User login
Children’s upper airways primed to combat SARS-CoV-2 infection
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antidepressant helps prevent hospitalization in COVID patients: Study
A handful of studies have suggested that for newly infected COVID-19 patients, risk for serious illness may be reduced with a short course of fluvoxamine (Luvox), a decades-old pill typically prescribed for depression or obsessive-compulsive disorder (OCD). But those were small studies involving just a few hundred people.
This week, researchers reported promising data from a large, randomized phase 3 trial that enrolled COVID-19 patients from 11 sites in Brazil. In this study, in which 1,472 people were assigned to receive either a 10-day course of fluvoxamine or placebo pills, the antidepressant cut emergency department and hospital admissions by 29%.
Findings from the new study, which have not yet been peer reviewed, were published August 23 in MedRxiv.
Around the globe, particularly in countries without access to vaccines, “treatment options that are cheap and available and supported by good-quality evidence are the only hope we’ve got to reduce mortality within high-risk populations,” said Edward Mills, PhD, professor in the department of health research methods, evidence, and impact, McMaster University, Hamilton, Ont.
The new findings came from TOGETHER, a large platform trial coordinated by Dr. Mills and colleagues to evaluate the use of fluvoxamine and other repurposed drug candidates for symptomatic, high-risk, adult outpatients with confirmed cases of COVID-19.
The trial’s adaptive format allows multiple agents to be added and tested alongside placebo in a single master protocol – similar to the United Kingdom’s Recovery trial, which found that the common steroid dexamethasone could reduce deaths among hospitalized COVID-19 patients.
In platform trials, treatment arms can be dropped for futility, as was the case with hydroxychloroquine and lopinavir-ritonavir, neither of which did better than placebo at preventing hospitalization in an earlier TOGETHER trial analysis.
Study details
In the newly reported analysis, patients were randomly assigned to receive fluvoxamine or placebo between January and August 2021. Participants took fluvoxamine 100 mg twice daily for 10 days. By comparison, the U.S. Food and Drug Administration recommends a maximum daily dose of 300 mg of fluvoxamine for patients with OCD; full psychiatric benefits occur after 6 weeks.
For the primary outcome, the investigators assessed whether the conditions of patients with COVID worsened over a 28-day period so as to require either hospitalization or observation in the emergency department for more than 6 hours. In the placebo group, 108 of 733 patients’ conditions deteriorated to this extent (14.7%); by contrast, only 77 of 739 patients in the fluvoxamine group (10.4%) met these primary criteria – a relative risk reduction of 29%.
The treatment effect was greater (34%) in the per protocol analysis of participants who completed their course of pills.
The investigators also collected data on vital signs, including temperature and oxygen saturation, as well as adverse events reported at clinic visits or through video conferencing, phone calls, or social media applications. Side effects were mild, most commonly nausea and fatigue, and did not differ significantly between active treatment and control groups, Dr. Mills said in an interview.
Amid scores of studies evaluating repurposed drugs for COVID-19, the data on fluvoxamine are “looking much more favorable than anyone could have guessed – at least anyone in infectious disease,” said Paul Sax, MD, clinical director of the Division of Infectious Diseases at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
The new TOGETHER trial results augment supportive data published in JAMA last November from a phase 2 randomized trial that was small but “very well done,” Dr. Sax told this news organization.
Those results got a boost from a subsequent study of 65 racetrack workers who chose to take fluvoxamine during a COVID-19 outbreak in the San Francisco Bay area. Forty-eight persons opted against taking the drug. In this small, nonrandomized study, “the people who chose to be treated with fluvoxamine were sicker [at baseline] than the people who didn’t go on it, and yet the [treated group] ended up better,” said Dr. Sax, who discussed accumulating data on the use of fluvoxamine for COVID-19 in a recent New England Journal of Medicine blog post.
Anti-inflammatory effect?
After reviewing the new findings, Frank Domino, MD, professor of family medicine and community health at the University of Massachusetts, Worcester, said he would encourage patients with high-risk COVID-19 to consider taking fluvoxamine to lower their risk of being hospitalized. “But I would make it clear this was not a ‘cure,’ “ he said, “and we are unsure how it helps.”
At this point, U.S. treatment guidelines do not recommend fluvoxamine as the standard of care for nonhospitalized COVID-19 patients, but the National Institutes of Health is “very aware of the data,” Dr. Sax told this news organization.
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) – a class of drugs that includes the more commonly prescribed antidepressant fluoxetine (Prozac). If prescribed off-label to COVID-19 patients, fluvoxamine should not be used within 2 weeks of starting treatment with other SSRI or monoamine oxidase inhibitor antidepressants and should be used with caution with other QT-interval prolonging medications, Dr. Sax said.
In addition, fluvoxamine can enhance the effect of antiplatelet and anticoagulant drugs, potentially triggering bleeding.
On the basis of in vitro and mouse studies of fluvoxamine, “we think it has an anti-inflammatory effect,” said child psychiatrist Angela Reiersen, MD, of Washington University, St. Louis, who came up with the idea for testing fluvoxamine in last year’s phase 2 trial and coauthored a recent article describing the drug’s potential mechanisms of action in COVID-19.
She and other researchers believe fluvoxamine’s anti-inflammatory effects derive from the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, which regulates cellular responses to stress and infection.
Fluvoxamine also inhibits the activation of platelets. “In COVID-19, there does seem to be a problem with hyperactivation of platelets and excessive blood clots forming, so it is possible this could be another mechanism where it might be helping,” Dr. Reiersen said.
If sigma-1 activation turns out to be the main mechanism underlying fluvoxamine’s benefits in COVID-19, other sigma-1 agonists, such as fluoxetine, may also help. In a retrospective analysis of thousands of adults hospitalized for COVID-19 in France early in the pandemic, those who were taking antidepressants had a 44% lower risk for intubation or death.
And in a study under review, researchers at Stanford (Calif.) University and the University of California, San Francisco, analyzed electronic health records to explore a potential link between fluoxetine use and COVID outcomes among more than 80,000 patients from over 80 institutions across the United States. Other research suggests that antipsychotics could also have protective effects for patients with COVID-19.
Long COVID, long-term challenges
On the basis of its potential mechanisms of action, fluvoxamine may be able to prevent or treat long COVID, Dr. Reiersen said. That possibility will be assessed among other secondary endpoints in two ongoing studies of repurposed drugs: the NIH’s ACTIV-6, and the University of Minnesota’s COVID-OUT, an at-home trial of ivermectin, metformin, and fluvoxamine.
Dr. Reiersen and Washington University colleagues are also analyzing longer-term outcomes of participants in their own phase 3 trial of fluvoxamine (Stop COVID 2), which was discontinued when enrollment slowed to a trickle during the U.S. vaccine rollout. Logistical hurdles and scant funding have greatly hampered efforts to test the use of off-patent drugs for COVID-19 outpatients during the pandemic.
U.S. efforts face other obstacles as well. Elsewhere in the world – including Brazil, where the TOGETHER trial was run – vaccines are scarce, and there are no monoclonal antibodies.
“People have a great sense of community duty, and they’re participating in the trials,” Dr. Mills said. “You’re in a much more political environment in the U.S. on these outpatient trials.”
The TOGETHER trial was funded by Fast Grants and the Rainwater Foundation. Dr. Reiersen is an inventor on a patent application related to methods of treating COVID-19, which was filed by Washington University, St. Louis. Dr. Mills, Dr. Domino, and Dr. Sax report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A handful of studies have suggested that for newly infected COVID-19 patients, risk for serious illness may be reduced with a short course of fluvoxamine (Luvox), a decades-old pill typically prescribed for depression or obsessive-compulsive disorder (OCD). But those were small studies involving just a few hundred people.
This week, researchers reported promising data from a large, randomized phase 3 trial that enrolled COVID-19 patients from 11 sites in Brazil. In this study, in which 1,472 people were assigned to receive either a 10-day course of fluvoxamine or placebo pills, the antidepressant cut emergency department and hospital admissions by 29%.
Findings from the new study, which have not yet been peer reviewed, were published August 23 in MedRxiv.
Around the globe, particularly in countries without access to vaccines, “treatment options that are cheap and available and supported by good-quality evidence are the only hope we’ve got to reduce mortality within high-risk populations,” said Edward Mills, PhD, professor in the department of health research methods, evidence, and impact, McMaster University, Hamilton, Ont.
The new findings came from TOGETHER, a large platform trial coordinated by Dr. Mills and colleagues to evaluate the use of fluvoxamine and other repurposed drug candidates for symptomatic, high-risk, adult outpatients with confirmed cases of COVID-19.
The trial’s adaptive format allows multiple agents to be added and tested alongside placebo in a single master protocol – similar to the United Kingdom’s Recovery trial, which found that the common steroid dexamethasone could reduce deaths among hospitalized COVID-19 patients.
In platform trials, treatment arms can be dropped for futility, as was the case with hydroxychloroquine and lopinavir-ritonavir, neither of which did better than placebo at preventing hospitalization in an earlier TOGETHER trial analysis.
Study details
In the newly reported analysis, patients were randomly assigned to receive fluvoxamine or placebo between January and August 2021. Participants took fluvoxamine 100 mg twice daily for 10 days. By comparison, the U.S. Food and Drug Administration recommends a maximum daily dose of 300 mg of fluvoxamine for patients with OCD; full psychiatric benefits occur after 6 weeks.
For the primary outcome, the investigators assessed whether the conditions of patients with COVID worsened over a 28-day period so as to require either hospitalization or observation in the emergency department for more than 6 hours. In the placebo group, 108 of 733 patients’ conditions deteriorated to this extent (14.7%); by contrast, only 77 of 739 patients in the fluvoxamine group (10.4%) met these primary criteria – a relative risk reduction of 29%.
The treatment effect was greater (34%) in the per protocol analysis of participants who completed their course of pills.
The investigators also collected data on vital signs, including temperature and oxygen saturation, as well as adverse events reported at clinic visits or through video conferencing, phone calls, or social media applications. Side effects were mild, most commonly nausea and fatigue, and did not differ significantly between active treatment and control groups, Dr. Mills said in an interview.
Amid scores of studies evaluating repurposed drugs for COVID-19, the data on fluvoxamine are “looking much more favorable than anyone could have guessed – at least anyone in infectious disease,” said Paul Sax, MD, clinical director of the Division of Infectious Diseases at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
The new TOGETHER trial results augment supportive data published in JAMA last November from a phase 2 randomized trial that was small but “very well done,” Dr. Sax told this news organization.
Those results got a boost from a subsequent study of 65 racetrack workers who chose to take fluvoxamine during a COVID-19 outbreak in the San Francisco Bay area. Forty-eight persons opted against taking the drug. In this small, nonrandomized study, “the people who chose to be treated with fluvoxamine were sicker [at baseline] than the people who didn’t go on it, and yet the [treated group] ended up better,” said Dr. Sax, who discussed accumulating data on the use of fluvoxamine for COVID-19 in a recent New England Journal of Medicine blog post.
Anti-inflammatory effect?
After reviewing the new findings, Frank Domino, MD, professor of family medicine and community health at the University of Massachusetts, Worcester, said he would encourage patients with high-risk COVID-19 to consider taking fluvoxamine to lower their risk of being hospitalized. “But I would make it clear this was not a ‘cure,’ “ he said, “and we are unsure how it helps.”
At this point, U.S. treatment guidelines do not recommend fluvoxamine as the standard of care for nonhospitalized COVID-19 patients, but the National Institutes of Health is “very aware of the data,” Dr. Sax told this news organization.
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) – a class of drugs that includes the more commonly prescribed antidepressant fluoxetine (Prozac). If prescribed off-label to COVID-19 patients, fluvoxamine should not be used within 2 weeks of starting treatment with other SSRI or monoamine oxidase inhibitor antidepressants and should be used with caution with other QT-interval prolonging medications, Dr. Sax said.
In addition, fluvoxamine can enhance the effect of antiplatelet and anticoagulant drugs, potentially triggering bleeding.
On the basis of in vitro and mouse studies of fluvoxamine, “we think it has an anti-inflammatory effect,” said child psychiatrist Angela Reiersen, MD, of Washington University, St. Louis, who came up with the idea for testing fluvoxamine in last year’s phase 2 trial and coauthored a recent article describing the drug’s potential mechanisms of action in COVID-19.
She and other researchers believe fluvoxamine’s anti-inflammatory effects derive from the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, which regulates cellular responses to stress and infection.
Fluvoxamine also inhibits the activation of platelets. “In COVID-19, there does seem to be a problem with hyperactivation of platelets and excessive blood clots forming, so it is possible this could be another mechanism where it might be helping,” Dr. Reiersen said.
If sigma-1 activation turns out to be the main mechanism underlying fluvoxamine’s benefits in COVID-19, other sigma-1 agonists, such as fluoxetine, may also help. In a retrospective analysis of thousands of adults hospitalized for COVID-19 in France early in the pandemic, those who were taking antidepressants had a 44% lower risk for intubation or death.
And in a study under review, researchers at Stanford (Calif.) University and the University of California, San Francisco, analyzed electronic health records to explore a potential link between fluoxetine use and COVID outcomes among more than 80,000 patients from over 80 institutions across the United States. Other research suggests that antipsychotics could also have protective effects for patients with COVID-19.
Long COVID, long-term challenges
On the basis of its potential mechanisms of action, fluvoxamine may be able to prevent or treat long COVID, Dr. Reiersen said. That possibility will be assessed among other secondary endpoints in two ongoing studies of repurposed drugs: the NIH’s ACTIV-6, and the University of Minnesota’s COVID-OUT, an at-home trial of ivermectin, metformin, and fluvoxamine.
