User login
Creating an Intensive Care Unit From a Postanesthesia Care Unit for the COVID-19 Surge at the Veterans Affairs Ann Arbor Healthcare System
The rise in prevalence of the community spread of coronavirus disease 2019 (COVID-19) in the US in early March 2020 led to hospital systems across the country preparing for an increase in critically ill patients.1 The US Department of Veterans Affairs (VA) Ann Arbor Healthcare System (VAAAHS) anticipated an increased census of veterans who would need hospital admission for severe COVID-19 as well as the potential need to receive patients from community hospitals in Southeast Michigan, the location of one of the worst outbreaks in the US at that time.2
Through the facility’s incident command center, a hospital operations group identified the postanesthesia care unit (PACU) as a space to convert to an intensive care unit (ICU) for patients with COVID-19 needing mechanical ventilation. Other hospitals throughout the world have created similar makeshift ICUs to help care for the surge of patients with COVID-19, recognizing the high level of monitoring and resources available in the perioperative setting.3-5 These ICUs have been successfully created in operating rooms,3 recovery rooms,5 and procedural settings.4
Between March 27, 2020 and April 25, 2020, a great multidisciplinary effort enabled the VAAAHS PACU-ICU to care for critically ill veterans with COVID-19 from Southeast Michigan as well as civilian transfers from overwhelmed neighboring community hospitals. This article will discuss planning considerations, including facility preparation, equipment, and staffing models. The unique challenges faced in managing an open-plan surge-capacity ICU also will be discussed as well as the solutions that were enacted.
Methods
Hospital Preparation
Maintaining a 2-zone model in which patients with COVID-19 and without COVID-19 could be cared for separately was of major importance. The VAAAHS traditional ICU was converted into a 16-bed COVID-19 ICU and staffed by the Pulmonary Critical Care Service. A separate wing of the hospital was converted into a 19-bed non-COVID-19 ICU, which also was staffed by the Pulmonary Critical Care Service that increased its staffing of residents, fellows, and attending physicians to meet the increasing clinical demands. Elective major surgery cases were postponed, and surgeons managed the care of postoperative surgical ICU patients. This arrangement allowed the existing 4 anesthesiologist intensivists to staff the PACU COVID-19 ICU.
Considerations, including space requirements, staffing, equipment, infection control requirements, and ability for facilities to engineer a negative pressure space were factored into the decision to convert the PACU to an additional 12-bed ICU. This effectively tripled the VAAAHS ICU capacity, enabling patient transfers from the John D. Dingell VA Medical Center in Detroit, Michigan, which was being impacted by a surge of cases in Detroit. In addition, this allowed for the opening of the hospital for both COVID-19 and non-COVID-19 ICU transfers from hospitals in Southeast Michigan in order to fulfill the fourth VA mission to provide care and support to state and local communities for emergency management, public health, and safety.
PACU Preparation
PACU was selected as an overflow ICU due to its open floor plan, allowing patients on ventilators to be seen from a central nursing station. This would allow for the safe use of ventilators without central alarm capabilities (especially anesthesia machines). Given the risk of a circuit disconnect, all ventilators without central alarm capabilities needed to be seen and heard within the space to ensure patient safety.
Facilities Management was able to construct temporary barriers with vinyl covered sheetrock and plexiglass to partition the central nursing workstation from the patient area in a U-shape (Figure 1). The patient area was turned into a negative pressure space where strict airborne precautions could be observed. Although the air handling unit serving this space is equipped with high efficiency particulate air (HEPA) filters, it was mechanically manipulated to ensure that all air coming from the space was discharged through exhaust and not recirculated into another occupied space within the hospital. Total air exchange rates were measured and calculated for both the positive and negative spaces to ensure they met or exceeded at least 6 air changes per hour, as recommended by Occupational Safety and Health Administration guidance.6,7 A differential pressure indicator was installed to provide staff with the ability to monitor the pressure relationship between the 2 spaces in real time.
Twelve patient care beds were created. A traditionally engineered airborne infection isolation room in PACU served as a procedure room for aerosol-generating procedures, especially intubation, extubation, use of high-flow nasal cannula, and tracheostomy placement. Strict airborne precautions were taken within the patient area. The area inside the nursing station was positively pressurized to allow for surgical masks only to be required for the comfort of health care workers (Figure 2). A clear donning and doffing workflow was created for movement between the nursing area and the patient care area.
Personal Protective Equipment
Personal protective equipment (PPE) was of paramount importance in this open care unit. Airborne precautions were used in the entire patient care area. Powered air-purifying respirators (PAPRs) were used when possible to conserve the supply of N95 masks. Each health care worker was issued a reusable PAPR hood, which was cleaned by the user after each use by wiping the exterior of the entire hood with virucidal wipes. The brand and active ingredient of the virucidal wipes varied by availability of supplies, but the “virus kill time” was clearly labeled on each container. Each health care worker had a paper bag for storing his or her PAPR hood between usage to allow drying and ventilation. PAPR units were charged in between uses and shared by all clinical staff. Two layers of nonsterile gloves were worn.
Because of the open care area, attention had to be given to adhere to infection control policies if health care workers wanted to care for multiple patients while in the area. A new gown was placed over the existing gown, and the outer layer of gloves was removed. The under layer of gloves was then sanitized with hand sanitizer, and a new pair of outer gloves was then worn.
Equipment
Much of the ICU-level equipment needed was already present within the operating room (OR) area. Existing patient monitors were used and connected to a central monitoring station present in the nurses station. Relevant contents of the ICU storage room were duplicated and placed on shelves in the patient care area. Out-of-use anesthesia carts were used for a dedicated COVID-19 invasive line cart. A designated ultrasound with cardiac and vascular access probes was assigned to the PACU-ICU. Anesthesia machines were brought into the PACU-ICU and prepared with viral filters in line to prevent contamination of the machines, in keeping with national guidance from the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation.8
Multidisciplinary Staffing Model
With the reduced surgical and procedural case load due to halting nonemergent operations, the Anesthesiology and Perioperative Care Service was able to staff the PACU-ICU with critical care anesthesiologists, nurse anesthetists, residents, and PACU and procedural nurses without hindering access to emergent surgeries. A separate preoperative area was maintained with an 8-bed capacity for both preoperative and postoperative management of non-COVID-19 surgical patients.
The staffing model was designed using guidance on the expansion of ICU staffing with non-ICU resources from the Society of Critical Care Medicine as well as local guidance on appropriate nursing ratios (Figure 3).9 Given the high acuity and dynamic nature of COVID-19 coupled with the unique considerations that exist using anesthesia machines as long-term ICU ventilators, 24-hour inhospital attending intensivist coverage was provided in the ICU by 4 critical care anesthesiologists who rotated between 12-hour day and night shifts. The critical care anesthesiologists led a team of anesthesiology and surgery residents and ICU advanced practice providers dedicated solely to the PACU-ICU. Non-ICU anesthesiologists helped with procedures such as intubation and invasive line placement and provided coverage of the ICU patients during sign-out and rounding. Certified registered nurse anesthetists (CRNAs) performed intubations and helped offload respiratory therapists (one of the resources most in shortage) by managing and weaning ventilators and were instrumental in prone positioning of patients. Dedicated ICU nurses were deployed every shift to oversee the unit and act as a resource to the PACU nurses. Fortunately, many PACU nurses had prior ICU training and experience, and nurses from outpatient areas also were recruited to help with patient care. Together, they provided direct patient care. OR nurses assisted with delivering supplies, medications and transporting specimens to the laboratory, as no formal hospital tube station was present in the PACU.
Because of the open-unit setting, nurses practiced bundled care and staggered their turns in the patient care area. For example, a nurse who entered to administer medication to patient A, could then receive communication to check the urine output for patient B and do so without completely doffing and redonning. This allowed preservation of PPE and reduced time in PPE for the health care providers (HCPs).
A scheduled daily meeting included staff from PACU-ICU; Medical ICU (MICU), which also treated patients with COVID-19; and the Palliative Care Service (Figure 4). Patients with single-organ failure were preferentially sent to PACU-ICU, as the ability to do renal replacement therapy (RRT) in an open unit proved difficult. The palliative care team and VAAAHS social workers assisted both MICU and PACU-ICU with communicating with patients’ families, which provided a great help during a clinically demanding time. Physical therapists increased their staffing of the ICU to specifically help with mobilization of patients with COVID-19 and acute respiratory distress syndrome, given the prolonged mechanical ventilation courses that were seen. Other consulting services frequently involved included infectious disease and nephrology.
Challenges and Solutions
Communication between staff located within the patient area and staff located in the nursing station was difficult given the loud noise generated by a PAPR and the plexiglass walls that separated the areas. Multiple techniques were attempted to overcome this. Dry erase boards were placed within the space to facilitate requests, but these were found to be time consuming. Two-way radios worked well if the users were wearing N95s but were harder to communicate when users were wearing PAPRs. Baby monitors were purchased to facilitate 2-way communication and were useful at times although quieter than desired. Vocera B3000N Communication Badges, which were already utilized in the perioperative period at the facility, could be utilized underneath PPE and were ultimately the best form of clear communication between staff within the patient care area and outside the negative pressure zone. In accordance with company guidance, these mobile devices were cleaned with virucidal wipes after use.10
Communication with patients’ families was critically important. The ICU team, palliative care team, or social workers made daily telephone calls to family members. The facility telehealth coordinator provided a designated tablet device to enable the intensivists to video conference with the patients’ families at bedside, utilizing virtual care manager appointments. This allowed families to see and interact with their loved ones despite the prohibition of family visitors. Every effort was made to utilize video calling daily; however, clinical demands as well as Internet and technological constraints from individual family members intermittently precluded video calls.
Clinical Challenges
Patients with severe COVID-19 infections requiring mechanical ventilation have proven to be exceptionally high-acuity patients with myriad organ-based complications reported.11 Specific to our PACU-ICU, we determined that it was impractical to arrange for continuous RRT given the amount of training PACU nursing staff would have required and the limited ICU nursing staff in the PACU-ICU. Intermittent hemodialysis required replumbing for water supply and drainage but was ultimately not required as our facility expanded the number of continuous RRT machines available, allowing all patients in the COVID-19 ICU who required RRT to stay in the 16-bed ICU. Daily communication with the MICU allowed for safe transfer of patients with imminent needs for RRT to the MICU, providing a coordinated strategy for the deployment of scarce resources across our expanded ICU footprint.
Using anesthesia machines as ICU ventilators proved challenging, despite following best practice guidance.8 Notably, anesthesia machines are not actively humidified and require very high fresh gas flows, necessitating the addition of heat moisture exchangers (HME) to the circuit. Also, viral filters were placed in the circuit to prevent machine contamination. The addition of the HME and viral filters to each circuit increased the present dead space and led todifficulty in providing adequate ventilation to patients who already may have had a high proportion of physiologic dead space. The high fresh gas flows used still seemed inadequate in preventing moisture buildup in the machine parts, necessitating frequent exchanges of viral filters, HMEs, and circuits to prevent high peak airway pressures. In addition, anesthesia machines directly sample gas from the patient's breathing circuit, creating the risk for contamination of the space. This required a reconfiguration to allow for a suction scavenging system by VAAAHS biomedical engineers. Also, anesthesia machines are not designed for long-term ventilation and have different ventilation modes compared with modern ICU ventilators. Although they were used for several patients when the PACU-ICU opened, the hospital was able to acquire additional ICU ventilators, and extensive or prolonged use of anesthesia machine ventilators was avoided.
Infection Control
The open care setting provided unique infection control issues that had to be addressed.12 The open setting allowed preservation of PPE and the ability for bundled care to be delivered easily. The VAAAHS infection control team worked closely with the ICU team to develop practices to ensure both patient and health care worker protection. Notable challenges included donning new gowns between patients when a PAPR was already being worn, leading to draping of new gowns over existing gowns when going between patients. True hand hygiene was also difficult, as health care workers did not want to completely remove gloves while in the patient care area. Layering of 2 pairs of gloves allowed the outer gloves to be removed after care of each patient, at which time alcohol gel was applied to the inner gloves, a new gown was placed over the existing gown, and a new pair of gloves was layered on top.
Although patients were intubated for long periods in the PACU-ICU, there was concern for increased risk of exposure of health care workers after extubation given the inability to contain the coughing patients within a private room. If a patient did well, they were transferred to a private room on the general medical floors within 24 hours of extubation to minimize this risk.
Privacy
The open care design meant less privacy for patients than would be provided in a private room. Curtains were drawn around patient beds as much as possible, especially for nursing care, but priority was given to visualization of the ventilator when a HCP was not present to ensure safety at all times. The majority of patients cared for in the PACU-ICU were intubated and sedated on arrival, but thankfully many were extubated. After extubation privacy in the open care area became more of an issue and may have led to more nighttime disturbances and substandard delirium prevention measures. Priority was given to expediting the transfer of these patients to private rooms on the general medical floor once their respiratory status was deemed stable.
Conclusions
The COVID-19 pandemic is truly an unprecedented event in our nation’s history, which has led to the first nationwide authorization of the fourth mission of VA to provide support for national, state, and local public health. The PACU-ICU was designed, engineered, built, and staffed by perioperative HCPs through an exceptional multidisciplinary effort in a matter of days. Through this dedication of health care workers and staff, the VAAAHS was able to care for critically ill veterans from Southeast Michigan and serve the community during a time of overwhelming demand on the national health care system.
Acknowledgments
The authors thank the outstanding team of administrators, engineers, physical therapists, pharmacists, nurses, advanced practice providers, CRNAs, respiratory therapists, and physicians who made it possible to respond to our veterans’ and our community’s needs in a time of unprecedented demand on our health care system. A special thank you to Eric Deters, Chief Strategy Officer; Brittany McClure, ICU Nurse Manager; and Mark Dotson, Chief Supply Chain Officer. It was a privilege to serve on this mission together.
1. Murray CJL; IHME COVID-19 Health Service Utilization Forecasting Team. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator days and deaths by US state in the next 4 months. https://www.medrxiv.org/content/10.1101/2020.03.27.20043752v1.full.pdf. Accessed July 17, 2020.
2. Johns Hopkins University and Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/data/state-timeline/new-confirmed-cases/michigan. Updated July 17, 2020. Accessed July 17, 2020.
3. Mojoli F, Mongodi S, Grugnetti G, et al. Setup of a dedicated coronavirus intensive care unit: logistical aspects. Anesthesiology. 2020;133(1):244-246. doi:10.1097/ALN.0000000000003325
4. Peters AW, Chawla KS, Turnbull ZA. Transforming ORs into ICUs. N Engl J Med. 2020;382(19):e52. doi:10.1056/NEJMc2010853
5. Lund E, Whitten A, Middleton R, Phlippeau N, Flynn DN. Converting peri-anesthesia care units into COVID-19 critical care units: one community hospital’s response. Anesthesiology News. April 30, 2020. https://www.anesthesiologynews.com/Online-First/Article/04-20/Converting-Peri-Anesthesia-Care-Units-Into-COVID-19-Critical-Care-Units/58167. Accessed July 14, 2020.
6. American Institute of Architects. Guidelines for Design and Construction of Hospitals and Healthcare Facilities. Washington, DC: American Institute of Architects Press; 2001.
7. Garner JS. The CDC Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1993;21(3):160-162. doi:10.1016/0196-6553(93)90009-s
8. American Society of Anesthesiologists. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Updated May 7, 2020. Accessed July 14, 2020.
9. Halpern NA, Tan KS. United States Resource Availability for COVID-19. https://sccm.org/getattachment/Blog/March-2020/United-States-Resource-Availability-for-COVID-19/United-States-Resource-Availability-for-COVID-19.pdf. Updated May 12, 2020. Accessed July 14, 2020.
10. Vocera. Vocera devices and accessories cleaning guide. http://pubs.vocera.com/device/vseries/production/docs/vseries_device_cleaning_guide.pdf. Updated June 24, 2020. Accessed July 14, 2020.
11. Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19 [published online ahead of print, 2020 Mar 26]. JAMA. 2020;10.1001/jama.2020.4914. doi:10.1001/jama.2020.4914
12. O’Connell NH, Humphreys H. Intensive care unit design and environmental factors in the acquisition of infection. J Hosp Infect. 2000;45(4):255-262. doi:10.1053/jhin.2000.0768
The rise in prevalence of the community spread of coronavirus disease 2019 (COVID-19) in the US in early March 2020 led to hospital systems across the country preparing for an increase in critically ill patients.1 The US Department of Veterans Affairs (VA) Ann Arbor Healthcare System (VAAAHS) anticipated an increased census of veterans who would need hospital admission for severe COVID-19 as well as the potential need to receive patients from community hospitals in Southeast Michigan, the location of one of the worst outbreaks in the US at that time.2
Through the facility’s incident command center, a hospital operations group identified the postanesthesia care unit (PACU) as a space to convert to an intensive care unit (ICU) for patients with COVID-19 needing mechanical ventilation. Other hospitals throughout the world have created similar makeshift ICUs to help care for the surge of patients with COVID-19, recognizing the high level of monitoring and resources available in the perioperative setting.3-5 These ICUs have been successfully created in operating rooms,3 recovery rooms,5 and procedural settings.4
Between March 27, 2020 and April 25, 2020, a great multidisciplinary effort enabled the VAAAHS PACU-ICU to care for critically ill veterans with COVID-19 from Southeast Michigan as well as civilian transfers from overwhelmed neighboring community hospitals. This article will discuss planning considerations, including facility preparation, equipment, and staffing models. The unique challenges faced in managing an open-plan surge-capacity ICU also will be discussed as well as the solutions that were enacted.
Methods
Hospital Preparation
Maintaining a 2-zone model in which patients with COVID-19 and without COVID-19 could be cared for separately was of major importance. The VAAAHS traditional ICU was converted into a 16-bed COVID-19 ICU and staffed by the Pulmonary Critical Care Service. A separate wing of the hospital was converted into a 19-bed non-COVID-19 ICU, which also was staffed by the Pulmonary Critical Care Service that increased its staffing of residents, fellows, and attending physicians to meet the increasing clinical demands. Elective major surgery cases were postponed, and surgeons managed the care of postoperative surgical ICU patients. This arrangement allowed the existing 4 anesthesiologist intensivists to staff the PACU COVID-19 ICU.
Considerations, including space requirements, staffing, equipment, infection control requirements, and ability for facilities to engineer a negative pressure space were factored into the decision to convert the PACU to an additional 12-bed ICU. This effectively tripled the VAAAHS ICU capacity, enabling patient transfers from the John D. Dingell VA Medical Center in Detroit, Michigan, which was being impacted by a surge of cases in Detroit. In addition, this allowed for the opening of the hospital for both COVID-19 and non-COVID-19 ICU transfers from hospitals in Southeast Michigan in order to fulfill the fourth VA mission to provide care and support to state and local communities for emergency management, public health, and safety.
PACU Preparation
PACU was selected as an overflow ICU due to its open floor plan, allowing patients on ventilators to be seen from a central nursing station. This would allow for the safe use of ventilators without central alarm capabilities (especially anesthesia machines). Given the risk of a circuit disconnect, all ventilators without central alarm capabilities needed to be seen and heard within the space to ensure patient safety.
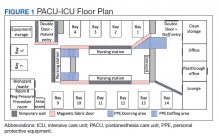
Facilities Management was able to construct temporary barriers with vinyl covered sheetrock and plexiglass to partition the central nursing workstation from the patient area in a U-shape (Figure 1). The patient area was turned into a negative pressure space where strict airborne precautions could be observed. Although the air handling unit serving this space is equipped with high efficiency particulate air (HEPA) filters, it was mechanically manipulated to ensure that all air coming from the space was discharged through exhaust and not recirculated into another occupied space within the hospital. Total air exchange rates were measured and calculated for both the positive and negative spaces to ensure they met or exceeded at least 6 air changes per hour, as recommended by Occupational Safety and Health Administration guidance.6,7 A differential pressure indicator was installed to provide staff with the ability to monitor the pressure relationship between the 2 spaces in real time.
Twelve patient care beds were created. A traditionally engineered airborne infection isolation room in PACU served as a procedure room for aerosol-generating procedures, especially intubation, extubation, use of high-flow nasal cannula, and tracheostomy placement. Strict airborne precautions were taken within the patient area. The area inside the nursing station was positively pressurized to allow for surgical masks only to be required for the comfort of health care workers (Figure 2). A clear donning and doffing workflow was created for movement between the nursing area and the patient care area.
Personal Protective Equipment
Personal protective equipment (PPE) was of paramount importance in this open care unit. Airborne precautions were used in the entire patient care area. Powered air-purifying respirators (PAPRs) were used when possible to conserve the supply of N95 masks. Each health care worker was issued a reusable PAPR hood, which was cleaned by the user after each use by wiping the exterior of the entire hood with virucidal wipes. The brand and active ingredient of the virucidal wipes varied by availability of supplies, but the “virus kill time” was clearly labeled on each container. Each health care worker had a paper bag for storing his or her PAPR hood between usage to allow drying and ventilation. PAPR units were charged in between uses and shared by all clinical staff. Two layers of nonsterile gloves were worn.
Because of the open care area, attention had to be given to adhere to infection control policies if health care workers wanted to care for multiple patients while in the area. A new gown was placed over the existing gown, and the outer layer of gloves was removed. The under layer of gloves was then sanitized with hand sanitizer, and a new pair of outer gloves was then worn.
Equipment
Much of the ICU-level equipment needed was already present within the operating room (OR) area. Existing patient monitors were used and connected to a central monitoring station present in the nurses station. Relevant contents of the ICU storage room were duplicated and placed on shelves in the patient care area. Out-of-use anesthesia carts were used for a dedicated COVID-19 invasive line cart. A designated ultrasound with cardiac and vascular access probes was assigned to the PACU-ICU. Anesthesia machines were brought into the PACU-ICU and prepared with viral filters in line to prevent contamination of the machines, in keeping with national guidance from the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation.8
Multidisciplinary Staffing Model
With the reduced surgical and procedural case load due to halting nonemergent operations, the Anesthesiology and Perioperative Care Service was able to staff the PACU-ICU with critical care anesthesiologists, nurse anesthetists, residents, and PACU and procedural nurses without hindering access to emergent surgeries. A separate preoperative area was maintained with an 8-bed capacity for both preoperative and postoperative management of non-COVID-19 surgical patients.
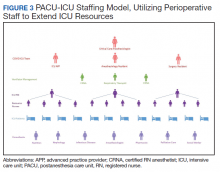
The staffing model was designed using guidance on the expansion of ICU staffing with non-ICU resources from the Society of Critical Care Medicine as well as local guidance on appropriate nursing ratios (Figure 3).9 Given the high acuity and dynamic nature of COVID-19 coupled with the unique considerations that exist using anesthesia machines as long-term ICU ventilators, 24-hour inhospital attending intensivist coverage was provided in the ICU by 4 critical care anesthesiologists who rotated between 12-hour day and night shifts. The critical care anesthesiologists led a team of anesthesiology and surgery residents and ICU advanced practice providers dedicated solely to the PACU-ICU. Non-ICU anesthesiologists helped with procedures such as intubation and invasive line placement and provided coverage of the ICU patients during sign-out and rounding. Certified registered nurse anesthetists (CRNAs) performed intubations and helped offload respiratory therapists (one of the resources most in shortage) by managing and weaning ventilators and were instrumental in prone positioning of patients. Dedicated ICU nurses were deployed every shift to oversee the unit and act as a resource to the PACU nurses. Fortunately, many PACU nurses had prior ICU training and experience, and nurses from outpatient areas also were recruited to help with patient care. Together, they provided direct patient care. OR nurses assisted with delivering supplies, medications and transporting specimens to the laboratory, as no formal hospital tube station was present in the PACU.
Because of the open-unit setting, nurses practiced bundled care and staggered their turns in the patient care area. For example, a nurse who entered to administer medication to patient A, could then receive communication to check the urine output for patient B and do so without completely doffing and redonning. This allowed preservation of PPE and reduced time in PPE for the health care providers (HCPs).
A scheduled daily meeting included staff from PACU-ICU; Medical ICU (MICU), which also treated patients with COVID-19; and the Palliative Care Service (Figure 4). Patients with single-organ failure were preferentially sent to PACU-ICU, as the ability to do renal replacement therapy (RRT) in an open unit proved difficult. The palliative care team and VAAAHS social workers assisted both MICU and PACU-ICU with communicating with patients’ families, which provided a great help during a clinically demanding time. Physical therapists increased their staffing of the ICU to specifically help with mobilization of patients with COVID-19 and acute respiratory distress syndrome, given the prolonged mechanical ventilation courses that were seen. Other consulting services frequently involved included infectious disease and nephrology.
Challenges and Solutions
Communication between staff located within the patient area and staff located in the nursing station was difficult given the loud noise generated by a PAPR and the plexiglass walls that separated the areas. Multiple techniques were attempted to overcome this. Dry erase boards were placed within the space to facilitate requests, but these were found to be time consuming. Two-way radios worked well if the users were wearing N95s but were harder to communicate when users were wearing PAPRs. Baby monitors were purchased to facilitate 2-way communication and were useful at times although quieter than desired. Vocera B3000N Communication Badges, which were already utilized in the perioperative period at the facility, could be utilized underneath PPE and were ultimately the best form of clear communication between staff within the patient care area and outside the negative pressure zone. In accordance with company guidance, these mobile devices were cleaned with virucidal wipes after use.10
Communication with patients’ families was critically important. The ICU team, palliative care team, or social workers made daily telephone calls to family members. The facility telehealth coordinator provided a designated tablet device to enable the intensivists to video conference with the patients’ families at bedside, utilizing virtual care manager appointments. This allowed families to see and interact with their loved ones despite the prohibition of family visitors. Every effort was made to utilize video calling daily; however, clinical demands as well as Internet and technological constraints from individual family members intermittently precluded video calls.
Clinical Challenges
Patients with severe COVID-19 infections requiring mechanical ventilation have proven to be exceptionally high-acuity patients with myriad organ-based complications reported.11 Specific to our PACU-ICU, we determined that it was impractical to arrange for continuous RRT given the amount of training PACU nursing staff would have required and the limited ICU nursing staff in the PACU-ICU. Intermittent hemodialysis required replumbing for water supply and drainage but was ultimately not required as our facility expanded the number of continuous RRT machines available, allowing all patients in the COVID-19 ICU who required RRT to stay in the 16-bed ICU. Daily communication with the MICU allowed for safe transfer of patients with imminent needs for RRT to the MICU, providing a coordinated strategy for the deployment of scarce resources across our expanded ICU footprint.
Using anesthesia machines as ICU ventilators proved challenging, despite following best practice guidance.8 Notably, anesthesia machines are not actively humidified and require very high fresh gas flows, necessitating the addition of heat moisture exchangers (HME) to the circuit. Also, viral filters were placed in the circuit to prevent machine contamination. The addition of the HME and viral filters to each circuit increased the present dead space and led todifficulty in providing adequate ventilation to patients who already may have had a high proportion of physiologic dead space. The high fresh gas flows used still seemed inadequate in preventing moisture buildup in the machine parts, necessitating frequent exchanges of viral filters, HMEs, and circuits to prevent high peak airway pressures. In addition, anesthesia machines directly sample gas from the patient's breathing circuit, creating the risk for contamination of the space. This required a reconfiguration to allow for a suction scavenging system by VAAAHS biomedical engineers. Also, anesthesia machines are not designed for long-term ventilation and have different ventilation modes compared with modern ICU ventilators. Although they were used for several patients when the PACU-ICU opened, the hospital was able to acquire additional ICU ventilators, and extensive or prolonged use of anesthesia machine ventilators was avoided.
Infection Control
The open care setting provided unique infection control issues that had to be addressed.12 The open setting allowed preservation of PPE and the ability for bundled care to be delivered easily. The VAAAHS infection control team worked closely with the ICU team to develop practices to ensure both patient and health care worker protection. Notable challenges included donning new gowns between patients when a PAPR was already being worn, leading to draping of new gowns over existing gowns when going between patients. True hand hygiene was also difficult, as health care workers did not want to completely remove gloves while in the patient care area. Layering of 2 pairs of gloves allowed the outer gloves to be removed after care of each patient, at which time alcohol gel was applied to the inner gloves, a new gown was placed over the existing gown, and a new pair of gloves was layered on top.
Although patients were intubated for long periods in the PACU-ICU, there was concern for increased risk of exposure of health care workers after extubation given the inability to contain the coughing patients within a private room. If a patient did well, they were transferred to a private room on the general medical floors within 24 hours of extubation to minimize this risk.
Privacy
The open care design meant less privacy for patients than would be provided in a private room. Curtains were drawn around patient beds as much as possible, especially for nursing care, but priority was given to visualization of the ventilator when a HCP was not present to ensure safety at all times. The majority of patients cared for in the PACU-ICU were intubated and sedated on arrival, but thankfully many were extubated. After extubation privacy in the open care area became more of an issue and may have led to more nighttime disturbances and substandard delirium prevention measures. Priority was given to expediting the transfer of these patients to private rooms on the general medical floor once their respiratory status was deemed stable.
