User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
The good old days
“It’s good to be in something from the ground floor. I came too late for that. ... But lately, I’m getting the feeling that I came in at the end. The best is over.” –Tony Soprano
For me, I’m unsure. Sometimes it feels like our best days are behind us. When I was a kid, we explored life in pond water, watching water fleas and hydra swim under our Child World toy microscopes. Today, kids learn to eat Tide Pods from TikTok. Back when I was young, a doctor’s appointment was a special occasion! My brothers and I had a bath and got dressed in our Sunday best for our appointment with Dr. Bellin, a genteel, gray-haired pediatrician who worked out of his Victorian office with wooden floors and crystal door handles. Contrast that with the appointment I had with a patient the other day, done by telephone while she was in line ordering at Starbucks. I waited patiently for her to give her order.
This ache I feel for the past is called nostalgia. At one time, it was a diagnosable condition. It was first used by Dr. Johannes Hofer in the 17th century to describe Swiss soldiers fighting in foreign lands. From the Greek, it means “homecoming pain.” Although over time nostalgia has lost its clinical meaning, the feeling of yearning for the past has dramatically gained in prevalence. The word “nostalgia” appears more in print now than at any point since 1800. We are most nostalgic during times of duress, it seems. This, no doubt, is because it’s comforting to think we’d be better off back in pastoral, idyll times, back when work ended at 5 p.m. and cotton balls were soaked in alcohol and office visits ended with a lollipop on a loop.
Of course, the good old days weren’t really better. We have a selective view of history – as many things were contemptible or bad then as now. Yes, Dr. Bellin was the consummate professional, but thank goodness, I didn’t have acute lymphocytic leukemia or Haemophilus influenzae type B or even suffocate under a pile of blankets while sleeping on my stomach. Without doubt, clinically we’re much better today. Also back then, there was hardly a consideration for atrocious racial disparities in care. We’ve not come far, but we are at least better off today than a few decades ago. And what about medicine as a profession? Although he had loads of autonomy and respect, Dr. Bellin also started every day of his 50-year career at 6 a.m. rounding in the newborn nursery before seeing patients in the office 6 days a week. Not many of us would trade our practice for his.
Yet, there’s reasons to be nostalgic. Chart notes might have been barely legible, but at least they served a purpose. The problem-oriented medical record was intended to logically capture and organize data. SOAP notes were invented to help us think better, to get diagnoses correct, to succinctly see progress. Today, notes are written for administrators and payers and patients. As a result, they’re often useless to us.
And although it may have been inconvenient to sit in the waiting room reading Highlights magazine, I’m unsure it was a worse user experience, compared with a chain pharmacy “virtual” doctor visit. (Particularly because you could always drop pennies down the large hot-air iron floor grate in the corner).
The thrumming undercurrent of progress promises artificial intelligence and genomics and wearable diagnostics in our future. But the assumption is the new things will be better suited to our needs than the old. Sometimes, they are not. Sometimes technology diminishes us instead of enhancing us.
I cannot count how many times I’ve hit my head or whacked my shin because our Tesla Model X doors open by magic and of their own accord. Back when I was young, we opened car doors by pulling on the door handle. I sometimes miss those days.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“It’s good to be in something from the ground floor. I came too late for that. ... But lately, I’m getting the feeling that I came in at the end. The best is over.” –Tony Soprano
For me, I’m unsure. Sometimes it feels like our best days are behind us. When I was a kid, we explored life in pond water, watching water fleas and hydra swim under our Child World toy microscopes. Today, kids learn to eat Tide Pods from TikTok. Back when I was young, a doctor’s appointment was a special occasion! My brothers and I had a bath and got dressed in our Sunday best for our appointment with Dr. Bellin, a genteel, gray-haired pediatrician who worked out of his Victorian office with wooden floors and crystal door handles. Contrast that with the appointment I had with a patient the other day, done by telephone while she was in line ordering at Starbucks. I waited patiently for her to give her order.
This ache I feel for the past is called nostalgia. At one time, it was a diagnosable condition. It was first used by Dr. Johannes Hofer in the 17th century to describe Swiss soldiers fighting in foreign lands. From the Greek, it means “homecoming pain.” Although over time nostalgia has lost its clinical meaning, the feeling of yearning for the past has dramatically gained in prevalence. The word “nostalgia” appears more in print now than at any point since 1800. We are most nostalgic during times of duress, it seems. This, no doubt, is because it’s comforting to think we’d be better off back in pastoral, idyll times, back when work ended at 5 p.m. and cotton balls were soaked in alcohol and office visits ended with a lollipop on a loop.
Of course, the good old days weren’t really better. We have a selective view of history – as many things were contemptible or bad then as now. Yes, Dr. Bellin was the consummate professional, but thank goodness, I didn’t have acute lymphocytic leukemia or Haemophilus influenzae type B or even suffocate under a pile of blankets while sleeping on my stomach. Without doubt, clinically we’re much better today. Also back then, there was hardly a consideration for atrocious racial disparities in care. We’ve not come far, but we are at least better off today than a few decades ago. And what about medicine as a profession? Although he had loads of autonomy and respect, Dr. Bellin also started every day of his 50-year career at 6 a.m. rounding in the newborn nursery before seeing patients in the office 6 days a week. Not many of us would trade our practice for his.
Yet, there’s reasons to be nostalgic. Chart notes might have been barely legible, but at least they served a purpose. The problem-oriented medical record was intended to logically capture and organize data. SOAP notes were invented to help us think better, to get diagnoses correct, to succinctly see progress. Today, notes are written for administrators and payers and patients. As a result, they’re often useless to us.
And although it may have been inconvenient to sit in the waiting room reading Highlights magazine, I’m unsure it was a worse user experience, compared with a chain pharmacy “virtual” doctor visit. (Particularly because you could always drop pennies down the large hot-air iron floor grate in the corner).
The thrumming undercurrent of progress promises artificial intelligence and genomics and wearable diagnostics in our future. But the assumption is the new things will be better suited to our needs than the old. Sometimes, they are not. Sometimes technology diminishes us instead of enhancing us.
I cannot count how many times I’ve hit my head or whacked my shin because our Tesla Model X doors open by magic and of their own accord. Back when I was young, we opened car doors by pulling on the door handle. I sometimes miss those days.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“It’s good to be in something from the ground floor. I came too late for that. ... But lately, I’m getting the feeling that I came in at the end. The best is over.” –Tony Soprano
For me, I’m unsure. Sometimes it feels like our best days are behind us. When I was a kid, we explored life in pond water, watching water fleas and hydra swim under our Child World toy microscopes. Today, kids learn to eat Tide Pods from TikTok. Back when I was young, a doctor’s appointment was a special occasion! My brothers and I had a bath and got dressed in our Sunday best for our appointment with Dr. Bellin, a genteel, gray-haired pediatrician who worked out of his Victorian office with wooden floors and crystal door handles. Contrast that with the appointment I had with a patient the other day, done by telephone while she was in line ordering at Starbucks. I waited patiently for her to give her order.
This ache I feel for the past is called nostalgia. At one time, it was a diagnosable condition. It was first used by Dr. Johannes Hofer in the 17th century to describe Swiss soldiers fighting in foreign lands. From the Greek, it means “homecoming pain.” Although over time nostalgia has lost its clinical meaning, the feeling of yearning for the past has dramatically gained in prevalence. The word “nostalgia” appears more in print now than at any point since 1800. We are most nostalgic during times of duress, it seems. This, no doubt, is because it’s comforting to think we’d be better off back in pastoral, idyll times, back when work ended at 5 p.m. and cotton balls were soaked in alcohol and office visits ended with a lollipop on a loop.
Of course, the good old days weren’t really better. We have a selective view of history – as many things were contemptible or bad then as now. Yes, Dr. Bellin was the consummate professional, but thank goodness, I didn’t have acute lymphocytic leukemia or Haemophilus influenzae type B or even suffocate under a pile of blankets while sleeping on my stomach. Without doubt, clinically we’re much better today. Also back then, there was hardly a consideration for atrocious racial disparities in care. We’ve not come far, but we are at least better off today than a few decades ago. And what about medicine as a profession? Although he had loads of autonomy and respect, Dr. Bellin also started every day of his 50-year career at 6 a.m. rounding in the newborn nursery before seeing patients in the office 6 days a week. Not many of us would trade our practice for his.
Yet, there’s reasons to be nostalgic. Chart notes might have been barely legible, but at least they served a purpose. The problem-oriented medical record was intended to logically capture and organize data. SOAP notes were invented to help us think better, to get diagnoses correct, to succinctly see progress. Today, notes are written for administrators and payers and patients. As a result, they’re often useless to us.
And although it may have been inconvenient to sit in the waiting room reading Highlights magazine, I’m unsure it was a worse user experience, compared with a chain pharmacy “virtual” doctor visit. (Particularly because you could always drop pennies down the large hot-air iron floor grate in the corner).
The thrumming undercurrent of progress promises artificial intelligence and genomics and wearable diagnostics in our future. But the assumption is the new things will be better suited to our needs than the old. Sometimes, they are not. Sometimes technology diminishes us instead of enhancing us.
I cannot count how many times I’ve hit my head or whacked my shin because our Tesla Model X doors open by magic and of their own accord. Back when I was young, we opened car doors by pulling on the door handle. I sometimes miss those days.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Incorporating self-care, wellness into routines can prevent doctors’ burnout
Gradually, we are emerging from the chaos, isolation, and anxiety of COVID-19. As the Centers for Disease Control and Prevention adjusts its recommendations and vaccinations become more widely available, our communities are beginning to return to normalcy. We are encouraged to put aside our masks if vaccinated and rejoin society, to venture out with less hesitancy and anxiety. As family and friends reunite, memories of confusion, frustration, and fear are beginning to fade to black. Despite the prevailing belief that we should move on, look forward, and remember the past to safeguard our future, remnants of the pandemic remain.
Unvaccinated individuals, notably children under the age of 12, are quite significant in number. The use of telehealth is now standard practice.
For several years, we were warned about the looming “mental health crisis.” The past year has demonstrated that a crisis no longer looms – it has arrived. Our patients can reveal the vulnerability COVID-19 has wrought – from the devastation of lives lost, supply shortages, loss of employment and financial stability – to a lack of access to computers and thereby, the risk of educational decline. Those factors, coupled with isolation and uncertainty about the future, have led to an influx of individuals with anxiety, depression, and other mood disorders seeking mental health treatment.
Doctors, others suffering
As result of a medical culture guided by the sacred oath to which care, compassion, and dedication held as true in ancient Greece as it does today, the focus centers on those around us – while signs of our own weariness are waved away as “a bad day.” Even though several support groups are readily available to offer a listening ear and mental health physicians who focus on the treatment of health care professionals are becoming more ubiquitous, the vestiges of past doctrine remain.
