User login
IV fluid weaning unnecessary after gastroenteritis rehydration
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Intravenous fluids can simply be stopped after children with acute viral gastroenteritis are rehydrated in the hospital; there’s no need for a slow wean, according to a review at the Connecticut Children’s Medical Center, Hartford.
Researchers found that children leave the hospital hours sooner, with no ill effects. “This study suggests that slowly weaning IV fluids may not be necessary,” said lead investigator Danielle Klima, DO, a University of Connecticut pediatrics resident.
The team at Connecticut Children’s noticed that weaning practices after gastroenteritis rehydration varied widely on the pediatric floors, and appeared to be largely provider dependent, with “much subjective decision making.” The team wanted to see if it made a difference one way or the other, Dr. Klima said at Pediatric Hospital Medicine.
During respiratory season, “our pediatric floors are surging. Saving even a couple hours to get these kids out” quicker matters, she said, noting that it’s likely the first time the issue has been studied.
The team reviewed 153 children aged 2 months to 18 years, 95 of whom had IV fluids stopped once physicians deemed they were fluid resuscitated and ready for an oral feeding trial; the other 58 were weaned, with at least two reductions by half before final discontinuation.
There were no significant differences in age, gender, race, or insurance type between the two groups. The mean age was 2.6 years, and there were slightly more boys. The ED triage level was a mean of 3.2 points in both groups on a scale of 1-5, with 1 being the most urgent. Children with serious comorbidities, chronic diarrhea, feeding tubes, severe electrolyte abnormalities, or feeding problems were among those excluded.
Overall length of stay was 36 hours in the stop group versus 40.5 hours in the weaning group (P = .004). Children left the hospital about 6 hours after IV fluids were discontinued, versus 26 hours after weaning was started (P less than .001).
Electrolyte abnormalities on admission were more common in the weaning group (65% versus 57%), but not significantly so (P = .541). Electrolyte abnormalities were also more common at the end of fluid resuscitation in the weaning arm, but again not significantly (65% 42%, P = .077).
Fluid resuscitation needed to be restarted in 15 children in the stop group (16%), versus 11 (19%) in the wean arm (P = .459). One child in the stop group (1%) versus four (7%) who were weaned were readmitted to the hospital within a week for acute viral gastroenteritis (P = .067).
“I expected we were taking a more conservative weaning approach in younger infants,” but age didn’t seem to affect whether patients were weaned or not, Dr. Klima said.
With the results in hand, “our group is taking a closer look at exactly what we are doing,” perhaps with an eye toward standardization or even a randomized trial, she said.
She noted that weaning still makes sense for a fussy toddler who refuses to take anything by mouth.
There was no external funding, and Dr. Klima had no disclosures. The conference was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
REPORTING FROM PHM 2019
Older patients who stop statins may be increasing their cardiovascular risk
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
Discontinuing statins was associated with an increased risk of hospital admission for a cardiovascular event, according to a study of elderly French patients with no history of heart disease.
“The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention,” wrote Philippe Giral, MD, of Hôpital La Pitié Salpêtrière (France) and coauthors. The study was published in the European Heart Journal.
To determine if statins are a cardiovascular benefit or detriment to older people, the researchers reviewed data from 120,173 patients in French health care databases who turned 75 during 2012-2014. Patients with a diagnosis of cardiovascular disease in the previous 2 years were excluded, and all eligible patients were required to have a statin medication possession ratio of at least 80% in each of the previous 2 years.
Over a follow-up period that averaged 2.4 years, 17,204 patients (14.3%) discontinued statins and 5,396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for admissions after statin discontinuation were 1.33 (95% confidence interval, 1.18-1.50) for a cardiovascular event, 1.46 (95% CI, 1.21-1.75) for a coronary event, 1.26 (95% CI, 1.05-1.51) for a cerebrovascular event, and 1.02 (95% CI, 0.74-1.40) for other vascular events, respectively.
The coauthors acknowledged their study’s limitations, including being unable to account for certain cardiovascular risk factors such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers. In addition, no information was available as to why patients discontinued statins. However, the presence of other major cardiovascular risk factors was investigated and accounted for, as was discontinuation of other cardiovascular drug therapies.
The study was not funded, and the authors declared no conflicts of interest.
SOURCE: Giral P at al. Eur Heart J. 2019 July 31. doi: 10.1093/eurheartj/ehz458.
