User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
FDA Approves Polyarticular JIA Indication for Sarilumab
The US Food and Drug Administration (FDA) has approved sarilumab (Kevzara) for the treatment of polyarticular juvenile idiopathic arthritis (pJIA) for patients weighing ≥ 63 kg (139 lb).
“Polyarticular juvenile idiopathic arthritis (JIA) can be a painful disease for children where multiple joints are impacted by this chronic inflammation,” said George D. Yancopoulos, MD, PhD, president and chief scientific officer at Regeneron in a press release.
It is estimated that nearly 300,000 children in the United States have JIA, and 1 in 4 of them have pJIA, according to the Arthritis Foundation.
“Not only are their daily lives impacted, but their futures can be disrupted without adequate treatment,” Dr. Yancopoulos continued. “The approval of Kevzara in polyarticular juvenile idiopathic arthritis provides these vulnerable patients and their families a new FDA-approved treatment option to help navigate this disease.”
Sarilumab, jointly developed by Sanofi and Regeneron, is an interleukin 6 receptor blocker. It was first approved in 2017 for the treatment of moderate to severely active rheumatoid arthritis (RA) in adults who had inadequate response or intolerance to at least one other disease-modifying antirheumatic drug (DMARD).
In 2023, the FDA approved sarilumab as the first biologic treatment for polymyalgia rheumatica in adults who had inadequate response to corticosteroids and could not tolerate a corticosteroid taper.
For pJIA, sarilumab is administered subcutaneously using a 200-mg/1.14-mL prefilled syringe once every 2 weeks. The medication can be used alone or in combination with other conventional DMARDs.
“Use of KEVZARA in pediatric patients with pJIA is supported by evidence from adequate and well-controlled studies of KEVZARA in adults with RA, pharmacokinetic data from adult patients with RA,” and pharmacokinetic comparability in 101 pediatric patients aged 2-17 years treated with sarilumab, according to the prescribing information. Sarilumab is not approved for pediatric patients < 63 kg “because of a lack of an appropriate dosage form.”
The most common reported adverse reactions for sarilumab in pJIA are nasopharyngitis, neutropenia, upper respiratory tract infection, and injection site erythema. The pJIA trial recorded no new adverse reactions or safety concerns, compared with patients with RA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved sarilumab (Kevzara) for the treatment of polyarticular juvenile idiopathic arthritis (pJIA) for patients weighing ≥ 63 kg (139 lb).
“Polyarticular juvenile idiopathic arthritis (JIA) can be a painful disease for children where multiple joints are impacted by this chronic inflammation,” said George D. Yancopoulos, MD, PhD, president and chief scientific officer at Regeneron in a press release.
It is estimated that nearly 300,000 children in the United States have JIA, and 1 in 4 of them have pJIA, according to the Arthritis Foundation.
“Not only are their daily lives impacted, but their futures can be disrupted without adequate treatment,” Dr. Yancopoulos continued. “The approval of Kevzara in polyarticular juvenile idiopathic arthritis provides these vulnerable patients and their families a new FDA-approved treatment option to help navigate this disease.”
Sarilumab, jointly developed by Sanofi and Regeneron, is an interleukin 6 receptor blocker. It was first approved in 2017 for the treatment of moderate to severely active rheumatoid arthritis (RA) in adults who had inadequate response or intolerance to at least one other disease-modifying antirheumatic drug (DMARD).
In 2023, the FDA approved sarilumab as the first biologic treatment for polymyalgia rheumatica in adults who had inadequate response to corticosteroids and could not tolerate a corticosteroid taper.
For pJIA, sarilumab is administered subcutaneously using a 200-mg/1.14-mL prefilled syringe once every 2 weeks. The medication can be used alone or in combination with other conventional DMARDs.
“Use of KEVZARA in pediatric patients with pJIA is supported by evidence from adequate and well-controlled studies of KEVZARA in adults with RA, pharmacokinetic data from adult patients with RA,” and pharmacokinetic comparability in 101 pediatric patients aged 2-17 years treated with sarilumab, according to the prescribing information. Sarilumab is not approved for pediatric patients < 63 kg “because of a lack of an appropriate dosage form.”
The most common reported adverse reactions for sarilumab in pJIA are nasopharyngitis, neutropenia, upper respiratory tract infection, and injection site erythema. The pJIA trial recorded no new adverse reactions or safety concerns, compared with patients with RA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved sarilumab (Kevzara) for the treatment of polyarticular juvenile idiopathic arthritis (pJIA) for patients weighing ≥ 63 kg (139 lb).
“Polyarticular juvenile idiopathic arthritis (JIA) can be a painful disease for children where multiple joints are impacted by this chronic inflammation,” said George D. Yancopoulos, MD, PhD, president and chief scientific officer at Regeneron in a press release.
It is estimated that nearly 300,000 children in the United States have JIA, and 1 in 4 of them have pJIA, according to the Arthritis Foundation.
“Not only are their daily lives impacted, but their futures can be disrupted without adequate treatment,” Dr. Yancopoulos continued. “The approval of Kevzara in polyarticular juvenile idiopathic arthritis provides these vulnerable patients and their families a new FDA-approved treatment option to help navigate this disease.”
Sarilumab, jointly developed by Sanofi and Regeneron, is an interleukin 6 receptor blocker. It was first approved in 2017 for the treatment of moderate to severely active rheumatoid arthritis (RA) in adults who had inadequate response or intolerance to at least one other disease-modifying antirheumatic drug (DMARD).
In 2023, the FDA approved sarilumab as the first biologic treatment for polymyalgia rheumatica in adults who had inadequate response to corticosteroids and could not tolerate a corticosteroid taper.
For pJIA, sarilumab is administered subcutaneously using a 200-mg/1.14-mL prefilled syringe once every 2 weeks. The medication can be used alone or in combination with other conventional DMARDs.
“Use of KEVZARA in pediatric patients with pJIA is supported by evidence from adequate and well-controlled studies of KEVZARA in adults with RA, pharmacokinetic data from adult patients with RA,” and pharmacokinetic comparability in 101 pediatric patients aged 2-17 years treated with sarilumab, according to the prescribing information. Sarilumab is not approved for pediatric patients < 63 kg “because of a lack of an appropriate dosage form.”
The most common reported adverse reactions for sarilumab in pJIA are nasopharyngitis, neutropenia, upper respiratory tract infection, and injection site erythema. The pJIA trial recorded no new adverse reactions or safety concerns, compared with patients with RA.
A version of this article appeared on Medscape.com.
USPSTF Draft Recommendations Support More Options for Osteoporosis Screening, Seek More Research in Men
An influential US panel may largely reaffirm its current recommendation in favor of screening older women to prevent osteoporotic fractures, while also repeating its call for more research to try to determine whether men would benefit from this kind of routine testing.
The US Preventive Services Task Force (USPSTF) on June 11 released a draft update of its recommendations on osteoporosis screening. The task force will accept comments on the draft through July 8. Federal law gives the USPSTF recommendations extra clout, requiring insurers to cover — without co-pay — services that get top marks “A” or “B” from the task force.
The task force intends to maintain a “B” recommendation on screening of older women, indicating that the evidence gathered to date suggests a moderate net benefit. But the draft includes a shift in the approach to this screening.
The USPSTF proposed saying that it recommends screening for osteoporosis in both women aged 65 years and older and postmenopausal women younger than 65 years who are at an increased risk for an osteoporotic fracture. The current recommendation, finalized in 2018, advises “screening for osteoporosis with bone measurement testing [emphasis added]” for these groups.
The proposed change in language — dropping the phrase “with bone measurement testing” — is intended to expand flexibility for clinicians, Esa Davis, MD, MPH, a member of USPSTF and a professor at the University of Maryland School of Medicine, Baltimore, told this news organization.
“It provides them with more options instead of telling them, ‘You have to do it this way,’ ” Dr. Davis said.
The task force’s draft recommendation is not meant to apply to people with secondary osteoporosis due to an underlying medical condition such as cancer, metabolic bone diseases or hyperthyroidism, or chronic use of a medication associated with bone loss.
Rajesh K. Jain, MD, who was not involved with the USPSTF work, read the draft recommendations at the request of this news organization. In an email, he said he generally agreed with the decision to largely stick to the 2018 recommendations for women.
He also noted that there’s still a lack of a clear direction for physicians about assessing osteoporosis risk in men. But multiple randomized control trials of osteoporosis drugs seem to suggest these medicines work for both sexes, said Dr. Jain, who is the endocrinology fellowship program director at University of Chicago Medicine, Chicago.
The USPSTF draft also would reiterate the current “I” grade about screening men for osteoporosis.
An “I” grade means the task force found the current body of available evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis to prevent osteoporotic fractures in men.
“Since there is no recommendation right now, it would have seemed sensible to include a recommendation to screen men with prior fracture or other risk factors for osteoporosis, much like they do for younger women,” Dr. Jain said.
Insufficient Evidence
The USPSTF’s “I” grade is different from a “D” grade, which is what the task force uses to recommend against the use of a service.
A “D” grade means the USPSTF says there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. (The USPSTF makes it easy to search online for grades given to preventive services, including those that got a “D.”)
The USPSTF is calling for more studies on the benefits and harms of screening for osteoporosis to prevent fractures and related morbidity and mortality in men.
“Men do get osteoporosis,” Dr. Davis said. “But unfortunately, the evidence isn’t there” to allow USPSTF to make a recommendation on screening approaches.
“Any man who has concerns about bone health should certainly talk to his clinician and figure out what is the best form of screening” he might want to do, she said.
There’s been a growing interest in the question of whether to screen men for osteoporosis and bone health. For example, Osteoporosis Canada last year updated a guideline to emphasize the need to assess older patients of both sexes for the risk for fractures. But the Canadian Task Force on Preventive Health Care in 2023 came to a conclusion in line with the USPSTF draft.
The Canadian task force recommended against routine screening in men, while adding that clinicians should be alert to changes in health that may indicate the patient has experienced or is at a higher risk for fragility fracture.
Risk Factors, Concerns About Tests
The USPSTF said that risk factors associated with fragility fractures are similar in men and women. These include:
- Increasing age
- Low body mass index
- Excessive alcohol intake
- Current smoking
- Chronic corticosteroid use
- History of prior fractures, falls within the past year, cerebrovascular accident, and diabetes
- Hypogonadism
The process of updating the USPSTF recommendations can serve as a chance to expand public awareness about osteoporosis, as many men may not know to raise the question of their fracture risk during medical appointments, Dr. Davis said.
“Clinicians need to be aware of the risk factors and to be able to have conversations with men,” she said.
Dr. Davis also cautioned about the need to be aware of limitations with clinical risk assessment tools. In the draft recommendation statement, the USPSTF noted that some tools and approaches may be less likely to identify Black, Hispanic, and Asian people as high risk, and subsequently, clinicians may be less likely to offer treatment to them compared with White people of the same age, bone mineral density, and clinical risk profile.
Dr. Davis had no relevant financial relationships. Dr. Jain received research funding from the Amgen Foundation.
A version of this article appeared on Medscape.com.
An influential US panel may largely reaffirm its current recommendation in favor of screening older women to prevent osteoporotic fractures, while also repeating its call for more research to try to determine whether men would benefit from this kind of routine testing.
The US Preventive Services Task Force (USPSTF) on June 11 released a draft update of its recommendations on osteoporosis screening. The task force will accept comments on the draft through July 8. Federal law gives the USPSTF recommendations extra clout, requiring insurers to cover — without co-pay — services that get top marks “A” or “B” from the task force.
The task force intends to maintain a “B” recommendation on screening of older women, indicating that the evidence gathered to date suggests a moderate net benefit. But the draft includes a shift in the approach to this screening.
The USPSTF proposed saying that it recommends screening for osteoporosis in both women aged 65 years and older and postmenopausal women younger than 65 years who are at an increased risk for an osteoporotic fracture. The current recommendation, finalized in 2018, advises “screening for osteoporosis with bone measurement testing [emphasis added]” for these groups.
The proposed change in language — dropping the phrase “with bone measurement testing” — is intended to expand flexibility for clinicians, Esa Davis, MD, MPH, a member of USPSTF and a professor at the University of Maryland School of Medicine, Baltimore, told this news organization.
“It provides them with more options instead of telling them, ‘You have to do it this way,’ ” Dr. Davis said.
The task force’s draft recommendation is not meant to apply to people with secondary osteoporosis due to an underlying medical condition such as cancer, metabolic bone diseases or hyperthyroidism, or chronic use of a medication associated with bone loss.
Rajesh K. Jain, MD, who was not involved with the USPSTF work, read the draft recommendations at the request of this news organization. In an email, he said he generally agreed with the decision to largely stick to the 2018 recommendations for women.
He also noted that there’s still a lack of a clear direction for physicians about assessing osteoporosis risk in men. But multiple randomized control trials of osteoporosis drugs seem to suggest these medicines work for both sexes, said Dr. Jain, who is the endocrinology fellowship program director at University of Chicago Medicine, Chicago.
The USPSTF draft also would reiterate the current “I” grade about screening men for osteoporosis.
An “I” grade means the task force found the current body of available evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis to prevent osteoporotic fractures in men.
“Since there is no recommendation right now, it would have seemed sensible to include a recommendation to screen men with prior fracture or other risk factors for osteoporosis, much like they do for younger women,” Dr. Jain said.