In this modern age of medical training, there is often as much sacrifice as there is attainment of knowledge. This philosophy is so ingrained that throughout training and practice one may come across colleagues experiencing an abundance of guilt when leave is needed for personal reasons. We are quick to recommend such steps for our patients, family, and friends, but hesitant to consider such for ourselves. Yet, of all the lessons this past year has wrought, the importance of mental health and self-care cannot be overstated. This raises the question:
It is vital to accept our humanity as something not to repair, treat, or overcome but to understand. There is strength and power in vulnerability. If we do not perceive and validate this process within ourselves, how can we do so for others? In other words, the oxygen mask must be placed on us first before we can place it on anyone else – patients or otherwise.
Chiefly and above all else, the importance of identifying individual signs of stress is essential. Where do you hold tension? Are you prone to GI distress or headaches when taxed? Do you tend toward irritability, apathy, or exhaustion?
Once this is determined, it is important to assess your stress on a numerical scale, such as those used for pain. Are you a 5 or an 8? Finally, are there identifiable triggers or reliable alleviators? Is there a time of day or day of the week that is most difficult to manage? Can you anticipate potential stressors? Understanding your triggers, listening to your body, and practicing the language of self is the first step toward wellness.
Following introspection and observation, the next step is inventory. Take stock of your reserves. What replenishes? What depletes? What brings joy? What brings dread? Are there certain activities that mitigate stress? If so, how much time do they entail? Identify your number on a scale and associate that number with specific strategies or techniques. Remember that decompression for a 6 might be excessive for a 4. Furthermore, what is the duration of these feelings? Chronic stressors may incur gradual change verses sudden impact if acute. Through identifying personal signs, devising and using a scale, as well as escalating or de-escalating factors, individuals become more in tune with their bodies and therefore, more likely to intervene before burnout takes hold.
With this process well integrated, one can now consider stylized approaches for stress management. For example, those inclined toward mindfulness practices may find yoga, meditation, and relaxation exercises beneficial. Others may thrive on positive affirmations, gratitude, and thankfulness. While some might find relief in physical activity, be it strenuous or casual, the creative arts might appeal to those who find joy in painting, writing, or doing crafts. In addition, baking, reading, dancing, and/or listening to music might help lift stress.
Along with those discoveries, or in some cases, rediscoveries, basic needs such as dietary habits and nutrition, hydration, and sleep are vital toward emotional regulation, physiological homeostasis, and stress modulation. Remember HALT: Hungry, Angry, Lonely, Tired, Too hot, Too cold, Sad or Stressed. Those strategies are meant to guide self-care and highlight the importance of allowing time for self-awareness. Imagine yourself as if you are meeting a new patient. Establish rapport, identify symptoms, and explore options for treatment. When we give time to ourselves, we can give time more freely to others. With this in mind, try following the 5-minute wellness check that I formulated:
1. How am I feeling? What am I feeling?
2. Assess HALTS.
3. Identify the number on your scale.
4. Methods of quick de-escalation:
- Designate and schedule personal time.
- Write down daily goals.
- Repeat positive affirmations or write down words of gratitude.
- Use deep breathing exercises.
- Stretch or take a brief walk.
- Engage in mindfulness practices, such as meditation.
Once we develop a habit of monitoring, assessing, and practicing self-care, the process becomes more efficient and effective. Think of the way a seasoned attending can manage workflow with ease, compared with an intern. Recognizing signs and using these strategies routinely can become a quick daily measure of well-being.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
Gradually, we are emerging from the chaos, isolation, and anxiety of COVID-19. As the Centers for Disease Control and Prevention adjusts its recommendations and vaccinations become more widely available, our communities are beginning to return to normalcy. We are encouraged to put aside our masks if vaccinated and rejoin society, to venture out with less hesitancy and anxiety. As family and friends reunite, memories of confusion, frustration, and fear are beginning to fade to black. Despite the prevailing belief that we should move on, look forward, and remember the past to safeguard our future, remnants of the pandemic remain.
Unvaccinated individuals, notably children under the age of 12, are quite significant in number. The use of telehealth is now standard practice.
For several years, we were warned about the looming “mental health crisis.” The past year has demonstrated that a crisis no longer looms – it has arrived. Our patients can reveal the vulnerability COVID-19 has wrought – from the devastation of lives lost, supply shortages, loss of employment and financial stability – to a lack of access to computers and thereby, the risk of educational decline. Those factors, coupled with isolation and uncertainty about the future, have led to an influx of individuals with anxiety, depression, and other mood disorders seeking mental health treatment.
Doctors, others suffering
As result of a medical culture guided by the sacred oath to which care, compassion, and dedication held as true in ancient Greece as it does today, the focus centers on those around us – while signs of our own weariness are waved away as “a bad day.” Even though several support groups are readily available to offer a listening ear and mental health physicians who focus on the treatment of health care professionals are becoming more ubiquitous, the vestiges of past doctrine remain.
In this modern age of medical training, there is often as much sacrifice as there is attainment of knowledge. This philosophy is so ingrained that throughout training and practice one may come across colleagues experiencing an abundance of guilt when leave is needed for personal reasons. We are quick to recommend such steps for our patients, family, and friends, but hesitant to consider such for ourselves. Yet, of all the lessons this past year has wrought, the importance of mental health and self-care cannot be overstated. This raises the question:
It is vital to accept our humanity as something not to repair, treat, or overcome but to understand. There is strength and power in vulnerability. If we do not perceive and validate this process within ourselves, how can we do so for others? In other words, the oxygen mask must be placed on us first before we can place it on anyone else – patients or otherwise.
Chiefly and above all else, the importance of identifying individual signs of stress is essential. Where do you hold tension? Are you prone to GI distress or headaches when taxed? Do you tend toward irritability, apathy, or exhaustion?
Once this is determined, it is important to assess your stress on a numerical scale, such as those used for pain. Are you a 5 or an 8? Finally, are there identifiable triggers or reliable alleviators? Is there a time of day or day of the week that is most difficult to manage? Can you anticipate potential stressors? Understanding your triggers, listening to your body, and practicing the language of self is the first step toward wellness.
Following introspection and observation, the next step is inventory. Take stock of your reserves. What replenishes? What depletes? What brings joy? What brings dread? Are there certain activities that mitigate stress? If so, how much time do they entail? Identify your number on a scale and associate that number with specific strategies or techniques. Remember that decompression for a 6 might be excessive for a 4. Furthermore, what is the duration of these feelings? Chronic stressors may incur gradual change verses sudden impact if acute. Through identifying personal signs, devising and using a scale, as well as escalating or de-escalating factors, individuals become more in tune with their bodies and therefore, more likely to intervene before burnout takes hold.
With this process well integrated, one can now consider stylized approaches for stress management. For example, those inclined toward mindfulness practices may find yoga, meditation, and relaxation exercises beneficial. Others may thrive on positive affirmations, gratitude, and thankfulness. While some might find relief in physical activity, be it strenuous or casual, the creative arts might appeal to those who find joy in painting, writing, or doing crafts. In addition, baking, reading, dancing, and/or listening to music might help lift stress.
Along with those discoveries, or in some cases, rediscoveries, basic needs such as dietary habits and nutrition, hydration, and sleep are vital toward emotional regulation, physiological homeostasis, and stress modulation. Remember HALT: Hungry, Angry, Lonely, Tired, Too hot, Too cold, Sad or Stressed. Those strategies are meant to guide self-care and highlight the importance of allowing time for self-awareness. Imagine yourself as if you are meeting a new patient. Establish rapport, identify symptoms, and explore options for treatment. When we give time to ourselves, we can give time more freely to others. With this in mind, try following the 5-minute wellness check that I formulated:
1. How am I feeling? What am I feeling?
2. Assess HALTS.
3. Identify the number on your scale.
4. Methods of quick de-escalation:
- Designate and schedule personal time.
- Write down daily goals.
- Repeat positive affirmations or write down words of gratitude.
- Use deep breathing exercises.
- Stretch or take a brief walk.
- Engage in mindfulness practices, such as meditation.
Once we develop a habit of monitoring, assessing, and practicing self-care, the process becomes more efficient and effective. Think of the way a seasoned attending can manage workflow with ease, compared with an intern. Recognizing signs and using these strategies routinely can become a quick daily measure of well-being.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
Gradually, we are emerging from the chaos, isolation, and anxiety of COVID-19. As the Centers for Disease Control and Prevention adjusts its recommendations and vaccinations become more widely available, our communities are beginning to return to normalcy. We are encouraged to put aside our masks if vaccinated and rejoin society, to venture out with less hesitancy and anxiety. As family and friends reunite, memories of confusion, frustration, and fear are beginning to fade to black. Despite the prevailing belief that we should move on, look forward, and remember the past to safeguard our future, remnants of the pandemic remain.
Unvaccinated individuals, notably children under the age of 12, are quite significant in number. The use of telehealth is now standard practice.
For several years, we were warned about the looming “mental health crisis.” The past year has demonstrated that a crisis no longer looms – it has arrived. Our patients can reveal the vulnerability COVID-19 has wrought – from the devastation of lives lost, supply shortages, loss of employment and financial stability – to a lack of access to computers and thereby, the risk of educational decline. Those factors, coupled with isolation and uncertainty about the future, have led to an influx of individuals with anxiety, depression, and other mood disorders seeking mental health treatment.
Doctors, others suffering
As result of a medical culture guided by the sacred oath to which care, compassion, and dedication held as true in ancient Greece as it does today, the focus centers on those around us – while signs of our own weariness are waved away as “a bad day.” Even though several support groups are readily available to offer a listening ear and mental health physicians who focus on the treatment of health care professionals are becoming more ubiquitous, the vestiges of past doctrine remain.
In this modern age of medical training, there is often as much sacrifice as there is attainment of knowledge. This philosophy is so ingrained that throughout training and practice one may come across colleagues experiencing an abundance of guilt when leave is needed for personal reasons. We are quick to recommend such steps for our patients, family, and friends, but hesitant to consider such for ourselves. Yet, of all the lessons this past year has wrought, the importance of mental health and self-care cannot be overstated. This raises the question:
It is vital to accept our humanity as something not to repair, treat, or overcome but to understand. There is strength and power in vulnerability. If we do not perceive and validate this process within ourselves, how can we do so for others? In other words, the oxygen mask must be placed on us first before we can place it on anyone else – patients or otherwise.
Chiefly and above all else, the importance of identifying individual signs of stress is essential. Where do you hold tension? Are you prone to GI distress or headaches when taxed? Do you tend toward irritability, apathy, or exhaustion?
Once this is determined, it is important to assess your stress on a numerical scale, such as those used for pain. Are you a 5 or an 8? Finally, are there identifiable triggers or reliable alleviators? Is there a time of day or day of the week that is most difficult to manage? Can you anticipate potential stressors? Understanding your triggers, listening to your body, and practicing the language of self is the first step toward wellness.
