User login
Evaluation of the American Academy of Orthopaedic Surgeons Appropriate Use Criteria for the Nonarthroplasty Treatment of Knee Osteoarthritis in Veterans
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
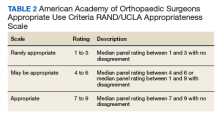
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
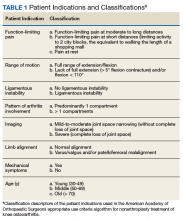
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
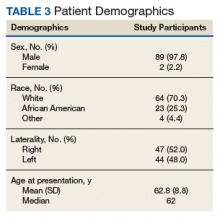
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
The Dyad Model for Interprofessional Academic Patient Aligned Care Teams
Background
In 2011, 5 US Department of Veterans Affairs (VA) medical centers were selected by the VA Office of Academic Affiliations (OAA) to establish Centers of Excellence in Primary Care Education (CoEPCE). As part of VA’s New Models of Care initiative, the 5 CoEPCEs are using VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurses (APRNs), undergraduate nursing students, and other health professions trainees (such as pharmacy, social work, psychology, physician assistants) for primary care practice. The CoEPCE sites are developing, implementing, and evaluating curricula to prepare learners from relevant professions to practice in patientcentered, interprofessional team-based primary care settings. Patient aligned care teams (PACTs) that have 2 or more health professions trainees engaged in learning, working, and teaching are known as interprofessional academic PACTs (iAPACTs), which is the preferred model for the VA.
The Cleveland Transforming Outpatient Care (TOPC)-CoEPCE was designed for collaborative learning among nurse practitioner (NP) students and physician residents. Its robust curriculum consists of a dedicated half-day of didactics for all learners, interprofessional quality improvement projects, panel management sessions, and primary care clinical sessions for nursing and physician learners that include the dyad workplace learning model.
In 2015, the OAA lead evaluator observed the TOPC-CoEPCE dyad model process, reviewed background documents, and conducted 10 open-ended interviews with TOPC-CoEPCE staff, participating trainees, faculty, and affiliate leadership. Informants described their involvement, challenges encountered, and benefits of the TOPCCoEPC dyad model to participants, veterans, VA, and affiliates.
Lack of Interprofessional Learning Opportunities
Current health care professional education models typically do not have many workplace learning settings where physician and nursing trainees learn together and provide patient-centered care. Often in a shared clinical environment, trainees may engage in “parallel play,” which can result in physician trainees and NP students learning independently and being ill-prepared to practice effectively together.
Moreover, trainees from different professions have different learning needs. For example, less experienced NP students require greater time, supervision, and evaluation of their patient care skills. On the other hand, senior physician residents, who require less clinical instruction, need to be engaged in ways that provide opportunities to enhance their ambulatory teaching skills. Although enhancement of resident teaching skills occurs in the inpatient hospital setting, there have been limited teaching experiences for residents in a primary care setting where the instruction is traditionally faculty-based. The TOPCCoEPCE dyad model offers an opportunity to simultaneously provide trainees with a true interprofessional experience through advancement of skills in primary care, teamwork, and teaching, while addressing health care needs.
The Dyad Model
In 2011, the OAA directed COEPCE sites to develop innovative curriculum and workplace learning strategies to create more opportunities for physician and NP trainees to work as a team. There is evidence demonstrating that when students develop a shared understanding of each other’s skill set, care procedures, and values, patient care is improved.1 Further, training in pairs can be an effective strategy in education of preclerkship medical students.2 In April 2013, TOPC-CoEPCE staff asked representatives from the Student-Run Clinic at Case Western Reserve University (CWRU) in Cleveland, Ohio, to present their approach to pairing nursing and medical students in clinic under supervision by volunteer faculty. However, formal structure and curricular objectives were lacking. To address diverse TOPCCoEPCE trainee needs and create a team approach to patient care, the staff formalized and developed a workplace curriculum called the dyad model. Specifically, the model pairs 1 NP student with a senior (PGY2 or PGY3) physician resident to care for ambulatory patients as a dyad teaching/learning team. The dyad model has 3 goals: improving clinical performance, learning team dynamics, and improving the physician resident’s teaching skills in an ambulatory setting.
Planning and Implementation
Planning the dyad model took 4 months. Initial conceptualization of the model was discussed at TOPC-CoEPCE infrastructure meetings. Workgroups with representatives from medicine, nursing, evaluation and medical center administration were formed to finalize the model. The workgroups met weekly or biweekly to develop protocols for scheduling, ongoing monitoring and assessment, microteaching session curriculum development, and logistics. A pilot program was initiated for 1 month with 2 dyads to monitor learner progress and improve components, such as adjusting the patient exam start times and curriculum. In maintaining the program, the workgroups continue to meet monthly to check for areas for further improvement and maintain dissemination activities.
Curriculum
The dyad model is a novel opportunity to have trainees from different professions not only collaborate in the care of the same patient at the same time, but also negotiate their respective responsibilities preand postvisit. The experience focuses on interprofessional relationships and open communication. TOPC-CoEPCE used a modified version of the RIME (Reporter-Interpreter-Manager-Educator) model called the O-RIME model (Table 1), which includes an observer (O) phase as the first component for clarification about a beginners’ role.3,4
Four dyad pairs provide collaborative clinical care for veterans during one halfday session per week. The dyad conducts 4 hour-long patient visits per session. To be a dyad participant, the physician residents must be at least a PGY2, and their schedule must align with the NP student clinic schedule. Participation is mandatory for both NP students and physician residents. TOPC staff assemble the pairs.
The dyad model requires knowledge of the clinical and curricular interface and when to block the dyad team members’ schedules for 4 patients instead of 6. Physician residents are in the TOPC-CoEPCE for 12 weeks and then on inpatient for 12 weeks. Depending on the nursing school affiliate, NP student trainees are scheduled for either a 6- or 12-month TOPC-CoEPCE experience. For the 12-month NP students, they are paired with up to 4 internal medicine residents over the course of their dyad participation so they can experience different teaching styles of each resident while developing more varied interprofessional communication skills.
Faculty Roles and Development
The dyad model also seeks to address the paucity of deliberate interprofessional precepting in academic primary care settings. The TOPC-CoEPCE staff decided to use the existing primary care clinic faculty development series bimonthly for 1 hour each. The dyad model team members presented sessions covering foundational material in interprofessional teaching and precepting skills, which prepare faculty to precept for different professions and the dyad teams. It is important for preceptors to develop awareness of learners from different professions and the corresponding educational trajectories, so they can communicate with paired trainees of differing professions and academic levels who may require different levels of discussion.
Resources
By utilizing advanced residents as teachers, faculty were able to increase the number of learners in the clinic without increasing the number preceptors. For example, precepting a student typically requires more preceptor time, especially when we consider that the preceptor must also see the patient. The TOPC-CoEPCE faculty run the microteaching sessions, and an evaluator monitors and evaluates the program. The microteaching sessions were derived from several teaching resources.
Monitoring and Assessment
The Cleveland TOPC administered 2 different surveys developed by the Dyad Model Infrastructure and Evaluation workgroup. A 7-item survey assesses dyad team communication and interprofessional team functioning, and an 8-item survey assesses the teaching/mentoring of the resident as teacher. Both were collected from all participants to evaluate the residents’ and students’ point of view. Surveys are collected in the first and last weeks of the dyad experience. Feedback from participants has been used to make improvements to the program (eg, monitoring how the dyad teams are functioning, coaching individual learners).
Partnerships
In addition to TOPC staff and faculty support and engagement, the initiative has benefited from partnerships with VA clinic staff and with the associated academic affiliates. In particular, the Associate Chief of General Internal Medicine at the Cleveland VA medical center and interim clinic director helped institute changes to the primary care clinic structure. Additionally, buy-in from the clinic nurse manager was needed to make adjustments with staff schedules and clinic resources. To implement the dyad model, the clinic director had to approve reductions in the residents’ clinic loads for the mornings when they participated.
The NP affiliates’ faculty at the schools of nursing are integral partners who assist with student recruitment and participate in the planning and refinement of TOPCCoEPCE components. The Frances Payne Bolton School of Nursing at CWRU and the Breen School of Nursing of Ursuline College in Pepper Pike, Ohio, were involved in the planning stages and continue to receive monthly updates from TOPC-CoEPCE. Similarly, the CWRU School of Medicine and Cleveland Clinic Foundation affiliates contribute on an ongoing basis to the improvement and implementation process.
Discussion
One challenge has been advancing aspects of a nonhierarchical team approach while it is a teacher-student relationship. The dyad model is viewed as an opportunity to recognize nonhierarchical structures and teach negotiation and communication skills as well as increase interprofessional understanding of each other’s education, expertise, and scope of practice.
Another challenge is accommodating the diversity in NP training and clinical expertise. The NP student participants are in either the first or second year of their academic program. This is a challenge since both physician residents and physician faculty preceptors need to assess the NP students’ skills before providing opportunities to build on their skill level. Staff members have learned the value of checking in weekly on this issue.
Factors for Success
VA facility support and TOPC-CoEPCE leadership with the operations/academic partnership remain critical to integrating and sustaining the model into the Cleveland primary care clinic. The expertise of TOPC-CoEPCE dyad model faculty who serve as facilitators has been crucial, as they oversee team development concepts such as developing problem solving and negotiation skills. The workgroups ensured that faculty were skilled in understanding the different types of learners and provided guidance to dyad teams. Another success factor was the continual monitoring of the process and real-time evaluation of the program to adapt the model as needed.
Accomplishments and Benefits
There is evidence that the dyad model is achieving its goals: Trainees are using team skills during and outside formal dyad pairs; NP students report improvements in skill levels and comfort; and physician residents feel the teaching role in the dyad pair is an opportunity for them to improve their practice.
Interprofessional Educational Capacity
The dyad model complements the curriculum components and advances trainee understanding of 4 core domains: shared decision-making (SDM), sustained relationships (SR), interprofessional collaboration (IPC), and performance improvement (PI) (Table 2). The dyad model supports the other CoEPCE interprofessional education activities and is reinforced by these activities. The model is a learning laboratory for studying team dynamics and developing a curriculum that strengthens a team approach to patient-centered care.
Participants’ Knowledge, Attitudes, Skills, and Competencies
As of May 2015, 35 trainees (21 internal medicine physician residents and 14 NP students) have participated in dyads. Because physician residents participate over 2 years and may partner with more than 1 NP student, this has resulted in 27 dyad pairs in this time frame. Findings from an analysis of evaluations suggest that the dyad pair trainees learn from one another, and the model provides a safe space where trainees can practice and increase their confidence.1,6,7 The NP students seem to increase clinical skills quickly—expanding physical exam skills, building a differential diagnosis, and formulating therapeutic plans—and progressing to the Interpreter and Manager levels in the O-RIME model. The physician resident achieves the Educator level.
As of September 2015, the results from the pairs who completed beginning and end evaluations show that the physician residents increased the amount of feedback they provided about performance to the student, and likewise the student NPs also felt they received an increased amount of feedback about performance from the physician resident. In addition, physician residents reported improving the most in the following areas: allowing the student to make commitments in diagnoses and treatment plans and asking the student to provide supporting evidence for their commitment to the diagnoses. NP students reported the largest increases in receiving weekly feedback about their performance from the physician and their ability to listen to the patient.1,6,7
Interprofessional Collaboration
The TOPC-CoEPCE staff observed strengthened dyad pair relationships and mutual respect between the dyad partners. Trainees communicate with each other and work together to provide care of the patient. Second, dyad pair partners are learning about the other profession—their trajectory, their education model, and their differences. The physician resident develops an awareness of the partner NP student’s knowledge and expertise, such as their experience of social and psychological factors to become a more effective teacher, contributing to patient-centered care. The evaluation results illustrate increased ability of trainees to give and receive feedback and the change in roles for providing diagnosis and providing supporting evidence within the TOPCCoEPCE dyad team.6-8
The Future
The model has broad applicability for interprofessional education in the VA since it enhances skills that providers need to work in a PACT/PCMH model. Additionally, the TOPC-CoEPCE dyad model has proven to be an effective interprofessional training experience for its affiliates and may have applicability in other VA/affiliate training programs. The dyad model can be adapted to different trainee types in the ambulatory care setting. The TOPCCoEPCE is piloting a version of the dyad with NP residents (postgraduate) and first-year medical students. Additionally, the TOPCCoEPCE is paving the way for integrating improvement of physician resident teaching skills into the primary care setting and facilitating bidirectional teaching among different professions. TOPC-CoEPCE intends to develop additional resources to facilitate use of the model application in other settings such as the dyad implementation template.
1. Billett SR. Securing intersubjectivity through interprofessional workplace learning experiences. J Interprof Care. 2014;28(3):206-211.
2. Tolsgaard MG, Bjørck S, Rasmussen MB, Gustafsson A, Ringsted C. Improving efficiency of clinical skills training: a randomized trial. J Gen Intern Med. 2013;28(8);1072-1077.
3. Pangaro L. A new vocabulary and other innovations for improving descriptive in-training evaluations. Acad Med. 1999;74(11):1203-1207.
4. Tham KY. Observer-Reporter-Interpreter-Manager-Educator (O-RIME) framework to guide formative assessment of medical students. Ann Acad Med Singapore. 2013;42(11):603-607.
6. Clementz L, Dolansky MA, Lawrence RH, et al. Dyad teams: interprofessional collaboration and learning in ambulatory setting. Poster session presented: 38th Annual Meeting of the Society of General Internal Medicine; April 2015:Toronto, Canada. www.pcori.org/sites/default/files /SGIM-Conference-Program-2015.pdf. Accessed August 29, 2018.
7. Singh M, Clementz L, Dolansky MA, et al. MD-NP learning dyad model: an innovative approach to interprofessional teaching and learning. Workshop presented at: Annual Meeting of the Midwest Society of General Internal Medicine; August 27, 2015: Cleveland, Ohio.
8. Lawrence RH, Dolansky MA, Clementz L, et al. Dyad teams: collaboration and learning in the ambulatory care setting. Poster session presented at: AAMC meeting, Innovations in Academic Medicine; November 7-11, 2014: Chicago, IL.
Background
In 2011, 5 US Department of Veterans Affairs (VA) medical centers were selected by the VA Office of Academic Affiliations (OAA) to establish Centers of Excellence in Primary Care Education (CoEPCE). As part of VA’s New Models of Care initiative, the 5 CoEPCEs are using VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurses (APRNs), undergraduate nursing students, and other health professions trainees (such as pharmacy, social work, psychology, physician assistants) for primary care practice. The CoEPCE sites are developing, implementing, and evaluating curricula to prepare learners from relevant professions to practice in patientcentered, interprofessional team-based primary care settings. Patient aligned care teams (PACTs) that have 2 or more health professions trainees engaged in learning, working, and teaching are known as interprofessional academic PACTs (iAPACTs), which is the preferred model for the VA.
The Cleveland Transforming Outpatient Care (TOPC)-CoEPCE was designed for collaborative learning among nurse practitioner (NP) students and physician residents. Its robust curriculum consists of a dedicated half-day of didactics for all learners, interprofessional quality improvement projects, panel management sessions, and primary care clinical sessions for nursing and physician learners that include the dyad workplace learning model.
In 2015, the OAA lead evaluator observed the TOPC-CoEPCE dyad model process, reviewed background documents, and conducted 10 open-ended interviews with TOPC-CoEPCE staff, participating trainees, faculty, and affiliate leadership. Informants described their involvement, challenges encountered, and benefits of the TOPCCoEPC dyad model to participants, veterans, VA, and affiliates.
Lack of Interprofessional Learning Opportunities
Current health care professional education models typically do not have many workplace learning settings where physician and nursing trainees learn together and provide patient-centered care. Often in a shared clinical environment, trainees may engage in “parallel play,” which can result in physician trainees and NP students learning independently and being ill-prepared to practice effectively together.
Moreover, trainees from different professions have different learning needs. For example, less experienced NP students require greater time, supervision, and evaluation of their patient care skills. On the other hand, senior physician residents, who require less clinical instruction, need to be engaged in ways that provide opportunities to enhance their ambulatory teaching skills. Although enhancement of resident teaching skills occurs in the inpatient hospital setting, there have been limited teaching experiences for residents in a primary care setting where the instruction is traditionally faculty-based. The TOPCCoEPCE dyad model offers an opportunity to simultaneously provide trainees with a true interprofessional experience through advancement of skills in primary care, teamwork, and teaching, while addressing health care needs.
The Dyad Model
In 2011, the OAA directed COEPCE sites to develop innovative curriculum and workplace learning strategies to create more opportunities for physician and NP trainees to work as a team. There is evidence demonstrating that when students develop a shared understanding of each other’s skill set, care procedures, and values, patient care is improved.1 Further, training in pairs can be an effective strategy in education of preclerkship medical students.2 In April 2013, TOPC-CoEPCE staff asked representatives from the Student-Run Clinic at Case Western Reserve University (CWRU) in Cleveland, Ohio, to present their approach to pairing nursing and medical students in clinic under supervision by volunteer faculty. However, formal structure and curricular objectives were lacking. To address diverse TOPCCoEPCE trainee needs and create a team approach to patient care, the staff formalized and developed a workplace curriculum called the dyad model. Specifically, the model pairs 1 NP student with a senior (PGY2 or PGY3) physician resident to care for ambulatory patients as a dyad teaching/learning team. The dyad model has 3 goals: improving clinical performance, learning team dynamics, and improving the physician resident’s teaching skills in an ambulatory setting.
Planning and Implementation
Planning the dyad model took 4 months. Initial conceptualization of the model was discussed at TOPC-CoEPCE infrastructure meetings. Workgroups with representatives from medicine, nursing, evaluation and medical center administration were formed to finalize the model. The workgroups met weekly or biweekly to develop protocols for scheduling, ongoing monitoring and assessment, microteaching session curriculum development, and logistics. A pilot program was initiated for 1 month with 2 dyads to monitor learner progress and improve components, such as adjusting the patient exam start times and curriculum. In maintaining the program, the workgroups continue to meet monthly to check for areas for further improvement and maintain dissemination activities.
Curriculum
The dyad model is a novel opportunity to have trainees from different professions not only collaborate in the care of the same patient at the same time, but also negotiate their respective responsibilities preand postvisit. The experience focuses on interprofessional relationships and open communication. TOPC-CoEPCE used a modified version of the RIME (Reporter-Interpreter-Manager-Educator) model called the O-RIME model (Table 1), which includes an observer (O) phase as the first component for clarification about a beginners’ role.3,4
Four dyad pairs provide collaborative clinical care for veterans during one halfday session per week. The dyad conducts 4 hour-long patient visits per session. To be a dyad participant, the physician residents must be at least a PGY2, and their schedule must align with the NP student clinic schedule. Participation is mandatory for both NP students and physician residents. TOPC staff assemble the pairs.
The dyad model requires knowledge of the clinical and curricular interface and when to block the dyad team members’ schedules for 4 patients instead of 6. Physician residents are in the TOPC-CoEPCE for 12 weeks and then on inpatient for 12 weeks. Depending on the nursing school affiliate, NP student trainees are scheduled for either a 6- or 12-month TOPC-CoEPCE experience. For the 12-month NP students, they are paired with up to 4 internal medicine residents over the course of their dyad participation so they can experience different teaching styles of each resident while developing more varied interprofessional communication skills.
Faculty Roles and Development
The dyad model also seeks to address the paucity of deliberate interprofessional precepting in academic primary care settings. The TOPC-CoEPCE staff decided to use the existing primary care clinic faculty development series bimonthly for 1 hour each. The dyad model team members presented sessions covering foundational material in interprofessional teaching and precepting skills, which prepare faculty to precept for different professions and the dyad teams. It is important for preceptors to develop awareness of learners from different professions and the corresponding educational trajectories, so they can communicate with paired trainees of differing professions and academic levels who may require different levels of discussion.
Resources
By utilizing advanced residents as teachers, faculty were able to increase the number of learners in the clinic without increasing the number preceptors. For example, precepting a student typically requires more preceptor time, especially when we consider that the preceptor must also see the patient. The TOPC-CoEPCE faculty run the microteaching sessions, and an evaluator monitors and evaluates the program. The microteaching sessions were derived from several teaching resources.
Monitoring and Assessment
The Cleveland TOPC administered 2 different surveys developed by the Dyad Model Infrastructure and Evaluation workgroup. A 7-item survey assesses dyad team communication and interprofessional team functioning, and an 8-item survey assesses the teaching/mentoring of the resident as teacher. Both were collected from all participants to evaluate the residents’ and students’ point of view. Surveys are collected in the first and last weeks of the dyad experience. Feedback from participants has been used to make improvements to the program (eg, monitoring how the dyad teams are functioning, coaching individual learners).
Partnerships
In addition to TOPC staff and faculty support and engagement, the initiative has benefited from partnerships with VA clinic staff and with the associated academic affiliates. In particular, the Associate Chief of General Internal Medicine at the Cleveland VA medical center and interim clinic director helped institute changes to the primary care clinic structure. Additionally, buy-in from the clinic nurse manager was needed to make adjustments with staff schedules and clinic resources. To implement the dyad model, the clinic director had to approve reductions in the residents’ clinic loads for the mornings when they participated.
The NP affiliates’ faculty at the schools of nursing are integral partners who assist with student recruitment and participate in the planning and refinement of TOPCCoEPCE components. The Frances Payne Bolton School of Nursing at CWRU and the Breen School of Nursing of Ursuline College in Pepper Pike, Ohio, were involved in the planning stages and continue to receive monthly updates from TOPC-CoEPCE. Similarly, the CWRU School of Medicine and Cleveland Clinic Foundation affiliates contribute on an ongoing basis to the improvement and implementation process.
Discussion
One challenge has been advancing aspects of a nonhierarchical team approach while it is a teacher-student relationship. The dyad model is viewed as an opportunity to recognize nonhierarchical structures and teach negotiation and communication skills as well as increase interprofessional understanding of each other’s education, expertise, and scope of practice.
Another challenge is accommodating the diversity in NP training and clinical expertise. The NP student participants are in either the first or second year of their academic program. This is a challenge since both physician residents and physician faculty preceptors need to assess the NP students’ skills before providing opportunities to build on their skill level. Staff members have learned the value of checking in weekly on this issue.
Factors for Success
VA facility support and TOPC-CoEPCE leadership with the operations/academic partnership remain critical to integrating and sustaining the model into the Cleveland primary care clinic. The expertise of TOPC-CoEPCE dyad model faculty who serve as facilitators has been crucial, as they oversee team development concepts such as developing problem solving and negotiation skills. The workgroups ensured that faculty were skilled in understanding the different types of learners and provided guidance to dyad teams. Another success factor was the continual monitoring of the process and real-time evaluation of the program to adapt the model as needed.
Accomplishments and Benefits
There is evidence that the dyad model is achieving its goals: Trainees are using team skills during and outside formal dyad pairs; NP students report improvements in skill levels and comfort; and physician residents feel the teaching role in the dyad pair is an opportunity for them to improve their practice.
Interprofessional Educational Capacity
The dyad model complements the curriculum components and advances trainee understanding of 4 core domains: shared decision-making (SDM), sustained relationships (SR), interprofessional collaboration (IPC), and performance improvement (PI) (Table 2). The dyad model supports the other CoEPCE interprofessional education activities and is reinforced by these activities. The model is a learning laboratory for studying team dynamics and developing a curriculum that strengthens a team approach to patient-centered care.
