User login
Successful accelerated taper for sleeping aid
THE CASE
A 49-year-old man with chronic insomnia was referred to the pharmacist authors (LF and DP) to initiate and manage the tapering of nightly zolpidem use. Per chart review, the patient had complaints of insomnia for more than 30 years. His care had been transferred to a Nebraska clinic 5 years earlier, with a medication list that included zolpidem controlled release (CR) 12.5 mg nightly. Since then, multiple interventions to achieve cessation had been tried, including counseling on sleep hygiene, adjunct antidepressant use, and abrupt discontinuation. Each of these methods was unsuccessful. So, his family physician (SS) reached out to the pharmacist authors (LF and DP).
THE APPROACH
Due to the patient’s long history of zolpidem use, a lack of literature on the topic, and worry for withdrawal symptoms, a taper schedule was designed utilizing various benzodiazepine taper resources for guidance. The proposed taper utilized 5-mg immediate release (IR) tablets to ensure ease of tapering. The taper ranged from 20% to 43% weekly reductions based on the ability to split the zolpidem tablet in half.
DISCUSSION
Zolpidem is a sedative-hypnotic medication indicated for the treatment of insomnia when used at therapeutic dosing (ie, 5 to 10 mg nightly). Anecdotal efficacy, accompanied by weak chronic insomnia guideline recommendations, has led prescribers to use zolpidem as a chronic medication to treat insomnia.1,2 There is evidence of dependence and possible seizures from supratherapeutic zolpidem doses in the hundreds of milligrams, raising safety concerns regarding abuse, dependence, and withdrawal seizures in chronic use.2,3
Additionally, there is limited evidence regarding the appropriate process of discontinuing zolpidem after chronic use.2 Often a taper schedule—similar to those used with benzodiazepine medications—is used as a reference for discontinuation.1 The hypothetical goal of a taper is to prevent withdrawal effects such as rebound insomnia, anxiety, palpitations, and seizures.3 However, an extended taper may not actually be necessary with chronic zolpidem patients.
Tapering with minimal adverse effects
Pharmacokinetic and pharmacodynamic studies have suggested minimal, if not complete, absence of rebound or withdrawal effects with short-term zolpidem use.4 The same appears to be true of patients with long-term use. In a study, Roehrs and colleagues5 explored whether long-term treatment (defined as 8 months) caused rebound insomnia upon abrupt withdrawal. The investigators concluded that people with primary insomnia do not experience rebound insomnia or withdrawal symptoms with chronic, therapeutic dosing.
Another study involving 92 elderly patients on long-term treatment of zolpidem (defined as > 1 month, with average around 9.9 ± 6.2 years) experienced only 1 or 2 nights of rebound insomnia during a month-long taper.1,6 Following that, they experienced improvements in initiation and staying asleep.
A possible explanation for the lack of dependence or withdrawal symptoms in patients chronically treated with zolpidem is the pharmacokinetic profile. While the selectivity of the binding sites differentiates this medication from benzodiazepines, the additional fact of a short half-life, and no repeated dosing throughout the day, likely limit the risk of experiencing withdrawal symptoms.1 The daily periods of minimal zolpidem exposure in the body may limit the amount of physical dependence.
Continue to: Discontinuation of zolpidem
Discontinuation of zolpidem
The 49-year-old man had a history of failed abrupt discontinuation of zolpidem in the past (without noted withdrawal symptoms). Thus, various benzodiazepine taper resources were consulted to develop a taper schedule.
We switched our patient from the zolpidem CR 12.5 mg nightly to 10 mg of the IR formulation, and the pharmacists proposed 20% to 43% weekly decreases in dosing based on dosage strengths. At the initial 3-day follow-up (having taken 10 mg nightly for 3 days), the patient reported a quicker onset of sleep but an inability to sleep through the night. The patient denied withdrawal symptoms or any significant impact to his daily routines. These results encouraged a progression to the next step of the taper. For the next 9 days, the patient took 5 mg nightly, rather than the pharmacist-advised dosing of alternating 5 mg and 10 mg nightly, and reported similar outcomes at his next visit.
This success led to the discontinuation of scheduled zolpidem. The patient was also given a prescription of 2.5 mg, as needed, if insomnia rebounded. No adverse effects were noted despite the accelerated taper. Based on patient response and motivation, the taper had progressed more quickly than scheduled, resulting in 3 days of 10 mg, 9 days of 5 mg, and 1 final day of 2.5 mg that was used when the patient had trouble falling asleep. At the 6-month follow-up, the patient informed the physician that he had neither experienced insomnia nor used any further medication.
THE TAKEAWAY
This case documents a successfully accelerated taper for a patient with a chronic history (> 5 years) of zolpidem use. Although withdrawal is often patient specific, this case suggests the risk is low despite the chronic usage. This further adds to the literature suggesting against the need for an extended taper, and possibly a taper at all, when using recommended doses of chronic zolpidem. This is a significant difference compared to past practices that drew from literature-based benzodiazepine tapers.6 This case serves as an observational point of reference for clinicians who are assisting patients with chronic zolpidem tapers.
CORRESPONDENCE
Logan Franck, PharmD, 986145 Nebraska Medical Center, Omaha, NE 68198-6145; [email protected]
1. Lähteenmäki R, Neuvonen PJ, Puustinen J, et al. Withdrawal from long-term use of zopiclone, zolpidem and temazepam may improve perceived sleep and quality of life in older adults with primary insomnia. Basic Clin Pharmacol Toxicol. 2019;124:330-340. doi: 10.1111/bcpt.13144
2. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
3. Haji Seyed Javadi SA, Hajiali F, Nassiri-Asl M. Zolpidem dependency and withdrawal seizure: a case report study. Iran Red Crescent Med J. 2014;16:e19926. doi: 10.5812/ircmj.19926
4. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29:142-153. doi: 10.2165/00003088-199529030-00002
5. Roehrs TA, Randall S, Harris E, et al. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol. 2012;26:1088-1095. doi: 10.1177/0269881111424455
6. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol 2014;77:295-301. doi: 10.1111/j.1365-2125.2012.04418.x
THE CASE
A 49-year-old man with chronic insomnia was referred to the pharmacist authors (LF and DP) to initiate and manage the tapering of nightly zolpidem use. Per chart review, the patient had complaints of insomnia for more than 30 years. His care had been transferred to a Nebraska clinic 5 years earlier, with a medication list that included zolpidem controlled release (CR) 12.5 mg nightly. Since then, multiple interventions to achieve cessation had been tried, including counseling on sleep hygiene, adjunct antidepressant use, and abrupt discontinuation. Each of these methods was unsuccessful. So, his family physician (SS) reached out to the pharmacist authors (LF and DP).
THE APPROACH
Due to the patient’s long history of zolpidem use, a lack of literature on the topic, and worry for withdrawal symptoms, a taper schedule was designed utilizing various benzodiazepine taper resources for guidance. The proposed taper utilized 5-mg immediate release (IR) tablets to ensure ease of tapering. The taper ranged from 20% to 43% weekly reductions based on the ability to split the zolpidem tablet in half.
DISCUSSION
Zolpidem is a sedative-hypnotic medication indicated for the treatment of insomnia when used at therapeutic dosing (ie, 5 to 10 mg nightly). Anecdotal efficacy, accompanied by weak chronic insomnia guideline recommendations, has led prescribers to use zolpidem as a chronic medication to treat insomnia.1,2 There is evidence of dependence and possible seizures from supratherapeutic zolpidem doses in the hundreds of milligrams, raising safety concerns regarding abuse, dependence, and withdrawal seizures in chronic use.2,3
Additionally, there is limited evidence regarding the appropriate process of discontinuing zolpidem after chronic use.2 Often a taper schedule—similar to those used with benzodiazepine medications—is used as a reference for discontinuation.1 The hypothetical goal of a taper is to prevent withdrawal effects such as rebound insomnia, anxiety, palpitations, and seizures.3 However, an extended taper may not actually be necessary with chronic zolpidem patients.
Tapering with minimal adverse effects
Pharmacokinetic and pharmacodynamic studies have suggested minimal, if not complete, absence of rebound or withdrawal effects with short-term zolpidem use.4 The same appears to be true of patients with long-term use. In a study, Roehrs and colleagues5 explored whether long-term treatment (defined as 8 months) caused rebound insomnia upon abrupt withdrawal. The investigators concluded that people with primary insomnia do not experience rebound insomnia or withdrawal symptoms with chronic, therapeutic dosing.
Another study involving 92 elderly patients on long-term treatment of zolpidem (defined as > 1 month, with average around 9.9 ± 6.2 years) experienced only 1 or 2 nights of rebound insomnia during a month-long taper.1,6 Following that, they experienced improvements in initiation and staying asleep.
A possible explanation for the lack of dependence or withdrawal symptoms in patients chronically treated with zolpidem is the pharmacokinetic profile. While the selectivity of the binding sites differentiates this medication from benzodiazepines, the additional fact of a short half-life, and no repeated dosing throughout the day, likely limit the risk of experiencing withdrawal symptoms.1 The daily periods of minimal zolpidem exposure in the body may limit the amount of physical dependence.
Continue to: Discontinuation of zolpidem
Discontinuation of zolpidem
The 49-year-old man had a history of failed abrupt discontinuation of zolpidem in the past (without noted withdrawal symptoms). Thus, various benzodiazepine taper resources were consulted to develop a taper schedule.
We switched our patient from the zolpidem CR 12.5 mg nightly to 10 mg of the IR formulation, and the pharmacists proposed 20% to 43% weekly decreases in dosing based on dosage strengths. At the initial 3-day follow-up (having taken 10 mg nightly for 3 days), the patient reported a quicker onset of sleep but an inability to sleep through the night. The patient denied withdrawal symptoms or any significant impact to his daily routines. These results encouraged a progression to the next step of the taper. For the next 9 days, the patient took 5 mg nightly, rather than the pharmacist-advised dosing of alternating 5 mg and 10 mg nightly, and reported similar outcomes at his next visit.
This success led to the discontinuation of scheduled zolpidem. The patient was also given a prescription of 2.5 mg, as needed, if insomnia rebounded. No adverse effects were noted despite the accelerated taper. Based on patient response and motivation, the taper had progressed more quickly than scheduled, resulting in 3 days of 10 mg, 9 days of 5 mg, and 1 final day of 2.5 mg that was used when the patient had trouble falling asleep. At the 6-month follow-up, the patient informed the physician that he had neither experienced insomnia nor used any further medication.
THE TAKEAWAY
This case documents a successfully accelerated taper for a patient with a chronic history (> 5 years) of zolpidem use. Although withdrawal is often patient specific, this case suggests the risk is low despite the chronic usage. This further adds to the literature suggesting against the need for an extended taper, and possibly a taper at all, when using recommended doses of chronic zolpidem. This is a significant difference compared to past practices that drew from literature-based benzodiazepine tapers.6 This case serves as an observational point of reference for clinicians who are assisting patients with chronic zolpidem tapers.
CORRESPONDENCE
Logan Franck, PharmD, 986145 Nebraska Medical Center, Omaha, NE 68198-6145; [email protected]
THE CASE
A 49-year-old man with chronic insomnia was referred to the pharmacist authors (LF and DP) to initiate and manage the tapering of nightly zolpidem use. Per chart review, the patient had complaints of insomnia for more than 30 years. His care had been transferred to a Nebraska clinic 5 years earlier, with a medication list that included zolpidem controlled release (CR) 12.5 mg nightly. Since then, multiple interventions to achieve cessation had been tried, including counseling on sleep hygiene, adjunct antidepressant use, and abrupt discontinuation. Each of these methods was unsuccessful. So, his family physician (SS) reached out to the pharmacist authors (LF and DP).
THE APPROACH
Due to the patient’s long history of zolpidem use, a lack of literature on the topic, and worry for withdrawal symptoms, a taper schedule was designed utilizing various benzodiazepine taper resources for guidance. The proposed taper utilized 5-mg immediate release (IR) tablets to ensure ease of tapering. The taper ranged from 20% to 43% weekly reductions based on the ability to split the zolpidem tablet in half.
DISCUSSION
Zolpidem is a sedative-hypnotic medication indicated for the treatment of insomnia when used at therapeutic dosing (ie, 5 to 10 mg nightly). Anecdotal efficacy, accompanied by weak chronic insomnia guideline recommendations, has led prescribers to use zolpidem as a chronic medication to treat insomnia.1,2 There is evidence of dependence and possible seizures from supratherapeutic zolpidem doses in the hundreds of milligrams, raising safety concerns regarding abuse, dependence, and withdrawal seizures in chronic use.2,3
Additionally, there is limited evidence regarding the appropriate process of discontinuing zolpidem after chronic use.2 Often a taper schedule—similar to those used with benzodiazepine medications—is used as a reference for discontinuation.1 The hypothetical goal of a taper is to prevent withdrawal effects such as rebound insomnia, anxiety, palpitations, and seizures.3 However, an extended taper may not actually be necessary with chronic zolpidem patients.
Tapering with minimal adverse effects
Pharmacokinetic and pharmacodynamic studies have suggested minimal, if not complete, absence of rebound or withdrawal effects with short-term zolpidem use.4 The same appears to be true of patients with long-term use. In a study, Roehrs and colleagues5 explored whether long-term treatment (defined as 8 months) caused rebound insomnia upon abrupt withdrawal. The investigators concluded that people with primary insomnia do not experience rebound insomnia or withdrawal symptoms with chronic, therapeutic dosing.
Another study involving 92 elderly patients on long-term treatment of zolpidem (defined as > 1 month, with average around 9.9 ± 6.2 years) experienced only 1 or 2 nights of rebound insomnia during a month-long taper.1,6 Following that, they experienced improvements in initiation and staying asleep.
A possible explanation for the lack of dependence or withdrawal symptoms in patients chronically treated with zolpidem is the pharmacokinetic profile. While the selectivity of the binding sites differentiates this medication from benzodiazepines, the additional fact of a short half-life, and no repeated dosing throughout the day, likely limit the risk of experiencing withdrawal symptoms.1 The daily periods of minimal zolpidem exposure in the body may limit the amount of physical dependence.
Continue to: Discontinuation of zolpidem
Discontinuation of zolpidem
The 49-year-old man had a history of failed abrupt discontinuation of zolpidem in the past (without noted withdrawal symptoms). Thus, various benzodiazepine taper resources were consulted to develop a taper schedule.
We switched our patient from the zolpidem CR 12.5 mg nightly to 10 mg of the IR formulation, and the pharmacists proposed 20% to 43% weekly decreases in dosing based on dosage strengths. At the initial 3-day follow-up (having taken 10 mg nightly for 3 days), the patient reported a quicker onset of sleep but an inability to sleep through the night. The patient denied withdrawal symptoms or any significant impact to his daily routines. These results encouraged a progression to the next step of the taper. For the next 9 days, the patient took 5 mg nightly, rather than the pharmacist-advised dosing of alternating 5 mg and 10 mg nightly, and reported similar outcomes at his next visit.
This success led to the discontinuation of scheduled zolpidem. The patient was also given a prescription of 2.5 mg, as needed, if insomnia rebounded. No adverse effects were noted despite the accelerated taper. Based on patient response and motivation, the taper had progressed more quickly than scheduled, resulting in 3 days of 10 mg, 9 days of 5 mg, and 1 final day of 2.5 mg that was used when the patient had trouble falling asleep. At the 6-month follow-up, the patient informed the physician that he had neither experienced insomnia nor used any further medication.
THE TAKEAWAY
This case documents a successfully accelerated taper for a patient with a chronic history (> 5 years) of zolpidem use. Although withdrawal is often patient specific, this case suggests the risk is low despite the chronic usage. This further adds to the literature suggesting against the need for an extended taper, and possibly a taper at all, when using recommended doses of chronic zolpidem. This is a significant difference compared to past practices that drew from literature-based benzodiazepine tapers.6 This case serves as an observational point of reference for clinicians who are assisting patients with chronic zolpidem tapers.
CORRESPONDENCE
Logan Franck, PharmD, 986145 Nebraska Medical Center, Omaha, NE 68198-6145; [email protected]
1. Lähteenmäki R, Neuvonen PJ, Puustinen J, et al. Withdrawal from long-term use of zopiclone, zolpidem and temazepam may improve perceived sleep and quality of life in older adults with primary insomnia. Basic Clin Pharmacol Toxicol. 2019;124:330-340. doi: 10.1111/bcpt.13144
2. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
3. Haji Seyed Javadi SA, Hajiali F, Nassiri-Asl M. Zolpidem dependency and withdrawal seizure: a case report study. Iran Red Crescent Med J. 2014;16:e19926. doi: 10.5812/ircmj.19926
4. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29:142-153. doi: 10.2165/00003088-199529030-00002
5. Roehrs TA, Randall S, Harris E, et al. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol. 2012;26:1088-1095. doi: 10.1177/0269881111424455
6. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol 2014;77:295-301. doi: 10.1111/j.1365-2125.2012.04418.x
1. Lähteenmäki R, Neuvonen PJ, Puustinen J, et al. Withdrawal from long-term use of zopiclone, zolpidem and temazepam may improve perceived sleep and quality of life in older adults with primary insomnia. Basic Clin Pharmacol Toxicol. 2019;124:330-340. doi: 10.1111/bcpt.13144
2. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307-349. doi: 10.5664/jcsm.6470
3. Haji Seyed Javadi SA, Hajiali F, Nassiri-Asl M. Zolpidem dependency and withdrawal seizure: a case report study. Iran Red Crescent Med J. 2014;16:e19926. doi: 10.5812/ircmj.19926
4. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29:142-153. doi: 10.2165/00003088-199529030-00002
5. Roehrs TA, Randall S, Harris E, et al. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol. 2012;26:1088-1095. doi: 10.1177/0269881111424455
6. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol 2014;77:295-301. doi: 10.1111/j.1365-2125.2012.04418.x
Long QT and Cardiac Arrest After Symptomatic Improvement of Pulmonary Edema
A case of extreme QT prolongation induced following symptomatic resolution of acute pulmonary edema is both relatively unknown and poorly understood.
Abnormalities in the T-wave morphology of an electrocardiogram (ECG) are classically attributed to ischemic cardiac disease. However, these changes can be seen in a variety of other etiologies, including noncardiac pathology, which should be considered whenever reviewing an ECG: central nervous system disease, including stroke and subarachnoid hemorrhage; hypothermia; pulmonary disease, such as pulmonary embolism or chronic obstructive pulmonary disease; myopericarditis; drug effects; and electrolyte abnormalities.
Prolongation of the QT interval, on the other hand, can be precipitated by medications, metabolic derangements, or genetic phenotypes. The QT interval is measured from the beginning of the QRS complex to the termination of the T wave and represents the total time for ventricular depolarization and repolarization. The QT interval must be corrected based on the patient’s heart rate, known as the QTc. As the QTc interval lengthens, there is increased risk of R-on-T phenomena, which may result in Torsades de Pointes (TdP). Typical features of TdP include an antecedent prolonged QTc, cyclic polymorphic ventricular tachycardia on the surface ECG, and either a short-lived spontaneously terminating course or degeneration into ventricular fibrillation (VF) and sudden cardiac death.1 These dysrhythmias become more likely as the QTc interval exceeds 500 msec.2
The combination of new-onset global T-wave inversions with prolongation of the QT interval has been reported in only a few limited conditions. Some known causes of these QT T changes include cardiac ischemia, status epilepticus, pheochromocytoma, and acute cocaine intoxication.3 One uncommon and rarely reported cause of extreme QT prolongation and T-wave inversion is acute pulmonary edema. The ECG findings are not present on initial patient presentation; rather the dynamic changes occur after resolution of the pulmonary symptoms. Despite significant ECG changes, all prior reported cases describe ECG normalization without significant morbidity.4,5 We report a case of extreme QT prolongation following acute pulmonary edema that resulted in cardiac arrest secondary to VF.
Case Presentation
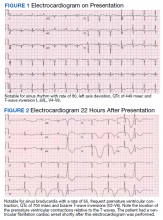
A 72-year-old male with medical history of combined systolic and diastolic heart failure, ischemic cardiomyopathy, coronary artery disease, cerebral vascular accident, hypertension, hyperlipidemia, type 2 diabetes mellitus, and tobacco dependence presented to the emergency department (ED) by emergency medical services after awaking with acute onset of dyspnea and diaphoresis. On arrival at the ED, the patient was noted to be in respiratory distress (ie, unable to speak single words) and was extremely diaphoretic. His initial vital signs included blood pressure, 186/113 mm Hg, heart rate, 104 beats per minute, respiratory rate, 40 breaths per minute, and temperature, 36.4 °C. The patient was quickly placed on bilevel positive airway pressure and given sublingual nitroglycerin followed by transdermal nitroglycerin with a single dose of 40 mg IV furosemide, which improved his respiratory status. A chest X-ray was consistent with pulmonary edema, and his brain natriuretic peptide was 1654 pg/mL. An ECG demonstrated new T-wave inversions, and his troponin increased from 0.04 to 0.24 ng/mL during his ED stay (Figure 1). He was started on a heparin infusion and admitted to the hospital for hypertensive emergency with presumed acute decompensated heart failure and non-ST-elevated myocardial infarction.
Throughout the patient’s first night, the troponin level started to down-trend after peaking at 0.24 ng/mL, and his oxygen requirements decreased allowing transition to nasal cannula. However, his repeat ECGs demonstrated significant T-wave abnormalities, new premature ventricular contractions, bradycardia, and a prolonging QTc interval to 703 msec (Figure 2). At this time, the patient’s electrolytes were normal, specifically a potassium level of 4.4 mEq/L, calcium 8.8 mg/dL, magnesium 2.0 mg/dL, and phosphorus 2.6 mg/dL. Given the worsening ECG changes, a computed tomography scan of his head was ordered to rule out intracranial pathology. While in the scanner, the patient went into pulseless VF, prompting defibrillation with 200 J. In addition, he was given 75 mg IV lidocaine, 2 g IV magnesium, and 1 ampule of both calcium chloride and sodium bicarbonate. With treatment, he had return of spontaneous circulation and was taken promptly to cardiac catheterization. The catheterization showed no significant obstructive coronary artery disease, and no interventions were performed. The patient was transferred to the cardiac intensive care unit for continued care.
During his course in the intensive care unit, the patient’s potassium and magnesium levels were maintained at high-normal levels. The patient was started on a dobutamine infusion to increase his heart rate and attempt to decrease his QTc. The patient also underwent cardiac magnetic resonance imaging (MRI) to evaluate for possible myocarditis, which showed no evidence of acute inflammation. Echocardiogram demonstrated an ejection fraction of 40% and global hypokinesis but no specific regional abnormalities and no change from prior echocardiogram performed 1 year earlier. Over the course of 3 days, his ECG normalized and his QTc shortened to 477 msec. Genetic testing was performed and did not reveal any mutations associated with long QT syndrome. Ultimately, an automated internal cardiac defibrillator (AICD) was placed, and the patient was discharged home.
Over the 2 years since his initial event, the patient has not experienced recurrent VF and his AICD has not fired. The patient continues to have ED presentations for heart-failure symptoms, though he has been stable from an electrophysiologic standpoint and his QTc remains less than 500 msec.
Discussion
Prolongation of the QT interval as a result of deep, global T-wave inversions after resolution of acute pulmonary edema has been minimally reported.4,5 This phenomenon has been described in the cardiology literature but has not been discussed in the emergency medicine literature and bears consideration in this case.4,5 As noted, an extensive evaluation did not reveal another cause of QTc prolongation. The patient had normal electrolytes and temperature, his neurologic examination and computed tomography were not remarkable. The patient had no obstructive coronary artery disease on catheterization, no evidence of acute myocarditis on cardiac MRI, no prescribed medications associated with QT prolongation, and no evidence of genetic mutations associated with QT prolongation on testing. The minimal troponin elevation was felt to represent a type II myocardial infarction related to ischemia due to supply-demand mismatch rather than acute plaque rupture.
Littmann published a case series of 9 cases of delayed onset T-wave inversion and extreme QTc prolongation in the 24 to 48 hours following treatment and symptomatic improvement in acute pulmonary edema.4 In each of his patients, an ischemic cardiac insult was ruled out as the etiology of the pulmonary edema by laboratory assessment, echocardiography, and left heart catheterization.All of the patients in this case series recovered without incident and with normalization of the QTc interval.4 Similarly, in our patient, significant QT T changes occurred approximately 22 hours after presentation and with resolution of symptoms of pulmonary edema. Pascale and colleagues also published a series of 3 patients developing similar ECG patterns following a hypertensive crisis with resolution of ECG findings and without any morbidity.5 In contrast, our patient experienced significant morbidity secondary to the extreme QTc prolongation.
Conclusions
We believe this is the first reported case of excessive prolongation of the QTc with VF arrest secondary to resolution of acute pulmonary edema. The pattern observed in our patient follows the patterns outlined in the previous case series—patients present with acute pulmonary edema and hypertensive crisis but develop significant ECG abnormalities about 24 hours after the resolution of the high catecholamine state. Our patient did have a history of prior cardiac insult, given the QTc changes developed acutely, with frequent premature ventricular contractions, and the cardiac arrest occurred at maximal QTc prolongation, yet after resolution of the high catecholamine state, the treatment team felt there was likely an uncaptured and short-lived episode of TdP that degenerated into VF. This theory is further supported by the lack of recurrent VF episodes, confirmed by AICD interrogation, after normalization of the QTc in our patient.
1. Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001;85(2):321-341. doi:10.1016/s0025-7125(05)70318-7
2. Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE. Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review. ScientificWorldJournal. 2012;2012:212178. doi:10.1100/2012/212178
3. Miller MA, Elmariah S, Fischer A. Giant T-wave inversions and extreme QT prolongation. Circ Arrhythm Electrophysiol. 2009;2(6):e42-e43. doi:10.1161/CIRCEP.108.825729
4. Littmann L. Large T wave inversion and QT prolongation associated with pulmonary edema: a report of nine cases. J Am Coll Cardiol. 1999;34(4):1106-1110. doi:10.1016/s0735-1097(99)00311-3
5. Pascale P, Quartenoud B, Stauffer JC. Isolated large inverted T wave in pulmonary edema due to hypertensive crisis: a novel electrocardiographic phenomenon mimicking ischemia?. Clin Res Cardiol. 2007;96(5):288-294. doi:10.1007/s00392-007-0504-1
A case of extreme QT prolongation induced following symptomatic resolution of acute pulmonary edema is both relatively unknown and poorly understood.
A case of extreme QT prolongation induced following symptomatic resolution of acute pulmonary edema is both relatively unknown and poorly understood.
Abnormalities in the T-wave morphology of an electrocardiogram (ECG) are classically attributed to ischemic cardiac disease. However, these changes can be seen in a variety of other etiologies, including noncardiac pathology, which should be considered whenever reviewing an ECG: central nervous system disease, including stroke and subarachnoid hemorrhage; hypothermia; pulmonary disease, such as pulmonary embolism or chronic obstructive pulmonary disease; myopericarditis; drug effects; and electrolyte abnormalities.
Prolongation of the QT interval, on the other hand, can be precipitated by medications, metabolic derangements, or genetic phenotypes. The QT interval is measured from the beginning of the QRS complex to the termination of the T wave and represents the total time for ventricular depolarization and repolarization. The QT interval must be corrected based on the patient’s heart rate, known as the QTc. As the QTc interval lengthens, there is increased risk of R-on-T phenomena, which may result in Torsades de Pointes (TdP). Typical features of TdP include an antecedent prolonged QTc, cyclic polymorphic ventricular tachycardia on the surface ECG, and either a short-lived spontaneously terminating course or degeneration into ventricular fibrillation (VF) and sudden cardiac death.1 These dysrhythmias become more likely as the QTc interval exceeds 500 msec.2
The combination of new-onset global T-wave inversions with prolongation of the QT interval has been reported in only a few limited conditions. Some known causes of these QT T changes include cardiac ischemia, status epilepticus, pheochromocytoma, and acute cocaine intoxication.3 One uncommon and rarely reported cause of extreme QT prolongation and T-wave inversion is acute pulmonary edema. The ECG findings are not present on initial patient presentation; rather the dynamic changes occur after resolution of the pulmonary symptoms. Despite significant ECG changes, all prior reported cases describe ECG normalization without significant morbidity.4,5 We report a case of extreme QT prolongation following acute pulmonary edema that resulted in cardiac arrest secondary to VF.
Case Presentation
A 72-year-old male with medical history of combined systolic and diastolic heart failure, ischemic cardiomyopathy, coronary artery disease, cerebral vascular accident, hypertension, hyperlipidemia, type 2 diabetes mellitus, and tobacco dependence presented to the emergency department (ED) by emergency medical services after awaking with acute onset of dyspnea and diaphoresis. On arrival at the ED, the patient was noted to be in respiratory distress (ie, unable to speak single words) and was extremely diaphoretic. His initial vital signs included blood pressure, 186/113 mm Hg, heart rate, 104 beats per minute, respiratory rate, 40 breaths per minute, and temperature, 36.4 °C. The patient was quickly placed on bilevel positive airway pressure and given sublingual nitroglycerin followed by transdermal nitroglycerin with a single dose of 40 mg IV furosemide, which improved his respiratory status. A chest X-ray was consistent with pulmonary edema, and his brain natriuretic peptide was 1654 pg/mL. An ECG demonstrated new T-wave inversions, and his troponin increased from 0.04 to 0.24 ng/mL during his ED stay (Figure 1). He was started on a heparin infusion and admitted to the hospital for hypertensive emergency with presumed acute decompensated heart failure and non-ST-elevated myocardial infarction.
Throughout the patient’s first night, the troponin level started to down-trend after peaking at 0.24 ng/mL, and his oxygen requirements decreased allowing transition to nasal cannula. However, his repeat ECGs demonstrated significant T-wave abnormalities, new premature ventricular contractions, bradycardia, and a prolonging QTc interval to 703 msec (Figure 2). At this time, the patient’s electrolytes were normal, specifically a potassium level of 4.4 mEq/L, calcium 8.8 mg/dL, magnesium 2.0 mg/dL, and phosphorus 2.6 mg/dL. Given the worsening ECG changes, a computed tomography scan of his head was ordered to rule out intracranial pathology. While in the scanner, the patient went into pulseless VF, prompting defibrillation with 200 J. In addition, he was given 75 mg IV lidocaine, 2 g IV magnesium, and 1 ampule of both calcium chloride and sodium bicarbonate. With treatment, he had return of spontaneous circulation and was taken promptly to cardiac catheterization. The catheterization showed no significant obstructive coronary artery disease, and no interventions were performed. The patient was transferred to the cardiac intensive care unit for continued care.
During his course in the intensive care unit, the patient’s potassium and magnesium levels were maintained at high-normal levels. The patient was started on a dobutamine infusion to increase his heart rate and attempt to decrease his QTc. The patient also underwent cardiac magnetic resonance imaging (MRI) to evaluate for possible myocarditis, which showed no evidence of acute inflammation. Echocardiogram demonstrated an ejection fraction of 40% and global hypokinesis but no specific regional abnormalities and no change from prior echocardiogram performed 1 year earlier. Over the course of 3 days, his ECG normalized and his QTc shortened to 477 msec. Genetic testing was performed and did not reveal any mutations associated with long QT syndrome. Ultimately, an automated internal cardiac defibrillator (AICD) was placed, and the patient was discharged home.
Over the 2 years since his initial event, the patient has not experienced recurrent VF and his AICD has not fired. The patient continues to have ED presentations for heart-failure symptoms, though he has been stable from an electrophysiologic standpoint and his QTc remains less than 500 msec.
Discussion
Prolongation of the QT interval as a result of deep, global T-wave inversions after resolution of acute pulmonary edema has been minimally reported.4,5 This phenomenon has been described in the cardiology literature but has not been discussed in the emergency medicine literature and bears consideration in this case.4,5 As noted, an extensive evaluation did not reveal another cause of QTc prolongation. The patient had normal electrolytes and temperature, his neurologic examination and computed tomography were not remarkable. The patient had no obstructive coronary artery disease on catheterization, no evidence of acute myocarditis on cardiac MRI, no prescribed medications associated with QT prolongation, and no evidence of genetic mutations associated with QT prolongation on testing. The minimal troponin elevation was felt to represent a type II myocardial infarction related to ischemia due to supply-demand mismatch rather than acute plaque rupture.
Littmann published a case series of 9 cases of delayed onset T-wave inversion and extreme QTc prolongation in the 24 to 48 hours following treatment and symptomatic improvement in acute pulmonary edema.4 In each of his patients, an ischemic cardiac insult was ruled out as the etiology of the pulmonary edema by laboratory assessment, echocardiography, and left heart catheterization.All of the patients in this case series recovered without incident and with normalization of the QTc interval.4 Similarly, in our patient, significant QT T changes occurred approximately 22 hours after presentation and with resolution of symptoms of pulmonary edema. Pascale and colleagues also published a series of 3 patients developing similar ECG patterns following a hypertensive crisis with resolution of ECG findings and without any morbidity.5 In contrast, our patient experienced significant morbidity secondary to the extreme QTc prolongation.
Conclusions
We believe this is the first reported case of excessive prolongation of the QTc with VF arrest secondary to resolution of acute pulmonary edema. The pattern observed in our patient follows the patterns outlined in the previous case series—patients present with acute pulmonary edema and hypertensive crisis but develop significant ECG abnormalities about 24 hours after the resolution of the high catecholamine state. Our patient did have a history of prior cardiac insult, given the QTc changes developed acutely, with frequent premature ventricular contractions, and the cardiac arrest occurred at maximal QTc prolongation, yet after resolution of the high catecholamine state, the treatment team felt there was likely an uncaptured and short-lived episode of TdP that degenerated into VF. This theory is further supported by the lack of recurrent VF episodes, confirmed by AICD interrogation, after normalization of the QTc in our patient.
Abnormalities in the T-wave morphology of an electrocardiogram (ECG) are classically attributed to ischemic cardiac disease. However, these changes can be seen in a variety of other etiologies, including noncardiac pathology, which should be considered whenever reviewing an ECG: central nervous system disease, including stroke and subarachnoid hemorrhage; hypothermia; pulmonary disease, such as pulmonary embolism or chronic obstructive pulmonary disease; myopericarditis; drug effects; and electrolyte abnormalities.
Prolongation of the QT interval, on the other hand, can be precipitated by medications, metabolic derangements, or genetic phenotypes. The QT interval is measured from the beginning of the QRS complex to the termination of the T wave and represents the total time for ventricular depolarization and repolarization. The QT interval must be corrected based on the patient’s heart rate, known as the QTc. As the QTc interval lengthens, there is increased risk of R-on-T phenomena, which may result in Torsades de Pointes (TdP). Typical features of TdP include an antecedent prolonged QTc, cyclic polymorphic ventricular tachycardia on the surface ECG, and either a short-lived spontaneously terminating course or degeneration into ventricular fibrillation (VF) and sudden cardiac death.1 These dysrhythmias become more likely as the QTc interval exceeds 500 msec.2
The combination of new-onset global T-wave inversions with prolongation of the QT interval has been reported in only a few limited conditions. Some known causes of these QT T changes include cardiac ischemia, status epilepticus, pheochromocytoma, and acute cocaine intoxication.3 One uncommon and rarely reported cause of extreme QT prolongation and T-wave inversion is acute pulmonary edema. The ECG findings are not present on initial patient presentation; rather the dynamic changes occur after resolution of the pulmonary symptoms. Despite significant ECG changes, all prior reported cases describe ECG normalization without significant morbidity.4,5 We report a case of extreme QT prolongation following acute pulmonary edema that resulted in cardiac arrest secondary to VF.
Case Presentation
A 72-year-old male with medical history of combined systolic and diastolic heart failure, ischemic cardiomyopathy, coronary artery disease, cerebral vascular accident, hypertension, hyperlipidemia, type 2 diabetes mellitus, and tobacco dependence presented to the emergency department (ED) by emergency medical services after awaking with acute onset of dyspnea and diaphoresis. On arrival at the ED, the patient was noted to be in respiratory distress (ie, unable to speak single words) and was extremely diaphoretic. His initial vital signs included blood pressure, 186/113 mm Hg, heart rate, 104 beats per minute, respiratory rate, 40 breaths per minute, and temperature, 36.4 °C. The patient was quickly placed on bilevel positive airway pressure and given sublingual nitroglycerin followed by transdermal nitroglycerin with a single dose of 40 mg IV furosemide, which improved his respiratory status. A chest X-ray was consistent with pulmonary edema, and his brain natriuretic peptide was 1654 pg/mL. An ECG demonstrated new T-wave inversions, and his troponin increased from 0.04 to 0.24 ng/mL during his ED stay (Figure 1). He was started on a heparin infusion and admitted to the hospital for hypertensive emergency with presumed acute decompensated heart failure and non-ST-elevated myocardial infarction.
Throughout the patient’s first night, the troponin level started to down-trend after peaking at 0.24 ng/mL, and his oxygen requirements decreased allowing transition to nasal cannula. However, his repeat ECGs demonstrated significant T-wave abnormalities, new premature ventricular contractions, bradycardia, and a prolonging QTc interval to 703 msec (Figure 2). At this time, the patient’s electrolytes were normal, specifically a potassium level of 4.4 mEq/L, calcium 8.8 mg/dL, magnesium 2.0 mg/dL, and phosphorus 2.6 mg/dL. Given the worsening ECG changes, a computed tomography scan of his head was ordered to rule out intracranial pathology. While in the scanner, the patient went into pulseless VF, prompting defibrillation with 200 J. In addition, he was given 75 mg IV lidocaine, 2 g IV magnesium, and 1 ampule of both calcium chloride and sodium bicarbonate. With treatment, he had return of spontaneous circulation and was taken promptly to cardiac catheterization. The catheterization showed no significant obstructive coronary artery disease, and no interventions were performed. The patient was transferred to the cardiac intensive care unit for continued care.
During his course in the intensive care unit, the patient’s potassium and magnesium levels were maintained at high-normal levels. The patient was started on a dobutamine infusion to increase his heart rate and attempt to decrease his QTc. The patient also underwent cardiac magnetic resonance imaging (MRI) to evaluate for possible myocarditis, which showed no evidence of acute inflammation. Echocardiogram demonstrated an ejection fraction of 40% and global hypokinesis but no specific regional abnormalities and no change from prior echocardiogram performed 1 year earlier. Over the course of 3 days, his ECG normalized and his QTc shortened to 477 msec. Genetic testing was performed and did not reveal any mutations associated with long QT syndrome. Ultimately, an automated internal cardiac defibrillator (AICD) was placed, and the patient was discharged home.
Over the 2 years since his initial event, the patient has not experienced recurrent VF and his AICD has not fired. The patient continues to have ED presentations for heart-failure symptoms, though he has been stable from an electrophysiologic standpoint and his QTc remains less than 500 msec.
Discussion
Prolongation of the QT interval as a result of deep, global T-wave inversions after resolution of acute pulmonary edema has been minimally reported.4,5 This phenomenon has been described in the cardiology literature but has not been discussed in the emergency medicine literature and bears consideration in this case.4,5 As noted, an extensive evaluation did not reveal another cause of QTc prolongation. The patient had normal electrolytes and temperature, his neurologic examination and computed tomography were not remarkable. The patient had no obstructive coronary artery disease on catheterization, no evidence of acute myocarditis on cardiac MRI, no prescribed medications associated with QT prolongation, and no evidence of genetic mutations associated with QT prolongation on testing. The minimal troponin elevation was felt to represent a type II myocardial infarction related to ischemia due to supply-demand mismatch rather than acute plaque rupture.
Littmann published a case series of 9 cases of delayed onset T-wave inversion and extreme QTc prolongation in the 24 to 48 hours following treatment and symptomatic improvement in acute pulmonary edema.4 In each of his patients, an ischemic cardiac insult was ruled out as the etiology of the pulmonary edema by laboratory assessment, echocardiography, and left heart catheterization.All of the patients in this case series recovered without incident and with normalization of the QTc interval.4 Similarly, in our patient, significant QT T changes occurred approximately 22 hours after presentation and with resolution of symptoms of pulmonary edema. Pascale and colleagues also published a series of 3 patients developing similar ECG patterns following a hypertensive crisis with resolution of ECG findings and without any morbidity.5 In contrast, our patient experienced significant morbidity secondary to the extreme QTc prolongation.
Conclusions
We believe this is the first reported case of excessive prolongation of the QTc with VF arrest secondary to resolution of acute pulmonary edema. The pattern observed in our patient follows the patterns outlined in the previous case series—patients present with acute pulmonary edema and hypertensive crisis but develop significant ECG abnormalities about 24 hours after the resolution of the high catecholamine state. Our patient did have a history of prior cardiac insult, given the QTc changes developed acutely, with frequent premature ventricular contractions, and the cardiac arrest occurred at maximal QTc prolongation, yet after resolution of the high catecholamine state, the treatment team felt there was likely an uncaptured and short-lived episode of TdP that degenerated into VF. This theory is further supported by the lack of recurrent VF episodes, confirmed by AICD interrogation, after normalization of the QTc in our patient.
1. Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001;85(2):321-341. doi:10.1016/s0025-7125(05)70318-7
2. Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE. Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review. ScientificWorldJournal. 2012;2012:212178. doi:10.1100/2012/212178
3. Miller MA, Elmariah S, Fischer A. Giant T-wave inversions and extreme QT prolongation. Circ Arrhythm Electrophysiol. 2009;2(6):e42-e43. doi:10.1161/CIRCEP.108.825729
4. Littmann L. Large T wave inversion and QT prolongation associated with pulmonary edema: a report of nine cases. J Am Coll Cardiol. 1999;34(4):1106-1110. doi:10.1016/s0735-1097(99)00311-3
5. Pascale P, Quartenoud B, Stauffer JC. Isolated large inverted T wave in pulmonary edema due to hypertensive crisis: a novel electrocardiographic phenomenon mimicking ischemia?. Clin Res Cardiol. 2007;96(5):288-294. doi:10.1007/s00392-007-0504-1
1. Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001;85(2):321-341. doi:10.1016/s0025-7125(05)70318-7
2. Kallergis EM, Goudis CA, Simantirakis EN, Kochiadakis GE, Vardas PE. Mechanisms, risk factors, and management of acquired long QT syndrome: a comprehensive review. ScientificWorldJournal. 2012;2012:212178. doi:10.1100/2012/212178
3. Miller MA, Elmariah S, Fischer A. Giant T-wave inversions and extreme QT prolongation. Circ Arrhythm Electrophysiol. 2009;2(6):e42-e43. doi:10.1161/CIRCEP.108.825729
4. Littmann L. Large T wave inversion and QT prolongation associated with pulmonary edema: a report of nine cases. J Am Coll Cardiol. 1999;34(4):1106-1110. doi:10.1016/s0735-1097(99)00311-3
5. Pascale P, Quartenoud B, Stauffer JC. Isolated large inverted T wave in pulmonary edema due to hypertensive crisis: a novel electrocardiographic phenomenon mimicking ischemia?. Clin Res Cardiol. 2007;96(5):288-294. doi:10.1007/s00392-007-0504-1
Emphysematous Aortitis due to Klebsiella Pneumoniae in a Patient With Poorly Controlled Diabetes Mellitus
Patients with poorly controlled diabetes mellitus and an infectious source can be predisposed to infectious aortitis.
Aortitis is the all-encompassing term ascribed to the inflammatory process in the aortic wall that can be either infective or noninfective in origin, commonly autoimmune or inflammatory large-vessel vasculitis.1 Infectious aortitis, also known as bacterial, microbial, or cryptogenic aortitis, as well as mycotic or infected aneurysm, is a rare entity in the current antibiotic era but potentially a life-threatening disorder.2 The potential complications of infectious aortitis include emphysematous aortitis (EA), pseudoaneurysm, aortic rupture, septic emboli, and fistula formation (eg, aorto-enteric fistula).2,3
EA is a rare but serious inflammatory condition of the aorta with a nonspecific clinical presentation associated with high morbidity and mortality.2-6 The condition is characterized by a localized collection of gas and purulent exudate at the aortic wall.1,3 A few cases of EA have previously been reported; however, no known cases have been reported in the literature due to Klebsiella pneumoniae (K pneumoniae).
The pathophysiology of EA is the presence of underlying damage to the arterial wall caused by a hematogenously inoculated gas-producing organism.2,3 Most reported cases of EA are due to endovascular graft complications. Under normal circumstances, the aortic intima is highly resistant to infectious pathogens; however, certain risk factors, such as diabetes mellitus (DM), atherosclerotic disease, preexisting aneurysm, cystic medial necrosis, vascular malformation, presence of medical devices, surgery, or impaired immunity can alter the integrity of the aortic intimal layer and predispose the aortic intima to infection.1,4-7 Bacteria are the most common causative organisms that can infect the aorta, especially Staphylococcus, Enterococcus, Streptococcus, Salmonella, and spirochete Treponema pallidum (syphilis).1,2,4,8 The site of the primary infection remains unclear in some patients.2,3,5,6 Infection of the aorta can arise by several mechanisms: direct extension of a local infection to an existing intimal injury or atherosclerotic plaque (the most common mechanism), septic embolism from endocarditis, direct bacterial inoculation from traumatic contamination, contiguous infection extending to the aorta wall, or a distant source of bacteremia.2,3
Clinical manifestations of EA depend on the site and the extent of infection. The diagnosis should be considered in patients with atherosclerosis, fever, abdominal pain, and leukocytosis.2,4-8 The differential diagnosis for EA includes (1) noninfective causes of aortitis, including rheumatoid arthritis and systemic lupus erythematosus; (2) tuberculous aortitis; (3) syphilitic aortitis; and (4) idiopathic isolated aortitis. Establishing an early diagnosis of infectious aortitis is extremely important because this condition is associated with a high rate of morbidity and mortality secondary to aortic rupture.2-7
Imaging is critical for a reliable and quick diagnosis of acute aortic pathology. Noninvasive cross-sectional imaging modalities, such as contrast-enhanced computed tomography (CT), magnetic resonance imaging, nuclear medicine, or positron emission tomography, are used for both the initial diagnosis and follow-up of aortitis.1 CT is the primary imaging method in most medical centers because it is widely available with short acquisition time in critically ill patients.3 CT allows rapid detection of abnormalities in wall thickness, diameter, and density, and enhancement of periaortic structures, enabling reliable exclusion of other aortic pathologies that may resemble acute aortitis. Also, CT aids in planning the optimal therapeutic approach.1,3,5-8
This case illustrates EA associated with infection by K pneumoniae in a patient with poorly controlled type 2 DM (T2DM). In this single case, our patient presented to the Bay Pines Veterans Affairs Healthcare System (BPVAHS) in Florida with recent superficial soft tissue injury, severe hyperglycemia, worsening abdominal pain, and leukocytosis without fever or chills. The correct diagnosis of EA was confirmed by characteristic CT findings.