Following introspection and observation, the next step is inventory. Take stock of your reserves. What replenishes? What depletes? What brings joy? What brings dread? Are there certain activities that mitigate stress? If so, how much time do they entail? Identify your number on a scale and associate that number with specific strategies or techniques. Remember that decompression for a 6 might be excessive for a 4. Furthermore, what is the duration of these feelings? Chronic stressors may incur gradual change verses sudden impact if acute. Through identifying personal signs, devising and using a scale, as well as escalating or de-escalating factors, individuals become more in tune with their bodies and therefore, more likely to intervene before burnout takes hold.
With this process well integrated, one can now consider stylized approaches for stress management. For example, those inclined toward mindfulness practices may find yoga, meditation, and relaxation exercises beneficial. Others may thrive on positive affirmations, gratitude, and thankfulness. While some might find relief in physical activity, be it strenuous or casual, the creative arts might appeal to those who find joy in painting, writing, or doing crafts. In addition, baking, reading, dancing, and/or listening to music might help lift stress.
Along with those discoveries, or in some cases, rediscoveries, basic needs such as dietary habits and nutrition, hydration, and sleep are vital toward emotional regulation, physiological homeostasis, and stress modulation. Remember HALT: Hungry, Angry, Lonely, Tired, Too hot, Too cold, Sad or Stressed. Those strategies are meant to guide self-care and highlight the importance of allowing time for self-awareness. Imagine yourself as if you are meeting a new patient. Establish rapport, identify symptoms, and explore options for treatment. When we give time to ourselves, we can give time more freely to others. With this in mind, try following the 5-minute wellness check that I formulated:
1. How am I feeling? What am I feeling?
2. Assess HALTS.
3. Identify the number on your scale.
4. Methods of quick de-escalation:
- Designate and schedule personal time.
- Write down daily goals.
- Repeat positive affirmations or write down words of gratitude.
- Use deep breathing exercises.
- Stretch or take a brief walk.
- Engage in mindfulness practices, such as meditation.
Once we develop a habit of monitoring, assessing, and practicing self-care, the process becomes more efficient and effective. Think of the way a seasoned attending can manage workflow with ease, compared with an intern. Recognizing signs and using these strategies routinely can become a quick daily measure of well-being.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
AMA acknowledges medical education racism of past, vows better future
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
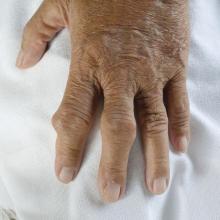
‘COVID toes’ chilblain-like lesions not related to COVID-19
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New biomarkers may predict interstitial lung disease progression in patients with systemic sclerosis
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Western and proinflammatory diets are important drivers of gout risk
Diets high in red meats, saturated fats, and sugars, relative to diets dominated by fruits, vegetables, and legumes, are associated with an increased risk of gout independent of an underlying genetic risk, according to independent sets of data presented at the annual European Congress of Rheumatology.
Only one of the two retrospective analyses evaluated diet in the context of a genetic risk score, but “no evidence of an additional or multiplicative interaction” was seen when genetic risk was evaluated on top of the risk already known to be associated with a Western diet, reported Chio Yokose, MD, a researcher and clinician in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital, Boston.
A parallel study presented at the EULAR Congress looked at the impact of a proinflammatory diet. Although genetic predisposition was not considered in this analysis, this diet, too, was associated with increased risk of gout independent of a long list of other variables. Each of the studies supports the potential for diet to be a target for risk reduction.
“Adhering to a diet with low inflammatory potential may mediate systemic and metabolic inflammation,” reported Natalie McCormick, PhD, a research fellow at Massachusetts General Hospital. She said the association of an inflammatory diet with gout is analogous to previous studies linking this type of diet to type 2 diabetes mellitus and cardiovascular disease because the inflammatory response is a pathogenic factor.
The two retrospective studies evaluated different but overlapping sets of data. Dr. Yokose and Dr. McCormick collaborated on both studies.
In the study of Western diet, which was restricted to women, the focus was on both diet and genes. Using food frequency questionnaires completed by 18,512 women participating in the Nurses’ Health Study (NHS), subjects were placed in quintiles for relative exposure to Western diets and for an interventional diet called DASH (Dietary Approaches to Stop Hypertension) that is high in fruits and vegetables.
A genetic risk score (GRS) was developed for participants using 114 serum urate single-nucleotide polymorphisms from a genomewide association study.
For the Western diet, there was a stepwise increased risk of gout per quintile associated with greater exposure. For the DASH diet, the same phenomenon was seen in reverse so that risk of gout was incrementally lower per quintile defining greater adherence.
When considered as a variable, GRS altered these basic relationships only for the DASH diet. After adjusting for multiple factors, such as age, menopause, use of hormone therapy, and hypertension, there was no significant interaction observed for genetic predisposition in relation to the Western diet.
For the DASH diet, there was an even greater reduction in the relative risk of gout among those with a high GRS if they were in the quintile defining greatest adherence to the DASH diet. Although this association fell just short of reaching statistical significance (P = .056), Dr. Yokose indicated that it was a strong trend.
Gout similarly associated with proinflammatory diet
The proinflammatory diet shares many food items with the Western diet, including refined carbohydrates, sweetened beverages, red meat, and fried foods. The study that evaluated its impact used dietary history collected from in 164,090 women in the NHS and 40,598 men in the Health Professionals Follow-up Study. In both, participants completed dietary questionnaires every 4 years. Patients were assigned an Empirical Dietary Index of Inflammatory Potential (EDIP) score on the basis of these questionnaires.
When the 2,874 incident gout cases were evaluated by EDIP quintile, those in the highest had a 50% greater risk of gout than did those in the lowest when adjusted for multiple potential confounders. When stratified by intake of alcohol, the impact of being in the highest quintile of inflammatory diet was even greater, producing a 2.37-fold increased risk of gout.
Impact of weight on risk for gout
The impact of proinflammatory diet was detectable even after adjusting for adiposity, a gout risk factor reconfirmed in a third study presented at EULAR by this same team of investigators. In that study, presented by Dr. Yokose, a GRS above the mean was associated with a further increased likelihood of gout among those with elevated body mass index. However, obesity remained a risk factor for gout even among those with a low GRS.
The data from this study indicate “maintaining healthy weight is an important gout prevention strategy, regardless of underlying genetic risk,” Dr. Yokose reported.
All three studies reinforce diet as a modifiable risk factor for gout. According to both Dr. Yokose and Dr. McCormick, healthy diets should be considered as a gout prevention strategy.
Annelies Boonen, MD, PhD, professor of internal medicine (rheumatology) at the University of Maastricht (the Netherlands), did not challenge these conclusions. However, she cautioned that it is “very difficult to evaluate food questionnaires.” She further noted that retrospective analyses complicate efforts to control for the many potential confounders.
Ultimately, healthy diets can be recommended for many reasons, particularly in individuals with other risk factors for gout. For this reason, Dr. Boonen indicated that it will be difficult to prove definitively that gout can be prevented by avoiding Western diets and other diets high in proinflammatory foods. However, definitive proof of this benefit might not be essential for the purpose of a general recommendation to eat healthy foods.
Dr. Yokose and Dr. McCormick reported no potential conflicts of interest.
Diets high in red meats, saturated fats, and sugars, relative to diets dominated by fruits, vegetables, and legumes, are associated with an increased risk of gout independent of an underlying genetic risk, according to independent sets of data presented at the annual European Congress of Rheumatology.
Only one of the two retrospective analyses evaluated diet in the context of a genetic risk score, but “no evidence of an additional or multiplicative interaction” was seen when genetic risk was evaluated on top of the risk already known to be associated with a Western diet, reported Chio Yokose, MD, a researcher and clinician in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital, Boston.
A parallel study presented at the EULAR Congress looked at the impact of a proinflammatory diet. Although genetic predisposition was not considered in this analysis, this diet, too, was associated with increased risk of gout independent of a long list of other variables. Each of the studies supports the potential for diet to be a target for risk reduction.
“Adhering to a diet with low inflammatory potential may mediate systemic and metabolic inflammation,” reported Natalie McCormick, PhD, a research fellow at Massachusetts General Hospital. She said the association of an inflammatory diet with gout is analogous to previous studies linking this type of diet to type 2 diabetes mellitus and cardiovascular disease because the inflammatory response is a pathogenic factor.
The two retrospective studies evaluated different but overlapping sets of data. Dr. Yokose and Dr. McCormick collaborated on both studies.
In the study of Western diet, which was restricted to women, the focus was on both diet and genes. Using food frequency questionnaires completed by 18,512 women participating in the Nurses’ Health Study (NHS), subjects were placed in quintiles for relative exposure to Western diets and for an interventional diet called DASH (Dietary Approaches to Stop Hypertension) that is high in fruits and vegetables.
A genetic risk score (GRS) was developed for participants using 114 serum urate single-nucleotide polymorphisms from a genomewide association study.
For the Western diet, there was a stepwise increased risk of gout per quintile associated with greater exposure. For the DASH diet, the same phenomenon was seen in reverse so that risk of gout was incrementally lower per quintile defining greater adherence.
When considered as a variable, GRS altered these basic relationships only for the DASH diet. After adjusting for multiple factors, such as age, menopause, use of hormone therapy, and hypertension, there was no significant interaction observed for genetic predisposition in relation to the Western diet.
For the DASH diet, there was an even greater reduction in the relative risk of gout among those with a high GRS if they were in the quintile defining greatest adherence to the DASH diet. Although this association fell just short of reaching statistical significance (P = .056), Dr. Yokose indicated that it was a strong trend.
Gout similarly associated with proinflammatory diet
The proinflammatory diet shares many food items with the Western diet, including refined carbohydrates, sweetened beverages, red meat, and fried foods. The study that evaluated its impact used dietary history collected from in 164,090 women in the NHS and 40,598 men in the Health Professionals Follow-up Study. In both, participants completed dietary questionnaires every 4 years. Patients were assigned an Empirical Dietary Index of Inflammatory Potential (EDIP) score on the basis of these questionnaires.
When the 2,874 incident gout cases were evaluated by EDIP quintile, those in the highest had a 50% greater risk of gout than did those in the lowest when adjusted for multiple potential confounders. When stratified by intake of alcohol, the impact of being in the highest quintile of inflammatory diet was even greater, producing a 2.37-fold increased risk of gout.
Impact of weight on risk for gout
The impact of proinflammatory diet was detectable even after adjusting for adiposity, a gout risk factor reconfirmed in a third study presented at EULAR by this same team of investigators. In that study, presented by Dr. Yokose, a GRS above the mean was associated with a further increased likelihood of gout among those with elevated body mass index. However, obesity remained a risk factor for gout even among those with a low GRS.
The data from this study indicate “maintaining healthy weight is an important gout prevention strategy, regardless of underlying genetic risk,” Dr. Yokose reported.
All three studies reinforce diet as a modifiable risk factor for gout. According to both Dr. Yokose and Dr. McCormick, healthy diets should be considered as a gout prevention strategy.