Insufficient Evidence
The USPSTF’s “I” grade is different from a “D” grade, which is what the task force uses to recommend against the use of a service.
A “D” grade means the USPSTF says there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. (The USPSTF makes it easy to search online for grades given to preventive services, including those that got a “D.”)
The USPSTF is calling for more studies on the benefits and harms of screening for osteoporosis to prevent fractures and related morbidity and mortality in men.
“Men do get osteoporosis,” Dr. Davis said. “But unfortunately, the evidence isn’t there” to allow USPSTF to make a recommendation on screening approaches.
“Any man who has concerns about bone health should certainly talk to his clinician and figure out what is the best form of screening” he might want to do, she said.
There’s been a growing interest in the question of whether to screen men for osteoporosis and bone health. For example, Osteoporosis Canada last year updated a guideline to emphasize the need to assess older patients of both sexes for the risk for fractures. But the Canadian Task Force on Preventive Health Care in 2023 came to a conclusion in line with the USPSTF draft.
The Canadian task force recommended against routine screening in men, while adding that clinicians should be alert to changes in health that may indicate the patient has experienced or is at a higher risk for fragility fracture.
Risk Factors, Concerns About Tests
The USPSTF said that risk factors associated with fragility fractures are similar in men and women. These include:
- Increasing age
- Low body mass index
- Excessive alcohol intake
- Current smoking
- Chronic corticosteroid use
- History of prior fractures, falls within the past year, cerebrovascular accident, and diabetes
- Hypogonadism
The process of updating the USPSTF recommendations can serve as a chance to expand public awareness about osteoporosis, as many men may not know to raise the question of their fracture risk during medical appointments, Dr. Davis said.
“Clinicians need to be aware of the risk factors and to be able to have conversations with men,” she said.
Dr. Davis also cautioned about the need to be aware of limitations with clinical risk assessment tools. In the draft recommendation statement, the USPSTF noted that some tools and approaches may be less likely to identify Black, Hispanic, and Asian people as high risk, and subsequently, clinicians may be less likely to offer treatment to them compared with White people of the same age, bone mineral density, and clinical risk profile.
Dr. Davis had no relevant financial relationships. Dr. Jain received research funding from the Amgen Foundation.
A version of this article appeared on Medscape.com.
An influential US panel may largely reaffirm its current recommendation in favor of screening older women to prevent osteoporotic fractures, while also repeating its call for more research to try to determine whether men would benefit from this kind of routine testing.
The US Preventive Services Task Force (USPSTF) on June 11 released a draft update of its recommendations on osteoporosis screening. The task force will accept comments on the draft through July 8. Federal law gives the USPSTF recommendations extra clout, requiring insurers to cover — without co-pay — services that get top marks “A” or “B” from the task force.
The task force intends to maintain a “B” recommendation on screening of older women, indicating that the evidence gathered to date suggests a moderate net benefit. But the draft includes a shift in the approach to this screening.
The USPSTF proposed saying that it recommends screening for osteoporosis in both women aged 65 years and older and postmenopausal women younger than 65 years who are at an increased risk for an osteoporotic fracture. The current recommendation, finalized in 2018, advises “screening for osteoporosis with bone measurement testing [emphasis added]” for these groups.
The proposed change in language — dropping the phrase “with bone measurement testing” — is intended to expand flexibility for clinicians, Esa Davis, MD, MPH, a member of USPSTF and a professor at the University of Maryland School of Medicine, Baltimore, told this news organization.
“It provides them with more options instead of telling them, ‘You have to do it this way,’ ” Dr. Davis said.
The task force’s draft recommendation is not meant to apply to people with secondary osteoporosis due to an underlying medical condition such as cancer, metabolic bone diseases or hyperthyroidism, or chronic use of a medication associated with bone loss.
Rajesh K. Jain, MD, who was not involved with the USPSTF work, read the draft recommendations at the request of this news organization. In an email, he said he generally agreed with the decision to largely stick to the 2018 recommendations for women.
He also noted that there’s still a lack of a clear direction for physicians about assessing osteoporosis risk in men. But multiple randomized control trials of osteoporosis drugs seem to suggest these medicines work for both sexes, said Dr. Jain, who is the endocrinology fellowship program director at University of Chicago Medicine, Chicago.
The USPSTF draft also would reiterate the current “I” grade about screening men for osteoporosis.
An “I” grade means the task force found the current body of available evidence is insufficient to assess the balance of benefits and harms of screening for osteoporosis to prevent osteoporotic fractures in men.
“Since there is no recommendation right now, it would have seemed sensible to include a recommendation to screen men with prior fracture or other risk factors for osteoporosis, much like they do for younger women,” Dr. Jain said.
Insufficient Evidence
The USPSTF’s “I” grade is different from a “D” grade, which is what the task force uses to recommend against the use of a service.
A “D” grade means the USPSTF says there is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. (The USPSTF makes it easy to search online for grades given to preventive services, including those that got a “D.”)
The USPSTF is calling for more studies on the benefits and harms of screening for osteoporosis to prevent fractures and related morbidity and mortality in men.
“Men do get osteoporosis,” Dr. Davis said. “But unfortunately, the evidence isn’t there” to allow USPSTF to make a recommendation on screening approaches.
“Any man who has concerns about bone health should certainly talk to his clinician and figure out what is the best form of screening” he might want to do, she said.
There’s been a growing interest in the question of whether to screen men for osteoporosis and bone health. For example, Osteoporosis Canada last year updated a guideline to emphasize the need to assess older patients of both sexes for the risk for fractures. But the Canadian Task Force on Preventive Health Care in 2023 came to a conclusion in line with the USPSTF draft.
The Canadian task force recommended against routine screening in men, while adding that clinicians should be alert to changes in health that may indicate the patient has experienced or is at a higher risk for fragility fracture.
Risk Factors, Concerns About Tests
The USPSTF said that risk factors associated with fragility fractures are similar in men and women. These include:
- Increasing age
- Low body mass index
- Excessive alcohol intake
- Current smoking
- Chronic corticosteroid use
- History of prior fractures, falls within the past year, cerebrovascular accident, and diabetes
- Hypogonadism
The process of updating the USPSTF recommendations can serve as a chance to expand public awareness about osteoporosis, as many men may not know to raise the question of their fracture risk during medical appointments, Dr. Davis said.
“Clinicians need to be aware of the risk factors and to be able to have conversations with men,” she said.
Dr. Davis also cautioned about the need to be aware of limitations with clinical risk assessment tools. In the draft recommendation statement, the USPSTF noted that some tools and approaches may be less likely to identify Black, Hispanic, and Asian people as high risk, and subsequently, clinicians may be less likely to offer treatment to them compared with White people of the same age, bone mineral density, and clinical risk profile.
Dr. Davis had no relevant financial relationships. Dr. Jain received research funding from the Amgen Foundation.
A version of this article appeared on Medscape.com.
Having More Tender Than Swollen Joints Worsens Outcomes in Early Rheumatoid Arthritis
TOPLINE:
Having more tender than swollen joints is linked to worse patient-reported outcomes (PROs), particularly in pain interference, social participation, and fatigue, in patients with rheumatoid arthritis (RA).
METHODOLOGY:
- In early RA, understanding the impact of tender-swollen joint differences (TSJDs) on PROs across multiple domains of health-related quality of life is important to customize personalized therapeutic strategies.
- This study evaluated the impact of TSJDs on PROs over 1 year in 547 patients (mean age, 56 years; 70% women; mean symptom duration, 5.3 months) with early RA across 18 centers in Canada between January 2016 and August 2022.
- TSJDs were assessed for 28 joints (six large and 22 small) at baseline and at 3-, 6-, and 12-month visits using the PRO Measurement Information System (PROMIS-29), covering seven domains of health. Higher PROMIS T-scores indicated better health outcomes.
TAKEAWAY:
- A one-point increase of TSJD was significantly associated with worse PROMIS T-scores in physical function (adjusted regression coefficient [β], −0.27; 95% CI, −0.39 to −0.15) and social participation (β, −0.34; 95% CI, −0.50 to −0.19).
- A one-point increase in TSJD was also linked to worsened PROMIS symptoms in pain interference (β, 0.49), fatigue (β, 0.34), sleep problems (β, 0.29), anxiety (β, 0.23), and depression (β, 0.20).
- Large-joint TSJD was particularly associated with worse PROs than small-joint TSJD.
- The sensitivity analysis validated the reliability of the primary findings regarding the association between joint counts and PROs evaluated by PROMIS-29, even when accounting for C-reactive protein levels in various scenarios or assumptions.
IN PRACTICE:
“Patients with more tender than swollen joints may experience worsening of all seven domains of health, especially pain interference, social participation, and fatigue. Rheumatologists should be alerted to their patients with early RA having more tender than swollen joints, particularly in large joints,” the authors wrote.
SOURCE:
The study was led by Charis F. Meng, MD, Division of Rheumatology, Hospital for Special Surgery, New York. It was published online on May 1, 2024, in Journal of Clinical Rheumatology.
LIMITATIONS:
The study was observational with missing data, which could have impacted the reliability of the results. Most participants were women and White individuals, which could restrict the generalizability of results. The absence of ultrasound for synovitis limited the clinical assessment of patients with RA for information beyond physical examination alone.
DISCLOSURES:
The study was supported and funded by the Inflammatory Arthritis Center and Division of Rheumatology at the Hospital for Special Surgery, New York. One author reported receiving funding from the National Institutes of Health.
A version of this article appeared on Medscape.com.
TOPLINE:
Having more tender than swollen joints is linked to worse patient-reported outcomes (PROs), particularly in pain interference, social participation, and fatigue, in patients with rheumatoid arthritis (RA).
METHODOLOGY:
- In early RA, understanding the impact of tender-swollen joint differences (TSJDs) on PROs across multiple domains of health-related quality of life is important to customize personalized therapeutic strategies.
- This study evaluated the impact of TSJDs on PROs over 1 year in 547 patients (mean age, 56 years; 70% women; mean symptom duration, 5.3 months) with early RA across 18 centers in Canada between January 2016 and August 2022.
- TSJDs were assessed for 28 joints (six large and 22 small) at baseline and at 3-, 6-, and 12-month visits using the PRO Measurement Information System (PROMIS-29), covering seven domains of health. Higher PROMIS T-scores indicated better health outcomes.
TAKEAWAY:
- A one-point increase of TSJD was significantly associated with worse PROMIS T-scores in physical function (adjusted regression coefficient [β], −0.27; 95% CI, −0.39 to −0.15) and social participation (β, −0.34; 95% CI, −0.50 to −0.19).
- A one-point increase in TSJD was also linked to worsened PROMIS symptoms in pain interference (β, 0.49), fatigue (β, 0.34), sleep problems (β, 0.29), anxiety (β, 0.23), and depression (β, 0.20).
- Large-joint TSJD was particularly associated with worse PROs than small-joint TSJD.
- The sensitivity analysis validated the reliability of the primary findings regarding the association between joint counts and PROs evaluated by PROMIS-29, even when accounting for C-reactive protein levels in various scenarios or assumptions.
IN PRACTICE:
“Patients with more tender than swollen joints may experience worsening of all seven domains of health, especially pain interference, social participation, and fatigue. Rheumatologists should be alerted to their patients with early RA having more tender than swollen joints, particularly in large joints,” the authors wrote.
SOURCE:
The study was led by Charis F. Meng, MD, Division of Rheumatology, Hospital for Special Surgery, New York. It was published online on May 1, 2024, in Journal of Clinical Rheumatology.
LIMITATIONS:
The study was observational with missing data, which could have impacted the reliability of the results. Most participants were women and White individuals, which could restrict the generalizability of results. The absence of ultrasound for synovitis limited the clinical assessment of patients with RA for information beyond physical examination alone.
DISCLOSURES:
The study was supported and funded by the Inflammatory Arthritis Center and Division of Rheumatology at the Hospital for Special Surgery, New York. One author reported receiving funding from the National Institutes of Health.
A version of this article appeared on Medscape.com.
TOPLINE:
Having more tender than swollen joints is linked to worse patient-reported outcomes (PROs), particularly in pain interference, social participation, and fatigue, in patients with rheumatoid arthritis (RA).
METHODOLOGY:
- In early RA, understanding the impact of tender-swollen joint differences (TSJDs) on PROs across multiple domains of health-related quality of life is important to customize personalized therapeutic strategies.
- This study evaluated the impact of TSJDs on PROs over 1 year in 547 patients (mean age, 56 years; 70% women; mean symptom duration, 5.3 months) with early RA across 18 centers in Canada between January 2016 and August 2022.
- TSJDs were assessed for 28 joints (six large and 22 small) at baseline and at 3-, 6-, and 12-month visits using the PRO Measurement Information System (PROMIS-29), covering seven domains of health. Higher PROMIS T-scores indicated better health outcomes.
TAKEAWAY:
- A one-point increase of TSJD was significantly associated with worse PROMIS T-scores in physical function (adjusted regression coefficient [β], −0.27; 95% CI, −0.39 to −0.15) and social participation (β, −0.34; 95% CI, −0.50 to −0.19).