FROM THE EUROPEAN HEART JOURNAL
Too many blood cultures ordered for pediatric SSTIs
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
SEATTLE – Blood cultures were ordered for over half of pediatric skin infection encounters across 38 children’s hospitals, with rates varying from about 20% to 80% between hospitals, according to a review of almost 50,000 encounters in the Pediatric Health Information System database.
It was a surprising finding, because current guidelines from the Infectious Diseases Society of America do not recommend blood cultures as part of the routine evaluation of uncomplicated pediatric skin and soft-tissue infections (SSTIs), meaning infections in children who are otherwise healthy without neutropenia or other complicating factors.
Just 0.6% of the cultures were positive in the review, and it’s likely some of those were caused by contamination. After adjustment for demographics, complex chronic conditions, and severity of illness, culture draws were associated with a 20% increase in hospital length of stay (LOS), hospital costs, and 30-day readmission rates.
“Our data provide more evidence that [routine] blood cultures for children with SSTI represents low-value practice and should be avoided,” said lead investigator John Stephens, MD, a pediatrics professor and hospitalist at the University of North Carolina at Chapel Hill.
Dr. Stephens became curious about how common the practice was across hospitals after he and a friend penned an article about the issue for the Journal of Hospital Medicine’s “Things We Do for No Reason” series. The single-center studies they reviewed showed similarly high rates of both testing and negative cultures (J Hosp Med. 2018 Jul;13[7]:496-9).
Dr. Stephens and his team queried the Pediatric Health Information System database for encounters in children aged 2 months to 18 years with the diagnostic code 383, “cellulitis and other skin infections,” from 2012 to 2017, during which time “there really wasn’t a change” in IDSA guidance, he noted. Transfers, encounters with ICU care, and immunocompromised children were excluded.
Hospital admissions were included in the review if they had an additional code for erysipelas, cellulitis, impetigo, or other localized skin infection. The rate of positive cultures was inferred from subsequent codes for bacteremia or septicemia.
Across 49,291 encounters, the median rate of blood culture for skin infection was 51.6%, with tremendous variation between hospitals. With blood cultures, the hospital LOS was about 1.9 days, the hospital cost was $4,030, and the 30-day readmission rate was 1.3%. Without cultures, LOS was 1.6 days, the cost was $3,291, and the readmission rate was 1%.
Although infrequent, it’s likely that positive cultures triggered additional work-up, time in the hospital, and other measures, which might help account for the increase in LOS and costs.
As for why blood testing was so common, especially in some hospitals, “I think it’s just institutional culture. No amount of clinical variation in patient population could explain” a 20%-80% “variation across hospitals. It’s really just ingrained habits,” Dr. Stephens said at Pediatric Hospital Medicine.
“The rate of positive blood culture was really low, and the association was for higher cost and utilization. I think this really reinforces the IDSA guidelines. We need to focus on quality improvement efforts to do this better,” he said, noting that he hopes to do so at his own institution.
“I’d also like to know more on the positives. In the single center studies, we know more than half of them are contaminants. Often, there’s more contamination than true positives,” he said at the meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Instead of routine blood culture, Dr. Stephens recommended in his article to send pus for a Gram stain and culture and sensitivity, while noting that blood cultures remain reasonable for complicated infections, immunocompromised patients, and neonates.
There was no external funding, and Dr. Stephens didn’t report any disclosures.
REPORTING FROM PHM 2019
Dispatch from HM19: COPD updates
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
Session presenter
Cathy Grossman MD, FCCP, CHSE
Session title
COPD Updates 2019
Session summary
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death in the United States and accounts for close to 730,000 admissions and 120,000 deaths per year.1 That correlates to one death every 4 minutes. By 2020, the adjusted cost of COPD in the United States was projected to be approximately $50 billion.2
Every COPD exacerbation is associated with economic, social, and mortality burdens. The probability of survival decreases to 20% by the end of 5 years in patients with frequent readmissions, compared with patients with no acute exacerbations of COPD.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recently released its 2019 report and gave fresh guidance on medication changes to consider in patients who have had a COPD exacerbation.
At HM19, Cathy Grossman, MD, assistant professor of medicine in the division of pulmonary and critical care medicine at Virginia Commonwealth University, Richmond, discussed the updates. She explained that most of the patients who are treated by hospitalists are GOLD group C or group D, and stressed the importance of involving the pulmonology team in the care of these patients.