Conclusions
The COVID-19 pandemic is truly an unprecedented event in our nation’s history, which has led to the first nationwide authorization of the fourth mission of VA to provide support for national, state, and local public health. The PACU-ICU was designed, engineered, built, and staffed by perioperative HCPs through an exceptional multidisciplinary effort in a matter of days. Through this dedication of health care workers and staff, the VAAAHS was able to care for critically ill veterans from Southeast Michigan and serve the community during a time of overwhelming demand on the national health care system.
Acknowledgments
The authors thank the outstanding team of administrators, engineers, physical therapists, pharmacists, nurses, advanced practice providers, CRNAs, respiratory therapists, and physicians who made it possible to respond to our veterans’ and our community’s needs in a time of unprecedented demand on our health care system. A special thank you to Eric Deters, Chief Strategy Officer; Brittany McClure, ICU Nurse Manager; and Mark Dotson, Chief Supply Chain Officer. It was a privilege to serve on this mission together.
The rise in prevalence of the community spread of coronavirus disease 2019 (COVID-19) in the US in early March 2020 led to hospital systems across the country preparing for an increase in critically ill patients.1 The US Department of Veterans Affairs (VA) Ann Arbor Healthcare System (VAAAHS) anticipated an increased census of veterans who would need hospital admission for severe COVID-19 as well as the potential need to receive patients from community hospitals in Southeast Michigan, the location of one of the worst outbreaks in the US at that time.2
Through the facility’s incident command center, a hospital operations group identified the postanesthesia care unit (PACU) as a space to convert to an intensive care unit (ICU) for patients with COVID-19 needing mechanical ventilation. Other hospitals throughout the world have created similar makeshift ICUs to help care for the surge of patients with COVID-19, recognizing the high level of monitoring and resources available in the perioperative setting.3-5 These ICUs have been successfully created in operating rooms,3 recovery rooms,5 and procedural settings.4
Between March 27, 2020 and April 25, 2020, a great multidisciplinary effort enabled the VAAAHS PACU-ICU to care for critically ill veterans with COVID-19 from Southeast Michigan as well as civilian transfers from overwhelmed neighboring community hospitals. This article will discuss planning considerations, including facility preparation, equipment, and staffing models. The unique challenges faced in managing an open-plan surge-capacity ICU also will be discussed as well as the solutions that were enacted.
Methods
Hospital Preparation
Maintaining a 2-zone model in which patients with COVID-19 and without COVID-19 could be cared for separately was of major importance. The VAAAHS traditional ICU was converted into a 16-bed COVID-19 ICU and staffed by the Pulmonary Critical Care Service. A separate wing of the hospital was converted into a 19-bed non-COVID-19 ICU, which also was staffed by the Pulmonary Critical Care Service that increased its staffing of residents, fellows, and attending physicians to meet the increasing clinical demands. Elective major surgery cases were postponed, and surgeons managed the care of postoperative surgical ICU patients. This arrangement allowed the existing 4 anesthesiologist intensivists to staff the PACU COVID-19 ICU.
Considerations, including space requirements, staffing, equipment, infection control requirements, and ability for facilities to engineer a negative pressure space were factored into the decision to convert the PACU to an additional 12-bed ICU. This effectively tripled the VAAAHS ICU capacity, enabling patient transfers from the John D. Dingell VA Medical Center in Detroit, Michigan, which was being impacted by a surge of cases in Detroit. In addition, this allowed for the opening of the hospital for both COVID-19 and non-COVID-19 ICU transfers from hospitals in Southeast Michigan in order to fulfill the fourth VA mission to provide care and support to state and local communities for emergency management, public health, and safety.
PACU Preparation
PACU was selected as an overflow ICU due to its open floor plan, allowing patients on ventilators to be seen from a central nursing station. This would allow for the safe use of ventilators without central alarm capabilities (especially anesthesia machines). Given the risk of a circuit disconnect, all ventilators without central alarm capabilities needed to be seen and heard within the space to ensure patient safety.
Facilities Management was able to construct temporary barriers with vinyl covered sheetrock and plexiglass to partition the central nursing workstation from the patient area in a U-shape (Figure 1). The patient area was turned into a negative pressure space where strict airborne precautions could be observed. Although the air handling unit serving this space is equipped with high efficiency particulate air (HEPA) filters, it was mechanically manipulated to ensure that all air coming from the space was discharged through exhaust and not recirculated into another occupied space within the hospital. Total air exchange rates were measured and calculated for both the positive and negative spaces to ensure they met or exceeded at least 6 air changes per hour, as recommended by Occupational Safety and Health Administration guidance.6,7 A differential pressure indicator was installed to provide staff with the ability to monitor the pressure relationship between the 2 spaces in real time.
Twelve patient care beds were created. A traditionally engineered airborne infection isolation room in PACU served as a procedure room for aerosol-generating procedures, especially intubation, extubation, use of high-flow nasal cannula, and tracheostomy placement. Strict airborne precautions were taken within the patient area. The area inside the nursing station was positively pressurized to allow for surgical masks only to be required for the comfort of health care workers (Figure 2). A clear donning and doffing workflow was created for movement between the nursing area and the patient care area.
Personal Protective Equipment
Personal protective equipment (PPE) was of paramount importance in this open care unit. Airborne precautions were used in the entire patient care area. Powered air-purifying respirators (PAPRs) were used when possible to conserve the supply of N95 masks. Each health care worker was issued a reusable PAPR hood, which was cleaned by the user after each use by wiping the exterior of the entire hood with virucidal wipes. The brand and active ingredient of the virucidal wipes varied by availability of supplies, but the “virus kill time” was clearly labeled on each container. Each health care worker had a paper bag for storing his or her PAPR hood between usage to allow drying and ventilation. PAPR units were charged in between uses and shared by all clinical staff. Two layers of nonsterile gloves were worn.
Because of the open care area, attention had to be given to adhere to infection control policies if health care workers wanted to care for multiple patients while in the area. A new gown was placed over the existing gown, and the outer layer of gloves was removed. The under layer of gloves was then sanitized with hand sanitizer, and a new pair of outer gloves was then worn.
Equipment
Much of the ICU-level equipment needed was already present within the operating room (OR) area. Existing patient monitors were used and connected to a central monitoring station present in the nurses station. Relevant contents of the ICU storage room were duplicated and placed on shelves in the patient care area. Out-of-use anesthesia carts were used for a dedicated COVID-19 invasive line cart. A designated ultrasound with cardiac and vascular access probes was assigned to the PACU-ICU. Anesthesia machines were brought into the PACU-ICU and prepared with viral filters in line to prevent contamination of the machines, in keeping with national guidance from the American Society of Anesthesiologists and Anesthesia Patient Safety Foundation.8
Multidisciplinary Staffing Model
With the reduced surgical and procedural case load due to halting nonemergent operations, the Anesthesiology and Perioperative Care Service was able to staff the PACU-ICU with critical care anesthesiologists, nurse anesthetists, residents, and PACU and procedural nurses without hindering access to emergent surgeries. A separate preoperative area was maintained with an 8-bed capacity for both preoperative and postoperative management of non-COVID-19 surgical patients.
The staffing model was designed using guidance on the expansion of ICU staffing with non-ICU resources from the Society of Critical Care Medicine as well as local guidance on appropriate nursing ratios (Figure 3).9 Given the high acuity and dynamic nature of COVID-19 coupled with the unique considerations that exist using anesthesia machines as long-term ICU ventilators, 24-hour inhospital attending intensivist coverage was provided in the ICU by 4 critical care anesthesiologists who rotated between 12-hour day and night shifts. The critical care anesthesiologists led a team of anesthesiology and surgery residents and ICU advanced practice providers dedicated solely to the PACU-ICU. Non-ICU anesthesiologists helped with procedures such as intubation and invasive line placement and provided coverage of the ICU patients during sign-out and rounding. Certified registered nurse anesthetists (CRNAs) performed intubations and helped offload respiratory therapists (one of the resources most in shortage) by managing and weaning ventilators and were instrumental in prone positioning of patients. Dedicated ICU nurses were deployed every shift to oversee the unit and act as a resource to the PACU nurses. Fortunately, many PACU nurses had prior ICU training and experience, and nurses from outpatient areas also were recruited to help with patient care. Together, they provided direct patient care. OR nurses assisted with delivering supplies, medications and transporting specimens to the laboratory, as no formal hospital tube station was present in the PACU.
Because of the open-unit setting, nurses practiced bundled care and staggered their turns in the patient care area. For example, a nurse who entered to administer medication to patient A, could then receive communication to check the urine output for patient B and do so without completely doffing and redonning. This allowed preservation of PPE and reduced time in PPE for the health care providers (HCPs).
A scheduled daily meeting included staff from PACU-ICU; Medical ICU (MICU), which also treated patients with COVID-19; and the Palliative Care Service (Figure 4). Patients with single-organ failure were preferentially sent to PACU-ICU, as the ability to do renal replacement therapy (RRT) in an open unit proved difficult. The palliative care team and VAAAHS social workers assisted both MICU and PACU-ICU with communicating with patients’ families, which provided a great help during a clinically demanding time. Physical therapists increased their staffing of the ICU to specifically help with mobilization of patients with COVID-19 and acute respiratory distress syndrome, given the prolonged mechanical ventilation courses that were seen. Other consulting services frequently involved included infectious disease and nephrology.
Challenges and Solutions
Communication between staff located within the patient area and staff located in the nursing station was difficult given the loud noise generated by a PAPR and the plexiglass walls that separated the areas. Multiple techniques were attempted to overcome this. Dry erase boards were placed within the space to facilitate requests, but these were found to be time consuming. Two-way radios worked well if the users were wearing N95s but were harder to communicate when users were wearing PAPRs. Baby monitors were purchased to facilitate 2-way communication and were useful at times although quieter than desired. Vocera B3000N Communication Badges, which were already utilized in the perioperative period at the facility, could be utilized underneath PPE and were ultimately the best form of clear communication between staff within the patient care area and outside the negative pressure zone. In accordance with company guidance, these mobile devices were cleaned with virucidal wipes after use.10
Communication with patients’ families was critically important. The ICU team, palliative care team, or social workers made daily telephone calls to family members. The facility telehealth coordinator provided a designated tablet device to enable the intensivists to video conference with the patients’ families at bedside, utilizing virtual care manager appointments. This allowed families to see and interact with their loved ones despite the prohibition of family visitors. Every effort was made to utilize video calling daily; however, clinical demands as well as Internet and technological constraints from individual family members intermittently precluded video calls.
Clinical Challenges
Patients with severe COVID-19 infections requiring mechanical ventilation have proven to be exceptionally high-acuity patients with myriad organ-based complications reported.11 Specific to our PACU-ICU, we determined that it was impractical to arrange for continuous RRT given the amount of training PACU nursing staff would have required and the limited ICU nursing staff in the PACU-ICU. Intermittent hemodialysis required replumbing for water supply and drainage but was ultimately not required as our facility expanded the number of continuous RRT machines available, allowing all patients in the COVID-19 ICU who required RRT to stay in the 16-bed ICU. Daily communication with the MICU allowed for safe transfer of patients with imminent needs for RRT to the MICU, providing a coordinated strategy for the deployment of scarce resources across our expanded ICU footprint.
Using anesthesia machines as ICU ventilators proved challenging, despite following best practice guidance.8 Notably, anesthesia machines are not actively humidified and require very high fresh gas flows, necessitating the addition of heat moisture exchangers (HME) to the circuit. Also, viral filters were placed in the circuit to prevent machine contamination. The addition of the HME and viral filters to each circuit increased the present dead space and led todifficulty in providing adequate ventilation to patients who already may have had a high proportion of physiologic dead space. The high fresh gas flows used still seemed inadequate in preventing moisture buildup in the machine parts, necessitating frequent exchanges of viral filters, HMEs, and circuits to prevent high peak airway pressures. In addition, anesthesia machines directly sample gas from the patient's breathing circuit, creating the risk for contamination of the space. This required a reconfiguration to allow for a suction scavenging system by VAAAHS biomedical engineers. Also, anesthesia machines are not designed for long-term ventilation and have different ventilation modes compared with modern ICU ventilators. Although they were used for several patients when the PACU-ICU opened, the hospital was able to acquire additional ICU ventilators, and extensive or prolonged use of anesthesia machine ventilators was avoided.
Infection Control
The open care setting provided unique infection control issues that had to be addressed.12 The open setting allowed preservation of PPE and the ability for bundled care to be delivered easily. The VAAAHS infection control team worked closely with the ICU team to develop practices to ensure both patient and health care worker protection. Notable challenges included donning new gowns between patients when a PAPR was already being worn, leading to draping of new gowns over existing gowns when going between patients. True hand hygiene was also difficult, as health care workers did not want to completely remove gloves while in the patient care area. Layering of 2 pairs of gloves allowed the outer gloves to be removed after care of each patient, at which time alcohol gel was applied to the inner gloves, a new gown was placed over the existing gown, and a new pair of gloves was layered on top.
Although patients were intubated for long periods in the PACU-ICU, there was concern for increased risk of exposure of health care workers after extubation given the inability to contain the coughing patients within a private room. If a patient did well, they were transferred to a private room on the general medical floors within 24 hours of extubation to minimize this risk.
Privacy
The open care design meant less privacy for patients than would be provided in a private room. Curtains were drawn around patient beds as much as possible, especially for nursing care, but priority was given to visualization of the ventilator when a HCP was not present to ensure safety at all times. The majority of patients cared for in the PACU-ICU were intubated and sedated on arrival, but thankfully many were extubated. After extubation privacy in the open care area became more of an issue and may have led to more nighttime disturbances and substandard delirium prevention measures. Priority was given to expediting the transfer of these patients to private rooms on the general medical floor once their respiratory status was deemed stable.
Conclusions
The COVID-19 pandemic is truly an unprecedented event in our nation’s history, which has led to the first nationwide authorization of the fourth mission of VA to provide support for national, state, and local public health. The PACU-ICU was designed, engineered, built, and staffed by perioperative HCPs through an exceptional multidisciplinary effort in a matter of days. Through this dedication of health care workers and staff, the VAAAHS was able to care for critically ill veterans from Southeast Michigan and serve the community during a time of overwhelming demand on the national health care system.
Acknowledgments
The authors thank the outstanding team of administrators, engineers, physical therapists, pharmacists, nurses, advanced practice providers, CRNAs, respiratory therapists, and physicians who made it possible to respond to our veterans’ and our community’s needs in a time of unprecedented demand on our health care system. A special thank you to Eric Deters, Chief Strategy Officer; Brittany McClure, ICU Nurse Manager; and Mark Dotson, Chief Supply Chain Officer. It was a privilege to serve on this mission together.
1. Murray CJL; IHME COVID-19 Health Service Utilization Forecasting Team. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator days and deaths by US state in the next 4 months. https://www.medrxiv.org/content/10.1101/2020.03.27.20043752v1.full.pdf. Accessed July 17, 2020.
2. Johns Hopkins University and Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/data/state-timeline/new-confirmed-cases/michigan. Updated July 17, 2020. Accessed July 17, 2020.
3. Mojoli F, Mongodi S, Grugnetti G, et al. Setup of a dedicated coronavirus intensive care unit: logistical aspects. Anesthesiology. 2020;133(1):244-246. doi:10.1097/ALN.0000000000003325
4. Peters AW, Chawla KS, Turnbull ZA. Transforming ORs into ICUs. N Engl J Med. 2020;382(19):e52. doi:10.1056/NEJMc2010853
5. Lund E, Whitten A, Middleton R, Phlippeau N, Flynn DN. Converting peri-anesthesia care units into COVID-19 critical care units: one community hospital’s response. Anesthesiology News. April 30, 2020. https://www.anesthesiologynews.com/Online-First/Article/04-20/Converting-Peri-Anesthesia-Care-Units-Into-COVID-19-Critical-Care-Units/58167. Accessed July 14, 2020.
6. American Institute of Architects. Guidelines for Design and Construction of Hospitals and Healthcare Facilities. Washington, DC: American Institute of Architects Press; 2001.
7. Garner JS. The CDC Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1993;21(3):160-162. doi:10.1016/0196-6553(93)90009-s
8. American Society of Anesthesiologists. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Updated May 7, 2020. Accessed July 14, 2020.
9. Halpern NA, Tan KS. United States Resource Availability for COVID-19. https://sccm.org/getattachment/Blog/March-2020/United-States-Resource-Availability-for-COVID-19/United-States-Resource-Availability-for-COVID-19.pdf. Updated May 12, 2020. Accessed July 14, 2020.
10. Vocera. Vocera devices and accessories cleaning guide. http://pubs.vocera.com/device/vseries/production/docs/vseries_device_cleaning_guide.pdf. Updated June 24, 2020. Accessed July 14, 2020.
11. Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19 [published online ahead of print, 2020 Mar 26]. JAMA. 2020;10.1001/jama.2020.4914. doi:10.1001/jama.2020.4914
12. O’Connell NH, Humphreys H. Intensive care unit design and environmental factors in the acquisition of infection. J Hosp Infect. 2000;45(4):255-262. doi:10.1053/jhin.2000.0768
1. Murray CJL; IHME COVID-19 Health Service Utilization Forecasting Team. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator days and deaths by US state in the next 4 months. https://www.medrxiv.org/content/10.1101/2020.03.27.20043752v1.full.pdf. Accessed July 17, 2020.
2. Johns Hopkins University and Medicine. Coronavirus resource center. https://coronavirus.jhu.edu/data/state-timeline/new-confirmed-cases/michigan. Updated July 17, 2020. Accessed July 17, 2020.
3. Mojoli F, Mongodi S, Grugnetti G, et al. Setup of a dedicated coronavirus intensive care unit: logistical aspects. Anesthesiology. 2020;133(1):244-246. doi:10.1097/ALN.0000000000003325
4. Peters AW, Chawla KS, Turnbull ZA. Transforming ORs into ICUs. N Engl J Med. 2020;382(19):e52. doi:10.1056/NEJMc2010853
5. Lund E, Whitten A, Middleton R, Phlippeau N, Flynn DN. Converting peri-anesthesia care units into COVID-19 critical care units: one community hospital’s response. Anesthesiology News. April 30, 2020. https://www.anesthesiologynews.com/Online-First/Article/04-20/Converting-Peri-Anesthesia-Care-Units-Into-COVID-19-Critical-Care-Units/58167. Accessed July 14, 2020.
6. American Institute of Architects. Guidelines for Design and Construction of Hospitals and Healthcare Facilities. Washington, DC: American Institute of Architects Press; 2001.
7. Garner JS. The CDC Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1993;21(3):160-162. doi:10.1016/0196-6553(93)90009-s
8. American Society of Anesthesiologists. APSF/ASA Guidance on Purposing Anesthesia Machines as ICU Ventilators. https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators. Updated May 7, 2020. Accessed July 14, 2020.
9. Halpern NA, Tan KS. United States Resource Availability for COVID-19. https://sccm.org/getattachment/Blog/March-2020/United-States-Resource-Availability-for-COVID-19/United-States-Resource-Availability-for-COVID-19.pdf. Updated May 12, 2020. Accessed July 14, 2020.
10. Vocera. Vocera devices and accessories cleaning guide. http://pubs.vocera.com/device/vseries/production/docs/vseries_device_cleaning_guide.pdf. Updated June 24, 2020. Accessed July 14, 2020.
11. Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With COVID-19 [published online ahead of print, 2020 Mar 26]. JAMA. 2020;10.1001/jama.2020.4914. doi:10.1001/jama.2020.4914
12. O’Connell NH, Humphreys H. Intensive care unit design and environmental factors in the acquisition of infection. J Hosp Infect. 2000;45(4):255-262. doi:10.1053/jhin.2000.0768
Contrasting qSOFA and SIRS Criteria for Early Sepsis Identification in a Veteran Population (FULL)
Sepsis is a major public health concern: 10% of patients with sepsis die, and mortality quadruples with progression to septic shock.1 Systemic inflammatory response syndrome (SIRS) criteria, originally published in 1992, are commonly used to detect sepsis, but as early as 2001, these criteria were recognized as lacking specificity.2 Nonetheless, the use of SIRS criteria has persisted in practice. Sepsis was redefined in Sepsis-3 (2016) to guide earlier and more appropriate identification and treatment, which has been shown to greatly improve patient outcomes.1,3 Key recommendations in Sepsis 3 included eliminating SIRS criteria, defining organ dysfunction by the Sequential Organ Failure Assessment (SOFA) score, and introducing the quick SOFA (qSOFA) score.1
The qSOFA combines 3 clinical variables to provide a rapid, simple bedside score that measures the likelihood of poor outcomes, such as admission to an intensive care unit (ICU) or mortality in adults with suspected infection.1,3 The qSOFA score is intended to aid healthcare professionals in more timely stratification of those patients who need escalated care to prevent deterioration.1 The assessment also has been explored as a screening tool for sepsis in clinical practice; however, limited data exists concerning the comparative utility of qSOFA and SIRS in this capacity, and study results are inconsistent.4-6
The most important attribute of a screening tool is high sensitivity, but high specificity also is desired. The qSOFA could supplant SIRS as a screening tool for sepsis if it maintained similarly high sensitivity but achieved superior specificity. Therefore, our primary objective for this study was to determine the effectiveness of qSOFA as a screening assessment for sepsis in the setting of a general inpatient medicine service by contrasting the sensitivity and specificity of qSOFA with SIRS in predicting sepsis, using a retrospective chart review design.
Methods
Administrative data from the Department of Veterans Affairs (VA) Corporate Data Warehouse were accessed via the VA Informatics and Computing Infrastructure (VINCI) and used to identify VA inpatient admissions and obtain the laboratory and vital sign data necessary to calculate SIRS, qSOFA, and SOFA scores. The data were supplemented by manual review of VA health records to obtain information that was not readily available in administrative records, including septic shock outcomes and laboratory and vital sign data obtained in the ICU. This study was approved by the institutional review board at the University of Iowa and the research and development committee at the Iowa City VA Medical Center (ICVAMC).
Patients
The study population included veterans admitted to the nonsurgical medicine unit at ICVAMC between August 1, 2014 and August 1, 2016 who were transferred to an ICU after admission; direct ICU admissions were not included as the qSOFA has been shown in studies to be more beneficial and offer better predictive validity outside the ICU. Excluding these direct admissions prevented any potential skewing of the data. To control for possible selection bias, veterans also were excluded if they transferred from another facility, were admitted under observation status, or if they had been admitted within the prior 30 days. These patients may have been more critically ill than those who presented directly to our facility and any prior treatment could affect the clinical status of the patient and assessment for sepsis at the time of presentation to the VA. Veterans were further required to have evidence of suspected infection based on manual review of the health record, which was determined by receipt of an antibiotic relevant to the empiric treatment of sepsis within 48 hours of admission.
Sepsis and Septic Shock Assessment Tools
As outlined in the Sepsis-3 guidelines, sepsis was defined as suspected or confirmed infection with an acute change in the SOFA score of ≥ 2 points, which is assumed to be 0 in those not known to have preexisting dysfunction.1 The SOFA score includes variables from the respiratory, coagulation, hepatic, cardiovascular, renal, and central nervous systems.1 Septic shock was defined as vasopressor administration and a serum lactic acid level > 2 mmol/L occurring up to 24 hours apart and within 3 days of the first antibiotic dose administered.
The SIRS assessment includes 4 clinical variables (temperature, heart rate, respiratory rate, and white blood cell count) while qSOFA is comprised of 3 variables (respiratory rate, systolic blood pressure, and altered mental status).1 With both assessments, a score ≥ 2 is considered positive, which indicates increased risk for sepsis in patients with suspected infection.1 In keeping with existing studies, qSOFA and SIRS assessments were scored using maximum values found within 48 hours before and 24 hours after the first administered antibiotic dose.3
Outcomes
The primary outcome variable was the presence of sepsis in adults with evidence of infection within 48 hours of admission. Secondary outcome measures included 30-day mortality and septic shock.
Performance between the SIRS and qSOFA assessments was contrasted using sensitivity, specificity, and positive and negative predictive value measurements. Associations of qSOFA and SIRS with septic shock and 30-day mortality were evaluated using a 2-tailed Fisher’s exact test with a threshold of α = 0.05 to determine statistical significance.
Results
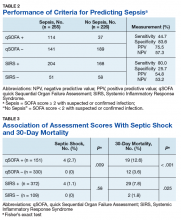
The study sample of 481 veterans had a mean age of 67.4 years, 94% were male, and 91.1% were white (Table 1).
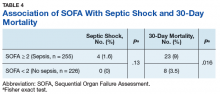
Scores for qSOFA, but not SIRS, were significantly associated with septic shock (Fisher’s exact test; qSOFA: P = .009; SIRS: P = .58) (Table 3).
Discussion
High sensitivity is critical for a sepsis screening tool. To be clinically useful, it has been suggested that biomarkers predicting poor outcomes for sepsis should have a sensitivity of > 80%.4 Although qSOFA demonstrated greater specificity than SIRS in our study (83.6% vs 25.7%), qSOFA showed lower sensitivity (44.7% vs 80.0%), which resulted in a greater potential for false negatives; 55.3% of those with sepsis would go undetected. Therefore, our study does not support qSOFA as a better screening assessment than SIRS for sepsis in the veteran population.
Most studies concur with our findings of low sensitivity and high specificity of qSOFA. In a systematic review and meta-analysis, Serafim and colleagues identified 10 studies published after Sepsis-3 that reported sensitivity or specificity of qSOFA and SIRS for sepsis diagnosis.5 Seven of the 10 studies reported sensitivities and favored SIRS in the diagnosis of sepsis (Relative risk: 1.32; 95% CI: 0.40-2.24; P < .0001; I2 = 100%). The authors noted that substantial heterogeneity among studies, including differences in study design, sample size, and criteria for determination of infection, was an important limitation. In addition, most studies that contrast qSOFA and SIRS center on prognostic value in predicting mortality, rather than as a screening test for a diagnosis of sepsis.
We concluded SIRS was more sensitive and thus superior to qSOFA when used as a screening tool for sepsis but conceded that more prospective and homogenous investigations were necessary. To our knowledge, only 1 published study has deviated from this conclusion and reported comparable sensitivity between SIRS (92%) and qSOFA (90%).6 Our study adds to existing literature as it is the first conducted in a veteran population. Additionally, we performed our investigation in a general medicine population with methods similar to existing literature, including the key study validating clinical criteria for sepsis by Seymour and colleagues.3
Limitations
This study is not without limitations, including potential misclassification of cases if essential data points were not available during data collection via health record review or the data points were not representative of a true change from baseline (eg, the Glasgow Coma Scale score for altered mental status in the qSOFA or the SOFA score for organ dysfunction). Generalizability of the results also may be limited due to our retrospective, single-center design and characteristics typical of a veteran population (eg, older, white males). Additionally, many veterans were excluded from the study if they transferred from another facility. These veterans may have been more critically ill than those who presented directly to our facility, which possibly introduced selection bias.
Conclusion
Our findings do not support use of the qSOFA as a suitable replacement for SIRS as a sepsis screening tool among patients with suspected infection in the general medicine inpatient setting. The clinical concern with SIRS is that unfavorable specificity leads to unnecessary antibiotic exposure among patients who are falsely positive. While qSOFA has demonstrated higher specificity, its use would cause many sepsis cases to go undetected due to the technique’s low sensitivity. Frequent false negative qSOFA results could thus serve to impede, rather than enhance, early recognition and intervention for sepsis.
The ideal sepsis screening tool is rapid and possesses high sensitivity and specificity to promptly identify and manage sepsis and avert unfavorable outcomes such as septic shock and death. While the SIRS criteria do not satisfy these ideal features, its measurement characteristics are more suitable for the application of sepsis screening than the qSOFA and should thus remain the standard tool in this setting. Future prospectively designed studies with more uniform methodologies are necessary to ascertain the most effective approach to identify sepsis for which novel screening approaches with more clinically suitable measurement properties are greatly needed.
Acknowledgements
This research was supported by the Iowa City VA Health Care System, Department of Pharmacy Services. Additional support was provided by the Health Services Research and Development Service, Department of Veterans Affairs.
1. Singer M, Deutchman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256.
3. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774.
4. Giamorellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al; Hellenic Sepsis Study Group. Validation of the new Sepsis-3 definitions: proposal for improvement of early risk identification. Clin Microbiol Infect. 2016;23(2):104-109.
5. Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646-655.
6. Forward E, Konecny P, Burston J, Adhikari S, Doolan H, Jensen T. Predictive validity of qSOFA criteria for sepsis in non-ICU patients. Intensive Care Med. 2017;43(6):945-946.
