insulin delivery and glucose monitoring, a reassessment of potential benefits of [intensive insulin therapy] should once again be evaluated in a prospective randomized trial,” they wrote.
Dr. Hersh and his coauthors declared no financial or nonfinancial disclosures related to the study.
SOURCE: Hersh AM et al. CHEST 2018. doi: 10.1016/j.chest.2018.04.025.
compared with patients with a target of 90-140 mg/dL, according to results of a retrospective cohort analysis.
With the computerized intravenous insulin protocol used in the study, the strict target could be achieved with a low rate of hypoglycemia, the authors wrote. The analysis was published in the journal CHEST®.
However, it does raise the possibility that earlier investigations finding an association between intensive insulin therapy and excess mortality “may have been accurate only in the setting of technologies which led to high rates of severe hypoglycemia,” they wrote.
The retrospective cohort analysis by Dr. Hersh and his colleagues included 1,809 adult patients treated at three different ICUs in two hospitals between January 2010 and December 2015. Treatment was delivered with a computerized ICU insulin infusion protocol that allows clinicians to choose between two blood glucose targets: 80-110 mg/dL or 90-140 mg/dL. The lower target was chosen for 951 patients, and the moderate target for 858 patients.
The most common primary admission diagnoses in the cohort included chest pain or acute coronary syndrome in 43.3%, cardiothoracic surgery in 31.9%, heart failure (including cardiogenic shock) in 6.8%, and vascular surgery in 6.0%.
While patients in the low blood glucose target group had a higher rate of moderate hypoglycemia, both groups had a low rate of severe hypoglycemia, at 1.16% in the low target group and 0.35% in the moderate target group (P = .051).
Unadjusted 30-day mortality was significantly lower in the 80-110–mg/dL group compared with the 90-140–mg/dL group (4.3% vs. 9.2%, respectively; P less than .001), according to the investigators.
Furthermore, logistic regression analysis showed that patients treated with a target of 80-110 mg/dL had a lower risk of 30-day mortality compared with patients with a target of 90-140 mg/dL (odds ratio 0.65; 95% confidence interval, 0.43-0.98; P = .04).
These results advance the debate over appropriate blood glucose targets in critically ill patients, as they suggest that the effects of targeting blood glucose and the effects of severe hypoglycemia “can be separated,” the investigators wrote.
Current guidelines on intensive insulin therapy are based in part on findings of the NICE-SUGAR trial, which found that among adults treated in the ICU, intensive glucose control increased mortality. However, a post hoc analysis suggested the mortality increase in NICE-SUGAR was “largely driven by a significant incidence of moderate hypoglycemia, and to a greater degree severe hypoglycemia,” Dr. Hersh and his coauthors noted in their report.
“Given improvements in insulin delivery and glucose monitoring, a reassessment of potential benefits of [intensive insulin therapy] should once again be evaluated in a prospective randomized trial,” they wrote.
Dr. Hersh and his coauthors declared no financial or nonfinancial disclosures related to the study.
SOURCE: Hersh AM et al. CHEST 2018. doi: 10.1016/j.chest.2018.04.025.
FROM THE JOURNAL CHEST®
Key clinical point: Among critically ill cardiac and cardiothoracic patients, a lower glucose target was associated with improved 30-day mortality.
Major finding: Patients treated with a target of 80-110 mg/dL had a lower risk of 30-day mortality compared with patients with a target of 90-140 mg/dL (odds ratio 0.65; 95% confidence interval, 0.43-0.98; P = .04).
Study details: A retrospective cohort analysis of 1,809 adult patients treated at three ICUs from two hospitals between January 2010 and December 2015.
Disclosures: The authors declared no disclosures.
Source: Hersh AM et al. CHEST 2018. doi: 10.1016/j.chest.2018.04.025.