Dr. Grossman explained that GOLD 2019 recommended using eosinophil counts to predict the effect of inhaled corticosteroids (ICS), added to regular maintenance bronchodilator treatment, in preventing future exacerbations. These effects are observed to be incrementally increasing at higher eosinophil counts. For patients who are taking a long-acting beta2-agonist or muscarinic antagonist (LABA or LAMA), and have a high eosinophil count (at least 300 cells/mcL, or at least 100 cells/mcL plus a history of several exacerbations), one could consider adding an ICS.4 For patients who don’t fulfill these criteria, one could try a LABA plus a LAMA. However, one has to be cautious as some of these patients get intravenous dexamethasone by EMS and admission labs may not show eosinophils.
A caveat to using ICS is that, in some of these of the patients, ICS may lead to bacterial overgrowth and therefore more pneumonias, and that may be contributing to frequent admissions of these patients. In such patients, discontinuation might be a viable option. The guidelines recommend starting GOLD group C and D patients with LAMA or LAMA/LABA combination inhalers, and ICS if they have high eosinophil counts. If patients are already on triple therapy, one could add roflumilast5 or a macrolide.
The effectiveness of noninvasive positive-pressure ventilation (NIV) in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure remains unclear, although there is some data to support the use of home NIV in patients with COPD and obstructive sleep apnea, both with and without hypercapnia. Dr. Grossman mentioned that there are still many unanswered questions, like identifying the right patient, right time, and right settings, and more studies are underway.
Dr. Grossman concluded that bread-and-butter topics like smoking cessation counseling, inhaler instruction, and referral to pulmonary rehab are still the most important tools to decrease COPD exacerbations.
Dr. Jonnalagadda is a physician advisor, and Dr. Medarametla is medical director, of hospital medicine at Baystate Medical Center in Springfield, Mass.
References
1. Guarascio AJ et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013 Jun 17;5:235-45.
2. Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Disease. National Institutes of Health and National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf.
3. Soler-Cataluña JJ et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease; Thorax. 2005;60:925-31.
4. Cheng SL. Blood eosinophils and inhaled corticosteroids in patients with COPD: Systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018 Sept 6;13:2775-84.
5. FJ Martinez et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicenter, randomized, controlled trial. Lancet. 2015;385(9971):857-66.
AI technology meets AFib detection
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
This artificial intelligence-enabled ECG interpretation is groundbreaking in creating an algorithm to reveal the likelihood of atrial fibrillation in ECGs showing sinus rhythm.
AFib is now considered a global pandemic and needs to be detected not only to manage the arrhythmia but also to prevent comorbidities and death.
A 10-second, 12-lead ECG in current clinical practice is unlikely to reveal possible AFib if not present in this short monitoring time. However, the findings have clinical importance, particularly in identifying silent AFib and may have important implications for secondary prevention of patients with embolic stroke of undetermined source in terms of providing appropriate oral anticoagulation to prevent recurrences of stroke. The AI-enabled algorithm would require further validation in a different patient cohort, testing a healthier out-of-hospital population, as well as a rigorous prospective clinical trial assessment.
Future research areas include combining ECG algorithms with demographic variables, clinical features, and biomarkers, as well as exploring the use of wearable devices linking these variables and AI for smart monitoring to diagnose AFib.
Jeroen Hendriks, MD, of the University of Adelaide (Australia), and Larissa Fabritz, MD, of the University of Birmingham (England), made these comments in an accompanying editorial. Dr. Hendriks disclosed lecture or consulting fees from Medtronic and Pfizer/Bristol-Myers Squibb. Dr. Fabritz is the inventor of two patents and disclosed research grants and nonfinancial support from European research institutions and Gilead.
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
An artificial intelligence-enabled ECG model identified patients with intermittent atrial fibrillation in a 10-second test with 83% accuracy, based on data from more than 180,000 individuals.
“We have previously shown convolution neural networks can evaluate the resting ECG for detection of antiarrhythmic drug levels, abnormal electrolytes levels, and detection of asymptomatic left ventricular dysfunction, providing proof of concept that clinically important phenomena can be detected with artificial intelligence (AI) applications to the ECG,” wrote Zachi I. Attia, an electrical engineer and a primary author of the study, is with the Mayo Clinic, Rochester, Minn., and colleagues.
In a study published in the Lancet, the researchers reviewed data from 649,931 normal sinus rhythm ECGs collected from 180,922 adults between December 1993 and July 2017.