Participants’ Knowledge, Attitudes, Skills, and Competencies
As of May 2015, 35 trainees (21 internal medicine physician residents and 14 NP students) have participated in dyads. Because physician residents participate over 2 years and may partner with more than 1 NP student, this has resulted in 27 dyad pairs in this time frame. Findings from an analysis of evaluations suggest that the dyad pair trainees learn from one another, and the model provides a safe space where trainees can practice and increase their confidence.1,6,7 The NP students seem to increase clinical skills quickly—expanding physical exam skills, building a differential diagnosis, and formulating therapeutic plans—and progressing to the Interpreter and Manager levels in the O-RIME model. The physician resident achieves the Educator level.
As of September 2015, the results from the pairs who completed beginning and end evaluations show that the physician residents increased the amount of feedback they provided about performance to the student, and likewise the student NPs also felt they received an increased amount of feedback about performance from the physician resident. In addition, physician residents reported improving the most in the following areas: allowing the student to make commitments in diagnoses and treatment plans and asking the student to provide supporting evidence for their commitment to the diagnoses. NP students reported the largest increases in receiving weekly feedback about their performance from the physician and their ability to listen to the patient.1,6,7
Interprofessional Collaboration
The TOPC-CoEPCE staff observed strengthened dyad pair relationships and mutual respect between the dyad partners. Trainees communicate with each other and work together to provide care of the patient. Second, dyad pair partners are learning about the other profession—their trajectory, their education model, and their differences. The physician resident develops an awareness of the partner NP student’s knowledge and expertise, such as their experience of social and psychological factors to become a more effective teacher, contributing to patient-centered care. The evaluation results illustrate increased ability of trainees to give and receive feedback and the change in roles for providing diagnosis and providing supporting evidence within the TOPCCoEPCE dyad team.6-8
The Future
The model has broad applicability for interprofessional education in the VA since it enhances skills that providers need to work in a PACT/PCMH model. Additionally, the TOPC-CoEPCE dyad model has proven to be an effective interprofessional training experience for its affiliates and may have applicability in other VA/affiliate training programs. The dyad model can be adapted to different trainee types in the ambulatory care setting. The TOPCCoEPCE is piloting a version of the dyad with NP residents (postgraduate) and first-year medical students. Additionally, the TOPCCoEPCE is paving the way for integrating improvement of physician resident teaching skills into the primary care setting and facilitating bidirectional teaching among different professions. TOPC-CoEPCE intends to develop additional resources to facilitate use of the model application in other settings such as the dyad implementation template.
Background
In 2011, 5 US Department of Veterans Affairs (VA) medical centers were selected by the VA Office of Academic Affiliations (OAA) to establish Centers of Excellence in Primary Care Education (CoEPCE). As part of VA’s New Models of Care initiative, the 5 CoEPCEs are using VA primary care settings to develop and test innovative approaches to prepare physician residents and students, advanced practice nurses (APRNs), undergraduate nursing students, and other health professions trainees (such as pharmacy, social work, psychology, physician assistants) for primary care practice. The CoEPCE sites are developing, implementing, and evaluating curricula to prepare learners from relevant professions to practice in patientcentered, interprofessional team-based primary care settings. Patient aligned care teams (PACTs) that have 2 or more health professions trainees engaged in learning, working, and teaching are known as interprofessional academic PACTs (iAPACTs), which is the preferred model for the VA.
The Cleveland Transforming Outpatient Care (TOPC)-CoEPCE was designed for collaborative learning among nurse practitioner (NP) students and physician residents. Its robust curriculum consists of a dedicated half-day of didactics for all learners, interprofessional quality improvement projects, panel management sessions, and primary care clinical sessions for nursing and physician learners that include the dyad workplace learning model.
In 2015, the OAA lead evaluator observed the TOPC-CoEPCE dyad model process, reviewed background documents, and conducted 10 open-ended interviews with TOPC-CoEPCE staff, participating trainees, faculty, and affiliate leadership. Informants described their involvement, challenges encountered, and benefits of the TOPCCoEPC dyad model to participants, veterans, VA, and affiliates.
Lack of Interprofessional Learning Opportunities
Current health care professional education models typically do not have many workplace learning settings where physician and nursing trainees learn together and provide patient-centered care. Often in a shared clinical environment, trainees may engage in “parallel play,” which can result in physician trainees and NP students learning independently and being ill-prepared to practice effectively together.
Moreover, trainees from different professions have different learning needs. For example, less experienced NP students require greater time, supervision, and evaluation of their patient care skills. On the other hand, senior physician residents, who require less clinical instruction, need to be engaged in ways that provide opportunities to enhance their ambulatory teaching skills. Although enhancement of resident teaching skills occurs in the inpatient hospital setting, there have been limited teaching experiences for residents in a primary care setting where the instruction is traditionally faculty-based. The TOPCCoEPCE dyad model offers an opportunity to simultaneously provide trainees with a true interprofessional experience through advancement of skills in primary care, teamwork, and teaching, while addressing health care needs.
The Dyad Model
In 2011, the OAA directed COEPCE sites to develop innovative curriculum and workplace learning strategies to create more opportunities for physician and NP trainees to work as a team. There is evidence demonstrating that when students develop a shared understanding of each other’s skill set, care procedures, and values, patient care is improved.1 Further, training in pairs can be an effective strategy in education of preclerkship medical students.2 In April 2013, TOPC-CoEPCE staff asked representatives from the Student-Run Clinic at Case Western Reserve University (CWRU) in Cleveland, Ohio, to present their approach to pairing nursing and medical students in clinic under supervision by volunteer faculty. However, formal structure and curricular objectives were lacking. To address diverse TOPCCoEPCE trainee needs and create a team approach to patient care, the staff formalized and developed a workplace curriculum called the dyad model. Specifically, the model pairs 1 NP student with a senior (PGY2 or PGY3) physician resident to care for ambulatory patients as a dyad teaching/learning team. The dyad model has 3 goals: improving clinical performance, learning team dynamics, and improving the physician resident’s teaching skills in an ambulatory setting.
Planning and Implementation
Planning the dyad model took 4 months. Initial conceptualization of the model was discussed at TOPC-CoEPCE infrastructure meetings. Workgroups with representatives from medicine, nursing, evaluation and medical center administration were formed to finalize the model. The workgroups met weekly or biweekly to develop protocols for scheduling, ongoing monitoring and assessment, microteaching session curriculum development, and logistics. A pilot program was initiated for 1 month with 2 dyads to monitor learner progress and improve components, such as adjusting the patient exam start times and curriculum. In maintaining the program, the workgroups continue to meet monthly to check for areas for further improvement and maintain dissemination activities.
Curriculum
The dyad model is a novel opportunity to have trainees from different professions not only collaborate in the care of the same patient at the same time, but also negotiate their respective responsibilities preand postvisit. The experience focuses on interprofessional relationships and open communication. TOPC-CoEPCE used a modified version of the RIME (Reporter-Interpreter-Manager-Educator) model called the O-RIME model (Table 1), which includes an observer (O) phase as the first component for clarification about a beginners’ role.3,4
Four dyad pairs provide collaborative clinical care for veterans during one halfday session per week. The dyad conducts 4 hour-long patient visits per session. To be a dyad participant, the physician residents must be at least a PGY2, and their schedule must align with the NP student clinic schedule. Participation is mandatory for both NP students and physician residents. TOPC staff assemble the pairs.
The dyad model requires knowledge of the clinical and curricular interface and when to block the dyad team members’ schedules for 4 patients instead of 6. Physician residents are in the TOPC-CoEPCE for 12 weeks and then on inpatient for 12 weeks. Depending on the nursing school affiliate, NP student trainees are scheduled for either a 6- or 12-month TOPC-CoEPCE experience. For the 12-month NP students, they are paired with up to 4 internal medicine residents over the course of their dyad participation so they can experience different teaching styles of each resident while developing more varied interprofessional communication skills.
Faculty Roles and Development
The dyad model also seeks to address the paucity of deliberate interprofessional precepting in academic primary care settings. The TOPC-CoEPCE staff decided to use the existing primary care clinic faculty development series bimonthly for 1 hour each. The dyad model team members presented sessions covering foundational material in interprofessional teaching and precepting skills, which prepare faculty to precept for different professions and the dyad teams. It is important for preceptors to develop awareness of learners from different professions and the corresponding educational trajectories, so they can communicate with paired trainees of differing professions and academic levels who may require different levels of discussion.
Resources
By utilizing advanced residents as teachers, faculty were able to increase the number of learners in the clinic without increasing the number preceptors. For example, precepting a student typically requires more preceptor time, especially when we consider that the preceptor must also see the patient. The TOPC-CoEPCE faculty run the microteaching sessions, and an evaluator monitors and evaluates the program. The microteaching sessions were derived from several teaching resources.
Monitoring and Assessment
The Cleveland TOPC administered 2 different surveys developed by the Dyad Model Infrastructure and Evaluation workgroup. A 7-item survey assesses dyad team communication and interprofessional team functioning, and an 8-item survey assesses the teaching/mentoring of the resident as teacher. Both were collected from all participants to evaluate the residents’ and students’ point of view. Surveys are collected in the first and last weeks of the dyad experience. Feedback from participants has been used to make improvements to the program (eg, monitoring how the dyad teams are functioning, coaching individual learners).
Partnerships
In addition to TOPC staff and faculty support and engagement, the initiative has benefited from partnerships with VA clinic staff and with the associated academic affiliates. In particular, the Associate Chief of General Internal Medicine at the Cleveland VA medical center and interim clinic director helped institute changes to the primary care clinic structure. Additionally, buy-in from the clinic nurse manager was needed to make adjustments with staff schedules and clinic resources. To implement the dyad model, the clinic director had to approve reductions in the residents’ clinic loads for the mornings when they participated.
The NP affiliates’ faculty at the schools of nursing are integral partners who assist with student recruitment and participate in the planning and refinement of TOPCCoEPCE components. The Frances Payne Bolton School of Nursing at CWRU and the Breen School of Nursing of Ursuline College in Pepper Pike, Ohio, were involved in the planning stages and continue to receive monthly updates from TOPC-CoEPCE. Similarly, the CWRU School of Medicine and Cleveland Clinic Foundation affiliates contribute on an ongoing basis to the improvement and implementation process.
Discussion
One challenge has been advancing aspects of a nonhierarchical team approach while it is a teacher-student relationship. The dyad model is viewed as an opportunity to recognize nonhierarchical structures and teach negotiation and communication skills as well as increase interprofessional understanding of each other’s education, expertise, and scope of practice.
Another challenge is accommodating the diversity in NP training and clinical expertise. The NP student participants are in either the first or second year of their academic program. This is a challenge since both physician residents and physician faculty preceptors need to assess the NP students’ skills before providing opportunities to build on their skill level. Staff members have learned the value of checking in weekly on this issue.
Factors for Success
VA facility support and TOPC-CoEPCE leadership with the operations/academic partnership remain critical to integrating and sustaining the model into the Cleveland primary care clinic. The expertise of TOPC-CoEPCE dyad model faculty who serve as facilitators has been crucial, as they oversee team development concepts such as developing problem solving and negotiation skills. The workgroups ensured that faculty were skilled in understanding the different types of learners and provided guidance to dyad teams. Another success factor was the continual monitoring of the process and real-time evaluation of the program to adapt the model as needed.
Accomplishments and Benefits
There is evidence that the dyad model is achieving its goals: Trainees are using team skills during and outside formal dyad pairs; NP students report improvements in skill levels and comfort; and physician residents feel the teaching role in the dyad pair is an opportunity for them to improve their practice.
Interprofessional Educational Capacity
The dyad model complements the curriculum components and advances trainee understanding of 4 core domains: shared decision-making (SDM), sustained relationships (SR), interprofessional collaboration (IPC), and performance improvement (PI) (Table 2). The dyad model supports the other CoEPCE interprofessional education activities and is reinforced by these activities. The model is a learning laboratory for studying team dynamics and developing a curriculum that strengthens a team approach to patient-centered care.
Participants’ Knowledge, Attitudes, Skills, and Competencies
As of May 2015, 35 trainees (21 internal medicine physician residents and 14 NP students) have participated in dyads. Because physician residents participate over 2 years and may partner with more than 1 NP student, this has resulted in 27 dyad pairs in this time frame. Findings from an analysis of evaluations suggest that the dyad pair trainees learn from one another, and the model provides a safe space where trainees can practice and increase their confidence.1,6,7 The NP students seem to increase clinical skills quickly—expanding physical exam skills, building a differential diagnosis, and formulating therapeutic plans—and progressing to the Interpreter and Manager levels in the O-RIME model. The physician resident achieves the Educator level.
As of September 2015, the results from the pairs who completed beginning and end evaluations show that the physician residents increased the amount of feedback they provided about performance to the student, and likewise the student NPs also felt they received an increased amount of feedback about performance from the physician resident. In addition, physician residents reported improving the most in the following areas: allowing the student to make commitments in diagnoses and treatment plans and asking the student to provide supporting evidence for their commitment to the diagnoses. NP students reported the largest increases in receiving weekly feedback about their performance from the physician and their ability to listen to the patient.1,6,7
Interprofessional Collaboration
The TOPC-CoEPCE staff observed strengthened dyad pair relationships and mutual respect between the dyad partners. Trainees communicate with each other and work together to provide care of the patient. Second, dyad pair partners are learning about the other profession—their trajectory, their education model, and their differences. The physician resident develops an awareness of the partner NP student’s knowledge and expertise, such as their experience of social and psychological factors to become a more effective teacher, contributing to patient-centered care. The evaluation results illustrate increased ability of trainees to give and receive feedback and the change in roles for providing diagnosis and providing supporting evidence within the TOPCCoEPCE dyad team.6-8
The Future
The model has broad applicability for interprofessional education in the VA since it enhances skills that providers need to work in a PACT/PCMH model. Additionally, the TOPC-CoEPCE dyad model has proven to be an effective interprofessional training experience for its affiliates and may have applicability in other VA/affiliate training programs. The dyad model can be adapted to different trainee types in the ambulatory care setting. The TOPCCoEPCE is piloting a version of the dyad with NP residents (postgraduate) and first-year medical students. Additionally, the TOPCCoEPCE is paving the way for integrating improvement of physician resident teaching skills into the primary care setting and facilitating bidirectional teaching among different professions. TOPC-CoEPCE intends to develop additional resources to facilitate use of the model application in other settings such as the dyad implementation template.
1. Billett SR. Securing intersubjectivity through interprofessional workplace learning experiences. J Interprof Care. 2014;28(3):206-211.
2. Tolsgaard MG, Bjørck S, Rasmussen MB, Gustafsson A, Ringsted C. Improving efficiency of clinical skills training: a randomized trial. J Gen Intern Med. 2013;28(8);1072-1077.
3. Pangaro L. A new vocabulary and other innovations for improving descriptive in-training evaluations. Acad Med. 1999;74(11):1203-1207.
4. Tham KY. Observer-Reporter-Interpreter-Manager-Educator (O-RIME) framework to guide formative assessment of medical students. Ann Acad Med Singapore. 2013;42(11):603-607.
6. Clementz L, Dolansky MA, Lawrence RH, et al. Dyad teams: interprofessional collaboration and learning in ambulatory setting. Poster session presented: 38th Annual Meeting of the Society of General Internal Medicine; April 2015:Toronto, Canada. www.pcori.org/sites/default/files /SGIM-Conference-Program-2015.pdf. Accessed August 29, 2018.
7. Singh M, Clementz L, Dolansky MA, et al. MD-NP learning dyad model: an innovative approach to interprofessional teaching and learning. Workshop presented at: Annual Meeting of the Midwest Society of General Internal Medicine; August 27, 2015: Cleveland, Ohio.
8. Lawrence RH, Dolansky MA, Clementz L, et al. Dyad teams: collaboration and learning in the ambulatory care setting. Poster session presented at: AAMC meeting, Innovations in Academic Medicine; November 7-11, 2014: Chicago, IL.
1. Billett SR. Securing intersubjectivity through interprofessional workplace learning experiences. J Interprof Care. 2014;28(3):206-211.
2. Tolsgaard MG, Bjørck S, Rasmussen MB, Gustafsson A, Ringsted C. Improving efficiency of clinical skills training: a randomized trial. J Gen Intern Med. 2013;28(8);1072-1077.
3. Pangaro L. A new vocabulary and other innovations for improving descriptive in-training evaluations. Acad Med. 1999;74(11):1203-1207.
4. Tham KY. Observer-Reporter-Interpreter-Manager-Educator (O-RIME) framework to guide formative assessment of medical students. Ann Acad Med Singapore. 2013;42(11):603-607.
6. Clementz L, Dolansky MA, Lawrence RH, et al. Dyad teams: interprofessional collaboration and learning in ambulatory setting. Poster session presented: 38th Annual Meeting of the Society of General Internal Medicine; April 2015:Toronto, Canada. www.pcori.org/sites/default/files /SGIM-Conference-Program-2015.pdf. Accessed August 29, 2018.
7. Singh M, Clementz L, Dolansky MA, et al. MD-NP learning dyad model: an innovative approach to interprofessional teaching and learning. Workshop presented at: Annual Meeting of the Midwest Society of General Internal Medicine; August 27, 2015: Cleveland, Ohio.
8. Lawrence RH, Dolansky MA, Clementz L, et al. Dyad teams: collaboration and learning in the ambulatory care setting. Poster session presented at: AAMC meeting, Innovations in Academic Medicine; November 7-11, 2014: Chicago, IL.
Advances in CAR T-Cell Therapies (FULL)
Gene therapies, especially chimeric antigen receptor (CAR) T-cell therapies, experienced significant growth in 2017. The CAR T-cell therapies are among the most clinically important of the adoptive cell transfer therapies. In August, the FDA approved tisagenlecleucel for patients aged < 26 years with acute or relapsed lymphoblastic leukemia (ALL). In October, the FDA approved axicabtagene ciloleucel for treatment of adult patients nonresponsive to, or relapsed from treatment of, certain types of large B-cell lymphoma. And in November, the FDA granted breakthrough therapy designation to Celgene and Bluebird Bio for the bb2121 anti-B-cell maturation antigen (BCMA) CAR T-cell therapy for relapsed and refractory multiple myeloma (MM).
Chimeric antigen receptor T-cells circumvent the human major histocompatibility complex that T-cell receptors must navigate, shifting cell-based therapy away from identification of existing cells and toward creating T-cell products through genetic engineering. This broadens the potential for CAR T-cell applications and allows for rapid manufacture of tumor and patient-specific agents.1 Both Novartis’ Kymriah and Kite Pharma’s Yescarta are derived from investigations into anti-CD19 CAR therapy, which has been the most heavily researched of the CARs due to its links with B-cell malignancies, expression in most tumor cells, and absence from vital tissues.2 Studied in relation to a number of cancers, CD19 has not shown much success in either MM or solid tumor cancers.
Targeting the right antigen for myeloma is complicated: first because common MM antigens—CD38, CD56, CD138—also are expressed on essential normal cells, and second, because myeloma cells are synonymous with heterogeneity. The FDA based its designation of bb2121, or BCMA CAR T-cell therapy, on preliminary data from an ongoing phase 1 CRB-401 trial that, as of December 2017, concluded that 94% of 21 patients with MM treated with the highest doses showed complete or partial remissions and high rates of progression-free survival.3 The trial also showed that cytokine-release toxicity (CRS), although severe in some patients, was generally reversible and short lived.
Multiple myeloma BCMA is only one of several CAR targets under consideration for MM treatment; others include CD138, CD38, signaling lymphocyte-activating molecule 7, and κ light chain. However, B-cell maturation antigen is attractive to researchers because BCMA–specific CAR-expressing T lymphocytes recognize and kill B-cell maturation antigen–expressing tumor cells. Also, BCMA acts as a receptor for both a proliferation-inducing ligand and as a B-cell–activating factor and is a member of the tumor necrosis factor receptor superfamily, playing a key role in plasma cell survival. B-cell maturation antigen is expressed in most, if not all, myeloma cells but not in epithelial tissues. Finally, integration of CAR-Ts with other myeloma therapies is an important area of future research.4
Most of the 23 trials looking at CAR T-cell therapy for MM are in the U.S. or China, and several deal jointly with MM, leukemia, and lymphoma. The THINK (THerapeutic Immunotherapy with NKR-2) multinational open-label phase 1 study stands alone in assessing the safety and clinical activity of multiple administrations of autologous NKR-2 cells in 7 refractory cancers, including 5 solid tumors (colorectal, ovarian, bladder, triple-negative breast and pancreatic cancers) and 2 hematologic tumors (acute myeloid leukemia and MM). Unlike traditional CAR T-cell therapy, which targets only 1 tumor antigen, NK cell receptors enable a single receptor to recognize multiple tumor antigens.
Despite challenges of toxicity, costs, and restricted availability for immunotherapies, CAR T-cell therapies seem to offer great possibilities of groundbreaking treatments and possible cures for formerly hard to treat cancers, including MM.5
Click here to read the digital edition.
1. Almåsbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602.
2. Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125(9):3392-3400.
3. C
4. Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017;130(24):2594-2602.
5. Vallet S, Pecherstorfer M, Podar K. Adoptive cell therapy in multiple myeloma. Expert Opin Biol Ther. 2017;17(12):1511-1522.
Gene therapies, especially chimeric antigen receptor (CAR) T-cell therapies, experienced significant growth in 2017. The CAR T-cell therapies are among the most clinically important of the adoptive cell transfer therapies. In August, the FDA approved tisagenlecleucel for patients aged < 26 years with acute or relapsed lymphoblastic leukemia (ALL). In October, the FDA approved axicabtagene ciloleucel for treatment of adult patients nonresponsive to, or relapsed from treatment of, certain types of large B-cell lymphoma. And in November, the FDA granted breakthrough therapy designation to Celgene and Bluebird Bio for the bb2121 anti-B-cell maturation antigen (BCMA) CAR T-cell therapy for relapsed and refractory multiple myeloma (MM).
Chimeric antigen receptor T-cells circumvent the human major histocompatibility complex that T-cell receptors must navigate, shifting cell-based therapy away from identification of existing cells and toward creating T-cell products through genetic engineering. This broadens the potential for CAR T-cell applications and allows for rapid manufacture of tumor and patient-specific agents.1 Both Novartis’ Kymriah and Kite Pharma’s Yescarta are derived from investigations into anti-CD19 CAR therapy, which has been the most heavily researched of the CARs due to its links with B-cell malignancies, expression in most tumor cells, and absence from vital tissues.2 Studied in relation to a number of cancers, CD19 has not shown much success in either MM or solid tumor cancers.
Targeting the right antigen for myeloma is complicated: first because common MM antigens—CD38, CD56, CD138—also are expressed on essential normal cells, and second, because myeloma cells are synonymous with heterogeneity. The FDA based its designation of bb2121, or BCMA CAR T-cell therapy, on preliminary data from an ongoing phase 1 CRB-401 trial that, as of December 2017, concluded that 94% of 21 patients with MM treated with the highest doses showed complete or partial remissions and high rates of progression-free survival.3 The trial also showed that cytokine-release toxicity (CRS), although severe in some patients, was generally reversible and short lived.