Dr. Reiersen and Washington University colleagues are also analyzing longer-term outcomes of participants in their own phase 3 trial of fluvoxamine (Stop COVID 2), which was discontinued when enrollment slowed to a trickle during the U.S. vaccine rollout. Logistical hurdles and scant funding have greatly hampered efforts to test the use of off-patent drugs for COVID-19 outpatients during the pandemic.
U.S. efforts face other obstacles as well. Elsewhere in the world – including Brazil, where the TOGETHER trial was run – vaccines are scarce, and there are no monoclonal antibodies.
“People have a great sense of community duty, and they’re participating in the trials,” Dr. Mills said. “You’re in a much more political environment in the U.S. on these outpatient trials.”
The TOGETHER trial was funded by Fast Grants and the Rainwater Foundation. Dr. Reiersen is an inventor on a patent application related to methods of treating COVID-19, which was filed by Washington University, St. Louis. Dr. Mills, Dr. Domino, and Dr. Sax report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A handful of studies have suggested that for newly infected COVID-19 patients, risk for serious illness may be reduced with a short course of fluvoxamine (Luvox), a decades-old pill typically prescribed for depression or obsessive-compulsive disorder (OCD). But those were small studies involving just a few hundred people.
This week, researchers reported promising data from a large, randomized phase 3 trial that enrolled COVID-19 patients from 11 sites in Brazil. In this study, in which 1,472 people were assigned to receive either a 10-day course of fluvoxamine or placebo pills, the antidepressant cut emergency department and hospital admissions by 29%.
Findings from the new study, which have not yet been peer reviewed, were published August 23 in MedRxiv.
Around the globe, particularly in countries without access to vaccines, “treatment options that are cheap and available and supported by good-quality evidence are the only hope we’ve got to reduce mortality within high-risk populations,” said Edward Mills, PhD, professor in the department of health research methods, evidence, and impact, McMaster University, Hamilton, Ont.
The new findings came from TOGETHER, a large platform trial coordinated by Dr. Mills and colleagues to evaluate the use of fluvoxamine and other repurposed drug candidates for symptomatic, high-risk, adult outpatients with confirmed cases of COVID-19.
The trial’s adaptive format allows multiple agents to be added and tested alongside placebo in a single master protocol – similar to the United Kingdom’s Recovery trial, which found that the common steroid dexamethasone could reduce deaths among hospitalized COVID-19 patients.
In platform trials, treatment arms can be dropped for futility, as was the case with hydroxychloroquine and lopinavir-ritonavir, neither of which did better than placebo at preventing hospitalization in an earlier TOGETHER trial analysis.
Study details
In the newly reported analysis, patients were randomly assigned to receive fluvoxamine or placebo between January and August 2021. Participants took fluvoxamine 100 mg twice daily for 10 days. By comparison, the U.S. Food and Drug Administration recommends a maximum daily dose of 300 mg of fluvoxamine for patients with OCD; full psychiatric benefits occur after 6 weeks.
For the primary outcome, the investigators assessed whether the conditions of patients with COVID worsened over a 28-day period so as to require either hospitalization or observation in the emergency department for more than 6 hours. In the placebo group, 108 of 733 patients’ conditions deteriorated to this extent (14.7%); by contrast, only 77 of 739 patients in the fluvoxamine group (10.4%) met these primary criteria – a relative risk reduction of 29%.
The treatment effect was greater (34%) in the per protocol analysis of participants who completed their course of pills.
The investigators also collected data on vital signs, including temperature and oxygen saturation, as well as adverse events reported at clinic visits or through video conferencing, phone calls, or social media applications. Side effects were mild, most commonly nausea and fatigue, and did not differ significantly between active treatment and control groups, Dr. Mills said in an interview.
Amid scores of studies evaluating repurposed drugs for COVID-19, the data on fluvoxamine are “looking much more favorable than anyone could have guessed – at least anyone in infectious disease,” said Paul Sax, MD, clinical director of the Division of Infectious Diseases at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, Boston.
The new TOGETHER trial results augment supportive data published in JAMA last November from a phase 2 randomized trial that was small but “very well done,” Dr. Sax told this news organization.
Those results got a boost from a subsequent study of 65 racetrack workers who chose to take fluvoxamine during a COVID-19 outbreak in the San Francisco Bay area. Forty-eight persons opted against taking the drug. In this small, nonrandomized study, “the people who chose to be treated with fluvoxamine were sicker [at baseline] than the people who didn’t go on it, and yet the [treated group] ended up better,” said Dr. Sax, who discussed accumulating data on the use of fluvoxamine for COVID-19 in a recent New England Journal of Medicine blog post.
Anti-inflammatory effect?
After reviewing the new findings, Frank Domino, MD, professor of family medicine and community health at the University of Massachusetts, Worcester, said he would encourage patients with high-risk COVID-19 to consider taking fluvoxamine to lower their risk of being hospitalized. “But I would make it clear this was not a ‘cure,’ “ he said, “and we are unsure how it helps.”
At this point, U.S. treatment guidelines do not recommend fluvoxamine as the standard of care for nonhospitalized COVID-19 patients, but the National Institutes of Health is “very aware of the data,” Dr. Sax told this news organization.
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) – a class of drugs that includes the more commonly prescribed antidepressant fluoxetine (Prozac). If prescribed off-label to COVID-19 patients, fluvoxamine should not be used within 2 weeks of starting treatment with other SSRI or monoamine oxidase inhibitor antidepressants and should be used with caution with other QT-interval prolonging medications, Dr. Sax said.
In addition, fluvoxamine can enhance the effect of antiplatelet and anticoagulant drugs, potentially triggering bleeding.
On the basis of in vitro and mouse studies of fluvoxamine, “we think it has an anti-inflammatory effect,” said child psychiatrist Angela Reiersen, MD, of Washington University, St. Louis, who came up with the idea for testing fluvoxamine in last year’s phase 2 trial and coauthored a recent article describing the drug’s potential mechanisms of action in COVID-19.
She and other researchers believe fluvoxamine’s anti-inflammatory effects derive from the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, which regulates cellular responses to stress and infection.
Fluvoxamine also inhibits the activation of platelets. “In COVID-19, there does seem to be a problem with hyperactivation of platelets and excessive blood clots forming, so it is possible this could be another mechanism where it might be helping,” Dr. Reiersen said.
If sigma-1 activation turns out to be the main mechanism underlying fluvoxamine’s benefits in COVID-19, other sigma-1 agonists, such as fluoxetine, may also help. In a retrospective analysis of thousands of adults hospitalized for COVID-19 in France early in the pandemic, those who were taking antidepressants had a 44% lower risk for intubation or death.
And in a study under review, researchers at Stanford (Calif.) University and the University of California, San Francisco, analyzed electronic health records to explore a potential link between fluoxetine use and COVID outcomes among more than 80,000 patients from over 80 institutions across the United States. Other research suggests that antipsychotics could also have protective effects for patients with COVID-19.
Long COVID, long-term challenges
On the basis of its potential mechanisms of action, fluvoxamine may be able to prevent or treat long COVID, Dr. Reiersen said. That possibility will be assessed among other secondary endpoints in two ongoing studies of repurposed drugs: the NIH’s ACTIV-6, and the University of Minnesota’s COVID-OUT, an at-home trial of ivermectin, metformin, and fluvoxamine.
Dr. Reiersen and Washington University colleagues are also analyzing longer-term outcomes of participants in their own phase 3 trial of fluvoxamine (Stop COVID 2), which was discontinued when enrollment slowed to a trickle during the U.S. vaccine rollout. Logistical hurdles and scant funding have greatly hampered efforts to test the use of off-patent drugs for COVID-19 outpatients during the pandemic.
U.S. efforts face other obstacles as well. Elsewhere in the world – including Brazil, where the TOGETHER trial was run – vaccines are scarce, and there are no monoclonal antibodies.
“People have a great sense of community duty, and they’re participating in the trials,” Dr. Mills said. “You’re in a much more political environment in the U.S. on these outpatient trials.”
The TOGETHER trial was funded by Fast Grants and the Rainwater Foundation. Dr. Reiersen is an inventor on a patent application related to methods of treating COVID-19, which was filed by Washington University, St. Louis. Dr. Mills, Dr. Domino, and Dr. Sax report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Advocates seek to reframe masks as a disability accommodation
As governors and legislatures in states such as Texas, Florida, South Carolina, and Arkansas have banned schools and other entities from implementing mask mandates, disability rights advocates have pushed back. In federal civil rights lawsuits, they argue that bans on mask mandates violate antidiscrimination laws protecting people with disabilities.
For unvaccinated and immunosuppressed individuals, masks can provide crucial protection from SARS-CoV-2.
argues Mical Raz, MD, PhD, a professor at the University of Rochester (N.Y.) and a physician at Strong Memorial Hospital, also in Rochester, New York, in an article published in JAMA with coauthor Doron Dorfman, LLB, JSD.
This news organization talked with Dr. Raz about approaching mask requirements as disability accommodation during the COVID-19 pandemic. The following interview was lightly edited for length and clarity.
How did you come to think about mask requirements as a form of disability accommodation?
I saw a tweet from a professor at a university who said they couldn’t ask students about their vaccination status or to wear a mask. All agency was removed from the professor to take care of and protect themselves. I thought, well, that can’t be right. And ostensibly, that would be particularly dangerous for somebody with immunosuppression for whom the vaccine is not adequately protective. So, I called my friend, Doron Dorfman, and asked him to help me think through the legal part of this. We fleshed it out and wrote the article that same night.
How novel is it to view accommodations for people who are immunosuppressed through the lens of disability accommodation?
I think there has not been enough focus during the pandemic on individuals with disabilities or on how disability law can be mobilized during this pandemic to help supplement the public health law. This framework should be used a lot more because it’s good for everybody, not just for individuals with disabilities.
For example, take what’s called the “curb effect.” If you expand sidewalks, yes, it helps individuals who use a wheelchair. But it also helps me as a mom with a stroller. It helps somebody with a shopping cart, or a kid with a bike. If we adopt policies that are inclusive to those who are disadvantaged, it’s good for everybody. We should always strive to be an inclusive society, not just because it’s the right thing to do but because it really makes our society better.
How can mask requirements be used as a form of disability accommodation, as you argue in the JAMA article?
The ADA requires employers to provide reasonable accommodations for disability. In this case, the disability is your immunosuppressive status. We have an abundance of evidence showing individuals who are immunocompromised and vaccinated are still inadequately protected from the SARS-CoV-2 virus. So, there is absolute data to show individuals with immunosuppression have a disability that requires accommodation.
The ADA has a mandate requiring employers to adjust or modify policies in order to accommodate a disability. There are certain situations in which you cannot or do not need to accommodate a disability, when it would fundamentally alter the kind of employment you offer or if it’s an undue burden or hardship. But given that we’ve been wearing masks and working remotely for a year now, arguing that somehow these accommodations are no longer possible seems disingenuous.
In that way, allowing a person who’s immunocompromised to require those around them to mask is a form of modified protective policies. And in this case, those policies line up with a public health good, masking in the face of the highly contagious Delta variant ravaging our country right now.
In your view, can this argument be used in the mask debates happening right now across the country?
This argument can and should be useful for a couple of different lawsuits that are now underway in different states. I hope our article will provide further support for those suits. And I hope in school board hearings, when parents and teachers are talking about their concerns, this could be one way to argue for why we should allow mask mandates in classes. I’ve received emails from parents who said they’re going to bring this article to their school board hearing.
I also hope this could shift the narrative around the pandemic. Instead of focusing on individual responsibility – I got my vaccine shot so I’m fine – let’s focus on how we create an inclusive environment where we protect everybody, including those who cannot be vaccinated because of age or disability, or those who are vaccinated but inadequately protected because of their underlying conditions.
In the JAMA article, you talk about how our pandemic response has focused on individual health and how that individual focus can be ableist. Can you explain that point?
I think this idea that we just make our choices – like whether to get vaccinated or wear a mask, or not – and live with it really perpetuates a highly individualistic and ableist mindset. It doesn’t consider the people I admit to the hospital who are vaccinated but have a heart transplant and didn’t mount the sufficient immune response. Or even the people who chose not to be vaccinated because they were exposed to hours and hours of misinformation on TV.
We like to individualize everything, focusing on personal responsibility and choices, but a pandemic is one of those moments where everybody’s choices affect everybody else. Laying responsibility at the doorstep of each person, rather than thinking about what steps we as a society could be taking, is cheap and politically expedient. There is no public health rationale behind the bans on mask requirements in states like Texas, Iowa, and Florida. These choices are about politics. And the price is always borne by the most disadvantaged among us.
A version of this article first appeared on Medscape.com.
As governors and legislatures in states such as Texas, Florida, South Carolina, and Arkansas have banned schools and other entities from implementing mask mandates, disability rights advocates have pushed back. In federal civil rights lawsuits, they argue that bans on mask mandates violate antidiscrimination laws protecting people with disabilities.
For unvaccinated and immunosuppressed individuals, masks can provide crucial protection from SARS-CoV-2.
argues Mical Raz, MD, PhD, a professor at the University of Rochester (N.Y.) and a physician at Strong Memorial Hospital, also in Rochester, New York, in an article published in JAMA with coauthor Doron Dorfman, LLB, JSD.
This news organization talked with Dr. Raz about approaching mask requirements as disability accommodation during the COVID-19 pandemic. The following interview was lightly edited for length and clarity.
How did you come to think about mask requirements as a form of disability accommodation?
I saw a tweet from a professor at a university who said they couldn’t ask students about their vaccination status or to wear a mask. All agency was removed from the professor to take care of and protect themselves. I thought, well, that can’t be right. And ostensibly, that would be particularly dangerous for somebody with immunosuppression for whom the vaccine is not adequately protective. So, I called my friend, Doron Dorfman, and asked him to help me think through the legal part of this. We fleshed it out and wrote the article that same night.
