User login
FDA approves omadacycline for pneumonia and skin infections
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
The, for treating community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults, the manufacturer, Paratek, announced in a press release.
The company expects that omadacycline will be available in the first quarter of 2019. Administered once-daily in either oral or IV formulations, the antibiotic was effective and well tolerated across multiple trials, which altogether included almost 2,000 patients, according to Paratek. As part of the approval, the company has agreed to conduct postmarketing studies, specifically, more studies in CABP and in pediatric populations. “To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nuzyra and other antibacterial drugs, Nuzyra should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria,” according to a statement in the indications section of the prescribing information.
Omadacycline is contraindicated for patients with a known hypersensitivity to the drug or any members of the tetracycline class of antibacterial drugs; hypersensitivity reactions have been observed, so use should be discontinued if one is suspected. Use of this drug during later stages of pregnancy can lead to irreversible discoloration of the infant’s teeth and inhibition of bone growth; it should also not be used during breastfeeding.
Because omadacycline is structurally similar to tetracycline class drugs, some adverse reactions to those drugs may be seen with this one, such as photosensitivity, pseudotumor cerebri, and antianabolic action. Adverse reactions known to have an association with omadacycline include nausea, vomiting, hypertension, insomnia, diarrhea, constipation, and increases of alanine aminotransferase, aspartate aminotransferase, and/or gamma-glutamyl transferase.
Drug interactions may occur with anticoagulants, so dosage of those drugs may need to be reduced while treating with omadacycline. Antacids also are believed to have a drug interaction – specifically, impairing absorption of omadacycline
Perioperative diabetes and HbA1c in mortality
Clinical question: Do preoperative hemoglobin A1c (HbA1c) and perioperative glucose predict outcomes in patients undergoing noncardiac and cardiac surgeries?
Background: Hyperglycemia in the perioperative period has been associated with infection, delayed wound healing, and postoperative mortality. Studies have investigated the effects of HbA1c or hyperglycemia on postoperative outcomes, but none have been performed to assess the effect of one while controlling for the other.
Study design: Retrospective analysis.
Setting: Single-center, Duke University Health System.
Synopsis: Using a database of electronic health records at Duke University Health System, Durham, N.C., investigators reviewed 13,077 surgeries (6,684 noncardiac and 6,393 cardiac) to determine the association of preoperative HbA1c with perioperative glucose and 30-day mortality. For noncardiac surgery, increased average perioperative glucose was associated with increased mortality (P = .04). In cardiac surgery both low and high average glucose was associated with increased mortality (P = .001). By contrast, HbA1c was not a significant predictor of postoperative mortality in cardiac surgery (P = .08), and in noncardiac surgery, HbA1C was negatively associated with 30-day mortality (P = .01). Overall, perioperative glucose was predictive of 30-day mortality, but HbA1c was not associated with 30-day mortality after researchers controlled for glucose.
Because the study is retrospective, no causal relationship can be established. Hospitalists involved in perioperative care should aim for optimization of glucose control regardless of preoperative HbA1c.
Bottom line: Perioperative glucose is related to surgical outcomes, but HbA1c is a less useful indicator of 30-day postoperative mortality.
Citation: Van den Boom W et al. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018 Feb;41:782-8.
Clinical question: Do preoperative hemoglobin A1c (HbA1c) and perioperative glucose predict outcomes in patients undergoing noncardiac and cardiac surgeries?
Background: Hyperglycemia in the perioperative period has been associated with infection, delayed wound healing, and postoperative mortality. Studies have investigated the effects of HbA1c or hyperglycemia on postoperative outcomes, but none have been performed to assess the effect of one while controlling for the other.
Study design: Retrospective analysis.
Setting: Single-center, Duke University Health System.
Synopsis: Using a database of electronic health records at Duke University Health System, Durham, N.C., investigators reviewed 13,077 surgeries (6,684 noncardiac and 6,393 cardiac) to determine the association of preoperative HbA1c with perioperative glucose and 30-day mortality. For noncardiac surgery, increased average perioperative glucose was associated with increased mortality (P = .04). In cardiac surgery both low and high average glucose was associated with increased mortality (P = .001). By contrast, HbA1c was not a significant predictor of postoperative mortality in cardiac surgery (P = .08), and in noncardiac surgery, HbA1C was negatively associated with 30-day mortality (P = .01). Overall, perioperative glucose was predictive of 30-day mortality, but HbA1c was not associated with 30-day mortality after researchers controlled for glucose.
Because the study is retrospective, no causal relationship can be established. Hospitalists involved in perioperative care should aim for optimization of glucose control regardless of preoperative HbA1c.
Bottom line: Perioperative glucose is related to surgical outcomes, but HbA1c is a less useful indicator of 30-day postoperative mortality.
Citation: Van den Boom W et al. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018 Feb;41:782-8.
Clinical question: Do preoperative hemoglobin A1c (HbA1c) and perioperative glucose predict outcomes in patients undergoing noncardiac and cardiac surgeries?
Background: Hyperglycemia in the perioperative period has been associated with infection, delayed wound healing, and postoperative mortality. Studies have investigated the effects of HbA1c or hyperglycemia on postoperative outcomes, but none have been performed to assess the effect of one while controlling for the other.
Study design: Retrospective analysis.
Setting: Single-center, Duke University Health System.
Synopsis: Using a database of electronic health records at Duke University Health System, Durham, N.C., investigators reviewed 13,077 surgeries (6,684 noncardiac and 6,393 cardiac) to determine the association of preoperative HbA1c with perioperative glucose and 30-day mortality. For noncardiac surgery, increased average perioperative glucose was associated with increased mortality (P = .04). In cardiac surgery both low and high average glucose was associated with increased mortality (P = .001). By contrast, HbA1c was not a significant predictor of postoperative mortality in cardiac surgery (P = .08), and in noncardiac surgery, HbA1C was negatively associated with 30-day mortality (P = .01). Overall, perioperative glucose was predictive of 30-day mortality, but HbA1c was not associated with 30-day mortality after researchers controlled for glucose.
Because the study is retrospective, no causal relationship can be established. Hospitalists involved in perioperative care should aim for optimization of glucose control regardless of preoperative HbA1c.
Bottom line: Perioperative glucose is related to surgical outcomes, but HbA1c is a less useful indicator of 30-day postoperative mortality.
Citation: Van den Boom W et al. Effect of A1C and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018 Feb;41:782-8.
Predicting failure of nonoperative management of spinal epidural abscess
Clinical question: Can one predict whether nonoperative management of spinal epidural abscesses will fail?
Background: Even though spinal epidural abscesses have a low incidence and nonspecific presentation, a delay in treatment can lead to significant morbidity. Previously, operative management was the preferred treatment; however, improvements in imaging and timing of diagnosis have led to an increased interest in nonoperative management. Few studies have identified possible predictors of failure for nonoperative management, and no algorithm exists for weighing the different possible predictors with the outcome of nonoperative management failure.
Study design: Retrospective cohort study.
Setting: A Massachusetts hospital system with two tertiary academic medical centers and three regional community hospitals.
Synopsis: The study evaluated 1,053 patients admitted with a spinal epidural abscess during 1993-2016. Of these, 432 patients were managed nonoperatively, and 367 were included in the analysis. Failure of nonoperative management occurred in 99 patients (27%). These patients were compared with 266 patients with successful nonoperative management with more than 60 days of follow-up. Six independent factors were associated with failure of nonoperative management including motor deficit at presentation (odds ratio, 7.85), pathological or compression fractures (OR, 6.12), active malignancy (OR, 3.32), diabetes (OR, 2.92), sensory changes at presentation (3.48), and location of the abscess dorsal to the thecal sac (OR, 0.29). Subsequently, a clinical algorithm was created to predict the likelihood of failure of nonoperative management.
Because of its retrospective design, the study was unable to assess the efficacy of surgery versus nonoperative management.
Bottom line: Specific measures of general health, neurologic status at presentation, and anatomical data of a patient with a spinal epidural abscess have led to the development of a clinical algorithm to determine the risk of failure in nonoperative management of spinal epidural abscesses.
Citation: Shah AA et al. Nonoperative management of spinal epidural abscess: Development of a predictive algorithm for failure. J Bone Joint Surg Am. 2018;100(7):546-55.
Dr. Tsien is a hospitalist in the division of hospital medicine in the department of medicine at Loyola University Chicago, Maywood, Ill.
Clinical question: Can one predict whether nonoperative management of spinal epidural abscesses will fail?
Background: Even though spinal epidural abscesses have a low incidence and nonspecific presentation, a delay in treatment can lead to significant morbidity. Previously, operative management was the preferred treatment; however, improvements in imaging and timing of diagnosis have led to an increased interest in nonoperative management. Few studies have identified possible predictors of failure for nonoperative management, and no algorithm exists for weighing the different possible predictors with the outcome of nonoperative management failure.
Study design: Retrospective cohort study.
Setting: A Massachusetts hospital system with two tertiary academic medical centers and three regional community hospitals.
Synopsis: The study evaluated 1,053 patients admitted with a spinal epidural abscess during 1993-2016. Of these, 432 patients were managed nonoperatively, and 367 were included in the analysis. Failure of nonoperative management occurred in 99 patients (27%). These patients were compared with 266 patients with successful nonoperative management with more than 60 days of follow-up. Six independent factors were associated with failure of nonoperative management including motor deficit at presentation (odds ratio, 7.85), pathological or compression fractures (OR, 6.12), active malignancy (OR, 3.32), diabetes (OR, 2.92), sensory changes at presentation (3.48), and location of the abscess dorsal to the thecal sac (OR, 0.29). Subsequently, a clinical algorithm was created to predict the likelihood of failure of nonoperative management.
Because of its retrospective design, the study was unable to assess the efficacy of surgery versus nonoperative management.
Bottom line: Specific measures of general health, neurologic status at presentation, and anatomical data of a patient with a spinal epidural abscess have led to the development of a clinical algorithm to determine the risk of failure in nonoperative management of spinal epidural abscesses.
Citation: Shah AA et al. Nonoperative management of spinal epidural abscess: Development of a predictive algorithm for failure. J Bone Joint Surg Am. 2018;100(7):546-55.
Dr. Tsien is a hospitalist in the division of hospital medicine in the department of medicine at Loyola University Chicago, Maywood, Ill.
Clinical question: Can one predict whether nonoperative management of spinal epidural abscesses will fail?
