User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
In newly diagnosed hypertension with OSA, adding CPAP augmented the benefits of losartan
In patients with new-onset hypertension and obstructive sleep apnea, continuous positive airway pressure (CPAP) therapy plus antihypertensive treatment with losartan led to reductions in systolic blood pressure beyond those achieved with losartan alone, a two-phase study found.
“Adding CPAP treatment to losartan may reduce blood pressure in a clinically relevant way if the patients are compliant with the device,” said Dr. Erik Thunström of the Sahlgrenska Academy at the University of Gothenburg, Sweden, and his associates.
In their open-label study, 89 men and women with new-onset untreated hypertension – 54 of whom were found to have obstructive sleep apnea (OSA) through a home sleep study and 35 of whom were determined to not have OSA – were treated for 6 weeks with losartan, 50 mg daily. Ambulatory 24-hour blood pressure monitoring was performed before and after treatment.
The patients with OSA were then randomized to receive 6 weeks of nightly add-on CPAP therapy or to continue losartan alone. Ambulatory 24-hour blood pressure monitoring was performed again.
Losartan alone reduced blood pressure in patients with hypertension and concomitant OSA, but the effect was smaller than that seen in patients without OSA. Statistically significant differences were seen in the mean net reduction in morning systolic blood pressure and morning mean arterial pressure. Overall, losartan appeared to be less effective at night and during the early morning hours in patients with OSA, the researchers reported.
After 6 weeks of losartan alone, a blood pressure less than 130/80 mm Hg was achieved by 12.5% of the patients with OSA and by 29% of the patients without OSA.
After 6 weeks of add-on CPAP therapy, 25% of patients with OSA achieved blood pressures less than 130/80 mm Hg. The differences in blood pressures for the OSA patients receiving CPAP plus losartan and those receiving losartan alone were 4.4 mm Hg for 24-hour systolic blood pressure, 1.9 mm Hg for diastolic, and 2.5 mm Hg for mean arterial pressure.
The most “robust” blood pressure changes were seen in the patients who used CPAP therapy for more than 4 hours every night, reducing the mean 24-hour systolic blood pressure by 6.5 mm Hg, the diastolic pressure by 3.8 mm Hg, and the mean arterial blood pressure by 4.6 mm Hg, the researchers reported (Am J Respir Crit Care Med. 2016 Feb.;193:310-20). “Adding CPAP to treatment with losartan reduced the mean 24-hour systolic blood pressure by 6.5 mm Hg in the subgroup of patients with OSA who were adherent with CPAP,” they wrote.
Patients included in the study all had a body mass index of 35 kg/m2; those with OSA had slightly higher BMIs that did not differ significantly from those without OSA.
That CPAP seems to have additive blood pressure–lowering effect when used concomitantly with losartan “favors the idea that it contributes to a further down-regulation of RAAS [renin-angiotensin-aldosterone system] activity in new-onset hypertension and OSA,” the authors wrote.
RAAS activity is often changed in hypertension, and in animal studies it has been shown to be up-regulated by intermittent hypoxia. Angiotensin II receptor antagonists are thus viewed as a good choice in the treatment of patients with OSA and new-onset hypertension, they wrote.
Treating OSA may make hypertension easier to address pharmacologically. The effect of CPAP on blood pressure is relatively small when all patients are considered but is more substantial and clinically important for those who use CPAP for more than 4 hours per night.
Can treatment of OSA effectively reduce blood pressure in an otherwise asymptomatic hypertensive patient with OSA? I believe the study would suggest that the answer remains “maybe.”
Most of the patients in the study would require a higher dose of losartan or an additional antihypertensive drug, even while using CPAP, to get to target blood pressures. Getting patients to use CPAP is a difficult task, as is adherence with any long-term pharmacologic management.
All in all, however, CPAP could contribute to blood pressure control while also improving quality of life and possibly reducing the risk for cardiovascular disease.
Dr. David P. White is with Harvard Medical School in Boston. His comments are excerpted from an accompanying editorial (Am J Respir Crit Care Med. 2016 Feb;193:238-9).
Treating OSA may make hypertension easier to address pharmacologically. The effect of CPAP on blood pressure is relatively small when all patients are considered but is more substantial and clinically important for those who use CPAP for more than 4 hours per night.
Can treatment of OSA effectively reduce blood pressure in an otherwise asymptomatic hypertensive patient with OSA? I believe the study would suggest that the answer remains “maybe.”
Most of the patients in the study would require a higher dose of losartan or an additional antihypertensive drug, even while using CPAP, to get to target blood pressures. Getting patients to use CPAP is a difficult task, as is adherence with any long-term pharmacologic management.
All in all, however, CPAP could contribute to blood pressure control while also improving quality of life and possibly reducing the risk for cardiovascular disease.
Dr. David P. White is with Harvard Medical School in Boston. His comments are excerpted from an accompanying editorial (Am J Respir Crit Care Med. 2016 Feb;193:238-9).
Treating OSA may make hypertension easier to address pharmacologically. The effect of CPAP on blood pressure is relatively small when all patients are considered but is more substantial and clinically important for those who use CPAP for more than 4 hours per night.
Can treatment of OSA effectively reduce blood pressure in an otherwise asymptomatic hypertensive patient with OSA? I believe the study would suggest that the answer remains “maybe.”
Most of the patients in the study would require a higher dose of losartan or an additional antihypertensive drug, even while using CPAP, to get to target blood pressures. Getting patients to use CPAP is a difficult task, as is adherence with any long-term pharmacologic management.
All in all, however, CPAP could contribute to blood pressure control while also improving quality of life and possibly reducing the risk for cardiovascular disease.
Dr. David P. White is with Harvard Medical School in Boston. His comments are excerpted from an accompanying editorial (Am J Respir Crit Care Med. 2016 Feb;193:238-9).
In patients with new-onset hypertension and obstructive sleep apnea, continuous positive airway pressure (CPAP) therapy plus antihypertensive treatment with losartan led to reductions in systolic blood pressure beyond those achieved with losartan alone, a two-phase study found.
“Adding CPAP treatment to losartan may reduce blood pressure in a clinically relevant way if the patients are compliant with the device,” said Dr. Erik Thunström of the Sahlgrenska Academy at the University of Gothenburg, Sweden, and his associates.
In their open-label study, 89 men and women with new-onset untreated hypertension – 54 of whom were found to have obstructive sleep apnea (OSA) through a home sleep study and 35 of whom were determined to not have OSA – were treated for 6 weeks with losartan, 50 mg daily. Ambulatory 24-hour blood pressure monitoring was performed before and after treatment.
The patients with OSA were then randomized to receive 6 weeks of nightly add-on CPAP therapy or to continue losartan alone. Ambulatory 24-hour blood pressure monitoring was performed again.
Losartan alone reduced blood pressure in patients with hypertension and concomitant OSA, but the effect was smaller than that seen in patients without OSA. Statistically significant differences were seen in the mean net reduction in morning systolic blood pressure and morning mean arterial pressure. Overall, losartan appeared to be less effective at night and during the early morning hours in patients with OSA, the researchers reported.
After 6 weeks of losartan alone, a blood pressure less than 130/80 mm Hg was achieved by 12.5% of the patients with OSA and by 29% of the patients without OSA.
After 6 weeks of add-on CPAP therapy, 25% of patients with OSA achieved blood pressures less than 130/80 mm Hg. The differences in blood pressures for the OSA patients receiving CPAP plus losartan and those receiving losartan alone were 4.4 mm Hg for 24-hour systolic blood pressure, 1.9 mm Hg for diastolic, and 2.5 mm Hg for mean arterial pressure.
The most “robust” blood pressure changes were seen in the patients who used CPAP therapy for more than 4 hours every night, reducing the mean 24-hour systolic blood pressure by 6.5 mm Hg, the diastolic pressure by 3.8 mm Hg, and the mean arterial blood pressure by 4.6 mm Hg, the researchers reported (Am J Respir Crit Care Med. 2016 Feb.;193:310-20). “Adding CPAP to treatment with losartan reduced the mean 24-hour systolic blood pressure by 6.5 mm Hg in the subgroup of patients with OSA who were adherent with CPAP,” they wrote.
Patients included in the study all had a body mass index of 35 kg/m2; those with OSA had slightly higher BMIs that did not differ significantly from those without OSA.
That CPAP seems to have additive blood pressure–lowering effect when used concomitantly with losartan “favors the idea that it contributes to a further down-regulation of RAAS [renin-angiotensin-aldosterone system] activity in new-onset hypertension and OSA,” the authors wrote.
RAAS activity is often changed in hypertension, and in animal studies it has been shown to be up-regulated by intermittent hypoxia. Angiotensin II receptor antagonists are thus viewed as a good choice in the treatment of patients with OSA and new-onset hypertension, they wrote.
In patients with new-onset hypertension and obstructive sleep apnea, continuous positive airway pressure (CPAP) therapy plus antihypertensive treatment with losartan led to reductions in systolic blood pressure beyond those achieved with losartan alone, a two-phase study found.
“Adding CPAP treatment to losartan may reduce blood pressure in a clinically relevant way if the patients are compliant with the device,” said Dr. Erik Thunström of the Sahlgrenska Academy at the University of Gothenburg, Sweden, and his associates.
In their open-label study, 89 men and women with new-onset untreated hypertension – 54 of whom were found to have obstructive sleep apnea (OSA) through a home sleep study and 35 of whom were determined to not have OSA – were treated for 6 weeks with losartan, 50 mg daily. Ambulatory 24-hour blood pressure monitoring was performed before and after treatment.
The patients with OSA were then randomized to receive 6 weeks of nightly add-on CPAP therapy or to continue losartan alone. Ambulatory 24-hour blood pressure monitoring was performed again.
Losartan alone reduced blood pressure in patients with hypertension and concomitant OSA, but the effect was smaller than that seen in patients without OSA. Statistically significant differences were seen in the mean net reduction in morning systolic blood pressure and morning mean arterial pressure. Overall, losartan appeared to be less effective at night and during the early morning hours in patients with OSA, the researchers reported.
After 6 weeks of losartan alone, a blood pressure less than 130/80 mm Hg was achieved by 12.5% of the patients with OSA and by 29% of the patients without OSA.
After 6 weeks of add-on CPAP therapy, 25% of patients with OSA achieved blood pressures less than 130/80 mm Hg. The differences in blood pressures for the OSA patients receiving CPAP plus losartan and those receiving losartan alone were 4.4 mm Hg for 24-hour systolic blood pressure, 1.9 mm Hg for diastolic, and 2.5 mm Hg for mean arterial pressure.
The most “robust” blood pressure changes were seen in the patients who used CPAP therapy for more than 4 hours every night, reducing the mean 24-hour systolic blood pressure by 6.5 mm Hg, the diastolic pressure by 3.8 mm Hg, and the mean arterial blood pressure by 4.6 mm Hg, the researchers reported (Am J Respir Crit Care Med. 2016 Feb.;193:310-20). “Adding CPAP to treatment with losartan reduced the mean 24-hour systolic blood pressure by 6.5 mm Hg in the subgroup of patients with OSA who were adherent with CPAP,” they wrote.
Patients included in the study all had a body mass index of 35 kg/m2; those with OSA had slightly higher BMIs that did not differ significantly from those without OSA.
That CPAP seems to have additive blood pressure–lowering effect when used concomitantly with losartan “favors the idea that it contributes to a further down-regulation of RAAS [renin-angiotensin-aldosterone system] activity in new-onset hypertension and OSA,” the authors wrote.
RAAS activity is often changed in hypertension, and in animal studies it has been shown to be up-regulated by intermittent hypoxia. Angiotensin II receptor antagonists are thus viewed as a good choice in the treatment of patients with OSA and new-onset hypertension, they wrote.
FROM AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Key clinical point: In patients with new-onset hypertension and obstructive sleep apnea, adding continuous positive airway pressure may reduce blood pressure levels further than achieved with losartan alone.
Major finding: In adherent patients, CPAP reduced the mean 24-hour systolic blood pressure by an additional 6.5 mm Hg as compared to the levels seen in patients on losartan alone.
Data source: A study of 89 men and women with new-onset untreated hypertension who were treated with losartan for 6 weeks and tested for OSA. In a second 6-week study, patients found to have OSA were randomized to receive CPAP or no CPAP.
Disclosures: The researchers had no relevant financial disclosures.
Sleep apnea found in 57% of veterans with PTSD
Obstructive sleep apnea syndrome (OSAS) was diagnosed in more than half of 200 active duty service members with combat-related post-traumatic stress disorder (PTSD) who were studied at Walter Reed Army Medical Center in Washington.
Compared with age-matched peers with just one of these disorders, the service members with PTSD and OSAS had poorer somnolence and sleep-related quality of life and were less adherent and responsive to positive airway pressure therapy.
The findings “highlight the need for a high index of suspicion and a comprehensive approach to identifying and treating sleep-disordered breathing in these patients,” Dr. Christopher J. Lettieri of the Uniformed Services University in Bethesda, Md., and his associates wrote (Chest. 2016 Feb;149[2]:483-90). “Given the prevalence of OSAS in patients with PTSD and its adverse impact on symptoms and adherence, early identification may improve outcomes.”
In the observational cohort study, 200 consecutive active duty service members who were diagnosed with PTSD as part of post-deployment screening underwent sleep evaluations regardless of whether there was clinical suspicion of sleep-disordered breathing. More than half – about 57% – were diagnosed with OSAS. Almost 60% of the study group had mild traumatic brain injury, which has been connected in prior research to obstructive sleep apnea, and many had comorbid insomnia. Those who were diagnosed with OSAS were older and had higher BMIs than those not found to have OSAS.
All 200 patients were compared with 50 consecutive age-matched control patients who had OSAS but had not been deployed and did not have PTSD, as well as with 50 age-matched service members without prior deployment or either of the two disorders. All of the patients diagnosed with OSAS were prescribed positive airway pressure (PAP) therapy and evaluated after a month.
Sleep quality was poor in the majority of patients with PTSD, and OSAS and PTSD were both independently associated with increased daytime sleepiness and lower quality-of-life index scores. However, patients with both conditions fared significantly worse, particularly with respect to quality of life as measured by the Functional Outcomes of Sleep Questionnaire (FOSQ).
