User login
Accuracy of Endoscopic Ultrasound in Staging of Early Rectal Cancer (FULL)
Endoscopic ultrasound can be highly accurate for the staging of neoplasms in early rectal cancer.
Colorectal cancer is the second most common cause of cancer death in the US, with one-third of all colorectal cancers occurring within the rectum. Each year, an estimated 40000 Americans are diagnosed with rectal cancer (RC).1,2 The prognosis and treatment of RC depends on both T and N stage at the time of diagnosis.3-5 According to the most recent National Comprehensive Cancer Network guidelines from May 2019, patients with T1 to T2N0 tumors should undergo transanal or transabdominal surgery upfront, whereas patients with T3 to T4N0 or any TN1 to 2 should start with neoadjuvant therapy for better locoregional control, followed by surgery.6 Therefore, the appropriate management of RC requires adequate staging.
Endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and computed tomography (CT) are the imaging techniques currently used to stage RC. In a meta-analysis of 90 articles published between 1985 and 2002 that compared the 3 radiologic modalities, Bipat and colleagues found that MRI and EUS had a similar sensitivity of 94%, whereas the specificity of EUS (86%) was significantly higher than that of MRI (69%) for muscularis propria invasion.7 CT was performed only in a limited number of trials because CT was considered inadequate to assess early T stage. For perirectal tissue invasion, the sensitivity of EUS was statistically higher than that of CT and MRI imaging: 90% compared with 79% and 82%, respectively. The specificity estimates for EUS, CT, and MRI were comparable: 75%, 78%, and 76%, respectively. The respective sensitivity and specificity of the 3 imaging modalities to evaluate lymph nodes were also comparable: EUS, 67% and 78%; CT, 55% and 74%; and MRI, 66% and 76%.
The role of EUS in the diagnosis and treatment of RC has long been validated.1,2-5 A meta-analysis of 42 studies involving 5039 patients found EUS to be highly accurate for differentiating various T stages.8 However, EUS cannot assess iliac and mesenteric lymph nodes or posterior tumor extension beyond endopelvic fascia in advanced RC. Notable heterogeneity was found among the studies in the meta-analyses with regard to the type of equipment used for staging, as well as the criteria used to assess the depth of penetration and nodal status. The recent introduction of phased-array coils and the development of T2-weighted fast spin sequences have improved the resolution of MRI. The MERCURY trial showed that extension of tumor to within 1 mm of the circumferential margin on high-resolution MRI correctly predicted margin involvement at the time of surgery in 92% of the patients.9 In the retrospective study by Balyasnikova and colleagues, MRI was found to correctly identify partial submucosal invasion and suitability for local excision in 89% of the cases.10
Therefore, both EUS and MRI are useful, more so than CT, in assessment of the depth of tumor invasion, nodal staging, and predicting the circumferential resection margin. The use of EUS, however, does not preclude the use of MRI, or vice versa. Rather, the 2 modalities can complement each other in staging and proper patient selection for treatment.11
Despite data supporting the value of EUS in staging RC, its use is limited by a high degree of operator dependence and a substantial learning curve,12-17 which may explain the low EUS accuracy observed in some reports.7,13,15 Given the presence of recognized alternatives such as MRI, we decided to reevaluate EUS accuracy for the staging of RC outside high-volume specialized centers and prospective clinical trials.
Methods
A retrospective chart review was performed that included all consecutive patients undergoing rectal ultrasound from January 2011 to August 2015 at the US Department of Veterans Affairs Medical Center (VAMC) in Memphis, Tennessee. Sixty-five patients with short-stocked or sessile lesions < 15 cm from anal margin staged T2N0M0 or lower by endorectal ultrasound (ERUS) were included. The patients with neoplasms staged in excess of T2 or N0 were excluded from the study because treatment protocol dictates immediate neoadjuvant treatment, the administration of which would affect subsequent histopathology.
For the 37 patients included in the final analysis, ERUS results were compared with surgical pathology to ascertain accuracy. The resections were performed endoscopically or surgically with a goal of obtaining clear margins. The choice of procedure depended on size, shape, location, and depth of invasion. All patients underwent clinical and endoscopic surveillance with flexible sigmoidoscopy/EUS every 3 to 6 months for the first 2 years. We used 2 different gold standards for surveillance depending on the type of procedure performed to remove the lesion. A pathology report was the gold standard used for patients who underwent surgery. In patients who underwent endoscopic resection, we used the lack of recurrent disease, determined by normal endoscopic and endoscopic ultrasound examination, to signify complete endoscopic resection and therefore adequate staging as an early neoplasm.
Results
From January 2011 to August 2015, 65 rectal ultrasounds were performed. All EUS procedures were performed by 1 physician (C Ruben Tombazzi). All patients had previous endoscopic evaluation and tissue diagnoses. Twenty-eight patients were excluded: 18 had T3 or N1 disease, 2 had T2N0 but refused surgery, 2 had anal cancer, 3 patients with suspected cancer had benign nonneoplastic disease (2 radiation proctitis, 1 normal rectal wall), and 3 underwent EUS for benign tumors (1 ganglioneuroma and 2 lipomas).
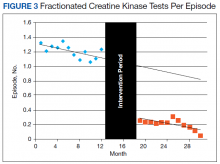
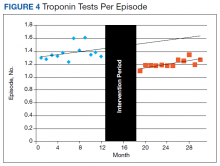
Thirty-seven patients were included in the study, 3 of whom were staged as T2N0 and 34 as T1N0 or lower by EUS. All patients were men ranging in age from 43 to 73 years (mean, 59 years). All 37 patients underwent endoscopic or surgical resection of their early rectal neoplasm. The final pathologic evaluation of the specimens demonstrated 14 carcinoid tumors, 11 adenocarcinomas, 6 tubular adenomas with high-grade dysplasia, and 6 benign adenomas. The preoperative EUS staging was confirmed for all patients, with 100% sensitivity, specificity, and accuracy. None of the patients who underwent endoscopic or surgical transanal resection had recurrence, determined by normal endoscopic and endoscopic ultrasound appearance, during a mean of 32.6 months surveillance.
Discussion
EUS has long been a recognized method for T and N staging of RC.1,3-5,7,8 Our data confirm that, in experienced hands, EUS is highly accurate in the staging of early rectal cancers.
The impact of EUS on the management of RC was demonstrated in a Mayo Clinic prospective blinded study.1 In that cohort of 80 consecutive patients who had previously had a CT for staging, EUS altered patient management in about 30% of cases. The most common change precipatated by EUS was the indication for additional neoadjuvant treatment.
However, the results have not been as encouraging when ERUS is performed outside of strict research protocol. A multicenter, prospective, country-wide quality assurance study from > 300 German hospitals was designed to assess the diagnostic accuracy of EUS in RC.13 Of 29206 patients, 7096 underwent surgery, without neoadjuvant treatment, and were included in the final analysis. The correspondence of tumor invasion with histopathology was 64.7%, with understaging of 18% and overstaging of 17.3%.13 These numbers were better in hospitals with greater experience performing ERUS: 73% accuracy in the centers with a case load of > 30 cases per year compared with 63.2% accuracy for the centers with < 10 cases a year. Marusch and colleagues had previously demonstrated an EUS accuracy of 63.3% in a study of 1463 patients with RC in Germany.14 Another study based out of the UK had similar findings. Ashraf and colleagues performed a database analyses from 20 UK centers and identified 165 patients with RC who underwent ERUS and endoscopic microsurgery.15 Compared with histopathology, EUS had 57.1% sensitivity, 73% specificity, and 42.9% accuracy for T1 cancers; EUS accuracy was 50% for T2 and 58% for T3 tumors. The authors concluded that the general accuracy of EUS in determining stage was around 50%, the statistical equivalent of flipping a coin.
The low accuracy of EUS observed by German and British multicenter studies13-15 was attributed to the difference that may exist in clinical trials at specialized centers compared with wider use of EUS in a community setting. As seen by our data, the Memphis VAMC is not a high-volume center for the treatment of RC. However, all our EUS procedures were performed and interpreted by a single operator (C. Ruben Tombazzi) with 18 years of EUS experience. We cannot conclude that no patient was overstaged, as patients receiving a stage of T3N0 or T > N0 received neoadjuvant treatment and were not included. However, we can conclude that no patient was understaged. All patients deemed to be T1 to T2N0 included in our study received accurate staging. Our results are consistent with the high accuracy of EUS reported from other centers with experience in diagnosis and treatment of RC.1,3-5,17,18
Although EUS is accurate in differentiating T1 from T2 tumors, it cannot reliably differentiate T1 from T0 lesions. In one study, 57.6% of adenomas and 30.7% of carcinomas in situ were staged as T1 on EUS, while almost half of T1 cancers were interpreted as T0.17 This drawback is a well-known limitation of EUS; although, the misinterpretation does not affect treatment, as both T0 and T1 lesions can be treated successfully by local excision alone, which was the algorithm used for our patients. The choice of the specific procedure for local excision was left to the clinicians and included transanal endoscopic or surgical resections. At a mean follow-up of 32.6 months, none of the 37 patients who underwent endoscopic or surgical transanal resection had evidence of recurrent disease.
A limitation of EUS, or any other imaging modality, is differentiating tumor invasion from peritumoral inflammation. The inflammation can render images of tumor borders ill-defined and irregular, which hinders precise staging. However, the accurate identification of tumors with deep involvement of the submucosa (T1sm3) is of importance, because these tumors are more advanced than the superficial and intermediate T1 lesions (T1sm1 and T1sm2, respectively).
Patients with RC whose lesions are considered T1sm3 are at higher risk of harboring lymph node metastases.18 Nascimbeni and colleagues had shown that the invasion into the lower third of the submucosa (sm3) was an independent risk factor for lower cancer-free survival among patients with T1 RC.19
Unlike rectal adenocarcinomas, the prognosis for carcinoid tumors correlates not only with the depth of invasion but also with the size of the tumor. The other adverse prognostic features include poor differentiation, high mitosis index, and lymphovascular invasion.20
EUS had been shown to be highly accurate in determining the precise carcinoid tumor size, depth of invasion, and lymph node metastases.20,21 In a study of 66 resected rectal carcinoid tumors by Ishii and colleagues, 57 lesions had a diameter of ≤ 10 mm and 9 lesions had a diameter of > 10 mm.21 All of the 57 carcinoid tumors with a diameter of ≤ 10 mm were confined to the submucosa. In contrast, 5 of the 9 lesions > 10 mm invaded the muscularis propria, 6 had a lymphovascular invasion, 4 were lymph node metastases, and 1 was a liver metastasis.
In our series, 4 of the 14 carcinoid tumors were > 10 mm but none were > 20 mm. None of the carcinoids with a diameter ≤ 10 mm invaded the muscularis propria. Of the 4 carcinoids > 10 mm, 1 was T2N0 and 3 were T1N0. All carcinoid tumors in our series were low grade and with low proliferation indexes, and all were treated successfully by local excision.
Conclusion
We believe our study shows that EUS can be highly accurate in staging rectal lesions, specifically lesions that are T1-T2N0, be they adenocarcinoma or carcinoid. Although we could not assess overstaging for lesions that were staged > T2 or > N0, we w
1. Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123(1):24-32.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
3. Ahuja NK, Sauer BG, Wang AY, et al. Performance of endoscopic ultrasound in staging rectal adenocarcinoma appropriate for primary surgical resection. Clin Gastroenterol Hepatol. 2015;13:339-44.
4. Doornebosch PG, Bronkhorst PJ, Hop WC, Bode WA, Sing AK, de Graaf EJ. The role of endorectal ultrasound in therapeutic decision-making for local vs. transabdominal resection of rectal tumors. Dis Colon Rectum. 2008;51(1):38-42.
5. Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G. The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis Colon Rectum. 2009;52(11):1837-1843.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published May 15, 2019. Accessed July 19, 2019.
7. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773-783.
8. Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254-265.
9. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779.
10. Balyasnikova S, Read J, Wotherspoon A, et al. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patient with early rectal cancer. BMJ Open Gastroenterol. 2017;4(1):e000151.
11. Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer. 2011;117(14):3118-3125.
12. Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43(4):440-446.
13. Marusch F, Ptok H, Sahm M, et al. Endorectal ultrasound in rectal carcinoma – do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43(5):425-431.
14. Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34(5):385-390.
15. Ashraf S, Hompes R, Slater A, et al; Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14(7):821-826.
16. Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99(4):623-627.
17. Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23(6):1384-1389.
18. Akasu T, Kondo H, Moriya Y, et al. Endoscopic ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24(9):1061-1068.
19. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47(11):1773-1779.
20. Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43(9):790-795.
21. Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24(6):1413-1419.
Endoscopic ultrasound can be highly accurate for the staging of neoplasms in early rectal cancer.
Endoscopic ultrasound can be highly accurate for the staging of neoplasms in early rectal cancer.
Colorectal cancer is the second most common cause of cancer death in the US, with one-third of all colorectal cancers occurring within the rectum. Each year, an estimated 40000 Americans are diagnosed with rectal cancer (RC).1,2 The prognosis and treatment of RC depends on both T and N stage at the time of diagnosis.3-5 According to the most recent National Comprehensive Cancer Network guidelines from May 2019, patients with T1 to T2N0 tumors should undergo transanal or transabdominal surgery upfront, whereas patients with T3 to T4N0 or any TN1 to 2 should start with neoadjuvant therapy for better locoregional control, followed by surgery.6 Therefore, the appropriate management of RC requires adequate staging.
Endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and computed tomography (CT) are the imaging techniques currently used to stage RC. In a meta-analysis of 90 articles published between 1985 and 2002 that compared the 3 radiologic modalities, Bipat and colleagues found that MRI and EUS had a similar sensitivity of 94%, whereas the specificity of EUS (86%) was significantly higher than that of MRI (69%) for muscularis propria invasion.7 CT was performed only in a limited number of trials because CT was considered inadequate to assess early T stage. For perirectal tissue invasion, the sensitivity of EUS was statistically higher than that of CT and MRI imaging: 90% compared with 79% and 82%, respectively. The specificity estimates for EUS, CT, and MRI were comparable: 75%, 78%, and 76%, respectively. The respective sensitivity and specificity of the 3 imaging modalities to evaluate lymph nodes were also comparable: EUS, 67% and 78%; CT, 55% and 74%; and MRI, 66% and 76%.
The role of EUS in the diagnosis and treatment of RC has long been validated.1,2-5 A meta-analysis of 42 studies involving 5039 patients found EUS to be highly accurate for differentiating various T stages.8 However, EUS cannot assess iliac and mesenteric lymph nodes or posterior tumor extension beyond endopelvic fascia in advanced RC. Notable heterogeneity was found among the studies in the meta-analyses with regard to the type of equipment used for staging, as well as the criteria used to assess the depth of penetration and nodal status. The recent introduction of phased-array coils and the development of T2-weighted fast spin sequences have improved the resolution of MRI. The MERCURY trial showed that extension of tumor to within 1 mm of the circumferential margin on high-resolution MRI correctly predicted margin involvement at the time of surgery in 92% of the patients.9 In the retrospective study by Balyasnikova and colleagues, MRI was found to correctly identify partial submucosal invasion and suitability for local excision in 89% of the cases.10
Therefore, both EUS and MRI are useful, more so than CT, in assessment of the depth of tumor invasion, nodal staging, and predicting the circumferential resection margin. The use of EUS, however, does not preclude the use of MRI, or vice versa. Rather, the 2 modalities can complement each other in staging and proper patient selection for treatment.11
Despite data supporting the value of EUS in staging RC, its use is limited by a high degree of operator dependence and a substantial learning curve,12-17 which may explain the low EUS accuracy observed in some reports.7,13,15 Given the presence of recognized alternatives such as MRI, we decided to reevaluate EUS accuracy for the staging of RC outside high-volume specialized centers and prospective clinical trials.
Methods
A retrospective chart review was performed that included all consecutive patients undergoing rectal ultrasound from January 2011 to August 2015 at the US Department of Veterans Affairs Medical Center (VAMC) in Memphis, Tennessee. Sixty-five patients with short-stocked or sessile lesions < 15 cm from anal margin staged T2N0M0 or lower by endorectal ultrasound (ERUS) were included. The patients with neoplasms staged in excess of T2 or N0 were excluded from the study because treatment protocol dictates immediate neoadjuvant treatment, the administration of which would affect subsequent histopathology.
For the 37 patients included in the final analysis, ERUS results were compared with surgical pathology to ascertain accuracy. The resections were performed endoscopically or surgically with a goal of obtaining clear margins. The choice of procedure depended on size, shape, location, and depth of invasion. All patients underwent clinical and endoscopic surveillance with flexible sigmoidoscopy/EUS every 3 to 6 months for the first 2 years. We used 2 different gold standards for surveillance depending on the type of procedure performed to remove the lesion. A pathology report was the gold standard used for patients who underwent surgery. In patients who underwent endoscopic resection, we used the lack of recurrent disease, determined by normal endoscopic and endoscopic ultrasound examination, to signify complete endoscopic resection and therefore adequate staging as an early neoplasm.
Results
From January 2011 to August 2015, 65 rectal ultrasounds were performed. All EUS procedures were performed by 1 physician (C Ruben Tombazzi). All patients had previous endoscopic evaluation and tissue diagnoses. Twenty-eight patients were excluded: 18 had T3 or N1 disease, 2 had T2N0 but refused surgery, 2 had anal cancer, 3 patients with suspected cancer had benign nonneoplastic disease (2 radiation proctitis, 1 normal rectal wall), and 3 underwent EUS for benign tumors (1 ganglioneuroma and 2 lipomas).
Thirty-seven patients were included in the study, 3 of whom were staged as T2N0 and 34 as T1N0 or lower by EUS. All patients were men ranging in age from 43 to 73 years (mean, 59 years). All 37 patients underwent endoscopic or surgical resection of their early rectal neoplasm. The final pathologic evaluation of the specimens demonstrated 14 carcinoid tumors, 11 adenocarcinomas, 6 tubular adenomas with high-grade dysplasia, and 6 benign adenomas. The preoperative EUS staging was confirmed for all patients, with 100% sensitivity, specificity, and accuracy. None of the patients who underwent endoscopic or surgical transanal resection had recurrence, determined by normal endoscopic and endoscopic ultrasound appearance, during a mean of 32.6 months surveillance.
Discussion
EUS has long been a recognized method for T and N staging of RC.1,3-5,7,8 Our data confirm that, in experienced hands, EUS is highly accurate in the staging of early rectal cancers.
The impact of EUS on the management of RC was demonstrated in a Mayo Clinic prospective blinded study.1 In that cohort of 80 consecutive patients who had previously had a CT for staging, EUS altered patient management in about 30% of cases. The most common change precipatated by EUS was the indication for additional neoadjuvant treatment.
However, the results have not been as encouraging when ERUS is performed outside of strict research protocol. A multicenter, prospective, country-wide quality assurance study from > 300 German hospitals was designed to assess the diagnostic accuracy of EUS in RC.13 Of 29206 patients, 7096 underwent surgery, without neoadjuvant treatment, and were included in the final analysis. The correspondence of tumor invasion with histopathology was 64.7%, with understaging of 18% and overstaging of 17.3%.13 These numbers were better in hospitals with greater experience performing ERUS: 73% accuracy in the centers with a case load of > 30 cases per year compared with 63.2% accuracy for the centers with < 10 cases a year. Marusch and colleagues had previously demonstrated an EUS accuracy of 63.3% in a study of 1463 patients with RC in Germany.14 Another study based out of the UK had similar findings. Ashraf and colleagues performed a database analyses from 20 UK centers and identified 165 patients with RC who underwent ERUS and endoscopic microsurgery.15 Compared with histopathology, EUS had 57.1% sensitivity, 73% specificity, and 42.9% accuracy for T1 cancers; EUS accuracy was 50% for T2 and 58% for T3 tumors. The authors concluded that the general accuracy of EUS in determining stage was around 50%, the statistical equivalent of flipping a coin.
The low accuracy of EUS observed by German and British multicenter studies13-15 was attributed to the difference that may exist in clinical trials at specialized centers compared with wider use of EUS in a community setting. As seen by our data, the Memphis VAMC is not a high-volume center for the treatment of RC. However, all our EUS procedures were performed and interpreted by a single operator (C. Ruben Tombazzi) with 18 years of EUS experience. We cannot conclude that no patient was overstaged, as patients receiving a stage of T3N0 or T > N0 received neoadjuvant treatment and were not included. However, we can conclude that no patient was understaged. All patients deemed to be T1 to T2N0 included in our study received accurate staging. Our results are consistent with the high accuracy of EUS reported from other centers with experience in diagnosis and treatment of RC.1,3-5,17,18
Although EUS is accurate in differentiating T1 from T2 tumors, it cannot reliably differentiate T1 from T0 lesions. In one study, 57.6% of adenomas and 30.7% of carcinomas in situ were staged as T1 on EUS, while almost half of T1 cancers were interpreted as T0.17 This drawback is a well-known limitation of EUS; although, the misinterpretation does not affect treatment, as both T0 and T1 lesions can be treated successfully by local excision alone, which was the algorithm used for our patients. The choice of the specific procedure for local excision was left to the clinicians and included transanal endoscopic or surgical resections. At a mean follow-up of 32.6 months, none of the 37 patients who underwent endoscopic or surgical transanal resection had evidence of recurrent disease.
A limitation of EUS, or any other imaging modality, is differentiating tumor invasion from peritumoral inflammation. The inflammation can render images of tumor borders ill-defined and irregular, which hinders precise staging. However, the accurate identification of tumors with deep involvement of the submucosa (T1sm3) is of importance, because these tumors are more advanced than the superficial and intermediate T1 lesions (T1sm1 and T1sm2, respectively).
Patients with RC whose lesions are considered T1sm3 are at higher risk of harboring lymph node metastases.18 Nascimbeni and colleagues had shown that the invasion into the lower third of the submucosa (sm3) was an independent risk factor for lower cancer-free survival among patients with T1 RC.19
Unlike rectal adenocarcinomas, the prognosis for carcinoid tumors correlates not only with the depth of invasion but also with the size of the tumor. The other adverse prognostic features include poor differentiation, high mitosis index, and lymphovascular invasion.20
EUS had been shown to be highly accurate in determining the precise carcinoid tumor size, depth of invasion, and lymph node metastases.20,21 In a study of 66 resected rectal carcinoid tumors by Ishii and colleagues, 57 lesions had a diameter of ≤ 10 mm and 9 lesions had a diameter of > 10 mm.21 All of the 57 carcinoid tumors with a diameter of ≤ 10 mm were confined to the submucosa. In contrast, 5 of the 9 lesions > 10 mm invaded the muscularis propria, 6 had a lymphovascular invasion, 4 were lymph node metastases, and 1 was a liver metastasis.
In our series, 4 of the 14 carcinoid tumors were > 10 mm but none were > 20 mm. None of the carcinoids with a diameter ≤ 10 mm invaded the muscularis propria. Of the 4 carcinoids > 10 mm, 1 was T2N0 and 3 were T1N0. All carcinoid tumors in our series were low grade and with low proliferation indexes, and all were treated successfully by local excision.
Conclusion
We believe our study shows that EUS can be highly accurate in staging rectal lesions, specifically lesions that are T1-T2N0, be they adenocarcinoma or carcinoid. Although we could not assess overstaging for lesions that were staged > T2 or > N0, we w
Colorectal cancer is the second most common cause of cancer death in the US, with one-third of all colorectal cancers occurring within the rectum. Each year, an estimated 40000 Americans are diagnosed with rectal cancer (RC).1,2 The prognosis and treatment of RC depends on both T and N stage at the time of diagnosis.3-5 According to the most recent National Comprehensive Cancer Network guidelines from May 2019, patients with T1 to T2N0 tumors should undergo transanal or transabdominal surgery upfront, whereas patients with T3 to T4N0 or any TN1 to 2 should start with neoadjuvant therapy for better locoregional control, followed by surgery.6 Therefore, the appropriate management of RC requires adequate staging.
Endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and computed tomography (CT) are the imaging techniques currently used to stage RC. In a meta-analysis of 90 articles published between 1985 and 2002 that compared the 3 radiologic modalities, Bipat and colleagues found that MRI and EUS had a similar sensitivity of 94%, whereas the specificity of EUS (86%) was significantly higher than that of MRI (69%) for muscularis propria invasion.7 CT was performed only in a limited number of trials because CT was considered inadequate to assess early T stage. For perirectal tissue invasion, the sensitivity of EUS was statistically higher than that of CT and MRI imaging: 90% compared with 79% and 82%, respectively. The specificity estimates for EUS, CT, and MRI were comparable: 75%, 78%, and 76%, respectively. The respective sensitivity and specificity of the 3 imaging modalities to evaluate lymph nodes were also comparable: EUS, 67% and 78%; CT, 55% and 74%; and MRI, 66% and 76%.
The role of EUS in the diagnosis and treatment of RC has long been validated.1,2-5 A meta-analysis of 42 studies involving 5039 patients found EUS to be highly accurate for differentiating various T stages.8 However, EUS cannot assess iliac and mesenteric lymph nodes or posterior tumor extension beyond endopelvic fascia in advanced RC. Notable heterogeneity was found among the studies in the meta-analyses with regard to the type of equipment used for staging, as well as the criteria used to assess the depth of penetration and nodal status. The recent introduction of phased-array coils and the development of T2-weighted fast spin sequences have improved the resolution of MRI. The MERCURY trial showed that extension of tumor to within 1 mm of the circumferential margin on high-resolution MRI correctly predicted margin involvement at the time of surgery in 92% of the patients.9 In the retrospective study by Balyasnikova and colleagues, MRI was found to correctly identify partial submucosal invasion and suitability for local excision in 89% of the cases.10
Therefore, both EUS and MRI are useful, more so than CT, in assessment of the depth of tumor invasion, nodal staging, and predicting the circumferential resection margin. The use of EUS, however, does not preclude the use of MRI, or vice versa. Rather, the 2 modalities can complement each other in staging and proper patient selection for treatment.11
Despite data supporting the value of EUS in staging RC, its use is limited by a high degree of operator dependence and a substantial learning curve,12-17 which may explain the low EUS accuracy observed in some reports.7,13,15 Given the presence of recognized alternatives such as MRI, we decided to reevaluate EUS accuracy for the staging of RC outside high-volume specialized centers and prospective clinical trials.
Methods
A retrospective chart review was performed that included all consecutive patients undergoing rectal ultrasound from January 2011 to August 2015 at the US Department of Veterans Affairs Medical Center (VAMC) in Memphis, Tennessee. Sixty-five patients with short-stocked or sessile lesions < 15 cm from anal margin staged T2N0M0 or lower by endorectal ultrasound (ERUS) were included. The patients with neoplasms staged in excess of T2 or N0 were excluded from the study because treatment protocol dictates immediate neoadjuvant treatment, the administration of which would affect subsequent histopathology.
For the 37 patients included in the final analysis, ERUS results were compared with surgical pathology to ascertain accuracy. The resections were performed endoscopically or surgically with a goal of obtaining clear margins. The choice of procedure depended on size, shape, location, and depth of invasion. All patients underwent clinical and endoscopic surveillance with flexible sigmoidoscopy/EUS every 3 to 6 months for the first 2 years. We used 2 different gold standards for surveillance depending on the type of procedure performed to remove the lesion. A pathology report was the gold standard used for patients who underwent surgery. In patients who underwent endoscopic resection, we used the lack of recurrent disease, determined by normal endoscopic and endoscopic ultrasound examination, to signify complete endoscopic resection and therefore adequate staging as an early neoplasm.
Results
From January 2011 to August 2015, 65 rectal ultrasounds were performed. All EUS procedures were performed by 1 physician (C Ruben Tombazzi). All patients had previous endoscopic evaluation and tissue diagnoses. Twenty-eight patients were excluded: 18 had T3 or N1 disease, 2 had T2N0 but refused surgery, 2 had anal cancer, 3 patients with suspected cancer had benign nonneoplastic disease (2 radiation proctitis, 1 normal rectal wall), and 3 underwent EUS for benign tumors (1 ganglioneuroma and 2 lipomas).
Thirty-seven patients were included in the study, 3 of whom were staged as T2N0 and 34 as T1N0 or lower by EUS. All patients were men ranging in age from 43 to 73 years (mean, 59 years). All 37 patients underwent endoscopic or surgical resection of their early rectal neoplasm. The final pathologic evaluation of the specimens demonstrated 14 carcinoid tumors, 11 adenocarcinomas, 6 tubular adenomas with high-grade dysplasia, and 6 benign adenomas. The preoperative EUS staging was confirmed for all patients, with 100% sensitivity, specificity, and accuracy. None of the patients who underwent endoscopic or surgical transanal resection had recurrence, determined by normal endoscopic and endoscopic ultrasound appearance, during a mean of 32.6 months surveillance.
Discussion
EUS has long been a recognized method for T and N staging of RC.1,3-5,7,8 Our data confirm that, in experienced hands, EUS is highly accurate in the staging of early rectal cancers.
The impact of EUS on the management of RC was demonstrated in a Mayo Clinic prospective blinded study.1 In that cohort of 80 consecutive patients who had previously had a CT for staging, EUS altered patient management in about 30% of cases. The most common change precipatated by EUS was the indication for additional neoadjuvant treatment.
However, the results have not been as encouraging when ERUS is performed outside of strict research protocol. A multicenter, prospective, country-wide quality assurance study from > 300 German hospitals was designed to assess the diagnostic accuracy of EUS in RC.13 Of 29206 patients, 7096 underwent surgery, without neoadjuvant treatment, and were included in the final analysis. The correspondence of tumor invasion with histopathology was 64.7%, with understaging of 18% and overstaging of 17.3%.13 These numbers were better in hospitals with greater experience performing ERUS: 73% accuracy in the centers with a case load of > 30 cases per year compared with 63.2% accuracy for the centers with < 10 cases a year. Marusch and colleagues had previously demonstrated an EUS accuracy of 63.3% in a study of 1463 patients with RC in Germany.14 Another study based out of the UK had similar findings. Ashraf and colleagues performed a database analyses from 20 UK centers and identified 165 patients with RC who underwent ERUS and endoscopic microsurgery.15 Compared with histopathology, EUS had 57.1% sensitivity, 73% specificity, and 42.9% accuracy for T1 cancers; EUS accuracy was 50% for T2 and 58% for T3 tumors. The authors concluded that the general accuracy of EUS in determining stage was around 50%, the statistical equivalent of flipping a coin.
The low accuracy of EUS observed by German and British multicenter studies13-15 was attributed to the difference that may exist in clinical trials at specialized centers compared with wider use of EUS in a community setting. As seen by our data, the Memphis VAMC is not a high-volume center for the treatment of RC. However, all our EUS procedures were performed and interpreted by a single operator (C. Ruben Tombazzi) with 18 years of EUS experience. We cannot conclude that no patient was overstaged, as patients receiving a stage of T3N0 or T > N0 received neoadjuvant treatment and were not included. However, we can conclude that no patient was understaged. All patients deemed to be T1 to T2N0 included in our study received accurate staging. Our results are consistent with the high accuracy of EUS reported from other centers with experience in diagnosis and treatment of RC.1,3-5,17,18
Although EUS is accurate in differentiating T1 from T2 tumors, it cannot reliably differentiate T1 from T0 lesions. In one study, 57.6% of adenomas and 30.7% of carcinomas in situ were staged as T1 on EUS, while almost half of T1 cancers were interpreted as T0.17 This drawback is a well-known limitation of EUS; although, the misinterpretation does not affect treatment, as both T0 and T1 lesions can be treated successfully by local excision alone, which was the algorithm used for our patients. The choice of the specific procedure for local excision was left to the clinicians and included transanal endoscopic or surgical resections. At a mean follow-up of 32.6 months, none of the 37 patients who underwent endoscopic or surgical transanal resection had evidence of recurrent disease.
A limitation of EUS, or any other imaging modality, is differentiating tumor invasion from peritumoral inflammation. The inflammation can render images of tumor borders ill-defined and irregular, which hinders precise staging. However, the accurate identification of tumors with deep involvement of the submucosa (T1sm3) is of importance, because these tumors are more advanced than the superficial and intermediate T1 lesions (T1sm1 and T1sm2, respectively).
Patients with RC whose lesions are considered T1sm3 are at higher risk of harboring lymph node metastases.18 Nascimbeni and colleagues had shown that the invasion into the lower third of the submucosa (sm3) was an independent risk factor for lower cancer-free survival among patients with T1 RC.19
Unlike rectal adenocarcinomas, the prognosis for carcinoid tumors correlates not only with the depth of invasion but also with the size of the tumor. The other adverse prognostic features include poor differentiation, high mitosis index, and lymphovascular invasion.20
EUS had been shown to be highly accurate in determining the precise carcinoid tumor size, depth of invasion, and lymph node metastases.20,21 In a study of 66 resected rectal carcinoid tumors by Ishii and colleagues, 57 lesions had a diameter of ≤ 10 mm and 9 lesions had a diameter of > 10 mm.21 All of the 57 carcinoid tumors with a diameter of ≤ 10 mm were confined to the submucosa. In contrast, 5 of the 9 lesions > 10 mm invaded the muscularis propria, 6 had a lymphovascular invasion, 4 were lymph node metastases, and 1 was a liver metastasis.
In our series, 4 of the 14 carcinoid tumors were > 10 mm but none were > 20 mm. None of the carcinoids with a diameter ≤ 10 mm invaded the muscularis propria. Of the 4 carcinoids > 10 mm, 1 was T2N0 and 3 were T1N0. All carcinoid tumors in our series were low grade and with low proliferation indexes, and all were treated successfully by local excision.
Conclusion
We believe our study shows that EUS can be highly accurate in staging rectal lesions, specifically lesions that are T1-T2N0, be they adenocarcinoma or carcinoid. Although we could not assess overstaging for lesions that were staged > T2 or > N0, we w
1. Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123(1):24-32.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
3. Ahuja NK, Sauer BG, Wang AY, et al. Performance of endoscopic ultrasound in staging rectal adenocarcinoma appropriate for primary surgical resection. Clin Gastroenterol Hepatol. 2015;13:339-44.
4. Doornebosch PG, Bronkhorst PJ, Hop WC, Bode WA, Sing AK, de Graaf EJ. The role of endorectal ultrasound in therapeutic decision-making for local vs. transabdominal resection of rectal tumors. Dis Colon Rectum. 2008;51(1):38-42.
5. Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G. The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis Colon Rectum. 2009;52(11):1837-1843.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published May 15, 2019. Accessed July 19, 2019.
7. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773-783.
8. Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254-265.
9. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779.
10. Balyasnikova S, Read J, Wotherspoon A, et al. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patient with early rectal cancer. BMJ Open Gastroenterol. 2017;4(1):e000151.
11. Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer. 2011;117(14):3118-3125.
12. Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43(4):440-446.
13. Marusch F, Ptok H, Sahm M, et al. Endorectal ultrasound in rectal carcinoma – do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43(5):425-431.
14. Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34(5):385-390.
15. Ashraf S, Hompes R, Slater A, et al; Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14(7):821-826.
16. Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99(4):623-627.
17. Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23(6):1384-1389.
18. Akasu T, Kondo H, Moriya Y, et al. Endoscopic ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24(9):1061-1068.
19. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47(11):1773-1779.
20. Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43(9):790-795.
21. Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24(6):1413-1419.
1. Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123(1):24-32.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
3. Ahuja NK, Sauer BG, Wang AY, et al. Performance of endoscopic ultrasound in staging rectal adenocarcinoma appropriate for primary surgical resection. Clin Gastroenterol Hepatol. 2015;13:339-44.
4. Doornebosch PG, Bronkhorst PJ, Hop WC, Bode WA, Sing AK, de Graaf EJ. The role of endorectal ultrasound in therapeutic decision-making for local vs. transabdominal resection of rectal tumors. Dis Colon Rectum. 2008;51(1):38-42.
5. Santoro GA, Gizzi G, Pellegrini L, Battistella G, Di Falco G. The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis Colon Rectum. 2009;52(11):1837-1843.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer, version 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published May 15, 2019. Accessed July 19, 2019.
7. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology. 2004;232(3):773-783.
8. Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16(2):254-265.
9. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779.
10. Balyasnikova S, Read J, Wotherspoon A, et al. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patient with early rectal cancer. BMJ Open Gastroenterol. 2017;4(1):e000151.
11. Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer. 2011;117(14):3118-3125.
12. Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43(4):440-446.
13. Marusch F, Ptok H, Sahm M, et al. Endorectal ultrasound in rectal carcinoma – do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011;43(5):425-431.
14. Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy. 2002;34(5):385-390.
15. Ashraf S, Hompes R, Slater A, et al; Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012;14(7):821-826.
16. Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99(4):623-627.
17. Zorcolo L, Fantola G, Cabras F, Marongiu L, D’Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23(6):1384-1389.
18. Akasu T, Kondo H, Moriya Y, et al. Endoscopic ultrasonography and treatment of early stage rectal cancer. World J Surg. 2000;24(9):1061-1068.
19. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47(11):1773-1779.
20. Park CH, Cheon JH, Kim JO, et al. Criteria for decision making after endoscopic resection of well-differentiated rectal carcinoids with regard to potential lymphatic spread. Endoscopy. 2011;43(9):790-795.
21. Ishii N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection and preoperative assessment with endoscopic ultrasonography for the treatment of rectal carcinoid tumors. Surg Endosc. 2010;24(6):1413-1419.
Sacroiliac Joint Dysfunction in Patients With Low Back Pain
Patients experiencing sacroiliac joint (SIJ) dysfunction might show symptoms that overlap with those seen in lumbar spine pathology. This article reviews diagnostic tools that assist practitioners to discern the true pain generator in patients with low back pain (LBP) and therapeutic approaches when the cause is SIJ dysfunction.
Prevalence
Most of the US population will experience LBP at some point in their lives. A 2002 National Health Interview survey found that more than one-quarter (26.4%) of 31 044 respondents had complained of LBP in the previous 3 months.1 About 74 million individuals in the US experienced LBP in the past 3 months.1 A full 10% of the US population is expected to suffer from chronic LBP, and it is estimated that 2.3% of all visits to physicians are related to LBP.1
The etiology of LBP often is unclear even after thorough clinical and radiographic evaluation because of the myriad possible mechanisms. Degenerative disc disease, facet arthropathy, ligamentous hypertrophy, muscle spasm, hip arthropathy, and SIJ dysfunction are potential pain generators and exact clinical and radiographic correlation is not always possible. Compounding this difficulty is the lack of specificity with current diagnostic techniques. For example, many patients will have disc desiccation or herniation without any LBP or radicular symptoms on radiographic studies, such as X-rays, computed tomography (CT), and magnetic resonance imaging (MRI). As such, providers of patients with diffuse radiographic abnormalities often have to identify a specific pain generator, which might not have any role in the patient’s pain.
Other tests, such as electromyographic studies, positron emission tomography (PET) scans, discography, and epidural steroid injections, can help pinpoint a specific pain generator. These tests might help determine whether the patient has a surgically treatable condition and could help predict whether a patient’s symptoms will respond to surgery.
However, the standard spine surgery workup often fails to identify an obvious pain generator in many individuals. The significant number of patients that fall into this category has prompted spine surgeons to consider other potential etiologies for LBP, and SIJ dysfunction has become a rapidly developing field of research.
Sacroiliac Joint Dysfunction
The SIJ is a bilateral, C-shaped synovial joint surrounded by a fibrous capsule and affixes the sacrum to the ilia. Several sacral ligaments and pelvic muscles support the SIJ. The L5 nerve ventral ramus and lumbosacral trunk pass anteriorly and the S1 nerve ventral ramus passes inferiorly to the joint capsule. The SIJ is innervated by the dorsal rami of L4-S3 nerve roots, transmitting nociception and temperature. Mechanisms of injury to the SIJ could arise from intra- and extra-articular etiologies, including capsular disruption, ligamentous tension, muscular inflammation, shearing, fractures, arthritis, and infection.2 Patients could develop SIJ pain spontaneously or after a traumatic event or repetitive shear.3 Risk factors for developing SIJ dysfunction include a history of lumbar fusion, scoliosis, leg length discrepancies, sustained athletic activity, pregnancy, seronegative HLA-B27 spondyloarthropathies, or gait abnormalities. Inflammation of the SIJ and surrounding structures secondary to an environmental insult in susceptible individuals is a common theme among these etiologies.2
Pain from the SIJ is localized to an area of approximately 3 cm × 10 cm that is inferior to the ipsilateral posterior superior iliac spine.4 Referred pain maps from SIJ dysfunction extend in the L5-S1 nerve distributions, commonly seen in the buttocks, groin, posterior thigh, and lower leg with radicular symptoms. However, this pain distribution demonstrates extensive variability among patients and bears strong similarities to discogenic or facet joint sources of LBP.5-7 Direct communication has been shown between the SIJ and adjacent neural structures, namely the L5 nerve, sacral foramina, and the lumbosacral plexus. These direct pathways could explain an inflammatory mechanism for lower extremity symptoms seen in SIJ dysfunction.8
The prevalence of SIJ dysfunction among patients with LBP is estimated to be 15% to 30%, an extraordinary number given the total number of patients presenting with LBP every year.9 These patients might represent a significant segment of patients with an unrevealing standard spine evaluation. Despite the large number of patients who experience SIJ dysfunction, there is disagreement about optimal methods for diagnosis and treatment.
Diagnosis
The International Association for the Study of Pain has proposed criteria for evaluating patients who have suspected SIJ dysfunction: Pain must be in the SIJ area, should be reproducible by performing specific provocative maneuvers, and must be relieved by injection of local anesthetic into the SIJ.10 These criteria provide a sound foundation, but in clinical practice, patients often defy categorization.