Case Presentation
A 72-year-old male with a history of T2DM, hypertension, atherosclerotic vascular disease, obstructive lung disease, and smoking 1.5 packs per day for 40 years presented with diabetic ketoacidosis, a urinary tract infection, and abdominal pain of 1-week duration that started to worsen the morning he arrived at the BPVAHS emergency department. He reported no nausea, vomiting, diarrhea, constipation, chest pain, shortness of breath, fever, chills, fatigue, or dysuria. He had a nonhealing laceration on his left medial foot that occurred 18 days before admission and was treated at an outside hospital.
The patient’s surgical history included a left common femoral endarterectomy and a left femoral popliteal above-knee reverse saphenous vein bypass 4 years ago for severe critical limb ischemia due to occlusion of his left superficial femoral artery with distal embolization to the first and fifth toes. About 6 months later, he developed disabling claudication in his left lower extremity due to distal popliteal artery occlusion and had another bypass surgery to the below-knee popliteal artery with a reverse saphenous vein graft harvested from the right thigh.
On initial examination, his vital signs were within normal limits except for a blood pressure of 177/87 mm Hg. His physical examination demonstrated a nondistended abdomen with normal bowel sounds, mild lower quadrant tenderness on the left more than on the right, intermittent abdominal pain located around umbilicus with radiation to the back, and a negative psoas sign. His left medial foot had a nonhealing laceration with black sutures in place, with minimal erythema in the surrounding tissue and scab formation. He also had mild costovertebral tenderness on the left.
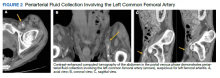
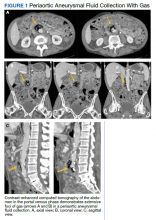
Initial laboratory investigation results were notable for a glucose level of 609 mg/dL and a white blood cell count of 14.6 × 103 cells/mcL with 86.5% of neutrophils. A CT scan of his abdomen revealed extensive atherosclerosis of the abdominal aorta and periaortic aneurysmal fluid collection with multiple foci of gas (Figure 1). Additionally, the aneurysmal fluid collection involved the proximal segment of the left common femoral artery, suspicious for left femoral arteritis (Figure 2). The patient was started on broad-spectrum antibiotics, morphine, and an insulin drip. Both urine and blood cultures were positive for K pneumoniae susceptible to multiple antibiotics. He was transferred to a tertiary medical center and was referred for a vascular surgery consultation.
The patient underwent surgical resection of the infected infrarenal EA and infected left common femoral artery with right axillary-bifemoral bypass with an 8-mm PTFE (polytetrafluoroethylene) graft. During the surgery, excision of the wall of the left common femoral artery and infrarenal aorta revealed frank pus with purulent fluid, which was sent to cytology for analysis and culture. His intraoperative cultures grew K pneumoniae sensitive to multiple antibiotics, including ceftriaxone, sulfamethoxazole/trimethoprim, and ampicillin/sulbactam. The vascular surgery team recommended inpatient admission and administration of 6 weeks of IV antibiotics postoperatively with ceftriaxone, followed by outpatient oral suppression therapy after discharge. The patient tolerated the surgery well and was discharged after 6 weeks of IV ceftriaxone followed by outpatient oral suppression therapy. However, the patient was transferred back to BPVAHS for continued care and rehabilitation placement.
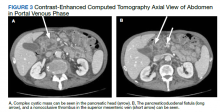
The patient’s subsequent course was complicated by multiple hospital admissions, including aspiration pneumonia, hypoglycemia, diarrhea, and anemia. On one of his CT abdomen/pelvic examinations, a cysticlike mass was noted in the pancreatic head with a possible pancreatic duodenal fistula (this mass was not mentioned on the initial presurgical CT, although it can be seen in retrospect (Figure 3). Gastroenterology was consulted.
An upper endoscopy was performed that confirmed the fistula at the second portion of the duodenum. Findings from an endoscopic ultrasonography performed at an outside institution were concerning for a main duct intraductal papillary mucinous neoplasm (IPMN) with fistula, with biopsy results pending.
Discussion
This case contributes to the evidence that poorly controlled T2DM can be a predisposing factor for multiple vascular complications, including the infection of the aortic wall with progression to EA. Klebsiella species are considered opportunistic, Gram-negative pathogens that may disseminate to other tissues, causing life-threatening infections, including pneumonia, UTIs, bacteremia, and sepsis.9K pneumoniae infections are particularly challenging in neonates, the elderly, and immunocompromised individuals.9 CT is sensitive and specific in the detection of this pathologic entity.1,3 In patients with a suspected infectious etiology, the presence of foci of gas on CT in solid organ tissues is usually associated with an anaerobic infection. Gas can also be produced by Gram-negative facultative anaerobes that can ferment glucose in necrotic tissues.9
Although any microorganism can infect the aorta, K pneumoniae cultured from the blood specimen, urine culture, and intraoperative specimens in our patient was responsible for the formed gas in the aortic wall. Occurrence of spontaneous gas by this microorganism is usually associated with conditions leading to either increased vulnerability to infections and/or enhanced bacterial virulence.9 Although a relationship between EA and T2DM has not been proved, it is well known that patients with T2DM have a defect in their host-defense mechanisms, making them more susceptible to infections such as EA. Furthermore, because patients with T2DM are prone to the development of Gram-negative sepsis, organisms such as K pneumoniae would tend to emerge. Patients with poorly controlled T2DM and the presence of an infectious source can be predisposed to infectious aortitis, eventually leading to a gas-forming infection of the aorta.5,7
We postulate that the hematogenous spread of bacteria from a laceration in the leg as well as the presence of the pancreaticoduodenal fistula was likely the cause of the infectious EA in this case, considering the patient’s underlying uncontrolled T2DM. The patient’s prior left lower extremity vascular graft also may have provided a nidus for spreading to the aorta. Other reported underlying diseases of EA include aortic atherosclerosis, T2DM, diverticulitis, colon cancer, underlying aneurysm, immune-compromised status, and the presence of a medical device or open surgery.4-7,9
To our knowledge, this is the first case of EA associated with a pancreaticoduodenal fistula related to intraductal papillary mucinous neoplasm (IPMN). Fistulation of a main duct IPMN is rare, occurring in just 6.6% of cases.10 It can occur both before and after malignant degeneration.
EA requires rapid diagnosis, antibiotic therapy, and consultation with a vascular surgeon for immediate resection of the infected artery and graft bypass. The initial treatment of suspected infectious aortitis is IV antibiotics with broad antimicrobial coverage of the most likely pathologic organisms, particularly staphylococcal species and Gram-negative rods. Surgical debridement and revascularization should be completed early because of the high mortality rate of this condition. The intent of surgery is to control sepsis and reconstruct the arterial vasculature. Patients should remain on parenteral or oral antibiotics for at least 6 weeks to ensure full clearance of the infection.8 They should be followed up closely with serial blood cultures and CT scans.8 The rarity of the disorder, low level of awareness, varying presentations, and lack of evidence delineating pathogenesis and causality contribute to the challenge of recognizing, diagnosing, and treating EA in patients with T2DM and inflammation.
Conclusions
This case report can help bring awareness of this rare and potentially life-threatening condition in patients with T2DM. Clinicians should be aware of the risk of AE, particularly in patients with several additional risk factors: recent skin/soft tissue trauma, prior vascular graft surgery, and an underlying pancreatic mass. CT is the imaging method of choice that helps to rapidly choose a necessary emergent treatment approach.
1. Litmanovich DE, Yıldırım A, Bankier AA. Insights into imaging of aortitis. Insights Imaging. 2012;3(6):545-560. doi:10.1007/s13244-012-0192-x
2. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32(9):488-490. doi:10.1002/clc.20578
3. Murphy DJ, Keraliya AR, Agrawal MD, Aghayev A, Steigner ML. Cross-sectional imaging of aortic infections. Insights Imaging. 2016;7(6):801-818. doi:10.1007/s13244-016-0522-5
4. Md Noh MSF, Abdul Rashid AM, Ar A, B N, Mohammed Y, A RE. Emphysematous aortitis: report of two cases and CT imaging findings. BJR Case Rep. 2017;3(3):20170006. doi:10.1259/bjrcr.20170006
5. Harris C, Geffen J, Rizg K, et al. A rare report of infectious emphysematous aortitis secondary to Clostridium septicum without prior vascular intervention. Case Rep Vasc Med. 2017;2017:4984325. doi:10.1155/2017/4984325
6. Ito F, Inokuchi R, Matsumoto A, et al. Presence of periaortic gas in Clostridium septicum-infected aortic aneurysm aids in early diagnosis: a case report and systematic review of the literature. J Med Case Rep. 2017;11(1):268. doi:10.1186/s13256-017-1422-0
7. Urgiles S, Matos-Casano H, Win KZ, Berardo J, Bhatt U, Shah J. Emphysematous aortitis due to Clostridium septicum in an 89-year-old female with ileus. Case Rep Infect Dis. 2019;2019:1094837. doi:10.1155/2019/1094837
8. Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89-97. doi:10.1007/s11936-005-0010-6
9. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629-661. doi:10.1128/mmbr.00078-15
10. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45(10):1080-1089. doi:10.1007/s00535-010-0263-z
Patients with poorly controlled diabetes mellitus and an infectious source can be predisposed to infectious aortitis.
Patients with poorly controlled diabetes mellitus and an infectious source can be predisposed to infectious aortitis.
Aortitis is the all-encompassing term ascribed to the inflammatory process in the aortic wall that can be either infective or noninfective in origin, commonly autoimmune or inflammatory large-vessel vasculitis.1 Infectious aortitis, also known as bacterial, microbial, or cryptogenic aortitis, as well as mycotic or infected aneurysm, is a rare entity in the current antibiotic era but potentially a life-threatening disorder.2 The potential complications of infectious aortitis include emphysematous aortitis (EA), pseudoaneurysm, aortic rupture, septic emboli, and fistula formation (eg, aorto-enteric fistula).2,3
EA is a rare but serious inflammatory condition of the aorta with a nonspecific clinical presentation associated with high morbidity and mortality.2-6 The condition is characterized by a localized collection of gas and purulent exudate at the aortic wall.1,3 A few cases of EA have previously been reported; however, no known cases have been reported in the literature due to Klebsiella pneumoniae (K pneumoniae).
The pathophysiology of EA is the presence of underlying damage to the arterial wall caused by a hematogenously inoculated gas-producing organism.2,3 Most reported cases of EA are due to endovascular graft complications. Under normal circumstances, the aortic intima is highly resistant to infectious pathogens; however, certain risk factors, such as diabetes mellitus (DM), atherosclerotic disease, preexisting aneurysm, cystic medial necrosis, vascular malformation, presence of medical devices, surgery, or impaired immunity can alter the integrity of the aortic intimal layer and predispose the aortic intima to infection.1,4-7 Bacteria are the most common causative organisms that can infect the aorta, especially Staphylococcus, Enterococcus, Streptococcus, Salmonella, and spirochete Treponema pallidum (syphilis).1,2,4,8 The site of the primary infection remains unclear in some patients.2,3,5,6 Infection of the aorta can arise by several mechanisms: direct extension of a local infection to an existing intimal injury or atherosclerotic plaque (the most common mechanism), septic embolism from endocarditis, direct bacterial inoculation from traumatic contamination, contiguous infection extending to the aorta wall, or a distant source of bacteremia.2,3
Clinical manifestations of EA depend on the site and the extent of infection. The diagnosis should be considered in patients with atherosclerosis, fever, abdominal pain, and leukocytosis.2,4-8 The differential diagnosis for EA includes (1) noninfective causes of aortitis, including rheumatoid arthritis and systemic lupus erythematosus; (2) tuberculous aortitis; (3) syphilitic aortitis; and (4) idiopathic isolated aortitis. Establishing an early diagnosis of infectious aortitis is extremely important because this condition is associated with a high rate of morbidity and mortality secondary to aortic rupture.2-7
Imaging is critical for a reliable and quick diagnosis of acute aortic pathology. Noninvasive cross-sectional imaging modalities, such as contrast-enhanced computed tomography (CT), magnetic resonance imaging, nuclear medicine, or positron emission tomography, are used for both the initial diagnosis and follow-up of aortitis.1 CT is the primary imaging method in most medical centers because it is widely available with short acquisition time in critically ill patients.3 CT allows rapid detection of abnormalities in wall thickness, diameter, and density, and enhancement of periaortic structures, enabling reliable exclusion of other aortic pathologies that may resemble acute aortitis. Also, CT aids in planning the optimal therapeutic approach.1,3,5-8
This case illustrates EA associated with infection by K pneumoniae in a patient with poorly controlled type 2 DM (T2DM). In this single case, our patient presented to the Bay Pines Veterans Affairs Healthcare System (BPVAHS) in Florida with recent superficial soft tissue injury, severe hyperglycemia, worsening abdominal pain, and leukocytosis without fever or chills. The correct diagnosis of EA was confirmed by characteristic CT findings.
Case Presentation
A 72-year-old male with a history of T2DM, hypertension, atherosclerotic vascular disease, obstructive lung disease, and smoking 1.5 packs per day for 40 years presented with diabetic ketoacidosis, a urinary tract infection, and abdominal pain of 1-week duration that started to worsen the morning he arrived at the BPVAHS emergency department. He reported no nausea, vomiting, diarrhea, constipation, chest pain, shortness of breath, fever, chills, fatigue, or dysuria. He had a nonhealing laceration on his left medial foot that occurred 18 days before admission and was treated at an outside hospital.
The patient’s surgical history included a left common femoral endarterectomy and a left femoral popliteal above-knee reverse saphenous vein bypass 4 years ago for severe critical limb ischemia due to occlusion of his left superficial femoral artery with distal embolization to the first and fifth toes. About 6 months later, he developed disabling claudication in his left lower extremity due to distal popliteal artery occlusion and had another bypass surgery to the below-knee popliteal artery with a reverse saphenous vein graft harvested from the right thigh.
On initial examination, his vital signs were within normal limits except for a blood pressure of 177/87 mm Hg. His physical examination demonstrated a nondistended abdomen with normal bowel sounds, mild lower quadrant tenderness on the left more than on the right, intermittent abdominal pain located around umbilicus with radiation to the back, and a negative psoas sign. His left medial foot had a nonhealing laceration with black sutures in place, with minimal erythema in the surrounding tissue and scab formation. He also had mild costovertebral tenderness on the left.
Initial laboratory investigation results were notable for a glucose level of 609 mg/dL and a white blood cell count of 14.6 × 103 cells/mcL with 86.5% of neutrophils. A CT scan of his abdomen revealed extensive atherosclerosis of the abdominal aorta and periaortic aneurysmal fluid collection with multiple foci of gas (Figure 1). Additionally, the aneurysmal fluid collection involved the proximal segment of the left common femoral artery, suspicious for left femoral arteritis (Figure 2). The patient was started on broad-spectrum antibiotics, morphine, and an insulin drip. Both urine and blood cultures were positive for K pneumoniae susceptible to multiple antibiotics. He was transferred to a tertiary medical center and was referred for a vascular surgery consultation.
The patient underwent surgical resection of the infected infrarenal EA and infected left common femoral artery with right axillary-bifemoral bypass with an 8-mm PTFE (polytetrafluoroethylene) graft. During the surgery, excision of the wall of the left common femoral artery and infrarenal aorta revealed frank pus with purulent fluid, which was sent to cytology for analysis and culture. His intraoperative cultures grew K pneumoniae sensitive to multiple antibiotics, including ceftriaxone, sulfamethoxazole/trimethoprim, and ampicillin/sulbactam. The vascular surgery team recommended inpatient admission and administration of 6 weeks of IV antibiotics postoperatively with ceftriaxone, followed by outpatient oral suppression therapy after discharge. The patient tolerated the surgery well and was discharged after 6 weeks of IV ceftriaxone followed by outpatient oral suppression therapy. However, the patient was transferred back to BPVAHS for continued care and rehabilitation placement.
The patient’s subsequent course was complicated by multiple hospital admissions, including aspiration pneumonia, hypoglycemia, diarrhea, and anemia. On one of his CT abdomen/pelvic examinations, a cysticlike mass was noted in the pancreatic head with a possible pancreatic duodenal fistula (this mass was not mentioned on the initial presurgical CT, although it can be seen in retrospect (Figure 3). Gastroenterology was consulted.
An upper endoscopy was performed that confirmed the fistula at the second portion of the duodenum. Findings from an endoscopic ultrasonography performed at an outside institution were concerning for a main duct intraductal papillary mucinous neoplasm (IPMN) with fistula, with biopsy results pending.
Discussion
This case contributes to the evidence that poorly controlled T2DM can be a predisposing factor for multiple vascular complications, including the infection of the aortic wall with progression to EA. Klebsiella species are considered opportunistic, Gram-negative pathogens that may disseminate to other tissues, causing life-threatening infections, including pneumonia, UTIs, bacteremia, and sepsis.9K pneumoniae infections are particularly challenging in neonates, the elderly, and immunocompromised individuals.9 CT is sensitive and specific in the detection of this pathologic entity.1,3 In patients with a suspected infectious etiology, the presence of foci of gas on CT in solid organ tissues is usually associated with an anaerobic infection. Gas can also be produced by Gram-negative facultative anaerobes that can ferment glucose in necrotic tissues.9
Although any microorganism can infect the aorta, K pneumoniae cultured from the blood specimen, urine culture, and intraoperative specimens in our patient was responsible for the formed gas in the aortic wall. Occurrence of spontaneous gas by this microorganism is usually associated with conditions leading to either increased vulnerability to infections and/or enhanced bacterial virulence.9 Although a relationship between EA and T2DM has not been proved, it is well known that patients with T2DM have a defect in their host-defense mechanisms, making them more susceptible to infections such as EA. Furthermore, because patients with T2DM are prone to the development of Gram-negative sepsis, organisms such as K pneumoniae would tend to emerge. Patients with poorly controlled T2DM and the presence of an infectious source can be predisposed to infectious aortitis, eventually leading to a gas-forming infection of the aorta.5,7
We postulate that the hematogenous spread of bacteria from a laceration in the leg as well as the presence of the pancreaticoduodenal fistula was likely the cause of the infectious EA in this case, considering the patient’s underlying uncontrolled T2DM. The patient’s prior left lower extremity vascular graft also may have provided a nidus for spreading to the aorta. Other reported underlying diseases of EA include aortic atherosclerosis, T2DM, diverticulitis, colon cancer, underlying aneurysm, immune-compromised status, and the presence of a medical device or open surgery.4-7,9
To our knowledge, this is the first case of EA associated with a pancreaticoduodenal fistula related to intraductal papillary mucinous neoplasm (IPMN). Fistulation of a main duct IPMN is rare, occurring in just 6.6% of cases.10 It can occur both before and after malignant degeneration.
EA requires rapid diagnosis, antibiotic therapy, and consultation with a vascular surgeon for immediate resection of the infected artery and graft bypass. The initial treatment of suspected infectious aortitis is IV antibiotics with broad antimicrobial coverage of the most likely pathologic organisms, particularly staphylococcal species and Gram-negative rods. Surgical debridement and revascularization should be completed early because of the high mortality rate of this condition. The intent of surgery is to control sepsis and reconstruct the arterial vasculature. Patients should remain on parenteral or oral antibiotics for at least 6 weeks to ensure full clearance of the infection.8 They should be followed up closely with serial blood cultures and CT scans.8 The rarity of the disorder, low level of awareness, varying presentations, and lack of evidence delineating pathogenesis and causality contribute to the challenge of recognizing, diagnosing, and treating EA in patients with T2DM and inflammation.
Conclusions
This case report can help bring awareness of this rare and potentially life-threatening condition in patients with T2DM. Clinicians should be aware of the risk of AE, particularly in patients with several additional risk factors: recent skin/soft tissue trauma, prior vascular graft surgery, and an underlying pancreatic mass. CT is the imaging method of choice that helps to rapidly choose a necessary emergent treatment approach.
Aortitis is the all-encompassing term ascribed to the inflammatory process in the aortic wall that can be either infective or noninfective in origin, commonly autoimmune or inflammatory large-vessel vasculitis.1 Infectious aortitis, also known as bacterial, microbial, or cryptogenic aortitis, as well as mycotic or infected aneurysm, is a rare entity in the current antibiotic era but potentially a life-threatening disorder.2 The potential complications of infectious aortitis include emphysematous aortitis (EA), pseudoaneurysm, aortic rupture, septic emboli, and fistula formation (eg, aorto-enteric fistula).2,3
EA is a rare but serious inflammatory condition of the aorta with a nonspecific clinical presentation associated with high morbidity and mortality.2-6 The condition is characterized by a localized collection of gas and purulent exudate at the aortic wall.1,3 A few cases of EA have previously been reported; however, no known cases have been reported in the literature due to Klebsiella pneumoniae (K pneumoniae).
The pathophysiology of EA is the presence of underlying damage to the arterial wall caused by a hematogenously inoculated gas-producing organism.2,3 Most reported cases of EA are due to endovascular graft complications. Under normal circumstances, the aortic intima is highly resistant to infectious pathogens; however, certain risk factors, such as diabetes mellitus (DM), atherosclerotic disease, preexisting aneurysm, cystic medial necrosis, vascular malformation, presence of medical devices, surgery, or impaired immunity can alter the integrity of the aortic intimal layer and predispose the aortic intima to infection.1,4-7 Bacteria are the most common causative organisms that can infect the aorta, especially Staphylococcus, Enterococcus, Streptococcus, Salmonella, and spirochete Treponema pallidum (syphilis).1,2,4,8 The site of the primary infection remains unclear in some patients.2,3,5,6 Infection of the aorta can arise by several mechanisms: direct extension of a local infection to an existing intimal injury or atherosclerotic plaque (the most common mechanism), septic embolism from endocarditis, direct bacterial inoculation from traumatic contamination, contiguous infection extending to the aorta wall, or a distant source of bacteremia.2,3
Clinical manifestations of EA depend on the site and the extent of infection. The diagnosis should be considered in patients with atherosclerosis, fever, abdominal pain, and leukocytosis.2,4-8 The differential diagnosis for EA includes (1) noninfective causes of aortitis, including rheumatoid arthritis and systemic lupus erythematosus; (2) tuberculous aortitis; (3) syphilitic aortitis; and (4) idiopathic isolated aortitis. Establishing an early diagnosis of infectious aortitis is extremely important because this condition is associated with a high rate of morbidity and mortality secondary to aortic rupture.2-7
Imaging is critical for a reliable and quick diagnosis of acute aortic pathology. Noninvasive cross-sectional imaging modalities, such as contrast-enhanced computed tomography (CT), magnetic resonance imaging, nuclear medicine, or positron emission tomography, are used for both the initial diagnosis and follow-up of aortitis.1 CT is the primary imaging method in most medical centers because it is widely available with short acquisition time in critically ill patients.3 CT allows rapid detection of abnormalities in wall thickness, diameter, and density, and enhancement of periaortic structures, enabling reliable exclusion of other aortic pathologies that may resemble acute aortitis. Also, CT aids in planning the optimal therapeutic approach.1,3,5-8
This case illustrates EA associated with infection by K pneumoniae in a patient with poorly controlled type 2 DM (T2DM). In this single case, our patient presented to the Bay Pines Veterans Affairs Healthcare System (BPVAHS) in Florida with recent superficial soft tissue injury, severe hyperglycemia, worsening abdominal pain, and leukocytosis without fever or chills. The correct diagnosis of EA was confirmed by characteristic CT findings.
Case Presentation
A 72-year-old male with a history of T2DM, hypertension, atherosclerotic vascular disease, obstructive lung disease, and smoking 1.5 packs per day for 40 years presented with diabetic ketoacidosis, a urinary tract infection, and abdominal pain of 1-week duration that started to worsen the morning he arrived at the BPVAHS emergency department. He reported no nausea, vomiting, diarrhea, constipation, chest pain, shortness of breath, fever, chills, fatigue, or dysuria. He had a nonhealing laceration on his left medial foot that occurred 18 days before admission and was treated at an outside hospital.
The patient’s surgical history included a left common femoral endarterectomy and a left femoral popliteal above-knee reverse saphenous vein bypass 4 years ago for severe critical limb ischemia due to occlusion of his left superficial femoral artery with distal embolization to the first and fifth toes. About 6 months later, he developed disabling claudication in his left lower extremity due to distal popliteal artery occlusion and had another bypass surgery to the below-knee popliteal artery with a reverse saphenous vein graft harvested from the right thigh.
On initial examination, his vital signs were within normal limits except for a blood pressure of 177/87 mm Hg. His physical examination demonstrated a nondistended abdomen with normal bowel sounds, mild lower quadrant tenderness on the left more than on the right, intermittent abdominal pain located around umbilicus with radiation to the back, and a negative psoas sign. His left medial foot had a nonhealing laceration with black sutures in place, with minimal erythema in the surrounding tissue and scab formation. He also had mild costovertebral tenderness on the left.
Initial laboratory investigation results were notable for a glucose level of 609 mg/dL and a white blood cell count of 14.6 × 103 cells/mcL with 86.5% of neutrophils. A CT scan of his abdomen revealed extensive atherosclerosis of the abdominal aorta and periaortic aneurysmal fluid collection with multiple foci of gas (Figure 1). Additionally, the aneurysmal fluid collection involved the proximal segment of the left common femoral artery, suspicious for left femoral arteritis (Figure 2). The patient was started on broad-spectrum antibiotics, morphine, and an insulin drip. Both urine and blood cultures were positive for K pneumoniae susceptible to multiple antibiotics. He was transferred to a tertiary medical center and was referred for a vascular surgery consultation.
The patient underwent surgical resection of the infected infrarenal EA and infected left common femoral artery with right axillary-bifemoral bypass with an 8-mm PTFE (polytetrafluoroethylene) graft. During the surgery, excision of the wall of the left common femoral artery and infrarenal aorta revealed frank pus with purulent fluid, which was sent to cytology for analysis and culture. His intraoperative cultures grew K pneumoniae sensitive to multiple antibiotics, including ceftriaxone, sulfamethoxazole/trimethoprim, and ampicillin/sulbactam. The vascular surgery team recommended inpatient admission and administration of 6 weeks of IV antibiotics postoperatively with ceftriaxone, followed by outpatient oral suppression therapy after discharge. The patient tolerated the surgery well and was discharged after 6 weeks of IV ceftriaxone followed by outpatient oral suppression therapy. However, the patient was transferred back to BPVAHS for continued care and rehabilitation placement.
The patient’s subsequent course was complicated by multiple hospital admissions, including aspiration pneumonia, hypoglycemia, diarrhea, and anemia. On one of his CT abdomen/pelvic examinations, a cysticlike mass was noted in the pancreatic head with a possible pancreatic duodenal fistula (this mass was not mentioned on the initial presurgical CT, although it can be seen in retrospect (Figure 3). Gastroenterology was consulted.
An upper endoscopy was performed that confirmed the fistula at the second portion of the duodenum. Findings from an endoscopic ultrasonography performed at an outside institution were concerning for a main duct intraductal papillary mucinous neoplasm (IPMN) with fistula, with biopsy results pending.
Discussion
This case contributes to the evidence that poorly controlled T2DM can be a predisposing factor for multiple vascular complications, including the infection of the aortic wall with progression to EA. Klebsiella species are considered opportunistic, Gram-negative pathogens that may disseminate to other tissues, causing life-threatening infections, including pneumonia, UTIs, bacteremia, and sepsis.9K pneumoniae infections are particularly challenging in neonates, the elderly, and immunocompromised individuals.9 CT is sensitive and specific in the detection of this pathologic entity.1,3 In patients with a suspected infectious etiology, the presence of foci of gas on CT in solid organ tissues is usually associated with an anaerobic infection. Gas can also be produced by Gram-negative facultative anaerobes that can ferment glucose in necrotic tissues.9
Although any microorganism can infect the aorta, K pneumoniae cultured from the blood specimen, urine culture, and intraoperative specimens in our patient was responsible for the formed gas in the aortic wall. Occurrence of spontaneous gas by this microorganism is usually associated with conditions leading to either increased vulnerability to infections and/or enhanced bacterial virulence.9 Although a relationship between EA and T2DM has not been proved, it is well known that patients with T2DM have a defect in their host-defense mechanisms, making them more susceptible to infections such as EA. Furthermore, because patients with T2DM are prone to the development of Gram-negative sepsis, organisms such as K pneumoniae would tend to emerge. Patients with poorly controlled T2DM and the presence of an infectious source can be predisposed to infectious aortitis, eventually leading to a gas-forming infection of the aorta.5,7
We postulate that the hematogenous spread of bacteria from a laceration in the leg as well as the presence of the pancreaticoduodenal fistula was likely the cause of the infectious EA in this case, considering the patient’s underlying uncontrolled T2DM. The patient’s prior left lower extremity vascular graft also may have provided a nidus for spreading to the aorta. Other reported underlying diseases of EA include aortic atherosclerosis, T2DM, diverticulitis, colon cancer, underlying aneurysm, immune-compromised status, and the presence of a medical device or open surgery.4-7,9
To our knowledge, this is the first case of EA associated with a pancreaticoduodenal fistula related to intraductal papillary mucinous neoplasm (IPMN). Fistulation of a main duct IPMN is rare, occurring in just 6.6% of cases.10 It can occur both before and after malignant degeneration.
EA requires rapid diagnosis, antibiotic therapy, and consultation with a vascular surgeon for immediate resection of the infected artery and graft bypass. The initial treatment of suspected infectious aortitis is IV antibiotics with broad antimicrobial coverage of the most likely pathologic organisms, particularly staphylococcal species and Gram-negative rods. Surgical debridement and revascularization should be completed early because of the high mortality rate of this condition. The intent of surgery is to control sepsis and reconstruct the arterial vasculature. Patients should remain on parenteral or oral antibiotics for at least 6 weeks to ensure full clearance of the infection.8 They should be followed up closely with serial blood cultures and CT scans.8 The rarity of the disorder, low level of awareness, varying presentations, and lack of evidence delineating pathogenesis and causality contribute to the challenge of recognizing, diagnosing, and treating EA in patients with T2DM and inflammation.
Conclusions
This case report can help bring awareness of this rare and potentially life-threatening condition in patients with T2DM. Clinicians should be aware of the risk of AE, particularly in patients with several additional risk factors: recent skin/soft tissue trauma, prior vascular graft surgery, and an underlying pancreatic mass. CT is the imaging method of choice that helps to rapidly choose a necessary emergent treatment approach.
1. Litmanovich DE, Yıldırım A, Bankier AA. Insights into imaging of aortitis. Insights Imaging. 2012;3(6):545-560. doi:10.1007/s13244-012-0192-x
2. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32(9):488-490. doi:10.1002/clc.20578
3. Murphy DJ, Keraliya AR, Agrawal MD, Aghayev A, Steigner ML. Cross-sectional imaging of aortic infections. Insights Imaging. 2016;7(6):801-818. doi:10.1007/s13244-016-0522-5
4. Md Noh MSF, Abdul Rashid AM, Ar A, B N, Mohammed Y, A RE. Emphysematous aortitis: report of two cases and CT imaging findings. BJR Case Rep. 2017;3(3):20170006. doi:10.1259/bjrcr.20170006
5. Harris C, Geffen J, Rizg K, et al. A rare report of infectious emphysematous aortitis secondary to Clostridium septicum without prior vascular intervention. Case Rep Vasc Med. 2017;2017:4984325. doi:10.1155/2017/4984325
6. Ito F, Inokuchi R, Matsumoto A, et al. Presence of periaortic gas in Clostridium septicum-infected aortic aneurysm aids in early diagnosis: a case report and systematic review of the literature. J Med Case Rep. 2017;11(1):268. doi:10.1186/s13256-017-1422-0
7. Urgiles S, Matos-Casano H, Win KZ, Berardo J, Bhatt U, Shah J. Emphysematous aortitis due to Clostridium septicum in an 89-year-old female with ileus. Case Rep Infect Dis. 2019;2019:1094837. doi:10.1155/2019/1094837
8. Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89-97. doi:10.1007/s11936-005-0010-6
9. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629-661. doi:10.1128/mmbr.00078-15
10. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45(10):1080-1089. doi:10.1007/s00535-010-0263-z
1. Litmanovich DE, Yıldırım A, Bankier AA. Insights into imaging of aortitis. Insights Imaging. 2012;3(6):545-560. doi:10.1007/s13244-012-0192-x
2. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. 2009;32(9):488-490. doi:10.1002/clc.20578
3. Murphy DJ, Keraliya AR, Agrawal MD, Aghayev A, Steigner ML. Cross-sectional imaging of aortic infections. Insights Imaging. 2016;7(6):801-818. doi:10.1007/s13244-016-0522-5
4. Md Noh MSF, Abdul Rashid AM, Ar A, B N, Mohammed Y, A RE. Emphysematous aortitis: report of two cases and CT imaging findings. BJR Case Rep. 2017;3(3):20170006. doi:10.1259/bjrcr.20170006
5. Harris C, Geffen J, Rizg K, et al. A rare report of infectious emphysematous aortitis secondary to Clostridium septicum without prior vascular intervention. Case Rep Vasc Med. 2017;2017:4984325. doi:10.1155/2017/4984325
6. Ito F, Inokuchi R, Matsumoto A, et al. Presence of periaortic gas in Clostridium septicum-infected aortic aneurysm aids in early diagnosis: a case report and systematic review of the literature. J Med Case Rep. 2017;11(1):268. doi:10.1186/s13256-017-1422-0
7. Urgiles S, Matos-Casano H, Win KZ, Berardo J, Bhatt U, Shah J. Emphysematous aortitis due to Clostridium septicum in an 89-year-old female with ileus. Case Rep Infect Dis. 2019;2019:1094837. doi:10.1155/2019/1094837
8. Foote EA, Postier RG, Greenfield RA, Bronze MS. Infectious aortitis. Curr Treat Options Cardiovasc Med. 2005;7(2):89-97. doi:10.1007/s11936-005-0010-6
9. Paczosa MK, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629-661. doi:10.1128/mmbr.00078-15
10. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010;45(10):1080-1089. doi:10.1007/s00535-010-0263-z
72-year-old man • fever • new-onset urinary frequency • altered mental state • Dx?
THE CASE
A 72-year-old man was admitted to our Dallas hospital with a 4-day history of fevers and new-onset urinary frequency. He did not report any joint pain, sick contacts, or recent travel or recall any skin findings (rashes, insect bites). Past medical history was significant for hypertension, hyperlipidemia, diabetes, benign prostatic hyperplasia, recurrent urinary tract infections, and lumbar radiculopathy.
Initial signs and symptoms were suggestive of sepsis: a temperature of 102.7 °F, tachycardia, and a suspected genitourinary infection. This was supported by initial labs concerning for end-organ damage: elevated creatinine of 1.58 mg/dL (reference range, 0.67-1.17 mg/dL), elevated international normalized ratio (INR) of 1.6 (reference range, 0.9-1.1), hemoglobin of 12.8 g/dL (reference range, 13.5 - 17.5 g/dL), and platelet count of 99 ×109/L (reference range, 160-383 ×109/L).
Over the next several days, the patient’s condition worsened, and he experienced a decline in mental status, despite initiation of broad-spectrum antibiotics and fluid resuscitation. Although lumbar puncture was warranted, neither Neurology nor Interventional Radiology were willing to risk the procedure given the patient’s worsening hemoglobin (8.3 g/dL) and platelet count (51 ×109/L).
Preliminary work-up included a urinalysis negative for leukocytes, nitrites, and bacteria—despite a urine culture that showed gram-positive cocci. His chest x-ray was unremarkable, and computed tomography of his brain showed generalized atrophy without acute changes. The work-up was expanded to fungal cultures and immunochemical assays. Empiric treatment with micafungin and acyclovir was started without improvement.
Further conversation with family revealed that the patient liked to spend time outdoors and he’d had a similar episode in which he’d been diagnosed with an unknown disease from an insect bite. Pertinent negative tests included: HIV, syphilis, rapid heterophile antibody, influenza, respiratory virus panel, blood culture, fungal culture, antineutrophil cytoplasmic antibodies, histoplasmosis, brucellosis, malaria, Epstein-Barr virus, cytomegalovirus, and parvovirus. Coxiella burnetii and West Nile virus immunoglobulin (Ig) G were positive, suggesting a prior exposure.
THE DIAGNOSIS
Given these new findings and reported outdoor activities, Infectious Diseases recommended we start our patient on doxycycline for possible rickettsia infection. On Day 8, doxycycline 200 mg IV once daily was started. (The IV form was initiated due to the patient’s altered mentation.) The patient started to show improvement, and on Day 14, an immunofluorescence antibody (IFA) assay revealed Rickettsia typhi IgM titers 1:512 (< 1:64) and IgG titers 1:256 (< 1:64), consistent with a diagnosis of murine (endemic) typhus.
DISCUSSION
Murine typhus is an acute febrile disease caused by R typhi, an obligate, intracellular gram-negative organism.1 Worldwide, transmission to humans occurs mainly from infected rat fleas harbored by rodents. In the United States, it’s been suggested that opossums serve as an important reservoir in peri-domestic settings, with cat fleas as vectors.2-4 The disease is endemic to southern California and south Texas.4
Continue to: Incidence of murine typhus
Incidence of murine typhus has declined in the United States since 1945 with the use of the insecticide dichlorodiphenyltrichloroethane (DDT). However, a recent rise in murine typhus cases—likely due to ecological changes—makes timely diagnosis and treatment essential.5 An epidemiologic study of 1762 confirmed cases in Texas from 2003 to 2013 found an increase in the number of cases and an expansion of the geographic areas impacted.3 Thus, in the work-up of acute fever of unknown origin, it is not unreasonable to include murine typhus in the differential.
Murine typhus can be difficult to diagnose due to nonspecific clinical manifestation.3,4 A 2016 systematic review of 2074 patients reported common symptoms of fever, headache, malaise, chills, and myalgia.6 The most common laboratory abnormalities in adults were elevated aminotransferases, lactate dehydrogenase, hypoalbuminemia, and thrombocytopenia.6 A 4-fold increase in typhus group IgM or IgG-specific antibody titer by IFA is supportive of diagnosis.4
The differential diagnosis included urosepsis, prostatitis, syphilis, HIV, and meningitis. However, lack of response to broad-spectrum antibiotics and antifungals made a diagnosis of urologic infection unlikely. A negative sexually transmitted infection screen ruled out syphilis and HIV.
Treatment may begin without a definitive diagnosis
Serologic testing with IFA is the preferred diagnostic method; however, a definitive diagnosis is not needed before treatment can be initiated. Doxycycline is the first-line therapy for all rickettsioses. Adults are advised to take doxycycline 200 mg orally once, followed by 100 mg twice daily until the patient improves, has been afebrile for 48 hours, and has received treatment for at least 7 days.7 Oral chloramphenicol is considered a second-line treatment; however it is not available in the United States and is associated with adverse hematologic effects.7
Our patient responded remarkably well to the doxycycline. After a 14-day course was completed, he was discharged to a skilled nursing facility for physical rehabilitation.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Rickettsia diseases, such as murine typhus, should be considered in the differential if a patient presents with a worsening clinical picture of unresolved delirium; fever despite use of broad-spectrum antibiotics, antifungals, and antivirals; and a history of potential outdoor exposure. Sources include opossums or cats when flea contact is likely. Rickettsia diseases belong in the differential when there is a history of travel to tropical areas, as well. All suspected cases should be reported to the local health department.
CORRESPONDENCE
Tenzin Tsewang MD, 5200 Harry Hines Boulevard, Dallas, TX 75235; [email protected]
1. Afzal Z, Kallumadanda S, Wang F, et al. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis. 2017;23:1268-1273. doi: 10.3201/eid2308.161861
2. Stern RM, Luskin MR, Clark RP, et al. A headache of a diagnosis. N Engl J Med. 2018;379:475-479. doi: 10.1056/NEJMcps1803584
3. Murray KO, Evert N, Mayes B, et al. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Iinfect Dis. 2017;23:645-648. doi: 10.3201/eid2304.160958
4. Blanton LS, Idowu BM, Tatsch TN, et al. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am J Trop Med Hyg. 2016;95:457-461. doi: 10.4269/ajtmh.16-0197
5. Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918. doi: 10.1086/527443
6. Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24. doi: 10.1016/j.actatropica.2016.10.018
7. Petri WA Jr. Murine (Endemic) Typhus. Merck Manual Professional Version. Modified July 2020. Accessed October 25, 2021. www.merckmanuals.com/professional/infectious-diseases/rickettsiae-and-related-organisms/murine-endemic-typhus
THE CASE
A 72-year-old man was admitted to our Dallas hospital with a 4-day history of fevers and new-onset urinary frequency. He did not report any joint pain, sick contacts, or recent travel or recall any skin findings (rashes, insect bites). Past medical history was significant for hypertension, hyperlipidemia, diabetes, benign prostatic hyperplasia, recurrent urinary tract infections, and lumbar radiculopathy.
Initial signs and symptoms were suggestive of sepsis: a temperature of 102.7 °F, tachycardia, and a suspected genitourinary infection. This was supported by initial labs concerning for end-organ damage: elevated creatinine of 1.58 mg/dL (reference range, 0.67-1.17 mg/dL), elevated international normalized ratio (INR) of 1.6 (reference range, 0.9-1.1), hemoglobin of 12.8 g/dL (reference range, 13.5 - 17.5 g/dL), and platelet count of 99 ×109/L (reference range, 160-383 ×109/L).
Over the next several days, the patient’s condition worsened, and he experienced a decline in mental status, despite initiation of broad-spectrum antibiotics and fluid resuscitation. Although lumbar puncture was warranted, neither Neurology nor Interventional Radiology were willing to risk the procedure given the patient’s worsening hemoglobin (8.3 g/dL) and platelet count (51 ×109/L).
Preliminary work-up included a urinalysis negative for leukocytes, nitrites, and bacteria—despite a urine culture that showed gram-positive cocci. His chest x-ray was unremarkable, and computed tomography of his brain showed generalized atrophy without acute changes. The work-up was expanded to fungal cultures and immunochemical assays. Empiric treatment with micafungin and acyclovir was started without improvement.
Further conversation with family revealed that the patient liked to spend time outdoors and he’d had a similar episode in which he’d been diagnosed with an unknown disease from an insect bite. Pertinent negative tests included: HIV, syphilis, rapid heterophile antibody, influenza, respiratory virus panel, blood culture, fungal culture, antineutrophil cytoplasmic antibodies, histoplasmosis, brucellosis, malaria, Epstein-Barr virus, cytomegalovirus, and parvovirus. Coxiella burnetii and West Nile virus immunoglobulin (Ig) G were positive, suggesting a prior exposure.
THE DIAGNOSIS
Given these new findings and reported outdoor activities, Infectious Diseases recommended we start our patient on doxycycline for possible rickettsia infection. On Day 8, doxycycline 200 mg IV once daily was started. (The IV form was initiated due to the patient’s altered mentation.) The patient started to show improvement, and on Day 14, an immunofluorescence antibody (IFA) assay revealed Rickettsia typhi IgM titers 1:512 (< 1:64) and IgG titers 1:256 (< 1:64), consistent with a diagnosis of murine (endemic) typhus.
DISCUSSION
Murine typhus is an acute febrile disease caused by R typhi, an obligate, intracellular gram-negative organism.1 Worldwide, transmission to humans occurs mainly from infected rat fleas harbored by rodents. In the United States, it’s been suggested that opossums serve as an important reservoir in peri-domestic settings, with cat fleas as vectors.2-4 The disease is endemic to southern California and south Texas.4
Continue to: Incidence of murine typhus
Incidence of murine typhus has declined in the United States since 1945 with the use of the insecticide dichlorodiphenyltrichloroethane (DDT). However, a recent rise in murine typhus cases—likely due to ecological changes—makes timely diagnosis and treatment essential.5 An epidemiologic study of 1762 confirmed cases in Texas from 2003 to 2013 found an increase in the number of cases and an expansion of the geographic areas impacted.3 Thus, in the work-up of acute fever of unknown origin, it is not unreasonable to include murine typhus in the differential.
Murine typhus can be difficult to diagnose due to nonspecific clinical manifestation.3,4 A 2016 systematic review of 2074 patients reported common symptoms of fever, headache, malaise, chills, and myalgia.6 The most common laboratory abnormalities in adults were elevated aminotransferases, lactate dehydrogenase, hypoalbuminemia, and thrombocytopenia.6 A 4-fold increase in typhus group IgM or IgG-specific antibody titer by IFA is supportive of diagnosis.4
The differential diagnosis included urosepsis, prostatitis, syphilis, HIV, and meningitis. However, lack of response to broad-spectrum antibiotics and antifungals made a diagnosis of urologic infection unlikely. A negative sexually transmitted infection screen ruled out syphilis and HIV.
Treatment may begin without a definitive diagnosis
Serologic testing with IFA is the preferred diagnostic method; however, a definitive diagnosis is not needed before treatment can be initiated. Doxycycline is the first-line therapy for all rickettsioses. Adults are advised to take doxycycline 200 mg orally once, followed by 100 mg twice daily until the patient improves, has been afebrile for 48 hours, and has received treatment for at least 7 days.7 Oral chloramphenicol is considered a second-line treatment; however it is not available in the United States and is associated with adverse hematologic effects.7
Our patient responded remarkably well to the doxycycline. After a 14-day course was completed, he was discharged to a skilled nursing facility for physical rehabilitation.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Rickettsia diseases, such as murine typhus, should be considered in the differential if a patient presents with a worsening clinical picture of unresolved delirium; fever despite use of broad-spectrum antibiotics, antifungals, and antivirals; and a history of potential outdoor exposure. Sources include opossums or cats when flea contact is likely. Rickettsia diseases belong in the differential when there is a history of travel to tropical areas, as well. All suspected cases should be reported to the local health department.
CORRESPONDENCE
Tenzin Tsewang MD, 5200 Harry Hines Boulevard, Dallas, TX 75235; [email protected]
THE CASE
A 72-year-old man was admitted to our Dallas hospital with a 4-day history of fevers and new-onset urinary frequency. He did not report any joint pain, sick contacts, or recent travel or recall any skin findings (rashes, insect bites). Past medical history was significant for hypertension, hyperlipidemia, diabetes, benign prostatic hyperplasia, recurrent urinary tract infections, and lumbar radiculopathy.
Initial signs and symptoms were suggestive of sepsis: a temperature of 102.7 °F, tachycardia, and a suspected genitourinary infection. This was supported by initial labs concerning for end-organ damage: elevated creatinine of 1.58 mg/dL (reference range, 0.67-1.17 mg/dL), elevated international normalized ratio (INR) of 1.6 (reference range, 0.9-1.1), hemoglobin of 12.8 g/dL (reference range, 13.5 - 17.5 g/dL), and platelet count of 99 ×109/L (reference range, 160-383 ×109/L).
Over the next several days, the patient’s condition worsened, and he experienced a decline in mental status, despite initiation of broad-spectrum antibiotics and fluid resuscitation. Although lumbar puncture was warranted, neither Neurology nor Interventional Radiology were willing to risk the procedure given the patient’s worsening hemoglobin (8.3 g/dL) and platelet count (51 ×109/L).
Preliminary work-up included a urinalysis negative for leukocytes, nitrites, and bacteria—despite a urine culture that showed gram-positive cocci. His chest x-ray was unremarkable, and computed tomography of his brain showed generalized atrophy without acute changes. The work-up was expanded to fungal cultures and immunochemical assays. Empiric treatment with micafungin and acyclovir was started without improvement.
