User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
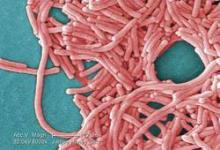
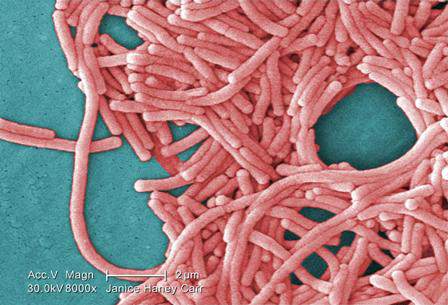
Legionellosis cases continue to increase nationwide
Data from the first 3 years of the Center for Disease Control and Prevention’s Active Bacterial Core surveillance (ABCs) program on legionellosis confirm that incidences of disease caused by the bacteria are increasing across the United States, according to the latest Morbidity and Mortality Weekly Report (MMWR. 2015 Oct 30;64[42]:1190-1193).
The ABCs program, which launched in 2011, identified 1,426 cases of legionellosis over the 3-year time span, and incidence rates of 1.3 (2011), 1.1 (2012), and 1.4 (2013) cases per 100,000 individuals in the general population. This corroborates similar findings made by the National Notifiable Diseases Surveillance System (NNDSS) between 2000 and 2011, which reported an increase in the crude incidence rate of legionellosis nationwide from 0.39 to 1.36 cases per 100,000 individuals.
The two main clinical syndromes associated with legionellosis are Legionnaires’ disease, which is a severe form of pneumonia; and Pontiac fever, a milder illness without pneumonia.
“During 2000-2011, passive surveillance for legionellosis in the United States demonstrated a 249% increase in crude incidence, although little was known about the clinical course and method of diagnosis,” says the report, led by Dr. Kathleen I. Dooling of the CDC’s National Center for Immunization and Respiratory Diseases.
The report also states that “ABCs data during 2011-2013 showed that approximately 44% of patients with legionellosis required intensive care, and 9% died.” Furthermore, incidence increased with age among the ABCs program cohort. Those younger than 50 years of age had a 0.4 incidence rate per 100,000 individuals; patients between 50 and 64 years old had a 2.5 per 100,000 incidence rate; those who were 65-79 years old had a 3.6 per 100,000 incidence rate; and individuals 80 years and older had an incidence rate of 4.7 per 100,000 individuals.
“Among cases identified during 2011-2013, 79% occurred in persons aged >50 years, 65% were in males, and 72% of patients were white,” says the report, adding that “1,300 (91%) received a diagnosis of legionellosis on the basis of urine antigen testing, which only detects Lp1 species.”
The ABCs program defined a confirmed case of legionellosis as “the isolation of Legionella from respiratory culture, detection of Legionella antigen in urine, or seroconversion (a more than fourfold rise in antibody titer between acute and convalescent sera) to Lp1.” Unlike NNDSS, ABCs recorded clinical and race data for each patient found to have legionellosis, finding that incidence rates among blacks were higher than among whites per 100,000 individuals: 1.0 vs. 1.5, respectively.
The report concludes by calling for further research into the disparities in legionellosis cases based on race, age, and geography, as well as the need for “more sensitive laboratory tests for legionellosis because proper diagnosis is needed for treatment and public health action.”
This study was supported by the CDC.
Data from the first 3 years of the Center for Disease Control and Prevention’s Active Bacterial Core surveillance (ABCs) program on legionellosis confirm that incidences of disease caused by the bacteria are increasing across the United States, according to the latest Morbidity and Mortality Weekly Report (MMWR. 2015 Oct 30;64[42]:1190-1193).
The ABCs program, which launched in 2011, identified 1,426 cases of legionellosis over the 3-year time span, and incidence rates of 1.3 (2011), 1.1 (2012), and 1.4 (2013) cases per 100,000 individuals in the general population. This corroborates similar findings made by the National Notifiable Diseases Surveillance System (NNDSS) between 2000 and 2011, which reported an increase in the crude incidence rate of legionellosis nationwide from 0.39 to 1.36 cases per 100,000 individuals.
The two main clinical syndromes associated with legionellosis are Legionnaires’ disease, which is a severe form of pneumonia; and Pontiac fever, a milder illness without pneumonia.
“During 2000-2011, passive surveillance for legionellosis in the United States demonstrated a 249% increase in crude incidence, although little was known about the clinical course and method of diagnosis,” says the report, led by Dr. Kathleen I. Dooling of the CDC’s National Center for Immunization and Respiratory Diseases.
The report also states that “ABCs data during 2011-2013 showed that approximately 44% of patients with legionellosis required intensive care, and 9% died.” Furthermore, incidence increased with age among the ABCs program cohort. Those younger than 50 years of age had a 0.4 incidence rate per 100,000 individuals; patients between 50 and 64 years old had a 2.5 per 100,000 incidence rate; those who were 65-79 years old had a 3.6 per 100,000 incidence rate; and individuals 80 years and older had an incidence rate of 4.7 per 100,000 individuals.
“Among cases identified during 2011-2013, 79% occurred in persons aged >50 years, 65% were in males, and 72% of patients were white,” says the report, adding that “1,300 (91%) received a diagnosis of legionellosis on the basis of urine antigen testing, which only detects Lp1 species.”
The ABCs program defined a confirmed case of legionellosis as “the isolation of Legionella from respiratory culture, detection of Legionella antigen in urine, or seroconversion (a more than fourfold rise in antibody titer between acute and convalescent sera) to Lp1.” Unlike NNDSS, ABCs recorded clinical and race data for each patient found to have legionellosis, finding that incidence rates among blacks were higher than among whites per 100,000 individuals: 1.0 vs. 1.5, respectively.
The report concludes by calling for further research into the disparities in legionellosis cases based on race, age, and geography, as well as the need for “more sensitive laboratory tests for legionellosis because proper diagnosis is needed for treatment and public health action.”
This study was supported by the CDC.
Data from the first 3 years of the Center for Disease Control and Prevention’s Active Bacterial Core surveillance (ABCs) program on legionellosis confirm that incidences of disease caused by the bacteria are increasing across the United States, according to the latest Morbidity and Mortality Weekly Report (MMWR. 2015 Oct 30;64[42]:1190-1193).
The ABCs program, which launched in 2011, identified 1,426 cases of legionellosis over the 3-year time span, and incidence rates of 1.3 (2011), 1.1 (2012), and 1.4 (2013) cases per 100,000 individuals in the general population. This corroborates similar findings made by the National Notifiable Diseases Surveillance System (NNDSS) between 2000 and 2011, which reported an increase in the crude incidence rate of legionellosis nationwide from 0.39 to 1.36 cases per 100,000 individuals.
The two main clinical syndromes associated with legionellosis are Legionnaires’ disease, which is a severe form of pneumonia; and Pontiac fever, a milder illness without pneumonia.
“During 2000-2011, passive surveillance for legionellosis in the United States demonstrated a 249% increase in crude incidence, although little was known about the clinical course and method of diagnosis,” says the report, led by Dr. Kathleen I. Dooling of the CDC’s National Center for Immunization and Respiratory Diseases.
The report also states that “ABCs data during 2011-2013 showed that approximately 44% of patients with legionellosis required intensive care, and 9% died.” Furthermore, incidence increased with age among the ABCs program cohort. Those younger than 50 years of age had a 0.4 incidence rate per 100,000 individuals; patients between 50 and 64 years old had a 2.5 per 100,000 incidence rate; those who were 65-79 years old had a 3.6 per 100,000 incidence rate; and individuals 80 years and older had an incidence rate of 4.7 per 100,000 individuals.
“Among cases identified during 2011-2013, 79% occurred in persons aged >50 years, 65% were in males, and 72% of patients were white,” says the report, adding that “1,300 (91%) received a diagnosis of legionellosis on the basis of urine antigen testing, which only detects Lp1 species.”
The ABCs program defined a confirmed case of legionellosis as “the isolation of Legionella from respiratory culture, detection of Legionella antigen in urine, or seroconversion (a more than fourfold rise in antibody titer between acute and convalescent sera) to Lp1.” Unlike NNDSS, ABCs recorded clinical and race data for each patient found to have legionellosis, finding that incidence rates among blacks were higher than among whites per 100,000 individuals: 1.0 vs. 1.5, respectively.
The report concludes by calling for further research into the disparities in legionellosis cases based on race, age, and geography, as well as the need for “more sensitive laboratory tests for legionellosis because proper diagnosis is needed for treatment and public health action.”
This study was supported by the CDC.
FROM MMWR
Key clinical point: Data from the first 3 years of the CDC’s Active Bacterial Core surveillance program provides evidence confirming that legionellosis rates across the United States are steadily rising.
Major finding: ABCs identified 1,426 legionellosis cases during 2011-2013, for an incidence of 1.3 cases per 100,000 population over the 3 years.
Data source: Analysis of 1,426 legionellosis cases from 2011-2013 collected by the CDC’s ABCs program.
Disclosures: Study supported by the CDC.
Statins may inhibit flu vaccine response
According to a pair of studies published online Oct. 29 in the Journal of Infectious Diseases, statins – a class of drugs widely utilized by older adults to reduce cholesterol – may have the unintended consequence of reducing immunotherapeutic response to and effectiveness of influenza vaccination.
In a post-hoc analysis (J Infect Dis. 2015 Oct 29. doi: 10.1093/infdis/jiv456), Dr. Steven Black of Cincinnati Children’s Hospital Medical Center and colleagues derived data from an international, multisite, randomized, controlled, influenza vaccine clinical trial population of 6,961 subjects over the age of 65. At 3 weeks post vaccination, the researchers measured the level of antibodies to flu vaccine strains in the blood of both statin and non–statin taking participants. The investigators discovered that hemagglutination-inhibiting geometric mean titers to influenza A (H1NI), A (H3N2), and B strains were 38% (95% confidence interval, 27%-50%), 67% (95% CI, 54%-80%) and 38% (95% CI, 28%-29%) lower, respectively, in the statin therapy arm as compared with the non–statin therapy cohort. The effect was most dramatic in patients on synthetic (as opposed to natural, fermentation-derived) statin treatment regimens.
“Apparently, statins interfere with the response to influenza vaccine and lower the immune response, and this would seem to also result in a lower effectiveness of influenza vaccines,” Dr. Black and his colleagues wrote. Potential mitigation strategies for statin-induced immunosuppression suggested by the research team include preferential use of high-dose or adjuvanted vaccines.
In addition, a separate retrospective investigation (J Infect Dis. 2015 Oct 29. doi: 10.1093/infdis/jiv457.) tracking 137,488 patients from a Georgia managed care organization database over nine flu seasons (from 2002 to 2011) also generated data implying a connection between statin use and compromised influenza vaccine efficacy and immune response. Dr. Saad Omer of the Emory Vaccine Center at Emory University in Atlanta and his colleagues analyzed the impact of statins on influenza vaccine efficacy against medically attended acute respiratory illness (MAARI). MAARI incidence is routinely employed as an influenza impact marker, although not all MAARI incidence is influenza related.
The Emory University research team found that influenza vaccine effectiveness against MAARI was decreased in statin users, compared with nonusers during periods of local (14.1% vs. 22.9%; mean difference, 11.4%; 95% CI, −1.7%-26.1%) and widespread (12.6% vs. 26.2%; mean difference, 18.4%; 95% CI, 2.9%-36.2%) influenza circulation. “Even after adjustment for several covariates … the observed reduction in influenza vaccine effectiveness among statin users remained statistically significant for periods of widespread influenza circulation with a nonsignificant trend toward reduced vaccine effectiveness during periods of local circulation, as well,” noted Dr. Omer and his coauthors. Said results, wrote the researchers, “have potential implications for clinical guidelines regarding statin use around the time of routine vaccinations.”
Dr. Black is a consultant for Novartis Vaccines, GSK, Takeda Vaccines, Protein Sciences, and the World Health Organization. His coauthors – Dr. Uwe Nicolay, Dr. Giuseppe Del Giudice, and Dr. Rino Rappuoli – are employees of Novartis Vaccines.
Novartis Vaccines funded the post-hoc analysis conducted by Dr. Black and his colleagues, as well as the original clinical trial that developed the data utilized for the analysis. Dr. Omer and his colleagues were funded by Emory University and the National Institute of Allergy and Infectious Diseases and reported no relevant disclosures.
The findings that statin use adversely affects IIV (inactivated influenza vaccine) immunogenicity and vaccine effectiveness are biologically plausible, based on known immunomodulatory effects of these drugs and raise important questions about the use of these important medications. Should these results affect a physician’s care of patients? Should statins be stopped for a period while influenza vaccine is administered? Should IIV not be administered to statin users? The answer to all of these questions is no.
Instead the results of these studies should be viewed as hypothesis generating and should prompt further investigation. If statins are found to reduce immunogenicity, then potentially transient interruption of statin therapy could be considered for testing. The effect of chronic statin use on the immunogenicity of other vaccines also needs to be evaluated further. Future studies could also evaluate whether alternative vaccination strategies with improved immunogenicity, such as high-dose, intradermally delivered, or adjuvanted vaccines will overcome the effects of statin use (if any).
The results also underscore the need for the development of influenza vaccines with improved efficacy and effectiveness.
Dr. Robert L. Atmar is a professor of medicine and interim chief of medicine-infectious disease at the Baylor College of Medicine, Houston, Texas. Dr. Wendy A. Keitel is an associate professor of molecular virology and microbiology at the Baylor College of Medicine, Houston, Texas. Dr. Atmar reported receiving grants from Takeda Vaccines. Dr. Keitel reported no relevant disclosures. Dr. Atmar and Dr. Keitel made these remarks in an editorial commentary (J Infect Dis. 2015 Oct 29. doi: 10.1093/infdis/jiv459.) that accompanied the data furnished by Dr. Black and colleagues and Dr. Omer and colleagues.
The findings that statin use adversely affects IIV (inactivated influenza vaccine) immunogenicity and vaccine effectiveness are biologically plausible, based on known immunomodulatory effects of these drugs and raise important questions about the use of these important medications. Should these results affect a physician’s care of patients? Should statins be stopped for a period while influenza vaccine is administered? Should IIV not be administered to statin users? The answer to all of these questions is no.
Instead the results of these studies should be viewed as hypothesis generating and should prompt further investigation. If statins are found to reduce immunogenicity, then potentially transient interruption of statin therapy could be considered for testing. The effect of chronic statin use on the immunogenicity of other vaccines also needs to be evaluated further. Future studies could also evaluate whether alternative vaccination strategies with improved immunogenicity, such as high-dose, intradermally delivered, or adjuvanted vaccines will overcome the effects of statin use (if any).
The results also underscore the need for the development of influenza vaccines with improved efficacy and effectiveness.
Dr. Robert L. Atmar is a professor of medicine and interim chief of medicine-infectious disease at the Baylor College of Medicine, Houston, Texas. Dr. Wendy A. Keitel is an associate professor of molecular virology and microbiology at the Baylor College of Medicine, Houston, Texas. Dr. Atmar reported receiving grants from Takeda Vaccines. Dr. Keitel reported no relevant disclosures. Dr. Atmar and Dr. Keitel made these remarks in an editorial commentary (J Infect Dis. 2015 Oct 29. doi: 10.1093/infdis/jiv459.) that accompanied the data furnished by Dr. Black and colleagues and Dr. Omer and colleagues.
