User login
Microneedling With Bimatoprost to Treat Hypopigmented Skin Caused by Burn Scars
To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
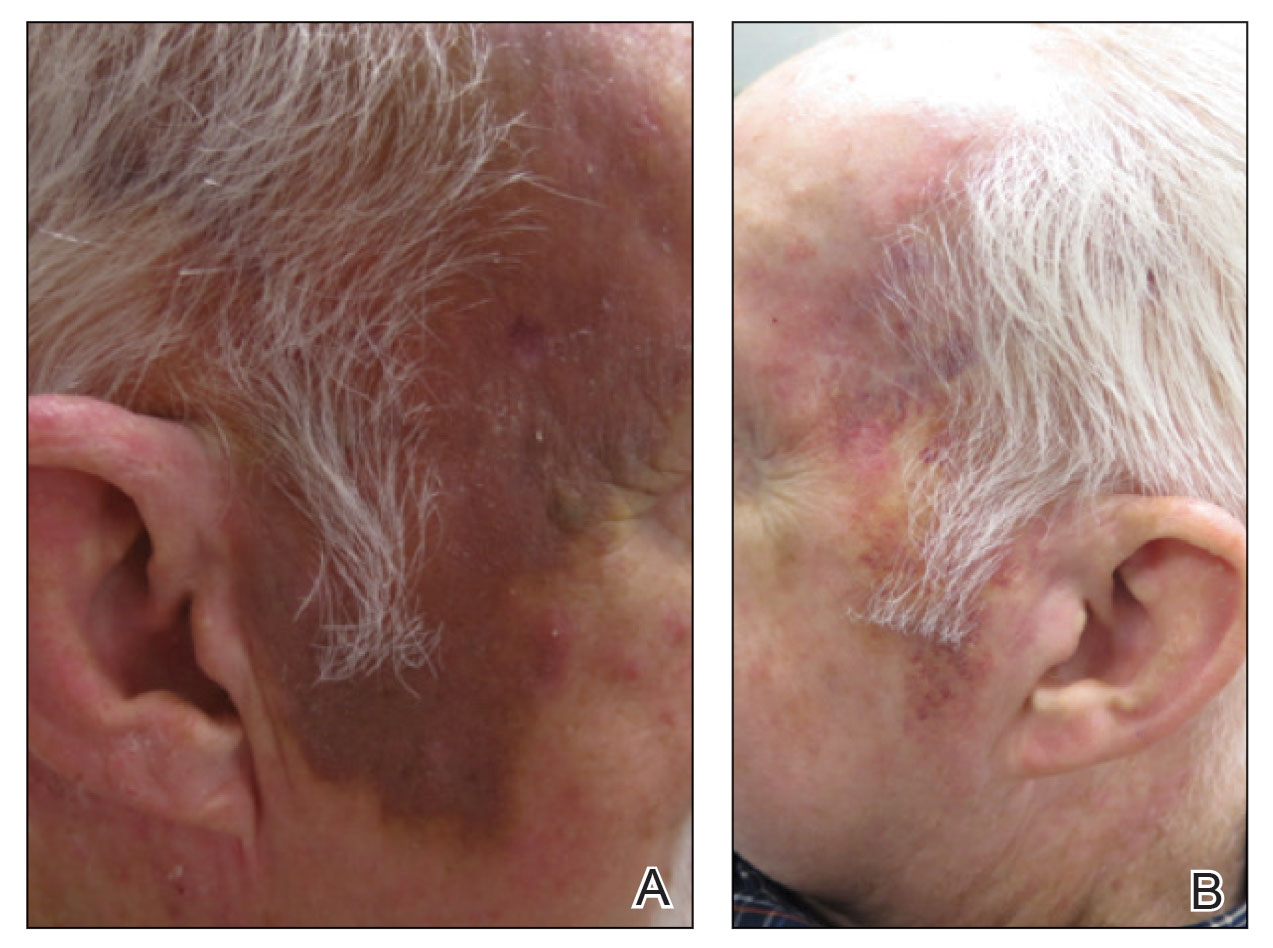
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
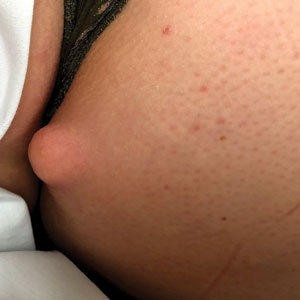
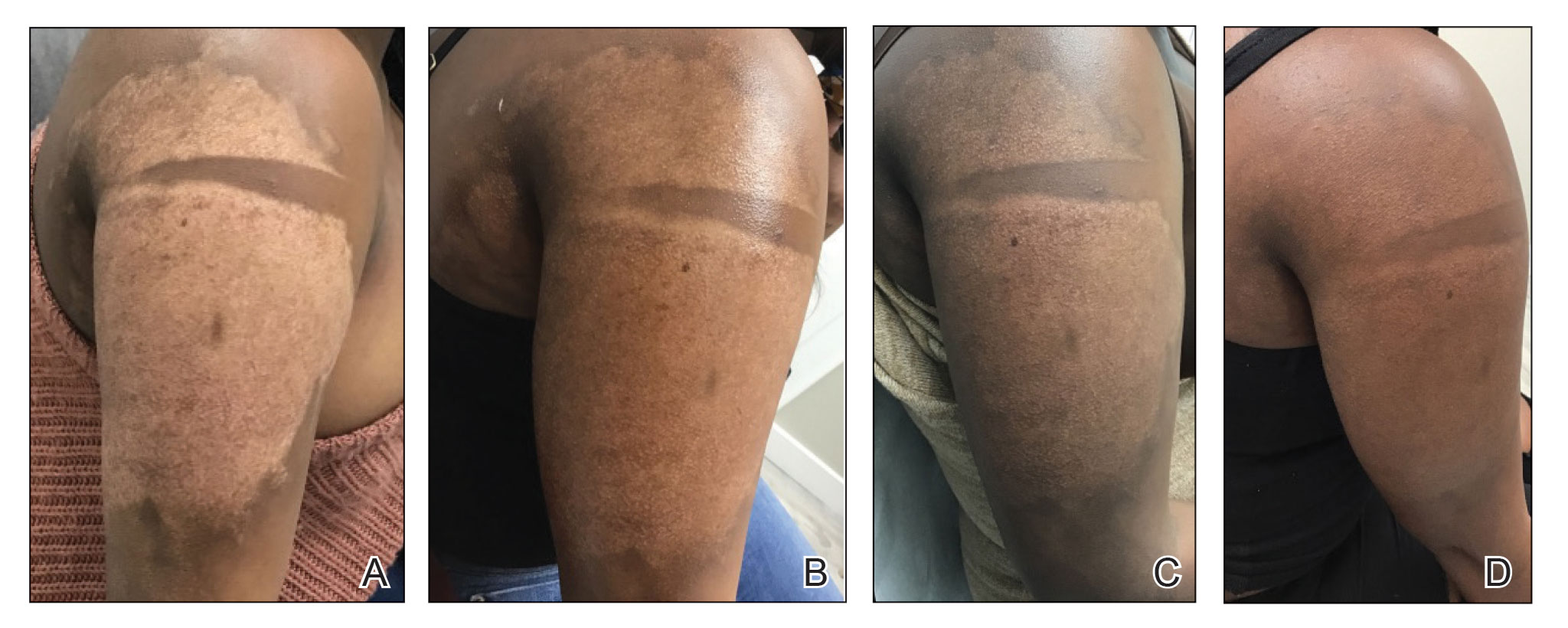
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.
Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
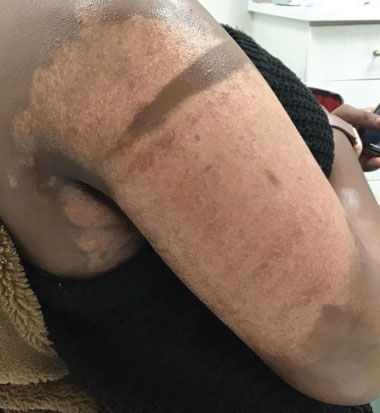
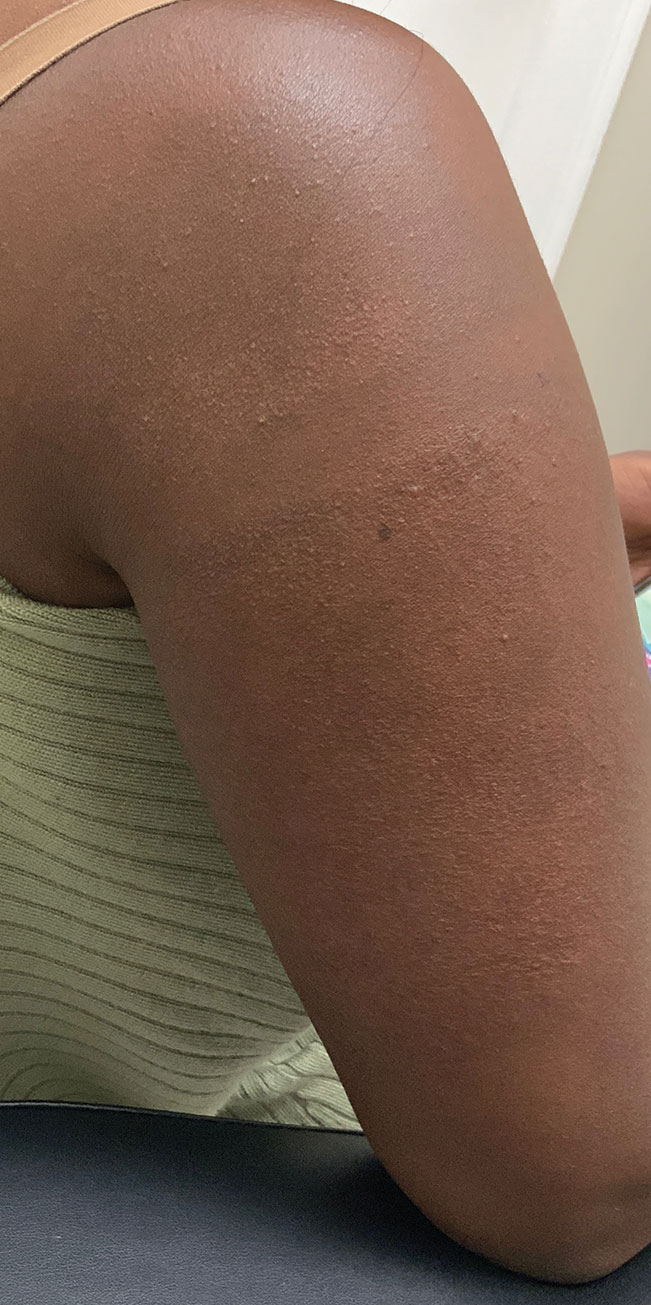
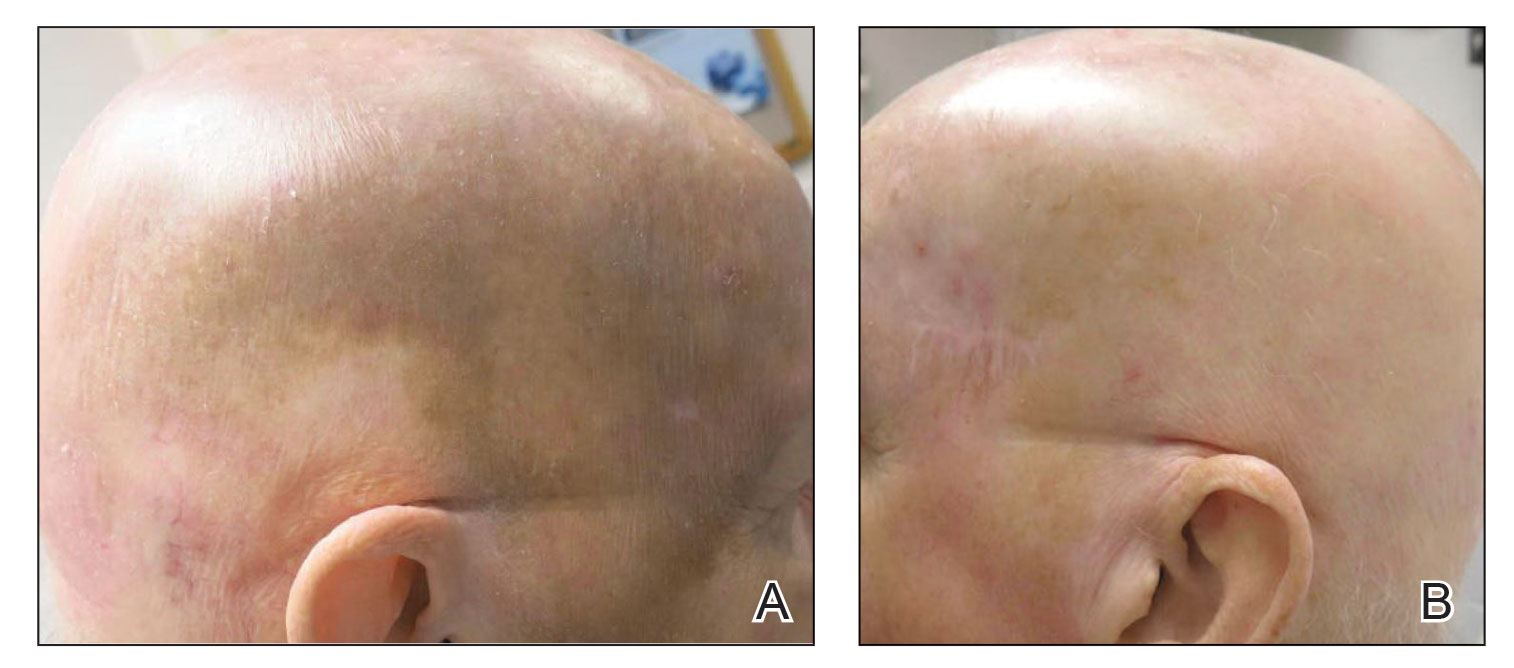
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).
We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.
- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.

Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).

We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.

To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.

Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).

We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.

- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
PRACTICE POINTS
- Microneedling is a percutaneous collagen induction therapy that also may be used in drug delivery.
- Hypopigmentation can cause considerable distress for patients with skin of color.
- Percutaneous drug delivery of bimatoprost may be helpful in skin repigmentation.
Methacrylate Polymer Powder Dressing for a Lower Leg Surgical Defect
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
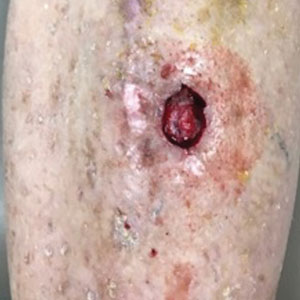
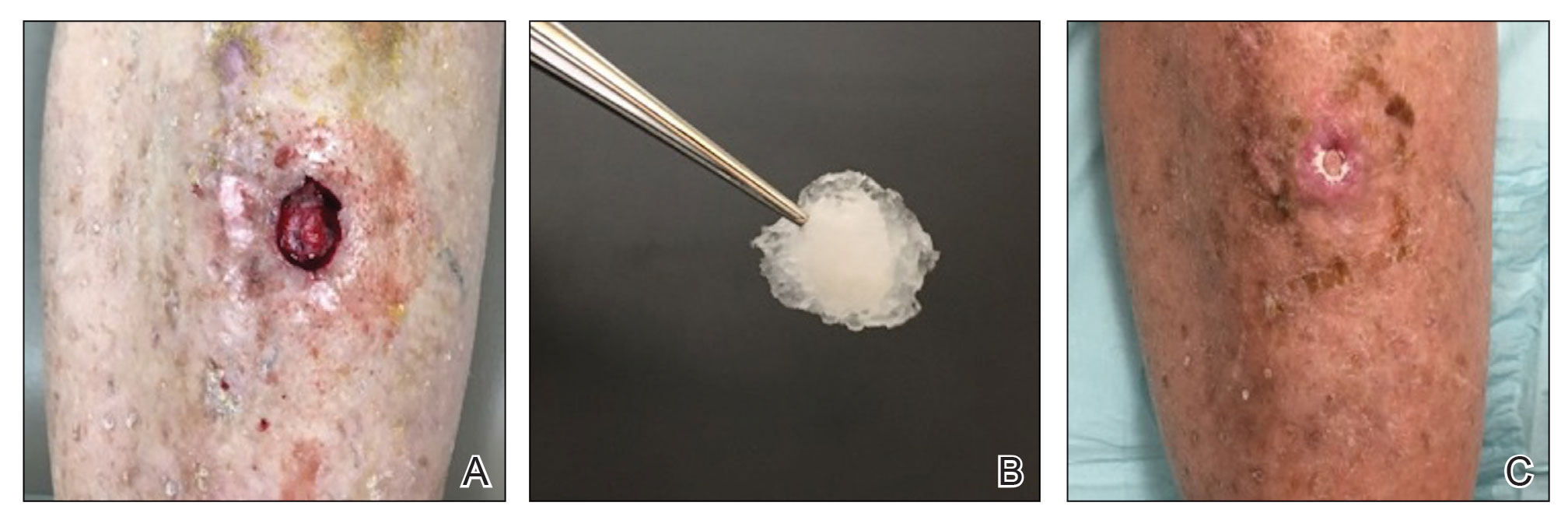
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.
Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1
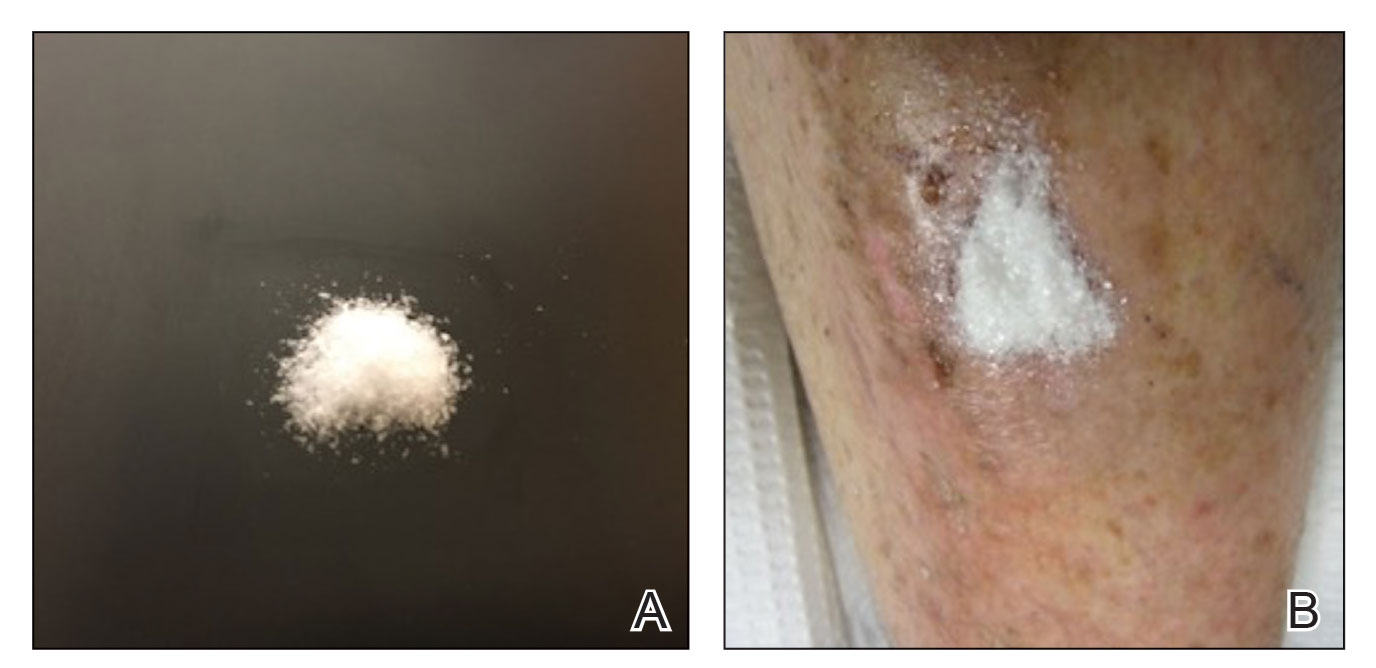
Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1
This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.

Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1

Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1

This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
To the Editor:
Surgical wounds on the lower leg are challenging to manage because venous stasis, bacterial colonization, and high tension may contribute to protracted healing. Advances in technology led to the development of novel, polymer-based wound-healing modalities that hold promise for the management of these wounds.
A 75-year-old man presented with a well-differentiated squamous cell carcinoma with a 3-mm depth of invasion on the left pretibial region. His comorbidities were notable for hypertension, hypercholesterolemia, varicose veins, myocardial infarction, peripheral vascular disease, and a 32 pack-year cigarette smoking history. Current medications included clopidogrel bisulfate and warfarin sodium to manage a recently placed coronary artery stent.
The tumor was cleared after 2 stages of Mohs micrographic surgery with excision down to tibialis anterior fascia (Figure 1A). The resultant defect measured 43×33 mm in area and 9 mm in depth (wound size, 12,771 mm3). Reconstructive options were discussed, including random-pattern flap repair and skin graft. Given the patient’s risk of bleeding, the decision was made to forego a flap repair. Additionally, the patient was a heavy smoker and could not comply with the wound care and elevation and ambulation restrictions required for optimal skin graft care. Therefore, a decision was made to proceed with secondary intention healing using a methacrylate polymer powder dressing.
After achieving hemostasis, a novel 10-mg sterile, biologically inert methacrylate polymer powder dressing was poured over the wound in a uniform layer to fill and seal the entire wound surface (Figure 1B). Sterile normal saline 0.1 mL was sprayed onto the powder to activate particle aggregation. No secondary dressing was used, and the patient was permitted to get the dressing wet after 48 hours.
The dressing was changed in a similar fashion 4 weeks after application, following gentle debridement with gauze and normal saline. Eight weeks after surgery, the wound exhibited healthy granulation tissue and measured 5×6 mm in area and 2 mm in depth (wound size, 60 mm3), which represented a 99.5% reduction in wound size (Figure 1C). The dressing was not painful, and there were no reported adverse effects. The patient continued to smoke and ambulate fully throughout this period. No antibiotics were used.

Methacrylate polymer powder dressings are a novel and sophisticated dressing modality with great promise for the management of surgical wounds on the lower limb. The dressing is a sterile powder consisting of 84.8% poly-2-hydroxyethylmethacrylate, 14.9% poly-2-hydroxypropylmethacrylate, and 0.3% sodium deoxycholate. These hydrophilic polymers have a covalent methacrylate backbone with a hydroxyl aliphatic side chain. When saline or wound exudate contacts the powder, the spheres hydrate and nonreversibly aggregate to form a moist, flexible dressing that conforms to the topography of the wound and seals it (Figure 2).1

Once the spheres have aggregated, they are designed to orient in a honeycomb formation with 4- to 10-nm openings that serve as capillary channels (Figure 3). This porous architecture of the polymer is essential for adequate moisture management. It allows for vapor transpiration at a rate of 12 L/m2 per day, which ensures the capillary flow from the moist wound surface is evenly distributed through the dressing, contributing to its 68% water content. Notably, this approximately three-fifths water composition is similar to the water makeup of human skin. Optimized moisture management is theorized to enhance epithelial migration, stimulate angiogenesis, retain growth factors, promote autolytic debridement, and maintain ideal voltage and oxygen gradients for wound healing. The risk for infection is not increased by the existence of these pores, as their small size does not allow for bacterial migration.1