Multiple myeloma BCMA is only one of several CAR targets under consideration for MM treatment; others include CD138, CD38, signaling lymphocyte-activating molecule 7, and κ light chain. However, B-cell maturation antigen is attractive to researchers because BCMA–specific CAR-expressing T lymphocytes recognize and kill B-cell maturation antigen–expressing tumor cells. Also, BCMA acts as a receptor for both a proliferation-inducing ligand and as a B-cell–activating factor and is a member of the tumor necrosis factor receptor superfamily, playing a key role in plasma cell survival. B-cell maturation antigen is expressed in most, if not all, myeloma cells but not in epithelial tissues. Finally, integration of CAR-Ts with other myeloma therapies is an important area of future research.4
Most of the 23 trials looking at CAR T-cell therapy for MM are in the U.S. or China, and several deal jointly with MM, leukemia, and lymphoma. The THINK (THerapeutic Immunotherapy with NKR-2) multinational open-label phase 1 study stands alone in assessing the safety and clinical activity of multiple administrations of autologous NKR-2 cells in 7 refractory cancers, including 5 solid tumors (colorectal, ovarian, bladder, triple-negative breast and pancreatic cancers) and 2 hematologic tumors (acute myeloid leukemia and MM). Unlike traditional CAR T-cell therapy, which targets only 1 tumor antigen, NK cell receptors enable a single receptor to recognize multiple tumor antigens.
Despite challenges of toxicity, costs, and restricted availability for immunotherapies, CAR T-cell therapies seem to offer great possibilities of groundbreaking treatments and possible cures for formerly hard to treat cancers, including MM.5
Click here to read the digital edition.
Gene therapies, especially chimeric antigen receptor (CAR) T-cell therapies, experienced significant growth in 2017. The CAR T-cell therapies are among the most clinically important of the adoptive cell transfer therapies. In August, the FDA approved tisagenlecleucel for patients aged < 26 years with acute or relapsed lymphoblastic leukemia (ALL). In October, the FDA approved axicabtagene ciloleucel for treatment of adult patients nonresponsive to, or relapsed from treatment of, certain types of large B-cell lymphoma. And in November, the FDA granted breakthrough therapy designation to Celgene and Bluebird Bio for the bb2121 anti-B-cell maturation antigen (BCMA) CAR T-cell therapy for relapsed and refractory multiple myeloma (MM).
Chimeric antigen receptor T-cells circumvent the human major histocompatibility complex that T-cell receptors must navigate, shifting cell-based therapy away from identification of existing cells and toward creating T-cell products through genetic engineering. This broadens the potential for CAR T-cell applications and allows for rapid manufacture of tumor and patient-specific agents.1 Both Novartis’ Kymriah and Kite Pharma’s Yescarta are derived from investigations into anti-CD19 CAR therapy, which has been the most heavily researched of the CARs due to its links with B-cell malignancies, expression in most tumor cells, and absence from vital tissues.2 Studied in relation to a number of cancers, CD19 has not shown much success in either MM or solid tumor cancers.
Targeting the right antigen for myeloma is complicated: first because common MM antigens—CD38, CD56, CD138—also are expressed on essential normal cells, and second, because myeloma cells are synonymous with heterogeneity. The FDA based its designation of bb2121, or BCMA CAR T-cell therapy, on preliminary data from an ongoing phase 1 CRB-401 trial that, as of December 2017, concluded that 94% of 21 patients with MM treated with the highest doses showed complete or partial remissions and high rates of progression-free survival.3 The trial also showed that cytokine-release toxicity (CRS), although severe in some patients, was generally reversible and short lived.
Multiple myeloma BCMA is only one of several CAR targets under consideration for MM treatment; others include CD138, CD38, signaling lymphocyte-activating molecule 7, and κ light chain. However, B-cell maturation antigen is attractive to researchers because BCMA–specific CAR-expressing T lymphocytes recognize and kill B-cell maturation antigen–expressing tumor cells. Also, BCMA acts as a receptor for both a proliferation-inducing ligand and as a B-cell–activating factor and is a member of the tumor necrosis factor receptor superfamily, playing a key role in plasma cell survival. B-cell maturation antigen is expressed in most, if not all, myeloma cells but not in epithelial tissues. Finally, integration of CAR-Ts with other myeloma therapies is an important area of future research.4
Most of the 23 trials looking at CAR T-cell therapy for MM are in the U.S. or China, and several deal jointly with MM, leukemia, and lymphoma. The THINK (THerapeutic Immunotherapy with NKR-2) multinational open-label phase 1 study stands alone in assessing the safety and clinical activity of multiple administrations of autologous NKR-2 cells in 7 refractory cancers, including 5 solid tumors (colorectal, ovarian, bladder, triple-negative breast and pancreatic cancers) and 2 hematologic tumors (acute myeloid leukemia and MM). Unlike traditional CAR T-cell therapy, which targets only 1 tumor antigen, NK cell receptors enable a single receptor to recognize multiple tumor antigens.
Despite challenges of toxicity, costs, and restricted availability for immunotherapies, CAR T-cell therapies seem to offer great possibilities of groundbreaking treatments and possible cures for formerly hard to treat cancers, including MM.5
Click here to read the digital edition.
1. Almåsbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602.
2. Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125(9):3392-3400.
3. C
4. Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017;130(24):2594-2602.
5. Vallet S, Pecherstorfer M, Podar K. Adoptive cell therapy in multiple myeloma. Expert Opin Biol Ther. 2017;17(12):1511-1522.
1. Almåsbak H, Aarvak T, Vemuri MC. CAR T cell therapy: a game changer in cancer treatment. J Immunol Res. 2016;2016:5474602.
2. Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125(9):3392-3400.
3. C
4. Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017;130(24):2594-2602.
5. Vallet S, Pecherstorfer M, Podar K. Adoptive cell therapy in multiple myeloma. Expert Opin Biol Ther. 2017;17(12):1511-1522.
Barriers and Facilitators to the Use of Genomic-Based Targeted Therapy in the VA: Qualitative Findings(FULL)
Lung cancer is the most frequent cause of cancer-related mortality worldwide.1 The most prevalent type of lung cancer is non-small cell lung cancer (NSCLC), which comprises about 85% of lung cancer cases.2 As there are no cost-effective approaches to screening for lung cancer, most lung cancers are identified at an advanced stage (stage IIIB or IV).
New approaches to managing advanced lung cancer have emerged in recent years, including drugs designed to target specific genetic mutations in some tumors.3 The National Comprehensive Cancer Network (NCCN) recommends erlotinib, a receptor tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) for first-line treatment of advanced NSCLC with EGFR mutation.4 Crizotinib is recommended to treat cancers that test positive for the anaplastic lymphoma kinase (ALK) mutation.4 Utilization of targeting agents has been found to extend the survival times for patients with the specified mutations.5 Both erlotinib and crizotinib are available at the VHA.
Previous research showed that VHA providers expressed overall favorable attitudes about genomic medicine.6 Providers perceived genomic medicine to have an important and possibly transformative role in medicine. Barriers to utilization of genomic medicine involved concerns about coordination of care, changes in workload, and increased length of patient visits. In addition to these system-level barriers, many providers had concerns about the proficiency of VHA-based practitioners to appropriately use genomic medicine.
Previous research has evaluated utilization of genomic testing and genomic-based targeted therapy (GBTT) in VA and community settings.5-8 It is unclear whether VHA-based providers are following clinical guidelines regarding genomic testing and utilization of GBTT.4 The authors set out to identify factors that impede and encourage guideline-consistent care in the management of NSCLC at the VHA. The authors specifically sought information about oncologists’ perceptions and experiences with EGFR and ALK mutation testing in patients with advanced NSCLC, as well as use of erlotinib and crizotinib in treating such patients.
Methods
This study was approved by the institutional review boards at Michael E. DeBakey VAMC in Houston, Texas and Baylor College of Medicine. In-depth qualitative interviews were conducted with VHA oncologists to examine their reported barriers and facilitators to mutation testing and prescribing of genomic-based treatment in patients with advanced NSCLC.
The sample of participants was recruited from a list of VHA medical oncologists, compiled by the study project coordinator. Investigators stratified the list by American College of Surgeons Commission on Cancer (CoC) accreditation status (yes/no) and used a stratified purposive sampling technique to recruit participants from CoC-accredited facilities and nonaccredited facilities. Recruitment and data collection occurred between March 2015 and February 2016. Oncologists were considered for inclusion if they (1) were specialists in oncology; (2) practiced at the VHA during the time of recruitment; and (3) had experience treating lung cancer at a VHA facility. During recruitment, potential participants were told that the investigators were interested in learning about oncologists’ experiences and decisions about using GBTT to treat advanced lung cancer in the VHA. Participants were scheduled for telephone-based interviews, and verbal consent was obtained prior to all interviews. Interviews ranged from 19 to 90 minutes (average, 40 min).
Recruitment was stopped at the point of thematic saturation, defined a priori as the point when 2 independent coders agreed that 3 consecutive transcripts for a given interview category (see below) rendered no new thematic concepts.9,10 Consistent with the theoretical framework developed by Cabana and colleagues, interviews were designed to elicit information about oncologists’ knowledge, attitudes, intent to use GBTT, and perceived facilitators and barriers to using GBTT in the VHA.11 Additional findings are presented elsewhere.12 The interview guide was pilot tested and revised prior to initiating data collection. All interviews were recorded, transcribed, and analyzed for content.
Analysis
Data were analyzed using framework analysis methodology, which allows for the inclusion of existing concepts as well as emergent themes within an established theoretical framework.13 Two independent coders with expertise in framework analysis independently created codes and indexed the data using Atlas.ti 6.2 (Scientific Software Development, Berlin, Germany). Disagreements about coding decisions were resolved through group consensus. Coding centered on 2 themes:
- Barriers and facilitators to mutation testing. This includes system or facility factors and testing weaknesses that act as barriers to ordering mutation testing, system or facility factors that facilitate ordering mutation testing, and oncologists’ suggestions for ways to encourage more testing in the VHA.
- Barriers and facilitators to prescribing GBTT. This includes system or facility factors that act as barriers to prescribing GBTT, system or facility factors that facilitate prescribing GBTT, and oncologists’ suggestions for ways to encourage more prescribing of GBTT in the VHA.
Thirty medical oncologists were interviewed. Participant demographics are presented in the Table.
Barriers to testing
The 2 most commonly cited barriers to ordering mutation testing can be considered weaknesses in the testing process: lack of tissue and wait time for results. Almost all providers identified lack of tissue as a barrier to ordering a mutation test.
Another frequently cited testing weakness involved the wait time for results. Because the mutation analysis is not conducted in the VHA facility, providers often must wait 2 to 4 weeks to receive results. This can present a problem because some providers do not want to wait for the results before recommending a course of treatment.
Several providers cited system and facility factors as barriers to mutation testing. The most common of these involves the ordering process. Oncology providers often remarked that ordering the mutation test is cumbersome or inconvenient because there is no ordering mechanism in the Computerized Patient Record System (CPRS). Many different approaches for ordering a mutation test exist, including e-mailing the pathology department, calling to place the order, or requesting the test in person. As providers can order many, if not most, other tests via CPRS, it is clear that this presents an inconvenient exception.
Budgetary constraints were another frequently cited system or facility-level barrier. Providers sometimes were unable to access the test due to the cost
Finally, several providers noted that in some cases patients did not wish to undergo a biopsy. Thus, patient preference can act as a barrier to mutation testing. Some patients wish to forgo treatment, which eliminates the need for a mutation test. Other patients believe that due to their smoking history, they are unlikely to have an ALK or EGFR mutation and instead immediately opt for chemotherapy. Only a small minority of participants identified no barriers to mutation testing.
Facilitators for Testing
Many providers complimented the availability of the mutation test. Interestingly, while some providers mentioned that lack of CPRS ordering was a barrier to testing, several also listed access to a CPRS order as a facilitator. These providers commented that ordering a test was streamlined and easy, given the mechanism in CPRS. Some VHA facilities offer CPRS order capabilities, and others do not. Other oncologists commented more generally on the cooperativeness of the pathology department in ordering mutation tests. It seems that facilities may use different ordering procedures, but in most of these facilities, a high degree of cooperation exists between departments to send out for tests that are requested.
Providers offered many ideas for ways to improve mutation testing or to facilitate the testing. By far, the most commonly cited way to improve the testing process was to make mutation testing reflexive for metastatic nonsquamous NSCLC. Some acknowledged that to achieve this would require a change to the budgeting process such that the test would not drain the pathology department’s budget. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address several of the barriers that were identified in this study. Other providers recommended standardizing the ordering procedure and location of results. Specifically, providers recommended creating a button in CPRS for ordering and always reporting the results in the same place in CPRS.
Barriers to GBTT Prescribing
The clear majority of providers identified no barriers to prescribing GBTTs. A few mentioned that they were required to submit a nonformulary consult. A representative quote described this as “more out of a formality, and the pharmacist basically is there with me and he approves it on the spot and provides the prescription on the day, right when I’m seeing the patient.” Only a very small minority of providers identified medication cost as a barrier, but even those respondents did not indicate that cost prevented them from offering GBTTs to their patients. Rather, cost consciousness simply made them more mindful and judicious when making decisions about prescribing GBTTs.
Facilitators to GBTT Prescribing
Several providers listed availability of the costly medication in the VHA as a facilitator to prescribing. Veterans can obtain GBTTs with little to no insurance cost or copayment, which is not always the case outside the VHA.
One recommendation for further facilitating prescribing of GBTTs involved eliminating the preauthorization requirement, particularly in first-line use for patients testing positive for ALK or EGFR mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
Discussion
Although in some cases, testing weaknesses (lack of tissue, wait time to receive results) can interrupt a treatment trajectory, many of the barriers identified in this study are modifiable. Overwhelmingly, oncologists recommended making mutation testing reflexive for metastatic nonsquamous NSCLC. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address issues related to variable utilization of genomic testing in VHA.12 Additionally, in response to system and facility barriers to mutation testing, other providers recommended standardizing the ordering procedure and location of results. Utilization of GBTT can be facilitated by eliminating the preauthorization requirement, particularly in first-line use for patients with positive mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
This study extends previous research that identified underuse of genomic testing in community-based practices. The authors sought to interview a broad sample of providers from various facilities (small, large, CoC accredited, nonaccredited) to understand the range of conditions faced by VA providers. Some providers face more barriers than do others, whereas some face few or no barriers. This wide range of experiences can help to better understand the factors that facilitate guideline-adherent care.
Limitations
The authors recognize that availability of resources and testing and prescribing practices are constantly evolving and perhaps have improved since the data were collected. Thus, the age of the study data might be a limitation to the study. Like most qualitative studies, these findings are limited in their generalizability beyond the study population. Additionally, the authors were limited to recruiting oncologists with reliable contact information listed in the VHA directory. Although this could have introduced some degree of sampling bias, the authors are confident that the sample sufficiently represents the population of VHA-based medical oncologists who treat lung cancer. Despite these limitations, these findings provide novel perspectives on barriers and facilitators to genomic testing GBTT prescribing in the VHA. The authors identify modifiable barriers to testing and prescribing that can be addressed to improve and standardize care of advanced lung cancer in the VHA.
Conclusion
Efforts should be made to address modifiable barriers to mutation testing and guideline-consistent prescribing of GBTT in the VA setting. Implementation of specific practices like reflexive testing for all metastatic nonsquamous NSCLC, standardization of the mutation test ordering procedure, standardization of results reporting, and elimination of the preauthorization to prescribe GBTT could impact the utilization of GBTT in VHA.
Click here to read the digital edition.
. , , , Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. What is non-small cell lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Updated May 16, 2016. Accessed January 19, 2018.
3. , , . New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1097-1104.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). non-small cell lung cancer 2. 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated December 19, 2017. Accessed Jan
5. Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967.
6. Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Per Med. 2010;7(5):485-494.
7. Lynch JA, Berse B, Dotson D, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017; 19(8):890-899.
8. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer, 2017;18(6):651-659.
9. Morse JM. The significance of saturation. Qual Health Res.1995;5(2):147-149.
10. Aita VA, McIlvain HE. An armchair adventure in case study research. In: Crabtree BF, Miller WF, eds. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999:253-268.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
12. Arney JB, Helm A, Crook T, Braun U, Chen GJ, Hayes TG. Utilization of genomic testing in advanced non-small cell lung cancer among oncologists in the Veterans Health Administration. Lung Cancer, 2018;116:25-29.
13. Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, eds. Analyzing Qualitative Data. New York, NY: Routledge; 1994:173-194.
Lung cancer is the most frequent cause of cancer-related mortality worldwide.1 The most prevalent type of lung cancer is non-small cell lung cancer (NSCLC), which comprises about 85% of lung cancer cases.2 As there are no cost-effective approaches to screening for lung cancer, most lung cancers are identified at an advanced stage (stage IIIB or IV).
New approaches to managing advanced lung cancer have emerged in recent years, including drugs designed to target specific genetic mutations in some tumors.3 The National Comprehensive Cancer Network (NCCN) recommends erlotinib, a receptor tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) for first-line treatment of advanced NSCLC with EGFR mutation.4 Crizotinib is recommended to treat cancers that test positive for the anaplastic lymphoma kinase (ALK) mutation.4 Utilization of targeting agents has been found to extend the survival times for patients with the specified mutations.5 Both erlotinib and crizotinib are available at the VHA.
Previous research showed that VHA providers expressed overall favorable attitudes about genomic medicine.6 Providers perceived genomic medicine to have an important and possibly transformative role in medicine. Barriers to utilization of genomic medicine involved concerns about coordination of care, changes in workload, and increased length of patient visits. In addition to these system-level barriers, many providers had concerns about the proficiency of VHA-based practitioners to appropriately use genomic medicine.
Previous research has evaluated utilization of genomic testing and genomic-based targeted therapy (GBTT) in VA and community settings.5-8 It is unclear whether VHA-based providers are following clinical guidelines regarding genomic testing and utilization of GBTT.4 The authors set out to identify factors that impede and encourage guideline-consistent care in the management of NSCLC at the VHA. The authors specifically sought information about oncologists’ perceptions and experiences with EGFR and ALK mutation testing in patients with advanced NSCLC, as well as use of erlotinib and crizotinib in treating such patients.
Methods
This study was approved by the institutional review boards at Michael E. DeBakey VAMC in Houston, Texas and Baylor College of Medicine. In-depth qualitative interviews were conducted with VHA oncologists to examine their reported barriers and facilitators to mutation testing and prescribing of genomic-based treatment in patients with advanced NSCLC.
The sample of participants was recruited from a list of VHA medical oncologists, compiled by the study project coordinator. Investigators stratified the list by American College of Surgeons Commission on Cancer (CoC) accreditation status (yes/no) and used a stratified purposive sampling technique to recruit participants from CoC-accredited facilities and nonaccredited facilities. Recruitment and data collection occurred between March 2015 and February 2016. Oncologists were considered for inclusion if they (1) were specialists in oncology; (2) practiced at the VHA during the time of recruitment; and (3) had experience treating lung cancer at a VHA facility. During recruitment, potential participants were told that the investigators were interested in learning about oncologists’ experiences and decisions about using GBTT to treat advanced lung cancer in the VHA. Participants were scheduled for telephone-based interviews, and verbal consent was obtained prior to all interviews. Interviews ranged from 19 to 90 minutes (average, 40 min).
Recruitment was stopped at the point of thematic saturation, defined a priori as the point when 2 independent coders agreed that 3 consecutive transcripts for a given interview category (see below) rendered no new thematic concepts.9,10 Consistent with the theoretical framework developed by Cabana and colleagues, interviews were designed to elicit information about oncologists’ knowledge, attitudes, intent to use GBTT, and perceived facilitators and barriers to using GBTT in the VHA.11 Additional findings are presented elsewhere.12 The interview guide was pilot tested and revised prior to initiating data collection. All interviews were recorded, transcribed, and analyzed for content.
Analysis
Data were analyzed using framework analysis methodology, which allows for the inclusion of existing concepts as well as emergent themes within an established theoretical framework.13 Two independent coders with expertise in framework analysis independently created codes and indexed the data using Atlas.ti 6.2 (Scientific Software Development, Berlin, Germany). Disagreements about coding decisions were resolved through group consensus. Coding centered on 2 themes:
- Barriers and facilitators to mutation testing. This includes system or facility factors and testing weaknesses that act as barriers to ordering mutation testing, system or facility factors that facilitate ordering mutation testing, and oncologists’ suggestions for ways to encourage more testing in the VHA.
- Barriers and facilitators to prescribing GBTT. This includes system or facility factors that act as barriers to prescribing GBTT, system or facility factors that facilitate prescribing GBTT, and oncologists’ suggestions for ways to encourage more prescribing of GBTT in the VHA.
Thirty medical oncologists were interviewed. Participant demographics are presented in the Table.
Barriers to testing
The 2 most commonly cited barriers to ordering mutation testing can be considered weaknesses in the testing process: lack of tissue and wait time for results. Almost all providers identified lack of tissue as a barrier to ordering a mutation test.
Another frequently cited testing weakness involved the wait time for results. Because the mutation analysis is not conducted in the VHA facility, providers often must wait 2 to 4 weeks to receive results. This can present a problem because some providers do not want to wait for the results before recommending a course of treatment.
Several providers cited system and facility factors as barriers to mutation testing. The most common of these involves the ordering process. Oncology providers often remarked that ordering the mutation test is cumbersome or inconvenient because there is no ordering mechanism in the Computerized Patient Record System (CPRS). Many different approaches for ordering a mutation test exist, including e-mailing the pathology department, calling to place the order, or requesting the test in person. As providers can order many, if not most, other tests via CPRS, it is clear that this presents an inconvenient exception.
Budgetary constraints were another frequently cited system or facility-level barrier. Providers sometimes were unable to access the test due to the cost
Finally, several providers noted that in some cases patients did not wish to undergo a biopsy. Thus, patient preference can act as a barrier to mutation testing. Some patients wish to forgo treatment, which eliminates the need for a mutation test. Other patients believe that due to their smoking history, they are unlikely to have an ALK or EGFR mutation and instead immediately opt for chemotherapy. Only a small minority of participants identified no barriers to mutation testing.
Facilitators for Testing
Many providers complimented the availability of the mutation test. Interestingly, while some providers mentioned that lack of CPRS ordering was a barrier to testing, several also listed access to a CPRS order as a facilitator. These providers commented that ordering a test was streamlined and easy, given the mechanism in CPRS. Some VHA facilities offer CPRS order capabilities, and others do not. Other oncologists commented more generally on the cooperativeness of the pathology department in ordering mutation tests. It seems that facilities may use different ordering procedures, but in most of these facilities, a high degree of cooperation exists between departments to send out for tests that are requested.
Providers offered many ideas for ways to improve mutation testing or to facilitate the testing. By far, the most commonly cited way to improve the testing process was to make mutation testing reflexive for metastatic nonsquamous NSCLC. Some acknowledged that to achieve this would require a change to the budgeting process such that the test would not drain the pathology department’s budget. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address several of the barriers that were identified in this study. Other providers recommended standardizing the ordering procedure and location of results. Specifically, providers recommended creating a button in CPRS for ordering and always reporting the results in the same place in CPRS.
Barriers to GBTT Prescribing
The clear majority of providers identified no barriers to prescribing GBTTs. A few mentioned that they were required to submit a nonformulary consult. A representative quote described this as “more out of a formality, and the pharmacist basically is there with me and he approves it on the spot and provides the prescription on the day, right when I’m seeing the patient.” Only a very small minority of providers identified medication cost as a barrier, but even those respondents did not indicate that cost prevented them from offering GBTTs to their patients. Rather, cost consciousness simply made them more mindful and judicious when making decisions about prescribing GBTTs.
Facilitators to GBTT Prescribing
Several providers listed availability of the costly medication in the VHA as a facilitator to prescribing. Veterans can obtain GBTTs with little to no insurance cost or copayment, which is not always the case outside the VHA.
