User login
FDA approves drug combo to treat highly resistant TB
The U.S. Food and Drug Administration granted special approval to a new drug combo intended for the treatment of “a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug-resistant pulmonary” tuberculosis, according to an FDA news release.
The effectiveness of the combination treatment of pretomanid tablets with bedaquiline and linezolid was shown in a clinical study of patients with extensively drug-resistant, treatment-intolerant, or nonresponsive multidrug-resistant pulmonary tuberculosis of the lungs. Of 107 infected patients who were evaluated 6 months after the end of therapy, 95 (89%) were deemed successes, which significantly exceeded the historical success rates for treatment of extensively drug-resistant TB, the FDA reported. The trial is sponsored by the Global Alliance for TB Drug Development.
The most common adverse effects reported included peripheral neuropathy, anemia, nausea, vomiting, headache, increased liver enzymes, dyspepsia, rash, visual impairment, low blood sugar, and diarrhea, according to the release.
“Multidrug-resistant TB and extensively drug-resistant TB are public health threats due to limited treatment options. New treatments are important to meet patient national and global health needs,” stated FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD, in the release. She also explained that the approval marked the second time a drug was approved under the “Limited Population Pathway for Antibacterial and Antifungal Drugs, a pathway advanced by Congress to spur development of drugs targeting infections that lack effective therapies.”
In 2016, the World Health Organization reported that there were an estimated 490,000 new cases of multidrug-resistant TB worldwide, with a smaller portion of cases of extensively drug-resistant TB, according to the release, demonstrating the need for new therapeutics.
SOURCE: U.S. Food and Drug Administration. Aug. 14, 2019. News release.
The U.S. Food and Drug Administration granted special approval to a new drug combo intended for the treatment of “a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug-resistant pulmonary” tuberculosis, according to an FDA news release.
The effectiveness of the combination treatment of pretomanid tablets with bedaquiline and linezolid was shown in a clinical study of patients with extensively drug-resistant, treatment-intolerant, or nonresponsive multidrug-resistant pulmonary tuberculosis of the lungs. Of 107 infected patients who were evaluated 6 months after the end of therapy, 95 (89%) were deemed successes, which significantly exceeded the historical success rates for treatment of extensively drug-resistant TB, the FDA reported. The trial is sponsored by the Global Alliance for TB Drug Development.
The most common adverse effects reported included peripheral neuropathy, anemia, nausea, vomiting, headache, increased liver enzymes, dyspepsia, rash, visual impairment, low blood sugar, and diarrhea, according to the release.
“Multidrug-resistant TB and extensively drug-resistant TB are public health threats due to limited treatment options. New treatments are important to meet patient national and global health needs,” stated FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD, in the release. She also explained that the approval marked the second time a drug was approved under the “Limited Population Pathway for Antibacterial and Antifungal Drugs, a pathway advanced by Congress to spur development of drugs targeting infections that lack effective therapies.”
In 2016, the World Health Organization reported that there were an estimated 490,000 new cases of multidrug-resistant TB worldwide, with a smaller portion of cases of extensively drug-resistant TB, according to the release, demonstrating the need for new therapeutics.
SOURCE: U.S. Food and Drug Administration. Aug. 14, 2019. News release.
The U.S. Food and Drug Administration granted special approval to a new drug combo intended for the treatment of “a limited and specific population of adult patients with extensively drug resistant, treatment-intolerant or nonresponsive multidrug-resistant pulmonary” tuberculosis, according to an FDA news release.
The effectiveness of the combination treatment of pretomanid tablets with bedaquiline and linezolid was shown in a clinical study of patients with extensively drug-resistant, treatment-intolerant, or nonresponsive multidrug-resistant pulmonary tuberculosis of the lungs. Of 107 infected patients who were evaluated 6 months after the end of therapy, 95 (89%) were deemed successes, which significantly exceeded the historical success rates for treatment of extensively drug-resistant TB, the FDA reported. The trial is sponsored by the Global Alliance for TB Drug Development.
The most common adverse effects reported included peripheral neuropathy, anemia, nausea, vomiting, headache, increased liver enzymes, dyspepsia, rash, visual impairment, low blood sugar, and diarrhea, according to the release.
“Multidrug-resistant TB and extensively drug-resistant TB are public health threats due to limited treatment options. New treatments are important to meet patient national and global health needs,” stated FDA Principal Deputy Commissioner Amy Abernethy, MD, PhD, in the release. She also explained that the approval marked the second time a drug was approved under the “Limited Population Pathway for Antibacterial and Antifungal Drugs, a pathway advanced by Congress to spur development of drugs targeting infections that lack effective therapies.”
In 2016, the World Health Organization reported that there were an estimated 490,000 new cases of multidrug-resistant TB worldwide, with a smaller portion of cases of extensively drug-resistant TB, according to the release, demonstrating the need for new therapeutics.
SOURCE: U.S. Food and Drug Administration. Aug. 14, 2019. News release.
NEWS FROM THE FDA
Study: Cardiac biomarkers predicted CV events in CAP
in a recently conducted study.
These biomarkers were also used to predict late cardiovascular events at day 30 of community-acquired pneumonia (CAP) in patients who did not have a history of cardiovascular disease, according to Rosario Menéndez, MD, from the Hospital Universitario y Politécnico La Fe and Instituto de Investigación Sanitaria La Fe in Valencia, Spain, and colleagues.
“Some patients have still high levels of inflammatory and cardiac biomarkers at 30 days, when they are usually referred to primary care without receiving any specific additional recommendations,” Dr. Menéndez and colleagues wrote in CHEST. “Our results suggest that a change in usual practice is needed to reduce current and further cardiovascular CAP complications.”
Dr. Menéndez and colleagues prospectively followed 730 patients for 1 year who were hospitalized for CAP, measuring the cardiac biomarkers proadrenomedullin (proADM), pro b-type natriuretic peptide (proBNP), proendothelin-1, and troponin T, and the inflammatory biomarkers interleukin 6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT). The researchers also collected data on age, gender, smoking status, and vaccination history, as well as whether patients had any cardiac, renal, pulmonary, neurological or diabetes-related comorbidities.
Overall, 95 patients experienced early cardiovascular events, 67 patients had long-term cardiovascular events, and 20 patients experienced both early and late events. In hospital, the mortality rate was 4.7%; the 30-day mortality rate was 5.3%, and the 1-year mortality rate was 9.9%.
With regard to biomarkers, patients who experienced both early and late cardiovascular events had significantly higher initial levels of proADM, proendothelin-1, troponin, proBNP, and IL-6. Patients who experienced later events had consistent levels of these biomarkers until day 30, except for a decrease at day 4 or day 5.
After adjustment for age, sepsis, previous cardiac disease, and a partial pressure of oxygen in the alveoli to fractional inspired oxygen ratio (PaO2/FiO2) of less than 250mm Hg, cardiac biomarkers proendothelin-1 (odds ratio, 2.25; 95% confidence interval, 1.34-3.79), proADM (OR, 2.53; 95% CI, 1.53-4.20), proBNP (OR, 2.67; 95% CI, 1.59-4.49), and troponin T (OR, 2.70; 95% CI, 1.62-4.49) significantly predicted early cardiovascular events, while proendothelin-1 (OR, 3.13; 95% CI, 1.41-7.80), proADM (2.29; 95% CI, 1.01-5.19) and proBNP (OR, 2.34; 95% CI, 1.01-5.56) significantly predicted late cardiovascular events. For day 30 results, when researchers added IL-6 levels to proendothelin-1, the odds ratio for late events increased to 3.53, and when they added IL-6 levels to proADM, the odds ratio increased to 2.80.
Researchers noted the limitations of the study included that they did not analyze cardiac biomarkers to predict specific cardiovascular events, did not identify the cause for mortality at 1 year in most patients, and did not include a control group.
This study was supported in part by funding from Instituto de Salud Carlos III, Sociedad Española de Neumología y Cirugía Torácica, and the Center for Biomedical Research Network in Respiratory Diseases. The authors reported no relevant conflicts of interest.
SOURCE: Menéndez R et al. Chest. 2019 Aug 2. doi: 10.1016/j.chest.2019.06.040.
in a recently conducted study.
These biomarkers were also used to predict late cardiovascular events at day 30 of community-acquired pneumonia (CAP) in patients who did not have a history of cardiovascular disease, according to Rosario Menéndez, MD, from the Hospital Universitario y Politécnico La Fe and Instituto de Investigación Sanitaria La Fe in Valencia, Spain, and colleagues.
“Some patients have still high levels of inflammatory and cardiac biomarkers at 30 days, when they are usually referred to primary care without receiving any specific additional recommendations,” Dr. Menéndez and colleagues wrote in CHEST. “Our results suggest that a change in usual practice is needed to reduce current and further cardiovascular CAP complications.”
Dr. Menéndez and colleagues prospectively followed 730 patients for 1 year who were hospitalized for CAP, measuring the cardiac biomarkers proadrenomedullin (proADM), pro b-type natriuretic peptide (proBNP), proendothelin-1, and troponin T, and the inflammatory biomarkers interleukin 6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT). The researchers also collected data on age, gender, smoking status, and vaccination history, as well as whether patients had any cardiac, renal, pulmonary, neurological or diabetes-related comorbidities.
Overall, 95 patients experienced early cardiovascular events, 67 patients had long-term cardiovascular events, and 20 patients experienced both early and late events. In hospital, the mortality rate was 4.7%; the 30-day mortality rate was 5.3%, and the 1-year mortality rate was 9.9%.
With regard to biomarkers, patients who experienced both early and late cardiovascular events had significantly higher initial levels of proADM, proendothelin-1, troponin, proBNP, and IL-6. Patients who experienced later events had consistent levels of these biomarkers until day 30, except for a decrease at day 4 or day 5.
After adjustment for age, sepsis, previous cardiac disease, and a partial pressure of oxygen in the alveoli to fractional inspired oxygen ratio (PaO2/FiO2) of less than 250mm Hg, cardiac biomarkers proendothelin-1 (odds ratio, 2.25; 95% confidence interval, 1.34-3.79), proADM (OR, 2.53; 95% CI, 1.53-4.20), proBNP (OR, 2.67; 95% CI, 1.59-4.49), and troponin T (OR, 2.70; 95% CI, 1.62-4.49) significantly predicted early cardiovascular events, while proendothelin-1 (OR, 3.13; 95% CI, 1.41-7.80), proADM (2.29; 95% CI, 1.01-5.19) and proBNP (OR, 2.34; 95% CI, 1.01-5.56) significantly predicted late cardiovascular events. For day 30 results, when researchers added IL-6 levels to proendothelin-1, the odds ratio for late events increased to 3.53, and when they added IL-6 levels to proADM, the odds ratio increased to 2.80.
Researchers noted the limitations of the study included that they did not analyze cardiac biomarkers to predict specific cardiovascular events, did not identify the cause for mortality at 1 year in most patients, and did not include a control group.
This study was supported in part by funding from Instituto de Salud Carlos III, Sociedad Española de Neumología y Cirugía Torácica, and the Center for Biomedical Research Network in Respiratory Diseases. The authors reported no relevant conflicts of interest.
SOURCE: Menéndez R et al. Chest. 2019 Aug 2. doi: 10.1016/j.chest.2019.06.040.
in a recently conducted study.
These biomarkers were also used to predict late cardiovascular events at day 30 of community-acquired pneumonia (CAP) in patients who did not have a history of cardiovascular disease, according to Rosario Menéndez, MD, from the Hospital Universitario y Politécnico La Fe and Instituto de Investigación Sanitaria La Fe in Valencia, Spain, and colleagues.
“Some patients have still high levels of inflammatory and cardiac biomarkers at 30 days, when they are usually referred to primary care without receiving any specific additional recommendations,” Dr. Menéndez and colleagues wrote in CHEST. “Our results suggest that a change in usual practice is needed to reduce current and further cardiovascular CAP complications.”