FOSQ scores were abnormal at baseline in 60% of those with PTSD and OSAS, 43% with PTSD alone, 24% with OSAS alone, and 7% of those with neither condition.
Service members with both conditions also were less likely to adhere to therapy; 30% regularly used continuous PAP therapy, compared with 55% of those who had OSAS alone.
And while continuous PAP therapy improved daytime sleepiness and quality of life in patients with both PTSD and OSAS, the degree of improvement was less than that experienced by those with OSAS alone. PTSD “represents an independent barrier to the effective treatment of OSAS and should prompt multipronged and individualized care,” they wrote.
The researchers reported having no financial disclosures.
Obstructive sleep apnea syndrome (OSAS) was diagnosed in more than half of 200 active duty service members with combat-related post-traumatic stress disorder (PTSD) who were studied at Walter Reed Army Medical Center in Washington.
Compared with age-matched peers with just one of these disorders, the service members with PTSD and OSAS had poorer somnolence and sleep-related quality of life and were less adherent and responsive to positive airway pressure therapy.
The findings “highlight the need for a high index of suspicion and a comprehensive approach to identifying and treating sleep-disordered breathing in these patients,” Dr. Christopher J. Lettieri of the Uniformed Services University in Bethesda, Md., and his associates wrote (Chest. 2016 Feb;149[2]:483-90). “Given the prevalence of OSAS in patients with PTSD and its adverse impact on symptoms and adherence, early identification may improve outcomes.”
In the observational cohort study, 200 consecutive active duty service members who were diagnosed with PTSD as part of post-deployment screening underwent sleep evaluations regardless of whether there was clinical suspicion of sleep-disordered breathing. More than half – about 57% – were diagnosed with OSAS. Almost 60% of the study group had mild traumatic brain injury, which has been connected in prior research to obstructive sleep apnea, and many had comorbid insomnia. Those who were diagnosed with OSAS were older and had higher BMIs than those not found to have OSAS.
All 200 patients were compared with 50 consecutive age-matched control patients who had OSAS but had not been deployed and did not have PTSD, as well as with 50 age-matched service members without prior deployment or either of the two disorders. All of the patients diagnosed with OSAS were prescribed positive airway pressure (PAP) therapy and evaluated after a month.
Sleep quality was poor in the majority of patients with PTSD, and OSAS and PTSD were both independently associated with increased daytime sleepiness and lower quality-of-life index scores. However, patients with both conditions fared significantly worse, particularly with respect to quality of life as measured by the Functional Outcomes of Sleep Questionnaire (FOSQ).
FOSQ scores were abnormal at baseline in 60% of those with PTSD and OSAS, 43% with PTSD alone, 24% with OSAS alone, and 7% of those with neither condition.
Service members with both conditions also were less likely to adhere to therapy; 30% regularly used continuous PAP therapy, compared with 55% of those who had OSAS alone.
And while continuous PAP therapy improved daytime sleepiness and quality of life in patients with both PTSD and OSAS, the degree of improvement was less than that experienced by those with OSAS alone. PTSD “represents an independent barrier to the effective treatment of OSAS and should prompt multipronged and individualized care,” they wrote.
The researchers reported having no financial disclosures.
Obstructive sleep apnea syndrome (OSAS) was diagnosed in more than half of 200 active duty service members with combat-related post-traumatic stress disorder (PTSD) who were studied at Walter Reed Army Medical Center in Washington.
Compared with age-matched peers with just one of these disorders, the service members with PTSD and OSAS had poorer somnolence and sleep-related quality of life and were less adherent and responsive to positive airway pressure therapy.
The findings “highlight the need for a high index of suspicion and a comprehensive approach to identifying and treating sleep-disordered breathing in these patients,” Dr. Christopher J. Lettieri of the Uniformed Services University in Bethesda, Md., and his associates wrote (Chest. 2016 Feb;149[2]:483-90). “Given the prevalence of OSAS in patients with PTSD and its adverse impact on symptoms and adherence, early identification may improve outcomes.”
In the observational cohort study, 200 consecutive active duty service members who were diagnosed with PTSD as part of post-deployment screening underwent sleep evaluations regardless of whether there was clinical suspicion of sleep-disordered breathing. More than half – about 57% – were diagnosed with OSAS. Almost 60% of the study group had mild traumatic brain injury, which has been connected in prior research to obstructive sleep apnea, and many had comorbid insomnia. Those who were diagnosed with OSAS were older and had higher BMIs than those not found to have OSAS.
All 200 patients were compared with 50 consecutive age-matched control patients who had OSAS but had not been deployed and did not have PTSD, as well as with 50 age-matched service members without prior deployment or either of the two disorders. All of the patients diagnosed with OSAS were prescribed positive airway pressure (PAP) therapy and evaluated after a month.
Sleep quality was poor in the majority of patients with PTSD, and OSAS and PTSD were both independently associated with increased daytime sleepiness and lower quality-of-life index scores. However, patients with both conditions fared significantly worse, particularly with respect to quality of life as measured by the Functional Outcomes of Sleep Questionnaire (FOSQ).
FOSQ scores were abnormal at baseline in 60% of those with PTSD and OSAS, 43% with PTSD alone, 24% with OSAS alone, and 7% of those with neither condition.
Service members with both conditions also were less likely to adhere to therapy; 30% regularly used continuous PAP therapy, compared with 55% of those who had OSAS alone.
And while continuous PAP therapy improved daytime sleepiness and quality of life in patients with both PTSD and OSAS, the degree of improvement was less than that experienced by those with OSAS alone. PTSD “represents an independent barrier to the effective treatment of OSAS and should prompt multipronged and individualized care,” they wrote.
The researchers reported having no financial disclosures.
FROM CHEST
Key clinical point: Obstructive sleep apnea is prevalent in service members with PTSD.
Major finding: More than 57% of active duty service members with combat-related PTSD were diagnosed with OSAS.
Data source: A case-controlled observational cohort study conducted at an academic military medical center and involving 200 consecutive patients with PTSD.
Disclosures: Dr. Lettieri and his colleagues did not report any conflicts of interest.
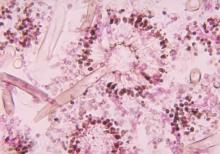
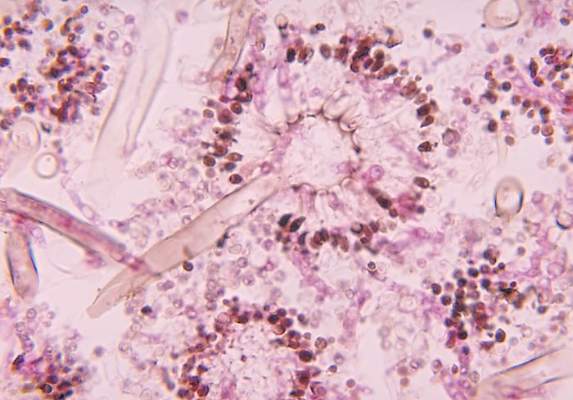
New drug comparable to voriconazole for aspergillosis
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
FROM THE LANCET
Key clinical point: Isavuconazole is an appropriate alternative for primary treatment of suspected invasive aspergillosis.
Major finding: All-cause mortality at 6 weeks in the intention-to-treat group of 516 patients was 19% with isavuconazole and 20% with voriconazole. Fewer drug-related adverse events were reported with isavuconazole, however (42% vs. 60% of patients).
Data source: A phase III randomized, double-blind noninferiority trial – the SECURE trial – comparing the safety and efficacy of intravenous and oral formulations of isavuconazole and voriconazole for the primary treatment of invasive aspergillosis and disease caused by other molds.
Disclosures: Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International. Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees, and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer, during the study.
Bedside asthma medication delivery tied to lower ED readmissions
A bedside medication delivery service increased the percentage of asthma patients discharged with medications in hand from 0% to 75%, which helped prevent emergency department readmissions within the next month, according to an exploratory, retrospective analysis.
“To our knowledge this report is the first to detail specific strategies to reliably discharge patients with meds in hand,” said Dr. Jonathan Hatoun of Boston University Medical Center and his associates. “Although this study was not designed to detect adherence, families may feel more comfortable administering medications after receiving teaching from and having questions answered by their inpatient nurse, using the same medications and equipment they will use at home.”
Before the intervention, the hospital previously had routinely discharged asthma patients without medications in hand, and in 2011, a survey showed that 37% never filled their prescriptions. Concerned that patients were “unnecessarily suffering,” Dr. Hatoun and his associates assembled a multidisciplinary team that worked for 2 years to improve this outcome measure. They initially asked residents to write prescriptions at least a day before discharge, but they were concerned that treatment plans could change. Next, they asked families to pick up medications at the hospital pharmacy, but parents were reluctant to leave their sick child’s bedside. Therefore, the researchers designed an in-room service in which pharmacists delivered the medications to the child’s room when a parent was present (Pediatrics 2016 Feb 24. doi: 10.1542/peds.2015-0461). “Copayments were collected in the room, either in cash or with a mobile credit payment system purchased by the pharmacy,” the researchers explained. “Unlike traditional pharmacy pickup, the delivery service allows the patient, parent, nurse, and pharmacist to be together in the patient’s hospital room for teaching with the actual medications available for demonstration.”
The delivery service not only met the project goal to increase the “meds in hand” rate from 0% to 75%, but an analysis of patients with complete insurance claims showed that patients discharged with medications in hand were significantly less likely to return to the emergency department within 30 days of discharge, for any reason, compared with patients who received usual care (odds ratio, 0.22; 95% confidence interval, 0.05-0.99).
“Although more evidence on the impact of being discharged in possession of discharge medications is needed, a service that provides admitted patients with their outpatient medications before they leave the hospital has many potential benefits,” the investigators noted. “Additional areas of exploration could include how the Meds-in-Hand service affects the patient experience, hospital finances, and clinical outcomes for other medical conditions.”
The authors had no external funding sources or disclosures.
A bedside medication delivery service increased the percentage of asthma patients discharged with medications in hand from 0% to 75%, which helped prevent emergency department readmissions within the next month, according to an exploratory, retrospective analysis.
“To our knowledge this report is the first to detail specific strategies to reliably discharge patients with meds in hand,” said Dr. Jonathan Hatoun of Boston University Medical Center and his associates. “Although this study was not designed to detect adherence, families may feel more comfortable administering medications after receiving teaching from and having questions answered by their inpatient nurse, using the same medications and equipment they will use at home.”
Before the intervention, the hospital previously had routinely discharged asthma patients without medications in hand, and in 2011, a survey showed that 37% never filled their prescriptions. Concerned that patients were “unnecessarily suffering,” Dr. Hatoun and his associates assembled a multidisciplinary team that worked for 2 years to improve this outcome measure. They initially asked residents to write prescriptions at least a day before discharge, but they were concerned that treatment plans could change. Next, they asked families to pick up medications at the hospital pharmacy, but parents were reluctant to leave their sick child’s bedside. Therefore, the researchers designed an in-room service in which pharmacists delivered the medications to the child’s room when a parent was present (Pediatrics 2016 Feb 24. doi: 10.1542/peds.2015-0461). “Copayments were collected in the room, either in cash or with a mobile credit payment system purchased by the pharmacy,” the researchers explained. “Unlike traditional pharmacy pickup, the delivery service allows the patient, parent, nurse, and pharmacist to be together in the patient’s hospital room for teaching with the actual medications available for demonstration.”
The delivery service not only met the project goal to increase the “meds in hand” rate from 0% to 75%, but an analysis of patients with complete insurance claims showed that patients discharged with medications in hand were significantly less likely to return to the emergency department within 30 days of discharge, for any reason, compared with patients who received usual care (odds ratio, 0.22; 95% confidence interval, 0.05-0.99).
“Although more evidence on the impact of being discharged in possession of discharge medications is needed, a service that provides admitted patients with their outpatient medications before they leave the hospital has many potential benefits,” the investigators noted. “Additional areas of exploration could include how the Meds-in-Hand service affects the patient experience, hospital finances, and clinical outcomes for other medical conditions.”
The authors had no external funding sources or disclosures.
A bedside medication delivery service increased the percentage of asthma patients discharged with medications in hand from 0% to 75%, which helped prevent emergency department readmissions within the next month, according to an exploratory, retrospective analysis.
“To our knowledge this report is the first to detail specific strategies to reliably discharge patients with meds in hand,” said Dr. Jonathan Hatoun of Boston University Medical Center and his associates. “Although this study was not designed to detect adherence, families may feel more comfortable administering medications after receiving teaching from and having questions answered by their inpatient nurse, using the same medications and equipment they will use at home.”
Before the intervention, the hospital previously had routinely discharged asthma patients without medications in hand, and in 2011, a survey showed that 37% never filled their prescriptions. Concerned that patients were “unnecessarily suffering,” Dr. Hatoun and his associates assembled a multidisciplinary team that worked for 2 years to improve this outcome measure. They initially asked residents to write prescriptions at least a day before discharge, but they were concerned that treatment plans could change. Next, they asked families to pick up medications at the hospital pharmacy, but parents were reluctant to leave their sick child’s bedside. Therefore, the researchers designed an in-room service in which pharmacists delivered the medications to the child’s room when a parent was present (Pediatrics 2016 Feb 24. doi: 10.1542/peds.2015-0461). “Copayments were collected in the room, either in cash or with a mobile credit payment system purchased by the pharmacy,” the researchers explained. “Unlike traditional pharmacy pickup, the delivery service allows the patient, parent, nurse, and pharmacist to be together in the patient’s hospital room for teaching with the actual medications available for demonstration.”
The delivery service not only met the project goal to increase the “meds in hand” rate from 0% to 75%, but an analysis of patients with complete insurance claims showed that patients discharged with medications in hand were significantly less likely to return to the emergency department within 30 days of discharge, for any reason, compared with patients who received usual care (odds ratio, 0.22; 95% confidence interval, 0.05-0.99).
“Although more evidence on the impact of being discharged in possession of discharge medications is needed, a service that provides admitted patients with their outpatient medications before they leave the hospital has many potential benefits,” the investigators noted. “Additional areas of exploration could include how the Meds-in-Hand service affects the patient experience, hospital finances, and clinical outcomes for other medical conditions.”
The authors had no external funding sources or disclosures.