One recommendation for further facilitating prescribing of GBTTs involved eliminating the preauthorization requirement, particularly in first-line use for patients testing positive for ALK or EGFR mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
Discussion
Although in some cases, testing weaknesses (lack of tissue, wait time to receive results) can interrupt a treatment trajectory, many of the barriers identified in this study are modifiable. Overwhelmingly, oncologists recommended making mutation testing reflexive for metastatic nonsquamous NSCLC. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address issues related to variable utilization of genomic testing in VHA.12 Additionally, in response to system and facility barriers to mutation testing, other providers recommended standardizing the ordering procedure and location of results. Utilization of GBTT can be facilitated by eliminating the preauthorization requirement, particularly in first-line use for patients with positive mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
This study extends previous research that identified underuse of genomic testing in community-based practices. The authors sought to interview a broad sample of providers from various facilities (small, large, CoC accredited, nonaccredited) to understand the range of conditions faced by VA providers. Some providers face more barriers than do others, whereas some face few or no barriers. This wide range of experiences can help to better understand the factors that facilitate guideline-adherent care.
Limitations
The authors recognize that availability of resources and testing and prescribing practices are constantly evolving and perhaps have improved since the data were collected. Thus, the age of the study data might be a limitation to the study. Like most qualitative studies, these findings are limited in their generalizability beyond the study population. Additionally, the authors were limited to recruiting oncologists with reliable contact information listed in the VHA directory. Although this could have introduced some degree of sampling bias, the authors are confident that the sample sufficiently represents the population of VHA-based medical oncologists who treat lung cancer. Despite these limitations, these findings provide novel perspectives on barriers and facilitators to genomic testing GBTT prescribing in the VHA. The authors identify modifiable barriers to testing and prescribing that can be addressed to improve and standardize care of advanced lung cancer in the VHA.
Conclusion
Efforts should be made to address modifiable barriers to mutation testing and guideline-consistent prescribing of GBTT in the VA setting. Implementation of specific practices like reflexive testing for all metastatic nonsquamous NSCLC, standardization of the mutation test ordering procedure, standardization of results reporting, and elimination of the preauthorization to prescribe GBTT could impact the utilization of GBTT in VHA.
Click here to read the digital edition.
Lung cancer is the most frequent cause of cancer-related mortality worldwide.1 The most prevalent type of lung cancer is non-small cell lung cancer (NSCLC), which comprises about 85% of lung cancer cases.2 As there are no cost-effective approaches to screening for lung cancer, most lung cancers are identified at an advanced stage (stage IIIB or IV).
New approaches to managing advanced lung cancer have emerged in recent years, including drugs designed to target specific genetic mutations in some tumors.3 The National Comprehensive Cancer Network (NCCN) recommends erlotinib, a receptor tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) for first-line treatment of advanced NSCLC with EGFR mutation.4 Crizotinib is recommended to treat cancers that test positive for the anaplastic lymphoma kinase (ALK) mutation.4 Utilization of targeting agents has been found to extend the survival times for patients with the specified mutations.5 Both erlotinib and crizotinib are available at the VHA.
Previous research showed that VHA providers expressed overall favorable attitudes about genomic medicine.6 Providers perceived genomic medicine to have an important and possibly transformative role in medicine. Barriers to utilization of genomic medicine involved concerns about coordination of care, changes in workload, and increased length of patient visits. In addition to these system-level barriers, many providers had concerns about the proficiency of VHA-based practitioners to appropriately use genomic medicine.
Previous research has evaluated utilization of genomic testing and genomic-based targeted therapy (GBTT) in VA and community settings.5-8 It is unclear whether VHA-based providers are following clinical guidelines regarding genomic testing and utilization of GBTT.4 The authors set out to identify factors that impede and encourage guideline-consistent care in the management of NSCLC at the VHA. The authors specifically sought information about oncologists’ perceptions and experiences with EGFR and ALK mutation testing in patients with advanced NSCLC, as well as use of erlotinib and crizotinib in treating such patients.
Methods
This study was approved by the institutional review boards at Michael E. DeBakey VAMC in Houston, Texas and Baylor College of Medicine. In-depth qualitative interviews were conducted with VHA oncologists to examine their reported barriers and facilitators to mutation testing and prescribing of genomic-based treatment in patients with advanced NSCLC.
The sample of participants was recruited from a list of VHA medical oncologists, compiled by the study project coordinator. Investigators stratified the list by American College of Surgeons Commission on Cancer (CoC) accreditation status (yes/no) and used a stratified purposive sampling technique to recruit participants from CoC-accredited facilities and nonaccredited facilities. Recruitment and data collection occurred between March 2015 and February 2016. Oncologists were considered for inclusion if they (1) were specialists in oncology; (2) practiced at the VHA during the time of recruitment; and (3) had experience treating lung cancer at a VHA facility. During recruitment, potential participants were told that the investigators were interested in learning about oncologists’ experiences and decisions about using GBTT to treat advanced lung cancer in the VHA. Participants were scheduled for telephone-based interviews, and verbal consent was obtained prior to all interviews. Interviews ranged from 19 to 90 minutes (average, 40 min).
Recruitment was stopped at the point of thematic saturation, defined a priori as the point when 2 independent coders agreed that 3 consecutive transcripts for a given interview category (see below) rendered no new thematic concepts.9,10 Consistent with the theoretical framework developed by Cabana and colleagues, interviews were designed to elicit information about oncologists’ knowledge, attitudes, intent to use GBTT, and perceived facilitators and barriers to using GBTT in the VHA.11 Additional findings are presented elsewhere.12 The interview guide was pilot tested and revised prior to initiating data collection. All interviews were recorded, transcribed, and analyzed for content.
Analysis
Data were analyzed using framework analysis methodology, which allows for the inclusion of existing concepts as well as emergent themes within an established theoretical framework.13 Two independent coders with expertise in framework analysis independently created codes and indexed the data using Atlas.ti 6.2 (Scientific Software Development, Berlin, Germany). Disagreements about coding decisions were resolved through group consensus. Coding centered on 2 themes:
- Barriers and facilitators to mutation testing. This includes system or facility factors and testing weaknesses that act as barriers to ordering mutation testing, system or facility factors that facilitate ordering mutation testing, and oncologists’ suggestions for ways to encourage more testing in the VHA.
- Barriers and facilitators to prescribing GBTT. This includes system or facility factors that act as barriers to prescribing GBTT, system or facility factors that facilitate prescribing GBTT, and oncologists’ suggestions for ways to encourage more prescribing of GBTT in the VHA.
Thirty medical oncologists were interviewed. Participant demographics are presented in the Table.
Barriers to testing
The 2 most commonly cited barriers to ordering mutation testing can be considered weaknesses in the testing process: lack of tissue and wait time for results. Almost all providers identified lack of tissue as a barrier to ordering a mutation test.
Another frequently cited testing weakness involved the wait time for results. Because the mutation analysis is not conducted in the VHA facility, providers often must wait 2 to 4 weeks to receive results. This can present a problem because some providers do not want to wait for the results before recommending a course of treatment.
Several providers cited system and facility factors as barriers to mutation testing. The most common of these involves the ordering process. Oncology providers often remarked that ordering the mutation test is cumbersome or inconvenient because there is no ordering mechanism in the Computerized Patient Record System (CPRS). Many different approaches for ordering a mutation test exist, including e-mailing the pathology department, calling to place the order, or requesting the test in person. As providers can order many, if not most, other tests via CPRS, it is clear that this presents an inconvenient exception.
Budgetary constraints were another frequently cited system or facility-level barrier. Providers sometimes were unable to access the test due to the cost
Finally, several providers noted that in some cases patients did not wish to undergo a biopsy. Thus, patient preference can act as a barrier to mutation testing. Some patients wish to forgo treatment, which eliminates the need for a mutation test. Other patients believe that due to their smoking history, they are unlikely to have an ALK or EGFR mutation and instead immediately opt for chemotherapy. Only a small minority of participants identified no barriers to mutation testing.
Facilitators for Testing
Many providers complimented the availability of the mutation test. Interestingly, while some providers mentioned that lack of CPRS ordering was a barrier to testing, several also listed access to a CPRS order as a facilitator. These providers commented that ordering a test was streamlined and easy, given the mechanism in CPRS. Some VHA facilities offer CPRS order capabilities, and others do not. Other oncologists commented more generally on the cooperativeness of the pathology department in ordering mutation tests. It seems that facilities may use different ordering procedures, but in most of these facilities, a high degree of cooperation exists between departments to send out for tests that are requested.
Providers offered many ideas for ways to improve mutation testing or to facilitate the testing. By far, the most commonly cited way to improve the testing process was to make mutation testing reflexive for metastatic nonsquamous NSCLC. Some acknowledged that to achieve this would require a change to the budgeting process such that the test would not drain the pathology department’s budget. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address several of the barriers that were identified in this study. Other providers recommended standardizing the ordering procedure and location of results. Specifically, providers recommended creating a button in CPRS for ordering and always reporting the results in the same place in CPRS.
Barriers to GBTT Prescribing
The clear majority of providers identified no barriers to prescribing GBTTs. A few mentioned that they were required to submit a nonformulary consult. A representative quote described this as “more out of a formality, and the pharmacist basically is there with me and he approves it on the spot and provides the prescription on the day, right when I’m seeing the patient.” Only a very small minority of providers identified medication cost as a barrier, but even those respondents did not indicate that cost prevented them from offering GBTTs to their patients. Rather, cost consciousness simply made them more mindful and judicious when making decisions about prescribing GBTTs.
Facilitators to GBTT Prescribing
Several providers listed availability of the costly medication in the VHA as a facilitator to prescribing. Veterans can obtain GBTTs with little to no insurance cost or copayment, which is not always the case outside the VHA.
One recommendation for further facilitating prescribing of GBTTs involved eliminating the preauthorization requirement, particularly in first-line use for patients testing positive for ALK or EGFR mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
Discussion
Although in some cases, testing weaknesses (lack of tissue, wait time to receive results) can interrupt a treatment trajectory, many of the barriers identified in this study are modifiable. Overwhelmingly, oncologists recommended making mutation testing reflexive for metastatic nonsquamous NSCLC. Implementing reflexive testing of patients, as recommended by guidelines, would understandably address issues related to variable utilization of genomic testing in VHA.12 Additionally, in response to system and facility barriers to mutation testing, other providers recommended standardizing the ordering procedure and location of results. Utilization of GBTT can be facilitated by eliminating the preauthorization requirement, particularly in first-line use for patients with positive mutations. Although the preauthorization was not seen as a significant barrier, removal of this formality could make prescribing easier.
This study extends previous research that identified underuse of genomic testing in community-based practices. The authors sought to interview a broad sample of providers from various facilities (small, large, CoC accredited, nonaccredited) to understand the range of conditions faced by VA providers. Some providers face more barriers than do others, whereas some face few or no barriers. This wide range of experiences can help to better understand the factors that facilitate guideline-adherent care.
Limitations
The authors recognize that availability of resources and testing and prescribing practices are constantly evolving and perhaps have improved since the data were collected. Thus, the age of the study data might be a limitation to the study. Like most qualitative studies, these findings are limited in their generalizability beyond the study population. Additionally, the authors were limited to recruiting oncologists with reliable contact information listed in the VHA directory. Although this could have introduced some degree of sampling bias, the authors are confident that the sample sufficiently represents the population of VHA-based medical oncologists who treat lung cancer. Despite these limitations, these findings provide novel perspectives on barriers and facilitators to genomic testing GBTT prescribing in the VHA. The authors identify modifiable barriers to testing and prescribing that can be addressed to improve and standardize care of advanced lung cancer in the VHA.
Conclusion
Efforts should be made to address modifiable barriers to mutation testing and guideline-consistent prescribing of GBTT in the VA setting. Implementation of specific practices like reflexive testing for all metastatic nonsquamous NSCLC, standardization of the mutation test ordering procedure, standardization of results reporting, and elimination of the preauthorization to prescribe GBTT could impact the utilization of GBTT in VHA.
Click here to read the digital edition.
. , , , Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. What is non-small cell lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Updated May 16, 2016. Accessed January 19, 2018.
3. , , . New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1097-1104.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). non-small cell lung cancer 2. 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated December 19, 2017. Accessed Jan
5. Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967.
6. Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Per Med. 2010;7(5):485-494.
7. Lynch JA, Berse B, Dotson D, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017; 19(8):890-899.
8. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer, 2017;18(6):651-659.
9. Morse JM. The significance of saturation. Qual Health Res.1995;5(2):147-149.
10. Aita VA, McIlvain HE. An armchair adventure in case study research. In: Crabtree BF, Miller WF, eds. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999:253-268.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
12. Arney JB, Helm A, Crook T, Braun U, Chen GJ, Hayes TG. Utilization of genomic testing in advanced non-small cell lung cancer among oncologists in the Veterans Health Administration. Lung Cancer, 2018;116:25-29.
13. Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, eds. Analyzing Qualitative Data. New York, NY: Routledge; 1994:173-194.
. , , , Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. What is non-small cell lung cancer? https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Updated May 16, 2016. Accessed January 19, 2018.
3. , , . New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1097-1104.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). non-small cell lung cancer 2. 2018. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Updated December 19, 2017. Accessed Jan
5. Rosell R, Moran T, Queralt C, et al; Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958-967.
6. Arar N, Seo J, Abboud HE, Parchman M, Noel P. Providers’ behavioral beliefs regarding the delivery of genomic medicine at the Veterans Health Administration. Per Med. 2010;7(5):485-494.
7. Lynch JA, Berse B, Dotson D, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: analysis of gene-specific billing in Medicare claims data. Genet Med. 2017; 19(8):890-899.
8. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer, 2017;18(6):651-659.
9. Morse JM. The significance of saturation. Qual Health Res.1995;5(2):147-149.
10. Aita VA, McIlvain HE. An armchair adventure in case study research. In: Crabtree BF, Miller WF, eds. Doing Qualitative Research. Thousand Oaks, CA: Sage; 1999:253-268.
11. Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458-1465.
12. Arney JB, Helm A, Crook T, Braun U, Chen GJ, Hayes TG. Utilization of genomic testing in advanced non-small cell lung cancer among oncologists in the Veterans Health Administration. Lung Cancer, 2018;116:25-29.
13. Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess RG, eds. Analyzing Qualitative Data. New York, NY: Routledge; 1994:173-194.
A National WestlawNext Database Analysis of Malpractice Litigation in Radiation Oncology (FULL)
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.
Click here to read the digital edition.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.
Click here to read the digital edition.
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.
Click here to read the digital edition.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
Mohs Micrographic Surgery in the VHA (FULL)
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
AGA Clinical Practice Update: Functional gastrointestinal symptoms in patients with inflammatory bowel disease
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Implementing the 2017 VA/DoD Diabetes Clinical Practice Guideline (FULL)
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Paul Conlin, MD. Thank you all for joining us to talk about the recently released VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (CPG). We’ve gathered together a group of experts who were part of the CPG development committee. We’re going to talk about some topics that were highlighted in the CPG that might provide additional detail to those in primary-care practices and help them in their management of patients with diabetes.
A unique feature of the VA/DoD CPG is that it emphasizes shared decision making as an important tool that clinicians should employ in their patient encounters. Dr. Watts, health care providers may wonder how they can make time for an intervention involving shared decision making using the SHARE approach, (ie, seek, help, assess, reach, and evaluate). Can you give us some advice on this?
Sharon Watts, DNP. Shared decision making is really crucial to success in diabetes. It’s been around for a while. We are trying to make an emphasis on this. The SHARE approach is from the Agency for Healthcare Research and Quality (AHRQ). The AHRQ has a wealth of information on its website. What AHRQ emphasizes is making it brief but conversational when you’re using the SHARE approach with your patient. Most importantly, the patient needs to be in the center of this dialogue, expressing his or her values and preferences of what’s most important to the whole team. This is a team effort. It’s not just with a provider. That’s where providers get overwhelmed. You can ask your nurse to advise the patient to write down 1 or 2 questions that are really important about diabetes before they come to see you, before the encounter. We can refer patients to diabetes classes where a lot of this information is given. The patient can talk to the dietitian or the pharmacist. There’s a whole team out there that will help in SHARE decision making. It’s crucial in the end for the provider to help the patient reach the decision and determine how best to treat the diabetes with them.
Dr. Conlin. Can you give a brief description of the key components of the SHARE approach?
Dr. Watts. Breaking it down simply, providers can start off by asking permission to go over the condition or treatment options because this immediately sets the stage as a signal to the patient that they are important in controlling the dialogue. It’s not the provider giving a discourse. You’re asking for permission. The next step would be to explore the benefits and risks of any course taken. Use decision aids if you have them. Keep in mind your patient’s current health literacy, numeracy, and other limitations.
Next ask about values, preferences, or barriers to whatever treatment you’re talking about. For instance, will this work with your work schedule?
Then the last thing would be ask what the patient wants to do next. Reach a decision on treatment, whatever it is, and make sure that you revisit that decision. Follow up later to see if it’s really working.
Dr. Conlin. If I’m a busy clinician and I have a limited amount of time with a patient, when are the appropriate times to employ the SHARE approach? Can I break it into components, where I address some elements during one visit and other elements in another visit?
Dr. Watts. Absolutely. It can be spread out. Your team is probably already providing information that will help in the SHARE approach. Just chart that you’ve done it. We know the SHARE approach is important because people
tend to be adherent if they came up with part of the plan.
Dr. Conlin. Where does diabetes self-management education and diabetes self-management support fall into this framework?
Dr. Watts. Diabetes is a complex disease for providers and for the team and even more so for our patients. Invite them to diabetes classes. There’s so much to understand. The classes go over medications and blood sugar ranges, though you still may have to review it with the patient in your office. It saves the provider time if you have an informed and activated patient. It’s the same with sending a patient to a dietitian. I do all of the above.
Dr. Conlin. Many providers may not be familiar with this type of approach. How can I tell whether or not I’m doing it correctly?
Dr. Watts. The AHRQ website has conversation starters (www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/index.html). Then make sure when you are with the patient to use Teach-Back. Have that conversation and say, “I want to make sure I understood correctly what we decided would work best for you.” Ask patients to say in their own words what they understand. Then I think you’re off to a great start.
Dr. Conlin. Many patients tend to be deferential to their health care providers. They were brought up in an era where they needed to listen to and respect clinicians rather than participate in discussions about their ongoing care. How do you engage with these patients?
Dr. Watts. That is a tough one. Before the patient leaves the office, I ask them: Are there any barriers? Does this work for your schedule? Is this a preference and value that you have? Is there anything that might get in the way of this working when you go home? I try to pull out a little bit more, making sure to give them some decision aids and revisit it at the next visit to make sure it’s working.
Dr. Conlin. We’ll now turn to a discussion of using hemoglobin A1c (HbA1c) measurements in clinical practice. Dr. Aron, what factors can impact the relationship between HbA1c and blood glucose? How should we use HbA1c in the treatment of patients who come from varied ethnic and racial backgrounds, where the relationship to average blood glucose may be different?
David C. Aron, MD, MS. The identification of HbA1c has been a tremendous advance in our ability to manage patients with diabetes. It represents an average blood glucose over the preceding 3 months but like everything in this world, it has issues. One is the fact that there is a certain degree of inaccuracy in measurement, and that’s true of any measurement that you make of anything. Just as you allow a little bit of wiggle room when you’re driving down the New Jersey Turnpike and watching your speedometer, which is not 100% accurate. It says you are going 65 but it could, for example be 68 or 62. You don’t want to go too fast or you’ll get a speeding ticket. You don’t want to go too slowly or the person behind you will start honking at you. You want to be at the speed limit plus or minus. The first thing to think about in using HbA1c is the issue of accuracy. Rather than choose a specific target number, health care providers should choose a range between this and that. There’ll be more detail on that later.
The second thing is that part of the degree to which HbA1c represents the average blood glucose depends on a lot of factors, and some of these factors are things that we can do absolutely nothing about because we are born with them. African Americans tend to have higher HbA1c levels than do whites for the same glucose. That difference is as much as 0.4. An HbA1c of 6.2 in African Americans gets you a 5.8 in whites for the same average blood glucose. Similarly, Native Americans have somewhat higher HbA1c, although not quite as high as African Americans. Hispanics and Asians do as well, so you have to take your patient’s ethnicity into account.
The second has to do with the way that HbA1c is measured and the fact that there are many things that can affect the measurement. An HbA1c is dependent upon the lifespan of the red blood cell, so if there are alterations in red cell lifespan or if someone has anemia, that can affect HbA1c. Certain hemoglobin variants, for example, hemoglobin F, which is typically elevated in someone with thalassemia, migrates with some assays in the same place as thalassemia, so the assay can’t tell the difference between thalassemia and hemoglobin F. There are drugs and other conditions that can also affect HbA1c. You should think about HbA1c as a guide, but no number should be considered to be written in stone.
Dr. Conlin. I can imagine that this would be particularly important if you were using HbA1c as a criterion for diagnosing diabetes.
Dr. Aron. Quite right. The effects of race and ethnicity on HbA1c account for one of the differences between the VA/DoD guidelines and those of the American Diabetes Association (ADA).
Dr. Conlin. Isn’t < 8% HbA1c a national performance measure that people are asked to adhere to?
Dr. Aron. Not in the VA. In fact, the only performance measure that the VA has with a target is percent of patients with HbA1c > 9%, and we don’t want any of those or very few of them anyway. We have specifically avoided targets like < 8% HbA1c or < 7% HbA1c, which was prevalent some years ago, because the choice of HbA1c is very dependent upon the needs and desires of the individual patient. The VA has had stratified targets based on life expectancy and complications going back more than 15 years.
Dr. Conlin. Another issue that can confuse clinicians is when the HbA1c is in the target range but actually reflects an average of glucose levels that are at times very high and very low. How do we address this problem clinically?
Dr. Aron. In managing patients, you use whatever data you can get. The HbA1c gives you a general indication of average blood glucose, but particularly for those patients who are on insulin, it’s not a complete substitute for measuring blood glucose at appropriate times and taking the degree of glucose variability into account. We don’t want patients getting hypoglycemic, and particularly if they’re elderly, falling, or getting into a car accident. Similarly, we don’t want people to have very high blood sugars, even for limited periods of time, because they can lead to dehydration and other symptoms as well. We use a combination of both HbA1c and individual measures of blood glucose, like finger-stick blood sugar testing, typically.
Dr. Conlin. The VA/DoD CPG differs from other published guidelines in that we proposed patients are treated to HbA1c target ranges, whereas most other guidelines propose upper limits or threshold values, such as the HbA1c should be < 7% or < 8% but without lower bounds. Dr. Colburn, what are the target ranges that are recommended in the CPG? How were they determined?
Maj. Jeffrey A. Colburn, MD. It may be helpful to pull up the Determination of Average Target HbA1c Level Over Time table (page S17), which lays out risk for patients of treatment as well as the benefits of treatment. We first look at the patient’s state of health and whether they have a major comorbidity, a physiologic age that could be high risk, or advanced physiologic age with a diminished life expectancy. In controlling the levels of glucose, we’re often trying to benefit the microvascular health of the patient, realizing also that eventually poor management over time will lead to macrovascular disease as well. The main things that we see in child data is that the benefits of tight glucose control for younger patients with shorter duration of type 2 diabetes mellitus (T2DM) is the prevention of retinopathy, nephropathy, and peripheral neuropathy. Those patients that already have advanced microvascular disease are less likely to benefit from tight control. Trying to push glucose very low can harm the patient. It’s a delicate balance between the possible benefit vs the real harm.