Background: Even though spinal epidural abscesses have a low incidence and nonspecific presentation, a delay in treatment can lead to significant morbidity. Previously, operative management was the preferred treatment; however, improvements in imaging and timing of diagnosis have led to an increased interest in nonoperative management. Few studies have identified possible predictors of failure for nonoperative management, and no algorithm exists for weighing the different possible predictors with the outcome of nonoperative management failure.
Study design: Retrospective cohort study.
Setting: A Massachusetts hospital system with two tertiary academic medical centers and three regional community hospitals.
Synopsis: The study evaluated 1,053 patients admitted with a spinal epidural abscess during 1993-2016. Of these, 432 patients were managed nonoperatively, and 367 were included in the analysis. Failure of nonoperative management occurred in 99 patients (27%). These patients were compared with 266 patients with successful nonoperative management with more than 60 days of follow-up. Six independent factors were associated with failure of nonoperative management including motor deficit at presentation (odds ratio, 7.85), pathological or compression fractures (OR, 6.12), active malignancy (OR, 3.32), diabetes (OR, 2.92), sensory changes at presentation (3.48), and location of the abscess dorsal to the thecal sac (OR, 0.29). Subsequently, a clinical algorithm was created to predict the likelihood of failure of nonoperative management.
Because of its retrospective design, the study was unable to assess the efficacy of surgery versus nonoperative management.
Bottom line: Specific measures of general health, neurologic status at presentation, and anatomical data of a patient with a spinal epidural abscess have led to the development of a clinical algorithm to determine the risk of failure in nonoperative management of spinal epidural abscesses.
Citation: Shah AA et al. Nonoperative management of spinal epidural abscess: Development of a predictive algorithm for failure. J Bone Joint Surg Am. 2018;100(7):546-55.
Dr. Tsien is a hospitalist in the division of hospital medicine in the department of medicine at Loyola University Chicago, Maywood, Ill.
SPRINT: Pill burden affects ability to reach systolic BP control
CHICAGO –
“Five or more medications” meant total drug burden, both for hypertension and comorbidities. “When you are treating a patient for hypertension, you care about blood pressure, but you also need to care about” what else they are on, and their total drug burden, “because it affects their ability to get to their blood pressure goal, especially if you’re targeting intensive control,” said lead investigator Catherine Derington, PharmD, a postdoctoral fellow at the University of Colorado, Aurora.
Good old-fashioned exercise and weight loss remain potent non–pill options, she noted at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The take-home message is to use more combination drugs for hypertension “to get patients on fewer pills.” Also, “eliminate drugs [patients] don’t need,” said SPRINT investigator William C. Cushman, MD, professor of medicine and physiology at the University of Tennessee, Memphis, when asked what he thought of the new findings.
SPRINT [Systolic Blood Pressure Intervention Trial] found that targeting systolic blood pressures below 120 mm Hg, as compared with less than 140 mm Hg, led to lower rates of MI, stroke, heart failure, and death from any cause.
Medication burden had no effect on patients in the 140 mm Hg group; they achieved a mean systolic blood pressure (SBP) of 136 mm Hg at 1 year, whether they were on fewer than five drugs a day or more. Hitting that target took an average of 1.8 hypertension medications.
Reaching 120 mm Hg generally took one extra drug (an average of 2.8), and the overall pill burden did matter; 2,463 patients in the intensive arm on fewer than five medications dropped their mean SBP 20 mm Hg at one year, while 1,698 taking more than five had a 15–mm Hg reduction. The group with the lower pill burden had a mean SBP of 120.6 mm Hg and those taking five or more had a mean SBP of 122.5 mm Hg. It’s a small difference but likely important.
Comorbidities in SPRINT included kidney and cardiovascular disease, among others, but the trial excluded patients with diabetes. Many patients were on statins and aspirin.
Pharmacy review is especially a good idea in the elderly.
Patients on five or more medications had higher rates of significant adverse events in the post hoc analysis (about 50% versus about 30%), including hypotension, syncope, electrolyte abnormalities, and acute kidney injury, regardless if they were in the 120–mm Hg or 140–mm Hg group.
About 45% in the intensive arm reported high adherence (Morisky Medication Adherence Scale-8 score greater than 8 at 12 months); medication burden made no difference. Oddly, in the standard-treatment arm, more patients on five or more drugs reported high adherence, 44.5% versus 38.1% among patients taking fewer,
The post hoc analysis was based on pill bottle review at baseline. Medication count did not affect hypertension treatment satisfaction, which was about 84% in the intensive and 77% in the standard arm.
There was no industry funding for the work. Dr. Derington didn’t have any disclosures.
CHICAGO –
“Five or more medications” meant total drug burden, both for hypertension and comorbidities. “When you are treating a patient for hypertension, you care about blood pressure, but you also need to care about” what else they are on, and their total drug burden, “because it affects their ability to get to their blood pressure goal, especially if you’re targeting intensive control,” said lead investigator Catherine Derington, PharmD, a postdoctoral fellow at the University of Colorado, Aurora.
Good old-fashioned exercise and weight loss remain potent non–pill options, she noted at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The take-home message is to use more combination drugs for hypertension “to get patients on fewer pills.” Also, “eliminate drugs [patients] don’t need,” said SPRINT investigator William C. Cushman, MD, professor of medicine and physiology at the University of Tennessee, Memphis, when asked what he thought of the new findings.
SPRINT [Systolic Blood Pressure Intervention Trial] found that targeting systolic blood pressures below 120 mm Hg, as compared with less than 140 mm Hg, led to lower rates of MI, stroke, heart failure, and death from any cause.
Medication burden had no effect on patients in the 140 mm Hg group; they achieved a mean systolic blood pressure (SBP) of 136 mm Hg at 1 year, whether they were on fewer than five drugs a day or more. Hitting that target took an average of 1.8 hypertension medications.
Reaching 120 mm Hg generally took one extra drug (an average of 2.8), and the overall pill burden did matter; 2,463 patients in the intensive arm on fewer than five medications dropped their mean SBP 20 mm Hg at one year, while 1,698 taking more than five had a 15–mm Hg reduction. The group with the lower pill burden had a mean SBP of 120.6 mm Hg and those taking five or more had a mean SBP of 122.5 mm Hg. It’s a small difference but likely important.
Comorbidities in SPRINT included kidney and cardiovascular disease, among others, but the trial excluded patients with diabetes. Many patients were on statins and aspirin.
Pharmacy review is especially a good idea in the elderly.
Patients on five or more medications had higher rates of significant adverse events in the post hoc analysis (about 50% versus about 30%), including hypotension, syncope, electrolyte abnormalities, and acute kidney injury, regardless if they were in the 120–mm Hg or 140–mm Hg group.
About 45% in the intensive arm reported high adherence (Morisky Medication Adherence Scale-8 score greater than 8 at 12 months); medication burden made no difference. Oddly, in the standard-treatment arm, more patients on five or more drugs reported high adherence, 44.5% versus 38.1% among patients taking fewer,
The post hoc analysis was based on pill bottle review at baseline. Medication count did not affect hypertension treatment satisfaction, which was about 84% in the intensive and 77% in the standard arm.
There was no industry funding for the work. Dr. Derington didn’t have any disclosures.
CHICAGO –
“Five or more medications” meant total drug burden, both for hypertension and comorbidities. “When you are treating a patient for hypertension, you care about blood pressure, but you also need to care about” what else they are on, and their total drug burden, “because it affects their ability to get to their blood pressure goal, especially if you’re targeting intensive control,” said lead investigator Catherine Derington, PharmD, a postdoctoral fellow at the University of Colorado, Aurora.
Good old-fashioned exercise and weight loss remain potent non–pill options, she noted at the joint scientific sessions of AHA Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The take-home message is to use more combination drugs for hypertension “to get patients on fewer pills.” Also, “eliminate drugs [patients] don’t need,” said SPRINT investigator William C. Cushman, MD, professor of medicine and physiology at the University of Tennessee, Memphis, when asked what he thought of the new findings.
SPRINT [Systolic Blood Pressure Intervention Trial] found that targeting systolic blood pressures below 120 mm Hg, as compared with less than 140 mm Hg, led to lower rates of MI, stroke, heart failure, and death from any cause.
Medication burden had no effect on patients in the 140 mm Hg group; they achieved a mean systolic blood pressure (SBP) of 136 mm Hg at 1 year, whether they were on fewer than five drugs a day or more. Hitting that target took an average of 1.8 hypertension medications.
Reaching 120 mm Hg generally took one extra drug (an average of 2.8), and the overall pill burden did matter; 2,463 patients in the intensive arm on fewer than five medications dropped their mean SBP 20 mm Hg at one year, while 1,698 taking more than five had a 15–mm Hg reduction. The group with the lower pill burden had a mean SBP of 120.6 mm Hg and those taking five or more had a mean SBP of 122.5 mm Hg. It’s a small difference but likely important.
Comorbidities in SPRINT included kidney and cardiovascular disease, among others, but the trial excluded patients with diabetes. Many patients were on statins and aspirin.
Pharmacy review is especially a good idea in the elderly.
Patients on five or more medications had higher rates of significant adverse events in the post hoc analysis (about 50% versus about 30%), including hypotension, syncope, electrolyte abnormalities, and acute kidney injury, regardless if they were in the 120–mm Hg or 140–mm Hg group.
About 45% in the intensive arm reported high adherence (Morisky Medication Adherence Scale-8 score greater than 8 at 12 months); medication burden made no difference. Oddly, in the standard-treatment arm, more patients on five or more drugs reported high adherence, 44.5% versus 38.1% among patients taking fewer,
The post hoc analysis was based on pill bottle review at baseline. Medication count did not affect hypertension treatment satisfaction, which was about 84% in the intensive and 77% in the standard arm.
There was no industry funding for the work. Dr. Derington didn’t have any disclosures.
REPORTING FROM JOINT HYPERTENSION 2018
Key clinical point: Consider overall pill burden when managing hypertension.
Major finding: Patients in the SPRINT trial were less likely to meet the intensive treatment goal – systolic blood pressure below 120 mm Hg – if they were taking five or more medications a day.
Study details: Post hoc analysis of the SPRINT trial
Disclosures: There was no industry funding, and the lead investigator didn’t have any disclosures.
Source: Derington C et al. Joint Hypertension 2018, Abstract P208.
Low-dose ketamine controls pain from severe chest injury, while sparing opioid consumption
SAN DIEGO – while reducing opioid consumption.
The anesthetic didn’t make much difference in pain control or opioid use overall in a randomized study of 93 patients with thoracic injury Nathan Kugler, MD, said at the annual meeting of the American Association for the Surgery of Trauma. But among severely injured patients, it cut the opioid mean equivalency dose by about 164 mg over the 48-hour infusion and by 328 mg over a mean hospital stay while maintaining pain control, said Dr. Kugler, a surgical resident at the Medical College of Wisconsin, Milwaukee.