FROM PEDIATRICS
Key clinical point: A bedside medication delivery service ensured that most children hospitalized with asthma left with medications in hand, helping prevent 30-day readmissions.
Major finding: The rate of discharge with medications in hand rose from 0% to 75%. Discharge with medications in hand was associated with significantly decreased odds of 30-day all-cause emergency department readmission, compared with usual care (odds ratio, 0.22; 95% confidence interval, 0.05-0.99).
Data source: A single-center exploratory retrospective study.
Disclosures: The researchers had no external funding sources and no disclosures.
Helping patients with cystic fibrosis live longer
› Prescribe inhaled dornase alpha and inhaled tobramycin for maintenance pulmonary treatment of moderate to severe cystic fibrosis (CF). A
› Give aggressive nutritional supplementation to maintain a patient’s body mass index and blood sugar control and to attain maximal forced expiratory volume in one second (FEV1). B
› Consider prescribing cystic fibrosis transmembrane conductance regulator modulators, which have demonstrated a 5% to 10% improvement in FEV1 for CF patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The focus of treatment. CF is not limited to the classic picture of lung and pancreas destruction with subsequent loss of function. The underlying pathology can occur in body epithelial tissues from the intestinal lining to sweat glands. These tissues contain cystic fibrosis transmembrane conductance regulator (CFTR), a protein that allows for the transport of chloride across epithelial cell membranes.2 In individuals homozygous for mutated CFTR genes, chloride transport can be impaired. In addition to regulating chloride transport, CFTR is part of a larger, complex interaction of ion transport proteins such as the epithelial sodium channel (ENaC) and others that regulate bicarbonate secretion.2 Decreased chloride ion transport in mutant CFTR negatively affects the ion transport complex; the result is a higher-than-normal viscosity of secreted body fluids.
Reason for hope. It is this impairment of chloride ion transport that leads to the classic phenotypic features of CF (eg, pulmonary function decline, pancreatic insufficiency, malnutrition, chronic respiratory infection), and is the target of both established and emerging therapies3—both of which I will review here.
When to consider a CF diagnosis
Cystic fibrosis remains a clinical diagnosis when evidence of at least one phenotypic feature of the disease (TABLE 1) exists in the presence of laboratory evidence of a CFTR abnormality.4 Confirmation of CFTR dysfunction is demonstrated by an abnormality on sweat testing or identification of a CF-causing mutation in each copy of CFTR (ie, one on each chromosome).5 All 50 states now have neonatal laboratory screening programs;4 despite this, 30% of cases in 2012 were still diagnosed in those older than 1 year of age, with 3% to 5% diagnosed after age 18.1
A sweat chloride reading in the abnormal range (>60 mmol/L) is present in 90% of patients diagnosed with CF in adulthood; this test remains the gold standard in the diagnosis of CF and the initial test of choice in suspected cases.4 Newborn screening programs identify those at risk by detecting persistent hypertrypsinogenemia and referring those with positive results for definitive testing with sweat chloride evaluation. Keep CF in mind when evaluating adolescents and adults who have chronic sinusitis, chronic/recurrent pulmonary infections, chronic/recurrent pancreatitis, or infertility from absence of the vas deferens.4 When features of the CF phenotype are present, especially if there is a known positive family history of CF or CF carrier status, order sweat chloride testing.
Traditional therapies
Both maintenance and acute therapies are directed throughout the body at decreasing fluid viscosity, clearing fluid with a high viscosity, or treating the tissue destruction that results from highly-viscous fluid.3 The traditional classic picture of CF is one of lung and pancreas destruction with subsequent loss of function. However, CF is, in reality, a full-body disease.
Respiratory system: Lungs
CFTR dysfunction in the lungs results in thick pulmonary secretions as the aqueous surface layer (ASL) lining the alveolar epithelium becomes dehydrated and creates a prime environment for the development of chronic infection. What ensues is a recurrent cycle of chronic infection, inflammation, and tissue destruction with loss of lung volume and function. Current therapies interrupt this cycle at multiple points.6
Airway clearance is one of the hallmarks of CF therapy, using both chemical and mechanical treatments. Daily, most patients will use either a therapy vest that administers sheering forces to the chest cavity or an airflow device that creates positive expiratory pressure and laminar flow to aid in expectorating pulmonary secretions.7 Because exercise has yielded comparable results to mechanical or airflow clearance devices, it is recommended that all CF patients who are not otherwise prohibited engage in regular, vigorous exercise in accordance with standard recommendations for the general public.7
Mechanical therapies are often preceded by airway dilation with short- and long-acting bronchodilators and inhaled steroids that open airways for optimal airway clearance.4 Thick secretions can be treated directly and enzymatically with nebulized dornase alpha,4,8 which is also best administered before mechanical clearance therapy. Finally, viscosity of airway secretions can be decreased by improving the hydration of the ASL with nebulized 7% hypertonic saline.4,8
Infection suppression. Thickened pulmonary secretions create a fertile environment for the development of chronic infection. By the time most CF patients reach adulthood, many are colonized with mucoid producing strains of Pseudomonas aeruginosa.4,8-10 Many may also have chronic infection with Staphylococcus aureus, some strains of which may be methicillin-resistant. Quarterly culture and sensitivity results can be essential in directing acute antibiotic therapy, both in the hospital and ambulatory settings. In addition, in the case of Pseudomonas, inhaled antibiotics suppress chronic infection, improve lung function, decrease pulmonary secretions, and reduce inflammation.
Formulations are available for tobramycin and aztreonam, both of which are administered every other month to reduce toxicities and to deter antibiotic resistance. Some patients may use a single agent or may alternate agents every month. When acute antibiotic therapy is necessary for a pulmonary exacerbation, the inhaled agent is generally withheld. If outpatient treatment is warranted, the only available oral antibiotics with anti-pseudomonal activity are ciprofloxacin and levofloxacin.4,8-10S aureus can be treated with trimethoprim/sulfamethoxazole or doxycycline.4,8-10
Inflammation reduction is addressed with high-dose ibuprofen twice daily, azithromycin daily or 3 times weekly, or both. Children up to age 18 benefit from ibuprofen, which also improves forced expiratory volume in one second (FEV1) to a greater extent than azithromycin.8 Adults, however, face the risk of gastrointestinal bleeding and renal dysfunction with ibuprofen, which must be weighed against its potential anti-inflammatory benefit. Both populations, however, benefit from chronic azithromycin, whose mechanism of action in this setting is believed to be more anti-inflammatory than bacterial suppression, since it has no direct bactericidal effect on the primary colonizing microbe, P aeruginosa.4,11
Gastrointestinal system: Pancreas
Cystic fibrosis was first comprehensively described in 1938 and was named for the diseased appearance of the pancreas.12 As happens in the lungs, thick pancreatic duct secretions create a cycle of tissue destruction, inflammation, and dysfunction.2 CF patients lack adequate secretion of pancreatic enzymes and bicarbonate into the small bowel, which progressively leads to pancreatic dysfunction in most patients.
As malabsorption of nutrients advances, patients suffer varying degrees of malnutrition and vitamin deficiency, especially of the fat-soluble vitamins A, D, E, and K. Over 85% of CF patients have deficient pancreatic function, requiring pancreatic enzyme supplementation with all food intake and daily vitamin supplementation.2
Ensuring adequate nutrition. Most CF patients experience a chronic mismatch of dietary intake against caloric expenditure and benefit from aggressive nutritional management featuring a high-calorie diet with supplementation in the form of nutrition shakes or bars.2 There is a well-documented linear relationship between BMI and FEV1. Lung function declines in CF when a man’s body mass index (BMI) falls below 23 kg/m2 and a woman’s BMI drops below 22 kg/m2.2 For this reason, the goal for caloric intake can be as high as 200% of the customary recommended daily allowance.2
Watch for CF-related diabetes. Since the pancreas is also the major source of endogenous insulin, nearly half of adults with CF will develop cystic fibrosis-related diabetes (CFRD) as pancreatic deficiency progresses.13 Similar to the relationship between BMI and FEV1, there is a relationship between glucose intolerance and FEV1. For this reason, annual diabetes screening is recommended for all CF patients ages 10 years and older.13 Because glycated hemoglobin (HbA1c) may not accurately reflect low levels of glucose intolerance, screen for CFRD with a 2-hour 75-g oral glucose tolerance test.13 Early insulin therapy can help maintain BMI and lower average blood sugar in support of FEV1. Once CFRD is diagnosed, the goals and recommendations for control are largely the same as those recommended by the American Diabetes Association for other forms of diabetes.13
Cystic Fibrosis Resources
Cystic Fibrosis Foundation
www.cff.org
Consensus report on cystic fibrosis management
Yankaskas JR, Marchall BC, Sufian B, et al. Cystic fibrosis adult care. Chest. 2004;125:1S-39S.
Consensus report on cystic fibrosis diagnostic guidelines
Farrell PM, Rosenstein BJ, White RB. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation Consensus Report. J Pediatr. 2008;153:S4-S14.
Gastrointestinal system: Alimentary canal
CF is often mistakenly believed to be primarily a pulmonary disease since 85% of the mortality is due to lung dysfunction,7 but intermittent abdominal pain is a common experience for most patients, and disorders can range from gastroesophageal reflux disease (GERD) to small bowel bacterial overgrowth (SBBO) to constipation. Up to 85% of adult patients experience symptoms of reflux, with as many as 40% of cases occurring silently.2 Proton pump inhibitors are a first-line treatment, but they can also contribute to intestinal bacterial overgrowth and pulmonary infections.
In SBBO, gram-negative colonic bacteria colonize the small bowel and can contribute to abdominal pain and malabsorption, weight loss, and malnutrition. Treatment requires antibiotics with activity against gram-negative organisms, or non-absorbable agents such as rifamyxin, sometimes on a chronic, recurrent, or rotating basis.2
Chronic constipation is also quite common among CF patients and many require daily administration of poly-ethylene-glycol. Before newborn screening programs were introduced, infants would on occasion present with complete distal intestinal obstruction. Adults are not immune to obstructive complications and may require hospitalization for bowel cleansing.
Gastrointestinal system: Liver
Liver disease is relatively common in CF, with up to 24% of adults experiencing hepatomegaly or persistently elevated liver function tests (LFT).4 Progressive biliary fibrosis and cirrhosis are encountered more often as the median survival age has increased. There is evidence that ursodeoxycholic acid (UDCA) can be a useful adjunct in the treatment of cholestasis, but it is not clear if it alters mortality or progression to cirrhosis. Only CF patients with elevated LFTs should be started on UDCA.4
Other areas of concern: Sinuses, serum sodium levels
Chronic, symptomatic sinus disease in CF patients—chiefly polyposis—is common and may require repeat surgery, although most patients with extensive nasal polyps find symptom relief with daily sinus rinses. Intranasal steroids and intranasal antibiotics are also often employed, and many CF patients need to be in regular contact with an otolaryngologist.14 For symptoms of allergic rhinitis, recommend OTC antihistamines in standard dosages.
Exercise is recommended for all CF patients, as noted earlier, and as life expectancy increases, many are engaging in more strenuous and longer duration activities.15 Due to high sweat sodium loss, CF patients are at risk for hyponatremia, especially when exercising on days with high temperatures and humidity. CF patients need to replace sodium losses in these conditions and when exercising for extended periods.
There are no evidence-based guidelines for sodium replacement. The Cystic Fibrosis Foundation (CFF) recommends that patients increase salt in the diet when under conditions likely to result in increased sodium loss, such as exercise. It has been thought that CF patients can easily dehydrate due to an impaired thirst mechanism and, when exercising, should consume fluids beyond the need to quench thirst.16,17 More recent evidence suggests, however, that the thirst mechanism in those with CF remains normally intact and that overconsumption of fluids beyond the level of thirst may predispose the individual to exercise-associated hyponatremia as serum sodium is diluted.15
New therapies
Small-molecule CFTR-modulating compounds are a promising development in the treatment of CF. The first such available medication was ivacaftor in 2012. Because these molecules are mutation specific, ivacaftor was available at first only for patients with at least one copy of the G551D mutation,18 which means about 5% of patients with CF.3
Ivacaftor increases the likelihood that the CFTR chloride channel will open and patients will exhibit a reduction in sweat chloride levels. In the first reported clinical trial of ivacaftor involving patients with the G551D mutation, FEV1 improvements of 10% occurred by the second week of therapy and persisted for 48 weeks.18 The drug has now been approved by the US Food and Drug Administration (FDA) for patients 12 years of age and older with at least one of the following mutations: R117H, G551D, G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, or G1349D.19
A medication combining ivacaftor with lumacaftor is also now available for patients with a copy of F508del on both chromosomes. F508del is the most common CFTR mutation, with one copy present in almost 87% of people with CF in the United States.1 Since 47% of CF patients have 2 copies of F508del,1 about half of those with CF in the United States are now eligible for small-molecule therapy. Lumacaftor acts by facilitating transport of a misfolded CFTR to the cell membrane where ivacaftor then increases the probability of an open chloride channel. This combination medication has improved lung function by about 5%.
The ivacaftor/lumacaftor combination was approved by the FDA in July 2015. Both ivacaftor and the ivacaftor/lumacaftor combination were deemed by the FDA to demonstrate statistically significant and sustained FEV1 improvements over placebo.
The CFF was instrumental in providing financial support for the development of both ivacaftor and the ivacaftor/lumacaftor combination and continues to provide significant research advancement. According to the CFF (www.cff.org), medications currently in the development pipeline include compounds that provide CFTR modulation, surface airway liquid restoration, anti-inflammation, inhaled anti-infection, and pancreatic enzyme function. For more on CFF, see "The traditional CF care model.”4,20
The traditional CF care model
The Cystic Fibrosis Foundation (CFF) has been a driving force behind the increased life expectancy CF patients have seen over the last 3 decades. Its contributions include the development of medication through the CFF Therapeutics Development Network (TDN) and disease management through a network of CF Care Centers throughout the United States. The CFF recommends a minimum of quarterly visits to a CF Care Center, and the primary care physician can play a critical role alongside the multidisciplinary CF team.20
At every CF Care Center encounter, the entire team (nurse, physician, dietician, social worker, psychologist) interacts with each patient and their families to maximize overall medical care. Respiratory cultures are generally obtained at each visit. Dual-energy x-ray absorptiometry is performed biannually. Lab work (complete blood count, comprehensive metabolic panel, glycated hemoglobin, vitamins A, D, E, and K, 2-hour glucose tolerance test), and chest x-ray are obtained at least annually (TABLE 2).4
Since CF generally involves both restrictive and obstructive lung components, complete spirometry evaluation is performed annually in the pulmonary function lab, with static lung volumes in addition to airflow measurement. Office spirometry to measure airflow alone is performed at each visit. FEV1 is tracked both as an indicator of disease progression and as a measure of current pulmonary status.
