User login
Permanent Alopecia in Breast Cancer Patients: Role of Taxanes and Endocrine Therapies
Anagen effluvium during chemotherapy is common, typically beginning within 1 month of treatment onset and resolving by 6 months after the final course.1 Permanent chemotherapy-induced alopecia (PCIA), in which hair loss persists beyond 6 months after chemotherapy without recovery to original density, was first reported in patients following high-dose chemotherapy regimens for allogeneic bone marrow transplantation.2 There are now increasing reports of PCIA in patients with breast cancer; at least 400 such cases have been documented.3-16 In addition to chemotherapy, patients often receive adjuvant endocrine therapy with selective estrogen receptor modulators, aromatase inhibitors, or gonadotropin-releasing hormone agonists.5-16 Endocrine therapies also can lead to alopecia, but their role in PCIA has not been well defined.15,16 We describe 3 patients with breast cancer who experienced PCIA following chemotherapy with taxanes with or without endocrine therapies. We also review the literature on non–bone marrow transplantation PCIA to better characterize this entity and explore the role of endocrine therapies in PCIA.
Case Reports
Patient 1
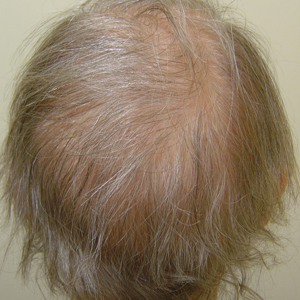
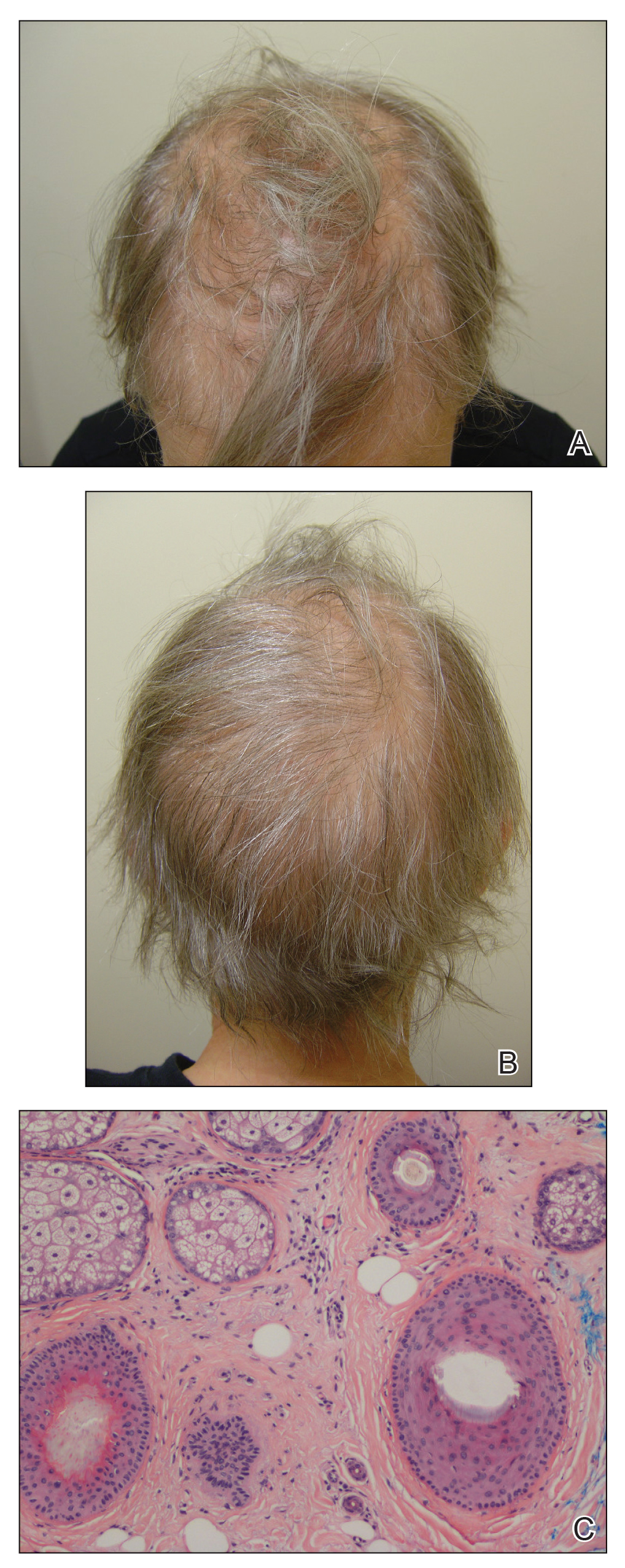
A 62-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 5 years after completing chemotherapy. She underwent 6 cycles of docetaxel and carboplatin along with radiation therapy as well as 1 year of trastuzumab and did not receive endocrine therapy. At the current presentation, she reported patchy hair regrowth that gradually filled in but failed to return to full density. Physical examination revealed the hair was diffusely thin, especially bitemporally (Figures 1A and 1B), and she did not experience any loss of body hair. She had no family history of hair loss. Her medical history was notable for hypertension, chronic obstructive bronchitis, osteopenia, and depression. Her thyroid stimulating hormone (TSH) level was within reference range. Medications included lisinopril, metoprolol, escitalopram, and trazodone. A biopsy from the occipital scalp showed nonscarring alopecia with variation of hair follicle size, a decreased number of hair follicles, and a decreased anagen to telogen ratio (Figure 1C). She was treated with clobetasol solution and minoxidil solution 5% for 1 year with mild improvement. She experienced no further hair loss but did not regain original hair density.
Patient 2
A 35-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 10 months after chemotherapy. She underwent 4 cycles of doxorubicin and cyclophosphamide followed by 4 cycles of paclitaxel and was started on trastuzumab. Tamoxifen was initiated 1 month after completing chemotherapy. She received radiation therapy the following month and continued trastuzumab for 1 year. At the current presentation, the patient noted that hair regrowth had started 1 month after the last course of chemotherapy but had progressed slowly. She denied body hair loss. Physical examination revealed diffuse thinning, especially over the crown, with scattered broken hairs throughout the scalp and several miniaturized hairs over the crown. She was evaluated as grade 3 on the Sinclair clinical grading scale used to evaluate female pattern hair loss (FPHL).17 Her family history was remarkable for FPHL in her maternal grandmother. She had no notable medical history, her TSH was normal, and she was taking tamoxifen and trastuzumab. Biopsy was not performed. The patient was started on minoxidil solution 2% and had mild improvement with no further broken-off hairs after 10 months. At that point, she was evaluated as grade 2 to 3 on the Sinclair scale.17
Patient 3
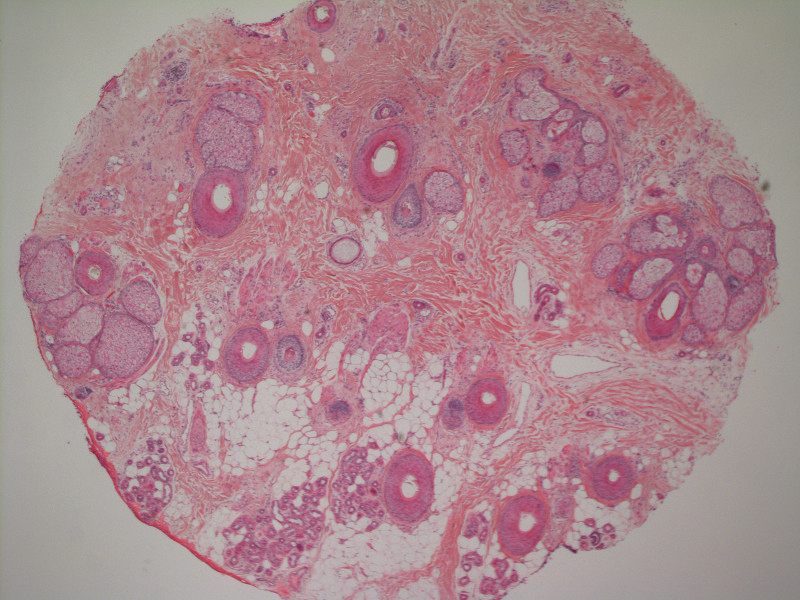
A 51-year-old woman with a history of papillary carcinoma and extensive ductal carcinoma in situ presented with persistent hair loss for 3.5 years following chemotherapy for recurrent breast cancer. After her initial diagnosis in the left breast, she received cyclophosphamide, methotrexate, and 5-fluorouracil but did not receive endocrine therapy. Her hair thinned during chemotherapy but returned to normal density within 1 year. She had a recurrence of the cancer in the right breast 14 years later and received 6 cycles of chemotherapy with cyclophosphamide and docetaxel followed by radiation therapy. After this course, her hair loss incompletely recovered. One year after chemotherapy, she underwent bilateral salpingo-oophorectomy and started anastrozole. Three months later, she noticed increased shedding and progressive thinning of the hair. Physical examination revealed diffuse thinning that was most pronounced over the crown. She also experienced lateral thinning of the eyebrows, decreased eyelashes, and dystrophic fingernails. Fluocinonide solution was discontinued by the patient due to scalp burning. She had a brother with bitemporal recession. Her medical history was notable for Hashimoto thyroiditis, vitamin D deficiency, and peripheral neuropathy. Her TSH occasionally was elevated, and she was intermittently on levothyroxine; however, her free T4 was maintained within reference range on all records. Her medications at the time of evaluation were anastrozole and gabapentin. Biopsies taken from the right and left temporal scalp revealed decreased follicle density with a majority of follicles in anagen, scattered miniaturized follicles, and a mild perivascular and perifollicular lymphoid infiltrate. Mild dermal fibrosis was present without evidence of frank scarring (Figure 2). She declined treatment, and there was no change in her condition over 3 years of follow-up.
Comment
Classification of Chemotherapy-Induced Hair Loss
Chemotherapy-induced alopecia is typically an anagen effluvium that is reversed within 6 months following the final course of chemotherapy. When incomplete regrowth persists, the patient is considered to have PCIA.1 The pathophysiology of PCIA is unclear.
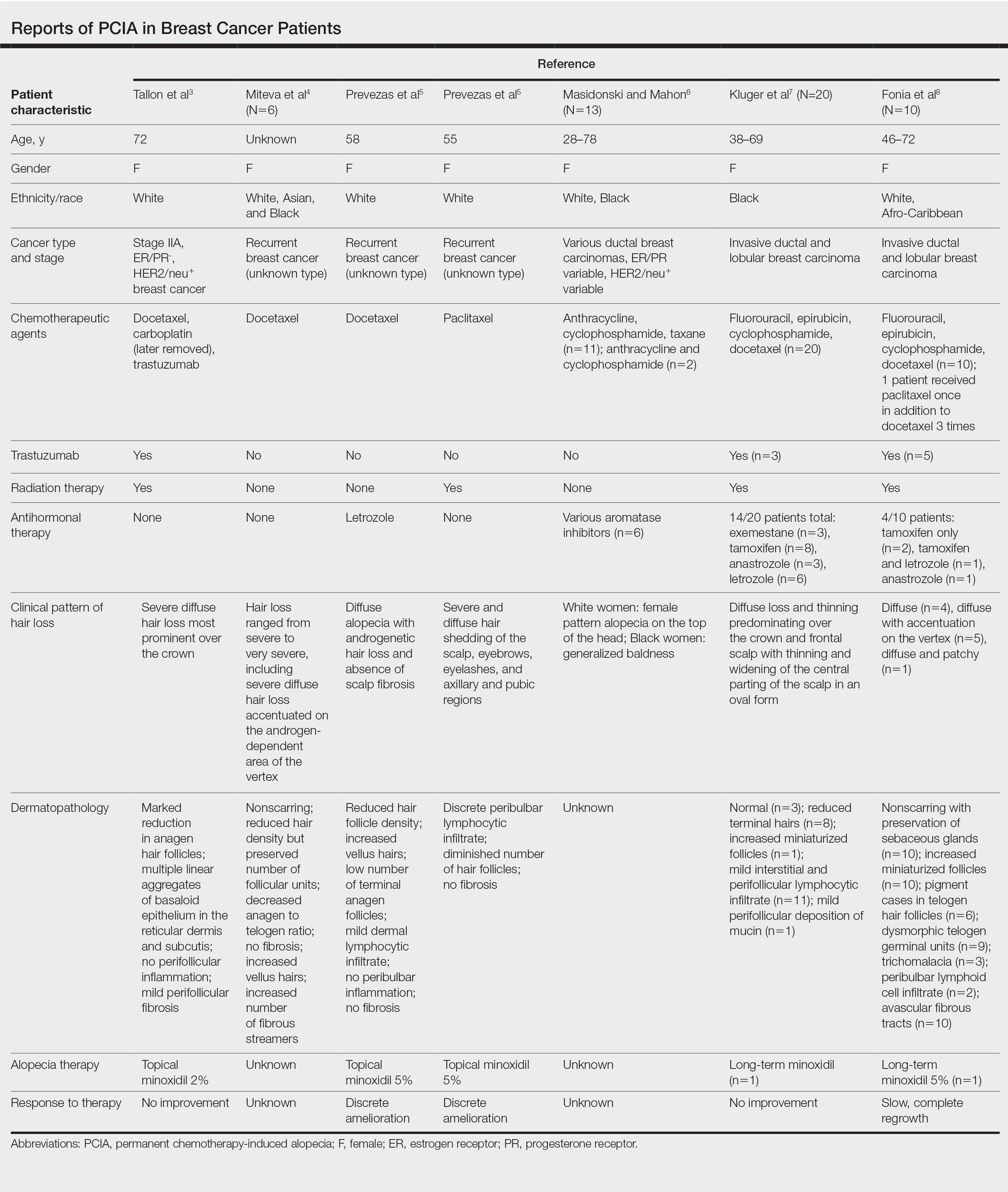
Traditional grading for chemotherapy-induced alopecia does not account for the patterns of loss seen in PCIA, of which the most common appears to be a female pattern with accentuated hair loss in androgen-dependent regions of the scalp.18 Other patterns include a diffuse type with body hair loss, patchy alopecia, and complete alopecia with or without body hair loss (Table).3-8 Whether these patterns all can be attributed to chemotherapy remains to be explored.
Breast Cancer Therapies Causing PCIA
The main agents thought to be responsible for PCIA in breast cancer patients are taxanes. The role of endocrine therapies has not been well explored. Trastuzumab lacks several of the common side effects of chemotherapy due to its specificity for the HER2/neu receptor and has not been found to increase the rate of hair loss when combined with standard chemotherapy.19,20 Although radiation therapy has the potential to damage hair follicles, and a dose-dependent relationship has been described for temporary and permanent alopecia at irradiated sites, permanent alopecia predominantly has been reported with cranial radiation used in the treatment of intracranial malignancies.21 The role of radiation therapy of the breasts in PCIA is unclear, as its inclusion in therapy has not been consistently reported in the literature.
Docetaxel is known to cause chemotherapy-induced alopecia, with an 83.4% incidence in phase 2 trials; however, it also appears to be related to PCIA.20 A PubMed search of articles indexed for MEDLINE was performed using the terms permanent chemotherapy induced alopecia, chemotherapy, docetaxel, endocrine therapies, hair loss, alopecia, and breast cancer. More than 400 cases of PCIA related to chemotherapy in breast cancer patients have been reported in the literature from a combination of case reports/series, retrospective surveys, and at least one prospective study. Data from some of the more detailed reports (n=52) are summarized in the Table. In the single-center, 3-year prospective study of women given adjuvant taxane-based or non–taxane-based chemotherapy, those who received taxane therapy were more likely to develop PCIA (odds ratio, 8.01).9
All 3 of our patients received taxanes. Interestingly, patient 3 underwent 2 rounds of chemotherapy 14 years apart and experienced full regrowth of the hair after the first course of taxane-free chemotherapy but experienced persistent hair loss following docetaxel treatment. Adjuvant endocrine therapies also may contribute to PCIA. A review of the side effects of endocrine therapies revealed an incidence of alopecia that was higher than expected; tamoxifen was the greatest offender. Additionally, using endocrine treatments in combination was found to have a synergistic effect on alopecia.18 Adjuvant endocrine therapy was used in patients 2 and 3. Although endocrine therapies appear to have a milder effect on hair loss compared to chemotherapy, these medications are continued for a longer duration, potentially contributing to the severity of hair loss and prolonging the time to regrowth.
Furthermore, endocrine therapies used in breast cancer treatment decrease estrogen levels or antagonize estrogen receptors, creating an environment of relative hyperandrogenism that may contribute to FPHL in genetically susceptible women.18 Although taxanes may cause irreversible hair loss in these patients, the action of endocrine therapies on the remaining hair follicles may affect the typical female pattern seen clinically. Patients 2 and 3 who presented with FPHL received adjuvant endocrine therapies and had positive family history, while patient 1 did not. Of note, patient 3 experienced worsening hair loss following the addition of anastrozole, which suggests a contribution of endocrine therapy to her PCIA. Our limited cases do not allow for evaluation of a worsened outcome with the combination of taxanes and endocrine therapies; however, we suggest further evaluation for a synergistic effect that may be contributing to PCIA.
Conclusion
Permanent alopecia in breast cancer patients appears to be a true potential adverse effect of taxanes and endocrine therapies, and it is important to characterize it appropriately so that its mechanism can be understood and appropriate treatment and counseling can take place. Although it may not influence clinical decision-making, patients should be informed that hair loss with chemotherapy can be permanent. Treatment with scalp cooling can reduce the risk for severe chemotherapy-induced alopecia, but it is unclear if it reduces risk for PCIA.12,15 Topical or oral minoxidil may be helpful in the treatment of PCIA once it has developed.7,8,15,22 Better characterization of these cases may elucidate risk factors for developing permanent alopecia, allowing for more appropriate risk stratification, counseling, and treatment.
- Dorr VJ. A practitioner’s guide to cancer-related alopecia. Semin Oncol. 1998;25:562-570.
- Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:979-982.
- Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63:333-336.
- Miteva M, Misciali C, Fanti PA, et al. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33:345-350.
- Prevezas C, Matard B, Pinquier L, et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160:883-885.
- Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clin J Oncol Nurs. 2009;13:13-14.
- Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23:2879-2884.
- Fonia A, Cota C, Setterfield JF, et al. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76:948-957.
- Kang D, Kim IR, Choi EK, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study [published online August 17, 2018]. Oncologist. 2019;24:414-420.
- Chan J, Adderley H, Alameddine M, et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: a retrospective survey at two tertiary UK cancer centres [published online December 22, 2020]. Eur J Cancer Care (Engl). doi:10.1111/ecc.13395
- Bourgeois H, Denis F, Kerbrat P, et al. Long term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: alert for an emerging side effect: ALOPERS Observatory. Cancer Res. 2009;69(24 suppl). doi:10.1158/0008-5472.SABCS-09-3174
- Bertrand M, Mailliez A, Vercambre S, et al. Permanent chemotherapy induced alopecia in early breast cancer patients after (neo)adjuvant chemotherapy: long term follow up. Cancer Res. 2013;73(24 suppl). doi:10.1158/0008-5472.SABCS13-P3-09-15
- Kim S, Park HS, Kim JY, et al. Irreversible chemotherapy-induced alopecia in breast cancer patient. Cancer Res. 2016;76(4 suppl). doi:10.1158/1538-7445.SABCS15-P1-15-04
- Thorp NJ, Swift F, Arundell D, et al. Long term hair loss in patients with early breast cancer receiving docetaxel chemotherapy. Cancer Res. 2015;75(9 suppl). doi:10.1158/1538-7445.SABCS14-P5-17-04
- Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80:1199-1213.
- Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155:724-728.
- Sinclair R, Jolley D, Mallari R, et al. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189-199.
- Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18:1126-1134.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Baselga J. Clinical trials of single-agent trastuzumab (Herceptin). Semin Oncol. 2000;27(5 suppl 9):20-26.
- Lawenda BD, Gagne HM, Gierga DP, et al. Permanent alopecia after cranial irradiation: dose-response relationship. Int J Radiat Oncol Biol Phys. 2004;60:879-887.
- Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil [published online May 13, 2015]. Australas J Dermatol. 2016;57:E130-E132.
Anagen effluvium during chemotherapy is common, typically beginning within 1 month of treatment onset and resolving by 6 months after the final course.1 Permanent chemotherapy-induced alopecia (PCIA), in which hair loss persists beyond 6 months after chemotherapy without recovery to original density, was first reported in patients following high-dose chemotherapy regimens for allogeneic bone marrow transplantation.2 There are now increasing reports of PCIA in patients with breast cancer; at least 400 such cases have been documented.3-16 In addition to chemotherapy, patients often receive adjuvant endocrine therapy with selective estrogen receptor modulators, aromatase inhibitors, or gonadotropin-releasing hormone agonists.5-16 Endocrine therapies also can lead to alopecia, but their role in PCIA has not been well defined.15,16 We describe 3 patients with breast cancer who experienced PCIA following chemotherapy with taxanes with or without endocrine therapies. We also review the literature on non–bone marrow transplantation PCIA to better characterize this entity and explore the role of endocrine therapies in PCIA.
Case Reports
Patient 1
A 62-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 5 years after completing chemotherapy. She underwent 6 cycles of docetaxel and carboplatin along with radiation therapy as well as 1 year of trastuzumab and did not receive endocrine therapy. At the current presentation, she reported patchy hair regrowth that gradually filled in but failed to return to full density. Physical examination revealed the hair was diffusely thin, especially bitemporally (Figures 1A and 1B), and she did not experience any loss of body hair. She had no family history of hair loss. Her medical history was notable for hypertension, chronic obstructive bronchitis, osteopenia, and depression. Her thyroid stimulating hormone (TSH) level was within reference range. Medications included lisinopril, metoprolol, escitalopram, and trazodone. A biopsy from the occipital scalp showed nonscarring alopecia with variation of hair follicle size, a decreased number of hair follicles, and a decreased anagen to telogen ratio (Figure 1C). She was treated with clobetasol solution and minoxidil solution 5% for 1 year with mild improvement. She experienced no further hair loss but did not regain original hair density.

Patient 2
A 35-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 10 months after chemotherapy. She underwent 4 cycles of doxorubicin and cyclophosphamide followed by 4 cycles of paclitaxel and was started on trastuzumab. Tamoxifen was initiated 1 month after completing chemotherapy. She received radiation therapy the following month and continued trastuzumab for 1 year. At the current presentation, the patient noted that hair regrowth had started 1 month after the last course of chemotherapy but had progressed slowly. She denied body hair loss. Physical examination revealed diffuse thinning, especially over the crown, with scattered broken hairs throughout the scalp and several miniaturized hairs over the crown. She was evaluated as grade 3 on the Sinclair clinical grading scale used to evaluate female pattern hair loss (FPHL).17 Her family history was remarkable for FPHL in her maternal grandmother. She had no notable medical history, her TSH was normal, and she was taking tamoxifen and trastuzumab. Biopsy was not performed. The patient was started on minoxidil solution 2% and had mild improvement with no further broken-off hairs after 10 months. At that point, she was evaluated as grade 2 to 3 on the Sinclair scale.17
Patient 3
A 51-year-old woman with a history of papillary carcinoma and extensive ductal carcinoma in situ presented with persistent hair loss for 3.5 years following chemotherapy for recurrent breast cancer. After her initial diagnosis in the left breast, she received cyclophosphamide, methotrexate, and 5-fluorouracil but did not receive endocrine therapy. Her hair thinned during chemotherapy but returned to normal density within 1 year. She had a recurrence of the cancer in the right breast 14 years later and received 6 cycles of chemotherapy with cyclophosphamide and docetaxel followed by radiation therapy. After this course, her hair loss incompletely recovered. One year after chemotherapy, she underwent bilateral salpingo-oophorectomy and started anastrozole. Three months later, she noticed increased shedding and progressive thinning of the hair. Physical examination revealed diffuse thinning that was most pronounced over the crown. She also experienced lateral thinning of the eyebrows, decreased eyelashes, and dystrophic fingernails. Fluocinonide solution was discontinued by the patient due to scalp burning. She had a brother with bitemporal recession. Her medical history was notable for Hashimoto thyroiditis, vitamin D deficiency, and peripheral neuropathy. Her TSH occasionally was elevated, and she was intermittently on levothyroxine; however, her free T4 was maintained within reference range on all records. Her medications at the time of evaluation were anastrozole and gabapentin. Biopsies taken from the right and left temporal scalp revealed decreased follicle density with a majority of follicles in anagen, scattered miniaturized follicles, and a mild perivascular and perifollicular lymphoid infiltrate. Mild dermal fibrosis was present without evidence of frank scarring (Figure 2). She declined treatment, and there was no change in her condition over 3 years of follow-up.