Dr. Menéndez and colleagues prospectively followed 730 patients for 1 year who were hospitalized for CAP, measuring the cardiac biomarkers proadrenomedullin (proADM), pro b-type natriuretic peptide (proBNP), proendothelin-1, and troponin T, and the inflammatory biomarkers interleukin 6 (IL-6), C-reactive protein (CRP), and procalcitonin (PCT). The researchers also collected data on age, gender, smoking status, and vaccination history, as well as whether patients had any cardiac, renal, pulmonary, neurological or diabetes-related comorbidities.
Overall, 95 patients experienced early cardiovascular events, 67 patients had long-term cardiovascular events, and 20 patients experienced both early and late events. In hospital, the mortality rate was 4.7%; the 30-day mortality rate was 5.3%, and the 1-year mortality rate was 9.9%.
With regard to biomarkers, patients who experienced both early and late cardiovascular events had significantly higher initial levels of proADM, proendothelin-1, troponin, proBNP, and IL-6. Patients who experienced later events had consistent levels of these biomarkers until day 30, except for a decrease at day 4 or day 5.
After adjustment for age, sepsis, previous cardiac disease, and a partial pressure of oxygen in the alveoli to fractional inspired oxygen ratio (PaO2/FiO2) of less than 250mm Hg, cardiac biomarkers proendothelin-1 (odds ratio, 2.25; 95% confidence interval, 1.34-3.79), proADM (OR, 2.53; 95% CI, 1.53-4.20), proBNP (OR, 2.67; 95% CI, 1.59-4.49), and troponin T (OR, 2.70; 95% CI, 1.62-4.49) significantly predicted early cardiovascular events, while proendothelin-1 (OR, 3.13; 95% CI, 1.41-7.80), proADM (2.29; 95% CI, 1.01-5.19) and proBNP (OR, 2.34; 95% CI, 1.01-5.56) significantly predicted late cardiovascular events. For day 30 results, when researchers added IL-6 levels to proendothelin-1, the odds ratio for late events increased to 3.53, and when they added IL-6 levels to proADM, the odds ratio increased to 2.80.
Researchers noted the limitations of the study included that they did not analyze cardiac biomarkers to predict specific cardiovascular events, did not identify the cause for mortality at 1 year in most patients, and did not include a control group.
This study was supported in part by funding from Instituto de Salud Carlos III, Sociedad Española de Neumología y Cirugía Torácica, and the Center for Biomedical Research Network in Respiratory Diseases. The authors reported no relevant conflicts of interest.
SOURCE: Menéndez R et al. Chest. 2019 Aug 2. doi: 10.1016/j.chest.2019.06.040.
FROM CHEST
Novel score spots high-risk febrile children in ED
LJUBLJANA, SLOVENIA – A new age-adjusted quick Sequential Organ Failure Assessment (qSOFA) score designed for use in children presenting to the ED with fever showed good predictive value for admission to critical care within the next 48 hours, Aakash Khanijau, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In the needle-in-a-haystack scenario that’s seen in pediatric emergency departments, our novel, age-adjusted qSOFA score could potentially improve the rapid identification and treatment of children with suspected sepsis presenting to the ED,” said Dr. Khanijau of the University of Liverpool (England).
He presented an exceptionally large retrospective validation study of the score’s performance in 12,393 children (median age, 2.5 years) who presented to EDs with fever, of whom 1,521 were admitted for suspected sepsis. Of the hospitalized children, 145 were admitted to critical care within the first 48 hours.
The pediatric qSOFA score had 72% sensitivity and 85% specificity for critical care admission within 48 hours, with a positive predictive value of 5.4% and, more importantly, a whopping negative predictive value of 99.6%.
“That very high negative predictive value underlines the powerful discriminatory nature of our tool in the emergency department setting,” Dr. Khanijau observed, adding that the score’s area under the receiver operating characteristic curve was 0.81, which is considered a good predictive value.
The impetus for developing an age-adjusted pediatric qSOFA score stems from the fact that the original qSOFA score was designed for rapid assessment of adults with suspected sepsis and isn’t applicable in children. Other existing scores, including SIRS (the Systemic Inflammatory Response Syndrome criteria), the full SOFA, and PELOD-2 (the Pediatric Logistic Organ Dysfunction score), take longer to determine than the adapted qSOFA in a setting where speed is of the essence, he explained.
The original qSOFA components are altered mentation, systolic blood pressure, and respiratory rate. The novel score developed by Dr. Khanijau and coworkers swaps out systolic BP in favor of capillary refill time and age-adjusted heart rate using the thresholds previously established in a landmark study from the Children’s Hospital of Philadelphia (Pediatrics. 2013 Apr;131[4]:e1150-7.)
“Our reasoning here is that arterial hypertension is known to be a much later sign of circulatory compromise in children and may provide less discriminatory value than signs such as delayed capillary refill time and tachycardia early in presentation in the emergency department,” according to Dr. Khanijau.
The novel scoring system features four criteria. One point each is given for a capillary refill time of 3 seconds or longer; anything less than “Alert” on the Alert, Responds to Voice, Respond to Pain, and Unresponsive scale; a heart rate above the 99th percentile on the age-adjusted curves; and a respiratory rate above the age-adjusted 99th percentile. Thus, scores can range from 0 to 4. In the validation study, a score of 2 or more spelled a 890% increased likelihood of being admitted to a critical care setting within 48 hours. It was also associated with a 100-fold increased likelihood of death during the hospitalization, which occurred in 10 children.
Asked how the new predictive score could change clinical management, Dr. Khanijau replied, “I think the key thing it does here is it identifies the children at risk of requiring critical care and should therefore motivate us in the children achieving that threshold to promptly investigate thoroughly for suspected sepsis using the more comprehensive tools, like the full SOFA.”
He reported having no financial conflicts of interest regarding his study.
SOURCE: Khanijau A et al. ESPID 2019, Abstract.
LJUBLJANA, SLOVENIA – A new age-adjusted quick Sequential Organ Failure Assessment (qSOFA) score designed for use in children presenting to the ED with fever showed good predictive value for admission to critical care within the next 48 hours, Aakash Khanijau, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In the needle-in-a-haystack scenario that’s seen in pediatric emergency departments, our novel, age-adjusted qSOFA score could potentially improve the rapid identification and treatment of children with suspected sepsis presenting to the ED,” said Dr. Khanijau of the University of Liverpool (England).
He presented an exceptionally large retrospective validation study of the score’s performance in 12,393 children (median age, 2.5 years) who presented to EDs with fever, of whom 1,521 were admitted for suspected sepsis. Of the hospitalized children, 145 were admitted to critical care within the first 48 hours.
The pediatric qSOFA score had 72% sensitivity and 85% specificity for critical care admission within 48 hours, with a positive predictive value of 5.4% and, more importantly, a whopping negative predictive value of 99.6%.
“That very high negative predictive value underlines the powerful discriminatory nature of our tool in the emergency department setting,” Dr. Khanijau observed, adding that the score’s area under the receiver operating characteristic curve was 0.81, which is considered a good predictive value.
The impetus for developing an age-adjusted pediatric qSOFA score stems from the fact that the original qSOFA score was designed for rapid assessment of adults with suspected sepsis and isn’t applicable in children. Other existing scores, including SIRS (the Systemic Inflammatory Response Syndrome criteria), the full SOFA, and PELOD-2 (the Pediatric Logistic Organ Dysfunction score), take longer to determine than the adapted qSOFA in a setting where speed is of the essence, he explained.
The original qSOFA components are altered mentation, systolic blood pressure, and respiratory rate. The novel score developed by Dr. Khanijau and coworkers swaps out systolic BP in favor of capillary refill time and age-adjusted heart rate using the thresholds previously established in a landmark study from the Children’s Hospital of Philadelphia (Pediatrics. 2013 Apr;131[4]:e1150-7.)
“Our reasoning here is that arterial hypertension is known to be a much later sign of circulatory compromise in children and may provide less discriminatory value than signs such as delayed capillary refill time and tachycardia early in presentation in the emergency department,” according to Dr. Khanijau.
The novel scoring system features four criteria. One point each is given for a capillary refill time of 3 seconds or longer; anything less than “Alert” on the Alert, Responds to Voice, Respond to Pain, and Unresponsive scale; a heart rate above the 99th percentile on the age-adjusted curves; and a respiratory rate above the age-adjusted 99th percentile. Thus, scores can range from 0 to 4. In the validation study, a score of 2 or more spelled a 890% increased likelihood of being admitted to a critical care setting within 48 hours. It was also associated with a 100-fold increased likelihood of death during the hospitalization, which occurred in 10 children.
Asked how the new predictive score could change clinical management, Dr. Khanijau replied, “I think the key thing it does here is it identifies the children at risk of requiring critical care and should therefore motivate us in the children achieving that threshold to promptly investigate thoroughly for suspected sepsis using the more comprehensive tools, like the full SOFA.”
He reported having no financial conflicts of interest regarding his study.
SOURCE: Khanijau A et al. ESPID 2019, Abstract.
LJUBLJANA, SLOVENIA – A new age-adjusted quick Sequential Organ Failure Assessment (qSOFA) score designed for use in children presenting to the ED with fever showed good predictive value for admission to critical care within the next 48 hours, Aakash Khanijau, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“In the needle-in-a-haystack scenario that’s seen in pediatric emergency departments, our novel, age-adjusted qSOFA score could potentially improve the rapid identification and treatment of children with suspected sepsis presenting to the ED,” said Dr. Khanijau of the University of Liverpool (England).
He presented an exceptionally large retrospective validation study of the score’s performance in 12,393 children (median age, 2.5 years) who presented to EDs with fever, of whom 1,521 were admitted for suspected sepsis. Of the hospitalized children, 145 were admitted to critical care within the first 48 hours.
The pediatric qSOFA score had 72% sensitivity and 85% specificity for critical care admission within 48 hours, with a positive predictive value of 5.4% and, more importantly, a whopping negative predictive value of 99.6%.
“That very high negative predictive value underlines the powerful discriminatory nature of our tool in the emergency department setting,” Dr. Khanijau observed, adding that the score’s area under the receiver operating characteristic curve was 0.81, which is considered a good predictive value.
The impetus for developing an age-adjusted pediatric qSOFA score stems from the fact that the original qSOFA score was designed for rapid assessment of adults with suspected sepsis and isn’t applicable in children. Other existing scores, including SIRS (the Systemic Inflammatory Response Syndrome criteria), the full SOFA, and PELOD-2 (the Pediatric Logistic Organ Dysfunction score), take longer to determine than the adapted qSOFA in a setting where speed is of the essence, he explained.
The original qSOFA components are altered mentation, systolic blood pressure, and respiratory rate. The novel score developed by Dr. Khanijau and coworkers swaps out systolic BP in favor of capillary refill time and age-adjusted heart rate using the thresholds previously established in a landmark study from the Children’s Hospital of Philadelphia (Pediatrics. 2013 Apr;131[4]:e1150-7.)
“Our reasoning here is that arterial hypertension is known to be a much later sign of circulatory compromise in children and may provide less discriminatory value than signs such as delayed capillary refill time and tachycardia early in presentation in the emergency department,” according to Dr. Khanijau.
The novel scoring system features four criteria. One point each is given for a capillary refill time of 3 seconds or longer; anything less than “Alert” on the Alert, Responds to Voice, Respond to Pain, and Unresponsive scale; a heart rate above the 99th percentile on the age-adjusted curves; and a respiratory rate above the age-adjusted 99th percentile. Thus, scores can range from 0 to 4. In the validation study, a score of 2 or more spelled a 890% increased likelihood of being admitted to a critical care setting within 48 hours. It was also associated with a 100-fold increased likelihood of death during the hospitalization, which occurred in 10 children.
Asked how the new predictive score could change clinical management, Dr. Khanijau replied, “I think the key thing it does here is it identifies the children at risk of requiring critical care and should therefore motivate us in the children achieving that threshold to promptly investigate thoroughly for suspected sepsis using the more comprehensive tools, like the full SOFA.”
He reported having no financial conflicts of interest regarding his study.
SOURCE: Khanijau A et al. ESPID 2019, Abstract.
REPORTING FROM ESPID 2019
Procalcitonin advocated to help rule out bacterial infections
SEATTLE – Procalcitonin, a marker of bacterial infection, rises and peaks sooner than C-reactive protein (CRP), and is especially useful to help rule out invasive bacterial infections in young infants and pediatric community acquired pneumonia due to typical bacteria, according to a presentation at the 2019 Pediatric Hospital Medicine Conference.