The CFF recommends that each patient receive full genetic testing and encourages patient participation in the CFF Registry, where mutation data are documented among other disease parameters to ensure that patients receive mutation specific therapies as they become available.4 The vaccine schedule recommended for CF patients is the same as for the general population.
CORRESPONDENCE
Douglas Lewis, MD, 1121 S. Clifton, Wichita, KS 67218; [email protected].
1. Cystic Fibrosis Foundation. Patient registry 2012 annual data report. Cystic Fibrosis Foundation Web site. Available at: http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf. Accessed August 14, 2014.
2. Haller W, Ledder O, Lewindon PJ, et al. Cystic fibrosis: An update for clinicians. Part 1: Nutrition and gastrointestinal complications. J Gastroenterol Hepatol. 2014;29:1344-1355.
3. Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics: the road ahead. Chest. 2013;143:207-213.
4. Yankaskas JR, Marshall BC, Sufian B, et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S.
5. Farrell PM, Rosenstein BJ, White TB, et al; Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4-S14.
6. Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631-1636.
7. Flume PA, Robinson KA, O’Sullivan BP, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522-537.
8. Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957-969.
9. Döring G, Flume P, Heijerman H, et al; Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461-479.
10. Flume PA, Mogayzel PJ Jr, Robinson KA, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802-808.
11. Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24:834-838.
12. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344-399.
13. Moran A, Brunzell C, Cohen RC, et al; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708.
14. Kerem E, Conway S, Elborn S, et al; Consensus Committee. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4:7-26.
15. Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25:303-320.
16. Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1177-R1185.
17. Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39:155-160.
18. Ramsey BW, Davies J, McElvaney NG, et al; VX08-770-102 Study Group. ACFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663-1672.
19. Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39:500-511.
20. Lewis D. Role of the family physician in the management of cystic fibrosis. Am Fam Physician. 2015;91:822-824.
› Prescribe inhaled dornase alpha and inhaled tobramycin for maintenance pulmonary treatment of moderate to severe cystic fibrosis (CF). A
› Give aggressive nutritional supplementation to maintain a patient’s body mass index and blood sugar control and to attain maximal forced expiratory volume in one second (FEV1). B
› Consider prescribing cystic fibrosis transmembrane conductance regulator modulators, which have demonstrated a 5% to 10% improvement in FEV1 for CF patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The focus of treatment. CF is not limited to the classic picture of lung and pancreas destruction with subsequent loss of function. The underlying pathology can occur in body epithelial tissues from the intestinal lining to sweat glands. These tissues contain cystic fibrosis transmembrane conductance regulator (CFTR), a protein that allows for the transport of chloride across epithelial cell membranes.2 In individuals homozygous for mutated CFTR genes, chloride transport can be impaired. In addition to regulating chloride transport, CFTR is part of a larger, complex interaction of ion transport proteins such as the epithelial sodium channel (ENaC) and others that regulate bicarbonate secretion.2 Decreased chloride ion transport in mutant CFTR negatively affects the ion transport complex; the result is a higher-than-normal viscosity of secreted body fluids.
Reason for hope. It is this impairment of chloride ion transport that leads to the classic phenotypic features of CF (eg, pulmonary function decline, pancreatic insufficiency, malnutrition, chronic respiratory infection), and is the target of both established and emerging therapies3—both of which I will review here.
When to consider a CF diagnosis
Cystic fibrosis remains a clinical diagnosis when evidence of at least one phenotypic feature of the disease (TABLE 1) exists in the presence of laboratory evidence of a CFTR abnormality.4 Confirmation of CFTR dysfunction is demonstrated by an abnormality on sweat testing or identification of a CF-causing mutation in each copy of CFTR (ie, one on each chromosome).5 All 50 states now have neonatal laboratory screening programs;4 despite this, 30% of cases in 2012 were still diagnosed in those older than 1 year of age, with 3% to 5% diagnosed after age 18.1
A sweat chloride reading in the abnormal range (>60 mmol/L) is present in 90% of patients diagnosed with CF in adulthood; this test remains the gold standard in the diagnosis of CF and the initial test of choice in suspected cases.4 Newborn screening programs identify those at risk by detecting persistent hypertrypsinogenemia and referring those with positive results for definitive testing with sweat chloride evaluation. Keep CF in mind when evaluating adolescents and adults who have chronic sinusitis, chronic/recurrent pulmonary infections, chronic/recurrent pancreatitis, or infertility from absence of the vas deferens.4 When features of the CF phenotype are present, especially if there is a known positive family history of CF or CF carrier status, order sweat chloride testing.
Traditional therapies
Both maintenance and acute therapies are directed throughout the body at decreasing fluid viscosity, clearing fluid with a high viscosity, or treating the tissue destruction that results from highly-viscous fluid.3 The traditional classic picture of CF is one of lung and pancreas destruction with subsequent loss of function. However, CF is, in reality, a full-body disease.
Respiratory system: Lungs
CFTR dysfunction in the lungs results in thick pulmonary secretions as the aqueous surface layer (ASL) lining the alveolar epithelium becomes dehydrated and creates a prime environment for the development of chronic infection. What ensues is a recurrent cycle of chronic infection, inflammation, and tissue destruction with loss of lung volume and function. Current therapies interrupt this cycle at multiple points.6
Airway clearance is one of the hallmarks of CF therapy, using both chemical and mechanical treatments. Daily, most patients will use either a therapy vest that administers sheering forces to the chest cavity or an airflow device that creates positive expiratory pressure and laminar flow to aid in expectorating pulmonary secretions.7 Because exercise has yielded comparable results to mechanical or airflow clearance devices, it is recommended that all CF patients who are not otherwise prohibited engage in regular, vigorous exercise in accordance with standard recommendations for the general public.7
Mechanical therapies are often preceded by airway dilation with short- and long-acting bronchodilators and inhaled steroids that open airways for optimal airway clearance.4 Thick secretions can be treated directly and enzymatically with nebulized dornase alpha,4,8 which is also best administered before mechanical clearance therapy. Finally, viscosity of airway secretions can be decreased by improving the hydration of the ASL with nebulized 7% hypertonic saline.4,8
Infection suppression. Thickened pulmonary secretions create a fertile environment for the development of chronic infection. By the time most CF patients reach adulthood, many are colonized with mucoid producing strains of Pseudomonas aeruginosa.4,8-10 Many may also have chronic infection with Staphylococcus aureus, some strains of which may be methicillin-resistant. Quarterly culture and sensitivity results can be essential in directing acute antibiotic therapy, both in the hospital and ambulatory settings. In addition, in the case of Pseudomonas, inhaled antibiotics suppress chronic infection, improve lung function, decrease pulmonary secretions, and reduce inflammation.
Formulations are available for tobramycin and aztreonam, both of which are administered every other month to reduce toxicities and to deter antibiotic resistance. Some patients may use a single agent or may alternate agents every month. When acute antibiotic therapy is necessary for a pulmonary exacerbation, the inhaled agent is generally withheld. If outpatient treatment is warranted, the only available oral antibiotics with anti-pseudomonal activity are ciprofloxacin and levofloxacin.4,8-10S aureus can be treated with trimethoprim/sulfamethoxazole or doxycycline.4,8-10
Inflammation reduction is addressed with high-dose ibuprofen twice daily, azithromycin daily or 3 times weekly, or both. Children up to age 18 benefit from ibuprofen, which also improves forced expiratory volume in one second (FEV1) to a greater extent than azithromycin.8 Adults, however, face the risk of gastrointestinal bleeding and renal dysfunction with ibuprofen, which must be weighed against its potential anti-inflammatory benefit. Both populations, however, benefit from chronic azithromycin, whose mechanism of action in this setting is believed to be more anti-inflammatory than bacterial suppression, since it has no direct bactericidal effect on the primary colonizing microbe, P aeruginosa.4,11
Gastrointestinal system: Pancreas
Cystic fibrosis was first comprehensively described in 1938 and was named for the diseased appearance of the pancreas.12 As happens in the lungs, thick pancreatic duct secretions create a cycle of tissue destruction, inflammation, and dysfunction.2 CF patients lack adequate secretion of pancreatic enzymes and bicarbonate into the small bowel, which progressively leads to pancreatic dysfunction in most patients.
As malabsorption of nutrients advances, patients suffer varying degrees of malnutrition and vitamin deficiency, especially of the fat-soluble vitamins A, D, E, and K. Over 85% of CF patients have deficient pancreatic function, requiring pancreatic enzyme supplementation with all food intake and daily vitamin supplementation.2
Ensuring adequate nutrition. Most CF patients experience a chronic mismatch of dietary intake against caloric expenditure and benefit from aggressive nutritional management featuring a high-calorie diet with supplementation in the form of nutrition shakes or bars.2 There is a well-documented linear relationship between BMI and FEV1. Lung function declines in CF when a man’s body mass index (BMI) falls below 23 kg/m2 and a woman’s BMI drops below 22 kg/m2.2 For this reason, the goal for caloric intake can be as high as 200% of the customary recommended daily allowance.2
Watch for CF-related diabetes. Since the pancreas is also the major source of endogenous insulin, nearly half of adults with CF will develop cystic fibrosis-related diabetes (CFRD) as pancreatic deficiency progresses.13 Similar to the relationship between BMI and FEV1, there is a relationship between glucose intolerance and FEV1. For this reason, annual diabetes screening is recommended for all CF patients ages 10 years and older.13 Because glycated hemoglobin (HbA1c) may not accurately reflect low levels of glucose intolerance, screen for CFRD with a 2-hour 75-g oral glucose tolerance test.13 Early insulin therapy can help maintain BMI and lower average blood sugar in support of FEV1. Once CFRD is diagnosed, the goals and recommendations for control are largely the same as those recommended by the American Diabetes Association for other forms of diabetes.13
Cystic Fibrosis Resources
Cystic Fibrosis Foundation
www.cff.org
Consensus report on cystic fibrosis management
Yankaskas JR, Marchall BC, Sufian B, et al. Cystic fibrosis adult care. Chest. 2004;125:1S-39S.
Consensus report on cystic fibrosis diagnostic guidelines
Farrell PM, Rosenstein BJ, White RB. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation Consensus Report. J Pediatr. 2008;153:S4-S14.
Gastrointestinal system: Alimentary canal
CF is often mistakenly believed to be primarily a pulmonary disease since 85% of the mortality is due to lung dysfunction,7 but intermittent abdominal pain is a common experience for most patients, and disorders can range from gastroesophageal reflux disease (GERD) to small bowel bacterial overgrowth (SBBO) to constipation. Up to 85% of adult patients experience symptoms of reflux, with as many as 40% of cases occurring silently.2 Proton pump inhibitors are a first-line treatment, but they can also contribute to intestinal bacterial overgrowth and pulmonary infections.
In SBBO, gram-negative colonic bacteria colonize the small bowel and can contribute to abdominal pain and malabsorption, weight loss, and malnutrition. Treatment requires antibiotics with activity against gram-negative organisms, or non-absorbable agents such as rifamyxin, sometimes on a chronic, recurrent, or rotating basis.2
Chronic constipation is also quite common among CF patients and many require daily administration of poly-ethylene-glycol. Before newborn screening programs were introduced, infants would on occasion present with complete distal intestinal obstruction. Adults are not immune to obstructive complications and may require hospitalization for bowel cleansing.
Gastrointestinal system: Liver
Liver disease is relatively common in CF, with up to 24% of adults experiencing hepatomegaly or persistently elevated liver function tests (LFT).4 Progressive biliary fibrosis and cirrhosis are encountered more often as the median survival age has increased. There is evidence that ursodeoxycholic acid (UDCA) can be a useful adjunct in the treatment of cholestasis, but it is not clear if it alters mortality or progression to cirrhosis. Only CF patients with elevated LFTs should be started on UDCA.4
Other areas of concern: Sinuses, serum sodium levels
Chronic, symptomatic sinus disease in CF patients—chiefly polyposis—is common and may require repeat surgery, although most patients with extensive nasal polyps find symptom relief with daily sinus rinses. Intranasal steroids and intranasal antibiotics are also often employed, and many CF patients need to be in regular contact with an otolaryngologist.14 For symptoms of allergic rhinitis, recommend OTC antihistamines in standard dosages.
Exercise is recommended for all CF patients, as noted earlier, and as life expectancy increases, many are engaging in more strenuous and longer duration activities.15 Due to high sweat sodium loss, CF patients are at risk for hyponatremia, especially when exercising on days with high temperatures and humidity. CF patients need to replace sodium losses in these conditions and when exercising for extended periods.
There are no evidence-based guidelines for sodium replacement. The Cystic Fibrosis Foundation (CFF) recommends that patients increase salt in the diet when under conditions likely to result in increased sodium loss, such as exercise. It has been thought that CF patients can easily dehydrate due to an impaired thirst mechanism and, when exercising, should consume fluids beyond the need to quench thirst.16,17 More recent evidence suggests, however, that the thirst mechanism in those with CF remains normally intact and that overconsumption of fluids beyond the level of thirst may predispose the individual to exercise-associated hyponatremia as serum sodium is diluted.15
New therapies
Small-molecule CFTR-modulating compounds are a promising development in the treatment of CF. The first such available medication was ivacaftor in 2012. Because these molecules are mutation specific, ivacaftor was available at first only for patients with at least one copy of the G551D mutation,18 which means about 5% of patients with CF.3
Ivacaftor increases the likelihood that the CFTR chloride channel will open and patients will exhibit a reduction in sweat chloride levels. In the first reported clinical trial of ivacaftor involving patients with the G551D mutation, FEV1 improvements of 10% occurred by the second week of therapy and persisted for 48 weeks.18 The drug has now been approved by the US Food and Drug Administration (FDA) for patients 12 years of age and older with at least one of the following mutations: R117H, G551D, G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, or G1349D.19
A medication combining ivacaftor with lumacaftor is also now available for patients with a copy of F508del on both chromosomes. F508del is the most common CFTR mutation, with one copy present in almost 87% of people with CF in the United States.1 Since 47% of CF patients have 2 copies of F508del,1 about half of those with CF in the United States are now eligible for small-molecule therapy. Lumacaftor acts by facilitating transport of a misfolded CFTR to the cell membrane where ivacaftor then increases the probability of an open chloride channel. This combination medication has improved lung function by about 5%.