This case demonstrates the effectiveness of using a methacrylate polymer powder dressing to promote timely wound healing in a poorly vascularized lower leg surgical wound. The low maintenance, user-friendly dressing was changed at monthly intervals, which spared the patient the inconvenience and pain associated with the repeated application of more conventional primary and secondary dressings. The dressing was well tolerated and resulted in a 99.5% reduction in wound size. Further studies are needed to investigate the utility of this promising technology.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
1. Fitzgerald RH, Bharara M, Mills JL, et al. Use of a nanoflex powder dressing for wound management following debridement for necrotising fasciitis in the diabetic foot. Int Wound J. 2009;6:133-139.
PRACTICE POINTS
- Lower leg surgical wounds are difficult to manage, as venous stasis, bacterial colonization, and high tension may contribute to protracted healing.
- A methacrylate polymer powder dressing is user friendly and facilitates granulation and reduction in size of difficult lower leg wounds.
Oral Propranolol Used as Adjunct Therapy in Cutaneous Angiosarcoma
To the Editor:
Angiosarcoma is a malignancy of the vascular endothelium that most commonly presents on the skin.1 Patients diagnosed with cutaneous angiosarcoma, which is a rare and aggressive malignancy, have a 5-year survival rate of approximately 30%.2,3 Angiosarcoma can be seen in the setting of chronic lymphedema; radiation therapy; and sporadically in elderly patients, where it is commonly seen on the head and neck. Presentation on the head and neck has been associated with worse outcomes, with a projected overall 10-year survival rate of 13.8%; the survival rate is lower if the tumor is surgically unresectable or larger in size. Metastasis can occur via both lymphatic and hematogenous routes, with pulmonary and hepatic metastases most frequently observed.1 Prognostications of poor outcomes for patients with head and neck cutaneous angiosarcoma via a 5-year survival rate were identified in a meta-analysis and included the following: patient age older than 70 years, larger tumors, tumor location of scalp vs face, nonsurgical treatments, and lack of clear margins on histology.2
Treatment of angiosarcoma historically has encompassed both surgical resection and adjuvant radiation therapy with suboptimal success. Evidence supporting various treatment regimens remains sparse due to the low incidence of the neoplasm. Although surgical resection is the only documented curative treatment, cutaneous angiosarcomas frequently are found to have positive surgical margins and require adjuvant radiation. Use of high-dose radiation (>50 Gy) with application over a wide treatment area such as total scalp irradiation is recommended.4 Although radiation has been found to diminish local recurrence rates, it has not substantially affected rates of distant disease recurrence.1 Cytotoxic chemotherapy has clinical utility in minimizing progression, but standard regimens afford a progression-free survival of only months.3 Adjuvant treatment with paclitaxel has been shown to have improved efficacy in scalp angiosarcoma vs other visceral sites, showing a nonprogression rate of 42% at 4 months after treatment.5 More recently, targeted chemotherapeutics, including the vascular endothelial growth factor inhibitor bevacizumab and tyrosine kinase inhibitor sorafenib, have shown some survival benefit, but it is unclear if these agents are superior to traditional cytotoxic agents.4,6-10 A phase 2 study of paclitaxel administered weekly with or without bevacizumab showed similar progression-free survival and overall survival, albeit at the expense of added toxicity experienced by participants in the combined group.10
The addition of the nonselective β-adrenergic blocker propranolol to the treatment armamentarium, which was pursued due to its utility in the treatment of benign infantile hemangioma and demonstrated ability to limit the expression of adrenergic receptors in angiosarcoma, has gained clinical attention for possible augmentation of cutaneous angiosarcoma therapy.11-14 Propranolol has been shown to reduce metastasis in other neoplasms—both vascular and nonvascular—and may play a role as an adjuvant treatment to current therapies in angiosarcoma.15-20 We report a patient with cutaneous angiosarcoma (T2 classification) with disease-free survival of nearly 6 years without evidence of recurrence in the setting of continuous propranolol use supplementary to chemotherapy and radiation.
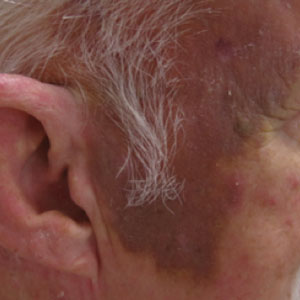
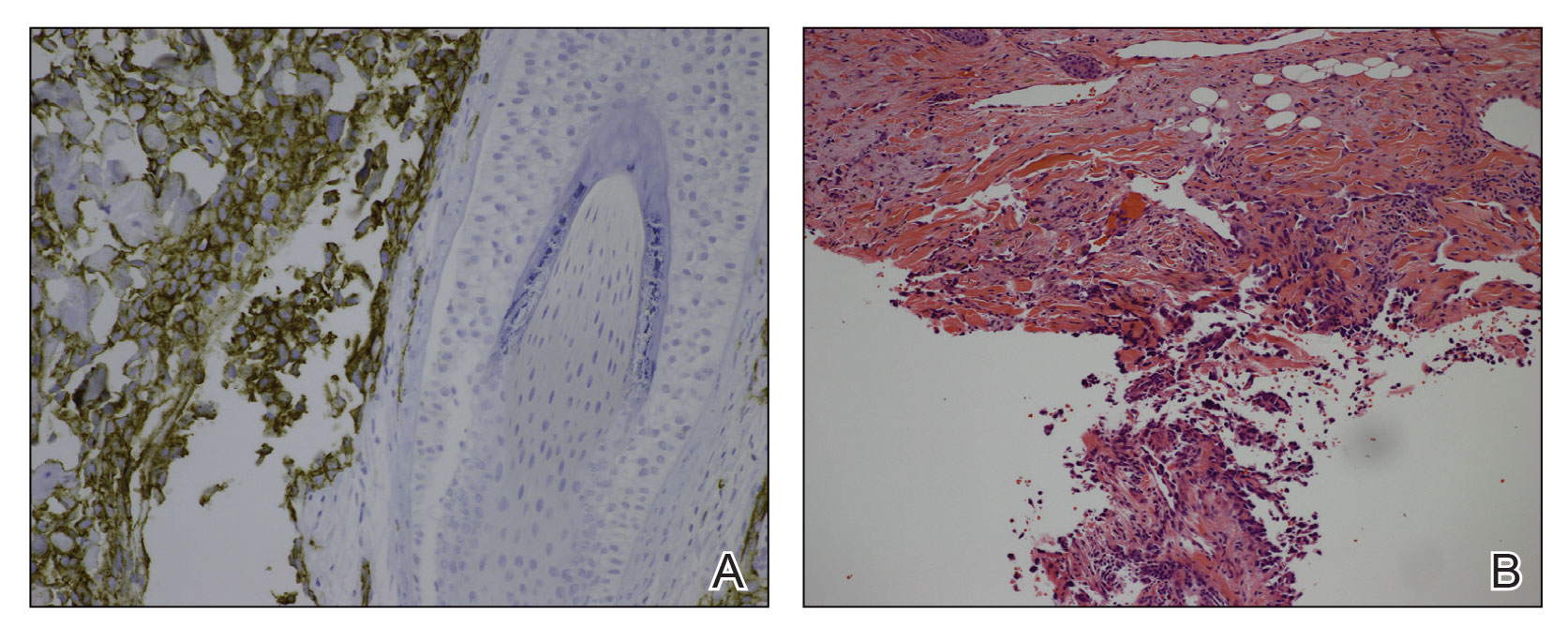
A 78-year-old man with a history of multiple basal cell carcinomas, hypertension, and remote smoking history presented to the dermatology clinic with an enlarging red-brown plaque on the scalp of 2 months’ duration. The lesion had grown rapidly to involve the forehead, right temple, preauricular region, and parietal scalp. At presentation, the tumor measured more than 20 cm in diameter at its greatest point (Figure 1). Physical examination revealed a 6-mm purple nodule within the lesion on the patient’s right parietal scalp. No clinical lymphadenopathy was appreciated at the time of diagnosis. Punch biopsies of the right parietal scalp nodule and right temple patch showed findings consistent with angiosarcoma with diffuse cytoplasmic staining of CD31 in atypical endothelial cells and no staining for human herpesvirus 8 (Figure 2). Concurrent computed tomography of the head showed thickening of the right epidermis, dermis, and deeper scalp tissues, but there was no evidence of skull involvement. Computed tomography of the thorax, abdomen, and pelvis showed no evidence of metastatic disease. After a diagnostic workup, the patient was diagnosed with T2bN0M0 angiosarcoma.
The lesion was determined to be nonresectable due to the extent of the patient’s cutaneous disease. The patient was started on a regimen of paclitaxel, scalp radiation, and oral propranolol. Propranolol 40 mg twice daily was initiated at the time of diagnosis with a plan to continue indefinitely. Starting 1 month after staging, the patient completed 10 weekly cycles of paclitaxel, and he was treated with 60 Gy of scalp radiation in 30 fractions, starting with the second cycle of paclitaxel. He tolerated both well with no reported adverse events. Repeat computed tomography performed 1 month after completion of chemotherapy and radiation showed no evidence of a mass or fluid collection in subcutaneous scalp tissues and no evidence of metastatic disease. This correlated with an observed clinical regression at 1 month and complete clinical response at 5 months with residual hemosiderin and radiation changes. The area of prior disease involvement subsequently evolved from violet to dusky gray in appearance to an eventual complete resolution 26 months after diagnosis, accompanied by atrophic radiation-induced sequelae (Figure 3).
The patient’s postchemotherapy course was complicated by hospitalization for a suspected malignant pleural effusion. Analysis revealed growing ground-glass opacities and nodules in the right lower lung lobe. A thoracentesis with cytology studies was negative for malignancy. Continued monitoring over 19 months demonstrated eventual resolution of those findings. He experienced notable complication from local radiation therapy to the scalp with chronic cutaneous ulceration refractory to wound care and surgical intervention. The patient did not exhibit additional signs or symptoms concerning for recurrence or metastasis and was followed by dermatology and oncology until he died nearly 5 years after initial diagnosis due to complications from acute hypoxic respiratory failure secondary to COVID-19. The last imaging obtained showed no convincing evidence of metastasis, though spinal imaging within a month of his death showed lesions favored to represent benign angiomatous growths. His survival after diagnosis ultimately reached 57 months without confirmed disease recurrence and cause of death unrelated to malignancy history, which is a markedly long documented survival for this extent of disease.
Cutaneous angiosarcoma is an aggressive yet rare malignancy without effective treatments for prolonging survival or eradicating disease. Cutaneous angiosarcoma of the head and neck has a reported 10-year survival rate of 13.8%.1 Although angiosarcoma in any location holds a bleak prognosis, cutaneous angiosarcoma of the scalp with a T2 classification has a 2-year survival rate of 0%. Moreover, even if remission is achieved, disease is highly recurrent, typically within months with the current standard of care.3,21,22
Emerging evidence for the possible role of β-adrenergic receptor blockade in the treatment of malignant vascular neoplasms is promising. Microarrays from a host of vascular growths have demonstrated expression of β-adrenergic receptors in 77% of sampled angiosarcoma specimens in addition to strong expression in infantile hemangiomas, hemangiomas, hemangioendotheliomas, and vascular malformations.19 Research findings have further verified the validity of this approach with the demonstration of b1-, b2-, and b3- adrenergic receptor expression by angiosarcoma cell lines. Propranolol subsequently was shown to effectively target proliferation of these cells and induce apoptosis in a dose-dependent manner and moreover be synergistic in effect with other chemotherapies.15 Several genes have exhibited differential expression between control tumor cells and propranolol-treated cells. Specifically, target genes including AXL (a receptor tyrosine kinase associated with cell adhesion, proliferation, and apoptosis and found to upregulated in melanoma and leukemia) and ERBB receptor feedback inhibitor 1 (receptor tyrosine kinase, with ERBB family members commonly overexpressed or mutated in the setting malignancy) have been posited as possible explanatory factors in the observed angiosarcoma response to propranolol.23
Several cases describing propranolol use as an adjunctive therapy for angiosarcoma suggest a beneficial role in clinical medicine. One case report described propranolol monotherapy for lesion to our patient, with a resultant reduction in Ki-67 as a measure of proliferative index within 1 week of initiating propranolol therapy.13 Propranolol also has been shown to halt or slow progression of metastatic disease in visceral and metastatic angiosarcomas.12-14 In combination with oral etoposide and cyclophosphamide, maintenance propranolol therapy in 7 cases of advanced cutaneous angiosarcoma resulted in 1 complete response and 3 very good partial responses, with a median progression-free survival of 11 months.11 Larger-scale studies have not been published, but the growing number of case reports and case series warrants further investigation of the utility of propranolol as an adjunct to current therapies in advanced angiosarcoma.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fury MG, Antonescu CR, Zee KJV, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer. 2005;11:241-247.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol. 2008;26:5269-5274.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Ishida Y, Otsuka A, Kabashima K. Cutaneous angiosarcoma: update on biology and latest treatment. Curr Opin Oncol. 2018;30:107-112.
- Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II trial from the French Sarcoma Group (GSF/GETO). Oncologist. 2012;17:260-266.
- Ray-Coquard IL, Domont J, Tresch-Bruneel E, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial. J Clin Oncol. 2015;33:2797-2802.
- Pasquier E, Andre N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Banavali S, Pasquier E, Andre N. Targeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience. 2015;9:499.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated beta-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Daguze J, Saint-Jean M, Peuvrel L, et al. Visceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. JAAD Case Rep. 2016;2:497-499.
- Stiles JM, Amaya C, Rains S, et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013;8:e60021.
- Chang PY, Chung CH, Chang WC, et al. The effect of propranolol on the prognosis of hepatocellular carcinoma: a nationwide population-based study. PLoS One. 2019;14:e0216828.
- De Giorgi V, Grazzini M, Benemei S, et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4:e172908.
- Rico M, Baglioni M, Bondarenko M, et al. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget. 2017;8:2874-2889.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Zhou S, Liu P, Jiang W, et al. Identification of potential target genes associated with the effect of propranolol on angiosarcoma via microarray analysis. Oncol Lett. 2017;13:4267-4275.
To the Editor:
Angiosarcoma is a malignancy of the vascular endothelium that most commonly presents on the skin.1 Patients diagnosed with cutaneous angiosarcoma, which is a rare and aggressive malignancy, have a 5-year survival rate of approximately 30%.2,3 Angiosarcoma can be seen in the setting of chronic lymphedema; radiation therapy; and sporadically in elderly patients, where it is commonly seen on the head and neck. Presentation on the head and neck has been associated with worse outcomes, with a projected overall 10-year survival rate of 13.8%; the survival rate is lower if the tumor is surgically unresectable or larger in size. Metastasis can occur via both lymphatic and hematogenous routes, with pulmonary and hepatic metastases most frequently observed.1 Prognostications of poor outcomes for patients with head and neck cutaneous angiosarcoma via a 5-year survival rate were identified in a meta-analysis and included the following: patient age older than 70 years, larger tumors, tumor location of scalp vs face, nonsurgical treatments, and lack of clear margins on histology.2
Treatment of angiosarcoma historically has encompassed both surgical resection and adjuvant radiation therapy with suboptimal success. Evidence supporting various treatment regimens remains sparse due to the low incidence of the neoplasm. Although surgical resection is the only documented curative treatment, cutaneous angiosarcomas frequently are found to have positive surgical margins and require adjuvant radiation. Use of high-dose radiation (>50 Gy) with application over a wide treatment area such as total scalp irradiation is recommended.4 Although radiation has been found to diminish local recurrence rates, it has not substantially affected rates of distant disease recurrence.1 Cytotoxic chemotherapy has clinical utility in minimizing progression, but standard regimens afford a progression-free survival of only months.3 Adjuvant treatment with paclitaxel has been shown to have improved efficacy in scalp angiosarcoma vs other visceral sites, showing a nonprogression rate of 42% at 4 months after treatment.5 More recently, targeted chemotherapeutics, including the vascular endothelial growth factor inhibitor bevacizumab and tyrosine kinase inhibitor sorafenib, have shown some survival benefit, but it is unclear if these agents are superior to traditional cytotoxic agents.4,6-10 A phase 2 study of paclitaxel administered weekly with or without bevacizumab showed similar progression-free survival and overall survival, albeit at the expense of added toxicity experienced by participants in the combined group.10
The addition of the nonselective β-adrenergic blocker propranolol to the treatment armamentarium, which was pursued due to its utility in the treatment of benign infantile hemangioma and demonstrated ability to limit the expression of adrenergic receptors in angiosarcoma, has gained clinical attention for possible augmentation of cutaneous angiosarcoma therapy.11-14 Propranolol has been shown to reduce metastasis in other neoplasms—both vascular and nonvascular—and may play a role as an adjuvant treatment to current therapies in angiosarcoma.15-20 We report a patient with cutaneous angiosarcoma (T2 classification) with disease-free survival of nearly 6 years without evidence of recurrence in the setting of continuous propranolol use supplementary to chemotherapy and radiation.