The findings that statin use adversely affects IIV (inactivated influenza vaccine) immunogenicity and vaccine effectiveness are biologically plausible, based on known immunomodulatory effects of these drugs and raise important questions about the use of these important medications. Should these results affect a physician’s care of patients? Should statins be stopped for a period while influenza vaccine is administered? Should IIV not be administered to statin users? The answer to all of these questions is no.
Instead the results of these studies should be viewed as hypothesis generating and should prompt further investigation. If statins are found to reduce immunogenicity, then potentially transient interruption of statin therapy could be considered for testing. The effect of chronic statin use on the immunogenicity of other vaccines also needs to be evaluated further. Future studies could also evaluate whether alternative vaccination strategies with improved immunogenicity, such as high-dose, intradermally delivered, or adjuvanted vaccines will overcome the effects of statin use (if any).
The results also underscore the need for the development of influenza vaccines with improved efficacy and effectiveness.
Dr. Robert L. Atmar is a professor of medicine and interim chief of medicine-infectious disease at the Baylor College of Medicine, Houston, Texas. Dr. Wendy A. Keitel is an associate professor of molecular virology and microbiology at the Baylor College of Medicine, Houston, Texas. Dr. Atmar reported receiving grants from Takeda Vaccines. Dr. Keitel reported no relevant disclosures. Dr. Atmar and Dr. Keitel made these remarks in an editorial commentary (J Infect Dis. 2015 Oct 29. doi: 10.1093/infdis/jiv459.) that accompanied the data furnished by Dr. Black and colleagues and Dr. Omer and colleagues.
According to a pair of studies published online Oct. 29 in the Journal of Infectious Diseases, statins – a class of drugs widely utilized by older adults to reduce cholesterol – may have the unintended consequence of reducing immunotherapeutic response to and effectiveness of influenza vaccination.
In a post-hoc analysis (J Infect Dis. 2015 Oct 29. doi: 10.1093/infdis/jiv456), Dr. Steven Black of Cincinnati Children’s Hospital Medical Center and colleagues derived data from an international, multisite, randomized, controlled, influenza vaccine clinical trial population of 6,961 subjects over the age of 65. At 3 weeks post vaccination, the researchers measured the level of antibodies to flu vaccine strains in the blood of both statin and non–statin taking participants. The investigators discovered that hemagglutination-inhibiting geometric mean titers to influenza A (H1NI), A (H3N2), and B strains were 38% (95% confidence interval, 27%-50%), 67% (95% CI, 54%-80%) and 38% (95% CI, 28%-29%) lower, respectively, in the statin therapy arm as compared with the non–statin therapy cohort. The effect was most dramatic in patients on synthetic (as opposed to natural, fermentation-derived) statin treatment regimens.
“Apparently, statins interfere with the response to influenza vaccine and lower the immune response, and this would seem to also result in a lower effectiveness of influenza vaccines,” Dr. Black and his colleagues wrote. Potential mitigation strategies for statin-induced immunosuppression suggested by the research team include preferential use of high-dose or adjuvanted vaccines.
In addition, a separate retrospective investigation (J Infect Dis. 2015 Oct 29. doi: 10.1093/infdis/jiv457.) tracking 137,488 patients from a Georgia managed care organization database over nine flu seasons (from 2002 to 2011) also generated data implying a connection between statin use and compromised influenza vaccine efficacy and immune response. Dr. Saad Omer of the Emory Vaccine Center at Emory University in Atlanta and his colleagues analyzed the impact of statins on influenza vaccine efficacy against medically attended acute respiratory illness (MAARI). MAARI incidence is routinely employed as an influenza impact marker, although not all MAARI incidence is influenza related.
The Emory University research team found that influenza vaccine effectiveness against MAARI was decreased in statin users, compared with nonusers during periods of local (14.1% vs. 22.9%; mean difference, 11.4%; 95% CI, −1.7%-26.1%) and widespread (12.6% vs. 26.2%; mean difference, 18.4%; 95% CI, 2.9%-36.2%) influenza circulation. “Even after adjustment for several covariates … the observed reduction in influenza vaccine effectiveness among statin users remained statistically significant for periods of widespread influenza circulation with a nonsignificant trend toward reduced vaccine effectiveness during periods of local circulation, as well,” noted Dr. Omer and his coauthors. Said results, wrote the researchers, “have potential implications for clinical guidelines regarding statin use around the time of routine vaccinations.”
Dr. Black is a consultant for Novartis Vaccines, GSK, Takeda Vaccines, Protein Sciences, and the World Health Organization. His coauthors – Dr. Uwe Nicolay, Dr. Giuseppe Del Giudice, and Dr. Rino Rappuoli – are employees of Novartis Vaccines.
Novartis Vaccines funded the post-hoc analysis conducted by Dr. Black and his colleagues, as well as the original clinical trial that developed the data utilized for the analysis. Dr. Omer and his colleagues were funded by Emory University and the National Institute of Allergy and Infectious Diseases and reported no relevant disclosures.
According to a pair of studies published online Oct. 29 in the Journal of Infectious Diseases, statins – a class of drugs widely utilized by older adults to reduce cholesterol – may have the unintended consequence of reducing immunotherapeutic response to and effectiveness of influenza vaccination.
In a post-hoc analysis (J Infect Dis. 2015 Oct 29. doi: 10.1093/infdis/jiv456), Dr. Steven Black of Cincinnati Children’s Hospital Medical Center and colleagues derived data from an international, multisite, randomized, controlled, influenza vaccine clinical trial population of 6,961 subjects over the age of 65. At 3 weeks post vaccination, the researchers measured the level of antibodies to flu vaccine strains in the blood of both statin and non–statin taking participants. The investigators discovered that hemagglutination-inhibiting geometric mean titers to influenza A (H1NI), A (H3N2), and B strains were 38% (95% confidence interval, 27%-50%), 67% (95% CI, 54%-80%) and 38% (95% CI, 28%-29%) lower, respectively, in the statin therapy arm as compared with the non–statin therapy cohort. The effect was most dramatic in patients on synthetic (as opposed to natural, fermentation-derived) statin treatment regimens.
“Apparently, statins interfere with the response to influenza vaccine and lower the immune response, and this would seem to also result in a lower effectiveness of influenza vaccines,” Dr. Black and his colleagues wrote. Potential mitigation strategies for statin-induced immunosuppression suggested by the research team include preferential use of high-dose or adjuvanted vaccines.
In addition, a separate retrospective investigation (J Infect Dis. 2015 Oct 29. doi: 10.1093/infdis/jiv457.) tracking 137,488 patients from a Georgia managed care organization database over nine flu seasons (from 2002 to 2011) also generated data implying a connection between statin use and compromised influenza vaccine efficacy and immune response. Dr. Saad Omer of the Emory Vaccine Center at Emory University in Atlanta and his colleagues analyzed the impact of statins on influenza vaccine efficacy against medically attended acute respiratory illness (MAARI). MAARI incidence is routinely employed as an influenza impact marker, although not all MAARI incidence is influenza related.
The Emory University research team found that influenza vaccine effectiveness against MAARI was decreased in statin users, compared with nonusers during periods of local (14.1% vs. 22.9%; mean difference, 11.4%; 95% CI, −1.7%-26.1%) and widespread (12.6% vs. 26.2%; mean difference, 18.4%; 95% CI, 2.9%-36.2%) influenza circulation. “Even after adjustment for several covariates … the observed reduction in influenza vaccine effectiveness among statin users remained statistically significant for periods of widespread influenza circulation with a nonsignificant trend toward reduced vaccine effectiveness during periods of local circulation, as well,” noted Dr. Omer and his coauthors. Said results, wrote the researchers, “have potential implications for clinical guidelines regarding statin use around the time of routine vaccinations.”
Dr. Black is a consultant for Novartis Vaccines, GSK, Takeda Vaccines, Protein Sciences, and the World Health Organization. His coauthors – Dr. Uwe Nicolay, Dr. Giuseppe Del Giudice, and Dr. Rino Rappuoli – are employees of Novartis Vaccines.
Novartis Vaccines funded the post-hoc analysis conducted by Dr. Black and his colleagues, as well as the original clinical trial that developed the data utilized for the analysis. Dr. Omer and his colleagues were funded by Emory University and the National Institute of Allergy and Infectious Diseases and reported no relevant disclosures.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Key clinical point: Statin therapy may adversely impact the efficacy of influenza vaccine.
Major findings: Hemagglutination-inhibiting geometric mean titers to influenza A (H1NI), A (H3N2), and B strains were 38% , 67%, and 38% lower, respectively, in the chronic statin therapy cohort (compared with the non–statin therapy group). Influenza vaccine effectiveness against acute respiratory illness was decreased in statin users, compared with nonusers during periods of local and widespread influenza circulation.
Data source: A retrospective analysis of 6,961 subjects over the age of 65 from a randomized, controlled influenza vaccine trial; a retrospective cohort study of 137,488 Georgia managed care patients over nine flu seasons from 2002/2003 to 2010/2011 (totaling 447,588 person-seasons).
Disclosures: Dr. Steven Black is a consultant for Novartis Vaccines, GSK, Takeda Vaccines, Protein Sciences, and the World Health Organization. His coauthors – Dr. Uwe Nicolay, Dr. Giuseppe Del Giudice, and Dr. Rino Rappuoli – are employees of Novartis Vaccines. Novartis Vaccines funded the post-hoc analysis conducted by Dr. Black and colleagues and also the original clinical trial that developed the data utilized for said analysis.
Dr. Saad Omer and colleagues were funded by Emory University and the National Institute of Allergy and Infectious Diseases and reported no relevant disclosures.
HFSA: Emphasizing "acute" in acute decompensated heart failure
NATIONAL HARBOR, MD. – Acute decompensated heart failure is becoming more of an emergency.
Traditionally, it has been seen as a lumbering event that could be treated at a relatively leisurely pace, but heart failure physicians increasingly see the moment when patients arrive in the hospital with an episode of acute decompensated heart failure as a time-sensitive event that requires rapid intervention in a manner much more akin to an acute MI than to chronic heart failure.
While the tide is slowly shifting to put a premium on rapid treatment to try to decongest acute heart failure patients, the treatment options clinicians have available for these patients often remain inadequate.
“Development of drugs for acutely decompensated heart failure has been extremely difficult. We have done a really horrible job treating this disease,” Dr. Milton Packer said at the HFSA annual scientific meeting.
Treatment of acute heart failure patients has generally focused on relieving dyspnea, but part of the new appreciation of this state as an emergency event involves understanding that the pathology patients have when they reach the hospital is much more global and has profoundly morbid consequences.
“We want more from treatment than for patients to feel a little bit better an hour or two sooner” by relieving dyspnea, said Dr. Packer, professor of medicine and a heart failure specialist at the University of Texas Southwestern Medical Center in Dallas. “In the first 6 hours [of acute heart failure hospitalization], many patients are spilling troponin. We don’t know what this means, but the patients who spill troponin have a markedly increased risk for a more complicated hospital course.” About 10%-25% of patients hospitalized with acute heart failure have recurrent worsening heart failure, and many of these are also the patients who have a spike in their troponin level during initial hospitalization, he noted.
The troponin release in many patients and its association with worse outcomes is a clue that these patients are experiencing an ischemic myocardial event similar to an acute MI, possibly caused by myocardial-wall stretch, Dr. Packer said in an interview.
“If we can reduce this early acute cardiac dilatation, maybe we can reduce myocardial injury, reduce troponin release, and have favorable effects on clinically relevant events both short-term and long-term,” he said. “That’s why in trials [of investigational drugs for acute heart failure] we are treating patients earlier. Before we said we could enroll patients [into acute-treatment trials] within 48 hours of hospital admission. Now we enroll within 16 hours, or within 12 hours. We’ve learned that early intervention is important. That makes acute heart failure a lot more similar to an acute MI.”
European Society recommends faster acute heart failure management
European heart failure specialists have also become convinced that acute heart failure is an emergency that needs a rapid response. In June, the Heart Failure Association of the European Society of Cardiology published new recommendations on the in-hospital management of patients with acute heart failure (Eur J Heart Fail 2015 June;17[6];544-58). In the document, the association’s writing panel said, “The potentially greater benefit of early treatment is of conceptual importance in many cardiovascular presentations (e.g., myocardial infarction). Unfortunately, acute heart failure has not been considered with this regard until recently.” Breaking with the past, the association’s new recommendations now say that “all acute heart failure patients should receive appropriate therapy as early as possible,” an approach that involves starting acute management in the prehospital setting.
One member of the writing group for these recommendations put it more succinctly while speaking at the annual congress of the European Society of Cardiology in London in August: “Time is muscle in acute heart failure,” said Dr. Piotr Ponikowski, professor and heart failure specialist at the Medical University in Wroclaw, Poland. “When a patient has acute coronary syndrome everyone rushes, but we have patients with acute heart failure and no one rushes. We give furosemide, maybe something else, and then we wait and see.” He recommended adhering to a schedule that would have a patient assessed and initially treated within the first hour of hospitalization, and even sooner if treatment could start at the prehospital stage.
Like Dr. Packer, Dr. Ponikowski also lamented the inadequate tools now available for treating acute heart failure and the pressing need to identify better approaches to treatment, especially for selected acute heart failure patients.
“It is too simple to think that one drug or one treatment will help the entire spectrum of acute heart failure patients,” he said. “Our hypothesis is that profiling patients at every step of acute heart failure is crucial.”
He itemized five distinct types of acute heart failure patients based on their precipitating triggers of decompensation:
• Rapid arrhythmia or rhythm disturbance.
• Hypertension emergency.
• Pulmonary embolism.
• Pulmonary infection.
• Mechanical cause of acute heart failure.
“We need to clinically profile” patients into these subgroups to better tailor management, he said.
Another important aspect of patient heterogeneity is that fluid congestion may be less important in many patients compared with fluid redistribution from the splanchnic circulation. This distinction is important because fluid redistribution may be better treated with a vasodilator than with a diuretic, he noted. He voiced hope that two phase III trials now in progress with two unique vasodilator drugs, the TRUE-AHF trial of ularitide, and the RELAX-AHF-2 trial of serelaxin, may identify two new vasodilators with “unique effects” that could potentially launch a new era in management of selected patients with acute heart failure. Dr. Packer, the principal investigator for the ularitide trial, offered similar hope.
The responsiveness of acute heart failure patients to in-hospital treatment may vary depending on what end-organ damage they experience, Dr. Ponikowski said.
This end-organ damage is often an acute process occurring during hospitalization caused by the fluid congestion and redistribution that occurs during acute heart failure, said Dr. Alexandre Mebazaa, professor of anesthesiology and critical care medicine at Lariboisière Hospital in Paris.
“Fluid overload leads to organ dysfunction. In the past, we thought that kidney dysfunction [occurring during acute heart failure] was due to low cardiac output, but we know that dysfunction in the kidney and liver is due to congestion, and diuretics do not remove water from the liver and kidney,” Dr. Mebazaa said in an interview. “Diuretics may remove fluid from vessels, but not from organs. We need new approaches to remove fluid from organs – from the kidney, liver, and lungs” – during acute heart failure. This is another reason why heart failure physicians are excited about the possibility of finding new vasodilators, such a ularitide and serelaxin, that might address the issue of venous congestion in peripheral organs.
Faster management endorsed by U.S. clinicians, too
“We used to think that the reason why patients with acute heart failure were not voiding well and became diuretic resistant was because of poor cardiac output. Now we know that there is a lot of venous congestion with an impact on the liver and kidneys,” agreed Dr. Mariell L. Jessup, professor and medical director of the Penn Heart and Vascular Center at the University of Pennsylvania in Philadelphia. “We’ve begun to appreciate how important venous congestion is in causing high pressures on the right side” of the circulatory system, she said in an interview.