The presence of pain in the area inferior to the posterior superior iliac spine and lateral to the gluteal fold with pain referral patterns in the L5-S1 nerve distributions is highly sensitive for identifying patients with SIJ dysfunction. Furthermore, pain arising from the SIJ will not be above the level of the L5 nerve sensory distribution. However, this diagnostic finding alone is not specific and might represent other etiologies known to produce similar pain, such as intervertebral discs and facet joints. Patients with SIJ dysfunction often describe their pain as sciatica-like, recurrent, and triggered with bending or twisting motions. It is worsened with any activity loading the SIJ, such as walking, climbing stairs, standing, or sitting upright. SIJ pain might be accompanied by dyspareunia and changes in bladder function because of the nerves involved.11
The use of provocative maneuvers for testing SIJ dysfunction is controversial because of the high rate of false positives and the inability to distinguish whether the SIJ or an adjacent structure is affected. However, the diagnostic utility of specific stress tests has been studied, and clusters of tests are recommended if a health care provider (HCP) suspects SIJ dysfunction. A diagnostic algorithm should first focus on using the distraction test and the thigh thrust test. Distraction is done by applying vertically oriented pressure to the anterior superior iliac spine while aiming posteriorly, therefore distracting the SIJ. During the thigh thrust test the examiner fixates the patient’s sacrum against the table with the left hand and applies a vertical force through the line of the femur aiming posteriorly, producing a posterior shearing force at the SIJ. Studies show that the thigh thrust test is the most sensitive, and the distraction test is the most specific. If both tests are positive, there is reasonable evidence to suggest SIJ dysfunction as the source of LBP.
If there are not 2 positive results, the addition of the compression test, followed by the sacral thrust test also can point to the diagnosis. The compression test is performed with vertical downward force applied to the iliac crest with the patient lying on each side, compressing the SIJ by transverse pressure across the pelvis. The sacral thrust test is performed with vertical force applied to the midline posterior sacrum at its apex directed anteriorly with the patient lying prone, producing a shearing force at the SIJs. The Gaenslen test uses a torsion force by applying a superior and posterior force to the right knee and posteriorly directed force to the left knee. Omitting the Gaenslen test has not been shown to compromise diagnostic efficacy of the other tests and can be safely excluded.12
A HCP can rule out SIJ dysfunction if these provocation tests are negative. However, the diagnostic predictive value of these tests is subject to variability among HCPs, and their reliability is increased when used in clusters.9,13
Imaging for the SIJ should begin with anterior/posterior, oblique, and lateral view plain X-rays of the pelvis (Figures 1 and 2), which will rule out other pathologies by identifying other sources of LBP, such as spondylolisthesis or hip osteoarthritis. HCPs should obtain lumbar and pelvis CT images to identify inflammatory or degenerative changes within the SIJ. CT images provide the high resolution that is needed to identify pathologies, such as fractures and tumors within the pelvic ring that could cause similar pain. MRI does not reliably depict a dysfunctional ligamentous apparatus within the SIJ; however, it can help identify inflammatory sacroiliitis, such as is seen in the spondyloarthropathies.11,14 Recent studies show combined single photon emission tomography and CT (SPECT-CT) might be the most promising imaging modality to reveal mechanical failure of load transfer with increased scintigraphic uptake in the posterior and superior SIJ ligamentous attachments. The joint loses its characteristic “dumbbell” shape in affected patients with about 50% higher uptake than unaffected joints. These findings were evident in patients who experienced pelvic trauma or during the peripartum period.15,16
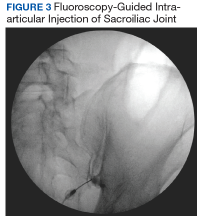
Fluoroscopy-guided intra-articular injection of a local anesthetic (lidocaine) and/or a corticosteroid (triamcinolone) has the dual functionality of diagnosis and treatment (Figure 3). It often is considered the most reliable method to diagnose SIJ dysfunction and has the benefit of pain relief for up to 1 year. However, intra-articular injections lack diagnostic validity because the solution often extravasates to extracapsular structures. This confounds the source of the pain and makes it difficult to interpret these diagnostic injections. In addition, the injection might not reach the entire SIJ capsule and could result in a false-negative diagnosis.17,18 Periarticular injections have been shown to result in better pain relief in patients diagnosed with SIJ dysfunction than intra-articular injections. Periarticular injections also are easier to perform and could be a first-step option for these patients.19
Treatment
Nonoperative management of SIJ dysfunction includes exercise programs, physical therapy, manual manipulation therapy, sacroiliac belts, and periodic articular injections. Efficacy of these methods is variable, and analgesics often do not significantly benefit this type of pain. Another nonoperative approach is radiofrequency ablation (RFA) of the lumbar dorsal rami and lateral sacral branches, which can vary based on the number of rami treated as well as the technique used. About two-thirds of patients report pain relief after RFA.2 When successful, pain is relieved for 6 to 12 months, which is a temporary yet effective option for patients experiencing SIJ dysfunction.14,20
Fusion Surgery
Cadaver studies show that biomechanical stabilization of the SIJ leads to decreased range of motion in flexion/extension, lateral bending, and axial rotation. This results in a decreased need for periarticular muscular and ligamentous support, therefore facilitating load transfer across the SIJ.21,22 Patients undergoing minimally invasive surgery report better pain relief compared with those receiving open surgery at 12 months postoperatively.23 The 2 main SIJ fusion approaches used are the lateral transarticular and the dorsal approaches. In the dorsal approach, the SIJ is distracted and allograft dowels or titanium cages with graft are inserted into the joint space posteriorly through the back. When approaching laterally, hollow screw implants filled with graft or triangular titanium implants are placed across the joint, accessing the SIJ through the iliac bones using imaging guidance. This lateral transiliac approach using porous titanium triangular rods currently is the most studied technique.24
A recent prospective, multicenter trial included 423 patients with SIJ dysfunction who were randomized to receive SIJ fusion with triangular titanium implants vs a control group who received nonoperative management. Patients in the SIJ fusion group showed substantially greater improvement in pain (81.4%) compared with that of the nonoperative group (26.1%) 6 months after surgery. Pain relief in the SIJ fusion group was maintained at > 80% at 1 and 2 year follow-up, while the nonoperative group’s pain relief decreased to < 10% at the follow-ups. Measures of quality of life and disability also improved for the SIJ fusion group compared with that of the nonoperative group. Patients who were crossed over from conservative management to SIJ fusion after 6 months demonstrated improvements that were similar to those in the SIJ fusion group by the end of the study. Only 3% of patients required surgical revision. The strongest predictor of pain relief after surgery was a diagnostic SIJ anesthetic block of 30 to 60 minutes, which resulted in > 75% pain reduction.21,25 Additional predictors of successful SIJ fusion include nonsmokers, nonopioid users, and older patients who have a longer time course of SIJ pain.26
Another study investigating the outcomes of SIJ fusion, RFA, and conservative management with a 6-year follow-up demonstrated similar results.27 This further confirms the durability of the surgical group’s outcome, which sustained significant improvement compared with RFA and conservative management group in pain relief, daily function, and opioid use.
HCPs should consider SIJ fusion for patients who have at least 6 months of unsuccessful nonoperative management, significant SIJ pain (> 5 in a 10-point scale), ≥ 3 positive provocation tests, and at least 50% pain relief (> 75% preferred) with diagnostic intra-articular anesthetic injection.14 It is reasonable for primary care providers to refer these patients to a neurosurgeon or orthopedic spine surgeon for possible fusion. Patients with earlier lumbar/lumbosacral spinal fusions and persistent LBP should be evaluated for potential SIJ dysfunction. SIJ dysfunction after lumbosacral fusion could be considered a form of distal pseudarthrosis resulting from increased motion at the joint. One study found its incidence correlated with the number of segments fused in the lumbar spine.28 Another study found that about one-third of patients with persistent LBP after lumbosacral fusion could be attributed to SIJ dysfunction.29
Case Presentation
A 27-year-old female army veteran presented with bilateral buttock pain, which she described as a dull, aching pain across her sacral region, 8 out of 10 in severity. The pain was in a L5-S1 pattern. The pain was bilateral, with the right side worse than the left, and worsened with lateral bending and load transferring. She reported no numbness, tingling, or weakness.
On physical examination, she had full strength in her lower extremities and intact sensation. She reported tenderness to palpation of the sacrum and SIJ. Her gait was normal. The patient had positive thigh thrust and distraction tests. Lumbar spine X-ray, CT, MRI, and electromyographic studies did not show any pathology. She described little or no relief with analgesics or physical therapy. Previous L4-L5 and L5-S1 facet anesthetic injections and transforaminal epidural steroid injections provided minimal pain relief immediately after the procedures. Bilateral SIJ anesthetic injections under fluoroscopic guidance decreased her pain severity from a 7 to 3 out of 10 for 2 to 3 months before returning to her baseline. Radiofrequency ablation of the right SIJ under fluoroscopy provided moderate relief for about 4 months.
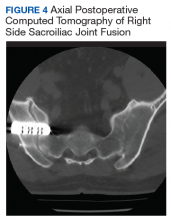
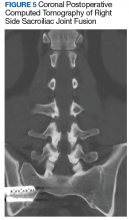
After exhausting nonoperative management for SIJ dysfunction without adequate pain control, the patient was referred to neurosurgery for surgical fusion. The patient was deemed an appropriate surgical candidate and underwent a right-sided SIJ fusion (Figures 4 and 5). At her 6-month and 1-year follow-up appointments, she had lasting pain relief, 2 out of 10.
Conclusion
SIJ dysfunction is widely overlooked because of the difficulty in distinguishing it from other similarly presenting syndromes. However, with a detailed history, appropriate physical maneuvers, imaging, and adequate response to intra-articular anesthetic, providers can reach an accurate diagnosis that will inform subsequent treatments. After failure of nonsurgical methods, patients with SIJ dysfunction should be considered for minimally invasive fusion techniques, which have proven to be a safe, effective, and viable treatment option.
1. Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59-66.
2. Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440-1453.
3. Chou LH, Slipman CW, Bhagia SM, et al. Inciting events initiating injection‐proven sacroiliac joint syndrome. Pain Med. 2004;5(1):26-32.
4. Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255-265.
5. Buijs E, Visser L, Groen G. Sciatica and the sacroiliac joint: a forgotten concept. Br J Anaesth. 2007;99(5):713-716.
6. Fortin JD, Dwyer AP, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19(13):1475-1482.
7. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
8. Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. ANJR Am J Neuroradiol. 1999;20(8):1429-1434.
9. Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368.
10. Merskey H, Bogduk N, eds. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
11. Cusi MF. Paradigm for assessment and treatment of SIJ mechanical dysfunction. J Bodyw Mov Ther. 2010;14(2):152-161.
12. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
13. Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16(3):142-152.
14. Polly DW Jr. The sacroiliac joint. Neurosurg Clin N Am. 2017;28(3):301-312.
15. Cusi M, Van Der Wall H, Saunders J, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22(7):1674-1682.
16. Tofuku K, Koga H, Komiya S. The diagnostic value of single-photon emission computed tomography/computed tomography for severe sacroiliac joint dysfunction. Eur Spine J. 2015;24(4):859-863.
17. Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra‐articular sacroiliac joint injections: a systematic review. Pain Med. 2015;16(8):1500-1518.
18. Schneider BJ, Huynh L, Levin J, Rinkaekan P, Kordi R, Kennedy DJ. Does immediate pain relief after an injection into the sacroiliac joint with anesthetic and corticosteroid predict subsequent pain relief? Pain Med. 2018;19(2):244-251.
19. Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007;12(3):274-280.
20. Cohen SP, Hurley RW, Buckenmaier CC 3rd, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
21. Polly DW, Cher DJ, Wine KD, et al; INSITE Study Group. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-690.
22. Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, et al. The effect of implant placement on sacroiliac joint range of motion: posterior versus transarticular. Spine. 2015;40(9):E525-E530.
23. Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14.
24. Rashbaum RF, Ohnmeiss DD, Lindley EM, Kitchel SH, Patel VV. Sacroiliac joint pain and its treatment. Clin Spine Surg. 2016;29(2):42-48.
25. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28.
26. Dengler J, Duhon B, Whang P, et al. Predictors of outcome in conservative and minimally invasive surgical management of pain originating from the sacroiliac joint: a pooled analysis. Spine (Phila Pa 1976). 2017;42(21):1664-1673.
27. Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally invasive sacroiliac joint fusion, radiofrequency denervation, and conservative management for sacroiliac joint pain: 6-year comparative case series. Neurosurgery. 2018;82(1):48-55.
28. Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine (Phila Pa 1976). 2016;41(12):999-1005.
29. Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96-99.
Patients experiencing sacroiliac joint (SIJ) dysfunction might show symptoms that overlap with those seen in lumbar spine pathology. This article reviews diagnostic tools that assist practitioners to discern the true pain generator in patients with low back pain (LBP) and therapeutic approaches when the cause is SIJ dysfunction.
Prevalence
Most of the US population will experience LBP at some point in their lives. A 2002 National Health Interview survey found that more than one-quarter (26.4%) of 31 044 respondents had complained of LBP in the previous 3 months.1 About 74 million individuals in the US experienced LBP in the past 3 months.1 A full 10% of the US population is expected to suffer from chronic LBP, and it is estimated that 2.3% of all visits to physicians are related to LBP.1
The etiology of LBP often is unclear even after thorough clinical and radiographic evaluation because of the myriad possible mechanisms. Degenerative disc disease, facet arthropathy, ligamentous hypertrophy, muscle spasm, hip arthropathy, and SIJ dysfunction are potential pain generators and exact clinical and radiographic correlation is not always possible. Compounding this difficulty is the lack of specificity with current diagnostic techniques. For example, many patients will have disc desiccation or herniation without any LBP or radicular symptoms on radiographic studies, such as X-rays, computed tomography (CT), and magnetic resonance imaging (MRI). As such, providers of patients with diffuse radiographic abnormalities often have to identify a specific pain generator, which might not have any role in the patient’s pain.
Other tests, such as electromyographic studies, positron emission tomography (PET) scans, discography, and epidural steroid injections, can help pinpoint a specific pain generator. These tests might help determine whether the patient has a surgically treatable condition and could help predict whether a patient’s symptoms will respond to surgery.
However, the standard spine surgery workup often fails to identify an obvious pain generator in many individuals. The significant number of patients that fall into this category has prompted spine surgeons to consider other potential etiologies for LBP, and SIJ dysfunction has become a rapidly developing field of research.
Sacroiliac Joint Dysfunction
The SIJ is a bilateral, C-shaped synovial joint surrounded by a fibrous capsule and affixes the sacrum to the ilia. Several sacral ligaments and pelvic muscles support the SIJ. The L5 nerve ventral ramus and lumbosacral trunk pass anteriorly and the S1 nerve ventral ramus passes inferiorly to the joint capsule. The SIJ is innervated by the dorsal rami of L4-S3 nerve roots, transmitting nociception and temperature. Mechanisms of injury to the SIJ could arise from intra- and extra-articular etiologies, including capsular disruption, ligamentous tension, muscular inflammation, shearing, fractures, arthritis, and infection.2 Patients could develop SIJ pain spontaneously or after a traumatic event or repetitive shear.3 Risk factors for developing SIJ dysfunction include a history of lumbar fusion, scoliosis, leg length discrepancies, sustained athletic activity, pregnancy, seronegative HLA-B27 spondyloarthropathies, or gait abnormalities. Inflammation of the SIJ and surrounding structures secondary to an environmental insult in susceptible individuals is a common theme among these etiologies.2
Pain from the SIJ is localized to an area of approximately 3 cm × 10 cm that is inferior to the ipsilateral posterior superior iliac spine.4 Referred pain maps from SIJ dysfunction extend in the L5-S1 nerve distributions, commonly seen in the buttocks, groin, posterior thigh, and lower leg with radicular symptoms. However, this pain distribution demonstrates extensive variability among patients and bears strong similarities to discogenic or facet joint sources of LBP.5-7 Direct communication has been shown between the SIJ and adjacent neural structures, namely the L5 nerve, sacral foramina, and the lumbosacral plexus. These direct pathways could explain an inflammatory mechanism for lower extremity symptoms seen in SIJ dysfunction.8
The prevalence of SIJ dysfunction among patients with LBP is estimated to be 15% to 30%, an extraordinary number given the total number of patients presenting with LBP every year.9 These patients might represent a significant segment of patients with an unrevealing standard spine evaluation. Despite the large number of patients who experience SIJ dysfunction, there is disagreement about optimal methods for diagnosis and treatment.
Diagnosis
The International Association for the Study of Pain has proposed criteria for evaluating patients who have suspected SIJ dysfunction: Pain must be in the SIJ area, should be reproducible by performing specific provocative maneuvers, and must be relieved by injection of local anesthetic into the SIJ.10 These criteria provide a sound foundation, but in clinical practice, patients often defy categorization.
The presence of pain in the area inferior to the posterior superior iliac spine and lateral to the gluteal fold with pain referral patterns in the L5-S1 nerve distributions is highly sensitive for identifying patients with SIJ dysfunction. Furthermore, pain arising from the SIJ will not be above the level of the L5 nerve sensory distribution. However, this diagnostic finding alone is not specific and might represent other etiologies known to produce similar pain, such as intervertebral discs and facet joints. Patients with SIJ dysfunction often describe their pain as sciatica-like, recurrent, and triggered with bending or twisting motions. It is worsened with any activity loading the SIJ, such as walking, climbing stairs, standing, or sitting upright. SIJ pain might be accompanied by dyspareunia and changes in bladder function because of the nerves involved.11
The use of provocative maneuvers for testing SIJ dysfunction is controversial because of the high rate of false positives and the inability to distinguish whether the SIJ or an adjacent structure is affected. However, the diagnostic utility of specific stress tests has been studied, and clusters of tests are recommended if a health care provider (HCP) suspects SIJ dysfunction. A diagnostic algorithm should first focus on using the distraction test and the thigh thrust test. Distraction is done by applying vertically oriented pressure to the anterior superior iliac spine while aiming posteriorly, therefore distracting the SIJ. During the thigh thrust test the examiner fixates the patient’s sacrum against the table with the left hand and applies a vertical force through the line of the femur aiming posteriorly, producing a posterior shearing force at the SIJ. Studies show that the thigh thrust test is the most sensitive, and the distraction test is the most specific. If both tests are positive, there is reasonable evidence to suggest SIJ dysfunction as the source of LBP.
If there are not 2 positive results, the addition of the compression test, followed by the sacral thrust test also can point to the diagnosis. The compression test is performed with vertical downward force applied to the iliac crest with the patient lying on each side, compressing the SIJ by transverse pressure across the pelvis. The sacral thrust test is performed with vertical force applied to the midline posterior sacrum at its apex directed anteriorly with the patient lying prone, producing a shearing force at the SIJs. The Gaenslen test uses a torsion force by applying a superior and posterior force to the right knee and posteriorly directed force to the left knee. Omitting the Gaenslen test has not been shown to compromise diagnostic efficacy of the other tests and can be safely excluded.12
A HCP can rule out SIJ dysfunction if these provocation tests are negative. However, the diagnostic predictive value of these tests is subject to variability among HCPs, and their reliability is increased when used in clusters.9,13
Imaging for the SIJ should begin with anterior/posterior, oblique, and lateral view plain X-rays of the pelvis (Figures 1 and 2), which will rule out other pathologies by identifying other sources of LBP, such as spondylolisthesis or hip osteoarthritis. HCPs should obtain lumbar and pelvis CT images to identify inflammatory or degenerative changes within the SIJ. CT images provide the high resolution that is needed to identify pathologies, such as fractures and tumors within the pelvic ring that could cause similar pain. MRI does not reliably depict a dysfunctional ligamentous apparatus within the SIJ; however, it can help identify inflammatory sacroiliitis, such as is seen in the spondyloarthropathies.11,14 Recent studies show combined single photon emission tomography and CT (SPECT-CT) might be the most promising imaging modality to reveal mechanical failure of load transfer with increased scintigraphic uptake in the posterior and superior SIJ ligamentous attachments. The joint loses its characteristic “dumbbell” shape in affected patients with about 50% higher uptake than unaffected joints. These findings were evident in patients who experienced pelvic trauma or during the peripartum period.15,16
Fluoroscopy-guided intra-articular injection of a local anesthetic (lidocaine) and/or a corticosteroid (triamcinolone) has the dual functionality of diagnosis and treatment (Figure 3). It often is considered the most reliable method to diagnose SIJ dysfunction and has the benefit of pain relief for up to 1 year. However, intra-articular injections lack diagnostic validity because the solution often extravasates to extracapsular structures. This confounds the source of the pain and makes it difficult to interpret these diagnostic injections. In addition, the injection might not reach the entire SIJ capsule and could result in a false-negative diagnosis.17,18 Periarticular injections have been shown to result in better pain relief in patients diagnosed with SIJ dysfunction than intra-articular injections. Periarticular injections also are easier to perform and could be a first-step option for these patients.19
Treatment
Nonoperative management of SIJ dysfunction includes exercise programs, physical therapy, manual manipulation therapy, sacroiliac belts, and periodic articular injections. Efficacy of these methods is variable, and analgesics often do not significantly benefit this type of pain. Another nonoperative approach is radiofrequency ablation (RFA) of the lumbar dorsal rami and lateral sacral branches, which can vary based on the number of rami treated as well as the technique used. About two-thirds of patients report pain relief after RFA.2 When successful, pain is relieved for 6 to 12 months, which is a temporary yet effective option for patients experiencing SIJ dysfunction.14,20
Fusion Surgery
Cadaver studies show that biomechanical stabilization of the SIJ leads to decreased range of motion in flexion/extension, lateral bending, and axial rotation. This results in a decreased need for periarticular muscular and ligamentous support, therefore facilitating load transfer across the SIJ.21,22 Patients undergoing minimally invasive surgery report better pain relief compared with those receiving open surgery at 12 months postoperatively.23 The 2 main SIJ fusion approaches used are the lateral transarticular and the dorsal approaches. In the dorsal approach, the SIJ is distracted and allograft dowels or titanium cages with graft are inserted into the joint space posteriorly through the back. When approaching laterally, hollow screw implants filled with graft or triangular titanium implants are placed across the joint, accessing the SIJ through the iliac bones using imaging guidance. This lateral transiliac approach using porous titanium triangular rods currently is the most studied technique.24
A recent prospective, multicenter trial included 423 patients with SIJ dysfunction who were randomized to receive SIJ fusion with triangular titanium implants vs a control group who received nonoperative management. Patients in the SIJ fusion group showed substantially greater improvement in pain (81.4%) compared with that of the nonoperative group (26.1%) 6 months after surgery. Pain relief in the SIJ fusion group was maintained at > 80% at 1 and 2 year follow-up, while the nonoperative group’s pain relief decreased to < 10% at the follow-ups. Measures of quality of life and disability also improved for the SIJ fusion group compared with that of the nonoperative group. Patients who were crossed over from conservative management to SIJ fusion after 6 months demonstrated improvements that were similar to those in the SIJ fusion group by the end of the study. Only 3% of patients required surgical revision. The strongest predictor of pain relief after surgery was a diagnostic SIJ anesthetic block of 30 to 60 minutes, which resulted in > 75% pain reduction.21,25 Additional predictors of successful SIJ fusion include nonsmokers, nonopioid users, and older patients who have a longer time course of SIJ pain.26
Another study investigating the outcomes of SIJ fusion, RFA, and conservative management with a 6-year follow-up demonstrated similar results.27 This further confirms the durability of the surgical group’s outcome, which sustained significant improvement compared with RFA and conservative management group in pain relief, daily function, and opioid use.
HCPs should consider SIJ fusion for patients who have at least 6 months of unsuccessful nonoperative management, significant SIJ pain (> 5 in a 10-point scale), ≥ 3 positive provocation tests, and at least 50% pain relief (> 75% preferred) with diagnostic intra-articular anesthetic injection.14 It is reasonable for primary care providers to refer these patients to a neurosurgeon or orthopedic spine surgeon for possible fusion. Patients with earlier lumbar/lumbosacral spinal fusions and persistent LBP should be evaluated for potential SIJ dysfunction. SIJ dysfunction after lumbosacral fusion could be considered a form of distal pseudarthrosis resulting from increased motion at the joint. One study found its incidence correlated with the number of segments fused in the lumbar spine.28 Another study found that about one-third of patients with persistent LBP after lumbosacral fusion could be attributed to SIJ dysfunction.29
Case Presentation
A 27-year-old female army veteran presented with bilateral buttock pain, which she described as a dull, aching pain across her sacral region, 8 out of 10 in severity. The pain was in a L5-S1 pattern. The pain was bilateral, with the right side worse than the left, and worsened with lateral bending and load transferring. She reported no numbness, tingling, or weakness.
On physical examination, she had full strength in her lower extremities and intact sensation. She reported tenderness to palpation of the sacrum and SIJ. Her gait was normal. The patient had positive thigh thrust and distraction tests. Lumbar spine X-ray, CT, MRI, and electromyographic studies did not show any pathology. She described little or no relief with analgesics or physical therapy. Previous L4-L5 and L5-S1 facet anesthetic injections and transforaminal epidural steroid injections provided minimal pain relief immediately after the procedures. Bilateral SIJ anesthetic injections under fluoroscopic guidance decreased her pain severity from a 7 to 3 out of 10 for 2 to 3 months before returning to her baseline. Radiofrequency ablation of the right SIJ under fluoroscopy provided moderate relief for about 4 months.
After exhausting nonoperative management for SIJ dysfunction without adequate pain control, the patient was referred to neurosurgery for surgical fusion. The patient was deemed an appropriate surgical candidate and underwent a right-sided SIJ fusion (Figures 4 and 5). At her 6-month and 1-year follow-up appointments, she had lasting pain relief, 2 out of 10.
Conclusion
SIJ dysfunction is widely overlooked because of the difficulty in distinguishing it from other similarly presenting syndromes. However, with a detailed history, appropriate physical maneuvers, imaging, and adequate response to intra-articular anesthetic, providers can reach an accurate diagnosis that will inform subsequent treatments. After failure of nonsurgical methods, patients with SIJ dysfunction should be considered for minimally invasive fusion techniques, which have proven to be a safe, effective, and viable treatment option.
Patients experiencing sacroiliac joint (SIJ) dysfunction might show symptoms that overlap with those seen in lumbar spine pathology. This article reviews diagnostic tools that assist practitioners to discern the true pain generator in patients with low back pain (LBP) and therapeutic approaches when the cause is SIJ dysfunction.
Prevalence
Most of the US population will experience LBP at some point in their lives. A 2002 National Health Interview survey found that more than one-quarter (26.4%) of 31 044 respondents had complained of LBP in the previous 3 months.1 About 74 million individuals in the US experienced LBP in the past 3 months.1 A full 10% of the US population is expected to suffer from chronic LBP, and it is estimated that 2.3% of all visits to physicians are related to LBP.1
The etiology of LBP often is unclear even after thorough clinical and radiographic evaluation because of the myriad possible mechanisms. Degenerative disc disease, facet arthropathy, ligamentous hypertrophy, muscle spasm, hip arthropathy, and SIJ dysfunction are potential pain generators and exact clinical and radiographic correlation is not always possible. Compounding this difficulty is the lack of specificity with current diagnostic techniques. For example, many patients will have disc desiccation or herniation without any LBP or radicular symptoms on radiographic studies, such as X-rays, computed tomography (CT), and magnetic resonance imaging (MRI). As such, providers of patients with diffuse radiographic abnormalities often have to identify a specific pain generator, which might not have any role in the patient’s pain.
Other tests, such as electromyographic studies, positron emission tomography (PET) scans, discography, and epidural steroid injections, can help pinpoint a specific pain generator. These tests might help determine whether the patient has a surgically treatable condition and could help predict whether a patient’s symptoms will respond to surgery.
However, the standard spine surgery workup often fails to identify an obvious pain generator in many individuals. The significant number of patients that fall into this category has prompted spine surgeons to consider other potential etiologies for LBP, and SIJ dysfunction has become a rapidly developing field of research.
Sacroiliac Joint Dysfunction
The SIJ is a bilateral, C-shaped synovial joint surrounded by a fibrous capsule and affixes the sacrum to the ilia. Several sacral ligaments and pelvic muscles support the SIJ. The L5 nerve ventral ramus and lumbosacral trunk pass anteriorly and the S1 nerve ventral ramus passes inferiorly to the joint capsule. The SIJ is innervated by the dorsal rami of L4-S3 nerve roots, transmitting nociception and temperature. Mechanisms of injury to the SIJ could arise from intra- and extra-articular etiologies, including capsular disruption, ligamentous tension, muscular inflammation, shearing, fractures, arthritis, and infection.2 Patients could develop SIJ pain spontaneously or after a traumatic event or repetitive shear.3 Risk factors for developing SIJ dysfunction include a history of lumbar fusion, scoliosis, leg length discrepancies, sustained athletic activity, pregnancy, seronegative HLA-B27 spondyloarthropathies, or gait abnormalities. Inflammation of the SIJ and surrounding structures secondary to an environmental insult in susceptible individuals is a common theme among these etiologies.2
Pain from the SIJ is localized to an area of approximately 3 cm × 10 cm that is inferior to the ipsilateral posterior superior iliac spine.4 Referred pain maps from SIJ dysfunction extend in the L5-S1 nerve distributions, commonly seen in the buttocks, groin, posterior thigh, and lower leg with radicular symptoms. However, this pain distribution demonstrates extensive variability among patients and bears strong similarities to discogenic or facet joint sources of LBP.5-7 Direct communication has been shown between the SIJ and adjacent neural structures, namely the L5 nerve, sacral foramina, and the lumbosacral plexus. These direct pathways could explain an inflammatory mechanism for lower extremity symptoms seen in SIJ dysfunction.8
The prevalence of SIJ dysfunction among patients with LBP is estimated to be 15% to 30%, an extraordinary number given the total number of patients presenting with LBP every year.9 These patients might represent a significant segment of patients with an unrevealing standard spine evaluation. Despite the large number of patients who experience SIJ dysfunction, there is disagreement about optimal methods for diagnosis and treatment.
Diagnosis
The International Association for the Study of Pain has proposed criteria for evaluating patients who have suspected SIJ dysfunction: Pain must be in the SIJ area, should be reproducible by performing specific provocative maneuvers, and must be relieved by injection of local anesthetic into the SIJ.10 These criteria provide a sound foundation, but in clinical practice, patients often defy categorization.
The presence of pain in the area inferior to the posterior superior iliac spine and lateral to the gluteal fold with pain referral patterns in the L5-S1 nerve distributions is highly sensitive for identifying patients with SIJ dysfunction. Furthermore, pain arising from the SIJ will not be above the level of the L5 nerve sensory distribution. However, this diagnostic finding alone is not specific and might represent other etiologies known to produce similar pain, such as intervertebral discs and facet joints. Patients with SIJ dysfunction often describe their pain as sciatica-like, recurrent, and triggered with bending or twisting motions. It is worsened with any activity loading the SIJ, such as walking, climbing stairs, standing, or sitting upright. SIJ pain might be accompanied by dyspareunia and changes in bladder function because of the nerves involved.11
The use of provocative maneuvers for testing SIJ dysfunction is controversial because of the high rate of false positives and the inability to distinguish whether the SIJ or an adjacent structure is affected. However, the diagnostic utility of specific stress tests has been studied, and clusters of tests are recommended if a health care provider (HCP) suspects SIJ dysfunction. A diagnostic algorithm should first focus on using the distraction test and the thigh thrust test. Distraction is done by applying vertically oriented pressure to the anterior superior iliac spine while aiming posteriorly, therefore distracting the SIJ. During the thigh thrust test the examiner fixates the patient’s sacrum against the table with the left hand and applies a vertical force through the line of the femur aiming posteriorly, producing a posterior shearing force at the SIJ. Studies show that the thigh thrust test is the most sensitive, and the distraction test is the most specific. If both tests are positive, there is reasonable evidence to suggest SIJ dysfunction as the source of LBP.
If there are not 2 positive results, the addition of the compression test, followed by the sacral thrust test also can point to the diagnosis. The compression test is performed with vertical downward force applied to the iliac crest with the patient lying on each side, compressing the SIJ by transverse pressure across the pelvis. The sacral thrust test is performed with vertical force applied to the midline posterior sacrum at its apex directed anteriorly with the patient lying prone, producing a shearing force at the SIJs. The Gaenslen test uses a torsion force by applying a superior and posterior force to the right knee and posteriorly directed force to the left knee. Omitting the Gaenslen test has not been shown to compromise diagnostic efficacy of the other tests and can be safely excluded.12
A HCP can rule out SIJ dysfunction if these provocation tests are negative. However, the diagnostic predictive value of these tests is subject to variability among HCPs, and their reliability is increased when used in clusters.9,13
Imaging for the SIJ should begin with anterior/posterior, oblique, and lateral view plain X-rays of the pelvis (Figures 1 and 2), which will rule out other pathologies by identifying other sources of LBP, such as spondylolisthesis or hip osteoarthritis. HCPs should obtain lumbar and pelvis CT images to identify inflammatory or degenerative changes within the SIJ. CT images provide the high resolution that is needed to identify pathologies, such as fractures and tumors within the pelvic ring that could cause similar pain. MRI does not reliably depict a dysfunctional ligamentous apparatus within the SIJ; however, it can help identify inflammatory sacroiliitis, such as is seen in the spondyloarthropathies.11,14 Recent studies show combined single photon emission tomography and CT (SPECT-CT) might be the most promising imaging modality to reveal mechanical failure of load transfer with increased scintigraphic uptake in the posterior and superior SIJ ligamentous attachments. The joint loses its characteristic “dumbbell” shape in affected patients with about 50% higher uptake than unaffected joints. These findings were evident in patients who experienced pelvic trauma or during the peripartum period.15,16
Fluoroscopy-guided intra-articular injection of a local anesthetic (lidocaine) and/or a corticosteroid (triamcinolone) has the dual functionality of diagnosis and treatment (Figure 3). It often is considered the most reliable method to diagnose SIJ dysfunction and has the benefit of pain relief for up to 1 year. However, intra-articular injections lack diagnostic validity because the solution often extravasates to extracapsular structures. This confounds the source of the pain and makes it difficult to interpret these diagnostic injections. In addition, the injection might not reach the entire SIJ capsule and could result in a false-negative diagnosis.17,18 Periarticular injections have been shown to result in better pain relief in patients diagnosed with SIJ dysfunction than intra-articular injections. Periarticular injections also are easier to perform and could be a first-step option for these patients.19
Treatment
Nonoperative management of SIJ dysfunction includes exercise programs, physical therapy, manual manipulation therapy, sacroiliac belts, and periodic articular injections. Efficacy of these methods is variable, and analgesics often do not significantly benefit this type of pain. Another nonoperative approach is radiofrequency ablation (RFA) of the lumbar dorsal rami and lateral sacral branches, which can vary based on the number of rami treated as well as the technique used. About two-thirds of patients report pain relief after RFA.2 When successful, pain is relieved for 6 to 12 months, which is a temporary yet effective option for patients experiencing SIJ dysfunction.14,20
Fusion Surgery
Cadaver studies show that biomechanical stabilization of the SIJ leads to decreased range of motion in flexion/extension, lateral bending, and axial rotation. This results in a decreased need for periarticular muscular and ligamentous support, therefore facilitating load transfer across the SIJ.21,22 Patients undergoing minimally invasive surgery report better pain relief compared with those receiving open surgery at 12 months postoperatively.23 The 2 main SIJ fusion approaches used are the lateral transarticular and the dorsal approaches. In the dorsal approach, the SIJ is distracted and allograft dowels or titanium cages with graft are inserted into the joint space posteriorly through the back. When approaching laterally, hollow screw implants filled with graft or triangular titanium implants are placed across the joint, accessing the SIJ through the iliac bones using imaging guidance. This lateral transiliac approach using porous titanium triangular rods currently is the most studied technique.24
A recent prospective, multicenter trial included 423 patients with SIJ dysfunction who were randomized to receive SIJ fusion with triangular titanium implants vs a control group who received nonoperative management. Patients in the SIJ fusion group showed substantially greater improvement in pain (81.4%) compared with that of the nonoperative group (26.1%) 6 months after surgery. Pain relief in the SIJ fusion group was maintained at > 80% at 1 and 2 year follow-up, while the nonoperative group’s pain relief decreased to < 10% at the follow-ups. Measures of quality of life and disability also improved for the SIJ fusion group compared with that of the nonoperative group. Patients who were crossed over from conservative management to SIJ fusion after 6 months demonstrated improvements that were similar to those in the SIJ fusion group by the end of the study. Only 3% of patients required surgical revision. The strongest predictor of pain relief after surgery was a diagnostic SIJ anesthetic block of 30 to 60 minutes, which resulted in > 75% pain reduction.21,25 Additional predictors of successful SIJ fusion include nonsmokers, nonopioid users, and older patients who have a longer time course of SIJ pain.26
Another study investigating the outcomes of SIJ fusion, RFA, and conservative management with a 6-year follow-up demonstrated similar results.27 This further confirms the durability of the surgical group’s outcome, which sustained significant improvement compared with RFA and conservative management group in pain relief, daily function, and opioid use.
HCPs should consider SIJ fusion for patients who have at least 6 months of unsuccessful nonoperative management, significant SIJ pain (> 5 in a 10-point scale), ≥ 3 positive provocation tests, and at least 50% pain relief (> 75% preferred) with diagnostic intra-articular anesthetic injection.14 It is reasonable for primary care providers to refer these patients to a neurosurgeon or orthopedic spine surgeon for possible fusion. Patients with earlier lumbar/lumbosacral spinal fusions and persistent LBP should be evaluated for potential SIJ dysfunction. SIJ dysfunction after lumbosacral fusion could be considered a form of distal pseudarthrosis resulting from increased motion at the joint. One study found its incidence correlated with the number of segments fused in the lumbar spine.28 Another study found that about one-third of patients with persistent LBP after lumbosacral fusion could be attributed to SIJ dysfunction.29
Case Presentation
A 27-year-old female army veteran presented with bilateral buttock pain, which she described as a dull, aching pain across her sacral region, 8 out of 10 in severity. The pain was in a L5-S1 pattern. The pain was bilateral, with the right side worse than the left, and worsened with lateral bending and load transferring. She reported no numbness, tingling, or weakness.
On physical examination, she had full strength in her lower extremities and intact sensation. She reported tenderness to palpation of the sacrum and SIJ. Her gait was normal. The patient had positive thigh thrust and distraction tests. Lumbar spine X-ray, CT, MRI, and electromyographic studies did not show any pathology. She described little or no relief with analgesics or physical therapy. Previous L4-L5 and L5-S1 facet anesthetic injections and transforaminal epidural steroid injections provided minimal pain relief immediately after the procedures. Bilateral SIJ anesthetic injections under fluoroscopic guidance decreased her pain severity from a 7 to 3 out of 10 for 2 to 3 months before returning to her baseline. Radiofrequency ablation of the right SIJ under fluoroscopy provided moderate relief for about 4 months.
After exhausting nonoperative management for SIJ dysfunction without adequate pain control, the patient was referred to neurosurgery for surgical fusion. The patient was deemed an appropriate surgical candidate and underwent a right-sided SIJ fusion (Figures 4 and 5). At her 6-month and 1-year follow-up appointments, she had lasting pain relief, 2 out of 10.
Conclusion
SIJ dysfunction is widely overlooked because of the difficulty in distinguishing it from other similarly presenting syndromes. However, with a detailed history, appropriate physical maneuvers, imaging, and adequate response to intra-articular anesthetic, providers can reach an accurate diagnosis that will inform subsequent treatments. After failure of nonsurgical methods, patients with SIJ dysfunction should be considered for minimally invasive fusion techniques, which have proven to be a safe, effective, and viable treatment option.
1. Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59-66.
2. Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440-1453.
3. Chou LH, Slipman CW, Bhagia SM, et al. Inciting events initiating injection‐proven sacroiliac joint syndrome. Pain Med. 2004;5(1):26-32.
4. Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255-265.
5. Buijs E, Visser L, Groen G. Sciatica and the sacroiliac joint: a forgotten concept. Br J Anaesth. 2007;99(5):713-716.
6. Fortin JD, Dwyer AP, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19(13):1475-1482.
7. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
8. Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. ANJR Am J Neuroradiol. 1999;20(8):1429-1434.
9. Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368.
10. Merskey H, Bogduk N, eds. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
11. Cusi MF. Paradigm for assessment and treatment of SIJ mechanical dysfunction. J Bodyw Mov Ther. 2010;14(2):152-161.
12. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
13. Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16(3):142-152.
14. Polly DW Jr. The sacroiliac joint. Neurosurg Clin N Am. 2017;28(3):301-312.
15. Cusi M, Van Der Wall H, Saunders J, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22(7):1674-1682.
16. Tofuku K, Koga H, Komiya S. The diagnostic value of single-photon emission computed tomography/computed tomography for severe sacroiliac joint dysfunction. Eur Spine J. 2015;24(4):859-863.
17. Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra‐articular sacroiliac joint injections: a systematic review. Pain Med. 2015;16(8):1500-1518.
18. Schneider BJ, Huynh L, Levin J, Rinkaekan P, Kordi R, Kennedy DJ. Does immediate pain relief after an injection into the sacroiliac joint with anesthetic and corticosteroid predict subsequent pain relief? Pain Med. 2018;19(2):244-251.
19. Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007;12(3):274-280.
20. Cohen SP, Hurley RW, Buckenmaier CC 3rd, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
21. Polly DW, Cher DJ, Wine KD, et al; INSITE Study Group. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-690.
22. Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, et al. The effect of implant placement on sacroiliac joint range of motion: posterior versus transarticular. Spine. 2015;40(9):E525-E530.
23. Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14.