Comment
Classification of Chemotherapy-Induced Hair Loss
Chemotherapy-induced alopecia is typically an anagen effluvium that is reversed within 6 months following the final course of chemotherapy. When incomplete regrowth persists, the patient is considered to have PCIA.1 The pathophysiology of PCIA is unclear.
Traditional grading for chemotherapy-induced alopecia does not account for the patterns of loss seen in PCIA, of which the most common appears to be a female pattern with accentuated hair loss in androgen-dependent regions of the scalp.18 Other patterns include a diffuse type with body hair loss, patchy alopecia, and complete alopecia with or without body hair loss (Table).3-8 Whether these patterns all can be attributed to chemotherapy remains to be explored.

Breast Cancer Therapies Causing PCIA
The main agents thought to be responsible for PCIA in breast cancer patients are taxanes. The role of endocrine therapies has not been well explored. Trastuzumab lacks several of the common side effects of chemotherapy due to its specificity for the HER2/neu receptor and has not been found to increase the rate of hair loss when combined with standard chemotherapy.19,20 Although radiation therapy has the potential to damage hair follicles, and a dose-dependent relationship has been described for temporary and permanent alopecia at irradiated sites, permanent alopecia predominantly has been reported with cranial radiation used in the treatment of intracranial malignancies.21 The role of radiation therapy of the breasts in PCIA is unclear, as its inclusion in therapy has not been consistently reported in the literature.
Docetaxel is known to cause chemotherapy-induced alopecia, with an 83.4% incidence in phase 2 trials; however, it also appears to be related to PCIA.20 A PubMed search of articles indexed for MEDLINE was performed using the terms permanent chemotherapy induced alopecia, chemotherapy, docetaxel, endocrine therapies, hair loss, alopecia, and breast cancer. More than 400 cases of PCIA related to chemotherapy in breast cancer patients have been reported in the literature from a combination of case reports/series, retrospective surveys, and at least one prospective study. Data from some of the more detailed reports (n=52) are summarized in the Table. In the single-center, 3-year prospective study of women given adjuvant taxane-based or non–taxane-based chemotherapy, those who received taxane therapy were more likely to develop PCIA (odds ratio, 8.01).9
All 3 of our patients received taxanes. Interestingly, patient 3 underwent 2 rounds of chemotherapy 14 years apart and experienced full regrowth of the hair after the first course of taxane-free chemotherapy but experienced persistent hair loss following docetaxel treatment. Adjuvant endocrine therapies also may contribute to PCIA. A review of the side effects of endocrine therapies revealed an incidence of alopecia that was higher than expected; tamoxifen was the greatest offender. Additionally, using endocrine treatments in combination was found to have a synergistic effect on alopecia.18 Adjuvant endocrine therapy was used in patients 2 and 3. Although endocrine therapies appear to have a milder effect on hair loss compared to chemotherapy, these medications are continued for a longer duration, potentially contributing to the severity of hair loss and prolonging the time to regrowth.
Furthermore, endocrine therapies used in breast cancer treatment decrease estrogen levels or antagonize estrogen receptors, creating an environment of relative hyperandrogenism that may contribute to FPHL in genetically susceptible women.18 Although taxanes may cause irreversible hair loss in these patients, the action of endocrine therapies on the remaining hair follicles may affect the typical female pattern seen clinically. Patients 2 and 3 who presented with FPHL received adjuvant endocrine therapies and had positive family history, while patient 1 did not. Of note, patient 3 experienced worsening hair loss following the addition of anastrozole, which suggests a contribution of endocrine therapy to her PCIA. Our limited cases do not allow for evaluation of a worsened outcome with the combination of taxanes and endocrine therapies; however, we suggest further evaluation for a synergistic effect that may be contributing to PCIA.
Conclusion
Permanent alopecia in breast cancer patients appears to be a true potential adverse effect of taxanes and endocrine therapies, and it is important to characterize it appropriately so that its mechanism can be understood and appropriate treatment and counseling can take place. Although it may not influence clinical decision-making, patients should be informed that hair loss with chemotherapy can be permanent. Treatment with scalp cooling can reduce the risk for severe chemotherapy-induced alopecia, but it is unclear if it reduces risk for PCIA.12,15 Topical or oral minoxidil may be helpful in the treatment of PCIA once it has developed.7,8,15,22 Better characterization of these cases may elucidate risk factors for developing permanent alopecia, allowing for more appropriate risk stratification, counseling, and treatment.
Anagen effluvium during chemotherapy is common, typically beginning within 1 month of treatment onset and resolving by 6 months after the final course.1 Permanent chemotherapy-induced alopecia (PCIA), in which hair loss persists beyond 6 months after chemotherapy without recovery to original density, was first reported in patients following high-dose chemotherapy regimens for allogeneic bone marrow transplantation.2 There are now increasing reports of PCIA in patients with breast cancer; at least 400 such cases have been documented.3-16 In addition to chemotherapy, patients often receive adjuvant endocrine therapy with selective estrogen receptor modulators, aromatase inhibitors, or gonadotropin-releasing hormone agonists.5-16 Endocrine therapies also can lead to alopecia, but their role in PCIA has not been well defined.15,16 We describe 3 patients with breast cancer who experienced PCIA following chemotherapy with taxanes with or without endocrine therapies. We also review the literature on non–bone marrow transplantation PCIA to better characterize this entity and explore the role of endocrine therapies in PCIA.
Case Reports
Patient 1
A 62-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 5 years after completing chemotherapy. She underwent 6 cycles of docetaxel and carboplatin along with radiation therapy as well as 1 year of trastuzumab and did not receive endocrine therapy. At the current presentation, she reported patchy hair regrowth that gradually filled in but failed to return to full density. Physical examination revealed the hair was diffusely thin, especially bitemporally (Figures 1A and 1B), and she did not experience any loss of body hair. She had no family history of hair loss. Her medical history was notable for hypertension, chronic obstructive bronchitis, osteopenia, and depression. Her thyroid stimulating hormone (TSH) level was within reference range. Medications included lisinopril, metoprolol, escitalopram, and trazodone. A biopsy from the occipital scalp showed nonscarring alopecia with variation of hair follicle size, a decreased number of hair follicles, and a decreased anagen to telogen ratio (Figure 1C). She was treated with clobetasol solution and minoxidil solution 5% for 1 year with mild improvement. She experienced no further hair loss but did not regain original hair density.

Patient 2
A 35-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 10 months after chemotherapy. She underwent 4 cycles of doxorubicin and cyclophosphamide followed by 4 cycles of paclitaxel and was started on trastuzumab. Tamoxifen was initiated 1 month after completing chemotherapy. She received radiation therapy the following month and continued trastuzumab for 1 year. At the current presentation, the patient noted that hair regrowth had started 1 month after the last course of chemotherapy but had progressed slowly. She denied body hair loss. Physical examination revealed diffuse thinning, especially over the crown, with scattered broken hairs throughout the scalp and several miniaturized hairs over the crown. She was evaluated as grade 3 on the Sinclair clinical grading scale used to evaluate female pattern hair loss (FPHL).17 Her family history was remarkable for FPHL in her maternal grandmother. She had no notable medical history, her TSH was normal, and she was taking tamoxifen and trastuzumab. Biopsy was not performed. The patient was started on minoxidil solution 2% and had mild improvement with no further broken-off hairs after 10 months. At that point, she was evaluated as grade 2 to 3 on the Sinclair scale.17
Patient 3
A 51-year-old woman with a history of papillary carcinoma and extensive ductal carcinoma in situ presented with persistent hair loss for 3.5 years following chemotherapy for recurrent breast cancer. After her initial diagnosis in the left breast, she received cyclophosphamide, methotrexate, and 5-fluorouracil but did not receive endocrine therapy. Her hair thinned during chemotherapy but returned to normal density within 1 year. She had a recurrence of the cancer in the right breast 14 years later and received 6 cycles of chemotherapy with cyclophosphamide and docetaxel followed by radiation therapy. After this course, her hair loss incompletely recovered. One year after chemotherapy, she underwent bilateral salpingo-oophorectomy and started anastrozole. Three months later, she noticed increased shedding and progressive thinning of the hair. Physical examination revealed diffuse thinning that was most pronounced over the crown. She also experienced lateral thinning of the eyebrows, decreased eyelashes, and dystrophic fingernails. Fluocinonide solution was discontinued by the patient due to scalp burning. She had a brother with bitemporal recession. Her medical history was notable for Hashimoto thyroiditis, vitamin D deficiency, and peripheral neuropathy. Her TSH occasionally was elevated, and she was intermittently on levothyroxine; however, her free T4 was maintained within reference range on all records. Her medications at the time of evaluation were anastrozole and gabapentin. Biopsies taken from the right and left temporal scalp revealed decreased follicle density with a majority of follicles in anagen, scattered miniaturized follicles, and a mild perivascular and perifollicular lymphoid infiltrate. Mild dermal fibrosis was present without evidence of frank scarring (Figure 2). She declined treatment, and there was no change in her condition over 3 years of follow-up.

Comment
Classification of Chemotherapy-Induced Hair Loss
Chemotherapy-induced alopecia is typically an anagen effluvium that is reversed within 6 months following the final course of chemotherapy. When incomplete regrowth persists, the patient is considered to have PCIA.1 The pathophysiology of PCIA is unclear.
Traditional grading for chemotherapy-induced alopecia does not account for the patterns of loss seen in PCIA, of which the most common appears to be a female pattern with accentuated hair loss in androgen-dependent regions of the scalp.18 Other patterns include a diffuse type with body hair loss, patchy alopecia, and complete alopecia with or without body hair loss (Table).3-8 Whether these patterns all can be attributed to chemotherapy remains to be explored.

Breast Cancer Therapies Causing PCIA
The main agents thought to be responsible for PCIA in breast cancer patients are taxanes. The role of endocrine therapies has not been well explored. Trastuzumab lacks several of the common side effects of chemotherapy due to its specificity for the HER2/neu receptor and has not been found to increase the rate of hair loss when combined with standard chemotherapy.19,20 Although radiation therapy has the potential to damage hair follicles, and a dose-dependent relationship has been described for temporary and permanent alopecia at irradiated sites, permanent alopecia predominantly has been reported with cranial radiation used in the treatment of intracranial malignancies.21 The role of radiation therapy of the breasts in PCIA is unclear, as its inclusion in therapy has not been consistently reported in the literature.
Docetaxel is known to cause chemotherapy-induced alopecia, with an 83.4% incidence in phase 2 trials; however, it also appears to be related to PCIA.20 A PubMed search of articles indexed for MEDLINE was performed using the terms permanent chemotherapy induced alopecia, chemotherapy, docetaxel, endocrine therapies, hair loss, alopecia, and breast cancer. More than 400 cases of PCIA related to chemotherapy in breast cancer patients have been reported in the literature from a combination of case reports/series, retrospective surveys, and at least one prospective study. Data from some of the more detailed reports (n=52) are summarized in the Table. In the single-center, 3-year prospective study of women given adjuvant taxane-based or non–taxane-based chemotherapy, those who received taxane therapy were more likely to develop PCIA (odds ratio, 8.01).9
All 3 of our patients received taxanes. Interestingly, patient 3 underwent 2 rounds of chemotherapy 14 years apart and experienced full regrowth of the hair after the first course of taxane-free chemotherapy but experienced persistent hair loss following docetaxel treatment. Adjuvant endocrine therapies also may contribute to PCIA. A review of the side effects of endocrine therapies revealed an incidence of alopecia that was higher than expected; tamoxifen was the greatest offender. Additionally, using endocrine treatments in combination was found to have a synergistic effect on alopecia.18 Adjuvant endocrine therapy was used in patients 2 and 3. Although endocrine therapies appear to have a milder effect on hair loss compared to chemotherapy, these medications are continued for a longer duration, potentially contributing to the severity of hair loss and prolonging the time to regrowth.
Furthermore, endocrine therapies used in breast cancer treatment decrease estrogen levels or antagonize estrogen receptors, creating an environment of relative hyperandrogenism that may contribute to FPHL in genetically susceptible women.18 Although taxanes may cause irreversible hair loss in these patients, the action of endocrine therapies on the remaining hair follicles may affect the typical female pattern seen clinically. Patients 2 and 3 who presented with FPHL received adjuvant endocrine therapies and had positive family history, while patient 1 did not. Of note, patient 3 experienced worsening hair loss following the addition of anastrozole, which suggests a contribution of endocrine therapy to her PCIA. Our limited cases do not allow for evaluation of a worsened outcome with the combination of taxanes and endocrine therapies; however, we suggest further evaluation for a synergistic effect that may be contributing to PCIA.
Conclusion
Permanent alopecia in breast cancer patients appears to be a true potential adverse effect of taxanes and endocrine therapies, and it is important to characterize it appropriately so that its mechanism can be understood and appropriate treatment and counseling can take place. Although it may not influence clinical decision-making, patients should be informed that hair loss with chemotherapy can be permanent. Treatment with scalp cooling can reduce the risk for severe chemotherapy-induced alopecia, but it is unclear if it reduces risk for PCIA.12,15 Topical or oral minoxidil may be helpful in the treatment of PCIA once it has developed.7,8,15,22 Better characterization of these cases may elucidate risk factors for developing permanent alopecia, allowing for more appropriate risk stratification, counseling, and treatment.
- Dorr VJ. A practitioner’s guide to cancer-related alopecia. Semin Oncol. 1998;25:562-570.
- Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:979-982.
- Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63:333-336.
- Miteva M, Misciali C, Fanti PA, et al. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33:345-350.
- Prevezas C, Matard B, Pinquier L, et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160:883-885.
- Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clin J Oncol Nurs. 2009;13:13-14.
- Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23:2879-2884.
- Fonia A, Cota C, Setterfield JF, et al. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76:948-957.
- Kang D, Kim IR, Choi EK, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study [published online August 17, 2018]. Oncologist. 2019;24:414-420.
- Chan J, Adderley H, Alameddine M, et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: a retrospective survey at two tertiary UK cancer centres [published online December 22, 2020]. Eur J Cancer Care (Engl). doi:10.1111/ecc.13395
- Bourgeois H, Denis F, Kerbrat P, et al. Long term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: alert for an emerging side effect: ALOPERS Observatory. Cancer Res. 2009;69(24 suppl). doi:10.1158/0008-5472.SABCS-09-3174
- Bertrand M, Mailliez A, Vercambre S, et al. Permanent chemotherapy induced alopecia in early breast cancer patients after (neo)adjuvant chemotherapy: long term follow up. Cancer Res. 2013;73(24 suppl). doi:10.1158/0008-5472.SABCS13-P3-09-15
- Kim S, Park HS, Kim JY, et al. Irreversible chemotherapy-induced alopecia in breast cancer patient. Cancer Res. 2016;76(4 suppl). doi:10.1158/1538-7445.SABCS15-P1-15-04
- Thorp NJ, Swift F, Arundell D, et al. Long term hair loss in patients with early breast cancer receiving docetaxel chemotherapy. Cancer Res. 2015;75(9 suppl). doi:10.1158/1538-7445.SABCS14-P5-17-04
- Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80:1199-1213.
- Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155:724-728.
- Sinclair R, Jolley D, Mallari R, et al. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189-199.
- Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18:1126-1134.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Baselga J. Clinical trials of single-agent trastuzumab (Herceptin). Semin Oncol. 2000;27(5 suppl 9):20-26.
- Lawenda BD, Gagne HM, Gierga DP, et al. Permanent alopecia after cranial irradiation: dose-response relationship. Int J Radiat Oncol Biol Phys. 2004;60:879-887.
- Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil [published online May 13, 2015]. Australas J Dermatol. 2016;57:E130-E132.
- Dorr VJ. A practitioner’s guide to cancer-related alopecia. Semin Oncol. 1998;25:562-570.
- Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:979-982.
- Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63:333-336.
- Miteva M, Misciali C, Fanti PA, et al. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33:345-350.
- Prevezas C, Matard B, Pinquier L, et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160:883-885.
- Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clin J Oncol Nurs. 2009;13:13-14.
- Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23:2879-2884.
- Fonia A, Cota C, Setterfield JF, et al. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76:948-957.
- Kang D, Kim IR, Choi EK, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study [published online August 17, 2018]. Oncologist. 2019;24:414-420.
- Chan J, Adderley H, Alameddine M, et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: a retrospective survey at two tertiary UK cancer centres [published online December 22, 2020]. Eur J Cancer Care (Engl). doi:10.1111/ecc.13395
- Bourgeois H, Denis F, Kerbrat P, et al. Long term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: alert for an emerging side effect: ALOPERS Observatory. Cancer Res. 2009;69(24 suppl). doi:10.1158/0008-5472.SABCS-09-3174
- Bertrand M, Mailliez A, Vercambre S, et al. Permanent chemotherapy induced alopecia in early breast cancer patients after (neo)adjuvant chemotherapy: long term follow up. Cancer Res. 2013;73(24 suppl). doi:10.1158/0008-5472.SABCS13-P3-09-15
- Kim S, Park HS, Kim JY, et al. Irreversible chemotherapy-induced alopecia in breast cancer patient. Cancer Res. 2016;76(4 suppl). doi:10.1158/1538-7445.SABCS15-P1-15-04
- Thorp NJ, Swift F, Arundell D, et al. Long term hair loss in patients with early breast cancer receiving docetaxel chemotherapy. Cancer Res. 2015;75(9 suppl). doi:10.1158/1538-7445.SABCS14-P5-17-04
- Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80:1199-1213.
- Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155:724-728.
- Sinclair R, Jolley D, Mallari R, et al. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189-199.
- Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18:1126-1134.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Baselga J. Clinical trials of single-agent trastuzumab (Herceptin). Semin Oncol. 2000;27(5 suppl 9):20-26.
- Lawenda BD, Gagne HM, Gierga DP, et al. Permanent alopecia after cranial irradiation: dose-response relationship. Int J Radiat Oncol Biol Phys. 2004;60:879-887.
- Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil [published online May 13, 2015]. Australas J Dermatol. 2016;57:E130-E132.
Practice Points
- Permanent chemotherapy-induced alopecia (PCIA) is defined as hair loss that persists beyond 6 months after treatment with chemotherapy. It may be complicated by the addition of endocrine therapies.
- Patients and clinicians should be aware that PCIA can occur and appears to be a higher risk with taxane therapy.
Treatment of Generalized Pustular Psoriasis of Pregnancy With Infliximab
Generalized pustular psoriasis of pregnancy (GPPP), formerly known as impetigo herpetiformis, is a rare dermatosis that causes maternal and fetal morbidity and mortality. It is characterized by widespread, circular, erythematous plaques with pustules at the periphery.1 Conventional first-line treatment includes systemic corticosteroids and cyclosporine. The National Psoriasis Foundation Medical Board also has included infliximab among the first-line treatment options for GPPP.2 Herein, we report a case of GPPP treated with infliximab at 30 weeks’ gestation and during the postpartum period.
Case Report
A 22-year-old woman was admitted to our inpatient clinic at 20 weeks’ gestation in her second pregnancy for evaluation of cutaneous eruptions covering the entire body. The lesions first appeared 3 to 4 days prior to her admission and dramatically progressed. She had a history of psoriasis vulgaris diagnosed during her first pregnancy 2 years prior that was treated with topical steroids throughout the pregnancy and methotrexate during lactation for a total of 11 months. She then was started on cyclosporine, which she used for 6 months due to ineffectiveness of the methotrexate, but she stopped treatment 4 months before the second pregnancy.
At the current presentation, physical examination revealed erythroderma and widespread pustules on the chest, abdomen, arms, and legs, including the intertriginous regions, that tended to coalesce and form lakes of pus over an erythematous base (Figure 1). The mucosae were normal. She exhibited a low blood pressure (85/50 mmHg) and high body temperature (102 °F [38.9 °C]). Routine laboratory examination revealed anemia and a normal leukocyte count. Her erythrocyte sedimentation rate (57 mm/h [reference range, <20 mm/h]) and C-reactive protein level (102 mg/L [reference range, <6 mg/L]) were elevated, whereas total calcium (8.11 mg/dL [reference range, 8.2–10.6 mg/dL]) and albumin (3.15 g/dL [reference range, >4.0 g/dL]) levels were low.
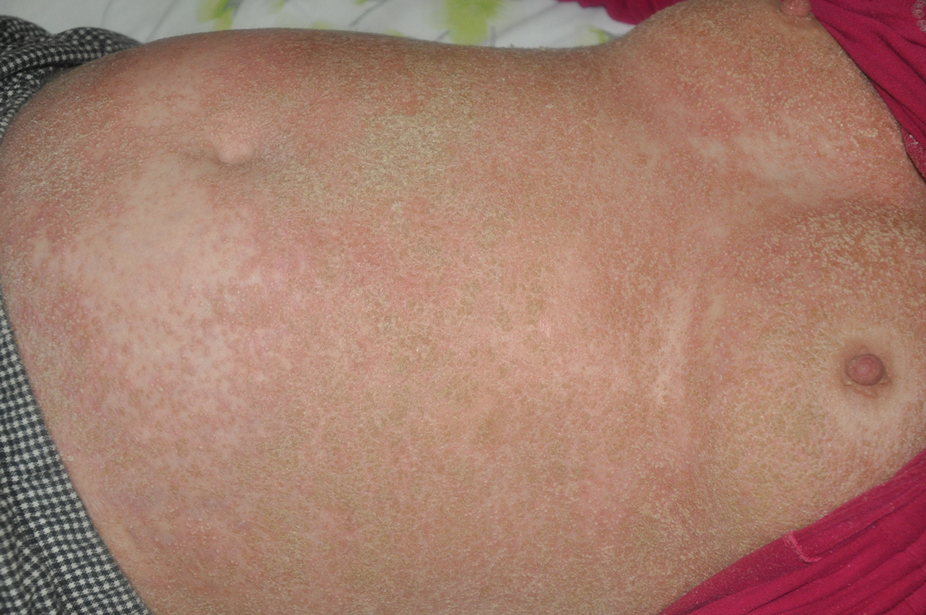
Empirical intravenous piperacillin/tazobactam was started due to hypotension, high fever, and elevated C-reactive protein levels; however, treatment was stopped after 4 days when microbiological cultures taken from blood and pustules revealed no bacterial growth, and therefore the fever was assumed to be caused by erythroderma. A skin biopsy before the start of topical and systemic treatment revealed changes consistent with GPPP.
Because her disease was extensive, systemic methylprednisolone 1.5 mg/kg once daily was started, and the dose was increased up to 2.5 mg/kg once daily on the tenth day of treatment to control new crops of eruptions. The dose was tapered to 2 mg/kg once daily when the lesions subsided 4 weeks into the treatment. The patient was discharged after 7 weeks at 27 weeks’ gestation.
Twelve days later, the patient was readmitted to the clinic in an erythrodermic state. The lesions were not controlled with increased doses of systemic corticosteroids. Treatment with cyclosporine was considered, but the patient refused; thus, infliximab treatment was planned. Isoniazid 300 mg once daily was started due to a risk of latent Mycobacterium tuberculosis infection revealed by a tuberculosis blood test. Other evaluations revealed no contraindications, and an infusion of infliximab 300 mg (5 mg/kg) was administered at 30 weeks’ gestation. There was visible improvement in the erythroderma and pustular lesions within the same day of treatment, and the lesions were completely cleared within 2 days of the infusion. The methylprednisolone dose was reduced to 1.5 mg/kg once daily.