How novel is it to view accommodations for people who are immunosuppressed through the lens of disability accommodation?
I think there has not been enough focus during the pandemic on individuals with disabilities or on how disability law can be mobilized during this pandemic to help supplement the public health law. This framework should be used a lot more because it’s good for everybody, not just for individuals with disabilities.
For example, take what’s called the “curb effect.” If you expand sidewalks, yes, it helps individuals who use a wheelchair. But it also helps me as a mom with a stroller. It helps somebody with a shopping cart, or a kid with a bike. If we adopt policies that are inclusive to those who are disadvantaged, it’s good for everybody. We should always strive to be an inclusive society, not just because it’s the right thing to do but because it really makes our society better.
How can mask requirements be used as a form of disability accommodation, as you argue in the JAMA article?
The ADA requires employers to provide reasonable accommodations for disability. In this case, the disability is your immunosuppressive status. We have an abundance of evidence showing individuals who are immunocompromised and vaccinated are still inadequately protected from the SARS-CoV-2 virus. So, there is absolute data to show individuals with immunosuppression have a disability that requires accommodation.
The ADA has a mandate requiring employers to adjust or modify policies in order to accommodate a disability. There are certain situations in which you cannot or do not need to accommodate a disability, when it would fundamentally alter the kind of employment you offer or if it’s an undue burden or hardship. But given that we’ve been wearing masks and working remotely for a year now, arguing that somehow these accommodations are no longer possible seems disingenuous.
In that way, allowing a person who’s immunocompromised to require those around them to mask is a form of modified protective policies. And in this case, those policies line up with a public health good, masking in the face of the highly contagious Delta variant ravaging our country right now.
In your view, can this argument be used in the mask debates happening right now across the country?
This argument can and should be useful for a couple of different lawsuits that are now underway in different states. I hope our article will provide further support for those suits. And I hope in school board hearings, when parents and teachers are talking about their concerns, this could be one way to argue for why we should allow mask mandates in classes. I’ve received emails from parents who said they’re going to bring this article to their school board hearing.
I also hope this could shift the narrative around the pandemic. Instead of focusing on individual responsibility – I got my vaccine shot so I’m fine – let’s focus on how we create an inclusive environment where we protect everybody, including those who cannot be vaccinated because of age or disability, or those who are vaccinated but inadequately protected because of their underlying conditions.
In the JAMA article, you talk about how our pandemic response has focused on individual health and how that individual focus can be ableist. Can you explain that point?
I think this idea that we just make our choices – like whether to get vaccinated or wear a mask, or not – and live with it really perpetuates a highly individualistic and ableist mindset. It doesn’t consider the people I admit to the hospital who are vaccinated but have a heart transplant and didn’t mount the sufficient immune response. Or even the people who chose not to be vaccinated because they were exposed to hours and hours of misinformation on TV.
We like to individualize everything, focusing on personal responsibility and choices, but a pandemic is one of those moments where everybody’s choices affect everybody else. Laying responsibility at the doorstep of each person, rather than thinking about what steps we as a society could be taking, is cheap and politically expedient. There is no public health rationale behind the bans on mask requirements in states like Texas, Iowa, and Florida. These choices are about politics. And the price is always borne by the most disadvantaged among us.
A version of this article first appeared on Medscape.com.
As governors and legislatures in states such as Texas, Florida, South Carolina, and Arkansas have banned schools and other entities from implementing mask mandates, disability rights advocates have pushed back. In federal civil rights lawsuits, they argue that bans on mask mandates violate antidiscrimination laws protecting people with disabilities.
For unvaccinated and immunosuppressed individuals, masks can provide crucial protection from SARS-CoV-2.
argues Mical Raz, MD, PhD, a professor at the University of Rochester (N.Y.) and a physician at Strong Memorial Hospital, also in Rochester, New York, in an article published in JAMA with coauthor Doron Dorfman, LLB, JSD.
This news organization talked with Dr. Raz about approaching mask requirements as disability accommodation during the COVID-19 pandemic. The following interview was lightly edited for length and clarity.
How did you come to think about mask requirements as a form of disability accommodation?
I saw a tweet from a professor at a university who said they couldn’t ask students about their vaccination status or to wear a mask. All agency was removed from the professor to take care of and protect themselves. I thought, well, that can’t be right. And ostensibly, that would be particularly dangerous for somebody with immunosuppression for whom the vaccine is not adequately protective. So, I called my friend, Doron Dorfman, and asked him to help me think through the legal part of this. We fleshed it out and wrote the article that same night.
How novel is it to view accommodations for people who are immunosuppressed through the lens of disability accommodation?
I think there has not been enough focus during the pandemic on individuals with disabilities or on how disability law can be mobilized during this pandemic to help supplement the public health law. This framework should be used a lot more because it’s good for everybody, not just for individuals with disabilities.
For example, take what’s called the “curb effect.” If you expand sidewalks, yes, it helps individuals who use a wheelchair. But it also helps me as a mom with a stroller. It helps somebody with a shopping cart, or a kid with a bike. If we adopt policies that are inclusive to those who are disadvantaged, it’s good for everybody. We should always strive to be an inclusive society, not just because it’s the right thing to do but because it really makes our society better.
How can mask requirements be used as a form of disability accommodation, as you argue in the JAMA article?
The ADA requires employers to provide reasonable accommodations for disability. In this case, the disability is your immunosuppressive status. We have an abundance of evidence showing individuals who are immunocompromised and vaccinated are still inadequately protected from the SARS-CoV-2 virus. So, there is absolute data to show individuals with immunosuppression have a disability that requires accommodation.
The ADA has a mandate requiring employers to adjust or modify policies in order to accommodate a disability. There are certain situations in which you cannot or do not need to accommodate a disability, when it would fundamentally alter the kind of employment you offer or if it’s an undue burden or hardship. But given that we’ve been wearing masks and working remotely for a year now, arguing that somehow these accommodations are no longer possible seems disingenuous.
In that way, allowing a person who’s immunocompromised to require those around them to mask is a form of modified protective policies. And in this case, those policies line up with a public health good, masking in the face of the highly contagious Delta variant ravaging our country right now.
In your view, can this argument be used in the mask debates happening right now across the country?
This argument can and should be useful for a couple of different lawsuits that are now underway in different states. I hope our article will provide further support for those suits. And I hope in school board hearings, when parents and teachers are talking about their concerns, this could be one way to argue for why we should allow mask mandates in classes. I’ve received emails from parents who said they’re going to bring this article to their school board hearing.
I also hope this could shift the narrative around the pandemic. Instead of focusing on individual responsibility – I got my vaccine shot so I’m fine – let’s focus on how we create an inclusive environment where we protect everybody, including those who cannot be vaccinated because of age or disability, or those who are vaccinated but inadequately protected because of their underlying conditions.
In the JAMA article, you talk about how our pandemic response has focused on individual health and how that individual focus can be ableist. Can you explain that point?
I think this idea that we just make our choices – like whether to get vaccinated or wear a mask, or not – and live with it really perpetuates a highly individualistic and ableist mindset. It doesn’t consider the people I admit to the hospital who are vaccinated but have a heart transplant and didn’t mount the sufficient immune response. Or even the people who chose not to be vaccinated because they were exposed to hours and hours of misinformation on TV.
We like to individualize everything, focusing on personal responsibility and choices, but a pandemic is one of those moments where everybody’s choices affect everybody else. Laying responsibility at the doorstep of each person, rather than thinking about what steps we as a society could be taking, is cheap and politically expedient. There is no public health rationale behind the bans on mask requirements in states like Texas, Iowa, and Florida. These choices are about politics. And the price is always borne by the most disadvantaged among us.
A version of this article first appeared on Medscape.com.
NIH to study COVID vaccine booster in people with autoimmune disease
In the wake of the Centers for Disease Control and Prevention’s recommendation for a third COVID-19 mRNA vaccine dose for immunocompromised people and the Food and Drug Administration’s authorization of the third dose, the according to an announcement.
The investigators of the trial, called COVID‐19 Booster Vaccine in Autoimmune Disease Non‐Responders, also want to determine if pausing immunosuppressive therapy for autoimmune disease improves the antibody response to an extra dose of a COVID-19 vaccine.
The trial will specifically look at the effects of mycophenolate mofetil (MMF) or mycophenolic acid (MPA), and methotrexate (MTX), or receipt of B cell–depletion therapy such as rituximab within the past 12 months on immune response to a booster dose in people with systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, systemic sclerosis, or pemphigus. They have to have either no serologic response to their initial COVID-19 vaccine regimen or a suboptimal response, defined as a Roche Elecsys Anti-SARS-CoV-2 S (RBD) result greater than or equal to 50 U/mL.
The results of studies conducted in solid-organ transplant recipients who take immunosuppressants showed that an extra dose of vaccine could improve the immune response to the vaccine in many of the individuals, which suggests that the same approach might work in people with autoimmune disease who need treatment with immunosuppressive drugs. Improving the immune response of people with autoimmune disease to COVID-19 vaccines is important because higher rates of severe COVID-19 and death have been reported in this group of patients than in the general population, and it is unclear whether this is attributable to the autoimmune disease, the immunosuppressive medications taken to treat it, or both.
The open-label trial, conducted by the NIAID-funded Autoimmunity Centers of Excellence, aims to enroll 600 people aged 18 years and older with those conditions at 15-20 sites in the United States.
Because medications commonly taken by people with these conditions have been associated with poorer immune responses to vaccines, the trial will randomize the following two cohorts to stop or continue taking their immunosuppressive medication(s) or stop them before and after the booster according to protocol:
- Cohort 1 includes people who are taking MMF or MPA, without additional B cell–depleting medications or MTX.
- Cohort 2 includes people who are taking MTX without additional B cell–depleting medications or MMF/MPA.
A third, nonrandomized cohort consists of people who have received B cell–depletion therapy within the past 12 months regardless of whether they are also taking MMF/MPA or MTX.
Besides the cohort-specific exclusions, other rheumatic disease medications, including biologics, are allowed in the groups.
The primary outcome of the trial is the proportion of participants who have a protective antibody response at week 4. Secondary outcomes will examine various antibody responses at intervals, changes in disease activity across autoimmune diseases, adverse events, and SARS-CoV-2 infections out to 48 weeks.
Study participants will be followed for a total of 13 months. Preliminary results are expected in November 2021, according to the National Institutes of Health.
The trial is being led by Judith James, MD, PhD; Meggan Mackay, MD, MS; Dinesh Khanna, MBBS, MSc; and Amit Bar-Or, MD.
In the wake of the Centers for Disease Control and Prevention’s recommendation for a third COVID-19 mRNA vaccine dose for immunocompromised people and the Food and Drug Administration’s authorization of the third dose, the according to an announcement.
The investigators of the trial, called COVID‐19 Booster Vaccine in Autoimmune Disease Non‐Responders, also want to determine if pausing immunosuppressive therapy for autoimmune disease improves the antibody response to an extra dose of a COVID-19 vaccine.
The trial will specifically look at the effects of mycophenolate mofetil (MMF) or mycophenolic acid (MPA), and methotrexate (MTX), or receipt of B cell–depletion therapy such as rituximab within the past 12 months on immune response to a booster dose in people with systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, systemic sclerosis, or pemphigus. They have to have either no serologic response to their initial COVID-19 vaccine regimen or a suboptimal response, defined as a Roche Elecsys Anti-SARS-CoV-2 S (RBD) result greater than or equal to 50 U/mL.
The results of studies conducted in solid-organ transplant recipients who take immunosuppressants showed that an extra dose of vaccine could improve the immune response to the vaccine in many of the individuals, which suggests that the same approach might work in people with autoimmune disease who need treatment with immunosuppressive drugs. Improving the immune response of people with autoimmune disease to COVID-19 vaccines is important because higher rates of severe COVID-19 and death have been reported in this group of patients than in the general population, and it is unclear whether this is attributable to the autoimmune disease, the immunosuppressive medications taken to treat it, or both.
The open-label trial, conducted by the NIAID-funded Autoimmunity Centers of Excellence, aims to enroll 600 people aged 18 years and older with those conditions at 15-20 sites in the United States.
Because medications commonly taken by people with these conditions have been associated with poorer immune responses to vaccines, the trial will randomize the following two cohorts to stop or continue taking their immunosuppressive medication(s) or stop them before and after the booster according to protocol:
- Cohort 1 includes people who are taking MMF or MPA, without additional B cell–depleting medications or MTX.
- Cohort 2 includes people who are taking MTX without additional B cell–depleting medications or MMF/MPA.
A third, nonrandomized cohort consists of people who have received B cell–depletion therapy within the past 12 months regardless of whether they are also taking MMF/MPA or MTX.
Besides the cohort-specific exclusions, other rheumatic disease medications, including biologics, are allowed in the groups.
The primary outcome of the trial is the proportion of participants who have a protective antibody response at week 4. Secondary outcomes will examine various antibody responses at intervals, changes in disease activity across autoimmune diseases, adverse events, and SARS-CoV-2 infections out to 48 weeks.
Study participants will be followed for a total of 13 months. Preliminary results are expected in November 2021, according to the National Institutes of Health.
The trial is being led by Judith James, MD, PhD; Meggan Mackay, MD, MS; Dinesh Khanna, MBBS, MSc; and Amit Bar-Or, MD.
In the wake of the Centers for Disease Control and Prevention’s recommendation for a third COVID-19 mRNA vaccine dose for immunocompromised people and the Food and Drug Administration’s authorization of the third dose, the according to an announcement.
The investigators of the trial, called COVID‐19 Booster Vaccine in Autoimmune Disease Non‐Responders, also want to determine if pausing immunosuppressive therapy for autoimmune disease improves the antibody response to an extra dose of a COVID-19 vaccine.