Further conversation with family revealed that the patient liked to spend time outdoors and he’d had a similar episode in which he’d been diagnosed with an unknown disease from an insect bite. Pertinent negative tests included: HIV, syphilis, rapid heterophile antibody, influenza, respiratory virus panel, blood culture, fungal culture, antineutrophil cytoplasmic antibodies, histoplasmosis, brucellosis, malaria, Epstein-Barr virus, cytomegalovirus, and parvovirus. Coxiella burnetii and West Nile virus immunoglobulin (Ig) G were positive, suggesting a prior exposure.
THE DIAGNOSIS
Given these new findings and reported outdoor activities, Infectious Diseases recommended we start our patient on doxycycline for possible rickettsia infection. On Day 8, doxycycline 200 mg IV once daily was started. (The IV form was initiated due to the patient’s altered mentation.) The patient started to show improvement, and on Day 14, an immunofluorescence antibody (IFA) assay revealed Rickettsia typhi IgM titers 1:512 (< 1:64) and IgG titers 1:256 (< 1:64), consistent with a diagnosis of murine (endemic) typhus.
DISCUSSION
Murine typhus is an acute febrile disease caused by R typhi, an obligate, intracellular gram-negative organism.1 Worldwide, transmission to humans occurs mainly from infected rat fleas harbored by rodents. In the United States, it’s been suggested that opossums serve as an important reservoir in peri-domestic settings, with cat fleas as vectors.2-4 The disease is endemic to southern California and south Texas.4
Continue to: Incidence of murine typhus
Incidence of murine typhus has declined in the United States since 1945 with the use of the insecticide dichlorodiphenyltrichloroethane (DDT). However, a recent rise in murine typhus cases—likely due to ecological changes—makes timely diagnosis and treatment essential.5 An epidemiologic study of 1762 confirmed cases in Texas from 2003 to 2013 found an increase in the number of cases and an expansion of the geographic areas impacted.3 Thus, in the work-up of acute fever of unknown origin, it is not unreasonable to include murine typhus in the differential.
Murine typhus can be difficult to diagnose due to nonspecific clinical manifestation.3,4 A 2016 systematic review of 2074 patients reported common symptoms of fever, headache, malaise, chills, and myalgia.6 The most common laboratory abnormalities in adults were elevated aminotransferases, lactate dehydrogenase, hypoalbuminemia, and thrombocytopenia.6 A 4-fold increase in typhus group IgM or IgG-specific antibody titer by IFA is supportive of diagnosis.4
The differential diagnosis included urosepsis, prostatitis, syphilis, HIV, and meningitis. However, lack of response to broad-spectrum antibiotics and antifungals made a diagnosis of urologic infection unlikely. A negative sexually transmitted infection screen ruled out syphilis and HIV.
Treatment may begin without a definitive diagnosis
Serologic testing with IFA is the preferred diagnostic method; however, a definitive diagnosis is not needed before treatment can be initiated. Doxycycline is the first-line therapy for all rickettsioses. Adults are advised to take doxycycline 200 mg orally once, followed by 100 mg twice daily until the patient improves, has been afebrile for 48 hours, and has received treatment for at least 7 days.7 Oral chloramphenicol is considered a second-line treatment; however it is not available in the United States and is associated with adverse hematologic effects.7
Our patient responded remarkably well to the doxycycline. After a 14-day course was completed, he was discharged to a skilled nursing facility for physical rehabilitation.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Rickettsia diseases, such as murine typhus, should be considered in the differential if a patient presents with a worsening clinical picture of unresolved delirium; fever despite use of broad-spectrum antibiotics, antifungals, and antivirals; and a history of potential outdoor exposure. Sources include opossums or cats when flea contact is likely. Rickettsia diseases belong in the differential when there is a history of travel to tropical areas, as well. All suspected cases should be reported to the local health department.
CORRESPONDENCE
Tenzin Tsewang MD, 5200 Harry Hines Boulevard, Dallas, TX 75235; [email protected]
1. Afzal Z, Kallumadanda S, Wang F, et al. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis. 2017;23:1268-1273. doi: 10.3201/eid2308.161861
2. Stern RM, Luskin MR, Clark RP, et al. A headache of a diagnosis. N Engl J Med. 2018;379:475-479. doi: 10.1056/NEJMcps1803584
3. Murray KO, Evert N, Mayes B, et al. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Iinfect Dis. 2017;23:645-648. doi: 10.3201/eid2304.160958
4. Blanton LS, Idowu BM, Tatsch TN, et al. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am J Trop Med Hyg. 2016;95:457-461. doi: 10.4269/ajtmh.16-0197
5. Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918. doi: 10.1086/527443
6. Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24. doi: 10.1016/j.actatropica.2016.10.018
7. Petri WA Jr. Murine (Endemic) Typhus. Merck Manual Professional Version. Modified July 2020. Accessed October 25, 2021. www.merckmanuals.com/professional/infectious-diseases/rickettsiae-and-related-organisms/murine-endemic-typhus
1. Afzal Z, Kallumadanda S, Wang F, et al. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerg Infect Dis. 2017;23:1268-1273. doi: 10.3201/eid2308.161861
2. Stern RM, Luskin MR, Clark RP, et al. A headache of a diagnosis. N Engl J Med. 2018;379:475-479. doi: 10.1056/NEJMcps1803584
3. Murray KO, Evert N, Mayes B, et al. Typhus group rickettsiosis, Texas, USA, 2003–2013. Emerg Iinfect Dis. 2017;23:645-648. doi: 10.3201/eid2304.160958
4. Blanton LS, Idowu BM, Tatsch TN, et al. Opossums and cat fleas: new insights in the ecology of murine typhus in Galveston, Texas. Am J Trop Med Hyg. 2016;95:457-461. doi: 10.4269/ajtmh.16-0197
5. Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918. doi: 10.1086/527443
6. Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24. doi: 10.1016/j.actatropica.2016.10.018
7. Petri WA Jr. Murine (Endemic) Typhus. Merck Manual Professional Version. Modified July 2020. Accessed October 25, 2021. www.merckmanuals.com/professional/infectious-diseases/rickettsiae-and-related-organisms/murine-endemic-typhus
The Meaning of Words and Why They Matter During End-of-Life Conversations
Effective communication during end-of-life is crucial for health care delivery, but misinterpretation can influence how the quality of the care is rendered and perceived.
When I was a new palliative care nurse practitioner (NP), I remember my mentor telling me that communication in our field is equivalent to surgical procedures in general surgery. Conversations need to be handled with accuracy and precision, conducted in a timely fashion, and require skills that take practice to sharpen. Over the years, I learned that unlike surgery, we do not have control over how the procedure will flow. We approach patients with a blank canvas, open to receive messages that will be shared and reacted to accordingly. The ability to communicate effectively also requires compassion, which is a trait that tends to be inherent in humans and typically is not learned from textbooks but can be cultivated with training and application.
Among the barriers identified to effective communication are avoiding emotional issues and focusing on technical topics due in part to the fear of lengthy encounters, not allowing patients or families enough time to speak, and reframing instead of validating emotions.1 Many years later, I had the chance to help care for a patient whose story reminds me of how our choice of words and how our interpretation of what we are told can influence the way we care for patients and their families.
Case Presentation
Mr. P, aged 86 years, was admitted to a teaching hospital for pneumonia and heart failure exacerbation. He was treated with diuretics and antibiotics and discharged home on room air after 3 days. He returned to the hospital after 8 days, reporting labored breathing. He was found to be hypoxic, and a further workup revealed acute hypoxic respiratory failure that was likely from severe pulmonary hypertension and exacerbation of his heart failure. Left heart disease is a common cause of pulmonary hypertension, which can lead to right ventricular failure and increased mortality.2
After meeting with his pulmonologist and cardiologist, Mr. P elected for a do-not-resuscitate code status and declined to be intubated. He also refused further diagnostics and life-prolonging treatments for his conditions, including a stress test, cardiac catheterization, and a right heart catheterization. He required bilevel positive airway pressure (BPAP) support at bedtime, which he also declined. He agreed to the use of supplemental oxygen through a nasal cannula and always needed 5 liters of oxygen.
Palliative care was consulted to assist with goals of care discussion. This visit took place during the COVID-19 pandemic, but Mr. P had tested negative for the COVID virus, so the palliative care NP was able to meet with Mr. P in person. He shared his understanding of the serious nature of his condition and the likelihood of a limited life expectancy without further diagnostics and possible life-prolonging treatments. He said his goal was to go home and spend the remainder of his life with his wife. He had not been out of bed since his hospitalization except to transfer to a nearby chair with the help of his nurse due to exertional dyspnea and generalized weakness. Prior to his recent hospitalizations, he was independently ambulating and had no dyspnea when performing strenuous activities. Mr. P shared that his wife was aged in her 70s and was legally blind. He added that she did not require physical assistance, but he was unsure whether she could help him because they had not been in such a situation previously. They had a daughter who visited frequently and helped with driving them to doctors’ appointments and shopping. Mr. P shared that he wanted to go home. After explaining the option of home hospice, Mr. P decided he wanted to receive hospice services at home and asked palliative care NP to contact his daughter to let her know his wishes and to tell her more about how hospice can help with his care.
The palliative care NP met with Mr. P’s nurse and shared the outcome of her visit. His nurse asked the palliative care NP whether she was familiar with his daughter. The nurse added that she wanted the palliative care NP to know that Mr. P’s daughter was quite angry and upset with his doctors after being told about his prognosis. His doctors’ notes also indicated that Mr. P wanted them to contact his daughter regarding his condition and plans for discharge, concluding that he deferred to his daughter for medical decision making.
As Mr. P’s hospitalization took place during the COVID pandemic, a face-to-face meeting with his family was not possible. The NP spoke with Mr. P’s daughter over the phone to relay his wishes and goals for his care. Mr. P’s daughter cried at times during the conversation and asked whether his condition was really that serious. The NP allowed Mr. P’s daughter to express her sadness and allowed for periods of silence during the conversation while his daughter gathered her composure. The NP reinforced the clinical information she had been provided by the medical team. Mr. P’s daughter added that he was completely independent, not requiring supplemental oxygen and was otherwise healthy just a month prior. She also asked whether there was truly nothing else that could be done to prolong his life. The NP acknowledged her observations and explained how Mr. P’s body and organs had not been able to bounce back from the recent insults to his overall physical condition.
After being told that Mr. P’s options for treatment were limited not only by his advanced age and comorbidities, but also the limitations and goals for his care he had identified, his daughter supported her father’s decision. The palliative care NP provided her information on how home hospice assists in her father’s care at home, including symptom management, nursing visits, home equipment, family support, among others. Mr. P’s daughter also said she would relay the information to her mother and call the palliative care NP if they had additional questions or concerns.
The outcome of her visit with Mr. P and his daughter were relayed by the palliative care NP to his acute health care team through an official response to the consultation request via his electronic health record. The palliative care NP also alerted the palliative care social worker to follow-up with Mr. P, his daughter, and his acute health care team to coordinate hospice services at the time of his discharge from the hospital.
Mr. P was discharged from the hospital with home hospice services after a few days. Three weeks later, Mr. P passed away peacefully on the in-patient unit of his home hospice agency as his physical care needs became too much for his family to provide at home a few days before his death. The palliative care social worker later shared with the NP that Mr. P’s daughter shared her gratitude and satisfaction with the care he had received not only from palliative care, but also from everyone during his hospitalization.
Discussion
Key themes found in end-of-life (EOL) communication with families and caregivers include highlighting clinical deterioration, involvement in decision making, continuation of high-quality care after cessation of aggressive measures, tailoring to individuals, clarity, honesty, and use of techniques in delivery.2 Some of the techniques identified were pacing, staging, and repetition.3 Other techniques that can be beneficial include allowing for time to express one’s feelings, being comfortable with brief periods of silence, validating observations shared, among others. These themes were evident in the interactions that his health care team had with Mr. P and his daughter. With honesty and clarity, various members of the health care team repeatedly shared information regarding his clinical deterioration.
Family Influence
EOL decision-making roles within a family tend to originate from family interactional histories, familial roles as well as decision-making situations the family faces.4 The US medical and legal systems also recognize formal role assignments for surrogate decision makers.4 In the case of Mr. P, his advance directive (AD) identified his daughter as his surrogate decision maker. ADs are written statements made in advance by patients expressing their wishes and limitations for treatment as well as appointing surrogate decision makers when they become unable to decide for themselves in the future.5
During discussions about the goals for his care, Mr. P made his own medical decisions and elected to pursue a comfort-focused approach to care. His request for his health care team to reach out to his daughter was largely due to his need for assistance in explaining the complexity of his clinical condition to her and how hospice services would be helpful with his EOL care. Mr. P depended on his daughter to bring him to the hospital or to his doctors’ appointments, and she had been a major source of support for him and his wife. Contrary to the belief of some of his health care practitioners, Mr. P was not deferring his medical decisions to his daughter but rather allowing for her participation as his health care partner.
Communication between nurses and patients has been found to be challenging to both parties. Nurses express difficulties in areas that include supporting patients and families after they have had a difficult conversation with their physicians and responding to patients and family members’ emotions like anger.6 EOL care issues, such as family barriers to prognostic understanding, can interfere with psychosocial care.6 Families of patients approaching the EOL describe feeling mentally worn down and being unable to think straight, leading to feelings of being overwhelmed.7 They feel the need to be in a place where they can accept the content of difficult EOL conversations to be able to effectively engage.7
Studies have shown that family members of patients at the EOL experience stress, anxiety, fatigue and depression.8 Reactions that can be perceived as anger may not be so nor directed to the health care team. Questions raised regarding the accuracy of prognostication and treatment recommendations may not necessarily reflect concerns about the quality of care received but an exercise of advocacy in exploring other options on behalf of the patient. Allowing time for families to process the information received and react freely are necessary steps to facilitate reaching a place where they can acknowledge the information being relayed.
Communication Skills Training
Every member of the health care team should be equipped with the basic skills to have these conversations. The academic curricula for members of the health care team focuses on developing communication skills, but there has been a lack of content on palliative and EOL care.9
Due to time constraints and limited opportunities in the clinical setting, there has been an increasing use of simulation-based learning activities (SBLA) to enhance communication skills among nursing students.9 At this time, the impact of SBLA in enhancing communication competency is not fully known, but given the lack of clinical opportunities for students, this option is worth considering.9 When asked, nurses recognized the need for improved EOL communication education, training, and guidelines.10 They also felt that a multidisciplinary approach in EOL communication is beneficial. The inclusion of the End-of-Life Nursing Education Consortium (ELNEC) Core training in Bachelor of Science in Nursing programs have led to improved insight on palliative care and nurses’ role in palliative care and hospice among nursing students.11
The Palliative Care and Hospice Education and Training Act of 2017 amended the Public Health Service Act to include improving EOL training for health care providers, including talking about death and dying.12 Even though the Liaison Committee of Medical Education asked medical schools to incorporate EOL care education in the medical school curricula, there is still a lack of developmentally appropriate and supervised EOL education in medical schools.12 Training on grief also has been lacking and less likely to be mandatory among medical students and residents: Workshops are mostly conducted before they can be applied in the clinical setting.13 Meanwhile, resources are available to assist physicians in EOL conversations with patient and families, such as the Serious Illness Conversation Guide, The Conversation Project, and Stanford’s Letter Project.12
Conclusions
Palliative consultation is associated with an overall improvement in EOL care, communication, and support, according to families of deceased patients.14 It has also been shown to enhance patients’ quality of life and mood, improve documentation of resuscitation preferences, and lead to less aggressive care at the EOL, including less chemotherapy.15 Integration of palliative care in the care of patients hospitalized with acute heart failure has been associated with improved quality of life, decreased symptom burden and depressive symptoms, and increased participation in advance care planning.16
The involvement of palliative care in the care of patients and their families at EOL enhances goals of care discussions that improve understanding for everyone involved. It helps provide consistency with the message being delivered by the rest of the health care team to patients and families regarding prognosis and recommendations. Palliative care can provide an alternative when all other aggressive measures are no longer helpful and allow for the continuation of care with a shift in focus from prolonging life to promoting its quality. Furthermore, palliative care involvement for care of patients with life-limiting illness also has been found to improve symptom control, decrease hospitalizations and health care costs, and even improve mortality.17A multidisciplinary approach to palliative care EOL conversations is beneficial, but every member of the health care team should have the training, education, and skills to be ready to have these difficult conversations. These health care team members include physicians, advance practice clinicians, nurses, social workers, and chaplains, among others. Patients and families are likely to be in contact with different members of the health care team who should be able to carry out therapeutic conversations. Using validated tools and resources on communication techniques through evidence-based practice is helpful and should be encouraged. This provides a framework on how EOL conversations should be conducted in the clinical setting to augment the identified lack of training on EOL communication in schools. Repeated opportunities for its use over time will help improve the ability of clinicians to engage in effective EOL communication.
1. MacKenzie AR, Lasota M. Bringing life to death: the need for honest, compassionate, and effective end-of-life conversations. Am Soc Clin Oncol Educ Book. 2020;40:476-484. doi:10.1200/EDBK_279767
2. Krishnan U, Horn E. Pulmonary hypertension due to left heart disease (group 2 pulmonary hypertension) in adults. Accessed September 17, 2021. https://www.uptodate.com/contents/pulmonary-hypertension-due-to-left-heart-disease-group-2-pulmonary-hypertension-in-adults
3. Anderson RJ, Bloch S, Armstrong M, Stone PC, Low JT. Communication between healthcare professionals and relatives of patients approaching the end-of-life: a systematic review of qualitative evidence. Palliat Med. 2019;33(8):926-941. doi:10.1177/0269216319852007
4. Trees AR, Ohs JE, Murray MC. Family communication about end-of-life decisions and the enactment of the decision-maker role. Behav Sci (Basel). 2017;7(2):36. doi:10.3390/bs7020036 5. Arruda LM, Abreu KPB, Santana LBC, Sales MVC. Variables that influence the medical decision regarding advance directives and their impact on end-of-life care. Einstein (Sao Paulo). 2019;18:eRW4852. doi:10.31744/einstein_journal/2020RW4852
6. Banerjee SC, Manna R, Coyle N, et al. The implementation and evaluation of a communication skills training program for oncology nurses. Transl Behav Med. 2017;7(3):615-623. doi:10.1007/s13142-017-0473-5
7. Mitchell S, Spry JL, Hill E, Coad J, Dale J, Plunkett A. Parental experiences of end of life care decision-making for children with life-limiting conditions in the paediatric intensive care unit: a qualitative interview study. BMJ Open. 2019;9(5):e028548. doi:10.1136/bmjopen-2018-028548
8. Laryionava K, Pfeil TA, Dietrich M, Reiter-Theil S, Hiddemann W, Winkler EC. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
9. Smith MB, Macieira TGR, Bumbach MD, et al. The use of simulation to teach nursing students and clinicians palliative care and end-of-life communication: a systematic review. Am J Hosp Palliat Care. 2018;35(8):1140-1154. doi:10.1177/1049909118761386
10. Griffiths I. What are the challenges for nurses when providing end-of-life care in intensive care units? Br J Nurs. 2019;28(16):1047-1052. doi:10.12968/bjon.2019.28.16.1047
11. Li J, Smothers A, Fang W, Borland M. Undergraduate nursing students’ perception of end-of-life care education placement in the nursing curriculum. J Hosp Palliat Nurs. 2019;21(5):E12-E18. doi:10.1097/NJH.0000000000000533
12. Sutherland R. Dying well-informed: the need for better clinical educationsurrounding facilitating end-of-life conversations. Yale J Biol Med. 2019;92(4):757-764.
13. Sikstrom L, Saikaly R, Ferguson G, Mosher PJ, Bonato S, Soklaridis S. Being there: a scoping review of grief support training in medical education. PLoS One. 2019;14(11):e0224325. doi:10.1371/journal.pone.0224325
14. Yefimova M, Aslakson RA, Yang L, et al. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020;155(2):138-146. doi:10.1001/jamasurg.2019.5083
15. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-42. doi:10.1056/NEJMoa1000678.
16. Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18(2):134-142. doi:org/10.1089/jpm.2014.0192
17. Diop MS, Rudolph JL, Zimmerman KM, Richter MA, Skarf LM. Palliative careinterventions for patients with heart failure: a systematic review and meta-analysis. J Palliat Med. 2017;20(1):84-92. doi:10.1089/jpm.2016.0330
Effective communication during end-of-life is crucial for health care delivery, but misinterpretation can influence how the quality of the care is rendered and perceived.
Effective communication during end-of-life is crucial for health care delivery, but misinterpretation can influence how the quality of the care is rendered and perceived.
When I was a new palliative care nurse practitioner (NP), I remember my mentor telling me that communication in our field is equivalent to surgical procedures in general surgery. Conversations need to be handled with accuracy and precision, conducted in a timely fashion, and require skills that take practice to sharpen. Over the years, I learned that unlike surgery, we do not have control over how the procedure will flow. We approach patients with a blank canvas, open to receive messages that will be shared and reacted to accordingly. The ability to communicate effectively also requires compassion, which is a trait that tends to be inherent in humans and typically is not learned from textbooks but can be cultivated with training and application.
Among the barriers identified to effective communication are avoiding emotional issues and focusing on technical topics due in part to the fear of lengthy encounters, not allowing patients or families enough time to speak, and reframing instead of validating emotions.1 Many years later, I had the chance to help care for a patient whose story reminds me of how our choice of words and how our interpretation of what we are told can influence the way we care for patients and their families.
Case Presentation
Mr. P, aged 86 years, was admitted to a teaching hospital for pneumonia and heart failure exacerbation. He was treated with diuretics and antibiotics and discharged home on room air after 3 days. He returned to the hospital after 8 days, reporting labored breathing. He was found to be hypoxic, and a further workup revealed acute hypoxic respiratory failure that was likely from severe pulmonary hypertension and exacerbation of his heart failure. Left heart disease is a common cause of pulmonary hypertension, which can lead to right ventricular failure and increased mortality.2
After meeting with his pulmonologist and cardiologist, Mr. P elected for a do-not-resuscitate code status and declined to be intubated. He also refused further diagnostics and life-prolonging treatments for his conditions, including a stress test, cardiac catheterization, and a right heart catheterization. He required bilevel positive airway pressure (BPAP) support at bedtime, which he also declined. He agreed to the use of supplemental oxygen through a nasal cannula and always needed 5 liters of oxygen.
Palliative care was consulted to assist with goals of care discussion. This visit took place during the COVID-19 pandemic, but Mr. P had tested negative for the COVID virus, so the palliative care NP was able to meet with Mr. P in person. He shared his understanding of the serious nature of his condition and the likelihood of a limited life expectancy without further diagnostics and possible life-prolonging treatments. He said his goal was to go home and spend the remainder of his life with his wife. He had not been out of bed since his hospitalization except to transfer to a nearby chair with the help of his nurse due to exertional dyspnea and generalized weakness. Prior to his recent hospitalizations, he was independently ambulating and had no dyspnea when performing strenuous activities. Mr. P shared that his wife was aged in her 70s and was legally blind. He added that she did not require physical assistance, but he was unsure whether she could help him because they had not been in such a situation previously. They had a daughter who visited frequently and helped with driving them to doctors’ appointments and shopping. Mr. P shared that he wanted to go home. After explaining the option of home hospice, Mr. P decided he wanted to receive hospice services at home and asked palliative care NP to contact his daughter to let her know his wishes and to tell her more about how hospice can help with his care.
The palliative care NP met with Mr. P’s nurse and shared the outcome of her visit. His nurse asked the palliative care NP whether she was familiar with his daughter. The nurse added that she wanted the palliative care NP to know that Mr. P’s daughter was quite angry and upset with his doctors after being told about his prognosis. His doctors’ notes also indicated that Mr. P wanted them to contact his daughter regarding his condition and plans for discharge, concluding that he deferred to his daughter for medical decision making.
As Mr. P’s hospitalization took place during the COVID pandemic, a face-to-face meeting with his family was not possible. The NP spoke with Mr. P’s daughter over the phone to relay his wishes and goals for his care. Mr. P’s daughter cried at times during the conversation and asked whether his condition was really that serious. The NP allowed Mr. P’s daughter to express her sadness and allowed for periods of silence during the conversation while his daughter gathered her composure. The NP reinforced the clinical information she had been provided by the medical team. Mr. P’s daughter added that he was completely independent, not requiring supplemental oxygen and was otherwise healthy just a month prior. She also asked whether there was truly nothing else that could be done to prolong his life. The NP acknowledged her observations and explained how Mr. P’s body and organs had not been able to bounce back from the recent insults to his overall physical condition.
After being told that Mr. P’s options for treatment were limited not only by his advanced age and comorbidities, but also the limitations and goals for his care he had identified, his daughter supported her father’s decision. The palliative care NP provided her information on how home hospice assists in her father’s care at home, including symptom management, nursing visits, home equipment, family support, among others. Mr. P’s daughter also said she would relay the information to her mother and call the palliative care NP if they had additional questions or concerns.
The outcome of her visit with Mr. P and his daughter were relayed by the palliative care NP to his acute health care team through an official response to the consultation request via his electronic health record. The palliative care NP also alerted the palliative care social worker to follow-up with Mr. P, his daughter, and his acute health care team to coordinate hospice services at the time of his discharge from the hospital.
Mr. P was discharged from the hospital with home hospice services after a few days. Three weeks later, Mr. P passed away peacefully on the in-patient unit of his home hospice agency as his physical care needs became too much for his family to provide at home a few days before his death. The palliative care social worker later shared with the NP that Mr. P’s daughter shared her gratitude and satisfaction with the care he had received not only from palliative care, but also from everyone during his hospitalization.
Discussion
Key themes found in end-of-life (EOL) communication with families and caregivers include highlighting clinical deterioration, involvement in decision making, continuation of high-quality care after cessation of aggressive measures, tailoring to individuals, clarity, honesty, and use of techniques in delivery.2 Some of the techniques identified were pacing, staging, and repetition.3 Other techniques that can be beneficial include allowing for time to express one’s feelings, being comfortable with brief periods of silence, validating observations shared, among others. These themes were evident in the interactions that his health care team had with Mr. P and his daughter. With honesty and clarity, various members of the health care team repeatedly shared information regarding his clinical deterioration.
Family Influence
EOL decision-making roles within a family tend to originate from family interactional histories, familial roles as well as decision-making situations the family faces.4 The US medical and legal systems also recognize formal role assignments for surrogate decision makers.4 In the case of Mr. P, his advance directive (AD) identified his daughter as his surrogate decision maker. ADs are written statements made in advance by patients expressing their wishes and limitations for treatment as well as appointing surrogate decision makers when they become unable to decide for themselves in the future.5
During discussions about the goals for his care, Mr. P made his own medical decisions and elected to pursue a comfort-focused approach to care. His request for his health care team to reach out to his daughter was largely due to his need for assistance in explaining the complexity of his clinical condition to her and how hospice services would be helpful with his EOL care. Mr. P depended on his daughter to bring him to the hospital or to his doctors’ appointments, and she had been a major source of support for him and his wife. Contrary to the belief of some of his health care practitioners, Mr. P was not deferring his medical decisions to his daughter but rather allowing for her participation as his health care partner.
Communication between nurses and patients has been found to be challenging to both parties. Nurses express difficulties in areas that include supporting patients and families after they have had a difficult conversation with their physicians and responding to patients and family members’ emotions like anger.6 EOL care issues, such as family barriers to prognostic understanding, can interfere with psychosocial care.6 Families of patients approaching the EOL describe feeling mentally worn down and being unable to think straight, leading to feelings of being overwhelmed.7 They feel the need to be in a place where they can accept the content of difficult EOL conversations to be able to effectively engage.7
Studies have shown that family members of patients at the EOL experience stress, anxiety, fatigue and depression.8 Reactions that can be perceived as anger may not be so nor directed to the health care team. Questions raised regarding the accuracy of prognostication and treatment recommendations may not necessarily reflect concerns about the quality of care received but an exercise of advocacy in exploring other options on behalf of the patient. Allowing time for families to process the information received and react freely are necessary steps to facilitate reaching a place where they can acknowledge the information being relayed.
Communication Skills Training
Every member of the health care team should be equipped with the basic skills to have these conversations. The academic curricula for members of the health care team focuses on developing communication skills, but there has been a lack of content on palliative and EOL care.9
Due to time constraints and limited opportunities in the clinical setting, there has been an increasing use of simulation-based learning activities (SBLA) to enhance communication skills among nursing students.9 At this time, the impact of SBLA in enhancing communication competency is not fully known, but given the lack of clinical opportunities for students, this option is worth considering.9 When asked, nurses recognized the need for improved EOL communication education, training, and guidelines.10 They also felt that a multidisciplinary approach in EOL communication is beneficial. The inclusion of the End-of-Life Nursing Education Consortium (ELNEC) Core training in Bachelor of Science in Nursing programs have led to improved insight on palliative care and nurses’ role in palliative care and hospice among nursing students.11
The Palliative Care and Hospice Education and Training Act of 2017 amended the Public Health Service Act to include improving EOL training for health care providers, including talking about death and dying.12 Even though the Liaison Committee of Medical Education asked medical schools to incorporate EOL care education in the medical school curricula, there is still a lack of developmentally appropriate and supervised EOL education in medical schools.12 Training on grief also has been lacking and less likely to be mandatory among medical students and residents: Workshops are mostly conducted before they can be applied in the clinical setting.13 Meanwhile, resources are available to assist physicians in EOL conversations with patient and families, such as the Serious Illness Conversation Guide, The Conversation Project, and Stanford’s Letter Project.12
Conclusions
Palliative consultation is associated with an overall improvement in EOL care, communication, and support, according to families of deceased patients.14 It has also been shown to enhance patients’ quality of life and mood, improve documentation of resuscitation preferences, and lead to less aggressive care at the EOL, including less chemotherapy.15 Integration of palliative care in the care of patients hospitalized with acute heart failure has been associated with improved quality of life, decreased symptom burden and depressive symptoms, and increased participation in advance care planning.16
The involvement of palliative care in the care of patients and their families at EOL enhances goals of care discussions that improve understanding for everyone involved. It helps provide consistency with the message being delivered by the rest of the health care team to patients and families regarding prognosis and recommendations. Palliative care can provide an alternative when all other aggressive measures are no longer helpful and allow for the continuation of care with a shift in focus from prolonging life to promoting its quality. Furthermore, palliative care involvement for care of patients with life-limiting illness also has been found to improve symptom control, decrease hospitalizations and health care costs, and even improve mortality.17A multidisciplinary approach to palliative care EOL conversations is beneficial, but every member of the health care team should have the training, education, and skills to be ready to have these difficult conversations. These health care team members include physicians, advance practice clinicians, nurses, social workers, and chaplains, among others. Patients and families are likely to be in contact with different members of the health care team who should be able to carry out therapeutic conversations. Using validated tools and resources on communication techniques through evidence-based practice is helpful and should be encouraged. This provides a framework on how EOL conversations should be conducted in the clinical setting to augment the identified lack of training on EOL communication in schools. Repeated opportunities for its use over time will help improve the ability of clinicians to engage in effective EOL communication.
When I was a new palliative care nurse practitioner (NP), I remember my mentor telling me that communication in our field is equivalent to surgical procedures in general surgery. Conversations need to be handled with accuracy and precision, conducted in a timely fashion, and require skills that take practice to sharpen. Over the years, I learned that unlike surgery, we do not have control over how the procedure will flow. We approach patients with a blank canvas, open to receive messages that will be shared and reacted to accordingly. The ability to communicate effectively also requires compassion, which is a trait that tends to be inherent in humans and typically is not learned from textbooks but can be cultivated with training and application.
Among the barriers identified to effective communication are avoiding emotional issues and focusing on technical topics due in part to the fear of lengthy encounters, not allowing patients or families enough time to speak, and reframing instead of validating emotions.1 Many years later, I had the chance to help care for a patient whose story reminds me of how our choice of words and how our interpretation of what we are told can influence the way we care for patients and their families.
Case Presentation
Mr. P, aged 86 years, was admitted to a teaching hospital for pneumonia and heart failure exacerbation. He was treated with diuretics and antibiotics and discharged home on room air after 3 days. He returned to the hospital after 8 days, reporting labored breathing. He was found to be hypoxic, and a further workup revealed acute hypoxic respiratory failure that was likely from severe pulmonary hypertension and exacerbation of his heart failure. Left heart disease is a common cause of pulmonary hypertension, which can lead to right ventricular failure and increased mortality.2
After meeting with his pulmonologist and cardiologist, Mr. P elected for a do-not-resuscitate code status and declined to be intubated. He also refused further diagnostics and life-prolonging treatments for his conditions, including a stress test, cardiac catheterization, and a right heart catheterization. He required bilevel positive airway pressure (BPAP) support at bedtime, which he also declined. He agreed to the use of supplemental oxygen through a nasal cannula and always needed 5 liters of oxygen.
Palliative care was consulted to assist with goals of care discussion. This visit took place during the COVID-19 pandemic, but Mr. P had tested negative for the COVID virus, so the palliative care NP was able to meet with Mr. P in person. He shared his understanding of the serious nature of his condition and the likelihood of a limited life expectancy without further diagnostics and possible life-prolonging treatments. He said his goal was to go home and spend the remainder of his life with his wife. He had not been out of bed since his hospitalization except to transfer to a nearby chair with the help of his nurse due to exertional dyspnea and generalized weakness. Prior to his recent hospitalizations, he was independently ambulating and had no dyspnea when performing strenuous activities. Mr. P shared that his wife was aged in her 70s and was legally blind. He added that she did not require physical assistance, but he was unsure whether she could help him because they had not been in such a situation previously. They had a daughter who visited frequently and helped with driving them to doctors’ appointments and shopping. Mr. P shared that he wanted to go home. After explaining the option of home hospice, Mr. P decided he wanted to receive hospice services at home and asked palliative care NP to contact his daughter to let her know his wishes and to tell her more about how hospice can help with his care.
The palliative care NP met with Mr. P’s nurse and shared the outcome of her visit. His nurse asked the palliative care NP whether she was familiar with his daughter. The nurse added that she wanted the palliative care NP to know that Mr. P’s daughter was quite angry and upset with his doctors after being told about his prognosis. His doctors’ notes also indicated that Mr. P wanted them to contact his daughter regarding his condition and plans for discharge, concluding that he deferred to his daughter for medical decision making.
As Mr. P’s hospitalization took place during the COVID pandemic, a face-to-face meeting with his family was not possible. The NP spoke with Mr. P’s daughter over the phone to relay his wishes and goals for his care. Mr. P’s daughter cried at times during the conversation and asked whether his condition was really that serious. The NP allowed Mr. P’s daughter to express her sadness and allowed for periods of silence during the conversation while his daughter gathered her composure. The NP reinforced the clinical information she had been provided by the medical team. Mr. P’s daughter added that he was completely independent, not requiring supplemental oxygen and was otherwise healthy just a month prior. She also asked whether there was truly nothing else that could be done to prolong his life. The NP acknowledged her observations and explained how Mr. P’s body and organs had not been able to bounce back from the recent insults to his overall physical condition.
After being told that Mr. P’s options for treatment were limited not only by his advanced age and comorbidities, but also the limitations and goals for his care he had identified, his daughter supported her father’s decision. The palliative care NP provided her information on how home hospice assists in her father’s care at home, including symptom management, nursing visits, home equipment, family support, among others. Mr. P’s daughter also said she would relay the information to her mother and call the palliative care NP if they had additional questions or concerns.
The outcome of her visit with Mr. P and his daughter were relayed by the palliative care NP to his acute health care team through an official response to the consultation request via his electronic health record. The palliative care NP also alerted the palliative care social worker to follow-up with Mr. P, his daughter, and his acute health care team to coordinate hospice services at the time of his discharge from the hospital.
Mr. P was discharged from the hospital with home hospice services after a few days. Three weeks later, Mr. P passed away peacefully on the in-patient unit of his home hospice agency as his physical care needs became too much for his family to provide at home a few days before his death. The palliative care social worker later shared with the NP that Mr. P’s daughter shared her gratitude and satisfaction with the care he had received not only from palliative care, but also from everyone during his hospitalization.
Discussion
Key themes found in end-of-life (EOL) communication with families and caregivers include highlighting clinical deterioration, involvement in decision making, continuation of high-quality care after cessation of aggressive measures, tailoring to individuals, clarity, honesty, and use of techniques in delivery.2 Some of the techniques identified were pacing, staging, and repetition.3 Other techniques that can be beneficial include allowing for time to express one’s feelings, being comfortable with brief periods of silence, validating observations shared, among others. These themes were evident in the interactions that his health care team had with Mr. P and his daughter. With honesty and clarity, various members of the health care team repeatedly shared information regarding his clinical deterioration.
Family Influence
EOL decision-making roles within a family tend to originate from family interactional histories, familial roles as well as decision-making situations the family faces.4 The US medical and legal systems also recognize formal role assignments for surrogate decision makers.4 In the case of Mr. P, his advance directive (AD) identified his daughter as his surrogate decision maker. ADs are written statements made in advance by patients expressing their wishes and limitations for treatment as well as appointing surrogate decision makers when they become unable to decide for themselves in the future.5
During discussions about the goals for his care, Mr. P made his own medical decisions and elected to pursue a comfort-focused approach to care. His request for his health care team to reach out to his daughter was largely due to his need for assistance in explaining the complexity of his clinical condition to her and how hospice services would be helpful with his EOL care. Mr. P depended on his daughter to bring him to the hospital or to his doctors’ appointments, and she had been a major source of support for him and his wife. Contrary to the belief of some of his health care practitioners, Mr. P was not deferring his medical decisions to his daughter but rather allowing for her participation as his health care partner.
Communication between nurses and patients has been found to be challenging to both parties. Nurses express difficulties in areas that include supporting patients and families after they have had a difficult conversation with their physicians and responding to patients and family members’ emotions like anger.6 EOL care issues, such as family barriers to prognostic understanding, can interfere with psychosocial care.6 Families of patients approaching the EOL describe feeling mentally worn down and being unable to think straight, leading to feelings of being overwhelmed.7 They feel the need to be in a place where they can accept the content of difficult EOL conversations to be able to effectively engage.7
Studies have shown that family members of patients at the EOL experience stress, anxiety, fatigue and depression.8 Reactions that can be perceived as anger may not be so nor directed to the health care team. Questions raised regarding the accuracy of prognostication and treatment recommendations may not necessarily reflect concerns about the quality of care received but an exercise of advocacy in exploring other options on behalf of the patient. Allowing time for families to process the information received and react freely are necessary steps to facilitate reaching a place where they can acknowledge the information being relayed.
Communication Skills Training
Every member of the health care team should be equipped with the basic skills to have these conversations. The academic curricula for members of the health care team focuses on developing communication skills, but there has been a lack of content on palliative and EOL care.9
Due to time constraints and limited opportunities in the clinical setting, there has been an increasing use of simulation-based learning activities (SBLA) to enhance communication skills among nursing students.9 At this time, the impact of SBLA in enhancing communication competency is not fully known, but given the lack of clinical opportunities for students, this option is worth considering.9 When asked, nurses recognized the need for improved EOL communication education, training, and guidelines.10 They also felt that a multidisciplinary approach in EOL communication is beneficial. The inclusion of the End-of-Life Nursing Education Consortium (ELNEC) Core training in Bachelor of Science in Nursing programs have led to improved insight on palliative care and nurses’ role in palliative care and hospice among nursing students.11
The Palliative Care and Hospice Education and Training Act of 2017 amended the Public Health Service Act to include improving EOL training for health care providers, including talking about death and dying.12 Even though the Liaison Committee of Medical Education asked medical schools to incorporate EOL care education in the medical school curricula, there is still a lack of developmentally appropriate and supervised EOL education in medical schools.12 Training on grief also has been lacking and less likely to be mandatory among medical students and residents: Workshops are mostly conducted before they can be applied in the clinical setting.13 Meanwhile, resources are available to assist physicians in EOL conversations with patient and families, such as the Serious Illness Conversation Guide, The Conversation Project, and Stanford’s Letter Project.12
Conclusions
Palliative consultation is associated with an overall improvement in EOL care, communication, and support, according to families of deceased patients.14 It has also been shown to enhance patients’ quality of life and mood, improve documentation of resuscitation preferences, and lead to less aggressive care at the EOL, including less chemotherapy.15 Integration of palliative care in the care of patients hospitalized with acute heart failure has been associated with improved quality of life, decreased symptom burden and depressive symptoms, and increased participation in advance care planning.16