“With increasing focus on multimodal pain strategies, opioid-based regimens continue to be the backbone of pain control,” he said. “We have used ketamine effectively for failure of maximum therapy and demonstrated an opioid-sparing effect.” This new research shows that the drug can be an effective adjunct for acute pain control for severely injured patients in the emergency setting.
The study recruited 93 patients with thoracic injury; they had a mean of six broken ribs, mostly caused by motor vehicle accidents. Most of the patients were male (75%), and their mean age was 46 years. The mean Injury Severity Score was about 15; about 30% had flail chest.
All patients received a standardized acute pain medication regime comprising acetaminophen, nonsteroidal anti-inflammatories, methocarbamol (Robaxin), and intravenous opioids. Regional therapies included rib block with an epidural catheter. In addition, they were randomized to placebo infusions or to 48 hours of IV ketamine at 2.5 mcg/kg per minute. “To put this in perspective, for a 70-kg patient, that is a mean of 10.5 mg/hour,” Dr. Kugler said.
The primary endpoint was a reduction of at least 2 points on an 11-point pain scale. Secondary endpoints included opioid use in oral morphine equivalents (OME); respiratory complications; and psychoactive events. The primary outcome was assessed with an area under the curve model.
In the overall group, there was no significant between-group difference in pain score. Nor were there differences in the total OME at 12-24 hours (184 mg ketamine vs. 230 mg placebo), or at 48 hours (86 vs. 113 mg).
Dr. Kugler also looked at these outcomes in patients who had only rib fractures independent of other chest injury. He saw no significant differences in pain scores or OME at 24 or 48 hours.
However, significant differences did emerge in the group of severely injured patients with an Injury Severity Score of more than 15. There were no differences in pain scores at either time point. However, ketamine allowed patients to achieve the same level of pain control with significantly less opioid medication. The OME at 12-24 hours was 50.5 mg vs 94 mg. At 24-48 hours, it was 87 mg vs. 64 mg.
This worked out to a mean OME savings of 148 mg over a patient’s entire hospitalization.
“We saw a very nice separation of opioid consumption that began early and continued to separate over the 48-hour infusion and even after it was discontinued,” Dr. Kugler said.
This benefit was achieved without any additional adverse events, he added. There were no significant differences in confusion; epidural placement; length of stay; respiratory event, sedation, hallucinations, delusions or disturbing dreams; or unplanned transfers to the ICU.
Dr. Kugler disclosed that he and primary investigator Thomas Carver, MD, also of the Medical College of Wisconsin, Milwaukee, are both paid consultants for InnoVital Systems.
SAN DIEGO – while reducing opioid consumption.
The anesthetic didn’t make much difference in pain control or opioid use overall in a randomized study of 93 patients with thoracic injury Nathan Kugler, MD, said at the annual meeting of the American Association for the Surgery of Trauma. But among severely injured patients, it cut the opioid mean equivalency dose by about 164 mg over the 48-hour infusion and by 328 mg over a mean hospital stay while maintaining pain control, said Dr. Kugler, a surgical resident at the Medical College of Wisconsin, Milwaukee.
“With increasing focus on multimodal pain strategies, opioid-based regimens continue to be the backbone of pain control,” he said. “We have used ketamine effectively for failure of maximum therapy and demonstrated an opioid-sparing effect.” This new research shows that the drug can be an effective adjunct for acute pain control for severely injured patients in the emergency setting.
The study recruited 93 patients with thoracic injury; they had a mean of six broken ribs, mostly caused by motor vehicle accidents. Most of the patients were male (75%), and their mean age was 46 years. The mean Injury Severity Score was about 15; about 30% had flail chest.
All patients received a standardized acute pain medication regime comprising acetaminophen, nonsteroidal anti-inflammatories, methocarbamol (Robaxin), and intravenous opioids. Regional therapies included rib block with an epidural catheter. In addition, they were randomized to placebo infusions or to 48 hours of IV ketamine at 2.5 mcg/kg per minute. “To put this in perspective, for a 70-kg patient, that is a mean of 10.5 mg/hour,” Dr. Kugler said.
The primary endpoint was a reduction of at least 2 points on an 11-point pain scale. Secondary endpoints included opioid use in oral morphine equivalents (OME); respiratory complications; and psychoactive events. The primary outcome was assessed with an area under the curve model.
In the overall group, there was no significant between-group difference in pain score. Nor were there differences in the total OME at 12-24 hours (184 mg ketamine vs. 230 mg placebo), or at 48 hours (86 vs. 113 mg).
Dr. Kugler also looked at these outcomes in patients who had only rib fractures independent of other chest injury. He saw no significant differences in pain scores or OME at 24 or 48 hours.
However, significant differences did emerge in the group of severely injured patients with an Injury Severity Score of more than 15. There were no differences in pain scores at either time point. However, ketamine allowed patients to achieve the same level of pain control with significantly less opioid medication. The OME at 12-24 hours was 50.5 mg vs 94 mg. At 24-48 hours, it was 87 mg vs. 64 mg.
This worked out to a mean OME savings of 148 mg over a patient’s entire hospitalization.
“We saw a very nice separation of opioid consumption that began early and continued to separate over the 48-hour infusion and even after it was discontinued,” Dr. Kugler said.
This benefit was achieved without any additional adverse events, he added. There were no significant differences in confusion; epidural placement; length of stay; respiratory event, sedation, hallucinations, delusions or disturbing dreams; or unplanned transfers to the ICU.
Dr. Kugler disclosed that he and primary investigator Thomas Carver, MD, also of the Medical College of Wisconsin, Milwaukee, are both paid consultants for InnoVital Systems.
SAN DIEGO – while reducing opioid consumption.
The anesthetic didn’t make much difference in pain control or opioid use overall in a randomized study of 93 patients with thoracic injury Nathan Kugler, MD, said at the annual meeting of the American Association for the Surgery of Trauma. But among severely injured patients, it cut the opioid mean equivalency dose by about 164 mg over the 48-hour infusion and by 328 mg over a mean hospital stay while maintaining pain control, said Dr. Kugler, a surgical resident at the Medical College of Wisconsin, Milwaukee.
“With increasing focus on multimodal pain strategies, opioid-based regimens continue to be the backbone of pain control,” he said. “We have used ketamine effectively for failure of maximum therapy and demonstrated an opioid-sparing effect.” This new research shows that the drug can be an effective adjunct for acute pain control for severely injured patients in the emergency setting.
The study recruited 93 patients with thoracic injury; they had a mean of six broken ribs, mostly caused by motor vehicle accidents. Most of the patients were male (75%), and their mean age was 46 years. The mean Injury Severity Score was about 15; about 30% had flail chest.
All patients received a standardized acute pain medication regime comprising acetaminophen, nonsteroidal anti-inflammatories, methocarbamol (Robaxin), and intravenous opioids. Regional therapies included rib block with an epidural catheter. In addition, they were randomized to placebo infusions or to 48 hours of IV ketamine at 2.5 mcg/kg per minute. “To put this in perspective, for a 70-kg patient, that is a mean of 10.5 mg/hour,” Dr. Kugler said.
The primary endpoint was a reduction of at least 2 points on an 11-point pain scale. Secondary endpoints included opioid use in oral morphine equivalents (OME); respiratory complications; and psychoactive events. The primary outcome was assessed with an area under the curve model.
In the overall group, there was no significant between-group difference in pain score. Nor were there differences in the total OME at 12-24 hours (184 mg ketamine vs. 230 mg placebo), or at 48 hours (86 vs. 113 mg).
Dr. Kugler also looked at these outcomes in patients who had only rib fractures independent of other chest injury. He saw no significant differences in pain scores or OME at 24 or 48 hours.
However, significant differences did emerge in the group of severely injured patients with an Injury Severity Score of more than 15. There were no differences in pain scores at either time point. However, ketamine allowed patients to achieve the same level of pain control with significantly less opioid medication. The OME at 12-24 hours was 50.5 mg vs 94 mg. At 24-48 hours, it was 87 mg vs. 64 mg.
This worked out to a mean OME savings of 148 mg over a patient’s entire hospitalization.
“We saw a very nice separation of opioid consumption that began early and continued to separate over the 48-hour infusion and even after it was discontinued,” Dr. Kugler said.
This benefit was achieved without any additional adverse events, he added. There were no significant differences in confusion; epidural placement; length of stay; respiratory event, sedation, hallucinations, delusions or disturbing dreams; or unplanned transfers to the ICU.
Dr. Kugler disclosed that he and primary investigator Thomas Carver, MD, also of the Medical College of Wisconsin, Milwaukee, are both paid consultants for InnoVital Systems.
REPORTING FROM THE AAST ANNUAL MEETING
Key clinical point: Low-dose ketamine controlled pain while reducing opioid use among patients with severe thoracic injury.
Major finding: Compared with placebo, ketamine reduced opioids conferred OME savings of 148 mg over a patient’s entire hospitalization.
Study details: The randomized study comprised 93 patients with thoracic injury.
Disclosures: Dr. Kugler disclosed that he and primary investigator Thomas Carver, MD, are both paid consultants for InnoVital Systems.
Source: Carver T et al. AAST 2018, Oral abstract 2
Palliative care consultations reduce hospital costs
Background: Health care costs are on the rise, and previous studies have found that PCC can reduce hospital costs. Timing of consultation and allocation of palliative care intervention to a certain population of patients may reveal a more significant cost reduction.
Study design: Meta-analysis.
Setting: English peer reviewed articles.
Synopsis: A systematic search was performed for articles that provided economic evaluation of PCC for adult inpatients in acute care hospitals. Patients were included if they had least one of seven conditions: cancer, heart failure, liver failure, kidney failure, chronic obstructive pulmonary disease, AIDS/HIV, or neurodegenerative conditions. Six data sets were reviewed, which included 133,118 patients altogether. There was a significant reduction in costs with PCC within 3 days of admission, regardless of the diagnosis (–$3,237; 95% confidence interval, –$3,581 to –$2,893). In the stratified analysis, the pooled meta-analysis suggested a statistically significant reduction in costs for both cancer (–$4,251; 95% CI, –$4,664 to –$3,837; P less than .001) and noncancer (–$2,105; 95% CI, –$2,698 to –$1,511; P less than .001) subsamples. In patients with cancer, the treatment effect was greater for patients with four or more comorbidities than it was for those with two or fewer.