Annelies Boonen, MD, PhD, professor of internal medicine (rheumatology) at the University of Maastricht (the Netherlands), did not challenge these conclusions. However, she cautioned that it is “very difficult to evaluate food questionnaires.” She further noted that retrospective analyses complicate efforts to control for the many potential confounders.
Ultimately, healthy diets can be recommended for many reasons, particularly in individuals with other risk factors for gout. For this reason, Dr. Boonen indicated that it will be difficult to prove definitively that gout can be prevented by avoiding Western diets and other diets high in proinflammatory foods. However, definitive proof of this benefit might not be essential for the purpose of a general recommendation to eat healthy foods.
Dr. Yokose and Dr. McCormick reported no potential conflicts of interest.
Diets high in red meats, saturated fats, and sugars, relative to diets dominated by fruits, vegetables, and legumes, are associated with an increased risk of gout independent of an underlying genetic risk, according to independent sets of data presented at the annual European Congress of Rheumatology.
Only one of the two retrospective analyses evaluated diet in the context of a genetic risk score, but “no evidence of an additional or multiplicative interaction” was seen when genetic risk was evaluated on top of the risk already known to be associated with a Western diet, reported Chio Yokose, MD, a researcher and clinician in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital, Boston.
A parallel study presented at the EULAR Congress looked at the impact of a proinflammatory diet. Although genetic predisposition was not considered in this analysis, this diet, too, was associated with increased risk of gout independent of a long list of other variables. Each of the studies supports the potential for diet to be a target for risk reduction.
“Adhering to a diet with low inflammatory potential may mediate systemic and metabolic inflammation,” reported Natalie McCormick, PhD, a research fellow at Massachusetts General Hospital. She said the association of an inflammatory diet with gout is analogous to previous studies linking this type of diet to type 2 diabetes mellitus and cardiovascular disease because the inflammatory response is a pathogenic factor.
The two retrospective studies evaluated different but overlapping sets of data. Dr. Yokose and Dr. McCormick collaborated on both studies.
In the study of Western diet, which was restricted to women, the focus was on both diet and genes. Using food frequency questionnaires completed by 18,512 women participating in the Nurses’ Health Study (NHS), subjects were placed in quintiles for relative exposure to Western diets and for an interventional diet called DASH (Dietary Approaches to Stop Hypertension) that is high in fruits and vegetables.
A genetic risk score (GRS) was developed for participants using 114 serum urate single-nucleotide polymorphisms from a genomewide association study.
For the Western diet, there was a stepwise increased risk of gout per quintile associated with greater exposure. For the DASH diet, the same phenomenon was seen in reverse so that risk of gout was incrementally lower per quintile defining greater adherence.
When considered as a variable, GRS altered these basic relationships only for the DASH diet. After adjusting for multiple factors, such as age, menopause, use of hormone therapy, and hypertension, there was no significant interaction observed for genetic predisposition in relation to the Western diet.
For the DASH diet, there was an even greater reduction in the relative risk of gout among those with a high GRS if they were in the quintile defining greatest adherence to the DASH diet. Although this association fell just short of reaching statistical significance (P = .056), Dr. Yokose indicated that it was a strong trend.
Gout similarly associated with proinflammatory diet
The proinflammatory diet shares many food items with the Western diet, including refined carbohydrates, sweetened beverages, red meat, and fried foods. The study that evaluated its impact used dietary history collected from in 164,090 women in the NHS and 40,598 men in the Health Professionals Follow-up Study. In both, participants completed dietary questionnaires every 4 years. Patients were assigned an Empirical Dietary Index of Inflammatory Potential (EDIP) score on the basis of these questionnaires.
When the 2,874 incident gout cases were evaluated by EDIP quintile, those in the highest had a 50% greater risk of gout than did those in the lowest when adjusted for multiple potential confounders. When stratified by intake of alcohol, the impact of being in the highest quintile of inflammatory diet was even greater, producing a 2.37-fold increased risk of gout.
Impact of weight on risk for gout
The impact of proinflammatory diet was detectable even after adjusting for adiposity, a gout risk factor reconfirmed in a third study presented at EULAR by this same team of investigators. In that study, presented by Dr. Yokose, a GRS above the mean was associated with a further increased likelihood of gout among those with elevated body mass index. However, obesity remained a risk factor for gout even among those with a low GRS.
The data from this study indicate “maintaining healthy weight is an important gout prevention strategy, regardless of underlying genetic risk,” Dr. Yokose reported.
All three studies reinforce diet as a modifiable risk factor for gout. According to both Dr. Yokose and Dr. McCormick, healthy diets should be considered as a gout prevention strategy.
Annelies Boonen, MD, PhD, professor of internal medicine (rheumatology) at the University of Maastricht (the Netherlands), did not challenge these conclusions. However, she cautioned that it is “very difficult to evaluate food questionnaires.” She further noted that retrospective analyses complicate efforts to control for the many potential confounders.
Ultimately, healthy diets can be recommended for many reasons, particularly in individuals with other risk factors for gout. For this reason, Dr. Boonen indicated that it will be difficult to prove definitively that gout can be prevented by avoiding Western diets and other diets high in proinflammatory foods. However, definitive proof of this benefit might not be essential for the purpose of a general recommendation to eat healthy foods.
Dr. Yokose and Dr. McCormick reported no potential conflicts of interest.
FROM THE EULAR 2021 CONGRESS
Secukinumab provides clinical benefit in phase 3 juvenile arthritis trial
Favorable safety sustained at 104 weeks
Secukinumab (Cosentyx), an interleukin-17A inhibitor, is effective and reasonably well tolerated for treatment of enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) in children and adolescents, according to a phase 3 trial presented at a late breaking abstracts session of the annual European Congress of Rheumatology.
On the primary outcome of time to flare, the curves for secukinumab and placebo separated almost immediately, with fewer than half the number of flares occurring in the experimental arm over the course of the study, according to Nicolino Ruperto, MD, senior research scientist at IRCCS Istituto Giannina Gaslini in Genoa, Italy.
The trial, called JUNIPERA, was conducted over 2 years and included an open-label treatment period (TP1) and then a randomized, placebo-controlled comparison (TP2). In TP1, 86 children were initiated on open-label secukinumab administered subcutaneously on weeks 1, 2, 3, 4, 8, and 12. The dose was 75 mg for children less than 50 kg and 150 kg for those heavier.
Average patient age was 13.1 years
Of these 86 children, 52 had ERA and 34 had JPsA. Disease duration of at least 6 months was required for entry. Patients up to the age of 18 years were permitted to enroll. The average age was 13.1 years. Most patients, two-thirds of whom were male, had received an immunomodulator prior to study entry.
At the end of TP1, 69.9% of patients had achieved 70% improvement in the Juvenile Idiopathic Arthritis American College of Rheumatology joint score (JIA ACR70). The 90.4% of patients who achieved JIA ACR30 were invited to enroll in TP2. A total of 75 patients did so.
At the end of TP2, response rates strongly favored secukinumab over placebo for JIA ACR30 (89.2% vs. 64.9%; P = .014) and JIA ACR70 (67.7% vs. 43.2%; P = .042). Higher but not statistically significant differences in response rates were seen for secukinumab over placebo for JIA ACR50 (78.4% vs. 62.2%; P = .152), JIA ACR90 (51.4% vs. 40.5%; P = .431) and JIA ACR100 (43.2% vs. 37.8%; P = .755).
During TP2, there were 10 flares in the group randomized to secukinumab versus 21 flares in the placebo group, translating by hazard ratio (HR) into a 72% risk reduction (HR, 0.28; P < .001).
Side effects similar to those in adults
The types and rates of serious adverse events were similar to those reported previously in adult patients, according to Dr. Ruperto. Although the rate of serious adverse events (14.6% vs. 10.6%) was only moderately higher in the experimental arm, more patients randomized to secukinumab than placebo discontinued therapy (13.2% vs. 6.3%) before the end of follow-up.
The side effects that occurred more commonly on secukinumab included gastrointestinal complaints, such as diarrhea (22.9% vs. 15.8%). Other adverse events occurring in more than 10% of patients included headache and nasopharyngitis, but most side effects were mild and resolved.
Although the proportion of patients with flare increased over time in both groups, Dr. Ruperto reported that protection against flares and relative improvement in clinical markers of disease activity relative to placebo “were sustained out to 2 years of follow-up.”
The submission of these data to regulatory agencies is anticipated. If secukinumab is given an indication for these forms of arthritis, it will join an indication for plaque psoriasis in children that was granted just a few days before these data were presented. The psoriasis indication is the only current use approved for children in the United States.
More biologics needed for JPsA
Additional biologics will be helpful for children with arthritis who are poorly controlled on available treatments, according to Natasha M. Ruth, MD, director of the division of pediatric rheumatology at the Medical University of South Carolina, Charleston. Dr. Ruth was senior author of a case study published 2 years ago in which secukinumab was used to control psoriatic arthritis and nail manifestations of psoriasis.
“It was a girl who had already failed to improve adequately to TNF inhibitors,” reported Dr. Ruth, who had said the child and her parent were very concerned about the nail appearance.
“The nail involvement completely resolved, so it was a very good result in a difficult situation,” Dr. Ruth explained. She said that the decision to try secukinumab was made collaboratively in a clinic in which dermatologists and rheumatologists at her institution work together on difficult cases.
“There is a need for more biologics with different mechanisms of action,” Dr. Ruth said. Based on her experience, secukinumab could be an important addition to treatment options.
Dr. Ruperto reported having financial relationships with more than 20 pharmaceutical companies, including Novartis, which provided financial support for this trial. Many coauthors had financial relationships with multiple companies, including Novartis, and some were employees of the company. Dr. Ruth reported having no potential conflicts of interest.
Favorable safety sustained at 104 weeks
Favorable safety sustained at 104 weeks
Secukinumab (Cosentyx), an interleukin-17A inhibitor, is effective and reasonably well tolerated for treatment of enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) in children and adolescents, according to a phase 3 trial presented at a late breaking abstracts session of the annual European Congress of Rheumatology.
On the primary outcome of time to flare, the curves for secukinumab and placebo separated almost immediately, with fewer than half the number of flares occurring in the experimental arm over the course of the study, according to Nicolino Ruperto, MD, senior research scientist at IRCCS Istituto Giannina Gaslini in Genoa, Italy.
The trial, called JUNIPERA, was conducted over 2 years and included an open-label treatment period (TP1) and then a randomized, placebo-controlled comparison (TP2). In TP1, 86 children were initiated on open-label secukinumab administered subcutaneously on weeks 1, 2, 3, 4, 8, and 12. The dose was 75 mg for children less than 50 kg and 150 kg for those heavier.
Average patient age was 13.1 years
Of these 86 children, 52 had ERA and 34 had JPsA. Disease duration of at least 6 months was required for entry. Patients up to the age of 18 years were permitted to enroll. The average age was 13.1 years. Most patients, two-thirds of whom were male, had received an immunomodulator prior to study entry.