- A one-point increase in TSJD was also linked to worsened PROMIS symptoms in pain interference (β, 0.49), fatigue (β, 0.34), sleep problems (β, 0.29), anxiety (β, 0.23), and depression (β, 0.20).
- Large-joint TSJD was particularly associated with worse PROs than small-joint TSJD.
- The sensitivity analysis validated the reliability of the primary findings regarding the association between joint counts and PROs evaluated by PROMIS-29, even when accounting for C-reactive protein levels in various scenarios or assumptions.
IN PRACTICE:
“Patients with more tender than swollen joints may experience worsening of all seven domains of health, especially pain interference, social participation, and fatigue. Rheumatologists should be alerted to their patients with early RA having more tender than swollen joints, particularly in large joints,” the authors wrote.
SOURCE:
The study was led by Charis F. Meng, MD, Division of Rheumatology, Hospital for Special Surgery, New York. It was published online on May 1, 2024, in Journal of Clinical Rheumatology.
LIMITATIONS:
The study was observational with missing data, which could have impacted the reliability of the results. Most participants were women and White individuals, which could restrict the generalizability of results. The absence of ultrasound for synovitis limited the clinical assessment of patients with RA for information beyond physical examination alone.
DISCLOSURES:
The study was supported and funded by the Inflammatory Arthritis Center and Division of Rheumatology at the Hospital for Special Surgery, New York. One author reported receiving funding from the National Institutes of Health.
A version of this article appeared on Medscape.com.
EULAR 2024 Preview: Therapeutics in Development Take Center Stage
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
Chronotherapy: Why Timing Drugs to Our Body Clocks May Work
Do drugs work better if taken by the clock?
A new analysis published in The Lancet journal’s eClinicalMedicine suggests: Yes, they do — if you consider the patient’s individual body clock. The study is the first to find that timing blood pressure drugs to a person’s personal “chronotype” — that is, whether they are a night owl or an early bird — may reduce the risk for a heart attack.
The findings represent a significant advance in the field of circadian medicine or “chronotherapy” — timing drug administration to circadian rhythms. A growing stack of research suggests this approach could reduce side effects and improve the effectiveness of a wide range of therapies, including vaccines, cancer treatments, and drugs for depression, glaucoma, pain, seizures, and other conditions. Still, despite decades of research, time of day is rarely considered in writing prescriptions.
“We are really just at the beginning of an exciting new way of looking at patient care,” said Kenneth A. Dyar, PhD, whose lab at Helmholtz Zentrum München’s Institute for Diabetes and Cancer focuses on metabolic physiology. Dr. Dyar is co-lead author of the new blood pressure analysis.
“Chronotherapy is a rapidly growing field,” he said, “and I suspect we are soon going to see more and more studies focused on ‘personalized chronotherapy,’ not only in hypertension but also potentially in other clinical areas.”
The ‘Missing Piece’ in Chronotherapy Research
Blood pressure drugs have long been chronotherapy’s battleground. After all, blood pressure follows a circadian rhythm, peaking in the morning and dropping at night.
That healthy overnight dip can disappear in people with diabetes, kidney disease, and obstructive sleep apnea. Some physicians have suggested a bed-time dose to restore that dip. But studies have had mixed results, so “take at bedtime” has become a less common recommendation in recent years.
But the debate continued. After a large 2019 Spanish study found that bedtime doses had benefits so big that the results drew questions, an even larger, 2022 randomized, controlled trial from the University of Dundee in Dundee, Scotland — called the TIME study — aimed to settle the question.
Researchers assigned over 21,000 people to take morning or night hypertension drugs for several years and found no difference in cardiovascular outcomes.
“We did this study thinking nocturnal blood pressure tablets might be better,” said Thomas MacDonald, MD, professor emeritus of clinical pharmacology and pharmacoepidemiology at the University of Dundee and principal investigator for the TIME study and the recent chronotype analysis. “But there was no difference for heart attacks, strokes, or vascular death.”
So, the researchers then looked at participants’ chronotypes, sorting outcomes based on whether the participants were late-to-bed, late-to-rise “night owls” or early-to-bed, early-to-rise “morning larks.”
Their analysis of these 5358 TIME participants found the following results: Risk for hospitalization for a heart attack was at least 34% lower for “owls” who took their drugs at bedtime. By contrast, owls’ heart attack risk was at least 62% higher with morning doses. For “larks,” the opposite was true. Morning doses were associated with an 11% lower heart attack risk and night doses with an 11% higher risk, according to supplemental data.
The personalized approach could explain why some previous chronotherapy studies have failed to show a benefit. Those studies did not individualize drug timing as this one did. But personalization could be key to circadian medicine’s success.
“Our ‘internal personal time’ appears to be an important variable to consider when dosing antihypertensives,” said co-lead author Filippo Pigazzani, MD, PhD, clinical senior lecturer and honorary consultant cardiologist at the University of Dundee School of Medicine. “Chronotherapy research has been going on for decades. We knew there was something important with time of day. But researchers haven’t considered the internal time of individual people. I think that is the missing piece.”
The analysis has several important limitations, the researchers said. A total of 95% of participants were White. And it was an observational study, not a true randomized comparison. “We started it late in the original TIME study,” Dr. MacDonald said. “You could argue we were reporting on those who survived long enough to get into the analysis.” More research is needed, they concluded.
Looking Beyond Blood Pressure
What about the rest of the body? “Almost all the cells of our body contain ‘circadian clocks’ that are synchronized by daily environmental cues, including light-dark, activity-rest, and feeding-fasting cycles,” said Dr. Dyar.
An estimated 50% of prescription drugs hit targets in the body that have circadian patterns. So, experts suspect that syncing a drug with a person’s body clock might increase effectiveness of many drugs.
A handful of US Food and Drug Administration–approved drugs already have time-of-day recommendations on the label for effectiveness or to limit side effects, including bedtime or evening for the insomnia drug Ambien, the HIV antiviral Atripla, and cholesterol-lowering Zocor. Others are intended to be taken with or after your last meal of the day, such as the long-acting insulin Levemir and the cardiovascular drug Xarelto. A morning recommendation comes with the proton pump inhibitor Nexium and the attention-deficit/hyperactivity disorder drug Ritalin.
Interest is expanding. About one third of the papers published about chronotherapy in the past 25 years have come out in the past 5 years. The May 2024 meeting of the Society for Research on Biological Rhythms featured a day-long session aimed at bringing clinicians up to speed. An organization called the International Association of Circadian Health Clinics is trying to bring circadian medicine findings to clinicians and their patients and to support research.
Moreover, while recent research suggests minding the clock could have benefits for a wide range of treatments, ignoring it could cause problems.
In a Massachusetts Institute of Technology study published in April in Science Advances, researchers looked at engineered livers made from human donor cells and found more than 300 genes that operate on a circadian schedule, many with roles in drug metabolism. They also found that circadian patterns affected the toxicity of acetaminophen and atorvastatin. Identifying the time of day to take these drugs could maximize effectiveness and minimize adverse effects, the researchers said.
Timing and the Immune System
Circadian rhythms are also seen in immune processes. In a 2023 study in The Journal of Clinical Investigation of vaccine data from 1.5 million people in Israel, researchers found that children and older adults who got their second dose of the Pfizer mRNA COVID vaccine earlier in the day were about 36% less likely to be hospitalized with SARS-CoV-2 infection than those who got an evening shot.
“The sweet spot in our data was somewhere around late morning to late afternoon,” said lead researcher Jeffrey Haspel, MD, PhD, associate professor of medicine in the division of pulmonary and critical care medicine at Washington University School of Medicine in St. Louis.
In a multicenter, 2024 analysis of 13 studies of immunotherapy for advanced cancers in 1663 people, researchers found treatment earlier in the day was associated with longer survival time and longer survival without cancer progression.
“Patients with selected metastatic cancers seemed to largely benefit from early [time of day] infusions, which is consistent with circadian mechanisms in immune-cell functions and trafficking,” the researchers noted. But “retrospective randomized trials are needed to establish recommendations for optimal circadian timing.”
Other research suggests or is investigating possible chronotherapy benefits for depression, glaucoma, respiratory diseases, stroke treatment, epilepsy, and sedatives used in surgery. So why aren’t healthcare providers adding time of day to more prescriptions? “What’s missing is more reliable data,” Dr. Dyar said.
Should You Use Chronotherapy Now?
Experts emphasize that more research is needed before doctors use chronotherapy and before medical organizations include it in treatment recommendations. But for some patients, circadian dosing may be worth a try:
Night owls whose blood pressure isn’t well controlled. Dr. Dyar and Dr. Pigazzani said night-time blood pressure drugs may be helpful for people with a “late chronotype.” Of course, patients shouldn’t change their medication schedule on their own, they said. And doctors may want to consider other concerns, like more overnight bathroom visits with evening diuretics.
In their study, the researchers determined participants’ chronotype with a few questions from the Munich Chronotype Questionnaire about what time they fell asleep and woke up on workdays and days off and whether they considered themselves “morning types” or “evening types.” (The questions can be found in supplementary data for the study.)
If a physician thinks matching the timing of a dose with chronotype would help, they can consider it, Dr. Pigazzani said. “However, I must add that this was an observational study, so I would advise healthcare practitioners to wait for our data to be confirmed in new RCTs of personalized chronotherapy of hypertension.”
Children and older adults getting vaccines. Timing COVID shots and possibly other vaccines from late morning to mid-afternoon could have a small benefit for individuals and a bigger public-health benefit, Dr. Haspel said. But the most important thing is getting vaccinated. “If you can only get one in the evening, it’s still worthwhile. Timing may add oomph at a public-health level for more vulnerable groups.”
A version of this article appeared on Medscape.com.
Do drugs work better if taken by the clock?
A new analysis published in The Lancet journal’s eClinicalMedicine suggests: Yes, they do — if you consider the patient’s individual body clock. The study is the first to find that timing blood pressure drugs to a person’s personal “chronotype” — that is, whether they are a night owl or an early bird — may reduce the risk for a heart attack.
The findings represent a significant advance in the field of circadian medicine or “chronotherapy” — timing drug administration to circadian rhythms. A growing stack of research suggests this approach could reduce side effects and improve the effectiveness of a wide range of therapies, including vaccines, cancer treatments, and drugs for depression, glaucoma, pain, seizures, and other conditions. Still, despite decades of research, time of day is rarely considered in writing prescriptions.
“We are really just at the beginning of an exciting new way of looking at patient care,” said Kenneth A. Dyar, PhD, whose lab at Helmholtz Zentrum München’s Institute for Diabetes and Cancer focuses on metabolic physiology. Dr. Dyar is co-lead author of the new blood pressure analysis.
“Chronotherapy is a rapidly growing field,” he said, “and I suspect we are soon going to see more and more studies focused on ‘personalized chronotherapy,’ not only in hypertension but also potentially in other clinical areas.”
The ‘Missing Piece’ in Chronotherapy Research
Blood pressure drugs have long been chronotherapy’s battleground. After all, blood pressure follows a circadian rhythm, peaking in the morning and dropping at night.
That healthy overnight dip can disappear in people with diabetes, kidney disease, and obstructive sleep apnea. Some physicians have suggested a bed-time dose to restore that dip. But studies have had mixed results, so “take at bedtime” has become a less common recommendation in recent years.
But the debate continued. After a large 2019 Spanish study found that bedtime doses had benefits so big that the results drew questions, an even larger, 2022 randomized, controlled trial from the University of Dundee in Dundee, Scotland — called the TIME study — aimed to settle the question.
Researchers assigned over 21,000 people to take morning or night hypertension drugs for several years and found no difference in cardiovascular outcomes.
“We did this study thinking nocturnal blood pressure tablets might be better,” said Thomas MacDonald, MD, professor emeritus of clinical pharmacology and pharmacoepidemiology at the University of Dundee and principal investigator for the TIME study and the recent chronotype analysis. “But there was no difference for heart attacks, strokes, or vascular death.”
So, the researchers then looked at participants’ chronotypes, sorting outcomes based on whether the participants were late-to-bed, late-to-rise “night owls” or early-to-bed, early-to-rise “morning larks.”
Their analysis of these 5358 TIME participants found the following results: Risk for hospitalization for a heart attack was at least 34% lower for “owls” who took their drugs at bedtime. By contrast, owls’ heart attack risk was at least 62% higher with morning doses. For “larks,” the opposite was true. Morning doses were associated with an 11% lower heart attack risk and night doses with an 11% higher risk, according to supplemental data.
The personalized approach could explain why some previous chronotherapy studies have failed to show a benefit. Those studies did not individualize drug timing as this one did. But personalization could be key to circadian medicine’s success.
“Our ‘internal personal time’ appears to be an important variable to consider when dosing antihypertensives,” said co-lead author Filippo Pigazzani, MD, PhD, clinical senior lecturer and honorary consultant cardiologist at the University of Dundee School of Medicine. “Chronotherapy research has been going on for decades. We knew there was something important with time of day. But researchers haven’t considered the internal time of individual people. I think that is the missing piece.”
The analysis has several important limitations, the researchers said. A total of 95% of participants were White. And it was an observational study, not a true randomized comparison. “We started it late in the original TIME study,” Dr. MacDonald said. “You could argue we were reporting on those who survived long enough to get into the analysis.” More research is needed, they concluded.