The ECGs were divided into three groups: training (454,789 ECGs from 126,526 patients) internal validation (64,340 ECGs from 18,116 patients) and testing (130,802 ECGs from 36,280 patients). The primary outcome was whether the AI-programmed ECG could identify AFib in a total of 3,051 patients in the testing data set who had verified AFib before being tested with the AI device. The AI-enabled ECG was designed to detect subtle changes using neural network technology previously used by the researchers to identify ventricular dysfunction.
Overall, a single ECG scan identified AFib with an accuracy of 79.4%, an area under the curve (AUC) of 0.87, sensitivity of 79.0%, and specificity of 79.5%. When researchers reviewed multiple ECGs from a 1-month window of either the study start date or 31 days before the first AFib, the accuracy increased to 83.3%, with an AUC of 0.90, sensitivity of 82.3%, and specificity of 83.4%.
The results support the use of subtle changes on normal sinus rhythm ECG to identify patient with potentially undetected AFib, and suggest that AI-enabled ECGs could be used at the point of care to identify patients at risk after unexplained strokes, also known as embolic stroke of undetermined source (ESUS), or heart failure, the researchers noted.
“Although it would require further study, it is possible that this algorithm could identify a high-risk subset of patients with ESUS who could benefit from empirical anticoagulation,” the researchers said.
The study findings were limited by several factors, including possible mislabeling of patients with unidentified atrial fibrillation who were classified negative. In addition, the prevalence of AFib in the study population may be higher than in the general population, they said.
However, the results suggest that use a noninvasive, widely available, point of care test to identify AFib “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” they concluded.
This study was funded by internal resources of the Mayo Clinic. The researchers had no financial conflicts to disclose.
SOURCE: Attia ZI et al. Lancet. 2019 Aug 1. doi. org/10.1016/S0140-6736(19)31721-0.
FROM THE LANCET
Enteral feeding is safe during bronchiolitis HFNC
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – There were no cases of aspiration with enteric feeds of 60 children aged up to 2 years on high flow nasal cannula (HFNC) for bronchiolitis at the University of Oklahoma Children’s Hospital, Oklahoma City, according to research presented at the 2019 Pediatric Hospital Medicine Conference.
HFNC has become common for bronchiolitis management; it often saves infants from intubation. However, many providers opt for total parenteral nutrition during therapy instead of enteral feeding because of concerns about aspiration pneumonia.
Pediatricians at the children’s hospital began to wonder if the concern was really necessary. There have been reports of safe feeding during HFNC, and “clinical care literature has shown that feeding the gut throughout illness improves outcomes,” said lead investigator, Sarah Walter, MD, a third-year pediatrics resident at the hospital.
So her team took a leap of faith. They consulted the HFNC literature, asked their fellow providers what they would be comfortable with, and instituted a pediatric HFNC enteral feeding protocol at the children’s hospital for use on inpatient floors, pediatric ICUs, and elsewhere.
Feedings – formula or breast milk – are triggered by stable respiratory Tal scores over 8 hours, meaning that respiratory rates, breath sounds, and accessory muscle use were stable or improving. Children on a flow of 6 L/min or less, with a respiratory rate below 60 breaths per minute, are started on oral feeds, and those on higher flows on nasogastric (NG) tube feeds.
Feeds are started at 1 mL/kg per hour and advanced by the same amount every 3 hours until volume goals are reached; IV fluids are tapered accordingly. It’s a standing order, so nurses are able to initiate and advance feeding as indicated, any time of day.
Feeding was temporarily suspended in only 17 children: 6 for emesis, 6 for worsening respiratory scores, and the rest for dislodged NG tubes, procedures, or other issues. Enteric feeds were restarted with two stable scores below 7 points, at half the rate at which they were stopped.
NG tubes were used in over half of the 478 nursing shifts during which the 60 children – the majority aged 4-24 months – were fed; oral feeds in more than a third; and gastric tubes and other options in the rest. IV nutrition was used during just 1.8% of the shifts.
Enteric feeds were given up to a flow rate of 3.5 L/kg. There were no aspirations, even when children vomited. “We have seen good results so far that feeding is safe in these children,” Dr. Walters said.
“Our hospitalist team has been very receptive; they have been using the order set pretty continuously.” Parents also feel better when they know their children were “getting food in their belly,” even if by NG tube. “It’s important for family satisfaction,” she said.