At the end of TP1, 69.9% of patients had achieved 70% improvement in the Juvenile Idiopathic Arthritis American College of Rheumatology joint score (JIA ACR70). The 90.4% of patients who achieved JIA ACR30 were invited to enroll in TP2. A total of 75 patients did so.
At the end of TP2, response rates strongly favored secukinumab over placebo for JIA ACR30 (89.2% vs. 64.9%; P = .014) and JIA ACR70 (67.7% vs. 43.2%; P = .042). Higher but not statistically significant differences in response rates were seen for secukinumab over placebo for JIA ACR50 (78.4% vs. 62.2%; P = .152), JIA ACR90 (51.4% vs. 40.5%; P = .431) and JIA ACR100 (43.2% vs. 37.8%; P = .755).
During TP2, there were 10 flares in the group randomized to secukinumab versus 21 flares in the placebo group, translating by hazard ratio (HR) into a 72% risk reduction (HR, 0.28; P < .001).
Side effects similar to those in adults
The types and rates of serious adverse events were similar to those reported previously in adult patients, according to Dr. Ruperto. Although the rate of serious adverse events (14.6% vs. 10.6%) was only moderately higher in the experimental arm, more patients randomized to secukinumab than placebo discontinued therapy (13.2% vs. 6.3%) before the end of follow-up.
The side effects that occurred more commonly on secukinumab included gastrointestinal complaints, such as diarrhea (22.9% vs. 15.8%). Other adverse events occurring in more than 10% of patients included headache and nasopharyngitis, but most side effects were mild and resolved.
Although the proportion of patients with flare increased over time in both groups, Dr. Ruperto reported that protection against flares and relative improvement in clinical markers of disease activity relative to placebo “were sustained out to 2 years of follow-up.”
The submission of these data to regulatory agencies is anticipated. If secukinumab is given an indication for these forms of arthritis, it will join an indication for plaque psoriasis in children that was granted just a few days before these data were presented. The psoriasis indication is the only current use approved for children in the United States.
More biologics needed for JPsA
Additional biologics will be helpful for children with arthritis who are poorly controlled on available treatments, according to Natasha M. Ruth, MD, director of the division of pediatric rheumatology at the Medical University of South Carolina, Charleston. Dr. Ruth was senior author of a case study published 2 years ago in which secukinumab was used to control psoriatic arthritis and nail manifestations of psoriasis.
“It was a girl who had already failed to improve adequately to TNF inhibitors,” reported Dr. Ruth, who had said the child and her parent were very concerned about the nail appearance.
“The nail involvement completely resolved, so it was a very good result in a difficult situation,” Dr. Ruth explained. She said that the decision to try secukinumab was made collaboratively in a clinic in which dermatologists and rheumatologists at her institution work together on difficult cases.
“There is a need for more biologics with different mechanisms of action,” Dr. Ruth said. Based on her experience, secukinumab could be an important addition to treatment options.
Dr. Ruperto reported having financial relationships with more than 20 pharmaceutical companies, including Novartis, which provided financial support for this trial. Many coauthors had financial relationships with multiple companies, including Novartis, and some were employees of the company. Dr. Ruth reported having no potential conflicts of interest.
Secukinumab (Cosentyx), an interleukin-17A inhibitor, is effective and reasonably well tolerated for treatment of enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) in children and adolescents, according to a phase 3 trial presented at a late breaking abstracts session of the annual European Congress of Rheumatology.
On the primary outcome of time to flare, the curves for secukinumab and placebo separated almost immediately, with fewer than half the number of flares occurring in the experimental arm over the course of the study, according to Nicolino Ruperto, MD, senior research scientist at IRCCS Istituto Giannina Gaslini in Genoa, Italy.
The trial, called JUNIPERA, was conducted over 2 years and included an open-label treatment period (TP1) and then a randomized, placebo-controlled comparison (TP2). In TP1, 86 children were initiated on open-label secukinumab administered subcutaneously on weeks 1, 2, 3, 4, 8, and 12. The dose was 75 mg for children less than 50 kg and 150 kg for those heavier.
Average patient age was 13.1 years
Of these 86 children, 52 had ERA and 34 had JPsA. Disease duration of at least 6 months was required for entry. Patients up to the age of 18 years were permitted to enroll. The average age was 13.1 years. Most patients, two-thirds of whom were male, had received an immunomodulator prior to study entry.
At the end of TP1, 69.9% of patients had achieved 70% improvement in the Juvenile Idiopathic Arthritis American College of Rheumatology joint score (JIA ACR70). The 90.4% of patients who achieved JIA ACR30 were invited to enroll in TP2. A total of 75 patients did so.
At the end of TP2, response rates strongly favored secukinumab over placebo for JIA ACR30 (89.2% vs. 64.9%; P = .014) and JIA ACR70 (67.7% vs. 43.2%; P = .042). Higher but not statistically significant differences in response rates were seen for secukinumab over placebo for JIA ACR50 (78.4% vs. 62.2%; P = .152), JIA ACR90 (51.4% vs. 40.5%; P = .431) and JIA ACR100 (43.2% vs. 37.8%; P = .755).
During TP2, there were 10 flares in the group randomized to secukinumab versus 21 flares in the placebo group, translating by hazard ratio (HR) into a 72% risk reduction (HR, 0.28; P < .001).
Side effects similar to those in adults
The types and rates of serious adverse events were similar to those reported previously in adult patients, according to Dr. Ruperto. Although the rate of serious adverse events (14.6% vs. 10.6%) was only moderately higher in the experimental arm, more patients randomized to secukinumab than placebo discontinued therapy (13.2% vs. 6.3%) before the end of follow-up.
The side effects that occurred more commonly on secukinumab included gastrointestinal complaints, such as diarrhea (22.9% vs. 15.8%). Other adverse events occurring in more than 10% of patients included headache and nasopharyngitis, but most side effects were mild and resolved.
Although the proportion of patients with flare increased over time in both groups, Dr. Ruperto reported that protection against flares and relative improvement in clinical markers of disease activity relative to placebo “were sustained out to 2 years of follow-up.”
The submission of these data to regulatory agencies is anticipated. If secukinumab is given an indication for these forms of arthritis, it will join an indication for plaque psoriasis in children that was granted just a few days before these data were presented. The psoriasis indication is the only current use approved for children in the United States.
More biologics needed for JPsA
Additional biologics will be helpful for children with arthritis who are poorly controlled on available treatments, according to Natasha M. Ruth, MD, director of the division of pediatric rheumatology at the Medical University of South Carolina, Charleston. Dr. Ruth was senior author of a case study published 2 years ago in which secukinumab was used to control psoriatic arthritis and nail manifestations of psoriasis.
“It was a girl who had already failed to improve adequately to TNF inhibitors,” reported Dr. Ruth, who had said the child and her parent were very concerned about the nail appearance.
“The nail involvement completely resolved, so it was a very good result in a difficult situation,” Dr. Ruth explained. She said that the decision to try secukinumab was made collaboratively in a clinic in which dermatologists and rheumatologists at her institution work together on difficult cases.
“There is a need for more biologics with different mechanisms of action,” Dr. Ruth said. Based on her experience, secukinumab could be an important addition to treatment options.
Dr. Ruperto reported having financial relationships with more than 20 pharmaceutical companies, including Novartis, which provided financial support for this trial. Many coauthors had financial relationships with multiple companies, including Novartis, and some were employees of the company. Dr. Ruth reported having no potential conflicts of interest.
FROM THE EULAR 2021 CONGRESS
Minnesota named best place to practice in 2021
For physicians who are just starting out or thinking about moving, the “Land of 10,000 Lakes” could be the land of opportunity, according to a recent Medscape analysis.

In a ranking of the 50 states, Minnesota “claimed top marks for livability, low incidence of adverse actions against doctors, and the performance of its health system,” Shelly Reese wrote in Medscape’s “Best & Worst Places to Practice 2021.”
Minnesota is below average where it’s good to be below average – share of physicians reporting burnout and/or depression – but above average in the share of physicians who say they’re “very happy” outside of work, Medscape said in the annual report.
and adverse actions and a high level of livability. Third place went to Washington (called the most livable state in the country by U.S. News and World Report), fourth to Colorado (physicians happy at and outside of work, high retention rate for residents), and fifth to Utah (low crime rate, high quality of life), Medscape said.
At the bottom of the list for 2021 is West Virginia, where physicians “may confront a bevy of challenges” in the form of low livability, a high rate of adverse actions, and relatively high malpractice payouts, Ms. Reese noted in the report.
State number 49 is Louisiana, where livability is low, malpractice payouts are high, and more than half of physicians say that they’re burned out and/or depressed. New Mexico is 48th (very high rate of adverse actions, poor resident retention), Nevada is 47th (low marks for avoidable hospital use and disparity in care), and Rhode Island is 46th (high malpractice payouts, low physician compensation), Medscape said.
Continuing with the group-of-five theme, America’s three most populous states finished in the top half of the ranking – California 16th, Texas 11th, and Florida 21st – but New York and Pennsylvania, numbers four and five by population size, did not.
The rankings are based on states’ performance in 10 different measures, three of which were sourced from Medscape surveys – happiness at work, happiness outside of work, and burnout/depression – and seven from other organizations: adverse actions against physicians, malpractice payouts, compensation (adjusted for cost of living), overall health, health system performance, overall livability, resident retention.
For physicians who are just starting out or thinking about moving, the “Land of 10,000 Lakes” could be the land of opportunity, according to a recent Medscape analysis.

In a ranking of the 50 states, Minnesota “claimed top marks for livability, low incidence of adverse actions against doctors, and the performance of its health system,” Shelly Reese wrote in Medscape’s “Best & Worst Places to Practice 2021.”
Minnesota is below average where it’s good to be below average – share of physicians reporting burnout and/or depression – but above average in the share of physicians who say they’re “very happy” outside of work, Medscape said in the annual report.
and adverse actions and a high level of livability. Third place went to Washington (called the most livable state in the country by U.S. News and World Report), fourth to Colorado (physicians happy at and outside of work, high retention rate for residents), and fifth to Utah (low crime rate, high quality of life), Medscape said.
At the bottom of the list for 2021 is West Virginia, where physicians “may confront a bevy of challenges” in the form of low livability, a high rate of adverse actions, and relatively high malpractice payouts, Ms. Reese noted in the report.
State number 49 is Louisiana, where livability is low, malpractice payouts are high, and more than half of physicians say that they’re burned out and/or depressed. New Mexico is 48th (very high rate of adverse actions, poor resident retention), Nevada is 47th (low marks for avoidable hospital use and disparity in care), and Rhode Island is 46th (high malpractice payouts, low physician compensation), Medscape said.
Continuing with the group-of-five theme, America’s three most populous states finished in the top half of the ranking – California 16th, Texas 11th, and Florida 21st – but New York and Pennsylvania, numbers four and five by population size, did not.