Looking Beyond Blood Pressure
What about the rest of the body? “Almost all the cells of our body contain ‘circadian clocks’ that are synchronized by daily environmental cues, including light-dark, activity-rest, and feeding-fasting cycles,” said Dr. Dyar.
An estimated 50% of prescription drugs hit targets in the body that have circadian patterns. So, experts suspect that syncing a drug with a person’s body clock might increase effectiveness of many drugs.
A handful of US Food and Drug Administration–approved drugs already have time-of-day recommendations on the label for effectiveness or to limit side effects, including bedtime or evening for the insomnia drug Ambien, the HIV antiviral Atripla, and cholesterol-lowering Zocor. Others are intended to be taken with or after your last meal of the day, such as the long-acting insulin Levemir and the cardiovascular drug Xarelto. A morning recommendation comes with the proton pump inhibitor Nexium and the attention-deficit/hyperactivity disorder drug Ritalin.
Interest is expanding. About one third of the papers published about chronotherapy in the past 25 years have come out in the past 5 years. The May 2024 meeting of the Society for Research on Biological Rhythms featured a day-long session aimed at bringing clinicians up to speed. An organization called the International Association of Circadian Health Clinics is trying to bring circadian medicine findings to clinicians and their patients and to support research.
Moreover, while recent research suggests minding the clock could have benefits for a wide range of treatments, ignoring it could cause problems.
In a Massachusetts Institute of Technology study published in April in Science Advances, researchers looked at engineered livers made from human donor cells and found more than 300 genes that operate on a circadian schedule, many with roles in drug metabolism. They also found that circadian patterns affected the toxicity of acetaminophen and atorvastatin. Identifying the time of day to take these drugs could maximize effectiveness and minimize adverse effects, the researchers said.
Timing and the Immune System
Circadian rhythms are also seen in immune processes. In a 2023 study in The Journal of Clinical Investigation of vaccine data from 1.5 million people in Israel, researchers found that children and older adults who got their second dose of the Pfizer mRNA COVID vaccine earlier in the day were about 36% less likely to be hospitalized with SARS-CoV-2 infection than those who got an evening shot.
“The sweet spot in our data was somewhere around late morning to late afternoon,” said lead researcher Jeffrey Haspel, MD, PhD, associate professor of medicine in the division of pulmonary and critical care medicine at Washington University School of Medicine in St. Louis.
In a multicenter, 2024 analysis of 13 studies of immunotherapy for advanced cancers in 1663 people, researchers found treatment earlier in the day was associated with longer survival time and longer survival without cancer progression.
“Patients with selected metastatic cancers seemed to largely benefit from early [time of day] infusions, which is consistent with circadian mechanisms in immune-cell functions and trafficking,” the researchers noted. But “retrospective randomized trials are needed to establish recommendations for optimal circadian timing.”
Other research suggests or is investigating possible chronotherapy benefits for depression, glaucoma, respiratory diseases, stroke treatment, epilepsy, and sedatives used in surgery. So why aren’t healthcare providers adding time of day to more prescriptions? “What’s missing is more reliable data,” Dr. Dyar said.
Should You Use Chronotherapy Now?
Experts emphasize that more research is needed before doctors use chronotherapy and before medical organizations include it in treatment recommendations. But for some patients, circadian dosing may be worth a try:
Night owls whose blood pressure isn’t well controlled. Dr. Dyar and Dr. Pigazzani said night-time blood pressure drugs may be helpful for people with a “late chronotype.” Of course, patients shouldn’t change their medication schedule on their own, they said. And doctors may want to consider other concerns, like more overnight bathroom visits with evening diuretics.
In their study, the researchers determined participants’ chronotype with a few questions from the Munich Chronotype Questionnaire about what time they fell asleep and woke up on workdays and days off and whether they considered themselves “morning types” or “evening types.” (The questions can be found in supplementary data for the study.)
If a physician thinks matching the timing of a dose with chronotype would help, they can consider it, Dr. Pigazzani said. “However, I must add that this was an observational study, so I would advise healthcare practitioners to wait for our data to be confirmed in new RCTs of personalized chronotherapy of hypertension.”
Children and older adults getting vaccines. Timing COVID shots and possibly other vaccines from late morning to mid-afternoon could have a small benefit for individuals and a bigger public-health benefit, Dr. Haspel said. But the most important thing is getting vaccinated. “If you can only get one in the evening, it’s still worthwhile. Timing may add oomph at a public-health level for more vulnerable groups.”
A version of this article appeared on Medscape.com.
Do drugs work better if taken by the clock?
A new analysis published in The Lancet journal’s eClinicalMedicine suggests: Yes, they do — if you consider the patient’s individual body clock. The study is the first to find that timing blood pressure drugs to a person’s personal “chronotype” — that is, whether they are a night owl or an early bird — may reduce the risk for a heart attack.
The findings represent a significant advance in the field of circadian medicine or “chronotherapy” — timing drug administration to circadian rhythms. A growing stack of research suggests this approach could reduce side effects and improve the effectiveness of a wide range of therapies, including vaccines, cancer treatments, and drugs for depression, glaucoma, pain, seizures, and other conditions. Still, despite decades of research, time of day is rarely considered in writing prescriptions.
“We are really just at the beginning of an exciting new way of looking at patient care,” said Kenneth A. Dyar, PhD, whose lab at Helmholtz Zentrum München’s Institute for Diabetes and Cancer focuses on metabolic physiology. Dr. Dyar is co-lead author of the new blood pressure analysis.
“Chronotherapy is a rapidly growing field,” he said, “and I suspect we are soon going to see more and more studies focused on ‘personalized chronotherapy,’ not only in hypertension but also potentially in other clinical areas.”
The ‘Missing Piece’ in Chronotherapy Research
Blood pressure drugs have long been chronotherapy’s battleground. After all, blood pressure follows a circadian rhythm, peaking in the morning and dropping at night.
That healthy overnight dip can disappear in people with diabetes, kidney disease, and obstructive sleep apnea. Some physicians have suggested a bed-time dose to restore that dip. But studies have had mixed results, so “take at bedtime” has become a less common recommendation in recent years.
But the debate continued. After a large 2019 Spanish study found that bedtime doses had benefits so big that the results drew questions, an even larger, 2022 randomized, controlled trial from the University of Dundee in Dundee, Scotland — called the TIME study — aimed to settle the question.
Researchers assigned over 21,000 people to take morning or night hypertension drugs for several years and found no difference in cardiovascular outcomes.
“We did this study thinking nocturnal blood pressure tablets might be better,” said Thomas MacDonald, MD, professor emeritus of clinical pharmacology and pharmacoepidemiology at the University of Dundee and principal investigator for the TIME study and the recent chronotype analysis. “But there was no difference for heart attacks, strokes, or vascular death.”
So, the researchers then looked at participants’ chronotypes, sorting outcomes based on whether the participants were late-to-bed, late-to-rise “night owls” or early-to-bed, early-to-rise “morning larks.”
Their analysis of these 5358 TIME participants found the following results: Risk for hospitalization for a heart attack was at least 34% lower for “owls” who took their drugs at bedtime. By contrast, owls’ heart attack risk was at least 62% higher with morning doses. For “larks,” the opposite was true. Morning doses were associated with an 11% lower heart attack risk and night doses with an 11% higher risk, according to supplemental data.
The personalized approach could explain why some previous chronotherapy studies have failed to show a benefit. Those studies did not individualize drug timing as this one did. But personalization could be key to circadian medicine’s success.
“Our ‘internal personal time’ appears to be an important variable to consider when dosing antihypertensives,” said co-lead author Filippo Pigazzani, MD, PhD, clinical senior lecturer and honorary consultant cardiologist at the University of Dundee School of Medicine. “Chronotherapy research has been going on for decades. We knew there was something important with time of day. But researchers haven’t considered the internal time of individual people. I think that is the missing piece.”
The analysis has several important limitations, the researchers said. A total of 95% of participants were White. And it was an observational study, not a true randomized comparison. “We started it late in the original TIME study,” Dr. MacDonald said. “You could argue we were reporting on those who survived long enough to get into the analysis.” More research is needed, they concluded.
Looking Beyond Blood Pressure
What about the rest of the body? “Almost all the cells of our body contain ‘circadian clocks’ that are synchronized by daily environmental cues, including light-dark, activity-rest, and feeding-fasting cycles,” said Dr. Dyar.
An estimated 50% of prescription drugs hit targets in the body that have circadian patterns. So, experts suspect that syncing a drug with a person’s body clock might increase effectiveness of many drugs.
A handful of US Food and Drug Administration–approved drugs already have time-of-day recommendations on the label for effectiveness or to limit side effects, including bedtime or evening for the insomnia drug Ambien, the HIV antiviral Atripla, and cholesterol-lowering Zocor. Others are intended to be taken with or after your last meal of the day, such as the long-acting insulin Levemir and the cardiovascular drug Xarelto. A morning recommendation comes with the proton pump inhibitor Nexium and the attention-deficit/hyperactivity disorder drug Ritalin.
Interest is expanding. About one third of the papers published about chronotherapy in the past 25 years have come out in the past 5 years. The May 2024 meeting of the Society for Research on Biological Rhythms featured a day-long session aimed at bringing clinicians up to speed. An organization called the International Association of Circadian Health Clinics is trying to bring circadian medicine findings to clinicians and their patients and to support research.
Moreover, while recent research suggests minding the clock could have benefits for a wide range of treatments, ignoring it could cause problems.
In a Massachusetts Institute of Technology study published in April in Science Advances, researchers looked at engineered livers made from human donor cells and found more than 300 genes that operate on a circadian schedule, many with roles in drug metabolism. They also found that circadian patterns affected the toxicity of acetaminophen and atorvastatin. Identifying the time of day to take these drugs could maximize effectiveness and minimize adverse effects, the researchers said.
Timing and the Immune System
Circadian rhythms are also seen in immune processes. In a 2023 study in The Journal of Clinical Investigation of vaccine data from 1.5 million people in Israel, researchers found that children and older adults who got their second dose of the Pfizer mRNA COVID vaccine earlier in the day were about 36% less likely to be hospitalized with SARS-CoV-2 infection than those who got an evening shot.
“The sweet spot in our data was somewhere around late morning to late afternoon,” said lead researcher Jeffrey Haspel, MD, PhD, associate professor of medicine in the division of pulmonary and critical care medicine at Washington University School of Medicine in St. Louis.
In a multicenter, 2024 analysis of 13 studies of immunotherapy for advanced cancers in 1663 people, researchers found treatment earlier in the day was associated with longer survival time and longer survival without cancer progression.
“Patients with selected metastatic cancers seemed to largely benefit from early [time of day] infusions, which is consistent with circadian mechanisms in immune-cell functions and trafficking,” the researchers noted. But “retrospective randomized trials are needed to establish recommendations for optimal circadian timing.”
Other research suggests or is investigating possible chronotherapy benefits for depression, glaucoma, respiratory diseases, stroke treatment, epilepsy, and sedatives used in surgery. So why aren’t healthcare providers adding time of day to more prescriptions? “What’s missing is more reliable data,” Dr. Dyar said.
Should You Use Chronotherapy Now?
Experts emphasize that more research is needed before doctors use chronotherapy and before medical organizations include it in treatment recommendations. But for some patients, circadian dosing may be worth a try:
Night owls whose blood pressure isn’t well controlled. Dr. Dyar and Dr. Pigazzani said night-time blood pressure drugs may be helpful for people with a “late chronotype.” Of course, patients shouldn’t change their medication schedule on their own, they said. And doctors may want to consider other concerns, like more overnight bathroom visits with evening diuretics.
In their study, the researchers determined participants’ chronotype with a few questions from the Munich Chronotype Questionnaire about what time they fell asleep and woke up on workdays and days off and whether they considered themselves “morning types” or “evening types.” (The questions can be found in supplementary data for the study.)
If a physician thinks matching the timing of a dose with chronotype would help, they can consider it, Dr. Pigazzani said. “However, I must add that this was an observational study, so I would advise healthcare practitioners to wait for our data to be confirmed in new RCTs of personalized chronotherapy of hypertension.”
Children and older adults getting vaccines. Timing COVID shots and possibly other vaccines from late morning to mid-afternoon could have a small benefit for individuals and a bigger public-health benefit, Dr. Haspel said. But the most important thing is getting vaccinated. “If you can only get one in the evening, it’s still worthwhile. Timing may add oomph at a public-health level for more vulnerable groups.”
A version of this article appeared on Medscape.com.
The Appendix: Is It ’Useless,’ or a Safe House and Immune Training Ground?
When doctors and patients consider the appendix, it’s often with urgency. In cases of appendicitis, the clock could be ticking down to a life-threatening burst. Thus, despite recent research suggesting antibiotics could be an alternative therapy, appendectomy remains standard for uncomplicated appendicitis.
But what if removing the appendix could raise the risk for gastrointestinal (GI) diseases like irritable bowel syndrome and colorectal cancer? That’s what some emerging science suggests. And though the research is early and mixed, it’s enough to give some health professionals pause.
“If there’s no reason to remove the appendix, then it’s better to have one,” said Heather Smith, PhD, a comparative anatomist at Midwestern University, Glendale, Arizona. Preemptive removal is not supported by the evidence, she said.