It’s “excellent for identifying low risk patients” and has the potential to decrease lumbar punctures and antibiotic exposure, but “the specificity isn’t great,” so there’s the potential for false positives, said Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
There was great interest in procalcitonin at the meeting; the presentation room was packed, with a line out the door. It’s used mostly in Europe at this point. Testing is available in many U.S. hospitals, but a large majority of audience members, when polled, said they don’t currently use it in clinical practice, and that it’s not a part of diagnostic algorithms at their institutions.
Levels of procalcitonin, a calcitonin precursor normally produced by the thyroid, are low or undetectable in healthy people, but inflammation, be it from infectious or noninfectious causes, triggers production by parenchymal cells throughout the body.
Levels began to rise as early as 2.5 hours after healthy subjects in one study were injected with bacterial endotoxins, and peaked as early as 6 hours; CRP, in contrast, started to rise after 12 hours, and peaked at 30 hours. Procalcitonin levels also seem to correlate with bacterial load and severity of infection, said Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University (J Pediatr Intensive Care. 2016 Dec;5[4]:162-71).
Due to time, the presenters focused their talk on community acquired pneumonia (CAP) and invasive bacterial infections (IBI) in young infants, meaning essentially bacteremia and meningitis.
Different studies use different cutoffs, but a procalcitonin below, for instance, 0.5 ng/mL is “certainly more sensitive [for IBI] than any single biomarker we currently use,” including CRP, white blood cells, and absolute neutrophil count (ANC). “If it’s negative, you’re really confident it’s negative,” but “a positive test does not necessarily indicate the presence of IBI,” Dr. McCulloh said (Pediatrics. 2012 Nov;130[5]:815-22).
“Procalcitonin works really well as part of a validated step-wise rule” that includes, for instance, CRP and ANC; “I think that’s where its utility is. On its own, it is not a substitute for you examining the patient and doing your basic risk stratification, but it may enhance your decision making incrementally above what we currently have,” he said.
Meanwhile, in a study of 532 children a median age of 2.4 years with radiographically confirmed CAP, procalcitonin levels were a median of 6.1 ng/mL in children whose pneumonia was caused by Streptococcus pneumoniae or other typical bacteria, and no child infected with typical bacteria had a level under 0.1 ng/mL. Below that level, “you can be very sure you do not have typical bacteria pneumonia,” said Marie Wang, MD, also a pediatric infectious disease specialist at Stanford (J Pediatric Infect Dis Soc. 2018 Feb 19;7[1]:46-53).
As procalcitonin levels went up, the likelihood of having bacterial pneumonia increased; at 2 ng/mL, 26% of subjects were infected with typical bacteria, “but even in that group, 58% still had viral infection, so you are still detecting a lot of viral” disease, she said.
Prolcalcitonin-guided therapy – antibiotics until patients fall below a level of 0.25 ng/ml, for instance – has also been associated with decreased antibiotic exposure (Respir Med. 2011 Dec;105[12]:1939-45).
The speakers had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Procalcitonin, a marker of bacterial infection, rises and peaks sooner than C-reactive protein (CRP), and is especially useful to help rule out invasive bacterial infections in young infants and pediatric community acquired pneumonia due to typical bacteria, according to a presentation at the 2019 Pediatric Hospital Medicine Conference.
It’s “excellent for identifying low risk patients” and has the potential to decrease lumbar punctures and antibiotic exposure, but “the specificity isn’t great,” so there’s the potential for false positives, said Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
There was great interest in procalcitonin at the meeting; the presentation room was packed, with a line out the door. It’s used mostly in Europe at this point. Testing is available in many U.S. hospitals, but a large majority of audience members, when polled, said they don’t currently use it in clinical practice, and that it’s not a part of diagnostic algorithms at their institutions.
Levels of procalcitonin, a calcitonin precursor normally produced by the thyroid, are low or undetectable in healthy people, but inflammation, be it from infectious or noninfectious causes, triggers production by parenchymal cells throughout the body.
Levels began to rise as early as 2.5 hours after healthy subjects in one study were injected with bacterial endotoxins, and peaked as early as 6 hours; CRP, in contrast, started to rise after 12 hours, and peaked at 30 hours. Procalcitonin levels also seem to correlate with bacterial load and severity of infection, said Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University (J Pediatr Intensive Care. 2016 Dec;5[4]:162-71).
Due to time, the presenters focused their talk on community acquired pneumonia (CAP) and invasive bacterial infections (IBI) in young infants, meaning essentially bacteremia and meningitis.
Different studies use different cutoffs, but a procalcitonin below, for instance, 0.5 ng/mL is “certainly more sensitive [for IBI] than any single biomarker we currently use,” including CRP, white blood cells, and absolute neutrophil count (ANC). “If it’s negative, you’re really confident it’s negative,” but “a positive test does not necessarily indicate the presence of IBI,” Dr. McCulloh said (Pediatrics. 2012 Nov;130[5]:815-22).
“Procalcitonin works really well as part of a validated step-wise rule” that includes, for instance, CRP and ANC; “I think that’s where its utility is. On its own, it is not a substitute for you examining the patient and doing your basic risk stratification, but it may enhance your decision making incrementally above what we currently have,” he said.
Meanwhile, in a study of 532 children a median age of 2.4 years with radiographically confirmed CAP, procalcitonin levels were a median of 6.1 ng/mL in children whose pneumonia was caused by Streptococcus pneumoniae or other typical bacteria, and no child infected with typical bacteria had a level under 0.1 ng/mL. Below that level, “you can be very sure you do not have typical bacteria pneumonia,” said Marie Wang, MD, also a pediatric infectious disease specialist at Stanford (J Pediatric Infect Dis Soc. 2018 Feb 19;7[1]:46-53).
As procalcitonin levels went up, the likelihood of having bacterial pneumonia increased; at 2 ng/mL, 26% of subjects were infected with typical bacteria, “but even in that group, 58% still had viral infection, so you are still detecting a lot of viral” disease, she said.
Prolcalcitonin-guided therapy – antibiotics until patients fall below a level of 0.25 ng/ml, for instance – has also been associated with decreased antibiotic exposure (Respir Med. 2011 Dec;105[12]:1939-45).
The speakers had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
SEATTLE – Procalcitonin, a marker of bacterial infection, rises and peaks sooner than C-reactive protein (CRP), and is especially useful to help rule out invasive bacterial infections in young infants and pediatric community acquired pneumonia due to typical bacteria, according to a presentation at the 2019 Pediatric Hospital Medicine Conference.
It’s “excellent for identifying low risk patients” and has the potential to decrease lumbar punctures and antibiotic exposure, but “the specificity isn’t great,” so there’s the potential for false positives, said Russell McCulloh, MD, a pediatric infectious disease specialist at the University of Nebraska Medical Center, Omaha.
There was great interest in procalcitonin at the meeting; the presentation room was packed, with a line out the door. It’s used mostly in Europe at this point. Testing is available in many U.S. hospitals, but a large majority of audience members, when polled, said they don’t currently use it in clinical practice, and that it’s not a part of diagnostic algorithms at their institutions.
Levels of procalcitonin, a calcitonin precursor normally produced by the thyroid, are low or undetectable in healthy people, but inflammation, be it from infectious or noninfectious causes, triggers production by parenchymal cells throughout the body.
Levels began to rise as early as 2.5 hours after healthy subjects in one study were injected with bacterial endotoxins, and peaked as early as 6 hours; CRP, in contrast, started to rise after 12 hours, and peaked at 30 hours. Procalcitonin levels also seem to correlate with bacterial load and severity of infection, said Nivedita Srinivas, MD, a pediatric infectious disease specialist at Stanford (Calif.) University (J Pediatr Intensive Care. 2016 Dec;5[4]:162-71).
Due to time, the presenters focused their talk on community acquired pneumonia (CAP) and invasive bacterial infections (IBI) in young infants, meaning essentially bacteremia and meningitis.
Different studies use different cutoffs, but a procalcitonin below, for instance, 0.5 ng/mL is “certainly more sensitive [for IBI] than any single biomarker we currently use,” including CRP, white blood cells, and absolute neutrophil count (ANC). “If it’s negative, you’re really confident it’s negative,” but “a positive test does not necessarily indicate the presence of IBI,” Dr. McCulloh said (Pediatrics. 2012 Nov;130[5]:815-22).
“Procalcitonin works really well as part of a validated step-wise rule” that includes, for instance, CRP and ANC; “I think that’s where its utility is. On its own, it is not a substitute for you examining the patient and doing your basic risk stratification, but it may enhance your decision making incrementally above what we currently have,” he said.
Meanwhile, in a study of 532 children a median age of 2.4 years with radiographically confirmed CAP, procalcitonin levels were a median of 6.1 ng/mL in children whose pneumonia was caused by Streptococcus pneumoniae or other typical bacteria, and no child infected with typical bacteria had a level under 0.1 ng/mL. Below that level, “you can be very sure you do not have typical bacteria pneumonia,” said Marie Wang, MD, also a pediatric infectious disease specialist at Stanford (J Pediatric Infect Dis Soc. 2018 Feb 19;7[1]:46-53).
As procalcitonin levels went up, the likelihood of having bacterial pneumonia increased; at 2 ng/mL, 26% of subjects were infected with typical bacteria, “but even in that group, 58% still had viral infection, so you are still detecting a lot of viral” disease, she said.
Prolcalcitonin-guided therapy – antibiotics until patients fall below a level of 0.25 ng/ml, for instance – has also been associated with decreased antibiotic exposure (Respir Med. 2011 Dec;105[12]:1939-45).
The speakers had no disclosures. The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
EXPERT ANALYSIS FROM PHM 2019
Algorithm boosts MACE prediction in patients with chest pain
Adding electrocardiogram findings and clinical assessment to high-sensitivity cardiac troponin measurements in patients presenting with chest pain could improve predictions of their risk of 30-day major adverse cardiac events, particularly unstable angina, research suggests.
Investigators reported outcomes of a prospective study involving 3,123 patients with suspected acute myocardial infarction. The findings are in the Journal of the American College of Cardiology.
The aim of the researchers was to validate an extended algorithm that combined the European Society of Cardiology’s high-sensitivity cardiac troponin measurement at presentation and after 1 hour (ESC hs-cTn 0/1 h algorithm) with clinical assessment and ECG findings to aid prediction of major adverse cardiac events (MACE) within 30 days.
The clinical assessment involved the treating ED physician’s use of a visual analog scale to assess the patient’s pretest probability for an acute coronary syndrome (ACS), with a score above 70% qualifying as high likelihood.
The researchers found that the ESC hs-cTn 0/1 h algorithm alone triaged significantly more patients toward rule-out for MACE than did the extended algorithm (60% vs. 45%, P less than .001). This resulted in 487 patients being reclassified toward “observe” by the extended algorithm, and among this group the 30-day MACE rate was 1.1%.
However, the 30-day MACE rates were similar in the two groups – 0.6% among those ruled out by the ESC hs-cTn 0/1 h algorithm alone and 0.4% in those ruled out by the extended algorithm – resulting in a similar negative predictive value.
“These estimates will help clinicians to appropriately manage patients triaged toward rule-out according to the ESC hs-cTnT 0/1 h algorithm, in whom either the [visual analog scale] for ACS or the ECG still suggests the presence of an ACS,” wrote Thomas Nestelberger, MD, of the Cardiovascular Research Institute Basel (Switzerland) at the University of Basel, and coinvestigators.
The ESC hs-cTn 0/1 h algorithm also ruled in fewer patients than did the extended algorithm (16% vs. 26%, P less than .001), giving it a higher positive predictive value.
When the researchers added unstable angina to the major adverse cardiac event outcome, they found the ESC hs-cTn 0/1 h algorithm had a lower negative predictive value and a higher negative likelihood ratio compared with the extended algorithm for patients ruled out, but a higher positive predictive value and positive likelihood ratio for patients ruled in.
“Our findings corroborate and extend previous research regarding the development and validation of algorithms for the safe and effective rule-out and rule-in of MACE in patients with symptoms suggestive of AMI,” the authors wrote.