The rankings are based on states’ performance in 10 different measures, three of which were sourced from Medscape surveys – happiness at work, happiness outside of work, and burnout/depression – and seven from other organizations: adverse actions against physicians, malpractice payouts, compensation (adjusted for cost of living), overall health, health system performance, overall livability, resident retention.
For physicians who are just starting out or thinking about moving, the “Land of 10,000 Lakes” could be the land of opportunity, according to a recent Medscape analysis.

In a ranking of the 50 states, Minnesota “claimed top marks for livability, low incidence of adverse actions against doctors, and the performance of its health system,” Shelly Reese wrote in Medscape’s “Best & Worst Places to Practice 2021.”
Minnesota is below average where it’s good to be below average – share of physicians reporting burnout and/or depression – but above average in the share of physicians who say they’re “very happy” outside of work, Medscape said in the annual report.
and adverse actions and a high level of livability. Third place went to Washington (called the most livable state in the country by U.S. News and World Report), fourth to Colorado (physicians happy at and outside of work, high retention rate for residents), and fifth to Utah (low crime rate, high quality of life), Medscape said.
At the bottom of the list for 2021 is West Virginia, where physicians “may confront a bevy of challenges” in the form of low livability, a high rate of adverse actions, and relatively high malpractice payouts, Ms. Reese noted in the report.
State number 49 is Louisiana, where livability is low, malpractice payouts are high, and more than half of physicians say that they’re burned out and/or depressed. New Mexico is 48th (very high rate of adverse actions, poor resident retention), Nevada is 47th (low marks for avoidable hospital use and disparity in care), and Rhode Island is 46th (high malpractice payouts, low physician compensation), Medscape said.
Continuing with the group-of-five theme, America’s three most populous states finished in the top half of the ranking – California 16th, Texas 11th, and Florida 21st – but New York and Pennsylvania, numbers four and five by population size, did not.
The rankings are based on states’ performance in 10 different measures, three of which were sourced from Medscape surveys – happiness at work, happiness outside of work, and burnout/depression – and seven from other organizations: adverse actions against physicians, malpractice payouts, compensation (adjusted for cost of living), overall health, health system performance, overall livability, resident retention.
Risankizumab shows efficacy, tolerability in patients with PsA
Risankizumab (Skyrizi) was effective for treating psoriatic arthritis (PsA) in patients who did not respond to or who could not tolerate other biologics or standard disease-modifying antirheumatic drugs (DMARDs), according to a study presented at the annual European Congress of Rheumatology. It was also well tolerated.
“Treatment with risankizumab resulted in significantly greater improvements in signs and symptoms of psoriatic arthritis, including assessments of disease activity in joints and skin and patient-reported outcomes, compared with placebo, in patients who did not respond to or were intolerant to biologics or DMARDs,” reported Andrew Ostor, MD, of Monash University and Cabrini Hospital, both in Melbourne,. The safety profile was “consistent with that established for risankizumab in the treatment moderate to severe psoriasis,” he told attendees.
Risankizumab is approved in the United States for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It is a humanized immunoglobulin G1 monoclonal antibody that selectively inhibits cytokine interleukin-23 by binding to its p19 subunit. IL-23 has been implicated in the development of PsA.
This was a phase 3 trial with “promising results in line with the ACR 20 response [at least 20% improvement in American College of Rheumatology response criteria] of other biologics in psoriatic arthritis,” according to Gaëlle Varkas, MD, PhD, of the Ghent (the Netherlands) University VIB Center for Inflammation Research and the department of rheumatology, Ghent University Hospital. “Especially in patients with severe and/or refractory skin disease or inadequate response at the level of the joint to other DMARDs or biologics, risankizumab is filling a void,” Dr. Varkas, who was not involved in the research, said in an interview.
There were no major safety problems, although long-term data, especially in regard to cancer and cardiovascular effects, “are always of interest, as they can be missed in randomized, controlled trials,” she said. In addition, “efficacy in concomitant axial disease, uveitis, and inflammatory bowel disease might favor one treatment over the other.” Another clinically significant takeaway was risankizumab’s “better effect on skin psoriasis while maintaining the effect on joint manifestations.”
Details of 24-week trial results
The phase 3, randomized, placebo-controlled, double-blind KEEPSAKE 2 trial involved 444 patients who had active PsA, defined as at least five swollen joints and at least five tender joints. All the patients either had an inadequate response to or were intolerant of one or two biologics or at least one conventional synthetic DMARD.
A total of 224 patients were randomly assigned to receive 150 mg of subcutaneous risankizumab at baseline and at 4 and 16 weeks after baseline; 220 participants received placebo injections. The primary endpoint was the proportion of patients who had at least 20% improvement in American College of Rheumatology response criteria at week 24.
Demographic and clinical characteristics were similar in both groups at baseline. Among the participants, the total mean number of swollen joints was 13.3, and the total mean number of tender joints was 22.6. The participants had PsA for an average of 8.2 years. The proportions of patients previously treated with biologics and DMARDs were similar in both groups, as were the proportions of patients currently taking glucocorticoids, NSAIDs, or methotrexate or another DMARD. At week 24, there remained 199 patients in the placebo group and 215 in the risankizumab group.
Just over half (51.3%) of patients who took risankizumab achieved at least 20% improvement in their ACR 20 score, compared with just over a quarter (26.5%) of those who received placebo (P < .001). All secondary endpoints also showed statistically significant improvements (P < .001 for all except P < .009 for the Fatigue Functional Assessment of Chronic Illness Therapy–Fatigue [FACIT-Fatigue] secondary endpoint).
Scores on the Health Assessment Questionnaire–Disability Index were –0.22 in the risankizumab group and –0.05 in the placebo group (P < .001). In the risankizumab group, 55% of patients achieved at least a 90% reduction in scores on the Psoriasis Area Severity Index, compared with 10.2% of patients who received placebo. Similarly, 25.6% of patients who took risankizumab and 11.4% of patients who received placebo had minimal disease activity 24 weeks after baseline.
In the 36-item Short Form Health Survey Physical Component Summary, the score change among risankizumab patients was 5.9, compared with 2 among the patients who received placebo. The change in FACIT-Fatigue score was 4.9 for patients who took risankizumab and 2.6 for patients who received placebo.
The researchers also assessed how many patients achieved higher levels of response to treatment. At least a 50% improvement in ACR response criteria occurred among 26.3% of patients taking risankizumab and 9.3% of patients taking placebo (P < .001). ACR 70 responses were seen in 12% of patients receiving risankizumab, compared with 5.9% of patients receiving placebo (P < .02). In the risankizumab group, 72.5% of patients had resolution of dactylitis and 42.9% had resolution of enthesitis, compared with 42.1% and 30.4%, respectively, in the placebo group.
Serious adverse events occurred in 4% of patients who received risankizumab and 5.5% of patients who received placebo. Serious infections occurred in 0.9% of those receiving risankizumab and 2.3% of those receiving placebo. Rates of treatment-emergent adverse events were also similar in the risankizumab (55.4%) and placebo (54.8%) groups.
In response to a question about whether it was possible to identify patients who might respond better to IL-23 inhibitors, compared with IL-17 inhibitors, Dr. Ostor acknowledged that rheumatologic practice is not yet proficient at using biomarkers to direct therapy, so the benefit from these drugs lay elsewhere.
“What I think is great is the luxury of choice these days,” Dr. Ostor told attendees. “We have these agents now, including risankizumab, that do work very effectively across the spectrum of the clinical features. It’s just lovely to have these agents available that can truly make a difference to the clinical picture of the individual.”
The trial was sponsored by AbbVie. Dr. Ostor has received research grants or speaking or consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, and UCB. Dr. Varkas has received research grants or speaker fees from AbbVie and Pfizer.
A version of this article first appeared on Medscape.com.
Risankizumab (Skyrizi) was effective for treating psoriatic arthritis (PsA) in patients who did not respond to or who could not tolerate other biologics or standard disease-modifying antirheumatic drugs (DMARDs), according to a study presented at the annual European Congress of Rheumatology. It was also well tolerated.
“Treatment with risankizumab resulted in significantly greater improvements in signs and symptoms of psoriatic arthritis, including assessments of disease activity in joints and skin and patient-reported outcomes, compared with placebo, in patients who did not respond to or were intolerant to biologics or DMARDs,” reported Andrew Ostor, MD, of Monash University and Cabrini Hospital, both in Melbourne,. The safety profile was “consistent with that established for risankizumab in the treatment moderate to severe psoriasis,” he told attendees.
Risankizumab is approved in the United States for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It is a humanized immunoglobulin G1 monoclonal antibody that selectively inhibits cytokine interleukin-23 by binding to its p19 subunit. IL-23 has been implicated in the development of PsA.
This was a phase 3 trial with “promising results in line with the ACR 20 response [at least 20% improvement in American College of Rheumatology response criteria] of other biologics in psoriatic arthritis,” according to Gaëlle Varkas, MD, PhD, of the Ghent (the Netherlands) University VIB Center for Inflammation Research and the department of rheumatology, Ghent University Hospital. “Especially in patients with severe and/or refractory skin disease or inadequate response at the level of the joint to other DMARDs or biologics, risankizumab is filling a void,” Dr. Varkas, who was not involved in the research, said in an interview.
There were no major safety problems, although long-term data, especially in regard to cancer and cardiovascular effects, “are always of interest, as they can be missed in randomized, controlled trials,” she said. In addition, “efficacy in concomitant axial disease, uveitis, and inflammatory bowel disease might favor one treatment over the other.” Another clinically significant takeaway was risankizumab’s “better effect on skin psoriasis while maintaining the effect on joint manifestations.”
Details of 24-week trial results
The phase 3, randomized, placebo-controlled, double-blind KEEPSAKE 2 trial involved 444 patients who had active PsA, defined as at least five swollen joints and at least five tender joints. All the patients either had an inadequate response to or were intolerant of one or two biologics or at least one conventional synthetic DMARD.
A total of 224 patients were randomly assigned to receive 150 mg of subcutaneous risankizumab at baseline and at 4 and 16 weeks after baseline; 220 participants received placebo injections. The primary endpoint was the proportion of patients who had at least 20% improvement in American College of Rheumatology response criteria at week 24.
Demographic and clinical characteristics were similar in both groups at baseline. Among the participants, the total mean number of swollen joints was 13.3, and the total mean number of tender joints was 22.6. The participants had PsA for an average of 8.2 years. The proportions of patients previously treated with biologics and DMARDs were similar in both groups, as were the proportions of patients currently taking glucocorticoids, NSAIDs, or methotrexate or another DMARD. At week 24, there remained 199 patients in the placebo group and 215 in the risankizumab group.
Just over half (51.3%) of patients who took risankizumab achieved at least 20% improvement in their ACR 20 score, compared with just over a quarter (26.5%) of those who received placebo (P < .001). All secondary endpoints also showed statistically significant improvements (P < .001 for all except P < .009 for the Fatigue Functional Assessment of Chronic Illness Therapy–Fatigue [FACIT-Fatigue] secondary endpoint).