The next step is to assess impact on length of stay, and education efforts to encourage broader use of the order set.
There was no external funding, and Dr. Walter had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
REPORTING FROM PHM 2019
U.S. infant mortality continued slow decline in 2017
according to data released Aug. 1 by the National Center for Health Statistics, based on data from the National Vital Statistics System.
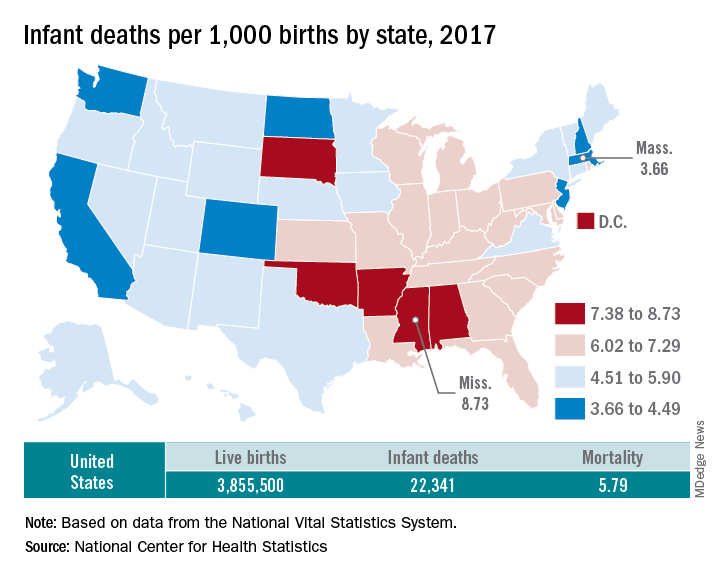
The rate for 2017 was 5.79 deaths per 1,000 live births, which was not statistically different from the rate of 5.87 in 2016, the National Center for Health Statistics said in a new report. Neonatal and postneonatal mortality – 3.85 and 1.94 per 1,000, respectively – both showed the same nonsignificant drop from 2016 to 2017.
About two-thirds of the infants who died in 2017 were children born preterm (less than 37 weeks’ gestation), the NCHS said, and “the mortality rate for infants born before 28 weeks of gestation [389.4 per 1,000] was 183 times the rate for term infants” born at 37-41 weeks.
Rates at the state level in 2017 ranged from a low of 3.66 deaths/1,000 live births in Massachusetts to a high of 8.73/1,000 in Mississippi. Washington (3.88) was the only other state with a rate below 4.0, while Arkansas (8.10) was the only other state above 8.0 (The District of Columbia had a rate of 8.16.). Infant mortality was significantly lower than the national rate in 11 states and significantly higher in 15 states and D.C., according to the report.
Overall, in 2017, 3,855,500 live births occurred, with 22,341 infants having died before the age of 1 year, data from the National Vital Statistics System’s linked birth/infant death file show. In 1995, the first year that the linked file was available, the corresponding numbers were 3,899,589 births and 29,505 deaths, for a rate of 7.57 deaths/1,000 live births.
according to data released Aug. 1 by the National Center for Health Statistics, based on data from the National Vital Statistics System.

The rate for 2017 was 5.79 deaths per 1,000 live births, which was not statistically different from the rate of 5.87 in 2016, the National Center for Health Statistics said in a new report. Neonatal and postneonatal mortality – 3.85 and 1.94 per 1,000, respectively – both showed the same nonsignificant drop from 2016 to 2017.
About two-thirds of the infants who died in 2017 were children born preterm (less than 37 weeks’ gestation), the NCHS said, and “the mortality rate for infants born before 28 weeks of gestation [389.4 per 1,000] was 183 times the rate for term infants” born at 37-41 weeks.
Rates at the state level in 2017 ranged from a low of 3.66 deaths/1,000 live births in Massachusetts to a high of 8.73/1,000 in Mississippi. Washington (3.88) was the only other state with a rate below 4.0, while Arkansas (8.10) was the only other state above 8.0 (The District of Columbia had a rate of 8.16.). Infant mortality was significantly lower than the national rate in 11 states and significantly higher in 15 states and D.C., according to the report.
Overall, in 2017, 3,855,500 live births occurred, with 22,341 infants having died before the age of 1 year, data from the National Vital Statistics System’s linked birth/infant death file show. In 1995, the first year that the linked file was available, the corresponding numbers were 3,899,589 births and 29,505 deaths, for a rate of 7.57 deaths/1,000 live births.
according to data released Aug. 1 by the National Center for Health Statistics, based on data from the National Vital Statistics System.