This study was supported by the Swiss National Science Foundation, the Swiss Heart Foundation, the European Union, the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, Beckman Coulter, Biomerieux, BRAHMS, Roche, Nanosphere, Siemens, Ortho Diagnostics, and Singulex. Several authors reported grants and support from the pharmaceutical sector.
SOURCE: Nestelberger T et al. J Am Coll Cardiol. 2019 Aug 20. doi: 10.1016/j.jacc.2019.06.025.
In patients presenting at the emergency department with chest pain, it’s important not only to diagnose acute myocardial infarction, but also to predict short-term risk of cardiac events to help guide management. This thoughtful and comprehensive analysis is the largest study assessing the added value of clinical and ECG assessment to the prognostication by high-sensitivity cardiac troponin algorithms in patients evaluated for chest pain. It reinforces the accuracy of hs-cTn at presentation and after 1 hour (ESC hs-cTn 0/1 h) algorithms to predict AMI and 30-day AMI-related events.
It is important to note that if unstable angina had been included as a major adverse cardiac event, the study would have found that the extended algorithm performs better than the hs-cTn 0/1 h algorithm in the prediction of this endpoint.
Germán Cediel, MD, is from Hospital Universitari Germans Trias i Pujol in Spain, Alfredo Bardají, MD, is from the Joan XXIII University Hospital in Spain, and José A. Barrabés, MD, is from Vall d’Hebron University Hospital and Research Institute, Universitat Autònoma de Barcelona. The comments are adapted from an editorial (J Am Coll Cardiol. 2019 Aug 20. doi: 10.1016/j.jacc.2019.05.065). The authors declared support from Instituto de Salud Carlos III, Spain, cofinanced by the European Regional Development Fund, and declared consultancies and educational activities with the pharmaceutical sector.
In patients presenting at the emergency department with chest pain, it’s important not only to diagnose acute myocardial infarction, but also to predict short-term risk of cardiac events to help guide management. This thoughtful and comprehensive analysis is the largest study assessing the added value of clinical and ECG assessment to the prognostication by high-sensitivity cardiac troponin algorithms in patients evaluated for chest pain. It reinforces the accuracy of hs-cTn at presentation and after 1 hour (ESC hs-cTn 0/1 h) algorithms to predict AMI and 30-day AMI-related events.
It is important to note that if unstable angina had been included as a major adverse cardiac event, the study would have found that the extended algorithm performs better than the hs-cTn 0/1 h algorithm in the prediction of this endpoint.
Germán Cediel, MD, is from Hospital Universitari Germans Trias i Pujol in Spain, Alfredo Bardají, MD, is from the Joan XXIII University Hospital in Spain, and José A. Barrabés, MD, is from Vall d’Hebron University Hospital and Research Institute, Universitat Autònoma de Barcelona. The comments are adapted from an editorial (J Am Coll Cardiol. 2019 Aug 20. doi: 10.1016/j.jacc.2019.05.065). The authors declared support from Instituto de Salud Carlos III, Spain, cofinanced by the European Regional Development Fund, and declared consultancies and educational activities with the pharmaceutical sector.
In patients presenting at the emergency department with chest pain, it’s important not only to diagnose acute myocardial infarction, but also to predict short-term risk of cardiac events to help guide management. This thoughtful and comprehensive analysis is the largest study assessing the added value of clinical and ECG assessment to the prognostication by high-sensitivity cardiac troponin algorithms in patients evaluated for chest pain. It reinforces the accuracy of hs-cTn at presentation and after 1 hour (ESC hs-cTn 0/1 h) algorithms to predict AMI and 30-day AMI-related events.
It is important to note that if unstable angina had been included as a major adverse cardiac event, the study would have found that the extended algorithm performs better than the hs-cTn 0/1 h algorithm in the prediction of this endpoint.
Germán Cediel, MD, is from Hospital Universitari Germans Trias i Pujol in Spain, Alfredo Bardají, MD, is from the Joan XXIII University Hospital in Spain, and José A. Barrabés, MD, is from Vall d’Hebron University Hospital and Research Institute, Universitat Autònoma de Barcelona. The comments are adapted from an editorial (J Am Coll Cardiol. 2019 Aug 20. doi: 10.1016/j.jacc.2019.05.065). The authors declared support from Instituto de Salud Carlos III, Spain, cofinanced by the European Regional Development Fund, and declared consultancies and educational activities with the pharmaceutical sector.
Adding electrocardiogram findings and clinical assessment to high-sensitivity cardiac troponin measurements in patients presenting with chest pain could improve predictions of their risk of 30-day major adverse cardiac events, particularly unstable angina, research suggests.
Investigators reported outcomes of a prospective study involving 3,123 patients with suspected acute myocardial infarction. The findings are in the Journal of the American College of Cardiology.
The aim of the researchers was to validate an extended algorithm that combined the European Society of Cardiology’s high-sensitivity cardiac troponin measurement at presentation and after 1 hour (ESC hs-cTn 0/1 h algorithm) with clinical assessment and ECG findings to aid prediction of major adverse cardiac events (MACE) within 30 days.
The clinical assessment involved the treating ED physician’s use of a visual analog scale to assess the patient’s pretest probability for an acute coronary syndrome (ACS), with a score above 70% qualifying as high likelihood.
The researchers found that the ESC hs-cTn 0/1 h algorithm alone triaged significantly more patients toward rule-out for MACE than did the extended algorithm (60% vs. 45%, P less than .001). This resulted in 487 patients being reclassified toward “observe” by the extended algorithm, and among this group the 30-day MACE rate was 1.1%.
However, the 30-day MACE rates were similar in the two groups – 0.6% among those ruled out by the ESC hs-cTn 0/1 h algorithm alone and 0.4% in those ruled out by the extended algorithm – resulting in a similar negative predictive value.
“These estimates will help clinicians to appropriately manage patients triaged toward rule-out according to the ESC hs-cTnT 0/1 h algorithm, in whom either the [visual analog scale] for ACS or the ECG still suggests the presence of an ACS,” wrote Thomas Nestelberger, MD, of the Cardiovascular Research Institute Basel (Switzerland) at the University of Basel, and coinvestigators.
The ESC hs-cTn 0/1 h algorithm also ruled in fewer patients than did the extended algorithm (16% vs. 26%, P less than .001), giving it a higher positive predictive value.
When the researchers added unstable angina to the major adverse cardiac event outcome, they found the ESC hs-cTn 0/1 h algorithm had a lower negative predictive value and a higher negative likelihood ratio compared with the extended algorithm for patients ruled out, but a higher positive predictive value and positive likelihood ratio for patients ruled in.
“Our findings corroborate and extend previous research regarding the development and validation of algorithms for the safe and effective rule-out and rule-in of MACE in patients with symptoms suggestive of AMI,” the authors wrote.
This study was supported by the Swiss National Science Foundation, the Swiss Heart Foundation, the European Union, the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, Beckman Coulter, Biomerieux, BRAHMS, Roche, Nanosphere, Siemens, Ortho Diagnostics, and Singulex. Several authors reported grants and support from the pharmaceutical sector.
SOURCE: Nestelberger T et al. J Am Coll Cardiol. 2019 Aug 20. doi: 10.1016/j.jacc.2019.06.025.
Adding electrocardiogram findings and clinical assessment to high-sensitivity cardiac troponin measurements in patients presenting with chest pain could improve predictions of their risk of 30-day major adverse cardiac events, particularly unstable angina, research suggests.
Investigators reported outcomes of a prospective study involving 3,123 patients with suspected acute myocardial infarction. The findings are in the Journal of the American College of Cardiology.
The aim of the researchers was to validate an extended algorithm that combined the European Society of Cardiology’s high-sensitivity cardiac troponin measurement at presentation and after 1 hour (ESC hs-cTn 0/1 h algorithm) with clinical assessment and ECG findings to aid prediction of major adverse cardiac events (MACE) within 30 days.
The clinical assessment involved the treating ED physician’s use of a visual analog scale to assess the patient’s pretest probability for an acute coronary syndrome (ACS), with a score above 70% qualifying as high likelihood.
The researchers found that the ESC hs-cTn 0/1 h algorithm alone triaged significantly more patients toward rule-out for MACE than did the extended algorithm (60% vs. 45%, P less than .001). This resulted in 487 patients being reclassified toward “observe” by the extended algorithm, and among this group the 30-day MACE rate was 1.1%.
However, the 30-day MACE rates were similar in the two groups – 0.6% among those ruled out by the ESC hs-cTn 0/1 h algorithm alone and 0.4% in those ruled out by the extended algorithm – resulting in a similar negative predictive value.
“These estimates will help clinicians to appropriately manage patients triaged toward rule-out according to the ESC hs-cTnT 0/1 h algorithm, in whom either the [visual analog scale] for ACS or the ECG still suggests the presence of an ACS,” wrote Thomas Nestelberger, MD, of the Cardiovascular Research Institute Basel (Switzerland) at the University of Basel, and coinvestigators.
The ESC hs-cTn 0/1 h algorithm also ruled in fewer patients than did the extended algorithm (16% vs. 26%, P less than .001), giving it a higher positive predictive value.
When the researchers added unstable angina to the major adverse cardiac event outcome, they found the ESC hs-cTn 0/1 h algorithm had a lower negative predictive value and a higher negative likelihood ratio compared with the extended algorithm for patients ruled out, but a higher positive predictive value and positive likelihood ratio for patients ruled in.
“Our findings corroborate and extend previous research regarding the development and validation of algorithms for the safe and effective rule-out and rule-in of MACE in patients with symptoms suggestive of AMI,” the authors wrote.
This study was supported by the Swiss National Science Foundation, the Swiss Heart Foundation, the European Union, the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, Beckman Coulter, Biomerieux, BRAHMS, Roche, Nanosphere, Siemens, Ortho Diagnostics, and Singulex. Several authors reported grants and support from the pharmaceutical sector.
SOURCE: Nestelberger T et al. J Am Coll Cardiol. 2019 Aug 20. doi: 10.1016/j.jacc.2019.06.025.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point:
Major finding: High-sensitivity cardiac troponin measurements combined with ECG and clinical assessment can help rule out MACE and unstable angina.
Study details: A prospective study of 3,123 patients with suspected acute myocardial infarction.
Disclosures: This study was supported by the Swiss National Science Foundation, the Swiss Heart Foundation, the European Union, the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, Beckman Coulter, Biomerieux, BRAHMS, Roche, NanoSphere, Siemens, Ortho Diagnostics, and Singulex. Several authors reported grants and support from the pharmaceutical sector.
Source: Nestelberger T et al. J Am Coll Cardiol. 2019 Aug 20. doi: 10.1016/j.jacc.2019.06.025.