Scores on the Health Assessment Questionnaire–Disability Index were –0.22 in the risankizumab group and –0.05 in the placebo group (P < .001). In the risankizumab group, 55% of patients achieved at least a 90% reduction in scores on the Psoriasis Area Severity Index, compared with 10.2% of patients who received placebo. Similarly, 25.6% of patients who took risankizumab and 11.4% of patients who received placebo had minimal disease activity 24 weeks after baseline.
In the 36-item Short Form Health Survey Physical Component Summary, the score change among risankizumab patients was 5.9, compared with 2 among the patients who received placebo. The change in FACIT-Fatigue score was 4.9 for patients who took risankizumab and 2.6 for patients who received placebo.
The researchers also assessed how many patients achieved higher levels of response to treatment. At least a 50% improvement in ACR response criteria occurred among 26.3% of patients taking risankizumab and 9.3% of patients taking placebo (P < .001). ACR 70 responses were seen in 12% of patients receiving risankizumab, compared with 5.9% of patients receiving placebo (P < .02). In the risankizumab group, 72.5% of patients had resolution of dactylitis and 42.9% had resolution of enthesitis, compared with 42.1% and 30.4%, respectively, in the placebo group.
Serious adverse events occurred in 4% of patients who received risankizumab and 5.5% of patients who received placebo. Serious infections occurred in 0.9% of those receiving risankizumab and 2.3% of those receiving placebo. Rates of treatment-emergent adverse events were also similar in the risankizumab (55.4%) and placebo (54.8%) groups.
In response to a question about whether it was possible to identify patients who might respond better to IL-23 inhibitors, compared with IL-17 inhibitors, Dr. Ostor acknowledged that rheumatologic practice is not yet proficient at using biomarkers to direct therapy, so the benefit from these drugs lay elsewhere.
“What I think is great is the luxury of choice these days,” Dr. Ostor told attendees. “We have these agents now, including risankizumab, that do work very effectively across the spectrum of the clinical features. It’s just lovely to have these agents available that can truly make a difference to the clinical picture of the individual.”
The trial was sponsored by AbbVie. Dr. Ostor has received research grants or speaking or consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, and UCB. Dr. Varkas has received research grants or speaker fees from AbbVie and Pfizer.
A version of this article first appeared on Medscape.com.
Risankizumab (Skyrizi) was effective for treating psoriatic arthritis (PsA) in patients who did not respond to or who could not tolerate other biologics or standard disease-modifying antirheumatic drugs (DMARDs), according to a study presented at the annual European Congress of Rheumatology. It was also well tolerated.
“Treatment with risankizumab resulted in significantly greater improvements in signs and symptoms of psoriatic arthritis, including assessments of disease activity in joints and skin and patient-reported outcomes, compared with placebo, in patients who did not respond to or were intolerant to biologics or DMARDs,” reported Andrew Ostor, MD, of Monash University and Cabrini Hospital, both in Melbourne,. The safety profile was “consistent with that established for risankizumab in the treatment moderate to severe psoriasis,” he told attendees.
Risankizumab is approved in the United States for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. It is a humanized immunoglobulin G1 monoclonal antibody that selectively inhibits cytokine interleukin-23 by binding to its p19 subunit. IL-23 has been implicated in the development of PsA.
This was a phase 3 trial with “promising results in line with the ACR 20 response [at least 20% improvement in American College of Rheumatology response criteria] of other biologics in psoriatic arthritis,” according to Gaëlle Varkas, MD, PhD, of the Ghent (the Netherlands) University VIB Center for Inflammation Research and the department of rheumatology, Ghent University Hospital. “Especially in patients with severe and/or refractory skin disease or inadequate response at the level of the joint to other DMARDs or biologics, risankizumab is filling a void,” Dr. Varkas, who was not involved in the research, said in an interview.
There were no major safety problems, although long-term data, especially in regard to cancer and cardiovascular effects, “are always of interest, as they can be missed in randomized, controlled trials,” she said. In addition, “efficacy in concomitant axial disease, uveitis, and inflammatory bowel disease might favor one treatment over the other.” Another clinically significant takeaway was risankizumab’s “better effect on skin psoriasis while maintaining the effect on joint manifestations.”
Details of 24-week trial results
The phase 3, randomized, placebo-controlled, double-blind KEEPSAKE 2 trial involved 444 patients who had active PsA, defined as at least five swollen joints and at least five tender joints. All the patients either had an inadequate response to or were intolerant of one or two biologics or at least one conventional synthetic DMARD.
A total of 224 patients were randomly assigned to receive 150 mg of subcutaneous risankizumab at baseline and at 4 and 16 weeks after baseline; 220 participants received placebo injections. The primary endpoint was the proportion of patients who had at least 20% improvement in American College of Rheumatology response criteria at week 24.
Demographic and clinical characteristics were similar in both groups at baseline. Among the participants, the total mean number of swollen joints was 13.3, and the total mean number of tender joints was 22.6. The participants had PsA for an average of 8.2 years. The proportions of patients previously treated with biologics and DMARDs were similar in both groups, as were the proportions of patients currently taking glucocorticoids, NSAIDs, or methotrexate or another DMARD. At week 24, there remained 199 patients in the placebo group and 215 in the risankizumab group.
Just over half (51.3%) of patients who took risankizumab achieved at least 20% improvement in their ACR 20 score, compared with just over a quarter (26.5%) of those who received placebo (P < .001). All secondary endpoints also showed statistically significant improvements (P < .001 for all except P < .009 for the Fatigue Functional Assessment of Chronic Illness Therapy–Fatigue [FACIT-Fatigue] secondary endpoint).
Scores on the Health Assessment Questionnaire–Disability Index were –0.22 in the risankizumab group and –0.05 in the placebo group (P < .001). In the risankizumab group, 55% of patients achieved at least a 90% reduction in scores on the Psoriasis Area Severity Index, compared with 10.2% of patients who received placebo. Similarly, 25.6% of patients who took risankizumab and 11.4% of patients who received placebo had minimal disease activity 24 weeks after baseline.
In the 36-item Short Form Health Survey Physical Component Summary, the score change among risankizumab patients was 5.9, compared with 2 among the patients who received placebo. The change in FACIT-Fatigue score was 4.9 for patients who took risankizumab and 2.6 for patients who received placebo.
The researchers also assessed how many patients achieved higher levels of response to treatment. At least a 50% improvement in ACR response criteria occurred among 26.3% of patients taking risankizumab and 9.3% of patients taking placebo (P < .001). ACR 70 responses were seen in 12% of patients receiving risankizumab, compared with 5.9% of patients receiving placebo (P < .02). In the risankizumab group, 72.5% of patients had resolution of dactylitis and 42.9% had resolution of enthesitis, compared with 42.1% and 30.4%, respectively, in the placebo group.
Serious adverse events occurred in 4% of patients who received risankizumab and 5.5% of patients who received placebo. Serious infections occurred in 0.9% of those receiving risankizumab and 2.3% of those receiving placebo. Rates of treatment-emergent adverse events were also similar in the risankizumab (55.4%) and placebo (54.8%) groups.
In response to a question about whether it was possible to identify patients who might respond better to IL-23 inhibitors, compared with IL-17 inhibitors, Dr. Ostor acknowledged that rheumatologic practice is not yet proficient at using biomarkers to direct therapy, so the benefit from these drugs lay elsewhere.
“What I think is great is the luxury of choice these days,” Dr. Ostor told attendees. “We have these agents now, including risankizumab, that do work very effectively across the spectrum of the clinical features. It’s just lovely to have these agents available that can truly make a difference to the clinical picture of the individual.”
The trial was sponsored by AbbVie. Dr. Ostor has received research grants or speaking or consulting fees from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, and UCB. Dr. Varkas has received research grants or speaker fees from AbbVie and Pfizer.
A version of this article first appeared on Medscape.com.
The Cures Act: Is the “cure” worse than the disease?
There is a sudden spill of icy anxiety down your spine as you pick up your phone in your shaking hands. It’s 6 p.m.; your doctor’s office is closed. You open the message, and your worst fears are confirmed ... the cancer is back.
Or is it? You’re not sure. The biopsy sure sounds bad. But you’re an English teacher, not a doctor, and you spend the rest of the night Googling words like “tubulovillous” and “high-grade dysplasia.” You sit awake, terrified in front of the computer screen desperately trying to make sense of the possibly life-changing results. You wish you knew someone who could help you understand; you consider calling your doctor’s emergency line, or your cousin who is an ophthalmologist – anybody who can help you make sense of the results.
Or imagine another scenario: you’re a trans teen who has asked your doctor to refer to you by your preferred pronouns. You’re still presenting as your birth sex, in part because your family would disown you if they knew, and you’re not financially or emotionally ready for that step. You feel proud of yourself for advocating for your needs to your long-time physician, and excited about the resources they’ve included in your after visit summary and the referrals they’d made to gender-confirming specialists.
When you get home, you are confronted with a terrible reality that your doctor’s notes, orders, and recommendations are immediately viewable to anybody with your MyChart login – your parents knew the second your doctor signed the note. They received the notification, logged on as your guardians, and you have effectively been “outed” by the physician who took and oath to care for you and who you trusted implicitly.
How the Cures Act is affecting patients
While these examples may sound extreme, they are becoming more and more commonplace thanks to a recently enacted 21st Century Cures Act. The act was originally written to improve communication between physicians and patients. Part of the act stipulates that nearly all medical information – from notes to biopsies to lab results – must be available within 24 hours, published to a patient portal and a notification be sent to the patient by phone.
Oftentimes, this occurs before the ordering physician has even seen the results, much less interpreted them and made a plan for the patient. What happens now, not long after its enactment date, when it has become clear that the Cures Act is causing extreme harm to our patients?
Take, for example, the real example of a physician whose patient found out about her own intrauterine fetal demise by way of an EMR text message alert of “new imaging results!” sent directly to her phone. Or a physician colleague who witnessed firsthand the intrusive unhelpfulness of the Cures Act when she was informed via patient portal releasing her imaging information that she had a large, possibly malignant breast mass. “No phone call,” she said. “No human being for questions or comfort. Just a notification on my phone.”
The stories about the impact of the Cures Act across the medical community are an endless stream of anxiety, hurt, and broken trust. The relationship between a physician and a patient should be sacred, bolstered by communication and mutual respect.
In many ways, the new act feels like a third party to the patient-physician relationship – a digital imposter, oftentimes blurting out personal and life-altering medical information without any of the finesse, context, and perspective of an experienced physician.
Breaking ‘bad news’ to a patient
In training, some residents are taught how to “break bad news” to a patient. Some good practices for doing this are to have information available for the patient, provide emotional support, have a plan for their next steps already formulated, and call the appropriate specialist ahead of time if you can.