The involvement of palliative care in the care of patients and their families at EOL enhances goals of care discussions that improve understanding for everyone involved. It helps provide consistency with the message being delivered by the rest of the health care team to patients and families regarding prognosis and recommendations. Palliative care can provide an alternative when all other aggressive measures are no longer helpful and allow for the continuation of care with a shift in focus from prolonging life to promoting its quality. Furthermore, palliative care involvement for care of patients with life-limiting illness also has been found to improve symptom control, decrease hospitalizations and health care costs, and even improve mortality.17A multidisciplinary approach to palliative care EOL conversations is beneficial, but every member of the health care team should have the training, education, and skills to be ready to have these difficult conversations. These health care team members include physicians, advance practice clinicians, nurses, social workers, and chaplains, among others. Patients and families are likely to be in contact with different members of the health care team who should be able to carry out therapeutic conversations. Using validated tools and resources on communication techniques through evidence-based practice is helpful and should be encouraged. This provides a framework on how EOL conversations should be conducted in the clinical setting to augment the identified lack of training on EOL communication in schools. Repeated opportunities for its use over time will help improve the ability of clinicians to engage in effective EOL communication.
1. MacKenzie AR, Lasota M. Bringing life to death: the need for honest, compassionate, and effective end-of-life conversations. Am Soc Clin Oncol Educ Book. 2020;40:476-484. doi:10.1200/EDBK_279767
2. Krishnan U, Horn E. Pulmonary hypertension due to left heart disease (group 2 pulmonary hypertension) in adults. Accessed September 17, 2021. https://www.uptodate.com/contents/pulmonary-hypertension-due-to-left-heart-disease-group-2-pulmonary-hypertension-in-adults
3. Anderson RJ, Bloch S, Armstrong M, Stone PC, Low JT. Communication between healthcare professionals and relatives of patients approaching the end-of-life: a systematic review of qualitative evidence. Palliat Med. 2019;33(8):926-941. doi:10.1177/0269216319852007
4. Trees AR, Ohs JE, Murray MC. Family communication about end-of-life decisions and the enactment of the decision-maker role. Behav Sci (Basel). 2017;7(2):36. doi:10.3390/bs7020036 5. Arruda LM, Abreu KPB, Santana LBC, Sales MVC. Variables that influence the medical decision regarding advance directives and their impact on end-of-life care. Einstein (Sao Paulo). 2019;18:eRW4852. doi:10.31744/einstein_journal/2020RW4852
6. Banerjee SC, Manna R, Coyle N, et al. The implementation and evaluation of a communication skills training program for oncology nurses. Transl Behav Med. 2017;7(3):615-623. doi:10.1007/s13142-017-0473-5
7. Mitchell S, Spry JL, Hill E, Coad J, Dale J, Plunkett A. Parental experiences of end of life care decision-making for children with life-limiting conditions in the paediatric intensive care unit: a qualitative interview study. BMJ Open. 2019;9(5):e028548. doi:10.1136/bmjopen-2018-028548
8. Laryionava K, Pfeil TA, Dietrich M, Reiter-Theil S, Hiddemann W, Winkler EC. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
9. Smith MB, Macieira TGR, Bumbach MD, et al. The use of simulation to teach nursing students and clinicians palliative care and end-of-life communication: a systematic review. Am J Hosp Palliat Care. 2018;35(8):1140-1154. doi:10.1177/1049909118761386
10. Griffiths I. What are the challenges for nurses when providing end-of-life care in intensive care units? Br J Nurs. 2019;28(16):1047-1052. doi:10.12968/bjon.2019.28.16.1047
11. Li J, Smothers A, Fang W, Borland M. Undergraduate nursing students’ perception of end-of-life care education placement in the nursing curriculum. J Hosp Palliat Nurs. 2019;21(5):E12-E18. doi:10.1097/NJH.0000000000000533
12. Sutherland R. Dying well-informed: the need for better clinical educationsurrounding facilitating end-of-life conversations. Yale J Biol Med. 2019;92(4):757-764.
13. Sikstrom L, Saikaly R, Ferguson G, Mosher PJ, Bonato S, Soklaridis S. Being there: a scoping review of grief support training in medical education. PLoS One. 2019;14(11):e0224325. doi:10.1371/journal.pone.0224325
14. Yefimova M, Aslakson RA, Yang L, et al. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020;155(2):138-146. doi:10.1001/jamasurg.2019.5083
15. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-42. doi:10.1056/NEJMoa1000678.
16. Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18(2):134-142. doi:org/10.1089/jpm.2014.0192
17. Diop MS, Rudolph JL, Zimmerman KM, Richter MA, Skarf LM. Palliative careinterventions for patients with heart failure: a systematic review and meta-analysis. J Palliat Med. 2017;20(1):84-92. doi:10.1089/jpm.2016.0330
1. MacKenzie AR, Lasota M. Bringing life to death: the need for honest, compassionate, and effective end-of-life conversations. Am Soc Clin Oncol Educ Book. 2020;40:476-484. doi:10.1200/EDBK_279767
2. Krishnan U, Horn E. Pulmonary hypertension due to left heart disease (group 2 pulmonary hypertension) in adults. Accessed September 17, 2021. https://www.uptodate.com/contents/pulmonary-hypertension-due-to-left-heart-disease-group-2-pulmonary-hypertension-in-adults
3. Anderson RJ, Bloch S, Armstrong M, Stone PC, Low JT. Communication between healthcare professionals and relatives of patients approaching the end-of-life: a systematic review of qualitative evidence. Palliat Med. 2019;33(8):926-941. doi:10.1177/0269216319852007
4. Trees AR, Ohs JE, Murray MC. Family communication about end-of-life decisions and the enactment of the decision-maker role. Behav Sci (Basel). 2017;7(2):36. doi:10.3390/bs7020036 5. Arruda LM, Abreu KPB, Santana LBC, Sales MVC. Variables that influence the medical decision regarding advance directives and their impact on end-of-life care. Einstein (Sao Paulo). 2019;18:eRW4852. doi:10.31744/einstein_journal/2020RW4852
6. Banerjee SC, Manna R, Coyle N, et al. The implementation and evaluation of a communication skills training program for oncology nurses. Transl Behav Med. 2017;7(3):615-623. doi:10.1007/s13142-017-0473-5
7. Mitchell S, Spry JL, Hill E, Coad J, Dale J, Plunkett A. Parental experiences of end of life care decision-making for children with life-limiting conditions in the paediatric intensive care unit: a qualitative interview study. BMJ Open. 2019;9(5):e028548. doi:10.1136/bmjopen-2018-028548
8. Laryionava K, Pfeil TA, Dietrich M, Reiter-Theil S, Hiddemann W, Winkler EC. The second patient? Family members of cancer patients and their role in end-of-life decision making. BMC Palliat Care. 2018;17(1):29. doi:10.1186/s12904-018-0288-2
9. Smith MB, Macieira TGR, Bumbach MD, et al. The use of simulation to teach nursing students and clinicians palliative care and end-of-life communication: a systematic review. Am J Hosp Palliat Care. 2018;35(8):1140-1154. doi:10.1177/1049909118761386
10. Griffiths I. What are the challenges for nurses when providing end-of-life care in intensive care units? Br J Nurs. 2019;28(16):1047-1052. doi:10.12968/bjon.2019.28.16.1047
11. Li J, Smothers A, Fang W, Borland M. Undergraduate nursing students’ perception of end-of-life care education placement in the nursing curriculum. J Hosp Palliat Nurs. 2019;21(5):E12-E18. doi:10.1097/NJH.0000000000000533
12. Sutherland R. Dying well-informed: the need for better clinical educationsurrounding facilitating end-of-life conversations. Yale J Biol Med. 2019;92(4):757-764.
13. Sikstrom L, Saikaly R, Ferguson G, Mosher PJ, Bonato S, Soklaridis S. Being there: a scoping review of grief support training in medical education. PLoS One. 2019;14(11):e0224325. doi:10.1371/journal.pone.0224325
14. Yefimova M, Aslakson RA, Yang L, et al. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020;155(2):138-146. doi:10.1001/jamasurg.2019.5083
15. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-42. doi:10.1056/NEJMoa1000678.
16. Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18(2):134-142. doi:org/10.1089/jpm.2014.0192
17. Diop MS, Rudolph JL, Zimmerman KM, Richter MA, Skarf LM. Palliative careinterventions for patients with heart failure: a systematic review and meta-analysis. J Palliat Med. 2017;20(1):84-92. doi:10.1089/jpm.2016.0330
Pembrolizumab-Induced Type 1 Diabetes in a 95-Year-Old Veteran With Metastatic Melanoma
Immune checkpoint inhibitors (CPIs) have revolutionized cancer therapy and improved the prognosis for a variety of advanced solid tumors and Hodgkin lymphoma, but evidence is growing regarding severe endocrine disturbances.1,2 CPIs block inhibitory molecules on activated T cells to increase tumor cell destruction but also can breach normal tolerance, resulting in a spectrum of immune-related adverse events (irAE).1,2 Programmed cell death-1 (PD-1) inhibitors are one type of CPIs. Pembrolizumab is a humanized monoclonal antibody that targets the PD-1 checkpoint pathway and is approved for the treatment of malignant melanoma and non-small cell lung cancer.3,4 When the PD-1 checkpoint pathway is inhibited, T cells targeting cancer are activated, as are autoreactive T cells, such as those regulating pancreatic islet cell survival, which can lead to type 1 diabetes mellitus (T1DM).5
Case Presentation
A 95-year-old male veteran with long-standing, stable prediabetes was treated with pembrolizumab for stage 4 melanoma. Four months after treatment initiation and 3 weeks after completion of his sixth treatment cycle of pembrolizumab (2 mg/kg every 3 weeks), he presented for surveillance positron emission tomography (PET) and was incidentally found to have a serum glucose of 423 mg/dL. Hypothesis-driven history taking revealed polyuria, polydipsia, and a 12-lb weight loss during the previous 3 months. The patient reported no abdominal pain, nausea, or vomiting. He showed no evidence of pancreatic metastases on recent imaging. His family history was notable for a daughter with T1DM diagnosed at a young age.
On examination, the patient’s vital signs were normal aside from a blood pressure of 80/40 mm Hg. His body mass index was 30. He was alert and oriented with comfortable respirations and no Kussmaul breathing. He exhibited dry mucous membranes and poor skin turgor. Laboratory studies revealed 135 mmol/L sodium (reference, 135-145), 4.6 mmol/L potassium (reference, 3.6-5.2), 100 mmol/L chloride (reference, 99-106), bicarbonate of 26.5 mmol/L (reference, 23-29), serum blood urea nitrogen 27 mg/dL (reference, 6-24), 1.06 mg/dL creatinine (reference, 0.74-1.35), and 423 mg/dL glucose (reference, 70-100), with negative urine ketones. Further studies demonstrated 462 µmol/L fructosamine (reference, 190-270), correlating with hemoglobin A1c (
Dicussion
Immunotherapy is now an integral part of cancer treatment and can result in endocrine disturbances.1,2 Life-threatening irAEs are rare and may mimic more common conditions; thus, there is growing recognition of the need to educate health care professionals in appropriate screening and management of these conditions. CPI-induced T1DM is an uncommon but clinically significant event with an incidence of 0.4 to 1.27% and a median onset of 20 weeks after initiation of therapy (range, 1-228 weeks).8-12In case seriesfrom 3 academic centers, 59 to81% of patients with CPI-induced T1DM presented with diabetic ketoacidosis (DKA), and only 40 to 71% of patients were autoantibody positive.13-16 These patients are older than those presenting with classic T1DM, often require intensive care unit admission, and nearly invariably require exogenous insulin injections for metabolic control.13-16
Based on the later age of onset of cancers that may be treated with CPI, patients with CPI-induced T1DM may be misdiagnosed with T2DM or hyperglycemia from other causes, such as medications or acute illness in the outpatient setting, risking suboptimal treatment.
Given the infrequent incidence and lack of controlled trials, screening and treatment recommendations for CPI-induced T1DM are based on principles derived from case series and expert opinion. Development of polyuria, polydipsia, weight loss, nausea, and/or vomiting should prompt investigation for possible development or worsening of hyperglycemia, suggestive of development of T1DM.17 American Society of Clinical Oncology (ASCO) guidelines recommend that serum glucose be assessed at baseline and with each treatment cycle during induction for 12 weeks, then every 3 to 6 weeks thereafter.17 There is no reported association between the number of CPI treatments and the development of DM.8,9 Following our patient’s fifth pembrolizumab cycle, a random glucose reading was noted to be 186 mg/dL (Figure 1). Under the ASCO guidelines, ideally the patient would have received close clinical follow-up given the striking increase in plasma glucose compared with prior baseline lower values and perhaps been further evaluated with an anti-GAD antibody titer to screen for T1DM.17
This patient's case adds to the published reports of CPI-induced T1DM without DKA and represents the oldest patient experiencing this irAE in the literature.13-16 The degree of elevation of his initial fructosamine, which is comparable to an average plasma glucose of approximately 270 mg/dL, belied the rapid rate of rise of his recent plasma glucose. Given the trajectory of glycemic markers and symptoms, one could certainly be concerned about imminent decompensation to DKA. However, fortuitous point-of-care glucose reading prior to surveillance PET resulted in a new critical diagnosis and initiation of treatment.
Assessing the need for inpatient evaluation includes obtaining urine ketones and acid-base status as screening for DKA.17 Antibodies and C-peptide can be sent to support diagnosis of new onset T1DM, although the initiation of therapy should not be delayed for these results.17 As noted before, many of these patients also are antibody negative.13-16 Low C-peptide levels should prompt a high suspicion for CPI-induced T1DM, and initiation of insulin therapy should be strongly considered.17 In a case series of 27 patients, 85% exhibited a rapid loss of β-cell function, evidenced by the acute progression to hyperglycemia and low or undetectable levels of C-peptide at diagnosis.9 Likewise, our patient had a low C-peptide level and negative anti-GAD antibody titer but was treated before these results were available. Inpatient admission for close glycemic monitoring may be reasonable; several cases reported prompt diagnosis and avoidance of DKA in this setting.17
In contrast to other irAEs, there is no available evidence that high-dose corticosteroids alter the course of pembrolizumab-induced T2DM.18 Depending on the degree of hyperglycemia, endocrinology consultation and insulin treatment are appropriate where the diagnosis of T1DM is suspected even without evidence of DKA.17 For patients with T2DM, there may be a positive synergistic effect of metformin in combination with CPIs in tumor control.19 Our patient’s C-peptide improved with insulin treatment, consistent with correction of glucose toxicity and a honeymoon period in his course. However, in patients reported with pembrolizumab-induced T1DM, insulin requirement for treatment generally persists despite cessation of pembrolizumab therapy.13-16
Conclusions
Pembrolizumab-induced T1DM is a rare, but potentially life-threatening irAE. The acute risk of DKA requires early recognition and prompt treatment of patients taking CPIs. More than 90% of primary care physicians (PCPs) fulfill general medical care roles for patients with cancer; therefore, they play an essential role in evaluating symptoms during therapy.20 Further studies evaluating the role of PCPs and outcomes when PCPs are involved in oncologic care should be conducted.
With increased index of suspicion, this clinical scenario presents an opportunity for PCPs that may help reduce irAE-associated morbidity and mortality of patients on CPIs, like pembrolizumab. Figure 2 illustrates an example addendum that can be used to alert and tag a PCP of a mutual patient after initiation of CPI therapy. Determining the optimal interface between PCPs, oncologists, and endocrinologists in delivering and coordinating high-quality cancer care in the setting of immunotherapy is an important area for ongoing quality improvement.
Acknowledgment
The authors thank all the staff and health care professionals at VA Greater Los Angeles Healthcare System who were involved in the care of this patient.
1. Puzanov I, Diab A, Abdallah K, et al; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi:10.1186/s40425-017-0300-z
2. Villa NM, Farahmand A, Du L, et al. Endocrinopathies with use of cancer immunotherapies. Clin Endocrinol (Oxf). 2018;88(2):327-332. doi:10.1111/cen.13483
3. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853-1862. doi:10.1016/S0140-6736(17)31601-X
4. Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 Study. J Clin Oncol. 2019;37(28):2518-2527. doi:10.1200/JCO.19.00934
5. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517-2519. doi:10.1056/NEJMe1205943
6. Malmstrom H, Walldius G, Grill V, Jungner I, Gudbjomsdottir S, Hammar N. Frustosamine is a useful indicator of hyperglycemia and glucose control in clinical and epidemiological studies- cross-sectional and longitudinal experience from the AMORIS cohort. PLoS One. 2014;9(10):e111463. doi:10.1371/journal.pone.0111463
7. Skinner S, Diaw M, Mbaye MN, et al. Evaluation of agreement between hemoglobin A1c, fasting glucose, and fructosamine in Senagalese individuals with and without sickle-cell trait. PLoS One. 2019;14(2):e0212552. doi:10.1371/journal.pone.0212552
8. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy-immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195-207. doi:10.1038/nrendo.2016.205
9. Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67(8):1471-1480. doi:10.2337/dbi18-0002
10. Liu J, Zhou H, Zhang Y, et al. Reporting of immune checkpoint inhibitor therapy-associated diabetes, 2015-2019. Diabetes Care. 2020;43(7):e79-e80. [Published online ahead of print, 2020 May 11]. doi:10.2337/dc20-0459
11. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173-182. doi:10.1001/jamaoncol.2017.3064
12. de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51(3):145-156. doi:10.1055/a-0843-3366
13. Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55-e57. doi:10.2337/dc14-2349
14. Clotman K, Janssens K, Specenier P, Weets I, De block CEM. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab. 2018;103(9):3144-3154. doi:10.1210/jc.2018-00728
15. Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care. 2019;7(1):e000591. doi:10.1136/bmjdrc-2018-000591
16. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40(1):17-65. doi:10.1210/er.2018-00006
17. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-1768. doi:10.1200/JCO.2017.77.6385
18. Aleksova J, Lau PK, Soldatos G, Mcarthur G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep. 2016;2016:bcr2016217454. doi:10.1136/bcr-2016-217454
19. Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J Immunother Cancer. 2018;6(1):64. doi:10.1186/s40425-018-0375-1
20. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24(9):1029-1036. doi:10.1007/s11606-009-1058-x
Immune checkpoint inhibitors (CPIs) have revolutionized cancer therapy and improved the prognosis for a variety of advanced solid tumors and Hodgkin lymphoma, but evidence is growing regarding severe endocrine disturbances.1,2 CPIs block inhibitory molecules on activated T cells to increase tumor cell destruction but also can breach normal tolerance, resulting in a spectrum of immune-related adverse events (irAE).1,2 Programmed cell death-1 (PD-1) inhibitors are one type of CPIs. Pembrolizumab is a humanized monoclonal antibody that targets the PD-1 checkpoint pathway and is approved for the treatment of malignant melanoma and non-small cell lung cancer.3,4 When the PD-1 checkpoint pathway is inhibited, T cells targeting cancer are activated, as are autoreactive T cells, such as those regulating pancreatic islet cell survival, which can lead to type 1 diabetes mellitus (T1DM).5
Case Presentation
A 95-year-old male veteran with long-standing, stable prediabetes was treated with pembrolizumab for stage 4 melanoma. Four months after treatment initiation and 3 weeks after completion of his sixth treatment cycle of pembrolizumab (2 mg/kg every 3 weeks), he presented for surveillance positron emission tomography (PET) and was incidentally found to have a serum glucose of 423 mg/dL. Hypothesis-driven history taking revealed polyuria, polydipsia, and a 12-lb weight loss during the previous 3 months. The patient reported no abdominal pain, nausea, or vomiting. He showed no evidence of pancreatic metastases on recent imaging. His family history was notable for a daughter with T1DM diagnosed at a young age.
On examination, the patient’s vital signs were normal aside from a blood pressure of 80/40 mm Hg. His body mass index was 30. He was alert and oriented with comfortable respirations and no Kussmaul breathing. He exhibited dry mucous membranes and poor skin turgor. Laboratory studies revealed 135 mmol/L sodium (reference, 135-145), 4.6 mmol/L potassium (reference, 3.6-5.2), 100 mmol/L chloride (reference, 99-106), bicarbonate of 26.5 mmol/L (reference, 23-29), serum blood urea nitrogen 27 mg/dL (reference, 6-24), 1.06 mg/dL creatinine (reference, 0.74-1.35), and 423 mg/dL glucose (reference, 70-100), with negative urine ketones. Further studies demonstrated 462 µmol/L fructosamine (reference, 190-270), correlating with hemoglobin A1c (
Dicussion
Immunotherapy is now an integral part of cancer treatment and can result in endocrine disturbances.1,2 Life-threatening irAEs are rare and may mimic more common conditions; thus, there is growing recognition of the need to educate health care professionals in appropriate screening and management of these conditions. CPI-induced T1DM is an uncommon but clinically significant event with an incidence of 0.4 to 1.27% and a median onset of 20 weeks after initiation of therapy (range, 1-228 weeks).8-12In case seriesfrom 3 academic centers, 59 to81% of patients with CPI-induced T1DM presented with diabetic ketoacidosis (DKA), and only 40 to 71% of patients were autoantibody positive.13-16 These patients are older than those presenting with classic T1DM, often require intensive care unit admission, and nearly invariably require exogenous insulin injections for metabolic control.13-16
Based on the later age of onset of cancers that may be treated with CPI, patients with CPI-induced T1DM may be misdiagnosed with T2DM or hyperglycemia from other causes, such as medications or acute illness in the outpatient setting, risking suboptimal treatment.
Given the infrequent incidence and lack of controlled trials, screening and treatment recommendations for CPI-induced T1DM are based on principles derived from case series and expert opinion. Development of polyuria, polydipsia, weight loss, nausea, and/or vomiting should prompt investigation for possible development or worsening of hyperglycemia, suggestive of development of T1DM.17 American Society of Clinical Oncology (ASCO) guidelines recommend that serum glucose be assessed at baseline and with each treatment cycle during induction for 12 weeks, then every 3 to 6 weeks thereafter.17 There is no reported association between the number of CPI treatments and the development of DM.8,9 Following our patient’s fifth pembrolizumab cycle, a random glucose reading was noted to be 186 mg/dL (Figure 1). Under the ASCO guidelines, ideally the patient would have received close clinical follow-up given the striking increase in plasma glucose compared with prior baseline lower values and perhaps been further evaluated with an anti-GAD antibody titer to screen for T1DM.17
This patient's case adds to the published reports of CPI-induced T1DM without DKA and represents the oldest patient experiencing this irAE in the literature.13-16 The degree of elevation of his initial fructosamine, which is comparable to an average plasma glucose of approximately 270 mg/dL, belied the rapid rate of rise of his recent plasma glucose. Given the trajectory of glycemic markers and symptoms, one could certainly be concerned about imminent decompensation to DKA. However, fortuitous point-of-care glucose reading prior to surveillance PET resulted in a new critical diagnosis and initiation of treatment.
Assessing the need for inpatient evaluation includes obtaining urine ketones and acid-base status as screening for DKA.17 Antibodies and C-peptide can be sent to support diagnosis of new onset T1DM, although the initiation of therapy should not be delayed for these results.17 As noted before, many of these patients also are antibody negative.13-16 Low C-peptide levels should prompt a high suspicion for CPI-induced T1DM, and initiation of insulin therapy should be strongly considered.17 In a case series of 27 patients, 85% exhibited a rapid loss of β-cell function, evidenced by the acute progression to hyperglycemia and low or undetectable levels of C-peptide at diagnosis.9 Likewise, our patient had a low C-peptide level and negative anti-GAD antibody titer but was treated before these results were available. Inpatient admission for close glycemic monitoring may be reasonable; several cases reported prompt diagnosis and avoidance of DKA in this setting.17
In contrast to other irAEs, there is no available evidence that high-dose corticosteroids alter the course of pembrolizumab-induced T2DM.18 Depending on the degree of hyperglycemia, endocrinology consultation and insulin treatment are appropriate where the diagnosis of T1DM is suspected even without evidence of DKA.17 For patients with T2DM, there may be a positive synergistic effect of metformin in combination with CPIs in tumor control.19 Our patient’s C-peptide improved with insulin treatment, consistent with correction of glucose toxicity and a honeymoon period in his course. However, in patients reported with pembrolizumab-induced T1DM, insulin requirement for treatment generally persists despite cessation of pembrolizumab therapy.13-16
Conclusions
Pembrolizumab-induced T1DM is a rare, but potentially life-threatening irAE. The acute risk of DKA requires early recognition and prompt treatment of patients taking CPIs. More than 90% of primary care physicians (PCPs) fulfill general medical care roles for patients with cancer; therefore, they play an essential role in evaluating symptoms during therapy.20 Further studies evaluating the role of PCPs and outcomes when PCPs are involved in oncologic care should be conducted.
With increased index of suspicion, this clinical scenario presents an opportunity for PCPs that may help reduce irAE-associated morbidity and mortality of patients on CPIs, like pembrolizumab. Figure 2 illustrates an example addendum that can be used to alert and tag a PCP of a mutual patient after initiation of CPI therapy. Determining the optimal interface between PCPs, oncologists, and endocrinologists in delivering and coordinating high-quality cancer care in the setting of immunotherapy is an important area for ongoing quality improvement.
Acknowledgment
The authors thank all the staff and health care professionals at VA Greater Los Angeles Healthcare System who were involved in the care of this patient.
Immune checkpoint inhibitors (CPIs) have revolutionized cancer therapy and improved the prognosis for a variety of advanced solid tumors and Hodgkin lymphoma, but evidence is growing regarding severe endocrine disturbances.1,2 CPIs block inhibitory molecules on activated T cells to increase tumor cell destruction but also can breach normal tolerance, resulting in a spectrum of immune-related adverse events (irAE).1,2 Programmed cell death-1 (PD-1) inhibitors are one type of CPIs. Pembrolizumab is a humanized monoclonal antibody that targets the PD-1 checkpoint pathway and is approved for the treatment of malignant melanoma and non-small cell lung cancer.3,4 When the PD-1 checkpoint pathway is inhibited, T cells targeting cancer are activated, as are autoreactive T cells, such as those regulating pancreatic islet cell survival, which can lead to type 1 diabetes mellitus (T1DM).5
Case Presentation
A 95-year-old male veteran with long-standing, stable prediabetes was treated with pembrolizumab for stage 4 melanoma. Four months after treatment initiation and 3 weeks after completion of his sixth treatment cycle of pembrolizumab (2 mg/kg every 3 weeks), he presented for surveillance positron emission tomography (PET) and was incidentally found to have a serum glucose of 423 mg/dL. Hypothesis-driven history taking revealed polyuria, polydipsia, and a 12-lb weight loss during the previous 3 months. The patient reported no abdominal pain, nausea, or vomiting. He showed no evidence of pancreatic metastases on recent imaging. His family history was notable for a daughter with T1DM diagnosed at a young age.
On examination, the patient’s vital signs were normal aside from a blood pressure of 80/40 mm Hg. His body mass index was 30. He was alert and oriented with comfortable respirations and no Kussmaul breathing. He exhibited dry mucous membranes and poor skin turgor. Laboratory studies revealed 135 mmol/L sodium (reference, 135-145), 4.6 mmol/L potassium (reference, 3.6-5.2), 100 mmol/L chloride (reference, 99-106), bicarbonate of 26.5 mmol/L (reference, 23-29), serum blood urea nitrogen 27 mg/dL (reference, 6-24), 1.06 mg/dL creatinine (reference, 0.74-1.35), and 423 mg/dL glucose (reference, 70-100), with negative urine ketones. Further studies demonstrated 462 µmol/L fructosamine (reference, 190-270), correlating with hemoglobin A1c (
Dicussion
Immunotherapy is now an integral part of cancer treatment and can result in endocrine disturbances.1,2 Life-threatening irAEs are rare and may mimic more common conditions; thus, there is growing recognition of the need to educate health care professionals in appropriate screening and management of these conditions. CPI-induced T1DM is an uncommon but clinically significant event with an incidence of 0.4 to 1.27% and a median onset of 20 weeks after initiation of therapy (range, 1-228 weeks).8-12In case seriesfrom 3 academic centers, 59 to81% of patients with CPI-induced T1DM presented with diabetic ketoacidosis (DKA), and only 40 to 71% of patients were autoantibody positive.13-16 These patients are older than those presenting with classic T1DM, often require intensive care unit admission, and nearly invariably require exogenous insulin injections for metabolic control.13-16
Based on the later age of onset of cancers that may be treated with CPI, patients with CPI-induced T1DM may be misdiagnosed with T2DM or hyperglycemia from other causes, such as medications or acute illness in the outpatient setting, risking suboptimal treatment.
Given the infrequent incidence and lack of controlled trials, screening and treatment recommendations for CPI-induced T1DM are based on principles derived from case series and expert opinion. Development of polyuria, polydipsia, weight loss, nausea, and/or vomiting should prompt investigation for possible development or worsening of hyperglycemia, suggestive of development of T1DM.17 American Society of Clinical Oncology (ASCO) guidelines recommend that serum glucose be assessed at baseline and with each treatment cycle during induction for 12 weeks, then every 3 to 6 weeks thereafter.17 There is no reported association between the number of CPI treatments and the development of DM.8,9 Following our patient’s fifth pembrolizumab cycle, a random glucose reading was noted to be 186 mg/dL (Figure 1). Under the ASCO guidelines, ideally the patient would have received close clinical follow-up given the striking increase in plasma glucose compared with prior baseline lower values and perhaps been further evaluated with an anti-GAD antibody titer to screen for T1DM.17
This patient's case adds to the published reports of CPI-induced T1DM without DKA and represents the oldest patient experiencing this irAE in the literature.13-16 The degree of elevation of his initial fructosamine, which is comparable to an average plasma glucose of approximately 270 mg/dL, belied the rapid rate of rise of his recent plasma glucose. Given the trajectory of glycemic markers and symptoms, one could certainly be concerned about imminent decompensation to DKA. However, fortuitous point-of-care glucose reading prior to surveillance PET resulted in a new critical diagnosis and initiation of treatment.
Assessing the need for inpatient evaluation includes obtaining urine ketones and acid-base status as screening for DKA.17 Antibodies and C-peptide can be sent to support diagnosis of new onset T1DM, although the initiation of therapy should not be delayed for these results.17 As noted before, many of these patients also are antibody negative.13-16 Low C-peptide levels should prompt a high suspicion for CPI-induced T1DM, and initiation of insulin therapy should be strongly considered.17 In a case series of 27 patients, 85% exhibited a rapid loss of β-cell function, evidenced by the acute progression to hyperglycemia and low or undetectable levels of C-peptide at diagnosis.9 Likewise, our patient had a low C-peptide level and negative anti-GAD antibody titer but was treated before these results were available. Inpatient admission for close glycemic monitoring may be reasonable; several cases reported prompt diagnosis and avoidance of DKA in this setting.17
In contrast to other irAEs, there is no available evidence that high-dose corticosteroids alter the course of pembrolizumab-induced T2DM.18 Depending on the degree of hyperglycemia, endocrinology consultation and insulin treatment are appropriate where the diagnosis of T1DM is suspected even without evidence of DKA.17 For patients with T2DM, there may be a positive synergistic effect of metformin in combination with CPIs in tumor control.19 Our patient’s C-peptide improved with insulin treatment, consistent with correction of glucose toxicity and a honeymoon period in his course. However, in patients reported with pembrolizumab-induced T1DM, insulin requirement for treatment generally persists despite cessation of pembrolizumab therapy.13-16
Conclusions
Pembrolizumab-induced T1DM is a rare, but potentially life-threatening irAE. The acute risk of DKA requires early recognition and prompt treatment of patients taking CPIs. More than 90% of primary care physicians (PCPs) fulfill general medical care roles for patients with cancer; therefore, they play an essential role in evaluating symptoms during therapy.20 Further studies evaluating the role of PCPs and outcomes when PCPs are involved in oncologic care should be conducted.
With increased index of suspicion, this clinical scenario presents an opportunity for PCPs that may help reduce irAE-associated morbidity and mortality of patients on CPIs, like pembrolizumab. Figure 2 illustrates an example addendum that can be used to alert and tag a PCP of a mutual patient after initiation of CPI therapy. Determining the optimal interface between PCPs, oncologists, and endocrinologists in delivering and coordinating high-quality cancer care in the setting of immunotherapy is an important area for ongoing quality improvement.
Acknowledgment
The authors thank all the staff and health care professionals at VA Greater Los Angeles Healthcare System who were involved in the care of this patient.
1. Puzanov I, Diab A, Abdallah K, et al; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi:10.1186/s40425-017-0300-z
2. Villa NM, Farahmand A, Du L, et al. Endocrinopathies with use of cancer immunotherapies. Clin Endocrinol (Oxf). 2018;88(2):327-332. doi:10.1111/cen.13483
3. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853-1862. doi:10.1016/S0140-6736(17)31601-X
4. Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 Study. J Clin Oncol. 2019;37(28):2518-2527. doi:10.1200/JCO.19.00934
5. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517-2519. doi:10.1056/NEJMe1205943
6. Malmstrom H, Walldius G, Grill V, Jungner I, Gudbjomsdottir S, Hammar N. Frustosamine is a useful indicator of hyperglycemia and glucose control in clinical and epidemiological studies- cross-sectional and longitudinal experience from the AMORIS cohort. PLoS One. 2014;9(10):e111463. doi:10.1371/journal.pone.0111463
7. Skinner S, Diaw M, Mbaye MN, et al. Evaluation of agreement between hemoglobin A1c, fasting glucose, and fructosamine in Senagalese individuals with and without sickle-cell trait. PLoS One. 2019;14(2):e0212552. doi:10.1371/journal.pone.0212552
8. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy-immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195-207. doi:10.1038/nrendo.2016.205
9. Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67(8):1471-1480. doi:10.2337/dbi18-0002
10. Liu J, Zhou H, Zhang Y, et al. Reporting of immune checkpoint inhibitor therapy-associated diabetes, 2015-2019. Diabetes Care. 2020;43(7):e79-e80. [Published online ahead of print, 2020 May 11]. doi:10.2337/dc20-0459
11. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173-182. doi:10.1001/jamaoncol.2017.3064
12. de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51(3):145-156. doi:10.1055/a-0843-3366
13. Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55-e57. doi:10.2337/dc14-2349
14. Clotman K, Janssens K, Specenier P, Weets I, De block CEM. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab. 2018;103(9):3144-3154. doi:10.1210/jc.2018-00728
15. Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care. 2019;7(1):e000591. doi:10.1136/bmjdrc-2018-000591
16. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40(1):17-65. doi:10.1210/er.2018-00006
17. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-1768. doi:10.1200/JCO.2017.77.6385
18. Aleksova J, Lau PK, Soldatos G, Mcarthur G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep. 2016;2016:bcr2016217454. doi:10.1136/bcr-2016-217454
19. Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J Immunother Cancer. 2018;6(1):64. doi:10.1186/s40425-018-0375-1
20. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24(9):1029-1036. doi:10.1007/s11606-009-1058-x
1. Puzanov I, Diab A, Abdallah K, et al; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi:10.1186/s40425-017-0300-z
2. Villa NM, Farahmand A, Du L, et al. Endocrinopathies with use of cancer immunotherapies. Clin Endocrinol (Oxf). 2018;88(2):327-332. doi:10.1111/cen.13483
3. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853-1862. doi:10.1016/S0140-6736(17)31601-X
4. Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 Study. J Clin Oncol. 2019;37(28):2518-2527. doi:10.1200/JCO.19.00934
5. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517-2519. doi:10.1056/NEJMe1205943
6. Malmstrom H, Walldius G, Grill V, Jungner I, Gudbjomsdottir S, Hammar N. Frustosamine is a useful indicator of hyperglycemia and glucose control in clinical and epidemiological studies- cross-sectional and longitudinal experience from the AMORIS cohort. PLoS One. 2014;9(10):e111463. doi:10.1371/journal.pone.0111463
7. Skinner S, Diaw M, Mbaye MN, et al. Evaluation of agreement between hemoglobin A1c, fasting glucose, and fructosamine in Senagalese individuals with and without sickle-cell trait. PLoS One. 2019;14(2):e0212552. doi:10.1371/journal.pone.0212552
8. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy-immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195-207. doi:10.1038/nrendo.2016.205
9. Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67(8):1471-1480. doi:10.2337/dbi18-0002
10. Liu J, Zhou H, Zhang Y, et al. Reporting of immune checkpoint inhibitor therapy-associated diabetes, 2015-2019. Diabetes Care. 2020;43(7):e79-e80. [Published online ahead of print, 2020 May 11]. doi:10.2337/dc20-0459
11. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173-182. doi:10.1001/jamaoncol.2017.3064
12. de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51(3):145-156. doi:10.1055/a-0843-3366
13. Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55-e57. doi:10.2337/dc14-2349
14. Clotman K, Janssens K, Specenier P, Weets I, De block CEM. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab. 2018;103(9):3144-3154. doi:10.1210/jc.2018-00728
15. Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care. 2019;7(1):e000591. doi:10.1136/bmjdrc-2018-000591
16. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40(1):17-65. doi:10.1210/er.2018-00006
17. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-1768. doi:10.1200/JCO.2017.77.6385
18. Aleksova J, Lau PK, Soldatos G, Mcarthur G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep. 2016;2016:bcr2016217454. doi:10.1136/bcr-2016-217454
19. Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J Immunother Cancer. 2018;6(1):64. doi:10.1186/s40425-018-0375-1
20. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24(9):1029-1036. doi:10.1007/s11606-009-1058-x
Annular Erythema of Infancy With Reactive Helper T Lymphocytes
Annular erythemas of infancy (AEIs) are rare benign skin eruptions characterized by annular or circinate, erythematous patches and plaques that arise in patients younger than 1 year.1 Annular erythemas of infancy originally were described by Peterson and Jarratt2 in 1981. Relatively few cases of AEIs have been reported in the literature (eTable).2-15
Case Report
An 11-month-old girl presented to dermatology for a rash characterized by annular erythematous patches and plaques on the back, arms, and legs (Figure 1). Three months prior, the rash was more diffuse, monomorphic, and papular. Based on physical examination, the differential diagnosis included a gyrate erythema such as erythema annulare centrifugum (EAC), neonatal lupus, a viral exanthem, leukemia cutis, and AEI. A skin punch biopsy was performed.
Histologically, the biopsy revealed a superficial to mid dermal, tight, coat sleeve–like, perivascular lymphohistiocytic infiltrate admixed with rare neutrophils in eosinophils within the dermis (Figure 2A). The infiltrate also contained numerous large mononuclear cells with enlarged nuclei, fine loose chromatin, rare nucleoli, and a thin rim of cytoplasm (Figure 2B). There were associated apoptotic bodies with karyorrhectic debris. Immunohistochemistry exhibited enlarged cells that were strong staining with CD3 and CD4, which was consistent with reactive helper T cells (Figure 3). A myeloperoxidase stain highlighted few neutrophils. Stains for terminal deoxynucleotidyl transferase, CD1a, CD117, and CD34 were negative. These findings along with the clinical presentation yielded a diagnosis of AEI with reactive helper T cells.
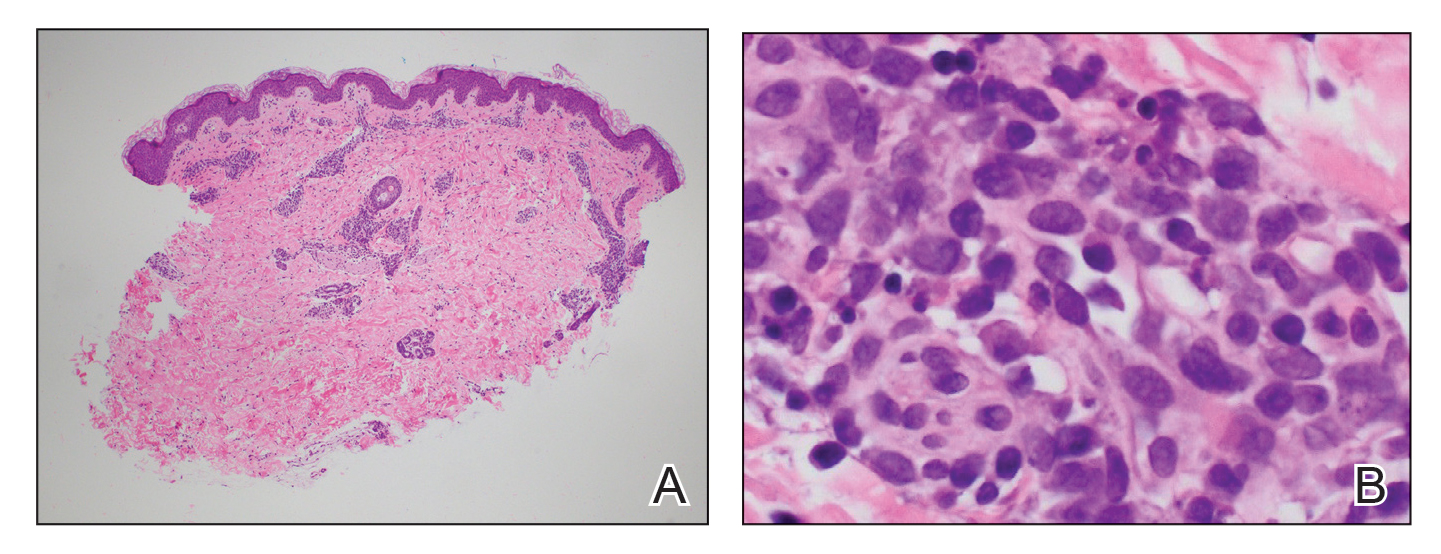
Comment
Clinical Presentation of AEIs—Annular erythemas of infancy are rare benign skin eruptions that develop in the first few months of life.1,16 Few cases have been reported (eTable). Clinically, AEIs are characterized by annular or circinate, erythematous patches and plaques. They can occur on the face, trunk, and extremities, and they completely resolve by 1 year of age in most cases. One case was reported to persist in a patient from birth until 15 years of age.9 It is thought that AEIs may occur as a hypersensitivity reaction to an unrecognized antigen.
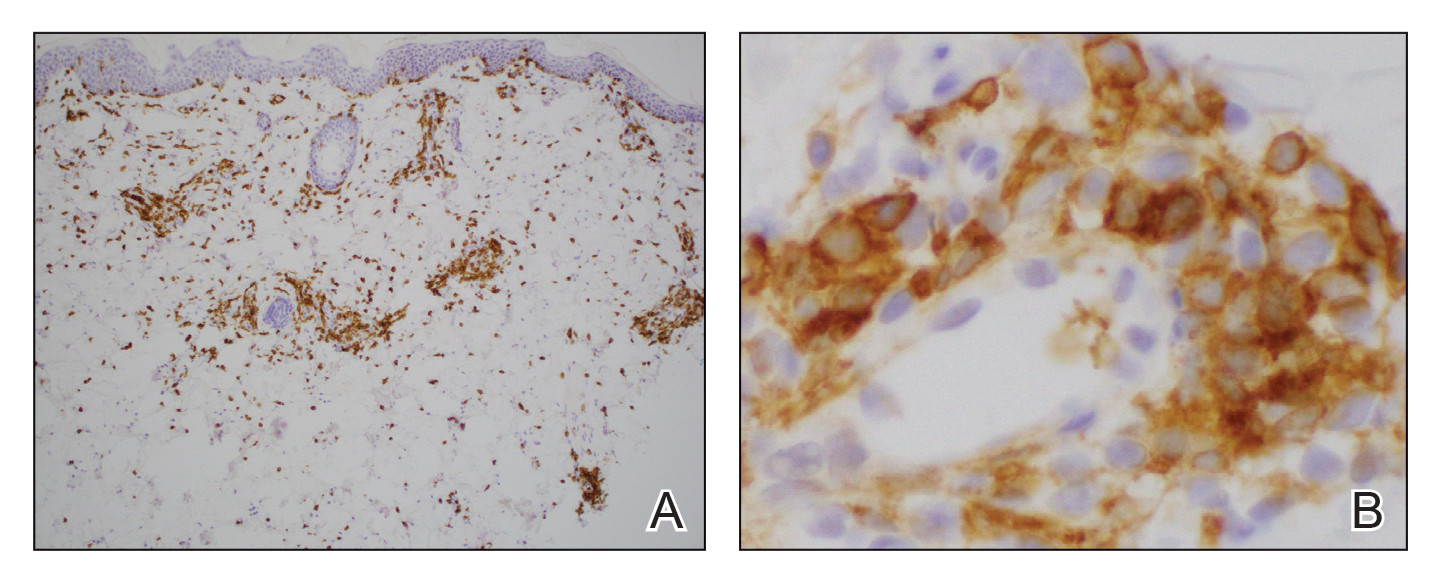
Histopathology—Histologically, AEIs demonstrate a superficial and deep, perivascular, inflammatory infiltrate in the dermis composed of small lymphocytes, some neutrophils, and eosinophils.16 Less common variants of AEI include eosinophilic annular erythema, characterized by a diffuse dermal infiltrate of eosinophils and some lymphocytes, and neutrophilic figurate erythema of infancy, characterized by a dermal infiltrate with neutrophils and leukocytoclasis without vasculitis.1
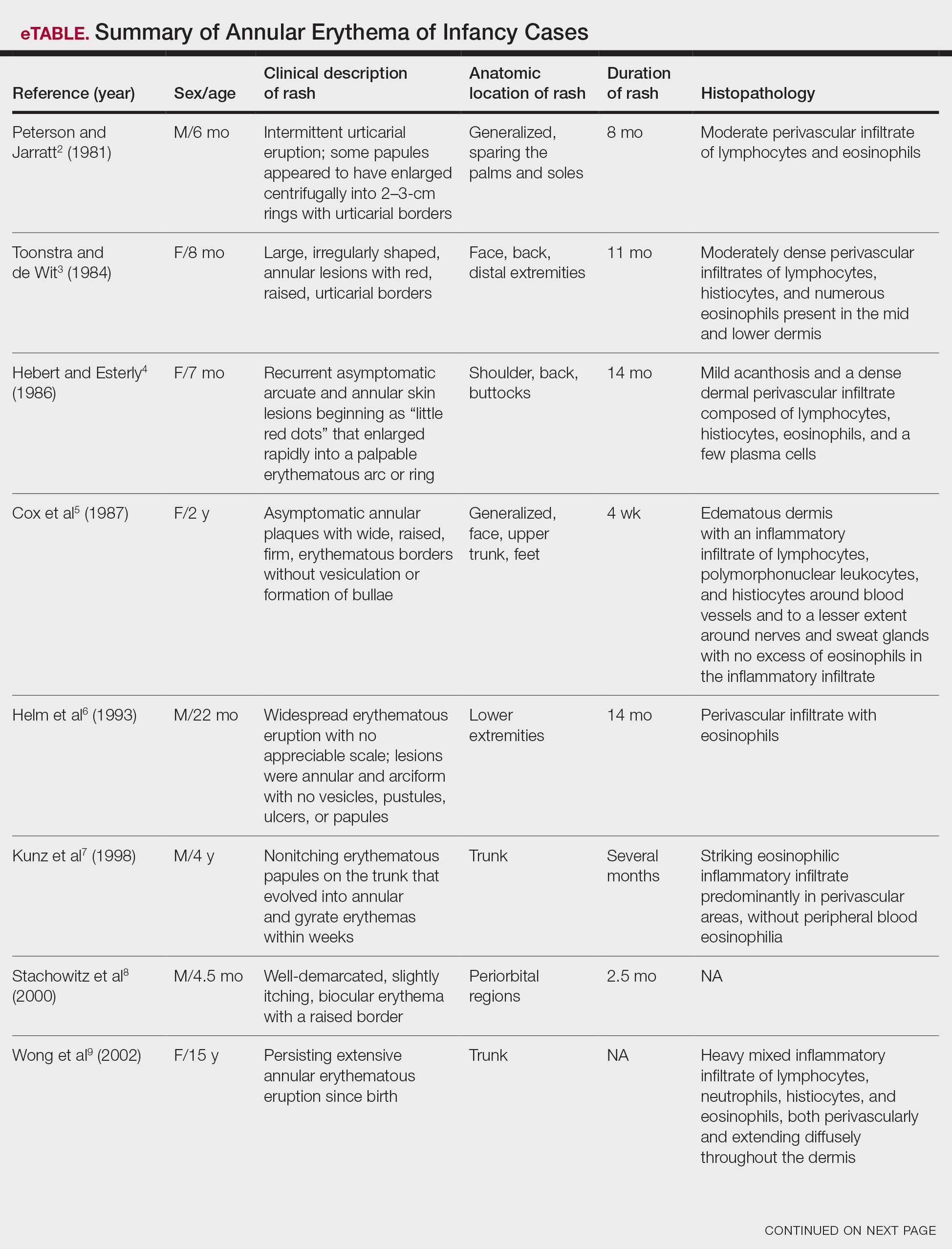
Our patient’s skin rash was unusual in that the biopsy demonstrated few neutrophils, rare eosinophils, and larger mononuclear cells consistent with reactive helper T lymphocytes. Although these cells may raise concern for an atypical lymphoid infiltrate, recognition of areas with more conventional histopathology of AEIs can facilitate the correct diagnosis.
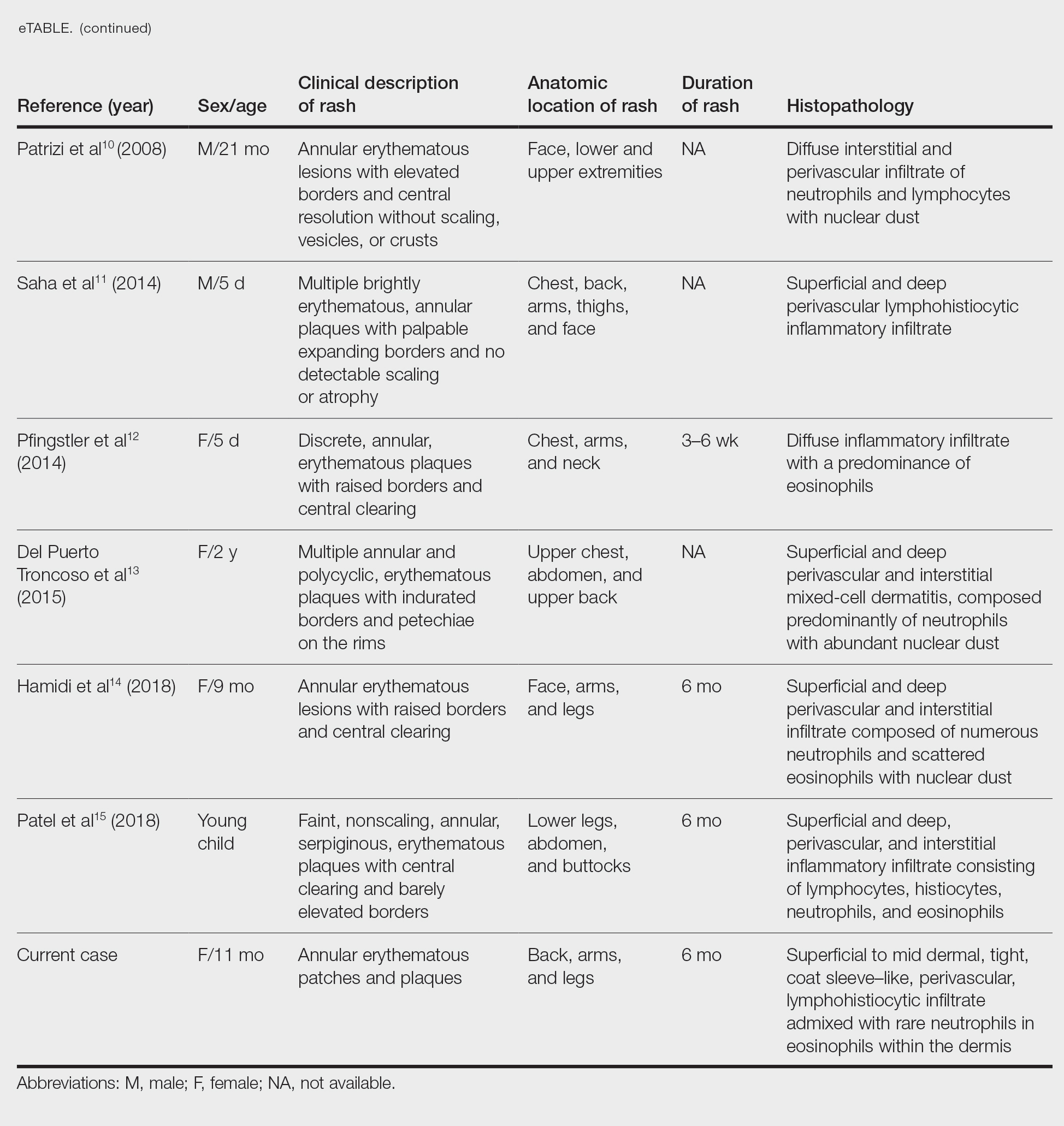
Differential Diagnosis—The main considerations in the differential diagnosis for AEIs include the following: EAC, familial annular erythema, erythema gyratum atrophicans transiens neonatale, erythema chronicum migrans, urticaria, tinea corporis, neonatal lupus erythematosus, viral exanthems, and leukemia cutis.16
Erythema annulare centrifugum typically begins in middle age and follows a course of 2 or more years.2 It occurs in association with an underlying infection or neoplasm, and it can develop on the trunk and proximal extremities. Morphologically, EAC can present with arcuate or polycyclic lesions with trailing scale. Histologically, a skin biopsy shows a tight, coat sleeve–like, perivascular, lymphohistiocytic infiltrate in the dermis, with variable epidermal spongiosis and parakeratosis.16 Our patient’s biopsy did show a tight perivascular infiltrate, raising suspicion for EAC. However, the eruption occurred in infancy, and she had no clinical evidence of infection or neoplasm.
Familial annular erythemas can arise within a few days after birth and can present on any part of the body, including the tongue.2 Individual lesions can persist for 4 to 5 days and can accompany congenital malformations. Morphologically, they can present as papules that slowly enlarge to form arcuate lesions with central hyperpigmentation. Histologically, there can be a mild, perivascular, lymphocytic infiltrate in the dermis.16 Our patient’s lesions showed no scale or pigmentation and occurred without a family history or associated malformations.
Erythema gyratum atrophicans transiens neonatale also can arise in the first few days of life and can affect the trunk, neck, and lips.16 Morphologically, the skin lesions can present as arcuate erythematous patches (3–20 mm) with raised borders and central atrophy. Histologically, there is epidermal atrophy with a dermal perivascular mononuclear cell infiltrate with edema. Our patient’s clinical presentation was not classic for this condition, and the lesions showed no atrophy.
Erythema chronicum migrans can arise in children, often with a history of an arthropod bite.13 Morphologically, lesions can evolve over weeks to months and rarely are multiple. Erythema chronicum migrans most commonly occurs in the United States in association with Lyme disease from infection with Borrelia burgdorferi. Histologically, erythema chronicum migrans shows a superficial and deep, perivascular lymphocytic infiltrate in the dermis with plasma cells and eosinophils. A silver stain can demonstrate dermal spirochetes. Our patient had no history of an arthropod bite. A Warthin-Starry stain performed on the biopsy was negative for spirochetes, and serologies for Lyme disease were negative.
Urticaria is rare in neonates and can occur on any part of the body.2 Morphologically, the skin lesions can present as arcuate, erythematous, and polycyclic plaques that wax and wane. Histologically, there is dermal edema with a mild, perivascular and interstitial, mixed inflammatory infiltrate.16 Our patient’s biopsy did not reveal notable edema, and the perivascular infiltrate was coat sleeve–like with few neutrophils and eosinophils. The patient did not respond to initial treatment with antihistamines, making urticaria less likely.
Tinea corporis is rare in neonates and can occur on any part of the body.13 Morphologically, it can present as scaly annular lesions that are fixed and more persistent. Histologically, there are fungal hyphae and/or yeast in the stratum corneum with spongiotic dermatitis and parakeratosis. Our patient’s lesions were not scaly, and the biopsy demonstrated minimal spongiosis. A periodic acid–Schiff special stain was negative for fungal microorganisms.
Neonatal lupus erythematosus can arise at birth or during the first few weeks of life.16 Morphologically, the skin lesions occur on the scalp, forehead, or neck in a periorbital or malar distribution. They can present as erythematous, annular, scaly patches and plaques. Transplacental transmission of material autoantibodies has been implicated in the etiology, and a complication is infantile heart block. Histologically, a skin biopsy typically shows interface/lichenoid dermatitis. However, our patient’s biopsy did not demonstrate interface changes, and serologically she was negative for autoantibodies.
Viral exanthems are skin eruptions that accompany underlying viral infections.17 Morphologically, patients can present with an erythematous maculopapular rash, sometimes with vesicular, petechial, and urticarial lesions. Laboratory confirmation is made by virus-specific serologies. Histologically, viral exanthems can show a superficial, perivascular, lymphocytic infiltrate in the dermis, with reactive T cells and epidermal spongiosis. Our patient was afebrile and had no known sick contacts. A cytomegalovirus immunohistochemical study on the biopsy was negative, and an Epstein-Barr encoding region in situ hybridization study was negative.
Leukemia cutis is the infiltration of the skin by leukemic cells, most often in conjunction with systemic leukemia.18 In infants and children, the most common leukemia is B-cell acute lymphoblastic leukemia. Morphologically, the skin lesions are characterized by single or multiple violaceous papules, nodules, and plaques. Histologically, there is a perivascular to interstitial infiltrate of atypical mononuclear cells in the dermis and sometimes subcutis. The leukemic cells demonstrate enlarged nuclei with coarse chromatin and prominent nucleoli. Increased mitotic activity may be seen with karyorrhectic debris. Immunohistochemically, the tumor cells can be positive for myeloperoxidase, CD43, CD68, CD34, and CD117.18 Although our patient’s biopsy demonstrated mononuclear cells with karyorrhexis, the cells did not have striking atypia and were negative for blast markers. A recent complete blood cell count on the patient was normal.
Conclusion
We report an unusual case of AEI with mononuclear cells consistent with helper T cells. One must keep these cells in mind when evaluating a biopsy of AEI, as they are benign and not suggestive of an atypical lymphoid infiltrate or leukemia cutis. This will prevent misdiagnosis and ensure that the patient receives appropriate management.
- Ríos-Martín JJ, Ferrándiz-Pulido L, Moreno-Ramírez D. Approaches to the dermatopathologic diagnosis of figurate lesions [in Spanish]. Actas Dermosifiliogr. 2011;102:316-324. doi:10.1016/j.ad.2010.12.009
- Peterson AO, Jarratt M. Annular erythema of infancy. Arch Dermatol. 1981;117:145-148.
- Toonstra J, de Wit RF. “Persistent” annular erythema of infancy. Arch Dermatol.1984;120:1069-1072.
- Hebert AA, Esterly NB. Annular erythema of infancy. J Am Acad Dermatol. 1986;14:339-343.
- Cox NH, McQueen A, Evans TJ, et al. An annular erythema of infancy. Arch Dermatol. 1987;123:510-513.
- Helm TN, Bass J, Chang LW, et al. Persistent annular erythema of infancy. Pediatr Dermatol. 1993;10:46-48.
- Kunz M, Hamm K, Bröcker EB, et al. Annular erythema in childhood—a new eosinophilic dermatosis [in German]. Hautarzt. 1998;49:131-134.
- Stachowitz S, Abeck D, Schmidt T, et al. Persistent annular erythema of infancy associated with intestinal Candida colonization. Clin Exp Dermatol. 2000;25:404-405.
- Wong L-C, Kakakios A, Rogers M. Congenital annular erythema persisting in a 15-year-old girl. Australas J Dermatol. 2002;43:55-61.
- Patrizi A, Savoia F, Varotti E, et al. Neutrophilic figurate erythema of infancy. Pediatr Dermatol. 2008;25:255-260. doi:10.1111/j.1525-1470.2008.00646.x
- Saha A, Seth J, Mukherjee S, et al. Annular erythema of infancy: a diagnostic challenge. Indian J Paediatr Dermatol. 2014;15:147-149. doi:10.4103/2319-7250.143678
- Pfingstler LF, Miller KP, Pride H. Recurring diffuse annular erythematous plaques in a newborn. JAMA Dermatol. 2014;150:565-566. doi:10.1001/jamadermatol.2013.8059
- Del Puerto Troncoso C, Curi Tuma M, González Bombardiere S, et al. Neutrophilic figurate erythema of infancy associated with juvenile myelomonocytic leukemia. Actas Dermosifiliogr. 2015;106:431-433. doi:10.1016/j.ad.2014.09.013
- Hamidi S, Prose NS, Selim MA. Neutrophilic figurate erythema of infancy: a diagnostic challenge [published online December 26, 2018]. J Cutan Pathol. 2019;46:216-220. doi:10.1111/cup.13394
- Patel N, Goldbach H, Hogeling M. An annular eruption in a young child. JAMA Dermatol. 2018;154:1213-1214. doi:10.1001/jamadermatol.2018.1174
- Palit A, Inamadar AC. Annular, erythematous skin lesions in a neonate. Indian Dermatol Online J. 2012;3:45-47. doi:10.4103/2229-5178.93504
- Keighley CL, Saunderson RB, Kok J, et al. Viral exanthems. Curr Opin Infect Dis. 2015;28:139-150. doi:10.1097/QCO.0000000000000145
- Cronin DMP, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132:101-110. doi:10.1309/AJCP6GR8BDEXPKHR
Annular erythemas of infancy (AEIs) are rare benign skin eruptions characterized by annular or circinate, erythematous patches and plaques that arise in patients younger than 1 year.1 Annular erythemas of infancy originally were described by Peterson and Jarratt2 in 1981. Relatively few cases of AEIs have been reported in the literature (eTable).2-15
Case Report
An 11-month-old girl presented to dermatology for a rash characterized by annular erythematous patches and plaques on the back, arms, and legs (Figure 1). Three months prior, the rash was more diffuse, monomorphic, and papular. Based on physical examination, the differential diagnosis included a gyrate erythema such as erythema annulare centrifugum (EAC), neonatal lupus, a viral exanthem, leukemia cutis, and AEI. A skin punch biopsy was performed.