24. Rashbaum RF, Ohnmeiss DD, Lindley EM, Kitchel SH, Patel VV. Sacroiliac joint pain and its treatment. Clin Spine Surg. 2016;29(2):42-48.
25. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28.
26. Dengler J, Duhon B, Whang P, et al. Predictors of outcome in conservative and minimally invasive surgical management of pain originating from the sacroiliac joint: a pooled analysis. Spine (Phila Pa 1976). 2017;42(21):1664-1673.
27. Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally invasive sacroiliac joint fusion, radiofrequency denervation, and conservative management for sacroiliac joint pain: 6-year comparative case series. Neurosurgery. 2018;82(1):48-55.
28. Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine (Phila Pa 1976). 2016;41(12):999-1005.
29. Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96-99.
1. Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59-66.
2. Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440-1453.
3. Chou LH, Slipman CW, Bhagia SM, et al. Inciting events initiating injection‐proven sacroiliac joint syndrome. Pain Med. 2004;5(1):26-32.
4. Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255-265.
5. Buijs E, Visser L, Groen G. Sciatica and the sacroiliac joint: a forgotten concept. Br J Anaesth. 2007;99(5):713-716.
6. Fortin JD, Dwyer AP, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19(13):1475-1482.
7. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
8. Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. ANJR Am J Neuroradiol. 1999;20(8):1429-1434.
9. Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368.
10. Merskey H, Bogduk N, eds. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
11. Cusi MF. Paradigm for assessment and treatment of SIJ mechanical dysfunction. J Bodyw Mov Ther. 2010;14(2):152-161.
12. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
13. Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16(3):142-152.
14. Polly DW Jr. The sacroiliac joint. Neurosurg Clin N Am. 2017;28(3):301-312.
15. Cusi M, Van Der Wall H, Saunders J, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22(7):1674-1682.
16. Tofuku K, Koga H, Komiya S. The diagnostic value of single-photon emission computed tomography/computed tomography for severe sacroiliac joint dysfunction. Eur Spine J. 2015;24(4):859-863.
17. Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra‐articular sacroiliac joint injections: a systematic review. Pain Med. 2015;16(8):1500-1518.
18. Schneider BJ, Huynh L, Levin J, Rinkaekan P, Kordi R, Kennedy DJ. Does immediate pain relief after an injection into the sacroiliac joint with anesthetic and corticosteroid predict subsequent pain relief? Pain Med. 2018;19(2):244-251.
19. Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007;12(3):274-280.
20. Cohen SP, Hurley RW, Buckenmaier CC 3rd, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
21. Polly DW, Cher DJ, Wine KD, et al; INSITE Study Group. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-690.
22. Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, et al. The effect of implant placement on sacroiliac joint range of motion: posterior versus transarticular. Spine. 2015;40(9):E525-E530.
23. Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14.
24. Rashbaum RF, Ohnmeiss DD, Lindley EM, Kitchel SH, Patel VV. Sacroiliac joint pain and its treatment. Clin Spine Surg. 2016;29(2):42-48.
25. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28.
26. Dengler J, Duhon B, Whang P, et al. Predictors of outcome in conservative and minimally invasive surgical management of pain originating from the sacroiliac joint: a pooled analysis. Spine (Phila Pa 1976). 2017;42(21):1664-1673.
27. Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally invasive sacroiliac joint fusion, radiofrequency denervation, and conservative management for sacroiliac joint pain: 6-year comparative case series. Neurosurgery. 2018;82(1):48-55.
28. Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine (Phila Pa 1976). 2016;41(12):999-1005.
29. Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96-99.
Using Optical Coherence Tomography in the Management of Postoperative Wound Leaks After Cataract Surgery
The term cataract is derived from the Latin word “catarractes,” which means “waterfall,” as the foamy white opacity of an advanced cataract can be likened to a tempestuous cascade. Cataract is the leading cause of preventable blindness worldwide.1,2 It is no surprise, therefore, that cataract surgery is the most frequently performed ophthalmic surgical procedure worldwide. Cataract surgeries may reach 30 million annual cases by 2020.3 Given the large number of surgeries being performed, postsurgical complications are not uncommon.
Early postoperative complications from lens exchange (cataract) surgery include increased intraocular pressure (IOP), corneal edema, and corneal wound leakage.4 Corneal wound leakage is not uncommon; one study showed that, in 100 cases, almost one-third of incisions leaked.5 A 2014 prospective study of 500 postcataract surgery eyes revealed that 48.8% had fluid egress.6 Early detection is important so that efforts to restore corneal integrity can immediately be implemented. If not caught early, patients are at risk for developing a cascade of sequelae, including endophthalmitis.
The majority of corneal wound leaks postphacoemulsification are self-limiting and self-sealing. Moderate wound leaks require treatment, as in the following case. Strategies to detect, image, and treat wound leaks are covered in this discussion.
Case Presentation
A 69-year-old male veteran presented with no complaints for a 1-day postoperative visit following right eye phacoemulsification cataract extraction. His best corrected visual acuity in the right eye was 20/40, and his pinhole visual acuity was 20/25+2. On slit-lamp examination, the temporally located main incision appeared well-adhered and was found to be Seidel negative; however, the inferior paracentesis wound was found to be Seidel positive, demonstrating a slow leak. Intraocular pressure (IOP) measured with tonopen was 9 mm Hg.
A bandage soft contact lens was placed on the eye. The patient was instructed not to rub or place any pressure on the eye and to avoid bending and heavy lifting. He was also instructed to continue his postoperative medications (prednisolone 1% every 2 hours and polymyxin B sulfate 4 times daily) in his right eye. A follow-up appointment was scheduled for the next day.
The patient presented for his postoperative day-2 visit with a best corrected visual acuity in the right eye of 20/20. He reported no visual problems, no eye pain, and mentioned that he had had a comfortable night sleep. A slit-lamp examination revealed trace diffuse injection in the operative eye, predominantly central Descemet membrane folds, 1+ stromal edema, and a Seidel negative main incision wound. However, the inferior paracentesis wound showed a moderate leak (Seidel positive), and the anterior chamber showed a 1+ cell and flare. Goldmann tonometry revealed an IOP of 5 mm Hg, indicating hypotony.
Anterior segment cube 512 x 128 optical coherence tomography (OCT) was obtained with the bandage contact lens (Figures 1 and 2), and then repeated with the bandage contact lens removed (Figures 3 and 4). OCT imaging confirmed epithelial and endothelial gaping, loss of coaptation, and a localized detachment of the Descemet membrane. The veteran was referred to his surgeon that same day, and 2 limbal vicryl sutures were placed. The patient was instructed to continue prednisolone 1% 4 times daily and polymyxin B sulfate every 2 hours; erythromycin ointment 3 times daily was added to his regimen.
He was scheduled for a follow-up examination 1 week later. At that visit, the wound was no longer leaking and IOP had risen to a preoperative value of 17 mm Hg. The corneal sutures were removed at the 1-month postoperative examination and a follow-up was scheduled for 4 months later. An anterior segment OCT was obtained (Figure 5).
Discussion
In July 1967, Charles Kelman, MD, suggested using a dental ultrasonic tool, normally employed to clean teeth, to fragment the nucleus of the crystalline lens. Dr. Kelman’s first operation using phacoemulsification on a human eye took 3 hours.7 As the procedure for cataract removal has been refined, complication rates and surgical times have vastly improved.
Phacoemulsification is the most commonly performed outpatient surgery in the US; about 3 million cases are performed annually. Due to the high volume of cases, adverse events (AEs) are not uncommon. The incidence of complications following phacoemulsification is < 5%; the frequency of severe complications has been estimated at < 0.7%.8 Severe complications include endophthalmitis, suprachoroidal hemorrhage, and/or retinal detachment.9 Studies have shown a decline in rates of sight-threatening AEs from 1994 to 2006.9 A retrospective study of 45,082 veterans from 2005 to 2007 identified that a preoperative disease burden such as diabetes mellitus, chronic pulmonary disease, age-related macular degeneration, and diabetes with ophthalmic manifestations, was positively associated with a greater risk of cataract surgical complications.10
Complications
The level of a surgeon’s proficiency with phacoemulsification is directly correlated to the number of operations performed; there is a lower complication rate among more experienced surgeons, including those who work in high-volume settings.11,12 One study identified that the AE rate within 14 days of surgery was 0.8% for surgeons performing 50 to 250 cataract surgeries per year, but only 0.1% for those performing > 1000 cataract surgeries annually.12
Potential postoperative lens exchange complications include increased IOP, corneal wound leakage, corneal edema, bullous keratopathy, cystoid macular edema, retinal detachment, and endophthalmitis (Table 1). A corneal wound leak can provide a potential ingress for bacteria, putting the patient at risk for endophthalmitis, perhaps the most devastating complication following cataract surgery.
Endophthalmitis
Endophthalmitis has been reported to occur in .001% to .327% of patients during postoperative care.5,13-17 Early detection is important to maintain corneal integrity and prevent a cascade of detrimental ocular sequalae including the potential for endophthalmitis. According to Zaida and colleagues, endophthalmitis occurred in fewer than 1 of 1000 consecutive cases.14 A leaking clear corneal incision wound on the first day postoperatively has been associated with a 44-fold increased risk of endophthalmitis.13
Causes of endophthalmitis
In a retrospective case-controlled series of 57 patients with postcataract endophthalmitis, implantation of an intraocular lens with a resultant wound abnormality was thought to be the causative factor in 5%.17 Another source of endophthalmitis can be the intraocular lens (IOL), which may act as a vector for bacteria. By placing the IOL against the conjunctiva or exposing it to the theater air during surgery, bacteria can be introduced prior to implantation.17 Immunosuppressive treatment is the only patient antecedent factor that can be considered a predictor for endopthalmitis.17
The internal corneal seal is IOP dependent, and postoperative ocular hypotony may cause a seemingly watertight wound to leak. Taban and colleagues used anterior segment OCT to image numerous self-sealing incisions. They found that the corneal incision wound more tightly seals at higher IOPs. Additionally, more perpendicular (larger angle) incisions seal better at a lower IOP while less perpendicular (smaller angle) incisions seal better at a higher IOP (Figure 6).18
Incision Placement
Studies have shown that the main incision site is more clinically competent than is the side port incision site, as in our case study.19 Side-port incisions have a 1- or 2-plane architectural profile in contrast to the 3-plane profile typical of a main incision.19 Recent advances including the conversion to clear-corneal incisions of diminishing size, techniques used for wound construction, phacoemulsification machine design, and small-incision IOLs, should further reduce the prevalence and complications of wound compromise.20
Seidel Testing
Seidel testing is the most common method to evaluate corneal wound integrity and identify leaks. A drop of topical anesthetic is instilled in the eye and then a fluorescein strip (not fluorescein sodium and benoxinate hydrochloride ophthalmic solution, which may become less sterile since it has a multiuse container) is applied to the superior conjunctiva. The clinician then looks for evidence of fluid egress using the cobalt blue filter. The patient is instructed to blink once. Fluid egress appears as a black stream as the fluorescein dye becomes diluted by aqueous humor escaping the nonintact wound and the appearance of bright green dye surrounds the leak site. The term Seidel positive indicates a leak. An estimate should be made of the rate and volume of fluid exiting the wound.
Gonioscopy
Gonioscopy can be used to evaluate the postsurgical incision, more specifically for identification and management of internal incision wound gape. On gonioscopy, internal wound gape appears as an elongated oval opening resembling a fish mouth. If internal incision wound gape is identified gonioscopically before surgery is complete, the leak can be managed intraoperatively. The surgeon can irrigate along the length of the incision to remove cortical fragments or viscoelastic that may cause internal wound gaping. If unsuccessful, rapidly deepening the anterior chamber with balanced salt solution through the paracentesis incision may be employed. These methods may improve wound stability, reduce risk of postoperative hyphema, lower the incidence of endophthalmitis, and lessen the likelihood of late against-the-rule drift.21
Anterior Segment Optical Coherence Tomography
Instances when Seidel testing was negative despite actual wound gaping have been described.22,23 Anterior segment OCT is useful to evaluate incision architecture. A 2007 United Kingdom study investigated the corneal architecture in the immediate postoperative period following phacoemulsification using anterior segment OCT. This study showed the benefits of identifying architectural features such as epithelial gaping, endothelial gaping, stripping of Descemet membrane, and loss of coaptation. These features were found to be more common at low IOP and could represent a significant risk factor for endophthalmitis.24 Another study published by Behrens and colleagues indicated that a localized detachment of Descemet membrane may be more common than observed with slit-lamp (Figure 7). Corneal gaping, especially if along the entire length of the surgical wound, may lead to inadvertent bacterial access into the anterior chamber.25
Anterior segment OCT imaging was first described by Izatt and colleagues in 1994.26 Unlike posterior segment OCT, anterior segment OCT requires a greater depth of field and higher energy levels as images are commonly distorted by refraction at boundaries where the refractive index changes. Longer infrared wavelengths improve the penetration through tissues that scatter light, such as the sclera and limbus, which allows visualization, for example, of the iridocorneal angle.27,28
Two main scan patterns are used for anterior segment OCT: 512 x 128 cube scan (4-mm width x 4-mm length) and 5-line raster (3-mm length) with adjustable rotation and spacing. A recent software update allows measurement of corneal thickness, visualization of anterior chamber angle structures along with topographic analysis, anterior and posterior elevation maps of the cornea, and reliable pachymetric maps.29,30 The anterior segment cube acquires a series of 128 horizontal scan lines each composed of 512 A-scans. These high-definition scans acquire vertical and horizontal directions composed of 1024 A-scans each. This cube may be used to measure corneal thickness and visualize corneal architecture, creating a 3-D image of the data (Figure 8). The anterior segment 5-line raster scans through 5 parallel lines of equal length to view high-resolution images of the anterior chamber angle and cornea. Each line, fixed at 3-mm in length, is composed of 4096 A-scans.31 Anterior segment cube OCT allows identification of subtle variations in incision architecture at different locations across the width of the OCT image.
Bandage Soft Contact Lens
Upon reviewing the anterior segment OCT images of our patient with the bandage contact lens in place, it was evident that the adherent ocular bandage was protecting the incision. A tighter fitting bandage contact lens is ideal and adheres firmly to any area of epithelial damage and epithelial gaping to help seal the incision, protecting the wound and improving structural integrity. The bandage contact lens is gradually replaced by new cells via re-epithelialization; thus, it behaves as an adjunct to natural wound healing. A bandage contact lens also improves patient comfort.
It is hypothesized that a bandage contact lens improves the structural integrity of the incision site and helps prevent leaking, hypotony, and minor wound leaks. One study revealed a statistically significant lower IOP in nonbandage contact lens patients by an average of 6 mm Hg (mean [SD] 13.4 mm Hg [5.3]; range, 5 - 23 mm Hg) vs patients with a bandage contact lens (mean [SD] 19.4 mm Hg [5.9]; range, 11 - 29 mm Hg) in the immediate postoperative period.32 The authors suggested that the bandage contact lens may prevent microleaks, resulting in a higher IOP.
Aqueous Suppressants
Aqueous suppressants are a great option when IOP is abnormally elevated by decreasing the IOP and allowing the cornea to heal and self-seal.Effective aqueous suppressants are β blockers and carbonic anhydrase inhibitors.
After phacoemulsification ocular hypotony (< 6 mm Hg) occurs most commonly due to wound leakage or excessive intraocular inflammation. However, with the presence of corneal wound leakage and ocular hypotony, aqueous suppressants are not the best option.
Further Management of Wound Leaks
Management of a postoperative wound leak will vary based on severity. The majority of mild leaks are self-sealing. Anterior segment OCT helps the clinician to identify microleaks in an otherwise Seidel negative eye. If wound leakage is moderate with a formed anterior chamber, the use of a bandage contact lens is a good option, as can be the prescription of aqueous suppressants, depending on IOP.33
If the anterior chamber is flat, iris prolapse is apparent, or extremely low IOP exists, the patient needs to be referred to the surgeon. Current standard of care directs the surgeon to use sutures to further manage corneal wound leak. However, several studies have recognized the increased risk of suture-related complications, such as induced astigmatism, corneal opacities, incomplete wound closure, and corneal neovascularization.6,34-38 Other wound closure options include polyethylene glycol-based products, corneal welding, cyanoacrylate, or fibrin (Table 2).39 Traditionally nylon sutures have been used for clear corneal incision wound closure. However, tissue adhesives are gaining popularity as a substitute for sutures in wound closure.40
Cyanoacrylate
Numerous studies have been published on the efficacy of cyanoacrylate as a substitute for sutures, specifically in clear corneal incisions. AEs of cyanoacrylate include a transient foreign-body sensation and diffuse or focal bulbar conjunctival hyperemia.41,42 Shigemitsu and Majima found that fibrin and cyanoacrylate glue had tensile strength similar to sutures when used in cataract surgery.39 Polyethylene glycol-based products, also used in artificial tears and contact lens materials, may also help seal wound leaks. Another agent is ReSure (Ocular Therapeutix, Bedford, MA), an FDA-approved synthetic, polyethylene glycol hydrogel sealant that is 90% water after polymerization. ReSure has been shown to be safe and effective in sealing cataract surgical clear corneal incisions.6,43 ReSure takes about 20 seconds to prepare, and placement is aided by the use of a blue dye that dissipates within hours. This hydrogel will gradually slough off in the tears once the tissue has fully regenerated; there is no need to remove the sealant.44
Rossi and colleagues evaluated the efficacy of corneal welding to close wounds after cataract surgery. The technique involves laser-assisted closure of the corneal wound(s) by a diode laser that welds the stroma.45 Corneal welding takes seconds to achieve good closure without significant astigmatism or inflammation; however very careful application of the light absorbing dyes is required as they are toxic if allowed to enter the anterior chamber.45-47
Conclusion
Optometrists may be called to manage patients during both the preoperative and postoperative phases of cataract surgical care. Those who participate in postoperative care should carefully evaluate for the presence of wound leak or wound gape as a potential complication. The OCT may be employed to evaluate patients suspected of having these leaks or gapes. Proficiency in the interpretation of OCT results and more traditional evaluation methods allows for successful detection of wound leaks or gapes. The timely diagnosis and treatment of postoperative wound leaks allow for the best possible outcomes for cataract surgery patients.
1. Thylefors B, Négrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995;73(1):115-121.
2. Flaxman SR, Bourne RRA, Resnikoff S, et al; Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-e1224.
3. Congdon N, Vingerling JR, Klein BE, et al; Eye Diseases Prevalence Research Group. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. Kurt E, Mayalı H. Early post-operative complications in cataract surgery. In: Zaidi FH, ed. Cataract Surgery. IntechOpen; 2013. https://www.intechopen.com/books/cataract-surgery/post-operative-infections-associated-with-cataract-surgery. Accessed July 15, 2019.
5. Chee SP. Clear corneal incision leakage after phacoemulsification--detection using povidone iodine 5%. Int Ophthalmol. 2005;26(4-5):175-179.
6. Masket S, Hovanesian JA, Levenson J, et al. Hydrogel sealant versus sutures to prevent fluid egress after cataract surgery. J Cataract Refract Surg. 2014;40(12):2057-2066.
7. Kelman CD. Phaco-emulsification and aspiration: a new technique of cataract removal. A preliminary report. Am J Ophthalmol. 1967;64(1):23-35.
8. Powe NR, Schein OD, Gieser SC, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team [published correction appears in Arch Ophthalmol. 1994;112(7):889]. Arch Ophthalmol. 1994;112(2):239-252.
9. Stein JD, Grossman DS, Mundy KM, Sugar A, Sloan FA. Severe adverse events after cataract surgery among medicare beneficiaries. Ophthalmology. 2011;118(9):1716-1723.
10. Greenberg PB, Tseng VL, Wu WC, et al. Prevalence and predictors of ocular complications associated with cataract surgery in United States veterans. Ophthalmology. 2011;118(3):507-514.
11. Mangan MS, Atalay E, Anci C, Tuncer I, Bilqec MD. Comparison of different types of complications in the phacoemulsification surgery learning curve according to number of operations performed. Turk J Ophthalmol. 2016;46(1):7-10.
12. Bell CM, Hatch WV, Cernat G, Urbach DR. Surgeon volumes and selected patient outcomes in cataract surgery: a population-based analysis. Ophthalmology. 2007;114(3):405-410.
13. Wallin T, Parker J, Jin Y, Kefalopoulos G, Olson RJ. Cohort study of 27 cases of endophthalmitis at a single institution. J Cataract Refract Surg. 2005;31(4):735-741.
14. Zaidi FH, Corbett MC, Burton BJ, Bloom PA. Raising the benchmark for the 21st century--the 1000 cataract operations audit and survey: outcomes, consultant-supervised training and sourcing NHS choice. Br J Ophthalmol. 2007;91(6):731-736.
15. Nichamin LD, Chang DF, Johnson SH, et al; American Society of Cataract and Refractive Surgery Cataract Clinical Committee. ASCRS white paper: what is the association between clear corneal cataract incisions and postoperative endophthalmitis? J Cataract Refract Surg. 2006;32(9):1556-1559.
16. Packer M, Chang DF, Dewey SH, et al; ASCRS Cataract Clinical Committee. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37(9):1699-1714.
17. Montan PG, Koranyi G, Setterquist HE, Stridh A, Philipson BT, Wiklund K. Endophthalmitis after cataract surgery: risk factors relating to technique and events of the operation and patient history: a retrospective case-control study. Ophthalmology. 1998;105(12):2171-2177.
18. Taban M, Rao B, Reznik J, Zhang J, Chen Z, McDonnell PJ. Dynamic morphology of sutureless cataract wounds—effect of incision angle and location. Surv Ophthalmol. 2004;49(suppl 2):S62-S72.
19. Chee SP, Ti SE, Lim L, Chan AS, Jap A. Anterior segment optical coherence tomography evaluation of the integrity of clear corneal incisions: a comparison between 2.2-mm and 2.65-mm main incisions. Am J Ophthalmol. 2010;149(5):768-776.e1.
20. Koch DD, Nacke RE, Wang L, Novak KD. Issues in wound management. In: Steinert R, ed. Cataract Surgery. 3rd ed. New York: Elsevier; 2009:581-588.
21. Gimbel HV, Sun R, DeBroff GM. Recognition and management of internal wound gape. J Cataract Refract Surg. 1995;21(2):121-124.
22. May WN, Castro-Combs J, Quinto GG, Kashiwabuchi R, Gower EW, Behrens A. Standardized Seidel test to evaluate different sutureless cataract incision configurations. J Cataract Refract Surg. 2010;36(6):1011-1017.
23. Kashiwabuchi FK, Khan YA, Rodrigues MW Jr, Wang J, McDonnell PJ, Daoud YJ. Seidel and India ink tests assessment of different clear cornea side-port incision configurations. Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1961-1965.
24. Calladine D, Packard R. Clear corneal incision architecture in the immediate postoperative period evaluated using optical coherence tomography. J Cataract Refract Surg. 2007;33(8):1429-1435.
25. Behrens WJ, Stark KA, Pratzer, McDonnell PJ. Dynamics of small-incision clear cornea wounds after phacoemulsification surgery using optical coherence tomography in the early postoperative period. J Refractive Surgery. 2008;24(1):46-49.
26. Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994;112(12):1584-1589.
27. Hurmeric V, Yoo SH, Mutlu FM. Optical coherence tomography in cornea and refractive surgery. Expert Rev Ophthalmol. 2012;7(3):241-250.
28. Schuman JS, Puliafito CA, Fujimoto JG, Duker JS. Optical Coherence Tomography of Ocular Diseases. 3rd ed. Thorofare, NJ: Slack Inc; 2013.
29. Salim S. The role of anterior segment optical coherence tomography in glaucoma. J Ophthalmol. 2012;2012:476801.
30. Kharousi NA, Wali UK, Azeem S. Current applications of optical coherence tomography in ophthalmology. In: Kawasaki M, ed. Optical Coherence Tomography. IntechOpen; 2013. https://www.intechopen.com/books/optical-coherence-tomography. Accessed July 31, 2019.
31. Rodrigues EB, Johanson M, Penha FM. Anterior segment tomography with the cirrus optical coherence tomography. J Ophthalmol. 2012;2012:806989.
32. Calladine D, Ward M, Packard R. Adherent ocular bandage for clear corneal incisions used in cataract surgery. J Cataract Refract Surg. 2010;36(11):1839-1848.
33. Haldar K, Saraff R. Closure technique for leaking wound resulting from thermal injury during phacoemulsification. J Cataract Refract Surg. 2014;40(9):1412-1414.
34. Zoghby JT, Cohen KL. Phacoemulsification-related corneal incision contracture. https://www.aao.org/eyenet/article/phacoemulsification-related-corneal-incision-contr. Published December 2012. Accessed June 16, 2019.
35. Bhatia SS. Ocular surface sealants and adhesives. Ocul Surf. 2006;4(3):146-154.
36. May WN, Castro-Combs J, Kashiwabuchi RT, et al. Bacterial-sized particle inflow through sutured clear corneal incisions in a laboratory human model. J Cataract Refract Surg. 2011;37(6):1140-1146.
37. Meskin SW, Ritterband DC, Shapiro DE, et al. Liquid bandage (2-octyl cyanoacrylate) as a temporary wound barrier in clear corneal cataract surgery. Ophthalmology. 2005;112(11):2015-2021.
38. Heaven CJ, Davison CR, Cockcroft PM. Bacterial contamination of nylon corneal sutures. Eye (Lond). 1995;9(pt 1):116-118.
39. Shigemitsu T, Majima Y. The utilization of a biological adhesive for wound treatment: comparison of suture, self-sealing sutureless and cyanoacrylate closure in the tensile strength test. Int Ophthalmol. 1996-1997;20:323-328.
40. Uy HS, Kenyon KR. Surgical outcomes after application of a liquid adhesive ocular bandage to clear corneal incisions during cataract surgery. J Cataract Refract Surg. 2013;39(11):1668-1674.
41. Meskin SW, Ritterband DC, Shapiro DE, et al. Liquid bandage (2-octyl cyanoacrylate) as a temporary wound barrier in clear corneal cataract surgery. Ophthalmology. 2005;112(11):2015-2021.
42. Tong AY, Gupta PK, Kim T. Wound closure and tissue adhesives in clear corneal incision cataract surgery. Curr Opin Ophthalmol. 2018;29(1):14-18.
43. US Food and Drug Administration. Summary of Safety and Effectiveness Data. Ophthalmic sealant: ReSure Sealant. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130004b.pdf. Published September 13, 2013. Accessed July 9, 2019.
44. About ReSure sealant. https://www.resuresealant.com/overview. Accessed July 31, 2019.
45. Menabuoni L, Pini R, Rossi F, Lenzetti I, Yoo SH, Parel JM. Laser-assisted corneal welding in cataract surgery: retrospective study. J Cataract Refract Surg. 2007;33(9):1608-1612.
46. Rasier R, Ozeren M, Artunay O, et al. Corneal tissue welding with infrared laser irradiation after clear corneal incision. Cornea. 2010;29(9):985-990.
47. Rossi F, Matteini P, Ratto F, Menabuoni L, Lenzetti I, Pini R. Laser tissue welding in ophthalmic surgery. J Biophotonics. 2008;1(4):331-342.
48. Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch Ophthalmol. 2005;123(5):613-620.
49. Taylor DM, Atlas BF, Romanchuk KG, Stern AL. Pseudophakic bullous keratopathy. Ophthalmology. 1983;90(1):19-24.
50. Lobo CL, Faria PM, Soares MA, Bernardes RC, Cunha-Vaz JG. Macular alterations after small-incision cataract surgery. J Cataract Refract Surg. 2004;30(4):752-760.
51. Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557-634.
52. Wright PL, Wilkinson CP, Balyeat HD, Popham J, Reinke M. Angiographic cystoid macular edema after posterior chamber lens implantation. Arch Ophthalmol. 1988;106(6):740-744.
53. Kim SJ, Belair ML, Bressler NM, et al. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina. 2008;28(6):870-876.
54. Alio JL, Ruiz-Moreno JM, Shabayek MH, Lugo FL, Abd El Rahman AM. The risk of retinal detachment in high myopia after small incision coaxial phacoemulsification. Am J Ophthalmol. 2007;144(1):93-98.
55. Bhagwandien AC, Cheng YY, Wolfs RC, van Meurs JC, Luyten GP. Relationship between retinal detachment and biometry in 4262 cataractous eyes. Ophthalmology. 2006;113(4):643-649.
56. Boberg-Ans G, Henning V, Villumsen J, la Cour M. Longterm incidence of rhegmatogenous retinal detachment and survival in a defined population undergoing standardized phacoemulsification surgery. Acta Ophthalmol Scand. 2006;84(5):613-618.
57. Jakobsson G, Montan P, Zetterberg M, Stenevi U, Behndig A, Lundström M. Capsule complication during cataract surgery: retinal detachment after cataract surgery with capsule complication: Swedish Capsule Rupture Study Group report 4. J Cataract Refract Surg. 2009;35(10):1699-1705.
58. Neuhann IM, Neuhann TF, Heimann H, Schmickler S, Gerl RH, Foerster MH. Retinal detachment after phacoemulsification in high myopia: analysis of 2356 cases. J Cataract Refract Surg. 2008;34(10):1644-1657.
59. Russell M, Gaskin B, Russell D, Polkinghorne PJ. Pseudophakic retinal detachment after phacoemulsification cataract surgery: ten-year retrospective review. J Cataract Refract Surg. 2006;32(3):442-445.
60. Apple DJ, Solomon KD, Tetz MR, et al. Posterior capsule opacification. Surv Ophthalmol. 1992;37(2):73-116.
61. Wu S, Tong N, Pan L, et al. Retrospective analyses of potential risk factors for posterior capsule opacification after cataract surgery. J Ophthalmol. 2018;2018:9089285.
62. Clark A, Morlet N, Ng JQ, Preen DB, Semmens JB. Whole population trends in complications of cataract surgery over 22 years in Western Australia. Ophthalmology. 2011;118(6):1055-1061.
63. Adhikari S, Shrestha UD. Pediatric cataract surgery with hydrophilic acrylic intraocular lens implantation in Nepalese Children. Clin Ophthalmol. 2017;12:7-11.
64. Lee BJ, Smith SD, Jeng BH. Suture-related corneal infections after clear corneal cataract surgery. J Cataract Refract Surg. 2009;35(5):939-942.
65. May WN, Castro-Combs J, Kashiwabuchi RT, et al. Sutured clear corneal incision: wound apposition and permeability to bacterial-sized particles. Cornea. 2013;32(3):319-325.
66. Hillier RJ, Ajit RR, Kelly SP. Suture-related complications after cataract surgery: a patient safety issue. J Cataract Refract Surg. 2009;35(11):2035-2036.
67. Hovanesian JA, Karageozian VH. Watertight cataract incision closure using fibrin tissue adhesive. J Cataract Refract Surg. 2007;33(8):1461-1463.
The term cataract is derived from the Latin word “catarractes,” which means “waterfall,” as the foamy white opacity of an advanced cataract can be likened to a tempestuous cascade. Cataract is the leading cause of preventable blindness worldwide.1,2 It is no surprise, therefore, that cataract surgery is the most frequently performed ophthalmic surgical procedure worldwide. Cataract surgeries may reach 30 million annual cases by 2020.3 Given the large number of surgeries being performed, postsurgical complications are not uncommon.
Early postoperative complications from lens exchange (cataract) surgery include increased intraocular pressure (IOP), corneal edema, and corneal wound leakage.4 Corneal wound leakage is not uncommon; one study showed that, in 100 cases, almost one-third of incisions leaked.5 A 2014 prospective study of 500 postcataract surgery eyes revealed that 48.8% had fluid egress.6 Early detection is important so that efforts to restore corneal integrity can immediately be implemented. If not caught early, patients are at risk for developing a cascade of sequelae, including endophthalmitis.
The majority of corneal wound leaks postphacoemulsification are self-limiting and self-sealing. Moderate wound leaks require treatment, as in the following case. Strategies to detect, image, and treat wound leaks are covered in this discussion.
Case Presentation
A 69-year-old male veteran presented with no complaints for a 1-day postoperative visit following right eye phacoemulsification cataract extraction. His best corrected visual acuity in the right eye was 20/40, and his pinhole visual acuity was 20/25+2. On slit-lamp examination, the temporally located main incision appeared well-adhered and was found to be Seidel negative; however, the inferior paracentesis wound was found to be Seidel positive, demonstrating a slow leak. Intraocular pressure (IOP) measured with tonopen was 9 mm Hg.
A bandage soft contact lens was placed on the eye. The patient was instructed not to rub or place any pressure on the eye and to avoid bending and heavy lifting. He was also instructed to continue his postoperative medications (prednisolone 1% every 2 hours and polymyxin B sulfate 4 times daily) in his right eye. A follow-up appointment was scheduled for the next day.
The patient presented for his postoperative day-2 visit with a best corrected visual acuity in the right eye of 20/20. He reported no visual problems, no eye pain, and mentioned that he had had a comfortable night sleep. A slit-lamp examination revealed trace diffuse injection in the operative eye, predominantly central Descemet membrane folds, 1+ stromal edema, and a Seidel negative main incision wound. However, the inferior paracentesis wound showed a moderate leak (Seidel positive), and the anterior chamber showed a 1+ cell and flare. Goldmann tonometry revealed an IOP of 5 mm Hg, indicating hypotony.
Anterior segment cube 512 x 128 optical coherence tomography (OCT) was obtained with the bandage contact lens (Figures 1 and 2), and then repeated with the bandage contact lens removed (Figures 3 and 4). OCT imaging confirmed epithelial and endothelial gaping, loss of coaptation, and a localized detachment of the Descemet membrane. The veteran was referred to his surgeon that same day, and 2 limbal vicryl sutures were placed. The patient was instructed to continue prednisolone 1% 4 times daily and polymyxin B sulfate every 2 hours; erythromycin ointment 3 times daily was added to his regimen.
He was scheduled for a follow-up examination 1 week later. At that visit, the wound was no longer leaking and IOP had risen to a preoperative value of 17 mm Hg. The corneal sutures were removed at the 1-month postoperative examination and a follow-up was scheduled for 4 months later. An anterior segment OCT was obtained (Figure 5).
Discussion
In July 1967, Charles Kelman, MD, suggested using a dental ultrasonic tool, normally employed to clean teeth, to fragment the nucleus of the crystalline lens. Dr. Kelman’s first operation using phacoemulsification on a human eye took 3 hours.7 As the procedure for cataract removal has been refined, complication rates and surgical times have vastly improved.
Phacoemulsification is the most commonly performed outpatient surgery in the US; about 3 million cases are performed annually. Due to the high volume of cases, adverse events (AEs) are not uncommon. The incidence of complications following phacoemulsification is < 5%; the frequency of severe complications has been estimated at < 0.7%.8 Severe complications include endophthalmitis, suprachoroidal hemorrhage, and/or retinal detachment.9 Studies have shown a decline in rates of sight-threatening AEs from 1994 to 2006.9 A retrospective study of 45,082 veterans from 2005 to 2007 identified that a preoperative disease burden such as diabetes mellitus, chronic pulmonary disease, age-related macular degeneration, and diabetes with ophthalmic manifestations, was positively associated with a greater risk of cataract surgical complications.10
Complications
The level of a surgeon’s proficiency with phacoemulsification is directly correlated to the number of operations performed; there is a lower complication rate among more experienced surgeons, including those who work in high-volume settings.11,12 One study identified that the AE rate within 14 days of surgery was 0.8% for surgeons performing 50 to 250 cataract surgeries per year, but only 0.1% for those performing > 1000 cataract surgeries annually.12
Potential postoperative lens exchange complications include increased IOP, corneal wound leakage, corneal edema, bullous keratopathy, cystoid macular edema, retinal detachment, and endophthalmitis (Table 1). A corneal wound leak can provide a potential ingress for bacteria, putting the patient at risk for endophthalmitis, perhaps the most devastating complication following cataract surgery.
Endophthalmitis
Endophthalmitis has been reported to occur in .001% to .327% of patients during postoperative care.5,13-17 Early detection is important to maintain corneal integrity and prevent a cascade of detrimental ocular sequalae including the potential for endophthalmitis. According to Zaida and colleagues, endophthalmitis occurred in fewer than 1 of 1000 consecutive cases.14 A leaking clear corneal incision wound on the first day postoperatively has been associated with a 44-fold increased risk of endophthalmitis.13
Causes of endophthalmitis
In a retrospective case-controlled series of 57 patients with postcataract endophthalmitis, implantation of an intraocular lens with a resultant wound abnormality was thought to be the causative factor in 5%.17 Another source of endophthalmitis can be the intraocular lens (IOL), which may act as a vector for bacteria. By placing the IOL against the conjunctiva or exposing it to the theater air during surgery, bacteria can be introduced prior to implantation.17 Immunosuppressive treatment is the only patient antecedent factor that can be considered a predictor for endopthalmitis.17
The internal corneal seal is IOP dependent, and postoperative ocular hypotony may cause a seemingly watertight wound to leak. Taban and colleagues used anterior segment OCT to image numerous self-sealing incisions. They found that the corneal incision wound more tightly seals at higher IOPs. Additionally, more perpendicular (larger angle) incisions seal better at a lower IOP while less perpendicular (smaller angle) incisions seal better at a higher IOP (Figure 6).18
Incision Placement
Studies have shown that the main incision site is more clinically competent than is the side port incision site, as in our case study.19 Side-port incisions have a 1- or 2-plane architectural profile in contrast to the 3-plane profile typical of a main incision.19 Recent advances including the conversion to clear-corneal incisions of diminishing size, techniques used for wound construction, phacoemulsification machine design, and small-incision IOLs, should further reduce the prevalence and complications of wound compromise.20
Seidel Testing
Seidel testing is the most common method to evaluate corneal wound integrity and identify leaks. A drop of topical anesthetic is instilled in the eye and then a fluorescein strip (not fluorescein sodium and benoxinate hydrochloride ophthalmic solution, which may become less sterile since it has a multiuse container) is applied to the superior conjunctiva. The clinician then looks for evidence of fluid egress using the cobalt blue filter. The patient is instructed to blink once. Fluid egress appears as a black stream as the fluorescein dye becomes diluted by aqueous humor escaping the nonintact wound and the appearance of bright green dye surrounds the leak site. The term Seidel positive indicates a leak. An estimate should be made of the rate and volume of fluid exiting the wound.
Gonioscopy
Gonioscopy can be used to evaluate the postsurgical incision, more specifically for identification and management of internal incision wound gape. On gonioscopy, internal wound gape appears as an elongated oval opening resembling a fish mouth. If internal incision wound gape is identified gonioscopically before surgery is complete, the leak can be managed intraoperatively. The surgeon can irrigate along the length of the incision to remove cortical fragments or viscoelastic that may cause internal wound gaping. If unsuccessful, rapidly deepening the anterior chamber with balanced salt solution through the paracentesis incision may be employed. These methods may improve wound stability, reduce risk of postoperative hyphema, lower the incidence of endophthalmitis, and lessen the likelihood of late against-the-rule drift.21
Anterior Segment Optical Coherence Tomography
Instances when Seidel testing was negative despite actual wound gaping have been described.22,23 Anterior segment OCT is useful to evaluate incision architecture. A 2007 United Kingdom study investigated the corneal architecture in the immediate postoperative period following phacoemulsification using anterior segment OCT. This study showed the benefits of identifying architectural features such as epithelial gaping, endothelial gaping, stripping of Descemet membrane, and loss of coaptation. These features were found to be more common at low IOP and could represent a significant risk factor for endophthalmitis.24 Another study published by Behrens and colleagues indicated that a localized detachment of Descemet membrane may be more common than observed with slit-lamp (Figure 7). Corneal gaping, especially if along the entire length of the surgical wound, may lead to inadvertent bacterial access into the anterior chamber.25
Anterior segment OCT imaging was first described by Izatt and colleagues in 1994.26 Unlike posterior segment OCT, anterior segment OCT requires a greater depth of field and higher energy levels as images are commonly distorted by refraction at boundaries where the refractive index changes. Longer infrared wavelengths improve the penetration through tissues that scatter light, such as the sclera and limbus, which allows visualization, for example, of the iridocorneal angle.27,28
Two main scan patterns are used for anterior segment OCT: 512 x 128 cube scan (4-mm width x 4-mm length) and 5-line raster (3-mm length) with adjustable rotation and spacing. A recent software update allows measurement of corneal thickness, visualization of anterior chamber angle structures along with topographic analysis, anterior and posterior elevation maps of the cornea, and reliable pachymetric maps.29,30 The anterior segment cube acquires a series of 128 horizontal scan lines each composed of 512 A-scans. These high-definition scans acquire vertical and horizontal directions composed of 1024 A-scans each. This cube may be used to measure corneal thickness and visualize corneal architecture, creating a 3-D image of the data (Figure 8). The anterior segment 5-line raster scans through 5 parallel lines of equal length to view high-resolution images of the anterior chamber angle and cornea. Each line, fixed at 3-mm in length, is composed of 4096 A-scans.31 Anterior segment cube OCT allows identification of subtle variations in incision architecture at different locations across the width of the OCT image.
Bandage Soft Contact Lens
Upon reviewing the anterior segment OCT images of our patient with the bandage contact lens in place, it was evident that the adherent ocular bandage was protecting the incision. A tighter fitting bandage contact lens is ideal and adheres firmly to any area of epithelial damage and epithelial gaping to help seal the incision, protecting the wound and improving structural integrity. The bandage contact lens is gradually replaced by new cells via re-epithelialization; thus, it behaves as an adjunct to natural wound healing. A bandage contact lens also improves patient comfort.