The ivacaftor/lumacaftor combination was approved by the FDA in July 2015. Both ivacaftor and the ivacaftor/lumacaftor combination were deemed by the FDA to demonstrate statistically significant and sustained FEV1 improvements over placebo.
The CFF was instrumental in providing financial support for the development of both ivacaftor and the ivacaftor/lumacaftor combination and continues to provide significant research advancement. According to the CFF (www.cff.org), medications currently in the development pipeline include compounds that provide CFTR modulation, surface airway liquid restoration, anti-inflammation, inhaled anti-infection, and pancreatic enzyme function. For more on CFF, see "The traditional CF care model.”4,20
The traditional CF care model
The Cystic Fibrosis Foundation (CFF) has been a driving force behind the increased life expectancy CF patients have seen over the last 3 decades. Its contributions include the development of medication through the CFF Therapeutics Development Network (TDN) and disease management through a network of CF Care Centers throughout the United States. The CFF recommends a minimum of quarterly visits to a CF Care Center, and the primary care physician can play a critical role alongside the multidisciplinary CF team.20
At every CF Care Center encounter, the entire team (nurse, physician, dietician, social worker, psychologist) interacts with each patient and their families to maximize overall medical care. Respiratory cultures are generally obtained at each visit. Dual-energy x-ray absorptiometry is performed biannually. Lab work (complete blood count, comprehensive metabolic panel, glycated hemoglobin, vitamins A, D, E, and K, 2-hour glucose tolerance test), and chest x-ray are obtained at least annually (TABLE 2).4
Since CF generally involves both restrictive and obstructive lung components, complete spirometry evaluation is performed annually in the pulmonary function lab, with static lung volumes in addition to airflow measurement. Office spirometry to measure airflow alone is performed at each visit. FEV1 is tracked both as an indicator of disease progression and as a measure of current pulmonary status.
The CFF recommends that each patient receive full genetic testing and encourages patient participation in the CFF Registry, where mutation data are documented among other disease parameters to ensure that patients receive mutation specific therapies as they become available.4 The vaccine schedule recommended for CF patients is the same as for the general population.
CORRESPONDENCE
Douglas Lewis, MD, 1121 S. Clifton, Wichita, KS 67218; [email protected].
› Prescribe inhaled dornase alpha and inhaled tobramycin for maintenance pulmonary treatment of moderate to severe cystic fibrosis (CF). A
› Give aggressive nutritional supplementation to maintain a patient’s body mass index and blood sugar control and to attain maximal forced expiratory volume in one second (FEV1). B
› Consider prescribing cystic fibrosis transmembrane conductance regulator modulators, which have demonstrated a 5% to 10% improvement in FEV1 for CF patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The focus of treatment. CF is not limited to the classic picture of lung and pancreas destruction with subsequent loss of function. The underlying pathology can occur in body epithelial tissues from the intestinal lining to sweat glands. These tissues contain cystic fibrosis transmembrane conductance regulator (CFTR), a protein that allows for the transport of chloride across epithelial cell membranes.2 In individuals homozygous for mutated CFTR genes, chloride transport can be impaired. In addition to regulating chloride transport, CFTR is part of a larger, complex interaction of ion transport proteins such as the epithelial sodium channel (ENaC) and others that regulate bicarbonate secretion.2 Decreased chloride ion transport in mutant CFTR negatively affects the ion transport complex; the result is a higher-than-normal viscosity of secreted body fluids.
Reason for hope. It is this impairment of chloride ion transport that leads to the classic phenotypic features of CF (eg, pulmonary function decline, pancreatic insufficiency, malnutrition, chronic respiratory infection), and is the target of both established and emerging therapies3—both of which I will review here.
When to consider a CF diagnosis
Cystic fibrosis remains a clinical diagnosis when evidence of at least one phenotypic feature of the disease (TABLE 1) exists in the presence of laboratory evidence of a CFTR abnormality.4 Confirmation of CFTR dysfunction is demonstrated by an abnormality on sweat testing or identification of a CF-causing mutation in each copy of CFTR (ie, one on each chromosome).5 All 50 states now have neonatal laboratory screening programs;4 despite this, 30% of cases in 2012 were still diagnosed in those older than 1 year of age, with 3% to 5% diagnosed after age 18.1
A sweat chloride reading in the abnormal range (>60 mmol/L) is present in 90% of patients diagnosed with CF in adulthood; this test remains the gold standard in the diagnosis of CF and the initial test of choice in suspected cases.4 Newborn screening programs identify those at risk by detecting persistent hypertrypsinogenemia and referring those with positive results for definitive testing with sweat chloride evaluation. Keep CF in mind when evaluating adolescents and adults who have chronic sinusitis, chronic/recurrent pulmonary infections, chronic/recurrent pancreatitis, or infertility from absence of the vas deferens.4 When features of the CF phenotype are present, especially if there is a known positive family history of CF or CF carrier status, order sweat chloride testing.
Traditional therapies
Both maintenance and acute therapies are directed throughout the body at decreasing fluid viscosity, clearing fluid with a high viscosity, or treating the tissue destruction that results from highly-viscous fluid.3 The traditional classic picture of CF is one of lung and pancreas destruction with subsequent loss of function. However, CF is, in reality, a full-body disease.
Respiratory system: Lungs
CFTR dysfunction in the lungs results in thick pulmonary secretions as the aqueous surface layer (ASL) lining the alveolar epithelium becomes dehydrated and creates a prime environment for the development of chronic infection. What ensues is a recurrent cycle of chronic infection, inflammation, and tissue destruction with loss of lung volume and function. Current therapies interrupt this cycle at multiple points.6
Airway clearance is one of the hallmarks of CF therapy, using both chemical and mechanical treatments. Daily, most patients will use either a therapy vest that administers sheering forces to the chest cavity or an airflow device that creates positive expiratory pressure and laminar flow to aid in expectorating pulmonary secretions.7 Because exercise has yielded comparable results to mechanical or airflow clearance devices, it is recommended that all CF patients who are not otherwise prohibited engage in regular, vigorous exercise in accordance with standard recommendations for the general public.7
Mechanical therapies are often preceded by airway dilation with short- and long-acting bronchodilators and inhaled steroids that open airways for optimal airway clearance.4 Thick secretions can be treated directly and enzymatically with nebulized dornase alpha,4,8 which is also best administered before mechanical clearance therapy. Finally, viscosity of airway secretions can be decreased by improving the hydration of the ASL with nebulized 7% hypertonic saline.4,8
Infection suppression. Thickened pulmonary secretions create a fertile environment for the development of chronic infection. By the time most CF patients reach adulthood, many are colonized with mucoid producing strains of Pseudomonas aeruginosa.4,8-10 Many may also have chronic infection with Staphylococcus aureus, some strains of which may be methicillin-resistant. Quarterly culture and sensitivity results can be essential in directing acute antibiotic therapy, both in the hospital and ambulatory settings. In addition, in the case of Pseudomonas, inhaled antibiotics suppress chronic infection, improve lung function, decrease pulmonary secretions, and reduce inflammation.
Formulations are available for tobramycin and aztreonam, both of which are administered every other month to reduce toxicities and to deter antibiotic resistance. Some patients may use a single agent or may alternate agents every month. When acute antibiotic therapy is necessary for a pulmonary exacerbation, the inhaled agent is generally withheld. If outpatient treatment is warranted, the only available oral antibiotics with anti-pseudomonal activity are ciprofloxacin and levofloxacin.4,8-10S aureus can be treated with trimethoprim/sulfamethoxazole or doxycycline.4,8-10
Inflammation reduction is addressed with high-dose ibuprofen twice daily, azithromycin daily or 3 times weekly, or both. Children up to age 18 benefit from ibuprofen, which also improves forced expiratory volume in one second (FEV1) to a greater extent than azithromycin.8 Adults, however, face the risk of gastrointestinal bleeding and renal dysfunction with ibuprofen, which must be weighed against its potential anti-inflammatory benefit. Both populations, however, benefit from chronic azithromycin, whose mechanism of action in this setting is believed to be more anti-inflammatory than bacterial suppression, since it has no direct bactericidal effect on the primary colonizing microbe, P aeruginosa.4,11
Gastrointestinal system: Pancreas
Cystic fibrosis was first comprehensively described in 1938 and was named for the diseased appearance of the pancreas.12 As happens in the lungs, thick pancreatic duct secretions create a cycle of tissue destruction, inflammation, and dysfunction.2 CF patients lack adequate secretion of pancreatic enzymes and bicarbonate into the small bowel, which progressively leads to pancreatic dysfunction in most patients.
As malabsorption of nutrients advances, patients suffer varying degrees of malnutrition and vitamin deficiency, especially of the fat-soluble vitamins A, D, E, and K. Over 85% of CF patients have deficient pancreatic function, requiring pancreatic enzyme supplementation with all food intake and daily vitamin supplementation.2
Ensuring adequate nutrition. Most CF patients experience a chronic mismatch of dietary intake against caloric expenditure and benefit from aggressive nutritional management featuring a high-calorie diet with supplementation in the form of nutrition shakes or bars.2 There is a well-documented linear relationship between BMI and FEV1. Lung function declines in CF when a man’s body mass index (BMI) falls below 23 kg/m2 and a woman’s BMI drops below 22 kg/m2.2 For this reason, the goal for caloric intake can be as high as 200% of the customary recommended daily allowance.2
Watch for CF-related diabetes. Since the pancreas is also the major source of endogenous insulin, nearly half of adults with CF will develop cystic fibrosis-related diabetes (CFRD) as pancreatic deficiency progresses.13 Similar to the relationship between BMI and FEV1, there is a relationship between glucose intolerance and FEV1. For this reason, annual diabetes screening is recommended for all CF patients ages 10 years and older.13 Because glycated hemoglobin (HbA1c) may not accurately reflect low levels of glucose intolerance, screen for CFRD with a 2-hour 75-g oral glucose tolerance test.13 Early insulin therapy can help maintain BMI and lower average blood sugar in support of FEV1. Once CFRD is diagnosed, the goals and recommendations for control are largely the same as those recommended by the American Diabetes Association for other forms of diabetes.13
Cystic Fibrosis Resources
Cystic Fibrosis Foundation
www.cff.org
Consensus report on cystic fibrosis management
Yankaskas JR, Marchall BC, Sufian B, et al. Cystic fibrosis adult care. Chest. 2004;125:1S-39S.
Consensus report on cystic fibrosis diagnostic guidelines
Farrell PM, Rosenstein BJ, White RB. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation Consensus Report. J Pediatr. 2008;153:S4-S14.
Gastrointestinal system: Alimentary canal
CF is often mistakenly believed to be primarily a pulmonary disease since 85% of the mortality is due to lung dysfunction,7 but intermittent abdominal pain is a common experience for most patients, and disorders can range from gastroesophageal reflux disease (GERD) to small bowel bacterial overgrowth (SBBO) to constipation. Up to 85% of adult patients experience symptoms of reflux, with as many as 40% of cases occurring silently.2 Proton pump inhibitors are a first-line treatment, but they can also contribute to intestinal bacterial overgrowth and pulmonary infections.
In SBBO, gram-negative colonic bacteria colonize the small bowel and can contribute to abdominal pain and malabsorption, weight loss, and malnutrition. Treatment requires antibiotics with activity against gram-negative organisms, or non-absorbable agents such as rifamyxin, sometimes on a chronic, recurrent, or rotating basis.2
Chronic constipation is also quite common among CF patients and many require daily administration of poly-ethylene-glycol. Before newborn screening programs were introduced, infants would on occasion present with complete distal intestinal obstruction. Adults are not immune to obstructive complications and may require hospitalization for bowel cleansing.
Gastrointestinal system: Liver
Liver disease is relatively common in CF, with up to 24% of adults experiencing hepatomegaly or persistently elevated liver function tests (LFT).4 Progressive biliary fibrosis and cirrhosis are encountered more often as the median survival age has increased. There is evidence that ursodeoxycholic acid (UDCA) can be a useful adjunct in the treatment of cholestasis, but it is not clear if it alters mortality or progression to cirrhosis. Only CF patients with elevated LFTs should be started on UDCA.4
Other areas of concern: Sinuses, serum sodium levels
Chronic, symptomatic sinus disease in CF patients—chiefly polyposis—is common and may require repeat surgery, although most patients with extensive nasal polyps find symptom relief with daily sinus rinses. Intranasal steroids and intranasal antibiotics are also often employed, and many CF patients need to be in regular contact with an otolaryngologist.14 For symptoms of allergic rhinitis, recommend OTC antihistamines in standard dosages.
Exercise is recommended for all CF patients, as noted earlier, and as life expectancy increases, many are engaging in more strenuous and longer duration activities.15 Due to high sweat sodium loss, CF patients are at risk for hyponatremia, especially when exercising on days with high temperatures and humidity. CF patients need to replace sodium losses in these conditions and when exercising for extended periods.