The major trials are the ADVANCE, ACCORD, and the VADT trial, which was done in a VA population. To generalize the results, you are looking at an intensive control, which was trying to keep the HbA1c in general down below the 7% threshold. The patients enrolled in those trials all had microvascular and macrovascular disease and typically longer durations of diabetes at the time of the study. The studies revealed that we were not preventing macrovascular disease, heart attacks, strokes, the types of things that kill patients with diabetes. Individuals at higher HbA1c levels that went down to better HbA1c levels saw some improvement in the microvascular risk. Individuals already at the lower end didn’t see as much improvement. What we saw though that was surprising and concerning was that hypoglycemia, particularly severe hypoglycemia in the VADT trial was a lot more frequent when you try and target the HbA1c on the lower end. Because of these findings, we proposed the table with a set of ranges. As Dr. Aron noted, HbA1c is not a perfect test. It does have some variance in the number it presents. The CPG proposed to give individuals target ranges. They should be individualized based upon physiologic age, comorbidities, and life expectancy.
A criticism of the table that I commonly hear is what’s the magic crystal ball for determining somebody’s life expectancy? We don’t have one. This is a clinician’s judgment. The findings might actually change over time with the patient. A target HbA1c range is something that should be adapted and evolve along with the clinician and patient experience of the diabetes.
There are other important studies. For example, the UKPDS trials that included patients with shorter durations of diabetes and lesser disease to try and get their HbA1c levels on the lower end. We included that in the chart. Another concept we put forward is the idea of relative risk (RR) vs absolute risk. The RR reduction doesn’t speak to what the actual beginning risk is lowered to for a patient. The UKPDS is often cited for RR reduction of microvascular disease as 37% when an HbA1c of 7.9% is targeted down to 7.0%. The absolute risk reduction is actually 5 with the number needed to treat to do so is 20 patients. When we present the data, we give it a fair shake. We want individuals to guide therapy that is going to be both beneficial to preventing outcomes but also not harmful to the patient. I would highly recommend clinicians and patients look at this table together when making their decisions.
Dr. Conlin. In the VA/DoD CPG, the HbA1c target range for individuals with limited life expectancy extends to 9%. That may seem high for some, since most other guidelines propose lower HbA1c levels. How strong are the data that a person with limited life expectancy, say with end-stage renal disease or advanced complications, could be treated to a range of 8% to 9%? Shouldn’t lower levels actually improve life expectancy in such people?
Dr. Colburn. There’s much less data to support this level, which is why it’s cited in CPG as having weaker evidence. The reason it’s proposed is the experience of the workgroup and the evidence that is available of a high risk for patients with low life expectancy when they reduce their HbA1c greatly. One of the concerns about being at that level might be the real issue of renal glycosuria for individuals when their blood glucose is reaching above 180 mg/dL, which correlates to the 8% to 9% HbA1c range. You may have renal loss and risk of dehydration. It is an area where the clinician should be cautious in monitoring a patient in the 8% to 9% HbA1c range. With that being said, a patient who is having a lot of challenges in their health and extremely advanced conditions could be in that range. We would not expect a reversing of a micro- or macrovascular disease with glycemia control. We’re not going to go back from that level of disease they have. The idea about keeping them there is to prevent the risks of overtreatment and harm to the patient.
Dr. Conlin. Since patients with diabetes can progress over their lifetime from no complications to mild-to-moderate complications to advanced complications, how does the HbA1c target range evolve as a patient’s condition changes?
Dr. Colburn. As we check for evidence of microvascular disease or neuropathy signs, that evidence often is good for discussion between the clinician and patient to advise them that better control early on may help stem off or reverse some of that change. As those changes solidify, the patient is challenged by microvascular conditions. I would entertain allowing more relaxed HbA1c ranges to prevent harm to the patient given that we’re not going back. But you have to be careful. We have to consider benefits to the patient and the challenges for controlling glucose.
I hope that this table doesn’t make providers throw up their hands and give up. It’s meant to start a conversation on safety and benefits. With newer agents coming out that can help us control glucose quite well, without as much hypoglycemia risk, clinicians and patients potentially can try and get that HbA1c into a well-managed range.
Dr. Conlin. The CPG discusses various treatment options that might be available for patients who require pharmacologic therapy. The number of agents available is growing quite markedly. Dr. Colburn, can you describe how the CPG put together the pharmacologic therapy preferences.
Dr. Colburn. The CPG expressively stayed away from trying to promote specific regimens of medications. For example, other guidelines promote starting with certain agents followed by a second-line agent by a third-line agents. The concern that we had about that approach is that the medication landscape is rapidly evolving. The options available to clinicians and patients are really diverse at this moment, and the data are not concrete regarding what works best for a single patient.
Rather than trying to go from one agent to the next, we thought it best to discuss with patients using the SHARE decision-making model, the adverse effects (AEs) and relative benefits that are involved with each medication class to determine what might be best for the person. We have many new agents with evidence for possible reductions in cardiovascular outcomes outside of their glycemic control properties. As those evidences promote a potentially better option for a patient, we wanted to allow the room in management tomake a decision together. I will say the CPG as well as all of the other applicable diabetes guidelines for T2DM promote metformin as the first therapy to consider for somebody with newly diagnosed T2DM because of safety and availability and the benefit that’s seen with that medication class. We ask clinicians to access the AHRQ website for updates as the medicines evolve.
In a rapidly changing landscape with new drugs coming into the market, each agency has on their individual website information about individual agents and their formulary status, criteria for use, and prior authorization requirements. We refer clinicians to the appropriate website for more information.
Dr. Conlin. There are a series of new medications that have recently come to market that seem to mitigate risk for hypoglycemia. Dr. Lugo, which treatment options carry greater risk? Which treatment options seem to have lesser risk for hypoglycemia?
Amy M. Lugo, PharmD. Insulin and the sulfonylureas have the highest risk of hypoglycemia. The sulfonylureas have fallen out of favor somewhat. One reason is that there are many newer agents that do not cause weight gain or increase the risk of hypoglycemia. Some of the newer insulins may have a lower risk of hypoglycemia and nocturnal hypoglycemia, in particular; however, it is difficult to conclude emphatically that one basal insulin analog is less likely to cause clinically relevant severe or nocturnal hypoglycemia events. This is due to the differences in the definitions of hypoglycemia used in the individual clinical trials, the open label study designs, and the different primary endpoints.
Dr. Conlin. How much affect on HbA1c might I expect to see using SGLT2 inhibitors or GLP-1 agonists? What would be some of the potential AEs I have to be aware of and therefore could counsel patients about?
Dr. Lugo. Let’s start with SGLT2 inhibitors. It depends on whether they are used as monotherapy or in combination. We prefer that patients start on metformin unless they have a contraindication. When used as monotherapy, the SGLT2s may decrease HbA1c from 0.4% to 1% from baseline. When combined with additional agents, they can have > 1% improvement in HbA1c from baseline. There are no head-to-head trials between any of the SGLT2 inhibitors. We cannot say that one is more efficacious than another in lowering HbA1c. The most common AEs include genital mycotic infections and urinary tract infections. The SGLT2 inhibitors also should be avoided in renal impairment. There was a recent FDA safety alert for the class for risk of ketoacidosis. Additionally, the FDA warned that patients
with a history of bladder cancer should avoid dapagliflozin, and canagliflozin has a warning for increased risk of bone fractures, amputation, and decreased bone density.
Other actions of the SGLT2 inhibitors include a reduction in triglycerides and a modest increase in both low-density lipoprotein cholesterol and highdensity lipoprotein cholesterol. The SGLT2 inhibitors also slightly decrease systolic blood pressure (by 4 mm Hg to 6 mm Hg) and body weight (reduction of 1.8 kg)
The GLP-1s are likely to be more efficacious in reducing HbA1c. Typically we see 1% or greater lowering in HbA1c from baseline. As a class, the GLP-1 agonists have a lower risk of hypoglycemia; however, the risk increases when combined with sulfonylureas or insulin. The dose of insulin or sulfonylurea will likely need to be decreased when used concomitantly.
Patients are likely to experience weight loss when on a GLP-1 agonist, which is a great benefit. Gastrointestinal AEs such as nausea are common. Adverse effects may differ somewhat between the agents.
Dr. Conlin. Patients’ experience of care is integral to their engagement with treatment as well as their adherence. Ms. Decesare, what are patients looking for from their health care team?
Elaine M. Decesare. Patients are looking for a knowledgeable and compassionate health care team that has a consistent approach and a consistent message and that the team is updated on the knowledge of appropriate treatments and appropriate lifestyle modifications and targets for the care of diabetes.
Also, I think that the team needs to have some empathy for the challenges of living with diabetes. It’s a 24-hour-a-day disorder, 7 days a week. They can’t take vacation from it. They just can’t take a pill and forget about it. It’s a fairly demanding disorder, and sometimes just acknowledging that with the patient can help you with the dialog.
The second thing I think patients want is an effective treatment plan that’s tailored to their needs and lifestyles. That goes in with the shared decision-making approach, but the plan itself really has to be likely to achieve the targets and the goals that you’ve set up. Sometimes I see patients who are doing all they can with their lifestyle changes, but they can’t get to goal, because there isn’t enough medication in the plan. The plan has to be adequate so that the patient can manage their diabetes. In the shared approach, the patient has to buy in to the plan. With the shared decision making they’re more likely to take the plan on as their plan.
Dr. Conlin. How do you respond to patients who feel treatment burnout from having a new dietary plan, an exercise program, regular monitoring of glucose through finger sticks, and in many cases multiple medications and or injections, while potentially not achieving the goals that you and the patient have arrived at?
Ms. Decesare. First, I want to assess their mood. Sometimes patients are depressed, and they actually need help with that. If they have trouble with just the management, we do have behavioral health psychologists on our team that work with patients to get through some of the barriers and discuss some of the feelings that they have about diabetes and diabetes management.
Sometimes we look at the plan again and see if there’s something we can do to make the plan easier. Occasionally, something has happened in their life. Maybe they’re taking care of an elderly parent or they’ve had other health problems that have come about that we need to reassess the plan and make sure that it’s actually doable for them at this point in time.
Certainly diabetes self-management education can be helpful. Some of those approaches can be helpful for finding something that’s going to work for patients in the long run, because it can be a very difficult disorder to manage as time goes on.
Dr. Colburn. Type 2 DM disproportionately affects individuals who are ≥ 65 years compared with younger individuals. Such older patients also are more likely to have cognitive impairment or visual issues. How do we best manage such patients?
Ms. Decesare. When I’m looking at the care plan, social support is very important. If someone has social support and they have a spouse or a son or daughter or someone else that can help them with their diabetes, we oftentimes will get them involved with the plan, as long as it’s fine with the patient, to offer some help, especially with the patients with cognitive problems, because sometimes the patients just cognitively cannot manage diabetes on their own. Prandial insulin could be a really dangerous product for someone who has cognitive disease.
I think you have to look at all the resources that are available. Sometimes you have to change your HbA1c target range to something that’s going to be manageable for that patient at that time. It might not be perfect, but it would be better to have no hypoglycemia rather than a real aggressive HbA1c target or a target range, if that’s what’s going to keep the patient safe.
Dr. Conlin. We thank our discussants for sharing very practical advice on how to implement the CPG. We hope this information supports clinicians as they develop treatment plans based on each patient's unique characteristics and goals of care.
Click here to read the digital edition.
Huddling for High-Performing Teams
In short team huddles, trainees and PACT teamlets meet to coordinate care and identify ways to improve team processes under the guidance of faculty members who reinforce collaborative practice and continuous improvement.
In 2011, 5 US Department of Veteran Affairs (VA) medical centers were selected by the VA Office of Academic Affiliations (OAA) to establish Centers of Excellence in Primary Care Education (CoEPCE). Part of the VA New Models of Care initiative, the 5 CoEPCEs (Boise, Cleveland, San Francisco, Seattle and West Haven) are utilizing VA primary care settings to develop and test innovative approaches to prepare physician residents, nurse practitioner (NP) students and residents (postgraduate), and other health professions trainees, such as pharmacy, social work, psychology, physician assistants (PAs), dieticians, etc for primary care practice.
The CoEPCEs are interprofessional academic patient aligned care teams (PACTs) defined by VA as a PACT that has at least 2 professions of trainees on the team engaged in learning.
The San Francisco VA Health Care System (SFVAHCS) Education in PACT (EdPACT)/CoEPCE developed and implemented a workplace learning model that embeds trainees into PACT teamlets and clinic workflow.1 Trainees are organized in practice partner triads with 2 second- or third-year internal medicine residents (R2s and R3s) and 1 NP student or resident. Physician residents rotate every 2 months between inpatient and outpatient settings and NP trainees are present continuously for 12 months. In this model, each trainee in the triad has his/her own patient panel and serves as a partner who delivers care to his/her partners’ patients when they are unavailable. Didactic sessions on clinical content and on topics related to the core domains occur 3-times weekly during pre- and postclinic conferences.2
Methods
In 2015, evaluators from the OAA reviewed background documents and conducted open-ended interviews with 9 CoEPCE staff, participating trainees, VA faculty, VA facility leadership, and affiliate faculty. Informants described their involvement, challenges encountered, and benefits of the huddle to participants, veterans, and the VA.
The Huddle
With the emphasis on patient-centered medical homes and team-based care in the Affordable Care Act, there is an urgent need to develop new training models that provide future health professionals with skills that support interprofessional communication and collaborative practice.2,3 A key aim of the CoEPCE is to expand workplace learning strategies and clinical opportunities for interprofessional trainees to work together as a team to anticipate and address the health care needs of veterans. Research suggests that patient care improves when team members develop a shared understanding of each other’s skill sets, care procedures, and values.4 In 2010, the SFVAHCS began phasing in VA-mandated PACTs. Each patient-aligned care teamlet serves about 1,200 patients and is composed of physician or NP primary care provider(s) (PCPs) and a registered nurse (RN) care manager, a licensed vocational nurse (LVN), and a medical support assistant (MSA). About every 3 teamlets also work with a profession-specific team member from the Social Work and Pharmacy departments. The implementation of PACT created an opportunity for the CoEPCE to add trainees of various professions to 13 preexisting PACTs in 3 SFVAHCS primary care clinics. This arrangement benefits both trainees and teamlets: trainees learn how to collaborate with clinic staff while the clinic PACT teamlets benefit from coaching by faculty skilled in team-based care.
As part of routine clinical activities, huddles provide opportunities for workplace learning related to coordination of care, building relationships, and developing a sense of camaraderie that is essential for team-based, patient-centered care. In their ideal state, huddles are “…the hub of interprofessional, team-based care”; they provide a venue where trainees can learn communication skills, team member roles, systems issues and resources, and clinical knowledge expected of full-time providers and staff.5 Embedding faculty in huddles as huddle coaches help ensure trainees are learning and applying these skills.
Planning and Implementation
After OAA funded the CoEPCE in 2011, faculty had 6 months to develop the EdPACT curriculum, which included a team building retreat, interactive didactic sessions, and workplace learning activities (ie, huddles). In July 2011, 10 trainee triads (each consisting of 2 physician residents and either a student NP or resident NP) were added to preexisting PACTs at the San Francisco VA Medical Center primary care clinic and 2 community-based outpatient clinics.
These trainee triads partnered with their PACT teamlets and huddled for 15 minutes at the beginning of each clinic day to plan for the day’s patients and future scheduled patients and to coordinate care needs for their panel of patients. CoEPCE staff built on this basic huddle model and made the following lasting modifications:
- Developed and implemented a huddle coach model and a huddle checklist to provide structure and feedback to the huddle (Online Resources);
- Scheduled huddles in NP student/resident’s exam room to reduce the hierarchy in the trainee triad;
- Incorporated trainees from other professions and levels into the huddle (psychology fellows, pharmacy residents, social work); and
- Linked the PACT teamlet (staff) to quality improvement projects that are discussed periodically in huddles and didactics.
Curriculum. The huddle allows for practical application of the 4 core domains: interprofessional collaboration (IPC), performance improvement (PI), sustained relationships (SR), and shared decision making (SDM) that shape the CoE curriculum.
Interprofessional collaboration (IPC) is the primary domain reinforced in the huddle. Trainees learn key content in half-day team retreats held at the beginning of the academic year and in interactive didactic sessions. These sessions, which draw on concepts from the Agency for Healthcare Research and Quality’s TeamSTEPPS, an evidence-based teamwork training program for health care workers, teach skills like closed-loop communication, check-backs, negotiation, and conflict resolution.
The CoE trainee triads also lead quality improvement (QI) projects, and the huddle is a venue for getting input, which reinforces the CoE’s performance improvement (PI) curriculum. For example, PACT teamlet staff members provide trainees with feedback on proposed QI interventions, such as increasing the use of telephone visits. The huddle supports SR among team members that enhance patient care while improving the quality of the clinic experience for team members. Strengthened communications and increased understanding of team member roles and system resources supports a patient-centered approach to care and lays the foundation for SDM between patients and team members.
Faculty Roles and Development. The CoEPCE physician and NP faculty members who precept and function as huddle coaches participate in monthly 2-hour faculty development sessions to address topics related to IPE. At least 1 session each year covers review of the items on the huddle checklist, tips on how to coach a huddle, discussions of the role of huddle coaches, and feedback and mentoring skills. Many huddle coach activities are inherent to clinical precepting, such as identifying appropriate clinical resources and answering clinical questions, but the core function of the huddle coach is to facilitate effective communication among team members.
Initially, a coach may guide the huddle by rounding up team members or directing the agenda of the huddle (ie, prompting the LVN to present the day’s patients and suggesting the group identify and discuss high-risk patients). As the year progresses, coaches often take a backseat, and the huddle may be facilitated by the trainees, the RN, LVN, or a combination of all members. During the huddle, coaches also may reinforce specific communication skills, such as a “check back” or ISBAR ( Identify who you are, describe the Situation, provide Background information, offer an Assessment of the situation/needs, make a Recommendation or Request)—skills that are taught during CoE didactic sessions.
The coach may call attention to particular feedback points, such as clarification of the order as an excellent example of a check-back. Each preceptor coaches 1 huddle per precepting session. After the teams huddle, preceptors do a smaller, shorter huddle in the precepting room to share successes, such as interprofessional trainees demonstrating backup behavior (eg, “in today’s huddle, I saw a great example of backup behavior when the medicine resident offered to show the NP student how to consent someone”) and discuss challenges (eg, getting all team members to the huddle).
Resources. The CoE staff schedule at least 20 huddles per week and coordinate preceptor and room schedules. The other required resources are clinic staff (RNs, LVNs, and MSAs) and exam rooms large enough to accommodate 8 or more people. Sufficient staffing coverage and staggered huddles also are important to allow cross-coverage for other clinical duties while team members and faculty are huddling.
Monitoring and Assessment. The CoE staff administer the Team Development Measure (TDM) twice yearly and a modified version of the TEAM 360 feedback survey once per year.6-9 The TDM member gages perceptions of team functioning (cohesiveness, communication, role clarity, and clarity of goals and means). Teams meet with a facilitator to debrief their TDM results and discuss ways to improve their team processes. Three-quarters of the way through the academic year, team members also complete the modified TEAM 360 survey on trainees. Each trainee receives a report describing his/her self-ratings and aggregate team member ratings on leadership, communication, interpersonal skills, and feedback.
Partnerships
In addition to CoEPCE staff and faculty support and engagement, huddles at SFVAHCS have benefited from partnerships with VA primary care leadership and with academic affiliates. In particular, support from the VA clinic directors and nurse managers was key to instituting changes to the clinics’ structure to include interprofessional trainees in huddles.
The affiliates—the University of California, San Francisco (UCSF) School of Medicine and School of Nursing—are integral partners and assist with NP student and medicine resident recruitment. These affiliates also participate in planning and refinement of CoEPCE curricular activities. The UCSF School of Nursing, School of Medicine, and Center for Faculty Educators were involved in the planning stages of the huddle model.
Challenges and Solutions
Having a staffing ratio that supports trainee participation in and learning through huddles is critical. Preceptor coverage must be in sufficient numbers to allow preceptors to coach the huddles, and clinical staff must be adequate to create cohesive and consistent teams for trainee providers. Clinic staff turnover and changes in teamlet staff can be very disruptive. Over time, teamlet staff often know key details and helpful contextual information about particular patients and clinic processes. This knowledge may be lost with turnover among teamlet staff. If team members miss huddles due to staffing shortages and clinical duties, there may be delays and errors in patient care. For example, if information discussed in the huddle is not relayed to the absent team member in a timely or accurate manner, care may be impacted. However, potential disruptions can be mitigated by a high-functioning team with strong communication skills and situational awareness who readily assist and distribute the workload.
Consistent huddling, huddle coaches, and checklists all help stabilize the group. Integration of trainees in the PACT team initially requires extra work because trainees are part-time and have panels significantly smaller than 1,200 (which means the teamlet staff are assigned to multiple trainee and provider huddles). However, teamlet staff find working with trainee teams personally rewarding, and developing highly functioning teams helps prevent burnout. Integration of pharmacy, psychology, and social work trainees takes time and thoughtful planning of activities and contributions that enhance team functioning while not overburdening trainees with additional responsibilities. If these other professions of health trainees are joining several teams’ huddles, their role may be to weigh in as needed vs preparing for and reviewing several PCPs’ schedules and patients in advance.
Factors for Success
The VA facility and primary care clinic leadership’s commitment to supporting staff participation in huddles was critical for integrating trainees into PACTs. Additionally, VA facility commitment to implementation of PACT was a key facilitating factor. Implementation of PACT, including huddles, has not been consistent at all VA facilities.10 The CoE’s approach to integrating trainees into the huddle was an opportunity to strengthen the huddle and to teach new staff members how to participate with team members in huddles. CoEPCE leadership, which has embraced change, meets regularly with facility leadership as well as an advisory board of UCSF leaders to update them on CoE activities. A critical factor for success was CoE expertise in interprofessional education and its ability to integrate concepts from the 4 core domains into an effective workplace learning experience, including attention to the physical space, scheduling, and the development and implementation of the huddle coach role and checklist.
Accomplishments and Benefits
There is evidence that SFVAHCS team huddles are achieving their goals and CoE trainees are being trained to provide team-based, patient-centered care to veterans. Key outcomes of the CoE’s approach to huddles include components in the next sections.
Interprofessional Educational Capacity. The CoEPCE faculty and staff consider the huddle to be one of the best ways to teach interprofessional communication and collaboration, team functioning, and clinical performance. Unlike a traditional didactic, classroom-based session on interprofessional collaboration, the huddle is an opportunity for health care professionals to work together to provide care in a clinic setting. It also is an activity in which the CoE has continued innovative activities, such as adding a preceptor huddle, incorporating additional professions, and encouraging panel management activities during huddles. The CoE has received significant interest and visibility and has been invited to share the model in numerous presentations.
Participants’ Knowledge, Attitudes, Skills, and Competencies. An aim of the CoE approach to huddles was to provide trainees with general skills in the core domain interprofessional collaboration, including teamwork and communication that transfer to other settings, such as inpatient teams and specialty clinics. Learning about other professions and their scopes of practice and areas of expertise can be helpful beyond huddles and primary care. Trainees also learn concepts and practices from the other core domains:
- Performance Improvement: The huddle is a venue for utilizing clinic metrics as well as focusing on QI projects that benefit from a team approach to solving problems;
- Sustained Relationships: The huddles support and teach the importance of relationships among the team. Trainees learn about the roles of clinic staff members, and clinic staff have more opportunities to interact with trainees and become comfortable with them, supporting coordinated care; and
- Shared Decision Making: The huddle is a venue for discussing options for providing patient decision-making support, such as discussing the pros and cons of colon cancer screening with a patient, improving patient-centered care.