Only six samples were evaluated, and causation could not be established because all samples had observational designs. There also was potential interpretation bias because the private investigator for each of the samples contributed to interpretation of the data and participated as an author. Overall evaluation of the economic value of PCC in this study was limited because analysis was focused to a single index hospital admission rather than including additional hospitalizations and outpatient costs.
Bottom line: Acute care hospitals might reduce hospital costs by increasing resources to allow palliative care consultations in patients with serious illnesses.
Citation: May P et al. Economics of palliative care for hospitalized adults with serious illness. JAMA Intern Med. 2018;178(6):820-9.
Dr. Libot is a hospitalist in the division of hospital medicine in the department of medicine at Loyola University Chicago, Maywood, Ill.
Background: Health care costs are on the rise, and previous studies have found that PCC can reduce hospital costs. Timing of consultation and allocation of palliative care intervention to a certain population of patients may reveal a more significant cost reduction.
Study design: Meta-analysis.
Setting: English peer reviewed articles.
Synopsis: A systematic search was performed for articles that provided economic evaluation of PCC for adult inpatients in acute care hospitals. Patients were included if they had least one of seven conditions: cancer, heart failure, liver failure, kidney failure, chronic obstructive pulmonary disease, AIDS/HIV, or neurodegenerative conditions. Six data sets were reviewed, which included 133,118 patients altogether. There was a significant reduction in costs with PCC within 3 days of admission, regardless of the diagnosis (–$3,237; 95% confidence interval, –$3,581 to –$2,893). In the stratified analysis, the pooled meta-analysis suggested a statistically significant reduction in costs for both cancer (–$4,251; 95% CI, –$4,664 to –$3,837; P less than .001) and noncancer (–$2,105; 95% CI, –$2,698 to –$1,511; P less than .001) subsamples. In patients with cancer, the treatment effect was greater for patients with four or more comorbidities than it was for those with two or fewer.
Only six samples were evaluated, and causation could not be established because all samples had observational designs. There also was potential interpretation bias because the private investigator for each of the samples contributed to interpretation of the data and participated as an author. Overall evaluation of the economic value of PCC in this study was limited because analysis was focused to a single index hospital admission rather than including additional hospitalizations and outpatient costs.
Bottom line: Acute care hospitals might reduce hospital costs by increasing resources to allow palliative care consultations in patients with serious illnesses.
Citation: May P et al. Economics of palliative care for hospitalized adults with serious illness. JAMA Intern Med. 2018;178(6):820-9.
Dr. Libot is a hospitalist in the division of hospital medicine in the department of medicine at Loyola University Chicago, Maywood, Ill.
Background: Health care costs are on the rise, and previous studies have found that PCC can reduce hospital costs. Timing of consultation and allocation of palliative care intervention to a certain population of patients may reveal a more significant cost reduction.
Study design: Meta-analysis.
Setting: English peer reviewed articles.
Synopsis: A systematic search was performed for articles that provided economic evaluation of PCC for adult inpatients in acute care hospitals. Patients were included if they had least one of seven conditions: cancer, heart failure, liver failure, kidney failure, chronic obstructive pulmonary disease, AIDS/HIV, or neurodegenerative conditions. Six data sets were reviewed, which included 133,118 patients altogether. There was a significant reduction in costs with PCC within 3 days of admission, regardless of the diagnosis (–$3,237; 95% confidence interval, –$3,581 to –$2,893). In the stratified analysis, the pooled meta-analysis suggested a statistically significant reduction in costs for both cancer (–$4,251; 95% CI, –$4,664 to –$3,837; P less than .001) and noncancer (–$2,105; 95% CI, –$2,698 to –$1,511; P less than .001) subsamples. In patients with cancer, the treatment effect was greater for patients with four or more comorbidities than it was for those with two or fewer.
Only six samples were evaluated, and causation could not be established because all samples had observational designs. There also was potential interpretation bias because the private investigator for each of the samples contributed to interpretation of the data and participated as an author. Overall evaluation of the economic value of PCC in this study was limited because analysis was focused to a single index hospital admission rather than including additional hospitalizations and outpatient costs.
Bottom line: Acute care hospitals might reduce hospital costs by increasing resources to allow palliative care consultations in patients with serious illnesses.
Citation: May P et al. Economics of palliative care for hospitalized adults with serious illness. JAMA Intern Med. 2018;178(6):820-9.
Dr. Libot is a hospitalist in the division of hospital medicine in the department of medicine at Loyola University Chicago, Maywood, Ill.
Home telemonitoring for heart failure cuts mortality
MUNICH – A comprehensive home telemonitoring program paid off big for selected patients with heart failure in a large, German nationwide masked randomization trial.
First, TIM-HF2 didn’t rely on passive monitoring of the patients’ daily electronically submitted home data. Instead, the data went straight to a central telemonitoring center staffed 24/7 by physicians and nurses with heart failure expertise. There, the information was immediately analyzed using proprietary telemedical analytic software known as the Fontane system. The software employs individually tailored, self-adapting algorithms in order to alert staff when trouble is brewing.
But the telemonitoring intervention doesn’t merely detect early clinical deterioration. It’s also a vehicle for ongoing patient education, outpatient adjustment of drugs, management of major comorbid conditions, and hospital admissions as needed. The patient’s local primary care physician was also plugged into the remote monitoring system and kept abreast of the patient’s condition.
Second, TIM-HF2 focused on a carefully selected subgroup of heart failure patients whom prior studies suggested were particularly likely to benefit from home telemedical management. All participants were NYHA class II or III with a left ventricular ejection fraction of 45% or less, a hospitalization for heart failure within 12 months prior to randomization, and free of moderate or severe depression as evidenced by a baseline Patient Health Questionnaire-9 score of 9 or less, explained Dr. Koehler, head of the center for cardiovascular telemedicine at Charite University in Berlin.
Why exclude patients with depression?
“In this concept, with wholistic remote patient management, we need an active patient who is able to measure every day, who is able to communicate with the telemedical center, and who is able to communicate in this network created between the telemedical center and local caregivers. If someone is really depressed, unable to act, lying in bed saying it makes no sense to take drugs or do anything, then we cannot help. That is for us, I think, the most important thing. We’ve seen it now in two trials,” according to the cardiologist.
The all-cause mortality rate was 7.86 per 100 person-years in the home-telemonitoring group versus 11.34 in usual-care controls. Patients in the active intervention arm lost a mean of 17.8 days per year because of unplanned cardiovascular hospital admissions, compared with 24.2 days per year in controls.
Importantly, outcomes were equally good in the remote patient-management group regardless of whether patients were among the 40% of participants living in urban Germany or the 60% in rural areas. Thus, the telemonitoring intervention eliminated the geographic disparity in health care outcomes which is a prominent issue in Germany, as well as the United States.
A formal cost-benefit analysis of the TIM-HF2 results is in the works, Dr. Koehler said.
Simultaneous with his presentation in Munich, the TIM-HF2 study was published online in the Lancet.
In an accompanying editorial, two prominent heart failure experts – John F.G. Cleland, MD, of the University of Glasgow and Robin A. Clark, MD, of Flinders University in Adelaide – hailed TIM-HF2 as a major advance and indicated in sharp terms that it’s time for guideline writers to sit up and take notice.
“Despite much clinical skepticism and feeble support from most guidelines, in our view the growing weight of evidence suggests that home telemonitoring does reduce mortality for patients with heart failure, and this effect might be substantial. These and other trials also show that the emphasis placed on hospital admission for heart failure might be misplaced, at least from a patient’s perspective, because the proportion of days lost due to hospital admission is small, compared with those lost to death,” the physicians wrote in the editorial.
They also noted that, even though the between-group difference in the number of days during which patients were hospitalized for cardiovascular causes was relatively small, it’s clear that home telemonitoring triggered some potentially life-saving hospitalizations. “Home telemonitoring puts the patient back in the center of health care, ensuring that they know what the health professional is trying to achieve and that they agree with those aims. Ultimately, patients and their families are a large and relatively untapped health care resource,” they wrote.
The TIM-HF 2 trial was funded by the German Federal Ministry of Education and Research. Dr. Koehler reported receiving speaking and/or consultant fees from Novartis, Abbott, and Medtronic.
[email protected]
SOURCE: Koehler F et al. ESC Congress 2018. Lancet. 2018 Sep 22;392(10152):1047-57.
MUNICH – A comprehensive home telemonitoring program paid off big for selected patients with heart failure in a large, German nationwide masked randomization trial.
First, TIM-HF2 didn’t rely on passive monitoring of the patients’ daily electronically submitted home data. Instead, the data went straight to a central telemonitoring center staffed 24/7 by physicians and nurses with heart failure expertise. There, the information was immediately analyzed using proprietary telemedical analytic software known as the Fontane system. The software employs individually tailored, self-adapting algorithms in order to alert staff when trouble is brewing.
But the telemonitoring intervention doesn’t merely detect early clinical deterioration. It’s also a vehicle for ongoing patient education, outpatient adjustment of drugs, management of major comorbid conditions, and hospital admissions as needed. The patient’s local primary care physician was also plugged into the remote monitoring system and kept abreast of the patient’s condition.
Second, TIM-HF2 focused on a carefully selected subgroup of heart failure patients whom prior studies suggested were particularly likely to benefit from home telemedical management. All participants were NYHA class II or III with a left ventricular ejection fraction of 45% or less, a hospitalization for heart failure within 12 months prior to randomization, and free of moderate or severe depression as evidenced by a baseline Patient Health Questionnaire-9 score of 9 or less, explained Dr. Koehler, head of the center for cardiovascular telemedicine at Charite University in Berlin.
Why exclude patients with depression?
“In this concept, with wholistic remote patient management, we need an active patient who is able to measure every day, who is able to communicate with the telemedical center, and who is able to communicate in this network created between the telemedical center and local caregivers. If someone is really depressed, unable to act, lying in bed saying it makes no sense to take drugs or do anything, then we cannot help. That is for us, I think, the most important thing. We’ve seen it now in two trials,” according to the cardiologist.
The all-cause mortality rate was 7.86 per 100 person-years in the home-telemonitoring group versus 11.34 in usual-care controls. Patients in the active intervention arm lost a mean of 17.8 days per year because of unplanned cardiovascular hospital admissions, compared with 24.2 days per year in controls.
Importantly, outcomes were equally good in the remote patient-management group regardless of whether patients were among the 40% of participants living in urban Germany or the 60% in rural areas. Thus, the telemonitoring intervention eliminated the geographic disparity in health care outcomes which is a prominent issue in Germany, as well as the United States.