PTSD in the inpatient setting
A problem hiding in plain sight
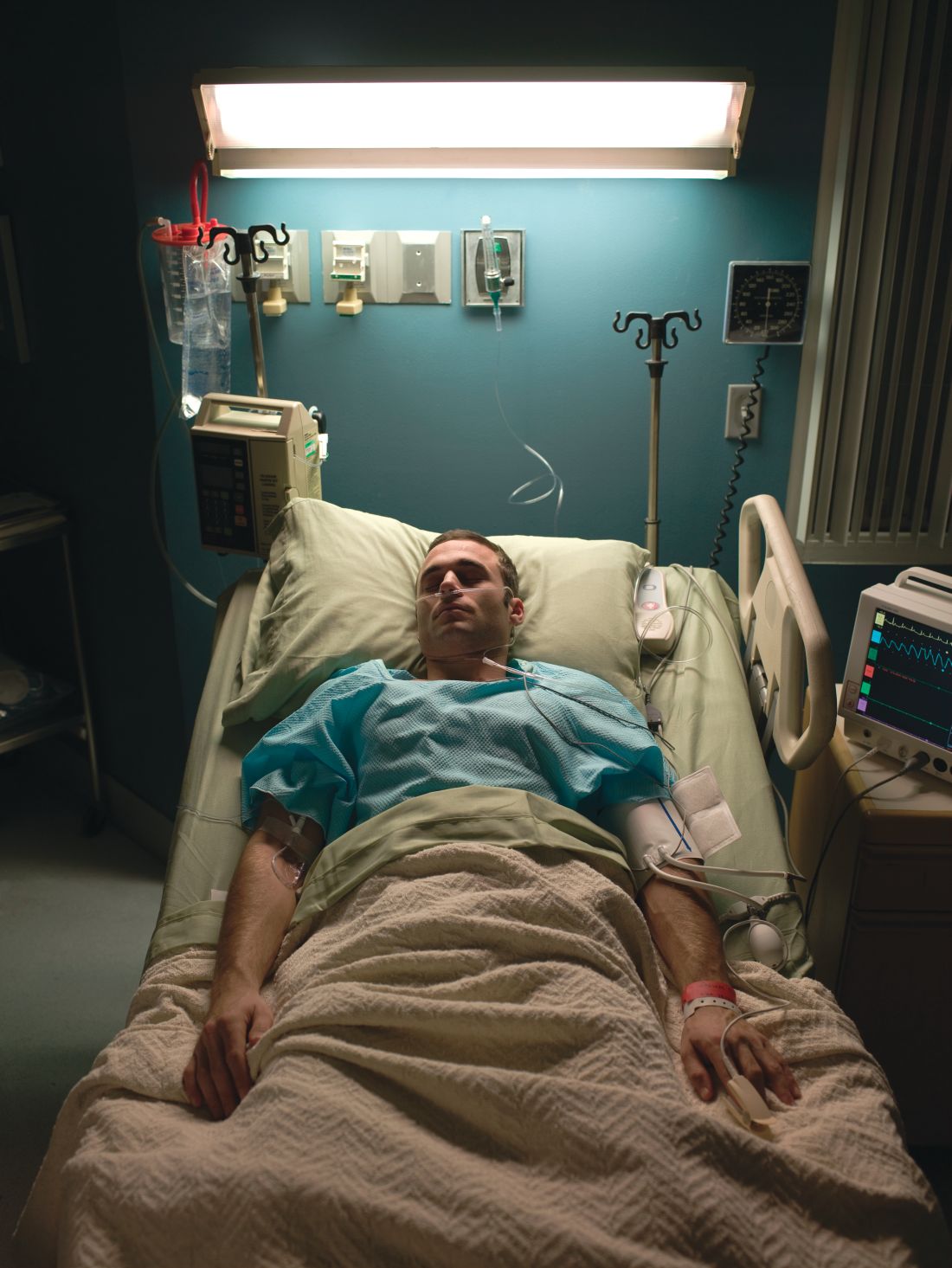
“I need to get out of here! I haven’t gotten any sleep, my medications never come on time, and I feel like a pincushion. I am leaving NOW!” The commotion interrupts your intern’s meticulous presentation as your team quickly files into the room. You find a disheveled, visibly frustrated man tearing at his intravenous line, surrounded by his half-eaten breakfast and multiple urinals filled to various levels. His IV pump is beeping, and telemetry wires hang haphazardly off his chest.
Mr. Smith had been admitted for a heart failure exacerbation. You’d been making steady progress with diuresis but are now faced with a likely discharge against medical advice if you can’t defuse the situation.
As hospitalists, this scenario might feel eerily familiar. Perhaps Mr. Smith had enough of being in the hospital and just wanted to go home to see his dog, or maybe the food was not up to his standards.
However, his next line stops your team dead in its tracks. “I feel like I am in Vietnam all over again. I am tied up with all these wires and feel like a prisoner! Please let me go.” It turns out that Mr. Smith had a comorbidity that was overlooked during his initial intake: posttraumatic stress disorder.
Impact of PTSD
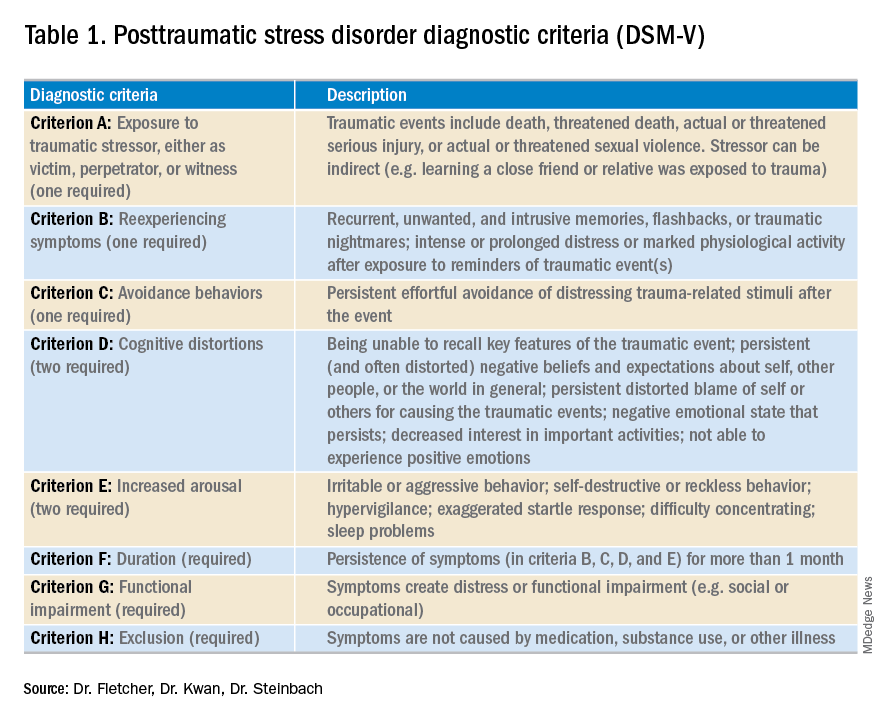
PTSD is a diagnosis characterized by intrusive recurrent thoughts, dreams, or flashbacks that follow exposure to a traumatic event or series of events (see Table 1). While more common among veterans (for example, Vietnam veterans have an estimated lifetime prevalence of PTSD of 30.9% for men and 26.9% for women),1 a national survey of U.S. households estimated the lifetime prevalence of PTSD among adult Americans to be 6.8%.2 PTSD is often underdiagnosed and underreported by patients in the outpatient setting, leading to underrecognition and undertreatment of these patients in the inpatient setting.
Although it may not be surprising that patients with PTSD use more mental health services, they are also more likely to use nonmental health services. In one study, total utilization of outpatient nonmental health services was 91% greater in veterans with PTSD, and these patients were three times more likely to be hospitalized than those without any mental health diagnoses.3 Additionally, they are likely to present later and stay longer when compared with patients without PTSD. One study estimated the cost of PTSD-related hospitalization in the United States from 2002 to 2011 as being $34.9 billion.4 Notably, close to 95% of hospitalizations in this study listed PTSD as a secondary rather than primary diagnosis, suggesting that the vast majority of these admitted patients are cared for by frontline providers who are not trained mental health professionals.
How PTSD manifests in the hospital
But, how exactly can the hospital environment contribute to decompensation of PTSD symptoms? Unfortunately, there is little empiric data to guide us. Based on what we do know of PTSD, we offer the following hypotheses.
Patients with PTSD may feel a loss of control or helplessness when admitted to the inpatient setting. For example, they cannot control when they receive their medications or when they get their meals. The act of showering or going outside requires approval. In addition, they might perceive they are being “ordered around” by staff and may be carted off to a study without knowing why the study is being done in the first place.
Triggers in the hospital environment may contribute to PTSD flares. Think about the loud, beeping IV pump that constantly goes off at random intervals, disrupting sleep. What about a blood draw in the early morning where the phlebotomist sticks a needle into the arm of a sleeping patient? Or the well-intentioned provider doing prerounds who wakes a sleeping patient with a shake of the shoulder or some other form of physical touch? The multidisciplinary team crowding around their hospital bed? For a patient suffering from PTSD, any of these could easily set off a cascade of escalating symptoms.
Knowing that these triggers exist, can anything be done to ameliorate their effects? We propose some practical suggestions for improving the hospital experience for patients with PTSD.
Strategies to combat PTSD in the inpatient setting
Perhaps the most practical place to start is with preserving sleep in hospitalized patients with PTSD. The majority of patients with PTSD have sleep disturbances, and interrupted sleep routines in these patients can exacerbate nightmares and underlying psychiatric issues.5 Therefore, we should strive to avoid unnecessary awakenings.
While this principle holds true for all hospitalized patients, it must be especially prioritized in patients with PTSD. Ask yourself these questions during your next admission: Must intravenous fluids run 24 hours a day, or could they be stopped at 6 p.m.? Are vital signs needed overnight? Could the last dose of furosemide occur at 4 p.m. to avoid nocturia?
Another strategy involves bedtime routines. Many of these patients may already follow a home sleep routine as part of their chronic PTSD management. To honor these habits in the hospital might mean that staff encourage turning the lights and the television off at a designated time. Additionally, the literature suggests music therapy can have a significant impact on enhanced sleep quality. When available, music therapy may reduce insomnia and decrease the amount of time prior to falling asleep.6
Other methods to counteract PTSD fall under the general principle of “trauma-informed care.” Trauma-informed care comprises practices promoting a culture of safety, empowerment, and healing.7 It is a mindful and sensitive approach that acknowledges the pervasive nature of trauma exposure, the reality of ongoing adverse effects in trauma survivors, and the fact that recovery is highly personal and complex.8
By definition, patients with PTSD have endured some traumatic event. Therefore, ideal care teams will ask patients about things that may trigger their anxiety and then work to mitigate them. For example, some patients with PTSD have a severe startle response when woken up by someone touching them. When patients feel that they can share their concerns with their care team and their team honors that observation by waking them in a different way, trust and control may be gained. This process of asking for patient guidance and adjusting accordingly is consistent with a trauma-informed care approach.9 A true trauma-informed care approach involves the entire practice environment but examining and adjusting our own behavior and assumptions are good places to start.
Summary of recommended treatments
Psychotherapy is preferable over pharmacotherapy, but both can be combined as needed. Individual trauma-focused psychotherapies utilizing a primary component of exposure and/or cognitive restructuring have strong evidence for effectiveness but are primarily outpatient based.
For pharmacologic treatment, selective serotonin reuptake inhibitors (for example, sertraline, paroxetine, or fluoxetine) and serotonin norepinephrine reuptake inhibitors (for example, venlafaxine) monotherapy have strong evidence for effectiveness and can be started while inpatient. However, these medications typically take weeks to produce benefits. Recent trials studying prazosin, an alpha1-adrenergic receptor antagonist used to alleviate nightmares associated with PTSD, have demonstrated inefficacy or even harm,leading experts to caution against its use.10,11 Finally, benzodiazepine and atypical antipsychotic usage should be restricted and used as a last resort.12
In summary, PTSD is common among veterans and nonveterans. While hospitalists may rarely admit patients because of their PTSD, they will often take care of patients who have PTSD as a comorbidity. Therefore, understanding the basics of PTSD and how hospitalization may exacerbate its symptoms can meaningfully improve care for these patients.
Dr. Fletcher is a hospitalist at the Milwaukee Veterans Affairs Medical Center and Froedtert Hospital in Wauwatosa, Wis. She is professor of internal medicine and program director for the internal medicine residency program at the Medical College of Wisconsin, Milwaukee. She is also faculty mentor for the VA’s Chief Resident for Quality and Safety. Dr. Kwan is a hospitalist at the VA San Diego Healthcare System and is associate professor at the University of California, San Diego, in the division of hospital medicine. He serves as an associate clerkship director of both the internal medicine clerkship and the medicine subinternship. He is the chair of SHM’s Physicians in Training committee. Dr. Steinbach is chief of hospital medicine at the Atlanta VA Medical Center and assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
References
1. Kang HK et al. Posttraumatic stress disorder and chronic fatigue syndrome–like illness among Gulf War veterans: A population-based survey of 30,000 veterans. Am J Epidemiol. 2003;157(2):141-8.
2. Kessler RC et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005; 62(6):593-602.
3. Cohen BE et al. Mental health diagnoses and utilization of VA nonmental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
4. Haviland MG et al. Posttraumatic stress disorder–related hospitalizations in the United States (2002-2011): Rates, co-occurring illnesses, suicidal ideation/self-harm, and hospital charges. J Nerv Ment Dis. 2016; 204(2):78-86.