Histologically, the biopsy revealed a superficial to mid dermal, tight, coat sleeve–like, perivascular lymphohistiocytic infiltrate admixed with rare neutrophils in eosinophils within the dermis (Figure 2A). The infiltrate also contained numerous large mononuclear cells with enlarged nuclei, fine loose chromatin, rare nucleoli, and a thin rim of cytoplasm (Figure 2B). There were associated apoptotic bodies with karyorrhectic debris. Immunohistochemistry exhibited enlarged cells that were strong staining with CD3 and CD4, which was consistent with reactive helper T cells (Figure 3). A myeloperoxidase stain highlighted few neutrophils. Stains for terminal deoxynucleotidyl transferase, CD1a, CD117, and CD34 were negative. These findings along with the clinical presentation yielded a diagnosis of AEI with reactive helper T cells.

Comment
Clinical Presentation of AEIs—Annular erythemas of infancy are rare benign skin eruptions that develop in the first few months of life.1,16 Few cases have been reported (eTable). Clinically, AEIs are characterized by annular or circinate, erythematous patches and plaques. They can occur on the face, trunk, and extremities, and they completely resolve by 1 year of age in most cases. One case was reported to persist in a patient from birth until 15 years of age.9 It is thought that AEIs may occur as a hypersensitivity reaction to an unrecognized antigen.