Other U.S. physicians echo the call by Dr. Packer and the European cardiologists for faster treatment of acute heart failure. “I collected data at U.S. hospitals and found it took an average of 22 hours for decompensated heart failure patients to receive treatment,” said Dr. Maria Rosa Costanzo, medical director of the heart failure and pulmonary hypertension program at Advocate Heart Institute in Naperville, Ill. “I have tried to convey the message that these patients must be treated early, and this is associated with better outcomes,” she said in an interview during the HFSA meeting.
“Early treatment means at least two doses of intravenous diuretic in the emergency department. We’ve seen that the two immediate doses can make a big difference, producing shorter lengths of stay in the intensive care unit, fewer rehospitalizations, and fewer deaths,” according to data collected in the ADHERE (Acute Decompensated Heart Failure National Registry), she said. “But this has not yet been picked up in a lot of U.S. practice.” Although the hemodynamic abnormalities that lead up to an acute decompensation event can take several weeks of steady worsening before severe symptoms drive a patient to the hospital, once the patient requires hospitalization “it should be treated as an emergency,” she said.
Dr. Costanzo is a major advocate for using ultrafiltration as a second-line treatment for acute decompensated heart failure patients who do not adequately respond to diuretic treatment, but for the time being, ultrafiltration remains a controversial option that at least some other heart failure physicians do not endorse, and it can involve reimbursement issues as many insurers consider it investigational.
“Try to get the patient decongested within the first 6 hours [after arrival at the hospital] or even sooner, within the first 1-2 hours,” recommended Dr. Christopher M. O’Connor, chief executive officer of Inova Heart and Vascular Institute in Falls Church, Va. He suggested treating patients with a combination of diuretics and vasodilators. “Some people are talking about instituting a performance measure for treating acute heart failure within the first 6 hours,” Dr. O’Connor said in an interview.
Currently, vasodilator treatment is limited to standard agents such as intravenous nitroglycerin, but Dr. O’Connor shared the hope that sometime soon a new vasodilator may be shown effective for acutely decompensated patients. He is a coinvestigator on the TRUE-AHF study of ularitide. “We hope that these new vasodilators, ularitide and serelaxin, will be good complements to diuretics, he said. Dr. O’Connor also recommended that clinicians shy away from using ultrafiltration as a back-up therapy, believing that it was shown ineffective and potentially harmful in results from the CARRESS-HF trial (N Engl J Med 2012 Dec 13;367[24]:2296-304).
But not all heart failure specialists see acute heart failure as a new frontier for early treatment and new drug discovery.
“So much energy has already been spent on acute heart failure with very little return,” said Dr. Clyde W. Yancy, professor and chief of cardiology at Northwestern University in Chicago. “I think that our best opportunities in heart failure are in prevention and in better chronic care. The hospitalized patient is so broad and complex; if we’re looking at how to best spend our resources I think it’s best to focus on prevention,” he said in an interview.
“The hospital experience needs to shift toward better use of systems of care and focus less on the biology. The biggest challenge is how to coordinate all the systems to make sure that patients have access to the resources and can obtain [existing] medications. Patients don’t often have the literacy to understand discharge instructions, and our systems are overwhelmed by trying to have 7-day follow-up visits. Focusing on management of the hospitalized patient does not give us a good return on the investment. There is no question that acute heart failure is an unmet need, but the greater unmet need is prevention and improved chronic care. No single intervention will dramatically change the acute heart failure experience. Focusing on the hospitalization does not offer us management opportunities that are as robust as we once thought,” Dr. Yancy said.
Dr. Packer has been a consultant to 22 companies. Dr. Ponikowski has been a consultant to, speaker for, or has received research grants from 11 companies. Dr. Mebazaa has received speaking honoraria and consulting fees from 11 companies. Dr. Jessup, Dr. Costanzo, and Dr. Yancy had no disclosures. Dr. O’Connor has been a consultant to ResMed, Roche Diagnostics, Cardiorentis, Bayer, and Actelion and has received research grants from Otsuka, ResMed, and Roche Diagnostics.
On Twitter@mitchelzoler
NATIONAL HARBOR, MD. – Acute decompensated heart failure is becoming more of an emergency.
Traditionally, it has been seen as a lumbering event that could be treated at a relatively leisurely pace, but heart failure physicians increasingly see the moment when patients arrive in the hospital with an episode of acute decompensated heart failure as a time-sensitive event that requires rapid intervention in a manner much more akin to an acute MI than to chronic heart failure.
While the tide is slowly shifting to put a premium on rapid treatment to try to decongest acute heart failure patients, the treatment options clinicians have available for these patients often remain inadequate.
“Development of drugs for acutely decompensated heart failure has been extremely difficult. We have done a really horrible job treating this disease,” Dr. Milton Packer said at the HFSA annual scientific meeting.
Treatment of acute heart failure patients has generally focused on relieving dyspnea, but part of the new appreciation of this state as an emergency event involves understanding that the pathology patients have when they reach the hospital is much more global and has profoundly morbid consequences.
“We want more from treatment than for patients to feel a little bit better an hour or two sooner” by relieving dyspnea, said Dr. Packer, professor of medicine and a heart failure specialist at the University of Texas Southwestern Medical Center in Dallas. “In the first 6 hours [of acute heart failure hospitalization], many patients are spilling troponin. We don’t know what this means, but the patients who spill troponin have a markedly increased risk for a more complicated hospital course.” About 10%-25% of patients hospitalized with acute heart failure have recurrent worsening heart failure, and many of these are also the patients who have a spike in their troponin level during initial hospitalization, he noted.
The troponin release in many patients and its association with worse outcomes is a clue that these patients are experiencing an ischemic myocardial event similar to an acute MI, possibly caused by myocardial-wall stretch, Dr. Packer said in an interview.
“If we can reduce this early acute cardiac dilatation, maybe we can reduce myocardial injury, reduce troponin release, and have favorable effects on clinically relevant events both short-term and long-term,” he said. “That’s why in trials [of investigational drugs for acute heart failure] we are treating patients earlier. Before we said we could enroll patients [into acute-treatment trials] within 48 hours of hospital admission. Now we enroll within 16 hours, or within 12 hours. We’ve learned that early intervention is important. That makes acute heart failure a lot more similar to an acute MI.”
European Society recommends faster acute heart failure management
European heart failure specialists have also become convinced that acute heart failure is an emergency that needs a rapid response. In June, the Heart Failure Association of the European Society of Cardiology published new recommendations on the in-hospital management of patients with acute heart failure (Eur J Heart Fail 2015 June;17[6];544-58). In the document, the association’s writing panel said, “The potentially greater benefit of early treatment is of conceptual importance in many cardiovascular presentations (e.g., myocardial infarction). Unfortunately, acute heart failure has not been considered with this regard until recently.” Breaking with the past, the association’s new recommendations now say that “all acute heart failure patients should receive appropriate therapy as early as possible,” an approach that involves starting acute management in the prehospital setting.
One member of the writing group for these recommendations put it more succinctly while speaking at the annual congress of the European Society of Cardiology in London in August: “Time is muscle in acute heart failure,” said Dr. Piotr Ponikowski, professor and heart failure specialist at the Medical University in Wroclaw, Poland. “When a patient has acute coronary syndrome everyone rushes, but we have patients with acute heart failure and no one rushes. We give furosemide, maybe something else, and then we wait and see.” He recommended adhering to a schedule that would have a patient assessed and initially treated within the first hour of hospitalization, and even sooner if treatment could start at the prehospital stage.
Like Dr. Packer, Dr. Ponikowski also lamented the inadequate tools now available for treating acute heart failure and the pressing need to identify better approaches to treatment, especially for selected acute heart failure patients.
“It is too simple to think that one drug or one treatment will help the entire spectrum of acute heart failure patients,” he said. “Our hypothesis is that profiling patients at every step of acute heart failure is crucial.”
He itemized five distinct types of acute heart failure patients based on their precipitating triggers of decompensation:
• Rapid arrhythmia or rhythm disturbance.
• Hypertension emergency.
• Pulmonary embolism.
• Pulmonary infection.
• Mechanical cause of acute heart failure.
“We need to clinically profile” patients into these subgroups to better tailor management, he said.
Another important aspect of patient heterogeneity is that fluid congestion may be less important in many patients compared with fluid redistribution from the splanchnic circulation. This distinction is important because fluid redistribution may be better treated with a vasodilator than with a diuretic, he noted. He voiced hope that two phase III trials now in progress with two unique vasodilator drugs, the TRUE-AHF trial of ularitide, and the RELAX-AHF-2 trial of serelaxin, may identify two new vasodilators with “unique effects” that could potentially launch a new era in management of selected patients with acute heart failure. Dr. Packer, the principal investigator for the ularitide trial, offered similar hope.
The responsiveness of acute heart failure patients to in-hospital treatment may vary depending on what end-organ damage they experience, Dr. Ponikowski said.
This end-organ damage is often an acute process occurring during hospitalization caused by the fluid congestion and redistribution that occurs during acute heart failure, said Dr. Alexandre Mebazaa, professor of anesthesiology and critical care medicine at Lariboisière Hospital in Paris.
“Fluid overload leads to organ dysfunction. In the past, we thought that kidney dysfunction [occurring during acute heart failure] was due to low cardiac output, but we know that dysfunction in the kidney and liver is due to congestion, and diuretics do not remove water from the liver and kidney,” Dr. Mebazaa said in an interview. “Diuretics may remove fluid from vessels, but not from organs. We need new approaches to remove fluid from organs – from the kidney, liver, and lungs” – during acute heart failure. This is another reason why heart failure physicians are excited about the possibility of finding new vasodilators, such a ularitide and serelaxin, that might address the issue of venous congestion in peripheral organs.
Faster management endorsed by U.S. clinicians, too
“We used to think that the reason why patients with acute heart failure were not voiding well and became diuretic resistant was because of poor cardiac output. Now we know that there is a lot of venous congestion with an impact on the liver and kidneys,” agreed Dr. Mariell L. Jessup, professor and medical director of the Penn Heart and Vascular Center at the University of Pennsylvania in Philadelphia. “We’ve begun to appreciate how important venous congestion is in causing high pressures on the right side” of the circulatory system, she said in an interview.
Other U.S. physicians echo the call by Dr. Packer and the European cardiologists for faster treatment of acute heart failure. “I collected data at U.S. hospitals and found it took an average of 22 hours for decompensated heart failure patients to receive treatment,” said Dr. Maria Rosa Costanzo, medical director of the heart failure and pulmonary hypertension program at Advocate Heart Institute in Naperville, Ill. “I have tried to convey the message that these patients must be treated early, and this is associated with better outcomes,” she said in an interview during the HFSA meeting.
“Early treatment means at least two doses of intravenous diuretic in the emergency department. We’ve seen that the two immediate doses can make a big difference, producing shorter lengths of stay in the intensive care unit, fewer rehospitalizations, and fewer deaths,” according to data collected in the ADHERE (Acute Decompensated Heart Failure National Registry), she said. “But this has not yet been picked up in a lot of U.S. practice.” Although the hemodynamic abnormalities that lead up to an acute decompensation event can take several weeks of steady worsening before severe symptoms drive a patient to the hospital, once the patient requires hospitalization “it should be treated as an emergency,” she said.
Dr. Costanzo is a major advocate for using ultrafiltration as a second-line treatment for acute decompensated heart failure patients who do not adequately respond to diuretic treatment, but for the time being, ultrafiltration remains a controversial option that at least some other heart failure physicians do not endorse, and it can involve reimbursement issues as many insurers consider it investigational.
“Try to get the patient decongested within the first 6 hours [after arrival at the hospital] or even sooner, within the first 1-2 hours,” recommended Dr. Christopher M. O’Connor, chief executive officer of Inova Heart and Vascular Institute in Falls Church, Va. He suggested treating patients with a combination of diuretics and vasodilators. “Some people are talking about instituting a performance measure for treating acute heart failure within the first 6 hours,” Dr. O’Connor said in an interview.
Currently, vasodilator treatment is limited to standard agents such as intravenous nitroglycerin, but Dr. O’Connor shared the hope that sometime soon a new vasodilator may be shown effective for acutely decompensated patients. He is a coinvestigator on the TRUE-AHF study of ularitide. “We hope that these new vasodilators, ularitide and serelaxin, will be good complements to diuretics, he said. Dr. O’Connor also recommended that clinicians shy away from using ultrafiltration as a back-up therapy, believing that it was shown ineffective and potentially harmful in results from the CARRESS-HF trial (N Engl J Med 2012 Dec 13;367[24]:2296-304).
But not all heart failure specialists see acute heart failure as a new frontier for early treatment and new drug discovery.
“So much energy has already been spent on acute heart failure with very little return,” said Dr. Clyde W. Yancy, professor and chief of cardiology at Northwestern University in Chicago. “I think that our best opportunities in heart failure are in prevention and in better chronic care. The hospitalized patient is so broad and complex; if we’re looking at how to best spend our resources I think it’s best to focus on prevention,” he said in an interview.
“The hospital experience needs to shift toward better use of systems of care and focus less on the biology. The biggest challenge is how to coordinate all the systems to make sure that patients have access to the resources and can obtain [existing] medications. Patients don’t often have the literacy to understand discharge instructions, and our systems are overwhelmed by trying to have 7-day follow-up visits. Focusing on management of the hospitalized patient does not give us a good return on the investment. There is no question that acute heart failure is an unmet need, but the greater unmet need is prevention and improved chronic care. No single intervention will dramatically change the acute heart failure experience. Focusing on the hospitalization does not offer us management opportunities that are as robust as we once thought,” Dr. Yancy said.
Dr. Packer has been a consultant to 22 companies. Dr. Ponikowski has been a consultant to, speaker for, or has received research grants from 11 companies. Dr. Mebazaa has received speaking honoraria and consulting fees from 11 companies. Dr. Jessup, Dr. Costanzo, and Dr. Yancy had no disclosures. Dr. O’Connor has been a consultant to ResMed, Roche Diagnostics, Cardiorentis, Bayer, and Actelion and has received research grants from Otsuka, ResMed, and Roche Diagnostics.
On Twitter@mitchelzoler
NATIONAL HARBOR, MD. – Acute decompensated heart failure is becoming more of an emergency.
Traditionally, it has been seen as a lumbering event that could be treated at a relatively leisurely pace, but heart failure physicians increasingly see the moment when patients arrive in the hospital with an episode of acute decompensated heart failure as a time-sensitive event that requires rapid intervention in a manner much more akin to an acute MI than to chronic heart failure.
While the tide is slowly shifting to put a premium on rapid treatment to try to decongest acute heart failure patients, the treatment options clinicians have available for these patients often remain inadequate.
“Development of drugs for acutely decompensated heart failure has been extremely difficult. We have done a really horrible job treating this disease,” Dr. Milton Packer said at the HFSA annual scientific meeting.
Treatment of acute heart failure patients has generally focused on relieving dyspnea, but part of the new appreciation of this state as an emergency event involves understanding that the pathology patients have when they reach the hospital is much more global and has profoundly morbid consequences.
“We want more from treatment than for patients to feel a little bit better an hour or two sooner” by relieving dyspnea, said Dr. Packer, professor of medicine and a heart failure specialist at the University of Texas Southwestern Medical Center in Dallas. “In the first 6 hours [of acute heart failure hospitalization], many patients are spilling troponin. We don’t know what this means, but the patients who spill troponin have a markedly increased risk for a more complicated hospital course.” About 10%-25% of patients hospitalized with acute heart failure have recurrent worsening heart failure, and many of these are also the patients who have a spike in their troponin level during initial hospitalization, he noted.