There are no evidence-based guidelines for sodium replacement. The Cystic Fibrosis Foundation (CFF) recommends that patients increase salt in the diet when under conditions likely to result in increased sodium loss, such as exercise. It has been thought that CF patients can easily dehydrate due to an impaired thirst mechanism and, when exercising, should consume fluids beyond the need to quench thirst.16,17 More recent evidence suggests, however, that the thirst mechanism in those with CF remains normally intact and that overconsumption of fluids beyond the level of thirst may predispose the individual to exercise-associated hyponatremia as serum sodium is diluted.15
New therapies
Small-molecule CFTR-modulating compounds are a promising development in the treatment of CF. The first such available medication was ivacaftor in 2012. Because these molecules are mutation specific, ivacaftor was available at first only for patients with at least one copy of the G551D mutation,18 which means about 5% of patients with CF.3
Ivacaftor increases the likelihood that the CFTR chloride channel will open and patients will exhibit a reduction in sweat chloride levels. In the first reported clinical trial of ivacaftor involving patients with the G551D mutation, FEV1 improvements of 10% occurred by the second week of therapy and persisted for 48 weeks.18 The drug has now been approved by the US Food and Drug Administration (FDA) for patients 12 years of age and older with at least one of the following mutations: R117H, G551D, G178R, S549N, S549R, G551S, G1244E, S1251N, S1255P, or G1349D.19
A medication combining ivacaftor with lumacaftor is also now available for patients with a copy of F508del on both chromosomes. F508del is the most common CFTR mutation, with one copy present in almost 87% of people with CF in the United States.1 Since 47% of CF patients have 2 copies of F508del,1 about half of those with CF in the United States are now eligible for small-molecule therapy. Lumacaftor acts by facilitating transport of a misfolded CFTR to the cell membrane where ivacaftor then increases the probability of an open chloride channel. This combination medication has improved lung function by about 5%.
The ivacaftor/lumacaftor combination was approved by the FDA in July 2015. Both ivacaftor and the ivacaftor/lumacaftor combination were deemed by the FDA to demonstrate statistically significant and sustained FEV1 improvements over placebo.
The CFF was instrumental in providing financial support for the development of both ivacaftor and the ivacaftor/lumacaftor combination and continues to provide significant research advancement. According to the CFF (www.cff.org), medications currently in the development pipeline include compounds that provide CFTR modulation, surface airway liquid restoration, anti-inflammation, inhaled anti-infection, and pancreatic enzyme function. For more on CFF, see "The traditional CF care model.”4,20
The traditional CF care model
The Cystic Fibrosis Foundation (CFF) has been a driving force behind the increased life expectancy CF patients have seen over the last 3 decades. Its contributions include the development of medication through the CFF Therapeutics Development Network (TDN) and disease management through a network of CF Care Centers throughout the United States. The CFF recommends a minimum of quarterly visits to a CF Care Center, and the primary care physician can play a critical role alongside the multidisciplinary CF team.20
At every CF Care Center encounter, the entire team (nurse, physician, dietician, social worker, psychologist) interacts with each patient and their families to maximize overall medical care. Respiratory cultures are generally obtained at each visit. Dual-energy x-ray absorptiometry is performed biannually. Lab work (complete blood count, comprehensive metabolic panel, glycated hemoglobin, vitamins A, D, E, and K, 2-hour glucose tolerance test), and chest x-ray are obtained at least annually (TABLE 2).4
Since CF generally involves both restrictive and obstructive lung components, complete spirometry evaluation is performed annually in the pulmonary function lab, with static lung volumes in addition to airflow measurement. Office spirometry to measure airflow alone is performed at each visit. FEV1 is tracked both as an indicator of disease progression and as a measure of current pulmonary status.
The CFF recommends that each patient receive full genetic testing and encourages patient participation in the CFF Registry, where mutation data are documented among other disease parameters to ensure that patients receive mutation specific therapies as they become available.4 The vaccine schedule recommended for CF patients is the same as for the general population.
CORRESPONDENCE
Douglas Lewis, MD, 1121 S. Clifton, Wichita, KS 67218; [email protected].
1. Cystic Fibrosis Foundation. Patient registry 2012 annual data report. Cystic Fibrosis Foundation Web site. Available at: http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf. Accessed August 14, 2014.
2. Haller W, Ledder O, Lewindon PJ, et al. Cystic fibrosis: An update for clinicians. Part 1: Nutrition and gastrointestinal complications. J Gastroenterol Hepatol. 2014;29:1344-1355.
3. Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics: the road ahead. Chest. 2013;143:207-213.
4. Yankaskas JR, Marshall BC, Sufian B, et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S.
5. Farrell PM, Rosenstein BJ, White TB, et al; Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4-S14.
6. Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631-1636.
7. Flume PA, Robinson KA, O’Sullivan BP, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522-537.
8. Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957-969.
9. Döring G, Flume P, Heijerman H, et al; Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461-479.
10. Flume PA, Mogayzel PJ Jr, Robinson KA, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802-808.
11. Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24:834-838.
12. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344-399.
13. Moran A, Brunzell C, Cohen RC, et al; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708.
14. Kerem E, Conway S, Elborn S, et al; Consensus Committee. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4:7-26.
15. Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25:303-320.
16. Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1177-R1185.
17. Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39:155-160.
18. Ramsey BW, Davies J, McElvaney NG, et al; VX08-770-102 Study Group. ACFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663-1672.
19. Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39:500-511.
20. Lewis D. Role of the family physician in the management of cystic fibrosis. Am Fam Physician. 2015;91:822-824.
1. Cystic Fibrosis Foundation. Patient registry 2012 annual data report. Cystic Fibrosis Foundation Web site. Available at: http://www.cff.org/UploadedFiles/research/ClinicalResearch/PatientRegistryReport/2012-CFF-Patient-Registry.pdf. Accessed August 14, 2014.
2. Haller W, Ledder O, Lewindon PJ, et al. Cystic fibrosis: An update for clinicians. Part 1: Nutrition and gastrointestinal complications. J Gastroenterol Hepatol. 2014;29:1344-1355.
3. Hoffman LR, Ramsey BW. Cystic fibrosis therapeutics: the road ahead. Chest. 2013;143:207-213.
4. Yankaskas JR, Marshall BC, Sufian B, et al. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S.
5. Farrell PM, Rosenstein BJ, White TB, et al; Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4-S14.
6. Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631-1636.
7. Flume PA, Robinson KA, O’Sullivan BP, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522-537.
8. Flume PA, O’Sullivan BP, Robinson KA, et al; Cystic Fibrosis Foundation, Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957-969.
9. Döring G, Flume P, Heijerman H, et al; Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461-479.
10. Flume PA, Mogayzel PJ Jr, Robinson KA, et al; Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802-808.
11. Southern KW, Barker PM. Azithromycin for cystic fibrosis. Eur Respir J. 2004;24:834-838.
12. Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am J Dis Child. 1938;56:344-399.
13. Moran A, Brunzell C, Cohen RC, et al; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708.
14. Kerem E, Conway S, Elborn S, et al; Consensus Committee. Standards of care for patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2005;4:7-26.
15. Hew-Butler T, Rosner MH, Fowkes-Godek S, et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin J Sport Med. 2015;25:303-320.
16. Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1177-R1185.
17. Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exerc Sport Sci Rev. 2011;39:155-160.
18. Ramsey BW, Davies J, McElvaney NG, et al; VX08-770-102 Study Group. ACFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663-1672.
19. Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39:500-511.
20. Lewis D. Role of the family physician in the management of cystic fibrosis. Am Fam Physician. 2015;91:822-824.
Asthma hospitalizations linked to prevalence of common cold in children
Daily viral prevalence was the greatest predictor of asthma hospitalizations in children, according to Rosalind M. Eggo and her associates.
Asthma hospitalizations in children occurred most frequently during the school year, and dropped off dramatically when school was out. Increases were not driven by triggers within the school environment, but by increased transmission and prevalence of viruses such as the common cold, the researchers noted. Common cold transmission rates were 45% lower during school holidays and breaks, and asthma hospitalizations decreased accordingly.
In adults, asthma hospitalizations were greatest during the winter and were most influenced by influenza prevalence. Low temperatures were an important risk factor for both groups; however, ozone and particulate matter were not.
“In general, future risk assessments and interventions for asthma, particularly in children, should explicitly consider both the school calendar and the seasonal dynamic of infectious triggers, either through spatiotemporal modeling or when possible, viral surveillance data,” the investigators concluded.
Find the study in PNAS (doi: 10.1073/pnas.1518677113).
Daily viral prevalence was the greatest predictor of asthma hospitalizations in children, according to Rosalind M. Eggo and her associates.
Asthma hospitalizations in children occurred most frequently during the school year, and dropped off dramatically when school was out. Increases were not driven by triggers within the school environment, but by increased transmission and prevalence of viruses such as the common cold, the researchers noted. Common cold transmission rates were 45% lower during school holidays and breaks, and asthma hospitalizations decreased accordingly.
In adults, asthma hospitalizations were greatest during the winter and were most influenced by influenza prevalence. Low temperatures were an important risk factor for both groups; however, ozone and particulate matter were not.
“In general, future risk assessments and interventions for asthma, particularly in children, should explicitly consider both the school calendar and the seasonal dynamic of infectious triggers, either through spatiotemporal modeling or when possible, viral surveillance data,” the investigators concluded.
Find the study in PNAS (doi: 10.1073/pnas.1518677113).
Daily viral prevalence was the greatest predictor of asthma hospitalizations in children, according to Rosalind M. Eggo and her associates.
Asthma hospitalizations in children occurred most frequently during the school year, and dropped off dramatically when school was out. Increases were not driven by triggers within the school environment, but by increased transmission and prevalence of viruses such as the common cold, the researchers noted. Common cold transmission rates were 45% lower during school holidays and breaks, and asthma hospitalizations decreased accordingly.
In adults, asthma hospitalizations were greatest during the winter and were most influenced by influenza prevalence. Low temperatures were an important risk factor for both groups; however, ozone and particulate matter were not.
“In general, future risk assessments and interventions for asthma, particularly in children, should explicitly consider both the school calendar and the seasonal dynamic of infectious triggers, either through spatiotemporal modeling or when possible, viral surveillance data,” the investigators concluded.
Find the study in PNAS (doi: 10.1073/pnas.1518677113).
FROM PNAS
U.S. flu activity continues steady climb
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
LUNG SAFE: Globally, ARDS is under-recognized, undertreated
ORLANDO – Many patients meeting the criteria for acute respiratory distress syndrome (ARDS) went unrecognized in a global sample of ICU patients, and those ARDS patients did not receive adjusted ventilator management or positioning and pharmacologic adjunctive treatments, based on the results of the LUNG SAFE study.
Enrolling nearly 30,000 patients in 50 countries on five continents, the LUNG SAFE study (Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure) looked for real-world answers to whether and how patients with ARDS are treated. The LUNG SAFE results were published concurrently with the presentation of results in a late-breaking session at the Critical Care Congress, sponsored by the Society of Critical Care Medicine (JAMA. 2016;315[8]:759-61). The first author is Dr. Giacomo Bellani, professor of medicine at the University of Milan-Bicocca, Monza, Italy, and Dr. John Laffey, professor of anesthesia, critical care, and physiology at the University of Toronto, presented the results at the meeting.
About 10% of the enrolled patients met ARDS criteria; of those, less than two-thirds received ventilator tidal volumes of 8 mL/kg or less of predicted body weight. Fewer than 18% of patients received positive end–expiratory pressure (PEEP) of more than 12 cm H2O, and clinicians used prone positioning for about 16% of patients with severe ARDS.
Clinicians recognized 60.2% of ARDS cases overall; recognition ranged from 51.3% of the cases of mild ARDS to 78.5% of the severe ARDS cases. For all patients, ARDS was associated with an in-hospital mortality rate of 40%. Nearly half of those with severe ARDS died, as did over a third of those with mild ARDS.
To this end, the LUNG SAFE investigators chose 4 consecutive weeks in the winter to enroll patients from a convenience sample of ICUs that they attempted to make broadly representative. They enrolled during February and March 2014 in the Northern hemisphere and July and August 2014 in the Southern hemisphere, and included all patients 16 years and older who were admitted to a participating ICU and received invasive or noninvasive ventilation.
Enrolled patients received daily evaluation for acute hypoxemic respiratory failure. Patients who met these criteria were then tracked with expanded data collection up to day 28 after respiratory failure was identified, or until ICU discharge or death. Overall, 3,022 patients met the Berlin Definition for ARDS. All but 436 patients (85.4%) received invasive ventilation, and those who did not were excluded from most data analysis.
One unexpected finding, said Dr. Laffey in an interview, was how common ARDS was in this ICU population. “Based on prior studies, we had anticipated finding an incidence of ARDS of approximately half of what we actually found in the LUNG SAFE study. We think that the difference is explained by the fact that we did not rely on clinician recognition of ARDS, but rather collected data directly on each of the Berlin diagnostic criteria, enabling us to make the diagnosis directly.” One possibility is that choosing the winter months for data collection may have resulted in overrepresentation of ARDS.
But Dr. Laffey said that LUNG SAFE’s most surprising finding was the low percentage of clinicians using higher PEEP levels. “It appeared that clinicians used lower-than-expected levels of PEEP, and that the use of PEEP didn’t increase in patients with the more severe forms of ARDS,” he said. “We think we need to increase our efforts to find more reliable ways to diagnose ARDS,” said Dr. Laffey. “While the reasons underlying clinician failure to recognize ARDS in critically ill patients are complex, the fact that there is no single test for diagnosing ARDS is a likely contributing factor.”
“This finding likely reflects the lack of a clear evidence base for the effectiveness of higher levels of PEEP in patients with ARDS” said Dr. Laffey. “It emphasizes the need for additional research to answer this and other important questions relating to the optimal treatment of patients with ARDS.”
However, if physicians did recognize ARDS, then they were more likely to use higher PEEPs (mean 8.9 cm H2O vs. 7.5 cm H2O for nonrecognized ARDS; P less than .001), prone positioning, and neuromuscular blockade (43.9% adjunctive treatment vs. 21.7% adjunctive treatment for nonrecognized ARDS; P less than .001), though they didn’t adjust the breath size used in ventilation.
In multivariable analysis, factors that made it more likely that ARDS would be recognized were higher nurse-to-patient and physician-to-patient ratios, younger patient age, lower Pa02/Fi02 ratios, and a pneumonia or pancreatitis diagnosis. Patients without an identified risk factor, and those with heart failure, were less likely to be diagnosed with ARDS.
The study was supported by the European Society of Intensive Care Medicine, by St. Michael’s Hospital, Toronto, and by the University of Milan-Bicocca, Monza, Italy. The authors reported no conflicts of interest.
On Twitter @karioakes
ORLANDO – Many patients meeting the criteria for acute respiratory distress syndrome (ARDS) went unrecognized in a global sample of ICU patients, and those ARDS patients did not receive adjusted ventilator management or positioning and pharmacologic adjunctive treatments, based on the results of the LUNG SAFE study.