Sepsis is a major public health concern: 10% of patients with sepsis die, and mortality quadruples with progression to septic shock.1 Systemic inflammatory response syndrome (SIRS) criteria, originally published in 1992, are commonly used to detect sepsis, but as early as 2001, these criteria were recognized as lacking specificity.2 Nonetheless, the use of SIRS criteria has persisted in practice. Sepsis was redefined in Sepsis-3 (2016) to guide earlier and more appropriate identification and treatment, which has been shown to greatly improve patient outcomes.1,3 Key recommendations in Sepsis 3 included eliminating SIRS criteria, defining organ dysfunction by the Sequential Organ Failure Assessment (SOFA) score, and introducing the quick SOFA (qSOFA) score.1
The qSOFA combines 3 clinical variables to provide a rapid, simple bedside score that measures the likelihood of poor outcomes, such as admission to an intensive care unit (ICU) or mortality in adults with suspected infection.1,3 The qSOFA score is intended to aid healthcare professionals in more timely stratification of those patients who need escalated care to prevent deterioration.1 The assessment also has been explored as a screening tool for sepsis in clinical practice; however, limited data exists concerning the comparative utility of qSOFA and SIRS in this capacity, and study results are inconsistent.4-6
The most important attribute of a screening tool is high sensitivity, but high specificity also is desired. The qSOFA could supplant SIRS as a screening tool for sepsis if it maintained similarly high sensitivity but achieved superior specificity. Therefore, our primary objective for this study was to determine the effectiveness of qSOFA as a screening assessment for sepsis in the setting of a general inpatient medicine service by contrasting the sensitivity and specificity of qSOFA with SIRS in predicting sepsis, using a retrospective chart review design.
Methods
Administrative data from the Department of Veterans Affairs (VA) Corporate Data Warehouse were accessed via the VA Informatics and Computing Infrastructure (VINCI) and used to identify VA inpatient admissions and obtain the laboratory and vital sign data necessary to calculate SIRS, qSOFA, and SOFA scores. The data were supplemented by manual review of VA health records to obtain information that was not readily available in administrative records, including septic shock outcomes and laboratory and vital sign data obtained in the ICU. This study was approved by the institutional review board at the University of Iowa and the research and development committee at the Iowa City VA Medical Center (ICVAMC).
Patients
The study population included veterans admitted to the nonsurgical medicine unit at ICVAMC between August 1, 2014 and August 1, 2016 who were transferred to an ICU after admission; direct ICU admissions were not included as the qSOFA has been shown in studies to be more beneficial and offer better predictive validity outside the ICU. Excluding these direct admissions prevented any potential skewing of the data. To control for possible selection bias, veterans also were excluded if they transferred from another facility, were admitted under observation status, or if they had been admitted within the prior 30 days. These patients may have been more critically ill than those who presented directly to our facility and any prior treatment could affect the clinical status of the patient and assessment for sepsis at the time of presentation to the VA. Veterans were further required to have evidence of suspected infection based on manual review of the health record, which was determined by receipt of an antibiotic relevant to the empiric treatment of sepsis within 48 hours of admission.
Sepsis and Septic Shock Assessment Tools
As outlined in the Sepsis-3 guidelines, sepsis was defined as suspected or confirmed infection with an acute change in the SOFA score of ≥ 2 points, which is assumed to be 0 in those not known to have preexisting dysfunction.1 The SOFA score includes variables from the respiratory, coagulation, hepatic, cardiovascular, renal, and central nervous systems.1 Septic shock was defined as vasopressor administration and a serum lactic acid level > 2 mmol/L occurring up to 24 hours apart and within 3 days of the first antibiotic dose administered.
The SIRS assessment includes 4 clinical variables (temperature, heart rate, respiratory rate, and white blood cell count) while qSOFA is comprised of 3 variables (respiratory rate, systolic blood pressure, and altered mental status).1 With both assessments, a score ≥ 2 is considered positive, which indicates increased risk for sepsis in patients with suspected infection.1 In keeping with existing studies, qSOFA and SIRS assessments were scored using maximum values found within 48 hours before and 24 hours after the first administered antibiotic dose.3
Outcomes
The primary outcome variable was the presence of sepsis in adults with evidence of infection within 48 hours of admission. Secondary outcome measures included 30-day mortality and septic shock.
Performance between the SIRS and qSOFA assessments was contrasted using sensitivity, specificity, and positive and negative predictive value measurements. Associations of qSOFA and SIRS with septic shock and 30-day mortality were evaluated using a 2-tailed Fisher’s exact test with a threshold of α = 0.05 to determine statistical significance.
Results
The study sample of 481 veterans had a mean age of 67.4 years, 94% were male, and 91.1% were white (Table 1).
Scores for qSOFA, but not SIRS, were significantly associated with septic shock (Fisher’s exact test; qSOFA: P = .009; SIRS: P = .58) (Table 3).
Discussion
High sensitivity is critical for a sepsis screening tool. To be clinically useful, it has been suggested that biomarkers predicting poor outcomes for sepsis should have a sensitivity of > 80%.4 Although qSOFA demonstrated greater specificity than SIRS in our study (83.6% vs 25.7%), qSOFA showed lower sensitivity (44.7% vs 80.0%), which resulted in a greater potential for false negatives; 55.3% of those with sepsis would go undetected. Therefore, our study does not support qSOFA as a better screening assessment than SIRS for sepsis in the veteran population.
Most studies concur with our findings of low sensitivity and high specificity of qSOFA. In a systematic review and meta-analysis, Serafim and colleagues identified 10 studies published after Sepsis-3 that reported sensitivity or specificity of qSOFA and SIRS for sepsis diagnosis.5 Seven of the 10 studies reported sensitivities and favored SIRS in the diagnosis of sepsis (Relative risk: 1.32; 95% CI: 0.40-2.24; P < .0001; I2 = 100%). The authors noted that substantial heterogeneity among studies, including differences in study design, sample size, and criteria for determination of infection, was an important limitation. In addition, most studies that contrast qSOFA and SIRS center on prognostic value in predicting mortality, rather than as a screening test for a diagnosis of sepsis.
We concluded SIRS was more sensitive and thus superior to qSOFA when used as a screening tool for sepsis but conceded that more prospective and homogenous investigations were necessary. To our knowledge, only 1 published study has deviated from this conclusion and reported comparable sensitivity between SIRS (92%) and qSOFA (90%).6 Our study adds to existing literature as it is the first conducted in a veteran population. Additionally, we performed our investigation in a general medicine population with methods similar to existing literature, including the key study validating clinical criteria for sepsis by Seymour and colleagues.3
Limitations
This study is not without limitations, including potential misclassification of cases if essential data points were not available during data collection via health record review or the data points were not representative of a true change from baseline (eg, the Glasgow Coma Scale score for altered mental status in the qSOFA or the SOFA score for organ dysfunction). Generalizability of the results also may be limited due to our retrospective, single-center design and characteristics typical of a veteran population (eg, older, white males). Additionally, many veterans were excluded from the study if they transferred from another facility. These veterans may have been more critically ill than those who presented directly to our facility, which possibly introduced selection bias.
Conclusion
Our findings do not support use of the qSOFA as a suitable replacement for SIRS as a sepsis screening tool among patients with suspected infection in the general medicine inpatient setting. The clinical concern with SIRS is that unfavorable specificity leads to unnecessary antibiotic exposure among patients who are falsely positive. While qSOFA has demonstrated higher specificity, its use would cause many sepsis cases to go undetected due to the technique’s low sensitivity. Frequent false negative qSOFA results could thus serve to impede, rather than enhance, early recognition and intervention for sepsis.
The ideal sepsis screening tool is rapid and possesses high sensitivity and specificity to promptly identify and manage sepsis and avert unfavorable outcomes such as septic shock and death. While the SIRS criteria do not satisfy these ideal features, its measurement characteristics are more suitable for the application of sepsis screening than the qSOFA and should thus remain the standard tool in this setting. Future prospectively designed studies with more uniform methodologies are necessary to ascertain the most effective approach to identify sepsis for which novel screening approaches with more clinically suitable measurement properties are greatly needed.
Acknowledgements
This research was supported by the Iowa City VA Health Care System, Department of Pharmacy Services. Additional support was provided by the Health Services Research and Development Service, Department of Veterans Affairs.
Sepsis is a major public health concern: 10% of patients with sepsis die, and mortality quadruples with progression to septic shock.1 Systemic inflammatory response syndrome (SIRS) criteria, originally published in 1992, are commonly used to detect sepsis, but as early as 2001, these criteria were recognized as lacking specificity.2 Nonetheless, the use of SIRS criteria has persisted in practice. Sepsis was redefined in Sepsis-3 (2016) to guide earlier and more appropriate identification and treatment, which has been shown to greatly improve patient outcomes.1,3 Key recommendations in Sepsis 3 included eliminating SIRS criteria, defining organ dysfunction by the Sequential Organ Failure Assessment (SOFA) score, and introducing the quick SOFA (qSOFA) score.1
The qSOFA combines 3 clinical variables to provide a rapid, simple bedside score that measures the likelihood of poor outcomes, such as admission to an intensive care unit (ICU) or mortality in adults with suspected infection.1,3 The qSOFA score is intended to aid healthcare professionals in more timely stratification of those patients who need escalated care to prevent deterioration.1 The assessment also has been explored as a screening tool for sepsis in clinical practice; however, limited data exists concerning the comparative utility of qSOFA and SIRS in this capacity, and study results are inconsistent.4-6
The most important attribute of a screening tool is high sensitivity, but high specificity also is desired. The qSOFA could supplant SIRS as a screening tool for sepsis if it maintained similarly high sensitivity but achieved superior specificity. Therefore, our primary objective for this study was to determine the effectiveness of qSOFA as a screening assessment for sepsis in the setting of a general inpatient medicine service by contrasting the sensitivity and specificity of qSOFA with SIRS in predicting sepsis, using a retrospective chart review design.
Methods
Administrative data from the Department of Veterans Affairs (VA) Corporate Data Warehouse were accessed via the VA Informatics and Computing Infrastructure (VINCI) and used to identify VA inpatient admissions and obtain the laboratory and vital sign data necessary to calculate SIRS, qSOFA, and SOFA scores. The data were supplemented by manual review of VA health records to obtain information that was not readily available in administrative records, including septic shock outcomes and laboratory and vital sign data obtained in the ICU. This study was approved by the institutional review board at the University of Iowa and the research and development committee at the Iowa City VA Medical Center (ICVAMC).
Patients
The study population included veterans admitted to the nonsurgical medicine unit at ICVAMC between August 1, 2014 and August 1, 2016 who were transferred to an ICU after admission; direct ICU admissions were not included as the qSOFA has been shown in studies to be more beneficial and offer better predictive validity outside the ICU. Excluding these direct admissions prevented any potential skewing of the data. To control for possible selection bias, veterans also were excluded if they transferred from another facility, were admitted under observation status, or if they had been admitted within the prior 30 days. These patients may have been more critically ill than those who presented directly to our facility and any prior treatment could affect the clinical status of the patient and assessment for sepsis at the time of presentation to the VA. Veterans were further required to have evidence of suspected infection based on manual review of the health record, which was determined by receipt of an antibiotic relevant to the empiric treatment of sepsis within 48 hours of admission.
Sepsis and Septic Shock Assessment Tools
As outlined in the Sepsis-3 guidelines, sepsis was defined as suspected or confirmed infection with an acute change in the SOFA score of ≥ 2 points, which is assumed to be 0 in those not known to have preexisting dysfunction.1 The SOFA score includes variables from the respiratory, coagulation, hepatic, cardiovascular, renal, and central nervous systems.1 Septic shock was defined as vasopressor administration and a serum lactic acid level > 2 mmol/L occurring up to 24 hours apart and within 3 days of the first antibiotic dose administered.
The SIRS assessment includes 4 clinical variables (temperature, heart rate, respiratory rate, and white blood cell count) while qSOFA is comprised of 3 variables (respiratory rate, systolic blood pressure, and altered mental status).1 With both assessments, a score ≥ 2 is considered positive, which indicates increased risk for sepsis in patients with suspected infection.1 In keeping with existing studies, qSOFA and SIRS assessments were scored using maximum values found within 48 hours before and 24 hours after the first administered antibiotic dose.3
Outcomes
The primary outcome variable was the presence of sepsis in adults with evidence of infection within 48 hours of admission. Secondary outcome measures included 30-day mortality and septic shock.
Performance between the SIRS and qSOFA assessments was contrasted using sensitivity, specificity, and positive and negative predictive value measurements. Associations of qSOFA and SIRS with septic shock and 30-day mortality were evaluated using a 2-tailed Fisher’s exact test with a threshold of α = 0.05 to determine statistical significance.
Results
The study sample of 481 veterans had a mean age of 67.4 years, 94% were male, and 91.1% were white (Table 1).
Scores for qSOFA, but not SIRS, were significantly associated with septic shock (Fisher’s exact test; qSOFA: P = .009; SIRS: P = .58) (Table 3).
Discussion
High sensitivity is critical for a sepsis screening tool. To be clinically useful, it has been suggested that biomarkers predicting poor outcomes for sepsis should have a sensitivity of > 80%.4 Although qSOFA demonstrated greater specificity than SIRS in our study (83.6% vs 25.7%), qSOFA showed lower sensitivity (44.7% vs 80.0%), which resulted in a greater potential for false negatives; 55.3% of those with sepsis would go undetected. Therefore, our study does not support qSOFA as a better screening assessment than SIRS for sepsis in the veteran population.
Most studies concur with our findings of low sensitivity and high specificity of qSOFA. In a systematic review and meta-analysis, Serafim and colleagues identified 10 studies published after Sepsis-3 that reported sensitivity or specificity of qSOFA and SIRS for sepsis diagnosis.5 Seven of the 10 studies reported sensitivities and favored SIRS in the diagnosis of sepsis (Relative risk: 1.32; 95% CI: 0.40-2.24; P < .0001; I2 = 100%). The authors noted that substantial heterogeneity among studies, including differences in study design, sample size, and criteria for determination of infection, was an important limitation. In addition, most studies that contrast qSOFA and SIRS center on prognostic value in predicting mortality, rather than as a screening test for a diagnosis of sepsis.
We concluded SIRS was more sensitive and thus superior to qSOFA when used as a screening tool for sepsis but conceded that more prospective and homogenous investigations were necessary. To our knowledge, only 1 published study has deviated from this conclusion and reported comparable sensitivity between SIRS (92%) and qSOFA (90%).6 Our study adds to existing literature as it is the first conducted in a veteran population. Additionally, we performed our investigation in a general medicine population with methods similar to existing literature, including the key study validating clinical criteria for sepsis by Seymour and colleagues.3
Limitations
This study is not without limitations, including potential misclassification of cases if essential data points were not available during data collection via health record review or the data points were not representative of a true change from baseline (eg, the Glasgow Coma Scale score for altered mental status in the qSOFA or the SOFA score for organ dysfunction). Generalizability of the results also may be limited due to our retrospective, single-center design and characteristics typical of a veteran population (eg, older, white males). Additionally, many veterans were excluded from the study if they transferred from another facility. These veterans may have been more critically ill than those who presented directly to our facility, which possibly introduced selection bias.
Conclusion
Our findings do not support use of the qSOFA as a suitable replacement for SIRS as a sepsis screening tool among patients with suspected infection in the general medicine inpatient setting. The clinical concern with SIRS is that unfavorable specificity leads to unnecessary antibiotic exposure among patients who are falsely positive. While qSOFA has demonstrated higher specificity, its use would cause many sepsis cases to go undetected due to the technique’s low sensitivity. Frequent false negative qSOFA results could thus serve to impede, rather than enhance, early recognition and intervention for sepsis.
The ideal sepsis screening tool is rapid and possesses high sensitivity and specificity to promptly identify and manage sepsis and avert unfavorable outcomes such as septic shock and death. While the SIRS criteria do not satisfy these ideal features, its measurement characteristics are more suitable for the application of sepsis screening than the qSOFA and should thus remain the standard tool in this setting. Future prospectively designed studies with more uniform methodologies are necessary to ascertain the most effective approach to identify sepsis for which novel screening approaches with more clinically suitable measurement properties are greatly needed.
Acknowledgements
This research was supported by the Iowa City VA Health Care System, Department of Pharmacy Services. Additional support was provided by the Health Services Research and Development Service, Department of Veterans Affairs.
1. Singer M, Deutchman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256.
3. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774.
4. Giamorellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al; Hellenic Sepsis Study Group. Validation of the new Sepsis-3 definitions: proposal for improvement of early risk identification. Clin Microbiol Infect. 2016;23(2):104-109.
5. Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646-655.
6. Forward E, Konecny P, Burston J, Adhikari S, Doolan H, Jensen T. Predictive validity of qSOFA criteria for sepsis in non-ICU patients. Intensive Care Med. 2017;43(6):945-946.
1. Singer M, Deutchman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256.
3. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762-774.
4. Giamorellos-Bourboulis EJ, Tsaganos T, Tsangaris I, et al; Hellenic Sepsis Study Group. Validation of the new Sepsis-3 definitions: proposal for improvement of early risk identification. Clin Microbiol Infect. 2016;23(2):104-109.
5. Serafim R, Gomes JA, Salluh J, Póvoa P. A Comparison of the Quick-SOFA and Systemic Inflammatory Response Syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646-655.
6. Forward E, Konecny P, Burston J, Adhikari S, Doolan H, Jensen T. Predictive validity of qSOFA criteria for sepsis in non-ICU patients. Intensive Care Med. 2017;43(6):945-946.
CRC task force updates colonoscopy follow-up guidance
The U.S. Multi-Society Task Force on Colorectal Cancer (CRC) recently updated recommendations for patient follow-up after colonoscopy and polypectomy.
The new guidance was based on advancements in both research and technology since the last recommendations were published in 2012, reported lead author Samir Gupta, MD, AGAF, of the University of California, San Diego, and colleagues.
“[Since 2012,] a number of articles have been published on risk of CRC based on colonoscopy findings and patient characteristics, as well as the potential impact of screening and surveillance colonoscopy on outcomes, such as incident CRC and polyps,” the investigators wrote in Gastroenterology. “Further, recent studies increasingly reflect the modern era of colonoscopy with more awareness of the importance of quality factors (e.g., adequate bowel preparation, cecal intubation, adequate adenoma detection, and complete polyp resection), and utilization of state of the art technologies (e.g., high-definition colonoscopes).”
The task force, which comprised the American College of Gastroenterology, the American Gastroenterological Association, and the American Society of Gastrointestinal Endoscopy, identified key topics using PICO (patient, intervention, comparison, and outcome) questions before conducting a comprehensive literature review that included 136 articles. Based on these findings, two task force members generated recommendations that were further refined through consensus discussion. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
According to Dr. Gupta and colleagues, some of the new recommendations, particularly those that advise less stringent follow-up, may encounter resistance from various stakeholders.
“Patients, primary care physicians, and colonoscopists may have concerns about lengthening a previously recommended interval, and will need to engage in shared decision making regarding whether to lengthen the follow-up interval based upon the guidance here or utilize the recommendation made at the time of the prior colonoscopy,” the task force wrote.
The most prominent recommendations of this kind concern patients who undergo removal of tubular adenomas less than 10 mm in size. For patients who have 1-2 of these adenomas removed, the task force now recommends follow-up after 7-10 years, instead of the previously recommended interval of 5-10 years.
“[This decision was] based on the growing body of evidence to support low risk for metachronous advanced neoplasia,” the task force wrote. “In this population, the risk for metachronous advanced neoplasia is similar to that for individuals with no adenoma. Importantly, the observed risk for fatal CRC among individuals with 1-10 adenomas less than 10 mm is lower than average for the general population.”
Along similar lines, patients who undergo removal of 3-4 small adenomas now have a recommended 3-5 year follow-up window, instead of the previously strict recommendation for follow-up at 3 years.
But not all of the new guidance is less stringent. While the task force previously recommended a follow-up period of less than 3 years after removal of more than 10 adenomas, they now recommend follow-up at 1 year. This change was made to simplify guidance, the investigators wrote, noting that the evidence base in this area “has not been markedly strengthened” since 2012.
Compared with the old guidance, the updated publication offers more detailed recommendations for follow-up after removal of serrated polyps. On this topic, 10 clinical scenarios are presented, with follow-up ranging from 6 months after piecemeal resection of a sessile serrated polyp greater than 20 mm to 10 years after removal of 20 or fewer hyperplastic polyps less than 10 mm that were located in the rectum or sigmoid colon. Incidentally, these two recommendations are strong and based on moderate evidence, whereas the remaining recommendations for serrated polyps are weak and based on very-low-quality evidence.
Because of such knowledge gaps, the investigators emphasized the need for more data. The publication includes extensive discussion of pressing research topics and appropriate methods of investigation.
“Our review highlights several opportunities for research to clarify risk stratification and management of patients post-polypectomy,” the task force wrote. “In order to optimize risk-reduction strategies, the mechanisms driving metachronous advanced neoplasia after baseline polypectomy and their relative frequency need to be better understood through studies that include large numbers of patients with interval cancers and/or advanced neoplasia after baseline polypectomy. Mechanisms may include new/incident growth, incomplete baseline resection, and missed neoplasia; each of these potential causes may require different interventions for improvement.”
The task force also suggested that some basic questions beyond risk stratification remain unanswered, such as the impact of surveillance on CRC incidence and mortality.
“Such evidence is needed given the increasing proportion of patients who are having adenomas detected as part of increased participation in CRC screening,” the task force wrote.
Other suggested topics of investigation include age-related analyses that incorporate procedural risk, cost-effectiveness studies, and comparisons of nonendoscopic methods of surveillance, such as fecal immunochemical testing.
The study was funded by the National Institutes of Health and the Department of Veterans Affairs. The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Gupta S et al. Gastroenterology. 2020 Feb 7. doi: 10.1053/j.gastro.2019.10.026.
The U.S. Multi-Society Task Force on Colorectal Cancer (CRC) recently updated recommendations for patient follow-up after colonoscopy and polypectomy.
The new guidance was based on advancements in both research and technology since the last recommendations were published in 2012, reported lead author Samir Gupta, MD, AGAF, of the University of California, San Diego, and colleagues.
“[Since 2012,] a number of articles have been published on risk of CRC based on colonoscopy findings and patient characteristics, as well as the potential impact of screening and surveillance colonoscopy on outcomes, such as incident CRC and polyps,” the investigators wrote in Gastroenterology. “Further, recent studies increasingly reflect the modern era of colonoscopy with more awareness of the importance of quality factors (e.g., adequate bowel preparation, cecal intubation, adequate adenoma detection, and complete polyp resection), and utilization of state of the art technologies (e.g., high-definition colonoscopes).”
The task force, which comprised the American College of Gastroenterology, the American Gastroenterological Association, and the American Society of Gastrointestinal Endoscopy, identified key topics using PICO (patient, intervention, comparison, and outcome) questions before conducting a comprehensive literature review that included 136 articles. Based on these findings, two task force members generated recommendations that were further refined through consensus discussion. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
According to Dr. Gupta and colleagues, some of the new recommendations, particularly those that advise less stringent follow-up, may encounter resistance from various stakeholders.
“Patients, primary care physicians, and colonoscopists may have concerns about lengthening a previously recommended interval, and will need to engage in shared decision making regarding whether to lengthen the follow-up interval based upon the guidance here or utilize the recommendation made at the time of the prior colonoscopy,” the task force wrote.
The most prominent recommendations of this kind concern patients who undergo removal of tubular adenomas less than 10 mm in size. For patients who have 1-2 of these adenomas removed, the task force now recommends follow-up after 7-10 years, instead of the previously recommended interval of 5-10 years.
“[This decision was] based on the growing body of evidence to support low risk for metachronous advanced neoplasia,” the task force wrote. “In this population, the risk for metachronous advanced neoplasia is similar to that for individuals with no adenoma. Importantly, the observed risk for fatal CRC among individuals with 1-10 adenomas less than 10 mm is lower than average for the general population.”
Along similar lines, patients who undergo removal of 3-4 small adenomas now have a recommended 3-5 year follow-up window, instead of the previously strict recommendation for follow-up at 3 years.
But not all of the new guidance is less stringent. While the task force previously recommended a follow-up period of less than 3 years after removal of more than 10 adenomas, they now recommend follow-up at 1 year. This change was made to simplify guidance, the investigators wrote, noting that the evidence base in this area “has not been markedly strengthened” since 2012.
Compared with the old guidance, the updated publication offers more detailed recommendations for follow-up after removal of serrated polyps. On this topic, 10 clinical scenarios are presented, with follow-up ranging from 6 months after piecemeal resection of a sessile serrated polyp greater than 20 mm to 10 years after removal of 20 or fewer hyperplastic polyps less than 10 mm that were located in the rectum or sigmoid colon. Incidentally, these two recommendations are strong and based on moderate evidence, whereas the remaining recommendations for serrated polyps are weak and based on very-low-quality evidence.
Because of such knowledge gaps, the investigators emphasized the need for more data. The publication includes extensive discussion of pressing research topics and appropriate methods of investigation.
“Our review highlights several opportunities for research to clarify risk stratification and management of patients post-polypectomy,” the task force wrote. “In order to optimize risk-reduction strategies, the mechanisms driving metachronous advanced neoplasia after baseline polypectomy and their relative frequency need to be better understood through studies that include large numbers of patients with interval cancers and/or advanced neoplasia after baseline polypectomy. Mechanisms may include new/incident growth, incomplete baseline resection, and missed neoplasia; each of these potential causes may require different interventions for improvement.”
The task force also suggested that some basic questions beyond risk stratification remain unanswered, such as the impact of surveillance on CRC incidence and mortality.
“Such evidence is needed given the increasing proportion of patients who are having adenomas detected as part of increased participation in CRC screening,” the task force wrote.
Other suggested topics of investigation include age-related analyses that incorporate procedural risk, cost-effectiveness studies, and comparisons of nonendoscopic methods of surveillance, such as fecal immunochemical testing.
The study was funded by the National Institutes of Health and the Department of Veterans Affairs. The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Gupta S et al. Gastroenterology. 2020 Feb 7. doi: 10.1053/j.gastro.2019.10.026.
The U.S. Multi-Society Task Force on Colorectal Cancer (CRC) recently updated recommendations for patient follow-up after colonoscopy and polypectomy.
The new guidance was based on advancements in both research and technology since the last recommendations were published in 2012, reported lead author Samir Gupta, MD, AGAF, of the University of California, San Diego, and colleagues.
“[Since 2012,] a number of articles have been published on risk of CRC based on colonoscopy findings and patient characteristics, as well as the potential impact of screening and surveillance colonoscopy on outcomes, such as incident CRC and polyps,” the investigators wrote in Gastroenterology. “Further, recent studies increasingly reflect the modern era of colonoscopy with more awareness of the importance of quality factors (e.g., adequate bowel preparation, cecal intubation, adequate adenoma detection, and complete polyp resection), and utilization of state of the art technologies (e.g., high-definition colonoscopes).”
The task force, which comprised the American College of Gastroenterology, the American Gastroenterological Association, and the American Society of Gastrointestinal Endoscopy, identified key topics using PICO (patient, intervention, comparison, and outcome) questions before conducting a comprehensive literature review that included 136 articles. Based on these findings, two task force members generated recommendations that were further refined through consensus discussion. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
According to Dr. Gupta and colleagues, some of the new recommendations, particularly those that advise less stringent follow-up, may encounter resistance from various stakeholders.
“Patients, primary care physicians, and colonoscopists may have concerns about lengthening a previously recommended interval, and will need to engage in shared decision making regarding whether to lengthen the follow-up interval based upon the guidance here or utilize the recommendation made at the time of the prior colonoscopy,” the task force wrote.
The most prominent recommendations of this kind concern patients who undergo removal of tubular adenomas less than 10 mm in size. For patients who have 1-2 of these adenomas removed, the task force now recommends follow-up after 7-10 years, instead of the previously recommended interval of 5-10 years.
“[This decision was] based on the growing body of evidence to support low risk for metachronous advanced neoplasia,” the task force wrote. “In this population, the risk for metachronous advanced neoplasia is similar to that for individuals with no adenoma. Importantly, the observed risk for fatal CRC among individuals with 1-10 adenomas less than 10 mm is lower than average for the general population.”
Along similar lines, patients who undergo removal of 3-4 small adenomas now have a recommended 3-5 year follow-up window, instead of the previously strict recommendation for follow-up at 3 years.
But not all of the new guidance is less stringent. While the task force previously recommended a follow-up period of less than 3 years after removal of more than 10 adenomas, they now recommend follow-up at 1 year. This change was made to simplify guidance, the investigators wrote, noting that the evidence base in this area “has not been markedly strengthened” since 2012.
Compared with the old guidance, the updated publication offers more detailed recommendations for follow-up after removal of serrated polyps. On this topic, 10 clinical scenarios are presented, with follow-up ranging from 6 months after piecemeal resection of a sessile serrated polyp greater than 20 mm to 10 years after removal of 20 or fewer hyperplastic polyps less than 10 mm that were located in the rectum or sigmoid colon. Incidentally, these two recommendations are strong and based on moderate evidence, whereas the remaining recommendations for serrated polyps are weak and based on very-low-quality evidence.