Additionally, huddles can address differences in trainee clinical expertise. For example, new physician interns with less experience in the clinic receive more coaching on system resources and patient histories than they might otherwise. Nurse practitioner residents often participate in more than 1 huddle team and transition to a coaching role.
Sustained Relationships, Role Clarity, and Collaboration. The huddles are structured to facilitate SRs among trainees from different professions and among the PACT teamlet in detail as a team. The huddle increases team efficiency by educating trainees and staff about team member roles. For example, trainees learn how the LVNs and MSAs prepare for patient visits. Moreover, an opportunity exists to learn how provider and clinic staff expertise may overlap. Registered nurse care managers, who have their own hypertension clinics, can help manage a patient’s medication titration. Similarly, pharmacy trainees can suggest a referral for a complicated patient with diabetes to pharmacy clinic, where clinical pharmacists can adjust medications and provide patient education for hypertension, hyperlipidemia, and hyperglycemia. In this way, role clarity is improved and trainees learn how team members work within their scope of practice and are better able to “share the care.”
There is evidence that huddles have resulted in expanded participant interprofessional collaboration. The CoE administers the TDM twice a year and the huddle teams rate themselves on several dimensions—cohesiveness, communications, role clarity, and goals.6,7 The 2011/2012 findings showed that nearly all teams showed improvement, with the mean scores for all teams combined increasing from 59.4 in the fall to 64.6 in the spring (max score is 100).5 These scores increased again from 62.2 to 70.3 in 2012/2013, from 66.6 to 70.2 in 2013/2014, and from 64.6 to 69.9 in 2014/2015.
Expanding Clinical Knowledge. At the individual level, the huddle is an opportunity for a trainees to expand their clinical expertise in real time. The huddle provides exposure to a variety of patients and corresponding patient care needs. Trainees are encouraged to complete patient prerounds before the huddle in order to focus the huddle discussion on patients with chronic conditions, complex needs, recent hospitalizations, and upcoming appointments. The CoEPCE trainees tap into the expertise and experience of their team members and coach.
The clinic staff can get information from trainees about their plan of care while trainees get a more complete picture of a patient’s situation—for example, medical or social history or communication preferences. Additionally, trainees learn team skills, such as communication techniques and warm handoffs, which can be used in other clinical settings outside primary care and beyond the VA. As trainees advance, the huddle helps them learn to delegate appropriately, practice conflict negotiation, and develop leadership skills.
Participants’ Satisfaction With Interventions. There is qualitative evidence that clinic RNs and LVNs like huddles and appreciate having the opportunity to communicate in person with providers as well as to teach trainees how to work interprofessionally. Faculty members who are huddle coaches report that they develop a richer understanding of the skill set of trainees, information that can inform CoE curriculum design. Trainees appreciate the opportunity to develop relationships with team members. In end-of-year interviews, they describe their teams as their families, making them feel more connected to the clinic. They also enjoyed starting their day with familiar faces.
Primary Care Delivery System. The huddle is an important component of a system-wide transformation to provide team-based, patient-centered care to veterans. The efforts to strengthen and standardize the huddle have the potential to hasten this transformation while improving relationships and quality of care. Additionally, the CoE approach to integrating trainees into huddles has broader applicability and is being considered for adoption by other VA centers of excellence in primary care education.Primary Care Services. The huddle may contribute to efficiencies in a busy clinic setting. For example, the RN care manager can have upward of 1,200 patients on his/her panel and, between staff and trainees, as many as 12 health care providers with whom to communicate. The huddle strengthens the communications with providers and is an opportunity to touch base on the patients, coordinate care, and keep track of high-risk patients who might fall off the radar otherwise. The huddle is flexible and can occur with various clinic staff and providers. A 2-person huddle can occur between an RN and the primary provider. The QI projects that have been developed as a result of a huddle have improved clinic primary care services, such as completing opiate consents and urine toxicology or improving continuity through increased telephone clinic usage.Patient Outcomes. The huddle results in a more robust plan of care than might be developed by an individual provider who might not have time to consider options outside the individual’s scope of practice or expertise. While there are few clinical outcomes that are directly influenced by huddles alone, huddles may help indirectly improve patient outcomes on many fronts, including:
- Increased continuity of care because the patient now has a team focusing on care. At times throughout the day when team members cannot talk face to face with one another or with the patient, they know about the patient’s situation and are better able to establish a rapport when the patient calls or comes in for the visit. Trainees also become familiar with their practice partners’ patients, which allows them to ensure continuity when the patient’s primary trainee provider is out of clinic;
- Panel management and identifying and tracking sicker patients;
- Increased access, such as identifying patients who could receive care by a telephone visit, decreasing the number of no shows by making extra efforts to remind patients about appointments and improving follow up; and
- Improved population health outcomes from process improvements, such as the development of a process for having patients on opioids sign new contracts or identifying diabetics who might benefit from a group approach to care.
The Future
The huddle coach concept and checklist have been shared broadly and have applicability in other teaching settings where providers and clinic staff are learning how to implement huddles. A video and resources on “How to Huddle” are available at suzannecgordon.com/how-to-huddle/.
Under stage 2 of the CoEPCE program, the CoE will develop a huddle coaching program implementation kit composed of a huddle how-to guide and a coach training manual. The CoE team huddle is one of many VA huddles and an example of how the huddle continues to evolve. It is a versatile tool that can be used to focus on different topics and include different professions. Currently, it is being adapted to specialty care where there is large patient volume, such as cardiology and orthopedics.
1. Rugen KW, Watts SA, Janson SL, et al. Veteran Affairs Centers of Excellence in Primary Care Education: transforming nurse practitioner education. Nurs Outlook. 2014;62(2):78-88.
2. Chang A, Bowen JL, Buranosky RA, et al. Transforming primary care training--patient-centered medical home entrustable professional activities for internal medicine residents. J Gen Intern Med. 2013;28(6):801-809.
3. Zabar S, Adams J, Kurland S, et al. Charting a key competency domain: understanding resident physician interprofessional collaboration (IPC) skills. J Gen Intern Med. 2016;31(8):846-853.
4. Institute of Medicine. Measuring the Impact of Interprofessional Education (IPE) on Collaborative Practice and Patient Outcomes. Washington, DC: The National Academies Press; 2015.
5. Shunk R, Dulay M, Chou C, Janson S, O’Brien BC. Huddle-coaching: a dynamic intervention for trainees and staff to support team-based care. Acad Med. 2014;89(2):244-250.
6. Stock R, Mahoney E, Carney PA. Measuring team development in clinical care settings. Fam Med. 2013;45(10):691-700.
7. PeaceHealth. Team development measure. https://www.peacehealth.org/about-peacehealth/medical-professionals/eugene-springfield-cottage-grove/team-measure/Pages/measure. Accessed August 16, 2018.
8. American Board of Internal Medicine. Teamwork effectiveness assessment module. https://team.abim.org. Accessed August 16, 2018.
9. Chesluk BJ, Bernabeo E, Hess B, Lynn LA, Reddy S, Holmboe ES. A new tool to give hospitalists feedback to improve interprofessional teamwork and advance patient care. Health Aff (Millwood). 2012;31(11):2485-2492.
10. Rodriguez HP, Meredith LS, Hamilton AB, Yano EM, Rubenstein LV. Huddle up!: the adoption and use of structured team communication for VA medical home implementation. Health Care Manage Rev. 2015;40(4):286-299.
In short team huddles, trainees and PACT teamlets meet to coordinate care and identify ways to improve team processes under the guidance of faculty members who reinforce collaborative practice and continuous improvement.
In short team huddles, trainees and PACT teamlets meet to coordinate care and identify ways to improve team processes under the guidance of faculty members who reinforce collaborative practice and continuous improvement.
In 2011, 5 US Department of Veteran Affairs (VA) medical centers were selected by the VA Office of Academic Affiliations (OAA) to establish Centers of Excellence in Primary Care Education (CoEPCE). Part of the VA New Models of Care initiative, the 5 CoEPCEs (Boise, Cleveland, San Francisco, Seattle and West Haven) are utilizing VA primary care settings to develop and test innovative approaches to prepare physician residents, nurse practitioner (NP) students and residents (postgraduate), and other health professions trainees, such as pharmacy, social work, psychology, physician assistants (PAs), dieticians, etc for primary care practice.
The CoEPCEs are interprofessional academic patient aligned care teams (PACTs) defined by VA as a PACT that has at least 2 professions of trainees on the team engaged in learning.
The San Francisco VA Health Care System (SFVAHCS) Education in PACT (EdPACT)/CoEPCE developed and implemented a workplace learning model that embeds trainees into PACT teamlets and clinic workflow.1 Trainees are organized in practice partner triads with 2 second- or third-year internal medicine residents (R2s and R3s) and 1 NP student or resident. Physician residents rotate every 2 months between inpatient and outpatient settings and NP trainees are present continuously for 12 months. In this model, each trainee in the triad has his/her own patient panel and serves as a partner who delivers care to his/her partners’ patients when they are unavailable. Didactic sessions on clinical content and on topics related to the core domains occur 3-times weekly during pre- and postclinic conferences.2
Methods
In 2015, evaluators from the OAA reviewed background documents and conducted open-ended interviews with 9 CoEPCE staff, participating trainees, VA faculty, VA facility leadership, and affiliate faculty. Informants described their involvement, challenges encountered, and benefits of the huddle to participants, veterans, and the VA.
The Huddle
With the emphasis on patient-centered medical homes and team-based care in the Affordable Care Act, there is an urgent need to develop new training models that provide future health professionals with skills that support interprofessional communication and collaborative practice.2,3 A key aim of the CoEPCE is to expand workplace learning strategies and clinical opportunities for interprofessional trainees to work together as a team to anticipate and address the health care needs of veterans. Research suggests that patient care improves when team members develop a shared understanding of each other’s skill sets, care procedures, and values.4 In 2010, the SFVAHCS began phasing in VA-mandated PACTs. Each patient-aligned care teamlet serves about 1,200 patients and is composed of physician or NP primary care provider(s) (PCPs) and a registered nurse (RN) care manager, a licensed vocational nurse (LVN), and a medical support assistant (MSA). About every 3 teamlets also work with a profession-specific team member from the Social Work and Pharmacy departments. The implementation of PACT created an opportunity for the CoEPCE to add trainees of various professions to 13 preexisting PACTs in 3 SFVAHCS primary care clinics. This arrangement benefits both trainees and teamlets: trainees learn how to collaborate with clinic staff while the clinic PACT teamlets benefit from coaching by faculty skilled in team-based care.
As part of routine clinical activities, huddles provide opportunities for workplace learning related to coordination of care, building relationships, and developing a sense of camaraderie that is essential for team-based, patient-centered care. In their ideal state, huddles are “…the hub of interprofessional, team-based care”; they provide a venue where trainees can learn communication skills, team member roles, systems issues and resources, and clinical knowledge expected of full-time providers and staff.5 Embedding faculty in huddles as huddle coaches help ensure trainees are learning and applying these skills.
Planning and Implementation
After OAA funded the CoEPCE in 2011, faculty had 6 months to develop the EdPACT curriculum, which included a team building retreat, interactive didactic sessions, and workplace learning activities (ie, huddles). In July 2011, 10 trainee triads (each consisting of 2 physician residents and either a student NP or resident NP) were added to preexisting PACTs at the San Francisco VA Medical Center primary care clinic and 2 community-based outpatient clinics.
These trainee triads partnered with their PACT teamlets and huddled for 15 minutes at the beginning of each clinic day to plan for the day’s patients and future scheduled patients and to coordinate care needs for their panel of patients. CoEPCE staff built on this basic huddle model and made the following lasting modifications:
- Developed and implemented a huddle coach model and a huddle checklist to provide structure and feedback to the huddle (Online Resources);
- Scheduled huddles in NP student/resident’s exam room to reduce the hierarchy in the trainee triad;
- Incorporated trainees from other professions and levels into the huddle (psychology fellows, pharmacy residents, social work); and
- Linked the PACT teamlet (staff) to quality improvement projects that are discussed periodically in huddles and didactics.
Curriculum. The huddle allows for practical application of the 4 core domains: interprofessional collaboration (IPC), performance improvement (PI), sustained relationships (SR), and shared decision making (SDM) that shape the CoE curriculum.
Interprofessional collaboration (IPC) is the primary domain reinforced in the huddle. Trainees learn key content in half-day team retreats held at the beginning of the academic year and in interactive didactic sessions. These sessions, which draw on concepts from the Agency for Healthcare Research and Quality’s TeamSTEPPS, an evidence-based teamwork training program for health care workers, teach skills like closed-loop communication, check-backs, negotiation, and conflict resolution.
The CoE trainee triads also lead quality improvement (QI) projects, and the huddle is a venue for getting input, which reinforces the CoE’s performance improvement (PI) curriculum. For example, PACT teamlet staff members provide trainees with feedback on proposed QI interventions, such as increasing the use of telephone visits. The huddle supports SR among team members that enhance patient care while improving the quality of the clinic experience for team members. Strengthened communications and increased understanding of team member roles and system resources supports a patient-centered approach to care and lays the foundation for SDM between patients and team members.
Faculty Roles and Development. The CoEPCE physician and NP faculty members who precept and function as huddle coaches participate in monthly 2-hour faculty development sessions to address topics related to IPE. At least 1 session each year covers review of the items on the huddle checklist, tips on how to coach a huddle, discussions of the role of huddle coaches, and feedback and mentoring skills. Many huddle coach activities are inherent to clinical precepting, such as identifying appropriate clinical resources and answering clinical questions, but the core function of the huddle coach is to facilitate effective communication among team members.
Initially, a coach may guide the huddle by rounding up team members or directing the agenda of the huddle (ie, prompting the LVN to present the day’s patients and suggesting the group identify and discuss high-risk patients). As the year progresses, coaches often take a backseat, and the huddle may be facilitated by the trainees, the RN, LVN, or a combination of all members. During the huddle, coaches also may reinforce specific communication skills, such as a “check back” or ISBAR ( Identify who you are, describe the Situation, provide Background information, offer an Assessment of the situation/needs, make a Recommendation or Request)—skills that are taught during CoE didactic sessions.
The coach may call attention to particular feedback points, such as clarification of the order as an excellent example of a check-back. Each preceptor coaches 1 huddle per precepting session. After the teams huddle, preceptors do a smaller, shorter huddle in the precepting room to share successes, such as interprofessional trainees demonstrating backup behavior (eg, “in today’s huddle, I saw a great example of backup behavior when the medicine resident offered to show the NP student how to consent someone”) and discuss challenges (eg, getting all team members to the huddle).
Resources. The CoE staff schedule at least 20 huddles per week and coordinate preceptor and room schedules. The other required resources are clinic staff (RNs, LVNs, and MSAs) and exam rooms large enough to accommodate 8 or more people. Sufficient staffing coverage and staggered huddles also are important to allow cross-coverage for other clinical duties while team members and faculty are huddling.
Monitoring and Assessment. The CoE staff administer the Team Development Measure (TDM) twice yearly and a modified version of the TEAM 360 feedback survey once per year.6-9 The TDM member gages perceptions of team functioning (cohesiveness, communication, role clarity, and clarity of goals and means). Teams meet with a facilitator to debrief their TDM results and discuss ways to improve their team processes. Three-quarters of the way through the academic year, team members also complete the modified TEAM 360 survey on trainees. Each trainee receives a report describing his/her self-ratings and aggregate team member ratings on leadership, communication, interpersonal skills, and feedback.
Partnerships
In addition to CoEPCE staff and faculty support and engagement, huddles at SFVAHCS have benefited from partnerships with VA primary care leadership and with academic affiliates. In particular, support from the VA clinic directors and nurse managers was key to instituting changes to the clinics’ structure to include interprofessional trainees in huddles.
The affiliates—the University of California, San Francisco (UCSF) School of Medicine and School of Nursing—are integral partners and assist with NP student and medicine resident recruitment. These affiliates also participate in planning and refinement of CoEPCE curricular activities. The UCSF School of Nursing, School of Medicine, and Center for Faculty Educators were involved in the planning stages of the huddle model.
Challenges and Solutions
Having a staffing ratio that supports trainee participation in and learning through huddles is critical. Preceptor coverage must be in sufficient numbers to allow preceptors to coach the huddles, and clinical staff must be adequate to create cohesive and consistent teams for trainee providers. Clinic staff turnover and changes in teamlet staff can be very disruptive. Over time, teamlet staff often know key details and helpful contextual information about particular patients and clinic processes. This knowledge may be lost with turnover among teamlet staff. If team members miss huddles due to staffing shortages and clinical duties, there may be delays and errors in patient care. For example, if information discussed in the huddle is not relayed to the absent team member in a timely or accurate manner, care may be impacted. However, potential disruptions can be mitigated by a high-functioning team with strong communication skills and situational awareness who readily assist and distribute the workload.
Consistent huddling, huddle coaches, and checklists all help stabilize the group. Integration of trainees in the PACT team initially requires extra work because trainees are part-time and have panels significantly smaller than 1,200 (which means the teamlet staff are assigned to multiple trainee and provider huddles). However, teamlet staff find working with trainee teams personally rewarding, and developing highly functioning teams helps prevent burnout. Integration of pharmacy, psychology, and social work trainees takes time and thoughtful planning of activities and contributions that enhance team functioning while not overburdening trainees with additional responsibilities. If these other professions of health trainees are joining several teams’ huddles, their role may be to weigh in as needed vs preparing for and reviewing several PCPs’ schedules and patients in advance.
Factors for Success
The VA facility and primary care clinic leadership’s commitment to supporting staff participation in huddles was critical for integrating trainees into PACTs. Additionally, VA facility commitment to implementation of PACT was a key facilitating factor. Implementation of PACT, including huddles, has not been consistent at all VA facilities.10 The CoE’s approach to integrating trainees into the huddle was an opportunity to strengthen the huddle and to teach new staff members how to participate with team members in huddles. CoEPCE leadership, which has embraced change, meets regularly with facility leadership as well as an advisory board of UCSF leaders to update them on CoE activities. A critical factor for success was CoE expertise in interprofessional education and its ability to integrate concepts from the 4 core domains into an effective workplace learning experience, including attention to the physical space, scheduling, and the development and implementation of the huddle coach role and checklist.
Accomplishments and Benefits
There is evidence that SFVAHCS team huddles are achieving their goals and CoE trainees are being trained to provide team-based, patient-centered care to veterans. Key outcomes of the CoE’s approach to huddles include components in the next sections.
Interprofessional Educational Capacity. The CoEPCE faculty and staff consider the huddle to be one of the best ways to teach interprofessional communication and collaboration, team functioning, and clinical performance. Unlike a traditional didactic, classroom-based session on interprofessional collaboration, the huddle is an opportunity for health care professionals to work together to provide care in a clinic setting. It also is an activity in which the CoE has continued innovative activities, such as adding a preceptor huddle, incorporating additional professions, and encouraging panel management activities during huddles. The CoE has received significant interest and visibility and has been invited to share the model in numerous presentations.
Participants’ Knowledge, Attitudes, Skills, and Competencies. An aim of the CoE approach to huddles was to provide trainees with general skills in the core domain interprofessional collaboration, including teamwork and communication that transfer to other settings, such as inpatient teams and specialty clinics. Learning about other professions and their scopes of practice and areas of expertise can be helpful beyond huddles and primary care. Trainees also learn concepts and practices from the other core domains:
- Performance Improvement: The huddle is a venue for utilizing clinic metrics as well as focusing on QI projects that benefit from a team approach to solving problems;
- Sustained Relationships: The huddles support and teach the importance of relationships among the team. Trainees learn about the roles of clinic staff members, and clinic staff have more opportunities to interact with trainees and become comfortable with them, supporting coordinated care; and
- Shared Decision Making: The huddle is a venue for discussing options for providing patient decision-making support, such as discussing the pros and cons of colon cancer screening with a patient, improving patient-centered care.
Additionally, huddles can address differences in trainee clinical expertise. For example, new physician interns with less experience in the clinic receive more coaching on system resources and patient histories than they might otherwise. Nurse practitioner residents often participate in more than 1 huddle team and transition to a coaching role.
Sustained Relationships, Role Clarity, and Collaboration. The huddles are structured to facilitate SRs among trainees from different professions and among the PACT teamlet in detail as a team. The huddle increases team efficiency by educating trainees and staff about team member roles. For example, trainees learn how the LVNs and MSAs prepare for patient visits. Moreover, an opportunity exists to learn how provider and clinic staff expertise may overlap. Registered nurse care managers, who have their own hypertension clinics, can help manage a patient’s medication titration. Similarly, pharmacy trainees can suggest a referral for a complicated patient with diabetes to pharmacy clinic, where clinical pharmacists can adjust medications and provide patient education for hypertension, hyperlipidemia, and hyperglycemia. In this way, role clarity is improved and trainees learn how team members work within their scope of practice and are better able to “share the care.”
There is evidence that huddles have resulted in expanded participant interprofessional collaboration. The CoE administers the TDM twice a year and the huddle teams rate themselves on several dimensions—cohesiveness, communications, role clarity, and goals.6,7 The 2011/2012 findings showed that nearly all teams showed improvement, with the mean scores for all teams combined increasing from 59.4 in the fall to 64.6 in the spring (max score is 100).5 These scores increased again from 62.2 to 70.3 in 2012/2013, from 66.6 to 70.2 in 2013/2014, and from 64.6 to 69.9 in 2014/2015.
Expanding Clinical Knowledge. At the individual level, the huddle is an opportunity for a trainees to expand their clinical expertise in real time. The huddle provides exposure to a variety of patients and corresponding patient care needs. Trainees are encouraged to complete patient prerounds before the huddle in order to focus the huddle discussion on patients with chronic conditions, complex needs, recent hospitalizations, and upcoming appointments. The CoEPCE trainees tap into the expertise and experience of their team members and coach.
The clinic staff can get information from trainees about their plan of care while trainees get a more complete picture of a patient’s situation—for example, medical or social history or communication preferences. Additionally, trainees learn team skills, such as communication techniques and warm handoffs, which can be used in other clinical settings outside primary care and beyond the VA. As trainees advance, the huddle helps them learn to delegate appropriately, practice conflict negotiation, and develop leadership skills.
Participants’ Satisfaction With Interventions. There is qualitative evidence that clinic RNs and LVNs like huddles and appreciate having the opportunity to communicate in person with providers as well as to teach trainees how to work interprofessionally. Faculty members who are huddle coaches report that they develop a richer understanding of the skill set of trainees, information that can inform CoE curriculum design. Trainees appreciate the opportunity to develop relationships with team members. In end-of-year interviews, they describe their teams as their families, making them feel more connected to the clinic. They also enjoyed starting their day with familiar faces.