It is hypothesized that a bandage contact lens improves the structural integrity of the incision site and helps prevent leaking, hypotony, and minor wound leaks. One study revealed a statistically significant lower IOP in nonbandage contact lens patients by an average of 6 mm Hg (mean [SD] 13.4 mm Hg [5.3]; range, 5 - 23 mm Hg) vs patients with a bandage contact lens (mean [SD] 19.4 mm Hg [5.9]; range, 11 - 29 mm Hg) in the immediate postoperative period.32 The authors suggested that the bandage contact lens may prevent microleaks, resulting in a higher IOP.
Aqueous Suppressants
Aqueous suppressants are a great option when IOP is abnormally elevated by decreasing the IOP and allowing the cornea to heal and self-seal.Effective aqueous suppressants are β blockers and carbonic anhydrase inhibitors.
After phacoemulsification ocular hypotony (< 6 mm Hg) occurs most commonly due to wound leakage or excessive intraocular inflammation. However, with the presence of corneal wound leakage and ocular hypotony, aqueous suppressants are not the best option.
Further Management of Wound Leaks
Management of a postoperative wound leak will vary based on severity. The majority of mild leaks are self-sealing. Anterior segment OCT helps the clinician to identify microleaks in an otherwise Seidel negative eye. If wound leakage is moderate with a formed anterior chamber, the use of a bandage contact lens is a good option, as can be the prescription of aqueous suppressants, depending on IOP.33
If the anterior chamber is flat, iris prolapse is apparent, or extremely low IOP exists, the patient needs to be referred to the surgeon. Current standard of care directs the surgeon to use sutures to further manage corneal wound leak. However, several studies have recognized the increased risk of suture-related complications, such as induced astigmatism, corneal opacities, incomplete wound closure, and corneal neovascularization.6,34-38 Other wound closure options include polyethylene glycol-based products, corneal welding, cyanoacrylate, or fibrin (Table 2).39 Traditionally nylon sutures have been used for clear corneal incision wound closure. However, tissue adhesives are gaining popularity as a substitute for sutures in wound closure.40
Cyanoacrylate
Numerous studies have been published on the efficacy of cyanoacrylate as a substitute for sutures, specifically in clear corneal incisions. AEs of cyanoacrylate include a transient foreign-body sensation and diffuse or focal bulbar conjunctival hyperemia.41,42 Shigemitsu and Majima found that fibrin and cyanoacrylate glue had tensile strength similar to sutures when used in cataract surgery.39 Polyethylene glycol-based products, also used in artificial tears and contact lens materials, may also help seal wound leaks. Another agent is ReSure (Ocular Therapeutix, Bedford, MA), an FDA-approved synthetic, polyethylene glycol hydrogel sealant that is 90% water after polymerization. ReSure has been shown to be safe and effective in sealing cataract surgical clear corneal incisions.6,43 ReSure takes about 20 seconds to prepare, and placement is aided by the use of a blue dye that dissipates within hours. This hydrogel will gradually slough off in the tears once the tissue has fully regenerated; there is no need to remove the sealant.44
Rossi and colleagues evaluated the efficacy of corneal welding to close wounds after cataract surgery. The technique involves laser-assisted closure of the corneal wound(s) by a diode laser that welds the stroma.45 Corneal welding takes seconds to achieve good closure without significant astigmatism or inflammation; however very careful application of the light absorbing dyes is required as they are toxic if allowed to enter the anterior chamber.45-47
Conclusion
Optometrists may be called to manage patients during both the preoperative and postoperative phases of cataract surgical care. Those who participate in postoperative care should carefully evaluate for the presence of wound leak or wound gape as a potential complication. The OCT may be employed to evaluate patients suspected of having these leaks or gapes. Proficiency in the interpretation of OCT results and more traditional evaluation methods allows for successful detection of wound leaks or gapes. The timely diagnosis and treatment of postoperative wound leaks allow for the best possible outcomes for cataract surgery patients.
The term cataract is derived from the Latin word “catarractes,” which means “waterfall,” as the foamy white opacity of an advanced cataract can be likened to a tempestuous cascade. Cataract is the leading cause of preventable blindness worldwide.1,2 It is no surprise, therefore, that cataract surgery is the most frequently performed ophthalmic surgical procedure worldwide. Cataract surgeries may reach 30 million annual cases by 2020.3 Given the large number of surgeries being performed, postsurgical complications are not uncommon.
Early postoperative complications from lens exchange (cataract) surgery include increased intraocular pressure (IOP), corneal edema, and corneal wound leakage.4 Corneal wound leakage is not uncommon; one study showed that, in 100 cases, almost one-third of incisions leaked.5 A 2014 prospective study of 500 postcataract surgery eyes revealed that 48.8% had fluid egress.6 Early detection is important so that efforts to restore corneal integrity can immediately be implemented. If not caught early, patients are at risk for developing a cascade of sequelae, including endophthalmitis.
The majority of corneal wound leaks postphacoemulsification are self-limiting and self-sealing. Moderate wound leaks require treatment, as in the following case. Strategies to detect, image, and treat wound leaks are covered in this discussion.
Case Presentation
A 69-year-old male veteran presented with no complaints for a 1-day postoperative visit following right eye phacoemulsification cataract extraction. His best corrected visual acuity in the right eye was 20/40, and his pinhole visual acuity was 20/25+2. On slit-lamp examination, the temporally located main incision appeared well-adhered and was found to be Seidel negative; however, the inferior paracentesis wound was found to be Seidel positive, demonstrating a slow leak. Intraocular pressure (IOP) measured with tonopen was 9 mm Hg.
A bandage soft contact lens was placed on the eye. The patient was instructed not to rub or place any pressure on the eye and to avoid bending and heavy lifting. He was also instructed to continue his postoperative medications (prednisolone 1% every 2 hours and polymyxin B sulfate 4 times daily) in his right eye. A follow-up appointment was scheduled for the next day.
The patient presented for his postoperative day-2 visit with a best corrected visual acuity in the right eye of 20/20. He reported no visual problems, no eye pain, and mentioned that he had had a comfortable night sleep. A slit-lamp examination revealed trace diffuse injection in the operative eye, predominantly central Descemet membrane folds, 1+ stromal edema, and a Seidel negative main incision wound. However, the inferior paracentesis wound showed a moderate leak (Seidel positive), and the anterior chamber showed a 1+ cell and flare. Goldmann tonometry revealed an IOP of 5 mm Hg, indicating hypotony.
Anterior segment cube 512 x 128 optical coherence tomography (OCT) was obtained with the bandage contact lens (Figures 1 and 2), and then repeated with the bandage contact lens removed (Figures 3 and 4). OCT imaging confirmed epithelial and endothelial gaping, loss of coaptation, and a localized detachment of the Descemet membrane. The veteran was referred to his surgeon that same day, and 2 limbal vicryl sutures were placed. The patient was instructed to continue prednisolone 1% 4 times daily and polymyxin B sulfate every 2 hours; erythromycin ointment 3 times daily was added to his regimen.
He was scheduled for a follow-up examination 1 week later. At that visit, the wound was no longer leaking and IOP had risen to a preoperative value of 17 mm Hg. The corneal sutures were removed at the 1-month postoperative examination and a follow-up was scheduled for 4 months later. An anterior segment OCT was obtained (Figure 5).
Discussion
In July 1967, Charles Kelman, MD, suggested using a dental ultrasonic tool, normally employed to clean teeth, to fragment the nucleus of the crystalline lens. Dr. Kelman’s first operation using phacoemulsification on a human eye took 3 hours.7 As the procedure for cataract removal has been refined, complication rates and surgical times have vastly improved.
Phacoemulsification is the most commonly performed outpatient surgery in the US; about 3 million cases are performed annually. Due to the high volume of cases, adverse events (AEs) are not uncommon. The incidence of complications following phacoemulsification is < 5%; the frequency of severe complications has been estimated at < 0.7%.8 Severe complications include endophthalmitis, suprachoroidal hemorrhage, and/or retinal detachment.9 Studies have shown a decline in rates of sight-threatening AEs from 1994 to 2006.9 A retrospective study of 45,082 veterans from 2005 to 2007 identified that a preoperative disease burden such as diabetes mellitus, chronic pulmonary disease, age-related macular degeneration, and diabetes with ophthalmic manifestations, was positively associated with a greater risk of cataract surgical complications.10
Complications
The level of a surgeon’s proficiency with phacoemulsification is directly correlated to the number of operations performed; there is a lower complication rate among more experienced surgeons, including those who work in high-volume settings.11,12 One study identified that the AE rate within 14 days of surgery was 0.8% for surgeons performing 50 to 250 cataract surgeries per year, but only 0.1% for those performing > 1000 cataract surgeries annually.12
Potential postoperative lens exchange complications include increased IOP, corneal wound leakage, corneal edema, bullous keratopathy, cystoid macular edema, retinal detachment, and endophthalmitis (Table 1). A corneal wound leak can provide a potential ingress for bacteria, putting the patient at risk for endophthalmitis, perhaps the most devastating complication following cataract surgery.
Endophthalmitis
Endophthalmitis has been reported to occur in .001% to .327% of patients during postoperative care.5,13-17 Early detection is important to maintain corneal integrity and prevent a cascade of detrimental ocular sequalae including the potential for endophthalmitis. According to Zaida and colleagues, endophthalmitis occurred in fewer than 1 of 1000 consecutive cases.14 A leaking clear corneal incision wound on the first day postoperatively has been associated with a 44-fold increased risk of endophthalmitis.13
Causes of endophthalmitis
In a retrospective case-controlled series of 57 patients with postcataract endophthalmitis, implantation of an intraocular lens with a resultant wound abnormality was thought to be the causative factor in 5%.17 Another source of endophthalmitis can be the intraocular lens (IOL), which may act as a vector for bacteria. By placing the IOL against the conjunctiva or exposing it to the theater air during surgery, bacteria can be introduced prior to implantation.17 Immunosuppressive treatment is the only patient antecedent factor that can be considered a predictor for endopthalmitis.17
The internal corneal seal is IOP dependent, and postoperative ocular hypotony may cause a seemingly watertight wound to leak. Taban and colleagues used anterior segment OCT to image numerous self-sealing incisions. They found that the corneal incision wound more tightly seals at higher IOPs. Additionally, more perpendicular (larger angle) incisions seal better at a lower IOP while less perpendicular (smaller angle) incisions seal better at a higher IOP (Figure 6).18
Incision Placement
Studies have shown that the main incision site is more clinically competent than is the side port incision site, as in our case study.19 Side-port incisions have a 1- or 2-plane architectural profile in contrast to the 3-plane profile typical of a main incision.19 Recent advances including the conversion to clear-corneal incisions of diminishing size, techniques used for wound construction, phacoemulsification machine design, and small-incision IOLs, should further reduce the prevalence and complications of wound compromise.20
Seidel Testing
Seidel testing is the most common method to evaluate corneal wound integrity and identify leaks. A drop of topical anesthetic is instilled in the eye and then a fluorescein strip (not fluorescein sodium and benoxinate hydrochloride ophthalmic solution, which may become less sterile since it has a multiuse container) is applied to the superior conjunctiva. The clinician then looks for evidence of fluid egress using the cobalt blue filter. The patient is instructed to blink once. Fluid egress appears as a black stream as the fluorescein dye becomes diluted by aqueous humor escaping the nonintact wound and the appearance of bright green dye surrounds the leak site. The term Seidel positive indicates a leak. An estimate should be made of the rate and volume of fluid exiting the wound.
Gonioscopy
Gonioscopy can be used to evaluate the postsurgical incision, more specifically for identification and management of internal incision wound gape. On gonioscopy, internal wound gape appears as an elongated oval opening resembling a fish mouth. If internal incision wound gape is identified gonioscopically before surgery is complete, the leak can be managed intraoperatively. The surgeon can irrigate along the length of the incision to remove cortical fragments or viscoelastic that may cause internal wound gaping. If unsuccessful, rapidly deepening the anterior chamber with balanced salt solution through the paracentesis incision may be employed. These methods may improve wound stability, reduce risk of postoperative hyphema, lower the incidence of endophthalmitis, and lessen the likelihood of late against-the-rule drift.21
Anterior Segment Optical Coherence Tomography
Instances when Seidel testing was negative despite actual wound gaping have been described.22,23 Anterior segment OCT is useful to evaluate incision architecture. A 2007 United Kingdom study investigated the corneal architecture in the immediate postoperative period following phacoemulsification using anterior segment OCT. This study showed the benefits of identifying architectural features such as epithelial gaping, endothelial gaping, stripping of Descemet membrane, and loss of coaptation. These features were found to be more common at low IOP and could represent a significant risk factor for endophthalmitis.24 Another study published by Behrens and colleagues indicated that a localized detachment of Descemet membrane may be more common than observed with slit-lamp (Figure 7). Corneal gaping, especially if along the entire length of the surgical wound, may lead to inadvertent bacterial access into the anterior chamber.25
Anterior segment OCT imaging was first described by Izatt and colleagues in 1994.26 Unlike posterior segment OCT, anterior segment OCT requires a greater depth of field and higher energy levels as images are commonly distorted by refraction at boundaries where the refractive index changes. Longer infrared wavelengths improve the penetration through tissues that scatter light, such as the sclera and limbus, which allows visualization, for example, of the iridocorneal angle.27,28
Two main scan patterns are used for anterior segment OCT: 512 x 128 cube scan (4-mm width x 4-mm length) and 5-line raster (3-mm length) with adjustable rotation and spacing. A recent software update allows measurement of corneal thickness, visualization of anterior chamber angle structures along with topographic analysis, anterior and posterior elevation maps of the cornea, and reliable pachymetric maps.29,30 The anterior segment cube acquires a series of 128 horizontal scan lines each composed of 512 A-scans. These high-definition scans acquire vertical and horizontal directions composed of 1024 A-scans each. This cube may be used to measure corneal thickness and visualize corneal architecture, creating a 3-D image of the data (Figure 8). The anterior segment 5-line raster scans through 5 parallel lines of equal length to view high-resolution images of the anterior chamber angle and cornea. Each line, fixed at 3-mm in length, is composed of 4096 A-scans.31 Anterior segment cube OCT allows identification of subtle variations in incision architecture at different locations across the width of the OCT image.
Bandage Soft Contact Lens
Upon reviewing the anterior segment OCT images of our patient with the bandage contact lens in place, it was evident that the adherent ocular bandage was protecting the incision. A tighter fitting bandage contact lens is ideal and adheres firmly to any area of epithelial damage and epithelial gaping to help seal the incision, protecting the wound and improving structural integrity. The bandage contact lens is gradually replaced by new cells via re-epithelialization; thus, it behaves as an adjunct to natural wound healing. A bandage contact lens also improves patient comfort.
It is hypothesized that a bandage contact lens improves the structural integrity of the incision site and helps prevent leaking, hypotony, and minor wound leaks. One study revealed a statistically significant lower IOP in nonbandage contact lens patients by an average of 6 mm Hg (mean [SD] 13.4 mm Hg [5.3]; range, 5 - 23 mm Hg) vs patients with a bandage contact lens (mean [SD] 19.4 mm Hg [5.9]; range, 11 - 29 mm Hg) in the immediate postoperative period.32 The authors suggested that the bandage contact lens may prevent microleaks, resulting in a higher IOP.
Aqueous Suppressants
Aqueous suppressants are a great option when IOP is abnormally elevated by decreasing the IOP and allowing the cornea to heal and self-seal.Effective aqueous suppressants are β blockers and carbonic anhydrase inhibitors.
After phacoemulsification ocular hypotony (< 6 mm Hg) occurs most commonly due to wound leakage or excessive intraocular inflammation. However, with the presence of corneal wound leakage and ocular hypotony, aqueous suppressants are not the best option.
Further Management of Wound Leaks
Management of a postoperative wound leak will vary based on severity. The majority of mild leaks are self-sealing. Anterior segment OCT helps the clinician to identify microleaks in an otherwise Seidel negative eye. If wound leakage is moderate with a formed anterior chamber, the use of a bandage contact lens is a good option, as can be the prescription of aqueous suppressants, depending on IOP.33
If the anterior chamber is flat, iris prolapse is apparent, or extremely low IOP exists, the patient needs to be referred to the surgeon. Current standard of care directs the surgeon to use sutures to further manage corneal wound leak. However, several studies have recognized the increased risk of suture-related complications, such as induced astigmatism, corneal opacities, incomplete wound closure, and corneal neovascularization.6,34-38 Other wound closure options include polyethylene glycol-based products, corneal welding, cyanoacrylate, or fibrin (Table 2).39 Traditionally nylon sutures have been used for clear corneal incision wound closure. However, tissue adhesives are gaining popularity as a substitute for sutures in wound closure.40
Cyanoacrylate
Numerous studies have been published on the efficacy of cyanoacrylate as a substitute for sutures, specifically in clear corneal incisions. AEs of cyanoacrylate include a transient foreign-body sensation and diffuse or focal bulbar conjunctival hyperemia.41,42 Shigemitsu and Majima found that fibrin and cyanoacrylate glue had tensile strength similar to sutures when used in cataract surgery.39 Polyethylene glycol-based products, also used in artificial tears and contact lens materials, may also help seal wound leaks. Another agent is ReSure (Ocular Therapeutix, Bedford, MA), an FDA-approved synthetic, polyethylene glycol hydrogel sealant that is 90% water after polymerization. ReSure has been shown to be safe and effective in sealing cataract surgical clear corneal incisions.6,43 ReSure takes about 20 seconds to prepare, and placement is aided by the use of a blue dye that dissipates within hours. This hydrogel will gradually slough off in the tears once the tissue has fully regenerated; there is no need to remove the sealant.44
Rossi and colleagues evaluated the efficacy of corneal welding to close wounds after cataract surgery. The technique involves laser-assisted closure of the corneal wound(s) by a diode laser that welds the stroma.45 Corneal welding takes seconds to achieve good closure without significant astigmatism or inflammation; however very careful application of the light absorbing dyes is required as they are toxic if allowed to enter the anterior chamber.45-47
Conclusion
Optometrists may be called to manage patients during both the preoperative and postoperative phases of cataract surgical care. Those who participate in postoperative care should carefully evaluate for the presence of wound leak or wound gape as a potential complication. The OCT may be employed to evaluate patients suspected of having these leaks or gapes. Proficiency in the interpretation of OCT results and more traditional evaluation methods allows for successful detection of wound leaks or gapes. The timely diagnosis and treatment of postoperative wound leaks allow for the best possible outcomes for cataract surgery patients.
1. Thylefors B, Négrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995;73(1):115-121.
2. Flaxman SR, Bourne RRA, Resnikoff S, et al; Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-e1224.
3. Congdon N, Vingerling JR, Klein BE, et al; Eye Diseases Prevalence Research Group. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. Kurt E, Mayalı H. Early post-operative complications in cataract surgery. In: Zaidi FH, ed. Cataract Surgery. IntechOpen; 2013. https://www.intechopen.com/books/cataract-surgery/post-operative-infections-associated-with-cataract-surgery. Accessed July 15, 2019.
5. Chee SP. Clear corneal incision leakage after phacoemulsification--detection using povidone iodine 5%. Int Ophthalmol. 2005;26(4-5):175-179.
6. Masket S, Hovanesian JA, Levenson J, et al. Hydrogel sealant versus sutures to prevent fluid egress after cataract surgery. J Cataract Refract Surg. 2014;40(12):2057-2066.
7. Kelman CD. Phaco-emulsification and aspiration: a new technique of cataract removal. A preliminary report. Am J Ophthalmol. 1967;64(1):23-35.
8. Powe NR, Schein OD, Gieser SC, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team [published correction appears in Arch Ophthalmol. 1994;112(7):889]. Arch Ophthalmol. 1994;112(2):239-252.
9. Stein JD, Grossman DS, Mundy KM, Sugar A, Sloan FA. Severe adverse events after cataract surgery among medicare beneficiaries. Ophthalmology. 2011;118(9):1716-1723.
10. Greenberg PB, Tseng VL, Wu WC, et al. Prevalence and predictors of ocular complications associated with cataract surgery in United States veterans. Ophthalmology. 2011;118(3):507-514.
11. Mangan MS, Atalay E, Anci C, Tuncer I, Bilqec MD. Comparison of different types of complications in the phacoemulsification surgery learning curve according to number of operations performed. Turk J Ophthalmol. 2016;46(1):7-10.
12. Bell CM, Hatch WV, Cernat G, Urbach DR. Surgeon volumes and selected patient outcomes in cataract surgery: a population-based analysis. Ophthalmology. 2007;114(3):405-410.
13. Wallin T, Parker J, Jin Y, Kefalopoulos G, Olson RJ. Cohort study of 27 cases of endophthalmitis at a single institution. J Cataract Refract Surg. 2005;31(4):735-741.
14. Zaidi FH, Corbett MC, Burton BJ, Bloom PA. Raising the benchmark for the 21st century--the 1000 cataract operations audit and survey: outcomes, consultant-supervised training and sourcing NHS choice. Br J Ophthalmol. 2007;91(6):731-736.
15. Nichamin LD, Chang DF, Johnson SH, et al; American Society of Cataract and Refractive Surgery Cataract Clinical Committee. ASCRS white paper: what is the association between clear corneal cataract incisions and postoperative endophthalmitis? J Cataract Refract Surg. 2006;32(9):1556-1559.
16. Packer M, Chang DF, Dewey SH, et al; ASCRS Cataract Clinical Committee. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37(9):1699-1714.
17. Montan PG, Koranyi G, Setterquist HE, Stridh A, Philipson BT, Wiklund K. Endophthalmitis after cataract surgery: risk factors relating to technique and events of the operation and patient history: a retrospective case-control study. Ophthalmology. 1998;105(12):2171-2177.
18. Taban M, Rao B, Reznik J, Zhang J, Chen Z, McDonnell PJ. Dynamic morphology of sutureless cataract wounds—effect of incision angle and location. Surv Ophthalmol. 2004;49(suppl 2):S62-S72.
19. Chee SP, Ti SE, Lim L, Chan AS, Jap A. Anterior segment optical coherence tomography evaluation of the integrity of clear corneal incisions: a comparison between 2.2-mm and 2.65-mm main incisions. Am J Ophthalmol. 2010;149(5):768-776.e1.
20. Koch DD, Nacke RE, Wang L, Novak KD. Issues in wound management. In: Steinert R, ed. Cataract Surgery. 3rd ed. New York: Elsevier; 2009:581-588.
21. Gimbel HV, Sun R, DeBroff GM. Recognition and management of internal wound gape. J Cataract Refract Surg. 1995;21(2):121-124.
22. May WN, Castro-Combs J, Quinto GG, Kashiwabuchi R, Gower EW, Behrens A. Standardized Seidel test to evaluate different sutureless cataract incision configurations. J Cataract Refract Surg. 2010;36(6):1011-1017.
23. Kashiwabuchi FK, Khan YA, Rodrigues MW Jr, Wang J, McDonnell PJ, Daoud YJ. Seidel and India ink tests assessment of different clear cornea side-port incision configurations. Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1961-1965.
24. Calladine D, Packard R. Clear corneal incision architecture in the immediate postoperative period evaluated using optical coherence tomography. J Cataract Refract Surg. 2007;33(8):1429-1435.
25. Behrens WJ, Stark KA, Pratzer, McDonnell PJ. Dynamics of small-incision clear cornea wounds after phacoemulsification surgery using optical coherence tomography in the early postoperative period. J Refractive Surgery. 2008;24(1):46-49.
26. Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994;112(12):1584-1589.
27. Hurmeric V, Yoo SH, Mutlu FM. Optical coherence tomography in cornea and refractive surgery. Expert Rev Ophthalmol. 2012;7(3):241-250.
28. Schuman JS, Puliafito CA, Fujimoto JG, Duker JS. Optical Coherence Tomography of Ocular Diseases. 3rd ed. Thorofare, NJ: Slack Inc; 2013.
29. Salim S. The role of anterior segment optical coherence tomography in glaucoma. J Ophthalmol. 2012;2012:476801.
30. Kharousi NA, Wali UK, Azeem S. Current applications of optical coherence tomography in ophthalmology. In: Kawasaki M, ed. Optical Coherence Tomography. IntechOpen; 2013. https://www.intechopen.com/books/optical-coherence-tomography. Accessed July 31, 2019.
31. Rodrigues EB, Johanson M, Penha FM. Anterior segment tomography with the cirrus optical coherence tomography. J Ophthalmol. 2012;2012:806989.
32. Calladine D, Ward M, Packard R. Adherent ocular bandage for clear corneal incisions used in cataract surgery. J Cataract Refract Surg. 2010;36(11):1839-1848.
33. Haldar K, Saraff R. Closure technique for leaking wound resulting from thermal injury during phacoemulsification. J Cataract Refract Surg. 2014;40(9):1412-1414.
34. Zoghby JT, Cohen KL. Phacoemulsification-related corneal incision contracture. https://www.aao.org/eyenet/article/phacoemulsification-related-corneal-incision-contr. Published December 2012. Accessed June 16, 2019.
35. Bhatia SS. Ocular surface sealants and adhesives. Ocul Surf. 2006;4(3):146-154.
36. May WN, Castro-Combs J, Kashiwabuchi RT, et al. Bacterial-sized particle inflow through sutured clear corneal incisions in a laboratory human model. J Cataract Refract Surg. 2011;37(6):1140-1146.
37. Meskin SW, Ritterband DC, Shapiro DE, et al. Liquid bandage (2-octyl cyanoacrylate) as a temporary wound barrier in clear corneal cataract surgery. Ophthalmology. 2005;112(11):2015-2021.
38. Heaven CJ, Davison CR, Cockcroft PM. Bacterial contamination of nylon corneal sutures. Eye (Lond). 1995;9(pt 1):116-118.
39. Shigemitsu T, Majima Y. The utilization of a biological adhesive for wound treatment: comparison of suture, self-sealing sutureless and cyanoacrylate closure in the tensile strength test. Int Ophthalmol. 1996-1997;20:323-328.
40. Uy HS, Kenyon KR. Surgical outcomes after application of a liquid adhesive ocular bandage to clear corneal incisions during cataract surgery. J Cataract Refract Surg. 2013;39(11):1668-1674.
41. Meskin SW, Ritterband DC, Shapiro DE, et al. Liquid bandage (2-octyl cyanoacrylate) as a temporary wound barrier in clear corneal cataract surgery. Ophthalmology. 2005;112(11):2015-2021.
42. Tong AY, Gupta PK, Kim T. Wound closure and tissue adhesives in clear corneal incision cataract surgery. Curr Opin Ophthalmol. 2018;29(1):14-18.
43. US Food and Drug Administration. Summary of Safety and Effectiveness Data. Ophthalmic sealant: ReSure Sealant. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130004b.pdf. Published September 13, 2013. Accessed July 9, 2019.
44. About ReSure sealant. https://www.resuresealant.com/overview. Accessed July 31, 2019.
45. Menabuoni L, Pini R, Rossi F, Lenzetti I, Yoo SH, Parel JM. Laser-assisted corneal welding in cataract surgery: retrospective study. J Cataract Refract Surg. 2007;33(9):1608-1612.
46. Rasier R, Ozeren M, Artunay O, et al. Corneal tissue welding with infrared laser irradiation after clear corneal incision. Cornea. 2010;29(9):985-990.
47. Rossi F, Matteini P, Ratto F, Menabuoni L, Lenzetti I, Pini R. Laser tissue welding in ophthalmic surgery. J Biophotonics. 2008;1(4):331-342.
48. Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch Ophthalmol. 2005;123(5):613-620.
49. Taylor DM, Atlas BF, Romanchuk KG, Stern AL. Pseudophakic bullous keratopathy. Ophthalmology. 1983;90(1):19-24.
50. Lobo CL, Faria PM, Soares MA, Bernardes RC, Cunha-Vaz JG. Macular alterations after small-incision cataract surgery. J Cataract Refract Surg. 2004;30(4):752-760.
51. Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557-634.
52. Wright PL, Wilkinson CP, Balyeat HD, Popham J, Reinke M. Angiographic cystoid macular edema after posterior chamber lens implantation. Arch Ophthalmol. 1988;106(6):740-744.
53. Kim SJ, Belair ML, Bressler NM, et al. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina. 2008;28(6):870-876.
54. Alio JL, Ruiz-Moreno JM, Shabayek MH, Lugo FL, Abd El Rahman AM. The risk of retinal detachment in high myopia after small incision coaxial phacoemulsification. Am J Ophthalmol. 2007;144(1):93-98.
55. Bhagwandien AC, Cheng YY, Wolfs RC, van Meurs JC, Luyten GP. Relationship between retinal detachment and biometry in 4262 cataractous eyes. Ophthalmology. 2006;113(4):643-649.
56. Boberg-Ans G, Henning V, Villumsen J, la Cour M. Longterm incidence of rhegmatogenous retinal detachment and survival in a defined population undergoing standardized phacoemulsification surgery. Acta Ophthalmol Scand. 2006;84(5):613-618.
57. Jakobsson G, Montan P, Zetterberg M, Stenevi U, Behndig A, Lundström M. Capsule complication during cataract surgery: retinal detachment after cataract surgery with capsule complication: Swedish Capsule Rupture Study Group report 4. J Cataract Refract Surg. 2009;35(10):1699-1705.
58. Neuhann IM, Neuhann TF, Heimann H, Schmickler S, Gerl RH, Foerster MH. Retinal detachment after phacoemulsification in high myopia: analysis of 2356 cases. J Cataract Refract Surg. 2008;34(10):1644-1657.
59. Russell M, Gaskin B, Russell D, Polkinghorne PJ. Pseudophakic retinal detachment after phacoemulsification cataract surgery: ten-year retrospective review. J Cataract Refract Surg. 2006;32(3):442-445.
60. Apple DJ, Solomon KD, Tetz MR, et al. Posterior capsule opacification. Surv Ophthalmol. 1992;37(2):73-116.
61. Wu S, Tong N, Pan L, et al. Retrospective analyses of potential risk factors for posterior capsule opacification after cataract surgery. J Ophthalmol. 2018;2018:9089285.
62. Clark A, Morlet N, Ng JQ, Preen DB, Semmens JB. Whole population trends in complications of cataract surgery over 22 years in Western Australia. Ophthalmology. 2011;118(6):1055-1061.
63. Adhikari S, Shrestha UD. Pediatric cataract surgery with hydrophilic acrylic intraocular lens implantation in Nepalese Children. Clin Ophthalmol. 2017;12:7-11.
64. Lee BJ, Smith SD, Jeng BH. Suture-related corneal infections after clear corneal cataract surgery. J Cataract Refract Surg. 2009;35(5):939-942.
65. May WN, Castro-Combs J, Kashiwabuchi RT, et al. Sutured clear corneal incision: wound apposition and permeability to bacterial-sized particles. Cornea. 2013;32(3):319-325.
66. Hillier RJ, Ajit RR, Kelly SP. Suture-related complications after cataract surgery: a patient safety issue. J Cataract Refract Surg. 2009;35(11):2035-2036.
67. Hovanesian JA, Karageozian VH. Watertight cataract incision closure using fibrin tissue adhesive. J Cataract Refract Surg. 2007;33(8):1461-1463.
1. Thylefors B, Négrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995;73(1):115-121.
2. Flaxman SR, Bourne RRA, Resnikoff S, et al; Vision Loss Expert Group of the Global Burden of Disease Study. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-e1224.
3. Congdon N, Vingerling JR, Klein BE, et al; Eye Diseases Prevalence Research Group. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487-494.
4. Kurt E, Mayalı H. Early post-operative complications in cataract surgery. In: Zaidi FH, ed. Cataract Surgery. IntechOpen; 2013. https://www.intechopen.com/books/cataract-surgery/post-operative-infections-associated-with-cataract-surgery. Accessed July 15, 2019.
5. Chee SP. Clear corneal incision leakage after phacoemulsification--detection using povidone iodine 5%. Int Ophthalmol. 2005;26(4-5):175-179.
6. Masket S, Hovanesian JA, Levenson J, et al. Hydrogel sealant versus sutures to prevent fluid egress after cataract surgery. J Cataract Refract Surg. 2014;40(12):2057-2066.
7. Kelman CD. Phaco-emulsification and aspiration: a new technique of cataract removal. A preliminary report. Am J Ophthalmol. 1967;64(1):23-35.
8. Powe NR, Schein OD, Gieser SC, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team [published correction appears in Arch Ophthalmol. 1994;112(7):889]. Arch Ophthalmol. 1994;112(2):239-252.
9. Stein JD, Grossman DS, Mundy KM, Sugar A, Sloan FA. Severe adverse events after cataract surgery among medicare beneficiaries. Ophthalmology. 2011;118(9):1716-1723.
10. Greenberg PB, Tseng VL, Wu WC, et al. Prevalence and predictors of ocular complications associated with cataract surgery in United States veterans. Ophthalmology. 2011;118(3):507-514.
11. Mangan MS, Atalay E, Anci C, Tuncer I, Bilqec MD. Comparison of different types of complications in the phacoemulsification surgery learning curve according to number of operations performed. Turk J Ophthalmol. 2016;46(1):7-10.
12. Bell CM, Hatch WV, Cernat G, Urbach DR. Surgeon volumes and selected patient outcomes in cataract surgery: a population-based analysis. Ophthalmology. 2007;114(3):405-410.
13. Wallin T, Parker J, Jin Y, Kefalopoulos G, Olson RJ. Cohort study of 27 cases of endophthalmitis at a single institution. J Cataract Refract Surg. 2005;31(4):735-741.
14. Zaidi FH, Corbett MC, Burton BJ, Bloom PA. Raising the benchmark for the 21st century--the 1000 cataract operations audit and survey: outcomes, consultant-supervised training and sourcing NHS choice. Br J Ophthalmol. 2007;91(6):731-736.
15. Nichamin LD, Chang DF, Johnson SH, et al; American Society of Cataract and Refractive Surgery Cataract Clinical Committee. ASCRS white paper: what is the association between clear corneal cataract incisions and postoperative endophthalmitis? J Cataract Refract Surg. 2006;32(9):1556-1559.
16. Packer M, Chang DF, Dewey SH, et al; ASCRS Cataract Clinical Committee. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37(9):1699-1714.
17. Montan PG, Koranyi G, Setterquist HE, Stridh A, Philipson BT, Wiklund K. Endophthalmitis after cataract surgery: risk factors relating to technique and events of the operation and patient history: a retrospective case-control study. Ophthalmology. 1998;105(12):2171-2177.
18. Taban M, Rao B, Reznik J, Zhang J, Chen Z, McDonnell PJ. Dynamic morphology of sutureless cataract wounds—effect of incision angle and location. Surv Ophthalmol. 2004;49(suppl 2):S62-S72.
19. Chee SP, Ti SE, Lim L, Chan AS, Jap A. Anterior segment optical coherence tomography evaluation of the integrity of clear corneal incisions: a comparison between 2.2-mm and 2.65-mm main incisions. Am J Ophthalmol. 2010;149(5):768-776.e1.
20. Koch DD, Nacke RE, Wang L, Novak KD. Issues in wound management. In: Steinert R, ed. Cataract Surgery. 3rd ed. New York: Elsevier; 2009:581-588.
21. Gimbel HV, Sun R, DeBroff GM. Recognition and management of internal wound gape. J Cataract Refract Surg. 1995;21(2):121-124.
22. May WN, Castro-Combs J, Quinto GG, Kashiwabuchi R, Gower EW, Behrens A. Standardized Seidel test to evaluate different sutureless cataract incision configurations. J Cataract Refract Surg. 2010;36(6):1011-1017.
23. Kashiwabuchi FK, Khan YA, Rodrigues MW Jr, Wang J, McDonnell PJ, Daoud YJ. Seidel and India ink tests assessment of different clear cornea side-port incision configurations. Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1961-1965.
24. Calladine D, Packard R. Clear corneal incision architecture in the immediate postoperative period evaluated using optical coherence tomography. J Cataract Refract Surg. 2007;33(8):1429-1435.
25. Behrens WJ, Stark KA, Pratzer, McDonnell PJ. Dynamics of small-incision clear cornea wounds after phacoemulsification surgery using optical coherence tomography in the early postoperative period. J Refractive Surgery. 2008;24(1):46-49.
26. Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994;112(12):1584-1589.
27. Hurmeric V, Yoo SH, Mutlu FM. Optical coherence tomography in cornea and refractive surgery. Expert Rev Ophthalmol. 2012;7(3):241-250.
28. Schuman JS, Puliafito CA, Fujimoto JG, Duker JS. Optical Coherence Tomography of Ocular Diseases. 3rd ed. Thorofare, NJ: Slack Inc; 2013.
29. Salim S. The role of anterior segment optical coherence tomography in glaucoma. J Ophthalmol. 2012;2012:476801.
30. Kharousi NA, Wali UK, Azeem S. Current applications of optical coherence tomography in ophthalmology. In: Kawasaki M, ed. Optical Coherence Tomography. IntechOpen; 2013. https://www.intechopen.com/books/optical-coherence-tomography. Accessed July 31, 2019.
31. Rodrigues EB, Johanson M, Penha FM. Anterior segment tomography with the cirrus optical coherence tomography. J Ophthalmol. 2012;2012:806989.
32. Calladine D, Ward M, Packard R. Adherent ocular bandage for clear corneal incisions used in cataract surgery. J Cataract Refract Surg. 2010;36(11):1839-1848.
33. Haldar K, Saraff R. Closure technique for leaking wound resulting from thermal injury during phacoemulsification. J Cataract Refract Surg. 2014;40(9):1412-1414.
34. Zoghby JT, Cohen KL. Phacoemulsification-related corneal incision contracture. https://www.aao.org/eyenet/article/phacoemulsification-related-corneal-incision-contr. Published December 2012. Accessed June 16, 2019.
35. Bhatia SS. Ocular surface sealants and adhesives. Ocul Surf. 2006;4(3):146-154.
36. May WN, Castro-Combs J, Kashiwabuchi RT, et al. Bacterial-sized particle inflow through sutured clear corneal incisions in a laboratory human model. J Cataract Refract Surg. 2011;37(6):1140-1146.
37. Meskin SW, Ritterband DC, Shapiro DE, et al. Liquid bandage (2-octyl cyanoacrylate) as a temporary wound barrier in clear corneal cataract surgery. Ophthalmology. 2005;112(11):2015-2021.
38. Heaven CJ, Davison CR, Cockcroft PM. Bacterial contamination of nylon corneal sutures. Eye (Lond). 1995;9(pt 1):116-118.
39. Shigemitsu T, Majima Y. The utilization of a biological adhesive for wound treatment: comparison of suture, self-sealing sutureless and cyanoacrylate closure in the tensile strength test. Int Ophthalmol. 1996-1997;20:323-328.
40. Uy HS, Kenyon KR. Surgical outcomes after application of a liquid adhesive ocular bandage to clear corneal incisions during cataract surgery. J Cataract Refract Surg. 2013;39(11):1668-1674.
41. Meskin SW, Ritterband DC, Shapiro DE, et al. Liquid bandage (2-octyl cyanoacrylate) as a temporary wound barrier in clear corneal cataract surgery. Ophthalmology. 2005;112(11):2015-2021.
42. Tong AY, Gupta PK, Kim T. Wound closure and tissue adhesives in clear corneal incision cataract surgery. Curr Opin Ophthalmol. 2018;29(1):14-18.
43. US Food and Drug Administration. Summary of Safety and Effectiveness Data. Ophthalmic sealant: ReSure Sealant. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130004b.pdf. Published September 13, 2013. Accessed July 9, 2019.
44. About ReSure sealant. https://www.resuresealant.com/overview. Accessed July 31, 2019.
45. Menabuoni L, Pini R, Rossi F, Lenzetti I, Yoo SH, Parel JM. Laser-assisted corneal welding in cataract surgery: retrospective study. J Cataract Refract Surg. 2007;33(9):1608-1612.
46. Rasier R, Ozeren M, Artunay O, et al. Corneal tissue welding with infrared laser irradiation after clear corneal incision. Cornea. 2010;29(9):985-990.
47. Rossi F, Matteini P, Ratto F, Menabuoni L, Lenzetti I, Pini R. Laser tissue welding in ophthalmic surgery. J Biophotonics. 2008;1(4):331-342.
48. Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch Ophthalmol. 2005;123(5):613-620.
49. Taylor DM, Atlas BF, Romanchuk KG, Stern AL. Pseudophakic bullous keratopathy. Ophthalmology. 1983;90(1):19-24.
50. Lobo CL, Faria PM, Soares MA, Bernardes RC, Cunha-Vaz JG. Macular alterations after small-incision cataract surgery. J Cataract Refract Surg. 2004;30(4):752-760.
51. Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc. 1998;96:557-634.
52. Wright PL, Wilkinson CP, Balyeat HD, Popham J, Reinke M. Angiographic cystoid macular edema after posterior chamber lens implantation. Arch Ophthalmol. 1988;106(6):740-744.
53. Kim SJ, Belair ML, Bressler NM, et al. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina. 2008;28(6):870-876.
54. Alio JL, Ruiz-Moreno JM, Shabayek MH, Lugo FL, Abd El Rahman AM. The risk of retinal detachment in high myopia after small incision coaxial phacoemulsification. Am J Ophthalmol. 2007;144(1):93-98.
55. Bhagwandien AC, Cheng YY, Wolfs RC, van Meurs JC, Luyten GP. Relationship between retinal detachment and biometry in 4262 cataractous eyes. Ophthalmology. 2006;113(4):643-649.
56. Boberg-Ans G, Henning V, Villumsen J, la Cour M. Longterm incidence of rhegmatogenous retinal detachment and survival in a defined population undergoing standardized phacoemulsification surgery. Acta Ophthalmol Scand. 2006;84(5):613-618.
57. Jakobsson G, Montan P, Zetterberg M, Stenevi U, Behndig A, Lundström M. Capsule complication during cataract surgery: retinal detachment after cataract surgery with capsule complication: Swedish Capsule Rupture Study Group report 4. J Cataract Refract Surg. 2009;35(10):1699-1705.