The troponin release in many patients and its association with worse outcomes is a clue that these patients are experiencing an ischemic myocardial event similar to an acute MI, possibly caused by myocardial-wall stretch, Dr. Packer said in an interview.
“If we can reduce this early acute cardiac dilatation, maybe we can reduce myocardial injury, reduce troponin release, and have favorable effects on clinically relevant events both short-term and long-term,” he said. “That’s why in trials [of investigational drugs for acute heart failure] we are treating patients earlier. Before we said we could enroll patients [into acute-treatment trials] within 48 hours of hospital admission. Now we enroll within 16 hours, or within 12 hours. We’ve learned that early intervention is important. That makes acute heart failure a lot more similar to an acute MI.”
European Society recommends faster acute heart failure management
European heart failure specialists have also become convinced that acute heart failure is an emergency that needs a rapid response. In June, the Heart Failure Association of the European Society of Cardiology published new recommendations on the in-hospital management of patients with acute heart failure (Eur J Heart Fail 2015 June;17[6];544-58). In the document, the association’s writing panel said, “The potentially greater benefit of early treatment is of conceptual importance in many cardiovascular presentations (e.g., myocardial infarction). Unfortunately, acute heart failure has not been considered with this regard until recently.” Breaking with the past, the association’s new recommendations now say that “all acute heart failure patients should receive appropriate therapy as early as possible,” an approach that involves starting acute management in the prehospital setting.
One member of the writing group for these recommendations put it more succinctly while speaking at the annual congress of the European Society of Cardiology in London in August: “Time is muscle in acute heart failure,” said Dr. Piotr Ponikowski, professor and heart failure specialist at the Medical University in Wroclaw, Poland. “When a patient has acute coronary syndrome everyone rushes, but we have patients with acute heart failure and no one rushes. We give furosemide, maybe something else, and then we wait and see.” He recommended adhering to a schedule that would have a patient assessed and initially treated within the first hour of hospitalization, and even sooner if treatment could start at the prehospital stage.
Like Dr. Packer, Dr. Ponikowski also lamented the inadequate tools now available for treating acute heart failure and the pressing need to identify better approaches to treatment, especially for selected acute heart failure patients.
“It is too simple to think that one drug or one treatment will help the entire spectrum of acute heart failure patients,” he said. “Our hypothesis is that profiling patients at every step of acute heart failure is crucial.”
He itemized five distinct types of acute heart failure patients based on their precipitating triggers of decompensation:
• Rapid arrhythmia or rhythm disturbance.
• Hypertension emergency.
• Pulmonary embolism.
• Pulmonary infection.
• Mechanical cause of acute heart failure.
“We need to clinically profile” patients into these subgroups to better tailor management, he said.
Another important aspect of patient heterogeneity is that fluid congestion may be less important in many patients compared with fluid redistribution from the splanchnic circulation. This distinction is important because fluid redistribution may be better treated with a vasodilator than with a diuretic, he noted. He voiced hope that two phase III trials now in progress with two unique vasodilator drugs, the TRUE-AHF trial of ularitide, and the RELAX-AHF-2 trial of serelaxin, may identify two new vasodilators with “unique effects” that could potentially launch a new era in management of selected patients with acute heart failure. Dr. Packer, the principal investigator for the ularitide trial, offered similar hope.
The responsiveness of acute heart failure patients to in-hospital treatment may vary depending on what end-organ damage they experience, Dr. Ponikowski said.
This end-organ damage is often an acute process occurring during hospitalization caused by the fluid congestion and redistribution that occurs during acute heart failure, said Dr. Alexandre Mebazaa, professor of anesthesiology and critical care medicine at Lariboisière Hospital in Paris.
“Fluid overload leads to organ dysfunction. In the past, we thought that kidney dysfunction [occurring during acute heart failure] was due to low cardiac output, but we know that dysfunction in the kidney and liver is due to congestion, and diuretics do not remove water from the liver and kidney,” Dr. Mebazaa said in an interview. “Diuretics may remove fluid from vessels, but not from organs. We need new approaches to remove fluid from organs – from the kidney, liver, and lungs” – during acute heart failure. This is another reason why heart failure physicians are excited about the possibility of finding new vasodilators, such a ularitide and serelaxin, that might address the issue of venous congestion in peripheral organs.
Faster management endorsed by U.S. clinicians, too
“We used to think that the reason why patients with acute heart failure were not voiding well and became diuretic resistant was because of poor cardiac output. Now we know that there is a lot of venous congestion with an impact on the liver and kidneys,” agreed Dr. Mariell L. Jessup, professor and medical director of the Penn Heart and Vascular Center at the University of Pennsylvania in Philadelphia. “We’ve begun to appreciate how important venous congestion is in causing high pressures on the right side” of the circulatory system, she said in an interview.
Other U.S. physicians echo the call by Dr. Packer and the European cardiologists for faster treatment of acute heart failure. “I collected data at U.S. hospitals and found it took an average of 22 hours for decompensated heart failure patients to receive treatment,” said Dr. Maria Rosa Costanzo, medical director of the heart failure and pulmonary hypertension program at Advocate Heart Institute in Naperville, Ill. “I have tried to convey the message that these patients must be treated early, and this is associated with better outcomes,” she said in an interview during the HFSA meeting.
“Early treatment means at least two doses of intravenous diuretic in the emergency department. We’ve seen that the two immediate doses can make a big difference, producing shorter lengths of stay in the intensive care unit, fewer rehospitalizations, and fewer deaths,” according to data collected in the ADHERE (Acute Decompensated Heart Failure National Registry), she said. “But this has not yet been picked up in a lot of U.S. practice.” Although the hemodynamic abnormalities that lead up to an acute decompensation event can take several weeks of steady worsening before severe symptoms drive a patient to the hospital, once the patient requires hospitalization “it should be treated as an emergency,” she said.
Dr. Costanzo is a major advocate for using ultrafiltration as a second-line treatment for acute decompensated heart failure patients who do not adequately respond to diuretic treatment, but for the time being, ultrafiltration remains a controversial option that at least some other heart failure physicians do not endorse, and it can involve reimbursement issues as many insurers consider it investigational.
“Try to get the patient decongested within the first 6 hours [after arrival at the hospital] or even sooner, within the first 1-2 hours,” recommended Dr. Christopher M. O’Connor, chief executive officer of Inova Heart and Vascular Institute in Falls Church, Va. He suggested treating patients with a combination of diuretics and vasodilators. “Some people are talking about instituting a performance measure for treating acute heart failure within the first 6 hours,” Dr. O’Connor said in an interview.
Currently, vasodilator treatment is limited to standard agents such as intravenous nitroglycerin, but Dr. O’Connor shared the hope that sometime soon a new vasodilator may be shown effective for acutely decompensated patients. He is a coinvestigator on the TRUE-AHF study of ularitide. “We hope that these new vasodilators, ularitide and serelaxin, will be good complements to diuretics, he said. Dr. O’Connor also recommended that clinicians shy away from using ultrafiltration as a back-up therapy, believing that it was shown ineffective and potentially harmful in results from the CARRESS-HF trial (N Engl J Med 2012 Dec 13;367[24]:2296-304).
But not all heart failure specialists see acute heart failure as a new frontier for early treatment and new drug discovery.
“So much energy has already been spent on acute heart failure with very little return,” said Dr. Clyde W. Yancy, professor and chief of cardiology at Northwestern University in Chicago. “I think that our best opportunities in heart failure are in prevention and in better chronic care. The hospitalized patient is so broad and complex; if we’re looking at how to best spend our resources I think it’s best to focus on prevention,” he said in an interview.
“The hospital experience needs to shift toward better use of systems of care and focus less on the biology. The biggest challenge is how to coordinate all the systems to make sure that patients have access to the resources and can obtain [existing] medications. Patients don’t often have the literacy to understand discharge instructions, and our systems are overwhelmed by trying to have 7-day follow-up visits. Focusing on management of the hospitalized patient does not give us a good return on the investment. There is no question that acute heart failure is an unmet need, but the greater unmet need is prevention and improved chronic care. No single intervention will dramatically change the acute heart failure experience. Focusing on the hospitalization does not offer us management opportunities that are as robust as we once thought,” Dr. Yancy said.
Dr. Packer has been a consultant to 22 companies. Dr. Ponikowski has been a consultant to, speaker for, or has received research grants from 11 companies. Dr. Mebazaa has received speaking honoraria and consulting fees from 11 companies. Dr. Jessup, Dr. Costanzo, and Dr. Yancy had no disclosures. Dr. O’Connor has been a consultant to ResMed, Roche Diagnostics, Cardiorentis, Bayer, and Actelion and has received research grants from Otsuka, ResMed, and Roche Diagnostics.
On Twitter@mitchelzoler
EXPERT ANALYSIS FROM THE HFSA ANNUAL SCIENTIFIC MEETING
Fewer doses of PCV13 could save money – but at what cost?
Streptococcus pneumoniae is the most common bacterial cause of pneumonia, sinusitis, and acute otitis media (AOM). It also causes invasive pneumococcal disease (IPD), such as bacteremia and meningitis, and it is the leading cause of vaccine-preventable death in children younger than 5 years of age. Pneumococcal conjugate vaccines (PCVs) are effective in infants and young children against IPD, non-IPD, and the acquisition of vaccine serotype nasopharyngeal carriage (contagion). PCV7 was licensed and introduced in 2000 on a schedule that matched the schedule of other routine infant immunizations of three primary doses at 2, 4, and 6 months, and a booster at 12-15 months. Later in 2010, PCV13 was licensed on that same “3+1” schedule. Different pneumococcal vaccination schedules are recommended across Europe and other countries, after consideration of the epidemiology, disease burden, immunogenicity of the vaccine, its compatibility with other vaccines, and its cost. The World Health Organization recently updated its PCV policy to support the use of three doses on either 3+0 or 2+1 schedules. Most European countries have adopted the 2+1 schedule used for routine infant immunizations.
In light of the escalating costs of providing current vaccines, and the anticipated need for additional vaccines, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) has convened a working group to evaluate the transition from a 3+1 to a 2+1 schedule for PCV administration to infants and children. This is not a trivial decision. In the United States, cost must be considered in the context of an additional focus on non-IPD disease prevention, especially AOM, where serotypes and immune protection levels differ from IPD. A 2+1 schedule may be effective to prevent IPD, compared with a 3+1 schedule, but its impact on non-IPD may be compromised, especially for AOM, for some serotypes of pneumococci, and for control of nasopharyngeal carriage.
Immunogenicity studies show that antibody responses from a vaccine regimen consisting of two doses in the primary series are less immunogenic, compared with those for a three-dose regimen, yet both regimens are effective for the prevention of IPD. Immunogenicity data that support the use of reduced-dose schedules for most, but not all, vaccine serotypes, were based on IPD. The degree to which higher antibody concentrations are important for protecting against nonbacteremic pneumonia, sinusitis, and AOM, and for preventing nasopharyngeal carriage, is not established.
However, clinical outcomes since the introduction of PCVs indicate that the true threshold will vary by serotype and host and disease condition, with higher concentrations required for certain serotypes, in immunologically less mature hosts, and in mucosal infections like nonbacteremic pneumonia, sinusitis, and AOM, compared with IPD. Also, higher IgG levels clearly are important in protecting against nasopharyngeal colonization, thereby conferring herd immunity, prolonging individual protection, and possibly correlating at the individual level with disease protection. Studies that evaluated the correlation of antibody concentration and protection against nasopharyngeal colonization have shown that a greater than 10-fold higher antibody concentration is needed, compared with levels in blood, to protect against IPD. Similarly protection against AOM require higher levels of antibody than are needed to protect against IPD, as evidenced by the lower efficacy of PCVs against AOM, compared with IPD.
Epidemiology and risk factors differ among countries of the world. Therefore, even among developed countries, there is a need for caution in accepting that what works in one country will work as well in another. For example, attendance at day care is the highest risk factor for both IPD and non-IPD. In the United States, we have many types of day care, including relatively large day care centers, and many infants enter day care at 2 months of age. In other developed countries, the size of day care centers is much smaller, and children may not enter day care until 1 or even 2 years of age. Those differences may have implications for protective efficacy with a reduced-dose vaccine schedule.
Siblings under the age of 8 years are also at significant risk. Again, the family size may differ among developed countries. Breastfeeding is protective for pneumococcal infections. Breastfeeding duration may differ among countries. The theme of this concern is apparent: Even evidence of adequate protection with a reduced-dose schedule in Finland, France, Germany, the United Kingdom, or elsewhere should not be interpreted to be completely applicable to the United States.
Whether reduced-dose schedules can provide equivalent protection against vaccine type IPD equivalent to a 3+1 schedule for all serotypes and for non-IPD when introduced into a national immunization program is unclear. Do we have enough data to inform the decision process, and specifically do we have a clear understanding of the full impact of reduced-dose schedules on non-IPD relative to 3+1? How would you vote?
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Pfizer, which makes PCV vaccine, has funded an investigator-initiated grant and a postmarketing study to Dr. Pichichero’s institution, and he is the primary investigator of both grants.
Streptococcus pneumoniae is the most common bacterial cause of pneumonia, sinusitis, and acute otitis media (AOM). It also causes invasive pneumococcal disease (IPD), such as bacteremia and meningitis, and it is the leading cause of vaccine-preventable death in children younger than 5 years of age. Pneumococcal conjugate vaccines (PCVs) are effective in infants and young children against IPD, non-IPD, and the acquisition of vaccine serotype nasopharyngeal carriage (contagion). PCV7 was licensed and introduced in 2000 on a schedule that matched the schedule of other routine infant immunizations of three primary doses at 2, 4, and 6 months, and a booster at 12-15 months. Later in 2010, PCV13 was licensed on that same “3+1” schedule. Different pneumococcal vaccination schedules are recommended across Europe and other countries, after consideration of the epidemiology, disease burden, immunogenicity of the vaccine, its compatibility with other vaccines, and its cost. The World Health Organization recently updated its PCV policy to support the use of three doses on either 3+0 or 2+1 schedules. Most European countries have adopted the 2+1 schedule used for routine infant immunizations.
In light of the escalating costs of providing current vaccines, and the anticipated need for additional vaccines, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) has convened a working group to evaluate the transition from a 3+1 to a 2+1 schedule for PCV administration to infants and children. This is not a trivial decision. In the United States, cost must be considered in the context of an additional focus on non-IPD disease prevention, especially AOM, where serotypes and immune protection levels differ from IPD. A 2+1 schedule may be effective to prevent IPD, compared with a 3+1 schedule, but its impact on non-IPD may be compromised, especially for AOM, for some serotypes of pneumococci, and for control of nasopharyngeal carriage.
Immunogenicity studies show that antibody responses from a vaccine regimen consisting of two doses in the primary series are less immunogenic, compared with those for a three-dose regimen, yet both regimens are effective for the prevention of IPD. Immunogenicity data that support the use of reduced-dose schedules for most, but not all, vaccine serotypes, were based on IPD. The degree to which higher antibody concentrations are important for protecting against nonbacteremic pneumonia, sinusitis, and AOM, and for preventing nasopharyngeal carriage, is not established.