Histopathology—Histologically, AEIs demonstrate a superficial and deep, perivascular, inflammatory infiltrate in the dermis composed of small lymphocytes, some neutrophils, and eosinophils.16 Less common variants of AEI include eosinophilic annular erythema, characterized by a diffuse dermal infiltrate of eosinophils and some lymphocytes, and neutrophilic figurate erythema of infancy, characterized by a dermal infiltrate with neutrophils and leukocytoclasis without vasculitis.1

Our patient’s skin rash was unusual in that the biopsy demonstrated few neutrophils, rare eosinophils, and larger mononuclear cells consistent with reactive helper T lymphocytes. Although these cells may raise concern for an atypical lymphoid infiltrate, recognition of areas with more conventional histopathology of AEIs can facilitate the correct diagnosis.

Differential Diagnosis—The main considerations in the differential diagnosis for AEIs include the following: EAC, familial annular erythema, erythema gyratum atrophicans transiens neonatale, erythema chronicum migrans, urticaria, tinea corporis, neonatal lupus erythematosus, viral exanthems, and leukemia cutis.16
Erythema annulare centrifugum typically begins in middle age and follows a course of 2 or more years.2 It occurs in association with an underlying infection or neoplasm, and it can develop on the trunk and proximal extremities. Morphologically, EAC can present with arcuate or polycyclic lesions with trailing scale. Histologically, a skin biopsy shows a tight, coat sleeve–like, perivascular, lymphohistiocytic infiltrate in the dermis, with variable epidermal spongiosis and parakeratosis.16 Our patient’s biopsy did show a tight perivascular infiltrate, raising suspicion for EAC. However, the eruption occurred in infancy, and she had no clinical evidence of infection or neoplasm.
Familial annular erythemas can arise within a few days after birth and can present on any part of the body, including the tongue.2 Individual lesions can persist for 4 to 5 days and can accompany congenital malformations. Morphologically, they can present as papules that slowly enlarge to form arcuate lesions with central hyperpigmentation. Histologically, there can be a mild, perivascular, lymphocytic infiltrate in the dermis.16 Our patient’s lesions showed no scale or pigmentation and occurred without a family history or associated malformations.
Erythema gyratum atrophicans transiens neonatale also can arise in the first few days of life and can affect the trunk, neck, and lips.16 Morphologically, the skin lesions can present as arcuate erythematous patches (3–20 mm) with raised borders and central atrophy. Histologically, there is epidermal atrophy with a dermal perivascular mononuclear cell infiltrate with edema. Our patient’s clinical presentation was not classic for this condition, and the lesions showed no atrophy.
Erythema chronicum migrans can arise in children, often with a history of an arthropod bite.13 Morphologically, lesions can evolve over weeks to months and rarely are multiple. Erythema chronicum migrans most commonly occurs in the United States in association with Lyme disease from infection with Borrelia burgdorferi. Histologically, erythema chronicum migrans shows a superficial and deep, perivascular lymphocytic infiltrate in the dermis with plasma cells and eosinophils. A silver stain can demonstrate dermal spirochetes. Our patient had no history of an arthropod bite. A Warthin-Starry stain performed on the biopsy was negative for spirochetes, and serologies for Lyme disease were negative.
Urticaria is rare in neonates and can occur on any part of the body.2 Morphologically, the skin lesions can present as arcuate, erythematous, and polycyclic plaques that wax and wane. Histologically, there is dermal edema with a mild, perivascular and interstitial, mixed inflammatory infiltrate.16 Our patient’s biopsy did not reveal notable edema, and the perivascular infiltrate was coat sleeve–like with few neutrophils and eosinophils. The patient did not respond to initial treatment with antihistamines, making urticaria less likely.
Tinea corporis is rare in neonates and can occur on any part of the body.13 Morphologically, it can present as scaly annular lesions that are fixed and more persistent. Histologically, there are fungal hyphae and/or yeast in the stratum corneum with spongiotic dermatitis and parakeratosis. Our patient’s lesions were not scaly, and the biopsy demonstrated minimal spongiosis. A periodic acid–Schiff special stain was negative for fungal microorganisms.
Neonatal lupus erythematosus can arise at birth or during the first few weeks of life.16 Morphologically, the skin lesions occur on the scalp, forehead, or neck in a periorbital or malar distribution. They can present as erythematous, annular, scaly patches and plaques. Transplacental transmission of material autoantibodies has been implicated in the etiology, and a complication is infantile heart block. Histologically, a skin biopsy typically shows interface/lichenoid dermatitis. However, our patient’s biopsy did not demonstrate interface changes, and serologically she was negative for autoantibodies.
Viral exanthems are skin eruptions that accompany underlying viral infections.17 Morphologically, patients can present with an erythematous maculopapular rash, sometimes with vesicular, petechial, and urticarial lesions. Laboratory confirmation is made by virus-specific serologies. Histologically, viral exanthems can show a superficial, perivascular, lymphocytic infiltrate in the dermis, with reactive T cells and epidermal spongiosis. Our patient was afebrile and had no known sick contacts. A cytomegalovirus immunohistochemical study on the biopsy was negative, and an Epstein-Barr encoding region in situ hybridization study was negative.
Leukemia cutis is the infiltration of the skin by leukemic cells, most often in conjunction with systemic leukemia.18 In infants and children, the most common leukemia is B-cell acute lymphoblastic leukemia. Morphologically, the skin lesions are characterized by single or multiple violaceous papules, nodules, and plaques. Histologically, there is a perivascular to interstitial infiltrate of atypical mononuclear cells in the dermis and sometimes subcutis. The leukemic cells demonstrate enlarged nuclei with coarse chromatin and prominent nucleoli. Increased mitotic activity may be seen with karyorrhectic debris. Immunohistochemically, the tumor cells can be positive for myeloperoxidase, CD43, CD68, CD34, and CD117.18 Although our patient’s biopsy demonstrated mononuclear cells with karyorrhexis, the cells did not have striking atypia and were negative for blast markers. A recent complete blood cell count on the patient was normal.
Conclusion
We report an unusual case of AEI with mononuclear cells consistent with helper T cells. One must keep these cells in mind when evaluating a biopsy of AEI, as they are benign and not suggestive of an atypical lymphoid infiltrate or leukemia cutis. This will prevent misdiagnosis and ensure that the patient receives appropriate management.
Annular erythemas of infancy (AEIs) are rare benign skin eruptions characterized by annular or circinate, erythematous patches and plaques that arise in patients younger than 1 year.1 Annular erythemas of infancy originally were described by Peterson and Jarratt2 in 1981. Relatively few cases of AEIs have been reported in the literature (eTable).2-15
Case Report
An 11-month-old girl presented to dermatology for a rash characterized by annular erythematous patches and plaques on the back, arms, and legs (Figure 1). Three months prior, the rash was more diffuse, monomorphic, and papular. Based on physical examination, the differential diagnosis included a gyrate erythema such as erythema annulare centrifugum (EAC), neonatal lupus, a viral exanthem, leukemia cutis, and AEI. A skin punch biopsy was performed.

Histologically, the biopsy revealed a superficial to mid dermal, tight, coat sleeve–like, perivascular lymphohistiocytic infiltrate admixed with rare neutrophils in eosinophils within the dermis (Figure 2A). The infiltrate also contained numerous large mononuclear cells with enlarged nuclei, fine loose chromatin, rare nucleoli, and a thin rim of cytoplasm (Figure 2B). There were associated apoptotic bodies with karyorrhectic debris. Immunohistochemistry exhibited enlarged cells that were strong staining with CD3 and CD4, which was consistent with reactive helper T cells (Figure 3). A myeloperoxidase stain highlighted few neutrophils. Stains for terminal deoxynucleotidyl transferase, CD1a, CD117, and CD34 were negative. These findings along with the clinical presentation yielded a diagnosis of AEI with reactive helper T cells.

Comment
Clinical Presentation of AEIs—Annular erythemas of infancy are rare benign skin eruptions that develop in the first few months of life.1,16 Few cases have been reported (eTable). Clinically, AEIs are characterized by annular or circinate, erythematous patches and plaques. They can occur on the face, trunk, and extremities, and they completely resolve by 1 year of age in most cases. One case was reported to persist in a patient from birth until 15 years of age.9 It is thought that AEIs may occur as a hypersensitivity reaction to an unrecognized antigen.

Histopathology—Histologically, AEIs demonstrate a superficial and deep, perivascular, inflammatory infiltrate in the dermis composed of small lymphocytes, some neutrophils, and eosinophils.16 Less common variants of AEI include eosinophilic annular erythema, characterized by a diffuse dermal infiltrate of eosinophils and some lymphocytes, and neutrophilic figurate erythema of infancy, characterized by a dermal infiltrate with neutrophils and leukocytoclasis without vasculitis.1

Our patient’s skin rash was unusual in that the biopsy demonstrated few neutrophils, rare eosinophils, and larger mononuclear cells consistent with reactive helper T lymphocytes. Although these cells may raise concern for an atypical lymphoid infiltrate, recognition of areas with more conventional histopathology of AEIs can facilitate the correct diagnosis.