Enrolling nearly 30,000 patients in 50 countries on five continents, the LUNG SAFE study (Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure) looked for real-world answers to whether and how patients with ARDS are treated. The LUNG SAFE results were published concurrently with the presentation of results in a late-breaking session at the Critical Care Congress, sponsored by the Society of Critical Care Medicine (JAMA. 2016;315[8]:759-61). The first author is Dr. Giacomo Bellani, professor of medicine at the University of Milan-Bicocca, Monza, Italy, and Dr. John Laffey, professor of anesthesia, critical care, and physiology at the University of Toronto, presented the results at the meeting.
About 10% of the enrolled patients met ARDS criteria; of those, less than two-thirds received ventilator tidal volumes of 8 mL/kg or less of predicted body weight. Fewer than 18% of patients received positive end–expiratory pressure (PEEP) of more than 12 cm H2O, and clinicians used prone positioning for about 16% of patients with severe ARDS.
Clinicians recognized 60.2% of ARDS cases overall; recognition ranged from 51.3% of the cases of mild ARDS to 78.5% of the severe ARDS cases. For all patients, ARDS was associated with an in-hospital mortality rate of 40%. Nearly half of those with severe ARDS died, as did over a third of those with mild ARDS.
To this end, the LUNG SAFE investigators chose 4 consecutive weeks in the winter to enroll patients from a convenience sample of ICUs that they attempted to make broadly representative. They enrolled during February and March 2014 in the Northern hemisphere and July and August 2014 in the Southern hemisphere, and included all patients 16 years and older who were admitted to a participating ICU and received invasive or noninvasive ventilation.
Enrolled patients received daily evaluation for acute hypoxemic respiratory failure. Patients who met these criteria were then tracked with expanded data collection up to day 28 after respiratory failure was identified, or until ICU discharge or death. Overall, 3,022 patients met the Berlin Definition for ARDS. All but 436 patients (85.4%) received invasive ventilation, and those who did not were excluded from most data analysis.
One unexpected finding, said Dr. Laffey in an interview, was how common ARDS was in this ICU population. “Based on prior studies, we had anticipated finding an incidence of ARDS of approximately half of what we actually found in the LUNG SAFE study. We think that the difference is explained by the fact that we did not rely on clinician recognition of ARDS, but rather collected data directly on each of the Berlin diagnostic criteria, enabling us to make the diagnosis directly.” One possibility is that choosing the winter months for data collection may have resulted in overrepresentation of ARDS.
But Dr. Laffey said that LUNG SAFE’s most surprising finding was the low percentage of clinicians using higher PEEP levels. “It appeared that clinicians used lower-than-expected levels of PEEP, and that the use of PEEP didn’t increase in patients with the more severe forms of ARDS,” he said. “We think we need to increase our efforts to find more reliable ways to diagnose ARDS,” said Dr. Laffey. “While the reasons underlying clinician failure to recognize ARDS in critically ill patients are complex, the fact that there is no single test for diagnosing ARDS is a likely contributing factor.”
“This finding likely reflects the lack of a clear evidence base for the effectiveness of higher levels of PEEP in patients with ARDS” said Dr. Laffey. “It emphasizes the need for additional research to answer this and other important questions relating to the optimal treatment of patients with ARDS.”
However, if physicians did recognize ARDS, then they were more likely to use higher PEEPs (mean 8.9 cm H2O vs. 7.5 cm H2O for nonrecognized ARDS; P less than .001), prone positioning, and neuromuscular blockade (43.9% adjunctive treatment vs. 21.7% adjunctive treatment for nonrecognized ARDS; P less than .001), though they didn’t adjust the breath size used in ventilation.
In multivariable analysis, factors that made it more likely that ARDS would be recognized were higher nurse-to-patient and physician-to-patient ratios, younger patient age, lower Pa02/Fi02 ratios, and a pneumonia or pancreatitis diagnosis. Patients without an identified risk factor, and those with heart failure, were less likely to be diagnosed with ARDS.
The study was supported by the European Society of Intensive Care Medicine, by St. Michael’s Hospital, Toronto, and by the University of Milan-Bicocca, Monza, Italy. The authors reported no conflicts of interest.
On Twitter @karioakes
ORLANDO – Many patients meeting the criteria for acute respiratory distress syndrome (ARDS) went unrecognized in a global sample of ICU patients, and those ARDS patients did not receive adjusted ventilator management or positioning and pharmacologic adjunctive treatments, based on the results of the LUNG SAFE study.
Enrolling nearly 30,000 patients in 50 countries on five continents, the LUNG SAFE study (Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure) looked for real-world answers to whether and how patients with ARDS are treated. The LUNG SAFE results were published concurrently with the presentation of results in a late-breaking session at the Critical Care Congress, sponsored by the Society of Critical Care Medicine (JAMA. 2016;315[8]:759-61). The first author is Dr. Giacomo Bellani, professor of medicine at the University of Milan-Bicocca, Monza, Italy, and Dr. John Laffey, professor of anesthesia, critical care, and physiology at the University of Toronto, presented the results at the meeting.
About 10% of the enrolled patients met ARDS criteria; of those, less than two-thirds received ventilator tidal volumes of 8 mL/kg or less of predicted body weight. Fewer than 18% of patients received positive end–expiratory pressure (PEEP) of more than 12 cm H2O, and clinicians used prone positioning for about 16% of patients with severe ARDS.
Clinicians recognized 60.2% of ARDS cases overall; recognition ranged from 51.3% of the cases of mild ARDS to 78.5% of the severe ARDS cases. For all patients, ARDS was associated with an in-hospital mortality rate of 40%. Nearly half of those with severe ARDS died, as did over a third of those with mild ARDS.
To this end, the LUNG SAFE investigators chose 4 consecutive weeks in the winter to enroll patients from a convenience sample of ICUs that they attempted to make broadly representative. They enrolled during February and March 2014 in the Northern hemisphere and July and August 2014 in the Southern hemisphere, and included all patients 16 years and older who were admitted to a participating ICU and received invasive or noninvasive ventilation.
Enrolled patients received daily evaluation for acute hypoxemic respiratory failure. Patients who met these criteria were then tracked with expanded data collection up to day 28 after respiratory failure was identified, or until ICU discharge or death. Overall, 3,022 patients met the Berlin Definition for ARDS. All but 436 patients (85.4%) received invasive ventilation, and those who did not were excluded from most data analysis.
One unexpected finding, said Dr. Laffey in an interview, was how common ARDS was in this ICU population. “Based on prior studies, we had anticipated finding an incidence of ARDS of approximately half of what we actually found in the LUNG SAFE study. We think that the difference is explained by the fact that we did not rely on clinician recognition of ARDS, but rather collected data directly on each of the Berlin diagnostic criteria, enabling us to make the diagnosis directly.” One possibility is that choosing the winter months for data collection may have resulted in overrepresentation of ARDS.
But Dr. Laffey said that LUNG SAFE’s most surprising finding was the low percentage of clinicians using higher PEEP levels. “It appeared that clinicians used lower-than-expected levels of PEEP, and that the use of PEEP didn’t increase in patients with the more severe forms of ARDS,” he said. “We think we need to increase our efforts to find more reliable ways to diagnose ARDS,” said Dr. Laffey. “While the reasons underlying clinician failure to recognize ARDS in critically ill patients are complex, the fact that there is no single test for diagnosing ARDS is a likely contributing factor.”
“This finding likely reflects the lack of a clear evidence base for the effectiveness of higher levels of PEEP in patients with ARDS” said Dr. Laffey. “It emphasizes the need for additional research to answer this and other important questions relating to the optimal treatment of patients with ARDS.”
However, if physicians did recognize ARDS, then they were more likely to use higher PEEPs (mean 8.9 cm H2O vs. 7.5 cm H2O for nonrecognized ARDS; P less than .001), prone positioning, and neuromuscular blockade (43.9% adjunctive treatment vs. 21.7% adjunctive treatment for nonrecognized ARDS; P less than .001), though they didn’t adjust the breath size used in ventilation.
In multivariable analysis, factors that made it more likely that ARDS would be recognized were higher nurse-to-patient and physician-to-patient ratios, younger patient age, lower Pa02/Fi02 ratios, and a pneumonia or pancreatitis diagnosis. Patients without an identified risk factor, and those with heart failure, were less likely to be diagnosed with ARDS.
The study was supported by the European Society of Intensive Care Medicine, by St. Michael’s Hospital, Toronto, and by the University of Milan-Bicocca, Monza, Italy. The authors reported no conflicts of interest.
On Twitter @karioakes
AT THE CRITICAL CARE CONGRESS
Key clinical point: Acute respiratory distress syndrome (ARDS) was under-recognized and did not receive adjusted management in a global sample of ICU patients.
Major finding: 10.4% of ICU patients fulfilled ARDS criteria; clinician recognition ranged from 51% to 78% of cases.
Data source: Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNG SAFE), an international multicenter prospective cohort study of 29,144 patients receiving invasive or noninvasive ventilation in 459 ICUs from 50 countries, over a period of 4 weeks in 2014.
Disclosures: The study was supported by the European Society of Intensive Care Medicine, by St. Michael’s Hospital, Toronto, and by the University of Milan-Bicocca, Monza, Italy. The authors reported no conflicts of interest.
2015-2016 flu vaccine 59% effective, CDC says
The overall rate of effectiveness for the 2015-2016 season’s influenza vaccine is 59%, according to preliminary data shared at a Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices meeting.
“This means that getting a flu vaccine this season reduced the risk of having to go to the doctor because of flu by nearly 60%,” Dr. Joseph Bresee – who heads the CDC’s Epidemiology and Prevention Branch – stated in a press release. “It’s good news and underscores the importance and the benefit of both annual and ongoing vaccination efforts this season.”
These findings are based on data collected by the U.S. Flu Vaccine Effectiveness Network between November 2, 2015, and February 12, 2016. A 59% effectiveness rate would be roughly in line with what previous years’ vaccines have rated when similar strains of influenza have been prevalent, according to the CDC.
Against the H1N1 strain, which was “responsible for most flu illness this season,” the influenza vaccine was 51% effective. Dr. Bresee noted that the CDC has received reports of unvaccinated individuals this season dying or falling seriously ill because of infection from the H1N1 strain.
The vaccine was 76% effective against all influenza B virus strains, and 79% effective against the B/Yamagata virus strains. There are not enough data at this time, however, to determine the vaccine’s effectiveness against B/Victoria or H3N2 strains.
The CDC cautions that, because the current influenza season is still weeks away from being over, the final rate of vaccine effectiveness for 2015-2016 may change once all the data are analyzed. On average, influenza seasons last 13 weeks.
“Flu activity this season started a bit later and has been lower so far than we’ve seen during the previous three seasons, but activity is still on the upswing and expected to continue for several weeks,” Dr. Bresee stated.
The overall rate of effectiveness for the 2015-2016 season’s influenza vaccine is 59%, according to preliminary data shared at a Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices meeting.
“This means that getting a flu vaccine this season reduced the risk of having to go to the doctor because of flu by nearly 60%,” Dr. Joseph Bresee – who heads the CDC’s Epidemiology and Prevention Branch – stated in a press release. “It’s good news and underscores the importance and the benefit of both annual and ongoing vaccination efforts this season.”
These findings are based on data collected by the U.S. Flu Vaccine Effectiveness Network between November 2, 2015, and February 12, 2016. A 59% effectiveness rate would be roughly in line with what previous years’ vaccines have rated when similar strains of influenza have been prevalent, according to the CDC.
Against the H1N1 strain, which was “responsible for most flu illness this season,” the influenza vaccine was 51% effective. Dr. Bresee noted that the CDC has received reports of unvaccinated individuals this season dying or falling seriously ill because of infection from the H1N1 strain.
The vaccine was 76% effective against all influenza B virus strains, and 79% effective against the B/Yamagata virus strains. There are not enough data at this time, however, to determine the vaccine’s effectiveness against B/Victoria or H3N2 strains.
The CDC cautions that, because the current influenza season is still weeks away from being over, the final rate of vaccine effectiveness for 2015-2016 may change once all the data are analyzed. On average, influenza seasons last 13 weeks.
“Flu activity this season started a bit later and has been lower so far than we’ve seen during the previous three seasons, but activity is still on the upswing and expected to continue for several weeks,” Dr. Bresee stated.
The overall rate of effectiveness for the 2015-2016 season’s influenza vaccine is 59%, according to preliminary data shared at a Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices meeting.
“This means that getting a flu vaccine this season reduced the risk of having to go to the doctor because of flu by nearly 60%,” Dr. Joseph Bresee – who heads the CDC’s Epidemiology and Prevention Branch – stated in a press release. “It’s good news and underscores the importance and the benefit of both annual and ongoing vaccination efforts this season.”
These findings are based on data collected by the U.S. Flu Vaccine Effectiveness Network between November 2, 2015, and February 12, 2016. A 59% effectiveness rate would be roughly in line with what previous years’ vaccines have rated when similar strains of influenza have been prevalent, according to the CDC.
Against the H1N1 strain, which was “responsible for most flu illness this season,” the influenza vaccine was 51% effective. Dr. Bresee noted that the CDC has received reports of unvaccinated individuals this season dying or falling seriously ill because of infection from the H1N1 strain.
The vaccine was 76% effective against all influenza B virus strains, and 79% effective against the B/Yamagata virus strains. There are not enough data at this time, however, to determine the vaccine’s effectiveness against B/Victoria or H3N2 strains.
The CDC cautions that, because the current influenza season is still weeks away from being over, the final rate of vaccine effectiveness for 2015-2016 may change once all the data are analyzed. On average, influenza seasons last 13 weeks.
“Flu activity this season started a bit later and has been lower so far than we’ve seen during the previous three seasons, but activity is still on the upswing and expected to continue for several weeks,” Dr. Bresee stated.
FROM AN ACIP MEETING
Brief measure helpful for insomnia complaints
LAS VEGAS – For patients who present with complaints of insomnia, consider using the principles of the Brief Behavioral Treatment of Insomnia tool as your frontline approach.
“It’s a pragmatic approach to treating insomnia complaints but also provides a useful foundation for the behavioral management of sleep disorders generally, when combined with other pharmacologic approaches,” Dr. Charles F. Reynolds III said at the annual psychopharmacology update held by the Nevada Psychiatric Association. “It involves relatively little time on the part of the clinician to apply.”