Because of such knowledge gaps, the investigators emphasized the need for more data. The publication includes extensive discussion of pressing research topics and appropriate methods of investigation.
“Our review highlights several opportunities for research to clarify risk stratification and management of patients post-polypectomy,” the task force wrote. “In order to optimize risk-reduction strategies, the mechanisms driving metachronous advanced neoplasia after baseline polypectomy and their relative frequency need to be better understood through studies that include large numbers of patients with interval cancers and/or advanced neoplasia after baseline polypectomy. Mechanisms may include new/incident growth, incomplete baseline resection, and missed neoplasia; each of these potential causes may require different interventions for improvement.”
The task force also suggested that some basic questions beyond risk stratification remain unanswered, such as the impact of surveillance on CRC incidence and mortality.
“Such evidence is needed given the increasing proportion of patients who are having adenomas detected as part of increased participation in CRC screening,” the task force wrote.
Other suggested topics of investigation include age-related analyses that incorporate procedural risk, cost-effectiveness studies, and comparisons of nonendoscopic methods of surveillance, such as fecal immunochemical testing.
The study was funded by the National Institutes of Health and the Department of Veterans Affairs. The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Gupta S et al. Gastroenterology. 2020 Feb 7. doi: 10.1053/j.gastro.2019.10.026.
FROM GASTROENTEROLOGY
U.S. Multi-Society Task Force publishes polypectomy guidance
The U.S. Multi-Society Task Force (USMSTF) on Colorectal Cancer recently published recommendations for endoscopic removal of precancerous colorectal lesions.
According to lead author Tonya Kaltenbach, MD, of the University of California, San Francisco, and fellow panelists, the publication aims to improve complete resection rates, which can vary widely between endoscopists; almost one out of four lesions (22.7%) may be incompletely removed by some practitioners, leading to higher rates of colorectal cancer.
“[A]lthough the majority (50%) of postcolonoscopy colon cancers [are] likely due to missed lesions, close to one-fifth of incident cancers [are] related to incomplete resection,” the panelists wrote in Gastroenterology, referring to a pooled analysis of eight surveillance studies.
The panelists’ recommendations, which were based on both evidence and clinical experience, range from specific polyp removal techniques to guidance for institution-wide quality assurance of polypectomies. Each statement is described by both strength of recommendation and level of evidence, the latter of which was determined by Grading of Recommendations, Assessment, Development, and Evaluation Ratings of Evidence (GRADE) criteria. Recommendations were written by a panel of nine experts and approved by the governing boards of the three societies they represented – the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
Central to the publication are recommended polypectomy techniques for specific types of lesions.
“Polypectomy techniques vary widely in clinical practice,” the panelists wrote. “They are often driven by physician preference based on how they were taught and on trial and error, due to the lack of standardized training and the paucity of published evidence. In the past decade, evidence has evolved on the superiority of specific methods.”
“Optimal techniques encompass effectiveness, safety, and efficiency,” they wrote. “Colorectal lesion characteristics, including location, size, morphology, and histology, influence the optimal removal method.”
For lesions up to 9 mm, the panelists recommended cold snare polypectomy “due to high complete resection rates and safety profile.” In contrast, they recommended against both cold and hot biopsy forceps, which have been associated with higher rates of incomplete resection. Furthermore, they cautioned that hot biopsy forceps may increase risks of complications and produce inadequate tissue samples for histopathology.
For nonpedunculated lesions between 10 and 19 mm, guidance is minimal. The panelists recommended cold or hot snare polypectomy, although this statement was conditional and based on low-quality evidence.
Recommendations were more extensive for large nonpedunculated lesions (at least 20 mm). For such lesions, the panelists strongly recommended endoscopic mucosal resection (EMR). They emphasized that large lesions should be removed in the fewest possible pieces by an appropriately experienced endoscopist during a single colonoscopy session. The panelists recommended the use of a viscous injection solution with a contrast agent and adjuvant thermal ablation of the post-EMR margin. They recommended against the use of tattoo as a submucosal injection solution, and ablation of residual lesion tissue that is endoscopically visible. Additional recommendations for large lesions, including prophylactic closure of resection defects and coagulation techniques, were based on low-quality evidence.
For pedunculated lesions greater than 10 mm, the panelists recommended hot snare polypectomy. For pedunculated lesions with a head greater than 20 mm or a stalk thickness greater than 5 mm, they recommended prophylactic mechanical ligation.
Beyond lesion assessment and removal, recommendations addressed lesion marking, equipment, surveillance, and quality of polypectomy.
Concerning quality, the panelists recommended that endoscopists participate in a quality assurance program that documents adverse events, and that institutions use standardized polypectomy competency assessments, such as Cold Snare Polypectomy Competency Assessment Tool and/or Direct Observation of Polypectomy Skills.
“Focused teaching is needed to ensure the optimal endoscopic management of colorectal lesions,” the panelists wrote. They went on to suggest that “development and implementation of polypectomy quality metrics may be necessary to optimize practice and outcomes.”
“For example, the type of resection method used for the colorectal lesion removal in the procedure report should be documented, and the inclusion of adequate resection technique as a quality indicator in colorectal cancer screening programs should be considered,” they wrote. “Adverse events, including bleeding, perforation, hospital admissions, and the number of benign colorectal lesions referred for surgical management, should be measured and reported. Finally, standards for pathology preparation and reporting of lesions suspicious for submucosal invasion should be in place to provide accurate staging and management.”
The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Kaltenbach T et al. Gastroenterology. 2020 Jan 18. doi: 10.1053/j.gastro.2019.12.018.
The U.S. Multi-Society Task Force (USMSTF) on Colorectal Cancer recently published recommendations for endoscopic removal of precancerous colorectal lesions.
According to lead author Tonya Kaltenbach, MD, of the University of California, San Francisco, and fellow panelists, the publication aims to improve complete resection rates, which can vary widely between endoscopists; almost one out of four lesions (22.7%) may be incompletely removed by some practitioners, leading to higher rates of colorectal cancer.
“[A]lthough the majority (50%) of postcolonoscopy colon cancers [are] likely due to missed lesions, close to one-fifth of incident cancers [are] related to incomplete resection,” the panelists wrote in Gastroenterology, referring to a pooled analysis of eight surveillance studies.
The panelists’ recommendations, which were based on both evidence and clinical experience, range from specific polyp removal techniques to guidance for institution-wide quality assurance of polypectomies. Each statement is described by both strength of recommendation and level of evidence, the latter of which was determined by Grading of Recommendations, Assessment, Development, and Evaluation Ratings of Evidence (GRADE) criteria. Recommendations were written by a panel of nine experts and approved by the governing boards of the three societies they represented – the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
Central to the publication are recommended polypectomy techniques for specific types of lesions.
“Polypectomy techniques vary widely in clinical practice,” the panelists wrote. “They are often driven by physician preference based on how they were taught and on trial and error, due to the lack of standardized training and the paucity of published evidence. In the past decade, evidence has evolved on the superiority of specific methods.”
“Optimal techniques encompass effectiveness, safety, and efficiency,” they wrote. “Colorectal lesion characteristics, including location, size, morphology, and histology, influence the optimal removal method.”
For lesions up to 9 mm, the panelists recommended cold snare polypectomy “due to high complete resection rates and safety profile.” In contrast, they recommended against both cold and hot biopsy forceps, which have been associated with higher rates of incomplete resection. Furthermore, they cautioned that hot biopsy forceps may increase risks of complications and produce inadequate tissue samples for histopathology.
For nonpedunculated lesions between 10 and 19 mm, guidance is minimal. The panelists recommended cold or hot snare polypectomy, although this statement was conditional and based on low-quality evidence.
Recommendations were more extensive for large nonpedunculated lesions (at least 20 mm). For such lesions, the panelists strongly recommended endoscopic mucosal resection (EMR). They emphasized that large lesions should be removed in the fewest possible pieces by an appropriately experienced endoscopist during a single colonoscopy session. The panelists recommended the use of a viscous injection solution with a contrast agent and adjuvant thermal ablation of the post-EMR margin. They recommended against the use of tattoo as a submucosal injection solution, and ablation of residual lesion tissue that is endoscopically visible. Additional recommendations for large lesions, including prophylactic closure of resection defects and coagulation techniques, were based on low-quality evidence.
For pedunculated lesions greater than 10 mm, the panelists recommended hot snare polypectomy. For pedunculated lesions with a head greater than 20 mm or a stalk thickness greater than 5 mm, they recommended prophylactic mechanical ligation.
Beyond lesion assessment and removal, recommendations addressed lesion marking, equipment, surveillance, and quality of polypectomy.
Concerning quality, the panelists recommended that endoscopists participate in a quality assurance program that documents adverse events, and that institutions use standardized polypectomy competency assessments, such as Cold Snare Polypectomy Competency Assessment Tool and/or Direct Observation of Polypectomy Skills.
“Focused teaching is needed to ensure the optimal endoscopic management of colorectal lesions,” the panelists wrote. They went on to suggest that “development and implementation of polypectomy quality metrics may be necessary to optimize practice and outcomes.”
“For example, the type of resection method used for the colorectal lesion removal in the procedure report should be documented, and the inclusion of adequate resection technique as a quality indicator in colorectal cancer screening programs should be considered,” they wrote. “Adverse events, including bleeding, perforation, hospital admissions, and the number of benign colorectal lesions referred for surgical management, should be measured and reported. Finally, standards for pathology preparation and reporting of lesions suspicious for submucosal invasion should be in place to provide accurate staging and management.”
The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Kaltenbach T et al. Gastroenterology. 2020 Jan 18. doi: 10.1053/j.gastro.2019.12.018.
The U.S. Multi-Society Task Force (USMSTF) on Colorectal Cancer recently published recommendations for endoscopic removal of precancerous colorectal lesions.
According to lead author Tonya Kaltenbach, MD, of the University of California, San Francisco, and fellow panelists, the publication aims to improve complete resection rates, which can vary widely between endoscopists; almost one out of four lesions (22.7%) may be incompletely removed by some practitioners, leading to higher rates of colorectal cancer.
“[A]lthough the majority (50%) of postcolonoscopy colon cancers [are] likely due to missed lesions, close to one-fifth of incident cancers [are] related to incomplete resection,” the panelists wrote in Gastroenterology, referring to a pooled analysis of eight surveillance studies.
The panelists’ recommendations, which were based on both evidence and clinical experience, range from specific polyp removal techniques to guidance for institution-wide quality assurance of polypectomies. Each statement is described by both strength of recommendation and level of evidence, the latter of which was determined by Grading of Recommendations, Assessment, Development, and Evaluation Ratings of Evidence (GRADE) criteria. Recommendations were written by a panel of nine experts and approved by the governing boards of the three societies they represented – the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
Central to the publication are recommended polypectomy techniques for specific types of lesions.
“Polypectomy techniques vary widely in clinical practice,” the panelists wrote. “They are often driven by physician preference based on how they were taught and on trial and error, due to the lack of standardized training and the paucity of published evidence. In the past decade, evidence has evolved on the superiority of specific methods.”
“Optimal techniques encompass effectiveness, safety, and efficiency,” they wrote. “Colorectal lesion characteristics, including location, size, morphology, and histology, influence the optimal removal method.”
For lesions up to 9 mm, the panelists recommended cold snare polypectomy “due to high complete resection rates and safety profile.” In contrast, they recommended against both cold and hot biopsy forceps, which have been associated with higher rates of incomplete resection. Furthermore, they cautioned that hot biopsy forceps may increase risks of complications and produce inadequate tissue samples for histopathology.
For nonpedunculated lesions between 10 and 19 mm, guidance is minimal. The panelists recommended cold or hot snare polypectomy, although this statement was conditional and based on low-quality evidence.
Recommendations were more extensive for large nonpedunculated lesions (at least 20 mm). For such lesions, the panelists strongly recommended endoscopic mucosal resection (EMR). They emphasized that large lesions should be removed in the fewest possible pieces by an appropriately experienced endoscopist during a single colonoscopy session. The panelists recommended the use of a viscous injection solution with a contrast agent and adjuvant thermal ablation of the post-EMR margin. They recommended against the use of tattoo as a submucosal injection solution, and ablation of residual lesion tissue that is endoscopically visible. Additional recommendations for large lesions, including prophylactic closure of resection defects and coagulation techniques, were based on low-quality evidence.
For pedunculated lesions greater than 10 mm, the panelists recommended hot snare polypectomy. For pedunculated lesions with a head greater than 20 mm or a stalk thickness greater than 5 mm, they recommended prophylactic mechanical ligation.
Beyond lesion assessment and removal, recommendations addressed lesion marking, equipment, surveillance, and quality of polypectomy.
Concerning quality, the panelists recommended that endoscopists participate in a quality assurance program that documents adverse events, and that institutions use standardized polypectomy competency assessments, such as Cold Snare Polypectomy Competency Assessment Tool and/or Direct Observation of Polypectomy Skills.
“Focused teaching is needed to ensure the optimal endoscopic management of colorectal lesions,” the panelists wrote. They went on to suggest that “development and implementation of polypectomy quality metrics may be necessary to optimize practice and outcomes.”
“For example, the type of resection method used for the colorectal lesion removal in the procedure report should be documented, and the inclusion of adequate resection technique as a quality indicator in colorectal cancer screening programs should be considered,” they wrote. “Adverse events, including bleeding, perforation, hospital admissions, and the number of benign colorectal lesions referred for surgical management, should be measured and reported. Finally, standards for pathology preparation and reporting of lesions suspicious for submucosal invasion should be in place to provide accurate staging and management.”
The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Kaltenbach T et al. Gastroenterology. 2020 Jan 18. doi: 10.1053/j.gastro.2019.12.018.
FROM GASTROENTEROLOGY
Understanding Principles of High Reliability Organizations Through the Eyes of VIONE, A Clinical Program to Improve Patient Safety by Deprescribing Potentially Inappropriate Medications and Reducing Polypharmacy
High reliability organizations (HROs) incorporate continuous process improvement through leadership commitment to create a safety culture that works toward creating a zero-harm environment.1 The Veterans Health Administration (VHA) has set transformational goals for becoming an HRO. In this article, we describe VIONE, an expanding medication deprescribing clinical program, which exemplifies the translation of HRO principles into health care system models. Both VIONE and HRO are globally relevant.
Reducing medication errors and related adverse drug events are important for achieving zero harm. Preventable medical errors rank behind heart disease and cancer as the third leading cause of death in the US.2 The simultaneous use of multiple medications can lead to dangerous drug interactions, adverse outcomes, and challenges with adherence. When a person is taking multiple medicines, known as polypharmacy, it is more likely that some are potentially inappropriate medications (PIM). Current literature highlights the prevalence and dangers of polypharmacy, which ranks among the top 10 common causes of death in the US, as well as suggestions to address preventable adverse outcomes from polypharmacy and PIM.3-5
Deprescribing of PIM frequently results in better disease management with improved health outcomes and quality of life.4 Many health care settings lack standardized approaches or set expectations to proactively deprescribe PIM. There has been insufficient emphasis on how to make decisions for deprescribing medications when therapeutic benefits are not clear and/or when the adverse effects may outweigh the therapeutic benefits.5
It is imperative to provide practice guidance for deprescribing nonessential medications along with systems-based infrastructure to enable integrated and effective assessments during opportune moments in the health care continuum. Multimodal approaches that include education, risk stratification, population health management interventions, research and resource allocation can help transform organizational culture in health care facilities toward HRO models of care, aiming at zero harm to patients.
The practical lessons learned from VIONE implementation science experiences on various scales and under diverse circumstances, cumulative wisdom from hindsight, foresight and critical insights gathered during nationwide spread of VIONE over the past 3 years continues to propel us toward the desirable direction and core concepts of an HRO.
The VIONE program facilitates practical, real-time interventions that could be tailored to various health care settings, organizational needs, and available resources. VIONE implements an electronic Computerized Patient Record System (CPRS) tool to enable planned cessation of nonessential medications that are potentially harmful, inappropriate, not indicated, or not necessary. The VIONE tool supports systematic, individualized assessment and adjustment through 5 filters (Figure 1). It prompts providers to assign 1 of these filters intuitively and objectively. VIONE combines clinical evidence for best practices, an interprofessional team approach, patient engagement, adapted use of existing medical records systems, and HRO principles for effective implementation.
As a tool to support safer prescribing practices, VIONE aligns closely with HRO principles (Table 1) and core pillars (Table 2).6-8 A zero-harm safety culture necessitates that medications be used for correct reasons, over a correct duration of time, and following a correct schedule while monitoring for adverse outcomes. However, reality generally falls significantly short of this for a myriad of reasons, such as compromised health literacy, functional limitations, affordability, communication gaps, patients seen by multiple providers, and an accumulation of prescriptions due to comorbidities, symptom progression, and management of adverse effects. Through a sharpened focus on both precision medicine and competent prescription management, VIONE is a viable opportunity for investing in the zero-harm philosophy that is integral to an HRO.
Design and Implementation
Initially launched in 2016 in a 15-bed inpatient, subacute rehabilitation unit within a VHA tertiary care facility, VIONE has been sustained and gradually expanded to 38 other VHA facility programs (Figure 2). Recognizing the potential value if adopted into widespread use, VIONE was a Gold Status winner in the VHA Under Secretary for Health Shark Tank-style competition in 2017 and was selected by the VHA Diffusion of Excellence as an innovation worthy of scale and spread through national dissemination.9 A toolkit for VIONE implementation, patient and provider brochures, VIONE vignette, and National Dialog template also have been created.10
Implementing VIONE in a new facility requires an actively engaged core team committed to patient safety and reduction of polypharmacy and PIM, interest and availability to lead project implementation strategies, along with meaningful local organizational support. The current structure for VIONE spread is as follows:
- Interested VHA participants review information and contact [email protected].
- The VIONE team orients implementing champions, mainly pharmacists, physicians, nurse practitioners, and physician assistants at a facility program level, offering guidance and available resources.
- Clinical Application Coordinators at Central Arkansas VA Healthcare System and participating facilities collaborate to add deprescribing menu options in CPRS and install the VIONE Polypharmacy Reminder Dialog template.
- Through close and ongoing collaborations, medical providers and clinical pharmacists proceed with deprescribing, aiming at planned cessation of nonessential and PIM, using the mnemonic prompt of VIONE. Vital and Important medications are continued and consolidated while a methodical plan is developed to deprescribe any medications that could lead to more harm than benefit and qualify based on the filters of Optional, Not indicated, and Every medicine has a diagnosis/reason. They select the proper discontinuation reasons in the CPRS medication menu (Figure 3) and document the rationale in the progress notes. It is highly encouraged that the collaborating pharmacists and health care providers add each other as cosigners and communicate effectively. Clinical pharmacy specialists also use the VIONE Polypharmacy Reminder Dialog Template (RDT) to document complete medication reviews with veterans to include deprescribing rationale and document shared decision making.
- A VIONE national dashboard captures deprescribing data in real time and automates reporting with daily updates that are readily accessible to all implementing facilities. Minimum data captured include the number of unique veterans impacted, number of medications deprescribed, cumulative cost avoidance to date, and number of prescriptions deprescribed per veteran. The dashboard facilitates real-time use of individual patient data and has also been designed to capture data from VHA administrative data portals and Corporate Data Warehouse.
Results
As of October 31, 2019, the assessment of polypharmacy using the VIONE tool across VHA sites has benefited > 60,000 unique veterans, of whom 49.2% were in urban areas, 47.7% in rural areas, and 3.1% in highly rural areas. Elderly male veterans comprised a clear majority. More than 128,000 medications have been deprescribed. The top classes of medications deprescribed are antihypertensives, over-the-counter medications, and antidiabetic medications. An annualized cost avoidance of > $4.0 million has been achieved. Cost avoidance is the cost of medications that otherwise would have continued to be filled and paid for by the VHA if they had not been deprescribed, projected for a maximum of 365 days. The calculation methodology can be summarized as follows:
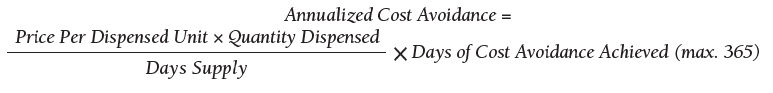
The calculations reported in Table 3 and Figure 4 are conservative and include only chronic outpatient prescriptions and do not account for medications deprescribed in inpatient units, nursing home, community living centers, or domiciliary populations. Data tracked separately from inpatient and community living center patient populations indicated an additional 25,536 deprescribed medications, across 28 VA facilities, impacting 7,076 veterans with an average 2.15 medications deprescribed per veteran. The additional achieved cost avoidance was $370,272 (based on $14.50 average cost per prescription). Medications restarted within 30 days of deprescribing are not included in these calculations.
The cost avoidance calculation further excludes the effects of VIONE implementation on many other types of interventions. These interventions include, but are not limited to, changing from aggressive care to end of life, comfort care when strongly indicated; reduced emergency department visits or invasive diagnostic and therapeutic approaches, when not indicated; medical supplies, antimicrobial preparations; labor costs related to packaging, mailing, and administering prescriptions; reduced/prevented clinical waste; reduced decompensation of systemic illnesses and subsequent health care needs precipitated by iatrogenic disturbances and prolonged convalescence; and overall changes to prescribing practices through purposeful and targeted interactions with colleagues across various disciplines and various hierarchical levels.
Discussion
The VIONE clinical program exemplifies the translation of HRO principles into health care system practices. VIONE offers a systematic approach to improve medication management with an emphasis on deprescribing nonessential medications across various health care settings, facilitating VHA efforts toward zero harm. It demonstrates close alignment with the key building blocks of an HRO. Effective VIONE incorporation into an organizational culture reflects leadership commitment to safety and reliability in their vision and actions. By empowering staff to proactively reduce inappropriate medications and thereby prevent patient harm, VIONE contributes to enhancing an enterprise-wide culture of safety, with fewer errors and greater reliability. As a standardized decision support tool for the ongoing practice of assessment and planned cessation of potentially inappropriate medications, VIONE illustrates how continuous process improvement can be a part of staff-engaged, veteran-centered, highly reliable care. The standardization of the VIONE tool promotes achievement and sustainment of desired HRO principles and practices within health care delivery systems.
Conclusions
The VIONE program was launched not as a cost savings or research program but as a practical, real-time bedside or ambulatory care intervention to improve patient safety. Its value is reflected in the overwhelming response from scholarly and well-engaged colleagues expressing serious interests in expanding collaborations and tailoring efforts to add more depth and breadth to VIONE related efforts.
Acknowledgments
The authors express their gratitude to Central Arkansas VA Healthcare System leadership, Clinical Applications Coordinators, and colleagues for their unconditional support, to the Diffusion of Excellence programs at US Department of Veterans Affairs Central Office for their endorsement, and to the many VHA participants who renew our optimism and energy as we continue this exciting journey. We also thank Bridget B. Kelly for her assistance in writing and editing of the manuscript.
1. Chassin MR, Jerod ML. High-reliability health care: getting there from here. The Joint Commission. Milbank Q. 2013;91(3):459-490.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data. 2017;4:170167.
4. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
5. Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med. 2016;176(4):482-483.
6. US Department of Veterans Affairs. High reliability. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx. [Nonpublic source, not verified.]
7. Gordon S, Mendenhall P, O’Connor BB. Beyond the Checklist: What Else Health Care Can Learn from Aviation Teamwork and Safety. Ithaca, NY: Cornell University Press; 2013.
8. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
9. US Department of Veterans Affairs. Diffusion of Excellence. https://www.va.gov/HEALTHCAREEXCELLENCE/diffusion-of-excellence/. Updated August 10, 2018. Accessed June 26, 2019.
10. US Department of Veterans Affairs. VIONE program toolkit. https://www.vapulse.net/docs/DOC-259375. [Nonpublic source, not verified.]
High reliability organizations (HROs) incorporate continuous process improvement through leadership commitment to create a safety culture that works toward creating a zero-harm environment.1 The Veterans Health Administration (VHA) has set transformational goals for becoming an HRO. In this article, we describe VIONE, an expanding medication deprescribing clinical program, which exemplifies the translation of HRO principles into health care system models. Both VIONE and HRO are globally relevant.
Reducing medication errors and related adverse drug events are important for achieving zero harm. Preventable medical errors rank behind heart disease and cancer as the third leading cause of death in the US.2 The simultaneous use of multiple medications can lead to dangerous drug interactions, adverse outcomes, and challenges with adherence. When a person is taking multiple medicines, known as polypharmacy, it is more likely that some are potentially inappropriate medications (PIM). Current literature highlights the prevalence and dangers of polypharmacy, which ranks among the top 10 common causes of death in the US, as well as suggestions to address preventable adverse outcomes from polypharmacy and PIM.3-5
Deprescribing of PIM frequently results in better disease management with improved health outcomes and quality of life.4 Many health care settings lack standardized approaches or set expectations to proactively deprescribe PIM. There has been insufficient emphasis on how to make decisions for deprescribing medications when therapeutic benefits are not clear and/or when the adverse effects may outweigh the therapeutic benefits.5
It is imperative to provide practice guidance for deprescribing nonessential medications along with systems-based infrastructure to enable integrated and effective assessments during opportune moments in the health care continuum. Multimodal approaches that include education, risk stratification, population health management interventions, research and resource allocation can help transform organizational culture in health care facilities toward HRO models of care, aiming at zero harm to patients.
The practical lessons learned from VIONE implementation science experiences on various scales and under diverse circumstances, cumulative wisdom from hindsight, foresight and critical insights gathered during nationwide spread of VIONE over the past 3 years continues to propel us toward the desirable direction and core concepts of an HRO.
The VIONE program facilitates practical, real-time interventions that could be tailored to various health care settings, organizational needs, and available resources. VIONE implements an electronic Computerized Patient Record System (CPRS) tool to enable planned cessation of nonessential medications that are potentially harmful, inappropriate, not indicated, or not necessary. The VIONE tool supports systematic, individualized assessment and adjustment through 5 filters (Figure 1). It prompts providers to assign 1 of these filters intuitively and objectively. VIONE combines clinical evidence for best practices, an interprofessional team approach, patient engagement, adapted use of existing medical records systems, and HRO principles for effective implementation.
As a tool to support safer prescribing practices, VIONE aligns closely with HRO principles (Table 1) and core pillars (Table 2).6-8 A zero-harm safety culture necessitates that medications be used for correct reasons, over a correct duration of time, and following a correct schedule while monitoring for adverse outcomes. However, reality generally falls significantly short of this for a myriad of reasons, such as compromised health literacy, functional limitations, affordability, communication gaps, patients seen by multiple providers, and an accumulation of prescriptions due to comorbidities, symptom progression, and management of adverse effects. Through a sharpened focus on both precision medicine and competent prescription management, VIONE is a viable opportunity for investing in the zero-harm philosophy that is integral to an HRO.
Design and Implementation
Initially launched in 2016 in a 15-bed inpatient, subacute rehabilitation unit within a VHA tertiary care facility, VIONE has been sustained and gradually expanded to 38 other VHA facility programs (Figure 2). Recognizing the potential value if adopted into widespread use, VIONE was a Gold Status winner in the VHA Under Secretary for Health Shark Tank-style competition in 2017 and was selected by the VHA Diffusion of Excellence as an innovation worthy of scale and spread through national dissemination.9 A toolkit for VIONE implementation, patient and provider brochures, VIONE vignette, and National Dialog template also have been created.10
Implementing VIONE in a new facility requires an actively engaged core team committed to patient safety and reduction of polypharmacy and PIM, interest and availability to lead project implementation strategies, along with meaningful local organizational support. The current structure for VIONE spread is as follows:
- Interested VHA participants review information and contact [email protected].
- The VIONE team orients implementing champions, mainly pharmacists, physicians, nurse practitioners, and physician assistants at a facility program level, offering guidance and available resources.
- Clinical Application Coordinators at Central Arkansas VA Healthcare System and participating facilities collaborate to add deprescribing menu options in CPRS and install the VIONE Polypharmacy Reminder Dialog template.
- Through close and ongoing collaborations, medical providers and clinical pharmacists proceed with deprescribing, aiming at planned cessation of nonessential and PIM, using the mnemonic prompt of VIONE. Vital and Important medications are continued and consolidated while a methodical plan is developed to deprescribe any medications that could lead to more harm than benefit and qualify based on the filters of Optional, Not indicated, and Every medicine has a diagnosis/reason. They select the proper discontinuation reasons in the CPRS medication menu (Figure 3) and document the rationale in the progress notes. It is highly encouraged that the collaborating pharmacists and health care providers add each other as cosigners and communicate effectively. Clinical pharmacy specialists also use the VIONE Polypharmacy Reminder Dialog Template (RDT) to document complete medication reviews with veterans to include deprescribing rationale and document shared decision making.
- A VIONE national dashboard captures deprescribing data in real time and automates reporting with daily updates that are readily accessible to all implementing facilities. Minimum data captured include the number of unique veterans impacted, number of medications deprescribed, cumulative cost avoidance to date, and number of prescriptions deprescribed per veteran. The dashboard facilitates real-time use of individual patient data and has also been designed to capture data from VHA administrative data portals and Corporate Data Warehouse.