To be fair, we’ve come a long way since 1928, when American physician Miles Breuer, MD, suggested that people with infected appendixes should be left to perish, so as to remove their inferior DNA from the gene pool (he called such people “uncivilized” and “candidates for extinction”). Charles Darwin, while less radical, believed the appendix was at best useless — a mere vestige of our ancestors switching diets from leaves to fruits.
What we know now is that the appendix isn’t just a troublesome piece of worthless flesh. Instead, it may act as a safe house for friendly gut bacteria and a training camp for the immune system. It also appears to play a role in several medical conditions, from ulcerative colitis and colorectal cancer to Parkinson’s disease and lupus. The roughly 300,000 Americans who undergo appendectomy each year should be made aware of this, some experts say. But the frustrating truth is, scientists are still trying to figure out in which cases having an appendix is protective and in which we may be better off without it.
A ‘Worm’ as Intestinal Protection
The appendix is a blind pouch (meaning its ending is closed off) that extends from the large intestine. Not all mammals have one; it’s been found in several species of primates and rodents, as well as in rabbits, wombats, and Florida manatees, among others (dogs and cats don’t have it). While a human appendix “looks like a little worm,” Dr. Smith said, these anatomical structures come in various sizes and shapes. Some are thick, as in a beaver, while others are long and spiraling, like a rabbit’s.
Comparative anatomy studies reveal that the appendix has evolved independently at least 29 times throughout mammalian evolution. This suggests that “it has some kind of an adaptive function,” Dr. Smith said. When French scientists analyzed data from 258 species of mammals, they discovered that those that possess an appendix live longer than those without one. A possible explanation, the researchers wrote, may lie with the appendix’s role in preventing diarrhea.
Their 2023 study supported this hypothesis. Based on veterinary records of 45 different species of primates housed in a French zoo, the scientists established that primates with appendixes are far less likely to suffer severe diarrhea than those that don’t possess this organ. The appendix, it appears, might be our tiny weapon against bowel troubles.
For immunologist William Parker, PhD, a visiting scholar at the University of North Carolina at Chapel Hill, these data are “about as good as we could hope for” in support of the idea that the appendix might protect mammals from GI problems. An experiment on humans would be unethical, Dr. Parker said. But observational studies offer clues.
One study showed that compared with people with an intact appendix, young adults with a history of appendectomy have more than double the risk of developing a serious infection with non-typhoidal Salmonella of the kind that would require hospitalization.
A ‘Safe House’ for Bacteria
Such studies add weight to a theory that Dr. Parker and his colleagues developed back in 2007: That the appendix acts as a “safe house” for beneficial gut bacteria.
Think of the colon as a wide pipe, Dr. Parker said, that may become contaminated with a pathogen such as Salmonella. Diarrhea follows, and the pipe gets repeatedly flushed, wiping everything clean, including your friendly gut microbiome. Luckily, “you’ve got this little offshoot of that pipe,” where the flow can’t really get in “because it’s so constricted,” Dr. Parker said. The friendly gut microbes can survive inside the appendix and repopulate the colon once diarrhea is over. Dr. Parker and his colleagues found that the human appendix contains a thick layer of beneficial bacteria. “They were right where we predicted they would be,” he said.
This safe house hypothesis could explain why the gut microbiome may be different in people who no longer have an appendix. In one small study, people who’d had an appendectomy had a less diverse microbiome, with a lower abundance of beneficial strains such as Butyricicoccus and Barnesiella, than did those with intact appendixes.
The appendix likely has a second function, too, Dr. Smith said: It may serve as a training camp for the immune system. “When there is an invading pathogen in the gut, it helps the GI system to mount the immune response,” she said. The human appendix is rich in special cells known as M cells. These act as scouts, detecting and capturing invasive bacteria and viruses and presenting them to the body’s defense team, such as the T lymphocytes.
If the appendix shelters beneficial bacteria and boosts immune response, that may explain its links to various diseases. According to an epidemiological study from Taiwan,patients who underwent an appendectomy have a 46% higher risk of developing irritable bowel syndrome (IBS) — a disease associated with a low abundance of Butyricicoccus bacteria. This is why, the study authors wrote, doctors should pay careful attention to people who’ve had their appendixes removed, monitoring them for potential symptoms of IBS.
The same database helped uncover other connections between appendectomy and disease. For one, there was type 2 diabetes: Within 3 years of the surgery, patients under 30 had double the risk of developing this disorder. Then there was lupus: While those who underwent appendectomy generally had higher risk for this autoimmune disease, women were particularly affected.
The Contentious Connections
The most heated scientific discussion surrounds the links between the appendix and conditions such as Parkinson’s disease, ulcerative colitis, and colorectal cancer. A small 2019 study showed, for example, that appendectomy may improve symptoms of certain forms of ulcerative colitis that don’t respond to standard medical treatments. A third of patients improved after their appendix was removed, and 17% fully recovered.
Why? According to Dr. Parker, appendectomy may work for ulcerative colitis because it’s “a way of suppressing the immune system, especially in the lower intestinal areas.” A 2023 meta-analysis found that people who’d had their appendix removed before being diagnosed with ulcerative colitis were less likely to need their colon removed later on.
Such a procedure may have a serious side effect, however: Colorectal cancer. French scientists discovered that removing the appendix may reduce the numbers of certain immune cells called CD3+ and CD8+ T cells, causing a weakened immune surveillance. As a result, tumor cells might escape detection.
Yet the links between appendix removal and cancer are far from clear. A recent meta-analysis found that while people with appendectomies generally had a higher risk for colorectal cancer, for Europeans, these effects were insignificant. In fact, removal of the appendix actually protected European women from this particular form of cancer. For Parker, such mixed results may stem from the fact that treatments and populations vary widely. The issue “may depend on complex social and medical factors,” Dr. Parker said.
Things also appear complicated with Parkinson’s disease — another condition linked to the appendix. A large epidemiological study showed that appendectomy is associated with a lower risk for Parkinson’s disease and a delayed age of Parkinson’s onset. It also found that a normal appendix contains α-synuclein, a protein that may accumulate in the brain and contribute to the development of Parkinson’s. “Although α-synuclein is toxic when in the brain, it appears to be quite normal when present in the appendix,” said Luis Vitetta, PhD, MD, a clinical epidemiologist at the University of Sydney, Camperdown, Australia. Yet, not all studies find that removing the appendix lowers the risk for Parkinson’s. In fact, some show the opposite results.
How Should Doctors View the Appendix?
Even with these mysteries and contradictions, Dr. Vitetta said, a healthy appendix in a healthy body appears to be protective. This is why, he said, when someone is diagnosed with appendicitis, careful assessment is essential before surgery is performed.
“Perhaps an antibiotic can actually help fix it,” he said. A 2020 study published in The New England Journal of Medicine showed that antibiotics may indeed be a good alternative to surgery for the treatment of appendicitis. “We don’t want necessarily to remove an appendix that could be beneficial,” Dr. Smith said.
The many links between the appendix and various diseases mean that doctors should be more vigilant when treating patients who’ve had this organ removed, Dr. Parker said. “When a patient loses an appendix, depending on their environment, there may be effects on infection and cancer. So they might need more regular checkups,” he said. This could include monitoring for IBS and colorectal cancer.
What’s more, Dr. Parker believes that research on the appendix puts even more emphasis on the need to protect the gut microbiome — such as taking probiotics with antibiotics. And while we are still a long way from understanding how exactly this worm-like structure affects various diseases, one thing appears quite certain: The appendix is not useless. “If Darwin had the information that we have, he would not have drawn these conclusions,” Dr. Parker said.
A version of this article first appeared on Medscape.com.
When doctors and patients consider the appendix, it’s often with urgency. In cases of appendicitis, the clock could be ticking down to a life-threatening burst. Thus, despite recent research suggesting antibiotics could be an alternative therapy, appendectomy remains standard for uncomplicated appendicitis.
But what if removing the appendix could raise the risk for gastrointestinal (GI) diseases like irritable bowel syndrome and colorectal cancer? That’s what some emerging science suggests. And though the research is early and mixed, it’s enough to give some health professionals pause.
“If there’s no reason to remove the appendix, then it’s better to have one,” said Heather Smith, PhD, a comparative anatomist at Midwestern University, Glendale, Arizona. Preemptive removal is not supported by the evidence, she said.
To be fair, we’ve come a long way since 1928, when American physician Miles Breuer, MD, suggested that people with infected appendixes should be left to perish, so as to remove their inferior DNA from the gene pool (he called such people “uncivilized” and “candidates for extinction”). Charles Darwin, while less radical, believed the appendix was at best useless — a mere vestige of our ancestors switching diets from leaves to fruits.
What we know now is that the appendix isn’t just a troublesome piece of worthless flesh. Instead, it may act as a safe house for friendly gut bacteria and a training camp for the immune system. It also appears to play a role in several medical conditions, from ulcerative colitis and colorectal cancer to Parkinson’s disease and lupus. The roughly 300,000 Americans who undergo appendectomy each year should be made aware of this, some experts say. But the frustrating truth is, scientists are still trying to figure out in which cases having an appendix is protective and in which we may be better off without it.
A ‘Worm’ as Intestinal Protection
The appendix is a blind pouch (meaning its ending is closed off) that extends from the large intestine. Not all mammals have one; it’s been found in several species of primates and rodents, as well as in rabbits, wombats, and Florida manatees, among others (dogs and cats don’t have it). While a human appendix “looks like a little worm,” Dr. Smith said, these anatomical structures come in various sizes and shapes. Some are thick, as in a beaver, while others are long and spiraling, like a rabbit’s.
Comparative anatomy studies reveal that the appendix has evolved independently at least 29 times throughout mammalian evolution. This suggests that “it has some kind of an adaptive function,” Dr. Smith said. When French scientists analyzed data from 258 species of mammals, they discovered that those that possess an appendix live longer than those without one. A possible explanation, the researchers wrote, may lie with the appendix’s role in preventing diarrhea.
Their 2023 study supported this hypothesis. Based on veterinary records of 45 different species of primates housed in a French zoo, the scientists established that primates with appendixes are far less likely to suffer severe diarrhea than those that don’t possess this organ. The appendix, it appears, might be our tiny weapon against bowel troubles.
For immunologist William Parker, PhD, a visiting scholar at the University of North Carolina at Chapel Hill, these data are “about as good as we could hope for” in support of the idea that the appendix might protect mammals from GI problems. An experiment on humans would be unethical, Dr. Parker said. But observational studies offer clues.
One study showed that compared with people with an intact appendix, young adults with a history of appendectomy have more than double the risk of developing a serious infection with non-typhoidal Salmonella of the kind that would require hospitalization.
A ‘Safe House’ for Bacteria
Such studies add weight to a theory that Dr. Parker and his colleagues developed back in 2007: That the appendix acts as a “safe house” for beneficial gut bacteria.
Think of the colon as a wide pipe, Dr. Parker said, that may become contaminated with a pathogen such as Salmonella. Diarrhea follows, and the pipe gets repeatedly flushed, wiping everything clean, including your friendly gut microbiome. Luckily, “you’ve got this little offshoot of that pipe,” where the flow can’t really get in “because it’s so constricted,” Dr. Parker said. The friendly gut microbes can survive inside the appendix and repopulate the colon once diarrhea is over. Dr. Parker and his colleagues found that the human appendix contains a thick layer of beneficial bacteria. “They were right where we predicted they would be,” he said.
This safe house hypothesis could explain why the gut microbiome may be different in people who no longer have an appendix. In one small study, people who’d had an appendectomy had a less diverse microbiome, with a lower abundance of beneficial strains such as Butyricicoccus and Barnesiella, than did those with intact appendixes.
The appendix likely has a second function, too, Dr. Smith said: It may serve as a training camp for the immune system. “When there is an invading pathogen in the gut, it helps the GI system to mount the immune response,” she said. The human appendix is rich in special cells known as M cells. These act as scouts, detecting and capturing invasive bacteria and viruses and presenting them to the body’s defense team, such as the T lymphocytes.
If the appendix shelters beneficial bacteria and boosts immune response, that may explain its links to various diseases. According to an epidemiological study from Taiwan,patients who underwent an appendectomy have a 46% higher risk of developing irritable bowel syndrome (IBS) — a disease associated with a low abundance of Butyricicoccus bacteria. This is why, the study authors wrote, doctors should pay careful attention to people who’ve had their appendixes removed, monitoring them for potential symptoms of IBS.
The same database helped uncover other connections between appendectomy and disease. For one, there was type 2 diabetes: Within 3 years of the surgery, patients under 30 had double the risk of developing this disorder. Then there was lupus: While those who underwent appendectomy generally had higher risk for this autoimmune disease, women were particularly affected.
The Contentious Connections
The most heated scientific discussion surrounds the links between the appendix and conditions such as Parkinson’s disease, ulcerative colitis, and colorectal cancer. A small 2019 study showed, for example, that appendectomy may improve symptoms of certain forms of ulcerative colitis that don’t respond to standard medical treatments. A third of patients improved after their appendix was removed, and 17% fully recovered.
Why? According to Dr. Parker, appendectomy may work for ulcerative colitis because it’s “a way of suppressing the immune system, especially in the lower intestinal areas.” A 2023 meta-analysis found that people who’d had their appendix removed before being diagnosed with ulcerative colitis were less likely to need their colon removed later on.