The trial will specifically look at the effects of mycophenolate mofetil (MMF) or mycophenolic acid (MPA), and methotrexate (MTX), or receipt of B cell–depletion therapy such as rituximab within the past 12 months on immune response to a booster dose in people with systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, systemic sclerosis, or pemphigus. They have to have either no serologic response to their initial COVID-19 vaccine regimen or a suboptimal response, defined as a Roche Elecsys Anti-SARS-CoV-2 S (RBD) result greater than or equal to 50 U/mL.
The results of studies conducted in solid-organ transplant recipients who take immunosuppressants showed that an extra dose of vaccine could improve the immune response to the vaccine in many of the individuals, which suggests that the same approach might work in people with autoimmune disease who need treatment with immunosuppressive drugs. Improving the immune response of people with autoimmune disease to COVID-19 vaccines is important because higher rates of severe COVID-19 and death have been reported in this group of patients than in the general population, and it is unclear whether this is attributable to the autoimmune disease, the immunosuppressive medications taken to treat it, or both.
The open-label trial, conducted by the NIAID-funded Autoimmunity Centers of Excellence, aims to enroll 600 people aged 18 years and older with those conditions at 15-20 sites in the United States.
Because medications commonly taken by people with these conditions have been associated with poorer immune responses to vaccines, the trial will randomize the following two cohorts to stop or continue taking their immunosuppressive medication(s) or stop them before and after the booster according to protocol:
- Cohort 1 includes people who are taking MMF or MPA, without additional B cell–depleting medications or MTX.
- Cohort 2 includes people who are taking MTX without additional B cell–depleting medications or MMF/MPA.
A third, nonrandomized cohort consists of people who have received B cell–depletion therapy within the past 12 months regardless of whether they are also taking MMF/MPA or MTX.
Besides the cohort-specific exclusions, other rheumatic disease medications, including biologics, are allowed in the groups.
The primary outcome of the trial is the proportion of participants who have a protective antibody response at week 4. Secondary outcomes will examine various antibody responses at intervals, changes in disease activity across autoimmune diseases, adverse events, and SARS-CoV-2 infections out to 48 weeks.
Study participants will be followed for a total of 13 months. Preliminary results are expected in November 2021, according to the National Institutes of Health.
The trial is being led by Judith James, MD, PhD; Meggan Mackay, MD, MS; Dinesh Khanna, MBBS, MSc; and Amit Bar-Or, MD.
Stop blaming the unvaccinated
As politicians battle over masks and mandates, heated rhetoric has been used to describe the fourth heartbreaking surge in COVID as a “pandemic of the unvaccinated.”
While it may serve to further divide red and blue states, I disagree with the assertion that the current surge in cases is driven simply by the unvaccinated. Why? First, the premise would assume complete efficacy with our vaccinated population, which is statistically incorrect (at least 15 million of the U.S. population never completed a second round of injections), which means they were not considered “fully vaccinated.”
Alternately, we need to examine what has occurred in nations with significantly higher vaccination rates than ours (the United Kingdom and Israel) to realize that variants have overrun the dramatic success achieved in those countries as well. Israel, once considered to be the most vaccinated country in the world, is facing a brutal fourth wave of COVID that has sent the country spiraling into another heartbreaking lockdown.
The unvaccinated could hardly be blamed for what is happening in either of these highly vaccinated countries.
The concept of blame
So why use blame? It defeats the purpose of encouraging those who are hesitant or possibly misinformed or disenfranchised to move forward. It lacks compassion. It does not encompass the art and science of nursing (for example, the University of Southern Indiana), such as those that hospitals have used to frame optimal nursing care. I abhor the idea of labeling because it denies the prospect of future comprehension.
Labeling reminds me of one of the saddest cases in my career.
An unfortunate case
I was the nurse caring for a man from a motor vehicular accident where an entire family was brutally killed. My patient was alleged to be the cause, with a blood alcohol level of 0.40%+ post hydration, intubated and ventilated, with a flailed chest and multiple orthopedic injuries as well as blunt head trauma. He was secured to the bed with handcuffs, although that was unnecessary. Multiple times I was asked how I could possibly care for such an individual, by the police and even a few colleagues. But it was not my place to judge the man.
He was in pain, and he was dying. I comforted him for the 2 weeks it took his battered body to pass into the next realm. No one visited him except the police, eagerly waiting for the man to wake up to explain the tragic events that occurred. It was my job to ease what pain I could and protect him from labels. Did he deserve the labels? Who knew? I did not care. I cared about his writhing and his physical anguish.
The comparison
Blame did not help the situation then, nor does it help us move forward now. As nurses, we seek to work within a framework of understanding. As we tire of caring for thousands of COVID patients, we do not stop to ask if they “deserve” care or if they have taken precautions and lived reasonably prior to seeking assistance for disease. We would not be nurses if we did this.
Think about Gov. Greg Abbott, who has asked that Texans not be allowed to mandate masks for children returning to school. He has recently been diagnosed with COVID, despite assuring the public he is fully vaccinated. Politically, his diagnosis could be visualized as a fiasco for a purple state where he has been adamant in denying the efficacy of masks for children.
Yet, his diagnosis should not be fodder for the press. The first concern should be his health and well-being, similar for any man of his age and potential comorbidity.
Conclusion
We should be people first, human beings that remain interconnected by our need for care and survival, not conservatives, independents, or liberals, not “vaccinated or unvaccinated,” not seen as “breakthrough” infections, or the immunosuppressed possibly unable to mount a robust response to COVID.
Labels do not define the ability to effectively defeat coronavirus or variants, as highly vaccinated countries have demonstrated in recent months. We are in the midst of a global pandemic, and the battle is raging onward.
In fact, the longer this pandemic continues, the more likely it is we will need to live with this as an endemic disease, so we should stop blaming those who become ill and need support.
It could be any of us.
A version of this article first appeared on Medscape.com.
As politicians battle over masks and mandates, heated rhetoric has been used to describe the fourth heartbreaking surge in COVID as a “pandemic of the unvaccinated.”
While it may serve to further divide red and blue states, I disagree with the assertion that the current surge in cases is driven simply by the unvaccinated. Why? First, the premise would assume complete efficacy with our vaccinated population, which is statistically incorrect (at least 15 million of the U.S. population never completed a second round of injections), which means they were not considered “fully vaccinated.”
Alternately, we need to examine what has occurred in nations with significantly higher vaccination rates than ours (the United Kingdom and Israel) to realize that variants have overrun the dramatic success achieved in those countries as well. Israel, once considered to be the most vaccinated country in the world, is facing a brutal fourth wave of COVID that has sent the country spiraling into another heartbreaking lockdown.
The unvaccinated could hardly be blamed for what is happening in either of these highly vaccinated countries.
The concept of blame
So why use blame? It defeats the purpose of encouraging those who are hesitant or possibly misinformed or disenfranchised to move forward. It lacks compassion. It does not encompass the art and science of nursing (for example, the University of Southern Indiana), such as those that hospitals have used to frame optimal nursing care. I abhor the idea of labeling because it denies the prospect of future comprehension.
Labeling reminds me of one of the saddest cases in my career.
An unfortunate case
I was the nurse caring for a man from a motor vehicular accident where an entire family was brutally killed. My patient was alleged to be the cause, with a blood alcohol level of 0.40%+ post hydration, intubated and ventilated, with a flailed chest and multiple orthopedic injuries as well as blunt head trauma. He was secured to the bed with handcuffs, although that was unnecessary. Multiple times I was asked how I could possibly care for such an individual, by the police and even a few colleagues. But it was not my place to judge the man.
He was in pain, and he was dying. I comforted him for the 2 weeks it took his battered body to pass into the next realm. No one visited him except the police, eagerly waiting for the man to wake up to explain the tragic events that occurred. It was my job to ease what pain I could and protect him from labels. Did he deserve the labels? Who knew? I did not care. I cared about his writhing and his physical anguish.
The comparison
Blame did not help the situation then, nor does it help us move forward now. As nurses, we seek to work within a framework of understanding. As we tire of caring for thousands of COVID patients, we do not stop to ask if they “deserve” care or if they have taken precautions and lived reasonably prior to seeking assistance for disease. We would not be nurses if we did this.
Think about Gov. Greg Abbott, who has asked that Texans not be allowed to mandate masks for children returning to school. He has recently been diagnosed with COVID, despite assuring the public he is fully vaccinated. Politically, his diagnosis could be visualized as a fiasco for a purple state where he has been adamant in denying the efficacy of masks for children.
Yet, his diagnosis should not be fodder for the press. The first concern should be his health and well-being, similar for any man of his age and potential comorbidity.
Conclusion
We should be people first, human beings that remain interconnected by our need for care and survival, not conservatives, independents, or liberals, not “vaccinated or unvaccinated,” not seen as “breakthrough” infections, or the immunosuppressed possibly unable to mount a robust response to COVID.
Labels do not define the ability to effectively defeat coronavirus or variants, as highly vaccinated countries have demonstrated in recent months. We are in the midst of a global pandemic, and the battle is raging onward.
In fact, the longer this pandemic continues, the more likely it is we will need to live with this as an endemic disease, so we should stop blaming those who become ill and need support.
It could be any of us.
A version of this article first appeared on Medscape.com.
As politicians battle over masks and mandates, heated rhetoric has been used to describe the fourth heartbreaking surge in COVID as a “pandemic of the unvaccinated.”
While it may serve to further divide red and blue states, I disagree with the assertion that the current surge in cases is driven simply by the unvaccinated. Why? First, the premise would assume complete efficacy with our vaccinated population, which is statistically incorrect (at least 15 million of the U.S. population never completed a second round of injections), which means they were not considered “fully vaccinated.”
Alternately, we need to examine what has occurred in nations with significantly higher vaccination rates than ours (the United Kingdom and Israel) to realize that variants have overrun the dramatic success achieved in those countries as well. Israel, once considered to be the most vaccinated country in the world, is facing a brutal fourth wave of COVID that has sent the country spiraling into another heartbreaking lockdown.
The unvaccinated could hardly be blamed for what is happening in either of these highly vaccinated countries.
The concept of blame
So why use blame? It defeats the purpose of encouraging those who are hesitant or possibly misinformed or disenfranchised to move forward. It lacks compassion. It does not encompass the art and science of nursing (for example, the University of Southern Indiana), such as those that hospitals have used to frame optimal nursing care. I abhor the idea of labeling because it denies the prospect of future comprehension.
Labeling reminds me of one of the saddest cases in my career.
An unfortunate case
I was the nurse caring for a man from a motor vehicular accident where an entire family was brutally killed. My patient was alleged to be the cause, with a blood alcohol level of 0.40%+ post hydration, intubated and ventilated, with a flailed chest and multiple orthopedic injuries as well as blunt head trauma. He was secured to the bed with handcuffs, although that was unnecessary. Multiple times I was asked how I could possibly care for such an individual, by the police and even a few colleagues. But it was not my place to judge the man.
He was in pain, and he was dying. I comforted him for the 2 weeks it took his battered body to pass into the next realm. No one visited him except the police, eagerly waiting for the man to wake up to explain the tragic events that occurred. It was my job to ease what pain I could and protect him from labels. Did he deserve the labels? Who knew? I did not care. I cared about his writhing and his physical anguish.
The comparison
Blame did not help the situation then, nor does it help us move forward now. As nurses, we seek to work within a framework of understanding. As we tire of caring for thousands of COVID patients, we do not stop to ask if they “deserve” care or if they have taken precautions and lived reasonably prior to seeking assistance for disease. We would not be nurses if we did this.
Think about Gov. Greg Abbott, who has asked that Texans not be allowed to mandate masks for children returning to school. He has recently been diagnosed with COVID, despite assuring the public he is fully vaccinated. Politically, his diagnosis could be visualized as a fiasco for a purple state where he has been adamant in denying the efficacy of masks for children.
Yet, his diagnosis should not be fodder for the press. The first concern should be his health and well-being, similar for any man of his age and potential comorbidity.
Conclusion
We should be people first, human beings that remain interconnected by our need for care and survival, not conservatives, independents, or liberals, not “vaccinated or unvaccinated,” not seen as “breakthrough” infections, or the immunosuppressed possibly unable to mount a robust response to COVID.
Labels do not define the ability to effectively defeat coronavirus or variants, as highly vaccinated countries have demonstrated in recent months. We are in the midst of a global pandemic, and the battle is raging onward.
In fact, the longer this pandemic continues, the more likely it is we will need to live with this as an endemic disease, so we should stop blaming those who become ill and need support.
It could be any of us.
A version of this article first appeared on Medscape.com.
Pandemic unveils growing suicide crisis for communities of color
This story is a collaboration between KHN and “Science Friday.”
Rafiah Maxie has been a licensed clinical social worker in the Chicago area for a decade. Throughout that time, she’d viewed suicide as a problem most prevalent among middle-aged white men.
Until May 27, 2020.
That day, Maxie’s 19-year-old son, Jamal Clay – who loved playing the trumpet and participating in theater, who would help her unload groceries from the car and raise funds for the March of the Dimes – killed himself in their garage.
“Now I cannot blink without seeing my son hanging,” said Maxie, who is Black.
Clay’s death, along with the suicides of more than 100 other Black residents in Illinois last year, has led locals to call for new prevention efforts focused on Black communities. In 2020, during the pandemic’s first year, suicides among White residents decreased compared with previous years, while they increased among Black residents, according to state data.
But this is not a local problem. Nor is it limited to the pandemic.
Interviews with a dozen suicide researchers, data collected from states across the country, and a review of decades of research revealed that suicide is a growing crisis for communities of color – one that plagued them well before the pandemic and has only been exacerbated since.