5. Aurora RN et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
6. Blanaru M et al. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress disorder. Ment Illn. 2012;4(2):e13.
7. Tello M. (2018, Oct 16). Trauma-informed care: What it is, and why it’s important. Retrieved March 18, 2019, from https://www.health.harvard.edu/blog/trauma-informed-care-what-it-is-and-why-its-important-2018101613562.
8. Harris M et al. Using trauma theory to design service systems. San Francisco: 2001.
9. Substance abuse and mental health services administration. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. HHS publication no. SMA 14-4884. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
10. Raskind MA et al. Trial of prazosin for posttraumatic stress disorder in military veterans. N Engl J Med. 2018 Feb 8;378(6):507-7.
11. McCall WV et al. A pilot, randomized clinical trial of bedtime doses of prazosin versus placebo in suicidal posttraumatic stress disorder patients with nightmares. J Clin Psychopharmacol. 2018 Dec;38(6):618-21.
12. U.S. Department of Veterans Affairs/U.S. Department of Defense. Clinical practice guideline for the management of posttraumatic stress disorder and acute stress reaction 2017. Accessed February 18, 2019.
A problem hiding in plain sight
A problem hiding in plain sight
“I need to get out of here! I haven’t gotten any sleep, my medications never come on time, and I feel like a pincushion. I am leaving NOW!” The commotion interrupts your intern’s meticulous presentation as your team quickly files into the room. You find a disheveled, visibly frustrated man tearing at his intravenous line, surrounded by his half-eaten breakfast and multiple urinals filled to various levels. His IV pump is beeping, and telemetry wires hang haphazardly off his chest.
Mr. Smith had been admitted for a heart failure exacerbation. You’d been making steady progress with diuresis but are now faced with a likely discharge against medical advice if you can’t defuse the situation.
As hospitalists, this scenario might feel eerily familiar. Perhaps Mr. Smith had enough of being in the hospital and just wanted to go home to see his dog, or maybe the food was not up to his standards.
However, his next line stops your team dead in its tracks. “I feel like I am in Vietnam all over again. I am tied up with all these wires and feel like a prisoner! Please let me go.” It turns out that Mr. Smith had a comorbidity that was overlooked during his initial intake: posttraumatic stress disorder.
Impact of PTSD
PTSD is a diagnosis characterized by intrusive recurrent thoughts, dreams, or flashbacks that follow exposure to a traumatic event or series of events (see Table 1). While more common among veterans (for example, Vietnam veterans have an estimated lifetime prevalence of PTSD of 30.9% for men and 26.9% for women),1 a national survey of U.S. households estimated the lifetime prevalence of PTSD among adult Americans to be 6.8%.2 PTSD is often underdiagnosed and underreported by patients in the outpatient setting, leading to underrecognition and undertreatment of these patients in the inpatient setting.

Although it may not be surprising that patients with PTSD use more mental health services, they are also more likely to use nonmental health services. In one study, total utilization of outpatient nonmental health services was 91% greater in veterans with PTSD, and these patients were three times more likely to be hospitalized than those without any mental health diagnoses.3 Additionally, they are likely to present later and stay longer when compared with patients without PTSD. One study estimated the cost of PTSD-related hospitalization in the United States from 2002 to 2011 as being $34.9 billion.4 Notably, close to 95% of hospitalizations in this study listed PTSD as a secondary rather than primary diagnosis, suggesting that the vast majority of these admitted patients are cared for by frontline providers who are not trained mental health professionals.
How PTSD manifests in the hospital
But, how exactly can the hospital environment contribute to decompensation of PTSD symptoms? Unfortunately, there is little empiric data to guide us. Based on what we do know of PTSD, we offer the following hypotheses.
Patients with PTSD may feel a loss of control or helplessness when admitted to the inpatient setting. For example, they cannot control when they receive their medications or when they get their meals. The act of showering or going outside requires approval. In addition, they might perceive they are being “ordered around” by staff and may be carted off to a study without knowing why the study is being done in the first place.
Triggers in the hospital environment may contribute to PTSD flares. Think about the loud, beeping IV pump that constantly goes off at random intervals, disrupting sleep. What about a blood draw in the early morning where the phlebotomist sticks a needle into the arm of a sleeping patient? Or the well-intentioned provider doing prerounds who wakes a sleeping patient with a shake of the shoulder or some other form of physical touch? The multidisciplinary team crowding around their hospital bed? For a patient suffering from PTSD, any of these could easily set off a cascade of escalating symptoms.
Knowing that these triggers exist, can anything be done to ameliorate their effects? We propose some practical suggestions for improving the hospital experience for patients with PTSD.
Strategies to combat PTSD in the inpatient setting
Perhaps the most practical place to start is with preserving sleep in hospitalized patients with PTSD. The majority of patients with PTSD have sleep disturbances, and interrupted sleep routines in these patients can exacerbate nightmares and underlying psychiatric issues.5 Therefore, we should strive to avoid unnecessary awakenings.
While this principle holds true for all hospitalized patients, it must be especially prioritized in patients with PTSD. Ask yourself these questions during your next admission: Must intravenous fluids run 24 hours a day, or could they be stopped at 6 p.m.? Are vital signs needed overnight? Could the last dose of furosemide occur at 4 p.m. to avoid nocturia?
Another strategy involves bedtime routines. Many of these patients may already follow a home sleep routine as part of their chronic PTSD management. To honor these habits in the hospital might mean that staff encourage turning the lights and the television off at a designated time. Additionally, the literature suggests music therapy can have a significant impact on enhanced sleep quality. When available, music therapy may reduce insomnia and decrease the amount of time prior to falling asleep.6
Other methods to counteract PTSD fall under the general principle of “trauma-informed care.” Trauma-informed care comprises practices promoting a culture of safety, empowerment, and healing.7 It is a mindful and sensitive approach that acknowledges the pervasive nature of trauma exposure, the reality of ongoing adverse effects in trauma survivors, and the fact that recovery is highly personal and complex.8
By definition, patients with PTSD have endured some traumatic event. Therefore, ideal care teams will ask patients about things that may trigger their anxiety and then work to mitigate them. For example, some patients with PTSD have a severe startle response when woken up by someone touching them. When patients feel that they can share their concerns with their care team and their team honors that observation by waking them in a different way, trust and control may be gained. This process of asking for patient guidance and adjusting accordingly is consistent with a trauma-informed care approach.9 A true trauma-informed care approach involves the entire practice environment but examining and adjusting our own behavior and assumptions are good places to start.
Summary of recommended treatments
Psychotherapy is preferable over pharmacotherapy, but both can be combined as needed. Individual trauma-focused psychotherapies utilizing a primary component of exposure and/or cognitive restructuring have strong evidence for effectiveness but are primarily outpatient based.
For pharmacologic treatment, selective serotonin reuptake inhibitors (for example, sertraline, paroxetine, or fluoxetine) and serotonin norepinephrine reuptake inhibitors (for example, venlafaxine) monotherapy have strong evidence for effectiveness and can be started while inpatient. However, these medications typically take weeks to produce benefits. Recent trials studying prazosin, an alpha1-adrenergic receptor antagonist used to alleviate nightmares associated with PTSD, have demonstrated inefficacy or even harm,leading experts to caution against its use.10,11 Finally, benzodiazepine and atypical antipsychotic usage should be restricted and used as a last resort.12
In summary, PTSD is common among veterans and nonveterans. While hospitalists may rarely admit patients because of their PTSD, they will often take care of patients who have PTSD as a comorbidity. Therefore, understanding the basics of PTSD and how hospitalization may exacerbate its symptoms can meaningfully improve care for these patients.
Dr. Fletcher is a hospitalist at the Milwaukee Veterans Affairs Medical Center and Froedtert Hospital in Wauwatosa, Wis. She is professor of internal medicine and program director for the internal medicine residency program at the Medical College of Wisconsin, Milwaukee. She is also faculty mentor for the VA’s Chief Resident for Quality and Safety. Dr. Kwan is a hospitalist at the VA San Diego Healthcare System and is associate professor at the University of California, San Diego, in the division of hospital medicine. He serves as an associate clerkship director of both the internal medicine clerkship and the medicine subinternship. He is the chair of SHM’s Physicians in Training committee. Dr. Steinbach is chief of hospital medicine at the Atlanta VA Medical Center and assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
References
1. Kang HK et al. Posttraumatic stress disorder and chronic fatigue syndrome–like illness among Gulf War veterans: A population-based survey of 30,000 veterans. Am J Epidemiol. 2003;157(2):141-8.
2. Kessler RC et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005; 62(6):593-602.
3. Cohen BE et al. Mental health diagnoses and utilization of VA nonmental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
4. Haviland MG et al. Posttraumatic stress disorder–related hospitalizations in the United States (2002-2011): Rates, co-occurring illnesses, suicidal ideation/self-harm, and hospital charges. J Nerv Ment Dis. 2016; 204(2):78-86.
5. Aurora RN et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
6. Blanaru M et al. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress disorder. Ment Illn. 2012;4(2):e13.
7. Tello M. (2018, Oct 16). Trauma-informed care: What it is, and why it’s important. Retrieved March 18, 2019, from https://www.health.harvard.edu/blog/trauma-informed-care-what-it-is-and-why-its-important-2018101613562.
8. Harris M et al. Using trauma theory to design service systems. San Francisco: 2001.
9. Substance abuse and mental health services administration. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. HHS publication no. SMA 14-4884. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
10. Raskind MA et al. Trial of prazosin for posttraumatic stress disorder in military veterans. N Engl J Med. 2018 Feb 8;378(6):507-7.
11. McCall WV et al. A pilot, randomized clinical trial of bedtime doses of prazosin versus placebo in suicidal posttraumatic stress disorder patients with nightmares. J Clin Psychopharmacol. 2018 Dec;38(6):618-21.
12. U.S. Department of Veterans Affairs/U.S. Department of Defense. Clinical practice guideline for the management of posttraumatic stress disorder and acute stress reaction 2017. Accessed February 18, 2019.
“I need to get out of here! I haven’t gotten any sleep, my medications never come on time, and I feel like a pincushion. I am leaving NOW!” The commotion interrupts your intern’s meticulous presentation as your team quickly files into the room. You find a disheveled, visibly frustrated man tearing at his intravenous line, surrounded by his half-eaten breakfast and multiple urinals filled to various levels. His IV pump is beeping, and telemetry wires hang haphazardly off his chest.
Mr. Smith had been admitted for a heart failure exacerbation. You’d been making steady progress with diuresis but are now faced with a likely discharge against medical advice if you can’t defuse the situation.
As hospitalists, this scenario might feel eerily familiar. Perhaps Mr. Smith had enough of being in the hospital and just wanted to go home to see his dog, or maybe the food was not up to his standards.
However, his next line stops your team dead in its tracks. “I feel like I am in Vietnam all over again. I am tied up with all these wires and feel like a prisoner! Please let me go.” It turns out that Mr. Smith had a comorbidity that was overlooked during his initial intake: posttraumatic stress disorder.
Impact of PTSD
PTSD is a diagnosis characterized by intrusive recurrent thoughts, dreams, or flashbacks that follow exposure to a traumatic event or series of events (see Table 1). While more common among veterans (for example, Vietnam veterans have an estimated lifetime prevalence of PTSD of 30.9% for men and 26.9% for women),1 a national survey of U.S. households estimated the lifetime prevalence of PTSD among adult Americans to be 6.8%.2 PTSD is often underdiagnosed and underreported by patients in the outpatient setting, leading to underrecognition and undertreatment of these patients in the inpatient setting.

Although it may not be surprising that patients with PTSD use more mental health services, they are also more likely to use nonmental health services. In one study, total utilization of outpatient nonmental health services was 91% greater in veterans with PTSD, and these patients were three times more likely to be hospitalized than those without any mental health diagnoses.3 Additionally, they are likely to present later and stay longer when compared with patients without PTSD. One study estimated the cost of PTSD-related hospitalization in the United States from 2002 to 2011 as being $34.9 billion.4 Notably, close to 95% of hospitalizations in this study listed PTSD as a secondary rather than primary diagnosis, suggesting that the vast majority of these admitted patients are cared for by frontline providers who are not trained mental health professionals.