However, clinical outcomes since the introduction of PCVs indicate that the true threshold will vary by serotype and host and disease condition, with higher concentrations required for certain serotypes, in immunologically less mature hosts, and in mucosal infections like nonbacteremic pneumonia, sinusitis, and AOM, compared with IPD. Also, higher IgG levels clearly are important in protecting against nasopharyngeal colonization, thereby conferring herd immunity, prolonging individual protection, and possibly correlating at the individual level with disease protection. Studies that evaluated the correlation of antibody concentration and protection against nasopharyngeal colonization have shown that a greater than 10-fold higher antibody concentration is needed, compared with levels in blood, to protect against IPD. Similarly protection against AOM require higher levels of antibody than are needed to protect against IPD, as evidenced by the lower efficacy of PCVs against AOM, compared with IPD.
Epidemiology and risk factors differ among countries of the world. Therefore, even among developed countries, there is a need for caution in accepting that what works in one country will work as well in another. For example, attendance at day care is the highest risk factor for both IPD and non-IPD. In the United States, we have many types of day care, including relatively large day care centers, and many infants enter day care at 2 months of age. In other developed countries, the size of day care centers is much smaller, and children may not enter day care until 1 or even 2 years of age. Those differences may have implications for protective efficacy with a reduced-dose vaccine schedule.
Siblings under the age of 8 years are also at significant risk. Again, the family size may differ among developed countries. Breastfeeding is protective for pneumococcal infections. Breastfeeding duration may differ among countries. The theme of this concern is apparent: Even evidence of adequate protection with a reduced-dose schedule in Finland, France, Germany, the United Kingdom, or elsewhere should not be interpreted to be completely applicable to the United States.
Whether reduced-dose schedules can provide equivalent protection against vaccine type IPD equivalent to a 3+1 schedule for all serotypes and for non-IPD when introduced into a national immunization program is unclear. Do we have enough data to inform the decision process, and specifically do we have a clear understanding of the full impact of reduced-dose schedules on non-IPD relative to 3+1? How would you vote?
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Pfizer, which makes PCV vaccine, has funded an investigator-initiated grant and a postmarketing study to Dr. Pichichero’s institution, and he is the primary investigator of both grants.
Streptococcus pneumoniae is the most common bacterial cause of pneumonia, sinusitis, and acute otitis media (AOM). It also causes invasive pneumococcal disease (IPD), such as bacteremia and meningitis, and it is the leading cause of vaccine-preventable death in children younger than 5 years of age. Pneumococcal conjugate vaccines (PCVs) are effective in infants and young children against IPD, non-IPD, and the acquisition of vaccine serotype nasopharyngeal carriage (contagion). PCV7 was licensed and introduced in 2000 on a schedule that matched the schedule of other routine infant immunizations of three primary doses at 2, 4, and 6 months, and a booster at 12-15 months. Later in 2010, PCV13 was licensed on that same “3+1” schedule. Different pneumococcal vaccination schedules are recommended across Europe and other countries, after consideration of the epidemiology, disease burden, immunogenicity of the vaccine, its compatibility with other vaccines, and its cost. The World Health Organization recently updated its PCV policy to support the use of three doses on either 3+0 or 2+1 schedules. Most European countries have adopted the 2+1 schedule used for routine infant immunizations.
In light of the escalating costs of providing current vaccines, and the anticipated need for additional vaccines, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) has convened a working group to evaluate the transition from a 3+1 to a 2+1 schedule for PCV administration to infants and children. This is not a trivial decision. In the United States, cost must be considered in the context of an additional focus on non-IPD disease prevention, especially AOM, where serotypes and immune protection levels differ from IPD. A 2+1 schedule may be effective to prevent IPD, compared with a 3+1 schedule, but its impact on non-IPD may be compromised, especially for AOM, for some serotypes of pneumococci, and for control of nasopharyngeal carriage.
Immunogenicity studies show that antibody responses from a vaccine regimen consisting of two doses in the primary series are less immunogenic, compared with those for a three-dose regimen, yet both regimens are effective for the prevention of IPD. Immunogenicity data that support the use of reduced-dose schedules for most, but not all, vaccine serotypes, were based on IPD. The degree to which higher antibody concentrations are important for protecting against nonbacteremic pneumonia, sinusitis, and AOM, and for preventing nasopharyngeal carriage, is not established.
However, clinical outcomes since the introduction of PCVs indicate that the true threshold will vary by serotype and host and disease condition, with higher concentrations required for certain serotypes, in immunologically less mature hosts, and in mucosal infections like nonbacteremic pneumonia, sinusitis, and AOM, compared with IPD. Also, higher IgG levels clearly are important in protecting against nasopharyngeal colonization, thereby conferring herd immunity, prolonging individual protection, and possibly correlating at the individual level with disease protection. Studies that evaluated the correlation of antibody concentration and protection against nasopharyngeal colonization have shown that a greater than 10-fold higher antibody concentration is needed, compared with levels in blood, to protect against IPD. Similarly protection against AOM require higher levels of antibody than are needed to protect against IPD, as evidenced by the lower efficacy of PCVs against AOM, compared with IPD.
Epidemiology and risk factors differ among countries of the world. Therefore, even among developed countries, there is a need for caution in accepting that what works in one country will work as well in another. For example, attendance at day care is the highest risk factor for both IPD and non-IPD. In the United States, we have many types of day care, including relatively large day care centers, and many infants enter day care at 2 months of age. In other developed countries, the size of day care centers is much smaller, and children may not enter day care until 1 or even 2 years of age. Those differences may have implications for protective efficacy with a reduced-dose vaccine schedule.
Siblings under the age of 8 years are also at significant risk. Again, the family size may differ among developed countries. Breastfeeding is protective for pneumococcal infections. Breastfeeding duration may differ among countries. The theme of this concern is apparent: Even evidence of adequate protection with a reduced-dose schedule in Finland, France, Germany, the United Kingdom, or elsewhere should not be interpreted to be completely applicable to the United States.
Whether reduced-dose schedules can provide equivalent protection against vaccine type IPD equivalent to a 3+1 schedule for all serotypes and for non-IPD when introduced into a national immunization program is unclear. Do we have enough data to inform the decision process, and specifically do we have a clear understanding of the full impact of reduced-dose schedules on non-IPD relative to 3+1? How would you vote?
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Pfizer, which makes PCV vaccine, has funded an investigator-initiated grant and a postmarketing study to Dr. Pichichero’s institution, and he is the primary investigator of both grants.
CHEST: Yoga performs like pulmonary rehab for COPD patients
MONTREAL – A structured regimen of regular yoga exercises was as effective as a standard pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease for improving lung function, exercise tolerance, dyspnea severity, and quality of life in a single-center, randomized comparison of the two strategies with 60 patients.
In addition, chronic obstructive pulmonary disease (COPD) patients had a higher level of acceptance of yoga and were more comfortable doing it, compared with standard pulmonary rehabilitation, and it is a cost-effective approach given the minimal equipment required, Dr. Randeep Guleria said at the annual meeting of the American College of Chest Physicians.
“Patients with difficulty walking, osteoarthritis, knee problems, or unable to do exercises like cycling or treadmill found yoga to be much more acceptable,” said Dr. Guleria in an interview. Acceptance of yoga was also higher than standard rehabilitation among patients with more severe COPD, said Dr. Guleria, professor and head of the department of pulmonary medicine and sleep disorders at the All India Institute of Medical Sciences in New Delhi.
“I think that yoga could be a very valuable adjunct” to pulmonary rehabilitation in COPD patients, commented Dr. Roger S. Goldstein, director of the divisional program in respiratory rehabilitation at the University of Toronto. Dr. Goldstein speculated that even better than comparing yoga against conventional pulmonary rehabilitation would be a study that compared a combined yoga plus rehabilitation program against standard rehabilitation alone. Yoga is “a tremendous opportunity,” he said.
The 12-week study enrolled 60 patients who averaged 56 years old who had been diagnosed with COPD for an average of 8 years. Just under a third of the patients had moderate COPD, 42% had severe COPD, and 28% had very severe COPD.
Dr. Guleria and his associates randomized 30 patients into a yoga program that included 4 weeks of biweekly 1-hour sessions that instructed patients in a series of specially designed yoga exercises. That was followed by 8 weeks during which patients were mostly left to perform their learned exercises on their own, but with a supervised session once every 2 weeks. The other 30 patients participated in a standard pulmonary rehabilitation program for 12 weeks.
The researchers measured several parameters at baseline and after 12 weeks, including two measures of dyspnea severity, 6-minute walk distance, a quality of life assessment, and two serum markers of inflammation, C-reactive protein and interleukin 6.
Both interventions resulted in modest but statistically significant improvements, such as increases in 6-minute walk distance and a reduced modified Borg scale assessment. The Borg scale score fell from an average of 1.5 at baseline to 1.0 after 12 weeks in the yoga patients, and from an average 3.0 at baseline to 0.5 after 12 weeks in the rehabilitation patients.
A score that measured total quality of life improved by an average of 32% in the yoga patients and by an average of 21% in the rehabilitation patients, changes that approached statistical significance in both subgroups.
Comparing the assessment measures after 12 weeks in both arms of the study showed no statistically significant between-group differences, Dr. Guleria reported.
The researchers commissioned a specially designed yoga program from a professional yoga instructor who had been briefed about COPD. The yoga exercises included physical postures, breathing technique, and meditation and relaxation. The pulmonary rehabilitation program included patient education, upper and lower limb exercises, and breathing exercises.
Dr. Guleria said he believed the opportunity exists to further modify the yoga program to better optimize its potential to benefit COPD patients.
Dr. Guleria had no relevant disclosures. Dr. Goldstein had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
MONTREAL – A structured regimen of regular yoga exercises was as effective as a standard pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease for improving lung function, exercise tolerance, dyspnea severity, and quality of life in a single-center, randomized comparison of the two strategies with 60 patients.
In addition, chronic obstructive pulmonary disease (COPD) patients had a higher level of acceptance of yoga and were more comfortable doing it, compared with standard pulmonary rehabilitation, and it is a cost-effective approach given the minimal equipment required, Dr. Randeep Guleria said at the annual meeting of the American College of Chest Physicians.
“Patients with difficulty walking, osteoarthritis, knee problems, or unable to do exercises like cycling or treadmill found yoga to be much more acceptable,” said Dr. Guleria in an interview. Acceptance of yoga was also higher than standard rehabilitation among patients with more severe COPD, said Dr. Guleria, professor and head of the department of pulmonary medicine and sleep disorders at the All India Institute of Medical Sciences in New Delhi.
“I think that yoga could be a very valuable adjunct” to pulmonary rehabilitation in COPD patients, commented Dr. Roger S. Goldstein, director of the divisional program in respiratory rehabilitation at the University of Toronto. Dr. Goldstein speculated that even better than comparing yoga against conventional pulmonary rehabilitation would be a study that compared a combined yoga plus rehabilitation program against standard rehabilitation alone. Yoga is “a tremendous opportunity,” he said.
The 12-week study enrolled 60 patients who averaged 56 years old who had been diagnosed with COPD for an average of 8 years. Just under a third of the patients had moderate COPD, 42% had severe COPD, and 28% had very severe COPD.
Dr. Guleria and his associates randomized 30 patients into a yoga program that included 4 weeks of biweekly 1-hour sessions that instructed patients in a series of specially designed yoga exercises. That was followed by 8 weeks during which patients were mostly left to perform their learned exercises on their own, but with a supervised session once every 2 weeks. The other 30 patients participated in a standard pulmonary rehabilitation program for 12 weeks.
The researchers measured several parameters at baseline and after 12 weeks, including two measures of dyspnea severity, 6-minute walk distance, a quality of life assessment, and two serum markers of inflammation, C-reactive protein and interleukin 6.
Both interventions resulted in modest but statistically significant improvements, such as increases in 6-minute walk distance and a reduced modified Borg scale assessment. The Borg scale score fell from an average of 1.5 at baseline to 1.0 after 12 weeks in the yoga patients, and from an average 3.0 at baseline to 0.5 after 12 weeks in the rehabilitation patients.
A score that measured total quality of life improved by an average of 32% in the yoga patients and by an average of 21% in the rehabilitation patients, changes that approached statistical significance in both subgroups.
Comparing the assessment measures after 12 weeks in both arms of the study showed no statistically significant between-group differences, Dr. Guleria reported.
The researchers commissioned a specially designed yoga program from a professional yoga instructor who had been briefed about COPD. The yoga exercises included physical postures, breathing technique, and meditation and relaxation. The pulmonary rehabilitation program included patient education, upper and lower limb exercises, and breathing exercises.
Dr. Guleria said he believed the opportunity exists to further modify the yoga program to better optimize its potential to benefit COPD patients.
Dr. Guleria had no relevant disclosures. Dr. Goldstein had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
MONTREAL – A structured regimen of regular yoga exercises was as effective as a standard pulmonary rehabilitation program in patients with chronic obstructive pulmonary disease for improving lung function, exercise tolerance, dyspnea severity, and quality of life in a single-center, randomized comparison of the two strategies with 60 patients.
In addition, chronic obstructive pulmonary disease (COPD) patients had a higher level of acceptance of yoga and were more comfortable doing it, compared with standard pulmonary rehabilitation, and it is a cost-effective approach given the minimal equipment required, Dr. Randeep Guleria said at the annual meeting of the American College of Chest Physicians.
“Patients with difficulty walking, osteoarthritis, knee problems, or unable to do exercises like cycling or treadmill found yoga to be much more acceptable,” said Dr. Guleria in an interview. Acceptance of yoga was also higher than standard rehabilitation among patients with more severe COPD, said Dr. Guleria, professor and head of the department of pulmonary medicine and sleep disorders at the All India Institute of Medical Sciences in New Delhi.
“I think that yoga could be a very valuable adjunct” to pulmonary rehabilitation in COPD patients, commented Dr. Roger S. Goldstein, director of the divisional program in respiratory rehabilitation at the University of Toronto. Dr. Goldstein speculated that even better than comparing yoga against conventional pulmonary rehabilitation would be a study that compared a combined yoga plus rehabilitation program against standard rehabilitation alone. Yoga is “a tremendous opportunity,” he said.
The 12-week study enrolled 60 patients who averaged 56 years old who had been diagnosed with COPD for an average of 8 years. Just under a third of the patients had moderate COPD, 42% had severe COPD, and 28% had very severe COPD.
Dr. Guleria and his associates randomized 30 patients into a yoga program that included 4 weeks of biweekly 1-hour sessions that instructed patients in a series of specially designed yoga exercises. That was followed by 8 weeks during which patients were mostly left to perform their learned exercises on their own, but with a supervised session once every 2 weeks. The other 30 patients participated in a standard pulmonary rehabilitation program for 12 weeks.
The researchers measured several parameters at baseline and after 12 weeks, including two measures of dyspnea severity, 6-minute walk distance, a quality of life assessment, and two serum markers of inflammation, C-reactive protein and interleukin 6.
Both interventions resulted in modest but statistically significant improvements, such as increases in 6-minute walk distance and a reduced modified Borg scale assessment. The Borg scale score fell from an average of 1.5 at baseline to 1.0 after 12 weeks in the yoga patients, and from an average 3.0 at baseline to 0.5 after 12 weeks in the rehabilitation patients.
A score that measured total quality of life improved by an average of 32% in the yoga patients and by an average of 21% in the rehabilitation patients, changes that approached statistical significance in both subgroups.
Comparing the assessment measures after 12 weeks in both arms of the study showed no statistically significant between-group differences, Dr. Guleria reported.