58. Neuhann IM, Neuhann TF, Heimann H, Schmickler S, Gerl RH, Foerster MH. Retinal detachment after phacoemulsification in high myopia: analysis of 2356 cases. J Cataract Refract Surg. 2008;34(10):1644-1657.
59. Russell M, Gaskin B, Russell D, Polkinghorne PJ. Pseudophakic retinal detachment after phacoemulsification cataract surgery: ten-year retrospective review. J Cataract Refract Surg. 2006;32(3):442-445.
60. Apple DJ, Solomon KD, Tetz MR, et al. Posterior capsule opacification. Surv Ophthalmol. 1992;37(2):73-116.
61. Wu S, Tong N, Pan L, et al. Retrospective analyses of potential risk factors for posterior capsule opacification after cataract surgery. J Ophthalmol. 2018;2018:9089285.
62. Clark A, Morlet N, Ng JQ, Preen DB, Semmens JB. Whole population trends in complications of cataract surgery over 22 years in Western Australia. Ophthalmology. 2011;118(6):1055-1061.
63. Adhikari S, Shrestha UD. Pediatric cataract surgery with hydrophilic acrylic intraocular lens implantation in Nepalese Children. Clin Ophthalmol. 2017;12:7-11.
64. Lee BJ, Smith SD, Jeng BH. Suture-related corneal infections after clear corneal cataract surgery. J Cataract Refract Surg. 2009;35(5):939-942.
65. May WN, Castro-Combs J, Kashiwabuchi RT, et al. Sutured clear corneal incision: wound apposition and permeability to bacterial-sized particles. Cornea. 2013;32(3):319-325.
66. Hillier RJ, Ajit RR, Kelly SP. Suture-related complications after cataract surgery: a patient safety issue. J Cataract Refract Surg. 2009;35(11):2035-2036.
67. Hovanesian JA, Karageozian VH. Watertight cataract incision closure using fibrin tissue adhesive. J Cataract Refract Surg. 2007;33(8):1461-1463.
The 10 Commandments of Internships
Partners in Oncology Care: Coordinated Follicular Lymphoma Management (FULL)
Four case examples illustrate the important role of multidisciplinary medical care for the optimal long-term care of patients with follicular lymphoma.
Patients benefit from multidisciplinary care that coordinates management of complex medical problems. Traditionally, multidisciplinary cancer care involves oncology specialty providers in fields that include medical oncology, radiation oncology, and surgical oncology. Multidisciplinary cancer care intends to improve patient outcomes by bringing together different health care providers (HCPs) who are involved in the treatment of patients with cancer. Because new therapies are more effective and allow patients with cancer to live longer, adverse effects (AEs) are more likely to impact patients’ well-being, both while receiving treatment and long after it has completed. Thus, this population may benefit from an expanded approach to multidisciplinary care that includes input from specialty and primary care providers (PCPs), clinical pharmacy specialists (CPS), physical and occupational therapists, and patient navigators and educators.
We present 4 hypothetical cases, based on actual patients, that illustrate opportunities where multidisciplinary care coordination may improve patient experiences. These cases draw on current quality initiatives from the National Cancer Institute Community Cancer Centers Program, which has focused on improving the quality of multidisciplinary cancer care at selected community centers, and the Veterans Health Administration (VHA) patient-aligned care team (PACT) model, which brings together different health professionals to optimize primary care coordination.1,2 In addition, the National Committee for Quality Assurance has introduced an educational initiative to facilitate implementation of an oncologic medical home.3 This initiative stresses increased multidisciplinary communication, patient-centered care delivery, and reduced fragmentation of care for this population. Despite these guidelines and experiences from other medical specialties, models for integrated cancer care have not been implemented in a prospective fashion within the VHA.
In this article, we focus on opportunities to take collaborative care approaches for the treatment of patients with follicular lymphoma (FL): a common, incurable, and often indolent B-cell non-Hodgkin lymphoma.4 FL was selected because these patients may be treated numerous times and long-term sequalae can accumulate throughout their cancer continuum (a series of health events encompassing cancer screening, diagnosis, treatment, survivorship, relapse, and death).5 HCPs in distinct roles can assist patients with cancer in optimizing their health outcomes and overall wellbeing.6
Case Example 1
A 70-year-old male was diagnosed with stage IV FL. Because of his advanced disease, he began therapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). Prednisone was administered at 100 mg daily on the first 5 days of each 21-day cycle. On day 4 of the first treatment cycle, the patient notified his oncologist that he had been very thirsty and his random blood sugar values on 2 different days were 283 mg/dL and 312 mg/dL. A laboratory review revealed his hemoglobin A1c (HbA1c) 7 months prior was 5.6%.
Discussion
The high-dose prednisone component of this and other lymphoma therapy regimens can worsen diabetes mellitus (DM) control and/or worsen prediabetes. Patient characteristics that increase the risk of developing glucocorticoid-induced DM after CHOP chemotherapy include age ≥ 60 years, HbA1c > 6.1%, and body mass index > 30.7 This patient did not have DM prior to the FL therapy initiation, but afterwards he met diagnostic criteria for DM. For completeness, other causes for elevated blood glucose should be ruled out (ie, infection, laboratory error, etc.). An oncologist often will triage acute hyperglycemia, treating immediately with IV fluids and/or insulin. Thereafter, ongoing chronic disease management for DM may be best managed by PCPs, certified DM educators, and registered dieticians.
Several programs involving multidisciplinary DM care, comprised of physicians, advanced practice providers, nurses, certified DM educators, and/or pharmacists have been shown to improve HbA1c, cardiovascular outcomes, and all-cause mortality, while reducing health care costs.8 In addition, patient navigators can assist patients with coordinating visits to disease-state specialists and identifying further educational needs. For example, in 1 program, nonclinical peer navigators were shown to improve the number of appointments attended and reduce HbA1c in a population of patients with DM who were primarily minority, urban, and of low socioeconomic status.9 Thus, integrating DM care shows potential to improve outcomes for patients with lymphoma who develop glucocorticoid
Case Example 2
A 75-year-old male was diagnosed with FL. He was treated initially with bendamustine and rituximab. He required reinitiation of therapy 20 months later when he developed lymphadenopathy, fatigue, and night sweats and began treatment with oral idelalisib, a second-line therapy. Later, the patient presented to his PCP for a routine visit, and on medication reconciliation review, the patient reported regular use of trimethoprim-sulfamethoxazole.
Discussion
Upon consultation with the CPS and the patient’s oncologist, the PCP confirmed trimethoprim-sulfamethoxazole should be continued during therapy and for about 6 months following completion of therapy. Trimethoprim-sulfamethoxazole is used for prophylaxis against Pneumocystis jirovecii (formerly Pneumocystis carinii). While use of prophylactic therapy is not necessary for all patients with FL, idelalisib impairs the function of circulating lymphoid B-cells and thus has been associated with an increased risk of serious infection.10 A CPS can provide insight that maximizes medication adherence and efficacy while minimizing food-drug, drug-drug interactions, and AEs. CPS have been shown to: improve adherence to oral therapies, increase prospective monitoring required for safe therapy dose selection, and document assessment of chemotherapy-related AEs.11,12 Thus, multidisciplinary, integrated care is an important component of providing quality oncology care.
Case Example 3
A 60-year-old female presented to her PCP with a 2-week history of shortness of breath and leg swelling. She was treated for FL 4 years previously with 6 cycles of R-CHOP. She reported no chest pain and did not have a prior history of hypertension, DM, or heart disease. On physical exam, she had elevated jugular venous pressure to jaw at 45°, bilateral pulmonary rales, and 2+ pitting pretibial edema. Laboratory tests that included complete blood count, basic chemistries, and thyroid stimulating hormone were unremarkable, though brain natriuretic peptide (BNP) was elevated at 425 pg/mL.
As this patient’s laboratory results and physical examination suggested new-onset congestive heart failure, the PCP obtained an echocardiogram, which demonstrated an ejection fraction of 35% and global hypokinesis. Because the patient was symptomatic, she was admitted to the hospital to begin guideline-directed medical therapy (GDMT) including IV diuresis.
Discussion
Given the absence of significant risk factors and prior history of coronary artery disease, the most probable cause for this patient’s cardiomyopathy is doxorubicin. Doxorubicin is an anthracycline chemotherapy that can cause nonischemic, dilated cardiomyopathy, particularly when cumulative doses > 400 mg/m2 are administered, or when combined with chest radiation.13 This patient benefited from GDMT for reduced ejection-fraction heart failure (HFrEF). Studies have demonstrated positive outcomes when HFrEF patients are cared for by a multidisciplinary team who focus of volume management as well as uptitration of therapies to target doses.14
Case Example 4
An 80-year-old female was diagnosed with stage III FL but did not require immediate therapy. After developing discomfort due to enlarging lymphadenopathy, she initiated therapy with rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP). She presented to her oncologist for consideration of her fifth cycle of R-CVP and reported a burning sensation on the soles of her feet and numbness in her fingertips and toes. On examination, her pulses were intact and there were no signs of infection, reduced blood flow, or edema. The patient demonstrated decreased sensation on monofilament testing. She had no history of DM and a recent HbA1c test was 4.9% An evaluation for other causes of neuropathy, such as hypothyroidism and vitamin B12 deficiency was negative. Thus, vincristine therapy was identified as the most likely etiology for her peripheral neuropathy. The oncologist decided to proceed with cycle 5 of chemotherapy but reduced the dose of vincristine by 50%.
Discussion
Vincristine is a microtubule inhibitor used in many chemotherapy regimens and may cause reversible or permanent neuropathy, including autonomic (constipation), sensory (stocking-glove distribution), or motor (foot-drop).15 A nerve conduction study may be indicated as part of the diagnostic evaluation. Treatment for painful sensory neuropathy may include pharmacologic therapy (such as gabapentin, pregabalin, capsaicin cream).16 Podiatrists can provide foot care and may provide shoes and inserts if appropriate. Physical therapists may assist with safety and mobility evaluations and can provide therapeutic exercises and assistive devices that improve function and quality of life.17
Conclusion
As cancer becomes more curable and more manageable, patients with cancer and survivors no longer rely exclusively on their oncologists for medical care. This is increasingly prevalent for patients with incurable but indolent cancers that may be present for years to decades, as acute and cumulative toxicities may complicate existing comorbidities. Thus, in this era of increasingly complex cancer therapies, multidisciplinary medical care that involves PCPs, specialists, and allied medical professionals, is essential for providing care that optimizes health and fully addresses patients’ needs.
1. Friedman EL, Chawla N, Morris PT, et al. Assessing the development of multidisciplinary care: experience of the National Cancer Institute community cancer centers program. J Oncol Pract. 2015;11(1):e36-e43.
2. Peterson K, Helfand M, Humphrey L, Christensen V, Carson S. Evidence brief: effectiveness of intensive primary care programs. https://www.hsrd.research.va.gov/publications/esp/Intensive-Primary-Care-Supplement.pdf. Published February 2013. Accessed April 5, 2019.
3. National Committee for Quality Assurance. Oncology medical home recognition. https://www.ncqa.org/programs/health-care-providers-practices/oncology-medical-home. Accessed April 5, 2019.
4. Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055-2063.
5. Dulaney C, Wallace AS, Everett AS, Dover L, McDonald A, Kropp L. Defining health across the cancer continuum. Cureus. 2017;9(2):e1029.
6. Hopkins J, Mumber MP. Patient navigation through the cancer care continuum: an overview. J Oncol Pract. 2009;5(4):150-152.
7. Lee SY, Kurita N, Yokoyama Y, et al. Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer. 2014;22(5):1385-1390.
8. McGill M, Blonde L, Juliana CN, et al; Global Partnership for Effective Diabetes Management. The interdisciplinary team in type 2 diabetes management: challenges and best practice solutions from real-world scenarios. J Clin Transl Endocrinol. 2017;7:21-27.
9. Horný M, Glover W, Gupte G, Saraswat A, Vimalananda V, Rosenzweig J. Patient navigation to improve diabetes outpatient care at a safety-net hospital: a retrospective cohort study. BMC Health Serv Res. 2017;17(1):759.
10. Reinwald M, Silva JT, Mueller NJ, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect. 2018;24(suppl 2):S53-S70.
11. Holle LM, Boehnke Michaud L. Oncology pharmacists in health care delivery: vital members of the cancer care team. J. Oncol. Pract. 2014;10(3):e142-e145.
12. Morgan KP, Muluneh B, Dean AM, Amerine LB. Impact of an integrated oral chemotherapy program on patient adherence. J Oncol Pharm Pract. 2018;24(5):332-336.
13. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869-2879.
14. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774-784.
15. Mora E, Smith EM, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. 2016;6(11):2416-2430.
16. Hershman DL, Lacchetti C, Dworkin RH, et al; American Society of Clinical Oncology. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967
17. Duregon F, Vendramin B, Bullo V, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90-100.
Four case examples illustrate the important role of multidisciplinary medical care for the optimal long-term care of patients with follicular lymphoma.
Four case examples illustrate the important role of multidisciplinary medical care for the optimal long-term care of patients with follicular lymphoma.
Patients benefit from multidisciplinary care that coordinates management of complex medical problems. Traditionally, multidisciplinary cancer care involves oncology specialty providers in fields that include medical oncology, radiation oncology, and surgical oncology. Multidisciplinary cancer care intends to improve patient outcomes by bringing together different health care providers (HCPs) who are involved in the treatment of patients with cancer. Because new therapies are more effective and allow patients with cancer to live longer, adverse effects (AEs) are more likely to impact patients’ well-being, both while receiving treatment and long after it has completed. Thus, this population may benefit from an expanded approach to multidisciplinary care that includes input from specialty and primary care providers (PCPs), clinical pharmacy specialists (CPS), physical and occupational therapists, and patient navigators and educators.
We present 4 hypothetical cases, based on actual patients, that illustrate opportunities where multidisciplinary care coordination may improve patient experiences. These cases draw on current quality initiatives from the National Cancer Institute Community Cancer Centers Program, which has focused on improving the quality of multidisciplinary cancer care at selected community centers, and the Veterans Health Administration (VHA) patient-aligned care team (PACT) model, which brings together different health professionals to optimize primary care coordination.1,2 In addition, the National Committee for Quality Assurance has introduced an educational initiative to facilitate implementation of an oncologic medical home.3 This initiative stresses increased multidisciplinary communication, patient-centered care delivery, and reduced fragmentation of care for this population. Despite these guidelines and experiences from other medical specialties, models for integrated cancer care have not been implemented in a prospective fashion within the VHA.
In this article, we focus on opportunities to take collaborative care approaches for the treatment of patients with follicular lymphoma (FL): a common, incurable, and often indolent B-cell non-Hodgkin lymphoma.4 FL was selected because these patients may be treated numerous times and long-term sequalae can accumulate throughout their cancer continuum (a series of health events encompassing cancer screening, diagnosis, treatment, survivorship, relapse, and death).5 HCPs in distinct roles can assist patients with cancer in optimizing their health outcomes and overall wellbeing.6
Case Example 1
A 70-year-old male was diagnosed with stage IV FL. Because of his advanced disease, he began therapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). Prednisone was administered at 100 mg daily on the first 5 days of each 21-day cycle. On day 4 of the first treatment cycle, the patient notified his oncologist that he had been very thirsty and his random blood sugar values on 2 different days were 283 mg/dL and 312 mg/dL. A laboratory review revealed his hemoglobin A1c (HbA1c) 7 months prior was 5.6%.
Discussion
The high-dose prednisone component of this and other lymphoma therapy regimens can worsen diabetes mellitus (DM) control and/or worsen prediabetes. Patient characteristics that increase the risk of developing glucocorticoid-induced DM after CHOP chemotherapy include age ≥ 60 years, HbA1c > 6.1%, and body mass index > 30.7 This patient did not have DM prior to the FL therapy initiation, but afterwards he met diagnostic criteria for DM. For completeness, other causes for elevated blood glucose should be ruled out (ie, infection, laboratory error, etc.). An oncologist often will triage acute hyperglycemia, treating immediately with IV fluids and/or insulin. Thereafter, ongoing chronic disease management for DM may be best managed by PCPs, certified DM educators, and registered dieticians.
Several programs involving multidisciplinary DM care, comprised of physicians, advanced practice providers, nurses, certified DM educators, and/or pharmacists have been shown to improve HbA1c, cardiovascular outcomes, and all-cause mortality, while reducing health care costs.8 In addition, patient navigators can assist patients with coordinating visits to disease-state specialists and identifying further educational needs. For example, in 1 program, nonclinical peer navigators were shown to improve the number of appointments attended and reduce HbA1c in a population of patients with DM who were primarily minority, urban, and of low socioeconomic status.9 Thus, integrating DM care shows potential to improve outcomes for patients with lymphoma who develop glucocorticoid
Case Example 2
A 75-year-old male was diagnosed with FL. He was treated initially with bendamustine and rituximab. He required reinitiation of therapy 20 months later when he developed lymphadenopathy, fatigue, and night sweats and began treatment with oral idelalisib, a second-line therapy. Later, the patient presented to his PCP for a routine visit, and on medication reconciliation review, the patient reported regular use of trimethoprim-sulfamethoxazole.
Discussion
Upon consultation with the CPS and the patient’s oncologist, the PCP confirmed trimethoprim-sulfamethoxazole should be continued during therapy and for about 6 months following completion of therapy. Trimethoprim-sulfamethoxazole is used for prophylaxis against Pneumocystis jirovecii (formerly Pneumocystis carinii). While use of prophylactic therapy is not necessary for all patients with FL, idelalisib impairs the function of circulating lymphoid B-cells and thus has been associated with an increased risk of serious infection.10 A CPS can provide insight that maximizes medication adherence and efficacy while minimizing food-drug, drug-drug interactions, and AEs. CPS have been shown to: improve adherence to oral therapies, increase prospective monitoring required for safe therapy dose selection, and document assessment of chemotherapy-related AEs.11,12 Thus, multidisciplinary, integrated care is an important component of providing quality oncology care.
Case Example 3
A 60-year-old female presented to her PCP with a 2-week history of shortness of breath and leg swelling. She was treated for FL 4 years previously with 6 cycles of R-CHOP. She reported no chest pain and did not have a prior history of hypertension, DM, or heart disease. On physical exam, she had elevated jugular venous pressure to jaw at 45°, bilateral pulmonary rales, and 2+ pitting pretibial edema. Laboratory tests that included complete blood count, basic chemistries, and thyroid stimulating hormone were unremarkable, though brain natriuretic peptide (BNP) was elevated at 425 pg/mL.
As this patient’s laboratory results and physical examination suggested new-onset congestive heart failure, the PCP obtained an echocardiogram, which demonstrated an ejection fraction of 35% and global hypokinesis. Because the patient was symptomatic, she was admitted to the hospital to begin guideline-directed medical therapy (GDMT) including IV diuresis.
Discussion
Given the absence of significant risk factors and prior history of coronary artery disease, the most probable cause for this patient’s cardiomyopathy is doxorubicin. Doxorubicin is an anthracycline chemotherapy that can cause nonischemic, dilated cardiomyopathy, particularly when cumulative doses > 400 mg/m2 are administered, or when combined with chest radiation.13 This patient benefited from GDMT for reduced ejection-fraction heart failure (HFrEF). Studies have demonstrated positive outcomes when HFrEF patients are cared for by a multidisciplinary team who focus of volume management as well as uptitration of therapies to target doses.14
Case Example 4
An 80-year-old female was diagnosed with stage III FL but did not require immediate therapy. After developing discomfort due to enlarging lymphadenopathy, she initiated therapy with rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP). She presented to her oncologist for consideration of her fifth cycle of R-CVP and reported a burning sensation on the soles of her feet and numbness in her fingertips and toes. On examination, her pulses were intact and there were no signs of infection, reduced blood flow, or edema. The patient demonstrated decreased sensation on monofilament testing. She had no history of DM and a recent HbA1c test was 4.9% An evaluation for other causes of neuropathy, such as hypothyroidism and vitamin B12 deficiency was negative. Thus, vincristine therapy was identified as the most likely etiology for her peripheral neuropathy. The oncologist decided to proceed with cycle 5 of chemotherapy but reduced the dose of vincristine by 50%.
Discussion
Vincristine is a microtubule inhibitor used in many chemotherapy regimens and may cause reversible or permanent neuropathy, including autonomic (constipation), sensory (stocking-glove distribution), or motor (foot-drop).15 A nerve conduction study may be indicated as part of the diagnostic evaluation. Treatment for painful sensory neuropathy may include pharmacologic therapy (such as gabapentin, pregabalin, capsaicin cream).16 Podiatrists can provide foot care and may provide shoes and inserts if appropriate. Physical therapists may assist with safety and mobility evaluations and can provide therapeutic exercises and assistive devices that improve function and quality of life.17
Conclusion
As cancer becomes more curable and more manageable, patients with cancer and survivors no longer rely exclusively on their oncologists for medical care. This is increasingly prevalent for patients with incurable but indolent cancers that may be present for years to decades, as acute and cumulative toxicities may complicate existing comorbidities. Thus, in this era of increasingly complex cancer therapies, multidisciplinary medical care that involves PCPs, specialists, and allied medical professionals, is essential for providing care that optimizes health and fully addresses patients’ needs.
Patients benefit from multidisciplinary care that coordinates management of complex medical problems. Traditionally, multidisciplinary cancer care involves oncology specialty providers in fields that include medical oncology, radiation oncology, and surgical oncology. Multidisciplinary cancer care intends to improve patient outcomes by bringing together different health care providers (HCPs) who are involved in the treatment of patients with cancer. Because new therapies are more effective and allow patients with cancer to live longer, adverse effects (AEs) are more likely to impact patients’ well-being, both while receiving treatment and long after it has completed. Thus, this population may benefit from an expanded approach to multidisciplinary care that includes input from specialty and primary care providers (PCPs), clinical pharmacy specialists (CPS), physical and occupational therapists, and patient navigators and educators.
We present 4 hypothetical cases, based on actual patients, that illustrate opportunities where multidisciplinary care coordination may improve patient experiences. These cases draw on current quality initiatives from the National Cancer Institute Community Cancer Centers Program, which has focused on improving the quality of multidisciplinary cancer care at selected community centers, and the Veterans Health Administration (VHA) patient-aligned care team (PACT) model, which brings together different health professionals to optimize primary care coordination.1,2 In addition, the National Committee for Quality Assurance has introduced an educational initiative to facilitate implementation of an oncologic medical home.3 This initiative stresses increased multidisciplinary communication, patient-centered care delivery, and reduced fragmentation of care for this population. Despite these guidelines and experiences from other medical specialties, models for integrated cancer care have not been implemented in a prospective fashion within the VHA.
In this article, we focus on opportunities to take collaborative care approaches for the treatment of patients with follicular lymphoma (FL): a common, incurable, and often indolent B-cell non-Hodgkin lymphoma.4 FL was selected because these patients may be treated numerous times and long-term sequalae can accumulate throughout their cancer continuum (a series of health events encompassing cancer screening, diagnosis, treatment, survivorship, relapse, and death).5 HCPs in distinct roles can assist patients with cancer in optimizing their health outcomes and overall wellbeing.6
Case Example 1
A 70-year-old male was diagnosed with stage IV FL. Because of his advanced disease, he began therapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone). Prednisone was administered at 100 mg daily on the first 5 days of each 21-day cycle. On day 4 of the first treatment cycle, the patient notified his oncologist that he had been very thirsty and his random blood sugar values on 2 different days were 283 mg/dL and 312 mg/dL. A laboratory review revealed his hemoglobin A1c (HbA1c) 7 months prior was 5.6%.
Discussion
The high-dose prednisone component of this and other lymphoma therapy regimens can worsen diabetes mellitus (DM) control and/or worsen prediabetes. Patient characteristics that increase the risk of developing glucocorticoid-induced DM after CHOP chemotherapy include age ≥ 60 years, HbA1c > 6.1%, and body mass index > 30.7 This patient did not have DM prior to the FL therapy initiation, but afterwards he met diagnostic criteria for DM. For completeness, other causes for elevated blood glucose should be ruled out (ie, infection, laboratory error, etc.). An oncologist often will triage acute hyperglycemia, treating immediately with IV fluids and/or insulin. Thereafter, ongoing chronic disease management for DM may be best managed by PCPs, certified DM educators, and registered dieticians.
Several programs involving multidisciplinary DM care, comprised of physicians, advanced practice providers, nurses, certified DM educators, and/or pharmacists have been shown to improve HbA1c, cardiovascular outcomes, and all-cause mortality, while reducing health care costs.8 In addition, patient navigators can assist patients with coordinating visits to disease-state specialists and identifying further educational needs. For example, in 1 program, nonclinical peer navigators were shown to improve the number of appointments attended and reduce HbA1c in a population of patients with DM who were primarily minority, urban, and of low socioeconomic status.9 Thus, integrating DM care shows potential to improve outcomes for patients with lymphoma who develop glucocorticoid
Case Example 2
A 75-year-old male was diagnosed with FL. He was treated initially with bendamustine and rituximab. He required reinitiation of therapy 20 months later when he developed lymphadenopathy, fatigue, and night sweats and began treatment with oral idelalisib, a second-line therapy. Later, the patient presented to his PCP for a routine visit, and on medication reconciliation review, the patient reported regular use of trimethoprim-sulfamethoxazole.
Discussion
Upon consultation with the CPS and the patient’s oncologist, the PCP confirmed trimethoprim-sulfamethoxazole should be continued during therapy and for about 6 months following completion of therapy. Trimethoprim-sulfamethoxazole is used for prophylaxis against Pneumocystis jirovecii (formerly Pneumocystis carinii). While use of prophylactic therapy is not necessary for all patients with FL, idelalisib impairs the function of circulating lymphoid B-cells and thus has been associated with an increased risk of serious infection.10 A CPS can provide insight that maximizes medication adherence and efficacy while minimizing food-drug, drug-drug interactions, and AEs. CPS have been shown to: improve adherence to oral therapies, increase prospective monitoring required for safe therapy dose selection, and document assessment of chemotherapy-related AEs.11,12 Thus, multidisciplinary, integrated care is an important component of providing quality oncology care.
Case Example 3
A 60-year-old female presented to her PCP with a 2-week history of shortness of breath and leg swelling. She was treated for FL 4 years previously with 6 cycles of R-CHOP. She reported no chest pain and did not have a prior history of hypertension, DM, or heart disease. On physical exam, she had elevated jugular venous pressure to jaw at 45°, bilateral pulmonary rales, and 2+ pitting pretibial edema. Laboratory tests that included complete blood count, basic chemistries, and thyroid stimulating hormone were unremarkable, though brain natriuretic peptide (BNP) was elevated at 425 pg/mL.
As this patient’s laboratory results and physical examination suggested new-onset congestive heart failure, the PCP obtained an echocardiogram, which demonstrated an ejection fraction of 35% and global hypokinesis. Because the patient was symptomatic, she was admitted to the hospital to begin guideline-directed medical therapy (GDMT) including IV diuresis.
Discussion
Given the absence of significant risk factors and prior history of coronary artery disease, the most probable cause for this patient’s cardiomyopathy is doxorubicin. Doxorubicin is an anthracycline chemotherapy that can cause nonischemic, dilated cardiomyopathy, particularly when cumulative doses > 400 mg/m2 are administered, or when combined with chest radiation.13 This patient benefited from GDMT for reduced ejection-fraction heart failure (HFrEF). Studies have demonstrated positive outcomes when HFrEF patients are cared for by a multidisciplinary team who focus of volume management as well as uptitration of therapies to target doses.14
Case Example 4
An 80-year-old female was diagnosed with stage III FL but did not require immediate therapy. After developing discomfort due to enlarging lymphadenopathy, she initiated therapy with rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP). She presented to her oncologist for consideration of her fifth cycle of R-CVP and reported a burning sensation on the soles of her feet and numbness in her fingertips and toes. On examination, her pulses were intact and there were no signs of infection, reduced blood flow, or edema. The patient demonstrated decreased sensation on monofilament testing. She had no history of DM and a recent HbA1c test was 4.9% An evaluation for other causes of neuropathy, such as hypothyroidism and vitamin B12 deficiency was negative. Thus, vincristine therapy was identified as the most likely etiology for her peripheral neuropathy. The oncologist decided to proceed with cycle 5 of chemotherapy but reduced the dose of vincristine by 50%.
Discussion
Vincristine is a microtubule inhibitor used in many chemotherapy regimens and may cause reversible or permanent neuropathy, including autonomic (constipation), sensory (stocking-glove distribution), or motor (foot-drop).15 A nerve conduction study may be indicated as part of the diagnostic evaluation. Treatment for painful sensory neuropathy may include pharmacologic therapy (such as gabapentin, pregabalin, capsaicin cream).16 Podiatrists can provide foot care and may provide shoes and inserts if appropriate. Physical therapists may assist with safety and mobility evaluations and can provide therapeutic exercises and assistive devices that improve function and quality of life.17
Conclusion
As cancer becomes more curable and more manageable, patients with cancer and survivors no longer rely exclusively on their oncologists for medical care. This is increasingly prevalent for patients with incurable but indolent cancers that may be present for years to decades, as acute and cumulative toxicities may complicate existing comorbidities. Thus, in this era of increasingly complex cancer therapies, multidisciplinary medical care that involves PCPs, specialists, and allied medical professionals, is essential for providing care that optimizes health and fully addresses patients’ needs.
1. Friedman EL, Chawla N, Morris PT, et al. Assessing the development of multidisciplinary care: experience of the National Cancer Institute community cancer centers program. J Oncol Pract. 2015;11(1):e36-e43.
2. Peterson K, Helfand M, Humphrey L, Christensen V, Carson S. Evidence brief: effectiveness of intensive primary care programs. https://www.hsrd.research.va.gov/publications/esp/Intensive-Primary-Care-Supplement.pdf. Published February 2013. Accessed April 5, 2019.
3. National Committee for Quality Assurance. Oncology medical home recognition. https://www.ncqa.org/programs/health-care-providers-practices/oncology-medical-home. Accessed April 5, 2019.
4. Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055-2063.
5. Dulaney C, Wallace AS, Everett AS, Dover L, McDonald A, Kropp L. Defining health across the cancer continuum. Cureus. 2017;9(2):e1029.
6. Hopkins J, Mumber MP. Patient navigation through the cancer care continuum: an overview. J Oncol Pract. 2009;5(4):150-152.
7. Lee SY, Kurita N, Yokoyama Y, et al. Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer. 2014;22(5):1385-1390.
8. McGill M, Blonde L, Juliana CN, et al; Global Partnership for Effective Diabetes Management. The interdisciplinary team in type 2 diabetes management: challenges and best practice solutions from real-world scenarios. J Clin Transl Endocrinol. 2017;7:21-27.
9. Horný M, Glover W, Gupte G, Saraswat A, Vimalananda V, Rosenzweig J. Patient navigation to improve diabetes outpatient care at a safety-net hospital: a retrospective cohort study. BMC Health Serv Res. 2017;17(1):759.
10. Reinwald M, Silva JT, Mueller NJ, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect. 2018;24(suppl 2):S53-S70.
11. Holle LM, Boehnke Michaud L. Oncology pharmacists in health care delivery: vital members of the cancer care team. J. Oncol. Pract. 2014;10(3):e142-e145.
12. Morgan KP, Muluneh B, Dean AM, Amerine LB. Impact of an integrated oral chemotherapy program on patient adherence. J Oncol Pharm Pract. 2018;24(5):332-336.
13. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869-2879.
14. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774-784.
15. Mora E, Smith EM, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. 2016;6(11):2416-2430.
16. Hershman DL, Lacchetti C, Dworkin RH, et al; American Society of Clinical Oncology. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967
17. Duregon F, Vendramin B, Bullo V, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90-100.
1. Friedman EL, Chawla N, Morris PT, et al. Assessing the development of multidisciplinary care: experience of the National Cancer Institute community cancer centers program. J Oncol Pract. 2015;11(1):e36-e43.
2. Peterson K, Helfand M, Humphrey L, Christensen V, Carson S. Evidence brief: effectiveness of intensive primary care programs. https://www.hsrd.research.va.gov/publications/esp/Intensive-Primary-Care-Supplement.pdf. Published February 2013. Accessed April 5, 2019.
3. National Committee for Quality Assurance. Oncology medical home recognition. https://www.ncqa.org/programs/health-care-providers-practices/oncology-medical-home. Accessed April 5, 2019.
4. Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055-2063.
5. Dulaney C, Wallace AS, Everett AS, Dover L, McDonald A, Kropp L. Defining health across the cancer continuum. Cureus. 2017;9(2):e1029.
6. Hopkins J, Mumber MP. Patient navigation through the cancer care continuum: an overview. J Oncol Pract. 2009;5(4):150-152.
7. Lee SY, Kurita N, Yokoyama Y, et al. Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer. 2014;22(5):1385-1390.
8. McGill M, Blonde L, Juliana CN, et al; Global Partnership for Effective Diabetes Management. The interdisciplinary team in type 2 diabetes management: challenges and best practice solutions from real-world scenarios. J Clin Transl Endocrinol. 2017;7:21-27.
9. Horný M, Glover W, Gupte G, Saraswat A, Vimalananda V, Rosenzweig J. Patient navigation to improve diabetes outpatient care at a safety-net hospital: a retrospective cohort study. BMC Health Serv Res. 2017;17(1):759.
10. Reinwald M, Silva JT, Mueller NJ, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Intracellular signaling pathways: tyrosine kinase and mTOR inhibitors). Clin Microbiol Infect. 2018;24(suppl 2):S53-S70.
11. Holle LM, Boehnke Michaud L. Oncology pharmacists in health care delivery: vital members of the cancer care team. J. Oncol. Pract. 2014;10(3):e142-e145.
12. Morgan KP, Muluneh B, Dean AM, Amerine LB. Impact of an integrated oral chemotherapy program on patient adherence. J Oncol Pharm Pract. 2018;24(5):332-336.
13. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869-2879.
14. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774-784.
15. Mora E, Smith EM, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. 2016;6(11):2416-2430.
16. Hershman DL, Lacchetti C, Dworkin RH, et al; American Society of Clinical Oncology. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967
17. Duregon F, Vendramin B, Bullo V, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90-100.
Occupational Hazard: Disruptive Behavior in Patients
While private or other public health care organizations can refuse to care for patients who have displayed disruptive behavior (DB), the VA Response to Disruptive Behavior of Patients law (38 CFR §17.107) prohibits the Veterans Health Administration (VHA) of the Department of Veterans Affairs (VA) from refusing care to veterans who display DB.1 The VHA defines DB as any behavior that is intimidating, threatening, or dangerous or that has, or could, jeopardize the health or safety of patients, VHA staff, or others.2
VA Response to DB Law
The VA Response to Disruptive Behavior of Patients requires the VHA to provide alternative care options that minimize risk while ensuring services; for example, providing care at a different location and/or time when additional staff are available to assist and monitor the patient. This can provide a unique opportunity to capture data on DB and the results of alternative forms of caring for this population.
The reason public health care organizations refuse care to persons who display DB is clear: DBs hinder business operations, are financially taxing, and put health care workers at risk.3-10 “In 2009, the VHA spent close to $5.5 million on workers’ compensation and medical expenditures for 425 incidents–or about $130,000 per DB incident (Hodgson M, Drummond D, Van Male L. Unpublished data, 2010).” In another study, 106 of 762 nurses in 1 hospital system reported an assault by a patient, and 30 required medical attention, which resulted in a total cost of $94,156.8 From 2002 to 2013, incidents of serious workplace violence requiring days off for an injured worker to recover on average were 4 times more common in health care than in other industries.6-11 Incidents of patient violence and aggression toward staff transcend specialization; however, hospital nurses and staff from the emergency, rehabilitation and gerontology departments, psychiatric unit, and home-based services are more susceptible and vulnerable to DB incidents than are other types of employees.8,10-19
Data reported by health care staff suggest that patients rather than staff members or visitors initiate > 70% of serious physical attacks against health care workers.9,13,20-23 A 2015 study of VHA health care providers (HCPs) found that > 60% had experienced some form of DB, verbal abuse being the most prevalent, followed by sexual abuse and physical abuse.20 Of 72,000 VHA staff responding to a nationwide survey, 13% experienced, on average, ≥ 1 assault by a veteran (eg, something was thrown at them; they were pushed, kicked, slapped; or were threatened or injured by a weapon).8,21
To meet its legal obligations and deliver empathetic care, the VHA documents and analyzes data on all patients who exhibit DB. A local DB Committee (DBC) reviews the data, whether it occurs in an inpatient or outpatient setting, such as community-based outpatient clinics. Once a DB incident is reported, the DBC begins an evidence-based risk evaluation, including the option of contacting the persons who displayed or experienced the DB. Goals are to (1) prevent future DB incidents; (2) detect vulnerabilities in the environment; and (3) collaborate with HCPs and patients to provide optimal care while improving the patient/provider interactions.
Effects of Disruptive Behavior
DB has negative consequences for both patients and health care workers and results in poor evaluations of care from both groups.27-32 Aside from interfering with safe medical care, DB also impacts care for other patients by delaying access to care and increasing appointment wait times due to employee absenteeism and staff shortages.3,4,20,32,33 For HCPs, patient violence is associated with unwillingness to provide care, briefer treatment periods, and decreases in occupational satisfaction, performance, and commitment
Harmful health effects experienced by HCPs who have been victims of DB include fear, mood disorders, anxiety, all symptoms of psychological distress and posttraumatic stress disorder (PTSD).10,22,30,34-36 In a study of the impact on productivity of PTSD triggered by job-related DB, PTSD symptoms were associated with withdrawal from or minimizing encounters with patients, job turnover, and troubles with thinking
Reporting Disruptive Behavior
The literature suggests that consistent and effective DB reporting is pivotal to improving the outcome and quality of care for those displaying DB.37-39 To provide high-quality health services to veterans who display DB, the VHA must promote the management and reporting of DB. Without knowledge of the full spectrum of DB events at VHA facilities, efforts to prevent or manage DB and ensure safety may have limited impact.7,37 Reports can be used for clinical decision making to optimize staff training in delivery of quality care while assuring staff safety. More than 80% of DB incidents occur during interactions with patients, thus this is a clinical issue that can affect the outcome of patient care.8,21
Documented DB reports are used to analyze the degree, frequency, and nature of incidents, which might reveal risk factors and develop preventive efforts and training for specific hazards.8,39 Some have argued that implementing a standardized DB reporting system is a crucial first step toward minimizing hazards and improving health care.38,40,41
When DB incidents were recorded through a hospital electronic reporting system and discussed in meetings, staff reported: (1) increased awareness of DB; (2) improved ability to manage DB incidents; and (3) amplified reporting of incidents.38,41,42 These findings support similar results from studies of an intervention implemented at VA Community Living Centers (CLCs) from 2013 to 2017: Staff Training in Assisted Living Residences (STAR-VA).4,12,19 The aim of STAR-VA was to minimize challenging dementia-related DB in CLCs. The intervention initially was established to train direct-care, assisted-living staff to provide better care to older patients displaying DB. Data revealed that documentation of DBs was, the first step to ensuring staff and patient safety.18,40
VHA Reporting System
In 2013, the VA Office of Inspector General (OIG) found no standardized documentation of DB events across the VA health care system.42 Instead, DB events were documented in multiple records in various locations, including administrative and progress notes in the electronic health record (EHR), police reports, e-mails, or letters submitted to DBC chairs.42 This situation reduced administrators’ ability to consider all relevant information and render appropriate decisions in DB cases.42 In 2015, based on OIG recommendations, the VHA implemented the Disruptive Behavior Reporting System (DBRS) nationwide, which allowed all VHA staff to report DB events. The DBRS was designed to address factors likely to impede reporting and management of DB, namely, complexity of and lack of access to a central reporting system.43,44 The DBRS is currently the primary VHA tool to document DB events.
The DBRS consists of 32 questions in 5 sections relating to the (1) location and time of DB event; (2) reporter; (3) disrupter; (4) DB event details; and (5) the person who experienced (experiencer) the event. The system also provides a list of the types of DB, such as inappropriate communication, bullying and/or intimidation, verbal or written threat of physical harm, physical violence, sexual harassment, sexual assault, and property damage. The DBRS has the potential to provide useful data on DB and DB reporting, such as the typical staff entering data and the number and/or types of DB occurring.
The DBRS complements the preexisting VHA policies and committees for care of veterans who display DB.1-3,14,21,24,25 The VHA Workplace Violence Prevention Program (WVPP) required facilities to submit data on DB events through a Workplace Behavioral Risk report. Data for the report were obtained from police reports, patient safety reports, DBC records, and notes in the EHR. Following implementations of DBRS, the number of DB events per year became a part of facility performance standards.
VHA is creating novel approaches to handling DB that allow health care workers to render care in a safe and effective manner guided by documented information. For example, DBCs can recommend the use of Category I Patient Record Flags (PRFs) following documented DB, which informs staff of the potential risk of DB and provides guidance on protective methods to use when meeting with the patient.2,21,24 A survey of 140 VA hospital chiefs of staff indicated that DBC procedures were related to a decrease in the rates of assaults.1 Additionally, VA provides training for staff in techniques to promote personal safety, such as identifying signs that precede DB, using verbal deescalation, and practicing therapeutic containment.