A 78-year-old man with a history of multiple basal cell carcinomas, hypertension, and remote smoking history presented to the dermatology clinic with an enlarging red-brown plaque on the scalp of 2 months’ duration. The lesion had grown rapidly to involve the forehead, right temple, preauricular region, and parietal scalp. At presentation, the tumor measured more than 20 cm in diameter at its greatest point (Figure 1). Physical examination revealed a 6-mm purple nodule within the lesion on the patient’s right parietal scalp. No clinical lymphadenopathy was appreciated at the time of diagnosis. Punch biopsies of the right parietal scalp nodule and right temple patch showed findings consistent with angiosarcoma with diffuse cytoplasmic staining of CD31 in atypical endothelial cells and no staining for human herpesvirus 8 (Figure 2). Concurrent computed tomography of the head showed thickening of the right epidermis, dermis, and deeper scalp tissues, but there was no evidence of skull involvement. Computed tomography of the thorax, abdomen, and pelvis showed no evidence of metastatic disease. After a diagnostic workup, the patient was diagnosed with T2bN0M0 angiosarcoma.

The lesion was determined to be nonresectable due to the extent of the patient’s cutaneous disease. The patient was started on a regimen of paclitaxel, scalp radiation, and oral propranolol. Propranolol 40 mg twice daily was initiated at the time of diagnosis with a plan to continue indefinitely. Starting 1 month after staging, the patient completed 10 weekly cycles of paclitaxel, and he was treated with 60 Gy of scalp radiation in 30 fractions, starting with the second cycle of paclitaxel. He tolerated both well with no reported adverse events. Repeat computed tomography performed 1 month after completion of chemotherapy and radiation showed no evidence of a mass or fluid collection in subcutaneous scalp tissues and no evidence of metastatic disease. This correlated with an observed clinical regression at 1 month and complete clinical response at 5 months with residual hemosiderin and radiation changes. The area of prior disease involvement subsequently evolved from violet to dusky gray in appearance to an eventual complete resolution 26 months after diagnosis, accompanied by atrophic radiation-induced sequelae (Figure 3).

The patient’s postchemotherapy course was complicated by hospitalization for a suspected malignant pleural effusion. Analysis revealed growing ground-glass opacities and nodules in the right lower lung lobe. A thoracentesis with cytology studies was negative for malignancy. Continued monitoring over 19 months demonstrated eventual resolution of those findings. He experienced notable complication from local radiation therapy to the scalp with chronic cutaneous ulceration refractory to wound care and surgical intervention. The patient did not exhibit additional signs or symptoms concerning for recurrence or metastasis and was followed by dermatology and oncology until he died nearly 5 years after initial diagnosis due to complications from acute hypoxic respiratory failure secondary to COVID-19. The last imaging obtained showed no convincing evidence of metastasis, though spinal imaging within a month of his death showed lesions favored to represent benign angiomatous growths. His survival after diagnosis ultimately reached 57 months without confirmed disease recurrence and cause of death unrelated to malignancy history, which is a markedly long documented survival for this extent of disease.
Cutaneous angiosarcoma is an aggressive yet rare malignancy without effective treatments for prolonging survival or eradicating disease. Cutaneous angiosarcoma of the head and neck has a reported 10-year survival rate of 13.8%.1 Although angiosarcoma in any location holds a bleak prognosis, cutaneous angiosarcoma of the scalp with a T2 classification has a 2-year survival rate of 0%. Moreover, even if remission is achieved, disease is highly recurrent, typically within months with the current standard of care.3,21,22
Emerging evidence for the possible role of β-adrenergic receptor blockade in the treatment of malignant vascular neoplasms is promising. Microarrays from a host of vascular growths have demonstrated expression of β-adrenergic receptors in 77% of sampled angiosarcoma specimens in addition to strong expression in infantile hemangiomas, hemangiomas, hemangioendotheliomas, and vascular malformations.19 Research findings have further verified the validity of this approach with the demonstration of b1-, b2-, and b3- adrenergic receptor expression by angiosarcoma cell lines. Propranolol subsequently was shown to effectively target proliferation of these cells and induce apoptosis in a dose-dependent manner and moreover be synergistic in effect with other chemotherapies.15 Several genes have exhibited differential expression between control tumor cells and propranolol-treated cells. Specifically, target genes including AXL (a receptor tyrosine kinase associated with cell adhesion, proliferation, and apoptosis and found to upregulated in melanoma and leukemia) and ERBB receptor feedback inhibitor 1 (receptor tyrosine kinase, with ERBB family members commonly overexpressed or mutated in the setting malignancy) have been posited as possible explanatory factors in the observed angiosarcoma response to propranolol.23
Several cases describing propranolol use as an adjunctive therapy for angiosarcoma suggest a beneficial role in clinical medicine. One case report described propranolol monotherapy for lesion to our patient, with a resultant reduction in Ki-67 as a measure of proliferative index within 1 week of initiating propranolol therapy.13 Propranolol also has been shown to halt or slow progression of metastatic disease in visceral and metastatic angiosarcomas.12-14 In combination with oral etoposide and cyclophosphamide, maintenance propranolol therapy in 7 cases of advanced cutaneous angiosarcoma resulted in 1 complete response and 3 very good partial responses, with a median progression-free survival of 11 months.11 Larger-scale studies have not been published, but the growing number of case reports and case series warrants further investigation of the utility of propranolol as an adjunct to current therapies in advanced angiosarcoma.
To the Editor:
Angiosarcoma is a malignancy of the vascular endothelium that most commonly presents on the skin.1 Patients diagnosed with cutaneous angiosarcoma, which is a rare and aggressive malignancy, have a 5-year survival rate of approximately 30%.2,3 Angiosarcoma can be seen in the setting of chronic lymphedema; radiation therapy; and sporadically in elderly patients, where it is commonly seen on the head and neck. Presentation on the head and neck has been associated with worse outcomes, with a projected overall 10-year survival rate of 13.8%; the survival rate is lower if the tumor is surgically unresectable or larger in size. Metastasis can occur via both lymphatic and hematogenous routes, with pulmonary and hepatic metastases most frequently observed.1 Prognostications of poor outcomes for patients with head and neck cutaneous angiosarcoma via a 5-year survival rate were identified in a meta-analysis and included the following: patient age older than 70 years, larger tumors, tumor location of scalp vs face, nonsurgical treatments, and lack of clear margins on histology.2
Treatment of angiosarcoma historically has encompassed both surgical resection and adjuvant radiation therapy with suboptimal success. Evidence supporting various treatment regimens remains sparse due to the low incidence of the neoplasm. Although surgical resection is the only documented curative treatment, cutaneous angiosarcomas frequently are found to have positive surgical margins and require adjuvant radiation. Use of high-dose radiation (>50 Gy) with application over a wide treatment area such as total scalp irradiation is recommended.4 Although radiation has been found to diminish local recurrence rates, it has not substantially affected rates of distant disease recurrence.1 Cytotoxic chemotherapy has clinical utility in minimizing progression, but standard regimens afford a progression-free survival of only months.3 Adjuvant treatment with paclitaxel has been shown to have improved efficacy in scalp angiosarcoma vs other visceral sites, showing a nonprogression rate of 42% at 4 months after treatment.5 More recently, targeted chemotherapeutics, including the vascular endothelial growth factor inhibitor bevacizumab and tyrosine kinase inhibitor sorafenib, have shown some survival benefit, but it is unclear if these agents are superior to traditional cytotoxic agents.4,6-10 A phase 2 study of paclitaxel administered weekly with or without bevacizumab showed similar progression-free survival and overall survival, albeit at the expense of added toxicity experienced by participants in the combined group.10
The addition of the nonselective β-adrenergic blocker propranolol to the treatment armamentarium, which was pursued due to its utility in the treatment of benign infantile hemangioma and demonstrated ability to limit the expression of adrenergic receptors in angiosarcoma, has gained clinical attention for possible augmentation of cutaneous angiosarcoma therapy.11-14 Propranolol has been shown to reduce metastasis in other neoplasms—both vascular and nonvascular—and may play a role as an adjuvant treatment to current therapies in angiosarcoma.15-20 We report a patient with cutaneous angiosarcoma (T2 classification) with disease-free survival of nearly 6 years without evidence of recurrence in the setting of continuous propranolol use supplementary to chemotherapy and radiation.