The researchers commissioned a specially designed yoga program from a professional yoga instructor who had been briefed about COPD. The yoga exercises included physical postures, breathing technique, and meditation and relaxation. The pulmonary rehabilitation program included patient education, upper and lower limb exercises, and breathing exercises.
Dr. Guleria said he believed the opportunity exists to further modify the yoga program to better optimize its potential to benefit COPD patients.
Dr. Guleria had no relevant disclosures. Dr. Goldstein had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
AT CHEST 2015
Key clinical point: COPD patients randomized to a 12-week program of yoga exercises had improvements similar to patients randomized to a standard pulmonary rehabilitation program.
Major finding: Average total quality of life scores improved by a relative 32% with yoga and 21% with standard rehabilitation.
Data source: Single-center, prospective, randomized trial with 60 patients.
Disclosures: Dr. Guleria had no relevant disclosures. Dr. Goldstein had no relevant disclosures.
CHEST: Oral solithromycin shows pneumonia pivotal-trial efficacy
MONTREAL – A new, next-generation macrolide, solithromycin, showed safety and efficacy as a once-daily oral agent that was noninferior to the comparator oral antibiotic, the fluoroquinolone moxifloxacin, in a phase III trial.
Macrolide resistance among strains of Streptococcus pneumoniae that cause many U.S. cases of severe community-acquired pneumonia has become common, complicating treatment of this common infection with a macrolide, Dr. Carlos M. Barrera explained at the annual meeting of the American College of Chest Physicians.
The SOLITAIRE-ORAL (Efficacy and Safety Study of Oral Solithromycin [CEM-101] Compared to Oral Moxifloxacin in Treatment of Patients With Community-Acquired Bacterial Pneumonia) trial enrolled 860 patients with moderate to moderately severe community-acquired pneumonia.
About half of the patients enrolled in the trial underwent microbiologic assessment of their infecting pathogen, and about 40% of cases in each treatment arm had infections caused by S. pneumoniae. In this subgroup, the 5-day regimen of solithromycin tested in the study succeeded in clearing the infection in 89% of patients, comparable to the 83% success rate achieved with a 7-day course of moxifloxacin (Avelox), said Dr. Barrera, a pulmonologist who practices in Miami.
The study’s primary endpoint for Food and Drug Administration approval of solithromycin was early clinical response, defined as an improvement in at least two listed symptoms at 72 hours after onset of treatment. That endpoint occurred in 78% of patients enrolled in each of the two arms of the study.
The data make solithromycin look like a promising way to once again have a macrolide available for empiric oral treatment of more severe community-acquired pneumonia, pending full peer review of the data, commented Dr. Muthiah P. Muthiah, a pulmonologist at the University of Tennessee Health Science Center in Memphis.
“A couple of decades ago, you could comfortably treat a patient with severe community-acquired pneumonia with a macrolide, but you can’t do that anymore,” Dr. Muthiah said in an interview.
If the newly reported data on oral solithromycin hold up under further review, it would mean that solithromycin was as effective as a potent quinolone, which remains an effective monotherapy for community-acquired pneumonia in patients who do not require treatment in an intensive care unit, Dr. Muthiah noted.
A companion study, SOLITAIRE-IV, is a phase III pivotal trial assessing the safety and efficacy of solithromycin when begun intravenously for treating community-acquired pneumonia, followed by a switch to oral dosing, in comparison with intravenous followed by oral treatment with moxifloxacin.
Once those data are fully collected and analyzed, the company will submit the information from both trials to the FDA, said Dr. David Oldach, chief medical officer for Cempra.
Results from the intravenous trial, reported in a preliminary way by Cempra Oct. 16 in a press release, showed that the solithromycin treatment regimen tested in SOLITAIRE-IV met its noninferiority targets, compared with moxifloxacin. The safety results, however, showed that solithromycin produced a higher number of patients with a liver-enzyme elevation, compared with patients treated with moxifloxacin.
In SOLITAIRE-IV, Cempra reported that grade 3 increase in levels of alanine transaminase (ALT) occurred in 8% of patients on solithromycin and in 3% of patients on moxifloxacin. Grade 4 increases in ALT occurred in less than 1% of patients in both treatment arms.
In the current, orally administered trial, grade 3 ALT increases occurred in 5% of patients treated with solithromycin and in 2% of patients treated with moxifloxacin, Dr. Barrera reported. Grade 4 ALT increases occurred in 0.5% of patients treated with solithromycin and in 1.2% of those treated with moxifloxacin. No patients in either arm developed an elevation of both ALT and bilirubin, and the ALT increases seen were reversible and asymptomatic, Dr. Barrera said.
By other assessments, the safety profiles of solithromycin and moxifloxacin were similar: 7% of patients on solithromycin and 6% on moxifloxacin had a serious adverse event, and 4% of patients in each study arm discontinued treatment because of an adverse event.
SOLITAIRE-ORAL was sponsored by Cempra, the company developing solithromycin. Dr. Barrera has received research funding from Cempra. Dr. Muthiah had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
MONTREAL – A new, next-generation macrolide, solithromycin, showed safety and efficacy as a once-daily oral agent that was noninferior to the comparator oral antibiotic, the fluoroquinolone moxifloxacin, in a phase III trial.
Macrolide resistance among strains of Streptococcus pneumoniae that cause many U.S. cases of severe community-acquired pneumonia has become common, complicating treatment of this common infection with a macrolide, Dr. Carlos M. Barrera explained at the annual meeting of the American College of Chest Physicians.
The SOLITAIRE-ORAL (Efficacy and Safety Study of Oral Solithromycin [CEM-101] Compared to Oral Moxifloxacin in Treatment of Patients With Community-Acquired Bacterial Pneumonia) trial enrolled 860 patients with moderate to moderately severe community-acquired pneumonia.
About half of the patients enrolled in the trial underwent microbiologic assessment of their infecting pathogen, and about 40% of cases in each treatment arm had infections caused by S. pneumoniae. In this subgroup, the 5-day regimen of solithromycin tested in the study succeeded in clearing the infection in 89% of patients, comparable to the 83% success rate achieved with a 7-day course of moxifloxacin (Avelox), said Dr. Barrera, a pulmonologist who practices in Miami.
The study’s primary endpoint for Food and Drug Administration approval of solithromycin was early clinical response, defined as an improvement in at least two listed symptoms at 72 hours after onset of treatment. That endpoint occurred in 78% of patients enrolled in each of the two arms of the study.
The data make solithromycin look like a promising way to once again have a macrolide available for empiric oral treatment of more severe community-acquired pneumonia, pending full peer review of the data, commented Dr. Muthiah P. Muthiah, a pulmonologist at the University of Tennessee Health Science Center in Memphis.
“A couple of decades ago, you could comfortably treat a patient with severe community-acquired pneumonia with a macrolide, but you can’t do that anymore,” Dr. Muthiah said in an interview.
If the newly reported data on oral solithromycin hold up under further review, it would mean that solithromycin was as effective as a potent quinolone, which remains an effective monotherapy for community-acquired pneumonia in patients who do not require treatment in an intensive care unit, Dr. Muthiah noted.
A companion study, SOLITAIRE-IV, is a phase III pivotal trial assessing the safety and efficacy of solithromycin when begun intravenously for treating community-acquired pneumonia, followed by a switch to oral dosing, in comparison with intravenous followed by oral treatment with moxifloxacin.
Once those data are fully collected and analyzed, the company will submit the information from both trials to the FDA, said Dr. David Oldach, chief medical officer for Cempra.
Results from the intravenous trial, reported in a preliminary way by Cempra Oct. 16 in a press release, showed that the solithromycin treatment regimen tested in SOLITAIRE-IV met its noninferiority targets, compared with moxifloxacin. The safety results, however, showed that solithromycin produced a higher number of patients with a liver-enzyme elevation, compared with patients treated with moxifloxacin.
In SOLITAIRE-IV, Cempra reported that grade 3 increase in levels of alanine transaminase (ALT) occurred in 8% of patients on solithromycin and in 3% of patients on moxifloxacin. Grade 4 increases in ALT occurred in less than 1% of patients in both treatment arms.
In the current, orally administered trial, grade 3 ALT increases occurred in 5% of patients treated with solithromycin and in 2% of patients treated with moxifloxacin, Dr. Barrera reported. Grade 4 ALT increases occurred in 0.5% of patients treated with solithromycin and in 1.2% of those treated with moxifloxacin. No patients in either arm developed an elevation of both ALT and bilirubin, and the ALT increases seen were reversible and asymptomatic, Dr. Barrera said.
By other assessments, the safety profiles of solithromycin and moxifloxacin were similar: 7% of patients on solithromycin and 6% on moxifloxacin had a serious adverse event, and 4% of patients in each study arm discontinued treatment because of an adverse event.
SOLITAIRE-ORAL was sponsored by Cempra, the company developing solithromycin. Dr. Barrera has received research funding from Cempra. Dr. Muthiah had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
MONTREAL – A new, next-generation macrolide, solithromycin, showed safety and efficacy as a once-daily oral agent that was noninferior to the comparator oral antibiotic, the fluoroquinolone moxifloxacin, in a phase III trial.
Macrolide resistance among strains of Streptococcus pneumoniae that cause many U.S. cases of severe community-acquired pneumonia has become common, complicating treatment of this common infection with a macrolide, Dr. Carlos M. Barrera explained at the annual meeting of the American College of Chest Physicians.
The SOLITAIRE-ORAL (Efficacy and Safety Study of Oral Solithromycin [CEM-101] Compared to Oral Moxifloxacin in Treatment of Patients With Community-Acquired Bacterial Pneumonia) trial enrolled 860 patients with moderate to moderately severe community-acquired pneumonia.
About half of the patients enrolled in the trial underwent microbiologic assessment of their infecting pathogen, and about 40% of cases in each treatment arm had infections caused by S. pneumoniae. In this subgroup, the 5-day regimen of solithromycin tested in the study succeeded in clearing the infection in 89% of patients, comparable to the 83% success rate achieved with a 7-day course of moxifloxacin (Avelox), said Dr. Barrera, a pulmonologist who practices in Miami.
The study’s primary endpoint for Food and Drug Administration approval of solithromycin was early clinical response, defined as an improvement in at least two listed symptoms at 72 hours after onset of treatment. That endpoint occurred in 78% of patients enrolled in each of the two arms of the study.
The data make solithromycin look like a promising way to once again have a macrolide available for empiric oral treatment of more severe community-acquired pneumonia, pending full peer review of the data, commented Dr. Muthiah P. Muthiah, a pulmonologist at the University of Tennessee Health Science Center in Memphis.
“A couple of decades ago, you could comfortably treat a patient with severe community-acquired pneumonia with a macrolide, but you can’t do that anymore,” Dr. Muthiah said in an interview.
If the newly reported data on oral solithromycin hold up under further review, it would mean that solithromycin was as effective as a potent quinolone, which remains an effective monotherapy for community-acquired pneumonia in patients who do not require treatment in an intensive care unit, Dr. Muthiah noted.
A companion study, SOLITAIRE-IV, is a phase III pivotal trial assessing the safety and efficacy of solithromycin when begun intravenously for treating community-acquired pneumonia, followed by a switch to oral dosing, in comparison with intravenous followed by oral treatment with moxifloxacin.
Once those data are fully collected and analyzed, the company will submit the information from both trials to the FDA, said Dr. David Oldach, chief medical officer for Cempra.
Results from the intravenous trial, reported in a preliminary way by Cempra Oct. 16 in a press release, showed that the solithromycin treatment regimen tested in SOLITAIRE-IV met its noninferiority targets, compared with moxifloxacin. The safety results, however, showed that solithromycin produced a higher number of patients with a liver-enzyme elevation, compared with patients treated with moxifloxacin.
In SOLITAIRE-IV, Cempra reported that grade 3 increase in levels of alanine transaminase (ALT) occurred in 8% of patients on solithromycin and in 3% of patients on moxifloxacin. Grade 4 increases in ALT occurred in less than 1% of patients in both treatment arms.
In the current, orally administered trial, grade 3 ALT increases occurred in 5% of patients treated with solithromycin and in 2% of patients treated with moxifloxacin, Dr. Barrera reported. Grade 4 ALT increases occurred in 0.5% of patients treated with solithromycin and in 1.2% of those treated with moxifloxacin. No patients in either arm developed an elevation of both ALT and bilirubin, and the ALT increases seen were reversible and asymptomatic, Dr. Barrera said.
By other assessments, the safety profiles of solithromycin and moxifloxacin were similar: 7% of patients on solithromycin and 6% on moxifloxacin had a serious adverse event, and 4% of patients in each study arm discontinued treatment because of an adverse event.
SOLITAIRE-ORAL was sponsored by Cempra, the company developing solithromycin. Dr. Barrera has received research funding from Cempra. Dr. Muthiah had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT CHEST 2015
Key clinical point: A next-generation, orally administered macrolide, solithromycin, showed efficacy that was noninferior to moxifloxacin against moderate to moderately severe community-acquired pneumonia in a phase III pivotal trial.
Major finding: Both solithromycin and moxifloxacin produced a 78% early clinical response rate, the study’s primary endpoint for Food and Drug Administration approval.
Data source: SOLITAIRE-ORAL, a multicenter, international phase III trial involving 860 patients with community-acquired pneumonia.
Disclosures: SOLITAIRE-ORAL was sponsored by Cempra, the company developing solithromycin. Dr. Barrera has received research funding from Cempra. Dr. Muthiah had no disclosures.
LABAs show no benefit over tiotropium in black patients with asthma
Black adults with asthma did not see significant differences in time to exacerbation after adding a long-acting beta-agonist to their inhaled corticosteroid treatment, compared with adding tiotropium, according to results from a randomized, open-label trial.
Moreover, genetic variations were not associated with differences in treatment response.
The study, published online Oct. 27 in JAMA, enrolled 1,070 patients already taking or eligible for combination therapy with inhaled corticosteroids (ICS). More than 75% of patients were women, and the mean age was 45 years.
Dr. Michael E. Wechsler of Brigham and Women’s Hospital, Boston, and colleagues carried out their research at 20 primary care sites in the United States, with information on variation in ADRB2 Arg16Gly alleles captured (JAMA 2015 Oct 27;314[16]:1720-30). Patients were followed an average of 310 days.
Of 538 patients randomized to salmeterol or formoterol plus ICS, the mean number of exacerbations was 0.42 per person-year, compared with 0.37 per person-year in the 532 patients of the tiotropium plus ICS group (rate ratio, 0.90; P = .31).
The likelihood of being exacerbation free at 1 year was 74% for a long-acting beta-agonist (LABA) plus ICS, vs. 75.7% for tiotropium plus ICS (hazard ratio, 0.91; P = .47).
Other measures – including patient-reported outcomes, spirometry, rescue medication use, asthma deteriorations, and adverse events – did not differ significantly between treatment assignments. When stratified by genotype, there were no significant differences in hazard ratios for time to first exacerbation, mean number of exacerbations, or lung function at 6, 12, or 18 months.
“The study was performed in a population that bears a disproportionate burden of asthma morbidity and in whom questions have been raised about the relative efficacy and safety of LABAs,” Dr. Wechsler and colleagues wrote in their analysis.
“Because questions have been raised about whether efficacy studies correctly capture all the causes of morbidity, we conducted a clinical effectiveness study primarily in practicing physician offices,” the authors explained. “We used an outcome important to patients, physicians, and policy makers – asthma exacerbations.”