A formal cost-benefit analysis of the TIM-HF2 results is in the works, Dr. Koehler said.
Simultaneous with his presentation in Munich, the TIM-HF2 study was published online in the Lancet.
In an accompanying editorial, two prominent heart failure experts – John F.G. Cleland, MD, of the University of Glasgow and Robin A. Clark, MD, of Flinders University in Adelaide – hailed TIM-HF2 as a major advance and indicated in sharp terms that it’s time for guideline writers to sit up and take notice.
“Despite much clinical skepticism and feeble support from most guidelines, in our view the growing weight of evidence suggests that home telemonitoring does reduce mortality for patients with heart failure, and this effect might be substantial. These and other trials also show that the emphasis placed on hospital admission for heart failure might be misplaced, at least from a patient’s perspective, because the proportion of days lost due to hospital admission is small, compared with those lost to death,” the physicians wrote in the editorial.
They also noted that, even though the between-group difference in the number of days during which patients were hospitalized for cardiovascular causes was relatively small, it’s clear that home telemonitoring triggered some potentially life-saving hospitalizations. “Home telemonitoring puts the patient back in the center of health care, ensuring that they know what the health professional is trying to achieve and that they agree with those aims. Ultimately, patients and their families are a large and relatively untapped health care resource,” they wrote.
The TIM-HF 2 trial was funded by the German Federal Ministry of Education and Research. Dr. Koehler reported receiving speaking and/or consultant fees from Novartis, Abbott, and Medtronic.
[email protected]
SOURCE: Koehler F et al. ESC Congress 2018. Lancet. 2018 Sep 22;392(10152):1047-57.
MUNICH – A comprehensive home telemonitoring program paid off big for selected patients with heart failure in a large, German nationwide masked randomization trial.
First, TIM-HF2 didn’t rely on passive monitoring of the patients’ daily electronically submitted home data. Instead, the data went straight to a central telemonitoring center staffed 24/7 by physicians and nurses with heart failure expertise. There, the information was immediately analyzed using proprietary telemedical analytic software known as the Fontane system. The software employs individually tailored, self-adapting algorithms in order to alert staff when trouble is brewing.
But the telemonitoring intervention doesn’t merely detect early clinical deterioration. It’s also a vehicle for ongoing patient education, outpatient adjustment of drugs, management of major comorbid conditions, and hospital admissions as needed. The patient’s local primary care physician was also plugged into the remote monitoring system and kept abreast of the patient’s condition.
Second, TIM-HF2 focused on a carefully selected subgroup of heart failure patients whom prior studies suggested were particularly likely to benefit from home telemedical management. All participants were NYHA class II or III with a left ventricular ejection fraction of 45% or less, a hospitalization for heart failure within 12 months prior to randomization, and free of moderate or severe depression as evidenced by a baseline Patient Health Questionnaire-9 score of 9 or less, explained Dr. Koehler, head of the center for cardiovascular telemedicine at Charite University in Berlin.
Why exclude patients with depression?
“In this concept, with wholistic remote patient management, we need an active patient who is able to measure every day, who is able to communicate with the telemedical center, and who is able to communicate in this network created between the telemedical center and local caregivers. If someone is really depressed, unable to act, lying in bed saying it makes no sense to take drugs or do anything, then we cannot help. That is for us, I think, the most important thing. We’ve seen it now in two trials,” according to the cardiologist.
The all-cause mortality rate was 7.86 per 100 person-years in the home-telemonitoring group versus 11.34 in usual-care controls. Patients in the active intervention arm lost a mean of 17.8 days per year because of unplanned cardiovascular hospital admissions, compared with 24.2 days per year in controls.
Importantly, outcomes were equally good in the remote patient-management group regardless of whether patients were among the 40% of participants living in urban Germany or the 60% in rural areas. Thus, the telemonitoring intervention eliminated the geographic disparity in health care outcomes which is a prominent issue in Germany, as well as the United States.
A formal cost-benefit analysis of the TIM-HF2 results is in the works, Dr. Koehler said.
Simultaneous with his presentation in Munich, the TIM-HF2 study was published online in the Lancet.
In an accompanying editorial, two prominent heart failure experts – John F.G. Cleland, MD, of the University of Glasgow and Robin A. Clark, MD, of Flinders University in Adelaide – hailed TIM-HF2 as a major advance and indicated in sharp terms that it’s time for guideline writers to sit up and take notice.
“Despite much clinical skepticism and feeble support from most guidelines, in our view the growing weight of evidence suggests that home telemonitoring does reduce mortality for patients with heart failure, and this effect might be substantial. These and other trials also show that the emphasis placed on hospital admission for heart failure might be misplaced, at least from a patient’s perspective, because the proportion of days lost due to hospital admission is small, compared with those lost to death,” the physicians wrote in the editorial.
They also noted that, even though the between-group difference in the number of days during which patients were hospitalized for cardiovascular causes was relatively small, it’s clear that home telemonitoring triggered some potentially life-saving hospitalizations. “Home telemonitoring puts the patient back in the center of health care, ensuring that they know what the health professional is trying to achieve and that they agree with those aims. Ultimately, patients and their families are a large and relatively untapped health care resource,” they wrote.
The TIM-HF 2 trial was funded by the German Federal Ministry of Education and Research. Dr. Koehler reported receiving speaking and/or consultant fees from Novartis, Abbott, and Medtronic.
[email protected]
SOURCE: Koehler F et al. ESC Congress 2018. Lancet. 2018 Sep 22;392(10152):1047-57.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Comprehensive home-telemonitoring program for heart failure saves lives.
Major finding: All-cause mortality was reduced by 30% at 1 year with a comprehensive home-telemonitoring program.
Study details: This prospective, masked randomization study included 1,538 German heart failure patients.
Disclosures: The TIM-HF2 trial was funded by the German Federal Ministry of Education and Research.
Source: Koehler F. ESC Congress 2018. Lancet. 2018 Sep 22;392(10152):1047-57.
Admission eosinopenia predicted severe CDI outcomes
For patients with even in the absence of hypotension and tachycardia, researchers wrote in JAMA Surgery.
“In animal models, peripheral eosinopenia is a biologically plausible predictive factor for adverse outcomes, and human data from this study indicate that this frequent addition to an admission complete blood cell count is an inexpensive, widely available risk index in the treatment of C. difficile infection,” wrote Audrey S. Kulaylat, MD, of Penn State University, Hershey, and her associates.
In their cohort study of 2,065 patients admitted to two tertiary referral centers with C. difficile infection, undetectable eosinophil counts at hospital admission were associated with significantly increased odds of in-hospital mortality in both a training dataset (odds ratio, 2.01; 95% confidence interval, 1.08-3.73; P = .03) and a validation dataset (OR, 2.26; 95% CI, 1.33-3.83; P = .002). Undetectable eosinophil counts also were associated with elevated odds of severe disease requiring intensive care, vasopressor use, and emergency total colectomy. Besides eosinopenia, significant predictors of mortality included having more comorbidities and lower systolic blood pressure at admission. Strikingly, when patients had no initial hypotension or tachycardia, an undetectable eosinophil count was the only identifiable predictor of in-hospital death (OR, 5.76; 95% CI, 1.99-16.64). An elevated white blood cell count was not a significant predictor of mortality in this subgroup.
Dr. Kulaylat and her associates are studying the microbiome in C. difficile infection. Their work has identified a host immune reaction marked by an “exaggerated inflammasome response” and peripheral eosinopenia, they explained. Two recent murine models have produced similar results.
Admission eosinophil counts “allow for an immediate assessment of mortality risk at admission that is inexpensive and part of a differential for a standard complete blood count available at any hospital,” they concluded. They are now prospectively evaluating a prognostic score for C. difficile infection that includes eosinopenia and other easily discernible admission factors. The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
SOURCE: Kulaylat AS et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
For patients with even in the absence of hypotension and tachycardia, researchers wrote in JAMA Surgery.
“In animal models, peripheral eosinopenia is a biologically plausible predictive factor for adverse outcomes, and human data from this study indicate that this frequent addition to an admission complete blood cell count is an inexpensive, widely available risk index in the treatment of C. difficile infection,” wrote Audrey S. Kulaylat, MD, of Penn State University, Hershey, and her associates.
In their cohort study of 2,065 patients admitted to two tertiary referral centers with C. difficile infection, undetectable eosinophil counts at hospital admission were associated with significantly increased odds of in-hospital mortality in both a training dataset (odds ratio, 2.01; 95% confidence interval, 1.08-3.73; P = .03) and a validation dataset (OR, 2.26; 95% CI, 1.33-3.83; P = .002). Undetectable eosinophil counts also were associated with elevated odds of severe disease requiring intensive care, vasopressor use, and emergency total colectomy. Besides eosinopenia, significant predictors of mortality included having more comorbidities and lower systolic blood pressure at admission. Strikingly, when patients had no initial hypotension or tachycardia, an undetectable eosinophil count was the only identifiable predictor of in-hospital death (OR, 5.76; 95% CI, 1.99-16.64). An elevated white blood cell count was not a significant predictor of mortality in this subgroup.
Dr. Kulaylat and her associates are studying the microbiome in C. difficile infection. Their work has identified a host immune reaction marked by an “exaggerated inflammasome response” and peripheral eosinopenia, they explained. Two recent murine models have produced similar results.
Admission eosinophil counts “allow for an immediate assessment of mortality risk at admission that is inexpensive and part of a differential for a standard complete blood count available at any hospital,” they concluded. They are now prospectively evaluating a prognostic score for C. difficile infection that includes eosinopenia and other easily discernible admission factors. The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
SOURCE: Kulaylat AS et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
For patients with even in the absence of hypotension and tachycardia, researchers wrote in JAMA Surgery.
“In animal models, peripheral eosinopenia is a biologically plausible predictive factor for adverse outcomes, and human data from this study indicate that this frequent addition to an admission complete blood cell count is an inexpensive, widely available risk index in the treatment of C. difficile infection,” wrote Audrey S. Kulaylat, MD, of Penn State University, Hershey, and her associates.
In their cohort study of 2,065 patients admitted to two tertiary referral centers with C. difficile infection, undetectable eosinophil counts at hospital admission were associated with significantly increased odds of in-hospital mortality in both a training dataset (odds ratio, 2.01; 95% confidence interval, 1.08-3.73; P = .03) and a validation dataset (OR, 2.26; 95% CI, 1.33-3.83; P = .002). Undetectable eosinophil counts also were associated with elevated odds of severe disease requiring intensive care, vasopressor use, and emergency total colectomy. Besides eosinopenia, significant predictors of mortality included having more comorbidities and lower systolic blood pressure at admission. Strikingly, when patients had no initial hypotension or tachycardia, an undetectable eosinophil count was the only identifiable predictor of in-hospital death (OR, 5.76; 95% CI, 1.99-16.64). An elevated white blood cell count was not a significant predictor of mortality in this subgroup.