A 78-year-old man with a history of multiple basal cell carcinomas, hypertension, and remote smoking history presented to the dermatology clinic with an enlarging red-brown plaque on the scalp of 2 months’ duration. The lesion had grown rapidly to involve the forehead, right temple, preauricular region, and parietal scalp. At presentation, the tumor measured more than 20 cm in diameter at its greatest point (Figure 1). Physical examination revealed a 6-mm purple nodule within the lesion on the patient’s right parietal scalp. No clinical lymphadenopathy was appreciated at the time of diagnosis. Punch biopsies of the right parietal scalp nodule and right temple patch showed findings consistent with angiosarcoma with diffuse cytoplasmic staining of CD31 in atypical endothelial cells and no staining for human herpesvirus 8 (Figure 2). Concurrent computed tomography of the head showed thickening of the right epidermis, dermis, and deeper scalp tissues, but there was no evidence of skull involvement. Computed tomography of the thorax, abdomen, and pelvis showed no evidence of metastatic disease. After a diagnostic workup, the patient was diagnosed with T2bN0M0 angiosarcoma.

The lesion was determined to be nonresectable due to the extent of the patient’s cutaneous disease. The patient was started on a regimen of paclitaxel, scalp radiation, and oral propranolol. Propranolol 40 mg twice daily was initiated at the time of diagnosis with a plan to continue indefinitely. Starting 1 month after staging, the patient completed 10 weekly cycles of paclitaxel, and he was treated with 60 Gy of scalp radiation in 30 fractions, starting with the second cycle of paclitaxel. He tolerated both well with no reported adverse events. Repeat computed tomography performed 1 month after completion of chemotherapy and radiation showed no evidence of a mass or fluid collection in subcutaneous scalp tissues and no evidence of metastatic disease. This correlated with an observed clinical regression at 1 month and complete clinical response at 5 months with residual hemosiderin and radiation changes. The area of prior disease involvement subsequently evolved from violet to dusky gray in appearance to an eventual complete resolution 26 months after diagnosis, accompanied by atrophic radiation-induced sequelae (Figure 3).