Results
As of October 31, 2019, the assessment of polypharmacy using the VIONE tool across VHA sites has benefited > 60,000 unique veterans, of whom 49.2% were in urban areas, 47.7% in rural areas, and 3.1% in highly rural areas. Elderly male veterans comprised a clear majority. More than 128,000 medications have been deprescribed. The top classes of medications deprescribed are antihypertensives, over-the-counter medications, and antidiabetic medications. An annualized cost avoidance of > $4.0 million has been achieved. Cost avoidance is the cost of medications that otherwise would have continued to be filled and paid for by the VHA if they had not been deprescribed, projected for a maximum of 365 days. The calculation methodology can be summarized as follows:
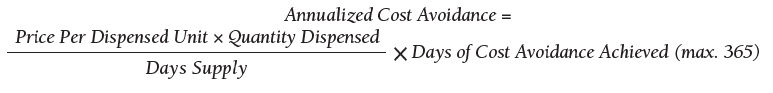
The calculations reported in Table 3 and Figure 4 are conservative and include only chronic outpatient prescriptions and do not account for medications deprescribed in inpatient units, nursing home, community living centers, or domiciliary populations. Data tracked separately from inpatient and community living center patient populations indicated an additional 25,536 deprescribed medications, across 28 VA facilities, impacting 7,076 veterans with an average 2.15 medications deprescribed per veteran. The additional achieved cost avoidance was $370,272 (based on $14.50 average cost per prescription). Medications restarted within 30 days of deprescribing are not included in these calculations.
The cost avoidance calculation further excludes the effects of VIONE implementation on many other types of interventions. These interventions include, but are not limited to, changing from aggressive care to end of life, comfort care when strongly indicated; reduced emergency department visits or invasive diagnostic and therapeutic approaches, when not indicated; medical supplies, antimicrobial preparations; labor costs related to packaging, mailing, and administering prescriptions; reduced/prevented clinical waste; reduced decompensation of systemic illnesses and subsequent health care needs precipitated by iatrogenic disturbances and prolonged convalescence; and overall changes to prescribing practices through purposeful and targeted interactions with colleagues across various disciplines and various hierarchical levels.
Discussion
The VIONE clinical program exemplifies the translation of HRO principles into health care system practices. VIONE offers a systematic approach to improve medication management with an emphasis on deprescribing nonessential medications across various health care settings, facilitating VHA efforts toward zero harm. It demonstrates close alignment with the key building blocks of an HRO. Effective VIONE incorporation into an organizational culture reflects leadership commitment to safety and reliability in their vision and actions. By empowering staff to proactively reduce inappropriate medications and thereby prevent patient harm, VIONE contributes to enhancing an enterprise-wide culture of safety, with fewer errors and greater reliability. As a standardized decision support tool for the ongoing practice of assessment and planned cessation of potentially inappropriate medications, VIONE illustrates how continuous process improvement can be a part of staff-engaged, veteran-centered, highly reliable care. The standardization of the VIONE tool promotes achievement and sustainment of desired HRO principles and practices within health care delivery systems.
Conclusions
The VIONE program was launched not as a cost savings or research program but as a practical, real-time bedside or ambulatory care intervention to improve patient safety. Its value is reflected in the overwhelming response from scholarly and well-engaged colleagues expressing serious interests in expanding collaborations and tailoring efforts to add more depth and breadth to VIONE related efforts.
Acknowledgments
The authors express their gratitude to Central Arkansas VA Healthcare System leadership, Clinical Applications Coordinators, and colleagues for their unconditional support, to the Diffusion of Excellence programs at US Department of Veterans Affairs Central Office for their endorsement, and to the many VHA participants who renew our optimism and energy as we continue this exciting journey. We also thank Bridget B. Kelly for her assistance in writing and editing of the manuscript.
High reliability organizations (HROs) incorporate continuous process improvement through leadership commitment to create a safety culture that works toward creating a zero-harm environment.1 The Veterans Health Administration (VHA) has set transformational goals for becoming an HRO. In this article, we describe VIONE, an expanding medication deprescribing clinical program, which exemplifies the translation of HRO principles into health care system models. Both VIONE and HRO are globally relevant.
Reducing medication errors and related adverse drug events are important for achieving zero harm. Preventable medical errors rank behind heart disease and cancer as the third leading cause of death in the US.2 The simultaneous use of multiple medications can lead to dangerous drug interactions, adverse outcomes, and challenges with adherence. When a person is taking multiple medicines, known as polypharmacy, it is more likely that some are potentially inappropriate medications (PIM). Current literature highlights the prevalence and dangers of polypharmacy, which ranks among the top 10 common causes of death in the US, as well as suggestions to address preventable adverse outcomes from polypharmacy and PIM.3-5
Deprescribing of PIM frequently results in better disease management with improved health outcomes and quality of life.4 Many health care settings lack standardized approaches or set expectations to proactively deprescribe PIM. There has been insufficient emphasis on how to make decisions for deprescribing medications when therapeutic benefits are not clear and/or when the adverse effects may outweigh the therapeutic benefits.5
It is imperative to provide practice guidance for deprescribing nonessential medications along with systems-based infrastructure to enable integrated and effective assessments during opportune moments in the health care continuum. Multimodal approaches that include education, risk stratification, population health management interventions, research and resource allocation can help transform organizational culture in health care facilities toward HRO models of care, aiming at zero harm to patients.
The practical lessons learned from VIONE implementation science experiences on various scales and under diverse circumstances, cumulative wisdom from hindsight, foresight and critical insights gathered during nationwide spread of VIONE over the past 3 years continues to propel us toward the desirable direction and core concepts of an HRO.
The VIONE program facilitates practical, real-time interventions that could be tailored to various health care settings, organizational needs, and available resources. VIONE implements an electronic Computerized Patient Record System (CPRS) tool to enable planned cessation of nonessential medications that are potentially harmful, inappropriate, not indicated, or not necessary. The VIONE tool supports systematic, individualized assessment and adjustment through 5 filters (Figure 1). It prompts providers to assign 1 of these filters intuitively and objectively. VIONE combines clinical evidence for best practices, an interprofessional team approach, patient engagement, adapted use of existing medical records systems, and HRO principles for effective implementation.
As a tool to support safer prescribing practices, VIONE aligns closely with HRO principles (Table 1) and core pillars (Table 2).6-8 A zero-harm safety culture necessitates that medications be used for correct reasons, over a correct duration of time, and following a correct schedule while monitoring for adverse outcomes. However, reality generally falls significantly short of this for a myriad of reasons, such as compromised health literacy, functional limitations, affordability, communication gaps, patients seen by multiple providers, and an accumulation of prescriptions due to comorbidities, symptom progression, and management of adverse effects. Through a sharpened focus on both precision medicine and competent prescription management, VIONE is a viable opportunity for investing in the zero-harm philosophy that is integral to an HRO.
Design and Implementation
Initially launched in 2016 in a 15-bed inpatient, subacute rehabilitation unit within a VHA tertiary care facility, VIONE has been sustained and gradually expanded to 38 other VHA facility programs (Figure 2). Recognizing the potential value if adopted into widespread use, VIONE was a Gold Status winner in the VHA Under Secretary for Health Shark Tank-style competition in 2017 and was selected by the VHA Diffusion of Excellence as an innovation worthy of scale and spread through national dissemination.9 A toolkit for VIONE implementation, patient and provider brochures, VIONE vignette, and National Dialog template also have been created.10
Implementing VIONE in a new facility requires an actively engaged core team committed to patient safety and reduction of polypharmacy and PIM, interest and availability to lead project implementation strategies, along with meaningful local organizational support. The current structure for VIONE spread is as follows:
- Interested VHA participants review information and contact [email protected].
- The VIONE team orients implementing champions, mainly pharmacists, physicians, nurse practitioners, and physician assistants at a facility program level, offering guidance and available resources.
- Clinical Application Coordinators at Central Arkansas VA Healthcare System and participating facilities collaborate to add deprescribing menu options in CPRS and install the VIONE Polypharmacy Reminder Dialog template.
- Through close and ongoing collaborations, medical providers and clinical pharmacists proceed with deprescribing, aiming at planned cessation of nonessential and PIM, using the mnemonic prompt of VIONE. Vital and Important medications are continued and consolidated while a methodical plan is developed to deprescribe any medications that could lead to more harm than benefit and qualify based on the filters of Optional, Not indicated, and Every medicine has a diagnosis/reason. They select the proper discontinuation reasons in the CPRS medication menu (Figure 3) and document the rationale in the progress notes. It is highly encouraged that the collaborating pharmacists and health care providers add each other as cosigners and communicate effectively. Clinical pharmacy specialists also use the VIONE Polypharmacy Reminder Dialog Template (RDT) to document complete medication reviews with veterans to include deprescribing rationale and document shared decision making.
- A VIONE national dashboard captures deprescribing data in real time and automates reporting with daily updates that are readily accessible to all implementing facilities. Minimum data captured include the number of unique veterans impacted, number of medications deprescribed, cumulative cost avoidance to date, and number of prescriptions deprescribed per veteran. The dashboard facilitates real-time use of individual patient data and has also been designed to capture data from VHA administrative data portals and Corporate Data Warehouse.
Results
As of October 31, 2019, the assessment of polypharmacy using the VIONE tool across VHA sites has benefited > 60,000 unique veterans, of whom 49.2% were in urban areas, 47.7% in rural areas, and 3.1% in highly rural areas. Elderly male veterans comprised a clear majority. More than 128,000 medications have been deprescribed. The top classes of medications deprescribed are antihypertensives, over-the-counter medications, and antidiabetic medications. An annualized cost avoidance of > $4.0 million has been achieved. Cost avoidance is the cost of medications that otherwise would have continued to be filled and paid for by the VHA if they had not been deprescribed, projected for a maximum of 365 days. The calculation methodology can be summarized as follows:
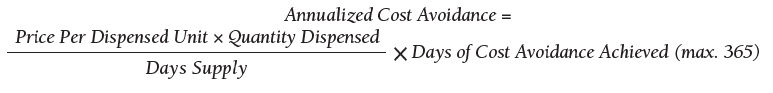
The calculations reported in Table 3 and Figure 4 are conservative and include only chronic outpatient prescriptions and do not account for medications deprescribed in inpatient units, nursing home, community living centers, or domiciliary populations. Data tracked separately from inpatient and community living center patient populations indicated an additional 25,536 deprescribed medications, across 28 VA facilities, impacting 7,076 veterans with an average 2.15 medications deprescribed per veteran. The additional achieved cost avoidance was $370,272 (based on $14.50 average cost per prescription). Medications restarted within 30 days of deprescribing are not included in these calculations.
The cost avoidance calculation further excludes the effects of VIONE implementation on many other types of interventions. These interventions include, but are not limited to, changing from aggressive care to end of life, comfort care when strongly indicated; reduced emergency department visits or invasive diagnostic and therapeutic approaches, when not indicated; medical supplies, antimicrobial preparations; labor costs related to packaging, mailing, and administering prescriptions; reduced/prevented clinical waste; reduced decompensation of systemic illnesses and subsequent health care needs precipitated by iatrogenic disturbances and prolonged convalescence; and overall changes to prescribing practices through purposeful and targeted interactions with colleagues across various disciplines and various hierarchical levels.
Discussion
The VIONE clinical program exemplifies the translation of HRO principles into health care system practices. VIONE offers a systematic approach to improve medication management with an emphasis on deprescribing nonessential medications across various health care settings, facilitating VHA efforts toward zero harm. It demonstrates close alignment with the key building blocks of an HRO. Effective VIONE incorporation into an organizational culture reflects leadership commitment to safety and reliability in their vision and actions. By empowering staff to proactively reduce inappropriate medications and thereby prevent patient harm, VIONE contributes to enhancing an enterprise-wide culture of safety, with fewer errors and greater reliability. As a standardized decision support tool for the ongoing practice of assessment and planned cessation of potentially inappropriate medications, VIONE illustrates how continuous process improvement can be a part of staff-engaged, veteran-centered, highly reliable care. The standardization of the VIONE tool promotes achievement and sustainment of desired HRO principles and practices within health care delivery systems.
Conclusions
The VIONE program was launched not as a cost savings or research program but as a practical, real-time bedside or ambulatory care intervention to improve patient safety. Its value is reflected in the overwhelming response from scholarly and well-engaged colleagues expressing serious interests in expanding collaborations and tailoring efforts to add more depth and breadth to VIONE related efforts.
Acknowledgments
The authors express their gratitude to Central Arkansas VA Healthcare System leadership, Clinical Applications Coordinators, and colleagues for their unconditional support, to the Diffusion of Excellence programs at US Department of Veterans Affairs Central Office for their endorsement, and to the many VHA participants who renew our optimism and energy as we continue this exciting journey. We also thank Bridget B. Kelly for her assistance in writing and editing of the manuscript.
1. Chassin MR, Jerod ML. High-reliability health care: getting there from here. The Joint Commission. Milbank Q. 2013;91(3):459-490.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data. 2017;4:170167.
4. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
5. Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med. 2016;176(4):482-483.
6. US Department of Veterans Affairs. High reliability. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx. [Nonpublic source, not verified.]
7. Gordon S, Mendenhall P, O’Connor BB. Beyond the Checklist: What Else Health Care Can Learn from Aviation Teamwork and Safety. Ithaca, NY: Cornell University Press; 2013.
8. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
9. US Department of Veterans Affairs. Diffusion of Excellence. https://www.va.gov/HEALTHCAREEXCELLENCE/diffusion-of-excellence/. Updated August 10, 2018. Accessed June 26, 2019.
10. US Department of Veterans Affairs. VIONE program toolkit. https://www.vapulse.net/docs/DOC-259375. [Nonpublic source, not verified.]
1. Chassin MR, Jerod ML. High-reliability health care: getting there from here. The Joint Commission. Milbank Q. 2013;91(3):459-490.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Quinn KJ, Shah NH. A dataset quantifying polypharmacy in the United States. Sci Data. 2017;4:170167.
4. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834.
5. Steinman MA. Polypharmacy—time to get beyond numbers. JAMA Intern Med. 2016;176(4):482-483.
6. US Department of Veterans Affairs. High reliability. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx. [Nonpublic source, not verified.]
7. Gordon S, Mendenhall P, O’Connor BB. Beyond the Checklist: What Else Health Care Can Learn from Aviation Teamwork and Safety. Ithaca, NY: Cornell University Press; 2013.
8. Institute of Medicine (US) Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press; 2000.
9. US Department of Veterans Affairs. Diffusion of Excellence. https://www.va.gov/HEALTHCAREEXCELLENCE/diffusion-of-excellence/. Updated August 10, 2018. Accessed June 26, 2019.
10. US Department of Veterans Affairs. VIONE program toolkit. https://www.vapulse.net/docs/DOC-259375. [Nonpublic source, not verified.]
Evaluating a Veterans Affairs Home-Based Primary Care Population for Patients at High Risk of Osteoporosis
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
Using Voogle to Search Within Patient Records in the VA Corporate Data Warehouse
Digitalization of patient-specific information over the past 2 decades has dramatically altered health care delivery. Nonetheless, this technology has yet to live up to its promise of improving patient outcomes, in part due to data storage challenges as well as the emphasis on data entry to support administrative and financial goals of the institution.1-4 Substantially less emphasis has been placed on the retrieval of information required for accurate diagnosis.
A new search engine, Voogle, is now available through Microsoft Internet Explorer (Redmond, WA) to all providers in the US Department of Veterans Affairs (VA) on any intranet-enabled computer behind the VA firewall. Voogle facilitates rapid query-based search and retrieval of patient-specific data in the VA Corporate Data Warehouse (CDW).
Case Example
A veteran presented requesting consideration for implantation of a new device for obstructive sleep apnea. Guidelines for implantation of the new device specify a narrow therapeutic window, so determination of his apnea-hypopnea index (AHI) was critical. The patient had received care at more than 20 VA facilities and knew the approximate year the test had been performed at a non-VA facility.
A health care provider (HCP) using Voogle from his VA computer indexed all Veterans Information Systems and Technology Architecture (VistA) notes for the desired date range. The indexing of > 200 notes was completed in seconds. The HCP opened the indexed records with Voogle and entered a query for “sleep apnea,” which displayed multiple instances of the term within the patient record notes. A VA HCP had previously entered the data from the outside sleep study into a note shortly after the study.
This information was found immediately by sorting the indexed notes by date. The total time required by Voogle to find and display the critical information from the sleep study entered at a different VA more than a dozen years earlier was about 1 minute. These data provided the information needed for decision making at the time of the current patient encounter, without which repeat (and unnecessary) testing would have been required.
Information Overload
Electronic health records (EHRs) such as VistA, upload, store, collate, and present data in near real-time across multiple locations. Although the availability of these data can potentially reduce the risk of error due to missing critical information, its sheer volume limits its utility for point-of-care decision making. Much patient-specific text data found in clinical notes are recorded for administrative, financial, and business purposes rather than to support patient care decision making.1-3 The majority of data documents processes of care rather than HCP observations, assessment of current status, or plans for care. Much of this text is inserted into templates, consists of imported structured data elements, and may contain repeated copy-and-paste free text.
Data uploaded to the CDW are aggregated from multiple hospitals, each with its own “instance” of VistA. Often the CDW contains thousands of text notes for a single patient. This volume of text may conceal critical historical information needed for patient care mixed with a plethora of duplicated or extraneous text entered to satisfy administrative requirements. The effects of information overload and poor system usability have been studied extensively in other disciplines, but this science has largely not been incorporated into EHR design.1,3,4
A position paper published recently by the American College of Physicians notes that physician cognitive work is adversely impacted by the incorporation of nonclinical information into the EHR for use by other administrative and financial functions.2
Information Chaos
Beasley and colleagues noted that information in an EHR needed for optimal care may be unavailable, inadequate, scattered, conflicting, lost, or inaccurate, a condition they term information chaos.5 Smith and colleagues reported that decision making in 1 of 7 primary care visits was impaired by missing critical information. Surveyed HCPs estimated that 44% of patients with missing information may receive compromised care as a result, including delayed or erroneous diagnosis and increased costs due to duplication of diagnostic testing.6
Even when technically available, the usability of patient-specific data needed for accurate diagnosis is compromised if the HCP cannot find the information. In most systems data storage paradigms mirror database design rather than provider cognitive models. Ultimately, the design of current EHR interaction paradigms squanders precious cognitive resources and time, particularly during patient encounters, leaving little available for the cognitive tasks necessary for accurate diagnosis and treatment decisions.1,3,4,7
VA Corporate Data Warehouse
VistA was implemented as a decentralized system with 130 instances, each of which is a freestanding EHR. However, as all systems share common data structures, the data can be combined from multiple instances when needed. The VA established a CDW more than 15 years ago in order to collate information from multiple sites to support operations as well as to seek new insights. The CDW currently updates nightly from all 130 EHR instances and is the only location in which patient information from all treating sites is combined. Voogle can access the CDW through the Veterans Informatics and Computing Infrastructure (VINCI), which is a mirror of the CDW databases and was established as a secure research environment.
The CDW contains information on 25 million veterans, with about 15 terabytes of text data. Approximately 4 billion data points, including 1 million text notes, are accrued nightly. The Integrated Control Number (ICN), a unique patient identifier, is assigned to each CDW record and is cross-indexed in the master patient index. All CDW data are tied to the ICN, facilitating access to and attribution of all patient data from all VA sites. Voogle relies on this identifier to build indexed files, or domains (which are document collections), of requested specific patient information to support its search algorithm.
Structured Data
Most of the data accrued in an EHR are structured data (such as laboratory test results and vital signs) and stored in a defined database framework. Voogle uses iFind (Intersystems Inc, Cambridge, MA) to index, count, and then search for requested information within structured data fields.
Unstructured Text
In contrast to structured data, text notes are stored as documents that are retrievable by patient, author, date, clinic, as well as numerous other fields. Unstructured (free) text notes are more information rich than either structured data or templated notes since their narrative format more closely parallels providers’ cognitive processes.1,7 The value of the narrative becomes even more critical in understanding complex clinical scenarios with multiple interacting disease processes. Narratives emphasize important details, reducing cognitive overload by reducing the salience of detail the author deems to be less critical. Narrative notes simultaneously assure availability through the use of unstandardized language, often including specialty and disease-specific abbreviations.1 Information needed for decision making in the illustrative case in this report was present only in HCP-entered free-text notes, as the structured data from which the free text was derived were not available.
Search
The introduction of search engines can be considered one of the major technologic disruptors of the 21st century.8 However, this advance has not yet made significant inroads into health care, despite advances in other domains. As of 2019, EHR users are still required to be familiar with the system’s data and menu structure in order to find needed information (or enter orders, code visits, or any of a number of tasks). Anecdotally, one of the authors (David Eibling) observed that the most common question from his trainees is “How do you . . .?” referring not to the care of the patient but rather to interaction with the EHR.
What is needed is a simple query-based application that finds the data on request. In addition to Voogle, other advances are being made in this arena such as the EMERSE, medical record search engine (project-emerse.org). Voogle was released to VA providers in 2017 and is available through the Internet Explorer browser on VA computers with VA intranet access. The goal of Voogle is to reduce HCP cognitive load by reducing the time and effort needed to seek relevant information for the care of a specific patient.
Natural Language Processing
Linguistic analysis of text seeking to understand its meaning constitutes a rapidly expanding field, with current heavy emphasis on the role of artificial intelligence and machine learning.1 Advances in processing both structured data and free-text notes in the health care domain is in its infancy, despite the investment of considerable resources. Undoubtedly, advances in this arena will dramatically change provider cognitive work in the next decades.
VistA is coded in MUMPS (Massachusetts General Hospital Utility Multi-Programming System, also known as M), which has been in use for more than 50 years. Voogle employs iKnow, a novel natural language processing (NLP) application that resides in Caché (Intersystems, Boston, MA), the vendor-supported MUMPS infrastructure VistA uses to perform text analysis. iKnow does not attempt to interpret the meaning of text as do other common NLP applications, but instead relies on the expert user to interpret the meaning of the analyzed text. iKnow initially divides sentences into relations (usually verbs) and concepts, and then generates an index of these entities. The efficiency of iKnow results in very rapid indexing—often several thousand notes (not an uncommon number) can be indexed in 20 to 30 seconds. iKnow responds to a user query by searching for specific terms or similar terms within the indexed text, and then displays these terms within the original source documents, similar to well-known commercial search engines. Structured data are indexed by the iFind program simultaneously with free-text indexing (Figure 1).
Security
Maintaining high levels of security of Health Insurance Portability and Accountability (HIPAA)-compliant information in an online application such as Voogle is critical to ensure trust of veterans and HCPs. All patient data accessed by Voogle reside within the secure firewall-protected VINCI environment. All moving information is protected with high-level encryption protocols (transport layer security [TLS]), and data at rest are also encrypted. As the application is online, no data are stored on the accessing device. Voogle uses a secure Microsoft Windows logon using VA Active Directory coupled with VistA authorization to regulate who can see the data and use the application. All access is audited, not only for “sensitive patients,” but also for specific data types. Users are reminded of this Voogle attribute on the home screen.
Accessing Voogle
Voogle is available on the VA intranet to all authorized users at https://voogle.vha.med.va.gov/voogle. To assure high-level security the application can only be accessed with the Internet Explorer browser using established user identification protocols to avoid unauthorized access or duplicative log-in tasks.
Indexing
Indexing is user-driven and is required prior to patient selection and term query. The user is prompted for a patient identifier and a date range. The CDW unique patient identifier is used for all internal processing. However, a social security number look-up table is incorporated to facilitate patient selection. The date field defaults to 3 years but can be extended to approximately the year 2000.
Queries
Entering the patient name in Lastname, Firstname (no space) format will yield a list of indexed patients. All access is audited in order to deter unauthorized queries. Data from a demonstration patient are displayed in Figures 2, 3, 4, 5,
and 6.
Structured Data Searches
Structured data categories that contain the queried term, as well as a term count, are displayed after the “Structured Data” toggle is selected (Figure 2). After the desired category (Figure 2: “Outpatient Rx”) is selected, Voogle accesses the data file and displays it as a grid (medication list, Figure 3). Filter and sort functions enable display of specific medications, drug classes, or date ranges (Figure 4).
Display of Terms Within Text Notes
Selecting a term from the drop-down list (Figure 5) opens a grid with the term highlighted in a snippet of text (Figure 6). Opening the document displays the context of the term, along with negation terms (ie, not, denies, no, etc) in red font if present. Voogle, unlike other NLP tools that attempt to interpret medical notes, relies on interpretation by the HCP user. Duplicate note fragments will be displayed in multiple notes, often across multiple screens, vividly demonstrating the pervasive use of the copy-and-paste text-entry strategy. Voogle satisfies 2 of the 4 recommendations of the recent report on copy-and-paste by Tsou and colleagues.9 The Voogle text display grid identifies copy-and-pasted text as well as establishes the provenance of the text (by sorting on the date column). Text can be copied from Voogle into an active Computerized Patient Record System (CPRS) note if needed for active patient care. Reindexing the following day and then repeating the search will demonstrate the newly copied-and-pasted text appended to the sequence.
Limitations
Voogle is unable to access all VA patient data currently. There are a dozen or so clinical domains that are indexed by Voogle that include prescriptions, problem lists, health factors, and others. More domains can be added with minimal effort and would then be available for fast search. The most critical deficiency is its inability to access, index, or query text reports stored as images within VistA Imaging. This includes nearly all reports from outside HCPs, emergency department visits or discharge summaries from unlinked hospitals, anesthesia reports, intensive care unit flow sheets, electrocardiograms, as well as numerous other text reports such as pulmonary function reports or sleep studies. Information that is transcribed by the provider into VistA as text (as in the case presented) is available within the CDW and can be found and displayed by Voogle search.
Voogle requires that the user initiates the indexing process prior to initiating the search process. Although Voogle defaults to 3 years prior to the current date, the user can specify a start date extending to close to the year 2000. The volume of data flowing into the CDW precludes automatic indexing of all patient data, as well as automatic updating of previously indexed data. We have explored the feasibility of queueing scheduled appointments for the following day, and although the strategy shows some promise, avoiding conflict with user-requested on-demand indexing remains challenging.
The current VA network architecture updates the CDW every night, resulting in up to a 24-hour delay in data availability. However, this delay should be reduced to several minutes after implementation of real-time data feeds accompanying the coming transition to a new EHR platform.
Conclusions
The recent introduction of the Joint Legacy Viewer (JLV) to the VA EHR desktop has enhanced the breadth of patient-specific information available to any VHA clinician, with recent enhancements providing access to some community care notes from outside HCPs. Voogle builds on this capability by enabling rapid search of text notes and structured data from multiple VA sites, over an extended time frame, and perhaps entered by hundreds of authors, as demonstrated in the case example. Formal usability and workload studies have not been performed; however, anecdotal reports indicate the application dramatically reduces the time required to search for critical information needed for care of complex patients who have been treated in multiple different VA hospitals and clinics.
The Voogle paradigm of leveraging patient information stored within a large enterprise-wide data warehouse through NLP techniques may be applicable to other systems as well, and warrants exploration. We believe that replacing traditional data search paradigms that require knowledge of data structure with a true query-based paradigm is a potential game changer for health information systems. Ultimately this strategy may help provide an antidote for the information chaos impacting HCP cognition. Moreover, reducing HCP cognitive load and time on task may lessen overall health care costs, reduce provider burn-out, and improve the quality of care received by patients.
Near real-time data feeds and adding additional clinical domains will potentially provide other benefits to patient care. For example, the authors plan to investigate whether sampling incoming data may assist with behind-the-scenes continuous monitoring of indicators of patient status to facilitate early warning of impending physiologic collapse.10 Other possible applications could include real-time scans for biosurveillance or other population screening requirements.
Acknowledgments
The authors express their sincere appreciation to Leslie DeYoung for documentation and Justin Wilson who constructed much of the graphical user interface for the Voogle application and design. Without their expertise, passion, and commitment the application would not be available as it is now.
1. Wachter RM. The Digital Doctor: Hope, Hype and Harm at the Dawn of the Computer Age New York: McGraw-Hill Education; 2017.
2. Erickson SM, Rockwern B, Koltov M, McLean RM; Medical Practice and Quality Committee of the American College of Physicians. Putting patients first by reducing administrative tasks in health care: a position paper of the American College of Physicians. Ann Intern Med. 2017;166(9):659-661.
3. Woods DD, Patterson ES, Roth EM. Can we ever escape from data overload? A cognitive systems diagnosis. Cogn Technol Work. 2002;4(1):22-36.
4. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156.
5. Beasley JW, Wetterneck TB, Temte J, et al. Information chaos in primary care: implications for physician performance and patient safety. J Am Board Fam Med. 2011;24(6):745-751.