Dr. Kulaylat and her associates are studying the microbiome in C. difficile infection. Their work has identified a host immune reaction marked by an “exaggerated inflammasome response” and peripheral eosinopenia, they explained. Two recent murine models have produced similar results.
Admission eosinophil counts “allow for an immediate assessment of mortality risk at admission that is inexpensive and part of a differential for a standard complete blood count available at any hospital,” they concluded. They are now prospectively evaluating a prognostic score for C. difficile infection that includes eosinopenia and other easily discernible admission factors. The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
SOURCE: Kulaylat AS et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
FROM JAMA SURGERY
Key clinical point: Undetectable peripheral eosinophils predicted severe outcomes in patients admitted with Clostridium difficile infection.
Major finding: In the training and validation datasets, odds of in-hospital mortality were 2.01 (95% CI, 1.08-3.73) and 2.26 (95% CI, 1.33-3.83), respectively.
Study details: Two-hospital cohort study of 2,065 patients admitted with C. difficile infection.
Disclosures: The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
Source: Kulaylat A et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
Treating cannabinoid hyperemesis syndrome
Incidence may increase as marijuana use rises
Case
WS is a 54-year-old African American male with a medical history of diabetes mellitus type 2, hypertension, obstructive sleep apnea, and gastroparesis. He has multiple admissions for intractable nausea, vomiting, and abdominal pain believed to be from diabetic gastroparesis despite a normal gastric-emptying study. Endoscopy done in prior admission showed duodenitis, gastritis, and esophagitis, and colonoscopy revealed diverticulosis. He had a negative gastric-emptying study of 6% retention at 4 hrs. His last hemoglobin A1c was 5 and his glucose has been well controlled. He is hospitalized again for intractable abdominal pain, nausea, and vomiting. His examination was unremarkable except for dry mucosa and epigastric tenderness. His labs were also insignificant except for prerenal azotemia. Upon further questioning he admitted to significant marijuana use, and his symptoms transiently improved with a hot shower in the hospital. He was diagnosed with cannabinoid hyperemesis syndrome (CHS) and admitted for further management.
Background
In the United States, 9 states and the District of Columbia have legalized recreational marijuana use, and 29 states and DC have legalized medical marijuana. Marijuana use is likely to rise, and with it may arise an increasing incidence of CHS.
The exact prevalence of CHS is not known. Diagnosis is often delayed as there is no reliable diagnostic test. A high index of suspicion is needed for prompt diagnosis.
CHS was first described in 2004 in South Australia and since then many case reports have been published. Marijuana has both proemetic and antiemetic effects. Unlike its antiemetic effect, the pathophysiology of the proemetic effect of marijuana is not well understood.
Key clinical features
CHS typically has three phases. Initially patients present with prodromal symptoms of abdominal discomfort and nausea. There is no emesis at this early phase. Patients are still able to tolerate a liquid diet in this prodromal phase.
This is followed by a more active phase of intractable vomiting, which is relieved by hot showers or baths. Most patients take compulsively long hot showers or baths many times a day. Also, they develop diaphoresis, restlessness, agitation, and weight loss.
The active phase is followed by a recovery phase when symptoms resolve and patients return to baseline, only to have it recur if marijuana use continues.
Diagnostic approach and management
CHS should be suspected in patients coming in with recurrent symptoms of abdominal pain, nausea and vomiting, and who have normal CBC, basic metabolic panel, lipase, and liver function tests. Patients should be directly questioned about marijuana use and whether symptoms are relieved with hot showers. A toxicology screen should be done. For patients with marijuana use and compulsive hot showers, further work up of their symptoms (e.g., upper endoscopy, abdominal ultrasound, and/or nuclear medicine emptying study) should be avoided. Figure 1 shows the suggested work-up.
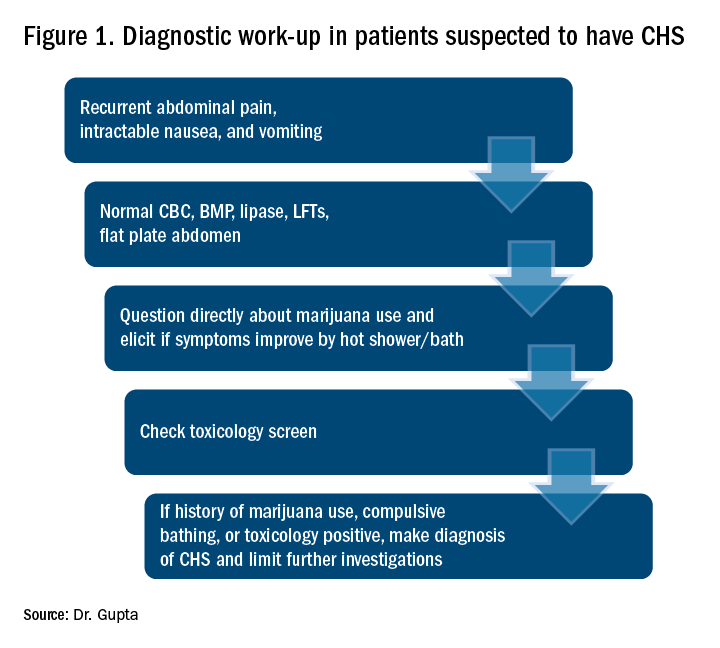
The differential diagnosis for recurrent abdominal pain, nausea, and vomiting is chronic pancreatitis, gastroparesis, severe gastritis, medication adverse effects (especially GLP1 receptor agonists), cyclic vomiting syndrome, psychogenic vomiting, and (with the rise of narcotic abuse) narcotic bowel syndrome.
Our patient had a history of diabetes with an HbA1c at goal and a normal nuclear medicine gastric-emptying study (6% retention at 4 hours). He was also on liraglutide, but his symptoms predated this medicine use.
The mainstay of treatment for CHS is supportive therapy with intravenous fluids and antiemetics like 5-HT3-receptor antagonists (ondansetron); D2-receptor antagonists (metoclopramide); and H1-receptor antagonists (diphenhydramine). The effectiveness of these agents is limited, which is also a clue for the diagnosis of CHS. If traditional agents fail in controlling the symptoms, haloperidol can be tried, but it has been used with limited success. Our patient did not respond to traditional antiemetics, but responded well to a small dose of lorazepam. Even though a benzodiazepine is not the mainstay of treatment, it may be tried if other agents fail. Acid-suppression therapy with a proton pump inhibitor should be used as esophagogastroduodenoscopy (EGD) usually reveals mild gastritis and esophagitis, as in our patient. Narcotic use should be avoided for management of abdominal pain.
Patients should be counseled against marijuana use. This may be difficult if marijuana is being used as an appetite stimulant or for treatment of chemotherapy-induced nausea and vomiting. If willing, patients should be referred to a substance abuse rehabilitation center.
Back to the case
In this case, after a diagnosis of CHS was made, the patient was counseled against marijuana use. His abdominal pain and intractable vomiting did not improve with conservative management of n.p.o status, prochlorperazine, metoclopramide, and ondansetron. He was given a trial of low-dose lorazepam with significant improvement in his symptoms. He was counseled extensively against marijuana use and discharged. A follow-up phone call at 3 months showed continued abstinence and no recurrence of symptoms.
Dr. Gupta is a hospitalist at Yale New Haven Health and Bridgeport (Conn.) Hospital.
References
1. Bajgoric S et al. Cannabinoid hyperemesis syndrome: A guide for the practising clinician. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210246.
2. Batke M et al. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: A report of eight cases in the united states. Dig Dis Sci. 2010 Nov;55(11):3113-9.
3. Iacopetti CL et al. Cannabinoid hyperemesis syndrome: a case report and review of pathophysiology. Clin Med Res. 2014 Sep;12(1-2):65-7.
4. Hickey JL et al. Haloperidol for treatment of cannabinoid hyperemesis syndrome. Am J Emerg Med. 2013 Jun. 31(6):1003.e5-6. Epub 2013 Apr 10.
Key points
Suspect CHS for patients with recurrent abdominal pain, nausea, and vomiting with negative initial work-up.
- Ask directly about marijuana use.
- Ask whether symptoms are relieved with hot shower/ bath.
- Send a toxicology screen.
- Make a diagnosis of CHS if:
1. Positive marijuana use.
2. Symptom improvement with hot baths or
3. Toxicology positive for marijuana.
- Manage conservatively with hydration and antiemetics.
- Suspect CHS if traditional antiemetics are not providing relief .
- If traditional antiemetics fail, trial of haloperidol or low-dose benzodiazepines.
- Avoid narcotics.
- Avoid unnecessary investigations.
- Counsel patients against marijuana use and refer to substance abuse center if patient agrees.
Incidence may increase as marijuana use rises
Incidence may increase as marijuana use rises
Case
WS is a 54-year-old African American male with a medical history of diabetes mellitus type 2, hypertension, obstructive sleep apnea, and gastroparesis. He has multiple admissions for intractable nausea, vomiting, and abdominal pain believed to be from diabetic gastroparesis despite a normal gastric-emptying study. Endoscopy done in prior admission showed duodenitis, gastritis, and esophagitis, and colonoscopy revealed diverticulosis. He had a negative gastric-emptying study of 6% retention at 4 hrs. His last hemoglobin A1c was 5 and his glucose has been well controlled. He is hospitalized again for intractable abdominal pain, nausea, and vomiting. His examination was unremarkable except for dry mucosa and epigastric tenderness. His labs were also insignificant except for prerenal azotemia. Upon further questioning he admitted to significant marijuana use, and his symptoms transiently improved with a hot shower in the hospital. He was diagnosed with cannabinoid hyperemesis syndrome (CHS) and admitted for further management.
Background
In the United States, 9 states and the District of Columbia have legalized recreational marijuana use, and 29 states and DC have legalized medical marijuana. Marijuana use is likely to rise, and with it may arise an increasing incidence of CHS.
The exact prevalence of CHS is not known. Diagnosis is often delayed as there is no reliable diagnostic test. A high index of suspicion is needed for prompt diagnosis.
CHS was first described in 2004 in South Australia and since then many case reports have been published. Marijuana has both proemetic and antiemetic effects. Unlike its antiemetic effect, the pathophysiology of the proemetic effect of marijuana is not well understood.