The rate for 2017 was 5.79 deaths per 1,000 live births, which was not statistically different from the rate of 5.87 in 2016, the National Center for Health Statistics said in a new report. Neonatal and postneonatal mortality – 3.85 and 1.94 per 1,000, respectively – both showed the same nonsignificant drop from 2016 to 2017.
About two-thirds of the infants who died in 2017 were children born preterm (less than 37 weeks’ gestation), the NCHS said, and “the mortality rate for infants born before 28 weeks of gestation [389.4 per 1,000] was 183 times the rate for term infants” born at 37-41 weeks.
Rates at the state level in 2017 ranged from a low of 3.66 deaths/1,000 live births in Massachusetts to a high of 8.73/1,000 in Mississippi. Washington (3.88) was the only other state with a rate below 4.0, while Arkansas (8.10) was the only other state above 8.0 (The District of Columbia had a rate of 8.16.). Infant mortality was significantly lower than the national rate in 11 states and significantly higher in 15 states and D.C., according to the report.
Overall, in 2017, 3,855,500 live births occurred, with 22,341 infants having died before the age of 1 year, data from the National Vital Statistics System’s linked birth/infant death file show. In 1995, the first year that the linked file was available, the corresponding numbers were 3,899,589 births and 29,505 deaths, for a rate of 7.57 deaths/1,000 live births.
Short-course azithromycin no benefit in pediatric asthma admissions
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
SEATTLE – Adding a 3-day course of azithromycin to treatment regimens of children hospitalized with asthma did not shorten length of stay or bring other benefits in a randomized, blinded trial of more than 150 youngsters at The Children’s Hospital at Montefiore, New York.
In recent years, some pediatricians at Montefiore had begun giving short-course azithromycin to hospitalized children who were not recovering as quickly as they had hoped, spurred by outpatient reports of reduced exacerbations and other benefits with long-term azithromycin (e.g., Lancet. 2017 Aug 12;390(10095):659-68).
“We had no evidence for doing that at all” in the hospital, and it might be going on elsewhere, said senior investigator Alyssa Silver, MD, assistant professor of pediatrics at Montefiore and Albert Einstein College of Medicine, New York. She and her colleagues, including primary investigator Lindsey Douglas, MD, assistant professor of pediatrics at the Icahn School of Medicine at Mount Sinai, New York, took a closer look.
The negative results mean that “we can stop doing this, giving kids unnecessary things. Word is starting to get out” at Montefiore. “People are not using it as much,” she said at Pediatric Hospital Medicine, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
The team had expected azithromycin to shorten length of stay (LOS) by about half a day, due to its anti-inflammatory effects, but that’s not what was found when they randomized 80 children aged 4-12 years with persistent asthma to oral azithromycin 10 mg/kg per day for 3 days within 12 hours of admission, and 79 to placebo.
LOS was 1.86 days in the placebo arm, and 1.69 days in the azithromycin group (P = .23). One placebo child was transferred to the pediatric ICU, versus none in the azithromycin arm (P = .50). The study was stopped short of its 214 subject enrollment goal because of futility, but even so, it was well powered to detect a difference in LOS, the primary outcome, Dr. Silver said.
At 1 week phone follow-up, 7 placebo children and 11 in the azithromycin arm had persistent asthma symptoms (P = .42), and 1 placebo child and 2 azithromycin children had been readmitted (P greater than .99). There were no differences in days of school missed, or work days missed among parents and guardians.
At one month, 23 placebo and 18 azithromycin children had persistent asthma symptoms (P = .5); 7 placebo and 6 azithromycin children had returned to the ED (P = .75).
In short, “we really found no difference” with short-course azithromycin. “Clinicians should consider [these] data before prescribing azithromycin [to] children hospitalized with asthma,” Dr. Silver and her team concluded.
Subjects were an average of about 7 years old, and about two-thirds were boys. They were not on azithromycin or other antibiotics prior to admission. About half had been admitted in the previous year, and about a quarter had at least one previous pediatric ICU admission. Over two-thirds had been on daily asthma medications. There were about 2 days of symptoms prior to admission.
There was no external funding, and Dr. Silver had no disclosures.