Above all, it’s most important to let the patient be the one to direct their own care. Give them time to ask questions and answer them honestly and clearly. Ask them how much they want to know and help them to understand the complex change in their usual state of health.
Now, unless physicians are keeping a very close eye on their inbox, results are slipping out to patients in a void. The bad news conversations aren’t happening at all, or if they are, they’re happening at 8 p.m. on a phone call after an exhausted physician ends their shift but has to slog through their results bin, calling all the patients who shouldn’t have to find out their results in solitude.
Reaching out to these patients immediately is an honorable, kind thing to, but for a physician, knowing they need to beat the patient to opening an email creates anxiety. Plus, making these calls at whatever hour the results are released to a patient is another burden added to doctors’ already-full plates.
Interpreting results
None of us want to harm our patients. All of us want to be there for them. But this act stands in the way of delivering quality, humanizing medical care.
It is true that patients have a right to access their own medical information. It is also true that waiting anxiously on results can cause undue harm to a patient. But the across-the-board, breakneck speed of information release mandated in this act causes irreparable harm not only to patients, but to the patient-physician relationship.
No patient should find out their cancer recurred while checking their emails at their desk. No patient should first learn of a life-altering diagnosis by way of scrolling through their smartphone in bed. The role of a physician is more than just a healer – we should also be educators, interpreters, partners and, first and foremost, advocates for our patients’ needs.
Our patients are depending on us to stand up and speak out about necessary changes to this act. Result releases should be delayed until they are viewed by a physician. Our patients deserve the dignity and opportunity of a conversation with their medical provider about their test results, and physicians deserve the chance to interpret results and frame the conversation in a way which is conducive to patient understanding and healing.
Dr. Persampiere is a first-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece. You can contact them at [email protected].
There is a sudden spill of icy anxiety down your spine as you pick up your phone in your shaking hands. It’s 6 p.m.; your doctor’s office is closed. You open the message, and your worst fears are confirmed ... the cancer is back.
Or is it? You’re not sure. The biopsy sure sounds bad. But you’re an English teacher, not a doctor, and you spend the rest of the night Googling words like “tubulovillous” and “high-grade dysplasia.” You sit awake, terrified in front of the computer screen desperately trying to make sense of the possibly life-changing results. You wish you knew someone who could help you understand; you consider calling your doctor’s emergency line, or your cousin who is an ophthalmologist – anybody who can help you make sense of the results.
Or imagine another scenario: you’re a trans teen who has asked your doctor to refer to you by your preferred pronouns. You’re still presenting as your birth sex, in part because your family would disown you if they knew, and you’re not financially or emotionally ready for that step. You feel proud of yourself for advocating for your needs to your long-time physician, and excited about the resources they’ve included in your after visit summary and the referrals they’d made to gender-confirming specialists.
When you get home, you are confronted with a terrible reality that your doctor’s notes, orders, and recommendations are immediately viewable to anybody with your MyChart login – your parents knew the second your doctor signed the note. They received the notification, logged on as your guardians, and you have effectively been “outed” by the physician who took and oath to care for you and who you trusted implicitly.
How the Cures Act is affecting patients
While these examples may sound extreme, they are becoming more and more commonplace thanks to a recently enacted 21st Century Cures Act. The act was originally written to improve communication between physicians and patients. Part of the act stipulates that nearly all medical information – from notes to biopsies to lab results – must be available within 24 hours, published to a patient portal and a notification be sent to the patient by phone.
Oftentimes, this occurs before the ordering physician has even seen the results, much less interpreted them and made a plan for the patient. What happens now, not long after its enactment date, when it has become clear that the Cures Act is causing extreme harm to our patients?
Take, for example, the real example of a physician whose patient found out about her own intrauterine fetal demise by way of an EMR text message alert of “new imaging results!” sent directly to her phone. Or a physician colleague who witnessed firsthand the intrusive unhelpfulness of the Cures Act when she was informed via patient portal releasing her imaging information that she had a large, possibly malignant breast mass. “No phone call,” she said. “No human being for questions or comfort. Just a notification on my phone.”
The stories about the impact of the Cures Act across the medical community are an endless stream of anxiety, hurt, and broken trust. The relationship between a physician and a patient should be sacred, bolstered by communication and mutual respect.
In many ways, the new act feels like a third party to the patient-physician relationship – a digital imposter, oftentimes blurting out personal and life-altering medical information without any of the finesse, context, and perspective of an experienced physician.
Breaking ‘bad news’ to a patient
In training, some residents are taught how to “break bad news” to a patient. Some good practices for doing this are to have information available for the patient, provide emotional support, have a plan for their next steps already formulated, and call the appropriate specialist ahead of time if you can.
Above all, it’s most important to let the patient be the one to direct their own care. Give them time to ask questions and answer them honestly and clearly. Ask them how much they want to know and help them to understand the complex change in their usual state of health.
Now, unless physicians are keeping a very close eye on their inbox, results are slipping out to patients in a void. The bad news conversations aren’t happening at all, or if they are, they’re happening at 8 p.m. on a phone call after an exhausted physician ends their shift but has to slog through their results bin, calling all the patients who shouldn’t have to find out their results in solitude.
Reaching out to these patients immediately is an honorable, kind thing to, but for a physician, knowing they need to beat the patient to opening an email creates anxiety. Plus, making these calls at whatever hour the results are released to a patient is another burden added to doctors’ already-full plates.
Interpreting results
None of us want to harm our patients. All of us want to be there for them. But this act stands in the way of delivering quality, humanizing medical care.
It is true that patients have a right to access their own medical information. It is also true that waiting anxiously on results can cause undue harm to a patient. But the across-the-board, breakneck speed of information release mandated in this act causes irreparable harm not only to patients, but to the patient-physician relationship.
No patient should find out their cancer recurred while checking their emails at their desk. No patient should first learn of a life-altering diagnosis by way of scrolling through their smartphone in bed. The role of a physician is more than just a healer – we should also be educators, interpreters, partners and, first and foremost, advocates for our patients’ needs.
Our patients are depending on us to stand up and speak out about necessary changes to this act. Result releases should be delayed until they are viewed by a physician. Our patients deserve the dignity and opportunity of a conversation with their medical provider about their test results, and physicians deserve the chance to interpret results and frame the conversation in a way which is conducive to patient understanding and healing.
Dr. Persampiere is a first-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece. You can contact them at [email protected].
There is a sudden spill of icy anxiety down your spine as you pick up your phone in your shaking hands. It’s 6 p.m.; your doctor’s office is closed. You open the message, and your worst fears are confirmed ... the cancer is back.
Or is it? You’re not sure. The biopsy sure sounds bad. But you’re an English teacher, not a doctor, and you spend the rest of the night Googling words like “tubulovillous” and “high-grade dysplasia.” You sit awake, terrified in front of the computer screen desperately trying to make sense of the possibly life-changing results. You wish you knew someone who could help you understand; you consider calling your doctor’s emergency line, or your cousin who is an ophthalmologist – anybody who can help you make sense of the results.
Or imagine another scenario: you’re a trans teen who has asked your doctor to refer to you by your preferred pronouns. You’re still presenting as your birth sex, in part because your family would disown you if they knew, and you’re not financially or emotionally ready for that step. You feel proud of yourself for advocating for your needs to your long-time physician, and excited about the resources they’ve included in your after visit summary and the referrals they’d made to gender-confirming specialists.
When you get home, you are confronted with a terrible reality that your doctor’s notes, orders, and recommendations are immediately viewable to anybody with your MyChart login – your parents knew the second your doctor signed the note. They received the notification, logged on as your guardians, and you have effectively been “outed” by the physician who took and oath to care for you and who you trusted implicitly.
How the Cures Act is affecting patients
While these examples may sound extreme, they are becoming more and more commonplace thanks to a recently enacted 21st Century Cures Act. The act was originally written to improve communication between physicians and patients. Part of the act stipulates that nearly all medical information – from notes to biopsies to lab results – must be available within 24 hours, published to a patient portal and a notification be sent to the patient by phone.
Oftentimes, this occurs before the ordering physician has even seen the results, much less interpreted them and made a plan for the patient. What happens now, not long after its enactment date, when it has become clear that the Cures Act is causing extreme harm to our patients?
Take, for example, the real example of a physician whose patient found out about her own intrauterine fetal demise by way of an EMR text message alert of “new imaging results!” sent directly to her phone. Or a physician colleague who witnessed firsthand the intrusive unhelpfulness of the Cures Act when she was informed via patient portal releasing her imaging information that she had a large, possibly malignant breast mass. “No phone call,” she said. “No human being for questions or comfort. Just a notification on my phone.”
The stories about the impact of the Cures Act across the medical community are an endless stream of anxiety, hurt, and broken trust. The relationship between a physician and a patient should be sacred, bolstered by communication and mutual respect.
In many ways, the new act feels like a third party to the patient-physician relationship – a digital imposter, oftentimes blurting out personal and life-altering medical information without any of the finesse, context, and perspective of an experienced physician.
Breaking ‘bad news’ to a patient
In training, some residents are taught how to “break bad news” to a patient. Some good practices for doing this are to have information available for the patient, provide emotional support, have a plan for their next steps already formulated, and call the appropriate specialist ahead of time if you can.
Above all, it’s most important to let the patient be the one to direct their own care. Give them time to ask questions and answer them honestly and clearly. Ask them how much they want to know and help them to understand the complex change in their usual state of health.
Now, unless physicians are keeping a very close eye on their inbox, results are slipping out to patients in a void. The bad news conversations aren’t happening at all, or if they are, they’re happening at 8 p.m. on a phone call after an exhausted physician ends their shift but has to slog through their results bin, calling all the patients who shouldn’t have to find out their results in solitude.
Reaching out to these patients immediately is an honorable, kind thing to, but for a physician, knowing they need to beat the patient to opening an email creates anxiety. Plus, making these calls at whatever hour the results are released to a patient is another burden added to doctors’ already-full plates.
Interpreting results
None of us want to harm our patients. All of us want to be there for them. But this act stands in the way of delivering quality, humanizing medical care.
It is true that patients have a right to access their own medical information. It is also true that waiting anxiously on results can cause undue harm to a patient. But the across-the-board, breakneck speed of information release mandated in this act causes irreparable harm not only to patients, but to the patient-physician relationship.
No patient should find out their cancer recurred while checking their emails at their desk. No patient should first learn of a life-altering diagnosis by way of scrolling through their smartphone in bed. The role of a physician is more than just a healer – we should also be educators, interpreters, partners and, first and foremost, advocates for our patients’ needs.
Our patients are depending on us to stand up and speak out about necessary changes to this act. Result releases should be delayed until they are viewed by a physician. Our patients deserve the dignity and opportunity of a conversation with their medical provider about their test results, and physicians deserve the chance to interpret results and frame the conversation in a way which is conducive to patient understanding and healing.
Dr. Persampiere is a first-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece. You can contact them at [email protected].