The patient’s postchemotherapy course was complicated by hospitalization for a suspected malignant pleural effusion. Analysis revealed growing ground-glass opacities and nodules in the right lower lung lobe. A thoracentesis with cytology studies was negative for malignancy. Continued monitoring over 19 months demonstrated eventual resolution of those findings. He experienced notable complication from local radiation therapy to the scalp with chronic cutaneous ulceration refractory to wound care and surgical intervention. The patient did not exhibit additional signs or symptoms concerning for recurrence or metastasis and was followed by dermatology and oncology until he died nearly 5 years after initial diagnosis due to complications from acute hypoxic respiratory failure secondary to COVID-19. The last imaging obtained showed no convincing evidence of metastasis, though spinal imaging within a month of his death showed lesions favored to represent benign angiomatous growths. His survival after diagnosis ultimately reached 57 months without confirmed disease recurrence and cause of death unrelated to malignancy history, which is a markedly long documented survival for this extent of disease.
Cutaneous angiosarcoma is an aggressive yet rare malignancy without effective treatments for prolonging survival or eradicating disease. Cutaneous angiosarcoma of the head and neck has a reported 10-year survival rate of 13.8%.1 Although angiosarcoma in any location holds a bleak prognosis, cutaneous angiosarcoma of the scalp with a T2 classification has a 2-year survival rate of 0%. Moreover, even if remission is achieved, disease is highly recurrent, typically within months with the current standard of care.3,21,22
Emerging evidence for the possible role of β-adrenergic receptor blockade in the treatment of malignant vascular neoplasms is promising. Microarrays from a host of vascular growths have demonstrated expression of β-adrenergic receptors in 77% of sampled angiosarcoma specimens in addition to strong expression in infantile hemangiomas, hemangiomas, hemangioendotheliomas, and vascular malformations.19 Research findings have further verified the validity of this approach with the demonstration of b1-, b2-, and b3- adrenergic receptor expression by angiosarcoma cell lines. Propranolol subsequently was shown to effectively target proliferation of these cells and induce apoptosis in a dose-dependent manner and moreover be synergistic in effect with other chemotherapies.15 Several genes have exhibited differential expression between control tumor cells and propranolol-treated cells. Specifically, target genes including AXL (a receptor tyrosine kinase associated with cell adhesion, proliferation, and apoptosis and found to upregulated in melanoma and leukemia) and ERBB receptor feedback inhibitor 1 (receptor tyrosine kinase, with ERBB family members commonly overexpressed or mutated in the setting malignancy) have been posited as possible explanatory factors in the observed angiosarcoma response to propranolol.23
Several cases describing propranolol use as an adjunctive therapy for angiosarcoma suggest a beneficial role in clinical medicine. One case report described propranolol monotherapy for lesion to our patient, with a resultant reduction in Ki-67 as a measure of proliferative index within 1 week of initiating propranolol therapy.13 Propranolol also has been shown to halt or slow progression of metastatic disease in visceral and metastatic angiosarcomas.12-14 In combination with oral etoposide and cyclophosphamide, maintenance propranolol therapy in 7 cases of advanced cutaneous angiosarcoma resulted in 1 complete response and 3 very good partial responses, with a median progression-free survival of 11 months.11 Larger-scale studies have not been published, but the growing number of case reports and case series warrants further investigation of the utility of propranolol as an adjunct to current therapies in advanced angiosarcoma.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fury MG, Antonescu CR, Zee KJV, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer. 2005;11:241-247.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol. 2008;26:5269-5274.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Ishida Y, Otsuka A, Kabashima K. Cutaneous angiosarcoma: update on biology and latest treatment. Curr Opin Oncol. 2018;30:107-112.
- Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II trial from the French Sarcoma Group (GSF/GETO). Oncologist. 2012;17:260-266.
- Ray-Coquard IL, Domont J, Tresch-Bruneel E, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial. J Clin Oncol. 2015;33:2797-2802.
- Pasquier E, Andre N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Banavali S, Pasquier E, Andre N. Targeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience. 2015;9:499.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated beta-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Daguze J, Saint-Jean M, Peuvrel L, et al. Visceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. JAAD Case Rep. 2016;2:497-499.
- Stiles JM, Amaya C, Rains S, et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013;8:e60021.
- Chang PY, Chung CH, Chang WC, et al. The effect of propranolol on the prognosis of hepatocellular carcinoma: a nationwide population-based study. PLoS One. 2019;14:e0216828.
- De Giorgi V, Grazzini M, Benemei S, et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4:e172908.
- Rico M, Baglioni M, Bondarenko M, et al. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget. 2017;8:2874-2889.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Zhou S, Liu P, Jiang W, et al. Identification of potential target genes associated with the effect of propranolol on angiosarcoma via microarray analysis. Oncol Lett. 2017;13:4267-4275.
- Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14:1953-1967.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fury MG, Antonescu CR, Zee KJV, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer. 2005;11:241-247.
- Dossett LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol. 2008;26:5269-5274.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24:257-263.
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133-3140.
- Ishida Y, Otsuka A, Kabashima K. Cutaneous angiosarcoma: update on biology and latest treatment. Curr Opin Oncol. 2018;30:107-112.
- Ray-Coquard I, Italiano A, Bompas E, et al. Sorafenib for patients with advanced angiosarcoma: a phase II trial from the French Sarcoma Group (GSF/GETO). Oncologist. 2012;17:260-266.
- Ray-Coquard IL, Domont J, Tresch-Bruneel E, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial. J Clin Oncol. 2015;33:2797-2802.
- Pasquier E, Andre N, Street J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine. 2016;6:87-95.
- Banavali S, Pasquier E, Andre N. Targeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience. 2015;9:499.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated beta-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Daguze J, Saint-Jean M, Peuvrel L, et al. Visceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. JAAD Case Rep. 2016;2:497-499.
- Stiles JM, Amaya C, Rains S, et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013;8:e60021.
- Chang PY, Chung CH, Chang WC, et al. The effect of propranolol on the prognosis of hepatocellular carcinoma: a nationwide population-based study. PLoS One. 2019;14:e0216828.
- De Giorgi V, Grazzini M, Benemei S, et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4:e172908.
- Rico M, Baglioni M, Bondarenko M, et al. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget. 2017;8:2874-2889.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Zhou S, Liu P, Jiang W, et al. Identification of potential target genes associated with the effect of propranolol on angiosarcoma via microarray analysis. Oncol Lett. 2017;13:4267-4275.
PRACTICE POINTS
- In one classic presentation, cutaneous angiosarcoma characteristically appears as a bruiselike patch on the head and neck of an elderly gentleman.
- Although cutaneous angiosarcoma typically portends a poor prognosis at the time of diagnosis, adjunctive oral propranolol may be a promising and relatively benign therapy, posited to afford benefit in a manner similar to its efficacy in the treatment of infantile hemangiomas.
Use of Dupilumab in Severe, Multifactorial, Chronic Itch for Geriatric Patients
To the Editor:
Today’s geriatric population is the fastest growing in history. The National Institutes of Health predicts there will be over 1.5 billion individuals aged 65 years and older by the year 2050: 17% of the world’s population.1 Pruritus—either acute or chronic (>6 weeks)—is defined as a sensory perception that leads to an intense desire to scratch.2 Chronic pruritus is an increasing health concern that impacts quality of life within the geriatric population. Elderly patients have various risk factors for developing chronic itch, including aging skin, polypharmacy, and increased systemic comorbidities.3-7
Although the therapeutic armamentarium for chronic itch continues to grow, health care providers often are hesitant to prescribe medications for geriatric patients because of comorbidities and potential drug-drug interactions. Novel biologic therapies now provide alternatives for this complex population. Dupilumab is a fully humanized, monoclonal antibody approved for treatment-resistant atopic dermatitis. This biologic prevents helper T-cell (TH2) signaling, IL-4 and IL-13 release, and subsequent effector cell (eg, mast cell, eosinophil) activity.8-10 The combined efficacy and safety of this medication has changed the treatment landscape of resistant atopic dermatitis. We present the use of dupilumab in a geriatric patient with severe and recalcitrant itch resistant to numerous topical and oral medications.
An 81-year-old man presented to the clinic with a long history of generalized pruritic rash. His medical history was significant for insulin-dependent type 2 diabetes mellitus (T2DM), hypertension, and renal cancer following a right nephrectomy. Laboratory results approximately 14 months prior to the visit revealed a blood urea nitrogen level of 31 mg/dL (reference range, 7–20 mg/dL), creatinine level of 2.20 mg/dL (reference range, 0.7–1.3 mg/dL), and glomerular filtration rate of 29 mL/min (reference range, 90–120 mL/min). Physical examination revealed numerous pink excoriated papules on the face, neck, trunk, and extremities. Lichenified plaques were present on both arms and legs. The patient received the diagnosis of severe atopic dermatitis with greater than 10% body surface area involvement. The investigator global assessment score was 4/4, indicating severe disease burden, and biopsy results reported spongiotic dermatitis. He proceeded to trial various topical corticosteroids, including hydrocortisone ointment 2.5%, betamethasone valerate ointment 0.01%, fluocinonide ointment 0.05%, and mupirocin ointment without benefit. Three subsequent courses of oral steroids failed to provide durable relief. At this point, the peak pruritus numerical rating scale (NRS) score was 7/10, indicating severe pruritus, with a negative impact on the patient’s quality of life and sleep.
Therapy was switched to tacrolimus acetonide ointment 0.1%, betamethasone dipropionate ointment 0.05%, and triamcinolone acetonide ointment 0.1%. Eleven days later, the patient denied experiencing any response to the topical regimen and sought alternative therapy for the itch and associated poor sleep; the NRS score was 10/10, indicating very severe pruritus. Prednisone 20 mg and doxepin 10 mg were initiated for symptom management until the intended transition to dupilumab. The patient began dupilumab with a loading dose of 600 mg, then 300 mg every other week thereafter. At 2- and 4-month follow-up, the patient reported notable relief in symptoms. The rash had improved, and the NRS score decreased from 10/10 to 3/10. He endorsed improved sleep and quality of life.
Pruritus may arise from a series of age-related mechanisms such as structural and chemical changes within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease (CKD).5,6,11 Identifying the underlying etiology often is difficult and involves a complete history and physical examination as well as an appropriate contextualized laboratory workup.
Our patient’s comorbid T2DM and renal disease may have contributed to the pruritus. Type 2 diabetes mellitus can cause diabetic neuropathy, a sequela known to lead to various complications, including pruritus. One study identified a 4-fold increase in pruritus in those with diabetic polyneuropathy compared with age-matched nondiabetics.12,13 An additional study found that pruritus was present in 70% of patients with small fiber neuropathy.14 We needed to consider the role of our patient’s insulin-dependent T2DM and potential underlying neuropathy when addressing the pruritic symptoms.
Furthermore, our patient’s stage IV CKD and elevated urea level also may factor into the pruritus. The pathophysiology of CKD-associated pruritus (also referred to as uremic pruritus) remains poorly understood. Suggested mechanisms include immune-mediated neural inflammation and erroneous nociceptive-receptor activity.15,16 Although uremic pruritus is appreciated primarily in late dialysis-dependent disease, research shows that a notable portion of those with lesser disease, similar to our patient, also experience a significant itch burden.17 Diminishing pruritus is difficult and often aided by management of the underlying renal disease.18
In addition to disease management, symptomatic treatment incorporates the use of emollients, corticosteroids, and antihistamines. Unfortunately, the clinical response in the elderly population to such regimens often is poor.19 Dupilumab is an optimistic therapeutic option for chronic pruritus. By inhibiting the IL-4α receptor found on helper T cells, this biologic inhibits TH2 differentiation and subsequent inflammatory activity. One report identified an optimistic response to dupilumab in the management of uremic pruritus.20 The remarkable improvement and absence of adverse effects in our patient confirmed the utility and safety of dupilumab in complex cases such as elderly patients with multiple comorbidities. Such relief may result from inhibition of proinflammatory cytokine activity as well as decreased afferent spinal cord itch stimuli.10 The positive results from this case cast a favorable outlook on the treatment of chronic itch in the complex geriatric population.
- World’s older population grows dramatically. News release. National Institute on Aging. Published March 28, 2016. Accessed December 23, 2022. http://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically
- Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23:1-11.
- Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450. doi:10.1001/jama.2013.282023
- Valdes-Rodriguez, R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95:417-421. doi:10.2340/00015555-1968
- Li J, Tang H, Hu X, et al. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106-112.
- Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847-2856.
- Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 pt 1):571-585.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140:633-643. doi:10.1016/j.jaci.2017.07.006
- Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3-11.
- Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500-508.
- Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging. 2015;32:201-215. doi:10.1007/s40266-015-0246-0
- Misery L, Brenaut E, Le Garrec R, et al. Neuropathic pruritus. Nat Rev Neurol. 2014;10:408-416.
- Yamaoka H, Sasaki H, Yamasaki H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care. 2010;33:150-155.
- Brenaut E, Marcorelles P, Genestet S, et al. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328-332.
- Adigun M, Badu LA, Berner NM, et al. Uremic pruritus review. US Pharm. 2015;40:HS12-HS15.
- Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70:638-655.
- Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014.
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26.
- Brummer GC, Wang LT, Sontheimer RD. A possible role for dupilumab (Dupixent) in the management of idiopathic chronic eczematous eruption of aging. Dermatol Online J. 2018;24:13030/qt55z1f6xh.
- Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019;5:339-341.
To the Editor:
Today’s geriatric population is the fastest growing in history. The National Institutes of Health predicts there will be over 1.5 billion individuals aged 65 years and older by the year 2050: 17% of the world’s population.1 Pruritus—either acute or chronic (>6 weeks)—is defined as a sensory perception that leads to an intense desire to scratch.2 Chronic pruritus is an increasing health concern that impacts quality of life within the geriatric population. Elderly patients have various risk factors for developing chronic itch, including aging skin, polypharmacy, and increased systemic comorbidities.3-7
Although the therapeutic armamentarium for chronic itch continues to grow, health care providers often are hesitant to prescribe medications for geriatric patients because of comorbidities and potential drug-drug interactions. Novel biologic therapies now provide alternatives for this complex population. Dupilumab is a fully humanized, monoclonal antibody approved for treatment-resistant atopic dermatitis. This biologic prevents helper T-cell (TH2) signaling, IL-4 and IL-13 release, and subsequent effector cell (eg, mast cell, eosinophil) activity.8-10 The combined efficacy and safety of this medication has changed the treatment landscape of resistant atopic dermatitis. We present the use of dupilumab in a geriatric patient with severe and recalcitrant itch resistant to numerous topical and oral medications.
An 81-year-old man presented to the clinic with a long history of generalized pruritic rash. His medical history was significant for insulin-dependent type 2 diabetes mellitus (T2DM), hypertension, and renal cancer following a right nephrectomy. Laboratory results approximately 14 months prior to the visit revealed a blood urea nitrogen level of 31 mg/dL (reference range, 7–20 mg/dL), creatinine level of 2.20 mg/dL (reference range, 0.7–1.3 mg/dL), and glomerular filtration rate of 29 mL/min (reference range, 90–120 mL/min). Physical examination revealed numerous pink excoriated papules on the face, neck, trunk, and extremities. Lichenified plaques were present on both arms and legs. The patient received the diagnosis of severe atopic dermatitis with greater than 10% body surface area involvement. The investigator global assessment score was 4/4, indicating severe disease burden, and biopsy results reported spongiotic dermatitis. He proceeded to trial various topical corticosteroids, including hydrocortisone ointment 2.5%, betamethasone valerate ointment 0.01%, fluocinonide ointment 0.05%, and mupirocin ointment without benefit. Three subsequent courses of oral steroids failed to provide durable relief. At this point, the peak pruritus numerical rating scale (NRS) score was 7/10, indicating severe pruritus, with a negative impact on the patient’s quality of life and sleep.
Therapy was switched to tacrolimus acetonide ointment 0.1%, betamethasone dipropionate ointment 0.05%, and triamcinolone acetonide ointment 0.1%. Eleven days later, the patient denied experiencing any response to the topical regimen and sought alternative therapy for the itch and associated poor sleep; the NRS score was 10/10, indicating very severe pruritus. Prednisone 20 mg and doxepin 10 mg were initiated for symptom management until the intended transition to dupilumab. The patient began dupilumab with a loading dose of 600 mg, then 300 mg every other week thereafter. At 2- and 4-month follow-up, the patient reported notable relief in symptoms. The rash had improved, and the NRS score decreased from 10/10 to 3/10. He endorsed improved sleep and quality of life.
Pruritus may arise from a series of age-related mechanisms such as structural and chemical changes within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease (CKD).5,6,11 Identifying the underlying etiology often is difficult and involves a complete history and physical examination as well as an appropriate contextualized laboratory workup.
Our patient’s comorbid T2DM and renal disease may have contributed to the pruritus. Type 2 diabetes mellitus can cause diabetic neuropathy, a sequela known to lead to various complications, including pruritus. One study identified a 4-fold increase in pruritus in those with diabetic polyneuropathy compared with age-matched nondiabetics.12,13 An additional study found that pruritus was present in 70% of patients with small fiber neuropathy.14 We needed to consider the role of our patient’s insulin-dependent T2DM and potential underlying neuropathy when addressing the pruritic symptoms.
Furthermore, our patient’s stage IV CKD and elevated urea level also may factor into the pruritus. The pathophysiology of CKD-associated pruritus (also referred to as uremic pruritus) remains poorly understood. Suggested mechanisms include immune-mediated neural inflammation and erroneous nociceptive-receptor activity.15,16 Although uremic pruritus is appreciated primarily in late dialysis-dependent disease, research shows that a notable portion of those with lesser disease, similar to our patient, also experience a significant itch burden.17 Diminishing pruritus is difficult and often aided by management of the underlying renal disease.18
In addition to disease management, symptomatic treatment incorporates the use of emollients, corticosteroids, and antihistamines. Unfortunately, the clinical response in the elderly population to such regimens often is poor.19 Dupilumab is an optimistic therapeutic option for chronic pruritus. By inhibiting the IL-4α receptor found on helper T cells, this biologic inhibits TH2 differentiation and subsequent inflammatory activity. One report identified an optimistic response to dupilumab in the management of uremic pruritus.20 The remarkable improvement and absence of adverse effects in our patient confirmed the utility and safety of dupilumab in complex cases such as elderly patients with multiple comorbidities. Such relief may result from inhibition of proinflammatory cytokine activity as well as decreased afferent spinal cord itch stimuli.10 The positive results from this case cast a favorable outlook on the treatment of chronic itch in the complex geriatric population.
To the Editor:
Today’s geriatric population is the fastest growing in history. The National Institutes of Health predicts there will be over 1.5 billion individuals aged 65 years and older by the year 2050: 17% of the world’s population.1 Pruritus—either acute or chronic (>6 weeks)—is defined as a sensory perception that leads to an intense desire to scratch.2 Chronic pruritus is an increasing health concern that impacts quality of life within the geriatric population. Elderly patients have various risk factors for developing chronic itch, including aging skin, polypharmacy, and increased systemic comorbidities.3-7
Although the therapeutic armamentarium for chronic itch continues to grow, health care providers often are hesitant to prescribe medications for geriatric patients because of comorbidities and potential drug-drug interactions. Novel biologic therapies now provide alternatives for this complex population. Dupilumab is a fully humanized, monoclonal antibody approved for treatment-resistant atopic dermatitis. This biologic prevents helper T-cell (TH2) signaling, IL-4 and IL-13 release, and subsequent effector cell (eg, mast cell, eosinophil) activity.8-10 The combined efficacy and safety of this medication has changed the treatment landscape of resistant atopic dermatitis. We present the use of dupilumab in a geriatric patient with severe and recalcitrant itch resistant to numerous topical and oral medications.
An 81-year-old man presented to the clinic with a long history of generalized pruritic rash. His medical history was significant for insulin-dependent type 2 diabetes mellitus (T2DM), hypertension, and renal cancer following a right nephrectomy. Laboratory results approximately 14 months prior to the visit revealed a blood urea nitrogen level of 31 mg/dL (reference range, 7–20 mg/dL), creatinine level of 2.20 mg/dL (reference range, 0.7–1.3 mg/dL), and glomerular filtration rate of 29 mL/min (reference range, 90–120 mL/min). Physical examination revealed numerous pink excoriated papules on the face, neck, trunk, and extremities. Lichenified plaques were present on both arms and legs. The patient received the diagnosis of severe atopic dermatitis with greater than 10% body surface area involvement. The investigator global assessment score was 4/4, indicating severe disease burden, and biopsy results reported spongiotic dermatitis. He proceeded to trial various topical corticosteroids, including hydrocortisone ointment 2.5%, betamethasone valerate ointment 0.01%, fluocinonide ointment 0.05%, and mupirocin ointment without benefit. Three subsequent courses of oral steroids failed to provide durable relief. At this point, the peak pruritus numerical rating scale (NRS) score was 7/10, indicating severe pruritus, with a negative impact on the patient’s quality of life and sleep.
Therapy was switched to tacrolimus acetonide ointment 0.1%, betamethasone dipropionate ointment 0.05%, and triamcinolone acetonide ointment 0.1%. Eleven days later, the patient denied experiencing any response to the topical regimen and sought alternative therapy for the itch and associated poor sleep; the NRS score was 10/10, indicating very severe pruritus. Prednisone 20 mg and doxepin 10 mg were initiated for symptom management until the intended transition to dupilumab. The patient began dupilumab with a loading dose of 600 mg, then 300 mg every other week thereafter. At 2- and 4-month follow-up, the patient reported notable relief in symptoms. The rash had improved, and the NRS score decreased from 10/10 to 3/10. He endorsed improved sleep and quality of life.
Pruritus may arise from a series of age-related mechanisms such as structural and chemical changes within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease (CKD).5,6,11 Identifying the underlying etiology often is difficult and involves a complete history and physical examination as well as an appropriate contextualized laboratory workup.
Our patient’s comorbid T2DM and renal disease may have contributed to the pruritus. Type 2 diabetes mellitus can cause diabetic neuropathy, a sequela known to lead to various complications, including pruritus. One study identified a 4-fold increase in pruritus in those with diabetic polyneuropathy compared with age-matched nondiabetics.12,13 An additional study found that pruritus was present in 70% of patients with small fiber neuropathy.14 We needed to consider the role of our patient’s insulin-dependent T2DM and potential underlying neuropathy when addressing the pruritic symptoms.
Furthermore, our patient’s stage IV CKD and elevated urea level also may factor into the pruritus. The pathophysiology of CKD-associated pruritus (also referred to as uremic pruritus) remains poorly understood. Suggested mechanisms include immune-mediated neural inflammation and erroneous nociceptive-receptor activity.15,16 Although uremic pruritus is appreciated primarily in late dialysis-dependent disease, research shows that a notable portion of those with lesser disease, similar to our patient, also experience a significant itch burden.17 Diminishing pruritus is difficult and often aided by management of the underlying renal disease.18
In addition to disease management, symptomatic treatment incorporates the use of emollients, corticosteroids, and antihistamines. Unfortunately, the clinical response in the elderly population to such regimens often is poor.19 Dupilumab is an optimistic therapeutic option for chronic pruritus. By inhibiting the IL-4α receptor found on helper T cells, this biologic inhibits TH2 differentiation and subsequent inflammatory activity. One report identified an optimistic response to dupilumab in the management of uremic pruritus.20 The remarkable improvement and absence of adverse effects in our patient confirmed the utility and safety of dupilumab in complex cases such as elderly patients with multiple comorbidities. Such relief may result from inhibition of proinflammatory cytokine activity as well as decreased afferent spinal cord itch stimuli.10 The positive results from this case cast a favorable outlook on the treatment of chronic itch in the complex geriatric population.
- World’s older population grows dramatically. News release. National Institute on Aging. Published March 28, 2016. Accessed December 23, 2022. http://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically
- Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23:1-11.
- Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450. doi:10.1001/jama.2013.282023
- Valdes-Rodriguez, R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95:417-421. doi:10.2340/00015555-1968
- Li J, Tang H, Hu X, et al. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106-112.
- Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847-2856.
- Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 pt 1):571-585.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140:633-643. doi:10.1016/j.jaci.2017.07.006
- Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3-11.
- Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500-508.
- Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging. 2015;32:201-215. doi:10.1007/s40266-015-0246-0
- Misery L, Brenaut E, Le Garrec R, et al. Neuropathic pruritus. Nat Rev Neurol. 2014;10:408-416.
- Yamaoka H, Sasaki H, Yamasaki H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care. 2010;33:150-155.
- Brenaut E, Marcorelles P, Genestet S, et al. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328-332.
- Adigun M, Badu LA, Berner NM, et al. Uremic pruritus review. US Pharm. 2015;40:HS12-HS15.
- Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70:638-655.
- Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014.
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26.
- Brummer GC, Wang LT, Sontheimer RD. A possible role for dupilumab (Dupixent) in the management of idiopathic chronic eczematous eruption of aging. Dermatol Online J. 2018;24:13030/qt55z1f6xh.
- Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019;5:339-341.
- World’s older population grows dramatically. News release. National Institute on Aging. Published March 28, 2016. Accessed December 23, 2022. http://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically
- Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23:1-11.
- Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450. doi:10.1001/jama.2013.282023
- Valdes-Rodriguez, R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95:417-421. doi:10.2340/00015555-1968
- Li J, Tang H, Hu X, et al. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106-112.
- Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847-2856.
- Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 pt 1):571-585.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140:633-643. doi:10.1016/j.jaci.2017.07.006
- Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3-11.
- Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500-508.
- Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging. 2015;32:201-215. doi:10.1007/s40266-015-0246-0
- Misery L, Brenaut E, Le Garrec R, et al. Neuropathic pruritus. Nat Rev Neurol. 2014;10:408-416.
- Yamaoka H, Sasaki H, Yamasaki H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care. 2010;33:150-155.
- Brenaut E, Marcorelles P, Genestet S, et al. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328-332.
- Adigun M, Badu LA, Berner NM, et al. Uremic pruritus review. US Pharm. 2015;40:HS12-HS15.
- Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70:638-655.
- Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014.
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26.
- Brummer GC, Wang LT, Sontheimer RD. A possible role for dupilumab (Dupixent) in the management of idiopathic chronic eczematous eruption of aging. Dermatol Online J. 2018;24:13030/qt55z1f6xh.
- Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019;5:339-341.
PRACTICE POINTS
- A series of age-related mechanisms within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease may contribute to pruritus in elderly patients.
- Patients with mild kidney disease may still experience a recalcitrant and notable itch burden.
- Dupilumab is efficacious and safe in the management of chronic pruritus, even in complex cases such as elderly patients with multiple comorbidities.
Juvenile Dermatomyositis–Associated Panniculitis
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.
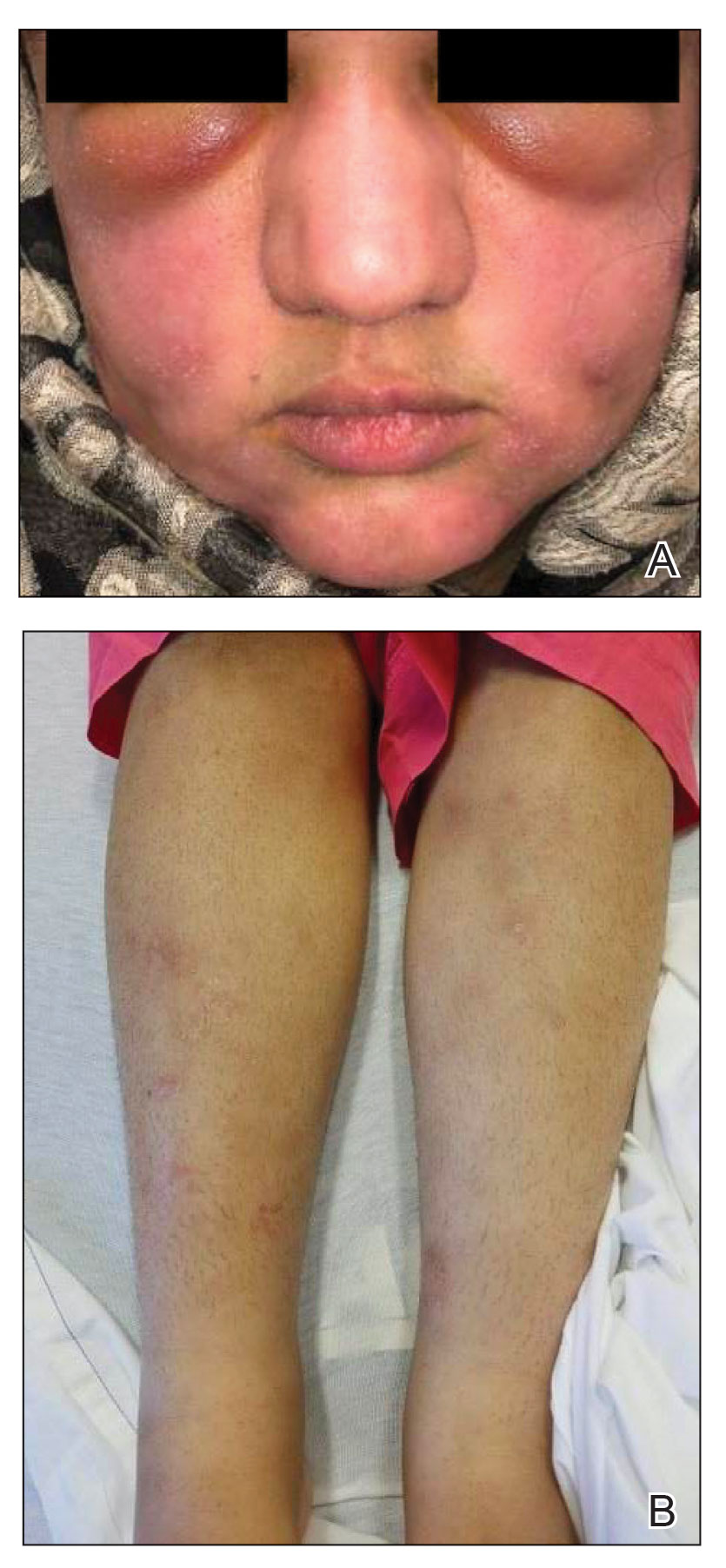
A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.
Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
Practice Points
- Juvenile dermatomyositis is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin.
- Juvenile dermatomyositis has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant, dermatomyositis (DM).
- Panniculitis is a rare but severe complication of DM, and this subset of DM may be challenging to treat, requiring aggressive therapy.
Skin Manifestations of Complex Regional Pain Syndrome
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.
To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
PRACTICE POINTS
- Common dermatologic manifestations of complex regional pain syndrome (CRPS), which often are nonspecific and often the presenting symptoms of the syndrome, include allodynia, edema, erythema, hypopigmentation or hyperpigmentation, and petechiae.
- Diagnosis and management of CRPS are the most important steps in treating dermatologic manifestations of the syndrome.
Primary Malignant Melanoma of the Middle Ear
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
PRACTICE POINTS
- Primary malignant melanoma of the middle ear is rare and has poor prognosis.
- Distant metastasis, including cutaneous metastasis, results from primary middle ear melanoma.
A Trauma-Induced Fatty Mass: The Facts About Posttraumatic Pseudolipomas
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.
In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5
Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.