How PTSD manifests in the hospital
But, how exactly can the hospital environment contribute to decompensation of PTSD symptoms? Unfortunately, there is little empiric data to guide us. Based on what we do know of PTSD, we offer the following hypotheses.
Patients with PTSD may feel a loss of control or helplessness when admitted to the inpatient setting. For example, they cannot control when they receive their medications or when they get their meals. The act of showering or going outside requires approval. In addition, they might perceive they are being “ordered around” by staff and may be carted off to a study without knowing why the study is being done in the first place.
Triggers in the hospital environment may contribute to PTSD flares. Think about the loud, beeping IV pump that constantly goes off at random intervals, disrupting sleep. What about a blood draw in the early morning where the phlebotomist sticks a needle into the arm of a sleeping patient? Or the well-intentioned provider doing prerounds who wakes a sleeping patient with a shake of the shoulder or some other form of physical touch? The multidisciplinary team crowding around their hospital bed? For a patient suffering from PTSD, any of these could easily set off a cascade of escalating symptoms.
Knowing that these triggers exist, can anything be done to ameliorate their effects? We propose some practical suggestions for improving the hospital experience for patients with PTSD.
Strategies to combat PTSD in the inpatient setting
Perhaps the most practical place to start is with preserving sleep in hospitalized patients with PTSD. The majority of patients with PTSD have sleep disturbances, and interrupted sleep routines in these patients can exacerbate nightmares and underlying psychiatric issues.5 Therefore, we should strive to avoid unnecessary awakenings.
While this principle holds true for all hospitalized patients, it must be especially prioritized in patients with PTSD. Ask yourself these questions during your next admission: Must intravenous fluids run 24 hours a day, or could they be stopped at 6 p.m.? Are vital signs needed overnight? Could the last dose of furosemide occur at 4 p.m. to avoid nocturia?
Another strategy involves bedtime routines. Many of these patients may already follow a home sleep routine as part of their chronic PTSD management. To honor these habits in the hospital might mean that staff encourage turning the lights and the television off at a designated time. Additionally, the literature suggests music therapy can have a significant impact on enhanced sleep quality. When available, music therapy may reduce insomnia and decrease the amount of time prior to falling asleep.6
Other methods to counteract PTSD fall under the general principle of “trauma-informed care.” Trauma-informed care comprises practices promoting a culture of safety, empowerment, and healing.7 It is a mindful and sensitive approach that acknowledges the pervasive nature of trauma exposure, the reality of ongoing adverse effects in trauma survivors, and the fact that recovery is highly personal and complex.8
By definition, patients with PTSD have endured some traumatic event. Therefore, ideal care teams will ask patients about things that may trigger their anxiety and then work to mitigate them. For example, some patients with PTSD have a severe startle response when woken up by someone touching them. When patients feel that they can share their concerns with their care team and their team honors that observation by waking them in a different way, trust and control may be gained. This process of asking for patient guidance and adjusting accordingly is consistent with a trauma-informed care approach.9 A true trauma-informed care approach involves the entire practice environment but examining and adjusting our own behavior and assumptions are good places to start.
Summary of recommended treatments
Psychotherapy is preferable over pharmacotherapy, but both can be combined as needed. Individual trauma-focused psychotherapies utilizing a primary component of exposure and/or cognitive restructuring have strong evidence for effectiveness but are primarily outpatient based.
For pharmacologic treatment, selective serotonin reuptake inhibitors (for example, sertraline, paroxetine, or fluoxetine) and serotonin norepinephrine reuptake inhibitors (for example, venlafaxine) monotherapy have strong evidence for effectiveness and can be started while inpatient. However, these medications typically take weeks to produce benefits. Recent trials studying prazosin, an alpha1-adrenergic receptor antagonist used to alleviate nightmares associated with PTSD, have demonstrated inefficacy or even harm,leading experts to caution against its use.10,11 Finally, benzodiazepine and atypical antipsychotic usage should be restricted and used as a last resort.12
In summary, PTSD is common among veterans and nonveterans. While hospitalists may rarely admit patients because of their PTSD, they will often take care of patients who have PTSD as a comorbidity. Therefore, understanding the basics of PTSD and how hospitalization may exacerbate its symptoms can meaningfully improve care for these patients.
Dr. Fletcher is a hospitalist at the Milwaukee Veterans Affairs Medical Center and Froedtert Hospital in Wauwatosa, Wis. She is professor of internal medicine and program director for the internal medicine residency program at the Medical College of Wisconsin, Milwaukee. She is also faculty mentor for the VA’s Chief Resident for Quality and Safety. Dr. Kwan is a hospitalist at the VA San Diego Healthcare System and is associate professor at the University of California, San Diego, in the division of hospital medicine. He serves as an associate clerkship director of both the internal medicine clerkship and the medicine subinternship. He is the chair of SHM’s Physicians in Training committee. Dr. Steinbach is chief of hospital medicine at the Atlanta VA Medical Center and assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
References
1. Kang HK et al. Posttraumatic stress disorder and chronic fatigue syndrome–like illness among Gulf War veterans: A population-based survey of 30,000 veterans. Am J Epidemiol. 2003;157(2):141-8.
2. Kessler RC et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005; 62(6):593-602.
3. Cohen BE et al. Mental health diagnoses and utilization of VA nonmental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
4. Haviland MG et al. Posttraumatic stress disorder–related hospitalizations in the United States (2002-2011): Rates, co-occurring illnesses, suicidal ideation/self-harm, and hospital charges. J Nerv Ment Dis. 2016; 204(2):78-86.
5. Aurora RN et al. Best practice guide for the treatment of nightmare disorder in adults. J Clin Sleep Med. 2010;6(4):389-401.
6. Blanaru M et al. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress disorder. Ment Illn. 2012;4(2):e13.
7. Tello M. (2018, Oct 16). Trauma-informed care: What it is, and why it’s important. Retrieved March 18, 2019, from https://www.health.harvard.edu/blog/trauma-informed-care-what-it-is-and-why-its-important-2018101613562.
8. Harris M et al. Using trauma theory to design service systems. San Francisco: 2001.
9. Substance abuse and mental health services administration. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. HHS publication no. SMA 14-4884. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
10. Raskind MA et al. Trial of prazosin for posttraumatic stress disorder in military veterans. N Engl J Med. 2018 Feb 8;378(6):507-7.
11. McCall WV et al. A pilot, randomized clinical trial of bedtime doses of prazosin versus placebo in suicidal posttraumatic stress disorder patients with nightmares. J Clin Psychopharmacol. 2018 Dec;38(6):618-21.
12. U.S. Department of Veterans Affairs/U.S. Department of Defense. Clinical practice guideline for the management of posttraumatic stress disorder and acute stress reaction 2017. Accessed February 18, 2019.
In newborns, concentrated urine helps rule out UTI
SEATTLE – according to investigators at the University of Texas Health Science Center, Houston.
The researchers found that urine testing negative for nitrites with a specific gravity above 1.015 in children up to 2 months old had a sensitivity of 53% for ruling out UTIs, but that urine with a specific gravity below that mark had a sensitivity of just 14%. The finding “should be taken into account when interpreting nitrite results ... in this high-risk population,” they concluded.
Bacteria in the bladder convert nitrates to nitrites, so positive results are pretty much pathognomonic for UTIs, with a specificity of nearly 100%, according to the researchers.
Negative results, however, don’t reliably rule out infection, and are even less reliable in infants because they urinate frequently, which means they usually flush out bacteria before they have enough time to make the conversion, which takes several hours, they said.
The lead investigator Raymond Parlar-Chun, MD, an assistant professor of pediatrics at the University of Texas McGovern Medical School in Houston, said he had a hunch that negative results might be more reliable when newborns urinate less frequently and have more concentrated urine.
He and his team reviewed data collected on 413 infants up to 2 months old who were admitted for fever workup and treated for UTIs both in the hospital and after discharge. Nitrite results were stratified by urine concentration. A specific gravity of 1.015 was used as the cutoff between concentrated and dilute urine, which was “midway between the parameters reported” in every urinalysis, Dr. Parlar-Chun said.
Although the sensitivity of concentrated urine was only 53%, “it’s a stark difference from” the 14% in dilute urine, he said.“You should take a look at specific gravity to interpret nitrites. If urine is concentrated, you have [more confidence] that you don’t have a UTI if you’re negative. It’s better than taking [nitrites] at face value.”
The subjects were 31 days old, on average, and 62% were boys; 112 had a specific gravity above 1.015, and 301 below.
There was no external funding, and Dr. Parlar-Chun didn’t have any disclosures.
SEATTLE – according to investigators at the University of Texas Health Science Center, Houston.
The researchers found that urine testing negative for nitrites with a specific gravity above 1.015 in children up to 2 months old had a sensitivity of 53% for ruling out UTIs, but that urine with a specific gravity below that mark had a sensitivity of just 14%. The finding “should be taken into account when interpreting nitrite results ... in this high-risk population,” they concluded.
Bacteria in the bladder convert nitrates to nitrites, so positive results are pretty much pathognomonic for UTIs, with a specificity of nearly 100%, according to the researchers.
Negative results, however, don’t reliably rule out infection, and are even less reliable in infants because they urinate frequently, which means they usually flush out bacteria before they have enough time to make the conversion, which takes several hours, they said.
The lead investigator Raymond Parlar-Chun, MD, an assistant professor of pediatrics at the University of Texas McGovern Medical School in Houston, said he had a hunch that negative results might be more reliable when newborns urinate less frequently and have more concentrated urine.
He and his team reviewed data collected on 413 infants up to 2 months old who were admitted for fever workup and treated for UTIs both in the hospital and after discharge. Nitrite results were stratified by urine concentration. A specific gravity of 1.015 was used as the cutoff between concentrated and dilute urine, which was “midway between the parameters reported” in every urinalysis, Dr. Parlar-Chun said.
Although the sensitivity of concentrated urine was only 53%, “it’s a stark difference from” the 14% in dilute urine, he said.“You should take a look at specific gravity to interpret nitrites. If urine is concentrated, you have [more confidence] that you don’t have a UTI if you’re negative. It’s better than taking [nitrites] at face value.”
The subjects were 31 days old, on average, and 62% were boys; 112 had a specific gravity above 1.015, and 301 below.
There was no external funding, and Dr. Parlar-Chun didn’t have any disclosures.
SEATTLE – according to investigators at the University of Texas Health Science Center, Houston.
The researchers found that urine testing negative for nitrites with a specific gravity above 1.015 in children up to 2 months old had a sensitivity of 53% for ruling out UTIs, but that urine with a specific gravity below that mark had a sensitivity of just 14%. The finding “should be taken into account when interpreting nitrite results ... in this high-risk population,” they concluded.
Bacteria in the bladder convert nitrates to nitrites, so positive results are pretty much pathognomonic for UTIs, with a specificity of nearly 100%, according to the researchers.
Negative results, however, don’t reliably rule out infection, and are even less reliable in infants because they urinate frequently, which means they usually flush out bacteria before they have enough time to make the conversion, which takes several hours, they said.
The lead investigator Raymond Parlar-Chun, MD, an assistant professor of pediatrics at the University of Texas McGovern Medical School in Houston, said he had a hunch that negative results might be more reliable when newborns urinate less frequently and have more concentrated urine.
He and his team reviewed data collected on 413 infants up to 2 months old who were admitted for fever workup and treated for UTIs both in the hospital and after discharge. Nitrite results were stratified by urine concentration. A specific gravity of 1.015 was used as the cutoff between concentrated and dilute urine, which was “midway between the parameters reported” in every urinalysis, Dr. Parlar-Chun said.
Although the sensitivity of concentrated urine was only 53%, “it’s a stark difference from” the 14% in dilute urine, he said.“You should take a look at specific gravity to interpret nitrites. If urine is concentrated, you have [more confidence] that you don’t have a UTI if you’re negative. It’s better than taking [nitrites] at face value.”
The subjects were 31 days old, on average, and 62% were boys; 112 had a specific gravity above 1.015, and 301 below.