Three days after treatment with infliximab, lesions with yellow encrustation appeared in the perioral region and on the oral mucosa and left ear. She was diagnosed with an oral herpes infection. Oral valacyclovir 1 g twice daily and topical mupirocin were started and the lesions subsided within 1 week. Twelve days after the infliximab infusion, new pustular lesions appeared, and a second infusion of infliximab was administered 13 days after the first, which cleared all lesions within 48 hours.
The patient’s methylprednisolone dose was tapered and stopped prior to delivery at 34 weeks’ gestation—2 weeks after the second dose of infliximab—as she did not have any new skin eruptions. A third infliximab infusion that normally would have occurred 4 weeks after the second treatment was postponed for a Cesarean section scheduled at 36 weeks’ gestation due to suspected intrauterine growth retardation. The patient stayed at the hospital until delivery without any new skin lesions. The gross and histopathologic examination of the placenta was normal. The neonate weighed 4.8 lb at birth and had neonatal jaundice that resolved spontaneously within 10 days but was otherwise healthy.
The patient returned to the clinic 3 weeks postpartum with a few pustules on erythematous plaques on the chest, abdomen, and back. At this time, she received a third infusion of infliximab 8 weeks after the second dose. For the past 5 years, the patient has been undergoing infliximab maintenance treatment, which she receives at the hospital every 8 weeks with excellent response. She has had no further pregnancies to date.
Comment
Generalized pustular psoriasis of pregnancy is a rare condition that typically occurs in the third trimester but also can start in the first and second trimesters. It may result in maternal and fetal morbidity by causing fluid and electrolyte imbalance and/or placental insufficiency, resulting in an increased risk for fetal abnormalities, stillbirth, and neonatal death.3 In subsequent pregnancies, GPPP has been observed to recur at an earlier gestational age with a more severe presentation.1,3
Generalized pustular psoriasis of pregnancy usually involves an eruption that begins symmetrically in the intertriginous areas and spreads to the rest of the body. The lesions present as erythematous annular plaques with pustules on the periphery and desquamation in the center due to older pustules.1,3 The mucous membranes also may be involved with erosive and exfoliative plaques, and there may be nail involvement. Patients often present with systemic symptoms such as fever, malaise, diarrhea, and vomiting.1 Laboratory investigations may reveal neutrophilic leukocytosis, high erythrocyte sedimentation rate, hypocalcemia, and hypoalbuminemia.4 Cultures from blood and pustules show no bacterial growth. A skin biopsy is helpful in diagnosis, with features similar to generalized pustular psoriasis, demonstrating spongiform pustules containing neutrophils, lymphocytic and neutrophilic infiltrates in the papillary dermis, and negative direct immunofluorescence.3
The differential diagnosis of GPPP includes subcorneal pustular dermatosis, dermatitis herpetiformis, herpes gestationis, impetigo, and acute generalized exanthematous pustulosis.1,3 Due to concerns of fetal implications, treatment options in GPPP are somewhat limited; however, the condition requires treatment because it may result in unfavorable pregnancy outcomes. Topical corticosteroids may be an option for limited disease.5,6 Systemic corticosteroids (eg, prednisone 60–80 mg/d) were previously considered as first-line agents, although they have shown limited efficacy in our case as well as in other case reports.7 Their ineffectiveness and risk for flare-up after dose tapering should be kept in mind when starting GPPP patients on systemic corticosteroids. Systemic cyclosporine (2–3 mg/kg/d) may be added to increase the efficacy of systemic steroids, which was done in several cases in literature.1,6,8 Although cyclosporine has been classified as a pregnancy category C drug, an analysis of pregnancy outcomes of 629 renal transplant patients revealed no association with adverse pregnancy outcomes compared to the general population and no increase in fetal malformations.9 Therefore, cyclosporine is a safe treatment option and was classified as a first-line drug for GPPP in a 2012 review by the National Psoriasis Foundation Medical Board.2 Narrowband UVB also has been reported to be used for the treatment of GPPP.10 Methotrexate and retinoids have been used in cases with lesions that persisted postpartum.1
Anti–tumor necrosis factor (TNF) α agents are another effective option for treatment of GPPP. Anti-TNF agents are classified as pregnancy category B due to results showing that anti-mouse TNF-α monoclonal antibodies did not cause embryotoxicity or teratogenicity in pregnant mice.11 Although Carter et al12 published a review of US Food and Drug Administration data on pregnant women receiving anti-TNF treatment and concluded that these agents were associated with the VACTERL group of malformations (vertebral defects, anal atresia, cardiac defect, tracheoesophageal fistula with esophageal atresia, cardiac defects, renal and limb anomalies), no such association was found in further studies. A 2014 study showed no difference in the rate of major malformations in infants born to women who were treated with anti-TNF drugs compared to the disease-matched group not treated with these agents and pregnant women counselled for nonteratogenic exposure.13 The same study detected an increase in preterm and low-birth-weight deliveries and suggested this might be caused by the increased severity of disease in patients requiring anti-TNF medication. The British Society of Rheumatology Biologics Register published data on pregnancy outcomes in 130 rheumatoid arthritis patients who had been exposed to anti-TNF agents.14 The results suggested an increased rate of spontaneous abortions in women exposed to anti-TNF treatment around the time of conception, especially in those taking these medications together with methotrexate or leflunomide; however, results also indicated that disease activity may have had an impact on the rate of spontaneous abortions in these patients. In a 2013 review of 462 women with inflammatory bowel disease who had been exposed to anti-TNF agents during pregnancy, the investigators concluded that pregnancy outcomes and the rate of congenital anomalies did not significantly differ from other inflammatory bowel disease patients not receiving anti-TNF drugs or the general population.15
In 2012, the National Board of the National Psoriasis Foundation put infliximab amongst the first-line treatment modalities for GPPP.2 In one case of GPPP in which the eruption persisted after delivery, the patient was treated with infliximab 7 weeks postpartum due to failure to control the disease with prednisolone 60 mg daily and cyclosporine 7.5 mg/kg daily. Unlike our patient, this patient was only started on an infliximab regimen after delivery.16 In another case reported in 2010, the patient was started on infliximab during the postpartum period of her first pregnancy following a pustular flare of previously diagnosed plaque psoriasis (not a generalized pustular psoriasis, as in our case).17 As a good response was obtained, infliximab treatment was continued in the patient throughout her second pregnancy.
Our case is unique in that infliximab was started during pregnancy because of intractable disease leading to systemic symptoms. Our patient showed an excellent response to infliximab after a 10-week disease course with repeated flare-ups and impairment to her overall condition. Delivery occurred at 36 weeks’ gestation due to suspected intrauterine growth retardation; however, the neonate was born with a 5-minute APGAR score of 10 and required no special medical care, which suggests that the low birth weight was constitutional due to the patient’s small frame (her height was 4 ft 11 in). The breast milk of patients with inflammatory bowel disease has been detected to contain very small amounts of infliximab (101 ng/mL, about 1/200 of the therapeutic blood level).18 Considering the large molecular weight of this agent and possible proteolysis in the stomach and intestines, infliximab is unlikely to affect the neonate.15 Thus, we encouraged our patient to breastfeed her baby. A case of fatal disseminated Bacille-Calmette-Guérin infection in an infant whose mother received infliximab treatment during pregnancy has been reported.19 It has been suggested that live vaccines should be avoided in neonates exposed to anti-TNF agents at least for the first 6 months of life or until the agent is no longer detectable in their blood.15 We therefore informed our patient’s family practitioner about this data.
Conclusion
We report a case of infliximab treatment for GPPP that was continued during the postpartum period. Infliximab was an effective treatment option in our patient with no detected serious adverse events and may be considered in other cases of GPPP that are not responsive to systemic steroids. However, further studies are warranted to evaluate the safety and efficacy of infliximab treatment for GPPP and psoriasis in pregnancy.
- Lerhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Gao QQ, Xi MR, Yao Q. Impetigo herpetiformis during pregnancy: a case report and literature review. Dermatology. 2013;226:35-40.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine [published May 12, 2011]. BMJ Case Rep. doi:10.1136/bcr.02.2011.3915
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Luan L, Han S, Zhang Z, et al. Personal treatment experience for severe generalized pustular psoriasis of pregnancy: two case reports. Dermatol Ther. 2014;27:174-177.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB. Cutan Ocul Toxicol. 2012;31:67-69.
- Treacy G. Using an analogous monoclonal antibody to evaluate the reproductive and chronic toxicity potential for a humanized anti-TNF alpha monoclonal antibody. Hum Exp Toxicol. 2000;19:226-228.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- Verstappen SM, King Y, Watson KD, et al. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823-826.
- Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013;108:1426-1438.
- Sheth N, Greenblatt DT, Acland K, et al. Generalized pustular psoriasis of pregnancy treated with infliximab. Clin Exp Dermatol. 2009;34:521-522.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2011;5:555-558.
- Cheent K, Nolan J, Shariq S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
Generalized pustular psoriasis of pregnancy (GPPP), formerly known as impetigo herpetiformis, is a rare dermatosis that causes maternal and fetal morbidity and mortality. It is characterized by widespread, circular, erythematous plaques with pustules at the periphery.1 Conventional first-line treatment includes systemic corticosteroids and cyclosporine. The National Psoriasis Foundation Medical Board also has included infliximab among the first-line treatment options for GPPP.2 Herein, we report a case of GPPP treated with infliximab at 30 weeks’ gestation and during the postpartum period.
Case Report
A 22-year-old woman was admitted to our inpatient clinic at 20 weeks’ gestation in her second pregnancy for evaluation of cutaneous eruptions covering the entire body. The lesions first appeared 3 to 4 days prior to her admission and dramatically progressed. She had a history of psoriasis vulgaris diagnosed during her first pregnancy 2 years prior that was treated with topical steroids throughout the pregnancy and methotrexate during lactation for a total of 11 months. She then was started on cyclosporine, which she used for 6 months due to ineffectiveness of the methotrexate, but she stopped treatment 4 months before the second pregnancy.
At the current presentation, physical examination revealed erythroderma and widespread pustules on the chest, abdomen, arms, and legs, including the intertriginous regions, that tended to coalesce and form lakes of pus over an erythematous base (Figure 1). The mucosae were normal. She exhibited a low blood pressure (85/50 mmHg) and high body temperature (102 °F [38.9 °C]). Routine laboratory examination revealed anemia and a normal leukocyte count. Her erythrocyte sedimentation rate (57 mm/h [reference range, <20 mm/h]) and C-reactive protein level (102 mg/L [reference range, <6 mg/L]) were elevated, whereas total calcium (8.11 mg/dL [reference range, 8.2–10.6 mg/dL]) and albumin (3.15 g/dL [reference range, >4.0 g/dL]) levels were low.

Empirical intravenous piperacillin/tazobactam was started due to hypotension, high fever, and elevated C-reactive protein levels; however, treatment was stopped after 4 days when microbiological cultures taken from blood and pustules revealed no bacterial growth, and therefore the fever was assumed to be caused by erythroderma. A skin biopsy before the start of topical and systemic treatment revealed changes consistent with GPPP.
Because her disease was extensive, systemic methylprednisolone 1.5 mg/kg once daily was started, and the dose was increased up to 2.5 mg/kg once daily on the tenth day of treatment to control new crops of eruptions. The dose was tapered to 2 mg/kg once daily when the lesions subsided 4 weeks into the treatment. The patient was discharged after 7 weeks at 27 weeks’ gestation.
Twelve days later, the patient was readmitted to the clinic in an erythrodermic state. The lesions were not controlled with increased doses of systemic corticosteroids. Treatment with cyclosporine was considered, but the patient refused; thus, infliximab treatment was planned. Isoniazid 300 mg once daily was started due to a risk of latent Mycobacterium tuberculosis infection revealed by a tuberculosis blood test. Other evaluations revealed no contraindications, and an infusion of infliximab 300 mg (5 mg/kg) was administered at 30 weeks’ gestation. There was visible improvement in the erythroderma and pustular lesions within the same day of treatment, and the lesions were completely cleared within 2 days of the infusion. The methylprednisolone dose was reduced to 1.5 mg/kg once daily.
Three days after treatment with infliximab, lesions with yellow encrustation appeared in the perioral region and on the oral mucosa and left ear. She was diagnosed with an oral herpes infection. Oral valacyclovir 1 g twice daily and topical mupirocin were started and the lesions subsided within 1 week. Twelve days after the infliximab infusion, new pustular lesions appeared, and a second infusion of infliximab was administered 13 days after the first, which cleared all lesions within 48 hours.
The patient’s methylprednisolone dose was tapered and stopped prior to delivery at 34 weeks’ gestation—2 weeks after the second dose of infliximab—as she did not have any new skin eruptions. A third infliximab infusion that normally would have occurred 4 weeks after the second treatment was postponed for a Cesarean section scheduled at 36 weeks’ gestation due to suspected intrauterine growth retardation. The patient stayed at the hospital until delivery without any new skin lesions. The gross and histopathologic examination of the placenta was normal. The neonate weighed 4.8 lb at birth and had neonatal jaundice that resolved spontaneously within 10 days but was otherwise healthy.
The patient returned to the clinic 3 weeks postpartum with a few pustules on erythematous plaques on the chest, abdomen, and back. At this time, she received a third infusion of infliximab 8 weeks after the second dose. For the past 5 years, the patient has been undergoing infliximab maintenance treatment, which she receives at the hospital every 8 weeks with excellent response. She has had no further pregnancies to date.
Comment
Generalized pustular psoriasis of pregnancy is a rare condition that typically occurs in the third trimester but also can start in the first and second trimesters. It may result in maternal and fetal morbidity by causing fluid and electrolyte imbalance and/or placental insufficiency, resulting in an increased risk for fetal abnormalities, stillbirth, and neonatal death.3 In subsequent pregnancies, GPPP has been observed to recur at an earlier gestational age with a more severe presentation.1,3
Generalized pustular psoriasis of pregnancy usually involves an eruption that begins symmetrically in the intertriginous areas and spreads to the rest of the body. The lesions present as erythematous annular plaques with pustules on the periphery and desquamation in the center due to older pustules.1,3 The mucous membranes also may be involved with erosive and exfoliative plaques, and there may be nail involvement. Patients often present with systemic symptoms such as fever, malaise, diarrhea, and vomiting.1 Laboratory investigations may reveal neutrophilic leukocytosis, high erythrocyte sedimentation rate, hypocalcemia, and hypoalbuminemia.4 Cultures from blood and pustules show no bacterial growth. A skin biopsy is helpful in diagnosis, with features similar to generalized pustular psoriasis, demonstrating spongiform pustules containing neutrophils, lymphocytic and neutrophilic infiltrates in the papillary dermis, and negative direct immunofluorescence.3
The differential diagnosis of GPPP includes subcorneal pustular dermatosis, dermatitis herpetiformis, herpes gestationis, impetigo, and acute generalized exanthematous pustulosis.1,3 Due to concerns of fetal implications, treatment options in GPPP are somewhat limited; however, the condition requires treatment because it may result in unfavorable pregnancy outcomes. Topical corticosteroids may be an option for limited disease.5,6 Systemic corticosteroids (eg, prednisone 60–80 mg/d) were previously considered as first-line agents, although they have shown limited efficacy in our case as well as in other case reports.7 Their ineffectiveness and risk for flare-up after dose tapering should be kept in mind when starting GPPP patients on systemic corticosteroids. Systemic cyclosporine (2–3 mg/kg/d) may be added to increase the efficacy of systemic steroids, which was done in several cases in literature.1,6,8 Although cyclosporine has been classified as a pregnancy category C drug, an analysis of pregnancy outcomes of 629 renal transplant patients revealed no association with adverse pregnancy outcomes compared to the general population and no increase in fetal malformations.9 Therefore, cyclosporine is a safe treatment option and was classified as a first-line drug for GPPP in a 2012 review by the National Psoriasis Foundation Medical Board.2 Narrowband UVB also has been reported to be used for the treatment of GPPP.10 Methotrexate and retinoids have been used in cases with lesions that persisted postpartum.1
Anti–tumor necrosis factor (TNF) α agents are another effective option for treatment of GPPP. Anti-TNF agents are classified as pregnancy category B due to results showing that anti-mouse TNF-α monoclonal antibodies did not cause embryotoxicity or teratogenicity in pregnant mice.11 Although Carter et al12 published a review of US Food and Drug Administration data on pregnant women receiving anti-TNF treatment and concluded that these agents were associated with the VACTERL group of malformations (vertebral defects, anal atresia, cardiac defect, tracheoesophageal fistula with esophageal atresia, cardiac defects, renal and limb anomalies), no such association was found in further studies. A 2014 study showed no difference in the rate of major malformations in infants born to women who were treated with anti-TNF drugs compared to the disease-matched group not treated with these agents and pregnant women counselled for nonteratogenic exposure.13 The same study detected an increase in preterm and low-birth-weight deliveries and suggested this might be caused by the increased severity of disease in patients requiring anti-TNF medication. The British Society of Rheumatology Biologics Register published data on pregnancy outcomes in 130 rheumatoid arthritis patients who had been exposed to anti-TNF agents.14 The results suggested an increased rate of spontaneous abortions in women exposed to anti-TNF treatment around the time of conception, especially in those taking these medications together with methotrexate or leflunomide; however, results also indicated that disease activity may have had an impact on the rate of spontaneous abortions in these patients. In a 2013 review of 462 women with inflammatory bowel disease who had been exposed to anti-TNF agents during pregnancy, the investigators concluded that pregnancy outcomes and the rate of congenital anomalies did not significantly differ from other inflammatory bowel disease patients not receiving anti-TNF drugs or the general population.15
In 2012, the National Board of the National Psoriasis Foundation put infliximab amongst the first-line treatment modalities for GPPP.2 In one case of GPPP in which the eruption persisted after delivery, the patient was treated with infliximab 7 weeks postpartum due to failure to control the disease with prednisolone 60 mg daily and cyclosporine 7.5 mg/kg daily. Unlike our patient, this patient was only started on an infliximab regimen after delivery.16 In another case reported in 2010, the patient was started on infliximab during the postpartum period of her first pregnancy following a pustular flare of previously diagnosed plaque psoriasis (not a generalized pustular psoriasis, as in our case).17 As a good response was obtained, infliximab treatment was continued in the patient throughout her second pregnancy.
Our case is unique in that infliximab was started during pregnancy because of intractable disease leading to systemic symptoms. Our patient showed an excellent response to infliximab after a 10-week disease course with repeated flare-ups and impairment to her overall condition. Delivery occurred at 36 weeks’ gestation due to suspected intrauterine growth retardation; however, the neonate was born with a 5-minute APGAR score of 10 and required no special medical care, which suggests that the low birth weight was constitutional due to the patient’s small frame (her height was 4 ft 11 in). The breast milk of patients with inflammatory bowel disease has been detected to contain very small amounts of infliximab (101 ng/mL, about 1/200 of the therapeutic blood level).18 Considering the large molecular weight of this agent and possible proteolysis in the stomach and intestines, infliximab is unlikely to affect the neonate.15 Thus, we encouraged our patient to breastfeed her baby. A case of fatal disseminated Bacille-Calmette-Guérin infection in an infant whose mother received infliximab treatment during pregnancy has been reported.19 It has been suggested that live vaccines should be avoided in neonates exposed to anti-TNF agents at least for the first 6 months of life or until the agent is no longer detectable in their blood.15 We therefore informed our patient’s family practitioner about this data.
Conclusion
We report a case of infliximab treatment for GPPP that was continued during the postpartum period. Infliximab was an effective treatment option in our patient with no detected serious adverse events and may be considered in other cases of GPPP that are not responsive to systemic steroids. However, further studies are warranted to evaluate the safety and efficacy of infliximab treatment for GPPP and psoriasis in pregnancy.
Generalized pustular psoriasis of pregnancy (GPPP), formerly known as impetigo herpetiformis, is a rare dermatosis that causes maternal and fetal morbidity and mortality. It is characterized by widespread, circular, erythematous plaques with pustules at the periphery.1 Conventional first-line treatment includes systemic corticosteroids and cyclosporine. The National Psoriasis Foundation Medical Board also has included infliximab among the first-line treatment options for GPPP.2 Herein, we report a case of GPPP treated with infliximab at 30 weeks’ gestation and during the postpartum period.
Case Report
A 22-year-old woman was admitted to our inpatient clinic at 20 weeks’ gestation in her second pregnancy for evaluation of cutaneous eruptions covering the entire body. The lesions first appeared 3 to 4 days prior to her admission and dramatically progressed. She had a history of psoriasis vulgaris diagnosed during her first pregnancy 2 years prior that was treated with topical steroids throughout the pregnancy and methotrexate during lactation for a total of 11 months. She then was started on cyclosporine, which she used for 6 months due to ineffectiveness of the methotrexate, but she stopped treatment 4 months before the second pregnancy.
At the current presentation, physical examination revealed erythroderma and widespread pustules on the chest, abdomen, arms, and legs, including the intertriginous regions, that tended to coalesce and form lakes of pus over an erythematous base (Figure 1). The mucosae were normal. She exhibited a low blood pressure (85/50 mmHg) and high body temperature (102 °F [38.9 °C]). Routine laboratory examination revealed anemia and a normal leukocyte count. Her erythrocyte sedimentation rate (57 mm/h [reference range, <20 mm/h]) and C-reactive protein level (102 mg/L [reference range, <6 mg/L]) were elevated, whereas total calcium (8.11 mg/dL [reference range, 8.2–10.6 mg/dL]) and albumin (3.15 g/dL [reference range, >4.0 g/dL]) levels were low.

Empirical intravenous piperacillin/tazobactam was started due to hypotension, high fever, and elevated C-reactive protein levels; however, treatment was stopped after 4 days when microbiological cultures taken from blood and pustules revealed no bacterial growth, and therefore the fever was assumed to be caused by erythroderma. A skin biopsy before the start of topical and systemic treatment revealed changes consistent with GPPP.
Because her disease was extensive, systemic methylprednisolone 1.5 mg/kg once daily was started, and the dose was increased up to 2.5 mg/kg once daily on the tenth day of treatment to control new crops of eruptions. The dose was tapered to 2 mg/kg once daily when the lesions subsided 4 weeks into the treatment. The patient was discharged after 7 weeks at 27 weeks’ gestation.
Twelve days later, the patient was readmitted to the clinic in an erythrodermic state. The lesions were not controlled with increased doses of systemic corticosteroids. Treatment with cyclosporine was considered, but the patient refused; thus, infliximab treatment was planned. Isoniazid 300 mg once daily was started due to a risk of latent Mycobacterium tuberculosis infection revealed by a tuberculosis blood test. Other evaluations revealed no contraindications, and an infusion of infliximab 300 mg (5 mg/kg) was administered at 30 weeks’ gestation. There was visible improvement in the erythroderma and pustular lesions within the same day of treatment, and the lesions were completely cleared within 2 days of the infusion. The methylprednisolone dose was reduced to 1.5 mg/kg once daily.
Three days after treatment with infliximab, lesions with yellow encrustation appeared in the perioral region and on the oral mucosa and left ear. She was diagnosed with an oral herpes infection. Oral valacyclovir 1 g twice daily and topical mupirocin were started and the lesions subsided within 1 week. Twelve days after the infliximab infusion, new pustular lesions appeared, and a second infusion of infliximab was administered 13 days after the first, which cleared all lesions within 48 hours.
The patient’s methylprednisolone dose was tapered and stopped prior to delivery at 34 weeks’ gestation—2 weeks after the second dose of infliximab—as she did not have any new skin eruptions. A third infliximab infusion that normally would have occurred 4 weeks after the second treatment was postponed for a Cesarean section scheduled at 36 weeks’ gestation due to suspected intrauterine growth retardation. The patient stayed at the hospital until delivery without any new skin lesions. The gross and histopathologic examination of the placenta was normal. The neonate weighed 4.8 lb at birth and had neonatal jaundice that resolved spontaneously within 10 days but was otherwise healthy.
The patient returned to the clinic 3 weeks postpartum with a few pustules on erythematous plaques on the chest, abdomen, and back. At this time, she received a third infusion of infliximab 8 weeks after the second dose. For the past 5 years, the patient has been undergoing infliximab maintenance treatment, which she receives at the hospital every 8 weeks with excellent response. She has had no further pregnancies to date.