In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5

Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.

In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5

Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
Practice Points
- Physicians should include pseudolipoma in the differential diagnosis when evaluating masses that develop in patients at sites of blunt or prolonged trauma.
- A pseudolipoma is an unencapsulated, round, or fusiform fatty mass that differs from a traditional lipoma by the absence of a capsule.
- Further research may elucidate the pathogenesis of these adiposities.
Dupilumab as a Therapeutic Approach in Alopecia Universalis
To the Editor:
Atopic diseases, specifically atopic dermatitis (AD) and alopecia areata (AA), are at the forefront of a new era in dermatology involving molecular-directed therapy. Dupilumab is one specific example, having received US Food and Drug Administration approval in March 2017 for the treatment of adults with moderate to severe AD.1 It currently is being investigated for use in pediatric AD. The most commonly reported side effects associated with the use of dupilumab include headaches, conjunctivitis, keratitis, blepharitis, nasopharyngitis, and injection-site reactions.2 We discuss a case of hair regrowth in a patient who was previously diagnosed with AA after treatment with dupilumab for refractory AD.
A 65-year-old White man presented with a history of AD since childhood. Additional medical history included hyperlipidemia; herpes simplex virus infection; asthma; and a diagnosis of AA 6 years prior, which eventually progressed to alopecia universalis. Physical examination demonstrated scattered erythematous lichenified plaques with excoriations involving the arms, legs, and trunk. The patient’s face and scalp were spared of lesions. Complete loss of body hair including the eyelashes and eyebrows also was noted, which was consistent with alopecia universalis.
The patient was started on dupilumab for refractory AD after multiple courses of topical and systemic steroids failed. Prior treatment for AD did not include immunosuppressive or light therapy. The standard dosage of dupilumab was administered, which consisted of a 600-mg subcutaneous loading dose, followed by 300 mg every 2 weeks. There was no concurrent topical corticosteroid or topical calcineurin inhibitor prescribed. After 1 month of treatment with dupilumab, near-complete resolution of the patient’s AD was noted, and after 10 months of treatment, the patient experienced regrowth of the eyelashes, terminal hairs of the beard area (Figure), and vellus hairs of the eyebrows. This hair regrowth persists today with continued dupilumab treatment, and the patient has experienced no additional side effects.