Such a procedure may have a serious side effect, however: Colorectal cancer. French scientists discovered that removing the appendix may reduce the numbers of certain immune cells called CD3+ and CD8+ T cells, causing a weakened immune surveillance. As a result, tumor cells might escape detection.
Yet the links between appendix removal and cancer are far from clear. A recent meta-analysis found that while people with appendectomies generally had a higher risk for colorectal cancer, for Europeans, these effects were insignificant. In fact, removal of the appendix actually protected European women from this particular form of cancer. For Parker, such mixed results may stem from the fact that treatments and populations vary widely. The issue “may depend on complex social and medical factors,” Dr. Parker said.
Things also appear complicated with Parkinson’s disease — another condition linked to the appendix. A large epidemiological study showed that appendectomy is associated with a lower risk for Parkinson’s disease and a delayed age of Parkinson’s onset. It also found that a normal appendix contains α-synuclein, a protein that may accumulate in the brain and contribute to the development of Parkinson’s. “Although α-synuclein is toxic when in the brain, it appears to be quite normal when present in the appendix,” said Luis Vitetta, PhD, MD, a clinical epidemiologist at the University of Sydney, Camperdown, Australia. Yet, not all studies find that removing the appendix lowers the risk for Parkinson’s. In fact, some show the opposite results.
How Should Doctors View the Appendix?
Even with these mysteries and contradictions, Dr. Vitetta said, a healthy appendix in a healthy body appears to be protective. This is why, he said, when someone is diagnosed with appendicitis, careful assessment is essential before surgery is performed.
“Perhaps an antibiotic can actually help fix it,” he said. A 2020 study published in The New England Journal of Medicine showed that antibiotics may indeed be a good alternative to surgery for the treatment of appendicitis. “We don’t want necessarily to remove an appendix that could be beneficial,” Dr. Smith said.
The many links between the appendix and various diseases mean that doctors should be more vigilant when treating patients who’ve had this organ removed, Dr. Parker said. “When a patient loses an appendix, depending on their environment, there may be effects on infection and cancer. So they might need more regular checkups,” he said. This could include monitoring for IBS and colorectal cancer.
What’s more, Dr. Parker believes that research on the appendix puts even more emphasis on the need to protect the gut microbiome — such as taking probiotics with antibiotics. And while we are still a long way from understanding how exactly this worm-like structure affects various diseases, one thing appears quite certain: The appendix is not useless. “If Darwin had the information that we have, he would not have drawn these conclusions,” Dr. Parker said.
A version of this article first appeared on Medscape.com.
When doctors and patients consider the appendix, it’s often with urgency. In cases of appendicitis, the clock could be ticking down to a life-threatening burst. Thus, despite recent research suggesting antibiotics could be an alternative therapy, appendectomy remains standard for uncomplicated appendicitis.
But what if removing the appendix could raise the risk for gastrointestinal (GI) diseases like irritable bowel syndrome and colorectal cancer? That’s what some emerging science suggests. And though the research is early and mixed, it’s enough to give some health professionals pause.
“If there’s no reason to remove the appendix, then it’s better to have one,” said Heather Smith, PhD, a comparative anatomist at Midwestern University, Glendale, Arizona. Preemptive removal is not supported by the evidence, she said.
To be fair, we’ve come a long way since 1928, when American physician Miles Breuer, MD, suggested that people with infected appendixes should be left to perish, so as to remove their inferior DNA from the gene pool (he called such people “uncivilized” and “candidates for extinction”). Charles Darwin, while less radical, believed the appendix was at best useless — a mere vestige of our ancestors switching diets from leaves to fruits.
What we know now is that the appendix isn’t just a troublesome piece of worthless flesh. Instead, it may act as a safe house for friendly gut bacteria and a training camp for the immune system. It also appears to play a role in several medical conditions, from ulcerative colitis and colorectal cancer to Parkinson’s disease and lupus. The roughly 300,000 Americans who undergo appendectomy each year should be made aware of this, some experts say. But the frustrating truth is, scientists are still trying to figure out in which cases having an appendix is protective and in which we may be better off without it.
A ‘Worm’ as Intestinal Protection
The appendix is a blind pouch (meaning its ending is closed off) that extends from the large intestine. Not all mammals have one; it’s been found in several species of primates and rodents, as well as in rabbits, wombats, and Florida manatees, among others (dogs and cats don’t have it). While a human appendix “looks like a little worm,” Dr. Smith said, these anatomical structures come in various sizes and shapes. Some are thick, as in a beaver, while others are long and spiraling, like a rabbit’s.
Comparative anatomy studies reveal that the appendix has evolved independently at least 29 times throughout mammalian evolution. This suggests that “it has some kind of an adaptive function,” Dr. Smith said. When French scientists analyzed data from 258 species of mammals, they discovered that those that possess an appendix live longer than those without one. A possible explanation, the researchers wrote, may lie with the appendix’s role in preventing diarrhea.
Their 2023 study supported this hypothesis. Based on veterinary records of 45 different species of primates housed in a French zoo, the scientists established that primates with appendixes are far less likely to suffer severe diarrhea than those that don’t possess this organ. The appendix, it appears, might be our tiny weapon against bowel troubles.
For immunologist William Parker, PhD, a visiting scholar at the University of North Carolina at Chapel Hill, these data are “about as good as we could hope for” in support of the idea that the appendix might protect mammals from GI problems. An experiment on humans would be unethical, Dr. Parker said. But observational studies offer clues.
One study showed that compared with people with an intact appendix, young adults with a history of appendectomy have more than double the risk of developing a serious infection with non-typhoidal Salmonella of the kind that would require hospitalization.
A ‘Safe House’ for Bacteria
Such studies add weight to a theory that Dr. Parker and his colleagues developed back in 2007: That the appendix acts as a “safe house” for beneficial gut bacteria.
Think of the colon as a wide pipe, Dr. Parker said, that may become contaminated with a pathogen such as Salmonella. Diarrhea follows, and the pipe gets repeatedly flushed, wiping everything clean, including your friendly gut microbiome. Luckily, “you’ve got this little offshoot of that pipe,” where the flow can’t really get in “because it’s so constricted,” Dr. Parker said. The friendly gut microbes can survive inside the appendix and repopulate the colon once diarrhea is over. Dr. Parker and his colleagues found that the human appendix contains a thick layer of beneficial bacteria. “They were right where we predicted they would be,” he said.
This safe house hypothesis could explain why the gut microbiome may be different in people who no longer have an appendix. In one small study, people who’d had an appendectomy had a less diverse microbiome, with a lower abundance of beneficial strains such as Butyricicoccus and Barnesiella, than did those with intact appendixes.
The appendix likely has a second function, too, Dr. Smith said: It may serve as a training camp for the immune system. “When there is an invading pathogen in the gut, it helps the GI system to mount the immune response,” she said. The human appendix is rich in special cells known as M cells. These act as scouts, detecting and capturing invasive bacteria and viruses and presenting them to the body’s defense team, such as the T lymphocytes.
If the appendix shelters beneficial bacteria and boosts immune response, that may explain its links to various diseases. According to an epidemiological study from Taiwan,patients who underwent an appendectomy have a 46% higher risk of developing irritable bowel syndrome (IBS) — a disease associated with a low abundance of Butyricicoccus bacteria. This is why, the study authors wrote, doctors should pay careful attention to people who’ve had their appendixes removed, monitoring them for potential symptoms of IBS.
The same database helped uncover other connections between appendectomy and disease. For one, there was type 2 diabetes: Within 3 years of the surgery, patients under 30 had double the risk of developing this disorder. Then there was lupus: While those who underwent appendectomy generally had higher risk for this autoimmune disease, women were particularly affected.
The Contentious Connections
The most heated scientific discussion surrounds the links between the appendix and conditions such as Parkinson’s disease, ulcerative colitis, and colorectal cancer. A small 2019 study showed, for example, that appendectomy may improve symptoms of certain forms of ulcerative colitis that don’t respond to standard medical treatments. A third of patients improved after their appendix was removed, and 17% fully recovered.
Why? According to Dr. Parker, appendectomy may work for ulcerative colitis because it’s “a way of suppressing the immune system, especially in the lower intestinal areas.” A 2023 meta-analysis found that people who’d had their appendix removed before being diagnosed with ulcerative colitis were less likely to need their colon removed later on.
Such a procedure may have a serious side effect, however: Colorectal cancer. French scientists discovered that removing the appendix may reduce the numbers of certain immune cells called CD3+ and CD8+ T cells, causing a weakened immune surveillance. As a result, tumor cells might escape detection.
Yet the links between appendix removal and cancer are far from clear. A recent meta-analysis found that while people with appendectomies generally had a higher risk for colorectal cancer, for Europeans, these effects were insignificant. In fact, removal of the appendix actually protected European women from this particular form of cancer. For Parker, such mixed results may stem from the fact that treatments and populations vary widely. The issue “may depend on complex social and medical factors,” Dr. Parker said.
Things also appear complicated with Parkinson’s disease — another condition linked to the appendix. A large epidemiological study showed that appendectomy is associated with a lower risk for Parkinson’s disease and a delayed age of Parkinson’s onset. It also found that a normal appendix contains α-synuclein, a protein that may accumulate in the brain and contribute to the development of Parkinson’s. “Although α-synuclein is toxic when in the brain, it appears to be quite normal when present in the appendix,” said Luis Vitetta, PhD, MD, a clinical epidemiologist at the University of Sydney, Camperdown, Australia. Yet, not all studies find that removing the appendix lowers the risk for Parkinson’s. In fact, some show the opposite results.
How Should Doctors View the Appendix?
Even with these mysteries and contradictions, Dr. Vitetta said, a healthy appendix in a healthy body appears to be protective. This is why, he said, when someone is diagnosed with appendicitis, careful assessment is essential before surgery is performed.
“Perhaps an antibiotic can actually help fix it,” he said. A 2020 study published in The New England Journal of Medicine showed that antibiotics may indeed be a good alternative to surgery for the treatment of appendicitis. “We don’t want necessarily to remove an appendix that could be beneficial,” Dr. Smith said.
The many links between the appendix and various diseases mean that doctors should be more vigilant when treating patients who’ve had this organ removed, Dr. Parker said. “When a patient loses an appendix, depending on their environment, there may be effects on infection and cancer. So they might need more regular checkups,” he said. This could include monitoring for IBS and colorectal cancer.
What’s more, Dr. Parker believes that research on the appendix puts even more emphasis on the need to protect the gut microbiome — such as taking probiotics with antibiotics. And while we are still a long way from understanding how exactly this worm-like structure affects various diseases, one thing appears quite certain: The appendix is not useless. “If Darwin had the information that we have, he would not have drawn these conclusions,” Dr. Parker said.
A version of this article first appeared on Medscape.com.
Clear Coverage Preference for Humira Over Biosimilars Seen in Most Medicare Part D Plans
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.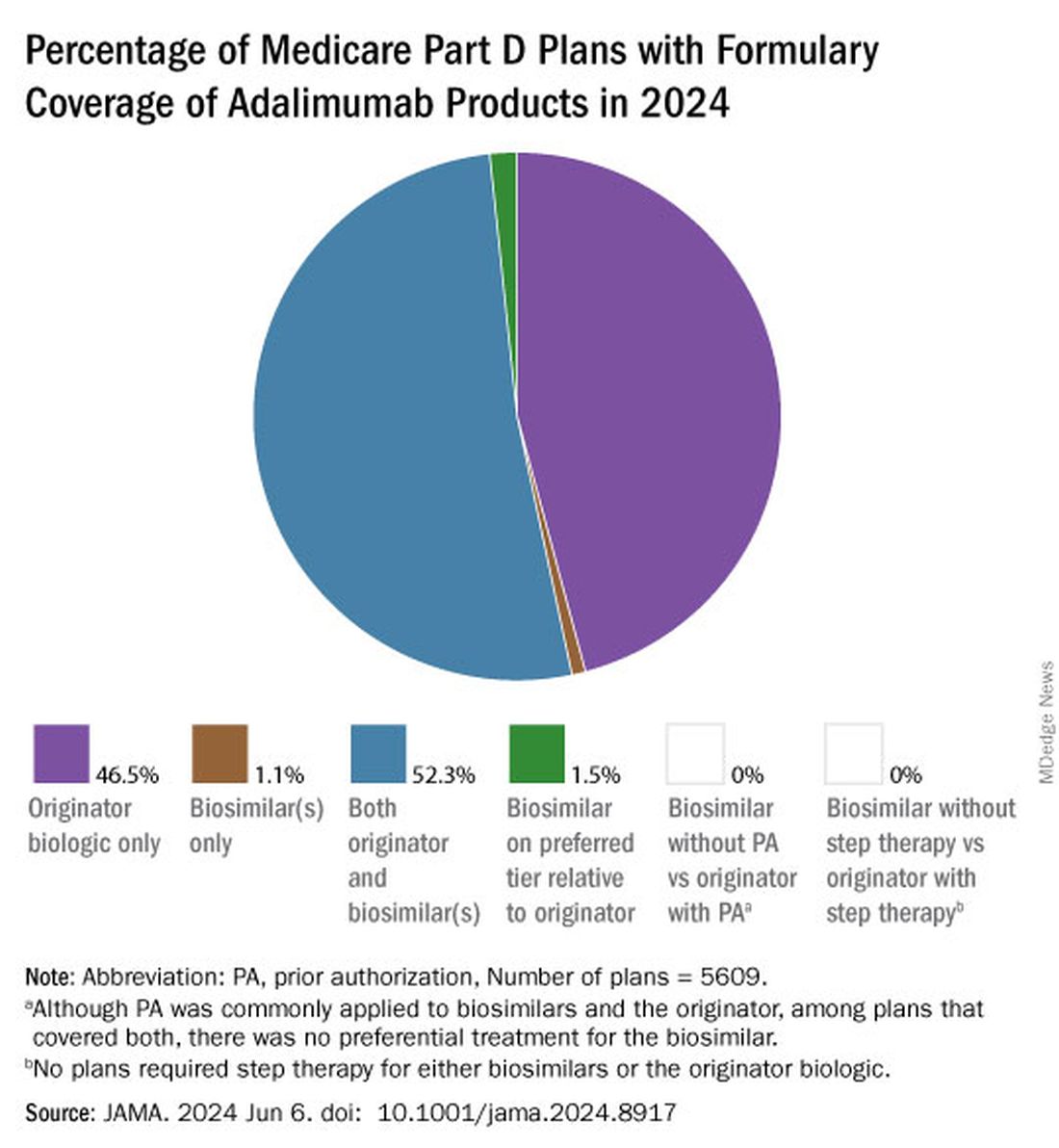
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
FROM JAMA
FDA Grants New Pediatric Arthritis Indications for Upadacitinib
Upadacitinib (Rinvoq) is now indicated for patients aged 2 years or older with active polyarticular juvenile idiopathic arthritis (pJIA) and psoriatic arthritis (PsA) who cannot tolerate or achieve adequate disease response with one or more tumor necrosis factor (TNF) blockers, according to a press release from manufacturer AbbVie.