6. Smith PC, Araya-Guerra R, Bublitz C, et al. Missing clinical information during primary care visits. JAMA. 2005;293(5):565-571.
7. Papadakos PJ, Berman E, eds. Distracted Doctoring: Returning to Patient-Centered Care in the Digital Age. New York: Springer International Publishing; 2017.
8. Battelle J. Search: How Google and its Rivals Rewrote the Rules of Business and Transformed Our Culture. New York: Penguin Group; 2005.
9. Tsou AY, Lehmann CU, Michel J, Solomon R, Possanza L, Gandhi T. Safe practices for copy and paste in the EHR. Systematic review, recommendations, and novel model for health IT collaboration. Appl Clin Inform. 2017;8(1):12-34.
10. Rothman MJ, Rothman SI, Beals J 4th. Development and validation of a continuous measure of patient condition using the electronic medical record. J Biomed Inform. 2013;46(5):837-848.
Digitalization of patient-specific information over the past 2 decades has dramatically altered health care delivery. Nonetheless, this technology has yet to live up to its promise of improving patient outcomes, in part due to data storage challenges as well as the emphasis on data entry to support administrative and financial goals of the institution.1-4 Substantially less emphasis has been placed on the retrieval of information required for accurate diagnosis.
A new search engine, Voogle, is now available through Microsoft Internet Explorer (Redmond, WA) to all providers in the US Department of Veterans Affairs (VA) on any intranet-enabled computer behind the VA firewall. Voogle facilitates rapid query-based search and retrieval of patient-specific data in the VA Corporate Data Warehouse (CDW).
Case Example
A veteran presented requesting consideration for implantation of a new device for obstructive sleep apnea. Guidelines for implantation of the new device specify a narrow therapeutic window, so determination of his apnea-hypopnea index (AHI) was critical. The patient had received care at more than 20 VA facilities and knew the approximate year the test had been performed at a non-VA facility.
A health care provider (HCP) using Voogle from his VA computer indexed all Veterans Information Systems and Technology Architecture (VistA) notes for the desired date range. The indexing of > 200 notes was completed in seconds. The HCP opened the indexed records with Voogle and entered a query for “sleep apnea,” which displayed multiple instances of the term within the patient record notes. A VA HCP had previously entered the data from the outside sleep study into a note shortly after the study.
This information was found immediately by sorting the indexed notes by date. The total time required by Voogle to find and display the critical information from the sleep study entered at a different VA more than a dozen years earlier was about 1 minute. These data provided the information needed for decision making at the time of the current patient encounter, without which repeat (and unnecessary) testing would have been required.
Information Overload
Electronic health records (EHRs) such as VistA, upload, store, collate, and present data in near real-time across multiple locations. Although the availability of these data can potentially reduce the risk of error due to missing critical information, its sheer volume limits its utility for point-of-care decision making. Much patient-specific text data found in clinical notes are recorded for administrative, financial, and business purposes rather than to support patient care decision making.1-3 The majority of data documents processes of care rather than HCP observations, assessment of current status, or plans for care. Much of this text is inserted into templates, consists of imported structured data elements, and may contain repeated copy-and-paste free text.
Data uploaded to the CDW are aggregated from multiple hospitals, each with its own “instance” of VistA. Often the CDW contains thousands of text notes for a single patient. This volume of text may conceal critical historical information needed for patient care mixed with a plethora of duplicated or extraneous text entered to satisfy administrative requirements. The effects of information overload and poor system usability have been studied extensively in other disciplines, but this science has largely not been incorporated into EHR design.1,3,4
A position paper published recently by the American College of Physicians notes that physician cognitive work is adversely impacted by the incorporation of nonclinical information into the EHR for use by other administrative and financial functions.2
Information Chaos
Beasley and colleagues noted that information in an EHR needed for optimal care may be unavailable, inadequate, scattered, conflicting, lost, or inaccurate, a condition they term information chaos.5 Smith and colleagues reported that decision making in 1 of 7 primary care visits was impaired by missing critical information. Surveyed HCPs estimated that 44% of patients with missing information may receive compromised care as a result, including delayed or erroneous diagnosis and increased costs due to duplication of diagnostic testing.6
Even when technically available, the usability of patient-specific data needed for accurate diagnosis is compromised if the HCP cannot find the information. In most systems data storage paradigms mirror database design rather than provider cognitive models. Ultimately, the design of current EHR interaction paradigms squanders precious cognitive resources and time, particularly during patient encounters, leaving little available for the cognitive tasks necessary for accurate diagnosis and treatment decisions.1,3,4,7
VA Corporate Data Warehouse
VistA was implemented as a decentralized system with 130 instances, each of which is a freestanding EHR. However, as all systems share common data structures, the data can be combined from multiple instances when needed. The VA established a CDW more than 15 years ago in order to collate information from multiple sites to support operations as well as to seek new insights. The CDW currently updates nightly from all 130 EHR instances and is the only location in which patient information from all treating sites is combined. Voogle can access the CDW through the Veterans Informatics and Computing Infrastructure (VINCI), which is a mirror of the CDW databases and was established as a secure research environment.
The CDW contains information on 25 million veterans, with about 15 terabytes of text data. Approximately 4 billion data points, including 1 million text notes, are accrued nightly. The Integrated Control Number (ICN), a unique patient identifier, is assigned to each CDW record and is cross-indexed in the master patient index. All CDW data are tied to the ICN, facilitating access to and attribution of all patient data from all VA sites. Voogle relies on this identifier to build indexed files, or domains (which are document collections), of requested specific patient information to support its search algorithm.
Structured Data
Most of the data accrued in an EHR are structured data (such as laboratory test results and vital signs) and stored in a defined database framework. Voogle uses iFind (Intersystems Inc, Cambridge, MA) to index, count, and then search for requested information within structured data fields.
Unstructured Text
In contrast to structured data, text notes are stored as documents that are retrievable by patient, author, date, clinic, as well as numerous other fields. Unstructured (free) text notes are more information rich than either structured data or templated notes since their narrative format more closely parallels providers’ cognitive processes.1,7 The value of the narrative becomes even more critical in understanding complex clinical scenarios with multiple interacting disease processes. Narratives emphasize important details, reducing cognitive overload by reducing the salience of detail the author deems to be less critical. Narrative notes simultaneously assure availability through the use of unstandardized language, often including specialty and disease-specific abbreviations.1 Information needed for decision making in the illustrative case in this report was present only in HCP-entered free-text notes, as the structured data from which the free text was derived were not available.
Search
The introduction of search engines can be considered one of the major technologic disruptors of the 21st century.8 However, this advance has not yet made significant inroads into health care, despite advances in other domains. As of 2019, EHR users are still required to be familiar with the system’s data and menu structure in order to find needed information (or enter orders, code visits, or any of a number of tasks). Anecdotally, one of the authors (David Eibling) observed that the most common question from his trainees is “How do you . . .?” referring not to the care of the patient but rather to interaction with the EHR.
What is needed is a simple query-based application that finds the data on request. In addition to Voogle, other advances are being made in this arena such as the EMERSE, medical record search engine (project-emerse.org). Voogle was released to VA providers in 2017 and is available through the Internet Explorer browser on VA computers with VA intranet access. The goal of Voogle is to reduce HCP cognitive load by reducing the time and effort needed to seek relevant information for the care of a specific patient.
Natural Language Processing
Linguistic analysis of text seeking to understand its meaning constitutes a rapidly expanding field, with current heavy emphasis on the role of artificial intelligence and machine learning.1 Advances in processing both structured data and free-text notes in the health care domain is in its infancy, despite the investment of considerable resources. Undoubtedly, advances in this arena will dramatically change provider cognitive work in the next decades.
VistA is coded in MUMPS (Massachusetts General Hospital Utility Multi-Programming System, also known as M), which has been in use for more than 50 years. Voogle employs iKnow, a novel natural language processing (NLP) application that resides in Caché (Intersystems, Boston, MA), the vendor-supported MUMPS infrastructure VistA uses to perform text analysis. iKnow does not attempt to interpret the meaning of text as do other common NLP applications, but instead relies on the expert user to interpret the meaning of the analyzed text. iKnow initially divides sentences into relations (usually verbs) and concepts, and then generates an index of these entities. The efficiency of iKnow results in very rapid indexing—often several thousand notes (not an uncommon number) can be indexed in 20 to 30 seconds. iKnow responds to a user query by searching for specific terms or similar terms within the indexed text, and then displays these terms within the original source documents, similar to well-known commercial search engines. Structured data are indexed by the iFind program simultaneously with free-text indexing (Figure 1).
Security
Maintaining high levels of security of Health Insurance Portability and Accountability (HIPAA)-compliant information in an online application such as Voogle is critical to ensure trust of veterans and HCPs. All patient data accessed by Voogle reside within the secure firewall-protected VINCI environment. All moving information is protected with high-level encryption protocols (transport layer security [TLS]), and data at rest are also encrypted. As the application is online, no data are stored on the accessing device. Voogle uses a secure Microsoft Windows logon using VA Active Directory coupled with VistA authorization to regulate who can see the data and use the application. All access is audited, not only for “sensitive patients,” but also for specific data types. Users are reminded of this Voogle attribute on the home screen.
Accessing Voogle
Voogle is available on the VA intranet to all authorized users at https://voogle.vha.med.va.gov/voogle. To assure high-level security the application can only be accessed with the Internet Explorer browser using established user identification protocols to avoid unauthorized access or duplicative log-in tasks.
Indexing
Indexing is user-driven and is required prior to patient selection and term query. The user is prompted for a patient identifier and a date range. The CDW unique patient identifier is used for all internal processing. However, a social security number look-up table is incorporated to facilitate patient selection. The date field defaults to 3 years but can be extended to approximately the year 2000.
Queries
Entering the patient name in Lastname, Firstname (no space) format will yield a list of indexed patients. All access is audited in order to deter unauthorized queries. Data from a demonstration patient are displayed in Figures 2, 3, 4, 5,
and 6.
Structured Data Searches
Structured data categories that contain the queried term, as well as a term count, are displayed after the “Structured Data” toggle is selected (Figure 2). After the desired category (Figure 2: “Outpatient Rx”) is selected, Voogle accesses the data file and displays it as a grid (medication list, Figure 3). Filter and sort functions enable display of specific medications, drug classes, or date ranges (Figure 4).
Display of Terms Within Text Notes
Selecting a term from the drop-down list (Figure 5) opens a grid with the term highlighted in a snippet of text (Figure 6). Opening the document displays the context of the term, along with negation terms (ie, not, denies, no, etc) in red font if present. Voogle, unlike other NLP tools that attempt to interpret medical notes, relies on interpretation by the HCP user. Duplicate note fragments will be displayed in multiple notes, often across multiple screens, vividly demonstrating the pervasive use of the copy-and-paste text-entry strategy. Voogle satisfies 2 of the 4 recommendations of the recent report on copy-and-paste by Tsou and colleagues.9 The Voogle text display grid identifies copy-and-pasted text as well as establishes the provenance of the text (by sorting on the date column). Text can be copied from Voogle into an active Computerized Patient Record System (CPRS) note if needed for active patient care. Reindexing the following day and then repeating the search will demonstrate the newly copied-and-pasted text appended to the sequence.
Limitations
Voogle is unable to access all VA patient data currently. There are a dozen or so clinical domains that are indexed by Voogle that include prescriptions, problem lists, health factors, and others. More domains can be added with minimal effort and would then be available for fast search. The most critical deficiency is its inability to access, index, or query text reports stored as images within VistA Imaging. This includes nearly all reports from outside HCPs, emergency department visits or discharge summaries from unlinked hospitals, anesthesia reports, intensive care unit flow sheets, electrocardiograms, as well as numerous other text reports such as pulmonary function reports or sleep studies. Information that is transcribed by the provider into VistA as text (as in the case presented) is available within the CDW and can be found and displayed by Voogle search.
Voogle requires that the user initiates the indexing process prior to initiating the search process. Although Voogle defaults to 3 years prior to the current date, the user can specify a start date extending to close to the year 2000. The volume of data flowing into the CDW precludes automatic indexing of all patient data, as well as automatic updating of previously indexed data. We have explored the feasibility of queueing scheduled appointments for the following day, and although the strategy shows some promise, avoiding conflict with user-requested on-demand indexing remains challenging.
The current VA network architecture updates the CDW every night, resulting in up to a 24-hour delay in data availability. However, this delay should be reduced to several minutes after implementation of real-time data feeds accompanying the coming transition to a new EHR platform.
Conclusions
The recent introduction of the Joint Legacy Viewer (JLV) to the VA EHR desktop has enhanced the breadth of patient-specific information available to any VHA clinician, with recent enhancements providing access to some community care notes from outside HCPs. Voogle builds on this capability by enabling rapid search of text notes and structured data from multiple VA sites, over an extended time frame, and perhaps entered by hundreds of authors, as demonstrated in the case example. Formal usability and workload studies have not been performed; however, anecdotal reports indicate the application dramatically reduces the time required to search for critical information needed for care of complex patients who have been treated in multiple different VA hospitals and clinics.
The Voogle paradigm of leveraging patient information stored within a large enterprise-wide data warehouse through NLP techniques may be applicable to other systems as well, and warrants exploration. We believe that replacing traditional data search paradigms that require knowledge of data structure with a true query-based paradigm is a potential game changer for health information systems. Ultimately this strategy may help provide an antidote for the information chaos impacting HCP cognition. Moreover, reducing HCP cognitive load and time on task may lessen overall health care costs, reduce provider burn-out, and improve the quality of care received by patients.
Near real-time data feeds and adding additional clinical domains will potentially provide other benefits to patient care. For example, the authors plan to investigate whether sampling incoming data may assist with behind-the-scenes continuous monitoring of indicators of patient status to facilitate early warning of impending physiologic collapse.10 Other possible applications could include real-time scans for biosurveillance or other population screening requirements.
Acknowledgments
The authors express their sincere appreciation to Leslie DeYoung for documentation and Justin Wilson who constructed much of the graphical user interface for the Voogle application and design. Without their expertise, passion, and commitment the application would not be available as it is now.
Digitalization of patient-specific information over the past 2 decades has dramatically altered health care delivery. Nonetheless, this technology has yet to live up to its promise of improving patient outcomes, in part due to data storage challenges as well as the emphasis on data entry to support administrative and financial goals of the institution.1-4 Substantially less emphasis has been placed on the retrieval of information required for accurate diagnosis.
A new search engine, Voogle, is now available through Microsoft Internet Explorer (Redmond, WA) to all providers in the US Department of Veterans Affairs (VA) on any intranet-enabled computer behind the VA firewall. Voogle facilitates rapid query-based search and retrieval of patient-specific data in the VA Corporate Data Warehouse (CDW).
Case Example
A veteran presented requesting consideration for implantation of a new device for obstructive sleep apnea. Guidelines for implantation of the new device specify a narrow therapeutic window, so determination of his apnea-hypopnea index (AHI) was critical. The patient had received care at more than 20 VA facilities and knew the approximate year the test had been performed at a non-VA facility.
A health care provider (HCP) using Voogle from his VA computer indexed all Veterans Information Systems and Technology Architecture (VistA) notes for the desired date range. The indexing of > 200 notes was completed in seconds. The HCP opened the indexed records with Voogle and entered a query for “sleep apnea,” which displayed multiple instances of the term within the patient record notes. A VA HCP had previously entered the data from the outside sleep study into a note shortly after the study.
This information was found immediately by sorting the indexed notes by date. The total time required by Voogle to find and display the critical information from the sleep study entered at a different VA more than a dozen years earlier was about 1 minute. These data provided the information needed for decision making at the time of the current patient encounter, without which repeat (and unnecessary) testing would have been required.
Information Overload
Electronic health records (EHRs) such as VistA, upload, store, collate, and present data in near real-time across multiple locations. Although the availability of these data can potentially reduce the risk of error due to missing critical information, its sheer volume limits its utility for point-of-care decision making. Much patient-specific text data found in clinical notes are recorded for administrative, financial, and business purposes rather than to support patient care decision making.1-3 The majority of data documents processes of care rather than HCP observations, assessment of current status, or plans for care. Much of this text is inserted into templates, consists of imported structured data elements, and may contain repeated copy-and-paste free text.
Data uploaded to the CDW are aggregated from multiple hospitals, each with its own “instance” of VistA. Often the CDW contains thousands of text notes for a single patient. This volume of text may conceal critical historical information needed for patient care mixed with a plethora of duplicated or extraneous text entered to satisfy administrative requirements. The effects of information overload and poor system usability have been studied extensively in other disciplines, but this science has largely not been incorporated into EHR design.1,3,4
A position paper published recently by the American College of Physicians notes that physician cognitive work is adversely impacted by the incorporation of nonclinical information into the EHR for use by other administrative and financial functions.2
Information Chaos
Beasley and colleagues noted that information in an EHR needed for optimal care may be unavailable, inadequate, scattered, conflicting, lost, or inaccurate, a condition they term information chaos.5 Smith and colleagues reported that decision making in 1 of 7 primary care visits was impaired by missing critical information. Surveyed HCPs estimated that 44% of patients with missing information may receive compromised care as a result, including delayed or erroneous diagnosis and increased costs due to duplication of diagnostic testing.6
Even when technically available, the usability of patient-specific data needed for accurate diagnosis is compromised if the HCP cannot find the information. In most systems data storage paradigms mirror database design rather than provider cognitive models. Ultimately, the design of current EHR interaction paradigms squanders precious cognitive resources and time, particularly during patient encounters, leaving little available for the cognitive tasks necessary for accurate diagnosis and treatment decisions.1,3,4,7
VA Corporate Data Warehouse
VistA was implemented as a decentralized system with 130 instances, each of which is a freestanding EHR. However, as all systems share common data structures, the data can be combined from multiple instances when needed. The VA established a CDW more than 15 years ago in order to collate information from multiple sites to support operations as well as to seek new insights. The CDW currently updates nightly from all 130 EHR instances and is the only location in which patient information from all treating sites is combined. Voogle can access the CDW through the Veterans Informatics and Computing Infrastructure (VINCI), which is a mirror of the CDW databases and was established as a secure research environment.
The CDW contains information on 25 million veterans, with about 15 terabytes of text data. Approximately 4 billion data points, including 1 million text notes, are accrued nightly. The Integrated Control Number (ICN), a unique patient identifier, is assigned to each CDW record and is cross-indexed in the master patient index. All CDW data are tied to the ICN, facilitating access to and attribution of all patient data from all VA sites. Voogle relies on this identifier to build indexed files, or domains (which are document collections), of requested specific patient information to support its search algorithm.
Structured Data
Most of the data accrued in an EHR are structured data (such as laboratory test results and vital signs) and stored in a defined database framework. Voogle uses iFind (Intersystems Inc, Cambridge, MA) to index, count, and then search for requested information within structured data fields.
Unstructured Text
In contrast to structured data, text notes are stored as documents that are retrievable by patient, author, date, clinic, as well as numerous other fields. Unstructured (free) text notes are more information rich than either structured data or templated notes since their narrative format more closely parallels providers’ cognitive processes.1,7 The value of the narrative becomes even more critical in understanding complex clinical scenarios with multiple interacting disease processes. Narratives emphasize important details, reducing cognitive overload by reducing the salience of detail the author deems to be less critical. Narrative notes simultaneously assure availability through the use of unstandardized language, often including specialty and disease-specific abbreviations.1 Information needed for decision making in the illustrative case in this report was present only in HCP-entered free-text notes, as the structured data from which the free text was derived were not available.
Search
The introduction of search engines can be considered one of the major technologic disruptors of the 21st century.8 However, this advance has not yet made significant inroads into health care, despite advances in other domains. As of 2019, EHR users are still required to be familiar with the system’s data and menu structure in order to find needed information (or enter orders, code visits, or any of a number of tasks). Anecdotally, one of the authors (David Eibling) observed that the most common question from his trainees is “How do you . . .?” referring not to the care of the patient but rather to interaction with the EHR.
What is needed is a simple query-based application that finds the data on request. In addition to Voogle, other advances are being made in this arena such as the EMERSE, medical record search engine (project-emerse.org). Voogle was released to VA providers in 2017 and is available through the Internet Explorer browser on VA computers with VA intranet access. The goal of Voogle is to reduce HCP cognitive load by reducing the time and effort needed to seek relevant information for the care of a specific patient.
Natural Language Processing
Linguistic analysis of text seeking to understand its meaning constitutes a rapidly expanding field, with current heavy emphasis on the role of artificial intelligence and machine learning.1 Advances in processing both structured data and free-text notes in the health care domain is in its infancy, despite the investment of considerable resources. Undoubtedly, advances in this arena will dramatically change provider cognitive work in the next decades.
VistA is coded in MUMPS (Massachusetts General Hospital Utility Multi-Programming System, also known as M), which has been in use for more than 50 years. Voogle employs iKnow, a novel natural language processing (NLP) application that resides in Caché (Intersystems, Boston, MA), the vendor-supported MUMPS infrastructure VistA uses to perform text analysis. iKnow does not attempt to interpret the meaning of text as do other common NLP applications, but instead relies on the expert user to interpret the meaning of the analyzed text. iKnow initially divides sentences into relations (usually verbs) and concepts, and then generates an index of these entities. The efficiency of iKnow results in very rapid indexing—often several thousand notes (not an uncommon number) can be indexed in 20 to 30 seconds. iKnow responds to a user query by searching for specific terms or similar terms within the indexed text, and then displays these terms within the original source documents, similar to well-known commercial search engines. Structured data are indexed by the iFind program simultaneously with free-text indexing (Figure 1).
Security
Maintaining high levels of security of Health Insurance Portability and Accountability (HIPAA)-compliant information in an online application such as Voogle is critical to ensure trust of veterans and HCPs. All patient data accessed by Voogle reside within the secure firewall-protected VINCI environment. All moving information is protected with high-level encryption protocols (transport layer security [TLS]), and data at rest are also encrypted. As the application is online, no data are stored on the accessing device. Voogle uses a secure Microsoft Windows logon using VA Active Directory coupled with VistA authorization to regulate who can see the data and use the application. All access is audited, not only for “sensitive patients,” but also for specific data types. Users are reminded of this Voogle attribute on the home screen.
Accessing Voogle
Voogle is available on the VA intranet to all authorized users at https://voogle.vha.med.va.gov/voogle. To assure high-level security the application can only be accessed with the Internet Explorer browser using established user identification protocols to avoid unauthorized access or duplicative log-in tasks.
Indexing
Indexing is user-driven and is required prior to patient selection and term query. The user is prompted for a patient identifier and a date range. The CDW unique patient identifier is used for all internal processing. However, a social security number look-up table is incorporated to facilitate patient selection. The date field defaults to 3 years but can be extended to approximately the year 2000.
Queries
Entering the patient name in Lastname, Firstname (no space) format will yield a list of indexed patients. All access is audited in order to deter unauthorized queries. Data from a demonstration patient are displayed in Figures 2, 3, 4, 5,
and 6.
Structured Data Searches
Structured data categories that contain the queried term, as well as a term count, are displayed after the “Structured Data” toggle is selected (Figure 2). After the desired category (Figure 2: “Outpatient Rx”) is selected, Voogle accesses the data file and displays it as a grid (medication list, Figure 3). Filter and sort functions enable display of specific medications, drug classes, or date ranges (Figure 4).
Display of Terms Within Text Notes
Selecting a term from the drop-down list (Figure 5) opens a grid with the term highlighted in a snippet of text (Figure 6). Opening the document displays the context of the term, along with negation terms (ie, not, denies, no, etc) in red font if present. Voogle, unlike other NLP tools that attempt to interpret medical notes, relies on interpretation by the HCP user. Duplicate note fragments will be displayed in multiple notes, often across multiple screens, vividly demonstrating the pervasive use of the copy-and-paste text-entry strategy. Voogle satisfies 2 of the 4 recommendations of the recent report on copy-and-paste by Tsou and colleagues.9 The Voogle text display grid identifies copy-and-pasted text as well as establishes the provenance of the text (by sorting on the date column). Text can be copied from Voogle into an active Computerized Patient Record System (CPRS) note if needed for active patient care. Reindexing the following day and then repeating the search will demonstrate the newly copied-and-pasted text appended to the sequence.
Limitations
Voogle is unable to access all VA patient data currently. There are a dozen or so clinical domains that are indexed by Voogle that include prescriptions, problem lists, health factors, and others. More domains can be added with minimal effort and would then be available for fast search. The most critical deficiency is its inability to access, index, or query text reports stored as images within VistA Imaging. This includes nearly all reports from outside HCPs, emergency department visits or discharge summaries from unlinked hospitals, anesthesia reports, intensive care unit flow sheets, electrocardiograms, as well as numerous other text reports such as pulmonary function reports or sleep studies. Information that is transcribed by the provider into VistA as text (as in the case presented) is available within the CDW and can be found and displayed by Voogle search.
Voogle requires that the user initiates the indexing process prior to initiating the search process. Although Voogle defaults to 3 years prior to the current date, the user can specify a start date extending to close to the year 2000. The volume of data flowing into the CDW precludes automatic indexing of all patient data, as well as automatic updating of previously indexed data. We have explored the feasibility of queueing scheduled appointments for the following day, and although the strategy shows some promise, avoiding conflict with user-requested on-demand indexing remains challenging.
The current VA network architecture updates the CDW every night, resulting in up to a 24-hour delay in data availability. However, this delay should be reduced to several minutes after implementation of real-time data feeds accompanying the coming transition to a new EHR platform.
Conclusions
The recent introduction of the Joint Legacy Viewer (JLV) to the VA EHR desktop has enhanced the breadth of patient-specific information available to any VHA clinician, with recent enhancements providing access to some community care notes from outside HCPs. Voogle builds on this capability by enabling rapid search of text notes and structured data from multiple VA sites, over an extended time frame, and perhaps entered by hundreds of authors, as demonstrated in the case example. Formal usability and workload studies have not been performed; however, anecdotal reports indicate the application dramatically reduces the time required to search for critical information needed for care of complex patients who have been treated in multiple different VA hospitals and clinics.
The Voogle paradigm of leveraging patient information stored within a large enterprise-wide data warehouse through NLP techniques may be applicable to other systems as well, and warrants exploration. We believe that replacing traditional data search paradigms that require knowledge of data structure with a true query-based paradigm is a potential game changer for health information systems. Ultimately this strategy may help provide an antidote for the information chaos impacting HCP cognition. Moreover, reducing HCP cognitive load and time on task may lessen overall health care costs, reduce provider burn-out, and improve the quality of care received by patients.
Near real-time data feeds and adding additional clinical domains will potentially provide other benefits to patient care. For example, the authors plan to investigate whether sampling incoming data may assist with behind-the-scenes continuous monitoring of indicators of patient status to facilitate early warning of impending physiologic collapse.10 Other possible applications could include real-time scans for biosurveillance or other population screening requirements.
Acknowledgments
The authors express their sincere appreciation to Leslie DeYoung for documentation and Justin Wilson who constructed much of the graphical user interface for the Voogle application and design. Without their expertise, passion, and commitment the application would not be available as it is now.
1. Wachter RM. The Digital Doctor: Hope, Hype and Harm at the Dawn of the Computer Age New York: McGraw-Hill Education; 2017.
2. Erickson SM, Rockwern B, Koltov M, McLean RM; Medical Practice and Quality Committee of the American College of Physicians. Putting patients first by reducing administrative tasks in health care: a position paper of the American College of Physicians. Ann Intern Med. 2017;166(9):659-661.
3. Woods DD, Patterson ES, Roth EM. Can we ever escape from data overload? A cognitive systems diagnosis. Cogn Technol Work. 2002;4(1):22-36.
4. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156.
5. Beasley JW, Wetterneck TB, Temte J, et al. Information chaos in primary care: implications for physician performance and patient safety. J Am Board Fam Med. 2011;24(6):745-751.
6. Smith PC, Araya-Guerra R, Bublitz C, et al. Missing clinical information during primary care visits. JAMA. 2005;293(5):565-571.
7. Papadakos PJ, Berman E, eds. Distracted Doctoring: Returning to Patient-Centered Care in the Digital Age. New York: Springer International Publishing; 2017.
8. Battelle J. Search: How Google and its Rivals Rewrote the Rules of Business and Transformed Our Culture. New York: Penguin Group; 2005.
9. Tsou AY, Lehmann CU, Michel J, Solomon R, Possanza L, Gandhi T. Safe practices for copy and paste in the EHR. Systematic review, recommendations, and novel model for health IT collaboration. Appl Clin Inform. 2017;8(1):12-34.
10. Rothman MJ, Rothman SI, Beals J 4th. Development and validation of a continuous measure of patient condition using the electronic medical record. J Biomed Inform. 2013;46(5):837-848.
1. Wachter RM. The Digital Doctor: Hope, Hype and Harm at the Dawn of the Computer Age New York: McGraw-Hill Education; 2017.
2. Erickson SM, Rockwern B, Koltov M, McLean RM; Medical Practice and Quality Committee of the American College of Physicians. Putting patients first by reducing administrative tasks in health care: a position paper of the American College of Physicians. Ann Intern Med. 2017;166(9):659-661.