Multiple retrospective and meta-analysis studies have demonstrated a high occurrence of AD comorbid with AA, which strongly suggests a common pathogenesis.3,4 Atopic dermatitis is an inflammatory skin disease mediated by IL-4, IL-5, and IL-13 of the helper T-cell type 2 (TH2) pathway.1 Dupilumab is a human monoclonal antibody that binds to IL-4Rα, which also is found in IL-13 receptors. Dupilumab prevents TH2 pathway-related downstream signaling effects of both cytokines. Although this effect was originally utilized to suppress the TH2-mediated signaling in AD, our patient and others have demonstrated successful hair regrowth with dupilumab, which likely stems from a similar TH2-related antagonism in AA.5,6
The cause of AA is unknown, but IL-4 and IL-13 of the TH2 pathway have been implicated, which renders support for the therapeutic effect of dupilumab in the treatment of AA. Scalp samples of patients with AA have demonstrated upregulation of TH2, helper T-cell type 1 (TH1), and IL-23 cytokines, suggesting efficacy with the use of anti-TH2, anti-TH1, and anti–IL-23 therapies.7 Polymerase chain reaction testing performed on serum samples in patients with AA displayed marked elevation of TH2 cytokines, notably IL-13, which were reduced following intralesional corticosteroid treatment.8 It also has been demonstrated that multiple TH2-related genes contribute to the genetic susceptibility of developing AA, specifically IL-4 and IL-13.9,10
Prior case reports have shown contradicting effects (dupilumab-induced AA), which are speculated to be caused by a stronger TH1 response from TH2 suppression.11,12 In one report, dupilumab was initiated for AD refractory to multiple topical and oral interventions. New-onset hair loss to the scalp was noted after 18 weeks of therapy. Twenty-six weeks into therapy with dupilumab, full hair regrowth was then reported.11 Despite this report, our patient’s hair regrowth after the use of dupilumab for refractory AD further strengthens support for the use of dupilumab as a potential therapy for alopecia universalis and other lymphocyte-mediated hair loss conditions. However, a large disparity in response time and an overall slower progression of hair regrowth reported in our case separate it from other reports of rapid voluminous hair regrowth.5,6 Our findings support the potential use of dupilumab in the treatment of patients with AA.
- Shirly M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Ou Z, Chen C, Chen A, et al. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303-310.
- Andersen YMF, Egeberg A, Gislason GH, et al. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-280.e1.
- Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2015;15:522-528.
- Penzi LR, Yasuda M, Manatis-Lornell A, et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154:1358-1360.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019;5:111-112.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Fuentes-Duculan J, Gulati N, Bonifacio KM, et al. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2016;25:282-286.
- Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132:2192-2197.
- Kalkan G, Karakus N, Bas¸ Y, et al. The association between interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. 2013;527:565-569.
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-56.
- Mitchell K, Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4:143-144.
To the Editor:
Atopic diseases, specifically atopic dermatitis (AD) and alopecia areata (AA), are at the forefront of a new era in dermatology involving molecular-directed therapy. Dupilumab is one specific example, having received US Food and Drug Administration approval in March 2017 for the treatment of adults with moderate to severe AD.1 It currently is being investigated for use in pediatric AD. The most commonly reported side effects associated with the use of dupilumab include headaches, conjunctivitis, keratitis, blepharitis, nasopharyngitis, and injection-site reactions.2 We discuss a case of hair regrowth in a patient who was previously diagnosed with AA after treatment with dupilumab for refractory AD.
A 65-year-old White man presented with a history of AD since childhood. Additional medical history included hyperlipidemia; herpes simplex virus infection; asthma; and a diagnosis of AA 6 years prior, which eventually progressed to alopecia universalis. Physical examination demonstrated scattered erythematous lichenified plaques with excoriations involving the arms, legs, and trunk. The patient’s face and scalp were spared of lesions. Complete loss of body hair including the eyelashes and eyebrows also was noted, which was consistent with alopecia universalis.
The patient was started on dupilumab for refractory AD after multiple courses of topical and systemic steroids failed. Prior treatment for AD did not include immunosuppressive or light therapy. The standard dosage of dupilumab was administered, which consisted of a 600-mg subcutaneous loading dose, followed by 300 mg every 2 weeks. There was no concurrent topical corticosteroid or topical calcineurin inhibitor prescribed. After 1 month of treatment with dupilumab, near-complete resolution of the patient’s AD was noted, and after 10 months of treatment, the patient experienced regrowth of the eyelashes, terminal hairs of the beard area (Figure), and vellus hairs of the eyebrows. This hair regrowth persists today with continued dupilumab treatment, and the patient has experienced no additional side effects.

Multiple retrospective and meta-analysis studies have demonstrated a high occurrence of AD comorbid with AA, which strongly suggests a common pathogenesis.3,4 Atopic dermatitis is an inflammatory skin disease mediated by IL-4, IL-5, and IL-13 of the helper T-cell type 2 (TH2) pathway.1 Dupilumab is a human monoclonal antibody that binds to IL-4Rα, which also is found in IL-13 receptors. Dupilumab prevents TH2 pathway-related downstream signaling effects of both cytokines. Although this effect was originally utilized to suppress the TH2-mediated signaling in AD, our patient and others have demonstrated successful hair regrowth with dupilumab, which likely stems from a similar TH2-related antagonism in AA.5,6
The cause of AA is unknown, but IL-4 and IL-13 of the TH2 pathway have been implicated, which renders support for the therapeutic effect of dupilumab in the treatment of AA. Scalp samples of patients with AA have demonstrated upregulation of TH2, helper T-cell type 1 (TH1), and IL-23 cytokines, suggesting efficacy with the use of anti-TH2, anti-TH1, and anti–IL-23 therapies.7 Polymerase chain reaction testing performed on serum samples in patients with AA displayed marked elevation of TH2 cytokines, notably IL-13, which were reduced following intralesional corticosteroid treatment.8 It also has been demonstrated that multiple TH2-related genes contribute to the genetic susceptibility of developing AA, specifically IL-4 and IL-13.9,10
Prior case reports have shown contradicting effects (dupilumab-induced AA), which are speculated to be caused by a stronger TH1 response from TH2 suppression.11,12 In one report, dupilumab was initiated for AD refractory to multiple topical and oral interventions. New-onset hair loss to the scalp was noted after 18 weeks of therapy. Twenty-six weeks into therapy with dupilumab, full hair regrowth was then reported.11 Despite this report, our patient’s hair regrowth after the use of dupilumab for refractory AD further strengthens support for the use of dupilumab as a potential therapy for alopecia universalis and other lymphocyte-mediated hair loss conditions. However, a large disparity in response time and an overall slower progression of hair regrowth reported in our case separate it from other reports of rapid voluminous hair regrowth.5,6 Our findings support the potential use of dupilumab in the treatment of patients with AA.
To the Editor:
Atopic diseases, specifically atopic dermatitis (AD) and alopecia areata (AA), are at the forefront of a new era in dermatology involving molecular-directed therapy. Dupilumab is one specific example, having received US Food and Drug Administration approval in March 2017 for the treatment of adults with moderate to severe AD.1 It currently is being investigated for use in pediatric AD. The most commonly reported side effects associated with the use of dupilumab include headaches, conjunctivitis, keratitis, blepharitis, nasopharyngitis, and injection-site reactions.2 We discuss a case of hair regrowth in a patient who was previously diagnosed with AA after treatment with dupilumab for refractory AD.
A 65-year-old White man presented with a history of AD since childhood. Additional medical history included hyperlipidemia; herpes simplex virus infection; asthma; and a diagnosis of AA 6 years prior, which eventually progressed to alopecia universalis. Physical examination demonstrated scattered erythematous lichenified plaques with excoriations involving the arms, legs, and trunk. The patient’s face and scalp were spared of lesions. Complete loss of body hair including the eyelashes and eyebrows also was noted, which was consistent with alopecia universalis.
The patient was started on dupilumab for refractory AD after multiple courses of topical and systemic steroids failed. Prior treatment for AD did not include immunosuppressive or light therapy. The standard dosage of dupilumab was administered, which consisted of a 600-mg subcutaneous loading dose, followed by 300 mg every 2 weeks. There was no concurrent topical corticosteroid or topical calcineurin inhibitor prescribed. After 1 month of treatment with dupilumab, near-complete resolution of the patient’s AD was noted, and after 10 months of treatment, the patient experienced regrowth of the eyelashes, terminal hairs of the beard area (Figure), and vellus hairs of the eyebrows. This hair regrowth persists today with continued dupilumab treatment, and the patient has experienced no additional side effects.

Multiple retrospective and meta-analysis studies have demonstrated a high occurrence of AD comorbid with AA, which strongly suggests a common pathogenesis.3,4 Atopic dermatitis is an inflammatory skin disease mediated by IL-4, IL-5, and IL-13 of the helper T-cell type 2 (TH2) pathway.1 Dupilumab is a human monoclonal antibody that binds to IL-4Rα, which also is found in IL-13 receptors. Dupilumab prevents TH2 pathway-related downstream signaling effects of both cytokines. Although this effect was originally utilized to suppress the TH2-mediated signaling in AD, our patient and others have demonstrated successful hair regrowth with dupilumab, which likely stems from a similar TH2-related antagonism in AA.5,6
The cause of AA is unknown, but IL-4 and IL-13 of the TH2 pathway have been implicated, which renders support for the therapeutic effect of dupilumab in the treatment of AA. Scalp samples of patients with AA have demonstrated upregulation of TH2, helper T-cell type 1 (TH1), and IL-23 cytokines, suggesting efficacy with the use of anti-TH2, anti-TH1, and anti–IL-23 therapies.7 Polymerase chain reaction testing performed on serum samples in patients with AA displayed marked elevation of TH2 cytokines, notably IL-13, which were reduced following intralesional corticosteroid treatment.8 It also has been demonstrated that multiple TH2-related genes contribute to the genetic susceptibility of developing AA, specifically IL-4 and IL-13.9,10
Prior case reports have shown contradicting effects (dupilumab-induced AA), which are speculated to be caused by a stronger TH1 response from TH2 suppression.11,12 In one report, dupilumab was initiated for AD refractory to multiple topical and oral interventions. New-onset hair loss to the scalp was noted after 18 weeks of therapy. Twenty-six weeks into therapy with dupilumab, full hair regrowth was then reported.11 Despite this report, our patient’s hair regrowth after the use of dupilumab for refractory AD further strengthens support for the use of dupilumab as a potential therapy for alopecia universalis and other lymphocyte-mediated hair loss conditions. However, a large disparity in response time and an overall slower progression of hair regrowth reported in our case separate it from other reports of rapid voluminous hair regrowth.5,6 Our findings support the potential use of dupilumab in the treatment of patients with AA.
- Shirly M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Ou Z, Chen C, Chen A, et al. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303-310.
- Andersen YMF, Egeberg A, Gislason GH, et al. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-280.e1.
- Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2015;15:522-528.
- Penzi LR, Yasuda M, Manatis-Lornell A, et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154:1358-1360.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019;5:111-112.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Fuentes-Duculan J, Gulati N, Bonifacio KM, et al. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2016;25:282-286.
- Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132:2192-2197.
- Kalkan G, Karakus N, Bas¸ Y, et al. The association between interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. 2013;527:565-569.
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-56.
- Mitchell K, Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4:143-144.
- Shirly M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Ou Z, Chen C, Chen A, et al. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303-310.
- Andersen YMF, Egeberg A, Gislason GH, et al. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76:274-280.e1.
- Mohan GC, Silverberg JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2015;15:522-528.
- Penzi LR, Yasuda M, Manatis-Lornell A, et al. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154:1358-1360.
- Alniemi DT, McGevna L. Dupilumab treatment for atopic dermatitis leading to unexpected treatment for alopecia universalis. JAAD Case Rep. 2019;5:111-112.
- Suárez-Fariñas M, Ungar B, Noda S, et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136:1277-1287.
- Fuentes-Duculan J, Gulati N, Bonifacio KM, et al. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp Dermatol. 2016;25:282-286.
- Jagielska D, Redler S, Brockschmidt FF, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132:2192-2197.
- Kalkan G, Karakus N, Bas¸ Y, et al. The association between interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. 2013;527:565-569.
- Flanagan K, Sperling L, Lin J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019;5:54-56.
- Mitchell K, Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4:143-144.
Practice Points
- Practicing dermatologists should be aware of the shared pathophysiology of alopecia areata and atopic dermatitis and the relief patients with these conditions can experience when treated with dupilumab.
- As molecular-directed biologic therapies emerge in the marketplace, their potential for targeting one atopic disease may offer notable benefits for use in the treatment of other atopic diseases.
Psoriasiform Dermatitis Associated With the Moderna COVID-19 Messenger RNA Vaccine
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
PRACTICE POINTS
- The differential diagnosis for a new-onset psoriasiform rash in an elderly patient should include a vaccine-related rash.
- A rash following vaccination that necessitates systemic corticosteroid therapy can decrease vaccine efficacy.