For the youngest patients, upadacitinib is also available as a weight-based oral solution (Rinvoq LQ) in addition to the previously available tablets, according to the company. JIA, which includes pJIA and juvenile PsA, affects nearly 300,000 children and adolescents in the United States, and alternatives to TNF inhibitor (TNFi) therapy are limited, according to the company.
“Pediatric patients with pJIA and PsA can be severely limited in their ability to complete daily physical tasks and participate in everyday activities. Understanding their needs today and knowing the likelihood of disease in adulthood underscores the need for additional treatment options,” Aarat Patel, MD, a pediatric rheumatologist at Bon Secours Rheumatology Center, Richmond, Virginia, said in the press release. “Having a treatment option available for patients who do not respond well to a TNFi addresses a need for the healthcare community, patients, and their families,” he said.
Upadacitinib, a Janus kinase (JAK) inhibitor, is being studied for multiple immune-mediated inflammatory diseases. The new indication was supported by data from adults with rheumatoid arthritis (RA) and PsA, 51 pediatric patients with pJIA and active polyarthritis, and safety data from 83 pediatric patients aged 2 years to younger than 18 years with pJIA and active polyarthritis.
In the studies, the drug’s safety in pediatric patients was similar to the known safety profile in adults, which includes increased risk for serious infections such as tuberculosis, cancer, immune system problems, blood clots, and serious allergic reactions to components of the drug, according to the press release. However, the safety and effectiveness of upadacitinib for pJIA and PsA in patients younger than 2 years are unknown.
“Upadacitinib plasma exposures in pediatric patients with pJIA and PsA at the recommended dosage are predicted to be comparable to those observed in adults with RA and PsA based on population pharmacokinetic modeling and simulation,” according to the press release.
Currently, upadacitinib’s only other pediatric indication is for moderate to severe atopic dermatitis in children aged 12 years or older. Upadacitinib also is indicated for treatment of adults with moderate to severe RA, active PsA, active ankylosing spondylitis, active nonradiographic axial spondyloarthritis, and moderate to severe ulcerative colitis and Crohn’s disease, but safety and efficacy for its use in treatment of these conditions in children and adolescents is unknown.
Upadacitinib also is being studied in phase 3 trials for treatment of conditions including alopecia areata, ankylosing spondylitis, atopic dermatitis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, hidradenitis suppurativa, psoriatic arthritis, RA, systemic lupus erythematosus, Takayasu arteritis, ulcerative colitis, and vitiligo, according to the press release.
Full prescribing information and safety data for upadacitinib are available here.
A version of this article appeared on Medscape.com.
Upadacitinib (Rinvoq) is now indicated for patients aged 2 years or older with active polyarticular juvenile idiopathic arthritis (pJIA) and psoriatic arthritis (PsA) who cannot tolerate or achieve adequate disease response with one or more tumor necrosis factor (TNF) blockers, according to a press release from manufacturer AbbVie.
For the youngest patients, upadacitinib is also available as a weight-based oral solution (Rinvoq LQ) in addition to the previously available tablets, according to the company. JIA, which includes pJIA and juvenile PsA, affects nearly 300,000 children and adolescents in the United States, and alternatives to TNF inhibitor (TNFi) therapy are limited, according to the company.
“Pediatric patients with pJIA and PsA can be severely limited in their ability to complete daily physical tasks and participate in everyday activities. Understanding their needs today and knowing the likelihood of disease in adulthood underscores the need for additional treatment options,” Aarat Patel, MD, a pediatric rheumatologist at Bon Secours Rheumatology Center, Richmond, Virginia, said in the press release. “Having a treatment option available for patients who do not respond well to a TNFi addresses a need for the healthcare community, patients, and their families,” he said.
Upadacitinib, a Janus kinase (JAK) inhibitor, is being studied for multiple immune-mediated inflammatory diseases. The new indication was supported by data from adults with rheumatoid arthritis (RA) and PsA, 51 pediatric patients with pJIA and active polyarthritis, and safety data from 83 pediatric patients aged 2 years to younger than 18 years with pJIA and active polyarthritis.
In the studies, the drug’s safety in pediatric patients was similar to the known safety profile in adults, which includes increased risk for serious infections such as tuberculosis, cancer, immune system problems, blood clots, and serious allergic reactions to components of the drug, according to the press release. However, the safety and effectiveness of upadacitinib for pJIA and PsA in patients younger than 2 years are unknown.
“Upadacitinib plasma exposures in pediatric patients with pJIA and PsA at the recommended dosage are predicted to be comparable to those observed in adults with RA and PsA based on population pharmacokinetic modeling and simulation,” according to the press release.
Currently, upadacitinib’s only other pediatric indication is for moderate to severe atopic dermatitis in children aged 12 years or older. Upadacitinib also is indicated for treatment of adults with moderate to severe RA, active PsA, active ankylosing spondylitis, active nonradiographic axial spondyloarthritis, and moderate to severe ulcerative colitis and Crohn’s disease, but safety and efficacy for its use in treatment of these conditions in children and adolescents is unknown.
Upadacitinib also is being studied in phase 3 trials for treatment of conditions including alopecia areata, ankylosing spondylitis, atopic dermatitis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, hidradenitis suppurativa, psoriatic arthritis, RA, systemic lupus erythematosus, Takayasu arteritis, ulcerative colitis, and vitiligo, according to the press release.
Full prescribing information and safety data for upadacitinib are available here.
A version of this article appeared on Medscape.com.
Upadacitinib (Rinvoq) is now indicated for patients aged 2 years or older with active polyarticular juvenile idiopathic arthritis (pJIA) and psoriatic arthritis (PsA) who cannot tolerate or achieve adequate disease response with one or more tumor necrosis factor (TNF) blockers, according to a press release from manufacturer AbbVie.
For the youngest patients, upadacitinib is also available as a weight-based oral solution (Rinvoq LQ) in addition to the previously available tablets, according to the company. JIA, which includes pJIA and juvenile PsA, affects nearly 300,000 children and adolescents in the United States, and alternatives to TNF inhibitor (TNFi) therapy are limited, according to the company.
“Pediatric patients with pJIA and PsA can be severely limited in their ability to complete daily physical tasks and participate in everyday activities. Understanding their needs today and knowing the likelihood of disease in adulthood underscores the need for additional treatment options,” Aarat Patel, MD, a pediatric rheumatologist at Bon Secours Rheumatology Center, Richmond, Virginia, said in the press release. “Having a treatment option available for patients who do not respond well to a TNFi addresses a need for the healthcare community, patients, and their families,” he said.
Upadacitinib, a Janus kinase (JAK) inhibitor, is being studied for multiple immune-mediated inflammatory diseases. The new indication was supported by data from adults with rheumatoid arthritis (RA) and PsA, 51 pediatric patients with pJIA and active polyarthritis, and safety data from 83 pediatric patients aged 2 years to younger than 18 years with pJIA and active polyarthritis.
In the studies, the drug’s safety in pediatric patients was similar to the known safety profile in adults, which includes increased risk for serious infections such as tuberculosis, cancer, immune system problems, blood clots, and serious allergic reactions to components of the drug, according to the press release. However, the safety and effectiveness of upadacitinib for pJIA and PsA in patients younger than 2 years are unknown.
“Upadacitinib plasma exposures in pediatric patients with pJIA and PsA at the recommended dosage are predicted to be comparable to those observed in adults with RA and PsA based on population pharmacokinetic modeling and simulation,” according to the press release.
Currently, upadacitinib’s only other pediatric indication is for moderate to severe atopic dermatitis in children aged 12 years or older. Upadacitinib also is indicated for treatment of adults with moderate to severe RA, active PsA, active ankylosing spondylitis, active nonradiographic axial spondyloarthritis, and moderate to severe ulcerative colitis and Crohn’s disease, but safety and efficacy for its use in treatment of these conditions in children and adolescents is unknown.
Upadacitinib also is being studied in phase 3 trials for treatment of conditions including alopecia areata, ankylosing spondylitis, atopic dermatitis, axial spondyloarthritis, Crohn’s disease, giant cell arteritis, hidradenitis suppurativa, psoriatic arthritis, RA, systemic lupus erythematosus, Takayasu arteritis, ulcerative colitis, and vitiligo, according to the press release.
Full prescribing information and safety data for upadacitinib are available here.
A version of this article appeared on Medscape.com.
Inebilizumab ‘MITIGATES’ Flare Risk in IgG4-Related Disease
TOPLINE:
Inebilizumab-cdon, a monoclonal antibody that depletes B cells, reduces the risk for flares without showing any new safety signals in patients with immunoglobulin G4-related disease (IgG4-RD) who have multiorgan disease and are on glucocorticoid therapy.
METHODOLOGY:
- IgG4-RD is an immune-mediated, fibroinflammatory condition that affects multiple organs, causing irreversible organ damage. MITIGATE is the first multinational, placebo-controlled trial involving patients with IgG4-RD.
- Researchers evaluated the efficacy and safety of inebilizumab in 135 adult patients at risk for flares due to a history of multiorgan disease and active disease requiring treatment with glucocorticoids.
- The patients were randomly assigned to receive 300-mg intravenous inebilizumab or placebo on day 1, day 15, and week 26.
- The primary endpoint was the time to the first treated and adjudicated IgG4-RD flare within 52 weeks.
- The secondary endpoints included the annualized flare rate, flare-free and treatment-free complete remission, and flare-free and corticosteroid-free complete remission.
TAKEAWAY:
- Compared with the placebo, inebilizumab reduced the risk for IgG4-RD flares by 87% during the 52-week trial period (hazard ratio, 0.13; P < .0001).
- All the secondary endpoints showed improvement following treatment with inebilizumab.
- The most common adverse reactions with inebilizumab, as observed in a previous trial for neuromyelitis optica spectrum disorder, were urinary tract infection and arthralgia.
- There were no new safety signals in the MITIGATE trial.
IN PRACTICE:
“These data mark a major milestone for the IgG4-RD community and provide substantial insight into not only how inebilizumab can help manage IgG4-RD but also key insights into the nature of this condition,” John Stone, MD, MPH, principal investigator, said in a news release.
SOURCE:
Dr. Stone, a professor of medicine at the Harvard Medical School and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital, Boston, led this study.
LIMITATIONS:
This press release did not discuss any limitations of the current study.
DISCLOSURES:
This study was funded by Mitsubishi Tanabe Pharma and Hansoh Pharma and sponsored by Amgen. The author disclosures were not available.
A version of this article appeared on Medscape.com.
TOPLINE:
Inebilizumab-cdon, a monoclonal antibody that depletes B cells, reduces the risk for flares without showing any new safety signals in patients with immunoglobulin G4-related disease (IgG4-RD) who have multiorgan disease and are on glucocorticoid therapy.
METHODOLOGY:
- IgG4-RD is an immune-mediated, fibroinflammatory condition that affects multiple organs, causing irreversible organ damage. MITIGATE is the first multinational, placebo-controlled trial involving patients with IgG4-RD.
- Researchers evaluated the efficacy and safety of inebilizumab in 135 adult patients at risk for flares due to a history of multiorgan disease and active disease requiring treatment with glucocorticoids.
- The patients were randomly assigned to receive 300-mg intravenous inebilizumab or placebo on day 1, day 15, and week 26.
- The primary endpoint was the time to the first treated and adjudicated IgG4-RD flare within 52 weeks.
- The secondary endpoints included the annualized flare rate, flare-free and treatment-free complete remission, and flare-free and corticosteroid-free complete remission.