Overall suicide rates in the U.S. decreased in 2019 and 2020. National and local studies attribute the trend to a drop among White Americans, who make up the majority of suicide deaths. Meanwhile, rates for Black, Hispanic, and Asian Americans – though lower than those of their white peers – continued to climb in many states. (Suicide rates have been consistently high for Native Americans.)
“COVID created more transparency regarding what we already knew was happening,” said Sonyia Richardson, a licensed clinical social worker who focuses on serving people of color, and assistant professor at the University of North Carolina–Charlotte, where she researches suicide. When you put the suicide rates of all communities in one bucket, “that bucket says it’s getting better and what we’re doing is working,” she said. “But that’s not the case for communities of color.”
Losing generations
Although the suicide rate is highest among middle-aged White men, young people of color are emerging as particularly at risk.
Research shows Black kids younger than 13 die by suicide at nearly twice the rate of White kids and, over time, their suicide rates have grown even as rates have decreased for White children. Among teenagers and young adults, suicide deaths have increased more than 45% for Black Americans and about 40% for Asian Americans in the 7 years ending in 2019. Other concerning trends in suicide attempts date to the ’90s.
“We have to pay attention now because if you’re out of the first decade of life and think life is not worth pursuing, that’s a signal to say something is going really wrong.”
These statistics also refute traditional ideas that suicide doesn’t happen in certain ethnic or minority populations because they’re “protected” and “resilient” or the “model minority,” said Kiara Alvarez, a researcher and psychologist at Massachusetts General Hospital who focuses on suicide among Hispanic and immigrant populations.
Although these groups may have had low suicide rates historically, that’s changing, she said.
Paul Chin lost his 17-year-old brother, Chris, to suicide in 2009. A poem Chris wrote in high school about his heritage has left Chin, 8 years his senior, wondering if his brother struggled to feel accepted in the U.S., despite being born and raised in New York.
Growing up, Asian Americans weren’t represented in lessons at school or in pop culture, said Chin, now 37. Even in clinical research on suicide as well as other health topics, kids like Chris are underrepresented, with less than 1% of federal research funding focused on Asian Americans.
It wasn’t until the pandemic, and the concurrent rise in hate crimes against Asian Americans, that Chin saw national attention on the community’s mental health. He hopes the interest is not short-lived.
Suicide is the leading cause of death for Asian Americans ages 15 to 24, yet “that doesn’t get enough attention,” Chin said. “It’s important to continue to share these stories.”
Kathy Williams, who is Black, has been on a similar mission since her 15-year-old son, Torian Graves, died by suicide in 1996. People didn’t talk about suicide in the Black community then, she said. So she started raising the topic at her church in Durham, N.C., and in local schools. She wanted Black families to know the warning signs and society at large to recognize the seriousness of the problem.
The pandemic may have highlighted this, Williams said, but “it has always happened. Always.”
Pandemic sheds light on the triggers
Pinpointing the root causes of rising suicide within communities of color has proved difficult. How much stems from mental illness? How much from socioeconomic changes like job losses or social isolation? Now, COVID-19 may offer some clues.
Recent decades have been marked by growing economic instability, a widening racial wealth gap, and more public attention on police killings of unarmed Black and Brown people, said Michael Lindsey, executive director of the New York University McSilver Institute for Poverty Policy and Research.
With social media, youths face racism on more fronts than their parents did, said Leslie Adams, assistant professor in the department of mental health at Johns Hopkins Bloomberg School of Public Health.
Each of these factors has been shown to affect suicide risk. For example, experiencing racism and sexism together is linked to a threefold increase in suicidal thoughts for Asian American women, said Brian Keum, assistant professor at UCLA, based on preliminary research findings.
COVID-19 intensified these hardships among communities of color, with disproportionate numbers of lost loved ones, lost jobs, and lost housing. The murder of George Floyd prompted widespread racial unrest, and Asian Americans saw an increase in hate crimes.
At the same time, studies in Connecticut and Maryland found that suicide rates rose within these populations and dropped for their White counterparts.
“It’s not just a problem within the person, but societal issues that need to be addressed,” said Shari Jager-Hyman, assistant professor of psychiatry at the University of Pennsylvania.
Lessons from Texas
In Texas, COVID-19 hit Hispanics especially hard. As of July 2021, they accounted for 45% of all COVID-19 deaths and disproportionately lost jobs. Individuals living in the U.S. without authorization were generally not eligible for unemployment benefits or federal stimulus checks.
During this time, suicide deaths among Hispanic Texans climbed from 847 deaths in 2019 to 962 deaths in 2020, according to preliminary state data. Suicide deaths rose for Black Texans and residents classified as “other” races or ethnicities, but decreased for White Texans.
The numbers didn’t surprise Marc Mendiola. The 20-year-old grew up in a majority-Hispanic community on the south side of San Antonio. Even before the pandemic, he often heard classmates say they were suicidal. Many faced dire finances at home, sometimes living without electricity, food, or water. Those who sought mental health treatment often found services prohibitively expensive or inaccessible because they weren’t offered in Spanish.
“These are conditions the community has always been in,” Mendiola said. “But with the pandemic, it’s even worse.”
Four years ago, Mendiola and his classmates at South San High School began advocating for mental health services. In late 2019, just months before COVID-19 struck, their vision became reality. Six community agencies partnered to offer free services to students and their families across three school districts.
Richard Davidson, chief operating officer of Family Service, one of the groups in the collaborative, said the number of students discussing economic stressors has been on the rise since April 2020. More than 90% of the students who received services in the first half of 2021 were Hispanic, and nearly 10% reported thoughts of suicide or self-harm, program data show. None died by suicide.
Many students are so worried about what’s for dinner the next day that they’re not able to see a future beyond that, Davidson said. That’s when suicide can feel like a viable option.
“One of the things we do is help them see … that despite this situation now, you can create a vision for your future,” Davidson said.
A good future
Researchers say the promise of a good future is often overlooked in suicide prevention, perhaps because achieving it is so challenging. It requires economic and social growth and breaking systemic barriers.
Tevis Simon works to address all those fronts. As a child in West Baltimore, Simon, who is Black, faced poverty and trauma. As an adult, she attempted suicide three times. But now she shares her story with youths across the city to inspire them to overcome challenges. She also talks to politicians, law enforcement agencies, and public policy officials about their responsibilities.
“We can’t not talk about race,” said Simon, 43. “We can’t not talk about systematic oppression. We cannot not talk about these conditions that affect our mental well-being and our feeling and desire to live.”
For Jamal Clay in Illinois, the systemic barriers started early. Before his suicide last year, he had tried to harm himself when he was 12 and the victim of bullies. At that time, he was hospitalized for a few days and told to follow up with outpatient therapy, said his mother, Maxie.
But it was difficult to find therapists who accepted Medicaid, she said. When Maxie finally found one, there was a 60-day wait. Other therapists canceled appointments, she said.
“So we worked on our own,” Maxie said, relying on church and community. Her son seemed to improve. “We thought we closed that chapter in our lives.”
But when the pandemic hit, everything got worse, she said. Clay came home from college and worked at an Amazon warehouse. On drives to and from work, he was frequently pulled over by police. He stopped wearing hats so officers would consider him less intimidating, Maxie said.
“He felt uncomfortable being out in the street,” she said.
Maxie is still trying to make sense of what happened the day Clay died. But she’s found meaning in starting a nonprofit called Soul Survivors of Chicago. Through the organization, she provides education, scholarships and shoes – including Jamal’s old ones – to those impacted by violence, suicide, and trauma.
“My son won’t be able to have a first interview in [those] shoes. He won’t be able to have a nice jump shot or go to church or even meet his wife,” Maxie said.
But she hopes his shoes will carry someone else to a good future.
[Editor’s note: For the purposes of this story, “people of color” or “communities of color” refers to any racial or ethnic populations whose members do not identify as White, including those who are multiracial. Hispanics can be of any race or combination of races.]
KHN senior correspondent JoNel Aleccia contributed to this report. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
This story is a collaboration between KHN and “Science Friday.”
Rafiah Maxie has been a licensed clinical social worker in the Chicago area for a decade. Throughout that time, she’d viewed suicide as a problem most prevalent among middle-aged white men.
Until May 27, 2020.
That day, Maxie’s 19-year-old son, Jamal Clay – who loved playing the trumpet and participating in theater, who would help her unload groceries from the car and raise funds for the March of the Dimes – killed himself in their garage.
“Now I cannot blink without seeing my son hanging,” said Maxie, who is Black.
Clay’s death, along with the suicides of more than 100 other Black residents in Illinois last year, has led locals to call for new prevention efforts focused on Black communities. In 2020, during the pandemic’s first year, suicides among White residents decreased compared with previous years, while they increased among Black residents, according to state data.
But this is not a local problem. Nor is it limited to the pandemic.
Interviews with a dozen suicide researchers, data collected from states across the country, and a review of decades of research revealed that suicide is a growing crisis for communities of color – one that plagued them well before the pandemic and has only been exacerbated since.
Overall suicide rates in the U.S. decreased in 2019 and 2020. National and local studies attribute the trend to a drop among White Americans, who make up the majority of suicide deaths. Meanwhile, rates for Black, Hispanic, and Asian Americans – though lower than those of their white peers – continued to climb in many states. (Suicide rates have been consistently high for Native Americans.)
“COVID created more transparency regarding what we already knew was happening,” said Sonyia Richardson, a licensed clinical social worker who focuses on serving people of color, and assistant professor at the University of North Carolina–Charlotte, where she researches suicide. When you put the suicide rates of all communities in one bucket, “that bucket says it’s getting better and what we’re doing is working,” she said. “But that’s not the case for communities of color.”
Losing generations
Although the suicide rate is highest among middle-aged White men, young people of color are emerging as particularly at risk.
Research shows Black kids younger than 13 die by suicide at nearly twice the rate of White kids and, over time, their suicide rates have grown even as rates have decreased for White children. Among teenagers and young adults, suicide deaths have increased more than 45% for Black Americans and about 40% for Asian Americans in the 7 years ending in 2019. Other concerning trends in suicide attempts date to the ’90s.
“We have to pay attention now because if you’re out of the first decade of life and think life is not worth pursuing, that’s a signal to say something is going really wrong.”
These statistics also refute traditional ideas that suicide doesn’t happen in certain ethnic or minority populations because they’re “protected” and “resilient” or the “model minority,” said Kiara Alvarez, a researcher and psychologist at Massachusetts General Hospital who focuses on suicide among Hispanic and immigrant populations.
Although these groups may have had low suicide rates historically, that’s changing, she said.
Paul Chin lost his 17-year-old brother, Chris, to suicide in 2009. A poem Chris wrote in high school about his heritage has left Chin, 8 years his senior, wondering if his brother struggled to feel accepted in the U.S., despite being born and raised in New York.
Growing up, Asian Americans weren’t represented in lessons at school or in pop culture, said Chin, now 37. Even in clinical research on suicide as well as other health topics, kids like Chris are underrepresented, with less than 1% of federal research funding focused on Asian Americans.
It wasn’t until the pandemic, and the concurrent rise in hate crimes against Asian Americans, that Chin saw national attention on the community’s mental health. He hopes the interest is not short-lived.
Suicide is the leading cause of death for Asian Americans ages 15 to 24, yet “that doesn’t get enough attention,” Chin said. “It’s important to continue to share these stories.”
Kathy Williams, who is Black, has been on a similar mission since her 15-year-old son, Torian Graves, died by suicide in 1996. People didn’t talk about suicide in the Black community then, she said. So she started raising the topic at her church in Durham, N.C., and in local schools. She wanted Black families to know the warning signs and society at large to recognize the seriousness of the problem.
The pandemic may have highlighted this, Williams said, but “it has always happened. Always.”
Pandemic sheds light on the triggers
Pinpointing the root causes of rising suicide within communities of color has proved difficult. How much stems from mental illness? How much from socioeconomic changes like job losses or social isolation? Now, COVID-19 may offer some clues.
Recent decades have been marked by growing economic instability, a widening racial wealth gap, and more public attention on police killings of unarmed Black and Brown people, said Michael Lindsey, executive director of the New York University McSilver Institute for Poverty Policy and Research.
With social media, youths face racism on more fronts than their parents did, said Leslie Adams, assistant professor in the department of mental health at Johns Hopkins Bloomberg School of Public Health.
Each of these factors has been shown to affect suicide risk. For example, experiencing racism and sexism together is linked to a threefold increase in suicidal thoughts for Asian American women, said Brian Keum, assistant professor at UCLA, based on preliminary research findings.
COVID-19 intensified these hardships among communities of color, with disproportionate numbers of lost loved ones, lost jobs, and lost housing. The murder of George Floyd prompted widespread racial unrest, and Asian Americans saw an increase in hate crimes.
At the same time, studies in Connecticut and Maryland found that suicide rates rose within these populations and dropped for their White counterparts.
“It’s not just a problem within the person, but societal issues that need to be addressed,” said Shari Jager-Hyman, assistant professor of psychiatry at the University of Pennsylvania.
Lessons from Texas
In Texas, COVID-19 hit Hispanics especially hard. As of July 2021, they accounted for 45% of all COVID-19 deaths and disproportionately lost jobs. Individuals living in the U.S. without authorization were generally not eligible for unemployment benefits or federal stimulus checks.
During this time, suicide deaths among Hispanic Texans climbed from 847 deaths in 2019 to 962 deaths in 2020, according to preliminary state data. Suicide deaths rose for Black Texans and residents classified as “other” races or ethnicities, but decreased for White Texans.
The numbers didn’t surprise Marc Mendiola. The 20-year-old grew up in a majority-Hispanic community on the south side of San Antonio. Even before the pandemic, he often heard classmates say they were suicidal. Many faced dire finances at home, sometimes living without electricity, food, or water. Those who sought mental health treatment often found services prohibitively expensive or inaccessible because they weren’t offered in Spanish.