Differential Diagnosis—The main considerations in the differential diagnosis for AEIs include the following: EAC, familial annular erythema, erythema gyratum atrophicans transiens neonatale, erythema chronicum migrans, urticaria, tinea corporis, neonatal lupus erythematosus, viral exanthems, and leukemia cutis.16
Erythema annulare centrifugum typically begins in middle age and follows a course of 2 or more years.2 It occurs in association with an underlying infection or neoplasm, and it can develop on the trunk and proximal extremities. Morphologically, EAC can present with arcuate or polycyclic lesions with trailing scale. Histologically, a skin biopsy shows a tight, coat sleeve–like, perivascular, lymphohistiocytic infiltrate in the dermis, with variable epidermal spongiosis and parakeratosis.16 Our patient’s biopsy did show a tight perivascular infiltrate, raising suspicion for EAC. However, the eruption occurred in infancy, and she had no clinical evidence of infection or neoplasm.
Familial annular erythemas can arise within a few days after birth and can present on any part of the body, including the tongue.2 Individual lesions can persist for 4 to 5 days and can accompany congenital malformations. Morphologically, they can present as papules that slowly enlarge to form arcuate lesions with central hyperpigmentation. Histologically, there can be a mild, perivascular, lymphocytic infiltrate in the dermis.16 Our patient’s lesions showed no scale or pigmentation and occurred without a family history or associated malformations.
Erythema gyratum atrophicans transiens neonatale also can arise in the first few days of life and can affect the trunk, neck, and lips.16 Morphologically, the skin lesions can present as arcuate erythematous patches (3–20 mm) with raised borders and central atrophy. Histologically, there is epidermal atrophy with a dermal perivascular mononuclear cell infiltrate with edema. Our patient’s clinical presentation was not classic for this condition, and the lesions showed no atrophy.
Erythema chronicum migrans can arise in children, often with a history of an arthropod bite.13 Morphologically, lesions can evolve over weeks to months and rarely are multiple. Erythema chronicum migrans most commonly occurs in the United States in association with Lyme disease from infection with Borrelia burgdorferi. Histologically, erythema chronicum migrans shows a superficial and deep, perivascular lymphocytic infiltrate in the dermis with plasma cells and eosinophils. A silver stain can demonstrate dermal spirochetes. Our patient had no history of an arthropod bite. A Warthin-Starry stain performed on the biopsy was negative for spirochetes, and serologies for Lyme disease were negative.
Urticaria is rare in neonates and can occur on any part of the body.2 Morphologically, the skin lesions can present as arcuate, erythematous, and polycyclic plaques that wax and wane. Histologically, there is dermal edema with a mild, perivascular and interstitial, mixed inflammatory infiltrate.16 Our patient’s biopsy did not reveal notable edema, and the perivascular infiltrate was coat sleeve–like with few neutrophils and eosinophils. The patient did not respond to initial treatment with antihistamines, making urticaria less likely.
Tinea corporis is rare in neonates and can occur on any part of the body.13 Morphologically, it can present as scaly annular lesions that are fixed and more persistent. Histologically, there are fungal hyphae and/or yeast in the stratum corneum with spongiotic dermatitis and parakeratosis. Our patient’s lesions were not scaly, and the biopsy demonstrated minimal spongiosis. A periodic acid–Schiff special stain was negative for fungal microorganisms.
Neonatal lupus erythematosus can arise at birth or during the first few weeks of life.16 Morphologically, the skin lesions occur on the scalp, forehead, or neck in a periorbital or malar distribution. They can present as erythematous, annular, scaly patches and plaques. Transplacental transmission of material autoantibodies has been implicated in the etiology, and a complication is infantile heart block. Histologically, a skin biopsy typically shows interface/lichenoid dermatitis. However, our patient’s biopsy did not demonstrate interface changes, and serologically she was negative for autoantibodies.
Viral exanthems are skin eruptions that accompany underlying viral infections.17 Morphologically, patients can present with an erythematous maculopapular rash, sometimes with vesicular, petechial, and urticarial lesions. Laboratory confirmation is made by virus-specific serologies. Histologically, viral exanthems can show a superficial, perivascular, lymphocytic infiltrate in the dermis, with reactive T cells and epidermal spongiosis. Our patient was afebrile and had no known sick contacts. A cytomegalovirus immunohistochemical study on the biopsy was negative, and an Epstein-Barr encoding region in situ hybridization study was negative.
Leukemia cutis is the infiltration of the skin by leukemic cells, most often in conjunction with systemic leukemia.18 In infants and children, the most common leukemia is B-cell acute lymphoblastic leukemia. Morphologically, the skin lesions are characterized by single or multiple violaceous papules, nodules, and plaques. Histologically, there is a perivascular to interstitial infiltrate of atypical mononuclear cells in the dermis and sometimes subcutis. The leukemic cells demonstrate enlarged nuclei with coarse chromatin and prominent nucleoli. Increased mitotic activity may be seen with karyorrhectic debris. Immunohistochemically, the tumor cells can be positive for myeloperoxidase, CD43, CD68, CD34, and CD117.18 Although our patient’s biopsy demonstrated mononuclear cells with karyorrhexis, the cells did not have striking atypia and were negative for blast markers. A recent complete blood cell count on the patient was normal.
Conclusion
We report an unusual case of AEI with mononuclear cells consistent with helper T cells. One must keep these cells in mind when evaluating a biopsy of AEI, as they are benign and not suggestive of an atypical lymphoid infiltrate or leukemia cutis. This will prevent misdiagnosis and ensure that the patient receives appropriate management.
- Ríos-Martín JJ, Ferrándiz-Pulido L, Moreno-Ramírez D. Approaches to the dermatopathologic diagnosis of figurate lesions [in Spanish]. Actas Dermosifiliogr. 2011;102:316-324. doi:10.1016/j.ad.2010.12.009
- Peterson AO, Jarratt M. Annular erythema of infancy. Arch Dermatol. 1981;117:145-148.
- Toonstra J, de Wit RF. “Persistent” annular erythema of infancy. Arch Dermatol.1984;120:1069-1072.
- Hebert AA, Esterly NB. Annular erythema of infancy. J Am Acad Dermatol. 1986;14:339-343.
- Cox NH, McQueen A, Evans TJ, et al. An annular erythema of infancy. Arch Dermatol. 1987;123:510-513.
- Helm TN, Bass J, Chang LW, et al. Persistent annular erythema of infancy. Pediatr Dermatol. 1993;10:46-48.
- Kunz M, Hamm K, Bröcker EB, et al. Annular erythema in childhood—a new eosinophilic dermatosis [in German]. Hautarzt. 1998;49:131-134.
- Stachowitz S, Abeck D, Schmidt T, et al. Persistent annular erythema of infancy associated with intestinal Candida colonization. Clin Exp Dermatol. 2000;25:404-405.
- Wong L-C, Kakakios A, Rogers M. Congenital annular erythema persisting in a 15-year-old girl. Australas J Dermatol. 2002;43:55-61.
- Patrizi A, Savoia F, Varotti E, et al. Neutrophilic figurate erythema of infancy. Pediatr Dermatol. 2008;25:255-260. doi:10.1111/j.1525-1470.2008.00646.x
- Saha A, Seth J, Mukherjee S, et al. Annular erythema of infancy: a diagnostic challenge. Indian J Paediatr Dermatol. 2014;15:147-149. doi:10.4103/2319-7250.143678
- Pfingstler LF, Miller KP, Pride H. Recurring diffuse annular erythematous plaques in a newborn. JAMA Dermatol. 2014;150:565-566. doi:10.1001/jamadermatol.2013.8059
- Del Puerto Troncoso C, Curi Tuma M, González Bombardiere S, et al. Neutrophilic figurate erythema of infancy associated with juvenile myelomonocytic leukemia. Actas Dermosifiliogr. 2015;106:431-433. doi:10.1016/j.ad.2014.09.013
- Hamidi S, Prose NS, Selim MA. Neutrophilic figurate erythema of infancy: a diagnostic challenge [published online December 26, 2018]. J Cutan Pathol. 2019;46:216-220. doi:10.1111/cup.13394
- Patel N, Goldbach H, Hogeling M. An annular eruption in a young child. JAMA Dermatol. 2018;154:1213-1214. doi:10.1001/jamadermatol.2018.1174
- Palit A, Inamadar AC. Annular, erythematous skin lesions in a neonate. Indian Dermatol Online J. 2012;3:45-47. doi:10.4103/2229-5178.93504
- Keighley CL, Saunderson RB, Kok J, et al. Viral exanthems. Curr Opin Infect Dis. 2015;28:139-150. doi:10.1097/QCO.0000000000000145
- Cronin DMP, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132:101-110. doi:10.1309/AJCP6GR8BDEXPKHR
- Ríos-Martín JJ, Ferrándiz-Pulido L, Moreno-Ramírez D. Approaches to the dermatopathologic diagnosis of figurate lesions [in Spanish]. Actas Dermosifiliogr. 2011;102:316-324. doi:10.1016/j.ad.2010.12.009
- Peterson AO, Jarratt M. Annular erythema of infancy. Arch Dermatol. 1981;117:145-148.
- Toonstra J, de Wit RF. “Persistent” annular erythema of infancy. Arch Dermatol.1984;120:1069-1072.
- Hebert AA, Esterly NB. Annular erythema of infancy. J Am Acad Dermatol. 1986;14:339-343.
- Cox NH, McQueen A, Evans TJ, et al. An annular erythema of infancy. Arch Dermatol. 1987;123:510-513.
- Helm TN, Bass J, Chang LW, et al. Persistent annular erythema of infancy. Pediatr Dermatol. 1993;10:46-48.
- Kunz M, Hamm K, Bröcker EB, et al. Annular erythema in childhood—a new eosinophilic dermatosis [in German]. Hautarzt. 1998;49:131-134.
- Stachowitz S, Abeck D, Schmidt T, et al. Persistent annular erythema of infancy associated with intestinal Candida colonization. Clin Exp Dermatol. 2000;25:404-405.
- Wong L-C, Kakakios A, Rogers M. Congenital annular erythema persisting in a 15-year-old girl. Australas J Dermatol. 2002;43:55-61.
- Patrizi A, Savoia F, Varotti E, et al. Neutrophilic figurate erythema of infancy. Pediatr Dermatol. 2008;25:255-260. doi:10.1111/j.1525-1470.2008.00646.x
- Saha A, Seth J, Mukherjee S, et al. Annular erythema of infancy: a diagnostic challenge. Indian J Paediatr Dermatol. 2014;15:147-149. doi:10.4103/2319-7250.143678
- Pfingstler LF, Miller KP, Pride H. Recurring diffuse annular erythematous plaques in a newborn. JAMA Dermatol. 2014;150:565-566. doi:10.1001/jamadermatol.2013.8059
- Del Puerto Troncoso C, Curi Tuma M, González Bombardiere S, et al. Neutrophilic figurate erythema of infancy associated with juvenile myelomonocytic leukemia. Actas Dermosifiliogr. 2015;106:431-433. doi:10.1016/j.ad.2014.09.013
- Hamidi S, Prose NS, Selim MA. Neutrophilic figurate erythema of infancy: a diagnostic challenge [published online December 26, 2018]. J Cutan Pathol. 2019;46:216-220. doi:10.1111/cup.13394
- Patel N, Goldbach H, Hogeling M. An annular eruption in a young child. JAMA Dermatol. 2018;154:1213-1214. doi:10.1001/jamadermatol.2018.1174
- Palit A, Inamadar AC. Annular, erythematous skin lesions in a neonate. Indian Dermatol Online J. 2012;3:45-47. doi:10.4103/2229-5178.93504
- Keighley CL, Saunderson RB, Kok J, et al. Viral exanthems. Curr Opin Infect Dis. 2015;28:139-150. doi:10.1097/QCO.0000000000000145
- Cronin DMP, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132:101-110. doi:10.1309/AJCP6GR8BDEXPKHR
Practice Points
- Annular erythemas of infancy (AEIs) are rare benign skin eruptions characterized by persistent, annular, urticarial, nonpruritic patches and plaques that develop in patients younger than 1 year.
- Although AEIs are benign, lesions with uncommon histologic features such as large mononuclear cells consistent with reactive helper T lymphocytes may pose diagnostic challenges.
Pediatric Subungual Exostosis
Exostosis is a type of benign bone tumor in which trabecular (spongy) bone overgrows its normal border in a nodular pattern. 1,2 Histologically, it usually is surrounded by a fibrocartilaginous cap. 3 It is most commonly found on the lateral or medial aspect of the foot and is thought to be caused by trauma, either physical pressure or infection. 4 When this lesion is found under the nail bed, it is termed subungual exostosis ( Dupuytren exostosis ) . 3 Sequelae of a subungual exostosis include nail dystrophy and lifting of the nail away from the toe, in addition to infection and possible loss of the toenail (onycholysis). There are only 2 genetic conditions related to exostosis: hereditary multiple exostosis and multiple exostoses-mental retardation syndrome.
An exostosis may appear to be a wart on first inspection. It may present similar to osteochondromas, and the only way to get a true diagnosis is by biopsy of the lesion. The treatment for an exostosis is surgery. The surgeon must remove the lesion at the base of the bone from which it grows to prevent recurrence of the lesion.5
Because exostosis may cause nail bed disruption, the differential diagnosis may include nail deformities, such as traumatic onycholysis, onychogryphosis, verrucae, subungual infection, or nail trauma.6,7
Case Report
A 7-year-old boy presented with changes of the right great toenail over the last 4 months. The patient noted that the affected nail was discolored, dystrophic, painful, and thickened. He did not recall prior trauma to the affected nail, and his mother stated that the lesion was growing and becoming more painful with a throbbing sensation at times. He described the pain as stabbing, which was exacerbated while walking and playing sports. Neither the patient nor his family had ever had any similar condition. He was not taking any medications, only a daily multivitamin. He had a history of eczematous dermatitis and keratosis pilaris without any other medical illnesses. He had a family history of psoriasis; however, no prior instances of exostosis had been reported. He had no medication allergies.
A full-body cutaneous and nail examination showed a well-developed, well-nourished boy who was in no acute distress. A firm, subungual, pink, pearly,hyperkeratotic nodule was appreciated on the right great toe (Figure 1). The lesion was tender to palpation. The rest of the examination and review of systems were normal.
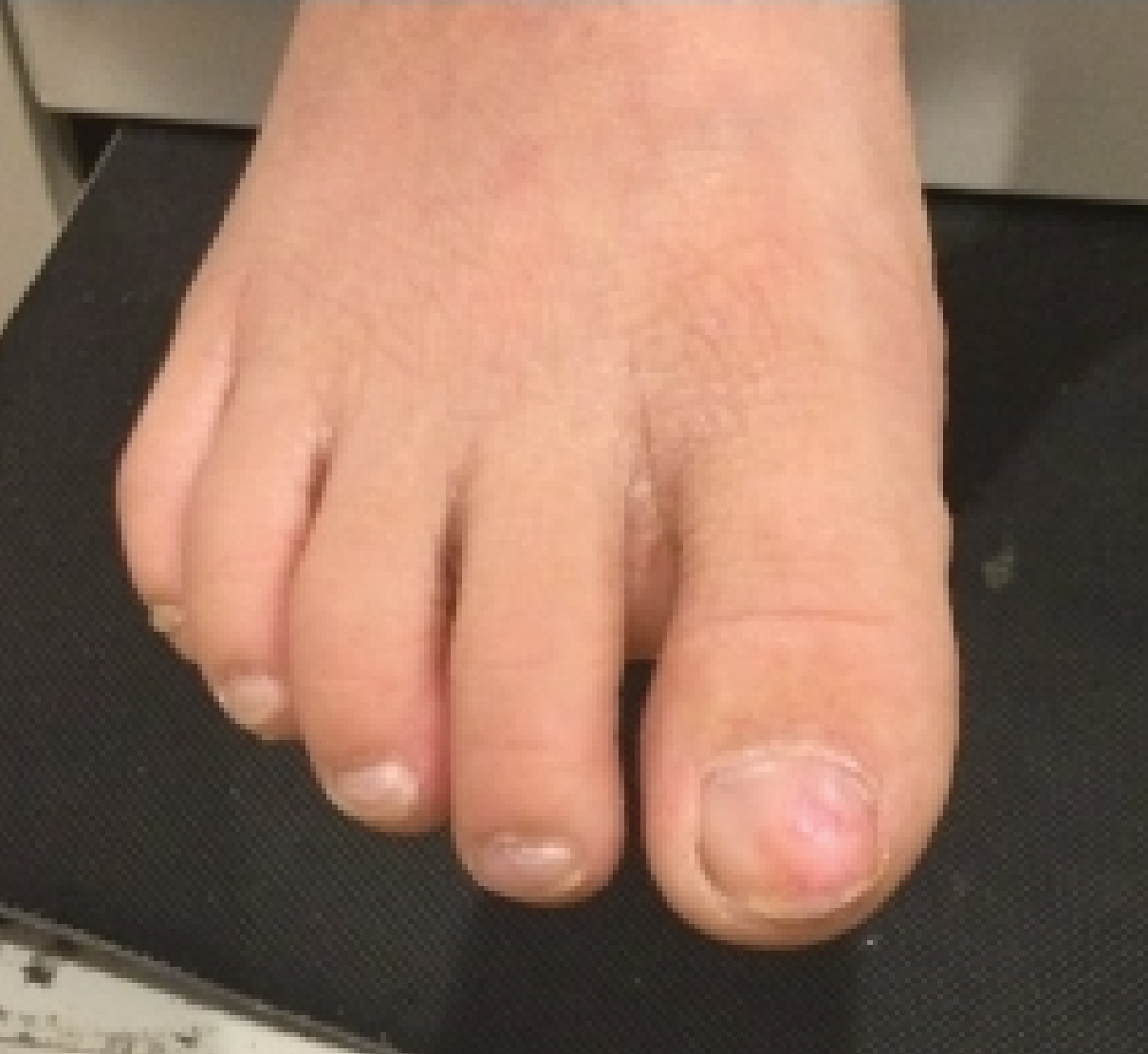
From the clinical findings, a differential diagnosis of glomus tumor, hemangioma, and infection was considered. Periodic acid–Schiff stain was negative, which ruled out fungal infection. Nail avulsion and a shave biopsy were performed under general anesthesia. There was an exostosis arising from the dorsal aspect of the great toe measuring approximately 5 mm in width at the base and approximately 1 mm in height, which endorsed a diagnosis of distal phalanx subungual exostosis. A postsurgery radiograph (Figure 2) showed residual bone below the level of shave removal at the nail bed.
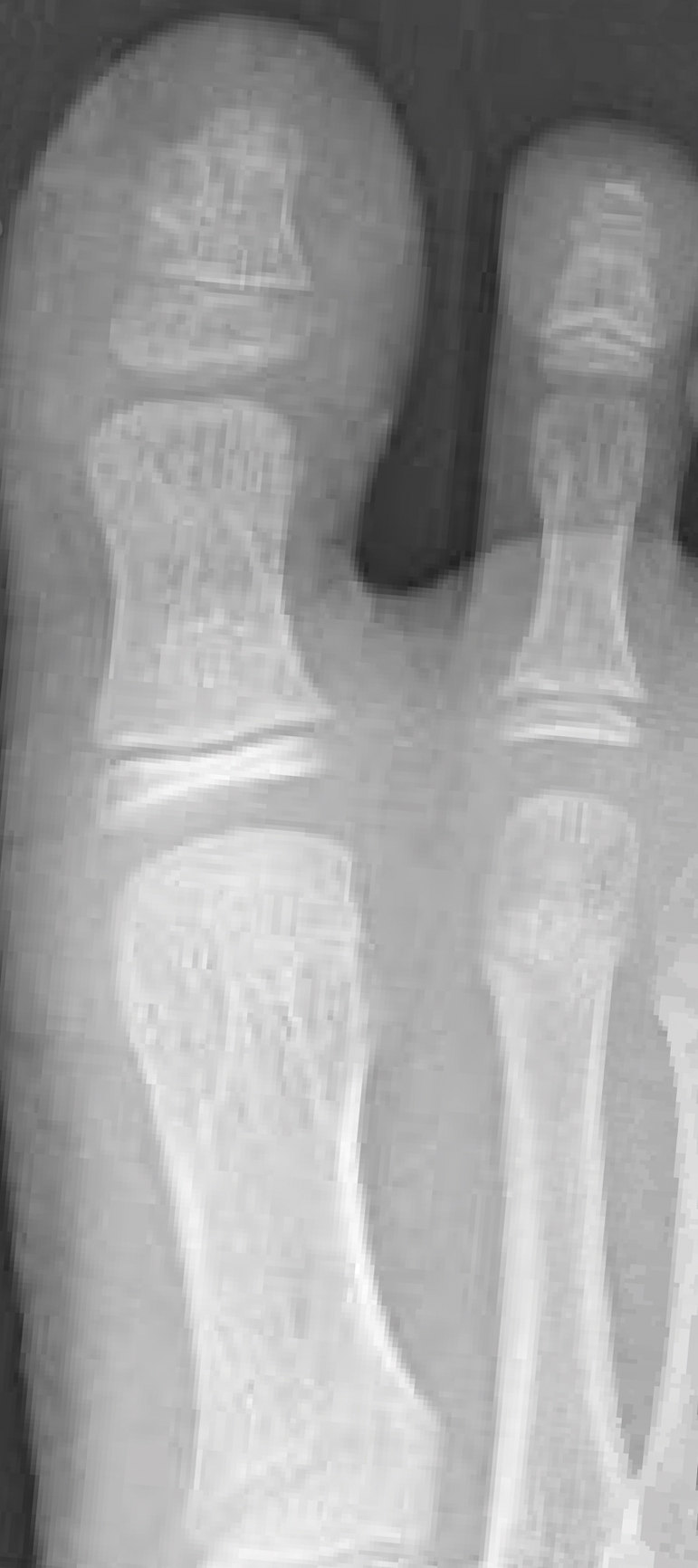
Comment
Exostosis is most commonly found on the lateral or medial aspect of the hallux (great toe) in patients younger than 18 years.8 Diagnosis often is obvious, even without a radiograph or biopsy, because the exostosis comes out from under the tip of the nail. Our case was interesting because the patient was a child, and the exostosis did not lift the nail or extrude from the distal tip of the nail bed. Evidence suggests that a greater-than-expected genetic influence contributes to an exostosis, though further investigation is needed to determine all of the causes and risk factors for subungual bony exostosis. Timely diagnosis and treatment are essential to the prevention of sequelae of the disease, such as toe infection or chronic pain.
- de Palma L, Gigante A, Specchia N. Subungual exostosis of the foot. Foot Ankle Int. 1996;17:758-763. doi:10.1177/107110079601701208
- Multhopp-Stephens H, Walling AK. Subungual (Dupuytren’s) exostosis. J Pediatr Orthop. 1995;15:582-584. doi:10.1097/01241398-199509000-00006
- Davis DA, Cohen PR. Subungual exostosis: case report and review of the literature. Pediatr Dermatol. 1996;13:212-218.
- Guarneri C, Guarneri F, Risitano G, et al. Solitary asymptomatic nodule of the great toe. Int J Dermatol. 2005;44:245-247.
- Letts M, Davidson D, Nizalik E. Subungual exostosis: diagnosis and treatment in children. J Trauma. 1998;44:346-349.
- Hoy NY, Leung AKC, Metelitsa AI, et al. New concepts in median nail dystrophy, onychomycosis, and hand, foot, and mouth disease nail pathology. ISRN Dermatol. 2012;2012:680163.
- Rich P, Scher RK. Examination of the nail and work-up of nail conditions. In: Rich P, Scher RK, eds. An Atlas of Diseases of the Nail. Parthenon Publishing; 2003.
- DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259. doi:10.1007/s11999-013-3345-4
Exostosis is a type of benign bone tumor in which trabecular (spongy) bone overgrows its normal border in a nodular pattern. 1,2 Histologically, it usually is surrounded by a fibrocartilaginous cap. 3 It is most commonly found on the lateral or medial aspect of the foot and is thought to be caused by trauma, either physical pressure or infection. 4 When this lesion is found under the nail bed, it is termed subungual exostosis ( Dupuytren exostosis ) . 3 Sequelae of a subungual exostosis include nail dystrophy and lifting of the nail away from the toe, in addition to infection and possible loss of the toenail (onycholysis). There are only 2 genetic conditions related to exostosis: hereditary multiple exostosis and multiple exostoses-mental retardation syndrome.
An exostosis may appear to be a wart on first inspection. It may present similar to osteochondromas, and the only way to get a true diagnosis is by biopsy of the lesion. The treatment for an exostosis is surgery. The surgeon must remove the lesion at the base of the bone from which it grows to prevent recurrence of the lesion.5
Because exostosis may cause nail bed disruption, the differential diagnosis may include nail deformities, such as traumatic onycholysis, onychogryphosis, verrucae, subungual infection, or nail trauma.6,7
Case Report
A 7-year-old boy presented with changes of the right great toenail over the last 4 months. The patient noted that the affected nail was discolored, dystrophic, painful, and thickened. He did not recall prior trauma to the affected nail, and his mother stated that the lesion was growing and becoming more painful with a throbbing sensation at times. He described the pain as stabbing, which was exacerbated while walking and playing sports. Neither the patient nor his family had ever had any similar condition. He was not taking any medications, only a daily multivitamin. He had a history of eczematous dermatitis and keratosis pilaris without any other medical illnesses. He had a family history of psoriasis; however, no prior instances of exostosis had been reported. He had no medication allergies.
A full-body cutaneous and nail examination showed a well-developed, well-nourished boy who was in no acute distress. A firm, subungual, pink, pearly,hyperkeratotic nodule was appreciated on the right great toe (Figure 1). The lesion was tender to palpation. The rest of the examination and review of systems were normal.

From the clinical findings, a differential diagnosis of glomus tumor, hemangioma, and infection was considered. Periodic acid–Schiff stain was negative, which ruled out fungal infection. Nail avulsion and a shave biopsy were performed under general anesthesia. There was an exostosis arising from the dorsal aspect of the great toe measuring approximately 5 mm in width at the base and approximately 1 mm in height, which endorsed a diagnosis of distal phalanx subungual exostosis. A postsurgery radiograph (Figure 2) showed residual bone below the level of shave removal at the nail bed.

Comment
Exostosis is most commonly found on the lateral or medial aspect of the hallux (great toe) in patients younger than 18 years.8 Diagnosis often is obvious, even without a radiograph or biopsy, because the exostosis comes out from under the tip of the nail. Our case was interesting because the patient was a child, and the exostosis did not lift the nail or extrude from the distal tip of the nail bed. Evidence suggests that a greater-than-expected genetic influence contributes to an exostosis, though further investigation is needed to determine all of the causes and risk factors for subungual bony exostosis. Timely diagnosis and treatment are essential to the prevention of sequelae of the disease, such as toe infection or chronic pain.
Exostosis is a type of benign bone tumor in which trabecular (spongy) bone overgrows its normal border in a nodular pattern. 1,2 Histologically, it usually is surrounded by a fibrocartilaginous cap. 3 It is most commonly found on the lateral or medial aspect of the foot and is thought to be caused by trauma, either physical pressure or infection. 4 When this lesion is found under the nail bed, it is termed subungual exostosis ( Dupuytren exostosis ) . 3 Sequelae of a subungual exostosis include nail dystrophy and lifting of the nail away from the toe, in addition to infection and possible loss of the toenail (onycholysis). There are only 2 genetic conditions related to exostosis: hereditary multiple exostosis and multiple exostoses-mental retardation syndrome.
An exostosis may appear to be a wart on first inspection. It may present similar to osteochondromas, and the only way to get a true diagnosis is by biopsy of the lesion. The treatment for an exostosis is surgery. The surgeon must remove the lesion at the base of the bone from which it grows to prevent recurrence of the lesion.5
Because exostosis may cause nail bed disruption, the differential diagnosis may include nail deformities, such as traumatic onycholysis, onychogryphosis, verrucae, subungual infection, or nail trauma.6,7
Case Report
A 7-year-old boy presented with changes of the right great toenail over the last 4 months. The patient noted that the affected nail was discolored, dystrophic, painful, and thickened. He did not recall prior trauma to the affected nail, and his mother stated that the lesion was growing and becoming more painful with a throbbing sensation at times. He described the pain as stabbing, which was exacerbated while walking and playing sports. Neither the patient nor his family had ever had any similar condition. He was not taking any medications, only a daily multivitamin. He had a history of eczematous dermatitis and keratosis pilaris without any other medical illnesses. He had a family history of psoriasis; however, no prior instances of exostosis had been reported. He had no medication allergies.
A full-body cutaneous and nail examination showed a well-developed, well-nourished boy who was in no acute distress. A firm, subungual, pink, pearly,hyperkeratotic nodule was appreciated on the right great toe (Figure 1). The lesion was tender to palpation. The rest of the examination and review of systems were normal.

From the clinical findings, a differential diagnosis of glomus tumor, hemangioma, and infection was considered. Periodic acid–Schiff stain was negative, which ruled out fungal infection. Nail avulsion and a shave biopsy were performed under general anesthesia. There was an exostosis arising from the dorsal aspect of the great toe measuring approximately 5 mm in width at the base and approximately 1 mm in height, which endorsed a diagnosis of distal phalanx subungual exostosis. A postsurgery radiograph (Figure 2) showed residual bone below the level of shave removal at the nail bed.

Comment
Exostosis is most commonly found on the lateral or medial aspect of the hallux (great toe) in patients younger than 18 years.8 Diagnosis often is obvious, even without a radiograph or biopsy, because the exostosis comes out from under the tip of the nail. Our case was interesting because the patient was a child, and the exostosis did not lift the nail or extrude from the distal tip of the nail bed. Evidence suggests that a greater-than-expected genetic influence contributes to an exostosis, though further investigation is needed to determine all of the causes and risk factors for subungual bony exostosis. Timely diagnosis and treatment are essential to the prevention of sequelae of the disease, such as toe infection or chronic pain.
- de Palma L, Gigante A, Specchia N. Subungual exostosis of the foot. Foot Ankle Int. 1996;17:758-763. doi:10.1177/107110079601701208
- Multhopp-Stephens H, Walling AK. Subungual (Dupuytren’s) exostosis. J Pediatr Orthop. 1995;15:582-584. doi:10.1097/01241398-199509000-00006
- Davis DA, Cohen PR. Subungual exostosis: case report and review of the literature. Pediatr Dermatol. 1996;13:212-218.
- Guarneri C, Guarneri F, Risitano G, et al. Solitary asymptomatic nodule of the great toe. Int J Dermatol. 2005;44:245-247.
- Letts M, Davidson D, Nizalik E. Subungual exostosis: diagnosis and treatment in children. J Trauma. 1998;44:346-349.
- Hoy NY, Leung AKC, Metelitsa AI, et al. New concepts in median nail dystrophy, onychomycosis, and hand, foot, and mouth disease nail pathology. ISRN Dermatol. 2012;2012:680163.
- Rich P, Scher RK. Examination of the nail and work-up of nail conditions. In: Rich P, Scher RK, eds. An Atlas of Diseases of the Nail. Parthenon Publishing; 2003.
- DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259. doi:10.1007/s11999-013-3345-4
- de Palma L, Gigante A, Specchia N. Subungual exostosis of the foot. Foot Ankle Int. 1996;17:758-763. doi:10.1177/107110079601701208
- Multhopp-Stephens H, Walling AK. Subungual (Dupuytren’s) exostosis. J Pediatr Orthop. 1995;15:582-584. doi:10.1097/01241398-199509000-00006
- Davis DA, Cohen PR. Subungual exostosis: case report and review of the literature. Pediatr Dermatol. 1996;13:212-218.
- Guarneri C, Guarneri F, Risitano G, et al. Solitary asymptomatic nodule of the great toe. Int J Dermatol. 2005;44:245-247.
- Letts M, Davidson D, Nizalik E. Subungual exostosis: diagnosis and treatment in children. J Trauma. 1998;44:346-349.
- Hoy NY, Leung AKC, Metelitsa AI, et al. New concepts in median nail dystrophy, onychomycosis, and hand, foot, and mouth disease nail pathology. ISRN Dermatol. 2012;2012:680163.
- Rich P, Scher RK. Examination of the nail and work-up of nail conditions. In: Rich P, Scher RK, eds. An Atlas of Diseases of the Nail. Parthenon Publishing; 2003.
- DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259. doi:10.1007/s11999-013-3345-4
Practice Points
- Nail dystrophy can have a variety of causes, most commonly trauma, onychomycosis, verrucae, or subungual exostosis.
- Exostosis is a benign osteochondral tumor commonly found on the lateral or medial aspect of the hallux (great toe) in pediatric and young adult patients.
- A radiograph can be used as a preliminary tool for diagnosis, but subungual exostosis must be confirmed by biopsy or tissue histology at the time of excision.
Early Pilomatrix Carcinoma: A Case Report With Emphasis on Molecular Pathology and Review of the Literature
Pilomatrix carcinoma is a rare adnexal tumor with origin from the germinative matrical cells of the hair follicle. Clinically, it presents as a solitary lesion commonly found in the head and neck region as well as the upper back. The tumors cannot be distinguished by their clinical appearance only and frequently are mistaken for cysts. Histopathologic examination provides the definitive diagnosis in most cases. These carcinomas are aggressive neoplasms with a high probability of local recurrence and distant metastasis. Assessment of the Wnt signaling pathway components such as β-catenin, lymphoid enhancer-binding factor 1 (LEF-1), and caudal-related homeobox transcription factor 2 (CDX-2) potentially can be used for diagnostic purposes and targeted therapy.
We report a rare and unique case of early pilomatrix carcinoma with intralesional melanocytes. We review the molecular pathology and pathogenesis of these carcinomas as well as the significance of early diagnosis.
Case Report
A 73-year-old man with a history of extensive sun exposure presented with a 1-cm, raised, rapidly growing, slightly irregular, purple lesion on the right forearm of 3 months’ duration with tendency to bleed. He did not have a history of skin cancers and was otherwise healthy. Excision was recommended due to the progressive and rapid growth of the lesion.
Histopathologic Findings—Gross examination revealed a 0.9×0.7-cm, raised, slightly irregular lesion located 1 mm away from the closest peripheral margin. Histologically, the lesion was a relatively circumscribed, dermal-based basaloid neoplasm with slightly ill-defined edges involving the superficial and deep dermis (Figure 1A). The neoplasm was formed predominantly of sheets of basaloid cells and small nests of ghost cells, in addition to some squamoid and transitional cells (Figure 1B). The basaloid cells exhibited severe nuclear atypia, pleomorphism, increased nuclear to cytoplasmic ratio (Figure 1C), minimal to moderate amounts of eosinophilic cytoplasm, enlarged nuclei, prominent nucleoli, and coarse chromatin pattern. Abundant mitotic activity and apoptotic bodies were present as well as focal area of central necrosis (Figure 1C). Also, melanophages and a multinucleated giant cell reaction was noted. Elastic trichrome special stain highlighted focal infiltration of the neoplastic cells into the adjacent desmoplastic stroma. Melanin stain was negative for melanin pigment within the neoplasm. Given the presence of severely atypical basaloid cells along with ghost cells indicating matrical differentiation, a diagnosis of pilomatrix carcinoma was rendered.
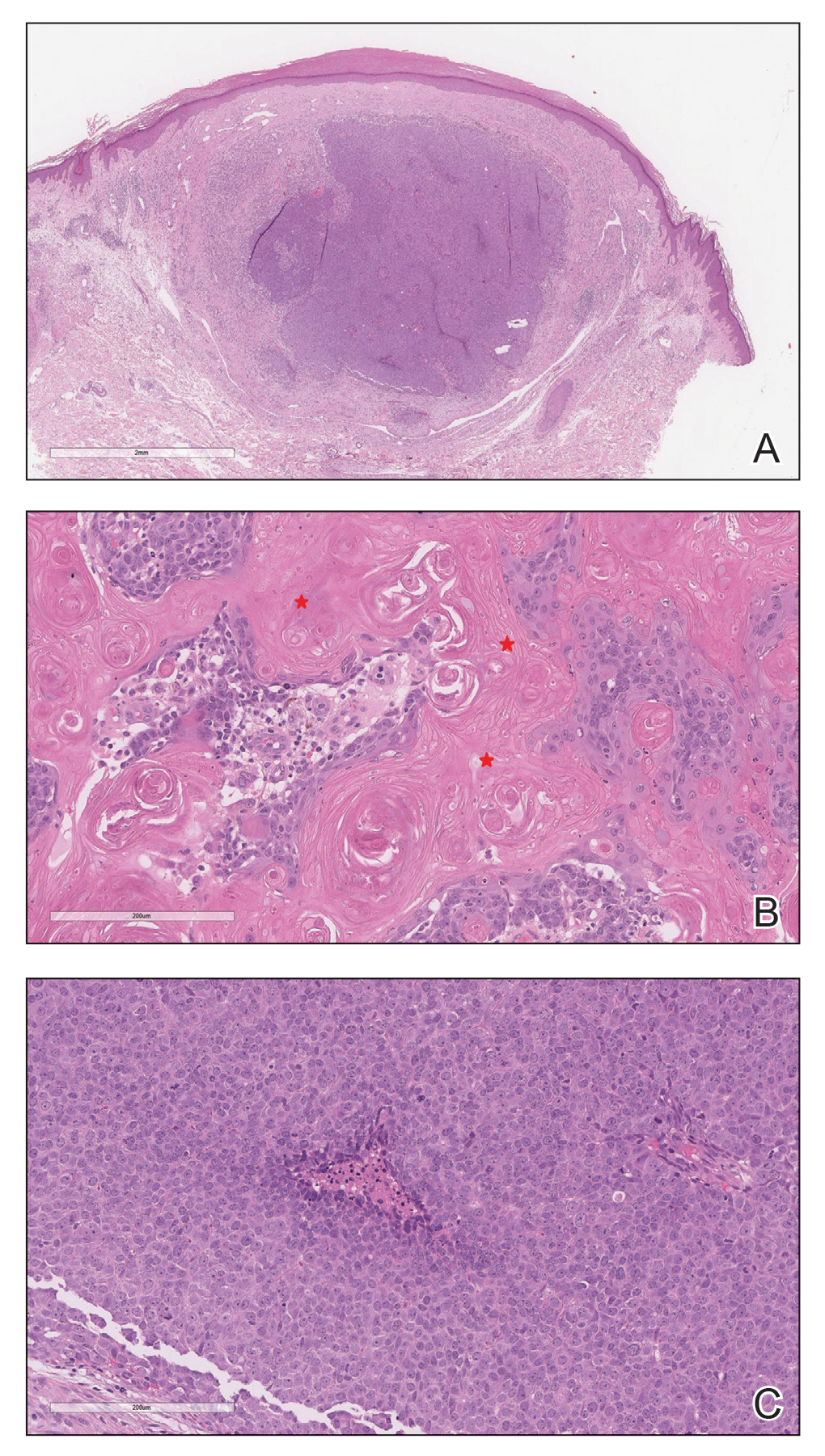
Immunohistochemistry—The neoplastic cells were diffusely positive for p63, CDX-2 (Figure 2A), β-catenin (Figure 2B), and CD10 (Figure 2C), and focally and weakly positive for cytokeratin (CK) 5, BerEP4 (staining the tumor periphery), androgen receptor, and CK18 (a low-molecular-weight keratin). They were negative for monoclonal carcinoembryonic antigen, epithelial membrane antigen, CK7, CK20, CD34, SOX-10, CD56, synaptophysin, and chromogranin. Cytokeratin 14 was positive in the squamoid cells but negative in the basaloid cells. SOX-10 and melanoma cocktail immunostains demonstrated few intralesional dendritic melanocytes.

Comment
Pilomatrix carcinoma is a rare malignant cutaneous adnexal neoplasm with origin from the germinative matrix of the hair bulb region of hair follicles. Pilomatrix carcinoma was first reported in 1980.1,2 These tumors are characterized by rapid growth and aggressive behavior. Their benign counterpart, pilomatrixoma, is a slow-growing, dermal or subcutaneous tumor that rarely recurs after complete excision.
As with pilomatrixoma, pilomatrix carcinomas are asymptomatic and present as solitary dermal or subcutaneous masses3,4 that most commonly are found in the posterior neck, upper back, and preauricular regions of middle-aged or elderly adults with male predominance.5 They range in size from 0.5 to 20 cm with a mean of 4 cm that is slightly larger than pilomatrixoma. Pilomatrix carcinomas predominantly are firm tumors with or without cystic components, and they exhibit a high probability of recurrence and have risk for distant metastasis.6-15
The differential diagnosis includes epidermal cysts, pilomatrixoma, basal cell carcinoma with matrical differentiation, trichoblastoma/trichoblastic carcinoma, and trichilemmal carcinoma. Pilomatrix carcinomas frequently are mistaken for epidermal cysts on clinical examination. Such a distinction can be easily resolved by histopathologic evaluation. The more challenging differential diagnosis is with pilomatrixoma. Histologically, pilomatrixomas consist of a distinct population of cells including basaloid, squamoid, transitional, and shadow cells in variable proportions. The basaloid cells transition to shadow cells in an organized zonal fashion.16 Compared to pilomatrixomas, pilomatrix carcinomas often show predominance of the basaloid cells; marked cytologic atypia and pleomorphism; numerous mitotic figures; deep infiltrative pattern into subcutaneous fat, fascia, and skeletal muscle; stromal desmoplasia; necrosis; and neurovascular invasion (Tables 1 and 2). Furthermore, the shadow cells tend to form a small nested pattern in pilomatrix carcinoma instead of the flat sheetlike pattern usually observed in pilomatrixoma.16 Basal cell carcinoma with matrical differentiation can pose a diagnostic challenge in the differential diagnosis; basal cell carcinoma usually exhibits a peripheral palisade of the basaloid cells accompanied by retraction spaces separating the tumor from the stroma. Trichoblastoma/trichoblastic carcinoma with matrical differentiation can be distinguished by its exuberant stroma, prominent primitive hair follicles, and papillary mesenchymal bodies. Trichilemmal carcinomas are recognized by their connection to the overlying epidermis, peripheral palisading, and presence of clear cells, while pilomatrix carcinoma lacks connection to the surface epithelium.