TAKEAWAY:
- Compared with the placebo, inebilizumab reduced the risk for IgG4-RD flares by 87% during the 52-week trial period (hazard ratio, 0.13; P < .0001).
- All the secondary endpoints showed improvement following treatment with inebilizumab.
- The most common adverse reactions with inebilizumab, as observed in a previous trial for neuromyelitis optica spectrum disorder, were urinary tract infection and arthralgia.
- There were no new safety signals in the MITIGATE trial.
IN PRACTICE:
“These data mark a major milestone for the IgG4-RD community and provide substantial insight into not only how inebilizumab can help manage IgG4-RD but also key insights into the nature of this condition,” John Stone, MD, MPH, principal investigator, said in a news release.
SOURCE:
Dr. Stone, a professor of medicine at the Harvard Medical School and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital, Boston, led this study.
LIMITATIONS:
This press release did not discuss any limitations of the current study.
DISCLOSURES:
This study was funded by Mitsubishi Tanabe Pharma and Hansoh Pharma and sponsored by Amgen. The author disclosures were not available.
A version of this article appeared on Medscape.com.
TOPLINE:
Inebilizumab-cdon, a monoclonal antibody that depletes B cells, reduces the risk for flares without showing any new safety signals in patients with immunoglobulin G4-related disease (IgG4-RD) who have multiorgan disease and are on glucocorticoid therapy.
METHODOLOGY:
- IgG4-RD is an immune-mediated, fibroinflammatory condition that affects multiple organs, causing irreversible organ damage. MITIGATE is the first multinational, placebo-controlled trial involving patients with IgG4-RD.
- Researchers evaluated the efficacy and safety of inebilizumab in 135 adult patients at risk for flares due to a history of multiorgan disease and active disease requiring treatment with glucocorticoids.
- The patients were randomly assigned to receive 300-mg intravenous inebilizumab or placebo on day 1, day 15, and week 26.
- The primary endpoint was the time to the first treated and adjudicated IgG4-RD flare within 52 weeks.
- The secondary endpoints included the annualized flare rate, flare-free and treatment-free complete remission, and flare-free and corticosteroid-free complete remission.
TAKEAWAY:
- Compared with the placebo, inebilizumab reduced the risk for IgG4-RD flares by 87% during the 52-week trial period (hazard ratio, 0.13; P < .0001).
- All the secondary endpoints showed improvement following treatment with inebilizumab.
- The most common adverse reactions with inebilizumab, as observed in a previous trial for neuromyelitis optica spectrum disorder, were urinary tract infection and arthralgia.
- There were no new safety signals in the MITIGATE trial.
IN PRACTICE:
“These data mark a major milestone for the IgG4-RD community and provide substantial insight into not only how inebilizumab can help manage IgG4-RD but also key insights into the nature of this condition,” John Stone, MD, MPH, principal investigator, said in a news release.
SOURCE:
Dr. Stone, a professor of medicine at the Harvard Medical School and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital, Boston, led this study.
LIMITATIONS:
This press release did not discuss any limitations of the current study.
DISCLOSURES:
This study was funded by Mitsubishi Tanabe Pharma and Hansoh Pharma and sponsored by Amgen. The author disclosures were not available.
A version of this article appeared on Medscape.com.
Over-the-Counter Arthritis Supplements Pose Adrenal Danger
BOSTON —
Patients who have been taking these supplements for prolonged periods must slowly taper off them with corticosteroid replacement, because abruptly stopping the supplement can precipitate AI, Kevin S. Wei, MD, said in a presentation of 12 cases — the largest such series to date of the phenomenon — at the annual meeting of the Endocrine Society.
The specific supplements used were Artri King in eight of the patients, Ardosons in two, and Ajo Rey in one. In April 2022, the US Food and Drug Administration issued a warning that Artri King contains diclofenac and dexamethasone not listed on the product label. In July 2023, the agency issued an expanded warning about that product and others including Ajo Rey.
The supplements are not believed to be sold in the United States, but they are available in Mexico and can be ordered online, said Dr. Wei, a second-year resident at the Keck School of Medicine at the University of California, Los Angeles.
“We found that quite a lot of patients after they’ve been on the Artri King or some other over the counter arthritis supplement, started developing these cushingoid features seen in the physical exam, such as rounded facial features or stretch marks of their abdomen,” he said.
And “when patients are abruptly taken off those supplements … sometimes this can cause them to go into signs or symptoms of adrenal insufficiency. That can occasionally be life-threatening if it’s not addressed in an inpatient setting,” Dr. Wei said.
In an interview, session moderator Sharon L. Wardlaw, MD, professor of medicine at Columbia University Irving Medical Center, New York, explained that when a person takes these drugs containing hidden glucocorticoids, “they won’t be picked up in a cortisol assay, but they’ll suppress the [adrenocorticotropic hormone] and the person’s own cortisol production. They look like they have Cushing, but when you measure their hormone levels, they’re undetectable. And then people wonder what’s going on. Well, their [hypothalamic-pituitary-adrenal] axis is suppressed.”
But if the product is suddenly stopped without cortisol replacement “If they get an infection they can die because they can’t mount a cortisol response.”
The takeaway message, she said, is “always ask patients to show you their supplements and look at them. In many cases, that’s why they work so well for pain relief because they have ingredients that people shouldn’t be taking.”
Twelve Patients Seen During 2022-2023
The 12 patients were seen during 2022-2023 at an endocrinology consult service in an urban safety net hospital. Their median age was 52 years, and one third were women. All had started using the supplements for joint pain, with a median of about 6 months of use prior to cessation.
Presenting symptoms included nausea/vomiting in 42%, fatigue in 42%, abdominal pain in 33%, and dizziness in 17%. Physical exam findings included moon facies in 66%, central adiposity in 66%, abdominal striae in 50%, dorsocervical fat pad in 33%, and bruising in 33%. Three required intensive care admission.
Cortisol testing was performed in 11 of the patients and was normal (≥ 16 mcg/dL) in just one. AI (≤ 3 mcg/dL) was found in three, while the rest had indeterminate results. Of those seven patients, subsequent cosyntropin-stimulation testing suggested AI (cortisol < 16 mcg/dL at 60 minutes post stimulation) in four patients, while the other two showed reduced but normal responses (cortisol 18.2-18.4 mcg/dL).
Ten of the 12 patients were prescribed glucocorticoid tapering replacements to avoid precipitating adrenal crisis, most commonly twice-daily hydrocortisone. Of those ten, eight continued to take the replacement steroids 1-2 years later, Dr. Wei said.
Dr. Wei and Dr. Wardlaw had no disclosures.
A version of this article appeared on Medscape.com.
BOSTON —
Patients who have been taking these supplements for prolonged periods must slowly taper off them with corticosteroid replacement, because abruptly stopping the supplement can precipitate AI, Kevin S. Wei, MD, said in a presentation of 12 cases — the largest such series to date of the phenomenon — at the annual meeting of the Endocrine Society.
The specific supplements used were Artri King in eight of the patients, Ardosons in two, and Ajo Rey in one. In April 2022, the US Food and Drug Administration issued a warning that Artri King contains diclofenac and dexamethasone not listed on the product label. In July 2023, the agency issued an expanded warning about that product and others including Ajo Rey.
The supplements are not believed to be sold in the United States, but they are available in Mexico and can be ordered online, said Dr. Wei, a second-year resident at the Keck School of Medicine at the University of California, Los Angeles.
“We found that quite a lot of patients after they’ve been on the Artri King or some other over the counter arthritis supplement, started developing these cushingoid features seen in the physical exam, such as rounded facial features or stretch marks of their abdomen,” he said.
And “when patients are abruptly taken off those supplements … sometimes this can cause them to go into signs or symptoms of adrenal insufficiency. That can occasionally be life-threatening if it’s not addressed in an inpatient setting,” Dr. Wei said.
In an interview, session moderator Sharon L. Wardlaw, MD, professor of medicine at Columbia University Irving Medical Center, New York, explained that when a person takes these drugs containing hidden glucocorticoids, “they won’t be picked up in a cortisol assay, but they’ll suppress the [adrenocorticotropic hormone] and the person’s own cortisol production. They look like they have Cushing, but when you measure their hormone levels, they’re undetectable. And then people wonder what’s going on. Well, their [hypothalamic-pituitary-adrenal] axis is suppressed.”
But if the product is suddenly stopped without cortisol replacement “If they get an infection they can die because they can’t mount a cortisol response.”
The takeaway message, she said, is “always ask patients to show you their supplements and look at them. In many cases, that’s why they work so well for pain relief because they have ingredients that people shouldn’t be taking.”
Twelve Patients Seen During 2022-2023
The 12 patients were seen during 2022-2023 at an endocrinology consult service in an urban safety net hospital. Their median age was 52 years, and one third were women. All had started using the supplements for joint pain, with a median of about 6 months of use prior to cessation.
Presenting symptoms included nausea/vomiting in 42%, fatigue in 42%, abdominal pain in 33%, and dizziness in 17%. Physical exam findings included moon facies in 66%, central adiposity in 66%, abdominal striae in 50%, dorsocervical fat pad in 33%, and bruising in 33%. Three required intensive care admission.
Cortisol testing was performed in 11 of the patients and was normal (≥ 16 mcg/dL) in just one. AI (≤ 3 mcg/dL) was found in three, while the rest had indeterminate results. Of those seven patients, subsequent cosyntropin-stimulation testing suggested AI (cortisol < 16 mcg/dL at 60 minutes post stimulation) in four patients, while the other two showed reduced but normal responses (cortisol 18.2-18.4 mcg/dL).
Ten of the 12 patients were prescribed glucocorticoid tapering replacements to avoid precipitating adrenal crisis, most commonly twice-daily hydrocortisone. Of those ten, eight continued to take the replacement steroids 1-2 years later, Dr. Wei said.
Dr. Wei and Dr. Wardlaw had no disclosures.
A version of this article appeared on Medscape.com.
BOSTON —
Patients who have been taking these supplements for prolonged periods must slowly taper off them with corticosteroid replacement, because abruptly stopping the supplement can precipitate AI, Kevin S. Wei, MD, said in a presentation of 12 cases — the largest such series to date of the phenomenon — at the annual meeting of the Endocrine Society.
The specific supplements used were Artri King in eight of the patients, Ardosons in two, and Ajo Rey in one. In April 2022, the US Food and Drug Administration issued a warning that Artri King contains diclofenac and dexamethasone not listed on the product label. In July 2023, the agency issued an expanded warning about that product and others including Ajo Rey.
The supplements are not believed to be sold in the United States, but they are available in Mexico and can be ordered online, said Dr. Wei, a second-year resident at the Keck School of Medicine at the University of California, Los Angeles.
“We found that quite a lot of patients after they’ve been on the Artri King or some other over the counter arthritis supplement, started developing these cushingoid features seen in the physical exam, such as rounded facial features or stretch marks of their abdomen,” he said.
And “when patients are abruptly taken off those supplements … sometimes this can cause them to go into signs or symptoms of adrenal insufficiency. That can occasionally be life-threatening if it’s not addressed in an inpatient setting,” Dr. Wei said.
In an interview, session moderator Sharon L. Wardlaw, MD, professor of medicine at Columbia University Irving Medical Center, New York, explained that when a person takes these drugs containing hidden glucocorticoids, “they won’t be picked up in a cortisol assay, but they’ll suppress the [adrenocorticotropic hormone] and the person’s own cortisol production. They look like they have Cushing, but when you measure their hormone levels, they’re undetectable. And then people wonder what’s going on. Well, their [hypothalamic-pituitary-adrenal] axis is suppressed.”
But if the product is suddenly stopped without cortisol replacement “If they get an infection they can die because they can’t mount a cortisol response.”
The takeaway message, she said, is “always ask patients to show you their supplements and look at them. In many cases, that’s why they work so well for pain relief because they have ingredients that people shouldn’t be taking.”
Twelve Patients Seen During 2022-2023
The 12 patients were seen during 2022-2023 at an endocrinology consult service in an urban safety net hospital. Their median age was 52 years, and one third were women. All had started using the supplements for joint pain, with a median of about 6 months of use prior to cessation.
Presenting symptoms included nausea/vomiting in 42%, fatigue in 42%, abdominal pain in 33%, and dizziness in 17%. Physical exam findings included moon facies in 66%, central adiposity in 66%, abdominal striae in 50%, dorsocervical fat pad in 33%, and bruising in 33%. Three required intensive care admission.
Cortisol testing was performed in 11 of the patients and was normal (≥ 16 mcg/dL) in just one. AI (≤ 3 mcg/dL) was found in three, while the rest had indeterminate results. Of those seven patients, subsequent cosyntropin-stimulation testing suggested AI (cortisol < 16 mcg/dL at 60 minutes post stimulation) in four patients, while the other two showed reduced but normal responses (cortisol 18.2-18.4 mcg/dL).
Ten of the 12 patients were prescribed glucocorticoid tapering replacements to avoid precipitating adrenal crisis, most commonly twice-daily hydrocortisone. Of those ten, eight continued to take the replacement steroids 1-2 years later, Dr. Wei said.
Dr. Wei and Dr. Wardlaw had no disclosures.
A version of this article appeared on Medscape.com.