Primary Care Delivery System. The huddle is an important component of a system-wide transformation to provide team-based, patient-centered care to veterans. The efforts to strengthen and standardize the huddle have the potential to hasten this transformation while improving relationships and quality of care. Additionally, the CoE approach to integrating trainees into huddles has broader applicability and is being considered for adoption by other VA centers of excellence in primary care education.Primary Care Services. The huddle may contribute to efficiencies in a busy clinic setting. For example, the RN care manager can have upward of 1,200 patients on his/her panel and, between staff and trainees, as many as 12 health care providers with whom to communicate. The huddle strengthens the communications with providers and is an opportunity to touch base on the patients, coordinate care, and keep track of high-risk patients who might fall off the radar otherwise. The huddle is flexible and can occur with various clinic staff and providers. A 2-person huddle can occur between an RN and the primary provider. The QI projects that have been developed as a result of a huddle have improved clinic primary care services, such as completing opiate consents and urine toxicology or improving continuity through increased telephone clinic usage.Patient Outcomes. The huddle results in a more robust plan of care than might be developed by an individual provider who might not have time to consider options outside the individual’s scope of practice or expertise. While there are few clinical outcomes that are directly influenced by huddles alone, huddles may help indirectly improve patient outcomes on many fronts, including:
- Increased continuity of care because the patient now has a team focusing on care. At times throughout the day when team members cannot talk face to face with one another or with the patient, they know about the patient’s situation and are better able to establish a rapport when the patient calls or comes in for the visit. Trainees also become familiar with their practice partners’ patients, which allows them to ensure continuity when the patient’s primary trainee provider is out of clinic;
- Panel management and identifying and tracking sicker patients;
- Increased access, such as identifying patients who could receive care by a telephone visit, decreasing the number of no shows by making extra efforts to remind patients about appointments and improving follow up; and
- Improved population health outcomes from process improvements, such as the development of a process for having patients on opioids sign new contracts or identifying diabetics who might benefit from a group approach to care.
The Future
The huddle coach concept and checklist have been shared broadly and have applicability in other teaching settings where providers and clinic staff are learning how to implement huddles. A video and resources on “How to Huddle” are available at suzannecgordon.com/how-to-huddle/.
Under stage 2 of the CoEPCE program, the CoE will develop a huddle coaching program implementation kit composed of a huddle how-to guide and a coach training manual. The CoE team huddle is one of many VA huddles and an example of how the huddle continues to evolve. It is a versatile tool that can be used to focus on different topics and include different professions. Currently, it is being adapted to specialty care where there is large patient volume, such as cardiology and orthopedics.
In 2011, 5 US Department of Veteran Affairs (VA) medical centers were selected by the VA Office of Academic Affiliations (OAA) to establish Centers of Excellence in Primary Care Education (CoEPCE). Part of the VA New Models of Care initiative, the 5 CoEPCEs (Boise, Cleveland, San Francisco, Seattle and West Haven) are utilizing VA primary care settings to develop and test innovative approaches to prepare physician residents, nurse practitioner (NP) students and residents (postgraduate), and other health professions trainees, such as pharmacy, social work, psychology, physician assistants (PAs), dieticians, etc for primary care practice.
The CoEPCEs are interprofessional academic patient aligned care teams (PACTs) defined by VA as a PACT that has at least 2 professions of trainees on the team engaged in learning.
The San Francisco VA Health Care System (SFVAHCS) Education in PACT (EdPACT)/CoEPCE developed and implemented a workplace learning model that embeds trainees into PACT teamlets and clinic workflow.1 Trainees are organized in practice partner triads with 2 second- or third-year internal medicine residents (R2s and R3s) and 1 NP student or resident. Physician residents rotate every 2 months between inpatient and outpatient settings and NP trainees are present continuously for 12 months. In this model, each trainee in the triad has his/her own patient panel and serves as a partner who delivers care to his/her partners’ patients when they are unavailable. Didactic sessions on clinical content and on topics related to the core domains occur 3-times weekly during pre- and postclinic conferences.2
Methods
In 2015, evaluators from the OAA reviewed background documents and conducted open-ended interviews with 9 CoEPCE staff, participating trainees, VA faculty, VA facility leadership, and affiliate faculty. Informants described their involvement, challenges encountered, and benefits of the huddle to participants, veterans, and the VA.
The Huddle
With the emphasis on patient-centered medical homes and team-based care in the Affordable Care Act, there is an urgent need to develop new training models that provide future health professionals with skills that support interprofessional communication and collaborative practice.2,3 A key aim of the CoEPCE is to expand workplace learning strategies and clinical opportunities for interprofessional trainees to work together as a team to anticipate and address the health care needs of veterans. Research suggests that patient care improves when team members develop a shared understanding of each other’s skill sets, care procedures, and values.4 In 2010, the SFVAHCS began phasing in VA-mandated PACTs. Each patient-aligned care teamlet serves about 1,200 patients and is composed of physician or NP primary care provider(s) (PCPs) and a registered nurse (RN) care manager, a licensed vocational nurse (LVN), and a medical support assistant (MSA). About every 3 teamlets also work with a profession-specific team member from the Social Work and Pharmacy departments. The implementation of PACT created an opportunity for the CoEPCE to add trainees of various professions to 13 preexisting PACTs in 3 SFVAHCS primary care clinics. This arrangement benefits both trainees and teamlets: trainees learn how to collaborate with clinic staff while the clinic PACT teamlets benefit from coaching by faculty skilled in team-based care.
As part of routine clinical activities, huddles provide opportunities for workplace learning related to coordination of care, building relationships, and developing a sense of camaraderie that is essential for team-based, patient-centered care. In their ideal state, huddles are “…the hub of interprofessional, team-based care”; they provide a venue where trainees can learn communication skills, team member roles, systems issues and resources, and clinical knowledge expected of full-time providers and staff.5 Embedding faculty in huddles as huddle coaches help ensure trainees are learning and applying these skills.
Planning and Implementation
After OAA funded the CoEPCE in 2011, faculty had 6 months to develop the EdPACT curriculum, which included a team building retreat, interactive didactic sessions, and workplace learning activities (ie, huddles). In July 2011, 10 trainee triads (each consisting of 2 physician residents and either a student NP or resident NP) were added to preexisting PACTs at the San Francisco VA Medical Center primary care clinic and 2 community-based outpatient clinics.
These trainee triads partnered with their PACT teamlets and huddled for 15 minutes at the beginning of each clinic day to plan for the day’s patients and future scheduled patients and to coordinate care needs for their panel of patients. CoEPCE staff built on this basic huddle model and made the following lasting modifications:
- Developed and implemented a huddle coach model and a huddle checklist to provide structure and feedback to the huddle (Online Resources);
- Scheduled huddles in NP student/resident’s exam room to reduce the hierarchy in the trainee triad;
- Incorporated trainees from other professions and levels into the huddle (psychology fellows, pharmacy residents, social work); and
- Linked the PACT teamlet (staff) to quality improvement projects that are discussed periodically in huddles and didactics.
Curriculum. The huddle allows for practical application of the 4 core domains: interprofessional collaboration (IPC), performance improvement (PI), sustained relationships (SR), and shared decision making (SDM) that shape the CoE curriculum.
Interprofessional collaboration (IPC) is the primary domain reinforced in the huddle. Trainees learn key content in half-day team retreats held at the beginning of the academic year and in interactive didactic sessions. These sessions, which draw on concepts from the Agency for Healthcare Research and Quality’s TeamSTEPPS, an evidence-based teamwork training program for health care workers, teach skills like closed-loop communication, check-backs, negotiation, and conflict resolution.
The CoE trainee triads also lead quality improvement (QI) projects, and the huddle is a venue for getting input, which reinforces the CoE’s performance improvement (PI) curriculum. For example, PACT teamlet staff members provide trainees with feedback on proposed QI interventions, such as increasing the use of telephone visits. The huddle supports SR among team members that enhance patient care while improving the quality of the clinic experience for team members. Strengthened communications and increased understanding of team member roles and system resources supports a patient-centered approach to care and lays the foundation for SDM between patients and team members.
Faculty Roles and Development. The CoEPCE physician and NP faculty members who precept and function as huddle coaches participate in monthly 2-hour faculty development sessions to address topics related to IPE. At least 1 session each year covers review of the items on the huddle checklist, tips on how to coach a huddle, discussions of the role of huddle coaches, and feedback and mentoring skills. Many huddle coach activities are inherent to clinical precepting, such as identifying appropriate clinical resources and answering clinical questions, but the core function of the huddle coach is to facilitate effective communication among team members.
Initially, a coach may guide the huddle by rounding up team members or directing the agenda of the huddle (ie, prompting the LVN to present the day’s patients and suggesting the group identify and discuss high-risk patients). As the year progresses, coaches often take a backseat, and the huddle may be facilitated by the trainees, the RN, LVN, or a combination of all members. During the huddle, coaches also may reinforce specific communication skills, such as a “check back” or ISBAR ( Identify who you are, describe the Situation, provide Background information, offer an Assessment of the situation/needs, make a Recommendation or Request)—skills that are taught during CoE didactic sessions.
The coach may call attention to particular feedback points, such as clarification of the order as an excellent example of a check-back. Each preceptor coaches 1 huddle per precepting session. After the teams huddle, preceptors do a smaller, shorter huddle in the precepting room to share successes, such as interprofessional trainees demonstrating backup behavior (eg, “in today’s huddle, I saw a great example of backup behavior when the medicine resident offered to show the NP student how to consent someone”) and discuss challenges (eg, getting all team members to the huddle).
Resources. The CoE staff schedule at least 20 huddles per week and coordinate preceptor and room schedules. The other required resources are clinic staff (RNs, LVNs, and MSAs) and exam rooms large enough to accommodate 8 or more people. Sufficient staffing coverage and staggered huddles also are important to allow cross-coverage for other clinical duties while team members and faculty are huddling.
Monitoring and Assessment. The CoE staff administer the Team Development Measure (TDM) twice yearly and a modified version of the TEAM 360 feedback survey once per year.6-9 The TDM member gages perceptions of team functioning (cohesiveness, communication, role clarity, and clarity of goals and means). Teams meet with a facilitator to debrief their TDM results and discuss ways to improve their team processes. Three-quarters of the way through the academic year, team members also complete the modified TEAM 360 survey on trainees. Each trainee receives a report describing his/her self-ratings and aggregate team member ratings on leadership, communication, interpersonal skills, and feedback.
Partnerships
In addition to CoEPCE staff and faculty support and engagement, huddles at SFVAHCS have benefited from partnerships with VA primary care leadership and with academic affiliates. In particular, support from the VA clinic directors and nurse managers was key to instituting changes to the clinics’ structure to include interprofessional trainees in huddles.
The affiliates—the University of California, San Francisco (UCSF) School of Medicine and School of Nursing—are integral partners and assist with NP student and medicine resident recruitment. These affiliates also participate in planning and refinement of CoEPCE curricular activities. The UCSF School of Nursing, School of Medicine, and Center for Faculty Educators were involved in the planning stages of the huddle model.
Challenges and Solutions
Having a staffing ratio that supports trainee participation in and learning through huddles is critical. Preceptor coverage must be in sufficient numbers to allow preceptors to coach the huddles, and clinical staff must be adequate to create cohesive and consistent teams for trainee providers. Clinic staff turnover and changes in teamlet staff can be very disruptive. Over time, teamlet staff often know key details and helpful contextual information about particular patients and clinic processes. This knowledge may be lost with turnover among teamlet staff. If team members miss huddles due to staffing shortages and clinical duties, there may be delays and errors in patient care. For example, if information discussed in the huddle is not relayed to the absent team member in a timely or accurate manner, care may be impacted. However, potential disruptions can be mitigated by a high-functioning team with strong communication skills and situational awareness who readily assist and distribute the workload.
Consistent huddling, huddle coaches, and checklists all help stabilize the group. Integration of trainees in the PACT team initially requires extra work because trainees are part-time and have panels significantly smaller than 1,200 (which means the teamlet staff are assigned to multiple trainee and provider huddles). However, teamlet staff find working with trainee teams personally rewarding, and developing highly functioning teams helps prevent burnout. Integration of pharmacy, psychology, and social work trainees takes time and thoughtful planning of activities and contributions that enhance team functioning while not overburdening trainees with additional responsibilities. If these other professions of health trainees are joining several teams’ huddles, their role may be to weigh in as needed vs preparing for and reviewing several PCPs’ schedules and patients in advance.
Factors for Success
The VA facility and primary care clinic leadership’s commitment to supporting staff participation in huddles was critical for integrating trainees into PACTs. Additionally, VA facility commitment to implementation of PACT was a key facilitating factor. Implementation of PACT, including huddles, has not been consistent at all VA facilities.10 The CoE’s approach to integrating trainees into the huddle was an opportunity to strengthen the huddle and to teach new staff members how to participate with team members in huddles. CoEPCE leadership, which has embraced change, meets regularly with facility leadership as well as an advisory board of UCSF leaders to update them on CoE activities. A critical factor for success was CoE expertise in interprofessional education and its ability to integrate concepts from the 4 core domains into an effective workplace learning experience, including attention to the physical space, scheduling, and the development and implementation of the huddle coach role and checklist.
Accomplishments and Benefits
There is evidence that SFVAHCS team huddles are achieving their goals and CoE trainees are being trained to provide team-based, patient-centered care to veterans. Key outcomes of the CoE’s approach to huddles include components in the next sections.
Interprofessional Educational Capacity. The CoEPCE faculty and staff consider the huddle to be one of the best ways to teach interprofessional communication and collaboration, team functioning, and clinical performance. Unlike a traditional didactic, classroom-based session on interprofessional collaboration, the huddle is an opportunity for health care professionals to work together to provide care in a clinic setting. It also is an activity in which the CoE has continued innovative activities, such as adding a preceptor huddle, incorporating additional professions, and encouraging panel management activities during huddles. The CoE has received significant interest and visibility and has been invited to share the model in numerous presentations.
Participants’ Knowledge, Attitudes, Skills, and Competencies. An aim of the CoE approach to huddles was to provide trainees with general skills in the core domain interprofessional collaboration, including teamwork and communication that transfer to other settings, such as inpatient teams and specialty clinics. Learning about other professions and their scopes of practice and areas of expertise can be helpful beyond huddles and primary care. Trainees also learn concepts and practices from the other core domains:
- Performance Improvement: The huddle is a venue for utilizing clinic metrics as well as focusing on QI projects that benefit from a team approach to solving problems;
- Sustained Relationships: The huddles support and teach the importance of relationships among the team. Trainees learn about the roles of clinic staff members, and clinic staff have more opportunities to interact with trainees and become comfortable with them, supporting coordinated care; and
- Shared Decision Making: The huddle is a venue for discussing options for providing patient decision-making support, such as discussing the pros and cons of colon cancer screening with a patient, improving patient-centered care.
Additionally, huddles can address differences in trainee clinical expertise. For example, new physician interns with less experience in the clinic receive more coaching on system resources and patient histories than they might otherwise. Nurse practitioner residents often participate in more than 1 huddle team and transition to a coaching role.
Sustained Relationships, Role Clarity, and Collaboration. The huddles are structured to facilitate SRs among trainees from different professions and among the PACT teamlet in detail as a team. The huddle increases team efficiency by educating trainees and staff about team member roles. For example, trainees learn how the LVNs and MSAs prepare for patient visits. Moreover, an opportunity exists to learn how provider and clinic staff expertise may overlap. Registered nurse care managers, who have their own hypertension clinics, can help manage a patient’s medication titration. Similarly, pharmacy trainees can suggest a referral for a complicated patient with diabetes to pharmacy clinic, where clinical pharmacists can adjust medications and provide patient education for hypertension, hyperlipidemia, and hyperglycemia. In this way, role clarity is improved and trainees learn how team members work within their scope of practice and are better able to “share the care.”
There is evidence that huddles have resulted in expanded participant interprofessional collaboration. The CoE administers the TDM twice a year and the huddle teams rate themselves on several dimensions—cohesiveness, communications, role clarity, and goals.6,7 The 2011/2012 findings showed that nearly all teams showed improvement, with the mean scores for all teams combined increasing from 59.4 in the fall to 64.6 in the spring (max score is 100).5 These scores increased again from 62.2 to 70.3 in 2012/2013, from 66.6 to 70.2 in 2013/2014, and from 64.6 to 69.9 in 2014/2015.
Expanding Clinical Knowledge. At the individual level, the huddle is an opportunity for a trainees to expand their clinical expertise in real time. The huddle provides exposure to a variety of patients and corresponding patient care needs. Trainees are encouraged to complete patient prerounds before the huddle in order to focus the huddle discussion on patients with chronic conditions, complex needs, recent hospitalizations, and upcoming appointments. The CoEPCE trainees tap into the expertise and experience of their team members and coach.
The clinic staff can get information from trainees about their plan of care while trainees get a more complete picture of a patient’s situation—for example, medical or social history or communication preferences. Additionally, trainees learn team skills, such as communication techniques and warm handoffs, which can be used in other clinical settings outside primary care and beyond the VA. As trainees advance, the huddle helps them learn to delegate appropriately, practice conflict negotiation, and develop leadership skills.
Participants’ Satisfaction With Interventions. There is qualitative evidence that clinic RNs and LVNs like huddles and appreciate having the opportunity to communicate in person with providers as well as to teach trainees how to work interprofessionally. Faculty members who are huddle coaches report that they develop a richer understanding of the skill set of trainees, information that can inform CoE curriculum design. Trainees appreciate the opportunity to develop relationships with team members. In end-of-year interviews, they describe their teams as their families, making them feel more connected to the clinic. They also enjoyed starting their day with familiar faces.
Primary Care Delivery System. The huddle is an important component of a system-wide transformation to provide team-based, patient-centered care to veterans. The efforts to strengthen and standardize the huddle have the potential to hasten this transformation while improving relationships and quality of care. Additionally, the CoE approach to integrating trainees into huddles has broader applicability and is being considered for adoption by other VA centers of excellence in primary care education.Primary Care Services. The huddle may contribute to efficiencies in a busy clinic setting. For example, the RN care manager can have upward of 1,200 patients on his/her panel and, between staff and trainees, as many as 12 health care providers with whom to communicate. The huddle strengthens the communications with providers and is an opportunity to touch base on the patients, coordinate care, and keep track of high-risk patients who might fall off the radar otherwise. The huddle is flexible and can occur with various clinic staff and providers. A 2-person huddle can occur between an RN and the primary provider. The QI projects that have been developed as a result of a huddle have improved clinic primary care services, such as completing opiate consents and urine toxicology or improving continuity through increased telephone clinic usage.Patient Outcomes. The huddle results in a more robust plan of care than might be developed by an individual provider who might not have time to consider options outside the individual’s scope of practice or expertise. While there are few clinical outcomes that are directly influenced by huddles alone, huddles may help indirectly improve patient outcomes on many fronts, including:
- Increased continuity of care because the patient now has a team focusing on care. At times throughout the day when team members cannot talk face to face with one another or with the patient, they know about the patient’s situation and are better able to establish a rapport when the patient calls or comes in for the visit. Trainees also become familiar with their practice partners’ patients, which allows them to ensure continuity when the patient’s primary trainee provider is out of clinic;
- Panel management and identifying and tracking sicker patients;
- Increased access, such as identifying patients who could receive care by a telephone visit, decreasing the number of no shows by making extra efforts to remind patients about appointments and improving follow up; and
- Improved population health outcomes from process improvements, such as the development of a process for having patients on opioids sign new contracts or identifying diabetics who might benefit from a group approach to care.
The Future
The huddle coach concept and checklist have been shared broadly and have applicability in other teaching settings where providers and clinic staff are learning how to implement huddles. A video and resources on “How to Huddle” are available at suzannecgordon.com/how-to-huddle/.
Under stage 2 of the CoEPCE program, the CoE will develop a huddle coaching program implementation kit composed of a huddle how-to guide and a coach training manual. The CoE team huddle is one of many VA huddles and an example of how the huddle continues to evolve. It is a versatile tool that can be used to focus on different topics and include different professions. Currently, it is being adapted to specialty care where there is large patient volume, such as cardiology and orthopedics.
1. Rugen KW, Watts SA, Janson SL, et al. Veteran Affairs Centers of Excellence in Primary Care Education: transforming nurse practitioner education. Nurs Outlook. 2014;62(2):78-88.
2. Chang A, Bowen JL, Buranosky RA, et al. Transforming primary care training--patient-centered medical home entrustable professional activities for internal medicine residents. J Gen Intern Med. 2013;28(6):801-809.
3. Zabar S, Adams J, Kurland S, et al. Charting a key competency domain: understanding resident physician interprofessional collaboration (IPC) skills. J Gen Intern Med. 2016;31(8):846-853.
4. Institute of Medicine. Measuring the Impact of Interprofessional Education (IPE) on Collaborative Practice and Patient Outcomes. Washington, DC: The National Academies Press; 2015.
5. Shunk R, Dulay M, Chou C, Janson S, O’Brien BC. Huddle-coaching: a dynamic intervention for trainees and staff to support team-based care. Acad Med. 2014;89(2):244-250.
6. Stock R, Mahoney E, Carney PA. Measuring team development in clinical care settings. Fam Med. 2013;45(10):691-700.
7. PeaceHealth. Team development measure. https://www.peacehealth.org/about-peacehealth/medical-professionals/eugene-springfield-cottage-grove/team-measure/Pages/measure. Accessed August 16, 2018.
8. American Board of Internal Medicine. Teamwork effectiveness assessment module. https://team.abim.org. Accessed August 16, 2018.
9. Chesluk BJ, Bernabeo E, Hess B, Lynn LA, Reddy S, Holmboe ES. A new tool to give hospitalists feedback to improve interprofessional teamwork and advance patient care. Health Aff (Millwood). 2012;31(11):2485-2492.
10. Rodriguez HP, Meredith LS, Hamilton AB, Yano EM, Rubenstein LV. Huddle up!: the adoption and use of structured team communication for VA medical home implementation. Health Care Manage Rev. 2015;40(4):286-299.
1. Rugen KW, Watts SA, Janson SL, et al. Veteran Affairs Centers of Excellence in Primary Care Education: transforming nurse practitioner education. Nurs Outlook. 2014;62(2):78-88.
2. Chang A, Bowen JL, Buranosky RA, et al. Transforming primary care training--patient-centered medical home entrustable professional activities for internal medicine residents. J Gen Intern Med. 2013;28(6):801-809.
3. Zabar S, Adams J, Kurland S, et al. Charting a key competency domain: understanding resident physician interprofessional collaboration (IPC) skills. J Gen Intern Med. 2016;31(8):846-853.
4. Institute of Medicine. Measuring the Impact of Interprofessional Education (IPE) on Collaborative Practice and Patient Outcomes. Washington, DC: The National Academies Press; 2015.
5. Shunk R, Dulay M, Chou C, Janson S, O’Brien BC. Huddle-coaching: a dynamic intervention for trainees and staff to support team-based care. Acad Med. 2014;89(2):244-250.
6. Stock R, Mahoney E, Carney PA. Measuring team development in clinical care settings. Fam Med. 2013;45(10):691-700.
7. PeaceHealth. Team development measure. https://www.peacehealth.org/about-peacehealth/medical-professionals/eugene-springfield-cottage-grove/team-measure/Pages/measure. Accessed August 16, 2018.
8. American Board of Internal Medicine. Teamwork effectiveness assessment module. https://team.abim.org. Accessed August 16, 2018.
9. Chesluk BJ, Bernabeo E, Hess B, Lynn LA, Reddy S, Holmboe ES. A new tool to give hospitalists feedback to improve interprofessional teamwork and advance patient care. Health Aff (Millwood). 2012;31(11):2485-2492.
10. Rodriguez HP, Meredith LS, Hamilton AB, Yano EM, Rubenstein LV. Huddle up!: the adoption and use of structured team communication for VA medical home implementation. Health Care Manage Rev. 2015;40(4):286-299.