Key clinical features
CHS typically has three phases. Initially patients present with prodromal symptoms of abdominal discomfort and nausea. There is no emesis at this early phase. Patients are still able to tolerate a liquid diet in this prodromal phase.
This is followed by a more active phase of intractable vomiting, which is relieved by hot showers or baths. Most patients take compulsively long hot showers or baths many times a day. Also, they develop diaphoresis, restlessness, agitation, and weight loss.
The active phase is followed by a recovery phase when symptoms resolve and patients return to baseline, only to have it recur if marijuana use continues.
Diagnostic approach and management
CHS should be suspected in patients coming in with recurrent symptoms of abdominal pain, nausea and vomiting, and who have normal CBC, basic metabolic panel, lipase, and liver function tests. Patients should be directly questioned about marijuana use and whether symptoms are relieved with hot showers. A toxicology screen should be done. For patients with marijuana use and compulsive hot showers, further work up of their symptoms (e.g., upper endoscopy, abdominal ultrasound, and/or nuclear medicine emptying study) should be avoided. Figure 1 shows the suggested work-up.

The differential diagnosis for recurrent abdominal pain, nausea, and vomiting is chronic pancreatitis, gastroparesis, severe gastritis, medication adverse effects (especially GLP1 receptor agonists), cyclic vomiting syndrome, psychogenic vomiting, and (with the rise of narcotic abuse) narcotic bowel syndrome.
Our patient had a history of diabetes with an HbA1c at goal and a normal nuclear medicine gastric-emptying study (6% retention at 4 hours). He was also on liraglutide, but his symptoms predated this medicine use.
The mainstay of treatment for CHS is supportive therapy with intravenous fluids and antiemetics like 5-HT3-receptor antagonists (ondansetron); D2-receptor antagonists (metoclopramide); and H1-receptor antagonists (diphenhydramine). The effectiveness of these agents is limited, which is also a clue for the diagnosis of CHS. If traditional agents fail in controlling the symptoms, haloperidol can be tried, but it has been used with limited success. Our patient did not respond to traditional antiemetics, but responded well to a small dose of lorazepam. Even though a benzodiazepine is not the mainstay of treatment, it may be tried if other agents fail. Acid-suppression therapy with a proton pump inhibitor should be used as esophagogastroduodenoscopy (EGD) usually reveals mild gastritis and esophagitis, as in our patient. Narcotic use should be avoided for management of abdominal pain.
Patients should be counseled against marijuana use. This may be difficult if marijuana is being used as an appetite stimulant or for treatment of chemotherapy-induced nausea and vomiting. If willing, patients should be referred to a substance abuse rehabilitation center.
Back to the case
In this case, after a diagnosis of CHS was made, the patient was counseled against marijuana use. His abdominal pain and intractable vomiting did not improve with conservative management of n.p.o status, prochlorperazine, metoclopramide, and ondansetron. He was given a trial of low-dose lorazepam with significant improvement in his symptoms. He was counseled extensively against marijuana use and discharged. A follow-up phone call at 3 months showed continued abstinence and no recurrence of symptoms.
Dr. Gupta is a hospitalist at Yale New Haven Health and Bridgeport (Conn.) Hospital.
References
1. Bajgoric S et al. Cannabinoid hyperemesis syndrome: A guide for the practising clinician. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210246.
2. Batke M et al. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: A report of eight cases in the united states. Dig Dis Sci. 2010 Nov;55(11):3113-9.
3. Iacopetti CL et al. Cannabinoid hyperemesis syndrome: a case report and review of pathophysiology. Clin Med Res. 2014 Sep;12(1-2):65-7.
4. Hickey JL et al. Haloperidol for treatment of cannabinoid hyperemesis syndrome. Am J Emerg Med. 2013 Jun. 31(6):1003.e5-6. Epub 2013 Apr 10.
Key points
Suspect CHS for patients with recurrent abdominal pain, nausea, and vomiting with negative initial work-up.
- Ask directly about marijuana use.
- Ask whether symptoms are relieved with hot shower/ bath.
- Send a toxicology screen.
- Make a diagnosis of CHS if:
1. Positive marijuana use.
2. Symptom improvement with hot baths or
3. Toxicology positive for marijuana.
- Manage conservatively with hydration and antiemetics.
- Suspect CHS if traditional antiemetics are not providing relief .
- If traditional antiemetics fail, trial of haloperidol or low-dose benzodiazepines.
- Avoid narcotics.
- Avoid unnecessary investigations.
- Counsel patients against marijuana use and refer to substance abuse center if patient agrees.
Case
WS is a 54-year-old African American male with a medical history of diabetes mellitus type 2, hypertension, obstructive sleep apnea, and gastroparesis. He has multiple admissions for intractable nausea, vomiting, and abdominal pain believed to be from diabetic gastroparesis despite a normal gastric-emptying study. Endoscopy done in prior admission showed duodenitis, gastritis, and esophagitis, and colonoscopy revealed diverticulosis. He had a negative gastric-emptying study of 6% retention at 4 hrs. His last hemoglobin A1c was 5 and his glucose has been well controlled. He is hospitalized again for intractable abdominal pain, nausea, and vomiting. His examination was unremarkable except for dry mucosa and epigastric tenderness. His labs were also insignificant except for prerenal azotemia. Upon further questioning he admitted to significant marijuana use, and his symptoms transiently improved with a hot shower in the hospital. He was diagnosed with cannabinoid hyperemesis syndrome (CHS) and admitted for further management.
Background
In the United States, 9 states and the District of Columbia have legalized recreational marijuana use, and 29 states and DC have legalized medical marijuana. Marijuana use is likely to rise, and with it may arise an increasing incidence of CHS.
The exact prevalence of CHS is not known. Diagnosis is often delayed as there is no reliable diagnostic test. A high index of suspicion is needed for prompt diagnosis.
CHS was first described in 2004 in South Australia and since then many case reports have been published. Marijuana has both proemetic and antiemetic effects. Unlike its antiemetic effect, the pathophysiology of the proemetic effect of marijuana is not well understood.
Key clinical features
CHS typically has three phases. Initially patients present with prodromal symptoms of abdominal discomfort and nausea. There is no emesis at this early phase. Patients are still able to tolerate a liquid diet in this prodromal phase.
This is followed by a more active phase of intractable vomiting, which is relieved by hot showers or baths. Most patients take compulsively long hot showers or baths many times a day. Also, they develop diaphoresis, restlessness, agitation, and weight loss.
The active phase is followed by a recovery phase when symptoms resolve and patients return to baseline, only to have it recur if marijuana use continues.
Diagnostic approach and management
CHS should be suspected in patients coming in with recurrent symptoms of abdominal pain, nausea and vomiting, and who have normal CBC, basic metabolic panel, lipase, and liver function tests. Patients should be directly questioned about marijuana use and whether symptoms are relieved with hot showers. A toxicology screen should be done. For patients with marijuana use and compulsive hot showers, further work up of their symptoms (e.g., upper endoscopy, abdominal ultrasound, and/or nuclear medicine emptying study) should be avoided. Figure 1 shows the suggested work-up.

The differential diagnosis for recurrent abdominal pain, nausea, and vomiting is chronic pancreatitis, gastroparesis, severe gastritis, medication adverse effects (especially GLP1 receptor agonists), cyclic vomiting syndrome, psychogenic vomiting, and (with the rise of narcotic abuse) narcotic bowel syndrome.
Our patient had a history of diabetes with an HbA1c at goal and a normal nuclear medicine gastric-emptying study (6% retention at 4 hours). He was also on liraglutide, but his symptoms predated this medicine use.
The mainstay of treatment for CHS is supportive therapy with intravenous fluids and antiemetics like 5-HT3-receptor antagonists (ondansetron); D2-receptor antagonists (metoclopramide); and H1-receptor antagonists (diphenhydramine). The effectiveness of these agents is limited, which is also a clue for the diagnosis of CHS. If traditional agents fail in controlling the symptoms, haloperidol can be tried, but it has been used with limited success. Our patient did not respond to traditional antiemetics, but responded well to a small dose of lorazepam. Even though a benzodiazepine is not the mainstay of treatment, it may be tried if other agents fail. Acid-suppression therapy with a proton pump inhibitor should be used as esophagogastroduodenoscopy (EGD) usually reveals mild gastritis and esophagitis, as in our patient. Narcotic use should be avoided for management of abdominal pain.
Patients should be counseled against marijuana use. This may be difficult if marijuana is being used as an appetite stimulant or for treatment of chemotherapy-induced nausea and vomiting. If willing, patients should be referred to a substance abuse rehabilitation center.
Back to the case
In this case, after a diagnosis of CHS was made, the patient was counseled against marijuana use. His abdominal pain and intractable vomiting did not improve with conservative management of n.p.o status, prochlorperazine, metoclopramide, and ondansetron. He was given a trial of low-dose lorazepam with significant improvement in his symptoms. He was counseled extensively against marijuana use and discharged. A follow-up phone call at 3 months showed continued abstinence and no recurrence of symptoms.
Dr. Gupta is a hospitalist at Yale New Haven Health and Bridgeport (Conn.) Hospital.
References
1. Bajgoric S et al. Cannabinoid hyperemesis syndrome: A guide for the practising clinician. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210246.
2. Batke M et al. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: A report of eight cases in the united states. Dig Dis Sci. 2010 Nov;55(11):3113-9.
3. Iacopetti CL et al. Cannabinoid hyperemesis syndrome: a case report and review of pathophysiology. Clin Med Res. 2014 Sep;12(1-2):65-7.
4. Hickey JL et al. Haloperidol for treatment of cannabinoid hyperemesis syndrome. Am J Emerg Med. 2013 Jun. 31(6):1003.e5-6. Epub 2013 Apr 10.
Key points
Suspect CHS for patients with recurrent abdominal pain, nausea, and vomiting with negative initial work-up.
- Ask directly about marijuana use.
- Ask whether symptoms are relieved with hot shower/ bath.
- Send a toxicology screen.
- Make a diagnosis of CHS if:
1. Positive marijuana use.
2. Symptom improvement with hot baths or
3. Toxicology positive for marijuana.
- Manage conservatively with hydration and antiemetics.
- Suspect CHS if traditional antiemetics are not providing relief .
- If traditional antiemetics fail, trial of haloperidol or low-dose benzodiazepines.
- Avoid narcotics.
- Avoid unnecessary investigations.
- Counsel patients against marijuana use and refer to substance abuse center if patient agrees.