Resistance to Reporting
Many health care employees and employers are reticent to report DBs.22,31,43,45-48 Studies suggest health care organizations can cultivate a culture that is resistant to reporting DB.49,50 This complicates the ability of the health care system to design and maintain safety protocols and safer treatment plans.3,41,51 Worldwide, < 30% of DBs are reported.47 One barrier may be that supervisors may not wish to acknowledge DBs on their units or may not provide sufficient staff time for training or reporting.31,46,47 HCPs may worry that a DB report will stigmatize patients, especially those who are elderly or have cognitive impairment, brain injury, psychological illness, or developmental disability. Patients with cognitive conditions are reportedly 20% more likely to be violent toward caregivers and providers.31 A dementia diagnosis, for example, is associated with a high likelihood for DB.30,52 More than 80% of DB events displayed by patients with dementia may go unreported.26,31,50,52
Some clinicians may attribute DB to physiologic conditions that need to be treated, not reported. However, employers can face various legal liabilities if steps are not taken to protect employees.47,51 Federal and state statutes require that organizations provide a healthy and safe employment environment for workers. This requires that employers institute reasonable protective measures, such as procedures to intervene, policies on addressing DB incidents, and/or training to minimize or deescalate DB.51,53 Also, employees may sue employers if security measures are inadequate or deficient in properly investigating current and past evidence of DB or identifying vulnerabilities in the workplace. Unwillingness to investigate DB and safety-related workplace concerns have contributed to increased workplace violence and legal liability.52,53 The mission of caring and trust is consistent with assuring a safe environment.
Training and Empathetic Care
To combat cultural resistance to reporting DBs, more and perhaps different contextual approaches to education and training may be needed that address ethical dilemmas and concerns of providers. The success of training relies on administrators supporting staff in reporting DB. Training must address providers’ conflicting beliefs and assist with identifying strategies to provide the best possible care for patients who display DB.1,38 HCPs are less likely to document a DB if they feel that administrators are creating documentation that will have negative consequences for a patient. Thus, leadership is responsible for ensuring that misconceptions are dispelled through training and other efforts and information on how reported DB data will be used is communicated through strategic channels.
Education and training must consider empathic care that attempts to understand why patients behave as they do through the information gathered.55 Empathy in health care is multifaceted: It involves comprehending a patient’s viewpoint, circumstances, and feelings and the capacity to analyze whether one is comprehending these accurately in order to demonstrate supportive care.54,55
Improving patient and staff interaction once a problematic behavior is identified is the aim of empathic care. Increasing empathic care can improve compassionate, patient-centered interactions that begin once the patient seeks care. This approach has proven to decrease DB by patients with dementia and improve their care, lessen staff problems during interactions, and increase staff morale.20 Experts call for the adoption of an interpersonal approach to patient encounters, and there is evidence that creating organizational change by moving toward compassionate care can lead to a positive impact for patients.54,55
Future Studies
There are growth opportunities in utilization of the DBRS. Analysis of the DBRS database by the VA Central Office (VACO) showed that the system is underutilized by facilities across the VA system.56 In response to this current underutilization, VACO is taking steps to close these gaps through increasing training to staff and promotion of the use of the DBRS. A 2015 pilot study of VHA providers showed that > 70% of providers had experienced a DB as defined by VHA, but only 34% of them reported their most recently experienced DB within the past 12 months.20 Thus, DBRS use must be studied within the context that patient-perpetrated DB is underreported in health care organizations.5,9,29,41,43,57,58 Studies addressing national DBRS utilization patterns and the cost associated with implementing the DBRS also are needed. One study suggests that there is an association between measures of facility complexity and staff perceptions of safety, which should be considered in analyzing DBRS usage.57 Studies addressing the role of the DBRS and misconceptions that the tool may represent a punitive tool also are needed. VHA should consider how the attribution “disruptive behavior” assigns a negative connotation and leads HCPs to avoid using the DBRS. Additionally, DB reporting may increase when HCPs understand that DB reporting is part of the comprehensive, consultative strategy to provide the best care to patients.
Conclusion
Accurate reporting of DB events enables the development of strategies for multidisciplinary teams to work together to minimize hazards and to provide interventions that provide for the safe delivery of health care to all patients. Improving reporting ensures there is an accurate representation of how disruptive events impact care provided within a facility—and what types of variables may be associated with increased risk for these types of events.
Additionally, ensuring that reporting is maximized also provides the VHA with opportunities for DBCs to offer evidence-based risk assessment of violence and consultation to staff members who may benefit from improved competencies in working with patients who display DB. These potential improvements are consistent with the VHA I CARE values and will provide data that can inform recommendations for health care in other agencies/health care organizations.
Acknowledgments
This work was supported by the Center of Innovation on Disability and Rehabilitation Research (CINDRR) of the Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs.
1. Hodgson MJ, Mohr DC, Drummond DJ, Bell M, Van Male L. Managing disruptive patients in health care: necessary solutions to a difficult problem. Am J Ind Med. 2012;55(11):1009-1017.
2. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2010-053. Patient Record Flags. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2341 Published December 3, 2010. Accessed March 29, 2019.
3. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2012-026. Sexual Assaults and Other Defined Public Safety Incidents in VHA Facilities. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2797. Published September 27, 2012. Accessed March 29, 2019.
4. Curyto KJ, McCurry SM, Luci K, Karlin BE, Teri L, Karel MJ. Managing challenging behaviors of dementia in veterans: identifying and changing activators and consequences using STAR-VA. J Gerontol Nurs. 2017;43(2):33-43.
5. Speroni KG, Fitch T, Dawson E, Dugan L, Atherton M. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors. J Emerg Nurs. 2014;40(3):218-228.
6. Phillips JP. Workplace violence against health care workers in the United States. NEJM. 2016;374(17):1661-1669.
7. Janocha JA, Smith RT. Workplace safety and health in the health care and social assistance industry, 2003–07. https://www.bls.gov/opub/mlr/cwc/workplace-safety-and-health-in-the-health-care-and-social-assistance-industry-2003-07.pdf. Published August 30, 2010. Accessed February 19, 2019.
8. US Department of Labor, Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. https://www.osha.gov/Publications/OSHA3826.pdf. Published December 2015. Accessed February 19, 2019.
9. US Department of Labor, Occupational Safety and Health Administration. Prevention of Workplace Violence in Healthcare and Social Assistance. Occupational Safety and Health Administration, https://www.govinfo.gov/content/pkg/FR-2016-12-07/pdf/2016-29197.pdf. Accessed January 20, 2017.
10. Gerberich SG, Church TR, McGovern PM, et al. An epidemiological study of the magnitude and consequences of work related violence: the Minnesota Nurses’ Study. Occup Environ Med. 2004;61(6):495-503.
11. Sherman MF, Gershon RRM, Samar SM, Pearson JM, Canton AN, Damsky MR. Safety factors predictive of job satisfaction and job retention among home healthcare aides. J Occup Environ Med. 2008;50(12):1430-1441.
12. Karel MJ, Teri L, McConnell E, Visnic S, Karlin BE. Effectiveness of expanded implementation of STAR-VA for managing dementia-related behaviors among veterans. Gerontologist. 2016;56(1):126-134.
13. US Department of Labor, Bureau of Labor Statistics. Nonfatal occupational injuries and illnesses requiring days away from work. https://www.bls.gov/news.release/archives/osh2_11192015.htm. Published November 19, 2015.
14. Beech B, Leather P. Workplace violence in the health care sector: A review of staff training and integration of training evaluation models. Aggression Violent Behav. 2006;11(1):27-43.
15. Campbell CL, McCoy S, Burg MA, Hoffman N. Enhancing home care staff safety through reducing client aggression and violence in noninstitutional care settings: a systematic review. Home Health Care Manage Pract. 2014;26(1):3-10.
16. Gallant-Roman MA. Strategies and tools to reduce workplace violence. AAOHNJ. 2008;56(11):449-454.
17. Weinberger LE, Sreenivasan S, Smee DE, McGuire J, Garrick T. Balancing safety against obstruction to health care access: an examination of behavioral flags in the VA health care system. J Threat Assess Manage. 2018;5(1):35-41.
18. Elbogen EB, Johnson SC, Wagner HR, et al. Protective factors and risk modification of violence in Iraq and Afghanistan war veterans. J Clin Psychiatry. 2012;73(6):e767-e773.
19. Karlin BE, Visnic S, McGee JS, Teri L. Results from the multisite implementation of STAR-VA: a multicomponent psychosocial intervention for managing challenging dementia-related behaviors of veterans. Psychol Serv. 2014;11(2):200-208.
20. Semeah LM, Campbell CL, Cowper DC, Peet AC. Serving our homeless veterans: patient perpetrated violence as a barrier to health care access. J Pub Nonprofit Aff. 2017;3(2):223-234.
21. Hodgson MJ, Reed R, Craig T, et al. Violence in healthcare facilities: lessons from the Veterans Health Administration. J Occup Environ Med. 2004;46(11):1158-1165.
22. Farrell GA, Bobrowski C, Bobrowski P. Scoping workplace aggression in nursing: findings from an Australian study. J Adv Nurs. 2006;55(6):778-787.
23. Barling J, Rogers AG, Kelloway EK. Behind closed doors: in-home workers’ experience of sexual harassment and workplace violence. J Occup Health Psychol. 2001;6(3):255-269.
24. Pompeii LA, Schoenfisch AL, Lipscomb HJ, Dement JM, Smith CD, Upadhyaya M. Physical assault, physical threat, and verbal abuse perpetrated against hospital workers by patients or visitors in six U.S. hospitals. Am J Ind Med. 2015;58(11):1194-1204.
25. Sippel LM, Mota NP, Kachadourian LK, et al. The burden of hostility in U.S. veterans: results from the National Health and Resilience in Veterans Study. Psychiatry Res. 2016;243(suppl C):421-430.
26. Campbell C. Patient Violence and Aggression in Non-Institutional Health Care Settings: Predictors of Reporting By Healthcare Providers [doctoral dissertation]. Orlando: University of Central Florida; 2016.
27. Galinsky T, Feng HA, Streit J, et al. Risk factors associated with patient assaults of home healthcare workers. Rehabil Nurs. 2010;35(5):206-215.
28. Campbell CL. Incident reporting by health-care workers in noninstitutional care settings. Trauma, Violence Abuse. 2017;18(4):445-456.
29. Arnetz JE, Arnetz BB. Violence towards health care staff and possible effects on the quality of patient care. Soc Sci Med. 2001;52(3):417-427.
30. Gates D, Fitzwater E, Succop P. Relationships of stressors, strain, and anger to caregiver assaults. Issues Ment Health Nurs. 2003;24(8):775-793.
31. Brillhart B, Kruse B, Heard L. Safety concerns for rehabilitation nurses in home care. Rehabil Nurs. 2004;29(6):227-229.
32. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10(3):40-42.
33. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. The Joint Commission releases results of surveys of the VA health care system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2808. Updated August 5, 2014. Accessed February 19, 2019.
34. Büssing A, Höge T. Aggression and violence against home care workers. J Occup Health Psychol. 2004;9(3):206-219.
35. Geiger-Brown J, Muntaner C, McPhaul K, Lipscomb J, Trinkoff A. Abuse and violence during home care work as predictor of worker depression. Home Health Care Serv Q. 2007;26(1):59-77.
36. Gates DM, Gillespie GL, Succop P. Violence against nurses and its impact on stress and productivity. Nurs Econ. 2011;29(2):59-66.
37. Petterson IL, Arnetz BB. Psychosocial stressors and well-being in health care workers: the impact of an intervention program. Soc Sci Med. 1998;47(11):1763-1772.
38. Arnetz JE, Arnetz BB. Implementation and evaluation of a practical intervention programme for dealing with violence towards health care workers. J Adv Nurs. 2000;31(3):668-680.
39. Arnetz JE, Hamblin L, Russell J, et al. Preventing patient-to-worker violence in hospitals: outcome of a randomized controlled intervention. J Occup Environ Med. 2017;59(1):18-27.
40. Elbogen EB, Tomkins AJ, Pothuloori AP, Scalora MJ. Documentation of violence risk information in psychiatric hospital patient charts: an empirical examination. J Am Acad Psychiatry Law. 2003;31(1):58-64.
41. Winsvold Prang I, Jelson-Jorgensen LP. Should I report? A qualitative study of barriers to incident reporting among nurses working in nursing homes. Geriatr Nurs. 2014;35(6):441-447.
42. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: management of disruptive patient behavior at VA medical facilities. Report No. 11-02585-129. https://www.va.gov/oig/pubs/VAOIG-11-02585-129.pdf. Published Mrach 7, 2013. Accessed February 21, 2019.
43. Lipscomb J, London M. Not Part of the Job: How to Take a Stand Against Violence in the Work Setting. Silver Spring, MD: American Nurses Association; 2015.
44. May DD, Grubbs LM. The extent, nature, and precipitating factors of nurse assault among three groups of registered nurses in a regional medical center. J Emerg Nurs. 2002;28(1):11-17.
45. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57(5):460-477.
46. Brennan C, Worrall-Davies A, McMillan D, Gilbody S, House A. The hospital anxiety and depression scale: a diagnostic meta-analysis of case-finding ability. J Psychosom Res. 2010;69(4):371-378.
47. McPhaul K, Lipscomb J, Johnson J. Assessing risk for violence on home health visits. Home Healthc Nurse. 2010;28(5):278-289.
48. McPhaul KM, London M, Murrett K, Flannery K, Rosen J, Lipscomb J. Environmental evaluation for workplace violence in healthcare and social services. J Safety Res. 2008;39(2):237-250.
49. Kelly JA, Somlai AM, DiFranceisco WJ, et al. Bridging the gap between the science and service of HIV prevention: transferring effective research-based HIV prevention interventions to community AIDS service providers. Am J Public Health. 2000;90(7):1082-1088.
50. Pawlin S. Reporting violence. Emerg Nurse. 2008;16(4):16-21.
51. Brakel SJ. Legal liability and workplace violence. J Am Acad Psychiatry Law. 1998;26(4):553-562.
52. Neuman JH, Baron RA. Workplace violence and workplace aggression: evidence concerning specific forms, potential causes, and preferred targets. J Manage. 1998;24(3):391-419.53. Ferns T, Chojnacka I. Angels and swingers, matrons and sinners: nursing stereotypes. Br J Nurs. 2005;14(19):1028-1032.
54. Mercer SW, Reynolds WJ. Empathy and quality of care. Br J Gen Pract 2002;52(suppl):S9-S12.
55. Lee TH. An Epidemic of Empathy in Healthcare: How to Deliver Compassionate, Connected Patient Care That Creates a Competitive Advantage. Columbus, OH: McGraw-Hill Education; 2015.
56. US Department of Veterans Affairs, Veterans Health Administrastion. Veterans Health Administration workplace violence prevention program (WVPP): disruptive behavior reporting system utilization report. Published 2017. https://vaww.portal2.va.gov/sites/wvpp/Shared%20Documents/DBRS%20Utilization%20Reports/FY2017%20DBRS%20Quarterly%20Utilization%20Report%20(Quarter%201).pdf. [Source not verified.]
57. Campbell CL, Burg, MA, Gammonley D. Measures for incident reporting of patient violence and aggression towards healthcare providers: a systematic review. Aggression Violent Behav. 2015;25(part B):314-322.
58. Carney PT, West P, Neily J, Mills PD, Bagian JP. The effect of facility complexity on perceptions of safety climate in the operating room: size matters. Am J Med Qual. 2010;25(6):457-461.
While private or other public health care organizations can refuse to care for patients who have displayed disruptive behavior (DB), the VA Response to Disruptive Behavior of Patients law (38 CFR §17.107) prohibits the Veterans Health Administration (VHA) of the Department of Veterans Affairs (VA) from refusing care to veterans who display DB.1 The VHA defines DB as any behavior that is intimidating, threatening, or dangerous or that has, or could, jeopardize the health or safety of patients, VHA staff, or others.2
VA Response to DB Law
The VA Response to Disruptive Behavior of Patients requires the VHA to provide alternative care options that minimize risk while ensuring services; for example, providing care at a different location and/or time when additional staff are available to assist and monitor the patient. This can provide a unique opportunity to capture data on DB and the results of alternative forms of caring for this population.
The reason public health care organizations refuse care to persons who display DB is clear: DBs hinder business operations, are financially taxing, and put health care workers at risk.3-10 “In 2009, the VHA spent close to $5.5 million on workers’ compensation and medical expenditures for 425 incidents–or about $130,000 per DB incident (Hodgson M, Drummond D, Van Male L. Unpublished data, 2010).” In another study, 106 of 762 nurses in 1 hospital system reported an assault by a patient, and 30 required medical attention, which resulted in a total cost of $94,156.8 From 2002 to 2013, incidents of serious workplace violence requiring days off for an injured worker to recover on average were 4 times more common in health care than in other industries.6-11 Incidents of patient violence and aggression toward staff transcend specialization; however, hospital nurses and staff from the emergency, rehabilitation and gerontology departments, psychiatric unit, and home-based services are more susceptible and vulnerable to DB incidents than are other types of employees.8,10-19
Data reported by health care staff suggest that patients rather than staff members or visitors initiate > 70% of serious physical attacks against health care workers.9,13,20-23 A 2015 study of VHA health care providers (HCPs) found that > 60% had experienced some form of DB, verbal abuse being the most prevalent, followed by sexual abuse and physical abuse.20 Of 72,000 VHA staff responding to a nationwide survey, 13% experienced, on average, ≥ 1 assault by a veteran (eg, something was thrown at them; they were pushed, kicked, slapped; or were threatened or injured by a weapon).8,21
To meet its legal obligations and deliver empathetic care, the VHA documents and analyzes data on all patients who exhibit DB. A local DB Committee (DBC) reviews the data, whether it occurs in an inpatient or outpatient setting, such as community-based outpatient clinics. Once a DB incident is reported, the DBC begins an evidence-based risk evaluation, including the option of contacting the persons who displayed or experienced the DB. Goals are to (1) prevent future DB incidents; (2) detect vulnerabilities in the environment; and (3) collaborate with HCPs and patients to provide optimal care while improving the patient/provider interactions.
Effects of Disruptive Behavior
DB has negative consequences for both patients and health care workers and results in poor evaluations of care from both groups.27-32 Aside from interfering with safe medical care, DB also impacts care for other patients by delaying access to care and increasing appointment wait times due to employee absenteeism and staff shortages.3,4,20,32,33 For HCPs, patient violence is associated with unwillingness to provide care, briefer treatment periods, and decreases in occupational satisfaction, performance, and commitment
Harmful health effects experienced by HCPs who have been victims of DB include fear, mood disorders, anxiety, all symptoms of psychological distress and posttraumatic stress disorder (PTSD).10,22,30,34-36 In a study of the impact on productivity of PTSD triggered by job-related DB, PTSD symptoms were associated with withdrawal from or minimizing encounters with patients, job turnover, and troubles with thinking
Reporting Disruptive Behavior
The literature suggests that consistent and effective DB reporting is pivotal to improving the outcome and quality of care for those displaying DB.37-39 To provide high-quality health services to veterans who display DB, the VHA must promote the management and reporting of DB. Without knowledge of the full spectrum of DB events at VHA facilities, efforts to prevent or manage DB and ensure safety may have limited impact.7,37 Reports can be used for clinical decision making to optimize staff training in delivery of quality care while assuring staff safety. More than 80% of DB incidents occur during interactions with patients, thus this is a clinical issue that can affect the outcome of patient care.8,21
Documented DB reports are used to analyze the degree, frequency, and nature of incidents, which might reveal risk factors and develop preventive efforts and training for specific hazards.8,39 Some have argued that implementing a standardized DB reporting system is a crucial first step toward minimizing hazards and improving health care.38,40,41
When DB incidents were recorded through a hospital electronic reporting system and discussed in meetings, staff reported: (1) increased awareness of DB; (2) improved ability to manage DB incidents; and (3) amplified reporting of incidents.38,41,42 These findings support similar results from studies of an intervention implemented at VA Community Living Centers (CLCs) from 2013 to 2017: Staff Training in Assisted Living Residences (STAR-VA).4,12,19 The aim of STAR-VA was to minimize challenging dementia-related DB in CLCs. The intervention initially was established to train direct-care, assisted-living staff to provide better care to older patients displaying DB. Data revealed that documentation of DBs was, the first step to ensuring staff and patient safety.18,40
VHA Reporting System
In 2013, the VA Office of Inspector General (OIG) found no standardized documentation of DB events across the VA health care system.42 Instead, DB events were documented in multiple records in various locations, including administrative and progress notes in the electronic health record (EHR), police reports, e-mails, or letters submitted to DBC chairs.42 This situation reduced administrators’ ability to consider all relevant information and render appropriate decisions in DB cases.42 In 2015, based on OIG recommendations, the VHA implemented the Disruptive Behavior Reporting System (DBRS) nationwide, which allowed all VHA staff to report DB events. The DBRS was designed to address factors likely to impede reporting and management of DB, namely, complexity of and lack of access to a central reporting system.43,44 The DBRS is currently the primary VHA tool to document DB events.
The DBRS consists of 32 questions in 5 sections relating to the (1) location and time of DB event; (2) reporter; (3) disrupter; (4) DB event details; and (5) the person who experienced (experiencer) the event. The system also provides a list of the types of DB, such as inappropriate communication, bullying and/or intimidation, verbal or written threat of physical harm, physical violence, sexual harassment, sexual assault, and property damage. The DBRS has the potential to provide useful data on DB and DB reporting, such as the typical staff entering data and the number and/or types of DB occurring.
The DBRS complements the preexisting VHA policies and committees for care of veterans who display DB.1-3,14,21,24,25 The VHA Workplace Violence Prevention Program (WVPP) required facilities to submit data on DB events through a Workplace Behavioral Risk report. Data for the report were obtained from police reports, patient safety reports, DBC records, and notes in the EHR. Following implementations of DBRS, the number of DB events per year became a part of facility performance standards.
VHA is creating novel approaches to handling DB that allow health care workers to render care in a safe and effective manner guided by documented information. For example, DBCs can recommend the use of Category I Patient Record Flags (PRFs) following documented DB, which informs staff of the potential risk of DB and provides guidance on protective methods to use when meeting with the patient.2,21,24 A survey of 140 VA hospital chiefs of staff indicated that DBC procedures were related to a decrease in the rates of assaults.1 Additionally, VA provides training for staff in techniques to promote personal safety, such as identifying signs that precede DB, using verbal deescalation, and practicing therapeutic containment.
Resistance to Reporting
Many health care employees and employers are reticent to report DBs.22,31,43,45-48 Studies suggest health care organizations can cultivate a culture that is resistant to reporting DB.49,50 This complicates the ability of the health care system to design and maintain safety protocols and safer treatment plans.3,41,51 Worldwide, < 30% of DBs are reported.47 One barrier may be that supervisors may not wish to acknowledge DBs on their units or may not provide sufficient staff time for training or reporting.31,46,47 HCPs may worry that a DB report will stigmatize patients, especially those who are elderly or have cognitive impairment, brain injury, psychological illness, or developmental disability. Patients with cognitive conditions are reportedly 20% more likely to be violent toward caregivers and providers.31 A dementia diagnosis, for example, is associated with a high likelihood for DB.30,52 More than 80% of DB events displayed by patients with dementia may go unreported.26,31,50,52
Some clinicians may attribute DB to physiologic conditions that need to be treated, not reported. However, employers can face various legal liabilities if steps are not taken to protect employees.47,51 Federal and state statutes require that organizations provide a healthy and safe employment environment for workers. This requires that employers institute reasonable protective measures, such as procedures to intervene, policies on addressing DB incidents, and/or training to minimize or deescalate DB.51,53 Also, employees may sue employers if security measures are inadequate or deficient in properly investigating current and past evidence of DB or identifying vulnerabilities in the workplace. Unwillingness to investigate DB and safety-related workplace concerns have contributed to increased workplace violence and legal liability.52,53 The mission of caring and trust is consistent with assuring a safe environment.
Training and Empathetic Care
To combat cultural resistance to reporting DBs, more and perhaps different contextual approaches to education and training may be needed that address ethical dilemmas and concerns of providers. The success of training relies on administrators supporting staff in reporting DB. Training must address providers’ conflicting beliefs and assist with identifying strategies to provide the best possible care for patients who display DB.1,38 HCPs are less likely to document a DB if they feel that administrators are creating documentation that will have negative consequences for a patient. Thus, leadership is responsible for ensuring that misconceptions are dispelled through training and other efforts and information on how reported DB data will be used is communicated through strategic channels.
Education and training must consider empathic care that attempts to understand why patients behave as they do through the information gathered.55 Empathy in health care is multifaceted: It involves comprehending a patient’s viewpoint, circumstances, and feelings and the capacity to analyze whether one is comprehending these accurately in order to demonstrate supportive care.54,55
Improving patient and staff interaction once a problematic behavior is identified is the aim of empathic care. Increasing empathic care can improve compassionate, patient-centered interactions that begin once the patient seeks care. This approach has proven to decrease DB by patients with dementia and improve their care, lessen staff problems during interactions, and increase staff morale.20 Experts call for the adoption of an interpersonal approach to patient encounters, and there is evidence that creating organizational change by moving toward compassionate care can lead to a positive impact for patients.54,55
Future Studies
There are growth opportunities in utilization of the DBRS. Analysis of the DBRS database by the VA Central Office (VACO) showed that the system is underutilized by facilities across the VA system.56 In response to this current underutilization, VACO is taking steps to close these gaps through increasing training to staff and promotion of the use of the DBRS. A 2015 pilot study of VHA providers showed that > 70% of providers had experienced a DB as defined by VHA, but only 34% of them reported their most recently experienced DB within the past 12 months.20 Thus, DBRS use must be studied within the context that patient-perpetrated DB is underreported in health care organizations.5,9,29,41,43,57,58 Studies addressing national DBRS utilization patterns and the cost associated with implementing the DBRS also are needed. One study suggests that there is an association between measures of facility complexity and staff perceptions of safety, which should be considered in analyzing DBRS usage.57 Studies addressing the role of the DBRS and misconceptions that the tool may represent a punitive tool also are needed. VHA should consider how the attribution “disruptive behavior” assigns a negative connotation and leads HCPs to avoid using the DBRS. Additionally, DB reporting may increase when HCPs understand that DB reporting is part of the comprehensive, consultative strategy to provide the best care to patients.
Conclusion
Accurate reporting of DB events enables the development of strategies for multidisciplinary teams to work together to minimize hazards and to provide interventions that provide for the safe delivery of health care to all patients. Improving reporting ensures there is an accurate representation of how disruptive events impact care provided within a facility—and what types of variables may be associated with increased risk for these types of events.
Additionally, ensuring that reporting is maximized also provides the VHA with opportunities for DBCs to offer evidence-based risk assessment of violence and consultation to staff members who may benefit from improved competencies in working with patients who display DB. These potential improvements are consistent with the VHA I CARE values and will provide data that can inform recommendations for health care in other agencies/health care organizations.
Acknowledgments
This work was supported by the Center of Innovation on Disability and Rehabilitation Research (CINDRR) of the Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs.
While private or other public health care organizations can refuse to care for patients who have displayed disruptive behavior (DB), the VA Response to Disruptive Behavior of Patients law (38 CFR §17.107) prohibits the Veterans Health Administration (VHA) of the Department of Veterans Affairs (VA) from refusing care to veterans who display DB.1 The VHA defines DB as any behavior that is intimidating, threatening, or dangerous or that has, or could, jeopardize the health or safety of patients, VHA staff, or others.2
VA Response to DB Law
The VA Response to Disruptive Behavior of Patients requires the VHA to provide alternative care options that minimize risk while ensuring services; for example, providing care at a different location and/or time when additional staff are available to assist and monitor the patient. This can provide a unique opportunity to capture data on DB and the results of alternative forms of caring for this population.
The reason public health care organizations refuse care to persons who display DB is clear: DBs hinder business operations, are financially taxing, and put health care workers at risk.3-10 “In 2009, the VHA spent close to $5.5 million on workers’ compensation and medical expenditures for 425 incidents–or about $130,000 per DB incident (Hodgson M, Drummond D, Van Male L. Unpublished data, 2010).” In another study, 106 of 762 nurses in 1 hospital system reported an assault by a patient, and 30 required medical attention, which resulted in a total cost of $94,156.8 From 2002 to 2013, incidents of serious workplace violence requiring days off for an injured worker to recover on average were 4 times more common in health care than in other industries.6-11 Incidents of patient violence and aggression toward staff transcend specialization; however, hospital nurses and staff from the emergency, rehabilitation and gerontology departments, psychiatric unit, and home-based services are more susceptible and vulnerable to DB incidents than are other types of employees.8,10-19
Data reported by health care staff suggest that patients rather than staff members or visitors initiate > 70% of serious physical attacks against health care workers.9,13,20-23 A 2015 study of VHA health care providers (HCPs) found that > 60% had experienced some form of DB, verbal abuse being the most prevalent, followed by sexual abuse and physical abuse.20 Of 72,000 VHA staff responding to a nationwide survey, 13% experienced, on average, ≥ 1 assault by a veteran (eg, something was thrown at them; they were pushed, kicked, slapped; or were threatened or injured by a weapon).8,21
To meet its legal obligations and deliver empathetic care, the VHA documents and analyzes data on all patients who exhibit DB. A local DB Committee (DBC) reviews the data, whether it occurs in an inpatient or outpatient setting, such as community-based outpatient clinics. Once a DB incident is reported, the DBC begins an evidence-based risk evaluation, including the option of contacting the persons who displayed or experienced the DB. Goals are to (1) prevent future DB incidents; (2) detect vulnerabilities in the environment; and (3) collaborate with HCPs and patients to provide optimal care while improving the patient/provider interactions.
Effects of Disruptive Behavior
DB has negative consequences for both patients and health care workers and results in poor evaluations of care from both groups.27-32 Aside from interfering with safe medical care, DB also impacts care for other patients by delaying access to care and increasing appointment wait times due to employee absenteeism and staff shortages.3,4,20,32,33 For HCPs, patient violence is associated with unwillingness to provide care, briefer treatment periods, and decreases in occupational satisfaction, performance, and commitment
Harmful health effects experienced by HCPs who have been victims of DB include fear, mood disorders, anxiety, all symptoms of psychological distress and posttraumatic stress disorder (PTSD).10,22,30,34-36 In a study of the impact on productivity of PTSD triggered by job-related DB, PTSD symptoms were associated with withdrawal from or minimizing encounters with patients, job turnover, and troubles with thinking
Reporting Disruptive Behavior
The literature suggests that consistent and effective DB reporting is pivotal to improving the outcome and quality of care for those displaying DB.37-39 To provide high-quality health services to veterans who display DB, the VHA must promote the management and reporting of DB. Without knowledge of the full spectrum of DB events at VHA facilities, efforts to prevent or manage DB and ensure safety may have limited impact.7,37 Reports can be used for clinical decision making to optimize staff training in delivery of quality care while assuring staff safety. More than 80% of DB incidents occur during interactions with patients, thus this is a clinical issue that can affect the outcome of patient care.8,21
Documented DB reports are used to analyze the degree, frequency, and nature of incidents, which might reveal risk factors and develop preventive efforts and training for specific hazards.8,39 Some have argued that implementing a standardized DB reporting system is a crucial first step toward minimizing hazards and improving health care.38,40,41
When DB incidents were recorded through a hospital electronic reporting system and discussed in meetings, staff reported: (1) increased awareness of DB; (2) improved ability to manage DB incidents; and (3) amplified reporting of incidents.38,41,42 These findings support similar results from studies of an intervention implemented at VA Community Living Centers (CLCs) from 2013 to 2017: Staff Training in Assisted Living Residences (STAR-VA).4,12,19 The aim of STAR-VA was to minimize challenging dementia-related DB in CLCs. The intervention initially was established to train direct-care, assisted-living staff to provide better care to older patients displaying DB. Data revealed that documentation of DBs was, the first step to ensuring staff and patient safety.18,40
VHA Reporting System
In 2013, the VA Office of Inspector General (OIG) found no standardized documentation of DB events across the VA health care system.42 Instead, DB events were documented in multiple records in various locations, including administrative and progress notes in the electronic health record (EHR), police reports, e-mails, or letters submitted to DBC chairs.42 This situation reduced administrators’ ability to consider all relevant information and render appropriate decisions in DB cases.42 In 2015, based on OIG recommendations, the VHA implemented the Disruptive Behavior Reporting System (DBRS) nationwide, which allowed all VHA staff to report DB events. The DBRS was designed to address factors likely to impede reporting and management of DB, namely, complexity of and lack of access to a central reporting system.43,44 The DBRS is currently the primary VHA tool to document DB events.
The DBRS consists of 32 questions in 5 sections relating to the (1) location and time of DB event; (2) reporter; (3) disrupter; (4) DB event details; and (5) the person who experienced (experiencer) the event. The system also provides a list of the types of DB, such as inappropriate communication, bullying and/or intimidation, verbal or written threat of physical harm, physical violence, sexual harassment, sexual assault, and property damage. The DBRS has the potential to provide useful data on DB and DB reporting, such as the typical staff entering data and the number and/or types of DB occurring.
The DBRS complements the preexisting VHA policies and committees for care of veterans who display DB.1-3,14,21,24,25 The VHA Workplace Violence Prevention Program (WVPP) required facilities to submit data on DB events through a Workplace Behavioral Risk report. Data for the report were obtained from police reports, patient safety reports, DBC records, and notes in the EHR. Following implementations of DBRS, the number of DB events per year became a part of facility performance standards.
VHA is creating novel approaches to handling DB that allow health care workers to render care in a safe and effective manner guided by documented information. For example, DBCs can recommend the use of Category I Patient Record Flags (PRFs) following documented DB, which informs staff of the potential risk of DB and provides guidance on protective methods to use when meeting with the patient.2,21,24 A survey of 140 VA hospital chiefs of staff indicated that DBC procedures were related to a decrease in the rates of assaults.1 Additionally, VA provides training for staff in techniques to promote personal safety, such as identifying signs that precede DB, using verbal deescalation, and practicing therapeutic containment.
Resistance to Reporting
Many health care employees and employers are reticent to report DBs.22,31,43,45-48 Studies suggest health care organizations can cultivate a culture that is resistant to reporting DB.49,50 This complicates the ability of the health care system to design and maintain safety protocols and safer treatment plans.3,41,51 Worldwide, < 30% of DBs are reported.47 One barrier may be that supervisors may not wish to acknowledge DBs on their units or may not provide sufficient staff time for training or reporting.31,46,47 HCPs may worry that a DB report will stigmatize patients, especially those who are elderly or have cognitive impairment, brain injury, psychological illness, or developmental disability. Patients with cognitive conditions are reportedly 20% more likely to be violent toward caregivers and providers.31 A dementia diagnosis, for example, is associated with a high likelihood for DB.30,52 More than 80% of DB events displayed by patients with dementia may go unreported.26,31,50,52
Some clinicians may attribute DB to physiologic conditions that need to be treated, not reported. However, employers can face various legal liabilities if steps are not taken to protect employees.47,51 Federal and state statutes require that organizations provide a healthy and safe employment environment for workers. This requires that employers institute reasonable protective measures, such as procedures to intervene, policies on addressing DB incidents, and/or training to minimize or deescalate DB.51,53 Also, employees may sue employers if security measures are inadequate or deficient in properly investigating current and past evidence of DB or identifying vulnerabilities in the workplace. Unwillingness to investigate DB and safety-related workplace concerns have contributed to increased workplace violence and legal liability.52,53 The mission of caring and trust is consistent with assuring a safe environment.
Training and Empathetic Care
To combat cultural resistance to reporting DBs, more and perhaps different contextual approaches to education and training may be needed that address ethical dilemmas and concerns of providers. The success of training relies on administrators supporting staff in reporting DB. Training must address providers’ conflicting beliefs and assist with identifying strategies to provide the best possible care for patients who display DB.1,38 HCPs are less likely to document a DB if they feel that administrators are creating documentation that will have negative consequences for a patient. Thus, leadership is responsible for ensuring that misconceptions are dispelled through training and other efforts and information on how reported DB data will be used is communicated through strategic channels.
Education and training must consider empathic care that attempts to understand why patients behave as they do through the information gathered.55 Empathy in health care is multifaceted: It involves comprehending a patient’s viewpoint, circumstances, and feelings and the capacity to analyze whether one is comprehending these accurately in order to demonstrate supportive care.54,55
Improving patient and staff interaction once a problematic behavior is identified is the aim of empathic care. Increasing empathic care can improve compassionate, patient-centered interactions that begin once the patient seeks care. This approach has proven to decrease DB by patients with dementia and improve their care, lessen staff problems during interactions, and increase staff morale.20 Experts call for the adoption of an interpersonal approach to patient encounters, and there is evidence that creating organizational change by moving toward compassionate care can lead to a positive impact for patients.54,55
Future Studies
There are growth opportunities in utilization of the DBRS. Analysis of the DBRS database by the VA Central Office (VACO) showed that the system is underutilized by facilities across the VA system.56 In response to this current underutilization, VACO is taking steps to close these gaps through increasing training to staff and promotion of the use of the DBRS. A 2015 pilot study of VHA providers showed that > 70% of providers had experienced a DB as defined by VHA, but only 34% of them reported their most recently experienced DB within the past 12 months.20 Thus, DBRS use must be studied within the context that patient-perpetrated DB is underreported in health care organizations.5,9,29,41,43,57,58 Studies addressing national DBRS utilization patterns and the cost associated with implementing the DBRS also are needed. One study suggests that there is an association between measures of facility complexity and staff perceptions of safety, which should be considered in analyzing DBRS usage.57 Studies addressing the role of the DBRS and misconceptions that the tool may represent a punitive tool also are needed. VHA should consider how the attribution “disruptive behavior” assigns a negative connotation and leads HCPs to avoid using the DBRS. Additionally, DB reporting may increase when HCPs understand that DB reporting is part of the comprehensive, consultative strategy to provide the best care to patients.
Conclusion
Accurate reporting of DB events enables the development of strategies for multidisciplinary teams to work together to minimize hazards and to provide interventions that provide for the safe delivery of health care to all patients. Improving reporting ensures there is an accurate representation of how disruptive events impact care provided within a facility—and what types of variables may be associated with increased risk for these types of events.
Additionally, ensuring that reporting is maximized also provides the VHA with opportunities for DBCs to offer evidence-based risk assessment of violence and consultation to staff members who may benefit from improved competencies in working with patients who display DB. These potential improvements are consistent with the VHA I CARE values and will provide data that can inform recommendations for health care in other agencies/health care organizations.
Acknowledgments
This work was supported by the Center of Innovation on Disability and Rehabilitation Research (CINDRR) of the Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs.
1. Hodgson MJ, Mohr DC, Drummond DJ, Bell M, Van Male L. Managing disruptive patients in health care: necessary solutions to a difficult problem. Am J Ind Med. 2012;55(11):1009-1017.
2. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2010-053. Patient Record Flags. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2341 Published December 3, 2010. Accessed March 29, 2019.
3. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2012-026. Sexual Assaults and Other Defined Public Safety Incidents in VHA Facilities. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2797. Published September 27, 2012. Accessed March 29, 2019.
4. Curyto KJ, McCurry SM, Luci K, Karlin BE, Teri L, Karel MJ. Managing challenging behaviors of dementia in veterans: identifying and changing activators and consequences using STAR-VA. J Gerontol Nurs. 2017;43(2):33-43.
5. Speroni KG, Fitch T, Dawson E, Dugan L, Atherton M. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors. J Emerg Nurs. 2014;40(3):218-228.
6. Phillips JP. Workplace violence against health care workers in the United States. NEJM. 2016;374(17):1661-1669.
7. Janocha JA, Smith RT. Workplace safety and health in the health care and social assistance industry, 2003–07. https://www.bls.gov/opub/mlr/cwc/workplace-safety-and-health-in-the-health-care-and-social-assistance-industry-2003-07.pdf. Published August 30, 2010. Accessed February 19, 2019.
8. US Department of Labor, Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. https://www.osha.gov/Publications/OSHA3826.pdf. Published December 2015. Accessed February 19, 2019.
9. US Department of Labor, Occupational Safety and Health Administration. Prevention of Workplace Violence in Healthcare and Social Assistance. Occupational Safety and Health Administration, https://www.govinfo.gov/content/pkg/FR-2016-12-07/pdf/2016-29197.pdf. Accessed January 20, 2017.
10. Gerberich SG, Church TR, McGovern PM, et al. An epidemiological study of the magnitude and consequences of work related violence: the Minnesota Nurses’ Study. Occup Environ Med. 2004;61(6):495-503.
11. Sherman MF, Gershon RRM, Samar SM, Pearson JM, Canton AN, Damsky MR. Safety factors predictive of job satisfaction and job retention among home healthcare aides. J Occup Environ Med. 2008;50(12):1430-1441.
12. Karel MJ, Teri L, McConnell E, Visnic S, Karlin BE. Effectiveness of expanded implementation of STAR-VA for managing dementia-related behaviors among veterans. Gerontologist. 2016;56(1):126-134.
13. US Department of Labor, Bureau of Labor Statistics. Nonfatal occupational injuries and illnesses requiring days away from work. https://www.bls.gov/news.release/archives/osh2_11192015.htm. Published November 19, 2015.
14. Beech B, Leather P. Workplace violence in the health care sector: A review of staff training and integration of training evaluation models. Aggression Violent Behav. 2006;11(1):27-43.
15. Campbell CL, McCoy S, Burg MA, Hoffman N. Enhancing home care staff safety through reducing client aggression and violence in noninstitutional care settings: a systematic review. Home Health Care Manage Pract. 2014;26(1):3-10.
16. Gallant-Roman MA. Strategies and tools to reduce workplace violence. AAOHNJ. 2008;56(11):449-454.
17. Weinberger LE, Sreenivasan S, Smee DE, McGuire J, Garrick T. Balancing safety against obstruction to health care access: an examination of behavioral flags in the VA health care system. J Threat Assess Manage. 2018;5(1):35-41.