Immunohistochemical stains have little to no role in the differential diagnosis, and morphology is the mainstay in making the diagnosis. Rarely, pilomatrix carcinoma can be confused with poorly differentiated sebaceous carcinoma and poorly differentiated squamous cell carcinoma. Although careful scrutiny of the histologic features may help identify mature sebocytes in sebaceous carcinoma, evidence of keratinization in squamous cell carcinoma and ghost cells in pilomatrix carcinoma, using a panel of immunohistochemical stains can be helpful in reaching the final diagnosis (Table 3).

The development of hair matrix tumors have been known to harbor mutations in exon 3 of the catenin beta-1 gene, CTNNB1, that encodes for β-catenin, a downstream effector in the Wnt signaling pathway responsible for differentiation, proliferation, and adhesion of epithelial stem cells.17-21 In a study conducted by Kazakov et al,22 DNA was extracted from 86 lesions: 4 were pilomatrixomas and 1 was a pilomatrix carcinoma. A polymerase chain reaction assay revealed 8 pathogenic variants of the β-catenin gene. D32Y (CTNNB1):c.94G>T (p.Asp32Tyr) and G34R (CTNNB1):c.100G>C (p.Gly34Arg) were the mutations present in pilomatrixoma and pilomatrix carcinoma, respectively.22 In addition, there are several proteins that are part of the Wnt pathway in addition to β-catenin—LEF-1 and CDX-2.
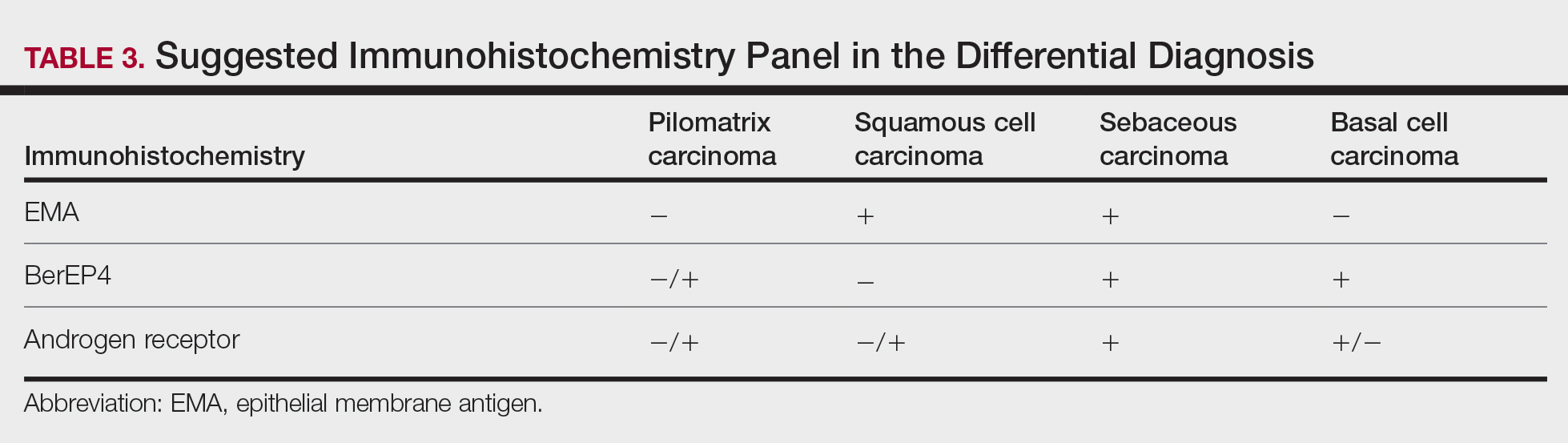
Tumminello and Hosler23 found that pilomatrixomas and pilomatrix carcinomas were positive for CDX-2, β-catenin, and LEF-1 by immunohistochemistry. These downstream molecules in the Wnt signaling pathway could have the potential to be used as diagnostic and prognostic markers.2,13,15,23
Although the pathogenesis is unclear, there are 2 possible mechanisms by which pilomatrix carcinomas develop. They can either arise as de novo tumors, or it is possible that initial mutations in β-catenin result in the formation of pilomatrixomas at an early age that may undergo malignant transformation in elderly patients over time with additional mutations.2
Our case was strongly and diffusely positive for β-catenin in a nuclear and cytoplasmic pattern and CDX-2 in a nuclear pattern, supporting the role of the Wnt signaling pathway in such tumors. Furthermore, our case demonstrated the presence of few intralesional normal dendritic melanocytes, a rare finding1,24,25 but not unexpected, as melanocytes normally are present within the hair follicle matrix.
Pilomatrix carcinomas are aggressive tumors with a high risk for local recurrence and tendency for metastasis. In a study of 13 cases of pilomatrix carcinomas, Herrmann et al13 found that metastasis was significantly associated with local tumor recurrence (P<.0413). They concluded that the combination of overall high local recurrence and metastatic rates of pilomatrix carcinoma as well as documented tumor-related deaths would warrant continued patient follow-up, especially for recurrent tumors.13 Rapid growth of a tumor, either de novo or following several months of stable size, should alert physicians to perform a diagnostic biopsy.
Management options of pilomatrix carcinoma include surgery or radiation with close follow-up. The most widely reported treatment of pilomatrix carcinoma is wide local excision with histologically confirmed clear margins. Mohs micrographic surgery is an excellent treatment option.2,13-15 Adjuvant radiation therapy may be necessary following excision. Currently there is no consensus on surgical management, and standard excisional margins have not been defined.26 Jones et al2 concluded that complete excision with wide margins likely is curative, with decreased rates of recurrence, and better awareness of this carcinoma would lead to appropriate treatment while avoiding unnecessary diagnostic tests.2
Conclusion
We report an exceptionally unique case of early pilomatrix carcinoma with a discussion on the pathogenesis and molecular pathology of hair matrix tumors. A large cohort of patients with longer follow-up periods and better molecular characterization is essential in drawing accurate information about their prognosis, identifying molecular markers that can be used as therapeutic targets, and determining ideal management strategy.
- Jani P, Chetty R, Ghazarian DM. An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature. Am J Dermatopathol. 2008;30:174-177.
- Jones C, Twoon M, Ho W, et al. Pilomatrix carcinoma: 12-year experience and review of the literature. J Cutan Pathol. 2018;45:33-38.
- Forbis R, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961;83:606.
- Elder D, Elenitsas R, Ragsdale BD. Tumors of epidermal appendages. In: Elder D, Elenitsas R, Jaworsky C, eds. Lever’s Histopathology of the Skin. 8th ed. Lippincott Raven; 1997:757-759.
- Aherne NJ, Fitzpatrick DA, Gibbons D, et al. Pilomatrix carcinoma presenting as an extra axial mass: clinicopathological features. Diagn Pathol. 2008;3:47.
- Papadakis M, de Bree E, Floros N, et al. Pilomatrix carcinoma: more malignant biological behavior than was considered in the past. Mol Clin Oncol. 2017;6:415-418.
- LeBoit PE, Parslow TG, Choy SH. Hair matrix differentiation: occurrence in lesions other than pilomatricoma. Am J Dermatopathol. 1987;9:399-405.
- Campoy F, Stiefel P, Stiefel E, et al. Pilomatrix carcinoma: role played by MR imaging. Neuroradiology. 1989;31:196-198.
- Tateyama H, Eimoto T, Tada T, et al. Malignant pilomatricoma: an immunohistochemical study with antihair keratin antibody. Cancer. 1992;69:127-132.
- O’Donovan DG, Freemont AJ, Adams JE, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1993;23:385-386.
- Cross P, Richmond I, Wells S, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1994;24:499-500.
- Niedermeyer HP, Peris K, Höfler H. Pilomatrix carcinoma with multiple visceral metastases: report of a case. Cancer. 1996;77:1311-1314.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Xing L, Marzolf SA, Vandergriff T, et al. Facial pilomatrix carcinomas treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:253-255.
- Fernandez-Flores A, Cassarino DS. Sarcomatoid pilomatrix carcinoma. J Cutan Pathol. 2018;45:508-514.
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993;71:2491-2498.
- Chan E, Gat U, McNiff JM, et al. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet. 1999;21:410-413.
- Huelsken J, Vogel R, Erdmann B, et al. β-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533-545.
- Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 2003;94:225-229.
- Durand M, Moles J. Beta-catenin mutations in a common skin cancer: pilomatricoma. Bull Cancer. 1999;86:725-726.
- Lazar AJF, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148-157.
- Kazakov DV, Sima R, Vanecek T, et al. Mutations in exon 3 of the CTNNB1 gene (β-catenin gene) in cutaneous adnexal tumors. Am J Dermatopathol. 2009;31:248-255.
- Tumminello K, Hosler GA. CDX2 and LEF-1 expression in pilomatrical tumors and their utility in the diagnosis of pilomatrical carcinoma. J Cutan Pathol. 2018;45:318-324.
- Rodic´ N, Taube JM, Manson P, et al Locally invasive dermal squamomelanocytic tumor with matrical differentiation: a peculiar case with review of the literature. Am J Dermatopathol. 2013;35:E72-E76.
- Perez C, Debbaneh M, Cassarino D. Preference for the term pilomatrical carcinoma with melanocytic hyperplasia: letter to the editor. J Cutan Pathol. 2017;44:655-657.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
Pilomatrix carcinoma is a rare adnexal tumor with origin from the germinative matrical cells of the hair follicle. Clinically, it presents as a solitary lesion commonly found in the head and neck region as well as the upper back. The tumors cannot be distinguished by their clinical appearance only and frequently are mistaken for cysts. Histopathologic examination provides the definitive diagnosis in most cases. These carcinomas are aggressive neoplasms with a high probability of local recurrence and distant metastasis. Assessment of the Wnt signaling pathway components such as β-catenin, lymphoid enhancer-binding factor 1 (LEF-1), and caudal-related homeobox transcription factor 2 (CDX-2) potentially can be used for diagnostic purposes and targeted therapy.
We report a rare and unique case of early pilomatrix carcinoma with intralesional melanocytes. We review the molecular pathology and pathogenesis of these carcinomas as well as the significance of early diagnosis.
Case Report
A 73-year-old man with a history of extensive sun exposure presented with a 1-cm, raised, rapidly growing, slightly irregular, purple lesion on the right forearm of 3 months’ duration with tendency to bleed. He did not have a history of skin cancers and was otherwise healthy. Excision was recommended due to the progressive and rapid growth of the lesion.
Histopathologic Findings—Gross examination revealed a 0.9×0.7-cm, raised, slightly irregular lesion located 1 mm away from the closest peripheral margin. Histologically, the lesion was a relatively circumscribed, dermal-based basaloid neoplasm with slightly ill-defined edges involving the superficial and deep dermis (Figure 1A). The neoplasm was formed predominantly of sheets of basaloid cells and small nests of ghost cells, in addition to some squamoid and transitional cells (Figure 1B). The basaloid cells exhibited severe nuclear atypia, pleomorphism, increased nuclear to cytoplasmic ratio (Figure 1C), minimal to moderate amounts of eosinophilic cytoplasm, enlarged nuclei, prominent nucleoli, and coarse chromatin pattern. Abundant mitotic activity and apoptotic bodies were present as well as focal area of central necrosis (Figure 1C). Also, melanophages and a multinucleated giant cell reaction was noted. Elastic trichrome special stain highlighted focal infiltration of the neoplastic cells into the adjacent desmoplastic stroma. Melanin stain was negative for melanin pigment within the neoplasm. Given the presence of severely atypical basaloid cells along with ghost cells indicating matrical differentiation, a diagnosis of pilomatrix carcinoma was rendered.

Immunohistochemistry—The neoplastic cells were diffusely positive for p63, CDX-2 (Figure 2A), β-catenin (Figure 2B), and CD10 (Figure 2C), and focally and weakly positive for cytokeratin (CK) 5, BerEP4 (staining the tumor periphery), androgen receptor, and CK18 (a low-molecular-weight keratin). They were negative for monoclonal carcinoembryonic antigen, epithelial membrane antigen, CK7, CK20, CD34, SOX-10, CD56, synaptophysin, and chromogranin. Cytokeratin 14 was positive in the squamoid cells but negative in the basaloid cells. SOX-10 and melanoma cocktail immunostains demonstrated few intralesional dendritic melanocytes.

Comment
Pilomatrix carcinoma is a rare malignant cutaneous adnexal neoplasm with origin from the germinative matrix of the hair bulb region of hair follicles. Pilomatrix carcinoma was first reported in 1980.1,2 These tumors are characterized by rapid growth and aggressive behavior. Their benign counterpart, pilomatrixoma, is a slow-growing, dermal or subcutaneous tumor that rarely recurs after complete excision.
As with pilomatrixoma, pilomatrix carcinomas are asymptomatic and present as solitary dermal or subcutaneous masses3,4 that most commonly are found in the posterior neck, upper back, and preauricular regions of middle-aged or elderly adults with male predominance.5 They range in size from 0.5 to 20 cm with a mean of 4 cm that is slightly larger than pilomatrixoma. Pilomatrix carcinomas predominantly are firm tumors with or without cystic components, and they exhibit a high probability of recurrence and have risk for distant metastasis.6-15
The differential diagnosis includes epidermal cysts, pilomatrixoma, basal cell carcinoma with matrical differentiation, trichoblastoma/trichoblastic carcinoma, and trichilemmal carcinoma. Pilomatrix carcinomas frequently are mistaken for epidermal cysts on clinical examination. Such a distinction can be easily resolved by histopathologic evaluation. The more challenging differential diagnosis is with pilomatrixoma. Histologically, pilomatrixomas consist of a distinct population of cells including basaloid, squamoid, transitional, and shadow cells in variable proportions. The basaloid cells transition to shadow cells in an organized zonal fashion.16 Compared to pilomatrixomas, pilomatrix carcinomas often show predominance of the basaloid cells; marked cytologic atypia and pleomorphism; numerous mitotic figures; deep infiltrative pattern into subcutaneous fat, fascia, and skeletal muscle; stromal desmoplasia; necrosis; and neurovascular invasion (Tables 1 and 2). Furthermore, the shadow cells tend to form a small nested pattern in pilomatrix carcinoma instead of the flat sheetlike pattern usually observed in pilomatrixoma.16 Basal cell carcinoma with matrical differentiation can pose a diagnostic challenge in the differential diagnosis; basal cell carcinoma usually exhibits a peripheral palisade of the basaloid cells accompanied by retraction spaces separating the tumor from the stroma. Trichoblastoma/trichoblastic carcinoma with matrical differentiation can be distinguished by its exuberant stroma, prominent primitive hair follicles, and papillary mesenchymal bodies. Trichilemmal carcinomas are recognized by their connection to the overlying epidermis, peripheral palisading, and presence of clear cells, while pilomatrix carcinoma lacks connection to the surface epithelium.

Immunohistochemical stains have little to no role in the differential diagnosis, and morphology is the mainstay in making the diagnosis. Rarely, pilomatrix carcinoma can be confused with poorly differentiated sebaceous carcinoma and poorly differentiated squamous cell carcinoma. Although careful scrutiny of the histologic features may help identify mature sebocytes in sebaceous carcinoma, evidence of keratinization in squamous cell carcinoma and ghost cells in pilomatrix carcinoma, using a panel of immunohistochemical stains can be helpful in reaching the final diagnosis (Table 3).

The development of hair matrix tumors have been known to harbor mutations in exon 3 of the catenin beta-1 gene, CTNNB1, that encodes for β-catenin, a downstream effector in the Wnt signaling pathway responsible for differentiation, proliferation, and adhesion of epithelial stem cells.17-21 In a study conducted by Kazakov et al,22 DNA was extracted from 86 lesions: 4 were pilomatrixomas and 1 was a pilomatrix carcinoma. A polymerase chain reaction assay revealed 8 pathogenic variants of the β-catenin gene. D32Y (CTNNB1):c.94G>T (p.Asp32Tyr) and G34R (CTNNB1):c.100G>C (p.Gly34Arg) were the mutations present in pilomatrixoma and pilomatrix carcinoma, respectively.22 In addition, there are several proteins that are part of the Wnt pathway in addition to β-catenin—LEF-1 and CDX-2.

Tumminello and Hosler23 found that pilomatrixomas and pilomatrix carcinomas were positive for CDX-2, β-catenin, and LEF-1 by immunohistochemistry. These downstream molecules in the Wnt signaling pathway could have the potential to be used as diagnostic and prognostic markers.2,13,15,23
Although the pathogenesis is unclear, there are 2 possible mechanisms by which pilomatrix carcinomas develop. They can either arise as de novo tumors, or it is possible that initial mutations in β-catenin result in the formation of pilomatrixomas at an early age that may undergo malignant transformation in elderly patients over time with additional mutations.2
Our case was strongly and diffusely positive for β-catenin in a nuclear and cytoplasmic pattern and CDX-2 in a nuclear pattern, supporting the role of the Wnt signaling pathway in such tumors. Furthermore, our case demonstrated the presence of few intralesional normal dendritic melanocytes, a rare finding1,24,25 but not unexpected, as melanocytes normally are present within the hair follicle matrix.
Pilomatrix carcinomas are aggressive tumors with a high risk for local recurrence and tendency for metastasis. In a study of 13 cases of pilomatrix carcinomas, Herrmann et al13 found that metastasis was significantly associated with local tumor recurrence (P<.0413). They concluded that the combination of overall high local recurrence and metastatic rates of pilomatrix carcinoma as well as documented tumor-related deaths would warrant continued patient follow-up, especially for recurrent tumors.13 Rapid growth of a tumor, either de novo or following several months of stable size, should alert physicians to perform a diagnostic biopsy.
Management options of pilomatrix carcinoma include surgery or radiation with close follow-up. The most widely reported treatment of pilomatrix carcinoma is wide local excision with histologically confirmed clear margins. Mohs micrographic surgery is an excellent treatment option.2,13-15 Adjuvant radiation therapy may be necessary following excision. Currently there is no consensus on surgical management, and standard excisional margins have not been defined.26 Jones et al2 concluded that complete excision with wide margins likely is curative, with decreased rates of recurrence, and better awareness of this carcinoma would lead to appropriate treatment while avoiding unnecessary diagnostic tests.2
Conclusion
We report an exceptionally unique case of early pilomatrix carcinoma with a discussion on the pathogenesis and molecular pathology of hair matrix tumors. A large cohort of patients with longer follow-up periods and better molecular characterization is essential in drawing accurate information about their prognosis, identifying molecular markers that can be used as therapeutic targets, and determining ideal management strategy.
Pilomatrix carcinoma is a rare adnexal tumor with origin from the germinative matrical cells of the hair follicle. Clinically, it presents as a solitary lesion commonly found in the head and neck region as well as the upper back. The tumors cannot be distinguished by their clinical appearance only and frequently are mistaken for cysts. Histopathologic examination provides the definitive diagnosis in most cases. These carcinomas are aggressive neoplasms with a high probability of local recurrence and distant metastasis. Assessment of the Wnt signaling pathway components such as β-catenin, lymphoid enhancer-binding factor 1 (LEF-1), and caudal-related homeobox transcription factor 2 (CDX-2) potentially can be used for diagnostic purposes and targeted therapy.
We report a rare and unique case of early pilomatrix carcinoma with intralesional melanocytes. We review the molecular pathology and pathogenesis of these carcinomas as well as the significance of early diagnosis.
Case Report
A 73-year-old man with a history of extensive sun exposure presented with a 1-cm, raised, rapidly growing, slightly irregular, purple lesion on the right forearm of 3 months’ duration with tendency to bleed. He did not have a history of skin cancers and was otherwise healthy. Excision was recommended due to the progressive and rapid growth of the lesion.
Histopathologic Findings—Gross examination revealed a 0.9×0.7-cm, raised, slightly irregular lesion located 1 mm away from the closest peripheral margin. Histologically, the lesion was a relatively circumscribed, dermal-based basaloid neoplasm with slightly ill-defined edges involving the superficial and deep dermis (Figure 1A). The neoplasm was formed predominantly of sheets of basaloid cells and small nests of ghost cells, in addition to some squamoid and transitional cells (Figure 1B). The basaloid cells exhibited severe nuclear atypia, pleomorphism, increased nuclear to cytoplasmic ratio (Figure 1C), minimal to moderate amounts of eosinophilic cytoplasm, enlarged nuclei, prominent nucleoli, and coarse chromatin pattern. Abundant mitotic activity and apoptotic bodies were present as well as focal area of central necrosis (Figure 1C). Also, melanophages and a multinucleated giant cell reaction was noted. Elastic trichrome special stain highlighted focal infiltration of the neoplastic cells into the adjacent desmoplastic stroma. Melanin stain was negative for melanin pigment within the neoplasm. Given the presence of severely atypical basaloid cells along with ghost cells indicating matrical differentiation, a diagnosis of pilomatrix carcinoma was rendered.

Immunohistochemistry—The neoplastic cells were diffusely positive for p63, CDX-2 (Figure 2A), β-catenin (Figure 2B), and CD10 (Figure 2C), and focally and weakly positive for cytokeratin (CK) 5, BerEP4 (staining the tumor periphery), androgen receptor, and CK18 (a low-molecular-weight keratin). They were negative for monoclonal carcinoembryonic antigen, epithelial membrane antigen, CK7, CK20, CD34, SOX-10, CD56, synaptophysin, and chromogranin. Cytokeratin 14 was positive in the squamoid cells but negative in the basaloid cells. SOX-10 and melanoma cocktail immunostains demonstrated few intralesional dendritic melanocytes.

Comment
Pilomatrix carcinoma is a rare malignant cutaneous adnexal neoplasm with origin from the germinative matrix of the hair bulb region of hair follicles. Pilomatrix carcinoma was first reported in 1980.1,2 These tumors are characterized by rapid growth and aggressive behavior. Their benign counterpart, pilomatrixoma, is a slow-growing, dermal or subcutaneous tumor that rarely recurs after complete excision.
As with pilomatrixoma, pilomatrix carcinomas are asymptomatic and present as solitary dermal or subcutaneous masses3,4 that most commonly are found in the posterior neck, upper back, and preauricular regions of middle-aged or elderly adults with male predominance.5 They range in size from 0.5 to 20 cm with a mean of 4 cm that is slightly larger than pilomatrixoma. Pilomatrix carcinomas predominantly are firm tumors with or without cystic components, and they exhibit a high probability of recurrence and have risk for distant metastasis.6-15
The differential diagnosis includes epidermal cysts, pilomatrixoma, basal cell carcinoma with matrical differentiation, trichoblastoma/trichoblastic carcinoma, and trichilemmal carcinoma. Pilomatrix carcinomas frequently are mistaken for epidermal cysts on clinical examination. Such a distinction can be easily resolved by histopathologic evaluation. The more challenging differential diagnosis is with pilomatrixoma. Histologically, pilomatrixomas consist of a distinct population of cells including basaloid, squamoid, transitional, and shadow cells in variable proportions. The basaloid cells transition to shadow cells in an organized zonal fashion.16 Compared to pilomatrixomas, pilomatrix carcinomas often show predominance of the basaloid cells; marked cytologic atypia and pleomorphism; numerous mitotic figures; deep infiltrative pattern into subcutaneous fat, fascia, and skeletal muscle; stromal desmoplasia; necrosis; and neurovascular invasion (Tables 1 and 2). Furthermore, the shadow cells tend to form a small nested pattern in pilomatrix carcinoma instead of the flat sheetlike pattern usually observed in pilomatrixoma.16 Basal cell carcinoma with matrical differentiation can pose a diagnostic challenge in the differential diagnosis; basal cell carcinoma usually exhibits a peripheral palisade of the basaloid cells accompanied by retraction spaces separating the tumor from the stroma. Trichoblastoma/trichoblastic carcinoma with matrical differentiation can be distinguished by its exuberant stroma, prominent primitive hair follicles, and papillary mesenchymal bodies. Trichilemmal carcinomas are recognized by their connection to the overlying epidermis, peripheral palisading, and presence of clear cells, while pilomatrix carcinoma lacks connection to the surface epithelium.

Immunohistochemical stains have little to no role in the differential diagnosis, and morphology is the mainstay in making the diagnosis. Rarely, pilomatrix carcinoma can be confused with poorly differentiated sebaceous carcinoma and poorly differentiated squamous cell carcinoma. Although careful scrutiny of the histologic features may help identify mature sebocytes in sebaceous carcinoma, evidence of keratinization in squamous cell carcinoma and ghost cells in pilomatrix carcinoma, using a panel of immunohistochemical stains can be helpful in reaching the final diagnosis (Table 3).

The development of hair matrix tumors have been known to harbor mutations in exon 3 of the catenin beta-1 gene, CTNNB1, that encodes for β-catenin, a downstream effector in the Wnt signaling pathway responsible for differentiation, proliferation, and adhesion of epithelial stem cells.17-21 In a study conducted by Kazakov et al,22 DNA was extracted from 86 lesions: 4 were pilomatrixomas and 1 was a pilomatrix carcinoma. A polymerase chain reaction assay revealed 8 pathogenic variants of the β-catenin gene. D32Y (CTNNB1):c.94G>T (p.Asp32Tyr) and G34R (CTNNB1):c.100G>C (p.Gly34Arg) were the mutations present in pilomatrixoma and pilomatrix carcinoma, respectively.22 In addition, there are several proteins that are part of the Wnt pathway in addition to β-catenin—LEF-1 and CDX-2.

Tumminello and Hosler23 found that pilomatrixomas and pilomatrix carcinomas were positive for CDX-2, β-catenin, and LEF-1 by immunohistochemistry. These downstream molecules in the Wnt signaling pathway could have the potential to be used as diagnostic and prognostic markers.2,13,15,23
Although the pathogenesis is unclear, there are 2 possible mechanisms by which pilomatrix carcinomas develop. They can either arise as de novo tumors, or it is possible that initial mutations in β-catenin result in the formation of pilomatrixomas at an early age that may undergo malignant transformation in elderly patients over time with additional mutations.2
Our case was strongly and diffusely positive for β-catenin in a nuclear and cytoplasmic pattern and CDX-2 in a nuclear pattern, supporting the role of the Wnt signaling pathway in such tumors. Furthermore, our case demonstrated the presence of few intralesional normal dendritic melanocytes, a rare finding1,24,25 but not unexpected, as melanocytes normally are present within the hair follicle matrix.
Pilomatrix carcinomas are aggressive tumors with a high risk for local recurrence and tendency for metastasis. In a study of 13 cases of pilomatrix carcinomas, Herrmann et al13 found that metastasis was significantly associated with local tumor recurrence (P<.0413). They concluded that the combination of overall high local recurrence and metastatic rates of pilomatrix carcinoma as well as documented tumor-related deaths would warrant continued patient follow-up, especially for recurrent tumors.13 Rapid growth of a tumor, either de novo or following several months of stable size, should alert physicians to perform a diagnostic biopsy.
Management options of pilomatrix carcinoma include surgery or radiation with close follow-up. The most widely reported treatment of pilomatrix carcinoma is wide local excision with histologically confirmed clear margins. Mohs micrographic surgery is an excellent treatment option.2,13-15 Adjuvant radiation therapy may be necessary following excision. Currently there is no consensus on surgical management, and standard excisional margins have not been defined.26 Jones et al2 concluded that complete excision with wide margins likely is curative, with decreased rates of recurrence, and better awareness of this carcinoma would lead to appropriate treatment while avoiding unnecessary diagnostic tests.2
Conclusion
We report an exceptionally unique case of early pilomatrix carcinoma with a discussion on the pathogenesis and molecular pathology of hair matrix tumors. A large cohort of patients with longer follow-up periods and better molecular characterization is essential in drawing accurate information about their prognosis, identifying molecular markers that can be used as therapeutic targets, and determining ideal management strategy.
- Jani P, Chetty R, Ghazarian DM. An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature. Am J Dermatopathol. 2008;30:174-177.
- Jones C, Twoon M, Ho W, et al. Pilomatrix carcinoma: 12-year experience and review of the literature. J Cutan Pathol. 2018;45:33-38.
- Forbis R, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961;83:606.
- Elder D, Elenitsas R, Ragsdale BD. Tumors of epidermal appendages. In: Elder D, Elenitsas R, Jaworsky C, eds. Lever’s Histopathology of the Skin. 8th ed. Lippincott Raven; 1997:757-759.
- Aherne NJ, Fitzpatrick DA, Gibbons D, et al. Pilomatrix carcinoma presenting as an extra axial mass: clinicopathological features. Diagn Pathol. 2008;3:47.
- Papadakis M, de Bree E, Floros N, et al. Pilomatrix carcinoma: more malignant biological behavior than was considered in the past. Mol Clin Oncol. 2017;6:415-418.
- LeBoit PE, Parslow TG, Choy SH. Hair matrix differentiation: occurrence in lesions other than pilomatricoma. Am J Dermatopathol. 1987;9:399-405.
- Campoy F, Stiefel P, Stiefel E, et al. Pilomatrix carcinoma: role played by MR imaging. Neuroradiology. 1989;31:196-198.
- Tateyama H, Eimoto T, Tada T, et al. Malignant pilomatricoma: an immunohistochemical study with antihair keratin antibody. Cancer. 1992;69:127-132.
- O’Donovan DG, Freemont AJ, Adams JE, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1993;23:385-386.
- Cross P, Richmond I, Wells S, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1994;24:499-500.
- Niedermeyer HP, Peris K, Höfler H. Pilomatrix carcinoma with multiple visceral metastases: report of a case. Cancer. 1996;77:1311-1314.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Xing L, Marzolf SA, Vandergriff T, et al. Facial pilomatrix carcinomas treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:253-255.
- Fernandez-Flores A, Cassarino DS. Sarcomatoid pilomatrix carcinoma. J Cutan Pathol. 2018;45:508-514.
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993;71:2491-2498.
- Chan E, Gat U, McNiff JM, et al. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet. 1999;21:410-413.
- Huelsken J, Vogel R, Erdmann B, et al. β-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533-545.
- Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 2003;94:225-229.
- Durand M, Moles J. Beta-catenin mutations in a common skin cancer: pilomatricoma. Bull Cancer. 1999;86:725-726.
- Lazar AJF, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148-157.
- Kazakov DV, Sima R, Vanecek T, et al. Mutations in exon 3 of the CTNNB1 gene (β-catenin gene) in cutaneous adnexal tumors. Am J Dermatopathol. 2009;31:248-255.
- Tumminello K, Hosler GA. CDX2 and LEF-1 expression in pilomatrical tumors and their utility in the diagnosis of pilomatrical carcinoma. J Cutan Pathol. 2018;45:318-324.
- Rodic´ N, Taube JM, Manson P, et al Locally invasive dermal squamomelanocytic tumor with matrical differentiation: a peculiar case with review of the literature. Am J Dermatopathol. 2013;35:E72-E76.
- Perez C, Debbaneh M, Cassarino D. Preference for the term pilomatrical carcinoma with melanocytic hyperplasia: letter to the editor. J Cutan Pathol. 2017;44:655-657.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Jani P, Chetty R, Ghazarian DM. An unusual composite pilomatrix carcinoma with intralesional melanocytes: differential diagnosis, immunohistochemical evaluation, and review of the literature. Am J Dermatopathol. 2008;30:174-177.
- Jones C, Twoon M, Ho W, et al. Pilomatrix carcinoma: 12-year experience and review of the literature. J Cutan Pathol. 2018;45:33-38.
- Forbis R, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961;83:606.
- Elder D, Elenitsas R, Ragsdale BD. Tumors of epidermal appendages. In: Elder D, Elenitsas R, Jaworsky C, eds. Lever’s Histopathology of the Skin. 8th ed. Lippincott Raven; 1997:757-759.
- Aherne NJ, Fitzpatrick DA, Gibbons D, et al. Pilomatrix carcinoma presenting as an extra axial mass: clinicopathological features. Diagn Pathol. 2008;3:47.
- Papadakis M, de Bree E, Floros N, et al. Pilomatrix carcinoma: more malignant biological behavior than was considered in the past. Mol Clin Oncol. 2017;6:415-418.
- LeBoit PE, Parslow TG, Choy SH. Hair matrix differentiation: occurrence in lesions other than pilomatricoma. Am J Dermatopathol. 1987;9:399-405.
- Campoy F, Stiefel P, Stiefel E, et al. Pilomatrix carcinoma: role played by MR imaging. Neuroradiology. 1989;31:196-198.
- Tateyama H, Eimoto T, Tada T, et al. Malignant pilomatricoma: an immunohistochemical study with antihair keratin antibody. Cancer. 1992;69:127-132.
- O’Donovan DG, Freemont AJ, Adams JE, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1993;23:385-386.
- Cross P, Richmond I, Wells S, et al. Malignant pilomatrixoma with bone metastasis. Histopathology. 1994;24:499-500.
- Niedermeyer HP, Peris K, Höfler H. Pilomatrix carcinoma with multiple visceral metastases: report of a case. Cancer. 1996;77:1311-1314.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
- Xing L, Marzolf SA, Vandergriff T, et al. Facial pilomatrix carcinomas treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:253-255.
- Fernandez-Flores A, Cassarino DS. Sarcomatoid pilomatrix carcinoma. J Cutan Pathol. 2018;45:508-514.
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993;71:2491-2498.
- Chan E, Gat U, McNiff JM, et al. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet. 1999;21:410-413.
- Huelsken J, Vogel R, Erdmann B, et al. β-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533-545.
- Kikuchi A. Tumor formation by genetic mutations in the components of the Wnt signaling pathway. Cancer Sci. 2003;94:225-229.
- Durand M, Moles J. Beta-catenin mutations in a common skin cancer: pilomatricoma. Bull Cancer. 1999;86:725-726.
- Lazar AJF, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148-157.
- Kazakov DV, Sima R, Vanecek T, et al. Mutations in exon 3 of the CTNNB1 gene (β-catenin gene) in cutaneous adnexal tumors. Am J Dermatopathol. 2009;31:248-255.
- Tumminello K, Hosler GA. CDX2 and LEF-1 expression in pilomatrical tumors and their utility in the diagnosis of pilomatrical carcinoma. J Cutan Pathol. 2018;45:318-324.
- Rodic´ N, Taube JM, Manson P, et al Locally invasive dermal squamomelanocytic tumor with matrical differentiation: a peculiar case with review of the literature. Am J Dermatopathol. 2013;35:E72-E76.
- Perez C, Debbaneh M, Cassarino D. Preference for the term pilomatrical carcinoma with melanocytic hyperplasia: letter to the editor. J Cutan Pathol. 2017;44:655-657.
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.
Practice Points
- Clinicians and pathologists should be aware of pilomatrix carcinoma to facilitate early detection.
- Early diagnosis and prompt treatment of pilomatrix carcinoma is crucial in lowering recurrence rate and avoiding a poor outcome.
- Caudal-related homeobox transcription factor 2 and β-catenin components of the Wnt signaling pathway play an important role in the pathogenesis of pilomatrix carcinoma.
- Although controversial, wide local excision is the treatment of choice for pilomatrix carcinoma.