Comment
Generalized pustular psoriasis of pregnancy is a rare condition that typically occurs in the third trimester but also can start in the first and second trimesters. It may result in maternal and fetal morbidity by causing fluid and electrolyte imbalance and/or placental insufficiency, resulting in an increased risk for fetal abnormalities, stillbirth, and neonatal death.3 In subsequent pregnancies, GPPP has been observed to recur at an earlier gestational age with a more severe presentation.1,3
Generalized pustular psoriasis of pregnancy usually involves an eruption that begins symmetrically in the intertriginous areas and spreads to the rest of the body. The lesions present as erythematous annular plaques with pustules on the periphery and desquamation in the center due to older pustules.1,3 The mucous membranes also may be involved with erosive and exfoliative plaques, and there may be nail involvement. Patients often present with systemic symptoms such as fever, malaise, diarrhea, and vomiting.1 Laboratory investigations may reveal neutrophilic leukocytosis, high erythrocyte sedimentation rate, hypocalcemia, and hypoalbuminemia.4 Cultures from blood and pustules show no bacterial growth. A skin biopsy is helpful in diagnosis, with features similar to generalized pustular psoriasis, demonstrating spongiform pustules containing neutrophils, lymphocytic and neutrophilic infiltrates in the papillary dermis, and negative direct immunofluorescence.3
The differential diagnosis of GPPP includes subcorneal pustular dermatosis, dermatitis herpetiformis, herpes gestationis, impetigo, and acute generalized exanthematous pustulosis.1,3 Due to concerns of fetal implications, treatment options in GPPP are somewhat limited; however, the condition requires treatment because it may result in unfavorable pregnancy outcomes. Topical corticosteroids may be an option for limited disease.5,6 Systemic corticosteroids (eg, prednisone 60–80 mg/d) were previously considered as first-line agents, although they have shown limited efficacy in our case as well as in other case reports.7 Their ineffectiveness and risk for flare-up after dose tapering should be kept in mind when starting GPPP patients on systemic corticosteroids. Systemic cyclosporine (2–3 mg/kg/d) may be added to increase the efficacy of systemic steroids, which was done in several cases in literature.1,6,8 Although cyclosporine has been classified as a pregnancy category C drug, an analysis of pregnancy outcomes of 629 renal transplant patients revealed no association with adverse pregnancy outcomes compared to the general population and no increase in fetal malformations.9 Therefore, cyclosporine is a safe treatment option and was classified as a first-line drug for GPPP in a 2012 review by the National Psoriasis Foundation Medical Board.2 Narrowband UVB also has been reported to be used for the treatment of GPPP.10 Methotrexate and retinoids have been used in cases with lesions that persisted postpartum.1
Anti–tumor necrosis factor (TNF) α agents are another effective option for treatment of GPPP. Anti-TNF agents are classified as pregnancy category B due to results showing that anti-mouse TNF-α monoclonal antibodies did not cause embryotoxicity or teratogenicity in pregnant mice.11 Although Carter et al12 published a review of US Food and Drug Administration data on pregnant women receiving anti-TNF treatment and concluded that these agents were associated with the VACTERL group of malformations (vertebral defects, anal atresia, cardiac defect, tracheoesophageal fistula with esophageal atresia, cardiac defects, renal and limb anomalies), no such association was found in further studies. A 2014 study showed no difference in the rate of major malformations in infants born to women who were treated with anti-TNF drugs compared to the disease-matched group not treated with these agents and pregnant women counselled for nonteratogenic exposure.13 The same study detected an increase in preterm and low-birth-weight deliveries and suggested this might be caused by the increased severity of disease in patients requiring anti-TNF medication. The British Society of Rheumatology Biologics Register published data on pregnancy outcomes in 130 rheumatoid arthritis patients who had been exposed to anti-TNF agents.14 The results suggested an increased rate of spontaneous abortions in women exposed to anti-TNF treatment around the time of conception, especially in those taking these medications together with methotrexate or leflunomide; however, results also indicated that disease activity may have had an impact on the rate of spontaneous abortions in these patients. In a 2013 review of 462 women with inflammatory bowel disease who had been exposed to anti-TNF agents during pregnancy, the investigators concluded that pregnancy outcomes and the rate of congenital anomalies did not significantly differ from other inflammatory bowel disease patients not receiving anti-TNF drugs or the general population.15
In 2012, the National Board of the National Psoriasis Foundation put infliximab amongst the first-line treatment modalities for GPPP.2 In one case of GPPP in which the eruption persisted after delivery, the patient was treated with infliximab 7 weeks postpartum due to failure to control the disease with prednisolone 60 mg daily and cyclosporine 7.5 mg/kg daily. Unlike our patient, this patient was only started on an infliximab regimen after delivery.16 In another case reported in 2010, the patient was started on infliximab during the postpartum period of her first pregnancy following a pustular flare of previously diagnosed plaque psoriasis (not a generalized pustular psoriasis, as in our case).17 As a good response was obtained, infliximab treatment was continued in the patient throughout her second pregnancy.
Our case is unique in that infliximab was started during pregnancy because of intractable disease leading to systemic symptoms. Our patient showed an excellent response to infliximab after a 10-week disease course with repeated flare-ups and impairment to her overall condition. Delivery occurred at 36 weeks’ gestation due to suspected intrauterine growth retardation; however, the neonate was born with a 5-minute APGAR score of 10 and required no special medical care, which suggests that the low birth weight was constitutional due to the patient’s small frame (her height was 4 ft 11 in). The breast milk of patients with inflammatory bowel disease has been detected to contain very small amounts of infliximab (101 ng/mL, about 1/200 of the therapeutic blood level).18 Considering the large molecular weight of this agent and possible proteolysis in the stomach and intestines, infliximab is unlikely to affect the neonate.15 Thus, we encouraged our patient to breastfeed her baby. A case of fatal disseminated Bacille-Calmette-Guérin infection in an infant whose mother received infliximab treatment during pregnancy has been reported.19 It has been suggested that live vaccines should be avoided in neonates exposed to anti-TNF agents at least for the first 6 months of life or until the agent is no longer detectable in their blood.15 We therefore informed our patient’s family practitioner about this data.
Conclusion
We report a case of infliximab treatment for GPPP that was continued during the postpartum period. Infliximab was an effective treatment option in our patient with no detected serious adverse events and may be considered in other cases of GPPP that are not responsive to systemic steroids. However, further studies are warranted to evaluate the safety and efficacy of infliximab treatment for GPPP and psoriasis in pregnancy.
- Lerhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Gao QQ, Xi MR, Yao Q. Impetigo herpetiformis during pregnancy: a case report and literature review. Dermatology. 2013;226:35-40.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine [published May 12, 2011]. BMJ Case Rep. doi:10.1136/bcr.02.2011.3915
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Luan L, Han S, Zhang Z, et al. Personal treatment experience for severe generalized pustular psoriasis of pregnancy: two case reports. Dermatol Ther. 2014;27:174-177.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB. Cutan Ocul Toxicol. 2012;31:67-69.
- Treacy G. Using an analogous monoclonal antibody to evaluate the reproductive and chronic toxicity potential for a humanized anti-TNF alpha monoclonal antibody. Hum Exp Toxicol. 2000;19:226-228.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- Verstappen SM, King Y, Watson KD, et al. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823-826.
- Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013;108:1426-1438.
- Sheth N, Greenblatt DT, Acland K, et al. Generalized pustular psoriasis of pregnancy treated with infliximab. Clin Exp Dermatol. 2009;34:521-522.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2011;5:555-558.
- Cheent K, Nolan J, Shariq S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Lerhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Gao QQ, Xi MR, Yao Q. Impetigo herpetiformis during pregnancy: a case report and literature review. Dermatology. 2013;226:35-40.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine [published May 12, 2011]. BMJ Case Rep. doi:10.1136/bcr.02.2011.3915
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Luan L, Han S, Zhang Z, et al. Personal treatment experience for severe generalized pustular psoriasis of pregnancy: two case reports. Dermatol Ther. 2014;27:174-177.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB. Cutan Ocul Toxicol. 2012;31:67-69.
- Treacy G. Using an analogous monoclonal antibody to evaluate the reproductive and chronic toxicity potential for a humanized anti-TNF alpha monoclonal antibody. Hum Exp Toxicol. 2000;19:226-228.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- Verstappen SM, King Y, Watson KD, et al. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823-826.
- Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013;108:1426-1438.
- Sheth N, Greenblatt DT, Acland K, et al. Generalized pustular psoriasis of pregnancy treated with infliximab. Clin Exp Dermatol. 2009;34:521-522.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2011;5:555-558.
- Cheent K, Nolan J, Shariq S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
Practice Points
- Generalized pustular psoriasis of pregnancy (GPPP) is a rare and severe condition that may lead to complications in both the mother and the fetus. Effective treatment with low impact on the fetus is essential.
- Infliximab, among other biologic agents, may be considered for the rapid clearing of skin lesions in GPPP.
Postoperative Neurologic Deficits in a Veteran With Recent COVID-19
Anesthesia providers should be aware of COVID-19 sensitive stroke code practices and maintain heightened vigilance for the need to implement perioperative stroke mitigation strategies.
The risk of perioperative stroke in noncardiac, nonneurologic, nonvascular surgery ranges from 0.1 to 1.9% and is associated with increased mortality.1,2 Stroke mechanisms include both ischemia (large and small vessel occlusion, cardioembolism, anemic-tissue hypoxia, cerebral hypoperfusion) and hemorrhage.1 Risk factors for perioperative stroke include prior cerebral vascular accident (CVA), hypertension, aged > 62 years, acute renal insufficiency, dialysis, and recent myocardial infarction (MI).2
Introduction
COVID-19 was declared a pandemic by the World Health Organization in March 2020.3 COVID-19 has certainly affected the veteran population; between February and May 2020, more than 60,000 veterans were tested for COVID-19 with a positive rate of about 9%.4 While primarily affecting the respiratory system, there are increasing reports of COVID-19 neurologic manifestations: headache, hypogeusia, hyposomia, seizure, encephalitis, and acute stroke.5 In an early case series from Wuhan, China, 36% of 214 patients with COVID-19 reported neurologic complications, and acute CVAs were more common in patients with severe (compared to milder) viral disease presentations (5.7% vs 0.8%).6 Large vessel stroke was a presenting feature in another report of 5 patients aged < 50 years.7
The mechanism of ischemic stroke in the setting of COVID-19 is unclear.8 Indeed, stroke and COVID-19 share similar risk factors (eg, hypertension, diabetes mellitus [DM], older age), and immobile critically ill patients may already be prone to developing stroke.5,9 However, COVID-19 is associated with arterial and venous thromboembolism, elevated D-dimer and fibrinogen levels, and antiphospholipid antibody production. This prothrombotic state may be linked to cytokine-induced endothelial damage, mononuclear cell activation, tissue factor expression, and ultimately thrombin propagation and platelet activation.8
The rates of perioperative stroke may change as more patients with COVID-19 present for surgery, and the anesthesiology care team must prioritize mitigation efforts in high-risk patients, including veterans. Reducing the elevated stroke burden within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) is a public health priority.10 We present the case of a veteran with prior CVA and recent positive COVID-19 testing who experienced transient weakness and dysarthria following plastic surgery. The patient discussed provided written Health Insurance Portability and Accountability Act consent for publication of this report.
Case Presentation
A 75-year-old male veteran presented to the Minneapolis VA Medical Center in Minnesota with chronic left foot ulceration necessitating debridement and flap coverage. His medical history was significant for hypertension, type 2 DM, anemia of chronic disease, and coronary artery disease (left ventricular ejection fraction, 50%). Additionally, he had prior ischemic strokes in the oculomotor nucleus (in 2004 with internuclear ophthalmoplegia) and left ventral medulla (in 2019 with right hemiparesis). During his 2019 poststroke rehabilitation, he was diagnosed with mild neurocognitive deficit not attributable to his strokes. The patient’s medications included amlodipine, lisinopril, atorvastatin, clopidogrel (lifelong for secondary stroke prevention), metformin, and glipizide. The debridement procedure was initially delayed 3 weeks due to positive routine preoperative COVID-19 nasopharyngeal testing, though he reported no respiratory symptoms or fever. During the delay, the primary team prescribed daily oral rivaroxaban for thrombosis prophylaxis in addition to clopidogrel. One week prior to surgery, his repeat COVID-19 test was negative and prophylactic anticoagulation stopped.
On the day of surgery, the patient was hemodynamically stable: heart rate 86 beats/min, blood pressure 167/93 mm Hg (baseline 120-150 mm Hg systolic pressure), respiratory rate 16 breaths/min, oxygen saturation 99% without supplemental oxygen, temperature 97.1 °F. He received amlodipine and clopidogrel, but not lisinopril, that morning. No focal neurologic deficits were appreciated on preoperative examination, and resolution of symptoms related to the 2 prior MIs was confirmed. Preoperative glucose was 163 mg/dL. Femoral and sciatic peripheral nerve blocks were done for postoperative analgesia. A preinduction arterial line was placed and 2 mg of midazolam was administered for anxiolysis. Induction of general anesthesia with oral endotracheal intubation proceeded uneventfully; he was positioned prone.
Given his stroke risk factors, mean arterial pressure was maintained > 70 mm Hg for the duration of surgery. No vasoactive infusions were necessary and no β-blocking agents were administered. Insulin infusion was required; the maximum-recorded glucose was 219 mg/dL. Arterial blood gas samples were routinely drawn; acid-base balance was well maintained, PaO2 was > 185 mm Hg, and PaCO2 ranged from 29.4 to 38.5 mm Hg. The patient received 2 units of packed red blood cells for nadir hemoglobin of 7.5 mg/dL. At surgery end, we fully reversed neuromuscular blockade with suggamadex. The patient was returned to a supine position and extubated uneventfully after demonstrating the ability to follow commands.
During postanesthesia care unit (PACU) handoff, the patient exhibited acute speech impairment. He was able to state his name on repetition but seemed confused and sedated. Prompt formal neurology evaluation (stroke code) was sought. Initial National Institutes of Health (NIH) stroke scale score was 8 (1 for level of consciousness, 1 for minor right facial droop, 1 for right arm drift, 3 for right leg with no effort against gravity, 1 for right partial sensory loss, and 1 for mild dysarthria). The patient was oriented only to self. Other findings included mild right facial droop and dysarthria. On a 5-point strength scale, he scored 4 for the right deltoid, biceps, triceps, wrist extensors, right knee flexion, right dorsiflexion, and plantarflexion, 2 for right hip flexion, and ≥ 4 for right knee extension. Positive sensory findings were notable for decreased pin prick sensation on the right limbs.
We obtained emergent head computed tomography (CT) that was negative for acute abnormalities; CT angiography was negative for large vessel occlusion or clinically significant stenosis (Figure). On returning to the PACU from the CT scanner, the patient regained symmetric strength in both arms, right leg was antigravity, and his speech had normalized. Prior to PACU discharge 2 hours later, the patient was back to his prehospitalization neurologic function and NIH stroke scale was 0. Given this rapid clinical resolution, no acute stroke interventions were done, though permissive hypertension was recommended by the neurologist during PACU recovery.
The neurology team concluded that the patient’s symptoms were likely secondary to recrudescence of previous stroke symptoms in the setting of brief postoperative delirium (POD). However, we could not exclude transient ischemic attack or new cardioembolism, therefore patient was started on dual antiplatelet therapy for 3 weeks. Unfortunately, elective confirmatory magnetic resonance imaging (MRI) was not sought to confirm new ischemic changes due hospital COVID-19 restrictions on nonessential scanning. Neurology did not recommend carotid duplex ultrasound given patent vasculature on the head and neck CT angiography. Finally, the patient had undergone surface echocardiography 3 weeks prior to surgery that showed a left ventricular ejection fraction of 50% without significant valvular abnormalities, thrombus, or interatrial shunting, so repeated study was deferred.
Formal neurology consultation did not extend beyond postoperative day 1. One month after surgery, the anesthesiology team visited the patient during inpatient rehabilitation; he had not developed further focal neurologic symptoms or delirium. His strength was equal bilaterally and no speech deficits were noted. Unfortunately, the patient was readmitted to the hospital for continued foot wound drainage 2 months postoperatively, though no focal neurologic deficits were documented on his medical admission history and physical. No long term sequalae of his COVID-19 infection have been suspected.
Discussion
We report a veteran with prior stroke and COVID-19 who experienced postoperative speech and motor deficit despite deliberate risk factor mitigation. This case calls for increased vigilance by anesthesia providers to employ proper perioperative stroke management and anticoagulation strategies, and to be prepared for prompt intervention with COVID-19-sensitive practices should the need for advanced airway management or thrombectomy arises.
The exact etiology of the postoperative neurologic deficit in our patient is unknown. The most likely possibility is that this represents poststroke recrudescence (PSR), knowing he had a previous left medullary infarct that presented similarly.11 PSR is a phenomenon in which prior stroke symptoms recur acutely and transiently in the setting of physiologic stressors—also known as locus minoris resistantiae.12 Triggers include γ aminobutyric acid (GABA) mediating anesthetic agents such as midazolam, opioids (eg, fentanyl or hydromorphone), infection, or relative cerebral hypoperfusion.11,13,14 The focality of our patient’s presentation favors PSR in the context of brief POD; of note, these entities share similar risk factors.15 Our patient did indeed receive low-dose preoperative midazolam in the context of mild preoperative neurocognitive deficit, which may have predisposed him to POD.
Though less likely, our patient’s presentation could have been explained by a new cerebrovascular event—transient ischemic attack vs new MI. Speech and right-sided motor/sensory deficits can localize to the left middle cerebral artery or small penetrating arteries of the left brainstem or deep white matter. MRI was not performed to exclude this possibility due to hospital-wide COVID-19 precautions minimizing nonessential MRIs unlikely to change clinical management. We speculate, however, that due to recent SARS-CoV-2 infection, our patient may have been at higher risk for cerebrovascular events due to subclinical endothelial damage and/or microclot in predisposed neurovasculature. Though our patient had interval COVID-19 negative tests, the timeframe of coronavirus procoagulant effects is unknown.16
There are well-established guidelines for perioperative stroke management published by the Society for Neuroscience in Anesthesiology and Critical Care (SNACC).17 This case exemplifies many recommendations including tight hemodynamic and glucose control, optimized oxygen delivery, avoidance of intraoperative β blockade, and prompt neurologic consultation. Additionally, special precaution was taken to ensure continuation of antiplatelet therapy on the day of surgery; in light of COVID-19 prothrombosis risk we considered this essential. Low-dose enoxaparin was also instituted on postoperative day 1. Prophylactic anticoagulation with low molecular weight heparin (LMWH) is recommended for hospitalized COVID-19–positive patients, though perioperatively, this must be weighed against hemorrhagic stroke transformation and surgical bleeding.8,16 Interestingly, the benefit of LMWH may partly relate to its anti-inflammatory effects, of which higher levels are observed in COVID-19.16,18
Though substantial health care provider energy and hospital resource utilization is presently focused on controlling the COVID-19 pandemic, the importance of appropriate stroke code processes must not be neglected. Recently, SNACC released anesthetic guidelines for endovascular ischemic stroke management that reflect COVID-19 precautions; highlights include personal protective equipment (PPE) utilization, risk-benefit analysis of general anesthesia (with early decision to intubate) vs sedation techniques for thrombectomy, and airway management strategies to minimize aerosolization exposure.19 Finally, negative pressure rooms relative to PACU and operating room locations need to be known and marked, as well as the necessary airway equipment and PPE to transfer patients safely to and from angiography suites.
Conclusions
We discuss a surgical patient with prior SARS-CoV-2 infection at elevated stroke risk that experienced recurrence of neurologic deficits postoperatively. This case informs anesthesia providers of the broad differential diagnosis for focal neurological deficits to include PSR and the possible contribution of COVID-19 to elevated acute stroke risk. Perioperative physicians, including VHA practitioners, with knowledge of current COVID-19 practices are primed to coordinate multidisciplinary efforts during stroke codes and ensuring appropriate anticoagulation.
Acknowledgments
The authors would like to thank perioperative care teams across the world caring for COVID-19 patients safely.
1. Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63(2):193-204. doi:10.1007/s12630-015-0494-9
2. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289-1296. doi:10.1097/ALN.0b013e318216e7f4
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160. Published 2020 Mar 19. doi:10.23750/abm.v91i1.9397
4. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States Veterans. Preprint. medRxiv. 2020;2020.05.12.20099135. Published 2020 May 18. doi:10.1101/2020.05.12.20099135
5. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi:10.1016/j.clineuro.2020.105921
6. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127
7. Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. doi:10.1056/NEJMc2009787
8. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi:10.1136/jnnp-2020-323586
9. Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32(3):667-671. doi:10.1007/s12028-020-00978-4
10. Lich KH, Tian Y, Beadles CA, et al. Strategic planning to reduce the burden of stroke among veterans: using simulation modeling to inform decision making. Stroke. 2014;45(7):2078-2084. doi:10.1161/STROKEAHA.114.004694
11. Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of Deficits After Stroke: Clinical and Imaging Phenotype, Triggers, and Risk Factors. JAMA Neurol. 2017;74(9):1048-1055. doi:10.1001/jamaneurol.2017.1668
12. Jun-O’connell AH, Henninger N, Moonis M, Silver B, Ionete C, Goddeau RP. Recrudescence of old stroke deficits among transient neurological attacks. Neurohospitalist. 2019;9(4):183-189. doi:10.1177/194187441982928813. Karnik HS, Jain RA. Anesthesia for patients with prior stroke. J Neuroanaesthesiology Crit Care. 2018;5(3):150-157. doi:10.1055/s-0038-1673549
14. Minhas JS, Rook W, Panerai RB, et al. Pathophysiological and clinical considerations in the perioperative care of patients with a previous ischaemic stroke: a multidisciplinary narrative review. Br J Anaesth. 2020;124(2):183-196. doi:10.1016/j.bja.2019.10.021
15. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium [published correction appears in Eur J Anaesthesiol. 2018 Sep;35(9):718-719]. Eur J Anaesthesiol. 2017;34(4):192-214. doi:10.1097/EJA.0000000000000594
16. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
17. Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273-285. doi:10.1097/ana.0000000000000087
18. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-3153. doi:10.1007/s00415-020-09990-2
19. Sharma D, Rasmussen M, Han R, et al. Anesthetic Management of Endovascular Treatment of Acute Ischemic Stroke During COVID-19 Pandemic: Consensus Statement From Society for Neuroscience in Anesthesiology & Critical Care (SNACC): Endorsed by Society of Vascular & Interventional Neurology (SVIN), Society of NeuroInterventional Surgery (SNIS), Neurocritical Care Society (NCS), European Society of Minimally Invasive Neurological Therapy (ESMINT) and American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) Cerebrovascular Section. J Neurosurg Anesthesiol. 2020;32(3):193-201. doi:10.1097/ANA.0000000000000688
Anesthesia providers should be aware of COVID-19 sensitive stroke code practices and maintain heightened vigilance for the need to implement perioperative stroke mitigation strategies.
Anesthesia providers should be aware of COVID-19 sensitive stroke code practices and maintain heightened vigilance for the need to implement perioperative stroke mitigation strategies.