Part of the Brief Behavioral Treatment of Insomnia (BBTI), which was developed by Dr. Daniel J. Buysse and his colleagues at the University of Pittsburgh (Arch Intern Med. 2011;171[10]:887-95 and Behav Sleep Med. 2012;10[4]:266-79), involves education about lifestyle choices and practices that can be helpful to sleep, such as physical activity, stability of daily routines, treating medical problems that can interfere with sleep, having a comfortable sleep environment, and keeping the bed only for sleep and sexual intimacy.
The BBTI also educates the patient about practices that can be harmful to sleep, including alcohol, caffeine, worries, a poor sleep environment, or using the bed for things other than sleep or sex. “You can go a long way toward helping people with sleep-wake problems if they’re well educated about these issues, and if they keep a diary so that they can engage in self-monitoring,” Dr. Reynolds said.
He went on to note that the principles of the BBTI are grounded within an understanding of the physiological factors that regulate sleep. “One factor that controls sleep is how long you’ve been awake, the so-called homeostatic drive to sleep,” said Dr. Reynolds, Endowed Professor of Geriatric Psychiatry at the University of Pittsburgh. “Another factor is the biological clock, the circadian rhythms deep within the brain stem that govern the timing of sleep onset and offset. These two physiological processes provide the basis for the behavioral prescriptions that we make in brief behavioral treatment for insomnia.”
The four steps of the BBTI involve the following:
• Reduce time in bed. This doesn’t mean decreasing the amount of sleep per se, but rather the amount of wakefulness that can occur during a night. “By reducing time in bed we’re trying to increase the homeostatic drive to sleep,” said Dr. Reynolds, also director of the University of Pittsburgh Medical Center Aging Institute. “Being awake longer leads to quicker, deeper, more solid sleep.”
• Get up at the same time every day of the week. This practice “provides a kind of circadian anchor to the brain’s sleep wave rhythms, and reinforces those rhythms, and hence the efficiency of sleep,” he explained. “Even if you’ve slept poorly, getting up at the same time helps you to sleep better the next night.”
• Don’t go to bed unless sleepy. This strategy helps to increase sleep drive by keeping you awake longer. “Going to bed when you’re not sleepy can lead to frustration and gives your brain the wrong message,” he said. “The principles of stimulus control and temporal control are at the behavioral root of BBTI prescriptions.”
• Don’t stay in bed unless asleep. “We are teaching patients how to associate lying in bed with sleeping, and not with worrying or other activities that may lead to frustration or hyperarousal,” he said.
Studies have demonstrated that BBTI produces improvement in 70%-80% of patients. “It is a briefer, less complicated approach than traditional cognitive-behavioral therapy for insomnia,” Dr. Reynolds said. “BBTI also provides a wonderful behavioral foundation for intelligent evidence-based pharmacotherapy for common sleep disorders.”
If it’s determined that pharmacologic treatment for insomnia is indicated, Dr. Reynolds cautioned that the margin of safety for benzodiazepines such as temazepam is wide. Contraindications include obstructive sleep apnea, substance abuse disorders, and advanced liver disease. Side effects may include daytime sedation, anterograde amnesia, sleepwalking, sleep-related eating disorder, respiratory depression, and in some cases, rebound insomnia.
“In my practice, I tend to use shorter-acting agents often as an augmentation pharmacotherapy,” he said. “For example, if I’m treating an older adult with depression, I may use an antidepressant as the primary pharmacotherapy and then add a low-dose benzodiazepine to help keep them more comfortable and help them sleep better during the initial phases of treatment.”
Studies of the orexin receptor antagonist suvorexant have shown improved sleep time, falling asleep faster, staying asleep longer, and sleeping more throughout the night. However, “there are some possibilities for daytime hangover,” Dr. Reynolds said. “I’m also not clear on how well suvorexant’s effects on breathing during sleep have been evaluated. In my view, that remains a somewhat open question.”
The two most widely used sedating antidepressants for insomnia problems include doxepin and trazodone. “They can be particularly helpful in patients with co-occurring depression,” he said. Doxepin in low doses is approved for sleep maintenance insomnia.
Ramelteon is the first melatonin receptor agonist approved for the treatment of insomnia. Contraindications include a history of angioedema with concurrent use of fluvoxamine.
“In general, I recommend to my colleagues not to use sedative antipsychotics like quetiapine or olanzapine, particularly in the context of elderly with dementing disorders and sleep-wake disturbances,” he said.
Dr. Reynolds reported having no relevant financial conflicts.
LAS VEGAS – For patients who present with complaints of insomnia, consider using the principles of the Brief Behavioral Treatment of Insomnia tool as your frontline approach.
“It’s a pragmatic approach to treating insomnia complaints but also provides a useful foundation for the behavioral management of sleep disorders generally, when combined with other pharmacologic approaches,” Dr. Charles F. Reynolds III said at the annual psychopharmacology update held by the Nevada Psychiatric Association. “It involves relatively little time on the part of the clinician to apply.”
Part of the Brief Behavioral Treatment of Insomnia (BBTI), which was developed by Dr. Daniel J. Buysse and his colleagues at the University of Pittsburgh (Arch Intern Med. 2011;171[10]:887-95 and Behav Sleep Med. 2012;10[4]:266-79), involves education about lifestyle choices and practices that can be helpful to sleep, such as physical activity, stability of daily routines, treating medical problems that can interfere with sleep, having a comfortable sleep environment, and keeping the bed only for sleep and sexual intimacy.
The BBTI also educates the patient about practices that can be harmful to sleep, including alcohol, caffeine, worries, a poor sleep environment, or using the bed for things other than sleep or sex. “You can go a long way toward helping people with sleep-wake problems if they’re well educated about these issues, and if they keep a diary so that they can engage in self-monitoring,” Dr. Reynolds said.
He went on to note that the principles of the BBTI are grounded within an understanding of the physiological factors that regulate sleep. “One factor that controls sleep is how long you’ve been awake, the so-called homeostatic drive to sleep,” said Dr. Reynolds, Endowed Professor of Geriatric Psychiatry at the University of Pittsburgh. “Another factor is the biological clock, the circadian rhythms deep within the brain stem that govern the timing of sleep onset and offset. These two physiological processes provide the basis for the behavioral prescriptions that we make in brief behavioral treatment for insomnia.”
The four steps of the BBTI involve the following:
• Reduce time in bed. This doesn’t mean decreasing the amount of sleep per se, but rather the amount of wakefulness that can occur during a night. “By reducing time in bed we’re trying to increase the homeostatic drive to sleep,” said Dr. Reynolds, also director of the University of Pittsburgh Medical Center Aging Institute. “Being awake longer leads to quicker, deeper, more solid sleep.”
• Get up at the same time every day of the week. This practice “provides a kind of circadian anchor to the brain’s sleep wave rhythms, and reinforces those rhythms, and hence the efficiency of sleep,” he explained. “Even if you’ve slept poorly, getting up at the same time helps you to sleep better the next night.”
• Don’t go to bed unless sleepy. This strategy helps to increase sleep drive by keeping you awake longer. “Going to bed when you’re not sleepy can lead to frustration and gives your brain the wrong message,” he said. “The principles of stimulus control and temporal control are at the behavioral root of BBTI prescriptions.”
• Don’t stay in bed unless asleep. “We are teaching patients how to associate lying in bed with sleeping, and not with worrying or other activities that may lead to frustration or hyperarousal,” he said.
Studies have demonstrated that BBTI produces improvement in 70%-80% of patients. “It is a briefer, less complicated approach than traditional cognitive-behavioral therapy for insomnia,” Dr. Reynolds said. “BBTI also provides a wonderful behavioral foundation for intelligent evidence-based pharmacotherapy for common sleep disorders.”
If it’s determined that pharmacologic treatment for insomnia is indicated, Dr. Reynolds cautioned that the margin of safety for benzodiazepines such as temazepam is wide. Contraindications include obstructive sleep apnea, substance abuse disorders, and advanced liver disease. Side effects may include daytime sedation, anterograde amnesia, sleepwalking, sleep-related eating disorder, respiratory depression, and in some cases, rebound insomnia.
“In my practice, I tend to use shorter-acting agents often as an augmentation pharmacotherapy,” he said. “For example, if I’m treating an older adult with depression, I may use an antidepressant as the primary pharmacotherapy and then add a low-dose benzodiazepine to help keep them more comfortable and help them sleep better during the initial phases of treatment.”
Studies of the orexin receptor antagonist suvorexant have shown improved sleep time, falling asleep faster, staying asleep longer, and sleeping more throughout the night. However, “there are some possibilities for daytime hangover,” Dr. Reynolds said. “I’m also not clear on how well suvorexant’s effects on breathing during sleep have been evaluated. In my view, that remains a somewhat open question.”
The two most widely used sedating antidepressants for insomnia problems include doxepin and trazodone. “They can be particularly helpful in patients with co-occurring depression,” he said. Doxepin in low doses is approved for sleep maintenance insomnia.
Ramelteon is the first melatonin receptor agonist approved for the treatment of insomnia. Contraindications include a history of angioedema with concurrent use of fluvoxamine.
“In general, I recommend to my colleagues not to use sedative antipsychotics like quetiapine or olanzapine, particularly in the context of elderly with dementing disorders and sleep-wake disturbances,” he said.
Dr. Reynolds reported having no relevant financial conflicts.
LAS VEGAS – For patients who present with complaints of insomnia, consider using the principles of the Brief Behavioral Treatment of Insomnia tool as your frontline approach.
“It’s a pragmatic approach to treating insomnia complaints but also provides a useful foundation for the behavioral management of sleep disorders generally, when combined with other pharmacologic approaches,” Dr. Charles F. Reynolds III said at the annual psychopharmacology update held by the Nevada Psychiatric Association. “It involves relatively little time on the part of the clinician to apply.”
Part of the Brief Behavioral Treatment of Insomnia (BBTI), which was developed by Dr. Daniel J. Buysse and his colleagues at the University of Pittsburgh (Arch Intern Med. 2011;171[10]:887-95 and Behav Sleep Med. 2012;10[4]:266-79), involves education about lifestyle choices and practices that can be helpful to sleep, such as physical activity, stability of daily routines, treating medical problems that can interfere with sleep, having a comfortable sleep environment, and keeping the bed only for sleep and sexual intimacy.
The BBTI also educates the patient about practices that can be harmful to sleep, including alcohol, caffeine, worries, a poor sleep environment, or using the bed for things other than sleep or sex. “You can go a long way toward helping people with sleep-wake problems if they’re well educated about these issues, and if they keep a diary so that they can engage in self-monitoring,” Dr. Reynolds said.
He went on to note that the principles of the BBTI are grounded within an understanding of the physiological factors that regulate sleep. “One factor that controls sleep is how long you’ve been awake, the so-called homeostatic drive to sleep,” said Dr. Reynolds, Endowed Professor of Geriatric Psychiatry at the University of Pittsburgh. “Another factor is the biological clock, the circadian rhythms deep within the brain stem that govern the timing of sleep onset and offset. These two physiological processes provide the basis for the behavioral prescriptions that we make in brief behavioral treatment for insomnia.”
The four steps of the BBTI involve the following:
• Reduce time in bed. This doesn’t mean decreasing the amount of sleep per se, but rather the amount of wakefulness that can occur during a night. “By reducing time in bed we’re trying to increase the homeostatic drive to sleep,” said Dr. Reynolds, also director of the University of Pittsburgh Medical Center Aging Institute. “Being awake longer leads to quicker, deeper, more solid sleep.”
• Get up at the same time every day of the week. This practice “provides a kind of circadian anchor to the brain’s sleep wave rhythms, and reinforces those rhythms, and hence the efficiency of sleep,” he explained. “Even if you’ve slept poorly, getting up at the same time helps you to sleep better the next night.”
• Don’t go to bed unless sleepy. This strategy helps to increase sleep drive by keeping you awake longer. “Going to bed when you’re not sleepy can lead to frustration and gives your brain the wrong message,” he said. “The principles of stimulus control and temporal control are at the behavioral root of BBTI prescriptions.”
• Don’t stay in bed unless asleep. “We are teaching patients how to associate lying in bed with sleeping, and not with worrying or other activities that may lead to frustration or hyperarousal,” he said.
Studies have demonstrated that BBTI produces improvement in 70%-80% of patients. “It is a briefer, less complicated approach than traditional cognitive-behavioral therapy for insomnia,” Dr. Reynolds said. “BBTI also provides a wonderful behavioral foundation for intelligent evidence-based pharmacotherapy for common sleep disorders.”
If it’s determined that pharmacologic treatment for insomnia is indicated, Dr. Reynolds cautioned that the margin of safety for benzodiazepines such as temazepam is wide. Contraindications include obstructive sleep apnea, substance abuse disorders, and advanced liver disease. Side effects may include daytime sedation, anterograde amnesia, sleepwalking, sleep-related eating disorder, respiratory depression, and in some cases, rebound insomnia.
“In my practice, I tend to use shorter-acting agents often as an augmentation pharmacotherapy,” he said. “For example, if I’m treating an older adult with depression, I may use an antidepressant as the primary pharmacotherapy and then add a low-dose benzodiazepine to help keep them more comfortable and help them sleep better during the initial phases of treatment.”
Studies of the orexin receptor antagonist suvorexant have shown improved sleep time, falling asleep faster, staying asleep longer, and sleeping more throughout the night. However, “there are some possibilities for daytime hangover,” Dr. Reynolds said. “I’m also not clear on how well suvorexant’s effects on breathing during sleep have been evaluated. In my view, that remains a somewhat open question.”
The two most widely used sedating antidepressants for insomnia problems include doxepin and trazodone. “They can be particularly helpful in patients with co-occurring depression,” he said. Doxepin in low doses is approved for sleep maintenance insomnia.
Ramelteon is the first melatonin receptor agonist approved for the treatment of insomnia. Contraindications include a history of angioedema with concurrent use of fluvoxamine.
“In general, I recommend to my colleagues not to use sedative antipsychotics like quetiapine or olanzapine, particularly in the context of elderly with dementing disorders and sleep-wake disturbances,” he said.
Dr. Reynolds reported having no relevant financial conflicts.
EXPERT ANALYSIS AT THE NPA PSYCHOPHARMACOLOGY UPDATE