18. Elbogen EB, Johnson SC, Wagner HR, et al. Protective factors and risk modification of violence in Iraq and Afghanistan war veterans. J Clin Psychiatry. 2012;73(6):e767-e773.
19. Karlin BE, Visnic S, McGee JS, Teri L. Results from the multisite implementation of STAR-VA: a multicomponent psychosocial intervention for managing challenging dementia-related behaviors of veterans. Psychol Serv. 2014;11(2):200-208.
20. Semeah LM, Campbell CL, Cowper DC, Peet AC. Serving our homeless veterans: patient perpetrated violence as a barrier to health care access. J Pub Nonprofit Aff. 2017;3(2):223-234.
21. Hodgson MJ, Reed R, Craig T, et al. Violence in healthcare facilities: lessons from the Veterans Health Administration. J Occup Environ Med. 2004;46(11):1158-1165.
22. Farrell GA, Bobrowski C, Bobrowski P. Scoping workplace aggression in nursing: findings from an Australian study. J Adv Nurs. 2006;55(6):778-787.
23. Barling J, Rogers AG, Kelloway EK. Behind closed doors: in-home workers’ experience of sexual harassment and workplace violence. J Occup Health Psychol. 2001;6(3):255-269.
24. Pompeii LA, Schoenfisch AL, Lipscomb HJ, Dement JM, Smith CD, Upadhyaya M. Physical assault, physical threat, and verbal abuse perpetrated against hospital workers by patients or visitors in six U.S. hospitals. Am J Ind Med. 2015;58(11):1194-1204.
25. Sippel LM, Mota NP, Kachadourian LK, et al. The burden of hostility in U.S. veterans: results from the National Health and Resilience in Veterans Study. Psychiatry Res. 2016;243(suppl C):421-430.
26. Campbell C. Patient Violence and Aggression in Non-Institutional Health Care Settings: Predictors of Reporting By Healthcare Providers [doctoral dissertation]. Orlando: University of Central Florida; 2016.
27. Galinsky T, Feng HA, Streit J, et al. Risk factors associated with patient assaults of home healthcare workers. Rehabil Nurs. 2010;35(5):206-215.
28. Campbell CL. Incident reporting by health-care workers in noninstitutional care settings. Trauma, Violence Abuse. 2017;18(4):445-456.
29. Arnetz JE, Arnetz BB. Violence towards health care staff and possible effects on the quality of patient care. Soc Sci Med. 2001;52(3):417-427.
30. Gates D, Fitzwater E, Succop P. Relationships of stressors, strain, and anger to caregiver assaults. Issues Ment Health Nurs. 2003;24(8):775-793.
31. Brillhart B, Kruse B, Heard L. Safety concerns for rehabilitation nurses in home care. Rehabil Nurs. 2004;29(6):227-229.
32. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10(3):40-42.
33. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. The Joint Commission releases results of surveys of the VA health care system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2808. Updated August 5, 2014. Accessed February 19, 2019.
34. Büssing A, Höge T. Aggression and violence against home care workers. J Occup Health Psychol. 2004;9(3):206-219.
35. Geiger-Brown J, Muntaner C, McPhaul K, Lipscomb J, Trinkoff A. Abuse and violence during home care work as predictor of worker depression. Home Health Care Serv Q. 2007;26(1):59-77.
36. Gates DM, Gillespie GL, Succop P. Violence against nurses and its impact on stress and productivity. Nurs Econ. 2011;29(2):59-66.
37. Petterson IL, Arnetz BB. Psychosocial stressors and well-being in health care workers: the impact of an intervention program. Soc Sci Med. 1998;47(11):1763-1772.
38. Arnetz JE, Arnetz BB. Implementation and evaluation of a practical intervention programme for dealing with violence towards health care workers. J Adv Nurs. 2000;31(3):668-680.
39. Arnetz JE, Hamblin L, Russell J, et al. Preventing patient-to-worker violence in hospitals: outcome of a randomized controlled intervention. J Occup Environ Med. 2017;59(1):18-27.
40. Elbogen EB, Tomkins AJ, Pothuloori AP, Scalora MJ. Documentation of violence risk information in psychiatric hospital patient charts: an empirical examination. J Am Acad Psychiatry Law. 2003;31(1):58-64.
41. Winsvold Prang I, Jelson-Jorgensen LP. Should I report? A qualitative study of barriers to incident reporting among nurses working in nursing homes. Geriatr Nurs. 2014;35(6):441-447.
42. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: management of disruptive patient behavior at VA medical facilities. Report No. 11-02585-129. https://www.va.gov/oig/pubs/VAOIG-11-02585-129.pdf. Published Mrach 7, 2013. Accessed February 21, 2019.
43. Lipscomb J, London M. Not Part of the Job: How to Take a Stand Against Violence in the Work Setting. Silver Spring, MD: American Nurses Association; 2015.
44. May DD, Grubbs LM. The extent, nature, and precipitating factors of nurse assault among three groups of registered nurses in a regional medical center. J Emerg Nurs. 2002;28(1):11-17.
45. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57(5):460-477.
46. Brennan C, Worrall-Davies A, McMillan D, Gilbody S, House A. The hospital anxiety and depression scale: a diagnostic meta-analysis of case-finding ability. J Psychosom Res. 2010;69(4):371-378.
47. McPhaul K, Lipscomb J, Johnson J. Assessing risk for violence on home health visits. Home Healthc Nurse. 2010;28(5):278-289.
48. McPhaul KM, London M, Murrett K, Flannery K, Rosen J, Lipscomb J. Environmental evaluation for workplace violence in healthcare and social services. J Safety Res. 2008;39(2):237-250.
49. Kelly JA, Somlai AM, DiFranceisco WJ, et al. Bridging the gap between the science and service of HIV prevention: transferring effective research-based HIV prevention interventions to community AIDS service providers. Am J Public Health. 2000;90(7):1082-1088.
50. Pawlin S. Reporting violence. Emerg Nurse. 2008;16(4):16-21.
51. Brakel SJ. Legal liability and workplace violence. J Am Acad Psychiatry Law. 1998;26(4):553-562.
52. Neuman JH, Baron RA. Workplace violence and workplace aggression: evidence concerning specific forms, potential causes, and preferred targets. J Manage. 1998;24(3):391-419.53. Ferns T, Chojnacka I. Angels and swingers, matrons and sinners: nursing stereotypes. Br J Nurs. 2005;14(19):1028-1032.
54. Mercer SW, Reynolds WJ. Empathy and quality of care. Br J Gen Pract 2002;52(suppl):S9-S12.
55. Lee TH. An Epidemic of Empathy in Healthcare: How to Deliver Compassionate, Connected Patient Care That Creates a Competitive Advantage. Columbus, OH: McGraw-Hill Education; 2015.
56. US Department of Veterans Affairs, Veterans Health Administrastion. Veterans Health Administration workplace violence prevention program (WVPP): disruptive behavior reporting system utilization report. Published 2017. https://vaww.portal2.va.gov/sites/wvpp/Shared%20Documents/DBRS%20Utilization%20Reports/FY2017%20DBRS%20Quarterly%20Utilization%20Report%20(Quarter%201).pdf. [Source not verified.]
57. Campbell CL, Burg, MA, Gammonley D. Measures for incident reporting of patient violence and aggression towards healthcare providers: a systematic review. Aggression Violent Behav. 2015;25(part B):314-322.
58. Carney PT, West P, Neily J, Mills PD, Bagian JP. The effect of facility complexity on perceptions of safety climate in the operating room: size matters. Am J Med Qual. 2010;25(6):457-461.
1. Hodgson MJ, Mohr DC, Drummond DJ, Bell M, Van Male L. Managing disruptive patients in health care: necessary solutions to a difficult problem. Am J Ind Med. 2012;55(11):1009-1017.
2. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2010-053. Patient Record Flags. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2341 Published December 3, 2010. Accessed March 29, 2019.
3. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 2012-026. Sexual Assaults and Other Defined Public Safety Incidents in VHA Facilities. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2797. Published September 27, 2012. Accessed March 29, 2019.
4. Curyto KJ, McCurry SM, Luci K, Karlin BE, Teri L, Karel MJ. Managing challenging behaviors of dementia in veterans: identifying and changing activators and consequences using STAR-VA. J Gerontol Nurs. 2017;43(2):33-43.
5. Speroni KG, Fitch T, Dawson E, Dugan L, Atherton M. Incidence and cost of nurse workplace violence perpetrated by hospital patients or patient visitors. J Emerg Nurs. 2014;40(3):218-228.
6. Phillips JP. Workplace violence against health care workers in the United States. NEJM. 2016;374(17):1661-1669.
7. Janocha JA, Smith RT. Workplace safety and health in the health care and social assistance industry, 2003–07. https://www.bls.gov/opub/mlr/cwc/workplace-safety-and-health-in-the-health-care-and-social-assistance-industry-2003-07.pdf. Published August 30, 2010. Accessed February 19, 2019.
8. US Department of Labor, Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. https://www.osha.gov/Publications/OSHA3826.pdf. Published December 2015. Accessed February 19, 2019.
9. US Department of Labor, Occupational Safety and Health Administration. Prevention of Workplace Violence in Healthcare and Social Assistance. Occupational Safety and Health Administration, https://www.govinfo.gov/content/pkg/FR-2016-12-07/pdf/2016-29197.pdf. Accessed January 20, 2017.
10. Gerberich SG, Church TR, McGovern PM, et al. An epidemiological study of the magnitude and consequences of work related violence: the Minnesota Nurses’ Study. Occup Environ Med. 2004;61(6):495-503.
11. Sherman MF, Gershon RRM, Samar SM, Pearson JM, Canton AN, Damsky MR. Safety factors predictive of job satisfaction and job retention among home healthcare aides. J Occup Environ Med. 2008;50(12):1430-1441.
12. Karel MJ, Teri L, McConnell E, Visnic S, Karlin BE. Effectiveness of expanded implementation of STAR-VA for managing dementia-related behaviors among veterans. Gerontologist. 2016;56(1):126-134.
13. US Department of Labor, Bureau of Labor Statistics. Nonfatal occupational injuries and illnesses requiring days away from work. https://www.bls.gov/news.release/archives/osh2_11192015.htm. Published November 19, 2015.
14. Beech B, Leather P. Workplace violence in the health care sector: A review of staff training and integration of training evaluation models. Aggression Violent Behav. 2006;11(1):27-43.
15. Campbell CL, McCoy S, Burg MA, Hoffman N. Enhancing home care staff safety through reducing client aggression and violence in noninstitutional care settings: a systematic review. Home Health Care Manage Pract. 2014;26(1):3-10.
16. Gallant-Roman MA. Strategies and tools to reduce workplace violence. AAOHNJ. 2008;56(11):449-454.
17. Weinberger LE, Sreenivasan S, Smee DE, McGuire J, Garrick T. Balancing safety against obstruction to health care access: an examination of behavioral flags in the VA health care system. J Threat Assess Manage. 2018;5(1):35-41.
18. Elbogen EB, Johnson SC, Wagner HR, et al. Protective factors and risk modification of violence in Iraq and Afghanistan war veterans. J Clin Psychiatry. 2012;73(6):e767-e773.
19. Karlin BE, Visnic S, McGee JS, Teri L. Results from the multisite implementation of STAR-VA: a multicomponent psychosocial intervention for managing challenging dementia-related behaviors of veterans. Psychol Serv. 2014;11(2):200-208.
20. Semeah LM, Campbell CL, Cowper DC, Peet AC. Serving our homeless veterans: patient perpetrated violence as a barrier to health care access. J Pub Nonprofit Aff. 2017;3(2):223-234.
21. Hodgson MJ, Reed R, Craig T, et al. Violence in healthcare facilities: lessons from the Veterans Health Administration. J Occup Environ Med. 2004;46(11):1158-1165.
22. Farrell GA, Bobrowski C, Bobrowski P. Scoping workplace aggression in nursing: findings from an Australian study. J Adv Nurs. 2006;55(6):778-787.
23. Barling J, Rogers AG, Kelloway EK. Behind closed doors: in-home workers’ experience of sexual harassment and workplace violence. J Occup Health Psychol. 2001;6(3):255-269.
24. Pompeii LA, Schoenfisch AL, Lipscomb HJ, Dement JM, Smith CD, Upadhyaya M. Physical assault, physical threat, and verbal abuse perpetrated against hospital workers by patients or visitors in six U.S. hospitals. Am J Ind Med. 2015;58(11):1194-1204.
25. Sippel LM, Mota NP, Kachadourian LK, et al. The burden of hostility in U.S. veterans: results from the National Health and Resilience in Veterans Study. Psychiatry Res. 2016;243(suppl C):421-430.
26. Campbell C. Patient Violence and Aggression in Non-Institutional Health Care Settings: Predictors of Reporting By Healthcare Providers [doctoral dissertation]. Orlando: University of Central Florida; 2016.
27. Galinsky T, Feng HA, Streit J, et al. Risk factors associated with patient assaults of home healthcare workers. Rehabil Nurs. 2010;35(5):206-215.
28. Campbell CL. Incident reporting by health-care workers in noninstitutional care settings. Trauma, Violence Abuse. 2017;18(4):445-456.
29. Arnetz JE, Arnetz BB. Violence towards health care staff and possible effects on the quality of patient care. Soc Sci Med. 2001;52(3):417-427.
30. Gates D, Fitzwater E, Succop P. Relationships of stressors, strain, and anger to caregiver assaults. Issues Ment Health Nurs. 2003;24(8):775-793.
31. Brillhart B, Kruse B, Heard L. Safety concerns for rehabilitation nurses in home care. Rehabil Nurs. 2004;29(6):227-229.
32. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10(3):40-42.
33. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. The Joint Commission releases results of surveys of the VA health care system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=2808. Updated August 5, 2014. Accessed February 19, 2019.
34. Büssing A, Höge T. Aggression and violence against home care workers. J Occup Health Psychol. 2004;9(3):206-219.
35. Geiger-Brown J, Muntaner C, McPhaul K, Lipscomb J, Trinkoff A. Abuse and violence during home care work as predictor of worker depression. Home Health Care Serv Q. 2007;26(1):59-77.
36. Gates DM, Gillespie GL, Succop P. Violence against nurses and its impact on stress and productivity. Nurs Econ. 2011;29(2):59-66.
37. Petterson IL, Arnetz BB. Psychosocial stressors and well-being in health care workers: the impact of an intervention program. Soc Sci Med. 1998;47(11):1763-1772.
38. Arnetz JE, Arnetz BB. Implementation and evaluation of a practical intervention programme for dealing with violence towards health care workers. J Adv Nurs. 2000;31(3):668-680.
39. Arnetz JE, Hamblin L, Russell J, et al. Preventing patient-to-worker violence in hospitals: outcome of a randomized controlled intervention. J Occup Environ Med. 2017;59(1):18-27.
40. Elbogen EB, Tomkins AJ, Pothuloori AP, Scalora MJ. Documentation of violence risk information in psychiatric hospital patient charts: an empirical examination. J Am Acad Psychiatry Law. 2003;31(1):58-64.
41. Winsvold Prang I, Jelson-Jorgensen LP. Should I report? A qualitative study of barriers to incident reporting among nurses working in nursing homes. Geriatr Nurs. 2014;35(6):441-447.
42. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection: management of disruptive patient behavior at VA medical facilities. Report No. 11-02585-129. https://www.va.gov/oig/pubs/VAOIG-11-02585-129.pdf. Published Mrach 7, 2013. Accessed February 21, 2019.
43. Lipscomb J, London M. Not Part of the Job: How to Take a Stand Against Violence in the Work Setting. Silver Spring, MD: American Nurses Association; 2015.
44. May DD, Grubbs LM. The extent, nature, and precipitating factors of nurse assault among three groups of registered nurses in a regional medical center. J Emerg Nurs. 2002;28(1):11-17.
45. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57(5):460-477.
46. Brennan C, Worrall-Davies A, McMillan D, Gilbody S, House A. The hospital anxiety and depression scale: a diagnostic meta-analysis of case-finding ability. J Psychosom Res. 2010;69(4):371-378.
47. McPhaul K, Lipscomb J, Johnson J. Assessing risk for violence on home health visits. Home Healthc Nurse. 2010;28(5):278-289.
48. McPhaul KM, London M, Murrett K, Flannery K, Rosen J, Lipscomb J. Environmental evaluation for workplace violence in healthcare and social services. J Safety Res. 2008;39(2):237-250.
49. Kelly JA, Somlai AM, DiFranceisco WJ, et al. Bridging the gap between the science and service of HIV prevention: transferring effective research-based HIV prevention interventions to community AIDS service providers. Am J Public Health. 2000;90(7):1082-1088.
50. Pawlin S. Reporting violence. Emerg Nurse. 2008;16(4):16-21.
51. Brakel SJ. Legal liability and workplace violence. J Am Acad Psychiatry Law. 1998;26(4):553-562.
52. Neuman JH, Baron RA. Workplace violence and workplace aggression: evidence concerning specific forms, potential causes, and preferred targets. J Manage. 1998;24(3):391-419.53. Ferns T, Chojnacka I. Angels and swingers, matrons and sinners: nursing stereotypes. Br J Nurs. 2005;14(19):1028-1032.
54. Mercer SW, Reynolds WJ. Empathy and quality of care. Br J Gen Pract 2002;52(suppl):S9-S12.
55. Lee TH. An Epidemic of Empathy in Healthcare: How to Deliver Compassionate, Connected Patient Care That Creates a Competitive Advantage. Columbus, OH: McGraw-Hill Education; 2015.
56. US Department of Veterans Affairs, Veterans Health Administrastion. Veterans Health Administration workplace violence prevention program (WVPP): disruptive behavior reporting system utilization report. Published 2017. https://vaww.portal2.va.gov/sites/wvpp/Shared%20Documents/DBRS%20Utilization%20Reports/FY2017%20DBRS%20Quarterly%20Utilization%20Report%20(Quarter%201).pdf. [Source not verified.]
57. Campbell CL, Burg, MA, Gammonley D. Measures for incident reporting of patient violence and aggression towards healthcare providers: a systematic review. Aggression Violent Behav. 2015;25(part B):314-322.
58. Carney PT, West P, Neily J, Mills PD, Bagian JP. The effect of facility complexity on perceptions of safety climate in the operating room: size matters. Am J Med Qual. 2010;25(6):457-461.
A Primary Care Provider’s Guide to Cataract Surgery in the Very Elderly
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
Effects of Process Improvement on Guideline-Concordant Cardiac Enzyme Testing
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
In recent years, driven by accelerating health care costs and desire for improved health care value, major specialty group guidelines have incorporated resource utilization and value calculations into their recommendations. High-value care has the characteristics of enhancing outcomes, safety, and patient satisfaction at a reasonable cost. As one example, the American College of Cardiology (ACC) recently published a consensus statement on its clinical practice guidelines with a specific focus on cost and value.1 This guideline acknowledges the difficulty in incorporating value into clinical decision making but stresses a need for increased transparency and consistency to boost value in everyday practice.
Chest pain and related symptoms were listed as the second leading principle reasons for emergency department visits in the US in 2011 with 14% of patients undergoing cardiac enzyme testing.2 The ACC guidelines advocate use of troponin as the preferred laboratory test for the initial evaluation of acute coronary syndrome (ACS). Fractionated creatine kinase (CK-MB) is an acceptable alternative only when a cardiac troponin test is not available.3 Furthermore, troponins should be obtained no more than 3 times for the initial evaluation of a single event, and further trending provides no additional benefit or prognostic information.
A recent study from an academic hospital showed that process improvement interventions focused on eliminating unnecessary cardiac enzyme testing led to a 1-year cost savings of $1.25 million while increasing the rate of ACS diagnosis.4 Common clinical practice at Naval Medical Center Portsmouth (NMCP) in Virginia still routinely includes both troponin as well as a CK panel comprised of CK, CK-MB, and a calculated CK-MB/CK index. Our study focuses on the implementation of quality improvement efforts described by Larochelle and colleagues at NMCP.4 The study aimed to determine the impact of implementing interventions designed to improve the ordering practices and reduce the cost of cardiac enzyme testing.
Methods
The primary focus of the intervention was on ordering practices of the emergency medicine department (EMD), internal medicine (IM) inpatient services, and cardiology inpatient services. Specific interventions were: (1) removal of the CK panel from the chest pain order set in the EMD electronic health record (EHR); (2) removal of the CK panel from the inpatient cardiology order set; (3) education of staff on the changes in CK panel utility via direct communication during IM academic seminars; (4) education of nursing staff ordering laboratory results on behalf of physicians on the cardiology service at the morning and evening huddles; and (5) addition of “max of 3 tests indicated” comment to the inpatient EHR ordering page of the troponin test
Data Source
The process improvement interventions were considered exempt from institutional review board (IRB) approval; however, we obtained expedited IRB approval with waiver of consent for the research aspect of the project. We obtained clinical administrative data from the Military Health System Data Repository (MDR). We identified all adult patients aged ≥ 18 years who had a troponin test, CK-MB, or both drawn at NMCP on the following services: the EMD, IM, and cardiology. A troponin or CK-MB test was defined using Current Procedural Terminology (CPT) codes and unique Logical Observation Identifiers Names and Codes (LOINC).
Measures
The study was divided into 3 periods: the preintervention period from August 1, 2013 to July 31, 2014; the intervention period from August 1, 2014 to January 31, 2015; and the postintervention period February 1, 2015 to January 31, 2016.
The primary outcomes measured were the frequency of guideline concordance and total costs for tests ordered per month using the Centers for Medicare and Medicaid Services (CMS) clinical laboratory fee schedule of $13.40 for troponin and $16.17 for CK-MB.5Concordance was defined as ≤ 3 troponin tests and no CK-MB tests ordered during 1 encounter for a patient without an ACS diagnosis in the preceding 7 days. Due to faster cellular release kinetics of CK-MB compared with that of troponin, this test has utility in evaluating new or worsening chest pain in the setting of a recent myocardial infarction (MI). Therefore, we excluded any patient who had a MI within the preceding 7 days of an order for either CK-MB or troponin tests. Additionally, the number of tests, both CK-MB and troponin, ordered per patient encounter (hereafter referred to as an episode) were measured. Finally, we measured the monthly prevalence of ACS diagnosis and percentage of visits having that diagnosis.
Data Analysis
Descriptive statistics were used to calculate population demographics of age group, sex, beneficiary category, sponsor service, and clinical setting. Monthly data were grouped into the preintervention and postintervention periods. The analysis was performed using t tests to compare mean values and CIs before and after the intervention. Simple linear regression with attention to correlation was used to create best fit lines with confidence bands before and after the intervention. Interrupted time series (ITS) regression was used to describe all data points throughout the study. Consistency between these various methods was verified. Mean values and CIs were reported from the t tests. Statistical significance was reported when appropriate. Equations and confidence predictions on the simple linear regressions were produced and reported. These were used to identify values at the start, midpoint, and end of the pre- and postintervention periods.
Results
There were a total of 6,281 patients in the study population. More patients were seen during the postintervention period than in the preintervention period. The mean age of patients was slightly higher during the preintervention period (Table 1).
Guideline Concordance
To determine whether ordering practices for cardiac enzyme testing improved, we assessed the changes in the frequency of guideline concordance during the pre- and postintervention period. On average during the preintervention year, the percentage of tests ordered that met guideline concordance was 10.1% (95% CI, 7.4%-12.9%), increasing by 0.80% (95% CI, 0.17%-1.42%) each month.
Costs
We assessed changes in total dollars spent on cardiac enzyme testing during the pre- and postintervention periods. During the preintervention year, $9,400 (95% CI, $8,700-$10,100) was spent on average each month, which did not change significantly throughout the period. During the postintervention year, the cost was stable at $5,000 (95% CI, $4,600-$5,300) on average each month, a reduction of $4,400 (95% CI, $3,700-$5,100) (Figure 2).
CK-MB and Troponin Tests per Patient
To further assess ordering practices for cardiac enzyme testing, we compared the changes in the monthly number of tests and the average number of CK-MB and troponin tests ordered per episode pre- and postintervention. On average during the preintervention year, 297 tests (95% CI, 278-315) were run per month, with an average of 1.21 CK tests (95% CI, 1.15-1.27) per episode (Table 2, Figure 3).
The changes in troponin testing were not as dramatic. The counts of tests each month remained similar, with a preintervention year average of 341 (95% CI, 306-377) and postintervention year average of 310 (95% CI, 287-332), which were not statistically different. However, there was a statistically significant decrease in the number of tests per episode. During the preintervention year, 1.38 troponin tests (95% CI, 1.31-1.45) were ordered per patient on average. This dropped by 0.17 (95% CI, 0.09-0.24) to the postintervention average of 1.21 (95% CI, 1.17-1.25) (Table 2, Figure 4).
ACS Prevalence
To determine whether there was an impact on ACS diagnoses, we looked at the numbers of ACS diagnoses and their prevalence among visits before and after the intervention. During the preintervention year, the average monthly number of diagnoses was 29.7 (95% CI, 26.1-33.2), and prevalence of ACS was 0.56% (95% CI, 0.48%-0.63%) of all episodes. Although the monthly rate was statistically decreasing by 0.022% (95% CI, 0.003-0.41), this has little meaning since the level of correlation (r2 = 0.2522, not displayed) was poor due to the essentially nonexistent correlation in number of visits each month (r2 = 0.0112, not displayed). During the postintervention year, the average number of diagnoses was 32.2 (95% CI, 27.9-36.6), and the prevalence of ACS was 0.62% (95% CI, 0.54-0.65). Neither of these values changed significantly between the pre- and postintervention period. All ICD-9 and ICD-10 diagnosis codes used for the analysis are available upon request from the authors.
Discussion
Our data demonstrate the ability of simple process improvement interventions to decrease unnecessary testing in the workup of ACS, increasing the rate of guideline concordant testing by > 70% at a single military treatment facility (MTF). In particular, with the now widespread use of EHR, the order set presents a high-yield target for process improvement in an easily implemented, durable fashion. We had expected to see some decrease in the efficacy of the intervention at a time of staff turnover in the summer of 2015 because ongoing dedicated teaching sessions were not performed. Despite that, the intervention remained effective without further dedicated teaching sessions. This outcome was certainly attributable to the hardwired interventions made (mainly via order sets), but possibly indicates an institutional memory that can take hold after an initial concerted effort is made.
We reduced the estimated preintervention annual cost of $113,000 by $53,000 (95% CI, $42,000-$64,000). Although on a much smaller scale than the study by Larochelle, our study represents a nearly 50% reduction in the total cost of initial testing for possible ACS and a > 80% reduction in unnecessary CK-MB testing.4 This result was achieved with no statistical change in the prevalence of ACS. The cost reduction does not account for the labor costs to clinically follow-up and address additional unnecessary lab results. The estimated cost of intervention was limited to the time required to educate residents, interns, and nursing staff as well as the implementation of the automated, reflexive laboratory results ordering process.
Unique to our study, we also demonstrated an intervention that satisfied all the major stakeholders in the ordering of these laboratory results. By instituting the reflexive ordering of CK-MB tests for positive troponins, we obtained the support of the facility’s interventional cardiology department, which finds value in that data. Appreciating the time-sensitive nature of an ACS diagnosis, the reflexive ordering minimized the delay in receiving these data while still greatly reducing the number of tests performed. That being said, if the current trend away from CK-MB in favor of exclusively testing troponin continues, removing the reflexive ordering for positive laboratory results protocol would be an easy follow-on intervention.
Limitations
Our study presented several limitations. First, reporting errors due to improper or insufficient medical coding as well as data entry errors may exist within the MDR; therefore, the results of this analysis may be over- or underestimated. Specifically, CPT codes for troponin and CK-MB were available only in 1 of the 2 data sets used for this study, which primarily contains outpatient patient encounters. For this reason, most of the laboratory testing comes from the EMD rather than from inpatient services. However, because we excluded all patients who eventually had an ACS diagnosis (patients who likely had more inpatient time and better indication for repeat troponin), we feel that our intervention was still thoroughly investigated. Second, the number of tests drawn per patient was significantly < 2, the expected minimum number of tests to rule out ACS in patients with appropriate symptoms.
This study was not designed to answer the source of variation from guidelines. Many patients had only 1 test, which we feel represents an opportunity for future study to identify other ways cardiac enzyme testing is being used clinically. These tests might be used for patients without convincing symptoms and signs of coronary syndromes or for patients with other primary problems. Third, by using the ITS analysis, we assumed that the outcome during each intervention period follows a linear pattern. However, changes may follow a nonlinear pattern over a long period. Finally, our intervention was limited to only a single MTF, which may limit generalizability to other facilities across military medicine. However, we feel this study should serve as a guide for other MTFs as well as US Department of Veterans Affairs facilities that could institute similar process improvements.
Conclusion
We made easily implemented and durable process improvement interventions that changed institution-wide ordering practices. These changes dramatically increased the rate of guideline-concordant testing, decreasing cost and furthering the goal of high-value medical care.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
1. Anderson JL, Heidenreich PA, Barnett PG, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129(22):2329-2345.
2. Centers for Disease Control and Prevention, National Center for Health Statistics. National hospital ambulatory medical care survey: 2010 emergency department summary tables. https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed March 15, 2019.
3. Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356-e375.
4. Larochelle MR, Knight AM, Pantle H, Riedel S, Trost JC. Reducing excess cardiac biomarker testing at an academic medical center. J Gen Intern Med. 2014;29(11):1468-1474.
5. Centers for Medicare and Medicaid Services. 2016 clinical laboratory fee schedule. https://www.cms.gov/Medicare/Medicare-Fee -for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files-Items/16CLAB.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending. Accessed March 15, 2019.
Clinical Pharmacist Credentialing and Privileging: A Process for Ensuring High-Quality Patient Care
The Red Lake Indian Health Service (IHS) health care facility is in north-central Minnesota within the Red Lake Nation. The facility supports primary care, emergency, urgent care, pharmacy, inpatient, optometry, dental, radiology, laboratory, physical therapy, and behavioral health services to about 10,000 Red Lake Band of Chippewa Indian patients. The Red Lake pharmacy provides inpatient and outpatient medication services and pharmacist-managed clinical patient care.
In 2013, the Red Lake IHS medical staff endorsed the implementation of comprehensive clinical pharmacy services to increase health care access and optimize clinical outcomes for patients. During the evolution of pharmacy-based patient-centric care, the clinical programs offered by Red Lake IHS pharmacy expanded from 1 anticoagulation clinic to multiple advanced-practice clinical pharmacy services. This included pharmacy primary care, medication-assisted therapy, naloxone, hepatitis C, and behavioral health medication management clinics.
The immense clinical growth of the pharmacy department demonstrated a need to assess and monitor pharmacist competency to ensure the delivery of quality patient care. Essential quality improvement processes were lacking. To fill these quality improvement gaps, a robust pharmacist credentialing and privileging program was implemented in 2015.
Patient Care
As efforts within health care establishments across the US focus on the delivery of efficient, high-quality, affordable health care, pharmacists have become increasingly instrumental in providing patient care within expanded clinical roles.1-8 Many clinical pharmacy models have evolved into interdisciplinary approaches to care.9 Within these models, abiding by state and federal laws, pharmacists practice under the indirect supervision of licensed independent practitioners (LIPs), such as physicians, nurse practitioners, and physician assistants.8 Under collaborative practice agreements (CPAs), patients are initially diagnosed by LIPs, then referred to clinical pharmacists for therapeutic management.5,7
Clinical pharmacist functions encompass comprehensive medication management (ie, prescribing, monitoring, and adjustment of medications), nonpharmacologic guidance, and coordination of care. Interdisciplinary collaboration allows pharmacists opportunities to provide direct patient care or consultations by telecommunication in many different clinical environments, including disease management, primary care, or specialty care. Pharmacists may manage chronic or acute illnesses associated with endocrine, cardiovascular, respiratory, gastrointestinal, or other systems.
Pharmacists may also provide comprehensive medication review services, such as medication therapy management (MTM), transitions of care, or chronic care management. Examples of specialized areas include psychiatric, opioid use disorder, palliative care, infectious disease, chronic pain, or oncology services. For hospitalized patients, pharmacists may monitor pharmacokinetics and adjust dosing, transition patients from IV to oral medications, or complete medication reconciliation.10 Within these clinical roles, pharmacists assist in providing patient care during shortages of other health care providers (HCPs), improve patient outcomes, decrease health care-associated costs by preventing emergency department and hospital admissions or readmissions, increase access to patient care, and increase revenue through pharmacist-managed clinics and services.11
Pharmacist Credentialing
With the advancement of modern clinical pharmacy practice, many pharmacists have undertaken responsibilities to fulfill the complex duties of clinical care and diverse patient situations, but with few or no requirements to prove initial or ongoing clinical competency.2 Traditionally, pharmacist credentialing is limited to a onetime or periodic review of education and licensure, with little to no involvement in privileging and ongoing monitoring of clinical proficiency.10 These quality assurance disparities can be met and satisfied through credentialing and privileging processes. Credentialing and privileging are systematic, evidence-based processes that provide validation to HCPs, employers, and patients that pharmacists are qualified to practice clinically. 2,9 According to the Council on Credentialing in Pharmacy, clinical pharmacists should be held accountable for demonstrating competency and providing quality care through credentialing and privileging, as required for other HCPs.2,12
Credentialing and recredentialing is a primary source verification process. These processes ensure that there are no license restrictions or revocations; certifications are current; mandatory courses, certificates, and continuing education are complete; training and orientation are satisfactory; and any disciplinary action, malpractice claims, or history of impairment is reported. Privileging is the review of credentials and evaluation of clinical training and competence by the Clinical Director and Medical Executive Committee to determine whether a clinical pharmacist is competent to practice within requested privileges.11
Credentialing and privileging processes are designed not only to initially confirm that a pharmacist is competent to practice clinically, but also monitor ongoing performance.2,13 Participation in professional practice evaluations, which includes peer reviews, ongoing professional practice evaluations, and focused professional practice evaluations, is required for all credentialed and privileged practitioners. These evaluations are used to identify, assess, and correct unsatisfactory trends. Individual practices, documentation, and processes are evaluated against existing department standards (eg, CPAs, policies, processes)11,13 The results of individual professional practice evaluations are reviewed with practitioners on a regular basis and performance improvement plans implemented as needed.
Since 2015, 17 pharmacists at the Red Lake IHS health care facility have been granted membership to the medical staff as credentialed and privileged practitioners. In a retrospective review of professional practice evaluations by the Red Lake IHS pharmacy clinical coordinator, 971 outpatient clinical peer reviews, including the evaluation of 21,526 peer-review elements were completed by pharmacists from fiscal year 2015 through 2018. Peer-review elements assessed
Conclusion
Pharmacists have become increasingly instrumental in providing effective, cost-efficient, and accessible clinical services by continuing to move toward expanding and evolving roles within comprehensive, patient-centered clinical pharmacy practice settings.5,6 Multifaceted clinical responsibilities associated with health care delivery necessitate assessment and monitoring of pharmacist performance. Credentialing and privileging is an established and trusted systematic process that assures HCPs, employers, and patients that pharmacists are qualified and competent to practice clinically.2,4,12 Implementation of professional practice evaluations suggest improved staff compliance with visit documentation, patient care standards, and clinic processes required by CPAs, policies, and department standards to ensure the delivery of safe, high-quality patient care.
1. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed March 15, 2019.
2. Rouse MJ, Vlasses PH, Webb CE; Council on Credentialing in Pharmacy. Credentialing and privileging of pharmacists: a resource paper from the Council on Credentialing in Pharmacy. Am J Health Syst Pharm. 2014;71(21):e109-e118.
3. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759-769.
4. Blair MM, Carmichael J, Young E, Thrasher K; Qualified Provider Model Ad Hoc Committee. Pharmacist privileging in a health system: report of the Qualified Provider Model Ad Hoc Committee. Am J Health Syst Pharm. 2007;64(22):2373-2381.
5. Claxton KI, Wojtal P. Design and implementation of a credentialing and privileging model for ambulatory care pharmacists. Am J Health Syst Pharm. 2006;63(17):1627-1632.
6. Jordan TA, Hennenfent JA, Lewin JJ III, Nesbit TW, Weber R. Elevating pharmacists’ scope of practice through a health-system clinical privileging process. Am J Health Syst Pharm. 2016;73(18):1395-1405.
7. Centers for Disease Control and Prevention. Collaborative practice agreements and pharmacists’ patient care services: a resource for doctors, nurses, physician assistants, and other providers. https://www.cdc.gov/dhdsp/pubs/docs/Translational_Tools_Providers.pdf. Published October 2013. Accessed March 18, 2019.
8. Council on Credentialing in Pharmacy, Albanese NP, Rouse MJ. Scope of contemporary pharmacy practice: roles, responsibilities, and functions of practitioners and pharmacy technicians. J Am Pharm Assoc (2003). 2010;50(2):e35-e69.
9. Philip B, Weber R. Enhancing pharmacy practice models through pharmacists’ privileging. Hosp Pharm. 2013; 48(2):160-165.
10. Galt KA. Credentialing and privileging of pharmacists. Am J Health Syst Pharm. 2004;61(7):661-670.
11. Smith ML, Gemelas MF; US Public Health Service; Indian Health Service. Indian Health Service medical staff credentialing and privileging guide. https://www.ihs.gov/riskmanagement/includes/themes/newihstheme/display_objects/documents/IHS-Medical-Staff-Credentialing-and-Privileging-Guide.pdf. Published September 2005. Accessed March 15, 2019.
12. US Department of Health and Human Services, Indian Health Service. Indian health manual: medical credentials and privileges review process. https://www.ihs.gov/ihm/pc/part-3/p3c1. Accessed March 15, 2019.
13. Holley SL, Ketel C. Ongoing professional practice evaluation and focused professional practice evaluation: an overview for advanced practice clinicians. J Midwifery Women Health. 2014;59(4):452-459.
The Red Lake Indian Health Service (IHS) health care facility is in north-central Minnesota within the Red Lake Nation. The facility supports primary care, emergency, urgent care, pharmacy, inpatient, optometry, dental, radiology, laboratory, physical therapy, and behavioral health services to about 10,000 Red Lake Band of Chippewa Indian patients. The Red Lake pharmacy provides inpatient and outpatient medication services and pharmacist-managed clinical patient care.
In 2013, the Red Lake IHS medical staff endorsed the implementation of comprehensive clinical pharmacy services to increase health care access and optimize clinical outcomes for patients. During the evolution of pharmacy-based patient-centric care, the clinical programs offered by Red Lake IHS pharmacy expanded from 1 anticoagulation clinic to multiple advanced-practice clinical pharmacy services. This included pharmacy primary care, medication-assisted therapy, naloxone, hepatitis C, and behavioral health medication management clinics.
The immense clinical growth of the pharmacy department demonstrated a need to assess and monitor pharmacist competency to ensure the delivery of quality patient care. Essential quality improvement processes were lacking. To fill these quality improvement gaps, a robust pharmacist credentialing and privileging program was implemented in 2015.
Patient Care
As efforts within health care establishments across the US focus on the delivery of efficient, high-quality, affordable health care, pharmacists have become increasingly instrumental in providing patient care within expanded clinical roles.1-8 Many clinical pharmacy models have evolved into interdisciplinary approaches to care.9 Within these models, abiding by state and federal laws, pharmacists practice under the indirect supervision of licensed independent practitioners (LIPs), such as physicians, nurse practitioners, and physician assistants.8 Under collaborative practice agreements (CPAs), patients are initially diagnosed by LIPs, then referred to clinical pharmacists for therapeutic management.5,7
Clinical pharmacist functions encompass comprehensive medication management (ie, prescribing, monitoring, and adjustment of medications), nonpharmacologic guidance, and coordination of care. Interdisciplinary collaboration allows pharmacists opportunities to provide direct patient care or consultations by telecommunication in many different clinical environments, including disease management, primary care, or specialty care. Pharmacists may manage chronic or acute illnesses associated with endocrine, cardiovascular, respiratory, gastrointestinal, or other systems.
Pharmacists may also provide comprehensive medication review services, such as medication therapy management (MTM), transitions of care, or chronic care management. Examples of specialized areas include psychiatric, opioid use disorder, palliative care, infectious disease, chronic pain, or oncology services. For hospitalized patients, pharmacists may monitor pharmacokinetics and adjust dosing, transition patients from IV to oral medications, or complete medication reconciliation.10 Within these clinical roles, pharmacists assist in providing patient care during shortages of other health care providers (HCPs), improve patient outcomes, decrease health care-associated costs by preventing emergency department and hospital admissions or readmissions, increase access to patient care, and increase revenue through pharmacist-managed clinics and services.11
Pharmacist Credentialing
With the advancement of modern clinical pharmacy practice, many pharmacists have undertaken responsibilities to fulfill the complex duties of clinical care and diverse patient situations, but with few or no requirements to prove initial or ongoing clinical competency.2 Traditionally, pharmacist credentialing is limited to a onetime or periodic review of education and licensure, with little to no involvement in privileging and ongoing monitoring of clinical proficiency.10 These quality assurance disparities can be met and satisfied through credentialing and privileging processes. Credentialing and privileging are systematic, evidence-based processes that provide validation to HCPs, employers, and patients that pharmacists are qualified to practice clinically. 2,9 According to the Council on Credentialing in Pharmacy, clinical pharmacists should be held accountable for demonstrating competency and providing quality care through credentialing and privileging, as required for other HCPs.2,12
Credentialing and recredentialing is a primary source verification process. These processes ensure that there are no license restrictions or revocations; certifications are current; mandatory courses, certificates, and continuing education are complete; training and orientation are satisfactory; and any disciplinary action, malpractice claims, or history of impairment is reported. Privileging is the review of credentials and evaluation of clinical training and competence by the Clinical Director and Medical Executive Committee to determine whether a clinical pharmacist is competent to practice within requested privileges.11
Credentialing and privileging processes are designed not only to initially confirm that a pharmacist is competent to practice clinically, but also monitor ongoing performance.2,13 Participation in professional practice evaluations, which includes peer reviews, ongoing professional practice evaluations, and focused professional practice evaluations, is required for all credentialed and privileged practitioners. These evaluations are used to identify, assess, and correct unsatisfactory trends. Individual practices, documentation, and processes are evaluated against existing department standards (eg, CPAs, policies, processes)11,13 The results of individual professional practice evaluations are reviewed with practitioners on a regular basis and performance improvement plans implemented as needed.