The risk of perioperative stroke in noncardiac, nonneurologic, nonvascular surgery ranges from 0.1 to 1.9% and is associated with increased mortality.1,2 Stroke mechanisms include both ischemia (large and small vessel occlusion, cardioembolism, anemic-tissue hypoxia, cerebral hypoperfusion) and hemorrhage.1 Risk factors for perioperative stroke include prior cerebral vascular accident (CVA), hypertension, aged > 62 years, acute renal insufficiency, dialysis, and recent myocardial infarction (MI).2
Introduction
COVID-19 was declared a pandemic by the World Health Organization in March 2020.3 COVID-19 has certainly affected the veteran population; between February and May 2020, more than 60,000 veterans were tested for COVID-19 with a positive rate of about 9%.4 While primarily affecting the respiratory system, there are increasing reports of COVID-19 neurologic manifestations: headache, hypogeusia, hyposomia, seizure, encephalitis, and acute stroke.5 In an early case series from Wuhan, China, 36% of 214 patients with COVID-19 reported neurologic complications, and acute CVAs were more common in patients with severe (compared to milder) viral disease presentations (5.7% vs 0.8%).6 Large vessel stroke was a presenting feature in another report of 5 patients aged < 50 years.7
The mechanism of ischemic stroke in the setting of COVID-19 is unclear.8 Indeed, stroke and COVID-19 share similar risk factors (eg, hypertension, diabetes mellitus [DM], older age), and immobile critically ill patients may already be prone to developing stroke.5,9 However, COVID-19 is associated with arterial and venous thromboembolism, elevated D-dimer and fibrinogen levels, and antiphospholipid antibody production. This prothrombotic state may be linked to cytokine-induced endothelial damage, mononuclear cell activation, tissue factor expression, and ultimately thrombin propagation and platelet activation.8
The rates of perioperative stroke may change as more patients with COVID-19 present for surgery, and the anesthesiology care team must prioritize mitigation efforts in high-risk patients, including veterans. Reducing the elevated stroke burden within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) is a public health priority.10 We present the case of a veteran with prior CVA and recent positive COVID-19 testing who experienced transient weakness and dysarthria following plastic surgery. The patient discussed provided written Health Insurance Portability and Accountability Act consent for publication of this report.
Case Presentation
A 75-year-old male veteran presented to the Minneapolis VA Medical Center in Minnesota with chronic left foot ulceration necessitating debridement and flap coverage. His medical history was significant for hypertension, type 2 DM, anemia of chronic disease, and coronary artery disease (left ventricular ejection fraction, 50%). Additionally, he had prior ischemic strokes in the oculomotor nucleus (in 2004 with internuclear ophthalmoplegia) and left ventral medulla (in 2019 with right hemiparesis). During his 2019 poststroke rehabilitation, he was diagnosed with mild neurocognitive deficit not attributable to his strokes. The patient’s medications included amlodipine, lisinopril, atorvastatin, clopidogrel (lifelong for secondary stroke prevention), metformin, and glipizide. The debridement procedure was initially delayed 3 weeks due to positive routine preoperative COVID-19 nasopharyngeal testing, though he reported no respiratory symptoms or fever. During the delay, the primary team prescribed daily oral rivaroxaban for thrombosis prophylaxis in addition to clopidogrel. One week prior to surgery, his repeat COVID-19 test was negative and prophylactic anticoagulation stopped.
On the day of surgery, the patient was hemodynamically stable: heart rate 86 beats/min, blood pressure 167/93 mm Hg (baseline 120-150 mm Hg systolic pressure), respiratory rate 16 breaths/min, oxygen saturation 99% without supplemental oxygen, temperature 97.1 °F. He received amlodipine and clopidogrel, but not lisinopril, that morning. No focal neurologic deficits were appreciated on preoperative examination, and resolution of symptoms related to the 2 prior MIs was confirmed. Preoperative glucose was 163 mg/dL. Femoral and sciatic peripheral nerve blocks were done for postoperative analgesia. A preinduction arterial line was placed and 2 mg of midazolam was administered for anxiolysis. Induction of general anesthesia with oral endotracheal intubation proceeded uneventfully; he was positioned prone.
Given his stroke risk factors, mean arterial pressure was maintained > 70 mm Hg for the duration of surgery. No vasoactive infusions were necessary and no β-blocking agents were administered. Insulin infusion was required; the maximum-recorded glucose was 219 mg/dL. Arterial blood gas samples were routinely drawn; acid-base balance was well maintained, PaO2 was > 185 mm Hg, and PaCO2 ranged from 29.4 to 38.5 mm Hg. The patient received 2 units of packed red blood cells for nadir hemoglobin of 7.5 mg/dL. At surgery end, we fully reversed neuromuscular blockade with suggamadex. The patient was returned to a supine position and extubated uneventfully after demonstrating the ability to follow commands.
During postanesthesia care unit (PACU) handoff, the patient exhibited acute speech impairment. He was able to state his name on repetition but seemed confused and sedated. Prompt formal neurology evaluation (stroke code) was sought. Initial National Institutes of Health (NIH) stroke scale score was 8 (1 for level of consciousness, 1 for minor right facial droop, 1 for right arm drift, 3 for right leg with no effort against gravity, 1 for right partial sensory loss, and 1 for mild dysarthria). The patient was oriented only to self. Other findings included mild right facial droop and dysarthria. On a 5-point strength scale, he scored 4 for the right deltoid, biceps, triceps, wrist extensors, right knee flexion, right dorsiflexion, and plantarflexion, 2 for right hip flexion, and ≥ 4 for right knee extension. Positive sensory findings were notable for decreased pin prick sensation on the right limbs.
We obtained emergent head computed tomography (CT) that was negative for acute abnormalities; CT angiography was negative for large vessel occlusion or clinically significant stenosis (Figure). On returning to the PACU from the CT scanner, the patient regained symmetric strength in both arms, right leg was antigravity, and his speech had normalized. Prior to PACU discharge 2 hours later, the patient was back to his prehospitalization neurologic function and NIH stroke scale was 0. Given this rapid clinical resolution, no acute stroke interventions were done, though permissive hypertension was recommended by the neurologist during PACU recovery.
The neurology team concluded that the patient’s symptoms were likely secondary to recrudescence of previous stroke symptoms in the setting of brief postoperative delirium (POD). However, we could not exclude transient ischemic attack or new cardioembolism, therefore patient was started on dual antiplatelet therapy for 3 weeks. Unfortunately, elective confirmatory magnetic resonance imaging (MRI) was not sought to confirm new ischemic changes due hospital COVID-19 restrictions on nonessential scanning. Neurology did not recommend carotid duplex ultrasound given patent vasculature on the head and neck CT angiography. Finally, the patient had undergone surface echocardiography 3 weeks prior to surgery that showed a left ventricular ejection fraction of 50% without significant valvular abnormalities, thrombus, or interatrial shunting, so repeated study was deferred.
Formal neurology consultation did not extend beyond postoperative day 1. One month after surgery, the anesthesiology team visited the patient during inpatient rehabilitation; he had not developed further focal neurologic symptoms or delirium. His strength was equal bilaterally and no speech deficits were noted. Unfortunately, the patient was readmitted to the hospital for continued foot wound drainage 2 months postoperatively, though no focal neurologic deficits were documented on his medical admission history and physical. No long term sequalae of his COVID-19 infection have been suspected.
Discussion
We report a veteran with prior stroke and COVID-19 who experienced postoperative speech and motor deficit despite deliberate risk factor mitigation. This case calls for increased vigilance by anesthesia providers to employ proper perioperative stroke management and anticoagulation strategies, and to be prepared for prompt intervention with COVID-19-sensitive practices should the need for advanced airway management or thrombectomy arises.
The exact etiology of the postoperative neurologic deficit in our patient is unknown. The most likely possibility is that this represents poststroke recrudescence (PSR), knowing he had a previous left medullary infarct that presented similarly.11 PSR is a phenomenon in which prior stroke symptoms recur acutely and transiently in the setting of physiologic stressors—also known as locus minoris resistantiae.12 Triggers include γ aminobutyric acid (GABA) mediating anesthetic agents such as midazolam, opioids (eg, fentanyl or hydromorphone), infection, or relative cerebral hypoperfusion.11,13,14 The focality of our patient’s presentation favors PSR in the context of brief POD; of note, these entities share similar risk factors.15 Our patient did indeed receive low-dose preoperative midazolam in the context of mild preoperative neurocognitive deficit, which may have predisposed him to POD.
Though less likely, our patient’s presentation could have been explained by a new cerebrovascular event—transient ischemic attack vs new MI. Speech and right-sided motor/sensory deficits can localize to the left middle cerebral artery or small penetrating arteries of the left brainstem or deep white matter. MRI was not performed to exclude this possibility due to hospital-wide COVID-19 precautions minimizing nonessential MRIs unlikely to change clinical management. We speculate, however, that due to recent SARS-CoV-2 infection, our patient may have been at higher risk for cerebrovascular events due to subclinical endothelial damage and/or microclot in predisposed neurovasculature. Though our patient had interval COVID-19 negative tests, the timeframe of coronavirus procoagulant effects is unknown.16
There are well-established guidelines for perioperative stroke management published by the Society for Neuroscience in Anesthesiology and Critical Care (SNACC).17 This case exemplifies many recommendations including tight hemodynamic and glucose control, optimized oxygen delivery, avoidance of intraoperative β blockade, and prompt neurologic consultation. Additionally, special precaution was taken to ensure continuation of antiplatelet therapy on the day of surgery; in light of COVID-19 prothrombosis risk we considered this essential. Low-dose enoxaparin was also instituted on postoperative day 1. Prophylactic anticoagulation with low molecular weight heparin (LMWH) is recommended for hospitalized COVID-19–positive patients, though perioperatively, this must be weighed against hemorrhagic stroke transformation and surgical bleeding.8,16 Interestingly, the benefit of LMWH may partly relate to its anti-inflammatory effects, of which higher levels are observed in COVID-19.16,18
Though substantial health care provider energy and hospital resource utilization is presently focused on controlling the COVID-19 pandemic, the importance of appropriate stroke code processes must not be neglected. Recently, SNACC released anesthetic guidelines for endovascular ischemic stroke management that reflect COVID-19 precautions; highlights include personal protective equipment (PPE) utilization, risk-benefit analysis of general anesthesia (with early decision to intubate) vs sedation techniques for thrombectomy, and airway management strategies to minimize aerosolization exposure.19 Finally, negative pressure rooms relative to PACU and operating room locations need to be known and marked, as well as the necessary airway equipment and PPE to transfer patients safely to and from angiography suites.
Conclusions
We discuss a surgical patient with prior SARS-CoV-2 infection at elevated stroke risk that experienced recurrence of neurologic deficits postoperatively. This case informs anesthesia providers of the broad differential diagnosis for focal neurological deficits to include PSR and the possible contribution of COVID-19 to elevated acute stroke risk. Perioperative physicians, including VHA practitioners, with knowledge of current COVID-19 practices are primed to coordinate multidisciplinary efforts during stroke codes and ensuring appropriate anticoagulation.
Acknowledgments
The authors would like to thank perioperative care teams across the world caring for COVID-19 patients safely.
The risk of perioperative stroke in noncardiac, nonneurologic, nonvascular surgery ranges from 0.1 to 1.9% and is associated with increased mortality.1,2 Stroke mechanisms include both ischemia (large and small vessel occlusion, cardioembolism, anemic-tissue hypoxia, cerebral hypoperfusion) and hemorrhage.1 Risk factors for perioperative stroke include prior cerebral vascular accident (CVA), hypertension, aged > 62 years, acute renal insufficiency, dialysis, and recent myocardial infarction (MI).2
Introduction
COVID-19 was declared a pandemic by the World Health Organization in March 2020.3 COVID-19 has certainly affected the veteran population; between February and May 2020, more than 60,000 veterans were tested for COVID-19 with a positive rate of about 9%.4 While primarily affecting the respiratory system, there are increasing reports of COVID-19 neurologic manifestations: headache, hypogeusia, hyposomia, seizure, encephalitis, and acute stroke.5 In an early case series from Wuhan, China, 36% of 214 patients with COVID-19 reported neurologic complications, and acute CVAs were more common in patients with severe (compared to milder) viral disease presentations (5.7% vs 0.8%).6 Large vessel stroke was a presenting feature in another report of 5 patients aged < 50 years.7
The mechanism of ischemic stroke in the setting of COVID-19 is unclear.8 Indeed, stroke and COVID-19 share similar risk factors (eg, hypertension, diabetes mellitus [DM], older age), and immobile critically ill patients may already be prone to developing stroke.5,9 However, COVID-19 is associated with arterial and venous thromboembolism, elevated D-dimer and fibrinogen levels, and antiphospholipid antibody production. This prothrombotic state may be linked to cytokine-induced endothelial damage, mononuclear cell activation, tissue factor expression, and ultimately thrombin propagation and platelet activation.8
The rates of perioperative stroke may change as more patients with COVID-19 present for surgery, and the anesthesiology care team must prioritize mitigation efforts in high-risk patients, including veterans. Reducing the elevated stroke burden within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) is a public health priority.10 We present the case of a veteran with prior CVA and recent positive COVID-19 testing who experienced transient weakness and dysarthria following plastic surgery. The patient discussed provided written Health Insurance Portability and Accountability Act consent for publication of this report.
Case Presentation
A 75-year-old male veteran presented to the Minneapolis VA Medical Center in Minnesota with chronic left foot ulceration necessitating debridement and flap coverage. His medical history was significant for hypertension, type 2 DM, anemia of chronic disease, and coronary artery disease (left ventricular ejection fraction, 50%). Additionally, he had prior ischemic strokes in the oculomotor nucleus (in 2004 with internuclear ophthalmoplegia) and left ventral medulla (in 2019 with right hemiparesis). During his 2019 poststroke rehabilitation, he was diagnosed with mild neurocognitive deficit not attributable to his strokes. The patient’s medications included amlodipine, lisinopril, atorvastatin, clopidogrel (lifelong for secondary stroke prevention), metformin, and glipizide. The debridement procedure was initially delayed 3 weeks due to positive routine preoperative COVID-19 nasopharyngeal testing, though he reported no respiratory symptoms or fever. During the delay, the primary team prescribed daily oral rivaroxaban for thrombosis prophylaxis in addition to clopidogrel. One week prior to surgery, his repeat COVID-19 test was negative and prophylactic anticoagulation stopped.
On the day of surgery, the patient was hemodynamically stable: heart rate 86 beats/min, blood pressure 167/93 mm Hg (baseline 120-150 mm Hg systolic pressure), respiratory rate 16 breaths/min, oxygen saturation 99% without supplemental oxygen, temperature 97.1 °F. He received amlodipine and clopidogrel, but not lisinopril, that morning. No focal neurologic deficits were appreciated on preoperative examination, and resolution of symptoms related to the 2 prior MIs was confirmed. Preoperative glucose was 163 mg/dL. Femoral and sciatic peripheral nerve blocks were done for postoperative analgesia. A preinduction arterial line was placed and 2 mg of midazolam was administered for anxiolysis. Induction of general anesthesia with oral endotracheal intubation proceeded uneventfully; he was positioned prone.
Given his stroke risk factors, mean arterial pressure was maintained > 70 mm Hg for the duration of surgery. No vasoactive infusions were necessary and no β-blocking agents were administered. Insulin infusion was required; the maximum-recorded glucose was 219 mg/dL. Arterial blood gas samples were routinely drawn; acid-base balance was well maintained, PaO2 was > 185 mm Hg, and PaCO2 ranged from 29.4 to 38.5 mm Hg. The patient received 2 units of packed red blood cells for nadir hemoglobin of 7.5 mg/dL. At surgery end, we fully reversed neuromuscular blockade with suggamadex. The patient was returned to a supine position and extubated uneventfully after demonstrating the ability to follow commands.
During postanesthesia care unit (PACU) handoff, the patient exhibited acute speech impairment. He was able to state his name on repetition but seemed confused and sedated. Prompt formal neurology evaluation (stroke code) was sought. Initial National Institutes of Health (NIH) stroke scale score was 8 (1 for level of consciousness, 1 for minor right facial droop, 1 for right arm drift, 3 for right leg with no effort against gravity, 1 for right partial sensory loss, and 1 for mild dysarthria). The patient was oriented only to self. Other findings included mild right facial droop and dysarthria. On a 5-point strength scale, he scored 4 for the right deltoid, biceps, triceps, wrist extensors, right knee flexion, right dorsiflexion, and plantarflexion, 2 for right hip flexion, and ≥ 4 for right knee extension. Positive sensory findings were notable for decreased pin prick sensation on the right limbs.
We obtained emergent head computed tomography (CT) that was negative for acute abnormalities; CT angiography was negative for large vessel occlusion or clinically significant stenosis (Figure). On returning to the PACU from the CT scanner, the patient regained symmetric strength in both arms, right leg was antigravity, and his speech had normalized. Prior to PACU discharge 2 hours later, the patient was back to his prehospitalization neurologic function and NIH stroke scale was 0. Given this rapid clinical resolution, no acute stroke interventions were done, though permissive hypertension was recommended by the neurologist during PACU recovery.
The neurology team concluded that the patient’s symptoms were likely secondary to recrudescence of previous stroke symptoms in the setting of brief postoperative delirium (POD). However, we could not exclude transient ischemic attack or new cardioembolism, therefore patient was started on dual antiplatelet therapy for 3 weeks. Unfortunately, elective confirmatory magnetic resonance imaging (MRI) was not sought to confirm new ischemic changes due hospital COVID-19 restrictions on nonessential scanning. Neurology did not recommend carotid duplex ultrasound given patent vasculature on the head and neck CT angiography. Finally, the patient had undergone surface echocardiography 3 weeks prior to surgery that showed a left ventricular ejection fraction of 50% without significant valvular abnormalities, thrombus, or interatrial shunting, so repeated study was deferred.
Formal neurology consultation did not extend beyond postoperative day 1. One month after surgery, the anesthesiology team visited the patient during inpatient rehabilitation; he had not developed further focal neurologic symptoms or delirium. His strength was equal bilaterally and no speech deficits were noted. Unfortunately, the patient was readmitted to the hospital for continued foot wound drainage 2 months postoperatively, though no focal neurologic deficits were documented on his medical admission history and physical. No long term sequalae of his COVID-19 infection have been suspected.
Discussion
We report a veteran with prior stroke and COVID-19 who experienced postoperative speech and motor deficit despite deliberate risk factor mitigation. This case calls for increased vigilance by anesthesia providers to employ proper perioperative stroke management and anticoagulation strategies, and to be prepared for prompt intervention with COVID-19-sensitive practices should the need for advanced airway management or thrombectomy arises.
The exact etiology of the postoperative neurologic deficit in our patient is unknown. The most likely possibility is that this represents poststroke recrudescence (PSR), knowing he had a previous left medullary infarct that presented similarly.11 PSR is a phenomenon in which prior stroke symptoms recur acutely and transiently in the setting of physiologic stressors—also known as locus minoris resistantiae.12 Triggers include γ aminobutyric acid (GABA) mediating anesthetic agents such as midazolam, opioids (eg, fentanyl or hydromorphone), infection, or relative cerebral hypoperfusion.11,13,14 The focality of our patient’s presentation favors PSR in the context of brief POD; of note, these entities share similar risk factors.15 Our patient did indeed receive low-dose preoperative midazolam in the context of mild preoperative neurocognitive deficit, which may have predisposed him to POD.
Though less likely, our patient’s presentation could have been explained by a new cerebrovascular event—transient ischemic attack vs new MI. Speech and right-sided motor/sensory deficits can localize to the left middle cerebral artery or small penetrating arteries of the left brainstem or deep white matter. MRI was not performed to exclude this possibility due to hospital-wide COVID-19 precautions minimizing nonessential MRIs unlikely to change clinical management. We speculate, however, that due to recent SARS-CoV-2 infection, our patient may have been at higher risk for cerebrovascular events due to subclinical endothelial damage and/or microclot in predisposed neurovasculature. Though our patient had interval COVID-19 negative tests, the timeframe of coronavirus procoagulant effects is unknown.16
There are well-established guidelines for perioperative stroke management published by the Society for Neuroscience in Anesthesiology and Critical Care (SNACC).17 This case exemplifies many recommendations including tight hemodynamic and glucose control, optimized oxygen delivery, avoidance of intraoperative β blockade, and prompt neurologic consultation. Additionally, special precaution was taken to ensure continuation of antiplatelet therapy on the day of surgery; in light of COVID-19 prothrombosis risk we considered this essential. Low-dose enoxaparin was also instituted on postoperative day 1. Prophylactic anticoagulation with low molecular weight heparin (LMWH) is recommended for hospitalized COVID-19–positive patients, though perioperatively, this must be weighed against hemorrhagic stroke transformation and surgical bleeding.8,16 Interestingly, the benefit of LMWH may partly relate to its anti-inflammatory effects, of which higher levels are observed in COVID-19.16,18
Though substantial health care provider energy and hospital resource utilization is presently focused on controlling the COVID-19 pandemic, the importance of appropriate stroke code processes must not be neglected. Recently, SNACC released anesthetic guidelines for endovascular ischemic stroke management that reflect COVID-19 precautions; highlights include personal protective equipment (PPE) utilization, risk-benefit analysis of general anesthesia (with early decision to intubate) vs sedation techniques for thrombectomy, and airway management strategies to minimize aerosolization exposure.19 Finally, negative pressure rooms relative to PACU and operating room locations need to be known and marked, as well as the necessary airway equipment and PPE to transfer patients safely to and from angiography suites.
Conclusions
We discuss a surgical patient with prior SARS-CoV-2 infection at elevated stroke risk that experienced recurrence of neurologic deficits postoperatively. This case informs anesthesia providers of the broad differential diagnosis for focal neurological deficits to include PSR and the possible contribution of COVID-19 to elevated acute stroke risk. Perioperative physicians, including VHA practitioners, with knowledge of current COVID-19 practices are primed to coordinate multidisciplinary efforts during stroke codes and ensuring appropriate anticoagulation.
Acknowledgments
The authors would like to thank perioperative care teams across the world caring for COVID-19 patients safely.
1. Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63(2):193-204. doi:10.1007/s12630-015-0494-9
2. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289-1296. doi:10.1097/ALN.0b013e318216e7f4
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160. Published 2020 Mar 19. doi:10.23750/abm.v91i1.9397
4. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States Veterans. Preprint. medRxiv. 2020;2020.05.12.20099135. Published 2020 May 18. doi:10.1101/2020.05.12.20099135
5. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi:10.1016/j.clineuro.2020.105921
6. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127
7. Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. doi:10.1056/NEJMc2009787
8. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi:10.1136/jnnp-2020-323586
9. Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32(3):667-671. doi:10.1007/s12028-020-00978-4
10. Lich KH, Tian Y, Beadles CA, et al. Strategic planning to reduce the burden of stroke among veterans: using simulation modeling to inform decision making. Stroke. 2014;45(7):2078-2084. doi:10.1161/STROKEAHA.114.004694
11. Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of Deficits After Stroke: Clinical and Imaging Phenotype, Triggers, and Risk Factors. JAMA Neurol. 2017;74(9):1048-1055. doi:10.1001/jamaneurol.2017.1668
12. Jun-O’connell AH, Henninger N, Moonis M, Silver B, Ionete C, Goddeau RP. Recrudescence of old stroke deficits among transient neurological attacks. Neurohospitalist. 2019;9(4):183-189. doi:10.1177/194187441982928813. Karnik HS, Jain RA. Anesthesia for patients with prior stroke. J Neuroanaesthesiology Crit Care. 2018;5(3):150-157. doi:10.1055/s-0038-1673549
14. Minhas JS, Rook W, Panerai RB, et al. Pathophysiological and clinical considerations in the perioperative care of patients with a previous ischaemic stroke: a multidisciplinary narrative review. Br J Anaesth. 2020;124(2):183-196. doi:10.1016/j.bja.2019.10.021
15. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium [published correction appears in Eur J Anaesthesiol. 2018 Sep;35(9):718-719]. Eur J Anaesthesiol. 2017;34(4):192-214. doi:10.1097/EJA.0000000000000594
16. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
17. Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273-285. doi:10.1097/ana.0000000000000087
18. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-3153. doi:10.1007/s00415-020-09990-2
19. Sharma D, Rasmussen M, Han R, et al. Anesthetic Management of Endovascular Treatment of Acute Ischemic Stroke During COVID-19 Pandemic: Consensus Statement From Society for Neuroscience in Anesthesiology & Critical Care (SNACC): Endorsed by Society of Vascular & Interventional Neurology (SVIN), Society of NeuroInterventional Surgery (SNIS), Neurocritical Care Society (NCS), European Society of Minimally Invasive Neurological Therapy (ESMINT) and American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) Cerebrovascular Section. J Neurosurg Anesthesiol. 2020;32(3):193-201. doi:10.1097/ANA.0000000000000688
1. Vlisides P, Mashour GA. Perioperative stroke. Can J Anaesth. 2016;63(2):193-204. doi:10.1007/s12630-015-0494-9
2. Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289-1296. doi:10.1097/ALN.0b013e318216e7f4
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157-160. Published 2020 Mar 19. doi:10.23750/abm.v91i1.9397
4. Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States Veterans. Preprint. medRxiv. 2020;2020.05.12.20099135. Published 2020 May 18. doi:10.1101/2020.05.12.20099135
5. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin Neurol Neurosurg. 2020;194:105921. doi:10.1016/j.clineuro.2020.105921
6. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127
7. Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. doi:10.1056/NEJMc2009787
8. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi:10.1136/jnnp-2020-323586
9. Needham EJ, Chou SH, Coles AJ, Menon DK. Neurological Implications of COVID-19 Infections. Neurocrit Care. 2020;32(3):667-671. doi:10.1007/s12028-020-00978-4
10. Lich KH, Tian Y, Beadles CA, et al. Strategic planning to reduce the burden of stroke among veterans: using simulation modeling to inform decision making. Stroke. 2014;45(7):2078-2084. doi:10.1161/STROKEAHA.114.004694
11. Topcuoglu MA, Saka E, Silverman SB, Schwamm LH, Singhal AB. Recrudescence of Deficits After Stroke: Clinical and Imaging Phenotype, Triggers, and Risk Factors. JAMA Neurol. 2017;74(9):1048-1055. doi:10.1001/jamaneurol.2017.1668
12. Jun-O’connell AH, Henninger N, Moonis M, Silver B, Ionete C, Goddeau RP. Recrudescence of old stroke deficits among transient neurological attacks. Neurohospitalist. 2019;9(4):183-189. doi:10.1177/194187441982928813. Karnik HS, Jain RA. Anesthesia for patients with prior stroke. J Neuroanaesthesiology Crit Care. 2018;5(3):150-157. doi:10.1055/s-0038-1673549
14. Minhas JS, Rook W, Panerai RB, et al. Pathophysiological and clinical considerations in the perioperative care of patients with a previous ischaemic stroke: a multidisciplinary narrative review. Br J Anaesth. 2020;124(2):183-196. doi:10.1016/j.bja.2019.10.021
15. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium [published correction appears in Eur J Anaesthesiol. 2018 Sep;35(9):718-719]. Eur J Anaesthesiol. 2017;34(4):192-214. doi:10.1097/EJA.0000000000000594
16. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/jth.14810
17. Mashour GA, Moore LE, Lele AV, Robicsek SA, Gelb AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in Anesthesiology and Critical Care*. J Neurosurg Anesthesiol. 2014;26(4):273-285. doi:10.1097/ana.0000000000000087
18. Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267(11):3135-3153. doi:10.1007/s00415-020-09990-2
19. Sharma D, Rasmussen M, Han R, et al. Anesthetic Management of Endovascular Treatment of Acute Ischemic Stroke During COVID-19 Pandemic: Consensus Statement From Society for Neuroscience in Anesthesiology & Critical Care (SNACC): Endorsed by Society of Vascular & Interventional Neurology (SVIN), Society of NeuroInterventional Surgery (SNIS), Neurocritical Care Society (NCS), European Society of Minimally Invasive Neurological Therapy (ESMINT) and American Association of Neurological Surgeons (AANS) and Congress of Neurological Surgeons (CNS) Cerebrovascular Section. J Neurosurg Anesthesiol. 2020;32(3):193-201. doi:10.1097/ANA.0000000000000688
Role of Speech Pathology in a Multidisciplinary Approach to a Patient With Mild Traumatic Brain Injury
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
14-year-old girl • history of bullying • lack of social support • multiple linear scars on breasts • Dx?
THE CASE
A 14-year-old girl with no significant medical history presented to the office accompanied by her mother for a routine well-adolescent visit. She attended school online due to a history of severe bullying and, when interviewed alone, admitted to a lack of a social life as a result. On questioning, she denied tobacco, alcohol, or illicit drug use. Her gender identity was female. Her sexual orientation was toward both males and females, but she was not sexually active. She denied exposure to physical or emotional violence at home and said she did not feel depressed or think about suicide.
Physical examination revealed multiple erythematous linear scars surrounding the areola of both breasts. When questioned about these lesions, she admitted to cutting herself on the breasts during the past several months but again denied suicidal intent. She believed that her behavior was a normal coping mechanism.
The physical exam was otherwise normal. Lab results, including thyroid-stimulating hormone and complete blood count, were within normal limits.
THE DIAGNOSIS
The physical exam findings and the patient’s self report pointed to a diagnosis of nonsuicidal self-injurious (NSSI) behavior involving cutting.
DISCUSSION
The NSSI behavior displayed by this patient is a common biopsychosocial disorder observed in adolescents. Self-injury is defined as the deliberate injuring of body tissues without suicidal intent.1 Self-injurious behavior typically begins when patients are 13 to 16 years of age, and cutting is the most common form. Most acts occur on the arms, legs, wrists, and stomach.2 Studies have shown that the prevalence of this behavior is on the rise among adolescents, from about 7% in 2014 to between 14% and 24% in 2015.3
Risk for suicide. Although a feature of NSSI is the lack of suicidal intent, this type of high-risk behavior is associated with past, present, and future suicide attempts. It is important for physicians to identify NSSI in an adolescent, as it is linked to a 7-fold increased risk for a suicide attempt.3
Screening for NSSI. Less than one-fifth of adolescents who injure themselves come to the attention of health care providers.4 We propose that primary care physicians add NSSI to the list of risky behaviors—including drug abuse, sexual activity, and depression—for which they screen during well-child visits.
Continue to: Identifying risk factors
Identifying risk factors. The case patient experienced bullying and reported a nonheterosexual orientation, both of which have been demonstrated as strong risk factors for NSSI.5 Female gender has also been identified as a risk factor for NSSI.3
In adolescent psychiatric samples, prevalence rates of NSSI were found to be as high as 60% for 1 incident of NSSI and around 50% for repetitive NSSI.6 NSSI coincides with other psychiatric comorbidities, including eating disorders, mood disorders (depression), anxiety disorders, posttraumatic stress disorder, and borderline personality disorder.3 In a study of 93 subjects, each of whom was a self-reported abuse survivor with a history of self-injury, 96% were in therapy for diagnoses that included posttraumatic stress disorder (73%), dissociative disorder (40%), borderline personality disorder (37%), and multiple personality disorder (29%).7
The experience of adverse childhood events also increases risk for NSSI. This includes parental neglect, abuse, or deprivation.6 Insecure paternal attachment and parental neglect are significant predictors for women, while childhood separation is a primary predictor for men.8 Indirect childhood maltreatment, such as witnessing domestic violence or increased parental critique, is also associated with NSSI.8 NSSI is also more prevalent among young people who identify with a subculture such as gothic or emo.6
Why they do it and how to help
In multiple studies aimed at identifying reasons for self-injury, converging evidence suggests that nearly all patients act with the intent of alleviating negative affect.9 Patients self-harm to regulate distress, anxiety, and frustration that they perceive to be intolerable.9 They may self-harm to generate feeling when emotionally empty or to avert suicidal intent.9 For others, self-harm is a way to communicate their distress.
How to proceed. After a physician identifies NSSI, the patient should be assessed for suicidality and medical severity of the injury.3 Factors associated with higher likelihood of suicidality in patients with NSSI include multiple self-injurious methods and locations, early age of onset, longer history of NSSI, recent worsening of the injuries, simultaneous substance use, and the perception that the patient is addicted to self-injury.10
Continue to: It is also important...
It is also important to ask the patient whether she or he has told anyone about the behavior. Participation in NSSI communities may reinforce it.3
Treatment found to be effective for NSSI involves dialectical behavioral therapy, cognitive behavioral therapy, and mentalization-based therapy.11
Our patient was admitted to the hospital several weeks after her well visit because she expressed suicidal ideation. After being discharged, she was referred to outpatient Psychiatry with a treatment plan that included cognitive behavioral therapy.
THE TAKEAWAY
While our patient may have concealed her self-injurious experience because of stigma and concern about others’ reactions, there were several risk factors for NSSI in her history that prompted further investigation with a skin exam.
If a patient presents with 1 or more risk factors, an initial assessment for possible NSSI should be performed with detailed history-taking and a skin exam. Once NSSI is identified, the initial response and tone of questioning toward the patient need to convey a sense of genuine curiosity about the patient’s experience. From there, the physician can avail the patient to the proper treatment modalities.
NSSI patients can be resistant to sharing and participating in support groups. However, a referred counselor can follow up with a stepwise approach to slowly gain the trust of the individual, find the root cause, and get the patient to a point where she or he is ready to start the necessary treatment.
1. Klonsky ED, Glenn CR. Resisting urges to self-injure. Behav Cogn Psychother. 2008;36:211-220. doi: 10.1017/S1352465808004128
2. Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939-1948. doi: 10.1542/peds.2005-2543
3. Lewis SP, Heath NL. Non-suicidal self-injury among youth. J Pediatr. 2015;166:526-630. doi: 10.1016/j.jpeds.2014.11.062
4. Ystgaard M, Arensman E, Hawton K, et al. Deliberate self-harm in adolescents: comparison between those who receive help following self-harm and those who do not. J Adolesc. 2009;32: 875-891.
5. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2:524-531. doi: 10.1016/S2215-0366(15)00165-0
6. Brown RC, Plener PL. Non-suicidal self-injury in adolescence. Curr Psychiatry Rep. 2017;19:20. doi: 10.1007/s11920-017-0767-9
7. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68:609-620. doi:10.1037/h0080369
8. Gratz KL, Conrad SD, Roemer L. Risk factors for deliberate self-harm among college students. Am J Orthopsychiatry. 2002;1:128-140. doi: 10.1037//0002-9432.72.1.128
9. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27:226-239.
10. Nock MK, Joiner Jr. TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65-72. doi: 10.1016/j.psychres.2006.05.010
11. Lewis SP, Baker TG. The possible risks of self-injury websites: a content analysis. Arch Suicide Res. 2011;15:390-396. doi: 10.1080/13811118.2011.616154
THE CASE
A 14-year-old girl with no significant medical history presented to the office accompanied by her mother for a routine well-adolescent visit. She attended school online due to a history of severe bullying and, when interviewed alone, admitted to a lack of a social life as a result. On questioning, she denied tobacco, alcohol, or illicit drug use. Her gender identity was female. Her sexual orientation was toward both males and females, but she was not sexually active. She denied exposure to physical or emotional violence at home and said she did not feel depressed or think about suicide.
Physical examination revealed multiple erythematous linear scars surrounding the areola of both breasts. When questioned about these lesions, she admitted to cutting herself on the breasts during the past several months but again denied suicidal intent. She believed that her behavior was a normal coping mechanism.
The physical exam was otherwise normal. Lab results, including thyroid-stimulating hormone and complete blood count, were within normal limits.
THE DIAGNOSIS
The physical exam findings and the patient’s self report pointed to a diagnosis of nonsuicidal self-injurious (NSSI) behavior involving cutting.
DISCUSSION
The NSSI behavior displayed by this patient is a common biopsychosocial disorder observed in adolescents. Self-injury is defined as the deliberate injuring of body tissues without suicidal intent.1 Self-injurious behavior typically begins when patients are 13 to 16 years of age, and cutting is the most common form. Most acts occur on the arms, legs, wrists, and stomach.2 Studies have shown that the prevalence of this behavior is on the rise among adolescents, from about 7% in 2014 to between 14% and 24% in 2015.3
Risk for suicide. Although a feature of NSSI is the lack of suicidal intent, this type of high-risk behavior is associated with past, present, and future suicide attempts. It is important for physicians to identify NSSI in an adolescent, as it is linked to a 7-fold increased risk for a suicide attempt.3
Screening for NSSI. Less than one-fifth of adolescents who injure themselves come to the attention of health care providers.4 We propose that primary care physicians add NSSI to the list of risky behaviors—including drug abuse, sexual activity, and depression—for which they screen during well-child visits.
Continue to: Identifying risk factors
Identifying risk factors. The case patient experienced bullying and reported a nonheterosexual orientation, both of which have been demonstrated as strong risk factors for NSSI.5 Female gender has also been identified as a risk factor for NSSI.3
In adolescent psychiatric samples, prevalence rates of NSSI were found to be as high as 60% for 1 incident of NSSI and around 50% for repetitive NSSI.6 NSSI coincides with other psychiatric comorbidities, including eating disorders, mood disorders (depression), anxiety disorders, posttraumatic stress disorder, and borderline personality disorder.3 In a study of 93 subjects, each of whom was a self-reported abuse survivor with a history of self-injury, 96% were in therapy for diagnoses that included posttraumatic stress disorder (73%), dissociative disorder (40%), borderline personality disorder (37%), and multiple personality disorder (29%).7
The experience of adverse childhood events also increases risk for NSSI. This includes parental neglect, abuse, or deprivation.6 Insecure paternal attachment and parental neglect are significant predictors for women, while childhood separation is a primary predictor for men.8 Indirect childhood maltreatment, such as witnessing domestic violence or increased parental critique, is also associated with NSSI.8 NSSI is also more prevalent among young people who identify with a subculture such as gothic or emo.6
Why they do it and how to help
In multiple studies aimed at identifying reasons for self-injury, converging evidence suggests that nearly all patients act with the intent of alleviating negative affect.9 Patients self-harm to regulate distress, anxiety, and frustration that they perceive to be intolerable.9 They may self-harm to generate feeling when emotionally empty or to avert suicidal intent.9 For others, self-harm is a way to communicate their distress.
How to proceed. After a physician identifies NSSI, the patient should be assessed for suicidality and medical severity of the injury.3 Factors associated with higher likelihood of suicidality in patients with NSSI include multiple self-injurious methods and locations, early age of onset, longer history of NSSI, recent worsening of the injuries, simultaneous substance use, and the perception that the patient is addicted to self-injury.10
Continue to: It is also important...
It is also important to ask the patient whether she or he has told anyone about the behavior. Participation in NSSI communities may reinforce it.3
Treatment found to be effective for NSSI involves dialectical behavioral therapy, cognitive behavioral therapy, and mentalization-based therapy.11
Our patient was admitted to the hospital several weeks after her well visit because she expressed suicidal ideation. After being discharged, she was referred to outpatient Psychiatry with a treatment plan that included cognitive behavioral therapy.
THE TAKEAWAY
While our patient may have concealed her self-injurious experience because of stigma and concern about others’ reactions, there were several risk factors for NSSI in her history that prompted further investigation with a skin exam.
If a patient presents with 1 or more risk factors, an initial assessment for possible NSSI should be performed with detailed history-taking and a skin exam. Once NSSI is identified, the initial response and tone of questioning toward the patient need to convey a sense of genuine curiosity about the patient’s experience. From there, the physician can avail the patient to the proper treatment modalities.
NSSI patients can be resistant to sharing and participating in support groups. However, a referred counselor can follow up with a stepwise approach to slowly gain the trust of the individual, find the root cause, and get the patient to a point where she or he is ready to start the necessary treatment.
THE CASE
A 14-year-old girl with no significant medical history presented to the office accompanied by her mother for a routine well-adolescent visit. She attended school online due to a history of severe bullying and, when interviewed alone, admitted to a lack of a social life as a result. On questioning, she denied tobacco, alcohol, or illicit drug use. Her gender identity was female. Her sexual orientation was toward both males and females, but she was not sexually active. She denied exposure to physical or emotional violence at home and said she did not feel depressed or think about suicide.
Physical examination revealed multiple erythematous linear scars surrounding the areola of both breasts. When questioned about these lesions, she admitted to cutting herself on the breasts during the past several months but again denied suicidal intent. She believed that her behavior was a normal coping mechanism.
The physical exam was otherwise normal. Lab results, including thyroid-stimulating hormone and complete blood count, were within normal limits.
THE DIAGNOSIS
The physical exam findings and the patient’s self report pointed to a diagnosis of nonsuicidal self-injurious (NSSI) behavior involving cutting.
DISCUSSION
The NSSI behavior displayed by this patient is a common biopsychosocial disorder observed in adolescents. Self-injury is defined as the deliberate injuring of body tissues without suicidal intent.1 Self-injurious behavior typically begins when patients are 13 to 16 years of age, and cutting is the most common form. Most acts occur on the arms, legs, wrists, and stomach.2 Studies have shown that the prevalence of this behavior is on the rise among adolescents, from about 7% in 2014 to between 14% and 24% in 2015.3
Risk for suicide. Although a feature of NSSI is the lack of suicidal intent, this type of high-risk behavior is associated with past, present, and future suicide attempts. It is important for physicians to identify NSSI in an adolescent, as it is linked to a 7-fold increased risk for a suicide attempt.3
Screening for NSSI. Less than one-fifth of adolescents who injure themselves come to the attention of health care providers.4 We propose that primary care physicians add NSSI to the list of risky behaviors—including drug abuse, sexual activity, and depression—for which they screen during well-child visits.
Continue to: Identifying risk factors
Identifying risk factors. The case patient experienced bullying and reported a nonheterosexual orientation, both of which have been demonstrated as strong risk factors for NSSI.5 Female gender has also been identified as a risk factor for NSSI.3
In adolescent psychiatric samples, prevalence rates of NSSI were found to be as high as 60% for 1 incident of NSSI and around 50% for repetitive NSSI.6 NSSI coincides with other psychiatric comorbidities, including eating disorders, mood disorders (depression), anxiety disorders, posttraumatic stress disorder, and borderline personality disorder.3 In a study of 93 subjects, each of whom was a self-reported abuse survivor with a history of self-injury, 96% were in therapy for diagnoses that included posttraumatic stress disorder (73%), dissociative disorder (40%), borderline personality disorder (37%), and multiple personality disorder (29%).7
The experience of adverse childhood events also increases risk for NSSI. This includes parental neglect, abuse, or deprivation.6 Insecure paternal attachment and parental neglect are significant predictors for women, while childhood separation is a primary predictor for men.8 Indirect childhood maltreatment, such as witnessing domestic violence or increased parental critique, is also associated with NSSI.8 NSSI is also more prevalent among young people who identify with a subculture such as gothic or emo.6
Why they do it and how to help
In multiple studies aimed at identifying reasons for self-injury, converging evidence suggests that nearly all patients act with the intent of alleviating negative affect.9 Patients self-harm to regulate distress, anxiety, and frustration that they perceive to be intolerable.9 They may self-harm to generate feeling when emotionally empty or to avert suicidal intent.9 For others, self-harm is a way to communicate their distress.
How to proceed. After a physician identifies NSSI, the patient should be assessed for suicidality and medical severity of the injury.3 Factors associated with higher likelihood of suicidality in patients with NSSI include multiple self-injurious methods and locations, early age of onset, longer history of NSSI, recent worsening of the injuries, simultaneous substance use, and the perception that the patient is addicted to self-injury.10
Continue to: It is also important...
It is also important to ask the patient whether she or he has told anyone about the behavior. Participation in NSSI communities may reinforce it.3
Treatment found to be effective for NSSI involves dialectical behavioral therapy, cognitive behavioral therapy, and mentalization-based therapy.11
Our patient was admitted to the hospital several weeks after her well visit because she expressed suicidal ideation. After being discharged, she was referred to outpatient Psychiatry with a treatment plan that included cognitive behavioral therapy.
THE TAKEAWAY
While our patient may have concealed her self-injurious experience because of stigma and concern about others’ reactions, there were several risk factors for NSSI in her history that prompted further investigation with a skin exam.
If a patient presents with 1 or more risk factors, an initial assessment for possible NSSI should be performed with detailed history-taking and a skin exam. Once NSSI is identified, the initial response and tone of questioning toward the patient need to convey a sense of genuine curiosity about the patient’s experience. From there, the physician can avail the patient to the proper treatment modalities.
NSSI patients can be resistant to sharing and participating in support groups. However, a referred counselor can follow up with a stepwise approach to slowly gain the trust of the individual, find the root cause, and get the patient to a point where she or he is ready to start the necessary treatment.
1. Klonsky ED, Glenn CR. Resisting urges to self-injure. Behav Cogn Psychother. 2008;36:211-220. doi: 10.1017/S1352465808004128
2. Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939-1948. doi: 10.1542/peds.2005-2543
3. Lewis SP, Heath NL. Non-suicidal self-injury among youth. J Pediatr. 2015;166:526-630. doi: 10.1016/j.jpeds.2014.11.062
4. Ystgaard M, Arensman E, Hawton K, et al. Deliberate self-harm in adolescents: comparison between those who receive help following self-harm and those who do not. J Adolesc. 2009;32: 875-891.
5. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2:524-531. doi: 10.1016/S2215-0366(15)00165-0
6. Brown RC, Plener PL. Non-suicidal self-injury in adolescence. Curr Psychiatry Rep. 2017;19:20. doi: 10.1007/s11920-017-0767-9
7. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68:609-620. doi:10.1037/h0080369
8. Gratz KL, Conrad SD, Roemer L. Risk factors for deliberate self-harm among college students. Am J Orthopsychiatry. 2002;1:128-140. doi: 10.1037//0002-9432.72.1.128
9. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27:226-239.
10. Nock MK, Joiner Jr. TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65-72. doi: 10.1016/j.psychres.2006.05.010
11. Lewis SP, Baker TG. The possible risks of self-injury websites: a content analysis. Arch Suicide Res. 2011;15:390-396. doi: 10.1080/13811118.2011.616154
1. Klonsky ED, Glenn CR. Resisting urges to self-injure. Behav Cogn Psychother. 2008;36:211-220. doi: 10.1017/S1352465808004128
2. Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939-1948. doi: 10.1542/peds.2005-2543
3. Lewis SP, Heath NL. Non-suicidal self-injury among youth. J Pediatr. 2015;166:526-630. doi: 10.1016/j.jpeds.2014.11.062
4. Ystgaard M, Arensman E, Hawton K, et al. Deliberate self-harm in adolescents: comparison between those who receive help following self-harm and those who do not. J Adolesc. 2009;32: 875-891.
5. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2:524-531. doi: 10.1016/S2215-0366(15)00165-0
6. Brown RC, Plener PL. Non-suicidal self-injury in adolescence. Curr Psychiatry Rep. 2017;19:20. doi: 10.1007/s11920-017-0767-9
7. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68:609-620. doi:10.1037/h0080369
8. Gratz KL, Conrad SD, Roemer L. Risk factors for deliberate self-harm among college students. Am J Orthopsychiatry. 2002;1:128-140. doi: 10.1037//0002-9432.72.1.128
9. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27:226-239.
10. Nock MK, Joiner Jr. TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65-72. doi: 10.1016/j.psychres.2006.05.010
11. Lewis SP, Baker TG. The possible risks of self-injury websites: a content analysis. Arch Suicide Res. 2011;15:390-396. doi: 10.1080/13811118.2011.616154
Glucosuria Is Not Always Due to Diabetes
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Truncus Bicaroticus With Arteria Lusoria: A Rare Combination of Aortic Root Anatomy Complicating Cardiac Catheterization
While most patients with arteria lusoria and common carotid trunk conditions are asymptomatic, discovery of such anomalies periprocedurally may affect the cardiac catheterization access site, catheter selection, and additional imaging.
Branching of the great vessels from the aorta normally progresses with the brachiocephalic trunk as the first takeoff followed by the left common carotid and left subclavian artery in approximately 85% of cases.1 Variants of great vessel branching patterns include the so-called bovine arch, arteria lusoria or aberrant right subclavian artery (ARSA), aberrant origin of the vertebral arteries, and truncus bicaroticus, or common origin of the carotid arteries (COCA). These aberrancies are quite rare, some with an incidence of < 1%.1,2
These vascular anomalies become clinically relevant when they pose difficulty for operators in surgical and interventional specialties, necessitating unique approaches, catheters, and techniques to overcome. We present a case of concomitant aortic arch abnormalities during a diagnostic workup for transcatheter aortic valve replacement (TAVR) in a patient with previous coronary artery bypass grafting (CABG).
Case Presentation
A 66-year-old woman with coronary artery disease (CAD) status post-CABG and stage D1 aortic stenosis (AS) presented with exertional dyspnea. She was referred for coronary angiography as part of a workup for TAVR. Echocardiography confirmed severe AS with a peak velocity of 4.1 m/s, mean pressure gradient of 50 mm Hg, and an aortic valve area of 0.7 cm2. The patient was scheduled for cardiac catheterization with anticipated left radial artery approach for intubation and opacification of the left internal mammary artery (LIMA). However, this approach was abandoned during the procedure due to discovery of aberrant left radial artery anatomy, and the procedure was completed via femoral access.