The investigators acknowledged as limitations of their study its open-label design and that asthma diagnoses were made by community physicians with no required objective testing. In addition, the study’s high discontinuation rate and poor medicine adherence were roughly equal between treatment groups and reflective of real-world practice, they noted.
The Agency for Heathcare Research and Quality funded the study. Dr. Wechsler and several coauthors disclosed consultant fees from multiple pharmaceutical manufacturers.
Black adults with asthma did not see significant differences in time to exacerbation after adding a long-acting beta-agonist to their inhaled corticosteroid treatment, compared with adding tiotropium, according to results from a randomized, open-label trial.
Moreover, genetic variations were not associated with differences in treatment response.
The study, published online Oct. 27 in JAMA, enrolled 1,070 patients already taking or eligible for combination therapy with inhaled corticosteroids (ICS). More than 75% of patients were women, and the mean age was 45 years.
Dr. Michael E. Wechsler of Brigham and Women’s Hospital, Boston, and colleagues carried out their research at 20 primary care sites in the United States, with information on variation in ADRB2 Arg16Gly alleles captured (JAMA 2015 Oct 27;314[16]:1720-30). Patients were followed an average of 310 days.
Of 538 patients randomized to salmeterol or formoterol plus ICS, the mean number of exacerbations was 0.42 per person-year, compared with 0.37 per person-year in the 532 patients of the tiotropium plus ICS group (rate ratio, 0.90; P = .31).
The likelihood of being exacerbation free at 1 year was 74% for a long-acting beta-agonist (LABA) plus ICS, vs. 75.7% for tiotropium plus ICS (hazard ratio, 0.91; P = .47).
Other measures – including patient-reported outcomes, spirometry, rescue medication use, asthma deteriorations, and adverse events – did not differ significantly between treatment assignments. When stratified by genotype, there were no significant differences in hazard ratios for time to first exacerbation, mean number of exacerbations, or lung function at 6, 12, or 18 months.
“The study was performed in a population that bears a disproportionate burden of asthma morbidity and in whom questions have been raised about the relative efficacy and safety of LABAs,” Dr. Wechsler and colleagues wrote in their analysis.
“Because questions have been raised about whether efficacy studies correctly capture all the causes of morbidity, we conducted a clinical effectiveness study primarily in practicing physician offices,” the authors explained. “We used an outcome important to patients, physicians, and policy makers – asthma exacerbations.”
The investigators acknowledged as limitations of their study its open-label design and that asthma diagnoses were made by community physicians with no required objective testing. In addition, the study’s high discontinuation rate and poor medicine adherence were roughly equal between treatment groups and reflective of real-world practice, they noted.
The Agency for Heathcare Research and Quality funded the study. Dr. Wechsler and several coauthors disclosed consultant fees from multiple pharmaceutical manufacturers.
Black adults with asthma did not see significant differences in time to exacerbation after adding a long-acting beta-agonist to their inhaled corticosteroid treatment, compared with adding tiotropium, according to results from a randomized, open-label trial.
Moreover, genetic variations were not associated with differences in treatment response.
The study, published online Oct. 27 in JAMA, enrolled 1,070 patients already taking or eligible for combination therapy with inhaled corticosteroids (ICS). More than 75% of patients were women, and the mean age was 45 years.
Dr. Michael E. Wechsler of Brigham and Women’s Hospital, Boston, and colleagues carried out their research at 20 primary care sites in the United States, with information on variation in ADRB2 Arg16Gly alleles captured (JAMA 2015 Oct 27;314[16]:1720-30). Patients were followed an average of 310 days.
Of 538 patients randomized to salmeterol or formoterol plus ICS, the mean number of exacerbations was 0.42 per person-year, compared with 0.37 per person-year in the 532 patients of the tiotropium plus ICS group (rate ratio, 0.90; P = .31).
The likelihood of being exacerbation free at 1 year was 74% for a long-acting beta-agonist (LABA) plus ICS, vs. 75.7% for tiotropium plus ICS (hazard ratio, 0.91; P = .47).
Other measures – including patient-reported outcomes, spirometry, rescue medication use, asthma deteriorations, and adverse events – did not differ significantly between treatment assignments. When stratified by genotype, there were no significant differences in hazard ratios for time to first exacerbation, mean number of exacerbations, or lung function at 6, 12, or 18 months.
“The study was performed in a population that bears a disproportionate burden of asthma morbidity and in whom questions have been raised about the relative efficacy and safety of LABAs,” Dr. Wechsler and colleagues wrote in their analysis.
“Because questions have been raised about whether efficacy studies correctly capture all the causes of morbidity, we conducted a clinical effectiveness study primarily in practicing physician offices,” the authors explained. “We used an outcome important to patients, physicians, and policy makers – asthma exacerbations.”
The investigators acknowledged as limitations of their study its open-label design and that asthma diagnoses were made by community physicians with no required objective testing. In addition, the study’s high discontinuation rate and poor medicine adherence were roughly equal between treatment groups and reflective of real-world practice, they noted.
The Agency for Heathcare Research and Quality funded the study. Dr. Wechsler and several coauthors disclosed consultant fees from multiple pharmaceutical manufacturers.
FROM JAMA
Key clinical point: In black patients, long-acting beta-agonists used with inhaled corticosteroids were not more effective than were tiotropium plus inhaled corticosteroids in lengthening time to asthma exacerbations.
Major finding: The risk ratio of a severe asthma exacerbation (requiring corticosteroids or hospitalization) with tiotropium plus inhaled corticosteroids vs. a LABA plus inhaled corticosteroids was 0.90 (P = .31).
Data source: A randomized, open-label study enrolling 1,070 black patients at 20 U.S. primary care sites.
Disclosures: The Agency for Healthcare Research and Quality sponsored the study. Dr. Wechsler and several coauthors disclosed consultant fees from multiple pharmaceutical manufacturers.
ACS: No pull-out pneumothorax with ‘party balloon Valsalva’
CHICAGO – Investigators have come up with a simple way to reduce and maybe even eliminate pull-out pneumothoraces during chest tube removal.
Instead of standard inhale or exhale Valsalva maneuvers, they have their patients blow up a party balloon as the tube is pulled.
That produces the same Valsalva effects as the standard maneuvers, but with two significant advantages. First, it’s easy to explain and for patients to understand and do – not much more instruction is required than “blow up the balloon” – and, secondly, the inflating balloon is a visual check to make sure patients are doing the maneuver correctly. “It’s easy. Everyone can do it,” said lead investigator Dr. Puwadon Thitivaraporn, who developed the technique with Dr. Kritaya Kritayakirana and colleagues at King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
To see how well it works, the team randomized 10 women and 38 men about equally to four removal techniques: the standard expire Valsalva, the standard inspire Valsalva, and two balloon maneuvers – blowing the balloon up after a deep breath and blowing it up with residual lung volume after an initial exhalation.
The subjects were trauma patients 15-64 years old, with a mean age of 38 years. Lung injuries, rib fractures, and tube suction were a bit more common in the standard maneuver groups. Patients with tracheotomies, chronic lung disease, and Glasgow Coma Scores below 13 were excluded from the study. Hemopneumothorax was the most common indication for tube placement.
Two patients in each of the standard groups (16%) developed a pull-out pneumothorax within 24 hours of tube removal, confirmed by x-ray. One required chest tube reinsertion, and all four ended up spending extra time in the hospital. Similar problems have been reported in American medicine (J Trauma. 2001 Apr;50[4]:674-7).
Meanwhile, not a single balloon patient had a lung collapse when their tube was pulled.
Because of the small number of subjects, the differences weren’t statistically significant, but they came close in a group comparison of standard patients with balloon patients (P = .11). The investigators estimated they would need almost 600 hundred subjects to reach statistical significance.
Even so, the party balloon technique appears to be “easier and safer” than standard maneuvers, as well as “reproducible and cheap, and it can prevent recurrent pneumothorax. It can be used as an alternative to the classic Valsalva,” said Dr. Thitivaraporn, a cardiothoracic surgery resident at the Bangkok hospital.
The balloon method is being used there now in nontrauma patients, as well, but the standard maneuvers are also being used until the balloon technique shows statistically significant benefits, he said.
With manometry, the team found that a party balloon’s internal pressure builds quickly as it’s inflated from a starting diameter of about 4.5 cm to about 9 cm, peaking at about 60 mm Hg; pressure trails off to about 40 mm Hg as inflation continues past 9 cm.
The investigators have no relevant disclosures.
CHICAGO – Investigators have come up with a simple way to reduce and maybe even eliminate pull-out pneumothoraces during chest tube removal.
Instead of standard inhale or exhale Valsalva maneuvers, they have their patients blow up a party balloon as the tube is pulled.
That produces the same Valsalva effects as the standard maneuvers, but with two significant advantages. First, it’s easy to explain and for patients to understand and do – not much more instruction is required than “blow up the balloon” – and, secondly, the inflating balloon is a visual check to make sure patients are doing the maneuver correctly. “It’s easy. Everyone can do it,” said lead investigator Dr. Puwadon Thitivaraporn, who developed the technique with Dr. Kritaya Kritayakirana and colleagues at King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
To see how well it works, the team randomized 10 women and 38 men about equally to four removal techniques: the standard expire Valsalva, the standard inspire Valsalva, and two balloon maneuvers – blowing the balloon up after a deep breath and blowing it up with residual lung volume after an initial exhalation.
The subjects were trauma patients 15-64 years old, with a mean age of 38 years. Lung injuries, rib fractures, and tube suction were a bit more common in the standard maneuver groups. Patients with tracheotomies, chronic lung disease, and Glasgow Coma Scores below 13 were excluded from the study. Hemopneumothorax was the most common indication for tube placement.
Two patients in each of the standard groups (16%) developed a pull-out pneumothorax within 24 hours of tube removal, confirmed by x-ray. One required chest tube reinsertion, and all four ended up spending extra time in the hospital. Similar problems have been reported in American medicine (J Trauma. 2001 Apr;50[4]:674-7).
Meanwhile, not a single balloon patient had a lung collapse when their tube was pulled.
Because of the small number of subjects, the differences weren’t statistically significant, but they came close in a group comparison of standard patients with balloon patients (P = .11). The investigators estimated they would need almost 600 hundred subjects to reach statistical significance.
Even so, the party balloon technique appears to be “easier and safer” than standard maneuvers, as well as “reproducible and cheap, and it can prevent recurrent pneumothorax. It can be used as an alternative to the classic Valsalva,” said Dr. Thitivaraporn, a cardiothoracic surgery resident at the Bangkok hospital.
The balloon method is being used there now in nontrauma patients, as well, but the standard maneuvers are also being used until the balloon technique shows statistically significant benefits, he said.
With manometry, the team found that a party balloon’s internal pressure builds quickly as it’s inflated from a starting diameter of about 4.5 cm to about 9 cm, peaking at about 60 mm Hg; pressure trails off to about 40 mm Hg as inflation continues past 9 cm.
The investigators have no relevant disclosures.
CHICAGO – Investigators have come up with a simple way to reduce and maybe even eliminate pull-out pneumothoraces during chest tube removal.
Instead of standard inhale or exhale Valsalva maneuvers, they have their patients blow up a party balloon as the tube is pulled.
That produces the same Valsalva effects as the standard maneuvers, but with two significant advantages. First, it’s easy to explain and for patients to understand and do – not much more instruction is required than “blow up the balloon” – and, secondly, the inflating balloon is a visual check to make sure patients are doing the maneuver correctly. “It’s easy. Everyone can do it,” said lead investigator Dr. Puwadon Thitivaraporn, who developed the technique with Dr. Kritaya Kritayakirana and colleagues at King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
To see how well it works, the team randomized 10 women and 38 men about equally to four removal techniques: the standard expire Valsalva, the standard inspire Valsalva, and two balloon maneuvers – blowing the balloon up after a deep breath and blowing it up with residual lung volume after an initial exhalation.
The subjects were trauma patients 15-64 years old, with a mean age of 38 years. Lung injuries, rib fractures, and tube suction were a bit more common in the standard maneuver groups. Patients with tracheotomies, chronic lung disease, and Glasgow Coma Scores below 13 were excluded from the study. Hemopneumothorax was the most common indication for tube placement.
Two patients in each of the standard groups (16%) developed a pull-out pneumothorax within 24 hours of tube removal, confirmed by x-ray. One required chest tube reinsertion, and all four ended up spending extra time in the hospital. Similar problems have been reported in American medicine (J Trauma. 2001 Apr;50[4]:674-7).
Meanwhile, not a single balloon patient had a lung collapse when their tube was pulled.
Because of the small number of subjects, the differences weren’t statistically significant, but they came close in a group comparison of standard patients with balloon patients (P = .11). The investigators estimated they would need almost 600 hundred subjects to reach statistical significance.
Even so, the party balloon technique appears to be “easier and safer” than standard maneuvers, as well as “reproducible and cheap, and it can prevent recurrent pneumothorax. It can be used as an alternative to the classic Valsalva,” said Dr. Thitivaraporn, a cardiothoracic surgery resident at the Bangkok hospital.
The balloon method is being used there now in nontrauma patients, as well, but the standard maneuvers are also being used until the balloon technique shows statistically significant benefits, he said.
With manometry, the team found that a party balloon’s internal pressure builds quickly as it’s inflated from a starting diameter of about 4.5 cm to about 9 cm, peaking at about 60 mm Hg; pressure trails off to about 40 mm Hg as inflation continues past 9 cm.
The investigators have no relevant disclosures.
AT THE ACS CLINICAL CONGRESS
Key clinical point: The next time you pull a chest tube, you might want to ask your patient to blow up a balloon.
Major finding: Sixteen percent of patients collapsed a lung with classic inhale/exhale Valsalva maneuvers during chest tube removal, but none did with the balloon technique.
Data source: Randomized, controlled trial of 48 chest tube patients.
Disclosures: The investigators have no relevant disclosures.
EADV: Another promising topical for atopic dermatitis
COPENHAGEN – A novel topical nonsteroidal inhibitor of phosphodiesterase-4 showed a favorable efficacy to side effect ratio in a phase II study of adolescents and adults with mild to moderate atopic dermatitis, Dr. Jon M. Hanifin reported at the annual congress of the European Academy of Dermatology and Venereology.
“For topical agents, I think a 30% improvement with few side effects is a desirable balance,” observed Dr. Hanifin of Oregon Health and Sciences University, Portland.
The investigational agent, known for now as OPA-15406, is formulated as a twice-daily ointment. It is highly selective for the phosphodiesterase-4 (PDE-4) B subtype.
Dr. Hanifin noted that these are “exciting times” in the development of new topical therapies for atopic dermatitis (AD). In addition to the successful phase II study of OPA-15406 he presented, a highlight of the EADV congress was the strongly positive pivotal phase III data presented for crisaborole, another nonsteroidal topical PDE-4 inhibitor, albeit with a different mechanism of action.
For Dr. Hanifin these developments are particularly satisfying personally because 33 years ago as a young investigator – before the term ‘translational science’ had come into vogue – he and his research team made the seminal observation that increased phosphodiesterase activity is “a basic biochemical characteristic relevant to skin immunocellular regulation in atopic disease” (J Allergy Clin Immunol. 1982 Dec;70[6]:452-7).
The 8-week, multicenter, randomized, double-blind phase II OPA-15406 dose-ranging study included 121 patients, 70% with moderate AD, the rest with mild disease. About 20% were adolescents, the rest adults. Their mean baseline Eczema Area Severity Index (EASI) score was 9.5, with a self-reported pruritus score of 61 on a 0-100 scale. Participants were randomized to twice-daily application of OPA-15406 at 0.3% or 1% or the vehicle ointment as a control.