“These are conditions the community has always been in,” Mendiola said. “But with the pandemic, it’s even worse.”
Four years ago, Mendiola and his classmates at South San High School began advocating for mental health services. In late 2019, just months before COVID-19 struck, their vision became reality. Six community agencies partnered to offer free services to students and their families across three school districts.
Richard Davidson, chief operating officer of Family Service, one of the groups in the collaborative, said the number of students discussing economic stressors has been on the rise since April 2020. More than 90% of the students who received services in the first half of 2021 were Hispanic, and nearly 10% reported thoughts of suicide or self-harm, program data show. None died by suicide.
Many students are so worried about what’s for dinner the next day that they’re not able to see a future beyond that, Davidson said. That’s when suicide can feel like a viable option.
“One of the things we do is help them see … that despite this situation now, you can create a vision for your future,” Davidson said.
A good future
Researchers say the promise of a good future is often overlooked in suicide prevention, perhaps because achieving it is so challenging. It requires economic and social growth and breaking systemic barriers.
Tevis Simon works to address all those fronts. As a child in West Baltimore, Simon, who is Black, faced poverty and trauma. As an adult, she attempted suicide three times. But now she shares her story with youths across the city to inspire them to overcome challenges. She also talks to politicians, law enforcement agencies, and public policy officials about their responsibilities.
“We can’t not talk about race,” said Simon, 43. “We can’t not talk about systematic oppression. We cannot not talk about these conditions that affect our mental well-being and our feeling and desire to live.”
For Jamal Clay in Illinois, the systemic barriers started early. Before his suicide last year, he had tried to harm himself when he was 12 and the victim of bullies. At that time, he was hospitalized for a few days and told to follow up with outpatient therapy, said his mother, Maxie.
But it was difficult to find therapists who accepted Medicaid, she said. When Maxie finally found one, there was a 60-day wait. Other therapists canceled appointments, she said.
“So we worked on our own,” Maxie said, relying on church and community. Her son seemed to improve. “We thought we closed that chapter in our lives.”
But when the pandemic hit, everything got worse, she said. Clay came home from college and worked at an Amazon warehouse. On drives to and from work, he was frequently pulled over by police. He stopped wearing hats so officers would consider him less intimidating, Maxie said.
“He felt uncomfortable being out in the street,” she said.
Maxie is still trying to make sense of what happened the day Clay died. But she’s found meaning in starting a nonprofit called Soul Survivors of Chicago. Through the organization, she provides education, scholarships and shoes – including Jamal’s old ones – to those impacted by violence, suicide, and trauma.
“My son won’t be able to have a first interview in [those] shoes. He won’t be able to have a nice jump shot or go to church or even meet his wife,” Maxie said.
But she hopes his shoes will carry someone else to a good future.
[Editor’s note: For the purposes of this story, “people of color” or “communities of color” refers to any racial or ethnic populations whose members do not identify as White, including those who are multiracial. Hispanics can be of any race or combination of races.]
KHN senior correspondent JoNel Aleccia contributed to this report. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
This story is a collaboration between KHN and “Science Friday.”
Rafiah Maxie has been a licensed clinical social worker in the Chicago area for a decade. Throughout that time, she’d viewed suicide as a problem most prevalent among middle-aged white men.
Until May 27, 2020.
That day, Maxie’s 19-year-old son, Jamal Clay – who loved playing the trumpet and participating in theater, who would help her unload groceries from the car and raise funds for the March of the Dimes – killed himself in their garage.
“Now I cannot blink without seeing my son hanging,” said Maxie, who is Black.
Clay’s death, along with the suicides of more than 100 other Black residents in Illinois last year, has led locals to call for new prevention efforts focused on Black communities. In 2020, during the pandemic’s first year, suicides among White residents decreased compared with previous years, while they increased among Black residents, according to state data.
But this is not a local problem. Nor is it limited to the pandemic.
Interviews with a dozen suicide researchers, data collected from states across the country, and a review of decades of research revealed that suicide is a growing crisis for communities of color – one that plagued them well before the pandemic and has only been exacerbated since.
Overall suicide rates in the U.S. decreased in 2019 and 2020. National and local studies attribute the trend to a drop among White Americans, who make up the majority of suicide deaths. Meanwhile, rates for Black, Hispanic, and Asian Americans – though lower than those of their white peers – continued to climb in many states. (Suicide rates have been consistently high for Native Americans.)
“COVID created more transparency regarding what we already knew was happening,” said Sonyia Richardson, a licensed clinical social worker who focuses on serving people of color, and assistant professor at the University of North Carolina–Charlotte, where she researches suicide. When you put the suicide rates of all communities in one bucket, “that bucket says it’s getting better and what we’re doing is working,” she said. “But that’s not the case for communities of color.”
Losing generations
Although the suicide rate is highest among middle-aged White men, young people of color are emerging as particularly at risk.
Research shows Black kids younger than 13 die by suicide at nearly twice the rate of White kids and, over time, their suicide rates have grown even as rates have decreased for White children. Among teenagers and young adults, suicide deaths have increased more than 45% for Black Americans and about 40% for Asian Americans in the 7 years ending in 2019. Other concerning trends in suicide attempts date to the ’90s.
“We have to pay attention now because if you’re out of the first decade of life and think life is not worth pursuing, that’s a signal to say something is going really wrong.”
These statistics also refute traditional ideas that suicide doesn’t happen in certain ethnic or minority populations because they’re “protected” and “resilient” or the “model minority,” said Kiara Alvarez, a researcher and psychologist at Massachusetts General Hospital who focuses on suicide among Hispanic and immigrant populations.
Although these groups may have had low suicide rates historically, that’s changing, she said.
Paul Chin lost his 17-year-old brother, Chris, to suicide in 2009. A poem Chris wrote in high school about his heritage has left Chin, 8 years his senior, wondering if his brother struggled to feel accepted in the U.S., despite being born and raised in New York.
Growing up, Asian Americans weren’t represented in lessons at school or in pop culture, said Chin, now 37. Even in clinical research on suicide as well as other health topics, kids like Chris are underrepresented, with less than 1% of federal research funding focused on Asian Americans.
It wasn’t until the pandemic, and the concurrent rise in hate crimes against Asian Americans, that Chin saw national attention on the community’s mental health. He hopes the interest is not short-lived.
Suicide is the leading cause of death for Asian Americans ages 15 to 24, yet “that doesn’t get enough attention,” Chin said. “It’s important to continue to share these stories.”
Kathy Williams, who is Black, has been on a similar mission since her 15-year-old son, Torian Graves, died by suicide in 1996. People didn’t talk about suicide in the Black community then, she said. So she started raising the topic at her church in Durham, N.C., and in local schools. She wanted Black families to know the warning signs and society at large to recognize the seriousness of the problem.
The pandemic may have highlighted this, Williams said, but “it has always happened. Always.”
Pandemic sheds light on the triggers
Pinpointing the root causes of rising suicide within communities of color has proved difficult. How much stems from mental illness? How much from socioeconomic changes like job losses or social isolation? Now, COVID-19 may offer some clues.
Recent decades have been marked by growing economic instability, a widening racial wealth gap, and more public attention on police killings of unarmed Black and Brown people, said Michael Lindsey, executive director of the New York University McSilver Institute for Poverty Policy and Research.
With social media, youths face racism on more fronts than their parents did, said Leslie Adams, assistant professor in the department of mental health at Johns Hopkins Bloomberg School of Public Health.
Each of these factors has been shown to affect suicide risk. For example, experiencing racism and sexism together is linked to a threefold increase in suicidal thoughts for Asian American women, said Brian Keum, assistant professor at UCLA, based on preliminary research findings.
COVID-19 intensified these hardships among communities of color, with disproportionate numbers of lost loved ones, lost jobs, and lost housing. The murder of George Floyd prompted widespread racial unrest, and Asian Americans saw an increase in hate crimes.
At the same time, studies in Connecticut and Maryland found that suicide rates rose within these populations and dropped for their White counterparts.
“It’s not just a problem within the person, but societal issues that need to be addressed,” said Shari Jager-Hyman, assistant professor of psychiatry at the University of Pennsylvania.
Lessons from Texas
In Texas, COVID-19 hit Hispanics especially hard. As of July 2021, they accounted for 45% of all COVID-19 deaths and disproportionately lost jobs. Individuals living in the U.S. without authorization were generally not eligible for unemployment benefits or federal stimulus checks.
During this time, suicide deaths among Hispanic Texans climbed from 847 deaths in 2019 to 962 deaths in 2020, according to preliminary state data. Suicide deaths rose for Black Texans and residents classified as “other” races or ethnicities, but decreased for White Texans.
The numbers didn’t surprise Marc Mendiola. The 20-year-old grew up in a majority-Hispanic community on the south side of San Antonio. Even before the pandemic, he often heard classmates say they were suicidal. Many faced dire finances at home, sometimes living without electricity, food, or water. Those who sought mental health treatment often found services prohibitively expensive or inaccessible because they weren’t offered in Spanish.
“These are conditions the community has always been in,” Mendiola said. “But with the pandemic, it’s even worse.”
Four years ago, Mendiola and his classmates at South San High School began advocating for mental health services. In late 2019, just months before COVID-19 struck, their vision became reality. Six community agencies partnered to offer free services to students and their families across three school districts.
Richard Davidson, chief operating officer of Family Service, one of the groups in the collaborative, said the number of students discussing economic stressors has been on the rise since April 2020. More than 90% of the students who received services in the first half of 2021 were Hispanic, and nearly 10% reported thoughts of suicide or self-harm, program data show. None died by suicide.
Many students are so worried about what’s for dinner the next day that they’re not able to see a future beyond that, Davidson said. That’s when suicide can feel like a viable option.
“One of the things we do is help them see … that despite this situation now, you can create a vision for your future,” Davidson said.
A good future
Researchers say the promise of a good future is often overlooked in suicide prevention, perhaps because achieving it is so challenging. It requires economic and social growth and breaking systemic barriers.
Tevis Simon works to address all those fronts. As a child in West Baltimore, Simon, who is Black, faced poverty and trauma. As an adult, she attempted suicide three times. But now she shares her story with youths across the city to inspire them to overcome challenges. She also talks to politicians, law enforcement agencies, and public policy officials about their responsibilities.
“We can’t not talk about race,” said Simon, 43. “We can’t not talk about systematic oppression. We cannot not talk about these conditions that affect our mental well-being and our feeling and desire to live.”
For Jamal Clay in Illinois, the systemic barriers started early. Before his suicide last year, he had tried to harm himself when he was 12 and the victim of bullies. At that time, he was hospitalized for a few days and told to follow up with outpatient therapy, said his mother, Maxie.
But it was difficult to find therapists who accepted Medicaid, she said. When Maxie finally found one, there was a 60-day wait. Other therapists canceled appointments, she said.
“So we worked on our own,” Maxie said, relying on church and community. Her son seemed to improve. “We thought we closed that chapter in our lives.”
But when the pandemic hit, everything got worse, she said. Clay came home from college and worked at an Amazon warehouse. On drives to and from work, he was frequently pulled over by police. He stopped wearing hats so officers would consider him less intimidating, Maxie said.
“He felt uncomfortable being out in the street,” she said.
Maxie is still trying to make sense of what happened the day Clay died. But she’s found meaning in starting a nonprofit called Soul Survivors of Chicago. Through the organization, she provides education, scholarships and shoes – including Jamal’s old ones – to those impacted by violence, suicide, and trauma.
“My son won’t be able to have a first interview in [those] shoes. He won’t be able to have a nice jump shot or go to church or even meet his wife,” Maxie said.
But she hopes his shoes will carry someone else to a good future.
[Editor’s note: For the purposes of this story, “people of color” or “communities of color” refers to any racial or ethnic populations whose members do not identify as White, including those who are multiracial. Hispanics can be of any race or combination of races.]
KHN senior correspondent JoNel Aleccia contributed to this report. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Fauci corrects prediction on when pandemic will be under control
The United States could get the COVID-19 pandemic under control by the spring of 2022 if enough Americans become vaccinated, Anthony S. Fauci, MD, said.
Speaking to Anderson Cooper on CNN, Dr. Fauci corrected the timeline he gave in an interview earlier with Mary Louise Kelly of NPR.
In the NPR interview, he had said that if “the overwhelming majority of the people vaccinated, I think as we get into the fall and the winter, we could start to really get some good control over this as we get into 2022.”
Dr. Fauci told Mr. Cooper that he listened to a recording of the NPR interview later and realized his mistake.
“I meant to say the spring of 2022,” Dr. Fauci told CNN. “I misspoke. My bad.”
Dr. Fauci, the head of the National Institute of Allergy and Infectious Diseases and the chief White House medical adviser, said the pandemic will be under control when the large majority of Americans have gotten vaccinated or been infected with COVID-19 and recovered, which offers some protection against the virus.
People who have been infected and recovered should still get vaccinated, he said.
“The degree of protection you could induce in someone who’s been infected and then recovered and then vaccinated is an enormous increase in the degree of protection,” Dr. Fauci said.
“I think we can get a degree of overall blanket protection of the community that as we get into the early part of 2022 ... we could start getting back to a degree of normality.”
A version of this article first appeared on WebMD.com.
The United States could get the COVID-19 pandemic under control by the spring of 2022 if enough Americans become vaccinated, Anthony S. Fauci, MD, said.
Speaking to Anderson Cooper on CNN, Dr. Fauci corrected the timeline he gave in an interview earlier with Mary Louise Kelly of NPR.
In the NPR interview, he had said that if “the overwhelming majority of the people vaccinated, I think as we get into the fall and the winter, we could start to really get some good control over this as we get into 2022.”
Dr. Fauci told Mr. Cooper that he listened to a recording of the NPR interview later and realized his mistake.
“I meant to say the spring of 2022,” Dr. Fauci told CNN. “I misspoke. My bad.”
Dr. Fauci, the head of the National Institute of Allergy and Infectious Diseases and the chief White House medical adviser, said the pandemic will be under control when the large majority of Americans have gotten vaccinated or been infected with COVID-19 and recovered, which offers some protection against the virus.
People who have been infected and recovered should still get vaccinated, he said.
“The degree of protection you could induce in someone who’s been infected and then recovered and then vaccinated is an enormous increase in the degree of protection,” Dr. Fauci said.
“I think we can get a degree of overall blanket protection of the community that as we get into the early part of 2022 ... we could start getting back to a degree of normality.”
A version of this article first appeared on WebMD.com.
The United States could get the COVID-19 pandemic under control by the spring of 2022 if enough Americans become vaccinated, Anthony S. Fauci, MD, said.
Speaking to Anderson Cooper on CNN, Dr. Fauci corrected the timeline he gave in an interview earlier with Mary Louise Kelly of NPR.
In the NPR interview, he had said that if “the overwhelming majority of the people vaccinated, I think as we get into the fall and the winter, we could start to really get some good control over this as we get into 2022.”
Dr. Fauci told Mr. Cooper that he listened to a recording of the NPR interview later and realized his mistake.
“I meant to say the spring of 2022,” Dr. Fauci told CNN. “I misspoke. My bad.”
Dr. Fauci, the head of the National Institute of Allergy and Infectious Diseases and the chief White House medical adviser, said the pandemic will be under control when the large majority of Americans have gotten vaccinated or been infected with COVID-19 and recovered, which offers some protection against the virus.
People who have been infected and recovered should still get vaccinated, he said.
“The degree of protection you could induce in someone who’s been infected and then recovered and then vaccinated is an enormous increase in the degree of protection,” Dr. Fauci said.
“I think we can get a degree of overall blanket protection of the community that as we get into the early part of 2022 ... we could start getting back to a degree of normality.”
A version of this article first appeared on WebMD.com.
COVID booster may benefit active-treatment cancer patients
A COVID-19 booster shot may be beneficial for patients with cancer who are undergoing treatment, according to new findings from an Israeli case-control study.
The seropositivity rate among the patients with cancer remained high (87%) about 4 months after the patients had received the second BNT162b2 (Pfizer/BioNTech) vaccination. However, the median IgG titer in the patients and the control persons who were without cancer decreased over time. Notably, in a previous analysis that the authors conducted and in the current one, the IgG titers were statistically significantly lower in the patients with cancer as compared to control persons.
The correlation between antibody levels following vaccination and clinical protection has yet to be proven, but the accumulating evidence supports antibody response as a possible correlate of disease protection.
“Our data can’t predict if a third booster dose is necessary,” said study author Salomon M. Stemmer, MD, professor at the Institute of Oncology of Rabin Medical Center, Petah Tikva, Israel. “It does seem quite logical that a booster dose will cause an increase in IgG levels.”
The findings were published Aug. 11, 2021, in a research letter in JAMA Oncology.
In their previous study, Dr. Stemmer and colleagues compared the rates of anti–spike antibody response to the initial shot of the BNT162b2 vaccine among 102 adults with solid-tumor cancers who were undergoing treatment with that of 78 healthy control persons. They found that a high percentage of patients undergoing treatment for cancer (90%) achieved a sufficient antibody response to the BNT162b2 vaccine.
Booster endorsed
Responses to COVID-19 vaccination have varied among patients with cancer. For patients with solid tumors, responses have been good even while the patients were receiving systemic therapy. However, among patients with blood cancers, particularly those receiving immunosuppressive therapies, responses have been poor. Studies have identified factors associated with a poor response, but it has been unclear whether to recommend booster shots.
In August the Food and Drug Administration authorized a third dose of either the Pfizer or the Moderna COVID-19 vaccine for all individuals with compromised immune systems. Those eligible for a third dose include solid-organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems.
IgG titers lower in cancer patients
In the current analysis, the authors evaluated the anti-S response in the patients with cancer approximately 4 months after they had received the second vaccine dose. They compared the responses in those patients with the responses in a control group.
The cohort included 95 patients from the prior study and 66 control persons. The most common malignancies were gastrointestinal (26%), lung (25%), and breast (18%).
All patients were receiving systemic therapy. Chemotherapy was the most common (28%), followed by immunotherapy (21%) and combination chemotherapy/biological therapy (20%).
At a median of 123 days after the second vaccination, 83 patients with cancer (87%) and all of the control patients (100%) were seropositive for anti-S IgG antibodies. The median titer levels were significantly lower among case patients as compared with control patients (417 AU/mL [interquartile range, 136-895] vs. 1,220 AU/mL [IQR, 588-1,987]; P < .001)
There was a 3.6-fold range in median titer values across tumor types and an even wider range (8.8-fold) across the different types of treatment. The lowest titers were observed among patients who had received immunotherapy plus chemotherapy/biological therapy (median [IQR], 94.4 [49.4-191] AU/mL vs. 147 [62.8-339] AU/mL).
In an exploratory multivariable analysis, treatments with chemotherapy plus immunotherapy and immunotherapy plus biological therapy were significantly associated with lower IgG titers.
No downside for cancer patients
The Biden administration announced a plan to begin booster COVID-19 vaccinations for all American adults in September, with recommendations that the third vaccine be given at least 8 months after the second mRNA vaccine dose.
Jeremy M. Levin, DPhil, the chairman and CEO of Ovid Therapeutics, explained that, concerning boosters, “it is inconceivable that we will have all data at this stage.
“Knowledge about how boosters work and don’t work and when you should ideally have them is imperfect,” he told this news organization. “However, we can have a lot of confidence in the fact that hundreds of millions of people have received the vaccine, so we know a lot about the safety and efficacy.”
Immunocompromised adults represent less than 5% of the total population, and most of the available data on vaccination are from patients who have undergone solid-organ transplant, Dr. Levin explained. Studies have shown that their response is less robust to vaccination in comparison with adults in the general population.
“Although it is still preliminary, the strongest data come from Israel,” he said, “where they found that the booster was highly effective and doubled the number of transplant patients who developed antibodies.”
But data are not yet available in the setting of cancer. “But even though we don’t have the data yet, the answer is that no matter, the booster process is essential,” he said. “The evidence we have is that boosters raise the immune response, and it is the best data we have now.”
Martin J. Edelman, MD, chair, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that the current recommendation is that patients who are immunocompromised receive a booster immediately.
At his health system, this is interpreted to include patients who have undergone the following treatments: Transplant (solid-organ and bone marrow transplant), hemodialysis, hematologic malignancy treatment, active immunosuppressive (chemotherapy, chemoimmunotherapy, and nonhormonal or single-agent immunotherapy) treatment, rheumatology treatments, and high-dose steroids.
“As for cancer patients, we are making arrangements to vaccinate patients who meet the above criteria now,” he said. “There is no known downside to receiving booster immediately. While there may be less of a response than waiting for completion of treatment, we know that patients on active therapy are frequently able to mount a response, and any response is better than none.”
Dr. Edelman added that this area is changing very rapidly. “We will modify our approach as information and guidance from appropriate organizations, such as the FDA and CDC, become available.”
Dr. Stemmer has received institutional research grants from CAN-FITE, AstraZeneca, Bioline RX, BMS, Halozyme, Clovis Oncology, CTG Pharma, Exelixis, Geicam, Incyte, Lilly, Moderna, Teva Pharmaceuticals, and Roche, and owns stocks and options in CTG Pharma, DocBoxMD, Tyrnovo, VYPE, Cytora, and CAN-FITE. Dr. Edelman has received personal fees and other compensation from Windmil, Biomarker Strategies, AstraZeneca, Takeda, GlaxoSmithKline, Apexigen, Nektar, Bristol-Myers Squibb, Armo, Bergen Bio, and Apexigen outside the submitted work. He has submitted a patent for epigenetic modifications to increase susceptibility to radiopharmaceuticals and is a paid adviser for Kanaph and Flame. Dr. Levin is chairman and CEO of Ovid Therapeutics.
A version of this article first appeared on Medscape.com.
A COVID-19 booster shot may be beneficial for patients with cancer who are undergoing treatment, according to new findings from an Israeli case-control study.
The seropositivity rate among the patients with cancer remained high (87%) about 4 months after the patients had received the second BNT162b2 (Pfizer/BioNTech) vaccination. However, the median IgG titer in the patients and the control persons who were without cancer decreased over time. Notably, in a previous analysis that the authors conducted and in the current one, the IgG titers were statistically significantly lower in the patients with cancer as compared to control persons.
The correlation between antibody levels following vaccination and clinical protection has yet to be proven, but the accumulating evidence supports antibody response as a possible correlate of disease protection.
“Our data can’t predict if a third booster dose is necessary,” said study author Salomon M. Stemmer, MD, professor at the Institute of Oncology of Rabin Medical Center, Petah Tikva, Israel. “It does seem quite logical that a booster dose will cause an increase in IgG levels.”
The findings were published Aug. 11, 2021, in a research letter in JAMA Oncology.
In their previous study, Dr. Stemmer and colleagues compared the rates of anti–spike antibody response to the initial shot of the BNT162b2 vaccine among 102 adults with solid-tumor cancers who were undergoing treatment with that of 78 healthy control persons. They found that a high percentage of patients undergoing treatment for cancer (90%) achieved a sufficient antibody response to the BNT162b2 vaccine.
Booster endorsed
Responses to COVID-19 vaccination have varied among patients with cancer. For patients with solid tumors, responses have been good even while the patients were receiving systemic therapy. However, among patients with blood cancers, particularly those receiving immunosuppressive therapies, responses have been poor. Studies have identified factors associated with a poor response, but it has been unclear whether to recommend booster shots.
In August the Food and Drug Administration authorized a third dose of either the Pfizer or the Moderna COVID-19 vaccine for all individuals with compromised immune systems. Those eligible for a third dose include solid-organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems.
IgG titers lower in cancer patients
In the current analysis, the authors evaluated the anti-S response in the patients with cancer approximately 4 months after they had received the second vaccine dose. They compared the responses in those patients with the responses in a control group.
The cohort included 95 patients from the prior study and 66 control persons. The most common malignancies were gastrointestinal (26%), lung (25%), and breast (18%).
All patients were receiving systemic therapy. Chemotherapy was the most common (28%), followed by immunotherapy (21%) and combination chemotherapy/biological therapy (20%).
At a median of 123 days after the second vaccination, 83 patients with cancer (87%) and all of the control patients (100%) were seropositive for anti-S IgG antibodies. The median titer levels were significantly lower among case patients as compared with control patients (417 AU/mL [interquartile range, 136-895] vs. 1,220 AU/mL [IQR, 588-1,987]; P < .001)
There was a 3.6-fold range in median titer values across tumor types and an even wider range (8.8-fold) across the different types of treatment. The lowest titers were observed among patients who had received immunotherapy plus chemotherapy/biological therapy (median [IQR], 94.4 [49.4-191] AU/mL vs. 147 [62.8-339] AU/mL).
In an exploratory multivariable analysis, treatments with chemotherapy plus immunotherapy and immunotherapy plus biological therapy were significantly associated with lower IgG titers.
No downside for cancer patients
The Biden administration announced a plan to begin booster COVID-19 vaccinations for all American adults in September, with recommendations that the third vaccine be given at least 8 months after the second mRNA vaccine dose.
Jeremy M. Levin, DPhil, the chairman and CEO of Ovid Therapeutics, explained that, concerning boosters, “it is inconceivable that we will have all data at this stage.
“Knowledge about how boosters work and don’t work and when you should ideally have them is imperfect,” he told this news organization. “However, we can have a lot of confidence in the fact that hundreds of millions of people have received the vaccine, so we know a lot about the safety and efficacy.”
Immunocompromised adults represent less than 5% of the total population, and most of the available data on vaccination are from patients who have undergone solid-organ transplant, Dr. Levin explained. Studies have shown that their response is less robust to vaccination in comparison with adults in the general population.
“Although it is still preliminary, the strongest data come from Israel,” he said, “where they found that the booster was highly effective and doubled the number of transplant patients who developed antibodies.”
But data are not yet available in the setting of cancer. “But even though we don’t have the data yet, the answer is that no matter, the booster process is essential,” he said. “The evidence we have is that boosters raise the immune response, and it is the best data we have now.”
Martin J. Edelman, MD, chair, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that the current recommendation is that patients who are immunocompromised receive a booster immediately.
At his health system, this is interpreted to include patients who have undergone the following treatments: Transplant (solid-organ and bone marrow transplant), hemodialysis, hematologic malignancy treatment, active immunosuppressive (chemotherapy, chemoimmunotherapy, and nonhormonal or single-agent immunotherapy) treatment, rheumatology treatments, and high-dose steroids.
“As for cancer patients, we are making arrangements to vaccinate patients who meet the above criteria now,” he said. “There is no known downside to receiving booster immediately. While there may be less of a response than waiting for completion of treatment, we know that patients on active therapy are frequently able to mount a response, and any response is better than none.”
Dr. Edelman added that this area is changing very rapidly. “We will modify our approach as information and guidance from appropriate organizations, such as the FDA and CDC, become available.”
Dr. Stemmer has received institutional research grants from CAN-FITE, AstraZeneca, Bioline RX, BMS, Halozyme, Clovis Oncology, CTG Pharma, Exelixis, Geicam, Incyte, Lilly, Moderna, Teva Pharmaceuticals, and Roche, and owns stocks and options in CTG Pharma, DocBoxMD, Tyrnovo, VYPE, Cytora, and CAN-FITE. Dr. Edelman has received personal fees and other compensation from Windmil, Biomarker Strategies, AstraZeneca, Takeda, GlaxoSmithKline, Apexigen, Nektar, Bristol-Myers Squibb, Armo, Bergen Bio, and Apexigen outside the submitted work. He has submitted a patent for epigenetic modifications to increase susceptibility to radiopharmaceuticals and is a paid adviser for Kanaph and Flame. Dr. Levin is chairman and CEO of Ovid Therapeutics.
A version of this article first appeared on Medscape.com.
A COVID-19 booster shot may be beneficial for patients with cancer who are undergoing treatment, according to new findings from an Israeli case-control study.
The seropositivity rate among the patients with cancer remained high (87%) about 4 months after the patients had received the second BNT162b2 (Pfizer/BioNTech) vaccination. However, the median IgG titer in the patients and the control persons who were without cancer decreased over time. Notably, in a previous analysis that the authors conducted and in the current one, the IgG titers were statistically significantly lower in the patients with cancer as compared to control persons.
The correlation between antibody levels following vaccination and clinical protection has yet to be proven, but the accumulating evidence supports antibody response as a possible correlate of disease protection.
“Our data can’t predict if a third booster dose is necessary,” said study author Salomon M. Stemmer, MD, professor at the Institute of Oncology of Rabin Medical Center, Petah Tikva, Israel. “It does seem quite logical that a booster dose will cause an increase in IgG levels.”
The findings were published Aug. 11, 2021, in a research letter in JAMA Oncology.
In their previous study, Dr. Stemmer and colleagues compared the rates of anti–spike antibody response to the initial shot of the BNT162b2 vaccine among 102 adults with solid-tumor cancers who were undergoing treatment with that of 78 healthy control persons. They found that a high percentage of patients undergoing treatment for cancer (90%) achieved a sufficient antibody response to the BNT162b2 vaccine.
Booster endorsed
Responses to COVID-19 vaccination have varied among patients with cancer. For patients with solid tumors, responses have been good even while the patients were receiving systemic therapy. However, among patients with blood cancers, particularly those receiving immunosuppressive therapies, responses have been poor. Studies have identified factors associated with a poor response, but it has been unclear whether to recommend booster shots.
In August the Food and Drug Administration authorized a third dose of either the Pfizer or the Moderna COVID-19 vaccine for all individuals with compromised immune systems. Those eligible for a third dose include solid-organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems.
IgG titers lower in cancer patients
In the current analysis, the authors evaluated the anti-S response in the patients with cancer approximately 4 months after they had received the second vaccine dose. They compared the responses in those patients with the responses in a control group.
The cohort included 95 patients from the prior study and 66 control persons. The most common malignancies were gastrointestinal (26%), lung (25%), and breast (18%).
All patients were receiving systemic therapy. Chemotherapy was the most common (28%), followed by immunotherapy (21%) and combination chemotherapy/biological therapy (20%).
At a median of 123 days after the second vaccination, 83 patients with cancer (87%) and all of the control patients (100%) were seropositive for anti-S IgG antibodies. The median titer levels were significantly lower among case patients as compared with control patients (417 AU/mL [interquartile range, 136-895] vs. 1,220 AU/mL [IQR, 588-1,987]; P < .001)
There was a 3.6-fold range in median titer values across tumor types and an even wider range (8.8-fold) across the different types of treatment. The lowest titers were observed among patients who had received immunotherapy plus chemotherapy/biological therapy (median [IQR], 94.4 [49.4-191] AU/mL vs. 147 [62.8-339] AU/mL).
In an exploratory multivariable analysis, treatments with chemotherapy plus immunotherapy and immunotherapy plus biological therapy were significantly associated with lower IgG titers.
No downside for cancer patients
The Biden administration announced a plan to begin booster COVID-19 vaccinations for all American adults in September, with recommendations that the third vaccine be given at least 8 months after the second mRNA vaccine dose.
Jeremy M. Levin, DPhil, the chairman and CEO of Ovid Therapeutics, explained that, concerning boosters, “it is inconceivable that we will have all data at this stage.
“Knowledge about how boosters work and don’t work and when you should ideally have them is imperfect,” he told this news organization. “However, we can have a lot of confidence in the fact that hundreds of millions of people have received the vaccine, so we know a lot about the safety and efficacy.”
Immunocompromised adults represent less than 5% of the total population, and most of the available data on vaccination are from patients who have undergone solid-organ transplant, Dr. Levin explained. Studies have shown that their response is less robust to vaccination in comparison with adults in the general population.
“Although it is still preliminary, the strongest data come from Israel,” he said, “where they found that the booster was highly effective and doubled the number of transplant patients who developed antibodies.”
But data are not yet available in the setting of cancer. “But even though we don’t have the data yet, the answer is that no matter, the booster process is essential,” he said. “The evidence we have is that boosters raise the immune response, and it is the best data we have now.”
Martin J. Edelman, MD, chair, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that the current recommendation is that patients who are immunocompromised receive a booster immediately.
At his health system, this is interpreted to include patients who have undergone the following treatments: Transplant (solid-organ and bone marrow transplant), hemodialysis, hematologic malignancy treatment, active immunosuppressive (chemotherapy, chemoimmunotherapy, and nonhormonal or single-agent immunotherapy) treatment, rheumatology treatments, and high-dose steroids.
“As for cancer patients, we are making arrangements to vaccinate patients who meet the above criteria now,” he said. “There is no known downside to receiving booster immediately. While there may be less of a response than waiting for completion of treatment, we know that patients on active therapy are frequently able to mount a response, and any response is better than none.”
Dr. Edelman added that this area is changing very rapidly. “We will modify our approach as information and guidance from appropriate organizations, such as the FDA and CDC, become available.”
Dr. Stemmer has received institutional research grants from CAN-FITE, AstraZeneca, Bioline RX, BMS, Halozyme, Clovis Oncology, CTG Pharma, Exelixis, Geicam, Incyte, Lilly, Moderna, Teva Pharmaceuticals, and Roche, and owns stocks and options in CTG Pharma, DocBoxMD, Tyrnovo, VYPE, Cytora, and CAN-FITE. Dr. Edelman has received personal fees and other compensation from Windmil, Biomarker Strategies, AstraZeneca, Takeda, GlaxoSmithKline, Apexigen, Nektar, Bristol-Myers Squibb, Armo, Bergen Bio, and Apexigen outside the submitted work. He has submitted a patent for epigenetic modifications to increase susceptibility to radiopharmaceuticals and is a paid adviser for Kanaph and Flame. Dr. Levin is chairman and CEO of Ovid Therapeutics.
A version of this article first appeared on Medscape.com.
Children and COVID: New cases soar to near-record level
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.
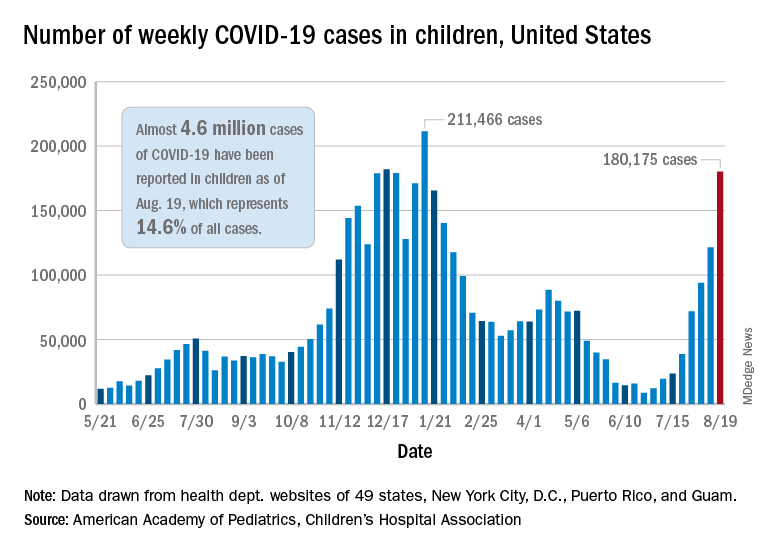
The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.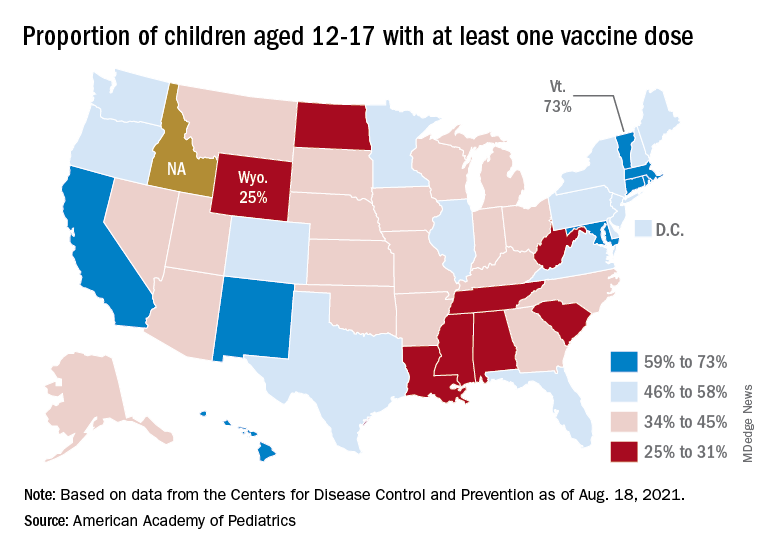
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Health care workers eager for COVID booster shots
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.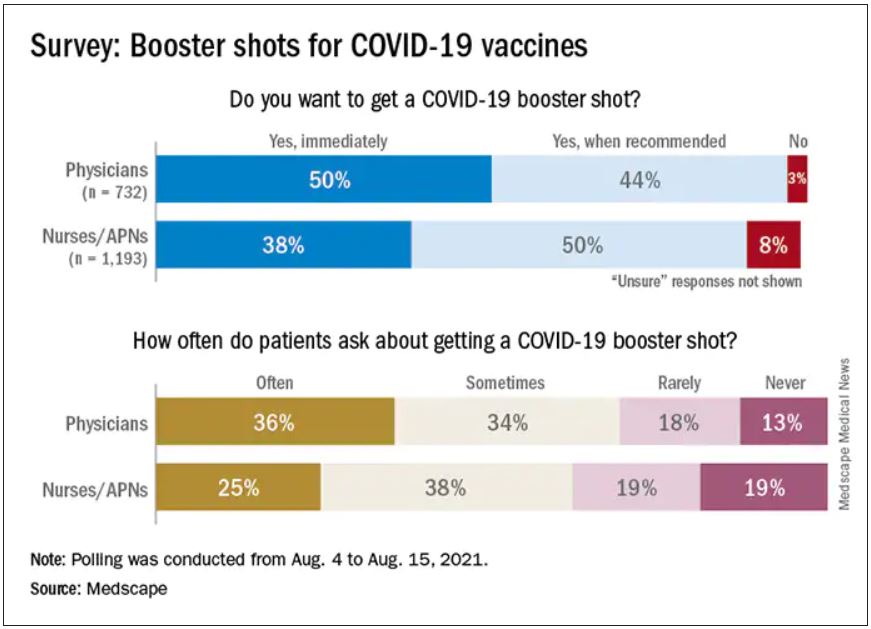
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.
As COVID vaccine boosters move closer to reality, most physicians and nurses are ready and willing to get another shot in the arm, according to a new Medscape survey.
Altogether, 93% of physicians and 87% of nurses/advanced practice nurses (APNs) said they wanted to get a booster, although the timing of when they wanted the shots differed somewhat between the two groups surveyed Aug. 4-15.
Among the 732 physicians polled, 50% wanted to get their shot immediately, compared with 38% of the 1,193 nurses/APNs who responded, while 44% of physicians and 50% of nurses/APNs said that they would wait until the vaccine booster was authorized and recommended.
At this point in time, almost all of the health care workers surveyed – 98% of physicians and 94% of nurses/APNs – have been fully vaccinated against COVID-19. A small proportion of each group, however, received the Johnson & Johnson vaccine (1% of physicians and 3% of nurses) and are not included in the current plan for booster shots.
The Medscape survey sample did include one group that is already eligible for a third dose: About 20% of physicians and 26% of nurses/ANPs said they have a condition or take a medication that compromises their immune system.
Respondents’ experiences with patient requests for boosters suggest a somewhat lower level of interest. About two-thirds of the health care workers (69% of physicians and 63% of nurses) said that patients frequently or sometimes asked about COVID boosters, compared with 13% (physicians) and 19% (nurses) who said their patients had never asked.
Interest lower among general population
In a separate survey conducted by WebMD, 82% of those who have been at least partially vaccinated said they want to get a COVID vaccine booster (14% immediately and 68% after authorization and recommendation). Of the remaining vaccinees, 7% said they do not want to get a booster and 11% were unsure.
The full sample of 592 respondents surveyed Aug. 5-10, however, included 19% who do not plan to get vaccinated and 6% who are planning to be vaccinated but have not yet done so.
The proportion of immunocompromised individuals in the two survey groups was similar, with about 25% of those in the WebMD survey reporting they have a condition or take a medication that compromises their immune system. Those respondents were more than twice as likely to want to get a booster immediately, compared to those with an uncompromised immune system (24% vs. 11%).
The distribution of vaccines received by brand was also comparable between the two groups surveyed. Of health care workers and readers, over half of each group received the Pfizer/BioNTech vaccine (59% vs. 54%), followed by Moderna (38% vs. 40%) and Johnson & Johnson (3% vs. 5%).
A version of this article first appeared on Medscape.com.