Subsequent coronary angiography revealed 3-vessel CAD, patent saphenous vein grafts (SVG) to the right coronary artery (RCA) and a diagonal branch vessel with an occluded SVG to the left circumflex. Difficulty was encountered when engaging the left subclavian artery using a JR 4.0 diagnostic catheter for LIMA angiography. Nonselective angiography of the aortic arch was performed and demonstrated an uncommon anatomical variant (Figure 1, left). The right common carotid artery (CCA) [A] and the left CCA [B] arose from a single trunk, consistent with truncus bicaroticus or COCA [C]. The right subclavian artery [D] originated distal to the left subclavian artery otherwise known as arteria lusoria or ARSA forming an incomplete vascular ring [E]. Selective engagement of the left subclavian artery remained problematic even with the use of specialty arch catheters (Headhunter and LIMA catheters). The procedure concluded without confirming patency of the LIMA graft. A total of 145 mL of Omnipaque (iohexol injection) contrast was used for the procedure, and no adverse events occurred.
Same-day access of the ipsilateral ulnar artery was not pursued because of the risk of hand ischemia. The patient underwent repeat catheterization utilizing left ulnar artery access after adequate recovery time from the initial left radial approach. Selective LIMA angiography was achieved and demonstrated a patent LIMA to LAD graft. A computed tomography (CT) aorta for purposes of TAVR planning was able to reconstruct the aortic arch vasculature (Figure 1, right) confirming the presence of both ARSA and COCA. The patient went on to undergo successful TAVR with subsequent improvement of clinical symptoms.
Discussion
Arteria lusoria is defined as an anomalous right subclavian artery arising distal to the origin of the left subclavian artery on the aortic arch. It has an estimated incidence of 0.5 to 2% and occurs as a consequence of abnormal embryologic involution of the right fourth aortic arch and right proximal dorsal aorta. This causes the origin of the right subclavian artery to shift onto the descending aorta and cross the mediastinum from left to right, passing behind the esophagus and the trachea.1,3-5
ARSA is often associated with other anatomic abnormalities, including COCA, right-sided aortic arch, interrupted aortic arch, aortic coarctation, tetralogy of Fallot, truncus arteriosus, transposition of the great arteries, atrial septal defects, and ventricular septal defects.Underlying genetic disorders, such as Edwards, Down, DiGeorge syndromes, aneurysms, and arterioesophageal fistulae can accompany these vascular malformations.6
COCA, such as we encountered, is the presence of a single branch from the aorta giving off both right and left common carotid arteries. It has an incidence of < 0.1% in isolation and is discovered most often in cadaveric dissections or incidentally on imaging.1 Its embryologic origin results from the third pair of cervical aortic arches persisting as a common bicarotid trunk.1,4,5 The combination of ARSA and COCA is rare. Of the 0.5 to 2% of ARSA cases discovered, only 20% of those cases present with associated COCA for a combined prevalence estimated at < 0.05%.7
The majority of patients with either anatomic abnormality are asymptomatic. However, a few classic clinical manifestations have been described. ARSA can rarely present with dysphagia lusoria, a condition resulting from an incomplete vascular ring formed by the abnormal course of the right subclavian compressing the esophagus. Although not seen in our patient, it should be considered in the differential diagnosis for dysphagia.1,2,7 Ortner syndrome can result from right laryngeal nerve compression and palsy resultant from the aberrant course of the right subclavian artery.8 Another clinically relevant feature of ARSA is the presence of a diverticulum of Kommerell or dilatation at the origin of the right subclavian artery. It is a type of retroesophageal diverticulum resulting from persistence of a segment of the right sixth aortic arch.9 Finally, the spatial arrangement of ARSA increases risk for injury during head and neck surgical procedures, such as thyroidectomy, tracheotomy, and lymph node dissection of the right paratracheal fossa.6 Although the incidence is not well described, COCA has been described in several case reports as causing tracheal compression with dyspnea and in some cases, ischemic stroke.4,5,10
Diagnosis
The diagnosis of ARSA and COCA is often made incidentally on diagnostic imaging studies such as endovascular imaging, CT angiography, magnetic resonance (MR) angiography, postmortem cadaveric dissections, or, as in our case, during cardiac catheterization.11,12 A classification system for aortic arch branching patterns exists published by Adachi and Williams.6 The classification includes ARSA and differentiates it into 4 subtypes (Figure 2). Our patient exhibited type H-1, indicating ARSA as the distal most branch of the aortic arch with coexistence of COCA.6 The primary clinical implication of ARSA and COCA in our case was increased difficulty and complexity when performing coronary angiography. Available literature has well characterized the challenges operators encounter when cannulating aberrant great vessel anatomy, often electing to perform nonselective aortography to define a patient’s anatomy.7,9,13 A comparison of diagnostic imaging techniques for vascular rings such as ARSA have shown MR, CT, and endovascular angiography to be the most reliable modalities to delineate vascular anatomy.14
Methods
Due to the presence of CABG in our patient, left radial and ulnar artery approaches were used rather than a right radial artery approach. Engagement of the LIMA is performed most commonly with left radial or femoral artery access using an internal mammary catheter that has a more steeply angled tip (80º-85º) compared with the standard JR catheter. An accessory left radial artery anatomic variant was encountered in our case precluding left radial approach. In addition, abnormal takeoffs of the great vessels thwarted multiple attempts at intubation of the LSA (Figure 1, right). Some data suggest CT imaging can be of assistance in establishing patency of bypass grafts in CABG patients.15 This can be considered an option if branch-vessel anatomy remains unclear. Our patient exhibited several risk factors for stroke, including female gender, hypertension, and prior CABG. These and other risk factors may influence clinical decisions such as continued catheter manipulation, choice of catheter type, and further contrast studies.16
Nonselective angiography in these cases often can require excessive iodinated contrast, exposing the patient to increased risk of contrast-induced nephropathy (CIN).7,17 Although the amount of contrast used in our case was average for diagnostic catheterization,the patient went on to undergo a second catheterization and CT angiography to establish LIMA graft patency.17 CT imaging reconstruction elucidated her aberrant branch-vessel anatomy. Patients are at increased risk of CIN with contrast loads < 200 mL per study, and this effect is compounded when the patient is elderly, has diabetes mellitus, and/or antecedent renal disease.18 Attention to the patient’s preoperative glomerular filtration rate, avoidance of nephrotoxic agents, and intraoperative left ventricular end-diastolic pressure during cardiac catheterization with postcontrast administration of IV isotonic fluids have been shown to prevent CIN.19,20 In the POSEIDON trial, fluid administration on a sliding scale based on the left ventricular end-diastolic pressure resulted in lower absolute risk of CIN postcatheterization vs standard postprocedure hydration in cardiac catheterization.21 Further, the now widespread use of low and iso-osmolar contrast agents further reduces the risk of CIN.22
For cardiac catheter laboratory operators, it is important to note that ARSA is more frequently encountered due to increased use of the transradial approach to coronary angiography.11 It should be suspected when accessing the ascending aorta proves exceptionally challenging and the catheter has a predilection for entering the descending aorta.11 While more technically demanding, 2 cases described by Allen and colleagues exhibited safe and successful entry into the ascending aorta with catheter rotation and hydrophilic support wires indicating the right radial approach is feasible despite presence of ARSA.12 Several patient-initiated maneuvers can be utilized to aid in accessing the ascending aorta. For example, deep inspiration to reduce the angulation between the aortic arch and ARSA. The use of curved catheters, such as Amplatz left, internal mammary catheter, or Simmons catheter may be considered to cannulate the ascending aorta if ARSA is encountered. Complications associated with a transradial approach include dissection and intramural hematoma. Minor bleeds and vasospasm also can occur secondary to increased procedural duration.6,8
Treatment
ARSA and COCA are considered normal anatomic variants and no treatment is indicated if the patient is asymptomatic. If symptoms are present, they often arise from aneurysmal or occlusive complications of the vascular anatomy. In patients with isolated ARSA and mild dysphasia or reflux symptoms, the use of prokinetics and antireflux medications may provide relief. It is important to note the coexistence of ARSA and COCA is more likely to produce esophageal compression compared to ARSA alone due to formation of a more complete vascular ring. Surgical management has been described in severe cases of ARSA involving risk of aneurysm rupture, right upper limb ischemia, or compression of the esophagus or trachea.
Several surgical approaches have been described, including simple ligation and division of ARSA and reimplantation of the RSA into the right CCA or ascending aorta.5 A recent review of 180 cases of ARSA diagnosed on CT angiography with concomitant common carotid trunk in half of studied individuals focused on a hybrid open and intravascular procedure. This procedure involved a double transposition or bypass (LSA to left common carotid artery and ARSA to the right CCA) followed by implantation of a thoracic stent graft. Few cases are eligible for these procedures or require them for definitive treatment.23
Conclusions
Recognition of aortic arch anatomical variants such as our case of ARSA with concomitant COCA may influence clinician decisions in various specialties, such as interventional cardiology, interventional neurology, cardiothoracic surgery, and gastroenterology. While most patients with these conditions are asymptomatic, some may present with dysphagia, dyspnea, and/or stroke symptoms. In our practice, discovery of such anomalies periprocedurally may affect cardiac catheterization access site, catheter selection, and additional imaging. The presence of arteria lusoria can be of critical importance when encountering a patient with myocardial infarction as switching from transradial to transfemoral approach may be required to gain access to the ascending aorta. Overall, transradial coronary angiography and percutaneous coronary intervention is not contraindicated in the setting of ARSA/COCA and can be safely performed by an experienced operator.
It is important for surgical specialists to be aware of the coexistence of anomalies where the discovery of one aberrancy can signal coexistent variant anatomy. If aortic arch anatomy is unclear, it is useful to perform nonselective angiography and/or further imaging with CT angiography. Knowledge of abnormal aortic arch anatomy can decrease fluoroscopy time and contrast load administered, thereby reducing potential periprocedural adverse events.
1. Kurt MA, An I, Ikiz I. A case with coincidence of aberrant right subclavian artery and common origin of the carotid arteries. Ann Anat. 1997;179(2):175-176. doi:10.1016/s0940-9602(97)80100-8
2. Klinkhamer AC. Aberrant right subclavian artery. Clinical and roentgenologic aspects. Am J Roentgenol Radium Ther Nucl Med. 1966;97(2):438-446. doi:10.2214/ajr.97.2.438
3. Türkvatan A, Büyükbayraktar FG, Olçer T, Cumhur T. Congenital anomalies of the aortic arch: evaluation with the use of multidetector computed tomography. Korean J Radiol. 2009;10(2):176-184. doi:10.3348/kjr.2009.10.2.176
4. Ozateş M, Nazaroglu H, Uyar A. MR angiography in diagnosis of aberrant right subclavian artery associated with common carotid trunk. Eur Radiol. 2000;10(9):1503. doi:10.1007/s003300000335
5. Poultsides GA, Lolis ED, Vasquez J, Drezner AD, Venieratos D. Common origins of carotid and subclavian arterial systems: report of a rare aortic arch variant. Ann Vasc Surg. 2004;18(5):597-600. doi:10.1007/s10016-004-0060-3
6. Leite TFO, Pires LAS, Cisne R, Babinski MA, Chagas CAA. Clinical discussion of the arteria lusoria: a case report. J Vasc Bras. 2017;16(4):339-342. doi:10.1590/1677-5449.007617
7. Tsai IC, Tzeng WS, Lee T, et al. Vertebral and carotid artery anomalies in patients with aberrant right subclavian arteries. Pediatr Radiol. 2007;37(10):1007-1012. doi:10.1007/s00247-007-0574-2
8. Rafiq A, Chutani S, Krim NR. Incidental finding of arteria lusoria during transradial coronary catheterization: significance in interventional cardiology. Catheter Cardiovasc Interv. 2018;91(7):1283-1286. doi:10.1002/ccd.27439
9. Priya S, Thomas R, Nagpal P, Sharma A, Steigner M. Congenital anomalies of the aortic arch. Cardiovasc Diagn Ther. 2018;8(suppl 1):S26-S44. doi:10.21037/cdt.2017.10.15
10. Khatri R, Maud A, Rodriguez GJ. Aberrant right subclavian artery and common carotid trunk. J Vasc Interv Neurol. 2010;3(1):33-34.
11. Valsecchi O, Vassileva A, Musumeci G, et al. Failure of transradial approach during coronary interventions: anatomic considerations. Catheter Cardiovasc Interv. 2006;67(6):870-878. doi:10.1002/ccd.20732
12. Allen D, Bews H, Vo M, Kass M, Jassal DS, Ravandi A. Arteria lusoria: an anomalous finding during right transradial coronary intervention. Case Rep Cardiol. 2016;2016:8079856. doi:10.1155/2016/8079856
13. Fineschi M, Iadanza A, Sinicropi G, Pierli C. Images in cardiology: angiographic evidence of aberrant right subclavian artery associated with common carotid trunk. Heart. 2002;88(2):158. doi:10.1136/heart.88.2.158
14. van Son JA, Julsrud PR, Hagler DJ, et al. Imaging strategies for vascular rings. Ann Thorac Surg. 1994;57(3):604-610. doi:10.1016/0003-4975(94)90552-5
15. Lee R, Lim J, Kaw G, Wan G, Ng K, Ho KT. Comprehensive noninvasive evaluation of bypass grafts and native coronary arteries in patients after coronary bypass surgery: accuracy of 64-slice multidetector computed tomography compared to invasive coronary angiography. J Cardiovasc Med (Hagerstown). 2010;11(2):81-90. doi:10.2459/JCM.0b013e32832f3e2e
16. Hamon M, Baron JC, Viader F, Hamon M. Periprocedural stroke and cardiac catheterization. Circulation. 2008;118(6): 678-683. doi:10.1161/CIRCULATIONAHA.108.784504
17. Hwang JR, D’Alfonso S, Kostuk WJ, et al. Contrast volume use in manual vs automated contrast injection systems for diagnostic coronary angiography and percutaneous coronary interventions. Can J Cardiol. 2013;29(3):372-376. doi:10.1016/j.cjca.2012.11.023
18. Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150(6):1237-1242.
19. Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-728. doi:10.1148/radiol.13122276
20. American College of Radiology. ACR Manual on Contrast Media 2020. American College of Radiology; 2020:33-34. Accessed January 15, 2021. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
21. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383(9931):1814-1823. doi:10.1016/S0140-6736(14)60689-9
22. Aoun J, Nicolas D, Brown JR, Jaber BL. Maximum allowable contrast dose and prevention of acute kidney injury following cardiovascular procedures. Curr Opin Nephrol Hypertens. 2018;27(2):121-129. doi:10.1097/MNH.0000000000000389
23. Settembre N, Saba C, Bouziane Z, Jeannon F, Mandry D, Malikov S. Hybrid treatment of the aberrant right subclavian artery (arteria lusoria): feasibility study on 180 angio-CTs. Ann Vasc Surg. 2017;44:229-233. doi:10.1016/j.avsg.2017.03.172
While most patients with arteria lusoria and common carotid trunk conditions are asymptomatic, discovery of such anomalies periprocedurally may affect the cardiac catheterization access site, catheter selection, and additional imaging.
While most patients with arteria lusoria and common carotid trunk conditions are asymptomatic, discovery of such anomalies periprocedurally may affect the cardiac catheterization access site, catheter selection, and additional imaging.
Branching of the great vessels from the aorta normally progresses with the brachiocephalic trunk as the first takeoff followed by the left common carotid and left subclavian artery in approximately 85% of cases.1 Variants of great vessel branching patterns include the so-called bovine arch, arteria lusoria or aberrant right subclavian artery (ARSA), aberrant origin of the vertebral arteries, and truncus bicaroticus, or common origin of the carotid arteries (COCA). These aberrancies are quite rare, some with an incidence of < 1%.1,2
These vascular anomalies become clinically relevant when they pose difficulty for operators in surgical and interventional specialties, necessitating unique approaches, catheters, and techniques to overcome. We present a case of concomitant aortic arch abnormalities during a diagnostic workup for transcatheter aortic valve replacement (TAVR) in a patient with previous coronary artery bypass grafting (CABG).
Case Presentation
A 66-year-old woman with coronary artery disease (CAD) status post-CABG and stage D1 aortic stenosis (AS) presented with exertional dyspnea. She was referred for coronary angiography as part of a workup for TAVR. Echocardiography confirmed severe AS with a peak velocity of 4.1 m/s, mean pressure gradient of 50 mm Hg, and an aortic valve area of 0.7 cm2. The patient was scheduled for cardiac catheterization with anticipated left radial artery approach for intubation and opacification of the left internal mammary artery (LIMA). However, this approach was abandoned during the procedure due to discovery of aberrant left radial artery anatomy, and the procedure was completed via femoral access.
Subsequent coronary angiography revealed 3-vessel CAD, patent saphenous vein grafts (SVG) to the right coronary artery (RCA) and a diagonal branch vessel with an occluded SVG to the left circumflex. Difficulty was encountered when engaging the left subclavian artery using a JR 4.0 diagnostic catheter for LIMA angiography. Nonselective angiography of the aortic arch was performed and demonstrated an uncommon anatomical variant (Figure 1, left). The right common carotid artery (CCA) [A] and the left CCA [B] arose from a single trunk, consistent with truncus bicaroticus or COCA [C]. The right subclavian artery [D] originated distal to the left subclavian artery otherwise known as arteria lusoria or ARSA forming an incomplete vascular ring [E]. Selective engagement of the left subclavian artery remained problematic even with the use of specialty arch catheters (Headhunter and LIMA catheters). The procedure concluded without confirming patency of the LIMA graft. A total of 145 mL of Omnipaque (iohexol injection) contrast was used for the procedure, and no adverse events occurred.
Same-day access of the ipsilateral ulnar artery was not pursued because of the risk of hand ischemia. The patient underwent repeat catheterization utilizing left ulnar artery access after adequate recovery time from the initial left radial approach. Selective LIMA angiography was achieved and demonstrated a patent LIMA to LAD graft. A computed tomography (CT) aorta for purposes of TAVR planning was able to reconstruct the aortic arch vasculature (Figure 1, right) confirming the presence of both ARSA and COCA. The patient went on to undergo successful TAVR with subsequent improvement of clinical symptoms.
Discussion
Arteria lusoria is defined as an anomalous right subclavian artery arising distal to the origin of the left subclavian artery on the aortic arch. It has an estimated incidence of 0.5 to 2% and occurs as a consequence of abnormal embryologic involution of the right fourth aortic arch and right proximal dorsal aorta. This causes the origin of the right subclavian artery to shift onto the descending aorta and cross the mediastinum from left to right, passing behind the esophagus and the trachea.1,3-5
ARSA is often associated with other anatomic abnormalities, including COCA, right-sided aortic arch, interrupted aortic arch, aortic coarctation, tetralogy of Fallot, truncus arteriosus, transposition of the great arteries, atrial septal defects, and ventricular septal defects.Underlying genetic disorders, such as Edwards, Down, DiGeorge syndromes, aneurysms, and arterioesophageal fistulae can accompany these vascular malformations.6
COCA, such as we encountered, is the presence of a single branch from the aorta giving off both right and left common carotid arteries. It has an incidence of < 0.1% in isolation and is discovered most often in cadaveric dissections or incidentally on imaging.1 Its embryologic origin results from the third pair of cervical aortic arches persisting as a common bicarotid trunk.1,4,5 The combination of ARSA and COCA is rare. Of the 0.5 to 2% of ARSA cases discovered, only 20% of those cases present with associated COCA for a combined prevalence estimated at < 0.05%.7
The majority of patients with either anatomic abnormality are asymptomatic. However, a few classic clinical manifestations have been described. ARSA can rarely present with dysphagia lusoria, a condition resulting from an incomplete vascular ring formed by the abnormal course of the right subclavian compressing the esophagus. Although not seen in our patient, it should be considered in the differential diagnosis for dysphagia.1,2,7 Ortner syndrome can result from right laryngeal nerve compression and palsy resultant from the aberrant course of the right subclavian artery.8 Another clinically relevant feature of ARSA is the presence of a diverticulum of Kommerell or dilatation at the origin of the right subclavian artery. It is a type of retroesophageal diverticulum resulting from persistence of a segment of the right sixth aortic arch.9 Finally, the spatial arrangement of ARSA increases risk for injury during head and neck surgical procedures, such as thyroidectomy, tracheotomy, and lymph node dissection of the right paratracheal fossa.6 Although the incidence is not well described, COCA has been described in several case reports as causing tracheal compression with dyspnea and in some cases, ischemic stroke.4,5,10
Diagnosis
The diagnosis of ARSA and COCA is often made incidentally on diagnostic imaging studies such as endovascular imaging, CT angiography, magnetic resonance (MR) angiography, postmortem cadaveric dissections, or, as in our case, during cardiac catheterization.11,12 A classification system for aortic arch branching patterns exists published by Adachi and Williams.6 The classification includes ARSA and differentiates it into 4 subtypes (Figure 2). Our patient exhibited type H-1, indicating ARSA as the distal most branch of the aortic arch with coexistence of COCA.6 The primary clinical implication of ARSA and COCA in our case was increased difficulty and complexity when performing coronary angiography. Available literature has well characterized the challenges operators encounter when cannulating aberrant great vessel anatomy, often electing to perform nonselective aortography to define a patient’s anatomy.7,9,13 A comparison of diagnostic imaging techniques for vascular rings such as ARSA have shown MR, CT, and endovascular angiography to be the most reliable modalities to delineate vascular anatomy.14
Methods
Due to the presence of CABG in our patient, left radial and ulnar artery approaches were used rather than a right radial artery approach. Engagement of the LIMA is performed most commonly with left radial or femoral artery access using an internal mammary catheter that has a more steeply angled tip (80º-85º) compared with the standard JR catheter. An accessory left radial artery anatomic variant was encountered in our case precluding left radial approach. In addition, abnormal takeoffs of the great vessels thwarted multiple attempts at intubation of the LSA (Figure 1, right). Some data suggest CT imaging can be of assistance in establishing patency of bypass grafts in CABG patients.15 This can be considered an option if branch-vessel anatomy remains unclear. Our patient exhibited several risk factors for stroke, including female gender, hypertension, and prior CABG. These and other risk factors may influence clinical decisions such as continued catheter manipulation, choice of catheter type, and further contrast studies.16
Nonselective angiography in these cases often can require excessive iodinated contrast, exposing the patient to increased risk of contrast-induced nephropathy (CIN).7,17 Although the amount of contrast used in our case was average for diagnostic catheterization,the patient went on to undergo a second catheterization and CT angiography to establish LIMA graft patency.17 CT imaging reconstruction elucidated her aberrant branch-vessel anatomy. Patients are at increased risk of CIN with contrast loads < 200 mL per study, and this effect is compounded when the patient is elderly, has diabetes mellitus, and/or antecedent renal disease.18 Attention to the patient’s preoperative glomerular filtration rate, avoidance of nephrotoxic agents, and intraoperative left ventricular end-diastolic pressure during cardiac catheterization with postcontrast administration of IV isotonic fluids have been shown to prevent CIN.19,20 In the POSEIDON trial, fluid administration on a sliding scale based on the left ventricular end-diastolic pressure resulted in lower absolute risk of CIN postcatheterization vs standard postprocedure hydration in cardiac catheterization.21 Further, the now widespread use of low and iso-osmolar contrast agents further reduces the risk of CIN.22
For cardiac catheter laboratory operators, it is important to note that ARSA is more frequently encountered due to increased use of the transradial approach to coronary angiography.11 It should be suspected when accessing the ascending aorta proves exceptionally challenging and the catheter has a predilection for entering the descending aorta.11 While more technically demanding, 2 cases described by Allen and colleagues exhibited safe and successful entry into the ascending aorta with catheter rotation and hydrophilic support wires indicating the right radial approach is feasible despite presence of ARSA.12 Several patient-initiated maneuvers can be utilized to aid in accessing the ascending aorta. For example, deep inspiration to reduce the angulation between the aortic arch and ARSA. The use of curved catheters, such as Amplatz left, internal mammary catheter, or Simmons catheter may be considered to cannulate the ascending aorta if ARSA is encountered. Complications associated with a transradial approach include dissection and intramural hematoma. Minor bleeds and vasospasm also can occur secondary to increased procedural duration.6,8
Treatment
ARSA and COCA are considered normal anatomic variants and no treatment is indicated if the patient is asymptomatic. If symptoms are present, they often arise from aneurysmal or occlusive complications of the vascular anatomy. In patients with isolated ARSA and mild dysphasia or reflux symptoms, the use of prokinetics and antireflux medications may provide relief. It is important to note the coexistence of ARSA and COCA is more likely to produce esophageal compression compared to ARSA alone due to formation of a more complete vascular ring. Surgical management has been described in severe cases of ARSA involving risk of aneurysm rupture, right upper limb ischemia, or compression of the esophagus or trachea.