A significant treatment effect was seen within the first week. At 1 week, the mean EASI score was reduced by 31% from baseline in the 1% OPA-15406 group, 15% with the 0.3% formulation, and 6% with vehicle. The active treatment remained significantly more effective than vehicle throughout the 8-week period.
Another measure of efficacy – an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 along with at least a 2-point improvement on the 0-5 scale at week 4 – was met by 21% of patients on 1% OPA-15406, 15% on the 0.3% formulation, and 2.7% on vehicle. By the less stringent standard of an IGA of 0 or 1 plus at least a 1-point reduction at week 4, the success rates were 30%, 24%, and 10%.
Dr. Hanifin said the 1% formulation is the one likely to advance to phase III studies, given its superior efficacy. This was particularly evident with regard to itch. At the first evaluation, after just 1 week of treatment, pruritus scores showed a mean 30% reduction with 1% OPA-15406 versus no significant change in controls. He added that his clinical impression is that many patients experience a significant improvement in itching within the first 24 hours on the 1% formulation, an observation that deserves formal study.
Scores on the Dermatology Life Quality Index and Children’s Dermatology Life Quality Index were significantly better in the active therapy arms than with vehicle as early as week 1.
The adverse events findings were “not very exciting,” according to the dermatologist. No treatment-related serious adverse events occurred. The two most common adverse events leading to treatment discontinuation – worsening AD and pruritus – occurred more often in the vehicle-treated controls.
Session cochair Dr. Jacek Szepietowski called the pruritus results particularly impressive.
“It’s very difficult to do successful clinical trials of topicals for atopic dermatitis because the vehicle alone, especially if it’s an ointment, can have favorable effects which increase over time both on EASI and pruritus,” commented Dr. Szepietowski, professor and head of the department of dermatology, venereology, and allergology at the Medical University of Wroclaw (Poland).
The study was sponsored by Otsuka Pharmaceuticals. Dr. Hanifin reported serving as a consultant to and paid investigator for the company.
COPENHAGEN – A novel topical nonsteroidal inhibitor of phosphodiesterase-4 showed a favorable efficacy to side effect ratio in a phase II study of adolescents and adults with mild to moderate atopic dermatitis, Dr. Jon M. Hanifin reported at the annual congress of the European Academy of Dermatology and Venereology.
“For topical agents, I think a 30% improvement with few side effects is a desirable balance,” observed Dr. Hanifin of Oregon Health and Sciences University, Portland.
The investigational agent, known for now as OPA-15406, is formulated as a twice-daily ointment. It is highly selective for the phosphodiesterase-4 (PDE-4) B subtype.
Dr. Hanifin noted that these are “exciting times” in the development of new topical therapies for atopic dermatitis (AD). In addition to the successful phase II study of OPA-15406 he presented, a highlight of the EADV congress was the strongly positive pivotal phase III data presented for crisaborole, another nonsteroidal topical PDE-4 inhibitor, albeit with a different mechanism of action.
For Dr. Hanifin these developments are particularly satisfying personally because 33 years ago as a young investigator – before the term ‘translational science’ had come into vogue – he and his research team made the seminal observation that increased phosphodiesterase activity is “a basic biochemical characteristic relevant to skin immunocellular regulation in atopic disease” (J Allergy Clin Immunol. 1982 Dec;70[6]:452-7).
The 8-week, multicenter, randomized, double-blind phase II OPA-15406 dose-ranging study included 121 patients, 70% with moderate AD, the rest with mild disease. About 20% were adolescents, the rest adults. Their mean baseline Eczema Area Severity Index (EASI) score was 9.5, with a self-reported pruritus score of 61 on a 0-100 scale. Participants were randomized to twice-daily application of OPA-15406 at 0.3% or 1% or the vehicle ointment as a control.
A significant treatment effect was seen within the first week. At 1 week, the mean EASI score was reduced by 31% from baseline in the 1% OPA-15406 group, 15% with the 0.3% formulation, and 6% with vehicle. The active treatment remained significantly more effective than vehicle throughout the 8-week period.
Another measure of efficacy – an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 along with at least a 2-point improvement on the 0-5 scale at week 4 – was met by 21% of patients on 1% OPA-15406, 15% on the 0.3% formulation, and 2.7% on vehicle. By the less stringent standard of an IGA of 0 or 1 plus at least a 1-point reduction at week 4, the success rates were 30%, 24%, and 10%.
Dr. Hanifin said the 1% formulation is the one likely to advance to phase III studies, given its superior efficacy. This was particularly evident with regard to itch. At the first evaluation, after just 1 week of treatment, pruritus scores showed a mean 30% reduction with 1% OPA-15406 versus no significant change in controls. He added that his clinical impression is that many patients experience a significant improvement in itching within the first 24 hours on the 1% formulation, an observation that deserves formal study.
Scores on the Dermatology Life Quality Index and Children’s Dermatology Life Quality Index were significantly better in the active therapy arms than with vehicle as early as week 1.
The adverse events findings were “not very exciting,” according to the dermatologist. No treatment-related serious adverse events occurred. The two most common adverse events leading to treatment discontinuation – worsening AD and pruritus – occurred more often in the vehicle-treated controls.
Session cochair Dr. Jacek Szepietowski called the pruritus results particularly impressive.
“It’s very difficult to do successful clinical trials of topicals for atopic dermatitis because the vehicle alone, especially if it’s an ointment, can have favorable effects which increase over time both on EASI and pruritus,” commented Dr. Szepietowski, professor and head of the department of dermatology, venereology, and allergology at the Medical University of Wroclaw (Poland).
The study was sponsored by Otsuka Pharmaceuticals. Dr. Hanifin reported serving as a consultant to and paid investigator for the company.
COPENHAGEN – A novel topical nonsteroidal inhibitor of phosphodiesterase-4 showed a favorable efficacy to side effect ratio in a phase II study of adolescents and adults with mild to moderate atopic dermatitis, Dr. Jon M. Hanifin reported at the annual congress of the European Academy of Dermatology and Venereology.
“For topical agents, I think a 30% improvement with few side effects is a desirable balance,” observed Dr. Hanifin of Oregon Health and Sciences University, Portland.
The investigational agent, known for now as OPA-15406, is formulated as a twice-daily ointment. It is highly selective for the phosphodiesterase-4 (PDE-4) B subtype.
Dr. Hanifin noted that these are “exciting times” in the development of new topical therapies for atopic dermatitis (AD). In addition to the successful phase II study of OPA-15406 he presented, a highlight of the EADV congress was the strongly positive pivotal phase III data presented for crisaborole, another nonsteroidal topical PDE-4 inhibitor, albeit with a different mechanism of action.
For Dr. Hanifin these developments are particularly satisfying personally because 33 years ago as a young investigator – before the term ‘translational science’ had come into vogue – he and his research team made the seminal observation that increased phosphodiesterase activity is “a basic biochemical characteristic relevant to skin immunocellular regulation in atopic disease” (J Allergy Clin Immunol. 1982 Dec;70[6]:452-7).
The 8-week, multicenter, randomized, double-blind phase II OPA-15406 dose-ranging study included 121 patients, 70% with moderate AD, the rest with mild disease. About 20% were adolescents, the rest adults. Their mean baseline Eczema Area Severity Index (EASI) score was 9.5, with a self-reported pruritus score of 61 on a 0-100 scale. Participants were randomized to twice-daily application of OPA-15406 at 0.3% or 1% or the vehicle ointment as a control.
A significant treatment effect was seen within the first week. At 1 week, the mean EASI score was reduced by 31% from baseline in the 1% OPA-15406 group, 15% with the 0.3% formulation, and 6% with vehicle. The active treatment remained significantly more effective than vehicle throughout the 8-week period.
Another measure of efficacy – an Investigator’s Global Assessment (IGA) score of 0 (clear) or 1 along with at least a 2-point improvement on the 0-5 scale at week 4 – was met by 21% of patients on 1% OPA-15406, 15% on the 0.3% formulation, and 2.7% on vehicle. By the less stringent standard of an IGA of 0 or 1 plus at least a 1-point reduction at week 4, the success rates were 30%, 24%, and 10%.
Dr. Hanifin said the 1% formulation is the one likely to advance to phase III studies, given its superior efficacy. This was particularly evident with regard to itch. At the first evaluation, after just 1 week of treatment, pruritus scores showed a mean 30% reduction with 1% OPA-15406 versus no significant change in controls. He added that his clinical impression is that many patients experience a significant improvement in itching within the first 24 hours on the 1% formulation, an observation that deserves formal study.
Scores on the Dermatology Life Quality Index and Children’s Dermatology Life Quality Index were significantly better in the active therapy arms than with vehicle as early as week 1.
The adverse events findings were “not very exciting,” according to the dermatologist. No treatment-related serious adverse events occurred. The two most common adverse events leading to treatment discontinuation – worsening AD and pruritus – occurred more often in the vehicle-treated controls.
Session cochair Dr. Jacek Szepietowski called the pruritus results particularly impressive.
“It’s very difficult to do successful clinical trials of topicals for atopic dermatitis because the vehicle alone, especially if it’s an ointment, can have favorable effects which increase over time both on EASI and pruritus,” commented Dr. Szepietowski, professor and head of the department of dermatology, venereology, and allergology at the Medical University of Wroclaw (Poland).
The study was sponsored by Otsuka Pharmaceuticals. Dr. Hanifin reported serving as a consultant to and paid investigator for the company.
AT THE EADV CONGRESS
Key clinical point: The pipeline for nonsteroidal topical therapies for pediatric and adult atopic dermatitis shows great promise.
Major finding: Atopic dermatitis patients showed a mean 31% reduction in Eczema Area Severity Index scores after 1 week on topical 1% OPA-15406 ointment, compared with a 6% decrease on vehicle alone.
Data source: This phase II multicenter, double-blind, 8-week randomized trial included 121 adolescents and adults with mild to moderate atopic dermatitis.
Disclosures: The study was sponsored by Otsuka Pharmaceuticals. The presenter reported serving as a consultant and paid investigator for the company.
Availability of flavored tobacco products drives tobacco use in youths
The availability of flavored tobacco products appears to be a major driver of tobacco use in youths, suggests an analysis of a nationally representative longitudinal cohort study from 2013 to 2014.
The 13,651 youth participants (aged 12-17 years) in the U.S. Population Assessment of Tobacco and Health (PATH) study responded to questions about ever and past 30-day use of various tobacco products. For each product ever used, youths indicated whether the first product they used was flavored. Users of noncigarette tobacco products, including e-cigarettes, reported past 30-day use of a flavored product or products; of those individuals, the ones that had used a tobacco product (including e-cigarettes) other than a cigarette within the past 30 days reported their reasons for engaging in such activities.
The majority of tobacco products users said that their inaugural taste of tobacco was in the form of a flavored product, including 88.7% of ever hookah users, 81.0% of ever e-cigarette users, 65.4% of ever users of any cigar type, and 50.1% of ever cigarette smokers. Among study participants who had used tobacco within the past 30 days, 79.8% had used a flavored tobacco product.
Furthermore, well over 50% of individuals who had used noncigarette tobacco products within the past 30 days reported the availability of flavored versions of such products as having been among their leading reasons for using them. Specifically, 81.5% of e-cigarette users, 78.9% of hookah users, 73.8% of cigar users, 69.3% of smokeless tobacco users, and 67.2% of snus pouch users said they liked their respective tobacco products “because they come in flavors.”
“Consistent with national school-based estimates, this study confirms widespread appeal of flavored products among youth tobacco users,” said Bridget K. Ambrose, Ph.D., of the Center for Tobacco Products, Food and Drug Administration, Silver Spring, Md., and her colleagues.
“Data from future PATH study waves can provide information on tobacco use trajectories following experimentation with flavored, compared with nonflavored products,” according to the researchers.
Read the full study in JAMA (doi: 10.1001/jama.2015.13802).
The availability of flavored tobacco products appears to be a major driver of tobacco use in youths, suggests an analysis of a nationally representative longitudinal cohort study from 2013 to 2014.
The 13,651 youth participants (aged 12-17 years) in the U.S. Population Assessment of Tobacco and Health (PATH) study responded to questions about ever and past 30-day use of various tobacco products. For each product ever used, youths indicated whether the first product they used was flavored. Users of noncigarette tobacco products, including e-cigarettes, reported past 30-day use of a flavored product or products; of those individuals, the ones that had used a tobacco product (including e-cigarettes) other than a cigarette within the past 30 days reported their reasons for engaging in such activities.
The majority of tobacco products users said that their inaugural taste of tobacco was in the form of a flavored product, including 88.7% of ever hookah users, 81.0% of ever e-cigarette users, 65.4% of ever users of any cigar type, and 50.1% of ever cigarette smokers. Among study participants who had used tobacco within the past 30 days, 79.8% had used a flavored tobacco product.
Furthermore, well over 50% of individuals who had used noncigarette tobacco products within the past 30 days reported the availability of flavored versions of such products as having been among their leading reasons for using them. Specifically, 81.5% of e-cigarette users, 78.9% of hookah users, 73.8% of cigar users, 69.3% of smokeless tobacco users, and 67.2% of snus pouch users said they liked their respective tobacco products “because they come in flavors.”
“Consistent with national school-based estimates, this study confirms widespread appeal of flavored products among youth tobacco users,” said Bridget K. Ambrose, Ph.D., of the Center for Tobacco Products, Food and Drug Administration, Silver Spring, Md., and her colleagues.
“Data from future PATH study waves can provide information on tobacco use trajectories following experimentation with flavored, compared with nonflavored products,” according to the researchers.
Read the full study in JAMA (doi: 10.1001/jama.2015.13802).
The availability of flavored tobacco products appears to be a major driver of tobacco use in youths, suggests an analysis of a nationally representative longitudinal cohort study from 2013 to 2014.
The 13,651 youth participants (aged 12-17 years) in the U.S. Population Assessment of Tobacco and Health (PATH) study responded to questions about ever and past 30-day use of various tobacco products. For each product ever used, youths indicated whether the first product they used was flavored. Users of noncigarette tobacco products, including e-cigarettes, reported past 30-day use of a flavored product or products; of those individuals, the ones that had used a tobacco product (including e-cigarettes) other than a cigarette within the past 30 days reported their reasons for engaging in such activities.
The majority of tobacco products users said that their inaugural taste of tobacco was in the form of a flavored product, including 88.7% of ever hookah users, 81.0% of ever e-cigarette users, 65.4% of ever users of any cigar type, and 50.1% of ever cigarette smokers. Among study participants who had used tobacco within the past 30 days, 79.8% had used a flavored tobacco product.
Furthermore, well over 50% of individuals who had used noncigarette tobacco products within the past 30 days reported the availability of flavored versions of such products as having been among their leading reasons for using them. Specifically, 81.5% of e-cigarette users, 78.9% of hookah users, 73.8% of cigar users, 69.3% of smokeless tobacco users, and 67.2% of snus pouch users said they liked their respective tobacco products “because they come in flavors.”
“Consistent with national school-based estimates, this study confirms widespread appeal of flavored products among youth tobacco users,” said Bridget K. Ambrose, Ph.D., of the Center for Tobacco Products, Food and Drug Administration, Silver Spring, Md., and her colleagues.
“Data from future PATH study waves can provide information on tobacco use trajectories following experimentation with flavored, compared with nonflavored products,” according to the researchers.
Read the full study in JAMA (doi: 10.1001/jama.2015.13802).
FROM JAMA