3. Woods DD, Patterson ES, Roth EM. Can we ever escape from data overload? A cognitive systems diagnosis. Cogn Technol Work. 2002;4(1):22-36.
4. Gupta A, Harrod M, Quinn M, et al. Mind the overlap: how system problems contribute to cognitive failure and diagnostic errors. Diagnosis (Berl). 2018;5(3):151-156.
5. Beasley JW, Wetterneck TB, Temte J, et al. Information chaos in primary care: implications for physician performance and patient safety. J Am Board Fam Med. 2011;24(6):745-751.
6. Smith PC, Araya-Guerra R, Bublitz C, et al. Missing clinical information during primary care visits. JAMA. 2005;293(5):565-571.
7. Papadakos PJ, Berman E, eds. Distracted Doctoring: Returning to Patient-Centered Care in the Digital Age. New York: Springer International Publishing; 2017.
8. Battelle J. Search: How Google and its Rivals Rewrote the Rules of Business and Transformed Our Culture. New York: Penguin Group; 2005.
9. Tsou AY, Lehmann CU, Michel J, Solomon R, Possanza L, Gandhi T. Safe practices for copy and paste in the EHR. Systematic review, recommendations, and novel model for health IT collaboration. Appl Clin Inform. 2017;8(1):12-34.
10. Rothman MJ, Rothman SI, Beals J 4th. Development and validation of a continuous measure of patient condition using the electronic medical record. J Biomed Inform. 2013;46(5):837-848.
Advancing Order Set Design
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
Heparin Drug Shortage Conservation Strategies
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Genomic Medicine and Genetic Counseling in the Department of Veterans Affairs and Department of Defense (FULL)
Vickie Venne, MS. What is the Genomic Medicine Service (GMS) at the US Department of Veterans Affairs (VA)?
Renee Rider, JD, MS, LCGC. GMS is a telehealth service. We are part of central office and field stationed at the George E. Wahlen VA Medical Center (VAMC) in Salt Lake City, Utah. We provide care to about 90 VAMCs and their associated clinics. Veterans are referred to us by entering an interfacility consult in the VA Computerized Patient Record System (CPRS). We review the consult to determine whether the patient needs to be seen, whether we can answer with an e-consult, or whether we need more information. For the patients who need an appointment, the telehealth department at the veteran’s VA facility will contact the patient to arrange a visit with us. At the time of the appointment, the facility has a staff member available to seat the patient and connect them to us using video equipment.
We provide genetic care for all specialties, including cancer, women’s health, cardiology and neurology. In today’s discussion, we are focusing on cancer care.
Vickie Venne. What do patients do at facilities that don’t get care through GMS?
Renee Rider. There are a handful of facilities that provide their own genetic care in-house. For example, VA Boston Healthcare System in Massachusetts and the Michael E. DeBakey VAMC in Houston, Texas each have their own programs. For veterans who are not at a VA facility that has an agreement with GMS and do not have a different genetics program, their providers need to make referrals to community care.
Vickie Venne. How do patients get referred and what happens at their facility when the patients return to the specialty and primary care providers (PCP)? Ishta, who do you refer to GMS and how do you define them initially?
Ishta Thakar, MD, FACP. Referrals can come at a couple of points during a veteran’s journey at the VA. The VA covers obstetrics care for women veterans. Whenever a PCP or a women’s health provider is doing the initial history and physical on a new patient, if the female veteran has an extensive family history of breast, ovarian, colon, or endometrial cancer, then we take more history and we send a consult to GMS. The second instance would be if she tells us that she has had a personal history of breast, ovarian, or endometrial cancer and she has never had genetic testing. The third instance would be whenever we have a female veteran who is diagnosed with breast, ovarian, endometrial, or colon cancer. We would definitely talk to her about genetic counseling and send a referral to GMS. We would ask for a GMS consult for a patient with advanced maternal age, with exposure to some kind of teratogens, with an abnormal ultrasound, a family history of chromosomal disorders, or if she’s seeing an obstetrician who wants her to be tested. And finally, if a patient has a constellation of multiple cancers in the family and we don’t know what’s going on, we would also refer the patient to GMS.
Vickie Venne. That would be why GMS fields over 150 referrals every week. It is a large list. We also see veterans with personal or family histories of neurologic or cardiologic concerns as well.
Renee, as somebody who fields many of these referrals from unaffected individuals, what is the family history process?
Renee Rider. We don’t expect the referring provider to be a genetic expert. When a provider is seeing a constellation of several different cancers and he or she doesn’t know if there’s anything going on genetically or even if it’s possible, absolutely they should put in a referral to GMS. We have a triage counselor who reviews every consult that comes into our service within 24 hours.
Many cancers are due to exposures that are not concerning for a genetic etiology. We can let you know that it is not concerning, and the PCP can counsel the patient that it is very unlikely to be genetic in nature. We still give feedback even if it’s not someone who is appropriate for genetic counseling and testing. It is important to reach out to GMS even if you don’t know whether a cancer is genetic in nature.
It also is important to take your time when gathering family histories. We get a lot of patients who say, “There’s a lot of cancer in my family. I have no idea who had cancer, but I know a lot of people had cancer.” That’s not the day to put in a referral to GMS. At that point, providers should tell the patient to get as much information as they can about the family history and then reassess. It’s important for us to have accurate information. We’ve had several times where we receive a referral because the veteran says that their sister had ovarian cancer. And then when our staff calls, they later find out it was cervical cancer. That’s not a good use of the veteran’s time, and it’s not a good use of VA resources.
The other important thing about family histories is keeping the questions open-ended. Often a PCP or specialist will ask about a certain type of cancer: “Does anyone in your family have breast cancer, ovarian cancer?” Or if the veteran
is getting a colonoscopy, they ask, “Does anybody have colon cancer?” Where really, we need to be a little bit more open-ended. We prefer questions like, “Has anyone in your family
had cancer?” because that’s the question that prompts a response of, “Yes, 3 people in my family have had thyroid cancer.” That’s very important for us to know, too.
If you do get a positive response, probe a little bit more: what kind of cancer did someone have, how old were they when they had their cancer? And how are they related? Is this an aunt on your mom’s side or on your dad’s side? Those are the types of information that we need to figure out if that person needs a referral.
Vickie Venne. It’s a different story when people already have a cancer diagnosis. Which hematology or oncology patients are good referrals and why?
Lisa Arfons, MD. When patients come in with newly diagnosed cancer, breast for example, it is an emotional diagnosis and psychologicallydistressing. Oftentimes, they want to know why this happened to them. The issues surrounding
genetic testing also becomes very emotional. They want to know whether their children are at risk as well.
Genetic discussions take a long time. I rarely do that on the first visit. I always record for myself in my clinic note if something strikes me regarding the patient’s diagnosis. I quickly run through the National Comprehensive Cancer Network (NCCN) guidelines to remind myself of what I need to go over with the patient at our next meeting. Most patients don’t need to be referred to GMS, and most patients don’t need to be tested once they’re seen.
I often save the referral discussion for after I have established a rapport with a patient, we have a treatment plan, or they already have had their first surgery. Therefore, we are not making decisions about their first surgery based on the genetic medicine results.
If I’m considering a referral, I do a deeper dive with the patient. Is the patient older or younger than 45 years? I pull up NCCN guidelines and we go through the entire checklist.
We have male breast cancer patients at the VA—probably more than the community—so we refer those patients. At the Louis Stokes Cleveland VAMC in Ohio, we have had some in-depth discussions about referring male breast cancer patients for genetic testing and whether it was beneficial to older patients with male breast cancer. Ultimately, we decided that it was important for our male veterans to be tested because it empowered them to have better understanding of their medical conditions that may not just have effect on them but on their offspring, and that that can be a source of psychological and emotional support.
I don’t refer most people to GMS once I go through the checklist. I appreciate the action for an e-consult within the CPRS telemedicine consult itself, as Renee noted. If it is not necessary, GMS makes it an e-consult. I try to communicate that I don’t know whether it is necessary or not so that GMS understands where I’m coming from.
Vickie Venne. In the US Department of Defense (DoD) the process is quite different. Mauricio, can you explain the clinical referral process, who is referred, and how that works from a laboratory perspective?
Maj De Castro, MD, FACMG, USAF. The VA has led the way in demonstrating how to best provide for the medical genetic needs of a large, decentralized population distributed all over the country. Over the last 5 to 10 years, the DoD has made strides in recognizing the role genetics plays in the practice of everyday medicine and redoubling efforts to meet the needs of servicemembers.
The way that it traditionally has worked in the DoD is that military treatment facilities (MTFs) that have dedicated geneticists and genetic counselors: Kessler Medical Center in Mississippi, Walter Reed National Military Medical
Center in Maryland, Tripler Army Medical Center in Hawaii, Madigan Army Medical Center in Washington, Brooke Army Medical Center in Texas, Naval Medical Center San Diego in California, and Naval Medical Center Portsmouth in Virginia. A patient seeking genetic evaluation, counseling, or testing in those larger facilities would be referred to the genetics service by their primary care manager. Wait times vary, but it would usually be weeks, maybe months. However, the great majority of MTFs do not have dedicated genetics support. Most of the time, those patients would have to be referred to the local civilian community—there was no process for them to be seen in in the military healthcare system—with wait times that exceed 6 to 8 months in some cases. This is due to just not a military but a national shortage of genetics professionals (counselors and physicians).
Last year we started the telegenetics initiative, which is small compared to the VA—it is comprised of 2 geneticists and 1 genetic counselor—but with the full intent of growing it over time. Its purpose is to extend the resources we
had to other MTFs. Genetics professionals stationed state-side can provide care to remote facilities with limited access to local genetics support such as Cannon Air Force Base (AFB) or overseas facilities such as Spangdahlem AFB in Germany.
We recognize there are military-specific needs for the DoD regarding the genetic counseling process that have to take into account readiness, genetic discrimination, continued ability to serve and fitness for duty. For this important reason, we are seeking to expand our telegenetics initiative. The goal is to be able to provide 100% of all genetic counseling in-house, so to speak.
Currently, providers at the 4 pilot sites (Cannon AFB, Fort Bragg, Spangdahlem AFB, and Guantanamo Bay) send us referrals. We triage them and assign the patient to see a geneticist or a counselor depending on the indication.
On the laboratory side, it has been a very interesting experience. Because we provide comprehensive germline cancer testing at very little cost to the provider at any MTF, we have had high numbers of test requests over the years.
In addition to saving the DoD millions of dollars in testing, we have learned some interesting lessons in the process. For instance, we have worked closely with several different groups to better understand how to educate providers on the genetic counseling and testing process. This has allowed us to craft a thorough and inclusive consent form that addresses the needs of the DoD. We have also learned valuable lessons about population-based screening vs evidence-based testing, and lessons surrounding narrow-based testing (BRCA1 and BRCA2 only testing) vs ordering a more comprehensive panel that includes other genes supported by strong evidence (such as PALB2, CHEK2, or TP53).
For example, we have found that in a significant proportion of individuals with and without family history, there are clinically relevant variants in genes other than BRCA1 or BRCA2. And so, we have made part of our consent process,
a statement on secondary findings. If the patient consents, we will report pathogenic variants in other genes known to be associated with cancer (with strong evidence) even if the provider ordered a narrow panel such as BRCA1 and BRCA2 testing only. In about 1% to 4% of patients that would otherwise not meet NCCN guidelines, we’ve reported variants that were clinically actionable and changed the medical management of that patient.
We feel strongly that this is a conversation that we need to have in our field, and we realize it’s a complex issue, maybe we need to expand who gets testing. Guideline based testing is missing some patients out there that could benefit from it.
Vickie Venne. There certainly are many sides to the conversation of population-based vs evidence-based genetic testing. Genetic testing policies are changing rapidly. There are teams exploring comprehensive gene sequencing for
newborns and how that potential 1-time test can provide information will be reinterpreted as a person goes from cradle to grave. However, unlike the current DoD process, in the VA there are patients who we don’t see.
Renee Rider. I want to talk about money. When we order a genetic test, that test is paid for by the pathology department at the patient’s VAMC. Most of the pathology departments we work with are clear that they only can provide
genetic testing that is considered medically necessary. Thus, we review each test to make sure it meets established guidelines for testing. We don’t do population genetic screening as there isn’t evidence or guidelines to support offering it. We are strict about who does and does not get genetic testing, partly because we have a responsibility to pathology departments and to the taxpayers.
GMS focuses on conditions that are inherited, that is to say, we deal with germline genetics. Therefore, we discontinue referrals for somatic requests, such as when an OncotypeDX test is requested. It is my understanding that pharmacogenetic referrals may be sent to the new PHASeR initiative, which is a joint collaboration between the VA and Sanford Health and is headed by Deepak Voora, MD.
We generally don’t see patients who still are having diagnostic procedures done. For example, if a veteran has a suspicious breast mass, we recommend that the provider workup the mass before referring to GMS. Regardless of a genetic test result, a suspicious mass needs to be worked up. And, knowing if the mass is cancerous could change how we would proceed with the genetic workup. For example, if the mass were not cancerous, we may recommend that an affected relative have the first genetic evaluation. Furthermore, knowing if the patient has cancer changes how we interpret negative test results.
Another group of patients we don’t see are those who already had genetic testing done by the referring provider. It’s a VA directive that if you order a test, you’re the person who is responsible for giving the results. We agree with
this directive. If you don’t feel comfortable giving back test results, don’t order the test. Often, when a provider sends a patient to us after the test was done, we discover that the patient didn’t have appropriate pretest counseling. A test result, such as a variant of uncertain significance (VUS), should never be a surprise to either the provider or the patient.
Ishta Thakar. For newly diagnosed cancers, the first call is to the patient to inform them that they have cancer. We usually bring up genetic counseling or testing, if applicable, when they are ready to accept the diagnosis and have a conversation about it. All our consults are via telehealth, so none of our patients physically come to GMS in Salt Lake City. All the consults are done virtually.
For newly diagnosed patients, we would send a consult in within a couple of weeks. For patients who had a family history, the referral would not be urgent: They can be seen within about 3 months. The turnaround times for GMS are so much better than what we have available in the community where it’s often at least 6 months, as previously noted.
Vickie Venne. Thank you. We continue to work on that. One of the interesting things that we’ve done, which is the brainchild of Renee, is shared medical appointments.
Renee Rider. We have now created 4 group appointments for people who have concerns surrounding cancer. One group is for people who don’t have cancer but have family members who have cancer who may be the best testing candidate. For example, that might be a 30-year old who tells you that her mother had breast cancer at age 45 years. Her mother is still living, but she’s never had genetic testing. We would put her in a group where we discuss the importance of talking to the family members and encouraging them to go get that first genetic evaluation in the family.
Our second group is for people who don’t have cancer themselves, but have a family history of cancer and those affected relatives have passed away. The family needs a genetic evaluation, and the veteran is the best living testing candidate.
That group is geared towards education about the test and informed consent.
The third group is for people with cancer who qualify for genetic testing. We provide all of the information that they need to make an informed decision on having (or not having) genetic testing.
The final group is for people who have family histories of known genetic mutations in cancer genes. Again, we provide them with all of the information that they need to make an informed decision regarding genetic testing.
With the shared medical appointments, we have been able to greatly increase the number of patients that we can see. Our first 3 groups all meet once a week and can have 10 or 12 veterans. Our last group meets every other week and has a maximum of 6 veterans. Wait times for our groups are generally ≤ 2 weeks. All veterans can choose to have an individual appointment if they prefer. We regularly get unsolicited feedback from veterans that they learn a lot during our groups and appreciate it.
Our group appointments have lowered the wait time for the people in the groups. And, they’ve lowered the wait time for the people who are seen individually. They’ve allowed us to address the backlog of patients waiting to see us in a more timely manner. Our wait time for individual appointment had been approaching 6 months, and it is now about 1.5 months.
We also think that being in a group normalizes the experience. Most people don’t know anyone who has had genetic testing. Now, they are in a group with others going through the same experience. In one of my groups, a male veteran talked about his breast cancer being really rare. Another male in the group volunteer that he had breast cancer, too. They both seemed to appreciate not feeling alone.
Vickie Venne. I want to move to our final piece. What do the referring providers tell the patients about a genetics referral and what should they expect?
Lisa Arfons. First and foremost, I tell the patient that it is a discussion with a genetic counselor. I make it clear that they understand that it is a discussion. They then can agree or not agree to accept genetic testing if it’s recommended.
I talk in general terms about why I think it can be important for them to have the discussion, but that we don’t have great data for decisionmaking. We understand that there are more options for preventive measures but then it ultimately will be a discussion between the PCP, the patient, and their family members about how they proceed about the preventive measures. I want them to start thinking about how the genetic test results, regardless of if they are positive, negative, or a variant that is not yet understood, can impact their offspring.
Probably I am biased, as my mom had breast cancer and she underwent genetic testing. So, I have a bit of an offspring focus as well. I already mentioned that you must discuss about whether or not it’s worth screening or doing any preventive measures on contralateral breast, or screening for things like prostate cancer at age 75 years. And so I focus more on the family members.
I try to stay in my lane. I am extremely uncomfortable when I hear about someone in our facility sending off a blood test and then asking someone else to interpret the results and discuss it with the patient. Just because it’s a blood test and it’s easy to order doesn’t mean that it is easy to know what to do with it, and it needs to be respected as such.
Ishta Thakar. Our PCPs let the patients know that GMS will contact the patient to schedule a video appointment and that if they want to bring any family members along with them, they’re welcome to. We also explain that certain cancers are genetically based and that if they have a genetic mutation, it can be passed on to their offspring. I also explain that if they have certain mutations, then we would be more vigilant in screening them for other kinds of cancers. That’s the reason that we refer that they get counseled. After counseling if they’re ready for the testing, then the counselor orders the test and does the posttest discussion with the patient.
Vickie Venne. In the VA, people are invited to attend a genetic counseling session but can certainly decline. Does the the DoD have a different approach?
Maj De Castro. I would say that the great majority of active duty patients have limited knowledge of what to expect out of a genetics appointment. One of the main things we do is educate them on their rights and protections and the potential risks associated with performing genetic testing, in particular when it comes to their continued ability to serve. Genetic testing for clinical purposes is not mandatory in the DoD, patients can certainly decline testing. Because genetic testing has the potential to alter someone’s career, it is critical we have a very thorough and comprehensive pre- and posttest counseling sessions that includes everything from career implications to the Genetic Information Nondiscrimination Act (GINA) and genetic discrimination in the military, in addition to the standard of care medical information.
Scenarios in which a servicemember is negatively impacted by pursuing a genetic diagnosis are very rare. More than 90% of the time, genetic counseling and/or testing has no adverse career effect. When they do, it is out of concern for the safety and wellbeing of a servicemember. For instance, if we diagnosis a patient with a genetic form of some arrhythmogenic disorder, part of the treatment plan can be to limit that person’s level of exertion, because it could potentially lead to death. We don’t want to put someone in a situation that may trigger that.
Vickie Venne. We also have a certain number of veterans who ask us about their service disability pay and the impact of genetic testing on it. One example is veterans with prostate cancer who were exposed to Agent Orange, which has been associated with increased risk for developing prostate cancer. I have had men who have been referred for genetic evaluation ask, “Well, if I have an identifiable mutation, how will that impact my service disability?” So we discuss the carcinogenic process that may include an inherited component as well as the environmental risk factors. I think that’s a unique issue for a population we’re honored to be able to serve.
Renee Rider. When we are talking about how the population of veterans is unique, I think it is also important to acknowledge mental health. I’ve had several patients tell me that they have posttraumatic stress disorder or anxiety and the idea of getting an indeterminant test result, such as VUS, would really weigh on them.
In the community, a lot of providers order the biggest panel they can, but for these patients who are worried about getting those indeterminant test results, I’ve been able to work with them to limit the size of the panel. I order a small panel that only has genes that have implications for that veteran’s clinical management. For example, in a patient with ductal breast cancer, I remove the genes that cause lobular breast cancer. This takes a bit of knowledge and critical thinking that our VA genetic counselors have because they have experience with veterans and their needs.
As our time draws to a close, I have one final thought. This has been a heartwarming conversation today. It is really nice to hear that GMS services are appreciated. We in GMS want to partner with our referring providers. Help us help you! When you enter a referral, please let us know how we can help you. The more we understand why you are sending your veteran to GMS, the more we can help meet your needs. If there are any questions or problems, feel free to send us an email or pick up the phone and call us.
Vickie Venne, MS. What is the Genomic Medicine Service (GMS) at the US Department of Veterans Affairs (VA)?
Renee Rider, JD, MS, LCGC. GMS is a telehealth service. We are part of central office and field stationed at the George E. Wahlen VA Medical Center (VAMC) in Salt Lake City, Utah. We provide care to about 90 VAMCs and their associated clinics. Veterans are referred to us by entering an interfacility consult in the VA Computerized Patient Record System (CPRS). We review the consult to determine whether the patient needs to be seen, whether we can answer with an e-consult, or whether we need more information. For the patients who need an appointment, the telehealth department at the veteran’s VA facility will contact the patient to arrange a visit with us. At the time of the appointment, the facility has a staff member available to seat the patient and connect them to us using video equipment.
We provide genetic care for all specialties, including cancer, women’s health, cardiology and neurology. In today’s discussion, we are focusing on cancer care.
Vickie Venne. What do patients do at facilities that don’t get care through GMS?
Renee Rider. There are a handful of facilities that provide their own genetic care in-house. For example, VA Boston Healthcare System in Massachusetts and the Michael E. DeBakey VAMC in Houston, Texas each have their own programs. For veterans who are not at a VA facility that has an agreement with GMS and do not have a different genetics program, their providers need to make referrals to community care.
Vickie Venne. How do patients get referred and what happens at their facility when the patients return to the specialty and primary care providers (PCP)? Ishta, who do you refer to GMS and how do you define them initially?
Ishta Thakar, MD, FACP. Referrals can come at a couple of points during a veteran’s journey at the VA. The VA covers obstetrics care for women veterans. Whenever a PCP or a women’s health provider is doing the initial history and physical on a new patient, if the female veteran has an extensive family history of breast, ovarian, colon, or endometrial cancer, then we take more history and we send a consult to GMS. The second instance would be if she tells us that she has had a personal history of breast, ovarian, or endometrial cancer and she has never had genetic testing. The third instance would be whenever we have a female veteran who is diagnosed with breast, ovarian, endometrial, or colon cancer. We would definitely talk to her about genetic counseling and send a referral to GMS. We would ask for a GMS consult for a patient with advanced maternal age, with exposure to some kind of teratogens, with an abnormal ultrasound, a family history of chromosomal disorders, or if she’s seeing an obstetrician who wants her to be tested. And finally, if a patient has a constellation of multiple cancers in the family and we don’t know what’s going on, we would also refer the patient to GMS.
Vickie Venne. That would be why GMS fields over 150 referrals every week. It is a large list. We also see veterans with personal or family histories of neurologic or cardiologic concerns as well.
Renee, as somebody who fields many of these referrals from unaffected individuals, what is the family history process?
Renee Rider. We don’t expect the referring provider to be a genetic expert. When a provider is seeing a constellation of several different cancers and he or she doesn’t know if there’s anything going on genetically or even if it’s possible, absolutely they should put in a referral to GMS. We have a triage counselor who reviews every consult that comes into our service within 24 hours.
Many cancers are due to exposures that are not concerning for a genetic etiology. We can let you know that it is not concerning, and the PCP can counsel the patient that it is very unlikely to be genetic in nature. We still give feedback even if it’s not someone who is appropriate for genetic counseling and testing. It is important to reach out to GMS even if you don’t know whether a cancer is genetic in nature.
It also is important to take your time when gathering family histories. We get a lot of patients who say, “There’s a lot of cancer in my family. I have no idea who had cancer, but I know a lot of people had cancer.” That’s not the day to put in a referral to GMS. At that point, providers should tell the patient to get as much information as they can about the family history and then reassess. It’s important for us to have accurate information. We’ve had several times where we receive a referral because the veteran says that their sister had ovarian cancer. And then when our staff calls, they later find out it was cervical cancer. That’s not a good use of the veteran’s time, and it’s not a good use of VA resources.
The other important thing about family histories is keeping the questions open-ended. Often a PCP or specialist will ask about a certain type of cancer: “Does anyone in your family have breast cancer, ovarian cancer?” Or if the veteran
is getting a colonoscopy, they ask, “Does anybody have colon cancer?” Where really, we need to be a little bit more open-ended. We prefer questions like, “Has anyone in your family
had cancer?” because that’s the question that prompts a response of, “Yes, 3 people in my family have had thyroid cancer.” That’s very important for us to know, too.
If you do get a positive response, probe a little bit more: what kind of cancer did someone have, how old were they when they had their cancer? And how are they related? Is this an aunt on your mom’s side or on your dad’s side? Those are the types of information that we need to figure out if that person needs a referral.
Vickie Venne. It’s a different story when people already have a cancer diagnosis. Which hematology or oncology patients are good referrals and why?
Lisa Arfons, MD. When patients come in with newly diagnosed cancer, breast for example, it is an emotional diagnosis and psychologicallydistressing. Oftentimes, they want to know why this happened to them. The issues surrounding
genetic testing also becomes very emotional. They want to know whether their children are at risk as well.
Genetic discussions take a long time. I rarely do that on the first visit. I always record for myself in my clinic note if something strikes me regarding the patient’s diagnosis. I quickly run through the National Comprehensive Cancer Network (NCCN) guidelines to remind myself of what I need to go over with the patient at our next meeting. Most patients don’t need to be referred to GMS, and most patients don’t need to be tested once they’re seen.
I often save the referral discussion for after I have established a rapport with a patient, we have a treatment plan, or they already have had their first surgery. Therefore, we are not making decisions about their first surgery based on the genetic medicine results.
If I’m considering a referral, I do a deeper dive with the patient. Is the patient older or younger than 45 years? I pull up NCCN guidelines and we go through the entire checklist.
We have male breast cancer patients at the VA—probably more than the community—so we refer those patients. At the Louis Stokes Cleveland VAMC in Ohio, we have had some in-depth discussions about referring male breast cancer patients for genetic testing and whether it was beneficial to older patients with male breast cancer. Ultimately, we decided that it was important for our male veterans to be tested because it empowered them to have better understanding of their medical conditions that may not just have effect on them but on their offspring, and that that can be a source of psychological and emotional support.
I don’t refer most people to GMS once I go through the checklist. I appreciate the action for an e-consult within the CPRS telemedicine consult itself, as Renee noted. If it is not necessary, GMS makes it an e-consult. I try to communicate that I don’t know whether it is necessary or not so that GMS understands where I’m coming from.
Vickie Venne. In the US Department of Defense (DoD) the process is quite different. Mauricio, can you explain the clinical referral process, who is referred, and how that works from a laboratory perspective?
Maj De Castro, MD, FACMG, USAF. The VA has led the way in demonstrating how to best provide for the medical genetic needs of a large, decentralized population distributed all over the country. Over the last 5 to 10 years, the DoD has made strides in recognizing the role genetics plays in the practice of everyday medicine and redoubling efforts to meet the needs of servicemembers.
The way that it traditionally has worked in the DoD is that military treatment facilities (MTFs) that have dedicated geneticists and genetic counselors: Kessler Medical Center in Mississippi, Walter Reed National Military Medical
Center in Maryland, Tripler Army Medical Center in Hawaii, Madigan Army Medical Center in Washington, Brooke Army Medical Center in Texas, Naval Medical Center San Diego in California, and Naval Medical Center Portsmouth in Virginia. A patient seeking genetic evaluation, counseling, or testing in those larger facilities would be referred to the genetics service by their primary care manager. Wait times vary, but it would usually be weeks, maybe months. However, the great majority of MTFs do not have dedicated genetics support. Most of the time, those patients would have to be referred to the local civilian community—there was no process for them to be seen in in the military healthcare system—with wait times that exceed 6 to 8 months in some cases. This is due to just not a military but a national shortage of genetics professionals (counselors and physicians).
Last year we started the telegenetics initiative, which is small compared to the VA—it is comprised of 2 geneticists and 1 genetic counselor—but with the full intent of growing it over time. Its purpose is to extend the resources we
had to other MTFs. Genetics professionals stationed state-side can provide care to remote facilities with limited access to local genetics support such as Cannon Air Force Base (AFB) or overseas facilities such as Spangdahlem AFB in Germany.
We recognize there are military-specific needs for the DoD regarding the genetic counseling process that have to take into account readiness, genetic discrimination, continued ability to serve and fitness for duty. For this important reason, we are seeking to expand our telegenetics initiative. The goal is to be able to provide 100% of all genetic counseling in-house, so to speak.
Currently, providers at the 4 pilot sites (Cannon AFB, Fort Bragg, Spangdahlem AFB, and Guantanamo Bay) send us referrals. We triage them and assign the patient to see a geneticist or a counselor depending on the indication.