Treatment and Management of Patients With Non-Small Cell Lung Cancer (FULL)
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.
Click here to read the digital edition.
Comorbidities
Joshua M. Bauml, MD, Corporal Michael J. Crescenz VAMC, Philadelphia, PA. One of the
In addition, kidney dysfunction is quite common as a result of comorbid cardiovascular and hypertensive diseases. Kidney dysfunction can negatively impact our ability to administer both cisplatin and other systemic therapies.
Millie Das, MD, Palo Alto Health Care System, CA. Another major comorbidity for a lot of our veterans is COPD (chronic obstructive pulmonary disease). It doesn’t complicate the chemotherapy choice, but it affects surgical candidacy for those patients who present with early stage disease. Many times if you obtain pulmonary function tests in patients with COPD, the tests are abnormal and can prohibit safe surgical resection. These are patients that I see in the clinic and refer for definitive radiation, usually SABR (stereotactic ablative radiotherapy)/SBRT (stereotactic body radiation therapy), at a local radiation facility that can offer specialized radiation treatment.
Dr. Bauml. The fact that the VA has so many patients who require stereotactic radiosurgery for their early stage lung cancer represents an opportunity. There is a newly opened study that is evaluating SBRT vs surgery for these early stage lung cancer patients within the VA system. That study model has previously failed in multiple health care settings, but the VA is uniquely suited to answer this question.
Kelly A. Tammaro, PharmD, BCOP, Boston VA Healthcare System, MA. I would add heart failure patients or patients who have cardiac comorbidities and fluid restrictions. These restrictions can affect hydration that is needed for cisplatin, for example, as well as final volumes used to mix other chemotherapeutic agents with narrow concentration maximums, such as etoposide.
Julie Beck, RN, MSN, MPH, APRN-BC, VA Connecticut Healthcare System West Haven Campus. As a lung cancer navigator, I find that psychosocial comorbidities are an impediment to getting patients to diagnosis and treatment. Patients will miss appointments because they don’t have rides or will be reluctant to get imaging or other diagnostic testing because of anxiety or because it triggers PTSD (posttraumatic stress disorder) or because they are concerned about cost.
Dr. Das. I couldn’t agree more.
Dr. Bauml. It’s a great point.
Ms. Beck. You have to think outside the box with this patient population. We treat patients from as far away as Western Massachusetts. We have a dedicated oncology social worker who helps to arrange transportation. We have our CLC ( community living center), which is a rehabilitation and hospice unit but is also a resource for patients who live alone or far away and are getting an aggressive daily treatment regimen such as combined chemotherapy and radiation. We admit some patients to the CLC during their treatment to ensure that they get their treatment on time, maintain their nutritional status, and to provide emotional support. This is not an acute medical bed. Patients will sometimes go home on the weekend, but the support of the CLC increases the chance that they will get through their treatment safely.
Cancer care requires a lot of handholding. We often have to make multiple telephone calls to persuade our patients to get imaging or biopsies. Some of our patients require admission following biopsy because they live alone and have no one to drive them home following the procedure.
Dr. Tammaro. Boston has a similar model. We have a social worker who is highly dedicated and is able address our patients needs immediately. We also have many patients with PTSD and other psychological comorbidities, and depending on the severity, may require admission for their treatment to avoid the overwhelming nature of the ambulatory setting. For those who have to travel long distances for treatment we the Huntington House, which is housing located next door to our ambulatory campus. This accommodation can be used by our patients and their caregivers. We also have long term care facilities and a hospice unit located at our Brockton facility.
Ms. Beck. In West Haven, we have both palliative care and health psychology providers embedded in our clinic. They assist with symptom management and issues related to coping with diagnosis, anxiety, sleep, pain, smoking cessation, and lifestyle changes. We have also been offering pet therapy through our social work team, which has been very helpful for many of our patients.
Dr. Bauml. Mental health issues also can affect the choice of the type of treatment. Patients who have severe claustrophobia associated with their PTSD may have difficulty undergoing radiation. This can impact their ability to comply with therapy, and we have to adjust the treatment accordingly. For instance, I have a patient who has a known brain metastasis that was treated with definitive intent, but this gentleman gets highly agitated doing a brain magnetic resonance image (MRI). Instead we have had to follow him with serial computed tomography (CAT) scans, which is suboptimal. We have discussed that, but the distress that it causes him is simply not worth it.
Dr. Das. In some instances, we have had to use IV sedation for some of our patients with severe claustrophobia just to be able to get them through a positron emission tomography (PET) scan as part of their staging workup. We discuss these types of challenging cases in a multidisciplinary setting in our thoracic tumor board in order to brainstorm and figure out a realistic plan with our radiology and anesthesia colleagues, with the goal of getting the patient through the necessary tests in order to establish a treatment recommendation.
Due to underlying mental health or other health issues, some of our patients may also have difficulty with breath holding or with following other necessary instructions during their radiation treatments. We sometimes have to get creative on an individual basis in order to help a patient get through the needed treatment.
We have a dedicated psychologist and social worker who are embedded in our clinics and work closely with the oncology providers to offer strategies that can help our patients comply and complete the recommended treatment plan.
Rural Care
Dr. Bauml. One of the questions that comes up frequently when you have a patient who is remote is the type of treatment that you can administer. It’s difficult to administer a weekly therapy if somebody’s traveling 3 hours to see you every time. That can play into your decision making as you’re choosing a chemotherapy. If there are equivalent treatment regimens and one involves visits every 3 weeks and one involves weekly visits, well, that will help sway your decision making after discussion with the patient.
We often have to balance things. For instance, when I give someone carboplatin and paclitaxel, my preference is to administer it weekly with 3 weeks on and 1 week off. However, if a patient tells me, “You know, I do not want to come in once a week,” then I will discuss with them my concern for the increased adverse effects (AEs) with the every-3-week dosing. We will do it and then watch them closely. Of course, this gets even more complicated when you consider the fact that many of these patients have multiple medical comorbidities, so you’d like to administer the treatments in the least toxic way possible.
Ms. Beck. We have overcome some of those challenges by partnering with the primary care doctors. We are very close to our primary care colleagues in Massachusetts. They will order labs for the patient the day before the patient's appointment, so if the patient has a long drive, we already have their lab work; and they are ready to go when they get here for their treatment. The nursing staff is very aware of who needs to get on a shuttle back to Massachusetts. For some patients, we will have them stay overnight before their treatment.
Precision Oncology
Dr. Tammaro. In Boston, we have integrated Precision Oncology to be part of clinical practice, which we started with metastatic lung cancer patients. The VA Precision Oncology Program (POP) began at our healthcare center. We had to evaluate the genetic testing platforms, the accuracy of the results, and amount of tissue necessary for the laboratories. We have since succeeded in sending high-quality samples to the laboratories that generate accurate results. However, for your standard mutation panel for identifying therapy for first line treatment in lung cancer, we still use our local send out laboratory.
The POP has rolled out nationwide, and it is another clinical tool, especially for patients who have already failed multiple lines of therapy. When we send for a precision oncology consult, the “N of 1” report provides annotation. The report will generate a review of relevant literature and provide available abstracts or phase 1 or 2 trials that support a targeted therapy against potential point mutation for your patient.
The POP also has a research component, known as Re-POP. The goal is to open bucket trials that assess targeted therapy off label. Re-POP allows us to recontact these patients in the future to say, “You had your tissue sent through precision oncology, and you were diagnosed with a certain point mutation. Now we have a clinical trial that’s available. Would you be interested?” The plan is to have those clinical trials open and available to our patients when we receive the results from precision oncology.
I have used POP for 2 metastatic prostate cancer patient who exhausted all lines of therapy in hopes to identify a potential BCRA 1/2 mutation in order for us to use a PARP inhibitor. Unfortunately, neither harbored this mutation. Precision oncology does not perform immunohistochemistry, therefore identifying HER-2 or PD-L1 status for example, would need to be done through your local laboratory. I have found POP to be helpful in identifying a patients potential therapeutic option after progression on first/second line therapy, by sending tissue to POP initially or at the time of relapse.
Dr. Das. In our clinical practice at the Palo Alto VA, we follow the National Comprehensive Cancer Network (NCCN) guidelines, and we routinely evaluate for the presence of an EGFR mutation and also for ALK and ROS1 translocations in all lung cancer patients with nonsquamous histology. We send our molecular testing through Quest Diagnostics (Madison, NJ), and we usually get results back within a week or so.
For those patients who do not have any of those targetable gene alterations, we will go ahead and send for next-generation sequencing through POP, which allows testing of a much broader gene panel. Those results can take about a month or so to come back. I usually don’t wait for these results in order to get someone started on treatment. For patients without EGFR, ALK, or ROS1 found on initial testing, I will go ahead and start them on IV systemic chemotherapy. It is often very useful when you do get the next-generation sequencing results back, since in almost all cases, a gene alteration can be detected and is provided in the accompanying report. In a large subset of lung cancer cases, a gene alteration is seen in KRAS, for which we still do not have an effective targeted therapy. Despite this, I still find it useful to obtain the results because we generally feel that the driving genetic alterations occur mutually exclusive of one another. When we do see KRAS reported from a patient’s tumor specimen, we’re not generally looking for other types of mutations, so I find it helpful to know what is the alteration that is driving the growth of a patient’s tumor. The trend moving forward is to perform next-generation sequencing on all tumor specimens regardless of tumor type or histology, which can hopefully enable us to get to the bottom of what the driving genetic alteration is and to see if there are any targeted treatment approaches that can be offered to the patient.
In a few lung cancer cases, I have seen alterations in HER2 and BRAF that have been detected and reported using a next-generation sequencing platform. Just recently the FDA approved the BRAF-directed therapies of dabrafenib and trametinib for patients with lung cancer who are found to have a BRAF V600E mutation. It is hoped that as the FDA continues to provide approvals for targeted drugs in patients with lung cancer, the VA formulary will be able to offer these therapies to our veteran patients with the ultimate goal of providing treatment that has increased efficacy and less toxicity compared to conventional IV chemotherapy.
One of my frustrations earlier on was when we did find these more rare targetable mutations, I would run into problems with the VA formulary in allowing me to prescribe certain targeted therapies. In many cases, if the drug was not FDA-approved for lung cancer, I was told that I couldn’t use it and would have to go through the appeal process, which was quite onerous. Moving forward, we are seeing more and more data and trials with newer targeted agents in lung cancer, leading to new FDA approvals. With these approvals, I think it will be easier to be able to offer these targeted therapies to our patients.
Dr. Bauml. One of the issues that arises when we’re discussing even the FDA-approved therapies, is that many of these targeted therapies are relatively rare, and they’re especially rare amongst veterans. Now others have mentioned BRAF and HER2, and these do have some overexpression and mutations that occur among smokers. But the more common targetable genetic aberrations, EGFR, ALK, and ROS1 are more common amongst never-smokers. Given the high prevalence of tobacco use among veterans, these changes are rare. The incidence of ALK translocation is 3% to 7%. The incidence amongst veterans is likely much lower than that, given the tobacco abuse—to the point that I actually had a patient who had an ALK translocation; and of course, I prescribed the patient crizotinib. This was prior to the ALEX Trial and alectinib data. I prescribed crizotinib and was told it wasn’t on the formulary. Initially I was surprised, but when I said, “Well, look, when was the last time someone within our VA has prescribed crizotinib?” The answer was never.
This is the difficulty: As we enter this era of molecularly targetable therapy, the way we structure our formularies and the way that we review these data is going to have to change. This year at the American Society of Clinical Oncology (ASCO) meeting there were some very exciting lung cancer abstracts that evaluated ado-trastuzumab emtansine, which is an antibody drug conjugate currently approved for the treatment of HER2 overexpressing breast cancer. The abstracts showed response rates of up to 40% in lung cancer with the administration of this drug in HER2-mutated lung cancer. The HER2-amplified still had a response rate of 20%, which given the toxicity profile of this agent, is quite appealing. Being able to explore these early phase studies, as was described through the personalized medicine pathway, is, a great step forward for VA care.
Dr. Tammaro. The PBM in collaboration with the POP Advisory Board, are developing different levels of evidence to support the use of targeted medications identified to be potential therapy in those diagnosed with a point mutation. Even if a medication does not have an FDA approval, it has to have some evidence to support its use in a particular cancer. If you identify a point mutation or biomarker in a patient and provide evidence to supports its use within that particular disease state, the VA pharmacy could approve its use based off of that evidence. VA pharmacy would not require an actual FDA approval for that indication.
What the VISNs, PBM, and precision oncology are trying to do is determine the level of evidence that we have to support or approve use of a targeted therapy. We are definitely moving forward and changing the horizon on how we actually treat our patients after they’ve gone through first-line therapy. We are trying to figure out where these point mutations come in, the line of the therapy, and how we actually treat these cancers. Pharmacy is making a step forward in conjunction with Michael Kelley, MD, the National Program Director for Oncology, Specialty Care Services, whose group is establishing those guidelines.
Dr. Bauml. I don’t mean to downplay the difficulty of that process. This is a huge, difficult process. One only needs to look at the long line of failed trials looking at PI3 kinase inhibitors to show that just knowing that a mutation exists does not necessarily mean that a targeted therapy works in that space.
Drawing that line is really complicated, both within the VA and, indeed, outside of the VA. It’s a really complicated process, and understanding the implications of different mutations is only going to get more complicated. Of course, now we have things like NTRK and even rarer genetic aberrations that are going to affect not only lung cancer, but also a wide range of malignancies.
Promising Research
Dr. Bauml. The pathways that are emerging as clear driver mutations for which we have available therapies, at least within lung cancer, are MET exon 14, RET, and NTRK. I am also intrigued by the emerging data in the HER2 space.
Dr. Das. The other therapy that has been getting a lot of press is immunotherapy, of course. And I’ve been seeing many really good responders to immunotherapy within the veteran population that I treat. It is felt that degree of PD-L1 expression correlates with responsiveness to the immune check point inhibitors that are being used in lung cancer, and we are tending to see higher rates of PD-L1 expression in patients who are prior or current smokers who have a higher overall tumor mutation burden.
I see patients both at Stanford and at the Palo Alto VA, and I have noticed that the patients that I have been treating at the VA tend to have higher levels of PD-L1 expression with better responses to the immunotherapy drugs, probably because most of the VA patients are former or current smokers. And, another interesting observation is that these veteran patients are, for whatever reason, having a lower incidence of some of the autoimmune AEs seen with these immune checkpoint inhibitors. I have been keeping an eye out for more data and information to support these observations I have had in my clinical practice and I specifically attended ASCO this year to learn more about what others have seen and studied with immune check point inhibition in lung cancer. We are learning now that PD-L1 is not a perfect marker for predicting response to the checkpoint inhibitors and the other immunotherapeutic agents, and there is a great deal of research going on to try to figure out what other biomarkers could be useful and which patients are most likely to benefit from these drugs.
I was excited to hear about the combination of nivolumab and ipilimumab that is being tested in both mesothelioma and in small-cell lung cancer where we really don’t have as many treatment options as we have in non-small cell lung cancer. That data was quite exciting, and interestingly, there does not seem to be a correlation with PD-L1 expression and responsiveness to treatment with the immunotherapeutic agents in those histologic subtypes. The story is still unfolding, and we await additional data to help guide us in our treatment decisions.
Dr. Tammaro. Immunotherapy is the new fad in oncology. We have just scheduled our first patient for first-line therapy due to PD-L1 tumor proportion score is > 50%. Recently, at ASCO KEYNOTE-021 researchers looked at using pembrolizumab in combination with carboplatin plus pemetrexed chemotherapy for first-line metastatic non-squamous NSCLC. The research suggested that patients treated with pembrolizumab + chemotherapy continued to derive a higher overall response rate and progression free survival when compare with those on chemotherapy alone despite a low or no PD-L1 tumor expression.
It’s very interesting that many clinical trials that we’re evaluating are now using some type of checkpoint inhibitor up front with cytotoxic chemotherapy. If they are positive trials, this could change how patients are treated up front.
Dr. Bauml. There was some really interesting data that were presented at ASCO this year by Matthew Hellmann, MD, which evaluated the predictive nature of PD-L1 vs tumor mutation burden and other biomarkers, including gene expression profiling. In this particular abstract, the PD-L1 and tumor mutation burden really do function as orthogonal biomarkers such that a patient who has high PD-L1 and high tumor mutation burden is the most likely to respond. Patients who are really low for both are unlikely to respond. We really need better biomarkers for immunotherapy, though. PD-L1 has a lot of limitations, namely, it is dynamic, so over time it changes. So I can do a biopsy at one point, then treat the patient and the PD-L1 may change.
More importantly, it’s heterogeneous. There was this great paper by McLaughlin and colleagues in JAMA Oncology (2016) who described a patient who had a small tumor biopsy. They took a micrograph of the tumor and showed that one part of the micrograph was completely floridly PD-L1 positive. At another site of the same biopsy it was completely stone-cold negative, which is humbling when you think about the fact that we stick small needles into tumors and make clinical decisions on the basis of that.
The KEYNOTE-024 study evaluated pembrolizumab vs chemotherapy in high PD-L1 expressers. It’s a very exciting study, but at the end of the day even in this highly select patient population, the response rate to immunotherapy was only about 50%, which is not the sort of biomarker-driven response that we’re used to seeing with our EGFR inhibitors. That’s really what we want to get to. More important even than that is being able to say the negative predictive value. One of the reasons that we’re probably seeing more responses among veterans is that we know that patients who are veterans who have high tobacco exposure have a higher tumor mutation burden. I’m surprised to hear about the immune-related AEs, actually, because one of the things that was reported this year at ASCO was some data that showed that patients who have immune-related AEs are more likely to have a better outcome, which is an interesting biomarker of response.
Dr. Das. I heard that as well, and I found that to be really interesting. The patients that I’ve had on nivolumab for over a year are doing very well. These are stage IV patients who have essentially had complete responses to treatment and have not had any or have had very minor immune-related AEs to date.
Overall, these are a small numbers of patients, but I have been curious to see why that might be the case. Anecdotally, my colleagues and I who treat patients at Stanford have seen significantly higher rates of grades 3 and 4 pneumonitis and other autoimmune toxicities, such as myocarditis and enterocolitis, in those lung cancer patients who are light or never-smokers treated with immune checkpoint inhibitors.
Dr. Bauml. I really feel that PD-L1 as a biomarker has significant limitations. I certainly hope that in at least 2 or 3 years we’re not going to be talking about PD-L1 anymore. I’m hopeful that we’ll be able to use better predictive biomarkers, such as mutational burden and gene expression profiling. In the data in head and neck that was presented this year at ASCO, patients who were low for both gene expression profiling and mutational burden had a very low response (Haddad et al, ASCO 2017).
That’s really what you want to be. You want to be able to say, “Here’s a person who will not benefit from this therapy.” From there you can identify, based upon these biomarkers, the combination that is going to be best for this person. Is it chemoimmunotherapy or combination immunotherapy with CTLA4, or another checkpoint blockade? That is really the way that we’re going to be able to fine-tune this, because the toxicity is substantial for some treatments, like the nivolumab/ipilimumab combination. Using them in a biomarker-blind fashion is just scary to me, honestly.
Managing Adverse Reactions
Dr. Tammaro. The increasing amount of oral chemotherapy has posed a significant challenge. As a clinical oncology pharmacist, it was difficult to grasp the most effective way to follow all these patients and ensure adherence, adverse drug event reporting/significance and adequate follow up. When patients are receiving IV chemotherapy, we know we will see them, we are assured compliance and are able to assess side effects in a timely manner. When we give oral chemotherapy, the tables are turned, where the responsibility is now on the patient. We are now depending on the patient to ensure they are taking the medication correctly and we may not see AEs if the patient misses an appointment or feels as though they are bothering the provider by calling.
In 2012, we started an oral oncology clinic here at the VA in Boston that I found to be extremely effective. When you’re sending a patient home with an oral chemotherapy, you have to make sure that you are counseling them correctly and encourage them to call at any time if they are experiencing any type of AE. One of the newest issues we have been seeing is bleeding with ibrutinib, especially in those patients on anticoagulation therapies.
A general strategy we employee for oral chemotherapy is to start at half dose and titrate slowly. This method has been effective in identifying AEs and preventing delays in therapy. We do this for the majority of oral chemotherapy. Patients are given a 2 week supply to start and then are reassessed on follow up for escalation to the target dose. We do not place refills on oral oncology prescriptions. They are instructed to call 10 days prior to running out if they are not scheduled to come in for an appointment. Having consistent dialogue with our patients allows us to assess for adherence, AEs, and tolerability. The other advantage to this clinic is ensuring our patients have someone to speak to at all times and answer all their questions. Direct lines of communication is what most of our patients are appreciative of when paying gratitude to the clinic.
Ms. Beck. We have an oral chemotherapy clinic staffed by dedicated oncology pharmacists. Patients meet with the pharmacist and have education prior to starting a new oral chemotherapy. They will then be followed by both the oncology provider and the pharmacist.
Dr. Das. One of the challenges we also face is with so many of our patients living so far away. When our patients do have AEs that require hospitalization, it can be very tricky to really get a sense for how they are being managed at the outside community (non-VA) facility. Sharing of electronic medical records can be a challenge in these cases, and I worry that the care teams at the more remote hospitals may not be as familiar with the newer cancer treatments and the toxicities associated with them, such as the autoimmune AEs associated with many of the immune checkpoint inhibitors.
I provide patients with pocket cards to keep in their wallets with my contact information and the name of the drug that they are getting because not all patients can remember or even pronounce the names of the drugs and may not be able to tell their local treating physician and care team what they are getting. I have been getting more frequent phone calls from emergency department physicians and hospitalists from the local communities where many of our veterans live, because they want guidance on how best to approach treatment for our patients when they show up with an AE related to their cancer treatment.
At times, the presenting symptoms may be vague or nonspecific, but for our patients being treated with immunotherapy, we always have to keep in mind the possibility of immune-related AEs because we know that prompt initiation of steroids is critical in these cases and can really help the patients feel better quickly.
Dr. Tammaro. You bring up a valid point. Our pharmacists meet with all the patients on checkpoint inhibitors. Specifically, when we started using ipilimumab it was uncharted territory for our team. We put together take home medication bag that included hydrocortisone cream, methylprednisolone dose pak, dipheydramine, and loperamide. This was utilized for all patients and specific attention was given to patients who lived far away from an emergency room. This bag system was accompanied by “what to do if I have this symptom” handout that outlined which medication to take depending on the severity of the AE. A direct line phone line to the oncology pharmacy also was supplied.
With the evolution to the PD-L1s and the anti-PD inhibitors, we haven’t seen the same level of AEs. Patients go home with wallet cards that includes our staff contact numbers/pagers. The wallet card also serves as information to a treating provider if the patient presents outside the VA, to ensure they understand the severity of a potential autoimmune AE, such as diarrhea.
Another challenge is shared-care patients. We have patients coming from outside hospitals, and at times they want to use this pharmacy like a CVS, and it just doesn’t operate that way. We want to collaborate with others. Most shared care patients present to our service for oral chemotherapy because the veteran just can’t afford the copays. So, we will see the patient concurrently. They can still see their outside hospital physician as well, but they have to fax us the laboratory results and progress notes on a monthly basis (or longer depending on where they are in there therapy). Before we fill their medications, we talk to the patients, the same way we would treat a veteran who was getting their oral chemotherapy here. In addition, they need to be seen by the VA physician at least every 3 months. We want our veterans to feel comfortable with the cancer care and help them out as best as we can.