Point-of-care test for respiratory viruses lowers antibiotic use
Routine testing in the ED is advocated
PARIS – Using a point-of-care test for viral pathogens, hospital admissions were avoided in about a third of emergency department patients with suspected respiratory infection when other clinical signs also suggested a low risk of a bacterial pathogen, according to a single-center experience presented at the annual congress of the European Respiratory Society.
“We found that when patients had point-of-care respiratory viral testing soon after they were admitted to the emergency department, we were able to reduce unnecessary admission and improve bed flow in our center,” reported Kay Roy, MBBS, consultant physician in respiratory medicine, West Hertfordshire (England) Hospital NHS Trust.
In a protocol that was launched at Dr. Kay’s institution in January 2018, the point-of-care viral test was combined with other clinical factors, particularly chest x-rays and elevated C-reactive protein (CRP), to determine whether patients had a viral pathogen and whether they could be discharged without antibiotics.
“Clinical judgment will always be required in individual patient decisions regarding antibiotic avoidance and early discharge,” Dr. Roy maintained. “But the point-of-care viral assay can be integrated into a strategy that permits more informed and rapid decision-making.”
This assertion is supported by the experience using a protocol anchored with the point-of-care viral test over a 4-month period. During this time, 901 patients with respiratory symptoms suspected of having a viral etiology were evaluated with the proprietary point-of-care device called FilmArray (bioMérieux).
From a sample taken with a nasopharyngeal swab, the test can identify a broad array of viruses using polymerase chain reaction technology in less than 45 minutes. However, the ED protocol for considering discharge without antibiotics requires additional evidence that the pathogen is viral, including a normal chest x-ray and a CRP less than 50 mg/L.
Of the 901 patients tested, a substantial proportion of whom had chronic obstructive pulmonary disease (COPD) or asthma, 507 (56%) tested positive for at least one virus, including influenza, rhinoviruses, coronaviruses, and adenovirus. Of these, 239 had normal chest x-rays and CRPs less than 50 mg/L. Because of the severity of symptoms or other clinical considerations, 154 patients were admitted, but 85 (36% of those meeting protocol criteria) were discharged without an antibiotic prescription.
“Antibiotics were continued in 90% of the patients who had an abnormal chest x-ray and abnormal CRP,” Dr. Roy reported. However, an objective strategy that permits clinicians to discharge patients at very low risk of a bacterial infection has many advantages even if it applies to a relatively modest proportion of those tested, according to Dr. Roy.
“Each respiratory admission can cost around [2,000 pounds] at our center,” reported Dr. Kay, referring to a figure equivalent to more than $2,600. In addition, she said that avoiding hospitalization frees up hospital beds and facilitates improved antimicrobial stewardship, which is vital to stem resistance.
Avoiding antibiotic use in patients with viral respiratory infections also is relevant to improved antibiotic stewardship in the community. For this reason, a randomized trial with a similar protocol involving the point-of-care viral test is planned in the outpatient setting. According to Dr. Roy, this will involve a community hub to which patients can be referred for testing and clinical evaluation.
“We hope that the quality of care can be improved with the point-of-care test for respiratory viruses as well as helping to reduce antibiotic resistance,” Dr. Roy said.
This approach is promising, according to Tobias Welte, MD, of the department of respiratory medicine at Hannover (Germany) Medical School, but he cautioned that it is not a standard approach.
“The protocol described by Dr. Roy will have to be compared to guidelines and recommended best clinical practice to confirm its usefulness,” he said, while conceding that any strategy that reduces unnecessary hospitalizations deserves further evaluation.
Routine testing in the ED is advocated
Routine testing in the ED is advocated
PARIS – Using a point-of-care test for viral pathogens, hospital admissions were avoided in about a third of emergency department patients with suspected respiratory infection when other clinical signs also suggested a low risk of a bacterial pathogen, according to a single-center experience presented at the annual congress of the European Respiratory Society.
“We found that when patients had point-of-care respiratory viral testing soon after they were admitted to the emergency department, we were able to reduce unnecessary admission and improve bed flow in our center,” reported Kay Roy, MBBS, consultant physician in respiratory medicine, West Hertfordshire (England) Hospital NHS Trust.
In a protocol that was launched at Dr. Kay’s institution in January 2018, the point-of-care viral test was combined with other clinical factors, particularly chest x-rays and elevated C-reactive protein (CRP), to determine whether patients had a viral pathogen and whether they could be discharged without antibiotics.
“Clinical judgment will always be required in individual patient decisions regarding antibiotic avoidance and early discharge,” Dr. Roy maintained. “But the point-of-care viral assay can be integrated into a strategy that permits more informed and rapid decision-making.”
This assertion is supported by the experience using a protocol anchored with the point-of-care viral test over a 4-month period. During this time, 901 patients with respiratory symptoms suspected of having a viral etiology were evaluated with the proprietary point-of-care device called FilmArray (bioMérieux).
From a sample taken with a nasopharyngeal swab, the test can identify a broad array of viruses using polymerase chain reaction technology in less than 45 minutes. However, the ED protocol for considering discharge without antibiotics requires additional evidence that the pathogen is viral, including a normal chest x-ray and a CRP less than 50 mg/L.
Of the 901 patients tested, a substantial proportion of whom had chronic obstructive pulmonary disease (COPD) or asthma, 507 (56%) tested positive for at least one virus, including influenza, rhinoviruses, coronaviruses, and adenovirus. Of these, 239 had normal chest x-rays and CRPs less than 50 mg/L. Because of the severity of symptoms or other clinical considerations, 154 patients were admitted, but 85 (36% of those meeting protocol criteria) were discharged without an antibiotic prescription.
“Antibiotics were continued in 90% of the patients who had an abnormal chest x-ray and abnormal CRP,” Dr. Roy reported. However, an objective strategy that permits clinicians to discharge patients at very low risk of a bacterial infection has many advantages even if it applies to a relatively modest proportion of those tested, according to Dr. Roy.
“Each respiratory admission can cost around [2,000 pounds] at our center,” reported Dr. Kay, referring to a figure equivalent to more than $2,600. In addition, she said that avoiding hospitalization frees up hospital beds and facilitates improved antimicrobial stewardship, which is vital to stem resistance.
Avoiding antibiotic use in patients with viral respiratory infections also is relevant to improved antibiotic stewardship in the community. For this reason, a randomized trial with a similar protocol involving the point-of-care viral test is planned in the outpatient setting. According to Dr. Roy, this will involve a community hub to which patients can be referred for testing and clinical evaluation.
“We hope that the quality of care can be improved with the point-of-care test for respiratory viruses as well as helping to reduce antibiotic resistance,” Dr. Roy said.
This approach is promising, according to Tobias Welte, MD, of the department of respiratory medicine at Hannover (Germany) Medical School, but he cautioned that it is not a standard approach.
“The protocol described by Dr. Roy will have to be compared to guidelines and recommended best clinical practice to confirm its usefulness,” he said, while conceding that any strategy that reduces unnecessary hospitalizations deserves further evaluation.
PARIS – Using a point-of-care test for viral pathogens, hospital admissions were avoided in about a third of emergency department patients with suspected respiratory infection when other clinical signs also suggested a low risk of a bacterial pathogen, according to a single-center experience presented at the annual congress of the European Respiratory Society.
“We found that when patients had point-of-care respiratory viral testing soon after they were admitted to the emergency department, we were able to reduce unnecessary admission and improve bed flow in our center,” reported Kay Roy, MBBS, consultant physician in respiratory medicine, West Hertfordshire (England) Hospital NHS Trust.
In a protocol that was launched at Dr. Kay’s institution in January 2018, the point-of-care viral test was combined with other clinical factors, particularly chest x-rays and elevated C-reactive protein (CRP), to determine whether patients had a viral pathogen and whether they could be discharged without antibiotics.
“Clinical judgment will always be required in individual patient decisions regarding antibiotic avoidance and early discharge,” Dr. Roy maintained. “But the point-of-care viral assay can be integrated into a strategy that permits more informed and rapid decision-making.”
This assertion is supported by the experience using a protocol anchored with the point-of-care viral test over a 4-month period. During this time, 901 patients with respiratory symptoms suspected of having a viral etiology were evaluated with the proprietary point-of-care device called FilmArray (bioMérieux).
From a sample taken with a nasopharyngeal swab, the test can identify a broad array of viruses using polymerase chain reaction technology in less than 45 minutes. However, the ED protocol for considering discharge without antibiotics requires additional evidence that the pathogen is viral, including a normal chest x-ray and a CRP less than 50 mg/L.
Of the 901 patients tested, a substantial proportion of whom had chronic obstructive pulmonary disease (COPD) or asthma, 507 (56%) tested positive for at least one virus, including influenza, rhinoviruses, coronaviruses, and adenovirus. Of these, 239 had normal chest x-rays and CRPs less than 50 mg/L. Because of the severity of symptoms or other clinical considerations, 154 patients were admitted, but 85 (36% of those meeting protocol criteria) were discharged without an antibiotic prescription.
“Antibiotics were continued in 90% of the patients who had an abnormal chest x-ray and abnormal CRP,” Dr. Roy reported. However, an objective strategy that permits clinicians to discharge patients at very low risk of a bacterial infection has many advantages even if it applies to a relatively modest proportion of those tested, according to Dr. Roy.
“Each respiratory admission can cost around [2,000 pounds] at our center,” reported Dr. Kay, referring to a figure equivalent to more than $2,600. In addition, she said that avoiding hospitalization frees up hospital beds and facilitates improved antimicrobial stewardship, which is vital to stem resistance.
Avoiding antibiotic use in patients with viral respiratory infections also is relevant to improved antibiotic stewardship in the community. For this reason, a randomized trial with a similar protocol involving the point-of-care viral test is planned in the outpatient setting. According to Dr. Roy, this will involve a community hub to which patients can be referred for testing and clinical evaluation.
“We hope that the quality of care can be improved with the point-of-care test for respiratory viruses as well as helping to reduce antibiotic resistance,” Dr. Roy said.
This approach is promising, according to Tobias Welte, MD, of the department of respiratory medicine at Hannover (Germany) Medical School, but he cautioned that it is not a standard approach.
“The protocol described by Dr. Roy will have to be compared to guidelines and recommended best clinical practice to confirm its usefulness,” he said, while conceding that any strategy that reduces unnecessary hospitalizations deserves further evaluation.
REPORTING FROM THE ERS CONGRESS 2018
Key clinical point:
Major finding: Of patients with a negative chest x-ray and low CRP level, 36% avoided hospital admission due to a positive test for a virus.
Study details: A case series.
Disclosures: Dr. Roy reports no financial relationships relevant to this study.