On the laboratory side, it has been a very interesting experience. Because we provide comprehensive germline cancer testing at very little cost to the provider at any MTF, we have had high numbers of test requests over the years.
In addition to saving the DoD millions of dollars in testing, we have learned some interesting lessons in the process. For instance, we have worked closely with several different groups to better understand how to educate providers on the genetic counseling and testing process. This has allowed us to craft a thorough and inclusive consent form that addresses the needs of the DoD. We have also learned valuable lessons about population-based screening vs evidence-based testing, and lessons surrounding narrow-based testing (BRCA1 and BRCA2 only testing) vs ordering a more comprehensive panel that includes other genes supported by strong evidence (such as PALB2, CHEK2, or TP53).
For example, we have found that in a significant proportion of individuals with and without family history, there are clinically relevant variants in genes other than BRCA1 or BRCA2. And so, we have made part of our consent process,
a statement on secondary findings. If the patient consents, we will report pathogenic variants in other genes known to be associated with cancer (with strong evidence) even if the provider ordered a narrow panel such as BRCA1 and BRCA2 testing only. In about 1% to 4% of patients that would otherwise not meet NCCN guidelines, we’ve reported variants that were clinically actionable and changed the medical management of that patient.
We feel strongly that this is a conversation that we need to have in our field, and we realize it’s a complex issue, maybe we need to expand who gets testing. Guideline based testing is missing some patients out there that could benefit from it.
Vickie Venne. There certainly are many sides to the conversation of population-based vs evidence-based genetic testing. Genetic testing policies are changing rapidly. There are teams exploring comprehensive gene sequencing for
newborns and how that potential 1-time test can provide information will be reinterpreted as a person goes from cradle to grave. However, unlike the current DoD process, in the VA there are patients who we don’t see.
Renee Rider. I want to talk about money. When we order a genetic test, that test is paid for by the pathology department at the patient’s VAMC. Most of the pathology departments we work with are clear that they only can provide
genetic testing that is considered medically necessary. Thus, we review each test to make sure it meets established guidelines for testing. We don’t do population genetic screening as there isn’t evidence or guidelines to support offering it. We are strict about who does and does not get genetic testing, partly because we have a responsibility to pathology departments and to the taxpayers.
GMS focuses on conditions that are inherited, that is to say, we deal with germline genetics. Therefore, we discontinue referrals for somatic requests, such as when an OncotypeDX test is requested. It is my understanding that pharmacogenetic referrals may be sent to the new PHASeR initiative, which is a joint collaboration between the VA and Sanford Health and is headed by Deepak Voora, MD.
We generally don’t see patients who still are having diagnostic procedures done. For example, if a veteran has a suspicious breast mass, we recommend that the provider workup the mass before referring to GMS. Regardless of a genetic test result, a suspicious mass needs to be worked up. And, knowing if the mass is cancerous could change how we would proceed with the genetic workup. For example, if the mass were not cancerous, we may recommend that an affected relative have the first genetic evaluation. Furthermore, knowing if the patient has cancer changes how we interpret negative test results.
Another group of patients we don’t see are those who already had genetic testing done by the referring provider. It’s a VA directive that if you order a test, you’re the person who is responsible for giving the results. We agree with
this directive. If you don’t feel comfortable giving back test results, don’t order the test. Often, when a provider sends a patient to us after the test was done, we discover that the patient didn’t have appropriate pretest counseling. A test result, such as a variant of uncertain significance (VUS), should never be a surprise to either the provider or the patient.
Ishta Thakar. For newly diagnosed cancers, the first call is to the patient to inform them that they have cancer. We usually bring up genetic counseling or testing, if applicable, when they are ready to accept the diagnosis and have a conversation about it. All our consults are via telehealth, so none of our patients physically come to GMS in Salt Lake City. All the consults are done virtually.
For newly diagnosed patients, we would send a consult in within a couple of weeks. For patients who had a family history, the referral would not be urgent: They can be seen within about 3 months. The turnaround times for GMS are so much better than what we have available in the community where it’s often at least 6 months, as previously noted.
Vickie Venne. Thank you. We continue to work on that. One of the interesting things that we’ve done, which is the brainchild of Renee, is shared medical appointments.
Renee Rider. We have now created 4 group appointments for people who have concerns surrounding cancer. One group is for people who don’t have cancer but have family members who have cancer who may be the best testing candidate. For example, that might be a 30-year old who tells you that her mother had breast cancer at age 45 years. Her mother is still living, but she’s never had genetic testing. We would put her in a group where we discuss the importance of talking to the family members and encouraging them to go get that first genetic evaluation in the family.
Our second group is for people who don’t have cancer themselves, but have a family history of cancer and those affected relatives have passed away. The family needs a genetic evaluation, and the veteran is the best living testing candidate.
That group is geared towards education about the test and informed consent.
The third group is for people with cancer who qualify for genetic testing. We provide all of the information that they need to make an informed decision on having (or not having) genetic testing.
The final group is for people who have family histories of known genetic mutations in cancer genes. Again, we provide them with all of the information that they need to make an informed decision regarding genetic testing.
With the shared medical appointments, we have been able to greatly increase the number of patients that we can see. Our first 3 groups all meet once a week and can have 10 or 12 veterans. Our last group meets every other week and has a maximum of 6 veterans. Wait times for our groups are generally ≤ 2 weeks. All veterans can choose to have an individual appointment if they prefer. We regularly get unsolicited feedback from veterans that they learn a lot during our groups and appreciate it.
Our group appointments have lowered the wait time for the people in the groups. And, they’ve lowered the wait time for the people who are seen individually. They’ve allowed us to address the backlog of patients waiting to see us in a more timely manner. Our wait time for individual appointment had been approaching 6 months, and it is now about 1.5 months.
We also think that being in a group normalizes the experience. Most people don’t know anyone who has had genetic testing. Now, they are in a group with others going through the same experience. In one of my groups, a male veteran talked about his breast cancer being really rare. Another male in the group volunteer that he had breast cancer, too. They both seemed to appreciate not feeling alone.
Vickie Venne. I want to move to our final piece. What do the referring providers tell the patients about a genetics referral and what should they expect?
Lisa Arfons. First and foremost, I tell the patient that it is a discussion with a genetic counselor. I make it clear that they understand that it is a discussion. They then can agree or not agree to accept genetic testing if it’s recommended.
I talk in general terms about why I think it can be important for them to have the discussion, but that we don’t have great data for decisionmaking. We understand that there are more options for preventive measures but then it ultimately will be a discussion between the PCP, the patient, and their family members about how they proceed about the preventive measures. I want them to start thinking about how the genetic test results, regardless of if they are positive, negative, or a variant that is not yet understood, can impact their offspring.
Probably I am biased, as my mom had breast cancer and she underwent genetic testing. So, I have a bit of an offspring focus as well. I already mentioned that you must discuss about whether or not it’s worth screening or doing any preventive measures on contralateral breast, or screening for things like prostate cancer at age 75 years. And so I focus more on the family members.
I try to stay in my lane. I am extremely uncomfortable when I hear about someone in our facility sending off a blood test and then asking someone else to interpret the results and discuss it with the patient. Just because it’s a blood test and it’s easy to order doesn’t mean that it is easy to know what to do with it, and it needs to be respected as such.
Ishta Thakar. Our PCPs let the patients know that GMS will contact the patient to schedule a video appointment and that if they want to bring any family members along with them, they’re welcome to. We also explain that certain cancers are genetically based and that if they have a genetic mutation, it can be passed on to their offspring. I also explain that if they have certain mutations, then we would be more vigilant in screening them for other kinds of cancers. That’s the reason that we refer that they get counseled. After counseling if they’re ready for the testing, then the counselor orders the test and does the posttest discussion with the patient.
Vickie Venne. In the VA, people are invited to attend a genetic counseling session but can certainly decline. Does the the DoD have a different approach?
Maj De Castro. I would say that the great majority of active duty patients have limited knowledge of what to expect out of a genetics appointment. One of the main things we do is educate them on their rights and protections and the potential risks associated with performing genetic testing, in particular when it comes to their continued ability to serve. Genetic testing for clinical purposes is not mandatory in the DoD, patients can certainly decline testing. Because genetic testing has the potential to alter someone’s career, it is critical we have a very thorough and comprehensive pre- and posttest counseling sessions that includes everything from career implications to the Genetic Information Nondiscrimination Act (GINA) and genetic discrimination in the military, in addition to the standard of care medical information.
Scenarios in which a servicemember is negatively impacted by pursuing a genetic diagnosis are very rare. More than 90% of the time, genetic counseling and/or testing has no adverse career effect. When they do, it is out of concern for the safety and wellbeing of a servicemember. For instance, if we diagnosis a patient with a genetic form of some arrhythmogenic disorder, part of the treatment plan can be to limit that person’s level of exertion, because it could potentially lead to death. We don’t want to put someone in a situation that may trigger that.
Vickie Venne. We also have a certain number of veterans who ask us about their service disability pay and the impact of genetic testing on it. One example is veterans with prostate cancer who were exposed to Agent Orange, which has been associated with increased risk for developing prostate cancer. I have had men who have been referred for genetic evaluation ask, “Well, if I have an identifiable mutation, how will that impact my service disability?” So we discuss the carcinogenic process that may include an inherited component as well as the environmental risk factors. I think that’s a unique issue for a population we’re honored to be able to serve.
Renee Rider. When we are talking about how the population of veterans is unique, I think it is also important to acknowledge mental health. I’ve had several patients tell me that they have posttraumatic stress disorder or anxiety and the idea of getting an indeterminant test result, such as VUS, would really weigh on them.
In the community, a lot of providers order the biggest panel they can, but for these patients who are worried about getting those indeterminant test results, I’ve been able to work with them to limit the size of the panel. I order a small panel that only has genes that have implications for that veteran’s clinical management. For example, in a patient with ductal breast cancer, I remove the genes that cause lobular breast cancer. This takes a bit of knowledge and critical thinking that our VA genetic counselors have because they have experience with veterans and their needs.
As our time draws to a close, I have one final thought. This has been a heartwarming conversation today. It is really nice to hear that GMS services are appreciated. We in GMS want to partner with our referring providers. Help us help you! When you enter a referral, please let us know how we can help you. The more we understand why you are sending your veteran to GMS, the more we can help meet your needs. If there are any questions or problems, feel free to send us an email or pick up the phone and call us.
Vickie Venne, MS. What is the Genomic Medicine Service (GMS) at the US Department of Veterans Affairs (VA)?
Renee Rider, JD, MS, LCGC. GMS is a telehealth service. We are part of central office and field stationed at the George E. Wahlen VA Medical Center (VAMC) in Salt Lake City, Utah. We provide care to about 90 VAMCs and their associated clinics. Veterans are referred to us by entering an interfacility consult in the VA Computerized Patient Record System (CPRS). We review the consult to determine whether the patient needs to be seen, whether we can answer with an e-consult, or whether we need more information. For the patients who need an appointment, the telehealth department at the veteran’s VA facility will contact the patient to arrange a visit with us. At the time of the appointment, the facility has a staff member available to seat the patient and connect them to us using video equipment.
We provide genetic care for all specialties, including cancer, women’s health, cardiology and neurology. In today’s discussion, we are focusing on cancer care.
Vickie Venne. What do patients do at facilities that don’t get care through GMS?
Renee Rider. There are a handful of facilities that provide their own genetic care in-house. For example, VA Boston Healthcare System in Massachusetts and the Michael E. DeBakey VAMC in Houston, Texas each have their own programs. For veterans who are not at a VA facility that has an agreement with GMS and do not have a different genetics program, their providers need to make referrals to community care.
Vickie Venne. How do patients get referred and what happens at their facility when the patients return to the specialty and primary care providers (PCP)? Ishta, who do you refer to GMS and how do you define them initially?
Ishta Thakar, MD, FACP. Referrals can come at a couple of points during a veteran’s journey at the VA. The VA covers obstetrics care for women veterans. Whenever a PCP or a women’s health provider is doing the initial history and physical on a new patient, if the female veteran has an extensive family history of breast, ovarian, colon, or endometrial cancer, then we take more history and we send a consult to GMS. The second instance would be if she tells us that she has had a personal history of breast, ovarian, or endometrial cancer and she has never had genetic testing. The third instance would be whenever we have a female veteran who is diagnosed with breast, ovarian, endometrial, or colon cancer. We would definitely talk to her about genetic counseling and send a referral to GMS. We would ask for a GMS consult for a patient with advanced maternal age, with exposure to some kind of teratogens, with an abnormal ultrasound, a family history of chromosomal disorders, or if she’s seeing an obstetrician who wants her to be tested. And finally, if a patient has a constellation of multiple cancers in the family and we don’t know what’s going on, we would also refer the patient to GMS.
Vickie Venne. That would be why GMS fields over 150 referrals every week. It is a large list. We also see veterans with personal or family histories of neurologic or cardiologic concerns as well.
Renee, as somebody who fields many of these referrals from unaffected individuals, what is the family history process?
Renee Rider. We don’t expect the referring provider to be a genetic expert. When a provider is seeing a constellation of several different cancers and he or she doesn’t know if there’s anything going on genetically or even if it’s possible, absolutely they should put in a referral to GMS. We have a triage counselor who reviews every consult that comes into our service within 24 hours.
Many cancers are due to exposures that are not concerning for a genetic etiology. We can let you know that it is not concerning, and the PCP can counsel the patient that it is very unlikely to be genetic in nature. We still give feedback even if it’s not someone who is appropriate for genetic counseling and testing. It is important to reach out to GMS even if you don’t know whether a cancer is genetic in nature.
It also is important to take your time when gathering family histories. We get a lot of patients who say, “There’s a lot of cancer in my family. I have no idea who had cancer, but I know a lot of people had cancer.” That’s not the day to put in a referral to GMS. At that point, providers should tell the patient to get as much information as they can about the family history and then reassess. It’s important for us to have accurate information. We’ve had several times where we receive a referral because the veteran says that their sister had ovarian cancer. And then when our staff calls, they later find out it was cervical cancer. That’s not a good use of the veteran’s time, and it’s not a good use of VA resources.
The other important thing about family histories is keeping the questions open-ended. Often a PCP or specialist will ask about a certain type of cancer: “Does anyone in your family have breast cancer, ovarian cancer?” Or if the veteran
is getting a colonoscopy, they ask, “Does anybody have colon cancer?” Where really, we need to be a little bit more open-ended. We prefer questions like, “Has anyone in your family
had cancer?” because that’s the question that prompts a response of, “Yes, 3 people in my family have had thyroid cancer.” That’s very important for us to know, too.
If you do get a positive response, probe a little bit more: what kind of cancer did someone have, how old were they when they had their cancer? And how are they related? Is this an aunt on your mom’s side or on your dad’s side? Those are the types of information that we need to figure out if that person needs a referral.
Vickie Venne. It’s a different story when people already have a cancer diagnosis. Which hematology or oncology patients are good referrals and why?
Lisa Arfons, MD. When patients come in with newly diagnosed cancer, breast for example, it is an emotional diagnosis and psychologicallydistressing. Oftentimes, they want to know why this happened to them. The issues surrounding
genetic testing also becomes very emotional. They want to know whether their children are at risk as well.
Genetic discussions take a long time. I rarely do that on the first visit. I always record for myself in my clinic note if something strikes me regarding the patient’s diagnosis. I quickly run through the National Comprehensive Cancer Network (NCCN) guidelines to remind myself of what I need to go over with the patient at our next meeting. Most patients don’t need to be referred to GMS, and most patients don’t need to be tested once they’re seen.
I often save the referral discussion for after I have established a rapport with a patient, we have a treatment plan, or they already have had their first surgery. Therefore, we are not making decisions about their first surgery based on the genetic medicine results.
If I’m considering a referral, I do a deeper dive with the patient. Is the patient older or younger than 45 years? I pull up NCCN guidelines and we go through the entire checklist.
We have male breast cancer patients at the VA—probably more than the community—so we refer those patients. At the Louis Stokes Cleveland VAMC in Ohio, we have had some in-depth discussions about referring male breast cancer patients for genetic testing and whether it was beneficial to older patients with male breast cancer. Ultimately, we decided that it was important for our male veterans to be tested because it empowered them to have better understanding of their medical conditions that may not just have effect on them but on their offspring, and that that can be a source of psychological and emotional support.
I don’t refer most people to GMS once I go through the checklist. I appreciate the action for an e-consult within the CPRS telemedicine consult itself, as Renee noted. If it is not necessary, GMS makes it an e-consult. I try to communicate that I don’t know whether it is necessary or not so that GMS understands where I’m coming from.
Vickie Venne. In the US Department of Defense (DoD) the process is quite different. Mauricio, can you explain the clinical referral process, who is referred, and how that works from a laboratory perspective?
Maj De Castro, MD, FACMG, USAF. The VA has led the way in demonstrating how to best provide for the medical genetic needs of a large, decentralized population distributed all over the country. Over the last 5 to 10 years, the DoD has made strides in recognizing the role genetics plays in the practice of everyday medicine and redoubling efforts to meet the needs of servicemembers.
The way that it traditionally has worked in the DoD is that military treatment facilities (MTFs) that have dedicated geneticists and genetic counselors: Kessler Medical Center in Mississippi, Walter Reed National Military Medical
Center in Maryland, Tripler Army Medical Center in Hawaii, Madigan Army Medical Center in Washington, Brooke Army Medical Center in Texas, Naval Medical Center San Diego in California, and Naval Medical Center Portsmouth in Virginia. A patient seeking genetic evaluation, counseling, or testing in those larger facilities would be referred to the genetics service by their primary care manager. Wait times vary, but it would usually be weeks, maybe months. However, the great majority of MTFs do not have dedicated genetics support. Most of the time, those patients would have to be referred to the local civilian community—there was no process for them to be seen in in the military healthcare system—with wait times that exceed 6 to 8 months in some cases. This is due to just not a military but a national shortage of genetics professionals (counselors and physicians).
Last year we started the telegenetics initiative, which is small compared to the VA—it is comprised of 2 geneticists and 1 genetic counselor—but with the full intent of growing it over time. Its purpose is to extend the resources we
had to other MTFs. Genetics professionals stationed state-side can provide care to remote facilities with limited access to local genetics support such as Cannon Air Force Base (AFB) or overseas facilities such as Spangdahlem AFB in Germany.
We recognize there are military-specific needs for the DoD regarding the genetic counseling process that have to take into account readiness, genetic discrimination, continued ability to serve and fitness for duty. For this important reason, we are seeking to expand our telegenetics initiative. The goal is to be able to provide 100% of all genetic counseling in-house, so to speak.
Currently, providers at the 4 pilot sites (Cannon AFB, Fort Bragg, Spangdahlem AFB, and Guantanamo Bay) send us referrals. We triage them and assign the patient to see a geneticist or a counselor depending on the indication.
On the laboratory side, it has been a very interesting experience. Because we provide comprehensive germline cancer testing at very little cost to the provider at any MTF, we have had high numbers of test requests over the years.
In addition to saving the DoD millions of dollars in testing, we have learned some interesting lessons in the process. For instance, we have worked closely with several different groups to better understand how to educate providers on the genetic counseling and testing process. This has allowed us to craft a thorough and inclusive consent form that addresses the needs of the DoD. We have also learned valuable lessons about population-based screening vs evidence-based testing, and lessons surrounding narrow-based testing (BRCA1 and BRCA2 only testing) vs ordering a more comprehensive panel that includes other genes supported by strong evidence (such as PALB2, CHEK2, or TP53).
For example, we have found that in a significant proportion of individuals with and without family history, there are clinically relevant variants in genes other than BRCA1 or BRCA2. And so, we have made part of our consent process,
a statement on secondary findings. If the patient consents, we will report pathogenic variants in other genes known to be associated with cancer (with strong evidence) even if the provider ordered a narrow panel such as BRCA1 and BRCA2 testing only. In about 1% to 4% of patients that would otherwise not meet NCCN guidelines, we’ve reported variants that were clinically actionable and changed the medical management of that patient.
We feel strongly that this is a conversation that we need to have in our field, and we realize it’s a complex issue, maybe we need to expand who gets testing. Guideline based testing is missing some patients out there that could benefit from it.
Vickie Venne. There certainly are many sides to the conversation of population-based vs evidence-based genetic testing. Genetic testing policies are changing rapidly. There are teams exploring comprehensive gene sequencing for
newborns and how that potential 1-time test can provide information will be reinterpreted as a person goes from cradle to grave. However, unlike the current DoD process, in the VA there are patients who we don’t see.
Renee Rider. I want to talk about money. When we order a genetic test, that test is paid for by the pathology department at the patient’s VAMC. Most of the pathology departments we work with are clear that they only can provide
genetic testing that is considered medically necessary. Thus, we review each test to make sure it meets established guidelines for testing. We don’t do population genetic screening as there isn’t evidence or guidelines to support offering it. We are strict about who does and does not get genetic testing, partly because we have a responsibility to pathology departments and to the taxpayers.
GMS focuses on conditions that are inherited, that is to say, we deal with germline genetics. Therefore, we discontinue referrals for somatic requests, such as when an OncotypeDX test is requested. It is my understanding that pharmacogenetic referrals may be sent to the new PHASeR initiative, which is a joint collaboration between the VA and Sanford Health and is headed by Deepak Voora, MD.
We generally don’t see patients who still are having diagnostic procedures done. For example, if a veteran has a suspicious breast mass, we recommend that the provider workup the mass before referring to GMS. Regardless of a genetic test result, a suspicious mass needs to be worked up. And, knowing if the mass is cancerous could change how we would proceed with the genetic workup. For example, if the mass were not cancerous, we may recommend that an affected relative have the first genetic evaluation. Furthermore, knowing if the patient has cancer changes how we interpret negative test results.
Another group of patients we don’t see are those who already had genetic testing done by the referring provider. It’s a VA directive that if you order a test, you’re the person who is responsible for giving the results. We agree with
this directive. If you don’t feel comfortable giving back test results, don’t order the test. Often, when a provider sends a patient to us after the test was done, we discover that the patient didn’t have appropriate pretest counseling. A test result, such as a variant of uncertain significance (VUS), should never be a surprise to either the provider or the patient.
Ishta Thakar. For newly diagnosed cancers, the first call is to the patient to inform them that they have cancer. We usually bring up genetic counseling or testing, if applicable, when they are ready to accept the diagnosis and have a conversation about it. All our consults are via telehealth, so none of our patients physically come to GMS in Salt Lake City. All the consults are done virtually.
For newly diagnosed patients, we would send a consult in within a couple of weeks. For patients who had a family history, the referral would not be urgent: They can be seen within about 3 months. The turnaround times for GMS are so much better than what we have available in the community where it’s often at least 6 months, as previously noted.
Vickie Venne. Thank you. We continue to work on that. One of the interesting things that we’ve done, which is the brainchild of Renee, is shared medical appointments.
Renee Rider. We have now created 4 group appointments for people who have concerns surrounding cancer. One group is for people who don’t have cancer but have family members who have cancer who may be the best testing candidate. For example, that might be a 30-year old who tells you that her mother had breast cancer at age 45 years. Her mother is still living, but she’s never had genetic testing. We would put her in a group where we discuss the importance of talking to the family members and encouraging them to go get that first genetic evaluation in the family.
Our second group is for people who don’t have cancer themselves, but have a family history of cancer and those affected relatives have passed away. The family needs a genetic evaluation, and the veteran is the best living testing candidate.
That group is geared towards education about the test and informed consent.
The third group is for people with cancer who qualify for genetic testing. We provide all of the information that they need to make an informed decision on having (or not having) genetic testing.
The final group is for people who have family histories of known genetic mutations in cancer genes. Again, we provide them with all of the information that they need to make an informed decision regarding genetic testing.
With the shared medical appointments, we have been able to greatly increase the number of patients that we can see. Our first 3 groups all meet once a week and can have 10 or 12 veterans. Our last group meets every other week and has a maximum of 6 veterans. Wait times for our groups are generally ≤ 2 weeks. All veterans can choose to have an individual appointment if they prefer. We regularly get unsolicited feedback from veterans that they learn a lot during our groups and appreciate it.
Our group appointments have lowered the wait time for the people in the groups. And, they’ve lowered the wait time for the people who are seen individually. They’ve allowed us to address the backlog of patients waiting to see us in a more timely manner. Our wait time for individual appointment had been approaching 6 months, and it is now about 1.5 months.
We also think that being in a group normalizes the experience. Most people don’t know anyone who has had genetic testing. Now, they are in a group with others going through the same experience. In one of my groups, a male veteran talked about his breast cancer being really rare. Another male in the group volunteer that he had breast cancer, too. They both seemed to appreciate not feeling alone.
Vickie Venne. I want to move to our final piece. What do the referring providers tell the patients about a genetics referral and what should they expect?
Lisa Arfons. First and foremost, I tell the patient that it is a discussion with a genetic counselor. I make it clear that they understand that it is a discussion. They then can agree or not agree to accept genetic testing if it’s recommended.
I talk in general terms about why I think it can be important for them to have the discussion, but that we don’t have great data for decisionmaking. We understand that there are more options for preventive measures but then it ultimately will be a discussion between the PCP, the patient, and their family members about how they proceed about the preventive measures. I want them to start thinking about how the genetic test results, regardless of if they are positive, negative, or a variant that is not yet understood, can impact their offspring.
Probably I am biased, as my mom had breast cancer and she underwent genetic testing. So, I have a bit of an offspring focus as well. I already mentioned that you must discuss about whether or not it’s worth screening or doing any preventive measures on contralateral breast, or screening for things like prostate cancer at age 75 years. And so I focus more on the family members.
I try to stay in my lane. I am extremely uncomfortable when I hear about someone in our facility sending off a blood test and then asking someone else to interpret the results and discuss it with the patient. Just because it’s a blood test and it’s easy to order doesn’t mean that it is easy to know what to do with it, and it needs to be respected as such.
Ishta Thakar. Our PCPs let the patients know that GMS will contact the patient to schedule a video appointment and that if they want to bring any family members along with them, they’re welcome to. We also explain that certain cancers are genetically based and that if they have a genetic mutation, it can be passed on to their offspring. I also explain that if they have certain mutations, then we would be more vigilant in screening them for other kinds of cancers. That’s the reason that we refer that they get counseled. After counseling if they’re ready for the testing, then the counselor orders the test and does the posttest discussion with the patient.
Vickie Venne. In the VA, people are invited to attend a genetic counseling session but can certainly decline. Does the the DoD have a different approach?
Maj De Castro. I would say that the great majority of active duty patients have limited knowledge of what to expect out of a genetics appointment. One of the main things we do is educate them on their rights and protections and the potential risks associated with performing genetic testing, in particular when it comes to their continued ability to serve. Genetic testing for clinical purposes is not mandatory in the DoD, patients can certainly decline testing. Because genetic testing has the potential to alter someone’s career, it is critical we have a very thorough and comprehensive pre- and posttest counseling sessions that includes everything from career implications to the Genetic Information Nondiscrimination Act (GINA) and genetic discrimination in the military, in addition to the standard of care medical information.
Scenarios in which a servicemember is negatively impacted by pursuing a genetic diagnosis are very rare. More than 90% of the time, genetic counseling and/or testing has no adverse career effect. When they do, it is out of concern for the safety and wellbeing of a servicemember. For instance, if we diagnosis a patient with a genetic form of some arrhythmogenic disorder, part of the treatment plan can be to limit that person’s level of exertion, because it could potentially lead to death. We don’t want to put someone in a situation that may trigger that.
Vickie Venne. We also have a certain number of veterans who ask us about their service disability pay and the impact of genetic testing on it. One example is veterans with prostate cancer who were exposed to Agent Orange, which has been associated with increased risk for developing prostate cancer. I have had men who have been referred for genetic evaluation ask, “Well, if I have an identifiable mutation, how will that impact my service disability?” So we discuss the carcinogenic process that may include an inherited component as well as the environmental risk factors. I think that’s a unique issue for a population we’re honored to be able to serve.
Renee Rider. When we are talking about how the population of veterans is unique, I think it is also important to acknowledge mental health. I’ve had several patients tell me that they have posttraumatic stress disorder or anxiety and the idea of getting an indeterminant test result, such as VUS, would really weigh on them.
In the community, a lot of providers order the biggest panel they can, but for these patients who are worried about getting those indeterminant test results, I’ve been able to work with them to limit the size of the panel. I order a small panel that only has genes that have implications for that veteran’s clinical management. For example, in a patient with ductal breast cancer, I remove the genes that cause lobular breast cancer. This takes a bit of knowledge and critical thinking that our VA genetic counselors have because they have experience with veterans and their needs.
As our time draws to a close, I have one final thought. This has been a heartwarming conversation today. It is really nice to hear that GMS services are appreciated. We in GMS want to partner with our referring providers. Help us help you! When you enter a referral, please let us know how we can help you. The more we understand why you are sending your veteran to GMS, the more we can help meet your needs. If there are any questions or problems, feel free to send us an email or pick up the phone and call us.