There was no external funding, and Dr. Parlar-Chun didn’t have any disclosures.
REPORTING FROM PHM 2019
Sepsis survivors’ persistent immunosuppression raises mortality risk
readmission after discharge, and mortality, according to a study published in JAMA Network Open.
“Individuals with persistent biomarkers of inflammation and immunosuppression had a higher risk of readmission and death due to cardiovascular disease and cancer compared with those with normal circulating biomarkers,” Sachin Yende, MD, of the VA Pittsburgh Healthcare System and the University of Pittsburgh and colleagues wrote in their study. “Our findings suggest that long-term immunomodulation strategies should be explored in patients hospitalized with sepsis.”
Dr. Yende and colleagues performed a multicenter, prospective cohort study of 483 patients who were hospitalized for sepsis at 12 different sites between January 2012 and May 2017. They measured inflammation using interleukin-6, high-sensitivity C-reactive protein (hs-CRP), and soluble programmed death-ligand 1 (sPD-L1); hemostasis using plasminogen activator inhibitor 1 and D-dimer; and endothelial dysfunction using intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin. The patients included were mean age 60.5 years, 54.9% were male, the mean Sequential Organ Failure Assessment score was 4.2, and a total of 376 patients (77.8%) had one or more chronic diseases.
Overall, there were 485 readmissions in 205 patients (42.5%). The mortality rate was 43 patients (8.9%) at 3 months, 56 patients (11.6%) at 6 months, and 85 patients (17.6%) at 12 months. At 3 months, 23 patients (25.8%) had elevated hs-CRP levels, which increased to 26 patients (30.2%) at 6 months and 40 patients (44.9%) at 12 months. sPD-L1 levels were elevated in 45 patients (46.4%) at 3 months, but the number of patients with elevated sPD-L1 did not appear to significantly increase at 6 months (40 patients; 44.9%) or 12 months (44 patients; 49.4%).
From these results, researchers developed a phenotype of hyperinflammation and immunosuppression that consisted of 326 of 477 (68.3%) patients with high hs-CRP and elevated sPD-L1 levels. Patients with this phenotype of hyperinflammation and immunosuppression had more than eight times the risk of 1-year mortality (odds ratio, 8.26; 95% confidence interval, 3.45-21.69; P less than .001) and more than five times the risk of readmission or mortality at 6 months related to cardiovascular disease (hazard ratio, 5.07; 95% CI, 1.18-21.84; P = .02) or cancer (hazard ratio, 5.15; 95% CI, 1.25-21.18; P = .02), compared with patients who had normal hs-CRP and sPD-L1 levels. This hyperinflammation and immunosuppression phenotype also was associated with greater risk of 6-month all-cause readmission or mortality (HR, 1.53; 95% CI, 1.10-2.13; P = .01), compared with patients who had the normal phenotype.
“The persistence of hyperinflammation in a large number of sepsis survivors and the increased risk of cardiovascular events among these patients may explain the association between infection and cardiovascular disease in a prior study,” the authors said. “Although prior trials tested immunomodulation strategies during only the early phase of hospitalization for sepsis, immunomodulation may be needed after hospital discharge,” and suggest points of future study for patients who survive sepsis and develop long-term sequelae.
This study was funded by grants from National Institutes of Health and resources from the VA Pittsburgh Healthcare System. The authors reported personal and institutional relationships in the form of personal fees, grants, and patents for Alung Technologies, Atox Bio, Bayer AG, Beckman Coulter, BristolMyers Squibb, Ferring, NIH, Roche, Selepressin, and the University of Pittsburgh.
SOURCE: Yende S et al. JAMA Netw Open. 2019 Aug 7. doi: 10.1001/jamanetworkopen.2019.8686.
readmission after discharge, and mortality, according to a study published in JAMA Network Open.
“Individuals with persistent biomarkers of inflammation and immunosuppression had a higher risk of readmission and death due to cardiovascular disease and cancer compared with those with normal circulating biomarkers,” Sachin Yende, MD, of the VA Pittsburgh Healthcare System and the University of Pittsburgh and colleagues wrote in their study. “Our findings suggest that long-term immunomodulation strategies should be explored in patients hospitalized with sepsis.”
Dr. Yende and colleagues performed a multicenter, prospective cohort study of 483 patients who were hospitalized for sepsis at 12 different sites between January 2012 and May 2017. They measured inflammation using interleukin-6, high-sensitivity C-reactive protein (hs-CRP), and soluble programmed death-ligand 1 (sPD-L1); hemostasis using plasminogen activator inhibitor 1 and D-dimer; and endothelial dysfunction using intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin. The patients included were mean age 60.5 years, 54.9% were male, the mean Sequential Organ Failure Assessment score was 4.2, and a total of 376 patients (77.8%) had one or more chronic diseases.
Overall, there were 485 readmissions in 205 patients (42.5%). The mortality rate was 43 patients (8.9%) at 3 months, 56 patients (11.6%) at 6 months, and 85 patients (17.6%) at 12 months. At 3 months, 23 patients (25.8%) had elevated hs-CRP levels, which increased to 26 patients (30.2%) at 6 months and 40 patients (44.9%) at 12 months. sPD-L1 levels were elevated in 45 patients (46.4%) at 3 months, but the number of patients with elevated sPD-L1 did not appear to significantly increase at 6 months (40 patients; 44.9%) or 12 months (44 patients; 49.4%).
From these results, researchers developed a phenotype of hyperinflammation and immunosuppression that consisted of 326 of 477 (68.3%) patients with high hs-CRP and elevated sPD-L1 levels. Patients with this phenotype of hyperinflammation and immunosuppression had more than eight times the risk of 1-year mortality (odds ratio, 8.26; 95% confidence interval, 3.45-21.69; P less than .001) and more than five times the risk of readmission or mortality at 6 months related to cardiovascular disease (hazard ratio, 5.07; 95% CI, 1.18-21.84; P = .02) or cancer (hazard ratio, 5.15; 95% CI, 1.25-21.18; P = .02), compared with patients who had normal hs-CRP and sPD-L1 levels. This hyperinflammation and immunosuppression phenotype also was associated with greater risk of 6-month all-cause readmission or mortality (HR, 1.53; 95% CI, 1.10-2.13; P = .01), compared with patients who had the normal phenotype.
“The persistence of hyperinflammation in a large number of sepsis survivors and the increased risk of cardiovascular events among these patients may explain the association between infection and cardiovascular disease in a prior study,” the authors said. “Although prior trials tested immunomodulation strategies during only the early phase of hospitalization for sepsis, immunomodulation may be needed after hospital discharge,” and suggest points of future study for patients who survive sepsis and develop long-term sequelae.
This study was funded by grants from National Institutes of Health and resources from the VA Pittsburgh Healthcare System. The authors reported personal and institutional relationships in the form of personal fees, grants, and patents for Alung Technologies, Atox Bio, Bayer AG, Beckman Coulter, BristolMyers Squibb, Ferring, NIH, Roche, Selepressin, and the University of Pittsburgh.
SOURCE: Yende S et al. JAMA Netw Open. 2019 Aug 7. doi: 10.1001/jamanetworkopen.2019.8686.
readmission after discharge, and mortality, according to a study published in JAMA Network Open.
“Individuals with persistent biomarkers of inflammation and immunosuppression had a higher risk of readmission and death due to cardiovascular disease and cancer compared with those with normal circulating biomarkers,” Sachin Yende, MD, of the VA Pittsburgh Healthcare System and the University of Pittsburgh and colleagues wrote in their study. “Our findings suggest that long-term immunomodulation strategies should be explored in patients hospitalized with sepsis.”
Dr. Yende and colleagues performed a multicenter, prospective cohort study of 483 patients who were hospitalized for sepsis at 12 different sites between January 2012 and May 2017. They measured inflammation using interleukin-6, high-sensitivity C-reactive protein (hs-CRP), and soluble programmed death-ligand 1 (sPD-L1); hemostasis using plasminogen activator inhibitor 1 and D-dimer; and endothelial dysfunction using intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin. The patients included were mean age 60.5 years, 54.9% were male, the mean Sequential Organ Failure Assessment score was 4.2, and a total of 376 patients (77.8%) had one or more chronic diseases.
Overall, there were 485 readmissions in 205 patients (42.5%). The mortality rate was 43 patients (8.9%) at 3 months, 56 patients (11.6%) at 6 months, and 85 patients (17.6%) at 12 months. At 3 months, 23 patients (25.8%) had elevated hs-CRP levels, which increased to 26 patients (30.2%) at 6 months and 40 patients (44.9%) at 12 months. sPD-L1 levels were elevated in 45 patients (46.4%) at 3 months, but the number of patients with elevated sPD-L1 did not appear to significantly increase at 6 months (40 patients; 44.9%) or 12 months (44 patients; 49.4%).
From these results, researchers developed a phenotype of hyperinflammation and immunosuppression that consisted of 326 of 477 (68.3%) patients with high hs-CRP and elevated sPD-L1 levels. Patients with this phenotype of hyperinflammation and immunosuppression had more than eight times the risk of 1-year mortality (odds ratio, 8.26; 95% confidence interval, 3.45-21.69; P less than .001) and more than five times the risk of readmission or mortality at 6 months related to cardiovascular disease (hazard ratio, 5.07; 95% CI, 1.18-21.84; P = .02) or cancer (hazard ratio, 5.15; 95% CI, 1.25-21.18; P = .02), compared with patients who had normal hs-CRP and sPD-L1 levels. This hyperinflammation and immunosuppression phenotype also was associated with greater risk of 6-month all-cause readmission or mortality (HR, 1.53; 95% CI, 1.10-2.13; P = .01), compared with patients who had the normal phenotype.
“The persistence of hyperinflammation in a large number of sepsis survivors and the increased risk of cardiovascular events among these patients may explain the association between infection and cardiovascular disease in a prior study,” the authors said. “Although prior trials tested immunomodulation strategies during only the early phase of hospitalization for sepsis, immunomodulation may be needed after hospital discharge,” and suggest points of future study for patients who survive sepsis and develop long-term sequelae.
This study was funded by grants from National Institutes of Health and resources from the VA Pittsburgh Healthcare System. The authors reported personal and institutional relationships in the form of personal fees, grants, and patents for Alung Technologies, Atox Bio, Bayer AG, Beckman Coulter, BristolMyers Squibb, Ferring, NIH, Roche, Selepressin, and the University of Pittsburgh.
SOURCE: Yende S et al. JAMA Netw Open. 2019 Aug 7. doi: 10.1001/jamanetworkopen.2019.8686.
FROM JAMA NETWORK OPEN
Key clinical point: Markers of inflammation and immunosuppression persist in over two-thirds of patients hospitalized for sepsis, which could explain worsened outcomes and mortality up to 1 year after hospitalization.
Major finding: Patients with signs of hyperinflammation and immunosuppression had significantly increased mortality after 1 year and were significantly more likely to be readmitted or die because of cardiovascular disease or cancer.
Study details: A prospective cohort study of 483 patients who were hospitalized because of sepsis at 12 different centers between January 2012 and May 2017.
Disclosures: This study was funded by grants from National Institutes of Health and resources from the Veterans Affairs Pittsburgh Healthcare System. The authors reported personal and institutional relationships in the form of personal fees, grants, and patents for Alung Technologies, Atox Bio, Bayer AG, Beckman Coulter, BristolMyers Squibb, Ferring, NIH, Roche, Selepressin, and the University of Pittsburgh.
Source: Yende S et al. JAMA Netw Open. 2019 Aug 7. doi: 10.1001/jamanetworkopen.2019.8686.
Hospital slashes S. aureus vancomycin resistance
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
REPORTING FROM ESPID 2019
Key clinical point: Staphylococcus aureus vancomycin MIC creep is reversible through dedicated antimicrobial stewardship.
Major finding: The prevalence of hGISA in MRSA and MSSA specimens improved from 25% during 2002-2009 to 6% during 2010-2017 at one German tertiary children’s hospital.
Study details: This was a retrospective single-center analysis of vancomycin resistance trends over time in 540 S. aureus specimens gathered in 2002-2017.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial support.
CDC finds that too little naloxone is dispensed
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT