User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Peramivir effective against most flu viruses circulating globally
SAN DIEGO – The neuraminidase inhibitor peramivir inhibited about 99% of seasonal influenza A and B viruses circulating globally during the 2013-2014 and 2014-2015 influenza seasons, a large analysis demonstrated.
“The frequency of H1N1pdm09 viruses carrying neuraminidase (NA) H275Y remained low during both seasons; this mutation confers resistance to oseltamivir and peramivir,” said Margaret Okomo-Adhiambo, Ph.D., at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. In addition, “a small proportion of viruses contained other neuraminidase changes that affect binding of peramivir to viral enzymes and may decrease virus susceptibility. These changes need to be closely monitored.”
Approved by the FDA in December of 2014, peramivir (Rapivab) is the only antiviral agent for influenza treatment to come to market in nearly 20 years. Approved for intravenous administration as a single dose, it is indicated for adults with acute uncomplicated influenza who may have trouble taking orally administered or inhaled neuraminidase (NA) inhibitors. Other NA inhibitors approved by the FDA for influenza infection include oseltamivir, which is orally administered, and zanamivir, which is inhaled.
For the current analysis, Dr. Okomo-Adhiambo of the influenza division at the Centers for Disease Control and Prevention, Atlanta, and her associates tested influenza virus susceptibility to peramivir during the 2013-2014 and 2014-2015 influenza seasons as part of the World Health Organization Global Influenza Surveillance and Response System. A total of 8,426 viruses were tested, 75% of which were circulating in the United States.
Dr. Okomo-Adhiambo reported that during the 2013-2014 and 2014-2015 influenza seasons, about 99% of influenza type A and B viruses were inhibited by peramivir, except for a few viruses belonging to subtype A(H1N1)pdm09 (1.5%), subtype A(H3N2) (0.2%), and type B (0.4%). In addition, NA activity of type A viruses was five to six times more sensitive to inhibition by peramivir, compared with type B NA.
She concluded her presentation by noting that studies “are needed to establish molecular markers of clinically relevant resistance to peramivir.”
The researchers reported having no financial disclosures.
SAN DIEGO – The neuraminidase inhibitor peramivir inhibited about 99% of seasonal influenza A and B viruses circulating globally during the 2013-2014 and 2014-2015 influenza seasons, a large analysis demonstrated.
“The frequency of H1N1pdm09 viruses carrying neuraminidase (NA) H275Y remained low during both seasons; this mutation confers resistance to oseltamivir and peramivir,” said Margaret Okomo-Adhiambo, Ph.D., at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. In addition, “a small proportion of viruses contained other neuraminidase changes that affect binding of peramivir to viral enzymes and may decrease virus susceptibility. These changes need to be closely monitored.”
Approved by the FDA in December of 2014, peramivir (Rapivab) is the only antiviral agent for influenza treatment to come to market in nearly 20 years. Approved for intravenous administration as a single dose, it is indicated for adults with acute uncomplicated influenza who may have trouble taking orally administered or inhaled neuraminidase (NA) inhibitors. Other NA inhibitors approved by the FDA for influenza infection include oseltamivir, which is orally administered, and zanamivir, which is inhaled.
For the current analysis, Dr. Okomo-Adhiambo of the influenza division at the Centers for Disease Control and Prevention, Atlanta, and her associates tested influenza virus susceptibility to peramivir during the 2013-2014 and 2014-2015 influenza seasons as part of the World Health Organization Global Influenza Surveillance and Response System. A total of 8,426 viruses were tested, 75% of which were circulating in the United States.
Dr. Okomo-Adhiambo reported that during the 2013-2014 and 2014-2015 influenza seasons, about 99% of influenza type A and B viruses were inhibited by peramivir, except for a few viruses belonging to subtype A(H1N1)pdm09 (1.5%), subtype A(H3N2) (0.2%), and type B (0.4%). In addition, NA activity of type A viruses was five to six times more sensitive to inhibition by peramivir, compared with type B NA.
She concluded her presentation by noting that studies “are needed to establish molecular markers of clinically relevant resistance to peramivir.”
The researchers reported having no financial disclosures.
SAN DIEGO – The neuraminidase inhibitor peramivir inhibited about 99% of seasonal influenza A and B viruses circulating globally during the 2013-2014 and 2014-2015 influenza seasons, a large analysis demonstrated.
“The frequency of H1N1pdm09 viruses carrying neuraminidase (NA) H275Y remained low during both seasons; this mutation confers resistance to oseltamivir and peramivir,” said Margaret Okomo-Adhiambo, Ph.D., at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. In addition, “a small proportion of viruses contained other neuraminidase changes that affect binding of peramivir to viral enzymes and may decrease virus susceptibility. These changes need to be closely monitored.”
Approved by the FDA in December of 2014, peramivir (Rapivab) is the only antiviral agent for influenza treatment to come to market in nearly 20 years. Approved for intravenous administration as a single dose, it is indicated for adults with acute uncomplicated influenza who may have trouble taking orally administered or inhaled neuraminidase (NA) inhibitors. Other NA inhibitors approved by the FDA for influenza infection include oseltamivir, which is orally administered, and zanamivir, which is inhaled.
For the current analysis, Dr. Okomo-Adhiambo of the influenza division at the Centers for Disease Control and Prevention, Atlanta, and her associates tested influenza virus susceptibility to peramivir during the 2013-2014 and 2014-2015 influenza seasons as part of the World Health Organization Global Influenza Surveillance and Response System. A total of 8,426 viruses were tested, 75% of which were circulating in the United States.
Dr. Okomo-Adhiambo reported that during the 2013-2014 and 2014-2015 influenza seasons, about 99% of influenza type A and B viruses were inhibited by peramivir, except for a few viruses belonging to subtype A(H1N1)pdm09 (1.5%), subtype A(H3N2) (0.2%), and type B (0.4%). In addition, NA activity of type A viruses was five to six times more sensitive to inhibition by peramivir, compared with type B NA.
She concluded her presentation by noting that studies “are needed to establish molecular markers of clinically relevant resistance to peramivir.”
The researchers reported having no financial disclosures.
AT ICAAC 2015
Key clinical point: Peramivir is potently effective against seasonal influenza viruses circulating globally.
Major finding: During the 2013-2014 and 2014-2015 influenza seasons, about 99% of influenza type A and B viruses were inhibited by peramivir.
Data source: An analysis of 8,426 influenza viruses that were tested during the 2013-2014 and 2014-2015 influenza seasons as part of the World Health Organization Global Influenza Surveillance and Response System.
Disclosures: The researchers reporting having no financial disclosures.
Simple breath test effectively detected TB
SAN DIEGO – Researchers in England used a novel gas analysis technique to detect tuberculosis in the breath, with a sensitivity of 93% and a specificity of 94%.
“Clearly these are promising results,” Dr. Amandip Sahota said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. “What interested me the most is that we were able to detect a significant difference in chemicals in both pulmonary and extra-pulmonary TB, which did indicate to us that the disease does not need to be limited to the lungs to be detectable in the breath.”
According to the latest data from the World Health Organization, there were 9 million active TB cases and 1.5 million deaths from the disease in 2013. Of these deaths, 80,000 were in children.
“TB remains a diagnostic challenge well into the 21st Century,” said Dr. Sahota, a consultant physician in infectious diseases at University Hospitals of Leicester, England. “We are still heavily reliant on the standard culture, which is both slow and resource-intensive throughout the world. Despite the advent of TB-PCR, we are still far away from a diagnostic test which is both available at point of care, at low cost, and is available throughout the world.”
In a study he conducted during his time as a research fellow at the University Hospitals of Coventry, in association with colleagues at the University of Warwick, Dr. Sahota and his associates used a field asymmetric ion mobility spectrometry device to collect samples of exhaled breath from 25 patients with suspected pulmonary or extra-pulmonary TB over a period of 6 months, before or within 1 week of treatment. For comparison, exhaled breath from 19 healthy controls was also obtained.
While ion mobility spectrometry has been used for years by the military and the security industry to detect explosives, for example, the technology has more recently been used to help diagnose medical conditions ranging from cancers to infections.
“Breath testing for TB is not new, but what is very exciting is the advent of newer gas sensor technologies which are being developed in line with a clinical need,” Dr. Sahota explained. “The point of interest here is volatile organic compounds: chemicals which are gaseous at ambient temperatures, often produce odors, and are endogenous products of metabolism in both health and disease states. So testing for breath can be quick, easy, and noninvasive. Clearly there’s plenty of sample. It’s rapid, and it allows access to chemicals in the blood, which are visible in the breath through ventilator processes.”
Patients in the study, which is believed to be the first of its kind, breathed into a 3L Tedlar air sample bag and the samples were tested within 2 hours with a portable field asymmetric ion mobility spectrometry device made by Oxford Immunotec, Inc. After measuring the ionic mobility of volatile organic compounds in the headspace, the researchers determined that the test was highly effective in detecting TB in the breath, with a sensitivity of 93% and a specificity of 94%.
“Clearly this is a small study and we do need to repeat this in a larger cohort to validate it further,” Dr. Sahota said. “We also need to investigate potential confounders such as other comorbidities and medications. Ideally, we’d like to use a smaller, more portable instrument which is ideally hand-held, so we’re exploring commercial partnerships.”
The study was funded by the Medical Research Council. The researchers reported having no financial disclosures.
*This story was updated on 10/5/2015.
SAN DIEGO – Researchers in England used a novel gas analysis technique to detect tuberculosis in the breath, with a sensitivity of 93% and a specificity of 94%.
“Clearly these are promising results,” Dr. Amandip Sahota said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. “What interested me the most is that we were able to detect a significant difference in chemicals in both pulmonary and extra-pulmonary TB, which did indicate to us that the disease does not need to be limited to the lungs to be detectable in the breath.”
According to the latest data from the World Health Organization, there were 9 million active TB cases and 1.5 million deaths from the disease in 2013. Of these deaths, 80,000 were in children.
“TB remains a diagnostic challenge well into the 21st Century,” said Dr. Sahota, a consultant physician in infectious diseases at University Hospitals of Leicester, England. “We are still heavily reliant on the standard culture, which is both slow and resource-intensive throughout the world. Despite the advent of TB-PCR, we are still far away from a diagnostic test which is both available at point of care, at low cost, and is available throughout the world.”
In a study he conducted during his time as a research fellow at the University Hospitals of Coventry, in association with colleagues at the University of Warwick, Dr. Sahota and his associates used a field asymmetric ion mobility spectrometry device to collect samples of exhaled breath from 25 patients with suspected pulmonary or extra-pulmonary TB over a period of 6 months, before or within 1 week of treatment. For comparison, exhaled breath from 19 healthy controls was also obtained.
While ion mobility spectrometry has been used for years by the military and the security industry to detect explosives, for example, the technology has more recently been used to help diagnose medical conditions ranging from cancers to infections.
“Breath testing for TB is not new, but what is very exciting is the advent of newer gas sensor technologies which are being developed in line with a clinical need,” Dr. Sahota explained. “The point of interest here is volatile organic compounds: chemicals which are gaseous at ambient temperatures, often produce odors, and are endogenous products of metabolism in both health and disease states. So testing for breath can be quick, easy, and noninvasive. Clearly there’s plenty of sample. It’s rapid, and it allows access to chemicals in the blood, which are visible in the breath through ventilator processes.”
Patients in the study, which is believed to be the first of its kind, breathed into a 3L Tedlar air sample bag and the samples were tested within 2 hours with a portable field asymmetric ion mobility spectrometry device made by Oxford Immunotec, Inc. After measuring the ionic mobility of volatile organic compounds in the headspace, the researchers determined that the test was highly effective in detecting TB in the breath, with a sensitivity of 93% and a specificity of 94%.
“Clearly this is a small study and we do need to repeat this in a larger cohort to validate it further,” Dr. Sahota said. “We also need to investigate potential confounders such as other comorbidities and medications. Ideally, we’d like to use a smaller, more portable instrument which is ideally hand-held, so we’re exploring commercial partnerships.”
The study was funded by the Medical Research Council. The researchers reported having no financial disclosures.
*This story was updated on 10/5/2015.
SAN DIEGO – Researchers in England used a novel gas analysis technique to detect tuberculosis in the breath, with a sensitivity of 93% and a specificity of 94%.
“Clearly these are promising results,” Dr. Amandip Sahota said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy. “What interested me the most is that we were able to detect a significant difference in chemicals in both pulmonary and extra-pulmonary TB, which did indicate to us that the disease does not need to be limited to the lungs to be detectable in the breath.”
According to the latest data from the World Health Organization, there were 9 million active TB cases and 1.5 million deaths from the disease in 2013. Of these deaths, 80,000 were in children.
“TB remains a diagnostic challenge well into the 21st Century,” said Dr. Sahota, a consultant physician in infectious diseases at University Hospitals of Leicester, England. “We are still heavily reliant on the standard culture, which is both slow and resource-intensive throughout the world. Despite the advent of TB-PCR, we are still far away from a diagnostic test which is both available at point of care, at low cost, and is available throughout the world.”
In a study he conducted during his time as a research fellow at the University Hospitals of Coventry, in association with colleagues at the University of Warwick, Dr. Sahota and his associates used a field asymmetric ion mobility spectrometry device to collect samples of exhaled breath from 25 patients with suspected pulmonary or extra-pulmonary TB over a period of 6 months, before or within 1 week of treatment. For comparison, exhaled breath from 19 healthy controls was also obtained.
While ion mobility spectrometry has been used for years by the military and the security industry to detect explosives, for example, the technology has more recently been used to help diagnose medical conditions ranging from cancers to infections.
“Breath testing for TB is not new, but what is very exciting is the advent of newer gas sensor technologies which are being developed in line with a clinical need,” Dr. Sahota explained. “The point of interest here is volatile organic compounds: chemicals which are gaseous at ambient temperatures, often produce odors, and are endogenous products of metabolism in both health and disease states. So testing for breath can be quick, easy, and noninvasive. Clearly there’s plenty of sample. It’s rapid, and it allows access to chemicals in the blood, which are visible in the breath through ventilator processes.”
Patients in the study, which is believed to be the first of its kind, breathed into a 3L Tedlar air sample bag and the samples were tested within 2 hours with a portable field asymmetric ion mobility spectrometry device made by Oxford Immunotec, Inc. After measuring the ionic mobility of volatile organic compounds in the headspace, the researchers determined that the test was highly effective in detecting TB in the breath, with a sensitivity of 93% and a specificity of 94%.
“Clearly this is a small study and we do need to repeat this in a larger cohort to validate it further,” Dr. Sahota said. “We also need to investigate potential confounders such as other comorbidities and medications. Ideally, we’d like to use a smaller, more portable instrument which is ideally hand-held, so we’re exploring commercial partnerships.”
The study was funded by the Medical Research Council. The researchers reported having no financial disclosures.
*This story was updated on 10/5/2015.
AT ICAAC 2015
Key clinical point: A quick breath test was highly effective in detecting tuberculosis.
Major finding: Using field asymmetric ion mobility spectrometry to detect tuberculosis had a sensitivity of 93% and a specificity of 94%.
Data source: An analysis of volatile active compounds in samples of exhaled breath from 25 patients with suspected pulmonary or extra-pulmonary TB over a period of 6 months.
Disclosures: The study was funded by the Medical Research Council. The researchers reported having no financial disclosures.
COPD: Optimizing treatment
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
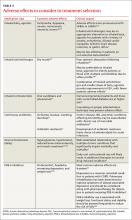
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
› Individualize treatment regimens based on severity of symptoms and risk for exacerbation, prescribing short-acting beta2-agonists, as needed, for all patients with chronic obstructive pulmonary disease (COPD). A
› Limit use of inhaled long-acting beta2-agonists to the recommended dosage; higher doses do not lead to better outcomes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Chronic obstructive pulmonary disease (COPD) carries a high disease burden. In 2012, it was the 4th leading cause of death worldwide.1,2 In 2015, the World Health Organization updated its Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, classifying patients with COPD based on disease burden as determined by symptoms, airflow obstruction, and exacerbation history.3 These revisions, coupled with expanded therapeutic options within established classes of medications and new combination drugs to treat COPD (TABLE 1),3-6 have led to questions about interclass differences and the best treatment regimen for particular patients.
Comparisons of various agents within a therapeutic class and their impact on lung function and rate of exacerbations address many of these concerns. In the text and tables that follow, we present the latest evidence highlighting differences in dosing, safety, and efficacy. We also include the updated GOLD classifications, evidence of efficacy for pulmonary rehabilitation, and practical implications of these findings for the optimal management of patients with COPD.
But first, a word about terminology.
Understanding COPD
COPD is a chronic lung disease characterized by progressive airflow limitation, usually measured by spirometry (TABLE 2),3 and chronic airway inflammation. Emphysema and chronic bronchitis are often used synonymously with COPD. In fact, there are important differences.
Individuals with chronic bronchitis do not necessarily have the airflow limitations found in those with COPD. And patients with COPD develop pathologic lung changes beyond the alveolar damage characteristic of emphysema, including airway fibrosis and inflammation, luminal plugging, and loss of elastic recoil.3
The medications included in this review aim to reduce both the morbidity and mortality associated with COPD. These drugs can also help relieve the symptoms of patients with chronic bronchitis and emphysema, but have limited effect on patient mortality.
Short- and long-acting beta2-agonists
Bronchodilator therapy with beta2-agonists improves forced expiratory volume in one second (FEV1) through relaxation of airway smooth muscle. Beta2-agonists have proven to be safe and effective when used as needed or scheduled for patients with COPD.7
Inhaled short-acting beta2-agonists (SABAs) improve FEV1 and symptoms within 10 minutes, with effects lasting up to 4 to 6 hours; long-acting beta2-agonists (LABAs) have a variable onset, with effects lasting 12 to 24 hours.8 Inhaled levalbuterol, the last SABA to receive US Food and Drug Administration approval, has not proven to be superior to conventional bronchodilators in ambulatory patients with stable COPD.3 In clinical trials, however, the slightly longer half-life of the nebulized formulation of levalbuterol was found to reduce both the frequency of administration and the overall cost of therapy in patients hospitalized with acute exacerbations of COPD.9,10
Recently approved LABAs
Clinical trials have studied the safety and efficacy of newer agents vs older LABAs in patients with moderate to severe COPD. Compared with theophylline, for example, formoterol 12 mcg inhaled every 12 hours for a 12-month period provided a clinically significant increase of >120 ml in FEV1 (P=.026).11 Higher doses of formoterol did not provide any additional improvement.
In a trial comparing indacaterol and tiotropium, an inhaled anticholinergic, both treatment groups had a clinically significant increase in FEV1, but patients receiving indacaterol achieved an additional increase of 40 to 50 mL at 12 weeks.12
Exacerbation rates for all LABAs range from 22% to 44%.5,12,13 In a study of patients receiving formoterol 12 mcg compared with 15-mcg and 25-mcg doses of arformoterol, those taking formoterol had a lower exacerbation rate than those on either strength of arformoterol (22% vs 32% and 31%, respectively).10 In various studies, doses greater than the FDA-approved regimens for indacaterol, arformoterol, and olodaterol did not result in a significant improvement in either FEV1 or exacerbation rates compared with placebo.5,12,14
Studies that assessed the use of rescue medication as well as exacerbation rates in patients taking LABAs reported reductions in the use of the rescue drugs ranging from 0.46 to 1.32 actuations per day, but the findings had limited clinical relevance.5,13 With the exception of indacaterol and olodaterol—both of which may be preferable because of their once-daily dosing regimen—no significant differences in safety and efficacy among LABAs have been found.5,12,13
Long-acting inhaled anticholinergics
Inhaled anticholinergic agents (IACs) can be used in place of, or in conjunction with, LABAs to provide bronchodilation for up to 24 hours.3 The introduction of long-acting IACs dosed once or twice daily has the potential to improve medication adherence over traditional short-acting ipratropium, which requires multiple daily doses for symptom control. Over 4 years, tiotropium has been shown to increase time to first exacerbation by approximately 4 months. It did not, however, significantly reduce the number of exacerbations compared with placebo.15
Long-term use of tiotropium appears to have the potential to preserve lung function. In one trial, it slowed the rate of decline in FEV1 by 5 mL per year, but this finding lacked clinical significance.13 In clinical trials of patients with moderate to severe COPD, however, once-daily tiotropium and umeclidinium provided clinically significant improvements in FEV1 (>120 mL; P<.01), regardless of the dose administered.6,16 In another trial, patients taking aclidinium 200 mcg or 400 mcg every 12 hours did not achieve a clinically significant improvement in FEV1 compared with placebo.17
In patients with moderate to severe COPD, the combination of umeclidinium/vilanterol, a LABA, administered once daily resulted in a clinically significant improvement in FEV1 (167 mL; P<.001) vs placebo—but was not significantly better than treatment with either agent alone.18
Few studies have evaluated time to exacerbation in patients receiving aclidinium or umeclidinium. In comparison to salmeterol, tiotropium reduced the time to first exacerbation by 42 days at one year (hazard ratio=0.83; 95% confidence interval [CI], 0.77-0.9; P<.001).19 The evidence suggests that when used in combination with LABAs, long-acting IACs have a positive impact on FEV1, but their effect on exacerbation rates has not been established.
Combination therapy with steroids and LABAs
The combination of inhaled corticosteroids (ICS) and LABAs has been found to improve FEV1 and symptoms in patients with moderate to severe COPD more than monotherapy with either drug class.20,21 In fact, ICS alone have not been proven to slow the progression of the disease or to lower mortality rates in patients with COPD.22
Fluticasone/salmeterol demonstrated a 25% reduction in exacerbation rates compared with placebo (P<.0001), a greater reduction than that of either drug alone.20 A retrospective observational study comparing fixed dose fluticasone/salmeterol with budesonide/formoterol reported a similar reduction in exacerbation rates, but the number of patients requiring the addition of an IAC was 16% lower in the latter group.23
The combination of fluticasone/vilanterol has the potential to improve adherence, given that it is dosed once daily, unlike other COPD combination drugs. Its clinical efficacy is comparable to that of fluticasone/salmeterol after 12 weeks of therapy, with similar improvements in FEV1,24 but fluticasone/vilanterol is associated with an increased risk of pneumonia.3
Chronic use of oral corticosteroids
Oral corticosteroids (OCS) are clinically indicated in individuals whose symptoms continue despite optimal therapy with inhaled agents that have demonstrated efficacy. Such patients are often referred to as “steroid dependent.”
While OCS are prescribed for both their anti-inflammatory activity and their ability to slow the progression of COPD,25,26 no well-designed studies have investigated their benefits for this patient population. One study concluded that patients who were slowly withdrawn from their OCS regimen had no more frequent exacerbations than those who maintained chronic usage. The withdrawal group did, however, lose weight.27
GOLD guidelines do not recommend OCS for chronic management of COPD due to the risk of toxicity.3 The well-established adverse effects of chronic OCS include hyperglycemia, hypertension, osteoporosis, and myopathy.28,29 A study of muscle function in 21 COPD patients receiving corticosteroids revealed decreases in quadriceps muscle strength and pulmonary function.30 Daily use of OCS will likely result in additional therapies to control drug-induced conditions, as well—another antihypertensive secondary to fluid retention caused by chronic use of OCS in patients with high blood pressure, for example, or additional medication to control elevated blood glucose levels in patients with diabetes.
Phosphodiesterase-4 inhibitors
The recommendation for roflumilast in patients with GOLD Class 2 to 4 symptoms remains unchanged since the introduction of this agent as a treatment option for COPD.3 Phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast reduce inflammation in the lungs and have no activity as a bronchodilator.31,32
Roflumilast has been shown to improve FEV1 in patients concurrently receiving a long-acting bronchodilator and to reduce exacerbations in steroid-dependent patients, a recent systematic review of 29 PDE-4 trials found.33 Patients taking roflumilast, however, suffered from more adverse events (nausea, appetite reduction, diarrhea, weight loss, sleep disturbances, and headache) than those on placebo.33
Antibiotics
GOLD guidelines do not recommend the use of antibiotics for patients with COPD, except to treat acute exacerbations.1 However, recent studies suggest that routine or pulsed dosing of prophylactic antibiotics can reduce the number of exacerbations.34-36 A 2013 review of 7 studies determined that continuous antibiotics, particularly macrolides, reduced the number of COPD exacerbations in patients with a mean age of 66 years (odds ratio [OR]=0.55; 95% CI, 0.39-0.77).37
A more recent trial randomized 92 patients with a history of ≥3 exacerbations in the previous year to receive either prophylactic azithromycin or placebo daily for 12 months. The treatment group experienced a significant decrease in the number of exacerbations (OR=0.58; 95% CI, 0.42-0.79; P=.001).38 This benefit must be weighed against the potential development of antibiotic resistance and adverse effects, so careful patient selection is important.
Pulmonary rehabilitation has proven benefits
GOLD, the American College of Chest Physicians, the American Thoracic Society, and the European Respiratory Society all recommend pulmonary rehabilitation for patients with COPD.39-41 In addition to reducing morbidity and mortality rates—including a reduction in number of hospitalizations and length of stay and improved post-discharge recovery—pulmonary rehabilitation has been shown to have other physical and psychological benefits.42 Specific benefits include improved exercise capacity, greater arm strength and endurance, reduced perception of intensity of breathlessness, and improved overall health-related quality of life.
Key features of rehab programs
Important components of pulmonary rehabilitation include counseling on tobacco cessation, nutrition, education—including correct inhalation technique—and exercise training. There are few contraindications to participation, and patients can derive benefit from both its non-exercise components and upper extremity training regardless of their mobility level.
A 2006 Cochrane review concluded that an effective pulmonary rehabilitation program should be at least 4 weeks in duration,43 and longer programs have been shown to produce greater benefits.44 However, there is no agreement on an optimal time frame. Studies are inconclusive on other specific aspects of pulmonary rehab programs, as well, such as the number of sessions per week, number of hours per session, duration and intensity of exercise regimens, and staff-to-patient ratios.
Home-based exercise training may produce many of the same benefits as a formal pulmonary rehabilitation program. A systematic review found improved quality of life and exercise capacity associated with patient care that lacked formal pulmonary rehabilitation, with no differences between results from home-based training and hospital-based outpatient pulmonary rehabilitation programs.45
Given the lack of availability of formal rehab programs in many communities, homebased training for patients with COPD is important to consider.
Implications for practice
What is the takeaway from this evidence-based review? Overall, it is clear that, with the possible exception of the effect of once-daily dosing on adherence, there is little difference among the therapeutic agents within a particular class of medications—and that more is not necessarily better. Indeed, evidence suggests that higher doses of LABAs may reduce their effectiveness, rendering them no better than placebo. In addition, there is no significant difference in the rate of exacerbations in patients taking ICS/LABA combinations and those receiving IACs alone.
Pulmonary rehabilitation should be recommended for all newly diagnosed patients, while appropriate drug therapies should be individualized based on the GOLD symptoms/risk evaluation categories (TABLE 3).3 While daily OCS and daily antibiotics have the potential to reduce exacerbation rates, for example, the risks of adverse effects and toxicities outweigh the benefits for patients whose condition is stable.
Determining the optimal treatment for a particular patient also requires an assessment of comorbidities, including potential adverse drug effects (TABLE 4).3,27-29,33,46-52 Selection of medication should be driven by patient and physician preference to optimize adherence and clinical outcomes, although cost and accessibility often play a significant role, as well.
CORRESPONDENCE
Nabila Ahmed-Sarwar, PharmD, BCPS, CDE, St. John Fisher College, Wegmans School of Pharmacy, 3690 East Avenue, Rochester, NY 14618; [email protected]
ACKNOWLEDGEMENTS
The authors thank the following people for their assistance in the preparation of this manuscript: Matthew Stryker, PharmD, Timothy Adler, PharmD, and Angela K. Nagel, PharmD, BCPS.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). Fact Sheet No. 315. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed January 29, 2015.
2. National Heart, Lung, and Blood Institute. Morbidity and mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Heart, Lung, and Blood Institute Web site. Available at: http://www.nhlbi.nih.gov/files/docs/research/2012_Chart-Book_508.pdf. Accessed January 29, 2015.
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2015. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed July 26, 2015.
4. Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD. 2008;5:25-34.
5. Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD. 2010;7:17-31.
6. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J. 2014;43:72-81.
7. Vathenen AS, Britton JR, Ebden P, et al. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138:850-855.
8. Cazzola M, Matera MG, Santangelo G, et al. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med. 1995;89:357-362.
9. Donohue JF, Hanania NA, Ciubotaru RL, et al. Comparison of levalbuterol and racemic albuterol in hospitalized patients with acute asthma or COPD: a 2-week, multicenter, randomized, open-label study. Clin Ther. 2008;30:989-1002.
10. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: efficacy and outcomes in patients hospitalized with COPD or asthma. Chest. 2003;123:128-135.
11. Rossi A, Kristufek P, Levine BE, et al; Formoterol in Chronic Obstructive Pulmonary Disease (FICOPD) II Study Group. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058-1069.
12. Donohue JF, Fogarty C, Lötvall J, et al; INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155-162.
13. Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629-645.
14. Boyd G, Morice AH, Pounsford JC, et al. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10:815-821.
15. Tashkin DP, Celli B, Senn S, et al; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554.
16. Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217-224.
17. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830-836.
18. Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538-1546.
19. Vogelmeier C, Hederer B, Glaab T, et al; POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093-1103.
20. Calverley P, Pauwels R, Vestbo J, et al; Trial of inhaled steroids and long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449-456.
21. Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74-81.
22. Calverley PM, Anderson JA, Celli B, et al; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789.
23. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273:584-594.
24. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108:1171-1179.
25. Davies L, Nisar M, Pearson MG, et al. Oral corticosteroid trials in the management of stable chronic obstructive pulmonary disease. QJM. 1999;92:395-400.
26. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;CD005374.
27. Rice KL, Rubins JB, Lebahn F, et al. Withdrawal of chronic systemic corticosteroids in patients with COPD: a randomized trial. Am J Respir Crit Care Med. 2000;162:174-178.
28. Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474.
29. McEvoy CE, Ensrud KE, Bender E, et al. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:704-709.
30. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11-16.
31. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al; M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695-703.
32. Calverley PM, Rabe KF, Goehring UM, et al; M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685-694.
33. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;11:CD002309.
34. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139-1147.
35. Sethi S, Jones PW, Theron MS, et al; PULSE study group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11:10.
36. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
37. Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2013;11:CD009764.
38. Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361-368.
39. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131:S4-S42.
40. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-e64.
41. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
42. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Global Initiative for Chronic Obstructive Lung Disease Web site. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed January 14, 2015.
43. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;CD003793.
44. Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, et al. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129-140.
45. Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:134-143.
46. Proair HFM (albuterol sulfate) [package insert]. Miami, FL: IVAX Laboratories; 2005.
47. Foradil (formoterol fumarate) [package insert]. Whitehouse Station, NJ: Merck & Co; 2012.
48. Spiriva (tiotropium bromide) [package insert]. Ridgefield, Conn: Boehringer Ingelheim Pharmaceuticals; 2014.
49. Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254-1263.
50. Flovent HFA (fluticasone propionate) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
51. Zithromax (azithromycin) [package insert]. New York, NY: Pfizer Labs; 2013.
52. Daliresp (roflumilast) [package insert]. St. Louis, Mo: Forest Pharmaceuticals; 2013.
This adjunct medication can speed CAP recovery
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Prescribe oral prednisone 50 mg/d to hospitalized patients with mild to moderate community-acquired pneumonia. It decreases time to clinical stability and length of hospital stay.1
Strength of recommendation
A: Based on a single good-quality randomized controlled trial and meta-analysis.
Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
Illustrative case
A 75-year-old woman with hypertension and diabetes mellitus presents to the emergency department with shortness of breath, cough, and fever that she’s had for 4 days. On examination, her temperature is 38.2°C (100.7°F), heart rate is 110 beats/min, respiratory rate is 28 breaths/min, oxygen saturation is 91%, and rhonchi are heard in her right lower lung field. A chest x-ray reveals an infiltrate in her right lower lobe. The patient is admitted and started on intravenous (IV) antibiotics, IV fluids, acetaminophen for fever, and oxygen. Can anything else be done to speed her recovery?
Community-acquired pneumonia (CAP) is responsible for more than one million hospitalizations annually in the United States, and is the 8th leading cause of death.2,3 Treatment of CAP typically consists of antibiotics and supportive measures such as IV fluids and antipyretics. Because the disease process of CAP involves extensive inflammation, adjunct treatment with corticosteroids may be beneficial.
Multiple studies have shown that treatment with corticosteroids can help patients with severe CAP, but the potential benefit in patients with less severe CAP has been uncertain.4,5 A Cochrane systematic review published in 2011 identified 6 small randomized controlled trials (RCTs) that evaluated the impact of corticosteroids on recovery from CAP.4 It suggested that corticosteroids may decrease time to recovery, but the studies that included patients with less severe CAP had a relatively high risk of bias.
Subsequently, a 2012 meta-analysis of 9 RCTs explored whether corticosteroids affected mortality in CAP; no benefit was observed in patients with less severe CAP.5 Most recently, a 2013 meta-analysis of 8 moderate-quality RCTs showed that corticosteroid use was associated with shorter hospital stays, but no change in mortality.6
The synthesis of small or moderate-quality studies suggests some potential benefit in treating less severe CAP with corticosteroids, but there has been a need for a large, definitive, high-quality RCT. This study investigated the impact of a short course of oral steroids on inpatients with less severe CAP.
STUDY SUMMARY: Prednisone hastens clinical stabilization, cuts length of hospital stay
In a multicenter, double-blind RCT, Blum et al1 enrolled 785 patients with CAP admitted to 7 tertiary care hospitals in Switzerland from 2009 to 2014. Patients were eligible for the study if they were ≥18 years old, had a new infiltrate on chest x-ray, and had at least one additional sign or symptom of respiratory illness (eg, cough, dyspnea, fever, abnormal breathing signs or rales, or elevated or decreased white blood cell count). Patients were excluded if they had one of several possible contraindications to corticosteroids, cystic fibrosis, or active tuberculosis.
Patients were randomized to receive either prednisone 50 mg/d or placebo for 7 days. They were treated with antibiotics according to accepted local guidelines; most patients received either amoxicillin/clavulanic acid or ceftriaxone. Antibiotic treatment was adjusted according to susceptibility whenever a specific pathogen was identified. Nurses assessed all patients every 12 hours during hospitalization, and laboratory tests were obtained on hospital Days 1, 3, 5, and 7, and before discharge. Follow-up telephone interviews were conducted on Day 30.
The primary outcome was length of time to clinical stability, which was defined as at least 24 hours of stable vital signs. Stable vital signs was a composite endpoint that required all of the following: temperature ≤37.8°C (≤100°F), heart rate ≤100 beats/min, spontaneous respiratory rate ≤24 breaths/min, systolic blood pressure ≥90 mm Hg (≥100 mm Hg for patients diagnosed with hypertension) without vasopressor support, mental status back to baseline, ability to take food by mouth, and adequate oxygenation on room air.
Secondary outcomes included length of hospital stay, pneumonia recurrence, hospital readmission, intensive care unit (ICU) admission, all-cause mortality, and duration of antibiotic treatment. Researchers also explored whether the rates of complications from pneumonia or corticosteroid use differed between the prednisone and placebo groups.
In an intention-to-treat analysis, the median time to clinical stability was shorter for the prednisone group at 3 days (interquartile range [IQR]=2.5-3.4) compared to the placebo group at 4.4 days (IQR=4-5; hazard ratio [HR]=1.33; 95% confidence interval [CI], 1.15-1.50; P<.0001). Median time to hospital discharge was also shorter for the prednisone group (6 days vs 7 days; HR=1.19; 95% CI, 1.04-1.38; P=.012) as was duration of IV antibiotic treatment (4 days vs 5 days, difference=-0.89 days; 95% CI, -1.57 to -0.20; P=.011).
There were no statistically significant differences in pneumonia recurrence, hospital readmission, ICU admission, or all-cause mortality. Patients treated with prednisone were more likely to experience hyperglycemia that required insulin treatment during admission (19% vs 11%; odds ratio=1.96; 95% CI, 1.31-2.93; P=.001).
WHAT'S NEW: This large, good-quality study reinforces previous evidence
This is the largest good-quality RCT to explore the impact of corticosteroid treatment on less severe CAP. Previous studies suggested that corticosteroids may decrease the duration of illness, but this is the first rigorous study to show a clear decrease in both time to clinical stability and length of hospital stay.
Also, this study used an easy-to-administer dose of oral steroids, instead of the several-day course of IV steroids used in most other studies. The findings from this study were incorporated into a 2015 meta-analysis that confirmed that corticosteroid treatment in patients with less severe CAP results in a shorter length of hospital stay and decreased time to clinical stability.7
CAVEATS: It's unclear whether steroids can benefit nonhospitalized patients
Because this study included hospitalized patients only, it’s not clear whether corticosteroids have a role in outpatient treatment of CAP. Additionally, while this was a large, well performed study, it did not have a sufficient number of patients to examine whether corticosteroids impact mortality among patients with CAP. Finally, the average length of hospital stay reported in this study was approximately 1.5 days longer than the typical length of stay in the United States.2 The average length of stay has varied widely in studies examining corticosteroids in CAP, but good-quality studies have consistently shown a median reduction in length of stay of one day.7
CHALLENGES TO IMPLEMENTATION: Steroids carry a risk of adverse events, including hyperglycemia
Treatment with prednisone increases the risk of corticosteroid-related adverse events, primarily hyperglycemia and the need for insulin. This may not be well received by patients or providers. However, these adverse effects appear to resolve quickly after treatment, and do not impact the overall time to clinical stability.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
1. Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomized, placebo-controlled trial. Lancet. 2015;385:1511-1518.
2. Centers for Disease Control and Prevention (CDC). FastStats: Pneumonia. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/fastats/pneumonia.htm. Accessed July 15, 2015.
3. Tejada-Vera B, Chong Y, Lu L, et al. Top 10 leading causes of death: United States, 1999–2013. Centers for Disease Control and Prevention National Center for Health Statistics Web site. Available at: http://blogs.cdc.gov/nchs-data-visualization/2015/06/01/leading-causes-of-death. Accessed September 10, 2015.
4. Chen Y, Li K, Pu H, et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2011;3:CD007720.
5. Nie W, Zhang Y, Cheng J, et al. Corticosteroids in the treatment of community-acquired pneumonia in adults: a meta-analysis. PLoS One. 2012;7:e47926.
6. Shafiq M, Mansoor MS, Khan AA, et al. Adjuvant steroid therapy in community-acquired pneumonia: a systematic review and meta-analysis. J Hosp Med. 2013;8:68-75.
7. Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015. [Epub ahead of print].
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reduced-nicotine cigarettes cut dependence, smoking
Reduced-nicotine cigarettes decreased tobacco dependence and the number of cigarettes smoked, with very little evidence of withdrawal or compensatory smoking, in a preliminary study reported online Oct. 1 in the New England Journal of Medicine.
Moreover, study participants who smoked very-low-nicotine cigarettes for the 6-week study were twice as likely to report that they attempted to quit 1 month later, compared with participants who smoked their usual brand or control cigarettes that had the usual nicotine content.
Reduced-nicotine cigarettes differ from “light” cigarettes in that the latter don’t actually reduce the nicotine content of the tobacco but instead increase ventilation of the cigarette – a strategy that is often circumvented by smokers who cover the ventilation holes or increase the number of cigarettes they smoke, said Eric C. Donny, Ph.D., of the department of psychology, University of Pittsburgh, and his associates.
The U.S. Food and Drug Administration recently was granted the authority to reduce, but not eliminate, nicotine in cigarettes if such action were deemed likely to benefit public health. However, no large-scale clinical trials have yet been performed to assess the potential benefit to public health.
Dr. Donny and his associates, supported by the National Institute on Drug Abuse and the FDA Center for Tobacco Products, conducted a double-blind, randomized trial at 10 sites comparing cigarettes with five levels of nicotine content among 839 adult smokers who were not planning to quit in the near future.
The study participants were assigned to smoke their usual brand of cigarettes (118 study subjects); control cigarettes containing the usual 15.8 mg of nicotine/g of tobacco (119 subjects); or experimental reduced-nicotine cigarettes containing 5.2 mg/g of nicotine (122 subjects), 2.4 mg/g (119 subjects), 1.3 mg/g (119 subjects), or 0.4 mg/g (242 subjects).
All the cigarettes were provided free of charge, and the smokers were paid for participating in the study. The dropout rate was only 8% at week 6 and did not differ significantly among the study groups.
The primary outcome – the average number of cigarettes smoked per day during week 6 – was markedly higher with the usual-brand group (22.2) and the control-cigarette group (21.3) than it was with the three lowest-nicotine groups (16.5, 16.3, and 14.9, respectively). That represents a reduction of 23%-30% in the number of cigarettes smoked in the latter three groups.
Tobacco dependence, as measured by the Wisconsin Inventory of Smoking Dependence Motives and the Fagerstrom Test for Nicotine Dependence, also was markedly lower with reduced-nicotine cigarettes.
Withdrawal symptoms did not increase; and during a brief voluntary abstinence period, smokers in the three lowest-nicotine groups actually reported fewer cravings than did those in the higher-nicotine groups, the investigators said (N Engl J Med. 2015 Oct 1;373[14]:1340-9).
At follow-up 30 days after completing the study, 34.7% of the participants who had smoked cigarettes with 0.4 mg/g of nicotine reported attempting to quit smoking, compared with 17% of those who had smoked cigarettes with 15.8 mg/g. In addition, participants who had smoked cigarettes with 1.3 mg/g or 0.4 mg/g of nicotine were still smoking significantly fewer cigarettes per day, even though the study had ended.
“In summary, these data suggest that if nicotine content is adequately reduced, smokers may benefit by smoking fewer cigarettes and experiencing less nicotine dependence, with few negative consequences,” Dr. Donny and his associates wrote. “If confirmed in longer-term studies, these findings suggest that, when combined with other tobacco-control policies (e.g., taxation and expanded access to treatment), limiting the nicotine content of cigarettes ... could improve public health.”
The study authors added that a longer clinical trial is now underway to further assess reduced-nicotine cigarettes.
NIDA and the FDA Center for Tobacco Products supported the study. Dr. Donny reported having no relevant financial disclosures. Two of his associates reported ties to Pfizer, and two reported serving as expert witnesses regarding addiction litigation against tobacco companies.
The findings of Dr. Donny and his colleagues justify exploration of a national nicotine-reduction policy and should encourage clinicians in practice to consider reduced-nicotine cigarettes as a potential resource for patients who want to quit smoking.
Given the number of current smokers in the United States, we can expect at least 20 million Americans to die prematurely if they continue to smoke. Reducing the nicotine content of cigarettes so that they are less addictive appears to be the most-promising regulatory policy option for preventing those 20 million premature deaths.
Dr. Michael Fiore and Timothy Baker, Ph.D., are at the Center for Tobacco Research and Intervention and the department of medicine at the University of Wisconsin, Madison. They reported having no relevant financial disclosures. Dr. Fiore and Dr. Baker made these remarks in an editorial accompanying Dr. Donny’s report (N Engl J Med. 2015 Oct 1; 373[14]:1289-91).
The findings of Dr. Donny and his colleagues justify exploration of a national nicotine-reduction policy and should encourage clinicians in practice to consider reduced-nicotine cigarettes as a potential resource for patients who want to quit smoking.
Given the number of current smokers in the United States, we can expect at least 20 million Americans to die prematurely if they continue to smoke. Reducing the nicotine content of cigarettes so that they are less addictive appears to be the most-promising regulatory policy option for preventing those 20 million premature deaths.
Dr. Michael Fiore and Timothy Baker, Ph.D., are at the Center for Tobacco Research and Intervention and the department of medicine at the University of Wisconsin, Madison. They reported having no relevant financial disclosures. Dr. Fiore and Dr. Baker made these remarks in an editorial accompanying Dr. Donny’s report (N Engl J Med. 2015 Oct 1; 373[14]:1289-91).
The findings of Dr. Donny and his colleagues justify exploration of a national nicotine-reduction policy and should encourage clinicians in practice to consider reduced-nicotine cigarettes as a potential resource for patients who want to quit smoking.
Given the number of current smokers in the United States, we can expect at least 20 million Americans to die prematurely if they continue to smoke. Reducing the nicotine content of cigarettes so that they are less addictive appears to be the most-promising regulatory policy option for preventing those 20 million premature deaths.
Dr. Michael Fiore and Timothy Baker, Ph.D., are at the Center for Tobacco Research and Intervention and the department of medicine at the University of Wisconsin, Madison. They reported having no relevant financial disclosures. Dr. Fiore and Dr. Baker made these remarks in an editorial accompanying Dr. Donny’s report (N Engl J Med. 2015 Oct 1; 373[14]:1289-91).
Reduced-nicotine cigarettes decreased tobacco dependence and the number of cigarettes smoked, with very little evidence of withdrawal or compensatory smoking, in a preliminary study reported online Oct. 1 in the New England Journal of Medicine.
Moreover, study participants who smoked very-low-nicotine cigarettes for the 6-week study were twice as likely to report that they attempted to quit 1 month later, compared with participants who smoked their usual brand or control cigarettes that had the usual nicotine content.
Reduced-nicotine cigarettes differ from “light” cigarettes in that the latter don’t actually reduce the nicotine content of the tobacco but instead increase ventilation of the cigarette – a strategy that is often circumvented by smokers who cover the ventilation holes or increase the number of cigarettes they smoke, said Eric C. Donny, Ph.D., of the department of psychology, University of Pittsburgh, and his associates.
The U.S. Food and Drug Administration recently was granted the authority to reduce, but not eliminate, nicotine in cigarettes if such action were deemed likely to benefit public health. However, no large-scale clinical trials have yet been performed to assess the potential benefit to public health.
Dr. Donny and his associates, supported by the National Institute on Drug Abuse and the FDA Center for Tobacco Products, conducted a double-blind, randomized trial at 10 sites comparing cigarettes with five levels of nicotine content among 839 adult smokers who were not planning to quit in the near future.
The study participants were assigned to smoke their usual brand of cigarettes (118 study subjects); control cigarettes containing the usual 15.8 mg of nicotine/g of tobacco (119 subjects); or experimental reduced-nicotine cigarettes containing 5.2 mg/g of nicotine (122 subjects), 2.4 mg/g (119 subjects), 1.3 mg/g (119 subjects), or 0.4 mg/g (242 subjects).
All the cigarettes were provided free of charge, and the smokers were paid for participating in the study. The dropout rate was only 8% at week 6 and did not differ significantly among the study groups.
The primary outcome – the average number of cigarettes smoked per day during week 6 – was markedly higher with the usual-brand group (22.2) and the control-cigarette group (21.3) than it was with the three lowest-nicotine groups (16.5, 16.3, and 14.9, respectively). That represents a reduction of 23%-30% in the number of cigarettes smoked in the latter three groups.
Tobacco dependence, as measured by the Wisconsin Inventory of Smoking Dependence Motives and the Fagerstrom Test for Nicotine Dependence, also was markedly lower with reduced-nicotine cigarettes.
Withdrawal symptoms did not increase; and during a brief voluntary abstinence period, smokers in the three lowest-nicotine groups actually reported fewer cravings than did those in the higher-nicotine groups, the investigators said (N Engl J Med. 2015 Oct 1;373[14]:1340-9).
At follow-up 30 days after completing the study, 34.7% of the participants who had smoked cigarettes with 0.4 mg/g of nicotine reported attempting to quit smoking, compared with 17% of those who had smoked cigarettes with 15.8 mg/g. In addition, participants who had smoked cigarettes with 1.3 mg/g or 0.4 mg/g of nicotine were still smoking significantly fewer cigarettes per day, even though the study had ended.
“In summary, these data suggest that if nicotine content is adequately reduced, smokers may benefit by smoking fewer cigarettes and experiencing less nicotine dependence, with few negative consequences,” Dr. Donny and his associates wrote. “If confirmed in longer-term studies, these findings suggest that, when combined with other tobacco-control policies (e.g., taxation and expanded access to treatment), limiting the nicotine content of cigarettes ... could improve public health.”
The study authors added that a longer clinical trial is now underway to further assess reduced-nicotine cigarettes.
NIDA and the FDA Center for Tobacco Products supported the study. Dr. Donny reported having no relevant financial disclosures. Two of his associates reported ties to Pfizer, and two reported serving as expert witnesses regarding addiction litigation against tobacco companies.
Reduced-nicotine cigarettes decreased tobacco dependence and the number of cigarettes smoked, with very little evidence of withdrawal or compensatory smoking, in a preliminary study reported online Oct. 1 in the New England Journal of Medicine.
Moreover, study participants who smoked very-low-nicotine cigarettes for the 6-week study were twice as likely to report that they attempted to quit 1 month later, compared with participants who smoked their usual brand or control cigarettes that had the usual nicotine content.
Reduced-nicotine cigarettes differ from “light” cigarettes in that the latter don’t actually reduce the nicotine content of the tobacco but instead increase ventilation of the cigarette – a strategy that is often circumvented by smokers who cover the ventilation holes or increase the number of cigarettes they smoke, said Eric C. Donny, Ph.D., of the department of psychology, University of Pittsburgh, and his associates.
The U.S. Food and Drug Administration recently was granted the authority to reduce, but not eliminate, nicotine in cigarettes if such action were deemed likely to benefit public health. However, no large-scale clinical trials have yet been performed to assess the potential benefit to public health.
Dr. Donny and his associates, supported by the National Institute on Drug Abuse and the FDA Center for Tobacco Products, conducted a double-blind, randomized trial at 10 sites comparing cigarettes with five levels of nicotine content among 839 adult smokers who were not planning to quit in the near future.
The study participants were assigned to smoke their usual brand of cigarettes (118 study subjects); control cigarettes containing the usual 15.8 mg of nicotine/g of tobacco (119 subjects); or experimental reduced-nicotine cigarettes containing 5.2 mg/g of nicotine (122 subjects), 2.4 mg/g (119 subjects), 1.3 mg/g (119 subjects), or 0.4 mg/g (242 subjects).
All the cigarettes were provided free of charge, and the smokers were paid for participating in the study. The dropout rate was only 8% at week 6 and did not differ significantly among the study groups.
The primary outcome – the average number of cigarettes smoked per day during week 6 – was markedly higher with the usual-brand group (22.2) and the control-cigarette group (21.3) than it was with the three lowest-nicotine groups (16.5, 16.3, and 14.9, respectively). That represents a reduction of 23%-30% in the number of cigarettes smoked in the latter three groups.
Tobacco dependence, as measured by the Wisconsin Inventory of Smoking Dependence Motives and the Fagerstrom Test for Nicotine Dependence, also was markedly lower with reduced-nicotine cigarettes.
Withdrawal symptoms did not increase; and during a brief voluntary abstinence period, smokers in the three lowest-nicotine groups actually reported fewer cravings than did those in the higher-nicotine groups, the investigators said (N Engl J Med. 2015 Oct 1;373[14]:1340-9).
At follow-up 30 days after completing the study, 34.7% of the participants who had smoked cigarettes with 0.4 mg/g of nicotine reported attempting to quit smoking, compared with 17% of those who had smoked cigarettes with 15.8 mg/g. In addition, participants who had smoked cigarettes with 1.3 mg/g or 0.4 mg/g of nicotine were still smoking significantly fewer cigarettes per day, even though the study had ended.
“In summary, these data suggest that if nicotine content is adequately reduced, smokers may benefit by smoking fewer cigarettes and experiencing less nicotine dependence, with few negative consequences,” Dr. Donny and his associates wrote. “If confirmed in longer-term studies, these findings suggest that, when combined with other tobacco-control policies (e.g., taxation and expanded access to treatment), limiting the nicotine content of cigarettes ... could improve public health.”
The study authors added that a longer clinical trial is now underway to further assess reduced-nicotine cigarettes.
NIDA and the FDA Center for Tobacco Products supported the study. Dr. Donny reported having no relevant financial disclosures. Two of his associates reported ties to Pfizer, and two reported serving as expert witnesses regarding addiction litigation against tobacco companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Reduced-nicotine cigarettes decreased dependence and the amount smoked, without producing withdrawal symptoms.
Major finding: The primary outcome – the average number of cigarettes smoked per day during week 6 – was markedly higher with the usual-brand group (22.2) and the control-cigarette group (21.3) than with the three lowest-nicotine groups (16.5, 16.3, and 14.9, respectively).
Data source: A preliminary 6-week, randomized, double-blind clinical trial with 839 smokers comparing cigarettes with five levels of nicotine content.
Disclosures: NIDA and the FDA Center for Tobacco Products supported the study. Dr. Donny reported having no relevant financial disclosures. Two of his associates reported ties to Pfizer, and two reported serving as expert witnesses regarding addiction litigation against tobacco companies.
Secondhand smoke increases hospitalization risk in asthmatic children
Children with asthma who are exposed to secondhand smoke (SHS) are at greater risk of being hospitalized because of asthma exacerbation, according to a systematic review by Zhen Wang, Ph.D., and associates.
The review identified 25 relevant studies, which found that asthmatic children exposed to SHS were nearly twice as likely to be hospitalized because of their asthma as were children who were not exposed, with an odds ratio of 1.85. The OR for visits to the emergency department or urgent care was also significantly higher in children exposed to SHS at 1.66.
Pulmonary function in children with asthma exposed to SHS was worse than in nonexposed children. The OR for wheeze symptoms was 1.32, and the forced expiratory volume in 1 second/forced vital capacity ratio was much lower, with an OR of –3.34.
“Assessment of SHS (subjective and objective measurements) should be an integral part of asthma care in children. This will help address and eliminate modifiable risk factors and improve the overall health of children with asthma,” the investigators concluded.
Find the study in Annals of Allergy, Asthma, and Immunology (doi: 10.1016/j.anai.2015.08.005).
Children with asthma who are exposed to secondhand smoke (SHS) are at greater risk of being hospitalized because of asthma exacerbation, according to a systematic review by Zhen Wang, Ph.D., and associates.
The review identified 25 relevant studies, which found that asthmatic children exposed to SHS were nearly twice as likely to be hospitalized because of their asthma as were children who were not exposed, with an odds ratio of 1.85. The OR for visits to the emergency department or urgent care was also significantly higher in children exposed to SHS at 1.66.
Pulmonary function in children with asthma exposed to SHS was worse than in nonexposed children. The OR for wheeze symptoms was 1.32, and the forced expiratory volume in 1 second/forced vital capacity ratio was much lower, with an OR of –3.34.
“Assessment of SHS (subjective and objective measurements) should be an integral part of asthma care in children. This will help address and eliminate modifiable risk factors and improve the overall health of children with asthma,” the investigators concluded.
Find the study in Annals of Allergy, Asthma, and Immunology (doi: 10.1016/j.anai.2015.08.005).
Children with asthma who are exposed to secondhand smoke (SHS) are at greater risk of being hospitalized because of asthma exacerbation, according to a systematic review by Zhen Wang, Ph.D., and associates.
The review identified 25 relevant studies, which found that asthmatic children exposed to SHS were nearly twice as likely to be hospitalized because of their asthma as were children who were not exposed, with an odds ratio of 1.85. The OR for visits to the emergency department or urgent care was also significantly higher in children exposed to SHS at 1.66.
Pulmonary function in children with asthma exposed to SHS was worse than in nonexposed children. The OR for wheeze symptoms was 1.32, and the forced expiratory volume in 1 second/forced vital capacity ratio was much lower, with an OR of –3.34.
“Assessment of SHS (subjective and objective measurements) should be an integral part of asthma care in children. This will help address and eliminate modifiable risk factors and improve the overall health of children with asthma,” the investigators concluded.
Find the study in Annals of Allergy, Asthma, and Immunology (doi: 10.1016/j.anai.2015.08.005).
HHS funds experimental flu drug for hospital patients
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) will provide “technical assistance and funding” to Janssen Pharmaceuticals for the development of an influenza antiviral drug that could be more potent and have a longer treatment window than do existing influenza drugs, according a statement from HHS.
The experimental drug, which Janssen intends to use to treat hospitalized patients with influenza, is called JNJ-872, also known as VX787, and may be the first in an entirely new class of influenza antivirals.
ASPR’s Biomedical Advanced Research and Development Authority (BARDA) specifically will provide up to $103.5 million over the next 4 years and 3 months to Janssen, and the contract leaves open the possibility of BARDA providing additional funding, capped at $131 million, for this project. BARDA’s contribution is part of its program for “advanced research and development, innovation, acquisition, and manufacturing of vaccines, drugs, diagnostic tools, and nonpharmaceutical products for public health emergency threats.”
Janssen will use the funds to conduct late stage clinical development of JNJ-872, which includes phase III studies in high-risk populations and hospitalized patients, and the final development of a validated commercial-scale manufacturing process. The pharmaceutical company will also explore the feasibility of an innovative approach to manufacturing, which allows for the continuous flow of materials throughout the manufacturing process, unlike the traditional manufacturing process, which is riddled with interruptions.
“BARDA’s goal for supporting JNJ-872 is development of a product that can be used not only to treat hospitalized influenza patients but also to treat patients for whom influenza poses high risks, such as the elderly, pediatrics, and those with chronic conditions such as COPD and heart disease ...,” the statement indicated.
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) will provide “technical assistance and funding” to Janssen Pharmaceuticals for the development of an influenza antiviral drug that could be more potent and have a longer treatment window than do existing influenza drugs, according a statement from HHS.
The experimental drug, which Janssen intends to use to treat hospitalized patients with influenza, is called JNJ-872, also known as VX787, and may be the first in an entirely new class of influenza antivirals.
ASPR’s Biomedical Advanced Research and Development Authority (BARDA) specifically will provide up to $103.5 million over the next 4 years and 3 months to Janssen, and the contract leaves open the possibility of BARDA providing additional funding, capped at $131 million, for this project. BARDA’s contribution is part of its program for “advanced research and development, innovation, acquisition, and manufacturing of vaccines, drugs, diagnostic tools, and nonpharmaceutical products for public health emergency threats.”
Janssen will use the funds to conduct late stage clinical development of JNJ-872, which includes phase III studies in high-risk populations and hospitalized patients, and the final development of a validated commercial-scale manufacturing process. The pharmaceutical company will also explore the feasibility of an innovative approach to manufacturing, which allows for the continuous flow of materials throughout the manufacturing process, unlike the traditional manufacturing process, which is riddled with interruptions.
“BARDA’s goal for supporting JNJ-872 is development of a product that can be used not only to treat hospitalized influenza patients but also to treat patients for whom influenza poses high risks, such as the elderly, pediatrics, and those with chronic conditions such as COPD and heart disease ...,” the statement indicated.
The U.S. Department of Health & Human Services’ Office of the Assistant Secretary for Preparedness and Response (ASPR) will provide “technical assistance and funding” to Janssen Pharmaceuticals for the development of an influenza antiviral drug that could be more potent and have a longer treatment window than do existing influenza drugs, according a statement from HHS.
The experimental drug, which Janssen intends to use to treat hospitalized patients with influenza, is called JNJ-872, also known as VX787, and may be the first in an entirely new class of influenza antivirals.
ASPR’s Biomedical Advanced Research and Development Authority (BARDA) specifically will provide up to $103.5 million over the next 4 years and 3 months to Janssen, and the contract leaves open the possibility of BARDA providing additional funding, capped at $131 million, for this project. BARDA’s contribution is part of its program for “advanced research and development, innovation, acquisition, and manufacturing of vaccines, drugs, diagnostic tools, and nonpharmaceutical products for public health emergency threats.”
Janssen will use the funds to conduct late stage clinical development of JNJ-872, which includes phase III studies in high-risk populations and hospitalized patients, and the final development of a validated commercial-scale manufacturing process. The pharmaceutical company will also explore the feasibility of an innovative approach to manufacturing, which allows for the continuous flow of materials throughout the manufacturing process, unlike the traditional manufacturing process, which is riddled with interruptions.
“BARDA’s goal for supporting JNJ-872 is development of a product that can be used not only to treat hospitalized influenza patients but also to treat patients for whom influenza poses high risks, such as the elderly, pediatrics, and those with chronic conditions such as COPD and heart disease ...,” the statement indicated.
Insomnia not always a clear-cut diagnosis
SAN DIEGO – As if having insomnia or sleep-disordered breathing isn’t challenging enough, some patients turn out to have “complex insomnia” – a combination of moderate/severe insomnia disorders and specific symptoms of sleep-disordered breathing.
“This overlap of insomnia and obstructive sleep apnea is interesting,” Dr. David N. Neubauer said at the annual U.S. Psychiatric and Mental Health Congress.
According to a study of 810 primary care outpatients with no sleep disorder history who presented for nonsleep-related complaints, 82% had at least one insomnia symptom, 36% met criteria for insomnia disorder, 60% had at least one symptom of sleep-disordered breathing, 51% had at least one insomnia symptom and one symptom of sleep-disordered breathing, while 11% had potential complex insomnia: a mix of symptoms meeting criteria for moderate/severe insomnia disorder and specific sleep-disordered breathing (Sleep Med. 2013;14[9]:814-23). Several other studies have demonstrated a strong prevalence of insomnia in obstructive sleep apnea (OSA) patients, “which is not that surprising,” said Dr. Neubauer, associate director of the Johns Hopkins Sleep Disorders Center, Baltimore. However, at least three sleep studies conducted in older patients with insomnia found a high prevalence of OSA. The largest study, of 394 postmenopausal women aged 55-70 years, found that 67% had an apnea-hypopnea index (AHI) of greater than 5.*
In a separate trial, veterans in the Los Angeles area who were at least 60 years old and had seen a Veterans Affairs outpatient provider in the past 2 years were recruited for an insomnia behavioral intervention trial (J Clin Sleep Med. 2013; 9[11]:1173-8). To be eligible for the trial, participants must have had a sleep disturbance with daytime consequences for at least 3 months; those with a history of sleep apnea diagnosis or treatment were excluded. Interventions included questionnaires, a phone interview, and in-home testing with the WatchPAT system, a portable device from Itamar Medical that can help diagnose sleep apnea. The mean age of the 435 community-dwelling participants was 72 years, and their mean body mass index was 28 kg/m2. The researchers found that the prevalence of OSA – defined as an AHI threshold of 15 or greater – was 47%.
In another study from Stanford (Calif.) University, researchers set out to evaluate the impact of a cognitive-behavioral intervention in people with insomnia and major depression (J Psychosom Res. 2009;67[2]:135-41). The screening consisted of a phone interview, in-person screening, and an overnight polysomnography test. The mean age of the 51 people who completed the screening was 48 years, and 57% were female. The researchers found that 69% of patients had an AHI of 5 or greater. Of those, 29% had an AHI between 5 and 15, 24% had an AHI between 15 and 25, while 16% had an AHI of greater than 25. “It must have been frustrating for these researchers to get a ‘clean’ insomnia population, because so many ended up having sleep apnea as part of their underlying problem,” Dr. Neubauer said.
Clinicians might think that the worse the OSA, the worse the insomnia, “but that’s not necessarily the case, because a lot of people with severe OSA are just really sleepy, and they’re sleeping through the next day,” Dr. Neubauer said. Patients with a combination of OSA and insomnia symptoms “tend to be some of the people with milder sleep apnea, or those who are under the radar, who wouldn’t even get diagnosed with OSA, but they have that same physiologic process of some inspiratory flow limitation.” This subset of patients might meet criteria for upper airway resistance syndrome, which was first described in 1993 and is characterized by repetitive increases in resistance to airflow, increased respiratory effort, absence of oxygen desaturation, brief sleep state changes or arousals, and daytime somnolence. “In the sleep community, the diagnosis of upper airway resistance syndrome is somewhat debatable, because some people think that if you don’t have absolute apnea events, they don’t count [as a sleep disorder],” Dr. Neubauer said. “But there are a lot of people who feel that these ‘under the radar’ events may still have a significant effect on sleep.” Compared with OSA patients, those with upper airway resistance syndrome tend to be younger, female, and have a lower body mass index (Respiration 2012;83[6]:559-66). In addition, he said, sleep-onset insomnia is common, and the condition is associated with functional somatic syndromes, such as headache, irritable bowel syndrome, gastroesophageal reflux, rhinitis, and orthostatic intolerance.
A recent analysis of 14 second-generation antidepressants based on Food and Drug Administration data and pharmaceutical company records found that the Top 5 most likely to cause insomnia, compared with placebo, are bupropion, desvenlafaxine, sertraline, fluvoxamine, and fluoxetine (J Clin Psychopharmacol. 2015;35[3]:296-303). The Top 5 most likely to cause somnolence, compared with placebo, are fluvoxamine, mirtazapine, reboxetine, paroxetine, and desvenlafaxine.
According to National Health and Nutrition Examination Survey data from more than 32,000 community-dwelling adults in the United States, 3% of adults took a medication commonly used for insomnia in the previous month – most often zolpidem and trazodone – and use increased between 1999 and 2010 (Sleep 2014;37[2]:343-9). More than half of NHANES participants taking a medication for insomnia (55%) reported taking at least one other sedating medicine concurrently, and 10% reported taking three or more sedating medicines. In addition, 25% reported taking opioids concomitantly, while 20% reported taking benzodiazepines not intended for insomnia. “Concurrent use with medications commonly used for insomnia is high,” Dr. Neubauer said.
He reported having no financial disclosures.
*An earlier version of this story misstated the apnea-hypopnea index (AHI) of 67% of participants in a study.
SAN DIEGO – As if having insomnia or sleep-disordered breathing isn’t challenging enough, some patients turn out to have “complex insomnia” – a combination of moderate/severe insomnia disorders and specific symptoms of sleep-disordered breathing.
“This overlap of insomnia and obstructive sleep apnea is interesting,” Dr. David N. Neubauer said at the annual U.S. Psychiatric and Mental Health Congress.
According to a study of 810 primary care outpatients with no sleep disorder history who presented for nonsleep-related complaints, 82% had at least one insomnia symptom, 36% met criteria for insomnia disorder, 60% had at least one symptom of sleep-disordered breathing, 51% had at least one insomnia symptom and one symptom of sleep-disordered breathing, while 11% had potential complex insomnia: a mix of symptoms meeting criteria for moderate/severe insomnia disorder and specific sleep-disordered breathing (Sleep Med. 2013;14[9]:814-23). Several other studies have demonstrated a strong prevalence of insomnia in obstructive sleep apnea (OSA) patients, “which is not that surprising,” said Dr. Neubauer, associate director of the Johns Hopkins Sleep Disorders Center, Baltimore. However, at least three sleep studies conducted in older patients with insomnia found a high prevalence of OSA. The largest study, of 394 postmenopausal women aged 55-70 years, found that 67% had an apnea-hypopnea index (AHI) of greater than 5.*
In a separate trial, veterans in the Los Angeles area who were at least 60 years old and had seen a Veterans Affairs outpatient provider in the past 2 years were recruited for an insomnia behavioral intervention trial (J Clin Sleep Med. 2013; 9[11]:1173-8). To be eligible for the trial, participants must have had a sleep disturbance with daytime consequences for at least 3 months; those with a history of sleep apnea diagnosis or treatment were excluded. Interventions included questionnaires, a phone interview, and in-home testing with the WatchPAT system, a portable device from Itamar Medical that can help diagnose sleep apnea. The mean age of the 435 community-dwelling participants was 72 years, and their mean body mass index was 28 kg/m2. The researchers found that the prevalence of OSA – defined as an AHI threshold of 15 or greater – was 47%.
In another study from Stanford (Calif.) University, researchers set out to evaluate the impact of a cognitive-behavioral intervention in people with insomnia and major depression (J Psychosom Res. 2009;67[2]:135-41). The screening consisted of a phone interview, in-person screening, and an overnight polysomnography test. The mean age of the 51 people who completed the screening was 48 years, and 57% were female. The researchers found that 69% of patients had an AHI of 5 or greater. Of those, 29% had an AHI between 5 and 15, 24% had an AHI between 15 and 25, while 16% had an AHI of greater than 25. “It must have been frustrating for these researchers to get a ‘clean’ insomnia population, because so many ended up having sleep apnea as part of their underlying problem,” Dr. Neubauer said.
Clinicians might think that the worse the OSA, the worse the insomnia, “but that’s not necessarily the case, because a lot of people with severe OSA are just really sleepy, and they’re sleeping through the next day,” Dr. Neubauer said. Patients with a combination of OSA and insomnia symptoms “tend to be some of the people with milder sleep apnea, or those who are under the radar, who wouldn’t even get diagnosed with OSA, but they have that same physiologic process of some inspiratory flow limitation.” This subset of patients might meet criteria for upper airway resistance syndrome, which was first described in 1993 and is characterized by repetitive increases in resistance to airflow, increased respiratory effort, absence of oxygen desaturation, brief sleep state changes or arousals, and daytime somnolence. “In the sleep community, the diagnosis of upper airway resistance syndrome is somewhat debatable, because some people think that if you don’t have absolute apnea events, they don’t count [as a sleep disorder],” Dr. Neubauer said. “But there are a lot of people who feel that these ‘under the radar’ events may still have a significant effect on sleep.” Compared with OSA patients, those with upper airway resistance syndrome tend to be younger, female, and have a lower body mass index (Respiration 2012;83[6]:559-66). In addition, he said, sleep-onset insomnia is common, and the condition is associated with functional somatic syndromes, such as headache, irritable bowel syndrome, gastroesophageal reflux, rhinitis, and orthostatic intolerance.
A recent analysis of 14 second-generation antidepressants based on Food and Drug Administration data and pharmaceutical company records found that the Top 5 most likely to cause insomnia, compared with placebo, are bupropion, desvenlafaxine, sertraline, fluvoxamine, and fluoxetine (J Clin Psychopharmacol. 2015;35[3]:296-303). The Top 5 most likely to cause somnolence, compared with placebo, are fluvoxamine, mirtazapine, reboxetine, paroxetine, and desvenlafaxine.
According to National Health and Nutrition Examination Survey data from more than 32,000 community-dwelling adults in the United States, 3% of adults took a medication commonly used for insomnia in the previous month – most often zolpidem and trazodone – and use increased between 1999 and 2010 (Sleep 2014;37[2]:343-9). More than half of NHANES participants taking a medication for insomnia (55%) reported taking at least one other sedating medicine concurrently, and 10% reported taking three or more sedating medicines. In addition, 25% reported taking opioids concomitantly, while 20% reported taking benzodiazepines not intended for insomnia. “Concurrent use with medications commonly used for insomnia is high,” Dr. Neubauer said.
He reported having no financial disclosures.
*An earlier version of this story misstated the apnea-hypopnea index (AHI) of 67% of participants in a study.
SAN DIEGO – As if having insomnia or sleep-disordered breathing isn’t challenging enough, some patients turn out to have “complex insomnia” – a combination of moderate/severe insomnia disorders and specific symptoms of sleep-disordered breathing.
“This overlap of insomnia and obstructive sleep apnea is interesting,” Dr. David N. Neubauer said at the annual U.S. Psychiatric and Mental Health Congress.
According to a study of 810 primary care outpatients with no sleep disorder history who presented for nonsleep-related complaints, 82% had at least one insomnia symptom, 36% met criteria for insomnia disorder, 60% had at least one symptom of sleep-disordered breathing, 51% had at least one insomnia symptom and one symptom of sleep-disordered breathing, while 11% had potential complex insomnia: a mix of symptoms meeting criteria for moderate/severe insomnia disorder and specific sleep-disordered breathing (Sleep Med. 2013;14[9]:814-23). Several other studies have demonstrated a strong prevalence of insomnia in obstructive sleep apnea (OSA) patients, “which is not that surprising,” said Dr. Neubauer, associate director of the Johns Hopkins Sleep Disorders Center, Baltimore. However, at least three sleep studies conducted in older patients with insomnia found a high prevalence of OSA. The largest study, of 394 postmenopausal women aged 55-70 years, found that 67% had an apnea-hypopnea index (AHI) of greater than 5.*
In a separate trial, veterans in the Los Angeles area who were at least 60 years old and had seen a Veterans Affairs outpatient provider in the past 2 years were recruited for an insomnia behavioral intervention trial (J Clin Sleep Med. 2013; 9[11]:1173-8). To be eligible for the trial, participants must have had a sleep disturbance with daytime consequences for at least 3 months; those with a history of sleep apnea diagnosis or treatment were excluded. Interventions included questionnaires, a phone interview, and in-home testing with the WatchPAT system, a portable device from Itamar Medical that can help diagnose sleep apnea. The mean age of the 435 community-dwelling participants was 72 years, and their mean body mass index was 28 kg/m2. The researchers found that the prevalence of OSA – defined as an AHI threshold of 15 or greater – was 47%.
In another study from Stanford (Calif.) University, researchers set out to evaluate the impact of a cognitive-behavioral intervention in people with insomnia and major depression (J Psychosom Res. 2009;67[2]:135-41). The screening consisted of a phone interview, in-person screening, and an overnight polysomnography test. The mean age of the 51 people who completed the screening was 48 years, and 57% were female. The researchers found that 69% of patients had an AHI of 5 or greater. Of those, 29% had an AHI between 5 and 15, 24% had an AHI between 15 and 25, while 16% had an AHI of greater than 25. “It must have been frustrating for these researchers to get a ‘clean’ insomnia population, because so many ended up having sleep apnea as part of their underlying problem,” Dr. Neubauer said.
Clinicians might think that the worse the OSA, the worse the insomnia, “but that’s not necessarily the case, because a lot of people with severe OSA are just really sleepy, and they’re sleeping through the next day,” Dr. Neubauer said. Patients with a combination of OSA and insomnia symptoms “tend to be some of the people with milder sleep apnea, or those who are under the radar, who wouldn’t even get diagnosed with OSA, but they have that same physiologic process of some inspiratory flow limitation.” This subset of patients might meet criteria for upper airway resistance syndrome, which was first described in 1993 and is characterized by repetitive increases in resistance to airflow, increased respiratory effort, absence of oxygen desaturation, brief sleep state changes or arousals, and daytime somnolence. “In the sleep community, the diagnosis of upper airway resistance syndrome is somewhat debatable, because some people think that if you don’t have absolute apnea events, they don’t count [as a sleep disorder],” Dr. Neubauer said. “But there are a lot of people who feel that these ‘under the radar’ events may still have a significant effect on sleep.” Compared with OSA patients, those with upper airway resistance syndrome tend to be younger, female, and have a lower body mass index (Respiration 2012;83[6]:559-66). In addition, he said, sleep-onset insomnia is common, and the condition is associated with functional somatic syndromes, such as headache, irritable bowel syndrome, gastroesophageal reflux, rhinitis, and orthostatic intolerance.
A recent analysis of 14 second-generation antidepressants based on Food and Drug Administration data and pharmaceutical company records found that the Top 5 most likely to cause insomnia, compared with placebo, are bupropion, desvenlafaxine, sertraline, fluvoxamine, and fluoxetine (J Clin Psychopharmacol. 2015;35[3]:296-303). The Top 5 most likely to cause somnolence, compared with placebo, are fluvoxamine, mirtazapine, reboxetine, paroxetine, and desvenlafaxine.
According to National Health and Nutrition Examination Survey data from more than 32,000 community-dwelling adults in the United States, 3% of adults took a medication commonly used for insomnia in the previous month – most often zolpidem and trazodone – and use increased between 1999 and 2010 (Sleep 2014;37[2]:343-9). More than half of NHANES participants taking a medication for insomnia (55%) reported taking at least one other sedating medicine concurrently, and 10% reported taking three or more sedating medicines. In addition, 25% reported taking opioids concomitantly, while 20% reported taking benzodiazepines not intended for insomnia. “Concurrent use with medications commonly used for insomnia is high,” Dr. Neubauer said.
He reported having no financial disclosures.
*An earlier version of this story misstated the apnea-hypopnea index (AHI) of 67% of participants in a study.
EXPERT ANALYSIS AT THE 2015 PSYCH CONGRESS
HHS funds development of experimental Ebola drug
The U.S. Department of Health & Human Services’ Office of Assistant Secretary for Preparedness and Response (ASPR) and Regeneron Pharmaceuticals have entered into an agreement for the purpose of advancing the development of a new experimental drug for treating the Ebola virus disease, according to a statement from the department.
The agreement represents the newest contribution of ASPR’s Biomedical Advance Research and Development Authority (BARDA) toward fighting the Ebola epidemic in West Africa. BARDA has promised to pay up to $38 million for the drug’s development and manufacturing, including the filing of an investigational new drug application with the Food and Drug Administration. All of the drug produced will be used in studies. “BARDA also could provide an additional $11.3 million to manufacture alternative monoclonal antibodies,” the statement indicates.
The experimental drug is based on three fully human monoclonal antibodies, which bind to a key Ebola viral protein and neutralize the virus. This decreases the amount of virus in the body that a patient’s immune system has to fight.
The drug company’s “technology facilitated the discovery and development of this monoclonal antibody therapeutic candidate in real time in just nine months as compared to the normal development cycle of several years, and the technology may have potential applications in future public health responses,” said BARDA Director Robin Robinson, Ph.D., in the statement.
The U.S. Department of Health & Human Services’ Office of Assistant Secretary for Preparedness and Response (ASPR) and Regeneron Pharmaceuticals have entered into an agreement for the purpose of advancing the development of a new experimental drug for treating the Ebola virus disease, according to a statement from the department.
The agreement represents the newest contribution of ASPR’s Biomedical Advance Research and Development Authority (BARDA) toward fighting the Ebola epidemic in West Africa. BARDA has promised to pay up to $38 million for the drug’s development and manufacturing, including the filing of an investigational new drug application with the Food and Drug Administration. All of the drug produced will be used in studies. “BARDA also could provide an additional $11.3 million to manufacture alternative monoclonal antibodies,” the statement indicates.
The experimental drug is based on three fully human monoclonal antibodies, which bind to a key Ebola viral protein and neutralize the virus. This decreases the amount of virus in the body that a patient’s immune system has to fight.
The drug company’s “technology facilitated the discovery and development of this monoclonal antibody therapeutic candidate in real time in just nine months as compared to the normal development cycle of several years, and the technology may have potential applications in future public health responses,” said BARDA Director Robin Robinson, Ph.D., in the statement.
The U.S. Department of Health & Human Services’ Office of Assistant Secretary for Preparedness and Response (ASPR) and Regeneron Pharmaceuticals have entered into an agreement for the purpose of advancing the development of a new experimental drug for treating the Ebola virus disease, according to a statement from the department.
The agreement represents the newest contribution of ASPR’s Biomedical Advance Research and Development Authority (BARDA) toward fighting the Ebola epidemic in West Africa. BARDA has promised to pay up to $38 million for the drug’s development and manufacturing, including the filing of an investigational new drug application with the Food and Drug Administration. All of the drug produced will be used in studies. “BARDA also could provide an additional $11.3 million to manufacture alternative monoclonal antibodies,” the statement indicates.
The experimental drug is based on three fully human monoclonal antibodies, which bind to a key Ebola viral protein and neutralize the virus. This decreases the amount of virus in the body that a patient’s immune system has to fight.
The drug company’s “technology facilitated the discovery and development of this monoclonal antibody therapeutic candidate in real time in just nine months as compared to the normal development cycle of several years, and the technology may have potential applications in future public health responses,” said BARDA Director Robin Robinson, Ph.D., in the statement.
ICAAC: Synergistic effects of two pediatric vaccines highlighted
SAN DIEGO – The potent synergistic benefits of coadministration of rotavirus vaccine and pneumococcal conjugate vaccine in young children are uniquely highlighted by a natural experiment conducted in southern Israel as described by Dr. Ron Dagan at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Dagan is professor of pediatrics and infectious diseases at Soroka University Medical Center in Beersheba, Israel. It’s the sole hospital in a large, well-defined area of southern Israel. All children in that part of Israel are born in the hospital and receive their health care there, making it possible to generate highly reliable disease incidence data.
At any given time, physicians at the hospital are responsible for the care of roughly 30,000 children less than 2 years of age. So there’s a huge study population, comprehensive follow-up, and – the final element in this prospective, population-based study – the rotavirus and pneumococcal conjugate vaccines were introduced in Israel just a few years ago and at roughly the same time. This enabled Dr. Dagan and his coinvestigators to compare hospitalization rates and pediatric emergency department outpatient visits for diarrheal and lower respiratory viral illnesses among children less than 2 years old during the prevaccine period of April 2006-March 2009 with rates during April 2013-March 2014, when uptake of the two vaccines in that age group exceeded 90%.
This was an unusual study in that it looked at the global impact of two major vaccines. In contrast, vaccine clinical trials and postmarketing studies typically evaluate only those outcomes directly related to that particular vaccine.
The results of the Israeli study were startling: in the 3 years prior to introduction of the vaccines, one in five children under age 2 years admitted to the hospital had as an admitting diagnosis either rotavirus gastroenteritis confirmed by a positive stool ELISA test or pneumococcal pneumonia as evidenced by alveolar pneumonia on chest x-ray. After the vaccines became available, the hospitalization rates for rotavirus gastroenteritis and alveolar pneumonia plummeted by 78% and 46%, respectively. Moreover, outpatient pediatric emergency department visits for rotavirus gastroenteritis dropped by 71% and visits for alveolar pneumonia fell by 67%.
But that’s not all. Smaller yet clinically meaningful reductions were also documented in nonrotavirus gastroenteritis and nonalveolar lower respiratory tract infections. Specifically, the hospitalization rate for nonrotavirus diarrheal illnesses and nonalveolar pneumonia lower respiratory infections dropped by 21% and 7%, respectively, while outpatient emergency visits for those disorders fell by 16% and 14%.
This translates to an estimated 1,890 fewer hospitalizations and 4,030 fewer outpatient emergency department visits for diarrheal disease or lower respiratory infection per 100,000 children under age 2 per year, Dr. Dagan reported.
Michael Schmidt, Ph.D., who chaired a press conference highlighting the Israeli study, declared, “These data are absolutely phenomenal. It really shows the global value of these vaccines for society.”
Dr. Schmidt, professor and vice chairman of microbiology and immunology at the Medical University of South Carolina, Charleston, posed a question: What’s the explanation for the reductions in diseases not directly addressed by those two vaccines?
“We believe that one success can favorably affect the other. If you are weakened by diarrhea, you may be more likely to get pneumonia, and vice versa,” according to Dr. Dagan.
He added that the results actually pack a significantly greater wallop than is apparent at first look because rotavirus gastroenteritis and pneumococcal pneumonia in young children are seasonal diseases. They occur chiefly during October-March. So those 5,920 fewer hospitalizations and outpatient visits/100,000 per young children per year are concentrated during pediatricians’ busiest half of the year.
“In most places in the world, winter is a time of so much illness that pediatricians can’t deliver appropriate care. We knew that in our hospital we couldn’t deliver appropriate care to children in the winter because there were so many sick children piled on top of each other. But now, because of these two vaccines, we are less crowded in the winter, we have more time for children, we make fewer mistakes,” he said.
The study was funded by vaccine manufacturers and the Israel Ministry of Health. Dr. Dagan reported serving as a consultant, adviser to, and/or recipient of research grants from GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, and Genocea.
SAN DIEGO – The potent synergistic benefits of coadministration of rotavirus vaccine and pneumococcal conjugate vaccine in young children are uniquely highlighted by a natural experiment conducted in southern Israel as described by Dr. Ron Dagan at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Dagan is professor of pediatrics and infectious diseases at Soroka University Medical Center in Beersheba, Israel. It’s the sole hospital in a large, well-defined area of southern Israel. All children in that part of Israel are born in the hospital and receive their health care there, making it possible to generate highly reliable disease incidence data.
At any given time, physicians at the hospital are responsible for the care of roughly 30,000 children less than 2 years of age. So there’s a huge study population, comprehensive follow-up, and – the final element in this prospective, population-based study – the rotavirus and pneumococcal conjugate vaccines were introduced in Israel just a few years ago and at roughly the same time. This enabled Dr. Dagan and his coinvestigators to compare hospitalization rates and pediatric emergency department outpatient visits for diarrheal and lower respiratory viral illnesses among children less than 2 years old during the prevaccine period of April 2006-March 2009 with rates during April 2013-March 2014, when uptake of the two vaccines in that age group exceeded 90%.
This was an unusual study in that it looked at the global impact of two major vaccines. In contrast, vaccine clinical trials and postmarketing studies typically evaluate only those outcomes directly related to that particular vaccine.
The results of the Israeli study were startling: in the 3 years prior to introduction of the vaccines, one in five children under age 2 years admitted to the hospital had as an admitting diagnosis either rotavirus gastroenteritis confirmed by a positive stool ELISA test or pneumococcal pneumonia as evidenced by alveolar pneumonia on chest x-ray. After the vaccines became available, the hospitalization rates for rotavirus gastroenteritis and alveolar pneumonia plummeted by 78% and 46%, respectively. Moreover, outpatient pediatric emergency department visits for rotavirus gastroenteritis dropped by 71% and visits for alveolar pneumonia fell by 67%.
But that’s not all. Smaller yet clinically meaningful reductions were also documented in nonrotavirus gastroenteritis and nonalveolar lower respiratory tract infections. Specifically, the hospitalization rate for nonrotavirus diarrheal illnesses and nonalveolar pneumonia lower respiratory infections dropped by 21% and 7%, respectively, while outpatient emergency visits for those disorders fell by 16% and 14%.
This translates to an estimated 1,890 fewer hospitalizations and 4,030 fewer outpatient emergency department visits for diarrheal disease or lower respiratory infection per 100,000 children under age 2 per year, Dr. Dagan reported.
Michael Schmidt, Ph.D., who chaired a press conference highlighting the Israeli study, declared, “These data are absolutely phenomenal. It really shows the global value of these vaccines for society.”
Dr. Schmidt, professor and vice chairman of microbiology and immunology at the Medical University of South Carolina, Charleston, posed a question: What’s the explanation for the reductions in diseases not directly addressed by those two vaccines?
“We believe that one success can favorably affect the other. If you are weakened by diarrhea, you may be more likely to get pneumonia, and vice versa,” according to Dr. Dagan.
He added that the results actually pack a significantly greater wallop than is apparent at first look because rotavirus gastroenteritis and pneumococcal pneumonia in young children are seasonal diseases. They occur chiefly during October-March. So those 5,920 fewer hospitalizations and outpatient visits/100,000 per young children per year are concentrated during pediatricians’ busiest half of the year.
“In most places in the world, winter is a time of so much illness that pediatricians can’t deliver appropriate care. We knew that in our hospital we couldn’t deliver appropriate care to children in the winter because there were so many sick children piled on top of each other. But now, because of these two vaccines, we are less crowded in the winter, we have more time for children, we make fewer mistakes,” he said.
The study was funded by vaccine manufacturers and the Israel Ministry of Health. Dr. Dagan reported serving as a consultant, adviser to, and/or recipient of research grants from GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, and Genocea.
SAN DIEGO – The potent synergistic benefits of coadministration of rotavirus vaccine and pneumococcal conjugate vaccine in young children are uniquely highlighted by a natural experiment conducted in southern Israel as described by Dr. Ron Dagan at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Dr. Dagan is professor of pediatrics and infectious diseases at Soroka University Medical Center in Beersheba, Israel. It’s the sole hospital in a large, well-defined area of southern Israel. All children in that part of Israel are born in the hospital and receive their health care there, making it possible to generate highly reliable disease incidence data.
At any given time, physicians at the hospital are responsible for the care of roughly 30,000 children less than 2 years of age. So there’s a huge study population, comprehensive follow-up, and – the final element in this prospective, population-based study – the rotavirus and pneumococcal conjugate vaccines were introduced in Israel just a few years ago and at roughly the same time. This enabled Dr. Dagan and his coinvestigators to compare hospitalization rates and pediatric emergency department outpatient visits for diarrheal and lower respiratory viral illnesses among children less than 2 years old during the prevaccine period of April 2006-March 2009 with rates during April 2013-March 2014, when uptake of the two vaccines in that age group exceeded 90%.
This was an unusual study in that it looked at the global impact of two major vaccines. In contrast, vaccine clinical trials and postmarketing studies typically evaluate only those outcomes directly related to that particular vaccine.
The results of the Israeli study were startling: in the 3 years prior to introduction of the vaccines, one in five children under age 2 years admitted to the hospital had as an admitting diagnosis either rotavirus gastroenteritis confirmed by a positive stool ELISA test or pneumococcal pneumonia as evidenced by alveolar pneumonia on chest x-ray. After the vaccines became available, the hospitalization rates for rotavirus gastroenteritis and alveolar pneumonia plummeted by 78% and 46%, respectively. Moreover, outpatient pediatric emergency department visits for rotavirus gastroenteritis dropped by 71% and visits for alveolar pneumonia fell by 67%.
But that’s not all. Smaller yet clinically meaningful reductions were also documented in nonrotavirus gastroenteritis and nonalveolar lower respiratory tract infections. Specifically, the hospitalization rate for nonrotavirus diarrheal illnesses and nonalveolar pneumonia lower respiratory infections dropped by 21% and 7%, respectively, while outpatient emergency visits for those disorders fell by 16% and 14%.
This translates to an estimated 1,890 fewer hospitalizations and 4,030 fewer outpatient emergency department visits for diarrheal disease or lower respiratory infection per 100,000 children under age 2 per year, Dr. Dagan reported.
Michael Schmidt, Ph.D., who chaired a press conference highlighting the Israeli study, declared, “These data are absolutely phenomenal. It really shows the global value of these vaccines for society.”
Dr. Schmidt, professor and vice chairman of microbiology and immunology at the Medical University of South Carolina, Charleston, posed a question: What’s the explanation for the reductions in diseases not directly addressed by those two vaccines?
“We believe that one success can favorably affect the other. If you are weakened by diarrhea, you may be more likely to get pneumonia, and vice versa,” according to Dr. Dagan.
He added that the results actually pack a significantly greater wallop than is apparent at first look because rotavirus gastroenteritis and pneumococcal pneumonia in young children are seasonal diseases. They occur chiefly during October-March. So those 5,920 fewer hospitalizations and outpatient visits/100,000 per young children per year are concentrated during pediatricians’ busiest half of the year.
“In most places in the world, winter is a time of so much illness that pediatricians can’t deliver appropriate care. We knew that in our hospital we couldn’t deliver appropriate care to children in the winter because there were so many sick children piled on top of each other. But now, because of these two vaccines, we are less crowded in the winter, we have more time for children, we make fewer mistakes,” he said.
The study was funded by vaccine manufacturers and the Israel Ministry of Health. Dr. Dagan reported serving as a consultant, adviser to, and/or recipient of research grants from GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, and Genocea.
AT ICAAC 2015
Key clinical point: Pediatric rotavirus and pneumococcal conjugate vaccines provide global benefits that extend well beyond the diseases they specifically target.
Major finding: When rotavirus and pneumococcal conjugate vaccines were introduced in southern Israel, hospitalization rates for rotavirus gastroenteritis and nonrotavirus gastroenteritis dropped by 78% and 21%, respectively, in children less than 2 years old, while hospitalizations for alveolar pneumonia, which is mainly pneumococcal, and other lower respiratory tract infections unlikely to be pneumococcal fell by 46% and 7%, respectively.
Data source: This study is part of an ongoing, prospective, population-based trial in southern Israel comparing rates of hospitalization and outpatient visits for diarrheal and lower respiratory tract illnesses in roughly 30,000 children, who were less than 2 years old during the 3 years just prior to introduction of the rotavirus and pneumococcal conjugate vaccines to rates after the vaccines became available.
Disclosures: The study was funded by vaccine manufacturers and the Israel Ministry of Health. The presenter reported serving as a consultant, advisor to, and/or recipient of research grants from GlaxoSmithKline, Merck Sharp & Dohme, Pfizer, and Genocea.