Since 2015, 17 pharmacists at the Red Lake IHS health care facility have been granted membership to the medical staff as credentialed and privileged practitioners. In a retrospective review of professional practice evaluations by the Red Lake IHS pharmacy clinical coordinator, 971 outpatient clinical peer reviews, including the evaluation of 21,526 peer-review elements were completed by pharmacists from fiscal year 2015 through 2018. Peer-review elements assessed
Conclusion
Pharmacists have become increasingly instrumental in providing effective, cost-efficient, and accessible clinical services by continuing to move toward expanding and evolving roles within comprehensive, patient-centered clinical pharmacy practice settings.5,6 Multifaceted clinical responsibilities associated with health care delivery necessitate assessment and monitoring of pharmacist performance. Credentialing and privileging is an established and trusted systematic process that assures HCPs, employers, and patients that pharmacists are qualified and competent to practice clinically.2,4,12 Implementation of professional practice evaluations suggest improved staff compliance with visit documentation, patient care standards, and clinic processes required by CPAs, policies, and department standards to ensure the delivery of safe, high-quality patient care.
The Red Lake Indian Health Service (IHS) health care facility is in north-central Minnesota within the Red Lake Nation. The facility supports primary care, emergency, urgent care, pharmacy, inpatient, optometry, dental, radiology, laboratory, physical therapy, and behavioral health services to about 10,000 Red Lake Band of Chippewa Indian patients. The Red Lake pharmacy provides inpatient and outpatient medication services and pharmacist-managed clinical patient care.
In 2013, the Red Lake IHS medical staff endorsed the implementation of comprehensive clinical pharmacy services to increase health care access and optimize clinical outcomes for patients. During the evolution of pharmacy-based patient-centric care, the clinical programs offered by Red Lake IHS pharmacy expanded from 1 anticoagulation clinic to multiple advanced-practice clinical pharmacy services. This included pharmacy primary care, medication-assisted therapy, naloxone, hepatitis C, and behavioral health medication management clinics.
The immense clinical growth of the pharmacy department demonstrated a need to assess and monitor pharmacist competency to ensure the delivery of quality patient care. Essential quality improvement processes were lacking. To fill these quality improvement gaps, a robust pharmacist credentialing and privileging program was implemented in 2015.
Patient Care
As efforts within health care establishments across the US focus on the delivery of efficient, high-quality, affordable health care, pharmacists have become increasingly instrumental in providing patient care within expanded clinical roles.1-8 Many clinical pharmacy models have evolved into interdisciplinary approaches to care.9 Within these models, abiding by state and federal laws, pharmacists practice under the indirect supervision of licensed independent practitioners (LIPs), such as physicians, nurse practitioners, and physician assistants.8 Under collaborative practice agreements (CPAs), patients are initially diagnosed by LIPs, then referred to clinical pharmacists for therapeutic management.5,7
Clinical pharmacist functions encompass comprehensive medication management (ie, prescribing, monitoring, and adjustment of medications), nonpharmacologic guidance, and coordination of care. Interdisciplinary collaboration allows pharmacists opportunities to provide direct patient care or consultations by telecommunication in many different clinical environments, including disease management, primary care, or specialty care. Pharmacists may manage chronic or acute illnesses associated with endocrine, cardiovascular, respiratory, gastrointestinal, or other systems.
Pharmacists may also provide comprehensive medication review services, such as medication therapy management (MTM), transitions of care, or chronic care management. Examples of specialized areas include psychiatric, opioid use disorder, palliative care, infectious disease, chronic pain, or oncology services. For hospitalized patients, pharmacists may monitor pharmacokinetics and adjust dosing, transition patients from IV to oral medications, or complete medication reconciliation.10 Within these clinical roles, pharmacists assist in providing patient care during shortages of other health care providers (HCPs), improve patient outcomes, decrease health care-associated costs by preventing emergency department and hospital admissions or readmissions, increase access to patient care, and increase revenue through pharmacist-managed clinics and services.11
Pharmacist Credentialing
With the advancement of modern clinical pharmacy practice, many pharmacists have undertaken responsibilities to fulfill the complex duties of clinical care and diverse patient situations, but with few or no requirements to prove initial or ongoing clinical competency.2 Traditionally, pharmacist credentialing is limited to a onetime or periodic review of education and licensure, with little to no involvement in privileging and ongoing monitoring of clinical proficiency.10 These quality assurance disparities can be met and satisfied through credentialing and privileging processes. Credentialing and privileging are systematic, evidence-based processes that provide validation to HCPs, employers, and patients that pharmacists are qualified to practice clinically. 2,9 According to the Council on Credentialing in Pharmacy, clinical pharmacists should be held accountable for demonstrating competency and providing quality care through credentialing and privileging, as required for other HCPs.2,12
Credentialing and recredentialing is a primary source verification process. These processes ensure that there are no license restrictions or revocations; certifications are current; mandatory courses, certificates, and continuing education are complete; training and orientation are satisfactory; and any disciplinary action, malpractice claims, or history of impairment is reported. Privileging is the review of credentials and evaluation of clinical training and competence by the Clinical Director and Medical Executive Committee to determine whether a clinical pharmacist is competent to practice within requested privileges.11
Credentialing and privileging processes are designed not only to initially confirm that a pharmacist is competent to practice clinically, but also monitor ongoing performance.2,13 Participation in professional practice evaluations, which includes peer reviews, ongoing professional practice evaluations, and focused professional practice evaluations, is required for all credentialed and privileged practitioners. These evaluations are used to identify, assess, and correct unsatisfactory trends. Individual practices, documentation, and processes are evaluated against existing department standards (eg, CPAs, policies, processes)11,13 The results of individual professional practice evaluations are reviewed with practitioners on a regular basis and performance improvement plans implemented as needed.
Since 2015, 17 pharmacists at the Red Lake IHS health care facility have been granted membership to the medical staff as credentialed and privileged practitioners. In a retrospective review of professional practice evaluations by the Red Lake IHS pharmacy clinical coordinator, 971 outpatient clinical peer reviews, including the evaluation of 21,526 peer-review elements were completed by pharmacists from fiscal year 2015 through 2018. Peer-review elements assessed
Conclusion
Pharmacists have become increasingly instrumental in providing effective, cost-efficient, and accessible clinical services by continuing to move toward expanding and evolving roles within comprehensive, patient-centered clinical pharmacy practice settings.5,6 Multifaceted clinical responsibilities associated with health care delivery necessitate assessment and monitoring of pharmacist performance. Credentialing and privileging is an established and trusted systematic process that assures HCPs, employers, and patients that pharmacists are qualified and competent to practice clinically.2,4,12 Implementation of professional practice evaluations suggest improved staff compliance with visit documentation, patient care standards, and clinic processes required by CPAs, policies, and department standards to ensure the delivery of safe, high-quality patient care.
1. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed March 15, 2019.
2. Rouse MJ, Vlasses PH, Webb CE; Council on Credentialing in Pharmacy. Credentialing and privileging of pharmacists: a resource paper from the Council on Credentialing in Pharmacy. Am J Health Syst Pharm. 2014;71(21):e109-e118.
3. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759-769.
4. Blair MM, Carmichael J, Young E, Thrasher K; Qualified Provider Model Ad Hoc Committee. Pharmacist privileging in a health system: report of the Qualified Provider Model Ad Hoc Committee. Am J Health Syst Pharm. 2007;64(22):2373-2381.
5. Claxton KI, Wojtal P. Design and implementation of a credentialing and privileging model for ambulatory care pharmacists. Am J Health Syst Pharm. 2006;63(17):1627-1632.
6. Jordan TA, Hennenfent JA, Lewin JJ III, Nesbit TW, Weber R. Elevating pharmacists’ scope of practice through a health-system clinical privileging process. Am J Health Syst Pharm. 2016;73(18):1395-1405.
7. Centers for Disease Control and Prevention. Collaborative practice agreements and pharmacists’ patient care services: a resource for doctors, nurses, physician assistants, and other providers. https://www.cdc.gov/dhdsp/pubs/docs/Translational_Tools_Providers.pdf. Published October 2013. Accessed March 18, 2019.
8. Council on Credentialing in Pharmacy, Albanese NP, Rouse MJ. Scope of contemporary pharmacy practice: roles, responsibilities, and functions of practitioners and pharmacy technicians. J Am Pharm Assoc (2003). 2010;50(2):e35-e69.
9. Philip B, Weber R. Enhancing pharmacy practice models through pharmacists’ privileging. Hosp Pharm. 2013; 48(2):160-165.
10. Galt KA. Credentialing and privileging of pharmacists. Am J Health Syst Pharm. 2004;61(7):661-670.
11. Smith ML, Gemelas MF; US Public Health Service; Indian Health Service. Indian Health Service medical staff credentialing and privileging guide. https://www.ihs.gov/riskmanagement/includes/themes/newihstheme/display_objects/documents/IHS-Medical-Staff-Credentialing-and-Privileging-Guide.pdf. Published September 2005. Accessed March 15, 2019.
12. US Department of Health and Human Services, Indian Health Service. Indian health manual: medical credentials and privileges review process. https://www.ihs.gov/ihm/pc/part-3/p3c1. Accessed March 15, 2019.
13. Holley SL, Ketel C. Ongoing professional practice evaluation and focused professional practice evaluation: an overview for advanced practice clinicians. J Midwifery Women Health. 2014;59(4):452-459.
1. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed March 15, 2019.
2. Rouse MJ, Vlasses PH, Webb CE; Council on Credentialing in Pharmacy. Credentialing and privileging of pharmacists: a resource paper from the Council on Credentialing in Pharmacy. Am J Health Syst Pharm. 2014;71(21):e109-e118.
3. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759-769.
4. Blair MM, Carmichael J, Young E, Thrasher K; Qualified Provider Model Ad Hoc Committee. Pharmacist privileging in a health system: report of the Qualified Provider Model Ad Hoc Committee. Am J Health Syst Pharm. 2007;64(22):2373-2381.
5. Claxton KI, Wojtal P. Design and implementation of a credentialing and privileging model for ambulatory care pharmacists. Am J Health Syst Pharm. 2006;63(17):1627-1632.
6. Jordan TA, Hennenfent JA, Lewin JJ III, Nesbit TW, Weber R. Elevating pharmacists’ scope of practice through a health-system clinical privileging process. Am J Health Syst Pharm. 2016;73(18):1395-1405.
7. Centers for Disease Control and Prevention. Collaborative practice agreements and pharmacists’ patient care services: a resource for doctors, nurses, physician assistants, and other providers. https://www.cdc.gov/dhdsp/pubs/docs/Translational_Tools_Providers.pdf. Published October 2013. Accessed March 18, 2019.
8. Council on Credentialing in Pharmacy, Albanese NP, Rouse MJ. Scope of contemporary pharmacy practice: roles, responsibilities, and functions of practitioners and pharmacy technicians. J Am Pharm Assoc (2003). 2010;50(2):e35-e69.
9. Philip B, Weber R. Enhancing pharmacy practice models through pharmacists’ privileging. Hosp Pharm. 2013; 48(2):160-165.
10. Galt KA. Credentialing and privileging of pharmacists. Am J Health Syst Pharm. 2004;61(7):661-670.
11. Smith ML, Gemelas MF; US Public Health Service; Indian Health Service. Indian Health Service medical staff credentialing and privileging guide. https://www.ihs.gov/riskmanagement/includes/themes/newihstheme/display_objects/documents/IHS-Medical-Staff-Credentialing-and-Privileging-Guide.pdf. Published September 2005. Accessed March 15, 2019.
12. US Department of Health and Human Services, Indian Health Service. Indian health manual: medical credentials and privileges review process. https://www.ihs.gov/ihm/pc/part-3/p3c1. Accessed March 15, 2019.
13. Holley SL, Ketel C. Ongoing professional practice evaluation and focused professional practice evaluation: an overview for advanced practice clinicians. J Midwifery Women Health. 2014;59(4):452-459.
Use of GBCA in MRIs for High-Risk Patients
To the Editor:
We read with interest the case report of nephrogenic systemic fibrosis (NSF) by Chuang, Kaneshiro, and Betancourt in the June 2018 issue of Federal Practitioner.1 It was reported that a 61-year-old Hispanic male patient with a history of IV heroin abuse with end-stage renal disease (ESRD) secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection received 15 mL gadoversetamide, a linear gadolinium-based contrast agent (GBCA) during magnetic resonance imaging (MRI) of the brain. Hemodialysis was performed 18 hours after the contrast administration.
Eight weeks after his initial presentation, the patient developed pyoderma gangrenosum on his right forearm, which was treated with high-dose steroids. He then developed thickening and induration of his bilateral forearm skin with peau d’orange appearance. NSF was confirmed by a skin biopsy. The patient developed contractures of his upper and lower extremities and was finally wheelchair bound.
This case is very concerning since no NSF cases in patients receiving GBCA have been published since 2009. Unfortunately, the authors give no information on the occurrence of this particular case. Thus, it is unclear whether this case was observed before or after the switch to macrocyclic agents in patients with reduced renal function. The reported patient with ESRD was on hemodialysis and received 15 mL gadoversetamide during MRI of the brain. In 2007 the ESUR (European Society of Urogenital Radiology) published guidelines indicating linear GBCA (gadodiamide, gadoversetamide, gadopentetate dimeglumine) as high-risk agents that may not be used in patients with eGFR < 30 mL/min/1.73 m2.2,3
Consequently in 2007, the European Medicines Agency contraindicated these linear GBCA in patients with chronic kidney disease grades 4 and 5. Also in 2007 the US Food and Drug Administration (FDA) requested a revision of the prescribing information for all 5 GBCA approved in the US.4 In response to accumulating more informative data, in 2010 the FDA again used this class labeling approach to more explicitly describe differences in NSF risks among the agents.4 FDA regulation and contraindication of the use of low-stability GBCA in patients with advanced renal impairment and robust local policies on the safe use of these agents have resulted in marked reduction in the prevalence of NSF in the US. This case report needs to clarify why a high-risk linear agent was administered to a patient with ESRD.
In 2006 Grobner and Marckmann and colleagues reported their observations of a previously unrecognized link between exposure to gadodiamide and the development of NSF.5,6 It soon became clear that NSF is a delayed adverse contrast reaction that may cause severe disability and even death. Advanced renal disease and high-risk linear GBCA are the main factors in the pathogenesis of NSF. Additionally, the dose of the agent may play a role. NSF can occur from hours to years after exposure to GBCA. Not all patients with severe kidney disease exposed to high-risk agents developed NSF. Thus, additional factors were proposed to play a role in the pathogenesis of NSF. Among those factors were erythropoietin, metabolic acidosis, anion gap, iron, increased phosphate, zinc loss, proinflammatory conditions/inflammation and angiotensin-converting enzyme (ACE) inhibitors.7 Although there is little proof with these assumptions, special care must be taken as shown by this reported patient with multiple inflammatory disorders.
- Gertraud Heinz, MD, MBA; Aart van der Molen, MD; and Giles Roditi, MD; on behalf of the ESUR Contrast Media Safety Committee
Author affiliations: Gertraud Heinz is former President ESUR and Head of the Department of Radiology, Diagnostics and Intervention University Hospital St. Pölten Karl Landsteiner University of Health Sciences.
Correspondence: Gertraud Heinz (gertraud.heinz@stpoelten .lknoe.at)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract. 2018;35(6):40-43.
2. Thomsen HS; European Society of Urogenital Radiology (ESUR). ESUR guideline: gadolinium based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17(10):2692-2696.
3. Thomsen HS, Morcos SK, Almén T, et al; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2013;23(2):307-318
4. Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based agents by the Food and Drug Administration. Radiology. 2012;265(1):248-253.
5. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
6. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
7. Thomsen HS, Bennett CL. Six years after. Acta Radiol. 2012;53(8):827-829.
To the Editor:
With great interest, I read the case report by Chuang, Kaneshiro, and Betancourt.1 Patients with nephrogenic systemic fibrosis (NSF) are of special interest because the disease is still unclear as mentioned by the authors. Although new cases may occur,2 this case raises some concerns that I would like to address.
First, it would be of great interest to know the date when the patient received the high-risk gadolinium-based contrast agent (GBCA) gadoversetamide. Unfortunately, the authors did not mention the date of the injection of the GBCA that probably caused NSF. Due to the obvious association between the applications of special GBCAs in 2006, the US Food and Drug Administration (FDA) warned physicians not to inject these contrast agents in patients with compromised kidney function.3 Moreover, in 2007 the American College of Radiology (ACR) published guidelines for the safe use of GBCAs in patients with renal failure.4 Also, the European Medicines Agency (EMA) demanded that companies provide warning in product inserts about the acquisition of NSF in patients with severe kidney injury.5
Second, the clinical illustration of the case is inadequate. In the manuscript, we read that the patient acquired NSF-characteristic lesions like peau d’orange skin lesions and contractures of his extremities, but unfortunately, Chuang, Kaneshiro, and Betancourt did not provide figures that show them. On the other hand, Figure 1 shows an uncharacteristic dermal induration around inflammatory and ulcerated skin lesion (pyoderma gangrenosum).1 Such clinical signs are well known and occur perilesional of different conditions independently of NSF.6-8
Third, the histological features described as presence of fibrotic tissue in the deep dermis in Figure 2, and dermal fibrosis with thick collagen deposition in Figure 31 do not confirm the existence of NSF.
Taken together, the case presented by Chuang, Kaneshiro, and Betancourt contains some unclear aspects; therefore, it is questionable whether the published case describes a patient with NSF or not. In the current presentation, the diagnosis NSF seems to be an overestimation.
NSF still is a poorly understood disorder. Therefore, exactly documented new cases could be of clinical value when providing interesting information. Even single cases could shed some light in the darkness of the pathological mechanisms of this entity. On the other hand, we should not mix the existing cohort of published NSF cases with other scleroderma-like diseases, because this will lead to a confusion. Moreover, such a practice could inhibit the discovery of the pathophysiology of NSF.
- Ingrid Böhm, MD
Author affiliations: Ingrid Böhm is a Physician in the Department of Diagnostics, Interventional and Pediatric Radiology at the University Hospital of Bern, Inselspital, University of Bern in Bern, Switzerland.
Correspondence: Ingrid Böhm ([email protected])
Disclosures: The author reports no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract . 2018;35(6):40-43.
2. Larson KN, Gagnon AL, Darling MD, Patterson JW, Cropley TG. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151(10):1117-1120.
3. US Food and Drug Administration. A Public Health Advisory. Gadolinium-containing contrast agents for magnetic resonance imaging (MRI). http://wayback.archive-it.org/7993/20170112033022/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformation forPatientsandProviders/ucm053112.htm. Published June 8, 2006. Accessed March 15, 2019.
4. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
5. European Medicines Agency. Public statement: Vasovist and nephrogenic systemic fibrosis (NSF). https://www.ema.europa.eu/en/news/public-statement-vasovist-nephrogenic-systemic-fibrosis-nsf. Published February 7, 2007. Accessed March 15, 2019.
6. Luke JC. The etiology and modern treatment of varicose ulcer. Can Med Assoc J. 1940;43(3):217-221.
7. Paulsen E, Bygum A. Keratin gel as an adjuvant in the treatment of recalcitrant pyoderma gangrenosum ulcers: a case report. Acta Derm Venereol. 2019;99(2):234-235.
8. Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000; 136(2):167-169.
Response:
We thank Drs. Heinz, van der Molen, and Roditi for their valuable response. The following is the opinion of the authors and is not representative of the views or policies of our institution. The patient in this case received a gadolinium-based contrast agent (GBCA) in 2015 and was diagnosed with nephrogenic systemic fibrosis (NSF) 8 weeks later. We agree with the correspondents that linear GBCAs should not be used in patients with eGFR < 30 mL/min/1.73 m2. To date, a few cases of patients who received GBCA and developed NSF since 2009 have unfortunately continued to be reported in the literature.1-3 Our intention in publishing this case was to provide ongoing education to the medical community regarding this serious condition to ensure prevention of future cases.
We thank Dr. Böhm for her important inquiry. The patient received a histopathologic diagnosis of NSF. The report from the patient’s left dorsal forearm skin punch biopsy was read by our pathologist as “fibrosis and inflammation consistent with nephrogenic systemic fibrosis,” a diagnosis agreed upon by our colleagues in the dermatology and rheumatology departments based on the rapidity of his symptom onset and progression. While we acknowledge that this patient had other inflammatory disorders of the skin that may have coexisted with the diagnosis, after weighing the preponderance of clinical evidence in support of the biopsy results, we believe that this represents a case of NSF, which is associated with high morbidity and mortality. Thankfully, the patient in this case engaged extensively in physical and occupational therapy and is still alive nearly 4 years later. We would like to thank all the letter writers for their correspondence.
Author Affiliations: Kelley Chuang and Casey Kaneshiro are Hospitalists and Jaime Betancourt is a Pulmonologist, all in the Department of Medicine at the VA Greater Los Angeles Healthcare System in California.
Correspondence: Kelley Chuang ([email protected])
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Aggarwal A, Froehlich AA, Essah P, Brinster N, High WA, Downs RW. Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report. J Med Case Rep. 2011;5:566.
2. Fuah KW, Lim CT. Erythema nodosum masking nephrogenic systemic fibrosis as initial skin manifestation. BMC Nephrol. 2017;18(1):249.
3. Koratala A, Bhatti V. Nephrogenic systemic fibrosis. Clin Case Rep. 2017;5(7):1184-1185.
To the Editor:
We read with interest the case report of nephrogenic systemic fibrosis (NSF) by Chuang, Kaneshiro, and Betancourt in the June 2018 issue of Federal Practitioner.1 It was reported that a 61-year-old Hispanic male patient with a history of IV heroin abuse with end-stage renal disease (ESRD) secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection received 15 mL gadoversetamide, a linear gadolinium-based contrast agent (GBCA) during magnetic resonance imaging (MRI) of the brain. Hemodialysis was performed 18 hours after the contrast administration.
Eight weeks after his initial presentation, the patient developed pyoderma gangrenosum on his right forearm, which was treated with high-dose steroids. He then developed thickening and induration of his bilateral forearm skin with peau d’orange appearance. NSF was confirmed by a skin biopsy. The patient developed contractures of his upper and lower extremities and was finally wheelchair bound.
This case is very concerning since no NSF cases in patients receiving GBCA have been published since 2009. Unfortunately, the authors give no information on the occurrence of this particular case. Thus, it is unclear whether this case was observed before or after the switch to macrocyclic agents in patients with reduced renal function. The reported patient with ESRD was on hemodialysis and received 15 mL gadoversetamide during MRI of the brain. In 2007 the ESUR (European Society of Urogenital Radiology) published guidelines indicating linear GBCA (gadodiamide, gadoversetamide, gadopentetate dimeglumine) as high-risk agents that may not be used in patients with eGFR < 30 mL/min/1.73 m2.2,3
Consequently in 2007, the European Medicines Agency contraindicated these linear GBCA in patients with chronic kidney disease grades 4 and 5. Also in 2007 the US Food and Drug Administration (FDA) requested a revision of the prescribing information for all 5 GBCA approved in the US.4 In response to accumulating more informative data, in 2010 the FDA again used this class labeling approach to more explicitly describe differences in NSF risks among the agents.4 FDA regulation and contraindication of the use of low-stability GBCA in patients with advanced renal impairment and robust local policies on the safe use of these agents have resulted in marked reduction in the prevalence of NSF in the US. This case report needs to clarify why a high-risk linear agent was administered to a patient with ESRD.
In 2006 Grobner and Marckmann and colleagues reported their observations of a previously unrecognized link between exposure to gadodiamide and the development of NSF.5,6 It soon became clear that NSF is a delayed adverse contrast reaction that may cause severe disability and even death. Advanced renal disease and high-risk linear GBCA are the main factors in the pathogenesis of NSF. Additionally, the dose of the agent may play a role. NSF can occur from hours to years after exposure to GBCA. Not all patients with severe kidney disease exposed to high-risk agents developed NSF. Thus, additional factors were proposed to play a role in the pathogenesis of NSF. Among those factors were erythropoietin, metabolic acidosis, anion gap, iron, increased phosphate, zinc loss, proinflammatory conditions/inflammation and angiotensin-converting enzyme (ACE) inhibitors.7 Although there is little proof with these assumptions, special care must be taken as shown by this reported patient with multiple inflammatory disorders.
- Gertraud Heinz, MD, MBA; Aart van der Molen, MD; and Giles Roditi, MD; on behalf of the ESUR Contrast Media Safety Committee
Author affiliations: Gertraud Heinz is former President ESUR and Head of the Department of Radiology, Diagnostics and Intervention University Hospital St. Pölten Karl Landsteiner University of Health Sciences.
Correspondence: Gertraud Heinz (gertraud.heinz@stpoelten .lknoe.at)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract. 2018;35(6):40-43.
2. Thomsen HS; European Society of Urogenital Radiology (ESUR). ESUR guideline: gadolinium based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17(10):2692-2696.
3. Thomsen HS, Morcos SK, Almén T, et al; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2013;23(2):307-318
4. Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based agents by the Food and Drug Administration. Radiology. 2012;265(1):248-253.
5. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
6. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
7. Thomsen HS, Bennett CL. Six years after. Acta Radiol. 2012;53(8):827-829.
To the Editor:
With great interest, I read the case report by Chuang, Kaneshiro, and Betancourt.1 Patients with nephrogenic systemic fibrosis (NSF) are of special interest because the disease is still unclear as mentioned by the authors. Although new cases may occur,2 this case raises some concerns that I would like to address.
First, it would be of great interest to know the date when the patient received the high-risk gadolinium-based contrast agent (GBCA) gadoversetamide. Unfortunately, the authors did not mention the date of the injection of the GBCA that probably caused NSF. Due to the obvious association between the applications of special GBCAs in 2006, the US Food and Drug Administration (FDA) warned physicians not to inject these contrast agents in patients with compromised kidney function.3 Moreover, in 2007 the American College of Radiology (ACR) published guidelines for the safe use of GBCAs in patients with renal failure.4 Also, the European Medicines Agency (EMA) demanded that companies provide warning in product inserts about the acquisition of NSF in patients with severe kidney injury.5
Second, the clinical illustration of the case is inadequate. In the manuscript, we read that the patient acquired NSF-characteristic lesions like peau d’orange skin lesions and contractures of his extremities, but unfortunately, Chuang, Kaneshiro, and Betancourt did not provide figures that show them. On the other hand, Figure 1 shows an uncharacteristic dermal induration around inflammatory and ulcerated skin lesion (pyoderma gangrenosum).1 Such clinical signs are well known and occur perilesional of different conditions independently of NSF.6-8
Third, the histological features described as presence of fibrotic tissue in the deep dermis in Figure 2, and dermal fibrosis with thick collagen deposition in Figure 31 do not confirm the existence of NSF.
Taken together, the case presented by Chuang, Kaneshiro, and Betancourt contains some unclear aspects; therefore, it is questionable whether the published case describes a patient with NSF or not. In the current presentation, the diagnosis NSF seems to be an overestimation.
NSF still is a poorly understood disorder. Therefore, exactly documented new cases could be of clinical value when providing interesting information. Even single cases could shed some light in the darkness of the pathological mechanisms of this entity. On the other hand, we should not mix the existing cohort of published NSF cases with other scleroderma-like diseases, because this will lead to a confusion. Moreover, such a practice could inhibit the discovery of the pathophysiology of NSF.
- Ingrid Böhm, MD
Author affiliations: Ingrid Böhm is a Physician in the Department of Diagnostics, Interventional and Pediatric Radiology at the University Hospital of Bern, Inselspital, University of Bern in Bern, Switzerland.
Correspondence: Ingrid Böhm ([email protected])
Disclosures: The author reports no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract . 2018;35(6):40-43.
2. Larson KN, Gagnon AL, Darling MD, Patterson JW, Cropley TG. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151(10):1117-1120.
3. US Food and Drug Administration. A Public Health Advisory. Gadolinium-containing contrast agents for magnetic resonance imaging (MRI). http://wayback.archive-it.org/7993/20170112033022/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformation forPatientsandProviders/ucm053112.htm. Published June 8, 2006. Accessed March 15, 2019.
4. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
5. European Medicines Agency. Public statement: Vasovist and nephrogenic systemic fibrosis (NSF). https://www.ema.europa.eu/en/news/public-statement-vasovist-nephrogenic-systemic-fibrosis-nsf. Published February 7, 2007. Accessed March 15, 2019.
6. Luke JC. The etiology and modern treatment of varicose ulcer. Can Med Assoc J. 1940;43(3):217-221.
7. Paulsen E, Bygum A. Keratin gel as an adjuvant in the treatment of recalcitrant pyoderma gangrenosum ulcers: a case report. Acta Derm Venereol. 2019;99(2):234-235.
8. Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000; 136(2):167-169.
Response:
We thank Drs. Heinz, van der Molen, and Roditi for their valuable response. The following is the opinion of the authors and is not representative of the views or policies of our institution. The patient in this case received a gadolinium-based contrast agent (GBCA) in 2015 and was diagnosed with nephrogenic systemic fibrosis (NSF) 8 weeks later. We agree with the correspondents that linear GBCAs should not be used in patients with eGFR < 30 mL/min/1.73 m2. To date, a few cases of patients who received GBCA and developed NSF since 2009 have unfortunately continued to be reported in the literature.1-3 Our intention in publishing this case was to provide ongoing education to the medical community regarding this serious condition to ensure prevention of future cases.
We thank Dr. Böhm for her important inquiry. The patient received a histopathologic diagnosis of NSF. The report from the patient’s left dorsal forearm skin punch biopsy was read by our pathologist as “fibrosis and inflammation consistent with nephrogenic systemic fibrosis,” a diagnosis agreed upon by our colleagues in the dermatology and rheumatology departments based on the rapidity of his symptom onset and progression. While we acknowledge that this patient had other inflammatory disorders of the skin that may have coexisted with the diagnosis, after weighing the preponderance of clinical evidence in support of the biopsy results, we believe that this represents a case of NSF, which is associated with high morbidity and mortality. Thankfully, the patient in this case engaged extensively in physical and occupational therapy and is still alive nearly 4 years later. We would like to thank all the letter writers for their correspondence.
Author Affiliations: Kelley Chuang and Casey Kaneshiro are Hospitalists and Jaime Betancourt is a Pulmonologist, all in the Department of Medicine at the VA Greater Los Angeles Healthcare System in California.
Correspondence: Kelley Chuang ([email protected])
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Aggarwal A, Froehlich AA, Essah P, Brinster N, High WA, Downs RW. Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report. J Med Case Rep. 2011;5:566.
2. Fuah KW, Lim CT. Erythema nodosum masking nephrogenic systemic fibrosis as initial skin manifestation. BMC Nephrol. 2017;18(1):249.
3. Koratala A, Bhatti V. Nephrogenic systemic fibrosis. Clin Case Rep. 2017;5(7):1184-1185.
To the Editor:
We read with interest the case report of nephrogenic systemic fibrosis (NSF) by Chuang, Kaneshiro, and Betancourt in the June 2018 issue of Federal Practitioner.1 It was reported that a 61-year-old Hispanic male patient with a history of IV heroin abuse with end-stage renal disease (ESRD) secondary to membranous glomerulonephritis on hemodialysis and chronic hepatitis C infection received 15 mL gadoversetamide, a linear gadolinium-based contrast agent (GBCA) during magnetic resonance imaging (MRI) of the brain. Hemodialysis was performed 18 hours after the contrast administration.
Eight weeks after his initial presentation, the patient developed pyoderma gangrenosum on his right forearm, which was treated with high-dose steroids. He then developed thickening and induration of his bilateral forearm skin with peau d’orange appearance. NSF was confirmed by a skin biopsy. The patient developed contractures of his upper and lower extremities and was finally wheelchair bound.
This case is very concerning since no NSF cases in patients receiving GBCA have been published since 2009. Unfortunately, the authors give no information on the occurrence of this particular case. Thus, it is unclear whether this case was observed before or after the switch to macrocyclic agents in patients with reduced renal function. The reported patient with ESRD was on hemodialysis and received 15 mL gadoversetamide during MRI of the brain. In 2007 the ESUR (European Society of Urogenital Radiology) published guidelines indicating linear GBCA (gadodiamide, gadoversetamide, gadopentetate dimeglumine) as high-risk agents that may not be used in patients with eGFR < 30 mL/min/1.73 m2.2,3
Consequently in 2007, the European Medicines Agency contraindicated these linear GBCA in patients with chronic kidney disease grades 4 and 5. Also in 2007 the US Food and Drug Administration (FDA) requested a revision of the prescribing information for all 5 GBCA approved in the US.4 In response to accumulating more informative data, in 2010 the FDA again used this class labeling approach to more explicitly describe differences in NSF risks among the agents.4 FDA regulation and contraindication of the use of low-stability GBCA in patients with advanced renal impairment and robust local policies on the safe use of these agents have resulted in marked reduction in the prevalence of NSF in the US. This case report needs to clarify why a high-risk linear agent was administered to a patient with ESRD.
In 2006 Grobner and Marckmann and colleagues reported their observations of a previously unrecognized link between exposure to gadodiamide and the development of NSF.5,6 It soon became clear that NSF is a delayed adverse contrast reaction that may cause severe disability and even death. Advanced renal disease and high-risk linear GBCA are the main factors in the pathogenesis of NSF. Additionally, the dose of the agent may play a role. NSF can occur from hours to years after exposure to GBCA. Not all patients with severe kidney disease exposed to high-risk agents developed NSF. Thus, additional factors were proposed to play a role in the pathogenesis of NSF. Among those factors were erythropoietin, metabolic acidosis, anion gap, iron, increased phosphate, zinc loss, proinflammatory conditions/inflammation and angiotensin-converting enzyme (ACE) inhibitors.7 Although there is little proof with these assumptions, special care must be taken as shown by this reported patient with multiple inflammatory disorders.
- Gertraud Heinz, MD, MBA; Aart van der Molen, MD; and Giles Roditi, MD; on behalf of the ESUR Contrast Media Safety Committee
Author affiliations: Gertraud Heinz is former President ESUR and Head of the Department of Radiology, Diagnostics and Intervention University Hospital St. Pölten Karl Landsteiner University of Health Sciences.
Correspondence: Gertraud Heinz (gertraud.heinz@stpoelten .lknoe.at)
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract. 2018;35(6):40-43.
2. Thomsen HS; European Society of Urogenital Radiology (ESUR). ESUR guideline: gadolinium based contrast media and nephrogenic systemic fibrosis. Eur Radiol. 2007;17(10):2692-2696.
3. Thomsen HS, Morcos SK, Almén T, et al; ESUR Contrast Medium Safety Committee. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2013;23(2):307-318
4. Yang L, Krefting I, Gorovets A, et al. Nephrogenic systemic fibrosis and class labeling of gadolinium-based agents by the Food and Drug Administration. Radiology. 2012;265(1):248-253.
5. Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104-1108.
6. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359-2362.
7. Thomsen HS, Bennett CL. Six years after. Acta Radiol. 2012;53(8):827-829.
To the Editor:
With great interest, I read the case report by Chuang, Kaneshiro, and Betancourt.1 Patients with nephrogenic systemic fibrosis (NSF) are of special interest because the disease is still unclear as mentioned by the authors. Although new cases may occur,2 this case raises some concerns that I would like to address.
First, it would be of great interest to know the date when the patient received the high-risk gadolinium-based contrast agent (GBCA) gadoversetamide. Unfortunately, the authors did not mention the date of the injection of the GBCA that probably caused NSF. Due to the obvious association between the applications of special GBCAs in 2006, the US Food and Drug Administration (FDA) warned physicians not to inject these contrast agents in patients with compromised kidney function.3 Moreover, in 2007 the American College of Radiology (ACR) published guidelines for the safe use of GBCAs in patients with renal failure.4 Also, the European Medicines Agency (EMA) demanded that companies provide warning in product inserts about the acquisition of NSF in patients with severe kidney injury.5
Second, the clinical illustration of the case is inadequate. In the manuscript, we read that the patient acquired NSF-characteristic lesions like peau d’orange skin lesions and contractures of his extremities, but unfortunately, Chuang, Kaneshiro, and Betancourt did not provide figures that show them. On the other hand, Figure 1 shows an uncharacteristic dermal induration around inflammatory and ulcerated skin lesion (pyoderma gangrenosum).1 Such clinical signs are well known and occur perilesional of different conditions independently of NSF.6-8
Third, the histological features described as presence of fibrotic tissue in the deep dermis in Figure 2, and dermal fibrosis with thick collagen deposition in Figure 31 do not confirm the existence of NSF.
Taken together, the case presented by Chuang, Kaneshiro, and Betancourt contains some unclear aspects; therefore, it is questionable whether the published case describes a patient with NSF or not. In the current presentation, the diagnosis NSF seems to be an overestimation.
NSF still is a poorly understood disorder. Therefore, exactly documented new cases could be of clinical value when providing interesting information. Even single cases could shed some light in the darkness of the pathological mechanisms of this entity. On the other hand, we should not mix the existing cohort of published NSF cases with other scleroderma-like diseases, because this will lead to a confusion. Moreover, such a practice could inhibit the discovery of the pathophysiology of NSF.
- Ingrid Böhm, MD
Author affiliations: Ingrid Böhm is a Physician in the Department of Diagnostics, Interventional and Pediatric Radiology at the University Hospital of Bern, Inselspital, University of Bern in Bern, Switzerland.
Correspondence: Ingrid Böhm ([email protected])
Disclosures: The author reports no conflict of interest with regard to this article.
References
1. Chuang K, Kaneshiro C, Betancourt J. Nephrogenic systemic fibrosis in a patient with multiple inflammatory disorders. Fed Pract . 2018;35(6):40-43.
2. Larson KN, Gagnon AL, Darling MD, Patterson JW, Cropley TG. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151(10):1117-1120.
3. US Food and Drug Administration. A Public Health Advisory. Gadolinium-containing contrast agents for magnetic resonance imaging (MRI). http://wayback.archive-it.org/7993/20170112033022/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformation forPatientsandProviders/ucm053112.htm. Published June 8, 2006. Accessed March 15, 2019.
4. Kanal E, Barkovich AJ, Bell C, et al; ACR Blue Ribbon Panel on MR Safety. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188(6):1447-1474.
5. European Medicines Agency. Public statement: Vasovist and nephrogenic systemic fibrosis (NSF). https://www.ema.europa.eu/en/news/public-statement-vasovist-nephrogenic-systemic-fibrosis-nsf. Published February 7, 2007. Accessed March 15, 2019.
6. Luke JC. The etiology and modern treatment of varicose ulcer. Can Med Assoc J. 1940;43(3):217-221.
7. Paulsen E, Bygum A. Keratin gel as an adjuvant in the treatment of recalcitrant pyoderma gangrenosum ulcers: a case report. Acta Derm Venereol. 2019;99(2):234-235.
8. Boehm I, Bauer R. Low-dose methotrexate controls a severe form of polyarteritis nodosa. Arch Dermatol. 2000; 136(2):167-169.
Response:
We thank Drs. Heinz, van der Molen, and Roditi for their valuable response. The following is the opinion of the authors and is not representative of the views or policies of our institution. The patient in this case received a gadolinium-based contrast agent (GBCA) in 2015 and was diagnosed with nephrogenic systemic fibrosis (NSF) 8 weeks later. We agree with the correspondents that linear GBCAs should not be used in patients with eGFR < 30 mL/min/1.73 m2. To date, a few cases of patients who received GBCA and developed NSF since 2009 have unfortunately continued to be reported in the literature.1-3 Our intention in publishing this case was to provide ongoing education to the medical community regarding this serious condition to ensure prevention of future cases.
We thank Dr. Böhm for her important inquiry. The patient received a histopathologic diagnosis of NSF. The report from the patient’s left dorsal forearm skin punch biopsy was read by our pathologist as “fibrosis and inflammation consistent with nephrogenic systemic fibrosis,” a diagnosis agreed upon by our colleagues in the dermatology and rheumatology departments based on the rapidity of his symptom onset and progression. While we acknowledge that this patient had other inflammatory disorders of the skin that may have coexisted with the diagnosis, after weighing the preponderance of clinical evidence in support of the biopsy results, we believe that this represents a case of NSF, which is associated with high morbidity and mortality. Thankfully, the patient in this case engaged extensively in physical and occupational therapy and is still alive nearly 4 years later. We would like to thank all the letter writers for their correspondence.
Author Affiliations: Kelley Chuang and Casey Kaneshiro are Hospitalists and Jaime Betancourt is a Pulmonologist, all in the Department of Medicine at the VA Greater Los Angeles Healthcare System in California.
Correspondence: Kelley Chuang ([email protected])
Disclosures: The authors report no conflict of interest with regard to this article.
References
1. Aggarwal A, Froehlich AA, Essah P, Brinster N, High WA, Downs RW. Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report. J Med Case Rep. 2011;5:566.
2. Fuah KW, Lim CT. Erythema nodosum masking nephrogenic systemic fibrosis as initial skin manifestation. BMC Nephrol. 2017;18(1):249.
3. Koratala A, Bhatti V. Nephrogenic systemic fibrosis. Clin Case Rep. 2017;5(7):1184-1185.