REPORTING FROM PHM 2019
Short Takes
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
Comparison of the number of major complications of laparoscopic cholecystectomy versus percutaneous catheter drainage in the treatment of acute cholecystitis
This randomized, controlled trial showed that 65% of high-risk patients (APACHE II score of at least 7) with acute cholecystitis experienced major complications after undergoing percutaneous catheter drainage, compared with 12% of patients who underwent laparoscopic cholecystectomy. Major complications included reintervention and recurrent biliary disease. The rate of death was the same in both groups.
Citation: Loozen CS et al. Laparoscopic cholecystectomy versus percutaneous catheter drainage for acute cholecystitis in high-risk patients (CHOCOLATE): Multicentre randomised clinical trial. BMJ. 2018 Aug 28;363:k3965.
Food and Drug Administration approves new drug to treat influenza
Two randomized, controlled trials showed that Xofluza (baloxavir marboxil) taken as a single dose decreased symptoms in uncomplicated influenza, compared with placebo. The medication also was associated with a lower viral load on day 1 after administration, compared with both placebo and oseltamivir, the most commonly used medication to treat influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018 Sep 6;379:913-23.
Effects of missed hemodialysis treatments
Researchers used a prospective cohort of 8,501 patients from hemodialysis (HD) centers in 20 countries to identify patients who missed one or more HD sessions in 4 months. In the United States, 24% of HD patients missed one or more sessions in 4 months, compared with 10% in Canada and 9% in the United Kingdom. Moreover, 12.2% of U.S. HD patients missed at least one session per month. All-cause mortality was 68% higher in patients who missed one or more sessions in 4 months. Risk factors associated with missing dialysis treatments were travel time of more than 1 hour to the facility, depression, younger age, being on dialysis for a shorter vintage, lower perceived burden of kidney disease, and shorter treatment times.
Citation: Al Salmi I et al. Missed hemodialysis treatments: International variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2018 Nov;72(5)634-43.
Do in situ mock codes affect in-hospital cardiac arrest mortality?
This ecological study included multiple hospital systems and showed that hospitals with a higher proportion of in situ mock codes had an in-hospital cardiac arrest survival rate of 42.8% versus 31.8% in hospitals with fewer mock codes (P greater than .0001).
Citation: Josey K et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018 Dec;133:47-52.
New oxygen guidelines
In patients admitted with acute stroke or MI, an international expert panel made a strong recommendation against initiating supplemental oxygen when the SpO2 is greater than 92% and a weak recommendation against initiating supplemental oxygen when the SpO2 is 90%-92%. In acutely ill medical patients receiving supplemental oxygen, the panel makes a strong recommendation to maintain an upper limit oxygen saturation of less than 96%.
Citation: Siemieniuk RAC et al. Oxygen therapy for acutely ill medical patients: A clinical practice guideline. BMJ. 2018 Oct 24;363:k4169.
FDA approvals permit double-immunoassay approach to Lyme disease diagnosis
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.
Concurrent or sequential enzyme immunoassays can now be conducted to diagnose Lyme disease, according to the U.S. Food and Drug Administration.
Four previously cleared tests are now approved by the agency for marketing with new indications as part of the revised diagnostic approach. Previously, the two-step diagnostic process consisted of an initial enzyme immunoassay followed by a Western blot test.
“With today’s action, clinicians have a new option to test for Lyme that is easier to interpret by a clinical laboratory due to the streamlined method of conducting the test. These tests may improve confidence in diagnosing a patient for a condition that requires the earliest possible treatment to ensure the best outcome for patients,” Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiologic Health, said in a press release announcing the newly approved approach.
The modified two-tier enzyme immunoassay approach was found to be as accurate for assessing exposure to Borrelia burgdorferi as the standard immunoassay followed by Western blot test in an FDA review of data from clinical studies using the following ZEUS Scientific ELISA Test Systems: Borrelia VlsE1/pepC10 IgG/IgM; Borrelia burgdorferi IgG/IgM; Borrelia burgdorferi IgM; and Borrelia burgdorferi IgG.
The recommendations of the Centers for Disease Control and Prevention should be followed for the diagnosis of Lyme disease and for determining when laboratory tests are appropriate, the FDA statement said. In 2017, the last year for which the CDC published data, a total of 42,743 confirmed and probable cases of Lyme disease were reported, an increase of 17% from 2016.
The FDA granted clearance of the ZEUS ELISA enzyme immunoassay tests to ZEUS Scientific.