Several surgical approaches have been described, including simple ligation and division of ARSA and reimplantation of the RSA into the right CCA or ascending aorta.5 A recent review of 180 cases of ARSA diagnosed on CT angiography with concomitant common carotid trunk in half of studied individuals focused on a hybrid open and intravascular procedure. This procedure involved a double transposition or bypass (LSA to left common carotid artery and ARSA to the right CCA) followed by implantation of a thoracic stent graft. Few cases are eligible for these procedures or require them for definitive treatment.23
Conclusions
Recognition of aortic arch anatomical variants such as our case of ARSA with concomitant COCA may influence clinician decisions in various specialties, such as interventional cardiology, interventional neurology, cardiothoracic surgery, and gastroenterology. While most patients with these conditions are asymptomatic, some may present with dysphagia, dyspnea, and/or stroke symptoms. In our practice, discovery of such anomalies periprocedurally may affect cardiac catheterization access site, catheter selection, and additional imaging. The presence of arteria lusoria can be of critical importance when encountering a patient with myocardial infarction as switching from transradial to transfemoral approach may be required to gain access to the ascending aorta. Overall, transradial coronary angiography and percutaneous coronary intervention is not contraindicated in the setting of ARSA/COCA and can be safely performed by an experienced operator.
It is important for surgical specialists to be aware of the coexistence of anomalies where the discovery of one aberrancy can signal coexistent variant anatomy. If aortic arch anatomy is unclear, it is useful to perform nonselective angiography and/or further imaging with CT angiography. Knowledge of abnormal aortic arch anatomy can decrease fluoroscopy time and contrast load administered, thereby reducing potential periprocedural adverse events.
Branching of the great vessels from the aorta normally progresses with the brachiocephalic trunk as the first takeoff followed by the left common carotid and left subclavian artery in approximately 85% of cases.1 Variants of great vessel branching patterns include the so-called bovine arch, arteria lusoria or aberrant right subclavian artery (ARSA), aberrant origin of the vertebral arteries, and truncus bicaroticus, or common origin of the carotid arteries (COCA). These aberrancies are quite rare, some with an incidence of < 1%.1,2
These vascular anomalies become clinically relevant when they pose difficulty for operators in surgical and interventional specialties, necessitating unique approaches, catheters, and techniques to overcome. We present a case of concomitant aortic arch abnormalities during a diagnostic workup for transcatheter aortic valve replacement (TAVR) in a patient with previous coronary artery bypass grafting (CABG).
Case Presentation
A 66-year-old woman with coronary artery disease (CAD) status post-CABG and stage D1 aortic stenosis (AS) presented with exertional dyspnea. She was referred for coronary angiography as part of a workup for TAVR. Echocardiography confirmed severe AS with a peak velocity of 4.1 m/s, mean pressure gradient of 50 mm Hg, and an aortic valve area of 0.7 cm2. The patient was scheduled for cardiac catheterization with anticipated left radial artery approach for intubation and opacification of the left internal mammary artery (LIMA). However, this approach was abandoned during the procedure due to discovery of aberrant left radial artery anatomy, and the procedure was completed via femoral access.
Subsequent coronary angiography revealed 3-vessel CAD, patent saphenous vein grafts (SVG) to the right coronary artery (RCA) and a diagonal branch vessel with an occluded SVG to the left circumflex. Difficulty was encountered when engaging the left subclavian artery using a JR 4.0 diagnostic catheter for LIMA angiography. Nonselective angiography of the aortic arch was performed and demonstrated an uncommon anatomical variant (Figure 1, left). The right common carotid artery (CCA) [A] and the left CCA [B] arose from a single trunk, consistent with truncus bicaroticus or COCA [C]. The right subclavian artery [D] originated distal to the left subclavian artery otherwise known as arteria lusoria or ARSA forming an incomplete vascular ring [E]. Selective engagement of the left subclavian artery remained problematic even with the use of specialty arch catheters (Headhunter and LIMA catheters). The procedure concluded without confirming patency of the LIMA graft. A total of 145 mL of Omnipaque (iohexol injection) contrast was used for the procedure, and no adverse events occurred.
Same-day access of the ipsilateral ulnar artery was not pursued because of the risk of hand ischemia. The patient underwent repeat catheterization utilizing left ulnar artery access after adequate recovery time from the initial left radial approach. Selective LIMA angiography was achieved and demonstrated a patent LIMA to LAD graft. A computed tomography (CT) aorta for purposes of TAVR planning was able to reconstruct the aortic arch vasculature (Figure 1, right) confirming the presence of both ARSA and COCA. The patient went on to undergo successful TAVR with subsequent improvement of clinical symptoms.
Discussion
Arteria lusoria is defined as an anomalous right subclavian artery arising distal to the origin of the left subclavian artery on the aortic arch. It has an estimated incidence of 0.5 to 2% and occurs as a consequence of abnormal embryologic involution of the right fourth aortic arch and right proximal dorsal aorta. This causes the origin of the right subclavian artery to shift onto the descending aorta and cross the mediastinum from left to right, passing behind the esophagus and the trachea.1,3-5
ARSA is often associated with other anatomic abnormalities, including COCA, right-sided aortic arch, interrupted aortic arch, aortic coarctation, tetralogy of Fallot, truncus arteriosus, transposition of the great arteries, atrial septal defects, and ventricular septal defects.Underlying genetic disorders, such as Edwards, Down, DiGeorge syndromes, aneurysms, and arterioesophageal fistulae can accompany these vascular malformations.6
COCA, such as we encountered, is the presence of a single branch from the aorta giving off both right and left common carotid arteries. It has an incidence of < 0.1% in isolation and is discovered most often in cadaveric dissections or incidentally on imaging.1 Its embryologic origin results from the third pair of cervical aortic arches persisting as a common bicarotid trunk.1,4,5 The combination of ARSA and COCA is rare. Of the 0.5 to 2% of ARSA cases discovered, only 20% of those cases present with associated COCA for a combined prevalence estimated at < 0.05%.7
The majority of patients with either anatomic abnormality are asymptomatic. However, a few classic clinical manifestations have been described. ARSA can rarely present with dysphagia lusoria, a condition resulting from an incomplete vascular ring formed by the abnormal course of the right subclavian compressing the esophagus. Although not seen in our patient, it should be considered in the differential diagnosis for dysphagia.1,2,7 Ortner syndrome can result from right laryngeal nerve compression and palsy resultant from the aberrant course of the right subclavian artery.8 Another clinically relevant feature of ARSA is the presence of a diverticulum of Kommerell or dilatation at the origin of the right subclavian artery. It is a type of retroesophageal diverticulum resulting from persistence of a segment of the right sixth aortic arch.9 Finally, the spatial arrangement of ARSA increases risk for injury during head and neck surgical procedures, such as thyroidectomy, tracheotomy, and lymph node dissection of the right paratracheal fossa.6 Although the incidence is not well described, COCA has been described in several case reports as causing tracheal compression with dyspnea and in some cases, ischemic stroke.4,5,10
Diagnosis
The diagnosis of ARSA and COCA is often made incidentally on diagnostic imaging studies such as endovascular imaging, CT angiography, magnetic resonance (MR) angiography, postmortem cadaveric dissections, or, as in our case, during cardiac catheterization.11,12 A classification system for aortic arch branching patterns exists published by Adachi and Williams.6 The classification includes ARSA and differentiates it into 4 subtypes (Figure 2). Our patient exhibited type H-1, indicating ARSA as the distal most branch of the aortic arch with coexistence of COCA.6 The primary clinical implication of ARSA and COCA in our case was increased difficulty and complexity when performing coronary angiography. Available literature has well characterized the challenges operators encounter when cannulating aberrant great vessel anatomy, often electing to perform nonselective aortography to define a patient’s anatomy.7,9,13 A comparison of diagnostic imaging techniques for vascular rings such as ARSA have shown MR, CT, and endovascular angiography to be the most reliable modalities to delineate vascular anatomy.14
Methods
Due to the presence of CABG in our patient, left radial and ulnar artery approaches were used rather than a right radial artery approach. Engagement of the LIMA is performed most commonly with left radial or femoral artery access using an internal mammary catheter that has a more steeply angled tip (80º-85º) compared with the standard JR catheter. An accessory left radial artery anatomic variant was encountered in our case precluding left radial approach. In addition, abnormal takeoffs of the great vessels thwarted multiple attempts at intubation of the LSA (Figure 1, right). Some data suggest CT imaging can be of assistance in establishing patency of bypass grafts in CABG patients.15 This can be considered an option if branch-vessel anatomy remains unclear. Our patient exhibited several risk factors for stroke, including female gender, hypertension, and prior CABG. These and other risk factors may influence clinical decisions such as continued catheter manipulation, choice of catheter type, and further contrast studies.16
Nonselective angiography in these cases often can require excessive iodinated contrast, exposing the patient to increased risk of contrast-induced nephropathy (CIN).7,17 Although the amount of contrast used in our case was average for diagnostic catheterization,the patient went on to undergo a second catheterization and CT angiography to establish LIMA graft patency.17 CT imaging reconstruction elucidated her aberrant branch-vessel anatomy. Patients are at increased risk of CIN with contrast loads < 200 mL per study, and this effect is compounded when the patient is elderly, has diabetes mellitus, and/or antecedent renal disease.18 Attention to the patient’s preoperative glomerular filtration rate, avoidance of nephrotoxic agents, and intraoperative left ventricular end-diastolic pressure during cardiac catheterization with postcontrast administration of IV isotonic fluids have been shown to prevent CIN.19,20 In the POSEIDON trial, fluid administration on a sliding scale based on the left ventricular end-diastolic pressure resulted in lower absolute risk of CIN postcatheterization vs standard postprocedure hydration in cardiac catheterization.21 Further, the now widespread use of low and iso-osmolar contrast agents further reduces the risk of CIN.22
For cardiac catheter laboratory operators, it is important to note that ARSA is more frequently encountered due to increased use of the transradial approach to coronary angiography.11 It should be suspected when accessing the ascending aorta proves exceptionally challenging and the catheter has a predilection for entering the descending aorta.11 While more technically demanding, 2 cases described by Allen and colleagues exhibited safe and successful entry into the ascending aorta with catheter rotation and hydrophilic support wires indicating the right radial approach is feasible despite presence of ARSA.12 Several patient-initiated maneuvers can be utilized to aid in accessing the ascending aorta. For example, deep inspiration to reduce the angulation between the aortic arch and ARSA. The use of curved catheters, such as Amplatz left, internal mammary catheter, or Simmons catheter may be considered to cannulate the ascending aorta if ARSA is encountered. Complications associated with a transradial approach include dissection and intramural hematoma. Minor bleeds and vasospasm also can occur secondary to increased procedural duration.6,8
Treatment
ARSA and COCA are considered normal anatomic variants and no treatment is indicated if the patient is asymptomatic. If symptoms are present, they often arise from aneurysmal or occlusive complications of the vascular anatomy. In patients with isolated ARSA and mild dysphasia or reflux symptoms, the use of prokinetics and antireflux medications may provide relief. It is important to note the coexistence of ARSA and COCA is more likely to produce esophageal compression compared to ARSA alone due to formation of a more complete vascular ring. Surgical management has been described in severe cases of ARSA involving risk of aneurysm rupture, right upper limb ischemia, or compression of the esophagus or trachea.
Several surgical approaches have been described, including simple ligation and division of ARSA and reimplantation of the RSA into the right CCA or ascending aorta.5 A recent review of 180 cases of ARSA diagnosed on CT angiography with concomitant common carotid trunk in half of studied individuals focused on a hybrid open and intravascular procedure. This procedure involved a double transposition or bypass (LSA to left common carotid artery and ARSA to the right CCA) followed by implantation of a thoracic stent graft. Few cases are eligible for these procedures or require them for definitive treatment.23
Conclusions
Recognition of aortic arch anatomical variants such as our case of ARSA with concomitant COCA may influence clinician decisions in various specialties, such as interventional cardiology, interventional neurology, cardiothoracic surgery, and gastroenterology. While most patients with these conditions are asymptomatic, some may present with dysphagia, dyspnea, and/or stroke symptoms. In our practice, discovery of such anomalies periprocedurally may affect cardiac catheterization access site, catheter selection, and additional imaging. The presence of arteria lusoria can be of critical importance when encountering a patient with myocardial infarction as switching from transradial to transfemoral approach may be required to gain access to the ascending aorta. Overall, transradial coronary angiography and percutaneous coronary intervention is not contraindicated in the setting of ARSA/COCA and can be safely performed by an experienced operator.
It is important for surgical specialists to be aware of the coexistence of anomalies where the discovery of one aberrancy can signal coexistent variant anatomy. If aortic arch anatomy is unclear, it is useful to perform nonselective angiography and/or further imaging with CT angiography. Knowledge of abnormal aortic arch anatomy can decrease fluoroscopy time and contrast load administered, thereby reducing potential periprocedural adverse events.
1. Kurt MA, An I, Ikiz I. A case with coincidence of aberrant right subclavian artery and common origin of the carotid arteries. Ann Anat. 1997;179(2):175-176. doi:10.1016/s0940-9602(97)80100-8
2. Klinkhamer AC. Aberrant right subclavian artery. Clinical and roentgenologic aspects. Am J Roentgenol Radium Ther Nucl Med. 1966;97(2):438-446. doi:10.2214/ajr.97.2.438
3. Türkvatan A, Büyükbayraktar FG, Olçer T, Cumhur T. Congenital anomalies of the aortic arch: evaluation with the use of multidetector computed tomography. Korean J Radiol. 2009;10(2):176-184. doi:10.3348/kjr.2009.10.2.176
4. Ozateş M, Nazaroglu H, Uyar A. MR angiography in diagnosis of aberrant right subclavian artery associated with common carotid trunk. Eur Radiol. 2000;10(9):1503. doi:10.1007/s003300000335
5. Poultsides GA, Lolis ED, Vasquez J, Drezner AD, Venieratos D. Common origins of carotid and subclavian arterial systems: report of a rare aortic arch variant. Ann Vasc Surg. 2004;18(5):597-600. doi:10.1007/s10016-004-0060-3
6. Leite TFO, Pires LAS, Cisne R, Babinski MA, Chagas CAA. Clinical discussion of the arteria lusoria: a case report. J Vasc Bras. 2017;16(4):339-342. doi:10.1590/1677-5449.007617
7. Tsai IC, Tzeng WS, Lee T, et al. Vertebral and carotid artery anomalies in patients with aberrant right subclavian arteries. Pediatr Radiol. 2007;37(10):1007-1012. doi:10.1007/s00247-007-0574-2
8. Rafiq A, Chutani S, Krim NR. Incidental finding of arteria lusoria during transradial coronary catheterization: significance in interventional cardiology. Catheter Cardiovasc Interv. 2018;91(7):1283-1286. doi:10.1002/ccd.27439
9. Priya S, Thomas R, Nagpal P, Sharma A, Steigner M. Congenital anomalies of the aortic arch. Cardiovasc Diagn Ther. 2018;8(suppl 1):S26-S44. doi:10.21037/cdt.2017.10.15
10. Khatri R, Maud A, Rodriguez GJ. Aberrant right subclavian artery and common carotid trunk. J Vasc Interv Neurol. 2010;3(1):33-34.
11. Valsecchi O, Vassileva A, Musumeci G, et al. Failure of transradial approach during coronary interventions: anatomic considerations. Catheter Cardiovasc Interv. 2006;67(6):870-878. doi:10.1002/ccd.20732
12. Allen D, Bews H, Vo M, Kass M, Jassal DS, Ravandi A. Arteria lusoria: an anomalous finding during right transradial coronary intervention. Case Rep Cardiol. 2016;2016:8079856. doi:10.1155/2016/8079856
13. Fineschi M, Iadanza A, Sinicropi G, Pierli C. Images in cardiology: angiographic evidence of aberrant right subclavian artery associated with common carotid trunk. Heart. 2002;88(2):158. doi:10.1136/heart.88.2.158
14. van Son JA, Julsrud PR, Hagler DJ, et al. Imaging strategies for vascular rings. Ann Thorac Surg. 1994;57(3):604-610. doi:10.1016/0003-4975(94)90552-5
15. Lee R, Lim J, Kaw G, Wan G, Ng K, Ho KT. Comprehensive noninvasive evaluation of bypass grafts and native coronary arteries in patients after coronary bypass surgery: accuracy of 64-slice multidetector computed tomography compared to invasive coronary angiography. J Cardiovasc Med (Hagerstown). 2010;11(2):81-90. doi:10.2459/JCM.0b013e32832f3e2e
16. Hamon M, Baron JC, Viader F, Hamon M. Periprocedural stroke and cardiac catheterization. Circulation. 2008;118(6): 678-683. doi:10.1161/CIRCULATIONAHA.108.784504
17. Hwang JR, D’Alfonso S, Kostuk WJ, et al. Contrast volume use in manual vs automated contrast injection systems for diagnostic coronary angiography and percutaneous coronary interventions. Can J Cardiol. 2013;29(3):372-376. doi:10.1016/j.cjca.2012.11.023
18. Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150(6):1237-1242.
19. Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-728. doi:10.1148/radiol.13122276
20. American College of Radiology. ACR Manual on Contrast Media 2020. American College of Radiology; 2020:33-34. Accessed January 15, 2021. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
21. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383(9931):1814-1823. doi:10.1016/S0140-6736(14)60689-9
22. Aoun J, Nicolas D, Brown JR, Jaber BL. Maximum allowable contrast dose and prevention of acute kidney injury following cardiovascular procedures. Curr Opin Nephrol Hypertens. 2018;27(2):121-129. doi:10.1097/MNH.0000000000000389
23. Settembre N, Saba C, Bouziane Z, Jeannon F, Mandry D, Malikov S. Hybrid treatment of the aberrant right subclavian artery (arteria lusoria): feasibility study on 180 angio-CTs. Ann Vasc Surg. 2017;44:229-233. doi:10.1016/j.avsg.2017.03.172
1. Kurt MA, An I, Ikiz I. A case with coincidence of aberrant right subclavian artery and common origin of the carotid arteries. Ann Anat. 1997;179(2):175-176. doi:10.1016/s0940-9602(97)80100-8
2. Klinkhamer AC. Aberrant right subclavian artery. Clinical and roentgenologic aspects. Am J Roentgenol Radium Ther Nucl Med. 1966;97(2):438-446. doi:10.2214/ajr.97.2.438
3. Türkvatan A, Büyükbayraktar FG, Olçer T, Cumhur T. Congenital anomalies of the aortic arch: evaluation with the use of multidetector computed tomography. Korean J Radiol. 2009;10(2):176-184. doi:10.3348/kjr.2009.10.2.176
4. Ozateş M, Nazaroglu H, Uyar A. MR angiography in diagnosis of aberrant right subclavian artery associated with common carotid trunk. Eur Radiol. 2000;10(9):1503. doi:10.1007/s003300000335
5. Poultsides GA, Lolis ED, Vasquez J, Drezner AD, Venieratos D. Common origins of carotid and subclavian arterial systems: report of a rare aortic arch variant. Ann Vasc Surg. 2004;18(5):597-600. doi:10.1007/s10016-004-0060-3
6. Leite TFO, Pires LAS, Cisne R, Babinski MA, Chagas CAA. Clinical discussion of the arteria lusoria: a case report. J Vasc Bras. 2017;16(4):339-342. doi:10.1590/1677-5449.007617
7. Tsai IC, Tzeng WS, Lee T, et al. Vertebral and carotid artery anomalies in patients with aberrant right subclavian arteries. Pediatr Radiol. 2007;37(10):1007-1012. doi:10.1007/s00247-007-0574-2
8. Rafiq A, Chutani S, Krim NR. Incidental finding of arteria lusoria during transradial coronary catheterization: significance in interventional cardiology. Catheter Cardiovasc Interv. 2018;91(7):1283-1286. doi:10.1002/ccd.27439
9. Priya S, Thomas R, Nagpal P, Sharma A, Steigner M. Congenital anomalies of the aortic arch. Cardiovasc Diagn Ther. 2018;8(suppl 1):S26-S44. doi:10.21037/cdt.2017.10.15
10. Khatri R, Maud A, Rodriguez GJ. Aberrant right subclavian artery and common carotid trunk. J Vasc Interv Neurol. 2010;3(1):33-34.
11. Valsecchi O, Vassileva A, Musumeci G, et al. Failure of transradial approach during coronary interventions: anatomic considerations. Catheter Cardiovasc Interv. 2006;67(6):870-878. doi:10.1002/ccd.20732
12. Allen D, Bews H, Vo M, Kass M, Jassal DS, Ravandi A. Arteria lusoria: an anomalous finding during right transradial coronary intervention. Case Rep Cardiol. 2016;2016:8079856. doi:10.1155/2016/8079856
13. Fineschi M, Iadanza A, Sinicropi G, Pierli C. Images in cardiology: angiographic evidence of aberrant right subclavian artery associated with common carotid trunk. Heart. 2002;88(2):158. doi:10.1136/heart.88.2.158
14. van Son JA, Julsrud PR, Hagler DJ, et al. Imaging strategies for vascular rings. Ann Thorac Surg. 1994;57(3):604-610. doi:10.1016/0003-4975(94)90552-5
15. Lee R, Lim J, Kaw G, Wan G, Ng K, Ho KT. Comprehensive noninvasive evaluation of bypass grafts and native coronary arteries in patients after coronary bypass surgery: accuracy of 64-slice multidetector computed tomography compared to invasive coronary angiography. J Cardiovasc Med (Hagerstown). 2010;11(2):81-90. doi:10.2459/JCM.0b013e32832f3e2e
16. Hamon M, Baron JC, Viader F, Hamon M. Periprocedural stroke and cardiac catheterization. Circulation. 2008;118(6): 678-683. doi:10.1161/CIRCULATIONAHA.108.784504
17. Hwang JR, D’Alfonso S, Kostuk WJ, et al. Contrast volume use in manual vs automated contrast injection systems for diagnostic coronary angiography and percutaneous coronary interventions. Can J Cardiol. 2013;29(3):372-376. doi:10.1016/j.cjca.2012.11.023
18. Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150(6):1237-1242.
19. Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-728. doi:10.1148/radiol.13122276
20. American College of Radiology. ACR Manual on Contrast Media 2020. American College of Radiology; 2020:33-34. Accessed January 15, 2021. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf
21. Brar SS, Aharonian V, Mansukhani P, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383(9931):1814-1823. doi:10.1016/S0140-6736(14)60689-9
22. Aoun J, Nicolas D, Brown JR, Jaber BL. Maximum allowable contrast dose and prevention of acute kidney injury following cardiovascular procedures. Curr Opin Nephrol Hypertens. 2018;27(2):121-129. doi:10.1097/MNH.0000000000000389
23. Settembre N, Saba C, Bouziane Z, Jeannon F, Mandry D, Malikov S. Hybrid treatment of the aberrant right subclavian artery (arteria lusoria): feasibility study on 180 angio-CTs. Ann Vasc Surg. 2017;44:229-233. doi:10.1016/j.avsg.2017.03.172
A Case Series of Catheter-Directed Thrombolysis With Mechanical Thrombectomy for Treating Severe Deep Vein Thrombosis
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066